Abstract
Cardiac computed tomography angiography (CCTA) has evolved into a versatile imaging modality that can depict atherosclerosis burden, determine functional significance of a stenotic lesion, and guide the management and treatment of stable coronary artery disease.1 With newer-generation scanners, diagnostic CCTA can be obtained in the majority of patients with a very acceptable radiation dose. We discuss the ability of CCTA to provide comprehensive assessment of a patient with suspected CAD, including functional techniques of stress-rest myocardial perfusion assessment using a vasodilator and a purely post-processing approach that assesses fractional flow reserve derived by CCTA. In addition, recent data validated the role of CCTA in managing stable patients with chest pain and suspected CAD, serving as a gatekeeper for invasive coronary angiogram as well as optimizing the preprocedural planning of percutaneous coronary revascularization and coronary artery bypass surgery.
Keywords: cardiac computed tomography angiography, coronary artery disease, CT fusion imaging
INTRODUCTION
A decade ago, cardiac computed tomography angiography (CCTA) was viewed primarily as an anatomic tool to exclude significant coronary artery luminal stenosis or as a gatekeeper for invasive coronary angiography (ICA). Since then, however, CCTA has evolved into a truly versatile imaging modality that can depict atherosclerosis burden, determine functional significance of a stenotic lesion, and guide the management and treatment of stable coronary artery disease.1 In November 2016, the National Institute for Health and Care Excellence (NICE) Clinical Guideline 95 recommended CCTA as the noninvasive test of choice to evaluate stable angina and the first-line investigation for all patients presenting with chest pain due to suspected coronary artery disease (CAD).2 Recently, the 2019 European Society of Cardiology guideline for the diagnosis and management of chronic coronary syndromes classified CCTA as a Class 1 recommendation for diagnosing CAD in symptomatic patients with suspected obstructive coronary artery disease. The guideline further states that CCTA should be preferentially considered if there is a low likelihood of obstructive CAD, patient characteristics suggest high image quality, local expertise is available, information on atherosclerosis is desired, and there is no history of CAD.
There are several reasons for the recommended broader and preferential use of CCTA as the initial test. First, CCTA has undergone remarkable technological advances that have led to improved image quality and a dramatic reduction in radiation dose. But the most important reason is its ability to extract functional information from routine CCTA and results from recent comparative effectiveness and outcome, which is described below.
TECHNOLOGICAL ADVANCES
Compared to the prior 64-slice multidetector CT, newer-generation scanners include different features such as improved spatial and temporal resolution, faster scan mode, and whole heart coverage with either wide-detector or dual-source CT. Wide-detector CT can acquire images of the entire heart in a single beat, and dual-source CT has ultra-high-pitch mode scan mode that can image the heart in less than 300 ms.3,4 Furthermore, thinner detectors with a spatial resolution of 250 microns along the XY planes, faster gantry rotation (220 ms), noise reduction with improved detector efficiency, and innovative electronic circuitry have made it possible to obtain diagnostic images in patients who were once deemed too challenging to image with prior-generation CT (eg, those with calcium score > 400 AU, large coronary artery stents, coronary artery bypass grafts, heart rate > 65 bpm, atrial fibrillation, and obesity/body mass index > 30 kg m−2).2,5
Although radiation is always a concern with CCTA, newer-generation scanners are equipped with many dose-saving features that, if used correctly, can achieve comparatively low radiation doses (Figure 1). Noise-reduction software, particularly the recent introduction of iterative reconstruction methods in CT, can achieve a given signal-to-noise ratio at a lower radiation dose. A low-kV x-ray is another dose-reduction strategy in smaller patients, improving the contrast-to-noise ratio in CCTA scans (the minimum kV available is generally 70 to 80 kV).6,7
Figure 1.
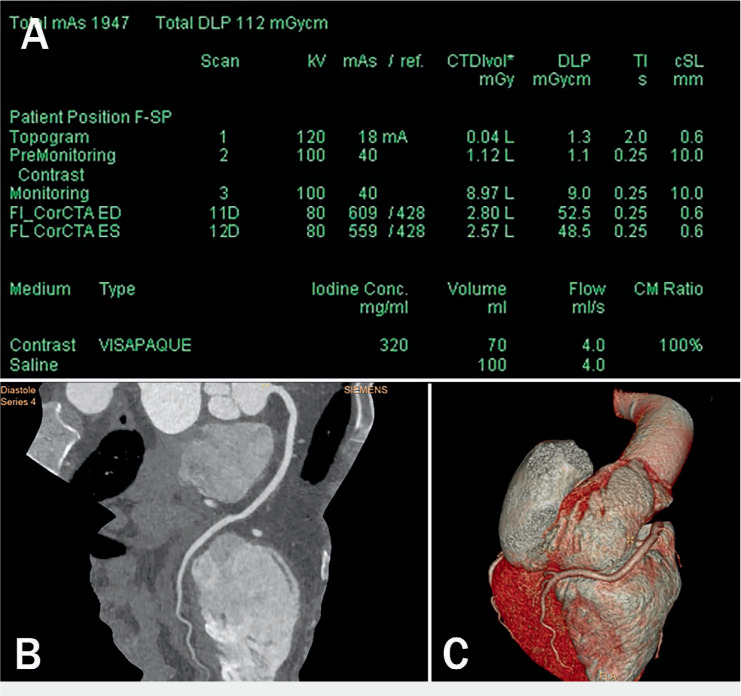
Technological advances. (A) Cardiac computed tomography angiography (CCTA) performed on a third-generation dual-source CT with the electrocardiograph-triggered high-pitch mode obtained at mid diastole (shown here) and systole with single 70-mL contrast bolus injection. Data acquisition of the entire z-axis of the adult heart was obtained within a fraction of one cardiac cycle (around 260 ms) with tube voltage of 80 kV and tube current of 560 mAs. The contrast injection rate was 4 mL/sec. Effective dose estimation by the dose length product (DLP) for each scan was around 50 mGy cm (effective dose of < 1 mSv). (B) Multiplanar reformation and (C) three-dimensional volume rendering of the right coronary artery in the same patient. The image quality of the coronary artery in spite of low-radiation-dose CT is optimal.
