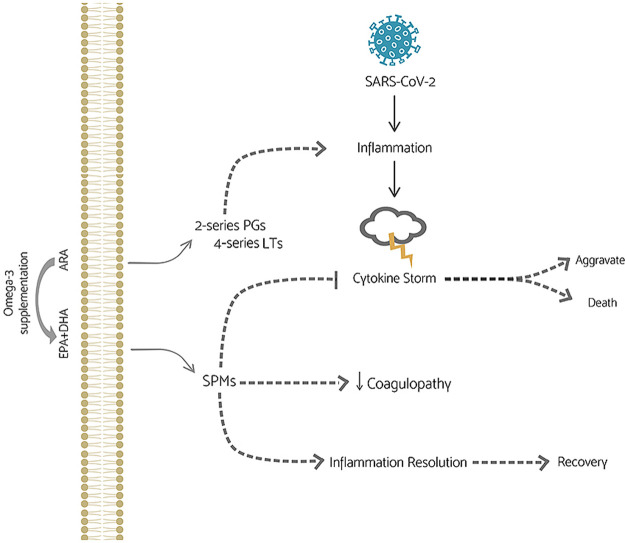
Abstract
Studies have shown that infection, excessive coagulation, cytokine storm, leukopenia, lymphopenia, hypoxemia and oxidative stress have also been observed in critically ill Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) patients in addition to the onset symptoms. There are still no approved drugs or vaccines. Dietary supplements could possibly improve the patient's recovery. Omega-3 fatty acids, specifically eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), present an anti-inflammatory effect that could ameliorate some patients need for intensive care unit (ICU) admission. EPA and DHA replace arachidonic acid (ARA) in the phospholipid membranes. When oxidized by enzymes, EPA and DHA contribute to the synthesis of less inflammatory eicosanoids and specialized pro-resolving lipid mediators (SPMs), such as resolvins, maresins and protectins. This reduces inflammation. In contrast, some studies have reported that EPA and DHA can make cell membranes more susceptible to non-enzymatic oxidation mediated by reactive oxygen species, leading to the formation of potentially toxic oxidation products and increasing the oxidative stress. Although the inflammatory resolution improved by EPA and DHA could contribute to the recovery of patients infected with SARS-CoV-2, Omega-3 fatty acids supplementation cannot be recommended before randomized and controlled trials are carried out.
Keywords: SARS-CoV-2, Inflammation, SPMs, Oxidative stress, EPA, DHA
Graphical abstract
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- ALI
acute lung injury
- AP-1
activator protein-1
- APCs
antigen-presenting cells
- ARA
arachidonic acid
- ASA
aspirins
- ARBS
ACE2 type I receptor blockers
- ARDS
acute respiratory distress syndrome
- cAMP
cyclic adenosine monophosphate
- CRP
C-reactive protein
- COX
ciclooxigenase
- DHA
docosahexaenoic acid
- DIC
disseminated intravascular coagulation
- EETs
epoxyeicosatrienoic acids
- EPA
eicosapentaenoic acid
- ESR
erythrocyte sedimentation rate
- GPCR
G protein-coupled receptor
- HNE
4-hydroxy-nonenal
- HHE
4-hydroxy-hexenal
- HETEs
hydroxyeicosatetraenoic acids
- HEPE
hydroxy-eicosapentaenoic acid
- ICU
intensive care unit
- ISoPs
isoprostane
- IFN-γ
interferon-γ
- IFN-α/β
interferon-α/β
- ISGs
IFN-stimulated genes
- Ig
immunoglobulin
- IL
interleukin
- LDH
lactate dehydrogenase
- LOX
lipoxigenase
- LTs
leukotrienes
- MDA
malondialdehyde
- M-CSF
macrophage colony-stimulating factor
- MERS-CoV
Middle East respiratory syndrome corona virus
- MCP-1
monocyte chemoattractant protein-1
- NK
Natural killer
- NF-kB
nuclear factor kappa B
- NTD
N-terminal domain
- NeuroPs
neuroprostanes
- PPARγ
peroxisome proliferator-activated receptor gamma
- PUFAs
polyunsaturated fatty acids
- PLA2
enzyme phospholipase A2
- PGE2
prostaglandin E2
- PD1
protectin D1
- RNA
ribonucleic acid
- RBD
receptor-binding domain
- ROS
reactive oxygen species
- RvE1
resolving E1
- SARS-CoV-2
severe acute respiratory syndrome corona virus 2
- SARS-CoV
severe acute respiratory syndrome corona virus
- SPMs
specialized pro-resolving mediators
- SIC
sepsis-induced coagulopathy
- TNF-α
tumor necrosis factor-α
- Th1
T-helper 1
- Th2
T-helper 2
- Th-17
T helper 17
- Treg
regulatory T cell
- TXs
thromboxanes
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- VTE
venous thromboembolism
- 4-ONE
4-oxononenal
- 17-HDHA
17-hydroxydocosahexaenoic acid
1. Introduction
The coronavirus disease 2019 (COVID-19) emerged for the first time in Wuhan, China, in December 2019 [1]. Two large-scale coronavirus outbreaks infected humans and caused fatalities previously: the severe acute respiratory syndrome coronavirus (SARS-CoV), in 2003, and the Middle East respiratory syndrome (MERS-CoV), in 2012, causing 813 and 845 deaths, respectively [2,3]. However, SARS-CoV-2 has shown a stronger transmission capacity, leading to more than 9.0 million infections and more than 469,000 deaths to date [4].
The most common symptoms at the onset of illness are fever, cough, dyspnea and myalgia, accompanied by leukopenia, lymphopenia, raised serum aspartate aminotransferase level, abnormalities on computer tomography of the chest (ground-glass opacity), bilateral respiratory distress and secondary infections [5]. In critically ill patients, there are also reports of abnormal coagulation, excessive inflammation, higher C-reactive protein (CRP) concentrations, lower oxygen saturation, hypoxemia and oxidative stress, leading to renal and hepatic failure [[6], [7], [8]].
No vaccine or treatments have been developed to date despite scientific efforts. This is due to the time and steps required for compounds to be considered efficient and safe [9]. The consumption of dietary supplements, such as omega-3 fatty acids, could help improve the treatment and recovery of severe SARS-CoV-2 infected patients by reducing inflammatory response and excessive blood coagulation. This hypothesis is based on the well-documented effect of marine omega-3 fatty acids, specifically eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), on inflammation resolution [10] and on their potential for coagulation reduction and arrhythmia improvement [11,12]. However, several studies have reported that high doses of EPA and DHA can increase host susceptibility to viruses [13,14]. It has also been reported that replacing ARA by EPA and DHA in phospholipid membranes makes the cells prone to oxidative stress when there is an increase in ROS concentration in the affected area [15,16].
Considering the potential benefits and risks of EPA and DHA supplementation for SARS-CoV-2 infected patient's prognosis, this review discusses the implications of omega-3 fatty acids alone and in combination with anti-inflammatory drugs, on inflammation resolution, coagulation and oxidative stress.
2. Pathogenesis of SARS-CoV-2 infection
Coronaviruses is a genus in the Coronaviridae family, from the Nidovirales order. They contain a positive sense single-stranded RNA as a nucleic material [17]. Its structure includes a lipid bilayer membrane containing the transmembrane protein, the spike protein and the envelope protein, surrounding a nucleocapsid [17]. Coronaviruses are comprised of a large number of viruses found broadly distributed among mammals and birds. They cause respiratory, enteric, hepatic and neurologic diseases.
SARS-CoV-2 entry into the cell is mediated by the angiotensin-converting enzyme 2 (ACE2), a membrane-bound aminopeptidase, part of the renin-angiotensin system, expressed in a small subset of cells in the lung and in other cell types, such as vascular endothelial cells and macrophages [5,18,19]. The first-line of defense of the immune system against viral infection involves the expression of type 1 interferons (IFN-α and IFN-β), which are cytokines secreted by various cell types, notably plasmacytoid dendritic cells [20]. Type 1 IFN activates transcription factors of IFN-stimulated genes (ISGs) which will act by several mechanisms to inhibit viral replication and dissemination at the early stage, such as destroying viral RNA [20].
Activation of the adaptive immune system is initiated with the presentation of virus antigen by dendritic cells to T lymphocytes. Cytotoxic CD8+ T cells are essential in killing viral infected cells, while CD4+ helper T cells, which can be divided into helper (e.g. Th1, Th2, Th17) and regulatory cells (Tregs) [21], are involved in the expression of type 1 INF, anti and pro-inflammatory cytokines, and play a central role in the maintenance of self-tolerance and immune homeostasis [22].
