Abstract
α-Melanocyte stimulating hormone (α-MSH) and adrenocorticotropic hormone (ACTH) possess properties suggesting that they may be involved in the pathogenesis of restless legs syndrome (RLS). We sought to determine if α-MSH and ACTH when administered centrally in rat recapitulate features reminiscent of RLS: increased activity, sleep fragmentation, and periodic movements during sleep. Rats were instrumented with electroencephalography, electromyography, and intracerebral cannulae and recorded for the measurement of sleep, periodic movements, and behavior following intracerebroventricular administration of α-MSH, ACTH, or saline. Studied behavior included grooming, locomotion, and rearing during wake and limb movements during sleep. Vigilance states included active wake (AW), quiet wake (QW), slow wave sleep I (SWSI), slow wave sleep II (SWSII), and paradoxical sleep (PS). All rats received normal saline acting as their own controls. Different rats received α-MSH in doses of 0.05, 0.5, 1.0, 2.0, and 6.0 μg or ACTH in doses of 0.5, 1.0, and 2.0 μg. Administered α-MSH caused an increase in waking behavior and prolongation of sleep latency, while ACTH stimulated waking behavior and fragmented sleep, yielding more AW and less SWSII and PS. Both hormones increased periodic movements during sleep. When administered centrally in rat, α-MSH and ACTH stimulate motor activity in wake, cause changes in sleep architecture, and increase periodic movements in sleep. These melanocortin hormones may play a role in the pathogenesis of RLS.
Keywords: RLS, animal, MSH, ACTH, hormone
Restless legs syndrome (RLS) is a common condition affecting between 5 and 10% of the general population.1–4 Essential RLS criteria include an urge to move often associated with discomfort, aggravation of symptoms with repose and in evening hours, and temporary symptom relief afforded by movement.5 Sleep-onset insomnia and periodic limb movements (PLMS) during sleep are additional but nonessential features of this syndrome.
Despite its clinical significance, the underlying physiologic mechanisms of RLS are poorly understood, in part due to a limited availability of suitable animal models. Challenges in creating an RLS animal model may stem from the lack of objective criteria and the verbal communication necessary to make the clinical diagnosis. In an animal model, the use of behavior and activity, surrogate for the central urge to move of RLS, becomes particularly important. Adjunctive features of RLS including PLMS and sleep fragmentation can be objectively measured and may aid in forming a credible RLS model. These three quantifiable features, activity, sleep, and PLMS, have each been studied in the past studies6–10 but have not been combined in a single animal model.
Currently, the majority of RLS animal work has focused on the dopaminergic system. Endocrine function and its potential association with RLS have not been studied in animals. A hormonal basis for RLS is suggested by its circadian variability and association with pregnancy. In a literature review, we found that adrenocorticotropic hormone (ACTH) and α-melanocyte stimulating hormone (α-MSH) possess properties congruent to features of RLS. Both melanocortin hormones possess similar physiologic properties as they share a 13 amino acid sequence (the entirety of α-MSH) and a common precursor protein, pro-opiomelanocortin (POMC).11
First, ACTH and α-MSH possess properties reminiscent of RLS diagnostic criteria. Both α-MSH and ACTH may cause an urge to move as they are well documented to stimulate activity in the rat, namely, locomotion, rearing, and grooming.12,13 Additionally, the melanocortins may contribute to the sensory discomfort common in RLS as both α-MSH and ACTH are involved in nociception, producing hyperalgesia when administered in the rat.14,15 α-MSH and ACTH exhibit a circadian pattern of release with hypothalamic levels of α-MSH increasing with darkness in the Texas toad.16 Finally, when administered intravenously in women, α-MSH causes “motor restlessness,”17 formerly a diagnostic criterion for RLS.
Levels of α-MSH and ACTH are elevated in conditions associated secondarily with RLS. Both hormones are elevated in the serum of pregnant women and end-stage renal patients undergoing hemodialysis.18–20 α-MSH and ACTH expressions are likely elevated in peripheral neuropathy as both hormones aid in processes of nerve remodeling and repair.21,22
Location of α-MSH and ACTH binding is also consistent with presumed RLS localization. Sensory discomfort in RLS is associated with increased activity in the thalamus and cerebellum as measured by functional MR1,23 whereas PLMS likely arise from the spinal cord.24 ACTH and α-MSH bind identical melanocortin receptors that are widely distributed throughout the central nervous system (CNS) but of note are found in hypothalamus, cerebellum, thalamus, and spinal cord.25,26 In an existing animal model of RLS, lesioning of diencephalic dopaminergic A11 neurons resulted in increased rearing and sleep latency in rat.6 The A11 dopaminergic system is particularly intriguing as it combines brain and spinal localizations, as it begins in the diencephalon with fibers in the hypothalamus and periventricular thalamus and projects to the spinal cord.27 Melanotropinergic neurons also reside in these areas, specifically arcuate nucleus, dorsomedial and posterior hypothalamus, periventricular thalamus, and dorsal horns of the spinal cord.11
Current understanding about RLS pathogenesis surrounds the dopaminergic and iron-handling systems. ACTH and α-MSH each have physiologic associations with dopamine and iron.28,29 Dopamine is involved in the regulation of α-MSH and ACTH. In rat hypothalamus, dopamine-2-receptor blockade results in increased mRNA levels of POMC,28 the precursor for both ACTH and α-MSH, suggesting that dopamine inhibits ACTH and α-MSH expression. Furthermore, exposure of red porgy and frog hypothalamic cells to dopamine inhibits α-MSH release.30,31
Iron and its handling are likely modulated by ACTH and α-MSH. Iron deposits in brain are found in deep pigmented nuclei, rich in neuromelanin. Iron is bound avidly by neuromelanin, the CNS analog of the skin melanocyte, the activity of which depends on ACTH or α-MSH binding.32,33 Neuropathologically, brains of RLS patients contain neuromelanin with decreased ferritin and transferrin receptor density.34 Decreased iron stores and iron-depleted neuromelanin may reflect quantitative or qualitative alterations in either ACTH or α-MSH; conversely primary iron abnormalities may cause alterations in ACTH and/or α-MSH expression. More acutely, ACTH when administered in rabbit causes a decrease in serum iron and iron-binding capacity.29
In total, that many features of RLS bear similarity to properties of ACTH and α-MSH make the melanocortins intriguing molecular candidates possibly underlying RLS pathogenesis. These similarities led us to pursue this study in which we attempt to capture features of RLS in rat by observing activity, sleep, and periodic movements during sleep. We expected to find that ACTH and α-MSH would stimulate grooming, locomotion, and rearing in wakefulness, affect sleep continuity, and produce periodic movements during sleep when administered centrally in rat.
