Abstract
Escherichia coli is known as one of the most important foodborne pathogens in humans, and contaminated chicken meat is an important source of foodborne infection with this bacterium. The occurrence of extended-spectrum β-lactamase (ESBL)-producing E. coli (ESBL-Ec), in particular, in chicken meat is considered a global health problem. This study aimed to determine the magnitude of E. coli, with special emphasis on ESBL-Ec, along with their phenotypic antimicrobial resistance pattern in frozen chicken meat. The study also focused on the determination of ESBL-encoding genes in E. coli. A total of 113 frozen chicken meat samples were purchased from 40 outlets of nine branded supershops in five megacities in Bangladesh. Isolation and identification of E. coli were done based on cultural and biochemical properties, as well as PCR assay. The resistance pattern was determined by the disc diffusion method. ESBL-encoding genes were determined by multiplex PCR. The results showed that 76.1% of samples were positive for E. coli, of which 86% were ESBL producers. All the isolates were multidrug-resistant (MDR). Resistance to 9–11 and 12–13 antimicrobial classes was observed in 38.4% and 17.4% isolates, respectively, while only 11.6% were resistant to 3–5 classes. Possible extensive drug resistance (pXDR) was found in 2.3% of isolates. High single resistance was observed for oxytetracycline (93%) and amoxicillin (91.9%), followed by ampicillin (89.5%), trimethoprim–sulfamethoxazole, and pefloxacin (88.4%), and tetracycline (84.9%). Most importantly, 89.6% of isolates were resistant to carbapenems. All the isolates were positive for the blaTEM gene. However, the blaSHV and blaCTX-M-2 genes were identified in two ESBL-non producer isolates. None of the isolates carried the blaCTX-M-1 gene. This study provided evidence of the existence of MDR and pXDR ESBL-Ec in frozen chicken meat in Bangladesh, which may pose a risk to human health if the meat is not properly cooked or pickled raw only. This emphasizes the importance of the implementation of good slaughtering and processing practices by the processors.
Keywords: Escherichia coli, antimicrobial resistance, ESBL, MDR, frozen chicken meat, Bangladesh
1. Introduction
Escherichia coli (E. coli), a member of the Enterobacteriaceae family, is a normal inhabitant of the gut of poultry and a frequent microbial contaminant of retail poultry meat [1]. E. coli is also known as one of the most important foodborne pathogens in humans, which may be associated with a diversity of acute and invasive infections in humans, and it can easily be disseminated in different ecosystems through the food chain [2,3]. It is a highly versatile bacterial species comprising both nonpathogenic strains and different pathogenic variants with the ability to cause either intestinal or extra-intestinal diseases [4]. While the majority of strains of E. coli are nonpathogenic in humans (e.g., uncomplicated urinary tract infections) or exist as part of the indigenous flora, often contributing to the vital tasks performed by the intestinal microflora, some strains of poultry-derived E. coli can also be opportunistic and pathogenic in nature (e.g., bloodstream infections) [4]. Chicken meat is frequently contaminated by E. coli during handling, improper dressing, cleaning, and unhygienic practices of selling meat. Contaminated chicken meat is considered as a potential source of infection with E. coli, either via direct contact during food preparation or via consumption of undercooked or raw meat products [3]. Although E. coli exhibits heat sensitivity to thermal treatment ranging from 60 to 80 °C, some strains of E. coli were reported to be highly resistant to heat [5]. It was reported that several strains of E. coli become resistant to heat by the addition of salt, and about 2% of E. coli-including food isolates harbor heat-resistant genes and show increased heat resistance [6]. The locus of heat resistance (LHR) can be transferred to another E. coli through lateral gene transfer [7]. Moreover, the duration of microwave exposure and the methods used for cooking can also result in failure of the thermal inactivation of E. coli [8]. E. coli can also survive low-temperature stress (cold shock) through different mechanisms. The synthesis of cold-shock proteins (CSPs) is one of the most important responses to cold temperature, and these are involved in a variety of essential functions such as transcription, translation, mRNA degradation, protein synthesis, and recombination in E. coli [9,10]. However, it was reported that rapid chilling (2000 °C∙min−1) induces an immediate loss of viability of up to more than 90% for exponentially growing cells of E. coli [11]. The most common symptoms of food poisoning due to E. coli are abdominal cramps, vomiting, and, in some cases, bloody diarrhea in humans. Sometimes, the infection caused by Shiga toxin-producing E. coli may lead to hemolytic–uremic syndrome that can cause kidney failure [12]. In most of the cases, E. coli infections are self-limiting, and antibiotic medication is discouraged [13].
Over the past few decades, antibiotic resistance trends increased at a faster rate among chicken isolates of E. coli than human clinical isolates [14]. Commensal E. coli were determined as an important reservoir of antimicrobial resistance genes that may spread to pathogenic strains [15]. One of the most common resistance mechanisms reported in the members of the family Enterobacteriaceae is the production of β-lactamase enzymes that hydrolyze β-lactam antibiotics [16]. Extended-spectrum β-lactamases (ESBLs), variants of β-lactamases, a heterogeneous group of enzymes, are encoded by genes which efficiently hydrolyze third- and fourth-generation cephalosporins and monobactams (e.g., aztreonam) but are inhibited by β-lactamase inhibitors such as clavulanic acid and tazobactam [17]. E. coli that produce ESBL are of particular concern because of the implications for human and food animal health worldwide [18]. The emergence of ESBLs is considered an important cause of transferable multidrug-resistant superbugs, particularly E. coli. Furthermore, ESBL-producing E. coli often exhibit co-resistance to multiple classes of antimicrobials, mainly fluoroquinolones, sulfonamides, aminoglycosides, chloramphenicol, trimethoprim, and tetracyclines, which may increase the risk of poor clinical outcomes due to lack of effective treatment options [18].
The major genes responsible for ESBL production include TEM genes (blaTEM), SHV genes (blaSHV), and CTX-M genes (blaCTX-M). The CTX-M type ESBL-producing E. coli is the most dominant globally [19]. In Bangladesh, blaCTX-M-1 (94.4%) and blaTEM (50–91.3%) ESBL-producing E. coli were reported in droppings of chickens [20,21,22]. Chickens are considered as a potential reservoir of ESBL-producing E. coli [23]. Chicken meat contaminated with ESBL-producing bacteria is thought to be one of the potential risk factors for the wide dissemination of ESBL-producing bacteria in humans [24].
The prevalence of multidrug-resistant superbugs and ESBL-producing bacteria is increasing in humans, as well as animals. Multidrug-resistant (MDR) and extensively drug-resistant (XDR) bacteria are well-defined by the European Center for Disease Control, and Centers for Disease Control and Prevention, Atlanta [25]. MDR is defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories, and XDR is defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (i.e., bacterial isolates remain susceptible to only one or two antimicrobial categories).
Currently, like in other countries, the lifestyle, preference, and demands of consumers in Bangladesh are changing rapidly. With the current shopping practice, supershops are now a necessity as they offer a unique shopping experience with all essential commodities under one roof. Consumers, especially city dwellers, are increasingly becoming more aware of their convenience and the lifestyle they allow, as they prefer to go to supershops rather than to wet markets to buy their everyday stuff, including frozen chicken meat. City dwellers tend to buy frozen chicken meat along with other frozen and ready-to-cook foodstuffs as these frozen items need minimal processing for cooking and, thus, they can save time [26]. However, the microbiological safety of this frozen chicken meat is an important concern in the context of public health hazards, as two studies reported bacterial contamination in frozen chicken meat in Dhaka city of Bangladesh [27,28]. Both studies were restricted to three to five supershops of Dhaka city only. Furthermore, none of these two reports investigated the multidrug resistance pattern of ESBL-producing E. coli. Therefore, a study is required to have an updated scenario of E. coli contamination along with the resistance pattern in frozen chicken meat covering more outlets of available branded supershops located in five megacities of Bangladesh. The present study determined the (i) prevalence and distribution of E. coli, with special emphasis on ESBL-producing E. coli, along with their phenotypic resistance pattern, in frozen chicken meat sold in various supershops located in five megacities of Bangladesh, and (ii) ESBL-encoding genes in E. coli in frozen chicken meat, which are yet to be investigated in Bangladesh.