Normal function of all stages in innate and adaptive immune systems leads to virus clearance and organism recovery. However, it has been suggested that in some cases, SARS-CoV-2 could compromise early viral control by blocking activation of IFN-α and IFN-β. This leads to uncontrolled virus proliferation [23,24] and may trigger a large influx of inflammatory neutrophils and macrophages in the lung tissue, promoting an exacerbated release of pro-inflammatory cytokines, such as tumor necrosis factor (TNF-α), interleukin 6 (IL-6), and interleukin 1 beta (IL-1β), chemokines and growth factors [6,24]. This condition has been reported as a “cytokine storm”. Huang et al. [6] demonstrated that critical SARS-CoV-2 infected patients showed higher levels of IL-1β, TNF-α, monocyte chemoattractant protein-1 (MCP-1) and IFN-γ than those found in mild patients.
In SARS-CoV-2 infection, the excessive and uncontrolled production of pro-inflammatory cytokines by the innate immune cells amplifies the secretion of pro-inflammatory chemoattractant factors, such as vascular endothelial growth factor (VEGF), MCP-1, interleukin 8 (IL-8) and reduced E-cadherin expression in endothelial cells [25]. VEGF and reduced E-cadherin expression contribute to vascular permeability and leakage, which are present in the pathophysiology of pulmonary dysfunction in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), leading to systemic inflammation and multiple organ failure characteristic of severe SARS-CoV-2 infected individuals [6,[25], [26], [27]]. Thus, the cytokine storm has been considered a key factor for disease control and an important cause for COVID-19 exacerbation or even mortality.
It has also been suggested that SARS-CoV-2 promotes lymphopenia, especially in severe patients, and functional exhaustion of T lymphocytes [6,[28], [29], [30]]. Zheng et al. [28] observed lower levels of Cytotoxic CD8+ lymphocytes in critically ill patients compared to mild ones. In another study, Qin et al. [30] observed a higher decrease of both CD4+ and CD8+ T lymphocytes in severe infection, mainly Treg cells. It has been suggested that these dysfunctions in T lymphocytes could result in aggravated inflammatory responses and slower virus clearance [[28], [29], [30]].
Many factors are involved in the cytokine storm observed in patients infected with SARS-CoV-2. The identification of which factors could be associated with healthy versus dysfunctional outcomes is essential to better deal with SARS-CoV-2 infection [19], and can influence the action of drugs and supplements.
3. Fatty acids and inflammation
Polyunsaturated fatty acids (PUFAs) play an important role in structural integrity and fluidity of membrane phospholipids. In addition, PUFAs influence gene expression and are substrate for the synthesis of lipid mediators, such as eicosanoids [31]. ARA, an omega-6 fatty acid, and EPA and DHA, omega-3 fatty acids, affect inflammatory and immune responses. In general, eicosanoids derived from EPA and DHA are less inflammatory than those derived from ARA [32]. For example, eicosanoid receptors typically have a lower affinity for the EPA-derived mediator than for the ARA-derived one [10,[33], [34], [35]].
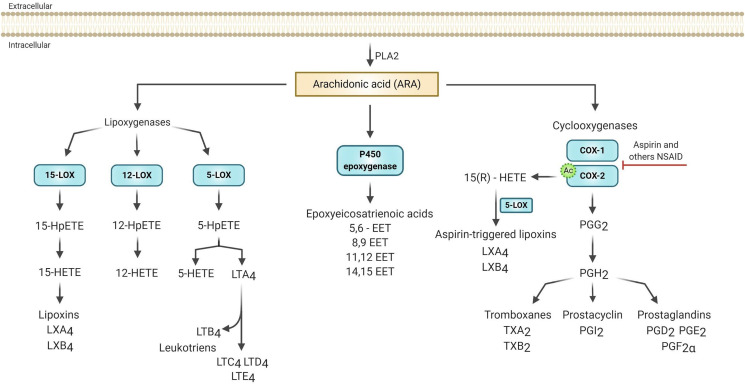
ARA is generally the main precursor of eicosanoid synthesis because the cell membrane composition of most immune cells contains a greater amount of ARA in comparison with other PUFAs, such as EPA [10]. The mobilization of ARA for eicosanoids formation occurs by the action of the enzyme phospholipase A2 (PLA2), which is activated by physiological or pathological stimuli [36]. The released ARA is then enzymatically oxidized through three enzymes: lipoxygenase (LOX), cytochrome P450 and cyclooxygenase (COX), that lead to leukotrienes (LTs), lipoxins, hydroxyeicosatetraenoic acids (HETEs), epoxyeicosatrienoic acids (EETs) and prostanoids, which include prostaglandins, prostacyclins and thromboxanes (TXs) (Fig. 1 ) [37].
Fig. 1.
Eicosanoid biosynthesis from arachidonic acid [ARA]. In response to various stimuli, ARA is released from cell membranes by phospholipase A2 [PLA2]. Free ARA can be metabolized to eicosanoids through the epoxygenase P-450, cyclooxygenase [COX 1 and 2], or lipoxygenases [5-LOX, 12-LOX and 15-LOX] pathways. In epoxygenase P-450, ARA is metabolized to epoxyeicosatrienoic acids [EETs]. The COX enzymes catalyze the conversion of ARA into intermediate prostaglandin G2 [PGG2] and then into prostaglandin H2 [PGH2]. PGH2 acts as a substrate for the generation of biologically active products such as prostaglandins [PGD2, PGE2, PGF2α], thromboxanes [TXA2 and TXB2] and the prostacyclin [PGI2], together these metabolites are called prostanoids. In the presence of aspirin, COX-2 is acetylated [indicated by ‘Ac'], which enhances the COX-2-catalyzed formation of 15-R-hydroxyeicosatetraenoic acid [15R -HETE] which can be converted by 5- LOX into the called aspirin-triggered lipoxins [LXA4 and LXB4]. LOX converts ARA first to the respective hydroperoxy-eicosatetraenoic acids [5, 12, and 15 - HpETEs] to produce the corresponding hydroxy-eicosatetraenoic acid [5, 12 and 15 - HETEs]. 5-HpETE is also further metabolized to form leukotriene [LT] A4 by 5-LOX. LTA4 is later converted to LTB4, LTC4, LTD4, and LTE4. The 15-HETE lead to the formation of lipoxins by 15-LOX. Based in Dennis & Norris [2015] and Harizi et al., [2008]. Figure made with biorender [https://biorender.com/].
The prostaglandin E2 (PGE2) is the most studied COX metabolite in immunological regulation. Its synthesis occurs in several cells, including macrophages, dendritic cells, fibroblasts and endothelial cells. PGE2 is involved in vasodilation, endothelial permeability and increase of pain [38]. PGE2 can exert pro-inflammatory and anti-inflammatory actions, and this heterogeneous effect depends in part, on the regulation of the expression of its specific receptors EP1 - EP4, which belong to the G protein-coupled receptor family (GPCR) [[38], [39], [40]].
The PGE2 has been shown to modulate both innate and adaptive immune cells and plays an important role in the link between the two systems, mediated by antigen-presenting cells (APCs) and T lymphocytes [39,41]. It contributes to the tissue influx of neutrophils, macrophages and mast cells, and can affect the differentiation and various functions of these cells, such as phagocytosis and degranulation [39]. PGE2 also acts as a regulator of APCs’ function by, for example, promoting activation, maturation and migration of dendritic cells [41]. In T cells, the PGE2 is important to control their differentiation, disrupting the Th1 response and improving the Th2 response, which leads to a reduction in protection against intracellular pathogens [42]. However, PGE2 can suppress the expression of MHC class II molecules, decrease natural killer (NK) cell activity and reduce the activation of T cells [34,41].
Coulombe et al. [43] showed that using animal model (C57BL6 mice) and in vitro assays (MDCK cells) the production of PGE2 during infection by the influenza A virus (H1N1 strain) led to inhibition of type 1 IFN and apoptosis of alveolar macrophages, promoting an increase in virus replication. In this context, PGE2 inhibition improved the antiviral response, suggesting that the specific suppression of PGE2 represents a therapeutic route for the treatment and prevention of influenza and potentially other viral infections. Furthermore, in vitro studies using fibroblasts and human chondrocytes, suggest that PGE2 can regulate the production of cytokines such as IL-6, IL-8 and macrophage colony-stimulating factor (M-CSF). The production of these pro-inflammatory mediators involves the binding of PGE2 with EP receptors and increased intracellular cAMP levels and nuclear factor kappa B (NF-κB) activation [[44], [45], [46]].
