Abstract
Background
Merging studies have reported the association of lipoprotein(a) [Lp(a)] with poor outcomes of coronary artery disease (CAD) in patients with type 2 diabetes mellitus (T2DM). However, the prognostic importance of Lp(a) for recurrent cardiovascular events (CVEs) is currently undetermined in patients with T2DM and prior CVEs.
Methods
From April 2011 to March 2017, we consecutively recruited 2284 T2DM patients with prior CVEs. Patients were categorized into low, medium, and high groups by Lp(a) levels and followed up for recurrent CVEs, including nonfatal acute myocardial infarction, stroke, and cardiovascular mortality. Kaplan–Meier, Cox regression and C-statistic analyses were performed.
Results
During 7613 patient-years’ follow-up, 153 recurrent CVEs occurred. Lp(a) levels were significantly higher in patients with recurrent CVEs than counterparts (20.44 vs. 14.71 mg/dL, p = 0.002). Kaplan–Meier analysis revealed that the event-free survival rate was dramatically lower in high and medium Lp(a) groups than that in low group irrespective of HBA1c status (< 7.0%; ≥ 7.0%, both p < 0.05). Furthermore, multivariate Cox regression models indicated that Lp(a) was independently associated with high risk of recurrent CVEs [HR(95% CI): 2.049 (1.308–3.212)], such data remains in different HBA1c status (HR(95% CI): < 7.0%, 2.009 (1.051–3.840); ≥ 7.0%, 2.162 (1.148–4.073)). Moreover, the results of C-statistic were significantly improved by 0.029 when added Lp(a) to the Cox model.
Conclusions
Our data, for the first time, confirmed that Lp(a) was an independent predictor for recurrent CVEs in T2DM patients with prior CVEs, suggesting that Lp(a) measurement may help to further risk stratification for T2DM patients after they suffered a first CVE.
Keywords: CAD, HBA1c, Lp(a), Recurrent CVEs, T2DM
Background
It has been demonstrated that atherosclerotic cardiovascular disease (ASCVD) is the leading causes of morbidity and mortality for individuals with type 2 diabetes mellitus (T2DM) [1, 2]. Common conditions coexisting with T2DM such as hypertension and dyslipidemia are clear risk factors for ASCVD [1, 2]. For the past decades, controlling multiple cardiovascular risk factors have shown the efficacy of reducing or slowing ASCVD in people with T2DM [3]. However, the risk of recurrent major cardiovascular events (CVEs) remains high despite the intensive statin treatment and other secondary prevention strategies were recommended [4, 5]. Therefore, searching potential risk factors contributing to this residual cardiovascular risk is crucial for improving the long-term prognosis in patients with T2DM and a first CVE.
Elevated lipoprotein(a) (Lp[a]) represents one of the most common genetic dyslipidemias worldwide, affecting 1 in 5 individuals [6]. Close attention to Lp(a), a particle containing of a low-density lipoprotein (LDL)-like particle bound to apolipoprotein(a), has emergingly been paid due to its pathogenic role in atherosclerosis and thrombosis formation [6]. Epidemiological and prospective data have suggested that a high level of Lp(a) is an independent risk factor for incident cardiovascular disease (CVD) [7, 8], particularly among those with DM [9, 10]. Simultaneously, in the secondary prevention setting, elevated Lp(a) values were also proved to be an independent predictor of CVEs in patients with established coronary artery disease (CAD) [11] or patients undergone percutaneous coronary intervention (PCI) [12, 13]. Data from our team also delivered that Lp(a) levels were strongly associated with the presence and severity of CAD in individuals with DM [14] and could predict higher risk of subsequent CVEs in stable CAD patients with DM or pre-DM [15]. However, it is currently undetermined whether Lp(a) plays a role in predicting recurrent CVEs in patients who had experienced prior CVEs [16, 17], and even more, there is no large-scale study specific to the T2DM population.
Therefore, in this prospective, observational cohort study, we, for the first time, investigated the predictive value of Lp(a) with recurrent worse outcomes in T2DM patients with prior CVEs.
Materials and methods
Study population
The study complied with the Declaration of Helsinki and was approved by the hospital’s ethical review board (Fu Wai Hospital & National Center for Cardiovascular Diseases, Beijing, China). All enrolled subjects provided informed written consent in the current study.
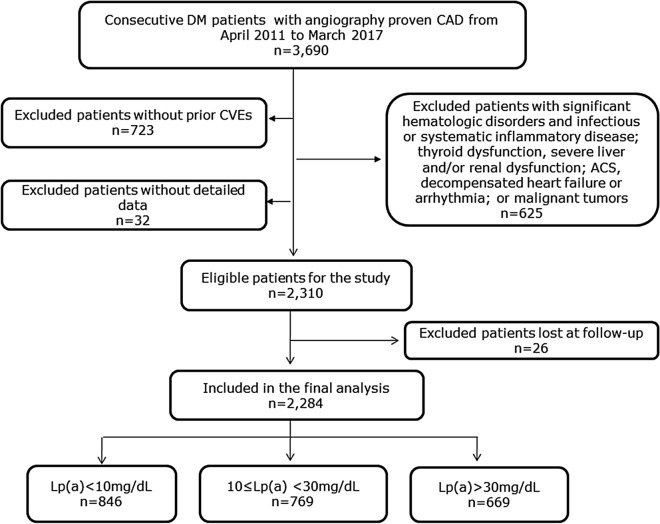
From April 2011 to March 2017 (as shown in Fig. 1), a total of 3690 T2DM patients with angiography proven stable CAD were consecutively recruited from three medical centers, including FuWai hospital, XuanWu Hospital, and AnZhen hospital according to the same protocol. The blood samples for testing Lp(a) were sent to FuWai hospital for unified measurement. The inclusion criteria were patients who had experienced a prior CVE [defined as myocardial infarction (MI), stroke, peripheral arterial disease, percutaneous coronary intervention (PCI), and coronary artery bypass grafting (CABG)] between 2 months to 1 year before admission. The exclusion criteria were patients with significant hematologic disorders and infectious or systematic inflammatory disease; thyroid dysfunction, severe liver and/or renal dysfunction; acute coronary syndrome (ACS), decompensated heart failure or arrhythmia; or malignant tumors, without detailed data. Finally, a total of 2310 eligible patients were enrolled in the current study. All study patients were prescribed secondary prevention medicine of ASCAD and followed up for adverse outcomes. Subsequently, a total of 26 patients were lost during the follow-up period. Therefore, there were 2284 T2DM patients with prior CVEs included in the final analysis, and were further divided into three groups according to Lp(a) levels.
