Abstract
Objective:
To validate kappa free light chain (KFLC) and lambda free light chain (LFLC) indices as a diagnostic biomarker in multiple sclerosis (MS).
Methods:
We performed a multicenter study including 745 patients from 18 centers (219 controls and 526 clinically isolated syndrome (CIS)/MS patients) with a known oligoclonal IgG band (OCB) status. KFLC and LFLC were measured in paired cerebrospinal fluid (CSF) and serum samples. Gaussian mixture modeling was used to define a cut-off for KFLC and LFLC indexes.
Results:
The cut-off for the KFLC index was 6.6 (95% confidence interval (CI) = 5.2–138.1). The cut-off for the LFLC index was 6.9 (95% CI = 4.5–22.2). For CIS/MS patients, sensitivity of the KFLC index (0.88; 95% CI = 0.85–0.90) was higher than OCB (0.82; 95%CI = 0.79–0.85; p < 0.001), but specificity (0.83; 95% CI = 0.78–0.88) was lower (OCB = 0.92; 95% CI = 0.89–0.96; p < 0.001). Both sensitivity and specificity for the LFLC index were lower than OCB.
Conclusion:
Compared with OCB, the KFLC index is more sensitive but less specific for diagnosing CIS/MS. Lacking an elevated KFLC index is more powerful for excluding MS compared with OCB but the latter is more important for ruling in a diagnosis of CIS/MS.
Keywords: Multiple sclerosis, KFLC, OCB, CSF, biomarkers
Introduction
Cerebrospinal fluid (CSF) assessment is often part of the diagnostic workup for multiple sclerosis (MS), and its value is supported by the latest 2017 revisions of the McDonalds criteria.1 CSF examination is frequently performed in diagnosing MS for excluding alternative diagnoses, although, in the current MS criteria,2 the assessment of oligoclonal IgG bands (OCB) plays a limited role. However a recent study showed the added value of OCB in the MS diagnostic criteria.3 In the 2017 revisions, the OCB have a prominent role in patients with a clinically isolated syndrome (CIS). The presence of both magnetic resonance imaging (MRI) criteria for dissemination in space (DIS) and CSF-specific OCB will enable to establish the MS diagnosis in patients with a single clinical episode suggestive of central nervous system (CNS) inflammatory demyelinating disease. The OCB assessment also has important prognostic value in CIS and MS.4,5 However, the assessment of OCB is labor-intensive, requires trained personnel, and is in some cases examiner- and method-dependent, which may affect its reliability.
Alongside intact immunoglobulins, which are composed of two heavy and two light chains, plasma cells produce and secrete immunoglobulin free light chains (FLC) of either kappa (KFLC) or lambda (LFLC) chains. KFLC and LFLC can be detected in both CSF and serum.6–10 Since the late 1970s, multiple studies have reported increased CSF levels of KFLC in MS.6–14 The analytical specificity of the earlier methods (e.g. radioimmunoassay,15,16 quantitative enzyme-linked immunosorbent assay)8,17 was insufficient, but with the recent emergence of the more sensitive nephelometric and turbidimetric FLC assays, research in this field has been revived. Nephelometric (and turbidimetric) FLC level determination has the additional advantage compared to OCB of being assessed by an automated procedure and being quantifiable.18
Using the FLC assay,19 recent studies showed that both CSF KFLC levels and the KFLC index are increased in patients with CIS or relapsing-remitting MS (RRMS) compared with controls.8,14,18,20–22 The use of an index measure is necessary, for example [CSF KFLC/serum KFLC]/[CSF albumin/serum albumin], to include blood-CSF barrier permeability.10,14 The KFLC index has comparable sensitivity and specificity to OCB for diagnosis of MS and CIS.14,21,23 However, large-scale studies comparing diagnostic performance of the two methods and to define the cut-off of FLC are lacking. The main aim of this study was to validate KFLC and LFLC indices as a diagnostic biomarker in MS compared with OCB in a large multicenter study including samples from 18 MS centers across Europe.
Methods
Patients and controls
Eighteen MS centers participated, located in the Netherlands, Spain, France, Belgium, Hungary, Italy, Poland, Turkey, Denmark, Serbia, Austria, and Switzerland. We selected 745 paired CSF/serum samples from patients with known OCB status, diagnosed as CIS (n = 242), RRMS (n = 235), primary-progressive MS (PPMS) (n = 41), and secondary-progressive MS (SPMS) (n = 8). We also included inflammatory neurological disease controls (INDC) (n = 67), non-inflammatory neurological disease controls (NINDC) (n = 76), symptomatic controls (SC) (n = 49), and healthy controls (HC) (n = 27) as defined previously.24 The different control groups were pooled into one control group (n = 219). The CIS and MS patients were also pooled (CIS/MS) (n = 526).
The large majority (84%) of the CIS/MS patients fulfilled the 2010 McDonald criteria2 but, in some cases, the patients were diagnosed according to the 2005 McDonald criteria25 (16%). Table 1 presents the demographic and clinical characteristics of the patients and controls.
Table 1.
Demographic and clinical characteristics of the included patients and controls at the time of lumbar puncture.
| Disease group | N = 745 | Sex (female) N (%) |
Age (years) Mean ± SD |
Stage/type of disease RR/SP/PP |
Disease duration (months) Median (IQR) |
No. of patients using treatment | No. of patients on corticosteriods Median (IQR) |
EDSS Median (IQR) |
OCB positive N (%) |
|---|---|---|---|---|---|---|---|---|---|
| CIS | 242 | 177 (73.1) | 35 ± 10 | n.a. | 0.8 (0.3–3.0) | 1 | 4 | 2.0 (1.0–2.5)a | 186 (76.9) |
| MS | 284 | 170 (59.9) | 38 ± 11 | 235/8/41 | 13.1 (2.4–48.1) | 3b | 10 | 2.0 (1.5–3.5)c | 245 (86.3) |
| Controls | 219 | 130 (59.4)d | 42 ± 12 | n.a. | n.d. | 1 | 1 | n.a. | 17 (7.8) |
| - NINDC | 76 | 49 (64.5) | 45 ± 13 | n.a. | n.d. | 0 | 0 | n.a. | 4 (5.3) |
| - INDC | 67 | 33 (49.3) | 42 ± 13 | n.a. | n.d. | 1e | 1e | n.a. | 13 (19.4) |
| - SC | 49 | 32 (65.3) | 39 ± 10 | n.a. | n.d. | 0 | 0 | n.a. | 0 (0) |
| – HC | 27 | 16 (59.3) | 41 ± 10 | n.a. | n.d. | 0 | 0 | n.a. | 0 (0) |
RR: relapsing-remitting; SP: secondary progressive; PP: primary progressive; disease duration: time between CSF lumbar puncture and date of onset first neurological complaints; EDSS: Expanded Disability Status Scale, OCB: oligoclonal IgG bands; CIS: clinically isolated syndrome; MS: multiple sclerosis; n.a.: not applicable; n.d.: not determined; NINDC: non-inflammatory neurological disease control; INDC: inflammatory neurological disease controls; SC: symptomatic control; HC: healthy control; CSF: cerebrospinal fluid; CLIPPERS: chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids.
