Abstract
Background:
Long-acting muscarinic antagonist (LAMA) monotherapy is recommended for chronic obstructive pulmonary disease (COPD) patients with high risk of exacerbations. It is unclear whether long-acting β2-agonist (LABA)/LAMA fixed-dose combinations (FDCs) are more effective than LAMAs alone in preventing exacerbations. The aim of this study was to systematically review the literature to investigate whether LABA/LAMA FDCs are more effective than LAMA monotherapy in preventing exacerbations.
Methods:
We searched several databases and manufacturers’ websites to identify relevant randomized clinical trials comparing LABA/LAMA FDC treatment with LAMAs alone ⩾24 weeks. Outcomes of interest were time to first exacerbation and rates of moderate to severe, severe and all exacerbations.
Results:
We included 10 trials in 9 articles from 2013 to 2018 with a total of 19,369 patients for analysis in this study. Compared with LAMA monotherapy, LABA/LAMA FDCs demonstrated similar efficacy in terms of time to first exacerbation [hazard ratio, 0.96; 95% confidence interval (CI) 0.79–1.18; p = 0.71], moderate to severe exacerbations [risk ratio (RR), 0.96; 95% CI 0.90–1.03; p = 0.28], severe exacerbations (RR, 0.92; 95% CI 0.81–1.03; p = 0.15), and a marginal superiority in terms of all exacerbations (RR, 0.92; 95% CI 0.86–1.00; p = 0.04). The incidence of all exacerbation events was lower in the LABA/LAMA FDC group for the COPD patients with a history of previous exacerbations and those with a longer treatment period (52–64 weeks).
Conclusion:
This study provides evidence that LABA/LAMA FDCs are marginally superior in the prevention of all exacerbations compared with LAMA monotherapy in patients with COPD.
The reviews of this paper are available via the supplemental material section.
Keywords: chronic obstructive pulmonary disease, COPD exacerbations, LABA/LAMA FDCs, LAMA
Introduction
Chronic obstructive pulmonary disease (COPD) represents a significant health burden and is currently the third leading cause of death worldwide.1 Long-acting bronchodilators, including long-acting β2 agonists (LABAs) and long-acting muscarinic antagonists (LAMAs), are the mainstay of treatment for symptom management in patients with COPD. In recent years, LABA/LAMA fixed-dose combinations (FDCs) have increasingly been used to treat COPD due to greater improvements in lung function, symptom scores, and health status when compared with LABA or LAMA monotherapy.2–5 There is solid evidence showing that LAMAs are superior to LABAs with regards to exacerbation prevention.6 However, the advantage of LABA/LAMA over LAMA in preventing exacerbations has not been consistently demonstrated, so the use of LABA/LAMA FDCs as initial treatment is currently guided by the level of symptoms but not the risk of exacerbations. According to the 2019 and 2020 revised Global Initiative for Chronic Obstructive Lung Disease (GOLD) reports, LAMA monotherapy is recommended as the initial choice of therapy for patients at high risk of COPD exacerbations (group C and D). LABA/LAMA FDC is an alternative choice for those with more severe symptoms. In addition, for patients with persistent exacerbations on long-acting bronchodilator monotherapy, escalation to LABA/LAMA is also recommended in current guidelines.7 The currently available LABA/LAMA FDCs, including olodaterol/tiotropium, indacaterol/glycopyrronium, formoterol/aclidinium, and vilanterol/umeclidinium, are widely used to treat COPD patients. However, few studies have investigated whether these LABA/LAMA FDCs offer additional benefits over LAMA monotherapy in exacerbation prevention. Recently, a large randomized controlled trial demonstrated that combining tiotropium and olodaterol did not reduce exacerbation rates as much as expected compared with tiotropium alone.8 Given the increased number of LABA/LAMA FDCs for clinical use in COPD patients, the initial choice of these agents or LAMA alone remains under debate in terms of exacerbation prevention. The aim of the current analysis was therefore to evaluate the comparative efficacy of LABA/LAMA FDCs and LAMA monotherapy in preventing exacerbations.
Methods
Search strategy and eligibility criteria
We performed a systematic literature search to identify randomized controlled trials (RCTs) evaluating the efficacy and safety of long-acting bronchodilators for COPD using PubMed, EMBASE, Cochrane Library, and Trip databases for relevant studies published up to August 1, 2019. In addition, reference lists of the included studies were scanned, and experts and physicians in this field were also consulted. This systematic review was drafted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols (PRISMA-P) guidelines.9 The inclusion criteria were: (a) patients with stable COPD according to the GOLD diagnostic criteria; (b) randomized controlled trials comparing LABA/LAMA FDCs and LAMAs; (c) at least 24 weeks of treatment duration; and (d) endpoints meeting any of our outcomes of interest (time to first exacerbation, rates of moderate to severe, severe and all exacerbations). We also included studies with subgroup analysis comparing LAMA/LABA FDCs with individual LAMA components.
