Abstract
Purpose
Individuals receiving botulinum toxin A (BoNT-A) injections in the head and neck for migraine treatment have reported decreases in photophobia and sensations of dryness, independent of ocular surface parameters. We hypothesized that patients without migraine but with similar ocular neuropathic-like symptoms would also experience symptomatic improvement with periocular BoNT-A injections, independent of ocular surface changes.
Observations
We identified four individuals without a history of migraine but with neuropathic ocular pain (symptoms of dryness, burning, and photophobia that were out of proportion to ocular surface findings and unresponsive to ongoing dry eye (DE) therapies). Individuals underwent 1 session of periocular BoNT-A injections. Validated questionnaires (Visual Light Sensitivity Questionnaire-8, Dry Eye Questionnaire-5) assessed photophobia and DE symptoms pre- and 1-month post-injections. All four reported improvements in frequency and severity of photophobia and eye discomfort following BoNT-A injections. Tear film parameters (phenol red thread test, tear break-up time, corneal staining, and Schirmer test) and eyelid (palpebral fissure height and levator palpebrae superioris function) and eyebrow (position) anatomy were also evaluated before and after injections. Despite a unanimous improvement in symptoms, there were no consistent changes in ocular surface parameters with BoNT-A injections across individuals.
Conclusions
Periocular BoNT-A shows promise in reducing photophobia and sensations of dryness in individuals with neuropathic-like DE symptoms without a history of migraine, independent of tear film, eyelid, or eyebrow parameters.
Keywords: Neuropathic-like dry eye syndrome, Neuropathic ocular pain, Dry eye syndrome, Photophobia, Botulinum toxin A
1. Introduction
Within the umbrella of dry eye (DE), there is a subset of individuals in whom the degree of ocular pain surpasses what might be expected based on objective exam findings.1,2 In this group, neuropathic mechanisms are presumed to underlie symptoms. Neuropathic ocular pain (NOP) is caused by pathogenic mechanisms in the peripheral and central nervous system.3 Various ocular surface insults, including trauma, tear hyperosmolarity and environmental toxins, can damage trigeminal nerve endings, potentially leading to maladaptive neuronal plasticity with resultant atypical neuronal activity and hyper-responsiveness to stimuli. Chronic peripheral nerve stimulation can alter higher-order signaling neuronal pathways, a process known as central sensitization,4 whereby altered pain sensations, such as hyperalgesia (increased perception of pain) and allodynia (painful response to non-painful stimuli), become common features in both somatic and ocular pain.3,5,6 Risk factors for NOP include comorbid pain conditions such as fibromyalgia and migraine, as well as depression and anxiety, with central sensitization likely serving as the link between these various conditions.3
Treatment of NOP remains an active topic of research. Therapies first attempt to target nociceptive sources of pain (e.g. inflammation). Secondarily, treatments that affect neuronal function have been used such as autologous serum tears,7 alpha 2 delta ligands (gabapentin and pregabalin),8 stimulation therapies,9,10 and nerve blocks,8,11 with varying degrees of success. An emerging therapy for NOP is botulinum toxin, which has been used to treat post-herpetic neuralgia, trigeminal neuralgia, posttraumatic neuralgia, and painful diabetic neuropathy.12,13 Regarding the eye, a case series reported that botulinum toxin A (BoNT-A) injections in the head and neck were effective in reducing refractory photophobia in individuals with traumatic brain injury.14 Similarly, individuals receiving BoNT-A injections for chronic migraine reported a significant improvement in photophobia and DE symptoms with BoNT-A treatment that was independent of improvements in tear volume.15,16
Given potentially shared mechanisms between migraine and NOP, we hypothesized that individuals with NOP but without migraine may experience similar symptomatic improvement with periocular BoNT-A injections, independent of ocular surface changes. We identified four individuals with presumed NOP who received 1 session of periocular BoNT-A. All four reported improvements in frequency and severity of photophobia and eye discomfort with varying ocular surface changes 1 month after injections.
2. Findings
Demographics for each patient are displayed in Table 1. None had histories of migraine, BoNT-A treatment for migraine, or cataract or refractive surgery. All individuals were advised to continue current DE treatment regimen.
Table 1.
