Abstract
Background
Preliminary data showed prognostic impact of contrast-enhanced computed tomography (DCE-CT) identified Blood Volume (BV) in patients with metastatic renal cell carcinoma (mRCC). BV as an independent prognostic factor remains to be assessed.
Materials and Methods
DCE-CT identified BV was prospectively quantified in patients with mRCC receiving first line therapies, adjusted for International mRCC Database Consortium (IMDC) individual features and treatments, and associated with overall survival (OS), progression-free survival (PFS) and objective response (ORR), using Cox and logistic regression, respectively.
Results
105 patients with mRCC were included. Median baseline BV was 32.87 mL × 100 g−1 (range 9.52 to 92.87 mL × 100 g−1). BV above median was associated with IMDC favorable risk category (P = 0.004), metastasis free interval ≥ 1 year (P = 0.007), male gender (P = 0.032), normal hemoglobin (P = 0.040) and normal neutrophils (P = 0.007), whereas low BV was associated with poor risk IMDC features (P < 0.05). Patients with high vs. low baseline BV had longer PFS (12.5 vs. 5.6 months, P = 0.015) and longer OS (42.2 vs. 22.4 months, P = 0.001), respectively. In multivariate analysis high baseline BV remained independent favorable for OS (HR 0.49, 95% CI 0.30–0.78, P = 0.003) and PFS (HR 0.64; 95% CI 0.42–0.97, P = 0.036). BV as a continuous variable was also associated with OS in the multivariate analysis (HR 0.98, 95% CI 0.96–1.00, P = 0.017). The estimated concordance index (c-index) was 0.688 using IMDC score and 0.701 when BV was added.
Conclusions
DCE-CT identified Blood Volume is a new, independent prognostic factor in mRCC, which may improve the prognostic accuracy of IMDC.
Abbreviations: BV, blood volume; BF, blood flow; mRCC, metastatic renal cell carcinoma; DCE-CT, dynamic contrast-enhanced computed tomography; IMDC, International mRCC Database Consortium (IMDC); OS, overall survival; PFS, progression-free survival; ORR, objective response rate; HR, hazard ratio; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1; CE-CT, contrast-enhanced computed tomography; DaRenCa-1, Danish Renal Cancer Group Study-1; AIS, Angiogenesis Inhibitor Study; IL-2, interleukin 2; INF-α, interferon alpha; VOI, volume of interest; C-index, concordance index
Introduction
Treatment response in patients with metastatic cancer is evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) (1,2) using routine contrast-enhanced computed tomography (CE-CT) at baseline and during therapy. However, RECIST v1.1 solely monitors changes in tumor size which not necessarily reflects biological and physiological changes within the tumor (1,3,4). Improved understanding of tumor biology has led to rapid development of new biological targeted therapies and immunotherapies, but the imaging response evaluation tool has not kept up with this development pace, and RECIST v1.1 is still being used despite its limitations (5).
Dynamic Contrast-Enhanced Computed Tomography (DCE-CT) is a novel functional imaging modality. By performing repeated scans over a single target lesion, functional information on the contrast enhancement in tissue can be used to calculate perfusion parameters, such as Blood Volume (BV, mL × 100 g−1). These perfusion parameters correlate with tissue vascularity and can therefore be used to assess tumor vascularity ([6], [7], [8]). The technique offers a non-invasive quantification of tumor perfusion by histogram analysis of a whole target lesion. The best way to analyze the tumor histogram has been analyzed in detail; among seven different assessment methods (median, mean, mode, standard deviation, interquartile range, skewness and kurtosis) the median value was the best parameter to reflect the histogram (9). In patients with metastatic renal cell carcinoma (mRCC), previous studies have identified baseline DCE-CT assessed BV as the strongest functional imaging parameter to correlate with patient outcome, based on univariate analyses ([9], [10], [11]). However, the strength of the functional imaging parameter BV has not formally been tested against established clinical prognostic factors.
In mRCC, robust clinical prognostic factors have been established and validated. Based on patients included in clinical trials evaluating cytokine immunotherapy and chemotherapy at the Memorial Sloan Kettering Cancer Center (MSKCC), a total of five risk factors for poor overall survival (OS) were identified: poor Karnofsky performance status (KPS), time from diagnosis to oncological treatment <1 year, anemia, hypercalcemia, and elevated lactate dehydrogenase (12). Four of five features were later validated in a targeted therapy cohort (13); the model was supplemented with elevated neutrophils (14) and elevated platelets as independent poor prognostic factors. This prognostic model was later validated in an international cohort (15), named the International Metastatic renal cell carcinoma Database Consortium (IMDC). This model was able to stratify patients into clinically meaningful survival outcomes and able to predict the choice of therapy (16). To date, the IMDC risk category includes the strongest prognostic factors known to date in mRCC.
The aim of the present study was to evaluate the independent association of the DCE-CT identified translational factor BV compared with strong, validated, clinical prognostic factors included in IMDC, in order to identify the relatively strength and clinical relevance of DCE-CT identified BV.
Patients and methods
Patients and treatment
Patients with biopsy verified mRCC and measurable disease according to RECIST1.1 criteria were included into two prospective conducted studies at Aarhus University Hospital, Denmark, the Danish Renal Cancer Group Study-1 (DaRenCa-1) and the Angiogenesis Inhibitor Study (AIS). AIS (n = 33) comprised patients with first-line pazopanib (n = 12), temsirolimus (n = 9) or sunitinib (n = 12) enrolled between January 2012 and September 2016. DaRenCa-1 (n = 89) was a randomized phase II clinical trial that compared the efficacy of subcutaneously administered Interleukin 2 (IL-2) and interferon alpha (INF-α) with or without intravenously bevacizumab (n = 45 and n = 44, respectively) enrolled between October 2009 and November 2014; the clinical results have been published (17). Functional imaging was prospectively integrated in both studies to obtain translational information.
