Abstract
Hepatocellular carcinoma (HCC) is the most common hepatobiliary malignancy with limited therapeutic options. On the other hand, melatonin is an indoleamine that modulates a variety of potential therapeutic effects. In addition to its important role in the regulation of sleep–wake rhythms, several previous studies linked the biologic effects of melatonin to various substantial endocrine, neural, immune and antioxidant functions, among others. Furthermore, the effects of melatonin could be influenced through receptor dependent and receptor independent manner. Among the other numerous physiological and therapeutic effects of melatonin, controlling the survival and differentiation of mesenchymal stem cells (MSCs) has been recently discussed. Given its controversial interaction, several previous reports revealed the therapeutic potential of MSCs in controlling the hepatocellular carcinoma (HCC). Taken together, the intention of the present review is to highlight the effects of melatonin and mesenchymal stem cells as a key for functional integrity for liver cancer treatment. We hope to provide solid piece of information that may be helpful in designing novel drug targets to control HCC.
Keywords: melatonin, mesenchymal stem cells, liver cancer, functional integrity
1. Introduction
The last few years have witnessed extraordinary increase in the reports of liver cancers worldwide [1,2]. In 2018, around 841,000 cases and 782,000 deaths were recorded due to these types of cancer [1,2]. Furthermore, this type of cancer has been considered the 5th cancer type between the male, the 7th between female and the 4th fatal between other cancers [1,2]. Therefore, it is not surprising to state that liver cancer has been considered one of the tumors with the fastest rising incidence and highest mortality in recent years [3,4]. Among others, hepatocellular carcinoma (HCC) has been considered the most common type of liver cancers.
HCC is mostly linked to miscellaneous predisposing etiologies including viral hepatitis or exposure to toxins such as aflatoxin [5,6,7]. Given its global distribution, most HCC cases are estimated to occur in Asia and sub-Saharan Africa [6,8]. Several factors are considered inclining agents for developing HCC such as hemochromatosis and alpha 1-antitrypsin deficiency and metabolic syndrome [2]. Taken into account, the prognosis of HCC mainly depends on several factors including the degree of tumor spread, size of tumor and the general healthy status, among others [6]. Despite the great progress achieved in understanding of HCC, resistance of apoptosis treatment is still challenged by apoptosis resistance [9]. Some recent studies showed that inhibitor of apoptosis proteins (IAPs) have been involved in resistance to apoptosis in HCC through inhibition of caspases activation [10,11]. Indeed, it seems mandatory searching effective novel therapeutic agents that can improve the treatment courses of HCC and future prognosis of such cases.
To our knowledge, melatonin has been identified as a natural antioxidant with numerous immunoenhancing properties, while mesenchymal stem cells has shown a potential promising strategy either in preventing or arresting neoplastic growth [12,13]. The intention of the following sections is to give an overview about mesenchymal stem cells (MSCs) and highlight several physiological and biologic effects of melatonin followed by discussing the potential promising effects of the combined use of melatonin and MSCs in treatment of HCC.
2. Mesenchymal Stem Cells (MSCs)
2.1. An Overview of Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells (MSCs) are multipotent cells capable for differentiation into cartilage, bone, muscle, tendon, ligament, fat and hepatocyte. MSCs population is one of the major stem cell populations in the adult bone marrow [14]. They represent only 0.01% to 0.001% of all mononuclear cells in the bone marrow [14], making the identification of a native MSC niche is difficult [15]. Several locations for in vivo MSC niche within the bone marrow have been proposed including the periosteal niche, the pericytic niche and the perivascular niche [16]. Surface marker expression studies referred to the perivascular niche as the true “home” for MSCs, allowing the easier access of MScs progeny to the circulation [17]. The recent years gave more attention towards pluripotent mesenchymal stem cells, which are found in bone marrow stem cells (BMSCs) and adipose tissue (AD-MSC) [18]. In this regard, MSCs have been proposed as promising sources for restoring tissue and organ function [19]. However, several potential health hazards for their clinical application were reported, including shortage in their availability, their sensitivity to toxic environments, senescence and tumorigenicity [19]. In addition, MSCs- based treatment has shown regeneration of organ function via the production of cytokines and other several anti-inflammatory mechanisms [20].
2.2. Isolation and Characterization of MSCs
To authors’ knowledge, MSCs are mostly isolated from bone barrow, fat and cord tissues [21,22]. However, pluripotent stem cells could be isolated from other numerous tissues and organs, including adipose tissue, skin, dental tissues, placenta, umbilical cord blood, liver, menstrual blood, dental tissue, perinatal tissues and ear [23]. Among others, isolation of MSCs from adipose connective tissue has been considered as an ubiquitous techniques in stem cell-based therapy with minimal invasive protocol [24]. In addition, the outer surface of the ear has plenty of MSCs expressing multiple stromal markers besides their ability to differentiate into different lineage including fat, cartilaginous and osseous tissues [25,26]. It is noteworthy to mention that some modifications have been carried out for isolation of mulitpotent MSCs, including alterations to culture media supplements and serum percentage, growth on various substrates such as collagen and fibronectin, and depletion of hematopoietic cell contaminants by surface marker-based negative selection [27]. These methods allow enrichment of a fibroblastic spindle-cell population [27]. Although a heterogeneous mixture of spindle cells, star-shaped cells and large flattened cells is frequently observed, a characteristic pattern of surface marker co-expression indicates the self-renewal and multipotence capabilities was noticed [28]. Additional surface markers reveal subpopulations of MSC that are differentially committed to various stromal cell types were also recorded [29]. Taking into account that human and murine MSC generally are not able to express hematopoietic markers cluster of differentiations (CD) as CD34 and CD45, however, subpopulations of cells express a low level of these markers [30]. Human MSC were reported to be positive for the following surface markers: CD44, CD73, CD90, CD105, CD106 and STRO-1, while murine MSC express stem cell antigen-1 (Sca-1 or Ly6A) and all these previously mentioned markers except STRO-1 [31]. Likewise, these markers have been used in combination to get pure MSCs from bone marrow isolates [32]. Additionally, it was reported that certain multipotent bone marrow stromal cells did not display these markers in vivo. Consequently, sorted MSCs populations may not contain all multipotent marrow stromal subtypes [33,34]. The absence of MSCs specific genes and markers is another hurdle faced in the characterization of these cells. The trypsin-resistant antigen denoted STRO-1 remains the best choice, since it is absent in peripheral tissues, the hematopoietic compartment or in mature mesenchymal cells, however, it is expressed on endothelial progenitors [35]. Unfortunately, the structural and functional characteristics of STRO-1 have yet to be determined combined with STRO-1 negativity in mice before its use in preclinical studies [36]. It is noteworthy to state that the pluripotent stem cell-derived MSCs overcome many disadvantages of adult MSCs such as reduced batch-to-batch variations and stem cell senescence and produced some unique cytokines different from bone marrow-or cord-derived MSCs [37,38,39]. Interestingly, GMP-grade MSCs are currently being used in clinical trials for various viable diseases, including industrial sectors. In this concern, Mayo Clinic initiated a phase I/II trial to find the side effects and best dose of MSCs infected with oncolytic measles virus encoding NIS (MV-NIS) and to observe its effect on patients with ovarian cancer. These studies suggested further future in-depth research about MSCs timing in patients [40,41].
2.3. Recruitment of MSCs
Tissue repair, inflammation and neoplasia represent few of the processes that encourage engraftment of circulating MSCs [42]. Tumor tropism of MSC can be examined by cell trafficking assays in vitro and in vivo. A number of modalities, such as intravenous injection, utilizing fluorescence, magnetic resonance and bioluminescence, can be used to track ex vivo expanded MSC [43]. No previous studies have deeply explored the migration of endogenous MSC into tumors, but some previous reports have shown that recruitment of labeled MSC home to tumor stroma following bone marrow engraftment in sublethally irradiated mice [44].
The process of MSCs recruitment into tumors follows a similar pattern of recruitment of the activated inflammatory cells during tissue repair [45]. MSCs show graded responses to leukocyte and endothelial activating Transforming growth factor-beta (TGFβ-1), interleukin-6 (IL-6), IL-8, IL-37 and neurotrophin 3 (NT-3) [46]. However, under hypoxic conditions, breast cancer cells produce high amounts of IL-6 which activates and attracts MSCs. It should be stressed that IL-6 also acts in a paracrine fashion on MSC, resulting in activation of STAT3 and MAPK signaling pathways that together trigger the survival of the cell and their migratory potential [47]. Furthermore, LL-37 (Leucine leucine-37), which presents in many tumors, stimulates the migratory activity of MSCs and facilitates the progression of ovarian tumor via recruitment of MSCs to act as pro-angiogenic factor-expressing tumor stromal cells [48].
In addition, tumor cells secrete many chemoattractants that promote the migratory activity of MSCs and MSCs express receptors of the four chemokine subfamilies: CC, CXC, CX(3)C and C [49]. It should be born in mind that several chemotaxis assays in vitro have shown that Dose-dependent migration of MSCs could be induced by chemokines like CCL2/MCP1 (monocyte chemoattractant protein-1), CCL25 (thymus expressed chemokine), CXCL8 (IL8), CXCL12/SDF1α and CXCL13 (BCA1) [50]. In addition, sphingosine 1 phosphate (S1P) exerted a strong chemoattraction on MSCs through matrix metalloproteinase (MMP)-mediated signaling events and the RhoA/ROCK and MEK1/ERK intracellular pathways [51].
