Abstract
Background
18F-FDOPA PET/CT was proved to be a highly sensitive imaging method for detecting head and neck paraganglioma (HNPGL). The primary aim of the study was to evaluate the relationship between tumor characteristics and the SDHx-mutational status in a large series of patients with HNPGL evaluated by 18F-FDOPA PET/CT.
Methods
A total of 104 patients with HNPGL (65 sporadic/39 SDHx-mutated) were included.
Results
In comparison to SDHB/SDC/SDHx-negative cases, patients with SDHD were younger at diagnosis and had a higher rate of multifocal, vagal, and carotid paraganglioma. In patients with SDHD, vagal paraganglia represented the primary site of tumor origin. Multicentric involvement of the vagus nerve alone or in association with other locations was found to be a typical feature of SDHD cases compared to other cases (odds ratio = 59.4).
Conclusion
The present study shows that tumor multifocality within the vagus nerve is a phenotypic marker of SDHD mutation. This information is essential in the choice of the therapeutic strategy.
Keywords: diagnostic imaging, genetics, paragangliomas, vagus nerve
1 |. INTRODUCTION
Head and neck paragangliomas (HNPGL; often referred to as “glomus tumors”) belong to the family of pheochromocytoma and paraganglioma. They are located in specific areas of the head and neck and more rarely in the anterior or middle mediastinum, in close relationship with the parasympathetic nervous system. Although these tumors have been less investigated than their sympathetic counterparts from an embryological standpoint, it is widely admitted that they derivate from the neural crest. HNPGL can be sporadic or can occur as components of paraganglioma (PGL) hereditary syndromes in up to 30%−40% with an increased risk for recurrent behavior and tumor multiplicity.
The molecular genetics era of HNPGL began in 2000, with the description of the first succinate dehydrogenase subunit D (SDHD) gene mutation (PGL1 syndrome). Most of SDHD-mutation carriers that carry mutation from their father (maternal imprinting) will develop HNPGL that could possibly coexist with sympathetic pheochromocytoma and paraganglioma (PPGL). Of all the known genetic mutations, SDHD mutations are currently the leading cause of hereditary HNPGLs, followed by SDHB and SDHC mutations.1–3 Clinical predictors of germline mutations in patients with HNPGL are multiple: family history, previous pheochromocytoma, multiple HNPGL, age ≤ 40 years, and male gender.3 The estimated overall disease penetrance in nonproband SDHD mutation carriers (paternally inherited) is at almost twice the penetrance in nonproband SDHB mutation carriers.4 As hereditary tumors can be millimetric in size, disease penetrance and diagnosis of multifocality patients is widely dependent on the use of high sensitive imaging.
Recently,5F-FDOPA and 68Ga-labeled somatostatin receptor analogs (SSAs) have proved to be the most sensitive positron emission tomography (PET) radiopharmaceuticals in sporadic cases.6–8 If not available, SRS using [111In]In-DTPA-pentetreotide may be used as an alternative approach, considering the limitations given by spatial resolution of SPECT. 18F-FDG PET has high sensitivity in the setting of SDHx-related HNPGLs but sporadic cases may exhibit low uptake pattern. Based on our longstanding experience in 18F-FDOPA imaging and literature, false-positive findings in the head and neck region are seen in very rare situations and mainly be related to thyroid tumors of follicular origin,9 medullary thyroid carcinoma that can coexist with PPGL in the setting of MEN2 cases and prolactinomas10 that can be sporadic or related to SDHx mutations.
The aim of the present study was to describe 18F-FDOPA findings in a series of patients with HNPGL and evaluate the potential relationships between tumor location and SDH mutational status.
2 |. PATIENTS AND METHODS
2.1 |. Study design and subjects
This retrospective study was conducted in the department of Nuclear Medicine at La Timone Hospital, Marseille, France. We performed a comprehensive search in our database to identify all patients evaluated by PET/CT from 2007 to 2017 for a presumed HNPGL (based on radiological and functional imaging studies). Among these patients, only those who fulfilled the following criteria were included: (1) final diagnosis of PGL (2) evaluation by 18F-DOPA PET/CT, and (3) genetic testing for at least germline mutations (including large deletions) in the SDHB, SDHD, and SDHC genes. The study was approved by the local ethical committee of Aix- Marseille University. All patients gave informed consent for the use of anonymous personal data extracted from their medical records for research purposes.