METHODS
Animals and Surgical Procedures
The protocol was approved by the Institutional Animal Care and Use Committee of the Veterans Administration Hospital in Cleveland. Male Long-Evans rats between 90 and 120 days old were used in the study and housed under standard 12-hour light-dark conditions. Much of the surgical technique is described elsewhere,35 but briefly animals were anesthetized with pentobarbital 50 mg/kg. Four stainless steel screws (080 × 1/8, Plastics One, Roanoke, VA) were implanted stereotactically in frontal, parietal, and occipital regions of the skull to derive electroencephalography (EEG). Steel springs 0.5 mm in diameter soldered to 50 gauge braided wire (Cooner Wire, Chatsworth, CA) were implanted subcutaneously overlying anterior tibialis and gastrocnemius muscles on both hindlimbs, deriving hindlimb electromyography (EMG). Nuchal EMG was constituted by placing steel springs affixed to wire deep to levator scapulae muscles bilaterally. Finally, a 22 gauge stainless steel cannula (Plastics One) was placed into the left cerebral ventricle for administration of agents. Electrodes were soldered to a 12 pin connector, which was secured to the skull with dental acrylic. Animals were left to recover for 9 days.
Intracerebroventricular (i.c.v.) Agents
All injections were given i.c.v., measured 6 μL, and occurred within 15 min of the initiation of subjective night (“lights on”). During injection, animals were removed from their cage and placed on a desktop, where they were stabilized by gentle handling while injections were given over 20 to 30 seconds. On postoperative day 2, 0.12 μg of angiotensin II was administered with an ensuing drinking behavior indicating correct cannula placement. Injections included normal saline, acetylated α-MSH in doses of 0.05, 0.5, 1.0, 2.0, and 6.0 μg and ACTM1–24 in doses of 0.5, 1.0, and 2.0 μg. The administering investigator (BK) was blinded to the injectant identity.
Behavioral Assessment
Behavior was observed and scored 10 min after injection for 1 hour by video. Behavioral assessment was carried out by an investigator (BK) blinded to the nature of the administered injectant. Noted behaviors included rearing and locomotion, quantified as episodes and grooming, which was timed. Rearing occurred if both forepaws were maintained off the cage floor, whereas a locomotive episode consisted of walking between adjacent or diagonal chamber comers. Grooming included scratching, face wiping, or licking of any body part.
Signal Recording
EEG was derived from the reference of parietal to occipital electrodes, while referencing neck electrodes to one another constituted nuchal EMG. Hindlimb EMG was derived from referencing contralateral anterior tibialis and gastrocnemius electrodes. EEG signals were amplified 5,000 times and filtered on the low and high end at 0.01 and 100 Hz, respectively, using a digital polygraph (Model 15, Astro-Grass). EMG signals were amplified 5,000 times, unfiltered at the high end, and filtered at 5 Hz on the low end. The amplified signals were converted digitally using a data acquisition board (PCI-6040E, National Instruments) and recorded using Somnologica software (Medcare, Buffalo, NY).
Recording of EEG, hindlimb EMG, nuchal EMG, and video was initiated on postoperative day 10 and occurred every other day thereafter. Recordings lasted between 11.75 and 12 hours, occurred only during the light phase and were initiated within 15 min of the same time each day.
Data Scoring
Sleep
Vigilance states were scored in 30-second epochs by an investigator (BK) blinded to the received injectant and included: active wake (AW), quiet wake (QW), slow wave sleep I (SWSI), slow wave sleep II (SWSII), and paradoxical sleep (PS). Sleep was scored for the remaining light period (11.75–12 hours) immediately following injection. AW and QW were differentiated by the presence or absence respectively of physical activity demonstrated by EMG. SWSI was differentiated from SWSII by the presence of delta activity less than or greater than 50% of an epoch, respectively. PS was characterized by low amplitude, high-frequency EEG with low nuchal EMG tone. Arousal was scored if an increase in EEG frequency occurred from a sleep state for ≥2 seconds.
Each vigilance state was presented as percentage of total recording time for a given period. Total number of arousals divided by total sleep in hours yielded arousal index. Data are presented in 12-hour periods and further presented in 3-hour quartiles.
