Abstract
Structural heart interventions (SHIs) are increasingly applicable in a wide range of heart defects, but the intricate and dynamic nature of cardiac structures can make SHIs challenging to perform. Three-dimensional (3D) printed modeling integrates advanced clinical imaging and 3D printing technology to replicate patient-specific anatomy for comprehensive planning and simulation of SHIs. This review discusses the basic principles of patient-specific 3D print model development, print material selection, and model fabrication and highlights how cardiovascular 3D printing can be used in preprocedural planning, device sizing, enhanced communication, and procedure simulation.
Keywords: structural heart interventions, 3D printing, patient-specific models, heart valves, congenital heart defects
INTRODUCTION
Advances in clinical imaging have fueled the expansion of structural heart interventions (SHIs), including transcatheter aortic valve replacement (TAVR), MitraClip (Abbott), transcatheter mitral valve replacement (TMVR), tricuspid valve repair, and catheter-based congenital heart interventions. However, SHIs can be challenging to perform due to the complex and dynamic nature of cardiac structures as well as unpredictable device interactions with surrounding cardiac tissue. It has been shown that patient-specific models can be useful tools for precise geometric observation, clinical education, and preprocedural planning and rehearsal of highly intricate interventional cases.1,2 Furthermore, 3-dimensional (3D) printed replicas have also proven to be instrumental in enhancing communication with patients and their families.3
DEVELOPMENT OF CARDIOVASCULAR 3D PRINTED MODELS
Medical 3D printing can translate volumetric clinical imaging into physical patient-specific 3D models. Basically, 3D printers build objects by depositing layers of material over the digital geometry. The development of patient-specific 3D printed anatomical models involves several steps (Figure 1), starting with the acquisition of high-quality clinical images that are exported in DICOM (Digital Imaging and Communication in Medicine)-standard format and transferred into segmentation software, which helps identify and delineate anatomical elements of interest. This segmentation process is followed by digital modeling and adjustments and, ultimately, generation of a stereolithography (STL) file. The print material should be selected before initiating the 3D print process.
Figure 1.
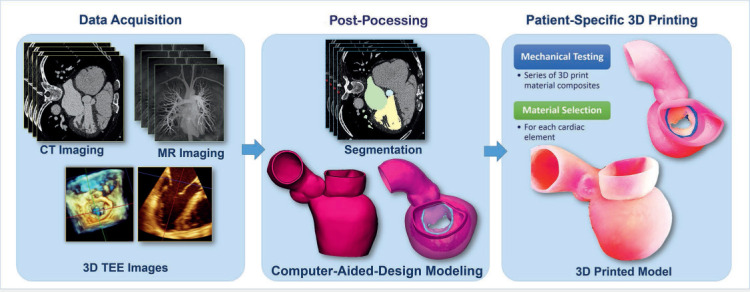
3-dimensional (3D) printed model development workflow. Patient-specific 3D printed modeling starts with high-quality data acquisition from computed tomography (CT), magnetic resonance (MR) imaging, and 3D transesophageal echocardiography (TEE) images. The next step is imaging post-processing that includes geometry segmentation and computer-aided design modeling. The models are 3D printed using multiple materials that were previously selected based on mechanical testing.
IMAGING AND SEGMENTATION
Imaging modalities most suitable for 3D print modeling include contrast-enhanced computed tomography (CT),4 cardiovascular magnetic resonance (CMR),3,5 and 3D transesophageal echocardiography (TEE).2,6 Ideal datasets for 3D reconstruction include images from multiple phases of the cardiac cycle, thus allowing the interventional team to select the cardiac phase of interest.7 Contrast-enhanced CT images are preferable for 3D digital reconstruction because of their superior spatial resolution, thin slices (0.625 mm), wide field of view, multidimensional reconstruction capabilities, and differentiation of soft tissue from bone or calcified structures. These images are particularly well suited for replicating cardiac chambers, large cardiac vessels, heart valves, and subvalvular apparatus morphologies.4,7–10 However, cardiac CT acquisition requires the use of radiation and iodinated contrast.
