Oral
OAA0102
Low levels of intact proviral DNA in HIV elite controllers associate with cell‐associated HIV RNA and protective HLA alleles
M. Peluso1, N. Iyer1, S. Kumar1, S. Munter1, L. Torres1, R. Hoh1, S. Deeks1, W. Trypsteen2, T. Henrich1
1University of California, San Francisco, United States, 2Ghent University, Ghent, Belgium
Background: The levels of intact and defective HIV provirus and their biological correlates in large cohorts of individuals who control HIV the absence of therapy (“elite controllers”) are unknown.
Methods: We used the intact proviral DNA assay (IPDA) to estimate the levels of intact and defective HIV provirus in cryopreserved PBMCs from 74 highly curated HIV elite controllers. We evaluated associations with clinical parameters, cell‐associated unspliced HIV RNA measured using quantitative PCR and the presence of protective HLA alleles (B*27, *57 and *58). Many individuals had no detectable intact proviruses. As DNA shearing is a known limitation of the IPDA, and as corrections traditionally require having detectable levels of intact HIV DNA, we applied the shearing index correction based on the lowest observed non‐intact concentration.
Results: Of the 74 controllers, 41 (55.4%) had undetectable levels of intact provirus. This is a greater proportion compared with a cohort of ART‐suppressed individuals that we have previously reported (7/81, 8.6%; p < 0.001). Detectable levels of intact provirus ranged from 10.5 to 3429.5 copies/106 cells. The median level of 3’ defective provirus was 80.9 (IQR 0 to 210), 5’ defective provirus was 38.5 (IQR 0 to 137.1) and combined defective provirus was 137.4 (IQR 89.0 to 391.0) copies/106 cells. The median ratio of intact/defective provirus was 0.17 (0 to 0.5), which is comparable to what we previously reported among those on ART (0.15, 0.05 to 0.33). Across all controllers, both the estimated intact provirus and combined defective provirus level directly correlated with higher levels of cell‐associated RNA (r = 0.41, p = 0.0014; r = 0.50, p < 0.001 respectively). Furthermore, individuals without detectable provirus were more likely to have at least one protective HLA allele (69% vs. 40%, p = 0.014). When the analysis was performed using the traditional shearing correction Methods, individuals without detectable provirus had higher CD4/CD8 ratios (1.14 vs. 0.90, p = 0.021).
Conclusions: Elite controllers have low levels of intact provirus, but the level of transcriptional activity is directly correlated with the frequency of intact virions. Protective alleles are associated with no detectable levels of intact HIV, arguing that potent and stringent T‐cell‐mediated control of the reservoir is possible.
OAA0103
In‐depth characterization of full‐length archived HIV genomes in long‐term post‐treatment and natural HIV controllers (ANRS CODEX/iVISCONTI Cohort)
P. Trémeaux1, F. Lemoine2, M. Gousset1, A. Mélard1, F. Boufassa3, O. Gascuel2, O. Lambotte4, L. Hocqueloux5, A. Saez‐Cirion2, C. Rouzioux6, V. Avettand‐Fenoel, ANRS CODEX/iVISCONTI Cohort Study Group
1University of Paris/Cochin Institute, Paris, France, 2Pasteur Institute, Paris, France, 3Université Paris Sud/INSERM CESP U1018, Paris, France, 4Université Paris Sud/INSERM UMR 1184, Paris, France, 5Orléans Hospital, Orléans, France, 6University of Paris, Paris, France
Background: Post‐treatment controllers (PTCs) and natural HIV controllers (HICs) are models of HIV remission but their mechanisms of control are different. We characterized HIV blood reservoir to better understand this control.
Methods: The reverse transcriptase (RT) gene viral diversity and the near‐full‐length proviral landscape of 9 PTCs were compared to those of 13 HICs (six aviraemic‐HICs and seven blipper‐HICs) and of individuals under efficient antiretroviral therapy initiated either at the primary infection (PHI, n = 6) or during the chronic phase (CHI, n = 6), by single‐genome amplification and deep‐sequencing. Bioinformatic tools were developed to identify genetic defects.
Results: Overall, more than 25000 RT sequences and 510 full‐length genomes were studied. The proviral diversity was lower in the PTC, PHI and aviraemic‐HIC groups than in the blipper‐HIC and CHI groups. The proportion of intact genomes was lower in the CHI (median (IQR): 2 (0 to 8)%) than the PHI (23 (13 to 34)%) group but similar among others, despite a high inter‐individual variability (HICs: 0 (0 to 28)%, PTCs: 4 (0 to 14)% 9.2 years (7.4 to 12.5) after treatment interruption). No difference was observed in the amounts of intact proviruses between groups. A subsequent sample taken four to six years later for three PTCs revealed no evolution of the proviral quasispecies and defects. The higher total HIV‐DNA loads in CHI were due to higher amounts of defective proviruses. HICs harboured lower proportions of hypermutated proviruses than the other three groups, suggesting that APOBEC3G/3F does not play a prominent role in them. A deletion in the nef gene was observed in every proviral sequence of two HICs, suggesting a role of these attenuated strains in the viral control in these HICs.
Conclusions: For the first time, we show the presence of intact proviruses and a stable and low viral diversity in PTCs after treatment interruption, reflecting a low residual replication over years. The absence of difference in the proviral landscape between PHIs and PTCs after treatment interruption suggests that post‐treatment control is mainly linked to non‐viral factors, contrary to some cases of natural control. The difference of defective (but not intact) proviruses amounts between groups suggests a role of these forms in the pathogenesis of HIV infection.
OAA0104
Suppression of HIV‐1 linked long non‐coding RNAs in viraemic HIV‐1‐positive individuals is associated with ongoing viral replication
C. Van Hecke1, S. Kinloch‐de Loes2, T. Schynkel1, E. Malatinkova1, K. Vervisch1, Y. Noppe1, S.L. Rutseart1, L. Vandekerckhove1, W. Trypsteen1
1Ghent University, Department of Internal Medicine and Pediatrics, HIV Cure Research Center, Gent, Belgium, 2Royal Free Hospital and University College London, Division of Infection and Immunity, London, United Kingdom
Background: Long non‐coding RNAs (lncRNAs) are recently established as a new layer in the HIV‐host response with the identification of several lncRNAs directly affecting HIV infection in vitro. However, their impact on HIV‐1 infection and replication in vivo remains largely unexplored and proves a necessity to further understand their clinical importance. Therefore, this cross‐sectional study has assessed expression levels of HIV‐1 linked lncRNAs in cohorts of infected individuals with different levels of virological control to determine their association with the HIV‐1 reservoir and host restriction factors.
Methods: The expression levels of five established HIV‐linked lncRNAs (MALAT1, NEAT1, NRON, GAS5 and linc01426) were evaluated by qPCR in peripheral blood mononuclear cells from 14 healthy individuals and 104 HIV‐1 positive individuals subdivided into five pre‐defined cohorts: recent seroconverters (n = 19), ART‐naïve progressors (n = 12), ART‐naïve long‐term non‐progressors (n = 17), early (n = 24) and late ART‐treated HIV‐1 positive individuals (n = 32). The levels of HIV‐1 markers were assessed via digital PCR assays for cell‐associated HIV RNA, total HIV‐1 DNA and 2LTR circles, together with qPCR profiling of host markers: IFIT and MX1. Next, lncRNA expression changes in these cohorts were determined via pairwise multiple comparisons testing (Kruskal–Wallis with Nemenyi test) and associations with HIV‐1 reservoir markers or host factors were explored via spearman correlation analysis.
Results: The expression of all five lncRNAs was significantly downregulated in ART‐naïve progressors with high HIV‐1 viral load (all p < 0.0003) and their expression levels were negatively correlated with viral load and total HIV‐1 DNA (all p < 0.01), indicating that the depletion of these lncRNAs is associated with ongoing viral replication and larger reservoir size. Only one lncRNA, GAS5, showed a negative correlation with HIV‐1 usRNA (p = 0.009), suggesting that individuals with lower levels of GAS5 have more ongoing viral transcription. Furthermore, one lncRNA NRON demonstrated a negative correlation with MX1 levels (p = 0.001), suggesting that interferon‐induction after infection is a possible driving factor for this lncRNA.
Conclusions: The present data characterized lncRNA expression in‐depth for the first time across HIV‐1 cohorts to address their link with the HIV‐1 reservoir and gained further evidence on their importance in HIV‐1 infection with possible implications for clinical follow‐up or future therapeutic strategies.
OAA0105
Assessing the T‐cell compartment of the extremely rare phenotype of elite control in children
V. Vieira1, E. Adland1, D. Malone1, J. Millar1, M. Muenchhoff2, C. Fortuny Guash3, C. Brander4, A. Prendergast5, G. Tudor‐Williams6, P. Goulder1
1University of Oxford, Department of Paediatrics, Oxford, United Kingdom, 2Max von Pettenkofer‐Institute, Department of Virology, Munich, Germany, 3Hospital Sant Joan de Déu, Universitat de Barcelona, Servicio de Pediatría, Barcelona, Spain, 4IrsiCaixa – AIDS Research Institute, Badalona, Spain, 5Queen Mary University of London, Centre for Genomics and Child Health, London, United Kingdom, 6Imperial College, Department of Paediatrics, London, United Kingdom
Background: Although important progress on prevention of mother‐to‐child transmission has been achieved, the incidence of HIV‐infected children is still a burden in low‐income countries. An intervention that leads to remission can be an important instrument in the epidemic control. However, due to the unique features of the immune system in children, strategies tailored in adults might not be applicable to this age group. Adults who spontaneously control viraemia (elite controllers) have been extensively investigated as a natural model of remission. This phenotype has not been studied in children, since paediatric elite controllers (PEC) are extremely rare, approximately 10‐fold lower than in adults.
Methods: In this study, we investigated the T‐cell compartment and the HIV‐specific response of four PEC, 13 non‐progressors, 10 progressors and 8 HIV‐exposed uninfected (EU) individuals matched by age. Peripheral mononuclear cells samples were analysed by flow cytometry.
Results: The CD4 T‐cell immunophenotype in PEC, non‐progressors and EU is similar, with a high naïve cells percentage and low expression of HLA‐DR, CD38, PD‐1 and CCR5. Clustering analysis shows a clear pattern of PEC grouping together with EU for activation markers on total, central memory and effector memory cells. The CD8 T‐cell compartment in PEC, however, shows increased frequency of more differentiated subsets and higher activation, but lower PD‐1 expression. Upon stimulation with HIV peptides pools Gag‐specific CD8 + and CD4 + T cells were more polyfunctional in PEC than in the non‐progressors and progressors. Unexpectedly, across all the groups studied, IFN‐γ expression on CD4 T cells negatively correlated with viral load.
Conclusions: Viraemic control in the paediatric population is only achieved after years of infection, compared to weeks in adults. Very low levels of immune activation in PEC and non‐progressors are important to maintain normal‐for‐age CD4 counts and preserve CD4 T‐cell function until antiviral immune activity has developed sufficiently to reduce viraemia. Although robust HIV‐specific CD8 + T‐cell responses are present among PECs, unlike adult EC these children are not enriched with the well‐described protective HLAs. Other mechanisms yet to be determined are also likely contributing to viraemic control among PECs.
OAA0106
Virological and immunological evaluation of individuals with spontaneous persistent viral control without ART
M. Khan1, J. Tosswill2, J. Haddow1, K. Pollock1, T. Elliott1, P. Patel2, A. Menezes2, T. Mbisa2, C. Brown2, D. Bradshaw2, G.P. Taylor1, S.J. Fidler1
1Imperial College London, Department of Medicine, Section of Virology, London, United Kingdom, 2Public Health England, Infectious Diseases, London, United Kingdom
Background: HIV elite controllers (EC) maintain undetectable viral loads (<20 HIV RNA copies/mL) and normal CD4/CD8 counts without ART. Despite WHO guidelines recommending ART irrespective of CD4 count and viral load, there remains a lack of consensus on best EC management. We have applied molecular and immunological assays to better understand mechanisms of natural viral control and possible negative immunological consequences.
Methods: A prospective study of 17 ECs attending a tertiary referral clinic (2017 to 2019) in London, measuring the following: NRTIs plasma concentrations by LC‐MS; nucleic acids by single copy assays RNA/mL and DNA/105 PBMCs, targeting gag, pol and int genes; CD4, CD8, CD25 and HLA‐DR by flow cytometry; HIV specific CD8 T‐cell responses using a pool of gag, env, nef and vif peptides in IFN‐γ ELISPOT; plasma cytokines (IL‐2,IL‐6, TNF‐α, MIP‐1β, CRP) by mesoscale Vplex.
Methods: A prospective study of 17 ECs attending a tertiary referral clinic (2017 to 2019) in London, measuring the following: NRTIs plasma concentrations by LC‐MS; nucleic acids by single copy assays RNA/mL and DNA/105 PBMCs, targeting gag, pol and int genes; CD4, CD8, CD25 and HLA‐DR by flow cytometry; HIV specific CD8 T‐cell responses using a pool of gag, env, nef and vif peptides in IFN‐γ ELISPOT; plasma cytokines (IL‐2,IL‐6, TNF‐α, MIP‐1β, CRP) by mesoscale Vplex.
Results: EC had a median age 42y (IQR = 37 to 54), 10 were female and NRTIs were not detected.
Abstract OAA0106‐Table 1. HIV nucleic acid was not detected in 5 (molecular‐negative) but detected in 12 (molecular‐positive); HIV RNA in 9/12 (median 5 cpm, range = 2 to 17), HIV DNA in 7/12
| CD4 cells/µL | CD8, cells/µL | CD4:8, ratio | T‐cell activation | |
|---|---|---|---|---|
| Molecular positive median (IQR) | 1015 (751 to 1369) | 553 (372 to 817) | 1.9 (1.3 to 2.5) | CD4 + CD25 + % 23 (18 to 32) CD8 + CD25 + % 8.5 (6.7 to 11) CD4 + HLA‐DR + % 7 (5.7 to 10) CD8 + HLA‐DR + % 19 (15 to 31) |
| Molecular negative EC median (IQR) | 785 (658 to 1138) | 779 (436 to 911) | 1.5 (0.8 to 2.5) | CD4 + CD25 + % 22 (11 to 24) CD8 + CD25 + % 6 (4.5 to 11) CD4 + HLA‐DR+% 8 (5 to 8) CD8 + HLA‐DR+% 16 (10 to 24) |
All had CD4 and CD8 counts within normal range and 16 had CD4:CD8 ratio >1. Neither T‐cell activation markers nor plasma cytokine concentrations differed significantly between groups.
Abstract OAA0106‐Figure 1.
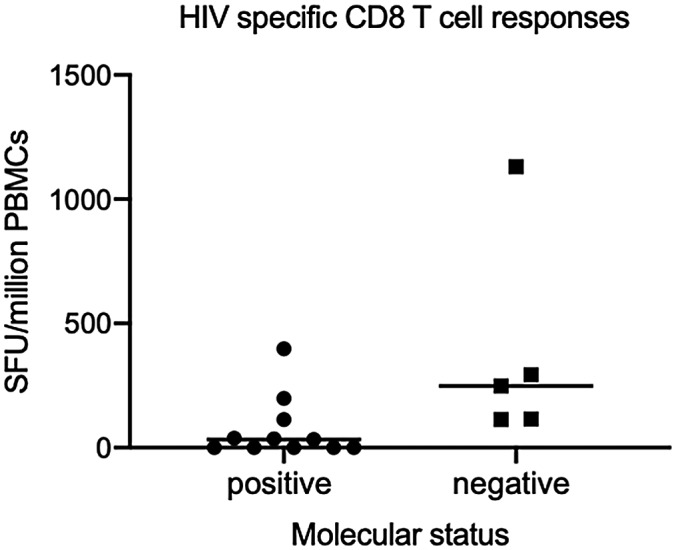
The frequency of CD8 responses was significantly higher (p = 0.01) in molecular‐negative (median = 248 SFU/106 PBMCs, IQR = 115 to 293) than molecular‐positive EC (median = 33 SFU/106 PBMCs, IQR = 0 to 75).
Conclusions: EC can be sub‐classified as molecular‐positive and molecular‐negative. Higher frequency of HIV‐specific CD8 responses in molecular‐negative suggests this may be important in the level of control. In this cohort, irrespective of detection of nucleic acids, there is no evidence of increased T‐cell activation or inflammation. Further studies are essential to determine the role of lifelong ART in such EC.
OAA0202
SMAC mimetic plus triple combination bispecific HIVxCD3 DART® molecules in SHIV.CH505‐infected, ART‐suppressed rhesus macaques
A. Dashti1, C. Waller1, N. Schoof1, M. Mavigner1, K. Bar2, G.M. Shaw2, T. Vanderford3, S. Liang3, J. Lifson4, G. Ferrari5, J.L. Nordstrom6, D. Margolis7, G. Silvestri3,8, A. Chahroudi1,3,9
1Emory University, Department of Pediatrics, Atlanta, United States, 2University of Pennsylvania, Department of Medicine, Philadelphia, United States, 3Yerkes National Primate Research Center, Emory University, Atlanta, United States, 4AIDS and Cancer Virus Program, Frederick National Laboratory for Cancer Research, Frederick, United States, 5Duke Human Vaccine Institute, Duke University Medical Center, Durham, United States, 6MacroGenics, Inc, Rockville, United States, 7University of North Carolina at Chapel Hill, Department of Medicine, Chapel Hill, United States, 8Emory Vaccine Center, Emory University, Atlanta, United States, 9Emory+Children's Center for Childhood Infections and Vaccines, Atlanta, United States
Background: “Kick‐and‐kill” HIV cure strategies involve latency reversal followed by immune‐mediated clearance of infected cells. Our prior work demonstrated strong latency reversal of SIV by AZD5582, a SMAC mimetic targeting the non‐canonical NF‐kB pathway. Here, we combined AZD5582 with bispecific HIVxCD3 DART molecules to reduce viral reservoirs in SHIV‐infected, ART‐suppressed rhesus macaques (RMs).
Methods: Thirteen RMs were infected with SHIV.C.CH505.375H.dCT. Triple ART (TDF+FTC+DTG) was initiated at 16 weeks. After 42 weeks, 8 ART‐suppressed RMs received a cocktail of 3 HIVxCD3 DART molecules with rhesusized Fc domains having A32, 7B2 or PGT145 anti‐HIV‐1 envelope specificities. For 10 weeks, DART molecules were administered weekly (1 mg/kg each) followed two days later by AZD5582 (0.1 mg/kg). Five RMs served as controls. Reservoir size was measured by cell‐associated SHIV‐DNA and ‐RNA and quantitative virus outgrowth.
Results: Peak viraemia (106 to 107 copies/mL) occurred two weeks after infection; two weeks of ART suppressed viral loads to below detection (<60 copies/mL). Three RMs showed transient control of viraemia < 60 copies/mL before ART. DART molecule serum levels declined after 3 to 5 doses coincident with development of anti‐drug antibodies, but Cmax levels > 100 ng/mL (sufficient for near‐maximal redirected killing of infected CD4 + cells in vitro) were maintained for 8 to 9 doses. AZD5582 did not increase on‐ART viraemia or cell‐associated SHIV‐RNA in blood or lymph node CD4 + T cells. SHIV‐DNA levels in blood or lymph node CD4 + T cells did not decline after treatment. Similarly, no differences were observed between experimental and control groups for SHIV‐DNA in GI tract or spleen CD4 + T cells, or replication‐competent virus in lymph node or spleen CD4 + T cells.
Conclusions: DART molecules did not reduce reservoir size in animals on ART, likely due to inadequate latency reversal. Lack of latency reversal in this system may be related to low pre‐ART viral loads (<105 copies/mL) and low pre‐LRA reservoir size (<102 SHIV‐DNA copies/million blood CD4 + T cells), which we have found to predict AZD5582‐induced on‐ART viraemia in SIV‐infected, ART‐suppressed RMs. Future studies to assess efficacy of Env‐targeting DART molecules to reduce viral reservoirs may be more suited to settings with greater viral burden.
OAA0203
Infection outcome in RT‐SHIV infected macaques treated early with antiretroviral therapy alone or in combination with the TLR7 agonist vesatolimod
M. Daly1, S. Ruone1, D. Rudolph1, C. Dinh1, A. Holder1, J. Mitchell1, M. Sterling1, K. Nishiura1, G. Khalil1, C. Tansey2, J. Weed2, K. Curtis3,1, V.V. Van Eygen4, W. Spreen5, W. Heneine1, J.G. García‐Lerma1
1Centers for Disease Control and Prevention, Division of HIV and AIDS Prevention, Atlanta, United States, 2Centers for Disease Control and Prevention, Division of Scientific Resources, Atlanta, United States, 3Centers for Disease Control and Prevention, National Center for HIV/AIDS Prevention, Atlanta, United States, 4Janssen Research & Development, Clinical Microbiology and Immunology, Beerse, Belgium, 5ViiV Healthcare, Medicine Development, Research Triangle Park, United States
Background: Early antiretroviral therapy (eART) preserves immune function and limits virus diversification but is not curative in people due to rapid viral reservoir establishment. We modelled in macaques the effect of a potent eART regimen (emtricitabine/tenofovir alafenamide (FTC/TAF) and long‐acting cabotegravir/rilpivirine (CAB‐LA/RPV‐LA)Eight rhesus macaques infected intrarectally with RT‐SHIV initiated treatment with human‐equivalent doses of oral FTC/TAF (20 and 1.5 mg/kg daily) and intramuscular CAB‐LA/RPV‐LA (50 and 200 mg/kg monthly) at 6 (range = 5 to 8) days post‐infection (dpi). Group I (n = 4) was treated for 12 months. Group II (n = 4) was treated for four months and also received weekly VES (0.15 mg/kg). Two untreated animals were used as controls. Plasma viraemia was monitored by RT‐PCR (limit of quantification = 50 copies). Antibody responses to p66, gp130, gp41, nef, gp36, gp140 and p27 were measured using an SIV/HIV Bio‐Plex assay. The Wilcoxon rank sum test was used to compare medians.
Results: Peak viraemia in the eART‐only and eART+VES groups were similar (3.4 (range = 2.7 to 4.3) and 4.2 (3.7 to 4.4) log10 RNA copies/mL, p = 0.111) and lower than the untreated controls (6.8 to 7.0 log10RNA copies/mL). Virus replication from treatment initiation until virus suppression was similar in the eART‐only and eART+VES animals (AUC = 42.6 (31.6 to 59.7) and 45.0 (38.4 to 51.07) RNA copies/mL/day, p = 0.886), although eART+VES suppressed replication earlier (18 (14 to 22) vs. 13 (11 to 13) dpi, p = 0.029). All macaques from the eART‐only group had undetectable viraemia during treatment and remain aviraemic 10 months after treatment interruption. Serologic responses in untreated controls were observed for the full panel tested. In contrast, responses in the eART and eART+VES groups were limited to gp140, albeit they developed at different rates (14 (14 to 17) vs. 36.5 (33 to 40) days post‐infection, respectively, p = 0.029). The eART‐VES animals are currently undergoing treatment interruption.
Conclusions: Using a relevant macaque model of mucosal RT‐SHIV infection we show that potent early ART leads to prolonged viral control after treatment interruption. Serologic responses, limited to gp140, were consistent with efficient virus control. The combination of eART and VES quickly suppressed viraemia and delayed serologic responses. Further characterization of immune function and virus dynamics will shed light on the immunomodulatory effect of VES during acute infection.
OAA0204
Tyrosine kinase inhibitors promote antiviral resistance in CD4 + T cells against HIV‐1 infection even after treatment withdrawal
L. Vigón1, S. Rodríguez‐Mora1, V. García2, A. Luna2, G. Bautista3, M. Galán1, J. Alcamí1, J.L. Steegmann4, A. Spivak5, V. Planelles5, M.R. López‐Huertas1, M. Coiras1
1Institute of Health Carlos III, AIDS Immunopathology, Majadahonda, Spain, 2Hospital Universitario Ramón y Cajal, Hematology, Madrid, Spain, 3Hospital Universitario Puerta de Hierro, Hematology, Majadahonda, Spain, 4Hospital Universitario La Princesa, Hematology, Madrid, Spain, 5University of Utah School of Medicine, Division of Microbiology and Immunology, Department of Pathology, Salt Lake City, United States
Background: Our group described previously that tyrosine kinase inhibitors (TKIs) used against chronic myeloid leukaemia (CML) show antiviral effect against HIV‐1 by interfering with SAMHD1 phosphorylation, HIV‐1 proviral integration and transcription and also decreasing viraemia and reservoir size in NSG mice engrafted with human CD34 + cells. By blocking T‐cell proliferation induced with homeostatic cytokines, TKIs might also impede reservoir replenishment, delaying viral rebound after controlled treatment interruption. Finally, TKIs showed immunomodulatory properties that may be preserved after treatment interruption (TI) due to deep molecular response (DMR) against cancerous cells.
Objective: To evaluate whether PBMCs from CML patients on TI are still resistant to HIV‐1 infection.
Methods: PBMCs from patients with CML on TI (Off‐TKI) (n = 17) and healthy donors (n = 30) were analysed by flow cytometry. Proviral integration was analysed by Alu‐qPCR and viral protein synthesis was quantified by chemiluminescence. Ex‐vivo infection was performed with NL4‐3_renilla strain.
Results: 1) Off‐TKI patients were 57% male, 43% female; mean age of CML diagnosis 61 ± 5.5 years; mean lymphocyte count 2.4 ± 0.3x103/mL; previously treated with imatinib, nilotinib and/or dasatinib for 5.3 ± 0.4 years; mean time off treatment 13.7 ± 3.5 months. 2) CD4 + T cells from Off‐TKI patients showed 2.1‐fold reduced levels of phosphorylated (p)SAMHD1 in non‐activated conditions, and CD4 + CD25 + CD69 + decreased 2.6‐fold, regarding healthy controls. After activation with PHA/IL‐2, CD4 + pSAMHD1 + and CD4 + CD25 + CD69 + populations were similar in Off‐TKI and controls. 3) Ex‐vivo HIV‐1 infection of PBMCs from Off‐TKI decreased 12.4‐ and 5.2‐fold proviral integration and viral proteins synthesis respectively. 4) Expression of Natural Killer (NK) activation marker CD56 increased 5‐fold in CML patients off treatment. Populations of cytotoxic cells CD56 + CD16 + CD107a+ and CD8 ± TCRgd+ increased 5‐ and 3‐fold in Off‐TKI respectively.
Conclusions: CD4 + T cells from CML patients on TI showed response to activating stimuli, with normal levels of pSAMHD1 and activation markers. However, these cells were resistant to HIV‐1 infection, even though patients withdrew treatment with TKIs more than one year ago. Cytotoxic cell populations with antiviral effect were detected in these patients. These results suggest that TKIs could be used temporarily as cART adjuvants in HIV‐infected patients to modulate the immune response in order to interfere with reservoir replenishment and reactivation.
OAA0205
Combining a conditional suicide gene with CCR5 knockout for anti‐HIV gene therapy
R. Yeh1, T. Mehmetoglu‐Gurbuz2, A. Joshi2, H. Garg2
1Paul L. Foster School of Medicine, El Paso, United States, 2Texas Tech University Health Sciences Center El Paso, Department of Molecular and Translational Medicine, El Paso, United States
Background: The recent success of the Berlin and London patients has attracted the attention of the scientific community worldwide to achieve an HIV cure for a wider group of patients. However, mathematic modelling has suggested that strategies targeting CCR5 alone will fail unless combined with a suicide gene. Hence, we developed a combined suicide gene therapy approach to target viral entry along with a conditional cytotoxic gene to specifically eliminate HIV‐infected cells.
Methods: We developed a 2‐step gene therapy approach involving the delivery of TKSR39 gene via vector 1 (integrating lentivirus) and CCR5 knock‐out combined with tat expression via vector 2 (non‐integrating vector). Vector 1 incorporated an internal ribosomal entry site (IRES) followed by the GFP sequence to allow for sorting of the transduced cells. This TZM‐TKSR39 CCR5 KO cell line was thoroughly characterized for resistance to HIV infection and specific killing of HIV‐infected cells in the presence of ganciclovir.
Results: TZM‐TKSR39 cells developed in the lab previously were transduced with CCR5 sgRNA packaged lentiviral particles. Through sorting, enrichment of the CCR5 KO population was achieved and potential CCR5 KO candidates were obtained by single cell cloning. HIV infection of TZM‐TKSR39 CCR5 KO cells resulted in negligent infection with R5 tropic HIV while still allowing infection with X4 tropic HIV. CCR5 deletion was further confirmed via a T7 endonuclease PCR. Moreover, the cells were susceptible to ganciclovir‐mediated cell killing after an X4 tropic virus infection.
Conclusions: Our study provides proof of principle for an HIV gene therapy to modify stem cells from an HIV infected patient to achieve a cure. Our combined gene therapy approach prevents viral entry via CCR5 knockout. However, in the event of X4 virus emergence, specific cell killing of infected cells can be achieved via ganciclovir. This approach is highly regulated and capable of targeting both X4 and R5 variants and has the potential to create an HIV proof immune system.
OAA0206
Gene therapy with an anti‐tat gene can strongly block HIV‐1 transcription and virus replication in mouse models of infection
D. Harrich1, H. Jin1, L. Rustanti2
1QIMR Berghofer Medical Research Institute, Cell and Molecular Biology, Brisbane, Australia, 2Australian Red Cross, Blood Research, Brisbane, Australia
Background: Nullbasic (NB) is a mutant protein of the HIV‐1 transcriptional activator protein, Tat. Our research has demonstrated that NB is a nontoxic, first‐in‐class antiviral agent that inhibits HIV production and viral spread in human T cells by independent mechanisms: 1) it inhibits the transcriptional activation function of Tat, 2) it disrupts HIV mRNA trafficking by interfering with the viral Rev regulatory protein, 3) it inhibits HIV reverse transcription. We have shown that with stable expression in cells, NB inhibits HIV replication in human cells and it also inhibits HIV reactivation from latently infected cells.
Methods: We used retroviral gene therapy vectors to deliver a Nullbasic‐ZsGreen1 fusion protein or ZsGreen1 to human CD4 + T cells, which were purified and transplanted into NOD‐SCID or BALB/c‐Rag2‐/‐γc‐/‐ (RAG2) mice. The mice were infected with HIV‐1 and virus replication was followed for up to eight weeks. As an adjunct method, we also trialled layered double hydroxide nanoparticles (LDH NPs) to deliver NB protein to primary human CD4 + T cells.
Results: Both mouse models showed that Nullbasic inhibited virus replication. In Rag2 mice, Nullbasic‐ZsGreen1 delayed replication and lowered viral titres by approximately 10 to 15 fold. Increased virus replication inversely correlated with Nullbasic‐ZsGreen1 expression in CD4 + T cells. Interestingly, NOD‐SCID mice had CD4 + T cells that showed robust expression of Nullbasic‐ZsGreen1 and up to 7000‐fold inhibition of HIV‐1. We observed that 100% of CD4 + T cells can be treated with NB‐LDH NPs. NB was detected in treated cells for three days.
Conclusions: Interest in strategies leading to a functional cure for HIV‐1 infection by long‐term or permanent viral suppression is growing. Here, we show that a mutant form of the HIV‐1 Tat protein, referred to as Nullbasic, inhibits HIV‐1 transcription in infected CD4 + cells in vivo. Analysis shows that stable expression of Nullbasic in CD4 + cells could lead to durable anti‐HIV‐1 activity. Nullbasic, as a gene therapy candidate, could be a part of a functional‐cure strategy to suppress HIV‐1 transcription and replication.
OAA0302
Multivariant HIV‐1 infection in infants with broadly neutralizing plasma antibodies: Implication for polyvalent vaccines
N. Mishra1, S. Sharma1, A. Dobhal1, M. Makhdoomi1, S. Kumar1, R. Singh2, B. Das2, R. Lodha3, S. Kabra3, K. Luthra1
1All India Institute of Medical Sciences, Biochemistry, New Delhi, India, 2All India Institute of Medical Sciences, Microbiology, New Delhi, India, 3All India Institute of Medical Sciences, Pediatrics, New Delhi, India
Background: An effective HIV‐1 vaccine that can curtail the AIDS pandemic is the need of the hour. Several second‐generation broad and potent neutralizing antibodies (bnAbs), mostly targeting distinct conserved regions of the viral envelope glycoprotein (env), have been isolated and shown to have a protective effect. Due to the extensive antigenic diversity of HIV‐1, bnAbs develop in a subset of infected individuals over two to three years of infection. Interestingly, infected infants have been shown to develop plasma bnAbs frequently and as early as one‐year post‐infection, with features atypical than adult bnAbs, suggesting the factors governing bnAb induction in infants are different than those in adults. Understanding the antigenic features in infants with early bnAb responses will provide key information on the antigenic triggers driving B‐cell maturation pathways towards the induction of bnAbs.
Methods: Plasma neutralization activity and bnAb susceptibility profiles were assessed by TZM‐bl‐based neutralization assays. HIV‐1 RNA was isolated from plasma samples of infants, and full‐length envelope genes were amplified, sequenced and cloned for the generation of pseudoviruses. Viral diversity, recombination and phylogeny analysis were performed using MEGAX, RAPR, HIV AnalyzeAlign and SimPlot. Antigenic characterization of candidate vaccine strains was done using surface‐binding assays, ELISAs and on‐cell sDC4 triggering assay.
Results: Herein, we evaluated the presence of plasma bnAbs in 51 infants of Indian origin perinatally infected with HIV‐1 clade C and identified the viral factors associated with early bnAb responses. A strong association of multivariant infection in infant elite neutralizers with development of plasma nAbs targeting diverse autologous viruses was observed. We observed the plasma nAbs in infants with multivariant infection to target both variants, suggesting env‐specific antibodies generated in context of two distinct viral variants can target epitopes on both envelopes. In addition, several viral strains capable of serving as potential vaccine candidates were identified from infant elite neutralizers.
Conclusions: Our data provide information supportive of polyvalent vaccination approaches for paediatric HIV‐1 vaccination.
OAA0303
Intradermal MVA vaccinations provide superior protection compared to intramuscular MVA vaccinations against a homologous tier 2 SHIV challenge
V.S. Bollimpelli1, P.B.J. Reddy1, S. Gangadhara1, T. Charles2, S. Burton2, C. Labranche3, G. Tharp2, T.M. Styles1, T. Legere1, S.P. Kasturi2, D. Montefiori3, G. Shaw4, J. Moore5, S. Bosinger2, P. Kozlowski6, B. Pulendran7, C. Derdeyn2, E. Hunter2, R.R. Amara1
1Emory University, Microbiology and Immunology, Atlanta, United States, 2Emory University, Pathology, Atlanta, United States, 3Duke University, Department of Surgery, Durham, United States, 4University of Pennsylvania, Philadelphia, United States, 5Weill Medical College of Cornell University, New York, United States, 6Louisiana State University Health Sciences Center, New Orleans, United States, 7Stanford University, Stanford, United States
Background: The composition of antigen‐presenting cells is different in different compartments and thus the route of immunization can markedly influence the magnitude and quality of evoked immune response and thereby vaccine efficacy. Here, we tested the influence of intradermal (ID) and intramuscular (IM) routes of MVA immunization on HIV vaccine efficacy.
Methods: We immunized two groups of rhesus macaques (n = 10/group) with DNA/MVA/Protein vaccine regimen. DNA and MVA vaccines expressed SIV Gag and membrane anchored trimeric HIV BG505 envelope (Env). Soluble BG505‐SOSIP.664 trimer protein plus 3M‐052 adjuvant encapsulated in nanoparticles was used as a protein boost. While both groups received DNA immunizations intradermally and protein immunizations subcutaneously, they differed only in the route of MVA immunization where one group received MVA via ID and the other via IM route.
Results: Both groups (ID and IM) showed strong binding antibody response to BG505‐SOSIP.664 gp140 in serum/vaginal secretions, and some animals generated autologous neutralizing antibody response against BG505.664 Env but these were comparable between the groups. IFNg+ SHIV‐specific CD8 T‐cell responses were marginally higher (not significant) in the IM group. However, the MVA‐ID vaccination induced significantly higher proliferating CD4 T cells in blood consisting of effector memory (CD45RA‐CCR7‐), circulating Tfh (CXCR5 + ) and non‐Th1 (CXCR3‐) cells compared to MVA‐IM. Similarly, the GC‐Tfh and GC‐B cells in the LNs were higher in the MVA‐ID group. Following 10 weekly BG505‐SHIV intravaginal challenges, protection was evident only in the MVA‐ID group (vaccine efficacy of 73% per exposure, p = 0.006 with 40% of the animals completely protected), but not in the MVA‐IM group. Analysis of DC and monocyte activation in blood after MVA immunization revealed markedly higher activation of non‐classical (CD16 + CD14‐) monocytes and CD11c+ DCs in the MVA‐IM group not in the MVA‐ID group. Analysis of RNA transcriptome in blood after MVA immunization revealed marked induction of inflammasome pathway in the MVA‐IM group but not in MVA‐ID group.
Conclusions: These results demonstrate that MVA‐ID vaccination is superior to MVA‐IM vaccination for protection against HIV and the route of MVA vaccination markedly influences the quality of T helper response and innate activation that are associated with difference in protection outcome.
OAA0304
Priming with DNA expressing trimeric V1V2A244 alters the immune hierarchy and favours the development of V2‐specific HIV antibodies in rhesus macaques
S. Devasundaram1, M. Rosati1, H.V. Trinh2, M. Rao2, X.‐P. Kong3, A. Valentin1, S. Zolla‐Pazner3, G.N. Pavlakis1, B.K. Felber1
1National Cancer Institute at Frederick, Frederick, United States, 2U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, United States, 3NYU School of Medicine, New York, United States
Background: The RV144 clinical vaccine trial showed that reduced risk of HIV infection is correlated with non‐neutralizing antibody (Ab) responses targeting the V1V2 region of the HIV gp120 Env, making this region an important vaccine target. To induce V2‐specific Abs, we tested the immunogenicity of a vaccine regimen that includes priming with DNA expressing the trimeric epitope‐scaffold V1V2A244 immunogen.
Methods: The vaccine regimen included two DNA primes followed by 3 DNA + protein co‐immunization boosts. The “V1V2 group” (N = 4) was primed with V1V2A244‐2J9C DNA (Jiang, J Virol 2016; Zolla‐Pazner, J Virol 2016) and the “gp145 group” (N = 4) was primed with gp145 DNA expressing membrane‐bound trimeric Env and soluble gp120. The booster vaccine in both groups consisted of gp145 DNA and GLA‐SE‐adjuvanted gp120. The V1V2 group also received V1V2A244 DNA in the boost. Antibodies were monitored after the prime and the boost.
Results: The V1V2 group developed robust Ab responses recognizing heterologous trimeric V1V2‐scaffold proteins and cyclic V2 from different clades (B,C,E), whereas only low levels of V2 Abs were induced by the gp145 DNA vaccine. The V1V2 DNA‐induced Abs also potently recognized gp120 by ELISA and trimeric clade A/ECM244 and clade CCH505 Env anchored on the cell surface of stably transduced HEK293 cells detected by flow cytometry. Peptide mapping showed greater Ab breadth within the V2 region in the V1V2 group, with Abs specific for the V2 peptide RDKKQKVHALFYKLDIVPIE (HXB2 AA166 to 185), a critical target identified in RV144, which was only found by immunization with the V1V2A244 DNA. Importantly, Ab responses to a V2 peptide with the K169V mutation were drastically reduced, mimicking the specificity of monoclonal and polyclonal Abs induced in RV144 (Liao, Immunity 2016; Zolla‐Pazner, PLoS One 2013). The magnitude and breadth of the V2‐specific responses were higher in V1V2 group with lower V3 responses.
Conclusions: Our results demonstrate that priming with DNA expressing trimeric V1V2 focuses the Ab response on the V1V2 region of gp120, inducing cross‐clade reactive Abs. This regimen alters the hierarchy of immunodominant Env epitopes, providing a selective advantage for induction the V1V2 Abs associated with protection from SHIV and HIV.
OAA0305
Sequence and structure guided HIV‐1 clade C trimeric immunogen design to induce neutralizing and V1V2 directed antibody responses
A. Sahoo1, C. LaBranche2, S. Shen2, T.M. Styles1, V. Velu1, S. Gangadhara1, D. Montefiori2, G.D. Tomaras2, R.R. Amara1
1Emory Vaccine Center, Yerkes National Primate Research Center, Emory University, Atlanta, United States, 2Duke Human Vaccine institute, Duke University School of Medicine, Duke University, Durham, United States
Background: About 50% of global HIV‐1 infections are due to clade C viruses and there is a great need for the development of stabilized natively like trimeric clade C gp140 protein immunogen for inducing neutralizing antibodies by vaccination. The C.1086 based gp140 trimer would be of interest as the monomeric gp120 version of this protein is currently being used in a Phase 2a/b clinical study (HVTN702). The unstabilized C.1086 K160N (to improve binding to bnAb PG9) gp140 protein does not induce autologous neutralizing antibodies.