Studies also showed that PGE2 inhibits 5-LOX and decreases leukotriene production [47]. The leukotriene series derived from ARA consists of B4 (LTB4) produced by neutrophils, monocytes and macrophages; C4 (LTC4), D4 (LTD4) and E4 (LTE4) produced by mast cells, basophils and eosinophils [36,47]. The synthesis of leukotrienes occurs mainly in the respiratory, microvascular and gastrointestinal systems. Bronchoconstriction is the most evident physiological action of leukotrienes and has been related to asthma and allergic diseases [36]. In inflammatory response, epithelial cells are also important sources of leukotrienes for stimulating the recruitment of circulating leukocytes, generation of cytokines and ROS, increased TNF-α expression and induce activation, differentiation and proliferation of B cells, that are involved with immune response. LTB4 has been studied due to its antimicrobial effect for inducing antimicrobial peptide release from neutrophils, and in some cases, it can inhibit viral replication [48,49].
Thus, eicosanoids play an important role for host immune response and their synthesis depends on the type of fatty acid and precursor.
4. Anti-inflammatory drugs in the treatment of patients with COVID-19
Therapeutic approaches used in patients with SARS-CoV-2 include antiviral, antibacterial and anti-inflammatory drugs [6,50]. Among the latter, corticosteroids are widely prescribed for the treatment of patients with severe illness for possible relief of inflammation and higher survival of patients with SARS-CoV [51]. However, recent evidence suggests that corticosteroids may aggravate lung injury associated with SARS-CoV-2 due to delayed viral clearance [6,26].
Corticosteroids, such as dexamethasone, act by suppressing the immune system due to the inhibitory action of transcription factors, such as the activator protein-1 (AP-1) and nuclear factor kB (NF-κB), responsible for the expression of pro-inflammatory genes involved in the synthesis of cytokines like IL-6, IL-2 and TNF-α. They also decrease the action of COX-2 and the production of chemokines. It should be noted that COX-2 catalyzes the synthesis of prostaglandins and thromboxanes, which are mediators responsible for many aspects of the inflammatory response [52]. However, continued use of corticosteroids can lead individuals to immunosuppression, making them more vulnerable to infections compared to healthy individuals [[52], [53], [54]].
Recently, several studies suggested that Ibuprofen, a non-steroidal anti-inflammatory drug, should not be used in suspected cases of SARS-CoV-2 infection [55]. Non-steroidal anti-inflammatory drugs act in the selective or non-selective inhibition of the COX-1 and COX-2 (Fig. 1), blocking the cascade of ARA and the formation of prostaglandins [10,55]. The inhibition of prostaglandin expression induces vasoconstriction, altering blood flow and favoring the activation of the “renin-angiotensin” system, which can lead to an increased expression of ACE2 in the lungs, kidneys and intestines. It has been hypothesized that this upregulation of ACE2 by Ibuprofen could increase the individual susceptibility to infection by the SARS-CoV-2 [55,56]. This hypothesis has not been confirmed, and further studies are needed to better evaluate the negative effects of Ibuprofen as well as other anti-inflammatory drugs of the same therapeutic class.
Aspirins (ASA, acetylsalicylic acid) have a different mechanism of action compared to Ibuprofen and other non-selective non-steroidal anti-inflammatory drugs, and are 170-fold more potent in inhibiting COX-1 than COX-2 [57]. In addition to the inactivation of COX-1, ASA promotes the conversion of ARA into 15-HETE [15-hydroxyeicosatetraenoic acid] through COX-2 pathway (Fig. 1), decreasing prostaglandins concentration, increasing leukotrienes and lipoxins, and favoring the inflammation resolution [36,57].
5. Omega-3 fatty acids and inflammation resolution
The anti-inflammatory effect of EPA and DHA has been associated with decreasing of arachidonic acid (ARA) in the membrane phospholipids, which leads to a reduced synthesis of ARA-lipid mediators and increased production of less inflammatory EPA-derived lipid mediators [33,58]. EPA and DHA supplementation can increase the proportion of both fatty acids in blood lipids, blood cells and many tissue compartments. The incorporation of EPA and DHA in phospholipids occurs in a dose and time-dependent manner, and the incorporation of EPA generally occurs faster than DHA [59]. Studies have observed that the rate of incorporation of EPA and DHA in humans after supplementation varies between cell types, with a rapid change in plasma fatty acid fractions, between 1 and 4 weeks, while changes in mononuclear cells were observed after months of supplementation [60,61]. A study conducted by Gerling et al. [62] demonstrated that healthy young men supplemented with fish oil (3g EPA + 2g DHA/day) for 12 weeks showed an increase in the content of EPA and DHA in whole muscle and mitochondrial membranes and also decreased the omega-6/omega-3 proportion. This result shows that supplementation with EPA and DHA can reduce the availability of ARA for synthesis of eicosanoids, affecting the actions regulated by these mediators [59,63].
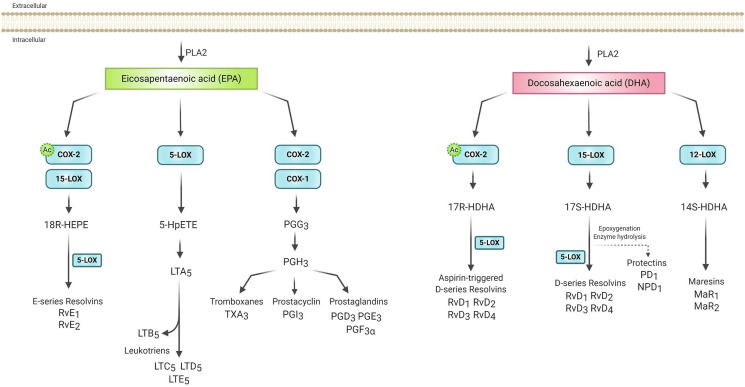
Mechanisms underlying the anti-inflammatory actions of EPA and DHA involve inhibition of leukocyte chemotaxis, reduction of adhesion molecule expression and leukocyte-endothelial adhesive interactions, disruption of lipid rafts, inhibition of activation of NF-κB, activation of anti-inflammatory transcription factors, such as Peroxisome Proliferator-Activated Receptor Gamma (PPARγ), and binding to the G protein-coupled receptor (GPCR120) [59,64,65]. Furthermore, the enzymatic oxidation of EPA and DHA leads to the synthesis of specialized pro-resolving mediators (SPMs), such as resolvins, protectins and maresins (Fig. 2 ). Resolvins produced from EPA (E-series) and DHA (D-series) and protectins produced from DHA involves the COX and LOX pathways and are inflammation-resolving, inhibiting transendothelial migration of neutrophils and cytokines (IL-1β and TNF-α) and chemokines production [64].
Fig. 2.
Eicosanoids biosynthesis from eicosapentaenoic [EPA] and docosahexaenoic [DHA]. Specialized pro-resolution mediators [SPMs] are produced by the LOX and COX enzymes. EPA can be converted by 15-LOX or in the presence of aspirin by acetylated COX2 [indicated by ‘Ac'] in 18-hydroxyeicosapentaenoic acid [18-HEPE], which can be converted by 5-LOX in E series resolvins [RvE1 and RvE2]. Also, EPA can be converted, through COX and 5-LOX respectively, into less inflammatory prostanoids and leukotrienes than ARA derivatives. DHA can be metabolized in the presence of aspirin by acetylated COX2, 15-LOX and 12-LOX. In the COX-2 pathway, DHA forms 17-hydroxydocosahexaenoic acid [17R-HDHA], which is metabolized by 5-LOX into aspirin-triggered D-series resolvins [RvD1-RvD4]. Through 15-LOX, DHA originates from the metabolite 17S-HDHA that can generate the D-series resolvins through 5-LOX or protectins [PD1] and neuroprotectins [NPD1], when generated in neural tissue, through other reactions. 12-LOX-derived 14-hydroxydocosahexaenoic acid [14S-HDHA] can be converted to maresins [MaR1 and MaR2]. Based in Basil & Levy [2016] and Serhan [2014]. Figure made with biorender [https://biorender.com/].
Several studies have investigated the use of ASA as a possible potentiator of EPA and DHA effects. This occurs due to ASA-triggered biosynthesis of resolvins and protectins from EPA and DHA, and the ability of ASA to modify the production of the SPMs [66,67]. After acetylation of the COX-2 induced by ASA, EPA is converted into 18-HEPE, ARA into 15-HETE and DHA into 17-hydroxydocosahexaenoic acid (17-HDHA) (Fig. 1, Fig. 2). These products are transformed into lipoxins, resolvins and protectins. The 18-HEPE is then released by the endothelial cells and taken up by adherent leukocytes, where 5-LOX converts it into E-series resolvins, mainly RvE1. Additionally, ASA can trigger the formation of an epimeric protectin D1 (PD1), a molecule derived from DHA through the action of the 15-LOX identified as AT-PD1. In a recent study, ApoE−/- mice were supplemented with EPA and DHA, or EPA and DHA combined with ASA, at levels equivalent to human doses. The combined group showed lower serum ARA-derived prostanoids and 15-HETE concentrations [67]. Thus, the endogenous metabolism of prostaglandins and SPMs may change in the presence of ASA, and its combination with EPA and/or DHA can prevent the onset of inflammation and even resolve it [68,69].
