Abstract
Cardiovascular magnetic resonance (CMR) has emerged as a key modality to assess nonischemic cardiomyopathies. Its ability to detect cardiac morphology and function with fast cine imaging, myocardial edema with T2-based techniques, and fibrosis with late gadolinium enhancement techniques has enabled noninvasive characterization of cardiac tissue, thus helping clinicians assess cardiovascular risk and determine the most effective management strategy. Active investigations into parametric imaging techniques will further expand the potential clinical applications of CMR for cardiac tissue characterization. This review discusses the use of CMR techniques in characterizing the major morphofunctional phenotypes of nonischemic cardiomyopathies.
Keywords: nonischemic cardiomyopathies, parametric mapping, tissue characterization, cardiovascular magnetic resonance
INTRODUCTION
Cardiovascular magnetic resonance (CMR) has become a key modality for diagnosing nonischemic cardiomyopathies. Its use of imaging and parametric mapping techniques weighted for magnetic properties and of gadolinium-based contrast agents allow clinicians to characterize myocardial tissue. With its fast cine imaging techniques, CMR not only enables clear visualization of cardiac morphology but also serves as a clinical reference for cardiac ventricular volumes, ejection fractions, and myocardial mass. This review provides a brief overview of CMR capabilities and summarizes significant CMR findings in nonischemic cardiomyopathies encountered in clinical practice.
CMR TECHNIQUES
Since the introduction of magnetic resonance imaging (MRI) in the 1970s, CMR methods have advanced significantly. In general, CMR allows the acquisition of high-contrast, high-spatiotemporal resolution cardiac cine images within a single breath hold and highly reproducible quantification of ventricular volumes and ejection fractions, with clinical implications for implantable cardiac defibrillators based on guideline-established ejection fraction criteria for implantation.1 Phase-contrast imaging techniques provide velocity and flow volume measures to assess coexisting valvular diseases. With appropriate adjustment to the MRI sequences, T1- and T2-weighted images inform clinicians on myocardial tissue components. These imaging techniques are now more frequently supplemented with T1, T2, and T2* parametric image maps for characterizing fibrosis, water content, and iron content, respectively. Native and post-contrast T1 parametric maps are also used to derive extracellular volume fractions (ECV), a surrogate for interstitial fibrosis. Lastly, late gadolinium enhancement (LGE) helps clinicians detect thrombus, microvascular obstruction, and scarring/replacement fibrosis (scar appearing as LGE).
CARDIOMYOPATHIES
The classification scheme for cardiomyopathies among professional societies has evolved over the past few decades. Previously, these diseases were classified based on genetic versus acquired forms. However, an increasing number of identifiable underlying genotypes has led to a shift in thinking toward a genotype-phenotype classification scheme. In 2013, the World Heart Federation proposed the MOGE(S) scheme that describes the morphofunctional phenotype, organ/system involvement, genetic inheritance pattern, etiology, and stage.2 Although discussion of the entire classification scheme is beyond the scope of this review, we highlight the major morphofunctional phenotype categories of cardiomyopathy and the significant CMR findings in clinical practice. In addition, phenotypic findings on CMR may prompt reevaluation of clinically presumed cardiomyopathies and lead to re-classification of disease entities, alterations in expected prognosis, and changes in treatment strategies.3
ISCHEMIC CARDIOMYOPATHIES
Although not formally considered a cardiomyopathy or intrinsic disorder of the myocardium itself by societal consensus, in clinical parlance “ischemic cardiomyopathy” refers to irreversible myocardial loss due to an infarction or to myocardial hibernation or stunning, usually resulting from significant coronary artery disease (CAD).4 CMR allows direct visualization of nonviable scar tissue changes from myocardial infarction (through the classic LGE watershed pattern) and detection of myocardial ischemia through stress imaging protocols.5,6 Moreover, the absence of any LGE has been described in patients with angiographically proven significant CAD stenoses,7 suggesting a nonischemic etiology to such cardiomyopathies in this population. Lastly, even in the absence of significant CAD (aka, nonobstructive coronary arteries), CMR has been shown to detect CAD-type LGE, a phenomenon called myocardial infarction with nonobstructive coronary arteries.8
HYPERTROPHIC CARDIOMYOPATHIES
Hypertrophic cardiomyopathy (HCM) has been defined in adults as a left ventricular (LV) wall thickness ≥ 15 mm in the absence of secondary causes such as hypertension or aortic stenosis,9 although an unexplained ≥ 13 mm thickness can be used for first-degree family members of patients with established disease.10 Prior echocardiographic work on asymmetric septal hypertrophy established the criteria of a septal-lateral wall thickness ratio of 1.3 in normotensive adults and 1.5 in hypertensive adults.11,12
CMR enables a full 3-dimensional assessment of myocardial morphology, allowing visualization of regional LV hypertrophy (Figure 1 A) and apical hypertrophy (Figure 1 B) that may be missed on echocardiography.13 In one small study (N=48), CMR was able to identify LV hypertrophy in 6% of cases missed by echocardiography. In addition, CMR allows for accurate quantification of mitral regurgitation in the setting of coexisting systolic anterior motion of the anterior mitral leaflet, as described in the accompanying article on valve assessment.