ANATOMICAL ASSESSMENT OF LUMINAL STENOSIS
While ICA remains the gold standard for diagnosing CAD, CCTA has become a viable noninvasive alternative.8,9 Multiple studies have established that CCTA has excellent sensitivity and high specificity (64–83%) for detecting coronary stenosis ≥ 50% compared to invasive angiogram. The extremely high negative predictive value of > 95% makes CCTA the test of choice for excluding obstructive coronary artery stenosis in patients without known CAD, particularly in the low-intermediate pre-test probability group. CCTA has similar diagnostic accuracy in the emergency room when used to evaluate patients with acute chest pain and low to intermediate risk of acute coronary syndrome.10,11
ATHEROSCLEROTIC PLAQUE IMAGING
Coronary calcified plaques can be readily detected and quantified using noncontrast gated MDCT. From there, coronary artery calcium (CAC) assessment can help diagnose atherosclerosis and obstructive disease and determine risk stratification for future cardiac events. CAC measured by CCTA has a high sensitivity and negative predictive value for obstructive CAD but with limited specificity. Multiple studies involving thousands of patients showed negative predictive values of 96% to 100%, providing physicians with a high level of confidence that an individual with a total calcium score of 0 does not have obstructive angiographic CAD. In addition, patients with high disease burden, such as a CAC score > 400, had a 3- to 4-fold higher risk of events (revascularization, myocardial infarction, or death) compared to patients with a minimal or no CAC score, irrespective of SPECT nuclear stress test.12 With administration of contrast material, CCTA allows the detection of nonobstructive lesions that may not be identified by existing stress testing modalities or even by ICA. CCTA also can provide an even better assessment of plaque composition (noncalcified, partially calcified, or calcified based on the amount of calcium in the lesion), akin to a combination of intravascular ultrasound and ICA. Although detailed plaque histology cannot be derived from CCTA, several plaque features have demonstrated a significant association with future acute coronary syndrome, including the “napkin-ring” sign (ring-like attenuation in the noncalcified portion of the mixed plaque), spotty calcification, low attenuation plaque, a large necrotic core, circular enhancement, and positive remodeling. The prevalence of these features is low, however, with limited sensitivity (∼40%) and positive predictive value (∼20 %) to predict plaque rupture.13,14 CCTA is ideal for noninvasive plaque characterization and can identify phenotypic alterations in plaque characteristics when serial studies are performed. Several studies observed favorable changes in plaque composition, including a decrease in total, noncalcified, and low-density noncalcified plaque volumes among patients who achieved > 10% reduction in low-density lipoprotein levels.8,15 Despite this, the most powerful predictor of outcomes is the level of disease burden rather than type of plaque. As with CAC, the extent of disease burden seen by CCTA is a very strong prognostic predictor as shown in multiple studies.13
Several recent studies have indicated that the plaque burden detected by CCTA is more effective than luminal stenosis at providing incremental predictive value of abnormal invasive fractional flow reserve (FFR) and predicting myocardial ischemia detected by stress positron emission tomography.11,16,17
PHYSIOLOGIC ASSESSMENT WITH CCTA
While CCTA is extremely sensitive in detecting CAD and excluding significant coronary luminal stenosis, it is limited by a relatively modest positive predictive value (mainly in very calcific lesions). Similar to ICA, the correlation between stenosis seen on CCTA versus functional testing (such as noninvasive SPECT or PET and invasive FFR) is at most modest. Fortunately, it is possible to extract functional information from the anatomical assessment provided by CCTA. The most validated techniques include stress-rest myocardial perfusion assessment using a vasodilator and a purely post-processing approach that assesses FFR derived by CCTA.
FRACTIONAL FLOW RESERVE ASSESSMENT
Fractional flow reserve derived by CCTA (FFRCT) is a noninvasive tool that evaluates coronary anatomy including plaque burden, stenosis, luminal diameter, and left ventricular mass; with this information, it then models a simulated hyperemic blood flow response from a resting CCTA by computerized post-processing without actually inducing hyperemia or using a pharmacologic stress agent. The cutoff for an abnormal study is < 0.8. In a recent large-scale multicenter trial, FFRCT accurately reclassified 68% of CCTA false positives to true negatives without a compromise in sensitivity.18 In patients with chronic stable angina, FFRCT is also a gatekeeper that helps determine who would benefit from ICA or revascularization. In the invasive arm of the PLATFORM (Prospective Longitudinal Trial of FFRCT: Outcome and Resource Impacts) trial, FFRCT led to safe cancellation of ICA in 61% of patients with chronic stable angina. FFRCT has similarly demonstrated safe cancellation of ICA in 75% of high-risk and 91% of low- to intermediate-risk patients with nonacute symptoms who were scheduled for ICA.19,20 Some studies have shown that FFRCT in emergency room patients with acute chest pain is feasible and may help determine which patients would benefit the most from ICA or revascularization.21
There are some disadvantages to FFRCT, such as increased cost, slower turnaround (from sending the data to be processed), lack of precision, and rejection of images with significant calcification and motion.22 FFRCT has not been tested in patients with a history of revascularization. The clinical significance of an intermediate or borderline value (0.75 to 0.80) or abnormal value in diffusely diseased vessels without discrete stenosis is not well established. The technology seems to have found its niche assessing CAD using CCTA in patients with intermediate lesions, particularly those with lesions in multiples vessels (Figures 2, 3).
