Abstract
The incidence and prevalence of ulcerative colitis (UC) has been reported to be rising in newly industrialised regions, such as Latin America. Here, we review data from published studies reporting demographics and clinical aspects of UC in Latin America to further understand epidemiology and disease burden. The incidence and prevalence of UC in Latin America varied between regions and studies, ranging between 0.04 to 8.00/100,000 and 0.23 to 76.1/100,000, respectively, and generally increased over the period from 1986 to 2015. The majority of patients with UC were female (53.6–72.6%) and urban residents (77.8–97.4%). Extraintestinal manifestations were reported in approximately 26–89.4% of patients. Use of biologic therapies was generally low (0.8–16.2%), with the exception of Mato Grosso do Sul, Brazil, with a greater proportion of patients tending to receive 5-aminosalicylates, immunosuppressants or corticosteroids; colectomy rates varied between studies (1.5–22%). A high proportion of patients had moderate to severe UC (45.9–73.0%) and, in 11 of 19 studies, the greatest proportion of patients had extensive disease (pancolitis). Colorectal cancer (0–1.7%) and mortality rates (0–7.6%) were low. This evaluation of published studies may influence therapeutic approaches and the development of strategies to improve healthcare access and patient outcomes, although further high-quality studies are required in patients with UC in Latin America.
Keywords: disease characteristics, incidence, Latin America, prevalence, therapies, ulcerative colitis
Introduction
Ulcerative colitis (UC) is a chronic, relapsing and remitting inflammatory disease of the colon, with characteristic symptoms including bloody stools, diarrhoea, urgency, incontinence, fatigue, mucus discharge, cramps, night-time bowel movements and an increased frequency in bowel movements.1 UC represents a significant burden on both patient quality of life and healthcare resource utilisation, with the full impact often being underestimated.2,3
The highest incidence and prevalence of UC between 1990 and 2016 was reported in Europe and North America, with UC prevalence exceeding 0.3% of the overall population in many countries.4 However, the incidence and prevalence of UC is increasing steadily in developing regions where cultures have become more westernised, such as Latin America.4,5 A recent review of population-based studies in Brazil observed that the incidence and prevalence of UC increased from 2000 onwards, and were higher in more developed regions.6 A recent systematic review reported that the incidence of UC in Brazil ranged from 0.74/100,000 in 1986–1990 to 6.76/100,000 in 1996–2000, and the prevalence of UC ranged between 0.99/100,000 in Brazil in 1986–1990 and 44.3/100,000 in Barbados in 2004.7 Additionally, a recent systematic review of inflammatory bowel disease (IBD) in Latin America reported that the incidence of UC ranged from 4.3 to 5.3/100,000, and the prevalence of UC ranged from 15.0 to 24.1/100,000.8
In order to further aid understanding of epidemiology and disease burden of UC in Latin America, here, we review data from published studies reporting demographics and clinical aspects of UC in Latin America.
Methods
A literature search was conducted in PubMed to retrieve studies published on or before 2 August 2019, based on the following search terms: ulcerative colitis; and incidence or prevalence; and Latin America, Latin American, Argentina, Argentinian, Brazil, Brazilian, Chile, Chilean, Colombia, Colombian, Mexico, Mexican or Peru.
Articles were limited to those written in English and published within the previous 11 years (to May 2008). Identified references were reviewed manually to evaluate their relevance. References reporting UC in countries other than those in Latin America, and references focussing on other forms of IBD only (such as Crohn’s disease), were excluded.
References in relevant articles were examined to identify additional articles, and the authors drew on their knowledge of the wider literature to provide context to guide appropriate interpretation of data within the identified references.
Results
Selected studies
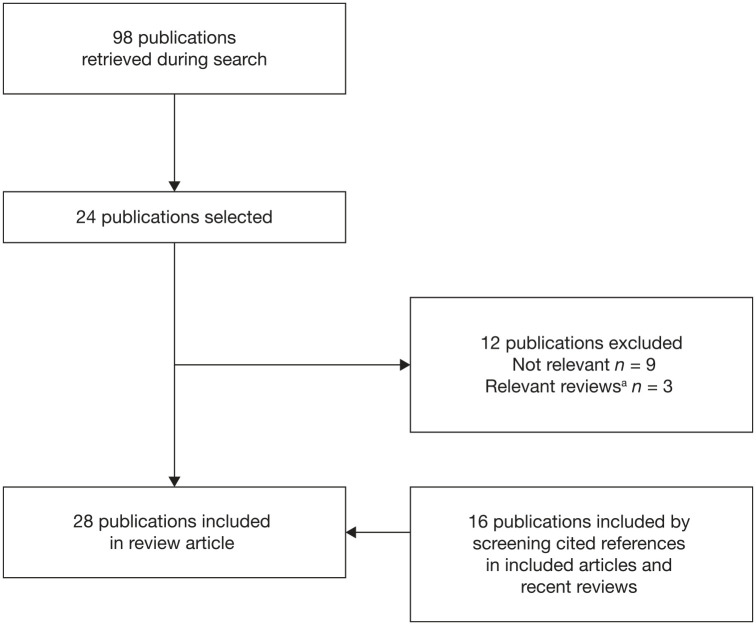
A total of 28 articles were included; 12 from Brazil, 4 from Mexico (1 abstract and 1 manuscript were from the same study), 4 from Puerto Rico, 2 from Uruguay, 2 from Chile, 1 from Argentina, 1 from both Argentina and Panama, 1 from Colombia and 1 from Barbados (Figure 1). Study designs and subject numbers for the included articles are shown in Table 1.
Figure 1.
Study selection.
aRelevant reviews that were identified are included in the Discussion, not in the Results section of the manuscript.
Table 1.
