Abstract
Purpose.
Ovarian serous carcinoma is an aggressive cancer that often presents with metastatic disease. Although primary tumor and established metastatic foci in the omentum are generally compared to identify proteins involved in drug resistance, we investigated a potential bridge, the malignant cells from ascites, as facilitator of drug resistance and recurrence.
Methods.
We evaluated the expression of drug resistance markers P-glycoprotein (P-gp), canalicular multispecific organic anion transporter (MRP2), and lung resistance-related protein (LRP) in malignant cells from ascites and matched omental metastasis from 25 patients with advanced-stage ovarian serous carcinoma who were chemotherapeutic naїve and undergoing initial cytoreductive surgery. Cell viability in vitro, patient response to chemotherapy, and patient survival were correlated with these biomarkers.
Results.
Of the 25 patients evaluated for a correlation of LRP to 1-year recurrence, we correctly predicted the 1-year recurrence of 24 patients based solely on the presence of LRP in ascitic tumor cells (p = 0.01). P-gp and MRP2 were not expressed in malignant cells of ascites or omental metastases. Malignant cells from ascites had higher expression of LRP and were found to be more resistant to carboplatin treatment than cells from omental metastasis (p = 0.00375) by in vitro assay. LRP expression in the malignant cells of ascites correlated with carboplatin resistance (p = 0.001) by in vitro assay and recurrence at 1 year (p = 0.0125).
Conclusions.
LRP expression in malignant cells of ascites is a promising marker to predict response to first-line chemotherapy in patients with advanced ovarian serous carcinoma.
The majority (75 %) of patients with ovarian carcinoma present with metastatic disease throughout the peritoneal cavity.1–3 Although treatment involves cytoreductive surgery with removal of as much of the primary tumor and metastatic disease as possible followed by combination chemotherapy, the majority of patients will have recurrent disease and the 5-year survival rate is only 30 %.3 Accordingly, there is a need for identification of biological markers that might provide useful prognostic information and predict outcomes, including response to therapy.
Currently, treatment responses are evaluated by the change in measurable disease, and most studies focus on the primary tumor and metastatic foci in the omentum.2 P-glycoprotein and canalicular multispecific organic anion transporter, MRP2, are plasma membrane proteins that actively pump drugs out of cells.4–8 Whereas P-glycoprotein overexpression and canalicular multispecific organic anion transporter overexpression have been associated with resistance to taxane or cisplatin respectively, both of which combined are the first-line treatment for ovarian cancer.9 Lung resistance-related protein is a new marker of drug resistance, which is thought to mediate intracellular transport of a wide variety of substrates, including cisplatin.10–12 At present, P-gp (7–76 %), MRP2 (16–56 %), and LRP (12–76 %) have not been a reliable predictor of response to chemotherapy and survival in either primary tumor or omental metastasis of epithelial ovarian carcinomas.4–15
However, with new evidence supporting the role of malignant cells in ascites in ovarian cancer dissemination, we chose to focus this study on the malignant cells in ascites.2,16 Therefore, the primary purpose of this study was to examine expression of P-gp, MRP2, and LRP in malignant cells of ascites of chemotherapy-naїve patients with advanced ovarian serous carcinoma. In addition, expression of LRP was correlated with cell survival after treatment with carboplatin, Taxol, and combination of carboplatin/Taxol in vitro, as well as association with patient response to chemotherapy and patient survival.
MATERIALS AND METHODS
Patient Selection and Follow-up
After institutional review board approval, ascites and omental metastasis were collected prospectively from patients with suspected advanced-stage ovarian serous carcinoma undergoing initial cytoreductive surgery and who were chemotherapy naїve. The histology of the tumors was confirmed by a diagnostic pathologist (WEG), and patients with papillary serous or mixed papillary serous carcinomas were included. The International Federation of Obstetricians and Gynecologists (FIGO) staging was obtained from the pathology report, and the extent of cytoreduction (optimal versus suboptimal cytoreduction) was obtained from the operative note. Additional clinical information, including patient demographics, initial chemotherapy regimen, presence/absence of recurrence, and outcomes were obtained from medical records. Patients received 6 AUC of Carboplatin by IV and Taxol at 175 mg/m2 over 3 h by IV. Patients received six cycles of chemotherapy (96 %), and any deviations were based on the decision of the gynecologic oncologist and/or patient. Recurrence was determined by CT imaging using RECIST criteria and/or Gynecologic Cancer Intergroup CA-125 progression criteria. After institutional review board approval, tumor cells from ascites fluid were isolated from six patients with recurrent ovarian cancer. These recurrent patients had received six cycles of chemotherapy before palliative removal of ascites. Recurrence was confirmed using CT imaging or CA-125 progression criteria.
Specimen Collection and Processing
The ascites and omental metastases were obtained within 30 min of the initiation of the surgery. The former was collected in sterile plastic containers and transported on ice. Malignant cells in the ascites were isolated by initially diluting with phosphate buffered saline (PBS) and centrifuging at 25–32 ×g for 5 min. Once a cell pellet was obtained, it was washed twice in PBS. To confirm the presence of malignant epithelial cells in the ascites, a cytopathologist (IEE) reviewed the hematoxylin and eosin slides of the ascites. Immunohistochemical stains, including cytokeratin 5/6, calretinin, MOC31, and CA-125, were performed.17,18 The ascites volume and color were noted. Eight-millimeter tumor slices were prepared from omental metastasis using a Krumdieck tissue slicer sequentially cut cores as previously described.19,20 Tumor cells from ascites of the six recurrent patients were collected as indicated above. The isolated tumor cells were evaluated for LRP using western blot as described below.