Signed informed consent was obtained before study entry. The Regional Ethics Committee and the Danish Data protection Agency approved the studies. The Darenca-1 study was approved by the Ethics Committee (case no. 1-10-72-472-12), the Data Protection Agency (journal no. 2012-41-0897), and the Danish Medicines Agency (journal no. 2612–4042); the study was registered at ClinicalTrials.gov (identifier NCT01274273). AIS was approved by the Ethics Committee (case no. 1-10-72-423-14) and the Data Protection Agency (journal no. 1-16-02-71-11).
Preliminary results have been published on 69 patients (DaRenCa-1, n = 50 and AIS, n = 19) evaluating various DCE-CT parameters (10,11) identifying BV as the best DCE-CT parameter using patient outcome as endpoint (9). For this study, more patients have been included, survival data has been updated, and all DCE-CT assessments have been fully repeated by an independent radiologist (ADN).
Patient medical files were used to retrieve information about treatment and baseline clinical factors such as age, gender, tumor histology, nephrectomy status and the IMDC risk features: low Karnofsky performance status (KPS) <80%, < 1 year from diagnosis to oncological treatment, hemoglobin <lower limit of normal (LLN) (serum hemoglobin <7.3 mmol/L for females and <8.3 mmol/L for males), albumin corrected calcium >upper limit of normal (ULN), neutrophils >ULN (neutrophils >7 * 109/L), and platelets >ULN (platelets >400*109/L for females and >350*109/L for males) (13).
CE-CT and DCE-CT
DCE-CT was performed at baseline over a single target lesion followed by a routine CE-CT of the thorax, abdomen and pelvis. An experienced radiologist was responsible for choosing the target lesion optimal for functional CT, MRI and ultrasound imaging, as well as core biopsy, based on prespecified criteria in the prospective studies (17). The current study is limited to the DCE-CT data.
Patients remained supine for 10 min between the DCE-CT and routine CE-CT. 60 mL iodixanol 270 mg I/mL at 6 mL/s was administered before the DCE-CT scan, and iodixanol 270 mg I/mL per kg body weight (maximum 180 mL) at 4 mL/s was given before the routine CE-CT scan.
A routine CE-CT scan was performed every three months until progression according to RECIST v1.1 (1,2), and was used for clinical decision making.
DCE-CT and routine CE-CT was performed using a Phillips Brilliance 64 or iCT 256, Phillips. DCE-CT was performed using 2 s scan cycles for 70 s.
Assessments
DCE-CT analysis was performed using the prototype software program Advanced Perfusion and Permeability Application, Philips, Best, The Netherlands. This method analyzed the volume of the target lesion by segmentation, and combined with the time dimension in DCE-CT due to repeated measurements, the assessment resulted in a 4-dimensional analysis. Firstly, the dynamic data was loaded in the software program, followed by a non-rigid registration used for spatial filtration and motion correction. Based on physiological principles, the software program processed the dynamic data and calculated the blood volume (BV, mL × 100 g−1) using the deconvolution method and displayed the corresponding blood volume maps ([6], [7], [8]). To assess the highest BV in the target lesion, the DCE-CT data was analyzed at arterial peak enhancement (PE). These data were loaded into Intellispace 6.0 Multimodality Tumor Tracking, Philips. The target lesion on the PE series was delineated on each CT scan slices using a semi-quantitative 3D sculpt-tool and defined as the volume of interest (VOI). All analyses were performed by a radiologist blinded to treatment and outcomes. Excellent interobserver correlations have been shown for this method (9).
Dynamic data using DCE-CT VOI were loaded and analyzed in MATLAB (v. R2015b, MathWorks Inc., Natick, MA, USA), where histogram values of BV were extracted based on DCE-CT VOI using in-house software. The median BV was calculated for each histogram, as this parameter was the most reproducible and had best correlation with patient outcome (9). Furthermore, Hounsfield Units for each target lesion was assessed at PE.
Statistical analysis
Progression-free survival (PFS) was defined as the time period between study inclusion and progression according to RECIST v1.1 or cancer-related death, whichever came first. Overall survival (OS) was defined as the time period between inclusion and death. Objective response rate (ORR) was defined as partial response (PR) or complete response (CR) as best overall response according to RECIST v1.1.
The association between baseline DCE-CT identified BV as a categorical (above/≤ median) variable and treatment group or clinical prognostic factors were tested using chi-squared test or Fischer exact test, as appropriate.
The correlation between BV as a continuous variable and the contrast enhancement measured as Hounsfield Units was assessed using Spearman rank correlation test.
Univariate Cox proportional Hazard model was used to assess the association of treatment, baseline clinical prognostic factors and baseline DCE-CT identified BV as a categorical and as a continuous variable with PFS and OS as endpoints. Univariate factors with P < 0.10 were tested for independence in a forward multivariate Cox proportional Hazard model, and results were expressed as a Hazard Ratio (HR) with 95% confidence intervals (CI). The assumptions of proportional Hazards were tested graphically at log(-logS(t)) vs. time. Interaction analysis in a Cox model was performed when relevant.
Concordance index (c-index) was calculated using the individual IMDC features followed by the addition of baseline BV as a categorical and continuous variable, respectively (18).
The association between baseline factors and ORR was analyzed using univariate logistic regression, and univariate factors with P < 0.10 were analyzed for independence using a forward multivariate logistic regression model and expressed as Odds Ratio (OR) with 95% CI.
All statistical tests were two-sided, and P < 0.05 was considered as statistically significant.
Analyses were performed using IBM SPSS Statistics for Windows (Version 25.0, Armonk, NY, USA, IBM Corp.) and STATA Statistical Software (Version 11.2, College Station, TX, USA, StataCorp LLC).
