Abstract
A 3-year-old castrated male domestic shorthair cat was presented for evaluation of acute onset tachypnea, dyspnea, and restlessness. Blood analysis revealed markedly elevated creatinine kinase, troponin, and D-dimers, together with azotemia and an inflammatory leukogram. Ultrasonography identified cardiomegaly with pericardial and pleural effusion. Thoracocentesis identified a high protein transudate. Cardiac computed tomographic angiography (CTA) identified an enlarged left auricle containing a non-contrast enhancing mass measuring 1.6 × 1.2 × 1.2 cm subsequently confirmed to be a thrombus. The cat underwent a left cardiac auriculectomy and was discharged on clopidogrel. Post-operative complications, including late-onset hemothorax and dyspnea, were managed to resolution.
Key clinical message:
A cardiac auriculectomy was effective in management of thromboembolic disease in a domestic cat.
Résumé
Auriculectomie pour une thrombose spontanée de l’oreillette gauche chez un chat domestique à poils courts. Un chat domestique à poils courts castré âgé de 3ans fut présenté pour évaluation suite à l’apparition soudaine de tachypnée, dyspnée et agitation. L’analyse sanguine révéla une augmentation marquée de la créatine kinase, de la troponine, des dimères-D avec également une azotémie et un leucogramme inflammatoire. L’échographie révéla une cardiomégalie avec effusions péricardique et pleurale. Une thoracocentèse identifia un transsudat élevé en protéine. Une angiographie par tomodensitométrie (CTA) identifia une oreillette gauche augmentée de volume contenant une masse non-contrastante mesurant 1,6 × 1,2 × 1,2 cm qui fut subséquemment confirmée être un thrombus. Le chat subit une auriculectomie cardiaque gauche et obtint son congé avec du clopidogrel. Des complications post-opératoires, incluant un hémothorax qui apparut tardivement et de la dyspnée, furent gérées jusqu’à leur résolution.
Message clinique clé :
Une auriculectomie cardiaque fut efficace pour gérer un problème thrombo-embolique chez un chat domestique.
(Traduit par Dr Serge Messier)
Here we report the presentation, diagnostic work-up, treatment, histopathologic diagnosis, post-operative management, and complications associated with a left auricular thrombus in a domestic shorthair cat. The cat underwent surgery for a left auriculectomy and subtotal pericardiectomy, with subsequent revision surgeries to place a porcine collagen patch, and later a PleuralPort (Norfolk Vet Products, Skokie, Illinois, USA). The cat was managed over the following weeks with coagulation modulation, repeat ultrasonographic scans, and aspiration of pleural effusion. This case presents an example of an unusual procedure successfully completed in a cat.
Case description
A 3-year-old, castrated male domestic shorthair cat was presented to the referring veterinarian for acute-onset tachypnea, dyspnea, and restlessness. Physical examination also identified hypothermia and bradycardia. Thoracic radiographs revealed an enlarged cardiac silhouette and pleural effusion. Tests for feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) were negative. The cat was treated for presumptive congestive heart failure with oxygen supplementation, butorphanol, furosemide, and diphenhydramine. After initial response to supportive therapy, the cat deteriorated with new onset tachycardia and altered mentation. Intravenous fluids were administered and a thoracocentesis identified pleural effusion consistent with a high-protein transudate (total protein = 31 g/L, nucleated cell count = 3.6 × 103/μL). The cat was then referred to Cornell University Hospital for Animals for further evaluation and care.
On presentation, the cat was bright, alert, and responsive, but was tachypneic (60 breaths/min) and had muffled heart and lung sounds on auscultation. No other abnormalities were detected. Initial Thoracic Focused Assessment with Sonography for Trauma (TFAST) revealed mild pleural effusion and scant pericardial effusion. A point-of-care cardiac troponin test was performed as a screening assessment to differentiate dyspnea secondary to cardiac failure from other causes. Serum cardiac troponin was markedly elevated (1.69 μg/L; RI: 0 to 0.11 μg/L), consistent with release from injured myocardial tissue. The cat was admitted for further diagnostic work-up of suspected cardiac disease and treated with intravenous fluid (Plasmalyte with 15 mEq/L of KCl; Baxter, Deerfield, Illinois, USA), 16 mL/h reduced to 8 mL/h after 4 h, and supplemental oxygen (40%).
