Abstract
Background:
In CARE-MS II (Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; NCT00548405), alemtuzumab (12 mg/day; baseline: 5 days; 12 months later: 3 days) significantly improved health-related quality of life (HRQL) outcomes versus subcutaneous interferon beta-1a (SC IFNB-1a) in relapsing-remitting multiple sclerosis (RRMS) patients over 2 years. Patients completing CARE-MS II could enter a 4-year extension study (NCT00930553).
Objective:
The aim of this study is to assess 6-year HRQL outcomes in alemtuzumab-treated CARE-MS II patients, including those with highly active disease (HAD).
Methods:
During extension, patients could receive additional alemtuzumab for clinical/magnetic resonance imaging (MRI) activity or other disease-modifying therapies per investigator’s discretion. Assessments include Functional Assessment of Multiple Sclerosis (FAMS), 36-Item Short-Form Health Survey (SF-36), and EQ-5D visual analog scale (EQ-VAS).
Results:
Alemtuzumab-treated patients improved or stabilized all HRQL measures over 6 years with significant improvements from baseline at all time points on EQ-VAS and for up to 5 years on FAMS, SF-36 MCS, and SF-36 PCS. Alemtuzumab-treated patients with HAD showed significant improvements versus baseline at Year 2 on all HRQL measures, and significant improvements versus SC IFNB-1a on SF-36 PCS and EQ-VAS; however, the improvements did not reach the threshold for clinical relevance.
Conclusion:
Alemtuzumab-treated CARE-MS II patients improved or stabilized HRQL versus baseline over 6 years. This is the first study to show long-term HRQL benefits in patients with HAD.
Keywords: Alemtuzumab, FAMS, SF-36, EQ-VAS, health-related quality of life, patient-reported outcomes, relapsing-remitting multiple sclerosis
Introduction
Multiple sclerosis (MS) is a chronic immune-mediated inflammatory disease of the central nervous system that affects more than 2 million people worldwide.1 The majority (85%) of patients are initially diagnosed with relapsing-remitting multiple sclerosis (RRMS), in which periods of active neurological deterioration are separated by periods of recovery.2,3
RRMS patients with highly active disease (HAD) have increased inflammatory activity compared with other RRMS patients. Frequent relapses in the first few years after clinical onset may lead to more rapid disease progression,4 so preventing relapses may help minimize disability worsening.5,6 Systematic reviews and meta-analyses have concluded that alemtuzumab is among the most efficacious disease-modifying therapies (DMTs) for reducing MS relapse rates.7,8
The CARE-MS II trial (Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; NCT00548405) evaluated the efficacy and safety of alemtuzumab treatment in patients with active RRMS who had inadequate response to prior DMTs.9 Alemtuzumab significantly reduced relapses (p < 0.001) and disability worsening (p = 0.0084) compared with subcutaneous interferon beta-1a (SC IFNB-1a) over 2 years.9 Other clinical and magnetic resonance imaging (MRI) outcomes, including 6-month confirmed disability improvement (CDI), and brain volume loss (BVL) were significantly favorable in patients treated with alemtuzumab versus SC IFNB-1a.9 The long-term efficacy of alemtuzumab was evaluated in an extension of the alemtuzumab clinical trials, including patients from CAMMS223 (NCT00050778), CARE-MS I (NCT00530348), and CARE-MS II. Results of the extension study (CAMMS03409; NCT00930553) showed that alemtuzumab maintained efficacy on clinical and MRI outcomes through Year 6.10,11
Adverse events (AEs) in alemtuzumab-treated patients include infusion-associated reactions (IARs), infections, and autoimmune AEs (mainly thyroid events, and less frequently, immune thrombocytopenia [ITP] and nephropathies [including anti-glomerular basement membrane disease]).9–11 The incidence of IARs decreased with the second course of alemtuzumab and each additional course thereafter.12 The risk of infections also decreased over time from ~60% in Year 1 to ~40% in Year 5; over 95% of infections were mild to moderate with serious infections occurring in less than 2% of patients per year.13 Thyroid events occurred in 42% of patients over 6 years, peaking in the third year and declining over time.11 Throughout the alemtuzumab clinical development program for MS (up to 12 years, depending on the study), ITP occurred in ~2% of patients, and the incidence of nephropathies was 0.34%.14,15
In CARE-MS II, patient-reported outcome (PRO) measures were used to evaluate the impact of alemtuzumab on patients’ health-related quality of life (HRQL). At 2 years, the alemtuzumab group showed statistically significant improvements compared with SC IFNB-1a on PRO scores.16 These results affirmed the importance of improvement in clinical and MRI endpoints to patients and demonstrated that alemtuzumab treatment can have a meaningful positive impact on patients’ lives.