Figure 1.
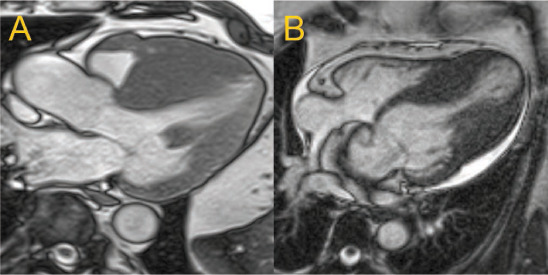
(A) A 3-chamber long-axis view at end diastole in one individual with asymmetric septal hypertrophy (A) with septal thickness > 3 cm. (B) A 4-chamber long-axis view at end diastole in another patient with mid and apical ventricular hypertrophy with apical thinning suggestive of an apical aneurysm.
Microvascular ischemia has been implicated as the pathogenesis of replacement fibrosis, which develops in later stages of HCM. Stress CMR protocols enable the detection of such ischemia through evidence of stress-inducible perfusion abnormalities.14 In the later stages of HCM remodeling, the detection of LGE on CMR has been firmly associated with an increased risk for sudden cardiac death and adverse LV remodeling, especially when the burden is ≥ 15% of the LV mass,15 which has implications when considering implantation of a cardiac defibrillator. CMR also enables the detection of HCM-associated LV apical aneurysms, reported in 4.8% of a 1,940-person HCM cohort and associated with an increased risk for sudden cardiac death and thromboembolic events.16 Because of these findings, the European Society of Cardiology has endorsed repeat CMR every 5 years in clinically stable HCM patients or every 2 to 3 years in those with progressive disease.10 The clinical implications of T1 parametric mapping and ECV quantification in HCM are still being explored and may allow for earlier detection of fibrotic changes in hypertrophied myocardium.
RESTRICTIVE CARDIOMYOPATHIES
Restrictive cardiomyopathies refer to cardiomyopathies with stiffened myocardial walls for reasons other than sarcomere hypertrophy and include infiltrative disorders, storage diseases, and endomyocardial fibrosis. Such cardiomyopathies are characterized by diastolic dysfunction with restrictive filling, reduced diastolic volume of either or both ventricles, preserved systolic function in their early stages, and normal or mildly increased wall thickness.17
Infiltrative cardiac disorders include amyloidosis and sarcoidosis. For amyloidosis, the classic diffuse subendocardial LGE pattern seen on CMR has become pathognomonic for its diagnosis by CMR and has been linked with deposition of amyloid protein in the myocardial interstitium (Figure 2 A), with a sensitivity of 80% and specificity of 94%.18,19 Atrial infiltration by amyloid protein can occur and be similarly detected on LGE imaging. Studies of CMR using T1 mapping techniques in this population have established that native T1 times and ECV in patients with amyloidosis are increased compared with controls, with native T1 times being usable even when gadolinium is contraindicated.20,21 Research to differentiate between light chain (AL) and transthyretin forms (ATTR) of cardiac amyloidosis using T1 mapping and LGE shows that the AL type has higher T1 times and ATTR has more transmural LGE involvement.22,23 Unfortunately, no universal cutoffs for T1 relaxation times have been established to date due to variability between individual scanners, between MRI sequences and postprocessing, and among local site reference ranges. Use of ECV attempts to circumvent physical T1 dependencies on scanners and sequences by more directly quantifying the extracellular volume.24,25 Although several small studies have explored ECV cutoffs for detecting cardiac amyloidosis, no universal ECV cutoff has yet been validated and established among the CMR community for this purpose. Both T1 mapping and ECV remain active areas of investigation for the diagnosis and management of cardiac amyloidosis.
Figure 2.
