Abstract
Purpose:
To report the clinical presentation, microbiology, and management outcome of endogenous endophthalmitis in Indian patients.
Methods:
Retrospective chart review of culture-positive (vitreous/urine/blood) endogenous endophthalmitis cases treated in tertiary eye care facility in India was done.
Results:
The study included 173 eyes of 117 patients. Mean patient age was 25.41 ± 20.46 years (median 24 years). Pre-disposing systemic illness could not be elicited in 79 (67.63%) patients. Commonest predisposing systemic condition in patients where it could be detected (n = 79) was pyrexia of unknown origin (25/79 = 32.0%). Following treatment, 45 out of 173 (26.0%) eyes regained vision of ≥20/400. Commonest isolated organism from vitreous was Streptococcus pneumoniae (36 eyes, 20.8%) and fungi were isolated in 24 (13.8%) eyes, the commonest being Candida spp. (8/24, 33.33%). Favorable functional outcome was seen in 26% eyes and favorable anatomic outcome in 43% eyes. Those with an underlying systemic illness were older (P = 0.02), had greater urine culture positivity (P = 0.003), lesser vitreous culture positivity (0.001), greater gram negative etiology (P = 0.0006), and greater fungal etiology (P = 0.01) as compared to those cases without underlying systemic illness.
Conclusion:
Endogenous endophthalmitis in India often presents in young immunocompetent individuals without any underlying systemic illness and with negative blood or urine microbiologic work up. Underlying systemic illness leads to greater gram-negative and fungal etiology. Overall visual outcome is poor inspite of prompt management.
Keywords: Endogenous, endophthalmitis, outcomes
Endogenous endophthalmitis (EE) is caused by a hematogenous spread of infection from a systemic focus to the eye. It accounts for 2-15% of all endophthalmitis cases reported in the world.[1,2,3] Etiology of EE is multifactorial. Both bacteria and fungi are implicated.[1,4] While the gram positive cocci (Streptococcus spp., Staphylococcus spp.) are more often reported from the West, gram-negative bacteria (Klebsiella spp.) are more often reported in the South-east Asia.[5,6,7,8,9,10,11,12,13,14] The largest series reported from India is description of 61 cases.[15] In this study we report 117 cases of endogenous endophthalmitis, currently the largest from India and compare the data with similar large series from different parts of the world.
Methods
Case records of all cases with endogenous endophthalmitis from January 2006 to July 2018 were identified by the institute medical record system and the microbiology laboratory records. All cases presenting with spontaneous onset redness, lid edema, hypopyon, decreased vision, and vitreous exudates were clinically defined as endogenous endophthalmitis. Of these, culture-positive cases were included in the study. Cases that did not fit into the above inclusion criteria were included. Institutional Review Board approval for the study was taken. Details of history, clinical examination, clinical features at presentation, microbiological evaluation including antibiotic susceptibility report and clinical response to therapy were obtained from the chart review. The essential clinical findings included presenting and final best corrected visual acuity, status of anterior segment, presence/absence of hypopyon, extent of fundal glow and status of the retinal vessels, if visible. Whenever the fundus was not visible by the binocular indirect ophthalmoscope using the highest illumination, B-scan ultrasonography was done to determine the extent and location of vitreous involvement and other posterior segment diseases, such as retinal detachment, choroidal thickening, or choroidal detachment. All patients received a detailed medical examination by an internist and a detailed laboratory work up. The work up included a complete blood picture with differential count, erythrocyte sedimentation rate, blood culture, urine microscopy with culture and a comprehensive clinical examination in all cases. Depending upon the history and clinical examination leads, specific investigations like X-ray chest, abdominal ultrasound and computerized tomography were done in selected patients.
Intervention
As per the institute protocol, the surgical management of endophthalmitis consisted of pars plana vitrectomy, direct microscopy and culture of undiluted vitreous, antimicrobial susceptibility testing of bacterial isolates, intravitreal antibiotics (vancomycin, 1 mg/0.1 ml + ceftazidime, 2.25 mg/0.1 ml) with or without dexamethasone (400 μg/0.1 ml). The medical treatment also included intensive topical antibiotics (ciprofloxacin 0.3% one hourly) and corticosteroid (prednisolone acetate 1% one hourly) and oral ciprofloxacin (750 mg two times per day) for 7-10 days. Additional procedures such as repeat intravitreal antibiotics or repeat pars plana vitreous lavage depended on the response to treatment and were left to the decision of the treating physicians. In cases with hazy view due to corneal involvement, a vitreous biopsy was performed instead of a vitrectomy procedure.
