Abstract
Salmonella enteritidis and Salmonella pullorum belonging to Group O9 Salmonella are major causative agents of infectious diseases in chicken. O9 antigen as a part of lipopolysaccharide (LPS) is a predominant detected target for Salmonella infection. To identify the infection, an anti-O9 monoclonal antibody (McAb)-based direct competitive enzyme-linked assay (O9 Dc-ELISA) was developed after constraints were optimized; the establishment and application of O9 Dc-ELISA, compared to two commercial kits and plate agglutination test (PAT), showed that O9 Dc-ELISA could screen out more positive samples than the PAT method could and produce the same agreement rates with commercial kits in terms of sensitivity in addition to strong specificity to clinical serum samples.
Keywords: O:9 Salmonella, McAb, O9 Dc-ELISA, specificity, PAT
Introduction
Salmonella, an important zoonotic pathogen, is one of the major causative agents of food-borne infectious diseases worldwide (1). Consumption of foods such as egg, chicken, pork, beef, and dairy products contaminated with Salmonella can cause salmonellosis in humans (2–4). This pathogen not only brings huge economic loss in the animal industry but also impacts human health, even death (5–8). Because of these disease harms and public health hazards, efficient surveillance is very important to reduce the prevalence of Salmonella and the risk of transmission to humans.
Salmonella enteritidis and Salmonella pullorum, which are important members in group O:9 Salmonella, are the main pathogens found in modern large-scale chicken farms in China (2, 9–11). In addition to their high morbidity and mortality in young broilers, they cause non-apparent infections in adult chickens without obvious clinical symptoms. Thus, it is difficult to find Salmonella infection in adult chickens. If Salmonella-infected chicken is not found on time, it may be a source of infection causing unlimited spread in chicken, even to humans, because of horizontal transmission and vertical transmission. It is necessary to carry out a seroepidemiological survey on Salmonella for healthy breeding and food safety.
Currently, plate agglutination test (PAT) is the main detection method used during Salmonella surveillance for its easy operation and low cost, but its sensitivity and specificity are poor and can easily cause false results because of antigen detection, visual observation, and subjective judgment.
LPS is the main antigen found on the Salmonella surface and the primary target for the immune system (12). After Salmonella infection, LPS can induce and keep a high level of antibody from early stages. Serotyping using serum/antibodies to the O-antigen of Salmonella lipopolysaccharide (LPS) (13, 14) is a critical basis of current Salmonella surveillance programs. Routine serotyping helps in monitoring public health response to the global challenge of salmonellosis and the effectiveness of control measures (9, 15–17).
Therefore, the development of readily available detection systems of the Salmonella antibody in chicken is important for mass-scale laboratory diagnosis. In this study, we developed an anti-O:9 Salmonella McAb-based direct competitive ELISA method to meet the requirements of accurate Salmonella surveillance.
Materials and Methods
Ethical Statement
The present study was conducted under the approval of Laboratory Animal Ethics Committee of Yangzhou University (Jiangsu province, China) in accordance with Laboratory Animal Guidelines for ethical review of animal welfare (GB/T 35892-2018, National Standards of the People's Republic of China).
Strain, Hybridoma Cell Line, and Animals
Salmonella enteritidis (C50041) and Salmonella pullorum (S06004) were stored by our laboratory. A 3-47-0 hybridoma cell line secreting anti-O9 McAb was developed and preserved by our laboratory. Thirty 10-week BALB/c female mice were purchased for ascites from Comparative Medical Center of Yangzhou University.
Primary Quantity of Coated LPS and HRP-Labeled O9 McAb for Competitive ELISA
Primary quantities of LPS and HRP-labeled O9 McAb were confirmed by chessboard titration to develop a direct ELISA following conventional ELISA protocol. Horizontal gradient dilution of HRP-labeled O:9 McAb and vertical gradient dilution of coating antigens were performed. The final concentration of a tested positive serum was diluted 1:10. According to the serum inhibition rate [inhibition rate = (1-detected serum OD value/blank control OD) ×100%], optimal balanced concentrations were selected.
