Abstract
Background
Coronavirus disease 2019 (COVID-19) is an emerging infectious disease that first appeared in Wuhan, China, and quickly spread throughout the world. We aimed to understand the relationship between diabetes mellitus and the prognosis of COVID-19.
Methods
Demographic, clinical, laboratory, radiologic, treatments, complications, and clinical outcomes data were extracted from electronic medical records and compared between diabetes (n = 84) and nondiabetes (n = 500) groups. Kaplan-Meier method and multivariate Cox analysis were applied to determine the risk factors for the prognosis of COVID-19.
Results
Compared with nondiabetic patients, diabetic patients had higher levels of neutrophils (P = .014), C-reactive protein (P = .008), procalcitonin (P < .01), and D-dimer (P = .033), and lower levels of lymphocytes (P = .032) and albumin (P = .035). Furthermore, diabetic patients had a significantly higher incidence of bilateral pneumonia (86.9%, P = .020). In terms of complications and clinical outcomes, the incidence of respiratory failure (36.9% vs 24.2%, P = .022), acute cardiac injury (47.4% vs 21.2%, P < .01), and death (20.2% vs 8.0%, P = .001) in the diabetes group was significantly higher than that in the nondiabetes group. Kaplan-Meier survival curve showed that COVID-19 patients with diabetes had a shorter overall survival time. Multivariate Cox analysis indicated that diabetes (hazard ratio 2.180, P = .031) was an independent risk factor for COVID-19 prognosis. In subgroup analysis, we divided diabetic patients into insulin-required and non-insulin-required groups according to whether they needed insulin, and found that diabetic patients requiring insulin may have a higher risk of disease progression and worse prognosis after the infection of severe acute respiratory syndrome coronavirus 2.
Conclusions
Diabetes is an independent risk factor for the prognosis of COVID-19. More attention should be paid to the prevention and treatment for diabetic patients, especially those who require insulin therapy.
Keywords: COVID-19, Diabetes mellitus, Prognosis, Retrospective
Clinical Significance.
-
•
Diabetes is an independent risk factor for the prognosis of COVID-19.
-
•
More attention should be paid to the prevention and treatment of diabetic patients, especially those who require insulin therapy.
Alt-text: Unlabelled box
Introduction
In December 2019, an acute infectious pneumonia of unknown cause broke out in Wuhan, and quickly spread throughout the world.1 , 2 The pathogen was identified to be a unique clade from the β-coronaviruses related to severe acute respiratory syndrome (SARS) and Middle East syndrome (MERS), and was officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).3 Subsequently, this coronavirus-caused pneumonia was defined as coronavirus disease 2019 (COVID-19) by the World Health Organization on February 12.4 On April 18, 2020, SARS-CoV-2 had caused 84,180 confirmed cases, and more than 4000 deaths in China. Globally, this virus has spread to more than 200 countries, including the United States, Britain, Italy, South Korea, and Japan.
COVID-19 has a higher transmission rate and a greater risk of mortality in comparison with influenza. Although the majority of the patients are expected to have a favorable outcome, older people with underlying diseases may have a poor prognosis.5 Diabetes mellitus is one of the most common conditions among the elderly and contributes greatly to the morbidity worldwide. Moreover, numerous studies have shown that diabetic patients are more susceptible to various pathogens, such as Mycobacterium tuberculosis, Streptococcus pneumoniae, and Staphylococcus aureus.6 Several retrospective studies in Wuhan indicated that diabetes was one of the most prevalent comorbidities in COVID-19 patients,7, 8, 9 and suggested that diabetics were probably more susceptible to SARS-CoV-2 and these patients might have a worse prognosis.
In order to understand whether COVID-19 patients with diabetes have a more severe disease progression and a worse prognosis, 584 COVID-19 patients were studied. The data of demographic, clinical, laboratory, radiologic, treatments, complications, as well as clinical outcomes were carefully collected and analyzed. Our results indicate that diabetes mellitus is significantly associated with poor prognosis of COVID-19.
Materials and Methods
Study Design and Participants
In this retrospective, single-center cohort study, we included 584 patients with COVID-19 from December 25, 2019 to March 20, 2020, at the Wuhan No.7 Hospital. Wuhan No.7 Hospital was temporarily taken over by Zhongnan Hospital of Wuhan University, and it was also the designated hospital for the treatment of COVID-19. We diagnosed the adult patients with COVID-19 according to the New Coronavirus Pneumonia Prevention and Control Guideline (7th edition).25 The study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (2020082K). All research procedures are performed according to the criteria of the Declaration of Helsinki.
Data Collection
We obtained all the data including demographic, clinical, laboratory, radiological characteristics, treatments, complications, and clinical outcomes from patients’ electronic medical records. All patients were followed up on March 30, 2020. The data were carefully reviewed by 3 independent researchers (JS, QW, and HZ). Discrepancies between the reviewers were resolved by discussion or a fourth researcher (JL).