Data extraction and risk of bias assessment
The title and abstract of each study were independently assessed by three authors (C-YC, W-CC, and Y-FW) to confirm the eligibility for analysis, and any difference in opinion was resolved by consensus. Data from the included studies were independently extracted and checked by C-YC and Y-FW Two reviewers (C-YC and W-CC) independently assessed the risk of bias of the included studies according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions 5.1. Disagreements were resolved by consensus or assessed by other authors (Y-PH and C-HH).
Outcomes of interest
The outcomes of interest were the frequency of acute exacerbations (time to first exacerbation, rates of moderate to severe, severe and all exacerbations). Frequencies of exacerbations were also analyzed according to the treatment duration, high-risk versus low-risk populations, and tiotropium versus non-tiotropium groups.
Statistical analysis
Studies were pooled using risk ratios (RRs) for dichotomous outcomes and hazard ratios (HRs) for time to event outcomes in random effect models, respectively.10 A 95% confidence interval (CI) was set to determine statistical significance. Between-study heterogeneity assessed using the I2 test was considered to be moderate to high at a p-value < 0.10 and I2 value > 50%. Publication bias was examined using eyeballing, funnel plots and Egger’s test. Sensitivity analyses were performed to assess the contribution of each study to the pooled estimate by excluding individual studies one at a time and recalculating the pooled HR estimates for the remaining studies (one-study-removed meta-analysis). For any three-arm trials (e.g. indacaterol/glycopyrronium versus glycopyrronium versus tiotropium), each pairwise comparison (i.e. indacaterol/glycopyrronium versus glycopyrronium, and indacaterol/glycopyrronium versus tiotropium) was used in the meta-analysis by dividing the sample size in half to match the total sample size when adding together. High-risk versus low-risk exacerbation patient groups were defined according to the previous exacerbation history (⩾1 versus no exacerbations) or lung function (⩾50% versus <50% of forced expiratory volume in the first second) for the majority patients in the study. The meta-analysis was performed using Review Manager Software version 5.3 (Cochrane Library Software, Oxford, UK).
Results
Study characteristics
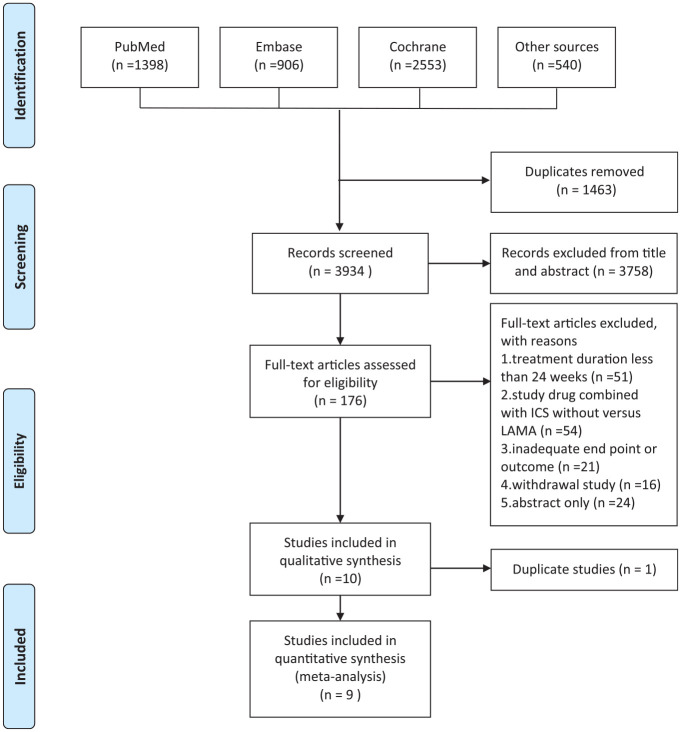
The relevant research and studies are shown in Figure 1. Two articles reported different outcomes from the same patients group so only one was included for analysis.11,12 Finally, a total of 19,369 patients were included in nine published articles from 2013 to 2018.8,12–19 Among these included articles, two compared indacaterol/glycopyrronium, glycopyrronium, and tiotropium; two compared tiotropium/olodaterol and tiotropium; two compared aclidinium/formoterol and aclidinium; one compared umeclidinium/vilanterol, umeclidinium, and tiotropium; one compared umeclidinium/vilanterol and tiotropium; and one compared glycopyrrolate/formoterol fumarate, glycopyrrolate, and tiotropium.
