Abstract
Background:
Data on vedolizumab (VDZ) use in inflammatory bowel disease (IBD) patients are still limited. We aimed to assess the effectiveness and tolerability of VDZ in a real-life clinical scenario.
Methods:
We retrospectively collected data of all consecutive IBD patients who started VDZ from September 2016 to December 2018 at our IBD Unit of the University Hospital of Padua and strictly followed them for 1 year. Clinical benefit (rate of clinical steroid-free remission plus clinical response), endoscopic and histological responses were evaluated over 1 year.
Results:
A total of 117 patients who started VDZ for Crohn’s disease (CD) and ulcerative colitis (UC) were included in the main analysis (69 CD patients, 48 UC patients). We obtained a clinical benefit in 68.1%, 68.1% and 59.4% of CD patients and in 68.7%, 54.2% and 54.1% of UC patients after induction, and at 30 weeks and 52 weeks, respectively. After 1 year, endoscopy response was observed in 47% of CD and 38.2% of UC patients, while the histological response was 19.6% and 23.5%, respectively. Finally, we found that 20.5% of patients needed treatment optimization, with 33.3% of them failing to respond despite this action. No deaths or serious adverse events requiring hospitalization were observed. The main cause of VDZ interruption was drug inefficacy. During the study, two patients developed new spondylarthritis, and two had a worsening of pre-existing arthralgia.
Conclusion:
Vedolizumab resulted in being effective and safe in CD as well as in UC patients.
Keywords: Crohn’s disease, effectiveness, IBD, ulcerative colitis, vedolizumab
Introduction
Vedolizumab (VDZ) is a humanized monoclonal immunoglobulin G (IgG)-1antibody that reduces intestinal inflammation by preventing the lymphocyte translocation from the blood into the inflamed gut tissue. This action is generated by the selective inhibition of the interaction between α4β7 integrin and mucosal-addressing cell-adhesion molecule-1.1–3 It has been approved for treating patients with moderate-to-severe active ulcerative colitis (UC) or Crohn’s disease (CD) and it is routinely administered as a 300 mg intravenous infusion. Its efficacy in terms of clinical, endoscopic and histological response in inflammatory bowel disease (IBD) patients has been demonstrated in several randomized controlled trials.4–10 Moreover, the same investigations showed that VDZ had a favorable long-term safety profile.11
However, as known, the drug efficacy evaluated in clinical trials is generally different and not comparable with that found in real life.12 This happens mainly because the general conditions of the patients are different and because we are allowed to intervene in a more robust way to achieve clinical or endoscopic remission. Thus, real-life studies are mandatory to fill in this gap of information and to help us in selecting the best treatment for our patients. For instance, due to the selective intestinal action of VDZ, its effect on extraintestinal manifestations is still unclear while an exacerbation of articular manifestation has been found.13 Recently, Macaluso et al. performed a prospective multicenter observational study on the effectiveness of VDZ in 163 IBD patients (84 CD and 79 UC patients) showing a good effectiveness after 10 and 22 weeks of treatment, and improvement of articular symptoms, likely due to the concomitant control of gut inflammation.14 To date, different studies have been conducted with variable sample sizes and lengths in follow up.15–24
We performed a retrospective evaluation at our tertiary referral center in order to assess the effectiveness and tolerability of VDZ in a large cohort of IBD patients.