Results
Patients and baseline characteristics
Of 122 patients with mRCC included in the study, 105 patients (DaRenCa-1, n = 76 and AIS, n = 29) were included in the final analysis. 17 patients were excluded, either due to motion artifacts caused by lack of breath-hold compliance (n = 8), the combination of the target lesion being too small for analysis and located adjacent to diaphragm causing motion artifacts (n = 3), or improper scan range (n = 5). One patient was excluded because oncological treatment was never initiated due to bowel obstruction. Patients were treated with sunitinib (n = 10), temsirolimus (n = 8), pazopanib (n = 11), interferon alpha (INF-α) in combination with interleukin-2 (IL-2) and bevacizumab (n = 39), and IFN-α in combination with IL-2 (n = 37). With a median follow-up of 42.2 months (range: 0.9 to 106.5 months), the median PFS was 10.8 months and the median OS was 31.0 months.
Baseline patient characteristics including median target lesion volume are shown in Table 1. The median age was 60.1 years, 73% (77/105) were male gender, and 53% (56/105) had intermediate IMDC risk. Approximately half of the patients had anemia, while neutrophilia, thrombocytosis and hypercalcemia were seen in 15% (16/105), 23% (24/105) and 11% (11/105), respectively. 95% had KPS ≥80% (100/105), 93% had clear cell tumor histology (98/105), 83% had prior nephrectomy (87/105) and 73% had metastasis free interval < 1 year (77/105).
Table 1.
Baseline characteristics by blood volume.
| Factor | N | BV below median, n/N (%) | BV above median, n/N (%) | P |
|---|---|---|---|---|
| Total | 105 | 53 (49.5) | 52 (50.5) | |
| IMDC | ||||
| IMDC group | ||||
| Favorable | 22 | 5 (22.7) | 17 (77.3) | 0.004 |
| Intermediate | 56 | 29 (51.8) | 27 (48.2) | |
| Poor | 27 | 19 (70.4) | 8 (29.6) | |
| Karnoffsky Performance status | ||||
| ≥ 80% | 100 | 51 (51.0) | 49 (49.0) | 0.678a |
| < 80% | 5 | 2 (40.0) | 3 (60.0) | |
| Metastasis-free interval | ||||
| ≥ 1 year | 28 | 8 (28.6) | 20 (71.4) | 0.007 |
| < 1 year | 77 | 45 (58.4) | 32 (41.6) | |
| Hemoglobin | ||||
| ≥LLN | 48 | 19 (39.6) | 29 (60.4) | 0.040 |
| <LLN | 57 | 34 (59.6) | 23 (40.4) | |
| Neutrophils | ||||
| ≤ULN | 89 | 40 (44.9) | 49 (55.1) | 0.007 |
| >ULN | 16 | 13 (81.2) | 3 (18.8) | |
| Thrombocytes | ||||
| ≤ULN | 81 | 37 (45.7) | 44 (54.3) | 0.071 |
| >ULN | 24 | 16 (66.7) | 8 (33.3) | |
| Albumin corrected calcium | ||||
| ≤ULN | 94 | 46 (48.9) | 48 (51.1) | 0.356 |
| >ULN | 11 | 7 (63.6) | 4 (36.4) | |
| Other factors | ||||
| Age | ||||
| Below median (≤60.1 years) | 52 | 29 (55.8) | 23 (44.2) | 0.283 |
| Above median (>60.1 years) | 53 | 24 (45.3) | 29 (54.7) | |
| Gender | ||||
| Male | 77 | 34 (44.2) | 43 (55.8) | 0.032 |
| Female | 28 | 19 (67.9) | 9 (32.1) | |
| Histology | 0.437a | |||
| Clear cell | 98 | 48 (49.0) | 50 (51.0) | |
| Non clear cell | 7 | 5 (71.4) | 2 (28.6) | |
| Prior nephrectomy | ||||
| Yes | 87 | 43 (49.4)) | 44 (50.6) | 0.636 |
| No | 18 | 10 (55.6) | 8 (44.4) | |
| Target lesion volume at baseline | ||||
| Below median (≤18.32 cm3) | 53 | 24 (45.3) | 29 (54.7) | 0.283 |
| Above median (>18.32 cm3) | 52 | 29 (55.8) | 23 (44.2) | |
| Treatment | ||||
| Sunitinib, pazopanib or temsirolimus | 29 | 15 (51.7) | 14 (48.3) | 0.511 |
| IL-2, INF-α and bevacizumab | 39 | 17 (43.6) | 22 (56.4) | |
| IL-2 and IFN-α | 37 | 21 (56.8) | 16 (43.2) |
Abbrevations: ULN = Upper Limit of Normal, LLN = Lower Limit of Normal, IL-2 = Interleukin-2, IFN-α = interferon
alpha, BV = Blood Volume, IMDC = International Metastatic Renal Cell Carcinoma Consortium. Bold P-values indicate statistical significance at the P < 0.05 level.
Fischers exact test was performed.
The median baseline DCE-CT identified BV for target lesions combined was 32.87 mL × 100 g−1 (range 9.52 to 92.87 mL × 100 g−1). High baseline DCE-CT identified BV (categorical) was associated with favorable IMDC group (77.3% vs. 22.3% respectively, P = 0.004), male gender (55.8% vs. 44.2%, P = 0.032), normal hemoglobin (60.4% vs. 39.6%; P = 0.040), normal neutrophils (55.1% vs. 44.9% respectively; P = 0.007) and a metastasis-free interval ≥ 1 year (71.4% vs. 28.6%, P = 0.007). Low baseline DCE-CT identified BV (categorical) was associated with poor IMDC group (70.4% vs. 29.6%; P = 0.004), anemia (59.6% vs. 40.4%; P = 0.040), neutrophilia (81.2% vs. 18.8% respectively; P = 0.007) and a metastasis-free interval < 1 year (58.4% vs. 41.6%, P = 0.007). No association was found between baseline DCE-CT identified BV and treatment (P = 0.511) or baseline target lesion volume (P = 0.283), Table 1.
The median baseline Hounsfield Unit was 90.0 (range, −1.0–250.0), and Spearman's correlation coefficient between Hounsfield Unit and DCE-CT identified BV was 0.51, (P < 0.001).
The median DCE-CT identified BV categorized by target lesion organ locations is shown in Table 2, illustrating relatively homogenous median BV in different target lesion locations, with overlapping ranges. Higher BV was observed in supradiaphragmatic lymph nodes, pancreas, lung, and soft tissue component of bone metastasis; lower BV was seen in the primary tumor and infradiaphragmatic lymph nodes.