All participants gave written informed consent at the center where the CSF/serum was conducted.
In 178 CIS patients, an EDSS was available.
Disease-modifying treatment in combination with corticosteroids was used in two MS patients. In one patient, only disease-modifying treatment.
In 107 MS patients, an EDSS was available.
In six control patients, the sex was unknown.
One INDC (CLIPPERS syndrome) patient had steroids and methotrexate at the time of lumbar puncture.
CSF and serum samples
Only CSF samples that were immediately centrifuged and stored in polypropylene tubes within 2 hours at −80°C, at the local center, were included. The assessment of OCB had been performed by isoelectric focusing (on agarose or polyacrylamide gel), followed by immunofixation by the centers as part of the diagnostic workup.
Samples were taken between 2005 and 2016, with a median age of 2.9 years (IQR = 1.7–5.7).
We used fresh aliquots, and in our lab, we did not freeze and thaw the samples during the analyses. As far as we know, no effects of freezing and thawing have been reported.
KFLC, LFLC, and albumin analysis
KFLC, LFLC, and albumin concentrations in CSF and serum samples were analyzed using the turbidimetric analyzer SPAplus® (The Binding Site, Birmingham, UK) with the serum free light chain immunoassay (Freelite®, The Binding Site, Birmingham, UK) according to the manufacturer’s instructions. All samples were measured centrally in the Neurochemistry Laboratory of the Department of Clinical Chemistry of the VU University Medical Center (VUmc), Amsterdam, the Netherlands. All samples were run blinded for the clinical data.
To verify the QC data supplied by the manufacturer, we calculated intra-assay coefficient of variation (CV) by taking the mean CVs of four replicates of five samples (CSF/serum) within one run. We calculated inter-assay CV based on n = 5 samples (CSF/serum) measured in 5 different days. CV values for KFLC and LFLC were all found to be lower than those supplied by the manufacturer (Supplemental Table 1). CV values for albumin were comparable to those supplied by the manufacturer. Assay linearity was experimentally confirmed for albumin (serum and CSF) and the FLC assays in serum and showed that recalculated values varied by 25.7% (KFLC) and 14.2% (LFLC) from the original value.
For 29 samples, CSF albumin results were below detection. Here, we assigned a random uniform value between 35 mg/mL (lowest detected value in undiluted rerun) and 175 mg/mL (formal detection limit).
FLC indices
We determined the CSF/serum quotients (Q FLC) of KFLC and LFLC and calculated indices in order to take possible blood-CSF diffusion into account. The FLC indices were defined as [Q FLC] × [serum albumin/CSF albumin].
Statistics
Differences in demographics, clinical characteristics, FLC concentrations, and FLC indices were tested via the Mann–Whitney U test for comparison of two groups of non-normally distributed data. For comparison of more than two groups, Kruskal–Wallis test with post hoc Dunn’s multiple comparison test was applied. For normally distributed continuous variables, an independent-samples t test or one-way analysis of variance (ANOVA) with post hoc Bonferroni correction was applied. For binary variables, a chi-square test was performed. These statistical analyzes were performed in SPSS 22.0 (IBM Crop., Armonk, NY, USA).
Gaussian mixture modeling was used to define cut-offs for abnormal FLC indices using the R statistical software program version 3.2.1 mixtools package. First, the number of distributions that best described the data was determined with the R boot.comp function. Next, we defined a data-driven cut-off as the point where the lines of two fitted normal distributions crossed each other. The main analyses included all subjects. Data were log-transformed because FLC indices were not normally distributed. Based on the defined cut-off, subjects were classified as positive or negative for kappa or lambda FLC as binary result, similar as the available results for OCB status. As extra comparisons, we combined the different tools to compare with the single measurement OCB. The three combinations were as follows: KFLC with OCB, LFLC with OCB, and KFLC with LFLC. We defined the outcome of the combination as followed: when one of the measurements is positive, the combination is positive, when both are negative, the combination is negative.
To compare the sensitivity, specificity, and accuracy between the two different diagnostic tools (OCB and FLC), the McNemar test was used (SPSS 22.0 (IBM Crop., Armonk, NY, USA)). The positive and negative predictive values (PPVs and NPVs) were compared using the R package DTcompair. The p values < 0.05 were considered statistically significant.
Results
Paired CSF and serum samples from a total of 745 patients were included in this multicenter study (see Supplemental Figure 1 for the flowchart of patient selection). The patient groups (n = 526) consisted of 242 CIS and 284 MS patients. The control group (n = 219) consisted of 76 NINDC, 67 INDC, 49 SC, and 27 HC. The control group was older (mean = 42 ± 12 years) than the CIS/MS patient group (mean = 35 ± 10 years, p < 0.001). There was no significant difference in sex distribution between the two groups (p = 0.20). Only 7.7% of the control group had a positive OCB status. The diagnoses of these patients are as follows: Tolosa hunt (n = 2), meningitis (n = 1), neurosarcoidosis (n = 2), lupus (n = 1), transient global amnesia (n = 1), stroke (n = 1), post-infectious myelitis (n = 1), myelitis (n = 1), encephalitis (n = 1), epilepsy (n = 1), ADEM (n = 1), neurodegeneration (n = 1), INDC, exact diagnosis unknown (n = 2), and neuritis optica (n = 1).
For a detailed list including the diagnoses of non-inflammatory neurological diseases and inflammatory neurological diseases included in the groups, see Supplemental Tables 2A and 2B.
FLC concentrations and FLC indices
Table 2 shows the levels and indices of the KFLC and LFLC in CIS/MS patients and controls. CSF KFLC and CSF LFLC concentrations were significantly increased in CSF of CIS/MS patients compared to controls (both p < 0.001). In addition, KFLC and LFLC concentrations in CSF were higher in MS patients than CIS patients (both p < 0.001).
Table 2.
