Abstract
BACKGROUND
Fluctuations in climate have been associated with variations in mosquito abundance.
OBJECTIVES
To analyse the influence of precipitation, temperature, solar radiation, wind speed and humidity on the oviposition dynamics of Aedes aegypti in three distinct environmental areas (Brasília Teimosa, Morro da Conceição/Alto José do Pinho and Dois Irmãos/Pintos) of the city of Recife and the Fernando de Noronha Archipelago northeastern Brazil.
METHODS
Time series study using a database of studies previously carried out in the areas. The eggs were collected using spatially distributed geo-referenced sentinel ovitraps (S-OVTs). Meteorological satellite data were obtained from the IRI climate data library. The association between meteorological variables and egg abundance was analysed using autoregressive models.
FINDINGS
Precipitation was positively associated with egg abundance in three of the four study areas with a lag of one month. Higher humidity (β = 45.7; 95% CI: 26.3 - 65.0) and lower wind speed (β = −125.2; 95% CI: −198.8 - −51.6) were associated with the average number of eggs in the hill area.
MAIN CONCLUSIONS
The effect of climate variables on oviposition varied according to local environmental conditions. Precipitation was a main predictor of egg abundance in the study settings.
Key words: Aedes, meteorological concepts, time series studies
Aedes aegypti, an arthropod of the Culicidae family, subgenus Stegomyia is the main vector responsible for the rapid spread and intensive transmission of arboviruses, such as dengue, Chikungunya and more recently Zika worldwide. 1 Recent studies analysing the distribution of Ae. aegypti in time and space point to the risk of expansion of this species to other regions of the world as a result of climate change. 2 In addition, greater human mobility, including sea and air cargo transport, has favored the global expansion of this mosquito, even in temperate climate zones. 3
There is strong evidence of the effect of climate variations, primarily temperature and precipitation, on the abundance of Ae. aegypti and, consequently, on the transmission dynamics of arboviruses such as dengue. 4 , 5 High temperatures have effect on the survival, development rate, mortality and spread of Aedes species while high humidity is associated with increased Ae. aegypti feeding activity survival and egg development. 4 , 6 Precipitation accelerates population growth of Aedes through the formation of new breeding sites, but when remarkably high can wash out the containers and negatively affect the abundance of these vectors. 7 Wind speed either favors mosquito dispersal or suppresses their flight activity interfering with feeding habits and oviposition. 8 Solar radiation may interfere with the egg-laying behavior of females, although studies present discordant results regarding the preference of females for shade or breeding sites exposed to the sun. 4 It has been demonstrated that fluctuations in climate and local environmental conditions, such as level of urbanisation, are associated with variations in mosquito abundance and arbovirus incidence (in general, dengue) between settings. 9
The Northeast region of Brazil has been affected by severe and repeated outbreaks of dengue and, more recently, Zika and Chikungunya, all diseases transmitted by Ae. aegypti 10 given the local effect of climate variations and physiographic characteristics on the population dynamics of Ae. aegypti (with consequent increased risk of transmission), it is important to investigate the population dynamics in different settings, with a view to establishing early warning systems and improving vector control measures in this region. In the present study, we examined the association between a set of meteorological variables (precipitation, temperature, humidity, wind velocity and solar radiation) and the oviposition dynamics of Ae. aegypti in four environmentally distinct urban settings in the State of Pernambuco, an area hyperendemic for arboviruses in Brazil.
MATERIALS AND METHODS
Study design and setting - A time series study was carried out using the databases created by one study conducted in the city of Recife (between April 2004 and May 2007), 11 and one carried out in the Fernando de Noronha Archipelago (between January 2011 and May 2013), 12 both in the State of Pernambuco in the Northeast region of Brazil (Fig. 1). The projects were reviewed and approved by the Research Ethics Committee of the Aggeu Magalhães Research Center ― Fiocruz Brazil (process: No. 14/04; CAAE No. 0095.0.095.000.10). Both studies were conducted with the aim of obtaining entomological parameters for an entomological surveillance system based on continuous collection of Ae. aegypti eggs by way of a sentinel ovitrap network (SMCP-Aedes - Aedes aegypti Monitoring and Population Control System).
Fig. 1: location of the study areas.