Surgical technique
Undiluted vitreous was collected at the beginning of the surgery in all cases, using a vitreous cutter. Topical 5% povidone iodine was used to prepare the eye before surgery and then instilled in the cul-de-sac in all cases at the end of surgery. A standard 3-port technique was used employing a vacuum setting of 200-300 mm Hg and a cutter setting of 3000-5000 cps. The cutter was connected to a 2 cc plastic disposable syringe. As the cutter-aspiration was started by the surgeon, simultaneously, the assistant aspirated the vitreous into the 2 cc syringe. Further handling and processing of the sample and the final interpretation was done as per the institute protocol.[7,8] Vitrectomy was done within 24 hours of presentation, either using a 20 G or 23/25 G system. In the former cases, the conjunctiva and scleral incisions were sutured with 7-0 polyglactin sutures.
Microbiological processing of vitreous, urine and blood
The microbiological processing of vitreous sample included direct microscopy by 0.1% Calcofluor white stain, Gram stain and Giemsa stain. Culture of the vitreous sample was done on 5% sheep blood chocolate agar, 5% sheep blood agar, brain heart infusion broth, thioglycollate broth, Robertson's cooked meat broth, Sabouraud dextrose agar (SDA) and potato dextrose agar (PDA). All media were incubated at 37°C for one week except SDA and PDA that were incubated at 27°C for two weeks. Blood sample (5-10 ml) was aseptically collected and inoculated in blood culture bottle (Hi Media, Mumbai, India) and incubated at 37°C for one week. Growth was checked by subculture on blood agar. Measured (1 μl) volume of mid-stream aseptically collected urine was inoculated on blood and MacConkey's agar (within one hour of collection) and incubated at 37°C for 48 hours. Growth of more than 105 cfu/ml was considered significant.
Bacterial and yeast isolates were identified by a combination of conventional microbiological and automated methods such as Vitek 2 compact system, (bioMérieux, France). The bacterial isolates were tested for susceptibility to various antibiotics using the Kirby–Bauer Disc-diffusion method on Mueller Hinton blood agar. The filamentous fungi were identified by culture characteristics and type of sporulation.
Outcome definition
The outcome at the last visit was considered for final analysis. A favorable anatomic outcome was defined as preservation of the globe, absence of hypotony (intraocular pressure ≥5 mm Hg), attached retina and absence of active inflammation. A favorable functional outcome was defined as an attached retina with a best-corrected visual acuity of ≥20/400.
Statistical analysis
The data were arranged on an Excel spread sheet and analyzed using the statistical software MedCalcver 12.2.1.0 (Ostend, Belgium). Percentage confidence intervals were calculated using online statistical calculators (https://www.allto.co.uk/tools/statistic-calculators). Odds ratio with appropriate confidence intervals was computed for possible risk variables. A P value < 0.05 was considered statistically significant. Comparison of continuous non-dependent variable with a categorical dependent variable was done using logistic regression. The functional outcome was the dependent factor for the logistic regression.
Results
The study included 173 consecutive eyes of 117 patients (male 55%; female 45%) with culture-proven EE. The mean age at presentation was 25.41 ± 20.46 years (median 24 years). The interval between the start of symptoms and the inciting event was a mean of 10.12 ± 8.8 days (median: 4 days; range: 1 to 24 days). The interval between the start of symptoms and presentation to the clinic was a mean of 13.61 ± 30.2 days (median: 7 days; range 1-90 days). The mean follow up was 20.35 ± 30.5 months (median: 7; range 2 to 131). There were 8 (4.62%) eyes with concurrent corneal infiltrates. In only 4 (2.31%) eyes, the presenting vision was ≥20/400. Seventy-six (43.9%) eyes registered anatomical success and 46 (26%) eyes registered functional success in this series. The demographic and clinical features of the cases are summarized in Table 1. Nineteen patients were diabetic at presentation but with well-controlled blood sugars (mean fasting blood sugar of 115 mg%; range 90 mg% to 183 mg%, and HbA1c of 6.8; range 6.1 to 7.2 mg %).
Table 1.