As an alternative, CMR provides good spatial and temporal resolution and enables noninvasive visualization of complex cardiac structures as well as quantitative assessment of flow features. In fact, CMR has been widely used to assess and characterize congenital cardiac defects and generate 3D printed cardiovascular replicas with congenital defects.
Three-dimensional TEE captures the motion of dynamic anatomical structures such as heart valve leaflets and subvalvular apparatus elements; this allows the clinical team to select and assess the imaging time window of greatest interest.7 While echo-based 3D printed replicas succeed in accurately representing regional anatomical features, the limited field of view and limited range of Hounsfield units that characterizes the grayscale can result in suboptimal reconstruction of small cardiac features or calcific structures. Additionally, 3D TEE images must be exported in Cartesian DICOM format (converting from a pyramidal to a cubic volume) for further processing.
3D PRINTING TECHNOLOGIES AND MATERIAL SELECTION
Rapid expansion of the 3D printing industry has fueled the technological innovation of 3D printers, decreased their costs, and improved overall efficiency and printing speeds. Today's practitioners can find a wide selection of 3D printers to meet their needs. The most critical elements to consider when selecting a printer are (1) machine resolution and surface finish, (2) the ability to print in multiple materials, (3) printing speeds, and (4) cost. The most commonly used 3D printing technologies to create full-scale models for medical applications include stereolithography, fused fiber filament or fused deposition modeling, selective laser sintering, and PolyJet technology.
PolyJet technology-based printers build 3D objects by spraying thin layers of photopolymers over digitally prescribed geometries and curing them using UV light. This technology allows for the simultaneous creation of models with multiple materials and colors and a gradual mixture of materials with different properties. In addition, it offers hundreds of different digital materials that can be used to create a variety of complex elements while preserving the surface smoothness and high resolution of the object.
Materials for 3D printing must be carefully selected and prescribed before printing fabrication takes place. Material selection is based on the required mechanical parameters of the model, with the goal of mimicking the quality and mechanical behavior of the native cardiac tissue. The ability to replicate the characteristics of living cardiovascular tissue requires careful combination of multiple materials.2,11 There are currently hundreds of different materials that can be selected to approximate the mechanical strength and deformation of various cardiovascular structures.
APPLICATIONS OF 3D PRINTED MODELING IN STRUCTURAL HEART INTERVENTIONS
Initially, 3D printed models were used only to improve visualization of complex anatomical structures, including the aortic and mitral valves and congenital heart defects. With the expansion of catheter-based interventions, personalized 3D prints have been increasingly used for preprocedure planning.
Aortic Valve 3D Modeling
Early 3D printed models of the aortic valve included the aortic root and valve. The aortic models were used to observe the aortic geometry, size the TAVR devices within stenosed and calcified aortic valves, and evaluate sites of paravalvular regurgitation (Figure 2 A). Maragiannis and colleagues advanced the application of 3D printed models by developing a series of functional aortic stenosis models that were inserted into the flow loop, allowing simulation of physiologic hemodynamic conditions found in patients (Figure 2 B) and echocardiographic assessment of flow features.12,13 Subsequently, Vukicevic and colleagues showed that a patient-specific model of aortic insufficiency with calcified structures could be used to simulate regurgitant flow when pressurized under tailored patient-specific conditions.14
Figure 2.

Patient-specific 3-dimensional (3D) printed aortic stenosis modeling. (A) Patient-specific aortic stenosis with calcific structures used for benchtop implantation and sizing of the CoreValve device. (B) Patient-specific 3D printed aortic stenosis model with left ventricle, left atrium, and left ventricular outflow tract (LVOT) used for functional analysis and echocardiographic assessment within the flow loop under pressurized flow conditions (left panel). Color Doppler data acquired in the model within the flow loop (right panel) compared favorably with the color Doppler of the actual patient (middle panel). Images adapted with permission from Vukicevic et al.10
Mitral Valve 3D Modeling
In 2016, Little and colleagues created a pioneering, patient-specific, multimaterial mitral valve model to plan the repair of a perforated posterior leaflet and severe central mitral regurgitation (Figure 3 A).1 Based on benchtop implantations of different occluder devices within the model, the team selected the accurate size and type of Amplatzer (Abbott) occluder. This case highlights the utility of 3D modeling to practice and perform SHIs that are unique and tailored to the individual patient.