On further work-up, a disseminated intravascular coagulation (DIC) panel [activated partial thromboplastin time (aPTT), partial thromboplastin time (PTT), fibrinogen, antithrombin, and D-dimer] yielded moderately elevated D-dimers (2305 nmol/L; RI: 0 to 1369 nmol/L) but was otherwise within reference intervals. The cat had a moderate leukocytosis [27.7 × 103/μL; reference interval (RI): 5.1 to 16.2 × 103/μL] with a normal hematocrit of 40% (RI: 31% to 48%), was hypokalemic (3.5 mmol/L; RI: 3.8 to 5.5 mmol/L), hypochloridemic (101 mmol/L; RI: 111 to 124 mmol/L), hypomagnesemic (0.75 mmol/L; RI: 0.85 to 1.1 mmol/L), had an elevated bicarbonate (29 mmol/L; RI: 14 to 20 mmol/L), elevated urea nitrogen (15.7 mmol/L; RI: 6.1 to 12.5 mmol/L), elevated creatinine (203.3 μmol/L; RI: 70.7 to 185.6 μmol/L), elevated aspartate aminotransferase (AST, 187 U/L; RI: 17 to 48 U/L), elevated alanine aminotransferase (ALT, 146 U/L; RI: 28 to 109 U/L), elevated creatine kinase (28 069 U/L; RI: 74 to 386 U/L), and elevated lactate dehydrogenase (LDH, 1369 U/L; RI: 71 to 406 U/L). Urinalysis revealed a urine specific gravity (USG) of 1.012 in the face of recent IV fluid therapy and diuretics. Toxoplasma titer was negative.
An echocardiogram revealed a hyperechoic mass close to the left atrium with mild pericardial effusion, and a mild-to-moderate amount of pleural effusion. Both atria and ventricles were of normal size with normal wall thickness, and cardiac function was assessed to be normal. The next day, the cat was sedated with butorphanol (Torbutrol; Zoetis, Kalamazoo, Michigan, USA), 0.23 mg/kg BW, SC; and underwent thoracic and abdominal ultrasound. No abnormalities were detected aside from the moderate pleural and pericardial effusion. Repeat blood gas and biochemistry analysis revealed a decreasing bicarbonate (23 mmol/L; RI: 14 to 20 mmol/L), increased hyper-magnesemia (1.2 mmol/L; RI: 0.85 to 1.1 mmol/L), increased creatinine kinase (30 784 U/L; RI: 74 to 386 U/L), and a decreasing LDH (681 U/L; RI: 71 to 406 U/L), ALT (120 U/L; RI: 28 to 109 U/L), and AST (151 U/L; RI: 17 to 48 U/L), in addition to a low urea nitrate (5.4 mmol/L; RI: 6.1 to 12.5 mmol/L), and hypoproteinemia (65 g/L; RI: 66 to 84 g/L). The initial azotemia had resolved.
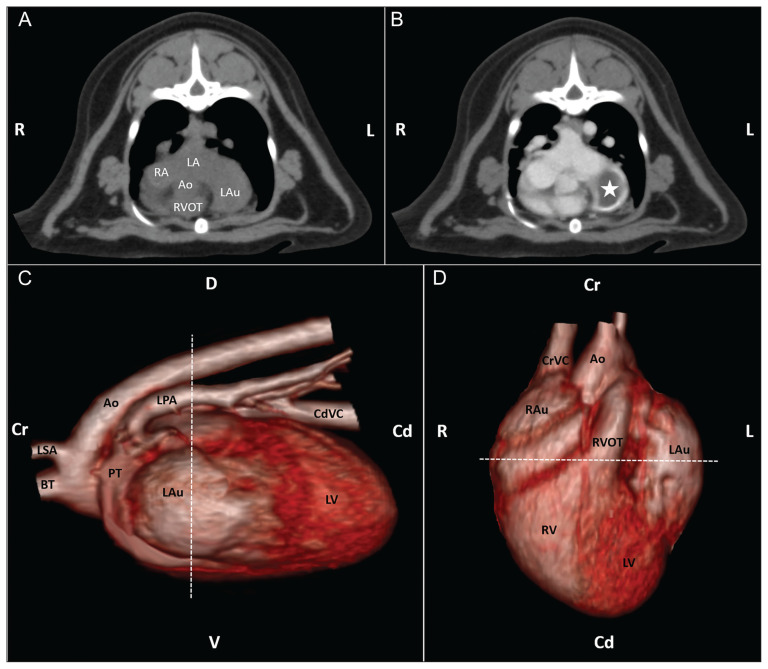