Demographic and clinical features of the cases in this series
| Total eyes included | n=173 |
|---|---|
| Age | Mean+SD: 25.41±20.46 y; Median: 24 y; Range: 0.1 to 87 y |
| Interval between inciting event and start of symptoms | Mean: 10.12±8.8 d; Median: 4 d; Range: 1 to 24 days |
| Interval between the start of symptoms and presentation to the clinic | Mean+SD: 13.61±30.2 d; Median: 7 d; Range 1-90 d |
| Cases with concurrent corneal infiltrates | n=8 (4.62%) |
| Favorable vision at presentation (>20/400) | n=4 (2.31%) |
| Favorable vision at last follow up | n=45 (26%) |
| Anatomic success at last follow up | n=76 (43.93%) |
| Mean follow up in months | + SD: Mean 20.35±30.51 m; Median 7 months |
d=Days, m=Months, y=Years
Microbiology
The broad distribution of infecting organisms was gram-positive bacteria 48%; gram-negative bacteria 37%; fungi 15%. The commonest organisms in these groups were Streptococcus pneumoniae (36 eyes, 20.8% of total; 44.5% of gram-positive cocci group) Pseudomonas aeruginosa (15 eyes, 8.67% of total; 23.4% of gram-negative bacilli group), and Candida spp. (8 eyes, 4.62% of total). The various organisms isolated are summarized in Table 2. The internist examination could not reveal an identifiable focus of infection in 117 (67.63%) patients. The foci of infection in the remaining 56 patients were: pyrexia of unknown origin (10.4%), post solid organ surgery (2.9%), complicated abortion (2.4%), and cellulitis (2.4%) [Table 3]. In gram-positive bacterial cases, antimicrobial susceptibility pattern was chloramphenicol (98.48%), vancomycin (98.38%) >ofloxacin (94.11%) > moxifloxacin (94%) and ciprofloxacin (91.52%). In gram-negative organisms, the antimicrobial susceptibility pattern was imipenem (86.95%) > ciprofloxacin (79.36%) >ofloxacin (78.12%) >amikacin (60%), and ceftazidime (54.38%) [Table 4].
Table 2.
Table showing the various micro -organisms isolated and cultured
| Organism | n (%) of total | |
|---|---|---|
| Gram-positive organism (n=83) | Streptococcus pneumonia | 37 (44.57%) |
| Streptococcus spp | 9 (10.84%) | |
| Staphylococcus epidermidis | 8 (9.63%) | |
| Staphylococcus spp | 7 (8.43%) | |
| Staphylococcus aureus | 6 (7.22%) | |
| Corynebacterium spp | 4 (4.81%) | |
| Unidentified gram-positive bacteria | 4 (4.81%) | |
| Bacillus spp | 3 (3.61%) | |
| Enterococcus spp | 2 (2.4%) | |
| Micrococcus spp | 1 (1.2%) | |
| Enterococcus fecalis | 1 (1.2%) | |
| Kocuria spp | 1 (1.2%) | |
| Gram negative organisms (n=64) | Pseudomonas aeruginosa | 15 (23.43%) |
| Escherichia coli | 8 (12.5%) | |
| Hemophilus influenzae | 7 (10.93%) | |
| Klebsiella pneumoniae | 6 (9.37%) | |
| Non-fermenting gram-negative bacilli | 6 (9.37%) | |
| Stenotrophomonas maltophilia | 5 (7.81%) | |
| Burkholderia cepacia | 3 (4.68%) | |
| Enterobacter spp | 3 (4.68%) | |
| Vibrio spp | 2 (3.13%) | |
| Alcaligenes spp | 1 (1.56%) | |
| Klebsiella spp | 1 (1.56%) | |
| Rhizobium spp | 1 (1.56%) | |
| Myroides spp | 1 (1.56%) | |
| Pantoea spp | 1 (1.56%) | |
| Aeromonas spp | 1 (1.56%) | |
| Salmonella spp | 1 (1.56%) | |
| Acenitobacter spp | 1 (1.56%) | |
| Citrobacter spp | 1 (1.56%) | |
| Fungi (n=24) | Candida spp | 8 (33.33%) |
| Aspergillus spp | 7 (29.16%) | |
| Fusarium spp | 3 (12.5%) | |
| Unidentified hyaline fungus | 2 (8.33%) | |
| Scedosporum spp | 1 (4.16%) | |
| Lasiodiplodia spp | 1 (4.16%) | |
| Curvularia spp | 1 (4.16%) | |
| Stephanoascus ciferrii | 1 (4.16%) | |
| Actinomycetes (n=2) | Nocardia | 2 (100%) |
Table 3.