To confirm SARS-CoV-2 infection, throat swab samples were collected from the upper respiratory tracts of all the patients and tested using reverse transcription polymerase chain reaction or next-generation sequencing technology. As described previously, pathogen detection was performed by the following 4 institutions: the Chinese Center for Disease Control and Prevention, the Chinese Academy of Medical Science, the Academy of Military Medical Sciences, and Wuhan Institute of Virology of the Chinese Academy of Sciences.10
Definition
Diabetes mellitus is diagnosed by meeting any one of the following:11 1) Fasting plasma glucose level ≥7.0 mmol/L; 2) Plasma glucose level ≥11.1 mmol/L 2 hours after a 75-g oral glucose load in a glucose tolerance test; 3) Casual plasma glucose level ≥11.1 mmol/L; 4) Glycated hemoglobin (HbA1c) ≥48 mmol/mol. Fever was defined as axillary temperature exceeding 37.3°C. In the assessment of disease severity, critical illness was identified if satisfying at least one of the following criteria: 1) respiratory failure requiring mechanical ventilation; 2) shock performance; 3) multiple organ failure requiring intensive care unit (ICU) monitoring. Respiratory failure was defined by an arterial partial pressure of oxygen (PaO2) below 60 mm Hg. Acute liver injury was defined by alanine aminotransferase increase to more than 3 times the upper limit of the reference, or aspartate aminotransferase increase to more than 3 times the upper limit of the reference, or total bilirubin increase to more than 2 times the upper limit of the reference, regardless of liver comorbidities.12 Acute myocardial injury was defined by serum level of myocardial biomarkers (high-sensitivity troponin I and creatine kinase-MB) exceeding the upper reference limit, or if there were any new abnormalities in an electrocardiogram.13 Acute kidney injury was defined based on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines.14
Statistical Analysis
Categorical variables were presented as frequency rates and percentages (%), and continuity variables were described using median and interquartile range. We used chi-squared test or Fisher's exact test to compare variables between diabetes and nondiabetes groups for categorical data. Otherwise, Mann-Whitney U test was applied for continuous variables. The Kaplan-Meier method and log-rank test were used to compare the prognosis of COVID-19 patients in different groups. Furthermore, multivariate Cox proportional hazards analysis was used to analyze the independent prognosis factors for survival in COVID-19 patients. All statistical analyses were performed using SPSS software (version 22.0; IBM, Armonk, NY). P value < .05 was considered statistically significant.
Results
Demographic and Clinical Characteristics
A total of 584 patients with COVID-19 were included in this retrospective study (Table 1 ). The median age was 59 years (interquartile range 25-75), ranging from 14 to 91 years, and 307 patients (52.6%) were female. The most common symptom at the beginning of the disease was fever (70.2%), followed by cough (60.6%), anorexia (37.2%), fatigue (35.1%), and dyspnea (32.4%). More than half of the patients had one or more comorbidities, including hypertension (34.1%), diabetes (14.4%), cardiovascular disease (10.6%), chronic respiratory disease (8.4%), chronic liver disease (5.8%), malignancy (4.8%), and chronic kidney disease (1.4%). According to the initial clinical data, 57 (9.8%) patients were critically ill.
Table 1.
Demographic and Clinical Characteristics of 584 COVID-19 Patients
| Variables | Total ( n = 584) | Diabetes (n = 84) | Nondiabetes (n = 500) | P Value |
|---|---|---|---|---|
| Age | .001 | |||
| >65 y | 209/584 (35.8%) | 44/84 (52.4%) | 165/500 (33.0%) | |
| <65 y | 375/584 (64.2%) | 40/84 (47.6%) | 335/500 (77.0%) | |
| Sex | .638 | |||
| Female | 307/584 (52.6%) | 42/84 (50.0%) | 265/500 (53.0%) | |
| Male | 277/584 (47.4%) | 42/84 (50.0%) | 235/500 (47.0%) | |
| Signs and symptoms | ||||
| Fever | 410/584 (70.2%) | 60/84 (71.4%) | 350/500 (70.0%) | .898 |
| Cough | 354/584 (60.6%) | 51/84 (60.7%) | 303/500 (60.6%) | 1.000 |
| Anorexia | 217/584 (37.2%) | 35/84 (41.7%) | 182/500 (36.4%) | .393 |
| Fatigue | 205/584 (35.1%) | 42/84 (50.0%) | 163/500 (32.6%) | .003 |
| Dyspnea | 189/584 (32.4%) | 36/84 (42.9%) | 153/500 (30.6%) | .030 |
| Diarrhea | 72/584 (12.3%) | 6/84 (7.1%) | 66/500 (13.2%) | .150 |
| Pharyngalgia | 64/584 (10.9%) | 8/84 (9.5%) | 56/500 (11.2%) | .850 |
| Nausea or vomiting | 60/584 (10.3%) | 8/84 (9.5%) | 51/500 (10.2%) | 1.000 |
| Myalgia | 53/584 (9.1%) | 8/84 (9.5%) | 45/500 (9.0%) | .838 |
| Comorbidities | ||||
| Hypertension | 199/584 (34.1%) | 53/84 (63.1%) | 146/500 (29.2%) | < .01 |
| Cardiovascular disease | 62/584 (10.6%) | 19/84 (22.6%) | 43/500 (8.6%) | < .01 |
| Chronic respiratory disease | 49/584 (8.4%) | 6/84 (7.1%) | 43/500 (8.6%) | .832 |
| Chronic liver disease | 34/584 (5.8%) | 7/84 (8.3%) | 27/500 (5.4%) | .310 |
| Malignancy | 28/584 (4.8%) | 4/84 (4.8%) | 24/500 (4.8%) | 1.000 |
| Chronic kidney disease | 8/584 (1.4%) | 3/84 (3.5%) | 5/500 (1.0%) | .090 |
| Heart rate, median (IQR), beats per minute | 88 (78, 98) | 88 (77, 101) | 88 (78, 98) | .951 |
| Respiratory rate, median (IQR) | 20 (20, 20) | 20 (20, 20.5) | 20 (20, 20) | .649 |
| Severity assessment on admission | .010 | |||
| Critical | 67/584 (11.5%) | 17/84 (20.2%) | 50/500 (10.0%) | |
| Noncritical | 517/584 (88.5%) | 67/84 (79.8%) | 450/500 (90.0%) |
NA = not available.