To extend and broaden these results, we report 6-year HRQL outcomes for alemtuzumab-treated patients followed from entry in CARE-MS II through completion of the CARE-MS extension study, including a subgroup analysis of patients with HAD.
Methods
Study design
The CARE-MS II study design has been published elsewhere.9 Briefly, CARE-MS II was a global, randomized, rater-blinded, active comparator, Phase 3 trial of patients with active RRMS (defined as ⩾2 relapses in prior 2 years and ⩾1 in prior year). Participation was limited to adult patients 18–55 years of age who had experienced MS symptom onset during the 10 years prior to enrollment, had at least one relapse during prior DMT (defined as ⩾6 months of treatment with IFNB or glatiramer acetate), and had an Expanded Disability Status Scale (EDSS) score of 0–5 at baseline.
Participants were randomized in a 2:1 ratio to receive intravenous (IV) alemtuzumab 12 mg administered once daily on five consecutive days at baseline and on three consecutive days 12 months later, or SC IFNB-1a 44 µg administered three times weekly. The core CARE-MS II study lasted 2 years (baseline to Month 24) and was followed by a 4-year extension study (Months 24–72). During the extension study, patients could receive as-needed treatment for relapse or MRI activity with alemtuzumab (three consecutive days, ⩾12 months from the previous course) or other DMTs at the investigator’s discretion. All procedures were approved by local institutional ethics review boards of participating sites. Patients provided written informed consent.
Study population
The populations of interest in this analysis were the RRMS patients who initiated alemtuzumab 12 mg at entry in CARE-MS II and the subgroup of those patients with HAD (characterized by ⩾2 relapses in the year prior to randomization and ⩾1 gadolinium-enhancing lesion at baseline, per definition in previous alemtuzumab publications).17
HRQL assessments
The main outcomes for this analysis were the changes from baseline scores in the following PRO instruments.
Functional Assessment of Multiple Sclerosis (version 4)
The Functional Assessment of Multiple Sclerosis (FAMS) is a validated, MS-specific, patient-reported HRQL questionnaire with 44 scored items comprising six scales (mobility, symptoms, emotional well-being, general contentment, thinking and fatigue, family/social well-being).18 FAMS total scores range from 0 to 176, with higher scores indicating better functioning. A clinically meaningful change in FAMS total scores in RRMS was not predetermined in the study protocol. However, in the absence of other information, 0.5 standard deviation (SD) is a conservative and scientific estimate of a clinically meaningful effect.19 The FAMS was administered at baseline, every 6 months during the core study and Year 1 of the extension period, and every 12 months thereafter.
Short-Form-36 (version 2)
The Short-Form-36 (SF-36) is a validated generic self-assessment questionnaire that captures patients’ perceptions of their health and how it affects their quality of life.20 It contains 36 items that are organized in eight scales and combined into two summary measures: the mental component summary (MCS; an aggregate score comprising the eight scales emphasizing the mental health, role-emotional, social functioning, and vitality [i.e. energy level/tiredness] scales) and the physical component summary (PCS; an aggregate of the eight scales emphasizing the physical function, role-physical, bodily pain, and general health scales). Norm-based scores ranged from 0 to 100 for both the MCS and PCS. A clinically meaningful change in PCS or MCS was defined as a decrease or increase of ⩾5 points from baseline norm-based scores.21–23 The SF-36 was administered at baseline, every 12 months during the core study, every 6 months during Year 1 of the extension period (per protocol), and every 12 months thereafter.
EuroQOL-Visual Analog Scale
The EuroQOL-Visual Analog Scale (EQ-VAS; part 2 of the EQ-5 Dimension 3-Level Questionnaire) is a validated, standardized, generic measure in which patients mark their self-rated current health status on a vertical scale ranging from 0 (worst imaginable health state) to 100 (best imaginable health state).24 A conservative approximation of what constitutes a clinically meaningful change in EQ-VAS scores for patients with RRMS is 0.5 SD.19 The EQ-VAS was administered at baseline, every 6 months during the core study and Year 1 of the extension period, and every 12 months thereafter.