2.4. Dual Roles of MSCs in Liver Cancer
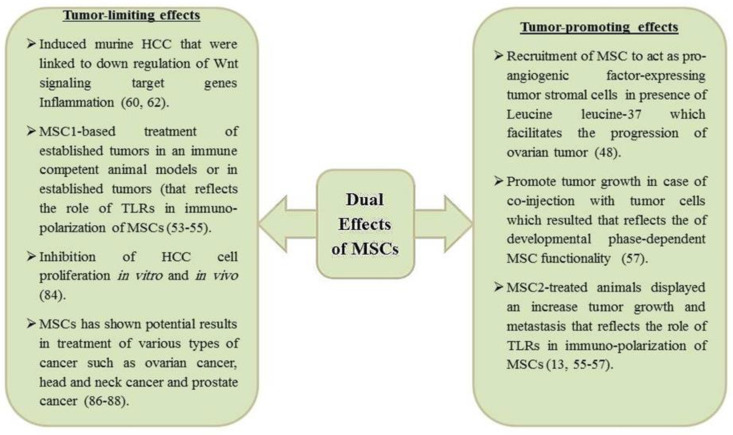
It is noteworthy to state that the considerable difference of intrahepatic microenvironment from other organs seems to influence the development of cancer [52]. MSCs constitute an important components within the microenvironment of both normal liver and liver with tumors, suggesting their pleiotropic functionality which is shown in Figure 1. Clearly, MSCs may exert a tumor-promoting or a tumor-limiting effects depending on the experimental circumstances [13]. Several hypotheses have been proposed to explore this dualistic behavior of MSCs in cancer [53,54]. One of these theories L is related to the role played by TLRs in immuno-polarization of MSCs. MSCs express several TLRs combined with their capabilities to migrate, invade and secrete immune modulating factors [13]. Interestingly, TLR4-primed MSCs and TLR3-primed MSCs are polarized into two phenotypes; a proinflammatory MSC1 and the classical immunosuppressive MSC2 phenotype, respectively [55]. In cancer models, MSC1-based treatment of established tumors in an immune competent animal models impaired the tumor growth and metastasis [56]. On the contrary, MSC2-treated animals displayed an increase tumor growth and metastasis [56]. The other theory proposes a developmental phase-dependent MSC functionality [57]. In this hypothesis, MSCs may promote tumor growth in case of co-injection with tumor cells, while their administration in established tumors inhibit progression of tumors. This means that the presence of MSCs during the early stage of tumorigenesis may contribute to angiogenesis [58]. Clearly, the tumor cells and their microenvironment may have an influence on the action of recruited MSCs [59]. Taken together, both postulations seem concomitantly true and therefore, it is very hard to make a prediction to the effects of MSCs on the cancerous process [60,61].
Figure 1.
The pleiotropic functionality of mesenchymal stem cells (MSCs) which tumor-limiting and tumor-promoting effects.
2.5. Mechanisms of MSC-Dependent Tumor Suppression in Liver Cancer
To author knowledge, MSCs have shown tumor suppressive effects in induced murine HCC that were linked to down regulation of Wnt signaling target genes [60,62]. There are several suggested mechanisms lying behind this action. In this concern, TLR signals can stimulate downstream effectors that may interfere with LPS–TLR4 pathway and the active secretion of Wnt inhibitors, including Dickkopf-1, combined with MSC-dependent inhibition of NF-kB signaling in cancer cells [13,63]. Additionally, MSCs release microvesicles that exert several actions include inhibition of both cell cycling and the growth of different established tumors in vivo, besides induction of the apoptosis of HCC cell lines in vitro, which in turn provides another antioncogenic pathway [62]. However, it should be borne in mind that the direct effectors are still unclear, but it seems that the secretome of MSCs play an crucial role in suppression of tumors [64]. It should be stressed that various molecular mechanisms seem to be involved in therapeutic MSC actions in vivo and increase their regenerative potential [65]. Among others, direct MSC differentiation into cardiac and endothelial cells and the paracrine activity mediated by MSC-derived soluble molecules and vesicles are currently considered as major factors mediating beneficial effects of MSC-based therapies in various viable diseases [65,66,67,68,69]. However, recent studies showed that paracrine function of MSCs is the main mechanism by which these cells participate in tissue repair [68]. Importantly, MSCs can promote normal tissue regeneration through enhancement of angiogenesis, tissue remodeling and activation of endogenous stem cells [70], which endorsed the paracrine actions rather than cell differentiation. MSCs paracrine functions seem tightly regulated by Rap1/nuclear factor-kappaB (NF-κB) signaling pathway. It is noteworthy to mention that the absence of Rap1 of MSCs markedly enhance the secretion profile of these cells and their resistance to any stressful challenge combined with reduction the production of proinflammatory cytokines [71]. Clearly, NF-kb signaling pathway play a critical role in HCC and therefore, the anti-inflammatory properties of MSCs were found remarkable in the earlier stages [72]. Another interesting mechanism of MSCs is through transfer of mitochondria as reported in several previous studies [73,74]. Mitochondria have been considered a key player involved in many biologic processes in health and disease, including in HCC [75,76,77,78,79]. Some proinflammatory cytokines such as Il-6 and TNF-α can induce MSCs skeletal rearrangement and form tunneling nanotubes (TNT) through which mitochondria mobility occurs from MSCs to neighbor cells [73,80]. Inflammation-driven mitochondrial transfer of MSC to neighbor cells including retinal cells and cancer cells were also reported recently [73]. It should be stressed that the therapeutic effects of MSCs and direction of mitochondrial transfer highly depend on the niche where MSCs is located, including in case of HCC [73]. It seems that the proinflammatory environment can enhance MSCs—mitochondrial transfer and MSC—mitochondrial transfer to T cells, which in turn trigger various immune cells, including CD4+T cells [81].
2.6. Therapeutic Application of MSCs in Liver Cancer
To our knowledge, several preclinical models showed that MSCs can migrate into different types of tumors and therefore, this notion has inspired many experts in the field about the potential use of MSCs in anticancer drug/gene delivery [82,83]. In this regards, the genetically modified MSCs demonstrated a clear inhibition the proliferation of HCC in vitro and in vivo [84]. Delivering oncolytic viruses such as Measles virus into the tumor cells via MSCs represent another approach to avoid pre-existing immunity against the virus [85]. These interesting findings seems very promising for designing novel trials for treating HCC using MSCs as a vector. In accordance with the clinical trials, MSCs have been extensively investigated in treatment of various types of cancer such as ovarian cancer, head and neck cancer and prostate cancer [86,87,88].
Moreover, adoptive immunotherapy which relies on transfer of naturally occurring or genetically engineered T cells represents another novel shape of cancer therapy [89,90]. This previously mentioned technique could be carried out using induced pluripotent stem cells (iPSCs) that may provide unlimited source of highly reactive antigen-specific cytotoxic T-lymphocytes, which in turn target, infiltrate and eradicate tumors upon their transfer into the patient [91]. Interestingly, bone marrow-derived MSCs transduced with a lentiviral vector stTRAIL have shown promising results in to treatment of heat-shocked residual cancer cells that target tumor growth inhibition [92].
3. Melatonin
3.1. Synthesis and Precursors of Melatonin
Melatonin (MLT), N-acetyl-5-methoxytryptamine, is a natural substance that has been recognized in all major living species including plants, animals, bacteria, other unicellular microorganisms and human being [44,93]. This natural substance is normally secreted during the dark phase of the daily light–dark cycle [94]. Given its lipophilic nature, MLT is mainly produced by the pineal gland, then released into the circulation and gains access to various fluids, tissues and cellular compartments [95,96]. Other peripheral organs and tissues rather than pineal gland are also get involved in secretion of melatonin including retina, Harderian gland, gastrointestinal tract, leukocytes, thymus and bone marrow cells, however, the chronobiotic properties is retained to the pineal secretions [97]. As result of its amphiphilic nature, melatonin gets infiltrated inside subcellular compartment, enabling it to cross all biologic barriers and gets free access to all cellular compartments.
Regarding its synthesis, MLT is synthesized from the amino acid tryptophan, taken up from blood and converted to serotonin [98]. Serotonin is then acetylated to N-acetylserotonin by arylalkylamine N-acetyltransferase enzyme. N-acetylserotonin is subsequently converted into MLT by hydroxyindole-O-methyltransferase (HIOMT) enzyme. Interestingly, the enzymes of MLT biosynthesis have recently been identified in human lymphocytes and therefore, locally synthesized MLT is probably modulate the immune system [99]. Among other extra-pineal major production sites of MLT, the gastrointestinal (GI) tract is of particular interest since it contains several hundred-fold of MLT exceeding those amounts of the pineal gland [100]. Taketo into consideration, GI MLT may release into circulation under certain circumstances such as under the influence of high dietary tryptophan levels [101]. The following section highlights some facts about the physiological and therapeutic implications of melatonin, particularly against cancer (Figure 2).
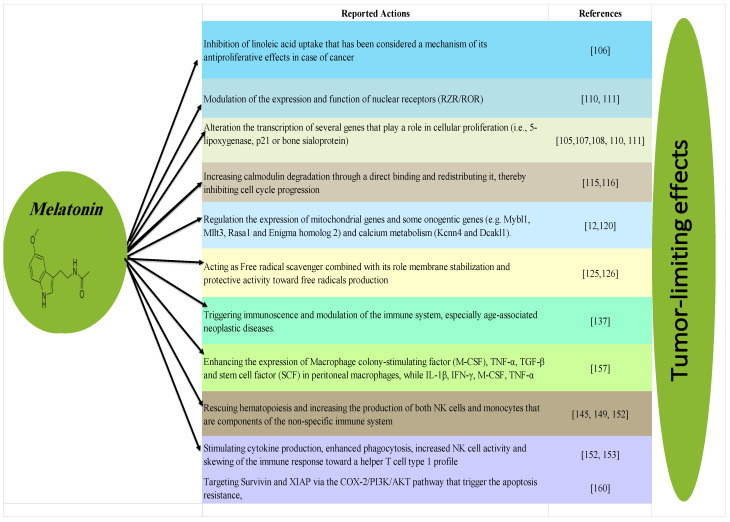
Figure 2.
Tumor-limiting effects of melatonin and their suggested mechanisms.
3.2. Signaling Mechanism of Melatonin
To best of author’s knowledge, two mammalian subtypes of G protein-coupled receptor (GPCR) binds to melatonin receptors; MT1 (Mel1a) and MT2 (Mel1b) [102]. These two mammalian subtypes play an important role in exerting some of MLT actions [102]. Moreover, MT3, has been identified initially as third binding site, then it was subsequently characterized as quinone reductase 2 enzyme [103]. It was reported that the decrease in Cyclic adenosine monophosphate (cAMP) production, caused by MLT via MT1 and MT2 receptor interaction, results in reduction the uptake of linoleic acid by affecting special fatty acid transporter [104,105]. Linoleic acid can be oxidized to 13- hydroxyoctadecadienoic acid by 15-lipoxygenase that serves as an energy source for tumor growth and tumor growth-signaling molecules. Moreover, inhibition of linoleic acid uptake by MLT has been considered a mechanism of the antiproliferative effects in case of cancer [106]. Taken into account, MLT also acts through binding to cytoplasmic proteins like the calcium binding proteins such as calmodulin or tubulin and to nuclear receptors like RZR/ROR [105,107,108]. Some studies also suggested that modulation of the expression and function of nuclear receptors (RZR/ROR) could influence the biologic effects of MLT [109,110]. By binding to nuclear receptors, MLT alters the process of transcription of several genes that play a role in cellular proliferation (i.e., 5-lipoxygenase, p21 or bone sialoprotein) [110,111].
