Abstract
Precision oncology is the field that places emphasis on the diagnosis and treatment of tumors that harbor specific genomic alterations susceptible to inhibition or modulation. Although most alterations are only present in a minority of patients, a substantial effect on survival can be observed in this subgroup. Mass genome sequencing has led to the identification of a specific driver in the translocations of the tropomyosin receptor kinase family (NTRK) in a subset of rare tumors both in children and in adults, and to the development and investigation of Larotrectinib. This medication was granted approval by the US Food and Drug Administration for NTRK-positive tumors, regardless of histology or age group, as such, larotrectinib was the first in its kind to be approved under the premise that molecular pattern is more important than histology in terms of therapeutic approach. It yielded significant results in disease control with good tolerability across a wide range of diseases including rare pediatric tumors, salivary gland tumors, gliomas, soft-tissue sarcomas, and thyroid carcinomas. In addition, and by taking different approaches in clinical trial design and conducting allocation based on biomarkers, the effects of target therapies can be isolated and quantified. Moreover, and considering developing nations and resource-limited settings, precision oncology could offer a tool to reduce cancer-related disability and hospital costs. In addition, developing nations also present patients with rare tumors that lack a chance of treatment, outside of clinical trials. This, in turn, offers the possibility for international collaboration, and contributes to employment, education, and health service provisions.
The reviews of this paper are available via the supplemental material section.
Keywords: larotrectinib, NTRK, precision medicine, tropomyosin receptor kinase family
Introduction
The development of molecular biology techniques in the last few decades has created the possibility for clinicians and researches to tap into the potentials of the unknown. Leading to advancements in genetics, the possibility for examining and dissecting the building blocks of the cells and, in this particular case, a wider understanding of the fundamental structure and functioning of tumor cells is now possible. Whole exome, genome, and RNA sequencing combined with leaps forward in bioinformatics have contributed to the discovery of several molecular alterations that affect the growth and spread of tumors.1,2 Two massive projects known as The Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium (ICGC) have accumulated information for all common cancers and rare tumors, suggesting that almost all driver genes have been discovered.3–6 The limits of applicability of these discoveries reach beyond research. Although these efforts initially attempted merely to describe the tumor genomic landscape, the idea of modification and intervention over these said discoveries has gained much ground in recent years and has permeated into clinical practice, especially in clinical oncology. This new prospect in medical care is known as precision medicine, and is explored extensively in this review.
What is precision oncology and what is its use?
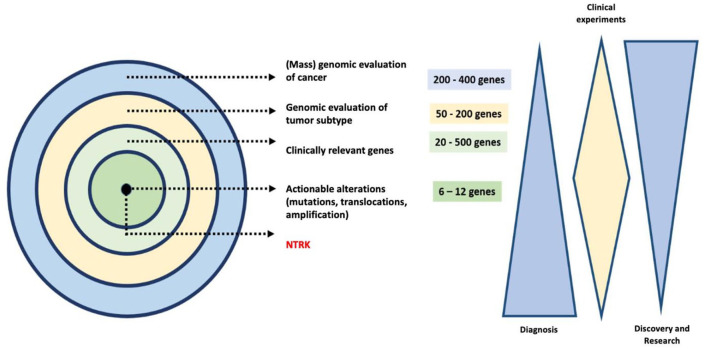
In oncology, precision medicine refers to the use of diagnostic and therapeutic activities combined for the benefit of a subset of patients whose tumors present specific genomic events that stem from molecular alterations that modify the biology of the tumor cell deregulating potentially actionable signaling pathways.7 The rapid evolution of technological tools that allow for polygenic evaluation through molecular profiles has allowed for the inclusion of predictive biomarkers that have radically modified the outlook of cancer care8 (Figure 1). Globally, 40% and 63% of the predictive and prognostic biomarkers in use are related to cancer.9 These figures translated into a net effect of precision technology that oscillates between 11% and 18% of the affected population.10 In the face of annual evidence of 32.6 million survivors with cancer globally, the correct use of precision medicine could modify the survival and quality of life of more than 5.5 million patients per year. In 2018, 101,893 cases of cancer were registered in Colombia,11 which in turn outlines that 18,340 people living with cancer in our area could benefit from the use of precision oncology. Considering a region-wide approach, approximately 6270 of the 1,412,732 cancer patients in Latin America12 will have tumors with different NTRK-1-3 translocations (neurological receptor of the kinase for tropomyosin), among which 20% are expected to be children.
Figure 1.
The use of precision oncology for the determination of potentially actionable genes with directed targeted therapy (a sequence from mass gene exploration until NTRK identification). Despite the cost of the generalized implementation of in-depth genomics, the directed use of state-of-the-art therapeutic strategies has demonstrated a global saving of 5.6% in the participating population. This care strategy based on value could reduce the global costs of directed oncological treatment after 18 months of intervention in more than 7.5%, a valid event for one in four patients with advanced cancer.13
Key points (1).
| • Precision oncology includes the use of diagnostic and therapeutic strategies combined to benefit a subgroup of patients whose tumors present specific genomic events that stem from molecular alterations susceptible to management with direct therapies. • Up to 18% of patients with cancer benefit from precision oncology. • NTRK1–3 translocations present in different tumors and their directed treatment constitute the most relevant example to demonstrate the usefulness of the value model based on precision oncology. |
What is the effect of precision oncology on rare tumors?
Many studies have demonstrated the relevance of the routine use of precision oncology, including some performed in community institutions not considered reference centers. Recently, Schram et al. determined that the inclusion of mass genome sequencing in regular clinical practice modified 20–25% of decisions. In the same manner, amongst the patients whose treatment was not rectified, attending physicians indicated the presence of at least one actionable genetic alternation in 55% of the cases. However, only 45% of these had a genomic variety validated by experts. In an attempt to optimize the use of high-precision and complex genomic tools, interactive genomic reports have been designed along with multidisciplinary meetings for results analysis and artificial intelligence platforms to accurately interpret the large amounts of data stemming from bioinformatics.14,15 In this situation tools such as the app ESCAT (ESMO Scale for Clinical Actionability of Molecular Targets) enable patient selection through a standardized classification system based on the evidence of genomic alterations with clinical implications.16
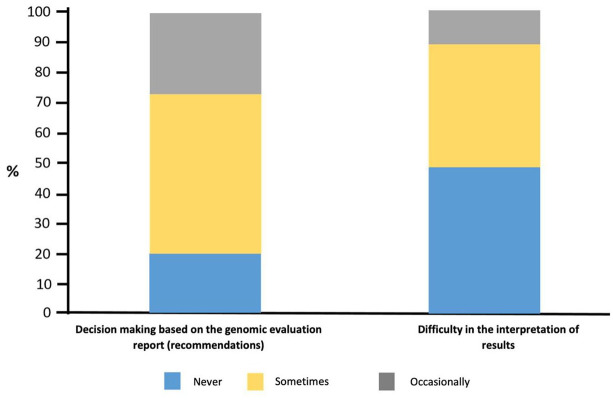
Recently, Freedman et al. carried out the National Survey on Precision Medicine in Cancer Treatment in the United States that included 1281 oncologists in community practice centers across the country.17 Among these, 75.6% said they had used mass genome sequencing tests to guide treatment options (Figure 2). In addition, 34% used the platform routinely to optimize the handling of patients with advanced refractory disease, 29.1% used it to determine the eligibility of subjects for clinical studies, and 17.5% to decide on the unapproved use of medicines admitted by the US Food and Drug Administration (FDA). The results of these tests provided useful recommendations for treatment in 26.8%, were occasionally useful in 52.4% and never or rarely useful for 20.8% of those consulted. Likewise, oncologists under 50 years old received training in genomics more frequently, usually treated more than 50 patients with direct targeted therapy per month, and had regular access to a multidisciplinary board that focused on the discussion of molecular tests.18
Figure 2.