Data are median (interquartile range [IQR]) or n/N (%). P values were calculated by chi-squared test, Fisher's exact test, or Mann-Whitney U test, as appropriate.
As shown in Table 1, COVID-19 patients were divided into a diabetes group (n = 84) and a nondiabetes group (n = 500), and there were more elderly people in the diabetes group. Patients with diabetes were more likely to have symptoms such as fatigue (50.0%, P = .003) and dyspnea (42.9%, P = .030). The proportions of diabetes patients with hypertension (63.1%, P < .01) and cardiovascular disease (22.6%, P < .01) were significantly higher than those of nondiabetes patients. Furthermore, more critically ill patients were in the diabetes group (20.2%, P = .010).
Laboratory and Radiologic Findings
Laboratory and radiologic findings of patients are described in Table 2 . On admission, many patients tended to have lymphopenia, elevated levels of infection-related biomarkers (C-reactive protein and procalcitonin), and abnormal levels of neutrophil, alanine aminotransferase, total bilirubin, albumin, blood urea nitrogen, serum creatinine, cardiac troponin I, and D-dimer. According to radiologic findings, 450 patients (77.1%) presented with bilateral pneumonia, and unilateral pneumonia occurred in 67 patients (11.5%).
Table 2.
Laboratory and Radiologic Findings of Patients on Admission
| Variables | Normal Range | Total (n = 584) | Diabetes (n = 84) |
Nondiabetes (n = 500) |
P Value |
|---|---|---|---|---|---|
| Blood routine | |||||
| White blood cells, × 109/L | 3.5-9.5 | 5.15 (3.95, 6.97) | 5.63 (4.31, 7.47) | 5.08 (3.89, 6.94) | .167 |
| Neutrophils, × 109/L | 1.8-6.3 | 3.39 (2.39, 5.04) | 3.86 (2.91, 6.31) | 3.29 (2.35, 4.94) | .014 |
| Lymphocytes, × 109/L | 1.1-3.2 | 1.02 (0.66, 1.51) | 0.84 (0.59, 1.42) | 1.04 (0.67, 1.54) | .032 |
| Platelets, × 109/L | 125-350 | 192 (149, 250) | 181 (139, 242) | 193 (150, 254) | .196 |
| Hemoglobin, × 109/L | 130-175 | 127 (116,137) | 126 (112, 134) | 127 (116, 137) | .432 |
| Biochemical | |||||
| Alanine aminotransferase, IU/L | 9-50 | 21 (13, 35) | 22 (16, 40.5) | 20 (13, 35) | .122 |
| Aspartate aminotransferase, IU/L | 15-40 | 26 (18, 38) | 26 (21, 38.5) | 25 (18, 38) | .191 |
| Total bilirubin, μmol/L | 2-23 | 7.9 (5.7, 11) | 9.7 (7.4, 13.05) | 7.5 (5.5, 10.7) | < .01 |
| Albumin, g/L | 40-55 | 37.3 (33.3, 41.5) | 35.5 (30.9, 41.5) | 37.5 (33.7, 41.5) | .035 |
| Blood urea nitrogen, mmol/L | 3.6-9.5 | 4.38 (3.43, 5.79) | 5.09 (3.68, 6.59) | 4.3 (3.41, 5.61) | .001 |
| Serum creatinine, μmol/L | 57-111 | 63 (53, 74) | 66 (55, 79.5) | 63 (52.7, 73) | .169 |
| Cardiac troponin I, ng/mL | 0-0.014 | 0.008 (0.006, 0.014) | 0.012 (0.008, 0.028) | 0.008 (0.005, 0.013) | < .01 |
| Creatine kinase-MB, ng/mL | 0-6.22 | 1.19 (0.74, 2.20) | 1.37 (0.78, 3.32) | 1.17 (0.74, 2.04) | .068 |
| Infection-related biomarkers | |||||
| C-reactive protein, mg/L | 0-3 | 18 (2.3, 57.4) | 33.5 (6.1, 84.3) | 15.45 (2.0, 51.7) | .008 |
| Procalcitonin, ng/mL | 0-0.1 | 0.05 (0.03, 0.13) | 0.10 (0.04, 0.23) | 0.05 (0.03, 0.11) | < .01 |
| Coagulation function | |||||
| Prothrombin time, s | 9.3-12.9 | 12.3 (11.5, 13.3) | 12.85 (11.6, 13.62) | 12.3 (11.5, 13.2) | .063 |
| D-dimer, μg/L | 0-0.243 | 0.21 (0.10, 0.61) | 0.31 (0.13, 1.06) | 0.19 (0.09, 0.52) | .033 |
| Imaging features | |||||
| Unilateral pneumonia | NA | 67/584 (11.5%) | 6/84 (7.1%) | 61/500 (12.2%) | .200 |
| Bilateral pneumonia | NA | 450/584 (77.1%) | 73/84 (86.9%) | 377/500 (75.4%) | .020 |
NA = not available.
Data are median (IQR) or n/N (%). P values were calculated by chi-squared test, Fisher's exact test, or Mann-Whitney U test, as appropriate.