Limb Movements
LM were scored by an investigator (BK) blinded to injectant identity. The record was scanned for EEG arousal and/or elevation of nuchal or hindlimb EMG activity during sleep. A LM was scored only if verified by video. LM included dorsiflexion of the foot, flexion at the knee, flexion at the hip, and less often flexion of the upper extremities. Head flexion and tail movement often co-occurred with LM. PLMS were scored if a sequence of at least four LM occurred with an intermovement interval between 2 and 90 seconds.
Limb movement index (LMI) was calculated by considering the number of LM per total hours of sleep. Periodic limb movement index (PLMI) was calculated similarly using PLMS.
Statistical Analysis
Data were analyzed using one-way analysis of variance and, when indicated, post hoc testing included one-way independent Student’s t testing with Sidak’s correction for the comparison of multiple variables. Pairwise t testing was used for analysis of PLMI, LMI, and AI as animals served as their own controls. Spearman’s correlational testing was used to determine dose-response relationships. Analyses were performed on the statistical software, R (University of Auckland) and SPSS (Chicago, IL).
RESULTS
α-MSH Behavioral Data
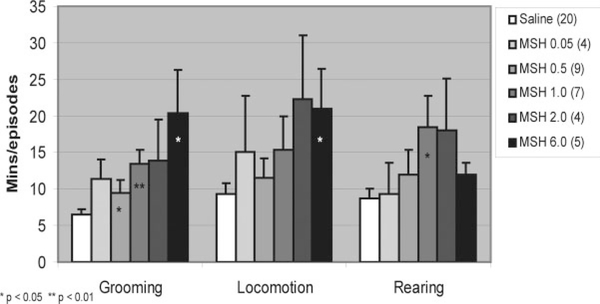
Increases were observed for each of the studied behaviors, grooming, locomotion, and rearing, subsequent to the administration of α-MSH compared to saline (see Fig. 1). Data are presented as means ± standard error as they are in all figures. Animals receiving i.c.v. normal saline (n = 20) groomed for an average of 6.5 ± 0.8 min and had episodes of locomotion and rearing averaging 9.4 ± 1.5 and 8.7 ± 1.3, respectively. Grooming time was increased by α-MSH in all doses, 0.05 μg (n = 4, 11.4 ± 2.7, P = 0.08), 0.5 μg (n = 9, 9.5 ± 1.8, P = 0.03), 1.0 μg (n = 7, 13.4 ± 2.0, P = 0.006), 2.0 μg (n = 4, 13.9 ± 5.6, P = 0.08), and 6.0 μg (n = 5, 20.3 ± 6.0, P = 0.04), but only significantly so for 1.0 μg.
FIG. 1.
Behavior after administration of α-MSH. Rats groomed significantly longer with 1.0 μg MSH and tended to groom more for 0.5 and 6.0 μg doses. Trends for increased locomotion with 6.0 μg MSH and rearing with 1.0 μg MSH were seen.
Trends toward an increase in locomotion were seen for α-MSH in all doses, 0.05 μg (15.0 ± 7.8, P = 0.24), 0.5 μg (11.6 ± 2.6, P = 0.12), 1.0 μg (15.4 ± 4.5, P = 0.10), 2.0 μg (22.3 ± 8.7, P = 0.06), and 6.0 μg (21.0 ± 5.4, P = 0.04). Trends toward an increase in rearing episodes were seen for 0.5 μg (12.0 ± 3.3, P = 0.10), 1.0 μg (18.4 ± 4.2, P = 0.03), 2.0 μg (18.0 ± 7.1, P = 0.07), and 6.0 μg (12.0 ± 1.6, P = 0 07)
Significant dose-response relationships were seen between α-MSH and grooming (ρ = 0.485, P < 0.001), locomotion (ρ = 0.382, P = 0.004), and rearing (ρ = 0.362, P = 0.007).
α-MSH Sleep Data
Sleep latency (min) was significantly prolonged after the administration of α-MSH in doses of 1.0 μg (n = 6, 36.9 ± 5.1, P = 0.01) and 6.0 μg (n = 5, 40.9 ± 6.9, P = 0.01) when compared with sleep latency after giving saline (n = 18, 22.2 ± 3.2). Neither vigilance states nor arousal index was significantly changed by any dose of α-MSH.
α-MSH Limb Movement Data
Limb movement data were analyzed pairwise as animals served as their own controls after receiving saline. Comparison of means was not appropriate because of heterogeneity in the number of LM for different rats given saline and subsequently hormone.
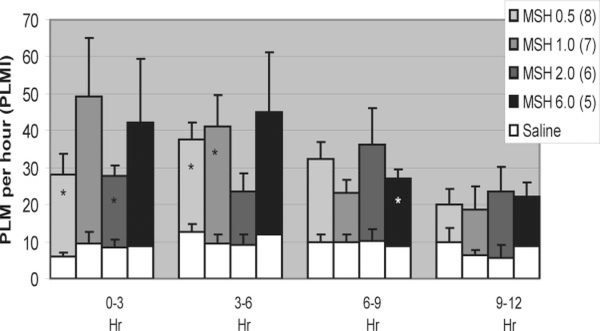
Differences in the LMI and PLMI were seen for differing α-MSH doses for the first three time periods (Fig. 2, only PLMI shown). In Figure 2, each column represents PLMI of animals given saline (white) as well as PLMI for the same animals given α-MSH (patterned) at the specified dose.
FIG. 2.
After MSH administration, PLMI increased significantly at 0 to 3 hours for 0.5 and 2.0 μg, at 3 to 6 hours for 0.5 and 1.0 μg and at 6 to 9 hours for 6.0 μg. No significant differences were seen in the time between 9 and 12 hours.