Figure 3.
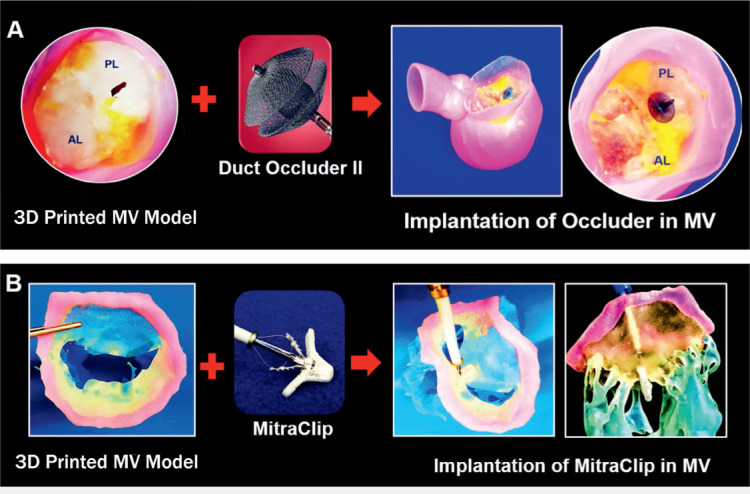
Patient-specific 3-dimensional (3D) printed models used for structural heart interventions planning. (A) Procedural planning of mitral valve (MV) with perforated posterior leaflet; occluding device selection and sizing was performed using 3D printed patient-specific mitral valve model. (B) Benchtop implantation of MitraClip device (Abbott) within the patient-specific 3D printed mitral valve model with chordae and papillary muscles. Figures adapted with permission from Vukicevic et al.10 AL: anterior leaflet; PL: posterior leaflet
Although the MitraClip procedure is a routine intervention, it can be challenging to select patients and accurately predict postprocedural outcomes. Consequently, Vukicevic and colleagues developed a comprehensive, multimaterial, patient-specific model of the mitral valve that included the entire subvalvular apparatus.15 With its flexible leaflets and hard subvalvular apparatus, we showed that such a mitral valve model could be useful for preprocedural planning of MitraClip deployment and for determining the optimal device landing zone (Figure 3 B). Such preoperative preparation can save time and help predict possible intraprocedural risks.
The rapid expansion of TAVR procedures has fueled the development of TMVR devices, many of which are in preclinical or clinical trials. However, their rapid adoption in clinical practice has been impeded due to anatomic challenges, such as the complex and dynamic nature of the mitral valve, the intricate bidirectional interaction between the TMVR device and mitral valve elements, and the risk of left ventricle outflow tract (LVOT) obstruction (Figure 4, left panel). To overcome these challenges, virtual TMVR platforms have been developed to facilitate more accurate device sizing, landing zone selection, and prediction of LVOT obstruction. Despite this, current virtual TMVR implantation tools are unable to accurately predict the realistic bidirectional interaction between the mitral tissue and the TMVR device.4,16 In contrast, 3D printed models fabricated with materials that closely mimic the mechanical properties of mitral tissue can more realistically predict the tissue-TMVR device interaction (Figure 4, right panels). In addition, the TMVR model construct can be scanned via CT, allowing the assessment of key parameters ahead of the procedure (Figure 4, right lower panels). Such comprehensive preoperative planning has the potential to prevent device under- or oversizing, facilitate improved patient selection, and ultimately allow more patients to qualify for the TMVR procedure.
Figure 4.