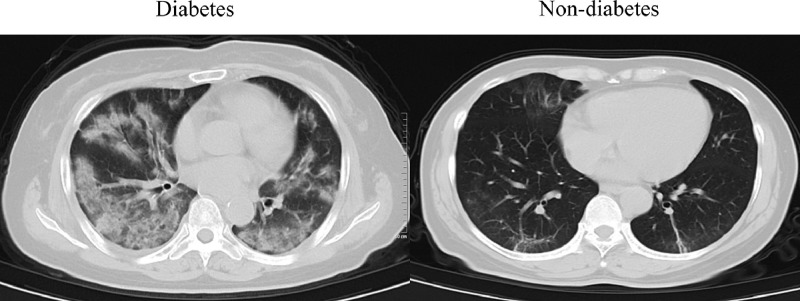
Compared with nondiabetic patients, diabetic patients had higher levels of neutrophils (P = .014), total bilirubin (P < .01), blood urea nitrogen (P = .001), cardiac troponin I (P < .01), C-reactive protein (P = .008), procalcitonin (P < .01), and D-dimer (P = .033), and lower levels of lymphocytes (P = .032) and albumin (P = .035). Furthermore, diabetic patients had a significantly higher incidence of bilateral pneumonia (86.9%, P = .020) (Figure 1 ). These results indicated that COVID-19 patients with diabetes had a more severe inflammatory response and lung infiltration, which might contribute to their poorer prognosis.
Figure 1.
Representative computed tomography images of diabetic patients and nondiabetic patients after the infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Treatments, Complications, and Clinical Outcomes
The data of treatments, complications, and clinical outcomes are summarized in Table 3 . Of the 584 patients, 435 (74.5%) received antiviral treatment, 418 (71.6%) received an antibiotic, 380 (65.1%) received traditional Chinese medicine, 175 (29.9%) received corticosteroid, and 107 patients (18.3%) received intravenous immunoglobin. A total of 57 patients (9.8%) required mechanical ventilation, and 38 patients (6.5%) were admitted to the ICU. In terms of complications, the most common complication was respiratory failure (26.1%), followed by acute cardiac injury (25.0%), acute kidney injury (9.0%), and acute liver injury (4.9%). In addition, a total of 57 patients (9.8%) eventually died.
Table 3.
Treatments, Complications, and Clinical Outcomes of 584 COVID-19 Patients
| Variables | Total (n = 584) | Diabetes (n = 84) | Nondiabetes (n = 500) | P Value |
|---|---|---|---|---|
| Treatments | ||||
| Antiviral treatment | 435/584 (74.5%) | 54 /84 (64.3%) | 381/500 (76.2%) | .030 |
| Antibiotics | 418/584 (71.6%) | 63/84 (75.0%) | 355/500 (71.0%) | .514 |
| Traditional Chinese medicine | 380/584 (65.1%) | 52/84 (61.9%) | 328/500 (65.6%) | .537 |
| Corticosteroids | 175/584 (29.9%) | 35/84 (41.7%) | 140/500 (28.0%) | .014 |
| Intravenous immunoglobin | 107/584 (18.3%) | 23/84 (27.4%) | 84/500 (16.8%) | .032 |
| Mechanical ventilation | 57/584 (9.8%) | 14/84 (16.7%) | 43/500 (8.6%) | .028 |
| ICU admission | 38/584 (6.5%) | 9/84 (10.7%) | 29/500 (5.8%) | .097 |
| Complications | ||||
| Respiratory failure | 152/583 (26.1%) | 31/84 (36.9%) | 121/499 (24.2%) | .022 |
| Acute cardiac injury | 120/480 (25.0%) | 33/70 (47.4%) | 87/410 (21.2%) | < .01 |
| Acute kidney injury | 49/543 (9.0%) | 11/81 (13.6%) | 38/462 (8.2%) | .139 |
| Acute liver injury | 27/542 (4.9%) | 6/81 (7.4%) | 21/461 (4.5%) | .270 |
| Clinical outcomes | ||||
| Death | 57/584 (9.8%) | 17/84 (20.2%) | 40/500 (8.0%) | .001 |
| Alive | 527/584 (90.2%) | 67/84 (79.8%) | 460/500 (92.0%) |
NA = not available.
Data are shown as n/N (%). P values were calculated by chi-squared test or Fisher's exact test.
Compared with nondiabetic patients, diabetic patients were more likely to receive corticosteroid (41.7% vs 28.0%, P = .014), intravenous immunoglobin (27.4% vs 16.8%, P = .032), and mechanical ventilation (16.7% vs 8.6%, P = .028). Patients with diabetes had a higher proportion of complications, including respiratory failure (36.9% vs 24.2%, P = .022) and acute cardiac injury (47.4% vs 21.2%, P < .01). Furthermore, more patients died in the diabetes groups (20.2% vs 8.0%, P = .001). These above data indicated that the clinical outcomes of diabetic patients were worse than those of nondiabetic patients.