6. Evidence of omega-3 fatty acids supplementation on inflammation resolution
EPA and DHA are known to play a key role in the prevention and treatment of coronary artery disease, diabetes, hypertension, arthritis, and other inflammatory and autoimune disorders. For example, De Souza et al. [70] evaluated the effect of fish oil supplementation (1.44 g EPA + 0.96g DHA/day) 9 for 8 weeks in overweight/obese type 2 diabetes patients and observed a reduction of inflammatory biomarkers (TNF-α, IL-1β and IL-6) and an improvement of insulin sensitivity. This data corroborated those found by Moghadam et al. [71] that observed reduction of the serum CRP, IL-2 and TNF-α concentrations in type 2 diabetes patients after 8 weeks of EPA and DHA supplementation (1.548 g EPA + 0.828 g DHA + 0.338 gother omega 3 fatty acids/day).
One feature of uncontrolled activation of the inflammatory response, such as that occurring in the cytokine storm present in critically ill SARS-CoV-2 infected patients, involves excessive production of pro-inflammatory cytokines and lipid-derived inflammatory mediator eicosanoids, accompanied by exacerbated oxidative stress [72]. For this reason, over the past years, several studies have investigated whether EPA and DHA administered during critical illness could modulate the inflammatory response and, in turn, benefit critically ill patients [[72], [73], [74]]. To date, promising results were obtained.
The resolvins derived from EPA and DHA have played an important role during sepsis. Pontes-Arruda et al. [75] investigated the effect of administering an enteral diet enriched with EPA + GLA + antioxidants for 28 days in patients with severe sepsis or septic shock who needed mechanical ventilation. The authors observed that the diet enriched with EPA and DHA contributed to improved clinical outcomes in the ICU individuals and that the treated group presented lower mortality rates when compared to the control group. Interestingly, Hosny et al. [76] evaluated the efficacy and safety of high-dose EPA and DHA supplementation (9 g/d added of 1g/d ascorbic acid + 400UI/12h alpha-tocopherol and 100μg/d selenium) in patients with early-stage sepsis for 7 days. It was observed that compared to the control group, the supplemented group exhibited lower levels of CRP, IL-6 and procalcitonin. In addition to these findings, the authors observed that the supplemented group also presented lesser need, shorter duration of mechanical ventilation and reduced development of severe sepsis.
Human and animal trials have also investigated the effect of EPA and DHA in ALI and ARDS, which is characteristic in severe SARS-CoV-2 patients. In a series of studies conducted by Mancuso et al. [77,78], male Long-Evans rats were fed enteral diets containing fish oil as a source of omega-3 fatty acids, or corn oil as a source of omega-6 fatty acids, for 21 days. After induction of acute inflammation caused by an intravenous injection of Salmonella enteritidis endotoxin (10 mg/Kg), the authors observed that the group receiving fish oil showed lower severity of pulmonary microvascular protein permeability and decreased pulmonary neutrophil accumulation. Furthermore, stimulated alveolar macrophages had reduced concentrations of ARA-derived mediators, such as PGE2 and TXB2, suggesting a beneficial effect of omega-3 fatty acids over omega-6 fatty acids in ALI attenuation [77,78]. More recently, the role of SPMs, specifically resolvins, in the resolution of inflammation has been demonstrated in several animal models of ALI and ARDS [[79], [80], [81], [82]]. These studies, carried out using rats and mice models infected with E.coli O55:B5 endotoxin LPS, suggested that the pro-resolving effects of these molecules could be attributed, for example, to the suppression of neutrophil infiltration due to reduction of expression and release of pro-inflammatory cytokines from alveolar macrophages [79,81].
Several trials have investigated the effect of EPA and DHA oral supplementation or parenteral nutrition formula, with or without antioxidants, in patients with ARDS. Prior trials utilized enteral formulations containing a high dose of EPA and DHA along with antioxidants and gamma-linolenic acid [75,[83], [84], [85]]. In general, these studies demonstrated favorable results in terms of multiple inflammatory, respiratory and clinical outcomes. A meta-analysis carried out by Pontes-Arruda et al. [86] also reported significant reduction in ventilator-free days, organ failures, length of stay in the intensive care unit and mortality in individuals with ARDS. More recently, a Cochrane review showed that patients with ARDS receiving EPA and DHA supplementation showed a significant improvement in blood oxygenation and reduction in ventilation requirement, organ failures, length of stay in the ICU and mortality at 28 days [87].
Thus, supplementation with EPA and DHA could have important implications in critically ill COVID-19 patients. Recently, several research reviews have corroborated this idea [58,[88], [89], [90], [91], [92]]. These studies foccused mainly on the anti-inflammatory properties of EPA and DHA, suggesting that the less inflammatory lipid mediators produced from these compounds along with EPA and DHA-derived SPMs could help in management of the cytokine storm, ameliorating inflammation and lung injury [58,87,88].
Bistrian [88] suggested parenteral supplementation of critically ill SARS-CoV-2 infected patients with fish oil. The author argued that dietary doses of EPA and DHA (about 1 g/d) could have modest anti-inflammatory effects. However, larger amounts achieved through supplementation (4–6 g/d) could show more potent effects on cytokine secretion and in the inflammatory response [88,93,94]. Later, Torrinhas et al. [92] agreed that immune modulatory properties of EPA and DHA provided in emulsions may be important to change clinical outcomes of SARS-CoV-2 infected patients. The authors suggested a prescription based on body weight (e.g. 0.2 g pure fish‐oil lipid emulsions/kg body weight/day) and also considering combining the parenteral administration of fish oil emulsion to low oral aspirin intake, in order to trigger resolvin synthesis from EPA and DHA. In another review, Torrinhas et al. [91] suggested parenteral supplementation as the best route for delivering emulsions enriched with EPA and DHA due to their faster incorporation into plasma phospholipids, blood cells and tissues.
The benefits expected based on the anti-inflammatory activity of EPA and DHA are anecdotal and need to be verified by rigorous clinical trials. To the best of our knowledge, there is an open-label, randomized, controlled study currently running in hospitalized subjects with confirmed SARS-CoV-2 (NCT04335032) [95]. The study comprises 240 participants, with one group receiving standard care, the other being provided daily 2 g of EPA capsules. Interventions will be carried out between 28 and 90 days, and the efficacy of EPA in the treatment of the disease, as well as its effect on oxygen saturation, levels of pro-inflammatory IL-6, mortality rate, ICU stays, hospitalization days, and need for mechanical ventilation will be determined. In fact, many additional rigorous trials must be conducted to verify the effects.
Although EPA and DHA has been widely used to reduce chronic inflammatory diseases, their effect on viral infections remains controversial [13]. Several studies have highlighted the role of SPMs in viral diseases. A study conducted by Morita et al. [96] showed that the SPM protectin D1 (PD1) exhibited antiviral activity against the influenza A virus (A/PuertoRico/8/34 –H1N1, A/California/04/2009 –H1N1 and A/Vietman/1203/04-H5N1, and a mutant H5N1 PB2-627E) using C57Bl/6J male mice as model, markedly attenuating virus replication. In addition, treatment with PD1 improved the survival and pathology of severe influenza in infected mice. In another study, Ramon et al. [97] evaluated the ability of 17-HDHA, a SPM derived from DHA that is metabolized to resolvins and protectins (Fig. 2), in improving the immune response to H1N1 influenza virus. The results showed that 17-HDHA was able to increase the levels of antibodies, which resulted in greater resistance to infection by the H1N1 (A/Brisbane/59/2007 or A/California/04/2009) virus [97]. More recently, it has been shown that pretreatment with DHA increases human SH-SY5Y cell viability and proliferation, restores mitochondrial function, reduces viral load and triggers an anti-inflammatory response in cells infected with Zika (KX197192) virus [98].
However, several studies have shown that EPA and DHA supplementation can have suppressive effects, which can reduce the immune response against viral infections. In C57BL/6J mice infected with H1N1 influenza virus (A/PuertoRico/8/34), supplemented with fish oil resulted in a 40% higher mortality rate and 70% higher viral load. Besides, the supplemented mice showed significantly less CD8+ T lymphocytes and decreased mRNA expression of inflammatory mediators, including IL-6 and TNF-α [14]. Similarly, BALB/c mice infected with the influenza virus (A/Queensland/6/72:H3N2) and fed a high-fat diet rich in EPA and DHA improved lymphocyte proliferation but had compromised levels of IFN-γ, serum immunoglobulin [Ig] G, lung IgA-specific antibodies and suppression of virus-specific lung T cell cytotoxicity. These results suggest that feeding a high-fat diet rich in EPA and DHA could impair immune response by delaying virus clearance, although the supplementation had not affected the ultimate outcome [99,100]. It is important to take into account that these contradictory results can be associated to other factors, for example, the initial weight loss usually observed when mice are supplemented with fish oil by diet [99] and physiological differences among animal models. In addition, rigorously controlled animal studies have not been conducted with the SARS-CoV-2 virus, and differences between this virus and other influenza viruses might offer some insight into the apparent contradictions. Therefore, further studies are needed to understand the role of EPA and DHA in the immune response related to viral infections.