Figure 2.
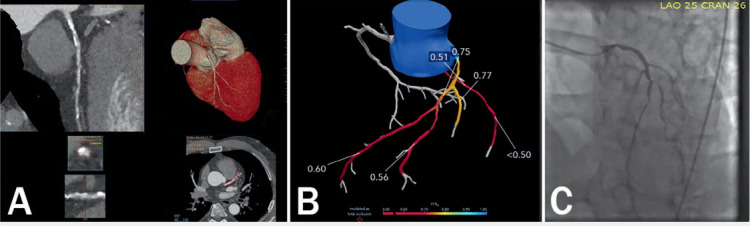
Fractional flow reserve computed tomography (FFRCT) case of a 58-year-old man. (A) Multiplanar reformation of the coronary computed tomography angiograph (CTA) demonstrating calcified and noncalcified atherosclerotic plaque in the proximal and mid left anterior descending artery associated with positive remodeling. Note the moderate stenosis in the proximal lesion with calcified plaque and moderate-to-severe stenosis in the mid lesion with predominantly noncalcified plaque. (B) FFRCT analysis added to coronary CTA to assess the hemodynamic significance. FFRCT across the proximal left anterior descending coronary artery (LAD) was 0.75 and across the mid and distal lesion was 0.60, suggesting the likelihood of hemodynamic significance. (C) Invasive coronary angiography showed sequential lesions from the proximal to mid LAD with the mid LAD lesion having an instantaneous wave-free ratio value of 0.72.
Figure 3.
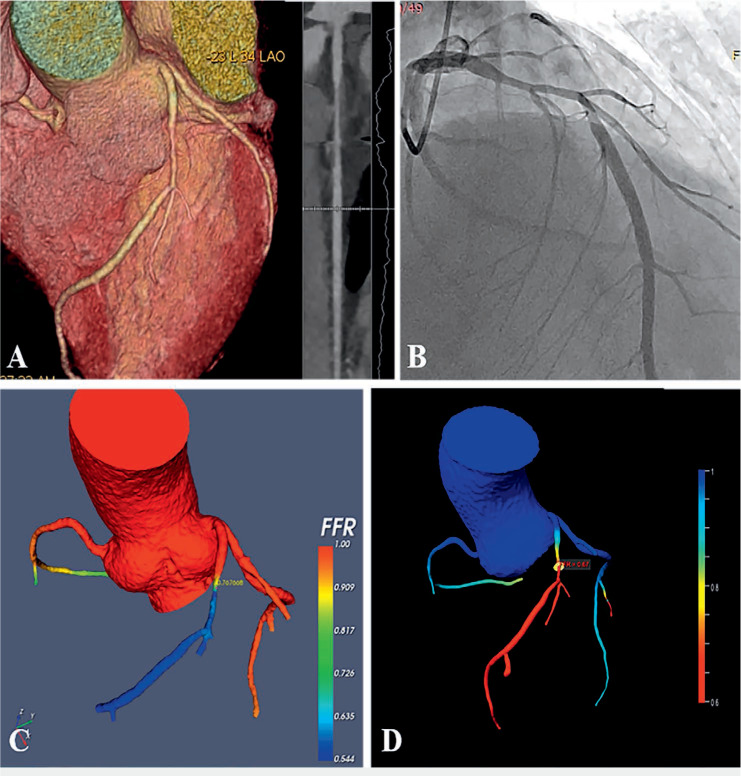
Fractional flow reserve computed tomography (FFRCT) case of a 58-year-old man with recurrent chest pain for 2 months. (A) Coronary computed tomography angiography (CCTA) showed the presence of noncalcified plaque and moderate stenosis (70%) in proximal and mid-left anterior descending artery (LAD). (B) Invasive coronary angiography (ICA) showed LAD had a long lesion with 70% stenosis. (C) FFRCT calculated with computational fluid dynamics (CFD). (D) FFRCT using machine learning algorithm. The FFRCT values with CFD and machine learning were 0.76 and 0.67, respectively. Both were positive with different value. The invasive catheter-derived FFR (as the gold standard) was 0.74. The patient was treated with a stent placed in the LAD.