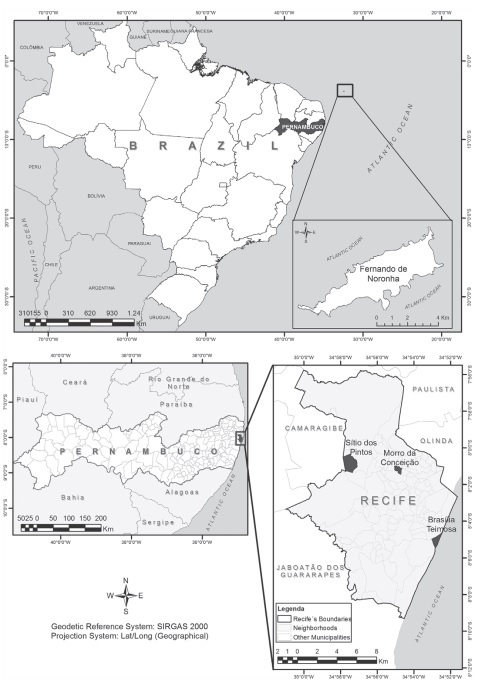
The city of Recife (08º03’14”S; 34º52’52”W), capital of the State of Pernambuco, has a territorial area of 218.4 km2 and a population of about 1.6 million inhabitants, corresponding to a population density of 7,039.6 inhabitants/ km2. 13 The regional climate is hot and humid with average annual temperatures around 25ºC, with higher averages in the months of January and February (≈ 27ºC) and lower ones in June and July (≈ 24ºC). Annual mean precipitation is 2,305 mm, with rainy seasons between April and July, and November and December are the driest months. Annual relative air humidity varies between 70% and 90% and the velocity of winds during the year ranges from 230 m/s to 340 m/s, with an annual average of 290 m/s. The archipelago of Fernando de Noronha (3º50’25 “S; 32º24’38” W) is composed of 21 islands in the Atlantic Ocean with a total area of 26 km2. Like the city of Recife, the climate is tropical humid with clearly defined dry and rainy seasons and a marked irregularity in interannual precipitation. The average temperature, of around 25ºC, varies little throughout the year and is slightly higher between November and April (≈ 30ºC) and slightly lower from May to October (≈ 28ºC). The average annual precipitation is 1,275 mm, with rainy seasons between February and July and drier seasons between October and November. The average speed of the winds is 660 m/s with the highest intensity between July and August. The relative humidity is around 80%. In Recife, the entomological data were collected in three urban sites with distinct physiographic characteristics: Brasília Teimosa, Morro da Conceição/Alto José do Pinho and Dois Irmãos/Sítio dos Pintos. In Fernando de Noronha, the data collection was carried out in 15 villages with size ranging from 14 to 172 households. Table I shows the main characteristics of the selected settings.
TABLE I. Main characteristics of the study settings, Pernambuco State, Brazil.
| Characteristics | Study areas | |||
| Brasília Teimosa | Morro da Conceição/ Alto José do Pinho | Dois Irmãos/ Sítio dos Pintos | Archipelago of Fernando de Noronha | |
| Urban landscape | Coastal area | Hill | Suburban area located near a remnant of Atlantic forest. | Marine biome |
| Territorial area (km2) | 0.61 | 0.38 | 1.80 | 17.017 |
| Total Population | 18,320 | 10,169 | 7,275 | 2,630 |
| Population density (inhabitants/km2) | 30,257.8 | 26,490.5 | 4,048.9 | 154.5 |
| Households connected to the water supply network (%) | 82.7 | 98.8 | 74.7 | 96.2 |
| Households connected to the sewage network (%) | 63.6 | 14.2 | 20.4 | 73.0 |
| Households with proper garbage disposal (%) | 99.6 | 99.0 | 97.3 | 100.0 |
Source: IBGE (2010).
Data collection - A detailed description of Ae. aegypti egg collection has already been provided. 12 A total of 368 geo-referenced S-OVTs (100 in Morro da Conceição/Alto José do Pinho, 80 in Brasília Teimosa, 85 in Dois Irmãos/Sítio dos Pintos and 103 in Fernando de Noronha) were installed at fixed points in the external parts of the residences one meter above ground level, in the shade and protected from rain. In Fernando de Noronha the S-OVTs were spatially distributed according to the number of households in each village: five in villages with 14 to 30 households seven to eight in villages with 56 to 89 households and 10 in villages with more than 100 households. Therefore, the number of S-OVTs varied from 63 to 129 S-OVT per km² in the different settings. The collection and exchange of the S-OVTs palletes for egg counting was carried out weekly in around 25 S-OVT per area, on a rotating basis. In Recife eggs, were counted using a stereoscopic microscope with the aid of a manual cell counter 12 whereas in Fernando de Noronha, a semi-automatic computerised system was employed. This computerised system is based on an optical platform that generates a digital image of the palletes. 14 The monthly average of eggs in each study area was obtained by dividing the total number of eggs collected by the number of OVTs inspected in their respective month. The Table II shows the frequency distribution of the average number of eggs per month.
TABLE II. Frequency distribution of the number of eggs collected monthly by ovitraps in the study areas, Pernambuco State, Brazil.
| Study areas | Mean | Median | Standard deviation | Minimum | Maximum |
| Brasilia Teimosa | 1445.6 | 1371.9 | 659.7 | 509.2 | 2947.0 |
| Morro da Conceição/Alto José do Pinho | 931.0 | 881.8 | 423.5 | 223.1 | 1903.2 |
| Dois Irmãos/Sítio dos Pintos | 487.1 | 443.2 | 215.9 | 159.3 | 1023.2 |
| Archipelago of Fernando de Noronha | 133.4 | 125.2 | 66.7 | 36.5 | 297.2 |
Meteorological data source - Monthly averages on precipitation (mm), temperature (ºC) relative air humidity (%), wind velocity (m/s) and solar radiation (W/m²) refer to the entire territorial area of Recife and Fernando de Noronha and were obtained from the International Research Institute for Climate and Society (IRI) climate satellite data library. Data on precipitation were specific to each of the three sites in Recife, while the data for Fernando de Noronha refer to the entire territorial area of the archipelago. 15
Data analysis - Data analysis was performed using the GRETL (Gnu Regression, Econometrics and Time-series Library) and R version 3.2.4 programs. First the frequency distribution of the meteorological variables and the number of Ae. aegypti eggs in each study setting were described. Time trend graphs were generated to evaluate the stationarity of the time series (no trend). The temporal dependence structure of the stationary series was analysed using autocorrelation functions, which provide moving averages (q) and partial autocorrelation (p) and indicate autoregression. The effect of the meteorological variables on the average number of eggs was analysed using an autoregressive model of order 1 (AR1). The association of each meteorological variable with the average number of eggs was estimate through first order autoregressive term - AR(1) model and using different lags (lag 0, 1, 2 e 3).