2. Results
2.1. Source of Chicken, and Processing and Packaging of Frozen Chicken Meat
The findings of the questionnaire survey, conducted in 40 outlets of nine branded supershops of five megacities in Bangladesh, revealed that supershops of all brands purchased chickens from their contract farms (Table 1). All the outlets of brands 4, 6, 8, and 9, and majority outlets of brands 1 to 3 had their chicken meat processed outside the supershops. Regarding the packaging of meat, it was observed that 100% of outlets of brands 6, 8, and 9, and the majority of brands 1 to 3 packaged their chicken meat inside the shops. However, all outlets of brand 5 processed and packaged chicken meat inside the shop; in contrast, all outlets of brand 7 did it outside the shop.
Table 1.
Demographic information of nine branded supershops in five megacities.
| Name of Supershops (N) | Source of Chicken (%) | Processing of Chicken | Packaging of Chicken | ||
|---|---|---|---|---|---|
| Inside Shop N (%) |
Outside Shop N (%) |
Inside Shop N (%) |
Outside Shop N (%) |
||
| Brand 1 (7) | Contract farm (100) | 1 (14.3) | 6 (85.7) | 6 (85.7) | 1 (14.3) |
| Brand 2 (15) | Contract farm (100) | 2 (13.3) | 13 (86.7) | 10 (66.7) | 5 (33.3) |
| Brand 3 (10) | Contract farm (100) | 2 (20.0) | 8 (80.0) | 8 (80.0) | 2 (20.0) |
| Brand 4 (3) | Contract farm (100) | 0 | 3 (100.0) | 2 (66.7) | 1 (33.3) |
| Brand 5 (1) | Contract farm (100) | 1 (100.0) | 0 | 1 (100.0) | 0 |
| Brand 6 (1) | Contract farm (100) | 0 | 1 (100.0) | 1 (100.0) | 0 |
| Brand 7 (1) | Contract farm (100) | 0 | 1 (100.0) | 0 | 1 (100.0) |
| Brand 8 (1) | Contract farm (100) | 0 | 1 (100.0) | 1 (100.0) | 0 |
| Brand 9 (1) | Contract farm (100) | 0 | 1 (100.0) | 1 (100.0) | 0 |
Contract farms: Farmers have the contract with the company (supershop authority) that the company provides the chickens, the feed, veterinary care, and technical advice, while the poultry farmers provide the day-to-day care of the birds, land, and housing, as well as utilities/maintenance of the housing.
2.2. Prevalence and Distribution of ESBL-Producing and ESBL-Non-Producing E. coli
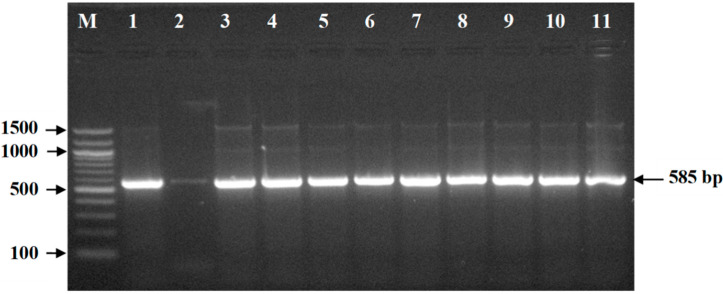
The overall prevalence of E. coli was 76.1% (86/113) in frozen chicken meat samples, and it varied from 33.3% to 100% among the nine different brands (Table 2). All E. coli isolates were confirmed by PCR as they generated a 585-bp fragment size on amplification (Figure 1). Out of 86 E. coli isolates, 74 (86%) were ESBL–producing E. coli (ESBL-Ec) and 14% (12/86) were ESBL-non-producing E. coli (non-ESBL-Ec) (Table 2). None of the E. coli isolates were recovered from one brand (brand 9). The prevalence of ESBL-Ec and non-ESBL-Ec varied significantly from brand to brand. The prevalence of ESBL-Ec in frozen chicken meat of different brands varied from 50% to 100%, while the prevalence of non-ESBL-Ec varied from 30% to 100% (Table 2).
Table 2.
Prevalence of extended-spectrum β-lactamase-producing Escherichia coli (ESBL-Ec) and non-ESBL-Ec isolated from frozen chicken meat in different supershops.
| Name of Supershops | Total No. of Samples | No. of E. coli-Positive Isolates (%) | ESBL-Ec No. (%) |
Non-ESBL-Ec No. (%) |
|---|---|---|---|---|
| Brand 1 | 23 | 21 (91.3) | 21 (100.0) a | 0 |
| Brand 2 | 40 | 30 (75.0) | 21 (70.0) b | 9 (30.0) b |
| Brand 3 | 28 | 24 (85.8) | 24 (100.0) a | 0 |
| Brand 4 | 8 | 3 (37.5) | 2 (66.7) b | 1 (33.3) b |
| Brand 5 | 2 | 2 (100.0) | 2 (100.0) a,b | 0 |
| Brand 6 | 2 | 2 (100.0) | 1 (50.0) b | 1 (50.0) b |
| Brand 7 | 5 | 3 (60.0) | 3 (100.0) a,b | 0 |
| Brand 8 | 3 | 1 (33.3) | 0 | 1 (100.0) a |
| Brand 9 | 2 | 0 | - | - |
| Total | 113 | 86 (76.1) | 74 (86.0) | 12 (14.0) |
ESBL-Ec = ESBL-producing E. coli; non-ESBL-Ec = ESBL- non producing E. coli; a,b values in the same column with different superscripts differ significantly (p ≤ 0.05).
Figure 1.
PCR amplified product of 585 bp from 16S rRNA gene of E. coli following 1.5% agarose gel electrophoresis and ethidium bromide staining. Legends: M = DNA marker (100 bp), Lane 1 = Positive control of E. coli, Lane 2 = Negative control, Lanes 3–11 = PCR product of tested E. coli isolates.
As shown in Table 3, the highest prevalence of ESBL-Ec was recorded in both Chattogram and Mymensingh divisions (100.0%), followed by the Dhaka (92.3%) division, which was significantly higher than that in the Rajshahi division (33.3%). On the other hand, the highest prevalence of non-ESBL-Ec was in the Sylhet (100.0%) division and the lowest was in the Dhaka division (7.7%). Moreover, in broiler and cockerel chickens, similar prevalence of ESBL-Ec (87.3% and 82.6%, respectively) and non-ESBL-Ec (12.7% and 17.4%, respectively) was observed (Table 3). We did not find any significant differences in the prevalence of both ESBL-Ec and non-ESBL-Ec between organic and non-organic chickens. Considering the types of meat sample, although the highest isolation rate of ESBL-Ec was found in leg muscle (100%) there were no significant differences between different types of meat sample. The isolation rate of non-ESBL-Ec was highest in breast muscle (18.2%) and lowest in drumstick (9.1%) (Table 3).
Table 3.
Distribution of ESBL-Ec and non-ESBL-Ec isolated from frozen chicken meat.
| Variables (N) | No. of E. coli-Positive Isolates (%) | ESBL-Ec No. (%) |
Non-ESBL-Ec No. (%) |
|---|---|---|---|
| Divisions | |||
| Dhaka (82) | 65 (79.3) | 60 (92.3) a | 5 (7.7) a |
| Chattogram (10) | 10 (100.0) | 10 (100.0) a | 0 |
| Sylhet (11) | 5 (45.5) | 0 | 5 (100.0) b |
| Mymensingh (5) | 3 (60.0) | 3 (100.0) a,b | 0 |
| Rajshahi (5) | 3 (60.0) | 1 (33.3) b | 2 (66.7) b |
| Chicken types | |||
| Broiler (82) | 63 (76.8) | 55 (87.3) a | 8 (12.7) a |
| Cockerel (31) | 23 (74.2) | 19 (82.6) a | 4 (17.4) a |
| Production types | |||
| Organic (10) | 5 (50.0) | 4 (80.0) a | 1 (20.0) a |
| Non-organic (103) | 81 (78.6) | 70 (86.4) a | 11 (13.6) a |
| Meat sample types | |||
| Breast (27) | 22 (81.5) | 18 (81.8) a | 4 (18.2) a |
| Drumstick (30) | 22 (73.3) | 20 (90.9) a | 2 (9.1) a |
| Leg (3) | 3 (100.0) | 3 (100.0) a | 0 |
| Wing (19) | 16 (84.2) | 14 (87.5) a | 2 (12.5) a |
| Whole-chicken pool sample (34) | 23 (67.6) | 19 (82.6) a | 4 (17.4) a |
| Total (113) | 86 (76.1) | 74 (86.0) | 12 (14.0) |
ESBL-Ec = ESBL-producing E. coli; non-ESBL-Ec = ESBL-non-producing E. coli; a,b values in the same column with different superscripts differ significantly (p ≤ 0.05).