In late 2019, the US Food and Drug Administration (FDA) approved the HeartFlow Planner (HeartFlow, Inc.), a virtual modeling tool for CAD. This technique appears to rely heavily on the quality of the underlying computational models and sophisticated boundary conditions and requires a few hours for computation. However, there are new methods of machine learning (ML) that accelerate the diagnostic processing time from hours to minutes. The ML-based FFRCT model was trained using a deep learning model to integrate the complex nonlinear relationship between the various features extracted from the coronary tree geometry. However, its clinical utility is not well tested.22–24
CT PERFUSION IMAGING
Cardiac CT perfusion imaging allows comprehensive noninvasive functional assessment of CAD, similar to nuclear perfusion imaging with SPECT or MRI. Using pharmacologic vasodilator agents such as regadenoson or adenosine, cardiac CT perfusion imaging shows the uptake of contrast material during first-pass acquisition. Since the distribution of contrast material depends on arterial blood supply, myocardial perfusion defects appear as areas of low contrast attenuation (Figure 4), thus allowing for accurate identification of ischemia.4,25–28 Infarcted or nonviable myocardium is seen as hypoattenuating areas with diminished contrast in rest and stress, whereas a perfusion defect in stress but not in rest indicates myocardial ischemia, likely both macro- and microvascular in origin. CT myocardial perfusion imaging can be performed in either static or dynamic mode. The more-studied static option is ECG gated at systole, which can be performed with single or dual energy CT. It captures a single time point (arterial phase) as iodinated contrast passes through the cardiac anatomy, allowing for qualitative and/or semi-quantitative assessment of myocardial CT perfusion. In contrast, dynamic or time-resolved myocardial CT perfusion imaging is done multiple times as the contrast transits from the aorta, coronary arteries, the myocardium, and cardiac veins, which allows quantitative assessment of myocardial blood volume and reserve.29
Figure 4.
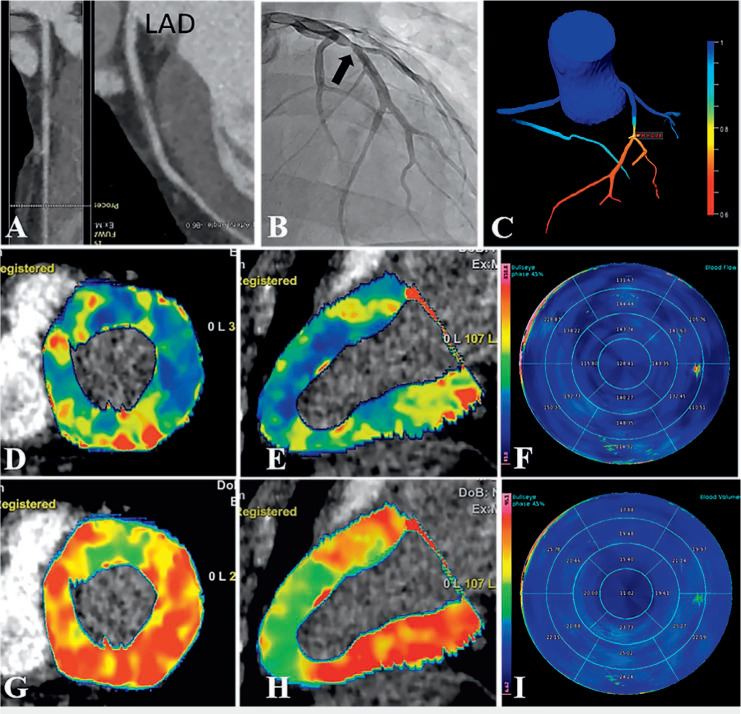
Functional coronary computed tomography angiography (CCTA) imaging: Case of 28-year-old man with precordial discomfort for 1 month. (A) CCTA images showed the presence of noncalcified plaque and > 70% stenosis in proximal left anterior descending artery. (B) Invasive coronary angiography images confirmed the left anterior descending artery stenosis as about 70%. (C) CT-derived fractional flow reserve (FFRCT) computed using machine learning algorithm; the FFRCT was positive of 0.74. (D, G) Short-axis images of myocardial blood flow (MBF) and myocardial blood volume (MBV) in adenosine-stress dynamic CT perfusion (CTP); the stressed dynamic CTP images manifested the reduced values of MBF and MBV in the apical and middle segments of the anterior and lateral wall. (E, H) Long-axis of CTP images showed reduced values of both MBF and MBV on apical and anterior wall of the left ventricle. (F, I) Bulls-eye diagram images show the quantitative values of both MBF and MBV. Courtesy of Drs. Na Zhao and Yang Gao.
Myocardial CT perfusion imaging has several advantages over FFRCT in that it is not affected by calcified plaque, patient motion, or inadequate patient cooperation during breath holding. Adding CT perfusion imaging to standard CCTA also improves diagnostic accuracy for identifying hemodynamically significant obstructive coronary artery stenosis.26,30 Multicenter trials have shown that both myocardial CT and MR perfusion imaging have comparable diagnostic accuracy in detecting CAD, with CT perfusion having higher sensitivity.23,29,31 However, a main disadvantage of CT perfusion imaging is patient exposure to higher radiation and dosing of an additional contrast agent. Also, it lacks clear optimal cut-off values of quantitative parameters to identify ischemia, such as myocardial blood volume and flow.
CCTA FOR MANAGING STABLE PATIENTS WITH CHEST PAIN AND SUSPECTED CAD
Past strategies using clinical and stress testing for evaluating and managing stable patients with chest pain and suspected CAD have been suboptimal in terms of referring for diagnostic ICA. A large national registry showed that two-thirds of patients referred for ICA had normal or nonobstructive (< 70%) stenosis on invasive angiography.9 In view of its versatility in assessing the coronary arteries, CCTA presents as a potentially superior initial strategy.