The cat was placed under general anesthesia for thoracic computed tomography (CT) and cardiac triple-phase computed tomographic angiography (CTA) to further interrogate the heart-based mass. The CT studies were performed via non-gated image acquisition before and after administration of 12 mL of Iohexol contrast medium (Omnipaque 350, Iohexol-350, 2.2 mL/kg; GE Healthcare, Marlborough, Massachussets, USA). Thoracic CT was acquired in 1-mm slices and reconstructed in 2-mm contiguous transverse pre- and post-contrast administration (delayed phase), in sagittal, and dorsal scans (Figure 1). Cardiac CTA was acquired in 0.5-mm slices and reconstructed in 1-mm contiguous transverse scans. A region of interest for bolus tracking was placed over the ascending aorta followed by 3 continuous acquisitions initiated after reaching an attenuation threshold of 80 Hounsfield Units. The left auricle was enlarged and contained a 1.6 × 1.2 × 1.2 cm soft tissue attenuating non-contrast-enhancing mass. Contrast material filled the left auricle at the periphery of the mass. The cranial aspect of the left cranial, right cranial, and middle lung lobes had mild-to-moderate, patchy attenuation that partially to completely effaced the pulmonary blood vessels. Based on the combined findings of the echocardiogram and CTA, the primary differential diagnosis was a thrombus within the distended left auricle with pulmonary atelectasis. Focal neoplasia could not be ruled out, but imaging had shown no evidence of systemic neoplasia. Lack of identification of a systemic cause of hypercoagulability (no evidence of protein-losing nephropathy; no systemic inflammatory disease identified) together with no history of cardiac trauma raised a concern for focal thrombotic disease arising in the left auricle due to a low flow/venous stasis state. Differentials for this included congenital stenosis of the left atrio-auricular ostium with secondary progressive auricular enlargement, or a congenital defect of the pericardial sac with herniation and partial strangulation of the auricle. Due to concerns for propagation or fragmentation of an intracardiac thrombus, exploratory thoracotomy with subtotal pericardiectomy and auriculectomy was recommended. In-house blood typing was performed in anticipation of the potential for hemorrhage and the need for a transfusion.
Figure 1.
Thoracic CT images in the transverse plane pre-contrast (A) and 40 s post-contrast (B), with the left auricular thrombus marked by a star. Images C and D are 3D reconstructions (C left lateral view, D ventral view) showing the enlarged left auricle — dashed line indicates the cutline for the upper images. (R — right; L — left; D — dorsal; V — ventral; Cr — cranial; Cd — caudal; LA — left atrium; RA — right atrium; Ao — aorta; RVOT — right ventricular outflow tract; LAu+ — left auricle; LSA — left subclavian artery; BT — brachiocephalic trunk; PT — pulmonary trunk; RAu — right auricle; LV — left ventricle; RV — right ventricle; CdVC — caudal vena cava; CrVC — cranial vena cava).