Diabetes is an Independent Risk Factor for COVID-19 Prognosis
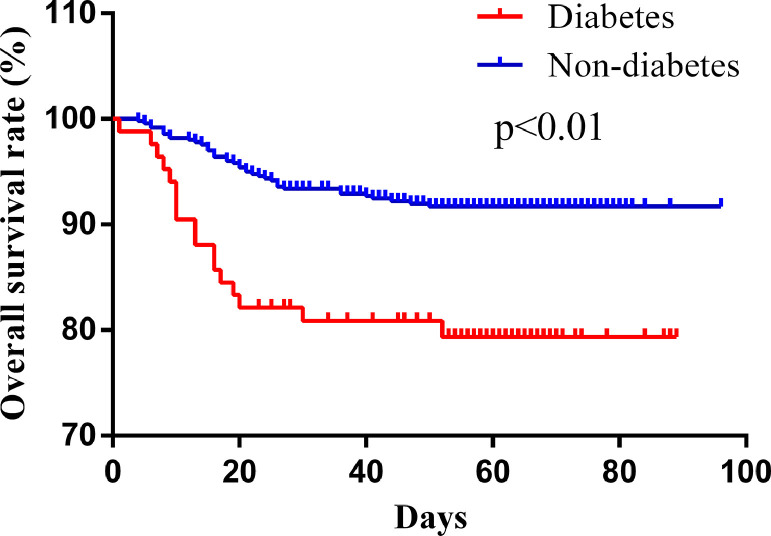
The Kaplan-Meier method and log-rank test were used in our study to investigate the relationship between diabetes and COVID-19 prognosis. The results indicated that diabetic patients had a significant lower overall survival rate than nondiabetic patients (hazard ratio [HR] 2.761, P = .0002) (Figure 2 ). Furthermore, multivariate Cox analysis was performed following adjustment for potential prognostic factors, which included the age, sex, hypertension, cardiovascular diseases, diabetes, chronic respiratory diseases, chronic kidney diseases, chronic liver diseases, acute kidney injury, acute liver injury, respiratory failure, and acute cardiac injury. The data showed that diabetes (HR 2.180, P = .031) and acute kidney injury (HR 3.520, P < .01) were the only 2 independent prognosis factors for mortality in COVID-19 patients (Table 4 ). These results indicate that diabetes is a potential risk factor for the prognosis of COVID-19.
Figure 2.
Kaplan-Meier survival curve of the patients with and without diabetes.
Table 4.
Multivariate Cox Proportional Hazards Analysis of Factors Associated with In-Hospital Death
| Variables | HR | 95% CI | P Value |
|---|---|---|---|
| Sex | 1.409 | 0.721-2.754 | .316 |
| Age | 1.835 | 0.826-4.077 | .136 |
| Hypertension | 0.700 | 0.364-1.349 | .287 |
| Cardiovascular diseases | 0.873 | 0.420-1.817 | .717 |
| Diabetes | 2.180 | 1.072-4.436 | .031 |
| Chronic respiratory diseases | 0.573 | 0.196-1.681 | .311 |
| Chronic kidney diseases | 12.301 | 0.902-167.823 | .060 |
| Chronic liver diseases | 1.365 | 0.452-4.119 | .581 |
| Acute kidney injury | 3.520 | 1.893-6.546 | < .01 |
| Acute liver injury | 0.913 | 0.371-2.247 | .843 |
| Respiratory failure | NA | NA | .769 |
| Acute cardiac injury | 2.085 | 0.957-4.543 | .064 |
CI = confidence interval; HR = hazard ratio; NA = not available.
Subgroup Analysis in Diabetes Patients
Of the 84 diabetic patients, 29 had uncontrollable plasma glucose levels and required insulin therapy. In subgroup analysis, we divided diabetic patients into insulin-required (n = 29) and non-insulin-required (n = 55) groups according to whether they needed insulin (Table 5 ). We found that there were more elderly (P = .011) and critically ill (P < .01) patients in the insulin-required group. Compared with the patients who did not need insulin, insulin-required diabetic patients had higher levels of aspartate aminotransferase (P = .015), C-reactive protein (P = .021), and D-dimer (P = .043), and a lower level of lymphocytes (P < .01).
Table 5.
Demographic, Clinical, Laboratory, Radiologic, Treatments, Complications, and Clinical Outcomes Data of Diabetic Patients in Subgroup Analysis
| Variables | Normal Range | Total (n = 84) |
Insulin Required (n = 29) |
Noninsulin Required (n = 55) |
P Value |
|---|---|---|---|---|---|
| Age | .011 | ||||
| >65 y | NA | 44/84 (52.4%) | 21/29 (72.4%) | 23/55 (41.8%) | |
| <65 y | NA | 40/84 (47.6%) | 8/29 (27.6%) | 32/55 (58.2%) | |
| Sex | .251 | ||||
| Female | NA | 42/84 (50%) | 12/29 (41.4%) | 30/55 (54.5%) | |
| Male | NA | 42/84 (50%) | 17/29 (58.6%) | 25/55 (45.5%) | |
| Severity assessment on admission | < .01 | ||||
| Critical | NA | 17/84 (20.2%) | 15/29 (51.7%) | 2/55 (3.6%) | |
| Noncritical | NA | 67/84 (79.8%) | 14/29 (48.3%) | 53/55 (96.4%) | |
| Blood routine | |||||
| White blood cells, × 109/L | 3.5-9.5 | 5.64 (4.78, 6.95) | 5.01 (4.08, 6.15) | 5.96 (5.20, 6.97) | .034 |
| Neutrophils, × 109/L | 1.8-6.3 | 3.43 (2.63, 4.21) | 3.39 (2.10, 4.32) | 3.54 (2.77, 4.16) | .370 |
| Lymphocytes, × 109/L | 1.1-3.2 | 1.56 (1.10, 2.04) | 1.09 (0.90, 1.55) | 1.72 (1.33, 2.09) | < .01 |