Several recent clinical trials using CCTA as the initial test in patients with chest pain have demonstrated similar or even superior clinical outcome benefit than standard of care. In the more recent SCOT-HEART (Scottish Computed Tomography of the HEART) and PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trials, the use of CCTA resulted in a higher rate of ICA than standard care alone (SCOT-HEART) or functional testing (PROMISE). In the SCOT-HEART trial, patients who underwent CCTA for assessment of stable chest pain had a lower subsequent risk of death from coronary heart disease or nonfatal myocardial infarction than those who received standard care alone. ICA and coronary revascularization are more likely to be used appropriately in patients who receive a correct diagnosis of coronary heart disease. In turn, patients who receive a correct diagnosis are also more likely to receive appropriate preventive therapies and may be more motivated to implement healthy lifestyle modifications. In addition, the SCOT-HEART trial encouraged initiation of secondary prevention strategies in patients with nonobstructive CAD and consequently increased prescribing of antiplatelet therapy in this group. The identification of disease is inextricably linked to downstream changes in lifestyle, initiation and intensification of preventative therapies, and the judicious use of coronary revascularization. Previous studies have suggested that the use of CCTA is associated with higher early rates of both ICA and coronary revascularization. However, beyond 12 months, rates of ICA and coronary revascularization were higher in the standard-care group than in the CCTA group.9,32–35
Before CCTA, patients without inducible ischemia were assumed to be free of obstructive CAD despite the possibility that they may have nonobstructive CAD. However, large-scale CCTA registries have since shown the cardiovascular risk of nonobstructive CAD. Given that statin and aspirin prescriptions significantly increase upon abnormal findings by CCTA like the presence of atherosclerosis, tailored treatment for nonobstructive CAD would improve the prognosis of these patients and contribute to the socioeconomic impact.10,20–22 Recent studies have demonstrated an improvement in event-free survival among patients with extensive nonobstructive CAD who take statins versus those who do not.36 In addition, there is compelling evidence that CCTA use in the emergency room to evaluate acute chest pain/possible ACS is safe, effective, and may be cost effective in terms of reducing hospital admissions, downstream testing, and associated costs for patients with low-to-intermediate-risk acute chest pain.15
ROLE OF CCTA FOR PATIENTS NEEDING BYPASS SURGERY
In a multicenter study of 223 patients with left main or triple-vessel CAD, Collet and colleagues randomized heart teams to evaluate the extent of CAD using either CCTA or conventional angiography, with each team blinded for the other modality. Treatment decision making based on CCTA showed high agreement with the decision derived from conventional coronary angiography. Coronary artery bypass grafting was recommended for 28% of patients on the basis of CCTA and 26% with conventional angiography, suggesting the potential feasibility of treatment decision making and planning based solely on CCTA and clinical information.37
ROLE OF CCTA FROM AN INTERVENTIONALIST PERSPECTIVE
The role of CCTA as a gatekeeper for ICA has increased the ratio of interventional to diagnostic procedures. Noninvasive CCTA is the only imaging technique that can quantify lesion length and vessel size before even initiating ICA. The detailed morphological characterization of coronary anatomy and plaque distribution provided by CCTA may also be useful in evaluating bifurcation lesions and provide insight as to the most appropriate PCI bifurcation technique.38 CCTA can predict possible complications during PCI (eg, slow-flow or no-reflow phenomenon) due to plaque characteristics such as low-attenuation plaque and a napkin ring-like appearance of culprit lesions shown on a CCTA CT-SYNTAX score.39 This score has been shown to correlate well with the invasive SYNTAX score, has high reproducibility,40 and reflects the severity of CAD, which has prognostic implications and can assist with decision making regarding myocardial revascularization.
Myocardial bridging is easily identified with CCTA. However, bridging of the left anterior descending coronary artery imposes a higher technical difficulty for bypass surgery and has been associated with higher rates of complications, including perforation of the right ventricle; therefore, preoperative identification with CCTA can potentially help with planning the revascularization procedure.41
Coronary chronic total occlusions (CTOs) are complex lesions that have > 99% obstruction for > 3 months and are responsible for a clinically significant decrease in blood flow (TIMI 0-1 flow). Several studies have demonstrated the value of CCTA in predicting recanalization success rates and optimizing the preprocedural planning of CTO percutaneous coronary revascularization through scoring similar to the KCCT (Korean Multicenter CTO CT Registry) and CT Rector (Computed Tomography Registry of Chronic Total Occlusion Revascularization) scores. This includes features such as length of the occluded segment, presence of more than two complete occlusions, amount of calcification, presence of a blunt stump, bending and tortuosity of the proximal vessel and/or occluded segment, and presence of a side branch.40,42–45 Preprocedural planning is key for the success and safety of the procedure as demonstrated in Figure 5. When co-registered with the angiogram, CCTA can also provide a live roadmap to help guide the wire towards the distal true lumen, avoid side branches, and determine the most suitable reentry zone, thus ensuring that the wire moves in the intended course and reducing the need for contralateral contrast injections.
Figure 5.
