Abstract
Objective
The aim of this pilot study was to determine if metabolic tumor volume (MTV) and total lesion glycolysis (TLG) could serve as predictors of biochemical remission and pharmacotherapy-free interval in patients with metastatic pheochromocytomas (PCCs) and paragangliomas (PGLs).
Background
Patients with metastatic PCCs/PGLs have a high rate of biochemical recurrence, which can be associated with increased cardiovascular morbidity. Predictors of biochemical response are needed to guide and select patients who may benefit from therapy.
Methods
Whole body MTV and TLG was calculated from preoperative 18F-FDG PET/CT scans and analyzed as marker of biochemical response and pharmacotherapy-free interval.
Results
Seventeen patients underwent a total of 19 procedures, with a median follow-up time of 26.4 months. Whole body MTV of patients with biochemical recurrence (n = 13, mean 73.8 mL) was higher than those who had a biochemical response (n = 6, mean 14.7 mL, P = 0.05). Patients with low MTV (<37.2mL) had an improved durable partial biochemical response (P <0.05), and a statistical trend for complete biochemical remission (P = 0.07) and pharmacotherapy-free interval (P = 0.06). In 8 patients with metastatic disease outside the abdomen, 4 patients had less than 35% of their disease burden outside the abdomen and these patients had a more durable partial biochemical response compared to patients with greater than 35% of their disease burden outside the abdomen (P < 0.05).
Conclusions
Whole body MTV and TLG represents novel and valuable predictors of biochemical response for patients with metastatic PCCs and PGLs. A larger prospective study should be performed to validate these findings.
Keywords: 18F-FDG PET/CT, metabolic tumor volume, metastatic pheochromocytoma, paraganglioma, total lesion glycolysis
Neuroendocrine tumors arising from chromaffin cells in the adrenal gland are termed pheochromocytomas (PCCs), whereas tumors arising from extra-adrenal sympathetic and parasympathetic locations are termed paragangliomas (PGLs). These tumors synthesize, store, metabolize, and secrete catecholamines or metanephrines.1 Although the majority of PCCs and PGLs are benign, at least 10% to 20% of PCCs and PGLs are metastatic depending on anatomical location of origin.2–4 Metastatic PCCs and PGLs are identified by the presence of tumor spread to sites devoid of chromaffin tissue such as lymph nodes, liver, lungs, and bones. Metastatic PCCs and PGLs are rare with an estimated annual incidence in the United States of 93 cases per 400 million persons.2 Patients with metastatic PCCs/PGLs are often associated with a primary tumor greater than 6 cm, necrosis, hemorrhage, and high mitotic index.5,6 SDHB mutation has been associated with metastatic disease, as well as a poor prognosis in both children and adults.7,8
Currently, there is no cure for metastatic PCCs and PGLs once metastases have developed if the tumor sites are not resectable.3 The prognosis is poor for patients with metastatic PCCs/PGLs, which have an overall 5-year survival rate between 34% and 60%.9 One of the main management issues in patients with biochemically active PCCs/PGLs who are symptomatic is to reduce the excess catecholamine levels for symptom palliation and to minimize adverse cardiovascular sequelae.10 There are several therapeutic options including surgical debulking; external-beam irradiation of bone metastases; radiofrequency ablation (RFA); chemotherapy with a combination of cyclophosphamide, vincristine, and dacarbazine; 131I-labeled metaiodobenzylguanidine; and metyrosine therapy.9,11–13 Given the rarity of the disease, management of these difficult cases has been based primarily on expert opinion and limited data. Predictors of treatment response in patients with metastatic PCCs/PGLs are needed to help guide management and when counseling patients with metastatic PCCs and PGLs.
