Abstract
Purpose
To investigate CT pulmonary angiography findings of pulmonary thromboembolism (PTE) in coronavirus disease 2019 (COVID-19) and its association with clinical and radiologic conditions.
Materials and Methods
This retrospective study includes 109 hospitalized patients with COVID-19 who underwent CT pulmonary angiography for suspected PTE from March 20 to May 3, 2020. Data were collected from our PACS. CT pulmonary angiography findings of PTE were evaluated. On the basis of the presence or absence of PTE, patients were divided into two groups, and their clinical and radiologic conditions were compared using the Mann–Whitney U test and χ2 test.
Results
The study population comprised 82 men and 19 women, with a mean age of 64.1 years ± 15.0 (95% confidence interval [CI]: 60.4, 67.6) years. CT pulmonary angiography was performed 19.8 days ± 6.1 (95% CI: 18.1, 20.2) after symptom onset and 10.5 days ± 3.8 (95% CI: 10.2, 12.9) after admission. Of 101 patients, 41 had PTE (40.6%). PTE was mostly bilateral or only right (37/41 [90.2%]), mainly involved segmental (37/41 [90.2%]) or subsegmental (25/41 [61.0%]) arteries and affected mainly the branches of the lower lobe (30/41 [73.2%]). Parenchymal segments supplied by segmental arteries with PTE showed a prevalent consolidation pattern (25/37 [67.6%]). Deep vein thrombosis was present only in five of 41 (12.2%) patients. Comparing groups with and without PTE, no significant difference was observed in age, sex, symptom onset, comorbidities, tumor history, use of respiratory supports, activated partial thromboplastin time, prothrombin time, and deep vein thrombosis. Conversely, differences were evaluated in CT lesion score (15.7 ± 1.4 [95% CI: 15.3, 16.1] vs 14.1 ± 1.1 [95% CI: 13.8, 14.4]; P = .035), d-dimer level (P < .001), lactate dehydrogenase level (P < .001), and C-reactive protein level (P = .042).
Conclusion
PTE in COVID-19 involves mainly the segmental and subsegmental arteries of segments affected by consolidations in patients with more severe lung disease. The authors hypothesize that the development of PTE in COVID-19 might be a pulmonary artery thrombosis because of severe lung inflammation and hypercoagulability rather than thromboembolism.
© RSNA, 2020
Summary
Pulmonary thromboembolism in hospitalized patients with COVID-19 involves mainly the segmental and subsegmental arteries of the segments affected by consolidations in patients with more severe COVID-19 pneumonia as scored by using CT and with increased laboratorial biomarkers (d-dimer, lactate dehydrogenase, and C-reactive protein levels).
Key Points
■ Pulmonary thromboembolism (PTE) is frequently observed in patients with COVID-19, mainly involving the segmental (90.2%) and subsegmental arteries (61.0%) of pulmonary segments affected by a consolidation pattern (67.6%).
■ Patients with more severe COVID-19 lung disease (higher CT lesion score and d-dimer, lactate dehydrogenase, and C-reactive protein levels) tend to be more affected by PTE.
■ The authors hypothesize that the development of PTE in COVID-19 could possibly be a pulmonary artery thrombosis because of severe lung inflammation and a hypercoagulable state rather than common thromboembolism.
Introduction
Coronavirus disease 2019 (COVID-19) is a viral disease caused by severe acute respiratory syndrome coronavirus 2, which was first detected in Wuhan, China, in December 2019 (1). Real-time reverse-transcription polymerase chain reaction of viral nucleic acid is regarded as the reference standard in the diagnosis of COVID-19 (2). CT imaging is helpful in the early detection of COVID-19 pneumonia, in the differentiation among clinical stages, and could provide information about disease progression (3,4).
As described by a recent study, blood hypercoagulability is common among hospitalized patients with COVID-19 (5). In particular, the effect of severe acute respiratory syndrome coronavirus 2 infection on pulmonary coagulation is regulated by different pathophysiologic mechanisms (6). It has been reported that coagulation is activated in response to proinflammatory cytokines and is accelerated by the dysfunction of endothelial cells induced by infection (7). In addition, hypoxia, a common finding in severe pneumonia, can stimulate thrombosis not only by increasing blood viscosity but also by a hypoxia-inducible transcription factor–dependent signaling pathway (8). Therefore, anticoagulation therapy has been recommended for patients with severe COVID-19 (9,10). Despite systematic thromboprophylaxis, Klok et al (11) reported an incidence of 31% for thrombotic complications in intensive care unit patients with COVID-19.
