Abstract
The main objective of this study was to evaluate the prevalence of human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), hepatitis C virus (HCV) and hepatitis B virus (HBV) and their co-infections among people who inject drugs (PWID) and female sex workers (FSWs). Data sources were searched from January 2008 to October 2018 in different databases. Data were analyzed in Stata 16 software using the Metaprop command. The results showed that the prevalence of HIV, HCV and HBV among PWID was 15%, 60% and 6%, respectively. The prevalence of HIV, HCV and HBV among FSWs was 5%, 1% and 3%, respectively. The prevalence of HIV/HCV, HIV/HBV, HCV/HBV and HIV/HCV/HBV co-infections among PWID was 13%, 2%, 3% and 2%, respectively. The prevalence of HIV/HCV and HIV/HBV co-infections among FSWs was 3% and 1%, respectively. The results show that the prevalence of HCV and HIV infections in PWID and the prevalence of HIV in FSWs is higher than their prevalence in the general population. Interventions for the prevention of HIV and HCV in PWID appear to be poor, and may not be sufficient to effectively prevent HIV and HCV transmission.
Keywords: human immunodeficiency virus (HIV), hepatitis C virus (HCV), hepatitis B virus (HBV), people who inject drugs (PWID), female sex workers (FSWs), Co-infection
1. Introduction
According to a World Health Organization (WHO) report in 2018, there are approximately 38 million people living with HIV/AIDS in the world, the largest number being in Africa, with 25.7 million [1]. The WHO’s goal is to identify 90% of HIV patients, treat 90% of those identified and virally suppress 90% of those treated by 2020 [2]. Hepatitis C virus (HCV) and hepatitis B virus (HBV) have affected 71 million and 257 million people worldwide, which can lead to cirrhosis and liver cancer, respectively. Removing these viruses, which requires significant economic and sanitary capital, will prevent more than 1.2 million deaths worldwide annually [3,4]. HCV has the highest prevalence in the Eastern Mediterranean region, followed by the European region, with a prevalence of 2.3% and 1.5%, respectively. It varies from 0.5% to 1% in other areas. HBV is most prevalent in the Western Pacific and African regions, with values of 6.2% and 6.1%, respectively. The lowest prevalence is reported in the US, with a value of 0.7% [5]. Viral hepatitis elimination program has been adapted by WHO in 2016 [6]. In line with this global program, several countries are working towards this elimination platform [7]. In this program, engagement of high-risk groups and marginal populations has been highlighted [8,9]. The efficient implementation of prevention and control measures incorporated in this program, requires detailed insight on the epidemiology of hepatitis viruses and other viral infectious agents circulating in these cohorts [9].
People who inject drugs (PWID) and female sex workers (FSWs) are key populations for blood-borne viral infections (BBVI), including HIV, HCV and HBV. PWID are usually infected through shared needles, syringes and other infected injection equipment, as well as other high-risk behaviors [10]. There are an estimated 15.6 million PWID aged 15–64 years worldwide. It is said that 23% of the new HCV cases and 33% of the annual HCV deaths are related to PWID [11,12]. According to the WHO report in 2018, HIV prevalence in PWID is estimated to be from less than 1.8% to 13.5%, but viral hepatitis has not been reported in many countries. HBV is 0.7% in some of these countries, including Afghanistan, Germany and Nepal, and 7.3% in Azerbaijan, and HCV is less than 7% in some countries, including Germany, Afghanistan and Madagascar, and more than 60% in Kazakhstan [13].
FSWs are also more exposed to high-risk behaviors, especially through sex. A study in a region with a high prevalence of HIV in India stated that about 77.5% of FSWs had drug injection history, and that they were at a higher risk of BBVI than those without drug injection. The prevalence of HIV in FSWs worldwide ranges from less than 1.4% to over 11%. The prevalence of viral hepatitis is not high in FSWs, but WHO reports that the prevalence of HBV in FSWs ranges from less than 0.3% in Brazil to over 3.6% in Peru, and hepatitis C prevalence ranges from less than 1.9% in Brazil to 6.2% in Kazakhstan in the same group. In fact, HIV is more prevalent among FSWs than HBV and HCV infections [14,15,16]. The prevalence of HIV is 22 times higher among PWID and 21 times higher among sex workers (SWs) than the general population. In 2018, about 54% of new HIV cases occurred among key populations and their sexual partners [17].
In patients co-infected with two or three HIV, HCV and HBV infections, HIV-induced immunodeficiency increases the likelihood of HBV and HCV persistence, and hepatotoxicity associated with anti-HIV treatment can worsen HBV-related liver diseases or HCV persistence. Evidence suggests that HIV infection increases the risk of HCV- and HBV-associated hepatocellular carcinoma. On the other hand, liver diseases associated with chronic HBV and HCV are leading to increased mortality and complications in HIV patients [18,19]. Adverse drug reactions (ADRs) related to antiretrovirals (ARVs) are higher in HIV patients co-infected with HBV or HCV than in HIV-monoinfected patients [20].
The purpose of this study was to conduct a systematic review and meta-analysis to estimate the prevalence of HIV (anti-HIV), HCV (anti-HCV), HBV (HBsAg) and their co-infections among PWID and FSWs, as separated by WHO geographical areas in the general population of these groups. The aim is to understand the current situation, in order to make better decisions regarding prevention, identification and treatment.
The protocol of this study had been registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42018115115).
2. Results
2.1. Search Results
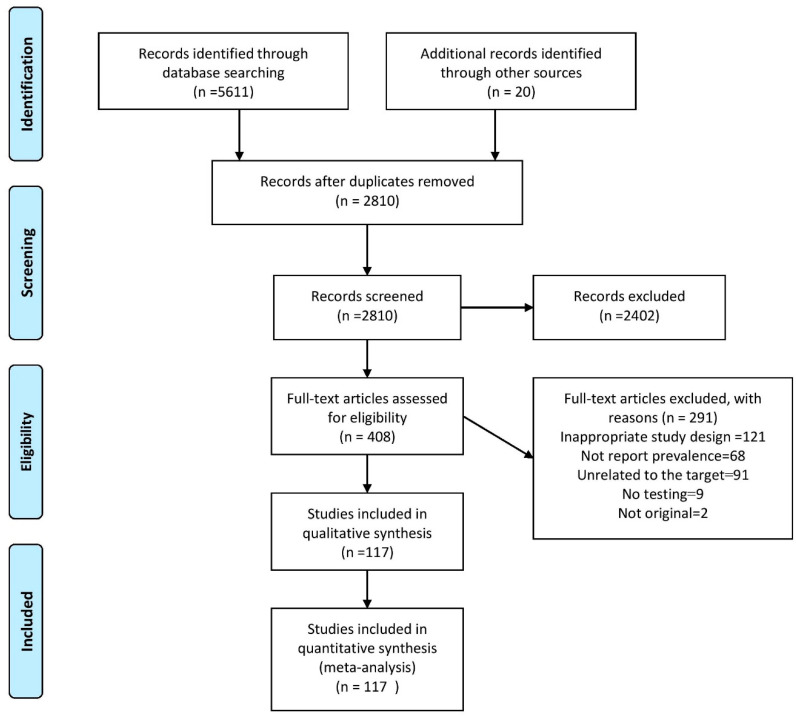
As showed in Figure 1, 5631 articles were found in the original search. After the removal of duplicate data, 2810 articles were selected for screening. After reviewing the title and abstract, 408 articles were selected for full text study. A total of 291 articles were excluded for various reasons, including inappropriate study design (121), failure to report prevalence (68), irrelevant target group (91), lack of proper testing (9) and non-originality (2). Eventually, after qualitative evaluation of data, 117 articles that had reported 161 records of HIV, HCV, HBV prevalence and their co-infections (HIV/HCV, HIV/HBV, HCV/HBV, HIV/HCV/HBV) among PWID and FSWs were analyzed. The characteristics of the studies are shown in Table 1. A total of 26 papers with 40 records had been published in the South-East Asia region, 25 papers with 32 records in the Americas region, 21 papers with 28 records in the Europe region, 17 papers with 19 records in the Africa region, 15 papers with 18 records in the Eastern Mediterranean region and 13 articles with 24 records in the Western Pacific region. A total of 89 papers with 119 records had been conducted on PWID and 33 papers with 42 records on FSWs.
Figure 1.
Flow-chart of systematic review of prevalence of hepatitis C virus (HCV), human immunodeficiency virus (HIV), hepatitis B virus (HBV) and their co-infections among people who inject drugs (PWID) and female sex workers (FSWs) worldwide; 2008–2018.
Table 1.
The characteristics of studies which entered into meta-analysis.
| Authors (Reference) | Year of Publication | Year of Study | Sampling Location | Study Population | Age (Mean or Median) | Method of Sampling | n/N (Prevalence of HCV) |
n/N (Prevalence of HBV) |
n/N (Prevalence of HIV) |
n/N (Prevalence of HCV/HBV) |
n/N (Prevalence of HCV/HIV) |
n/N (Prevalence of HIV/HBV) |
n/N (Prevalence of HCV/HIV/HBV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Toro-Tobón, D [21] |
2018 | 2014 | Colombia | PWID | 26 | RDS | 251/918 (27.3) |
47/918 (5.1) |
|||||
|
Rahman, M [22] |
2018 | 2011 | Bangladesh | PWID | 31.5 | 34/90 (37.8) |
|||||||
|
Rahman, M [22] |
2018 | 2011 | Bangladesh | FSWs | 31.5 | 0/15 (0) |
|||||||
|
Puga, M. A. M [23] |
2018 | 2009–2010 | Brazil | FSWs | 25 | RDS | 2/402 (0.5) |
||||||
|
Peach, E [24] |
2018 | 2014 | Australia | PWID | 37 | 118/127 (92.9) |
4/127 (3.1) |
5/128 (3.9) |
|||||
|
Patel, E. U [25] |
2018 | 2015–2016 | India | PWID | 41 | 213/541 (39.4) |
35/541 (6.5) |
86/541 (15.9) |
61/541 (11.3) |
||||
|
Oyaro, M [26] |
2018 | 2011–2012 | Kenya | PWID | RDS | 19/120 (15.8) |
13/120 (10.8) |
1/120 (0.8) |
1/120 (0.8) |
||||
|
Jõgeda, E. L [27] |
2018 | 2011 | Estonia | PWID | 30 | RDS | 306/345 (88.7) |
172/345 (49.9) |
169/345 (49) |
||||
|
Jarlais, D. C. D [28] |
2018 | 2011–2015 | USA | PWID | 40 | 493/796 (61.9) |
57/791 (7.2) |
||||||
|
Iakunchykova, O [29] |
2018 | 2014–2015 | Ukraine | PWID | 36 | RDS | 1002/1613 (62.1) |
668/1613 (41.4) |
441/1613 (27.3) |
||||
|
Haussig, J. M [30] |
2018 | 2011–2014 | German | PWID | RDS | 1361/2077 (65.5) |
22/2077 (1.1) |
100/2077 (4.8) |
20/2077 (1) |
3/2077 (0.1) |
|||
|
Ferreira, O. D [31] |
2018 | 2016 | Brazil | FSWs | RDS | 38/4154 (0.9) |
16/4154 (0.4) |
225/4154 (5.4) |
0/4154 (0) |
8/4154 (0.2) |
2/4154 (<0.1) |
||
|
Duong, H. T [32] |
2018 | 2014 | Vietnam | PWID | 38 | RDS | 410/603 (68) |
151/603 (25) |
|||||
|
Demissie, M [33] |
2018 | 2015 | Ethiopia | PWID | 26 | RDS | 8/237 (3.4) |
12/237 (5.1) |
15/237 (6.3) |
||||
|
Shengelia, N [34] |
2017 | 2014–2015 | Republic of Georgia | PWID | 39 | RDS | 44/2022 (2.2) |
||||||
|
Sharhani, A [35] |
2017 | 2017 | Iran | PWID | 36.7 | snowball | 332/606 (54.8) |
||||||
|
Salek, T. P [36] |
2017 | 2012 | USA | PWID (heroin) |
44 | randomly | 64/81 (79) |
||||||
|
Salek, T. P [36] |
2017 | 2012 | USA | PWID (other narcotics) |
44 | randomly | 41/58 (70.7) |
||||||
|
Niama, F. R [37] |
2017 | 2011–2012 | Republic of Congo | FSWs | 28.31 | RDS | 6/805 (0.7) |
34/805 (4.2) |
60/805 (7.5) |
||||
|
Neaigus, A [38] |
2017 | 2005 | USA | PWID | 43 | RDS | 341/500 (68.2) |
90/500 (18) |
|||||
|
Neaigus, A [38] |
2017 | 2009 | USA | PWID | 41 | RDS | 390/514 (75.9) |
64/514 (12.5) |
|||||
|
Neaigus, A [38] |
2017 | 2012 | USA | PWID | 45 | RDS | 352/525 (67) |
64/525 (12.2) |
|||||
|
Mutagoma, M [39] |
2017 | 2015 | Rwanda | FSWs | 26 | Venue-Day-Time | 28/1978 (1.4) |
49/1978 (2.5) |
849/1978 (42.9) |