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is an important imaging modality in the follow-up of patients with metastatic PCCs and PGLs,14 especially in patients with metastatic disease and SDHB gene mutation.15,16 Given the ability of 18F-FDG PET/CT to identify metastatic disease, there is a potential for quantification of disease burden for prognosis and treatment response by measuring the whole body metabolic tumor volume (MTV) as defined by 18F-FDG uptake, and total lesion glycolysis (TLG), which is the product of the mean standardized uptake value (SUV) and the volume of the tumor.17,18 Measurement of whole body MTV and TLG has been found to be a potentially valuable prognostic parameter and has been recently utilized to predict the prognosis of various cancers, such as esophageal cancer,19 multiple myeloma,20 and small cell lung cancer,21 and has been utilized to predict treatment response in pancreatic cancer 22,23 and non-small cell lung cancer.24 Furthermore, automated and semiautomated methods with various thresholds based on the percentage of the SUVmax have been developed to decrease inter- and intraobserver variation in tumor volume delineation, which allows data to be more accurate and generalizable.25
The aim of this pilot study was to determine if whole body MTV and TLG could serve as predictors of biochemical recurrence, and pharmacotherapy-free interval in patients with metastatic PCCs and PGLs undergoing cytoreductive surgery or ablation.
MATERIALS AND METHODS
Patients
Patient demographics, genetic tests, pathology, radiology, and operative history were reviewed in patients with metastatic PCCs and PGLs, who were evaluated at the National Institutes of Health Warren Magnuson Clinical Center on clinical protocols. Patients underwent genetic testing for mutations in SDHA, SDHB, SDHC, SDHD, SDHAF2, RET, VHL, MAX, and TMEM127. These genetic tests were performed in collaboration with the Mayo Clinic in Rochester, Minnesota. Preoperative and postoperative evaluation consisted of biochemical testing (plasma and urine catecholamines and metanephrines) and imaging studies [CT, magnetic resonance imaging (MRI), and 18F-FDG PET/CT imaging] as part of the clinical protocol. Seventeen patients undergoing a total of 19 intra-abdominal procedures with preoperative 18F-FDG PET/CT scans and adequate preoperative and postoperative follow-up data were included in the study.
Classification of Laboratory Values
PCC/PGL-specific biochemical test were used as the primary indicators of disease burden, remission, and recurrence. Any biochemical evidence of hormone elevation above the upper limit of normal was considered evidence of disease. Seven laboratory values were used as disease surrogates: plasma fractionated metanephrines (61 pg/mL), normetanephrines (112 pg/mL), epinephrine (83 pg/mL), norepinephrine (498 pg/mL), and dopamine (46 pg/mL); and 24-hour urinary fractionated metanephrine and normetanephrine (400 μg/24hours). Patients were instructed to discontinue use of medications that may result in false-positive results before laboratory testing, with blood pressure monitoring when off medications. Laboratory studies performed within 3 months of the intervention were used as surrogates for preoperative disease burden. Postoperative values were categorized into 3- and 6-month intervals and were recorded for the duration of follow-up (median: 26.4 months, range: 6–116 months). Only patients with preoperative laboratory values and postoperative laboratory samples drawn within 6 months of the intervention were included in the study cohort using the same assay and only those laboratory tests with known preoperative and postoperative values were considered for analysis.
18F-FDG PET/CT
All patients with a diagnosis of metastatic PCCs or PGLs underwent an 18F-FDG PET/CT within 6 months before intervention. The scans were performed approximately 60 minutes after intravenous administration of 10 mCi of 18F-FDG for patients less than 90 kg of weight and 15 mCi of intravenous 18F-FDG for patients of weight more than 90 kg. Blood glucose levels were less than 150 mg/dL for each patient before radiotracer administration. The patients were scanned from the base of the skull to the mid thighs. A noncontrast CT scan was used for attenuation and anatomical localization. All scans were compared with preintervention CT and MRI scans.