Fig. 1.
Flowchart of the enrolled study population
Follow-up
Patients were followed up at 6 months’ intervals through direct interviews or telephone by well-trained cardiologists or nurses who were blinded to the purpose of the study. The primary endpoints (recurrent CVEs) included cardiovascular death, non-fatal MI and stroke. For patients with suspected cardiovascular attacks, the medical records or emergency records were required to be sent to our centers. The endpoints were confirmed by at least two professional physicians.
Definition of clinical status
The diagnosis of Nonfatal MI included ST-segment–elevation MI (STEMI) and non–ST-segment–elevation MI (NSTEMI). STEMI was defined as elevated biomarkers and new or presumed new ST-segment elevation in 2 or more contiguous leads. NSTEMI was defined as the presence of elevated biomarkers and at least 1 of either ECG changes (ST-segment depression or T-wave abnormalities), or ischemic symptoms. Stroke was diagnosed by the presence of typical symptoms and imaging. DM was diagnosed by fasting plasma glucose ≥ 7.0 mmol/L, the 2-h plasma glucose of the oral glucose tolerance test (OGTT) ≥ 11.1 mmol/L (based on venous plasma glucose results before and 2 h after a 75 g oral glucose load), or current use of hypoglycemic drugs or insulin. Hypertension was defined as repeated systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg (at least two times in different environments) or currently taking anti-hypertensive drugs. Dyslipidemia was defined by medical history or fasting total cholesterol (TC) ≥ 5.18 mmol/L or triglyceride (TG) ≥ 1.7 mmol/L and/or high-density lipoprotein cholesterol (HDL-C) < 1.04 mmol/L (for male) or < 1.30 mmol/L (for female). Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Overweight was defined as patients with BMI ≥ 25 kg/m2. Current smokers were defined as having smoked a cigarette in the past 30 days and > 100 cigarettes in a lifetime. Family history of CAD was defined as CAD occurring in a first-degree relative including mother, father, siblings, or child.
Laboratory analysis
Blood samples were obtained from the cubital vein after at least 12 h of fasting in the current study. Concentrations of TC, TG, low density lipoprotein cholesterol (LDL-C), HDL-C levels were measured with an automatic biochemistry analyzer (7150; Hitachi, Tokyo, Japan) in an enzymatic assay. Apolipoprotein AI (apo AI), and apo B were measured by an immunoturbidimetric method (Tina-quant, Roche Diagnostics). Lp(a) was determined by immunoturbidimetry method [LASAY Lp(a) auto; SHIMA Laboratories Co., Ltd] with a normal value of < 30 mg/dL. An Lp(a) protein validated standard was used to calibrate the examination, and the coefficient of variation value of repetitive measurements was < 10%. The concentrations of glucose were measured by enzymatic hexokinase method, and HbA1c by a Tosoh Automated Glycohemoglobin Analyzer HLC-723G8.
Statistical analysis
The data were expressed as the mean ± SD or median (Q1–Q3) for the continuous variables and the number (percentage) for the categorical variables. The Kolmogorov–Smirnov test was used to test the distribution pattern. The differences between continuous variables were determined with the Student’s t test, analysis of variance, Mann–Whitney U test, Kruskal–Wallis H test, and between the categorical variables were analyzed by χ2-test or Fisher’s exact test where appropriate. The event-free survival rates among groups were calculated by the Kaplan–Meier analysis and compared by the log-rank test. Univariate and multivariate Cox proportional hazard models were used to calculate the hazard ratio (HR) and 95% confidence interval (CI). To identify whether Lp(a) could improve the prediction of recurrent CVEs based on the SMART risk score model [18], we calculated Harrell’s C-statistic in the current analysis. A p-values of less than 0.05 were considered statistically significant. The statistical analyses were performed with SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA) and R language version 3.6.3 (Feather Spray).
Results
Baseline characteristics
Consistent with previous researches [19, 20], the plasma lipoprotein(a) levels had a skewed distribution in the overall 2284 enrolled population (as shown in Fig. 2). Table 1 summarizes study sample characteristics stratified by Lp(a) levels (Low: Lp[a] < 10 mg/dL, n = 846; Medium: 10 mg/dL ≤ Lp[a] < 30 mg/dL, n = 769; High: Lp[a] ≥ 30 mg/dL, n = 669). Mean age of study participants was 58.54 years and 73.3% were male. Most participants were considered to have traditional CVD risk factors including hypertension (69.6%), dyslipidemia (79.6%), and current smokers (57.4%), while only 13.7% of the enrolled patients have family history of CAD. Participants in the high Lp(a) group (Lp[a] ≥ 30 mg/dL) had less male patients, higher TC, LDL-C, apolipoprotein B levels, lower plasma TG levels, and tended to have more multi-diseased vessels.
Fig. 2.
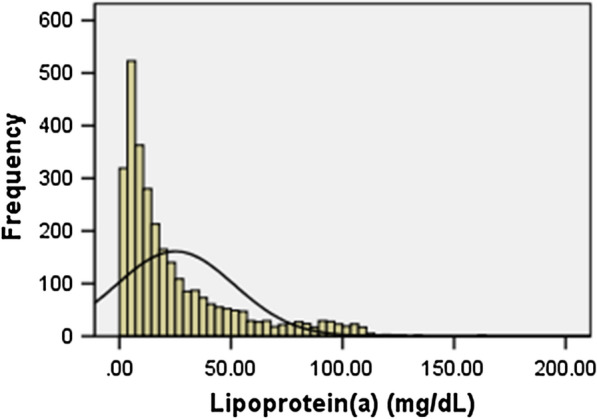
The distribution of lipoprotein(a) levels in patients with DM
Table 1.