Prior to surgery, the cat’s physical examination was unremarkable, vital parameters were within reference intervals, and no significant abnormalities were detected on recheck blood analysis. A recheck echocardiogram performed 6 d after initial presentation identified spontaneous resolution of the previously noted pleural and pericardial effusion, with persistent auricular thrombosis. The cat was placed under general anesthesia, positioned in right lateral recumbency, and the left hemithorax clipped, prepared, and draped in standard aseptic fashion. A thoracotomy was performed at the left fifth intercostal space and the left lung lobes were retracted caudally. Visualization of the pericardial surface showed no evidence of pericardial defects or auricular herniation. The pericardium was incised using Metzenbaum scissors ventral and parallel to the vagus nerve. Approximately 5 mL of serosanguinous pericardial fluid was present. To improve visualization of the heart, the pericardium was reflected with stay sutures which were then placed under tension and secured to the surrounding drapes thus elevating the heart into the incision. The left auricle was grossly enlarged and thickened, with a mottled surface. The auricle was isolated and the auricular base identified. A 3-row vascular stapler (30 mm-V3 Vascular Loading Unit with DST Series, Medtronic, Minneapolis, Minnesota, USA) was positioned across the base of the auricle and deployed. The auricular tissue distal to the staple line was excised using Metzenbaum scissors and placed in 10% formalin for histopathology. The vascular stapler was disengaged and removed, revealing the auricular stump. No hemorrhage was noted, and all staples appeared appropriately fired. A small area of redundant auricular tissue remained. This was addressed by placement of a 3-0 Vicryl ligating loop (Surgitie with Polysorb; Medtronic). The ligation was secured and trimmed. A subtotal pericardiectomy was performed using Metzenbaum scissors. The excised pericardial tissue was submitted for histopathology. Transesophageal echocardiography was performed to confirm that the suspect thrombus was completely removed and that there were no visible thrombi remaining extending into the atrium. The thoracic cavity was lavaged with warm, sterile saline. A small bore chest tube (14-gauge; MILA International, Florence, Kentucky, USA) was placed using the Seldinger technique. The thorax was closed routinely, and a splash block provided with 1.75 mL 0.5% bupivacaine (1.59 mg/kg BW; Hospira, Lake Forest, Illinois, USA). A non-adherent bandage was applied to the thoracotomy incision (Tegaderm; 3M, St. Paul, Minnesota, USA), and meloxicam (Henry Schein, Columbus, Ohio, USA), 0.05 mg/kg BW, was administered SQ. The cat recovered from anesthesia in the intensive care unit and was maintained on a regimen of IV fluids, buprenorphine, and meloxicam. Post-operative blood analysis and physical examination were unremarkable over the next 2 d. The cat’s thoracostomy tube and IV catheter were removed the day after surgery, and anticoagulant therapy was initiated (Clopidogrel 18.75 mg; Accord Healthcare, Durham, North Carolina, USA), 3.4 mg/kg BW, PO, q24h as a prophylactic measure against presumptive risk of atrial thrombosis at the surgical site. The cat was monitored for recrudescence of effusion, azotemia, and respiratory distress. Two days later, the cat was discharged from the hospital.
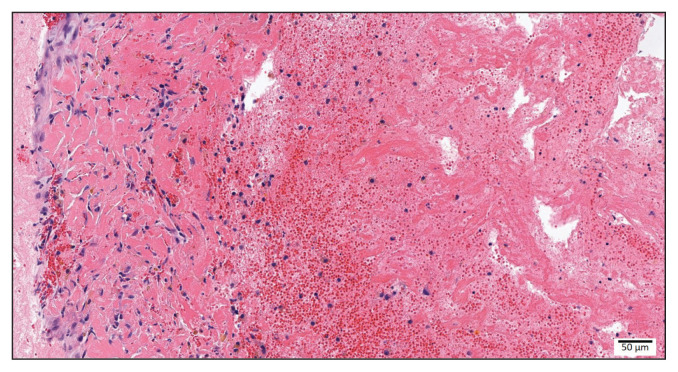
Histopathologic findings were consistent with a left auricular thrombus (Figure 2). The subjacent endocardium to which the thrombus was adhered was expanded by reactive fibroblasts, and there was evidence of myocardial hemorrhage with leukocytic infiltrate. The morphologic diagnosis was therefore determined to be left auricular thrombosis with reactive endocardial fibrosis.
Figure 2.
Cat, left auricle, 200× magnification: Attached to the endocardium were abundant streams of an eosinophilic fibrillar material (fibrin), interspersed with erythrocytes and mixed leukocytes (thrombus).
