Abstract
Background:
Soluble urokinase-type plasminogen activator receptor (suPAR) is positively correlated with immune system activity. Inflammation can promote the development of chronic obstructive pulmonary disease (COPD). Therefore, this study conducted a systematic review and meta-analysis to assess the association between suPAR levels and the pathogenesis of COPD, and further assess the exact clinical value of suPAR in COPD.
Methods:
PubMed, Excerpt Medica Database (Embase), Web of Science (WOS), and Cochrane Library databases were searched for studies that reported the value of suPAR diagnosis for adult COPD patients.
Results:
A total of 11 studies were included, involving 4520 participants. Both COPD patients with predicted forced expiratory volume in 1 s (FEV1)⩾80% [weighted mean difference (WMD) = 320.25; 95% confidence interval (CI): 99.79–540.71] and FEV1 < 80% (WMD = 2950.74; 95% CI: 2647.06–3254.43) showed higher suPAR level. The sensitivity and specificity of suPAR for diagnosis of COPD were 87% and 79%, respectively, and AUC was 84%. This can not only effectively identify acute exacerbation of COPD (AECOPD) in a healthy population (WMD = 3114.77; 95% CI: 2814.66–3414.88), but also has the potential to distinguish AECOPD from stable COPD (WMD = 351.40; 95% CI: 215.88–486.93). There was a significant decrease of suPAR level after treatment [WMD = –1226.97; 95% CI: –1380.91– (–1073.03)].
Conclusion:
suPAR as a novel biomarker has potential for early diagnosis of COPD and prediction of AECOPD. There is a potential correlation between the level of suPAR and the state of COPD, which may also indicate the early state and severity of COPD. When the suPAR level of COPD patients is further increased, the risk of acute exacerbation increases and should be highly valued. This also shows potential as a measure of treatment response, and as a guide to the clinical management in COPD.
The reviews of this paper are available via the supplemental material section.
Keywords: biomarker, chronic obstructive pulmonary disease (COPD), meta-analysis, systematic review, soluble urokinase-type plasminogen activator receptor (suPAR)
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation and recurrent acute exacerbations, and is associated with chronic inhalation of noxious particles and gas-induced pulmonary inflammation.1,2 Acute exacerbation of COPD (AECOPD) is characterized by deterioration of the respiratory symptoms beyond normal daily variations, usually leading to adverse outcomes.3 The World Health Organization predicts that, by 2030, COPD will be the third leading cause of death, and may rise to the fifth-largest leading cause of disability-adjusted life years.4,5 Accurate and timely diagnosis will provide a strong guarantee for screening high-risk groups and improving the clinical course and outcome of COPD. However, the biomarkers used to assist the diagnosis and prediction of COPD are still insufficient.6
Soluble urokinase-type plasminogen activator receptor (suPAR) is a soluble form of the urokinase plasminogen activator receptor (uPAR), which is released by membrane-bound plasminogen activator, and is positively correlated with the activation of the immune system.7 suPAR is found in various body fluids, including blood, urine, and cerebrospinal fluid.8 It is expressed by endothelial cells, macrophages, monocytes, neutrophils, lymphocytes, and fibroblasts, and is upregulated by infection and pro-inflammatory cytokines.8 suPAR can promote plasminogen activation, cell adhesion, chemotaxis, and immune cell activation.9 In recent years, it has become a potential inflammation biomarker.10 Airway inflammation plays an important role in the development of COPD. Inflammatory products are the main source of destructive and structural changes in the pathological process of COPD.11 Previous studies suggest that serum suPAR may reflect the inflammatory process of COPD, and this increase may be particularly effective for patients with stages III and IV of the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD).12,13 Therefore, the level of suPAR has the potential to guide the diagnosis of COPD.
Biomarkers for COPD should be able to help clinical diagnose, determine the early state of the disease, predict acute exacerbation, and monitor responses to existing and new treatment strategies. This systematic review and meta-analysis aims to explore the clinical role of suPAR as an emerging biomarker in the diagnosis and prediction of COPD.
Materials and methods
All methods of this systematic review and meta-analysis analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14,15
Data sources and searches
The review authors searched for medical literature before October 2019. The research was conducted in electronic databases including the Cochrane Library, PubMed, the Excerpt Medica Database (Embase), the Web of Science (WOS), and the reference lists from review articles, irrespective of publication date, status or language. The search was conducted with the following keywords: suPAR and COPD or AECOPD. Search strategies used in the Cochrane Library, PubMed, Embase, and WOS can be found in the Supplemental material.
This meta-analysis included studies that met the following criteria:
Adult patients with confirmed or suspected COPD or AECOPD (over 18 years of age).
The studies included the results of suPAR levels in patients with COPD or AECOPD, the diagnosis value of suPAR, the prediction value of suPAR for AECOPD, or the prediction value in COPD treatment effect. COPD or AECOPD was diagnosed based on the latest reference standard during the study, such as the GOLD criteria.
No publication date, status, or language restrictions were applied. Clinical original articles were included, whereas secondary studies, conference abstracts, editorials, and animal experiments were excluded.
Study selection
Two review authors (QH and HX) independently assessed the studies to be included based on the titles, abstracts, and keywords. If a study was found relevant to our topic, at least two reviewers further evaluated its full text to see if it met the inclusion criteria. In case of inconsistencies between the reviewers, a third reviewer (J Liu) was consulted. The authors consulted the original authors to further ensure the eligibility of a study, when additional information on the details of the results and methods or allocation concealment was needed. A study diagram was prepared to illustrate the entire literature research process and the selection of the studies.