| Platelets, × 109/L | 125-350 | 199 (173, 260) | 187 (165, 251) | 203 (175, 266) | .313 |
| Hemoglobin, × 109/L | 130-175 | 124 (114, 135) | 124 (115, 132) | 125 (113, 136) | .636 |
| Biochemical | |||||
| Alanine aminotransferase, IU/L | 9-50 | 17 (12, 33) | 21 (12, 38) | 16 (11, 29) | .209 |
| Aspartate aminotransferase, IU/L | 15-40 | 19 (15, 29) | 22 (18, 37) | 18 (14, 25) | .015 |
| Total bilirubin, μmol/L | 2-23 | 7.7 (5.6, 10.7) | 7.9 (6.3, 9.7) | 7.7 (5.1, 11.2) | .593 |
| Albumin, g/L | 40-55 | 41.0 (37.0, 44.5) | 39.2 (35.6, 43.6) | 42.5 (38.7, 44.7) | .126 |
| Blood urea nitrogen, mmol/L | 3.6-9.5 | 4.37 (3.46, 5.51) | 3.98 (3.27, 5.81) | 4.51 (3.67, 5.45) | .548 |
| Serum creatinine, μmol/L | 57-111 | 61 (52, 72) | 60 (51, 75) | 61 (52, 71) | .980 |
| Cardiac troponin I, ng/mL | 0-0.014 | 0.007 (0.004, 0.012) | 0.008 (0.004, 0.014) | 0.006 (0.004, 0.010) | .247 |
| Creatine kinase-MB, ng/mL | 0-6.22 | 1.23 (0.84, 1.92) | 1.36 (0.88, 2.72) | 1.08 (0.78, 1.71) | .280 |
| Infection-related biomarkers | |||||
| C-reactive protein, mg/L | 0-3 | 2.3 (0.4, 9.7) | 7.2 (0.7, 37.5) | 1.3 (0.4, 6.4) | .021 |
| Procalcitonin, ng/mL | 0-0.1 | 0.04 (0.03, 0.07) | 0.04 (0.03, 0.18) | 0.04 (0.03, 0.05) | .252 |
| Coagulation function | |||||
| Prothrombin time, s | 9.3-12.9 | 11.90 (11.15, 12.75) | 12.00 (11.30, 12.80) | 11.90 (11.08, 12.78) | .647 |
| D-dimer, μg/L | 0-0.243 | 0.14 (0.07, 0.23) | 0.19 (0.08, 0.50) | 0.12 (0.06, 0.19) | .043 |
| Imaging features | |||||
| Unilateral pneumonia | NA | 6/84 (7.1%) | 3/29 (10.3%) | 3/55 (5.5%) | .411 |
| Bilateral pneumonia | NA | 73/84 (86.9%) | 25/29 (86.2%) | 48/55 (87.3%) | 1.000 |
| Treatments | |||||
| Antiviral treatment | NA | 54/84 (64.3%) | 13/29 (44.8%) | 41/55 (74.5%) | .009 |
| Antibiotics | NA | 63/84 (75.0%) | 23/29 (79.3%) | 40/55 (72.7%) | .602 |
| Traditional Chinese medicine | NA | 52/84 (61.9%) | 16/29 (55.2%) | 36/55 (65.5%) | .479 |
| Corticosteroids | NA | 35/84 (41.7%) | 18/29 (62.1%) | 17/55 (30.9%) | .010 |
| Intravenous immunoglobin | NA | 23/84 (27.4%) | 11/29 (37.9%) | 12/55 (21.8%) | .130 |
| Mechanical ventilation | NA | 14/84 (16.7%) | 13/29 (44.8%) | 1/55 (1.8%) | < .01 |
| ICU admission | NA | 9/84 (10.7%) | 8/29 (27.6%) | 1/55 (1.8%) | .001 |
| Complications | |||||
| Respiratory failure | NA | 31/84 (36.9%) | 19/29 (65.5%) | 12/55 (21.8%) | < .01 |
| Acute cardiac injury | NA | 33/70 (47.1%) | 17/25 (68.0%) | 16/45 (35.6%) | .013 |
| Acute kidney injury | NA | 11/81 (13.6%) | 10/28 (35.7%) | 1/53 (1.9%) | < .01 |
| Acute liver injury | NA | 6/81 (7.4%) | 4/28 (14.3%) | 2/53 (3.8%) | .175 |
| Clinical outcomes | |||||
| Death | NA | 17/84 (20.2%) | 15/29 (51.7%) | 2/55 (3.6%) | < .01 |
| Alive | NA | 67/84 (79.8%) | 14/29 (48.3%) | 53/55 (96.4%) |
ICU = intensive care unit; NA = not available.
Data are median (interquartile range [IQR]) or n/N (%). P values were calculated by chi-squared test, Fisher's exact test or Mann-Whitney U test, as appropriate.
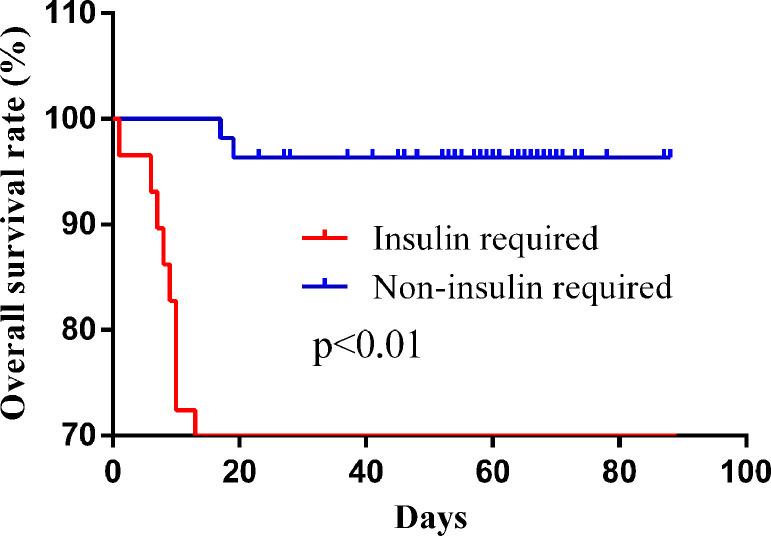
More patients in the insulin-required group received corticosteroid (62.1% vs 30.9%, P = .010), mechanical ventilation (44.8% vs 1.8%, P < .01), and ICU admission (27.6% vs 1.8%, P = .001). In terms of complications and clinical outcomes, the incidence of respiratory failure (65.5% vs 21.8%, P < .01), acute cardiac injury (68.0% vs 35.6%, P = .013), acute kidney injury (35.7% vs 1.9%, P < .01), and death (51.7% vs 3.6%, P < .01) in the insulin-required group was significantly higher than those in the non-insulin-required group. Kaplan-Meier survival curve showed that the insulin-required diabetic patients had a shorter overall survival time (HR = 20.55, P < .01) (Figure 3 ).