Another suggested mechanism of action of melatonin is modulation of intracellular calcium and calmodulin activity [112,113]. Calcium-activated calmodulin is linked to the initiation of the S and M phases of the cell cycle during the cell cycle-related gene expression regulation and in the reentry of quiescent cells from G0 back into the cell cycle [114]. Melatonin has shown to increase calmodulin degradation through a direct binding and redistributing it, thereby inhibiting cell cycle progression [115,116]. It also serves as a potent modulator of gene transcriptional activity and targets a considerable number of genes, in central or in peripheral tissues [117]. It was hypothesized that melatonin mediates the seasonal photoperiodic control via phasing clock genes expression in the pars tuberalis [118]. In addition, MLT downregulates the expression of integrin and integrin-associated protein encoding genes in rat retina, while upregulates the cAMP response element binding protein cAMP response element-binding (CREB) gene in retinal pigmentary cells [119]. Notably, melatonin has also shown a striking effects on the expression of certain genes related to oncogenesis (e.g., Mybl1, Mllt3, Rasa1 and Enigma homolog 2) and calcium metabolism (Kcnn4 and Dcakl1) [12,120]. It should be stressed that the exact mechanisms of melatonin underlying the suppression of these oncogenes are still unclear, however, some reports linked it to the direct interaction of with Bridging Integrator 1 (BIN1) that considers as HCC suppressant gene with c-Myc, leading to downregulation of c-Myc associated with HCC [121]. Interestingly, linked bridging integrator 1 (BIN1) or Myc box-dependent-interacting protein 1 is also highly expressed in pineal gland [122]. In addition, MLT has a significant effects on mitochondrial genes expression, like genes encoding cytochrome C oxidase subunits I and II (mt-Co1, mt-Co3), 16S ribosomal RNA (mt-RNr2), NADH dehydrogenase 1 (mt-Nd1),) and ATP synthase subunit 6 (mt-ATP6; downregulated) [123].
3.3. Antioxidant Effect of Melatonin
Interestingly, it seems that the function of melatonin in phylogeny is related to its antioxidant activity [124]. Several herbs have been used by Chinese in ancient ages due to its high levels of melatonin to retard aging and to treat diseases associated with the production of free radicals [114]. Besides its actions as free radical scavenger and its role in membrane stabilization, melatonin acts on enzymes that generate or metabolize reactive oxygen intermediates, there by further increasing its protective activity toward free radicals [125,126]. Furthermore, melatonin influences the antioxidant enzymes gene expression as it increases mRNA levels for both Cu–Zn–SOD and Mn–SOD in the Harderian gland and brain cortex of rodents [127]. Moreover, melatonin enhances the activity of glutathione peroxidase (GPx) to remove hydrogen peroxide (H2O2) from cells [128]. Therefore, several important antioxidative enzymes seem to be stimulated by MLT, protecting cells from oxidative damage [129]. Meanwhile, recent report indicated that the engineered MSC with overexpression of GPx can enhance the protection of hepatocytes [130].
3.4. Anticancer Effect of Melatonin
To our knowledge, many natural mechanisms are widely known to protect against carcinogenesis, and they fall into two main categories, immune and non-immune [131]. Importantly, immunosurveillance has been proposed as one of the major processes by which cancerous cells are detected and eliminated [132]. Studies of knockout mice have shown the important role played by the immune system in controlling the spontaneous generation of tumors [133]. Understanding the immune changes in the elderly can provide new insights into the complex relationship between immunity and cancer [134]. The age-related impairment of the immune system appears around the sixth decade of age coinciding with a normal decline in plasma MLT concentration [135]. Aging has been associated with a decrease in immune function and an increased incidence of cancer [136]. In this respect, the decline in the production of MLT with aging was suggested to play a crucial role in triggering immunosenescence, especially age-associated neoplastic diseases [137]. antitumor defense assumes a primary role among the various functions attributed to melatonin in modulation of the immune system. The nighttime physiological surge of MLT in blood or extracellular fluid has been proposed to serve as a “natural restraint” for tumor initiation, promotion and/or progressions [138]. The activation of lymphocytes and monocytes/macrophages by MLT can be one of the major mechanisms in preventing tumor development besides its crucial immunomodulatory role in the immunocompromised state [139]. Some previous reports have investigated the potential beneficial effects of melatonin in induction of HCC and HepG-2 cell death) and ovarian cancer in animal models through enhancement the apoptosis of cancerous cells [140,141,142].
Moreover, administration of melatonin increases the production of both NK cells and monocytes (which contain MLT receptors) in bone marrow and spleen within 7–14 days of treatment [143]. Since both cell types are components of the non-specific immune system, melatonin can be effective in prevent the growth of cancer [144]. Indeed, melatonin was able to rescue hematopoiesis from the toxic effect of cancer chemotherapy in several experimental models [145]. This evidence actually poses the basis for the therapeutic use of MLT as an adjuvant in combination with myelotoxic anticancer therapeutic protocols [146].
3.5. Regulation Effects of Melatonin on the Immune System
It seems that the level of melatonin secretion in human beings could be influenced along the different season of the year that reflects the significant role played by MLT on immune system modulation [147]. In addition, the synthesis of MLT by human lymphocytes support the hypothesis proposes that MLT has a role in the regulation of immune function [148,149]. Furthermore, melatonin can enhance the immune response that may be helpful in correction of the immunodeficiencies secondary to viral diseases or acute stress [150,151]. It should be stressed that melatonin plays an important role in modulation of hematopoiesis, immune cell production and function [149]. MLT also stimulates cytokine production, enhanced phagocytosis, increased NK cell activity and skewing of the immune response toward a helper T cell type 1 profile (Th1) [152]. Likewise, up regulation of cytokine production and immune function occur as a result of binding of melatonin to its receptors [153]. Both membrane and nuclear receptors have been identified on leukocytes. Importantly, membrane receptors were found mainly on CD4 T lymphocytes, but also on CD8 T and B cells and it was reported that melatonin modulates the proliferative response of stimulated lymphocytes via these receptors [154]. On the other hand, MLT induces cytokine production by mononuclear cells through its influence on the nuclear MLT receptors [155]. Indeed, treatment with MLT enhanced antigen presentation by splenic macrophages to T cells together with a concurrent increase in MHC class II expression and synthesis of the proinflammatory cytokines IL-1, IL-2 and Tumor Necrosis factor (TNF [156].
Additionally, treatment of mice with melatonin resulted in enhancing the expression of Macrophage colony-stimulating factor (M-CSF), TNF-α, TGF-β and stem cell factor (SCF) in peritoneal macrophages, while IL-1β, IFN-γ, M-CSF, TNF-α and SCF was increased in spleen cells of mice [157]. The presence of high levels of melatonin in cultured rat thymocytes and expression of mRNAs encoding for Arylalkylamine N-acetyltransferase (AANAT) and HIOMT in the rat and human thymus cells support the hypothesis that MLT is also synthesized by thymocytes [158]. Likewise, the pineal neurohormone MLT has been widely shown to exert an immunostimulatory and potent inhibitor of apoptosis in immune cells through its action on Th cells and on T- and B-cell precursors, respectively [156,159].
4. Potential Beneficial Effect of the Combination between Melatonin and MSCs on Triggering HCC
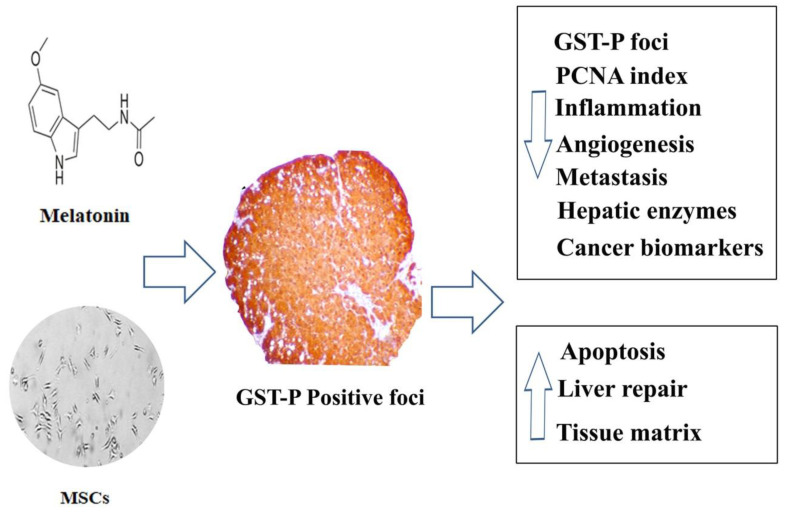
Given the above information, several previous works have suggested the potential contribution of melatonin to overcome HCC through various mechanisms including targeting the expression survivin and X-linked inhibitor of apoptosis (XIAP) via the cyclooxygenase-2 (COX-2)/phosphatidylinositol 3-kinase pathway (PI3K/Akt) pathway [160]. Furthermore, melatonin has shown to exerts numerous anticancer effects through enhancement the expression of various pro-apoptotic markers (mainly Bax and caspase 3) besides induction of apoptosis and inhibition of oxidative stress, inflammation and angiogenesis [143,161,162,163]. Given the fact that Mel receptors are expressed in bone marrow-derived MSCs, melatonin also exerted various receptor-mediated effects on MSCs including enhancement their survival, motility, engraftment and cell differentiation which seems linked to receptors/matrix enzymes interaction, and therefore higher homing effects of MSCs followed pre-administration of a combination of melatonin with MSCs [163,164,165]. On the other hand, various processes have been postulated in relation to MSC-dependent tumor suppression. In this regard, MSCs pulsed with tumor-derived microvesicles showed an enhanced antitumor activity in HCC [166]. Some previous report revealed the use of melatonin enhanced the potential therapeutic role MSCs in treatment various diseases such as acute kidney injury, metabolic syndromes including diabetes through various mechanisms that include through the activation of antioxidative pathways, inhibition of the inflammatory response and reduction of apoptosis and fibrosis [19,167,168,169,170]. More interestingly, few recent reports revealed the beneficial effects of the combination between melatonin and MSCs on targeting inflammation in HCC [171,172,173,174,175]. However, it should be stressed that there is a clear shortage in the available data about the combined use of melatonin and MSCs and their possible synergistic effects in treatment of HCC. In this concern, some of these reports favor the administration of melatonin before MSCs transplantation and it seems this method offer many advantages over the single use of either factor [172]. Interestingly, pre-administration of melatonin prior to MSCs transplantation in HCC resulted in series of actions include, promoted the homing potential of bone marrow-derived MSCs (BMMSCs) and decrease the carcinogenic effect induced by diethylnitrosamine (DEN) which is known as a potent liver carcinogen in rats [176]. Moreover, a significant decrease in the following parameters has been reported, proliferating cell nuclear antigen (PCNA) index, glutathione S-transferase placental positive foci (GST-P), tumor biomarkers in serum besides reduction of inflammation, angiogenesis and metastasis in HCC [171,172,176]. On the other hand, this combination resulted in induction of apoptosis and antioxidant enzymes, tissue matrix and liver repairs in HCC [171]. This was evidenced by lower level of apoptosis in liver tissues which indicated by marked increase in levels of PCNA immunoreactivity and decrease in levels of fragmented DNA and expression of p53, caspase 9 and caspase 3 genes [171,172,176]. Similarly, another study was carried out by Basyony et al. (2019) revealed that treatment using a combination of melatonin and MSCs were reported to decrease malondialdehyde (MDA) which is a known marker of oxidative stress and the antioxidant status in cancer [176]. In addition, in the same study, this combination increases the superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) combined with attenuation of PCNA, Bcl2 and programmed death ligand 1 (PD-L1) immunostain markers and down regulate the expression of inflammation and cell proliferation genes [171,176].