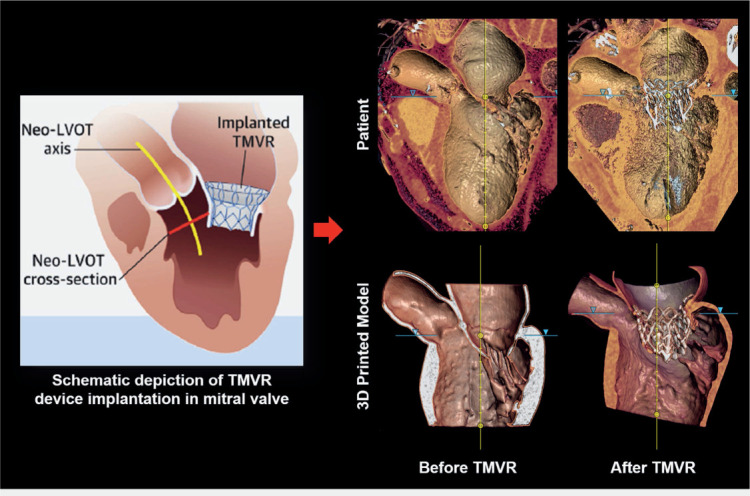
Transcatheter mitral valve replacement (TMVR) procedure planning using 3-dimensional (3D) printed model. Schematic depiction of TMVR device deployment and evaluation of neo-left ventricular outflow tract (LVOT) cross-section area (left panel; adapted with permission from Vukicevic et al.)4 Computed tomography imaging of the patient before and after the TMVR procedure (upper panel) compared well with the CT scan of the 3D printed model before and after the TMVR device implantation (lower panel).
Tricuspid Valve 3D Modeling
A growing interest in the treatment of tricuspid valve dysfunction and development of tricuspid repair and replacement devices has driven the advancement of 3D print modeling for the tricuspid valve. Comprehensive preoperative and intraoperative imaging of the tricuspid valve can be challenging due to its multiple leaflets, dense network of chordae, and varying number of papillary muscles. It has been shown that the tricuspid valve can be reconstructed from both high-quality CT images and volumetric echocardiographic images.17 In addition, a 3D printed tricuspid valve can be a useful tool for testing percutaneous devices. Vukicevic and colleagues showed that MitraClip preoperative planning can be successfully performed using a patient-specific 3D printed replica of the leaflets and annulus. An increasingly common issue for surgeons is determining where to deploy a MitraClip device within a three-leaflet structure. Preoperative rehearsal and device landing zone planning on a 3D patient-specific model may circumvent this problem during the clinical repair procedure.
Left Atrial Appendage 3D Modeling
Left atrial appendage (LAA) thrombosis is a dreaded complication of atrial fibrillation. Percutaneous closure of the LAA is a common treatment; however, the appropriate sizing and selection of devices using common clinical imaging can be challenging. Hachulla and colleagues reported that CT-based 3D printed models can be instrumental in preoperative planning (Figure 5, upper right), and that LAA device sizing based on 3D printed models is superior to 3D echocardiographic and CT images alone.18 Obasare and colleagues developed a series of CT-based 3D printed models of LAA for sizing the Watchman (Boston Scientific Corporation) device prior to its implantation in patients (Figure 5, upper left).19 They showed that patient-specific 3D printed LAA models reduced the catheterization time per patient, provided more accurate sizing than conventional 3D echocardiography imaging, and reduced the number of implanted devices. In addition, Robinson and colleagues showed that 3D print modeling can be used to design and develop permanent soft endocardial implants (Figure 5, lower panels).20
Figure 5.