Ten days after discharge, the cat was presented in respiratory distress. Point-of-care blood analysis revealed anemia (PCV: 22) and TFAST revealed pleural effusion. Thoracocentesis identified a hemorrhagic effusion, which was consistent with hemothorax (PCV: 23). The cat was again admitted to the intensive care unit and treated with supplemental oxygen support. The clopidogrel therapy was discontinued, and therapy with aminocaproic acid (104.5 mg/kg BW, IV, q6h; Hospira), was initiated. Twelve hours following admission, the cat remained tachypneic, had pale mucous membranes, and was increasingly anemic (PCV: 14). Thoracocentesis confirmed the ongoing hemothorax, and a whole blood transfusion was administered. The systemic PCV was 19% at 20 h post-transfusion. Repeat TFAST showed moderate amounts of pleural effusion; thoracocentesis produced 50 mL of serosanguinous fluid. Due to concerns of slow but active hemorrhage, the cat was placed under general anesthesia and taken to exploratory surgery. Following repeat left 5th intercostal thoracotomy, visualization of the auriculectomy site revealed active hemorrhage from the previous staple line. An additional staple line was placed using a TA30V stapler (30 mm-V3 Vascular Loading Unit with DST Series; Medtronic), proximal and parallel to the first, encroaching on atrial tissue. The adjacent pulmonary vasculature was not disturbed. To provide additional hemostatic reinforcement of the new staple line, an approximately 3 × 3 cm piece of porcine collagen (Porcine PERISeal Tissue Patch; Avalon Medical, Stillwater, Minnesota, USA) was applied over the site and staple line with surgical glue (Vetbond Tissue Adhesive; 3M, St. Paul, Minnesota, USA). A small bore chest tube was placed using Seldinger technique, and the thorax was closed routinely. The cat recovered from anesthesia in the intensive care unit on a combination of fentanyl (Pfizer, New York, New York, USA) and meloxicam (Henry Schein) for pain control. Echocardiogram and thoracic radiographs taken 48 h after surgery revealed tricuspid regurgitation with right atrial dilation consistent with pulmonary hypertension and scant pleural effusion, respectively. The cat was placed on sildenafil (0.5 mg/kg BW, PO, q24h; Amneal Pharmaceuticals, Bridgewater, New Jersey, USA) which was increased to q12h the next morning. Systemic blood pressure remained between 100 mmHg and 135 mmHg. The cat was transitioned from fentanyl to buprenorphine and remained comfortable. Small volumes of serosanguinous-to-clear fluid were removed via the chest tube regularly over the next 5 d; the cat’s chest tube was then removed. One day later, TFAST revealed scant pleural effusion, and the cat was discharged shortly after.
The cat was presented multiple times over the following month with a non-chylous modified transudate with recurrent pleural effusion. Analysis was consistent with a modified transudate with a PCV of 3%. The effusion was unresponsive to sildenafil and Yunnan Biayou, 1 capsule BID (Yunnan Baiyao Group, Chenggong District, Kunming, Yunnan, People’s Republic of China), requiring therapeutic thoracocentesis on 3 occasions. Twenty-four days after the revision surgery, a PleuralPort (Norfolk Vet Products) was placed to assist management of the recurrent pleural effusion. Sildenafil was discontinued at that time due to normal right atrial chamber size and function, determined via focused echocardiogram. The auriculectomy site was visualized and confirmed to be contracted and hyperechoic, consistent with scar formation. The cause of the persistent effusion was unknown; right-sided heart disease due to tricuspid insufficiency following surgery appeared to have been ruled out, and there was no evidence of ongoing hemorrhage. Persistent inflammation and pleuritis following surgery appeared unlikely.
At a 2-month recheck examination, the cat’s physical examination was unremarkable, vital parameters were within normal limits, and the surgical site had completely healed. A TFAST scan revealed scant pleural fluid and few B-lines. The PleuralPort was flushed with heparinized saline, and the cat was discharged to the care of its owners. No further removal of pleural fluid was required. The cat recovered full function, and the PleuralPort was removed 6 mo later, at which time the pleural effusion had resolved and thoracocentesis had not been required for 5 mo. Clinical signs did not recur, and the cat returned to normal activity.