2.2 |. 18F-DOPA PET/CT imaging protocol
A combined PET/CT scanner was used using Discovery ST or Discovery 710 GE Medical systems. All patients fasted for at least 3 hours. Image acquisition started 45 minutes (20–60 minutes, median 47.5 minutes) after injection of 3.5 Megabecquerels (MBq)/kilogram (kg) of IASOdopa (1.8–5 MBq/kg, median 3.4 MBq/kg). The acquisition protocol started with a craniocervical scanning (1 bed position of 10 minutes, headboard, arms along the body), followed by a whole body (WB) acquisition from the top of head to mid thigh (3 minutes/bed position, arms above the head). Each focus of increased extra physiologic radiotracer uptake was recorded and interpreted by an experienced nuclear physician (David Tai’eb). The precise embryological origin of the PPGL was also given.
2.3. Gold standard
Surgical findings and pathological examination were considered as the gold standard for PGL. In cases where no surgical resection was performed, the diagnosis of PGL and its location was made by the consensus between experienced radiologists and nuclear physicians taking into account all imaging studies (including MRI with angiographic sequences, petrous bone CT, cervico-thoraco-abdominal CT, PET with other radiopharmaceuticals) and follow-up. Vagal paraganglioma (VP) arises within or adjacent to the nerve fibers of the vagus nerve at various levels along its cervico-mediastinal route in the anterior angle formed by the posterolateral common carotid artery and posteromedial internal jugular vein walls. In the upper thorax, The vagus nerve runs in the mediastinum anteriorly to subclavian artery. Combination of 18F-FDOPA tumor uptake (on PET scan) and a typical anatomical location enable diagnosis of VP with a high accuracy. VP are mainly found just below the nodose ganglion. They may extend upward into the skull base through the jugular foramen but tumor volume is mainly located inferiorly in the cervical area. VP can be found at the level of the carotid bifurcation in the retrostyloid parapharyngeal space, splaying anteriorly the internal carotid artery from the internal jugular vein. VP can also rarely found in the lower neck or the mediastinum in typical locations.
2.4. Genetic analysis and in silico prediction
Genetic analysis was achieved by Sanger sequencing or by targeted Next-Generation Sequencing of blood leukocytes DNA between January 2008 and October 2017 in the laboratory of molecular biology at Conception Hospital. Based on the clinical presentation and the Endocrine Society Clinical Practice Guideline (Lenders et al, JCEM 2014), the genetic testing of the SDHB (NM_003000.2), SDHC (NM_003001.3), and SDHD (NM_003002.3) genes was performed for all the index cases after that their informed consents were obtained. The full coding sequences and exon-intron junctions of theses genes were analyzed. The variant changes were named using the HGVS nomenclature. The SDHB/SDHC/SDHD sequence variants were classified as pathogenic/likely pathogenic/variants of uncertain significance (VUS)/likely benign/benign according the ACMG-AMP Standards and guidelines for the interpretation of sequence variants.11 For unknown variants, silico analysis was performed using Alamut Visual (Interactive Biosoftware, Rouen, France). The splicing impact of the variations was evaluated using Human Splicing Finder 3.1 (MMG, Marseille, France). The bibliographic references of the different sequence variants were found using the Human Gene Mutation Database (HGMD).
2.5. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 20 (IBM SPSS Inc., Chicago, Illinois). Continuous variables are expressed as means ±SD and categorical variables are reported as count and percentages. Logistic regression model was used to find variables that were found to be associated with SDHD mutation status. Odds ratios were expressed with 95% confidence intervals. All the tests were two-sided. The statistical significance was defined as P < .05. 3 SDHD, single VP in another patient with SDHD and a single carotid body paraganglioma (CBP) in the latter patient with SDHC. None of the nonprobans cases received any specific prior therapy for their HNPGL.
The use of pathogenic variants prediction tools allowed us to reclassify two variants as likely benign variants (one of them initially classified as a SDHB mutation and the other one initially classified as a Von Hippel-Lindau (VHL) mutation). Thus, these variants were considered as sporadic cases. The mean age at diagnosis was 58.1 ± 15 years and 66.33% of patients with HNPGL were female.