Taken together, the combined use of melatonin and MSCs may provide promising beneficial effects via triggering the apoptosis resistance and as consequences target HCC Figure 3. It should be stressed that this action was confirmed in rat models where the combined treatment restored the liver function and decreased the HCC versus the treatment with either factor alone or in combination with preconditioning in HCC rats [171,172,176]. Given the lack of available data in this topic, further research seems mandatory to explore more about the effects of this combination together with investigation the main mechanisms underlying the reported actions for better understanding the potential use of targeted stems cell therapy in treatment of HCC.
Figure 3.
Effects of the combined use of melatonin and MSCs on hepatocellular carcinoma (HCC); ((GSTPS: glutathione S-transferases), (PCNA: proliferating cell nuclear antigen)) [171,172,173,174,175,176].
5. Conclusions
In conclusion, the present review highlights the main biologic, physiological and therapeutic effects of melatonin that could be very beneficial for controlling of HCC. Furthermore, an overview about MSCs was given with explaining their potential roles in controlling of HCC and their recruitments and suggested actions. It should be stressed that there is a shortage in the available data that explore the effects of the combined use of melatonin and MSCs-based treatments. The present review suggests further future research for exploring the possible beneficial effects of the combination of MSCs and melatonin in treatment of HCC, together with exploring the role of receptors in this possible underlying activity. We hope that this information may contribute to develop novel drug targets with anticancer activity.
Acknowledgments
The authors would like to thank Mahmoud Abbas for his technical support and help.
Abbreviations
| AKT | protein kinase B mybl1 MYB proto-oncogene like 1 |
| cAMP | cyclic adenosine monophosphate NF-kB nuclear factor kappa B |
| COX-2 | cyclooxygenase-2 NK natural killer cells |
| IFN-γ | interferon gamma PI3 K phosphoinositide 3-kinase |
| IL-1β | interleukin 1 beta rasa1 ras GTPase activating protein |
| Kcnn4 | potassium calcium-activated channel subfamily N member 4 ROR related orphan receptor |
| MAPK A | mitogen-activated protein kinase member 4 TLR Toll-like receptors |
| Mllt3 | protein AF-9 STAT3 Signal transducer and activator of transcription 3 |
| mt-Co1 | mitochondrially encoded cytochrome c oxidase I TGF-β Transforming growth factor beta |
| mt-Co3 | mitochondrially encoded cytochrome c oxidase 3 TNF-α tumor necrosis factor-alpha |
| mt-Nd1 | mitochondrially NADH-ubiquinone oxidoreductase chain 1 |
| mt-RNr2 | mitochondrially encoded 16S RNA TRAIL TNF-related apoptosis-inducing ligand |
| XIAP | X-linked inhibitor of apoptosis protein |
Author Contributions
Y.M. and E.K.E. designed the idea of the review, drafted the manuscript and submitted the manuscript to the journal. W.A. and T.Y. involved in the conception of the idea of the manuscript and contributed his scientific advice and revision of the manuscript. The manuscript was read and approved by all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Howlader M., Noone A., Krapcho M., Miller K.D., Brest A., Yu M.C., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., et al. SEER Cancer Statistics Review, 1975–2016. National Cancer Institute; Bethesda, MD, USA: 2019. [(accessed on 15 June 2020)]. Available online: https://seer.cancer.gov/csr/1975_2016/ [Google Scholar]
- 3.Rawla P., Sunkara T., Muralidharan P., Raj J.P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp. Oncol. (Pozn.) 2018;22:141–150. doi: 10.5114/wo.2018.78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver EASL–EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 6.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C., Lou T. Hypoxia inducible factors in hepatocellular carcinoma. Oncotarget. 2017;8:46691–46703. doi: 10.18632/oncotarget.17358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V., Abbas A.K., Aster J.C. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Elsevier; Amsterdam, The Netherlands: 2014. p. 1391. [Google Scholar]
- 9.Fan L., Song B., Sun G., Ma T., Zhong F., Wei W. Endoplasmic reticulum stress-induced resistance to doxorubicin is reversed by paeonol treatment in human hepatocellular carcinoma cells. PLoS ONE. 2013;8:e62627. doi: 10.1371/journal.pone.0062627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augello C., Caruso L., Maggioni M., Donadon M., Montorsi M., Santambrogio R., Torzilli G., Vaira V., Pellegrini C., Roncalli M., et al. Inhibitors of apoptosis proteins (IAPs) expression and their prognostic significance in hepatocellular carcinoma. BMC Cancer. 2009;9:125. doi: 10.1186/1471-2407-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straub C.S. Targeting IAPs as an approach to anti-cancer therapy. Curr. Top. Med. Chem. 2011;11:291–316. doi: 10.2174/156802611794072623. [DOI] [PubMed] [Google Scholar]
- 12.Finati E. Ph.D.Thesis. Università Degli Studi Di; Milano, Italy: 2013. Melatonin: A Pleiotropic Molecule of Natural Origin. Evaluation of the Different Therapeutic Activities in Animal Models and/or Human Patients and a Study of the Metabolic-Biochemical Pathways Related to Them. [Google Scholar]
- 13.Hernanda P.Y., Pedroza-Gonzalez A., Sprengers D., Peppelenbosch M.P., Pan Q. Multipotent mesenchymal stromal cells in liver cancer: Implications for tumor biology and therapy. Biochim. Biophys. Acta. 2014;1846:439–445. doi: 10.1016/j.bbcan.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Montemurro T., Vigano M., Parazzi V., Baluce B., Lavazza C., Budelli S., Montelatici E. Not all the stem cells meet all the clinical needs: Mesenchymal stem cells in regenerative medicine. Cytotherapy. 2014;16:S90. doi: 10.1016/j.jcyt.2014.01.335. [DOI] [Google Scholar]
- 15.Hsiao S.T., Asgari A., Lokmic Z., Sinclair R., Dusting G.J., Lim S.Y., Dilley R.J. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012;21:2189–2203. doi: 10.1089/scd.2011.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Da Silva Meirelles L., Caplan A.I., Nardi N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 17.Hu X., Garcia M., Weng L., Jung X., Murakami J.L., Kumar B., Warden C.D., Todorov I., Chen C.C. Identification of a common mesenchymal stromal progenitor for the adult haematopoietic niche. Nat. Commun. 2016;7:13095. doi: 10.1038/ncomms13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb T.L., Quimby J.M., Dow S.W. In vitro comparison of feline bone marrow-derived and adipose tissue-derived mesenchymal stem cells. J. Feline Med. Surg. 2012;14:165–168. doi: 10.1177/1098612X11429224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu C., Li L. Melatonin plays critical role in mesenchymal stem cell-based regenerative medicine in vitro and in vivo. Stem Cell Res. Ther. 2019;10:13. doi: 10.1186/s13287-018-1114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Lozano F.J., Garcia-Bernal D., Ros-Roca Mde L., Alguero Mdel C., Onate-Sanchez R.E., Camacho-Alonso F., Moraleda J.M. Cytoprotective effects of melatonin on zoledronic acid-treated human mesenchymal stem cells in vitro. J. Cranio-Maxillofac. Surg. 2015;43:855–862. doi: 10.1016/j.jcms.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Orbay H., Tobita M., Mizuno H. Mesenchymal stem cells isolated from adipose and other tissues: Basic biological properties and clinical applications. Stem Cells Int. 2012;2012:461718. doi: 10.1155/2012/461718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Y., Li X., Zhang Y., Chang F., Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8:886. doi: 10.3390/cells8080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayala-Cuellar A.P., Kang J.H., Jeung E.B., Choi K.C. Roles of Mesenchymal Stem Cells in Tissue Regeneration and Immunomodulation. Biomol. Ther. (Seoul) 2019;27:25–33. doi: 10.4062/biomolther.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mushahary D., Spittler A., Kasper C., Weber V., Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part A. 2018;93:19–31. doi: 10.1002/cyto.a.23242. [DOI] [PubMed] [Google Scholar]
- 25.Gawronska-Kozak B., Manuel J.A., Prpic V. Ear mesenchymal stem cells (EMSC) can differentiate into spontaneously contracting muscle cells. J. Cell Biochem. 2007;102:122–135. doi: 10.1002/jcb.21286. [DOI] [PubMed] [Google Scholar]
- 26.Rim J.S., Mynatt R.L., Gawronska-Kozak B. Mesenchymal stem cells from the outer ear: A novel adult stem cell model system for the study of adipogenesis. FASEB J. 2005;19:1205–1207. doi: 10.1096/fj.04-3204fje. [DOI] [PubMed] [Google Scholar]
- 27.Al-Nbaheen M., Vishnubalaji R., Ali D., Bouslimi A., Al-Jassir F., Megges M., Prigione A., Adjaye J., Kassem M., Aldahmash A. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. Rep. 2013;9:32–43. doi: 10.1007/s12015-012-9365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurung S., Deane J.A., Masuda H., Maruyama T., Gargett C.E. Stem Cells in Endometrial Physiology. Semin. Reprod. Med. 2015;33:326–332. doi: 10.1055/s-0035-1558405. [DOI] [PubMed] [Google Scholar]