Statistical analysis
Analyses were based on all available data (without imputation) for patients treated with alemtuzumab 12 mg from the first alemtuzumab dose in the CARE-MS II core study through 6 years. Subgroup analyses were performed on alemtuzumab-treated patients with HAD at core study baseline.
Baseline characteristics for all participants were summarized using descriptive statistics. Changes from baseline in HRQL measured by PRO questionnaires at each time point after baseline were analyzed using a mixed-effects model for repeated measurements (MMRM) including treatment, geographic region, visit, baseline HRQL value, and treatment-by-visit interaction as fixed effects with an unstructured variance-covariance structure. Visit was treated as a categorical variable. Results are presented as least-squares means with 95% confidence intervals (CIs).
Results
Study population and baseline characteristics
Of the 435 alemtuzumab-treated patients who completed 2 years in CARE-MS II, 393 (90%) enrolled in the extension study; of those, 338 (86%) remained on-study through end of Year 6. Of a total of 145 patients in the HAD subgroup, 103 were treated with alemtuzumab 12 mg in the CARE-MS II core study and 42 with SC IFNB-1a. Of the 103 alemtuzumab-treated HAD patients, 92 (89%) entered the extension study and 84 (91%) remained on-study through Year 6. Compared with the overall population, patients with HAD were slightly younger on average and had a higher mean number of relapses in the previous year (Table 1). Overall, 50% of patients received no additional alemtuzumab and no other DMT after completing the second alemtuzumab course at Month 12.
Table 1.
Baseline characteristics of overall CARE-MS II population and HAD subpopulation.
| Overall |
HAD subgroup |
|||
|---|---|---|---|---|
| Alemtuzumab (N = 435) | SC IFNB-1a (N = 202) | Alemtuzumab (n = 103) | SC IFNB-1a (n = 42) | |
| Demographics | ||||
| Age (years) | ||||
| Mean (SD) | 34.7 (8.3) | 35.8 (8.8) | 32.7 (7.7) | 34.1 (8.7) |
| Min–max | 18.0–55.0 | 18.0–54.0 | 18.0–50.0 | 18.0−54.0 |
| Sex, n (%) | ||||
| Female | 287 (66.0) | 131 (64.9) | 69 (67.0) | 27 (64.3) |
| Male | 148 (34.0) | 71 (35.1) | 34 (33.0) | 15 (35.7) |
| Region, n (%) | ||||
| United States, Canada, Australia | 220 (50.6) | 102 (50.5) | 56 (54.4) | 26 (61.9) |
| Latin America, Europe,a Israel | 215 (49.4) | 100 (49.5) | 47 (45.6) | 16 (38.1) |
| Race, n (%) | ||||
| White | 392 (90.1) | 187 (92.6) | 97 (94.2) | 40 (95.2) |
| Othersb | 43 (9.9) | 15 (7.4) | 6 (5.8) | 2 (4.8) |
| Relapses in previous year | ||||
| Mean (SD) | 1.7 (0.9) | 1.5 (0.8) | 2.4 (0.8) | 2.3 (0.5) |
| Median (range) | 1.0 (0.0−5.0) | 1.0 (0.0−4.0) | 2.0 (2.0–5.0) | 2.0 (2.0–3.0) |
| EDSS score | ||||
| Mean (SD) | 2.7 (1.3) | 2.7 (1.2) | 2.6 (1.2) | 2.7 (1.2) |
| Median (range) | 2.5 (0.0−6.5) | 2.5 (0.0−6.0) | 2.5 (0.0–5.0) | 2.5 (0.0–5.0) |
| Duration of previous MS drug use,c months | ||||
| Mean (SD) | 35.0 (24.5) | 36.0 (23.6) | ||
| Median (range) | 28 (2−131) | 29 (6−115) | ||
| Number of previous MS drugs used,c n (%) | ||||
| 1 | 304 (69.9) | 151 (74.8) | ||
| 2 | 96 (22.1) | 41 (20.3) | ||
| 3 | 24 (5.5) | 9 (4.5) | ||
| ⩾ 4 | 11 (2.5) | 1 (0.5) | ||
| Mean (SD) | 1 (0.7) | 1 (0.6) | ||
| Median (range) | 1 (1−5) | 1 (1−4) | ||
| Previous MS drugs used,c n (%) | ||||
| IFNB-1a | 237 (54.5) | 108 (53.5) | ||
| IM IFNB-1a | 125 (28.7) | 46 (22.8) | ||
| SC IFNB-1a (22 or 44 µg) | 146 (33.6) | 73 (36.1) | ||
| IFNB-1b | 157 (36.1) | 63 (31.2) | ||
| Glatiramer acetate | 149 (34.3) | 69 (34.2) | ||
| Natalizumab | 17 (3.9) | 7 (3.5) | ||
| Immunoglobulin | 9 (2.1) | 1 (0.5) | ||
| Azathioprine | 6 (1.4) | 5 (2.5) | ||
CARE-MS: Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; HAD: highly active disease; SD: standard deviation; SC IFNB-1a: subcutaneous interferon beta-1a; EDSS: Expanded Disability Status Scale; IM IFNB-1b: intramuscular interferon beta-1b; MS: multiple sclerosis; EU: European Union.