Material and methods
All consecutive IBD patients who started VDZ treatment at the IBD Unit of the University Hospital of Padua from September 2016 to December 2018 were retrospectively included in the analysis and strictly followed up for 1 year. Data collection for scientific purpose and dissemination was approved by the Ethics Committee of Padua (protocol number 4197/AO/17) on 25 July 2017. Informed consent was obtained from each patient included in the analysis. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Study population
According to international guidelines and treatment indications, VDZ was used in adult patients with UC or CD who had an inadequate or lost response to, or were intolerant to, either conventional therapy or tumor necrosis factor (TNF) inhibitors. The recommended dosage of 300 mg was infused intravenously at 0, 2 and 6 weeks, then every 8 weeks. In case of insufficient response, treatment optimization was performed every 4/6 weeks. Patients with ileal pouch rectal anastomosis (IPRA) were excluded from the analysis. Patients who were in clinical and endoscopic remission at baseline were segregated from the main analysis. These subjects started VDZ because of recent surgery and concomitant presence of risk factors associated with poor prognosis or because of adverse events to anti-TNF occurrence and need of switching to other treatment for maintaining the remission status, still in the presence of risk factors of poor prognosis.
The following data were available at baseline: age, sex, smoking habits, age at diagnosis, disease duration, disease extent, presence of extraintestinal manifestations, history of previous IBD-related surgery and medical treatments. As part of our standardized follow-up protocol, patients were clinically evaluated at baseline, after induction (between week 8 and 12), and at weeks 30 and 52: clinical activity was evaluated using the Harvey Bradshaw Index (HBI) for CD patients and the partial Mayo score (pMayo) for UC patients. C-reactive protein (CRP) levels (positive if >0.5 mg/dl) and fecal calprotectin (FC) values were also evaluated. Moreover, endoscopy was performed at baseline (within 3 months before induction) and week 52, and classified using the Simple Endoscopic Score (SES-CD) or Rutgeerts score for CD patients, and the Mayo endoscopic score for UC patients. Routine endoscopy is always requested for the patients after 1 year of treatment; however, is not mandatory as compared with the other examinations made during the follow up. Any requirement for treatment optimization and azathioprine or steroid prescription during the study period were reported. Finally, all adverse events requiring hospitalization or treatment interruption were recorded.
The steroid-free clinical remission was defined as a pMayo <2 or HBI ⩽4 without steroid use. We then reported the percentages of who obtained a clinical response (absence of steroid-free remission but ⩾2 points’ reduction of the baseline of pMayo or at least 3 points of the baseline HBI25). The clinical benefit was defined as the sum of steroid-free clinical remission plus clinical response. As secondary outcomes, we reported: the endoscopic response as a decrease of at least 1 point of the endoscopic Mayo score in the case of UC; a decrease of 50% from the baseline SES-CD and Rutgeerts scores at the endoscopic 1-year re-evaluation in the case of CD; and the histological response, as the evidence of inactive state at the histological evaluation according to a recent Italian position statement.26 The treatment failure was defined as discontinuation of biological therapy due to adverse events, lack of clinical response and need of hospitalization/surgery. A sub-analysis was conducted considering subjects who started VDZ in a remission state to evaluate the remission persistence.
Statistical analysis
Data were analyzed using STATA11 (Stata Corp., College Station, TX, USA) software. Continuous variables were reported as median with 25°–75° percentiles and categorical variables as frequency and percentage. The Wilcoxon signed-rank test was used to compare the change in FC and CRP over time. A survival curve graph was designed to show the treatment failure along the study in patients with CD and UC. We performed an intention-to-treat analysis. A p-value ⩽0.05 was considered statistically significant.
Results
Study population
During the study period, 137 patients were enrolled, 7 were excluded because of IPRA, while 13 patients were included only in a separate sub-analysis because they started VDZ in a remission state. So, 117 patients who started VDZ for a clinical and/or endoscopy moderate–severe disease were included in the main analysis (66 CD patients, 47 UC patients). The median age was 47, 59% were males. Table 1 shows the main characteristics of our study population. In particular, there were only 16 naïve patients who received VDZ as first biologic due to previous history of tumor or cardiovascular disease. Moreover, 33.3% started VDZ while taking steroids and 16.2% while taking azathioprine (Table 1).
Table 1.