Basic demographics.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age | 69 | 55 | 35 | 57 |
| Gender | Male | Male | Male | Female |
| Race | White | White | White | White |
| Ethnicity | Non-Hispanic | Hispanic | Non-Hispanic | Hispanic |
| Current smoker | No | Yes | No | No |
| Ever smoker | Yes | Yes | No | No |
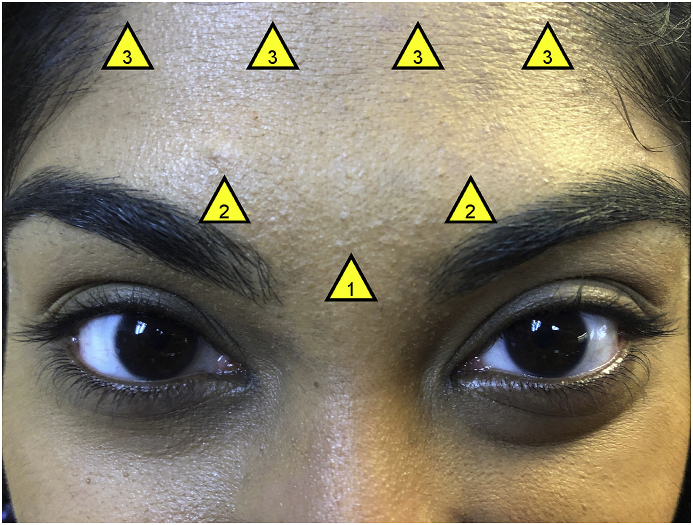
A modified migraine protocol using onabotulinumtoxinA was administered to all individuals (total 35 units, 7 injection sites: 5 units in procerus, 10 units in corrugators, 20 units in frontalis muscles), as shown in Fig. 1. Table 2, Table 3 display patient responses to validated questionnaires, Visual Light Sensitivity Questionnaire-8 (VLSQ-8)17 and Dry Eye Questionnaire-5 (DEQ-5)18, which assessed photophobia and DE symptoms pre- and 1-month post- BoNT-A injections. Tear film parameters (phenol red thread test, tear break-up time (TBUT), corneal staining according to the National Eye Institute's (NEI) scale, and Schirmer test) and eyelid (palpebral fissure height and levator palpebrae superioris function) and eyebrow (position as determined by two oculoplastic specialists) anatomy were also captured pre- and 1-month post-injection. One month was chosen as the follow-up timepoint as a previous study had detected significant reductions in photophobia and DE symptoms in individuals with chronic migraine about 1 month after injection with onabotulinumtoxinA.16
Fig. 1.
Injection Sites for Botulinum Toxin A Using a Modified Migraine Protocol. A total of 35 units were used, with 5 units at each injection site: (1) 5 units in procerus muscle, (2) 10 units in corrugator muscles, (3) 20 units in frontalis muscle. One of the authors is featured as the representative model.
Table 2.
Comparison of visual light sensitivity Questionnaire-8 (VLSQ-8) pre/post botulinum toxin A injection.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | ||
|---|---|---|---|---|---|
| Q1. In the past month, how often did you have visual light sensitivity outdoors during daylight? | Pre | 5 | 5 | 4 | 4 |
| Post | 3 | 3 | 3 | 2 | |
| Q2. In the past month, how often did you have a sense of glare in your eyes? | Pre | 5 | 5 | 4 | 3 |
| Post | 2 | 3 | 3 | 2 | |
| Q3. In the past month, how often did you have visual light sensitivity from flickering lights or bright colors? | Pre | 3 | 5 | 4 | 4 |
| Post | 2 | 3 | 4 | 2 | |
| Q4. Please rate the severity of the worst visual light sensitivity you experienced in the past month. | Pre | 5 | 5 | 5 | 4 |
| Post | 3 | 3 | 4 | 3 | |
| Q5. When you have sensitivity to light, do you also experience headache? | Pre | 1 | 5 | 3 | 3 |
| Post | 1 | 3 | 3 | 2 | |
| Q6. When you have sensitivity to light, how often is your vision blurry? | Pre | 1 | 5 | 2 | 2 |
| Post | 1 | 2 | 1 | 2 | |
| Q7. How often does sensitivity to light limit your ability to read, watch TV, or use the computer? | Pre | 5 | 5 | 4 | 4 |
| Post | 5 | 3 | 2 | 3 | |
| Q8. In the past month, how often did you need to wear dark glasses on cloudy days or indoors? | Pre | 5 | 5 | 4 | 2 |
| Post | 5 | 3 | 1 | 2 | |
| Total VLSQ-8 (scale 8–40) | Pre | 30 | 40 | 30 | 26 |
| Post | 22 | 23 | 21 | 18 | |
All questions except Question 4 were answered as 1 (never), 2 (rarely), 3 (sometimes), 4 (often), or 5 (always). Question 4 was answered from 1 to 5 with 1 as none, 3 as moderate, and 5 as severe.