The use of NGS tests during the last 12 months among United States oncologists.9
One of the greatest merits of mass genome sequencing is the identification of specific targets that maximize the benefit of intervention through simplifying the diagnosis. The best examples in this sphere are NTRK translocations found in a wide range of infrequent tumors amongst the adult and pediatric population. Once the molecular target was found linked to the response generated by larotrectinib, the diagnostic test was simplified with immunohistochemistry or fluorescence in situ hybridization (FISH) with prior validation of the information. In a study by Gatalica et al., the authors evaluated samples from 11,502 patients including 53 fusion genes and sequenced 592 additional genes in direct comparison with the classification by TrkA/B/C immunohistochemistry.19 Among the total cohort, 0.27% had NTRK alterations, with the most common fusions being ETV6:NTRK3 (n = 10) and TPM3:NTRK1 (n = 6). The greatest frequency of NTRK alterations was for patients with gliomas (1.4%), lesions that usually present an NTRK2 gene fusion. In parallel, 17 cases that were not related to the central nervous system were NTRK carriers, mainly lung, thyroid, breast, and nasal cavity carcinomas and diverse soft-tissue sarcomas. The uniform expression of the gene evaluated by immunohistochemistry found 7/8 NTRK lesions, 8/9 NTRK2 fusions, and 6/11 NTRK3.19 This information validates the usefulness and simplification of the sieving through immunohistochemistry.
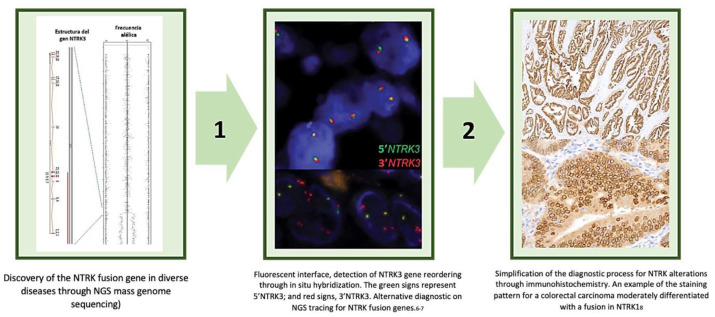
In a similar manner, Hechtman et al.20 found evidence that the Pan-Trk panel (rabbit recombinant monoclonal antibody, clone EPR17341, Abcam, Cambridge, MA) for the detection of fusion genes through immunohistochemistry has a sensitivity and diagnostic specificity of 95.2–100%, respectively. In this study, all cases that resulted positive through immunohistochemistry had a cytoplasmic staining, whilst specific pairing patterns were discovered for five LMNA-NTRK1 fusions that demonstrated accentuation of the nuclear membrane. In contrast, the four TPM3/4 fusions showed accentuation in the cellular membrane and half (3/6) of the NTRK3 fusions showed nuclear staining. In conclusion, Pan-Trk staining turned out to be a temporarily efficient test, easily implementable in tissue for the detection of NTRK fusions, particularly in specific advanced malignant tumors. In addition, Hung et al.21 went deeper on the use of immunohistochemistry as an initial diagnostic strategy in 210 cases of children, including 15 fibrosarcomas, 5 lipofibromatosis/lipofibromatosis-like neural tumors, 10 primitive myxoid/mesenchymal tumors of infancy (PMMTI), 15 fibrous hamartomas of infancy (FHI), miofibromatosis, desmoid fibromatosis and 20 synovial fibromyxoide sarcomas, rhabdomyosarcomas of spiculated dermatofibrosarcoma protuberans cells, and peripheric neural sheath nerves. The Pan-Trk immunohistochemistry panel confirmed positivity in the 15 infant fibrosarcomas (100%) and in the 5 subjects with lipofibromatosis/lipofibromatosis-like neutral tumors (100%), Lastly, Rudzinski et al.22 classified the sensitivity and specificity of the Pan-Trk panel (EPR17341) and the monoclonal antibody TrkA (EP1058Y) in a population of NTRK positive patients (n = 26). For the Pan-Trk panel (EPR17341) sensitivity and specificity were 97% and 98% respectively, whilst the TrkA IHC clone (EP1058Y) presented a sensitivity and specificity of 100% and 63%. In consensus, all this information supports the regular evaluation of NTRK alterations using immunohistochemistry, relegating the use of in depth genome sequencing for probable cases that initially turn out negative.23,24 Said strategy eases tackling and inter-population generalization, rapid access and reflection on the test, and the successful implementation of direct target treatment derived from rational knowledge of the fusion gene’s function through multiple tumors. Figure 3 demonstrates the evolution and simplification of diagnostic strategies for diverse NTRK carrier tumors.
Figure 3.
Diagnostic transition of NTRK fusion genes from their initial evaluation with polymerase chain reaction (PCR) sequencing techniques for mass genome sequencing (NGS)10,25–28 (Step 1), the use of fluorescence in situ hybridization (FISH)29,30 and sieving though immunohistochemistry20 (Step 2).
Key points (2).
| • Precision oncology permitted the identification of NTRK1–3 translocations in diverse tumors. Once the actionable nature of the fusion and the viability of the use of larotrectinib had been confirmed, immunohistochemistry was introduced (Pan-Trk panel) as a simplified diagnostic strategy. This test has demonstrated high yield among diverse tumors in children and adults. It presents an excellent cost–benefit relationship and a high sensitivity and specificity, findings that make it easily implementable at a local level. |
The importance of precision medicine in countries with limited resources
Precision oncology has demonstrated a balance in favor of economic aggregated value based on cost-effectiveness and use of the specific block of diverse molecular targets. Given that the net cost of the latest generation of oncology drugs represents between 12% and 20% of the care cost, precision oncology allows for the reduction of global investment by moderating the requirement for hospital care and institutional stay. For example, in 2015, the United Kingdom spent approximately £1.3 billion (USD $1.83 billion) in hospital services for patients with cancer who received inter-institutional treatment during their last year of life. In a similar way, Australia and the United States invested approximately 79% (AUD $3.6 billion, USD $2.6 billion) and 38% (USD $31.3 billion) on their total inter-institutional cancer care. Based on this profile there is convincing evidence that biomarkers, pharmacogenetic tests and specific targeted therapy significantly reduce visits to the emergency room and the requirement for hospitalized care.31,32 In the same way, the improvement in quality of life has a transcendental effect on the therapeutic course of infrequent diseases with limited alternative therapies that have not been very effective up until now. This is particularly the case in developing nations, where access to targeted therapies is not widespread, possibly due to economic conditions, limitations on coverage, and physical accessibility.33 International cooperative clinical trials offer a possibility to grant certain access to new effective medications. Taking into account the large number of newly diagnosed cancer cases in Latin America and the Caribbean, with the majority of these countries being cataloged as developing nations,34 a considerable number of patients with rare cancer diagnoses will suffer from these diseases without the possibility of a treatment opportunity. Nonetheless, international cooperation and accessibility to clinical trials offer an alternative. On the one hand, by recruiting these patients, clinical trial organizers gain possible research subjects. On the other hand, contribution to social value, intrinsically woven into the fabric of ethical clinical research, can be offered by administering participants otherwise inaccessible medications.35 Furthermore, additional benefits in participation, such as employment, training of community members, and increase of health care services, can be observed.36
It should be noted that one of the most important reasons that precision oncology is lagging behind in resource-limited settings, as compared with developed countries, is the lack of dedicated resources and research centers that help to promote the generation of new knowledge by training and educating young scientists, medical students, clinicians, etc. Implementation of personalized medicine in resource-limited environments would be stronger if research collaboration between developed and developing/resource-limited countries increases. To this end, developing countries will undoubtedly benefit from treatment access, training opportunities, knowledge transfer, and expanding transnational networks, while developed countries are likely to benefit through comparative work and multicenter projects on families with rare diseases or unique clinical features. To achieve this symbiosis, an enormous commitment should be made by researchers, physicians, and the pharmaceutical industry, to conduct high-quality clinical trials that will benefit patients at the same time as we gain substantial scientific knowledge.37,38
Another way in which patients with limited resources could access novel therapeutics is the compassionate use of drugs. Clinical trials were previously the only way to access new drugs under development. However, not every patient meets the enrolment criteria, and participation is difficult for patients with life-threatening, long-lasting, or seriously debilitating diseases such as cancer. Early access programs such as the “Compassionate Use Program” (CUP) have generated alternative channels for such patients, and offer another option for treatment access in developing countries.39
Key points (3).
