Abstract
Background:
Mitochondrial disease is a term used to describe a set of heterogeneous genetic diseases caused by impaired structure or function of mitochondria. Pyruvate therapy for mitochondrial disease is promising from a clinical point of view.
Methods:
According to PRISMA guidelines, the following databases were searched to identify studies regarding pyruvate therapy for mitochondrial disease: PubMed, EMBASE, Cochrane Library, and Clinicaltrials. The search was up to April 2019. The endpoints were specific biomarkers (plasma level of lactate, plasma level of pyruvate, L/P ratio) and clinical rating scales [Japanese mitochondrial disease-rating scale (JMDRS), Newcastle Mitochondrial Disease Adult Scale (NMDAS), and others]. Two researchers independently screened articles, extracted data, and assessed the quality of the studies.
Results:
A total of six studies were included. Considerable differences were noted between studies in terms of study design, patient information, and outcome measures. The collected evidence may indicate an effective potential of pyruvate therapy on the improvement of mitochondrial disease. The majority of the common adverse events of pyruvate therapy were diarrhea and short irritation of the stomach.
Conclusion:
Pyruvate therapy with no serious adverse events may be a potential therapeutic candidate for patients with incurable mitochondrial diseases, such as Leigh syndrome. However, recent evidence taken from case series and case reports, and theoretical supports of basic research are not sufficient. The use of global registries to collect patient data and more adaptive trial designs with larger numbers of participants are necessary to clarify the efficacy of pyruvate therapy.
Keywords: mitochondrial disease, pyruvate therapy, systematic review
Background
Mitochondrial disease is a term used to describe a set of heterogeneous genetic diseases caused by impaired structure or function of mitochondria encoded by nuclear DNA and mitochondrial DNA (mtDNA).1 As vital organelles in cells, mitochondria participate in cell metabolism, including the tricarboxylic acid (TCA) cycle, gluconeogenesis, oxidative phosphorylation, fatty acid oxidation, urea cycle, and ketogenesis.2 Most importantly, mitochondria as cell energy units produce 95% of ATP in order to meet cell energy requirements by the oxidative phosphorylation (OXPHOS) system. Therefore, mitochondrial disease often affects multiple systems, notably energy-consuming tissues or organs, such as brain, nerves, muscles, and heart.3 The clinical manifestations also range from asymptomatic manifestation to severe multiple organ injury, including central neurological features (including stroke-like episodes, encephalopathy, ataxia, seizures, and dementia), and peripheral neurological features (including peripheral neuropathy, myopathy, and ophthalmoplegia). The age of onset ranges from the neonatal period to adulthood. The complexity of mitochondrial disease not only seriously affects patients’ quality of life, but also increases the financial burden on patients’ families. It also presents some difficulties to the scientific research of mitochondrial diseases. At present, there are no authoritative epidemiological data in the world. The estimated prevalence of mitochondrial diseases is 1/8500–1/4300,4,5 in which the prevalence of adults ranges from 6.9/100,000 to 12.5/100,000,5,6 and the one of children is 4.7–15/100,000.3,7–12 The incidence may be higher in areas with similar consanguinity of specific pathogenetic genes.3
Currently, remarkable progress has been reported with regard to the mechanism and classification of mitochondrial disease, although no authoritative treatment has been recommended worldwide. Most studies have reported symptomatic and supportive treatment, including medication, diet therapy, and exercise therapy. In the field of medication, several drugs have been reported to enhance the function of mitochondria: antioxidants (coenzyme Q10 and vitamin C), respiratory chain auxiliary factors (nicotine, riboflavin, and coenzyme Q10), improvement of muscle lactic acid poisoning (dichloroacetate), and supplementation of deficient substances (creatine, levocarnitine).13 However, the effectiveness of these drugs has not been determined. The Consensus on Diagnosis and Treatment of the Mitochondrial Medicine Society states that case reports, a limited number of case series, and small open-labeled studies are the main supporting evidence for the treatment of mitochondrial disease.14,15 In addition, dichloroacetate was reported to cause toxic neuropathy in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes) in a randomized controlled trial,16 although it was effective for lactic acidosis. Therefore, medical treatments for mitochondrial disease are in urgent need of new discovery.
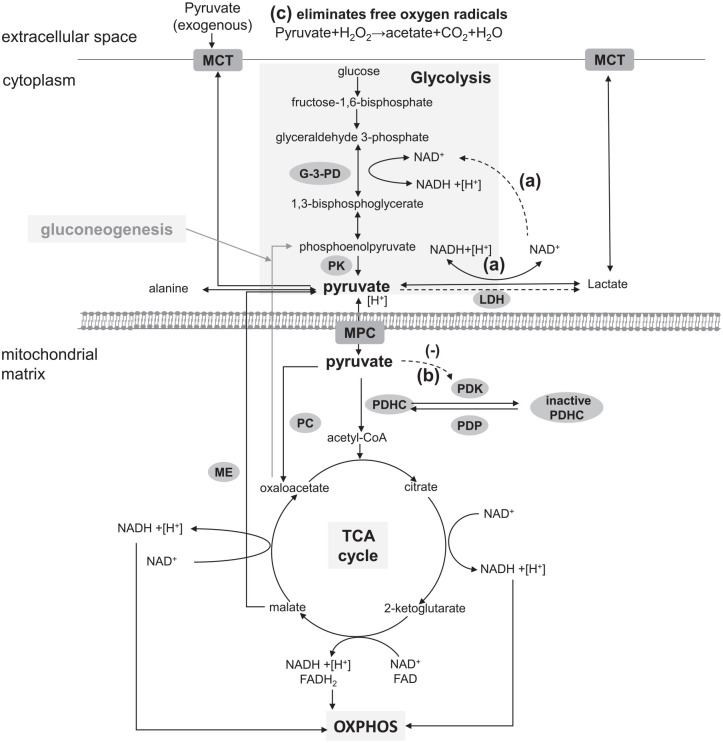
As an endogenous substance, pyruvate plays a crucial role in the metabolism of three principal substances, and is located at a metabolic branch point between glycolysis and mitochondrial oxidation. It is well known that pyruvate is synthesized mainly as the end product of glycolysis in the cytoplasm under normal physiological circumstances. In addition, pyruvate is also generated by amino acids (such as alanine, cysteine, serine, and glycine), malate, and lactate. In the cytoplasm, pyruvate can either be consumed as an amine receptor for alanine transaminase (ALT), metabolized to lactate and NAD+ by lactate dehydrogenase (LDH) to produce a few ATP for cells under hypoxic conditions, and excreted from the cell via the monocarboxylate transporter (MCT). Pyruvate can also enter into the mitochondrial matrix via the mitochondrial pyruvate carrier (MPC) and be converted to acetyl-CoA by pyruvate dehydrogenase complex (PDHC).17 Subsequently, acetyl-CoA can combine with oxaloacetate to form citrate and begin the TCA cycle to yield ATP, NADH, and FADH2, and the latter two molecules can transfer their energy to the electron transport chain.17 Alternatively, pyruvate may be transformed into oxaloacetate by pyruvate carboxylase (PC) in the mitochondria, which is an essential step in gluconeogenesis. Therefore, pyruvate play a central role in cellular homeostasis (Figure 1).