Data extraction and quality assessment
The data were independently extracted by two review authors (TS and CZ) and the resulting differences were resolved by a third reviewer (J Liu). The extracted data included the lead author, publication year, country of origin, participant characteristics [age, sex, hospitalization, predicted forced expiratory volume in 1 s (FEV1%), FEV1/forced vital capacity (FVC%), smokers number, and pack-years for ever-smokers], the measurement of suPAR levels and the suPAR levels of COPD patients and control group for studies including the results related to the diagnosis value of suPAR for COPD. The extracted data included the optimal cut-off threshold, values for sensitivity, specificity, true-positive, true-negative, false-positive, false-negative, and the area under the receiver operating characteristic (ROC) curve (AUC). If data were missing, a letter was written to the authors to request the data. If there was no response to the letter after 4 weeks, an email was sent. If there was no response to the email, estimates were made based on available data and used. The outcomes that cannot be pooled or analyzed are described in the literature.
Two review authors (MZ and J Lu) independently applied the guidelines of PRISMA statement16 to evaluate each involved study. The quality and bias of the included studies were assessed by two independent authors (MZ and J Lu). The Newcastle-Ottawa Scale (NOS) was used to assess the quality and bias of case control and cohort studies (Supplemental Table S1).17 As for the cross-sectional design, the Agency for Healthcare Research and Quality (AHRQ) was used for the quality and bias assessment (Table S2).18 If the included study contained the results of the diagnostic value of suPAR for COPD and the 2×2 contingency table, the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) was conducted to further assess the quality and bias (Table S3).19 In the case of any inconsistencies, an agreement was reached through discussion between all of the authors. The summary tables showed in supplemental material showed the assessment of the risk of bias.
Data synthesis and analysis
Extracted data were analyzed using Stata SE 14.0 (Stata Corp; College Station, TX, USA). Based on the outcomes, forest plots were made to demonstrate the cumulative effect of suPAR. Continuous variables were expressed as weighted mean difference (WMD) with a 95% confidence interval (CI). The pooled effect size was calculated by the fixed effect model. When significant heterogeneity (p < 0.05, I2 ⩾ 50%) was observed, a randomized effect model was applied. Subgroup analysis and sensitivity analysis were conducted to explore the source of heterogeneity.
Spearman’s correlation coefficient was used to evaluate the threshold of the diagnosis value of suPAR. The pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were calculated. The accuracy of the diagnostic effects was evaluated by constructing a summary receiver operating characteristic (SROC) curve (AUC).
Heterogeneity was assessed by the Q test (significant heterogeneity was indicated by p < 0.05) and the I2 test (significant heterogeneity was indicated by I2 > 50%). Funnel plots were generated to investigate the effects of smaller studies (the trend of intervention effects estimated in smaller studies may differ from that estimated in larger studies, which may be due to reporting biases, methodological or clinical heterogeneity, or other factors).20 If more than 10 studies are included, Egger’s test was prepared for publication bias.21 The value was set at 0.05.
Results
A total of 136 records were identified from the four electronic databases and the reference lists of review articles, with 70 remaining after the duplicates were removed. After screening the titles and abstracts, 34 articles were excluded. Of the 36 articles retrieved, 25 studies were excluded after full-text review because they did not meet the selection criteria. In summary, this systematic review and meta-analysis included 11 studies involving 4520 patients12,22–31 (Figure 1).
Figure 1.
PRISMA flow diagram and exclusion criteria.
PRISMA, preferred reporting items for systematic reviews and meta-analyses; suPAR, soluble urokinase-type plasminogen activator receptor; uPAR, urokinase-type plasminogen activator receptor.
The characteristics of the included trials are presented in Table 1. Three of the included studies were case-control studies,12,22,23 six prospective cohort studies,25–29,31 one retrospective cohort studies,24 and one cross-sectional study.30 Most trials diagnosed COPD based on the GOLD criteria. Five studies included participants from the outpatient department,12,22,25,27,28 and four included participants from the respiratory medicine.23,26,29,31 In terms of pulmonary function, six studies included patients with predicted FEV1% ⩾80%,12,22,23,26–28 and another five studies reported patients with the predicted FEV1% < 80%.12,25,29–31 The studies reported that the smoking status of patients ranged from 28 to 53 packs per year.
Table 1.
Characteristics of included studies.