Few studies and isolated clinical cases of COVID-19 pneumonia with pulmonary thromboembolism (PTE) have been recently published (12–16). Poissy et al (15) postulated an increased prevalence of PTE in patients with COVID-19, and Danzi et al (16) suggested that severe COVID-19 pneumonia could be a precipitant factor for acute PTE in the absence of major predisposing factors.
The awareness of such complications is essential because it could cause clinical deterioration (17). Therefore, the aim of this study is to investigate CT pulmonary angiography findings of PTE in patients with COVID-19 and its association with clinical and radiologic conditions.
Materials and Methods
Study Population
All procedures performed on studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Our study protocol was approved by the institutional review board of our hospital; written informed consent was waived. No industry support was given.
This retrospective study includes 109 hospitalized patients with COVID-19 confirmed by real-time reverse-transcription polymerase chain reaction on oropharyngeal swab, who underwent CT pulmonary angiography for suspected PTE from March 20 to May 3, 2020. Data were collected from the PACS of “Infermi” Hospital Rimini, a tertiary center in Northern Italy. All CT pulmonary angiographic examinations were performed because of the sudden onset of clinical deterioration with unexplained worsening of dyspnea, symptoms suggestive for PTE, d-dimer elevation, or in the case of mismatch between clinical worsening and chest radiograph stability. Eight patients with nondiagnostic CT pulmonary angiography findings were excluded: five because of motion artifacts and three because of attenuation values in the mean pulmonary artery <250 HU; this threshold is considered optimal for PTE evaluation (18,19). Our final cohort was composed of 101 patients.
CT Acquisition Protocol and Postprocessing
CT examinations were performed using a 64-row multidetector CT scanner (LightSpeed VCT; GE Healthcare, United States.); patients laid in supine position with arms elevated. A 20-gauge cannula was inserted into a superficial vein of the right antecubital fossa, connected to a two-way power injector. All patients were examined with the same standardized protocol, based on two acquisitions from the lung apices to the costophrenic recess, first a non–contrast-enhanced scan, then a contrast-enhanced scan. The scan parameters were as follows: tube voltage = 120 kVp, with automatic tube current modulation; pitch = 0.95; matrix = 512 × 512 pixels; and field of view = 350 mm × 350 mm. A volume of 70 mL of contrast medium (Iomeprol, 400 mgI/mL; Bracco Imaging, Milan, Italy) was injected at a flow rate of 4 mL/sec with a bolus tracking technique, followed by 40 mL of saline solution at the same rate. A circular region of interest was placed within the mean pulmonary artery; the acquisition started automatically when a threshold of 100 HU was reached. All images were reconstructed with a slice thickness of 0.625–1.250 mm with the same increment, using an iterative reconstruction algorithm. The lung parenchyma was evaluated using a sharp filter with a window/level of 1200/−600 HU, and mediastinal structures were evaluated using a softer filter with a window/ level of 350/50 HU. Multiplanar reconstruction and maximal intensity projection were also generated.
CT Evaluation
Two radiologists in consensus (one with >5 years [F.F.] and the other with >20 years [F.M.] of experience in chest imaging) retrospectively evaluated the CT examinations.
A semiquantitative scoring system was used to quantitatively estimate the pulmonary involvement of all these abnormalities based on the area involved. Each lobe was visually scored from 0 to 5 as follows: 0, no involvement; 1, <5% involvement; 2, 25% involvement; 3, 26%–49% involvement; 4, 50%–75% involvement; 5, >75% involvement. The total CT score was the sum of the individual lobar scores and ranged from 0 (no involvement) to 25 (maximum involvement) (20). The following diagnostic criteria for PTE were used: intraluminal filling defects or nonvisualization of the pulmonary arteries compared with the contralateral side. The obstruction CT index was calculated as described by Qanadli et al (21). Pulmonary artery diameter and right ventricle dilatation were evaluated.