Figure 1.
Pyruvate metabolic pathways and the potential mechanism for treating mitochondrial diseases: pyruvate is derived from glycolysis, amino acids (such as alanine, cysteine, serine, and glycine), malate, and lactate under different situations. In the cytoplasm, pyruvate can be transformed into alanine, metabolized to lactate and NAD+ by LDH, and excreted from the cell via MCT. Pyruvate can also enter into the mitochondria via MPC and convert to acetyl-CoA by PDHC. Subsequently, acetyl-CoA can combine with oxaloacetate to form citrate and begin the TCA cycle to yield ATP, NADH and FADH2, and the latter two molecules can transfer their energy to the electron transport chain. In other situations, pyruvate may be consumed by PC to generate oxaloacetate for gluconeogenesis. Dashed lines are used for the potential mechanism for treating mitochondrial diseases as figure shows: (a) Glycolysis activation: exogenous pyruvate was reduced by LDH to provide NAD+ for glycolysis; (b) PDHC activation: pyruvate inhibits PDK to activate PDHC for generation of acetyl-CoA; (c) eliminates free oxygen radicals by a non-enzymatic reaction.
G-3-PD, glyceraldehyde 3-phosphate dehydrogenase; LDH, lactate dehydrogenase; MCT, monocarboxylate transporter; MPC, mitochondrial pyruvate carrier; OXPHOS, oxidative phosphorylation; PC, pyruvate carboxylase; PDHC, pyruvate dehydrogenase complex; PDK, pyruvate dehydrogenase kinase; TCA, tricarboxylic acid.
In theory, the potential mechanism of pyruvate therapy to improve mitochondrial disease is as follows (Figure 1): (a) Glycolysis activation: in the cytosol, exogenous pyruvate is reduced to lactate by LDH to provide NAD+ for the oxidation of glyceraldehyde-3-phosphate in the glycolysis, and then activates glycolysis to protect ATP for sustaining cellular functions, even in the environment of high concentration of lactate or under a high lactate/pyruvate (L/P) ratio18; (b) PDHC activation: in the mitochondria, PDHC is inhibited by pyruvate dehydrogenase kinases (PDK) and activated by pyruvate dehydrogenase phosphatase (PDP). Exogenous pyruvate inhibits PDK to make PDP restore the activity of PDHC, which catalyzes more pyruvate convert to acetyl-CoA for the TCA cycle18; (c) antioxidant activity: in a stoichiometric manner, pyruvate eliminates free oxygen radicals (such as hydrogen peroxide) in hypoxia by a non-enzymatic reaction.19 Furthermore, pyruvate raises intracellular pH by consuming H+ under both anoxic and aerobic conditions so as to reduce intracellular acidosis effectively. Additionally, pyruvate consumes an additional H+ by PC reaction during gluconeogenesis.19 Studies in vitro and in vivo have indicated that pyruvate exhibits antioxidant effects and improved glucose metabolism disorders.20,21 A double-blind, placebo-controlled, crossover study concluded that pyruvate ingestion (0.1g/kg) 1 h before workout modified lactate production during exercise.22 In recent years, pyruvate therapy for mitochondrial disease has been reported.23–25 The present study integrated clinical studies to evaluate the efficacy and safety of pyruvate therapy and to provide a novel perspective on the clinical treatment of mitochondrial disease.
Methods
This study was registered in PROSPERO (CRD 141196). The study design complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).26,27
Search strategy
Relevant studies were systematically searched in the following four databases: Pubmed, Embase, Cochrane Library, and ClinicalTrials, from database inception to 23 April 2019. The search was carried out using both Medical Subject Headings (MeSH) and free text terms. Major search terms included “pyruvate”, “mitochondrial disease”, “mitochondrial”, “Leigh syndrome”, and “MELAS syndrome”. The following search strategy was used: (pyruvate OR sodium pyruvate OR pyruvate therapy) AND (mitochondrial disease OR mitochondrial OR Leigh syndrome OR MELAS syndrome).
Criteria for studies
All observational and experimental studies that assessed the efficacy and safety of pyruvate therapy in mitochondrial diseases were included. No restrictions were placed on the type of research, subtype of disease or publication language. Studies where the full text was not published, such as conference abstracts and letters to the editors were excluded. Moreover, all patients who were genetically, biochemically, and clinically diagnosed with mitochondrial disease, regardless of age and gender, were included.