2.3. Distribution of Possible Extensively Drug-Resistant (pXDR) E. coli
Notably, in this study, 2.3% (2/86) of E. coli isolates were pXDR (resistant to 13 out of 16 antimicrobial classes). The pXDR E. coli isolates were only susceptible to polymyxin, monobactam, and glycylcycline antimicrobial classes. One pXDR E. coli isolate was recovered from broiler meat of brand 5 in Dhaka division and another one was recovered from the cockerel meat of brand 7 in Mymensingh division. Both pXDR isolates originated from non-organically produced chickens.
2.4. Distribution of Multidrug-Resistant E. coli
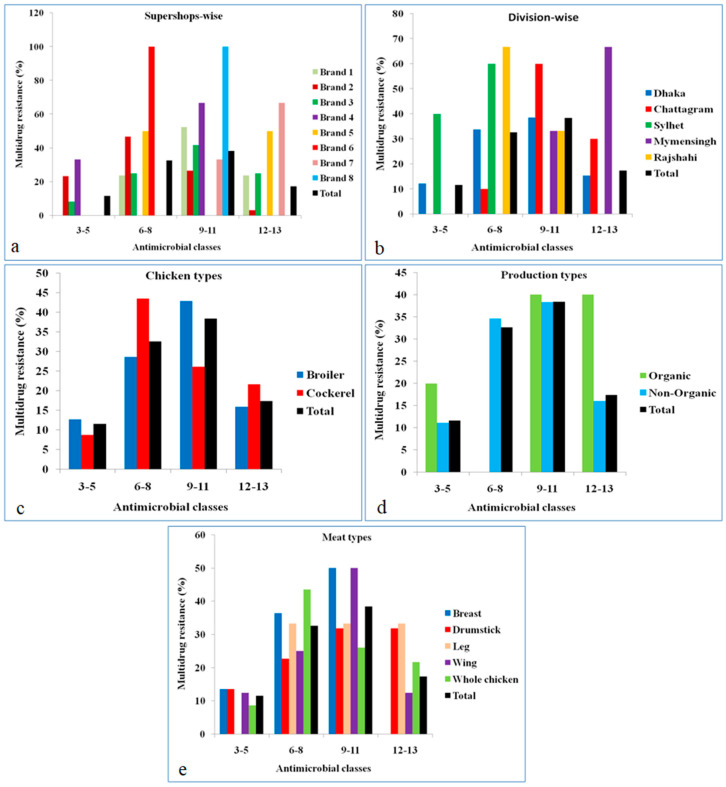
Of the 86 E. coli isolates tested, all the isolates were multidrug-resistant (MDR). In our study, we used 16 antimicrobial classes. The overall distributions of MDR E. coli are shown in Figure 2a–e. It was observed that 38.4% of isolates were resistant to 9–11 antimicrobial classes, 32.6% were resistant to 6–8 classes, and 11.6% were resistant to 3–5 classes. Notably, 17.4% of isolates were resistant to 12–13 antimicrobial classes. Multidrug-resistant E. coli were widespread among different brands, and all isolates from brand 6 and brand 8 showed a higher rate of resistance to 6–8 and 9–11 antimicrobial classes, respectively (Figure 2a). Regarding the division-wise distribution of MDR E. coli, the highest percentage of isolates, resistant to 6–8 and 12–13 antimicrobial classes, was observed in Rajshahi and Mymensingh divisions, respectively (Figure 2b). Considering chicken types, it was revealed that 43.5% of isolates from cockerel chicken meat, and 42.9% of isolates from broiler chicken meat were resistant to 6–8 and 9–11 antimicrobial classes, respectively (Figure 2c). Production type-wise MDR pattern results revealed that 40% of isolates from organically produced chickens were resistant to 9–11 and 12–13 antimicrobial classes, respectively, while 38.3% of isolates from non-organically produced chickens were resistant to 9–11 antimicrobial classes (Figure 2d). Looking at the meat sample type-wise distribution, 50% of the isolates, recovered from breast and wing muscle, were resistant to 9–11 antimicrobial classes (Figure 2e).
Figure 2.
(a–e) Antimicrobial class-wise distribution of multidrug resistance pattern of E. coli isolated from frozen chicken meat.
It is noted that, among the 86 E. coli isolates, all isolates were resistant to at least four, and up to 28 antimicrobials (Table 4). The frequency of resistance to 19–23 antimicrobials was observed in 22 (25.6%) isolates, while only 11 (12.8%) isolates were resistant to 4–8 antimicrobials. The percentage of resistance to 9–13 and 14–18 antimicrobials was the same (22.1%). Notably, 15 (17.4%) isolates were resistant to 24–28 antimicrobials. Most importantly, resistance to three or fewer antimicrobials was not observed in any of the isolates tested. Brand-wise resistance to antimicrobials revealed that the highest resistance to 19–23 antimicrobials was observed in 42.9% isolates from brand 1. Two (66.7%) isolates from brand 7, one (50%) from brand 5, and 7 (29.2%) from brand 3 were resistant to 24–28 antimicrobials (Table 4). Significant differences were observed in the resistance percentages to antimicrobial agents between brands.
Table 4.
Supershop-wise distribution of resistant E. coli isolated from frozen chicken meat.
| Name of Supershops (N) | No. (%) of Isolates Resistant to Antimicrobial Agents | ||||
|---|---|---|---|---|---|
| 4–8 | 9–13 | 14–18 | 19–23 | 24–28 | |
| Brand 1 (21) | 0 | 3 (14.3) a | 7 (33.3) a | 9 (42.9) a | 2 (9.5) a |
| Brand 2 (30) | 8 (26.7) a | 9 (30.0) a,b | 5 (16.7) b | 5 (16.7) b | 3 (10.0) a |
| Brand 3 (24) | 2 (8.3) b | 5 (20.8) a,b | 3 (12.5) b | 7 (29.2) a,b | 7 (29.2) b |
| Brand 4 (3) | 1 (33.3) a | 0 | 1 (33.3) a | 1 (33.3) a | 0 |
| Brand 5 (2) | 0 | 1 (50.0) a,b | 0 | 0 | 1 (50.0) c |
| Brand 6 (2) | 0 | 0 | 2 (100.0) c | 0 | 0 |
| Brand 7 (3) | 0 | 0 | 1 (33.3) a | 0 | 2 (66.7) c |
| Brand 8 (1) | 0 | 1 (100.0) b | 0 | 0 | 0 |
| Total | 11 (12.8) | 19 (22.1) | 19 (22.1) | 22 (25.6) | 15 (17.4) |
a,b,c Values in the same column with different superscripts differ significantly (p ≤ 0.05).
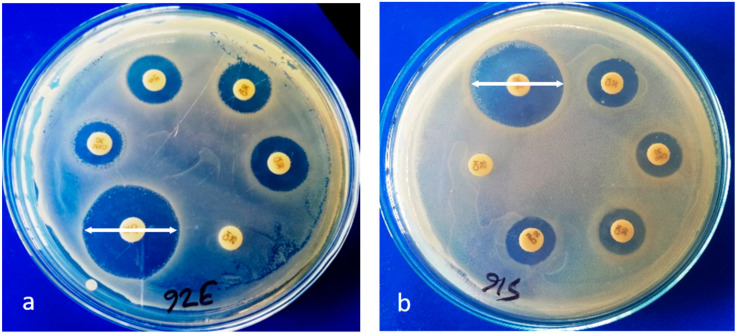
Overall analysis of disc diffusion results (Figure 3a,b) revealed that the highest single resistance in E. coli was detected against oxytetracycline (93%) and amoxicillin (91.9%). In addition, resistances to ampicillin (89.5%), trimethoprim–sulfamethoxazole and pefloxacin (88.4%), tetracycline (84.9%), cefepime (72.1%), and piperacillin–tazobactam (70.9%) were also very high in E. coli isolates (Table S1, Supplementary Materials). Among all the antibiotics, resistance to aztreonam was observed to be the lowest (1.2%), followed by ceftriaxone and tigecycline (2.3%) (Table S1, Supplementary Materials).