FLC in CIS/MS and controls.
| A | CIS/MS (n = 526) | Controls (n = 219) | p-value |
|---|---|---|---|
| CSF KFLC (mg/L) | 3.7 (1.0–10.1) | 0.2 (0.1–0.4) | <0.001 |
| Serum KFLC (mg/L) | 12.4 (9.8–15.7) | 13.7 (10.6–16.9) | 0.001 |
| KFLC index | 75.6 (21.8–197.0) | 2.8 (2.2–4.9) | <0.001 |
| KFLC index ⩾ 6.6 | 460 (87.5) | 38 (17.4) | – |
| B | CIS/MS (n = 543) | Controls (n = 202) | p-value |
| CSF LFLC (mg/L) | 0.5 (0.2–1.3) | 0.2 (0.2–0.3) | < 0.001 |
| Serum LFLC (mg/L) | 11.3 (9.2–13.7) | 12.1 (9.8–15.4) | 0.001 |
| LFLC index | 11.6 (5.3–33.3) | 3.3 (2.5–4.7) | < 0.001 |
| LFLC index ⩾ 6.9 | 359 (68.3) | 30 (13.7) | – |
FLC: free light chains; CIS: clinically isolated syndrome; MS: multiple sclerosis; A: KFLC in CIS/MS and controls; CSF: cerebrospinal fluid; KFLC: kappa free light chain; index: FLC quotient/albumin quotient; B: LFLC in CIS/MS and controls; LFLC: lambda free light chains; IQR: interquartile range.
Values are given as n (%) or as median (IQR).
KFLC and LFLC serum concentrations were significantly higher in the control group than CIS/MS (both, p = 0.001) but did not differ significantly between CIS and MS (KFLC p = 0.33, LFLC p = 1.00).
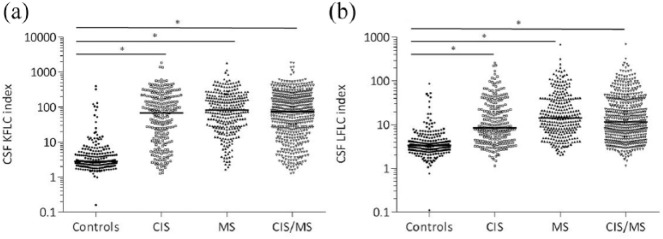
See Figure 1(a) for the KFLC indices per subgroup and Figure 1(b) for the LFLC indices per subgroup. KFLC and LFLC indices were significantly increased in CIS/MS compared to controls (both p < 0.001). In addition, FLC indices were higher in MS than in CIS (KFLC p = 0.07, LFLC p < 0.001).
Figure 1.
CSF KFLC and LFLC indices of CIS, MS, CIS/MS and controls. (a) Levels of KFLC indices, (b) levels of LFLC indices. Horizontal bars in the scatter dot plot represent the median.
CSF: cerebrospinal fluid; KFLC: kappa free light chains; LFLC: lambda free light chains; CIS: clinically isolated syndrome; MS: multiple sclerosis.
*p ⩽ 0.001.
FLC index cut-off
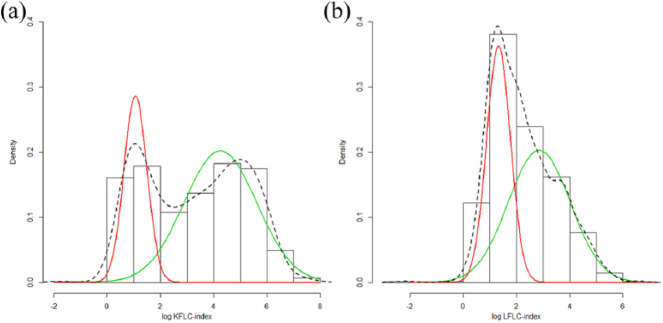
In the total cohort (n = 745) (all diagnostic subgroups), a bimodal distribution of the log-transformed KFLC index values fitted the data best. This yielded a cut-off for the log-KFLC index of 1.89 (95% confidence interval (CI) = 1.65–4.92) (Figure 2(a)), which corresponds to an KFLC index of 6.6 (95% CI = 5.2–138.1) on the original scale.
Figure 2.
Cerebrospinal fluid (a) KFLC and (b) LFLC cut-off values based on mixture modeling. The red (low values) and green (high values) lines are the individual components of the estimated mixture distributions, the dotted line is the combined estimated mixture distribution. The cut-off is defined as the point where the red and green lines cross.
For the LFLC index, a bimodal distribution yielded an optimal cut-off for the log-LFLC index of 1.9 (95% CI = 1.5–3.1) (Figure 2(b)), which corresponds to an LFLC index of 6.9 (95% CI = 4.5–22.2) on the original scale.
Diagnostic sensitivity and specificity of the indices compared to OCB
KFLC index
When pooling CIS and MS as one group (CIS/MS), the sensitivity to identify CIS/MS from controls of the KFLC index (0.88) was significantly higher than of OCB (0.82; p < 0.001) at the cost of a significantly lower specificity (KFLC index = 0.83, OCB = 0.92; p < 0.001) (see Table 3).
Table 3.
Diagnostic sensitivity and specificity of the FLC indices compared to OCB.
| OCB | KFLC | p-value | LFLC | p-value | |
|---|---|---|---|---|---|
| CIS/MS vs controls | |||||
| Sensitivity | 0.82 (95% CI = 0.79–0.85) | 0.88 (95% CI = 0.85–0.90) | < 0.001 | 0.66 (95% CI = 0.62–0.70) | < 0.001 |
| Specificity | 0.92 (95% CI = 0.89–0.96) | 0.83 (95% CI = 0.78–0.88) | < 0.001 | 0.86 (95% CI = 0.81–0.91) | 0.019 |
| Accuracy | 0.85 (95% CI = 0.82–0.88) | 0.86 (95% CI = 0.84–0.89) | 1.000 | 0.74 (95% CI = 0.70–0.77) | < 0.001 |
| PPV | 0.96 (95% CI = 0.94–0.98) | 0.92 (95% CI = 0.90–0.95) | < 0.001 | 0.92 (95% CI = 0.90–0.95) | 0.001 |
| NPV | 0.68 (95% CI = 0.63–0.73) | 0.73 (95% CI = 0.68–0.79) | 0.010 | 0.53 (95% CI = 0.48–0.58) | < 0.001 |
| CIS vs controls | |||||
| Sensitivity | 0.77 (95% CI = 0.72–0.82) | 0.81 (95% CI = 0.76–0.86) | 0.150 | 0.60 (95% CI = 0.54–0.66) | < 0.001 |
| Specificity | 0.92 (95% CI = 0.89–0.96) | 0.83 (95% CI = 0.78–0.88) | < 0.001 | 0.86 (95% CI = 0.82–0.91) | 0.019 |
| Accuracy | 0.84 (95% CI = 0.80–0.88) | 0.82 (95% CI = 0.78–0.85) | 0.149 | 0.73 (95% CI = 0.68–0.77) | < 0.001 |
| PPV | 0.92 (95% CI = 0.88–0.95) | 0.84 (95% CI = 0.79–0.88) | < 0.001 | 0.83 (95% CI = 0.77–0.89) | 0.001 |
| NPV | 0.78 (95% CI = 0.73–0.83) | 0.79 (95% CI = 0.74–0.84) | 0.56 | 0.66 (95% CI = 0.61–0.72) | < 0.001 |
| MS vs controls | |||||
| Sensitivity | 0.86 (95% CI = 0.82–0.90) | 0.93 (95% CI = 0.90–0.97) | < 0.001 | 0.75 (95% CI = 0.70–0.80) | < 0.001 |
| Specificity | 0.92 (95% CI = 0.89–0.96) | 0.83 (95% CI = 0.78–0.88) | < 0.001 | 0.86 (95% CI = 0.81–0.91) | 0.019 |
| Accuracy | 0.89 (95% CI = 0.86–0.92) | 0.89 (95% CI = 0.86–0.92) | 1.000 | 0.80 (95% CI = 0.77–0.84) | < 0.001 |
| PPV | 0.94 (95% CI = 0.91–0.97) | 0.88 (95% CI = 0.84–0.91) | < 0.001 | 0.88 (95% CI = 0.84–0.92) | 0.002 |
| NPV | 0.84 (95% CI = 0.79–0.88) | 0.91 (95% CI = 0.86–0.95) | 0.001 | 0.73 (95% CI = 0.68–0.78) | < 0.001 |
FLC: free light chains; OCB: oligoclonal IgG bands; KFLC: kappa free light chains; LFLC: lambda free light chains; CIS: clinically isolated syndrome; MS: multiple sclerosis; PPV: positive predictive value; NPV: negative predictive value.