Figure 3.
Kaplan-Meier survival curve of diabetic patients who needed and did not need insulin.
Discussion
Diabetes mellitus is a chronic inflammatory disease characterized by multiple macrovascular and microvascular abnormalities that can affect our body's response to pathogens.6 The relationship between diabetes and infection has always been an important concern of clinicians. Infectious diseases, especially influenza and pneumonia, are very common among elderly diabetic patients. In addition, previous studies had shown that diabetes was a risk factor for the morbidity and mortality of multiple viral infections, including 2009 influenza A (H1N1), MERS-CoV, and SARS-CoV.15, 16, 17 However, the relationship between diabetes and COVID-19 prognosis is rarely reported.
In this retrospective cohort study, we analyzed data from 584 patients with COVID-19, including 84 cases of diabetes and 500 cases of nondiabetes. First, we compared the differences in demographic and clinical characteristics between the 2 groups. The results indicate that the proportion of elderly patients in the diabetes group is significantly higher than that in the nondiabetes group. It is well known that diabetes is an aging disease, and previous studies have shown that aging is one of the important risk factors affecting the prognosis of COVID-19.7 Therefore, a high proportion of elderly patients in the diabetes group might suggest a poor clinical outcome. Furthermore, we analyzed the symptoms of the patients at the first visit and found that diabetic patients were more likely to have symptoms of fatigue and anorexia, which might eventually lead to poor nutritional status of the patients. According to the criteria in the methods, we evaluated the disease severity of all the patients and found that there were more critically ill patients in the diabetes group, which suggests that diabetic patients are more likely to progress to a severe condition after the infection with SARS-CoV-2.
Lymphopenia is common in the patients with COVID-19, but thrombocytopenia and leukopenia are relatively rare.18 Previous studies have shown that COVID-19 patients, especially those with severe conditions, have significantly increased levels of serum inflammation-related biomarkers, including interleukin-6, C-reactive protein, and procalcitonin, and these indicators are closely related to the prognosis of the disease.7 , 18 In diabetes, hyperglycemia and insulin resistance can promote the synthesis of numerous proinflammatory cytokines and adhesion molecules, which can exacerbate oxidative stress and inflammation in the body.19 However, it is not clear whether diabetes will further aggravate the inflammatory response in COVID-19 patients. In order to clarify this question, laboratory data were analyzed in our study, and the results showed that diabetic patients had higher levels of neutrophils, C-reactive protein, and procalcitonin, and a lower level of lymphocytes in comparison with nondiabetic patients. Besides lab findings, we also analyzed the radiologic data and found that diabetic patients had a significantly higher incidence of bilateral pneumonia than nondiabetic patients. These results indicate that COVID-19 patients with diabetes had more severe inflammatory responses and lung infiltration, which might contribute to the worse prognosis of SARS-CoV-2 infection.
D-dimer is a fibrin degradation product and is one of the main markers of coagulation activity.20 The high concentration of serum D-dimer is closely related to a variety of thrombotic diseases, including myocardial infarction, cerebral infarction, pulmonary embolism, and venous thrombosis.21 In our study, we found that the concentration of serum D-dimer of diabetic patients was significantly higher than that of nondiabetic patients, indicating that COVID-19 patients with diabetes are more likely to develop a hypercoagulable prothrombotic state.
Both SARS-CoV and SARS-CoV-2 share the same receptor angiotensin-converting enzyme 2.22 , 23 It is reported that angiotensin-converting enzyme 2 is widely expressed in multiple organs, including the heart, respiratory tract, liver, kidney, pancreas, and intestine,24 which might provide an explanation for why some COVID-19 patients have multiple organ dysfunctions. In our study, we observed that the incidence of respiratory failure and acute cardiac injury in the diabetes group were significantly higher than those in the nondiabetes group. These results suggest that more attention should be paid to respiratory support and heart protection in COVID-19 patients with diabetes.
So far, there is no clear evidence to indicate that antiviral treatment can significantly improve the prognosis of COVID-19 patients. The majority of the patients only received treatments such as oxygen therapy, fluid management, and respiratory support. Some received antibiotics, corticosteroids, and intravenous immunoglobin. Critically ill patients required ICU monitoring and mechanical ventilation. In our study, the diabetic patients were more likely to receive corticosteroids, intravenous immunoglobin, and mechanical ventilation than nondiabetic patients, which indicated that the diabetic patients had a more severe disease progression and needed more advanced therapy.