Treatment
The patient samples were treated with 30 or 50 μM of carboplatin, 600 or 1,000 nM of Taxol, and a combination of 30 μM/600 nM or 50 μM/1,000 nM of carboplatin/Taxol. These doses were chosen based on previously published results.19 The omental tumor slices were treated in 24-well plates, as previously described,19,20 and the malignant cells of the ascites were treated in replicates of 8. All samples were treated for 48 h and had vehicle controls. Assessment of cell viability was determined by measurement of cellular ATP levels using ATPLite luminescence-based assay (Perkin Elmer, Boston, MA).20
Western Blot Analysis
Both tumor cells from ascites of naїve and recurrent patients as well as omental metastasis from naїve patients were analyzed for LRP levels by Western blot. Samples were washed twice with PBS and lysed with 1 % NP-40 buffer with 0.2 mM of sodium orthovanadate with Complete-mini protease inhibitor (Roche, Indianapolis, IN) using standard procedures. The protein concentration of each sample was determined using Bradford assay (Bio-Rad, Hercules, CA). Samples (25 μg of protein) were resolved by SDS-gel electrophoresis and transferred onto PVDF membranes. Membranes were incubated with primary antibodies to LRP (BD Biosciences, Franklin Lakes, NJ) and B-actin overnight at 4 °C, then secondary antibodies (Bio-Rad, Hercules, CA) for 1 h. Proteins were visualized using chemiluminescence reagents (GE Healthcare, Piscataway, NJ) according to manufacturer’s instructions.
Fixation and Paraffinization
The malignant cells in the ascites were centrifuged in 15-mL tubes at 100 g for 5–10 min. The supernatant was decanted and the pellet was resuspended in 2.0 mL of PBS. To fix the cells, 4.0 mL of 10 % neutral-buffered formalin was added to cell suspension for 1 h at 25 °C. Cells were centrifuged and all supernatant was removed. Warmed Histogel (Richard-Allan Scientific, Kalamazoo, MI) was used to resuspend the cells, transferred to a small mold (cap of a 100-lL microfuge tube), and stored at 4 °C until Histogel solidified. The solid Histogel pellet was then transferred to a tissue-processing cassette and placed in cold 10 % neutral buffered formalin for 6–24 h. Samples were embedded in paraffin.
Immunohistochemistry
The methods used for bright-field immunohistochemistry (IHC) analysis have been previously described.21 Specifically, 5-micron sections of formalin-fixed, paraffin-embedded blocks of omental metastasis and malignant ascitic cells were mounted on Chrome Alum Gelatin treated slides. Primary antibodies were applied for 1 h at 25 °C (Pgp; BD Biosciences, dilution 1:1,000; MRP2; Millipore, Billerica, MA, dilution 1:1,000; LRP; BD Biosciences, dilution 1:750). The staining was determined by one of the coauthors (WEG), who was blinded to the clinical information. Cytoplasmic staining was deemed positive for P-gp and LRP and intercanalicular staining was deemed positive for MRP2. For all three antibodies, any degree of staining in the malignant cells/tumor was classified as positive.
Statistical Analysis
Data were analyzed using the statistical software package, SAS 9.1 (SAS Institute, Cary, NC). Wilcoxon sign-rank test was used to evaluate the cell viability of malignant ascitic cells and omental metastases in response to carboplatin, Taxol, and combination chemotherapy. In malignant ascitic cells and omental metastases, LRP correlation to cell viability was assessed using Pearson correlation and Spearman rank correlation. The rate of recurrence in LRP-positive and -negative patients was measured using Kaplan–Meier log-rank test and Chi square test to determine the goodness of fit for LRP prediction at 6 months after ending first-line chemotherapy and 1 year after surgery.
RESULTS
Patient Characteristics
From July 2009 to Dec 2010, we obtained consent from 45 newly diagnosed patients with primary epithelial ovarian carcinoma treated with cytoreductive surgery at the University of Alabama at Birmingham. Of those, 25 patients met all criteria, including a diagnosis of papillary serous or mixed papillary serous ovarian carcinoma, presence of malignant ascitic cells and omental tumor, and chemotherapy-naїve (Supplemental Table 1). The mean age of patients was 66 (range, 40–79) years, and the majority of patients were Caucasian (96 %). The majority of the patients had papillary serous histologic subtype (80 %) and stage III disease (84 %). The mean volume of ascites removed was 4 l (range, 0.5–8), and the color of the fluid ranged from yellow to red. The majority of the patients had suboptimal cytoreductive surgery (76 %).
Follow-up information was available on 23 (92 %) of the patients. All but one patient received standard adjuvant chemotherapy, including paclitaxel and carboplatin therapy (96 %). Median follow-up was 17 (range, 2–33) months. Fifteen (65 %) patients recurred with a median time to recurrence of 11 (range, 4–18) months. Eight (35 %) patients died with disease with a median time to death of 14 (range, 2–28) months. Except for the presence of recurrence, none of the clinicopathological data correlated with survival.