||||
|
Mmbaga, E. J [40] |
2017 | 2015 | Tanzania | PWID | 32 | RDS | 7/610 (1.1) |
94/610 (15.4) |
|||||
|
McFall, A. M [41] |
2017 | 2014 | India | PWID (women) |
30 | RDS | 177/796 (22.2) |
421/796 (52.9) |
87/796 (10.9) |
||||
|
McFall, A. M [41] |
2017 | 2014 | India | PWID (men) |
30 | RDS | 1721/5661 (30.4) |
985/5661 (17.4) |
|||||
|
Longo, J. D [42] |
2017 | 2013–2014 | Central African Republic | FSWs | 23 | 76/345 (22) |
66/345 (19.1) |
33/345 (9.6) |
|||||
|
Lambdin, B. H [43] |
2017 | 2011–2013 | Tanzania | PWID | 32 | 359/630 (57) |
187/630 (29.7) |
151/630 (24) |
|||||
|
Khatib, A [44] |
2017 | 2012 | Tanzania | PWID | 32 | RDS | 128/408 (31.4) |
25/408 (6.1) |
67/593 (16.4) |
13/408 (3.9) |
47/408 (11.5) |
7/408 (1.7) |
6/408 (1.5) |
|
Kaberg, M [45] |
2017 | 2013–2014 | Sweden | PWID | 39 | 1139/1386 (82.2) |
29/1386 (2.1) |
93/1386 (6.7) |
|||||
|
Jõgeda, E. L [46] |
2017 | 2011 | Estonia | PWID | 30 | RDS | 306/345 (88.7) |
18/345 (5.2) |
172/345 (49.9) |
||||
|
Ishizaki, A [47] |
2017 | 2007 | Vietnam | PWID | 34.1 | 81/760 (10.7) |
273/760 (35.9) |
8/760 (1.1) |
|||||
|
Ishizaki, A [47] |
2017 | 2008 | Vietnam | PWID | 32.5 | 34/302 (11.3) |
81/302 (26.8) |
4/302 (1.3) |
|||||
|
Ishizaki, A [47] |
2017 | 2012 | Vietnam | PWID | 32.2 | 43/389 (11.1) |
72/389 (18.5) |
4/389 (1) |
|||||
| Ishizaki, A (47) | 2017 | 2007 | Vietnam | FSWs | 24.8 | 10/91 (11) |
21/91 (23.1) |
1/91 (1.1) |
|||||
|
Ishizaki, A [47] |
2017 | 2008 | Vietnam | FSWs | 30.1 | 2/63 (3.2) |
13/63 (20.6) |
0/63 (0) |
|||||
|
Ishizaki, A [47] |
2017 | 2012 | Vietnam | FSWs | 29.8 | 2/51 (3.9) |
5/51 (9.8) |
0/51 (0) |
|||||
|
Handanagic, S [48] |
2017 | 2014–2015 | Republic of Croatia (Rijeka) |
PWID | 34 | RDS | 80/255 (31.4) |
||||||
|
Handanagic, S [48] |
2017 | 2014–2015 | Republic of Croatia (Split) |
PWID | 37 | RDS | 153/399 (38.3) |
||||||
|
Gupta, D [49] |
2017 | 2013–2014 | India | PWID | 80/194 (41.2) |
125/194 (64.4) |
51/194 (26.3) |
||||||
|
de Matos, M. A [50] |
2017 | 2009–2010 | Brazil | FSWs | RDS | 3/402 (0.7) |
6/402 (1.5) |
||||||
|
Iversen, J [51] |
2017 | Australia | PWID | 40 | 1173/2054 (57.1) |
||||||||
|
Pares-Badell, O [52] |
2017 | 2008–2012 | Spain | PWID | 1578/2243 (70.4) |
732/2243 (32.6) |
567/2243 (25.3) |
||||||
|
Wenz, B [53] |
2016 | 2011–2014 | Germany | PWID | RDS | 1361/2077 (65.5) |
101/2077 (4.9) |
84/2077 (4) |
|||||
|
Solomon, S. S [54] |
2016 | 2005 | India | PWID | 39 | 355/998 (35.6) |
71/998 (7.1) |
148/998 (14.8) |
26/998 (2.6) |
103/998 (10.3) |
|||
|
Skocibusic, S [55] |
2016 | Bosnia and Herzegovina | PWID | randomly | 63/120 (52.5) |
1/120 (0.8) |
|||||||
|
Nielsen, S [56] |
2016 | 2011–2014 | Germany | PWID | RDS | 854/2077 (41.1) |
100/2077 (4.8) |
||||||
|
Kermode, M [57] |
2016 | 2009–2010 | India | PWID | 29.8 | RDS | 607/821 (73.9) |
253/821 (30.8) |
241/821 (29.4) |
||||
|
Hsieh, M. H [58] |
2016 | 2008–2010 | Taiwan | PWID | 36.2 | 517/566 (91.3) |
87/566 (15.4) |
301/566 (53.2) |
|||||
|
Handanagic, S [59] |
2016 | 2014–2015 | Croatia (Zagreb) |
PWID | RDS | 55/176 (31.3) |
1/176 (0.6) |
||||||
|
Handanagic, S [59] |
2016 | 2014–2015 | Croatia (Rejika) |
PWID | RDS | 85/254 (33.5) |
2/254 (0.8) |
||||||
|
Handanagic, S [59] |
2016 | 2014–2015 | Croatia (Split) |
PWID | RDS | 173/387 (44.7) |
1/390 (0.3) |
||||||
|
Fotiou, A [60] |
2016 | 2013 | Greece | PWID | 36 | 447/563 (79.4) |
88/562 (15.7) |
81/541 (15) |
|||||
|
Folch, C [61] |
2016 | 2010–2011 | Spain | PWID | 548/761 (72) |
253/761 (33.2) |
|||||||
|
Fernandez-Lopez, L [62] |
2016 | 2011 | Spain | PWID | 35.6 | 35/172 (20.3) |
5/198 (2.5) |
||||||
|
Des Jarlais, D. C [63] |
2016 | 2014 | Vietnam | PWID | 37 | RDS | 403/603 (66.8) |
152/603 (25.2) |
|||||
|
Chen, Yi M. D [64] |
2016 | 2010–2015 | China (high tier) |
FSWs | 35 | 12/1832 (0.7) |
3/1832 (0.2) |
||||||
|
Chen, Yi M. D [64] |
2016 | 2010–2015 | China (middle tier) |
FSWs | 35 | 51/9938 (0.5) |
38/9938 (0.4) |
||||||
|
Chen, Yi M. D [64] |
2016 | 2010–2015 | China (low tier) |
FSWs | 35 | 79/10,686 (0.7) |
150/10,686 (1.4) |
||||||
|
Blackburn, N. A [65] |
2016 | 2012–2014 | USA | PWID | 37 | 3495/15,274 (22.9) |
576/15,274 (3.8) |
166/15,274 (1.1) |
|||||
|
Abadie, R [66] |
2016 | 2015 | Puerto Rico | PWID | 41.8 | RDS | 19/315 (6) |
||||||
|
Bouscaillou, J [67] |
2016 | 2014 | Ivory Coast | PWID | 33.5 | RDS | 4/73 (5.5) |
||||||
|
Bouscaillou, J [67] |
2016 | 2014 | Ivory Coast | FSWs | 33.5 | RDS | 9/49 (18.4) |
||||||
|
Tun, W [68] |
2015 | 2011 | Kenya | PWID | 31 | RDS | 50/269 (18.6) |
||||||
|
Tang, Z. Z [69] |
2015 | 2010 | China | FSWs | 28 | 128/12,622 (1) |
125/12,622 (1) |
||||||
|
Rosinska, M [70] |
2015 | 2004–2005 | Poland | PWID | 26 29 | snow-ball | 448/763 (58.7) |
137/763 (18) |
130/763 (17) |
||||
|
Mwatelah, R. S [71] |
2015 | Kenya | PWID | 33 | 25/152 (16.4) |
159/186 (85.5) |
24/152 (15.8) |
||||||
|
Kurth, A. E [72] |
2015 | 2012 | Kenya (Nairobi) |
PWID | 31.71 | RDS | 96/663 (14.5) |
||||||
|
Kurth, A. E [72] |
2015 | 2012 | Kenya (Coast region) |
PWID | 30.40 | RDS | 230/1122 (20.5) |
||||||
|
Jordan, A. E [73] |
2015 | 2006–2013 | USA | PWID | 41.2 | 1047/1535 (68.2) |
183/1535 (11.9) |
159/1535 (10.4) |
|||||
|
Iversen, J [74] |
2015 | 2004–2013 | Australia | PWID | 34 | 2879/5378 (53.5) |
29/5378 (0.5) |
||||||
|
Fan, Y. G [75] |
2015 | 2012–2013 | China | FSWs | 44/622 (7.1) |
7/622 (1.1) |
|||||||
|
Collier, M. G [76] |
2015 | 2009–2010 | USA | PWID | 26 | 135/519 (26) |
7/519 (1.3) |
||||||
|
Bugssa, G [77] |
2015 | 2013 | Ethiopia | FSWs | 32 | randomly | 19/319 (6) |
38/319 (11.9) |
|||||
|
Al-Tayyib, A. A [78] |
2015 | 2009 | USA (Colorado state) |
PWID | RDS | 289/395 (73.2) |
18/395 (4.6) |
15/395 (3.8) |
|||||
|
Al-Tayyib, A. A [78] |
2015 | 2009 | USA (Washington state) |
PWID | RDS | 189/260 (72.7) |
15/260 (5.8) |
11/260 (4.2) |
|||||
|
Zibbell, J. E [79] |
2014 | 2012 | USA | PWID | 30 | 34/100 (34) |
|||||||
|
Zhang, L [80] |
2014 | 2004 | China | FSWs | 24 | 2/343 (0.6) |
|||||||
|
Zhang, L [80] |
2014 | 2010 | China | FSWs | 26 | 6/404 (1.5) |
|||||||
|
Wang, L [81] |
2014 | 2008 | China | FSWs | randomly | 404/67,296 (0.6) |
|||||||
|
Wang, L [81] |
2014 | 2009 | China | FSW | randomly | 1328/147,528 (0.9) |
590/147,528 (0.4) |
||||||
|
Wang, L [81] |
2014 | 2010 | China | FSWs | randomly | 1596/199,571 (0.8) |
599/199,571 (0.3) |
||||||
|
Wang, L [81] |
2014 | 2011 | China | FSWs | randomly | 1434/204,873 (0.7) |
615/204,873 (0.3) |
||||||
|
Wang, L [81] |
2014 | 2012 | China | FSWs | randomly | 1662/207,811 (0.8) |
623/207,811 (0.3) |
||||||
|
Ruisenor-Escudero, H [82] |
2014 | 2009 | Afghanistan | PWID | 28 | RDS | 221/548 (40.3) |
39/548 (7.1) |
39/548 (7.1) |
37/548 (6.8) |
|||
|
Ramezani, A [83] |
2014 | 2012 | Iran | PWID | 33.3 | 56/100 (56) |
6/100 (6) |
19/100 (19) |
15/100 (15) |
||||
|
Palmateer, N. E [84] |
2014 | 2008–2009 | Scotland | PWID | 33.6 | 1420/2629 (54) |
|||||||
|
Palmateer, N. E [84] |
2014 | 2010 | Scotland | PWID | 34.6 | 1774/3168 (56) |
|||||||
|
Palmateer, N. E [84] |
2014 | 2011–2012 | Scotland | PWID | 35.3 | 1142/2154 (53) |
|||||||
|
Li, L [85] |
2014 | 2012 | China | PWID | 36.2 | RDS | 154/370 (41.6) |
68/370 (18.4) |
|||||
|
Javadi, A [86] |
2014 | 2008–2009 | Iran | PWID | 35.3 | simple | 6/539 (1.1) |
6/539 (1.1) |
|||||
|
Hsieh, M. H [87] |
2014 | 2008–2010 | Taiwan | PWID | 36.1 | 513/562 (91.3) |
86/562 (15.3) |
297/562 (52.8) |
78/562 (13.9) |
293/562 (52.1) |
56/562 (10) |
||
|
Broz, D [88] |
2014 | 2009 | USA | PWID | RDS | 397/9652 (4.1) |
|||||||
|
Goswami, P [89] |
2014 | 2008 | India (Manipour) |
PWID | RDS | 565/839 (67.3) |
51/839 (6.1) |
233/839 (27.8) |
|||||
|
Goswami, P [89] |
2014 | 2009 | India (Manipour) |
PWID | RDS | 601/860 (69.9) |
92/860 (10.7) |
251/860 (29.2) |
|||||
|
Goswami, P [89] |
2014 | 2008 | India (Nagaland) |
PWID | RDS | 121/821 (14.7) |
46/821 (5.6) |
12/821 (1.5) |
|||||
|
Goswami, P [89] |
2014 | 2009 | India (Nagaland) |
PWID | RDS | 125/829 (15.1) |
67/829 (8.1) |
13/829 (1.6) |
|||||
|
Seaberg, E. C [90] |
2014 | USA | PWID | 280/652 (42.9) |
43/640 (6.7) |
410/652 (62.9) |
13/640 (2) |
181/652 (27.8) |
|||||
|
Zhou, Y. J [91] |
2013 | 2010 | China | FSWs | 128/12,622 (1) |
125/12,622 (1) |
|||||||
|
Valadez, J. J [92] |
2013 | 2010 | Libya | FSWs | RDS | 5/69 (7.2) |
2/69 (2.9) |
7/69 (10.1) |
3/69 (4.3) |
0/69 (0) |
|||
|
Taylor, A [93] |
2013 | 2010–2011 | Scotland | PWID | 34 | 495/1625 (30.5) |
|||||||
|
Schuelter-Trevisol, F [94] |
2013 | 2009 | Brazil | FSWs | 28 | 13/147 (8.8) |
13/147 (8.8) |
3/147 (2) |
|||||
|
Salter, M. L [95] |
2013 | 2013 | USA | PWID | 47 | 1025/1191 (86.1) |
322/1191 (27) |
||||||
|
Prasetyo, A. A [96] |
2013 | 2009 | Indonesia | PWID | 32.3 | 47/94 (50) |
1/94 (1.1) |
13/94 (13.8) |
0/94 (0) |
12/94 (12.8) |
0/94 (0) |
||
|
Praseeda, S. D [16] |
2013 | 2007–2010 | India | FSWs | 31 | 7/250 (2.8) |
19/250 (7.6) |
105/250 (42.4) |
3/250 (1.2) |
7/250 (2.8) |
|||
|
Javadi, A [97] |
2013 | 2008–2009 | Iran | PWID | 35.3 | census | 6/539 (1.1) |
||||||
|
Huik, K [98] |
2013 | 2006–2007 | Estonia | PWID | 26 | RDS | 281/373 (75.3) |
205/373 (55) |
174/373 (46.6) |
||||
|
Hakre, S [99] |
2013 | 2009–2011 | Republic of Panama | FSWs | 29.4 | Venue-Day-Time | 2/999 (0.2) |
6/999 (0.6) |
7/999 (0.7) |
||||
|
Garfein, R. S [100] |
2013 | 2009–2010 | USA | PWID | 28 | RDS | 137/510 (26.9) |
21/510 (4.1) |
6/510 (1.2) |
||||
|
Chalana, H [101] |
2013 | 2009–2012 | India | PWID | 30.4 | 78/118 (66.1) |
6/118 (5.1) |
18/118 (15.2) |
2/118 (1.7) |
12/118 (10.2) |
2/118 (1.7) |
||
|
Bowring, A. L [102] |
2013 | 2011 | Tanzania | PWID | 30 | snowball and targeted | 74/267 (27.7) |
93/267 (34.8) |
45/267 (16.9) |
||||
|
Basu, D [103] |
2013 | 2008–2010 | India | PWID | 31.2 | 47/103 (45.6) |
2/103 (1.9) |
||||||
|
Alipour, A [104] |
2013 | 2010 | Iran | PWID | 37 | convenience | 86/226 (38.1) |
8/226 (3.5) |
21/226 (9.3) |
||||
|
Kotaki, T [105] |
2013 | 2012 | Indonesia | FSWs | 32 | randomly | 1/197 (0.5) |
8/197 (4.1) |
22/197 (11.2) |
0/197 (0) |
0/197 (0) |
||
|
Min, J. A [106] |
2013 | 2007–2010 | Korea | PWID | 41.9 | 154/318 (48.4) |
21/318 (6.6) |
0/318 (0) |
13/318 (4.1) |
||||
|
Johnston, L. G [107] |
2013 | 2012 | Republic of Mauritius | FSWs | 31 | RDS | 140/295 (47.5) |
0/295 (0) |
97/297 (32.7) |
84/295 (28.5) |
|||
|
Yen, Y. F [108] |