The inclusion criteria included: (1) study on any design; (2) patients diagnosed genetically, biochemically, and clinically with mitochondrial disease regardless of age and gender; (3) pyruvate therapy in any formula by any administration route; (4) included at least one of the following outcomes before and after pyruvate therapy: biomarkers as plasma lactate level, plasma pyruvate level, L/P ratio, serum concentrations of growth differentiation factor 15 (GDF 15) and fibroblast growth factor 21 (FGF21), and clinical rating scales as Japanese Mitochondrial Disease-Rating Scale (JMDRS),12 Newcastle Mitochondrial Disease Adult Scale (NMDAS),28 or Newcastle Pediatric Mitochondrial Disease Scale (NPMDS).29
Titles and abstracts were screened to exclude initially irrelevant articles. The full texts of the remaining articles were reviewed and assessed by two independent reviewers. Disagreements were discussed by consensus from all authors.
Data extraction and synthesis
Using a data extraction form, data collection was performed by two reviewers. The data extraction form included the following six categories: (1) study characteristics, such as author, year(s) of publication, country and study design; (2) treatment characteristics, including therapy period, follow-up period, sample size, administration route, and dose; (3) participant characteristics, such as number of participants, mean age/median age, gender ratio, subtype of mitochondrial disease, and baseline data; (4) study quality, namely quality evaluation, and explanation; (5) outcome measures, such as biomarkers (plasma level of lactate, plasma level of pyruvate, L/P ratio, serum GDF 15, and FGF21), clinical rating scales (JMDRS, NMDRS, and other); (6) adverse events reported.
The included studies were not easily comparable because of differences in study design, dosage of treatments, duration of study, and types of participants included. Due to these heterogeneities, a meta-analysis on the identified studies was not performed. Therefore, the data were only presented and described together.
Assessment of quality
Case series and case reports were assessed by The Joanna Briggs Institute Critical Appraisal tools.30 Non-randomized controlled studies of interventions were assessed by Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I).31 Randomized controlled trials were assessed by the Cochrane Risk of Bias tool.32 The studies were scored as low, moderate, or high risk of bias according to each criteria.
Results
Description of studies: results of the search/included studies/excluded studies
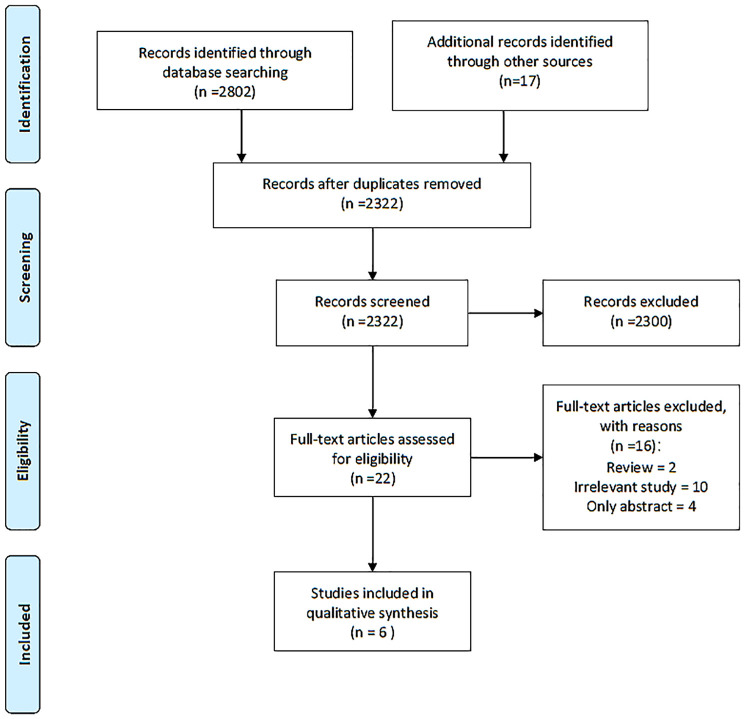
A total of 2322 articles were identified following removal of the duplicates. After screening of titles and abstracts, 2300 articles were excluded. The remaining 22 articles were evaluated by full text. A total of 10 studies were irrelevant, 2 articles were reviews and 4 studies were published only as abstracts.25,33–35 Overall, one non-randomized study of interventions and 5 case reports were eligible for inclusion and were included in the systematic review, outlined in the PRISMA flow diagram (Figure 2).23,24,37–39
Figure 2.
The PRISMA flow diagram.
PRISMA, preferred reporting items for systematic reviews and meta-analyses.
The main study characteristics of the included studies are presented in Table 1. There was variability across the results with respect to study design, intervention, and outcome measures. All the studies were from Japan. The total number of patients was 19. Six studies included participants with several types of mitochondrial diseases [mitochondria, including MELAS, Leigh syndrome (LS), Kearns-Sayre syndrome (KSS), mitochondrial diabetes mellitus, and mitochondrial DNA depletion syndrome]. No significant difference was noted in terms of drug intervention. The pyruvate therapy used in the selected studies chose sodium pyruvate (dose range = 0.25–0.5 g/kg/day) in different administrations. The duration of pyruvate therapy was variable, ranging from 2 months to 66 months.
Table 1.