Constraint Optimizations for O9 Dc-ELISA
Tested Positive Serum Dilution
Two Salmonella pullorum-positive sera, two S. enteritidis-positive sera, and two negative sera from specific pathogen-free (SPF) chicken were diluted 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, and 1:256. Based on the previous direct ELISA, each tested serum dilution was used as a competitor of positive serum and subjected to a competition ELISA. The serum dilution at the highest inhibition rate was selected as the serum dilution of the competitive ELISA method.
Quantity of Coated LPS and HRP-Labeled O:9 McAb
Based on the previous ELISA, LPS were divided into four groups, 480, 320, 190, and 160 ng/mL. By comparing the N/P values (negative serum OD value/positive serum OD value), the coating concentration at which the N/P value was the largest was chosen out as the optimal concentration.
Similarly, the antibody was divided into six groups, 56.8, 52.0, 48.0, 44.6, 41.6, and 39.1 ng/mL, to optimize the concentration of HRP-labeled McAb. The N/P values were compared (negative serum OD value/positive serum OD value) with the concentration of the HRP-labeled O9 McAb.
Time of LPS Being Coated Onto a Plate
Three ELISA plates were coated at 100 μL/well at an optimized coating concentration. The coating time of the three ELISA plates was 16, 24, and 36 h, respectively.
Time of HRP-labeled McAb Binding With LPS and Reacting With TMB
Competitive ELISA was performed with the LPS coating concentration and HRP-labeled McAb and serum dilution, which were optimized in the previous steps. To ensure McAb to bind with coated LPS as possible, the incubation time of the HRP-labeled McAb was set to 1.0, 1.5, 2.0, 2.5, and 3.0 h, respectively, for analysis based on the N/P value.
After optimization time of HRP-labeled McAb binding with LPS, the incubation time of HRP-labeled McAb to react with substrate 3,3′,5,5′-tetramethylbenzidine (TMB) was also optimized. The hydrolysis time for TMB substrate by HRP was set to 3, 5, and 10 min.
Setting Up of the Cutoff Value and Comparison With Commercial Kits and PAT
One hundred serum samples from artificially infected chickens at different time points as positive control and 100 serum samples from SPF chickens as negative control were detected using the France ID.vet Salmonella kit, and these 200 serum samples were detected by O9 Dc-ELISA; the receiver operating characteristic (ROC) curve was made according to the inhibition rate. Based on these results, the cutoff value which was the value of negative samples + 3SD as a negative/positive judgment boundary was set up.
Fifty random clinical serum samples were tested using a double blind test by O9 Dc-ELISA and compared to IDEXX ELISA kit (IDEXX USA, 99-0002040) and ID.vet ELISA kit (ID.vet France, SALSGPD-5P) to judge the accuracy of O9 Dc-ELISA in clinical application.
The coincidence rate was calculated by the following formula: number of [(+,+) + (-,-)]/total number %.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, USA). One-way ANOVA followed by Dunnett's multiple-comparison tests was used to determine the statistical differences between multiple experimental groups. All data are expressed as mean ± standard error of the mean (SEM) unless otherwise specified. P < 0.05 was considered statistically significant. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
Preparation of Coated LPS and HRP-Labeled O9 McAb
In this study, LPS were purified by the hot phenol–water method (18) and its concentration was calculated according to the standard sugar curve made by the anthracene ketone method. According to the measured OD620nm value and standard curve (Figure 1), the final concentration of LPS was 484.31 μg/mL. McAb against O9 LPS (O9 McAb) was purified from hybridoma supernatants by caprylic/ammonium sulfate precipitation (19) and labeled with horseradish peroxidase (HRP) (20). The titer of HRP-labeled O9 McAb (HRP-O9 McAb) was up to 51,200 by indirect ELISA.
Figure 1.
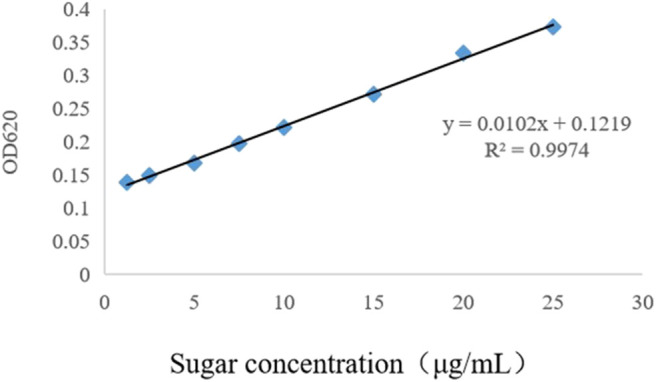
Standard curve of sugar concentration determined by the anthracene-ketone method.