| Author | Country (Year) | Study design | Clinical setting | Reference standard | Type of COPD | No. of participants | Average Age | FEV1 (% pred.) | FEV1/FVC (%) | Smokers (%) | Pack-years for ever-smokers | Tested sample | Measured time | Type of test used | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Böcskei et al.22 | Hungary (2019) | CC | OD | the GOLD criteria | SCOPD and AECOPD | 42 (45.2% male) | 59.0 | 87.80 ± 22.40 | 51.90 ± 12.70 | 76.2 | 33.90 ± 18.20 | plasma | NA | ELISA (ViroGates) | ①③④ |
| Godtfredsen et al.24 | Denmark (2018) | RC | ED | The ICD-10 diagnoses for COPD | SCOPD and AECOPD | 3290 (44.2% male) | 73.1 | NA | NA | NA | NA | plasma | NA | ELISA (ViroGates) | ④ |
| Liu and Li23 | China (2018) | CC | RM | the GOLD criteria | SCOPD | 130 (76.9% male) | 55.8 | NA | 63.10 ± 10.00 | NA | NA | Serum | D0 | ELISA (Raybiotech) | ① |
| AboEl-Magd and Mabrouk25 | Egypt (2018) | PC | OD or RM | the GOLD criteria | AECOPD | 65 (67.7% male) | 57.2 | 50.40 ± 19.83 | 54.53 ± 10.43 | 70.8 | 39.62 ± 9.56 | Serum | D1&D14 | ELISA (ViroGates) | ①②③ |
| Hu et al.26 | China (2017) | PC | RM | the GOLD criteria | SCOPD and AECOPD | 151 (69.5% male) | 61.4 | 80.65 ± 15.31 | 58.59 ± 18.92 | 70.9 | NA | Serum | D1 | NA | ①②④ |
| Wang et al.27 | China (2016) | PC | OD | the GOLD criteria | SCOPD | 135 (60.7% male) | 60.1 | 81.70 ± 19.24 | 57.67 ± 9.69 | 45.2 | 28.15 ± 20.48 | Serum | D1 | Luminex Screening Assay | ① |
| Böcskei et al.28 | Hungary (2016) | PC | OD | the GOLD criteria | SCOPD | 45 (84.4% male) | 59.4 | 81.37 ± 21.31 | NA | 55.6 | NA | plasma | D0 | ELISA (ViroGates) | ① |
| Kurtipek et al.29 | Turkey (2015) | PC | RM | Clinical symptoms | SCOPD and AECOPD | 107 (47.2% male) | 64.1 | 43.24 ± 14.29 | NA | NA | 44.51 ± 34.57 | plasma | D1 | ELISA (Eastbiopharm) | ②③④ |
| Gumus et al.30 | Turkey (2015) | CS | NA | the GOLD criteria | AECOPD | 73 (89.0% male) | 66.2 | 37.00 ± 12.00 | 51.00 ± 10.00 | 93.2 | 53.00 ± 20.00 | Serum | D0&D7 | ELISA (ViroGates) | ①②③ |
| Can et al.12 | Turkey (2014) | CC | OD | the GOLD criteria | SCOPD | 87 (80.5% male) | 55.9 | 45.32 ± 19.10 | NA | 59.8 | 39.63 ± 3.80 | Serum | D0 | ELISA (ViroGates) | ① |
| Portelli et al.31 | UK (2014) | PC | RM | the GOLD criteria | SCOPD | 323 (45.5% male) | 59.0 | 41.80 ± 18.52 | 57.53 ± 19.39 | NA | 31.25 ± 9.56 | Serum | D0 | Duoset ELISA (R&D Systems) | ① |
AECOPD, acute exacerbation of chronic obstructive lung disease; CC, case control study; COPD, chronic obstructive lung disease; CS, cross-sectional study; ED, emergency department; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GOLD, the Global Initiative for Chronic Obstructive Lung Disease; ICD-10, the International Classification of Diseases, 10th edition; NA, not available; OD, the Outpatient Department; PC, prospective cohort study; RC, retrospective cohort study; RM, Division of Respiratory Medicine; SCOPD, stable chronic obstructive lung disease.
①suPAR levels; ②diagnosis value of suPAR for COPD; ③value of suPAR for predicting COPD treatment effect; ④value of suPAR for predicting AECOPD.
The diagnosis value of suPAR in patients with COPD
In all, 10 studies used suPAR levels as the primary outcome.12,22,23,25–28,30,31 These studies provided the mean ± standard deviation (SD) of suPAR level and the number of subjects for the COPD patient group and the healthy control group. Among them, six studies included patients with predicted FEV1% ⩾ 80%, and five reported patients with predicted FEV1% < 80%. The randomized effect model presented that compared with the healthy group, the suPAR level of COPD patients was significantly increased (WMD = 1527.73; 95% CI: 763.78–2291.68; p < 0.001) (Figure 2). It was demonstrated that the elevated suPAR levels were effectively associated with a high risk of COPD.
Figure 2.
Forest plot of the suPAR level between COPD patients and healthy control.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, Forced expiratory volume in 1 s; suPAR, soluble urokinase-type plasminogen activator receptor; WMD, weighted mean difference.
In terms of the heterogeneity of the results (I2 = 97.1%; p < 0.001), we conducted a subgroup analysis based on the state of COPD. Compared with the healthy group, the suPAR level increased by 320.25 pg/ml in COPD patients with predicted FEV1% ⩾80% (WMD = 320.25; 95% CI: 99.79–540.71; p < 0.001), and the heterogeneity was I2 = 47.1% (p = 0.092). As for the patients with predicted FEV1% < 80%, the suPAR level increased by 2950.74 pg/ml (WMD = 2950.74; 95% CI: 2647.06–3254.43; p < 0.001), and the heterogeneity was insubstantial (I2 = 12.8%; p = 0.332) (Figure 2). Due to the different sample sizes of included studies, we generated funnel plots to investigate the effects of smaller studies. The result did not reveal evidence of statistical differences between the smaller trials and the larger trials (Figure S1). Egger’s test showed that the included studies were statistically unbiased (p = 0.060; Figure S2).