Europe includes EU and non-EU countries.
Includes Black, Asian, American Indian or Alaska Native, and Other.
This information was not available for patients with HAD.
HRQL outcomes over 6 years
Alemtuzumab arm in the overall population
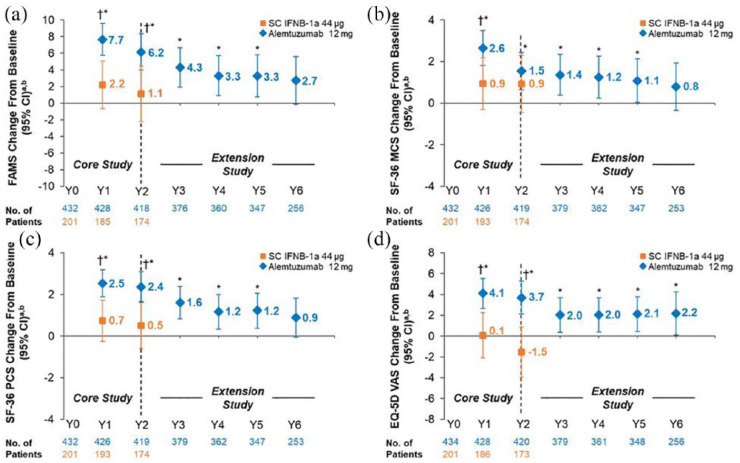
As previously reported,16 alemtuzumab-treated patients showed significant improvements from baseline on FAMS, SF-36 MCS, SF-36 PCS, and EQ-VAS at Year 2. Significant improvements were also observed versus SC IFNB-1a at Year 2 on FAMS, SF-36 PCS, and EQ-VAS (Figure 1). CARE-MS II patients maintained statistically significant improvements from baseline for up to 6 years on EQ-VAS, and up to 5 years on FAMS, SF-36 MCS, and SF-36 PCS; none of the observed improvements reached the threshold for clinical relevance. FAMS, and SF-36 MCS and PCS change from baseline scores showed numerical improvements at Year 6 in alemtuzumab-treated patients; however, the differences from baseline did not reach statistical significance or the threshold for clinical relevance (Figure 1 and Table 2).
Figure 1.
Change from baseline in HRQL over 6 years in RRMS patients from CARE-MS II. Change from core study baseline in (a) FAMS total, (b) SF-36 MCS, (c) SF-36 PCS, and (d) EQ-VAS scores. FAMS and EQ-VAS were administered at baseline (Month 0), every 6 months thereafter up to Year 3, and every 12 months from Years 3 to 6. The SF-36 was administered at baseline (Month 0), every 12 months thereafter up to Year 2, every 6 months from Years 2 to 3, and every 12 months from Years 3 to 6.
CARE-MS: Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; CI: confidence interval; EQ-VAS: European Quality of Life-5 Dimensions-3-level visual analog scale; FAMS: Functional Assessment of Multiple Sclerosis, version 4; HRQL: health-related quality of life; MCS: mental component summary; PCS: physical component summary; RRMS: relapsing-remitting multiple sclerosis; SC IFNB-1a: subcutaneous interferon beta-1a; SD: standard deviation; SF-36: 36-Item Short-Form Survey, version 2.
aData are least-squares means (95% CIs).
bAlemtuzumab baseline mean (SD) scores: FAMS total score, 119.1 (31.6); SF-36 MCS score, 44.9 (11.8); SF-36 PCS score, 42.7 (10.2); EQ-VAS score, 70.1 (19.1).
*p < 0.05 versus baseline (alemtuzumab-treated patients).
†p < 0.05 versus SC IFNB-1a.
Table 2.