7. Omega-3 fatty acids and oxidative stress
PUFAs are molecules highly susceptible to non-enzymatic oxidation, usually mediated by reactive species that are generated during normal metabolism. PUFAs esterifying glycerol, cholesterol or phospholipids can be oxidized by ROS forming α, β-polyunsaturated lipid aldehydes that play an important role in many cellular processes [101,102]. Membrane phospholipids and triglycerides are primary targets for ROS oxidation producing aldehydes such as malondialdehyde (MDA), 4-hydroxyl-hexenal (HHE), 4-hydroxyl-nonenal (HNE),4-oxononenal (4-ONE) and acrolein [103]. Under normal conditions, these aldehydes are detoxified by phase I and phase II metabolism [104], but under severe oxidative stress, they can initiate mitochondrial dysfunction eventually culminating in apoptosis [103].
Phospholipidperoxidation products are recognized as important bioactive lipid mediators playing an active role as modulators in signaling events in inflammation, immunity and infection [105]. Oxidized phospholipids have been suggested as biomarkers of atherosclerosis and other pathologies [101,106]. An important class of products formed from non-enzymatic PUFA oxidation are the isoprostanes [IsoPs] and neuroprostanes [NeuroPs]. The initially discovered IsoPs class derived from ARA oxidation was analogous to prostaglandins PGF2α. For this reason, these molecules were called as F2-IsoPs [107]. They are released in their free forms by platelet-activating factor acetlyhydrolase and other phospholipases, being a significant proportion conjugated as glucuronide and excreted in urine [108]. Among all raecemic compounds, the 5- and 15-series of F2-IsoPs formed through ROS mediated oxidation of ARA, F3-IsoPs from EPA and 4- and 20- series NeuroPs from DHA, have been reported as more abundant in vivo [108]. F2-IsoPs have also been recognized as biomarkers of oxidative stress, being associated with several chronic inflammatory diseases. Due to their chemical stability in biological samples, F2-IsoPs have been considered as realiable biomarkers of endogenous lipid peroxidation [109]. Actually, the molecules formed from ROS mediated oxidation of omega 6 and omega 3 fatty acids can act as bioactive molecules in physiological and/or pathological conditions, demonstrating both pro- or anti-inflammatory effects on different cells [101,110,111].
The hypoxemia caused by pneumonia reduces the energy supply by cell metabolism, increases the anaerobic fermentation, intracellular acidosis, and reactive oxygen species (ROS) to destroy the phospholipid layer of the cell membrane [1]. Oxidative stress via RNA virus infections can contribute to cellular events including apoptosis, loss of immune function and viral replication [112]. Higher ROS levels followed by depletion of antioxidant defense leads to the development of oxidative stress, chronic activation of immune responses and inflammation, which has been implicated in tissue damage [113]. Thus, considering the increase of ROS release during the cytokine storm due to SARS-CoV-2 infection, the increase in more unsaturated fatty acids esters of phospholipids, glycerol and cholesterol, with consequent higher susceptibility to non-enzymatic oxidation, must be investigated. For this reason, it has been suggested that supplementation with EPA and DHA should be accompanied by antioxidants, such as vitamin C and E [58,83,85]. In this regard, previous prospective, randomized and controlled studies investigating fish oil administration along with antioxidants via continuous enteral feeding formula as a therapy in ARDS and ALI patients have found benefits, such as reduced mortality, risk of developing organ failures, time on mechanical ventilation, and ICU length of stay [58,83,85].
8. Omega-3 fatty acids and coagulopathy
Besides the common biomarkers, SARS-CoV-2 infected patient's laboratory examinations included normal or reduced leukocyte count, reduced lymphocyte count, thrombocytopenia, elevated serum levels of transaminase, lactate dehydrogenase [LDH], creatine kinase and myoglobin [114]. In a recent work [107], from 183 pneumonia SARS-CoV-2 infected patients, 21 (11.5%) patients died. These non-survivors revealed significantly higher D-dimer and fibrin degradation product levels and longer prothrombin time compared to the survivors on admission. By the late hospitalization, the fibrinogen and antithrombin activity levels were also significantly lower in non-survivors, suggesting that conventional coagulation parameters during the course of pneumonia SARS-CoV-2 infection were significantly associated with their prognosis [115].
Coagulopathy, commonly found in severe cases of COVID-19 as disseminated intravascular coagulation (DIC), may exist in the majority of deaths. The International Society of Thrombosis and Haemostasis has proposed a new category identifying an earlier phase of sepsis-associated disseminated intravascular coagulation, called “sepsis-induced coagulopathy” (SIC) and patients who meet the diagnostic criteria of SIC benefiting from anticoagulant therapy, such as antithrombin, thrombomodulin (rsTM) and low-molecular-weight heparin, has been confirmed [116]. In addition, long-term bed rest and likely receiving hormone treatment also increase the risk of venous thromboembolism (VTE) in severely SARS-CoV-2 infected patients [116].
For these reasons, the active application of anticoagulants (such as heparin) for patients with severe SARS-CoV-2 infection has been recommended. However, its efficacy remains to be validated [115]. Tang et al. [115] have recently published that anticoagulant therapy mainly with low molecular weight heparin, appears to be associated with better prognosis in severe SARS-CoV-2 infected patients meeting SIC criteria or with markedly elevated D-dimer. In a study involving 449 SARS-CoV-2 infected patients, ninety-nine (22%) patients of SARS-CoV-2 group received heparin treatment for at least 7 days. The results showed that only the group with elevated D-dimer could benefit from heparin treatment in the study. They concluded that patients with severe pneumonia induced by SARS-CoV-2 had higher platelet count and only those with markedly elevated D-dimer may benefit from anticoagulant therapy mainly with low molecular weight heparin [117].
Wander and Patton [118] investigated the effect of consumption of moderate amounts (200 g/day) of three species of fish (salmon, sablefish, dover sole) by 23 healthy young men for 18 days on platelet fatty acid profile, bleeding time, platelet aggregation and ex vivo production of TXB2. They found that a moderate fish diet might cause modestly positive effects on platelet aggregation. Salmon and sablefish caused significant increases in the EPA content of the platelet fatty acid profile. Bleeding time increased moderately when salmon diets were consumed and platelet aggregation decreased with salmon and sablefish as part of a Western diet.
Dietary supplementation with EPA, DHA or ARA may alter platelet lipid membrane phospholipid composition and affect platelet function, which, in turn, may alter the progression and thrombotic complications of cardiovascular disease. Adili et al. [119] reported, with special emphasis on in vivo effects, that EPA and DHA act on the platelet membrane to reduce platelet aggregation and thromboxane release by acting on COX-1 and 12-LOX, involved in metabolizing fatty acids into oxylipins in platelets. Park and Harris [120] demonstrated that healthy subjects supplemented with EPA for 4 weeks showed reduction of platelet activation, an early step in platelet aggregation, whereas DHA did not show this effect. It has been assumed that EPA is more active than DHA in altering platelet function because it is a COX substrate. However, DHA appears to decrease TXA2/prostaglandins H2 receptor affinity [120]. Although supplementation of EPA and DHA has been shown to reduce platelet aggregation and activation in healthy subjects, a higher recommended dose of omega-3 fatty acids may be needed due to platelet hyperactivity prothrombotic conditions such as in cardiovascular disease [119].
While current antiplatelet therapies are under tests and its efficacy remains to be validated in experimental and clinical trials with COVID-19 patients, known omega-3 fatty acids anticoagulant properties make it possible only to speculate that supplementation could have an effect on the platelet aggregation in severe cases of SARS-CoV-2 infected subjects.