Study population description.
| n | All (n = 117) | Crohn’s patients (n = 69) | UC patients (n = 48) |
|---|---|---|---|
| % males | 69 (59) | 39 (56.5) | 30 (62.5) |
| Median age at diagnosis (25th–75th percentile) | 31 (20–47) | 25 (17–40) | 38.5 (28–57) |
| Median age at the time of study (25th–75th percentile) | 47 (36–6) | 44 (32–55) | 52 (43–67.5) |
| Median time from diagnosis (25th–75th percentile) | 11 (5–20) | 12 (8–21) | 8 (3.5–15) |
| Smoking: no smoker | 88 (75.2) | 48 (69.6) | 40 (83.3) |
| Ex-smoker | 14 (12) | 12 (17.4) | 2 (4.2) |
| Current smoker | 15 (12.8) | 9 (13.0) | 6 (12.5) |
| Crohn’s group: n (%) | |||
| Non-stenotic/non-penetrating | 18 (26.1) | ||
| Stenotic | 29 (42.0) | ||
| Penetrating | 16 (23.2) | ||
| Stenotic and penetrating | 6 (8.7) | ||
| Perianal disease | 19 (27.5) | ||
| Crohn’s group: n (%) | |||
| Ileum | 13 (18.8) | ||
| Colon | 19 (27.5) | ||
| Ileum–colon | 28 (40.6) | ||
| Upper GI + other | 9 (13.1) | ||
| HBI score (median, 25th–75th percentile) | 5 (2–7) | ||
| SES-CD (median, 25th–75th percentile) | 9 (8–13) | ||
| Rutgeerts score, 0/1/2/3/4 | 0/7/8/12/4 | ||
| UC group: n (%) | |||
| Proctitis | 1 (2.1) | ||
| Left colon | 16 (33.3) | ||
| Pancolitis | 31 (64.6) | ||
| pMayo score (median, 25th–75th percentile) | 6 (3–7) | ||
| Mayo endoscopic score, 0/1/2/3 | 0/5/14/29 | ||
| Calprotectin (median, 25th–75th percentile) | 1000 (71–6000) | 771 (71–2371) | 1725 (206–6000) |
| Pathological CRP n (%) | 38 (55.1) | 19 (39.6) | |
| Previous biologic therapy n (%) | |||
| Naïve | 16 (13.7) | 7 (10.2) | 9 (18.7) |
| Infliximab | 19 (16.2) | 5 (7.2) | 14 (29.2) |
| Adalimumab | 9 (7.7) | 5 (7.2) | 4 (8.3) |
| Inflixiamb and Adalimumab | 73 (62.4) | 52 (75.4) | 21 (43.8) |
| Steroids ongoing n (%) | 39 (33.3) | 17 (24.6) | 22 (45.8) |
| AZA ongoing n (%) | 19 (16.2) | 11 (15.9) | 8 (16.7) |
| Bowel Resection Surgery (yes) n (%) | 31 (44.9) | 0 | |
| EIM | |||
| Past history (inactive at initiation of VDZ) | |||
| Peripherical arthritis | 3 | 2 | 1 |
| Axial arthritis | 8 | 3 | 5 |
| Erythema nodosum | 1 | 1 | – |
| Active at initiation of VDZ | |||
| Peripherical arthritis | 1 | 1 | – |
| Axial arthritis | 6 | 3 | 3 |
AZA, azathioprine; CRP, C-reactive protein; EIM, extraintestinal manifestation; GI, gastrointestinal; HBI, Harvey Bradshaw Index; pMayo, partial Mayo score; SES-CD, Simple Endoscopic Score for Crohn’s disease; UC, ulcerative colitis; VDZ, vedolizumab.