| • NTRK inhibition (larotrectinib) has a high added therapeutic value and constitutes an unsatisfied medical therapeutic need. • There are no studies that have evaluated (locally or internationally) the cost-effectiveness of larotrectinib. However, the clear clinical benefit for diverse rare tumors that carry the NTRK1–3 fusion gene constitutes a balanced strategy for different sanitary models on a global scale. |
Approximation of NTRK tumors as an example of practice based on precision oncology
Mass genome sequencing permits the identification of three varieties of the NTRK fusion gene in 17 different tumor types. This active translocation in a constitutive form and dependent on the stimulus stemming from different ligands [neurotrophins, peripheral nerve growth factor (NGF) for TrkA, brain derived growth factor (BDGF), NT-4/5 for TrkB, and NT3 for TrkC] related to the tropomyosin receptor (TRK), The TRK is related to the growth, differentiation, maturity, and survival of neurons. For this reason, it is expressed as primary and normal tumors in the central nervous system. Regardless, NTRK fusion genomes represent a combination of chromosomic rearrangements expressed in segment 5 (more than 60 identified) in addition to the fraction 3 that codifies the tyrosine kinase intercellular segment.40
The binding of the TrkA receptor through NGF provokes the activation of the kinase Ras/Protein route activated by mitogens (MAPK) that lead to a greater proliferation and cellular growth through kinase signaling regulated by extracellular signals (ERK). Other routes such as phospholipase C-y (PLCy) and depending on PI3K are also activated in a parallel way. Likewise, the coupling of TrkC with NT3 provokes the preferential action of the PI3/AKT route, avoiding apoptosis and the increase of cellular survival, whilst TrkB transduces the BDNF signal through Ras-ERK, PI3K, and PLCy, resulting in differentiation and tumor surivival.23,24,31,32
The NTRK1 gene is located on the 1q21-q22 4 chromosome and its mutations interrupt the function of the TrkA protein found in patients affected by congenital insensitivity to pain with anhidrosis (CIPA). The NTRK2 gene is mapped on the 9q22.17 chromosome and contains 24 exons, 8 of which codify a protein of 822 amino acid residues (TrkB receptor). The NTRK3 gene is found on the 15q25,9 chromosome and its transcription product known as TrkC expressed on the human hippocampus, on the cerebral cortex, and on the layers of granular cerebellum cells.10,23,24,26,28,31,32,40
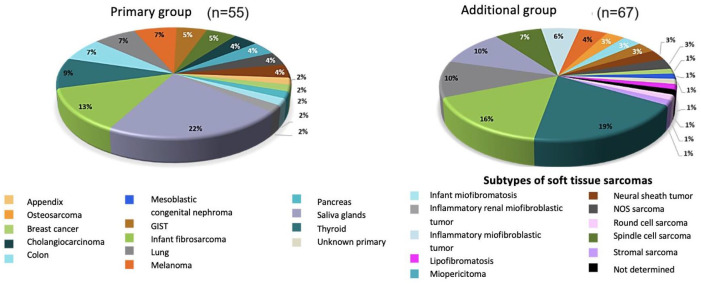
The family of NTRK gene rearrangements is implicated in the development of 1% of solid tumors, with an incremental frequency (between 5% and 7%) in some rare neoplasias of the pediatric population. Amongst others are infant fibrosarcoma, congenital mesoblastic nephroma, and diverse low-grade gliomas. All of these had minimal really effective alternative therapies, until the advent of precision oncology that permitted NTRK identification. The transition of biological information on NTRK amongst adults allowed for the documentation of alterations in secreting breast carcinomas, papillary thyroid carcinoma, bile duct adenocarcinoma, squamous head and neck carcinoma, acute myeloid leukemia, gastrointestinal stromal tumors, and saliva gland carcinomas.16 To the best of the authors’ current knowledge, there is no stablished workflow for screening NTRK mutations; therefore, we recommend that in resource-limited settings, NTRK should only be analyzed in malignancies in which it has been described that NTRK rearrangements are frequently present. Figure 4 summarizes the distribution of positive NTRK tumors.
Figure 4.
Distribution of positive NTRK tumors.
Polytopic clinical evaluation and new clinical experiments (perspective in the face of conventional experiments and new medications)
In 2017, the FDA approved for the first time a cancer treatment based on a common biomarker instead of a traditional neoplasia localization and its histological architecture. Since then, pembrolizumab has been authorized to treat any type of solid tumor that expresses microsatellite instability. This event illustrates a drastic evolution that altered the treatment panorama in oncology. The paradigm has changed from general-purpose cytotoxic medication towards precision medicine through which compounds are designed that attack specific tumors through the inhibition of their peculiar growth and/or survival mechanisms.41
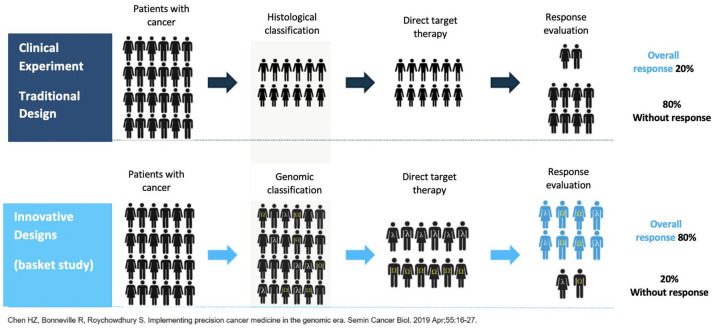
The proportion of studies that required the presence or absence of a genome alteration increased more than five times between 2006 and 2013. For 2017, clinical experiments that included biomarkers to stratify patients based on their possibility of response constituted 34% of cancer studies.42 The recruitment of sufficient patients with diverse tumor subtypes that presented a unique biology is a prevailing limit in performing such studies.43 Precision medicine continues in essence a focus based on the population, although it stratifies in subgroups of interest defined by the positivity or negativity of the biomarker. This tendency increases the prevalence of tumors with infrequent gene varieties susceptible to control. In the same area, the diversity of biomarkers combined with the unique evolution of malign tumors challenges the capacity of traditional studies to prove directed therapies with sufficient statistical power (Figure 5). In the last decade, various alternative designs have been proposed for clinical studies based on biomarkation. These are focused on answering questions on treatment in a more efficient way and in less time. The designs generally cover various sub-studies under a unique master protocol that follows a common hypothesis.44
Figure 5.
Comparison between the design of traditional clinical and innovative experiments for the evaluation of specific molecular targets in low incidence tumors.