18F-FDG PET/CT Metabolic Tumor Volume and SUVlbm Measurements
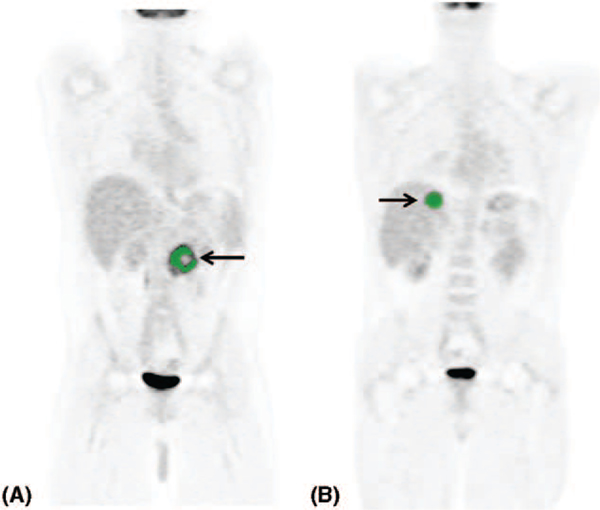
Image analyses were performed using custom software written in IDL (Excelis Visual Information Solutions, Boulder, CO). Regions were defined by a semiautomated method where the image in a hand-drawn volume was smoothed with a 5-mm full-width half max Gaussian filter. Forty-five percent of the maximum filtered value was used as a threshold to grow the region outward from the location of the maximum value. If needed, exclusion regions were defined to prevent the grown regions from expanding into high uptake areas that were obviously not tissue of interest. Metabolic tumor volumes and mean SUVlbm (SUV lean body mass) were measured and recorded for each tumor (Fig. 1). TLG was calculated by the product of the mean SUVlbm and the MTV for each tumor.26
FIGURE 1.
18F-FDG PET/CT scan MTV measurements. A, A patient with a history of primary adrenal PCC with locoregional recurrence. B, A patient with liver metastasis.
Statistical Analyses
The nonparametric Kolmogorov-Smirnov test was used to compare the MTV and TLG of patients with biochemical response and biochemical recurrence. Optimal cutoff values for differentiating patients with biochemical response and no response were determined by constructing receiver operator characteristic curves. The primary outcome variables analyzed were durations of biochemical response and pharmacotherapy-free intervals. Complete biochemical remission was defined when all indicative laboratory values returned to the normal range postoperatively. Partial biochemical response was defined as having at least 1, but not all, biochemical laboratory values returning to the normal range postoperatively. Pharmacotherapy-free interval was defined as the time interval postoperatively without blood pressure pharmacotherapy to the readdition of a blood pressure medication. The association between the length of biochemical response and pharmacotherapy-free interval was assessed using the Kaplan-Meier method. The statistical difference between Kaplan-Meier curves was determined using log-rank test. P <0.05 was used as a cutoff for statistical significance. All calculations were performed using GraphPad (GraphPad Software, Inc, La Jolla, CA).
RESULTS
Patient Characteristics
Seventeen patients underwent 19 preoperative 18F-FDG PET/CT scans before each procedure. Two patients developed recurrent disease and underwent a repeat 18F-FDG PET/CT scan before intervention. Clinical characteristics of the study cohort are summarized in Table 1. Interventions for disease control included surgery (n = 15) and RFA (n = 4) as well as preoperative systemic therapy (n = 2). Ten patients with disease confined to the abdomen underwent surgery, whereas 1 patient underwent RFA. Five patients with Patel et al metastatic disease outside the abdomen underwent 5 operations, whereas 2 patients underwent 3 RFAs. Median follow-up was 26.4 months (range: 6–116 months). Overall survival in the cohort was 94.1% at last follow-up. A comparison of 18F-FDG PET/CT to CT scan and MRI showed all 3 modalities were 100% concordant for detecting abdominal and thoracic lesions. However, CT scan and MRI did not detect all bone metastasis and had a sensitivity of 57.1% and 85.7% for detecting bone metastases, respectively. The mean number of laboratories elevated preoperatively was 3.4 ±0.9 (range: 1–7). Preoperative laboratory value elevations are summarized in detail in Table 2.
TABLE 1.