Summary of various systemic foci of infection determined in this series
| Predisposing illness | n (%) |
|---|---|
| None | 117 (67.63) |
| Medical conditions | |
| Thrombocytosis | 4 (2.3) |
| HIV | 3 (1.7) |
| Multiorgan failure | 1 (0.6) |
| Liver cirrhosis | 2 (1.2) |
| Typhoid fever | 3 (1.7) |
| Dialysis | 1 (0.6) |
| Urinary tract infection | 2 (1.2) |
| Pyrexia of unknown origin | 18 (10.4) |
| Intravenous fluid injection alone | 7 (4) |
| Cellulitis | 4 (2.4) |
| Surgical conditions | |
| Post solid organ surgery | 5 (2.9) |
| Post-organ transplantation | 2 (1.2) |
| Complicated abortion | 4 (2.4) |
Table 4.
Antimicrobial susceptibilities of various organisms tested
| Antibiotic | Isolates tested | Susceptibility | |
|---|---|---|---|
| Gram-positive organisms (n=83) | Amikacin | 58 | 50% |
| Cefazolin | 68 | 67.64% | |
| Chloramphenicol | 66 | 98.48% | |
| Ciprofloxacin | 59 | 91.52% | |
| Gatifloxacin | 62 | 93.54% | |
| Gentamicin | 22 | 77.27% | |
| Moxifloxacin | 50 | 94% | |
| Ofloxacin | 68 | 94.11% | |
| Vancomycin | 62 | 98.38% | |
| Gram-negative organisms (n=64) | Amikacin | 60 | 60% |
| Ceftazidime | 57 | 54.38% | |
| Chloramphenicol | 64 | 73.43% | |
| Ciprofloxacin | 63 | 79.36% | |
| Gatifloxacin | 61 | 77.04% | |
| Imipenem | 23 | 86.95% | |
| Ofloxacin | 64 | 78.12% | |
| Moxifloxacin | 49 | 73.46% |
Primary intervention
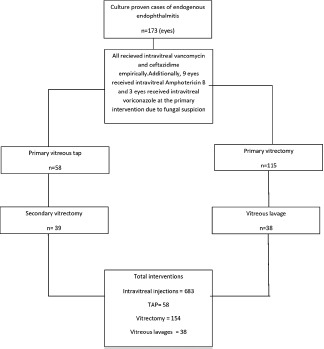
Primary vitreous tap alone was done in 58 eyes and 39 eyes underwent a pars plana vitrectomy at a later date. Primary vitrectomy was done in 115 eyes. All eyes received intravitreal vancomycin with ceftazidime in the primary intervention. Additionally, 9 eyes received intravitreal amphotericin B and 3 eyes received intravitreal voriconazole at the primary intervention due to high suspicion of fungal infection.
Secondary intervention
The treating physician decided the type and timing of secondary intervention. There were 620 repeat interventions—543 repeat intravitreal injection and 77 repeat vitreous surgery (lavage/vitrectomy). This accounted to mean of 3.6 repeat intervention—mean 3.1 repeat intravitreal injection and mean 0.4 repeat vitreous surgery. The intravitreal injection was repeated more often in bacterial infection (482 times in 149 bacterial infection eyes = 3.2 times; 61 times in 24 fungal infection eyes = 2.5 times) and vitreous surgery was more often repeated in fungal infection (49 times in 24 fungal infection eyes = 2.0 times; 28 times in 149 bacterial infection eyes = 0.2 times). The intravitreal injections in fungal infection were repeated every 48 hours for amphotericin B and every 24 hours for voriconazole as decided by the treating physicians.[12,13] The mean time interval between presentation and the first intravitreal antifungal antibiotic injection was 9.27 ± 9.03 days (Median 7 days). The cumulated intervention in these 173 eyes included 683 intravitreal injections, 58 taps, 154 vitrectomies, and 38 vitreous lavages [Chart 1] mean 3.94 intravitreal injections/eye, and mean 1.44 other surgical procedures/eye.
Chart 1.
