Abstract
Background/aim
This study was designed to determine the characteristic features of upper urinary system urothelial carcinomas (UUSUCs) and to evaluate the clinicopathological parameters associated with prognosis.
Materials and methods
A total of 74 cases of UUSUC were included, from three different centers. Demographic data and histopathological features such as tumor localization, concomitant tumor in the urinary system, distant metastasis with overall survival and disease-free survival obtained from the hospital records were evaluated retrospectively. Histopathologic prognostic features such as grade, perineural invasion, lymphovascular invasion, tumor necrosis, and surgical margin status were also evaluated.
Results
Seventy cases (94.6%) underwent open nephroureterectomy whereas 4 cases (5.4%) had laparoscopic nefroureterectomy. Thirty-eight (51.4%) cases were located in the pelvis, 7 (9.5%) in the ureter, 29 (39.2%) both in the pelvis and ureter. Fifty-six (75.7%) cases were alive; however, 18 (24.3%) patients were found to be dead. pTa, pT1, pT2, pT3, and pT4 tumors were reported in 16 (21.6%), 13 (17.6%), 4 (5.4%), 28 (37.8%), and 13 (17.6%) patients, respectively. Histopathologically, 17 cases (23%) were low-grade, 57 cases (77%) were high-grade. Statistically significant correlation was observed between overall survival and lymph node metastasis, distant metastasis, tumor necrosis, and differentiation by univariate analysis. Only distant metastasis was statistically associated with overall survival by multivariate analysis. We found no significant relationship between disease-free survival and all parameters.Conclusions: Differentiation and necrosis of tumor, lymph node involvement, and presence of distant metastasis is associated with the overall survival of urothelial carcinoma of the upper urinary system.
Keywords: Prognostic factors, upper tract urothelial carcinomas, upper urinary tract, urothelial carcinomas
1. Introduction
Urothelial carcinomas (UC) can arise in any part of the urinary tract lined by urothelium; however, the majority of cases are located in the lower tract (bladder, urethra) (1,2). Upper urinary system urothelial carcinomas (UUSUC), including renal pelvis and ureteral tumors, are known to be rare tumors, which constitute approximately 5%–10% of all UCs. The natural history and prognosis of UUSUC differ from bladder cancer (3–6). Among UUSUC, 60% of cases are invasive at diagnosis while only 15%–25% of bladder tumors are invasive at initial diagnosis. Moreover, the prognosis of UUSUC is poor and five year recurrence-free and overall survival (OS) rates are reported as 28% and 23%, respectively (1,2,7–13).
There are many studies in the literature evaluating the factors affecting the prognosis of the UC but the data about the prognostic factors of UUSUC are limited (1, 7, 9, 12-16). In this study, we evaluated the effect of clinicopathological factors including age, sex, tumor grade, tumor stage, tumor necrosis, lymphovascular invasion (LVI), perineural invasion (PNI), lymph node metastasis (LNM), and distant metastasis on OS of UUSUC.
2. Materials and methods
A total of 74 cases diagnosed with UUSUC from three different centers between February 2000 and December 2017 were included in the study. In patients with suspicion of UUSUC, diagnosis was obtained radiologically by using computed tomography urography which has the highest diagnostic accuracy among all of the clinically available imaging techniques. Radical nephroureterectomy with bladder cuff excision was performed without compromising oncological principles. Avoidance of entry into the urinary tract during surgery was taken into consideration in order to prevent tumor seeding in both open and laparoscopic nephroureterectomy cases. Approach to distal ureter was either performed with open or endoscopic techniques. Lymph node dissection was performed in case of clinical or radiological suspicion for metastasis. The demographic and clinicopathological features such as age, sex, tumor localization, distant metastasis, and concomitant tumor development in the urinary system and OS were obtained from urology records. Hematoxylin-eosin–stained slides were revised and histological grade, stage, differentiation, PNI, LVI, necrosis, LNM, and the status of the surgical margins were noted. World Health Organization (WHO) 2016 classification of urinary tumors was used for tumor grading and staging (17).
2.1. Statistical analysis
The correlation between OS and disease-free survival (DFS) and age, tumor size, tumor grade, tumor stage, tumor differentiation, concomitant UC, surgical margin, PNI, LVI, LNM, necrosis, and distant organ metastasis were investigated using Kaplan–Meier method, and log rank analysis. Multivariate analyses of OS were performed using the Cox regression method. P < 0.05 was considered to be the level of statistical significance. The OS and DFS of all patients during follow-up were assessed and statistical analysis was performed with SPSS version 24 (IBM Corp.; Armonk, NY, USA).