Incidence and prevalence of UC in Latin America.
| Country | Region | Study design | Number of patients with UC |
|---|---|---|---|
| Argentina | Partido General Pueyrredón9 | Hospital-based registry (multicentre) | 38 |
| −10 | HMO medical records | 112 | |
| Barbados | Nationwide11 | IBD clinic (single centre) | 121 |
| Brazil | São Paulo12 | Hospital records (single centre) | 75 |
| São Paulo13 | Government programme (dispensing free high-cost medicines) | 12,187 | |
| São Paulo14 | Hospital (single centre) | 803 | |
| Piauí15 | Hospital (single centre) | 152 | |
| Bahia16 | Hospitals (multicentre) | 267 | |
| Bahia17 | Referral centres (multicentre) | 68 | |
| Sergipe18 | Hospital (single centre) | 47 | |
| Mato Grosso do Sul19 | Referral centre (single centre) | 117 | |
| Espírito Santo20 | Government programme (dispensing high-cost medicines) | 935 | |
| Mato Grosso do Sul21 | Medical records | 185 | |
| Rio de Janeiro22 | Outpatient clinic (single centre) | 171 | |
| Alagoas23 | Outpatient clinic (single centre) | 13 | |
| Chile | Santiago24 | Registry (single centre) | 508 |
| Multicentre25 | Public health department records (multicentre) | 8951 | |
| Colombia | Nationwide26 | Database (insurance claims) | − |
| Mexico | Nationwide27,28 | Clinics (multicentre) | 2073 |
| Mexico City29 | Medical records (single centre) | 848 | |
| Nationwide30 | Hospitals (multicentre) | 908 | |
| Panama | District of Colón9 | Hospital-based registry (single centre) | 15 |
| USA | Puerto Rico31 | Registry (insured) | 499 |
| Puerto Rico32 | Insurance records | 291 | |
| Puerto Rico33 | Registry | 299 | |
| Puerto Rico34 | Registry | 241 | |
| Uruguay | Multicentre35 | Registry | 29 |
| Montevideo36 | Registry (multicentre) | 238 |
–, data not reported.
HMO, health maintenance organisation; IBD, inflammatory bowel disease; UC, ulcerative colitis.
Incidence and prevalence of UC in Latin America
We identified 3 articles that reported incidence rate only, 4 articles that reported prevalence rate only, and 4 articles that reported both incidence and prevalence rates.
The incidence of UC in Latin America ranged from 0.04 to 8.00/100,000 person-years and the prevalence of UC ranged from 0.23 to 76.1/100,000, with both incidence and prevalence generally increasing over time (Table 2).
Table 2.
Incidence and prevalence of UC in Latin America.
| Country | Data collection period | Incidence (per 100,000) | Prevalence (per 100,000) |
|---|---|---|---|
| Argentina | 1987–199310 | 2.2 | – |
| −10 | − | 76.1 | |
| Barbados | 1980–200411 | 1.85 | − |
| 1980–198411 | 1.30 | − | |
| 1985–198911 | 1.92 | − | |
| 1990–199411 | 2.30 | − | |
| 1995–199911 | 2.34 | − | |
| 2000–200411 | 1.58 | − | |
| 200411 | − | 44.3 | |
| Brazil | 1986–199012 | 0.74 | 0.99 |
| 1991–199512 | 3.86 | 4.77 | |
| 1996–200012 | 6.76 | 11.20 | |
| 2001–200512 | 4.48 | 14.81 | |
| 2012–201420 | − | 24.1 | |
| 2012–201513 | 7.16 | − | |
| 201213 | 6.73 | − | |
| 201313 | 6.92 | − | |
| 201420 | 5.3 | − | |
| 201413 | 6.99 | − | |
| 201513 | 8.00 | 28.3 | |
| Colombia | −26 | − | 51.77 |
| Mexico | 200027 | 0.04 | 0.23 |
| 200527 | 0.07 | 0.47 | |
| 201027 | 0.09 | 0.83 | |
| 201527 | 0.16 | 1.45 | |
| Panama | 1987–19939 | 1.2 | – |
| Puerto Rico | 199631 | − | 62.2 |
| 200232 | − | 21.72 | |
| 200332 | − | 20.46 | |
| 200432 | − | 24.33 | |
| 200532 | − | 23.32 | |
| Uruguay | 2007–200835 | 4.26 | – |
–, data not reported.
UC, ulcerative colitis.