Methods: Structure and sequence guided screening of 1086.C mutants and characterization by size‐exclusion chromatography, NE‐EM, improved antibody‐binding profile, immunogenicity in rabbits, characterization of the serum.
Results: To develop a stable trimer, we adopted recent structure guided strategies to design SOSIP, NFL (Native Flexible Linker) and UFO (Uncleaved Full‐Length Optimized) forms of the protein. The NFL and UFO versions yielded higher trimeric fractions than the SOSIP counterpart which predominantly formed aggregates. UFO design was further selected based on improved binding to V1V2‐specific bnAb PG16 than C.1086_NFL. Sequence guided mutational analysis of the V2 hotspot region (V2HS, 165 to 181) highlighted K166R to markedly improve binding to the V1V2 trimer‐specific bnAb PGT145. Additional structure guided modifications were adopted to improve the stability of the envelope. Variants at V2HS showed significant enhancement in binding to multiple V1V2 directed bnAbs. Alterations of residues at position 173 of the V2HS region was found to influence the immune responses. Following immunization in rabbits, one of the variants at 173 position improved Tier‐2 neutralization titres, recognition of membrane anchored envelopes and influenced V1V2 (displayed on gp70 scaffold) from envelopes across diverse clades. One of the neutralizers was able to induce antibodies targeting the V1V2 region which competed with known trimer‐specific V2‐directed bnAbs. We are currently analysing the neutralization specificity of the serum. Encouragingly, one of the immunogens elicited strong autologous neutralization titre (100 to 800) in macaques.
Conclusions: The stabilized C.1086 K160N UFO trimer protein can induce tier‐2 neutralizing antibodies and enhance binding antibodies specific to gp70‐V1V2 and membrane anchored trimeric Env. We are currently understanding mechanisms by which changes at position 173 would influence the immune responses.
OAA0306
Protective efficacy of a vaccine inducing Gag/Vif‐specific CD8 + T but not CD4 + T cells against repeated intrarectal low‐dose SIVmac239 challenges
H. Ishii1, K. Terahara2, T. Nomura1, T. Tokusumi3, T. Shu3, H. Suzuki4, D. Fujiwara4, H. Sakawaki5, T. Miura5, T. Matano1,6,7
1National Institute of Infectious Diseases, AIDS Research Center, Tokyo, Japan, 2National Institute of Infectious Diseases, Department of Immunology, Tokyo, Japan, 3ID Pharma Co., Ltd., Ibaraki, Japan, 4Kirin Holdings Co., Ltd., Yokohama, Japan, 5Kyoto University, Institute for Frontier Life and Medical Sciences, Kyoto, Japan, 6University of Tokyo, Institute of Medical Science, Tokyo, Japan, 7Kumamoto University, Joint Research Center for Human Retrovirus Infection, Kumamoto, Japan
Background: Virus‐specific CD4+ T‐cell responses are crucial for induction of effective CD8+ T‐cell responses against virus infection. Vaccine‐induced CD4+ T cells, however, can be preferential targets for HIV/SIV infection. Recent studies have indicated the detrimental effect of vaccine–induced CD4+ T cells on HIV vaccine efficacy (J Virol 88:14232, 2014; Sci Transl Med 11:eaav1800, 2019), supporting a rationale for vaccine design inducing HIV‐specific CD8 + T‐cell responses without HIV‐specific CD4+ T‐cell induction but with non‐HIV antigen‐specific CD4+ T‐cell help. Based on this concept, we have developed a novel immunogen, CaV11, consisting of tandemly connected overlapping 11‐mer peptides spanning viral Gag capsid (CA) and Vif. This CaV11 immunogen is expected to selectively elicit Gag/Vif‐specific CD8+ T cells with inefficient Gag/Vif‐specific CD4+ T‐cell induction, because the ideal length of CD4+ T‐cell epitopes is longer than 11 mers, whereas CD8+ T‐cell epitopes are 8 to 11 mers. In the present study, we evaluated the protective efficacy of a CaV11‐expressing vaccine against repeated intrarectal low‐dose SIV challenges in rhesus macaques.
Methods: Twelve rhesus macaques received four times of intramuscular CaV11‐expressing DNA vaccination at weeks 0, 1, 3 and 4 and four times of intranasal and intramuscular CaV11‐expressing Sendai virus vectors (SeV‐CaV11) at weeks 6, 7, 12 and 18. These twelve vaccinated and seven unvaccinated macaques were intrarectally challenged with low‐dose (200 TCID50) SIVmac239 repeatedly every two weeks starting from six weeks after the last vaccination.
Results: All the vaccinated animals efficiently induced Gag/Vif‐specific CD8+ T‐cell responses with inefficient Gag/Vif‐specific CD4+ T‐cell responses after SeV‐CaV11 vaccination. After eight times of SIV challenge, six of the seven unvaccinated macaques were infected, whereas eight of the twelve vaccinated were protected from SIV infection. Kaplan‐Meier analysis indicated a significant difference between unvaccinated and vaccinated (p = 0.0341 by Log‐rank test).
Conclusions: The present study for the first time indicates the potential of canonical CD8+ T cells induced by Env‐independent vaccination to protect HIV acquisition, suggesting that CD8+ T‐cell induction using this immunogen design is a promising HIV vaccine strategy.
OAA0307
Co‐immunization of DNA and protein in the same anatomical sites induces superior protective immune responses against SHIV challenge
B.K. Felber1, Z. Lu1, X. Hu1, A. Valentin1, M. Rosati1, J.A. Weiner2, X. Shen3, C.C. LaBranche3, D.C. Montefiori3, W.B. Williams3, K.O. Saunders3, H.V. Trinh4, M. Rao4, M.S. Alam3, S.G. Reed5, P.P. Aye6, B. Pahar6, P.A. Marx6, D.J. Venzon7, G.M. Shaw8, G. Ferrari3, M.E. Ackerman2, B.F. Haynes3, G.N. Pavlakis1
1National Cancer Institute at Frederick, Frederick, United States, 2Thayer School of Engineering, Dartmouth College, Hanover, United States, 3Duke University Medical Center, Durham, United States, 4U.S. Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, United States, 5IDRI, Seattle, United States, 6Tulane National Primate Research Center, Covington, United States, 7National Cancer Institute, Rockville, United States, 8University of Pennsylvania, Philadelphia, United States
Background: We compared immunogenicity and protective efficacy of an HIV vaccine comprised of DNA (env and gag) and Env proteins by co‐administration of DNA and Protein in the same muscle or by separate administration of the DNA and Protein components in contralateral sites.
Methods: Female rhesus macaques (20 animals/group) were immunized with a 6‐valent vaccine including DNA plasmids expressing membrane‐anchored gp145 Env sequentially isolated from a HIV‐1 infected individual (CH505). The DNA was delivered by IM injection followed by in vivo electroporation. The vaccine also included a gp120 Env protein component matching the sequences encoded by the plasmid DNA and adjuvanted in GLA‐SE. The DNA and protein vaccine components were administered in the same anatomical sites (“Co‐administration”) or in contralateral sites (“‘Separate Administration”) After six vaccinations in four‐month intervals, the macaques were challenged by weekly intravaginal exposures with low dose T/F tier‐hh2 SHIV CH505 stock.
Results: Only macaques in the co‐administration vaccine group were protected against SHIV CH505 acquisition, with a 67% risk reduction per exposure after 15 weekly IVAG challenges. Macaques in the co‐administration group developed higher Env‐specific humoral and cellular immune responses. Non‐neutralizing Env antibodies, ADCC and antibodies binding to Fc‐gamma Receptor IIIa were associated with decreased transmission risk. These data suggest that simultaneous recognition, processing and presentation of DNA + Env protein in the same draining lymph node play a critical role in the development of protective immunity.
Conclusions: Co‐immunization of DNA+Protein in the same muscle is superior for inducing protective immune responses against repeated tier‐2 SHIV challenge. The advantage of co‐immunization vaccine regimens targeting immunogens to the same draining LN could also be beneficial to other vaccine modalities and other pathogens.
OAA0402
Contribution of monocytes and CD4 T‐cell subsets in maintaining viral reservoirs in SIV‐infected macaques treated early after infection with antiretroviral drugs
H. Rabezanahary1, J. Clain1, G. Racine1, G. Andreani1, G. Benmadid‐Laktout1, O. Zghidi‐Abouzid1, G. Silvestri2, J. Estaquier1,3
1Centre de Recherche du CHU de Québec, Université Laval, Québec, Canada, 2Yerkes National Primate Research Center, Emory University, Atlanta, United States, 3INSERM U1124, Université Paris Descartes, Paris, France
Background: Although early antiretroviral therapy (ART) suppresses viral replication, ART discontinuation results in viral rebound, indicating early viral seeding and absence of full eradication. Therefore, identified the nature of infected cells and sanctuaries that contribute to viral rebound are crucial for HIV cure.
Methods: Rhesus macaques (RMs) were infected intravenously with SIVmac251 (20 AID50). Some of them were treated with ART at day 4 post infection. RMs were sacrificed at different time point post‐infection during natural infection (no ART), under ART (ART) and after ART interruption (ATi). Lymphoid tissues, including spleen, mesenteric and axillary/inguinal LNs and intestine (colon, ileum and jejunum parts) were recovered immediately after euthanasia. By flow cytometry, CD4 and monocyte cell subsets were sorted. Viral load and cell‐associated viral DNA and RNA were quantified by RT‐PCR as well as productive infectious viruses.
Results: We demonstrated that, in the absence of ART, monocyte cell subsets harbour viral DNA and RNA, and viruses produced after stimulation are infectious. We also demonstrated that TEM and TFH cells are the main preferential SIV target cells producing infectious SIV after T‐cell activation. We provided evidence that early ART, administrated at day 4 post‐infection, efficiently prevents viral dissemination. Furthermore, our results highlighted that early ART prevents infection of monocyte cell subsets in different tissues whereas ART did not prevent the establishment of viral reservoirs in TEM and TFH cells from visceral tissues including spleen and mesenteric LNs. We also observed that early ART drastically reduced inflammation. Consistent with previous reports, ART interruption is associated with viral rebound in less than two weeks, leading to viral dissemination and targeting both monocyte and T‐cell subsets.
Conclusions: Altogether, our results demonstrated that early ART prevents viral infection of monocytes but is unable to prevent infection of two major CD4 T‐cell subsets. Given the rapid dynamics of viral rebound after ATi, our results in RMs suggests that ART is actively suppressing viral production in infected cells, but once interrupted, these cells refill the pool of cells which are the main targets for SIV.
OAA0403
Cell proliferation contributes to the increase of genetically intact HIV over time
B. Horsburgh1, B. Hiener1, K. Fisher1, J.‐S. Eden1, E. Lee1, S. von Stockenstrom2, L. Odevall2, J. Milush3, T. Liegler3, R. Hoh3, R. Fromentin4, N. Chomont4, S. Deeks3, F. Hecht3, S. Palmer1
1The Westmead Institute for Medical Research, Centre for Virus Research, Westmead, Australia, 2Karolinska Institutet, Department of Microbiology, Tumor and Cell Biology, Stockholm, Sweden, 3University of California, Department of Medicine, San Francisco, United States, 4Université de Montréal, Centre de Recherche du CHUM and Department of Microbiology, Infectiology and Immunology, Montreal, Canada
Background: Effective HIV eradication strategies require an understanding of the mechanisms maintaining persistent HIV during therapy. Therefore, we examined the role of memory cell proliferation in maintaining genetically intact proviruses over four years of effective therapy.
Methods: Naïve (N), central (CM), transitional (TM) and effector (EM) memory CD4 + T‐cells were sorted from the peripheral blood of two participants on long‐term ART. Additional sequences from naïve, CM HLA‐DR+/DR‐, TM HLA‐DR+/DR‐ and EM HLA‐DR+/DR‐ T cells were obtained four years later. Full‐length individual proviral sequencing was used to characterise proviruses as intact or defective. Clusters of ≥ 2 100% genetically identical proviral sequences – indicative of host cell proliferation – were identified.
Results: A total of 287 and 448 sequences were isolated from the first and second time‐points, and 34 (12%) and 90 (20%) were considered intact. At both times the frequency of intact genomes differed between cell subsets, EM>TM>CM/N. In each subset, HLA‐DR+ memory T‐cells contained more intact provirus than HLA‐DR‐ memory T‐cells. The proportion of identical sequences was significantly higher in intact proviruses compared to defective at the second time‐point (85% vs. 41%, p = 0.03), but not the first. However, when the cell of origin was taken into account there was no significant difference in the proportion of 100% identical intact and defective genomes (p = 0.133). There was a significant correlation at the second time‐point between the proportion of identical sequences overall and the proportion of intact proviruses (R2=0.58 to 67, p = 0.02 to 0.04). The majority (44/51, 86%) of sequences observed at both time‐points (over four years) were found in cells of the same memory phenotype.
Conclusions: Genetically intact proviruses were found most frequently in the more differentiated EM cells. However, the frequency of intact proviruses was increased in each memory cell subset when the cell expressed HLA‐DR, highlighting the role of cellular activation in maintaining the reservoir. Moreover, the correlation between cellular proliferation and intact provirus highlights the importance of host cell proliferation in maintaining HIV over time. These findings demonstrate the importance of limiting cellular activation, differentiation and proliferation in strategies aimed at reducing the reservoir.
OAA0404
Multiple sanctuary sites for intact and “defective” HIV‐1 in post‐mortem tissues in individuals with suppressed HIV‐1 replication: Implications for HIV‐1 cure strategies
H. Imamichi1, V. Natarajan2, F. Scrimieri2, M. Smith1, Y. Badralmaa2, M. Bosche2, J. Hensien1, T. Buerkert1, W. Chang2, B. Sherman2, K. Singh1, H.C. Lane1
1NIAID, NIH, CMRS, LIR, Bethesda, United States, 2Frederick National Laboratory for Cancer Research, Frederick, United States
Background: The rapid viral rebound observed following treatment interruption, despite prolonged time on ART with plasma HIV‐RNA levels < 40 copies/mL, suggests persistent HIV‐1 reservoirs outside of blood. The purpose of the present study was to characterize post‐mortem tissues for HIV‐1 DNA and RNA in an effort to identify potential sanctuary sites in the body.
Methods: Autopsy specimens were collected from 8 donors with suppressed HIV‐1 replication at the time of death (blood HIV‐RNA levels < 5 copies/1 µg host‐genomic RNA). In addition to blood, tissue specimens were collected from lymph nodes, spleen, GI‐tract, CNS, lung, heart, kidney, liver pancreas and testes. Levels of HIV‐DNA and HIV‐RNA were determined using quantitative PCR. HIV‐1 proviruses were analysed by 5'LTR‐to‐3'LTR PCR single‐genome amplification of near full‐length HIV‐1 and direct amplicon sequencing.
Results: HIV‐DNA and HIV‐RNA species were detected in all 8 donors and ranged from <5 to 943 copies/2 µg gDNA and <5 to 102 copies/1 µg gRNA. While HIV‐1 provirus and cell‐associated HIV‐RNA could be found in all donors, no universal tissue hotspots were found across the donors. A total of 1329 HIV‐1 provirus sequences were obtained (average 222, range 50 to 745, per donor). Intact proviruses represented 5.1% (range 0 to 22.5%) of provirus in the blood and tissues. Lymph nodes had the greatest number of intact proviruses. “Defective” proviruses containing lethal genetic alterations or large internal deletions showed wide‐spread tissue distributions. The relative abundance varied by donor and much of the proviral DNA was associated with clonal expansions. Expanded provirus clones represented 43% (range 22 to 67.4%) of all HIV‐1 proviruses detected. Of note, similar findings were found in three donors with active HIV‐1 replication (blood HIV‐RNA levels ≥ 5 copies/1 µg gRNA, range: 15 to 3550) at the time of death.
Conclusions: We have demonstrated wide‐spread tissue distributions of HIV‐1 proviruses and viral RNA in an autopsy study of individuals with suppressed HIV‐1 replication. There were no universal hot spots with concentrations of HIV‐1 proviruses and/or viral RNA in tissues. These data demonstrate persistent HIV‐1 transcription at the tissue level, absence of common tissue sanctuary sites, and thus highlight the difficulties in designing effective HIV‐1 cure strategies.
OAA0405
High‐throughput characterization of HIV latent reservoir demonstrates integration into genes associated with inflammation, cell cycle, and nuclear envelope assembly, enrichment in accessible chromatin and large amounts of defective provirus
P. Roychoudhury1,2, K. Haworth2, H. Zhu1, C. Levy1,2, M.‐L. Huang1, C. Thanh3, T. Henrich3, R. Hoh3, S. Deeks3, F. Hladik21, H.‐P. Kiem21, K. Jerome12, S. Lee3
1University of Washington, Seattle, United States, 2Fred Hutchinson Cancer Research Center, Seattle, United States, 3University of California, San Francisco, United States
Background: HIV integration is a key step in the viral replication cycle. Prior in vivo studies have demonstrated that integration in specific genes may impact reservoir size and dynamics.
Methods: HIV integration sites (IS) were identified from bulk CD4‐enriched cryopreserved PBMCs from HIV+ ART‐suppressed individuals. Publicly available chromatin accessibility data (ATAC‐seq, DNase‐seq) and gene sets (MSigDB) were analysed in relation to IS data. Intact HIV DNA was estimated using a ddPCR assay detecting 5 regions of the HIV genome.
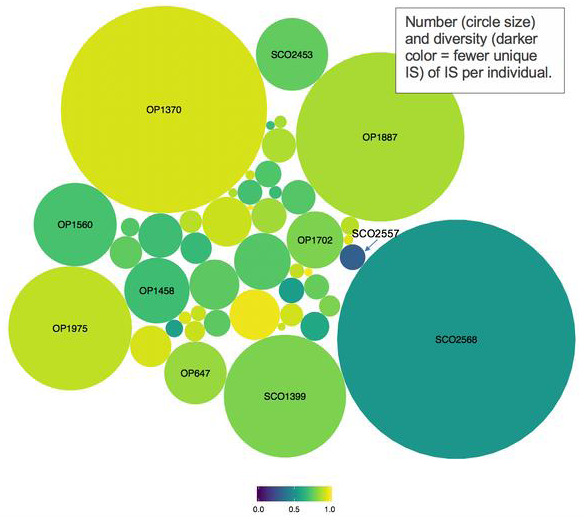
Results: Participants in this cross‐sectional study were mostly male (96%, n = 50) with median age 45 years, nadir CD4 + T cell count 364 cells/mm3, pre‐ART HIV RNA 4.7 log10 copies/mL, 4.7 years on ART and 1.4 years to ART initiation. We identified 38,214 unique IS with 80% in genes, and over‐representation of gene sets associated with chromatin accessibility, inflammation and nuclear envelope assembly. Although only 5% of IS (SD = 1.9%) were in known open chromatin regions, this exceeds the average amount of accessible chromatin in the genome. Most IS were seen 1 to two times; 26 individuals had clone sizes >3. The largest expanded clone was found in a participant (SCO2557) with low CD4 nadir and poor immune recovery (CD4 < 200); the associated IS was in the PIR gene involved in NF‐kB signalling. The only female participant (SCO2568) had expanded clones in RBM6 and CUL9, encoding tumour suppressor proteins and low CD4 nadir, but subsequent immune recovery. Across participants, the majority of proviral DNA was found to be defective.
Conclusions: In the largest in vivo HIV integration study to date, we observed enrichment of IS in open chromatin, genes regulating cell cycle, inflammation and nuclear envelope assembly. We showed that the majority of HIV DNA is defective viral sequences and highlight two unique clinical cases warranting further longitudinal studies in participants with poor immunologic recovery and in female participants.
Abstract OAA0405‐Figure 1.
OAA0406
The size of HIV reservoir is associated with telomere shortening and immunosenescence in early ART‐treated HIV‐infected children
A. De Rossi1, A. Dalzini1, G. Ballin1, S. Dominguez‐Rodriguez2, P. Rojo Conejo2, C. Foster3, P. Palma4, L. Sessa4, E. Nastouli5, S. Pahwa6, P. Rossi4, C. Giaquinto1, EPIICAL Consortium
1University of Padova, Department of Surgery, Oncology and Gastroenterology, Padova, Italy, 2Hospital Universitario 12 de Octubre, Madrid, Spain, 3Imperial College Healthcare NHS Trust, London, United Kingdom, 4Bambino Gesù Children's Hospital, Rome, Italy, 5UCL Great Ormond Street Institute of Child Health, London, United Kingdom, 6University of Miami, Miami, United States
Background: HIV infection is linked to premature senescence, with increased risk of ageing‐associated illnesses. Early ART has been associated with a reduced HIV reservoir in HIV‐perinatally infected children (PHIV), but its impact on the senescence process is an open question. Telomeres are critical for cellular replicative potential and their shortening is a marker of cellular senescence and ageing process. We investigated the relationship between immunosenescence and HIV reservoir in PHIV enrolled in a multicentre cross‐sectional study (CARMA, EPIICAL consortium).
Methods: Thirty‐seven PHIV, who started ART < 2 years of age and had undetectable viraemia for at least five years, were enrolled in this study. HIV‐DNA copies on CD4 cells and relative telomere length and levels of T‐cell receptor rearrangement excision circle (TREC, marker of thymic output) on CD4 and CD8 cells were quantified by qPCR. Senescent and activated CD4 and CD8 cells were estimated by flow cytometry. To explore the associations between cellular parameters, HIV reservoir and age at ART initiation, data were analysed using a multivariable Poisson regression (adjusted for baseline % CD4, plasmaviraemia, age at reservoir measurement and age at ART initiation as interaction term).
Results: HIV reservoir was significantly (p < 0.001) associated with immunosenescence (1.23 (1.21 to 1.26)) and telomere shortening (0.15 (0.13 to 0.17)) in CD4 cells, and immune activation (3.67 (3.49 to 3.85)) and TREC levels (1.08 (1.06 to 1.11)) in CD8 cells. These associations decreased by 1%, 10%, 6% and 6%, respectively, for each month ART was delayed. Early treated PHIV (ART initiation ≤6 months of age) displayed significantly lower HIV‐DNA level (89 (56 to 365) vs. 552 (303 to 1001) copies/106 cells) and % CD4 senescent cells (1.0 (0.5 to 2.7) vs. 2.9 (2.0 to 6.3)) than late treated ones (see Figure 1).
Abstract OAA0406‐Figure 1.
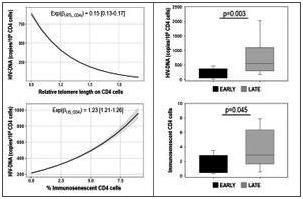
Conclusions: This is the first demonstration that HIV reservoir is directly associated with telomere shortening and immunosenescence on CD4 cells. Early ART initiation restricts the size of viral reservoir and the premature immunosenescence in PHIV.
OAB0102
Neurocognitive correlates of alcohol and cannabis use problems among adolescents and emerging adults living with HIV: ATN 129
B. Millar1, V. Dinaj‐Koci2, H. Budhwani3, S. Naar4, S. Nichols5, Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN)
1Hunter College, City University of New York (CUNY), PRIDE Health Research Consortium, New York, United States, 2Wayne State University, Department of Family Medicine and Public Health Sciences, Detroit, Michigan, United States, 3University of Alabama at Birmingham, School of Public Health, Department of Health Care Organization and Policy, Birmingham, Alabama, United States, 4Florida State University, Center for Translational Behavioral Science, Tallahassee, Florida, United States, 5University of California, San Diego, School of Medicine, Department of Neurosciences, La Jolla, United States
Background: Substance use represents an important health issue for youth living with HIV (YLWH). Accordingly, identifying neurocognitive factors influencing substance use among YLWH is vital. We tested associations between three often‐tested neurocognitive factors – inhibitory control (Flanker task), risk‐taking (Balloon Analogue Risk Task, BART) and delay discounting (Money Choice Questionnaire, MCQ) – and alcohol and cannabis use among YLWH aged 17 to 24. We adjusted for working memory, processing speed and episodic memory, areas commonly affected by HIV disease.
Methods: Participants enrolling for a U.S.‐based comparative effectiveness trial for alcohol‐using YLWH from 2014 to 2017 reported on demographics and completed computerized neurocognitive tasks: Flanker Task (NIH Toolbox), MCQ, BART, as well as Working Memory (NIH Toolbox List Sorting), Processing Speed (Visual Patterns; NIH Toolbox) and immediate recall on the Hopkins Verbal Learning Test‐Revised. Alcohol and cannabis use frequency and associated problems were summarized using the ASSISTs substance use involvement score (log‐transformed).
Results: Of the 179 participants (mean age, 21.4), 18 reported perinatal HIV infection. Most identified as Black (82%), and gay or bisexual males (72%). Overall, comparatively lower processing speed and immediate recall, though not working memory, were observed in this sample relative to age‐matched norms. Linear models, adjusting for age, gender and recent cannabis use, showed greater alcohol substance involvement was associated with lower Flanker (b = −0.01, p = 0.03) and BART (b = −0.01, p = .05), but not MCQ. Adding HIV‐related covariates (working memory, processing speed, immediate recall and whether perinatally infected), only Flanker remained significant (b = −0.01, p = .03). Among the 151 cannabis‐using participants, models adjusting for age and gender showed greater cannabis substance involvement was associated only with lower Flanker scores (b = −0.00, p = .03). Adding in the above HIV‐related covariates, Flanker remained significant (b = −0.00, p < .05). Scoring one standard deviation lower on Flanker was associated with a 1.23 point and 1.15 point increase on substance involvement scores (range: 0 to 39) for alcohol and cannabis respectively.
Conclusions: Greater alcohol and cannabis involvement was consistently associated with lower ability to inhibit attention to irrelevant stimuli, but not with risk‐taking (adjusted for HIV‐related covariates) or delay discounting. This highlights the importance of inhibitory control and executive functioning more generally for substance use prevention among YLWH.
OAB0103
Construct validity supports use of a novel, tablet‐based neurocognitive assessment for adolescents and young adults affected by perinatal HIV from vulnerable communities in the United States
R.N. Robbins1, J. Liu2, A.F. Santoro1, N. Nguyen1, J. Raymond1, E. Siegel1, S. Espinel1, C. Dolezal1, A. Wiznia3, E. Abrams4, C.A. Mellins1
1HIV Center for Clinical and Behavioral Studies at Columbia University and New York State Psychiatric Institute, New York, United States, 2New York State Psychiatric Institute, Psychiatry, New York, United States, 3Albert Einstein College of Medicine and Jacobi Medical Center, Department of Pediatrics, Bronx, United States, 4ICAP at Columbia University, Mailman School of Public Health, New York, United States
Background: Neurocognitive impairment is common among adolescents and young adults (AYA) living with perinatally acquired HIV (PHIV) and perinatal HIV‐exposure without HIV‐infection (PHEU). However, current assessment Methods are time‐consuming, require specialized forms, equipment and highly trained personnel to administer and score which precludes their use in many contexts. NeuroScreen is a novel, highly automated, relatively brief (25 minutes), easy‐to‐use by any staff, tablet‐based neurocognitive assessment tool that provides real‐time results, with potential to make neurocognitive assessments more available. This study examined how well (i.e. construct validity) the NeuroScreen app measures the neurocognitive domains of processing speed, working memory and executive functioning in AYA with PHIV and PHEU based on established gold‐standard, paper‐and‐pencil tests of those domains.
Methods: Participants came from an ongoing longitudinal study (CASAH) of AYA with PHIV and PHEU from vulnerable communities in New York City. To assess validity, at their last follow‐up, 62 AYA (33 PHIV, 29 PHEU) completed eight NeuroScreen tests of processing speed, working memory, executive functioning, as well as the gold‐standard Trail Making Tests A and B (TMT A (processing speed) and B (executive functioning)), and WAIS‐IV Digit Span Forwards and Backwards (working memory)). Pearson correlation coefficients were computed between the paper‐and‐pencil and NeuroScreen tests.
Results: Median age of participants was 24 years (IQR 22 to 26); 64% were male, 46% Latinx, and 44% African‐American. The paper‐and‐pencil and NeuroScreen tests of processing speed, working memory and executive functioning were all significantly correlated with each other respectively (Table 1).
Abstract OAB0103‐Table 1
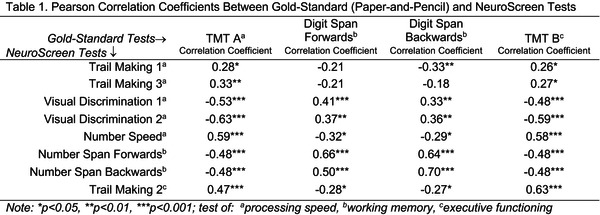
Conclusions: Results provide support for use of NeuroScreen with this population. Given its significant associations with gold‐standard tests, as well as ease‐of‐use, automation and real‐time results, NeuroScreen has great potential as a scalable assessment tool for clinical and research practice – providing access to much needed neurocognitive assessments for AYA at risk for neurocognitive deficits from HIV and other vulnerabilities.
OAB0104
High levels of mental health resilience among adolescents living with HIV in Thailand and Cambodia
R. Robbins1, K.M. Malee2, K. Chettra3, J. Sophononphan4, P. Kosalaraksa5, L. Aurpibul6, P. Ounchanum7, S. Kanjanavanit8, C. Ngampiyaskul9, P. Sun Ly3, K. Thongpibul10, R. Paul11, J. Ananworanich12, S.J. Kerr4, C.A. Mellins1, T. Puthanakit13
1HIV Center for Clinical and Behavioral Studies at Columbia University and New York State Psychiatric Institute, New York, United States, 2Northwestern University Feinberg School of Medicine, Department of Psychiatry and Behavioral Science, Chicago, United States, 3National Center for HIV/AIDS Dermatology and STDs, Phnom Penh, Cambodia, 4The HIV Netherlands Australia Thailand Research Collaboration (HIV‐NAT), The Thai Red Cross AIDS Research Centre, Bangkok, Thailand, 5Faculty of Medicine, Khon Kaen University, Department of Pediatrics, Khon Kaen, Thailand, 6Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand, 7Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand, 8Nakornping Hospital, Chiang Mai, Thailand, 9Prapokklao Hospital, Chanthaburi, Thailand, 10Faculty of Humanities, Chiang Mai University, Department of Psychology, Chiang Mai, Thailand, 11Missouri Institute of Mental Health/University of Missouri‐St. Louis, Missouri, St. Louis, United States, 12The Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, United States, 13Faculty of Medicine, Chulalongkorn University, Department of Pediatrics, Bangkok, Thailand
Background: Adolescents affected by HIV (i.e. perinatally acquired HIV (PHIV) and HIV‐exposed but uninfected (HEU)) are at risk for mental health (MH) problems; many, however, do not have MH problems. We used group‐based trajectory modelling to identify resilient MH trajectories and their predictors.
Methods: PHIV, HEU and HIV‐unexposed, ‐uninfected (HUU) Thai and Cambodian adolescents from the RESILIENCE Study underwent four yearly MH assessments. Resilient MH was defined as no MH problem at any study visit on the Child Behaviour Checklist, Children's Depression Inventory (<18 years) or Center for Epidemiological Studies‐Depression Scale (≥18 years). Resilience trajectory assignment was made through maximum likelihood estimation and Bayesian Information Criterion. Multinomial logistic regression examined baseline predictors of trajectories.
Results: 477 adolescents (201 PHIV, 131 HEU and 145 HUU; females 56%), median age 13 years (IQR 11 to 15) at enrolment, were evaluated over a median of 3 (IQR 2 to 4) visits. Analyses revealed a 3‐trajectory classification (Figure 1). Group 1 (n = 336) had consistently high resilience (91% to 97% of visits with no MH problems). Group 2 (n = 111) had consistently low resilience (25% to 35% of visits with no MH problems). Group 3 (n = 30) had increasing resilience from ages <11 to 15. Adolescents in Group 2 were more likely to: be PHIV than HUU (relative risk ratio (RRR) 1.46 (95% CI 1.00 to 2.12)), have lost any parent (RRR 1.74 (95% CI 1.25 to 2.43) and live with someone with MH problems (RRR 1.92 (95% CI 0.80 to 4.65)) than adolescents in Group1. Household income and sex were not associated with group membership.
Abstract OAB0104‐Figure 1.
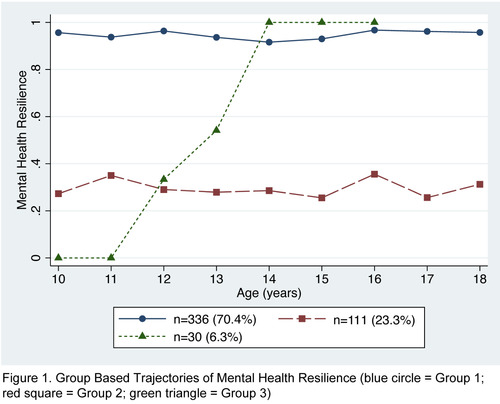
Conclusions: Most adolescents in the RESILIENCE Study exhibited MH resilience, including those with PHIV. The strongest predictors of low MH resilience were PHIV, losing any parent and living with a person with MH problems. MH interventions for AYA experiencing parental loss and other adverse family events may increase the likelihood of resilient MH outcomes as youth age.
OAB0105
Depo‐Provera worsens bone loss with TDF‐containing ART initiation in young women
F.K. Matovu1,2,3, J.A. Babirye1, M. Nabwana1, P. Musoke41, M. Beksinska5, J.M. Pettifor6, M.G. Fowler7, T.T. Brown8, for the BONE: CARE Study Team
1Makerere University‐Johns Hopkins University Research Collaboration, Kampala, Uganda, 2Makerere University College of Health Scienced, School of Public Health, Kampala, Uganda, 3Consortium for Advanced Research and Training in Africa/University of Witwatersrand, Johannesburg, South Africa, 4Makerere University College of Health Sciences, Department of Pediatrics and Child Health, Kampala, Uganda, 5Maternal Adolescent & Child Health (MatCH) Research, Johannesburg, South Africa, 6University of the Witwatersrand, Department of Paediatrics, Johannesburg, South Africa, 7Johns Hopkins University School of Medicine, Department of Pathology, Baltimore, United States, 8Johns Hopkins University, Division of Endocrinology, Diabetes, and Metabolism, Baltimore, United States
Background: Antiretroviral therapy (ART) initiation with tenofovir disoproxil fumarate (TDF) is associated with bone mineral density (BMD) loss. Among women of reproductive age, depot medroxyprogesterone acetate (DMPA, Depo Provera) also negatively impacts BMD. Our goal was to determine the combined BMD effects of DMPA and TDF initiation in young women over two years, compared to a matched HIV‐uninfected group.
Methods: Women were recruited from 11 HIV care centres and general health facilities around Kampala, Uganda and classified based on their combination of HIV status, TDF use and DMPA use. We compared three groups: women initiating TDF‐containing ART with (HIV+/DMPA+/TDF+) and without DMPA (HIV+/DMPA‐/TDF+) and an HIV‐uninfected control group not taking DMPA (HIV‐/‐DMPA‐/TDF)‐. All HIV+ women were ART‐naïve at baseline. BMD assessments of lumbar spine (LS), total hip (TH) and femoral neck (FN) were done using dual energy x‐ray absorptiometry at 6‐monthly intervals. We used repeated measures analyses to compare rate of change, calculated as percent (%) change in BMD/year.
Results: Between March 2015 and October 2017, we enrolled 265 HIV‐infected women initiating TDF‐containing ART (159 DMPA users, 106 non‐hormonal users), and 69 uninfected. Median age was 26 years. Baseline BMD was not significantly different from that of HIV‐uninfected controls. Annualized rates of BMD loss were higher in HIV‐infected women with greatest loss occurring in DMPA users compared to HIV‐infected non‐hormonal users, or uninfected controls at all sites: 4.0%(−4.4, −3.6) vs. −1.8%(−2.2, −1.4) vs. 0.8%(0.4, 1.1) at LS, −2.1%(−2.3, −1.9) vs. −0.9%(−1.1, −0.6) vs. −0.0%(−0.4, 0.3) TH and −2.5%(−2.8, −2.2) vs. −1.0%(−1.3, −0.7) vs. 0.1%(−0.3, 0.5) FN respectively. These changes were significantly different between the three groups, all p < 0.05 (Figure 1).
Abstract OAB0105‐Figure 1.

Conclusions: Concomitant DMPA use was associated with a doubling of BMD loss in young women initiating TDF‐containing ART. Newer treatment bone sparing regimens like tenofovir alafenamide‐based ART may mitigate BMD loss and early ageing among HIV‐infected women.
OAB0106
Optimization to dolutegravir‐based ART in a cohort of virally suppressed adolescents is associated with an increase in the rate of BMI change and odds of becoming overweight
N. Thivalapill1, N. Mthethwa2, S. Dlamini2, J. Petrus2,3, T. Simelane2, X. Katembo2, M. Abadie2,3, F. Anabwani2, S. Perry2,3, N. Mthethwa4, H.L. Kirchner3,5, A. Mandalakas3, B. Lukhele2, A. Kay2,3
1Harvard T.H. Chan School of Public Health, Epidemiology, Boston, United States, 2Baylor Children's Foundation‐Eswatini, Mbabane, Eswatini, 3Baylor College of Medicine, Pediatrics, Houston, United States, 4Eswatini National AIDS Program, Mbabane, Eswatini, 5Geisinger Health System, Department of Population Health Sciences, Geisinger, United States
Background: Antiretroviral therapy (ART) regimens that contain Dolutegravir (DTG) have been reported to be associated with increases in body mass index (BMI). However, this relationship has been poorly elucidated in adolescents, especially those in Sub‐Saharan Africa.
Methods: BMI measurements in a retrospective observational cohort of 605 virally suppressed (< 200 copies/mL3) adolescents living with HIV and enrolled in care at a clinical site in Eswatini, were analysed between one year prior to DTG initiation and up to one year after DTG initiation. 295 females and 310 males had an average of 6.4 visits and a total of 4040 visits within the study period. Two random‐effects linear spline models, with knots at DTG initiation, were used to model the rate of change in BMI and the odds of becoming obese or overweight, as defined by WHO BMI‐for‐age cutoffs, while adjusting for sex, DTG companion drugs, previous ART regimens and age at DTG initiation.
Results: In the first model, the rate of change in BMI was 0.316 kg/m2 per year prior to DTG initiation while the rate of change after DTG initiation was 0.941 kg/m2 per year (p < 0.0001). The second model reported no change in the odds of becoming overweight or obese prior to DTG initiation (OR = 0.998, p = 0.136). After DTG initiation, the odds of becoming overweight or obese increased by approximately 1% every day (OR = 1.010, p = 0.015). Patients on TDF‐3TC‐DTG compared with ABC‐3TC‐DTG had higher BMIs on average, as did females compared with males. BMI did not vary significantly by previous ART regimens (nevirapine or efavirenz).
Conclusions: The results suggest that DTG initiation is associated with an increase in the rate of BMI change and an increase in the odds of becoming either overweight or obese in adolescents living with HIV. Further investigation is required to assess how DTG impacts BMI in adolescents following a longer duration of treatment. Future work in a larger sample of this cohort is planned to estimate a predictive tool to identify adolescents who are most likely to become overweight or obese after being optimized to DTG.
OAB0202
Late‐onset opportunistic infections while on ART in Latin America
I. Núñez‐Saavedra1, B. Crabtree‐Ramirez1, B.E. Shepherd2, T.R. Sterling2, P. Cahn3, B. Grinsztejn4, C.P. Cortes5, D. Padgett6, J. Sierra‐Madero1, C. McGowan2, A.K. Person2, Y. Caro‐Vega1, Caribbean, Central, South America network for HIV epidemiology (CCASAnet) study group
1Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”, Mexico City, Mexico, 2Vanderbilt University, Nashville, United States, 3Fundación Huésped, Buenos Aires, Argentina, 4Instituto Nacional de Infectología Evandro Chagas, Río de Janeiro, Brazil, 5Fundación Arriarán, Santiago, Chile, 6Hospital Escuela Universitario, Tegucigalpa, Honduras
Background: Incidence of late‐onset (occurring after six months of antiretroviral therapy (ART) start) AIDS‐defining opportunistic infections (LOIs) and factors associated with them are largely unknown in resource‐limited settings. The aim of this study was to describe the incidence and risk factors of LOIs in five sites from Latin America as part of the Caribbean, Central and South America network for HIV epidemiology (CCASAnet).
Methods: We included all adults ART‐naive patients enrolled at CCASAnet sites in Argentina, Brazil, Chile, Honduras and Mexico from 2001 to 2015 who remained in care after year months of ART. We excluded patients with unavailable prior AIDS status. Among those who developed a clinical outcome, we report median time to LOI, death and LTFU. Using a Fine and Gray competing risk model (treating death and LTFU as competing events) we estimated the cumulative incidence of each outcome over time and calculated sub‐distribution hazard ratios.