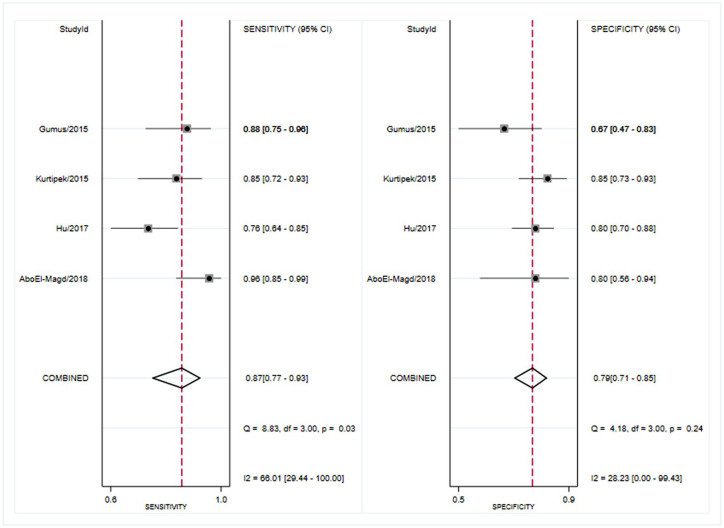
Four studies reported the results of the diagnosis value of suPAR.25,26,29,30 The pooled sensitivity of suPAR in the diagnosis of COPD was 0.87 [95% CI, 0.77–0.93; I2 = 66.01%, Q = 8.83 (p = 0.03)] and the specificity was 0.79 [95% CI, 0.71–0.85; I2 = 28.23%, Q = 4.18 (p = 0.24); Figure 3]. The PLR and NLR were 4.1 (95% CI, 3.0–5.6) and 0.17 (95% CI, 0.10–0.29), respectively, and the DOR was 24 (95% CI, 12–48). The AUC was 0.84 (95% CI, 0.81–0.87; Figure 4), indicating that suPAR has moderate diagnostic accuracy in COPD. In addition, there was no significant difference in threshold effect (Spearman correlation coefficient = 0.32; p = 0.68).
Figure 3.
Forest plot of the sensitivity and specificity of suPAR for the diagnosis of COPD.
COPD, chronic obstructive pulmonary disease.
Figure 4.
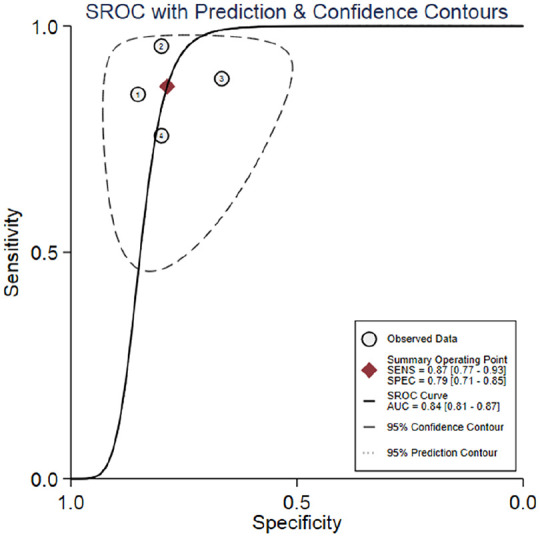
Summary receiver operating characteristics curve for studies evaluating the value of suPAR for the diagnosis of COPD.
AUC, area under the SROC curve; COPD, chronic obstructive pulmonary disease; SENS, sensitivity; SPEC, specificity; SROC, summary receiver operating characteristic; suPAR, soluble urokinase-type plasminogen activator receptor.
The predictive role of suPAR in COPD patients
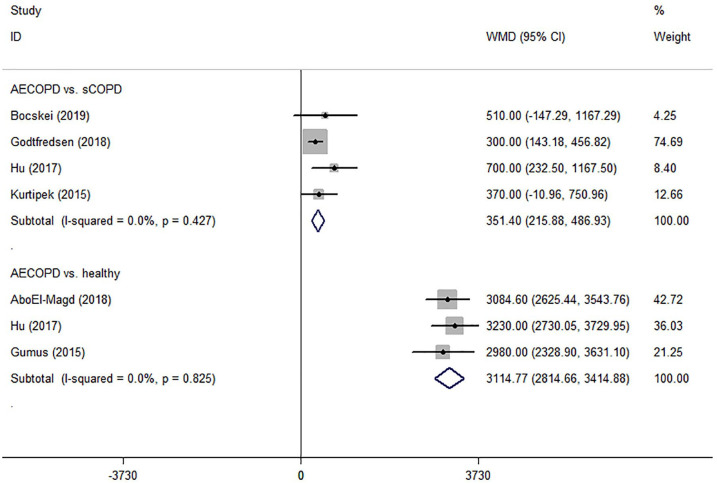
In terms of the prediction value of suPAR for AECOPD, six studies reported relevant results.22,24–26,29,30 Among them, four studies reported the value of suPAR in distinguishing AECOPD from stable COPD,22,24,26,29 and three reported the value in distinguishing AECOPD from healthy population.25,26,30 Compared with the stable COPD group, the level of suPAR increased by 351.40 pg/ml in AECOPD (WMD = 351.40; 95% CI: 215.88–486.93; p < 0.001), and no heterogeneity was found (I2 = 0.0%; p = 0.427). For the healthy population, suPAR levels increased notably, by 3114.77 pg/ml (WMD = 3114.77; 95% CI: 2814.66–3414.88; p < 0.001), and the heterogeneity was negligible (I2 = 0.0%; p = 0.825) (Figure 5). The results demonstrated that further increase in suPAR levels effectively indicates high risk of AECOPD and reflects the state of COPD.
Figure 5.
Forest plot of the prediction value of suPAR level for AECOPD.
AECOPD, Acute exacerbation of chronic obstructive pulmonary disease; CI, confidence interval; sCOPD, stable chronic obstructive lung disease; WMD, weighted mean difference.
In terms of the prediction value of suPAR for treatment response, four studies reported suPAR levels in COPD patients before and after treatment.22,25,29,30 Compared with the pre-treatment suPAR level, there was a significant decrease after treatment [WMD = –1226.97; 95% CI: –1380.91– (–1073.03); p < 0.001], and no heterogeneity was obtained (I2 = 0.0%, p = 0.635). This suggests that monitoring suPAR levels can be helpful in evaluating COPD treatment response (Table 2).