Table 2.
Tumor target lesions and corresponding median BV.
| Site of metastasis | N (%) | BV (mL × 100 g−1) | Range (mL × 100 g−1) |
|---|---|---|---|
| Lung | 21 (20.0) | 33.05 | 11.59–92.87 |
| Thoracic/supraclavicular lymph node | 16 (15.2) | 39.05 | 17.68–51.89 |
| Kidney | 14 (13.3) | 28.39 | 11.77–43.11 |
| Bone | 12 (11.4) | 39.04 | 10.03–70.03 |
| Liver | 8 (7.6) | 23.57 | 9.52–32.50 |
| Retroperitoneal lymph node | 8 (7.6) | 25.36 | 11.41–49.83 |
| Pancreas | 7(6.7) | 38.48 | 29.00–57.57 |
| Kidney bed | 6 (5.7) | 34.10 | 19.28–50.21 |
| Pleura | 5 (4.8) | 36.82 | 23.13–71.75 |
| Adrenal | 3 (2.9) | 27.71 | 13.75–41.04 |
| Soft Tissue | 5 (4.8) | 32.30 | 11.74–79.91 |
Abbreviation: BV = Blood Volume.
Univariate analyses of baseline characteristics and outcome
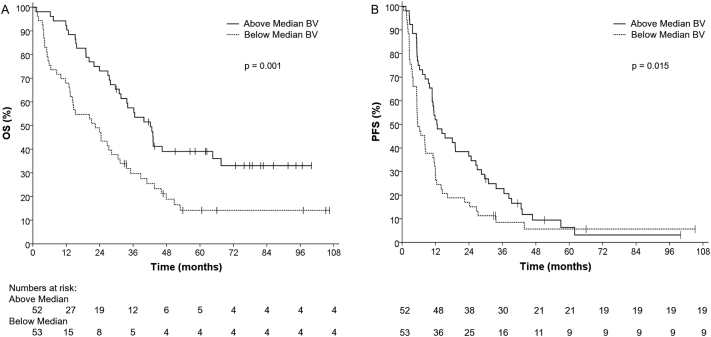
High baseline DCE-CT identified BV (categorical) was associated with favorable PFS (12.5 vs. 5.6 months; P = 0.015) and OS (42.2 vs. 22.4 months; P = 0.001), Fig. 1, Fig. 2. High baseline DCE-CT identified BV was also associated with favorable OS when assessed as a continuous variable (HR = 0.97; P = 0.003). Clinical risk factors associated with poor OS were KPS <80% (HR = 4.46; P = 0.002), anemia (HR = 2.15; P = 0.001), neutrophilia (HR = 4.42; P < 0.001) and thrombocytopenia (HR = 2.04; P = 0.007), Table 3.
Fig. 1.
Baseline high blood volume (BV) is associated with favorable patient outcome (A) Progression-free Survival and (B) Overall Survival.
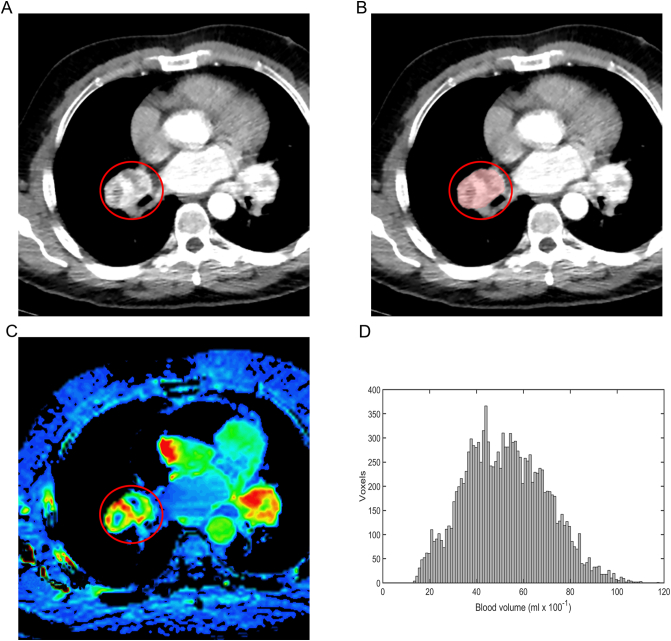
Fig. 2.
depicts a renal cell carcinoma metastasis located in a bronchopulmonary lymph node (marked with a red ring). Analysis showed a high baseline blood volume (BV) (51.89 mL × 100 g−1). The patient had progression free survival of 10.8 months and overall survival of 36.8 months. The metastasis is shown on a contrast-enhanced CT (A), delineated on a dynamic contrast enhanced CT (B) and shown on a BV map on a dynamic contrast enhanced CT (C). D depicts the corresponding BV histogram for the whole metastasis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Univariable analysis of the effect of baseline features on patient outcome.