Baseline clinical characteristics of the study participants according to Lp(a) categories
| Variables | All patients | Lp (a) categories (mg/dL) | p value | ||
|---|---|---|---|---|---|
| (n = 2284) | < 10 | 10 ~ 30 | ≥30 | ||
| (n = 846) | (n = 769) | (n = 669) | |||
| Clinical characteristics | |||||
| Age, years | 58.54 ± 10.47 | 57.99 ± 10.66 | 58.74 ± 10.61 | 59.00 ± 10.06 | 0.143 |
| Male, n (%) | 1674 (73.3) | 663 (78.4) | 550 (71.5) | 461 (68.9) | <0.001 |
| Hypertension, n (%) | 1589 (69.6) | 607 (71.8) | 529 (68.8) | 453 (67.8) | 0.199 |
| Dyslipidemia, n (%) | 1817 (79.6) | 677 (80.1) | 612 (79.7) | 528 (79.0) | 0.885 |
| Current smokers, n (%) | 1312 (57.4) | 507 (59.9) | 445 (57.9) | 360 (53.8) | 0.052 |
| Family history of CAD, n (%) | 312 (13.7) | 103 (12.2) | 109 (14.2) | 100 (14.9) | 0.281 |
| Body mass index, kg/m2 | 26.35 ± 3.15 | 26.64 ± 3.12 | 26.18 ± 3.27 | 26.19 ± 3.02 | 0.005 |
| SBP, mmHg | 128 ± 17 | 128 ± 17 | 128 ± 18 | 127 ± 16 | 0.288 |
| DBP, mmHg | 78 ± 16 | 78 ± 11 | 78 ± 22 | 77 ± 11 | 0.133 |
| Heart rate, bpm | 71 ± 10 | 72 ± 10 | 71 ± 10 | 71 ± 11 | 0.163 |
| Laboratory and clinical parameters | |||||
| FBG, mmol/L | 7.24 ± 2.31 | 7.33 ± 2.35 | 7.23 ± 2.34 | 7.12 ± 2.24 | 0.220 |
| HbA1c, % | 7.39 ± 1.26 | 7.35 ± 1.22 | 7.44 ± 1.29 | 7.38 ± 1.28 | 0.311 |
| TC, mmol/L | 4.08 ± 1.18 | 3.97 ± 1.21 | 4.02 ± 1.10 | 4.29 ± 1.20 | < 0.001 |
| HDL-C, mmol/L | 1.01 ± 0.27 | 1.00 ± 0.27 | 1.01 ± 0.26 | 1.03 ± 0.28 | 0.054 |
| LDL-C, mmol/L | 2.45 ± 0.97 | 2.28 ± 0.92 | 2.42 ± 0.90 | 2.69 ± 1.06 | < 0.001 |
| TG, mmol/L | 1.56 (1.17–2.20) | 1.65 (1.19–2.42) | 1.54 (1.16–2.14) | 1.48 (1.13–2.09) | < 0.001 |
| Lp (a), mg/dL | 15.01 (6.60–34.76) | 5.22 (3.32–7.33) | 17.19 (13.11–22.54) | 52.94 (38.85–79.41) | < 0.001 |
| ApoAI, g/L | 1.31 ± 0.30 | 1.32 ± 0.35 | 1.29 ± 0.26 | 1.31 ± 0.29 | 0.179 |
| ApoB, g/L | 0.92 ± 0.30 | 0.87 ± 0.29 | 0.90 ± 0.28 | 0.99 ± 0.31 | < 0.001 |
| Diseased vessels, n (%) | 0.016 | ||||
| One vessel | 430 (18.8) | 174 (20.6) | 151 (19.6) | 105 (15.7) | |
| Two vessels | 677 (29.6) | 273 (32.3) | 218 (28.4) | 186 (27.8) | |
| Multi-vessels | 1138 (49.8) | 382 (45.1) | 391 (50.8) | 365 (54.5) | |
| LVEF, % | 62.3 ± 8.7 | 62.3 ± 8.8 | 62.3 ± 9.1 | 62.2 ± 8.2 | 0.971 |
| Medications | |||||
| Aspirin, n (%) | 2227 (97.5) | 827 (97.7) | 753 (97.8) | 647 (96.8) | 0.418 |
| P2Y12 inhibitor, n (%) | 2040 (89.3) | 750 (88.6) | 703 (91.4) | 587 (87.8) | 0.070 |
| Statins, n (%) | 2133 (93.4) | 783 (92.7) | 730 (94.9) | 620 (92.7) | 0.150 |
| ACEI/ARB, n (%) | 1215 (53.2) | 453 (53.6) | 402 (52.3) | 360 (53.8) | 0.825 |
| β-blockers, n (%) | 1886 (82.6) | 711 (84.0) | 617 (80.2) | 558 (83.4) | 0.117 |
| CCB, n (%) | 875 (38.3) | 335 (39.6) | 295 (38.3) | 245 (36.7) | 0.513 |
| Anti-diabetes treatment | 0.757 | ||||
| Oral drugs | 1311 (57.4) | 488 (57.7) | 435 (56.6) | 388 (58.0) | |
| Insulin | 726 (31.8) | 259 (30.6) | 254 (33.0) | 213 (31.9) | |
Continuous values are summarized as mean ± SD, median (Q1–Q3) and categorical variables as n (%)
Lp(a) lipoprotein(a), CAD coronary artery disease, SBP systolic blood pressure, DBP diastolic blood pressure, FBG fasting blood glucose, HbA1c glycosylated hemoglobin, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TG triglyceride, ApoAI apolipoprotein AI, ApoB apolipoprotein B, LVEF left ventricular ejection fraction, ACEI angiotensin converting enzyme inhibitors, ARB angiotensin receptor blockers, CCB calcium channel blockers
Of these, 1311 (57.4%) was only on oral anti-diabetes drugs, and 726 (31.8%) had insulin treatment. Meanwhile, there was no significant difference with regard to anti-diabetes drug therapy as well as prescribed secondary prevention medicines such as aspirin, P2Y12 inhibitor, statins, angiotensin converting enzyme inhibitor/angiotensin receptor blockers (ACEI/ARB), β-blockers, and calcium channel blocker (CCB) among groups (Table 1).