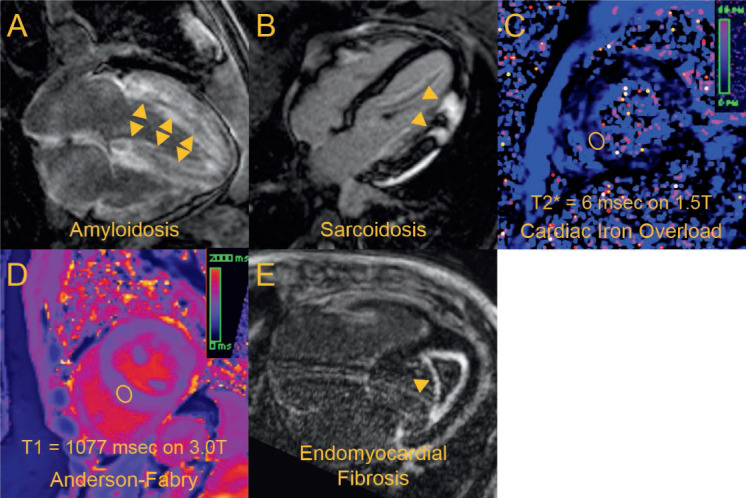
Each panel corresponds to a different patient with a different form of restrictive cardiomyopathy. (A) A 4-chamber view showing diffuse late gadolinium enhancement (arrows) consistent with amyloidosis. (B) A 4-chamber view showing focal thickening and late gadolinium enhancement of the lateral wall (arrows) in a nonischemic pattern suggestive of sarcoidosis. (C) Midventricular short-axis T2* parametric map showing very low T2* times in the left ventricle (sampled in ellipsoid region of interest) suggestive of cardiac iron overload. (D) Midventricular short-axis native T1 parametric map in an individual with confirmed Anderson-Fabry and no cardiac iron overload, with T1 times (sampled in ellipsoid region of interest) found to be significantly reduced compared with a reference healthy group (mean T1 1167 ± standard deviation 33 msec at 3.0 T). (E) A 4-chamber view showing an apical thrombus with surrounding fibrotic material by late gadolinium enhancement (arrow), consistent with endomyocardial fibrosis.
For cardiac sarcoidosis, the detection of LGE on CMR in a noninfarct pattern (Figure 2 B) has been ascribed to this disease entity and portends increased risk for cardiac death, although the entity could equally masquerade as an infarct pattern. In 81 patients with any sarcoidosis, 21 (26%) were found to have cardiac LGE, with basal and/or mid-ventricular septal involvement in 16 of these cases.26 Because of the variation in regional involvement of this disease, CMR may be useful in guiding endomyocardial biopsy for tissue diagnosis. Several sets of diagnostic criteria for cardiac sarcoidosis have been proposed. In its updated 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis, the Japanese Circulation Society considers CMR-detected LGE one of five major criteria for pathological/histopathological information since it is a key index of tissue damage and fibrosis in cardiac sarcoidos.27 In contrast, the Heart Rhythm Society relies heavily on either cardiac or extracardiac tissue diagnosis plus at least one of seven criteria that includes LGE plus exclusion of other etiologies.28 In either diagnostic scheme, CMR is considered useful for screening. With the introduction of parametric mapping, detection of increased T2 decay times may allow for detection of active sarcoidosis and response to immunosuppressive therapies.29
Storage diseases affecting the heart include cardiac iron overload and Anderson-Fabry disease. In cardiac iron overload, the increased iron content in myocardial tissue can be expected to shorten all the T1, T2, and T2* magnetic tissue properties. It should be noted that magnetic tissue properties within the same patient, and thus clinical criteria based on these properties, will differ with magnetic field strength. CMR-assessed T2* decay times of < 20 msec on 1.5 Tesla scanners have been previously validated for the detection of cardiac iron overload (Figure 2 C). Work originally done in thalassemia patients receiving multiple blood transfusions has proven its utility in the initiation of chelation therapy to improve survival.30 In the correct clinical context where cardiac iron overload may be strongly suspected, the detection of shortened T1 relaxation times on T1 parametric maps has also been proposed to supplement T2* measures in cardiac iron overload detection, especially in a third of cardiac iron overload cases where T2* may be > 20 msec on 1.5 Tesla scanners.31 The 2017 recommendations from the Society of Cardiovascular Magnetic Resonance have established the reporting of a 3-tier risk model of cardiac iron overload for myocardial T2* times on a 1.5 Tesla scanner: low risk, > 20 ms; intermediate risk, 10–20 ms; and high risk, < 10 ms.32 In Anderson-Fabry disease involving the heart, a mutation in the α-galactosidase leads to sphingolipid deposition in myocardial tissues. This process can lead to LGE typically detected in the inferolateral wall of affected individuals.33 However, with the advent of T1 parametric mapping, earlier detection of cardiac involvement for this disease entity is possible, as the myocardial lipid deposition has also been shown to lower T1 relaxation times in affected individuals (Figure 2 D) and may have a role in initiating early enzyme replacement therapy to delay this disease.34 Given the rarity of this disease entity, previously mentioned causes of shortened myocardial T1 times such as cardiac iron overload and chronic fibrofatty changes from a prior myocardial infarction should be excluded.