Figure 1.
Flow diagram of study identification.
ICS, inhaled corticosteroids; LAMA, long-acting muscarinic antagonist.
All the studies were RCTs and double-blinded, with a treatment period from 24 to 64 weeks. Three of the articles reported outcomes from two duplicate RCTs, and one of them reported outcomes of two trials separately.12,15,18 We paired the LAMA/LABA FDCs and compared them with individual or different LAMA components in the studies including three or more comparators. The characteristics of the included studies are summarized in Table 1.
Table 1.
Study characteristics of the included studies.
| Study | Study duration (weeks) | Number of patients | Treatment comparison/dose (µg) | Mean age (years) |
Male (%) |
Baseline FEV1
% predicted |
COPD severity by lung function | Exacerbation risk by lung function | Exacerbation history in previous year | Exacerbation risk by previous AE |
|---|---|---|---|---|---|---|---|---|---|---|
| Bateman et al.13 | 26 | 474 | Indacaterol 110/glycopyrronium 50 | 64.0 | 76.4 | 55.7 | Moderate (63.8%) Severe (36.1%) | Low | 0 (73.7%) 1 (19.5%) ⩾2 (6.8%) | Low |
| 473 | Glycopyrronium 50 | 64.3 | 77.2 | 55.1 | ||||||
| 480 | Tiotropium 18 | 63.5 | 75.0 | 55.1 | ||||||
| Wedzicha et al.14 | 64 | 729 | Indacaterol 110/glycopyrronium 50 | 63.1 | 76.0 | 37.0 | Severe (79.2%) Very severe (20.8%) |
High | 0 (1.5%) 1 (76.5%) ⩾2 (21.4%) | High |
| 740 | Glycopyrronium 50 | 63.1 | 73.0 | 37.3 | ||||||
| 737 | Tiotropium 18 | 63.6 | 75.0 | 37.4 | ||||||
| Decramer et al.15 study 1 | 24 | 212 | Umeclidinium 62.5/vilanterol 25 | 63.0 | 70.0 | 48.0 | Moderate (48.0%) Severe (41.2%) Very severe (10.8%) |
High | 0 (51.2%) ⩾1 (48.8%) | Low |
| 208 | Tiotropium 18 | 62.6 | 67.0 | 47.8 | ||||||
| Decramer et al.15 study 2 | 24 | 217 | Umeclidinium 62.5/vilanterol 25 | 65.0 | 65.0 | 47.7 | Moderate (43.9%) Severe (43.2%) Very severe (12.8%) |
High | 0 (66.5%) ⩾1 (33.5%) | Low |
| 222 | Umeclidinium 125 | 64.5 | 67.0 | 46.2 | ||||||
| 215 | Tiotropium 18 | 65.2 | 71.0 | 47.4 | ||||||
| Maleki-Yazdi et al.16 | 24 | 454 | Umeclidinium 62.5/vilanterol 25 | 61.9 | 68.0 | 46.2 | Moderate (41.4%) Severe (45.6%) Very severe (12.9%) |
High | N/A | N/A |
| 451 | Tiotropium 18 | 62.7 | 67.0 | 46.5 | ||||||
| Singh et al.17 | 24 | 385 | Aclidinium 400/formoterol 12 | 62.7 | 67.8 | 54.6 | Moderate (59.2%) Severe (40.8%) |
Low | N/A | N/A |
| 385 | Aclidinium 400 | 63.1 | 66.5 | 53.6 | ||||||
| Buhl et al.12 | 52 | 1029 | Tiotropium 5/olodaterol 5 | 63.8 | 71.2 | 49.3 | Moderate (49.4%) Severe (38.6%) Very severe (12.0%) |
High | N/A | N/A |
| 1033 | Tiotropium 5 | 63.9 | 73.1 | 49.7 | ||||||
| Hanania et al.18 | 52 | 1035 | Glycopyrrolate 18/formoterol 9.6 | 62.7 | 54.3 | 43.4 | Very severe (10.2%) | N/A | 0 (83.3%) 1 (9.5%) ⩾2 (7.2%) |
Low |
| 888 | Glycopyrrolate 18 | 62.8 | 55.9 | 42.6 | ||||||
| 450 | Tiotropium 18 | 62.9 | 59.6 | 42.7 | ||||||
| D’Urzo et al.19 | 52 | 335 | Aclidinium 400/formoterol 12 | 64.2 | 50.1 | 53.2 | Moderate (56.3%) Severe (43.7%) |
Low | 0 (79.2%) ⩾1 (20.8%) | Low |
| 337 | Aclidinium 400 | 64.4 | 55.8 | 53.0 | ||||||
| Calverley et al.8 | 52 | 3939 | Tiotropium 5/olodaterol 5 | 66.5 | 71.0 | 44.6 | N/A | N/A | 1 (55.7%) ⩾2 (44.3%) | High |
| 3941 | Tiotropium 5 | 66.3 | 72.0 | 44.5 |
AE, acute exacerbations; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in the first second.