3.2. Tumor locations across genotypes, with special emphasis on VP
The percentages of multifocality in patients with SDHB, SDHD, SDHC, and sporadic HNPGL were 0%, 78.2%, 25%, and 10.7%, respectively (Table 1). Regarding the PGL associated with the vagus nerve (VP), they were diagnosed in 0% of the SDHB mutation carriers, 86.9% (n = 20) of the SDHD mutation carriers, 12.5% (n = 1) of the SDHC mutation carriers, and 23.1% (n = 15) of the sporadic cases (Table 1, Figure 1). 69.5% (n =16) of SDHD mutations carrier patients had multifocal VP, whereas there were only 0%, 12.5% (n = 1), and 3.1% (n = 2) in SDHB, SDHC, and sporadic cases, respectively (Table 2). VP in SDHD typically occurs at the level of the nodose ganglion with additional tumors within the vagus nerve trunk (Table 2).
TABLE 1.
Patient’s and tumors’s characteristics across genotypes
| Gene mutated | SDHD (n = 23, 22.1%) | SDHB (n = 8, 7.7%) | SDHC (n = 8, 7.7%) | Negative genetic testing (n = 65, 62.5%) |
SDHD vs other |
||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | |||||
| Age | 45.1 ± 13.1 | 55.6 ± 13.7 | 53.2 ± 16.4 | 63.4 ± 12.6 | 0.9 | 0.88–0.96 | <.001 |
| Female, n (%) | 11 (47.8) | 2 (25) | 6 (75) | 50 (76.9) | 0.4 | 0.14–0.94 | .033 |
| Multifocality, n (%) | 18 (78.2) | 0 (0) | 2 (25) | 7 (10.7) | 28.8 | 8.6–96.49 | <.001 |
| VP, n (%) | 20 (86.9) | 0 (0) | 1 (12.5) | 15 (23.1) | 27.1 | 7.16–102.5 | <.001 |
| CBP, n (%) | 14 (60.8) | 2 (25) | 6 (75) | 18 (27.7) | 3.3 | 1.26–8.58 | .012 |
| JP, n (%) | 10 (43.4) | 6 (75) | 3 (37.5) | 34 (52.3) | 0.7 | 0.27–1.73 | .416 |
| Metastatic, n (%) | 1 (4.3) | 1 (12.5) | 0 (0) | 1 (1.5) | 1.8 | 0.16–20.73 | .531 |
Bold values indicate statistical significance (P < .05).
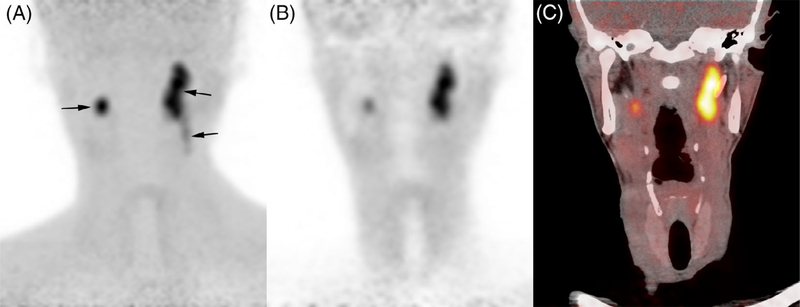
FIGURE 1.
Bilateral vagus nerve PGL in a patient with SDHD. 18F-FDOPA PET (maximal intensity projection), coronal 18F-FDOPA PET.
A, Craniocervical 18F-FDOPA PET image (maximal intensity projection) showing bilateral VP (arrows); B, Correction-attenuation coronal 18F-FDOPA PET image; C, Coronal 18F-FDOPA PET/CT fusion image
TABLE 2.