Clinical Characteristics of Study Cohort
| Characteristic | Data |
|---|---|
| No. patients | 17 |
| Sex (female/male) | 8/9 |
| Age, mean ± SD | 31.4 ± 15.7 |
| Procedures, n | 19 |
| Primary tumor site, n (%) | |
| Adrenal pheochromocytoma | 12 (70.6%) |
| Extra-adrenal paraganglioma | 3 (17.6%) |
| Periaortic paraganglioma | 1 (5.9%) |
| Bladder paraganglioma | 1 (5.9%) |
| Symptoms at presentation, n (%) | |
| Persistent hypertension | 12 (63.2%) |
| Headache | 7 (36.8%) |
| Palpitations | 8 (42.1%) |
| Diaphoresis | 6 (31.6%) |
| Family history and genetics, n (%)* | |
| Known family history | 4 (26.3%) |
| SDHB | 5 (35.3%) |
| SDHD | 1 (6.3%) |
| MAX | 1 (6.3%) |
| Sporadic without known mutation | 10 (52.6%) |
| Systemic therapy, n (%) | |
| MIBG | 1 (5.9%) |
| Multiple therapies, n (%) | |
| CVD + XRT | 1 (5.9%) |
Testing included frame shifts and deletions in SDHA, SDHB, SDHC, SDHD, SDHAF2, RET, VHL, MAX, and TMEM127.
CVD indicates cyclophosphamide, vincristine, dacarbazine; MIBG, metaiodobenzylguanidine; XRT, radiation therapy.
TABLE 2.
Biochemical Data
| Laboratory Test | Number With Elevated Levels | Mean ± SD (Range) |
|---|---|---|
| Dopamine, serum, pg/mL (ULN = 46) | 5 | 437.0 ± 770.8 |
| Epinephrine, serum, pg/mL (ULN = 83) | 3 | 373.0 ± 196.5 |
| Norepinephrine, serum, pg/mL (ULN = 498) | 13 | 4885.9 ± 5443.6 |
| Metanephrine, serum, pg/mL (ULN = 61) | 3 | 408.7 ± 252.2 |
| Normetanephrine, serum, pg/mL (ULN = 112) | 13 | 3484.5 ± 6018.8 |
| Fractionated metanephrines, urine, μg/24 h (ULN = 400) | 3 | 2739.7 ± 3292.9 |
ULN indicates upper limit of normal.
Metabolic Tumor Volume and Total Lesion Glycolysis
The number and type of interventions, disease location, and tumor burden are summarized in Table 3. Patients with a biochemical recurrence (n = 13) had a higher whole body MTV (mean: 73.8 mL, range: 0.9–365.0 mL) and TLG (mean: 310.7 SUVlbm*mL, range: 1.6–1.4 × 106 SUVlbm*mL) as compared with those who had a biochemical response (n = 6), total MTV (mean: 14.7 mL, range: 1.3–29.3 mL, P = 0.05) and TLG (mean: 67.9 SUVlbm*mL, range: 4.4–186.9 mL, P = 0.12). There was no difference in whole body MTV or TLG when comparing patients with SDHB mutations and non-SDHB mutations. Receiver operator characteristic curves were constructed to find optimal whole body MTV and TLG cutoffs to identify patients who benefited and had a biochemical response. The optimal whole body MTV was 37.2 mL and the optimal TLG was 190.8 SUVlbm*mL.
TABLE 3.
Treatment and Tumor Burden Characteristics
| Characteristic | Data |
|---|---|
| Age at procedure, median (range), yrs | 34 (13–68) |
| Intervention category, n | |
| Surgery, reoperation | 13 |
| Surgery, first operation | 2 |
| RFA | 4 |
| Disease location per patient, n (%) | |
| Abdominal metastases | |
| Liver | 3 (17.6%) |
| Retroperitoneal | 14 (82.4%) |
| Thoracic metastases | 2 (11.8%) |
| Bony metastases | 6 (35.3%) |
| No. tumors per disease location, n (range) | |
| Abdomen | 32 (1–6) |
| Bone | 128 (0–76) |
| Thoracic | 4 (0–3) |
| Preoperative pharmacotherapy, n (%)* | |
| None† | 2 (10.5%) |
| 1 drug | 3 (15.8%) |
| 2 drugs | 9 (47.4%) |
| >3 drugs | 5 (26.3%) |
| Postoperative biochemical response, n (%) | |
| Partial biochemical response‡ | 11 (57.9%) |
| Complete biochemical remission§ | 6 (31.6%) |
Drugs included alpha-blockers, beta-blockers, calcium channel blockers, and metyrosine.