Argentina
One hospital-based, multicentre study identified 38 cases of UC in Argentina between 1987 and 1993, with an incidence of 2.2/100,000 person-years,9 and a study using health maintenance organisation records identified 112 cases of UC in Argentina, with a prevalence of 76.1/100,000.10
Barbados
In a 25-year prospective study conducted between 1980 and 2004, in the only public hospital in Barbados, the crude incidence of UC was 1.90/100,000 person-years; the incidence of UC standardised to the global population was 1.85/100,000 person-years; the incidence of UC standardised to the European population was 2.05/100,000 person-years; and the incidence of UC standardised to the United States of America (USA) population was 2.09/100,000 person-years.11 The incidence of UC standardised to the global population increased from 1.30/100,000 person-years in 1980–1984 to 2.34/100,000 person-years in 1995–1999, and then decreased to 1.58/100,000 person-years in 2000–2004.11 Between 4.3 and 6.1 new cases of UC are expected each year in Barbados.11 The prevalence of UC in Barbados in 2004 was 44.3/100,000, and was higher in women versus men.11
Brazil
During 25 years of study at the Hospital of the Federal University of Piauí, a gradual increase in the incidence rate of IBD was observed each year, reaching 1.53/100,000 at its peak in 2007 and resulting in a prevalence of 12.8/100,000 in 2012.15 In this study, the incidence and prevalence of UC in Brazil were not reported separately from the overall incidence and prevalence of IBD.15 In line with this, a study conducted between 2003 and 2017 in Mato Grosso do Sul, Brazil reported that the number of cases of UC increased over time (p = 0.046) and first peaked in 2007 at 13.7% of new cases per year.19 Furthermore, in São Paulo, the incidence of UC increased steadily between 2012 and 2015 from 6.73 to 8.00/100,000 person-years.13 Another recent study in Espírito Santo, Brazil reported the prevalence of UC in 2012–2014 as 24.1/100,000 person-years and the incidence of UC in 2014 as 5.3/100,000.20
Chile
The number of patients diagnosed with UC in Santiago increased over time, with the majority of patients in the study diagnosed between 2001 and 2015, and the remainder diagnosed between 1971 and 2000.24 Data from the Department of Health Statistics showed a significant increase in the annual incidence rate of hospital discharges for UC, from 3.58/100,000 in 2001 to 5.53/100,000 in 2012 (p < 0.001).25
Colombia
One study, based on health insurance claims information, reported the prevalence of UC in Colombia as 51.77/100,000.26
Mexico
A large cohort study conducted in a referral hospital in Mexico City reported a 2.6-fold increase in the incidence of UC diagnosed from 1987–1996 to 1997–2006.29 In a nationwide study performed between 2000 and 2017, the highest incidence of UC was reported in 2015 (0.16/100,000 person-years) compared with previous years, the number of UC diagnoses increased by an average of 10.8 ± 6.3% per year, and the incidence of new UC cases increased 5.3-fold from 2000 to 2016.27
Panama
One hospital-based, single-centre study identified 15 cases of UC in Panama between 1987 and 1993, with an incidence of 1.2/100,000 person-years.9
Puerto Rico
In a large nationwide study of insured participants in Puerto Rico, there was an increase in the prevalence of IBD from 2002 to 2005, with the prevalence of UC increasing from 21.72 to 23.32/100,000.32
Uruguay
In a multicentre study in Uruguay between 2007 and 2008, the crude incidence of UC was 2.25/100,000 person-years, which increased to 4.26/100,000 person-years when standardised to the global population.35
Patient demographics
Patient demographics from 10 studies reporting these data are shown in Table 3. The mean age at disease onset/diagnosis ranged from 31.0 years in Puerto Rico to 41.7 years in Uruguay.33,35 The gender of patients with UC was reported in the majority of studies, with the proportion of males ranging from 27.4% in Mato Grosso do Sul, Brazil to 46.4% in Argentina,10,19 highlighting that UC is more prevalent in females in Latin America. The mean disease duration was 9.3 years in Puerto Rico and 7.6 years in Bahia, Brazil.16,33 The proportion of patients with a family history of IBD ranged from 2.5% in Barbados to 19.3% in Puerto Rico,11,33 and the vast majority of patients were urban residents, ranging from 77.8% in Sergipe, Brazil to 97.4% in Mato Grosso do Sul, Brazil.18,19 A low proportion of patients with UC were current smokers, ranging from 0.0% in Mexico City to 16.4% in Montevideo, Uruguay.29,36 The majority of patients were non-smokers (ranging from 60.1% in Montevideo, Uruguay to 91.3% in Mexico City), with the exception of Sergipe, Brazil, in which the majority of patients (76.1%) were ex-smokers.18,29,36
Table 3.
Demographics of patients with UC in Latin America.
| Country | Brazil | Chile | Mexico | Uruguay | Barbados | USA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Piauí15 | Bahia16 | Sergipe18 | Mato Grosso do Sul19 | Santiago24 | Mexico City29 | Montevideo36 | Multicentre35 | Nationwide11 | Puerto Rico33 |
| Mean age of disease onset, years | 36.8 | 39.4a | − | − | − | 31.3a | 35.2a | 41.7a | − | 31.0 |
| Male, % | 36.2 | 32.6 | 42.6 | 27.4 | − | 45.0 | 30.7 | 41.4 | 38.0 | 44.0 |
| Mean disease duration, years | − | 7.6 | − | − | − | − | − | − | − | 9.3 |
| Family history of IBD, % | 8.6 | 7.9 | 16.7 | 18.0 | 11.6 | 6.8 | − | − | 2.5 | 19.3 |
| Urban residents, % | 81.6 | 87.6 | 77.8 | 97.4 | − | 91.4 | − | − | − | − |
| Smoking status | ||||||||||
| Current smoker, % | − | 3.0 | 4.3 | 12.0 | 9.3 | 0.0 | 16.4 | − | 2.5 | 10.0 |
| Ex-smoker, % | − | 34.5 | 76.1 | − | 14.8 | 8.6 | 23.5 | − | − | − |
| Non-smoker, % | − | 62.5 | 19.6 | − | 76.0 | 91.3 | 60.1 | − | − | − |
Age at diagnosis.
–, data not reported.
IBD, inflammatory bowel disease; UC, ulcerative colitis.
Extraintestinal manifestations
A total of 14 studies reported the presence of extraintestinal manifestations (EIMs) in patients with UC (Table 4), with the proportion of patients with UC who experienced EIMs ranging from approximately 26% in Mexico and Puerto Rico to 89.4% in Sergipe, Brazil.18,27,33 The most prevalent EIMs that were reported were arthropathies/arthritis, articular EIMs and abnormal bone mineral density.
Table 4.