The selection of the meteorological variables in the final model was defined based on the use of the stepwise backward technique and the Akaike Information Criterion (AIC). 16
RESULTS
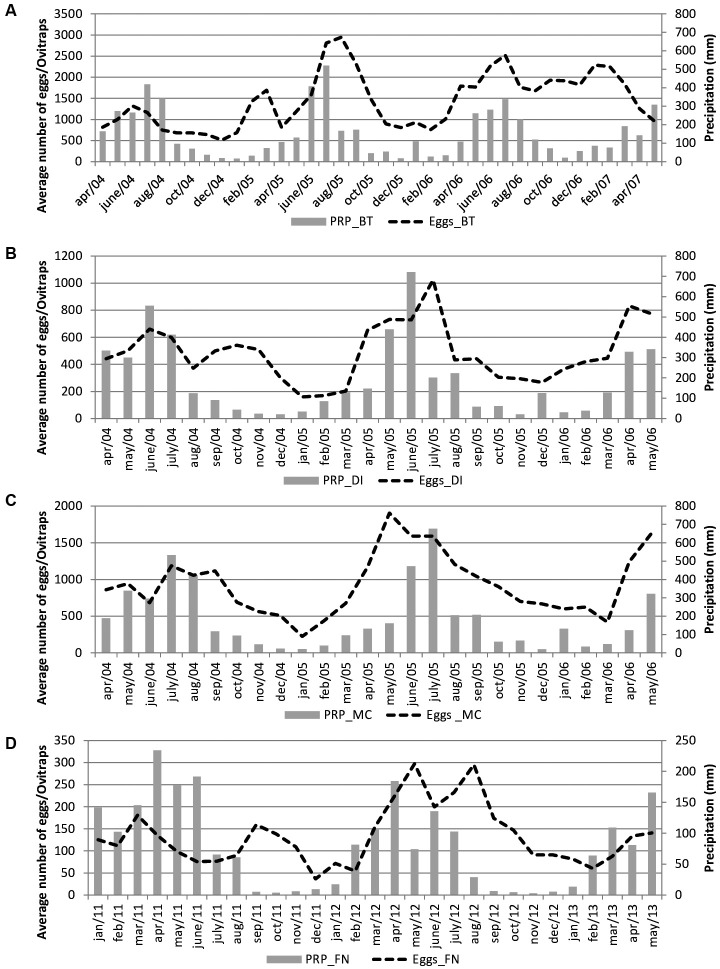
Descriptive analysis of meteorological and entomological data - The Fig. 2 and Table II show the monthly variation in precipitation (mm) and average number of eggs collected in all study areas. In Brasília Teimosa, Morro da Conceição/Alto José do Pinho, Dois Irmãos/Sítio dos Pintos and Fernando de Noronha, the mean monthly precipitation was 155.3 mm, 190.4 mm, 189 mm and 80.3 mm, respectively, during the study period. In all areas of Recife, peak precipitation occurred in July 2005, while the driest period was between November and February. In Fernando de Noronha, peak precipitation occurred in April 2011 (234 mm) and there were lower precipitation averages between September and December.
Fig. 2: monthly variation in the number of eggs and precipitation in the study areas. (A) Brasília Teimosa (BT); B: Dois Irmãos/Sítio dos Pintos (DI); C: Morro da Conceição/Alto José do Pinho (MC); D: Fernando de Noronha Archipelago (FN).