To compare the sensitivity and specificity between the two different diagnostic tools (OCB and FLC), the McNemar test was used.
When identifying CIS patients from controls, the sensitivities did not differ significantly between KFLC and OCB (KFLC index = 0.81, OCB = 0.77; p = 0.15), but the specificity of OCB was significantly higher (KFLC index = 0.83, OCB = 0.92, p < 0.001). Identifying MS patients from controls, the sensitivity of the KFLC index (0.93) was significantly higher than of OCB (0.86; p < 0.001) but the specificity (0.83) was significantly lower (0.92; p < 0.001).
The accuracies were similarly high for both biomarkers for all three comparisons. The PPVs were higher for OCB (in all three comparisons p < 0.001). The NPV was the same for both markers (p = 0.56) in CIS versus controls, but when pooling CIS/MS and in MS alone, the NPVs were slightly higher for KFLC (p = 0.010, p < 0.001) (see Table 3).
LFLC index
When pooling CIS and MS in one group (CIS/MS), the sensitivity to identify CIS/MS from controls of the LFLC index (0.66) was significantly lower than that of OCB (0.82; p < 0.001). The specificity to discriminate CIS/MS from controls was also significantly lower (0.86) than for OCD (0.92; p = 0.019).
When identifying CIS patients from controls, the sensitivities did differ; the LFLC index was significantly lower than OCB (LFLC index = 0.60; OCB = 0.77; p < 0.001). The specificity of LFLC in CIS was lower than OCB (LFLC index = 0.86; OCB = 0.92; p = 0.019). Identifying MS patients from controls, the sensitivity of the LFLC index (0.75) was significantly lower than OCB (0.86; p < 0.001). The specificity (0.86) was also significantly lower than OCB (0.92; p = 0.019). The accuracies and NPV were significantly lower for LFLC than OCB for all three comparisons. The PPV was significantly lower for LFLC than OCB when comparing MS with controls, and in the other comparisons, the PPV of LFLC was similar to OCB (see Table 3).
Combination of the different tools compared to single measurement
Three combinations were made, KFLC index–OCB, LFLC index–OCB, and KFLC index–LFLC index, and all were compared with the analysis of OCB alone. Sensitivity and specificity were calculated for all the subgroups compared to the control group. The sensitivity of the combination OCB with the KFLC index increased to 0.88, and the specificity of this combination decreased to 0.83. The same results were obtained for the combinations of LFLC index–OCB and KFLC index–LFLC index compared to single OCB, showing a slightly higher sensitivity (LFLC index–OCB = 0.87, KFLC–LFLC index = 0.87) and a lower specificity (0.83 and 0.80, respectively).
Sensitivities and specificities in alternative subgroups
Including patients with the diagnosis CIS (according to McDonald 2005 criteria) as RRMS patients resulted in lower sensitivities of OCB, KFLC, and LFLC that were seen when comparing CIS patients with controls. However, the p value did not change relevantly when comparing the new sensitivities for OCB, KFLC, and LFLC. No relevant differences were seen when comparing MS to control group.
No relevant differences were seen when we exclude patients with the CIS diagnosis according to McDonald 2005 criteria.
When excluding INDC from the control group, higher specificities were seen for OCB, KFLC, and LFLC when comparing CIS/MS with the controls. However, the p value did not change relevantly when comparing the new specificities of OCB, KFLC, and LFLC (data not shown).
Discussion
Our study indicates that the KFLC index is a valid test for diagnosing CIS/MS. Compared to OCB, the KFLC index is more sensitive at the cost of a lower specificity. This trade-off resulted in a higher NPV for the KFLC index compared to OCB, but a lower PPV. In addition, our results indicate that the LFLC index is not a valid test for diagnosing CIS/MS.
Our sensitivity and specificity for the KFLC index in CIS/MS were lower than a few much smaller previous studies,18,23,26 which reported sensitivity in the range of 0.93–0.95 and specificity in the range of 0.91–1.00. The study of Desplat-Jégo et al.9 showed a lower sensitivity of 0.70 and a lower specificity of 0.82 for the KFLC index in MS patients. The more recent study of Vasilj et al.27 showed a lower sensitivity of 0.71, however a higher specificity of 0.98. The results of the comparison of KFLC and OCB are in line with results of a recent multicenter study,14 where a cut-off of 5.9 was employed to validate KFLC in CSF as a diagnostic biomarker in 60 CIS patients, 60 MS patients compared to 60 OND, reporting a higher sensitivity of the KFLC index (0.78) compared to OCB (0.72) for diagnosis of CIS. In MS, the sensitivity of the KFLC and OCB was comparable (0.93 vs 0.93). However, the specificity (0.95) in CIS and MS was higher compared to our study. Another paper used the same 5.9 cut-off; this resulted in a sensitivity and specificity of 0.96 and 0.98, respectively, in MS patients.21
This discrepancy in the crude sensitivity and specificity of the KFLCs may be due to the more heterogeneous control group in our study compared to the previous studies, due to pooling of the CIS/MS group or the inclusion of not only clinical definite MS patients. For example, similar as for the OCB, KFLCs can be elevated in inflammatory controls,28 and thus specificity will be lower when included. Nevertheless, this is a very relevant control group in differential diagnosis of MS. The unprecedented large number of patients and the large heterogeneous control group in this study gave us a reflection of the real-life clinical situation, thus avoiding spectrum bias, and allowed us to give a more representative sensitivity and specificity for OCB, KFLC, and LFLC indices.