Clinical and biochemical data
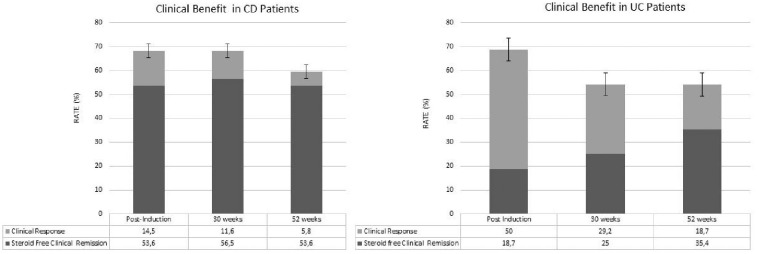
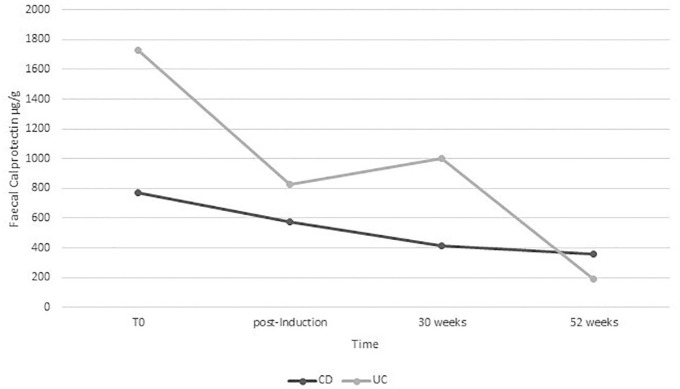
Steroid-free clinical remission was obtained in 37 CD patients (53.6%) post-induction and similar percentages were obtained after 30 weeks (56.5%) and after 52 weeks (53.6%). Moreover, adding the subjects that gained a clinical response, a clinical benefit was observed in 68.1%, 68.1% and 59.4% after induction, 30 weeks and 52 weeks, respectively. As Figure 1(b) shows, steroid-free clinical remission was instead obtained in nine UC patients (18.7%) and the percentages progressively increased after 30 weeks (25%), and after 52 weeks (35.4%). However, in this group, we observed higher percentages of clinical response, finally obtaining a clinical benefit in 68.7%, 54.2% and 54.1% [Figure 1(a, b)]. Fecal calprotectin progressively reduced from T0 to week 52 in both CD and UC groups (Figure 2). However, this reduction was only statistically significant from T0 to post-induction and between T0 and week 52 in both CD and UC. We did not observe a statistically significant reduction in CRP value along the study (p >0.05)
Figure 1.
Clinical benefit (rate of remission plus clinical response) among patients with Crohn’s disease (CD) and ulcerative colitis (UC) treated with vedolizumab at post-induction, week 30 and week 52.
Figure 2.
Fecal calprotectin values over time in patients with Crohn’s disease (CD) and ulcerative colitis (UC) treated with vedolizumab.
Treatment failure occurred in 45 patients during the study. Four interrupted the treatment after a single infusion (three with CD for need of surgery and one with UC for clinical intolerance). A total of 16 interrupted the treatment after induction (9 CD patients, 13% and 7 UC patients, 14.6%), while 12 at week 30 (6 CD patients, 8.7% and 6 UC patients, 12.5%) with 8 of them not achieving a clinical response or a clinical remission at post-induction. Finally, at week 52, 13 (8 CD patients, 11.6%, and 5 UC patients, 10.4%) interrupted the treatment, with 4 of them failing to achieve clinical response or clinical remission post-induction. The main reason for treatment failure was lack of clinical improvement, and no statistically significant difference was observed between CD and UC in treatment failure during the study (Figure 3).
Figure 3.