2012 | 2006–2010 | Taiwan | PWID | 40 | 1318/1447 (91.1) |
194/1447 (13.4) |
190/1447 (13.1) |
|||||
|
Sofian, M [109] |
2012 | 2009 | Iran | PWID | 30.7 | 91/153 (59.5) |
11/153 (7.2) |
9/153 (5.9) |
9/153 (5.9) |
8/153 (5.2) |
3/153 (2) |
2/153 (1.3) |
|
|
Goldenberg, S. M [110] |
2012 | 2008–2010 | Mexico | FSWs | 33 | 35/624 (5.6) |
|||||||
|
Ghosh, I [111] |
2012 | India | FSWs | 0/45 (0) |
7/45 (15.6) |
||||||||
|
Ghosh, I [111] |
2012 | India | PWID | 2/58 (3.4) |
2/58 (3.4) |
||||||||
|
Dunford, L [112] |
2012 | 2008–2009 | Vietnam | PWID | 45.7 | 556/1000 (55.6) |
|||||||
|
Dunford, L [113] |
2012 | 2008–2009 | Vietnam | PWID | 174/1000 (17.4) |
49/1000 (4.9) |
44/1000 (4.4) |
||||||
|
Barua, P [114] |
2012 | 2006 | India | FSWs | RDS | 41/426 (9.6) |
57/426 (13.4) |
21/426 (4.9) |
|||||
|
Wu, N [115] |
2011 | 2009 | China | PWID | 115/141 (81.6) |
||||||||
|
Pilon, R [116] |
2011 | 2007 | Canada | PWID | chain-referral | 246/407 (60.4) |
41/407 (10.1) |
40/407 (9.8) |
|||||
|
Mir-Nasseri, M. M [117] |
2011 | 2001–2002 | Iran | PWID | 35.24 | randomly | 359/518 (69.3) |
19/518 (3.7) |
70/452 (15.5) |
16/518 (3.1) |
58/452 (12.8) |
3/452 (0.7) |
3/452 (0.7) |
|
Kassak, K [118] |
2011 | 2007–2008 | Lebanon | FSWs | RDS | 0/103 (0) |
0/103 (0) |
0/103 (0) |
|||||
|
Kassaian, N [119] |
2011 | 2009–2010 | Iran | FSWs | 30.84 | snowball | 9/91 (9.9) |
1/91 (1.1) |
|||||
|
Johnston, L [120] |
2011 | 2009 | Mauritius | PWID | 31 | RDS | 495/511 (96.9) |
39/511 (7.6) |
230/511 (45) |
||||
|
Chang, S. Y [121] |
2011 | 2006–2009 | Taiwan | PWID | 37 | 36/211 (17.1) |
|||||||
|
Znazen, A [122] |
2011 | 2007 | Tunisia | FSWs | 34 | 2/183 (1.1) |
1/183 (0.5) |
0/183 (0) |
|||||
|
Telan, E. F. O [123] |
2011 | 2007 | Philippines | PWID | RDS | 219/250 (87.6) |
1/250 (0.4) |
||||||
|
Telan, E. F. O [123] |
2011 | 2009 | Philippines | PWID | RDS | 323/341 (94.7) |
2/341 (0.6) |
||||||
|
Telan, E. F. O [123] |
2011 | 2010 | Philippines | PWID | RDS | 59/59 (100) |
44/59 (74.6) |
||||||
|
Todd, C. S [124] |
2010 | 2006–2008 | Afghanistan | FSWs | 29 | 10/520 (1.9) |
34/520 (6.5) |
1/520 (0.2) |
|||||
|
Plitt, S. S [125] |
2010 | 2005 | Canada | PWID | 38 | 181/274 (66.1) |
65/272 (23.9) |
62/272 (22.8) |
|||||
|
Mahfoud, Z [126] |
2010 | 2007–2008 | Lebanon | FSWs | RDS | 0/95 (0) |
|||||||
|
Mahfoud, Z [126] |
2010 | 2007–2008 | Lebanon | PWID | RDS | 43/81 (53.1) |
2/81 (2.5) |
0/81 (0) |
|||||
|
Iversen, J [127] |
2010 | 1998–2008 | Australia | PWID | 31 | 8100/15,583 (52) |
|||||||
|
Alavi, S. M [128] |
2010 | 2002–2006 | Iran | PWID | 24.8 | 103/333 (30.9) |
12/333 (3.6) |
60/333 (18) |
|||||
|
Shethwala, N. D [129] |
2009 | India | FSWs | 10/300 (3.3) |
35/300 (11.7) |
2/300 (0.7) |
|||||||
|
Rehan, N [130] |
2009 | 2004 | Pakistan (Karachi) |
PWID | RDS | 347/399 (87) |
|||||||
|
Rehan, N [130] |
2009 | 2004 | Pakistan (Lahore) |
PWID | RDS | 348/380 (91.6) |
|||||||
|
Mahanta, J [131] |
2009 | 2004–2006 | India | PWID | 26 | 190/398 (47.7) |
15/397 (3.8) |
43/398 (10.8) |
8/398 (2) |
34/398 (8.5) |
3/398 (0.8) |
||
|
Lidman, C [132] |
2009 | 2004–2006 | Sweden | PWID | 35.6 | 268/310 (86.5) |
8/310 (2.6) |
3/310 (1) |
|||||
|
Dumchev, K. V [133] |
2009 | 2005 | Ukraine | PWID | 28.9 | snowball | 230/315 (73) |
44/315 (14) |
38/315(12.1) | ||||
|
Davoodian, P [134] |
2009 | 2002 | Iran | PWID | 35.4 | randomly | 163/249 (65.5) |
12/249 (4.8) |
38/249 (15.3) |
7/249 (2.8) |
36/249 (14.5) |
3/249 (1.2) |
3/249 (1.2) |
|
Chu, F. Y [135] |
2009 | 2005 | Taiwan | PWID | 32.4 | 172/192 (89.6) |
32/192 (16.7) |
49/192 (25.5) |
|||||
|
Uuskula, A [136] |
2008 | 2005–2006 | Estonia | FSWs | 29.5 | RDS | 15/191 (7.9) |
16/206 (7.8) |
5/185 (2.7) |
||||
|
Tseng, F. C [137] |
2008 | 1998–2000 | USA | PWID | 45 | 2092/2296 (91.1) |
73/2296 (3.2) |
273/2296 (11.9) |
|||||
|
Sunthornchart, S [138] |
2008 | 2003–2005 | Thailand | PWID | 551/1535 (35.9) |
||||||||
|
Solomon, S. S [139] |
2008 | 2004–2005 | India | PWID | 35 | convenience | 566/912 (62.1) |
101/912 (11.1) |
271/912 (29.7) |
235/912 (25.8) |
25/912 (2.7) |
||
|
Ngo, T. D [140] |
2008 | 2004 | China | FSWs | 26 | 24/310 (7.7) |
12/310 (3.9) |
8/310 (2.6) |
|||||
|
Neaigus, A [141] |
2008 | 2004–2006 | USA (Newark) |
PWID | 32.8 | 169/205 (82.4) |
52/199 (26.1) |
||||||
|
Neaigus, A [141] |
2008 | 2004–2006 | USA (NYC) |
PWID | 32.8 | 151/282 (53.5) |
15/288 (5.2) |
||||||
|
Kuniholm, M. H [142] |
2008 | 1997–1998 | Georgia | PWID | 539/926 (58.2) |
67/926(7.2) | 5/926 (0.5) |
4/926 (0.4) |
|||||
|
Jindal, N [143] |
2008 | India | PWID | 53/157 (33.8) |
28/157(17.8) | 26/157 (16.6) |
2/157 (1.3) |
11/157 (7) |
2/157 (1.3) |
2/157 (1.3) |
|||
|
Baumbach, J. P [144] |
2008 | 2005 | USA (Mexico) |
PWID | 38.3 | RDS | 194/203 (95.6) |
6/203 (3) |
|||||
|
Baumbach, J. P [144] |
2008 | 2005 | USA (Texas) |
PWID | 42 | RDS | 122/147 (83) |
9/155 (5.8) |
|||||
|
Baumbach, J. P [144] |
2008 | 2005 | USA (New Mexico) |
PWID | 42 | RDS | 76/95 (80) |
1/100 (1) |
RDS, respondent-driven sampling; PWID, people who injection drugs; FSWs, female sex workers.
2.2. Reports of Prevalence
2.2.1. Prevalence of HIV among PWID and FSWs
A total of 99 articles with 133 records and a sample size of 979,659 reported HIV prevalence in PWID and FSWs, of which 74 articles with 95 records were on PWID and 29 articles with 38 records were on FSWs. The overall prevalence of HIV among PWID was estimated to be 15% (95% CI: 12–18%, p = 0.00, I2 = 99.37%) and 5% among FSWs (95% CI: 4–5%, p = 0.00, I2 = 99.41%) (Supplementary Figures S1 and S2, Table 2).
Table 2.
The prevalence of HIV, HCV, HBV and their co-infections among PWID and FSWs by regions of the World Health Organization (WHO); 2008–2018.
| Prevalence | Americas, % (95% CI) |
Africa, % (95% CI) |
South-East Asia, % (95% CI) |
Europe, % (95% CI) |
Eastern Mediterranean, % (95% CI) |
Western Pacific, % (95% CI) |
Total, % (95% CI) |
|---|---|---|---|---|---|---|---|
| PWID | |||||||
| HIV | 10 (7–14) |
24 (16–34) |
22 (16–28) |
12 (6–20) |
8 (3–13) |
11 (2–26) |
15 (12–18) |
| HCV | 64 (51–77) |
38 (10–72) |
54 (43–66) |
59 (53–65) |
60 (46–73) |
75 (68–82) |
60 (55–64) |
| HBV | 3 (1–6) |
5 (2–9) |
9 (7–11) |
3 (1–5) |
5 (4–6) |
- | 6 (5–8) |
| HIV/HCV | 9 (3–18) |
16 (11–22) |
17 (11–24) |
16 (16–28) |
8 (4–14) |
- | 13 (9–18) |
| HIV/HBV | - | 1 (1–3) |
2 (1–5) |
- | 1 (0–2) |
- | 2 (1–3) |
| HBV/HCV | - | - | 3 (1–6) |
- | 3 (2–5) |
- | 3 (1–5) |
| HIV/HCV/HBV | - | 1 (0–2) |
2 (1–4) |
- | 1 (0–2) |
- | 2 (1–3) |
| FSWs | |||||||
| HIV | 4 (2–9) |
19 (8–34) |
18 (10–27) |
- | 0 (0–0) |
1 (0–1) |
5 (4–5) |
| HCV | 1 (0–2) |
9 (0–29) |
3 (0–8) |
- | 2 (0–5) |
1 (1–1) |
1 (1–2) |
| HBV | 1 (0–1) |
5 (1–10) |
4 (2–7) |
- | 1 (0–6) |
- | 3 (1–5) |
| HIV/HCV | 0 (0–0) |
23 (18–27) |
1 (0–6) |
- | - | - | 3 (0–9) |
| HIV/HBV | - | 7 (5–10) |
1 (0–2) |
- | - | - | 1 (0–3) |
2.2.2. Prevalence of HCV among PWID and FSWs
A total of 99 articles with 130 records and a sample size of 924,516 reported HCV prevalence in PWID and FSWs. A total of 76 papers with 101 records had been conducted on PWID and 24 papers with 29 records on FSWs. The overall prevalence of HCV in PWID and FSWs was 60% (95% CI: 55–64%, p = 0.00, I2 = 99.54%) and 1% (95% CI: 1–2%, p = 0.00, I2 = 97.04%), respectively (Supplementary Figures S3 and S4, Table 2).
2.2.3. Prevalence of HBV among PWID and FSWs
A total of 53 articles with 64 records and a sample size of 35,007 reported HBV prevalence in PWID and FSWs. A total of 37 papers with 44 records had been conducted on PWID and 18 papers with 20 records on FSWs. The overall prevalence of HBV in PWID and FSWs was 6% (95% CI: 5–8%, p = 0.00, I2 = 94.84%) and 3% (95% CI: 1–5%, p = 0.00, I2 = 95.37%), respectively (Supplementary Figures S5 and S6, Table 2).
2.2.4. Prevalence of Co-infections of HIV, HCV and HBV among PWID and FSWs
A total of 50 articles with 52 records and a sample size of 48,773 reported co-infection of HIV/HCV in PWID and FSWs. A total of 41 papers with 43 records had been conducted on PWID and nine papers with nine records on FSWs. The overall prevalence of HIV/HCV in PWID and FSWs was 13% (95% CI: 9–18%, p = 0.00, I2 = 99.36%) and 3% (95% CI: 0–9%, p = 0.00, I2 = 97.72%), respectively (Supplementary Figures S7 and S8, Table 2).
A total of 18 articles with 23 records and a sample size of 12,361 reported co-infection of HIV/HBV in PWID and FSWs, of which 12 articles with 14 records were on PWID and seven articles with nine records were on FSWs. The overall prevalence of HIV/HBV in PWID and FSWs was 2% (95% CI: 1–3%, p = 0.00, I2 = 93.26%) and 1% (95% CI: 0–3%, p = 0.00, I2 = 93.74%), respectively (Supplementary Figures S9 and S10, Table 2).
A total of 14 articles with 14 records and a sample size of 10,844 reported co-infection of HCV/HBV in PWID and FSWs, of which 13 articles with 13 records were on PWID and one article with one record was on FSWs [31]. The overall prevalence of HCV/HBV in PWID was 3% (95% CI: 1–5%, p = 0.00, I2 = 92.39%) (Supplementary Figure S11, Table 2).
A total of nine articles with nine records and a sample size of 3849 reported co-infection of HIV/HCV/HBV in PWID, but no articles reported this co-infection in FSWs. The overall prevalence of co-infection of HIV/HCV/HBV among PWID was estimated to be 2% (95% CI: 1–3%, p = 0.00, I2 = 75.19%) (Supplementary Figure S12, Table 2).
2.3. Subgroup Analysis by Regions of WHO
Subgroup analysis based on WHO regions is shown in Table 2. The highest prevalence of HIV in PWID is in the Africa region (24%) and the lowest prevalence is in the Eastern Mediterranean region (8%). The highest prevalence of HIV in FSWs is in the Africa (19%) and South-East Asia (18%) regions and the lowest prevalence is in Eastern Mediterranean (0%) and Western Pacific (1%) regions. The highest prevalence of HCV in PWID is in the Western Pacific (75%) and the lowest prevalence is in Africa (38%). The highest prevalence of HCV in FSWs is in Africa (9%) and the lowest prevalence is in Western Pacific and the Americas regions (1% each). The highest prevalence of HBV in PWID is in South-East Asia (9%) and the lowest prevalence is in the Americas and Eastern Mediterranean regions (1% each). The highest prevalence of HBV in FSWs is in Africa (5%) and South-East Asia (4%) regions and the lowest prevalence is in the Americas and Eastern Mediterranean (1% each).