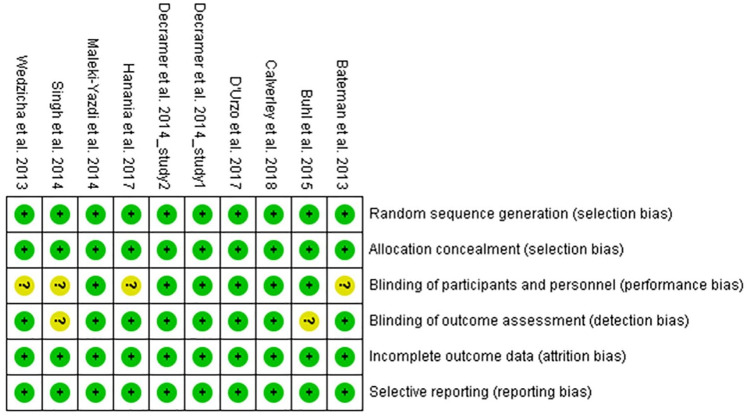
Risk of bias
Risk of bias assessments for the included studies were performed by the review authors independently. Most of the studies had a low risk of bias as shown by sufficient evidence of random sequence generation, double blinding protocol, and complete outcome assessment (Figure 2). However, an unclear risk of performance bias was found in four studies due to not mentioning the double dummy technique,13,14,17,18 and detection bias may have occurred in two studies due to incomplete descriptions of the blinding of outcome assessments.12,17
Figure 2.
Risk of bias graph: review authors’ judgement about the risk of each item of bias presented as percentages across all included studies.
Outcome assessments
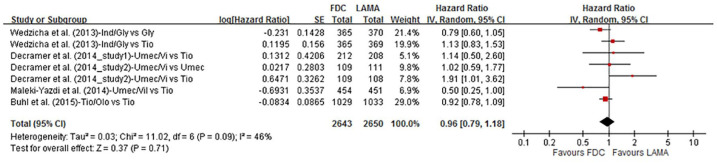
Time to first exacerbation
Four publications (including five RCTs, n = 5293) reported the time to first exacerbation as the endpoint (Figure 3). There was no statistical difference between the patients receiving LAMA/LABA FDCs compared with individual LAMAs (tiotropium, umeclidinium, and glycopyrronium). The HR for an exacerbation was 0.96 (95% CI 0.79–1.18; p = 0.71, I2 = 46%). Subgroup analyses according to different LAMAs (tiotropium and non-tiotropium), treatment duration (24 weeks and 52–64 weeks), and risk of exacerbations (by exacerbation history) were all not statistically significant (Supplemental Figures S1, S2, and S3).
Figure 3.
Forrest plot comparing LABA/LAMA FDCs and LAMAs on time to first exacerbation.
CI, confidence interval; FDC, fixed-dose combination; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
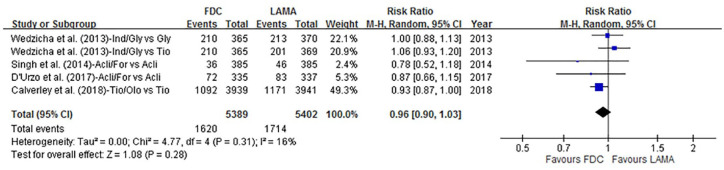
Moderate-to-severe exacerbations
Moderate-to-severe exacerbation data were available in four articles (n = 10,791). Overall, 30.0% (1620/5389) of the patients receiving LABA/LAMA FDCs experienced moderate-to-severe exacerbations, compared with 31.7% (1714/5402) of the patients receiving LAMAs alone (Figure 4). The RR was 0.96 (95% CI 0.90–1.03; p = 0.28, I2 = 16%), and no statistical difference was found. We then analyzed LABA/LAMA FDCs compared with different LAMAs (tiotropium and non-tiotropium), treatment duration (24 weeks and 52–64 weeks), and risk of exacerbations (by exacerbation history), but no statistically significant differences were found (Supplemental Figures S4, S5, and S6).
Figure 4.
Forrest plot comparing LABA/LAMA FDCs and LAMAs with events of moderate-to-severe exacerbations.