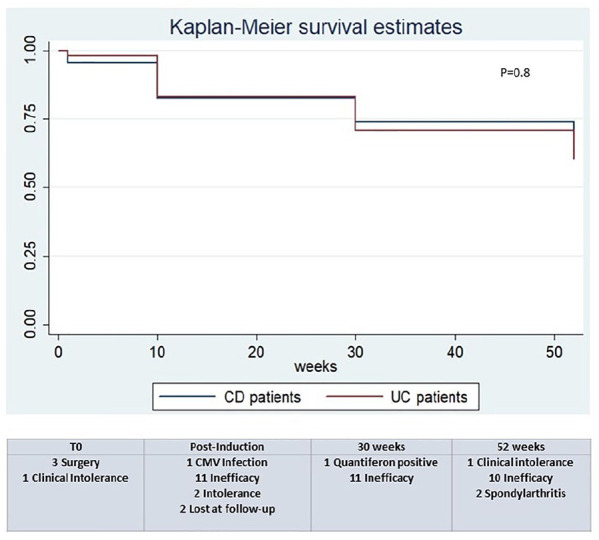
Treatment failure among patients with Crohn’s disease (CD) and ulcerative colitis (UC) treated with vedolizumab at post-induction, week 30 and week 52.
CMV, cytomegalovirus.
Endoscopy and histologic data
All patients who arrived at week 52 underwent endoscopy re-evaluation. We reported a per-protocol analysis using as denominator all patients in whom endoscopy was available at week 52 (51 CD patients and 34 UC patients). Endoscopy response was observed in 24/51 CD patients (47%) and 13/34 UC patients (38.2%). Histological response was instead obtained in 10/51 CD (19.6%) and 8/34 UC (23.5%) patients. All patients with histological response also showed endoscopy response.
Drug optimization and tolerability data
A total of 24 patients (20.5%) underwent treatment optimization (300 mg/6 or 4 weeks) during the first year of treatment; 10 of them were treated with a cycle of steroids at the same time and 3 of them added azathioprine (2.5 mg/kg) to their therapy. Despite the treatment optimization, eight patients interrupted the VDZ treatment for lack of response; two after the induction, one at week 30 and five at week 52.
No deaths or serious adverse events requiring hospitalization were observed. The main cause of VDZ interruption was drug inefficacy. Furthermore, VDZ treatment was interrupted in two cases for adverse event occurrence (re-activation of cytomegalovirus infection, evidence of QuantiFERON® positivization without clinical signs of infection), and two for new onset of spondylarthritis. The clinical intolerance in four cases was characterized by nausea, headache and malaise (Figure 2).
Extraintestinal manifestations and persistence data
Twelve patients had history of extraintestinal manifestations (EIMs) at baseline, while seven had an active articular involvement (Table 1). During the study, two patients with ileocolonic CD developed new EIMs (spondylarthritis), both after 30 weeks of infusion in parallel with a clinical gastrointestinal worsening. The treatment was interrupted at week 52 with full recovery from spondylarthritis and both were swapped to ustekinumab. Other two patients with ileocolonic CD had a worsening of pre-existing peripheral arthralgia, inactive at baseline, and did not respond to VDZ treatment. Indeed, VDZ treatment was suspended in both cases with rapid recovery from arthralgia. All other patients with active articular involvement at VDZ initiation had an improvement of these symptoms with the improvement of the intestinal disease.
Sub-analysis: remission group
The remission group was composed of 13 patients; out of them, 11 had CD. Treatment failure occurred in two patients (15.4%): one after induction for transient proteinuria, and one at week 52 due to the need for surgery. All the remaining patients maintained disease remission status with normal FC and endoscopy.
Discussion
Our study describes the effectiveness and tolerability of VDZ in patients from a same geographical area, followed by a single tertiary center with the same standardized protocol. We reported a good drug efficacy (steroid-free clinical remission plus clinical response), with about 68% of CD and UC patients achieving a clinical benefit after induction that became 59.4% and 54.1% in CD and UC, respectively, after 1 year. In particular, we reported higher percentages of steroid-free clinical remission in CD than in UC. Overall, about 20% of patients needed treatment optimization; however, this option was not always effective in preventing treatment discontinuation (8/24, 33.3% interrupted the VDZ in any case). Only few patients had EIMs at baseline: in two cases, we observed a worsening of arthralgia in parallel with the clinical relapse of CD, and in two cases, we had a new onset of spondylarthritis requiring treatment interruption. Finally, we confirmed the good safety profile of the drug.