Mean FAMS total, SF-36 MCS and PCS, and EQ-VAS scores at baseline and Year 6 in patients from the CARE-MS II study.
| Overall |
HAD subgroup |
|||
|---|---|---|---|---|
| Alemtuzumab (N = 435) | SC IFNB-1a (N = 202) | Alemtuzumab (n = 103) | SC IFNB-1a (n = 42) | |
| FAMS total | ||||
| Baseline | 119.1 (31.6) | 118.9 (32.7) | 122.0 (29.1) | 124.9 (34.5) |
| Year 6a | 123.4 (36.2) | 125.3 (34.6) | ||
| SF-36 MCS | ||||
| Baseline | 44.9 (11.8) | 43.9 (12.0) | 45.0 (11.6) | 44.7 (11.7) |
| Year 6a | 46.3 (12.7) | 48.7 (11.8) | ||
| SF-36 PCS | ||||
| Baseline | 42.7 (10.2) | 42.4 (10.2) | 43.3 (9.7) | 44.7 (10.4) |
| Year 6a | 44.1 (11.3) | 44.6 (10.8) | ||
| EQ-5D VAS | ||||
| Baseline | 70.1 (19.1) | 70.3 (17.7) | 72.0 (17.4) | 71.6 (18.4) |
| Year 6a | 74.0 (20.3) | 75.8 (19.3) | ||
FAMS: Functional Assessment of Multiple Sclerosis, version 4; SF-36: 36-Item Short-Form Survey, version 2; MCS: mental component summary; PCS: physical component summary; EQ-VAS: European Quality of Life-5 Dimensions-3-level visual analog scale; CARE-MS: Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; HAD: highly active disease; SC IFNB-1a: subcutaneous interferon beta-1a; SD: standard deviation.
Data are expressed as mean (SD).
Year 6 data were not available for SC IFNB-1a due to discontinuation of the active comparator arm after the 2-year core study period.
Alemtuzumab arm in the HAD subpopulation
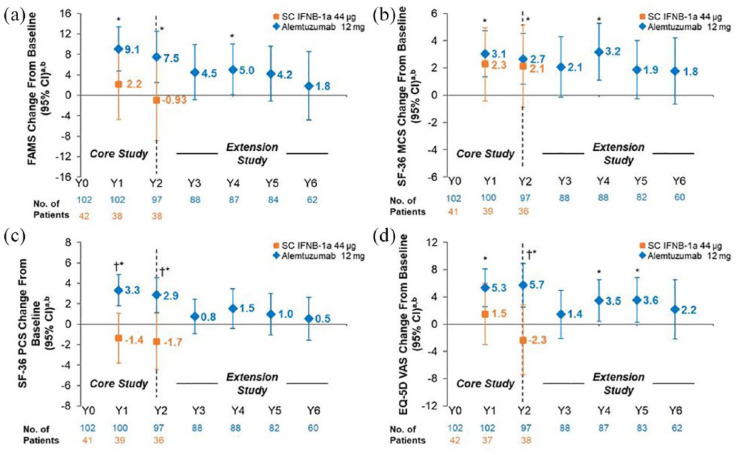
At Year 2, alemtuzumab-treated patients with HAD showed statistically significant improvements from baseline on FAMS, SF-36 MCS and PCS, and EQ-VAS, and statistically significant improvements versus SC IFNB-1a on SF-36 PCS and EQ-VAS (Figure 2). However, the improvements did not reach the threshold for clinical relevance. The change from baseline scores in all HRQL measures showed numerical improvements at Year 6 in patients with HAD; improvements did not reach statistical significance and were not clinically relevant (Figure 2 and Table 2).
Figure 2.
Change from baseline in HRQL over 6 years in the HAD subpopulation of RRMS patients from CARE-MS II. Change from core study baseline in (a) FAMS total, (b) SF-36 MCS, (c) SF-36 PCS, and (d) EQ-VAS scores. FAMS and EQ-VAS were administered at baseline (Month 0), every 6 months thereafter up to Year 3, and every 12 months from Years 3 to 6. The SF-36 was administered at baseline (Month 0), every 12 months thereafter up to Year 2, every 6 months from Years 2 to 3, and every 12 months from Years 3 to 6.