Noticeable is that the sensitivity and specificity of OCB to discriminate MS patients from controls were lower in our study than previously reported.10,29,30 However, a meta-analysis published in 201331 (13,467 patients) showed that the diagnostic specificity of OCB diminished if other inflammatory etiologies were considered. Therefore, the lower specificities in our study may also be due to inclusion of various control groups.
The cut-off in this study was calculated using a data-driven Gaussian mixture modeling approach. We chose a different approach compared to other studies that applied, for example, receiver operating characteristic (ROC) curve analysis and area under the curve (AUC) values,9,10,18,23,27,32 because we reasoned that the cut-off should be defined by biological levels (data driven) and not based on clinical diagnosis, which is an imperfect golden standard. We determined a cut-off of 6.6 for abnormal KFLC indices and 6.9 for abnormal LFLC indices. Our cut-off for KFLC is in line with a previous multicenter study showing a KFLC index cut-off of 5.9.10 This almost comparable cut-off for the KFLC index in two multicenter studies supports its robustness and implies that it can be used as an universal cut-off.
There are some limitations in this study. One limitation was that not all patients were diagnosed based on the same MS criteria; most patients by McDonald 2010 (84%) but a few with McDonald 2005, which may have influenced the diagnosis of CIS patients particularly. CIS patients diagnosed before 2010 may very well be MS patients according to McDonald 2010, because in the 2005 criteria, MS diagnosis was more stringent. None of the patients were diagnosed by the new 2017 criteria, because of the retrospective setup, and thus imaging information was not collected. We address this problem by pooling all CIS and MS patients. Another reason for pooling CIS and MS is that we did not have the data to test CIS converting to MS versus non-converting CIS, because we did not have follow-up data. We performed several sensitivity analyses in CIS or MS patients separately, and by reclassifying and excluding specific clinical groups (Table 3). In these analyses, similar results were observed, suggesting that our results are robust for the total population. Another limitation is that we did not repeat the OCB analysis per patient centrally, but relied on the original local outcomes. However, we received the samples and OCB status from expertise MS centers (participating in the BioMSeu consortium) using standardized protocols.33 Moreover, inter-laboratory agreement is reported to be good for OCB, for example an inter-laboratory agreement of kappa > 0.8 between 19 participating laboratories in Spain was observed.34
One more important note is that the best set up for the study would have been if the test population should be suspected MS cases and not already diagnosed with MS. Still, as provided in Supplemental Table 2B, we included various INDC and quite some patients initially suspected for demyelinating disease.
Alongside the sensitivity and specificity results of the indices, we found significantly increased FLC concentrations and quotients in CSF of CIS/MS patients compared to the control groups. However, our main focus in this study was in the FLC indices and not in the concentrations of the FLC. To control for blood-CSF barrier function, we used indices instead of concentrations.
Combination of different markers (KFLC index–OCB, LFLC index–OCB, and KFLC index–LFLC index) compared to the single measurement OCB, showed that the combination KFLC index–OCB compared to single OCB gave a slightly higher sensitivity (0.88). However, the specificity became lower (0.83). The same results were seen in the combination LFLC index–OCB and KFLC index–LFLC index compared to single OCB. By definition, the sensitivity become higher and the specificity lower when you decide beforehand that the combination test will be positive when the test is positive in one of the two.
For clinical practice, the KFLC index is more accurate in excluding CIS/MS compared to OCB but for ruling in a diagnosis of CIS/MS, analysis of OCB appears to be more accurate. If we replace OCB by KFLC in diagnostic practice, there is a slightly higher chance that a patient with a diagnosis different from MS will get the diagnosis of MS and maybe unnecessarily exposed to potential negative side effects of early treatment. Since the KFLC index is more sensitive at the cost of a lower specificity, we should stress that replacement of OCB by the KFLC index is not optimal to arrive at high diagnostic certainty. However, with the higher sensitivity of KFLC, an earlier treatment start may be considered. Whether it is an option to start treatment based on the KFLC result and clinical/MRI findings according to the novel McDonald criteria or whether the treatment may be adapted after a first-year evaluation is subject of further studies and discussions.
In conclusion, this study indicates that the KFLC index is a valid tool in the diagnostic process of MS. Since this marker is measured by a faster and rater-independent analytical procedure, it should be considered as a potential cost-effective replacement of the OCB, especially when CSF analysis will regain a more prominent role in the 2017 revisions of the McDonald criteria.
Supplemental Material
Supplemental material, MSJ845844_Supplementary_Figure_1 for Kappa free light chains is a valid tool in the diagnostics of MS: A large multicenter study by CE Leurs, HAM Twaalfhoven, BI Lissenberg-Witte, V van Pesch, I Dujmovic, J Drulovic, M Castellazzi, T Bellini, M Pugliatti, J Kuhle, LM Villar, JC Alvarez-Cermeño, R Alvarez-Lafuente, H Hegen, F Deisenhammer, LM Walchhofer, E Thouvenot, M Comabella, X Montalban, L Vécsei, C Rajda, D Galimberti, E Scarpini, A Altintas, K Rejdak, JL Frederiksen, G Pihl-Jensen, PEH Jensen, M Khalil, MM Voortman, F Fazekas, A Saiz, D La Puma, M Vercammen, L Vanopdenbosch, BMJ Uitdehaag, J Killestein, C Bridel and C Teunissen in Multiple Sclerosis Journal
Acknowledgments
Reagents for this study were provided by the Binding Site®. The authors would like to thank G. Oral and M. Atik for helping A. Altintas collecting clinical data and samples.