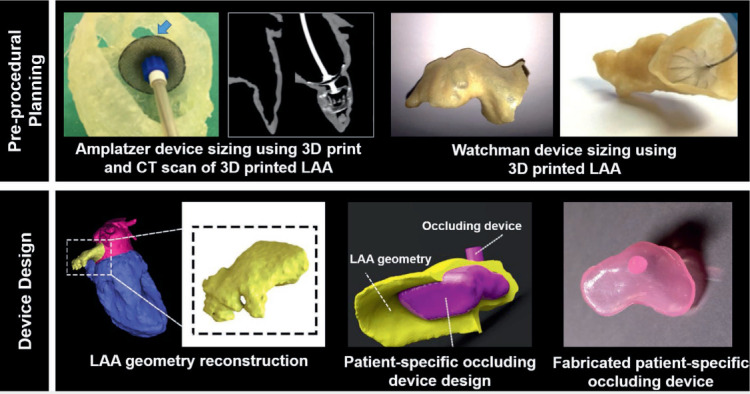
Preprocedural planning and device design based on patient-specific 3-dimensional (3D) printed left atrial appendage (LAA) models. (Upper left panel) Benchtop implantation of 25 Amplatzer (Abbott) occluding device in 3D printed LAA shows that the occluding device, which was selected based on measurements from computed tomography (CT) and echocardiographic images, is oversized. A smaller 22 Amplatzer device (shown in CT image of the model) was eventually implanted in the patient. Upper left panel figures adapted with permission from Hachulla et al.18 (Upper right panel) CT-based 3D printed model of LAA was developed for planning the Watchman (Boston Scientific Corporation) device implantation. Upper right panel figures adapted with permission from Obasare et al.19 Device design based on the patient's geometry was developed using the 3D printed modeling. Bottom panel adapted with permission from Robinson et al.20
3D Modeling of Congenital Heart Interventions
Anatomical structures in patients with congenital heart diseases can be very complex and are often difficult to assess using 2D imaging. Thus, patient-specific 3D printed modeling of complex congenital structures has a significant role in the planning of congenital heart interventions. 3D printed models are used to plan a range of congenital defect repairs, from relatively simple atrial and ventricular septal defects (Figure 6) to complicated Fontan reoperations, and to prepare for the implementation of mechanical left ventricular assist devices.21–23 Studies have described the feasibility of using hybrid 3D printing derived from two imaging modalities to enhance visualization of cardiac pathomorphology24 and have showed that intricate congenital heart interventions can be successfully planned using 3D printed replicas of unique congenital anatomy.25 In a pilot study by Biglino et al., 3D printed models of congenital defects were successfully used to enhance communication between physicians, patients, and families before and after structural procedures, with a majority of patients reporting that the models helped their understanding and improved their visit.26
Figure 6.
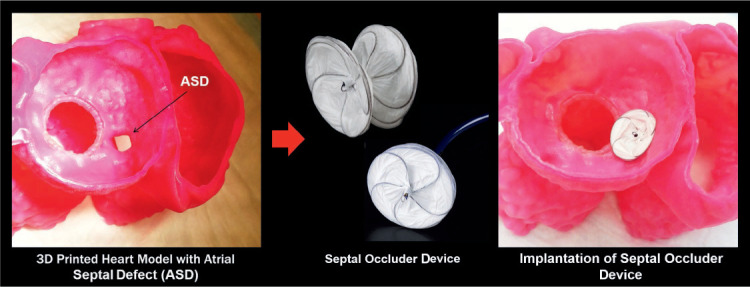
Preprocedural planning of an atrial septal defect (ASD) was performed using a patient-specific heart model. Adapted with permission from Vukicevic et al.4
FUTURE DEVELOPMENTS
Increasingly complicated SHIs require advanced visualization tools that optimize clinical and industrial teaching, facilitate communication with patients and their families, and allow enhanced preprocedural planning. To meet these challenges, 3D printed patient-specific models are ideal for simulating SHIs, implanting different devices and determining appropriate sizing, and understanding the interaction between percutaneous devices and surrounding anatomical structures. Furthermore, 3D replicas of patient-specific structural heart defects can undergo CT scanning before and after percutaneous device deployment, and the images can be used for realistic measurements and sizing of percutaneous devices. In addition, CT images of 3D printed models can be imported into the catheterization lab and used for procedural guidance during clinical interventions. Fusing these CT images with fluoroscopic images during actual clinical procedures has the potential to enhance device implantation, prevent complications, and improve procedural outcomes.27
KEY POINTS
Patient-specific 3-dimensional (3D) printed models can be created from any high-quality volumetric clinic image (echocardiogram, computed tomography, cardiac magnetic resonance imaging).
Such models can now be created using multiple images, materials, or material blends.
Functional patient-specific models can be created under tailored flow conditions to replicate hemodynamic situations including transvalvular pressure gradient, cardiac output, and regurgitant flow volume.
Procedure planning within a 3D printed model is a safe and a cost-effective strategy to simulate novel intracardiac procedures.