9. Conclusion
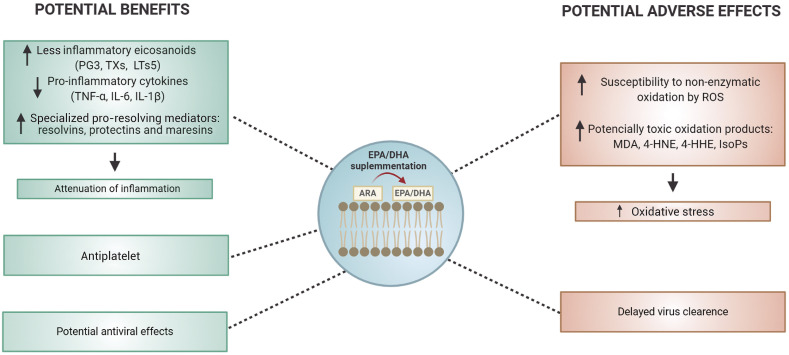
In the present contribution, we critically reviewed the potential benefits and adverse effects of omega-3 fatty acids supplementation as co-therapy for patients infected with SARS-CoV-2 (Fig. 3 ). Although the potential benefits of omega-3 fatty acids to reduce COVID-19 severity are based on well documented in vitro and in vivo experimental assays, the risk of supplementation with high doses before or during SARS-CoV-2 infection must be investigated. Actually, little is known about the virus pathology and how other characteristics of the patients are involved in their recovery. Thus, we suggest that randomized and controlled trials be carried out, taking into account the type of supplementation, doses, patient's characteristics, adverse effects and the combination of drugs or antioxidants. In addition, considering the consequences of the cytokines storm, oxidative stress and antiviral drugs to the progression of cardiovascular diseases, other studies about omega-3 fatty acids supplementation should also be performed with patients who survived SARS-CoV-2 infection, since the health of these individuals can be improved by adequate and controlled supplementation.
Fig. 3.
Potential effects of EPA and DHA supplementation in critical SARS-CoV-2 infected patients. PG3: prostaglandin E3, TXs: thromboxanes; LTs5: 5-series leukotrienes; TFN-α: tumor necrosis factor-α; IL-6: interleukin 6; IL-1β: interleukin 1β; ROS: reactive oxygen species; MDA: malondialdehyde; 4-HNE: hydroxyl-nonenal; 4-HHE: 4-hydroxy-hexenal; ISoPs: isoprostanes.
Funding
This work was supported by São Paulo Research Foundation – FAPESP (grants 2013/07914-8, 2017/08066-1 and 2019/24023-6), Brazil; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil and Food Research Center (FoRC), CEPID-FAPESP, Brazil.
Declaration of competing interest
The authors have no conflict of interest to declare.
References
- 1.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., Bi Z., Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO; 2018. WHO MERS Global Summary and Assessment of.https://www.who.int/publications-detail/who-mers-cov-global-summary-and-assessment-of-risk Available from. [Google Scholar]
- 3.World Health Organization . WHO; 2003. Cumulative Number of Reported Probable Cases of SARS.https://www.who.int/csr/sars/country/2003_07_11/en/ Available from. [Google Scholar]
- 4.World Health Organization . WHO; 2020. WHO Coronavirus Disease [COVID-19] Dashboard.https://covid19.who.int/ Available from. [Google Scholar]
- 5.Lake M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin. Med. 2020;20:124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Innes J.K., Calder P.C. Marine Omega-3 (N-3) Fatty acids for cardiovascular health: an update for 2020. Int. J. Mol. Sci. 2020;21:1362. doi: 10.3390/ijms21041362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tribulova N., Szeiffova Bacova B., Egan Benova T., Knezl V., Barancik M., Slezak J. Omega-3 Index and anti-arrhythmic potential of omega-3 PUFAs. Nutrients. 2017;9:1191. doi: 10.3390/nu9111191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiNicolantonio J.J., OKeefe J. Importance of maintaining a low omega-6/omega-3 ratio for reducing platelet aggregation, coagulation and thrombosis. Open Hear. 2019;6 doi: 10.1136/openhrt-2019-001011. e001011–e001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husson M.-O., Ley D., Portal C., Gottrand M., Hueso T., Desseyn J.-L., Gottrand F. Modulation of host defence against bacterial and viral infections by omega-3 polyunsaturated fatty acids. J. Infect. 2016;73:523–535. doi: 10.1016/j.jinf.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Schwerbrock N.M.J., Karlsson E.A., Shi Q., Sheridan P.A., Beck M.A. Fish oil-fed mice have impaired resistance to influenza infection. J. Nutr. 2009;139:1588–1594. doi: 10.3945/jn.109.108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundt H., Nilsen D.W.T., Mansoor M.A., Nordøy A. Increased lipid peroxidation during long-term intervention with high doses of n-3 fatty acids (PUFAs) following an acute myocardial infarction. Eur. J. Clin. Nutr. 2003;57:793–800. doi: 10.1038/sj.ejcn.1601730. [DOI] [PubMed] [Google Scholar]
- 16.Song J.H., Fujimoto K., Miyazawa T. Polyunsaturated (n-3) fatty acids susceptible to peroxidation are increased in plasma and tissue lipids of rats fed docosahexaenoic acid–containing oils. J. Nutr. 2000;130:3028–3033. doi: 10.1093/jn/130.12.3028. [DOI] [PubMed] [Google Scholar]
- 17.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 19.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallard E., Lescure F.-X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antivir. Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagliani N., Huber S. In: T-cell Diferentiation. Methods and Protocols. Lugli E., editor. Humana Press; New York, NY: 2017. Basic aspects of t helper cell differentiation. [Google Scholar]
- 22.Huber S.R., van Beek J., de Jonge J., Luytjes W., van Baarle D. T Cell responses to viral infections – opportunities for peptide vaccination. Front. Immunol. 2014;5:171. doi: 10.3389/fimmu.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frieman M., Heise M., Baric R. SARS coronavirus and innate immunity. Virus Res. 2008;133:101–112. doi: 10.1016/j.virusres.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’’ in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore B.J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 26.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target. Ther. 2020;5:16–18. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.-S. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calder P.C., Grimble R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002;56:S14–S19. doi: 10.1038/sj.ejcn.1601478. [DOI] [PubMed] [Google Scholar]
- 32.Natto Z.S., Yaghmoor W., Alshaeri H.K., Van Dyke T.E. Omega-3 fatty acids effects on inflammatory biomarkers and lipid profiles among diabetic and cardiovascular disease patients: a systematic review and meta-analysis. Sci. Rep. 2019;9:18867. doi: 10.1038/s41598-019-54535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calder P.C. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Lone A., Taskén K. Proinflammatory and immunoregulatory roles of eicosanoids in T Cells. Front. Immunol. 2013;4:130. doi: 10.3389/fimmu.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calder P.C., Yaqoob P. Marine omega-3 fatty acids and coronary heart disease. Curr. Opin. Cardiol. 2012;27:412–419. doi: 10.1097/HCO.0b013e328353febd. [DOI] [PubMed] [Google Scholar]
- 36.Dennis E.A., Norris P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calder P.C. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–1024. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- 38.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalinski P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harizi H., Corcuff J.-B., Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol. Med. 2008;14:461–469. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Harizi H. Reciprocal crosstalk between dendritic cells and natural killer cells under the effects of PGE2 in immunity and immunopathology. Cell. Mol. Immunol. 2013;10:213–221. doi: 10.1038/cmi.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sander W.J., O'Neill H.G., Pohl C.H. Prostaglandin E(2) as a modulator of viral infections. Front. Physiol. 2017;8:89. doi: 10.3389/fphys.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coulombe F., Jaworska J., Verway M., Tzelepis F., Massoud A., Gillard J., Wong G., Kobinger G., Xing Z., Couture C., Joubert P., Fritz J.H., Powell W.S., Divangahi M. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of yype I interferon and apoptosis in macrophages. Immunity. 2014;40:554–568. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Cho J.-S., Han I.-H., Lee H.R., Lee H.-M. Prostaglandin E2 induces IL-6 and IL-8 production by the EP Receptors/Akt/NF-κB Pathways in nasal polyp-derived fibroblasts. Allergy. Asthma Immunol. Res. 2014;6:449–457. doi: 10.4168/aair.2014.6.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue H., Takamori M., Shimoyama Y., Ishibashi H., Yamamoto S., Koshihara Y. Regulation by PGE2 of the production of interleukin-6, macrophage colony stimulating factor, and vascular endothelial growth factor in human synovial fibroblasts. Br. J. Pharmacol. 2002;136:287–295. doi: 10.1038/sj.bjp.0704705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang P., Zhu F., Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-κB activation. Am. J. Physiol. 2010;298:C1445–C1456. doi: 10.1152/ajpcell.00508.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calder P.C. Polyunsaturated fatty acids and inflammation, Prostaglandins. Leukot. Essent. Fat. Acids. 2006;75:197–202. doi: 10.1016/j.plefa.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Peters-Golden M., Canetti C., Mancuso P., Coffey M.J. Leukotrienes: underappreciated mediators of innate immune responses. J. Immunol. 2005;174:589–594. doi: 10.4049/jimmunol.174.2.589. [DOI] [PubMed] [Google Scholar]