The highest prevalence of HIV/HCV in PWID is in the South-East Asia, Africa and Europe regions (17%, 16% and 16%, respectively) and the lowest prevalence is in the Eastern Mediterranean region (8%); the highest prevalence in FSWs is in Africa (23%) and the lowest prevalence is in the Americas and South-East Asia regions (0% and 1%, respectively). The prevalence of HIV/HBV in PWID was estimated to be 2% in South-East Asia and 1% in Africa and the Eastern Mediterranean. The highest prevalence of HIV/HBV in FSWs was in Africa with 7% and the lowest prevalence was in South-East Asia with 1%. The prevalence of HCV/HBV in PWID in the South-East Asia and Eastern Mediterranean was 3% each, and the prevalence of HIV/HCV/HBV in PWID was 2% in South-East Asia and 1% in both Africa and the Eastern Mediterranean (Table 2).
2.4. Meta-Regression
Meta-regression results on heterogeneity of studies showed that sample size had a significant effect on the prevalence of HIV, HCV, HBV, HIV/HCV and HCV/HBV among PWID and HIV, HCV and HBV among FSWs, but the mean age of study participants has no significant effect (Table 3).
Table 3.
The results of met-regression on heterogeneity of pooled estimations.
| Study Population | Coefficient | [95% Confidence Interval] | Standard Error | t | p > t | ||
|---|---|---|---|---|---|---|---|
| PWID | HIV | ||||||
| age | 0.27 | −0.12 | 0.67 | 0.19 | 1.39 | 0.17 | |
| Sample size | 0.19 | 0.15 | 0.23 | 0.02 | 9.47 | 0.00 | |
| HCV | |||||||
| age | 0.07 | −0.29 | 0.45 | 0.18 | 0.43 | 0.67 | |
| Sample size | 0.59 | 0.54 | 0.64 | 0.02 | 23.96 | 0.00 | |
| HBV | |||||||
| age | 0.05 | −0.12 | 0.24 | 0.08 | 0.69 | 0.5 | |
| Sample size | 0.06 | 0.04 | 0.09 | 0.01 | 6.26 | 0.00 | |
| HIV/HCV | |||||||
| age | 0.18 | −0.30 | 0.66 | 0.22 | 0.81 | 0.43 | |
| Sample size | 0.16 | 0.12 | 0.20 | 0.02 | 7.76 | 0.00 | |
| HIV/HBV | |||||||
| age | −0.15 | −0.58 | 0.27 | 0.17 | −0.90 | 0.40 | |
| Sample size | 0.02 | 0.00 | 0.04 | 0.01 | 2.02 | 0.06 | |
| HBV/HCV | |||||||
| age | 0.00 | −0.51 | 0.50 | 0.18 | −0.01 | 0.99 | |
| Sample size | 0.04 | 0.00 | 0.07 | 0.01 | 2.75 | 0.02 | |
| HIV/HCV/HBV | |||||||
| age | 0.04 | −0.89 | 0.98 | 0.61 | 0.7 | 0.65 | |
| Sample size | 0.00 | −0.01 | 0.01 | 0.00 | 0.03 | 0.97 | |
| FSW s | HIV | ||||||
| age | −0.38 | −1.16 | 0.38 | 0.35 | −1.10 | 0.29 | |
| Sample size | 0.10 | 0.06 | 0.14 | 0.02 | 5.27 | 0.00 | |
| HCV | |||||||
| age | −0.01 | −0.38 | 0.34 | 0.15 | −0.11 | 0.91 | |
| Sample size | 0.05 | 0.01 | 0.08 | 0.01 | 2.71 | 0.01 | |
| HBV | |||||||
| age | 0.23 | −0.10 | 0.57 | 0.14 | 1.66 | 0.14 | |
| Sample size | 0.05 | 0.02 | 0.08 | 0.01 | 3.65 | 0.00 | |
| HIV/HCV | |||||||
| age | 0.12 | −1.55 | 1.80 | 0.13 | 0.96 | 0.51 | |
| Sample size | 0.06 | −0.02 | 0.16 | 0.03 | 1.73 | 0.13 | |
| HIV/HBV | |||||||
| Sample size | 0.03 | −0.03 | 0.10 | 0.02 | 1.61 | 0.20 | |
2.5. Publication Bias
Egger’s test results were significant for HIV prevalence among PWID and FSWs (p = 0.00), HCV among FSWs (p = 0.009), HBV among PWID and FSWs (p = 0.00), HIV/HCV among PWID (p = 0.00), HIV/HCV among FSWs (p = 0.029), HIV/HBV among PWID and FSWs (p = 0.003), and HCV/HBV among PWID (p = 0.008), pointing to publication bias. However, it was not significant for HIV/HCV/HBV prevalence (p = 0.432) and HCV among PWID (p = 0.078) indicating no publication bias.
3. Discussion
This systematic review and meta-analysis examined the prevalence of HIV, HCV, HBV and their co-infections among PWID and FSWs worldwide, and the results were shown by different WHO regions. The prevalence of HIV in PWID and FSWs was 15% and 5%, respectively. That means one of seven PWID and one of 20 FSWs get infected with HIV, with the highest prevalence in PWID being in Africa (24%), South-East Asia (22%) and Europe (12%), and in FSWs being in Africa (19%) and South-East Asia (18%). The number of people living with HIV worldwide in 2017 was estimated to be 36.8 million [145]. HIV is most likely transmitted through unprotected sex and syringes and needles used for injections. A total of 69.5% of HIV infection in the general population occurs through needle sharing and 10% through unprotected sex [146,147,148]. In a meta-analysis study in 2017, the prevalence of HIV among PWID worldwide was reported to be 17.8%, and the largest population under study was from sub-Saharan Africa. A total of 95% of new HIV cases were among the key populations in the Middle East and North Africa (MENA) [13]. In a 2019 study, the prevalence of HIV in PWID was 21% in Africa; in another study, HIV incidence in PWID in the MENA region was significant, with 75% of new cases occurring in PWID and their sexual partners [149,150]. The meta-analysis by Leung et al., in 2019, also estimated the highest prevalence of HIV in Africa and Asia, which is consistent with the results of our study [151]. The prevalence of HIV in Brazilian FSWs was 5%, and in part-time sex workers in Burkina Faso it was 6.5% [31,152].
The prevalence of HCV in PWID and FSWs was 60% and 1%, respectively. That means that almost one in every two PWID has HCV, with the highest prevalence of HCV in PWID being in the Western Pacific, the Americas and the Eastern Mediterranean, with 75%, 64% and 60%, respectively, and the lowest being in Africa (38%); the highest prevalence in FSWs is in Africa (9%), and the lowest prevalence is in the Americas and Western Pacific (1% each). In a 2007 meta-analysis study, the prevalence of HCV in PWID worldwide was estimated to be 50% [153]. In another study published by Nelson et al. in 2011, the prevalence of HCV in PWID was reported to be 60–80% in 25 countries and over 80% in 12 countries, with approximately 10 million PWID suffering from HCV, China and the US having the largest population [154]. Another meta-analysis study estimated the lowest prevalence of HCV was in the Middle East, North Africa, East and South-East Asia [152]. Worldwide, it is estimated that 14 million PWID are at risk for HCV exposure from contaminated blood. In 2011, the prevalence of hepatitis C in PWID worldwide was estimated to be 67%, the highest prevalence being in Eastern Europe (2.3 million) and East and South-East Asia (2.6 million) [154]. Degenhardt et al. (2017) reported a 52.3% prevalence of HCV among PWID worldwide [11]. In another European Union study, the prevalence of HCV among general population was estimated to be 0.54% to 1.50% in 2019, with the highest prevalence in PWID being in the range of 7.9–82% [155]. In a study in Brazil, the prevalence of hepatitis C in FSWs was estimated to be 0.9% [31]. The relatively high prevalence of needle/syringe sharing, low condom use, high levels of sex with sex workers, homosexual sex between men, and selling sex, indicate high-risk behaviors associated with HIV and HCV prevalence in different regions [156]. Another parameter influencing the prevalence and natural history of HCV infection is the host immune response-related genetics such as IFNL3/4 polymorphisms which impact the spontaneous clearance of HCV, and it was also observed that the genotypes associated with favorable outcome has different prevalence in ethnic groups [157,158].
In this study, the prevalence of HBV in PWID and FSWs was 6% and 3%, respectively, with the highest prevalence in PWID being in South-East Asia (9%) and Africa and the Eastern Mediterranean (5% each), and the lowest prevalence being in the Americas and Western Pacific (1% each). Moreover, the highest prevalence in FSWs was in Africa (5%) and South-East Asia (4%), and the lowest prevalence was in the Americas and Eastern Mediterranean (1% each). Nearly 3.6% of the world’s population (257 million people) have chronic hepatitis B, with a prevalence of 0.01–2% in the UK, the US, Canada, Western Europe, and Japan, and over 8% in most sub-Saharan areas in Africa and some countries in the Western Pacific region [5,159]. In high- and middle-income countries, HBV transmission is more perinatal and horizontal. In low-income countries, however, transmission occurs through drug injection and high-risk sexual behaviors [160]. Asia and Africa have the highest HBV endemicity, but highly effective vaccination programs in some countries have pushed the pattern towards moderate or low endemicity. Therefore, China is currently the only country in Asia where HBV is of paramount importance. Countries with moderate endemicity include India, Korea, the Philippines, Taiwan and Thailand. Countries with low endemicity include Japan, Pakistan, Bangladesh, Singapore, Sri Lanka and Malaysia. Most countries in Africa have high endemicity, with the exception of Tunisia and Morocco, which have moderate endemicity [161]. HBV vaccination is effective in reducing and eliminating HBV by 2030. According to the WHO, in 2017, 97% of blood donors were screened for HBV, but there are gaps in the program and strategies have been suggested to resolve the problems, including reducing insecure injections (it has reduced from 39% in 2000 to 5% in 2010), and safer sex practices, such as minimizing the number of sexual partners and using protection (condoms). On the other hand, according to WHO, 80% of people with hepatitis live without prevention, testing and treatment [5]. In a study from Germany, the coverage of three HBV vaccines was 58% for drug users, one of the influencing factors being injection drug use [162]. The prevalence of HBsAg in PWID in the study by Nelson et al is estimated to be 5–10% in 21 countries and over 10% in 10 other countries with a population of 1.2 million [153]. In a 2017 study, the prevalence of HBV among PWID worldwide was 9.1%. In another study, its prevalence was reported to be 5% in Africa [149,154].
The prevalence of HIV/HCV in PWID and FSWs was 13% and 3%, respectively, with the highest prevalence in PWID being in South-East Asia (17%), Africa and Europe (16% each), and the lowest in Eastern Mediterranean (8%); and the highest prevalence in FSWs being in Africa, and the lowest being in the Americas and South-East Asia (23%, 0% and 0%, respectively). The prevalence of HIV/HBV in PWID and FSWs was 2% and 1%, respectively, and the prevalence of HCV/HBV and HIV/HCV/HBV in PWID was 3% and 2%, respectively. The study by Larney et al. found a positive association between the high prevalence of anti-HCV and the prevalence of HIV in PWID worldwide [163]. Co-infection with viral hepatitis in HIV-positive patients may worsen the prognosis [164]. HBV reactivation is observed in patients with HBV/HCV co-infection during HCV treatment (direct acting antivirals (DAA)) or afterwards [165].
Strength and Limitations
Previous systematic review studies have investigated the prevalence of HIV, HCV and HBV in PWID worldwide, but we have investigated the prevalence of these infections and their co-infections in the high-risk groups PWID and FSWs as distinguished by geographical areas. One limitation of this study is the changing sensitivity of HIV, HCV and HBV diagnostic tests over time, therefore, the results of the 2018 surveys may be different from 2008. In this study, all the reviewed articles had used anti-HCV serology test to detect HCV, which does not differ between past and present infections. The articles reporting HCV-RNA were scarce and excluded. Another limitation of our study is the high heterogeneity in studies. As the analysis was performed in different geographical areas, the heterogeneity may be due to different inclusion and exclusion criteria (e.g., type of drug, minimum duration of injectable drug use, sampling location (prison, the Behavioral Disease Counseling Center, MMT Center, homeless people), frequency of sex during a specific time, number of sexual partners and condom use).
4. Materials and Methods
4.1. Search Strategy
This systematic review and meta-analysis was designed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA). Information sources from January 2008 to October 2018 were searched. Databases including PubMed, Scopus, Web of Science, Embase, Ovid, WHO and Google Scholar were searched, to find out the prevalence of HIV, HCV, HBV and their co-infections. The databases were searched using MESH keywords and the Boolean logic (AND, OR and NOT). The keywords included “Human Immunodeficiency Virus”, “HIV”, “Hepatitis C”, “HCV” “Hepatitis B”, “HBV”, “PWID”, “IDU”, “IVDU”, “FSWs” and “Co-infection”.
4.2. Study Selection and Data Extraction
Cross-sectional studies published in English that assessed anti-HIV, anti-HCV and HBsAg were selected. The studies that did not have a specific serology test to detect infections, those in which the prevalence rate was based on self-reports, the ones that used HBsAb, HBcAb, HBeAg and HBeAb tests to detect HBV and used HCV-RNA to detect HCV, and non-cross-sectional studies (case reports, reviews, case-control and cohort studies) were excluded.
After consulting with experts, two authors (RR and YM) extracted the data from the original articles. Extracted data include: (1) name of first author,(2) date of publication,(3) date of study,(4) country,(5) study subjects,(6) age of patients,(7) sample size,(8) method of sampling,(9) prevalence of HIV among PWID and FSWs,(10) prevalence of HCV among PWID and FSWs,(11) prevalence of HBV among PWID and FSWs,(12) prevalence of HIV/HCV among PWID and FSWs,(13) prevalence of HIV/HBV among PWID and FSWs,(14) prevalence of HCV/HBV among PWID and FSWs,(15) prevalence of HIV/HCV/HBV among PWID and FSWs.
All the steps ranging from searching to extracting data were independently performed by two researchers. In case of disagreement between the two, the problem was solved by referring to the article, discussing the problem and, if necessary, seeking help from a third reviewer (Kappa statistic for agreement for quality assessment; 0.75).
4.3. Quality Assessment
In this study, the Newcastle-Ottawa Quality Assessment Scale (NOS) checklist for cross-sectional studies was used to evaluate possible bias and quality of studies. This checklist was completed by two people. The quality of studies were judged based on such aspects as selection, comparability and outcome using the “star” rating system. Scores ranged from 0 stars (worst case) to 9 stars (best case). Studies with a score of 0–4 were categorized as low quality, 5–7 as moderate and more than 7 as high quality.