CARE-MS: Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; CI: confidence interval; EQ-VAS: European Quality of Life-5 Dimensions-3-level visual analog scale; FAMS: Functional Assessment of Multiple Sclerosis, version 4; HAD: highly active disease; HRQL: health-related quality of life; MCS: mental component summary; PCS: physical component summary; RRMS: relapsing-remitting multiple sclerosis; SC IFNB-1a: subcutaneous interferon beta-1a; SD: standard deviation; SF-36: 36-Item Short-Form Survey, version 2.
aData are least-squares means (95% CIs).
bAlemtuzumab baseline mean (SD) score: FAMS total score, 122.0 (29.1); SF-36 MCS score, 45.0 (11.6); SF-36 PCS score, 43.3 (9.7); and EQ-VAS score, 72.0 (17.4).
*p < 0.05 versus baseline.
†p < 0.05 versus SC IFNB-1a.
Discussion
Long-term HRQL evidence is critical to understanding treatment effects in chronic diseases such as RRMS and is an important indicator of treatment success from the patients’ perspective.25 Alemtuzumab-treated RRMS patients, including the HAD subpopulation, showed improvements from baseline in HRQL, which were maintained over 5 or 6 years, depending on the PRO measure. The Year 6 FAMS and SF-36 PCS changes from baseline scores were numerically greater than the 2-year changes for SC IFNB-1a-treated patients; however, the changes did not reach statistical significance and were not clinically relevant based on a responder definition of half an SD.19,22
Although the magnitude of improvement versus baseline was greatest in Years 1 and 2 after alemtuzumab treatment, patients’ scores did not fall below baseline in Years 4, 5, and 6 in each of the HRQL measures. This is an important finding given that patients with MS typically experience worsening in HRQL over time.26 Alemtuzumab-treated patients showed improvements, or at least stabilization, in both generic and disease-specific HRQL measures that were sustained over 6 years, and this was achieved with 50% of patients not receiving additional treatment (neither alemtuzumab nor another DMT) after Month 12. These HRQL results reflect the improvements in disability, reduced relapses, and slowed BVL reported previously for these patients.9–11
For the SF-36 PCS and MCS measures, the minimally important difference (MID) was established as a 5-point change, equivalent to half an SD.22 This MID threshold was established in healthy individuals rather than those with chronic disease, so it is unclear whether the threshold would be the same for patients with MS. An MID of half an SD has been suggested for the other PRO instruments as well.19 Although CARE-MS II patients did not reach this MID, their score increases were higher than those achieved in Phase 3 studies of natalizumab.23 Previous analyses to explore an MID in MS patients correlated changes in PRO scores with changes in EDSS.18 A 1-point change in SF-36 PCS and MCS scores and a 3-point change in FAMS score may represent MIDs for MS patients as those thresholds correlated with a 0.5-point EDSS change.27 In CARE-MS II, 43% of patients achieved 6-month CDI (defined as a ⩾1.0-point decrease in EDSS score from core study baseline confirmed over 6 months) over 6 years.11 Further analyses using an anchor-based approach to compare improvements in PRO scores with changes in EDSS are needed to determine the clinical relevance of this result.
There are few long-term HRQL studies of treatments for MS. Most studies were short-term (⩽2 years) and the majority used generic PRO instruments such as the SF-36,23 SF-12,28 or EQ-VAS.29 Few RRMS studies have randomized participants to two active treatments (rather than placebo) and used both disease-specific and generic PROs to evaluate HRQL. It is difficult to compare these studies due to differences in patient populations, PRO measures used, and length of follow-up.
This analysis is the first assessment of long-term HRQL in RRMS patients with a clearly defined HAD subpopulation. Although there is no standard definition of HAD, our definition (⩾2 relapses in the year prior to randomization and ⩾1 gadolinium-enhancing lesion at baseline) is consistent with definitions of rapidly evolving severe RRMS from the European Medicines Agency and the National Institute for Health and Care Excellence (⩾2 disabling relapses in 1 year plus ⩾1 gadolinium-enhancing lesions on brain MRI or a significant increase in T2 lesion load compared with a previous recent MRI).30,31 Other definitions include patients who had at least one relapse in the previous year while on therapy plus at least one gadolinium-enhancing lesion or nine or more T2-hyperintense lesions.32 HRQL data from randomized controlled trials are lacking for patients with HAD and represent a significant unmet need. The findings from this study support the hypothesis that use of effective therapies in patients with HAD can improve their quality of life outcomes.6
This study also had limitations. First, as discussed in our previous analysis,16 the SF-36 and EQ-VAS are generic instruments and therefore may not be as sensitive to MS-related HRQL changes as the disease-specific FAMS. Second, the CARE-MS II extension study was not powered to detect differences in HRQL outcomes (tertiary endpoints) among the alemtuzumab-treated RRMS population, and the HAD subgroup analyses were post hoc. Third, patients had knowledge of their treatment assignment and both groups used active treatments, which may have influenced patients’ HRQL responses. Fourth, not all patients had values for HRQL at each time point. Missing data over time can contribute to biased estimates of change by not accounting for the status of patients with incomplete/missing assessments. Finally, this analysis compared 6-year PROs with baseline scores for alemtuzumab-treated patients, as 6-year active comparator data were not available due to discontinuation of SC IFNB-1a after the 2-year core study period.