Clinical and Laboratory Analysis
For each patient, age, sex, onset symptoms (fever, cough, dyspnea, myalgia or fatigue, diarrhea, nausea, and vomiting), number of comorbidities, smoking history, and oncologic history were collected. Moreover, for each patient, the following laboratory values were collected within 48 hours after the CT examinations: activated partial thromboplastin time (aPTT), prothrombin time (PT), lactate dehydrogenase (LDH) level, C-reactive protein (CRP) level, and d-dimer level. The use of mechanical respiratory support, presence of deep venous thrombosis (DVT), and the type of therapy were annotated. Finally, the CT score of the pulmonary lesions at noncontrast acquisition was collected. Venous duplex US of the lower extremities was performed routinely in all patients.
Statistical Analysis
A dedicated statistical software was used (MedCalc v19.1.6; MedCalc Software, Ostend, Belgium) for statistical analysis. Continuous variables were reported as mean ± standard deviation, and categorical variables were reported as counts and percentages. CT pulmonary angiography findings suggestive of PTE were evaluated. On the basis of the presence or absence of PTE, patients were subdivided into two groups: group 1, with PTE and group 2, without PTE. Age, sex, onset symptoms (such as fever, cough, dyspnea), number of comorbidities, smoking history—oncologic history, use of ventilatory support, aPTT, PT, LDH, CRP, d-dimer, presence of DVT, and CT score were compared between two groups, using Mann–Whitney U test and χ2 test for continuous and categorical variables, respectively. This was a convenience sample; therefore, no statistical power analysis was performed a priori for comparing the distribution of variables between groups 1 and 2. A P value < .05 was defined as statistically significant.
Results
Patient Population
Among the 101 included patients, 82 (81.2%) were men and 19 (18.8%) were women, with a mean age of 64.1 years ± 15.0 (standard deviation) (95% confidence interval [CI]: 60.4, 67.6). The main onset symptoms were fever (70.3%) and cough (56.4%). Rates of other onset symptoms, number of comorbidities, smoking history, and tumor history are provided in Table 1.