3. Results
Seventy cases (94.6%) underwent open nephroureterectomy whereas four cases (5.4%) had laparoscopic nefroureterectomy. Thirty-eight (51.4%) tumors were located in the pelvis, 7 (9.5%) in the ureter, and 29 (39.2%) both in the pelvis and ureter. Sixty (81.1%) patients were male and 14 (18.9%) were female. The ages at the time of diagnosis ranged from 40 to 84 years with a median age of 63.8 years. The follow-up time of all patients was 43.5 months (±48.7) (min: 1 month-max: 204 months). Twenty-seven cases (36.5%) were ≥70 years of age whereas 47 cases (63.5%) were <70. Fifty-six (75.7%) cases were alive; however, 18 (24.3%) patients were found to be dead. The mean tumor size was 5.4 cm (0.3–17 cm). pTa, pT1, pT2, pT3, and pT4 tumors were reported in 16 (21.6%), 13 (17.6%), 4 (5.4%), 28 (37.8%), and 13 (17.6%) patients, respectively. Histopathologically, 17 cases (23%) were low-grade and 57 cases (77%) were high-grade (Table 1).
Table 1.
Univariate analysis of demographic, clinical, and pathological characteristics for overall survival.
| Clinicopathologic factors | Category | n (%) | P-values |
| Median age in years (range) | 63.8 (min 40-max 84) (SD ± 8.9) | 74 | |
| Age ≥ 70 | 27 (36.5) | 0.77 | |
| Age <70 | 47 (63.5) | ||
| Tumor size | 5.4 cm (min 0.3–max 17 cm) | 0.21 | |
| Sex | Male | 60 (81.1) | 0.11 |
| Female | 14 (18.9) | ||
| Survival | Live | 56 (75.7) | |
| Ex | 18 (24.3) | ||
| Initial surgery | Open nefroureterectomy | 70 (94.6) | |
| Laparoscopic nefroureterectomy | 4 (5.4) | ||
| Tumor location | Renal pelvis | 38 (51.4) | 0.324 |
| Ureter | 7 (9.5) | ||
| Renal pelvis and ureter | 29 (39.2) | ||
| Pathological T stage | pTa | 16 (21.6) | 0.19 |
| pT1 | 13 (17.6) | ||
| pT2 | 4 (5.4) | ||
| pT3 | 28 (37.8) | ||
| pT4 | 13 (17.6) | ||
| Tumor grade | High | 57 (77) | 0.14 |
| Low | 17 (23) | ||
| Differentiation | Absence | 52 (70.3) | <0.001 |
| Presence | 22 (29.8) | ||
| Squamous | 19 (25.7) | ||
| Sarcomatoid | 2 (2.7) | ||
| Glandular | 1 (1.4) | ||
| Lymph node metastasis | Absence | 64 (86.5) | 0.042 |
| Presence | 10 (13.5) | ||
| Lymphovascular invasion | Absence | 49 (66.2) | 0.74 |
| Presence | 25 (33.8) | ||
| Perineural invasion | Absence | 65 (87.8) | 0.13 |
| Presence | 9 (12.2) | ||
| Tumor necrosis | Absence | 45 (60.8) | <0.001 |
| Presence | 29 (39.2) | ||
| Surgical margins | Negative | 61 (82.4) | 0.28 |
| Positive | 13 (17.6) | ||
| Synchronous tumor | Absence | 42(56.8) | 0.45 |
| Presence | 32 (43.2) | ||
| Bladder | 31 (41.9) | ||
| Bladder and contralateral kidney | 1 (1.4) | ||
| Recurrent disease | Absence | 68 (91.9) | 0.57 |
| Presence | 6 (8.1) | ||
| Metastasis | Absence | 58 (78.4) | <0.001 |
| Presence | 16 (21.6) |
Twenty-two cases (29.7%) showed variant differentiation (19 squamous, 2 sarcomatoid, 1 glandular differentiation).
Necrosis, LVI, PNI, and LNM was observed in 29 (39.2%), 25 (33.8%), 9 (12.2%), and 10 (13.5%) cases, respectively. Furthermore, 13 cases (17.6%) showed positive surgical margin (Table 1).