Protein Expression
The first five patient samples were stained for P-gp, MRP2, and LRP by IHC. However, P-gp and MRP2 showed no staining in all five patients’ malignant ascitic cells and omental metastases, with the positive and negative controls staining appropriately (Fig. 1). Due to cost considerations, the remaining patient samples (n = 20) were only stained for LRP (Fig. 2). Fifteen (60 %) malignant ascites samples showed LRP expression and 6 (24 %) omental metastases samples showed LRP expression by IHC. All 6 of the positive omental metastases were matched with 1 of the 15 patients with LRP-positive malignant ascitic cells. In Supplemental Fig. 1, Western blot analysis of LRP correlated with LRP expression by IHC. Ascitic tumor cells from recurrent patients were LRP-positive.
FIG. 1.
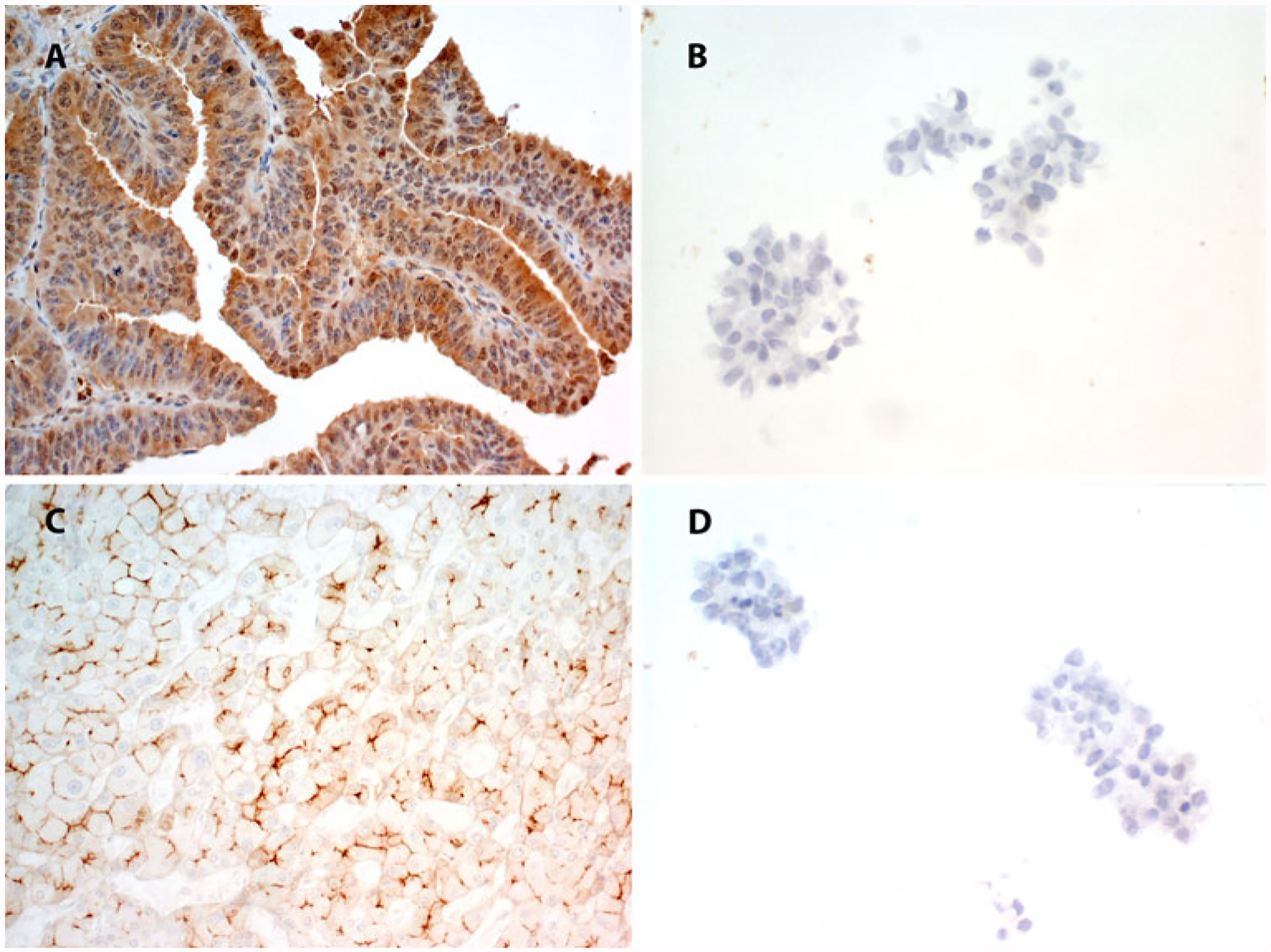
The positive controls stained appropriately for P-gp (a) and MRP2 (c), whereas the malignant cells in the ascites showed no staining for P-gp (b) or MRP2 (d)
FIG. 2.