4.4. Statistical Analysis
Meta-analysis was performed on the eligible data to determine the prevalence of HIV/HCV, HIV/HBV, HCV/HBV, HIV/HCV/HBV, HIV, HCV and HBV in PWID and FSWs. A chi-square test was used to investigate the heterogeneity of the studies. The heterogeneity results from random-effects model were used to analyze the data at 95% confidence level. The MetaProp command was used to estimate the prevalence.
Egger’s statistical test and funnel plot were used to evaluate publication bias. Subgroup analysis was performed to investigate any qualitative confounding factors that may influence the prevalence of the disease. Subgroup analysis was conducted for the two high risk groups of PWID and FSWs in WHO geographical areas. Meta-regression was performed for mean age and sample size. All two-way statistical tests were considered with α = 0.05. Meta-analysis was performed in STATA 16.
5. Conclusions
The results show that the prevalence of HCV and HIV infections in PWID, and the prevalence of HIV in FSWs are higher than in the general population. The results indicate that the coverage of interventions for HIV and HCV prevention in PWID appear to be poor, and may not be sufficient to effectively prevent HIV and HCV transmission. Additionally, the lack of political commitment and, as a result, inadequate investment, reluctance to address sensitive issues related to young people’s sexual and reproductive needs and rights, and issues related to key populations and harm reduction, and a lack of systematic prevention implementation, even with regard to policy, are three interconnected reasons that seem to underpin the failure to implement effective programs at scale. Increasing the interventions for PWID and FSWs, such as HBV vaccination for the prevention of HBV, and the use of harm reduction programs, such as reducing the number of sexual partners per person, condom distribution, the use of clean needles and syringes, opiate substitution therapy (e.g., methadone) and the treatment of people living with HIV to reduce viral load and prevent onward transmission of HCV, are still a top priority in stopping the HIV and HCV epidemics. For HCV patients, education and counselling on options for care and treatment; immunization with the hepatitis A and B vaccines to prevent co-infection from these hepatitis viruses, and to protect their liver; early and appropriate medical management, including antiviral therapy; and regular monitoring for early diagnosis of chronic liver disease are necessary. Key population should be regularly monitored and screened for these infections and their associated infections.
Acknowledgments
This work was supported by Deputy of Research of Kurdistan University of Medical Sciences. This article is a part of master’s degree dissertation in epidemiology in 2018 titled “Prevalence of HIV, Hepatitis C and B co-infection in People Who Inject Drug (PWID) and Female Sex Workers (FSWs) Worldwide; A Systematic Review and Meta-Analysis” which was recorded under code: IR.MUK.REC.1397/275.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/6/432/s1, Figure S1. Global prevalence of HIV among PWID worldwide; 2008–2018. Figure S2. Global prevalence of HIV among FSWs worldwide; 2008–2018. Figure S3. Global prevalence of HCV among PWID worldwide; 2008–2018. Figure S4. Global prevalence of HCV among FSWs worldwide; 2008–2018. Figure S5. Global prevalence of HBV among PWID worldwide; 2008–2018. Figure S6. Global prevalence of HBV among FSWs worldwide; 2008–2018. Figure S7. Global prevalence of HIV/HCV co-infection among PWID worldwide; 2008–2018. Figure S8. Global prevalence of HIV/HCV co-infection among FSWs worldwide; 2008–2018. Figure S9. Global prevalence of HIV/HBV co-infection among PWID worldwide; 2008–2018. Figure S10. Global prevalence of HIV/HBV co-infection among FSWs worldwide; 2008–2018. Figure S11. Global prevalence of HCV/HBV co-infection among PWID worldwide; 2008–2018. Figure S12. Global prevalence of HIV/HCV/HBV co-infection among PWID worldwide; 2008–2018.
Author Contributions
Conceptualization, G.M., H.S. and S.M.A.; methodology, R.R., Y.M. and H.S.; software, R.R., H.S. and Y.M.; validation, R.R., H.S. and Y.M.; formal analysis, R.R. and H.S.; investigation, R.R. and H.S.; resources, R.R.; data curation, R.R. and G.M.; writing—review and editing, R.R., G.M., H.S., and A.M.B.; visualization, G.M.; supervision, G.M.; project administration, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.WHO HIV/AIDS. [(accessed on 19 February 2020)]; Available online: https://www.who.int/hiv/en/
- 2.UNAIDS 90-90-90: Treatment for all. [(accessed on 19 February 2020)]; Available online: https://www.unaids.org/en/resources/909090.
- 3.WHO 90-90-90: Treatment for all. [(accessed on 19 February 2020)]; Available online: www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
- 4.Stanaway J.D., Flaxman A.D., Naghavi M., Fitzmaurice C., Vos T., Abubakar I., Abu-Raddad L.J., Assadi R., Bhala N., Cowie B., et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Hepatitis B. [(accessed on 19 February 2020)]; Available online: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b.
- 6.World Health Organization . Combating Hepatitis B and C to Reach Elimination by 2030: Advocacy Brief. World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- 7.Pourkarim M.R., Razavi H., Lemey P., Van Ranst M. Iran’s hepatitis elimination programme is under threat. Lancet. 2018;392:1009. doi: 10.1016/S0140-6736(18)31810-5. [DOI] [PubMed] [Google Scholar]
- 8.Thijssen M., Lemey P., Amini-Bavil-Olyaee S., Dellicour S., Alavian S.M., Tacke F., Verslype C., Nevens F., Pourkarim M.R. Mass migration to Europe: An opportunity for elimination of hepatitis B virus? Lancet Gastroenterol. Hepatol. 2019;4:315–323. doi: 10.1016/S2468-1253(19)30014-7. [DOI] [PubMed] [Google Scholar]
- 9.Hesamizadeh K., Sharafi H., Rezaee-Zavareh M.S., Behnava B., Alavian S.M. Next Steps Toward Eradication of Hepatitis C in the Era of Direct Acting Antivirals. Hepat. Mon. 2016;16 doi: 10.5812/hepatmon.37089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO HIV/AIDS. [(accessed on 19 February 2020)]; Available online: https://www.who.int/en/news-room/fact-sheets/detail/hiv-aids.
- 11.Degenhardt L., Peacock A., Colledge S., Leung J., Grebely J., Vickerman P., Stone J., Cunningham E.B., Trickey A., Dumchev K., et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review. Lancet Glob. Health. 2017;5:e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Hepatitis C. [(accessed on 19 February 2020)]; Available online: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c.
- 13.UNAIDS Global HIV & AIDS Statistics—2019 Fact Sheet. [(accessed on 19 February 2020)]; Available online: https://www.unaids.org/en/resources/fact-sheet.
- 14.UNAIDS AIDSinfo. [(accessed on 19 February 2020)]; Available online: http://aidsinfo.unaids.org/
- 15.Medhi G.K., Mahanta J., Kermode M., Paranjape R.S., Adhikary R., Phukan S.K., Ngully P. Factors associated with history of drug use among female sex workers (FSW) in a high HIV prevalence state of India. BMC Public Health. 2012;12:273. doi: 10.1186/1471-2458-12-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai Praseeda S., Anuradha D. A Study on the HBV and the HCV Infections in Female Sex Workers and their Co-Infection with HIV. J. Clin. Diagn. Res. 2013;7:234–237. doi: 10.7860/JCDR/2013/4322.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Publication. [(accessed on 19 February 2020)]; Available online: https://www.unaids.org/en/resources/documents/2019/aids-by-the-numbers.
- 18.Hu J., Liu K., Luo J. HIV/AIDS-Associated Viral Oncogenesis. Springer; Berlin/Heidelberg, Germany: 2019. HIV–HBV and HIV–HCV Coinfection and Liver Cancer Development; pp. 231–250. [DOI] [PubMed] [Google Scholar]
- 19.Easterbrook P., Sands A., Harmanci H. Challenges and Priorities in the Management of Hiv/Hbv and Hiv/Hcv Coinfection in Resource-Limited Settings. Seminars in Liver Disease, Thieme Medical Publishers; New York, NY, USA: 2012. pp. 147–157. [DOI] [PubMed] [Google Scholar]
- 20.French Network of Pharmacovigilance Centres. Bondon-Guitton E., Montastruc J.L., Lapeyre-Mestre M. Influence of HCV or HBV coinfection on adverse drug reactions to antiretroviral drugs in HIV patients. Eur. J. Clin. Pharmacol. 2006;62:243–249. doi: 10.1007/s00228-005-0080-0. [DOI] [PubMed] [Google Scholar]
- 21.Toro-Tobón D., Berbesi-Fernandez D., Mateu-Gelabert P., Segura-Cardona Á.M., Montoya-Vélez L.P. Prevalence of hepatitis C virus in young people who inject drugs in four Colombian cities: A cross-sectional study using Respondent Driven Sampling. Int. J. Drug Policy. 2018;60:56–64. doi: 10.1016/j.drugpo.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman M., Hossain M.E., Afrad M.H., Hasan R., Rahman M., Sarker S., Azim T. Hepatitis C virus infections among clients attending an HIV testing and counseling center in Dhaka, Bangladesh. J. Med. Virol. 2017;90:383–387. doi: 10.1002/jmv.24955. [DOI] [PubMed] [Google Scholar]
- 23.Puga M.A.M., Bandeira L.M., Weis S.M.D.S., Fernandes F.R.P., Castro L.S., Tanaka T.S.O., De Rezende G.R., Teles S., Castro V.D.O.L.D., Murat P.G., et al. High-risk behaviors for hepatitis B and C infections among female sex workers. Rev. Soc. Bras. Med. Trop. 2018;51:198–202. doi: 10.1590/0037-8682-0231-2017. [DOI] [PubMed] [Google Scholar]
- 24.Peach E., Cogger S., Byron K., Francis P., O’Keefe D., Higgs P., Stoove M., Elmore K., Dietze P., Hellard M. Blood-borne virus transmission in an urban, culturally diverse neighbourhood: Results from a cross-sectional bio-behavioural survey using innovative outreach methods in a hard-to-reach population. Sex. Health. 2018;15:54–60. doi: 10.1071/SH16219. [DOI] [PubMed] [Google Scholar]
- 25.Patel E.U., Solomon S.S., Mcfall A.M., Srikrishnan A.K., Pradeep A., Nandagopal P., Laeyendecker O., Tobian A.A., Thomas D.L., Sulkowski M.S. Hepatitis C care continuum and associated barriers among people who inject drugs in Chennai, India. Int. J. Drug Policy. 2018;57:5–60. doi: 10.1016/j.drugpo.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oyaro M., Wylie J., Chen C.-Y., Ondondo R.O., Kramvis A. Human immunodeficiency virus infection predictors and genetic diversity of hepatitis B virus and hepatitis C virus co-infections among drug users in three major Kenyan cities. South. Afr. J. HIV Med. 2018;19:737. doi: 10.4102/sajhivmed.v19i1.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jõgeda E.-L., Avi R., Pauskar M., Kallas E.G., Karki T., Jarlais N.D., Uusküla A., Toompere K., Lutsar I., Huik K. Association of IFNλ4 rs12979860 polymorphism with the acquisition of HCV and HIV infections among people who inject drugs. J. Med. Virol. 2018;90:1779–1783. doi: 10.1002/jmv.25258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarlais D.C.D., Cooper H.L.F., Arasteh K., Feelemyer J., McKnight C., Ross Z. Potential geographic “hotspots” for drug-injection related transmission of HIV and HCV and for initiation into injecting drug use in New York City, 2011–2015, with implications for the current opioid epidemic in the US. PLoS ONE. 2018;13:e0194799. doi: 10.1371/journal.pone.0194799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iakunchykova O., Meteliuk A., Zelenev A., Mazhnaya A., Tracy M., Altice F.L. Hepatitis C virus status awareness and test results confirmation among people who inject drugs in Ukraine. Int. J. Drug Policy. 2018;57:11–17. doi: 10.1016/j.drugpo.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haussig J., Nielsen S., Gassowski M., Bremer V., Marcus U., Wenz B., Bannert N., Bock C.-T., Zimmermann R. A large proportion of people who inject drugs are susceptible to hepatitis B: Results from a bio-behavioural study in eight German cities. Int. J. Infect. Dis. 2018;66:5–13. doi: 10.1016/j.ijid.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira-Júnior O.D.C., Guimarães M.D.C., Damacena G.N., Almeida W.D.S.D., De Souza-Júnior P.R.B., Szwarcwald C.L. Prevalence estimates of HIV, syphilis, hepatitis B and C among female sex workers (FSW) in Brazil, 2016. Medicine. 2018;97:S3–S8. doi: 10.1097/MD.0000000000009218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duong H.T., Jarlais D.D., Khuat O.H.T., Arasteh K., Feelemyer J., Khue P.M., Giang H.T., Laureillard D., Hai V.V., Drive Study Group et al. Risk Behaviors for HIV and HCV Infection Among People Who Inject Drugs in Hai Phong, Viet Nam, 2014. AIDS Behav. 2017;22:2161–2171. doi: 10.1007/s10461-017-1814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demissie M., Johnston L.G., Muleta M., Desyebelew D., Belete W., G/egxiabehre A., Gezahegn N., Kassa D., Aseffa Y. Prevalence of HIV and other infections and injection behaviours among people who inject drugs in Addis Ababa, Ethiopia. Afr. J. AIDS Res. 2018;17:1–6. doi: 10.2989/16085906.2018.1511604. [DOI] [PubMed] [Google Scholar]
- 34.Shengelia N., Chikovani I., Sulaberidze L. Human immunodeficiency virus prevalence and risk determinants among people who inject drugs in the Republic of Georgia. J. Infect. Dev. Ctries. 2017;11:772–780. doi: 10.3855/jidc.9103. [DOI] [PubMed] [Google Scholar]
- 35.Sharhani A., Mehrabi Y., Noroozi A., Nasirian M., Higgs P., Hajebi A., Hamzeh B., Khademi N., Noroozi M., Shakiba E. Hepatitis C virus seroprevalence and associated risk factors among male drug injectors in Kermanshah, Iran. Hepat. Mon. 2017;17 doi: 10.5812/hepatmon.58739. [DOI] [Google Scholar]