Table 3.
Comparison of dry eye Questionnaire-5 (DEQ-5) pre/post botulinum toxin a injection.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | ||
|---|---|---|---|---|---|
| Q1a. During a typical day in the past month, how often did your eyes feel discomfort? | Pre | 4 | 4 | 3 | 4 |
| Post | 2 | 1 | 2 | 1 | |
| Q1b. When your eyes felt discomfort, how intense was this feeling of discomfort at the end of the day, within 2 h of going to bed? | Pre | 5 | 5 | 4 | 4 |
| Post | 3 | 2 | 3 | 1 | |
| Q2a. During a typical day in the past month, how often did your eyes feel dry? | Pre | 2 | 4 | 3 | 4 |
| Post | 2 | 2 | 2 | 1 | |
| Q2b. When your eyes felt dry, how intense was this feeling of dryness at the end of the day, within 2 h of going to bed? | Pre | 3 | 5 | 3 | 5 |
| Post | 1 | 2 | 3 | 1 | |
| Q3a. During a typical day in the past month, how often did your eyes look or feel excessively watery? | Pre | 1 | 1 | 0 | 0 |
| Post | 0 | 1 | 1 | 1 | |
| Total DEQ-5 (scale 0–22) | Pre | 15 | 19 | 13 | 17 |
| Post | 8 | 8 | 11 | 5 | |
All “a” questions were answered as 0 (never), 1 (rarely), 2 (sometimes), 3 (frequently), or 4 (constantly). All “b” questions were answered from 0 to 5 with 0 as “never have it”, 1 as “not at all intense”, and 5 as “very intense”.
2.1. Case 1
The first patient presented to clinic with complaints of severe “dryness” in both eyes for two years. Six months of preservative-free artificial tears and cyclosporine drops provided only minimal improvement. Ocular history was remarkable for exudative age-related macular degeneration in the left eye, for which he received regular intravitreal injections, and he had no relevant medical history other than a 60-pack year smoking history.
Prior to injections, VLSQ-8 and DEQ-5 questionnaires revealed a main complaint of light sensitivity and eye discomfort with mild sensations of dryness. Light sensitivity was rated as severe, necessitating the use of sunglasses on cloudy days and indoors and limiting the patient's ability to read, watch television, and use the computer. All tear film parameters were normal except for minimal corneal staining (both eyes: NEI total score 1).
One month following periocular BoNT-A injections, the patient reported that his photophobia and dry eye symptoms were “better” without any adverse side effects from treatment. VLSQ-8 on follow-up demonstrated notable improvement in frequency of light sensitivity to outdoor daylight, flickering lights, and bright colors. Frequency in functional limitation (wearing sunglasses on cloudy days or indoors; limitations in reading, watching television, using the computer) did not change, however, severity of light sensitivity improved from severe to moderate. DEQ-5 responses improved concerning frequency and severity of eye discomfort and dryness. Interestingly, follow-up examination demonstrated lower TBUT (right eye: 3 seconds, left eye: 5 seconds) and Schirmer tests (right eye: 9 mm, left eye: 6 mm) despite symptomatic improvement. The patient developed minimal brow ptosis with corresponding decrease in palpebral fissure height by 2 mm. All other examination parameters remained unchanged.
2.2. Case 2
The second patient presented with severe “dryness” and “light sensitivity” in both eyes for 1 year with persistence after using artificial tears for two months. The patient had no significant ocular history and was not using other drops. Medical history was significant for HIV infection, which was well-controlled with antiretroviral therapy. In addition, the patient reported a history of depression and anxiety, for which he took antidepressant and anxiolytic medication including gabapentin.
