Abstract
Aggressive angiomyxoma is an interstitial tumour that is often misdiagnosed and is likely to recur. There have been few reported cases of angiomyxoma in pregnant women. We report a case of a woman who was previously diagnosed with a tumour in her vulva that increased in size during both of her pregnancies and spontaneously decreased postpartum. Local excision was performed and a gonadotropin-releasing hormone agonist was administered. According to a literature review, aggressive angiomyxoma is associated with good maternal and child outcomes. Caesarean section is not the delivery method of choice, but it is indicated if the tumour is preventing vaginal birth. Treatment for angiomyxoma is mainly postpartum local resection supplemented by hormone therapy. This tumour frequently recurs and patients should undergo long-term follow-up.
Keywords: Aggressive angiomyxoma, gonadotropin-releasing hormone agonist, pregnancy, genital tumour, labium majus, magnetic resonance imaging
Introduction
Aggressive angiomyxoma (AA) is a rare interstitial tumour that occurs in the vulva, vagina, pelvis, and perineum in women of childbearing age.1 Because of local invasion and recurrence of AA, it is relatively difficult to treat in women with fertility requirements and those affected during pregnancy. We report a case of AA during pregnancy. We also review AA cases reported to date in pregnant women to contribute to a better understanding of optimal treatment of these tumours.
Case report
A 25-year-old woman presented with a right vulvar tumour in June 2012 in the second month of her first pregnancy. The tumour was approximately 3 cm in size and gradually increased to 5 cm during pregnancy, with no pain or bleeding. Her medical history and physical examination were otherwise normal. No treatment or procedure was performed for the mass during her pregnancy. She delivered a healthy male neonate in March 2013 at 37 weeks’ gestation with the aid of a left lateral episiotomy. The mass was slightly reduced after delivery. In April 2014, the right vulvar mass was found to have increased to 7 cm. The tumour was partially removed under local anaesthesia. Postoperative pathology showed AA. In a follow-up, no tumour was found in her vulva.
In August 2017, at 2 months’ gestation in her second pregnancy, enlargement of the right labium majus was detected. On 30 January 2018, at more than 30 weeks’ gestation, colour Doppler ultrasound showed that the vulvar tumour was 6 × 4 × 3 cm. In March 2018, at 39 weeks’ gestation, the patient was admitted to hospital owing to a reduction in conscious foetal movement. At that time, the right vulvar mass was soft and approximately the size of an egg (Figure 1). A caesarean section was performed owing to foetal distress, and the patient delivered a healthy female neonate. In follow-up in May 2018, no obvious mass was found in the vulva.
Figure 1.
Right labium majus mass at 39 weeks’ gestation.
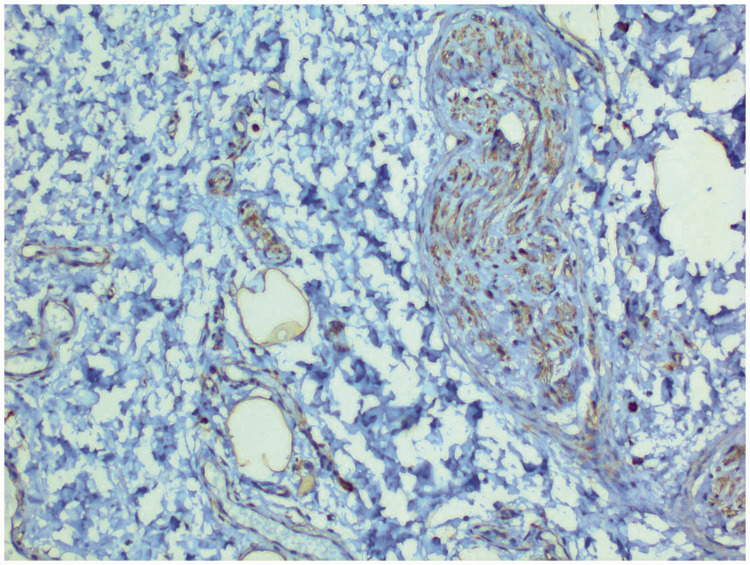
In January 2019, the mass spontaneously increased again, and blood tests showed normal ranges for CA125, CA199, CA153, carcinoembryonic antigen, and alpha fetoprotein levels. Magnetic resonance imaging (MRI) showed irregular, short T1 and long T2 signal shadows on the right side of the vulva. Diffusion-weighted imaging showed uneven, slightly high signal shadows with a range of 5.3 × 5.1 × 4.2 cm. The upper edge of the mass was close to the lower edge of the pubic symphysis. An enhanced scan showed homogeneous enhancement of the lesion (Figure 2). On 21 February 2019, the genital tumour was removed. The swelling of the mass was soft and irregular and a small area was connected to fat. Postoperative pathology suggested invasion of vascular myxoma (Figure 3). Immunohistochemistry showed that the tumour was vimentin +++ (Figure 4), oestrogen receptor ++, progesterone receptor ++, desmin +, Ki-67 + (<1%), S-100 (−), and alpha-smooth muscle actin (−). Postoperatively, three intramuscular injections of the gonadotropin-releasing hormone agonist triptorelin (3.75 mg) were administered, and no recurrence was observed in 9 months of follow-up.