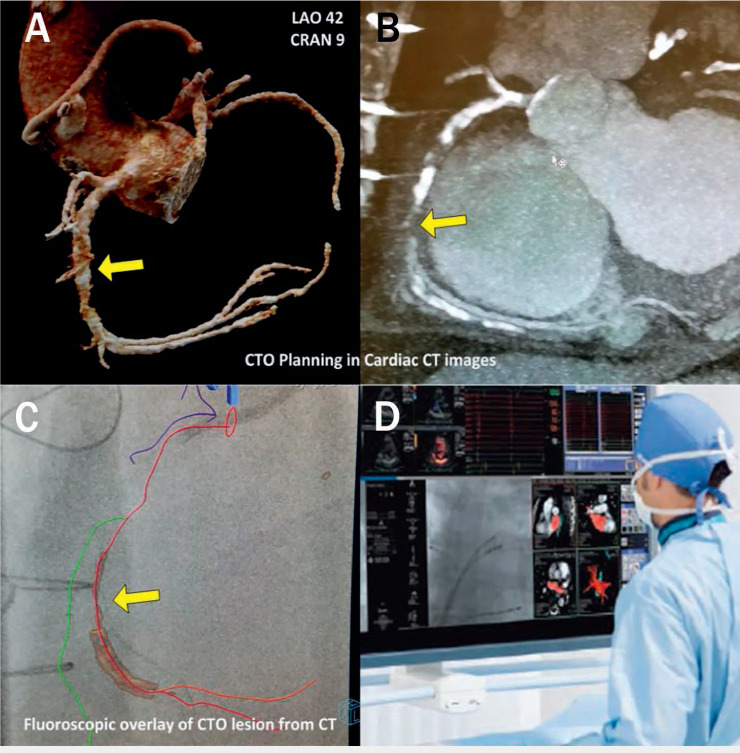
Computed tomography (CT)-guided percutaneous coronary intervention (PCI) of a chronic total occlusion (CTO) during a live case in the cath lab. A shows three-dimensional anatomy of the coronary arteries derived from CT angiography (CTA). B is the multiplanar reformation of a coronary CTA of the right coronary artery (RCA) demonstrating severe calcification with diffuse plaque in the entire RCA and chronic total occlusion in the mid segment. This facilitates identification of projections without foreshortening for each respective vessel segment. C demonstrates the CTA and fluoroscopic data combined to show coronary segments and visualization of the occluded segment in the cath lab. D shows this data in a road map during a CT-guided PCI of a CTO.
HYBRID APPROACH VIRTUAL STENTING
Ihdayhid and colleagues conceptualized an “interventional planner” in which a model is created to simulate a coronary artery after PCI, thus allowing the calculation of a post-PCI FFRCT. Using this preprocedural model to determine postprocedural physiology offers another opportunity to guide the interventionalist with revascularization decision making. We anticipate some feasibility studies in the future.46
LIMITATIONS
Controversy remains regarding the use of anatomical versus functional testing to guide revascularization in patients with stable ischemic heart disease.
In fact, there may be no difference in medically managing patients with intermediate stable ischemic heart disease after ruling out significant left main disease with CCTA. Furthermore, despite recent improvements in mechanical and software-based spatial and temporal resolution, CCTA is still inferior to coronary angiography, especially when evaluating intermediate stenosis in patients with atrial fibrillation or in the presence of high-degree calcifications.46,47
KEY POINTS
Cardiac computed tomography angiography (CCTA) is emerging as the initial test of choice in lowand intermediate-risk patients with suspected coronary artery disease (CAD).
In additional to stenosis assessment, CCTA can depict atherosclerosis burden, determine functional significance of a stenotic lesion, and guide the management and treatment of stable CAD.
Patients with suspected CAD who underwent CCTA as an initial test had similar or possibly better outcomes compared to standard of care.
Acknowledgments
The authors thank Yan Gao, MD, and Na Zhao, MD, for their contributions of cases using CTA perfusion and FFRCT with artificial intelligence.
Footnotes
Conflict of Interest Disclosure:
Dr. Mahmarian is a consultant and on the speakers bureau for Astellas Pharma US.
REFERENCES
- 1.Kolossváry M, Szilveszter B, Merkely B, Maurovich-Horvat P. Plaque imaging with CT-a comprehensive review on coronary CT angiography based risk assessment. Cardiovasc Diagn Ther. 2017 Oct;7(5):489–506. doi: 10.21037/cdt.2016.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moss AJ, Williams MC, Newby DE, Nicol ED. The Updated NICE Guidelines: Cardiac CT as the First-Line Test for Coronary Artery Disease. Curr Cardiovasc Imaging Rep. 2017;10(5):15. doi: 10.1007/s12410-017-9412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreini D, Pontone G, Mushtaq S et al. Image quality and radiation dose of coronary CT angiography performed with whole-heart coverage CT scanner with intra-cycle motion correction algorithm in patients with atrial fibrillation. Eur Radiol. 2018 Apr;28(4):1383–92. doi: 10.1007/s00330-017-5131-2. [DOI] [PubMed] [Google Scholar]
- 4.Chaikriangkrai K, Choi SY, Nabi F, Chang SM. Important advances in technology and unique applications to cardiovascular computed tomography. Methodist Debakey Cardiovasc J. 2014 Jul-Sep;10(3):152–8. doi: 10.14797/mdcj-10-3-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner JS, Smeeth L, Kendall JM, Adams PC, Timmis A, Chest Pain Guideline Development Group NICE guidance. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. Heart. 2010 Jun;96(12):974–8. doi: 10.1136/hrt.2009.190066. [DOI] [PubMed] [Google Scholar]
- 6.Lewis MA, Pascoal A, Keevil SF, Lewis CA. Selecting a CT scanner for cardiac imaging: the heart of the matter. Br J Radiol. 2016 Sep;89(1065):20160376. doi: 10.1259/bjr.20160376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halliburton SS, Abbara S, Chen MY et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr. 2011 Jul-Aug;5(4):198–224. doi: 10.1016/j.jcct.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmadi A, Argulian E, Leipsic J, Newby DE, Narula J. From Subclinical Atherosclerosis to Plaque Progression and Acute Coronary Events: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019 Sep 24;74(12):1608–17. doi: 10.1016/j.jacc.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann U, Udelson JE. Imaging Coronary Anatomy and Reducing Myocardial Infarction. N Engl J Med. 2018 Sep 6;379(10):977–8. doi: 10.1056/NEJMe1809203. [DOI] [PubMed] [Google Scholar]