Table 1:
Clinical Features of Patient Population
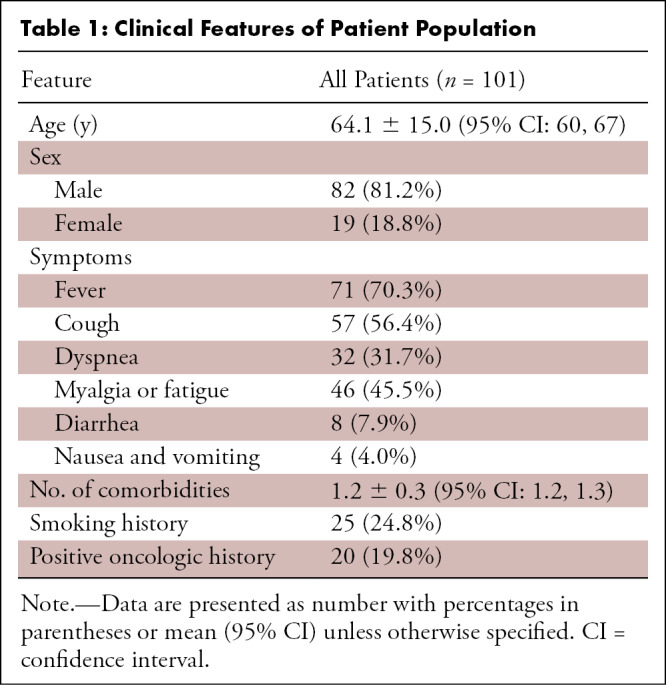
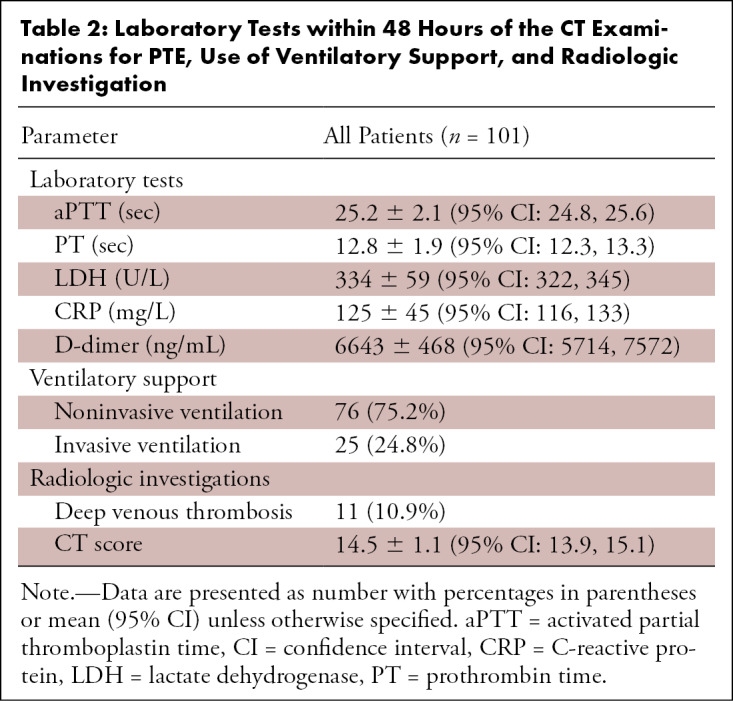
In both groups, the most frequent tumor in patients with positive oncologic history was prostate cancer. Laboratory tests within 48 hours after the CT examinations for PTE such as aPTT, PT, LDH, CRP, and d-dimer, the use of respiratory supports, the presence of DVT, and CT score are summarized in Table 2. All patients were treated with antivirals, anticoagulants, and hydroxychloroquine. Thromboembolism prophylaxis consisted of subcutaneous enoxaparin 4000 IU.
Table 2:
Laboratory Tests within 48 Hours of the CT Examinations for PTE, Use of Ventilatory Support, and Radiologic Investigation
PTE Findings
CT pulmonary angiographic examinations were performed 19.8 days ± 6.1 (95% CI: 18.1, 20.2) after symptom onset and 10.5 days ± 3.8 (95% CI: 10.2, 12.9) after admission (Figs 1–3). PTE was observed in 41 of 101 (40.6%) patients, 35 men and six women; it was bilateral in 20 (48.7%), only right in 17 (41.5%), and only left in four (9.8%) patients. PTE involved only the pulmonary trunk in one of 41 (2.4%), the main pulmonary arteries in nine of 41 (22.0%), and lobar arteries in 21 of 41 (51.2%) cases. Segmental arteries were involved in 37 of 41 (90.2%) and subsegmental arteries in 25 of 41 (61.0%) cases. The arteries for the upper lobes were involved in five of 41 (12.2%), for the middle lobe and the lingula in six of 41 (14.6%), and for the lower lobes in 30 of 41 (73.2%) patients. Parenchymal segments supplied by segmental arteries with PTE showed a predominant consolidation pattern in 25 of 37 (67.6%), predominant ground-glass pattern in 11 of 37 (29.7%), and no opacities in one of 37 (2.7%) patients. The number of cases with exclusive involvement of segmental arteries was 14 of 41 (34.1%) and that with exclusive involvement of subsegmental arteries was eight of 41 (19.5%). The mean obstruction CT index was 31.5% ± 14. Dilatation of the pulmonary artery was observed in seven of 41 (17.0%) patients and dilatation of the right ventricle in two of 41 (4.9%) patients.