Figure 3.
(a,b) Antimicrobial susceptibility tests of E. coli by disc diffusion method showing zone of inhibition (↔).
The variation in the resistance pattern of ESBL-Ec (n = 74) and non-ESBL-Ec (n = 12) isolates was determined (Table S1, Supplementary Materials). Resistances to oxytetracycline and amoxicillin (91.9%), ampicillin and trimethoprim–sulfamethoxazole (89.2%), pefloxacin (87.8%), cefepime (81.1%), piperacillin–tazobactam (73.0%), and doxycycline (70.3%) were found to be higher in ESBL-Ec isolates, while resistances to oxytetracycline (100.0%), tetracycline, pefloxacin, ampicillin, and amoxicillin (91.7%), and trimethoprim–sulfamethoxazole (83.3%) were observed to be higher in non-ESBL-Ec isolates. No significant differences were observed among these antimicrobial agents between ESBL-Ec and non-ESBL-Ec except for cefepime, streptomycin, and chloramphenicol. It is important to note that 77 (89.5%) isolates showed resistance to carbapenems, the antimicrobials used in human medicine, of which 76 isolates were ESBL-Ec. The resistance to imipenem was 47.7%, and that to meropenem was 41.9%.
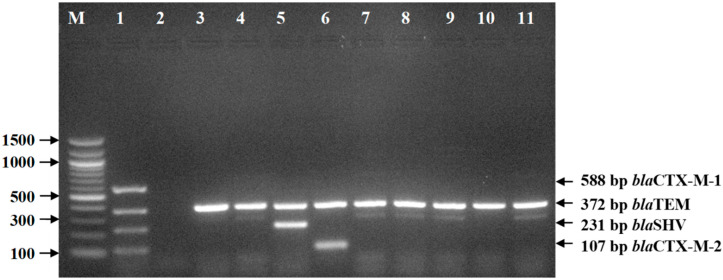
2.5. Genotypes of ESBL-Ec and Non-ESBL-Ec
The findings of ESBL genes, i.e., blaTEM, blaSHV, blaCTX-M-1, and blaCTX-M-2 genes, are presented in Table 5 and Figure 4. All the isolates were positive for the blaTEM gene. One isolate of non-ESBL-Ec was positive for the blaSHV gene, and another one isolate of non-ESBL-Ec was positive for the blaCTX-M-2 gene. None of the tested isolates harbored the blaCTX-M-1 gene.
Table 5.
Prevalence of ESBL-Ec and non-ESBL-Ec genotypes isolated from frozen chicken meat.
| Genotypes | ESBL-Ec (n = 74) |
non-ESBL-Ec (n = 12) |
Total (n = 86) |
|---|---|---|---|
| blaTEM | 74 (100.0) | 12 (100.0) | 86 (100.0) |
| blaSHV | 0 | 1 (8.3) | 1 (1.2) |
| blaCTX-M-1 | 0 | 0 | 0 |
| blaCTX-M-2 | 0 | 1 (8.3) | 1 (1.2) |
ESBL-Ec = ESBL-producing E. coli; non-ESBL-Ec = ESBL-non producing E. coli.
Figure 4.
ESBL-encoding genes of E. coli isolates from frozen chicken meat by multiplex PCR, followed by 1.5% agarose gel electrophoresis and ethidium bromide staining. Legends: M = DNA marker (100 bp), Lane 1 = Positive control, Lane 2 = Negative control, Lanes 3–11 = Positive for blaTEM gene; Lane 5 = Positive for blaSHV gene; Lane 6 = Positive for blaCTX-M-2 gene.
3. Discussion
E. coli is a common enteric pathogen, specific strains of which can cause human and animal disease. It is one of the groups of seven species that the World Health Organization (WHO) highlighted as of key antimicrobial resistance (AMR) concern, and it serves as a sentinel organism for the assessment of the development of antimicrobial resistance [29]. The emergence and spread of ESBL-Ec linked to chickens and other farm animals are of particular concern [23].
The present study reports the first comprehensive findings on the extent and distribution of ESBL-Ec and their antimicrobial resistance pattern including resistance genes in frozen chicken meat collected from almost all branded supershops located in five megacities of Bangladesh. This study showed the high prevalence (76.1%) of E. coli in frozen chicken meat compared with 49–53% prevalence in raw chicken meat as reported earlier in Bangladesh [30,31], 66.3% in India [32], 47.1% in Nepal [33], and 50.5% in Korea [34], and this may be a potential hazard to the consumers. The difference in the prevalence of E. coli may be attributed to several factors including the source of meat, sample number, isolation methods, possible cross-contamination during slaughtering, slaughterhouse sanitation, and personal hygiene, as well as other practices through to the food chain.
One of the main findings in this study was the high prevalence (86.0%) of ESBL-Ec in frozen chicken meat. These results corroborate the findings of similar studies conducted in Japan, in which the authors reported that 65–77% of frozen chicken meat samples were contaminated with ESBL-Ec [35,36]. The present study observed that the prevalence of ESBL-Ec in frozen chicken meat varied from brand to brand, which might be due to variation in processing, packaging, and personnel hygienic practices in different supershops. It is expected that different brands follow different types of management and, thus, there are different risks regarding the prevalence of ESBL-Ec. Contamination may also occur during the transportation of chicken meat from farm to supershops or during the steps involved in slaughtering, defeathering, plucking, and chilling of the chicken meat [37]. The distribution of ESBL-Ec was found to vary from division to division, with Chattogram, Mymensingh, and Dhaka divisions having the highest prevalence and the Rajshahi division having the lowest prevalence. The highest distribution of non-ESBL-Ec was observed in the Sylhet division of Bangladesh. An earlier study showed that 30% of ESBL-Ec was detected from droppings of domestic chickens in the Rajshahi division of Bangladesh [38]. In the present study, a considerably high percentage of ESBL-Ec was recovered from different types of meat samples. The pathogenic E. coli are usually absent in the muscle tissue and body fluids of healthy living chickens, but they can enter into the meat during slaughtering or at the time of processing and packaging from the gastrointestinal tract [39]. This high prevalence is very alarming and requires risk assessments and pertinent risk management to keep down the occurrence and spread of ESBL-Ec. This result also indicates that the contamination of frozen chicken meat with ESBL-Ec in Bangladesh is more frequent, which may rapidly raise the risk of humans being infected.
It is of particular concern that all the isolates of E. coli in this study were MDR, of which a substantial percentage of isolates showed resistance to 9–13 classes of antimicrobials, which is in line with previous observations among E. coli recovered from retail chicken meat in Korea [34], but which differed from some other reports [31,33]. The highest percentage of isolates from Rajshahi and Mymensingh divisions expressed MDR, which is in disagreement with previous reports in Bangladesh, in which 10–35% of E. coli isolates in retail chicken meat from Mymensingh and Dhaka divisions showed MDR [40,41]. Of note, the current study also observed that 2.3% of E. coli isolates were pXDR. An earlier report from Japan detected extensive MDR E. coli in 70% of frozen chicken meat samples [36]. The high rates of MDR and existence of pXDR in this study imply that this can reflect the frequent use or misuse of antimicrobials along with poor biosecurity and waste management systems in poultry production in Bangladesh, which creates a selection pressure, thus contributing to the emergence and spread of MDR bacteria in poultry production systems. Indeed, MDR in commensal bacteria develops naturally over time, usually through genetic changes and/or via the action of MDR efflux pumps; however, the massive use of antimicrobial agents for disease control and prevention causes an unprecedented increase in resistance [42,43]. Moreover, the use of disinfectants, particularly quaternary ammonium compounds (QACs), to limit infection in poultry may also induce the AMR through cross-resistance between QACs and a range of antimicrobials [44,45]. Another plausible explanation is that the high prevalence of MDR E. coli may be attributed to the possible cross-contamination during slaughtering, cutting, and further processing indirectly through contaminated equipment, as well as the use of stored water in containers that received minimal cleaning after frequent washing of carcasses [37]. These observations support the possibility that chicken meat might be one of the potential sources of MDR E. coli infections causing possible transmission of resistant bacteria to consumers, and they suggest that continued surveillance is important.