According to the follow-up data, 6.3% of cases with stage pTa, 23.1% of cases with stage pT1, 35.7% of cases with stage pT3, and 30.8% of the cases with stage pT4 were dead. All the cases with stage pT2 were alive. There was no statistically significant correlation between stage, tumor localization, positive surgical margin, and OS (P = 0.19, P = 0.324, P = 0.28, respectively).
Two of 17 low-grade carcinomas (11.8%) and 16 of 57 high-grade carcinomas (28.1%) were dead and no statistically significant correlation was found between tumor grade and OS (P = 0.14).
Concomitant bladder tumor was detected in 31 cases (41.9%). The tumor was located in bladder and contralateral kidney in one case (1.4%). However, the association between prognosis and concomitant urothelial cancer was not significant (P = 0.45).
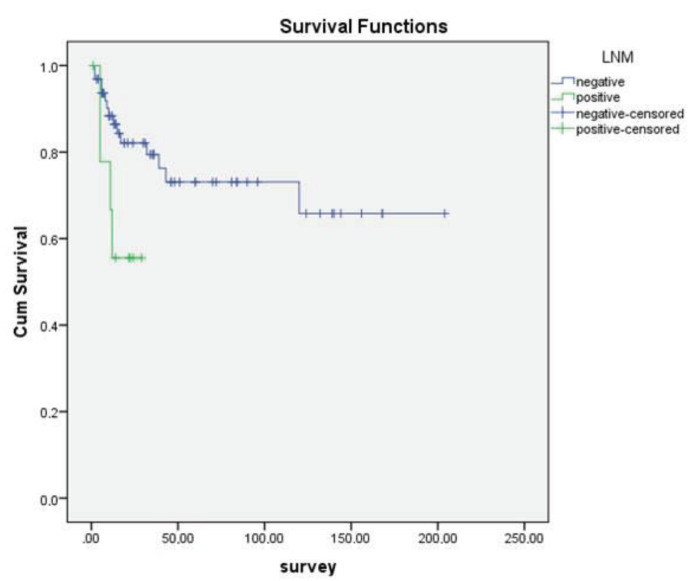
In addition, no statistically significant correlation was found between sex, age over or under 70, tumor size, PNI, LVI, and OS (P = 0.11, P = 0.774, P = 0.21, P = 0.13, P = 0.74, respectively). However, statistically significant association was observed between OS and differentiation (Figure 1), necrosis (Figure 2), LNM (Figure 3), and distant metastasis (Figure 4) (P < 0.001, P < 0.001, P < 0.001, P = 0.042, respectively).
Figure 1.
Kaplan–Meier curves of overall survival stratified according to the histological differentiation of tumors.
Figure 2.
Kaplan–Meier curves of overall survival stratified according to tumor necrosis.
Figure 3.
Kaplan–Meier curves of overall survival stratified according to lymph node metastasis. (LNM)
Figure 4.
Kaplan–Meier curves of overall survival stratified according to distant metastasis.
There was no significant correlation between synchronous tumor in the bladder (p=0.45) and OS as well as distant metastasis, differentiation, necrosis, LNM, and LVI (P = 0.96, P = 0.43, P = 0.79, P = 0.64, P = 0.92, respectively). Only distant metastasis was statistically associated with OS by multivariate analysis (P = 0.037). Table 2 demonstrates the multivariate analysis of parameters affecting OS.
Table 2.
Multivariate analysis of parameters predicting overall survival.
| Levels | Hazard ratio | 95% Cl Lower bound | 95% Cl Upper bound | P-value | |
| Tumor necrosis | NegativePositive | 5.483 | 0.895 | 33.583 | 0.066 |
| Tumor differentiation | NegativePositive | 1.825 | 0.627 | 5.314 | 0.270 |
| Lymph node metastasis | NegativePositive | 1.100 | 0.330 | 3.666 | 0.877 |
| Metastasis | NegativePositive | 4.200 | 1.087 | 16.227 | 0.037 |
Six of 74 cases had recurrence and 2 of these 6 cases were dead. We observed no significant relationship between recurrence and OS (P = 0.57).