Relationship between vagus nerve involvement and patient’s genotype
| Gene mutated | SDHD (n = 23, 22.1%) | SDHB (n = 8, 7.7%) | SDHC (n = 8, 7.7%) | Negative genetic testing (n = 65, 62.5%) |
SDHD vs others |
||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | |||||
| Number of patients with VP (%) | 20 (86.9) | 0 (0) | 1 (12.5) | 15 (23.1) | 27.1 | 7.16–102.5 | <.001 |
| Number of patients with multiple VP (%) | 16 (69.5) | 0 (0) | 1 (12.5) | 2 (3.1) | 59.4 | 13.8–254.7 | <.001 |
| Single VP without additional PPGL, number of patients: n (%) | 4 (17.3) | 0 (0) | 0 (0) | 13 (20) | 1.1 | 0.32–3.77 | .999 |
| Single VP with parasympathetic PGL only, n (%) | 0 (0) | 0 (0) | 1 (12.5) | 2 (3.1) | NA | NA | NA |
| Single VP with parasympathetic PGL and sympathetic PPGL, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA |
| Multiple VP without additional PPGL, n (%) | 1 (4.34) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA |
| Multiple VP with parasympathetic PGL only n (%) | 9 (39.1) | 0 (0) | 0 (0) | 0 (0) | 6.8 | 4.2–11.0 | <.001 |
| Multiple VP with parasympathetic PGL and sympathetic PPGL, n (%) | 6 (26.1) | 0 (0) | 0 (0) | 0 (0) | 5.8 | 3.74–8.88 | <.001 |
| Nodose VP only, n (%) | 9 (45) | 0 (0) | 1 (100) | 6 (40) | 1.05 | 0.2–3.9 | 1 |
| Nodose + trunk VP, n (%) | 10 (50) | 0 (0) | 0 (0) | 2 (13.3) | 7 | 1.2–39 | .03 |
| Trunk VP only, n (%) | 1 (5) | 0 (0) | 0 (0) | 7 (46.6) | 0.06 | 0.007–0.6 | .01 |
Bold values indicate statistical significance (P < .05).
3.3. Genotype-phenotype correlations in patients with SDHD
Eleven different variants were identified in SDHD mutation carriers. Among them, two nucleotidic variants were over represented: c.169+5G>A and c.129G>A, both located in exon 2. Mediastinal VP was only detected in patients with SDHD. Focusing on the c.129G>A variant, it appears clear that this genotype is more likely to express a phenotype with a mediastinal vagus location (OR = 7.14; P = 5 X 10−21). Indeed, among the 104 patients with HNPGL, 6 out the 7 patients with a mediastinal vagus location are patients with SDHD having a c.129G>A variant (Table 3). The two most represented variants: c.169+5G>A (n = 6) and c.129G>A (n = 6) have previously been reported in the literature (Cascon, et al.13; Timmers, et al.14). The c.169+5G>A variant has been described as potentially metastatic. Indeed, in our study, one out of the 6 c.169+5G>A variants (the only one among SDHD variants) presented distant metastasis at diagnosis, (Table 1) confirming that we should consider this variant as a potentially malignant condition. The c.129G>A variant was described once in the literature and presented a para-aortic localization; these results are in accordance with our findings. Four variants have never been reported in the literature: c.1A>G, c.446–449delinsA, c.64C>T, and c.433–438del (Table 3). Ultimately, among the three nonsense mutations coding for truncated proteins: c.129G>A (n = 6), c.325C>T (n = 3), and c.64C>T (n = 1); 100% of them were VP with multifocality (Table 3).
TABLE 3.
Relationship between genotype and phenotype in patients with SDHD
| Exon | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 4 | 4 | 4 |
| Nucleotidic change | c.1A>G | c.56G>T | c.64C>T | c.129G>A | c.169+5G>A | c.242C>T | c.274G>T | c.305A>C | c.325C>T | c.433_438del | c.446_449delinsA |
| Peptidic change | p.? | p.Arg17Leu | p.Arg22* | p.Trp43* | p.? | p.Pro81Leu | p.Asp92.Tyr | p.His102Pro | p.Gln109* | p.His145_Asp146del | p.Ile149_Cys150delinsAsn |
| Effect | Start loss | False-sense | Non-sense | Non-sense | Splice | False-sense | False-sense | False-sense | Non-sense | In frame deletion | In frame deletion |
| Interpretation | P | LP | P | P | P | P | P | LP | P | LP | LP |
| Affected patients | 1 | 1 | 1 | 6 | 6 | 1 | 1 | 1 | 3 | 1 | 1 |
| Unifocal | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 0 |
| Multifocality | 1 | 1 | 1 | 5 | 4 | 0 | 1 | 1 | 3 | 0 | 1 |
| Metastatic | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| VP (cervical only) | 1 | 1 | 1 | 0 | 3 | 1 | 1 | 0 | 3 | 1 | 1 |
| VP (mediastinal only) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| VP (cervical + mediastinal) | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Extra vagal PGL only | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Reference | Not reported | Lima et al.12 | Not reported | Cascon et al.13 | Timmers et al.14 | Baysal et al.15 | Baysal et al.15 | Burnichon et al.16 | Burnichon et al.16 | Not reported | Not reported |
= mutations that lead to the coding of a STOP codon.