Pharmacotherapy refused by a patient.
Partial biochemical response was defined as having at least 1, but not all, biochemical laboratory values returning to the normal range postoperatively.
Complete biochemical remission was defined when all indicative laboratory values returned to the normal range postoperatively.
Durability of Biochemical and Pharmacotherapy Response
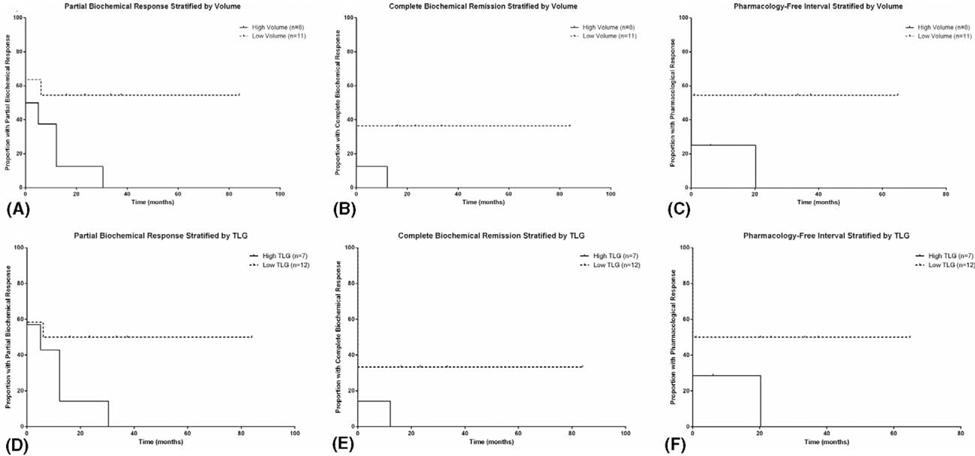
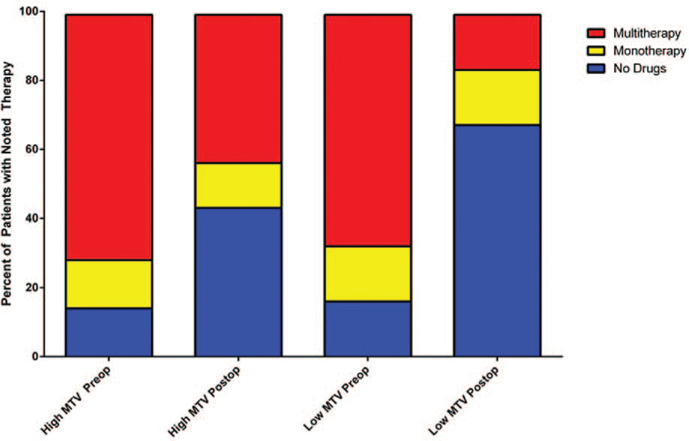
Patients were stratified by whole body MTV and TLG cutoffs to compare the durability of partial biochemical response, complete biochemical remission, and pharmacotherapy-free interval. Patients with a low whole body MTV of less than 37.2 mL showed an improved partial biochemical response (P < 0.05, Fig. 2A). A low whole body MTV had a trend toward statistically significant durable complete biochemical remission (P = 0.07, Fig. 2B) and longer pharmacotherapy-free interval (P = 0.06, Fig. 2C). A TLG cutoff of 190.8 SUVlbm*mL showed a trend toward statistical significance with improved partial biochemical response (P = 0.09, Fig. 2D). There was no statistical significance when comparing patients with low and high TLG for complete biochemical remission (P = 0.11, Fig. 2E), and pharmacotherapy-free interval (P = 0.12, Fig. 2F). Preoperative and postoperative pharmacotherapy was stratified by patients with low and high whole body MTV (Fig. 3). Of the 12 patients with low whole body MTV, 10 patients (84%) were on at least 1 medication for blood pressure control preoperatively. Postoperatively, 6 of those patients were medication free after intervention. Of the 7 patients with high whole body MTV, 6 patients (85%) were on at least 1 medication for blood pressure control. Postoperatively, 2 of those patients were medication free.