Lastly, endomyocardial fibrosis is a cardiomyopathy with a predilection to tropical and subtropical regions.35 Although classified as a “cardiomyopathy,” the myocardium itself is unaffected in this hypereosinophilic syndrome. Biventricular involvement should be suspected and excluded on cardiac imaging, as one population study of 211 confirmed cases reported both chambers to be involved in roughly 56% of cases. CMR can help confirm the classic pattern of LGE central thrombus with a surrounding hyperenhanced layer (Figure 2 E), although care should be taken with early and late gadolinium enhancement imaging to exclude gradual contrast permeation of a microvascular obstruction associated with an acute myocardial infarction. Despite being nonspecific, active inflammation of the mass can be detected on CMR in the form of edema using T2-based techniques
ARRHYTHMOGENIC RIGHT VENTRICULAR CARDIOMYOPATHY
As of 2010, the Task Force for Arrhythmogenic Right Ventricular Cardiomyopathy has established criteria for the diagnosis of this type of cardiomyopathy.36 Much of these criteria are based on clinical history, electrocardiographic findings, and endomyocardial biopsy results. For CMR, the following criteria have been recommended:
-
Major criteria: Regional RV akinesia or dyskinesia or dyssynchronous RV contraction (Figure 3) and one of the following:
RV end diastolic volume indexed for body surface area (≥ 110 mL/m2 in males; ≥ 100 mL/m2 in females), or
RV ejection fraction ≤ 40%
-
Minor criteria: Regional RV akinesia or dyskinesia or dyssynchronous RV contraction and one of the following:
Enlarged RV end diastolic volume indexed for body surface area (≥ 100 mL/m2 and < 110 mL/m2 in males; ≥ 90 mL/m2 and < 100 mL/m2 in females), or
RV ejection fraction > 40% and ≤ 45%
Less recognized but also a concern is the similar entity of left dominant arrhythmogenic cardiomyopathy. CMR may have a role in detecting fibrofatty changes in the LV myocardium of suspected cases using T1-based and LGE imaging techniques described.
Figure 3.
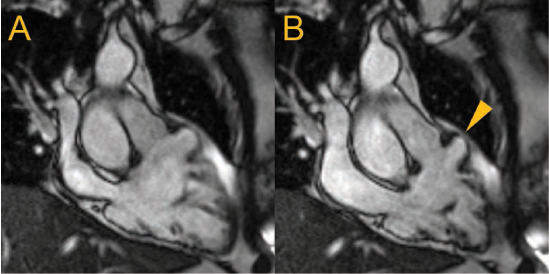
Cardiac magnetic resonance (CMR) right ventricular (RV) 3-chamber long-axis views are shown at (A) end-diastole and (B) end-systole with an anterior lateral free wall aneurysm (arrow) in an individual with previously unexplained frequent ventricular ectopy. Along with RV dilatation (115 mL/m2) and depressed systolic function (RV ejection fraction 38%), these findings fulfilled major CMR criteria for arrhythmogenic RV cardiomyopathy.
LEFT VENTRICULAR NONCOMPACTION
The hallmark of LV noncompaction (LVNC) is the presence of (A) prominent, noncompacted, spongiform trabeculae with (B) intertrabecular recesses in continuity with the LV cavity on (C) compacted myocardial tissue. These trabeculae are formed and then presumably become compacted during embryologic development.37 This feature can also involve the right ventricle, can be encountered in a multitude of patient populations (including those with dilated cardiomyopathy and healthy athletes), and can even be acquired and reversed.38–40 Thus, CMR can provide useful imaging criteria but should not be considered definitive for diagnosing LVNC cardiomyopathy. Three separate CMR criteria for LVNC have emerged in the literature using the noncompacted-to-compacted (NC/C) tissue relative proportions or trabecular border complexity:
Maximal end-diastolic NC/C thickness ratio > 2.3, with thickness perpendicular to compacted myocardium, on any cardiac long-axis views (Figure 4) (sensitivity 86%, specificity 99%)41
End-diastolic noncompacted tissue mass > 20% of total LV mass, where compacted tissue mass included the papillary muscles (sensitivity and specificity both 93.7%)42
End-diastolic maximal apical fractal dimension cutoff of ≥ 1.30 on cardiac short-axis views (Figure 5) (sensitivity and specificity both of 100%)43
The last method makes use of publicly available software (ImageJ, National Institutes of Health) but requires experience for its use. Lastly, delayed gadolinium enhancement imaging techniques allow for detection of intertrabecular thrombi, which can influence thromboembolic management.
Figure 4.
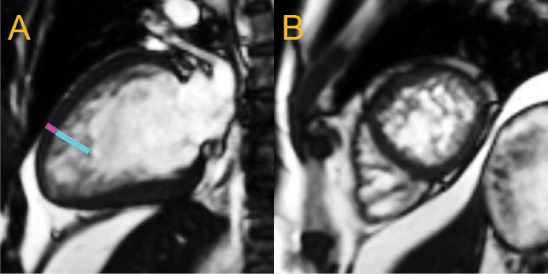
2-chamber long-axis (A) and apical short-axis (B) views of the same heart at end diastole show prominent, apical trabeculae in an individual with depressed left ventricular (LV) systolic function (LV ejection fraction 42%). The noncompacted (cyan line)-to-compacted (magenta line) myocardial thickness ratio was estimated to be 4.