The pathological stages of these recurrent cases were found as pTa (n=1), pT3 (n=4), and pT4 (n = 1). Five cases were high-grade whereas one case was low-grade. Two recurrent cases had LVI, concomitant bladder tumor and metastasis. None of them showed PNI. One case had tumor positive surgical margins.
We found no significant relationship between DFS and age (over/under 70), stage, tumor grade, LVI, PNI, surgical margin positivity, concomitant bladder tumor and metastasis. (P = 0.711, P = 0.436, P = 0.549, P = 0.918, P = 0.393, P = 0.900, P = 0.207, P = 0.100, respectively).
Three of 45 cases without necrosis had recurrence in addition to three cases with necrosis. Three of 52 cases without any additional differentiation had recurrence. Three tumors with additional differentiation had recurrent disease. None of the cases with recurrent disease had lymph node metastasis. Four cases with no metastasis and 2 cases with metastasis had recurrent disease. No significant relationship was observed between DFS and necrosis, differentiation and lymph node involvement (P = 0.254, P = 0.103, P = 0.458, respectively).
4. Discussion
UUSUC is rare but a potentially lethal disease (7). Upper urinary system tumors are generally multifocal affecting all urinary system lined by urothelium (1,9). The mean age of patients is reported as 65 years and the disease is more common in men (8). Various studies focused on the effect of clinical and pathologic parameters on UUSUC outcomes (1,5,7,10,12,18–24). Several studies reported tumor stage and grade as prognostic predictors in UUSUC (1,5,7,10,18–24).
In two different studies of Kikucki et al. and Bolenz et al., LVI was reported as an independent prognostic factor of DFS in UUSUCs (6,25). In our study, LVI was observed in 25 (33.8%) of 70 patients. There was no statistically significant correlation between LVI and OS. The presence of LVI should be stated in pathology reports in order to follow up the patients. (6).
Green et al. reported that the prognosis of bladder urothelial carcinoma is worse in women (10). Two multiinstitutional analyses performed by Fernandez et al. and Shariat et al. did not show any difference in pathologic characteristics and outcomes between sexes in UUSUC (1). Emamekhoo et al. reported that sex had no significant effect on OS and DFS of 454 cases (5). Most of our cases were men (n = 60) and no significant correlation was observed between sex and OS.
The studies about tumor localization and prognosis reported that ureteral tumors have a worse prognosis than renal pelvic tumors (1,5,7,18). The reason of this correlation is controversial while stage and treatment options may change (10). The protective effect of the renal parenchyma is thought to be associated with this finding. Also, the presence of a thin layer of adventitia surrounding the ureter, which contains an extensive plexus of blood and lymphatic vessels makes the invasion of tumor easier (21). However, several studies did not confirm the independent prognostic impact of tumor location on survival and showed the same recurrence-free survival and cancer-specific survival rates for renal pelvis and ureteral tumors (1,5). Similarly, we did not find a significant relation between OS and tumor localization. Nevertheless, this may be due to the unequal distribution of the cases for tumor localization.
Histopathologically, UUSUCs are generally high-grade tumors (9). Pathological tumor stage and histological grade are accepted as main indicators for prognosis similar to other malignant tumors (7,9,12,14,19–23). Most of the cases were high-grade in our study. Sixteen of 57 high-grade carcinomas were dead while only 2 of 17 low-grade carcinomas were found to be dead.
There was a correlation between grade or stage and OS; however, it was not statistically significant and this may be explained by the distribution numbers among the groups (P = 0.14, P = 0.19).
The presence of tumor necrosis is an indicator of aggressiveness in almost all malignancies (1,9,12). However, recent studies reported controversial results about the prognostic role of tumor necrosis in UUSUC (1). In the study of Zhang et al., tumor necrosis was found in 48 of 100 cases and was related to pathological stage, higher tumor grade, LNM, and LVI (12). Seitz et al. detected tumor necrosis in 165 of 754 cases (21.9%) from 9 different centers and showed that the prevalence of tumor necrosis increased as the pathological stage increased. Also, they reported that tumor necrosis was related to higher grade, LNM, LVI, sessile tumoral architecture and concomitant carcinoma in situ among UUSUC. However, tumor necrosis was not an independent predictor of clinical outcome (24). In our study, necrosis showed significant relation in univariate analysis; however, this relation was not proven by multivariate analysis (P < 0.001, P = 0.66, respectively). Larger studies are needed to prove this correlation.