4 |. DISCUSSION
To the best of our knowledge, this is the largest study that describes the locations of HNPGL across genotypes based on modern imaging, including 18F-DOPA PET/CT. Although our series was biased by the recruitment process from the department of Nuclear Medicine, the prevalence of SDHB/C/D in our population (37.5%) was in accordance with the literature. The unique missing location was tympanic PGL (ie, Fisch classes A and B). As these tumors are almost always sporadic and often cured by surgical resection, they are usually not evaluated by functional imaging in our institution.
The principal conclusions that can be drawn from this study include: (1) confirmation that HNPGL mainly occur in the context of sporadic and SDHD cases, (2) vagal location is the most common primary site of HNPGL in SDHD cases, and (3) presence of tumor multifocality with vagus nerve involvement should be considered as an important phenotypic marker of SDHD-related disease.
Several studies have shown that SDHD was the leading cause of hereditary HNPGL and this finding was recently illustrated in a large study of 876 patients with SDHB/C/D that reported genotype-phenotype correlations as well as penetrance disease across genotypes.4 However, detailed information regarding the locations of HNPGLs are lacking in most series. A large prospective study that evaluated 259 SDHx-mutations carriers with octreoscan and magnetic resonance angiography showed that VP mainly occurred in presence of SDHD-mutation, 33 of 37 and 10 of 10 being detected in SDHD index cases and relatives, respectively.17 In another study that evaluated 72 patients with SDHx by contrast-enhanced CT, 23 of 27 VP were observed in patients with SDHD.18 In both series, carotid body PGL was the most common location in patients with SDHD. In our series, we found that the vagus nerve paraganglia represented the most common location of the primary tumor in SDHD cases. This was probably due to the use of high sensitive PET/CT imaging. The continuing evolution of anatomical imaging and functional imaging has drastically increased the penetrance of HNPGL and prevalence of VP that could be millimetric in size, especially in the setting of hereditary PGL syndromes. In two prospective studies,7,8 68Ga- DOTATATE PET/CT was found to be slightly superior (not statistically) to F-FDOPA PET/CT in the detection of primary HNPGL in patients with SDHx, mainly due to the combination of a highly elevated tumor-background 68Ga- DOTATATE uptake ratio and the catecholaminergic differentiation pattern of SDHx-related PGL. However,5F-FDOPA PET/CT has excellent value in the location of HNPGL. The knowledge of genetic status and tumor multifocality has important clinical implications and is essential in the decision of the therapeutic strategy. In fact, surgical management of HNPGL can be challenging due to complex anatomy and nearly critical structures. Although subtotal surgical approaches have been developed to limit cranial nerve morbidity, surgical resection of HNPGL may lead to severe, permanent surgery-induced injuries (ie, dysphagia, chronic aspiration, vocal cord paralysis) and may compromise subsequent operations. Radiosurgery and conventional radiotherapy can control tumoral evolution but not without risk of cranial nerve morbidity. The present study shows that tumor multifocality with vagus nerve involvement is an important phenotypic marker of SDHD-related disease. As surgical management of HNPGL can be followed by secondarily neurological injuries, the presence of contralateral PGL especially VP may compromise further surgical and radiosurgical intervention. Surgical resection of VP almost always leads to the sacrifice of the vagus nerve, and sometimes palsy of other adjacent cranial nerves.19 It is important to recall that bilateral neurological injuries affect respiration, deglutition, and phonation and are associated with chronic complications and vital risks. Therefore, the impact of detecting VP at the primary workup is of a major clinical importance, especially in patients with tumor multifocality with bilateral VP or VP associated with contralateral CBP or jugular paraganglioma (JP). In patients with HNPGL, a conservative management should be proposed to prevent any neurological injuries until genetic testing and functional imaging results.
Interestingly, patients with SDHB and SDHC are unlikely to have multifocal disease, even when evaluated 5F-FDOPA PET/CT. Therefore, these patients should probably be treated as sporadic cases.