- 29.Lv F.J., Tuan R.S., Cheung K.M., Leung V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 30.Liu S., Hou K.D., Yuan M., Peng J., Zhang L., Sui X., Zhao B., Xu W., Wang A., Lu S., et al. Characteristics of mesenchymal stem cells derived from Wharton’s jelly of human umbilical cord and for fabrication of non-scaffold tissue-engineered cartilage. J. Biosci. Bioeng. 2014;117:229–235. doi: 10.1016/j.jbiosc.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Ramos T.L., Sanchez-Abarca L.I., Muntion S., Preciado S., Puig N., Lopez-Ruano G., Hernandez-Hernandez A., Redondo A., Ortega R., Rodriguez C., et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. 2016;14:2. doi: 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mabuchi Y., Houlihan D.D., Akazawa C., Okano H., Matsuzaki Y. Prospective isolation of murine and human bone marrow mesenchymal stem cells based on surface markers. Stem Cells Int. 2013;2013:507301. doi: 10.1155/2013/507301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitter S., Gronthos S., Ooi S.S., Zannettino A.C. The Mesenchymal Precursor Cell Marker Antibody STRO-1 Binds to Cell Surface Heat Shock Cognate 70. Stem Cells. 2017;35:940–951. doi: 10.1002/stem.2560. [DOI] [PubMed] [Google Scholar]
- 34.Lechanteur C., Briquet A., Giet O., Delloye O., Baudoux E., Beguin Y. Clinical-scale expansion of mesenchymal stromal cells: A large banking experience. J. Transl. Med. 2016;14:145. doi: 10.1186/s12967-016-0892-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karp J.M., Leng Teo G.S. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Isobe Y., Koyama N., Nakao K., Osawa K., Ikeno M., Yamanaka S., Okubo Y., Fujimura K., Bessho K. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int. J. Oral. Maxillofac. Surg. 2016;45:124–131. doi: 10.1016/j.ijom.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 37.Mastrolia I., Foppiani E.M., Murgia A., Candini O., Samarelli A.V., Grisendi G., Veronesi E., Horwitz E.M., Dominici M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl. Med. 2019;8:1135–1148. doi: 10.1002/sctm.19-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neri S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019;20:2406. doi: 10.3390/ijms20102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giuliani M., Oudrhiri N., Noman M.Z., Vernochet A., Chouaib S., Azzarone B., Durrbach A., Bennaceur-Griscelli A. Human mesenchymal stem cells derived from induced pluripotent stem cells down-regulate NK-cell cytolytic machinery. Blood. 2011;118:3254–3262. doi: 10.1182/blood-2010-12-325324. [DOI] [PubMed] [Google Scholar]
- 40.Schweizer M.T., Wang H., Bivalacqua T.J., Partin A.W., Lim S.J., Chapman C., Abdallah R., Levy O., Bhowmick N.A., Karp J.M., et al. A Phase I Study to Assess the Safety and Cancer-Homing Ability of Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells in Men with Localized Prostate Cancer. Stem Cells Transl. Med. 2019;8:441–449. doi: 10.1002/sctm.18-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galipeau J., Sensebe L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heissig B., Dhahri D., Eiamboonsert S., Salama Y., Shimazu H., Munakata S., Hattori K. Role of mesenchymal stem cell-derived fibrinolytic factor in tissue regeneration and cancer progression. Cell Mol. Life Sci. 2015;72:4759–4770. doi: 10.1007/s00018-015-2035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J.W., Krasnodembskaya A., McKenna D.H., Song Y., Abbott J., Matthay M.A. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am. J. Respir. Crit. Care Med. 2013;187:751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen S., Dooner M., Cheng Y., Papa E., Del Tatto M., Pereira M., Deng Y., Goldberg L., Aliotta J., Chatterjee D., et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 2016;30:2221–2231. doi: 10.1038/leu.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantovani A., Biswas S.K., Galdiero M.R., Sica A., Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 46.Lichtman M.K., Otero-Vinas M., Falanga V. Transforming growth factor beta (TGF-beta) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016;24:215–222. doi: 10.1111/wrr.12398. [DOI] [PubMed] [Google Scholar]
- 47.Bharti R., Dey G., Mandal M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: A snapshot of IL-6 mediated involvement. Cancer Lett. 2016;375:51–61. doi: 10.1016/j.canlet.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 48.Piktel E., Niemirowicz K., Wnorowska U., Watek M., Wollny T., Gluszek K., Gozdz S., Levental I., Bucki R. The Role of Cathelicidin LL-37 in Cancer Development. Arch Immunol. Exp. (Warsz.) 2016;64:33–46. doi: 10.1007/s00005-015-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roccaro A.M., Sacco A., Purschke W.G., Moschetta M., Buchner K., Maasch C., Zboralski D., Zollner S., Vonhoff S., Mishima Y., et al. SDF-1 inhibition targets the bone marrow niche for cancer therapy. Cell Rep. 2014;9:118–128. doi: 10.1016/j.celrep.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y., Chu N., Qiu X., Gober H.J., Li D., Wang L. The interconnected role of chemokines and estrogen in bone metabolism. Biosci. Trends. 2017;10:433–444. doi: 10.5582/bst.2016.01072. [DOI] [PubMed] [Google Scholar]
- 51.Geranmayeh M.H., Nourazarian A., Avci C.B., Rahbarghazi R., Farhoudi M. Stem Cells as a Promising Tool for the Restoration of Brain Neurovascular Unit and Angiogenic Orientation. Mol. Neurobiol. 2017;54:7689–7705. doi: 10.1007/s12035-016-0286-4. [DOI] [PubMed] [Google Scholar]
- 52.Montano-Loza A.J., Bhanji R.A., Wasilenko S., Mason A.L. Systematic review: Recurrent autoimmune liver diseases after liver transplantation. Aliment Pharm. Ther. 2017;45:485–500. doi: 10.1111/apt.13894. [DOI] [PubMed] [Google Scholar]
- 53.Frese L., Dijkman P.E., Hoerstrup S.P. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus. Med. Hemother. 2016;43:268–274. doi: 10.1159/000448180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holly J.M.P., Biernacka K., Perks C.M. The Neglected Insulin: IGF-II, a Metabolic Regulator with Implications for Diabetes, Obesity, and Cancer. Cells. 2019;8:1207. doi: 10.3390/cells8101207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng S.Z., Sun K., Wang R., Wang J. and Lin, Y. Fundamental Concepts and Features of Mesenchymal Stem Cells: Proliferation, Differentiation, Migration and Immunomodulatory Characteristics. Mesenchymal Stem Cells Craniofacial Regen. 2016;3:2896–2902. [Google Scholar]
- 56.Osiecki M.J. Ph.D. Thesis. Queensland University of Technology; Brisbane City, Australia: 2016. Isolation and Expansion of Placental Derived Mesenchymal Stromal Cells in a Packed Bed Bioreactor. [Google Scholar]
- 57.Zang W., Xie L.H., Zhu B.H., Cui D.W. Construction of human HepG-2 cells infected by lentivirus carrying green fluorescent protein gene. Int. J. Clin. Exp. Med. 2016;9:8161–8168. [Google Scholar]
- 58.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill B.S., Sarnella A., D’Avino G., Zannetti A. Recruitment of stromal cells into tumour microenvironment promote the metastatic spread of breast cancer. Semin. Cancer Biol. 2019 doi: 10.1016/j.semcancer.2019.07.028. [DOI] [PubMed] [Google Scholar]
- 60.Ai J., Ketabchi N., Verdi J., Gheibi N., Khadem Haghighian H., Kavianpour M. Mesenchymal stromal cells induce inhibitory effects on hepatocellular carcinoma through various signaling pathways. Cancer Cell Int. 2019;19:329. doi: 10.1186/s12935-019-1038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chowdhury R. Ph.D. Thesis. Cardiff University; Cardiff, UK: 2015. Prostate Cancer Exosomes Differentiate BM-MSCs into Pro-Angiogenic and Pro-Invasive Myofibroblasts. [Google Scholar]
- 62.Yuan Y., Zhou C., Chen X., Tao C., Cheng H., Lu X. Suppression of tumor cell proliferation and migration by human umbilical cord mesenchymal stem cells: A possible role for apoptosis and Wnt signaling. Oncol. Lett. 2018;15:8536–8544. doi: 10.3892/ol.2018.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parys M., Nelson N., Koehl K., Miller R., Kaneene J.B., Kruger J.M., Yuzbasiyan-Gurkan V. Safety of Intraperitoneal Injection of Adipose Tissue-Derived Autologous Mesenchymal Stem Cells in Cats. J. Vet. Intern. Med. 2016;30:157–163. doi: 10.1111/jvim.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marks D.L., Olson R.L., Fernandez-Zapico M.E. Epigenetic control of the tumor microenvironment. Epigenomics. 2016;8:1671–1687. doi: 10.2217/epi-2016-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jimenez-Puerta G.J., Marchal J.A., Lopez-Ruiz E., Galvez-Martin P. Role of Mesenchymal Stromal Cells as Therapeutic Agents: Potential Mechanisms of Action and Implications in Their Clinical Use. J. Clin. Med. 2020;9:445. doi: 10.3390/jcm9020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park K.S., Bandeira E., Shelke G.V., Lasser C., Lotvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019;10:288. doi: 10.1186/s13287-019-1398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park W.S., Ahn S.Y., Sung S.I., Ahn J.Y., Chang Y.S. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatric Res. 2018;83:214–222. doi: 10.1038/pr.2017.249. [DOI] [PubMed] [Google Scholar]