Lymph node involvement is generally accepted as an important prognostic factor (1). The lymph node status remains unknown because no lymphadenectomy procedures were done in many nephroureterectomy operations (5). Our clinical approach is the excision of lymph nodes when palpable lymph nodes were observed during the operation or when preoperative radiological studies reported a suspect of lymph node positivity.
However, there are many studies showing that LNM is an independent prognostic factor of UUSUC (1,7,12). The effect of lymph node dissection on survival is still controversial in nephroureterectomy for UUSUCs (26,27). In the metaanalysis of Guo et al., patients with LNM had worse prognosis (3). On the other hand, in the same study, it was reported that lymphadenectomy showed no significant difference on survival and recurrence in pN0 or pNx cases (3).
We observed a significant association between lymph node involvement and OS in univariate analysis; however, this correlation was not proven by multivariate analysis (P = 0.042, P = 0.877).
In the literature, distant metastasis is related to prognosis in UUSUC (12,28). We also found a significant relation between distant metastasis and OS by univariate and multivariate analysis (P < 0.001, P = 0.037).
Even though the prognostic role of squamous differentiation is accepted in UUSUC, the clinical importance is still controversial (7,13,16). Most of the studies revealed that squamous differentiation was related to higher tumor grade and advanced tumor stage in univariate analysis (13). Makise et al. observed that squamous differentiation was the most common histological variant among 140 primary UUSUC and related with poor prognosis in univariate analysis (13). Qin et al. suggested that differentiation was a poor prognostic factor especially in ureteral tumors (7). We observed that squamous differentiation is the most common variant and it showed a statistically significant correlation with OS but this was not proven by multivariate analysis (P < 0.001, P = 0.27, respectively).
The history of a bladder tumor is reported as a poor prognostic factor in UUSUC in the literature and such cases must be under more stringent follow-up regimens or being treated more aggressively (1,29,30). There are studies suggesting the effect of synchronous or metachronous bladder cancer on recurrence and survival among UUSUC (7). Novara et al. also reported that the presence of concomitant muscle invasive bladder cancer is a poor prognostic factor (23). Bladder was the most common localization of concomitant tumors (n = 8) in our study. One case had tumor in both bladder and kidney. However, the presence of concomitant bladder tumor had no effect on prognosis.
It was reported that the presence of bladder cancer before the diagnosis of UUSUC has no significant effect on prognosis (31). We could not subgroup our cases according to the occurrence time of the previous bladder tumor.
There are some studies supporting that cisplatin-based additional treatments after surgery prolongs survival in UUSUCs (4). In another study, adjuvant chemotherapy after surgery was found to be associated with longer cancer specific and recurrence-free survival in patients with pT3N0M0 UUSUCs (32). The metastatic cases in our study group had chemotherapy.
This study has several limitations that need to be considered in interpreting the findings. The first limitation is the retrospective nature of the study. Additionally, the number of patients and the follow-up period are not enough to fully interpret the results. Finally, surgical procedures were performed by different surgeons at different institutions, explaining both the variability of intraoperative management and extent of lymph node dissection. Despite these limitations, this study showed that squamous differentiation, lymph node metastasis, distant organ metastasis, and tumor necrosis have statistically significant correlation with OS by univariate analysis in patients with UUSUC. Nevertheless, distant metastasis was the only statistically significant prognostic factor of OS observed in multivariate analysis.
Finally, well-designed and larger multiinstitutional studies are still needed to provide stronger evidence and to promote the use of these prognostic factors in the management of the treatment.