Results: 5966 patients were elegible. Median follow‐up was 5.5 (IQR 3.01 to 9.06) years. 1837 (31%) patients had a clinical outcome (701 (38%) were LOIs). Estimated cumulative incidence at five years was of 9% for LOIs, 8% for LTFU and 4% for death (Figure 1). Commonest LOIs were tuberculosis (27%), oesophageal candidiasis (13%) and P. jirovecii pneumonia (12%). Median time to event was 2.5 years for LOIs, 3.4 for death and 4.5 for LTFU. Having an AIDS defining‐event during the first year months of treatment (HR:1.97 (1.57 to 2.46)), lower CD4 count at ART start (HR:1.33 (1.33 to 1.33) for CD4:100vsCD4:300), and starting ART earlier years (HR:1.35 (1.33 to 1.37) for 2005vs2015) were significantly associated with a higher risk of LOI.
Abstract OAB0202‐Figure 1.
Conclusions: In our cohort, LOIs continued to occur late during follow‐up. Risk factors were similar to those usually associated with early opportunistic infections. Closer long‐term follow‐up may be warranted in patients with lower CD4 at ART start and those initiating during the earlier years of the cohort.
OAB0203
Effects of immune‐check point inhibitors on anti‐HIV‐specific immune responses and HIV‐reservoir in people living with HIV (PLHIV) and cancer
M. Baron1, C. Soulié2, L. Assoumou3, J. Anna‐Tine3, B. Abbar1, A. Rousseau1, M. Veyri4, D. Costagliola3, C. Katlama5, B. Autran1, J. Cadranel6, A. Lavolé6, A. Saez‐Cirion7, O. Lambotte8, J.‐P. Spano4, A.‐G. Marcellin2, A. Guihot1, ANRS CO24 ONCOVIHAC Study Group
1CIMI, UMR 1135, Paris, France, 2INSERM‐Sorbonne Université, UMR_S 1136, Paris, France, 3INSERM‐Sorbonne Université, Institut Pierre Louis d'Epidémiologie et de Santé Publique, Paris, France, 4Hôpital Pitié‐Salpêtrière, Assistance‐Publique Hôpitaux de Paris, Medical Oncology, Paris, France, 5Hôpital Pitié‐Salpêtrière, Assistance‐Publique Hôpitaux de Paris, Infectious Diseases, Paris, France, 6Hôpital Tenon, Assistance‐Publique Hôpitaux de Paris, Pneumology, Paris, France, 7Institut Pasteur, Unité HIV, Inflammation et Persistance, Paris, France, 8Hôpital Kremlin‐Bicêtre, Assistance‐Publique Hôpitaux de Paris, Internal Medicine and Clinical Immunology, Paris, France
Background: Immune checkpoint inhibitors (ICPi) are a major advance in cancer treatment. Whether they can act as a latency reversal agent towards an HIV cure is yet unknown, with still sparse immune‐virological data.
Methods: OncoVIRIM is a biological sub‐study of the ongoing ANRS CO‐24 OncoVIHAC prospective multicentre cohort including virally suppressed PLHIV treated with anti‐PD1 for cancer. Blood samples were obtained at baseline and before different cycles (cycles 2, 3/4, 9, 15/18, 27/36, 51). Were evaluated by flow cytometry: T‐cell counts (CD3, CD4, CD8), T‐cell differentiation and activation markers (CD45RA, CCR7, CD27 and CD69, CD27, CD38, Ki67, HLA‐DR), ICP expression and HIV‐specific T cells measured by intracellular cytokine staining (ICS); by PCR: ultrasensitive plasma HIV‐RNA and total cell associated HIV‐DNA.
Results:
Abstract OAB0203‐Table 1
| Median baseline (range) | Median delta (D) or median fold change (FC) from C2 to D0 (n) | Median delta (D) or median fold change (FC) from last point to D0 (n) | p between C2 and D0 (Wilcoxon signed‐rank test) | p between last point and D0 (Wilcoxon signed‐rank test) | |
|---|---|---|---|---|---|
| CD4 HLA‐DR+ | 3.48% (0.52 to 6.17) | 2.91 (D, n = 10) | −0.26 (D, n = 12) | 0.0039 | 0.5693 |
| CD8 PD1+ | 28.8% (20 to 42) | 24.1 (D, n = 10) | −23.335 (D, n = 11) | 0.0195 | 0.001 |
| CD8 CTLA4+ | 0.05% (0.01 to 0.25) | −0.015 (D, n = 10) | 0.0005 (D, n = 12) | 0.625 | 0.6621 |
| CD8 TIM3+ | 5.66% (2 to 17) | −0.27 (D, n = 10) | 1.115 (D, n = 12) | 0.7695 | 0.2734 |
| HIV‐specific‐CD8+ | 1.42% (0.1 to 6.52) | 0.0025 (D, n = 5) | 0.0003 (D, n = 6) | 0.8125 | 0.8468 |
| HIV‐RNA | 20 copies/mL (1 to 47) | 1 (FC, n = 6) | 1 (FC, n = 7) | 0.75 | 0.8539 |
| HIV‐DNA | 218 copies/106 cells (40 to 620) | 0.486 (FC, n = 6) | 0.501 (FC, n = 7) | 0.625 | 0.4375 |
Fourteen patients had been enrolled (median age 61 years), from January 2018 to June 2019. The median follow‐up was six months (range 1 to 18), 4 patients stopped treatment and 6 died. The median baseline CD4‐cell count value was 373/mm3 (range 90 to 888), CD4/CD8 ratio was 0.8 (range 0.1 to 2.1). At C2 and at last time point, there was no significant change in CD4 and CD8‐cell counts or in T‐cell subsets distribution. However, proportions of HLA‐DR+ CD4 T cells increased by 85% at C2 without change in other activation markers. PD1 expression dramatically decreased by 95% at C2 without change in CTLA4 and TIM3 expression overtime. Frequencies of HIV‐specific‐CD8 T cells remained stable overtime, but with higher PD1 expression on IFN‐γ+ HIV‐specific‐CD8 T cells compared to non‐HIV‐specific‐CD8 T cells at baseline (70% vs. 38%, p = 0.0012). HIV‐RNA and DNA remained stable overtime (final median values: 10.5 copies/mL and 80 copies/106 cells).
Conclusions: In a context of HIV infection and cancer, these preliminary data on a limited number of patients suggest that ICPi used in monotherapy do not significantly impact the clinical biology of HIV infection.
OAB0204
A new tool for achieving the first goal of UNAIDS 90‐90‐90 targets – HIV self‐testing with urine
J. Ma1, Y. Wang2, T. Zhang3, Y. Wang1, Y. Lv1, C. Jin1, J. Wang2, Y. Jiang1
1National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China, 2Department of AIDS/STD Control and Prevention, Dehong Prefecture Center for Disease Control and Prevention, Yunnan, China, 3Center for Infectious Diseases, Beijing You'an Hospital, Capital Medical University, Beijing, China
Background: In view of insufficient HIV diagnosis in China, there is an urgent need of self‐testing. In August 2019, the first HIV self‐testing kit (colloidal gold) using urine specimen was approved by CFDA, which largely promoted the progress of HIV self‐testing in China. To provide scientific evidence for large scale application, the performance of HIV self‐testing with urine was evaluated in multicentre studies.
Methods: To evaluate the concordance of urine and blood colloidal gold kit, paired blood and urine specimen were collected from all 827 newly diagnosed HIV infected individuals and 214 healthy individuals in Dehong prefecture in 2018. To evaluate the performance of HIV self‐testing with urine in untrained individuals, 1066 individuals participated in a multicentre study of HIV self‐testing with questionnaire and testing in Beijing, Kunming and Zhenzhou including 92 from HIV positive people, 423 from key populations and 551 from general population.
Results: The HIV antibody detection concordance of colloidal gold kit with urine and blood was 98.07% (811/827) in HIV positive individuals and 100% (214/214) in healthy people. In the survey, 98.2% of untrained participants thought that they could complete HIV self‐testing with urine independently, and 97.84% thought that the experience of HIV self‐testing with urine was good. Overall, the antibody detection concordance between self‐testing by untrained individuals and professional testing was 99%. And the detection error rate was 0.66%, 6.86% and 2.16% in strong positive samples, weakly positive samples and negative samples respectively. The main causes of testing error included inaccurate volume of sample and inaccurate reacting time. The erroneous result was related with education level while it was unrelated to age, gender and location.
Conclusions: Our multicentre studies indicated that the innovative HIV self‐testing product using urine is acceptable, easy to use and has good detection concordance with professional testing using blood. As a new testing strategy in China, HIV self‐testing with urine provides an acceptable, convenient, and safe tool for people pursuing personal testing with better privacy, which will make important contribution to achieve the first goal of the UNAIDS 90‐90‐90 targets.
OAB0205
Vesatolimod, a toll‐like receptor 7 (TLR7) agonist, induces dose‐dependent immune responses in HIV controllers
J. Wallin1, J. Boice1, L. Zhang1, C. McDonald1, O. Podlaha1, P. Shweh1, M. Ramgopal2, C. Brinson3, E. DeJesus4, A. Mills5, P. Shalit6, S.G. Deeks7, J. Milush7, A. Ferre8, B. Shacklett8, D.M. Brainard1, D. SenGupta1
1Gilead Sciences Inc., Foster City, United States, 2Midway Research Center, Fort Pierce, United States, 3Central Texas Clinical Research, Austin, United States, 4Orlando Immunology Center, Orlando, United States, 5Southern California Men's Medical Group, Los Angeles, United States, 6Peter Shalit MD, Seattle, United States, 7University of California, San Francisco (UCSF), San Francisco, United States, 8University of California, Davis, United States
Background: An inadequate antiviral immune response occurs in People with HIV (PWH), requiring lifelong antiretroviral therapy (ART). Therefore, therapeutics designed to promote immune‐mediated ART‐free remission are under clinical evaluation. Vesatolimod (VES) is an oral, selective, small molecule TLR7 agonist shown to be safe and well tolerated in PWH. Dose‐dependent inductions of circulating serum cytokines and immune cell activation have been observed with VES treatment in HIV‐negative volunteers and PWH on ART. In addition, VES was associated with a modest delay in viral rebound and decrease in viral set‐point in HIV controllers in a placebo‐controlled Phase 1b trial. We further investigated the immune mechanisms of this effect.
Methods: We enrolled 25 HIV controllers (pre‐ART viral load 50 to 5000 c/mL) on ART. Seventeen participants were administered biweekly VES 4 to 8 mg (dose escalated within‐individual) for 10 doses, and eight participants received placebo. Participants were evaluated during an analytic treatment interruption (ATI) phase for up to 24 weeks to analyse the treatment effect on viral rebound. Peripheral blood mononuclear cells (PBMCs) and plasma were collected at baseline and on‐treatment (prior to ATI) for pharmacodynamic (PD) measurements and evaluation of HIV‐specific immune responses.
Results: Compared to placebo, VES induced a dose‐dependent increase of interferon stimulated mRNAs (ISGs: ISG15, MX1 and OAS1) and cytokines/chemokines (ITAC, IP‐10, IL1ra and IFN‐a), peaking 24 hours post‐dose and returning to baseline by seven‐day post‐dose. The ISG inductions plateaued at 6 mg with mean increase from baseline of 19‐, 8‐ and 6‐fold for ISG15, MX1 and OAS1 respectively. The minimal dose of VES where consistent and detectable cytokine/chemokines occurred was at 6 mg, with mean concentrations of 49, 368, 1834 and 0.6 pg/mL for ITAC, IP‐10, IL1ra and IFN‐a respectively. HIV‐specific T cell responses as assessed by intracellular cytokine staining (ICS) increased from baseline in some VES‐treated participants.
Conclusions: The PD and mechanistic activity of VES in PWH may play an important role in HIV cure innovations that include other agents such as CD8 + T‐cell‐inducing vaccines and monoclonal antibodies.
OAB0302
Analysis of protocol defined virologic failure through week 48 from a phase 2 trial (P011) of islatravir and doravirine in treatment‐naïve adults with HIV‐1 infection
C. Orkin1, J.‐M. Molina2, Y. Yazdanpanah3, C. Chahlin Anania4, E. DeJesus5, J. Eron6, S. Klopfer7, A. Grandhi7, K. Eves7, D. Rudd7, M. Robertson7, P. Sklar7, C. Hwang7, G. Hanna7, T. Correll7
1Queen Mary University of London, London, United Kingdom, 2St. Louis Hospital and University, Paris, France, 3Bichat Hospital, Paris, France, 4Hospital Hernan Henriaquez Aravena of Temuco, Temuco, Chile, 5Orlando Immunology Center, Orlando, United States, 6University of North Carolina, Chapel Hill, United States, 7Merck & Co., Inc., Kenilworth, United States
Background: Islatravir (ISL,MK‐8591) is the first nucleoside reverse transcriptase translocation inhibitor (NRTTI) in development for treatment and prevention of HIV‐1 infection. The objective of this analysis is to show detailed data of participants who discontinued with protocol‐defined virologic failure (PDVF) from the phase‐2 trial of islatravir (ISL) and doravirine (DOR).
Methods: Randomized, double‐blind, dose‐ranging trial participants initially received ISL (0.25, 0.75, or 2.25 mg) with DOR (100 mg) and lamivudine (3TC, 300 mg) or fixed‐dose combination of DOR, 3TC and tenofovir disoproxil fumarate (DOR/3TC/TDF) daily. Participants receiving ISL achieving HIV‐1 RNA < 50 copies/mL at week‐20 or later stopped 3TC at next visit. PDVF was conservatively defined as rebound with confirmed HIV‐1 RNA ≥ 50 copies/mL after suppression any time during the trial or non‐response with failure to achieve HIV‐1 RNA < 50 copies/mL by week‐48. Participants with PDVF were required to discontinue from the trial.
Results: 121 participants received study drug and were included in analyses. At week‐48, 89.7% (26/29), 90.0% (27/30), 77.4% (24/31) of randomized participants achieved HIV‐1 RNA < 50 copies/mL in the 0.25, 0.75 and 2.25 mg ISL groups, respectively, compared to 83.9%(26/31) with DOR/3TC/TDF. Six participants had PDVF; two rebounders each in the 0.25 and 0.75 mg ISL groups, one non‐responder in the 2.25 mg ISL group and one rebounder in the DOR/3TC/TDF group. All confirmatory HIV‐1 RNA Levels were < 80 copies/mL (Figure 1); none met criteria for resistance testing. Despite changing to new regimens, three of six participants (1 each from the 0.25 and 0.75 mg ISL groups and 1 from the DOR/3TC/TDF group) continued to have low‐level viraemia during 42‐day post‐discontinuation assessment.
Conclusions: Rates of PDVF were low and all participants who discontinued due to PDVF had HIV‐1 RNA levels below the clinically significant level of 200 copies/mL. The observed low‐level viraemia was comparable to levels detected in other treatment‐naïve studies.
HIV‐1 RNA levels Over Time for Participants with PDVF.
Abstract OAB0302‐Figure 1.
OAB0303
Adherence and factors associated with virologic success in HIV‐1 infected adults with tuberculosis receiving raltegravir or efavirenz in the ANRS 12300 Reflate TB2 trial
O. Marcy1, N. De Castro2, C. Chazallon1, E. Messou3, S. Eholié4, N. Bhatt5, C. Khosa5, D. Laureillard6, G. Do Chau7, V. Veloso8, C. Delaugerre2, X. Anglaret1, B. Grinsztejn8, J.‐M. Molina2, ANRS 12300 Reflate TB2 Study Group
1University of Bordeaux, Bordeaux Population Health ‐ Centre Inserm U1219, Bordeaux, France, 2Hôpital Saint Louis ‐ APHP, Paris, France, 3CEPREF, Abidjan, Cote D'Ivoire, 4CHU de Treichville, SMIT, Abidjan, Cote D'Ivoire, 5Instituto Nacional de Saude, Maputo, Mozambique, 6CHU de Nîmes, Nîmes, France, 7Pham Ngoc Thach Hospital, Ho Chi Minh City, Vietnam, 8Fiocruz, IPEC, Rio de Janeiro, Brazil
Background: Few studies have explored the use of integrase strand transfer inhibitors in HIV‐1 infected adults with tuberculosis. The Reflate TB2 trial failed to show non‐inferiority of raltegravir 400 mg BID compared to efavirenz 600 mg QD at week 48. We aimed to identify factors associated with virologic success, including adherence.
Methods: ANRS 12300 Reflate TB2 was an open‐label randomized trial conducted in Brazil, Côte d'Ivoire, France, Mozambique and Vietnam. ART‐naïve HIV1‐infected adults on tuberculosis treatment were randomized (1:1) to receive raltegravir 400 mg BID or efavirenz 600 mg QD with TDF 300 mg QD and 3TC 300 mg QD. We assessed adherence using pill counts. Poor adherence was defined as pill count ratio < 95%. We assessed determinants of virologic success (HIV‐1 RNA ≤ 50 copies/mL at 48 weeks) using logistic regression.
Results: 460 patients were enrolled (Brazil 43, Côte d'Ivoire 172, France 4, Mozambique 130, Vietnam 111; 230 in each trial arm): median age 35 (IQR 29 to 43) years, 40% female, median CD4 103 (IQR 38 to 239) cells/µL, median plasma HIV‐1 RNA 5.5 log/mL (IQR 5.0−5.8) with 340 (74%) patients having HIV‐1 RNA > 100,000 c/mL. Median pill count ratios over the study duration were 96.9% (IQR 89.4 to 100.0) and 100.0% (IQR 94.3 to 104.5) in the raltegravir and efavirenz arms, respectively, and poor adherence was seen in 96 (43%) and 60 (27%) patients, in the raltegravir and efavirenz arms respectively (p‐value < 0.001 for both). Overall, virologic success was achieved in 289/453 (64%) patients (excluding French), including 139/228 (61%) from raltegravir arm and 150/225 (67%) from efavirenz arm. In univariate analysis, gender, HIV‐1 RNA and adherence were associated with virologic success. In a multivariate model forcing country and study arm, female gender (OR: 1.77; 95% CI 1.16 to 2.72), HIV‐1 RNA < 100,000 c/mL (OR 2.29; 95% CI 1.33 to 3.96) and [100,000 c/mL to 500,000 c/mL (OR 1.62; 95% CI 1.02 to 2.57) versus > 500,000 c/mL, and pill count ratio ≥ 95% (OR 2.38; 95% CI 1.56 to 3.52) were independently associated with virologic success.
Conclusions: In the Reflate TB2 trial, higher adherence, lower baseline HIV‐1 RNA levels and female gender, but not treatment arm, were associated with virologic success. Lower treatment adherence to the raltegravir BID regimen might explain the failure to show non‐inferiority to the efavirenz regimen.
OAB0304
Reducing ART to less than 3‐ARV regimen linked to increased systemic inflammation
S. Serrano‐Villar1, M.R. López‐Huertas2, F. Gutiérrez3, M. Beltrán4, M. Pons5, P. Viciana6, J. Portilla7, F. García8, S. Moreno5, CoRIS
1University Hospital Ramón y Cajal, Infectious Diseases, Madrid, Spain, 2Carlos III Health Research Institute, Madrid, Spain, 3University Hospital of Elche, Elche, Spain, 4Carlos III Health Institute, Madrid, Spain, 5University Hospital Ramón y Cajal, Madrid, Spain, 6University Hospital Virgen del Rocío, Sevilla, Spain, 7University Hospital of Alicante, Alicante, Spain, 8University Hospital San Cecilio, Granada, Spain
Background: We assessed the long‐term consequences of changing triple therapy (3DR) to 2‐drug regimens (2DR) or PI‐based monotherapy (1DR) on virological failures, clinical events and systemic inflammation.
Methods: We selected ART‐naive patients initiating triple ART from 2004 to 2018 in the Spanish AIDS Research Network (CoRIS) who achieved virologic suppression (VS) in the first 48 weeks of ART and either remained on 3DR or were switched to 2DR (3TC+bPI; 3TC+DTG; DTG+RPV, CAB+RPV) or 1DR (bDRV or bLPV). We calculated cause‐specific cumulative incidence curves and used multivariate Cox proportional hazards models adjusted for potential confounders to estimate hazard ratios for the endpoints: 1) severe non‐AIDS events (NAE), 2) AIDS or AIDS‐related death, 3) all‐cause death, 4) virological failure, 5) composite endpoint of virological failure/serious NAE/death. In a nested study, we compared IL‐6, CRP, D‐dimers and IFABP trajectories during versus using multivariate mixed models and linear splines.
Results: From 14458 patients, 8416 met the inclusion criteria; 7665 remained on 3DR, 424 switched to 2DR and 327 to 1DR. The median time from enrolment to censoring was 4.9, 6.9 and 8.4 years in the 3DR, 2DR and 1DR groups respectively (P < 0.001).
No between‐group differences in the risk of endpoints 1 to 3 were detected. ART reduction after 24 months of therapy was associated with greater risk of virological failure (P = 0.003) and greater risk of the composite endpoint (p = 0.005), both driven by higher risk with 1DR but not with 2DR.
We analysed 710 samples from 174 subjects (3DR, N = 90; 2DR, N = 61; 1DR, N = 23). Compared to 3DR, 2DR was associated with increases of IL‐6 (p = 0.01), CRP (p = 0.003) and D‐dimers (p = 0.001) after year 3 from VS. A similar pattern was observed for the comparison between 3DR and 1DR, (only significant for D‐dimers trajectories, p = 0.002).
Conclusions: In this large cohort of virally suppressed individuals, 1DR was associated with a greater risk of virological failure, with no significant differences between 2DR and 3DR. However, maintaining 3DR was associated with a more favourable long‐term anti‐inflammatory profile than switching to 2DR or 1DR. The potential clinical implications of these findings on the development of non‐AIDS events deserve further investigation.
OAB0305
Islatravir safety analysis through week 48 from a phase 2 trial in treatment naïve adults with HIV‐1 infection
E. DeJesus1, J.‐M. Molina2, Y. Yazdanpanah3, A. Afani Saud4, C. Bettacchi5, C. Chahin Anania6, S. Klopfer7, A. Grandhi7, K. Eves7, M. Robertson7, P. Sklar7, C. Hwang7, G. Hanna7, T. Correll7
1Orlando Immunology Center, Orlando, United States, 2St. Louis Hospital and University, Paris, France, 3Bichat Hospital, Paris, France, 4University of Chile, Santiago, Chile, 5North Texas Infectious Diseases Consultants, Dallas, United States, 6Hospital Hernan Henriaquez Aravena of Temuco, Temuco, Chile, 7Merck & Co., Inc., Kenilworth, United States
Background: Islatravir (ISL, MK‐8591) is the first nucleoside reverse transcriptase translocation inhibitor (NRTTI) in development for treatment of HIV‐1 infection. We previously showed that ISL‐based regimens had similar efficacy to DOR/3TC/TDF in a phase 2 trial in treatment naïve adults. Here we present a detailed safety analysis of the week 48 results.
Methods: In this randomized, double‐blind, dose‐ranging trial, participants were initially assigned to receive ISL (0.25, 0.75, or 2.25 mg) with doravirine (DOR, 100 mg) and lamivudine (3TC, 300 mg) or a fixed‐dose combination of DOR, 3TC and tenofovir disoproxil fumarate (DOR/3TC/TDF) once daily. Participants receiving ISL with HIV‐1 RNA < 50 copies/mL at Week 20 or later stopped taking 3TC at their next visit and continued DOR+ISL at initial dosage; most participants stopped 3TC at Week 24. For the current analysis, we conducted a detailed review of adverse events (AE) examining the initial 24‐weeks, the 24‐week period after 3TC removal and the cumulative 0 through 48‐week study period.
Results: 121 participants received drug and were included in the analyses. Similar AE rates between treatment arms were observed across all arms of the trial for each time period. No dose‐dependent difference in the safety profile of ISL was observed. AEs were more frequent in the first 24 weeks of the trial as compared to the second 24‐week period for all treatment arms (Table 1). Overall, diarrhoea (most mild and transient) was more frequently reported for DOR/3TC/TDF (16.1%) as compared to ISL groups (combined 6.7%) while headache (most mild and transient) was more common in ISL groups (combined 11.1%) as compared to the DOR/3TC/TDF group (6.5%).
Conclusions: ISL was well tolerated regardless of dose through 48 weeks of treatment. Most AEs were mild and transient and did not result in study discontinuation.
Abstract OAB0305‐Table 1
OAB0402
Dolutegravir‐ versus low‐dose Efavirenz‐based regimen for the initial treatment of HIV‐1 infection in Cameroon: Week 96 Results of the ANRS 12313 – NAMSAL trial
C. Kouanfack1,2,3, T. Tovar Sanchez4, M. Lantche Wandji3, M. Mpoudi‐Etame5, P. Omgba Bassega6, S. Perrineau4, T. Abong Bwenda3, D. Tetsa Tata3, M. Tongo7, M. Varloteaux3, A. Montoyo8, M. Peeters4, J. Reynes9,4, A. Calmy10, E. Delaporte9,4, NAMSAL study group
1Faculty of Medicine and Pharmaceutical Sciences University of Dschang, Yaoundé, Cameroon, 2Central Hospital of Yaoundé, Yaoundé, Cameroon, 3Site ANRS, Yaoudé, Cameroon, 4TransVIHMI, Université Montpellier, IRD, INSERM, Montpellier, France, 5Military Hospital of Yaoundé, Yaoundé, Cameroon, 6Cité Verte Hospital, Yaoundé, Cameroon, 7CREMER, Yaoundé, Cameroon, 8Service de Vigilance des Recherches Cliniques ANRS Inserm, Paris, France, 9University Hospital Center of Montpellier, Montpellier, France, 10Geneva University Hospitals, Geneva, Switzerland
Background: The updated WHO 2019 guidelines for ARV treatment recommend a Dolutegravir (DTG)‐based regimen as the preferred first‐line regimen and low‐dose Efavirenz (EFV400) as an alternative option. The non‐inferior efficacy of DTG compared with EFV400 was prevoiously reported at W48. We report here the W96 data.
Methods: NAMSAL is a phase 3 randomized, open label, multicentre trial conducted in Yaoundé. HIV‐1 infected ARV‐naive adults with HIV‐RNA viral load (VL) > 1000 copies/mL were randomized (1:1) to DTG 50 mg or EFV 400 mg once daily, both with tenofovir disoproxil fumarate (TDF)/lamivudine (3TC). Randomization was stratified by screening VL and by site. The primary endpoint was the proportion of patients with VL < 50 copies/mL at W48 and extended at W96 (10% non‐inferiority margin).
Results: 613 participants (DTG arm: 310; EFV400 arm: 303) received at least one dose of study medication. In the ITT analysis at W96, the proportion of patients with HIV RNA < 50 copies/mL was 73.5% (228/310) and 72.3% (219/303) respectively (difference, 1.3%; 95% CI, ‐5.8 to 8.3; p‐value < 0.001). Figure 1 shows the viral suppression according to Baseline VL. The per‐protocol analysis showed similar results. Virological failure (WHO definition) was observed in 27 participants (DTG: 8; EFV400: 19), three were switched from DTG to EFV600 (May 2018 WHO signal). No resistance mutations to DTG was observed, unlike the EFV400 with 18 resistances (NNRTI±NRTI) in the 19 confirmed failure cases. Weight gain was greater in DTG arm (median weight gain: 5.0/3.0 Kg; incidence of obesity 12.3%/5.4%). 18 AE were observed (DTG: 8; EFV400: 10); one single participant in DTG arm had missing data.
Abstract OAB0402‐Figure 1.
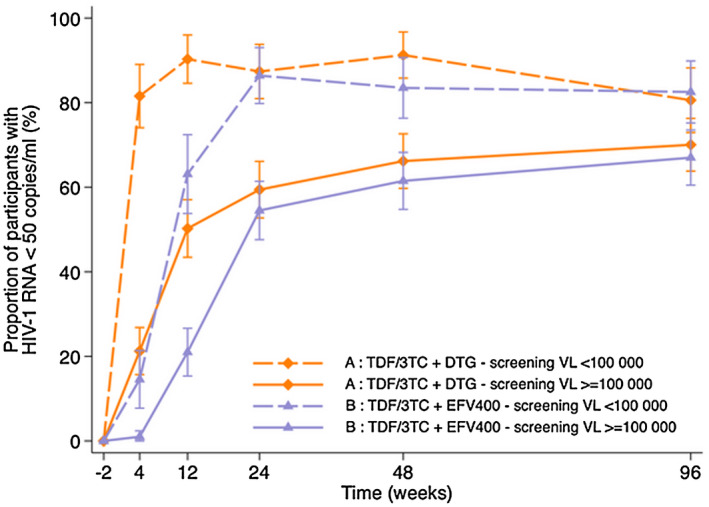
Conclusions: W96 results confirm the non‐inferior efficacy of the DTG‐based regimen and the no emergence of resistance to DTG. Virological success rate remains lower in patients with a high initial VL in both arms. We observed a continuous weight gain in the DTG arm.
OAB0403
Pooled analysis of 4 international trials of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) in adults aged > 65 or older demonstrating safety and efficacy: Week 48 Results
M. Ramgopal1, F. Maggiolo2, D. Ward3, B. Lebouche4, G. Rizzardini5, J.‐M. Molina6, C. Brinson7, H. Wang8, J. Gallant8, S. Collins8, I. McNicholl8, H. Martin8
1Midway Research, Fort Pierce, United States, 2Division of Infectious Diseases, ASST Papa Giovanni XXIII, Bergamo, Italy, 3Dupont Circle Physicians Group, Washington, D.C., United States, 4Research Institute, McGill University Health Centre, Montreal, Canada, 5Division of Infectious Diseases, Luigi Sacco Hospital, ASST Fatebenefratelli Sacco, Milan, Italy, 6Department of Infectious Diseases, Saint Louis Hospital, University Paris Diderot, Paris, France, 7Central Texas Clinical Research, Austin, United States, 8Gilead Sciences, Foster City, United States
Background: As life expectancy for people with HIV increases, optimizing antiretroviral therapy to fit the needs of older adults, including those with co‐morbidities and multiple medications, is paramount. B/F/TAF is a small single‐tablet regimen with few drug‐drug interactions, a high barrier to resistance and may provide a beneficial option for older patients.
Methods: In this pooled analysis of 4 international trials (Studies 1844, 1878, 4030 and 4449) of virologically suppressed (HIV‐1 RNA < 50 copies/mL), treatment‐experienced adults, we evaluated the efficacy and safety of switching to B/F/TAF for participants ≥ 65 years. Primary endpoint was HIV‐1 RNA < 50 copies/mL at Week 48 as defined by the FDA Snapshot algorithm.
Results: 140 participants were age ≥ 65 years at study enrolment. Median age (Q1, Q3) was 68 years (66, 72), 14% were female, and 88% were White. Medical history at baseline was significant for diabetes 22%, hypertension 55%, cardiovascular disease 24% and dyslipidaemia 59%. At W48, the proportion with HIV RNA < 50 copies/mL was 92% (129/140); 11 (8%) had no virologic data in window (5 discontinued study drug due to AE but had last available HIV‐1 RNA < 50 copies/mL; 6 had missing data but were still on study drug). No participant had virologic failure. Most common adverse events (AEs) were nasopharyngitis and arthralgia (7% each). Eleven participants (8%) had a study drug‐related AE, all were either Grade 1 or Grade 2. There were no Grade 3 to 4 study drug‐related AEs. Four participants had AEs that led to premature study drug discontinuation: abdominal discomfort, drug withdrawal syndrome, device‐related infection and alcohol withdrawal syndrome. Median changes from baseline in fasting lipids were: total fasting cholesterol (−7 mg/dL), LDL (−2 mg/dL), HDL (0 mg/dL), triglycerides (−15 mg/dL) and total cholesterol:HDL (−0.1). Median weight change was 1.0 kg (IQR −0.9, 3.0). Ten percent (14/140) of participants had Grade 3 or 4 laboratory abnormalities.
Conclusions: Switching to B/F/TAF in older adults was well tolerated and safe while maintaining high rates of virologic suppression through 48 weeks. These data support the use of B/F/TAF for treatment of adults ≥65 years who could benefit from a small tablet with few drug‐drug interactions and an established safety profile.
OAB0404
Third‐line antiretroviral therapy including raltegravir, darunavir/ritonavir and/or etravirine is well tolerated and achieves durable virologic suppression over 144 + weeks in resource‐limited settings ACTG: A5288 strategy trial
A. Avihingsanon1, M. Hughes2, R. Salata3, C. Godfrey4, C. McCarthy5, P. Mugyenyi6, E. Hogg7, R. Gross8, S.W. Cardoso9, A. Bukuru6, M. Makanga10, S. Faesen11, V. Mave12, B.W. Ndege13, S.N. Fontain14, W. Samaneka15, R. Secours14, M. van Schalkwyk16, R. Mngqibisa17, L. Mohapi18, J. Valencia19, P. Sugandhavesa20, E. Montalban21, J. Okanda22, C. Munyanga23, M.B. Chagomerana24, B.R. Santos25, N. Kumarasamy26, C. Kanyama27, R.T. Schooley28, J.W. Mellors29, C.L. Wallis30, A.C. Collier31, B. Grinsztejn9, for A5288 Study team
1Thai Red Cross AIDS Research Centre Treatment Clinical Research Site, Bangkok, Thailand, HIV‐NAT, Bangkok, Thailand, 2Harvard T H Chan School of Public Health, Boston, United States, 3Case Western Reserve University, Department of Medicine, Cleveland, United States, 4Senior Technical Advisor HIV Care and Treatment, PEPFAR/Office of the Global AIDS Coordinator, Washington DC, United States, 5Harvard School of Public Health, Statistical and Data Analysis Center,, Boston, United States, 6Joint Clinical Research Center, Kampala, Uganda, 7Social & Scientific Systems, Inc, Silver Spring, United States, 8Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, Philadelphia, United States, 9Instituto Nacional de Infectologia Evandro Chagas, Fundcao Oswaldo Cruz, Rio de Janeiro, Brazil, 10Kenya Medical Research Institute, Kisumu, Kenya, 11Wits HIV Clinical Research Site, Johannesburg, South Africa, 12BJ Medical College Clinical Research Site, Pune, India, 13AMPATH, Moi University Teaching Hospital Eldoret Clinical Research Site, Eldoret, Kenya, 14Les Centres GHESKIO Clinical Research Site, Port‐au‐Prince, Haiti, 15University of Zimbabwe College of Health Sciences Clinical Trials Research Centre, University of Zimbabwe, Harare, Zimbabwe, 16Family Clinical Research Unit Clinical Research Site, Stellenbosch University, Cape Town, South Africa, 17Durban Adult HIV Clinical Research Site, Enhancing Care Foundation, Durban, South Africa, 18Soweto AIDS Clinical Trials Group Clinical Research Site, University of the Witwatersrand, Johannesburg, South Africa, 19Barranco Clinical Research Site, Lima, Peru, 20Center for AIDS and STDs, Chiang Mai University, Chiang Mai, Thailand, 21San Miguel Clinical Research Site, Lima, Peru, 22Kisumu Clinical Research Site, Kisumu, Kenya, 23Malawi CRS, UNC‐project Malawi, Tidziwe Centre, Lilongwe, Malawi, 24UNC Project, Lilongwe, Malawi, 25Servico de Infectologia, Hospital Nossa Senhora da Conceicao, Grupo Hospitalar Conceicao, Porto Alegre, Brazil, 26Chennai Antiviral Research and Treatment Clinical Research Site, Chennai, India, 27University of North Carolina Project, Kamuzu Central Hospital, Lilongwe, Malawi, 28Division of Infectious Disease, University of California, San Diego, United States, 29Division of Infectious Diseases, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, United States, 30Bio Analytical Research Corporation South Africa, Lancet Laboratories, Johannesburg, South Africa, 31University of Washington School of Medicine, University of Washington, Seattle, United States
Background: ACTG A5288 was a strategy trial in resource‐limited settings (RLS) enrolling PLWH failing 2nd line PI‐based ART. Participants with resistance to LPV and/or all NRTIs were assigned to three different cohorts (B,C,D) according to resistance profiles (Figure 1) and started 3rd line regimens that included raltegravir (RAL), darunavir/ritonavir (DRV/r) and/or etravirine (ETR). At 48 weeks, 87% of participants in these cohorts achieved HIV‐1 RNA ≤ 200. At sites where RAL, DRV/r or ETR were not available outside the study, the drugs were provided via the study for 96 additional weeks. We report here long‐term outcomes over 144 + weeks, including all available follow‐up (FU) of participants in Cohorts B, C and D.
Methods: All participants were initially followed until 48 weeks after the last participant was enrolled. During the additional long‐term FU, HIV‐1 RNA was done every 48 weeks and CD4 count at 96 weeks; HIV‐1 RNA ≤ 200 copies/mL was imputed if necessary if both preceding and succeeding HIV‐1 RNA ≤ 200; CD4 count changes were estimated using loess regression.
Results: Of 257 participants, 38% were female. At study entry, median age 42y; CD4 count 179 cells/µL; HIV‐1 RNA: 4.6 log10 copies/mL. Median FU, 168 weeks (IQR: 156 to 204); 15 (6%) were lost to FU and 9 (4%) died. 27/246 (11%), 26/246 (11%) and 13/92 (14%) of PLWH who started RAL, DRV/r and ETR, respectively, discontinued these drugs, three due to adverse events. Estimated proportions with HIV‐1 RNA ≤ 200 copies/mL were 87%, 86%, 83% and 80% at weeks 48, 96, 144 and 168 (95% CI at week 168: 74% to 85%). Estimated mean increases in CD4 count were 150, 201, 245 and 265 cells/µL respectively (95% CI at week 168: 247 to 283).
Conclusions: Third‐line regimens containing RAL, DRV/r and/or ETR were very well tolerated and provided a high rate of durable virologic suppression among people living with HIV in RLS.
Abstract OAB0404‐Figure 1. Cohort definitions, assignment and antiretroviral regimens.
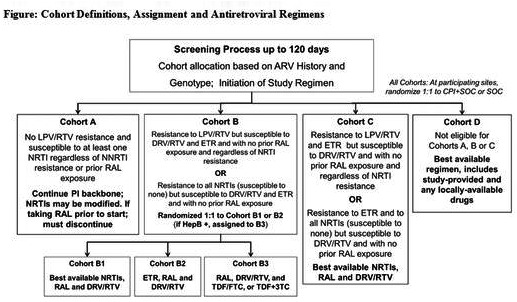
OAB0405
Sub‐optimal outcomes with switching to zidovudine vs. recycling tenofovir in second‐line treatment in Haiti
S. Pierre1, C. Nguyen2, B. Iryna2, F. Homeus1, V. Guillaume1, C.P.J. Pierre1, G. Sainvil1, A. Julien1, G. Julmiste1, Y. Macius1, G.P.‐L. Florestal1, V. Rouzier1, P. Severe1, P. Cremieux2, M.M. Deschamps1, B. Liautaud1, S. Koenig3, J.W. Pape1
1GHESKIO Centers, Research, Port‐au‐Prince, Haiti, 2Analysis Group, Boston, United States, 3Brigham Women Hospital, Boston, United States
Background: World Health Organization (WHO) guidelines recommend an optimized nucleoside reverse transcriptase inhibitor (NTRI) backbone for second‐line ART, including switching from tenofovir (TDF) to zidovudine (AZT), with presumed low‐level AZT resistance. However, many providers in resource‐poor countries recycle TDF in second‐line treatment due to concerns for toxicity and twice‐daily dosing of AZT and due to demonstrated efficacy of NRTIs (in spite of genotypic resistance found in several recent studies).
Methods: Using electronic medical records from GHESKIO (Port‐au‐Prince, Haiti), we identified adult patients who failed first‐line Efavirenz (EFV)/TDF/3TC and were switched to a second‐line regimen that included ritonavir‐boosted protease inhibitor (bPI), in combination with either TDF/3TC or AZT/3TC. Retention, adherence and viral suppression outcomes were evaluated at 12 month after initiation of second‐line regimen. Adherence was approximated using pharmacy refill data. Multivariable logistic regression was used to determine predictors of virologic suppression.
Results: From 2012 to 2018, 1017 patients met study criteria and were analysed. Of these, 509/1017 (50.0%) were women. Median patient age was 40.7 years. 733/1017 (72.1%) patients continued on TDF/3TC on second‐line, while 284/1017 (27.9%) were switched to AZT/3TC. Retention was similar in both groups with 612/733 (83.5%) in the TDF/3TC and 236/284 (83.1%) in the AZT/3TC group remaining in care. Of the patients with viral load at 12 months, 253/480 (52.7%) had VL < 200 copies/mL in TDF/3TC vs. 72/200 (36.0%) in the AZT/3TC group (p < 0.001). Viral suppression in patients with ≥ 90% adherence was also better in the TDF/3TC group with 166/230 (72.2%) compared to 43/75 (57.3%) in the AZT/3TC group (p < 0.016). Predictors of viral suppression included recycled TDF/3TC (odds ratio (OR): 2.08; 95% CI: 1.46, 2.97), secondary or higher education level (OR: 1.53; 95% CI: 1.10, 2.14) and being married/living together (OR 1.51; 95% CI: 1.00, 2.27).