- 68.Maacha S., Sidahmed H., Jacob S., Gentilcore G., Calzone R., Grivel J.C., Cugno C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020;2020:4356359. doi: 10.1155/2020/4356359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan X.L., Zhang Y., Li X., Fu Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol. Life Sci. 2020 doi: 10.1007/s00018-020-03454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siu C.W., Liao S.Y., Liu Y., Lian Q., Tse H.F. Stem cells for myocardial repair. Thromb. Haemost. 2010;104:6–12. doi: 10.1160/TH09-05-0336. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y., Chiu S., Liang X., Gao F., Zhang Z., Liao S., Liang Y., Chai Y.H., Low D.J., Tse H.F., et al. Rap1-mediated nuclear factor-kappaB (NF-kappaB) activity regulates the paracrine capacity of mesenchymal stem cells in heart repair following infarction. Cell Death Discov. 2015;1:15007. doi: 10.1038/cddiscovery.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zong C., Zhang H., Yang X., Gao L., Hou J., Ye F., Jiang J., Yang Y., Li R., Han Z., et al. The distinct roles of mesenchymal stem cells in the initial and progressive stage of hepatocarcinoma. Cell Death Dis. 2018;9:345. doi: 10.1038/s41419-018-0366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li C., Cheung M.K.H., Han S., Zhang Z., Chen L., Chen J., Zeng H., Qiu J. Mesenchymal stem cells and their mitochondrial transfer: A double-edged sword. Biosci. Rep. 2019;39 doi: 10.1042/BSR20182417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kholodenko I.V., Kurbatov L.K., Kholodenko R.V., Manukyan G.V., Yarygin K.N. Mesenchymal Stem Cells in the Adult Human Liver: Hype or Hope? Cells. 2019;8:1127. doi: 10.3390/cells8101127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ogunwobi O.O., Harricharran T., Huaman J., Galuza A., Odumuwagun O., Tan Y., Ma G.X., Nguyen M.T. Mechanisms of hepatocellular carcinoma progression. World J. Gastroenterol. 2019;25:2279–2293. doi: 10.3748/wjg.v25.i19.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Auger C., Alhasawi A., Contavadoo M., Appanna V.D. Dysfunctional mitochondrial bioenergetics and the pathogenesis of hepatic disorders. Front. Cell Dev. Biol. 2015;3:40. doi: 10.3389/fcell.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herst P.M., Rowe M.R., Carson G.M., Berridge M.V. Functional Mitochondria in Health and Disease. Front. Endocrinol. (Lausanne) 2017;8:296. doi: 10.3389/fendo.2017.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zimmermann A. Tumors and Tumor-Like Lesions of the Hepatobiliary Tract: General and Surgical Pathology. Springer International Publishing; Cham, Switzerland: 2017. Mitochondrial Biology in Hepatobiliary Tumors: Changes of the Cellular Energy Factory; pp. 3091–3124. [Google Scholar]
- 79.Léveillé M., Estall J. Mitochondrial Dysfunction in the Transition from NASH to HCC. Metabolites. 2019;9:233. doi: 10.3390/metabo9100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vignais M.L., Caicedo A., Brondello J.M., Jorgensen C. Cell Connections by Tunneling Nanotubes: Effects of Mitochondrial Trafficking on Target Cell Metabolism, Homeostasis, and Response to Therapy. Stem Cells Int. 2017;2017:6917941. doi: 10.1155/2017/6917941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Court A.C., Le-Gatt A., Luz-Crawford P., Parra E., Aliaga-Tobar V., Batiz L.F., Contreras R.A., Ortuzar M.I., Kurte M., Elizondo-Vega R., et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020;21:e48052. doi: 10.15252/embr.201948052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin W., Huang L., Li Y., Fang B., Li G., Chen L., Xu L. Mesenchymal Stem Cells and Cancer: Clinical Challenges and Opportunities. BioMed Res. Int. 2019;2019:2820853. doi: 10.1155/2019/2820853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Powell A.B., Williams K., Cruz C.R.Y. Gene-modified, cell-based therapies-an overview. Cytotherapy. 2016;18:1351–1359. doi: 10.1016/j.jcyt.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 84.Woodsworth D.J., Holt R.A. Cell-Based Therapeutics: Making a Faustian Pact with Biology. Trends Mol. Med. 2017;23:104–115. doi: 10.1016/j.molmed.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 85.Aurelian L. Oncolytic viruses as immunotherapy: Progress and remaining challenges. Onco. Targets Ther. 2016;9:2627–2637. doi: 10.2147/OTT.S63049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim N., Cho S.G. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int. J. Stem Cells. 2015;8:54–68. doi: 10.15283/ijsc.2015.8.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nowakowski A., Drela K., Rozycka J., Janowski M., Lukomska B. Engineered Mesenchymal Stem Cells as an Anti-Cancer Trojan Horse. Stem Cells Dev. 2016;25:1513–1531. doi: 10.1089/scd.2016.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hmadcha A., Martin-Montalvo A., Gauthier B.R., Soria B., Capilla-Gonzalez V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020;8:43. doi: 10.3389/fbioe.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao L., Cao Y.J. Engineered T Cell Therapy for Cancer in the Clinic. Front. Immunol. 2019;10:2250. doi: 10.3389/fimmu.2019.02250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Melief C.J., van Hall T., Arens R., Ossendorp F., van der Burg S.H. Therapeutic cancer vaccines. J. Clin. Investig. 2015;125:3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brauer P.M., Singh J., Xhiku S., Zuniga-Pflucker J.C. T Cell Genesis: In Vitro Veritas Est? Trends Immunol. 2016;37:889–901. doi: 10.1016/j.it.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niess H., Thomas M.N., Schiergens T.S., Kleespies A., Jauch K.W., Bruns C., Werner J., Nelson P.J., Angele M.K. Genetic engineering of mesenchymal stromal cells for cancer therapy: Turning partners in crime into Trojan horses. Innov. Surg. Sci. 2016;1:19–32. doi: 10.1515/iss-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hardeland R. Melatonin in Plants-Diversity of Levels and Multiplicity of Functions. Front. Plant Sci. 2016;7:198. doi: 10.3389/fpls.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tenorio F., Simoes Mde J., Teixeira V.W., Teixeira A.A. Effects of melatonin and prolactin in reproduction: Review of literature. Rev. Assoc. Med. Bras. 2015;61:269–274. doi: 10.1590/1806-9282.61.03.269. [DOI] [PubMed] [Google Scholar]
- 95.Malpaux B., Migaud M., Tricoire H., Chemineau P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J. Biol. Rhythm. 2001;16:336–347. doi: 10.1177/074873001129002051. [DOI] [PubMed] [Google Scholar]
- 96.Sanchez A., Calpena A.C., Clares B. Evaluating the Oxidative Stress in Inflammation: Role of Melatonin. Int. J. Mol. Sci. 2015;16:16981–17004. doi: 10.3390/ijms160816981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hardeland R., Cardinali D.P., Srinivasan V., Spence D.W., Brown G.M., Pandi-Perumal S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011;93:350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Brzęczek M.S.K., Hyla-Klekot L. Melatonina–hormon plejotropowym działaniu. Pediatr. Med. Rodz. 2016;12:127–133. doi: 10.15557/PiMR.2016.0011. [DOI] [Google Scholar]
- 99.Xin Z., Jiang S., Jiang P., Yan X., Fan C., Di S., Wu G., Yang Y., Reiter R.J., Ji G. Melatonin as a treatment for gastrointestinal cancer: A review. J. Pineal Res. 2015;58:375–387. doi: 10.1111/jpi.12227. [DOI] [PubMed] [Google Scholar]
- 100.Galano A. Indoleamines: Sources, Role in Biological Processes and Health Effects. Nova Science Publishers, Inc.; Hauppauge, NY, USA: 2015. The role of indoleamines in reducing free radical damage and oxidative stress: A physicochemical perspective; pp. 1–41. [Google Scholar]
- 101.Wongprayoon P., Govitrapong P. Melatonin Attenuates Methamphetamine-Induced Neurotoxicity. Curr. Pharm. Des. 2016;22:1022–1032. doi: 10.2174/1381612822666151214125657. [DOI] [PubMed] [Google Scholar]
- 102.Reppert S.M., Weaver D.R., Godson C. Melatonin receptors step into the light: Cloning and classification of subtypes. Trends Pharm. Sci. 1996;17:100–102. doi: 10.1016/0165-6147(96)10005-5. [DOI] [PubMed] [Google Scholar]
- 103.Zee P.C., Reid K.J. Diagnosis and Treatment. CRC Press; Boca Raton, FL, USA: 2016. 35 Melatonin in Sleep-Wake Regulation; p. 410. [Google Scholar]
- 104.Mektepbayeva D., Alibek K., Atinbayeva N., Irving S., Zhaisanbayeva B., Mussurova S., Mussakhan S. Anticancer Effects and uses of Melatonin: A Review. Austin J. Cancer Clin. Res. 2015;2:1052. [Google Scholar]
- 105.Boutin J.A. Melatonin binding site MT3 is QR2: State of the art. J. Soc. Biol. 2007;201:97–103. doi: 10.1051/jbio:2007011. [DOI] [PubMed] [Google Scholar]