Footnotes
Author Contributions: C.E.L. contributed to study design, collecting all data, statistical analysis, interpretation of the data, and drafting the manuscript. H.A.M.T. contributed to KFLC and LFLC data analyses, interpretation of the data, and revising the manuscript for intellectual content. B.I.L.-W. contributed to statistical analysis of the data (Biostatistician). B.M.J.U., J.K., C.B., and C.T. contributed to study design, interpretation of the data, and revising the manuscript for intellectual content. All other authors helped by selecting and collecting samples and clinical information. All other authors helped by revising the manuscript for intellectual content.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclosures: C.E. L. reports a grant from Stichting MS research. H.A.M. T., B.I.L.-W., M.C.,T.B., C.R., L.M.W., D. G., E.S., and P.E.H.J., and D.L.P. report no disclosures. V.V.P. received travel grants from Biogen, Bayer Schering, Genzyme, Merck, Teva, Sanofi and Roche. His institution receives honoraria for consultancy and lectures from Biogen, Bayer Schering, Sanofi, Merck, Roche, Teva and Novartis Pharma as well as research grants from Novartis Pharma, Bayer Schering, Sanofi, and Roche. I.D. received lecture fees and/or travel grants from Merck Serono, Rosche, Bayer, Medis, Teva, and Boehringer Ingelheim; received honoraria for acting as an advisor for Bayer; and was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 175031). J.D. received honoraria for consulting services, travel expenses for scientific meetings, and speaking honoraria from Bayer Schering Pharma, Merck Serono, Medis, Rosche, and Actavis and was supported by the Republic of Serbia Ministry of Education, Science and Technological Development grant support (No. 175031). M.P. participated in Advisory Board Meetings of Bayer Schering and Genzyme, and received travel or speaker honoraria from Almirall, Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi-Aventis, and Teva Neurosciences. J.K.’s institution (University Hospital Basel) received and used, exclusively for research support, consulting fees from Biogen, Novartis, Protagen AG, Roche, Teva; speaker fees from the Swiss MS Society, Biogen, Novartis, Roche, Genzyme; travel expenses from Merck Serono, Novartis, Roche; and grants from ECTRIMS Research Fellowship Program, University of Basel, Swiss MS Society, Swiss National Research Foundation (320030_160221), Bayer AG, Biogen, Genzyme, Merck, Novartis, Roche. L.M.V. received payment for lecturing, travel expenses, or research grants from Sanofi-Genzyme, Merck, Biogen, Novartis, Teva, Binding Site. J.C.A.-C. received payment for lecturing, travel expenses, or research grants from Bayer, Roche, Sanofi-Genzyme, Merck, Biogen, Novartis, Teva. R. A.-L. reported grants and personal fees from MercK Serono, personal fees, and non-financial support from Biogen Idec, grants, personal fees, and non-financial support from Novartis Pharmaceuticals S.A., grants and personal fees from Genzyme, non-financial support from Teva Pharma, S.L., non-financial support from Roche. H.H. has participated in meetings sponsored by and received speaker honoraria or travel funding from Bayer Schering, Biogen, Merck Serono, and Novartis, and received honoraria for acting as consultant for Teva Pharmaceuticals Europe. F.D. has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Biogen Idec, Genzyme-Sanofi, Merck, Novartis Pharma, Roche, and TEVA ratiopharm. E.T. has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Biogen Idec, Genzyme-Sanofi, Merck, Novartis, Roche, and TEVA. M.C. has received compensation for consulting services and speaking honoraria from Bayer Schering Pharma, Merk Serono, Biogen Idec, Teva Pharmaceuticals, Sanofi-Aventis, Genzyme, and Novartis. X.M. has received speaking honoraria and travel expenses for scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Bayer Schering Pharma, Biogen Idec, EMD Merck Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, Teva Phramaceuticals, Almirall and Roche. L.V.gave invited lectures and was the chair of symposia by Biogen, Genzyme, Teva, Roche, Merck, and Novartis. A.A. has received personal fees from received honoraria for giving educational presentations on multiple sclerosis and neuroimmunology at several national congresses or symposia from Teva Turkey, Merck Serono, Biogen Idec-Gen Pharma of Turkey, Novartis, Bayer, and Sanofi-Genzyme. She has received travel and registration coverage for attending several national and international congresses or symposia from Teva Turkey, Merck Serono, Biogen Idec-Gen Pharma of Turkey, Bayer, Sanofi-Genzyme. K.R. has received honoraria for consulting services and lecturing from Bayer Schering Pharma, Merk Serono, Biogen Idec, Teva Pharmaceuticals, Sanofi-Aventis, Genzyme, and Novartis. J. L.F. has no funding to support the presented work, but has served on scientific advisory boards for and received funding for travel related to these activities as well as honoraria from Biogen Idec, Merck Serono, Sanofi-Aventis, Teva, Novartis, and Almirall. She has received speaker honoraria from Biogen Idec, Teva, and Novartis. She has served as advisor on preclinical development for Takeda. G.P.-J. has no funding to support the presented work, but has received support from Biogen Idec for a study in optic neuritis and has received support for travel expenses to conferences from Genzyme, Novartis, Merck Serono, Roche, Teva, and Biogen. M.K. has received funding for travel and speaker honoraria from Bayer Schering Pharma, Novartis, Genzyme, Merck, Shire, Biogen Idec, and Teva Pharmaceutical Industries. M.M.V. received funding from the Austrian Federal Ministry of Science, Research and Economics and was trained within the frame of the PhD Program Molecular Medicine of the Medical University of Graz. F.F. served on scientific advisory boards for Biogen Idec, Genzyme, Merck, Novartis, Roche and Teva Pharmaceutical Industries Ltd.; serves on the editorial boards of Multiple Sclerosis, the Polish Journal of Neurology and Neurosurgery, Neurology and the Swiss Archives of Neurology and Psychiatry; provides services for Actelion and Parexel and has received speaker honoraria and support from Merck, Roche and Teva Pharmaceutical Industries Ltd. A.S. has received compensation for consulting services and speaker honoraria from Bayer Schering, Merck Serono, Biogen Idec, Sanofi-Aventis, Teva Pharmaceutical Industries Ltd, and Novartis. M.J.V. has received travel grants from the Binding Site and consumables from the Binding site, Siemens and Euroimmun. L.V. has received speaking honoraria and travel expenses for scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards by Biogen, Genzyme, Novartis, Merck, Roche. B.M.J.U. has received personal compensation for consulting from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche, and TEVA. J.K. has accepted speaker and consulting fees from Merck Serono, Biogen Idec, Teva, Genzyme, and Novartis. C.B. reported a grant from the Swiss MS Society. C.T. served on the advisory board of Fujirebio and Roche, received research consumables from Euroimmun, IBL, Fujirebio, Invitrogen and Mesoscale Discovery, performed contract research for IBL, Shire, Boehringer, Roche and Probiodrug, and received grants from the European Commission, the Dutch Research Council (ZonMW), Association of Frontotemporal Dementia/Alzheimer’s Drug Discovery Foundation, ISAO and the Alzheimer’s Drug Discovery Foundation.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs: M Castellazzi  https://orcid.org/0000-0001-6555-6075
https://orcid.org/0000-0001-6555-6075
R Alvarez-Lafuente  https://orcid.org/0000-0002-3132-1486
https://orcid.org/0000-0002-3132-1486
F Deisenhammer  https://orcid.org/0000-0003-4541-8841
https://orcid.org/0000-0003-4541-8841
M Khalil  https://orcid.org/0000-0002-5350-3328
https://orcid.org/0000-0002-5350-3328
MM Voortman  https://orcid.org/0000-0001-7357-2868
https://orcid.org/0000-0001-7357-2868
Contributor Information
CE Leurs, Department of Neurology, MS Center Amsterdam, VU University Medical Center, De Boelelaan 1118, Amsterdam 1081 HZ, The Netherlands.