| Factor | OS |
PFS |
ORR |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | OR (95% CI) | P | |
| Baseline BV above median (categorical) | 0.49 (0.31;0.77) | 0.002 | 0.61 (0.41;0.91) | 0.016 | 2.01 (0.89;4.52) | 0.091 |
| Baseline BV above median (continuous) | 0.97 (0.96;0.99) | 0.003 | 0.99 (0.97;1.00) | 0.106 | 1.02 (1.00;1.05) | 0.118 |
| Karnoffsky Performance status <80% | 4.46 (1.76;11.27) | 0.002 | 1.78 (0.72;4.41) | 0.210 | – | |
| Metastasisfree interval < 1 year | 1.63 (0.95;2.79) | 0.077 | 1.42 (0.90;2.24) | 0.135 | 1.03 (0.42;2.53) | 0.951 |
| Hemoglobin < LLN | 2.15 (1.35;3.42) | 0.001 | 1.22 (0.82;1.82) | 0.330 | 0.55 (0.24;1.22) | 0.141 |
| Neutrophils > ULN | 4.42 (2.46;7.96) | <0.001 | 2.76 (1.58;4.85) | <0.001 | 0.09 (0.01;0.74) | 0.025 |
| Thrombocytes > ULN | 2.04 (1.22;3.40) | 0.007 | 1.45 (0.91;2.32) | 0.119 | 0.85 (0.33:2.22) | 0.740 |
| Albumin corrected calcium > ULN | 1.34 (0.65;2.80) | 0.430 | 1.23 (0.64;2.38) | 0.531 | 0.63 (0.16;2.54) | 0.518 |
| Female gender | 0.74 (0.45;1.21) | 0.230 | 1.63 (1.04;2;56) | 0.034 | 1.28 (0.51;3.20) | 0.603 |
| Age below median | 0.95 (0.61;1.49) | 0.832 | 1.52 (1.01;2.28) | 0.044 | 0.97 (0.44;2.15) | 0.941 |
| Treatmentgroup | 0.092 | 0.019 | 0.397 | |||
| Sunitinib, pazopanib or temsirolimus | Reference | Reference | Reference | |||
| IL-2, INF-α and bevacizumab | 0.60 (0.35;1.06) | 1.65 (0.99;2.76) | 2.03 (0.72;5.69) | |||
| IL-2 and IFN-α | 0.55 (0.31;0.98) | 2.12 (1.25;3.58) | 1.42 (0.49;4.10) | |||
Abbreviations: ULN = Upper Limit of Normal, LLN = Lower Limit of Normal, IL-2 = Interleukin-2, IFN-α = interferon alpha, BV = Blood Volume. Bold P-values indicate statistical significance at the P < 0.10 level. These univariate factors were included in a forward multivariate Cox proportional Hazard model.
High baseline DCE-CT identified BV (categorical) was associated with favorable PFS (HR = 0.61; P = 0.016) whereas neutrophilia (HR = 2.76; P < 0.001), female gender (HR = 1.63; P = 0.034) and age below median (HR = 1.52; P = 0.044) were associated with poor PFS, Table 3.
Treatment with IL-2/INF-α/ bevacizumab or IL-2/IFN-α compared to treatment with pazopanib, sunitinib or temsirolimus were associated with shorter PFS (HR = 1.65 and HR = 2.12 respectively; P = 0.019), but were not associated with OS (P = 0.092), Table 3. Treatment with IL-2/IFN-α compared to the other treatments was associated with shorter PFS (HR = 1.61; P = 0.028), but was not associated with OS (HR = 0.76; P = 0.245).
Univariate analyses of baseline characteristics and ORR
CR rate was 2.9% and PR rate was 33.3% resulting in an ORR of 36.2%. The stable disease (SD) rate was 44.8%, and the progressive disease (PD) rate was 19.0%. Neutrophilia was associated with a lower ORR (OR = 0.09; P = 0.025). High baseline DCE-CT identified BV (categorical) was associated with higher ORR, but this was not statistically significant (OR = 2.01; P = 0.091), Table 3.
Multivariate analyses of baseline clinical factors and outcome
High baseline DCE-CT identified BV remained significantly associated with favorable OS, both as a categorical (HR = 0.49; P = 0.003) and as a continuous variable (HR 0.98; P = 0.017), Table 4. Neutrophilia and KPS <80% remained independently associated with poor OS (HR = 4.25; P < 0.001 and HR = 7.39; P < 0.001), respectively.
Table 4.
Multivariable analysis of the effect of baseline features on probability of overall survival and progression free survival.
| Factor | OS |
PFS |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| BV above median (categorical) | 0.49 (0.30;0.78) | 0.003 | 0.64 (0.42;0.97) | 0.036 |
| Karnofsky Performance status <80% | 7.39 (2.81;19.46) | <0.001 | – | |
| Neutrophils > ULN | 4.25 (2.32;7.76) | <0.001 | 3.15 (1.74;5.73) | <0.001 |
Abbreviations: ULN = Upper Limit of Normal, LLN = Lower Limit of Normal, IL-2 = Interleukin-2, IFN-α = interferon alpha. Bold P-values indicate statistical significance at the P < 0.05 level.
High baseline DCE-CT identified BV as a categorical variable remained significantly associated with favorable PFS (HR = 0.64; P = 0.036), but not as a continuous variable. Neutrophilia remained independently associated with poor PFS for DCE-CT identified BV as a categorical variable (HR = 3.15; P < 0.001) and as a continuous variable (HR = 3.18; P < 0.001), respectively.
The estimated c-index was 0.688 when assessed with each individual IMDC risk feature and improved to 0.698 when DCE-CT identified BV as a continuous variable was added and to 0.701 when DCE-CT identified BV as categorical variable was added.
A non-significant interaction indicated that the effect of DCE-CT identified BV as a categorical variable on PFS was independent of treatment group, regardless of the groups being stratified in IL-2/IFN-α compared to all the other treatments (P = 0.828), or treatment with IL-2 based therapies compared to treatment with angiogenesis inhibitors (P = 0.116). No association was found between treatment group and OS regardless of stratifying the treatment groups into IL-2/IFN-α compared to all other treatments, or treatment with IL-2 based therapies compared to treatment with angiogenesis inhibitors.
Multivariate analyses of baseline clinical factors and ORR
Patients with neutrophilia were 9 times less likely to respond to treatment (OR = 0.11; P = 0.038). Patients with a high baseline BV (categorical) were 1.6 times more likely to respond to treatment; this was, however, not statistically significant (OR = 1.56; P = 0.299).