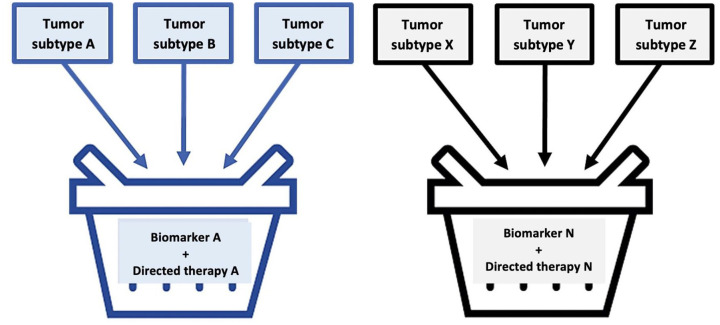
The basket study model includes patients with a certain common genetic mutation (e.g. NTRK), regardless of the location or origin of the neoplasia. This revolutionary clinical experiment model has also been described as cube, agnostic, or pan-tumor studies. Basket studies must be a simple design to include patients that possess a genome alteration in each segment. Sometimes, the combination of biomarkers can be considered or designed for the evaluation of multiple medicines in a selected number of generic alterations and their respective tumors (master study).44,45 These studies can be useful, quick, and less costly in determining a medicine directed at a determined genetic mutation related to a particular tumor they can be efficient for treating the same genome alteration found in the neoplasia originating from another location. As an example, vemurafenib (Zelboraf) is a tyrosine-kinase inhibitor that was originally approved by the FDA in 2011 for the treatment of BRAFV600E mutation carrier melanoma. After the basket study with diverse patients that presented some BRAF mutation, it was determined that vemurafenib was also effective in the treatment of low-frequency hematological neoplasia known as Erdheim–Chester disease (ECD), an occasion in which the BRAF mutation also presents. This study allowed for the final FDA approval in 2017 for vemurafenib as an elective therapeutic intervention for ECD.45
From March 2018 on, 38 basket model studies were included in the ClinicalTrials.gov platform. Eleven of these are intended to register the use of a specific medication for the treatment of diverse neoplasias before the FDA. The rest (27) were considered as exploratory. From 2017 on, the FDA and the EMA (European Medicines Agency) contemplated the use of basket studies as a registration study for pathology with a global prevalence of less than 200,000 patients/year or a density lower than 1 in 2000 people.45 In total, this type of study seems ideal to value the use of medicines in around 7000 low-frequency nosologic entities. In a similar vein, it promotes the valuation of an accompanying diagnostic test, allowing for the resolution of a hypothesis chain with clear and lineal responses.
The medication’s approval stems from a benefit analysis in a group population under the premise that molecular subtype is more important than histology. The wide majority of basket studies are designed with only one arm in order to evaluate the concept test in an early stage of development.45 In general, the number of participants in the individual sub-studies is between 20 and 50 and the hypotheses that can demonstrate statistical meaning are carried out only when there is an important therapeutic meaning.46 With regard to sub-study design, two stage or multiple stage models can be used. As such, basket model tests are optimal for the comprehensive evaluation of patients with rare or low-prevalence cancers. All basket studies require a precise capacity for the prediction of the response based on the selection of a tumor with particular molecular characteristics that favor target control under the concept of effective biological inhibition.46,47 In addition, the patients included in each sub-study are frequently made up of a heterogenous group in terms of tumor subtype, histology, or base patient characteristics. This makes it difficult to value temporary outcomes [progression-free survival (PFS) and global survival], that are regularly homogenized with the totality of the population after the response is estimated. Given the heterogeneity of the population, the rarity of the tumors studied and the absence of a control group, the therapeutic effect is usually confirmed with real life post-approval studies.48 Figure 6 simplifies a basket study in images.
Figure 6.
Pictorial description of a basket study.
Key points (4).
| • The designs of specific clinical experiments (basket and umbrella models) are more efficient for selecting particular populations than the use of multiple histological tests. • If efficiency in the treatment of a particular disease has been demonstrated, this can be easily translated to another pathology with the same genetic alteration. • With a single test, multiple pathologies can be tackled. • Innovative clinical studies allow for the demonstration of the usefulness of medications such as larotrectinib, with high rates of response and prolonged temporary outcomes. |
Larotrectinib as an example of precision oncology practice
The growing number of fusion genes discovered during the last decade (more than 100), including NTRK, increased interest within the scientific community in developing new medication with specific inhibitory capacity. In 2012, Bertrand et al. published the high-resolution crystalline structure of TrkA and TrkB in its Apo form, as well as the conformation of inhibitors in nanomolar fraction.49 At least 40 of the kinase dominion residues in their make-up Asp-Phe-Gly (DFG) potentially interact with the ligands of the place of ATP union, an occurrence that is highly conserved between the Trk proteins. Only 2 of the 30 residues are different between TrkA and TrkB, whilst the binding sites of ATP with TrkB and TrkC are identical.50 Thanks to these characteristics, it was easier to design pan-inhibitors for the three NTRK isoforms (1–3) instead of specific medication for each of these, intervention with a wider anti-tumor activity.
Larotrectinib (LOXO-101) is a pan-Trk inhibitor with a highly selective activity against the Trk kinase super family. Its pharmacokinetics demonstrated a good systemic exhibition through oral administration, reaching approximately 98% of the TrkA, B and C inhibition in maximum concentrations with a daily dose of between 50 and 100 mg.51 Information on larotrectinib’s clinical activity was initially provided in the case of a 41-year-old woman with a sarcoma registered in the phase I study that showed an impressive tumor response through exposure to the medication for less than 8 weeks.52 Another Trk pan-inhibitor with parallel action on ROS1 and ALK is entrectinib (RXDX-101 and NMS-E268), composed in development and with preliminary approval as an orphan molecule in the FDA for patients with lung and colon cancers that are NTRK fusion carriers.53,54 Altiratinib (DCC-2701) and sitravatinib (MGCD516)55 are inhibitors of multiple kinases with in vitro activity against TrkA and B. Both are in early development for patients with neoplasias that present fusion genes in NTKR. In addition, other compounds with anti-Trk activity in the course of preclinical research or phase I/II in patients with neurological illnesses and cancer include TSR-011, PLX7468, F17752, and cabozantinib (XL184).56
The recommended dose for larotrectinib in adult and pediatric patients with a body surface area (BSA) ⩾1 m2 is 100 mg twice a day and in pediatric patients with a BSA of <1 m2 it is 100 mg/m2 twice a day (medication taken with or without food). Larotrectinib must be continued until the disease progresses or in the event that an unacceptable toxicity is produced.57 As described, larotrectinib is a highly selective and potent Trk inhibitor (constant in vitro inhibitor of 50% 5–11 nmol/l), with minimal or null activity against other molecular targets. Trk inhibition avoids protein activation, which results both in the instigation of apoptosis as well as the inhibition of cellular growth in neoplasias that overexpress Trk.52 Larotrectinib demonstrates pharmacokinetics proportional to the dose in a range that oscillates between 100 and 400 mg (i.e. to say 1–4 recommended doses for adults). In adult patients, maximum plasmatic concentrations are reached in 1 h, with plasmatic levels in a stationary state after 3 days of intervention. In healthy volunteers, the average absolute bioavailability of oral larotrectinib was 34% and, after an intravenous dose, the average distribution volume was 48 l. Food had no clinically relevant effect on the medicine’s pharmacokinetics and adhesion to plasmatic proteins is close to 70%. The rate and grade of larotrectinib absorption was similar in pediatric patients that received 100 mg/m2 twice a day (maximum 100 mg twice a day).57,58
Larotrectinib’s pharmacokinetics are not affected in any clinically relevant grade by age (range between 28 days and 82 years), sex, body weight or the presence of renal insufficiency.57 With regard to patients with normal hepatic function, exposure to Larotrectinib was increased by 1.3, 2, and 3.2 times in patients with light (Child-Pugh A), moderate (Child-Pugh B), and severe (Child-Pugh C) hepatic affectation, respectively. The initial dose of larotrectinib must be reduced in 50% of patients with moderate or serious hepatic insufficiency.57 Clinically relevant pharmacological interactions can be produced when larotrectinib is co-administered with potent CYP3A4 inhibitors (itraconazole) or inducing medications (rifampicin). In these cases, the joint administration of these agents with the tyrosine-kinase inhibitor must be avoided. If joint administration cannot be avoided, the larotrectinib dose must be reduced 50% when administered with a CYP3A4 inhibitor and doubled when administered concomitantly with an enzyme inducer.57
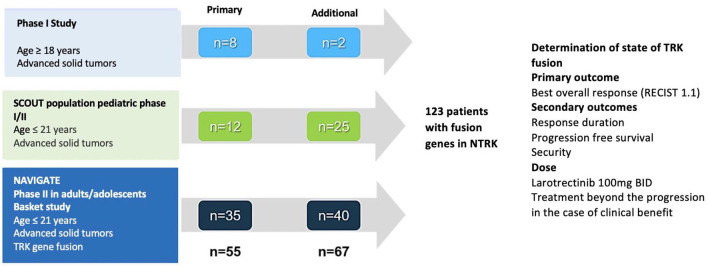
Figure 7 discriminates the evolution of clinical trials with larotrectinib in adult and pediatric populations. In the phase I study, larotrectinib is associated with an elevated rate of global response (93%, 14/15 patients), after a median monitoring period of 5.6 months in multiple solid tumors.58 The expansion to three cohorts with a multicentric character included patients from 1 month of life up to 21 years. Globally, these subjects had locally advanced or metastatic solid tumors, or recurring primary central nervous system tumors. In all cases an inadequate response had been documented to the therapies available in the absence of other standard systematic interventions.58 In 2016, the eligibility criteria were widened to include patients with locally advanced infant fibrosarcoma that required potentially disfiguring surgery to achieve complete tumor excision. Presurgical treatment with larotrectinib was a viable option for children (median age 2 years) who had locally advanced infant fibrosarcoma n = 3) or soft-tissue sarcomas (n = 2). Of these five patients, three had a complete or almost complete pathological response (>98%) and continued in the segment (minimum 7–15 months after the surgery) without treatment with larotrectinib. In the other two patients who had a viable tumor at the time of the excision, larotrectinib was continued until intolerance or progression59 (SCOUT [ClinicalTrials.gov identifier: NCT02637687]).