Increasing rates of antimicrobial resistance in both ESBL-Ec and non-ESBL-Ec are a growing public health problem that needs to be monitored continuously. Our study indicated that all isolates of E. coli exhibited absolute resistance (100%) to at least four antimicrobial agents. Of note, 17.4% isolates of E. coli showed resistance to more than 24 antimicrobials. A high percentage of antimicrobial-resistant E. coli from frozen chicken meat was also reported by several investigators [33,36]. In the current study, oxytetracycline resistance was the most frequently observed antimicrobial resistance in both ESBL-Ec and non-ESBL-Ec, which is consistent with several other studies in frozen chicken meat [33,46]. The finding is not surprising because, since its approval in 1948, oxytetracycline was widely used in veterinary practices, which probably led to this outcome [47].
A very high degree of resistance was also observed for amoxicillin, ampicillin, and trimethoprim–sulfamethoxazole in both ESBL-Ec and non-ESBL-Ec. A similar resistivity pattern was observed in Bangladesh [31,41], Japan [35], Korea [34], and Vietnam [48]. This may be attributed to the long-term and indiscriminate use of these antimicrobial agents in poultry production in Bangladesh [20]. As fluoroquinolones and cephalosporins are the drugs of choice for the treatment of bacterial infection in humans, E. coli resistant to these antimicrobials could represent a big challenge to animal and human therapeutic interventions, becoming a symbol a relevant public health implication [49]. Unfortunately, this study demonstrated that the prevalence of fluoroquinolone (mainly pefloxacin) resistance in both ESBL-Ec and non-ESBL-Ec was also very high. This result may imply the more frequent use of fluoroquinolones in poultry production in Bangladesh. Moreover, more than 80% isolates of ESBL-Ec showed resistance to cefepime, a fourth-generation cephalosporin antimicrobial, which is higher than a previous observation in retail chicken meat (4.8%) in Korea [34]. Cephalosporin resistance is a matter of concern because cefepime is not used in veterinary practices in Bangladesh, and it is worrisome to find these phenotypes in chicken meat. The rate of resistance to multiple antimicrobials among ESBL-Ec isolates is usually common due to the carrying of multi-resistance genes and plasmids [50]. These plasmids can also carry genes for co-resistance to multiple classes of antimicrobials including fluoroquinolones, sulfonamides, aminoglycosides, chloramphenicol, trimethoprim, and tetracyclines [18]. Surprisingly, remarkably high resistance prevalence was found against carbapenems (last-line therapeutics to treat multidrug-resistant superbugs), mainly imipenem and meropenem, although carbapenems are not used in poultry practices in Bangladesh. There was no clear explanation for these high levels of resistance, but it might be due to co-selection and/or cross-resistance generated by other antimicrobials [51].
On the other hand, a relatively low resistance rate to aztreonam, ceftriaxone, and tigecycline was observed, probably because these antimicrobials are not used in poultry practices in Bangladesh, resulting in a lack of selective pressure by these antimicrobials in poultry production. It also supports the contention that antimicrobial resistance, induced once, is difficult to eliminate, because of associated resistance to other related antimicrobials [52]. Therefore, resistance to these antimicrobials should be carefully monitored.
Among the prevalent ESBL-Ec genes from chicken meat, blaTEM, blaSHV, and blaCTX-M (blaCTX-M-1 and blaCTX-M-2) are considered to be most diverse. The ESBL genes are usually located on plasmids, which could promote the dissemination of ESBL genes in Gram-negative bacteria [23]. The most prevalent ESBL-encoding gene in the current study was blaTEM, which is consistent with a similar study conducted in Vietnam [48]. Interestingly, blaSHV and blaCTX-M-2 were detected in two non-ESBL-Ec isolates. No blaCTX-M-1 was detected in this study. These findings are inconsistent with earlier studies in Bangladesh, where more than 50% of E. coli isolates from droppings of chickens harbored the blaTEM gene and 94.4% carried the blaCTX-M-1 gene [21,38]. It may be hypothesized that frozen chicken meat which is sold to the consumers could potentially act as a major source of gut colonization by avian strains of E. coli that carry blaTEM ESBL genes.
It would be worthwhile if we could sample more outlets of supershops. However, frozen chicken meat samples were purchased from 40 outlets of almost all the renowned branded supershops located in five megacities of Bangladesh; thus, the data represent the scenario of all Bangladesh. This study seems to indicate the current status of contamination with ESBL-Ec in frozen chicken meat. It would be very important to investigate horizontal gene transfer, such as exchanges of plasmid or mobile genetic elements carrying genes for ESBLs, between bacteria isolated from chicken meat.
4. Materials and Methods
4.1. Sample Collection
A cross-sectional survey was conducted in 40 supershop outlets of nine brands available in five megacities (Dhaka, Sylhet, Mymensingh, Chattogram, and Rajshahi) of Bangladesh (Figure 5) from April to December 2019. A total of 113 frozen chicken meat samples (82 broiler chicken meat, 31 cockerel chicken meat) were purchased from these outlets. On availability, meat samples included whole chicken or chopped chicken comprising breast, drumstick, leg, and wing muscle. Samples were placed in separate sterile plastic bags, labeled, kept in an icebox, and transported to the laboratory and processed as soon as possible. Simultaneously, data on the brand name, source of chicken, processing and packaging of meat, and special labels (green chicken/organic) were collected.
Figure 5.
Map showing sampling sites in five megacities of Bangladesh.
4.2. Enrichment and Identification of E. coli
The preparation of the meat samples was based on the European standard ISO-16654:2001 [53]. During processing, frozen chicken meat was kept at room temperature until thawing; then, the meat surface was sterilized by stabbing with a hot spatula and the upper portion of meat was removed carefully. Then, about 25 g of the meat samples were chopped into very small fine pieces using sterile scissors and a scalpel, mixed with 225 mL of buffered peptone water, homogenized for two minutes with gentle shaking, and enriched overnight (18–24 h) at 37 °C. After pre-enrichment, 1 mL of diluted meat samples were taken using a sterile pipette and transferred into a test tube containing the nutrient broth and incubated overnight at 37 °C. Then, a loopful of this overnight culture was streaked onto Eosin Methylene Blue agar in duplicate and incubated at 37 °C for 18–24 h. Three presumptive E. coli colonies having a dark blue color with a characteristic metallic sheen from each selective agar plate were picked and then subcultured to obtain a pure culture, and identification was performed using standard microbiological and biochemical procedures including Gram staining, catalase, oxidase, indole, methyl red, Voges–Proskauer tests, and a sugar fermentation test using triple sugar iron agar. Positive isolates were stored in nutrient broth containing 50% (v/v) glycerol at −20 °C for further study.
4.3. Molecular Detection of E. coli
Bacterial DNA was extracted by boiling of 1 mL of overnight culture as described earlier [54]. The DNA concentration was measured by NanoDrop One (Thermo Fisher Scientific, Waltham, Massachusetts, USA). PCR was performed for the confirmation of E. coli using 16S rRNA gene-specific primers as described earlier [55]. The sequence of the forward primer was 5′–GACCTCGGTTTAGTTCACAGA–3 and that of the reverse primer was 5′–CACACGCTGACGCTGACCA–3′. Amplification reactions were done in a 25-μL volume containing 12.5 μL of PCR Master Mix (Thermo Scientific, Waltham, Massachusetts, USA), 1.5 μL (15 pmol) of each forward and reverse primer, 0.5 μL of template DNA (50 ng), and 9.0 μL of nuclease-free water. The PCR was run under the following conditions in a Veriti™ 96-Well Thermal Cycler (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA): initial denaturation at 95 °C for 5 min followed by 35 cycles of amplification, denaturation for 1 min at 94 °C, annealing at 58°C for 1 min, extension for 1 min at 72 °C, and final extension at 72 °C for 7 min. After amplification, the PCR product was subjected to electrophoresis on 1.5% agarose gel containing ethidium bromide (5µg/mL). The resulting band of the PCR product was examined under an ultraviolet (UV) transilluminator and documented.