In terms of steroid-free clinical remission, we reported higher rates in CD and similar in UC at week 52 compared with the clinical trials.4,5 Similarly, a recent systematic review27 of real-life studies on the efficacy and safety of VDZ reported lower percentage in CD and similar in UC compared with our results: a clinical remission of 22% and 25% after induction and of 32% and 39% at week 52, respectively, in CD and UC. In contrast, similar to our data, a recent Italian real-life study from Macaluso et al.14 described a similar percentage of clinical benefit: 64.3% and 68.4% at week 10, and 55.6% and 54.3% at week 22 in CD and UC, respectively. Our results, in terms of endoscopy response, were higher than those of two recent prospective studies evaluating the efficacy of VDZ in CD.28,29 We reported that 45/117 (38.4%) of patients, interrupted VDZ mainly for inefficacy, and these results are similar to the 42% found by Eriksson et al.22 In our study, only 16 subjects were naïve to biologic therapy, so we cannot define a better response in this sub-group as reported by previous studies.4,5,27 Moreover, we found that 20% of patients needed treatment optimization, with 33.3% of them failing to respond despite this action. These results are in agreement with those reported in the GEMINI long-term study.8,9 Regarding EIMs, our results confirm the theory that a worsening of articular disturbance can be observed in case of re-activation of intestinal activity.14,30 Furthermore, we observed a new onset of spondylarthritis in two patients, as previously reported in medical literature,18 with prompt resolution after drug withdrawal. Our study also confirms the good safety profile of VDZ.27,31
There are different strengths of our study to underline. We performed a mono-centric study where the study population was recruited and managed by the same outpatient unit, following a strict and standardized follow-up protocol. We provide biomarker data collected at strict and a priori defined time points (i.e. baseline, after induction, 6 and 12 months, or in case of relapse). We also reported all the drug optimization performed during the study. However, we have to acknowledge some limitations. First, the retrospective design that did not permit obtaining full data from our cohort. Second, the sample size was limited and the stratification of the patients according to the clinical conditions further reduced the number of patients included in each group. Another limitation is that our follow-up analysis was stopped at 1 year after VDZ introduction. Finally, data on therapeutic drug monitoring were lacking, giving the availability of them at our center.
Our study reported a good and persistent efficacy both in CD and UC patients. We also found that VDZ can be a good option for maintaining the clinical and endoscopic remission in patients who achieved clinical and endoscopic remission due to a different drug (i.e. anti-TNFα). Finally, the good safety profile confirms that VDZ can be a good choice in high-risk patients.
Acknowledgments
Fabiana Zingone, Brigida Barberio, Federico Compostella, Edoardo Vincenzo Savarino: study concept and design; collection, analysis and interpretation of data; drafting of the manuscript.
Giulia Girardin, Renata D’Iincà, Carla Marinelli, Ilaria Marsilio: analysis and interpretation of data; critical revision of the manuscript.
Footnotes
Conflict of interest statement: Edoardo Savarino: lecture and consultancy honoraria from Takeda, Janssen, MSD, Abbvie, Sofar, Malesci, Reckitt Benckiser, Medtronic. Renata D’Incà: lecture fees and/or advisory boards from Janssen, Mundipharma, Celltrion, Biogen, Amgen,Pfizer, Shire, Sandoz, Norgine,Takeda. Fabiana Zingone: lecture fees from Takeda, Alfasigma and Malesci.
Brigida Barberio, Federico Compostella, Giulia Girardin, Carla Marinelli, and Ilaria Marsilio had no conflicts of interest to report
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Fabiana Zingone  https://orcid.org/0000-0003-1133-1502
https://orcid.org/0000-0003-1133-1502
Brigida Barberio  https://orcid.org/0000-0002-3164-8243
https://orcid.org/0000-0002-3164-8243
Edoardo Vincenzo Savarino  https://orcid.org/0000-0002-3187-2894
https://orcid.org/0000-0002-3187-2894
Contributor Information
Fabiana Zingone, Department of Surgery, Division of Gastroenterology, Oncology and Gastroenterology, University of Padua, Via Giustiniani 2, Padua 35121, Italy.