- 49.McCarthy M.K., Weinberg J.B. Eicosanoids and respiratory viral infection: coordinators of inflammation and potential therapeutic targets. Mediat. Inflamm. 2012;2012:236345. doi: 10.1155/2012/236345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the 'Cytokine Storm’ in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L.S., Chan I.H.S., Lit L.C.W., Hui D.S.C., Chan M.H.M., Chung S.S.C., Sung J.J.Y. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anti S.M.A., Giorgi R.D.N., Chahade W.H. Steroidal antiinflammatory drugs: glucocorticoids. Einstein. 2008;6:159–165. [Google Scholar]
- 53.Becker D.E. Basic and clinical pharmacology of glucocorticosteroids. Anesth. Prog. 2013;60:25–32. doi: 10.2344/0003-3006-60.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramamoorthy S., Cidlowski J.A. Corticosteroids: mechanisms of action in health and disease. Rheum. Dis. Clin. 2016;42:15–31. doi: 10.1016/j.rdc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazaleuskaya L.L., Theken K.N., Gong L., Thorn C.F., FitzGerald G.A., Altman R.B., Klein T.E. PharmGKB summary: ibuprofen pathways. Pharmacogenetics Genom. 2015;25:96–106. doi: 10.1097/FPC.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Awtry E.H., Joseph L. Aspirin. Circulation. 2000;101:1206–1218. doi: 10.1161/01.CIR.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 58.Calder P.C. Nutrition , immunity and COVID-19, BMJ nutr. Prev. & Health. 2020 doi: 10.1136/bmjnph-2020-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calder P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012;142:592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 60.Browning L.M., Walker C.G., Mander A.P., West A.L., Madden J., Gambell J.M., Young S., Wang L., Jebb S.A., Calder P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012;96:748–758. doi: 10.3945/ajcn.112.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ottestad I., Hassani S., Borge G.I., Kohler A., Vogt G., Hyötyläinen T., Orešič M., Brønner K.W., Holven K.B., Ulven S.M., Myhrstad M.C.W. Fish oil supplementation alters the plasma lipidomic profile and increases long-chain PUFAs of phospholipids and triglycerides in healthy subjects. PloS One. 2012;7 doi: 10.1371/journal.pone.0042550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gerling C.J., Mukai K., Chabowski A., Heigenhauser G.J.F., Holloway G.P., Spriet L.L., Jannas-Vela S. Incorporation of omega-3 fatty acids into human skeletal muscle sarcolemmal and mitochondrial membranes following 12 weeks of fish oil supplementation. Front. Physiol. 2019;10:348. doi: 10.3389/fphys.2019.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murru E., Banni S., Carta G. Nutritional properties of dietary omega-3-enriched phospholipids. BioMed Res. Int. 2013:965417. doi: 10.1155/2013/965417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calder P.C. Long chain fatty acids and gene expression in inflammation and immunity. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:425–433. doi: 10.1097/MCO.0b013e3283620616. https://doi: 10.1097/MCO.0b013e3283620616 [DOI] [PubMed] [Google Scholar]
- 65.Rogero M.M., Calder P.C. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. 2018;10:1–19. doi: 10.3390/nu10040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spite M., Serhan C.N. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ. Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sorokin A.V., Yang Z.H., Remaley A.T. Anti-inflammatory and atheroprotective properties of omega-3 polyunsaturated fatty acids. J. Clin. Exp. Cardiol. 2016;7:5–7. doi: 10.4172/2155-9880.1000478. [DOI] [Google Scholar]
- 68.Ramkumar J., Sharma N. Low dose aspirin and omega-3 fatty acids in the pro-resolving pathway of cardiovascular disorders. Cardiol. Angiol.: Int. J. 2017;6:1–12. doi: 10.9734/ca/2017/36200. [DOI] [Google Scholar]
- 69.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Souza D.R., Pieri B.L. da S., Comim V.H., Marques S. de O., Luciano T.F., Rodrigues M.S., de Souza C.T. Fish oil reduces subclinical inflammation, insulin resistance, and atherogenic factors in overweight/obese type 2 diabetes mellitus patients: a pre-post pilot study. J. Diabet. Complicat. 2020;34:107553. doi: 10.1016/j.jdiacomp.2020.107553. [DOI] [PubMed] [Google Scholar]
- 71.Malekshahi Moghadam A., Saedisomeolia A., Djalali M., Djazayery A., Pooya S., Sojoudi F. Efficacy of omega-3 fatty acid supplementation on serum levels of tumour necrosis factor-alpha, C-reactive protein and interleukin-2 in type 2 diabetes mellitus patients. Singapore Med. J. 2012;53:615—619. [PubMed] [Google Scholar]
- 72.Stapleton R.D., Martin J.M., Mayer K. Fish oil in critical illness: mechanisms and clinical applications. Crit. Care Clin. 2010;26:501–514. doi: 10.1016/j.ccc.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calder P.C. n−3 Fatty acids, inflammation, and immunity— relevance to postsurgical and critically III patients. Lipids. 2004;39:1147–1161. doi: 10.1007/s11745-004-1342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parikh R., Bates J.H.T., Poynter M.E., Suratt B.T., Parsons P.E., Kien C.L., Heyland D.K., Crain K.I., Martin J., Garudathri J., Stapleton R.D. Pharmacokinetics of omega-3 fatty acids in patients with severe sepsis compared with healthy volunteers: a prospective cohort study. Clin. Nutr. 2020;39:958–965. doi: 10.1016/j.clnu.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pontes-Arruda A., Aragão A.M.A., Albuquerque J.D. Effects of enteral feeding with eicosapentaenoic acid, γ-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit. Care Med. 2006;34:2325‐2333. doi: 10.1097/01.CCM.0000234033.65657.B6. https://doi: 10.1097/01.CCM.0000234033.65657.B6 [DOI] [PubMed] [Google Scholar]
- 76.Hosny M., Nahas R., Ali S., Elshafei S.A., Khaled H. Impact of oral omega-3 fatty acids supplementation in early sepsis on clinical outcome and immunomodulation, Egypt. J. Crit. Care Med. 2013;1:119–126. doi: 10.1016/j.ejccm.2013.11.002. [DOI] [Google Scholar]
- 77.Mancuso P., Whelan J., DeMichele S.J., Snider C.C., Guszcza J.A., Claycombe K.J., Smith G.T., Gregory T.J., Karlstad M.D. Effects of eicosapentaenoic and gamma-linolenic acid on lung permeability and alveolar macrophage eicosanoid synthesis in endotoxic rats. Crit. Care Med. 1997;25:523–532. doi: 10.1097/00003246-199703000-00024. https://doi: 10.1097/00003246-199703000-00024 [DOI] [PubMed] [Google Scholar]
- 78.Mancuso P., Whelan J., DeMichele S.J., Snider C.C., Guszcza J.A., Karlstad M.D. Dietary fish oil and fish and borage oil suppress intrapulmonary proinflammatory eicosanoid biosynthesis and attenuate pulmonary neutrophil accumulation in endotoxic rats. Crit. Care Med. 1997;25:1198–1206. doi: 10.1097/00003246-199707000-00023. https://doi: 10.1097/00003246-199707000-00023 [DOI] [PubMed] [Google Scholar]
- 79.Uddin M., Levy B.D. Resolvins: natural agonists for resolution of pulmonary inflammation. Prog. Lipid Res. 2011;50:75–88. doi: 10.1016/j.plipres.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao Y., Zhang H., Luo L., Lin J., Li D., Zheng S., Huang H., Yan S., Yang J., Hao Y., Li H., Gao Smith F., Jin S. Resolvin D1 improves the resolution of inflammation via activating nf-κb p50/p50–mediated cyclooxygenase-2 expression in acute respiratory distress syndrome. J. Immunol. 2017;199:2043–2054. doi: 10.4049/jimmunol.1700315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang H.-W., Wang Q., Mei H.-X., Zheng S.-X., Ali A.M., Wu Q.-X., Ye Y., Xu H.-R., Xiang S.-Y., Jin S.-W. RvD1 ameliorates LPS-induced acute lung injury via the suppression of neutrophil infiltration by reducing CXCL2 expression and release from resident alveolar macrophages. Int. Immunopharm. 2019;76:105877. doi: 10.1016/j.intimp.2019.105877. [DOI] [PubMed] [Google Scholar]
- 82.Wang Q., Yan S.-F., Hao Y., Jin S.-W. Specialized pro-resolving mediators regulate alveolar fluid clearance during acute respiratory distress syndrome. Chin. Med. J. (Engl). 2018;131:982–989. doi: 10.4103/0366-6999.229890. https://doi: 10.4103/0366-6999.229890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singer P., Theilla M., Fisher H., Gibstein L., Grozovski E., Cohen J. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit. Care Med. 2006;34:1033‐1038. doi: 10.1097/01.CCM.0000206111.23629.0A. https://doi:10.1097/01.CCM.0000206111.23629.0A [DOI] [PubMed] [Google Scholar]