Prior to injections, the patient scored the maximum on the VLSQ-8, reporting highest severity and frequency of light sensitivity with resulting functional limitation (wearing sunglasses on cloudy days or indoors; limitations in reading, watching television, using the computer) and associated debilitating symptoms (headache, blurry vision, glare). The DEQ-5 also reflected maximal severity and frequency of eye discomfort and dryness. Discordant with the severity of these symptoms, ophthalmic examination was normal except for low TBUT (right eye: 5 seconds, left eye: 3 seconds). Brow ptosis was present prior to injections.
One month following periocular BoNT-A injections, the patient reported a “significant” improvement in visual health and reported that photophobia and DE symptoms were “better”. He denied any adverse effects from the injections. All VLSQ-8 responses improved in severity from “severe” to “moderate” and frequency of light sensitivity, functional impairment, and debilitating symptoms from “always” to “sometimes” or “rarely”. Similarly, all DEQ-5 questions pertaining to eye discomfort and dryness improved from severe to mild intensity and from constant frequency to intermittent or rare. All tear and eyelid parameters were normal on follow-up, with the only change from baseline being increased TBUT (right eye: 9 seconds, left eye: 12 seconds). Brow ptosis was unchanged.
2.3. Case 3
The third patient reported severe, bilateral, “burning pain” and “light sensitivity” for 1 year that started after a contact lens associated eye infection in the left eye. He had tried artificial tears, cyclosporine drops, lifitegrast drops, autologous serum tears, and punctal plugs with no improvement in pain. Additionally, he was treated for mild blepharitis and meibomian gland dysfunction with lid hygiene and warm compresses, both at home and with in-office procedures. He had no other significant ocular and medical history, and testing for Sjogren's antibodies was negative.
Prior to injections, the VLSQ-8 quantified light sensitivity of highest severity, resulting in frequent sunglass use on cloudy days and indoors and limitation in reading, watching television, and using the computer. Eye discomfort and dryness reported by the DEQ-5 was of moderate intensity and frequency. On clinical exam, TBUT (right eye: 8 seconds, left eye: 7 seconds) and Schirmer tests (right eye: 4 mm, left eye: 6 mm) were low with minimal corneal staining (both eyes: NEI total score 1), but all other tear film, eyelid, and eyebrow parameters were normal.
One month following periocular BoNT-A injections, the patient noted that his photophobia and DE symptoms were overall “better” without any adverse effects from the treatment. VSLQ-8 responses showed lower severity and frequency of light sensitivity, with largest improvement in areas of functional limitation from “often” to “never” wearing sunglasses on cloudy days and indoors and from “often” to “rarely” for limitation in reading, watching television, and using the computer. Likewise, DEQ-5 responses showed improvement in severity and frequency of eye discomfort and dryness. On exam, all tear film, eyelid and eyebrow parameters were normal, with increases in TBUT (both eyes: 20 seconds) and Schirmer tests (both eyes: 10 mm) and decreases in corneal staining (both eyes: NEI total score 0).
2.4. Case 4
The last patient had a history of receiving facial BoNT-A injections for cosmetic treatment of rhytids for five years, with the last treatment being 10 months prior. Interestingly, she noted that her DE symptoms seemed to improve with the injections. She was using artificial tears and cyclosporine drops with some improvement until symptoms significantly worsened after contracting viral conjunctivitis in both eyes. Following resolution of the conjunctivitis, her symptoms persisted and became intolerable despite minimal signs on clinical exam. She was thus offered periocular BoNT-A injections as treatment for her ocular pain. She had no other significant ocular history, and relevant medical history included hepatitis C infection treated four years prior.
Prior to injections, the patient reported moderate-to-severe intensity and frequency of light sensitivity via the VLSQ-8. She noted that the light sensitivity often limited her ability to read, watch television, and use the computer. Eye discomfort and dryness were reported with high severity and frequency through the DEQ-5. All ophthalmic examinations were normal except for a low TBUT (right eye: 5 seconds, left eye: 6 seconds).