In the 0- to 3-hour period, significant increases in LMI were seen for α-MSH in doses of 0.5 μg (n = 8, 43.0 ± 4.8 vs. 26.0 ± 1.7, P = 0.008) and in PLMI for 0.5 μg (n = 8, 22.3 ± 5.5 vs. 5.9 ± 1.2, P = 0.01) and 2.0 μg (n = 6, 19.4 ± 3.1 vs. 8.3 ± 2.2, P = 0.01). In the 3- to 6-hour period, significant increases in LMI were seen for 0.5 μg (n = 8, 43.6 ± 4.4 vs. 30.9 ± 2.4, P = 0.01), 1.0 μg (n = 7, 48.4 ± 7.0 vs. 28.6 ± 2.8, P = 0.006), and 2.0 μg (n = 6, 36.5 i 4.6 vs. 26.9 ± 3.2, P = 0.004) doses of α-MSH and in PLMI for 0.5 (n = 8, 25.0 ± 4.6 vs. 12.5 ± 2.4, P = 0.008) and 1.0 μg (n = 7, 31.7 ± 8.4 vs. 9.6 ± 2.4, P = 0.01) doses. In the 6- to 9-hour period, significant increases were seen for 6.0 μg of α-MSH in both LMI (n = 5, 39.1 ± 3.5 vs. 28.6 ± 2.2, P = 0.007) and PLMI (n = 5, 18.4 ± 2.4 vs. 8.8 ±2.1, P = 0.002). There were no significant increases in the 9- to 12-hour period.
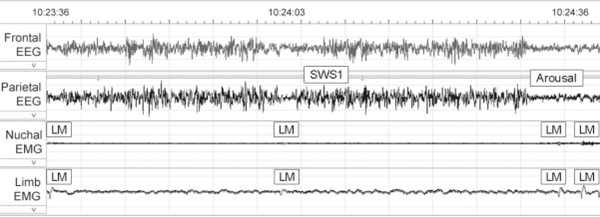
Dose-response relationships were seen between α-MSH and PLMI for the 0- to 3-hour time interval (ρ = 0.517, P = 0.001) and the 6- to 9-hour interval (ρ = 0.319, P = 0.04). Individual movements can be seen in Video 1 and correlate to signals shown in the polysomnographic epoch in Figure 3. Additional movements can be seen in Video 2.
FIG. 3.
Shown is a 1 min epoch showing electroencephalography (EEG) and electromyography (EMG) of both neck and hindlimbs. Correlate to video 1. Four limb movements occur associated with increased signal in both neck and hindlimb EMG and arousal as indicated by EEG. Times indicated on the EEG correspond to times on video 1 which has been edited. Note the subtle movements on the video which appear to involve multiple muscle groups.
ACTH Behavioral Data
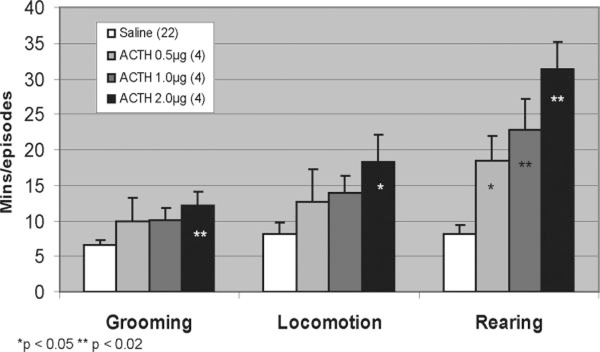
Increases in grooming, locomotion, and rearing were seen with all doses of ACTH (see Fig. 4). After receiving saline, rats (n = 23) groomed for 6.6 ± 0.8 min and had locomotive and rearing episodes averaging 8.2 ± 1.6 and 8.1 ± 1.3, respectively. With ACTH 0.5 μg (n = 4), trends were seen for increases in grooming time (10.0 ± 3.3 min, P = 0.19), number of locomotive episodes (12.8 ± 4.5, P = 0.23), and number of rearing episodes (18.5 ± 3.5, P = 0.03). For ACTH 1.0 μg (n = 4), a significant increase was seen for the number of rearing episodes (22.8 ± 4.4, P = 0.02), and trends were seen for increased grooming time (10.1 ± 1.7, P = 0.06) and locomotive episodes (14.0 ± 2.4, P = 0.06). For ACTH 2.0 μg (n = 4), significant elevations were seen for grooming time (12.1 ± 2.0 min, P = 0.02) and rearing episodes (31.3 ± 4.0, P = 0.003). A trend was observed for increased locomotive episodes (18.3 ± 3.8, P = 0.04).
FIG. 4.
With ACTH administration, increases in grooming, locomotion and rearing occurred in dose-dependent manners. ACTH at 2.0 μg significantly increased grooming, locomotion, and rearing, while lower doses only significantly increased rearing episodes.
Significant dose-response relationships were seen between ACTH and all studied behaviors: grooming (ρ = 0.373, P = 0.016), locomotion (ρ = 0.454, P = 0.004), and rearing (ρ = 0.696, P < 0.001).