EIMs in patients with UC in Latin America.
| Country | Brazil | Barbados | Chile | Mexico | Uruguay | USA | |||
|---|---|---|---|---|---|---|---|---|---|
| Region | São Paulo14 | Bahia16 | Bahia17 | Sergipe18 | Nationwide11 | Santiago24 | Mexico City29 | Montevideo36 | Puerto Rico33 |
| EIM, % | |||||||||
| Cutaneous | − | 3.4 | − | − | − | 2.2 | − | − | − |
| Pyoderma gangrenosum | − | − | − | − | 2.5 | − | 2.2 | − | 2.0 |
| Erythema nodosum | − | − | − | − | 1.7 | − | 4.4 | − | 1.6 |
| Hepatobiliary | − | 2.2 | − | − | − | − | − | − | − |
| Primary sclerosing cholangitis | 1.7 | − | − | 4.3 | 9.9 | 1.6 | 10.5 | 6.7 | 1.0 |
| Hepatitis | − | − | − | 4.3 | − | − | − | − | − |
| Colestasis | − | − | − | 4.3 | − | − | − | − | − |
| Ocular | − | 0.7 | − | − | 2.5 | 1.6 | − | − | − |
| Uveitis and/or episcleritis | − | − | − | − | − | − | 2.0 | − | 1.7 |
| Perineal disease | − | − | − | − | 3.3 | − | − | − | − |
| Arthropathy | − | − | − | − | − | − | − | 14.3 | 19.4 |
| Arthritis | − | − | − | 19.1 | − | − | 24.4 | − | − |
| Acute/peripheral arthropathy | − | − | − | − | 9.1 | − | − | − | 16.1 |
| Ankylosing spondylitis | − | − | − | 2.1 | 2.5 | − | 1.1 | − | 0.7 |
| Articular | − | 34.8 | − | − | − | 30.7 | − | − | − |
| Joint pain | − | − | − | 17.0 | − | − | − | − | − |
| Sacroiliitis | − | − | − | 4.3 | 0.8 | − | 3.1 | − | 2.6 |
| Abnormal bone mineral density | − | − | 41.2 | − | − | − | − | − | − |
| Osteoporosis | − | − | 2.9 | − | − | − | − | − | 2.0 |
| Extraintestinal neoplasia | 2.1 | − | − | − | − | − | − | − | − |
–, data not reported.
EIM, extraintestinal manifestation; UC, ulcerative colitis.
Treatment
A total of 10 studies reported treatment use by patients with UC in Latin America (Table 5). Generally, a low proportion of patients were treated with biologics, ranging from 0.8% in Puerto Rico to 16.2% in Mexico,30,34 with the exception of Mato Grosso do Sul, Brazil, in which 44.4% of patients received tumour necrosis factor inhibitors (TNFi; 35.0% received adalimumab and 19.7% received infliximab).19 The proportions of patients treated with 5-aminosalicylates, corticosteroids or immunosuppressants varied between studies, but were generally higher than the proportions of patients treated with biologics.
Table 5.
Treatment of patients with UC in Latin America.
| Country | Brazil | Chile | Mexico | Uruguay | USA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Bahia16 | Mato Grosso do Sul19 | Espírito Santo20 | Mato Grosso do Sul21 | Bahia17 | Santiago24 | Mexico City29 | Multiple states30 | Montevideo36 | Puerto Rico34 |
| Treatment, %a | ||||||||||
| 5-aminosalicylates | − | 43.6 | 62.2 (5-ASA oral) 44.4 (5-ASA topical) 62.5 (SLZ) |
82.7 (MES) 1.6 (SLZ) |
88.2 | 97.8 | 64.3 (5-ASA) 25.5 (SLZ) |
66.3 | 86.2b
94.8c |
74.7 |
| Corticosteroids | 62.8 | − | − | − | 17.6 | 58.5 | 33.3 (CS) 0.6 (BUD) |
28.7 | 53.4b
19.4c |
71.8 |
| Immunosuppressants | 19.5 | 29.1 (AZA) 1.7 (CYC) 0.9 (TAC) 0.0 (MTX) |
19.4 | 15.7 (AZA) 0.5 (MTX) |
11.8 | 32.7 (IMN) 1.4 (CYC) |
28.0 (AZA) 2.0 (6-MP) |
24.9 | 4.6b
17.8c |
14.5 |
| Biologics | 1.5 | 44.4 (TNFi) 35.0 (ADA) 19.7 (IFX) |
4.5 | 5.4 (ADA) 5.4 (IFX) |
− | 6.7 | 1.2 | 16.2 | 1.4b
1.0c |
0.8 |
Therapy is specified when more than one therapy is relevant to a single category.
Induction of remission.
Maintenance of remission.
–, data not reported.
5-ASA, 5-aminosalicylates; 6-MP, 6-mercaptopurine; ADA, adalimumab; AZA, azathioprine; BUD, budesonide; CS, corticosteroids; CYC, cyclosporine; IFX, infliximab; IMN, immunosuppressants; MES, mesalazine; MTX, methotrexate; SLZ, sulfasalazine; TAC, tacrolimus; TNFi, tumour necrosis factor inhibitor; UC, ulcerative colitis.
High hospitalisation rates were reported in Bahia, Brazil and Santiago, Chile, with 43.8% and 34.6% of patients requiring hospitalisation, respectively.16,24 Colectomy rates varied between countries, with 22% of patients in Puerto Rico, 10.5% in Montevideo, Uruguay, 10.1% in Mexico City and 1.5–3.4% in Bahia, Brazil requiring a colectomy.16,17,29,34,36 In Montevideo, Uruguay, 5.0% of patients underwent proctocolectomy (3.8% pouch and 1.3% definitive ileostomy) and 4.2% of patients required a colectomy.36
Disease extent and severity
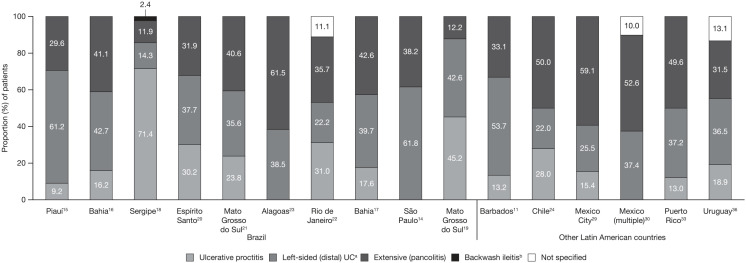
The extent of UC in all patients was reported in 19 articles: 10 from Brazil, 4 from Mexico, and 1 each from Chile, Puerto Rico, Argentina, Barbados and Uruguay (Figure 2). In 11 studies, the greatest proportion of patients had extensive (pancolitis).10,17,21–24,27–30,33 In a nationwide study in Mexico, which separated UC extension by age group, pancolitis was the most frequent extension of UC in children (64.4%), adult (62.2%) and elderly (57.7%) patients; distal/proctosigmoiditis was the second most frequent extension in children (22.2%), adult (24.8%) and elderly (26.8%) patients; and left-sided colitis was the least frequent extension in children (13.3%), adult (13.0%) and elderly (15.6%) patients.27,28 In line with this, the highest proportion of patients (43%) had pancolitis in a study in Argentina.10 Proximal extension developed in 17.4% of patients in Montevideo, Uruguay.36 Disease severity was only reported in 3 studies from Brazil, with moderate to severe UC accounting for 45.9–73.0% of all cases (Figure 3).15,18,19
Figure 2.