One month following periocular BoNT-A injections, the patient noted a “significant” change to her visual health, with photophobia and dry eye symptoms being “much better”. She did not report any adverse effects. According to the VLSQ-8, light sensitivity improved to moderate severity and rare frequency, resulting in less functional limitations. Severity and frequency of eye discomfort and dryness as reported by the DEQ-5 also improved to mild intensity and rare occurrence. Despite the symptomatic improvement, clinical examination showed mostly unchanged tear film, eyelid, and eyebrow parameters except for a slight increase in corneal staining (both eyes: NEI total score 1).
3. Discussion
Botulinum toxin A (BoNT-A) is a neurotoxin derived from Clostridium botulinum that has been used therapeutically for a wide variety of disorders including cervical dystonia, chronic migraine, hyperhidrosis, urinary incontinence, strabismus, and blepharospasm, as well as for cosmetic purposes.19,20 This neurotoxin inhibits acetylcholine neurotransmitter release at presynaptic nerve terminals by interfering with vesicle fusion, thereby reducing muscle fiber activity.21, 22, 23 In addition, BoNT-A may dampen neurogenic inflammation and peripheral sensitization by inhibiting release of local nociceptive neuropeptides, such as substance P, calcitonin gene-related peptide (cGRP) and glutamate and decreasing expression of transient receptor potential vanilloid 1 (TRPV1).22,24,25 Given these effects, BoNT-A has increasingly been used to treat a variety of neuropathic pain disorders, including post-herpetic neuralgia, diabetic neuropathy, post-traumatic neuralgia, complex regional pain syndrome, trigeminal neuralgia, and occipital neuralgia.13,26
In the arena of neuropathic-like DE symptoms, prior studies have shown that BoNT-A injections improved photophobia and DE symptoms in individuals with underlying chronic migraine, independent of an improvement in tear film volume.15,16 These improvements were thus hypothesized to be driven by modulation of vascular and neural pathways shared by migraine pain, photophobia, and DE symptoms.
We hypothesized that BoNT-A could also improve photophobia and DE symptoms in individuals without a history of chronic migraine by similar mechanisms. First, BoNT-A can decrease the release of several inflammatory mediators, including cGRP, from pathologically altered peripheral corneal nociceptors.21,24,30, 31, 32, 33, 34, 35 With time, dampening of peripheral nerve activation can stabilize the afferent system and can potentially reverse changes in peripheral and central sensitization.25,30,31 Second, BoNT-A can reduce facial muscle contractions through its action at the neuromuscular junction, which may decrease signaling through primary trigeminal afferents. Previous studies have demonstrated that persistent muscle fiber activation leads to mechanical hyperalgesia and cGRP mediated central sensitization, an effect that is alleviated by BoNT-A.32,36,37
For chronic migraine, standard Food and Drug Administration on-label protocols include 155–195 unit BoNT-A injections across 31 to 39 sites representing 7 head and neck muscle groups including the corrugators, procerus, frontalis, temporalis, occipitalis, cervical paraspinal and trapezius muscle groups.23 In this study, BoNT-A was injected using a modified migraine protocol, targeting only muscles in the periocular area (procerus, corrugators, and frontalis muscles) for chemodenervation, as it was felt that these muscles were closest in proximity to trigeminal afferents on the corneal surface, to allow for more directed therapy.
Limitations of this case series include the inability to objectively determine the placebo effect of receiving BoNT-A injections, as all individuals knew that BoNT-A injections were being administered as off-label treatment for neuropathic-like DE symptoms and this could have subconsciously influenced their perceived symptoms and responses to questionnaires. As all individuals were followed 1-month post-injection, long-term response is unknown, but similar to other indications, repeat injections would likely be needed to maintain effect. Future larger and, ultimately, masked, placebo-controlled studies will be warranted to better elucidate the effects of BoNT-A injections for the treatment of neuropathic-like DE symptoms. In addition, studies evaluating the effect of BoNT-A injections on individuals with other DE sub-types may provide interesting insights as well.
4. Conclusions
In this case series, we found that periocular BoNT-A injections appeared to improve neuropathic-like DE symptoms in individuals without a history of migraine independent of eyelid, eyebrow and tear parameters. Results of the VLSQ-8 and DEQ-5 demonstrated noticeable reductions in photophobia and DE symptoms, suggesting that the symptomatic improvement seen in this series is not attributable to post-injection anatomic or tear film changes that may have provided additional corneal protection. This case series demonstrates that periocular BoNT-A injections shows promise in the treatment of NOP in individuals without a history of migraine.