Constraint Determination of Competitive ELISA
A series of dilutions of LPS, HRP-labeled O9 McAb, and positive sera were prepared for chessboard titration and optimization (Figure 2). After optimization assay, 320 ng/ml LPS for coating (Figure 3), 41.6 ng/ml HRP-labeled O9 McAb (Figure 4), and positive serum dilution of 1:4 (Figure 5) were selected for developing O9 Dc-ELISA. The inhibition rate of positive serum by Salmonella pullorum and Salmonella enteritidis was up to 94 and 89%, respectively. On this basis, a standard operating procedure was formulated, after 96-well plates (Biofil company, Canada, FEP101896) were coated with ~100 μL purified LPS (320 ng/ml) in carbonate bicarbonate buffer (CBS, pH 9.4) at 4°C for 24 h (Figure 6) and washed with PBST (0.05% Tween 20 in phosphate-buffered saline) two times; 200 μL/well 2% BSA PBS solution was added again for blocking for 3 h at 37°C, then 50 μL 1:2 diluted chicken serum (PBS for blank control) and 50 μL HRP-labeled O9 McAb of 41.6 ng/ml were added at the same time. After incubation at 37°C for 2 h (Figure 7), all unbound materials were removed by washing with PBST six times. 100 μL of TMB chromogenic substrate was added to each well and incubated at 37°C for 3 min (Figure 8). After the color development was completed, 50 μL of 2 M H2SO4 was added to each well to terminate the color development, and the OD450nm absorption value was read.
Figure 2.
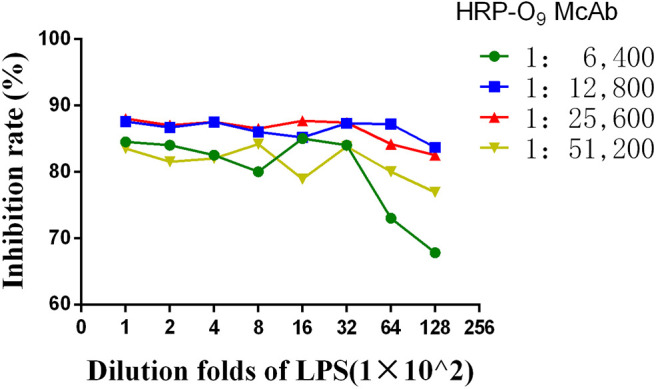
Curves of the HRP-labeled McAb binging to coated LPS.
Figure 3.
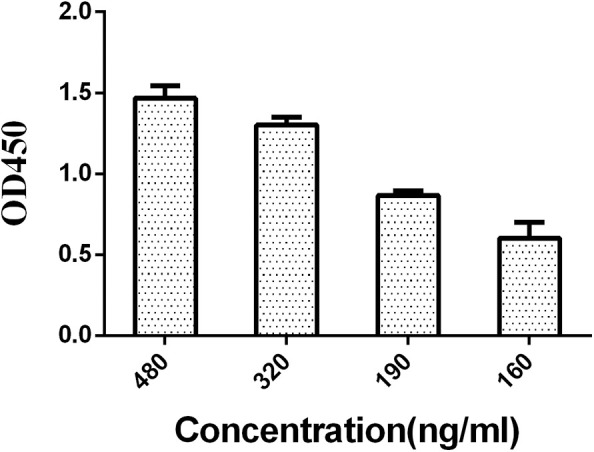
Concentration optimization of coated LPS in O9 Dc-ELISA.
Figure 4.