Summary of management
Outcome
Overall, a favorable anatomic outcome was seen in 76 eyes (43.93%) and a favorable functional outcome was seen in 45 eyes (26.01%). Multiple factors were assessed to look for those that affected the final outcome but only the type of intervention done primarily had a bearing on the final functional outcome. The odds of a favorable functional outcome when primary PPV was done instead of primary tap were 2.28 (95% Confidence Interval, CI: 1.06 to 4.88, P = 0.03). Adverse outcomes seen at the last visit were phthisis bulbi in 66 (38.2%) eyes, recurrent retinal detachment in 19 (10.9%) eyes and hypotony in 12 (6.9%) eyes. Overall, 50 (29%) eyes had no perception of light, 41 (23.7%) eyes had perception to hand motions vision, 45 (52%) eyes had ≥20/400 to 20/40 vision, and 19 (11%) eyes had ≥20/40 vision at the last examination visit. Certain differences were noted when cases with no underlying systemic illness were compared with those having underlying systemic illness [Table 5]. Lens status was as follows: 41% clear, 42% cataractous, 5% aphakic, 12% pseudophakic. Those with an underlying systemic illness were older (P = 0.02), had greater urine culture positivity (P = 0.003), lesser vitreous culture positivity (0.001), greater gram negative etiology (P = 0.0006), and greater fungal etiology (P = 0.01) as compared to those cases without underlying systemic illness.
Table 5.
Comparison of presentations and results of cases with and without underlying systemic illness
| No systemic illness | Underlying systemic illness | P | 95% C.I. | |
|---|---|---|---|---|
| Number of eyes | 117 | 56 | ||
| Male gender (%) | 64 (54.7%) | 32 (57.1%) | 0.76 | |
| Mean age (years) | 23.15±19.84 Median 20 | 30.14±24.09 Median 29 | 0.02 | 1-14 |
| Blood culture positivity (%) | 0 | 1 (1.78%) | 0.14 | |
| Urine culture positivity (%) | 3 (2.56%) | 8 (14.28%) | 0.003 | 3.4%-23.29% |
| Vitreous culture positivity (%) | 114 (97.43%) | 47 (83.92%) | 0.001 | 4.75%-25.36% |
| Gram-negative etiology (%) | 33 (28.2%) | 31 (55.35%) | 0.0006 | 11.52%-41.44% |
| Fungal etiology (%) | 11 (9.4%) | 13 (23.21%) | 0.01 | 2.52%-27% |
| Vitrectomy done (%) | 78 (67.2%) | 36 (64.3%) | 0.7 | |
| Favorable functional outcome (%) | 27 (23.07%) | 18 (32.14) | 0.2 | |
| Favorable anatomic outcome (%) | 54 (46.15%) | 22 (39.28%) | 0.39 | |
| Rate of evisceration or enucleation (%) | 2 (1.7%) | 7 (12.5%) | 0.002 | 3.16%-21.99% |
Discussion
The current communication describes the presentation, microbiology, management and outcome of culture-proven endogenous endophthalmitis patients treated at our institute. We compared this data with other recently reported largest series from south-east Asia[14] (n = 143), from India[15] (n = 61), and from the USA[16] (n = 34) [Table 6]. The following were the salient observations in the current series compared to other series:[14,15,16] (1) poor detection of an underlying systemic illness (32.3% in this series vs 75%, 53.4%, 97% respectively reported elsewhere[14,15,16]), (2) younger age at presentation (mean 25.4 years in this series vs 52.6 years, 34.6 years and 63.3 years respectively[14,15,16]), (3) lesser systemic symptoms (23.7% in this series vs 70%, 37.9% and 67.6% respectively[14,15,16]), (4) poor blood culture positivity (0.57% in this series vs 42%, 5.8% and 33.3% respectively[14,15,16]), (5) lesser urine culture positivity (6.3% in this series vs 41.3%, 11.6% and 25.9% respectively,[14,15,16] (6) high vitreous culture positivity (93.06% in this series vs 22.3%, 47.05% and 70.58% respectively[14,15,16]), (7) lower fungal infection (15% in this series vs 19.5% and 41.17% respectively[14,15,16]), and (8) higher rate of vitreous surgery (89% in this series vs 51.4%, 62.3% and 61.7% respectively[14,15,16]).
Table 6.