Figure 2.
Postpartum magnetic resonance imaging scan. In coronal fat-suppressed T2-weighted imaging sequences, lesions with a high signal (white arrow) are located in the deep part of the right vulva. The boundary is clear, the internal signal is not uniform, and a strip-shaped low signal shadow (black arrow) can be seen.
Figure 3.
Histopathology of the tumour. Haematoxylin and eosin staining (×100) shows spindle to stellate cells in a loose myxoid matrix (labelled as M) with variable sized blood vessels (labelled as V).
Figure 4.
Immunohistochemistry of the aggressive angiomyxoma. The tumour cells are positive for vimentin (×200).
The study was approved by Fujian Provincial Maternity and Children’s Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China. Details were de-identified to protect the identity of the patient. Verbal consent from the patient was obtained for publication of data and images.
Discussion
The first case of AA in pregnancy was reported in 1995, and only 16 cases have been reported in pregnancy to date (Table 1).2–15 The cause of AA is unknown. Our case was similar to most cases of AA in pregnancy. Our patient was asymptomatic and the mass was usually painless. These tumours tend to expand during pregnancy.
Table 1.
Characteristics of reported cases of aggressive angiomyxoma in pregnancy.
| Reference | Age (years) | History | Clinical symptoms | Site | Change and treatment of tumours with gestational age† |
|---|---|---|---|---|---|
| Current case | 32 | R | Tumour | Right vulva | The tumour increased in two pregnancies with a slight postpartum decrease at 1 year postpartum excision |
| Malukani, 20182 | 24 | P | Vaginal bleeding, difficulty in walking | Vaginal fornix | The tumour was 11.4 cm (17 weeks) with induced abortion and surgical removal of the tumour |
| Orfanelli, 20163 | 29 | P | Pudendal swelling | Right labium majus | The tumour increased from 2 cm (20 weeks) to 7 cm (37 weeks); caesarean section with surgical resection was performed (39 weeks) |
| Sampaio, 20164 | 25 | R | Vaginal swelling, dyspareunia, bleeding | Vaginal fornix | The tumour increased from 11 cm (9 weeks) to 12 cm (13 weeks) at an operation; full-term delivery occurred |
| Zangmo, 20165 | 21 | R | Tumour | Right labium majus | The tumour was the size of an almond (20 weeks) and then decreased to 8 cm (32 weeks) and then 15 cm (37 weeks, with pain); caesarean section was performed (38 weeks); the tumour increased to 18 cm at 6 weeks postpartum |
| Ashraf, 20146 | 24 | P | Tumour | Right labium majus | A tumour was found at 16 weeks and was 30 cm at 20 weeks at an operation; full-term caesarean section was performed |
| Goyal, 20147 | 25 | P | Tumour | Left labium majus | A tumour was found at 12 weeks and was 8 cm (18 weeks) at an operation; full-term delivery occurred |
| Vandana, 20148 | 43 | P | Difficulty in walking | Left labium majus | The tumour gradually increased for 9 years, but suddenly grew during pregnancy; caesarean section was performed and postpartum enlargement occurred; at 9 months postpartum, the tumour was 55 cm at an operation |
| Haldar, 20109 | / | R | / | / | The tumour size slightly increased during pregnancy |
| Aye, 200910 | 22 | R | Necrosis on the surface and mass | Right vestibule | The tumour was 3.1 cm (prenatal surgery and drug therapy) and increased to 5.1 cm (32 weeks); full-term caesarean section was performed |
| Bagga, 200711 | 25 | P | Swelling | Right labia majus | The tumour was 2 cm (12 weeks) and increased to 4 cm (16 weeks); mass excision with massive haemorrhage occurred, with compression haemostasis; full-term delivery occurred |
| Han-Geurts, 200612 | 31 | R | Tumour | Left buttock | The mass was larger in the second pregnancy than in the first pregnancy |
| Han-Geurts, 200612 | 34 | P | Tumour | Left and right labia majora | At 30 weeks’ gestation, left labial surgery was performed; several weeks later, right labial mass surgery was performed |
| Han-Geurts, 200612 | 27 | P | Tumour | Abdomen | During pregnancy, a mass was found in the front of the bladder and rectum during caesarean section |
| Wolf, 200313 | 32 | P | Tumour | Perineum | A tumour was found at 32 weeks and was 3 × 4 cm (36 weeks) at an operation; vaginal delivery occurred |
| Htwe, 199514 | 41 | P | Tumour | Left vulva | The tumour was 6 cm at 18 weeks and an operation was performed; full-term delivery occurred |
| Fishman, 199515 | 37 | R | Tumour | Right vulva | Pre-pregnancy excision of the tumour was performed; the tumour was 3 cm during pregnancy and increased to 40 cm in 3 years |
R: recurrence; P: primary.