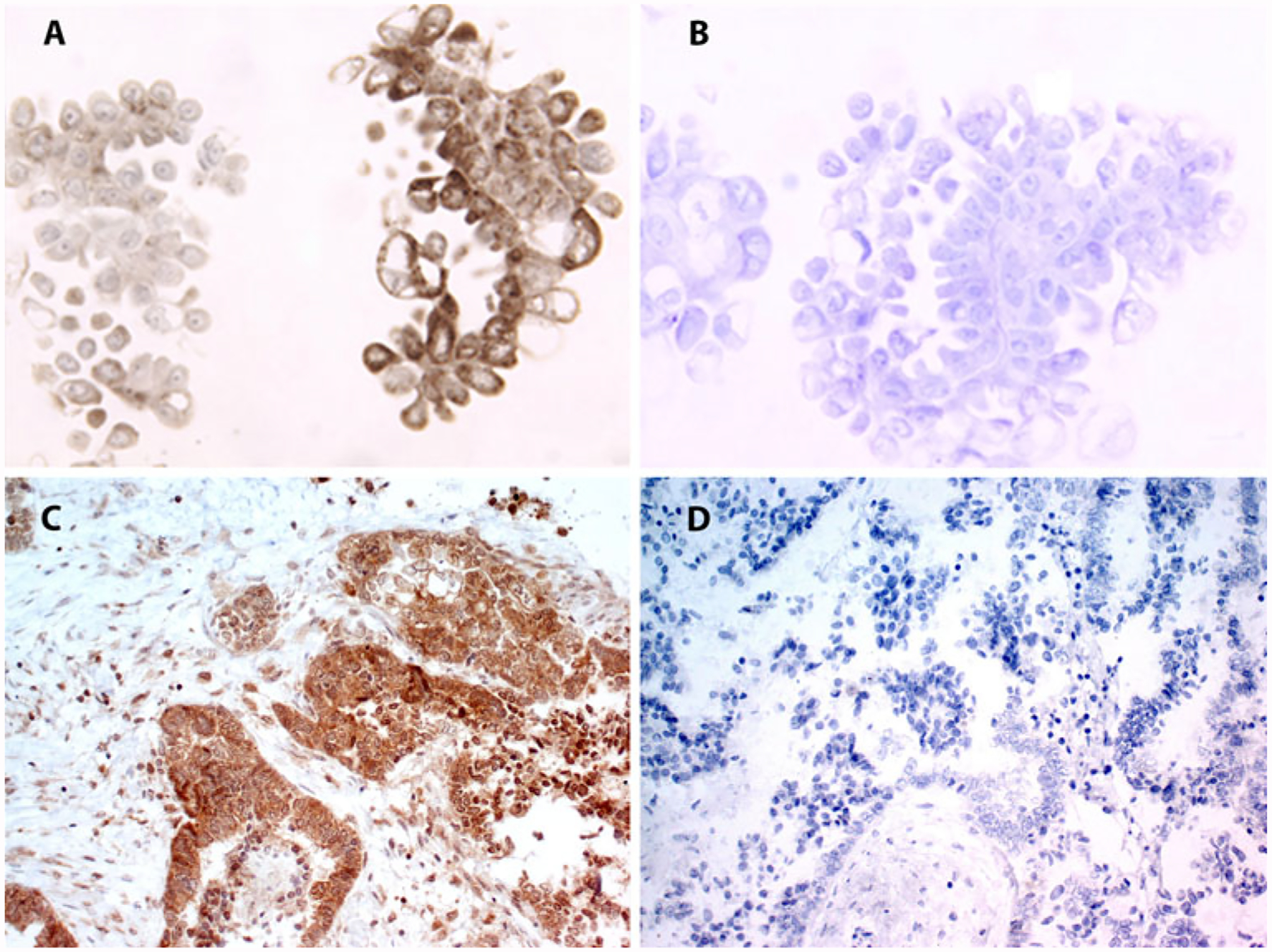
Positive LRP expression (a) and negative LRP expression (b) by IHC in malignant ascitic cells. Positive LRP expression (c) and negative LRP expression (d) by IHC in an omental metastasis
Cell Survival Assay and Protein Expression
The patient samples were treated with 30 μM of carboplatin, 50 μM of carboplatin, 600 nM of Taxol, 1,000 nM of Taxol, and combinations of 30 μM of carboplatin and 600 nM of Taxol, or 50 μM of carboplatin and 1,000 nM of Taxol. The malignant cells of ascites were significantly more resistant to carboplatin treatment than the omental metastasis (30 μM of carboplatin, p = 0.014; 50 μM carboplatin, p = 0.00375; Fig. 3). There was not a significant difference in response to 1,000 nM paclitaxel or a combination of 30 μM of carboplatin/600 nM of Taxol or 50 μM of carboplatin/1,000 nM of paclitaxel between the omental metastasis and the malignant ascitic cells (p < 0.05).
FIG. 3.