Characteristics of the included studies.
| Study | Study design | Patients |
Intervention (sodium pyruvate) |
Therapy duration | Inclusion criteria | Outcomes measures | |||
|---|---|---|---|---|---|---|---|---|---|
| Number | Age (years) | gender | Gene(n) | ||||||
| Koga et al.36 | Prospective, single-centre, exploratory, clinical study |
11 | 16–62 | M:F=6:5 | A3243G(9) Large deletions in mtDNA(2) |
Initial dose of 0.25 g/kg/day tid, orally; after 4 weeks, maintenance dose of 0.5 g/kg tid, orally | 48 weeks | Mitochondrial diseasesa (CM, MELAS, MELA, KSS) | Plasma lactate level, plasma pyruvate level, L/P ratio, serum GDF15 and FGF21, JMDRS, NMDAS |
| Fujii et al.37 | Case report | 4 | 8–100 months | M:F=2:2 | m.8993 T>G (1) m.9176 T>C (1) not published (1) mtDNA depletion (1) |
0.5 g/kg/day bid, oral or through a feeding tube; maintenance dose of 0.5~1.0 g/kg/d bid | 17–66 months | Mitochondrial diseasesb (Leigh syndrome, encephalomyopathy Myopathic mitochondrial depletion syndrome) |
Plasma lactate level, L/P ratio, JMDRS, NPMDS |
| Komaki et al.23 | Case report | 1 | 11 | F | not determined (1) | 0.5 g/kg/day, orally | 1 yearc | Leigh syndrome | Plasma lactate level, Plasma pyruvate level, L/P ratio |
| Koga et al.24 | Case report | 1 | 5 | M | PDH E1a c.559A>C (1) | 0.5 g/kg/day tid, orally | 3 yearsd | Leigh syndrome | Plasma lactate level, Plasma pyruvate level, L/P ratio |
| Saito et al.38 | Case report | 1 | 1 | F | mtDNA depletion (1) | 0.5 g/kg/day tid, through a nasogastric tube | 2 months | mitochondrial DNA depletion syndrome | Plasma lactate level, L/P ratio, NPMDS |
| Inoue et al.39 | Case report | 1 | 32 | M | m.14709T>C(1) | 0.5 g/kg/day tid, orally | 10 months | Mitochondrial diabetes mellitus | Plasma lactate level, Plasma pyruvate level, L/P ratio, JMDRS |
There were four subtypes of mitochondrial diseases in this study: 2 CM patients, 4 MELAS patients, 3 MELA patients, 2 KSS patients (with large deletions in mtDNA).
There were four subtypes of mitochondrial diseases in this study: 2 patients with Leigh syndrome, one patient with nonspecific encephalomyopathy associated with complex I and IV combined deficiency and another patient with myopathic mitochondrial DNA depletion syndrome. The common characteristics was OXPHOS disorders.
It is mentioned in the article that the follow-up time is 1 year, which refers to the treatment time through the full text analysis.
It is noted that the patient actually administrated pyruvate sodium for a 3-year period, and the measurement time of outcome was 58 weeks.
CM, cardiomyopathy; FGF21, fibroblast growth factor 21; JMDRS, Japanese Mitochondrial Disease-Rating Scale; KSS, Kearns-Sayre syndrome; L/P lactate/pyruvate; MELA, mitochondrial encephalopathy, and lactic acidosis; MELAS, mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes; mtDNA, mitochondrial DNA; NMDAS, Newcastle Pediatric Mitochondrial Disease Scale; PDH, pyruvate dehydrogenase.
Evaluation of quality of included studies
Due to case series and case reports included, the Joanna Briggs Institute Critical Appraisal tools was used to evaluate the included studies. The overall appraisal of the included trials was shown in Table 2. The study by Koga was included although it has insufficient demographic information and incomplete inclusion of participants.36 There is also lack of detailed method information about identification of the condition of the participants. The five included case reports were all included with some loss of data: Fujii 2014 did not describe clearly the medical history, clinical condition, and diagnostic methods37; Komaki 2010 recorded the intervention procedure clearly but only administration frequency; the time to clinical evaluation was also not reported.23
Table 2.
The quality checklist for the included studies.
| JBI critical appraisal tools - checklist for case reports | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Were patient’s demographic characteristics clearly described? | Was the patient’s history clearly described and presented as a timeline? | Was the current clinical condition of the patient on presentation clearly described? | Were diagnostic tests or assessment methods and the results clearly described? | Was the intervention(s) or treatment procedure(s) clearly described? | Was the post-intervention clinical condition clearly described? | Were adverse events (harms) or unanticipated events identified and described? | Does the case report provide takeaway lessons? | Overall appraisala | |
| Fujii et al.37 | Yes | No | Unclear | Unclear | Yes | Yes | Yes | Yes | Included | |
| Komaki et al.23 | Yes | Yes | Yes | Yes | Yes, only administration frequency was not reported | Yes, but the time to clinical evaluation was not reported | Yes | Yes | Included | |
| Koga et al.24 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Included | |
| Saito et al.38 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Included | |
| Inoue et al.39 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Included | |
The answer “Yes”, “Unclear” and “No” was marked as 2, 1 and 0 points. The total quality grade was marked: the score of ⩾ 12 as high quality, 12–8 as moderate quality, ⩽8 as low quality.
The answer “Yes”, “Unclear” and “No” was marked as 2, 1 and 0 points. The total quality grade was marked: the score of ⩾ 17 as high quality, 17–12 as moderate quality, ⩽12 as low quality.
JBI, Joanna Briggs Institute.
Effects of interventions
All the identified studies used more than one kind of outcome to evaluate the efficacy of pyruvate therapy. The most common outcomes of assessment were the plasma lactate levels and L/P ratio, which were used in six studies. Two out of six studies (Koga 2019 and Komaki 2010)23,36 demonstrated a remarkable decrease in plasma lactate levels and L/P ratio. Koga 2012 suggested that pyruvate therapy decreased lactate, pyruvate, and alanine levels,24 although the differences were not statistically significant. However, the other three case reports including six patients who reported no significant improvement with regard to these parameters.37–39 Besides, clinical rating scales were evaluated as secondary outcomes in five studies (outlined in Table 3). Furthermore, functional improvements in patients were represented in almost every article, despite the fact that the primary outcomes showed negative results. In the studies by Fujii and Saito,37,38 five patients showed mild improvements in the movement of extremities, at least in the short term. Komaki 2010 reported remarkable improvements in exercise intolerance and cardiac dysfunction so that the patient gained the ability of running.23 The improvement in cardiac function and diabetic parameters was reported in the studies of Koga and Inoue, respectively.24,39 There were too few homogeneous clinical studies to conduct a meta-analysis, so a narrative synthesis only was performed.