4.4. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility was determined by disc diffusion assay with 38 antimicrobials belonging to16 antimicrobial classes. The following antimicrobial discs (Biomaxima, Lublin, Lubelskie, Poland; Oxoid, Basingstoke, Hampshire, UK) were procured and used for the testing:
-
(A)Non-extended spectrum cephalosporins including
-
-First-generation cephalosporins: cephalexin (30 µg), cefradine (30 µg);
-
-Second-generation cephalosporins: cefuroxime (30 µg), cefaclor (30 µg);
-
-
-
(B)Extended-spectrum cephalosporins including
-
-Third-generation cephalosporins: cefotaxime (30 µg), ceftriaxone (30 µg), ceftazidime (30 µg), cefixime (5 µg);
-
-Fourth-generation cephalosporins: cefepime (30 µg);
-
-
-
(C)
Cephamycins: cefoxitin (30 µg);
-
(D)
Fluoroquinolones: nalidixic acid (30 µg), ciprofloxacin (5 µg), levofloxacin (5 µg), norfloxacin (10 µg), ofloxacin (5 µg), gatifloxacin (5 µg), pefloxacin (5 µg);
-
(E)
Penicillins: ampicillin (10 µg), amoxycillin (10 µg);
-
(F)
Penicillins + β-lactamase inhibitors: amoxicillin–clavulanic acid (30 µg);
-
(G)
Antipseudomonal penicillins + β-lactamase inhibitors: pipercillin–tazobactam (110 µg);
-
(H)
Carbapenems: imipenem (10 µg), meropenem (10 µg);
-
(I)
Polymyxins: colistin (10 µg), polymyxin B (300 units);
-
(J)
Monobactams: aztreonam (30 µg);
-
(K)
Aminoglycosides: gentamicin (10 µg), amikacin (30 µg), streptomycin (10 µg), neomycin (30 µg), tobramycin (10 µg);
-
(L)
Tetracyclines: tetracycline (30 µg), oxytetracycline (30 µg), doxycycline (10 µg);
-
(M)
Folate pathway inhibitors: trimethoprim–sulfamethoxazole (25 µg);
-
(N)
Glycylcyclines: tigecycline (15 µg);
-
(O)
Phenicols: chloramphenicol (30 µg);
-
(P)
Macrolides: azithromycin (15 µg).
After preparation of each bacterial suspension, the turbidity was adjusted equivalent to 0.5 McFarland standard and then inoculated onto Mueller–Hinton agar in duplicate. After overnight incubation at 37 °C, the diameter of the clear zone of inhibition around each antimicrobial disc was measured in millimeters. These results were interpreted as per the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [56]. The isolates were classified as susceptible, intermediate, and resistant. Isolates resistant to ≥ 1 agent in three or more antimicrobial classes were classed as multidrug-resistant (MDR), and isolates resistant to ≥ 1 agent in all but ≤ 2 antimicrobial classes were categorized as extensively drug resistant (XDR) [25].
4.5. Detection of ESBL-Producing E. coli
ESBL-producing E. coli were detected by a double-disc synergy technique, in which an amoxicillin/clavulanic acid disc (amoxicillin 20 µg and clavulanic acid 10 µg) was placed in the center of a plate, and cefotaxime (30 µg), ceftazidime (30 µg), and ceftriaxone (30 µg) discs were placed 30 mm (center to center) apart from the amoxicillin/clavulanic acid disc. The enhancement of the zone of inhibition of any one of the three discs toward the disc containing clavulanic acid suggested the presence of extended-spectrum β-lactamases [57]. The isolates that produced a zone of inhibition ≥ 22 mm for ceftazidime, ≥27 mm for cefotaxime, and ≥25 mm for ceftriaxone were considered as potential ESBL producers as recommended by CLSI [56].
4.6. Detection of ESBL-Encoding Genes
The ESBL-encoding genes (blaTEM, blaSHV, blaCTX-M-1, and blaCTX-M-2) were detected by multiplex PCR using specific primers as described in Table 6 [48]. Amplification reactions were set in a 25-μL volume containing 12.5 μL of PCR Master Mix (Thermo Scientific, Waltham, Massachusetts, USA), 1.0 μL (10 pmol) of each of the forward and reverse primers, 1 μL of DNA, and 3.5 μL of nuclease-free water. The multiplex PCR conditions used were as follows: initial denaturation at 95 °C for 5 min, followed by 25 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 1 min, and extension at 72 °C for 1 min, with a final extension at 72 °C for 10 min. Appropriate positive and negative controls (sterile phosphate buffer saline) were included in each PCR run. The PCR products were visualized by electrophoresis on a 1.5% agarose gel containing ethidium bromide. The DNA bands were photographed using a UV transilluminator.
Table 6.
Oligonucleotide primers used for the detection of ESBL-encoding genes.
| Gene | Name of Primers | Sequence 5′→3′ | Amplified Product (bp) |
|---|---|---|---|
| blaTEM | TEM-410F TEM-781R |
GGTCGCCGCATACACTATTCTC TTTATCCGCCTCCATCCAGTC |
372 |
| blaSHV | SHV-287F SHV-517R |
CCAGCAGGATCTGGTGGACTAC CCGGGAAGCGCCTCAT |
231 |
| blaCTX-M-1 | ctxm1-115F ctxm1-702R |
GAATTAGAGCGGCAGTCGGG CACAACCCAGGAAGCAGGC |
588 |
| blaCTX-M-2 | ctxm2-39F ctxm2-145R |
GATGGCGACGCTACCCC CAAGCCGACCTCCCGAAC |
107 |
4.7. Statistical Analysis
Descriptive statistics were used to compute the prevalence of E. coli and resistance percentage. The Z-test for proportions was done to find out the significant difference in the frequencies of E. coli and their resistance percentage among supershops, sampling area, chicken types, production types, meat types, etc. If any of the expected cell frequencies was less than five, Fisher’s exact tests were used. The level of significance was set at p < 0.05. SPSS version 22.0 software (IBM Corp., Armonk, N.Y., USA) was used for the analyses.
5. Conclusions
The presence of ESBL-producing E. coli in frozen chicken meat in Bangladesh poses a risk to human health. Our data indicate the presence of MDR and pXDR ESBL-producing E. coli in frozen chicken meat, to which people are regularly exposed, and it warrants the importance of immediate steps being taken to ensure good production and processing practices by the producers, as well as food processors, thus minimizing the contamination of frozen chicken meat in Bangladesh. Furthermore, frozen chicken meat should be properly handled and thoroughly cooked in order to make sure that safe products are consumed. Continuous monitoring and public health efforts targeting food safety management are warranted to proactively manage risks associated with the presence and spread of these antimicrobial-resistant E. coli in frozen chicken meat consumed by humans.
Acknowledgments
The authors would like to thank the Bangabandhu Science and Technology Fellowship Trust for technical support. We would also like to acknowledge Azimun Nahar, post-doctoral fellow, Graduate School of Life and Environmental Sciences, Osaka Prefecture University, Osaka 598-8531, Japan for providing the positive control of the ESBL gene in the current study.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/6/420/s1: Table S1. Antimicrobial susceptibility pattern of ESBL-Ec and non-ESBL-Ec isolated from frozen chicken meat.
Author Contributions
M.S.P. conceptualized the study design and was responsible for the literature search, acquisition of data, statistical analysis, and drafting of the manuscript. M.T.I. was involved in conceptualization, supervision, design, and coordination of the study, interpretation of the data, and critical revision of the manuscript. E.H.C. and M.T.R. participated in supervision, study design, and revision of the manuscript. S.T. and M.Y.A. greatly contributed to performing the experiments and analyzing the data. All authors have read and agreed to the published version of the manuscript.