Figure 7.
Studies developed to test larotrectinib in multiple pathologies; for pediatric and adult populations.
Figure 7 includes the sequence of studies developed for the evaluation of larotrectinib in multiple pathologies for pediatric and adult populations.
In the analysis intended to treat (n = 5) the overall response rate (ORR) evaluated by a central independent committee was 75% (95% CI 61–85; primary outcome for combined analysis), whilst that calculated by the researcher was 80% (95% CI 67–90).60 The responses occurred independently of the characteristics of the NTRK fusion gene, patient age or tumor type. By central evaluation, complete responses, partial responses, stable disease, and disease progression were achieved in 13%, 62%, 13%, and 9% of patients, respectively (the clinical benefit was 88% and only five patients progressed). Two patients included in the analysis temporarily withdrew due to clinical deterioration. The median response time was 1.8 months, the median response duration (DoR) and PFS were not reached after 8.3 and 9.9 months of monitoring, respectively. After a year, responses were maintained in 71% of patients, and 55% remained progression free, maintaining the benefit after 27 months of larotrectinib treatment (the response was maintained for at least 6 months in 73% and 9 months in 63%). Most patients (86%) with a response at the close of data continued receiving larotrectinib or had been subjected to surgery that was meant to be remedial.60
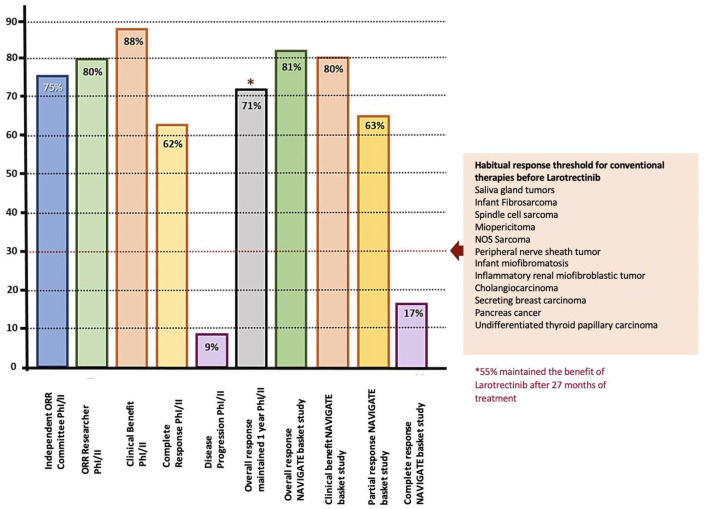
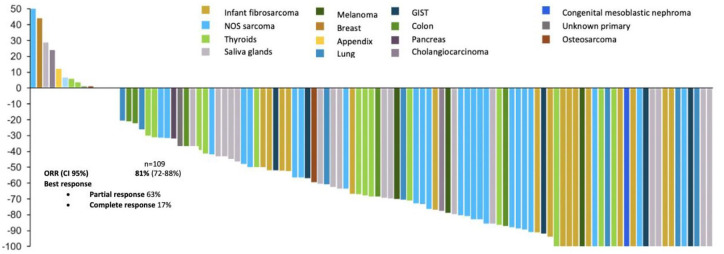
In the NAVIGATE [ClinicalTrials.gov identifier: NCT02576431] study all patients had a documented fusion of the NTRK gene, being TRKA, TRKB, and TRKC in 45%, 2%, and 53% of the patients, respectively. All cases were evaluated by sequencing (NGS) or FISH.60,61 For the cut-off period for data of July 2018, the ORR was 81% (95% CI 72–88) with complete and partial responses in 17% and 63% of patients, respectively. This was for an updated analysis of 109 pediatric and adult patients evaluable after a median monitoring of 17.6 months. In the combination of primary data (55 patients), the median was not reached for DoR.61 Figure 8 summarizes the response indexes in the study sequence of larotrectinib versus the threshold for other conventional therapies before the advent of the tyrosine kinase inhibitor. In the same way, Figure 9 discriminates individual response distribution in the integrated set of patients (diverse neoplasias, n = 109).61
Figure 8.
Characterization of the response with larotrectinib in the phase I and II study sequence (including the NAVIGATE basket study), with respect to the threshold obtained with previously used diverse conventional interventions.
Figure 9.
Efficiency of larotrectinib in diverse tumors (updated comprehensive data set, 2018). The overall response is 81% and the complete response 17%.