Table 3.
The outcomes of the included studies.
| Study | Primary outcomes |
Secondary outcomes |
Other qualitative outcomes |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma lactate level |
Plasma pyruvate level |
L/P ratio (<25.6) |
JMDRS |
NMDAS/NPMDSa |
Functional improvements | ||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | ||
| Koga et al.36b | 50.5 ± 12.5 mg/dl | 30.4 ± 5.08 mg/dlf | 1.9 ± 0.5 mg/dl | 1.5 ± 0.3 mg/dl | 28.1 ± 8.6 | 20.7 ± 3.5c | 24.1 ± 19.7 | 21.0 ± 22.4 | 38.6 ± 28.7 | 33.3 ± 30.7 | Significant decrease from the baseline values in serum GDF15 level and lactate cerebral ventricular levels |
| Fujii et al.37d | 1.2 mM | 0.85 mM | NA | NA | 19.7 | 20 | NA | NA | 42.3 | 38.6 | Able to roll over and raise the leg 90° |
| 2.8 mM | 2.4 mM | NA | NA | 23.2 | 23.1 | 52 | 53 | 34.2 | 53.7 | Able to roll over and full oral feeding | |
| 3.9 mM | 5.6 mM | NA | NA | 25 | 30.5 | NA | NA | 44.7 | 28.3 | Able to roll over bilaterally and dysphagia disappearede | |
| 2.3 mM | 2.5 mM | NA | NA | 16.9 | 17.3 | NA | NA | 35 | 64.8 | Mild improvement in the movement of extremitiesf | |
| Komaki et al.23 | 20.5 mg/dl | 10.3 mg/dl | 1.13 mg/dl | 0.88 mg/dl | 18.1 | 11.7 | NA | NA | NA | NA | capable of running; improvement in exercise intolerance and cardiac dysfunction |
| Koga et al.24 | 9.6 ± 0.54 mM | 5.28 ± 1.73 mM | 0.69 ± 0.13 mM | 0.42 ± 0.13mM | 14.5 ± 3.10 | 12.6 ± 1.52 | 58 | 57 | NA | NA | significantly decreased level of alanine improvement in the electroencephalogram |
| Saito et al.38 | 2.3 mM | 2.3 mM | NA | NA | 18 | 18 | NA | NA | 35 | 31 | Able to raise her forearm, lower leg and wrist against gravity |
| Inoue et al.39 | 1.86 mM | 2.94 mM | 0.12 mM | 0.26 mM | 15.4 | 11.3 | 23 | 26 | NA | NA | The improvement of diabetes parameters |
This scale has four age group classifications: 0–24 months, 2–11 years, and 12–18 years from NPMDS, and an adult age group classification from NMDAS.
In addition, a significant decrease from the baseline values in GDF-15 was reported. The JMDRS overall scores did not change significantly from the baseline values at weeks 48 of pyruvate therapy, although the JMDRS scores decreased significantly from the baseline values at weeks 12 of pyruvate therapy.
Significance between none and pyruvate therapy.
The report in detail monitored four bedridden patients with OXPHOS disorders in their treatment process.
The patient gained the ability to roll over bilaterally and the dysphagia disappeared after 2 months of pyruvate therapy, but a slow regression in motor function was observed over next 10 months.
In the included study, two units were used for lactic acid and pyruvate concentration: mg/dl and mM.
JMDRS, Japanese Mitochondrial Disease-Rating Scale; L/P lactate/pyruvate; NMDAS, Newcastle Pediatric Mitochondrial Disease Scale; NPMDS, Newcastle Pediatric Mitochondrial Disease Scale.
With regard to safety, diarrhea was observed as the most common adverse effect during pyruvate therapy.23,24,36,37 Moreover, nausea and irritation of the stomach shortly were reported in a 48-week, prospective, single-center, exploratory, clinical study.36 It is worth mentioning that no patients experienced adverse events, including diarrhea, in Saito 2012 and Inoue 2016.38,39
Discussion
Due to the rarity and complexity of mitochondrial diseases, clinical studies with pyruvate therapy are very limited. Only six studies were included to investigate the efficacy and safety of pyruvate therapy for mitochondrial diseases. Other than five case reports, the only clinical study was a prospective, single-center, exploratory study, which enrolled 11 Japanese adult patients with mitochondrial disease. This study mainly explored biomarkers and clinical rating scales, which may be applicable to the evaluation of pyruvate therapy. According to the results of this study, plasma lactate levels, plasma pyruvate levels, and L/P ratio were the primary outcomes. The remaining five studies were case reports and, in total, described eight patients with mitochondrial diseases treated with pyruvate therapy. Given the limitations of case report, their results must be interpreted cautiously. It is worth noting that four studies were excluded for the following reasons. Two studies were excluded due to the lack of full text.25,33 Although the case report by Kuroha was complete,34 the paper was written in Japanese and could not be translated because of password restrictions. In the Hirano study,33 13 participants were enrolled and the majority of the outcomes were consistent with ours. Unfortunately, we failed to contact the authors by email.