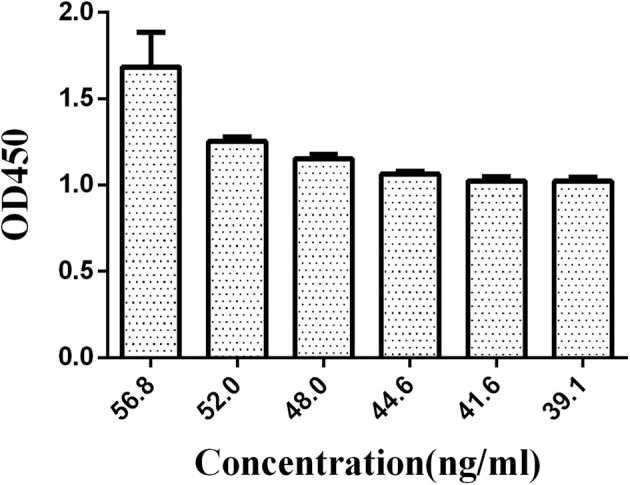
Concentration optimization of HRP-labeled O9 McAb in O9 Dc-ELISA.
Figure 5.
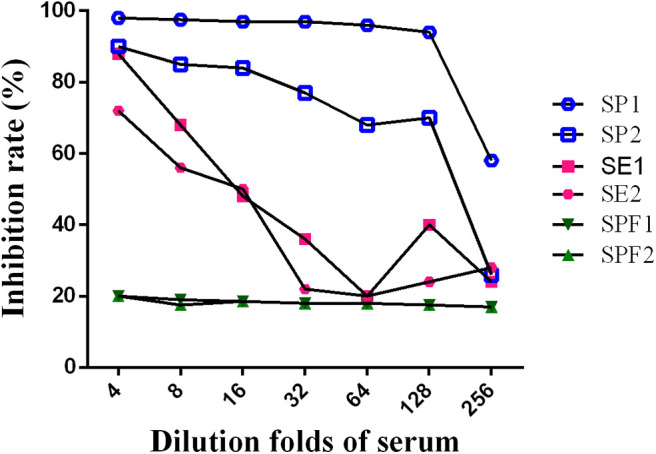
Optimization of positive serum dilution. (SE, SE2: serum by Salmonella enteritidis 1, 2; SP1, SP2: serum by Salmonella pullorum 1, 2; SPF1, SPF2: serum from SPF chickens).
Figure 6.
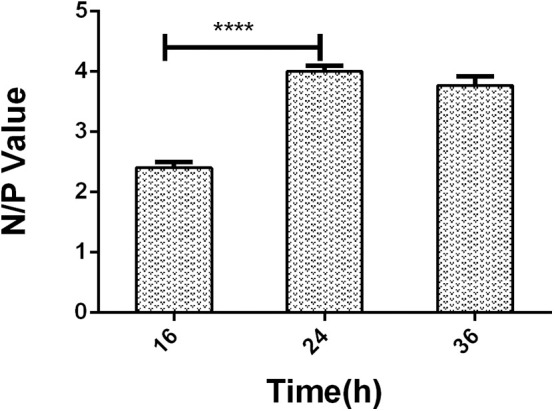
The negative/positive OD value of different LPS coating time in O9 Dc-ELISA. ****p < 0.0001.
Figure 7.
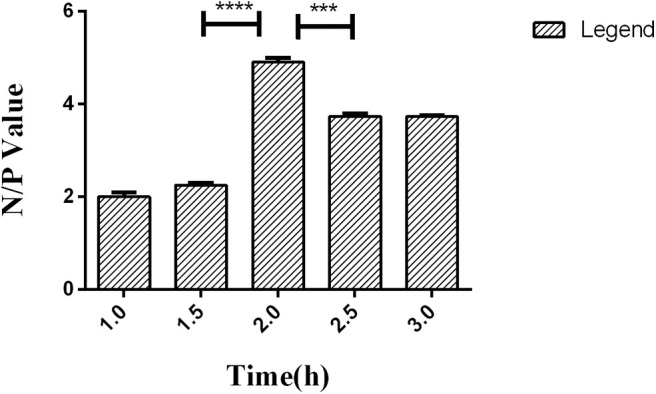
Time effect of HRP-labeled O9 McAb binding with coated LPS in O9 Dc-ELISA based on the negative/positive OD value. ***p < 0.001, ****p < 0.0001.
Figure 8.