- 10.Linde JJ, Kelbæk H, Hansen TF et al. Coronary CT Angiography in Patients With Non-ST-Segment Elevation Acute Coronary Syndrome. J Am Coll Cardiol. 2020 Feb 11;75(5):453–63. doi: 10.1016/j.jacc.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Park HB, Heo R, Ó Hartaigh B et al. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging. 2015 Jan;8(1):1–10. doi: 10.1016/j.jcmg.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang SM, Nabi F, Xu J et al. The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol. 2009 Nov 10;54(20):1872–82. doi: 10.1016/j.jacc.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 13.Thomas DM, Divakaran S, Villines TC et al. Management of Coronary Artery Calcium and Coronary CTA Findings. Curr Cardiovasc Imaging Rep. 2015;8(6):18. doi: 10.1007/s12410-015-9334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saremi F, Achenbach S. Coronary plaque characterization using CT. AJR Am J Roentgenol. 2015 Mar;204(3):W249–60. doi: 10.2214/AJR.14.13760. [DOI] [PubMed] [Google Scholar]
- 15.Tamarappoo B, Otaki Y, Doris M et al. Improvement in LDL is associated with decrease in non-calcified plaque volume on coronary CTA as measured by automated quantitative software. J Cardiovasc Comput Tomogr. 2018 Sep-Oct;12(5):385–90. doi: 10.1016/j.jcct.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Nakazato R, Shalev A, Doh JH et al. Aggregate plaque volume by coronary computed tomography angiography is superior and incremental to luminal narrowing for diagnosis of ischemic lesions of intermediate stenosis severity. J Am Coll Cardiol. 2013 Jul 30;62(5):460–7. doi: 10.1016/j.jacc.2013.04.062. [DOI] [PubMed] [Google Scholar]
- 17.Diaz-Zamudio M, Dey D, Schuhbaeck A et al. Automated Quantitative Plaque Burden from Coronary CT Angiography Noninvasively Predicts Hemodynamic Significance by using Fractional Flow Reserve in Intermediate Coronary Lesions. Radiology. 2015 Aug;276(2):408–15. doi: 10.1148/radiol.2015141648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nørgaard BL, Leipsic J, Gaur S et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps) J Am Coll Cardiol. 2014 Apr 1;63(12):1145–55. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 19.Douglas PS, De Bruyne B, Pontone G et al. 1-Year Outcomes of FFRCT-Guided Care in Patients With Suspected Coronary Disease: The PLATFORM Study. J Am Coll Cardiol. 2016 Aug 2;68(5):435–45. doi: 10.1016/j.jacc.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 20.Jensen JM, Bøtker HE, Mathiassen ON et al. Computed tomography derived fractional flow reserve testing in stable patients with typical angina pectoris: influence on downstream rate of invasive coronary angiography. Eur Heart J Cardiovasc Imaging. 2018 Apr 1;19(4):405–414. doi: 10.1093/ehjci/jex068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinnaiyan KM, Safian RD, Gallagher ML et al. Clinical Use of CT-Derived Fractional Flow Reserve in the Emergency Department. JACC Cardiovasc Imaging. 2020 Feb;13(2 Pt 1):452–61. doi: 10.1016/j.jcmg.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Hulten E, Blankstein R, Di Carli MF. The value of noninvasive computed tomography derived fractional flow reserve in our current approach to the evaluation of coronary artery stenosis. Curr Opin Cardiol. 2016 Nov;31(6):970–6. doi: 10.1097/HCO.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tesche C, De Cecco CN, Albrecht MH et al. Coronary CT Angiography-derived Fractional Flow Reserve. Radiology. 2017 Oct;285(1):17–33. doi: 10.1148/radiol.2017162641. [DOI] [PubMed] [Google Scholar]
- 24.Coenen A, Kim YH, Kruk M et al. Diagnostic Accuracy of a Machine-Learning Approach to Coronary Computed Tomographic Angiography-Based Fractional Flow Reserve: Result From the MACHINE Consortium. Circ Cardiovasc Imaging. 2018 Jun;11(6):e007217. doi: 10.1161/CIRCIMAGING.117.007217. [DOI] [PubMed] [Google Scholar]
- 25.Feuchtner GM, Plank F, Pena C et al. Evaluation of myocardial CT perfusion in patients presenting with acute chest pain to the emergency department: comparison with SPECT-myocardial perfusion imaging. Heart. 2012 Oct;98(20):1510–7. doi: 10.1136/heartjnl-2012-302531. [DOI] [PubMed] [Google Scholar]
- 26.Rochitte CE, George RT, Chen MY et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J. 2014 May;35(17):1120–30. doi: 10.1093/eurheartj/eht488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cury RC, Magalhães TA, Borges AC et al. Dipyridamole stress and rest myocardial perfusion by 64-detector row computed tomography in patients with suspected coronary artery disease. Am J Cardiol. 2010 Aug 1;106(3):310–5. doi: 10.1016/j.amjcard.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Ko SM, Choi JW, Song MG et al. Myocardial perfusion imaging using adenosine-induced stress dual-energy computed tomography of the heart: comparison with cardiac magnetic resonance imaging and conventional coronary angiography. Eur Radiol. 2011 Jan;21(1):26–35. doi: 10.1007/s00330-010-1897-1. [DOI] [PubMed] [Google Scholar]