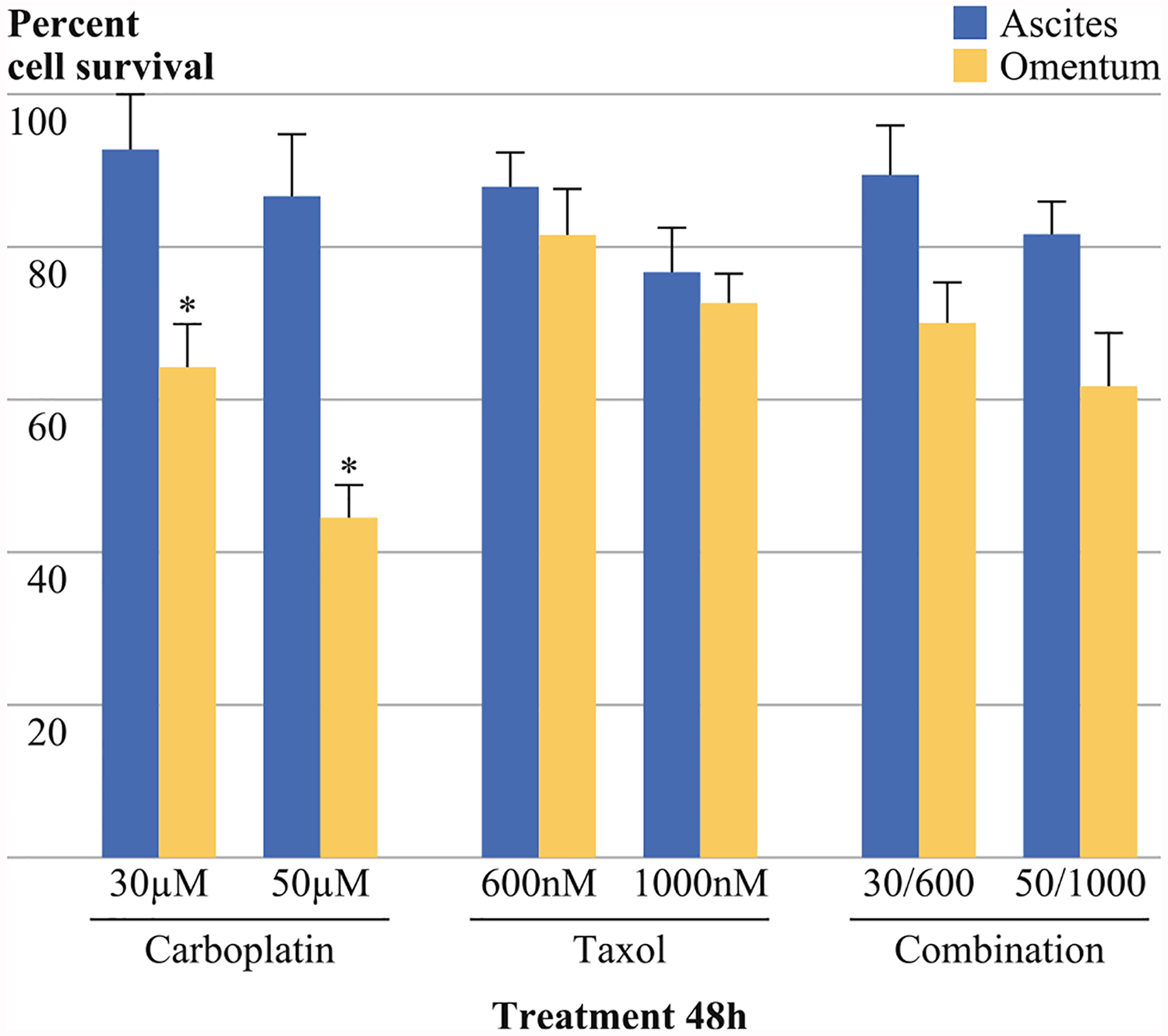
The malignant cells of ascites were significantly more resistant to treatment with 30 or 50 μM of carboplatin than the omental metastasis (p = 0.014; p = 0.00375 respectively). However, no significant difference was seen after treatment with Paclitaxel (600 or 1,000 nM) paclitaxel or a combination therapy of 30 μM carboplatin/600 nM taxlitaxel or 50 μM carboplatin/1,000 nM paclitaxel (p > 0.05). Samples were assayed using the ATP lite assay and samples were normalized to untreated control of the same tissue type
Next, we looked at LRP expression and cell viability after treatment with 50 μM of carboplatin. Malignant ascitic cells that expressed LRP were significantly more resistant to treatment with carboplatin than the malignant ascitic cells that were negative for LRP (p = 0.0043; Fig. 4). LRP expression correlated with carboplatin resistance (Pearson, p = 0.001; Spearman, p = 0.0012). LRP also correlated with resistance to combination (50/1,000) treatment (Pearson, p = 0.045, but not Spearman, p = 0.053). The malignant ascitic cells that expressed LRP also were significantly more resistant to treatment with carboplatin than the omental metastasis, which also expressed LRP (p = 0.00209). Expression of LRP by IHC in omental samples did not correlate with a more resistant phenotype (p = 0.18).
FIG. 4.
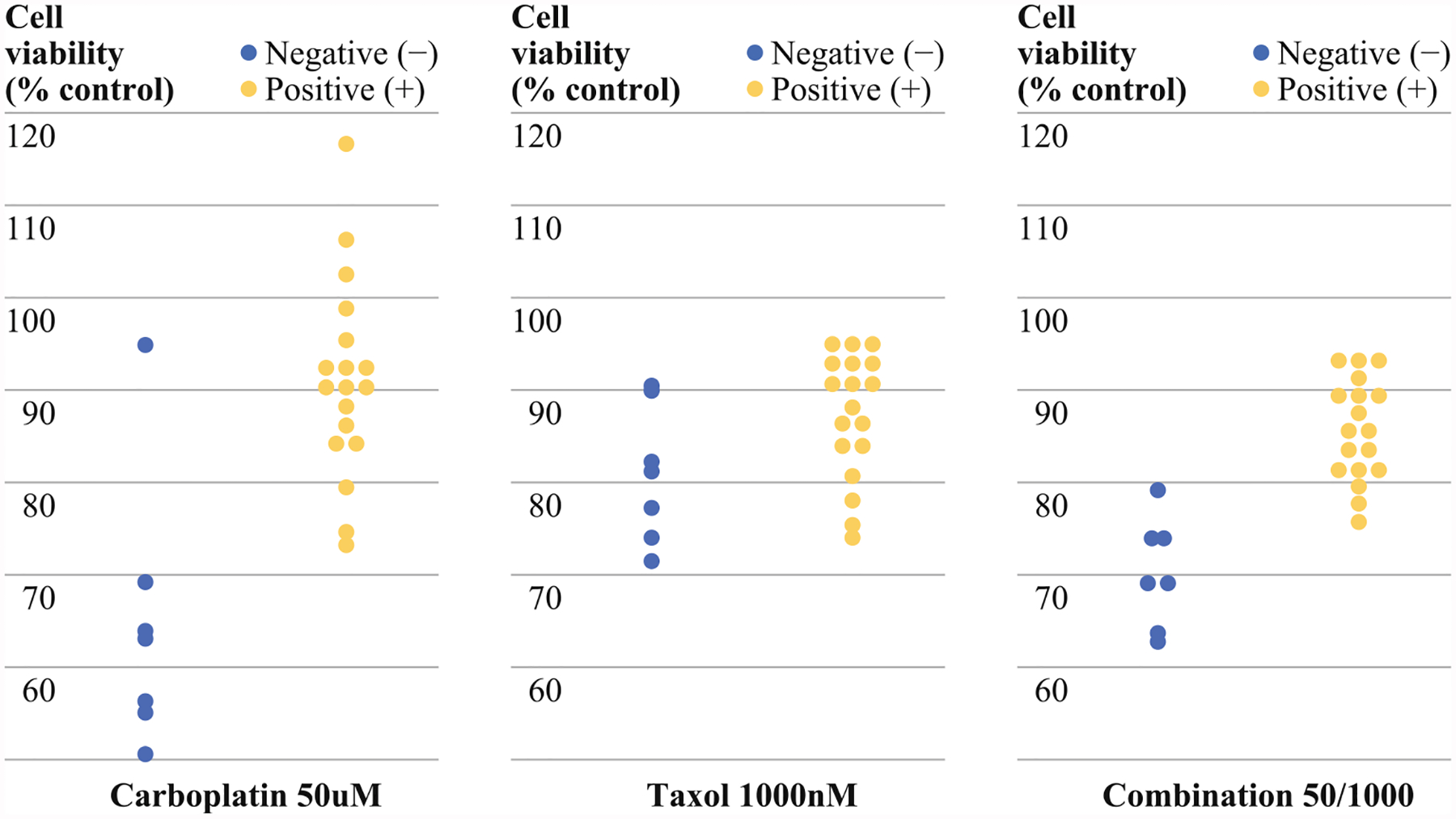
Malignant ascitic cells positive for LRP by IHC (+) showed greater resistance/cell viability to treatment with 50 μM carboplatin than malignant ascitic cells negative for LRP by IHC (−) (p = 0.0043). LRP stratification did not identify sensitivity to paclitaxel using cell viability assay (p > 0.05). Cells were more viable in LRP+ patients after combination (50/1,000) treatment than LRP–patient samples (p = 0.045)
Patient Follow-up and Protein Expression
LRP expression by IHC in malignant ascitic cells correlated with the risk of recurrence at one year (p = 0.0125; Fig. 5) and predicted recurrence (p = 0.0192). LRP expression by IHC in omental metastasis did not correlate with recurrence (p < 0.05).
FIG. 5.
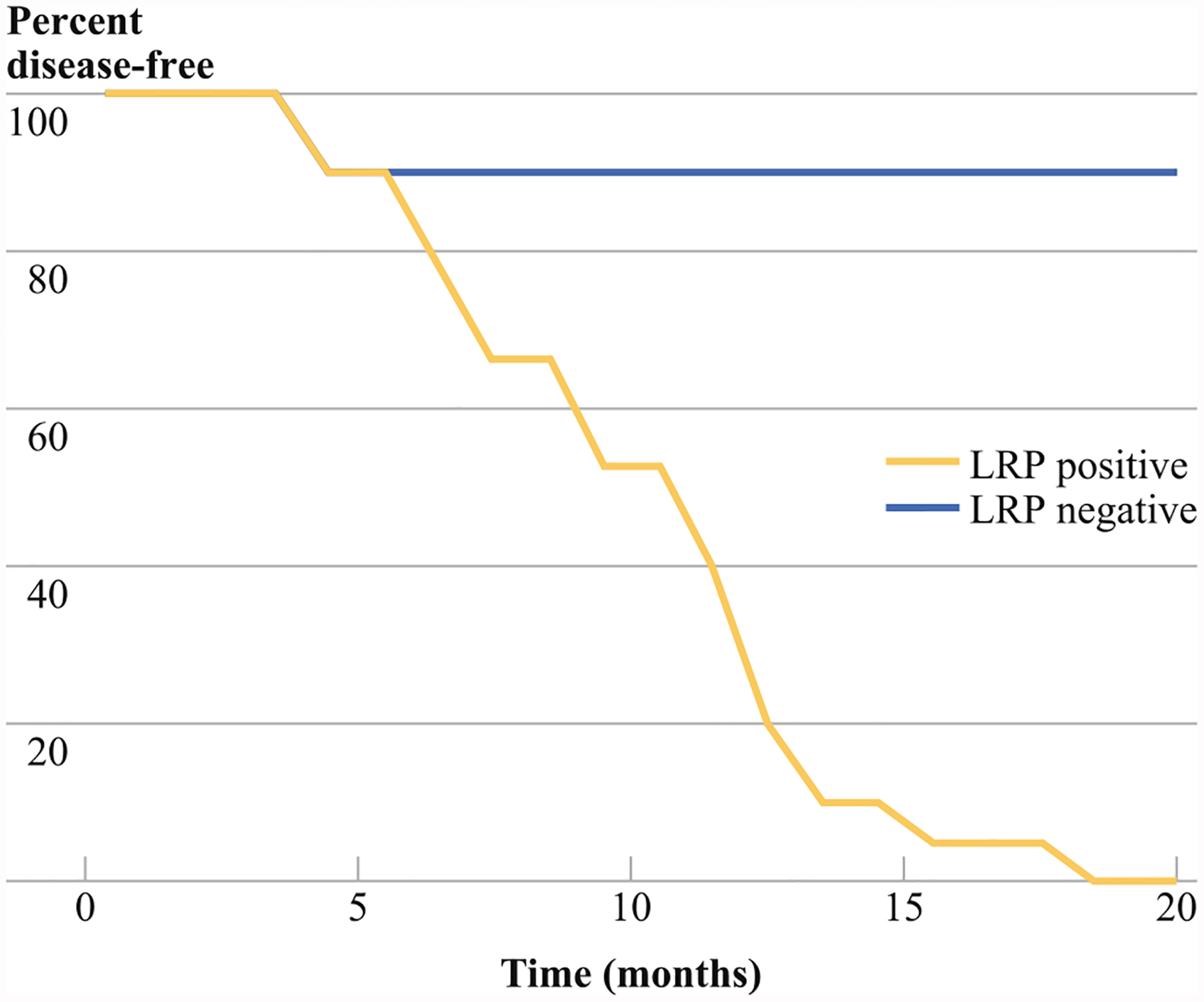
LRP expression in malignant ascitic cells by IHC correlates with recurrence at 1 year (p = 0.0125)
DISCUSSION