Relation of risk factors and recurrent CVEs
Over 7613 patient-years’ follow-up period, 153 recurrent CVEs occurred (68 been identified as cardiovascular death, 30 suffered nonfatal MI, and 55 had strokes) as shown in Table 2. Patients with recurrent CVEs were much older, with lower percentage of overweight. Of note, the Lp(a) levels were dramatically higher in patients with recurrent CVEs compared with those without recurrent CVEs (20.44 mg/dL vs. 14.71 mg/dL, p = 0.002). Nevertheless, the gender, blood pressure, heart rate, fasting blood glucose, HbA1c, TC, HDL-C, LDL-C, TG, apo A1, and apo B were balanced between patients with or without recurrent CVEs (all p > 0.05). The association of exposure and other variables with recurrent CVEs in patients with T2DM were shown in Additional file 1: Table S2.
Table 2.
Clinical and traditional risk factors in patients with and without recurrent CVEs
| Characteristics | With recurrent CVEs (n = 153) | Without recurrent CVEs (n = 2131) | p value |
|---|---|---|---|
| Age, years | 62.58 ± 9.17 | 58.25 ± 10.50 | < 0.001 |
| Male, n (%) | 115 (75.2) | 1559 (73.2) | 0.637 |
| Body mass index, kg/m2 | 25.85 ± 3.09 | 26.39 ± 3.15 | 0.042 |
| BMI < 25 kg/m2 | 70 (45.9) | 718 (33.7) | 0.003 |
| SBP, mmHg | 127 ± 18 | 128 ± 17 | 0.645 |
| SBP < 130 mmHg | 78 (51.2) | 1057 (49.6) | 0.785 |
| DBP, mmHg | 76 ± 10 | 78 ± 16 | 0.130 |
| DBP < 80 mmHg | 72 (47.3) | 889 (41.7) | 0.231 |
| Heart rate, bpm | 71 ± 10 | 71 ± 10 | 0.761 |
| Biochemical parameters | |||
| FBG, mmol/L | 7.12 ± 2.43 | 7.24 ± 2.31 | 0.526 |
| FBG 4.4 ~ 6.5 mmol/L | 60 (38.9) | 886 (41.6) | 0.548 |
| HbA1c, % | 7.53 ± 1.37 | 7.38 ± 1.25 | 0.150 |
| HbA1c < 7.0% | 74 (48.4) | 1072 (50.3) | 0.932 |
| TC, mmol/L | 4.03 ± 1.08 | 4.08 ± 1.19 | 0.618 |
| HDL-C, mmol/L | 0.99 ± 0.26 | 1.01 ± 0.27 | 0.325 |
| LDL-C, mmol/L | 2.44 ± 0.93 | 2.45 ± 0.97 | 0.942 |
| LDL-C < 1.4 mmol/L | 16 (10.6) | 232 (10.9) | 0.907 |
| TG, mmol/L | 1.54 (1.17–2.12) | 1.56 (1.17–2.20) | 0.502 |
| Lp(a), mg/dL | 20.44 (10.01–43.96) | 14.71 (6.43–34.16) | 0.002 |
| ApoAI, g/L | 1.29 ± 0.30 | 1.31 ± 0.30 | 0.407 |
| ApoB, g/L | 0.91 ± 0.30 | 0.92 ± 0.30 | 0.746 |
Continuous values are summarized as mean ± SD, median (Q1–Q3) and categorical variables as n (percentage)
CVEs cardiovascular events, SBP systolic blood pressure, DBP diastolic blood pressure, FBG fasting blood glucose, HbA1c glycosylated hemoglobin, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TG triglyceride, Lp(a) lipoprotein(a), ApoAI apolipoprotein AI, ApoB apolipoprotein B
Lp(a) levels and recurrent CVEs
The incidence of the composite recurrent CVEs in the low, medium, and high Lp(a) groups (based on the cut-off value of 10 and 30 mg/dL) was 4.4%, 7.7%, and 8.5%, respectively. As shown in Fig. 3a, the Kaplan–Meier analysis showed that subjects with medium and high Lp(a) value had a significantly lower cumulative event-free survival rate compared to those with low Lp(a) value (p = 0.001). Similar results were found in patients with HbA1c < 7.0% (p = 0.011, Fig. 3b) and HbA1c ≥ 7.0% (p = 0.040, Fig. 3c) group.
Fig. 3.