- 36.Salek T.P., Katz A.R., Lenze S.M., Lusk H.M., Li D., Jarlais D.C.D. Seroprevalence of HCV and HIV infection among clients of the nation’s longest-standing statewide syringe exchange program: A cross-sectional study of Community Health Outreach Work to Prevent AIDS (CHOW) Int. J. Drug Policy. 2017;48:34–43. doi: 10.1016/j.drugpo.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Niama R.F., Bongolo N.C.L., Mayengue P.I., Mboussou F.F., Bayonne E.S.K., Nzingoula F.M.K., Dossou-Yovo L.R., Louzolo I., Etoka-Beka M.K., Lanzy A., et al. A study on HIV, Syphilis, and Hepatitis B and C virus infections among female sex workers in the Republic of Congo. Arch. Public Health. 2017;75:21. doi: 10.1186/s13690-017-0189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neaigus A., Reilly K.H., Jenness S.M., Hagan H., Wendel T., Gelpi-Acosta C., Marshall D.M. Trends in HIV and HCV Risk Behaviors and Prevalent Infection Among People Who Inject Drugs in New York City, 2005–2012. JAIDS J. Acquir. Immune Defic. Syndr. 2017;75:S325–S332. doi: 10.1097/QAI.0000000000001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutagoma M., Nyirazinyoye L., Sebuhoro D., Riedel D.J., Ntaganira J. Syphilis and HIV prevalence and associated factors to their co-infection, hepatitis B and hepatitis C viruses prevalence among female sex workers in Rwanda. BMC Infect. Dis. 2017;17:525. doi: 10.1186/s12879-017-2625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mmbaga E.J., Moen K., Makyao N., Leshabari M. Prevalence and Predictors of Human Immunodeficiency Virus and Selected Sexually Transmitted Infections Among People Who Inject Drugs in Dar es Salaam, Tanzania. Sex. Transm. Dis. 2017;44:79–84. doi: 10.1097/OLQ.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 41.McFall A.M., Solomon S.S., Lucas G.M., Celentano D.D., Srikrishnan A.K., Kumar M.S., Mehta S.H. Epidemiology of HIV and hepatitis C infection among women who inject drugs in Northeast India: A respondent-driven sampling study. Addiction. 2017;112:1480–1487. doi: 10.1111/add.13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longo J.D.D., Simaleko M.M., Diemer H.S.-C., Grésenguet G., Brücker G., Bélec L. Risk factors for HIV infection among female sex workers in Bangui, Central African Republic. PLoS ONE. 2017;12:e0187654. doi: 10.1371/journal.pone.0187654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambdin B.H., Lorvick J., Mbwambo J.K., Rwegasha J., Hassan S., Lum P., Kral A.H. Prevalence and predictors of HCV among a cohort of opioid treatment patients in Dar es Salaam, Tanzania. Int. J. Drug Policy. 2017;45:64–69. doi: 10.1016/j.drugpo.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khatib A., Matiko E., Khalid F., Welty S., Ali A., Othman A., Haji S., Dahoma M., Rutherford G. HIV and hepatitis B and C co-infection among people who inject drugs in Zanzibar. BMC Public Health. 2017;17:917. doi: 10.1186/s12889-017-4933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kåberg M., Hammarberg A., Lidman C., Weiland O. Prevalence of hepatitis C and pre-testing awareness of hepatitis C status in 1500 consecutive PWID participants at the Stockholm needle exchange program. Infect. Dis. 2017;49:1–9. doi: 10.1080/23744235.2017.1334263. [DOI] [PubMed] [Google Scholar]
- 46.Jõgeda E.-L., Huik K., Pauskar M., Kallas E.G., Karki T., Jarlais D.D., Uusküla A., Lutsar I., Avi R. Prevalence and genotypes of GBV-C and its associations with HIV infection among persons who inject drugs in Eastern Europe. J. Med. Virol. 2016;89:632–638. doi: 10.1002/jmv.24683. [DOI] [PubMed] [Google Scholar]
- 47.Ishizaki A., Tran V.T., Nguyen C.H., Tanimoto T., Hoang H.T.T., Pham H.V., Phan C.T.T., Bi X., Van Pham T., Ichimura H. Discrepancies in prevalence trends for HIV, hepatitis B virus, and hepatitis C virus in Haiphong, Vietnam from 2007 to 2012. PLoS ONE. 2017;12:e0179616. doi: 10.1371/journal.pone.0179616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Handanagic S., Sevic S., Barbaric J., Dominkovic Z., Rode O.D., Begovac J., Bozicevic I. Correlates of anti-hepatitis C positivity and use of harm reduction services among people who inject drugs in two cities in Croatia. Drug Alcohol Depend. 2017;171:132–139. doi: 10.1016/j.drugalcdep.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 49.Gupta D., Saha K., Biswas A., Firdaus R., Ghosh M., Sadhukhan P.C. Recombination in hepatitis C virus is not uncommon among people who inject drugs in Kolkata, India. Infect. Genet. Evol. 2017;48:156–163. doi: 10.1016/j.meegid.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 50.De Matos M.A., França D.D.D.S., Carneiro M., Martins R.M.B., Kerr L., Caetano K.A.A., Pinheiro R.S., De Araújo L.A., Mota R.M.S., De Matos M.A.D., et al. Viral hepatitis in female sex workers using the Respondent-Driven Sampling. Rev. Saúde Pública. 2017;51:s1518–s8787. doi: 10.1590/s1518-8787.2017051006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iversen J., Grebely J., Catlett B., Cunningham P., Dore G.J., Maher L. Estimating the cascade of hepatitis C testing, care and treatment among people who inject drugs in Australia. Int. J. Drug Policy. 2017;47:77–85. doi: 10.1016/j.drugpo.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 52.Parés-Badell O., Espelt A., Folch C., Roca X.M., González V., Casabona J., Brugal M.T., González M.V. Undiagnosed HIV and Hepatitis C infection in people who inject drugs: From new evidence to better practice. J. Subst. Abuse Treat. 2017;77:13–20. doi: 10.1016/j.jsat.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Wenz B., Nielsen S., Gassowski M., Santos-Hövener C., Cai W., Roß S., Bock C.-T., Ratsch B.-A., Kücherer C., Bannert N., et al. High variability of HIV and HCV seroprevalence and risk behaviours among people who inject drugs: Results from a cross-sectional study using respondent-driven sampling in eight German cities (2011-14) BMC Public Health. 2016;16:927. doi: 10.1186/s12889-016-3545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solomon S.S., Srikrishnan A.K., McFall A.M., Kumar M.S., Saravanan S., Balakrishnan P., Solomon S., Thomas D.L., Sulkowski M.S., Mehta S.H. Burden of Liver Disease among Community-Based People Who Inject Drugs (PWID) in Chennai, India. PLoS ONE. 2016;11:e0147879. doi: 10.1371/journal.pone.0147879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skocibusic S., Martinac M., Arapovic J., Grgić S., Nikolic J., Hasanagic D., Bevanda M., Ravlija J. HBV and HCV serological monitoring among injection drugs users in opiate substitution treatment in Bosnia and Herzegovina. J. Infect. Dev. Ctries. 2016;10:968–972. doi: 10.3855/jidc.7445. [DOI] [PubMed] [Google Scholar]
- 56.Nielsen S., Gassowski M., Wenz B., Bannert N., Bock C.-T., Kücherer C., Roß S., Bremer V., Marcus U., Zimmermann R., et al. Concordance between self-reported and measured HIV and hepatitis C virus infection status among people who inject drugs in Germany. Hepatol. Med. Policy. 2016;1:8. doi: 10.1186/s41124-016-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kermode M., Nuken A., Medhi G.K., Akoijam B.S., Sharma H.U., Mahanta J. High burden of hepatitis C & HIV co-infection among people who inject drugs in Manipur, Northeast India. Indian J. Med. Res. 2016;143:348–356. doi: 10.4103/0971-5916.182626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsieh M.-H., Hsieh M.-Y., Huang C.-F., Yeh M.-L., Wang S.-C., Yang J.-F., Chang K., Lin W.-R., Lin C.-Y., Chen T.-C., et al. Anti-HIV seropositivity was related to HBsAg seropositivity among injecting drug users in Taiwan. Kaohsiung J. Med. Sci. 2016;32:96–102. doi: 10.1016/j.kjms.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Handanagic S., Bozicevic I., Civljak M., Dominkovic Z., Sevic S., Barbaric J., Blazic T.N., Rode O.D., Begovac J. HIV and hepatitis C prevalence, and related risk behaviours among people who inject drugs in three cities in Croatia: Findings from respondent-driven sampling surveys. Int. J. Drug Policy. 2016;32:57–63. doi: 10.1016/j.drugpo.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Fotiou A., Kanavou E., Antaraki A., Richardson C., Terzidou M., Kokkevi A. HCV/HIV coinfection among people who inject drugs and enter opioid substitution treatment in Greece: Prevalence and correlates. Hepatol. Med. Policy. 2016;1:9. doi: 10.1186/s41124-016-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Folch C., Casabona J., Espelt A., Majó X., Meroño M., Gonzalez V., Wiessing L., Colom J., Brugal M.T., Group R.S. High prevalence and incidence of HIV and HCV among new injecting drug users with a large proportion of migrants—Is prevention failing? Subst. Use Misuse. 2016;51:250–260. doi: 10.3109/10826084.2015.1092991. [DOI] [PubMed] [Google Scholar]
- 62.Fernàndez-López L., Folch C., Majó X., Gasulla L., Casabona J. Implementation of rapid HIV and HCV testing within harm reduction programmes for people who inject drugs: A pilot study. AIDS Care. 2016;28:1–5. doi: 10.1080/09540121.2016.1164290. [DOI] [PubMed] [Google Scholar]
- 63.Jarlais D.C.D., Huong D.T., Oanh K.T.H., Pham M.K., Giang H.T., Thanh N.T.T., Arasteh K., Feelemyer J., Hammett T., Peries M., et al. Prospects for ending the HIV epidemic among persons who inject drugs in Haiphong, Vietnam. Int. J. Drug Policy. 2016;32:50–56. doi: 10.1016/j.drugpo.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y., Bussell S.A., Shen Z., Tang Z., Lan G., Zhu Q., Liu W., Tang S., Li R., Huang W. Declining inconsistent condom use but increasing HIV and syphilis prevalence among older male clients of female sex workers: Analysis from sentinel surveillance sites (2010–2015), Guangxi, China. Medicine. 2016;95 doi: 10.1097/MD.0000000000003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blackburn N.A., Patel R.C., Zibbell J.E. Improving Screening Methods for Hepatitis C Among People Who Inject Drugs: Findings from the HepTLC Initiative, 2012–2014. Public Health Rep. 2016;131:91–97. doi: 10.1177/00333549161310S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abadie R., Welch-Lazoritz M., Gelpi-Acosta C., Reyes J.C., Dombrowski K. Understanding differences in HIV/HCV prevalence according to differentiated risk behaviors in a sample of PWID in rural Puerto Rico. Harm Reduct. J. 2016;13:10. doi: 10.1186/s12954-016-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bouscaillou J., Evanno J., Prouté M., Inwoley A., Kabran M., N’Guessan T., Djé-Bi S., Sidibé S., Thiam-Niangoin M., N’Guessan B.R., et al. Prevalence and risk factors associated with HIV and tuberculosis in people who use drugs in Abidjan, Ivory Coast. Int. J. Drug Policy. 2016;30:116–123. doi: 10.1016/j.drugpo.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 68.Tun W., Sheehy M., Broz D., Okal J., Muraguri N., Raymond H.F., Musyoki H., Kim A.A., Muthui M., Geibel S. HIV and STI Prevalence and Injection Behaviors Among People Who Inject Drugs in Nairobi: Results from a 2011 Bio-behavioral Study Using Respondent-Driven Sampling. AIDS Behav. 2014;19:24–35. doi: 10.1007/s10461-014-0936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang Z., Zhang C., Li X., Liu Y., Su S., Zhou Y., Shen Z. HIV risk among female sex workers with different patterns of drug use behaviors in Southwest China: A cross-sectional study. AIDS Care. 2014;27:293–300. doi: 10.1080/09540121.2014.980214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosinska M., Sieroslawski J., Wiessing L. High regional variability of HIV, HCV and injecting risks among people who inject drugs in Poland: Comparing a cross-sectional bio-behavioural study with case-based surveillance. BMC Infect. Dis. 2015;15:83. doi: 10.1186/s12879-015-0828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mwatelah R.S., Lwembe R.M., Osman S., Ogutu B.R., Aman R., Kitawi R.C., Wangai L.N., Oloo F.A., Kokwaro G.O., Ochieng W. Co-Infection Burden of Hepatitis C Virus and Human Immunodeficiency Virus among Injecting Heroin Users at the Kenyan Coast. PLoS ONE. 2015;10:e0132287. doi: 10.1371/journal.pone.0132287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurth A.E., Cleland C.M., Jarlais N.C.D., Musyoki H., Lizcano J.A., Chhun N., Cherutich P. HIV Prevalence, Estimated Incidence, and Risk Behaviors Among People Who Inject Drugs in Kenya. JAIDS J. Acquir. Immune Defic. Syndr. 2015;70:420–427. doi: 10.1097/QAI.0000000000000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jordan A.E., Jarlais N.C.D., Arasteh K., McKnight C., Nash D., Perlman D.C. Incidence and prevalence of hepatitis c virus infection among persons who inject drugs in New York City: 2006–2013. Drug Alcohol Depend. 2015;152:194–200. doi: 10.1016/j.drugalcdep.2015.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iversen J., Dolan K., Ezard N., Maher L. HIV and Hepatitis C Virus Infection and Risk Behaviors Among Heterosexual, Bisexual, and Lesbian Women Who Inject Drugs in Australia. LGBT Health. 2015;2:127–134. doi: 10.1089/lgbt.2014.0116. [DOI] [PubMed] [Google Scholar]
- 75.Fan Y.-G., Liu J.-J., Zhang Y.-J., Dai S.-Y., Li M., Ye N.-Q. HIV, other sexually transmitted infections, and risk behaviors among female sex workers in Liuzhou, China. Int. J. Gynecol. Obstet. 2014;128:18–22. doi: 10.1016/j.ijgo.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 76.Collier M., Drobeniuc J., Cuevas-Mota J., Garfein R.S., Kamili S., Teshale E.H. Hepatitis A and B among young persons who inject drugs—Vaccination, past, and present infection. Vaccine. 2015;33:2808–2812. doi: 10.1016/j.vaccine.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 77.Bugssa G., Dessalegn B., Dimtsu B., Berhane Y. Prevalence and factors associated with HIV and hepatitis B virus infections among female commercial sex workers in Mekelle, Ethiopia: Cross sectional study. Int. J. Pharm. Sci. Res. 2015;6:135. [Google Scholar]