Table 2.
The clinical role of suPAR for COPD patients.
| The role of suPAR for COPD | Studies, no. | Patients, no. | WMD | 95% CI | p value | I2 (p value) |
|---|---|---|---|---|---|---|
| Diagnostic value for COPD (overall) | 11 | 1136 | 1527.73 | 763.78–2291.68 | <0.001 | 97.1% (p < 0.001) |
| Diagnosis early state of COPD with predicted FEV1 ⩾ 80% | 6 | 458 | 320.25 | 99.79–540.71 | <0.001 | 47.1% (p = 0.092) |
| Prediction of AECOPD (versus sCOPD) | 4 | 3534 | 351.40 | 215.88–486.93 | <0.001 | 0.0% (p = 0.427) |
| Prediction of AECOPD (versus healthy) | 3 | 242 | 3114.77 | 2814.66–3414.88 | <0.001 | 0.0% (p = 0.825) |
| Treatment response | 4 | 317 | −1226.97 | −1380.91– (–1073.03) | <0.001 | 0.0% (p = 0.635) |
AECOPD, Acute exacerbation of chronic obstructive pulmonary disease; CI, confidence interval; COPD, chronic obstructive lung disease; FEV1, forced expiratory volume in 1 s; sCOPD, stable chronic obstructive lung disease; suPAR, soluble urokinase-type plasminogen activator receptor; WMD, weighted mean difference.
Discussion
This meta-analysis found that suPAR, as a notable biomarker, has the potential to help diagnose and predict COPD patients. Elevated levels of suPAR were effectively associated with a high risk of COPD (WMD = 1527.73; 95% CI: 763.78–2291.68; p<0.001). And suPAR can also help diagnosis in the early stages of COPD. Compared with the healthy group, suPAR levels increased by 320.25 pg/ml in COPD patients with predicted FEV1% ⩾ 80%. Further investigation on the accuracy of suPAR in the diagnosis of COPD found that suPAR has a promising diagnostic value in COPD (SEN: 0.87, SPE: 0.79 and AUC: 0.84). As for the clinical value of AECOPD prediction, suPAR can not only effectively identify AECOPD from healthy population, but also has the potential to distinguish AECOPD from stable COPD. In addition, the results showed that suPAR can assess the treatment response of COPD, and the levels of suPAR decreased by 1226.97 pg/ml approximately after effective treatment.
Urokinase-type plasminogen activator (uPA) system is composed mainly of uPA, urokinase-type plasminogen activator receptor (uPAR) and urokinase-type plasminogen activator inhibitor (PAI-1). The latter play a major role in activating the immune system and inflammation.6,32 SuPAR is released by uPAR (the membrane-bound receptor for uPA). The researchers underlined that suPAR has an important impact on lung disease. Wang et al. demonstrated that uPAR levels in small airway epithelium of COPD patients were significantly higher than that of healthy controls, and also found that uPAR was significantly associated with the expression of vimentin.33 The increase of uPAR level in COPD patients contributes to the activity of the epithelial-mesenchymal transition process, and uPAR is also associated with airflow limitation. The uPA system plays a critical role in the development and pathogenesis of COPD by inducing inflammation and tissue remodeling, including parenchymal destruction and small airway fibrosis.34–36 This indicates that suPAR has the potential to assist the clinical management of COPD patients.
Form the results of this meta-analysis, we found that suPAR was significantly correlated with the status and severity of COPD. Previous original studies have shown that serum suPAR may reflect the inflammatory process of COPD, and this increase is particularly effective in patients with stages GOLD III and IV.12,13 This is consistent with our results, and we found that patients with predicted FEV1% < 80% had a significant increase in suPAR levels, at 2950.74 pg/ml. However, in existing studies, the potential value of suPAR in early-stage diagnosis is unclear. We found that patients with stage GOLD I (predicted FEV1% ⩾ 80%) had a higher suPAR level of 320.25 pg/ml compared with the healthy population, which was statistically significant. Considering the importance of early prediction and diagnosis in improving the clinical course and outcome of COPD,37 and the effective value of suPAR in predicting the severity of COPD, we suggest that suPAR can be a promising biomarker for patients with COPD.
Currently, biomarkers, including serum C-reaction protein (CRP), IL-6 and fibrinogen, are used routinely to assist the diagnosis of COPD. CRP is the first acute phase reactant discovered, and can be detected in serum as early as 4 h after injury.12,38 The study performed by Mahsuk Taylan et al. Indicated that CRP is a promising diagnostic biomarker for COPD.39 The corresponding sensitivity and specificity were 82.3% and 72.6%, respectively, and the AUC was 80%. In our study, the AUC of suPAR used to diagnose COPD was 84%, showing better accuracy than CRP. In addition, suPAR also exhibited higher sensitivity and specificity (SEN = 87%, SPE = 79%) than CRP. This indicates that suPAR has an effective diagnostic value in COPD. Although suPAR has potential as an early biomarker and has a superior diagnostic tendency, considering that only four studies have reported the diagnostic accuracy of suPAR in COPD patients, more high-quality original studies are needed to prove it.25,29
COPD patients with acute exacerbation require longer hospital stays, higher costs, ICU admission, and even mechanical ventilation. In the worst case, a small number of patients may eventually die without remission. Previous studies have shown that early prediction and intervention of these COPD patients can reduce poor outcomes and mortality.40 Therefore, it is important to assess and predict the severity of acute exacerbations in COPD patients.41,42 However, there is currently no consensus on the assessment and prediction of the severity of acute exacerbation. In this meta-analysis, we found that suPAR has the potential to predict acute exacerbation of COPD patients. As an early and effective biomarker, suPAR can not only distinguish AECOPD patients from healthy population, but also distinguish acute exacerbations from stable COPD. When the suPAR level of COPD patients is further elevated, the risk of acute exacerbation increased and should be highly valued. By comparing the suPAR level in the healthy control, COPD patients with early stage (predicted FEV1%⩾80%), patients with predicted FEV1% < 80% and AECOPD patients, we found that suPAR levels increased with the severity of COPD. This further indicates that suPAR levels are closely associated with the state of COPD.