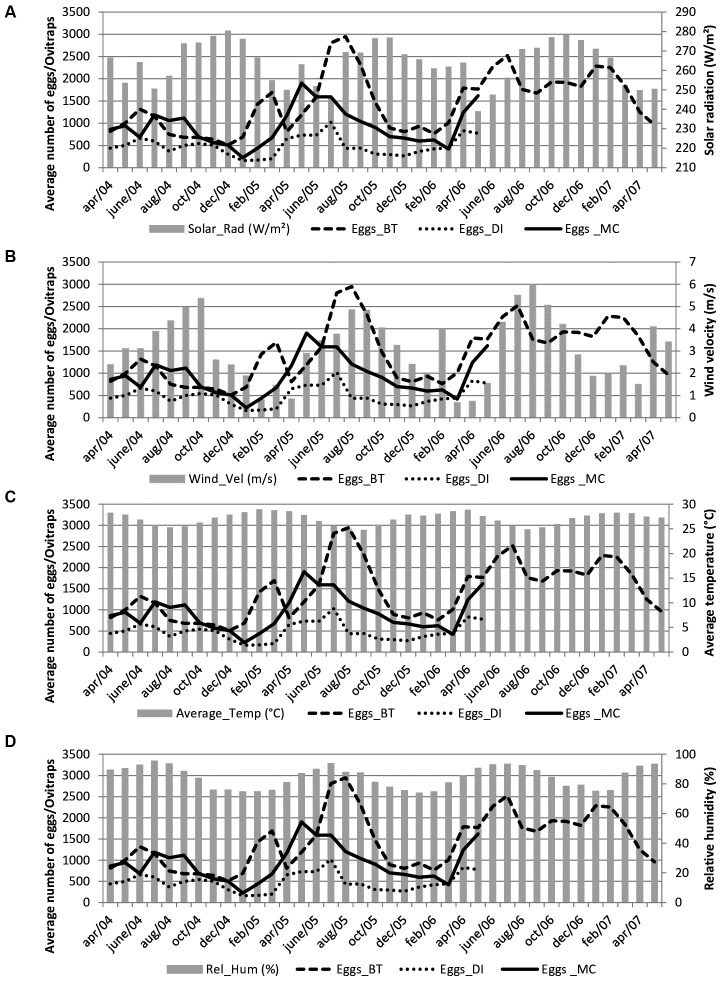
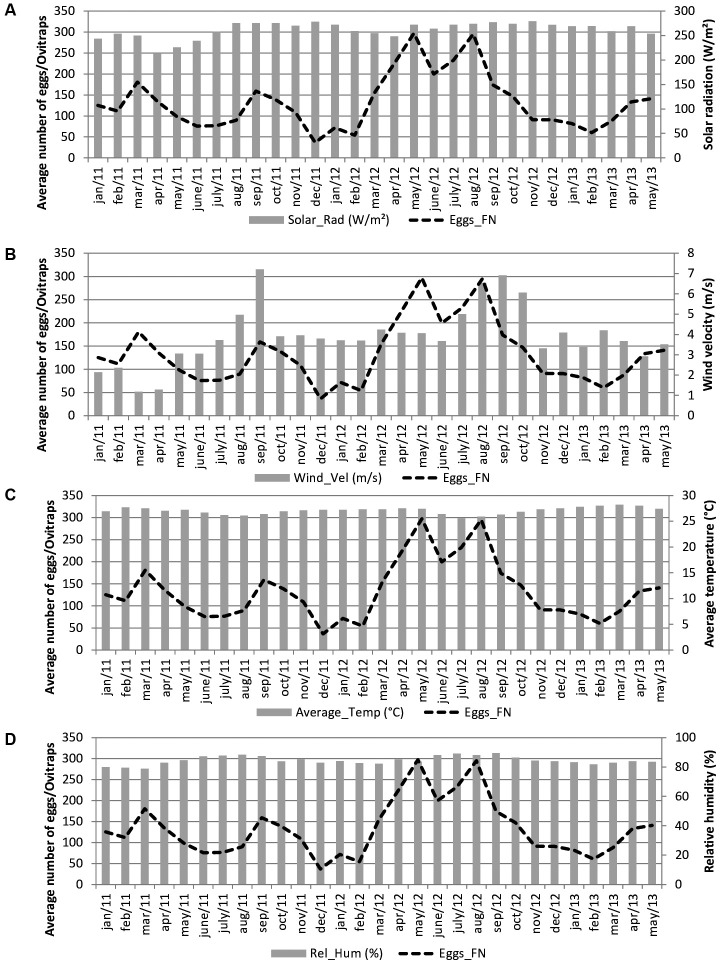
The monthly average temperatures, of around 27ºC (range 28ºC -26ºC), and the relative humidity, of around 80%, showed little variation throughout the year in either Recife or Fernando de Noronha. The monthly average solar radiation for all areas in Recife was 264 W/m², with the highest intensity in December 2004 (280.5 W/m²) and the lowest in May 2006 (239 W/m²) (Fig. 3A). In Fernando de Noronha, monthly solar radiation was 262 W/m² with a peak in November 2012 (279 W/m²) and lowest intensity in April 2011 (214 W/m²) (Fig. 4A).
Fig. 3: monthly variation in number of eggs and meteorological variables in Recife neighborhoods between April 2004 and May 2007. (A) solar radiation; (B) wind velocity; (C) average temperature; (D) relative humidity. Brasília Teimosa (BT); Dois Irmãos/Sítio dos Pintos (DI); Morro da Conceição/Alto José do Pinho (MC).
Fig. 4: monthly variation in number of eggs and meteorological variables in the Fernando de Noronha Archipelago between January 2011 and May 2013. (A) solar radiation; (B) wind velocity; (C) average temperature; (D) relative humidity.
The monthly average wind velocity in Recife was 3.1m/s with the lowest velocity in March 2006 (0.6 m/s) and highest in August 2006 (5.9 m/s) (Fig. 3B). In Fernando de Noronha, the monthly average wind velocity was 3.9 m/s with a minimum of 1.1 m/sin March 2011 and a maximum of 7.1 m/s in September 2011 (Fig. 4B).
The entomological data were as follows. The average numbers of Aedes eggs collected by month in the four study settings in Recife were 1,445.0 ± 659.7 in Brasília Teimosa, 931.0 ± 423.5 in Morro da Conceição and 487.1 ± 215.9 in Dois Irmãos/Sítio dos Pintos, with higher averages between April and August. In Fernando de Noronha, the monthly average number of eggs was 133.4 ± 66.7 with higher levels between March and August (Figs 2, 3, 4).