Footnotes
Conflict of Interest Disclosure:
Dr. Little is a formal advisor for Caption Health, Abbott US, and Medtronic and conducts research on behalf of Abbott US, Medronic, and Siemens Medical Solutions.
REFERENCES
- 1.Little SH, Vukicevic M, Avenatti E, Ramchandani M, Barker CM. 3D Printed Modeling for Patient-Specific Mitral Valve Intervention: Repair With a Clip and a Plug. JACC Cardiovasc Interv. 2016 May 9;9(9):973–5. doi: 10.1016/j.jcin.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 2.Vukicevic M, Puperi DS, Jane Grande-Allen K, Little SH. 3D Printed Modeling of the Mitral Valve for Catheter-Based Structural Interventions. Ann Biomed Eng. 2017 Feb;45(2):508–19. doi: 10.1007/s10439-016-1676-5. [DOI] [PubMed] [Google Scholar]
- 3.Biglino G, Schievano S, Taylor AM. The Use of Rapid Prototyping in Clinical Applications. In: Hoque Dr. M., editor. Advanced Applications of Rapid Prototyping Technology in Modern Engineering [Internet] London: InTechOpen Limited; c2011. editor. [Cited 2020 Jan 30]. Available from: http://cdn.intechweb.org/pdfs/20117.pdf. [Google Scholar]
- 4.Vukicevic M, Mosadegh B, Min JK, Little SH. Cardiac 3D Printing and its Future Directions. JACC Cardiovasc Imaging. 2017 Feb;10(2):171–84. doi: 10.1016/j.jcmg.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schievano S, Taylor AM, Capelli C et al. First-in-man implantation of a novel percutaneous valve: a new approach to medical device development. EuroIntervention. 2010 Jan;5(6):745–50. doi: 10.4244/eijv5i6a122. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood F, Owais K, Montealegre-Gallegos M et al. Echocardiography derived three-dimensional printing of normal and abnormal mitral annuli. Ann Card Anaesth. 2014 Oct-Dec;17(4):279–83. doi: 10.4103/0971-9784.142062. [DOI] [PubMed] [Google Scholar]
- 7.Wang DD, Gheewala N, Shah R et al. Three-Dimensional Printing for Planning of Structural Heart Interventions. Interv Cardiol Clin. 2018 Jul;7(3):415–23. doi: 10.1016/j.iccl.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Wang DD, Eng M, Greenbaum A et al. Predicting LVOT Obstruction After TMVR. JACC Cardiovasc Imaging. 2016 Nov;9(11):1349–52. doi: 10.1016/j.jcmg.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otton JM, Birbara NS, Hussain T, Greil G, Foley TA, Pather N. 3D printing from cardiovascular CT: a practical guide and review. Cardiovasc Diagn Ther. 2017 Oct;7(5):507–26. doi: 10.21037/cdt.2017.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vukicevic M, Vekilov DP, Grande-Allen J, Little SH. Patient-specific 3D Valve Modeling for Structural Intervention. Structural Heart. 2017 Nov;1(5–6):236–48. [Google Scholar]
- 11.Wang K, Wu C, Qian Z, Zhang C, Wang B, Vannan MA. Dual-material 3D printed metamaterials with tunable mechanical properties for patient-specific tissue-mimicking phantoms. Additive Manufacturing. 2016 Oct;12(Part A):31–7. [Google Scholar]
- 12.Maragiannis D, Jackson MS, Igo SR, Chang SM, Zoghbi WA, Little SH. Functional 3D printed patient-specific modeling of severe aortic stenosis. J Am Coll Cardiol. 2014 Sep 9;64(10):1066–8. doi: 10.1016/j.jacc.2014.05.058. [DOI] [PubMed] [Google Scholar]
- 13.Maragiannis D, Jackson MS, Igo SR et al. Replicating Patient-Specific Severe Aortic Valve Stenosis With Functional 3D Modeling. Circ Cardiovasc Imaging. 2015 Oct;8(10):e003626. doi: 10.1161/CIRCIMAGING.115.003626. [DOI] [PubMed] [Google Scholar]