References
- Lughezzani G Burger M Margulis V Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. 2012;62:100–114. doi: 10.1016/j.eururo.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Miyazaki J Nishiyama H Epidemiology of urothelial carcinoma. Int J Urol. 2017;24:730–734. doi: 10.1111/iju.13376. [DOI] [PubMed] [Google Scholar]
- Guo R Zhu Y Xiong G Li X Zhang K Role of lymph node dissection in the management of upper tract urothelial carcinomas: a meta-analysis. BMC Urol. 2018;18:24–24. doi: 10.1186/s12894-018-0336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow JJ Martin-Doyle W Fay AP Choueiri TK Chang SL Bellmunt J. A systematic review and metaanalysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol. 2014;66:529–541. doi: 10.1016/j.eururo.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Emamekhoo H Dhillon P Gopalakrishnan D Elson P Stephenson A Magi-Galluzzi C McKenney J Harper H Haber GP Kaouk J Prognostic factors and risk stratification in invasive upper tract urothelial carcinoma. Clin Genitourin Cancer. 2018;16:e751–e760. doi: 10.1016/j.clgc.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Bolenz C Fernández MI Trojan L Herrmann E Becker A Weiss C Alken P Ströbel P Michel MS Lymphovascular invasion and pathologic tumor stage are significant outcome predictors for patients with upper tract urothelial carcinoma. Urology. 2008;72:364–369. doi: 10.1016/j.urology.2008.04.032. [DOI] [PubMed] [Google Scholar]
- Prognostic significance of urothelial carcinoma with divergent differentiation in upper urinarytract after radical nephroureterectomy without metastatic diseases: A retrospective cohort study. u2f49. u2f52;96:e6945–e6945. doi: 10.1097/MD.0000000000006945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gümüş E Horasanlı K Tanrıverdi O Boylu U Çevik C Üst üriner sistem üretelyal tümörlerinde 10 yıllık klinik deneyimimiz. Turk J Urol. 2004;30:160–165. [Google Scholar]
- Humphrey PA urothelial carcinoma of the upper urinary tract. The Journal of Urology. 2014;192:1223–1224. doi: 10.1016/j.juro.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Green DA Rink M Xylinas E Matin SF Stenzl A Roupret M Karakiewicz PI Scherr DS Sharia SF urothelial carcinoma of the bladder and the upper tract: disparate twins. The Journal of Urology. 2013;189:1214–1221. doi: 10.1016/j.juro.2012.05.079. [DOI] [PubMed] [Google Scholar]
- Kikuchi E Oya M Clinical practice patterns for upper tract urothelial carcinoma: a nationwide survey in Japan. Japanese Journal of Clinical Oncology. 2016;46:768–774. doi: 10.1093/jjco/hyw072. [DOI] [PubMed] [Google Scholar]
- Zhang XK Zhang ZL Yang P Cai MY Hu WM Yun JP Zhou FJ Qian CN Cao Y Tumor necrosis predicts poor clinical outcomes in patients with node-negative upper urinary tract urothelial carcinoma. Jpn J Clin Oncol. 2015;45:1069–1075. doi: 10.1093/jjco/hyv127. [DOI] [PubMed] [Google Scholar]
- Makise N Morikawa T Kawai T Nakagawa T Kume H Homma Y Fukayama M Squamous differentiation and prognosis in upper urinary tract urothelial carcinoma. Int J Clin Exp Pathol. 2015;8:7203–7209. [PMC free article] [PubMed] [Google Scholar]
- Abdulmajed MI Sancak EB Reşorlu B Al-chalaby GZ What are the currently available and in development molecular markers for bladder cancer? Will they prove to be useful in the future? Turk J Urol. 2014;40:228–232. doi: 10.5152/tud.2014.60973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Osch FH Jochems SH van Schooten FJ Bryan RT Zeegers MP Significant role of lifetime cigarette smoking in worsening bladder cancer and upper tract urothelial carcinoma prognosis: a meta-analysis. J Urol. 2016;195:872–879. doi: 10.1016/j.juro.2015.10.139. [DOI] [PubMed] [Google Scholar]
- Kucuk U Pala EE Cakır E Sezer O Bayol U Divrik RT Cakmak O Clinical, demographic and histopathological prognostic factors for urothelial carcinoma of the bladder. Cent European J Urol. 2015;68:30–36. doi: 10.5173/ceju.2015.01.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Classification of Tumours of the Urinary System and Male Genital Organs 2016.