Conclusions: The WHO‐recommended optimized NTRI backbone for second‐line ART, which includes switching from TDF to AZT, was associated with lower rates of viral suppression than recycled TDF in Haiti. This may potentially be due to twice‐daily dosing and poor tolerability of AZT. ART adherence was found to be poor regardless of NRTI backbone, therefore additional interventions are needed to improve adherence in this population.
OAB0502
Efficacy and safety outcomes (HIV subgroup analysis) in the Nix‐TB Trial – bedaquiline, pretomanid and linezolid for treatment of extensively resistant, intolerant or non‐responsive pulmonary multidrug‐resistant tuberculosis
M. Olugbosi1, D. Everitt2, F. Conradie3, A. Crook4, G. Wills4, E. Sun2
1 TB Alliance, Clinical Development, Pretoria, South Africa, 2 TB Alliance, New York, United States, 3University of the Witwatersrand, Clinical HIV Research Unit, Johannesburg, South Africa, 4University College London, London, United Kingdom
Background: Nix‐TB achieved 92% treatment success among 109 XDR and treatment‐intolerant or non‐responsive (TI/NR) MDR TB patients in South Africa, with a 3‐drug, all‐oral, six‐month regimen of Bedaquiline, Pretomanid and Linezolid (BPaL). Half of the study population (51%) were HIV + .
Methods: Nix‐TB is an open label single arm study of extensively drug‐resistant (XDR) or treatment‐intolerant or non‐responsive (TI/NR) multidrug‐resistant (MDR)‐TB patients, with primary endpoint of relapse‐free microbiologic and clinical cure six months after end of therapy. Safety data analysis was descriptive with no inferential tests carried out. HIV+ patients were required to have CD4 + > 50 cells/μL and be able to receive allowed ARV regimens (NVP‐, LPV/r‐, or RAL‐based with NRTIs). Here we present a HIV subgroup analysis of the efficacy and safety data from the study.
Results: 56 HIV+ patients (of 109 total) were on ARV therapy prior to enrolment. 39 patients (69.6%) switched pre‐enrolment ARV to allowed regimens and these were all changes from EFV‐ regimens to either LPV/r‐ or NVP‐ regimens. CD4 count was available for 51 participants with mean, median and range of 394, 343, 55 to 1023 cells/µL respectively.
Success at the primary endpoint was 91% (95% CI, 80 to 97) in HIV+ and 92% (95% CI, 81 to 98) in HIV− patients. Results from a cox regression model showed that HIV status did not affect time to negative culture conversion status (hazard ratio 0.78 (95% CI, 0.50, 1.19); p = 0.249).
Among HIV+ and HIV− patients, grade 3 or 4 TEAEs were reported in 62.5% and 50.9%, hepatic TEAEs in 46.4% and 30.2%, peripheral neuropathy in 78.6% and 83%, haematopoietic cytopenias in 53.6% and 41.5% and serious TEAEs in 16.1% and 17.0% respectively. Of the eight reported deaths (7.3% of total study population), 5 (8.9%) and 3 (5.7%) were in the HIV+ and HIV− population respectively.
All surviving patients (irrespective of HIV status) were able to complete the full 26 weeks of therapy.
Conclusions: Results of this simplified, shortened all oral regimen for highly drug‐resistant TB show sustained high efficacy and manageable safety irrespective of HIV status.
OAB0503
Prevalence and incidence of tuberculosis infection and disease among household contacts exposed to rifampin‐resistant/multidrug resistant tuberculosis (RR/MDR‐TB)
A. Gupta1, X. Wu2, S. Kim2, L. Naini3, M. Hughes2, R. Dawson4, S. Gaikwad5, J. Sanchez6, A. Mendoza6, B. Smith7, S. Shah8, A. Hesseling9, G. Churchyard10, S. Swindells11, For the Acquired Immunodeficiency Syndrome (AIDS) Clinical Trials Group (ACTG) 5300/International Maternal Pediatric Adolescent AIDS Trials (IMPAACT) I2003 Protecting Households on Exposure to Newly Diagnosed Index Multidrug‐resistant Tuberculosis Patients (PHOENIx) Feasibility Study Team
1Johns Hopkins University, Medicine, Baltimore, United States, 2Harvard T.H. Chan School of Public Health, Boston, United States, 3Social & Scientific Systems, Silver Spring, United States, 4University of Cape Town, Cape Town, South Africa, 5Byramji Jeejeebhoy Government Medical College, Pune, India, 6Asociación Civil Impacta Salud y Educación, Lima, Peru, 7Division of AIDS, National Institute of Allergy and Infectious Diseases, NIAID, NIH, Bethesda, United States, 8Emory Rollins School of Public Health, Atlanta, United States, 9Stellenbosch University, Desmond Tutu TB Centre, Department of Paediatrics and Child Health, Cape Town, South Africa, 10Aurum Institute, Parktown, South Africa, 11University of Nebraska Medical Center, Medicine, Infectious Diseases, Omaha, United States
Background: To prepare for a clinical trial testing a novel TB preventive therapy (TPT) in high RR/MDR‐TB burden settings, we sought to quantify the incidence proportion of TB infection (TBI) and disease (TBD) among household contacts (HHCs) of RR/MDR‐TB cases.
Methods: RR/MDR‐TB HHCs in 8 high burden countries were enrolled in a cross‐sectional study and then reassessed one year later. TBI was assessed at baseline by tuberculin skin test (TST) and interferon gamma release assay (IGRA), QuantiFERON Gold/Gold In‐Tube; if IGRA‐negative or indeterminate at baseline, HHCs age ≥ 5 years had repeat IGRA at one year. TBD screening was performed using symptom screen, chest radiography and mycobacteriology at baseline and follow‐up. High‐risk groups were defined as children < 5 years, HIV‐infected, or TBI. Generalized Estimating Equations approach to fit logistic models was used to account for within household correlation.
Results: Of 1007 HHCs of 284 RR/MDR‐TB cases, baseline prevalence of TBI was 55.0% by TST, 65.6% by IGRA and prevalent TBD was 12%. At median of 51.4 weeks later, 850 (81.5%) HHCs from 247 households were traced; 6 HHCs (0.6%) had died (2 with TB). 253 (30%) HHCs were eligible for IGRA testing and 243 had it performed; 52 (21%) converted to IGRA‐positive and 1 was indeterminate. 1‐year cumulative TBI incidence among HHCs age < 5 years was 21.6%; 10.9% among 5 to 14 years; and 25.5% among ≥ 15 years, p = 0.007. There was no difference in IGRA conversion by HIV status (22.1% in HIV+ and 21.5% in HIV‐/unknown, p = 0.95). 1‐year cumulative TBD incidence was 2.3% (n = 16); 15 (93.7%) were within high‐risk groups. Cumulative TBD incidence was 2.7% in high‐risk groups compared to 0.5% in those not in high‐risk group (p = 0.006); higher in < 15 years than ≥ 15 years (4.9% vs. 1.3%, p = 0.023); higher but non‐significantly in HIV+ compared to HIV‐/unknown (6.6% vs. 1.9%, p = 0.21). Only 26 (5%) of 553 high‐risk HHCs received TPT; mostly isoniazid monotherapy.
Conclusions: By one year of follow‐up, most HHCs exposed to RR/MDR‐TB had TBI. All but one new TBD event occurred in a high‐risk group. Few received TPT, illustrating the enormous need for novel therapies and TPT scale up among this very high‐risk population.
OAB0504
Assessment of the tuberculosis clinical cascade among children living with HIV on antiretroviral therapy, 16 Sub‐Saharan PEPFAR‐supported programmes, October 1, 2018 to March 31, 2019
M.R. Patel1, M. Itoh1, K. Battey1, H. Kirking1, E. Click1, R. Golin2, T. Al‐Samarrai3, H.T. Wolf3, P. Agaba4, E. Rivadeneira1, S. Modi1
1US Centers for Disease Control and Prevention, Atlanta, United States, 2US Agency for International Development, Washington, United States, 3US Office of the Global AIDS Coordinator, Washington, United States, 4US Military HIV Research Program, Bethesda, United States
Background: Tuberculosis (TB) is underreported and contributes to substantial morbidity and mortality in children, particularly children living with HIV (CLHIV). We examined The US President's Emergency Plan for AIDS Relief (PEPFAR) data to identify opportunities to reduce TB burden among CLHIV.
Methods: We analysed PEPFAR data for CLHIV (<15 years) on antiretroviral treatment (ART) from October 1, 2018 to March 31, 2019. Of 21 PEPFAR‐supported countries in sub‐Saharan Africa, 5 were excluded due to non‐reporting of age‐disaggregated TB data. The remaining 16 were categorized by region, high paediatric TB incidence (≥25,000 per 100,000) and high CLHIV burden (≥60,000). We analysed these TB cascade indices: TB symptom screening coverage (percentage screened at least once), screening positivity (percentage with positive screen), proxy TB treatment initiation rate (percentage with positive screen initiating TB treatment), proxy TB preventive therapy (TPT) initiation rate (percentage with negative screen initiating TPT) and TPT completion (percentage initiating and completing TPT).
Results: In total, 555,851 CLHIV were included, representing 86% of CLHIV on ART across PEPFAR‐supported programmes. Of these, most were screened for TB (median 93%, interquartile range (IQR): 86%‐100%); however, few (median 3%, IQR:2%‐6%)) screened positive. Of those screening positive, median TB treatment initiation rate was 19% (IQR:15%‐29%). A median 8% (IQR:4% to 11%) of those screening negative initiated TPT. Of those initiating TPT, median completion was 72% (IQR:47%‐79%). TB cascade indices were similar by TB incidence and CLHIV burden; TPT completion was lower in Western/Central Africa (62%), despite higher TPT initiation (24%) (Figure 1).
Abstract OAB0504‐Figure 1.Median teberculosis clinical cascade indices among children living with HIV on antiretrovial therapy bt paediatric TB incidence, CLHIV burden and region, 16 sub‐Saharan PEPFAR‐supported programmes, October 1, 2018 to March 31, 2019 (n = 555,851).
Conclusions: TB screening coverage was high, but screening positivity was lower than expected, suggesting poor screening quality. Low TPT initiation and completion underscores that national TPT plans should address paediatric‐specific clinical guidance, supply chain and routine monitoring. Age‐disaggregated TB diagnosis data are needed, as proxies likely underestimate TB treatment initiation and overestimate TPT initiation rates.
Abstract OAB0504‐Figure 1.
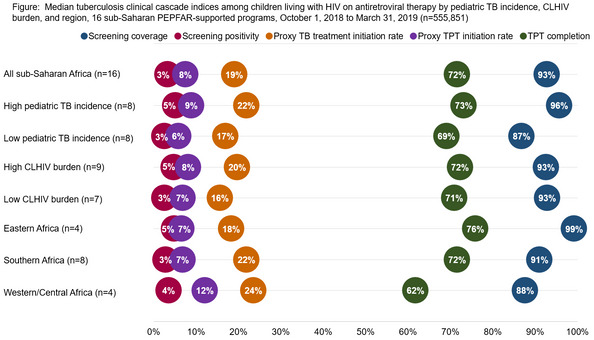
OAB0505
Risk factors for hepatotoxicity in HIV‐infected women receiving isoniazid preventive therapy in pregnancy and postpartum
A. Gupta1, L. Aaron2, G. Theron3, K. McCarthy4, S. Bradford4, T. Chipato5, T. Vhembo5, L. Stranix‐Chibanda5, C. Onyango‐Makumbi6, G. Masheto7, N. Chakhtoura8, P. Jean Phillippe9, R. Browning9, T. Sterling10, A. Weinberg11, G. Montipiedra2, for the IMPAACT P1078 TB APPRISE Study Team
1Johns Hopkins University, Medicine, Baltimore, United States, 2Harvard T.H. Chan School of Public Health, Boston, United States, 3Stellenbosch University, Obstetrics and Gynecology, Cape Town, South Africa, 4Family Health International FHI360, Durham, United States, 5University of Zimbabwe College of Health Sciences Clinical Trials Research Centre, Harare, Zimbabwe, 6Makerere University–Johns Hopkins University Research Collaboration, Kampala, Uganda, 7Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana, 8Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, Bethesda, United States, 9Division of AIDS, National Institute of Allergy and Infectious Diseases, NIAID, NIH, Bethesda, United States, 10Vanderbilt University Medical Center, Nashville, United States, 11University of Colorado Denver Anschutz Medical Campus, Aurora, United States
Background: IMPAACT P1078, a Phase IV randomized, double‐blind, placebo‐controlled non‐inferiority multi‐country trial assessing the safety of 28 weeks of isoniazid (INH) preventive therapy (IPT) initiated during pregnancy (immediate IPT) versus deferring to week 12 postpartum (deferred IPT) in HIV‐infected women on ART, showed higher than expected hepatotoxicity. We investigated hepatotoxicity risk factors.
Methods: We examined all‐cause hepatotoxicity defined as Grade ≥ 3 alanine transaminase (ALT) with or without symptoms or bilirubin > 2x upper limit of normal. We performed Poisson regression on study arm, country of enrolment, age, pharmacogenetics of INH and efavirenz (EFV) metabolism, timing of cotrimoxazole initiation and baseline status of the following: ARV regimen, Hepatitis B and C, CD4, HIV VL, BMI, mid‐upper arm circumference. Adjusted models included study arm and covariates with p < 0.25 in unadjusted model. Study arm effect modification by ARV regimen was also evaluated.
Results: Of 945 women with follow‐up ALT measurements, 63 (6%) experienced ≥ 1 hepatotoxicity event; 29 (6%) in immediate and 34 (7%) in deferred arm; 5 (8%) occurred in pregnancy, 5 (8%) within 1 week after delivery and 53 (84%) in postpartum > 1 week. ARV regimen and cotrimoxazole use ranged widely by country (66%‐100% taking EFV regimen; 0%‐31% taking NVP regimen and 5%‐95% taking cotrimoxazole) as did slow metabolizing status (20%‐70% for INH NAT2 genotype and 8%‐30% for EFV CYP2B6 genotype). There was a study arm, ARV interaction; higher hepatotoxicity was observed with nevirapine (NVP) in immediate arm, and with EFV in deferred arm. Hepatotoxicity was also associated with cotrimoxazole initiation and marginally with Hepatitis C (Table 1). There was significantly higher risk of hepatotoxicity among slow EFV metabolizers. All other participant characteristics analysed were not associated with hepatotoxicity.
Abstract‐OAB0505‐Table 1. Adjusted risk ratios for hepatotoxicity endpoint using poisson regression models
| Participant characteristics | Group | Estimated risk ratio | 95% confidence interval | p‐value |
|---|---|---|---|---|
| INH/ARV regimen interaction | ||||
| EFV: immediate versus deferred (ref) | 0.73 | (0.41, 1.27) | 0.028 | |
| NVP: immediate versus deferred (ref) | 8.67 | (1.06, 70.81) | ||
| Hepatitis C serology | 3.60 | (0.87, 14.88) | 0.077 | |
| Mid upper arm circumference (ref obesity) | Malnutrition <23 | 0.37 | (0.05, 2.77) | 0.420 |
| Normal 23 to 31 | 0.77 | (0.45, 1.32) | ||
| Initiated cotrimoxazole after week 12 postpartum (versus never initiated before week 12 postpartum) | 4.57 | (1.80, 11.47) | 0.001 | |
| CYP2B6 genotype (ref slow) | Fast | 0.37 | (0.16, 0.84) | 0.017 |
| Intermediate | 0.44 | (0.23, 0.82) |
Conclusions: It is critical to monitor for hepatotoxicity in the postpartum when most events occur. ARV regimen type and cotrimoxazole use should also be considered in decisions on when to optimally initiate IPT in pregnant and postpartum women.
OAB0506
Validation of a laboratory‐based reference test for TB‐LAM and FLOW‐TB, a novel point‐of‐care TB diagnostic assay, in a high‐HIV‐prevalence clinical cohort from KwaZulu‐Natal, South Africa
A.E. Shapiro1, M.W. Ngwane2, Z.P. Magcaba2, D. Boyle3, J. Cantera3, L. Lillis3, B. Mullins4, T. Wittwer4, H. Hannah5, J. Warrick4, S. Berry4, D.J. Beebe4, D.P.K. Wilson6,2, P.K. Drain1
1University of Washington, Global Health and Medicine (Infectious Diseases), Seattle, United States, 2Umkhuseli Innovation and Research Management, Pietermaritzburg, South Africa, 3PATH, Seattle, United States, 4Salus Discovery, Madison, United States, 5University of Washington, Global Health, Seattle, United States, 6University of KwaZulu‐Natal, Edendale Hospital, Internal Medicine, Pietermaritzburg, South Africa
Background: A first‐generation point‐of‐care urine lipoarabinomannan (LAM) assay has low sensitivity to diagnose active tuberculosis (TB). We developed and validated a second‐generation reference assay and point‐of‐care (POC) test to detect LAM for active TB diagnosis in people with and without HIV.
Methods: We selectively enrolled adults with and without pulmonary TB at Edendale Hospital in KwaZulu‐Natal, South Africa. We collected sputum for confirmatory TB testing and urine for LAM detection. We tested urine samples in the clinic laboratory using the Determine LAM Ag (Abbott) and FLOW‐TB assay (Salus Discovery) as POC tests. We also conducted reference quantitative LAM testing using the MesoScale Diagnostic (MSD) electrochemiluminescence assay with three separate capture/detection monoclonal antibody combinations. We calculated the diagnostic accuracy for each assay, using sputum Xpert MTB/RIF and/or TB culture as the reference test.
Results: Among 139 adults (45% female), 74% were HIV‐positive and 88% had microbiologically confirmed pulmonary TB. The lab‐based LAM reference assay had high diagnostic accuracy using the Otsuka S20/A194 (OA) antibody combination (83% sensitivity, 91% specificity) and the KI24/A194 (KA) antibody combination (81% sensitivity, 73% specificity). The MSD‐LAM reference assay had 86% sensitivity and 89% specificity for diagnosing pulmonary TB among the 70 participants with TB culture‐confirmed results. In LAM‐positive persons, the median LAM concentration measured by MSD‐LAM was 295 pg/mL (IQR 43 to 1541 pq/mL) using OA antibodies and 330 pg/mL (IQR 67 to 3909) using KA antibodies. FLOW‐TB sensitivity, compared to TB Xpert reference testing, was 71% (80% in HIV‐positive participants) and specificity was 67%. The FLOW‐TB assay detected LAM in 80% of samples with LAM as measured by the MSD‐LAM reference assay, whereas the Abbott LAM test detected LAM in 27% of samples with LAM as measured by the MSD‐LAM reference assay.
Abstract OAB0506‐Table 1.
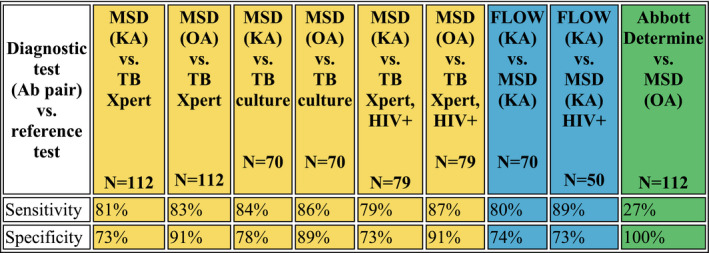
Conclusions: The MSD assay can accurately measure low concentrations of LAM in urine with high sensitivity and specificity for active TB in HIV‐positive and HIV‐negative persons, and is a suitable benchmark for evaluating novel POC LAM assays. The novel second‐generation FLOW‐TB assay had markedly improved sensitivity over the existing POC LAM assay.
OAB0602
Weight gain and hyperglycaemia during the dolutegravir transition in Africa
J. Ake1, A. Esber1,2, N. Dear1,2, M. Iroezindu1,3,4, E. Bahemana1,5, H. Kibuuka6, J. Owuoth1,7,8, J. Maswai1,7,8, C. Polyak1,2, T. Crowell12, AFRICOS Study Team
1Walter Reed Army Institute of Research, U.S. Military HIV Research Program, Silver Spring, United States, 2Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, United States, 3U.S. Army Medical Research Directorate, Nairobi, Kenya, 4Henry Jackson Foundation MRI, Abuja, Nigeria, 5Henry Jackson Foundation MRI, Mbeya, Tanzania, United Republic of, 6Makerere University Walter Reed Project, Kampala, Uganda, 7Kenya Medical Research Institute, Nairobi, Kenya, 8Henry Jackson Foundation MRI, Kisumu, Kenya
Background: Clinical trials demonstrated weight gain upon initiation of dolutegravir‐based regimens in sub‐Saharan Africa, and reports of hyperglycaemia have emerged during the programmatic rollout of TLD (tenofovir disoproxil fumarate/lamivudine/dolutegravir). We systematically examined the incidence of these conditions in the care and treatment setting.
Methods: The African Cohort Study (AFRICOS) enrolled HIV‐infected and uninfected participants at twelve PEPFAR‐supported clinics in Uganda, Kenya, Tanzania and Nigeria. BMI was assessed six‐monthly and glucose annually. Overweight/obese was defined as BMI > 25 kg/m2. Hyperglycaemia was defined as fasting glucose > 99, any glucose > 199 or taking hypoglycaemic medication. Incidence rates of becoming overweight/obese and developing hyperglycaemia were calculated overall and by HIV status and treatment groups. Among HIV‐infected participants without the conditions of interest upon enrolment, Cox proportional hazards models estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for TLD use and other potential risk factors for weight gain and hyperglycaemia.
Results: From January 2013‐November 2019, 3514 participants were enrolled including 2043 (58%) females and 2927 (83%) living with HIV with median age 38 (Interquartile range 31 to 46) years. Incidence for becoming overweight/obese was 72.33 (CI 66.22 to 78.99) cases/1000 PY overall (n = 2545) and 98.6 (CI 63.6 to 152.8) cases/1000 PY among participants on TLD (n = 528). Hyperglycaemia incidence was 52.01 (CI 47.28 to 57.21) cases/1000 PY overall (n = 3045) and 121.30 (CI 71.84 to 204.82) cases/1000 PY among participants on TLD (n = 373). For each condition, those taking TLD consistently demonstrated the highest incidence across sites, ART naïve participants had the lowest incidence, and the geographically highest rates were observed in Nigeria. In time‐to‐event analysis, 436 participants became overweight/obese and 380 developed hyperglycaemia. Those taking TLD had increased rates of becoming overweight/obese compared to those taking non‐TLD ART (HR 2.73; CI 1.67 to 4.48) after adjusting for site, gender, age and depression. While participants on TLD had an increased HR compared those on non‐TLD ART in the unadjusted model (1.81; CI 1.04 to 3.14), this difference was not statistically significant (HR 1.12; CI 0.65 to 1.93) after adjustment for site, gender, age and enrolment BMI.
Conclusions: TLD use was associated with increased incidence of weight gain and hyperglycaemia in this cohort. We observed regional differences in both conditions and an independent effect of TLD on becoming overweight/obese.
OAB0603
Changes in body mass index over time in persons with and without HIV
M. Silverberg1, W. Leyden1, S. Alexeeff1, J. Lam1, A. Anderson1, R. Hechter2, H. Hu3, J. Marcus4, Q. Yuan2, W. Towner2, M. Horberg3
1Kaiser Permanente Northern California, Division of Research, Oakland, United States, 2Kaiser Permanente Southern California, Research & Evaluation, Pasadena, United States, 3Kaiser Permanente Mid‐Atlantic States, Mid‐Atlantic Permanente Research Institute, Rockville, United States, 4Harvard Medical School and Harvard Pilgrim Health Care Institute, Population Medicine, Boston, United States
Background: Adults with HIV (HIV+) may experience increases in body mass index (BMI) over time after antiretroviral therapy (ART) initiation. It is unknown whether BMI in HIV+ adults has approached adults without HIV (HIV−).
Methods: We conducted a cohort study during 2005 to 2016 of HIV+ adults (≥18 years) who were members of Kaiser Permanente Northern California, Southern California, or Mid‐Atlantic States, integrated healthcare systems with longstanding HIV registries and electronic health records. HIV− were matched 10:1 to HIV+ by age, sex, race/ethnicity, medical centre and calendar year. We restricted analyses to those with recorded baseline BMI; HIV+ were further restricted to ART initiators. Using mixed effects models, we compared changes in BMI over time for HIV− (reference) and HIV+ adults, both overall and in baseline BMI subgroups: underweight/normal (<25 kg/m2); overweight (25 to 29.9 kg/m2); and obese (≥30 kg/m2). Multivariable models included terms for HIV status, time, HIV*time interaction, age, race/ethnicity, sex, year, substance use disorders, smoking, census‐based education/income, insurance type and common comorbidities.
Results: The study included 8256 HIV+ and 129,966 HIV− adults. Mean baseline BMI (kg/m2) was 29.3 for HIV− and 26.2 (p < 0.001) for HIV + . In adjusted models, the average annual change in BMI was 0.06 kg/m2 for HIV− (reference) and 0.16 kg/m2 (p < 0.001) for HIV + . Adjusted changes in BMI by HIV status with 95% confidence bands are presented in the Figure. For all patients (panel a), the average BMI at 12 years was 28.4 and 29.4 for HIV− and HIV+ adults respectively. For all baseline BMI categories (Figure, panels b‐d), HIV+ adults had faster BMI increases over time compared with changes for HIV− adults.
Abstract OAB0603‐Figure 1. Adjusted changes in BMI by HIV status and baseline BMI.
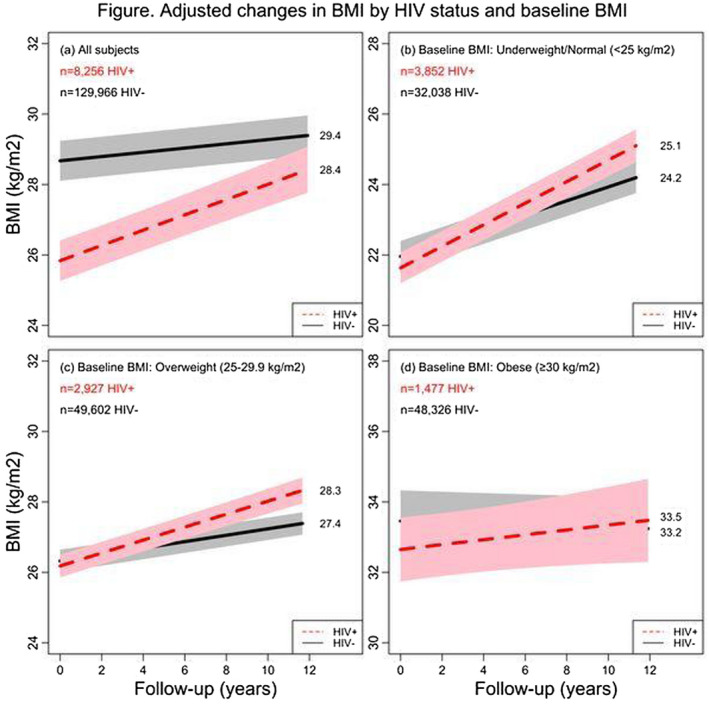
Conclusions: HIV+ adults initiating ART have more rapid increases in BMI over time compared with demographically similar HIV− adults. This may adversely impact efforts to reduce the risk of BMI‐related comorbidities in HIV+ adults, such as cardiovascular disease.
OAB0604
Weight gain before and after switch from TDF to TAF
P. Mallon1, L. Brunet2, R. Hsu34, J. Fusco2, K. Mounzer5, G. Prajapati6, A. Beyer6, M. Wohlfeiler3, G. Fusco2
1University College Dublin, School of Medicine, Dublin, Ireland, 2Epividian, Durham, United States, 3AIDS Healthcare Foundation, New York, United States, 4NYU Langone Health Center, Internal Medicine, New York, United States, 5Philadelphia FIGHT, Philadelphia, United States, 6Merck & Co., Inc., Kenilworth, United States
Background: Although significant weight gain has been reported with use of some integrase inhibitors (INSTI), concurrent use of tenofovir alafenamide (TAF) has also been implicated. We aimed to examine weight changes in people living with HIV (PLWH) who switched from tenofovir disoproxil fumarate (TDF) to TAF.
Methods: ARV‐experienced, virologically suppressed PLWH in the OPERA cohort who switched from TDF to TAF were included if they maintained all other ARVs or switched to an INSTI. We modelled weight change before/after switch to TAF using linear mixed models (random intercepts, restricted cubic splines on time), adjusting for age, sex, race, (age‐sex, race‐sex interactions), BMI, CD4 count, endocrine disorders and concurrent medications that could modify weight.
Results: Demographics of 6919 PLWH included were similar whether they maintained other ARVs or switched to INSTI (Table 1). Although modest weight gain over time was observed with TDF use (0.23 to 0.67 kg/year), switch to TAF was associated with early, pronounced weight gain (1.80 to 4.47 kg/year, Figure 1) in adjusted models. This effect with TAF switch was observed both in those who maintained other ARVs and those switching to an INSTI (regardless of which INSTI agent was used). Weight gain tended to slow down or plateau approximately nine months after switch to TAF; bictegravir lacked sufficient data beyond nine months.
Abstract OAB0604‐Table 1. Characteristics at switch from TDF to TAF
| Maintained NNRTI, n = 1454 | Maintained bPI, n = 747 | Maintained INSTI, n = 3288 | Switched to INSTI, n = 1430 | |
|---|---|---|---|---|
| Age, median (IQR) | 45 (34, 54) | 51 (42, 57) | 44 (33, 52) | 49 (39, 56) |
| Female, n (%) | 276 (19) | 155 (21) | 501 (15) | 253 (18) |
| Black, n (%) | 591 (41) | 292 (39) | 1206 (37) | 543 (38) |
| Hispanic, n (%) | 348 (24) | 190 (25) | 865 (26) | 373 (26) |
| BMI (kg/m2), median (IQR) | 27 (24, 31) | 27 (24, 31) | 26 (24, 30) | 27 (24, 30) |
| CD4‐cell count, median (IQR) | 717 (542, 939) | 608 (441, 826) | 654 (475, 868) | 668 (493, 875) |
| Endocrine disorders, n (%) | 272 (19) | 190 (25) | 677 (21) | 325 (23) |
| Medications associated with weight gain, n (%) | 404 (28) | 275 (37) | 989 (30) | 486 (34) |
| Medications associated with weight loss, n (%) | 268 (18) | 170 (23) | 652 (20) | 273 (19) |
Abstract OAB0604‐Figure 1. Predicted weight* over time on TDF and TAF and estimated rate† of weight gain (95% CI)‡. *Estimated with multivariable linear mixed model with restricted cubic splines in time. †Estimated with multivariable linear mixed model with linear splines on time. ‡Adjusted for age, sex, race, baseline BMI, CD4‐cell count, endocrine disorders, medications associated with weight gain/loss. INSTI, integrase inhibitor; bPI, boosted protease inhibitor; NNRTI, non‐nucleoside reverse transcriptase inhibitor, EVG/c, elvitegravir/cobicistat, DTG, dolutegravir; RAL, raltegravir; BIC, bictegravir.
Conclusions: In this large, diverse cohort of PLWH, switching from TDF to TAF was associated with pronounced weight gain immediately after switch, regardless of concurrent INSTI use. That this effect was observed across regimens suggests an independent effect of TAF on weight.
Abstract OAB0604‐Figure 1.
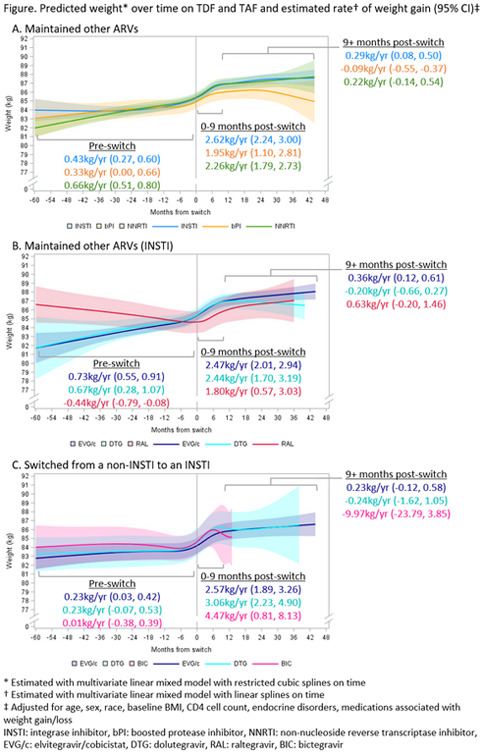
OAB0605
Weight changes after switching to doravirine/lamivudine/TDF in the DRIVE‐SHIFT trial
P. Kumar1, M. Johnson2, Z.J. Xu3, E. Martin3, P. Sklar3, W. Greaves3
1Georgetown University School of Medicine, Washington DC, United States, 2Royal Free Hospital, London, United Kingdom, 3Merck & Co., Inc., Kenilworth, United States
Background: Initiation of antiretroviral therapy (ART) often leads to weight gain. Greater weight gain has been observed with integrase inhibitors than with protease inhibitors (PI) or non‐nucleoside reverse transcriptase inhibitors (NNRTI), and with tenofovir alafenamide (TAF) versus tenofovir disoproxil fumarate (TDF). In treatment‐naïve clinical trials of doravirine (DOR), mean weight gain over 96 weeks was similar to the average change in adults without HIV. We conducted a post‐hoc analysis of weight changes in DRIVE‐SHIFT, a phase 3 trial in which adults with HIV‐1 who were virologically suppressed for ≥ 6 months switched to DOR/3TC/TDF on Day 1 (immediate switch group, ISG) or after continuing their prior regimen for 24 weeks (delayed switch group, DSG).
Methods: Mean weight change from time of switch was calculated for ISG and DSG at 24 weeks (24W) post‐switch and for ISG only at 48 weeks (48W) post‐switch, overall and by demographic subgroup (Men, Women, Black, White, Hispanic) and prior regimen (PI, NNRTI, Elvitegravir/TAF).
Results: 670 participants (447 ISG, 223 DSG) entered the trial (84.5% male, 76.4% white, mean age 43.3 years). Post‐switch weight data were available at 24W for 629 participants (ISG+DSG) and at 48W for 408 participants (ISG only). Weight gains after switch to DOR/3TC/TDF were small: mean 0.6 kg (95% CI: 0.4, 0.9) at 24W and 0.7 kg (0.4, 1.1) at 48W. Observed weight gain at 48W post‐switch was nominally lower in women (0.3 kg) versus men (0.8 kg), in white participants (0.6 kg) versus black (1.3 kg) or Hispanic participants (1.3 kg) and after switching from a boosted PI (0.6 kg) versus an NNRTI (1.3 kg). In the small group who switched from elvitegravir/TAF, mean weight change was ‐0.4 kg at 48W post‐switch.
Abstract OAB0605‐Table 1
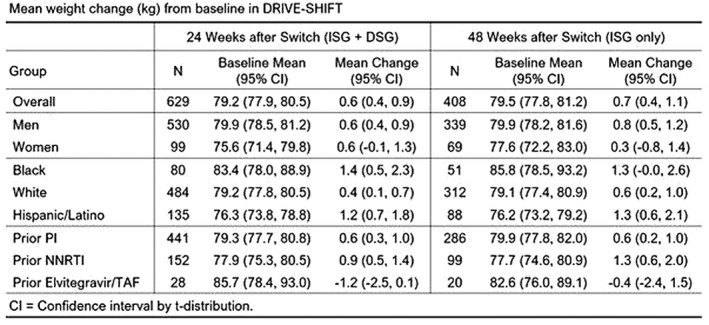
Conclusions: Weight changes among participants switching to DOR/3TC/TDF were modest and similar to the average change observed in adults without HIV in the US.
OAB0606
Improved metabolic parameters after switching from TAF‐based 3‐ or 4‐drug regimen to the 2‐drug regimen of DTG/3TC (dolutegravir/lamivudine): The TANGO study
J. van Wyk1, M. Ait‐Khaled1, J. Santos2, S. Scholten3, M. Wohlfeiler4, F. Ajana5, B. Jones1, M.C. Nascimento1, A. Tenorio6, D. Smith7, J. Wright8, B. Wynne6
1ViiV Healthcare ‐ UK, Brentford, United Kingdom, 2Hospital Virgen de la Victoria, Málaga, Spain, 3Praxis Hohenstaufenring, Cologne, Germany, 4AIDS Healthcare Foundation, Miami Beach, United States, 5Centre Hospitalier de Tourcoing, Tourcoing, France, 6ViiV Healthcare, Research Triangle Park, United States, 7Albion Centre, Sydney, Australia, 8GlaxoSmithKline, Stockley Park, United Kingdom
Background: Primary outcomes from TANGO demonstrated that switching to DTG/3TC is non‐inferior at 48 weeks to continuing a 3‐/4‐drug TAF‐based regimen (TBR) in virologically suppressed PLWH. Switching from TDF to TAF or using boosting agents has been associated with weight gain and dyslipidaemia.
Methods: Here we summarize changes over 48 weeks in weight, lipids, fasting glucose and insulin as well as prevalence at Week 48 of insulin resistance (IR) (defined as HOMA‐IR ≥ 2), and metabolic syndrome (MS) (International Diabetes Federation definition). Subgroup analyses by boosting status of the baseline regimen were performed.
Results: At baseline (BL), participants were randomized to either DTG/3TC (N = 369) or TBR (N = 372). Most participants were male (92%) and white (79%), median age was 40 years, and 74% received a boosting agent. Mean weight changes were small and comparable between arms. Changes in lipids, including TC:HDL ratio, generally favoured the DTG/3TC group. Changes in fasting glucose were small across arms; changes in fasting insulin favoured the DTG/3TC arm and were more pronounced in the unboosted group (Table 1). At Week 48, proportions with HOMA‐IR ≥ 2 were 65% (BL = 69%) and 74% (BL = 68%) in the DTG/3TC and TBR arms respectively (odds ratio 0.59 (CI:0.40, 0.87); p = 0.008), with differences favouring DTG/3TC more pronounced in the boosted group (Table 1). At Week 48, proportions with MS were 11% (BL = 10%) and 12% (BL = 11%) in the DTG/3TC and TBR arms respectively; adjusted treatment differences favoured DTG/3TC in the unboosted group (Table 1).
Abstract OAB0606‐Table 1
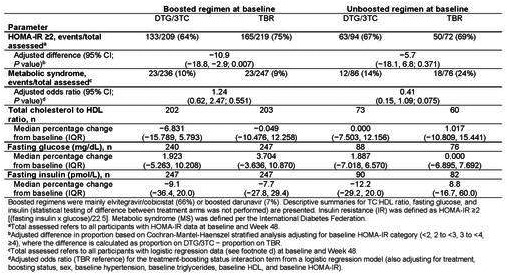
Conclusions: Switching from 3‐/4‐drug TAF‐based regimens to the 2‐drug regimen of DTG/3TC led to similar small increases in weight, but general improvements in other metabolic health parameters, over 48 weeks. More pronounced differences favouring DTG/3TC were noted compared to the unboosted TAF‐based group for fasting insulin and metabolic syndrome, and to the boosted TAF‐based group for lipids and insulin resistance.
OAB0702
Overlapping significant life events are associated with HIV viral non‐suppression among youth in clinics in rural East Africa
F. Mwangwa1, E. Charlebois2, W. Olio3, J. Ayieko3, D. Black2, J. Peng2, J. Kabami1, L. Balzer4, M. Petersen5, B. Kapogiannis6, M. Kamya7, D. Havlir2, T. Ruel2, SEARCH YOUTH Team
1Infectious Diseases Research Collaboration, Kampala, Uganda, 2University of California, San Francisco, San Francisco, United States, 3Kenya Medical Research Institute (KEMRI) Centre for Microbiology Research (CMR), Nairobi, Kenya, 4University of Massachusetts Amherst, Amherst, United States, 5University of California, Berkeley, Berkeley, United States, 6Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, United States, 7Makerere University College of Health Sciences, Kampala, Uganda
Background: The HIV‐care continuum among Youth Living with HIV (YLWH) is thought to be influenced by life events that may be part of normal psycho‐social development but affect engagement with treatment. However, data on the prevalence of disruptive life events among YLWH in rural sub‐Saharan Africa and their association with viral suppression are limited.
Methods: SEARCH Youth (NCT0384872) is a cluster‐randomized trial testing a package of youth‐focused interventions in 28 HIV clinics in rural Uganda and Kenya. In the intervention arm, a tablet‐based care‐planning tool is used to assess potential barriers to treatment including alcohol use, HIV disclosure status and major recent life‐events: start/stop of school or employment, change in residence, divorce/separation or relationship strife, new sexual partner, family death, sickness, incarceration, family strife and pregnancy or birth. We used multivariable logistic regression adjusted for clinic clustering to evaluate the association of potential barriers to treatment and age with viral suppression (<400 copies/mL, any ART status) at the time of enrolment.
Results: Among 900 participants (83% female), 885 (98%) completed HIV viral load testing. The age distribution (years) of subjects was 19% 15 to 17, 32% 18 to 20, 29% 21 to 22 and 20% 23 to 24. ART had been started at enrolment (12%), ≤6 months prior (21%), or >6 months prior and they remain in (62%) or have since disengaged from care (4%). The most common life events were pregnancy (16%), moving (16%), sickness (9%), start/stop job or school (9%), family death (8%), relationship strife or divorce/separation (8%) and a new sexual partner (8%). Overlapping (≥2) life‐events and alcohol were associated with viral non‐suppression, while increasing age and disclosure were associated with suppression (Table 1).