CA-125 as a tumor-progression criterion in relapsing ovarian cancer remains controversial.26,27 The most important finding in our study was the identification of LRP in ascitic tumor cells as a biomarker for carboplatin resistance and 1-year recurrence at the time of cytoreductive surgery in patients with advanced ovarian serous carcinoma. LRP expression on ascitic tumor cells had a PPV 83.3 % and NPV of 83.3 % at 12 months and PPV 100 % and NPV 83.3 % at 18 months of recurrence. A single marker that can be measured from readily obtainable tissue sampled at cytoreductive surgery can predict response to the most common adjuvant therapy used in ovarian serous carcinoma before initiation of therapy. The levels of alternative drug resistant P-gp and MRP2 in ascitic tumor cells did not correlate with survival or response to chemotherapy.
P-gp and MRP2 were not expressed by IHC in cellular specimens from our initial five untreated patients with papillary serous carcinomas. Conflicting data have been reported concerning the frequency of P-gp and MRP2 expression in ovarian carcinomas with a wide range of expression for P-gp (7–76 %) and for MRP2 (16–56 %).8,12–15 Due to the small number of samples evaluated, a role for these two molecules in the chemoresistance of ascitic cells of papillary serous carcinomas cannot be excluded; however, based on initial testing, it is unlikely that P-pg and MRP2 are predictive of recurrence.
The malignant cells of the ascites phenotypically expressed higher levels of LRP (60 %) than the cells of matched omental metastasis (24 %) respectively (Fig. 2). In addition, malignant ascitic cells were more resistant to treatment with carboplatin compared with the cells of omental metastases (p = 0.00375; Fig. 3). Malignant ascitic cells have anchorage and vascular independent structures, three-dimensional shape, and quiescent cellular states, which generate a metabolic density gradient making these cells more resistant to chemotherapeutic agents.25,26 Therefore, decreased sensitivity of the malignant ascitic cells to carboplatin compared with the cells of omental metastases could be in part due to unique three dimensional structures formed by the malignant ascitic cells. These ascitic cancer cells can remain within the patient after surgery and are likely to be responsible for metastatic colonization.22–24