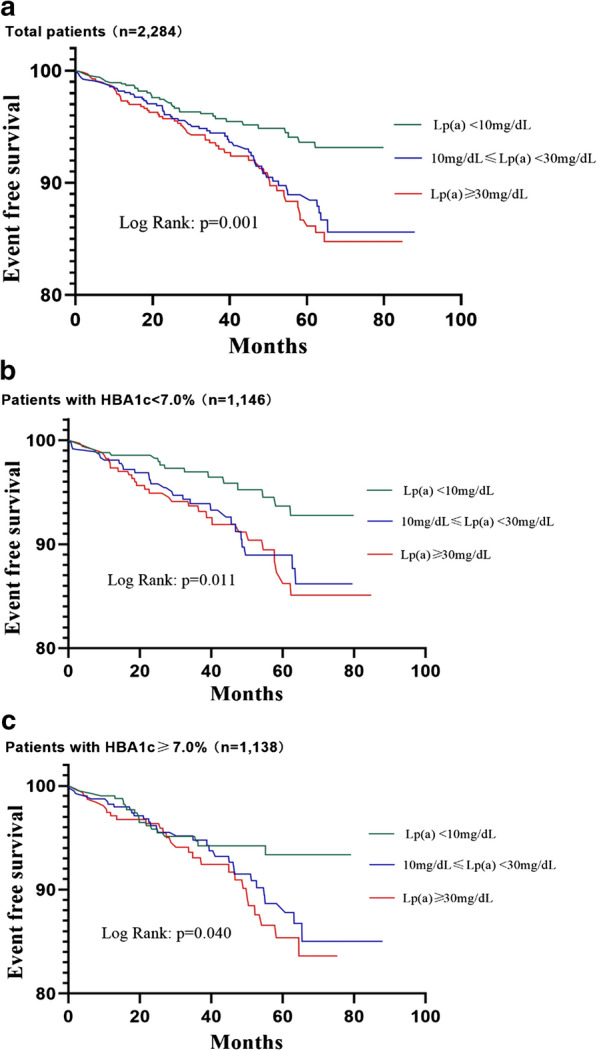
Kaplan-Meier analysis of Lp(a) categories for predicting Recurrent CVEs in subjects with different HBA1c status
As presented in Table 3, univariate Cox regression models showed that the hazard ratio of recurrent CVEs in patients with medium and high Lp(a) was 1.736-fold, 1.960-fold higher than ones with low Lp(a) values. Additional adjustment for other variables did not change the significance of high Lp(a) with recurrent CVEs (HR 2.049 [95% CI 1.308–3.212], p = 0.002). When divided the composite recurrent CVEs into three separate endpoints including non-fatal MI, stroke, and cardiovascular death, high Lp(a) group had a 3.016-fold hazard ratio of non-fatal MI (p = 0.026), and a 2.708-fold hazard ratio of cardiovascular death (p = 0.006) compared with low Lp(a) group. However, high Lp(a) group did not have an increase in stroke risk compared with the low Lp(a) group (p = 0.777). Furthermore, the relationship of Lp(a) levels with recurrent CVEs did not impacted by HBA1c status (as indicated in Table 4, p < 0.05). In a sensitivity analysis by excluding individuals with CABG, stroke and peripheral arterial disease (n = 1758), high Lp(a) remains an independent predictor of recurrent CVEs in this population (medium Lp(a) categories: HR 2.244 [95% CI 1.263–3.984], p = 0.006; high Lp(a) categories: HR 2.399 [95% CI 1.313–4.383], p = 0.004; respectively) after adjusting for potential confounding factors (shown in Additional file 1: Table S1).
Table 3.
Relation of Lp(a) levels with composite and separate recurrent CVEs in patients with T2DM
| Endpoints | Recurrent CVEs/Total | Crude model | Adjusted model | ||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Composite recurrent CVEs | 153/2284 | ||||
| Lp(a) per-SD increase | 1.007 (1.002–1.013) | 0.007 | 1.008 (1.002–1.014) | 0.006 | |
| Lp(a) < 10 | 37/846 | Reference | Reference | ||
| 10 ≤ Lp(a) < 30 | 59/769 | 1.736 (1.151–2.619) | 0.009 | 1.720 (1.099–2.692) | 0.018 |
| Lp(a) ≥ 30 | 57/669 | 1.960 (1.296–2.965) | 0.001 | 2.049 (1.308–3.212) | 0.002 |
| Non-fatal MI | 30/2284 | ||||
| Lp(a) per-SD increase | 1.012 (1.001–1.023) | 0.039 | 1.012 (1.000–1.024) | 0.050 | |
| Lp(a) < 10 | 7/846 | Reference | Reference | ||
| 10 ≤ Lp(a) < 30 | 8/769 | 1.259 (0.457–3.472) | 0.656 | 1.539 (0.529–4.474) | 0.428 |
| Lp(a) ≥ 30 | 15/669 | 2.737 (1.116–6.714) | 0.028 | 3.016 (1.144–7.949) | 0.026 |
| Stroke | 55/2284 | ||||
| Lp(a) per-SD increase | 1.002 (0.992–1.011) | 0.761 | 1.001 (0.990–1.012) | 0.815 | |
| Lp(a) < 10 | 17/846 | Reference | Reference | ||
| 10 ≤ Lp(a) < 30 | 22/769 | 1.408 (0.748–2.651) | 0.289 | 1.355 (0.674–2.724) | 0.394 |
| Lp(a) ≥ 30 | 16/669 | 1.199 (0.606–2.372) | 0.603 | 1.118 (0.518–2.413) | 0.777 |
| CVD deaths | 68/2284 | ||||
| Lp(a) per-SD increase | 1.009 (1.002–1.017) | 0.019 | 1.011 (1.003–1.020) | 0.011 | |
| Lp(a) < 10 | 13/846 | Reference | Reference | ||
| 10 ≤ Lp(a) < 30 | 29/769 | 2.419 (1.257–4.652) | 0.008 | 2.242 (1.108–4.535) | 0.025 |
| Lp(a) ≥ 30 | 26/669 | 2.539 (1.305–4.942) | 0.006 | 2.708 (1.340–5.475) | 0.006 |
The adjusted model including age, sex, body mass index, current smoking, hypertension, dyslipidemia, family history of coronary artery disease, diseased vessels, low-density lipoprotein cholesterol, fasting blood glucose, statin and anti-diabetes drugs use
CVEs cardiovascular events, MI myocardial infarction, CVD cardiovascular disease
Table 4.