Autocorrelation - The partial autocorrelation functions for the average number of eggs per S-OVT showed that the egg averages in a given month had a strong correlation with the abundance of eggs for the previous month (Fig. 5). The autocorrelograms show p = 1 and q = 0 corresponding to a first-order autoregressive model (p) of the time series for the number of eggs. The stationarity of the time series for eggs (outcome) was tested and not rejected there by eliminating the differentiation process.
Fig. 5: partial autocorrelation functions (PACFs) for the study areas. (A) Brasília Teimosa; (B) Dois Irmãos/Sítio dos Pintos; (C) Morro da Conceição; (D) Archipelago of Fernando de Noronha.
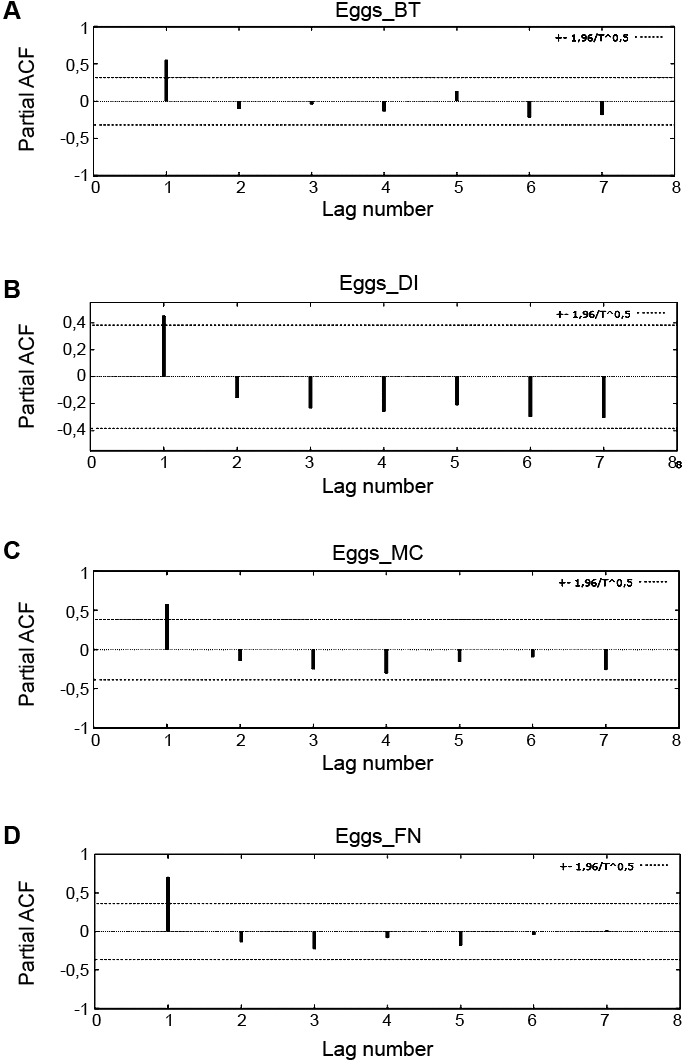
Autoregressive models AR (1) of the association of meteorological variables with average number of Ae. aegypti eggs - The Table III shows the adjusted estimates of the autoregressive models per study area. The time lags used in the model were those found in the analysis of the partial autocorrelation function of each meteorological variable with the egg averages. The time lags used in the model were those found in the analysis of the partial autocorrelation function of each meteorological variable with the egg averages. Precipitation remained positively associated with the abundance of eggs in Brasília Teimosa [β = 1.36; 95% confidence interval (CI): 0.04 - 2.68], Dois Irmãos (β = 0.69; 95% CI: 0.31 - 1.08) and in the Fernando de Noronha Archipelago (β = 0.38; 95% CI: 0.02 - 0.73). The humidity was positively associated with the average number of eggs only in Morro da Conceição/Alto José do Pinho (β = 45.68, 95% CI: 26.3 - 65.0), while wind speed remained inversely associated with the eggs only in Morro da Conceição/Alto José do Pinho (β = -125.2; 95% CI: -198.8 - -51.6).
TABLE III. Final autoregressive models AR (1) of the association between meteorological variables and number of Aedes aegypti eggs in the study setting of Recife and the Fernando de Noronha Archipelago. Pernambuco, Brazil.