- 29.Mushtaq S, Conte E, Pontone G et al. State-of-the-art-myocardial perfusion stress testing: Static CT perfusion. J Cardiovasc Comput Tomogr. 2019 Sep 12; doi: 10.1016/j.jcct.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Becker A, Becker C. CT imaging of myocardial perfusion: possibilities and perspectives. J Nucl Cardiol. 2013 Apr;20(2):289–96. doi: 10.1007/s12350-013-9681-7. [DOI] [PubMed] [Google Scholar]
- 31.Rief M, Chen MY, Vavere AL et al. Coronary Artery Disease: Analysis of Diagnostic Performance of CT Perfusion and MR Perfusion Imaging in Comparison with Quantitative Coronary Angiography and SPECT-Multicenter Prospective Trial. Radiology. 2018 Feb;286(2):461–70. doi: 10.1148/radiol.2017162447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fihn SD, Gardin JM, Abrams J et al2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2012 Dec 18;126(25):e354–471. doi: 10.1161/CIR.0b013e318277d6a0. .; American College of Cardiology Foundation/American Heart Association Task Force. [DOI] [PubMed] [Google Scholar]
- 33.Taylor AJ, Cerqueira M, Hodgson JM et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010 Nov 23;56(22):1864–94. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Li Yuehua, Yu Mengmeng, Dai Xu et al. Detection of Hemodynamically Significant Coronary Stenosis: CT Myocardial Perfusion versus Machine Learning CT Fractional Flow Reserve. Radiology. 2019 doi: 10.1148/radiol.2019190098. [DOI] [PubMed] [Google Scholar]
- 35.Newby DE, Adamson PD, Berry C et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med. 2018 Sep 6;379(10):924–33. doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 36.Hulten E, Bittencourt MS, Singh A et al. Coronary Artery Disease Detected by Coronary Computed Tomographic Angiography Is Associated With Intensification of Preventive Medical Therapy and Lower Low-Density Lipoprotein Cholesterol. Circ Cardiovasc Imaging. 2014 Jul;7(4):629–38. doi: 10.1161/CIRCIMAGING.113.001564. [DOI] [PubMed] [Google Scholar]
- 37.Collet C, Onuma Y, Andreini D et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J. 2018 Nov 1;39(41):3689–98. doi: 10.1093/eurheartj/ehy581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshitaka Goto Y, Kawasaki T, Koga N et al. Plaque distribution patterns in left main trunk bifurcations: prediction of branch vessel compromise by multidetector row computed topography after percutaneous coronary intervention. EuroIntervention. 2012 Oct;8(6):708–16. doi: 10.4244/EIJV8I6A110. [DOI] [PubMed] [Google Scholar]
- 39.Nakazawa G, Tanabe K, Onuma Y et al. Efficacy of culprit plaque assessment by 64-slice multidetector computed tomography to predict transient no-reflow phenomenon during percutaneous coronary intervention. Am Heart J. 2008 Jun;155(6):1150–7. doi: 10.1016/j.ahj.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 40.de Araújo Gonçalves P, Campos CA, Serruys PW, Garcia-Garcia HM. Computed tomography angiography for the interventional cardiologist. Eur Heart J Cardiovasc Imaging. 2014 Aug;15(8):842–54. doi: 10.1093/ehjci/jeu053. [DOI] [PubMed] [Google Scholar]
- 41.Konen E, Goitein O, Sternik L, Eshet Y, Shemesh J, Di Segni E. The pravalence and anatomical patterns of intramuscular coronary arteries: a coronary computed tomography angiographic study. J Am Coll Cardiol. 2007 Feb 6;49(5):587–93. doi: 10.1016/j.jacc.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 42.Choi JH, Kim EK, Kim SM et al. Noninvasive Discrimination of Coronary Chronic Total Occlusion and Subtotal Occlusion by Coronary Computed Tomography Angiography. JACC Cardiovasc Interv. 2015 Aug 17;8(9):1143–53. doi: 10.1016/j.jcin.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 43.Yu CW, Lee HJ, Suh J et al. Coronary Computed Tomography Angiography Predicts Guidewire Crossing and Success of Percutaneous Intervention for Chronic Total Occlusion: Korean Multicenter CTO CT Registry Score as a Tool for Assessing Difficulty in Chronic Total Occlusion Percutaneous Coronary Intervention. Circ Cardiovasc Imaging. 2017 Apr;10(4):e005800. doi: 10.1161/CIRCIMAGING.116.005800. [DOI] [PubMed] [Google Scholar]
- 44.Opolski MP, Achenbach S. CT Angiography for Revascularization of CTO: Crossing the Borders of Diagnosis and Treatment. JACC Cardiovasc Imaging. 2015 Jul;8(7):846–58. doi: 10.1016/j.jcmg.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Patel VG, Brayton KM, Tamayo A et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013 Feb;6(2):128–36. doi: 10.1016/j.jcin.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Ihdayhid AR, White A, Ko B. Assessment of Serial Coronary Stenoses With Noninvasive Computed Tomography-Derived Fractional Flow Reserve and Treatment Planning Using a Novel Virtual Stenting Application. JACC: Cardiovasc Interv. 2017 Dec 26;10(24):e223–e225. doi: 10.1016/j.jcin.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Colombo A, Giannini F. Is it time to replace conventional angiography with coronary computed tomography? Eur Heart J. 2018 Nov 1;39(41):3699–700. doi: 10.1093/eurheartj/ehy578. [DOI] [PubMed] [Google Scholar]