- 106.Goncalves Ndo N., Colombo J., Lopes J.R., Gelaleti G.B., Moschetta M.G., Sonehara N.M., Hellmen E., Zanon Cde F., Oliani S.M., Zuccari D.A. Effect of Melatonin in Epithelial Mesenchymal Transition Markers and Invasive Properties of Breast Cancer Stem Cells of Canine and Human Cell Lines. PLoS ONE. 2016;11:e0150407. doi: 10.1371/journal.pone.0150407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Panzer A., Viljoen M. The validity of melatonin as an oncostatic agent. J. Pineal Res. 1997;22:184–202. doi: 10.1111/j.1600-079X.1997.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 108.Pandi-Perumal S.R., Trakht I., Srinivasan V., Spence D.W., Maestroni G.J., Zisapel N., Cardinali D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008;85:335–353. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 109.Cutando A., Aneiros-Fernandez J., Lopez-Valverde A., Arias-Santiago S., Aneiros-Cachaza J., Reiter R.J. A new perspective in Oral health: Potential importance and actions of melatonin receptors MT1, MT2, MT3, and RZR/ROR in the oral cavity. Arch Oral. Biol. 2011;56:944–950. doi: 10.1016/j.archoralbio.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 110.Garcia-Maurino S., Pozo D., Calvo J.R., Guerrero J.M. Correlation between nuclear melatonin receptor expression and enhanced cytokine production in human lymphocytic and monocytic cell lines. J. Pineal Res. 2000;29:129–137. doi: 10.1034/j.1600-079X.2000.290301.x. [DOI] [PubMed] [Google Scholar]
- 111.Tuli H.S., Kashyap D., Sharma A.K., Sandhu S.S. Molecular aspects of melatonin (MLT)-mediated therapeutic effects. Life Sci. 2015;135:147–157. doi: 10.1016/j.lfs.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 112.Benitez-King G., Anton-Tay F. Calmodulin mediates melatonin cytoskeletal effects. Experientia. 1993;49:635–641. doi: 10.1007/BF01923944. [DOI] [PubMed] [Google Scholar]
- 113.Hardeland R. Melatonin: Signaling mechanisms of a pleiotropic agent. Biofactors. 2009;35:183–192. doi: 10.1002/biof.23. [DOI] [PubMed] [Google Scholar]
- 114.Srinivasan V., RPandi-Perumal S., Brzezinski A., PBhatnagar K., PCardinali D. Melatonin, immune function and cancer. Recent Pat. Endocr. Metab. Immune Drug Discov. 2011;5:109–123. doi: 10.2174/187221411799015408. [DOI] [PubMed] [Google Scholar]
- 115.Gupta B.B., Spessert R., Vollrath L. Molecular components and mechanism of adrenergic signal transduction in mammalian pineal gland: Regulation of melatonin synthesis. Indian J. Exp. Biol. 2005;43:115–149. [PubMed] [Google Scholar]
- 116.Dubocovich M.L., Yun K., Al-Ghoul W.M., Benloucif S., Masana M.I. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–1220. doi: 10.1096/fasebj.12.12.1211. [DOI] [PubMed] [Google Scholar]
- 117.Fischer T.W., Slominski A., Zmijewski M.A., Reiter R.J., Paus R. Melatonin as a major skin protectant: From free radical scavenging to DNA damage repair. Exp. Derm. 2008;17:713–730. doi: 10.1111/j.1600-0625.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 118.Loureiro R., Magalhaes-Novais S., Mesquita K.A., Baldeiras I., Sousa I.S., Tavares L.C., Barbosa I.A., Oliveira P.J., Vega-Naredo I. Melatonin antiproliferative effects require active mitochondrial function in embryonal carcinoma cells. Oncotarget. 2015;6:17081–17096. doi: 10.18632/oncotarget.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lopez-Munoz F., Molina J.D., Rubio G., Alamo C. An historical view of the pineal gland and mental disorders. J. Clin. Neurosci. 2011;18:1028–1037. doi: 10.1016/j.jocn.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 120.Lee H., Lee H.J., Jung J.H., Shin E.A., Kim S.H. Melatonin disturbs SUMOylation-mediated crosstalk between c-Myc and nestin via MT1 activation and promotes the sensitivity of paclitaxel in brain cancer stem cells. J. Pineal Res. 2018;65:e12496. doi: 10.1111/jpi.12496. [DOI] [PubMed] [Google Scholar]
- 121.Pan K., Liang X.-T., Zhang H.-k., Zhao J.-J., Wang D.-D., Li J.-J., Lian Q., Chang A.E., Li Q., Xia J.-C. Characterization of Bridging Integrator 1 (BIN1) as a Potential Tumor Suppressor and Prognostic Marker in Hepatocellular Carcinoma. Mol. Med. 2012;18:507–518. doi: 10.2119/molmed.2011.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Holler C.J., Davis P.R., Beckett T.L., Platt T.L., Webb R.L., Head E., Murphy M.P. Bridging integrator 1 (BIN1) protein expression increases in the Alzheimer’s disease brain and correlates with neurofibrillary tangle pathology. J. Alzheimers Dis. 2014;42:1221–1227. doi: 10.3233/JAD-132450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paradies G., Paradies V., Ruggiero F.M., Petrosillo G. Protective role of melatonin in mitochondrial dysfunction and related disorders. Arch. Toxicol. 2015;89:923–939. doi: 10.1007/s00204-015-1475-z. [DOI] [PubMed] [Google Scholar]
- 124.Reiter R.J., Tan D.X., Osuna C., Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J. BioMed. Sci. 2000;7:444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 125.Reiter R.J., Tan D.X., Cabrera J., D’Arpa D. Melatonin and tryptophan derivatives as free radical scavengers and antioxidants. Adv. Exp. Med. Biol. 1999;467:379–387. doi: 10.1007/978-1-4615-4709-9_48. [DOI] [PubMed] [Google Scholar]
- 126.Shyma M.S., Ansar E.B., Gayathri V., Varma H.K., Mohanan P.V. Attenuation of Cisplatin Induced Toxicity by Melatonin, Loaded on a Dextran Modified Iron Oxide Nanoparticles: An In Vitro Study. J. Forensic. Toxicol. Pharm. 2015;4 [Google Scholar]
- 127.Anisimov S.V., Popovic N. Genetic aspects of melatonin biology. Rev. Neurosci. 2004;15:209–230. doi: 10.1515/REVNEURO.2004.15.3.209. [DOI] [PubMed] [Google Scholar]
- 128.Maharaj D.S., Glass B.D., Daya S. Melatonin: New places in therapy. Biosci. Rep. 2007;27:299–320. doi: 10.1007/s10540-007-9052-1. [DOI] [PubMed] [Google Scholar]
- 129.Letra-Vilela R., Sanchez-Sanchez A.M., Rocha A.M., Martin V., Branco-Santos J., Puente-Moncada N., Santa-Marta M., Outeiro T.F., Antolin I., Rodriguez C., et al. Distinct roles of N-acetyl and 5-methoxy groups in the antiproliferative and neuroprotective effects of melatonin. Mol. Cell Endocrinol. 2016;434:238–249. doi: 10.1016/j.mce.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 130.Qi X., Ng K.T., Lian Q., Li C.X., Geng W., Ling C.C., Yeung W.H., Ma Y.Y., Liu X.B., Liu H., et al. Glutathione Peroxidase 3 Delivered by hiPSC-MSCs Ameliorated Hepatic IR Injury via Inhibition of Hepatic Senescence. Theranostics. 2018;8:212–222. doi: 10.7150/thno.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jakobisiak M., Lasek W., Golab J. Natural mechanisms protecting against cancer. Immunol. Lett. 2003;90:103–122. doi: 10.1016/j.imlet.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 132.Swann J.B., Smyth M.J. Immune surveillance of tumors. J. Clin. Investig. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hakim F.T., Flomerfelt F.A., Boyiadzis M., Gress R.E. Aging, immunity and cancer. Curr. Opin. Immunol. 2004;16:151–156. doi: 10.1016/j.coi.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 134.Deleidi M., Jaggle M., Rubino G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front. Neurosci. 2015;9:172. doi: 10.3389/fnins.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carrillo-Vico A., Lardone P.J., Alvarez-Sanchez N., Rodriguez-Rodriguez A., Guerrero J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013;14:8638–8683. doi: 10.3390/ijms14048638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Plackett T.P., Boehmer E.D., Faunce D.E., Kovacs E.J. Aging and innate immune cells. J. Leukoc. Biol. 2004;76:291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 137.Su S.C., Hsieh M.J., Yang W.E., Chung W.H., Reiter R.J., Yang S.F. Cancer metastasis: Mechanisms of inhibition by melatonin. J. Pineal Res. 2017;62 doi: 10.1111/jpi.12370. [DOI] [PubMed] [Google Scholar]
- 138.Guerrero J.M., Reiter R.J. Melatonin-immune system relationships. Curr. Top. Med. Chem. 2002;2:167–179. doi: 10.2174/1568026023394335. [DOI] [PubMed] [Google Scholar]
- 139.Medrano-Campillo P., Sarmiento-Soto H., Alvarez-Sanchez N., Alvarez-Rios A.I., Guerrero J.M., Rodriguez-Prieto I., Castillo-Palma M.J., Lardone P.J., Carrillo-Vico A. Evaluation of the immunomodulatory effect of melatonin on the T-cell response in peripheral blood from systemic lupus erythematosus patients. J. Pineal Res. 2015;58:219–226. doi: 10.1111/jpi.12208. [DOI] [PubMed] [Google Scholar]
- 140.Chuffa L.G., Lupi Junior L.A., Seiva F.R., Martinez M., Domeniconi R.F., Pinheiro P.F., Dos Santos L.D., Martinez F.E. Quantitative Proteomic Profiling Reveals That Diverse Metabolic Pathways Are Influenced by Melatonin in an in Vivo Model of Ovarian Carcinoma. J. Proteome Res. 2016;15:3872–3882. doi: 10.1021/acs.jproteome.6b00713. [DOI] [PubMed] [Google Scholar]
- 141.Martin-Renedo J., Mauriz J.L., Jorquera F., Ruiz-Andres O., Gonzalez P., Gonzalez-Gallego J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J. Pineal Res. 2008;45:532–540. doi: 10.1111/j.1600-079X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 142.Moreira A.J., Ordonez R., Cerski C.T., Picada J.N., Garcia-Palomo A., Marroni N.P., Mauriz J.L., González-Gallego J. Melatonin activates endoplasmic reticulum stress and apoptosis in rats with diethylnitrosamine-induced hepatocarcinogenesis. PLoS ONE. 2015;10:e0144517. doi: 10.1371/journal.pone.0144517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Currier N.L., Sun L.Z., Miller S.C. Exogenous melatonin: Quantitative enhancement in vivo of cells mediating non-specific immunity. J. Neuroimmunol. 2000;104:101–108. doi: 10.1016/S0165-5728(99)00271-4. [DOI] [PubMed] [Google Scholar]