| Final model | Study areas | |||||||||||
| Brasília Teimosa | Morro da Conceição/Alto José do Pinho | Dois Irmãos/Sítio dos Pintos | Fernando de Noronha Archipelago | |||||||||
| Coefficient (95%CI) | p | AIC | Coefficient (95%CI) | p | AIC | Coefficient (95%CI) | p | AIC | Coefficient (95%CI) | p | AIC | |
| AR1 | 0.74 (0.5 - 0.9) | 0.000 | 557.7 | 0.66 (0.36 - 0.95) | 0.000 | 346.9 | 0.42 (0.08 - 0.79) | 0.021 | 328.6 | 0.68 (0.42 - 0.93) | 0.000 | 301.4 |
| Precipitation (Lag 1) | 1.36 (0.04 - 2.68) | 0.042 | - | - | 0.69 (0.31 - 1.08) | 0.000 | 0.38 (0.02 - 0.73) | 0.033 | ||||
| Solar radiation (Lag0) | - | - | - | - | - | - | - | - | ||||
| Wind speed (Lag 1) | - | - | −125.2 (−198.8 - 51.6) | 0.000 | - | - | - | - | ||||
| Temperature (Lag 0) | - | - | - | - | - | - | - | - | ||||
| Humidity (Lag 0) | - | - | 45.68 (26.3 - 65.0) | 0.000 | - | - | - | - | ||||
| Constant | 1,190.6 (678.2 - 1,702.9) | 0.000 | −2.531 (−4,195.6 - 866.5) | 0.002 | 361.3 (236 - 486.7) | 0.000 | 101.5 (44.5 - 158.4) | 0.000 | ||||
AIC: akaike information criterion; CI: confidence interval.
DISCUSSION
The analysis showed that precipitation was a predictor of Ae. aegypti eggs abundance in all study areas, except for Morro da Conceição/Alto José do Pinho, a densely populated and higher altitude neighborhood in the city of Recife. Lower wind speed and higher humidity were only associated with egg means per S-OVT in Morro da Conceição/Alto José do Pinho. Temperature and solar radiation were not associated with variations in the average number of eggs in any of the study areas.
The average number of eggs per S-OVT in the areas of Recife was markedly higher than the average number of eggs observed in Fernando de Noronha, suggesting a lower population density of Ae. aegypti in the latter area. This result confirms data from previous studies that showed a much higher abundance of eggs in cities of Pernambuco where the same monitoring method was used, when compared to the averages obtained for Fernando de Noronha. 11 , 12 In addition to climatic factors, some peculiarities of Fernando de Noronha, such as its geographical characteristics, low population density and urban conformation, may limit the dispersion and reproduction of Aedes. It is also worth mentioning that Ae. aegypti was introduced in Fernando de Noronha later than in Recife. Instead, the city of Recife, with high population density and poor urban infrastructure and sanitation, has much more favorable conditions for the development of breeding and reproductive activity of this mosquito. On the other hand, the city of Recife has a high population density and large areas with poor urban and sanitation infrastructure that favor the development of breeding sites and proliferation of this mosquito.
Embryonic development period, hatching time of larvae, and development of immature forms, as well as the (extrinsic and intrinsic) incubation period of Ae. aegypti, are all parameters that influence the definition of the time lag in time series models. 17 Therefore, the observed variation in time lag between meteorological variables possibly explained by the differences in their effect on the distinct phases of the Aedes biological cycle.
According to data from several studies carried out in different regions of the world, 18 , 19 , 20 the analysis showed that precipitation was positively associated with the abundance of eggs in all areas, with the exception of Morro da Conceição/Alto José do Pinho. These results suggest that the occurrence of precipitation is one important factor that contributes to the increase of egg density in the study settings. The effect of precipitation on population growth of Ae. aegypti has mostly been related to local environmental characteristics, especially the availability and diversity of containers able to retain rainwater in the local environment. 18 , 19 , 20 Another possible explanation for the increase in the vector population would be the massive hatching of live eggs in the environment after the occurrence of precipitation. 11 , 12 It is reasonable to assume that both mechanisms may have contributed to the increase in egg production after the rains in the studied areas. In the area of Dois Irmãos/Sitio dos Pintos, with less population density and greater vegetation cover, it is possible that the second mechanism (hatching of live eggs) played a more important role.
Air temperature is one of the most important climatic variables that influence physiology, behavior, ecology and, by extension, insect survival. 21 Surprisingly, unlike the results obtained in other studies conducted in other region of Brazil, 17 no association was found of the temperature with egg production in the studied areas. In these settings, as in the entire Northeast region of Brazil, the temperature averages are between 20ºC and 28ºC throughout the year, levels of temperature considered ideal for the development and reproduction of Aedes. 21 Therefore, we suppose that this extremely low variability of this climatic data at ideal levels (26º - 28oC) for Aedes reproduction favors the maintenance of high population densities of this insect in the region throughout the year. Perhaps this fact explains the lack of seasonality of dengue transmission in our study setting, as observed by Cortez et al., 22 in a time series study comparing epidemiological data from Recife in Goiânia, in the Central-Western region of Brazil.
Similar phenomenon may have occurred for air humidity, which, like temperature, has a recognised effect in increasing the longevity of adult forms of Aedes species and facilitating blood-feeding, dispersion and egg-laying, 23 , 24 , 25 , 26 but was associated with the abundance of eggs in only one of the studied areas. In Recife, the monthly average relative humidity is around 80% throughout the year reaching slightly higher levels between May and August (above 80%), which coincides with the rainy season in the region. These levels of air humidity (as temperature), which are considered ideal for Aedes reproduction ,in combination with the slight variation throughout the year, may explain the lack of association between this meteorological variable and egg production in most of the areas studied.
In our study, we found a negative association between wind speed and egg abundance in Morro da Conceição/Alto José do Pinho. These results are different from previous studies conducted in the city of Colombo, Sri Lanka, 27 and the State of Florida, 28 where a positive association was observed between wind speed and egg production. Turbulence and wind speed are meteorological factors that can positively or negatively affect the development and reproduction of Aedes. It is assumed that higher wind speeds can favor the passive migration of mosquitoes and favor their flight over long distances from their breeding sites. 29 The negative effect of wind speed in a single study setting may be associated with local particularities of the environment that would interfere with the effect of this variable on Aedes oviposition activity.