FIGURE 2.
MTV and TLG and response to treatment in patients with metastatic PCCs/PGLs. Partial biochemical response (P < 0.05) (A), complete biochemical remission (P = 0.07) (B), and pharmacology-free interval (P = 0.06) (C) stratified by MTV cutoff of 37.2 mL. Partial biochemical response (P = 0.09) (D), complete biochemical remission (P = 0.11) (E), and pharmacology-free interval (P = 0.12) (F) stratified by TLG cutoff of 190.8 SUVlbm*mL.
FIGURE 3.
Pharmacotherapy comparison between patients with low and high whole body MTV preoperatively and postoperatively.
Extent of Disease and Amount of Disease Burden
Previous data published showed that the extent of disease was associated with biochemical response after cytoreductive surgery.27 In this study, extent of disease was analyzed for partial and full biochemical response. Patients with disease confined to the abdomen (n = 11) had a statistically trending improved partial and full biochemical response compared to patients with disease outside the abdomen (n = 8) (P = 0.09 and P = 0.07, respectively). Therefore, to determine whether patients with minimal tumor burden outside the abdomen could be better stratified for intervention, a subanalysis of patients with metastatic disease outside the abdomen was performed. Eight patients with metastatic PCC/PGL had disease outside the abdomen. Patients with less than 35% (based on total MTV) of their disease burden outside the abdomen as assessed by whole body MTV had an improved partial biochemical response (P < 0.05). One patient within this subset had a complete biochemical remission, which lasted 1 year, without resection of bone metastases, which were apparently not biochemically active.
DISCUSSION
Preoperative 18F-FDG PET/CT scans of 17 patients who underwent 19 procedures were analyzed to measure whole body MTV and TLG. Optimal whole body MTV and TLG cutoffs were calculated to determine if biochemical response could be predicted. The durability of partial biochemical response was shown to be associated with patients with a whole body MTV of less than 37.2 mL. The durability of complete biochemical remission and pharmacotherapy-free interval showed a statistically trending toward improvement in patients with whole body MTV of less than 37.2 mL. Furthermore, a subanalysis of patients with metastatic disease showed that a tumor burden less than 35% outside the abdomen was associated with an improved durable partial biochemical response.
To our knowledge, this is the first study that quantifies and utilizes tumor burden by measuring whole body MTV and TLG to attempt to identify patients with metastatic PCC/PGL who may benefit from therapy (cytoreductive surgery and ablative therapies). Previous studies with MTV have been disparate in terms of measuring total tumor burden and primary tumor. Oh et al21 used a similar method of whole body tumor volume measurement as in the current study, whereas others have focused on the main tumor burden. The novelty in this study is the measurement and quantification of whole body tumor burden in patients with metastatic PCC/PGL and determining whether such information was associated with biochemical response, which can be a surrogate for cardiovascular sequelae.10 There are very few studies examining predictors of biochemical response to help guide therapy in patients with malignant PCCs/PGLs. We previously showed that the completeness of resection and extent of disease were associated with biochemical response in patients undergoing surgical treatment for metastatic and recurrent PCCs/PGLs.27 In this study, we explored whether whole body MTV and TLG could be used as a predictive marker of which patients were more likely to have a durable biochemical response and pharmacotherapy-free interval as we observed biochemical response even in some of the patients who had extra-abdominal metastases. Given the significant morbidity associated with metastatic PCCs/PGLs, especially functional tumors, a multidisciplinary approach utilizing multiple therapies likely gives the patient the best prognosis. Novel targeted therapies such as tyrosine kinase inhibitors may improve prognosis, but require further study.28 Utilizing whole body MTV and TLG may allow researchers to quantify and determine the efficacy of these various treatments with respect to biochemical response and survival, and can guide which patient may benefit from cytoreductive surgery or other ablative therapies.