ACTH Sleep Data
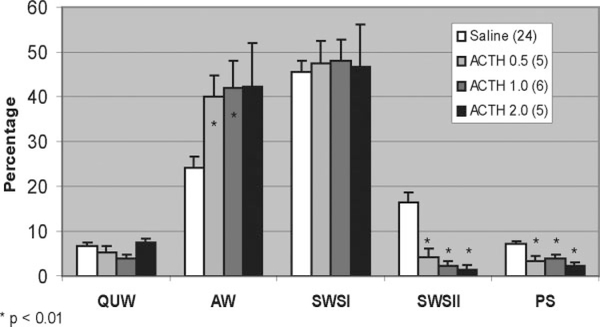
Changes in sleep staging occurred only in the first 3 hours of the sleeping period (see Fig. 5). In this 0- to 3-hour period, saline rats (n = 24) had a sleep latency of 16.7 ± 2.4 min, and their percentages of QW, AW, SWSI, SWSII, and PS were 6.6 ± 0.8, 24.3 ± 2.3, 45.7 ± 2.3, 16.2 ± 2.4, and 7.1 ± 0.6, respectively. Significant changes were seen in AW, SWSII, and PS for all ACTH doses except for 2.0 μg when considering AW.
FIG. 5.
In the initial 0- to 3-hour period, all doses of ACTH significantly reduced paradoxical sleep (PS) and slow wave sleep II (SWSII), while the lower doses significantly increased active wake (AW).
Animals receiving 0.5 μg of ACTH (n = 5) had significantly more AW (39.9 ± 4.7, P = 0.008) and significantly less SWSII (4.2 ± 2.0, P = 0.0001) and PS (3.2 ± 1.1, P = 0.004). For ACTH 1.0 μg (n = 6), rats had significantly more AW (42.0 ± 6.0, P = 0.015) and significantly less SWSII (2.2 ± 1.2, P = 0.000004) and PS (3.8 ± 0.9, P = 0.005). For ACTH 2.0 μg (n = 5), rats trended toward significant increases in AW (42.2 ± 9.8, P = 0.05) and had significantly reduced SWSII (1.52 ± 1.0. P = 0.000002) and PS (2.2 ± 0.9, P = 0.0003).
Arousal index that was significantly elevated for ACTH 1.0 μg (n = 6, 35.6 ± 2.2 vs. 23.3 ± 1.8,P = 0.006) and showed trends toward significant elevation at 0.5 μg (n = 5, 32.0 ± 4.0 vs. 22.3 ± 1.9, P = 0.025) and 2.0 μg (n = 4, 59.0 ±21.9 vs. 22.0 ± 2.6, P = 0.05) doses.
Dose-response relationships were significant between ACTH and AW (ρ = 0.523, P < 0.001), SWSII (ρ = −0.691, P < 0.001), and PS (ρ = −0.619, P < 0.001).
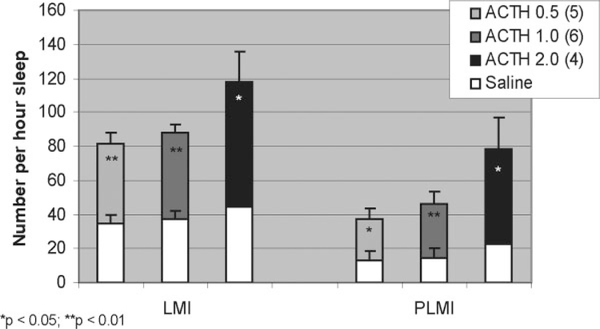
ACTH Limb Movement Data
As were sleep stages, LMI and PLMI were only significantly different in the 0- to 3-hour period (see Fig. 6). The format of this figure is the same as that for Figure 2. LMI and PLMI data for ACTH were analyzed pairwise. In the 0- to 3-hour period, LMI was significantly increased for ACTH at 0.5 (n = 5, 46.8 ± 6.2 vs. 34.7 ± 4.7, P = 0.014) and 1.0 μg (n = 6, 50.4 ± 5.6 vs. 37.3 ± 5.0, P = 0.004) and trended toward increases at 2.0 μg (n = 5, 72.0 ± 13.6 vs. 44.3 ± 1.9. P = 0.047). In this same period, PLMI was significantly increased for ACTH 1.0 (n = 6, 31.5 ± 6.9 vs. 14.8 ± 5.4, P = 0.006) and showed trends toward elevation at 0.5 (n = 5, 23.8 ± 6.6 vs. 13.1 ± 5.7, P = 0.03) and 2.0 μg (n = 5, 52.8 ± 15.0 vs. 22.2 ± 2.5, P = 0.03).
FIG. 6.
In the initial 0- to 3-hour period, both limb movement index (LMI) and periodic limb movement index (PLMI) were significantly elevated at all doses of ACTH.
Significant dose-response associations were observed for both LMI (ρ = 0.440, P = 0.02) and PLMI (ρ = 0.719, P < 0.001). A separate analysis of four animals for each administered dose of ACTH, α-MSH, and saline (n = 32) was carried out to separate PLMI in rapid-eye-movement (REM) and non-rapid-eye-movement (NREM) sleep. REM PLMI index was 2.9 ± 0.5, and NREM PLMI index was 23.9 ± 2.6. NREM PLMI was significantly higher than REM PLMI (P < 0.0001).