Abstract OAB0702‐Table 1
| Predictor of viral suppression | Prevalence in YLHIV | Adjusted odds ratios (95% CI) |
|---|---|---|
| Overlapping (2 or more) events | 17% (151/900) | 0.52 (035 to 0.77), p = 0.001 |
| Alcohol Use | 17 % (155/900) | 0.56 (0.38 to 0.84), p = 0.004 |
| Increasing age | n/a | 1.08 (1.02 to 1.15), p = 0.011 |
| Disclosure of HIV status to family members | 81% (727/900 ) | 2.00 (1.4 to 2.8), p < 0.001 |
| Disclosure of HIV status to partner | 54% (483/900 ) | 1.71 (1.2 to 2.4), p = 0.001 |
Conclusions: In this contemporary cohort of youth living with HIV in rural Africa, overlapping major life‐events, alcohol use and lack of disclosure were associated with viral non‐suppression. Systematic and routine assessment of life events could allow providers and patients to identify and address barriers to treatment, potentially improving clinical outcomes in this vulnerable population.
OAB0703
Improving paediatric index testing: Data from 12 PEPFAR‐supported countries in sub‐Saharan Africa
H.T. Wolf1, K. Battey2, M. Mujawar2, R. Bhatkoti2, E. Rivadeneira2, B. Phelps3, M. Grillo4, T. Al‐Samarrai1, J. Gross2
1Office of the Global AIDS Coordinator, Washington, United States, 2Centers for Disease Control, Division of Global HIV and Tuberculosis, Center for Global Health, Atlanta, United States, 3U.S. Agency for International Development, Washington, United States, 4U.S. Department of Defense, San Diego, United States
Background: Finding HIV‐positive children is critical to close the paediatric treatment gap in resource‐limited settings; 80% of children living with HIV (CLHIV) still not receiving treatment live in 12 PEPFAR‐supported sub‐Saharan African countries. Testing paediatric contacts of HIV‐positive persons yields high positivity rates, and often identifies asymptomatic CLHIV. This report describes the rollout of paediatric HIV index testing and resulting yield in PEPFAR‐supported countries.
Methods: We analysed PEPFAR HIV testing programme data for children one to fourteen years of age, disaggregated by age‐band (1 to 4, 5 to 9, 10 to 14), from 1 October 2017 to 30 September 2018 (FY18) and 1 October 2018 to 30 September 2019 (FY19) for 12 sub‐Saharan African countries. The change in proportion of index tests and resulting yield from FY18 to FY19 was assessed using a one‐sample Wilcoxon signed rank sum test.
Results: The testing yield across all modalities increased from 1.0% (FY18) to 1.4% (FY19) with 101,206 HIV‐positive tests in FY19. The proportion of index testing conducted increased from 9% (FY18) to 12% (FY19) (p < 0.001) and the proportion of HIV‐positive tests from index testing increased from 17% to 28% in FY18 to FY19 (p < 0.001). In FY19, 40% of all index testing occurred in five‐ to nine‐year‐olds who contributed 36% of all positives; index testing in one‐ to four‐year‐olds had the highest yield (4.5%). Eight countries had statistically significant increases in the proportions of index tests from FY18 to FY19: Cameroon (OR = 1.41, CI 1.38 to 1.44), Ethiopia (OR = 10.13, CI 9.91 to 10.36), Kenya (OR = 1.90, CI 1.89 to 1.92), Malawi (OR = 1.28, CI 1.24 to 1.31), Nigeria (OR = 2.13, CI 2.08 to 2.18), South Africa (OR = 2.36, CI 2.32 to 2.40), Tanzania (OR = 2.40, CI 2.38 to 2.42) and Zambia (OR = 1.98, CI 1.96 to 2.00). However, South Africa, Nigeria, Uganda and Malawi had less than 7% of HIV tests from index testing.
Abstract OAB0703‐Figure 1.
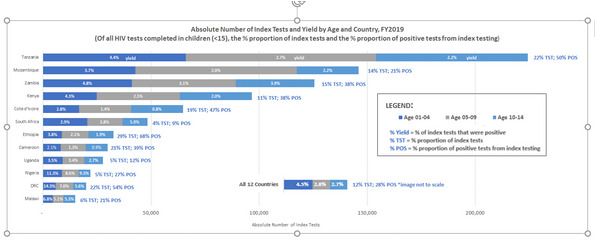
Conclusions: Index testing is a high‐yield approach to find CLHIV in PEPFAR supported settings. Implementation has improved but is sub‐optimal and must be prioritized, particularly in high‐burden settings.
OAB0704
Biomarker assessment of infant adherence to isoniazid prophylaxis in a primary TB infection prevention trial in Kenya
S. LaCourse1, D. Leon2, N. Panpradist2, B. Richardson3,4, E. Maleche‐Obimbo5, J. Mecha6, D. Matemo6, J. Escudero4, J. Kinuthia6,7, B. Lutz2, G. John‐Stewart1,4,8,9
1University of Washington, Department of Medicine, Division of Allergy and Infectious Diseases, Seattle, United States, 2University of Washington, Department of Bioengineering, Seattle, United States, 3University of Washington, Department of Biostatistics, Seattle, United States, 4University of Washington, Department of Global Health, Seattle, United States, 5University of Nairobi, Department of Pediatrics and Child Health, Nairobi, Kenya, 6Kenyatta National Hospital, Department of Research and Programs, Nairobi, Kenya, 7Kenyatta National Hospital, Department of Reproductive Health, Nairobi, Kenya, 8University of Washington, Department of Pediatrics, Seattle, United States, 9University of Washington, Department of Epidemiology, Seattle, United States
Background: Data are lacking regarding infant tuberculosis (TB) prevention therapy adherence. We assessed prevalence and cofactors of isoniazid (INH) prophylaxis (IPT) adherence using a low‐cost dipstick in a TB prevention trial of HIV‐exposed Kenyan infants.
Methods: Infants 6 weeks of age were randomized to 12 months daily INH versus no INH. For infants randomized to INH, standardized adherence questionnaires were administered to caregivers at follow‐up visits (10 weeks, 3, 6, 9, 12 months of age, and 12 months post‐randomization). Urine was collected for an INH dipstick test that changes colour with INH metabolite detection within 30 hours of ingestion. We compared self‐reported adherence to urine results and evaluated correlates of positive INH dipstick with relative risk regression using generalized linear models clustered by participant.
Results: Among 97 infants randomized to INH with ≥1 urine result, baseline median age was 6.3 weeks (IQR 6.0 to 6.4), 41 (43.3%) were female. All mothers were on ART; 69 (71.1%) initiated ART prior to pregnancy and 6 (6.7%) had HIV viral load (VL) >1000 copies/mL. Seventy‐three mothers (75.3%) previously received IPT; 10 (10.3%) reported history of TB.
One‐hundred fifty‐five urine tests were performed among 97 infants (54 (55.6%) 1 test, 29 (29.9%) 2 tests, 14 (14.4%) ≥3 tests) with 77 (49.7%) positive tests. Urine tests were positive in approximately 50% of infants with maternal‐reported optimal INH use (>90% pills taken since last visit) (48/94), INH taken ≤24 hours (69/134), or no missed doses past 3 days (72/136).
Positive urine INH test was associated with maternal secondary education (RR 1.5 (95% CI 1.1 to 2.2, p = 0.02)), increased household rooms (RR 1.2 per room (95% CI 1.0 to 1.5, p = 0.02)), maternal HIV VL < 1000 copies/mL (RR 2.1 (95% CI 1.1 to 4.0), p = 0.02)) and report of no missed doses past 3 days (RR 2.4 (95% CI 1.0 to 5.6), p = 0.05). Infant sex, age at visit, maternal history of TB or IPT were not associated with adherence.
Conclusions: Urine biomarker assessment suggests over‐reported infant INH adherence. Association of maternal education and viral suppression with increased infant INH adherence suggests maternal understanding of medication rationale and success in their own medication adherence predicts infant adherence. Biomarker monitoring may be useful to evaluate and motivate infant medication adherence.
OAB0705
Rates of cervical lesions by age and previous screening status and enhancing treatment among women living with HIV (WLHIV) in sub‐Saharan Africa within the Go Further partnership
D.H. Watts1, J. Albertini1, C. Cazier2, A. Shakarishvili3, A. Prainito1, H. Kusmich2, N.J. Heard1, E.P. Vallejo1, D. Birx1
1U. S. Department of State, Office of the Global AIDS Coordinator, Washington, United States, 2George W. Bush Institute, Dallas, United States, 3UN Joint Programme on AIDS, Geneva, Switzerland
Background: WLHIV are at increased risk of persistent HPV infection and invasive cervical cancer (ICC). Optimal age at initiation of screening and timing of follow‐up for WLHIV are unknown. We assessed the rates of abnormalities by age and screening status among WHLHIV and evaluated factors associated with treatment.
Methods: In May 2018, PEPFAR, the George W. Bush Institute and UNAIDS launched the Go Further partnership; Merck joined in 2019. PEPFAR provided support in eight countries with high HIV prevalence for rapid scaling of bi‐annual screening with visual inspection with acetic acid (VIA) for WLHIV aged 25 to 49 or per national guidelines and single screening for women > 49. Semi‐annual data included age, type of screening (first, rescreen or follow‐up one year after treatment), VIA findings (negative, positive – pre‐cancerous lesions, or suspect ICC) and treatment. Scale up began in Q4 of FY2018. Programmatic data, in country reviews and GIS mapping were used to assess factors associated with treatment rates.
Results: Through September 2019, 567,267 screenings were performed: 488,977 first screenings, 73,265 repeat and 5025 follow‐up after treatment. The rate of pre‐cancer and suspected ICC were 6.5% and 1.5% at first screen, 0.9% and 0.2% in rescreens and 11.9% and 7.1% after treatment. The rate of cervical pre‐cancerous lesions and suspected ICC by age are below.
Abstract OAB0705‐Table 1.
| Age | Negative n (%) | Positive n (%) | Suspected ICC n (%) |
|---|---|---|---|
| 15 to 19 | 6152 (93.8%) | 352 (5.4%) | 53 (0.8%) |
| 20 to 24 | 30,648 (92.0%) | 2306 (6.9%) | 355 (1.1%) |
| 25 to 29 | 94,106 (91.5%) | 7625 (7.4%) | 1091 (1.1%) |
| 30 to 34 | 100,823 (91.7%) | 7630 (6.9%) | 1463 (1.3%) |
| 35 to 39 | 87,065 (91.5%) | 6716 (7.1%) | 1419 (1.5%) |
| 40 to 44 | 74,356 (91.6%) | 5364 (6.6%) | 1435 (1.8%) |
| 45 to 49 | 56,178 (92.8%) | 3235 (5.3%) | 1119 (1.8%) |
| 50+ | 44,815 (92.4%) | 2110 (4.4%) | 1563 (3.2%) |
| Unknown age | 27,229 (93%) | 1627 (5.6%) | 432 (1.5%) |
Procurement issues, need for LEEP training and lack of space were associated with treatment delays; mapping site level results to equipment placement can alleviate treatment backlog.
Conclusions: Rates of pre‐cancerous lesions and suspected ICC after treatment were high, suggesting follow‐up screening sooner than one year. The rates of pre‐cancerous lesions and suspected ICC were high across age bands, suggesting screening should start before age 25 for WLHIV. Aligning treatment availability to sites with high VIA‐positive numbers can improve treatment rates.
OAB0706
Plasma exposure‐viral load response analysis for dolutegravir in children with HIV‐1: Results from IMPAACT P1093
R. Singh1, E. Acosta2, A. Buchanan3, J. Green4, C. Brothers3, A. Wiznia5, C. Alvero6, M. Farhad6, M. Bartlett7, S. Popson7, E. Townley8, R. Hazra9, K. George10, T. Ruel11, C. Vavro3, M. Baker12, The P1093 Team
1GlaxoSmithKline, Collegeville, United States, 2University of Alabama, Birmingham, United States, 3ViiV Healthcare, Research Triangle Park, United States, 4ViiV Healthcare, London, United Kingdom, 5Albert Einstein College of Medicine, Bronx, United States, 6Harvard T.H. Chan School of Public Health, Boston, United States, 7Frontier Science Foundation, Amherst, United States, 8National Institute of Allergy and Infectious Diseases, Rockville, United States, 9Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, United States, 10Family Health International, Durham, United States, 11University of California, San Francisco, United States, 12ViiV Healthcare, Nyon, Switzerland
Background: The approval of antiretroviral dosing in children is generally based on matching adult pharmacokinetic exposure parameters. However, higher variability in paediatric exposures suggests that efficacy may not be presumed to be identical to that in adults. Therefore, we evaluated the relationship between dolutegravir (DTG) exposure and virologic response in children.
Methods: P1093 is a Phase I/II, open‐label PK and safety study. The probability of virologic response (VR, HIV‐1 RNA <50 or <400 copies/mL at Weeks 4, 24 and 48) was modelled as a function of DTG exposure (C24, Cavg or AUC0‐24) based on sampling between days 5‐10, weeks 4, 12 and 24; covariates included baseline viral load (VL), CD4 + count, CDC HIV infection stage and baseline VL ≥100,000 copies/mL. Logistic regression analyses were performed using NONMEM (version 7.4.3).
Results: A total of 143, 135 and 112 VL observations were available at Weeks 4, 24 and 48 respectively. DTG exposure parameters (C24, AUC0‐24 and Cavg) were not predictive of VR within the dose ranges tested, suggesting that exposures were at the maximum of the exposure‐response curve. This may also be attributed to small sample size per dose and higher PK variability. Figure 1 shows exposure‐response relationships for short and long‐term (VR) versus C24. Baseline VL ≥100,000 copies/mL was a significant predictor of response and associated with a lower probability of achieving a VR of HIV‐1 RNA <50 copies/mL (p < 0.001).
Abstract OAB0706‐Figure 1. Viral load response rate versus C24.
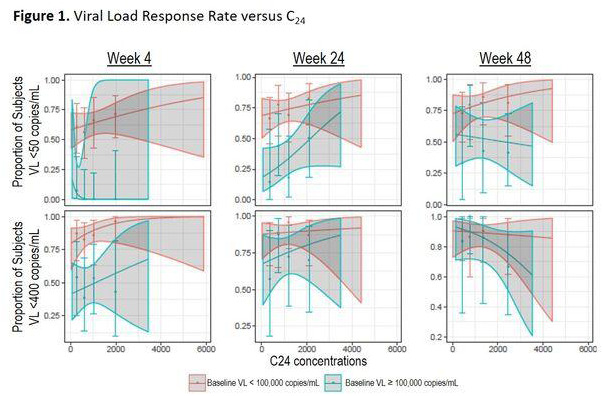
Conclusions: In IMPAACT P1093, a wide range of exposures (C24, AUC0‐24 and Cavg) were observed at tested doses. DTG exposure metrics did not predict VL response, suggesting that the doses tested maintained exposures near maximum drug effect, while baseline VL remained a significant predictor of response. These results suggest that matching paediatric PK exposure parameters to those in adults is a reasonable approach for dose determination of DTG‐containing formulations.
OAC0102
The impact of the DREAMS package on HIV incidence among young women who sell sex in Zimbabwe: A non‐randomized plausibility study
S.T. Chabata1, B. Hensen2, T. Chiyaka1, P. Mushati1, S. Musemburi1, J. Dirawo1, J. Busza2, S. Floyd2, I. Birdthistle2, J.R. Hargreaves2, F.M. Cowan1,3
1Centre for Sexual Health and HIV/AIDS Research (CeSHHAR), Harare, Zimbabwe, 2London School of Hygiene and Tropical Medicine, London, United Kingdom, 3Liverpool School of Tropical Medicine, Liverpool, United Kingdom
Background: DREAMS aims to reduce new HIV infections among adolescent girls and young women through a targeted evidence‐based intervention package. In a non‐randomized study, we estimated its impact on HIV incidence among young women who sell sex (YWSS) in Zimbabwe.
Methods: In two cities where DREAMS was implemented (2017 to 2019) and four towns without DREAMS implementation, respondent‐driven sampling was used to recruit YWSS aged 18 to 24. At enrolment in all sites, consenting YWSS were offered HIV testing and referred to existing services for sex workers. In DREAMS sites only, oral pre‐exposure prophylaxis (PrEP) and referral to other DREAMS services were available to YWSS. We followed up YWSS after two years. Using Poisson regression with follow‐up time estimated as the time between interviews or half of this for those who seroconverted, we compared HIV seroconversion rates among YWSS between DREAMS and non‐DREAMS sites. We adjusted for age, education, marital status, self‐identification as a sex worker, STI symptoms, sexual partners in the past month and HIV prevalence at enrolment. The study was powered to detect a 40% reduction in HIV incidence over two years.
Results: Of 1859 HIV‐negative women enrolled, 1019 (55%) were followed‐up for 1896 person‐years. Half of YWSS (48%) in DREAMS sites had been offered PrEP; 144 (28%) had ever started PrEP but few (12%) continued it (Figure 1). Social protection service uptake was minimal (<5%). Among YWSS from DREAMS sites, HIV incidence was 3.1/100 person‐years, compared to 5.3/100 person‐years in non‐DREAMS sites (RR = 0.59; 95% CI 0.38 to 0.93). In adjusted analyses, there was little difference in HIV incidence between the DREAMS and non‐DREAMS sites (RR = 0.74; 95% CI 0.43 to 1.29; p = 0.3).
Abstract OAC0102‐Table 1.
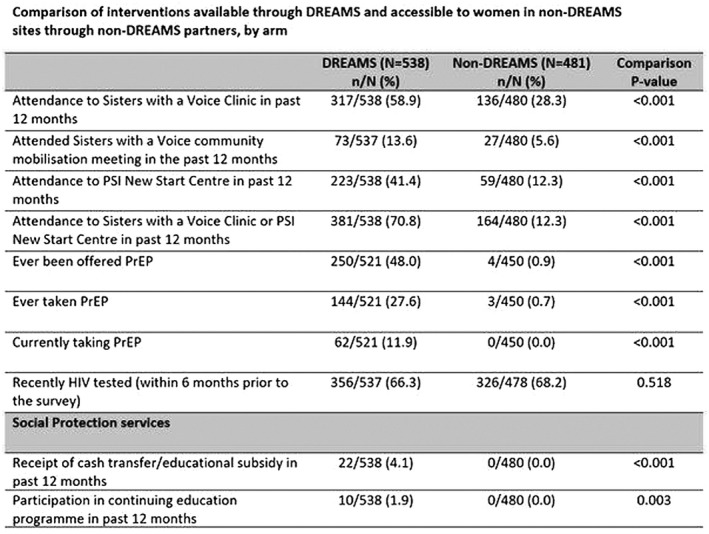
Conclusions: We found limited evidence of a large impact of DREAMS on HIV incidence among YWSS in two Zimbabwean cities. Identifying approaches that enhance access to social services combined with delivery of biomedical interventions including PrEP remains critical for YWSS.
OAC0103
Effects of economic support and community dialogue on adolescent sexual behaviour: Findings from a cluster‐randomized controlled trial in Zambia
H.K. Hegdahl1, I.F. Sandøy1
1University of Bergen, Department of Global Public Health and Primary Care, Bergen, Norway
Background: With a HIV prevalence twice that of their male counterparts and a high incidence of adolescent pregnancies, girls and young women in Sub‐Saharan Africa are disproportionately affected by sexual and reproductive health problems (SRH). The objective of this study was to measure the effectiveness of economic support alone or in combination with a community intervention, on sexual activity, contraceptive knowledge and contraceptive behaviour of adolescent girls.
Methods: Data come from a cluster‐randomized trial in rural Zambia. Recruitment was conducted between March and July 2016, and all girls from grade 7 in 157 selected schools were eligible to participate. Schools were randomized to either economic support, combined economic support and community dialogue, or control. Economic support consisted of cash transfers to girls and their parents, and payment of school fees for girls continuing to grade 8 and 9. The community dialogue consisted of community and youth meetings that aimed to enhance SRH knowledge and supportive community norms. The interventions lasted from 2016 to 2018, and outcomes were measured at the end of the intervention period. Comparisons between the arms were made using generalized estimating equations. All analyses were by intention‐to‐treat.
Results: In total 4922 girls assented to participate. The mean age was 13.6 years at baseline and 16.1 years at the end of the intervention period. The response rate at the end of the intervention period was 89.4%. The proportion of girls reporting recent sexual activity was markedly lower in the combined arm (RR 0.73; 95% C.I. 0.60 to 0.89) and slightly lower in the economic arm (0.85; 95% CI 0.69 to 1.05) than in the control arm. Knowledge of modern contraceptives was higher in the combined than in the other two arms, but only significantly different from the economic arm (combined vs. economic RR 1.17; 95% C.I. 1.00 to 1.36; combined vs. control RR 1.16; 95% C.I. 0.95 to 1.43). No intervention effect was found on reported current use of modern contraceptives.
Conclusions: Economic support combined with community dialogue increased contraceptive knowledge and reduced sexual activity more than economic support alone, and may in turn reduce the risk of SRH problems. However, contraceptive use was not affected.
OAC0104
What is the impact of DREAMS on HSV‐2 acquisition among AGYW in rural KwaZulu‐Natal, South Africa?
N. Mthiyane1, N. Chimbindi1, T. Zuma1, J. Dreyer1, I. Birdthistle2, S. Floyd2, A. Gourlay2, T. Smith1, K. Baisley2, N. McGrath3, G. Harling4, J. Seeley2, M. Shahmanesh4
1Africa Health Research Institute, Durban, South Africa, 2London School of Hygiene and Tropical Medicine, London, United Kingdom, 3University of Southampton, Southampton, United Kingdom, 4University College London, London, United Kingdom
Background: In South Africa, adolescent girls and young women (AGYW) are at high risk of acquiring HIV and other sexually transmitted infections such as Herpes Simplex Virus type‐2 (HSV‐2). HSV‐2 is a marker of unprotected sex and direct risk factor for HIV acquisition. We evaluated the impact of combination HIV prevention DREAMS (Determined, Resilient, Empowered, AIDS‐free, Mentored and Safe) Partnership introduced in 2016 on HSV‐2 infection in AGYW living in a rural area of South Africa where lifetime HIV acquisition risk is over 50%.
Methods: We analysed data collected from a representative cohort of AGYW aged 13 to 22 selected from the general population in uMkhanyakude, KwaZulu‐Natal. We collected data at three annual timepoints (2017 to 2019) on uptake of DREAMS interventions and collected dried blood spots for HSV‐2 testing. HSV‐2 seroconversion dates were estimated as the midpoint between date of last negative and first positive test; participants that remained negative throughout the study were censored at last visit date. We estimated HSV‐2 prevalence and incidence rates and used Poisson regression to compare rate ratios among DREAMS beneficiaries (AGYW who were invited at any timepoint in 2016 to 2018 to participle in any DREAMS activity) and non‐beneficiaries (AGYW never invited).
Results: Of 2184 AGYW enrolled and tested for HSV‐2 at baseline, 553 (25.3%) were HSV‐2 positive. Of the remaining 1631, 1397 (85.7%) provided at least one follow‐up test. HSV‐2 incidence was 15.4 per 100 person‐years (PY; 95% CI: 13.6 to 17.5). Incidence was non‐significantly lower (14.3/100 PY) among DREAMS beneficiaries compared to non‐DREAMS beneficiaries (16.9/100 PY). In age‐adjusted analyses incidence rates were not significantly different between DREAMS beneficiaries and non‐beneficiaries (overall: adjusted Rate Ratio (aRR) 0.97, 95% CI 0.75‐ 1.26; for 13 to 17 years: aRR 1.24, 95% CI 0.84 to 1.83; for 18 to 22 years: aRR 0.77, 95% CI 0.53 to 1.13).
Conclusions: We found little evidence of an impact of DREAMS on incidence of HSV‐2 among AGYW in this setting with high HSV‐2 and HIV prevalence. Sexual and reproductive health interventions need to be scaled up to reach vulnerable young people to prevent rapid acquisition of infections soon after sexual debut.
OAC0105
Incorporating PrEP into standard of prevention in a clinical trial is associated with reduced HIV incidence: Evidence from the ECHO Trial
D. Donnell1, I. Beesham2, J. Welch3, R. Heffron4, M. Pleaner2, L. Kidoguchi4, T. Palanee‐Phillips2, K. Ahmed5, D. Baron2, E. Bukusi4, C. Louw6, T. Mastro3, J. Smith2, J. Batting2, M. Malahleha5, V. Bailey5, M. Beksinska2, H. Reese2, J. Baeten4, ECHO Trial Consortium
1Fred Hutchinson Cancer Research Center, Vaccine and Infectious Diseases, Seattle, United States, 2University of Witwatersrand, Durban, South Africa, 3FHI 360, Durham, United States, 4University of Washington, Seattle, United States, 5Setshaba Research Center, Tshwane, South Africa, 6University of Pretoria, Pretoria, South Africa
Background: As oral PrEP becomes standard of prevention globally, its potential impact on HIV incidence in clinical trials of new prevention interventions is unknown. In the ECHO Trial, conducted between 2015 and 2018, PrEP was incorporated into standard of prevention from 2017. We assess the effect of access to PrEP on HIV incidence in this natural experiment.
Methods: At 12 sites in four countries (Eswatini, Kenya, South Africa, Zambia), women were randomized to receive one of three contraceptives (copper IUD, DMPA‐IM and levonorgestrol implant) and followed quarterly for up to 18 months to determine the impact of contraceptive use on HIV acquisition. The present analyses are limited to the South African sites (9 of 12 trial sites, 74% of trial enrolment), because PrEP access was offered on‐site by the study team in 2018, accompanied by additional staff training; in the other three sites PrEP was offered off‐site through demonstration and implementation projects. Using Poisson regression with GEE, we compared HIV incidence pre‐ versus post‐PrEP access, limited to quarterly visit months at which PrEP access was available on‐site and, in a sensitivity analysis, to the 180 days before and after access.
Results: 2043 women had follow‐up time after on‐site PrEP access began, of whom 543 (27%) initiated PrEP. A total of 12 HIV seroconversions were observed in 556 person‐years (incidence 2.16 per 100 person‐years) after PrEP access, compared to 133 HIV seroconversions in 2863 person‐years (4.65 per 100 person‐years) before PrEP access (IRR 0.451, p = 0.009). Limiting to the 180 days post‐ versus pre‐access showed similar results (incidence 2.29 vs. 5.00 per 100 person years, IRR 0.434, p = 0.016). Prior to PrEP access, HIV incidence was similar for women who did and did not have opportunity (based on enrolment date) to access PrEP on‐site.
Conclusions: Access to PrEP as part of standard of prevention in a clinical trial among women in South Africa was associated with a halving of HIV incidence, when about a quarter of women started PrEP. Providing access to PrEP on‐site as part of the standard of care package for prevention may result in decreased HIV incidence in future HIV prevention trials.
OAC0106
Consent comprehension and waiver of caregiver consent for minors participating in sensitive research: Views of adolescent girls and caregivers in Western Kenya
L. Oluoch1, K. Agot1, M. Ochillo1, G.‐N. Wango2, M. Omoya1, N. Ounda1, H. Thirumurthy3
1Impact Research and Development Organization, Research, Kisumu, Kenya, 2Nyanza Initiative for Girls’ Education & Empowerment, Kisumu, Kenya, 3University of Pennsylvania, Philadelphia, United States
Background: Adolescents under the age of consent often miss out on effective biomedical HIV interventions because they do not participate in trials. Researchers generally focus on adults to avoid dealing with the requirement of caregiver (CG) permission for minors to participate in research. We explored understanding of the consent by CG and adolescent girls (AG) and their views on waiver of CG consent.
Methods: We conducted in‐depth interviews (IDIs) with AG and CG, enrolled though the DREAMS programme. The topics elicited information on: how to administer the consent to ease comprehension; components of the consent difficult or easy to understand; views on waiver of caregiver consent on general and sensitive research topics such as on HIV, sexually transmitted infections, pregnancy and contraceptives. The sessions were audio‐recorded and transcribed; thematic approach was used to code the transcripts based on discussion topics.
Results: We conducted 33 IDIs with AG aged 15 to 17 years and 40 with CG aged 23 to 52 years. Although both AG and CG expressed fatigue with the “hard,” “compact” and “long” contents of the informed consent, elements found specifically difficult were: apparent contradictions e.g. with voluntarism (“you invite us to join a study then tell us we can withdraw or not answer questions”); confusion with multiple durations, e.g. for IDI, study, paper data storage, electronic data storage; subject matter of research, such as “how saliva can carry HIV.” AG also found research terms in local language difficult to understand, e.g. compensation for time, voluntarism, ethical oversight, etc. Both AG and CG preferred reading sub‐titles then staff reads text and asks questions; they suggested group discussion to aid understanding. Waiver of CG consent for minors on various reproductive health topics was rejected: 57.5% to 81.8% by AG and 37.5% to 100% by CG; however, if research topic is sensitive and may reveal sexual relationships of AG to their CG, 67% of AG preferred giving own consent.
Conclusions: AG and CG find consent documents generally long and technical. Waiver of CG consent was rejected by both AG and CG; however, if it leads to involuntary disclosure of AGs sexual behaviour, most AG but not CG recommend waiver.
OAC0202
Outreach‐based HIV testing approach from Test & Treat project in Tanzania: Mid‐term results
G. Martelli1, L. Van Duffel1, I. Amiri Salehe1, F. Cavallin2, E. Cosmas Kwezi1, G. Putoto3, G. Torelli1, T. Rinke de Wit4, A. Pozniak5, B. Mwesiga Desderius6, A. Bortolani3
1Doctors with Africa CUAMM, Shinyanga, Tanzania, United Republic of, 2Independent Statistician, Solagna, Italy, 3Doctors with Africa CUAMM, Padua, Italy, 4Amsterdam Institute for Global Health and Development (AIGHD), Amsterdam, Netherlands, 5Chelsea and Westminster Hospital NHS Foundation Trust, London, United Kingdom, 6Bugando Medical Centre, Mwanza, Tanzania, United Republic of
Background: The Test & Treat project is implemented in Tanzania by the Diocese of Shinyanga and CUAMM ‐ Doctors with Africa. This project offers universal HIV testing and access to decentralized antiretroviral treatment through 4 Care and Treatment Clinics in Shinyanga and Simiyu regions, where estimated HIV prevalence is 5.9% and 3.9% respectively (Tanzania HIV Impact Survey, 2017).
Methods: HIV testing and counselling activities were offered through extensive community outreaches, special events and facility‐based services. The current cross‐sectional study provides midterm results at 26 months (May 2017–June 2019), stratified by sex and age. Aggregated data were collected from governmental testing registers.
Results: A total 255,329 HIV tests were performed: 198,451 (77.7%) during testing campaigns in the villages, 44,286 (14.4%) in the health facilities and 12,592 (4.9%) during special events’ outreaches. Gender distribution varied among testing modalities: females represented 53.8% (23,809) among those who tested in the health facilities, while males were the majority in the community (54.4%, 114,835 among testing campaigns and special events). Over one third of tests (n = 102,427 41%) were performed among first‐time testers. At multivariable analysis, higher rate of first‐time testers was associated with being tested in the community versus in the facilities (RR 1.07, 95% CI 1.05 to 1.09; p < 0.0001), with males (RR 1.05, 95% CI 1.04 to 1.07; p < 0.0001) and with younger age (RRs ranging from 1.08 to 5.87 in age classes; p < 0.0001). The overall HIV positivity rate was 1.2%, ranging from 0.7% in the community to 3.8% in the health facilities. HIV positivity rate was higher in females, both in the community (0.9% vs. 0.5%, p < 0.0001) and in the health centres (4.2% vs. 3.4%, p < 0.0001), among those with higher age classes (RR 1.68, 95% CI 1.62 to 1.73; p < 0.0001) and the first‐time testers (RR 1.93, 95% CI 1.80 to 2.08; p < 0.0001).
Conclusions: Test &Treat project facilitated HIV testing in Shinyanga and Simiyu yielded relatively low numbers of newly identified HIV patients, complementary to ongoing efforts by National AIDS Control Program and others. More targeted efficient strategies to reach “the first 90” are recommended, such as index testing or hot spot testing.
OAC0203
Geographic hotspots of high population HIV viraemia and association with HIV incidence in a universal test‐and‐treat setting in rural Uganda and Kenya
J. Peng1, J. Kabami2, J. Ayieko3, F. Mwangwa2, M. Atukunda2, E. Charlebois1, G. Chamie1, D. Kwarisiima2, L. Balzer4, M. Kamya5, D. Havlir1, M. Petersen6
1University of California, Department of Medicine, San Francisco, United States, 2Infectious Disease Research Collaboration, Kampala, Uganda, 3Kenya Medical Research Institute, Nairobi, Kenya, 4University of Massachusetts, Amherst, United States, 5Makerere University, Kampala, Uganda, 6University of California, Berkeley, United States
Background: In the context of universal ART eligibility and increasing viral suppression, geospatial heterogeneity in HIV viraemia could clarify drivers of transmission and improve intervention targeting. We evaluated the geospatial distribution of viraemia before and after universal test‐and‐treat (UTT) implementation and its relation to HIV incidence.
Methods: In 2013 to 2014, 10 West Ugandan and 12 Kenyan communities in the SEARCH study (NCT01864603) were census‐enumerated with residential GPS coordinates recorded; 90% underwent HIV testing and HIV‐RNA measurement, with repeat testing after three years. All HIV+ persons were eligible for ART at or after baseline. A moving 2‐km Gaussian kernel was used to calculate local viraemia (% of all adults with HIV‐RNA > 1000 cps/mL) at baseline and endpoint. Geographic clusters were detected using Tango's scan statistic. Within‐community association between local viraemia and incidence was evaluated with cluster‐robust Poisson regression.
Results: Among 106,164 adults aged ≥15 years, median local viraemia was 2.0% in Uganda and 3.7% in Kenya at baseline and declined to 0.8% in Uganda and 1.8% in Kenya after three years (Figure 1). 11 communities (5 Uganda, 6 Kenya) had viraemia clusters at baseline; 4 of these (2 Uganda, 2 Kenya) plus 3 new communities had clusters at year 3. At baseline, persons living closer to a road or the Lake Victoria coast had higher local viraemia; these associations were attenuated after UTT. In Kenya, where HIV incidence declined by 43% during the study, every 1% absolute increase in local viraemia was associated with a 32% increase in HIV incidence (IRR: 1.32, 95% CI: 1.14 to 1.52). In Uganda, where HIV incidence did not decline despite similar reductions in viraemia, local viraemia did not predict incidence (IRR: 0.96, 95% CI: 0.78 to 1.17).
Abstract OAC0203‐Figure 1. Heatmap of baseline and year 3 follow‐up viraemia in Nyamrisa community in Kenya (A1 and A2) and Rubaare community in Uganda (B1 and B2), created using a 2‐kilometre Gaussian kernel.
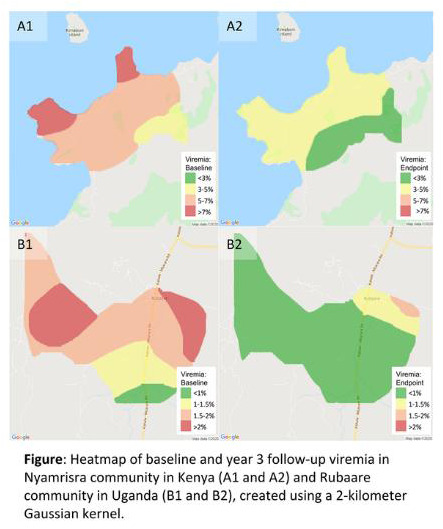
Conclusions: In the context of UTT, HIV viraemia declined but geographic hotspots of viraemia remained, suggesting a role for geospatially targeted testing and care engagement strategies.
OAC0204
Impact of universal testing and treatment on sexual risk behaviour and HSV‐2: Evidence from the HPTN 071 (PopART) trial in Zambia and South Africa
E. Wilson1, D. Donnell1, T. Skalland1, A. Moore2, N. Mandla3, J. Bwalya4, N. Kasese4, R. Dunbar3, K. Shanaube4, B. Kosloff5,4, O. Laeyendecker6, Y. Agyei6, D. Lennon6, G. Hoddinott3, P. Bock3, S. Fidler7, R. Hayes5, H. Ayles5,4
1Fred Hutchinson Cancer Research Center, Seattle, United States, 2FHI 360, Durham, United States, 3Stellenbosch University, Cape Town, South Africa, 4Zambart, Lusaka, Zambia, 5London School of Hygiene and Tropical Medicine, London, United Kingdom, 6Johns Hopkins Univ. School of Medicine, Baltimore, United States, 7Imperial College, London, United Kingdom
Background: HPTN 071 (PopART) was a cluster‐randomized trial of a combination HIV prevention strategy, including universal HIV testing and treatment (UTT) conducted between 2013 and 2018 in 21 high HIV prevalence communities in Zambia and South Africa. HIV incidence was significantly reduced in the trial arm which included universal HIV testing and treatment according to national guidelines (Arm B), with a lesser effect in the full UTT arm (Arm A), compared to standard of care (Arm C). We investigate if the intervention changed sexual behaviour.
Methods: A population cohort of approximately 2000 randomly selected adults (18 to 44) in each community (N = 48,301) was followed for three years to evaluate the impact of the trial on HIV, HSV‐2 and sexual behaviour (N = 27,501 completed final visit). Differences in self‐reported sexual behaviour were assessed using a two‐stage method for matched cluster‐randomized trials. HSV‐2 incidence, as a marker of sexual risk, was measured in participants negative at enrolment with blood drawn at the final visit.
Results:
Abstract OAC0204‐Table 1. Arm comparison of change in sexual risk outcomes
| Risk variable | Subgroup | Mean of community proportions at final study visit | Adj Prev ratio A versus C (95% CI) | p Values | Adj Prev ratio B versus C (95% CI) | p Values | ||
|---|---|---|---|---|---|---|---|---|
| Arm A | Arm B | Arm C | ||||||
| HSV‐2 incidence | Overall | 11.6% | 9.55% | 11.9% | 0.89 (0.73, 1.08) | 0.199 | 0.76 (0.63, 0.92) | 0.010 |
| Men | 7.48% | 5.25% | 7.82% | 0.93 (0.63, 1.38) | 0.700 | 0.64 (0.43, 0.95) | 0.030 | |
| Women | 14.1% | 12.4% | 14.8% | 0.89 (0.73, 1.07) | 0.190 | 0.81 (0.67, 0.98) | 0.035 | |
| Women | 5.18% | 4.93% | 4.71% | 1.01 (0.78, 1.30) | 0.950 | 1.02 (0.78, 1.31) | 0.902 | |
| Multiple sexual partners in last 12 mo. | Overall | 1.95% | 4.01% | 4.17% | 0.63 (0.29, 1.35) | 0.210 | 1.05 (0.49, 2.26) | 0.892 |
| Men | 5.35% | 9.12% | 10.8% | 0.65 (0.32, 1.31) | 0.205 | 0.87 (0.43, 1.75) | 0.661 | |
| Women | 1.08% | 2.25% | 1.63% | 0.68 (0.24, 1.99) | 0.450 | 1.53 (0.53, 4.45) | 0.396 | |
| No condom use at last sex | Overall | 60.3% | 62.6% | 62.4% | 0.89 (0.79, 1.01) | 0.064 | 0.98 (0.87, 1.11) | 0.778 |
| Sexual debut during PopART, if had never had sex at enrolment | Overall | 72.8% | 74.1% | 73.3% | 0.99 (0.84, 1.18) | 0.926 | 1.00 (0.85, 1.19) | 0.979 |
No significant changes in self‐reported sexual behaviour were observed as a result of the intervention (Arms A or B versus C) (Table 1). The percentage of HSV‐2‐negative participants who acquired HSV‐2 during the trial was 11.6% in Arm A, 9.6% in Arm B and 11.9% in Arm C. Mirroring the trial's HIV incidence result, HSV‐2 incidence was lower by 11% (95% CI ‐8%, 27%, p = 0.2) in Arm A versus C, and by 24% (95% CI 8%, 37%, p = 0.01) in Arm B versus Arm C. Similar results held for men and women, with fewer HSV‐2 infections observed in Arm B compared to Arm C.
Conclusions: There was no evidence that PopART interventions caused sexual risk disinhibition. HSV‐2 incidence mirrored HIV‐incidence, underscoring the potential importance of the correlation between HSV‐2 and HIV susceptibility.