Association of Lp(a) levels with recurrent CVEs in T2DM patients according to HBA1c status
| Lp(a) (mg/dL) | Recurrent CVEs/Total | Crude model | Adjusted model | ||
|---|---|---|---|---|---|
| (153/2284) | HR (95% CI) | p value | HR (95% CI) | p value | |
| Total patients | 153/2284 | ||||
| Lp(a) per-SD increase | 1.007 (1.002–1.013) | 0.007 | 1.008 (1.002–1.014) | 0.006 | |
| Lp(a) < 10 | 37/846 | Reference | Reference | ||
| 10 ≤ Lp(a) < 30 | 59/769 | 1.736 (1.151–2.619) | 0.009 | 1.720 (1.099–2.692) | 0.018 |
| Lp(a) ≥ 30 | 57/669 | 1.960 (1.296–2.965) | 0.001 | 2.049 (1.308–3.212) | 0.002 |
| HBA1c < 7.0% | 74/1146 | ||||
| Lp(a) per-SD increase | 1.008 (1.001–1.016) | 0.024 | 1.009 (1.001–1.018) | 0.023 | |
| Lp(a) < 10 | 17/427 | Reference | Reference | ||
| 10 ≤ Lp(a) < 30 | 28/373 | 1.968 (1.077–3.595) | 0.028 | 1.815 (0.949–3.473) | 0.072 |
| Lp(a) ≥ 30 | 29/346 | 2.156 (1.185–3.924) | 0.012 | 2.009 (1.051–3.840) | 0.035 |
| HBA1c ≥ 7.0% | 79/1138 | ||||
| Lp(a) per-SD increase | 1.006 (0.998–1.014) | 0.122 | 1.009 (1.000–1.018) | 0.050 | |
| Lp(a) < 10 | 20/418 | Reference | Reference | ||
| 10 ≤ Lp(a) < 30 | 31/402 | 1.520 (0.866–2.667) | 0.144 | 1.571 (0.842–2.932) | 0.155 |
| Lp(a) ≥ 30 | 28/318 | 1.816 (1.023–3.223) | 0.042 | 2.162 (1.148–4.073) | 0.017 |
The adjusted model including age, sex, body mass index, currentis smoking, hypertension, dyslipidemia, family history of coronary artery disease, diseased vessels, low-density lipoprotein cholesterol, fasting blood glucose, statin and anti-diabetes drugs use
CVEs cardiovascular events, MI myocardial infarction, CVD cardiovascular disease
Risk prediction for recurrent CVEs
As presented in Table 5, in the whole population, Cox prediction using the SMART risk score model, the C-statistic values were 0.660 (95% CI 0.595–0.726). Furthermore, adding Lp(a) categories to the original model resulted in a significant improvement in C-statistic (ΔC-statistic 0.029 [0.006–0.062], p = 0.047).
Table 5.
C-statistic of Lp(a) categories for predicting recurrent CVEs
| Models | C-statistic (95% CI) | ΔC-statistic (95% CI) | p value |
|---|---|---|---|
| Total patients (n = 2284) | |||
| Original model | 0.660 (0.595–0.726) | Reference | |
| Original model + Lp(a) categories | 0.689 (0.625–0.753) | 0.029 (0.006–0.062) | 0.047 |
Original model was using the SMART risk score model
Discussion
Our study enrolled a prospective cohort corresponding to diabetic individuals with prior established CVEs, who were at high risk for recurrent ischemic CVEs in the circumstance of following standard secondary prevention strategies recommended by the current guidelines [21, 22]. Data, for the first time, clearly confirmed that Lp(a) was an independent predictor for recurrent CVEs in T2DM patients with prior CVEs. When stratified by HBA1c levels (< 7.0%, or ≥ 7.0%), this association were significant in both HBA1c status independent of the level of the other risk factors. More importantly, in the overall cohort, the addition of Lp(a) to the model improved the risk prediction for recurrent CVEs. Thus, the present study strongly implied that Lp(a) might be a useful marker for further risk stratification in patients with T2DM after they suffered a first CVE.
The prevalence of T2DM has been increasing dramatically over the past few decades, with projections of an even greater growth over coming decades [23, 24]. Convincing evidence indicated that CAD is a common comorbidity in patients with T2DM and has been considered as a CAD risk equivalent based on multiple guidelines [25]. Currently, several clinical investigations indicated that despite aggressive multidisciplinary efforts have been made including revascularization and intensive management of LDL-C, glucose, blood pressure, and thrombotic risk, patients surviving an ACS event are at increased risk of recurrent CVEs, and this risk is further increased in patients with T2DM [26], raising the question of whether the treatment regimens are less effective in these patients. For decades, it has been well elucidated that abnormal lipid metabolism largely contributes to the additional cardiovascular risk for T2DM patients [27]. Therefore, the management of multiple risk factors especially lipid is of great significance for the prognosis. The recent guidelines have clearly recommended the target value of LDL-C [28], nonetheless, residual cardiovascular risk remains high for T2DM patients with a prior CVE compared with non-diabetic patients. Thus, it is essential to search additional modifiable lipid disorders to further improve the prognosis of these patients. Therefore, we consecutively recruited 2284 T2DM patients with prior CVEs and followed up for 7613 patient-years, attempting to seek plausible residual risk in terms of lipid disorders.