The most striking observation of this study is prognostic significance of LRP expression in malignant cells of ascites. Stratification by LRP expression in the malignant cells of ascites demonstrated significant differences in cell viability (Fig. 4) and disease-free survival (Fig. 5). Recurrence was correctly predicted for each of the 25 patients with the exception of one stage IV patient who was identified as LRP negative but had early recurrence and was carboplatin resistant. The CA-125 level at the time of cytoreductive surgery did not correlate with recurrence at 1 year (data not shown). Likewise, LRP status in omental metastases and the characteristics of the ascites, such as volume and color, did not correlate with patient outcome. Optimal cytoreductive surgery correlates strongly with recurrence and survival and may have affected the results of this study. Because our study consisted of 25 patients, a multivariate analysis was not performed. Determination of LRP status has potential to identify patients at high risk of adjuvant therapy failure and early recurrence, which may benefit early enrollment in clinical trials of novel adjuvant therapies.
CONCLUSIONS
We demonstrated that 1-year, disease-free survival in advanced ovarian serous carcinoma could be predicted at the time of cytoreductive surgery based solely on the presence of LRP on ascitic tumor cells. The high positive and negative predictive values of LRP expression in ascitic tumor cells to carboplatin resistance and recurrence exceed current standards. Malignant cells in ascites may represent chemoresistant niches, which contribute to high recurrence of carboplatin-treated ovarian carcinomas in part via expression of LRP as a mechanism of exporting this category of drugs. These results support further consideration in larger, multi-institutional, prospective studies.
Supplementary Material
ACKNOWLEDGMENT
This project was supported by grants from the Kaleidoscope of Hope Ovarian Cancer Foundation to LRM and the Lupton Family Fellowship to EHK.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1245/s10434-013-2878-9) contains supplementary material, which is available to authorized users.