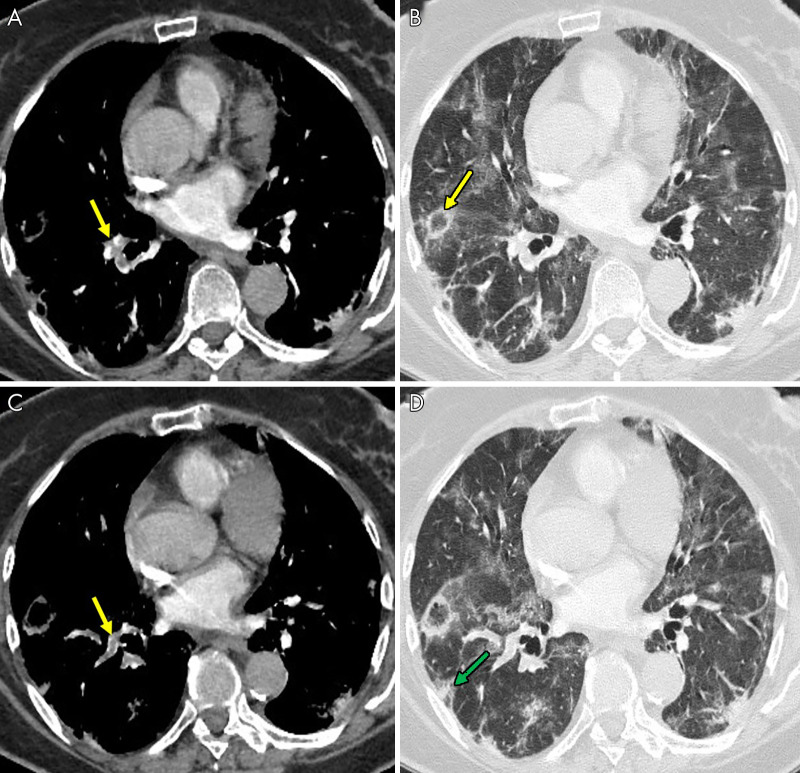
Figure 1:
An 85-year-old man, after 16 days of hospitalization in the intensive care unit with invasive ventilation, had a sudden increase in d-dimer value. The images show, A–C, pulmonary thromboembolism in the segmental arteries for the lower right lobe (yellow arrows). B, Lung parenchyma is characterized by reverse halo sign (yellow arrow) and, D, diffuse ground-glass and peripheral subpleural wedge-shaped consolidation in lower lung lobe (green arrow).
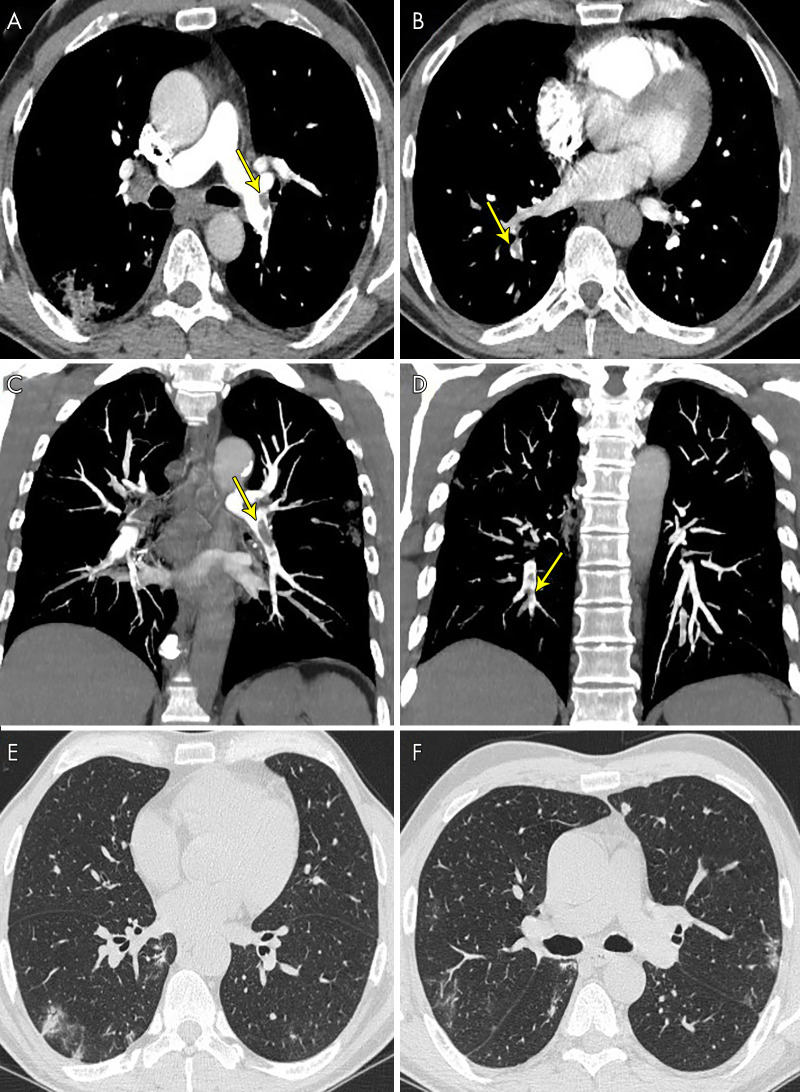
Figure 3:
A 63-year-old man, after 17 days of hospitalization without respiratory support, had a sudden increase in d-dimer value. The images show pulmonary thromboembolism, A, in the lobar arteries for the left lower lobe (yellow arrow) and, B, in the segmental arteries for the right lower lobe (yellow arrows), C, D, confirmed by maximum intensity projection reconstructions (yellow arrows). E, F, Lung parenchyma is characterized by some peripheral subpleural bilateral ground-glass opacities and consolidations.