Larotrectinib has a manageable safety and tolerability profile. Based on the joint analysis of three clinical experiments, LOXO-TRK-14001 (n = 70), SCOUT (n = 43), and NAVIGATOR (n = 63) it was found that 40% of the patients include had a larotrectinib exposure of >6 months and in 20% this was >1 year.62 The median patient age was 51 years (25% ⩽18 years) and 52% of the patients were male. The most common tumors were soft-tissue sarcomas (16%), saliva gland (11%), lung (10%), thyroid (9%), colon (8%), infant fibrosarcoma (8%), primary central nervous system (7%), and melanoma (5%). Most of the adults (80%) received larotrectinib 100 mg twice a day (dose range from 50 to 200 mg twice a day), whilst 68% of the pediatric patients (age ⩽ 18 years) received larotrectinib 100 mg/m2 twice a day (maximum dose of 100 mg twice a day, dose range dose 9.6–120 mg/m2 twice a day).57 The most common adverse reactions (frequency ⩾ 20%) of any grade that were produced in patients receiving larotrectinib were: increased AST level (45%), elevated ALT (45%), anemia (42%), fatigue (37%), nauseas (29%), dizziness (28%), vomiting (26%), cough (26%), constipation (23%), and diarrhea (22%). The adverse reactions that resulted in temporary interruptions or a reduction of the dose were increased ALT level (6%), increased AST level (6%) and dizziness (3%). The majority of these were produced during the first 3 months of exposure.62
Genomic driven tumors treated with inhibitors such as larotrectinib eventually acquire resistance to these medications. The majority of patients who experienced progression during or after treatment with larotrectinib in phase I/II studies were found to carry specific point mutations in NTRK3G263R, NTRK1F589L, NTRK1G595R, NTRK1G667S, NTRK3G623R, and NTRK3G696A, modifications which change the drug binding site, preventing its union to the kinase domain and other possible mechanisms.63 Detailed knowledge of the resistance mechanisms led to the design and development of LOXO-195, a highly potent TRK kinase inhibitor. Twenty patients enrolled in a phase I study of the medication and 11 participants of an FDA expanded access single patient protocol across 11 different cancer types who experienced progression after anti-TRK therapy were evaluated. An overall response rate of 34% was found, in patients with a known acquired resistance mutation, a response rate of 45% was achieved, giving encouraging results.64
Key points (5).
| • From a wide set of integrated data, larotrectinib has demonstrated an elevated efficiency in diverse tumors, achieving 81% ORR and a complete response of 17%. • Of the patients with NTRK fusions treated with larotrectinib, 9% required a reduction of the dose. All maintained a tumor regression. • Less than 1% of the patients with NTRK fusion suspended larotrectinib due to the presentation of an adverse event. |
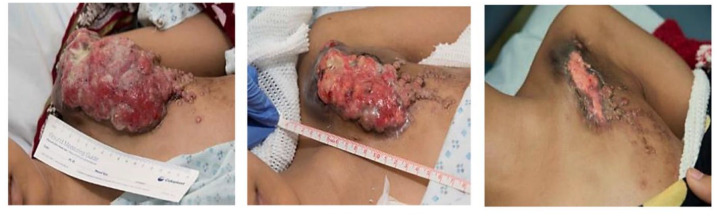
Figure 10 shows a representative case of a 14-year-old girl with a secreting breast carcinoma that is a carrier of ETV6-NTRK3 fusion gene, treated with five prior lines and multiple excisions without response. The patient received larotrectinib in the expanded access program (not available in Colombia) achieving rapid illness control.
Figure 10.
Representative case of a 14-year-old girl with a secreting breast tumor showing impressive response to larotrectinib.
Conclusion
Precision oncology has led to several changes in clinical trial designs, patient treatment, and improvement of outcomes. The approval of larotrectinib by the FDA for the treatment of NTREK gene fusion positive solid tumors regardless of pathology or age of presentation is the pinnacle representation of precision medicine and constituted a milestone in the advancement and transition. Not only does it constitute the first molecular-based drug approval, but also solidifies the importance of genomic classification of tumors and the necessity of expanding its use even to resource limited nations by offering results that would not be achieved otherwise, both in social and clinical aspects.
Supplemental Material
Supplemental material, Author_Response for Precision medicine and its implementation in patients with NTRK fusion genes: perspective from developing countries by Andrés F. Cardona, Oscar Arrieta, Alejandro Ruiz-Patiño, Carolina Sotelo, Nataly Zamudio-Molano, Zyanya Lucia Zatarain-Barrón, Luisa Ricaurte, Luis Raez, Marco Polo Peralta Álvarez, Feliciano Barrón, Leonardo Rojas, Christian Rolfo, Niki Karachaliou, Miguel Angel Molina-Vila and Rafael Rosell in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Precision medicine and its implementation in patients with NTRK fusion genes: perspective from developing countries by Andrés F. Cardona, Oscar Arrieta, Alejandro Ruiz-Patiño, Carolina Sotelo, Nataly Zamudio-Molano, Zyanya Lucia Zatarain-Barrón, Luisa Ricaurte, Luis Raez, Marco Polo Peralta Álvarez, Feliciano Barrón, Leonardo Rojas, Christian Rolfo, Niki Karachaliou, Miguel Angel Molina-Vila and Rafael Rosell in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Precision medicine and its implementation in patients with NTRK fusion genes: perspective from developing countries by Andrés F. Cardona, Oscar Arrieta, Alejandro Ruiz-Patiño, Carolina Sotelo, Nataly Zamudio-Molano, Zyanya Lucia Zatarain-Barrón, Luisa Ricaurte, Luis Raez, Marco Polo Peralta Álvarez, Feliciano Barrón, Leonardo Rojas, Christian Rolfo, Niki Karachaliou, Miguel Angel Molina-Vila and Rafael Rosell in Therapeutic Advances in Respiratory Disease
Acknowledgments
Reprinted from Annals of Oncology/29/(Supplement 9), D.S.W. Tan, U.N. Lassen, C.M. Albert, S. Kummar, C. van Tilburg, S.G. Dubois et al., Larotrectinib efficacy and safety in TRK fusion cancer: an expanded clinical dataset showing consistency in an age and tumor agnostic approach: ix23-ix27, Copyright (2018), with permission from Elsevier, License Number 4855550224674.
Footnotes
Author contribution(s): Andrés F. Cardona: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing-original draft; Writing-review & editing.
Oscar Arrieta: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Writing-original draft; Writing-review & editing.
Alejandro Ruiz-Patiño: Conceptualization; Data curation; Methodology; Writing-review & editing.
Carolina Sotelo: Formal analysis; Methodology; Visualization; Writing-review & editing.
Nataly Zamudio-Molano: Formal analysis; Writing-original draft.
Zyanya Lucia Zatarain-Barrón: Methodology; Writing-review & editing.
Luisa Ricaurte: Conceptualization; Investigation; Writing-review & editing.
Luis Raez: Conceptualization; Investigation; Writing-original draft.
Marco Polo Peralta Álvarez: Methodology; Validation; Writing-review & editing.
Feliciano Barrón: Data curation; Methodology; Supervision; Writing-original draft.
Leonardo Rojas: Conceptualization; Methodology; Validation; Writing-review & editing.
Christian Rolfo: Conceptualization; Visualization; Writing-review & editing.
Niki Karachaliou: Methodology; Validation; Writing-review & editing.
Miguel Angel Molina-Vila: Methodology; Writing-review & editing.
Rafael Rosell: Methodology; Writing-review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Andrés F. Cardona  https://orcid.org/0000-0003-3525-4126
https://orcid.org/0000-0003-3525-4126
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Andrés F. Cardona, Clinical and Translational Oncology Group, Clínica del Country, Calle 116 No. 9-72, c. 318, Bogotá, Colombia; Molecular Oncology and Biology Systems Research Group (FOX-G), Universidad el Bosque, Bogotá, Colombia; Foundation for Clinical and Applied Cancer Research (FICMAC), Bogotá, Colombia.
Oscar Arrieta, Thoracic Oncology Unit, Instituto Nacional de Cancerología (INCaN), México city, México.
Alejandro Ruiz-Patiño, Foundation for Clinical and Applied Cancer Research (FICMAC), Bogotá, Colombia; Molecular Oncology and Biology Systems Research Group (FOX-G), Universidad el Bosque, Bogotá, Colombia.
Carolina Sotelo, Foundation for Clinical and Applied Cancer Research (FICMAC), Bogotá, Colombia; Molecular Oncology and Biology Systems Research Group (FOX-G), Universidad el Bosque, Bogotá, Colombia.
Nataly Zamudio-Molano, Foundation for Clinical and Applied Cancer Research (FICMAC), Bogotá, Colombia.
Zyanya Lucia Zatarain-Barrón, Thoracic Oncology Unit, Instituto Nacional de Cancerología (INCaN), México city, México.