OAC0205
Increased targeted HIV testing and reduced undiagnosed HIV infections among gay and bisexual men in New South Wales, Australia 2010 to 2018
P. Keen1, P. Patel1, H. McManus1, T. Duck2, D. Callander3,1, C. Selvey2, C. Power2, R.T. Gray1, K. Vickie4,1, J. Asselin5, P. Read6,1, K. Johnson7, B.R. Bavinton1, A.E. Grulich1, R. Guy1, on behalf of the NSW HIV Prevention Partnership Project
1The Kirby Institute, UNSW Sydney, Faculty of Medicine, Sydney, Australia, 2NSW Ministry of Health, Sydney, Australia, 3Columbia University, Spatial Epidemiology Lab, New York City, United States, 4Sydney Sexual Health Centre, Sydney, Australia, 5Burnet Institute, Melbourne, Australia, 6Kirketon Road Centre, Sydney, Australia, 7ACON, Sydney, Australia
Background: In New South Wales (NSW), approximately 80% of HIV diagnoses occur among gay and bisexual men (GBM). In 2012 and 2016, the NSW Government released strategies aiming to increase HIV testing frequency among GBM and virtually eliminate HIV transmission. A range of HIV testing initiatives were introduced and expanded, and key indicators developed to evaluate their impact.
Methods: Seven HIV indicators were measured during 2010 to 2018: (1) state‐wide total HIV laboratory tests; (2) number of GBM attending cost‐free publicly funded HIV testing services; (3) 12‐monthly HIV testing uptake; (4) annual HIV testing frequency; (5) HIV testing concurrently with a STI diagnosis; (6) HIV positivity; and (7) proportion of men with undiagnosed HIV among GBM living with HIV. Data were collected from existing passive and sentinel surveillance systems and mathematical modelling. Indicators were stratified by Australian versus overseas‐born.
Results: Overall, 43,560 GBM attended the HIV testing services within the sentinel system (22,662 Australian‐born, 20,834 overseas‐born, 64 unknown) from 2010 to 2018. The number of attendees increased from 5186 in 2010 to 16,507 in 2018. There were increasing trends (p < 0.001 for all) in 12‐monthly HIV testing uptake (83.9% to 95.1%); concurrent HIV testing with a STI diagnosis (68.7% to 94.0%); annual HIV testing frequency (1.4 to 2.7); and a decreasing trend (p < 0.01) in HIV positivity (1.7% to 0.9%). Increases in testing were similar in Australian‐born GBM and overseas‐born GBM. However, among GBM living with HIV in NSW, there were decreasing trends in the estimated undiagnosed HIV proportion overall (9.5% to 7.7%) and in Australian‐born GBM (7.1% to 2.8%), but an increasing trend in overseas‐born GBM (15.3% to 16.9%) (p < 0.001 for all).
Conclusions: Over the nine‐year study period, more than three times more GBM attended the HIV testing services demonstrating increased demand for testing. Among these men, HIV testing was optimized reaching very high levels of uptake and frequency by 2018. The decline in the estimated undiagnosed proportions in GBM indicates HIV testing initiatives were well targeted in this group, reaching a very low level of undiagnosed HIV by 2018. Future initiatives should focus on addressing the higher undiagnosed proportion among overseas‐born GBM and achieving further increases in testing frequency.
OAC0206
Estimated time from HIV infection to diagnosis, 50 U.S. states and the District of Columbia, 2014 to 2017
N. Crepaz1, R. Song1, S. Lyss1, H.I. Hall1
1Centers for Disease Control and Prevention, Atlanta, United States
Background: In the United States, 38% of HIV transmissions occur from persons with undiagnosed HIV infection. Delayed diagnosis reduces opportunities to improve health outcomes of persons with HIV and to prevent HIV transmission. To inform local prevention efforts, we examined time between HIV infection and diagnosis (Infx‐to‐Dx) at the jurisdiction level.
Methods: We analysed data reported to the National HIV Surveillance System (NHSS) through June 2019 from 50 U.S. States and the District of Columbia for HIV diagnoses occurring among persons aged >13 years during 2014 to 2017. We calculated the interval between HIV infection and diagnosis by using HIV infection dates estimated based on a CD4 depletion model and HIV diagnosis dates reported to NHSS. Trends during 2014 to 2017 in the median number of months for Infx‐to‐Dx intervals were examined by using estimated annual percentage change.
Results: During 2014 to 2017 in the United States, 157,412 HIV diagnoses occurred. The median Infx‐to‐Dx interval decreased from 43 months for persons with HIV diagnosed in 2014 to 40 months for persons with HIV diagnosed in 2017, a 2.3% annual decrease (p < 0.001). Infx‐to‐Dx intervals shortened significantly during 2014 to 2017 in the South and the West (Table 1) which accounted for 71.2% of all HIV diagnoses during 2014 to 2017. In 41 jurisdictions with reliable estimates in 2017 (relative standard errors < 30%), median Infx‐to‐Dx intervals were <36 months for 10 (24.4%) jurisdictions, 36 to 47 months for 23 (56.1%) and ≥48 months for 8 (19.5%).
Abstract OAC0206‐Table 1.
| Region | 2014 Median Month (IQR) No. of HIV Diagnosis | 2015 Median Month (IQR) No. of HIV Diagnosis | 2016 Median Month (IQR) No. of HIV Diagnosis | 2017 Median Month (IQR) No. of HIV Diagnosis | Estimated Annual Percentage Chang p value |
|---|---|---|---|---|---|
| Northeast | 42 (0 to 106) N = 6671 | 46 (0 to 107) N = 6224 | 41 (0 to 105) N = 5861 | 42 (0 to 102) N = 5623 | –1.5 p = 0.428 |
| Midwest | 46 (0 to 108) N = 5111 | 39 (0 to 100) N = 5357 | 46 (0 to 104) N = 5292 | 41 (0 to 96) N = 5231 | –1.4 p = 0.645 |
| South | 45 (0 to 106) N = 20,294 | 42 (0 to 103) N = 20,487 | 41 (0 to 102) N = 20,399 | 41 (0 to 100) N = 19,874 | –3.1 p < 0.0001 |
| West | 38 (0 to 100) N = 7862 | 38 (0 to 98) N = 7711 | 37 (0 to 96) N = 7944 | 37 (0 to 95) N = 7473 | –1.6 p < 0.0001 |
| Total | 43 (0 to 106) N = 39,938 | 41 (0 to 102) N = 39,777 | 41 (0 to 102) N = 39,495 | 40 (0 to 99) N = 38,201 | –2.3 p < 0.0001 |
Conclusions: During 2014 to 2017, the median time from HIV infection to diagnosis shortened nationally and particularly in southern and western states, suggesting better access to testing. However, delayed HIV diagnosis is substantial; one in two persons with HIV diagnosed in 2017 was infected at least 40 months before diagnosis. That the median Infx‐to‐Dx interval was longer than 36 months for three‐quarters of jurisdictions underscores the importance of addressing local barriers to early diagnosis.
OAC0302
Drug overdoses are reducing the gains in life expectancy of people living with HIV (PLWH) in British Columbia, Canada
M. St‐Jean1, X. Dong1, D.M. Moore12, P. Sereda1, K. Salters1, R.S. Hogg1, T.L. Patterson3, R. Barrios1, J.S. Montaner1, V.D. Lima1
1British Columbia Centre for Excellence in HIV/AIDS, Epidemiology and Population Health, Vancouver, Canada, 2University of British Columbia, Department of Medicine, Vancouver, Canada, 3University of California, Department of Psychiatry, San Diego, United States
Background: Overdose deaths have substantially increased in British Columbia (BC) since 2014; a public health emergency was declared in 2016. People living with HIV (PLWH) are disproportionately affected by substance use. We assessed the impact of illicit and pharmaceutical drug overdoses on life expectancy (LE) among PLWH in BC and identified factors associated with overdose mortality using competing risk methodology.
Methods: PLWH were aged ≥20 years, initiated antiretroviral therapy (ART) between 1‐Apr‐1996 and 30‐Dec‐2017 in the Drug Treatment Program, and were followed until 31‐Dec‐2017, last contact date, or death date. We calculated all‐cause and overdose cause‐deleted LE at age 20 from abridged life tables. A subdistribution hazard model was built. Time‐fixed covariates (at ART initiation) included gender, HIV exposure category and ART initiation year (continuous). Time‐varying covariates (six‐month intervals) included age (continuous (years)), CD4 count (cells/mm3), % suppressed viral load (VL) and time period. Overdose mortality was the outcome in the presence of competing mortality of other causes.
Results: Overall, 10,362 PLWH had a median age of 40 (25th‐75th percentiles:33 to 47) years and follow‐up of 6.93 (2.84 to 12.39) years; 26% were people who injected drugs (PWID). The largest loss in LE attributed to overdose occurred during the current 2014 to 2017 overdose era (2.5‐ to 5‐fold higher than other periods). In 2014 to 2017, the estimated LE at age 20 is 55 years. However, when overdose deaths are deleted, the estimated LE becomes 65 years (10 years greater) (Figure 1). Factors with elevated overdose hazards included the current overdose era (adjusted subhazard ratio (aSHR) 4.73 95% Confidence Interval, 2.07 to 11.38) relative to the harm reduction era (2002 to 2007), PWID (aSHR 7.88, 4.82 to 12.87) relative to men who have sex with men, VL not tested (aSHR 4.73, 3.54 to 6.31) and < 100% suppression (aSHR 1.68, 1.20 to 2.36) relative to 100% suppression.
Abstract OAC0302‐Figure 1. Mortality and life expectancy trends for individuals initiating ART in British Columbia, Canada, 1996 to 2017.
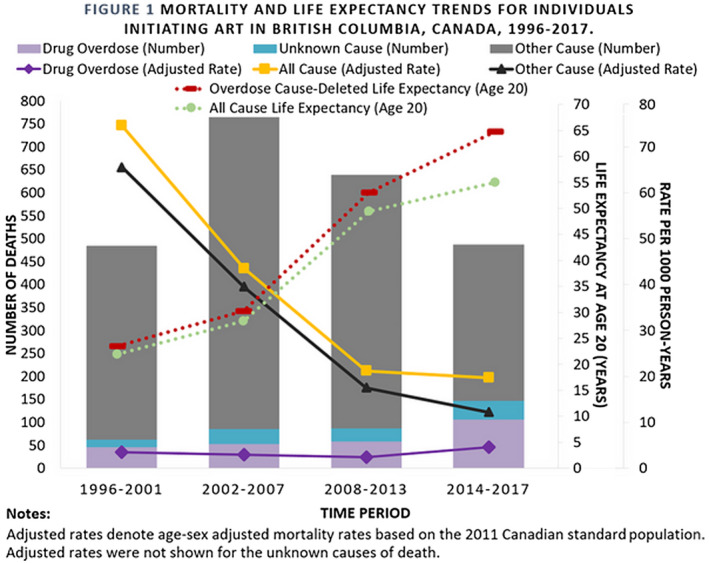
Conclusions: Survival gains, by virtue of combination ART, have been dramatically reduced due to the current overdose crisis.
OAC0303
Harm reduction revisited: The causal effect of the Dutch approach towards people who inject drugs on HIV, hepatitis B and C infection risk
D.K. van Santen1,2,3, A. Boyd1,4, A. Master1, S. Lodi5, M. Prins1,6
1Public Health Service of Amsterdam, Infectious Disease Research and Prevention, Amsterdam, Netherlands, 2Burnet Institute, Disease Elimination, Melbourne, Australia, 3Monash University, School of Population Health and Preventive Medicine, Melbourne, Australia, 4Stichting HIV Monitoring, Amsterdam, Netherlands, 5Boston University, Department of Biostatistics, Boston, United States, 6University of Amsterdam, Amsterdam, Netherlands
Background: Early implementation of low‐threshold harm‐reduction programmes (HRP) (opiate substitution therapy (OST) and needle and syringe exchange programmes (NSP)) in the Netherlands might have contributed to the major decline in incidence of human immunodeficiency virus (HIV), hepatitis C virus (HCV) and hepatitis B virus (HCV). We aimed to assess the causal effect of HRP participation on risk of these infections among persons who inject drugs (PWID).
Methods: We emulated a target trial using observational data from the Amsterdam Cohort Studies (1985 to 2014). We included PWID who ever used opioids, had a recent history of injecting drug use (IDU) and had a negative antibody test. Follow‐up was analysed in interval‐time risk‐sets with a maximum duration of two years. Follow‐up was calculated from the earliest date all eligibility criteria were met (i.e. baseline), until individuals were no longer compliant, HIV, HCV or HBV seroconversion, lost to follow‐up, reached administrative censoring date or completed the two‐year follow‐up interval; whichever occurred first. The intervention arms were: complete HRP participation (OST: ≥60 mg methadone and NSP: 100% coverage, or OST: any dose if no recent IDU) versus no/partial HRP participation (OST: <60 mg and/or NSP: <100% coverage). Marginal structural Cox‐regression models were used to estimate causal hazards ratios (HR) for each infection separately, including inverse probability weights of treatment and censoring.
Results: Of 983 PWID participants, 653, 143 and 310 PWID were HIV negative, HCV negative and HBV negative, respectively, and considered eligible. We observed 70 HIV, 48 HCV and 50 HBV seroconversions during follow‐up. Compared to no/partial HRP, complete HRP participation led to a decreased risk in HIV (HR = 0.56, 95% CI:0.33 to 0.92), HCV (HR = 0.12, 95% CI:0.05 to 0.29) and HBV (HR = 0.29, 95% CI:0.15 to 0.56) acquisition.
Conclusions: Harm reduction programmes led to a major decrease in HIV, HCV and HBV acquisition among PWID from Amsterdam. To the best of our knowledge this is the first study reporting causal estimates for HRP on infection risk. These findings reinforce the need to implement or scale up low‐threshold HRP to prevent ongoing transmission among PWID.
OAC0304
Are harm reduction projects for people who inject drugs in Ukraine improving HIV prevention and treatment outcomes?
A. Trickey1, N. Semchuk2, T. Salyuk2, Y. Sazonova2, O. Varetska2, J.G. Walker1, A.G. Lim1, J. Stone1, P. Vickerman1
1University of Bristol, Population Health Sciences, Bristol, United Kingdom, 2Alliance for Public Health, Kiev, Ukraine
Background: People who inject drugs (PWID) in Ukraine have high prevalences of HIV and hepatitis C virus (HCV). The Global Fund to fight AIDS, Tuberculosis and Malaria has funded non‐governmental organizations (NGOs) in Ukraine since 2003 to provide PWID with needle and syringe distribution, condoms, HIV and HCV testing, and to improve linkage to opioid substitution therapy (OST) and HIV treatment. However, due to policy changes the Global Fund is scaling back support in Ukraine. We investigated whether contact with these NGOs is associated with improved HIV prevention and treatment outcomes among PWID.
Methods: Five rounds of integrated bio‐behavioural survey data (2009 (N = 3962), 2011 (N = 9069), 2013 (N = 9502), 2015 (N = 9405) and 2017 (N = 10,076)) among PWID in Ukraine (including HIV/HCV testing and questionnaires) were analysed using mixed‐effect logistic regression models (mixed‐effects: city, year). These regression models assessed associations between being an NGO client and various behavioural, OST, HIV testing and HIV treatment outcomes, adjusting for demographic characteristics (age, gender, lifetime imprisonment, registration in a drug abuse clinic, education level). We also assessed associations between being an NGO client and being HIV‐positive or HCV‐positive, likewise adjusting for demographic characteristics (as above).
Results: NGO clients were more likely to have received HIV testing ever (adjusted odds ratio (aOR) 5.53, 95% confidence interval (95% CI): 5.10 to 6.00) or in the last year (aOR 3.44, 95% CI: 3.27 to 3.63), to have used condoms at last sexual intercourse (aOR 1.30, 95% CI: 1.23 to 1.37) and sterile needles at last injection (aOR 1.37, 95% CI: 1.20 to 1.57), to be currently (aOR 4.08, 95% CI: 3.38 to 4.93) or ever (aOR 2.76, 95% CI: 2.53 to 3.01) on OST, and to have in the last year received syringes (aOR 151.72, 95% CI: 136.56 to 168.57) or condoms (aOR 45.19, 95% CI: 42.24 to 48.35). PWID who were HIV‐positive (aOR 1.40, 95% CI: 1.32 to 1.48) or HCV‐positive (aOR 1.57, 95% CI: 1.49 to 1.64) were more likely to have contact with NGOs, with HIV‐positive PWID in contact with NGOs more likely to be registered at AIDS centres (aOR 2.30, 95% CI: 1.82 to 2.90) and to be on antiretroviral therapy (aOR 1.52, 95% CI: 1.32 to 1.76).
Conclusions: Contact with PWID targeted NGOs in Ukraine is associated with consistently better preventive, HIV testing and HIV treatment outcomes, suggesting a beneficial impact of Global Fund programming.
OAC0305
Assessing implementation and impact of an educational intervention for safer injection among people who inject drugs in Europe: A multi‐country mixed‐method study
P. Roux1, C. Donadille1, C. Magen1, E. Schatz2, R. Stranz3, A. Curado4, T. Tsiakou5, L. Verdes6, P. Carrieri1, A. Ben Charif7
1INSERM ‐ Aix Marseille University, SESSTIM, Marseille, France, 2Correlation Network, Amsterdam, Netherlands, 3AIDES, Paris, France, 4GAT, Lisbon, Portugal, 5PRAKSIS, Athens, Greece, 6ARAS, Bucarest, Romania, 7CERSSPL ‐ Université de Laval, Laval, Canada
Background: The implementation and scaling up of harm reduction (HR) interventions are essential to reduce HIV and HCV transmission among people who inject drugs (PWID) in Europe. The Individually Tailored Support and Education for Safer Injection (ITSESI) is an evidence‐based educational intervention for PWID. While ITSESI has been preliminarily evaluated in France (AERLI intervention in Roux et al, 2016), showing to reduce HIV and HCV risk practices, this study aimed to implement and evaluate ITSESI at European level.
Methods: We performed a mixed‐method implementation study. The quantitative component involved a non‐randomized controlled trial, while the qualitative component involved face‐to‐face interviews and focus groups. We conducted this study between 2018 and 2019 within HR programmes in Bulgaria, Greece, Portugal and Romania, by enrolling 307 adult PWID. Our intervention (ITSESI) consisted to observe injection practices of PWID and to provide an educational exchange with trained field workers. Participants were allocated to the usual services (control group) or the intervention group. Primary outcome was the effectiveness of ITSESI defined as the reduction of HIV and hepatitis C virus (HIV‐HCV) risk practices. We used RE‐AIM QuEST framework to assess effectiveness of ITSESI and other dimensions (e.g. reach, adaptation). We used a multivariable mixed logit model to analyse the primary outcome. Qualitative data was analysed thematically to provide future investigations.
Results: Out of 307 eligible PWID, 203 participated (66%) in the complete follow‐up. Among them, 60.6% received ITSESI. HIV‐HCV risk practices dropped from 27.1% to 14.8% in the intervention group, while it remained stable in the control group (20.0%). PWID who received ITSESI were less likely to report HIV‐HCV risk practices (adjusted odds ratio (95% confidence interval): 0.27 (0.11, 0.70)). Our qualitative data showed importance to adapt some components of ITSESI and involve stakeholders such as field workers and PWID as proactive research partner in order to make implementation of ITSESI more accessible and acceptable across Europe.
Conclusions: We demonstrated the effectiveness of ITSESI in reducing HIV‐HCV risk practices in the European context. Our findings provide important understandings of the adaptation and implementation of ITSESI that are relevant for a large‐scale implementation of ITSESI across Europe.
OAC0306
Progress in HIV prevention interventions uptakes among people who inject drugs in Unguja Island, Zanzibar: Analyses of bio‐behavioural surveys in 2007, 2012 and 2019
S. Haji1, F. Khalid1, C. Said2, S. Mohamed1, A.U. Khamis1, A.A. Othman1, E. Matiko3, S. Welty2, A. Khatib1, G. Mgomella3, W. McFarland2
1Zanzibar Integrated HIV, Hepatitis, Tuberculosis and Leprosy Programme (ZIHHTLP), Zanzibar Ministry of Health, Stonetown, Tanzania, United Republic of, 2University of California, Institute for Global Health Sciences, San Francisco, United States, 3US Centers for Disease Control and Prevention, Dar es Salaam, Tanzania, United Republic of
Background: Zanzibar has a concentrated HIV epidemic among key populations, including people who inject drugs (PWID) who are at elevated risk of acquiring HIV from both injection practices and exchange of sex for drugs. We aimed to measure HIV prevalence, HIV acquisition risk factors, and access to, and uptake of HIV prevention and care services over the last 12 years among PWID in Unguja, Tanzania.
Methods: We conducted cross‐sectional bio‐behavioural surveys (BBS) of PWID conducted in 2007, 2012 and 2019 in Unguja. PWID were recruited into BBS using respondent‐driven sampling (RDS). A total of 499, 408 and 419 PWID were surveyed in 2007, 2012 and 2019 respectively. Participants’ information was collected through an interviewer‐administered questionnaire among consenting PWIDs aged 15 + who reported to have injected in the last three months. HIV status was assessed using the national rapid test algorithm with return of results. Point estimates were adjusted for Respondent Driven Sampling. F‐test p‐values (2012 to 2019 comparison) and 95% CI confidence intervals were calculated.
Results: HIV prevalence among PWID in Unguja Island decreased from 16.0% (95% CI: 11.4 to 21.2) in 2007, to 11.3% (95% CI: 7.7 to 15.2) in 2012, to 5.1% (95% CI: 2.6 to 7.5) in 2019. The proportion of PWID who had tested for HIV and received their results in the past one year increased from 13.3% in 2007, to 38.0% in 2012, to 44.1% in 2019 (p < 0.001). Access to clean needles also increased over time, from 52.7% and 52.1% in 2007 and 2012, respectively, to 86.6% in 2019 (p < 0.001). Concurrently, the proportion of PWID who reported using a previously used needle in the past one month decreased from 53.8% in 2007 to 29.1% in 2012 to 18.7% in 2019 (p < 0.001).
Conclusions: We noted reduction of HIV prevalence and increase in self‐reported awareness of HIV status among PWID, which is key to linkage and retention in ART. Our results suggest that preventive interventions targeting PWID have been well taken up. Although significantly reduced, HIV prevalence and related risk behaviours persist at levels warranting enhanced efforts to reach all PWID with primary prevention and harm reduction services, especially, eliminating the use of non‐sterile needles.
OAC0402
Feasibility of implementation, acceptability and preliminary effects of a pilot, peer‐led HIV self‐testing intervention in a hyperendemic fishing community in rural Uganda
J.K. Matovu1,2, L. Bogart3, J. Nakabugo4, J.M. Gitta4, J. Kagaayi5, D. Serwadda4, R.K. Wanyenze4, A. Ko6, A. Kurth7
1Makerere University School of Public Health, Disease Control and Environmental Health, Kampala, Uganda, 2Busitema University Faculty of Health Sciences, Mbale, Uganda, 3RAND Corporation, Santa Monica, United States, 4Makerere University School of Public Health, Kampala, Uganda, 5Rakai Health Sciences Program, Kalisizo, Uganda, 6Yale University, Epidemiology of Microbial Diseases, New Haven, United States, 7Yale University, School of Nursing, New Haven, United States
Background: Novel interventions are urgently needed to reach young people and adult men, who continue to show low HIV testing and linkage to HIV care rates compared to other populations. We assessed the feasibility of implementation, acceptability and preliminary effects of a pilot, peer‐led oral HIV self‐testing (HIVST) intervention in Kasensero; a hyperendemic (HIV prevalence: 37%) fishing community along the shores of Lake Victoria in rural Uganda.
Methods: This prospective cohort study was conducted among young people (15 to 24 years) and adult men (25 + years) between May and August 2019. The intervention entailed distribution of HIVST kits by 34 trained “peer‐leaders” who were local people selected from existing social networks and trained in HIVST distribution processes. Each peer‐leader nominated up to 20 members from their social network who were screened for eligibility; up to 10 eligible members were enrolled into the study. Peer‐leaders received up to 10 kits (one for each member) to distribute to eligible members of their social networks. Eligible social network members were followed up at one‐month post‐baseline to assess uptake of HIVST and other associated outcomes. This intervention was deemed to be feasible if peer‐leaders distributed up to 70% of the kits they received; and acceptable if >80% of the respondents self‐tested for HIV. Data were analysed using STATA (version 14.1).
Results: Of 298 (87.6%) enrolled into the study, 56.4% (n = 168) were aged 15 to 24 years, 67.5% (n = 201) were males, while 21.1% were engaged in fishing or fishing‐related activities. Sixty‐nine percent (n = 206) had ever heard about oral HIVST. Peer‐leaders distributed 296 (99.3%) kits. Ninety‐seven percent (n = 286) of those who received the kits self‐tested for HIV, based on self‐reports and returned used kits. HIV prevalence was 7.4% (n = 21); 57.1% (n = 12) were first‐time HIV‐positive testers. One‐hundred per cent (n = 12) of first‐time HIV‐positive testers sought confirmatory HIV testing (as recommended) and 10 (83.3%) were confirmed as HIV‐positive. Nine of the ten (90%) confirmed first‐time HIV‐positive testers were linked to HIV care.
Conclusions: Our findings show that implementation of a social network‐based, peer‐led HIVST intervention in a hyperendemic fishing community is highly feasible, acceptable and achieves high linkage to HIV care among newly diagnosed HIV‐positive individuals.
OAC0403
HIV self‐tests free distribution in Brazil: An effective strategy for reaching undiagnosed key populations
M. Villares1, A. Bigolin1,2, L. Martins de Aquino1, P.C. Gaspar1,3, J. Boullosa Alonso Neto1, N. Denilse de Araujo1, G. Fernando Mendes Pereira1
1Brazilian Ministry of Health, Department of Diseases of Chronic Condition and Sexually Transmitted Infections, Brasilia, Brazil, 2Federal University of Santa Catarina, Postgraduate Program in Pharmacy, Florianópolis, Brazil, 3University of Brasília, Postgraduate Program in Collective Health, Brasilia, Brazil
Background: In 2018 there were 900.000 PLHIV in Brazil, of which 15% were unaware of their status. Innovative strategies are fundamental to increase access to testing, specially in countries with concentrated epidemics. In December 2018, Ministry of Health of Brazil (MoH) implemented a pilot strategy for free of charge distribution of 400,000 HIV self‐tests (HIVST) in the public health system, aiming to reach undiagnosed people. This strategy, carried out in eight states, consisted in delivering up to six HIVST in three situations: for PrEP users to give to peers or partners; at places of sociability of key population (KP) by health teams and civil society organization (CSO); and secondary distribution for people tested in Health Services (HS). This study aimed to presenting the profile of people reached by the strategy.
Methods: We collected HIVST distribution data through a form hosted in a monitoring system (SIMAV), with questions regarding demographics, sexual behaviour, previous testing and number of tests taken, filled upon test delivery, which were afterwards analysed.
Results: By 31 December 2019, MoH distributed 51,906 HIVST, of which 45,052 HIVST were distributed to 16,364 people who filled the forms. Out of those people, 22% were 18 to 24 years old and 21% were 25 to 29 years old; black people accounted for 43% of completed forms, MSM accounted for 54% and trans people accounted for 4%. 25% were in PrEP.
Among those not in PreP, 20% were first time testers (32% among those aged 18 to 24), 14% had last tested for HIV over two years before and 37% tested less than six months. People in PrEP took an average of 3.3 HIVST to peers and partners, while other people took an average of 2.4 HIVST.
Conclusions: Preliminary data suggest that strategy is reaching the target population for HIVST, including young people and first time testers, raising the potential to reach undiagnosed. People in PrEP are potential secondary distributors. These results encouraged expansion for another six states in 2020. Innovative efforts to reach trans people are of special interest and should be increased.
OAC0404
TRUST: Results of an HIV self‐testing intervention for Black or African‐American transgender women (TGW) and gay, bisexual and other men who have sex with men (MSM) in New York City
V. Frye1, V. Nandi2, M. Paige1, J. McCrossin2, G. Ortiz2, D. Lucy2, D. Usher2, D. Hoover3, M. Gwadz4, P. Sullivan5, L. Wilton6
1CUNY School of Medicine, Community Health and Social Medicine, New York, United States, 2New York Blood Center, Project ACHIEVE, New York, United States, 3Rutgers University, New Brunswick, United States, 4New York University, Silver School of Social Work, New York, United States, 5Emory University, Rollins School of Public Health, Atlanta, United States, 6Binghamton University, Department of Human Development, Binghamton, United States
Background: Increasing HIV testing, the gateway to prevention/care, is critical to eliminating racial disparities and ending the HIV epidemic in the United States. HIV self‐testing (HST), an alternative to clinic‐based testing, is private, convenient and acceptable and may increase consistent/frequent testing.
Methods: We evaluated a behavioural intervention to increase HST to support consistent HIV testing among Black or African‐American transgender women (TGW) and men who have sex with men (MSM) and/or TGW via a randomized controlled trial. We enrolled eligible “index” participants in “friend pairs” between (mid‐2016 to 2017) with every three‐month follow‐up over one year. The single‐session intervention arm provided counsellor‐delivered HIV testing (as friend pairs), training on HST and identification and practice of optimal peer support. The time/attention control arm provided counsellor‐delivered testing individually (results shared in pairs) and generic, didactic self‐screening (including HST) information. Both arms received HST kits and testing reminders every three months. A modified intent‐to‐treat analysis, using GEE models with an independent structure and time as a cluster, of 98 intervention and 99 control “index” participants (only) was conducted.
Results: Retention ranged from 78% to 82% at three and six months and 63% to 88% at 9 and 12 months across arms. In the intervention arm, the proportion of participants reporting HST in the past three months increased from baseline (2%) to three‐month (57%) and six‐month (54%) follow‐up. In the control arm, the proportion of participants reporting HST in the past three months increased from baseline (7%) to three‐month (42%) and six‐month (42%) follow‐up. The difference in the increases was statistically significant by arm at p < .05 and p ≤ 0.05 at three‐ and six‐month follow‐up, respectively, but not at 9‐ and 12‐month follow‐up. Intervention arm participants were approximately twice as likely to HST at three‐month (OR 2.24; 95% CI: 1.12 to 4.47) and six‐month (OR: 1.94; 95% CI: 1.00 to 3.75) follow‐up, compared with control arm participants.
Conclusions: The TRUST intervention increased HST over six months of follow‐up, but impact was attenuated at 9 and 12 months. The intervention integrates HST skills‐building and peer support to reduce barriers to HST, representing a promising approach to increasing consistent testing among subpopulations for whom consistent testing would be most beneficial.
OAC0405
Feasibility of index testing among incarcerated people: Early results from four correctional facilities in Zambia
C.N. Moonga1, H. Smith2, L.K. Mwango3, C. Zulu2, C. Masumba2, L. Kashela2, M. Chilinya2, K. Kaputula2, K. Nkwemu4, A. Mwila4, J. Okuku4, M. Mujansi3, C.W. Claassen3, M. Herce2,5
1Centre for Infectious Disease Research in Zambia, TB Department, Lusaka, Zambia, 2Centre for Infectious Disease Research in Zambia, Implementation Science, Lusaka, Zambia, 3University of Maryland—Baltimore (UMB), Centre for International Health, Education and Biosecurity (CIHEB), Lusaka, Zambia, 4U.S. Centers for Disease Control and Prevention (CDC), Lusaka, Zambia, 5University of North Carolina at Chapel Hill, Chapel Hill, United States
Background: Incarcerated people in Zambia face a disproportionately high HIV burden estimated at 27% to 30%. Until recently, targeted HIV testing services (HTS), such as index testing, have not been implemented in correctional settings. We began providing consensual and confidential HIV partner notification services in four Zambian correctional facilities and offered index testing services to HIV‐positive inmates with active community tracing of their partners. Traced contacts were offered HTS and HIV prevention and treatment services. We assessed the effectiveness of this programme.
Methods: In these four correctional facilities, entry and exit screening for HIV, tuberculosis and sexually transmitted infections is offered as part of routine care. All HIV‐positive inmates are immediately linked to HIV treatment, care and support. In February–September 2019, all inmates with new HIV diagnoses were offered index testing services by trained providers during post‐test counselling. Index patients were asked to provide contact information for their sexual partners per Ministry of Health guidance. Index contacts were traced by phone or home visit. Sexual contacts were counselled and tested for HIV. If HIV‐positive, they were linked to antiretroviral therapy (ART), and HIV‐negative contacts were linked to combination prevention.
Results: Of the 175 (Female‐27, Males‐148) inmates offered index testing, 166 (Female‐27, Male‐139) (94.9%) accepted (Table 1). 293 (Female‐223, Male‐70) sexual contacts were identified (elicitation ratio: 1:1.8). Of these, 109 (Female‐42, Male‐67) (37.2%) were contacted: 59 (Female‐23, Male‐36) (54.1%) already knew their status and were receiving ART, and 50 (Female‐19, Male‐31) (45.9%) tested for HIV. Of those tested, positivity was 30% (15/50). Of those with a new HIV diagnosis, 10 (Female‐6, Male‐4) (66.7%) were linked to ART, but 5 (Female‐3, Male‐2) (33.3%) declined treatment, citing preference for couples’ HTS or retesting at the clinic closest to home.
Abstract OAC0405‐Table 1.
| Steps in index testing cascade | Total (N, %) | Female (n, %) | Male (n,%) |
|---|---|---|---|
| Offered index | 175, 100% | 27, 15.4% | 148, 84.6% |
| Accepted index | 166, 94.9% | 27, 16.3% | 139, 83.7% |
| Contacts elicited | 293, 100% | 223, 76.1% | 70, 23.9% |
| Contacts traced | 109, 37.2% | 42, 38.5% | 67, 61.5% |
| Contacts known positive on ART | 59, 54.1% | 23, 39.0% | 36, 61.0% |
| Contacts tested for HIV | 50, 45.9% | 19, 38.0% | 31, 62.0% |
| New HIV‐positive contacts (testing yield) | 15, 30.0% | 9, 60.0% | 6, 40.0% |
| Contacts Linked to ART | 10, 67.7% | 6, 60.0% | 4, 40.0% |
| Contacts declining treatment | 5, 33.3% | 3, 60.0% | 2, 40.0% |
Conclusions: Index testing in correctional facilities is feasible and results in high testing yield.
OAC0406
What's a lab got to do with it? Alliances with private laboratories enhance HIV‐case finding among at‐risk MSM and transgender women in Central America
A. Cabrera1, S. Lungo1, C. Palma1
1Population Services International, Guatemala City, Guatemala
Background: Under the USAID Combination Prevention Program for HIV in Guatemala, El Salvador, Honduras, Nicaragua and Panama, the Pan American Social Marketing Organization (PASMO) implements offline and online interventions to increase HIV testing services (HTS) uptake among at‐risk MSM and transgender women (TW), and link reactive cases to care. HTS is performed by PASMO counsellors, laboratory technicians, or private laboratories, the latter of which must complete training and sensitization exercises to provide quality key population (KP)‐friendly services. Although the public sector generally provides HTS for free, difficult to access, “hidden” MSM and TW populations often prefer private health services due to fear of stigma, discrimination and confidentiality breaches in the public sector.
Description: PASMO uses a Unique Identifier Code (UIC) to track programme participants from initial engagement through entry in care. Print or online vouchers are used to refer to HTS. Vouchers received by private laboratory partners are collected by PASMO on a bi‐monthly basis. On a monthly basis, PASMO enters the monitoring data into its management information system, allowing it to track the number of individuals reached, percentage of individuals who receive HTS, HIV‐case finding yield (number of reactive cases identified per number of tests) and percentage linked to care.
Lessons learned: From October 2018 to September 2019, PASMO reached a total of 17,897 MSM and TW across the five countries through offline and online interventions of which 13,197 (748%) received HTS, and 720 were reactive (yield of 1 of every 17). PASMO counsellors performed 6877 of the tests with 298 reactive cases identified (1 of every 23), whereas private laboratories performed 6320 tests and detected 422 reactive cases (1 of every 15 tests). Private laboratories identified 59% of all reactive cases identified by the programme during the year.
Conclusions/Next steps: With this programme's focus on most at‐risk and “hidden” MSM and TW groups, the partnerships with private laboratories play a significant role in HIV case finding, producing improved yield and helping expand the access of difficult to access KPs to HTS services throughout the region.
OAC0407
Community mobilization to improve engagement in HIV testing, linkage to care and retention in care in 15 villages in South Africa: The Tsima cluster‐randomized controlled trial
S.A. Lippman1,2, A. Pettifor3,2, D. Rebombo4, M. Kang‐Dufour1, C. Whiteson Kabudula2, R. Twine2, R. Mathebula4, A. Julien2,3, R. West1, T.B. Neilands1, A. Gottert5, R. Wagner2, N. Haberland5, J. Pulerwitz5, I. Sanne6, F.X. Gómez‐Olivé2, D. Peacock4, K. Kahn2
1University of California, Department of Medicine, San Francisco, United States, 2MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), University of the Witwatersrand, School of Public Health, Faculty of Health Sciences, Johannesburg, South Africa, 3University of North Carolina, Epidemiology, Chapel Hill, United States, 4Sonke Gender Justice, Cape Town, South Africa, 5Population Council/Project SOAR, Washington, D.C. and New York, United States, 6University of the Witwatersrand, Clinical HIV Research Unit, Department of Medicine, Faculty of Health Sciences, Johannesburg, South Africa
Background: Increasing HIV testing and early treatment initiation is key to ending HIV. Community Mobilization (CM) ‐ which goes beyond service provision or outreach and engages communities in a process to collectively enact change ‐ has significant potential to increase HIV services uptake. CM interventions have rarely been rigorously evaluated.
Methods: We randomized 15 villages in the MRC/Wits‐Agincourt health and socio‐demographic surveillance site, South Africa, to intervention or control. The intervention engaged residents to address social barriers to HIV testing and treatment – poor awareness of HIV care (especially treatment as prevention); fear/stigma; and gender norms that deter accessing care. Activities were delivered through mobilizers and trained volunteers over three years in public spaces and homes. We assessed differences in HIV testing uptake, linkage to and retention in care among 18‐ to 49‐year‐old residents in intervention versus control villages over time (in three‐month increments) using data from 9 public clinics serving the area. Intention‐to‐treat analyses included generalized estimating equations stratified by sex and accounting for clustering.
Results: Among 38,392 residents, 13,404 had documented clinical visits between August 2015 and July 2018. HIV testing uptake increased quarterly by 13% and 11% in intervention men and women as compared to 9% and 10% among control men and women (p < 0.05); though annual testing among men never exceeded 10%. With more individuals entering care over time, retention fell approximately 2% per quarter among men and women, but less rapidly among intervention compared to control women (p < 0.01). There were no effects on linkage to care.
Conclusions
CM was associated with improvements in testing among men and women and retention among women, demonstrating that raising consciousness and activities addressing social barriers to HIV service engagement can increase HIV service use. However, even with extensive outreach among men, few accessed testing. CM programing should be paired with efforts to improve service delivery and bring services to the community.
OAC0502
Implementing a PrEP population management tool in the electronic health record of a large integrated healthcare system
C. Bruno1, G. Gallitero2, R. Herbers3, Y. Hussain4, M. Ballesca5, M.J. Silverberg6, L. Hurley6, D.P. Nguyen3, J.L. Marcus7, C.B. Hare3, J.E. Volk3
1Kaiser Permanente, Infectious Diseases, Vallejo, United States, 2Kaiser Permanente, Oakland, United States, 3Kaiser Permanente San Francisco, San Francisco, United States, 4The Permanente Medical Group Consulting Services, Emeryville, United States, 5Kaiser Permanente, Internal Medicine, Vacaville, United States, 6Kaiser Permanente Division of Research, Oakland, United States, 7Harvard Medical School and Harvard Pilgrim Health Care Institute, Population Medicine, Boston, United States
Background: As of 31 March 2019, over 10,000 patients had received a prescription for HIV preexposure prophylaxis (PrEP) at Kaiser Permanente Northern California, an integrated healthcare delivery system that includes clinical care, pharmacy services and insurance coverage for 4.2 million members. Given the rapid uptake of PrEP, efficient population management tools are needed to support PrEP adherence and monitor laboratory follow‐up.
Description: A PrEP population management tool was created and integrated into an EPIC electronic health record (EHR) in 2016. This tool uses real‐time pharmacy and clinical data to generate a list of patients who are prescribed tenofovir disoproxil fumarate/emtricitabine or tenofovir alafenamide/emtricitabine for PrEP. The tool captures demographic data, pharmacy information (e.g. PrEP refill dates) and laboratory data (e.g. HIV antibody and creatinine test dates and results), and allows providers to sort by these variables. Related clinical information such as sexually transmitted infection diagnoses and hepatitis B status are also included. Providers can send secure electronic messages to thousands of patients simultaneously with reminders for overdue medication refills, laboratory follow‐up, or new clinical updates regarding PrEP.
Lessons learned: Overall, the implementation of this tool has resulted in significant operational efficiencies, with thousands of PrEP users being safely monitored by only a few providers. PrEP users overdue for laboratory follow‐up and/or prescription refills are easily identified and contacted. However, the resources needed to develop the EHR‐based tool were substantial, including a team of technology experts, clinicians and project managers working in collaboration. An estimated 250 hours were spent in development. Many providers were initially reluctant to use the new technology given the additional training needed, initial investment of time to ensure data accuracy and adjustment in workflow. Modifications to the tool are possible, but require additional time and resources.