HAM Twaalfhoven, Neurochemistry Laboratory and Biobank, Department of Clinical Chemistry, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands.
BI Lissenberg-Witte, Department of Epidemiology and Biostatistics, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands.
V van Pesch, Department of Neurology, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Sint-Lambrechts-Woluwe, Belgium.
I Dujmovic, Clinic of Neurology, Clinical Centre of Serbia, School of Medicine, University of Belgrade, Belgrade, Serbia.
J Drulovic, Clinic of Neurology, Clinical Centre of Serbia, School of Medicine, University of Belgrade, Belgrade, Serbia.
M Castellazzi, Department of Biomedical and Specialty Surgical Sciences, University of Ferrara, Ferrara, Italy.
T Bellini, Department of Biomedical and Specialty Surgical Sciences, University of Ferrara, Ferrara, Italy.
M Pugliatti, Department of Biomedical and Specialty Surgical Sciences, University of Ferrara, Ferrara, Italy.
J Kuhle, Neurologic Clinic and Policlinic, Departments of Medicine, Clinical Research and Biomedicine, University Hospital Basel, University of Basel, Basel, Switzerland.
LM Villar, Department of Immunology, Hospital Ramón y Cajal, IRYCIS, Madrid, Spain/ Red Española de Esclerosis Múltiple (REEM), Madrid, Spain.
JC Alvarez-Cermeño, Red Española de Esclerosis Múltiple (REEM), Madrid, Spain/Department of Neurology, Hospital Ramón y Cajal, IRYICIS, Madrid, Spain.
R Alvarez-Lafuente, Red Española de Esclerosis Múltiple (REEM), Madrid, Spain/Grupo de Investigación de Esclerosis Múltiple, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria San Carlos (IdISSC), Madrid, Spain.
H Hegen, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
F Deisenhammer, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
LM Walchhofer, Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
E Thouvenot, Department of Neurology, CHU Nîmes, Hôpital Caremeau, Nîmes, France/Institut de Génomique Fonctionnelle, UMR5203, Université Montpellier, Montpellier, France.
M Comabella, Servei de Neurologia-Neuroimmunologia, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Institut de Recerca Vall d’Hebron (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
X Montalban, Servei de Neurologia-Neuroimmunologia, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Institut de Recerca Vall d’Hebron (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
L Vécsei, Department of Neurology, University of Szeged, Szeged, Hungary/MTA-SZTE Neuroscience Research Group, Szeged, Hungary.
C Rajda, Department of Neurology, University of Szeged, Szeged, Hungary.
D Galimberti, Multiple Sclerosis Centre, University of Milan, Dino Ferrari Centre, Fondazione Ca’ Granda, IRCCS Ospedale Policlinico, Milan, Italy.
E Scarpini, Multiple Sclerosis Centre, University of Milan, Dino Ferrari Centre, Fondazione Ca’ Granda, IRCCS Ospedale Policlinico, Milan, Italy.
A Altintas, Koc University, School of Medicine, Neurology Department, Istanbul, Turkey.
K Rejdak, Department of Neurology, Medical University of Lublin, Lublin, Poland.
JL Frederiksen, Department of Neurology, Rigshospitalet Glostrup and University of Copenhagen, Copenhagen, Denmark.
G Pihl-Jensen, Department of Neurology, Rigshospitalet Glostrup and University of Copenhagen, Copenhagen, Denmark.
PEH Jensen, Danish Multiple Sclerosis Centre, Department of Neurology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
M Khalil, Department of Neurology, Medical University of Graz, Graz, Austria.
MM Voortman, Department of Neurology, Medical University of Graz, Graz, Austria.
F Fazekas, Department of Neurology, Medical University of Graz, Graz, Austria.
A Saiz, Center of Neuroimmunology, Service of Neurology, Hospital Clinic, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Universitat de Barcelona, Barcelona, Spain.
D La Puma, Center of Neuroimmunology, Service of Neurology, Hospital Clinic, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and Universitat de Barcelona, Barcelona, Spain.
M Vercammen, Department of Laboratory Medicine, AZ Sint-Jan Brugge-Oostende, Brugge, Belgium.
L Vanopdenbosch, Department of Neurology, AZ Sint-Jan Brugge-Oostende, Brugge, Belgium.
BMJ Uitdehaag, Department of Neurology, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands.
J Killestein, Department of Neurology, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands.
C Bridel, Neurochemistry Laboratory and Biobank, Department of Clinical Chemistry, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands.
C Teunissen, Neurochemistry Laboratory and Biobank, Department of Clinical Chemistry, MS Center Amsterdam, Amsterdam Neuroscience, Amsterdam UMC, location VUmc, Amsterdam, The Netherlands.