Luisa Ricaurte, Foundation for Clinical and Applied Cancer Research (FICMAC), Bogotá, Colombia; Molecular Oncology and Biology Systems Research Group (FOX-G), Universidad el Bosque, Bogotá, Colombia; Pathology Department, Mayo Clinic, Rochester, Minnesota, Estados Unidos.
Luis Raez, Thoracic Oncology Program, Memorial Cancer Institute (MCI), Florida International University (FIU), Miami, Florida.
Marco Polo Peralta Álvarez, Thoracic Oncology Unit, Instituto Nacional de Cancerología (INCaN), México city, México.
Feliciano Barrón, Thoracic Oncology Unit, Instituto Nacional de Cancerología (INCaN), México city, México.
Leonardo Rojas, Foundation for Clinical and Applied Cancer Research (FICMAC), Bogotá, Colombia; Oncology Department, Clínica Colsanitas, Bogotá, Colombia.
Christian Rolfo, Thoracic Medical Oncology and Early Clinical Trials Unit, University of Maryland, Baltimore, MD, USA.
Niki Karachaliou, Global Clinical Development, Merck KGaA, Darmstadt, Germany.
Miguel Angel Molina-Vila, Pangaea Oncology, Laboratory of Molecular Biology, Quirón-Dexeus University Institute, Barcelona, Catalunya, Spain.
Rafael Rosell, Germans Trias i Pujol Research Institute and Hospital (IGTP), Badalona, Catalunya, Spain.
References
- 1. Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet 2010; 11: 685–696. [DOI] [PubMed] [Google Scholar]
- 2. Nakagawa H, Wardell CP, Furuta M, et al. Cancer whole-genome sequencing: present and future. Oncogene 2015; 34: 5943–5950. [DOI] [PubMed] [Google Scholar]
- 3. Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. International Cancer Genome Consortium; Hudson TJ, Anderson W, Artez A, et al. International network of cancer genome projects. Nature 2010; 464: 993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science 2013; 339: 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014; 505: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El Bairi K, Atanasov AG, Amrani M, et al. The arrival of predictive biomarkers for monitoring therapy response to natural compounds in cancer drug discovery. Biomed Pharmacother 2019; 109: 2492–2498. [DOI] [PubMed] [Google Scholar]
- 8. Yates LR, Seoane J, Le Tourneau C, et al. The European society for medical oncology (ESMO) precision medicine glossary. Ann Oncol 2018; 29: 30–35. [DOI] [PubMed] [Google Scholar]
- 9. Marquart J, Chen EY, Prasad V. Estimation of the percentage of US patients with cancer who benefit from genome-driven oncology. JAMA Oncol 2018; 4: 1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun 2014; 5: 4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Globocan. Colombia: cancer fact sheet [Internet], https://gco.iarc.fr/today/data/factsheets/populations/170-colombia-fact-sheets.pdf (2018, accessed 25 May 2019).
- 12. Globocan. Latin America and the Caribbean: cancer fact sheet [Internet], http://gco.iarc.fr/today/data/factsheets/populations/904-latin-america-and-the-caribbean-fact-sheets.pdf (2018, accessed 25 May 2019).
- 13. Pennic F. The state of value-based care in 2018: 10 key trends to know [Internet], https://hitconsultant.net/2018/06/18/value-based-care-trends/#.XK7LVOhKjIU (2018, 5 February 2019).
- 14. Schram AM, Reales D, Galle J, et al. Oncologist use and perception of large panel next-generation tumor sequencing. Ann Oncol 2017; 28: 2298–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rolfo C, Manca P, Salgado R, et al. Multidisciplinary molecular tumour board: a tool to improve clinical practice and selection accrual for clinical trials in patients with cancer. ESMO Open 2018; 3: e000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Freedman AN, Klabunde CN, Wiant K, et al. Use of next-generation sequencing tests to guide cancer treatment: results from a nationally representative survey of oncologists in the United States. JCO Precis Oncol 2018; 2: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marrone M, Filipski KK, Gillanders EM, et al. Multi-marker solid tumor panels using next-generation sequencing to direct molecularly targeted therapies. PLoS Curr 2014; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mansfield AS, Park BH, Mullane MP. Identification, prioritization, and treatment of mutations identified by next-generation sequencing. Am Soc Clin Oncol Educ Book 2018; 38: 873–880. [DOI] [PubMed] [Google Scholar]
- 19. Gatalica Z, Xiu J, Swensen J, et al. Molecular characterization of cancers with NTRK gene fusions. Mod Pathol 2019; 32: 147–153. [DOI] [PubMed] [Google Scholar]
- 20. Hechtman JF, Benayed R, Hyman DM, et al. Pan-Trk immunohistochemistry is an efficient and reliable screen for the detection of NTRK fusions. Am J Surg Pathol 2017; 41: 1547–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hung YP, Fletcher CDM, Hornick JL. Evaluation of pan-TRK immunohistochemistry in infantile fibrosarcoma, lipofibromatosis-like neural tumour and histological mimics. Histopathology 2018; 73: 634–644. [DOI] [PubMed] [Google Scholar]
- 22. Rudzinski ER, Lockwood CM, Stohr BA, et al. Pan-Trk immunohistochemistry identifies NTRK rearrangements in pediatric mesenchymal tumors. Am J Surg Pathol 2018; 42: 927–935. [DOI] [PubMed] [Google Scholar]
- 23. Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 2016; 1: e000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis JL, Lockwood CM, Stohr B, et al. Expanding the spectrum of pediatric NTRK-rearranged mesenchymal tumors. Am J Surg Pathol 2019; 43: 435–445. [DOI] [PubMed] [Google Scholar]
- 25. Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 1986; 319: 743–748. [DOI] [PubMed] [Google Scholar]
- 26. Créancier L, Vandenberghe I, Gomes B, et al. Chromosomal rearrangements involving the NTRK1 gene in colorectal carcinoma. Cancer Lett 2015; 365: 107–111. [DOI] [PubMed] [Google Scholar]
- 27. Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015; 17: 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vaishnavi A, Capelletti M, Le AT, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med 2013; 19: 1469–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi Y, Won Y-J, Lee S, et al. Cytoplasmic TrkA expression as a screen for detecting NTRK1 fusions in colorectal cancer. Transl Oncol 2018; 11: 764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang L, Busam KJ, Benayed R, et al. Identification of NTRK3 fusions in childhood melanocytic neoplasms. J Mol Diagn 2017; 19: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldman DP, Gupta C, Vasudeva E, et al. The value of diagnostic testing in personalized medicine [Internet]. Forum Health Econ Policy 16, https://www.degruyter.com/view/j/fhep.2013.16.issue-2/fhep-2013-0023/fhep-2013-0023.xml (2013; accessed 27 May 2019). [DOI] [PubMed] [Google Scholar]
- 32. Gavan SP, Thompson AJ, Payne K. The economic case for precision medicine. Expert Rev Precis Med Drug Dev 2018; 3: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calderon-Aparicio A, Orue A. Precision oncology in Latin America: current situation, challenges and perspectives [Internet]. Ecancer 13, https://ecancer.org/journal/13/full/920-precision-oncology-in-latin-america-current-situation-challenges-and-perspectives.php (2019; accessed 28 May 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Department of Economic and Social Affairs of the United Nations Secretariat. Country classification [Internet], https://www.un.org/en/development/desa/policy/wesp/wesp_current/2014wesp_country_classification.pdf (2014; accessed 25 May 2019).