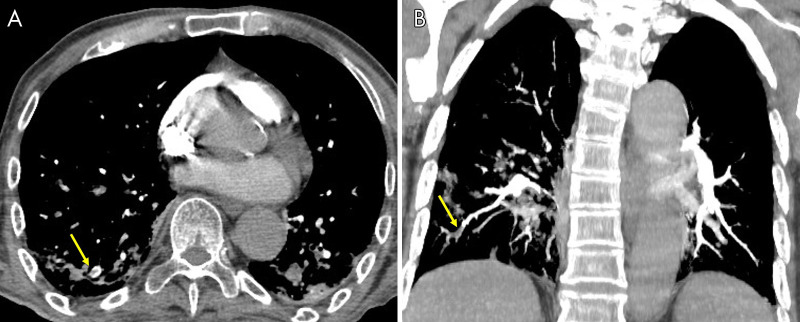
Figure 2:
A 71-year-old woman, after 21 days of hospitalization with noninvasive ventilation, had sudden dyspnea and an increase in d-dimer value. The images show, A, pulmonary thromboembolism in the segmental and subsegmental arteries for the lower right lobe (yellow arrow), B, confirmed by a maximum intensity projection reconstruction (yellow arrow).
Comparison between Groups with and without PTE
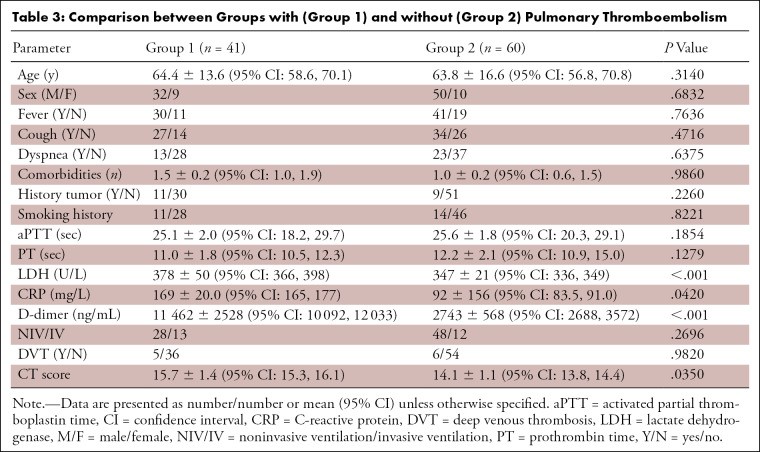
Comparing groups with and without PTE, no statistically significant difference was observed in age; sex; presentation of symptoms such as fever, cough, and dyspnea; number of comorbidities; smoking or oncologic histories; use of ventilatory support; aPTT; PT; or presence of DVT. Conversely, differences were noted in the CT score (15.7 ± 1.4 [95% CI: 15.3, 16.1] vs 14.1 ± 1.1 [95% CI: 13.8, 14.4]; P = .0350); d-dimer (P < .001) level, LDH (P < .001) level, and CRP level (P = .0420). Results are shown in Table 3.
Table 3:
Comparison between Groups with (Group 1) and without (Group 2) Pulmonary Thromboembolism
Discussion
Blood hypercoagulability is a common finding among hospitalized patients with COVID-19 (5). The effect of COVID-19 on pulmonary coagulation and the resulting development of PTE could be because of proinflammatory cytokines, endothelial dysfunction, and hypoxia (6,8). Several articles report high incidence of PTE in COVID-19, ranging from 22% to 39% (10–15,17,22): the awareness of such complication is of vital importance because it can lead to severe clinical deterioration.