Conclusions/Next steps: EHR‐based PrEP population management tools allow for efficient and targeted outreach to patients who are overdue for laboratory monitoring, need adherence support, may benefit from a change in PrEP medication, or have discontinued PrEP and may benefit from restarting. Ongoing technology resources and provider training will be needed as the tool evolves to accommodate emerging PrEP medications, dosing schedules and delivery mechanisms.
OAC0503
PrEP 2‐1‐1 education increases PrEP uptake and preserves effective PrEP coverage in a large nurse‐led community‐based sexual health clinic in San Francisco
J. Broussard1, J. Bena1, P.‐C. Crouch2, B. Taylor1, M. Chavez1, R.M. Grant1,2
1San Francisco AIDS Foundation, San Francisco, United States, 2University of California, San Francisco, United States
Background: PrEP 2‐1‐1 dosing (i.e. “on‐demand” dosing) with TDF/FTC for anal sex is not endorsed by the CDC and has limited utilization in the U.S., despite research and experience showing its effectiveness and appeal among people who otherwise might not take PrEP. To increase knowledge and use of PrEP 2‐1‐1, the sexual health clinic Magnet of San Francisco AIDS Foundation implemented a PrEP 2‐1‐1 programme.
Methods: Current and prospective PrEP clients were enrolled in a prospective cohort study, receiving an intervention about daily and 2‐1‐1 PrEP dosing with an educational handout and evidence that both dosing strategies are safe and effective for MSM, although 2‐1‐1 dosing had not been reviewed by the FDA. Participants selected their dosing and received standard of care, adherence counselling and HIV/STI/creatinine testing. PEP was offered within 72 hours if a potential HIV exposure not covered by PrEP occurred. Weekly online surveys collected sex and PrEP information.
Results: From 1 March 2019 to November 30, 2019, 3106 subjects (72% current PrEP clients; 28% new clients) received the intervention. Median age was 31 years; 98% were cis‐gender MSM. For new PrEP clients, 77% elected daily, and 23% elected 2‐1‐1. For current daily PrEP patients, 83% chose only daily dosing, 17% switched to 2‐1‐1. PEP use was rare in both groups: daily (0.8%), 2‐1‐1 (1.4%; p = 0.22). A higher proportion of 2‐1‐1 users (3.3%) reported PrEP‐less or condomless sex versus daily PrEP users (1.3%; p < .001). 63 people (2%) reported starting PrEP due to PrEP 2‐1‐1 and would not have accessed daily PrEP otherwise. PrEP 2‐1‐1 awareness increased across Magnet from 53% before the study to 69%. There were zero HIV infections in the 2‐1‐1 (262 years follow‐up) or daily PrEP (1231 years follow‐up) groups. Daily PrEP clients took more pills (4.85/week) than 2‐1‐1 clients (1.59/week, SD 2.01; p < 0.00, 001). During weeks with anal sex, daily clients took 5.36 tablets, 2‐1‐1 took 2.92 (p < 0.00, 001).
Conclusions: Providing 2‐1‐1 dosing information increased uptake of PrEP, was a popular dosing option, and reduced medication use by three‐fold while preserving high rates of effective use. This supports recent recommendations for 2‐1‐1 dosing among MSM from WHO and IAS‐USA.
OAC0504
Validation of self‐reported measures of optimal PrEP adherence among MSM in 4 U.S. cities
J. Chapin‐Bardales1, C. Sionean1, R. Haaland1, A. Holder1, V.A. Butts1, A. Martin1, E.K. Sey2, K.A. Brady3, H.F. Raymond4, J. Opoku5, I. Kuo6, G. Paz‐Bailey1, C. Wejnert1, for the NHBS Study Group
1U.S. Centers for Disease Control and Prevention, Atlanta, United States, 2Los Angeles County Department of Public Health, Los Angeles, United States, 3Philadelphia Department of Public Health, Philadelphia, United States, 4Rutgers University, Newark, United States, 5District of Columbia Department of Health, Washington D.C., United States, 6George Washington University, Washington D.C., United States
Background: Adherence to HIV pre‐exposure prophylaxis (PrEP) is key to its effectiveness as a prevention method. Self‐reported PrEP adherence measures could allow for monitoring adherence in non‐clinical‐trial settings where biological testing poses cost, time and logistical challenges. We evaluated validity of self‐reported PrEP adherence measures among men who have sex with men (MSM) in 4 U.S. cities.
Methods: In 2017 National HIV Behavioral Surveillance, eligible MSM aged ≥18 years were recruited via venue‐based sampling and completed a survey, HIV testing and dried blood spot (DBS) collection. DBS from HIV‐negative participants who reported PrEP use in the past month were tested for tenofovir diphosphate (TFV‐DP) by liquid chromatography mass spectrometry. Biological optimal adherence was defined as TFV‐DP ≥ 1250 fmol/punch (consistent with 7 doses/week) and considered gold standard. Three self‐reported optimal adherence measures were examined: (1) missed 0 doses in past 30 days, (2) missed 0 doses in past 7 days and (3) Wilson's 3‐item adherence scale. Focused on capturing optimal adherence prevalence and limiting false positives, we calculated positive predictive values (PPVs) and false‐positive rates (FPRs) overall and by population characteristics.
Results: PPVs were similar for the three measures of optimal adherence (approximately 73%) and FPRs were lowest for past‐30‐day missed 0 doses (41%) and Wilson scale (35%) measures. PPVs and FPRs of all optimal adherence measures varied by population characteristics; within each demographic subgroup, PPVs were similar across measures while FPRs were lowest and at similar magnitudes for the past‐30‐day missed 0 doses and Wilson scale measures.
Abstract OAC0504‐Table 1.
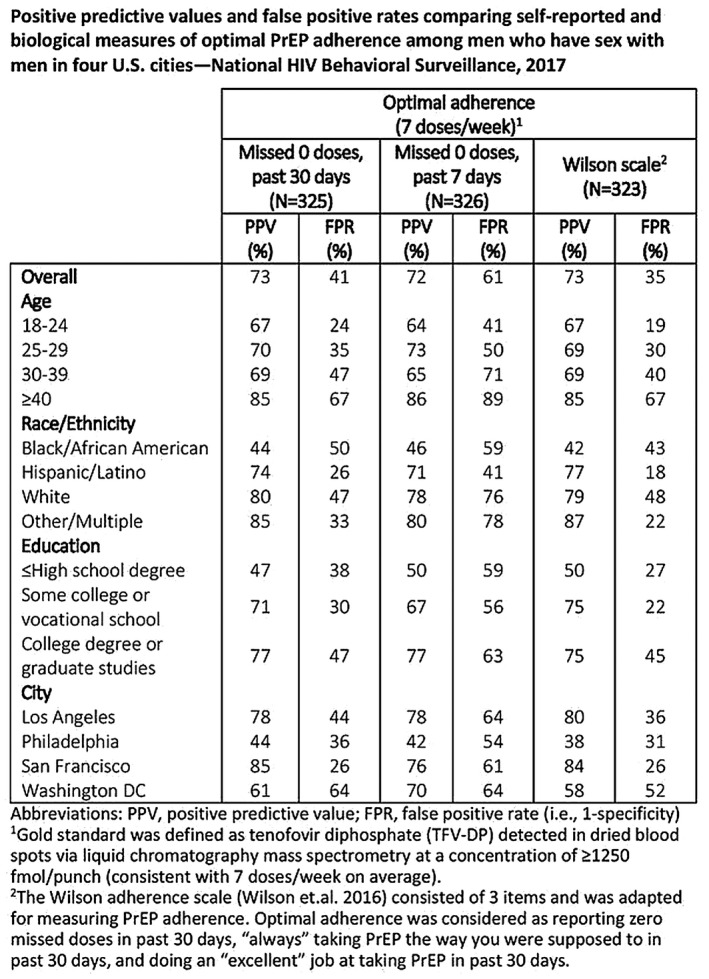
Conclusions: Self‐reported optimal PrEP adherence measures had moderate validity; no measure demonstrated high PPV or low FPR overall and all measures had PPVs and FPRs that varied by population characteristics. Of self‐reported measures, the past‐30‐day missed 0 doses item may be minimally sufficient to capture optimal adherence. Nevertheless, biological testing remains important to measuring PrEP adherence.
OAC0505
Factors associated with early continuation (EC) of pre‐exposure prophylaxis (PrEP) among young MSM (YMSM) in Brazil, Peru and Mexico: The ImPrEP Study
V.G. Veloso1, E.H. Vega‐Ramírez2, B. Hoagland1, K.A. Konda3, S. Bautista‐Arredondo4, J.V. Guanira5, T. Torres1, M.C.P. Oliveira6, H. Vernandere4, A. Farias7, M.V.G. Lacerda8, M. Benedetti1, S. Díaz9, P.M. Luz1, R.I. Moreira1, J. Moreira10, I.d.C. Leite1, B. Grinsztejn1, C. Cáceres3, ImPrEP Study Team
1National Institute of Infectious Diseases Evandro Chagas, (INI‐Fiocruz), Rio de Janeiro, Brazil, 2National Institute of Psychiatry Ramon de la Fuente Muñiz, Mexico City, Mexico, 3Centro de Investigación Interdisciplinaria en Sexualidad, Sida y Sociedad, Universidad Peruana Cayetano Heredia, Lima, Peru, 4National Institute of Public Health, Mexico City, Mexico, 5Investigaciones Medicas en Salud, Lima, Peru, 6Ministry of Health, Department of Chronic Conditions and STIs, Brasília, Brazil, 7Centro Estadual Especializado em Diagnóstico, Assistência e Pesquisa (CEDAP), Secretaria Estadual de Saúde, Salvador, Brazil, 8Fundação de Medicina Tropical Doutor Heitor Vieira Dourado, Manaus, Brazil, 9United Nations Population Fund, Mexico City, Mexico, 10Grupo Arco‐Íris de Cidadania LGBTI+, Rio de Janeiro, Brazil
Background: PrEP implementation in Latin America is very limited; awareness is lower among YMSM. ImPrEP is an ongoing demonstration study assessing safety and feasibility of same day PrEP for MSM and TGW in Brazil, Peru and Mexico. We report results on PrEP EC and associated factors among YMSM.
Methods: HIV uninfected, ≥18 years old, reporting 1 + risk criteria were enrolled and initiated PrEP on the same day; creatinine and STI testing were performed. Main outcome for this analysis was PrEP EC (attendance to first 2 follow‐up visits within 150 days of PrEP initiation) among YMSM (18 to 24).
Results: Among 7273 enrolled (February 2018–November 2019) 1843 (25.3%) were YMSM; 957 (51.9%), 607 (32.9%) and 279 (15.1%) from Brazil, Peru and Mexico; 1390 (75.4%) non‐white, 607 (33.0%) with < secondary level of education; Condomless receptive anal sex and having ≥4 sexual partners in the previous three months were reported by 1203 (65.3%) and 1053 (57.1%). Baseline active syphilis, rectal chlamydia and rectal gonorrhoea prevalence were 8.7% (95% CI: 7.3%‐10.2%), 13.1% (95% CI: 11.3%‐15.0%) and 10.3% (95% CI: 8.7%‐12.1%). Only 14 (0.9%) had eGFR < 60 mL/minutes. HIV incidence was 1.8%/100 PY (95% CI:1.0%‐2.9%) during 858.1 PY of PrEP use. Overall EC was 67.2%; Brazil: 77.3%; (95% CI: 74.3%‐80.0%), Mexico: 72.3%; (95% CI: 63.8%‐79.8%), Peru: 44.9%;(95% CI: 44.9%‐49.8%). Lower chance of PrEP EC was observed among nonwhite (aOR = 0.68; 95% CI:0.50 to 0.92), less educated (aOR = 0.66; 95% CI: 0.51 to 0.86), Peruvians (aOR = 0.24; 95% CI: 0.19 to 0.31), unaware of partner serostatus (aOR = 067; 95% CI:0.47 to 0.95), those coming to the site for reasons other than PrEP (aOR = 0.55; 95% CI:0.39 to 0.78), those reporting no condomless receptive anal sex (aOR = 0.74; 95% CI:0.57 to 0.95), with no prior PEP use (aOR = 0.61; 95% CI: 0.42 to 0.87).
Abstract OAC0505‐Table 1. Factors associated with PrEP EC among YMSM enroled in ImPrEP
| N (% EC) | OR unadj | p value | OR adj | p value | |
|---|---|---|---|---|---|
| Country | |||||
| Brazil | 897 (77.3) | 1 | 1 | ||
| Mexico | 130 (72.3) | 0.77 (0.51 to 1.16) | 0.21 | 0.90 (0.58 to 1.40) | 0.63 |
| Peru | 184 (44.9 | 0.24 (0.19 to 0.31) | <0.0001 | 0.39 (0.28 to 0.53) | <0.0001 |
| Education level | |||||
| Less than secondary/secondary | 431 (60.1) | 0.61 (0.48 to 0.79) | 0.0001 | 0.66 (0.51 to 0.86) | 0.002 |
| More than secondary | 957 (70.5) | 1 | 1 | ||
| Race | |||||
| White | 385 (78.2) | 1 | 1 | ||
| Non white | 1004 (63.0) | 0.68 (0.51 to 0.92) | 0.01 | 0.68 (0.50 to 0.92) | 0.01 |
| Reason to come to the site | |||||
| Looking for PrEP | 1152 (72.3) | 1 | 0.001 | 1 | 0.001 |
| Others | 237 (42.6) | 0.56 (0.40 to 0.78) | 0.55 (0.39 to 0.78) | ||
| Condomless receptive anal sex | |||||
| Yes | 907 (69.7) | 1 | 0.04 | 1 | 0.02 |
| No | 482 (62.7) | 0.77 (0.66 to 0.99) | 0.74 (0.57 to 0.95) | ||
| Sex work | |||||
| Yes | 186 (57.5) | 0.71 (0.51 to 1.00) | 0.05 | 0.84 (0.59 to 1.20) | 0.33 |
| No | 1203 (68.7) | 1 | 1 | ||
| Sex with HIV infected partners | |||||
| Yes | 260 (76.5) | 1 | 1 | ||
| No | 466 (66.1) | 0.78 (0.54 to 1.12) | 0.18 | 0.78 (0.54 to 1.14) | 0.20 |
| Unware | 663 (64.4) | 0.67 (0.47 to 0.94) | 0.02 | 0.67 (0.47 to 0.95) | 0.02 |
| PEP use | |||||
| Yes | 273 (82.0) | 1 | 1 | ||
| No | 1116 (63.6) | 0.61 (0.43 to 0.87) | 0.006 | 0.61 (0.42 to 0.87) | 0.006 |
| Cocaine | |||||
| Yes | 92 (63.0) | 0.64 (0.40 to 1.02) | 0.64 (0.39 to 1.04) | ||
| No | 1297 (67.5) | 1 | 0.06 | 1 | 0.07 |
Conclusions: ImPrEP successfully enrolled vulnerable YMSM. Efforts to increase awareness and strategies to support those at higher social vulnerability are urgently needed to increase PrEP benefits among YMSM in Latin America.
OAC0506
Factors associated with uptake of event‐driven and daily regimen of pre‐exposure prophylaxis among gay, bisexual and other men who have sex with men (GBMSM) in Taiwan: 2019 Hornet PrEP survey
S.W.‐W. Ku1,2, C.‐W. Li3, P. Huang4, C. Strong5, A. Garner6, S. Howell6, T.‐H. Wu2, J.C. Liao5, N.‐Y. Ko7, A. Bourne8
1Taipei City Hospital Renai Branch, Division of Infectious Diseases, Department of Medicine, Taipei, Taiwan, Province of China, 2HIV Education And Research Taiwan (H.E.A.R.T), Taipei, Taiwan, Province of China, 3National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Department of Internal Medicine, Tainan, Taiwan, Province of China, 4University of California, Department of Communication, La Jolla, United States, 5National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Department of Public Health, Tainan, Taiwan, Province of China, 6Hornet Networks Limited, Los Angeles, United States, 7National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Department of Nursing, Tainan, Taiwan, Province of China, 8La Trobe University, Australian Research Centre in Sex, Health& Society, Melbourne, Australia
Background: PrEP has been implemented in Taiwan since 2016, and a 2017 survey recruiting via gay app “Hornet” found that 1.3% and 1.7% of GBMSM respondents respectively reported using daily and event‐driven (ED) PrEP. In a repeat of the survey in 2019 we sought to establish factors associated with both daily and ED uptake.
Methods: We conducted a survey by convenience sampling of the users of a social networking application for GBMSM in Taiwan, with a design similar to the previous 2017 one. The survey was conducted between November 22nd and December 22th, 2019. The survey included 34 questions regarding basic demographics, HIV serostatus, risk behaviours, PrEP awareness, willingness and mode of use. Responses from the same IP address were excluded.
Results: There were a total of 3026 responses, of which 2554 were eligible for analysis. Among those who reported HIV‐negative or unknown serostatus, 227 respondents reported current PrEP use. Only 28.6% reported daily PrEP, use, while the remainder did so on an event‐driven basis. There were no statistical differences in the manner of use according to basic demographics, pre‐survey PrEP awareness, previous post‐exposure prophylaxis use, STI diagnosis, chemsex, or condomless anal intercourse. The major reasons given for daily use over ED regimen were: (1) “I can't plan having sex in advance” (67.2%); (2) “I feel more confident in protection” (56.3%); (3) “It's easier for me to remember taking pills” (53.1%). Contrastingly, respondents preferred ED over daily PrEP because: (1) “I have less frequent sex” (63.3%); (2) “It's more affordable” (56.3%); (3) “I can plan having sex in advance” (51.9%). Multivariable logistic regression revealed a greater number of sexual partners (more than 9 vs. 0 to 9 partners in the past 12 months) had significant correlation with current PrEP users adopting daily rather ED regimen (AOR 2.37, 95% CI 1.18 to 4.75, p = 0.015).
Conclusions: Our survey found ED PrEP has been adopted preferably over daily PrEP among GBMSM community in Taiwan. To scale up PrEP use further, we should consider prioritizing promotion of the ED regimen and address ED PrEP‐specific issues, including relevant knowledge pertaining to effective use and adherence.
OAC0602
Promoting anal health and pleasure with a community‐driven social marketing campaign
E. Land1, E. Jost1, A. Hattori1, R. Roybal1
1San Francisco AIDS Foundation, San Francisco, United States
Background: Anal fissures, rectal STIs, improper rectal douching and anal health concerns can increase HIV risk among people having receptive anal sex, including MSM. Yet stigma and embarrassment can keep MSM from seeking healthcare for these concerns, free anal health resources for MSM are not widely available online, and platforms like Facebook/Instagram restrict the kind of sexual health information that can be promoted. This social marketing campaign leveraged the lived experience of MSM community members and expertise of MSM providers to develop stigma‐free anal health resources to promote better health.
Description: From 2017 ‐ 2018, San Francisco AIDS Foundation (SFAF) developed: 1) a “Butt Health” webpage; 2) two community surveys on douching and pain during anal sex; 3) articles by MSM and clinicians giving first‐person perspectives and information on anal warts, fisting, anal douching, booty bumping and more; and, 4) a stylized cartoon “Douchie” mascot for articles, social media and printed materials. 575 people took the surveys, and personal experiences from surveys were shared in online articles. A modest advertising budget of $150 resulted in a 6.84% clickthrough rate (CTR) on Facebook ($0.10/click), performing better than any other SFAF paid campaign and far exceeding the average healthcare industry standard CTR of 0.83%. In the first two months, 76,481 individuals visited sfaf.org/butthealth, and today, campaign content generates 35% of all traffic to sfaf.org largely through organic search.
Lessons learned: Anal health topics are of high interest to MSM and other populations at risk for HIV. Elevating real‐world experiences of anal health conditions, pleasure and comfort alongside information from a trusted community health organization successfully engaged online audiences. Eye‐catching, playfully designed materials accounted for the wide reach of the campaign. Later iterations of this sex‐positive campaign were flagged as “pornographic” content on Facebook, Twitter and Google, limiting our ability to run paid promotions.
Conclusions/Next steps: Health campaigns promoting pleasure perform well for online audiences who may be at risk for or living with HIV. While online spaces are restrictive of sex‐positive content, optimizing audience targeting and graphics for organic sharing on social channels increases the reach of sexual health campaigns.
OAC0603
Deleterious effect of Truvada lawsuit advertisements on attitudes and decisions towards PrEP among sex and gender minority youth at risk for HIV
P.A. Serrano1, E. Daubert1, A. Muñoz1, S.G. Hosek1,2, A.L. French1,2
1Ruth M. Rothstein CORE Center, Chicago, United States, 2John H. Stroger, Jr. Hospital of Cook County, Chicago, United States
Background: In 2019, misleading lawsuit advertisements against Gilead Sciences regarding Truvada were launched and anecdotal evidence suggested the advertisements motivated some users to discontinue PrEP. This study aimed to ascertain the effects of the advertisements on attitudes and decisions about PrEP among participants in the Keeping it LITE study, an ongoing virtual cohort of sexual and gender minority youth vulnerable to HIV.
Methods: A 10‐item survey, including close and open‐ended questions regarding the advertisements, was administered online to participants enrolled in the cohort who were HIV uninfected, 13‐ to 34‐year‐olds who have sex with partners assigned male at birth. Participants met at least one of the following criteria in the last six months: oral sex; condomless anal sex; bacterial STI; or sex with an HIV+ partner. Quantitative and qualitative data were analysed using descriptive and inferential analysis in SAS, and thematic analysis respectively.
Results: From November to December 2019, 1485 (53.7%) of those eligible participated (mean age 26.8 (sd = 4.87); 54.9% White, 19.1% Latinx, 9.6% Black and 16.9% Other; 82% cisgender men, 10.6% transmasculine and 7.3% transfeminine). Prior PrEP use was reported by 43%, and use within the past six months was 32.7%. Almost half (722) were aware of the lawsuit and most (86.3%) had viewed an ad on social media. Of those aware who answered subsequent questions (n = 704), 18.7% reported quitting or deciding not to initiate PrEP use, and 32.1% reported the advertisements changed their opinions about PrEP. Participants with higher education were significantly less likely to quit or to decide against initiating PrEP use (OR = 0.29, 99% CI 0.14 to 0.61). In open ended responses, participants expressed safety concerns (75.25%), distrust towards the pharmaceutical industry (16.4%) and interest in alternative prevention options (8.4%).
Conclusions: The Truvada lawsuit advertisements reached a large, diverse group of youth at high risk of HIV throughout the USA. These advertisements produced hesitancy to initiate Truvada‐based PrEP and increased fears of potential side effects of Truvada. These results illustrate the deleterious public health effects of such direct advertising and distrust of the pharmaceutical industry and support the efforts by public health advocates to mitigate the negative effects of these advertisements.
OAC0604
Promoting uptake of HIV services using social media interventions among Men who have Sex with Men (MSM) in Ghana
S.E. Owusu1, S.K. Wosornu2, K.M. Diaba3
1Maritime Life Precious Foundation, Programs, Takoradi, Ghana, 2Maritime Life Precious Foundation, Executive Director, Takoradi, Ghana, 3WAPCAS, Programs, Accra, Ghana
Background: Social media is becoming a safe environment for communication among MSM in Ghana. MSM are increasingly soliciting potential sexual partners through social media platforms rather than geographic hotspots. Many MSM are “hidden” and engage in risky sexual behaviours, but are not reached by HIV programmes targeted at physical outreach locations. A differential approach to community mobilization on social media platforms was introduced to increase uptake of HIV testing among hidden MSM.
Description: A social media mobilizer was trained to engage MSM through social networking platforms such as Facebook and Grindr. IEC materials were developed and posted on selected social media platforms to raise awareness regarding HIV services among the hidden population. MSM who accessed these platforms were engaged through one–on‐one interaction and online counselling by the trained mobilizer. MSM recruited were given different timed appointments to access services at the Drop‐In‐Centre.
Lessons learned: Data from January to June 2019 show that social media reached out to more high risk MSM than through in‐person outreach at hotspots. Among 166 new MSM that were recruited through social media, 113 (68%) had not been tested for HIV within the last six months. Physical outreach reached 431 new MSM; 133 (31%) had not been tested within the last six months. 59% of MSM recruited through social media engaged in inconsistent use of condoms for casual anal sex, compared to 38% identified at hotspots.
HIV positivity rate was higher among those tested through social media outreach compared to hotspot outreach. 125 MSM tested through social media; 32 were diagnosed HIV positive (25.6% HIV+ yield). 396 MSM were tested through physical outreach at hotspots; 28 were diagnosed HIV positive (9% HIV+ yield).
Conclusions/Next steps: Confidential and accessible health services through social media encourages hidden MSM to seek HIV services themselves. There is high need to invest in newer approaches of HIV programming that take into account changing times and community dynamics.
Linking MSM to services through social media has shown to deliver higher HIV+ yield among hard to reach MSM. Hence, implementing partners should use social media as an effective tool for sharing behaviour change messages to reach hidden MSM.
OAC0605
Lynx: A pilot randomized controlled trial of a mobile health HIV testing and PrEP uptake intervention for young men who have sex with men
H. Scott1, K. Coleman1, J. Vinson1, A. Garcia2, R. Muench3, M.E. Enriquez‐Bruce2, K. Bojan3, P.A. Serrano3, T. Oyedele3, E. Enrique‐Bruce2, P. Emmanuel2, J. Jones4, K. Muessig5, C. Horvitz5, S. Mullin4, J. Roberts5, S. Buchbinder1, P. Sullivan4, L. Hightow‐Weidman5, A. Liu1
1San Francisco Department of Public Health, San Francisco, United States, 2University of South Florida, Tampa, United States, 3Ruth M. Rothstein CORE Center, Chicago, United States, 4Emory University, Atlanta, United States, 5University of North Carolina, Chapel Hill, United States
Background: HIV in the US disproportionately affects young men who have sex with men (YMSM), especially Black and Latinx YMSM. Delays in HIV testing, undiagnosed sexually transmitted infections (STI) and low uptake of pre‐exposure prophylaxis (PrEP) all contribute to this disparity. We developed and pilot tested an mHealth intervention to increase HIV testing and PrEP uptake among YMSM.
Methods: HIV‐uninfected YMSM aged 15 to 24 years who had not tested for HIV in the past three months and were not currently on PrEP were enrolled. Participants were randomized 2:1 to the LYNX mobile app intervention with the Sex Pro HIV risk assessment, a sexual diary, geo‐location of HIV/STI testing and PrEP clinics, PrEP information and videos, and access to home HIV/STI testing; or control (CDC HIV testing and PrEP information) and followed remotely for six months. The primary outcomes were feasibility and acceptability of Lynx, HIV testing and PrEP uptake assessed via CASI.
Results: From October 2018 to April 2019, 61 participants were enrolled and overall retention was 80% at six months. The median age was 20.5 years, 38% were White, 33% were Latinx and 24% were Black. At baseline, participants reported a median of 2 male sexual partners in the past three months. During the six‐month intervention, participants spent a median of 41 minutes using the app. App acceptability was high with a median System Usability Scale Score of 73.8; and 87% of intervention participants reporting they would recommend the app to a friend for HIV/STI testing and PrEP. Almost all (95%) intervention arm participants ordered an HIV test kit, and 50% reported testing at home. At six months, a higher proportion of intervention participants reported HIV testing although this was not statistically significant (70% versus 50%, p = 0.17). Overall, PrEP uptake was low with only 16% of YMSM initiating PrEP by six months with no difference between intervention and control (13% vs. 22%, p = 0.45).
Conclusions: The Lynx intervention showed high acceptability in YMSM, and shows promise for increasing HIV testing among this vulnerable population. However, PrEP uptake was low and more support is likely needed to remove barriers to PrEP access for YMSM in the US.
OAC0606
That's how we roll! Using human‐centred design to allow the community voice to design an educational campaign, social media and direct‐to‐consumer communication for PrEP rollout in Zambia
M. Njelesani1, S. Maher2, A. Chipukuma1, M. Nyumbu1, C. Madevu‐Matson2, A. Fullem2, M. Chikuba‐McLeod1
1JSI Research & Training Institute, Inc., Lusaka, Zambia, 2John Snow, Inc., Boston, United States
Background: In May 2018, USAID DISCOVER‐Health (DISCOVER), implemented by JSI Research & Training Institute (JSI) was among the first implementers to support MOH PrEP scale‐up in Zambia. At start‐up, PrEP rollout took place in an information vacuum, with little community access to credible PrEP information for individual decision‐making and/or collective action to create a supportive environment for PrEP. Using a Human‐Centred Design (HCD) process, DISCOVER extended its support to MOH to develop innovative strategies, interventions and products to ensure that PrEP rollout grounded in the realities of end‐users. DISCOVER leveraged its SBC technical capacities towards the development of media campaigns that support PrEP uptake and continuation. As an outcome of the HCD process, DISCOVER supported the MOH to develop a national HIV prevention brand and campaign: Zambia Ending AIDS, with a sub‐campaign for PrEP education and demand‐generation.
Description: DISCOVER developed digital innovations to support client management and client access to information, including SBC products such as the Zambia Ending AIDS Facebook page, direct‐to‐consumer communication platform through a free USSD short‐code service to enable access to basic information about PrEP and help end‐users find PrEP facility locations. On the provider‐side, DISCOVER designed and developed an end‐user‐informed HCW app, which provides guidance on PrEP administration, including counselling skills, and is electronically linked to PrEP management system.
Lessons learned: By developing and supporting direct‐to‐consumer communication (including Facebook and YouTube, USSD short‐code, and adverts on TV/radio), DISCOVER provided correct PrEP information to support rollout. These platforms allow people to privately and anonymously access reliable information. Between April and September 2019, DISCOVER saw 1.97M Facebook visits, 9.9M TV and radio ads seen/heard; 59,261 accesses to USSD short‐code; 27,889 inquiries about the nearest PrEP facility; sent 9018 PrEP auto‐appointment reminders; and sent 5038 PrEP adherence‐support messages, leading to 5175 new clients on PrEP at DISCOVER sites alone (242).
Conclusions/Next steps: Lack of information and misinformation can discourage PrEP uptake and derail effective HIV prevention. Use of HCD to inform communication and demand‐creation allows insightful participatory engagement with end‐users. Innovative direct‐to‐consumer communication platforms provide correct information; facilitate two‐way communication; increase PrEP utilization—all contributing to sustaining gains towards HIV epidemic control in Zambia.
OAC0702
Outcomes of HIV‐exposed but uninfected children in South Africa over five years: Comparison to un‐exposed peers
M. Rotheram‐Borus1, J. Stewart2, E. Almirol1, M. Tomlinson2
1Univ. of CA, Los Angeles, United States, 2Stellenbosch University, Stellenbosch, South Africa
Background: Researchers have documented extensively the benefits of mothers adhering to the tasks to Prevent Mother to Child Transmission (PMTCT), typically based on clinic samples. Yet, after 12 months, there is far less information on HIV exposed and uninfected children born to Mothers Living with HIV (MLH). This study examines a broad range of child outcomes over five years for HEU compared to their HIV‐unexposed and uninfected (HUU) peers living in the same communities.
Methods: Almost all (98%) of pregnant women in 24 neighbourhoods in Cape Town, South Africa were recruited in pregnancy and reassessed at multiple time points over five years with high retention (from 96% to 85.2% at 2 weeks post birth, 0.5, 1.5, 3 and 5 years). The growth, hospitalizations and cognitive and behavioural development of HEU children (n = 363) of MLH were compared to HUU children (n = 787) of mothers living without HIV over time.
Results: Approximately 9% of mothers and children died over five years, similar across maternal serostatus. Over time, HEU children had significantly lower weight‐for‐age z‐scores (WAZ) than HUU at the post‐birth and 18 month assessments, but not at any later time point. For height‐for‐age z‐scores (HAZ), we observed differences between HEU and HUU at 6 and 18‐months, but not at any later follow‐up. At five years, growth measures, such as HAZ scores, WAZ scores, and whether a child was stunted or malnourished, were similar among HEU children and HUU children. There were no other differences in cognitive abilities (based on the Bayley Scales at 1.5 years or the Kaufmann scales at 3 and 5 years), behavioural measures (the Achenbach Child Behavior Checklist and the Strengths and Difficulties Questionnaire), or hospitalizations among HEU and HUU children over five years.
Conclusions: Unexpectedly, the outcomes of HEU children were similar to their HUU peers. As broad diffusion of antiretroviral therapies occurs and mothers are surviving and living less symptomatic lives, their children appear to similar to peers not exposed to HIV.
OAC0703
Impact of PMTCT programmes on mother and child outcomes in Colombia
E. Martinez Buitrago1, M.P. Posada2, J. Pardo3, H.F. Mueses4, J.C. Alzate‐Ángel5, M. Mantilla6, L. Arévalo7, D. Alzamora8, J. Stand9, S. Valderrama‐Beltrán10, C. Gonzáles11, O. Sussmann12, M. García13, O.L. Ramos14, J. Franco15, J.W. Tobón16, GRUPO VIHCOL VIH de Colombia
1Universidad del Valle, Internal Medicine Department, Cali, Colombia, 2SIES Salud Medellín, Medellín, Colombia, 3CEPAÍN Cali, Cali, Colombia, 4Corporación de Lucha Contra el SIDA, Cali, Colombia, 5Corporación de Investigaciones Biológicas, Medellín, Colombia, 6CEPAÍN Colombia, Bogotá, Colombia, 7CEPAÍN Bogotá, Bogotá, Colombia, 8Vivir Bien IPS, Cartagena, Colombia, 9CEPAÍN Cúcuta, Cúcuta, Colombia, 10Universidad Pontificia Javeriana, Bogotá, Colombia, 11SIES Salud Cali, Cali, Colombia, 12Asistencia Cientifica de Alta Complejidad, Bogotá, Colombia, 13SIES Salud Armenia, Armenica, Colombia, 14CEPAÍN Neiva, Neiva, Colombia, 15SIES Salud Pereira, Pereira, Colombia, 16CEPAÍN Villavicencio, Villavicencio, Colombia
Background: Real‐world data on pregnancy complications and newborn outcomes in HIV pregnant women are scarce in Latin America. To characterize the effectiveness of HIV PMTCT programmes in Colombia, we search for pregnancies in HIV‐1 infected women that had delivery or pregnancy terminated before 31 December 2018, from 15 centres of the Colombian HIV Group (VIHCOL), an HIV nationwide network.
Methods: Retrospective univariate and bivariate descriptive analysis, using central trend and dispersion measures, frequencies and percentages, non‐parametric Kruskal–Wallis group comparison tests, and chi‐2 with Fisher's correction. All data on in Stata version 12.
Results: A total of 273 HIV‐positive pregnant women were included with a median age at the pregnancy diagnosis of 26.4 years (15.11‐ 43.3). An almost half (47.6%) had their HIV infection diagnosed following mandatory screening during pregnancy with a median gestational age of 17.5 weeks (p25‐p75 = 12 to 25.5), and started ART at 18 weeks (p25‐p75 = 14 to 27); 102 (37.4%) were known HIV positive before the pregnancy with a mean time of HIV diagnosis of 3.6 years (p25 to 75 = 0.99 to 6), of which 84 (30.8%) became pregnant on active ART; 9 women (3.3%) had diagnosis on delivery. Median CD4 count and viral load (p25‐p75) at the time of pregnancy diagnosis and at the end of pregnancy were 428 cells/mm3 (289 to 582), 3287 copies/mL (52 to 16,799), 500 cells/mm3 (349 to 683) and 0 copies/mL (0 to 40) respectively. 29 (18.5%) of 157 women with available data were late presenters (≥28 weeks of pregnancy). Viral load at the end of pregnancy was undetectable in 178 women (77.4%), and above 1000 copies/mL in 21 (9.1%). Lopinavir/ritonavir plus 2 NRTI was the cART most prescribed (n = 135, 52.1%). Cesarean section was the delivery method in 245 women (89.74%). Regarding birth outcomes, only two preterm deliveries (4.7%), one small‐for‐gestational‐age infant (4.33%), one birth defect (microcephaly) were reported, and there were no MTC transmissions.
Conclusions: This is the first report in Colombia of a nationwide HIV pregnant women and newborn cohort combined outcomes of PMTCT programmes. Our data confirmed that combining early diagnosis of HIV and referral to care in HIV centres results in few pregnancy‐related complications, rare poor birth outcomes, and no vertical transmissions.
OAC0704
Declining trend of HIV mother‐to‐child transmission in Brazil: A novel estimation method based on programmatic data
A.R.P. Pascom1, A.A.C.M. Ferreira1, L.N. da Silveira1, F.F. Fonseca1, M.A. de Freitas1, R.E.G.G. Pinho1, G.F.M. Pereira1, J.A. Pinto2
1Ministry of Health of Brazil, Department of Diseases of Chronic Condition and STI, Brasilia, Brazil, 2Universidade Federal de Minas Gerais, Faculdade de Medicina, Belo Horizonte, Brazil
Background: Prevention of mother‐to‐child transmission (PMTCT) of HIV has been a priority in Brazil. However, the challenge remains of having specific indicators to better guide public health policies. We aimed to present a novel method to estimate mother‐to‐child transmission rate (MTCTR) in Brazil, and to analyse its trends during 2010 to 2017.
Methods: We used programmatic data from antiretroviral therapy (ART) and HIV viral load (HIV‐VL) national systems to identify HIV‐exposed children (H‐EC) under one‐year old (yo). HIV infection criteria was: 1) having at least one ART dispensation; or 2) presented the first HIV‐VL ≥ 10,000copies/mL; or 3) presented at least two VL ≥ 5000copies/mL; or 4) had a single HIV‐VL ≥5000 copies/mL. In addition, all children aged ≤10 yo who had at least one ART dispensation were classified as vertically infected. We also estimated the number of H‐EC by subtracting the number of pregnancy losses from the number of pregnant women living with HIV. The MTCTR was calculated as the ratio between infected‐children and the exposed ones. We fitted generalized additive models to assess trends in the number of infected‐children and in the MTCTR.
Results: We estimated 107,734 H‐EC and identified 4765 HIV‐infected children; an overall MTCTR of 4.4%. The number of infected‐children decreased from 684 to 361, in 2010 and 2017 respectively (p < 0.001). Likewise, MTCTR declined 52% during the analysed period (p < 0.001), reaching 2.9% in 2017. MTCTR decline went from 15% to 38% in 2010 to 2013 and 2014 to 2017, respectively, coinciding with the ART scale‐up in Brazil.
Abstract OAC0704‐Table 1. Number of HIV‐infected, ‐exposed children and mother to child transmission rates by year of birth. Brazil, 2010 to 2018
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|---|---|---|---|
| HIV‐infected (n) | 684 | 651 | 607 | 602 | 556 | 517 | 507 | 361 |
| HIV‐exposed (n) | 11,354 | 11,503 | 11,654 | 11,807 | 11,962 | 12,119 | 12,278 | 12,454 |
| MTCT rate (%) | 6.0 | 5.7 | 5.2 | 5.1 | 4.6 | 4.3 | 4.1 | 2.9 |
Conclusions: We presented a more sensitive method to estimate MTCTR which is being used to monitor and guide public PMTCT policy. Since 2013, when Brazil implemented treatment for all, including pregnant women, there was a higher decrease in MTCTR. Therefore, we believe that the declining trends showed in this study will be persistent, aligned with public health polices, indicating that MTCT elimination is an attainable target in Brazil.
OAC0705
Prevention of mother‐to‐child‐transmission (PMTCT) of HIV in Khayelitsha, South Africa: A contemporary review of the service 20 years later
F. Phelanyane12, A. Boulle12, E. Kalk1
1University of Cape Town, Centre for Infectious Disease and Epidemiology Research, Cape Town, South Africa, 2Western Cape Government Health, Cape Town, South Africa
Background: The first Prevention of Mother‐To‐Child‐Transmission of HIV (PMTCT) pilot programme the Western Cape (WC), South Africa, was launched in Khayelitsha in 1999. All public health facilities in the WC share a unique patient identifier allowing linkage across all electronic health systems through the Provincial Health Data Centre (PHDC), an African Health Information Exchange within WC Department of Health. We aimed to describe recent PMTCT uptake and quantify MTCT risk factors based on routine data consolidated through the PHDC.
Methods: Retrospective observational cohort analysis of all live‐born linked mother‐infant pairs in which the HIV‐positive mother attended antenatal care in Khayelitsha in 2017. Descriptive statistics assessed coverage along the PMTCT cascade. Logistic regression analysis quantified risk factors associated with transmission, and a Cox‐proportional hazard model assessed time to and associations with maternal virologic failure.