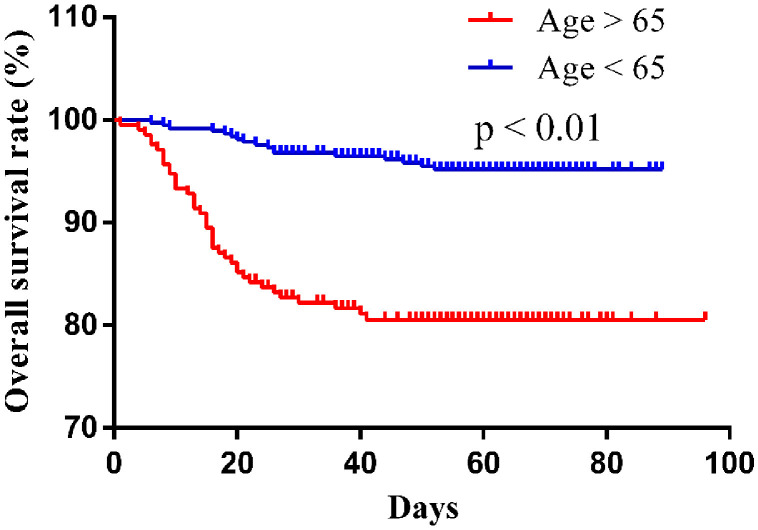
Despite receiving advanced treatment, the mortality of patients in the diabetes group was still significantly higher than that in the nondiabetes group. In order to determine the role of diabetes on the prognosis of COVID-19 patients, Kaplan–Meier method and survival curve were applied in our study, and the results indicate that the diabetic patients had a significantly lower overall survival rate than the nondiabetic patients. To exclude the influence of other factors, multivariate Cox analysis was performed and the data showed that diabetes was an independent prognosis factor for mortality in COVID-19 patients. Thus, more intensive monitors should be conducted in diabetic patients to prevent the disease from getting worse. Aging is one of the important factors affecting the prognosis of COVID-19 patients. In our study, Kaplan–Meier survival curve showed that patients older than 65 years had a shorter overall survival time (HR 5.260, P < .01) (Figure 4 ), suggesting that older age is associated with the poor prognosis of COVID-19. However, the results of the multivariate analysis do not show that aging is an independent risk factor for COVID-19 prognosis, probably due to the influence of multiple confounding factors and limited sample size in this study.
Figure 4.
Kaplan-Meier survival curve of COVID-19 patients over 65 years and under 65 years.
Diabetes patients with uncontrollable plasma glucose levels may have more complications and a worse prognosis, usually requiring insulin therapy. We divided diabetic patients into insulin-required and non-insulin-required groups according to whether they needed insulin. We found that the incidence of respiratory failure, acute cardiac injury, acute kidney injury, and death in the insulin-required group was significantly higher than that in the non-insulin-required group. Kaplan-Meier survival curve showed that the insulin-required diabetic patients had a shorter overall survival time. These results indicate that diabetic patients requiring insulin have a higher risk of disease progression and worse prognosis after the infection of SARS-CoV-2.
Our study has several limitations. First, a selection bias exits in this retrospective cohort study, and more prospective studies are needed. Second, our research is only based on a single-center study, and more large-scale multicenter research needs to be performed to validate our conclusions.
In conclusion, diabetes is an independent risk factor for the prognosis of COVID-19. Diabetic patients should be intensely monitored during treatment, especially those who require insulin therapy.
Footnotes
Funding: This study is supported by research gants from the National Major Scientific and Technological Special Project for Significant New Drugs Development (No. 2020ZX09201007) and National Natural Science Foundation of China (No. 81800494).
Conflict of Interest: None.
Authorship: All authors had access to the data and participated in writing the manuscript.
References
- 1.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020 Jan 30 doi: 10.1001/jama.2020.1097. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Lu R, Zhao X, Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu N, Zhang D, Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, Chen X, Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knapp S. Diabetes and infection: is there a link?–A mini-review. Gerontology. 2013;59(2):99–104. doi: 10.1159/000345107. [DOI] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mo P, Xing Y, Xiao Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020 Mar 16 doi: 10.1093/cid/ciaa270. [e-pub ahead of print] ci11270. [DOI] [Google Scholar]
- 10.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.[The protocol for prevention, diagnosis and treatment of liver injury in coronavirus disease 2019] Zhonghua Gan Zang Bing Za Zhi. 2020;28(3):217–221. doi: 10.3760/cma.j.cn501113-20200309-00095. [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi S, Qin M, Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 Mar 25 doi: 10.1001/jamacardio.2020.0950. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–e184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 15.Schoen K, Horvat N, Guerreiro N, de Castro I, de Giassi KS. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect Dis. 2019;19(1):964. doi: 10.1186/s12879-019-4592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang JK, Feng Y, Yuan MY. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 17.Banik GR, Alqahtani AS, Booy R, Rashid H. Risk factors for severity and mortality in patients with MERS-CoV: analysis of publicly available data from Saudi Arabia. Virol Sin. 2016;31(1):81–84. doi: 10.1007/s12250-015-3679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Gal G, Righini M, Wells PS. D-dimer for pulmonary embolism. JAMA. 2015;313(16):1668–1669. doi: 10.1001/jama.2015.3703. [DOI] [PubMed] [Google Scholar]
- 21.Frost SD, Brotman DJ, Michota FA. Rational use of D-dimer measurement to exclude acute venous thromboembolic disease. Mayo Clin Proc. 2003;78(11):1385–1391. doi: 10.4065/78.11.1385. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Moore MJ, Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Z, Xu Y, Bao L. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11(1):59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.China NHC. New Coronavirus Pneumonia Prevention and Control Protocol. 7th ed. National Health Commission of the People's Republic of China. 2020. [DOI] [PMC free article] [PubMed]