- Zigeuner RE Hutterer G Chromecki T Rehak P Langner C Bladder tumour development after urothelial carcinoma of the upper urinary tract is related to primary tumour location. BJU Int. 2006;98:1181–1186. doi: 10.1111/j.1464-410X.2006.06519.x. [DOI] [PubMed] [Google Scholar]
- Lughezzani G Jeldres C Isbarn H Sun M Shariat SF Alasker A Pharand D Widmer H Arjane P Graefen M Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: a population-based study of 2299 patients. Eur J Cancer. 2009;45:3291–3297. doi: 10.1016/j.ejca.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Akdogan B Dogan HS Eskicorapci SY Sahin A Erkan I Ozen H Prognostic significance of bladder tumor history and tumor location in upper tract transitional cell carcinoma. J Urol. 2006;176:48–52. doi: 10.1016/S0022-5347(06)00511-8. [DOI] [PubMed] [Google Scholar]
- Park J Ha SH Min GE Song C Hong B Hong JH Kim CS Ahn H. The protective role of renalparenchyma as a barrier to local tumor spread of upper tract transitional cell carcinoma and its impact on patient survival. J Urol. 2009;182:894–899. doi: 10.1016/j.juro.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Kamihira O Hattori R Yamaguchi A Kawa G Ogawa O Habuchi T Kawauchi A Uozumi J Yokoi S Tsujihata M Laparoscopic radical nephroureterectomy: a multicenter analysis in Japan. Eur Urol. 2009;55:1397–1409. doi: 10.1016/j.eururo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Novara G De Marco V Gottardo F Dalpiaz O Bouygues V Galfano A Martignoni G Patard JJ Artibani W Ficarra V Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: multi-institutional dataset from 3 European centers. Cancer. 2007;110:1715–1722. doi: 10.1002/cncr.22970. [DOI] [PubMed] [Google Scholar]
- Seitz C Gupta A Shariat SF Matsumoto K Kassouf W Walton TJ Fritsche HM Otto W Tritschler S Bastian PJ Association of tumor necrosis with pathological features and clinical outcome in 754 patients undergoing radical nephroureterectomy for upper tract urothelial carcinoma: an international validation study. J Urol. 2010;184:1895–1900. doi: 10.1016/j.juro.2010.06.106. [DOI] [PubMed] [Google Scholar]
- Kikuchi E Horiguchi Y Nakashima J Hatakeyama N Matsumoto M Nishiyama T Murai M. Lymphovascular invasion independently predicts increased disease specific survival in patients with transitional cell carcinoma of the upper urinary tract. J Urol. 2005;174:2120–2123. doi: 10.1097/01.ju.0000181801.22474.8b. [DOI] [PubMed] [Google Scholar]
- Mason RJ Kassouf W Bell DG Lacombe L Kapoor A Jacobsen N Fairey A Izawa J Black P Tanguay S The contemporary role of lymph node dissection during nephroureterectomy in the management of upper urinary tract urothelial carcinoma: the Canadian experience. Urology. 2012;79:840–845. doi: 10.1016/j.urology.2011.11.058. [DOI] [PubMed] [Google Scholar]
- Burger M Shariat SF Fritsche HM Martinez-Salamanca JI Matsumoto K Chromecki TF Ficarra V Kassouf W Seitz C Pycha A No overt influence of lymphadenectomy on cancer-specific survival in organ-confined versus locally advanced upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy: a retrospective international, multi-institutional study. World J Urol. 2011;29:465–472. doi: 10.1007/s00345-011-0705-0. [DOI] [PubMed] [Google Scholar]
- Li X Ma X Tang L Wang B Chen L Zhang F Zhang X Prognostic value of neutrophil-to-lymphocyte ratio in urothelial carcinoma of the upper urinary tract and bladder: a systematic review and meta-analysis. Oncotarget. 2016;8:62681–62692. doi: 10.18632/oncotarget.17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullerad M Russo P Golijanin D Chen HN Tsai HH Donat SM Bochner BH Herr HW Sheinfeld J Sogani PC Bladder cancer as a prognostic factor for upper tract transitional cell carcinoma. J Urol. 2004;172:2177–2181. doi: 10.1097/01.ju.0000144505.40915.98. [DOI] [PubMed] [Google Scholar]
- Li WM Li CC Ke HL Wu WJ Huang CN Huang CH The prognostic predictors of primary ureteral transitional cell carcinoma after radical nephroureterectomy. J Urol. 2009;182:451–458. doi: 10.1016/j.juro.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Cho DS Hong SY Kim YK Prognostic factors in transitional cell carcinoma of the upper urinary tract after radical nephroureterectomy. 2011;52:310–316. doi: 10.4111/kju.2011.52.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC Chen MF Shindel AW The efficacy of postoperative adjuvant chemotherapy for patients with pT3N0M0 upper tract urothelial carcinoma. 2015;194:323–329. doi: 10.1016/j.juro.2015.03.077. [DOI] [PubMed] [Google Scholar]