As for the clinical value of suPAR in predicting treatment response, we found significant differences in suPAR levels before and after treatment in COPD patients. This suggests that suPAR has potential as a biological indicator of effective treatment. After treatment, the content of suPAR in COPD patients decreased by approximately 1226.97 pg/ml. This is consistent with the conclusion of the review conducted by Can et al.,43 which demonstrated that suPAR has the potential in the follow-up of COPD treatment. Indicating inflammation in COPD and assessing suPAR levels can play a key role in the evaluation of the inflammatory process in COPD.43
For significant degree of heterogeneity, subgroup analyses revealed that the pulmonary function of COPD patients substantially affected the heterogeneity due to the association between suPAR and COPD. In the subgroup analysis, the heterogeneity in both of the predicted FEV1% ⩾80% subgroup and predicted FEV1% < 80% subgroup were insubstantial (I2 = 47.1%, p = 0.092 and I2 = 12.8%, p = 0.332, respectively). As the heterogeneity of the predicted FEV1% ⩾80% subgroup was I2 = 47.1%, we further conducted a sensitivity analysis to explore the source of heterogeneity. From the result of sensitivity analysis, it showed that the main source of heterogeneity derived from the study by Wang et al. (Figure S3).27 When the study by Wang et al. was excluded from the analysis, I2 decreased to 0%. Compared with other included studies, Wang’s study further divided COPD population and healthy population into smokers and non-smokers subgroups. However, they did not clearly indicate the inclusion criteria for the subgroup, so the inclusion population of this study may be potentially different from other studies, which may lead to the increasing heterogeneity. As for the analysis of diagnosis value in COPD, only four studies reported relevant results. There are no significant threshold effects in diagnostic studies (Spearman correlation coefficient = 0.32; p = 0.68), but the cut-off value of suPAR can also account for the heterogeneity. High quality original research is still needed to prove this.
There are several limitations in the current meta-analysis. First, the level of suPAR is associated with a variety of diseases, including COPD and malignant tumors, kidney damage, and inflammatory bowel disease. This can lead to confounding factors that are difficult to evaluate in the included studies. Because the population included in this meta-analysis was COPD patients, combining the clinical symptoms of this population with suPAR levels to reduce the effects of confounding factors can still indicate the clinical value of suPAR for COPD patients. Second, because it is difficult to obtain original data for each study, we are unable to determine the optimal cut-off point for suPAR for the diagnosis of COPD. Third, only four studies reported the accuracy of suPAR in the diagnosis of COPD. Although this can also indicate the trend of suPAR diagnosis superiority, more original research is still needed. In addition, future studies in a larger series of patients and a control group composed of healthy population may reflect the inflammatory process of COPD patients based on plasma suPAR levels. Therefore, before being used in clinic, further study including more patients are needed to assess the suPAR level of COPD and AECOPD patients.
Conclusion
From the results of this systematic review and meta-analysis, suPAR as a novel biomarker has potential in early diagnosis of COPD and prediction of AECOPD. There is a potential correlation between the level of suPAR and the state of COPD, which may also indicate the early state of COPD. With further clinical research, the application of suPAR will contribute to clinical decision-making. When the suPAR level of COPD patients is further increased, the risk of acute exacerbation increases and should be highly valued. In addition, suPAR shows potential to measure the response of COPD therapy. Considering that there is currently no consensus on the assessment and prediction of COPD severity, and the importance of early diagnosis and prediction for treatment, suPAR should be considered for COPD.
Supplemental Material
Supplemental material, Author_Response for The clinical value of suPAR in diagnosis and prediction for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis by Qiangru Huang, Huaiyu Xiong, Tiankui Shuai, Yalei Wang, Chuchu Zhang, Meng Zhang, Lei Zhu, Jiaju Lu and Jian Liu in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for The clinical value of suPAR in diagnosis and prediction for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis by Qiangru Huang, Huaiyu Xiong, Tiankui Shuai, Yalei Wang, Chuchu Zhang, Meng Zhang, Lei Zhu, Jiaju Lu and Jian Liu in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for The clinical value of suPAR in diagnosis and prediction for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis by Qiangru Huang, Huaiyu Xiong, Tiankui Shuai, Yalei Wang, Chuchu Zhang, Meng Zhang, Lei Zhu, Jiaju Lu and Jian Liu in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplemental_material for The clinical value of suPAR in diagnosis and prediction for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis by Qiangru Huang, Huaiyu Xiong, Tiankui Shuai, Yalei Wang, Chuchu Zhang, Meng Zhang, Lei Zhu, Jiaju Lu and Jian Liu in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors gratefully acknowledge the support of the First Clinical Hospital of Lanzhou University, the first clinical medical college of Lanzhou University, Evidence-based Medicine Center of Lanzhou University, and all the authors who participated in this study.
Footnotes
Author contribution(s): Qiangru Huang: Data curation; Investigation; Methodology; Writing-original draft; Writing-review & editing.