We did not find any association between solar radiation and Aedes oviposition in any study setting and this result is maybe explained by the low variation of this variable in the regions. However, data on the effects of solar radiation on egg production are scarce and its effect on Aedes oviposition activity may be attributed to the behavior of the mosquito, which prefers to lay eggs in the shade. 30
As a limitation of the present study it is worth noting that other factors possibly involved in egg production, such as pattern of urbanisation population density socioeconomic factors (per capita income) and geographical characteristics, such as altitude and vegetation were not included in the analysis. Likewise, the relatively short observation period (three years) and the time interval between egg collections (monthly) may have adversely affected the accuracy of the estimates.
The use of two different egg counting methods (manual cell counter and semi-automatic computerised system) could also lead to variations in the estimation of parameters if there were differences in precision between them. However, a validation study of the semi-automatic method in relation to the manual did not show differences in the egg count in the examined pallets 14 suggesting that this problem may not have occurred.
However, it is worth highlighting the advantages of the study design which allowed information to be obtained from areas with different geographical and environmental characteristics within the same region. Also noteworthy is the fact that meteorological variables were obtained from satellite data for each particular neighborhood there by enabling much more accurate analysis.
In summary the results suggest that the effect of precipitation on Aedes oviposition dynamics in the study areas. Moreover the climate characteristics and the narrow range of variation in meteorological variables in the region studied particularly for temperature and humidity make it ideal for the reproduction of the Aedes mosquito enabling continuous reproduction of the mosquito throughout the year and, consequently permanent transmission of arboviruses. We conclude that these meteorological data in statistical models to predict increases in the Ae. aegypti population may not be applicable in this region.
ACKNOWLEDGEMENTS
To Dr Constantino Silveira for providing entomological data from the SAUDAVEL and SMCP-Aedes projects, and to Dr Pietro Cecatto of The International Research Institute for Climate and Society, New York, for training in the use of satellite climate data.
Footnotes
Financial support: CNPq. CB (scholarship 303953/2018-7) and WVS (scholarship 306222/2013-2) receive partial support from the National Advisory Board of Scientific and Technological Development (CNPq).
REFERENCES
- 1.Donalisio MR, Freitas ARR, Zuben APBV. Arboviruses emerging in Brazil challenges for clinic and implications for public health. Rev Saude Publica. 2017;51(30):1–6. doi: 10.1590/S1518-8787.2017051006889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chadee DD, Martinez R. Aedes aegypti (L ) in Latin American and Caribbean Region: with growing evidence for vector adaptation to climate change? Acta Trop. 2016;156:137–143. doi: 10.1016/j.actatropica.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Mogi M, Tuno N. Impact of climate change on the distribution of Aedes albopictus (Diptera Culicidae) in northern Japan: retrospective analyses. J Med Entomol. 2014;51(3):572–579. doi: 10.1603/me13178. [DOI] [PubMed] [Google Scholar]
- 4.Morin CW, Comrie AC, Ernst K. Climate and dengue transmission evidence and implications. Environ Health Perspect. 2013;121(11-12):1264–1272. doi: 10.1289/ehp.1306556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver SC. Urbanization and geographic expansion of zoonotic arboviral diseases mechanisms and potential strategies for prevention. Trends Microbiol. 2013;21(8):360–363. doi: 10.1016/j.tim.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couret J, Dotson E, Benedict MQ. Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (Diptera Culicidae) PLoS One. 2014;9(2):1–9. doi: 10.1371/journal.pone.0087468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuang TW, Chaves LF, Chen PJ. Effects of local and regional climatic fluctuations on dengue outbreaks in southern Taiwan. PLoS One. 2017;12(6):1–20. doi: 10.1371/journal.pone.0178698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christophers SR. Aedes aegypti: the Yellow Fever mosquito: its life history, bionomics and structure. Cambridge University Press. 1960 [Google Scholar]
- 9.Simões TC, Codeço CT, Nobre AA, Eiras AE. Modeling the non-stationary climate dependent temporal dynamics of Aedes aegypti. PLoS One. 2013;8(8):1–10. doi: 10.1371/journal.pone.0064773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magalhães T, Braga C, Marli TC, Oliveira ALS, Castanha PMS, Maciel APR. Zika virus displacement by a Chikungunya outbreak in Recife, Brazil. PLoS Negl Trop Dis. 2017;11(11):1–25. doi: 10.1371/journal.pntd.0006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regis L, Monteiro AM, de Melo-Santos MAV, Silveira JC, Jr, Furtado AF, Acioli RV. Developing new approaches for detecting and preventing Aedes aegypti population outbreaks basis for surveillance, alert and control system. Mem Inst Oswaldo Cruz. 2008;103(1):50–59. doi: 10.1590/s0074-02762008000100008. [DOI] [PubMed] [Google Scholar]