References
- 1. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2017; 17(2): 162–173. [DOI] [PubMed] [Google Scholar]
- 2. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69(2): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arrambide G, Tintoré M, Espejo C, et al. The added value of oligoclonal bands in the multiple sclerosis diagnostic criteria. ECTRIMS 2017; 2017: 202450. [Google Scholar]
- 4. Kuhle J, Disanto G, Dobson R, et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult Scler 2015; 21(8): 1013–1024. [DOI] [PubMed] [Google Scholar]
- 5. Tintore M, Rovira A, Rio J, et al. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis. Neurology 2008; 70(13 Pt. 2): 1079–1083. [DOI] [PubMed] [Google Scholar]
- 6. Kaplan B, Golderman S, Yahalom G, et al. Free light chain monomer-dimer patterns in the diagnosis of multiple sclerosis. J Immunol Methods 2013; 390(1-2): 74–80. [DOI] [PubMed] [Google Scholar]
- 7. Kaplan B, Aizenbud BM, Golderman S, et al. Free light chain monomers in the diagnosis of multiple sclerosis. J Neuroimmunol 2010; 229(1-2): 263–271. [DOI] [PubMed] [Google Scholar]
- 8. Senel M, Tumani H, Lauda F, et al. Cerebrospinal fluid immunoglobulin kappa light chain in clinically isolated syndrome and multiple sclerosis. PLoS ONE 2014; 9(4): e88680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desplat-Jego S, Feuillet L, Pelletier J, et al. Quantification of immunoglobulin free light chains in cerebrospinal fluid by nephelometry. J Clin Immunol 2005; 25(4): 338–345. [DOI] [PubMed] [Google Scholar]
- 10. Presslauer S, Milosavljevic D, Brücke T, et al. Elevated levels of kappa free light chains in CSF support the diagnosis of multiple sclerosis. J Neurol 2008; 255(10): 1508–1514. [DOI] [PubMed] [Google Scholar]
- 11. Rudick RA, Peter DR, Bidlack JM, et al. Multiple sclerosis: Free light chains in cerebrospinal fluid. Neurology 1985; 35: 1443–1449. [DOI] [PubMed] [Google Scholar]
- 12. Bracco F, Gallo P, Menna R, et al. Free light chains in the CSF in multiple sclerosis. Journal of Neurology 1987; 234: 303–307. [DOI] [PubMed] [Google Scholar]
- 13. DeCarli C, Menegus MA, Rudick RA. Free light chains in multiple sclerosis and infections of the CNS. Neurology 1987; 37(8): 1334–1338. [DOI] [PubMed] [Google Scholar]
- 14. Presslauer S, Milosavljevic D, Huebl W, et al. Validation of kappa free light chains as a diagnostic biomarker in multiple sclerosis and clinically isolated syndrome: A multicenter study. Mult Scler 2016; 22: 102–110. [DOI] [PubMed] [Google Scholar]
- 15. Solling K. Free light chains of immunoglobulins in normal serum and urine determined by radioimmunoassay. Scand J Clin Lab Invest 1975; 35(5): 407–412. [PubMed] [Google Scholar]
- 16. Rudick RA, Pallant A, Bidlack JM, et al. Free kappa light chains in multiple sclerosis spinal fluid. Ann Neurol 1986; 20(1): 63–69. [DOI] [PubMed] [Google Scholar]
- 17. Makshakov G, Nazarov V, Kochetova O, et al. Diagnostic and prognostic value of the cerebrospinal fluid concentration of immunoglobulin free light chains in clinically isolated syndrome with conversion to multiple sclerosis. PLoS ONE 2015; 10(11): e0143375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duranti F, Pieri M, Centonze D, et al. Determination of κFLC and κ Index in cerebrospinal fluid: A valid alternative to assess intrathecal immunoglobulin synthesis. J Neuroimmunol 2013; 263: 116–120. [DOI] [PubMed] [Google Scholar]
- 19. Bradwell AR, Carr-Smith HD, Mead GP, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem 2001; 47(4): 673–680. [PubMed] [Google Scholar]
- 20. Presslauer S, Milosavljevic D, Huebl W, et al. Kappa free light chains: Diagnostic and prognostic relevance in MS and CIS. PLoS ONE 2014; 9(2): e89945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Voortman MM, Stojakovic T, Pirpamer L, et al. Prognostic value of free light chains lambda and kappa in early multiple sclerosis. Mult Scler 2016; 23: 1496–1505. [DOI] [PubMed] [Google Scholar]
- 22. Passerini G, Dalla Costa G, Sangalli F, et al. Free light chains and intrathecal B cells activity in multiple sclerosis: A prospective study and meta-analysis. Mult Scler Int 2016; 2016: 2303857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pieri M, Storto M, Pignalosa S, et al. KFLC Index utility in multiple sclerosis diagnosis: Further confirmation. J Neuroimmunol 2017; 309: 31–33. [DOI] [PubMed] [Google Scholar]
- 24. Teunissen C, Menge T, Altintas A, et al. Consensus definitions and application guidelines for control groups in cerebrospinal fluid biomarker studies in multiple sclerosis. Mult Scler 2013; 19(13): 1802–1809. [DOI] [PubMed] [Google Scholar]
- 25. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol 2005; 58(6): 840–846. [DOI] [PubMed] [Google Scholar]
- 26. Susse M, Hannich M, Petersmann A, et al. Kappa free light chains in cerebrospinal fluid to identify patients with oligoclonal bands. Eur J Neurol 2018; 25(9): 1134–1139. [DOI] [PubMed] [Google Scholar]
- 27. Vasilj M, Kes VB, Vrkic N, et al. Relevance of KFLC quantification to differentiate clinically isolated syndrome from multiple sclerosis at clinical onset. Clin Neurol Neurosurg 2018; 174: 220–229. [DOI] [PubMed] [Google Scholar]
- 28. Van der Heijden M, Kraneveld A, Redegeld F. Free immunoglobulin light chains as target in the treatment of chronic inflammatory diseases. Eur J Pharmacol 2006; 533(1-3): 319–326. [DOI] [PubMed] [Google Scholar]
- 29. Villar LM, Masjuan J, Sadaba MC, et al. Early differential diagnosis of multiple sclerosis using a new oligoclonal band test. Arch Neurol 2005; 62(4): 574–577. [DOI] [PubMed] [Google Scholar]
- 30. Petzold A. Markers for different glial cell responses in multiple sclerosis: Clinical and pathological correlations. Brain 2002; 125(Pt. 7): 1462–1473. [DOI] [PubMed] [Google Scholar]
- 31. Petzold A. Intrathecal oligoclonal IgG synthesis in multiple sclerosis. J Neuroimmunol 2013; 262: 1–10. [DOI] [PubMed] [Google Scholar]
- 32. Valencia-Vera E, Martinez-Escribano Garcia-Ripoll A, Enguix A, et al. Application of kappa free light chains in cerebrospinal fluid as a biomarker in multiple sclerosis diagnosis: Development of a diagnosis algorithm. Clin Chem Lab Med 2018; 56(4): 609–613. [DOI] [PubMed] [Google Scholar]
- 33. Gnanapavan S, Hegen H, Khalil M, et al. Guidelines for uniform reporting of body fluid biomarker studies in neurologic disorders. Neurology 2014; 83(13): 1210–1216. [DOI] [PubMed] [Google Scholar]
- 34. Abraira V, Alvarez-Cermeno JC, Arroyo R, et al. Utility of oligoclonal IgG band detection for MS diagnosis in daily clinical practice. J Immunol Methods 2011; 371(1–2): 170–173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ845844_Supplementary_Figure_1 for Kappa free light chains is a valid tool in the diagnostics of MS: A large multicenter study by CE Leurs, HAM Twaalfhoven, BI Lissenberg-Witte, V van Pesch, I Dujmovic, J Drulovic, M Castellazzi, T Bellini, M Pugliatti, J Kuhle, LM Villar, JC Alvarez-Cermeño, R Alvarez-Lafuente, H Hegen, F Deisenhammer, LM Walchhofer, E Thouvenot, M Comabella, X Montalban, L Vécsei, C Rajda, D Galimberti, E Scarpini, A Altintas, K Rejdak, JL Frederiksen, G Pihl-Jensen, PEH Jensen, M Khalil, MM Voortman, F Fazekas, A Saiz, D La Puma, M Vercammen, L Vanopdenbosch, BMJ Uitdehaag, J Killestein, C Bridel and C Teunissen in Multiple Sclerosis Journal