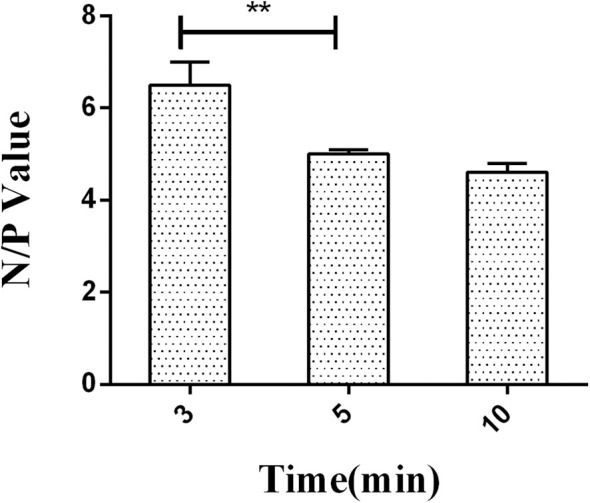
The negative/positive OD value of different reactive times of HRP labeled on O9 McAb with substrate TMB. **p < 0.01.
Specificity Analysis of O9 Dc-ELISA
We prepared the tested sera from chicken infected by Escherichia coli, Proteus mirabilis, non-O9 Salmonella [Salmonella typhimurium (O:4)], the negative sera from SPF chickens, and the positive sera from chickens infected with Salmonella pullorum and Salmonella enteritidis; the results showed that O9 Dc-ELISA could not check out the sera against non-Salmonella and non-O9 Salmonella. The value of negative sera was more than 1.0 whereas the OD value of positive sera was less than 0.25 as a control (Figure 9) based on P/N≥2.1.
Figure 9.
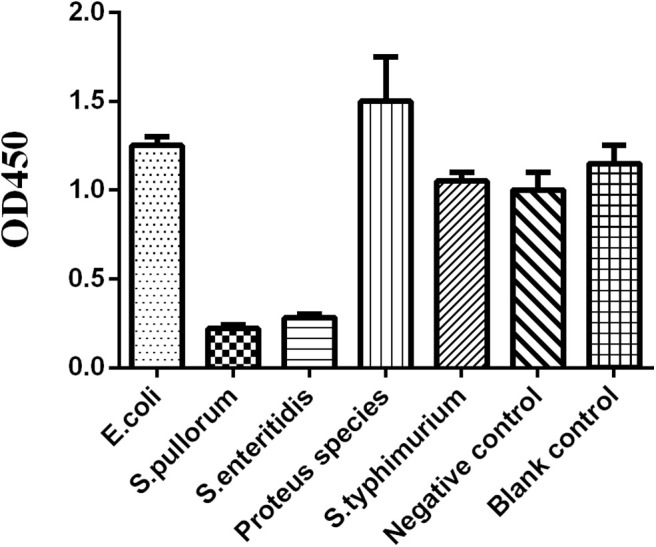
The OD value of chicken sera against different pathogens for specificity analysis.