The evidence gathered in the present review demonstrates an effective potential of pyruvate therapy in improving outcomes in mitochondrial disease. Three of the studies included indicated that pyruvate could significantly decrease plasma lactic acid levels and L/P ratio, 23,24,36 as manifested in two excluded studies,33,34 with the exception of the Koga study,36 where this decrease was not dramatically different. On the contrary, no change was found in the plasma lactic acid levels and L/P ratio in the other two studies,37,38 and there was even a tendency to increase.39 Although case reports did not demonstrate that pyruvate reduced lactic acid levels significantly, another excluded open study supported the possibility of pyruvate reducing lactic acid and the novel biomarker GDF-15.33 It is worth mentioning that functional improvements were common in both included and excluded studies, especially motor function and cardiac function. The patients involved in functional improvements were newborns or children, which suggested that pyruvate therapy may promote the growth and development of children with mitochondrial disease. Furthermore, pyruvate has indicated some advantages in the treatment of mitochondrial diabetes by improvement of diabetes parameters and endogenous insulin secretion.3,39
According to included study, patients at the early stages of mitochondrial diseases are more likely to improve their symptoms by pyruvate therapy. When patients were at advanced stages, disease development may exceed the efficacy of pyruvate therapy, e.g., patient 4 in the Fujii study.37 This may suggest that pyruvate may be more effective in the early stages than advanced stages. The complex pathogenesis of mitochondrial disease may be an additional factor that leads to the limited efficacy of pyruvate therapy. This type of treatment may be aimed at specific patients, such as those who present with LS and MELAS, which are associated with PDHC deficiency, rather than all patients with mitochondrial disease. According to data on the treatment status of the included studies, three patients with LS were more responsive to sodium pyruvate. Genetic data were extracted from the original literature and summarized, but failed to be analyzed due to the limited sample size and high heterogeneity. Furthermore, whether plasma lactic acid levels and L/P ratio can indicate the efficacy of pyruvate therapy is not fully explained and needs further verification. In recent years, several studies have reported that a novel biomarker named GDF-15 is expected to diagnose and monitor treatment.40–42 There was also no significant change in clinical score scales (JMDRS, NMDAS, and NPMDS) as secondary outcomes. Among them, NMDAS and NPMDS could not capture subtle changes of motor function, so improvement in children and severe patients could not be monitored effectively with these scales.23,37 Pyruvate is a physiological metabolite, which exhibits few adverse events and further provides a safety guarantee for further research. As suggested above, the present review has suggested that additional advanced clinical trials with large sample sizes and adaptive study designs are required. One report published recently highlighted that an investigator-initiated clinical trial of sodium pyruvate for MELAS and LS is currently ongoing in Japan.43
At present, the results and conclusions of pyruvate therapy are based on the above studies. Although well-designed case-series and case reports suggested potential efficacy of pyruvate therapy for treatment of mitochondrial disease, the overall certainty of the body of evidence is very low according to the GRADE system. In other words, the causal inferences for pyruvate’s effect on mitochondrial disease is still inadequate. Therefore, the following points need to be noted to further study of pyruvate therapy: (1) The improvement of patients may be attributed to the efficacy of pyruvate therapy, but it may also be due to natural motor development, especially functional improvement in children who are in a period of rapid growth. Similarly, the deterioration of condition may suggest that pyruvate therapy is ineffective, but it may be that the rapid deterioration overwhelms its efficacy. Thus, it is very important and necessary to distinguish between the effect of pyruvate therapy and the fluctuating course of the disease. (2) Although the mitochondrial clinical score scale did not manifest subtle changes, improvements in motor function and quality of life were recorded. These artificial records should be carefully considered for subjective and effective factors. (3) Another key issue is whether the statistical significance noted in the various biomarkers corresponds to the effectiveness of pyruvate treatment. For instance, whether plasma lactic acid levels, L/P ratio, and GDF-15 could represent effective treatment of patients remains to be elucidated. (4) According to the existing hypothesis, pyruvate may cause a further increase of lactate levels while improving mitochondrial disease by increasing NAD+. The potential mechanisms whereby pyruvate preferably adjusts the balance of lactate and itself have not been fully elucidated. But clearly, metabolism is not always a simple linear process and many metabolites may have multiple potential routes. For example, the role and function of lactate has been redefined and redescribed recently as the Lactate Shuttle Hypothesis.44,45 Thus, more basic research should further explore the function and relationship of lactate and pyruvate in mitochondrial disease.
To our knowledge, this is the first systematic review to examine the contribution of pyruvate therapy for mitochondrial disease. However, there are several limitations. Firstly, the present systematic review had small sample size and specific inconsistencies. Secondly, the types of included studies were case series and anecdotic case reports with patients with complicated conditions, which have too low homogeneity to sum up, so that the evidence quality is low and the analysis of the data is limited. Lastly, some published or unpublished reports may be missing although our search strategy was comprehensive. Based on the above limitations, a reminder is needed to take clinical situations into account when referring to the results of this review.
Conclusion
With no significant adverse effects, pyruvate therapy may be a potential therapeutic candidate for patients with incurable mitochondrial diseases, such as Leigh syndrome. However, recent evidence taken from case series and case reports and theoretical supports of basic research are not sufficient. Despite these challenges, there are many possibilities to understand the mechanism of pyruvate therapy through basic research, and to use global registries and more adaptive trial designs with larger numbers of participants to clarify the efficacy of pyruvate therapy.
Supplemental Material
Supplemental material, Supplement_material-ROBINS-I_tool_for_Koga,2019 for Therapeutic potential of pyruvate therapy for patients with mitochondrial diseases: a systematic review by Min Li, Shuang Zhou, Chaoyang Chen, Lingyun Ma, Daohuang Luo, Xin Tian, Xiu Dong, Ying Zhou, Yanling Yang and Yimin Cui in Therapeutic Advances in Endocrinology and Metabolism
Footnotes
Author contributions: Min Li: Data curation; Formal analysis; Investigation; Methodology; Writing-original draft; Writing-review & editing.