Huaiyu Xiong: Data curation; Formal analysis; Writing-original draft.
Tiankui Shuai: Investigation; Methodology; Writing-original draft.
Yalei Wang: Formal analysis; Methodology; Writing-review & editing.
Chuchu Zhang: Formal Analysis; Writing-review & editing.
Meng Zhang: Methodology; Writing-review & editing.
Lei Zhu: Conceptualization; Writing-review & editing.
Jiaju Lu: Investigation; Writing-review & editing.
Jian Liu: Investigation; Supervision; Validation; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Jian Liu  https://orcid.org/0000-0002-1825-571X
https://orcid.org/0000-0002-1825-571X
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Qiangru Huang, Department of Intensive Care Unit, The First Hospital of Lanzhou University, Lanzhou, China; The First Clinical Medical College of the First Hospital of Lanzhou University, Lanzhou, China.
Huaiyu Xiong, Department of Intensive Care Unit, The First Hospital of Lanzhou University, Lanzhou, China; The First Clinical Medical College of the First Hospital of Lanzhou University, Lanzhou, China.
Tiankui Shuai, Department of Intensive Care Unit, The First Hospital of Lanzhou University, Lanzhou, China; The First Clinical Medical College of the First Hospital of Lanzhou University, Lanzhou, China.
Yalei Wang, Department of Intensive Care Unit, The First Hospital of Lanzhou University, Lanzhou, China; The First Clinical Medical College of the First Hospital of Lanzhou University, Lanzhou, China.
Chuchu Zhang, Department of Intensive Care Unit, The First Hospital of Lanzhou University, Lanzhou, China; The First Clinical Medical College of the First Hospital of Lanzhou University, Lanzhou, China.
Meng Zhang, Department of Intensive Care Unit, The First Hospital of Lanzhou University, Lanzhou, China; The First Clinical Medical College of the First Hospital of Lanzhou University, Lanzhou, China.
Lei Zhu, Department of Intensive Care Unit, The First Hospital of Lanzhou University, Lanzhou, China; The First Clinical Medical College of the First Hospital of Lanzhou University, Lanzhou, China.
Jiaju Lu, Department of Intensive Care Unit, The First Hospital of Lanzhou University, Lanzhou, China; The First Clinical Medical College of the First Hospital of Lanzhou University, Lanzhou, China.
Jian Liu, Department of Intensive Care Unit, The First Hospital of Lanzhou University, Lanzhou, 730000, China; The First Clinical Medical College of the First Hospital of Lanzhou University, Lanzhou, 730000, China.
References
- 1. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Archivos de bronconeumologia 2017; 53: 128–149. [DOI] [PubMed] [Google Scholar]
- 2. Pauwels RA. National and international guidelines for COPD: the need for evidence. Chest 2000; 117: 20s–22s. [DOI] [PubMed] [Google Scholar]
- 3. Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000; 117: 398s–401s. [DOI] [PubMed] [Google Scholar]
- 4. Mathers CD, Ezzati M, Lopez AD. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl Trop Dis 2007; 1: e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dicker D, Nguyen G, Abate D, et al. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018: 1684–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang Y, Xiao W, Zhang Y, et al. Urokinase-type plasminogen activator system and human cationic antimicrobial protein 18 in serum and induced sputum of patients with chronic obstructive pulmonary disease. Respirology 2010; 15: 939–946. [DOI] [PubMed] [Google Scholar]
- 7. Backes Y, van der Sluijs KF, Tuip de, Boer AM, et al. Soluble urokinase-type plasminogen activator receptor levels in patients with burn injuries and inhalation trauma requiring mechanical ventilation: an observational cohort study. Crit Care 2011; 15: R270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thuno M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers 2009; 27: 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eugen-Olsen J. suPAR—a future risk marker in bacteremia. J Intern Med 2011; 270: 29–31. [DOI] [PubMed] [Google Scholar]
- 10. Backes Y, van der Sluijs KF, Mackie DP, et al. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med 2012; 38: 1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gompertz S, O’Brien C, Bayley DL, et al. Changes in bronchial inflammation during acute exacerbations of chronic bronchitis. Eur Respir J 2001; 17: 1112–1119. [DOI] [PubMed] [Google Scholar]
- 12. Can U, Güzelant A, Yerlikaya FH, et al. The role of serum soluble Urokinase-type plasminogen activator receptor in stable chronic obstructive pulmonary disease. J Investig Med 2014; 62: 938–943. [DOI] [PubMed] [Google Scholar]
- 13. Wang Q, Wang Y, Zhang Y, et al. The role of uPAR in epithelial-mesenchymal transition in small airway epithelium of patients with chronic obstructive pulmonary disease. Respir Res 2013; 14: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang X, Chen Y, Yao L, et al. Reporting of declarations and conflicts of interest in WHO guidelines can be further improved. J Clin Epidemiol 2018; 98: 1–8. [DOI] [PubMed] [Google Scholar]
- 15. Ge L, Tian JH, Li YN, et al. Association between prospective registration and overall reporting and methodological quality of systematic reviews: a meta-epidemiological study. J Clin Epidemiol 2018; 93: 45–55. [DOI] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62: e1–e34. [DOI] [PubMed] [Google Scholar]
- 17. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 2014; 14: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith SR. Preface to the AHRQ supplement. J Gen Intern Med 2014; 29(Suppl. 3): S712–S713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. [DOI] [PubMed] [Google Scholar]