Setting Up of the Cutoff Value
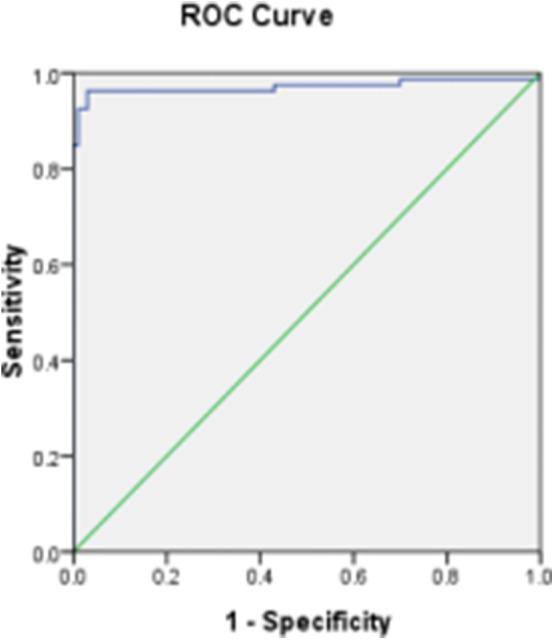
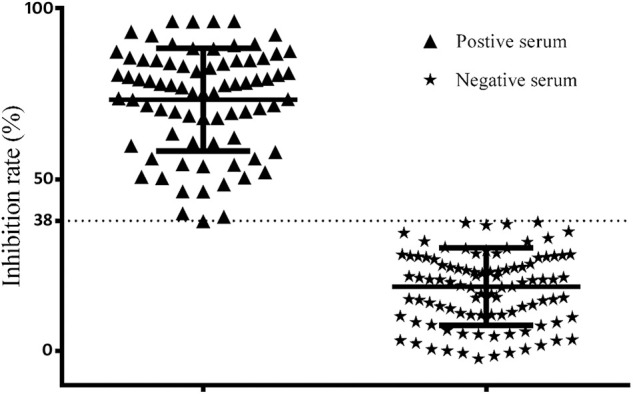
According to the results using the France ID.vet Salmonella kit as a reference of positive and negative chicken sera, and the percent inhibition (PI) values by O9 Dc-ELISA which were calculated using the formula PI (%) = (1−OD450 of test serum/OD450 of blank control) ×100%, the cutoff based on the ROC curve was 38% (Figure 10). Under PI of 38%, the specificity of O9 Dc-ELISA reached up to 99.7% and the sensitivity reached up to 96.2% in ROC. The distribution of 100 positive serum samples and the 100 negative serum samples determined by O9 Dc-ELISA showed that 38% of inhibiting rate was indeed a threshold which could distinguish positive serum and negative serum (Figure 11).
Figure 10.
The distribution of inhibiting rate by Dc-ELISA and setting up of the cut-off value.
Figure 11.
ROC analysis curve for competitive ELISA detecting chicken serum samples.
Comparison Among O9 Dc-ELISA, Three Commercial Kits, and PAT
To validate the test ability of O9 Dc-ELISA, we randomly collected 50 serum samples for comparison to the results using O9 Dc-ELISA, PAT, IDEXX ELISA kit, and ID.vet ELISA kit; the results revealed that their coincidence rates were 88% (44/50, Table 1), 98% (49/50, Table 2), and 98% (49/50, Table 3), respectively. Although four samples negative with PAT were positive with O9 Dc-ELISA and two commercial ELISA kits, and 1 sample negative with O9 Dc-ELISA, IDEXX, and ID.vet ELISA kit was positive with PAT, there was no statistical difference among 4 methods. The results showed that O9 Dc-ELISA could screen out more positive samples than the PAT method could and produced the same agreement rates with two commercial kits in terms of sensitivity in addition to strong specificity.
Table 1.
Comparison of the results between O9 Dc-ELISA and PAT.
| PAT | Total | |||
|---|---|---|---|---|
| + | - | |||
| O9 Dc-ELISA | + | 1 | 4 | 5 |
| - | 2 | 43 | 45 | |
| Total | 3 | 47 | 50 | |
Table 2.
Comparison of the results between O9 Dc-ELISA and IDEXX ELISA kit.
| IDEXX ELISA Kit | Total | |||
|---|---|---|---|---|
| + | - | |||
| O9 Dc-ELISA | + | 4 | 1 | 5 |
| - | 0 | 45 | 45 | |
| Total | 4 | 46 | 50 | |
Table 3.
Comparison of the results between O9 Dc-ELISA and ID.vet ELISA kit.
| ID.vet ELISA Kit | Total | |||
|---|---|---|---|---|
| + | - | |||
| O9 Dc-ELISA | + | 4 | 1 | 5 |
| - | 0 | 45 | 45 | |
| Total | 4 | 46 | 50 | |
Discussion
Salmonella enteritidis and Salmonella pullorum are two of the most important Salmonella spp. that threaten the poultry industry, and humans are infected by directly or indirectly eating contaminated water and food, which causes great hazard to human public health security (21, 22). In our study, a McAb-based competitive ELISA was established to detect O:9 Salmonella infection in chicken. In order to achieve a better reaction system, we explored various conditions, including concentration of LPS coating and HRP-labeled O9 McAb, serum dilution, LPS coating time, and reaction time of HRP-labeled McAb.