Patient consent
The was conducted in accordance with the principles of the Declaration of Helsinki and complied with the requirements of the United States Health Insurance Portability and Accountability Act. The University of Miami Institutional Review Board approved the retrospective examination of records for this study. Consent to publish the report was not obtained as this report does not contain any personal information that could lead to the identification of any of the study's participants.
Funding
Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research I01 CX002015 (Dr. Galor), Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Dr. Galor), Department of Defense GW190010 (Dr. Galor), R01EY026174 (Dr. Galor), NIH Center Core Grant P30EY014801 and Research to Prevent Blindness Unrestricted Grant. Funding sources had no involvement in study design; in the collection, analysis or interpretation of data; in the writing of this report; or in the decision to submit the article for publication.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
CRediT authorship contribution statement
Nandini Venkateswaran: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - original draft, Visualization, Project administration. Jodi Hwang: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - original draft, Visualization, Project administration. Andrew J. Rong: Formal analysis, Investigation. Alexandra E. Levitt: Investigation. Ryan J. Diel: Conceptualization, Methodology. Roy C. Levitt: Formal analysis, Funding acquisition. Konstantinos D. Sarantopoulos: Formal analysis. Wendy W. Lee: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Project administration. Anat Galor: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
There are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Galor A., Feuer W., Lee D.J., Florez H., Venincasa V.D., Perez V.L. Ocular surface parameters in older male veterans. Invest Ophthalmol Vis Sci. 2013;54(2):1426–1433. doi: 10.1167/iovs.12-10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenthal P., Baran I., Jacobs D.S. Corneal pain without stain: is it real? Ocul Surf. 2009;7(1):28–40. doi: 10.1016/s1542-0124(12)70290-2. [DOI] [PubMed] [Google Scholar]
- 3.Galor A., Moein H.R., Lee C. Neuropathic pain and dry eye. Ocul Surf. 2018;16(1):31–44. doi: 10.1016/j.jtos.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crane A.M., Feuer W., Felix E.R. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br J Ophthalmol. 2017;101(9):1238–1243. doi: 10.1136/bjophthalmol-2016-309658. [DOI] [PubMed] [Google Scholar]
- 5.von Hehn C.A., Baron R., Woolf C.J. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73(4):638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholz J., Woolf C.J. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10(11):1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal S., Kheirkhah A., Cavalcanti B.M. Autologous serum tears for treatment of photoallodynia in patients with corneal neuropathy: efficacy and evaluation with in vivo confocal microscopy. Ocul Surf. 2015;13(3):250–262. doi: 10.1016/j.jtos.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small L.R., Galor A., Felix E.R., Horn D.B., Levitt R.C., Sarantopoulos C.D. Oral gabapentinoids and nerve blocks for the treatment of chronic ocular pain. Eye Contact Lens. 2020;46(3):174–181. doi: 10.1097/ICL.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 9.Sivanesan E., Levitt R.C., Sarantopoulos C.D., Patin D., Galor A. Noninvasive electrical stimulation for the treatment of chronic ocular pain and photophobia. Neuromodulation. 2018;21(8):727–734. doi: 10.1111/ner.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zayan K., Aggarwal S., Felix E., Levitt R., Sarantopoulos K., Galor A. Transcutaneous electrical nerve stimulation for the long-term treatment of ocular pain. Neuromodulation. 2020 doi: 10.1111/ner.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duerr E.R., Chang A., Venkateswaran N. Resolution of pain with periocular injections in a patient with a 7-year history of chronic ocular pain. Am J Ophthalmol Case Rep. 2019;14:35–38. doi: 10.1016/j.ajoc.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng F., Peng K., Yang J.P., Ji F.H., Xia F., Meng X.W. Botulinum toxin-A for the treatment of neuralgia: a systematic review and meta-analysis. J Pain Res. 2018;11:2343–2351. doi: 10.2147/JPR.S168650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittal S.O., Safarpour D., Jabbari B. Botulinum toxin treatment of neuropathic pain. Semin Neurol. 2016;36(1):73–83. doi: 10.1055/s-0036-1571953. [DOI] [PubMed] [Google Scholar]