CI, confidence interval; FDC, fixed-dose combination; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
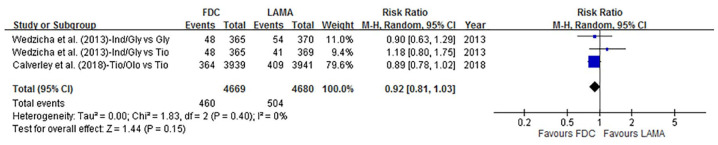
Severe exacerbations
Only two publications (n = 9349) reported severe exacerbations as one of the endpoints. There was no statistical difference between the LABA/LAMA FDCs and LAMA groups in terms of severe exacerbations [9.9% (460/4669) versus 10.8% (504/4680), respectively], with an RR of 0.92 (95% CI 0.81–1.03, p = 0.15, I2 = 0%) (Figure 5).
Figure 5.
Forrest plot comparing LABA/LAMA FDCs and LAMAs with events of severe exacerbations.
CI, confidence interval; FDC, fixed-dose combination; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
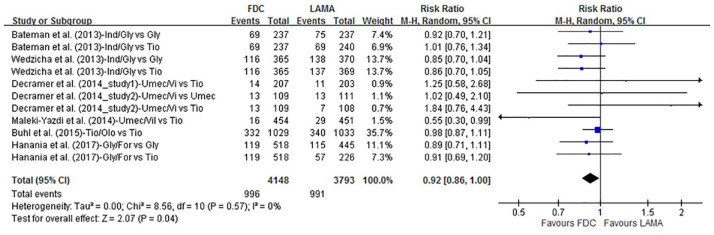
All exacerbations
The incidence of all exacerbations from six articles (including 9 RCTs, n = 7941) was lower in those treated with LABA/LAMA FDCs than in those treated with LAMAs [24.0% (996/4148) versus 26.1% (991/3799), respectively], with an RR of 0.92 (95% CI 0.86–1.00; p = 0.04, I2 = 0%) (Figure 6). Subgroup analyses showed similar efficacy in those treated with LABA/LAMA FDCs compared with those treated with different LAMAs, but slight superiority was demonstrated in those with a longer treatment duration (52–64 weeks) (RR, 0.92; 95% CI 0.85–1.00; p = 0.04) (Supplemental Figures S7 and S8). Other analyses according to the risk of exacerbations (high-risk versus low-risk, stratified by exacerbation history or lung function), demonstrated a lower rate of all exacerbations only in the high-risk population stratified by exacerbation history (RR, 0.85; 95% CI 0.74–0.98; p = 0.03) (Supplemental Figures S9 and S10).
Figure 6.
Forrest plot comparing LABA/LAMA FDCs and LAMAs with events of all exacerbations.
CI, confidence interval; FDC, fixed-dose combination; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
Discussion
The results of this meta-analysis demonstrated that LABA/LAMA FDCs were marginally beneficial in the prevention of all exacerbations compared with LAMA monotherapy in COPD patients. According to the revised GOLD reports, LAMA monotherapy is preferred as the initial treatment choice in group C and D COPD patients, and LABA/LAMA FDCs are an alternative choice for those with more symptoms.7 Escalation to LABA/LAMA is also recommended for patients with persistent exacerbations on LAMA monotherapy. Nevertheless, the 2019 updated National Institute for Health and Clinical Excellence (NICE) guidelines recommended LAMA+LABA dual therapy, but not monotherapy, as the preferred initial treatment in stable COPD patients who remain breathless or have exacerbations despite optimal non-pharmacological management and after using a short-acting bronchodilator.20 The NICE guidelines also state that LAMA/LABA dual therapy provides the greatest benefit to overall quality of life compared with monotherapy, and that dual therapy is better than other inhaled treatments for many individual outcomes (such as reducing the risk of moderate-to-severe exacerbations) and is the most cost-effective option. Nevertheless, the currently available evidence is not consistent with regards to whether LABA/LAMA combination therapy is more effective than LAMA monotherapy in preventing exacerbations.
Previous meta-analyses have focused on comparisons of LABA/LAMA combinations and LAMA monotherapy. One meta-analysis conducted by Rodrigo et al. demonstrated greater efficacy and comparable safety profiles with LABA/LAMA combinations versus LAMAs. However, the COPD exacerbation rate was not reported due to insufficient data.21 Rogliani et al. assessed the evidence for LABA/LAMA FDCs in the treatment of COPD. They analyzed trial results of the available LABA/LAMA FDCs and found that only indacaterol/glycopyrronium demonstrated superiority to glycopyrronium, but that there was no statistically significant difference versus tiotropium.22
Oba et al. conducted a comprehensive Cochrane review of different groups of inhalers (including LABA/LAMA combinations and LAMA monotherapy) in patients with moderate-to-severe COPD. They analyzed eight studies comparing different LABA/LAMA combinations and LAMA monotherapy, and demonstrated no statistical differences in terms of severe exacerbations [odds ratio (OR) 0.90; 95% CI 0.59–1.36; p = 0.61] and moderate-to-severe exacerbations (OR 0.96, CI 0.75–1.23; p = 0.77).23 Although this finding is consistent with our results, Oba’s review enrolled two studies with LABA/LAMA combinations in two separate inhalers and one study which was a 12-week study that was inappropriate to evaluate exacerbation outcomes. In addition, the largest DYNAGITO trial was not included for analysis in their review.