We carried out a retrospective study to investigate CT pulmonary angiography findings of PTE in hospitalized patients with COVID-19 and its association with radiologic and clinical parameters. In our study, thromboembolism was confirmed in a high percentage of patients (40.6%). The overall prevalence of PTE in our series is higher than that previously reported in the literature (22%–39%) (10–15,17,22). A possible explanation of this high frequency is that we collected patients from the beginning of the pandemic, who did not undergo appropriate prophylaxis. Our findings differ from the study of Grillet et al (12) who reported lower PTE incidence (23%) in patients with severe COVID-19. The two studies differ in some aspects; in the latter, information about d-dimer was not collected and association between CT severity score and PTE was not attempted. Our study confirmed that more severe COVID-19 pneumonia, as scored by using CT, and increased laboratorial biomarker levels (d-dimer, LDH, and CRP), were more commonly associated with PTE. However, Grillet et al (12) did report an association between invasive mechanical ventilation and the male sex with PTE, which was not evident in our cohort.
We hypothesized that PTE is a common complication of severe COVID-19 pneumonia occurring a few weeks after symptom onset. In support of our hypothesis, we carried out a literature review about PTE in previous similar pandemics caused by severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus. Severe acute respiratory syndrome coronavirus infection has been responsible for various coagulation abnormalities and multiple vascular thromboses commonly seen in lungs, suggesting that an underlying thrombophilic state can induce pulmonary artery thrombi formation (23). In the same way, hematologic manifestations of Middle East respiratory syndrome include thrombocytopenia and coagulopathy with an associated status of disseminated intravascular coagulation at different sites, including the pulmonary arteries (24).
In this study, PTE was mostly bilateral (48.7%) or only involving the right lung (41.5%); it often involved the small caliber peripheral pulmonary arteries, such as segmental (90.2%) or subsegmental (61.0%) branches, as already described by Chen et al (22), especially in the lower lung lobes. Owing to the small caliber of the vessels involved, the main PTE finding was vascular cutoff. Indeed, it is of interest to note that parenchymal segments supplied by segmental arteries with PTE often showed the prevalence of a CT consolidation pattern in 25 of 37 (67.6%) cases. Besides, the number of exclusively segmental arteries (34.1%) or only subsegmental arteries involved (19.5%) is higher than that reported in non–COVID-19 series (25,26). These sites seem to correspond to the most common distribution of COVID-19 opacities (27). Using a parallel rationale, Zotzmann et al (28) suggested that subpleural consolidations in COVID-19 should prompt a further diagnostic workup because of the potential occurrence of PTE.
In our study, lower extremity venous duplex US was performed in all hospitalized patients; interestingly, DVT was rarely concomitant with PTE (12.2%). When comparing groups with and without PTE, no differences were noted in the presence of DVT (P = .982). However, we cannot exclude that thrombi arising from different body regions could have contributed to the PTE observed in our series because duplex US would miss thrombi residing in the abdominopelvic or upper limbs. Besides, we cannot exclude that patients who had DVT with no PTE could have developed PTE later on because a hypercoagulable state is also present in these patients.
Moreover, between the two groups with and without PTE, no difference was observed according to symptoms at presentation of COVID-19, number of comorbidities, oncologic history, or use of respiratory support. Thus, we hypothesize that the development of PTE in severe COVID-19 pneumonia could be related to severe lung inflammation rather than to common risks factors for PTE or DVT. Considering potential limitations related to the narrow statistical power of our small sample, these preliminary findings—as also stated by previous authors (15)—may support the hypothesis that filling defects seen in pulmonary artery branches could be related to thrombosis rather than thromboembolic phenomena in COVID-19. This could have clinical implications as this hypothesis could support the indication of anticoagulant therapy as part of the first-line therapeutic approach, regardless of traditional risk factors for DVT (29,30). Our assumption seems to be confirmed by the significant higher values of d-dimer level (P <.001), LDH level (P <.001), CRP level (P = .042), and CT lesion score (P = .035). Further studies are still needed to settle the discussion between the thrombosis versus embolic hypothesis, as well as to evaluate the prognostic role of PTE in these patients and to evaluate the management of anticoagulants.
Our study has some limitations. First, it was retrospective and monocentric, leading to an increased risk for selection bias. Second, the influence of the therapies was not evaluated. Third, the study design and relatively small sample size of patients with PTE have limited power to definitively address the thrombosis versus embolism conundrum.
In conclusion, PTE is frequently observed in patients with COVID-19 and mainly involves the segmental and subsegmental arteries supplying the lung parenchyma affected by consolidation. Patients with more severe COVID-19 pneumonia, as scored by using CT, and with increased laboratorial biomarkers (d-dimer, LDH, and CRP levels), were more commonly affected by PTE. These findings are consistent with other observations showing that COVID-19 triggers a hypercoagulable state and also contribute to the discussion between pulmonary artery thrombosis versus venous thromboembolism as potential hypotheses for this phenomenon.