Conclusion
Over 6 years, alemtuzumab-treated RRMS patients with inadequate response to prior treatment reported improvement or stabilization in HRQL compared with baseline, including the subset of patients with HAD. Patients with HAD showed significant improvements in HRQL outcomes (as measured by SF-36 PCS and EQ-VAS) at 2 years after treatment with alemtuzumab compared with SC IFNB-1a; however, the improvements did not meet the threshold for clinical relevance.
Acknowledgments
Thanks go to Matthew Reaney, Luke Chung, and Colin Mitchell at Sanofi for their careful review and input. The authors also wish to thank Catherine Mirvis and Dipen Patel of Pharmerit International and Jaya Kolipaka of Eloquent Scientific Solutions for their assistance in writing this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.A. and D.C. have nothing to disclose. D.P.B. is currently employed by Sanofi on a contract basis (RWE/COA/PRO group). J.D.G. was an employee of Sanofi at the time of the analysis and is currently employed at Bristol-Myers Squibb, Lawrence Township, NJ, USA. D.H.M. was an employee of Sanofi at the time of the analysis and is currently employed at Cerevance, Inc., Boston, MA, USA, and reports personal fees from Sanofi outside the submitted work; in addition, he has patents US9664688 and US009498528 issued. M.M. and N.D. are employees of Sanofi.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Sanofi.
Contributor Information
Rafael Arroyo, Department of Neurology, Hospital Universitario Quirónsalud, Madrid, Spain.
Denise P Bury, Contractor to Sanofi Genzyme, Cambridge, MA, USA.
Jennifer D Guo, Sanofi, Cambridge, MA, USA; Bristol-Myers Squibb, Lawrence Township, NJ, USA.
David H Margolin, Sanofi, Cambridge, MA, USA; Cerevance, Inc., Boston, MA, USA.
Maria Melanson, Sanofi, Cambridge, MA, USA.
Nadia Daizadeh, Sanofi, Cambridge, MA, USA.
David Cella, Department of Medical Social Sciences, Center for Patient-Centered Outcomes, Institute for Public Health and Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
References
- 1. Global Burden of Disease Study 2015 (GBD 2015) Incidence, prevalence, and years lived with disability 1990–2015. Seattle, WA: Institute for Health Metrics and Evaluation, 2016. [Google Scholar]
- 2. Multiple sclerosis: Hope through research. Bethesda, MD: National Institute of Neurological Disorders and Stroke, 2012. [Google Scholar]
- 3. Confavreux C, Vukusic S. The natural history of multiple sclerosis. Rev Prat 2006; 56: 1313–1320. [PubMed] [Google Scholar]
- 4. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis, a geographically based study 10: Relapses and long-term disability. Brain 2010; 133: 1914–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubey D, Cano CA, Stuve O. Intractable and highly active relapsing multiple sclerosis—role of alemtuzumab. Neuropsychiatr Dis Treat 2015; 11: 2405–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernández Ó. Is there a change of paradigm towards more effective treatment early in the course of apparent high-risk MS? Mult Scler Relat Disord 2017; 17: 75–83. [DOI] [PubMed] [Google Scholar]
- 7. Canadian Agency for Drugs and Technologies in Health. Comparative clinical and cost-effectiveness of drug therapies for relapsing-remitting multiple sclerosis, 2013, https://www.cadth.ca/media/pdf/TR0004_RRMS_ScienceReport_e.pdf [PubMed]
- 8. Tramacere I, Del Giovane C, Salanti G, et al. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: A network meta-analysis. Cochrane Database Syst Rev 2015; (9): CD011381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012; 380(9856): 1829–1839. [DOI] [PubMed] [Google Scholar]
- 10. Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: Efficacy and safety findings. Neurology 2017; 89: 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ziemssen T, Thomas K. Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: An update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord 2017; 10(10): 343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mayer L, Fox E, LaGanke C, et al. Incidence of infusion-associated reactions decreases with subsequent courses of alemtuzumab: 5-year data from the CARE-MS extension study. Int J MS Care 2016; 18(s1): 1–11226917992 [Google Scholar]