Figure 5.
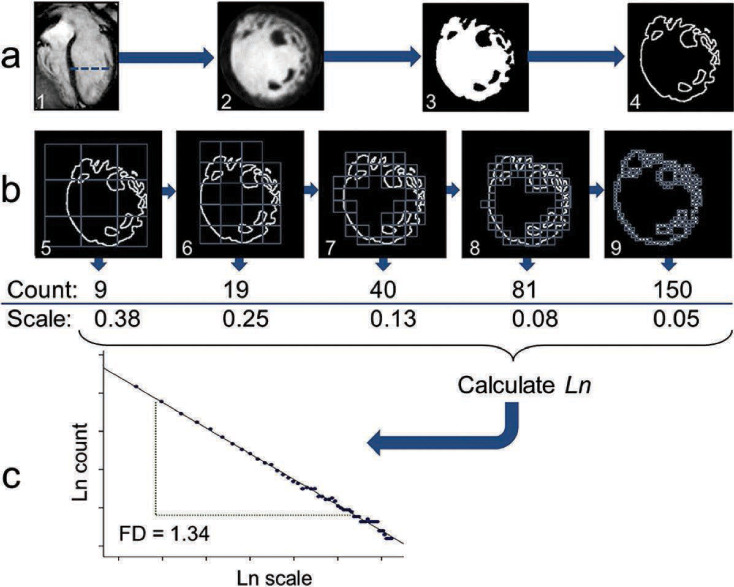
Steps are shown for fractal analysis of left ventricular (LV) trabeculae in a patient with established LV noncompaction. (A) The endocardial border of the LV in short-axis is segmented out, (B) progressively smaller boxes in grids (scale relative to total image size) are used to count boxes containing the border (boxes with any white pixel), (C) and the box count versus box scale are plotted using logarithmic scale and fitted with a regression line. The regression coefficient of the fitted line becomes the fractal dimension (FD), with higher values suggesting a more trabeculated endocardial border and lower values suggesting a smoother, less trabeculated border. (J Cardiovasc Magn Reason. 2013;10;15:36). Reproduction of the original, unmodified figure is permitted under a Creative Commons License as per BioMed Central copyright policies.
DILATED CARDIOMYOPATHIES
Dilated cardiomyopathy is broadly defined as the presence of LV dilation and systolic dysfunction with the exclusion of coronary artery disease and other cardiomyopathies and beyond the proportion expected with abnormal loading conditions (eg, aortic or mitral regurgitation) (Figure 6 A).44 Because of the enlarged ventricle, this entity can be accompanied by secondary or functional mitral regurgitation due to mitral annular dilatation and tethering of the mitral leaflets (as described in the accompanying article on valve assessment by Malahfji and Shah). When present, functional mitral regurgitation not only fosters adverse progression of this entity but also portends increased morbidity and mortality independent of LV ejection fraction.45 Similarly grim is the detection of replacement fibrosis (Figure 6 B), often found in a mid-mural location, seen in a third of dilated cardiomyopathies,46 and associated with ventricular tachycardia, increased morbidity and mortality, and decreased response to heart failure therapies.47–52
Figure 6.
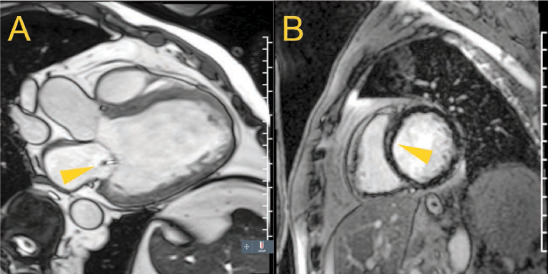
(A) A 3-chamber view of a heart with dilated cardiomyopathy in systole, illustrating tenting of the mitral valve with functional mitral regurgitation (arrow), (B) and a mid-ventricular short-axis view showing mid-mural scar (arrow) of the interventricular septum on late gadolinium enhancement imaging.
Screening for etiologies should include familial genetic cardiomyopathies, chronic tachyarrhythmias, alcohol intake, cardiotoxic chemotherapies, peripartum state, and viral illnesses or other inflammatory conditions. If a viral illness or other inflammatory condition is suspected, then the 2018 Modified Lake Louise Criteria can be applied to determine the likelihood of nonischemic myocardial inflammation on CMR.53 These criteria make use of the following CMR findings, with study authors advocating for a combination of both main criteria:
-
Two main:
Myocardial edema detected on T2 parametric maps or T2-weighted images (Figure 7)
Nonischemic myocardial injury detected on T1 parametric maps, regional ECV elevation, or LGE
-
Two supportive:
CMR evidence of pericarditis
Systolic LV dysfunction
Figure 7.