Discussion
This is the first study to demonstrate the functional imaging parameter blood volume (BV), identified by DCE-CT, as a strong and independent prognostic factor in patients with mRCC. This technique offers non-invasive quantification of tumor perfusion and may provide functional information in tumor monitoring. To our knowledge, no previous study has evaluated the association of baseline DCE-CT identified BV with strong, established, baseline clinical prognostic factors; we demonstrated BV to be of clinical relevance and statistical certainty. DCE-CT identified BV was associated with IMDC prognostic factors: high baseline BV with favorable IMDC prognostic factors, and low baseline BV with poor IMDC prognostic factors. These results indicate that baseline DCE-CT identified BV contains important information about tumor biology. Baseline BV assessed as a categorical variable was associated with OS and PFS, and baseline BV assessed as a continuous variable was associated with OS. The prognostic value of baseline BV was also independent from the treatment used. Our study suggests that baseline BV is a true prognostic factor, independent of individual IMDC risk features and treatments.
Heng et al. performed an external validation of the IMDC prognostic model and compared the performance of IMDC with four established prognostic risk scores and found IMDC to have the best prognostic accuracy, i.e., the highest c-index (15). We therefore used the individual IMDC factors for comparison in our study, and observed an improvement in c-index from 0.688 to 0.701 when adding DCE-CT identified BV to the IMDC risk score. Therefore, DCE-CT identified BV improved the prognostic accuracy compared with IMDC alone. The implication of our study is that DCE-CT identified BV represents a new category of prognostic factors in mRCC and has the potential to serve as a supplement to the IMDC risk stratification (13) for patient counseling and treatment decisions. However, the incorporation of DCE-CT identified BV on top of the IMDC criteria requires validation of the results in a prospective study with a larger population. Further research in functional imaging is encouraged, integrating functional imaging in a prospective clinical trial design, with the median baseline DCE-CT identified BV value of 32.87 mL × 100 g−1 used as a reference value.
Choi was the first to integrate functional information in response evaluation by combining morphological and functional information, by measuring CT contrast uptake (Hounsfield units), reflecting tumor vascularity in a target lesion (19). A great limitation to this method is that the CT contrast uptake is only measured on a single CT slice not taking in to account the intratumoral heterogenicity (19). Another limitation of the Choi method is that the CT contrast uptake in the target lesion is not assessed at peak arterial enhancement. However, the non-invasive 4D imaging technique DCE-CT (6) can assess the CT contrast uptake and functional parameters such as BV and blood flow (BF) in the entire target lesion at peak arterial enhancement. The difference in the Choi Criteria and the 4D DCE-CT technique can be illustrated by a single core biopsy, i.e., performing a small sampling from the entire lesion (the Choi method); not taking account for intratumoral heterogenicity and assessment of the entire tumor lesion (as the 4D DCE-CT technique does). Our study used the 4D DCE-CT technique, and only found a moderate correlation between Hounsfield Units and DCE-CT identified BV. Thus, Hounsfield Units cannot be used as a substitute for DCE-CT identified BV.
BV is correlated to tumor microvessel density (7) and reflects vascularity. BV is unaffected by cardiac output (7), which may be relevant since cardiovascular toxicity is a potential effect of angiogenesis inhibitors (20) and interleukin-2 (21). However, the use of the bolus tracking scanning technique used in our study compensated changes in cardiac output (22).
The median BV in this current study was 32.87 mL × 100 g−1 and the range of BV was wide (9.52 to 92.87 mL × 100 g−1). High BV reflected a high vascularization and correlated with better outcome, whereas low BV reflected low vascularization and correlated with worse outcome. These findings are consistent with data on other tumor types, where it has been established that tumor cells survive in a microenvironment with low hypoxia, leading to therapy resistance (23). Chen et al. found a significant difference in BV of the normal kidney cortex (mean 23.53 ± 5.71 mL × 100 g−1) and the primary kidney tumor (mean 17.17 ± 8.34 mL × 100 g−1) (24). The authors did not find an association between BV of normal kidney cortex and the primary kidney tumor, indicating that the vascularity of RCC distinguishes clearly from the vascularity of the normal kidney cortex.
Our measurements of baseline BV categorized by target lesion localization mainly reflect the clinical course based on the metastatic pattern in patients with mRCC. We observed higher BV in supradiaphragmatic than in infradiaphragmatic lymph node metastases. The IMDC showed that supradiaphragmatic lymph node metastasis had better outcome than infradiaphragmatic lymph nodes (25), consistent with our data. We showed high median baseline BV in pancreas and lung metastasis consistent with the clinical experience of better outcome in patients with these metastatic sites (26). We showed low BV in the primary kidney tumor and in patients with liver metastasis consistent with poor outcome in patients with the primary tumor in place (27) or liver metastasis (28). However, we found high baseline BV in the soft tissue component of bone metastasis. This seems in conflict with clinical data showing poor outcome in patients with bone metastasis (28). In our study, we only analyzed a bone lesion if a soft tissue component to the bone lesion was present; this might explain the difference in results. Overall, the baseline functional imaging parameter BV seemed to be a reflection of the underlying tumor biology.
The present DCE-CT study is based on data from the largest mRCC cohort examined to date. Fournier et al. found no association between baseline BV and BF and survival outcomes in 25 patients with mRCC treated with the angiogenesis inhibitors sorafenib and sunitinib, but found an association between high BV and BF and increased treatment response (29). The study population, however, was small, which could explain the lack of significant associations. Ng et al. found no association between baseline BV and PFS in 28 patients with mRCC treated with INF-α, but found that high BF was negatively associated to PFS (30) indicating that tumors with high vascularity at baseline have worse prognosis. In contrast, high baseline BV was positively associated with OS and PFS in our study, suggesting that tumors with high vascularity at baseline have better survival outcomes. Even though BV and BF were calculated differently, each parameter is correlated to tumor vascularity (7) and therefore approximate. In the study of Ng et al., where the patients were treated with INF-α only, the population had a minimal clinical effect with a median PFS of 5.3 months and only 6.7% of the population (one patient) achieved partial response (30). These findings indicate that the effect of the INF-α was marginal and could be compared with no therapy, which could explain the conflicting results. Furthermore, Ng. et al. based their analysis on the average of four CT slices not taking account for the intratumoral heterogenicity, whereas our study was based on 4-dimentional analysis of the entire target lesion, making our assessments of BV more accurate. It should be noted that none of these studies integrated and evaluated the functional imaging parameter with robust well-established clinical factors, as we did in the present study. Moreover, our previous work identified BV as a prognostic factor using quartiles, i.e. the strongest signal, as cutoffs (10,11). In the present study including more patients, we were able to identify the prognostic impact of BV using the median as cutoff, thereby improving the robustness of analyses.