Disclosures of Conflicts of Interest: E.C. disclosed no relevant relationships. F.M. disclosed no relevant relationships. F.F. disclosed no relevant relationships.
Abbreviations:
- aPTT
- activated partial thromboplastin time
- CI
- confidence interval
- COVID-19
- coronavirus disease 2019
- CRP
- C-reactive protein
- DVT
- deep vein thrombosis
- LDH
- lactate dehydrogenase
- PT
- prothrombin time
- PTE
- pulmonary thromboembolism
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382(8):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020. 10.1148/radiol.2020200642. Published online February 26, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Radiology 2020;296(1):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caruso D, Zerunian M, Polici M, et al. Chest CT features of COVID-19 in Rome, Italy. Radiology 2020. 10.1148/radiol.2020201237. Published online April 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol 2020;95(7):834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497–506 [Published correction appears in Lancet 2020;395(10223):496.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 2020;58(7):1116–1120. [DOI] [PubMed] [Google Scholar]
- 8.Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis 2020. 10.1007/s11239-020-02105-8. Published online April 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18(5):1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost 2020;18(7):1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology 2020. 10.1148/radiol.2020201544. Published online April 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leonard-Lorant I, Delabranche X, Severac F, et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology 2020. 10.1148/radiol.2020201561. Published online April 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Y, Wang X, Yang P, Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiol Cardiothorac Imaging 2020;2(2):e200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation 2020. 10.1161/CIRCULATIONAHA.120.047430. Published online April 24, 2020. [DOI] [PubMed] [Google Scholar]
- 16.Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J 2020;41(19):1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotzinger DC, Beigelman-Aubry C, von Garnier C, Qanadli SD. Pulmonary embolism in patients with COVID-19: time to change the paradigm of computed tomography. Thromb Res 2020;190:58–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palacio D, Benveniste MF, Betancourt-Cuellar SL, Gladish GW. Multidetector computed tomography pulmonary angiography pitfalls in the evaluation of pulmonary embolism with emphasis in technique. Semin Roentgenol 2015;50(3):217–225. [DOI] [PubMed] [Google Scholar]
- 19.Gilman MD, Kazerooni EA. Standardized Reporting of CT Pulmonary Angiography for Acute Pulmonary Embolism. Individual or Group PQI. https://www.rsna.org/uploadedFiles/RSNA/Content/Science_and_Education/Quality/Standardized%20Reporting%20of%20CT%20Pulmonary%20Angiography%20for%20Acute%20Pulmonary%20Embolism.pdf. Published 2015. Accessed June 2020. [Google Scholar]
- 20.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 2020;295(3):715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qanadli SD, El Hajjam M, Vieillard-Baron A, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol 2001;176(6):1415–1420. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Zhang X, Liu S, et al. Findings of acute pulmonary embolism in COVID-19 patients. Lancet Infect Dis 2020 (in press). [Google Scholar]
- 23.Ng KHL, Wu AKL, Cheng VCC, et al. Pulmonary artery thrombosis in a patient with severe acute respiratory syndrome. Postgrad Med J 2005;81(956):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh SK. Middle East respiratory syndrome virus pathogenesis. Semin Respir Crit Care Med 2016;37(4):572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med 2011;171(9):831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goy J, Lee J, Levine O, Chaudhry S, Crowther M. Sub-segmental pulmonary embolism in three academic teaching hospitals: a review of management and outcomes. J Thromb Haemost 2015;13(2):214–218. [DOI] [PubMed] [Google Scholar]
- 27.Cha SI, Shin KM, Lee JW, et al. Clinical characteristics of patients with peripheral pulmonary embolism. Respiration 2010;80(6):500–508. [DOI] [PubMed] [Google Scholar]
- 28.Zotzmann V, Lang CN, Bamberg F, Bode C, Staudacher DL. Are subpleural consolidations indicators for segmental pulmonary embolism in COVID-19? Intensive Care Med 2020;46(6):1109–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020;18(7):1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhai Z, Li C, Chen Y, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost 2020;120(6):937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]