- 13. Wray S, Havrdova E, Snydman DR, et al. Infection risk with alemtuzumab decreases over time: Pooled analysis of 6-year data from the CAMMS223, CARE-MS I, and CARE-MS II studies and the CAMMS03409 extension study. Mult Scler 2019; 25:1605–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cuker A, Bass A, Nadj C, et al. Immune thrombocytopenia in alemtuzumab-treated MS patients: Incidence, detection, and management. Mult Scler 2020; 26: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phelps R, Winston J, Wynn D, et al. Incidence, management, and outcomes of autoimmune nephropathies following alemtuzumab treatment in patients with multiple sclerosis. Mult Scler 2019; 25(9): 1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. González RA, Kita M, Crayton H, et al. Alemtuzumab improves quality-of-life outcomes compared with subcutaneous interferon beta-1a in patients with active relapsing-remitting multiple sclerosis. Mult Scler 2016: 23: 1367–1376. [DOI] [PubMed] [Google Scholar]
- 17. Singer B, Krieger S, Berkovich R, et al. Patients who had highly active RRMS and an inadequate response to prior therapy demonstrated durable efficacy with alemtuzumab: 5-year follow-up of the CARE-MS II study (p6.164). Neurology 2016; 86, https://n.neurology.org/content/86/16_Supplement/P6.164 [Google Scholar]
- 18. Cella DF, Dineen K, Arnason B, et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology 1996; 47: 129–139. [DOI] [PubMed] [Google Scholar]
- 19. Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: Another step toward consensus. J Clin Epidemiol 2005; 58(12): 1217–1219. [DOI] [PubMed] [Google Scholar]
- 20. Ware J, Kosinski M, Bjorner J, et al. Determining important differences in scores. User’s manual for the Sf-36v2® Health Survey. Lincoln, RI: QualityMetric Incorporated, 2007, pp. 125–133. [Google Scholar]
- 21. Johnston BC, Ebrahim S, Carrasco-Labra A, et al. Minimally important difference estimates and methods: A protocol. BMJ Open 2015; 5(10): e007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care 2003; 41(5): 582–592. [DOI] [PubMed] [Google Scholar]
- 23. Rudick RA, Miller D, Hass S, et al. Health-related quality of life in multiple sclerosis: Effects of natalizumab. Ann Neurol 2007; 62(4): 335–346. [DOI] [PubMed] [Google Scholar]
- 24. Van Reenen M, Oppe M. EQ-5D-3L User Guide. Rotterdam: EuroQol Research Foundation, 2015. [Google Scholar]
- 25. Ziemssen T, Hillert J, Butzkueven H. The importance of collecting structured clinical information on multiple sclerosis. BMC Med 2016; 14: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zwibel HL. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv Ther 2009; 26(12): 1043–1057. [DOI] [PubMed] [Google Scholar]
- 27. Steinman L, Wang H, Liu Y, et al. Defining clinical meaning of patient-reported outcomes with disability assessment in multiple sclerosis: An analysis of the CARE-MS II Study. Mult Scler 2014; 20(Suppl. 1): P802. [Google Scholar]
- 28. Svenningsson A, Falk E, Celius EG, et al. Natalizumab treatment reduces fatigue in multiple sclerosis. Results from the TYNERGY trial; a study in the real life setting. PLoS ONE 2013; 8(3): e58643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hersh CM, Hara-Cleaver C, Rudick RA, et al. Experience with fingolimod in clinical practice. Int J Neurosci 2015; 125: 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Technology appraisal guidance [TA127]: Natalizumab for the treatment of adults with highly active relapsing–remitting multiple sclerosis, https://www.nice.org.uk/guidance/ta127 (2007, accessed 25 August 2017).
- 31. Gilenya: European public assessment report—Product information, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002202/WC500104528.pdf (2011, accessed 25 August 2017).
- 32. Derfuss T, Bergvall NK, Sfikas N, et al. Efficacy of fingolimod in patients with highly active relapsing–remitting multiple sclerosis. Curr Med Res Opin 2015; 31(9): 1687–1691. [DOI] [PubMed] [Google Scholar]