- 14.Vukicevic M, Maragiannis D, Jackson M, Little SH. Abstract 18647: Functional Evaluation of a Patient-specific 3D Printed Model of Aortic Regurgitation. Circulation. 2015 Nov 10;132:A18647. [Google Scholar]
- 15.Vukicevic M, Mosadegh B, Min JK, Little SH. Cardiac 3D Printing and its Future Directions. JACC Cardiovasc Imaging. 2017 Feb;10(2):171–84. doi: 10.1016/j.jcmg.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harb SC, Rodriguez LL, Vukicevic M, Kapadia SR, Little SH. Three-Dimensional Printing Applications in Percutaneous Structural Heart Interventions. Circ Cardiovasc Imaging. 2019 Oct;12(10):e009014. doi: 10.1161/CIRCIMAGING.119.009014. [DOI] [PubMed] [Google Scholar]
- 17.Vukicevic M, Avenatti E, El-Tallawi KC, Barker CM, Little SH. 3D printed modeling of Tricuspid valve for structural heart valve interventions. J Am Soc Echocardiography. 2017;30(6):B37. [Google Scholar]
- 18.Hachulla AL, Noble S, Guglielmi G, Agulleiro D, Muller H, Vallee JP. 3D-printed heart model to guide LAA closure: useful in clinical practice? Eur Radiol. 2019 Jan;29(1):251–258. doi: 10.1007/s00330-018-5569-x. [DOI] [PubMed] [Google Scholar]
- 19.Obasare E, Mainigi SK, Morris DL et al. CT based 3D printing is superior to transesophageal echocardiography for pre-procedure planning in left atrial appendage device closure. Int J Cardiovasc Imaging. 2018 May;34(5):821–31. doi: 10.1007/s10554-017-1289-6. [DOI] [PubMed] [Google Scholar]
- 20.Robinson SS, Alaie S, Sidoti H et al. Patient-specific design of a soft occluder for the left atrial appendage. Nat Biomed Eng. 2018 Jan;2(1):8–16. doi: 10.1038/s41551-017-0180-z. [DOI] [PubMed] [Google Scholar]
- 21.Vukicevic M, Conover T, Jaeggli M et al. Control of respiration-driven retrograde flow in the subdiaphragmatic venous return of the Fontan circulation. ASAIO J. 2014 Jul-Aug;60(4):391–9. doi: 10.1097/MAT.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anwar S, Singh GK, Miller J et al. 3D Printing is a Transformative Technology in Congenital Heart Disease. JACC Basic Transl Sci. 2018 May 30;3(2):294–312. doi: 10.1016/j.jacbts.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuentes Rojas S, Vukicevic M, Thakkar AN, Little SH, Lin CH. 3D Cardiac Printing to Plan Transcatheter Closure of an Iatrogenic Primum Atrial Septal Defect. Structural Heart. 2019 Nov 2;3(6):512–3. [Google Scholar]
- 24.Gosnell J, Pietila T, Samuel BP, Kurup HK, Haw MP, Vettukattil JJ. Integration of Computed Tomography and Three-Dimensional Echocardiography for Hybrid Three-Dimensional Printing in Congenital Heart Disease. J Digit Imaging. 2016 Dec;29(6):665–9. doi: 10.1007/s10278-016-9879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivieri LJ, Krieger A, Loke YH, Nath DS, Kim PC, Sable CA. Three-dimensional printing of intracardiac defects from three-dimensional echocardiographic images: feasibility and relative accuracy. J Am Soc Echocardiogr. 2015 Apr;28(4):392–7. doi: 10.1016/j.echo.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Biglino G, Koniordou D, Gasparini M et al. Piloting the Use of Patient-Specific Cardiac Models as a Novel Tool to Facilitate Communication During Cinical Consultations. Pediatr Cardiol. 2017 Apr;38(4):813–8. doi: 10.1007/s00246-017-1586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thakkar AN, Chinnadurai P, Breinholt JP, Lin CH. Transcatheter closure of a sinus venosus atrial septal defect using 3D printing and image fusion guidance. Catheter Cardiovasc Interv. 2018 Aug 1;92(2):353–7. doi: 10.1002/ccd.27645. [DOI] [PMC free article] [PubMed] [Google Scholar]