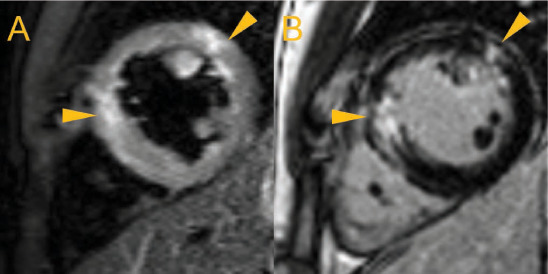
(A) Mid-ventricular short-axis views of an individual with known active myocarditis showing increased signal intensity suggestive of myocardial edema (white, arrows) on a dark blood T2-weighted image with fat suppression and (B) myocardial scar in the same mid-mural regions (white, arrows) on late gadolinium enhancement imaging.
Myocardial edema has been clinically presumed to represent the acute phase of myocarditis. The combinations of major CMR criteria have a reported median area under the curve for detecting myocarditis ranging from 0.75 to 0.90. However, as parametric mapping techniques have only recently begun to enter clinical use, their prognostic utility in such patients remains an active area of investigation.
LGE patterns for viral-type myocarditis have been described as confined to intramural septal wall or subepicardial lateral wall involvement but do not inform on the chronicity of the disease process alone.54
CONCLUSION
As illustrated, CMR provides a comprehensive set of methods for characterizing the heart at the structural and tissue level and, therefore, the morphofunctional phenotypic manifestations of nonischemic cardiomyopathies. Its findings are proving to be invaluable risk markers that influence clinical management and decision making in patients with these diseases. Thus, CMR has become a crucial imaging modality for the assessment of nonischemic cardiomyopathies and should be used routinely in the appropriate clinical setting.
KEY POINTS
Cardiovascular magnetic resonance (CMR) has enabled the characterization of morphofunctional phenotypes of nonischemic cardiomyopathies.
CMR use of imaging and parametric mapping techniques weighted for magnetic properties and of gadolinium-based contrast agents allow clinicians to characterize the myocardial tissues.
Key CMR findings are used in formal criteria for the diagnosis of various nonischemic cardiomyopathies.
Footnotes
Conflict of Interest Disclosure:
The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013 Oct 15;128(16):e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Arbustini E, Narula N, Tavazzi L et al. The MOGE(S) classification of cardiomyopathy for clinicians. J Am Coll Cardiol. 2014 Jul 22;64(3):304–18. doi: 10.1016/j.jacc.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Senthilkumar A, Majmudar MD, Shenoy C, Kim HW, Kim RJ. Identifying the etiology: a systematic approach using delayed-enhancement cardiovascular magnetic resonance. Heart Fail Clin. 2009 Jul;5(3):349–67. vi. doi: 10.1016/j.hfc.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster A, Morton G, Chiribiri A, Perera D, Vanoverschelde JL, Nagel E. Imaging in the management of ischemic cardiomyopathy: special focus on magnetic resonance. J Am Coll Cardiol. 2012 Jan 24;59(4):359–70. doi: 10.1016/j.jacc.2011.08.076. [DOI] [PubMed] [Google Scholar]
- 5.Kim RJ, Wu E, Rafael A et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000 Nov 16;343(20):1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 6.Manning WJ, Atkinson DJ, Grossman W, Paulin S, Edelman RR. First-pass nuclear magnetic resonance imaging studies using gadolinium-DTPA in patients with coronary artery disease. J Am Coll Cardiol. 1991 Oct;18(4):959–65. doi: 10.1016/0735-1097(91)90754-w. [DOI] [PubMed] [Google Scholar]
- 7.Soriano CJ, Ridocci F, Estornell J et al. Noninvasive Diagnosis of Coronary Artery Disease in Patients With Heart Failure and Systolic Dysfunction of Uncertain Etiology, Using Late Gadolinium-Enhanced Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2005 Mar 1;45(5):743–8. doi: 10.1016/j.jacc.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 8.Pasupathy S, Tavella R, Beltrame JF. The What, When, Who, Why, How and Where of Myocardial Infarction With Non-Obstructive Coronary Arteries (MINOCA) Circ J. 2016;80(1):11–6. doi: 10.1253/circj.CJ-15-1096. [DOI] [PubMed] [Google Scholar]
- 9.Gersh BJ, Maron BJ, Bonow RO et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011 Dec 13;124(24):2761–96. doi: 10.1161/CIR.0b013e318223e230. [DOI] [PubMed] [Google Scholar]
- 10.Elliott PM, Anastasakis A, Borger MA et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014 Oct 14;35(39):2733–79. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 11.Henry WL, Clark CE, Epstein SE. Asymmetric septal hypertrophy. Echocardiographic identification of the pathognomonic anatomic abnormality of IHSS. Circulation. 1973 Feb;47(2):225–33. doi: 10.1161/01.cir.47.2.225. [DOI] [PubMed] [Google Scholar]