Farne et al. reviewed 10 trials comparing tiotropium plus a LABA to tiotropium or a LABA alone and concluded that adding tiotropium to a LABA reduced exacerbations, but that adding a LABA to tiotropium did not.24 Another comprehensive systematic review reported by Halpin et al. demonstrated that tiotropium was beneficial in reducing the risk of exacerbations compared with other maintenance treatments. Their analysis showed that tiotropium provided similar efficacy to glycopyrronium and a LABA/LAMA FDC (glycopyrronium/indacaterol), although not all studies were sufficiently powered to demonstrate differences in exacerbation outcomes.25 However, the large DYNAGITO trial reported that a combination of tiotropium and olodaterol failed to reduce the exacerbation rate as expected when compared with tiotropium alone.8 Given the conflicting results, we stratified and analyzed different LAMAs as tiotropium and non-tiotropium. Our analysis showed that exacerbation events in those receiving LAMA/LABA FDCs were not significantly different compared with those receiving tiotropium or non-tiotropium therapy.
In the current study, we also compared LABA/LAMA FDC and LAMA therapy in patients grouped by the risk of exacerbations and treatment duration. We analyzed the COPD patients with a high or low risk of exacerbations in the studies according to lung function or previous exacerbation history. Our results demonstrated that only the incidence of all exacerbation (but not severe or moderate to severe exacerbation) events was lower in the LABA/LAMA FDCs for COPD patients with a history of previous exacerbation. However, this result was from the SPARK study in which most of the patients had a previous history of exacerbation (DYNAGITO study did not provide outcomes of all exacerbation).14 In addition, LABA/LAMA FDCs showed a small benefit in the incidence of all exacerbation events for those with a longer treatment period (52–64 weeks compared with 24–26 weeks), probably due to the long-term effects of LABA/LAMA combination therapy in terms of lung function improvements.
The GOLD reports recommend escalating to LABA/LAMA treatment for patients with persistent dyspnea or exacerbations on long-acting bronchodilator monotherapy. This escalation strategy is supported in part by this systematic review, in that LABA/LAMA FDCs may provide better efficacy in the prevention of all exacerbations compared to LAMA monotherapy, although the incremental benefit was small.
There are several limitations to this study. First, the results of this meta-analysis could not indicate differences between the various LABA/LAMA FDCs and LAMAs (although we stratified the LAMA group by tiotropium and non-tiotropium), and their efficacy may not be exactly the same. Second, assessments of the high- and low-risk patients in the included studies were not consistent, even though we stratified the majority of the study patients by lung function or previous exacerbation history. Third, exacerbation type (requiring antibiotic or systemic steroid therapy) was not analyzed due to insufficient data in the included studies.
In conclusion, our meta-analysis suggests that LAMA/LABA FDCs produce a small benefit in the prevention of all exacerbations compared to LAMA monotherapy, but similar efficacy in terms of time to first exacerbation, the rate of moderate-to-severe, and severe exacerbations. In addition to greater improvements in lung function, symptom scores, and health status, our findings provide evidence that LABA/LAMA FDCs are also better than LAMA monotherapy in terms of all exacerbation prevention and could be considered as the first-line treatment for COPD patients, especially in those with a history of previous exacerbations.