Comparison of features of EE in the current series with previous large literature around the world
| Current series | Muda R et al. (Malaysia/South-east Asia) | P and 95% C.I. | Ratra et al. (India/Asia) | P and 95% C.I. | Binder et al. (USA/North America) | P and 95% C.I. | |
|---|---|---|---|---|---|---|---|
| n | 173 | 143 | 61 | 34 | |||
| Males (%) | 96 (55.5) | 59 (49.2) | 0.26 | 36 (62.1) | 0.37 | 19 (55.5) | 0.98 |
| Mean age (years) | 25.41±20.46 | 52.6±15.1 | <0.0001, −31.24-−23.14 | 34.6±14.9 | 0.002, −14.81-−3.56 | 63.3 | - |
| Identification of primary source of infection (%) | 56 (32.37) | 90 (75) | <0.0001, 32.04%- 51.75% | 31 (53.4) | 0.003, 6.69%- 34.64% | 33 (97) | <0.0001. 50.66%- 71.67% |
| Systemic symptoms identified (%) | 41 (23.7) | 84 (70) | <0.0001 35.79%- 55.27% | 22 (37.9) | 0.03, 1.14%- 27.99% | 23 (67.64) | <0.0001, 25.78%- 58.34% |
| Complaints of blurry vision (%) | 161 (93) | 106 (74) | <0.0001, 10.89%- 27.23% | 60 (98.4) | 0.11 | 31 (92.6) | 0.93 |
| Blood culture positivity (%) | 1 (0.57) | 50 (42) | <0.0001, 33.22%- 49.63% | 2 (5.88) | 0.02, 0.3%- 18.52% | 9 (33.33) | <0.0001, 19.08%- 49.57% |
| Urine culture positivity (%) | 11 (6.35) | 19 (41.3) | <0.0001, 25.91%- 43.6% | 4 (11.6) | 0.88 | 7 (25.9) | 0.001, 5.97%- 38.5% |
| Vitreous culture positivity (%) | 161 (93.06) | 27 (22.3) | <0.0001, 61.79%- 77.42% | 16 (47.05) | <0.0001, 29.11%- 69.88% | 24 (70.58) | 0.0001, 9.01%- 39.49% |
| Gram-negative etiology (%) | 64 (37) | 66 (80.8) | <0.0001, 33.11%- 52.36% | 20 (58.82) | 0.01, 3.64%- 38.13% | 4 (11.76) | 0.004, 8.88%- 35.49% |
| Fungal etiology (%) | 24 (15) | 16 (19.5) | 0.3 | 5 (14.7) | 0.96 | 14 (41.17) | 0.0004, 10.16%- 43.38% |
| Vitrectomy done (%) | 154 (89) | 73 (51.4) | <0.0001, 27.83%- 46.58% | 38 (62.3) | <0.0001, 14.31%- 39.82% | 21 (61.7) | 0.0001, 14.31%- 39.82% |
| Favorable functional outcome (%) | 45 (26.01) | 100 (73) | <0.0001, 28.71%- 48.76% | 18 (29.5) | 0.59 | 12 (35.29) | 0.26 |
| Favorable anatomic outcome (%) | 76 (43.93) | No information | - | 33 (54.09) | 0.17 | ||
| Rate of evisceration or enucleation (%) | 9 (5.2) | 6 (4.19) | 0.67 | 12 (19.7) | 0.0007, 5.33%- 26.39% | 4 (11.76) | 0.15 |
The important features in EE are systemic factors, age, tissue fluid (blood and urine) culture, and the infecting organism. Systemic illness preceding and probably leading to endogenous endophthalmitis have been reported from various regions of the world. It includes indwelling urinary or intravenous catheters or systemic immunosuppression reported from the West,[2,4,17] and uncontrolled diabetes mellitus and hepato-biliary diseases reported from the South-east Asia.[18,19,20] While there are reports of endogenous endophthalmitis in neonates,[21,22] most patients in the world present in the fifth-sixth decade of life.[17,18,19,20] Endogenous endophthalmitis secondary to Niesseria spp. reported in younger health patients could be an exception.[2,23,24]
In all probability debilitating conditions like chronic immune-compromising illnesses (diabetes mellitus, renal failure), indwelling or long-term intravenous catheters, immunosuppressive diseases and therapy (malignancies, human immunodeficiency virus infection or HIV, chemotherapeutic agents), recent invasive surgery, endocarditis, gastrointestinal procedures, hepatobiliary tract infections, and intravenous drug abuse account for higher fungus and gram-negative infections. That the patients in this series were younger, immunocompetent, and without detectable systemic illness despite extensive work up explains more of bacterial infection and gram-positive bacterial infection. Even then, the visual outcome in the current study was poor. We suspect it was related to relatively more virulent infection in each group of microorganism—Streptococcus spp. in gram positive bacteria,[23,24,25,26] Pseudomonas spp. in gram negative bacteria[27], and Aspergillus spp in fungi.[28] The current study is in-sync with previous reports suggesting the possibility of endogenous endophthalmitis in otherwise healthy non-immunocompromised patients.[29,30] The proposed hypothesis is a possible usage of contaminated intravenous infusion set for a past illness which the patient may not necessarily elicit a history for. Such infusions especially when administered in indigenous rural settings were potential risk factors as per the reports. As these patients are largely not systemically sick, this could be a possible reason for low pathogens in the systemic circulation and resultant negative culture. Higher rate of vitreous surgery noted, may be dependent on surgeon's preference as few surgeons may be aggressive in the choice of modality. In view of this being a retrospective study this limitation may lead to analysis bias, since the treating doctor was variable.