DISCUSSION
Both ACTH and α-MSH stimulated motor activity, caused hyperarousal, and increased movements during sleep. The urge to move was estimated indirectly by measuring increased activity. For this dimension, three measures—locomotion, rearing, and grooming—were detected by video monitoring. Grooming consistently showed significant increases, rearing was increased significantly only by higher ACTH doses, and locomotion only trended toward elevation. All behaviors increased in significant dose-dependent manners for both ACTH and α-MSH. In our analysis of behavior, we confirmed well-established findings regarding ACTH, α-MSH, and their stimulatory effects on locomotion, rearing, and grooming,12,13,36–38 while newly applying these data to the urge to move of RLS. Speculatively, excessive grooming may additionally demonstrate sensory discomfort as animals displaying this behavior invariably scratch vigorously and physiologically, ACTH and α-MSH mediate nociception as central administration in rat produces hyperalgesia,14,15 a state present in RLS.39
Sleep was measured by EEG and EMG measurement. Both ACTH and α-MSH affected sleep architecture, α-MSH did not affect staging but significantly prolonged sleep latency. ACTH altered sleep staging by increasing AW and arousal index while decreasing SWSII and PS. Increases in AW may particularly germane in the study of RLS as patients with this condition often demonstrate activity near sleep onset and upon awakening. These observed effects are similar to reports in RLS patients, which demonstrate prolonged sleep latency, increased stage one and increased awakenings.40
Although not required for diagnosis, PLMS may be more specific to RLS than either activity or sleep fragmentation.41–44 Our measurement, LM as expressed by index (LMI), was significantly and dose-dependently increased by both ACTH and α-MSH. In further quantifying LM, we applied similar but refined human measurements of periodicity (PLMI), which too demonstrated significant and dose-dependent increases with the application of either ACTH or α-MSH.
As described previously, LM was heterogeneous and included dorsiflexion and plantarflexion of hindpaws and flexion of femorotibial, sacroiliac and radiohumeral joints, tail, head, and paraspinal muscles. This heterogeneity is not unlike the wide variation that has been described in human PLMS, even when measured in one individual. Muscles involved in human PLMS may include tibialis anterior, gastrocnemius, biceps femoris, triceps brachii, rectus abdominus, paraspinal muscles,45 and head muscles being involved anecdotally. Also consistent with PLMS in humans, the observed rat LM occurred significantly more often in NREM sleep than in REM sleep.46
All interesting issue to address was the appearance of LM in control animals in numbers higher than expected and observed by other groups.8 This apparent paradox might be explained by stress and endogenous release of ACTH, levels of which can be elevated up to 40 min by simple removal of rats from grouped housing to a ventilated jar for 3 min.47 Our work differs from the aforementioned study of LM in that it included placement of i.c.v. cannulae and i.c.v. injection of hormone. Direct injection into the ventricular system could have provoked the release of ACTH. This explanation is supported by the finding that control animals in addition to having increased LM spent a greater than expected amount of time grooming, a well-studied effect of i.c.v. ACTH in the rat.12,13,36–39
Although being novel and potentially relevant to RLS, these observations should be interpreted with some degree of caution. The possibility exists that the observed findings constitute a nonspecific stress response appropriately evoked by the application of stress hormones. Of most concern is the potential that the movements scored as LM and possibly assigned value as rodent PLMS could simply be nonspecific. Given the dearth of experience with PLMS in the rodent,8,10 presently, this issue is difficult to resolve; however, the scored LM could certainly represent an amalgam of PLMS, myoclonus, and additional nonspecific movement. Patients with RLS in addition to demonstrating increased numbers of PLMS may display supplementary propriospinal myoclonus characterized by trunk and limb jerking during quiet wakefulness.48 Future consensus on the scoring of rodent PLMS is certainly renuired to clarify this confusion.
In addition, hindlimb EMG, used to detect these movements, proved insensitive making dependence on video necessary. Although the scorer was blinded to the injectant identity, video scoring of LM undoubtedly increased subjectivity to levels greater than desired. Video scoring of PLMS in the rodent is suboptimal for several reasons: (1) rodent sleep posture may obscure limb visualization, possibly leading to count underestimation, (2) different camera angles and differing sleep posture add additional variability, again altering count, and (3) temporally, it is cumbersome. A consensus approach to the scoring of rodent LM would help in developing future RLS animal models.
Behavioral assessment also was carried out visually using video. Other studies have used laser technology taking into account the duration of movement.6 Last, our model used exogenous administration of hormone, unfortunately making testing of dopamine agonists unsuitable as dopamine acts upstream, inhibiting the release of these hormones.30,31 A model of endogenous overexpression and/or release of the studied peptides is more desirable.
Although our study is not a comprehensive RLS animal model, it may provide impetus for future animal work and bridge the present foci of RLS pathophysiology, the dopaminergic system6–8 and iron deficiency.9,49 The melanocortin system colocalizes with iron rich pigmented nuclei in the brain and dopaminergic A11 neurons in brain and spinal cord.11,27,33 Melanocortin release and expression are regulated by dopamine,28,30,31 while their binding may modulate iron levels in serum and brain.29,33 Undoubtedly, further investigation is needed to clarify interactions among these diverse systems. To this end, the current model may provide a framework for future animal and human work in RLS and at the same time open an alternative approach when considering the pathogenesis of RLS.
Acknowledgments
This work was supported bv a T-32 NIH training grant (HL 07913) and the Veterans Administration Research Service. We thank Nan Tian for his excellent technical assistance. We thank Yufen Hu and Drina Vurbic for their help with animal handling and the injections.
Footnotes
This article includes supplementary video clips, available online at http://www.interscienc.wiley.com/ipages/0885-3185/suppmat
LEGENDS TO VIDEO
Segment 1. Individual movements can be seen in Segment 1 and correlate to signals shown in the polysomnographic epoch in Figure 3. Additional movements can be seen in Segment 2.
Segment 2. This video consists of several different individual limb movements in different animals. Note the heterogeneity of movements which involve differing muscle groups which is further complicated by the assumption of various sleeping posture by the animal.