The optimal follow-up algorithm has not yet been validated in nonproband SDHx-PPGLs but most likely requires a more frequent and complete imaging workup than for their sporadic counterparts. The aim is to detect tumors at early stages of development, thereby minimizing tumor extension, and cranial nerve impairment as for example, published for SDHD-related HNPGLs,20 facilitating curative treatment, and potentially reducing the occurrence of local and distant metastatic spread, especially in more aggressive genotypes (eg, SDHB). At initial staging, the use of PET imaging should provide an adequate sensitivity and specificity at WB scale with limited radiation exposure. Based on clinical studies, 68Ga-DOTA-SSAs PET should be prioritized if available over 18F-FDOPA PET, although its indication has not been specifically studied in this setting nonproband SDHx cases. In absence of PPGL, follow-up should include annual biochemical screening, especially plasma methoxytyramine and MRI at regular time intervals.21 MRI may be done every 1–2 years for SDHD-related HNPGLs, but for SDHB ones, we would recommend to consider this imaging study to be done every year. For SDHA and SDHC HNPGLs, data are limited but perhaps these patients can follow the same guidelines as for patients with SDHD.
VPs arise from neural-crest derived endocrine cells associated with the vagus nerve. The so-called vagal body paraganglia has been originally described in birds by Muratori in 193222 and in humans by White in 1935.23 In fact, it is constituted by an average of 6–7 separate collections of paraganglia, mainly located at or just below (2.0 cm) the level of nodose ganglion (ie, inferior ganglion), within the nerve or ganglion itself.1 The nodose ganglion contains cell bodies of the sensory neurons that transmits visceral stimuli (taste buds, heart, lungs, and other visceral organs) to the nucleus of the solitary tract. Additional paraganglionic cells clusters could be found in distal portions of the vagus nerve. Despite similar architectural and cytochemical features between vagal body paraganglia and carotid bodies, its chemoreceptor role is still unclear despite possible observation of chief cell hyperplasia during chronic hypoxemia.5
Stout identified the first paraganglioma of the vagus nerve,24 and Birrell proposed the term vagal body tumor in 1953.25 During the past decades, several case series have been reported VP with possible multifocality with other PGL and malignant potential. Its possible familial occurrence has been formally recognized after it has been made for carotid and jugular PGL.26,27 Although, HNPGL can be related to SDH mutations, they are often sporadic. In these cases, mechanisms involved in tumorigenesis are still to be established. In SDHx-related PGL, SDH acts as a tumor suppressor in the paraganglionic system. Tumorigenesis requires a second hit that leads to SDH deficiency.
REFERENCES
- 1.Baysal BE, Willett-Brozick JE, Lawrence EC, et al. Prevalence of SDHB, SDHC, and SDHD germline mutations in clinic patients with head and neck paragangliomas. J Med Genet. 2002;39(3):178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piccini V, Rapizzi E, Bacca A, et al. Head and neck paragangliomas: genetic spectrum and clinical variability in 79 consecutive patients. Endocr Relat Cancer. 2012;19(2):149–155. [DOI] [PubMed] [Google Scholar]
- 3.Neumann HP, Erlic Z, Boedeker CC, et al. Clinical predictors for germline mutations in head and neck paraganglioma patients: cost reduction strategy in genetic diagnostic process as fall-out. Cancer Res. 2009;69(8):3650–3656. [DOI] [PubMed] [Google Scholar]
- 4.Andrews KA, Ascher DB, Pires DEV, et al. Tumour risks and genotype- phenotype correlations associated with germline variants in succinate dehydrogenase subunit genes SDHB, SDHC and SDHD. J Med Genet. 2018; 55(6):384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lack EE. Hyperplasia of vagal and carotid body paraganglia in patients with chronic hypoxemia. Am J Pathol. 1978;91(3):497–516. [PMC free article] [PubMed] [Google Scholar]
- 6.Taieb D, Pacak K. New insights into the nuclear imaging phenotypes of cluster 1 pheochromocytoma and paraganglioma. Trends Endocrinol Metab. 2017;28(11):807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archier A, Varoquaux A, Garrigue P, et al. Prospective comparison of (68) Ga-DOTATATE and (18)F-FDOPA PET/CT in patients with various pheochromocytomas and paragangliomas with emphasis on sporadic cases. Eur J Nucl Med Mol Imaging. 2016;43(7):1248–1257. [DOI] [PubMed] [Google Scholar]
