Abstract
For a developing fetus, the role of the placenta is simultaneously simple and complex. This ephemeral organ must allow nutrients and oxygen from the maternal bloodstream to reach the fetus but keep out pathogens and other harmful compounds. The placenta also secretes sex hormones, including estrogen and progesterone, to help direct fetal growth and development.1 Certain chemicals, including endocrine-disrupting chemicals (EDCs), can disrupt this delicate process. A recent article in Environmental Health Perspectives suggests that one such EDC, the fungal toxin zearalenone (ZEN),2 can readily cross the placenta and enter the fetal bloodstream.3
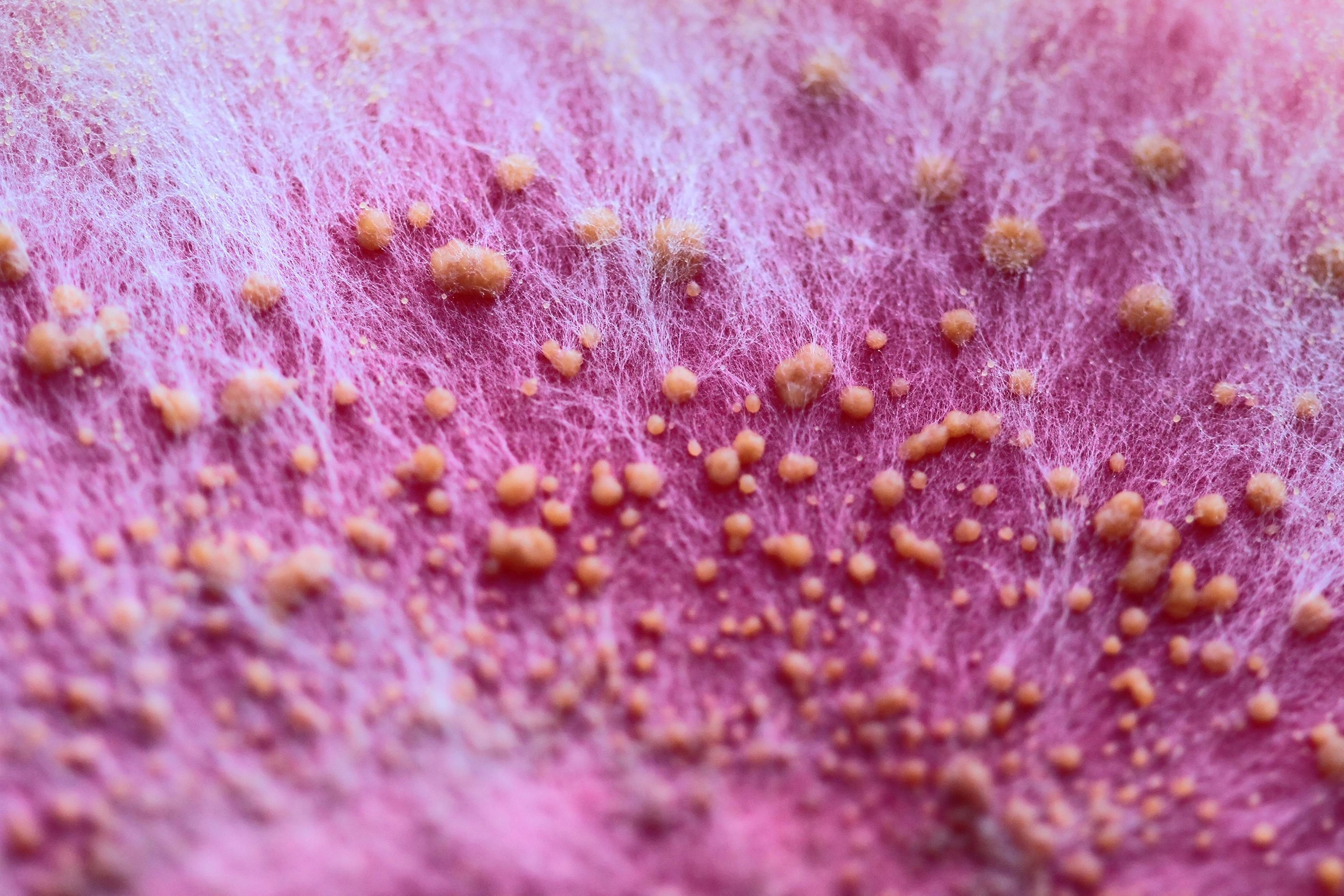
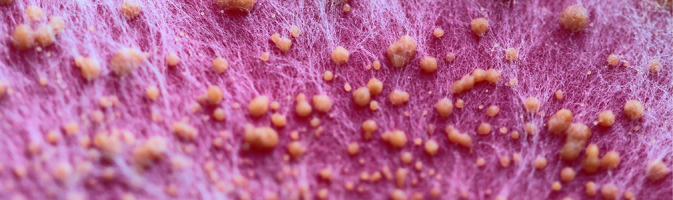
Fusarium culmorum, shown here, is one of several fungal species that produce ZEN. This micrograph shows the fungus’s fibrous white hyphae and globular orange sporodochia grown on pink medium. The sporodochia, which form from the hyphae, may comprise thousands of spores each. Image: © Lesny Ludek/Shutterstock.
The potential impact of disrupting the delicate balance of hormones during pregnancy is one of the major reasons that scientists are concerned about EDCs. “This work showed, in principle, that the transfer and metabolism of zearalenone across the placenta is possible,” says senior author Tina Buerki-Thurnherr, a toxicologist at the Swiss Federal Laboratories for Materials Science and Technology.