Discussion
This report describes a cardiac auriculectomy in a domestic cat, as well as the associated diagnostic work-up and post-operative management. There is a relative paucity of literature regarding cardiac surgery in domestic felids. However, cardiomyopathy, congestive heart failure, and thromboembolic disease are well-recognized, commonly diagnosed, and frequently managed in cats (1–9). Relative to dogs, cats are underrepresented for cardiovascular neoplasia, which is one of the major indications for canine cardiovascular surgery (5,10). Rather, hypertrophic cardiomyopathy (HCM) is the most commonly diagnosed cardiac disease in domestic cats (1,11). Cardiogenic thromboembolic disease generally occurs secondary to underlying disease that enables thrombus formation via derangement of one or more of the components of Virchow’s Triad (hypercoagulability, endothelial disruption, and blood stasis) (4,5,11,12). In cats, cardiogenic emboli arise most commonly in the left atrium and left auricle secondary to cardiomyopathies (7). Cardiogenic thromboembolic events are rare, but can markedly decrease the median survival time of cats with cardiac disease and are often immediately life-threatening (6,7,11). Therefore, high priority is indicated for prevention, diagnosis, and treatment of cardiogenic thrombi before they embolize. Although HCM is associated with cardiogenic embolism formation, it is not the only cause. The cat described herein did not have findings consistent with HCM, and there were no other significant findings on the echocardiogram aside from the presence of the auricular thrombus and pericardial effusion. The cause of the auricular thrombosis is unknown, but in view of the age of the cat and absence of other signs we speculate that the thrombosis was due to a congenital stenosis of the auricular orifice. Although this malformation specifically has not been described, there are reports of congenital defects involving the mitral valve and left auricle in domestic cats (13–15). The stenotic communication between the left atrium and left auricle would lead to high-pressure, turbulent blood flow into the auricle during atrial systole. Turbulence and stasis of blood within the auricle would be pro-thrombotic (12). Platelet activation and subsequent release of von Willebrand factor, combined with blood stasis caused by inability to adequately empty the auricle would promote formation of microthrombi. Additionally, histopathology suggested endocardial inflammation secondary to jet lesions produced as blood passed through the stenotic orifice into the auricle. Damage to the endocardium would expose subendothelial collagen, further promoting thrombosis. Finally, as the auricle continued to distend with thrombus formation, auricular emptying would be inhibited. As a result, blood would remain static within the auricle, thereby exacerbating thrombosis.
In this cat, CTA was used to characterize the intra-auricular mass and determine that it was a thrombus, rather than neoplasia. Additionally, the CTA images guided surgical planning. Although thromboprophylaxis is often used for prevention of emboli, and can reduce likelihood of thromboembolic recurrence (6), medical anti-thrombotic therapy alone was not considered the best option in this case as this typically fails to resolve existing thrombi. Surgery was recommended based on the young age of the cat and potential for resolution of both the thrombus and the inciting cause. We were not able to identify previous reports of this procedure in the domestic cat; therefore, surgical principles and approach were derived from literature published for the domestic dog. The lateral thoracotomy approach was chosen in this cat, as it provides adequate visualization of the ipsilateral side of the heart and would facilitate the exposure necessary to isolate the left auricle and place the vascular staple line. Post-operative complications encountered following lateral thoracotomy are often minor and related to the surgical approach; clinically significant complications are rare (16,17). Mortality is often associated with the underlying disease, particularly neoplasia, rather than the surgery itself (17). In this case, post-operative complications included hemorrhage from the surgical site, pleural effusion, pain, and tachypnea. Although a 3-row vascular stapler and a ligation loop were used for the initial ligation, bleeding occurred 10 d following the initial procedure. We hypothesized that the cause for the late onset bleeding was due to administration of clopidogrel. This was administered to prevent formation of arterial thromboemboli at the surgical site, but may have prevented appropriate clot formation and subsequent progression of cardiac wound healing.
At revision surgery, an additional staple line together with a pro-coagulant collagen patch was placed to prevent leakage. In addition, the cat was switched from anti-thrombotic therapy to pro-coagulant therapy. In the face of these measures, intrathoracic hemorrhage resolved, but a persistent pleural effusion developed. We suspected that the pleural fluid accumulation was multifactorial: tricuspid valve regurgitation was observed on initial post-operative echocardiography, suggesting pulmonary hypertension. Pulmonary hypertension would lead to increased hydrostatic pressure and pleural effusion. The porcine origin collagen patch may have served as an inflammatory focus contributing to an effusion that persisted for approximately 6 wk after surgery before resolving.