Brigida Barberio, Department of Surgery, Division of Gastroenterology, Oncology and Gastroenterology, University of Padua, Padua, Italy.
Federico Compostella, Department of Surgery, Division of Gastroenterology, Oncology and Gastroenterology, University of Padua, Padua, Italy.
Giulia Girardin, Department of Surgery, Division of Gastroenterology, Oncology and Gastroenterology, University of Padua, Padua, Italy.
Renata D’Incà, Department of Surgery, Division of Gastroenterology, Oncology and Gastroenterology, University of Padua, Padua, Italy.
Carla Marinelli, Department of Surgery, Division of Gastroenterology, Oncology and Gastroenterology, University of Padua, Padua, Italy.
Ilaria Marsilio, Department of Surgery, Division of Gastroenterology, Oncology and Gastroenterology, University of Padua, Padua, Italy.
Greta Lorenzon, Department of Surgery, Division of Gastroenterology, Oncology and Gastroenterology, University of Padua, Padua, Italy.
Edoardo Vincenzo Savarino, Department of Surgery, Division of Gastroenterology, Oncology and Gastroenterology, University of Padua, Padua, Italy.
References
- 1. Armuzzi A, Gionchetti P, Daperno M, et al. ; GIVI Group. Expert consensus paper on the use of vedolizumab for the management of patients with moderate-to-severe inflammatory bowel disease. Dig Liver Dis 2016; 48: 360–370. [DOI] [PubMed] [Google Scholar]
- 2. Scribano ML. Vedolizumab for inflammatory bowel disease: from randomized controlled trials to real-life evidence. World J Gastroenterol 2018; 24: 2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosario M, Dirks NL, Milch C, et al. A review of the clinical pharmacokinetics, pharmacodynamics, and immunogenicity of vedolizumab. Clin Pharmacokinet 2017; 56: 1287–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feagan BG, Rubin DT, Danese S, et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol 2017; 15: 229–239.e5. [DOI] [PubMed] [Google Scholar]
- 5. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 6. Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014; 147: 618–627.e3. [DOI] [PubMed] [Google Scholar]
- 7. Sands BE, Sandborn WJ, Van Assche G, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naive to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis 2017; 23: 97–106. [DOI] [PubMed] [Google Scholar]
- 8. Vermeire S, Loftus EV, Jr, Colombel JF, et al. Long-term efficacy of vedolizumab for Crohn’s disease. J Crohns Colitis 2017; 11: 412–424. [DOI] [PubMed] [Google Scholar]
- 9. Loftus EV, Jr, Colombel JF, Feagan BG, et al. Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis 2017; 11: 400–411. [DOI] [PubMed] [Google Scholar]
- 10. Noman M, Ferrante M, Bisschops R, et al. Vedolizumab induces long-term mucosal healing in patients with Crohn’s disease and ulcerative colitis. J Crohns Colitis 2017; 11: 1085–1089. [DOI] [PubMed] [Google Scholar]
- 11. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017; 66: 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ha C, Ullman TA, Siegel CA, et al. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol 2012; 10: 1002–1007; quiz e1078. [DOI] [PubMed] [Google Scholar]
- 13. Varkas G, Thevissen K, De Brabanter G, et al. An induction or flare of arthritis and/or sacroiliitis by vedolizumab in inflammatory bowel disease: a case series. Ann Rheum Dis 2017; 76: 878–881. [DOI] [PubMed] [Google Scholar]
- 14. Macaluso FS, Orlando R, Fries W, et al. The real-world effectiveness of vedolizumab on intestinal and articular outcomes in inflammatory bowel diseases. Dig Liver Dis 2018; 50: 675–681. [DOI] [PubMed] [Google Scholar]