ZEN, produced by multiple Fusarium species, is found in a broad range of foods, including cereal- and legume-based products.2 It mimics natural estrogens, says Buerki-Thurnherr, but it is not easily absorbed by the human body, and it is undetectable in the plasma of the majority of the population. A 2019 study found ZEN in the plasma of only 6.5% of participants, with concentrations of .4 This work is important in the context of the hypothesis that environmental exposures early in life can have an outsized impact on adult health.5 Importantly, however, the study did not assess potential health effects of ZEN exposure and is not intended as guidance for people to make dietary decisions. “We know the fetus is sensitive to xenoestrogens,” says University of Michigan environmental health scientist Rita Loch-Caruso, who was not involved in the study, “but we know very little about [the effects of] this particular mycotoxin.”
Nutrient, gas, and waste exchange between mother and fetus is both rapid and voluminous, with uterine blood flow reaching in late pregnancy.6 This allows low-molecular-weight compounds, those without an electric charge, and fat-soluble substances to cross the placental barrier more readily.7 ZEN has been shown to cross the placenta in rats.8 What the authors did not know was whether it could cross this barrier in humans, and if so, how quickly.
Buerki-Thurnherr had previous experience studying the placenta in human cell and tissue models. For this study, her team used a dually perfused ex vivo human placenta9 to model the transfer of ZEN and its nine key metabolites from mother to fetus. In this model, cannulae are inserted into blood vessels on both the maternal and fetal sides of the placenta. Investigators can then introduce compounds of interest on either side to observe how they transfer between mother and fetus. The process is labor-intensive and often fails due to placental leakage and other issues. “It’s definitely not high throughput,” Buerki-Thurnherr says.
The authors successfully perfused six placentas. The maternal circulation of three placentas received a control solution, and three were perfused with () ZEN for 6 hours. Both solutions were collected on the fetal side and analyzed using a new ultra-high-performance liquid chromatography–tandem mass spectrometry method developed by members of the team in a previous study.10 This method allowed the researchers to analyze up to 75 metabolites simultaneously instead of individually.
The new findings showed that two ZEN metabolites, including a highly estrogenically active form, were detected in fetal circulation within 15 minutes of being introduced on the maternal side. After 6 hours of ZEN perfusion, approximately 15% of the compound was present in the fetal circulation, 31% was present in the maternal circulation, and the rest was metabolized or present elsewhere in the placenta.
The results indicate that ZEN transfer was both fast and efficient, according to Loch-Caruso. She points out that the study is limited by its small sample size and the lack of demographic data about the mothers who donated their placentas. Still, she praised the work as “an important, early first study” in understanding how endocrine disruptors cross the placenta, with potential to impact fetal development.
Biography
Carrie Arnold is a freelance science writer living in Virginia. Her work has appeared in Scientific American, Discover, New Scientist, Smithsonian, and more.
References
- 1.Bukovsky A, Caudle MR, Cekanova M, Fernando RI, Wimalasena J, Foster JS, et al. 2003. Placental expression of estrogen receptor beta and its hormone binding variant—comparison with estrogen receptor alpha and a role for estrogen receptors in asymmetric division and differentiation of estrogen-dependent cells. Reprod Biol Endocrinol 1(1):36, PMID: 12740031, 10.1186/1477-7827-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Food Safety Authority Panel on Contaminants in the Food Chain. 2011. Scientific opinion on the risks for public health related to the presence of zearalenone in food. EFSA J 9(6):2197, 10.2903/j.efsa.2011.2197. [DOI] [Google Scholar]
- 3.Warth B, Preindl K, Manser P, Wick P, Marko D, Buerki-Thurnherr T. 2019. Transfer and metabolism of the xenoestrogen zearalenone in human perfused placenta. Environ Health Perspect 127(10):107004, PMID: 31596610, 10.1289/EHP4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan K, Xu J, Jiang K, Liu X, Meng J, Di Mavungu JD, et al. 2019. Determination of multiple mycotoxins in paired plasma and urine samples to assess human exposure in Nanjing, China. Environ Pollut 248:865–873, PMID: 30856502, 10.1016/j.envpol.2019.02.091. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ. 1995. Fetal origins of coronary heart disease. BMJ 311(6998):171–174, PMID: 7613432, 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Zhao S. 2010. Placental blood circulation In: Vascular Biology of the Placenta. San Rafael, CA: Morgan & Claypool Life Sciences. [PubMed] [Google Scholar]
- 7.Mitro SD, Johnson T, Zota AR. 2015. Cumulative chemical exposures during pregnancy and early development. Curr Environ Health Rep 2(4):367–378, PMID: 26341623, 10.1007/s40572-015-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernhoft A, Behrens GH, Ingebrigtsen K, Langseth W, Berndt S, Haugen TB, et al. 2001. Placental transfer of the estrogenic mycotoxin zearalenone in rats. Reprod Toxicol 15(5):545–550, PMID: 11780962, 10.1016/S0890-6238(01)00159-9. [DOI] [PubMed] [Google Scholar]
- 9.Hutson JR, Garcia-Bournissen F, Davis A, Koren G. 2011. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther 90(1):67–76, PMID: 21562489, 10.1038/clpt.2011.66. [DOI] [PubMed] [Google Scholar]
- 10.Preindl K, Braun D, Aichinger G, Sieri S, Fang M, Marko D, et al. 2019. A generic liquid chromatography–tandem mass spectrometry exposome method for the determination of xenoestrogens in biological matrices. Anal Chem 91(17):11334–11342, PMID: 31398002, 10.1021/acs.analchem.9b02446. [DOI] [PubMed] [Google Scholar]