Previous studies have found an association between Dynamic Contrast-Enhanced Ultrasound (DCE-US) parameters and survival outcome in mRCC (31,32). However, DCE-US cannot be used to evaluate metastasis in the lungs and brain, limiting this modality. As all patients have routine CT, the addition of DCE-CT functional imaging seems appealing.
Given recent advances in knowledge and the number of recent drug approvals, most patients in the current study would be treated differently today. Immune checkpoint inhibitors, such as nivolumab and ipilimumab, have largely replaced the use of IL-2 based immunotherapy and targeted therapy in mRCC. However, targeted therapy may still be offered to patients unfit for or failing checkpoint immunotherapy. Moreover, our data showed that baseline BV was independent of the treatments provided. Further studies in functional imaging are encouraged in patients treated with checkpoint inhibitors.
There were limitations to our study. Although patients were instructed in shallow breathing during the scan, motion artifacts occurred. The increased radiation dose of DCE-CT compared to routine CE-CT caused a larger stochastic risk for a radiation-induced cancer. However, the reduced life expectancy in patients with mRCC makes this risk minimal. The short z-axis of DCE-CT (maximum 8 cm) often resulted in only one lesion being scanned, potentially this lesions was not necessarily representative of other lesions due to intertumoral heterogeneity (33). A strength to our study is that we balanced the translational parameter BV with established clinical factors in multivariate testing and demonstrated statistical certainty and clinical relevance of the translational factor.
In conclusion, Dynamic Contrast-Enhanced Computed Tomography identified Blood Volume is a new independent prognostic factor in mRCC, which may improve the prognostic accuracy of IMDC.
Acknowledgments
Acknowledgements
We wish to acknowledge Phillips Healthcare, who provided the software used for the imaging analysis and Roche and Novartis, who supported the clinical part of the study financially, but were not involved in the imaging analysis. We also wish to acknowledge Ipsen and The Health Research Foundation of Central Denmark for supporting the study financially.
Funding
Phillips Healthcare (grant number not applicable) provided the software used for the imaging analysis, but were not involved in the imaging analysis.
Ipsen Pharma and The Health Research Fund of Central Denmark Region supported the study financially.
Author contribution
Aska Drljevic-Nielsen: Conceptualization, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing.
Finn Rasmussen: Conceptualization, Investigation, Writing - Review & Editing, Funding acquisition, Supervision.
Jill Mains: Conceptualization, Investigation, Formal analysis, Writing - Review & Editing, Supervision.
Kennet Thorup: Conceptualization, Software, Writing - Review & Editing.
Frede Donskov: Conceptualization, Formal analysis, Investigation, Writing - Review & Editing, Funding acquisition, Supervision.
Declaration of competing interest
A. Drljevic-Nielsen reports receiving research grants from Ipsen and The Health Research Foundation of Central Denmark Region and conference travels from Pfizer, Bristol-Meyer-Squibb and Ipsen. F. Donskov reports receiving a research grants from Ipsen, Pfizer and The Health Research Foundation of Central Denmark Region. JR. Mains reports receiving research grants from the Memorial Foundation of Eva and Henry Fraenkel. J.R and The Health Research Foundation of Central Denmark Region and conference travels from Pfizer. No potential conflicts of interest were disclosed by F. Rasmussen and K. Thorup.
Footnotes
Data were presented, in part, as a poster presentation at the European Society for Medical Oncology Congress, Barcelona September 2019.
References
- 1.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz L.H., Litiere S., de Vries E., Ford R., Gwyther S., Mandrekar S. RECIST 1.1-Update and clarification: from the RECIST committee. Eur. J. Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seymour L., Bogaerts J., Perrone A., Ford R., Schwartz L.H., Mandrekar S. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3) doi: 10.1016/S1470-2045(17)30074-8. (e143-e52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litiere S., Collette S., de Vries E.G., Seymour L., Bogaerts J. RECIST - learning from the past to build the future. Nat. Rev. Clin. Oncol. 2017;14(3):187–192. doi: 10.1038/nrclinonc.2016.195. [DOI] [PubMed] [Google Scholar]
- 5.Gerwing M., Herrmann K., Helfen A., Schliemann C., Berdel W.E., Eisenblatter M. The beginning of the end for conventional RECIST - novel therapies require novel imaging approaches. Nat. Rev. Clin. Oncol. 2019;16(7):442–458. doi: 10.1038/s41571-019-0169-5. [DOI] [PubMed] [Google Scholar]
- 6.Miles K.A. Perfusion imaging with computed tomography: brain and beyond. Eur. Radiol. 2006;16(Suppl. 7):M37–M43. doi: 10.1007/s10406-006-0194-1. [DOI] [PubMed] [Google Scholar]
- 7.Miles K.A., Lee T.Y., Goh V., Klotz E., Cuenod C., Bisdas S. Current status and guidelines for the assessment of tumour vascular support with dynamic contrast-enhanced computed tomography. Eur. Radiol. 2012;22(7):1430–1441. doi: 10.1007/s00330-012-2379-4. [DOI] [PubMed] [Google Scholar]
- 8.Miles K.A., Griffiths M.R. Perfusion CT: a worthwhile enhancement? Br. J. Radiol. 2003;74(904):220–31.2. doi: 10.1259/bjr/13564625. [DOI] [PubMed] [Google Scholar]