- 20. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Böcskei RM, Benczúr B, Losonczy G, et al. Soluble urokinase-type plasminogen activator receptor and arterial stiffness in patients with COPD. Lung 2019; 197: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Li R. Serum levels of suPAR, IL-8 and MMP-9 in patients with stable COPD and their significance. J Medicine 2018; 49: 50–53. [Google Scholar]
- 24. Godtfredsen NS, Jørgensen DV, Marsaa K, et al. Soluble urokinase plasminogen activator receptor predicts mortality in exacerbated COPD. Respir Res 2018; 19: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. AboEl-Magd GH, Mabrouk MM. Soluble urokinase-type plasminogen activator receptor as a measure of treatment response in acute exacerbation of COPD. J Bras Pneumol 2018; 44: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu Z, Wang J, Zhen J. Serum suPAR level in patients with AECOPD and its clinical diagnostic value. J Modern Medicine 2017; 45: 33–37. [Google Scholar]
- 27. Wang H, Yang T, Li D, et al. Elevated circulating PAI-1 levels are related to lung function decline, systemic inflammation, and small airway obstruction in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2016; 11: 2369–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Böcskei RM, Tamási L, Benczúr B, et al. Inflammatory markers (IL-6, suPAR) and arterial stiffness parameters in COPD patients. Eur Respir J 2016; 48: PA3670. [Google Scholar]
- 29. Kurtipek E, Kesli R, Bekçi TT, et al. Assessment of soluble urokinase-type plasminogen activator receptor (suPAR) in chronic obstructive pulmonary disease. Int Arch Med 2015; 8: 1–7. [Google Scholar]
- 30. Gumus A, Altintas N, Cinarka H, et al. Soluble urokinase-type plasminogen activator receptor is a novel biomarker predicting acute exacerbation in COPD. Int J Chron Obstruct Pulmon Dis 2015; 10: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Portelli MA, Siedlinski M, Stewart CE, et al. Genome-wide protein QTL mapping identifies human plasma kallikrein as a post-translational regulator of serum uPAR levels. Faseb J 2014; 28: 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Y, Xiao W, Jiang Y, et al. Levels of components of the urokinase-type plasminogen activator system are related to chronic obstructive pulmonary disease parenchymal destruction and airway remodelling. J Int Med Res 2012; 40: 976–985. [DOI] [PubMed] [Google Scholar]
- 33. Wang Q, Xiao W, Zhang Y, et al. The role of urokinase plasminogen activator receptor (UPAR) in epithelial-mesenchymal transition in small airway epithelium of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 189: A3017. [Google Scholar]
- 34. Wang IM, Stepaniants S, Boie Y, et al. Gene expression profiling in patients with chronic obstructive pulmonary disease and lung cancer. Am J Respir Crit Care Med 2008; 177: 402–411. [DOI] [PubMed] [Google Scholar]
- 35. Schuliga M, Harris T, Stewart AG. Plasminogen activation by airway smooth muscle is regulated by type I collagen. Am J Respir Cell Mol Biol 2011; 44: 831–839. [DOI] [PubMed] [Google Scholar]
- 36. Stewart CE, Hall IP, Parker SG, et al. PLAUR polymorphisms and lung function in UK smokers. BMC Med Genet 2009; 10: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agusti A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PloS One 2012; 7: e37483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lawn SD, Myer L, Bangani N, et al. Plasma levels of soluble urokinase-type plasminogen activator receptor (suPAR) and early mortality risk among patients enrolling for antiretroviral treatment in South Africa. BMC Infect Dis 2007; 7: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taylan M, Demir M, Kaya H, et al. Alterations of the neutrophil-lymphocyte ratio during the period of stable and acute exacerbation of chronic obstructive pulmonary disease patients. Clin Respir J 2017; 11: 311–317. [DOI] [PubMed] [Google Scholar]
- 40. Wilkinson TM, Donaldson GC, Hurst JR, et al. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 169: 1298–1303. [DOI] [PubMed] [Google Scholar]
- 41. Chandra D, Tsai CL, Camargo CA., Jr. Acute exacerbations of COPD: delay in presentation and the risk of hospitalization. COPD 2009; 6: 95–103. [DOI] [PubMed] [Google Scholar]
- 42. Seemungal TA, Wedzicha JA. Acute exacerbations of COPD: the challenge is early treatment. COPD 2009; 6: 79–81. [DOI] [PubMed] [Google Scholar]
- 43. Can U. The role of soluble urokinase-type plasminogen activator receptor (suPAR) in multiple respiratory diseases. Recept Clin Invest 2015; 2: e473. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response for The clinical value of suPAR in diagnosis and prediction for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis by Qiangru Huang, Huaiyu Xiong, Tiankui Shuai, Yalei Wang, Chuchu Zhang, Meng Zhang, Lei Zhu, Jiaju Lu and Jian Liu in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for The clinical value of suPAR in diagnosis and prediction for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis by Qiangru Huang, Huaiyu Xiong, Tiankui Shuai, Yalei Wang, Chuchu Zhang, Meng Zhang, Lei Zhu, Jiaju Lu and Jian Liu in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for The clinical value of suPAR in diagnosis and prediction for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis by Qiangru Huang, Huaiyu Xiong, Tiankui Shuai, Yalei Wang, Chuchu Zhang, Meng Zhang, Lei Zhu, Jiaju Lu and Jian Liu in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplemental_material for The clinical value of suPAR in diagnosis and prediction for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis by Qiangru Huang, Huaiyu Xiong, Tiankui Shuai, Yalei Wang, Chuchu Zhang, Meng Zhang, Lei Zhu, Jiaju Lu and Jian Liu in Therapeutic Advances in Respiratory Disease