In order to confirm that the established O9 Dc-ELISA did not cause a cross-reaction, we used O9 Dc-ELISA to test Escherichia coli, Proteus mirabilis, Salmonella typhimurium (O:4), and negative sera from SPF chicken, Salmonella pullorum, and Salmonella enteritidis. The tests showed that only sera from Salmonella pullorum and Salmonella enteritidis could cause significant inhibition.
By testing 100 artificial positive samples and 100 negative serum samples from SPF chickens and 50 random clinical serum samples, the sensitivity and specificity at different thresholds were compared, and the final selected inhibition rate was 38% as the critical value of the competition ELISA kit. According to ROC, the specificity of O9 Dc-ELISA was 99.7%, and the sensitivity was 96.2%. This O9 Dc-ELISA was compared with PAT, IDEXX ELISA kit, and ID.vet ELISA kit, respectively. The results showed that the coincidence rate of the O9 Dc-ELISA kit and ID.vet ELISA kit was 98%; the coincidence rate with the Salmonella enteritidis test kit was 98%; and the coincidence rate with PAT was 88%. The above results indicated that this O9 Dc-ELISA has a good detection effect on the O9 antibody and had better performance than the PAT method based on more positive samples being checked out and the same agreement rates with commercial kits in terms of sensitivity in addition to strong specificity in the detection of clinical samples. This kit offered a good base as a first-generation product; it will be further evaluated and optimized according to clinical detection performance based on more serum samples to develop a second-generation kit in the future.
Conclusion
O9 Dc-ELISA has good ability in O9 antibody detection and had better performance than the PAT method and agreement rates with commercial kits in terms of sensitivity during the detection of clinical chicken serum samples. It must play an important role in O:9 Salmonella detection for Salmonella clearance in China in the future.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by the Animal Welfare and Ethics Committees of Yangzhou University.
Author Contributions
SG, XJ, and HG designed the paper. HG, ZX, and DS performed the experiments. YC, JZ, and YW provided help during experiments. ZP and XJ made critical revisions to the paper and contributed to paper writing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the National Key Research and Development Program Special Project (2016YFD0501607), the Special Fund for Agroscientific Research in the Public Interest (201403054), and the Natural Science Foundation of Jiangsu Province of China (BK20151306).
References
- 1.Fei X, He X, Guo R, Yin C, Geng H, Wu K, et al. Analysis of prevalence and CRISPR typing reveals persistent antimicrobial-resistant Salmonella infection across chicken breeder farm production stages. Food Control. (2017) 77:102–9. 10.1016/j.foodcont.2017.01.023 [DOI] [Google Scholar]
- 2.Geimba MP, Tondo EC, de Oliveira, Canal CW, Brandelli A. Serological characterization and prevalence of spvR genes in Salmonella isolated from foods involved in outbreaks in Brazil. J Food Prot. (2004) 67:1229–33. 10.4315/0362-028X-67.6.1229 [DOI] [PubMed] [Google Scholar]
- 3.Meemken D, Tangemann AH, Meermeier D, Gundlach S, Mischok D, Greiner M, et al. Establishment of serological herd profiles for zoonoses and production diseases in pigs by “meat juice multi-serology”. Prev Vet Med. (2014) 113.4: 589–98. 10.1016/j.prevetmed.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 4.Vo TH, Le NH, Cao TT, Nuorti JP, Minh NN. An outbreak of food-borne salmonellosis linked to a bread takeaway shop in Ben Tre City Vietnam. Int J Infect Dis. (2014) 26:128–31. 10.1016/j.ijid.2014.05.023 [DOI] [PubMed] [Google Scholar]