Funding
The research work was partially funded by the BAS-USDA Program in Agriculture and Life Sciences (grant number BAS-USDA PALS LS-18).
Conflicts of Interest
The authors declare that they have no competing interests. The funders had no role in the study design, data collection and analysis, manuscript preparation, or decision to publish the manuscript.
References
- 1.Davis G.S., Waits K., Nordstrom L., Grande H., Weaver B., Papp K., Horwinski J., Koch B., Hungate B.A., Liu C.M., et al. Antibiotic-resistant Escherichia coli from retail poultry meat with different antibiotic use claims. BMC Microbiol. 2018;18:174. doi: 10.1186/s12866-018-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skurnik D., Ruimy R., Andremont A., Amorin C., Rouquet P., Picard B., Denamur E. Effect of human vicinity on antimicrobial resistance and integrons in animal faecal Escherichia coli. J. Antimicrob. Chemother. 2006;57:1215–1219. doi: 10.1093/jac/dkl122. [DOI] [PubMed] [Google Scholar]
- 3.Addis M., Sisay D. A review on major food borne bacterial illnesses. J. Trop. Dis. 2015;3:176–183. [Google Scholar]
- 4.Hammerum A.M., Heuer O.E. Human health hazards from antimicrobial-resistant Escherichia coli of animal origin. Clin. Infect. Dis. 2009;48:916–921. doi: 10.1086/597292. [DOI] [PubMed] [Google Scholar]
- 5.Li H., Ganzle M. Some like it hot: Heat resistance of Escherichia coli in food. Front. Microbiol. 2016;7:1763. doi: 10.3389/fmicb.2016.01763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Hernandez R., McMullen L., Gänzle M.G. Development and validation of a surrogate strain cocktail to evaluate bactericidal effects of pressure on verotoxigenic Escherichia coli. Int. J. Food Microbiol. 2015;205:16–22. doi: 10.1016/j.ijfoodmicro.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Mercer R., Zheng J., Garcia-Hernandez R., Ruan L., Gänzle M., McMullen L. Genetic determinants of heat resistance in Escherichia coli. Front. Microbiol. 2015;6:932. doi: 10.3389/fmicb.2015.00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goksoy E., James C., Corry J. The effect of short-time microwave exposures on inoculated pathogens on chicken and the shelf-life of uninoculated chicken meat. J. Food Eng. 2000;45:153–160. doi: 10.1016/S0260-8774(00)00054-6. [DOI] [Google Scholar]
- 9.Yamanaka K., Inouye M. Induction of CspA, an E. coli major cold-shock protein, upon nutritional upshift at 37° C. Genes Cells. 2001;6:279–290. doi: 10.1046/j.1365-2443.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- 10.Chung H.J., Bang W., Drake M.A. Stress Response of Escherichia coli. Compr. Rev. Food Sci. Food Saf. 2006;5:52–64. doi: 10.1111/j.1541-4337.2006.00002.x. [DOI] [Google Scholar]
- 11.Cao-Hoang L., Dumont F., Marechal P.-A., Thanh M., Gervais P. Rates of chilling to 0 °C: Implications for the survival of microorganisms and relationship with membrane fluidity modifications. Appl. Microbiol. Biotechnol. 2008;77:1379–1387. doi: 10.1007/s00253-007-1279-z. [DOI] [PubMed] [Google Scholar]
- 12.Switaj T.L., Winter K.J., Christensen S.R. Diagnosis and management of foodborne illness. Am. Fam. Physician. 2015;92:358–365. [PubMed] [Google Scholar]
- 13.Wong C.S., Jelacic S., Habeeb R.L., Watkins S.L., Tarr P.I. The risk of the hemolytic–uremic syndrome after antibiotic treatment of Escherichia coli O157: H7 infections. N. Engl. J. Med. 2000;342:1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tadesse D.A., Zhao S., Tong E., Ayers S., Singh A., Barthlomew M.J., McDermott P.F. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950–2002. Emerg. Infect. Dis. 2012;18:741–749. doi: 10.3201/eid1805.111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blake D.P., Hillman K., Fenlon D.R., Low J.C. Transfer of antibiotic resistance between commensal and pathogenic members of the Enterobacteriaceae under ileal conditions. J. Appl. Microbiol. 2003;95:428–436. doi: 10.1046/j.1365-2672.2003.01988.x. [DOI] [PubMed] [Google Scholar]
- 16.Pitout J.D., Thomson K.S., Hanson N.D., Ehrhardt A.F., Moland E.S., Sanders C.C. Beta-lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob. Agents Chemother. 1998;42:1350–1354. doi: 10.1128/AAC.42.6.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandes R., Amador P., Oliveira C., Prudencio C. Molecular characterization of ESBL-producing Enterobacteriaceae in northern Portugal. Sci. World J. 2014;2014:1–6. doi: 10.1155/2014/782897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnet R. Growing group of extended-spectrum β-lactamases: The CTX-M enzymes. Agents Chemother. 2004;48:1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeifer Y., Cullik A., Witte W. Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int. J. Med. Microbiol. 2010;300:371–379. doi: 10.1016/j.ijmm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Hasan B., Faruque R., Drobni M., Waldenström J., Sadique A., Ahmed K.U., Islam Z., Parvez M.B., Olsen B., Alam M. High prevalence of antibiotic resistance in pathogenic Escherichia coli from large- and small-scale poultry farms in Bangladesh. Avian Dis. 2011;55:689–692. doi: 10.1637/9686-021411-Reg.1. [DOI] [PubMed] [Google Scholar]
- 21.Parvez M.A.K., Marzan M., Liza S.M., Mou T.J., Azmi I.J., Rahman M.S., Mahmud Z.H. Prevalence of inhibitor resistant beta lactamase producing E. coli in human and poultry origin of Bangladesh. J. Bacteriol. Parasitol. 2016;7:1–3. [Google Scholar]
- 22.Al Azad M., Rahman A., Rahman M., Amin R., Begum M., Ara I., Fries R., Husna A., Khairalla A.S., Badruzzaman A. Susceptibility and multidrug resistance patterns of Escherichia coli isolated from cloacal swabs of live broiler chickens in Bangladesh. Pathogens. 2019;8:118. doi: 10.3390/pathogens8030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liebana E., Carattoli A., Coque T.M., Hasman H., Magiorakos A.-P., Mevius D., Peixe L., Poirel L., Schuepbach-Regula G., Torneke K. Public health risks of enterobacterial isolates producing extended-spectrum β-lactamases or AmpC β-lactamases in food and food-producing animals: An EU perspective of epidemiology, analytical methods, risk factors, and control options. Clin. Infect. Dis. 2013;56:1030–1037. doi: 10.1093/cid/cis1043. [DOI] [PubMed] [Google Scholar]
- 24.Ghodousi A., Bunora C., Maria Di Noto A., Mammina C. Extended-spectrum ß-lactamase, AmpC-producing, and fluoroquinolone-resistant Escherichia coli in retail broiler chicken meat, Italy. Foodborne Pathog. Dis. 2015;12:619–625. doi: 10.1089/fpd.2015.1936. [DOI] [PubMed] [Google Scholar]
- 25.Magiorakos A.-P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 26.Islam N., Tahsin N., Tarrannum N., Salihee R.Z., Tarannum S., Sujana J.T.M. Factors Influencing the Consumers’ Perceptions Towards Frozen and Ready-to-Cook Food Products in Bangladesh; Proceedings of the 1st Global International Conference 2019; Kathmandu, Nepal. 13–14 December 2019. [Google Scholar]
- 27.Alam S.T. Antibiogram of pre-processed raw chicken meat from different supershops of Dhaka city, Bangladesh. J. Allied Health Sci. 2015;2:45–52. [Google Scholar]
- 28.Uddin J., Hossain K., Hossain S., Saha K., Jubyda F.T., Haque R., Billah B., Talukder A.A., Parvez A.K., Dey S.K. Bacteriological assessments of foodborne pathogens in poultry meat at different super shops in Dhaka, Bangladesh. Ital. J. Food Saf. 2019;8:6720. doi: 10.4081/ijfs.2019.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim D.R., Dodd C.E.R., Stekel D.J., Ramsden S.J., Hobman J.L. Multidrug resistant, extended spectrum β-lactamase (ESBL)-producing Escherichia coli isolated from a dairy farm. FEMS Microbiol. Ecol. 2016;92 doi: 10.1093/femsec/fiw013. [DOI] [PubMed] [Google Scholar]