Extent of disease in patients with UC in Latin America.
aAlso includes proctosigmoiditis.
bDisease extent in one patient was classified as backwash ileitis.
UC, ulcerative colitis.
Figure 3.
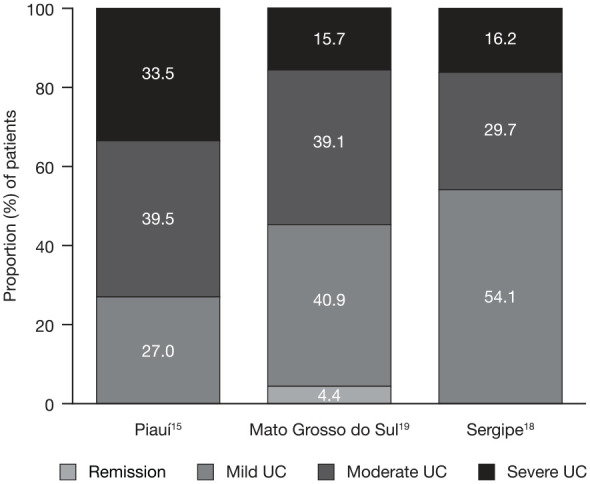
Disease severity in Brazilian patients with UC.
UC severity was determined in Piauí and Mato Grosso do Sul using the Montreal classification; the method for determining UC severity in Sergipe was not specified.
UC, ulcerative colitis.
Colorectal cancer and mortality
Few studies reported cancer (4 studies) and mortality rates (3 studies), and those that did reported generally low rates of cancer and mortality. In a study in Mexico City, 4 patients (0.5%) developed dysplasia or colorectal cancer, 15 years after initial diagnosis.29
In a study in São Paulo, Brazil, with a follow up that ranged from 1 to 22 years, 13 patients (1.7%) developed colorectal cancer, and 17 patients (2.1%) developed extraintestinal neoplasia; 13 patients (1.6%) died [9 of these patients (30%) died due to neoplasia].14 In a study in Mato Grosso do Sul, Brazil, no patients with UC developed neoplasia or died during the 15-year study period.19 In a study in Montevideo, Uruguay, 1.7% of patients developed colorectal cancer, and 7.6% of patients died.36
Discussion
Here, we reviewed the incidence and prevalence, patient demographics, EIMs, treatment, disease extent and severity, colorectal cancer rates and mortality rates in patients with UC in Latin America. Data from Argentina, Barbados, Brazil, Chile, Colombia, Mexico, Panama, Puerto Rico and Uruguay were included.
A previous systematic review highlighted that the incidence of UC ranged from 0.97–57.9/100,000 person-years in Europe to 8.8–23.14/100,000 person-years in the USA, and the prevalence of UC ranged from 2.42–505.0/100,000 in Europe to 139.8–286.3/100,000 in North America.4 Therefore, although the incidence and prevalence rates of UC in Latin America varied across countries and studies, they appeared to be much lower than in Europe and North America. However, the incidence and prevalence of UC in Latin America is generally increasing with time, which is in line with previous findings.5,6 The reasons behind the rising incidence and prevalence rates have not been fully elucidated, although industrialisation may be involved since these trends in newly industrialised regions, such as Latin America, are mimicking the patterns previously observed in developed countries.4–6 In addition, increased awareness and reporting, improved access to healthcare, the introduction of electronic medical records, genetic factors and additional environmental factors may play a role. Differences in diet may impair the gut microbiome, resulting in a condition called dysbiosis, which is frequently observed in IBD and can lead to changes in immune system homeostasis.37 This may prove of utmost importance in countries, such as Latin America, that are undergoing continuous diet and lifestyle changes over the years.38 However, until data regarding the precise genetic background and diet of people in Latin America are available, the reasons for the increasing incidence and prevalence of UC cannot be explored in causal depth. In contrast, the incidence of UC was found to decrease between later time intervals in Barbados (between 1995–1999 and 2000–2004) and São Paulo, Brazil (between 1996–2000 and 2001–2005),11,12 even though the incidence increased between earlier time intervals. This may be due to advances in IBD diagnosis criteria in these regions, and a higher patient demand at the beginning of the studies that later stabilised.12 However, 2 recent reviews have reported that the incidence and prevalence of UC are increasing in Brazil.6,8
This review article also offers insight into the characteristics of patients with UC in Latin America. Data from Barbados, Brazil, Mexico, Puerto Rico and Uruguay revealed UC to be more prevalent in females. Furthermore, in all countries with available data (Brazil and Mexico), more than three-quarters of patients with UC were urban residents, which may be due to a lack of access to healthcare and under-reporting in rural areas, and the mean age at UC diagnosis was 30–40 years, which is a poor prognostic factor.39 Findings in studies that have described the characteristics of patients with UC have previously contrasted with one another.40
EIMs were experienced by approximately 26% of patients in Mexico and Puerto Rico to 89.4% of patients in Sergipe, Brazil,18,27,33 with arthropathies/arthritis, articular EIMs and abnormal bone mineral density the most prevalent in patients from Latin America. These findings are generally in line with a previous Western study, which reported EIMs in 30.5% of patients with UC, with arthritis the most common EIM.41
A considerable proportion of patients had moderate to severe UC, however, it should be noted that the 3 studies reporting disease severity had low patient numbers, and although the Montreal classification was used to determine UC severity in 2 studies, the method for determining UC severity was not specified in the other study, and therefore definitive conclusions cannot be drawn. Across the 19 studies reporting disease extent, the proportion of patients with extensive (pancolitis) disease, which is also a poor prognostic factor in UC,39 ranged from 11.9 to 64.4%. Furthermore, in 11 of 19 studies, patients with extensive disease represented the largest proportion of the population by disease extent. Furthermore, hospitalisation rates were high in the 2 studies that reported these data, and up to approximately one-fifth of patients with UC required a colectomy. Hospitalisation is also a poor prognostic factor in patients with UC,39 with hospitalisation previously found to be associated with requirement for a colectomy.42 However, due to the limited data on hospitalisations, conclusions cannot be drawn on whether hospitalisations and healthcare provider visits are increasing across the general population. Therefore, the findings highlight that UC is not well controlled in many patients with UC in Latin America.