- 5.Schrader KN, Fernandez-Castro A, Cheung WKW, Crandall CM, Abbott SL. Evaluation of commercial Antisera for Salmonella serotyping. J Clin Microbiol. (2008) 46:685–8. 10.1128/JCM.01808-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendriksen RS, Mikoleit M, Carlson VP, Karlsmose S, Vieira AR, Jensen AB, et al. WHO Global Salm-Surv external quality assurance system for serotyping of Salmonella isolates from 2000 to 2007. J Clin Microbiol. (2009) 47:2729–36. 10.1128/JCM.02437-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoelzer K, Moreno Switt Al, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. (2011) 42:34. 10.1186/1297-9716-42-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mather AE, Reid SW, Maskell DJ, Parkhill J, Fookes MC, Harris SR, et al. Distinguishable epidemics within different hosts of the multidrug-resistant zoonotic pathogen Salmonella Typhimurium DT104. Science. (2013) 341:1514. 10.1126/science.1240578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herikstad H, Motarjemi Y, Tauxe RV. Salmonella surveillance: a global survey of public health serotyping. Epidemiol Infect. (2002) 129:1–8. 10.1017/S0950268802006842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh V. Salmonella serovars and their host specificity. J Vet Sci & Anim Husb. (2013) 1:301 10.15744/2348-9790.1.301 [DOI] [Google Scholar]
- 11.Gong J, Zhang J, Xu M, Zhu C, Yu Y, Liu X, et al. Prevalence and fimbrial genotype distribution of poultry Salmonella isolates in China (2006 to 2012). Appl Environ Microbiol. (2014) 80:687–93. 10.1128/AEM.03223-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko HJ, Yang JY, Shim DH, Yang H, Park SM, Curtiss R, III, et al. Innate immunity mediated by MyD88 signal is not essential for induction of lipopolysaccharide-specific B cell responses but is indispensable for protection against Salmonella enterica serovar Typhimurium infection. J Immunol. (2009) 182:2305–12. 10.4049/jimmunol.0801980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronholm J, Zhang Z, Cao X, Lin M. Monoclonal antibodies to lipopolysaccharide antigens of Salmonella enterica serotype Typhimurium DT104. Hybridoma. (2011) 30:43–52. 10.1089/hyb.2010.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eguchi M, Kikuchi Y. Binding of Salmonella-specific antibody facilitates specific T cell responses via augmentation of bacterial uptake and induction of apoptosis in macrophages. J Infect Dis. (2010) 201:62–70. 10.1086/648615 [DOI] [PubMed] [Google Scholar]
- 15.Wahlström H, Sternberg Lewerin S, Sundström K, Ivarsson S. Estimation of the expected change in domestic human Salmonella cases in Sweden in 2010, given a hypothetical relaxation of the current Salmonella control programme. PLoS ONE. (2014) 9:e89833. 10.1371/journal.pone.0089833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wattiau P, Boland C, Bertrand S. Methodologies for Salmonella enterica subsp. enterica subtyping: gold standards and alternatives. Appl Environ Microbiol. (2011) 77:7877–88. 10.1128/AEM.05527-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang YH, Lee SJ, Lim JG, Lee HS, Kim TJ, Park JH, et al. The rate of Salmonella spp. infection in zoo animals at Seoul Grand Park Korea. J Vet Sci. (2008) 9:177–81. 10.4142/jvs.2008.9.2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westphal O, Jann JK. Bacterial lipopolysaccharide extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. (1965) 5:83–92. [Google Scholar]
- 19.Fearnley E, Raupach J, Lagala F, Cameron S. Salmonella in chicken meat, eggs and humans; Adelaide, South Australia, 2008. Int J Food Microbiol. (2011) 146:219–27. 10.1016/j.ijfoodmicro.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 20.Almeida C, Cerqueira L, Azevedo NF, Vieira MJ. Detection of Salmonella enterica serovar enteritidis using real time PCR, immunocapture assay, PNA FISH and standard culture methods in different types of food samples. Int J Food Microbiol. (2013) 161:16–22. 10.1016/j.ijfoodmicro.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 21.Saeed AFUH, Ling S, Yuan J, Wang S. The Preparation and identification of a monoclonal antibody against domoic acid and establishment of detection by indirect competitive ELISA. Toxins (Basel). (2017) 9:250 10.3390/toxins9080250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YS, Zhou Y, Meng XY, Zhang YY, Liu JQ, Zhang Y, et al. Enzyme-antibody dual labeled gold nanoparticles probe for ultrasensitive detection of κ-casein in bovine milk samples. Biosens Bioelectron. (2014) 61:241–4. 10.1016/j.bios.2014.05.032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.