- 144.Srinivasan V., Maestroni G.J., Cardinali D.P., Esquifino A.I., Perumal S.R., Miller S.C. Melatonin, immune function and aging. Immun. Ageing. 2005;2:17. doi: 10.1186/1742-4933-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maestroni G.J. T-helper-2 lymphocytes as a peripheral target of melatonin. J. Pineal Res. 1995;18:84–89. doi: 10.1111/j.1600-079X.1995.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 146.Cardinali D.E., Acuña-Castroviejo D., Ortiz F. and Fernández-Gil, B. Melatonin-induced oncostasis, mechanisms and clinical relevance. J. Integ. Oncol. 2016 doi: 10.4172/2329-6771.S1-006. [DOI] [Google Scholar]
- 147.Fourtillan J.B., Brisson A.M., Fourtillan M., Ingrand I., Decourt J.P., Girault J. Melatonin secretion occurs at a constant rate in both young and older men and women. Am. J. Physiol. Endocrinol. Metab. 2001;280:E11–E22. doi: 10.1152/ajpendo.2001.280.1.E11. [DOI] [PubMed] [Google Scholar]
- 148.Hardeland R., Cardinali D.P., Brown G.M., Pandi-Perumal S.R. Melatonin and brain inflammaging. Prog. Neurobiol. 2015;127–128:46–63. doi: 10.1016/j.pneurobio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 149.Cutolo M., Maestroni G.J. The melatonin-cytokine connection in rheumatoid arthritis. Ann. Rheum. Dis. 2005;64:1109–1111. doi: 10.1136/ard.2005.038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maestroni G.J. Tryptophan, Serotonin, and Melatonin. Springer; Boston, MA, USA: 1999. Therapeutic potential of melatonin in immunodeficiency states, viral diseases, and cancer; pp. 217–226. [DOI] [PubMed] [Google Scholar]
- 151.Elmahallawy E.K., Luque J.O., Aloweidi A.S., Gutierrez-Fernandez J., Sampedro-Martinez A., Rodriguez-Granger J., Kaki A., Agil A. Potential Relevance of Melatonin Against Some Infectious Agents: A Review and Assessment of Recent Research. Curr. Med. Chem. 2015;22:3848–3861. doi: 10.2174/0929867322666150827093730. [DOI] [PubMed] [Google Scholar]
- 152.Reiter R.J., Mayo J.C., Tan D.X., Sainz R.M., Alatorre-Jimenez M., Qin L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 153.Garcia-Maurino S., Gonzalez-Haba M.G., Calvo J.R., Rafii-El-Idrissi M., Sanchez-Margalet V., Goberna R., Guerrero J.M. Melatonin enhances IL-2, IL-6, and IFN-gamma production by human circulating CD4+ cells: A possible nuclear receptor-mediated mechanism involving T helper type 1 lymphocytes and monocytes. J. Immunol. 1997;159:574–581. [PubMed] [Google Scholar]
- 154.Szczepanik M. Melatonin and its influence on immune system. J Physiol Pharm. 2007;58(Suppl. 6):115–124. [PubMed] [Google Scholar]
- 155.Lardone P.J., Carrillo-Vico A., Molinero P., Rubio A., Guerrero J.M. A novel interplay between membrane and nuclear melatonin receptors in human lymphocytes: Significance in IL-2 production. Cell Mol. Life Sci. 2009;66:516–525. doi: 10.1007/s00018-008-8601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Paterniti I., Cordaro M., Esposito E., Cuzzocrea S. The antioxidative property of melatonin against brain ischemia. Expert Rev. Neurother. 2016;16:841–848. doi: 10.1080/14737175.2016.1182020. [DOI] [PubMed] [Google Scholar]
- 157.Liu F., Ng T.B., Fung M.C. Pineal indoles stimulate the gene expression of immunomodulating cytokines. J. Neural. Transm. (Vienna) 2001;108:397–405. doi: 10.1007/s007020170061. [DOI] [PubMed] [Google Scholar]
- 158.Conti A., Conconi S., Hertens E., Skwarlo-Sonta K., Markowska M., Maestroni J.M. Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res. 2000;28:193–202. doi: 10.1034/j.1600-079X.2000.280401.x. [DOI] [PubMed] [Google Scholar]
- 159.Singh M., Jadhav H.R. Melatonin: Functions and ligands. Drug Discov. Today. 2014;19:1410–1418. doi: 10.1016/j.drudis.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 160.Fan L., Sun G., Ma T., Zhong F., Wei W. Melatonin overcomes apoptosis resistance in human hepatocellular carcinoma by targeting survivin and XIAP. J. Pineal Res. 2013;55:174–183. doi: 10.1111/jpi.12060. [DOI] [PubMed] [Google Scholar]
- 161.Wang Y., Wang P., Zheng X., Du X. Therapeutic strategies of melatonin in cancer patients: A systematic review and meta-analysis. Oncotargets Ther. 2018;11:7895. doi: 10.2147/OTT.S174100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rodriguez C., Martín V., Herrera F., García-Santos G., Rodriguez-Blanco J., Casado-Zapico S., Sánchez-Sánchez A.M., Suárez S., Puente-Moncada N., Anítua M.J. Mechanisms involved in the pro-apoptotic effect of melatonin in cancer cells. Int. J. Mol. Sci. 2013;14:6597–6613. doi: 10.3390/ijms14046597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee S.J., Jung Y.H., Oh S.Y., Yun S.P., Han H.J. Melatonin enhances the human mesenchymal stem cells motility via melatonin receptor 2 coupling with Galphaq in skin wound healing. J. Pineal Res. 2014;57:393–407. doi: 10.1111/jpi.12179. [DOI] [PubMed] [Google Scholar]
- 164.Mias C., Lairez O., Trouche E., Roncalli J., Calise D., Seguelas M.H., Ordener C., Piercecchi-Marti M.D., Auge N., Salvayre A.N., et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27:2734–2743. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 165.Zaminy A., Ragerdi Kashani I., Barbarestani M., Hedayatpour A., Mahmoudi R., Farzaneh Nejad A. Osteogenic differentiation of rat mesenchymal stem cells from adipose tissue in comparison with bone marrow mesenchymal stem cells: Melatonin as a differentiation factor. Iran BioMed J. 2008;12:133–141. [PubMed] [Google Scholar]
- 166.Zheng W., Yang Y., Sequeira R.C., Bishop C.E., Atala A., Gu Z., Zhao W. Effects of Extracellular Vesicles Derived from Mesenchymal Stem/Stromal Cells on Liver Diseases. Curr. Stem Cell Res. Ther. 2019;14:442–452. doi: 10.2174/1574888X14666190308123714. [DOI] [PubMed] [Google Scholar]
- 167.Cho Y.A., Noh K., Jue S.S., Lee S.Y., Kim E.C. Melatonin promotes hepatic differentiation of human dental pulp stem cells: Clinical implications for the prevention of liver fibrosis. J. Pineal Res. 2015;58:127–135. doi: 10.1111/jpi.12198. [DOI] [PubMed] [Google Scholar]
- 168.Kadry S.M., El-Dakdoky M.H., Haggag N.Z., Rashed L.A., Hassen M.T. Melatonin improves the therapeutic role of mesenchymal stem cells in diabetic rats. Toxicol. Mech. Methods. 2018;28:529–538. doi: 10.1080/15376516.2018.1471634. [DOI] [PubMed] [Google Scholar]
- 169.Wang X., Liang T., Qiu J., Qiu X., Gao B., Gao W., Lian C., Chen T., Zhu Y., Liang A., et al. Melatonin Reverses the Loss of Stemness Induced by TNF- α in Human Bone Marrow Mesenchymal Stem Cells through Upregulation of YAP Expression. Stem Cells Int. 2019;2019:1–16. doi: 10.1155/2019/6568394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jiang T., Xia C., Chen X., Hu Y., Wang Y., Wu J., Chen S., Gao Y. Melatonin promotes the BMP9-induced osteogenic differentiation of mesenchymal stem cells by activating the AMPK/β-catenin signalling pathway. Stem Cell Res. Ther. 2019;10:408. doi: 10.1186/s13287-019-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mohamed Y., Basyony M.A., El-Desouki N.I., Abdo W.S., El-Magd M.A. The potential therapeutic effect for melatonin and mesenchymal stem cells on hepatocellular carcinoma. BioMed (Taipei) 2019;9:24. doi: 10.1051/bmdcn/2019090424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.El-Magd M.A., Mohamed Y., El-Shetry E.S., Elsayed S.A., Abo Gazia M., Abdel-Aleem G.A., Shafik N.M., Abdo W.S., El-Desouki N.I., Basyony M.A. Melatonin maximizes the therapeutic potential of non-preconditioned MSCs in a DEN-induced rat model of HCC. BioMed Pharm. 2019;114:108732. doi: 10.1016/j.biopha.2019.108732. [DOI] [PubMed] [Google Scholar]
- 173.Mortezaee K., Khanlarkhani N., Sabbaghziarani F., Nekoonam S., Majidpoor J., Hosseini A., Pasbakhsh P., Kashani I.R., Zendedel A. Preconditioning with melatonin improves therapeutic outcomes of bone marrow-derived mesenchymal stem cells in targeting liver fibrosis induced by CCl4. Cell Tissue Res. 2017;369:303–312. doi: 10.1007/s00441-017-2604-1. [DOI] [PubMed] [Google Scholar]
- 174.Mortezaee K., Pasbakhsh P., Ragerdi Kashani I., Sabbaghziarani F., Omidi A., Zendedel A., Ghasemi S., Dehpour A.R. Melatonin Pretreatment Enhances the Homing of Bone Marrow-derived Mesenchymal Stem Cells Following Transplantation in a Rat Model of Liver Fibrosis. Iran BioMed J. 2016;20:207–216. doi: 10.7508/ibj.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen H.H., Lin K.C., Wallace C.G., Chen Y.T., Yang C.C., Leu S., Chen Y.C., Sun C.K., Tsai T.H., Chen Y.L., et al. Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. J. Pineal Res. 2014;57:16–32. doi: 10.1111/jpi.12140. [DOI] [PubMed] [Google Scholar]
- 176.Basyony M., Desouki N., Sobhy W., Hegazy R., Mohamed Y. Melatonin improves the anticancer effects of mesenchymal stem cell against HCC in rat. Egypt. J. Exp. Biol. (Zool.) 2019;1 doi: 10.5455/egysebz.20190630042451. [DOI] [Google Scholar]