- 9.Mains J.R., Donskov F., Pedersen E.M., Madsen H.H.T., Thygesen J., Thorup K. Use of patient outcome endpoints to identify the best functional CT imaging parameters in metastatic renal cell carcinoma patients. Br. J. Radiol. 2018;91(1082):20160795. doi: 10.1259/bjr.20160795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mains J.R., Donskov F., Pedersen E.M., Madsen H.H., Rasmussen F. Dynamic contrast-enhanced computed tomography as a potential biomarker in patients with metastatic renal cell carcinoma: preliminary results from the Danish Renal Cancer Group Study-1. Investig. Radiol. 2014;49(9):601–607. doi: 10.1097/RLI.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 11.Mains J.R., Donskov F., Pedersen E.M., Madsen H.H., Rasmussen F. Dynamic contrast-enhanced computed tomography-derived blood volume and blood flow correlate with patient outcome in metastatic renal cell carcinoma. Investig. Radiol. 2017;52(2):103–110. doi: 10.1097/RLI.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 12.Motzer R.J., Mazumdar M., Bacik J., Berg W., Amsterdam A., Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J. Clin. Oncol. 1999;17(8):2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 13.Heng D.Y., Xie W., Regan M.M., Warren M.A., Golshayan A.R., Sahi C. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J. Clin. Oncol. 2009;27(34):5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 14.Donskov F., von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J. Clin. Oncol. 2006;24(13):1997–2005. doi: 10.1200/JCO.2005.03.9594. [DOI] [PubMed] [Google Scholar]
- 15.Heng D.Y., Xie W., Regan M.M., Harshman L.C., Bjarnason G.A., Vaishampayan U.N. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer R.J., Tannir N.M., McDermott D.F., Aren Frontera O., Melichar B., Choueiri T.K. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donskov F, Jensen NV, Smidt-Hansen T, Brondum L, Geertsen P. A randomized phase II trial of interleukin-2 and interferon-alpha plus bevacizumab versus interleukin-2 and interferon-alpha in metastatic renal-cell carcinoma (mRCC): results from the Danish Renal Cancer Group (DaRenCa) study-1. Acta Oncologica (Stockholm, Sweden). 2018;57(5):589–94. [DOI] [PubMed]
- 18.Harrell F.E., Jr., Califf R.M., Pryor D.B., Lee K.L., Rosati R.A. Evaluating the yield of medical tests. JAMA. 1982;247(18):2543–2546. [PubMed] [Google Scholar]
- 19.van der Veldt A.A., Meijerink M.R., van den Eertwegh A.J., Haanen J.B., Boven E. Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br. J. Cancer. 2010;102(5):803–809. doi: 10.1038/sj.bjc.6605567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall P.S., Harshman L.C., Srinivas S., Witteles R.M. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Failure. 2013;1(1):72–78. doi: 10.1016/j.jchf.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Choueiri T.K., Motzer R.J. Systemic therapy for metastatic renal-cell carcinoma. N. Engl. J. Med. 2017;376(4):354–366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 22.Bae K.T. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology. 2010;256(1):32–61. doi: 10.1148/radiol.10090908. [DOI] [PubMed] [Google Scholar]
- 23.Horsman M.R., Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J. Radiat. Res. 2016;57(Suppl. 1) doi: 10.1093/jrr/rrw007. (i90-i8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y., Zhang J., Dai J., Feng X., Lu H., Zhou C. Angiogenesis of renal cell carcinoma: perfusion CT findings. Abdom. Imaging. 2010;35(5):622–628. doi: 10.1007/s00261-009-9565-0. [DOI] [PubMed] [Google Scholar]
- 25.Kroeger N., Pantuck A.J., Wells J.C., Lawrence N., Broom R., Kim J.J. Characterizing the impact of lymph node metastases on the survival outcome for metastatic renal cell carcinoma patients treated with targeted therapies. Eur. Urol. 2015;68(3):506–515. doi: 10.1016/j.eururo.2014.11.054. [DOI] [PubMed] [Google Scholar]
- 26.Turajlic S, Xu H, Litchfield K, Rowan A, Chambers T, Lopez JI, et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell. 2018;173(3):581–94.e12. [DOI] [PMC free article] [PubMed]
- 27.Donskov F., Xie W., Overby A., Wells J.C., Fraccon A.P., Sacco C.S. Synchronous versus metachronous metastatic disease: impact of time to metastasis on patient outcome-results from the international metastatic renal cell carcinoma database consortium. Eur Urol Oncol. 2020 doi: 10.1016/j.euo.2020.01.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.McKay R.R., Kroeger N., Xie W., Lee J.L., Knox J.J., Bjarnason G.A. Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. Eur. Urol. 2014;65(3):577–584. doi: 10.1016/j.eururo.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fournier L.S., Oudard S., Thiam R., Trinquart L., Banu E., Medioni J. Metastatic renal carcinoma: evaluation of antiangiogenic therapy with dynamic contrast-enhanced CT. Radiology. 2010;256(2):511–518. doi: 10.1148/radiol.10091362. [DOI] [PubMed] [Google Scholar]
- 30.Ng C.S., Wang X., Faria S.C., Lin E., Charnsangavej C., Tannir N.M. Perfusion CT in patients with metastatic renal cell carcinoma treated with interferon. AJR Am. J. Roentgenol. 2010;194(1):166–171. doi: 10.2214/AJR.09.3105. [DOI] [PubMed] [Google Scholar]
- 31.Hudson J.M., Bailey C., Atri M., Stanisz G., Milot L., Williams R. The prognostic and predictive value of vascular response parameters measured by dynamic contrast-enhanced-CT, -MRI and -US in patients with metastatic renal cell carcinoma receiving sunitinib. Eur. Radiol. 2018;28(6):2281–2290. doi: 10.1007/s00330-017-5220-2. [DOI] [PubMed] [Google Scholar]
- 32.Lassau N., Bonastre J., Kind M., Vilgrain V., Lacroix J., Cuinet M. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Investig. Radiol. 2014;49(12):794–800. doi: 10.1097/RLI.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beksac A.T., Paulucci D.J., Blum K.A., Yadav S.S., Sfakianos J.P., Badani K.K. Heterogeneity in renal cell carcinoma. Urol. Oncol. 2017;35(8):507–515. doi: 10.1016/j.urolonc.2017.05.006. [DOI] [PubMed] [Google Scholar]