- 84.Shirai K., Yoshida S., Matsumaru N., Toyoda I., Ogura S. Effect of enteral diet enriched with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with sepsis-induced acute respiratory distress syndrome. J. Intensive Care. 2015;3:24. doi: 10.1186/s40560-015-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gadek J.E., DeMichele S.J., Karlstad M.D., Pacht E.R., Donahoe M., Albertson T.E., Van Hoozen C., Wennberg A.K., Nelson J.L., Noursalehi M. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Crit. Care Med. 1999;27:1409‐1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 86.Pontes-Arruda A., DeMichele S., Seth A., Singer P. The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: a meta-analysis of outcome data. J. Parenter. Enteral Nutr. 2008;32:596–605. doi: 10.1177/0148607108324203. [DOI] [PubMed] [Google Scholar]
- 87.Dushianthan A., Cusack R., Burgess V.A., Grocott M.P., Calder P.C. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database Syst. Rev. 2019;1:CD012041. doi: 10.1002/14651858.CD012041.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bistrian B.R. Parenteral fish oil emulsions in critically ill COVID-19 emulsions. J. Parenter. Enteral Nutr. 2020 doi: 10.1002/jpen.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Messina G., Polito R., Monda V., Cipolloni L., Di Nunno N., Di Mizio G., Murabito P., Carotenuto M., Messina A., Pisanelli D., Valenzano A., Cibelli G., Scarinci A., Monda M., Sessa F. Functional role of dietary intervention to improve the outcome of COVID-19: a hypothesis of work. Int. J. Mol. Sci. 2020;21:E3104. doi: 10.3390/ijms21093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12:1181. doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Torrinhas R.S., Calder P., Lemos G.O., Waitzberg D.L. Parenteral fish oil, an adjuvant pharmacotherapy for COVID-19? Nutrition. 2020. http://eprints.soton.ac.uk/id/eprint/440710 [DOI] [PMC free article] [PubMed]
- 92.Torrinhas R.S., Calder P., Waitzberg D.L. Letter to the Editor in relation to Bistrian BR. Parenteral fish oil emulsions in critically ill COVID-19 emulsions [published online ahead of print, 2020 May 8] J. Parenter. Enteral Nutr. 2020 doi: 10.1002/jpen.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Calder P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Endres S., Ghorbani R., Kelley V.E., Georgilis K., Lonnemann G., van der Meer J.W.M., Cannon J.G., Rogers T.S., Klempner M.S., Weber P.C., Schaefer E.J., Wolff S.M., Dinarello C.A. The effect of dietary supplementation with n—3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells, N. Engl. J. Med. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 95.NCT04335032 A randomized controlled study of eicosapentaenoic acid (EPA-FFA) gastro-resistant capsules to treat hospitalized subjects with confirmed SARS-CoV-2. https://clinicaltrials.gov/ct2/show/NCT04335032 Available from. June 1st 2020.
- 96.Morita M., Kuba K., Ichikawa A., Nakayama M., Katahira J., Iwamoto R., Watanebe T., Sakabe S., Daidoji T., Nakamura S., Kadowaki A., Ohto T., Nakanishi H., Taguchi R., Nakaya T., Murakami M., Yoneda Y., Arai H., Kawaoka Y., Penninger J.M., Arita M., Imai Y. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 97.Ramon S., Baker S.F., Sahler J.M., Kim N., Feldsott E.A., Serhan C.N., Martínez-Sobrido L., Topham D.J., Phipps R.P. The specialized pro-resolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? J. Immunol. 2014;193:6031–6040. doi: 10.4049/jimmunol.1302795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Braz-De-Melo H.A., Pasquarelli-do-Nascimento G., Corrêa R., das Neves Almeida R., de Oliveira Santos I., Prado P.S., Picolo V., de Bem A.F., Pizato N., Magalhães K.G. Potential neuroprotective and anti-inflammatory effects provided by omega-3 (DHA) against Zika virus infection in human SH-SY5Y cells. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-56556-y. 20119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Byleveld P.M., Pang G.T., Clancy R.L., Roberts D.C.K. Fish oil feeding delays influenza virus clearance and impairs production of interferon-γ and virus-specific immunoglobulin a in the lungs of mice. J. Nutr. 1999;129:328–335. doi: 10.1093/jn/129.2.328. [DOI] [PubMed] [Google Scholar]
- 100.Byleveld P.M., Pang G.T., Clancy R.L., Roberts D.C. Fish oil feeding enhances lymphocyte proliferation but impairs virus-specific T lymphocyte cytotoxicity in mice following challenge with influenza virus. Clin. Exp. Immunol. 2000;119:287–292. doi: 10.1046/j.1365-2249.2000.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bochkov V., Gesslbauer B., Mauerhofer C., Philippova M., Erne P., Oskolkova O.V. Pleiotropic effects of oxidized phospholipids. Free Radic. Biol. Med. 2017;111:6–24. doi: 10.1016/j.freeradbiomed.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 102.Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 103.Hauck A.K., Bernlohr D.A. Oxidative stress and lipotoxicity. J. Lipid Res. 2016;57:1976–1986. doi: 10.1194/jlr.R066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moghe A., Ghare S., Lamoreau B., Mohammad M., Barve S., McClain C., Joshi-Barve S. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol. Sci. 2015;143:242–255. doi: 10.1093/toxsci/kfu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reis A. Oxidative phospholipidomics in health and disease: achievements, challenges and hopes. Free Radic. Biol. Med. 2017;111:25–37. doi: 10.1016/j.freeradbiomed.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 106.Que X., Hung M.-Y., Yeang C., Gonen A., Prohaska T.A., Sun X., Diehl C., Määttä A., Gaddis D.E., Bowden K., Pattison J., MacDonald J.G., Ylä-Herttuala S., Mellon P.L., Hedrick C.C., Ley K., Miller Y.I., Glass C.K., Peterson K.L., Binder C.J., Tsimikas S., Witztum J.L. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature. 2018;558:301–306. doi: 10.1038/s41586-018-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morrow J.D., Hill K.E., Burk R.F., Nammour T.M., Badr K.F., Roberts L.J. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. Unit. States Am. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vigor C., Bertrand-Michel J., Pinot E., Oger C., Vercauteren J., Le Faouder P., Galano J.-M., Lee J.C.-Y., Durand T. Non-enzymatic lipid oxidation products in biological systems: assessment of the metabolites from polyunsaturated fatty acids. J. Chromatogr. B. 2014;964:65–78. doi: 10.1016/j.jchromb.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 109.Milne G.L., Musiek E.S., Morrow J.D. F2-Isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;10:10–23. doi: 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- 110.Ayala A., Muñoz M.F., Argüelles S. Lipid Peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Milne G.L., Yin H., Hardy K.D., Davies S.S., Roberts L.J. Isoprostane generation and function. Chem. Rev. 2011;111:5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reshi M.L., Su Y.-C., Hong J.-R. RNA Viruses: ROS-mediated cell death. Int. J. Cell Biol. 2014:467452. doi: 10.1155/2014/467452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khomich O.A., Kochetkov S.N., Bartosch B., Ivanov A.V. Redox biology of respiratory viral infections. Viruses. 2018;10:392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang M.Q., Wang X.H., Chen Y.L., Zhao K.L., Cai Y.Q., An C.L., Lin M.G., Mu X.D. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Zhonghua Jiehe He Huxi Zazhi. 2020;43 doi: 10.3760/cma.j.issn.1001-0939.2020.0013. E013–E013. [DOI] [PubMed] [Google Scholar]
- 115.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemostasis. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iba T., Levy J.H., Warkentin T.E., Thachil J., van der Poll T., Levi M. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Thromb. Haemostasis. 2019;17:1989–1994. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]
- 117.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV-2 and non-SARS-CoV-2. J. Thromb. Thrombolysis. 2020:1–4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wander R.C., Patton B.D. Comparison of three species of fish consumed as part of a Western diet: effects on platelet fatty acids and function, hemostasis, and production of thromboxane. Am. J. Clin. Nutr. 1991;54:326–333. doi: 10.1093/ajcn/54.2.326. [DOI] [PubMed] [Google Scholar]
- 119.Adili R., Hawley M., Holinstat M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostag. Other Lipid Mediat. 2018;139:10–18. doi: 10.1016/j.prostaglandins.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park Y., Harris W. EPA, but not DHA, decreases mean platelet volume in normal subjects. Lipids. 2002;37:941. doi: 10.1007/s11745-006-0984-1. [DOI] [PubMed] [Google Scholar]