Recently, the relationship of elevated Lp(a) with CVD risk have been emergingly recognized in multiple investigations. Plasma concentrations of Lp(a) are mainly (90%) determined by the LPA gene, without significant dietary or environmental influences [29]. The association of Lp(a) with risk of CAD as well as mortality, which is independent of traditional cardiovascular risk factors, has been rapidly aware in series of studies [7, 30]. Lp(a) has been determined as the independent genetic risk factor of CVD and a causal role has been demonstrated by Mendelian randomization [31]. The Copenhagen City Heart Study demonstrated that compared to subjects with Lp(a) levels below 5 mg/dL, those with Lp(a) between 30 and 76 mg/dL had a 1.6-fold increased risk for incident MI. This risk increased to 1.90 for individuals with Lp(a) between 77 and 117 mg/dL and to 2.60 for individuals with Lp(a) concentrations above 117 mg/dL [8]. However, the data mainly based on investigations of apparently healthy participants in the general population rather than patients with a prior CVE. At the same time, among limited existing investigations related to patients with established CAD, inconsistent results were also observed. A recent cohort study support that in patients with stable CAD and chronic total occlusion, increased Lp(a) confers greater risk for poor coronary collateralization when TC, LDL-C or non-HDL-C are elevated especially in patients with T2DM [32]. In the previous study involving stable CAD patients with different glucose metabolism status, high Lp(a) were associated with significantly higher risk of subsequent CVEs in pre-DM and DM [15]. However, the enrolled population were restricted to patients with stable CAD but not those with prior CVEs. On the contrary, Schwartz GG, et al. enrolled 969 patients who experienced a recent ACS and treated with statins, Lp(a) concentration was not associated with adverse CVEs [16]. Additionally, the study conducted by Gencer et al. also suggested that high Lp(a) levels are not predictive for cardiovascular outcomes in patients otherwise medically well controlled, but might be useful to identify patients who would not be on LDL‐C targets 1 year after ACS [33]. The above two studies were localized in patients with the acute setting of ACS, and could not reflect the situation of DM with previous ASCVD attack. Therefore, studies concerning the prognosis of Lp(a) in patients with a prior CVE are of worth in the real-world, particularly in patients with T2DM.
Consequently, in our study, we observed that Lp(a) levels were significantly higher in patients suffered recurrent CVEs. Of note, our current data also demonstrated that the event-free survival rate was dramatically lower in medium and high Lp(a) groups. Significantly, compared with patients with low Lp(a) levels, those with high Lp(a) had a 2.049-fold higher hazard ratio of recurrent CVEs after adjusting for other variables including LDL-C, HBA1c, and so forth. Furthermore, when divided the population into two groups by HBA1c status, the predictive value of Lp(a) in risk of recurrent CVEs remains significant independent of the glucose control level. Finally, the C-statistic was significantly improved by 0.029 when added Lp(a) to the Cox model. Although the results were inconsistent with the study conducted by Schwartz GG [16], the different of enrolled population may partly explain the disparity. As far as we know, it is the first large study involved T2DM patients with a first CVE, which included the composite of MI, stroke, peripheral arterial disease, PCI, and CABG, instead of ACS or other specific status. Hence, the present study supported the opinion that Lp(a) was an independent predictor for recurrent CVEs in T2DM patients with prior CVEs in the stain era.
Till now, the mechanisms of Lp(a) potentiates CVD risk can be broadly classified in 3 categories: proatherogenic, proinflammatory, and potentially antifibrinolytic [29]. However, the exact mechanism of Lp(a) increasing CVD risk in DM status was not well clarified. The recent study assessed the relevance of biomarkers combined to pathway groups for the development of T2DM and coronary heart disease (CHD) during the median of 14 years follow‑up. The authors finally demonstrated that Lp(a) was inversely associated with T2DM and positively with CHD development [34]. However, the potentially causal mechanisms for both diseases, especially in relation to the observed opposite effect directions, are currently still obscure. More investigations were needed in the future.
Nevertheless, our study had several limitations. First of all, this is a study among Chinese population with T2DM and prior CVEs, and whether the data applied to other populations need to be testified. Secondly, the Lp(a) concentrations were only measured at baseline, and the alterations of the biomarkers may also be clinically significant during the follow-up period. Moreover, the method of Lp(a) measurement used in the study might be influenced by the apo(a) size due to the numbers of the KIV type 2 domain. Variations of apo(a) size between assay calibrators and patients’ samples might overestimate or underestimate the real concentration of Lp(a). Finally, as this was an observational study, further investigations are needed to clarify the underlying mechanism of the associations.
Conclusions
Our data for the first time indicated that Lp(a) was an independent predictor for recurrent CVEs in T2DM patients with prior CVEs, suggesting that Lp(a) measurement may help further risk stratification for T2DM patients after they suffered a first CVE.
Supplementary information
Additional file 1. Additional tables. Table S1. Relation of Lp(a) levels with recurrent CVEs in T2DM patients without CABG, peripheral arterial disease, and stroke. Table S2. Association of exposure and other variables with recurrent CVEs in patients with T2DM.
Acknowledgements
The authors wish to thank the participants and staff of this prospective population study.
Abbreviations
- ACS
Acute coronary syndrome
- ASCVD
Atherosclerotic cardiovascular disease
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- CVD
Cardiovascular disease
- CVEs
Cardiovascular events
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- Lp(a)
Lipoprotein(a)
- MI
Myocardial infarction
- PCI
Percutaneous coronary intervention
- TC
Total cholesterol
- TG
Triglyceride
Authors’ contributions
Y-Z completed the project, analyzed data, and wrote the manuscript. J-LJ, YX-C, H-WZ and R-XX contributed to data collection. QH and Y-FL contributed to the collections of data. Y-LG, N-QW, YG, and C-GZ contributed to recruitment of patients, and clinical diagnosis of disease. J-JL designed the study, interpreted data, and contributed to critically revising the manuscript. J-JL is the guarantor of this work and, and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Funding
This work was partially supported by the Capital Health Development Fund (201614035), CAMS Major Collaborative Innovation Project (2016-I2M-1-011) awarded by Dr. Jian-Jun Li, MD, PhD.
The study sponsors did not participate in the study design; the collection, analysis, or interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study complied with the principles of the Declaration of Helsinki and was approved by the ethical review board of Fuwai Hospital (Beijing, China). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12933-020-01083-8.