†Gestational weeks.
AA is often misdiagnosed as Bartholin’s gland cyst, lipoma, or a vaginal wall cyst. MRI is the preferred examination method of AA and it can show the scope of the tumour and its relationship with the pelvic diaphragm and surrounding organs. AA has certain characteristics on MRI, such as multiple twisted strip shadows, showing “whirlpool” or “stratified” changes. AA may also be confused with spindle cell lipoma, myxoid neurofibroma, intrauterine myxoma, and myxoid liposarcoma. Pathology is the preferred standard for diagnosing AA. This tumour can be variable in size, and its general characteristics usually include a lobulated or spherical appearance, soft texture with no capsule or a partial capsule, and frequently an unclear boundary within the surrounding tissue is observed. The tumour section is translucent, grey white, or grey brown, and it has a homogeneous jelly-like consistency, with cystic changes and a bleeding area. Microscopically, the tumour comprises short fusiform cells, with a uniform size and without obvious heteromorphism. The stroma is rich in an oedematous mucoid and collagen matrix, and there are many thin-walled and thick-walled vessels. Although there is no specific immunohistochemical marker in AA, vimentin and CD34 are strongly positive, and desmin, oestrogen receptor, and progesterone receptor are moderately positive. Additionally, S-100 is not generally expressed and Ki-67 has a low proliferation index (<1% tumour cells). These characteristics are helpful during differential diagnosis.
Most AAs are located in the vulva or vagina, and a tumour is the main clinical manifestation. Pain or bleeding are uncommon, but pain may occur if the tumour is significantly enlarged,5 and vaginal bleeding may be noted with sexual intercourse.4 In most cases, AA is found in the early and middle stages of pregnancy (n=11), and a few are found in late pregnancy.13 The extent of tumour enlargement during pregnancy varies from a slight increase9 to growth of several times the original tumour size.5 Similar to most pregnancies with AA, our patient had full-term delivery at both pregnancies, and had a good maternal and neonatal outcome. Although the majority of patients with AA choose caesarean section, vaginal delivery is feasible.13 In our patient, the lateral perineum was used for vaginal delivery in the first pregnancy.
AA has an abundant blood supply, and there is a risk of massive bleeding during pregnancy. If there is no pain or bleeding, tumours can be treated postpartum and may even be spontaneously reduced after delivery, as in our case. Surgical resection is the main treatment for AA. For AA with extensive and deep infiltration of pelvic organs, incomplete resection is recommended if complete resection is not feasible or may cause serious surgical injury. Studies have shown that surgical margins have no effect on recurrence of AA.16
The prevalence of AA in women of childbearing age, tumour growth during pregnancy, and the majority of cases with oestrogen and progesterone positive receptors suggest that AA tumours may be hormone-dependent. Therefore, hormone therapy is considered a possible effective treatment option. Some scholars believe that hormone therapy should be administered to patients with postoperative residual disease or in treatment of initial small lesions.17 However, whether long-term use of a gonadotropin-releasing hormone agonist can cure the disease or whether there is recurrence after stopping the drug is unclear.
Usually, the recurrence site is consistent with the initial site of AA. However, there is no clear relationship between the patient’s age, tumour size, number of pregnancies, and recurrence rate. Because the tumours are soft and can invade the pelvic organs, they may be missed on a clinical examination. Follow-up for AA should be supplemented by MRI studies.