- 15. Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis 2015; 21: 2879–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amiot A, Grimaud JC, Peyrin-Biroulet L, et al. ; Observatory on Efficacy and of Vedolizumab in Patients with Inflammatory Bowel Disease Study Group, Groupe d’Etude Therapeutique des Affections Inflammatoires du tube Digestif. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2016; 14: 1593–1601.e2. [DOI] [PubMed] [Google Scholar]
- 17. Vivio EE, Kanuri N, Gilbertsen JJ, et al. Vedolizumab effectiveness and safety over the first year of use in an IBD clinical practice. J Crohns Colitis 2016; 10: 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stallmach A, Langbein C, Atreya R, et al. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease - a prospective multicenter observational study. Aliment Pharmacol Ther 2016; 44: 1199–1212. [DOI] [PubMed] [Google Scholar]
- 19. Dulai PS, Singh S, Jiang X, et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY consortium. Am J Gastroenterol 2016; 111: 1147–1155. [DOI] [PubMed] [Google Scholar]
- 20. Kopylov U, Ron Y, Avni-Biron I, et al. Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease-the Israeli real-world experience. Inflamm Bowel Dis 2017; 23: 404–408. [DOI] [PubMed] [Google Scholar]
- 21. Samaan MA, Pavlidis P, Johnston E, et al. Vedolizumab: early experience and medium-term outcomes from two UK tertiary IBD centres. Frontline Gastroenterol 2017; 8: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eriksson C, Marsal J, Bergemalm D, et al. Long-term effectiveness of vedolizumab in inflammatory bowel disease: a national study based on the Swedish national quality registry for inflammatory bowel disease (SWIBREG). Scand J Gastroenterol 2017; 52: 722–729. [DOI] [PubMed] [Google Scholar]
- 23. Amiot A, Serrero M, Peyrin-Biroulet L, et al. ; OBSERV-IBD study group and the GETAID. One-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multicentre cohort study. Aliment Pharmacol Ther 2017; 46: 310–321. [DOI] [PubMed] [Google Scholar]
- 24. Amiot A, Serrero M, Peyrin-Biroulet L, et al. ; OBSERV-IBD study group and the GETAID. Three-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multi-centre cohort study. Aliment Pharmacol Ther 2019; 50: 40–53. [DOI] [PubMed] [Google Scholar]
- 25. Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 2002; 122: 512–530. [DOI] [PubMed] [Google Scholar]
- 26. Villanacci V, Reggiani-Bonetti L, Caprioli F, et al. Histopathology of inflammatory bowel disease - position statement of the pathologists of the Italian group for the study of inflammatory bowel disease (IG-IBD) and Italian group of gastrointestinal pathologists (GIPAD-SIAPEC). Dig Liver Dis 2020; 52: 262–267. [DOI] [PubMed] [Google Scholar]
- 27. Engel T, Ungar B, Yung DE, et al. Vedolizumab in IBD-lessons from real-world experience; a systematic review and pooled analysis. J Crohns Colitis 2018; 12: 245–257. [DOI] [PubMed] [Google Scholar]
- 28. Danese S, Sandborn WJ, Colombel JF, et al. Endoscopic, radiologic, and histologic healing with vedolizumab in patients with active Crohn’s disease. Gastroenterology 2019; 157: 1007–1018.e7. [DOI] [PubMed] [Google Scholar]
- 29. Löwenberg M, Vermeire S, Mostafavi N, et al. Vedolizumab induces endoscopic and histologic remission in patients with Crohn’s disease. Gastroenterology 2019; 157: 997–1006.e6. [DOI] [PubMed] [Google Scholar]
- 30. Fleisher M, Marsal J, Lee SD, et al. Effects of vedolizumab therapy on extraintestinal manifestations in inflammatory bowel disease. Dig Dis Sci 2018; 63: 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schreiber S, Dignass A, Peyrin-Biroulet L, et al. Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol 2018; 53: 1048–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]