Corresponding with this, uptake with advanced treatments (biologics) was relatively low in Latin America (with the exception of a private IBD centre in Mato Grosso do Sul, Brazil19), with less than one-fifth of patients receiving biologics. A recent review emphasised that the biologic penetration in treatment algorithms in Latin America is adequate in Crohn’s disease, but lower in UC.43 Therefore, the data from the private IBD centre in Mato Grosso do Sul, Brazil reflects a unique perspective on the continent and does not represent the reality observed in studies across different countries.19 In contrast, conventional therapies (5-aminosalicylates, corticosteroids, immunosuppressants) were generally the most widely utilised. These findings correlate with a previously published systematic review, which highlighted that corticosteroids and 5-aminosalicylates were widely used in Latin America, but TNFi use was low, which could potentially be due to difficulties in accessing these therapies.5 In addition, this is in line with previous findings in the USA, in which the proportion of patients using biologics ranged from 5.1 to 16.2%.44 It should be noted that differences in public medical insurance systems will affect the use of more expensive medications, such as biologics, in regions such as Latin America.43,45 Treatment guidelines for UC include sustained, steroid-free remission as an optimal goal of treatment.39,46 Accordingly, underutilisation of advanced therapies that may have a steroid-sparing effect (such as biologic and small molecule therapies) may result in sub-optimal treatment, and subsequently decreased patient quality of life, and an increased economic burden due to the potential requirement for hospitalisation, surgery and time off work.47
The low colorectal cancer and mortality rates observed may be due to the low prevalence of UC in Latin America until more recently. In addition, a lack of access to healthcare and under-reporting may result in UC-related cases of colorectal cancer and mortality not being identified. Colorectal cancer screening is currently only implemented in Colombia and Uruguay, with pilot screens underway in Argentina, Brazil and Chile,48 and therefore a lack of rigorous and standardised screening in the region may also account for the low rates reported.
Our literature search only included articles that were published in English and were included on PubMed, thus limiting the articles available for inclusion. Therefore, this review article includes a relatively small number of studies, and results should be interpreted with caution. Furthermore, there were limited data available on colectomy rates, hospitalisation rates and long-term complications such as colorectal cancer, and mortality rates. Recently, 2 systematic reviews of IBD in Latin America have been published that were not limited to articles identified using PubMed or by publication language, which were generally supportive of the conclusions of this article.5,7
Conclusion
In summary, consistent with other recent epidemiological reviews of IBD in Latin America,5,6,8 we noted an increase in the incidence and prevalence of UC in this population. Furthermore, despite a considerable proportion of patients with UC in Latin America having moderate to severe disease and requiring surgery, advanced therapies for the treatment of UC were underutilised. Improving access to such treatments and developing localised guidelines for their use are current challenges for the treatment of UC in Latin America. Knowledge gained from these studies may impact therapeutic approaches and the development of healthcare strategies to improve access and outcomes in patients with UC in Latin America. The public health system in Latin America faces a number of challenges, and health outcomes need to be improved by addressing the gaps in public health capacity.49 In agreement with previous review articles,6–8 additional high-quality standardised epidemiological studies are required to provide a clearer picture of the burden of UC in Latin America. This review may act as a starting point to better study UC epidemiology in a region that provides many opportunities for further local research.
Acknowledgments
Medical writing support, under the guidance of the authors, was provided by Pauline Craig, CMC Connect, McCann Health Medical Communications and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015; 163: 461–464).
Footnotes
Conference presentation: Data in this manuscript were presented at XVII Semana Brasileira do Aparelho Digestivo (SBAD), São Paulo, Brazil, 17–20 November 2018, and III Pan-American Congress of Crohn’s and Ulcerative Colitis (PANCCO), Cartagena, Colombia, 5–6 April 2019.
Funding: This work was funded by Pfizer Inc.