- 14.Katz B.J., Digre K.B. Diagnosis, pathophysiology, and treatment of photophobia. Surv Ophthalmol. 2016;61(4):466–477. doi: 10.1016/j.survophthal.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Diel R.J., Kroeger Z.A., Levitt R.C. Botulinum toxin A for the treatment of photophobia and dry eye. Ophthalmology. 2018;125(1):139–140. doi: 10.1016/j.ophtha.2017.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diel R.J., Hwang J., Kroeger Z.A. Photophobia and sensations of dryness in patients with migraine occur independent of baseline tear volume and improve following botulinum toxin A injections. Br J Ophthalmol. 2019;103(8):1024–1029. doi: 10.1136/bjophthalmol-2018-312649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verriotto J.D., Gonzalez A., Aguilar M.C. New methods for quantification of visual photosensitivity threshold and symptoms. Transl Vis Sci Technol. 2017;6(4):18. doi: 10.1167/tvst.6.4.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalmers R.L., Begley C.G., Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Contact Lens Anterior Eye. 2010;33(2):55–60. doi: 10.1016/j.clae.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Kao I., Drachman D.B., Price D.L. Botulinum toxin: mechanism of presynaptic blockade. Science. 1976;193(4259):1256–1258. doi: 10.1126/science.785600. [DOI] [PubMed] [Google Scholar]
- 20.Simpson L.L. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981;33(3):155–188. [PubMed] [Google Scholar]
- 21.Wheeler A., Smith H.S. Botulinum toxins: mechanisms of action, antinociception and clinical applications. Toxicology. 2013;306:124–146. doi: 10.1016/j.tox.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Oh H.M., Chung M.E. Botulinum toxin for neuropathic pain: a review of the literature. Toxins. 2015;7(8):3127–3154. doi: 10.3390/toxins7083127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dodick D.W., Turkel C.C., DeGryse R.E. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921–936. doi: 10.1111/j.1526-4610.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 24.Murata Y., Masuko S. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res. 2006;1085(1):87–94. doi: 10.1016/j.brainres.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 25.Aoki K.R. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26(5):785–793. doi: 10.1016/j.neuro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Jeynes L.C., Gauci C.A. Evidence for the use of botulinum toxin in the chronic pain setting--a review of the literature. Pain Pract Off J World Inst Pain. 2008;8(4):269–276. doi: 10.1111/j.1533-2500.2008.00202.x. [DOI] [PubMed] [Google Scholar]
- 30.Aoki K.R. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache. 2003;43(Suppl 1):S9–S15. doi: 10.1046/j.1526-4610.43.7s.3.x. [DOI] [PubMed] [Google Scholar]
- 31.Aoki K.R., Francis J. Updates on the antinociceptive mechanism hypothesis of botulinum toxin A. Park Relat Disord. 2011;17(Suppl 1):S28–S33. doi: 10.1016/j.parkreldis.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Durham P.L. Diverse physiological roles of calcitonin gene-related peptide in migraine pathology: modulation of neuronal-glial-immune cells to promote peripheral and central sensitization. Curr Pain Headache Rep. 2016;20(8):48. doi: 10.1007/s11916-016-0578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaiser E.A., Kuburas A., Recober A., Russo A.F. Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J Neurosci Off J Soc Neurosci. 2012;32(44):15439–15449. doi: 10.1523/JNEUROSCI.3265-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Recober A., Kuburas A., Zhang Z., Wemmie J.A., Anderson M.G., Russo A.F. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci : Off J Soc Neurosci. 2009;29(27):8798–8804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belmonte C., Acosta M.C., Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78(3):513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 36.Dessem D., Ambalavanar R., Evancho M., Moutanni A., Yallampalli C., Bai G. Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X(3) expression in a functionally relevant manner. Pain. 2010;149(2):284–295. doi: 10.1016/j.pain.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gazerani P., Au S., Dong X., Kumar U., Arendt-Nielsen L., Cairns B.E. Botulinum neurotoxin type A (BoNTA) decreases the mechanical sensitivity of nociceptors and inhibits neurogenic vasodilation in a craniofacial muscle targeted for migraine prophylaxis. Pain. 2010;151(3):606–616. doi: 10.1016/j.pain.2010.07.029. [DOI] [PubMed] [Google Scholar]