- 12.Regis L, Acioli RV, Silveira JC, Jr, de Melo-Santos MA, da Cunha MC, Souza F. Characterization of the spatial and temporal dynamics of the dengue vector population established in urban areas of Fernando de Noronha, a Brazilian oceanic island. Acta Trop. 2014;137:80–87. doi: 10.1016/j.actatropica.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Instituto Brasileiro de Geografia e Estatística https://www.ibge.gov.br
- 14.Silva MGNM, Rodrigues MAB, Araujo RE. Aedes aegypti egg counting system. In: 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC 11) Boston. 2011 doi: 10.1109/IEMBS.2011.6091679. [DOI] [PubMed] [Google Scholar]
- 15.Del Corral J, Blumenthal MB, Mantilla G, Ceccato P, Connor SJ, Thomson MC. Climate information for public health the role of the IRI climate data library in an integrated knowledge system. Geospatial Health. 2012;6(3):15–24. doi: 10.4081/gh.2012.118. [DOI] [PubMed] [Google Scholar]
- 16.Bozdogan H. Model selection and Akaike's Information Criterion (AIC) the general theory and its analytical extensions. Psychometrika. 1987;52(3):345–370. [Google Scholar]
- 17.Honório NA, Codeço CT, Alves FC, Magalhães MA, Lourenço-de-Oliveira R. Temporal Distribution of Aedes aegypti in different districts of Rio De Janeiro, Brazil, measured by two types of traps. J Med Entomol. 2009;46(5):1001–1014. doi: 10.1603/033.046.0505. [DOI] [PubMed] [Google Scholar]
- 18.Estallo EL, Ludueña-Almeida FF, Introini MV, Zaidenberg M, Almirón WR. Weather variability associated with Aedes (Stegomyia) aegypti (Dengue vector) oviposition dynamics in northwestern Argentina. PLoS One. 2015;10(5):1–11. doi: 10.1371/journal.pone.0127820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surendran SN, Kajatheepan A, Sanjeefkumar KFA, Jude PJ. Seasonality and insecticide susceptibility of dengue vectors an ovitrap based survey in a residential area of northern Sri Lanka, Southeast, Asian. J Trop Med Public Health. 2007;38(2):276–282. [PubMed] [Google Scholar]
- 20.Zahouli JBZ, Koudou BG, Müller P, Malone D, Tano Y, Utzinger J. Urbanization is a main driver for the larval ecology of Aedes mosquitoes in arbovirus-endemic settings in south-eastern Côte d'Ivoire. PLoS Negl Trop Dis. 2017;11(7):1–23. doi: 10.1371/journal.pntd.0005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinhold JM, Lazzari CR, Lahondère C. Effects of the environmental temperature on Aedes aegypti and Aedes albopictus mosquitoes a review. Insects. 2018;9(4):158–158. doi: 10.3390/insects9040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortes F, Martelli CMT, Ximenes RAA, Montarroyos UR, Siqueira JBJ, Cruz OG. Time series analysis of dengue surveillance data in two Brazilian cities. Acta Trop. 2018;182:190–197. doi: 10.1016/j.actatropica.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Dickerson CZ. The effects of temperature and humidity on the eggs of Aedes aegypti (L.) and Aedes albopictus (SKUSE) in Texas. Texas A&M University: Texas; 2007. [Google Scholar]
- 24.Wong J, Stoddard ST, Astete H, Morrison AC, Scott TW. Oviposition site selection by the dengue vector Aedes aegypti and its implications for dengue control. PLoS Negl Trop Dis. 2011;5(4):1–12. doi: 10.1371/journal.pntd.0001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cianci D, Hartemink N, Zeimes CB, Vanwambeke SO, Ienco A, Caputo B. High resolution spatial analysis of habitat preference of Aedes albopictus (Diptera Culicidae) in an urban environment. J Med Entomol. 2015;52(3):329–335. doi: 10.1093/jme/tjv026. [DOI] [PubMed] [Google Scholar]
- 26.Azil AH, Long SA, Ritchie SA, Williams CR. The development of predictive tools for pre-emptive dengue vector control a study of Aedes aegypti abundance and meteorological variables in North Queensland, Australia. Trop Med Int Health. 2010;15(10):1190–1197. doi: 10.1111/j.1365-3156.2010.02592.x. [DOI] [PubMed] [Google Scholar]
- 27.Jayathilake TA, Wickramasinghe MB, de Silva BG. Oviposition and vertical dispersal of Aedes mosquitoes in multiple storey buildings in Colombo district, Sri Lanka. J Vector Borne Dis. 2015;52(3):245–251. [PubMed] [Google Scholar]
- 28.Hribar LJ, Demay DJ, Lund UJ. The association between meteorological variables and the abundance of Aedes taeniorhynchus in the Florida Keys. J Vector Ecol. 2010;35(2):339–346. doi: 10.1111/j.1948-7134.2010.00092.x. [DOI] [PubMed] [Google Scholar]
- 29.Clements AN. The biology of mosquitoes: sensory reception and behviour. Vol. II. London: Chapman & Hall; 2006. [Google Scholar]
- 30.Barrera R, Amador M, Clark GG. Ecological factors influencing Aedes aegypti (Diptera Culicidae) productivity in artificial containers in Salinas, Puerto Rico. J Med Entomol. 2006;43(3):484–492. doi: 10.1603/0022-2585(2006)43[484:efiaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]