- 35. Emanuel EJ, Wendler D, Killen J, et al. What makes clinical research in developing countries ethical? The benchmarks of ethical research. J Infect Dis 2004; 189: 930–937. [DOI] [PubMed] [Google Scholar]
- 36. Participants in the 2001 Conference on Ethical Aspects of Research in Developing Countries: Ethics. Fair benefits for research in developing countries. Science 2002; 298: 2133–2134. [DOI] [PubMed] [Google Scholar]
- 37. Mitropoulos K, Cooper DN, Mitropoulou C, et al. Genomic medicine without borders: which strategies should developing countries employ to invest in precision medicine? A new fast-second winner strategy. OMICS 2017; 21: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cooper DN, Brand A, Dolzan V, et al. Bridging genomics research between developed and developing countries: the Genomic Medicine Alliance. Per Med 2014; 11: 615–623. [DOI] [PubMed] [Google Scholar]
- 39. Balasubramanian G, Morampudi S, Chhabra P, et al. An overview of compassionate use programs in the European union member states. Intractable Rare Dis Res 2016; 5: 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov 2015; 5: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heymach J, Krilov L, Alberg A, et al. Clinical cancer advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol 2018; 36: 1020–1044. [DOI] [PubMed] [Google Scholar]
- 42. Chen H-Z, Bonneville R, Roychowdhury S. Implementing precision cancer medicine in the genomic era. Semin Cancer Biol 2019; 55: 16–27. [DOI] [PubMed] [Google Scholar]
- 43. Roychowdhury S, Chinnaiyan AM. Translating cancer genomes and transcriptomes for precision oncology. CA Cancer J Clin 2016; 66: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hirakawa A, Asano J, Sato H, et al. Master protocol trials in oncology: review and new trial designs. Contemp Clin Trials Commun 2018; 12: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tao JJ, Schram AM, Hyman DM. Basket studies: redefining clinical trials in the era of genome-driven oncology. Annu Rev Med 2018; 69: 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dubuque C, Major P, Suarez S, et al. Oncology basket trials: an emerging paradigm shift in trial design treatment approach? [Internet], https://trinitylifesciences.com/wp-content/uploads/2019/05/Oncology_Basket_Trials_-_Trinity_Partners.pdf (2018; accessed 2 February 2019).
- 47. Cunanan KM, Gonen M, Shen R, et al. Basket trials in oncology: a trade-off between complexity and efficiency. J Clin Oncol 2017; 35: 271–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Polley E. Basket and umbrella trial designs in oncology [Internet], https://med.stanford.edu/content/dam/sm/cisd/symposium-May2017-slides/EricPolley-May2017-symposium.pdf (2017; accessed 2 February 2019).
- 49. Bertrand T, Kothe M, Liu J, et al. The crystal structures of TrkA and TrkB suggest key regions for achieving selective inhibition. J Mol Biol 2012; 423: 439–453. [DOI] [PubMed] [Google Scholar]
- 50. Raeppel SL, Gaudette F, Nguyen H, et al. Identification of a novel series of potent TrkA receptor tyrosine kinase inhibitors. Int J Med Chem 2012; 2012: 412614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Burris HA, Shaw AT, Bauer TM, et al. Pharmacokinetics (PK) of LOXO-101 during the first-in-human phase I study in patients with advanced solid tumors: interim update [Internet], p.4529. Experimental and Molecular Therapeutics, American Association for Cancer Research, http://cancerres.aacrjournals.org/lookup/doi/10.1158/1538-7445.AM2015-4529 (2015; accessed 27 May 2019).
- 52. Doebele RC, Davis LE, Vaishnavi A, et al. An oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101. Cancer Discov 2015; 5: 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Siena S, Drilon AE, Sai-Hong Ou I, et al. Entrectinib (RXDX-101), an oral pan-Trk, ROS1, and ALK inhibitor in patients with advanced solid tumors harboring gene rearrangements. Eur J Cancer 2015; 51: S724–S725. [Google Scholar]
- 54. De Braud FGM, Pilla L, Niger M, et al. Rxdx-101, an oral Pan-Trk, Ros1, and Alk inhibitor, in patients with advanced solid tumors with relevant molecular alterations. Ann Oncol 2014; 25: iv148–iv149. [Google Scholar]
- 55. Patwardhan PP, Ivy KS, Musi E, et al. Significant blockade of multiple receptor tyrosine kinases by MGCD516 (Sitravatinib), a novel small molecule inhibitor, shows potent anti-tumor activity in preclinical models of sarcoma. Oncotarget 2016; 7: 4093–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abrams J, Conley B, Mooney M, et al. National Cancer Institute’s precision medicine initiatives for the new National Clinical Trials Network. Am Soc Clin Oncol Educ Book 2014; 34: 71–76. [DOI] [PubMed] [Google Scholar]
- 57. FDA. Accelerated approval (COR-NDAACTION-04)—FDA: Vitrakvi (larotrectinib) [Internet], https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211710s000lbl.pdf (2018; accessed 2 February 2019).
- 58. Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol 2018; 19: 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. DuBois SG, Laetsch TW, Federman N, et al. The use of neoadjuvant larotrectinib in the management of children with locally advanced TRK fusion sarcomas. Cancer 2018; 124: 4241–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 2018; 378: 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tan DSW, Lassen UN, Albert CM, et al. Larotrectinib efficacy and safety in TRK fusion cancer: an expanded clinical dataset showing consistency in an age and tumor agnostic approach. Ann Oncol 29: ix23-ix27, https://academic.oup.com/annonc/article/doi/10.1093/annonc/mdy279.397/5140611 (2018; accessed 27 May 2019). [Google Scholar]
- 62. Hyman DM, Laetsch TW, Kummar S, et al. The efficacy of larotrectinib (LOXO-101), a selective tropomyosin receptor kinase (TRK) inhibitor, in adult and pediatric TRK fusion cancers. J Clin Oncol 2017; 35: LBA2501. [Google Scholar]
- 63. Ricciuti B, Genova C, Crinò L, et al. Antitumor activity of larotrectinib in tumors harboring NTRK gene fusions: a short review on the current evidence. OncoTargets Ther 2019; 12: 3171–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hyman D, Kummar S, Farago A, et al. CT127: phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi) [Internet]. Atlanta, https://www.abstractsonline.com/pp8/#!/6812/presentation/9845 (2019, accessed 2 February 2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response for Precision medicine and its implementation in patients with NTRK fusion genes: perspective from developing countries by Andrés F. Cardona, Oscar Arrieta, Alejandro Ruiz-Patiño, Carolina Sotelo, Nataly Zamudio-Molano, Zyanya Lucia Zatarain-Barrón, Luisa Ricaurte, Luis Raez, Marco Polo Peralta Álvarez, Feliciano Barrón, Leonardo Rojas, Christian Rolfo, Niki Karachaliou, Miguel Angel Molina-Vila and Rafael Rosell in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Precision medicine and its implementation in patients with NTRK fusion genes: perspective from developing countries by Andrés F. Cardona, Oscar Arrieta, Alejandro Ruiz-Patiño, Carolina Sotelo, Nataly Zamudio-Molano, Zyanya Lucia Zatarain-Barrón, Luisa Ricaurte, Luis Raez, Marco Polo Peralta Álvarez, Feliciano Barrón, Leonardo Rojas, Christian Rolfo, Niki Karachaliou, Miguel Angel Molina-Vila and Rafael Rosell in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Precision medicine and its implementation in patients with NTRK fusion genes: perspective from developing countries by Andrés F. Cardona, Oscar Arrieta, Alejandro Ruiz-Patiño, Carolina Sotelo, Nataly Zamudio-Molano, Zyanya Lucia Zatarain-Barrón, Luisa Ricaurte, Luis Raez, Marco Polo Peralta Álvarez, Feliciano Barrón, Leonardo Rojas, Christian Rolfo, Niki Karachaliou, Miguel Angel Molina-Vila and Rafael Rosell in Therapeutic Advances in Respiratory Disease