Conflict of interest statement: P.G. Kotze has received consultancy and speaker fees from AbbVie, Janssen, Pfizer Inc and Takeda; and speaker fees from UCB. F. Steinwurz has been an advisory board member for Pfizer Inc; and has received consultancy and speaker fees from Eurofarma, Janssen, Takeda and UCB. C. Francisconi has been an advisory board member for, and has received clinical investigator support from, Pfizer Inc; and has received clinical investigator support from AbbVie, Celgene, Janssen and Takeda. C. Zaltman has been an advisory board member for Pfizer Inc and UCB; has received speaker fees from AbbVie, Janssen, Takeda and UCB; and has received clinical investigator support from AbbVie, Janssen, Pfizer Inc and Takeda. M. Pinheiro, L. Salese and D. Ponce de Leon are employees and stockholders of Pfizer Inc.
ORCID iD: Paulo Gustavo Kotze  https://orcid.org/0000-0002-9632-6691
https://orcid.org/0000-0002-9632-6691
Contributor Information
Paulo Gustavo Kotze, Colorectal Surgery Unit, IBD Outpatient Clinics, Cajuru University Hospital, Catholic University of Paraná (PUCPR), Rua Bruno Filgueira, 369 cj.1205, Curitiba, PR, CEP 80440-220, Brazil.
Flavio Steinwurz, Unit of Inflammatory Bowel Disease, Hospital Israelita Albert Einstein, São Paulo, Brazil.
Carlos Francisconi, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil.
Cyrla Zaltman, IBD Outpatient Clinic, Division of Gastroenterology, Department of Internal Medicine, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Marcia Pinheiro, Pfizer Inc, São Paulo, Brazil.
Leonardo Salese, Pfizer Inc, Collegeville, PA, USA.
Dario Ponce de Leon, Pfizer Inc, Lima, Peru.
References
- 1. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017; 389: 1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Panés J, Louis E, Rutgeerts P. The full picture of ulcerative colitis: the burden, the patient, the treatment. EMJ Gastroenterol 2015; 4: 58–64. [Google Scholar]
- 3. Cohen RD, Yu AP, Wu EQ, et al. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther 2010; 31: 693–707. [DOI] [PubMed] [Google Scholar]
- 4. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017; 390: 2769–2778. [DOI] [PubMed] [Google Scholar]
- 5. Kotze PG, Underwood FE, Damião AOMC, et al. Progression of inflammatory bowel diseases throughout Latin America and the Caribbean: a systematic review. Clin Gastroenterol Hepatol 2020; 18: 304–312. [DOI] [PubMed] [Google Scholar]
- 6. Quaresma AB, Kaplan GG, Kotze PG. The globalization of inflammatory bowel disease: the incidence and prevalence of inflammatory bowel disease in Brazil. Curr Opin Gastroenterol. Epub ahead of print 9 April 2019. DOI: 10.1097/MOG.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 7. Calderón M, Minckas N, Nuñez S, et al. Inflammatory bowel disease in Latin America: a systematic review. Value Health Reg Issues 2018; 17: 126–134. [DOI] [PubMed] [Google Scholar]
- 8. Selvaratnam S, Gullino S, Shim L, et al. Epidemiology of inflammatory bowel disease in South America: a systematic review. World J Gastroenterol 2019; 25: 6866–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Linares de la Cal JA, Cantón C, Hermida C, et al. Estimated incidence of inflammatory bowel disease in Argentina and Panama (1987–1993). Rev Esp Enferm Dig 1999; 91: 277–286. [PubMed] [Google Scholar]
- 10. Sobrero MJ, Varela E, Gonzalez ML, et al. M1155 prevalence of inflammatory bowel disease in a university hospital health maintenance organization. Gastroenterol 2009; 136 (Suppl. 1): A-361–A-362. Abstract M1155. [Google Scholar]
- 11. Edwards CN, Griffith SG, Hennis AJ, et al. Inflammatory bowel disease: incidence, prevalence, and disease characteristics in Barbados, West Indies. Inflamm Bowel Dis 2008; 14: 1419–1424. [DOI] [PubMed] [Google Scholar]
- 12. Victoria CR, Sassak LY, Nunes HR. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq Gastroenterol 2009; 46: 20–25. [DOI] [PubMed] [Google Scholar]
- 13. Gasparini RG, Sassaki LY, Saad-Hossne R. Inflammatory bowel disease epidemiology in São Paulo State, Brazil. Clin Exp Gastroenterol 2018; 11: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campos FG, Teixeira MG, Scanavini A, et al. Intestinal and extraintestinal neoplasia in patients with inflammatory bowel disease in a tertiary care hospital. Arq Gastroenterol 2013; 50: 123–129. [DOI] [PubMed] [Google Scholar]
- 15. Parente JM, Coy CS, Campelo V, et al. Inflammatory bowel disease in an underdeveloped region of Northeastern Brazil. World J Gastroenterol 2015; 21: 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. da Silva BC, Lyra AC, Mendes CM, et al. The demographic and clinical characteristics of ulcerative colitis in a Northeast Brazilian population. Biomed Res Int 2015; 2015: 359130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lima CA, Lyra AC, Mendes CMC, et al. Bone mineral density and inflammatory bowel disease severity. Braz J Med Biol Res 2017; 50: e6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delmondes LM, Nunes MO, Azevedo AR, et al. Clinical and sociodemographic aspects of inflammatory bowel disease patients. Gastroenterol Res 2015; 8: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cury DB, Oliveira R, Cury MS. Inflammatory bowel diseases: time of diagnosis, environmental factors, clinical course, and management - a follow-up study in a private inflammatory bowel disease center (2003–2017). J Inflamm Res 2019; 12: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lima Martins A, Volpato RA, Zago-Gomes MDP. The prevalence and phenotype in Brazilian patients with inflammatory bowel disease. BMC Gastroenterol 2018; 18: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arantes JAV, dos Santos CHM, Delfino BM, et al. Epidemiological profile and clinical characteristics of patients with intestinal inflammatory disease. J Coloproctol 2017; 37: 273–278. [Google Scholar]