References
- 1.American Diabetes Association Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S1–S135. [Google Scholar]
- 2.American Diabetes Association (9) Cardiovascular Disease and Risk Management: standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S86–S104. doi: 10.2337/dc18-S009. [DOI] [PubMed] [Google Scholar]
- 3.Handelsman Y, Lepor NE. PCSK9 inhibitors in lipid management of patients with diabetes mellitus and high cardiovascular risk: a review. J Am Heart Assoc. 2018;7(13):e008953. doi: 10.1161/JAHA.118.008953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koskinas KC, Siontis GC, Piccolo R, et al. Impact of diabetic status on outcomes after revascularization with drug-eluting stents in relation to coronary artery disease complexity: patient-level pooled analysis of 6081 patients. Circ Cardiovasc Interv. 2016;9:e003255. doi: 10.1161/CIRCINTERVENTIONS.115.003255. [DOI] [PubMed] [Google Scholar]
- 5.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, et al. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 9.Waldeyer C, Makarova N, Zeller T, et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J. 2017;38:2490–2498. doi: 10.1093/eurheartj/ehx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeed A, Sun W, Agarwala A, et al. Lipoprotein(a) levels and risk of cardiovascular disease events in individuals with diabetes mellitus or prediabetes: the Atherosclerosis Risk in Communities study. Atherosclerosis. 2019;282:52–56. doi: 10.1016/j.atherosclerosis.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donoghue ML, Morrow DA, Tsimikas S, et al. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J Am Coll Cardiol. 2014;63:520–527. doi: 10.1016/j.jacc.2013.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konishi H, Miyauchi K, Kasai T, et al. Impact of lipoprotein(a) as residual risk on long-term outcomes in patients after percutaneous coronary intervention. Am J Cardiol. 2015;115:157–160. doi: 10.1016/j.amjcard.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Konishi H, Miyauchi K, Tsuboi S, et al. Plasma lipoprotein(a) predicts major cardiovascular events in patients with chronic kidney disease who undergo percutaneous coronary intervention. Int J Cardiol. 2016;205:50–53. doi: 10.1016/j.ijcard.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhang HW, Zhao X, Guo YL, et al. Elevated lipoprotein (a) levels are associated with the presence and severity of coronary artery disease in patients with type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2018;28:980–986. doi: 10.1016/j.numecd.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Jin JL, Cao YX, Zhang HW, et al. Lipoprotein(a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care. 2019;42(7):1312–1318. doi: 10.2337/dc19-0274. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GG, Ballantyne CM, Barter PJ, et al. Association of Lipoprotein(a) with risk of recurrent ischemic events following acute coronary syndrome: analysis of the dal-outcomes randomized clinical trial. JAMA Cardiol. 2018;3:164–168. doi: 10.1001/jamacardio.2017.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zewinger S, Kleber ME, Tragante V, et al. Relations Between Lipoprotein(a) concentrations, lpa genetic variants, and the risk of mortality in patients with established coronary heart disease: a molecular and genetic association study. Lancet Diabetes Endocrinol. 2017;5:534–543. doi: 10.1016/S2213-8587(17)30096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorresteijn JA, Visseren FL, Wassink AM, et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart. 2013;99:866–872. doi: 10.1136/heartjnl-2013-303640. [DOI] [PubMed] [Google Scholar]
- 19.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Wu NQ, Zhu CG, et al. Significance of lipoprotein(a) levels in familial hypercholesterolemia and coronary artery disease. Atherosclerosis. 2017;260:67–74. doi: 10.1016/j.atherosclerosis.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 22.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 23.Whiting DR, Guariguata L, Weil C, et al. global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Rawshani A, Franzén S, Eliasson B, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407–1418. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 25.Kavousi M, Leening MJ, Nanchen D, et al. Comparison of application of the ACC/AHA guidelines, Adult Treatment Panel III guidelines, and European Society of Cardiology guidelines for cardiovascular disease prevention in a European cohort. JAMA. 2014;311:1416–1423. doi: 10.1001/jama.2014.2632. [DOI] [PubMed] [Google Scholar]
- 26.Cziraky MJ, Reddy VS, Luthra R, et al. Clinical Outcomes and Medication Adherence in Acute Coronary Syndrome Patients With and Without Type 2 Diabetes Mellitus: a Longitudinal Analysis 2006-2011. J Manag Care Spec Pharm. 2015;21:470–477. doi: 10.18553/jmcp.2015.21.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A, Singh V. Atherogenic dyslipidemia and diabetes mellitus: what’s new in the management arena? Vasc Health Risk Manag. 2010;6:665–669. doi: 10.2147/VHRM.S5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;2019:1–78. [Google Scholar]
- 29.Gencer B, Kronenberg F, Stroes ES, et al. Lipoprotein(a): the revenant. Eur Heart J. 2017;38:1553–1560. doi: 10.1093/eurheartj/ehx033. [DOI] [PubMed] [Google Scholar]
- 30.Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and high risk of mortality. Eur Heart J. 2019;40:2760–2770. doi: 10.1093/eurheartj/ehy902. [DOI] [PubMed] [Google Scholar]
- 31.Clarke R, Peden JF, Hopewell JC, et al. Genetic Variants Associated With Lp(a) Lipoprotein Level and Coronary Disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 32.Shen Y, Chen S, Dai Y, et al. Lipoprotein (a) interactions with cholesterol-containing lipids on angiographic coronary collateralization in type 2 diabetic patients with chronic total occlusion. Cardiovasc Diabetol. 2019;18:82. doi: 10.1186/s12933-019-0888-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gencer B, Rigamonti F, Nanchen D, et al. Prognostic value of elevated lipoprotein(a) in patients with acute coronary syndromes. Eur J Clin Invest. 2019;49:e13117. doi: 10.1111/eci.13117. [DOI] [PubMed] [Google Scholar]
- 34.Huth C, Bauer A, Zierer A, et al. Biomarker-defined Pathways for Incident Type 2 Diabetes and Coronary Heart Disease-A Comparison in the MONICA/KORA Study. Cardiovasc Diabetol. 2020;19(1):32. doi: 10.1186/s12933-020-01003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional tables. Table S1. Relation of Lp(a) levels with recurrent CVEs in T2DM patients without CABG, peripheral arterial disease, and stroke. Table S2. Association of exposure and other variables with recurrent CVEs in patients with T2DM.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.