Supplemental Material
Supplemental material, Author_Response_1 for LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: a systematic review and meta-analysis by Ching-Yi Chen, Wang-Chun Chen, Chi-Hsien Huang, Yi-Ping Hsiang, Chau-Chyun Sheu, Yung-Che Chen, Meng-Chih Lin, Kuo-An Chu, Cheng-Hung Lee and Yu-Feng Wei in Therapeutic Advances in Respiratory Disease
Supplemental material, Author_Response_2 for LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: a systematic review and meta-analysis by Ching-Yi Chen, Wang-Chun Chen, Chi-Hsien Huang, Yi-Ping Hsiang, Chau-Chyun Sheu, Yung-Che Chen, Meng-Chih Lin, Kuo-An Chu, Cheng-Hung Lee and Yu-Feng Wei in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: a systematic review and meta-analysis by Ching-Yi Chen, Wang-Chun Chen, Chi-Hsien Huang, Yi-Ping Hsiang, Chau-Chyun Sheu, Yung-Che Chen, Meng-Chih Lin, Kuo-An Chu, Cheng-Hung Lee and Yu-Feng Wei in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.2 for LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: a systematic review and meta-analysis by Ching-Yi Chen, Wang-Chun Chen, Chi-Hsien Huang, Yi-Ping Hsiang, Chau-Chyun Sheu, Yung-Che Chen, Meng-Chih Lin, Kuo-An Chu, Cheng-Hung Lee and Yu-Feng Wei in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: a systematic review and meta-analysis by Ching-Yi Chen, Wang-Chun Chen, Chi-Hsien Huang, Yi-Ping Hsiang, Chau-Chyun Sheu, Yung-Che Chen, Meng-Chih Lin, Kuo-An Chu, Cheng-Hung Lee and Yu-Feng Wei in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplemental_Figures for LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: a systematic review and meta-analysis by Ching-Yi Chen, Wang-Chun Chen, Chi-Hsien Huang, Yi-Ping Hsiang, Chau-Chyun Sheu, Yung-Che Chen, Meng-Chih Lin, Kuo-An Chu, Cheng-Hung Lee and Yu-Feng Wei in Therapeutic Advances in Respiratory Disease
Acknowledgments
C.-Y. Chen and W.-C. Chen contributed equally to this work.
Footnotes
Author contribution(s): Ching-Yi Chen: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing-original draft.
Wang-Chun Chen: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing-original draft.
Chi-Hsien Huang: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing-review & editing.
Yi-Ping Hsiang: Conceptualization; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing-review & editing.
Chau-Chyun Sheu: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Validation; Writing-review & editing.
Yung-Che Chen: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Validation; Writing-review & editing.
Meng-Chih Lin: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Validation; Writing-review & editing.
Kuo-An Chu: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Validation; Writing-review & editing.
Cheng-Hung Lee: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Validation; Writing-review & editing.
Yu-Feng Wei: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision, Validation; Visualization; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by research grants from E-Da Hospital (EDAHP104042, EDAHP105058 and EDPJ105090).
ORCID iD: Yu-Feng Wei  https://orcid.org/0000-0002-6380-3527
https://orcid.org/0000-0002-6380-3527
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Ching-Yi Chen, Division of Chest Medicine, Department of Internal Medicine, E-Da Hospital, Kaohsiung.
Wang-Chun Chen, Department of Pharmacy, E-Da Hospital, I-Shou University, Kaohsiung.
Chi-Hsien Huang, Department of Family Medicine, E-Da Hospital, I-Shou University, Kaohsiung; Department of Community Healthcare & Geriatrics, Nagoya University Graduate School of Medicine, Nagoya, Aichi, Japan.
Yi-Ping Hsiang, Department of Pharmacy, E-Da Hospital, I-Shou University, Kaohsiung.
Chau-Chyun Sheu, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung; Department of Internal Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung.
Yung-Che Chen, Division of Pulmonary and Critical Care Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung.
Meng-Chih Lin, Division of Pulmonary and Critical Care Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung.
Kuo-An Chu, Division of Chest Medicine, Department of Internal Medicine, Kaohsiung Veterans General Hospital, Kaohsiung.
Cheng-Hung Lee, Division of Chest Medicine, Department of Internal Medicine, National Cheng Kung University Hospital, Tainan.
Yu-Feng Wei, Department of Internal Medicine, E-Da Hospital, I-Shou University, No. 1, Yida Road, Jiao-su Village, Yan-chao District, Kaohsiung 824; School of Medicine for International Students, College of Medicine, I-Shou University, Kaohsiung.