REFERENCES
- 1.Tison F, Crochard A, Leger D, Bouee S, Lainey E, El Hasnaoui A. Epidemiology of restless legs syndrome in French adults: a nationwide survey: the INSTANT study. Neurology 2005;65:239–246. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med 2005;165:1286–1292. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res 2002;53:547–554. [DOI] [PubMed] [Google Scholar]
- 4.Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini-Strambi L. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med 2004;5:237–246. [DOI] [PubMed] [Google Scholar]
- 5.Walters A. The international restless legs syndrome study group: toward a better definition of the restless legs syndrome. Mov Disord 1995;10:634–642. [DOI] [PubMed] [Google Scholar]
- 6.Ondo WG, He Y, Rajasekaran S, Le WD. Clinical correlates of 6-hydroxydopamine injections into A11 dopaminergic neurons in rats: a possible model for restless legs syndrome. Mov Disord 2002;15:154–158. [DOI] [PubMed] [Google Scholar]
- 7.Clemens S, Hochman S. Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J Neurosci 2004;24:11337–11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baier PC, Winkelmann J, Höhne A, Lancel M, Trenkwalder C. Assessment of spontaneously occurring periodic limb movements in sleep in the rat. J Neurol Sci 2002;198:71–77. [DOI] [PubMed] [Google Scholar]
- 9.Dean T Jr, Allen RP, O’Donnell CP, Earley CJ. The effects of dietary iron deprivation on murine circadian sleep architecture. Sleep Med 2006;7:634–640. [DOI] [PubMed] [Google Scholar]
- 10.Esteves AM, de Mello MT, Lancellotti CL, Natal CL, Tufik S. Occurrence of limb movement during sleep in rats with spinal cord injury. Brain Res 2004;1017:32–38. [DOI] [PubMed] [Google Scholar]
- 11.O’Donohue T. The opiomelanotropinergic neuronal and endocrine systems. Peptides 1982;3:353–395. [DOI] [PubMed] [Google Scholar]
- 12.Torre E, Celis ME. Cholinergic mediation in the ventral tegmental area of α-melanotropin induced excessive grooming: changes of the dopamine activity in the nucleus accumbens and caudate putamen. Life Sci 1988;42:1651–1657. [DOI] [PubMed] [Google Scholar]
- 13.Isaacson RL, Green EJ. The effect of ACTH1–24 on locomotion, exploration, rearing, and grooming. Behav Biol 1978;24:118–122. [DOI] [PubMed] [Google Scholar]
- 14.Sandman CA, Kastin AJ. Intraventricular administration of MSH induces hyperalgesia in rats. Peptides 1981;2:231–233. [DOI] [PubMed] [Google Scholar]
- 15.Gispen WH, van Wimersma Greidanus TB, Waters-Ezrin C, Zimmermann E, Krivoy WA, de Wied D. Influence of peptides on reduced response of rats to electric footshock after acute administration of morphine. Eur J Pharmacol 1975;33:99–105. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y, Carpenter A, Gregg K, Shahnaz Z, Carr J. Diurnal variation in α-melanocyte-stimulating hormone content of various brain regions and plasma of the Texas toad, Bufo speciosus. Gen Comp Endocrinol 1995;98:50–56. [DOI] [PubMed] [Google Scholar]
- 17.Kastin AJ. Extrapigmentary effects of melanocyte stimulating hormone in amenorrhoeic women. Lancet 1968;1:1107–1110. [DOI] [PubMed] [Google Scholar]
- 18.Altmeyer P, Bemd A, Holzmann H, Bacharach-Buhles M, Halberstadt E. α-MSH and pregnancy. Z Hautkr 1989;64:577–580. [PubMed] [Google Scholar]
- 19.Airaghi L, Garofalo L, Cutuli MG, et al. Plasma concentrations of α-melanocyte-stimulating hormone are elevated in patients on chronic haemodialysis. Nephrol Dial Transplant 2000; 8:1212–1216.. [DOI] [PubMed] [Google Scholar]
- 20.Letizia C, Mazzaferro S, De Ciocchis A, et al. Effects of haemodialysis session on plasma β-endorphin, ACTH and cortisol in patients with end-stage renal disease. Scand J Urol Nephrol 1996;30:399–402. [DOI] [PubMed] [Google Scholar]
- 21.Starowicz K, Przewlocka B. The role of melanocortins and their receptors in inflammatory processes, nerve regeneration and nociception. Life Sci 2003;73:823–847. [DOI] [PubMed] [Google Scholar]
- 22.Strand FL, Lee SJ, Lee TS, et al. Non-corticotropic ACTH peptides modulate nerve development and regeneration. Rev Neurosci 1993;4:321–363. [DOI] [PubMed] [Google Scholar]
- 23.Bucher SF, Seelos KC, Oertel WH, Reiser M, Trenkwalder C. Cerebral generators involved in the pathogenesis of the restless legs syndrome. Ann Neurol 1997;41:639–645. [DOI] [PubMed] [Google Scholar]
- 24.Rijsman RM, Stain CJ, de Weerd AW. Abnormal H-reflexes in periodic limb movement disorder; impact on understanding the pathophysiology of the disorder. Clin Neurophysiol 2005; 116: 204–210. [DOI] [PubMed] [Google Scholar]
- 25.Wikberg J, Muceniece R, Mandrika I, et al. New aspects on the melanocortins and their receptors. Pharmacol Res 2000;42:393–420.. [DOI] [PubMed] [Google Scholar]
- 26.Van der Kraan M, Tatro J, Wntwistle M, et al. Expression of melanocortin receptors and pro-opiomelanocortin in the rat spinal cord in relation to neurotrophic effects of melanocortins. Brain Res Mol Brain Res 1999;63:276–286. [DOI] [PubMed] [Google Scholar]