- 8.Janssen I, Chen CC, Taieb D, et al. 68Ga-DOTATATE PET/CT in the localization of head and neck paragangliomas compared with other functional imaging modalities and CT/MRI. J Nucl Med. 2016;57(2):186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pauleau G, Palazzo FF, Essamet W, Sebag F, Taieb D. Hurthle cell neoplasms: a new differential diagnosis for 18F-FDOPA-avid thyroid nodules? J Clin Endocrinol Metab. 2013;98(3):865–866. [DOI] [PubMed] [Google Scholar]
- 10.Ginet M, Cuny T, Schmitt E, Marie PY, Verger A. 18F-FDOPA PET imaging in prolactinoma. Clin Nucl Med. 2017;42(8):e383–e384. [DOI] [PubMed] [Google Scholar]
- 11.Group NGSiPS Toledo RA, Burnichon N, et al. Consensus Statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochro- mocytomas and paragangliomas. Nat Rev Endocrinol. 2017;13(4):233–247. [DOI] [PubMed] [Google Scholar]
- 12.Lima J, Feijao T, Ferreira da Silva A, et al. High frequency of germline succinate dehydrogenase mutations in sporadic cervical paragangliomas in northern Spain: mitochondrial succinate dehydrogenase structure-function relationships and clinical-pathological correlations. J Clin Endocrinol Metab. 2007;92(12):4853–4864. [DOI] [PubMed] [Google Scholar]
- 13.Cascon A, Ruiz-Llorente S, Cebrian A, et al. Identification of novel SDHD mutations in patients with phaeochromocytoma and/or paraganglioma. Eur J Hum Genet. 2002;10(8):457–461. [DOI] [PubMed] [Google Scholar]
- 14.Timmers HJ, Pacak K, Bertherat J, et al. Mutations associated with succinate dehydrogenase D-related malignant paragangliomas. Clin Endocrinol (Oxf ). 2008;68(4):561–566. [DOI] [PubMed] [Google Scholar]
- 15.Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000; 287(5454):848–851. [DOI] [PubMed] [Google Scholar]
- 16.Burnichon N, Rohmer V, Amar L, et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J Clin Endocrinol Metab. 2009;94(8):2817–2827. [DOI] [PubMed] [Google Scholar]
- 17.Gimenez-Roqueplo AP, Caumont-Prim A, Houzard C, et al. Imaging workup for screening of paraganglioma and pheochromocytoma in SDHx mutation carriers: a multicenter prospective study from the PGL.EVA investigators. J Clin Endocrinol Metab. 2013;98(1):E162–E173. [DOI] [PubMed] [Google Scholar]
- 18.Michalowska I, Lewczuk A, Cwikla J, et al. Evaluation of head and neck Paragangliomas by computed tomography in patients with Pheochromocytoma- Paraganglioma syndromes. Pol J Radiol 2016;81:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suarez C, Rodrigo JP, Bodeker CC, et al. Jugular and vagal paragangliomas: systematic study of management with surgery and radiotherapy. Head Neck. 2013;35(8):1195–1204. [DOI] [PubMed] [Google Scholar]
- 20.Heesterman BL, de Pont LMH, van der Mey AG, et al. Clinical progression and metachronous paragangliomas in a large cohort of SDHD germline variant carriers. Eur J Hum Genet. 2018;26:1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel E, Jones R, Bull M, Newell-Price J. Rapid-sequence MRI for long-term surveillance for paraganglioma and phaeochromocytoma in patients with succinate dehydrogenase mutations. Eur J Endocrinol. 2016;175(6): 561–570. [DOI] [PubMed] [Google Scholar]
- 22.Muratori G Contributo all’innervazione des tessuto paragangliare annesso al sistema del vago (glomo carotico, paragangli estravagali ed intravagali) e all’ innervazione del seno carotideo. Anat Anz 1932;75:115–123. [Google Scholar]
- 23.White EG. Die Struktur des Glomus caroticum, seine Pathologie und Physiologie und seine Beziehung zum Nervensystem. Beitr Pathol Anat Allgemienen Pathol. 1935;96:177–227. [Google Scholar]
- 24.Stout AP. The malignant tumors of the peripheral nerves. Am J Cancer. 1935;25:1–36. [Google Scholar]
- 25.Birrell JH. The vagal body and its tumour. Aust N Z J Surg. 1953;23(1): 48–54. [DOI] [PubMed] [Google Scholar]
- 26.Linn HJ, Proctor B. Tumor of the ganglion nodosum of the vagus nerve. Laryngoscope. 1956;66:1577–1581. [DOI] [PubMed] [Google Scholar]
- 27.Kahn LB. Vagal body tumor (nonchromaffin paraganglioma, chemodec- toma, and carotid body-like tumor) with cervical node metastasis and familial association: ultrastructural study and review. Cancer. 1976;38(6): 2367–2377. [DOI] [PubMed] [Google Scholar]