- 22. Santos RMD, Carvalho ATP, Silva KDS, et al. Inflammatory bowel disease: outpatient treatment profile. Arq Gastroenterol 2017; 54: 96–100. [DOI] [PubMed] [Google Scholar]
- 23. de Barros PAC, da Silva AMR, Lins Neto MÁdf. The epidemiological profile of inflammatory bowel disease patients on biologic therapy at a public hospital in Alagoas. J Coloproctol 2014; 34: 131–135. [Google Scholar]
- 24. Simian D, Fluxá D, Flores L, et al. Inflammatory bowel disease: a descriptive study of 716 local Chilean patients. World J Gastroenterol 2016; 22: 5267–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bellolio Roth F, Gómez J, Cerda J. Increase in hospital discharges for inflammatory bowel diseases in Chile between 2001 and 2012. Dig Dis Sci 2017; 62: 2311–2317. [DOI] [PubMed] [Google Scholar]
- 26. Juliao F, Calixto O. Prevalence of Crohn’s disease and ulcerative colitis in Colombia: analysis of the integral information system of social protection (Sispro). Am J Gastroenterol 2018; 113 (Suppl. 1): S9. Abstract P-034. [Google Scholar]
- 27. Yamamoto-Furusho JK, Sarmiento-Aguilar A, Toledo-Mauriño JJ, et al. Incidence and prevalence of inflammatory bowel disease in Mexico from a nationwide cohort study in a period of 15 years (2000–2017). Medicine (Baltimore) 2019; 98: e16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamamoto-Furusho J, Sarmiento A, Toledo-Mauriño J, et al. P839 clinical and sociodemographical characteristics of inflammatory bowel disease in Mexico: multicentric nation-wide study (EPIMEX-IBD). J Crohns Colitis 2018; 12 (Suppl. 1): S540. Abstract P839. [Google Scholar]
- 29. Yamamoto-Furusho JK. Clinical epidemiology of ulcerative colitis in Mexico: a single hospital-based study in a 20-year period (1987–2006). J Clin Gastroenterol 2009; 43: 221–224. [DOI] [PubMed] [Google Scholar]
- 30. Sarmiento A, Toledo J, Bozada K, et al. Clinical and sociodemographic characteristics of inflammatory bowel disease in Mexico: a multicenter and nationwide study (EPIMEX-IBD). Am J Gastroenterol 2018; 113 (Suppl. 1): S11. Abstract P-044. [Google Scholar]
- 31. Torres EA, De Jesús R, Pérez CM, et al. Prevalence of inflammatory bowel disease in an insured population in Puerto Rico during 1996. P R Health Sci J 2003; 22: 253–258. [PubMed] [Google Scholar]
- 32. Vendrell R, Venegas HL, Pérez CM, et al. Differences in prevalence of inflammatory bowel disease in Puerto Rico between commercial and government-sponsored managed health care insured individuals. Bol Asoc Med P R 2013; 105: 15–19. [PubMed] [Google Scholar]
- 33. Torres EA, Cruz A, Monagas M, et al. Inflammatory bowel disease in Hispanics: the University of Puerto Rico IBD registry. Int J Inflam 2012; 2012: 574079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meléndez JD, Larregui Y, Vázquez JM, et al. Medication profiles of patients in the University of Puerto Rico inflammatory bowel disease registry. P R Health Sci J 2011; 30: 3–8. [PubMed] [Google Scholar]
- 35. Buenavida G, Casañias A, Vásquez C, et al. Incidence of inflammatory bowel disease in five geographical areas of Uruguay in the biennial 2007–2008. Acta Gastroenterol Latinoam 2011; 41: 281–287. [PubMed] [Google Scholar]
- 36. Luciano MJ, Noria A, Iade B. Demographic, clinical, and therapeutic characteristics of a cohort of 238 patients with ulcerative colitis from two medical centres from Uruguay. J Crohns Colitis 2018; 12 (Suppl. 1): S458–S459. Abstract P688. [Google Scholar]
- 37. Ribaldone DG, Pellicano R, Actis GC. Inflammation in gastrointestinal disorders: prevalent socioeconomic factors. Clin Exp Gastroenterol 2019; 12: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Popkin BM, Reardon T. Obesity and the food system transformation in Latin America. Obes Rev 2018; 19: 1028–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019; 114: 384–413. [DOI] [PubMed] [Google Scholar]
- 40. da Silva BC, Lyra AC, Rocha R, et al. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J Gastroenterol 2014; 20: 9458–9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol 2011; 106: 110–119. [DOI] [PubMed] [Google Scholar]
- 42. Ananthakrishnan AN, Issa M, Beaulieu DB, et al. History of medical hospitalization predicts future need for colectomy in patients with ulcerative colitis. Inflamm Bowel Dis 2009; 15: 176–181. [DOI] [PubMed] [Google Scholar]
- 43. Quaresma AB, Coy CSR, Damião AOMC, et al. Biological therapy penetration for inflammatory bowel disease in Latin America: current status and future challenges. Arq Gastroenterol 2019; 56: 318–322. [DOI] [PubMed] [Google Scholar]
- 44. Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther 2018; 47: 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wei SC. Differences in the public medical insurance systems for inflammatory bowel disease treatment in Asian countries. Intest Res 2016; 14: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017; 11: 769–784. [DOI] [PubMed] [Google Scholar]
- 47. Khan HM, Mehmood F, Khan N. Optimal management of steroid-dependent ulcerative colitis. Clin Exp Gastroenterol 2015; 8: 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Young GP, Rabeneck L, Winawer SJ. The global paradigm shift in screening for colorectal cancer. Gastroenterology 2019; 156: 843–851e2. [DOI] [PubMed] [Google Scholar]
- 49. World Health Organization. Public health capacity in Latin America and the Caribbean: assessment and strengthening, https://www.who.int/management/publichealthcapacity.pdf?ua=1 (2007, accessed February 03, 2020).