- 12.Doi YL, Deanfield JE, McKenna WJ, Dargie HJ, Oakley CM, Goodwin JF. Echocardiographic differentiation of hypertensive heart disease and hypertrophic cardiomyopathy. Br Heart J. 1980 Oct;44(4):395–400. doi: 10.1136/hrt.44.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rickers C, Wilke NM, Jerosch-Herold M et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005 Aug 9;112(6):855–61. doi: 10.1161/CIRCULATIONAHA.104.507723. [DOI] [PubMed] [Google Scholar]
- 14.Ismail TF, Hsu LY, Greve AM et al. Coronary microvascular ischemia in hypertrophic cardiomyopathy - a pixel-wise quantitative cardiovascular magnetic resonance perfusion study. J Cardiovasc Magn Reson. 2014 Aug 12;16:49. doi: 10.1186/s12968-014-0049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan RH, Maron BJ, Olivotto I et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014 Aug 5;130(6):484–95. doi: 10.1161/CIRCULATIONAHA.113.007094. [DOI] [PubMed] [Google Scholar]
- 16.Rowin EJ, Maron BJ, Haas TS et al. Hypertrophic Cardiomyopathy With Left Ventricular Apical Aneurysm: Implications for Risk Stratification and Management. J Am Coll Cardiol. 2017 Feb 21;69(7):761–73. doi: 10.1016/j.jacc.2016.11.063. [DOI] [PubMed] [Google Scholar]
- 17.Muchtar E, Blauwet LA, Gertz MA. Restrictive Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res. 2017 Sep 15;121(7):819–37. doi: 10.1161/CIRCRESAHA.117.310982. [DOI] [PubMed] [Google Scholar]
- 18.Maceira AM, Joshi J, Prasad SK et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005 Jan 18;111(2):186–93. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 19.Vogelsberg H, Mahrholdt H, Deluigi CC et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008 Mar 11;51(10):1022–30. doi: 10.1016/j.jacc.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 20.Brooks J, Kramer CM, Salerno M. Markedly increased volume of distribution of gadolinium in cardiac amyloidosis demonstrated by T1 mapping. J Magn Reson Imaging. 2013 Dec;38(6):1591–5. doi: 10.1002/jmri.24078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banypersad SM, Sado DM, Flett AS et al. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circ Cardiovasc Imaging. 2013 Jan 1;6(1):34–9. doi: 10.1161/CIRCIMAGING.112.978627. [DOI] [PubMed] [Google Scholar]
- 22.Fontana M, Banypersad SM, Treibel TA et al. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014 Feb;7(2):157–65. doi: 10.1016/j.jcmg.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Dungu JN, Valencia O, Pinney JH et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014 Feb;7(2):133–42. doi: 10.1016/j.jcmg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson. 2012 Sep 10;14:63. doi: 10.1186/1532-429X-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellman P, Wilson JR, Xue H et al. Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson. 2012 Sep 11;14:64. doi: 10.1186/1532-429X-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel MR, Cawley PJ, Heitner JF et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009 Nov 17;120(20):1969–77. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terasaki F, Azuma A, Anzai T et al. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis-Digest Version. Circ J. 2019 Oct 25;83(11):2329–88. doi: 10.1253/circj.CJ-19-0508. [DOI] [PubMed] [Google Scholar]
- 28.Birnie DH, Sauer WH, Bogun F et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014 Jul;11(7):1305–23. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 29.Crouser ED, Ruden E, Julian MW, Raman SV. Resolution of abnormal cardiac MRI T2 signal following immune suppression for cardiac sarcoidosis. J Investig Med. 2016 Aug;64(6):1148–50. doi: 10.1136/jim-2016-000144. [DOI] [PubMed] [Google Scholar]
- 30.Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008 Sep 25;10:42. doi: 10.1186/1532-429X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sado DM, Maestrini V, Piechnik SK et al. Noncontrast myocardial T1 mapping using cardiovascular magnetic resonance for iron overload. J Magn Reson Imaging. 2015 Jun;41(6):1505–11. doi: 10.1002/jmri.24727. [DOI] [PubMed] [Google Scholar]
- 32.Messroghli DR, Moon JC, Ferreira VM et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI) J Cardiovasc Magn Reson. 2017 Oct 9;19(1):75. doi: 10.1186/s12968-017-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon JC, Sheppard M, Reed E, Lee P, Elliott PM, Pennell DJ. The histological basis of late gadolinium enhancement cardiovascular magnetic resonance in a patient with Anderson-Fabry disease. J Cardiovasc Magn Reson. 2006;8(3):479–82. doi: 10.1080/10976640600605002. [DOI] [PubMed] [Google Scholar]