- 27.Skagerberg G, Bjorklund A, Lindvall O, Schmidt RH. Origin and termination of the diencephalo-spinal dopamine system in the rat. Brain Res Bull 1982;9:237–244. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y. Effects of selective D1- or D2-like dopamine receptor antagonists with acute “binge” pattern cocaine on corticotropinreleasing hormone and proopiomelanocortin mRNA levels in the hypothalamus. Brain Res Mol Brain Res 2004;130:61–67. [DOI] [PubMed] [Google Scholar]
- 29.Akgun S, Rudman D, Wertheim A. Changes in plasma or serum concentrations of magnesium, iron, thyroxine, in leukocyte triglyceride content, and in peritoneal fluid protein concentration in rabbits injected with adrenocorticotropin or β-melanocyte-stimulating hormone. Endocrinology 1969;84:347–355. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez de Aguilar JL, Malagon MM, Vazquez-Martinez RM, et al. Differential effects of dopamine on two frog melanotrope cell subpopulations. Endocrinology 1999;140:159–164. [DOI] [PubMed] [Google Scholar]
- 31.VanderSalm AL. Differential release of α-melanophore stimulating hormone isoforms by the pituitary gland of red porgy, pagrus pagrus. Gen Comp Enodocrinol 2004;135:126–133. [DOI] [PubMed] [Google Scholar]
- 32.Wood JM, Gibbons NC, Schallreuter KU. Melanocortins in human melanocytes. Cell Mol Biol (Noisy-le-grand) 2006;52:75–78.. [PubMed] [Google Scholar]
- 33.Nicolaus BJ. A critical review of the function of neuromelanin and an attempt to provide a unified theory. Med Hypotheses 2005;65:791–796. [DOI] [PubMed] [Google Scholar]
- 34.Connor JR, Wang XS, Patton SM, et al. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology 2004;62:1563–1567. [DOI] [PubMed] [Google Scholar]
- 35.Feng P, Vurbic D, Wu Z, Strohl KP. Brain orexins and wake regulation in rats exposed to maternal deprivation. Brian Res C 2007;1154:163–172. [DOI] [PubMed] [Google Scholar]
- 36.O’Donohue TL, Handelmann GE, Loh YP, Olton DS, Leibowitz J, Jacobowitz DM. Comparison of biological and behavioral activities of alpha- and gamma-melanocyte stimulating hormones. Peptides 1981;2:101–104. [DOI] [PubMed] [Google Scholar]
- 37.Gispen WH, Isaacson RL. ACTH-induced excessive grooming in the rat. Pharmacol Ther 1981;12:209–246. [DOI] [PubMed] [Google Scholar]
- 38.Gispen WH, Wiegant VM, Greven HM, de Wied D. The induction of excessive grooming in the rat by intraventricular application of peptides derived from ACTH: structure-activity studies. Life Sci 1975;17:645–652. [DOI] [PubMed] [Google Scholar]
- 39.Stiasny-Kolster K, Magerl W, Oertel WH, Möller JC, Treede RD. Static mechanical hyperalgesia without dynamic tactile allodynia in patients with restless legs syndrome. Brain 2004; 127: 773–782. [DOI] [PubMed] [Google Scholar]
- 40.Saletu B, Gruber G, Saletu M, et al. Sleep laboratory studies in restless legs syndrome patients as compared with normals and acute effects of ropinirole. I. Findings on objective and subjective sleep and awakening quality. Neuropsychobiology 2000;41: 181–189. [DOI] [PubMed] [Google Scholar]
- 41.Hinton JM. Patterns of insomnia in depressive states. J Neurol Neurosurg Psychiatry 1963;26:184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemke MR, Puhl P, Broderick A. Motor activity and perception of sleep in depressed patients. J Psychiatr Res 1999;33:215–224.. [DOI] [PubMed] [Google Scholar]
- 43.Hyyppa MT, Kronholm E. Sleep movements and poor sleep in patients with non-specific somatic complaints. II. Affective disorders and sleep quality. J Psychosom Res 1987;31:631–637.. [DOI] [PubMed] [Google Scholar]
- 44.Gruber R, Sadeh A, Raviv A. Instability of sleep patterns in children with ADHD. J Am Acad Child Adolesc Psychiatry 2000;39: 495–501. [DOI] [PubMed] [Google Scholar]
- 45.Plazzi G, Vetrugno R, Meletti S, Provini F. Motor pattern of periodic limb movements in sleep in idiopathic RLS patients. Sleep Med 2002;3:S31–S34. [DOI] [PubMed] [Google Scholar]
- 46.Pollmacher T, Schulz H. Periodic leg movements (PLM): their relationship to sleep stages. Sleep 1993;16:572–577. [PubMed] [Google Scholar]
- 47.Le Mevel JC, Abitbol S, Beraud G, Maniey J. Temporal changes in plasma adrenocorticotropin concentration after repeated neurotropic stress in male and female rats. Endocrinology 1979; 105: 812–817. [DOI] [PubMed] [Google Scholar]
- 48.Vetrugno R, Provini F, Plazzi G, Cortelli P, Montagna P. Propriospinal myoclonus: a motor phenomenon found in restless legs syndrome different from periodic limb movements during sleep. Mov Disord 2005;20:1323–1329. [DOI] [PubMed] [Google Scholar]
- 49.Qu S, Le W, Zhang X, Xie W, Zhang A, Ondo WG. Locomotion is increased in A11 lesioned mice with iron deprivation: a possible animal model for restless legs syndrome. J Neuropath Exp Neurol 2007;66:383–388. [DOI] [PubMed] [Google Scholar]