Shuang Zhou: Data curation; Formal analysis; Writing-original draft.
Chaoyang Chen: Funding acquisition; Methodology; Resources; Writing-review & editing.
Lingyun Ma: Data curation; Formal analysis; Investigation; Writing-review & editing.
Daohuang Luo: Investigation; Methodology; Resources; Writing-original draft.
Xin Tian: Investigation; Methodology; Writing-original draft.
Xiu Dong: Formal analysis; Resources; Writing-review & editing.
Ying Zhou: Conceptualization; Project administration; Supervision; Writing-review & editing.
Yanling Yang: Conceptualization; Project administration; Resources; Writing-review & editing.
Yimin Cui: Conceptualization; Project administration; Resources; Writing-review & editing.
Corresponding authors had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Availability of data and materials: The data set supporting the results of this article are included within the article and other supplements.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This work was supported by the Development Center for Medical Science and Technology, National Health and Family Planning Commission of the People’s Republic of China (No.2018ZX09721003-008) and the 13th Five-Year Plan on National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2017ZX09304029-006-001)
ORCID iD: Yimin Cui  https://orcid.org/0000-0002-4186-1005
https://orcid.org/0000-0002-4186-1005
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Min Li, Department of Pharmacy, Peking University First Hospital, Beijing, China; Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Science, Peking University, Beijing, China.
Shuang Zhou, Department of Pharmacy, Peking University First Hospital, Beijing, China; Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Science, Peking University, Beijing, China.
Chaoyang Chen, Department of Pharmacy, Peking University First Hospital, Beijing, China; Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Science, Peking University, Beijing, China.
Lingyun Ma, Department of Pharmacy, Peking University First Hospital, Beijing, China.
Daohuang Luo, Department of Pharmacy, Peking University First Hospital, Beijing, China; Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Science, Peking University, Beijing, China.
Xin Tian, Department of Pharmacy, Peking University First Hospital, Beijing, China; Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Science, Peking University, Beijing, China.
Xiu Dong, Department of Pharmacy, Peking University First Hospital, Beijing, China; Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Science, Peking University, Beijing, China.
Ying Zhou, Department of Pharmacy, Peking University First Hospital, Beijing, China.
Yanling Yang, Department of Pediatrics, Peking University First Hospital, 8 Xishiku Street, Xicheng District, Beijing, 100034, China.
Yimin Cui, Department of Pharmacy, Peking University First Hospital, 6 Dahongluochang Street, Xicheng District, Beijing, 100034, China; Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Science, Peking University, Beijing, China.
References
- 1. Chow J, Rahman J, Achermann JC, et al. Mitochondrial disease and endocrine dysfunction. Nat Rev Endocrinol 2017; 13: 92–104. [DOI] [PubMed] [Google Scholar]
- 2. Craven L, Alston CL, Taylor RW, et al. Recent advances in mitochondrial disease. Annu Rev Genomics Hum Genet 2017; 18: 257–275. [DOI] [PubMed] [Google Scholar]
- 3. Skladal D, Halliday J, Thorburn DR. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain 2003; 126: 1905–1912. [DOI] [PubMed] [Google Scholar]
- 4. Chinnery PF. Mitochondrial Disorders Overview. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds.) GeneReviews®. Seattle (WA): University of Washington, Seattle; 1993. [Google Scholar]
- 5. Gorman GS, Schaefer AM, Ng Y, et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol 2015; 77: 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chinnery PF, Johnson MA, Wardell TM, et al. The epidemiology of pathogenic mitochondrial DNA mutations. Ann Neurol 2000; 48: 188–193. [PubMed] [Google Scholar]
- 7. Ryan E, King MD, Rustin P, et al. Mitochondrial cytopathies, phenotypic heterogeneity and a high incidence. Ir Med J 2006; 99: 262–264. [PubMed] [Google Scholar]
- 8. Darin N, Oldfors A, Moslemi AR, et al. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological, biochemical, and DNA abnormalities. Ann Neurol 2001; 49: 377–383. [PubMed] [Google Scholar]
- 9. Diogo L, Grazina M, Garcia P, et al. Pediatric mitochondrial respiratory chain disorders in the Centro region of Portugal. Pediatr Neurol 2009; 40: 351–356. [DOI] [PubMed] [Google Scholar]
- 10. Castro-Gago M, Blanco-Barca MO, Campos-González Y, et al. Epidemiology of pediatric mitochondrial respiratory chain disorders in Northwest Spain. Pediatr Neurol 2006; 34: 204–211. [DOI] [PubMed] [Google Scholar]
- 11. Uusimaa J, Remes AM, Rantala H, et al. Childhood encephalopathies and myopathies: a prospective study in a defined population to assess the frequency of mitochondrial disorders. Pediatrics 2000; 105: 598–603. [DOI] [PubMed] [Google Scholar]
- 12. Yatsuga S, Povalko N, Nishioka J, et al. ; Taro Matsuoka for MELAS Study Group in Japan. MELAS: a nationwide prospective cohort study of 96 patients in Japan. Biochim Biophys Acta 2012; 1820: 619–624. [DOI] [PubMed] [Google Scholar]