Conclusion
In conclusion, endogenous endophthalmitis in the Indian subcontinent presents a unique clinicodemographic profile. Most patients are young and immunocompetent without any underlying systemic focus of infection. Blood and urine culture could be negative and vitreous is the commonest sample to test positive for the infectious agent. The anatomic and visual outcomes are poor though an early treatment, particularly early vitrectomy is more useful.
Financial support and sponsorship
This research was funded by the Hyderabad Eye Research Foundation, Hyderabad.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Connell PP, O’Neill EC, Fabinyi D, Islam FM, Buttery R, McCombe M, et al. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye. 2011;25:66–72. doi: 10.1038/eye.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okada AA, Johnson RP, Liles WC, D’Amico DJ, Baker AS. Endogenous bacterial endophthalmitis: Report of a ten-year retrospective study. Ophthalmology. 1994;101:832–8. [PubMed] [Google Scholar]
- 3.Chee SP, Jap A. Endogenous endophthalmitis. Curr Opin Ophthalmol. 2001;12:464–70. doi: 10.1097/00055735-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Schiedler V, Scott IU, Flynn HW, Jr, Davis JL, Benz MS, Miller D. Culture-proven endogenous endophthalmitis: Clinical features and visual acuity outcomes. Am J Ophthalmol. 2004;137:725–31. doi: 10.1016/j.ajo.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: A 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48:403–23. doi: 10.1016/s0039-6257(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 6.Tsai AS, Lee SY, Jap AH. An unusual case of recurrent endogenous Klebsiella endophthalmitis. Eye. 2010;24:1630–1. doi: 10.1038/eye.2010.95. [DOI] [PubMed] [Google Scholar]
- 7.Das T, Choudhury K, Sharma S, Jalali S, Nuthethi R the Endophthalmitis Research Group. Clinical profile and outcome of Bacillus endophthalmitis. Ophthalmology. 2001;108:1819–25. doi: 10.1016/s0161-6420(01)00762-x. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Jalali S, Adiraju MV, Gopinathan U, Das T. Sensitivity and predictability of vitreous cytology, biopsy, and membrane filter culture in endophthalmitis. Retina. 1996;16:525–9. doi: 10.1097/00006982-199616060-00010. [DOI] [PubMed] [Google Scholar]
- 9.Mithal K, Pathengay A, Bawdekar A, Jindal A, Vira D, Relhan N, et al. Filamentous fungal endophthalmitis: Results of a combination therapy with intravitreal Amphotericin B and voriconazole. Clin Ophthalmol. 2015;13:649–55. doi: 10.2147/OPTH.S80387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radhika M, Mithal K, Bawdekar A, Dave V, Jindal A, Relhan N, et al. Pharmacokinetics of intravitreal antibiotics in endophthalmitis. J Ophthalmic Inflamm Infect. 2014;4:22. doi: 10.1186/s12348-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muda R, Vayavari V, Subbiah D, Ishak H, Adnan A, Mohamed SO. Endogenous endophthalmitis: A 9-year retrospective study at a tertiary referral hospital in Malaysia. J Ophthalmic Inflamm Infect. 2018;8:14. doi: 10.1186/s12348-018-0158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shenoy SB, Thotakura M, Kamath Y, Bekur R. Endogenous endophthalmitis in patient with MRSA septicemia: A case series and review of literature. Ocular Immunol Inflamm. 2016;24:515–20. doi: 10.3109/09273948.2015.1020173. [DOI] [PubMed] [Google Scholar]