References
- 1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calzetta L, Rogliani P, Matera MG, et al. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest 2016; 149: 1181–1196. [DOI] [PubMed] [Google Scholar]
- 3. Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting beta-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax 2016; 71: 15–25. [DOI] [PubMed] [Google Scholar]
- 4. Miravitlles M, Urrutia G, Mathioudakis AG, et al. Efficacy and safety of tiotropium and olodaterol in COPD: a systematic review and meta-analysis. Respir Res 2017; 18: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ni H, Moe S, Soe Z, et al. Combined aclidinium bromide and long-acting beta2-agonist for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 2018; 12: CD011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen WC, Huang CH, Sheu CC, et al. Long-acting beta2-agonists versus long-acting muscarinic antagonists in patients with stable COPD: a systematic review and meta-analysis of randomized controlled trials. Respirology 2017; 22: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 7. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J 2019; 53. [DOI] [PubMed] [Google Scholar]
- 8. Calverley PMA, Anzueto AR, Carter K, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med 2018; 6: 337–344. [DOI] [PubMed] [Google Scholar]
- 9. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015; 349: g7647. [DOI] [PubMed] [Google Scholar]
- 10. Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buhl R, Magder S, Bothner U, et al. Long-term general and cardiovascular safety of tiotropium/olodaterol in patients with moderate to very severe chronic obstructive pulmonary disease. Respir Med 2017; 122: 58–66. [DOI] [PubMed] [Google Scholar]
- 12. Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J 2015; 45: 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J 2013; 42: 1484–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med 2013; 1: 199–209. [DOI] [PubMed] [Google Scholar]
- 15. Decramer M, Anzueto A, Kerwin E, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med 2014; 2: 472–486. [DOI] [PubMed] [Google Scholar]
- 16. Maleki-Yazdi MR, Kaelin T, Richard N, et al. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24-week, randomized, controlled trial. Respir Med 2014; 108: 1752–1760. [DOI] [PubMed] [Google Scholar]
- 17. Singh D, Jones PW, Bateman ED, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm Med 2014; 14: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hanania NA, Tashkin DP, Kerwin EM, et al. Long-term safety and efficacy of glycopyrrolate/formoterol metered dose inhaler using novel co-suspension delivery technology in patients with chronic obstructive pulmonary disease. Respir Med 2017; 126: 105–115. [DOI] [PubMed] [Google Scholar]
- 19. D’Urzo A, Rennard S, Kerwin E, et al. A randomised double-blind, placebo-controlled, long-term extension study of the efficacy, safety and tolerability of fixed-dose combinations of aclidinium/formoterol or monotherapy in the treatment of chronic obstructive pulmonary disease. Respir Med 2017; 125: 39–48. [DOI] [PubMed] [Google Scholar]
- 20. National Institute for Health Care and Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. London, 2018. [PubMed] [Google Scholar]
- 21. Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2017; 12: 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rogliani P, Calzetta L, Braido F, et al. LABA/LAMA fixed-dose combinations in patients with COPD: a systematic review. Int J Chron Obstruct Pulmon Dis 2018; 13: 3115–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oba Y, Keeney E, Ghatehorde N, et al. Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta-analysis. Cochrane Database Syst Rev 2018; 12: CD012620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 10: CD008989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halpin DM, Vogelmeier C, Pieper MP, et al. Effect of tiotropium on COPD exacerbations: a systematic review. Respir Med 2016; 114: 1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: a systematic review and meta-analysis by Ching-Yi Chen, Wang-Chun Chen, Chi-Hsien Huang, Yi-Ping Hsiang, Chau-Chyun Sheu, Yung-Che Chen, Meng-Chih Lin, Kuo-An Chu, Cheng-Hung Lee and Yu-Feng Wei in Therapeutic Advances in Respiratory Disease
Supplemental material, Author_Response_2 for LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: a systematic review and meta-analysis by Ching-Yi Chen, Wang-Chun Chen, Chi-Hsien Huang, Yi-Ping Hsiang, Chau-Chyun Sheu, Yung-Che Chen, Meng-Chih Lin, Kuo-An Chu, Cheng-Hung Lee and Yu-Feng Wei in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: a systematic review and meta-analysis by Ching-Yi Chen, Wang-Chun Chen, Chi-Hsien Huang, Yi-Ping Hsiang, Chau-Chyun Sheu, Yung-Che Chen, Meng-Chih Lin, Kuo-An Chu, Cheng-Hung Lee and Yu-Feng Wei in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.2 for LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: a systematic review and meta-analysis by Ching-Yi Chen, Wang-Chun Chen, Chi-Hsien Huang, Yi-Ping Hsiang, Chau-Chyun Sheu, Yung-Che Chen, Meng-Chih Lin, Kuo-An Chu, Cheng-Hung Lee and Yu-Feng Wei in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: a systematic review and meta-analysis by Ching-Yi Chen, Wang-Chun Chen, Chi-Hsien Huang, Yi-Ping Hsiang, Chau-Chyun Sheu, Yung-Che Chen, Meng-Chih Lin, Kuo-An Chu, Cheng-Hung Lee and Yu-Feng Wei in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplemental_Figures for LABA/LAMA fixed-dose combinations versus LAMA monotherapy in the prevention of COPD exacerbations: a systematic review and meta-analysis by Ching-Yi Chen, Wang-Chun Chen, Chi-Hsien Huang, Yi-Ping Hsiang, Chau-Chyun Sheu, Yung-Che Chen, Meng-Chih Lin, Kuo-An Chu, Cheng-Hung Lee and Yu-Feng Wei in Therapeutic Advances in Respiratory Disease