- 13. El-Hattab AW, Zarante AM, Almannai M, et al. Therapies for mitochondrial diseases and current clinical trials. Mol Genet Metab 2017; 122: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parikh S, Goldstein A, Koenig MK, et al. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet Med 2015; 17: 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfeffer G, Majamaa K, Turnbull DM, et al. Treatment for mitochondrial disorders. Cochrane Database Syst Rev 2012; 2012: CD004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaufmann P, Engelstad K, Wei Y, et al. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology 2006; 66: 324–330. [DOI] [PubMed] [Google Scholar]
- 17. Olson KA, Schell JC, Rutter J. Pyruvate and metabolic flexibility: illuminating a path toward selective cancer therapies. Trends Biochem Sci 2016; 41: 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanaka M, Nishigaki Y, Fuku N, et al. Therapeutic potential of pyruvate for mitochondrial diseases. Mitochondrion 2007; 7: 399–401. [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Huang Y, Yang J, et al. Pyruvate is a prospective alkalizer to correct hypoxic lactic acidosis. Mil Med Res 2018; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramos-Ibeas P, Barandalla M, Colleoni S, et al. Pyruvate antioxidant roles in human fibroblasts and embryonic stem cells. Mol Cell Biochem 2017; 429: 137–150. [DOI] [PubMed] [Google Scholar]
- 21. Hegde KR, Kovtun S, Varma SD. Inhibition of glycolysis in the retina by oxidative stress: prevention by pyruvate. Mol Cell Biochem 2010; 343: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olek RA, Kujach S, Wnuk D, et al. Single sodium pyruvate ingestion modifies blood acid-base status and post-exercise lactate concentration in humans. Nutrients 2014; 6: 1981–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Komaki H, Nishigaki Y, Fuku N, et al. Pyruvate therapy for Leigh syndrome due to cytochrome C oxidase deficiency. Biochim Biophys Acta 2010; 1800: 313–315. [DOI] [PubMed] [Google Scholar]
- 24. Koga Y, Povalko N, Katayama K, et al. Beneficial effect of pyruvate therapy on Leigh syndrome due to a novel mutation in PDH E1α gene. Brain Dev 2012; 34: 87–91. [DOI] [PubMed] [Google Scholar]
- 25. Tanaka M, Wakamoto H, Yamamoto E, et al. Pyruvate therapy for Leigh syndrome with hypertrophic cardiomyopathy. Mitochondrion 2010; 10: 224. [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shamseer L, Moher D, Clarke M, et al. ; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015; 349: g7647. [DOI] [PubMed] [Google Scholar]
- 28. Schaefer AM, Phoenix C, Elson JL. Mitochondrial disease in adults: a scale to monitor progression and treatment. Neurology 2006; 66: 1932–1934. [DOI] [PubMed] [Google Scholar]
- 29. Phoenix C, Schaefer AM, Elson JL, et al. A scale to monitor progression and treatment of mitochondrial disease in children. Neuromuscul Disord 2006; 16: 814–820. [DOI] [PubMed] [Google Scholar]
- 30. The Joanna Briggs Institute. The Joanna Briggs Institute Critical appraisal tools, https://joannabriggs.org/ebp/critical_appraisal_tools (2017, accessed 1 March 2018).
- 31. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions, http://methods/cochrane.org/bias/assessing-riskbias-included-studies (2011, accessed 1 March 2018).
- 33. Hirano M. GDF-15, lactate as well as clinical grading scale was improved by sodium pyruvate therapy in mitochondrial myopathy. Neurology 2016; 87: E22–E23. [Google Scholar]
- 34. Kuroha Y, Tada M, Kawachi I, et al. Effect of sodium pyruvate on exercise intolerance and muscle weakness due to mitochondrial myopathy: a case report. Clinical Neurol 2015, 55: 412–416. [DOI] [PubMed] [Google Scholar]
- 35. Ayabe T, Inoue T. Sodium pyruvate treatment improved endogenous insulin secretion in a patient with mitochondrial diabetes. Pediatr Diabetes 2016; (Suppl. 24): 151. [Google Scholar]
- 36. Koga Y, Povalko N, Inoue E, et al. Biomarkers and clinical rating scales for sodium pyruvate therapy in patients with mitochondrial disease. Mitochondrion 2019; 48: 11–15. [DOI] [PubMed] [Google Scholar]
- 37. Fujii T, Nozaki F, Saito K, et al. Efficacy of pyruvate therapy in patients with mitochondrial disease: a semi-quantitative clinical evaluation study. Mol Genet Metab 2014; 112: 133–138. [DOI] [PubMed] [Google Scholar]
- 38. Saito K, Kimura N, Oda N, et al. Pyruvate therapy for mitochondrial DNA depletion syndrome. Biochim Biophys Acta 2012; 1820: 632–636. [DOI] [PubMed] [Google Scholar]
- 39. Inoue T, Murakami N, Ayabe T, et al. Pyruvate improved insulin secretion status in a mitochondrial diabetes mellitus patient. J Clin Endocrinol Metab 2016; 101: 1924–1926. [DOI] [PubMed] [Google Scholar]
- 40. Fujita Y, Ito M, Kojima T, et al. GDF15 is a novel biomarker to evaluate efficacy of pyruvate therapy for mitochondrial diseases. Mitochondrion 2015; 20: 34–42. [DOI] [PubMed] [Google Scholar]
- 41. Yatsuga S, Fujita Y, Ishii A, et al. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann Neurol 2015; 78: 814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xinbo J. Study on growth differentiation factor 15 (GDF15) as a diagnostic marker for mitochondrial diseases. China, Shandong: Shandong University, 2016. [Google Scholar]
- 43. Murayama K, Shimura M, Liu Z, et al. Recent topics: the diagnosis, molecular genesis, and treatment of mitochondrial diseases. J Hum Genet 2019; 64: 113–125. [DOI] [PubMed] [Google Scholar]
- 44. Brooks GA. Lactate as a fulcrum of metabolism. Redox Biol 2020; 35: 101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol 2009; 92: 329–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplement_material-ROBINS-I_tool_for_Koga,2019 for Therapeutic potential of pyruvate therapy for patients with mitochondrial diseases: a systematic review by Min Li, Shuang Zhou, Chaoyang Chen, Lingyun Ma, Daohuang Luo, Xin Tian, Xiu Dong, Ying Zhou, Yanling Yang and Yimin Cui in Therapeutic Advances in Endocrinology and Metabolism