- 30.Faruque O., Mahmud S., Munayem A., Sultana R., Molla T., Ali F., Wasim M., Sarker S., Evamoni F. Bacteriological analysis and public health impact of broiler meat: A study on Nalitabari Paurosova, Sherpur, Bangladesh. Adv. Microbiol. 2019;9:581–601. doi: 10.4236/aim.2019.97036. [DOI] [Google Scholar]
- 31.Rahman M.A., Rahman A., Islam M.A., Alam M.M. Antimicrobial resistance of Escherichia coli isolated from milk, beef and chicken meat in Bangladesh. Bangl. J. Vet. Med. 2017;15:141–146. doi: 10.3329/bjvm.v15i2.35525. [DOI] [Google Scholar]
- 32.Bhoomika S.S., Patyal A., Gade N.E. Occurrence and characteristics of extended-spectrum β-lactamases producing Escherichia coli in foods of animal origin and human clinical samples in Chhattisgarh, India. Vet. World. 2016;9:996. doi: 10.14202/vetworld.2016.996-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saud B., Paudel G., Khichaju S., Bajracharya D., Dhungana G., Awasthi M.S., Shrestha V. Multidrug-resistant bacteria from raw meat of buffalo and chicken, Nepal. Vet. Med. Int. 2019;2019:1–7. doi: 10.1155/2019/7960268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo K.W., Kim Y.B., Jeon H.Y., Lim S.-K., Lee Y.J. Comparative genetic characterization of third-generation cephalosporin-resistant Escherichia coli from chicken meat produced by integrated broiler operations in South Korea. Poult. Sci. 2018;97:2871–2879. doi: 10.3382/ps/pey127. [DOI] [PubMed] [Google Scholar]
- 35.Kawamura K., Goto K., Nakane K., Arakawa Y. Molecular epidemiology of extended-spectrum β-lactamases and Escherichia coli isolated from retail foods including chicken meat in Japan. Foodborne Pathog. Dis. 2014;11:104–110. doi: 10.1089/fpd.2013.1608. [DOI] [PubMed] [Google Scholar]
- 36.Nahar A., Awasthi S.P., Hatanaka N., Okuno K., Hoang P.H., Hassan J., Hinenoya A., Yamasaki S. Prevalence and characteristics of extended-spectrum β-lactamase-producing Escherichia coli in domestic and imported chicken meats in Japan. J. Vet. Med. Sci. 2018;80:510–517. doi: 10.1292/jvms.17-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouger A., Tresse O., Zagorec M. Bacterial contaminants of poultry meat: Sources, species, and dynamics. Microorganisms. 2017;5:E50. doi: 10.3390/microorganisms5030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasan B., Sandegren L., Melhus A., Drobni M., Hernandez J., Waldenstrom J., Alam M., Olsen B. Antimicrobial drug resistant Escherichia coli in wild birds and free-range poultry, Bangladesh. Emerg. Infect. Dis. 2012;18:2055–2058. doi: 10.3201/eid1812.120513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samaha I., Ibrahim H., Hamada M. Isolation of some enteropathogens from retailed poultry meat in Alexandria Province. Alex. J. Vet. Sci. 2012;37:17–22. [Google Scholar]
- 40.Islam M.K., Kabir S.L., Haque A.Z., Sarker Y., Sikder M. Molecular detection and characterization of Escherichia coli, Salmonella spp. and Campylobacter spp. isolated from broiler meat in Jamalpur, Tangail, Netrokona and Kishoreganj districts of Bangladesh. Afr. J. Microbiol. Res. 2018;12:761–770. [Google Scholar]
- 41.Al-Salauddin A.S., Hossain M.F., Dutta A., Mahmud S., Islam M.S., Saha S., Kabir S.M.L. Isolation, identification, and antibiogram studies of Salmonella species and Escherichia coli from boiler meat in some selected areas of Bangladesh. Int. J. Basic Clin. Pharm. 2015;4:999–1003. doi: 10.18203/2319-2003.ijbcp20150881. [DOI] [Google Scholar]
- 42.Nikaido H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy S. Reduced antibiotic use in livestock: How Denmark tackled resistance. Environ. Health Perspect. 2014;122:160–165. doi: 10.1289/ehp.122-A160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buffet-Bataillon S., Tattevin P., Bonnaure-Mallet M., Jolivet-Gougeon A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents. 2012;39:381–389. doi: 10.1016/j.ijantimicag.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Nhung N.T., Thuy C.T., Trung N.V., Campbell J., Baker S., Thwaites G., Hoa N.T., Carrique-Mas J. Induction of antimicrobial resistance in Escherichia coli and non-typhoidal Salmonella strains after adaptation to disinfectant commonly used on farms in Vietnam. Antibiotics. 2015;4:480–494. doi: 10.3390/antibiotics4040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adeyanju G.T., Ishola O. Salmonella and Escherichia coli contamination of poultry meat from a processing plant and retail markets in Ibadan, Oyo State, Nigeria. Springerplus. 2014;3:139. doi: 10.1186/2193-1801-3-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McEwen S.A., Fedorka-Cray P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002;34:S93–S106. doi: 10.1086/340246. [DOI] [PubMed] [Google Scholar]
- 48.Le Q.P., Ueda S., Nguyen T.N.H., Dao T.V.K., Van Hoang T.A., Tran T.T.N., Hirai I., Nakayama T., Kawahara R., Do T.H. Characteristics of extended-spectrum β-lactamase producing Escherichia coli in retail meats and shrimp at a local market in Vietnam. Foodborne Pathog. Dis. 2015;12:719–725. doi: 10.1089/fpd.2015.1954. [DOI] [PubMed] [Google Scholar]
- 49.Yang C.-M., Lin M.-F., Lin C.-H., Huang Y.-T., Hsu C.-T., Liou M.-L. Characterization of antimicrobial resistance patterns and integrons in human fecal Escherichia coli in Taiwan. Jpn. J. Infect. Dis. 2009;62:177–181. [PubMed] [Google Scholar]
- 50.Jacoby G.A., Sutton L. Properties of plasmids responsible for production of extended-spectrum beta-lactamases. Agents Chemother. 1991;35:164–169. doi: 10.1128/AAC.35.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guerra B., Fischer J., Helmuth R. An emerging public health problem: Acquired carbapenemase-producing microorganisms are present in food-producing animals, their environment, companion animals and wild birds. Vet. Microbiol. 2014;171:290–297. doi: 10.1016/j.vetmic.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 52.White D.G., Hudson C., Maurer J.J., Ayers S., Zhao S., Lee M.D., Bolton L., Foley T., Sherwood J. Characterization of chloramphenicol and florfenicol resistance in Escherichia coli associated with bovine diarrhea. J. Clin. Microbiol. 2000;38:4593–4598. doi: 10.1128/JCM.38.12.4593-4598.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.WHO . Isolation of Salmonella spp. From Food and Animal Faeces. 5th ed. Volume 13. WHO; Geneva, Switzerland: 2010. Laboratory protocol; pp. 4–8. [Google Scholar]
- 54.Dashti A., Jadaon M., Abdulsamad A., Dashti H. Heat treatment of bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med. J. 2009;41:117–122. [Google Scholar]
- 55.Schippa S., Iebba V., Barbato M., DiNardo G., Totino V., Checchi M.P., Longhi C., Maiella G., Cucchiara S., Conte M.P. A distinctive’ microbial signature’ in celiac pediatric patients. BMC Microbiol. 2010;10:175. doi: 10.1186/1471-2180-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. Wayne State University Press; Detroit, MI, USA: 2018. pp. 1–260. [Google Scholar]
- 57.Jarlier V., Nicolas M.H., Fournier G., Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: Hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.