- 34.Thompson RB, Chow K, Khan A et al. T(1) mapping with cardiovascular MRI is highly sensitive for Fabry disease independent of hypertrophy and sex. Circ Cardiovasc Imaging. 2013 Sep;6(5):637–45. doi: 10.1161/CIRCIMAGING.113.000482. [DOI] [PubMed] [Google Scholar]
- 35.Mocumbi AO, Ferreira MB, Sidi D, Yacoub MH. A population study of endomyocardial fibrosis in a rural area of Mozambique. N Engl J Med. 2008 Jul 3;359(1):43–9. doi: 10.1056/NEJMoa0708629. [DOI] [PubMed] [Google Scholar]
- 36.Marcus FI, McKenna WJ, Sherrill D et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010 Apr 6;121(13):1533–41. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samsa LA, Yang B, Liu J. Embryonic cardiac chamber maturation: Trabeculation, conduction, and cardiomyocyte proliferation. Am J Med Genet C Semin Med Genet. 2013 Aug;163C(3):157–68. doi: 10.1002/ajmg.c.31366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulusoy RE, Kucukarslan N, Kirilmaz A, Demiralp E. Noncompaction of ventricular myocardium involving both ventricles. Eur J Echocardiogr. 2006 Dec;7(6):457–60. doi: 10.1016/j.euje.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Hofer M, Stollberger C, Finsterer J. Acquired noncompaction associated with myopathy. Int J Cardiol. 2007 Oct 18;121(3):296–7. doi: 10.1016/j.ijcard.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 40.Gati S, Chandra N, Bennett RL et al. Increased left ventricular trabeculation in highly trained athletes: do we need more stringent criteria for the diagnosis of left ventricular noncompaction in athletes? Heart. 2013 Mar;99(6):401–8. doi: 10.1136/heartjnl-2012-303418. [DOI] [PubMed] [Google Scholar]
- 41.Petersen SE, Selvanayagam JB, Wiesmann F et al. Left ventricular noncompaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005 Jul 5;46(1):101–5. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 42.Jacquier A, Thuny F, Jop B et al. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular noncompaction. Eur Heart J. 2010 May;31(9):1098–104. doi: 10.1093/eurheartj/ehp595. [DOI] [PubMed] [Google Scholar]
- 43.Captur G, Muthurangu V, Cook C et al. Quantification of left ventricular trabeculae using fractal analysis. J Cardiovasc Magn Reson. 2013 May 10;15:36. doi: 10.1186/1532-429X-15-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elliott P. Cardiomyopathy. Diagnosis and management of dilated cardiomyopathy. Heart. 2000 Jul;84(1):106–12. doi: 10.1136/heart.84.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi A, Dini FL, Faggiano P et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. 2011 Oct;97(20):1675–80. doi: 10.1136/hrt.2011.225789. [DOI] [PubMed] [Google Scholar]
- 46.de Leeuw N, Ruiter DJ, Balk AH, de Jonge N, Melchers WJ, Galama JM. Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int. 2001 Sep;14(5):299–306. doi: 10.1007/s001470100339. [DOI] [PubMed] [Google Scholar]
- 47.Iles L, Pfluger H, Lefkovits L et al. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011 Feb 15;57(7):821–8. doi: 10.1016/j.jacc.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 48.Assomull RG, Prasad SK, Lyne J et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006 Nov 21;48(10):1977–85. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 49.Gulati A, Jabbour A, Ismail TF et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013 Mar 6;309(9):896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 50.Leong DP, Chakrabarty A, Shipp N et al. Effects of myocardial fibrosis and ventricular dyssynchrony on response to therapy in new-presentation idiopathic dilated cardiomyopathy: insights from cardiovascular magnetic resonance and echocardiography. Eur Heart J. 2012 Mar;33(5):640–8. doi: 10.1093/eurheartj/ehr391. [DOI] [PubMed] [Google Scholar]
- 51.Masci PG, Schuurman R, Andrea B et al. Myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy: a contrast-enhanced cardiovascular magnetic study. Circ Cardiovasc Imaging. 2013 Sep;6(5):790–9. doi: 10.1161/CIRCIMAGING.113.000438. [DOI] [PubMed] [Google Scholar]
- 52.Leyva F, Taylor RJ, Foley PW et al. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2012 Oct 23;60(17):1659–67. doi: 10.1016/j.jacc.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 53.Ferreira VM, Schulz-Menger J, Holmvang G et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018 Dec 18;72(24):3158–76. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 54.Mahrholdt H, Wagner A, Deluigi CC et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006 Oct 10;114(15):1581–90. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]