- 78.Al-Tayyib A., Thiede H., Burt R., Koester S. Unmet Health Care Needs and Hepatitis C Infection Among Persons Who Inject Drugs in Denver and Seattle, 2009. Prev. Sci. 2014;16:330–340. doi: 10.1007/s11121-014-0500-4. [DOI] [PubMed] [Google Scholar]
- 79.Zibbell J.E., Hart-Malloy R., Barry J., Fan L., Flanigan C. Risk Factors for HCV Infection Among Young Adults in Rural New York Who Inject Prescription Opioid Analgesics. Am. J. Public Health. 2014;104:2226–2232. doi: 10.2105/AJPH.2014.302142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang L., Liang S., Lu W., Pan S.W., Song B., Liu Q., Xu Y., Dong H., Xing H., Shao Y., et al. HIV, Syphilis, and Behavioral Risk Factors among Female Sex Workers before and after Implementation of Harm Reduction Programs in a High Drug-Using Area of China. PLoS ONE. 2014;9:e84950. doi: 10.1371/journal.pone.0084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang L., Tang W., Qian S., Li Y.-G., Xing J., Li D., Ding Z., Babu G.R., Wang N. The HIV, Syphilis, and HCV Epidemics Among Female Sex Workers in China: Results From a Serial Cross-Sectional Study Between 2008 and 2012. Clin. Infect. Dis. 2014;59:e1–e9. doi: 10.1093/cid/ciu245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruiseñor-Escudero H., Wirtz A.L., Berry M., Mfochive-Njindan I., Paikan F., Yousufi H.A., Yadav R.S., Burnham G., Vu A. Risky behavior and correlates of HIV and Hepatitis C Virus infection among people who inject drugs in three cities in Afghanistan. Drug Alcohol Depend. 2014;143:127–133. doi: 10.1016/j.drugalcdep.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 83.Ramezani A., Amirmoezi R., Volk J.E., Aghakhani A., Zarinfar N., McFarland W., Banifazl M., Mostafavi E., Eslamifar A., Sofian M. HCV, HBV, and HIV seroprevalence, coinfections, and related behaviors among male injection drug users in Arak, Iran. AIDS Care. 2014;26:1122–1126. doi: 10.1080/09540121.2014.882485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palmateer N.E., Taylor A., Goldberg D.J., Munro A., Aitken C., Shepherd S.J., McAllister G., Gunson R., Hutchinson S.J. Rapid Decline in HCV Incidence among People Who Inject Drugs Associated with National Scale-Up in Coverage of a Combination of Harm Reduction Interventions. PLoS ONE. 2014;9:e104515. doi: 10.1371/journal.pone.0104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li L., Assanangkornchai S., Duo L., McNeil E.B., Li J. Risk Behaviors, Prevalence of HIV and Hepatitis C Virus Infection and Population Size of Current Injection Drug Users in a China-Myanmar Border City: Results from a Respondent-Driven Sampling Survey in 2012. PLoS ONE. 2014;9:e106899. doi: 10.1371/journal.pone.0106899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Javadi A., Ataei B., Kassaian N., Nokhodian Z., Yaran M. Co-infection of human immunodeficiency virus, hepatitis C and hepatitis B virus among injection drug users in Drop in centers. J. Res. Med. Sci. 2014;19:S17–S21. [PMC free article] [PubMed] [Google Scholar]
- 87.Hsieh M.-H., Tsai J.-J., Hsieh M.-Y., Huang C.-F., Yeh M.-L., Yang J.-F., Chang K., Lin W.-R., Lin C.-Y., Chen T.-C., et al. Hepatitis C Virus Infection among Injection Drug Users with and without Human Immunodeficiency Virus Co-Infection. PLoS ONE. 2014;9:e94791. doi: 10.1371/journal.pone.0094791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Broz D., Wejnert C., Pham H.T., DiNenno E., Heffelfinger J.D., Cribbin M., Krishna N., Teshale E.H., Paz-Bailey G. HIV infection and risk, prevention, and testing behaviors among injecting drug users—National HIV Behavioral Surveillance System, 20 U.S. cities, 2009. MMWR. Surveill. Summ. 2014;63:1–51. [PubMed] [Google Scholar]
- 89.Goswami P., Medhi G.K., Armstrong G., Setia M.S., Mathew S., Thongamba G., Ramakrishnan L., George B., Singh R.K., Paranjape R.S., et al. An assessment of an HIV prevention intervention among People Who Inject Drugs in the states of Manipur and Nagaland, India. Int. J. Drug Policy. 2014;25:853–864. doi: 10.1016/j.drugpo.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 90.Seaberg E.C., Witt M.D., Jacobson L.P., Detels R., Rinaldo C.R., Young S., Phair J.P., Thio C.L. Differences in hepatitis C virus prevalence and clearance by mode of acquisition among men who have sex with men. J. Viral Hepat. 2013;21:696–705. doi: 10.1111/jvh.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou Y., Li X., Zhang C., Tan G., Stanton B., Zhang X., Cui Y. Rates of HIV, syphilis, and HCV infections among different demographic groups of female sex workers in Guangxi China: Evidence from 2010 national sentinel surveillance data. AIDS Care. 2013;25:1433–1441. doi: 10.1080/09540121.2013.772282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valadez J.J., Berendes S., Jeffery C., Thomson J., Ben Othman H., Danon L., Turki A.A., Saffialden R., Mirzoyan L. Filling the Knowledge Gap: Measuring HIV Prevalence and Risk Factors among Men Who Have Sex with Men and Female Sex Workers in Tripoli, Libya. PLoS ONE. 2013;8:e66701. doi: 10.1371/journal.pone.0066701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taylor A., Munro A., Allen E., Dunleavy K., Cameron S., Miller L., Hickman M. Low incidence of hepatitis C virus among prisoners in S cotland. Addiction. 2013;108:1296–1304. doi: 10.1111/add.12107. [DOI] [PubMed] [Google Scholar]
- 94.Trevisol D.J., Custódio G., Da Silva A.C.B., De Oliveira M.B., Wolfart A., Trevisol D.J. HIV, hepatitis B and C, and syphilis prevalence and coinfection among sex workers in Southern Brazil. Rev. Soc. Bras. Med. Trop. 2013;46:493–497. doi: 10.1590/0037-8682-1364-2013. [DOI] [PubMed] [Google Scholar]
- 95.Salter M.L., Lau B., Mehta S.H., Go V.F., Leng S., Kirk G.D. Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections. JAIDS J. Acquir. Immune Defic. Syndr. 2013;64:488–495. doi: 10.1097/QAI.0b013e3182a7ee2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prasetyo A.A., Dirgahayu P., Sari Y., Hudiyono H., Kageyama S. Molecular epidemiology of HIV, HBV, HCV, and HTLV-1/2 in drug abuser inmates in central Javan prisons, Indonesia. J. Infect. Dev. Ctries. 2013;7:453–467. doi: 10.3855/jidc.2965. [DOI] [PubMed] [Google Scholar]
- 97.Javadi A., Ataei B., Yaran M., Nokhodian Z., Kassaian N., Tayeri K., Meshkati M., Ali Z. Prevalence of HIV infection and related risk factors in Isfahan Drop in Centers. Pak. J. Med Sci. 2013;29 doi: 10.12669/pjms.291(Suppl).3531. [DOI] [Google Scholar]
- 98.Huik K., Avi R., Carrillo A., Harper N., Pauskar M., Sadam M., Karki T., Krispin T., Kongo U.-K., Jermilova T., et al. CCR5 Haplotypes Influence HCV Serostatus in Caucasian Intravenous Drug Users. PLoS ONE. 2013;8:e70561. doi: 10.1371/journal.pone.0070561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hakre S., Arteaga G., Núñez A.E., Bautista C.T., Bolen A., Villarroel M., Peel S.A., Paz-Bailey G., Scott P.T., Pascale J.M. Prevalence of HIV and other sexually transmitted infections and factors associated with syphilis among female sex workers in Panama. Sex. Transm. Infect. 2012;89:156–164. doi: 10.1136/sextrans-2012-050557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garfein R.S., Rondinelli A., Barnes R.F., Cuevas J., Metzner M., Velasquez M., Rodriguez D., Reilly M., Xing J., Teshale E.H. HCV infection prevalence lower than expected among 18–40-year-old injection drug users in San Diego, CA. J. Urban Health. 2013;90:516–528. doi: 10.1007/s11524-012-9728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chalana H., Singh H., Sachdeva J.K., Sharma S. Seroprevalence of human immunodeficiency virus, Hepatitis B surface antigen, and Hepatitis C in substance dependents admitted in a tertiary hospital at Amritsar, India. Asian J. Psychiatry. 2013;6:552–555. doi: 10.1016/j.ajp.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 102.Bowring A.L., Luhmann N., Pont S., Debaulieu C., Derozier S., Asouab F., Toufik A., Van Gemert C., Dietze P.M., Stoové M. An urgent need to scale-up injecting drug harm reduction services in Tanzania: Prevalence of blood-borne viruses among drug users in Temeke District, Dar-es-Salaam, 2011. Int. J. Drug Policy. 2013;24:78–81. doi: 10.1016/j.drugpo.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 103.Basu D., Kumar V., Sharma A.K., Barnwal P.K., Mattoo S.K. Seroprevalence of anti-hepatitis C virus (anti-HCV) antibody and HCV-related risk in injecting drug users in northern India: Comparison with non-injecting drug users. Asian J. Psychiatry. 2013;6:52–55. doi: 10.1016/j.ajp.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 104.Alipour A., Haghdoost A.A., Sajadi L., Zolala F. HIV prevalence and related risk behaviours among female partners of male injecting drugs users in Iran: Results of a bio-behavioural survey, 2010. Sex. Transm. Infect. 2013;89:iii41–iii44. doi: 10.1136/sextrans-2013-051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kotaki T., Khairunisa S.Q., Sukartiningrum S.D., Arfijanto M.V., Utsumi T., Normalina I., Handajani R., Widiyanti P., Rusli M., Rahayu R.P., et al. High Prevalence of HIV-1 CRF01_AE Viruses among Female Commercial Sex Workers Residing in Surabaya, Indonesia. PLoS ONE. 2013;8:e82645. doi: 10.1371/journal.pone.0082645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Min J.-A., Yoon Y., Lee H.J., Choi J., Kwon M., Kim K., Lee C.-U., Kim D.-J., Yun H. Prevalence and associated clinical characteristics of hepatitis B, C, and HIV infections among injecting drug users in Korea. J. Med. Virol. 2013;85:575–582. doi: 10.1002/jmv.23523. [DOI] [PubMed] [Google Scholar]
- 107.Johnston L.G., Corceal S. Unexpectedly High Injection Drug Use, HIV and Hepatitis C Prevalence Among Female Sex Workers in the Republic of Mauritius. AIDS Behav. 2012;17:574–584. doi: 10.1007/s10461-012-0278-y. [DOI] [PubMed] [Google Scholar]
- 108.Yen Y.-F., Yen M.-Y., Su L.-W., Li L.-H., Chuang P., Jiang X.-R., Deng C.-Y. Prevalences and associated risk factors of HCV/HIV co-infection and HCV mono-infection among injecting drug users in a methadone maintenance treatment program in Taipei, Taiwan. BMC Public Health. 2012;12:1066. doi: 10.1186/1471-2458-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sofian M., Aghakhani A., Banifazl M., Azadmanesh K., Farazi A., McFarland W., Eslamifar A., Ramezani A. Viral Hepatitis and HIV Infection Among Injection Drug Users in a Central Iranian City. J. Addict. Med. 2012;6:292–296. doi: 10.1097/ADM.0b013e3182659928. [DOI] [PubMed] [Google Scholar]
- 110.Goldenberg S.M., Rangel G., Harvey-Vera A., Patterson T.L., Abramovitz D., Silverman J.G., Raj A., Strathdee S.A. Exploring the Impact of Underage Sex Work Among Female Sex Workers in Two Mexico–US Border Cities. AIDS Behav. 2012;16:969–981. doi: 10.1007/s10461-011-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghosh I., Ghosh P., Bharti A.C., Mandal R., Biswas J., Basu P. Prevalence of human papillomavirus and co-existent sexually transmitted infections among female sex workers, men having sex with men and injectable drug abusers from eastern India. Asian Pac. J. Cancer Prev. 2012;13:799–802. doi: 10.7314/APJCP.2012.13.3.799. [DOI] [PubMed] [Google Scholar]
- 112.Dunford L., Carr M., Dean J., Waters A., Nguyen L.T., Thi T.H.T., Thi L.A.B., Do H.D., Thi T.T.D., Nguyen H.T., et al. Hepatitis C Virus in Vietnam: High Prevalence of Infection in Dialysis and Multi-Transfused Patients Involving Diverse and Novel Virus Variants. PLoS ONE. 2012;7:e41266. doi: 10.1371/journal.pone.0041266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dunford L., Carr M., Dean J., Nguyen L.T., Thi T.H.T., Nguyen B.T., Connell J., Coughlan S., Nguyen H.T., Hall W.W., et al. A Multicentre Molecular Analysis of Hepatitis B and Blood-Borne Virus Coinfections in Viet Nam. PLoS ONE. 2012;7:e39027. doi: 10.1371/journal.pone.0039027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barua P., Mahanta J., Medhi G.K., Dale J., Paranjape R.S., Thongamba G. Sexual activity as risk factor for hepatitis C virus (HCV) transmission among the female sex workers in Nagaland. Indian J. Med. Res. 2012;136:30. [Google Scholar]
- 115.Wu N., Ge Q., Feng Q., Zhang J., Liu X., Sun C., Xu Y., He G., Zhang C. High Prevalence of Hepatitis C virus Among Injection Drug Users in Zhenjiang, Jiangsu, China. Indian J. Virol. 2011;22:77–83. doi: 10.1007/s13337-011-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pilon R., Leonard L., Kim J., Vallée D., De Rubeis E., Jolly A.M., Wylie J., Pelude L., Sandstrom P. Transmission Patterns of HIV and Hepatitis C Virus among Networks of People Who Inject Drugs. PLoS ONE. 2011;6:e22245. doi: 10.1371/journal.pone.0022245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mir-Nasseri M.M., Mohammadkhani A., Tavakkoli H., Ansari E., Poustchi H. Incarceration is a major risk factor for blood-borne infection among intravenous drug users. Zahedan J. Res. Med. Sci. 2011;11:19–22. [PMC free article] [PubMed] [Google Scholar]
- 118.Kassak K., Mahfoud Z., Kreidieh K., Shamra S., Afifi R., Ramia S. Hepatitis B virus and hepatitis C virus infections among female sex workers and men who have sex with men in Lebanon: Prevalence, risk behaviour and immune status. Sex. Health. 2011;8:229–233. doi: 10.1071/SH10080. [DOI] [PubMed] [Google Scholar]
- 119.Kassaian N., Ataei B., Yaran M., Babak A., Shoaei P. Hepatitis B and C among women with illegal social behavior in Isfahan, Iran: Seroprevalence and associated factors. Zahedan J. Res. Med. Sci. 2011;11:368–371. [PMC free article] [PubMed] [Google Scholar]