CONFLICT OF INTEREST The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351: 2519–29. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein LJ, Pastan I, Gottesman MM. Multidrug resistance in human cancer. Crit Rev Oncol Hematol. 1992;12:243–53. [DOI] [PubMed] [Google Scholar]
- 5.Bradley G, Ling V. P-glycoprotein, multidrug resistance and tumor progression. Cancer Metastasis Rev. 1994;13:223–33. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi K, Wada M, Kohno K, et al. A human canalicular multispecific organic anion transporter (cMOAT) gene is over-expressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation. Cancer Res. 1996;56:4124–9. [PubMed] [Google Scholar]
- 7.Kool M, de Hass M, Scheffer GL, et al. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537–47. [PubMed] [Google Scholar]
- 8.Arts HJ, Katsaros D, de Vries EG, et al. Drug resistance-associated markers P-glycoprotein, multidrug resistance-associated protein 1, multidrug resistance-associated protein 2, and lung resistance protein as prognostic factors in ovarian carcinoma. Clin Cancer Res. 1999;5:2798–805. [PubMed] [Google Scholar]
- 9.Izquierdo MA, Scheffer GL, Schroeijers AB, et al. Vault-related resistance to anticancer drugs determined by the expression of the major vault protein LRP. Cytotechnology. 1998;27:137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kedersha NL, Rome LH, Isolation and characterization of a novel ribonucleoprotein particle: large structures contain a single species of small RNA. J Cell Biol. 1986;103:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rome L, Kedersha N, Chugani D, Unlocking vaults: organelles in search of a function. Trends Cell Biol. 1991;1:47–50. [DOI] [PubMed] [Google Scholar]
- 12.Oda Y, Ohishi Y, Basaki Y, et al. Prognostic implications of the nuclear localization of Y-box-binding protein-1 and CXCR4 expression in ovarian cancer: their correlation with activated Akt, LRP/MVP and P-glycoprotein expression. Cancer Sci. 2007;98: 1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yakirevich E, Sabo E, Naroditsky I, et al. Multidrug resistance-related phenotype and apoptosis-related protein expression in ovarian serous carcinomas. Gynecol Oncol. 2006;100:152–9. [DOI] [PubMed] [Google Scholar]
- 14.Goff BA, Paley PJ, Greer BE, et al. Evaluation of chemoresistance markers in women with epithelial ovarian carcinoma. Gynecol Oncol. 2001;81:18–24. [DOI] [PubMed] [Google Scholar]
- 15.Goff BA, Ries JA, Els LP, et al. Immunophenotype of ovarian cancer as predictor of clinical outcome: evaluation at primary surgery and second-look procedure. Gynecol Oncol. 1998;70: 378–85. [DOI] [PubMed] [Google Scholar]
- 16.Shield K, Ackland ML, Ahmed N, et al. Multicellular spheroids in ovarian cancer metastases: biology and pathology. Gynecol Oncol. 2009;113:143–8. [DOI] [PubMed] [Google Scholar]
- 17.Comin CE, Saieva C, Messerini L. h-Caldesmon, calretinin, estrogen receptor, and Ber-EP4: a useful combination of immunohistochemical markers for differentiating epithelioid peritoneal mesothelioma from serous papillary carcinoma of the ovary. Am J Surg Pathol. 2007;31:1139–48. [DOI] [PubMed] [Google Scholar]
- 18.Ordonez NG. The diagnostic utility of immunohistochemistry and electron microscopy in distinguishing between peritoneal mesotheliomas and serous carcinomas: a comparative study. Mod Pathol. 2006;19:34–48. [DOI] [PubMed] [Google Scholar]
- 19.Estes JM, Oliver PG, Straughn JM Jr, et al. Efficacy of anti-death receptor 5 (DR5) antibody (TRA-8) against primary human ovarian carcinoma using a novel ex vivo tissue slice model. Gynecol Oncol. 2007;105:291–8. [DOI] [PubMed] [Google Scholar]
- 20.Frederick PJ, Kendrick JE, Straughn JM Jr, et al. Effect of TRA-8 anti-death receptor 5 antibody in combination with chemotherapy in an ex vivo human ovarian cancer model. Int J Gynecol Cancer. 2009;19:814–9. [DOI] [PubMed] [Google Scholar]
- 21.Grizzle WE. Tissue resources in the detection and evaluation of markers In: Srivastava S, editor. Early detection of cancer: molecular markers. Armonk: Futura Publishing Co., 1997:69–76. [Google Scholar]
- 22.Casey RC, Burleson KM, Skubitz KM, et al. Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am J Pathol. 2001;159:2071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burleson KM, Casey RC, Skubitz KM, et al. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 2004;93:170–81. [DOI] [PubMed] [Google Scholar]
- 24.Burleson KM, Boente MP, Pambuccian SE, et al. Disaggregation and invasion of ovarian carcinoma ascites spheroids. J Transl Med. 2006;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inch WR, McCredie JA, Sutherland RM. Growth of nodular carcinomas in rodents compared with multi-cell spheroids in tissue culture. Growth. 1970;34:271–82. [PubMed] [Google Scholar]
- 26.Rustin GJS, Timmers P, Nelstrop A, et al. Comparison of CA-125 and standard definitions of progression of ovarian cancer in the Intergroup trial of cisplatin and paclitaxel versus cisplatin and cyclophosphamide. J Clin Oncol. 2006;24:45–51. [DOI] [PubMed] [Google Scholar]
- 27.Lee CK, Friedlander M, Brown C, et al. Absence of early decline in CA125 is a poor surrogate for benefit of liposomal doxorubicin and carboplatin treatment in the CALYPSO study. J Natl Cancer Inst. 2011;103:1338–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