In summary, the main clinical feature of AA in pregnancy is enlargement of the tumour. The outcomes for the mother and child are good. Although birth in most cases is by caesarean section, this is not the delivery method of choice. Caesarean section is indicated only if the tumour is preventing vaginal birth. Surgical resection is still the most important treatment and hormone therapy can be used as adjuvant therapy for AA. Although metastasis is rare, AA is prone to recurrence. Therefore, long-term, regular follow-up is important for this tumour.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Fujian Provincial Maternity and Children’s Hospital Research Fund Project (17-21).
ORCID iD
Haihua Xu https://orcid.org/0000-0002-5685-8677
References
- 1.Sun Y, Zhu L, Chang X, et al. Clinicopathological features and treatment analysis of rare aggressive angiomyxoma of the female pelvis and perineum – a retrospective study. Pathol Oncol Res 2017; 23: 131–137. [DOI] [PubMed] [Google Scholar]
- 2.Malukani K, Varma AV, Choudhary D, et al. Aggressive angiomyxoma in pregnancy: a rare and commonly misdiagnosed entity. J Lab Physicians 2018; 10: 245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orfanelli T, Kim CS, Vitez SF, et al. A case report of aggressive angiomyxoma in pregnancy: do hormones play a role? Case Rep Obstet Gynecol 2016; 2016: 6810368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampaio J, Sarmento-Gonçalves I, Ramada D, et al. Angiomyxoma in pregnancy: a rare condition, a common misdiagnosis. Case Rep Obstet Gynecol 2016; 2016: 8539704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zangmo R, Kumar S, Singh N, et al. Aggressive angiomyxoma of vulva in pregnancy: a case report. J Obstet Gynaecol India 2016; 66: 610–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashraf T, Haroon S. Aggressive angiomyxoma in pregnancy. J Coll Physicians Surg Pak 2014; 24: S24–S26. [PubMed] [Google Scholar]
- 7.Goyal P, Agrawal D, Sehgal S, et al. Aggressive angiomyxoma in pregnancy. Rare Tumors 2014; 6: 5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandana S, Dave KS, Bhansali RP, et al. Aggressive angiomyxoma of vulva which grew with pregnancy and attained a huge size rarely seen in literature. J Obstet Gynaecol India 2014; 64: 90–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haldar K, Martinek IE, Kehoe S. Aggressive angiomyxoma: a case series and literature review. Eur J Surg Oncol 2010; 36: 335–339. [DOI] [PubMed] [Google Scholar]
- 10.Aye C, Jefferis H, Chung DY, et al. A case of multi-modal managed vulval aggressive angiomyxoma diagnosed before conception and monitored during pregnancy. Gynecol Oncol 2009; 115: 170–171. [DOI] [PubMed] [Google Scholar]
- 11.Bagga R, Keepanasseril A, Suri V, et al. Aggressive angiomyxoma of the vulva in pregnancy: a case report and review of management options. MedGenMed 2007; 9: 16. [PMC free article] [PubMed] [Google Scholar]
- 12.Han-Geurts IJM, Van Geel AN, Van Doorn L, et al. Aggressive angiomyxoma: multimodality treatments can avoid mutilating surgery. Eur J Surg Oncol 2006; 32: 1217–1221. [DOI] [PubMed] [Google Scholar]
- 13.Wolf CA, Kurzeja R, Fietze E, et al. Aggressive angiomyxoma of the female perineum in pregnancy. Acta Obstet Gynecol Scand 2003; 82: 484–485. [DOI] [PubMed] [Google Scholar]
- 14.Htwe M, Deppisch LM, Saint-Julien JS. Hormone-dependent, aggressive angiomyxoma of the vulva. Obstet Gynecol 1995; 86: 697–699. [DOI] [PubMed] [Google Scholar]
- 15.Fishman A, Otey LP, Poindexter AN, 3rd, et al. Aggressive angiomyxoma of the pelvis and perineum. A case report. J Reprod Med 1995; 40: 665–669. [PubMed] [Google Scholar]
- 16.Chan IM, Hon E, Ngai SW. Aggressive angiomyxoma in females: is radical resection the only option?. Acta Obstet Gynecol Scand 2000; 79: 216–220. [PubMed] [Google Scholar]
- 17.Shinohara N, Nonomura KS, Ishikawa S, et al. Medical management of recurrent aggressive angiomyxoma with gonadotropin-releasing hormone agonist. Int J Urol 2004; 11: 432–435. [DOI] [PubMed] [Google Scholar]