There is no evidence in the literature that debulking procedures may reduce morbidity or increase survival in patients with metastatic PCCs/PGLs. Although this is a small study cohort, 8 patients had metastatic disease outside the abdomen, and of those 8, if the patients had less than 35% tumor burden outside the abdomen, they were more likely to achieve at least a partial biochemical response. One out of the 8 patients with metastatic disease in this subset achieved complete biochemical response and pharmacotherapy-free interval, which indicates that some patients have durable biochemical response and pharmacotherapy-free interval when the disease outside the abdomen is not biochemically active. This suggests that there may be a subset of patients with minimal tumor burden as assessed by whole body MTV that would benefit from cytoreductive therapies for biochemical control or in additional to other systemic therapy.
There are several limitations to this study. This is a retrospective study and is composed of patients with various interventions and systemic therapies and may not be completely generalizable to all patients with metastatic PCCs/PGLs. Furthermore, given the rarity of the disease, the sample size is small. Our findings will need to be validated in a larger cohort to further assess the value of whole body MTV and TLG with respect to biochemical response to therapy. Also, the use of biochemical response as surrogates of disease control may not translate to improved overall survival. However catecholamine excess increases the cardiovascular morbidity,10 and therefore one would expect improved overall survival in patients with better improved biochemical control and or an improved quality of life.
CONCLUSIONS
Whole body MTV and TLG was measured and calculated for patients with metastatic PCCs/PGLs. A low tumor volume was found to be indicative of a durable partial biochemical response and a statistically significant trend toward durable full biochemical remission and pharmacotherapy-free interval. A high tumor burden (≥35%) outside the abdomen was found to be associated with a poor biochemical response. 18F-FDG PET/CT measured whole body MTV and TLG have the potential to be a valuable prognostic tool but will require further validation in a larger cohort of patients with metastatic PCCs/PGLs.
Acknowledgments
This research was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
REFERENCES
- 1.Gimm O, DeMicco C, Perren A, et al. Malignant pheochromocytomas and paragangliomas: a diagnostic challenge. Langenbecks Arch Surg. 2012;397:155–177. [DOI] [PubMed] [Google Scholar]
- 2.Parenti G, Zampetti B, Rapizzi E, et al. Updated and new perspectives on diagnosis, prognosis, and therapy of malignant pheochromocytoma/paraganglioma. J Oncol. 2012;397:872713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adjalle R, Plouin PF, Pacak K, et al. Treatment of malignant pheochromocytoma. Horm Metab Res. 2009;41:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welander J, Soderkvist P, Gimm O. Genetics and clinical characteristics of hereditary pheochromocytomas and paragangliomas. Endocr Relat Cancer. 2011;18:R253–R276. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Song C, Park M, et al. Predictive characteristics of malignant pheochromocytoma. Korean J Urol. 2011;52:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strong VE, Kennedy T, Al-Ahmadie H, et al. Prognostic indicators of malignancy in adrenal pheochromocytomas: clinical, histopathologic, and cell cycle(apoptosis gene expression analysis. Surgery. 2008;143:759–768. [DOI] [PubMed] [Google Scholar]
- 7.Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B genemutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92:3822–3828. [DOI] [PubMed] [Google Scholar]
- 8.King KS, Prodanov T, Kantorovich V, et al. Metastatic pheochromocytoma (paraganglioma related to primary tumor development in childhood or adolescence: significant link to SDHB mutations. J Clin Oncol. 2011;29:4137–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacak K, Eisenhofer G, Ahlman H, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab. 2007;3:92–102. [DOI] [PubMed] [Google Scholar]