This case represents an unusual report of an ultimately successful auriculectomy in the domestic cat, and provides insight into the post-operative complications that may be encountered, as well as management and resolution of those complications. At a 6-month recheck the cat was active and comfortable with normal vital parameters. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Paige CF, Abbott JA, Elvinger F, Pyle RL. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc. 2009;234:1398–1403. doi: 10.2460/javma.234.11.1398. [DOI] [PubMed] [Google Scholar]
- 2.Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999) J Am Vet Med Assoc. 2002;220:202–207. doi: 10.2460/javma.2002.220.202. [DOI] [PubMed] [Google Scholar]
- 3.Häggström J, Fuentes V, Wess G. Screening for hypertrophic cardiomyopathy in cats. J Vet Cardiol. 2015;17:S134–S149. doi: 10.1016/j.jvc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Côté E, MacDonald KA, Meurs KM, Sleeper MM. Hypertrophic cardiomyopathy. In: Côté E, MacDonald KA, Meurs KM, Sleeper MM, editors. Feline Cardiology. 1st ed. Chichester, West Sussex, UK: John Wiley & Sons; 2011. pp. 103–175. [Google Scholar]
- 5.MacDonald K. Feline cardiomyopathy. In: Smith FW, Tilley LP, Oyama M, Sleeper MM, editors. Manual of Canine and Feline Cardiology. 5th ed. St. Louis, Missouri: Elsevier Health Sciences; 2015. pp. 153–80. [Google Scholar]
- 6.Payne J, Borgeat K, Brodbelt D, Connolly D, Fuentes VL. Risk factors associated with sudden death vs. congestive heart failure or arterial thromboembolism in cats with hypertrophic cardiomyopathy. J Vet Cardiol. 2015;17:S318–S328. doi: 10.1016/j.jvc.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Vititoe KP, Fries RC, Joslyn S, et al. Detection of intra-cardiac thrombi and congestive heart failure in cats using computed tomographic angiography. Vet Radiol Ultrasound. 2018;59:412–422. doi: 10.1111/vru.12616. [DOI] [PubMed] [Google Scholar]
- 8.Payne JR, Borgeat K, Connolly DJ, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2013;27:1427–1436. doi: 10.1111/jvim.12215. [DOI] [PubMed] [Google Scholar]
- 9.Fuentes VL. Arterial thromboembolism: Risks, realities and a rational first-line approach. J Feline Med Surg. 2012;14:459–470. doi: 10.1177/1098612X12451547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacPhail C. Surgery of the cardiovascular system. In: Fossum TW, Dewey CW, editors. Small Animal Surgery. 4th ed. St. Louis Missouri: Mosby; 2013. pp. 856–905. [Google Scholar]
- 11.Hogan DF, Brainard BM. Cardiogenic embolism in the cat. J Vet Cardiol. 2015;17:S202–S214. doi: 10.1016/j.jvc.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Stokol T, Brooks M, Rush J, et al. Hypercoagulability in cats with cardiomyopathy. J Vet Intern Med. 2008;22:546–552. doi: 10.1111/j.1939-1676.2008.0098.x. [DOI] [PubMed] [Google Scholar]
- 13.Fine DM, Tobias AH, Jacob KA. Supravalvular mitral stenosis in a cat. J Am Anim Hosp Assoc. 2002;38:403–406. doi: 10.5326/0380403. [DOI] [PubMed] [Google Scholar]
- 14.Campbell FE, Thomas WP. Congenital supravalvular mitral stenosis in 14 cats. J Vet Cardiol. 2012;14:281–292. doi: 10.1016/j.jvc.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Tidholm A, Ljungvall I, Michal J, Häggström J, Höglund K. Congenital heart defects in cats: A retrospective study of 162 cats (1996–2013) J Vet Cardiol. 2015;17:S215–S219. doi: 10.1016/j.jvc.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Weisse C, Soares N, Beal MW, Steffey MA, Drobatz KJ, Henry CJ. Survival times in dogs with right atrial hemangiosarcoma treated by means of surgical resection with or without adjuvant chemotherapy: 23 cases (1986–2000) J Am Vet Med Assoc. 2005;226:575–579. doi: 10.2460/javma.2005.226.575. [DOI] [PubMed] [Google Scholar]
- 17.Moores AL, Halfacree ZJ, Baines SJ, Lipscomb VJ. Indications, outcomes and complications following lateral thoracotomy in dogs and cats. J Small Anim Pract. 2007;48:695–698. doi: 10.1111/j.1748-5827.2007.00417.x. [DOI] [PubMed] [Google Scholar]