- 120.Johnston L.G., Saumtally A., Corceal S., Mahadoo I., Oodally F. High HIV and hepatitis C prevalence amongst injecting drug users in Mauritius: Findings from a population size estimation and respondent driven sampling survey. Int. J. Drug Policy. 2011;22:252–258. doi: 10.1016/j.drugpo.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 121.Chang S.-Y., Yang C.-L., Ko W.-S., Liu W.-C., Lin C.-Y., Wu C.-H., Su Y.-C., Chen M.-Y., Sheng W.-H., Hung C., et al. Molecular Epidemiology of Hepatitis D Virus Infection among Injecting Drug Users with and without Human Immunodeficiency Virus Infection in Taiwan. J. Clin. Microbiol. 2010;49:1083–1089. doi: 10.1128/JCM.01154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Znazen A., Frikha-Gargouri O., Berrajah L., Bellalouna S., Hakim H., Gueddana N., Hammami A. Sexually transmitted infections among female sex workers in Tunisia: High prevalence of Chlamydia trachomatis. Sex. Transm. Infect. 2010;86:500–505. doi: 10.1136/sti.2010.042770. [DOI] [PubMed] [Google Scholar]
- 123.Telan E.F.O., Samonte G.M.J., Abellanosa-Tac-An I.P., Alesna E.T., Leaño P.S.A., Emphasis Y.E.E., Tsuneki A., Matsumoto K., Kageyama S. The early phase of an HIV epidemic in a population exposed previously to HCV in the Philippines. J. Med. Virol. 2011;83:941–947. doi: 10.1002/jmv.22070. [DOI] [PubMed] [Google Scholar]
- 124.Todd C.S., Nasir A., Stanekzai M.R., Bautista C., Botros B.A., Scott P.T., Strathdee S.A., Tjaden J. HIV, hepatitis B, and hepatitis C prevalence and associated risk behaviors among female sex workers in three Afghan cities. AIDS. 2010;24:S69–S75. doi: 10.1097/01.aids.0000386736.25296.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Plitt S.S., Gratrix J., Hewitt S., Conroy P., Parnell T., Lucki B., Pilling V., Anderson B., Choudri Y., Archibald C.P., et al. Seroprevalence and Correlates of HIV and HCV among Injecting Drug Users in Edmonton, Alberta. Can. J. Public Health. 2010;101:50–55. doi: 10.1007/BF03405562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mahfoud Z., Afifi R., Ramia S., El Khoury D., Kassak K., El Barbir F., Ghanem M., El-Nakib M., DeJong J., Afifi R.A. HIV/AIDS among female sex workers, injecting drug users and men who have sex with men in Lebanon: Results of the first biobehavioral surveys. AIDS. 2010;24:S45–S54. doi: 10.1097/01.aids.0000386733.02425.98. [DOI] [PubMed] [Google Scholar]
- 127.Iversen J., Wand H., Gonnermann A., Maher L. Gender differences in hepatitis C antibody prevalence and risk behaviours amongst people who inject drugs in Australia 1998–2008. Int. J. Drug Policy. 2010;21:471–476. doi: 10.1016/j.drugpo.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 128.Alavi S.M., Behdad F. Seroprevalence Study of Hepatitis C and Hepatitis B Virus among Hospitalized Intravenous Drug Users in Ahvaz, Iran (2002–2006) Zahedan J. Res. Med. Sci. 2010;10:101–104. [PMC free article] [PubMed] [Google Scholar]
- 129.Mulla S.A., Kosambiya J.K., Desai V.K., Shethwala N.D. Sexually transmitted infections and reproductive tract infections in female sex workers. Indian J. Pathol. Microbiol. 2009;52:198. doi: 10.4103/0377-4929.48916. [DOI] [PubMed] [Google Scholar]
- 130.Rehan N., Bokhari A., Nizamani N.M., Jackson D., Naqvi H.R., Qayyum K., Mansoor S., Muzaffar R. National study of reproductive tract infections among high risk groups of Lahore and Karachi. J. Coll. Physicians Surg. Pak. 2009;19:228–231. [PubMed] [Google Scholar]
- 131.Mahanta J., Borkakoty B., Das H.K., Chelleng P.K. The risk of HIV and HCV infections among injection drug users in northeast India. AIDS Care. 2009;21:1420–1424. doi: 10.1080/09540120902862584. [DOI] [PubMed] [Google Scholar]
- 132.Lidman C., Nordén L., Kåberg M., Kall K., Franck J., Aleman S., Birk M. Hepatitis C infection among injection drug users in Stockholm Sweden: Prevalence and gender. Scand. J. Infect. Dis. 2009;41:679–684. doi: 10.1080/00365540903062143. [DOI] [PubMed] [Google Scholar]
- 133.Dumchev K., Soldyshev R., Qian H.-Z., Zezyulin O.O., Chandler S.D., Slobodyanyuk P., Moroz L., Schumacher J.E. HIV and hepatitis C virus infections among hanka injection drug users in central Ukraine: A cross-sectional survey. Harm. Reduct. J. 2009;6:23. doi: 10.1186/1477-7517-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davoodian P., Dadvand H., Mahouri K., Amoozandeh A., Salavati A. Prevalence of selected sexually and blood-borne infections in Injecting drug abuser inmates of bandar abbas and roodan correction facilities, Iran, 2002. Braz. J. Infect. Dis. 2009;13:356–358. doi: 10.1590/S1413-86702009000500008. [DOI] [PubMed] [Google Scholar]
- 135.Chu F.-Y., Chiang S.-C., Su F.-H., Chang Y.-Y., Cheng S.-H. Prevalence of human immunodeficiency virus and its association with hepatitis B, C, and D virus infections among incarcerated male substance abusers in Taiwan. J. Med. Virol. 2009;81:973–978. doi: 10.1002/jmv.21481. [DOI] [PubMed] [Google Scholar]
- 136.Uusküla A., Fischer K., Raudne R., Kilgi H., Krylov R., Salminen M., Brummer-Korvenkontio H., Lawrence J.S., Aral S. A study on HIV and hepatitis C virus among commercial sex workers in Tallinn. Sex. Transm. Infect. 2008;84:189–191. doi: 10.1136/sti.2007.027664. [DOI] [PubMed] [Google Scholar]
- 137.Tseng F.-C., Edlin B., Zhang M., Kral A., Busch M.P., Ortiz-Conde B.A., Welzel T.M., O’Brien T.R. The inverse relationship between chronic HBV and HCV infections among injection drug users is associated with decades of age and drug use. J. Viral Hepat. 2008;15:690–698. doi: 10.1111/j.1365-2893.2008.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sunthornchart S., Linkins R.W., Natephisarnwanish V., Levine W.C., Maneesinthu K., Lolekha R., Tappero J.W., Trirat N., Muktier S., Chancharastong P., et al. Prevalence of hepatitis B, tetanus, hepatitis A, human immunodeficiency virus and feasibility of vaccine delivery among injecting drug users in Bangkok, Thailand, 2003–2005. Addiction. 2008;103:1687–1695. doi: 10.1111/j.1360-0443.2008.02303.x. [DOI] [PubMed] [Google Scholar]
- 139.Solomon S.S., Srikrishnan A.K., Mehta S.H., Vasudevan C.K., Murugavel K.G., Thamburaj E., Anand S., Kumar M.S., Latkin C., Solomon S. High prevalence of HIV, HIV/hepatitis C virus co-infection and risk behaviors among IDUs in Chennai, India: A cause for concern. J. Acquir. Immune Defic. Syndr. 1999. 2008;49:327. doi: 10.1097/QAI.0b013e3181831e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ngo T.D., Laeyendecker O., Li C., Tai H., Cui M., Lai S., Quinn T.C. Herpes simplex virus type 2 infection among commercial sex workers in Kunming, Yunnan Province, China. Int. J. STD AIDS. 2008;19:694–697. doi: 10.1258/ijsa.2008.008072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neaigus A., Zhao M., Gyarmathy V.A., Cisek L., Friedman S.R., Baxter R.C. Greater Drug Injecting Risk for HIV, HBV, and HCV Infection in a City Where Syringe Exchange and Pharmacy Syringe Distribution are Illegal. J. Hered. 2008;85:309–322. doi: 10.1007/s11524-008-9271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kuniholm M.H., Aladashvili M., Del Rio C., Stvilia K., Gabelia N., Chitale R.A., Tsertsvadze T., Nelson K.E. Not All Injection Drug Users Are Created Equal: Heterogeneity of HIV, Hepatitis C Virus, and Hepatitis B Virus Infection in Georgia. Subst. Use Misuse. 2008;43:1424–1437. doi: 10.1080/10826080802108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jindal N., Arora U., Singh K. Prevalence of human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus in three groups of populations at high risk of HIV infection in Amritsar (Punjab), Northern India. Jpn. J. Infect. Dis. 2008;61:79. [PubMed] [Google Scholar]
- 144.Baumbach J., Foster L.N., Mueller M., Cruz M.F., Arbona S., Melville S., Ramos R.L., Strathdee S.A. Seroprevalence of select bloodborne pathogens and associated risk behaviors among injection drug users in the Paso del Norte region of the United States–Mexico border. Harm Reduct. J. 2008;5:33. doi: 10.1186/1477-7517-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frank T.D., Carter A., Jahagirdar D., Biehl M.H., Douwes-Schultz D., Larson S.L., Arora M., Dwyer-Lindgren L., Steuben K.M., Abbastabar H., et al. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: A systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV. 2019;6:e831–e859. doi: 10.1016/S2352-3018(19)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ronen K., Sharma A., Overbaugh J. HIV transmission biology: Translation for HIV prevention. AIDS. 2015;29:2219–2227. doi: 10.1097/QAD.0000000000000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jarlais D.C.D. HIV/STDs and drug use. AIDS/STD Health Promot. Exch. 1997;2:1–3. [PubMed] [Google Scholar]
- 148.Armstrong G., Humtsoe C., Kermode M. HIV risk behaviours among injecting drug users in Northeast India following scale-up of a targeted HIV prevention programme. BMC Public Health. 2011;11:S9. doi: 10.1186/1471-2458-11-S6-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scheibe A., Young K., Moses L., Basson R., Versfeld A., Spearman W., Sonderup M.W., Prabdial-Sing N., Manamela J., Puren A., et al. Understanding hepatitis B, hepatitis C and HIV among people who inject drugs in South Africa: Findings from a three-city cross-sectional survey. Harm Reduct. J. 2019;16:28. doi: 10.1186/s12954-019-0298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mumtaz G.R., Awad S.F., Feizzadeh A., Weiss H.A., Abu-Raddad L.J. HIV incidence among people who inject drugs in the Middle East and North Africa: Mathematical modelling analysis. J. Int. AIDS Soc. 2018;21:e25102. doi: 10.1002/jia2.25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leung J., Peacock A., Colledge S., Grebely J., Cunningham E.B., Hickman M., Vickerman P., Stone J., Trickey A., Dumchev K., et al. A Global Meta-analysis of the Prevalence of HIV, Hepatitis C Virus, and Hepatitis B Virus Among People Who Inject Drugs—Do Gender-Based Differences Vary by Country-Level Indicators? J. Infect. Dis. 2019;220:78–90. doi: 10.1093/infdis/jiz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Traore I.T., Hema N.M., Sanon A., Some F., Ouedraogo D., Some R., Niessougou J., Konate I., Mayaud P., Van De Perre P., et al. HIV risk and behaviour among part-time versus professional FSW: Baseline report of an interventional cohort in Burkina Faso: Table 1. Sex. Transm. Infect. 2016;92:550–553. doi: 10.1136/sextrans-2015-052038. [DOI] [PubMed] [Google Scholar]
- 153.Aceijas C., Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. Int. J. Drug Policy. 2007;18:352–358. doi: 10.1016/j.drugpo.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 154.Nelson P.K., Mathers B.M., Cowie B., Hagan H., Jarlais N.D., Horyniak D., Degenhardt L. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: Results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Han R., Zhou J., François C., Toumi M. Prevalence of hepatitis C infection among the general population and high-risk groups in the EU/EEA: A systematic review update. BMC Infect. Dis. 2019;19:655. doi: 10.1186/s12879-019-4284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mumtaz G.R., Weiss H.A., Thomas S.L., Riome S., Setayesh H., Riedner G., Semini I., Tawil O., Akala F.A., Wilson D., et al. HIV among People Who Inject Drugs in the Middle East and North Africa: Systematic Review and Data Synthesis. PLoS Med. 2014;11:e1001663. doi: 10.1371/journal.pmed.1001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sharafi H., Alavian S.M., Behnava B., Pouryasin A., Keshvari M. The Impact of IFNL4 rs12979860 Polymorphism on Spontaneous Clearance of Hepatitis C.; A Case-Control Study. Hepat Mon. 2014;14 doi: 10.5812/hepatmon.22649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Aalaei-Andabili S.H., Behnava B., Salimi S., Sharafi H., Alavian S.M. Mysterious Linkages Between Hepatitis C Virus Genotypes, Interleukin-28B Genotypes and Viral Clearance- A Meta-Analysis. Hepat Mon. 2014;14 doi: 10.5812/hepatmon.15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schweitzer A., Horn J., Mikolajczyk R., Krause G., Ott J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 160.Thio C.L., Hawkins C. Hepatitis B Virus. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier; Philadelphia, PA, USA: 2020. [Google Scholar]
- 161.André F. Hepatitis B epidemiology in Asia, the Middle East and Africa. Vaccine. 2000;18:S20–S22. doi: 10.1016/S0264-410X(99)00456-9. [DOI] [PubMed] [Google Scholar]
- 162.Raven S.F., Urbanus A., De Gee A., Hoebe C., Van Steenbergen J. Predictors of hepatitis B vaccination completion among people who use drugs participating in a national program of targeted vaccination. Vaccine. 2018;36:5282–5287. doi: 10.1016/j.vaccine.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 163.Larney S., Leung J., Grebely J., Hickman M., Vickerman P., Peacock A., Stone J., Trickey A., Dumchev K., Colledge S., et al. Global systematic review and ecological analysis of HIV in people who inject drugs: National population sizes and factors associated with HIV prevalence. Int. J. Drug Policy. 2020;77:102656. doi: 10.1016/j.drugpo.2019.102656. [DOI] [PubMed] [Google Scholar]
- 164.Mayaud P., McCartney D., Mabey D. 7-Sexually Transmitted Infections. In: Ryan E.T., Hill D.R., Solomon T., Aronson N.E., Endy T.P., editors. Hunter’s Tropical Medicine and Emerging Infectious Diseases. Content Repository Only!; London, UK: 2020. pp. 52–68. [DOI] [Google Scholar]
- 165.Mücke V.T., Mücke M.M., Peiffer K.-H., Weiler N., Welzel T.M., Sarrazin C., Zeuzem S., Berger A., Vermehren J. No evidence of hepatitis B virus reactivation in patients with resolved infection treated with direct-acting antivirals for hepatitis C in a large real-world cohort. Aliment. Pharmacol. Ther. 2017;46:432–439. doi: 10.1111/apt.14177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.