- 10.Stolk RF, Bakx C, Mulder J, et al. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab. 2013;98:1100–1106. [DOI] [PubMed] [Google Scholar]
- 11.Chrisoulidou A, Kaltsas G, Ilias I, et al. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2007;14:569–585. [DOI] [PubMed] [Google Scholar]
- 12.Serri O, Comtois R, Bettez P, et al. Reduction in the size of a pheochromocytoma pulmonary metastasis by metyrosine therapy. N Engl J Med. 1984;310:1264–1265. [DOI] [PubMed] [Google Scholar]
- 13.Tada K, Okuda Y, Yamashita K. Three cases of malignant pheochromocytoma treated with cyclophosphamide, vincristine, and dacarbazine combination chemotherapy and alpha-methyl-p-tyrosine to control hypercatecholaminemia. Hormone Res. 1998;49:295–297. [DOI] [PubMed] [Google Scholar]
- 14.Taieb D, Tessonnier L, Sebag F, et al. The role of 18F-FDOPA and 18F-FDG-PET in the management of malignant and multifocal phaeochromocytomas. Clin Endocrinol (Oxf). 2008;69:580–586. [DOI] [PubMed] [Google Scholar]
- 15.Zelinka T, Timmers HJ, Kozupa A, et al. Role of positron emission tomography and bone scintigraphy in the evaluation of bone involvement in metastatic pheochromocytoma and paraganglioma: specific implications for succinate dehydrogenase enzyme subunit B gene mutations. Endocr Relat Cancer. 2008;15:311–323. [DOI] [PubMed] [Google Scholar]
- 16.Timmers HJ, Chen CC, Carrasquillo JA, et al. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst. 2012;104:700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon SH, Choi JY, Lee HJ, et al. Prognostic value of 18F-FDG PET(CT inpatients with squamous cell carcinoma of the tonsil: comparisons of volume-based metabolic parameters. Head Neck. 2013;35:15–22. [DOI] [PubMed] [Google Scholar]
- 18.Kim K, Kim SJ, Kim IJ, et al. Prognostic value of volumetric parameters measured by F-18 FDG PET(CT in surgically resected non-small-cell lung cancer. Nucl Med Commun. 2012;33:613–620. [DOI] [PubMed] [Google Scholar]
- 19.F HS I, Kim SJ, Kim IJ, et al. Predictive value of metabolic tumor volume measured by 18F-FDG PET for regional lymph node status in patients with esophageal cancer. Clin Nucl Med. 2012;37:442–446. [DOI] [PubMed] [Google Scholar]
- 20.Fonti R, Larobina M, Del Vecchio S, et al. Metabolic tumor volume assessed by 18F-FDG PET(CT for the prediction of outcome in patients with multiple myeloma. J Nucl Med. 2012;53:1829–1835. [DOI] [PubMed] [Google Scholar]
- 21.Oh JR, Seo JH, Chong A, et al. Whole-body metabolic tumour volume of 18F-FDG PET(CT improves the prediction of prognosis in small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39:925–935. [DOI] [PubMed] [Google Scholar]
- 22.Dholakia AS, Chaudhry M, Leal JP, et al. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JW, Kang CM, Choi HJ, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET(CT in patients with pancreatic cancer. J Nucl Med. 2014;55:898–904. [DOI] [PubMed] [Google Scholar]
- 24.Satoh Y, Onishi H, Nambu A, et al. Volume-based parameters measured by using FDG PET(CT in patients with stage I NSCLC treated with stereotactic body radiation therapy: prognostic value. Radiology. 2014;270:275–281. [DOI] [PubMed] [Google Scholar]
- 25.Moon SH, Hyun SH, Choi JY. Prognostic significance of volume-based PET parameters in cancer patients. Korean J Radiol. 2013;14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai B, Bading J, Conti PS. Tumor quantification in clinical positron emission tomography. Theranostics. 2013;3:787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis RJ, Patel D, Prodanov T, et al. Response after surgical resection of metastatic pheochromocytoma and paraganglioma: can postoperative biochemical remission be predicted? J Am Coll Surg. 2013;217:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen KF, Altaf R, Krarup-Hansen A, et al. Malignant pheochromocytomas and paragangliomas: the importance of a multidisciplinary approach. Cancer Treat Rev. 2011;37:111–119. [DOI] [PubMed] [Google Scholar]