- 13.Jackson TL, Paraskevopoulos T, Georgalas I. Systematic review of 342 cases of endogenous endophthalmitis. Surv Ophthalmol. 2014;59:627–35. doi: 10.1016/j.survophthal.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Sadiq MA, Hassan M, Agarwal A, Sarwar S, Toufeeq S, Soliman MK, et al. Endogenous endophthalmitis: Diagnosis, management and prognosis. J Ophthalmic Inflamm Infect. 2015;5:32. doi: 10.1186/s12348-015-0063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratra D, Saurabh K, Das D, Nachiappan K, Nagpal A, Rishi E, et al. Endogenous endophthalmitis: A 10-year retrospective study at a tertiary hospital in South India. Asia Pac J Ophthalmol. 2015;4:286–92. doi: 10.1097/APO.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 16.Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM. Endogenous endophthalmitis: An 18-year review of culture-positive cases at a tertiary care center. Medicine. 2003;82:97–105. doi: 10.1097/00005792-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Essman TF, Flynn HW, Jr, Smiddy WE, Brod RD, Murray TG, Davis JL, et al. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers. 1997;28:185–94. [PubMed] [Google Scholar]
- 18.Wong JS, Chan TK, Lee HM, Chee SP. Endogenous bacterial endophthalmitis: An East Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology. 2000;107:1483–91. doi: 10.1016/s0161-6420(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 19.Chen YJ, Kuo HK, Wu PC, Kuo ML, Tsai HH, Liu CC, et al. A 10-year comparison of endogenous endophthalmitis outcomes: An East Asian experience with Klebsiella pneumoniae infection. Retina. 2004;24:383–90. doi: 10.1097/00006982-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Yang CS, Tsai HY, Sung CS, Lin KH, Lee FL, Hsu WM. Endogenous Klebsiella endophthalmitis associated with pyogenic liver abscess. Ophthalmology. 2007;114:876–80. doi: 10.1016/j.ophtha.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Jalali S, Pehere N, Rani PK, Bobbilli RB, Nalamada S, Motukupallly SR, et al. Treatment outcomes and clinicomicrobiological characteristics of a protocol-based approach for neonatal endogenous endophthalmitis. Eur J Ophthalmol. 2013;24:424–36. doi: 10.5301/ejo.5000395. [DOI] [PubMed] [Google Scholar]
- 22.Basu S, Kumar A, Kapoor K, Bagri NK, Chandra A. Neonatal endogenous endophthalmitis: A report of six cases. Pediatrics. 2013;131:e1292–7. doi: 10.1542/peds.2011-3391. [DOI] [PubMed] [Google Scholar]
- 23.Garg SP, Talwar D, Verma LK. Metastatic endophthalmitis: A reappraisal. Ann Ophthalmol. 1991;23:74–8. [PubMed] [Google Scholar]
- 24.Gonzalez GA, Whitcher JP, Irvine AR, O'Brien JM. A ring hypopyon in a patient with meningococcal endophthalmitis: A case report and review of the literature. J Pediatr Ophthalmol Strabismus. 1998;35:329–33. doi: 10.3928/0191-3913-19981101-08. [DOI] [PubMed] [Google Scholar]
- 25.Kuriyan AE, Weiss KD, Flynn HW, Jr, Smiddy WE, Berrocal AM, Albini TA. Endophthalmitis caused by Streptococcal species. Clinical settings, microbiology, management and outcomes. Am J Ophthalmol. 2014;157:774–80. doi: 10.1016/j.ajo.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yospaiboon Y, Meethongkam K, Sinawat S, Laovirojjanakul W, Ratanapakorn T, Sanguansak T, et al. Predictive factors in the treatment of streptococcal endophthalmitis. Clinical Ophthalmol. 2018;12:859–64. doi: 10.2147/OPTH.S161217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eifrig CW, Scott IU, Flynn HW, Jr, Miller D. Endophthalmitis caused by Pseudomonas aeruginosa. Ophthalmology. 2003;110:1714–17. doi: 10.1016/S0161-6420(03)00572-4. [DOI] [PubMed] [Google Scholar]
- 28.Sridhar J, Flynn WR, Jr, Kuriyan AE, Miller D, Albini T. Endogenous fungal endophthalmitis: Risk factors, clinical features, and treatment outcomes in mold and yeast infections. J Ophthalmic Inflamm Infect. 2013;3:60. doi: 10.1186/1869-5760-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta A, Gupta V, Dogra MR, Chakrabarti A, Ray P, Ram J, et al. Fungal endophthalmitis after a single intravenous administration of presumably contaminated dextrose infusion fluid. Retina. 2000;20:262–8. [PubMed] [Google Scholar]
- 30.Dogra M, Akella M, Dogra MR, Gupta A. Presumably contaminated intravenous infusion-induced Aspergillus terreus endogenous endophthalmitis presenting with posterior hypopyon. Indian J Ophthalmol. 2018;66:593–5. doi: 10.4103/ijo.IJO_695_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