Results: Antenatal prevalence in the cohort was 31.3%, MTCT (among live‐born linked infants with evidence of HIV outcome in the PHDC) was 1.8% at 12 months post‐partum. 88.3% of women knew they were HIV positive at their first antenatal visit, of whom 77.9% were already on ART; 74.9% of the entire cohort received a viral load test around birth (up to three months post‐partum), 70.1% were virologically suppressed. Early infant diagnosis coverage was sub‐optimal with birth HIV‐PCR (within 7 days of birth) coverage of 78.1%, and an even lower proportion (64.5%) of infants who tested negative had a repeat test around 10‐weeks. Older maternal age was protective against MTCT (a 10‐year increase in age reduced MTCT by 15%) and virologic failure (age < 25 almost doubled the risk of virologic failure). Post‐partum ART initiation (compared to antenatal initiation) increased MTCT risk by seven‐fold.
Conclusions: Although most women present to care already knowing their HIV status, ART initiation and uptake of viral load testing could still be improved. MTCT proportion, reliant on PCR alone, continues to be underestimated due to sub‐optimal HIV‐PCR coverage; HIV data from multiple sources, consolidated in an HIE suggested higher MTCT than programme‐reported HIV‐PCR testing alone. Further work is needed to determine whether women who initiated ART post‐partum seroconverted post‐partum or failed to link to ART during pregnancy.
OAC0706
Elimination of mother to child transmission of HIV: Practice and progress in Zhejiang province, China
X. Zhang1, L. Qiu2, D. Chen2
1Women's Hospital Zhejiang University, Women's Health, Hangzhou, China, 2Women's Hospital Zhejiang University, Hangzhou, China
Background: Background: Elimination of mother‐to‐child transmission (EMTCT) of HIV is globally advocated. In this study, we describe the progress and practice of EMTCT in Zhejiang province, China.
Methods: In Zhejiang, HIV screening is routinely provide to pregnant women during antenatal healthcare (ANC) . Early antiretroviral therapy (ART) is offered to women with HIV, including safe delivery. Early infant diagnose (EID) of HIV is tested at 42 days and three months. HIV antibody screening is offered to child with negative result of EID at 12 months and 18 months. Maternal and child healthcare activities, community support strategies, home visits are retention strategies. In the study, we analysed the progress and practice in EMTCT during 2015 to 2019.
Results: Totally, over 3 million pregnant women received HIV screening. HIV screening coverage has remained high level, with 99.02% in 2015, 98.82% in 2016, 99.15% in 2017, 99.01% in 2018 and 99.09% in 2019. HIV‐positive incidence in pregnant women was stable at 0.02%. ART coverages were 85.22%, 84.09%, 91.35%, 98.20% and 96.23% from 2015 to 2019, respectively, with significant rising trend (X2trend=22.112, p < 0.001). ART coverage gap between resident and migrant bridged obviously. During the period, ART coverage increased from 79.73% to 95.45% in migrant (χ2 trend =20.507, p < 0.001) and maintained over 98% in resident. EID proportion grew from 87.36% to 95.51% over years. HIV from mother to child transmission rates (MTCT) decreased from the highest level in 2016 with 4.48% to 1.18% in 2019. We has strongly integrated EMTCT with ANC, maternal and child healthcare and sexual disease prevention. Broad social mobilization is playing a crucial role in EMTCT.
Abstract OAC0706‐Figure 1. ART coverage for HIV pregnancy women
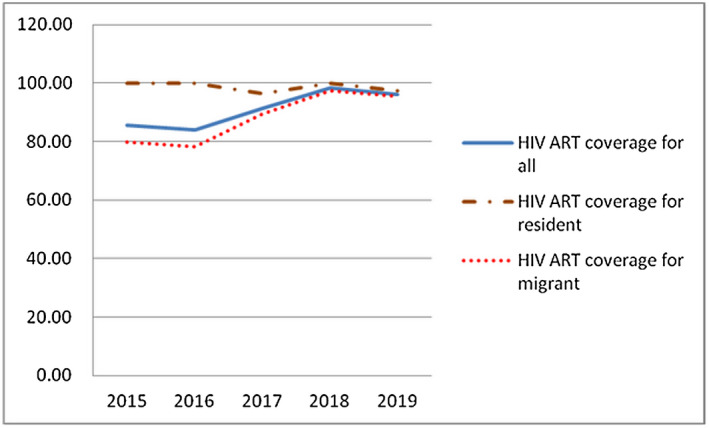
Conclusions: With the increases in ART and EID coverage, the improvement of social support, we observed a decrease rate of HIV MTCT and friend social atmosphere for HIV women and their infants.
OAC0802
Successful national PrEP scale‐up in Australia: Evaluation of uptake, adherence, discontinuation and HIV seroconversion from April 2018 to September 2019 using national dispensing data
N. Medland1, R. Guy1, A. Grulich1, B. Bavinton1, P. Keen1, J. Ellard2, F. Jin1, H. Paynter2, H. McManus1
1Kirby Institute, University of New South Wales, Sydney, Australia, 2Australian Federation of AIDS Organisations (AFAO), Sydney, Australia
Background: PrEP has been government subsidized in Australia since April 2018 and actively promoted to community and doctors. We used national dispensing data for PrEP and antiretroviral therapy (ART) to evaluate the success of the national programme.
Methods: Using linked de‐identified dispensing records of all government‐subsidized PrEP, for each patient we calculated days covered or without PrEP (assuming daily dosing) and proportion of days covered (PDC) for the most recent 90 days. We examined rates and predictors of recent nonadherence/intermittent use (90‐day PDC < 60%) and discontinuation (> 120 days without PrEP). We defined incident HIV infection as initiating ART > 60 days after initiating PrEP.
Results: Uptake was rapid and sustained with 6491 people initiating during the first quarter; declining to 3403 in the most recent quarter. Over 18 months 29,619 patients were dispensed 6,745,967 PrEP tablets; 98.7% were male and the median age was 35 years (IQR 28 to 45). Just above a quarter (25.9%) discontinued PrEP. The median 90‐day PDC was 93.3% (IQR 67% to 100%). Independent predictors of 90‐day PDC < 60% and/or discontinuation included female sex, younger age‐group, patient and doctor non‐inner‐urban location, lower doctor PrEP‐caseload and more disadvantaged patients (see Table 1).
The HIV incidence rate was 0.95/1000 PY (24 cases/25197 PY) and was higher during PrEP gaps than days covered (1.66/1000 PYs (14/8,443 PYs) versus 0.60/1000 PYs (10/16,754 PYs), incident rate ratio 2.78, p = 0.007).
Abstract OAC0802‐Table 1. Rates and predictors of 90‐day PDC < 60% or discontinuation
| n | 90 day PDC < 60% | aOR | p | Discontinued | aOR | p | |
|---|---|---|---|---|---|---|---|
| Total | 29,618 | 20.1% | 25.9% | ||||
| Sex | |||||||
| Male Female | 29,241 (98.7%) 377 (1.27%) | 20.9% 39.3% | ref 2.22 | – <0.001 | 25.5% 68.7% | ref 4.60 | – <0.001 |
| Age group | |||||||
| 18 to 29 30 to 39 40+ | 9085 (30.7%) 9899 (33.4%) 10,634 (35.9%) | 25.0% 20.1% 18.8% | 1.43 1.15 ref | <0.001 0.001 – | 33.5% 24.8% 21.0% | 1.77 1.32 ref | <0.001 <0.001 – |
| Patient location | |||||||
| Inner Urban Other | 15,292 (51.6%) 14,327 (48.4%) | 19.2% 23.1% | ref 1.09 | – .029 | 21.5% 31.1% | ref 1.13 | – <0.001 |
| Doctor location | |||||||
| Inner Urban Other | 21,239 (71.7%) 8380 (28.3) | 19.3% 25.9% | ref 1.30 | – <0.001 | 21.5% 38.9% | ref 1.69 | – <0.001 |
| Doctor PrEP caseload | |||||||
| ≤100 patients >100 patients | 13,741 (46.4%) 15,878 (53.6%) | 24.9% 18.0% | 1.52 ref | <0.001 – | 19.5% 34.9% | 1.73 ref | <0.001 – |
| Subsidy | |||||||
| Routine Additional | 26,506 (89.5%) 3112 (10.5%) | 20.6% 24.4% | ref 1.11 | – 0.07 | 24.9% 35.2% | ref 1.32 | – <0.001 |
Conclusions: Australia's national government‐subsidized PrEP programme has scaled up rapidly. The high proportion of patients using less‐than‐daily dosing may include appropriate intermittent PrEP. Uptake, adherence and discontinuation in women may reflect appropriate use under guidelines or the selective focus on promoting PrEP to gay and bisexual men. This study identified characteristics of patients and doctors to be targeted to improve retention/adherence and/or additional forms of HIV prevention.
OAC0803
Uptake of pre‐exposure prophylaxis among adolescent girls and young women in PEPFAR‐supported countries, 2017 to 2019
P. Patel1, E. Scholar2,3, K. Sato3,4, R. Eakle2, U. Kanagasbai1, C. Cooney3, J. Saul3,1, J. Albertini3
1Centers for Disease Control and Prevention, Division of Global HIV and TB, Atlanta, United States, 2US Agency for International Development, Crystal City, United States, 3US Department of State, Washington DC, United States, 4Peace Corps, Office of Global Health and HIV, Washington DC, United States
Background: The U.S. President's Emergency Plan for AIDS Relief's (PEPFAR) first implemented pre‐exposure prophylaxis (PrEP) for HIV prevention through the Determined, Resilient, Empowered, AIDS‐Free, Mentored and Safe (DREAMS) Initiative in 2016. Early research noted barriers for PrEP use among adolescent girls and young women (AGYW) were lack of policies, low demand, low risk perception, hesitancy by providers and stigma.
Description: PEPFAR supports PrEP implementation per the WHO guidelines. Programmes screened persons who tested HIV negative for eligibility and offered PrEP as part of combination prevention with follow‐up, including repeat HIV testing and counselling, at three‐month intervals. Platforms providing comprehensive services for AGYW were leveraged. We examined two PEPFAR indicators, using the FY19Q4 MER‐structured dataset, and narratives to understand the extent of and barriers to PrEP uptake from fiscal year 2017 to 2019.
Lessons learned: From 2017 to 2019, 265,770 total clients initiated PrEP and the number of countries offering PrEP doubled (Figure 1). Of 168,258 initiations among women, 51% were among AGYW with a significant increase per year: 8788 in 2017; 21,225 in 2018; 54,959 in 2019 (Figure 1). Among AGYW, 20‐ to 24‐year‐old women represented a significantly higher proportion of PrEP initiators than adolescents (15 to 19 years)(67% versus 33%, p < 0.001). Barriers to use were addressed through outreach efforts, including mobile sites, use of technology to educate and support AGYW, media campaigns and engaging peers in programme implementation. We saw a 2.5‐fold increase in PrEP use among AGYW from 2018 to 2019 (Figure 1); by 2019, all but one DREAMS country was implementing PrEP. Currently, 162,506 persons remain on PrEP; 29% are AGYW.
Abstract OAC0803‐Figure 1.
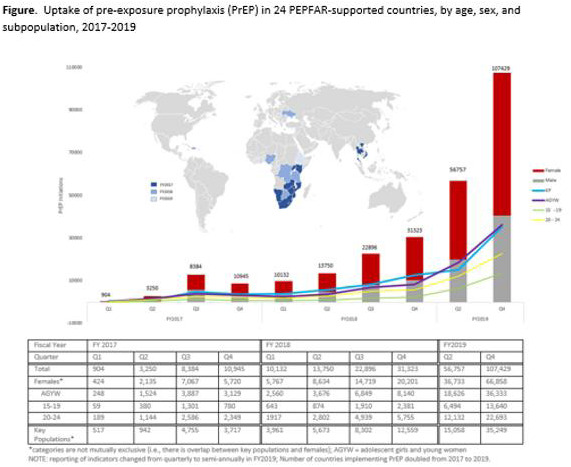
Conclusions/Next steps: Since 2016, PrEP use among AGYW has grown significantly. Adherence and low risk perception remain challenging and related tools are needed to improve PrEP use among AGYW. Still, AGYW represented a significant proportion of women who initiated and continued PrEP, contributing to epidemic control.
OAC0804
PrEP update in women in the US from 2012 to 2017
J. Guest1, J. Jones1, F. Mouhana1, A. Siegler1, R. Mera Giler2, P. Sullivan1
1Emory University, Epidemiology, Atlanta, United States, 2Gilead, San Francisco, United States
Background: Pre‐Exposure Prophylaxis (PrEP) is recommended for heterosexual women with an HIV‐positive partner, recent bacterial STI, high number of sex partners, inconsistent condom use, commercial sex work. PrEP can reduce HIV risk by > 92%. Reduction in incidence requires significant coverage of those at risk. These commercial data show the first five years of PrEP in uninfected women who initiate emtricitabine/tenofovir disoproxil fumarate (Truvada®) for PrEP.
Methods: Data are from linked pharmacy and claims data representing > 90% of US prescriptions. A validated algorithm was applied to exclude Truvada use for treatment of HIV or HBV infection or post‐exposure prophylaxis. Data from 2012 to 2017 are presented. 2017 HIV diagnoses were used as an epidemiological proxy for PrEP need. The PrEP‐to‐need ratio (PnR) (number of PrEP users divided by new HIV diagnoses) was used to describe distribution of prescriptions relative to need.
Results: Rates of PrEP use in women has steadily increased since 2012 (68.57/100,000 women) to a rate of 783.98/100,000 women in 2017 (p < 0.001 for trend). Rates are highest in 25‐ to 34‐year‐olds with 2770 women using PrEP in 2012, 27,556 in 2017. Rates are consistently highest in the Northeastern (NE) states. For 25‐ to 35‐year‐olds in 2017, the NE rate was 328.9/100,000 compared to rates of 158.6 (Midwest), 154.4 (West) and 139.6 (South); p < 0.0001. In comparison, HIV incidence in the NE is 15‐18% of total new infections across these years while 50‐51% of all new infections occurred in the South. The PnR was highest in the West (32.6), lowest in the South (8.4).
Conclusions: There has been > 1100% increase in PrEP utilization in the US in women. PrEP use is highest for ages 25 to 34 years, lowest in 55+. While PrEP uptake has been lowest in the Midwest and West, this is where HIV incidence is the lowest for women. PrEP use is significantly higher for women in the NE although incidence of HIV infections for women in the NE Is 1/3 that seen in the South where rates of PrEP are significantly lower. PrEP has the potential to substantially reduce the number of new HIV infections though we need continued advocacy for PrEP access and funding.
OAC0805
Lower than expected HIV incidence among men and women at elevated HIV risk in a population‐based PrEP study in rural Kenya and Uganda: Interim results from the SEARCH study
C.A. Koss1, D.V. Havlir1, J. Ayieko2, D. Kwarisiima3, J. Kabami3, M. Atukunda3, Y. Mwinike3, G. Chamie1, F. Mwangwa3, A. Owaraganise3, J. Peng1, W. Olilo2, K. Snyman1, B. Awuonda2, T.D. Clark1, D. Black1, J. Nugent4, L.B. Brown1, C. Marquez1, H. Okochi1, K. Zhang1, C.S. Camlin1, V. Jain1, M. Gandhi1, C.R. Cohen1, E.A. Bukusi2, E.D. Charlebois1, M.L. Petersen1, M.R. Kamya5, L.B. Balzer4, SEARCH Collaboration
1University of California, San Francisco, United States, 2Kenya Medical Research Institute, Nairobi, Kenya, 3Infectious Diseases Research Collaboration, Kampala, Uganda, 4University of Massachusetts, Amherst, United States, 5Makerere University College of Health Sciences, Kampala, Uganda
Background: Limited HIV incidence data exist among PrEP users in generalized epidemic settings, particularly outside of known high‐risk groups and with variable adherence. We sought to evaluate (1) HIV incidence and (2) clinical outcomes among seroconverters in a population‐based PrEP study in rural Kenya and Uganda.
Methods: During community‐wide and key population HIV testing of 76,132 individuals ≥15‐years in 16 communities in the ongoing SEARCH study (NCT01864603), PrEP was offered to persons at elevated HIV‐risk (based on serodifferent‐partnership, machine learning‐based risk‐score, or self‐identified HIV‐risk). Follow‐up occurred at facilities or community‐based sites at weeks 4, 12 and every 12 weeks. Among seroconverters, we offered same‐day ART initiation and analysed VL, tenofovir hair‐levels (LC‐MS/MS) and drug resistance. Using Poisson regression with cluster‐robust standard errors, we compared HIV incidence among PrEP initiators with repeat testing to incidence among propensity score‐matched historical controls (2015 to 2017; before PrEP availability) in the same communities, adjusted for risk‐group (serodifferent‐partners, women 15 to 24 years, widow(er)s, fishing/bar/transport workers, alcohol‐users).
Results: From June 2016 to April 2019, of 15,623 individuals at elevated HIV‐risk, 5447 (35%) initiated PrEP (51% male; median age 30‐years (IQR 24 to 39); 19% serodifferent‐partnership); 78% of PrEP initiators had subsequent HIV testing. At week 60, 54% (2778/5142 eligible) attended a follow‐up visit and 33% reported current HIV‐risk, of whom 75% self‐reported PrEP adherence (≥1 dose/last 3 days). Over 7143 person‐years of follow‐up, HIV incidence was 0.35% (95% CI:0.21% to 0.49%) among PrEP initiators versus 1.42% among matched controls, representing a 79% reduction in incidence (aIRR 0.21, 95% CI:0.08 to 0.55; p = 0.002). Of 25 seroconverters (68% women, 56% ≤30 years; median VL = 5871 copies/mL), 96% started ART (most same‐day); 18/18 (100%) of those with repeat VL after ART start achieved VL < 1000 copies/mL. Seven (28%) seroconverters reported taking PrEP ≤ 30 days before seroconversion; 6 had tenofovir hair‐levels indicating 4 to 7 doses/week taken. Of 10 participants with HIV genotyping, one with intermittent PrEP adherence confirmed by hair‐levels had transmitted NRTI/NNRTI mutations (D70N/K70R/K219Q/K103N/P225H), plus FTC resistance possibly related to PrEP use (M184V).
Conclusions: Population‐level PrEP offer (2016 to 2019) in 16 communities in rural Uganda and Kenya was associated with 79% lower HIV incidence among PrEP initiators with follow‐up HIV testing than among recent (2015 to 2017) matched controls in the absence of PrEP.
OAC0806
Programmatic outcomes of pre‐exposure prophylaxis (PrEP) in a resource constraint high HIV incidence setting in Eswatini
A. Aung1, B. Kerschberger1, C. Mamba1, Q. Mpala1, N. Ntshalintshali1, R. Nesbitt1, E. Mabhena1, M. Daka1, M. Nzima1, M.‐L. Tombo1, S. Matse2, A. Telnov3, B. Rusch3, A. Gonzalez3, I. Ciglenecki3
1Médecins Sans Frontières, Operational Center of Geneva (OCG), Nhlangano, Eswatini, 2Eswatini National AIDS Programme, Ministry of Health, Mbabane, Eswatini, 3Médecins Sans Frontières, Geneva, Switzerland
Background: Pre‐exposure prophylaxis (PrEP) is recommended for people at substantial risk of HIV infection, yet programmatic evidence from resource constraint high HIV incidence settings remains scarce. Eswatini national AIDS programme and Médecins Sans Frontières conducted a pilot implementation study in Shiselweni region of Eswatini to assess the programmatic feasibility of PrEP provision in public sector.
Methods: Between September 2017 and January 2019, HIV‐negative adults (≥16 years) were prospectively offered PrEP (tenofovir+lamivudine) in 12 community, primary and secondary health facilities of predominant rural setting. The target populations were young (16 to 25 years), pregnant, and lactating women, key populations (MSMs, sex‐workers), HIV‐negative partners of serodiscordant couples and patients with sexually transmitted disease. We used frequency statistics to characterize the PrEP cascade and multivariate regression analysis to describe predictors of PrEP initiation and continuation.
Results: Of 1824 clients assessed for PrEP eligibility, the majority were reached through primary care sites (80.8%), sexual reproductive health consultations (47.9%) and were women (79.6%). Almost half were aged 16 to 24 years (44.8%), most had finished secondary education (69.9%), and 16.2% lived in a serodiscordant relationship. A total of 497 (27%) clients initiated PrEP with 430 (86.5%) on the day of risk assessment and the remaining 67 within a median of 9 (IQR 3 to 32) days. Predictors of PrEP initiation were client's self‐interest in PrEP (adjusted odds ratio (aOR) 13.46; 95% CI 7.62 to 23.78), having an HIV‐infected partner (aOR 3.55; 95% CI 2.17 to 5.81), and lactating women (aOR 1.66; 95% CI 1.05 to 2.61). Cumulative hazard of PrEP continuation was 48.3%, 40.4%, 29.2% and 21.9% at 3, 6, 12 and 18 months. The risk of discontinuation was less in individuals with self‐interest in PrEP (adjusted hazard ratio (aHR) 0.61; 95% CI 0.41 to 0.89) and clients with a seropositive partner (aHR 0.43; 95% CI 0.28 to 0.65), while it was increased in lactating women (aHR 1.78; 95% CI 1.21 to 2.62) and same‐day PrEP initiation (aHR 1.52, 95% CI 1.04 to 2.22).
Conclusions: The provision of PrEP appeared feasible in this rural public sector. Self‐reflection of risky behaviour and living in serodiscordant relationship indicated strong engagement, yet initiation and retention rates were relatively low.
OAC0807
PrEP continuum of care and new HIV infections: Long‐term follow‐up in a large clinical cohort
J. Volk1, J.C. Hojilla2, L. Hurley2, M.J. Silverberg2, J. Skarbinski3, D.D. Satre2, C.B. Hare1, S. Alexeeff2, J.L. Marcus4
1Kaiser Permanente San Francisco, Infectious Diseases, San Francisco, United States, 2Kaiser Permanente Division of Research, Oakland, United States, 3Kaiser Permanente Oakland, Infectious Diseases, Oakland, United States, 4Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, United States
Background: Few studies have examined long‐term PrEP outcomes. We characterized the PrEP continuum of care and new HIV infections over more than five years of clinical follow‐up.
Methods: We used electronic health record data to identify Kaiser Permanente Northern California members who were linked to PrEP care during 16 July 2012 – 31 March 2019, defined as a PrEP referral or clinical encounter. PrEP prescription was defined as a prescription written by a provider, initiation as a pharmacy fill ≤6 months after linkage, and persistence as < 120 days since last day of PrEP in possession based on pharmacy fills. Persistence analyses were among those with ≥6 months of health plan enrolment, with censoring at death or disenrolment. We used unadjusted log‐binomial regression to identify factors associated with linkage, prescription, initiation and persistence.
Results: Among 12,963 patients linked to PrEP care, 95% were male, and 50% were White, with 21% Latinx, 15% Asian and 7% African American. Of those, 10,310 (80%) received a prescription and 8571 (66%) initiated. We observed 12,810 person‐years of PrEP use (mean 1.9 years/person). PrEP persistence was 73%, 64%, 60%, 57% and 56% at one, two, three, four and five years respectively. Of the 2525 who were not persistent on PrEP, 932 (37%) restarted. Compared to White patients, African Americans were less likely to receive a PrEP prescription (risk ratio (RR) 0.87; 95% CI 0.83 to 0.91) or initiate PrEP (RR 0.81; 0.76 to 0.86), and more likely to discontinue (RR 1.18; 1.04 to 1.34). There were 136 new HIV infections, including 42/12,963 (0.32%) at the time of PrEP linkage, 37/2653 (1.4%) among those who were linked to care but never received a prescription, 13/1739 (0.75%) among those who received a prescription but never initiated, 38/2525 (1.5%) among those who discontinued, and 6/4238 (0.14%) among those who were persistent on PrEP. The six diagnosed with HIV who were persistent on PrEP all self‐reported suboptimal adherence.
Conclusions: We observed high levels of PrEP uptake and persistence over five years and no new HIV infections with consistent use. Efforts are needed to reduce racial inequities and support persistence during periods of HIV risk.
OAD0102
“Living with HIV does not mean you should lose hope”: The importance of assessing resilience within the PLHIV Stigma Index 2.0
B.A. Friedland1, A. Gottert2, L. Nyblade3, S. Kentutsi4, D. Diouf5, U. Tamoufe6, J. Hows7, L. Sprague8, C. Mallouris9, S. Geibel2, U. Amenyeiwe10, F. Anam11, S.D. Baral12, J. Pulerwitz2
1Population Council, Center for Biomedical Research, New York, United States, 2Population Council, HIV and AIDS Program, Washington, DC, United States, 3RTI International, Washington, DC, United States, 4National Forum of PLHIV Networks in Uganda (NAFOPHANU), Kampala, Uganda, 5Enda Sante, Dakar, Senegal, 6Metabiota, Yaounde, Cameroon, 7D.R.A.G: Development Research Advocacy Governance, Amsterdam, Netherlands, 8UNAIDS, Geneva, Switzerland, 9UNAIDS, Johannesburg, South Africa, 10US Agency for International Development, Office of HIV/AIDS, Prevention, Care and Treatment (PCT) Division, Washington, DC, United States, 11Doctors Without Borders (MSF) Southern Africa, HIV/TB Advocacy Coordinator, Africa Region, Johannesburg, South Africa, 12Johns Hopkins University, Baltimore, United States
Background: The People Living with HIV (PLHIV) Stigma Index ‐‐ implemented by and among PLHIV ‐‐ is the most widely used survey documenting stigma and discrimination experienced by PLHIV globally. After nearly a decade of implementation experience, and reflecting revised treatment guidelines, the 2008 survey was updated through a consultative process (2016 to 2017). A key recommendation was to assess resilience – positive adaptation within the context of significant adversity – alongside stigma. A 10‐item PLHIV Resilience Scale (PLHIV‐RS) was therefore developed and validated. PLHIVs opinions of the new questions are presented.
Methods: The PLHIV‐RS assesses whether HIV status has had a positive/neutral/negative effect on meeting needs, such as ability to cope with stress, find love, contribute to community, or practice a religion. Along with testing the quantitative survey in Cameroon, Senegal and Uganda (n = 1207), 60 cognitive interviews (20 per country) and 8 focus groups (Uganda only) were conducted by PLHIV interviewers to assess face validity and perceived importance of survey questions, including the PLHIV‐RS. Respondents, including key populations such as men who have sex with men and sex workers, were purposively sampled to represent a broad range of opinions. Audio recorded interviews/focus groups were translated into English and analysed thematically.
Results: Respondents consistently said the resilience questions were important and relevant, and that the specific items were comprehensive. Several key themes emerged: being asked and answering the resilience questions was therapeutic, allowing respondents to reflect on the positive ways in which they are coping with, and even benefiting from their HIV‐positive status (“..(the questions) show that we can play an important role in society”); the questions imply that “PLHIV have the same desires as other people;” and the questions are important for capturing how well PLHIV are accepting their status, and that data generated can help providers know where additional support is needed.
Conclusions: This qualitative evaluation of the PLHIV‐RS underscored the importance to PLHIV of asking about resilience alongside adversities. Implementing the PLHIV‐RS as part of the Stigma Index 2.0 should be prioritized as a meaningful, appreciated experience for PLHIV, along with helping to inform and assess interventions to improve the lives of PLHIV.
OAD0103
Fragile lives. Gay shame, HIV stigma, substance use and structural factors greatly affect Latino gay men living with HIV in San Francisco, California: Implications for mental health interventions globally
A. Maiorana1, E. Santiago Rodriguez1, S. Hughes1, J. Sauceda1
1University of California, Center for AIDS Prevention Studies, San Francisco, United States
Background: In the USA, while the focus remains on HIV care engagement outcomes, little progress has been made in addressing the multiple intersections of stigma, mental health, substance use and structural factors affecting Latino gay men living with HIV. We explored these intersections to show where interventions should focus to improve quality of life and sustain HIV outcomes.
Methods: We conducted 16 semi‐structured interviews in English or Spanish with USA and foreign‐born Latino gay men living with HIV and depression (PHQ‐9 score > 10) at San Francisco General Hospital. Guided by an intersectional stigma framework and thematic analysis, we examined gay shame, HIV stigma, mental health, substance use and social inequities among participants.
Results: Participants (age 28 to 56) were lower income, and two‐thirds were monolingual Spanish speakers. Most participants reported viral suppression but some acknowledged poor adherence and potential disengagement from HIV care because of methamphetamine, cocaine, and alcohol use and ongoing challenges with depression and daily functioning. For most, their mental health states intersected with HIV stigma, feelings of shame, guilt and regret related to their sexual orientation and trauma from prior bullying, physical, emotional and/or sexual abuse. Their daily life was punctuated by different affective states: sadness, anxiety, fear and fatigue, which led to social isolation. Affective states and social isolation intertwined with the structural factors they faced: unstable/unsafe housing and limited income in an expensive city. Of those undocumented immigrants, finding employment and housing was difficult and intertwined with the fear of potential deportation. Some used the Spanish terms “angustia” (anguish) and “desesperación” (desperation/despair) to refer to their mental states. Although half had received prior depression treatment with mixed satisfaction, some used the Spanish term “desahogarse” to describe the chance to undrown themselves of their feelings during the study interviews, reflecting their need for other services.
Conclusions: Sustaining, not just achieving, optimal HIV care outcomes, requires that programmes and interventions take an intersectional approach to try to address the complex issues, including HIV stigma and internalized homophobia, that affect the wellbeing of Latino gay men. However, we also must address the structural barriers that negatively affect their circumstances and quality of life.
OAD0104
Family support as a source of resilience to counter HIV‐related stigma among adults on antiretroviral therapy in urban Zimbabwe
T.B. Masvawure1, J.E. Mantell2,3, M. Mapingure4, J. Zech5, C. Gwanzura6, T. Apollo6, G. Musuka4, R. Boccanera7, M. Msukwa8, G. George9, M. Strauss9, M. Rabkin5,10
1College of the Holy Cross, Health Studies Program, Center for Interdisciplinary Studies, Worcester, United States, 2Columbia University Division of Gender, Sexuality and Health, New York, United States, 3New York State Psychiatric Institute, New York, United States, 4ICAP Zimbabwe, Harare, Zimbabwe, 5ICAP at Columbia University, New York, United States, 6Ministry of Health and Child Care, Harare, Zimbabwe, 7Health Resources and Services Administration (HRSA), Rockville, United States, 8ICAP South Africa, Pretoria, South Africa, 9University of Kwa‐Zulu Natal, Health Economics and HIV and AIDS Research Division (HEARD), Durban, South Africa, 10Columbia University Mailman School of Public Health, Depts of Medicine and Epidemiology, New York, United States
Background: HIV‐related stigma continues to be a major threat to achieving HIV epidemic control as it can deter individuals from HIV testing, linkage to care, adherence to antiretroviral therapy (ART) and retention in treatment programmes. We present findings, from a PEPFAR‐funded study, on the role of family support in helping adults on ART manage HIV‐related stigma in Harare, Zimbabwe.
Methods: We conducted 8 focus group discussions (FGDs) with 26 women and 28 men aged 18 to 49 years on ART recruited from 7 high‐volume public‐sector health facilities in Harare and 35 interviews with healthcare workers (HCWs) at the same sites. Data were analysed in Dedoose using inductive and deductive approaches.
Results: Both female and male FGD participants reported pervasive HIV‐related stigma in their families and communities. Most thus preferred to keep their HIV status secret. Nevertheless, many had disclosed to a family member, usually a parent, spouse or sibling. These family members became key sources of psycho‐social support and often shielded participants from stigma. Family members provided protection from violence and rejection by disclosing on participants’ behalf (“I told him (my brother). My brother…talked to my husband and my husband accepted my status.”) and by keeping participants’ statuses hidden from other family members (“I told my mother…the rest do not know my status, so I'm not shy (ashamed) when I am with them.”). Family members also encouraged participants to enrol and remain in treatment despite others’ stigmatizing comments and offered practical support by collecting ARVs when participants could not get time off work without arousing suspicion or risking disclosure to employers. Most HCWs highlighted non‐disclosure to family members as a key barrier to retention in HIV care (“Maybe at home your family does not know that you are taking ARVs…so you can't explain to them that you have to go to the clinic.”).
Conclusions: Family members can act as key allies in managing HIV‐related stigma, fostering resilience among people living with HIV and supporting treatment adherence. However, it is equally important to ensure that supportive family members have access to psychosocial services to prevent burnout.
OAD0105
Symbolic violence in healthcare as a barrier to HIV prevention and care for young trans women in Brazil
E.C. Wilson1, E.M. Jalil2, N. Martinez Fernandez2, A.L. Ferreira2, C.V. Castro2, C.M. Jalil2, L. Kamel2, I. Moura2, C. Souza2, D. Bezerra2, L. Monteiro2, V.G. Veloso2, B. Grinsztejn2
1San Francisco Department of Public Health/University of California, Trans Research Unit for Equity/Epidemiology and Biostatistics, San Francisco, United States, 2Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil
Background: Examination of interactions that reinforce domination of oppressed groups is important for identifying intervention targets to address stigma. Bourdieu's concept of symbolic violence provides a framework for understanding experiences of marginalized groups in the healthcare setting. Young trans women are seen as transgressing current gender norms and face extremely high stigma in the Brazilian society, including in the universal healthcare system. This study was conducted to describe the ways symbolic violence manifests in healthcare interactions and structures for young trans women in Brazil who are at risk for or living with HIV.
Methods: We conducted content analysis of qualitative interview data collected with young trans women ages 18 to 24 years old and with clinical providers who practice in the universal healthcare system in Rio de Janeiro, Brazil. Ten young trans women and 10 providers were interviewed. Audio files were transcribed and translated from Portuguese into English. Findings are described.
Results: Most young trans women expressed distrust of the medical system based on prior discrimination and mistreatment by front line and clinical staff. Providers shared that colleagues knew little and had stigmatizing attitudes and beliefs about trans people. Overt discrimination manifested in individual behaviours like the unwillingness to use the social name of young trans women. Young trans women avoided the healthcare system, and thus had limited use of HIV prevention and care services. Young trans women also described limitations in the availability of medical services to meet their medical transition healthcare needs, resulting in structural violence wherein only a sub‐set of their medical needs could be met.
Conclusions: Young trans women avoid healthcare as a survival mechanism to prevent further experiences of discrimination and ostracization. Healthcare avoidance reinforced systems of exclusion and presents increased health risks as medical HIV prevention and care needs were not being met. Structural barriers to medical transition care presented further symbolic violence and eliminated an avenue for reaching young trans women to engage them in healthcare. Strategies aimed at sharing knowledge and building trust with young trans women, and availability of medical transition care can begin to dismantle the continuum of violence and promote healthcare engagement.
OAD0106
A counselling intervention to address HIV stigma at entry into antenatal care in Tanzania: Results from a parallel randomized controlled pilot study
M. Watt1, L. Minja2, S. Sao3, H. Osaki2, R. Mwamba3, G. Kisigo3, B. Knettel3, J. Ngocho2, J. Renju4, B. Mmbaga2
1University of Utah, Population Health Sciences, Salt Lake City, United States, 2Kilimanjaro Clinical Research Institute, Moshi, Tanzania, United Republic of, 3Duke University, Duke Global Health Institute, Durham, United States, 4London School of Hygiene and Tropical Medicine, London, United Kingdom
Background: Routine HIV testing and counselling during antenatal care (ANC) is an important catch point for new HIV diagnoses and can be an innovative site for addressing HIV stigmatizing attitudes among the general population. We developed Maisha, a counselling intervention implemented during routine ANC, to reduce HIV stigmatizing attitudes among women and their partners presenting for first ANC.
Methods: A parallel two‐arm pilot trial was conducted in two facilities in Moshi, Tanzania. Eligible consenting women and their partners attending their first ANC visit completed a baseline survey and were randomized to intervention or standard of care. Participants assigned to the intervention condition watched a short video followed by a brief counselling session delivered by trained lay counsellors that aimed to address misconceptions about HIV transmission and HIV stigmatizing attitudes and prepare participants for an HIV test. Participants with high stigmatizing attitudes were randomly selected for a three‐month follow‐up survey to measure the efficacy of Maisha. An 18‐item scale was used to measure stigmatizing attitudes, with subscales of moral judgement and social distancing. ANCOVA models were used to assess potential intervention effects.
Results: Between April and November 2019, we enrolled 1041 women and 494 men. At baseline, the intervention (n = 760) and control (n = 775) groups were statistically similar on all variables of interest (i.e. demographics and stigmatizing attitudes (p > 0.05)). To date, 218 participants (90 controls and 128 intervention) have completed the follow‐up survey. After controlling for baseline scores, intervention participants had significantly lower stigmatizing attitudes (F (1163) = 5.42, p = 0.021) and anticipated stigma scores (F (1158) = 4.35, p = 0.039) than control participants. In a subscale analysis, intervention participants had significantly lower moral judgment at follow‐up compared to the control (F (1163) = 12.34, p = 0.001), but there was no significant difference between conditions in interpersonal distancing (F (1163) = 2.28p = 0.133).
Conclusions: The Maisha intervention successfully reduced stigmatizing attitudes towards people living with HIV (PLWH) amongst HIV negative individuals, and reduced anticipations of stigmatizing reactions if they tested seropositive. Further research is needed to improve how Maisha content addresses the issue of interpersonal distancing from PLWH.
OAD0202
Zambia male characterization study: Insights to inform HIV programming to increase men's HIV service utilization
M. Chikuba‐McLeod1, M. Chisashi1, P. Chungulo1, L. Cicciò1, A. Fullem2, S. Illingworth3, C. Madevu‐Matson2, M. Musonda3, M. Njelesani1, H. O'Bra3
1JSI Research & Training Institute, Inc., Lusaka, Zambia, 2John Snow, Inc., Boston, United States, 3USAID, Lusaka, Zambia
Background: Male HIV service access/utilization is low in Zambia. The purpose of the Zambia male characterization study, conducted by the USAID DISCOVER‐Health project implemented by JSI, was to characterize and understand the male sexual partners of adolescent girls and young women (AGYW) at risk of HIV, in order to better target and improve HIV programmes for males, and reduce HIV transmission among AGYW.
Methods: The mixed methods study was conducted sequentially in 2017/18 in three urban DREAMS districts. A quantitative survey among AGYW characterized their male sexual‐partners. A subsequent qualitative survey among 123 males 20 to 34 years old (15 focus‐group‐discussions and 9 in‐depth‐interviews), defined men's health‐seeking behaviours and the interventions required to increase their access to and utilization of HIV services, including testing, treatment (ART), circumcision and condoms.
Results: Fear and apprehension about HIV and health system shut‐out emerged as the main barriers for men's access to/use of HIV services. The men in this study fear HIV. Most of the men living with HIV (MLHIV) they know were diagnosed late, with symptomatic HIV; they do not have many examples of MLHIV who are strong, healthy and well. They view HIV as emasculating, isolating and weak and HIV diagnosis as the start of embarrassing/stigmatizing ill‐health to early death. Many believe they have HIV from high‐risk behaviour, but are too afraid to test. Unlike women 20 to 34 who have significant health system contact, men feel shut‐out of the health system and have little access to reliable health/HIV information to inform their health choices. Equally ill‐informed peers are the primary source of information about HIV/health. Most do not know the benefits of early diagnosis or that with ART one can live healthy and strong. Men initially self‐medicate and/or use faith/traditional healers for healthcare. When the problem persists/worsens, they go to the clinic.
Conclusions: For Zambia to achieve HIV epidemic control by 2020, a key gap must be addressed: finding, engaging and sustaining the missing men, particularly men 20 to 34 (among the least virally suppressed) in HIV services. These insights should be used to improve HIV programmes to support men to access/utilize HIV services more, towards HIV epidemic control.
OAD0203
Unique and shared correlates of intimate partner violence perpetration and sexual risk behaviour among South African adolescent boys
N. Tarantino1,2, C. Matthews3,4, S. Sun1,2, L. Orchowski1,2, A. Harrison5,4, N. Abrahams3, A. Berkowitz6, M. Akande5, C. Kuo4,5
1Alpert Medical School of Brown University, Providence, United States, 2Rhode Island Hospital, Providence, United States, 3South Africa Medical Research Council, Cape Town, South Africa, 4University of Cape Town, Cape Town, South Africa, 5Brown University School of Public Health, Providence, United States, 6Alan Berkowitz Consulting, Mount Shasta, United States
Background: South Africa is a global priority setting for tackling the interacting HIV and sexual violence epidemics. Adult perpetration of intimate partner violence (IPV) is often associated with engagement in sexual risk behaviours (SRBs) relating to HIV acquisition or transmission. However, this relationship is less understood among adolescents. Guided by theory, this study explores the association between factors predictive of IPV perpetration and SRB among adolescent boys.
Methods: Boys (ages 15 to 17; N = 80) participated in a gender‐tailored intervention pilot trial focused on IPV perpetration and HIV risk behaviour prevention. Boys were recruited from a Cape Town community with high HIV prevalence. Baseline data associations among risk factors and target outcomes were analysed. Past‐year perpetration of IPV (i.e. forced sexual petting or oral, vaginal, or anal sex) and past 3‐month SRB (i.e. condomless sex, sex with multiple partners and sex while using alcohol/drugs) were measured. Significant bivariate correlates of IPV perpetration and SRB, including demographic/socio‐economic factors, violence/trauma exposure, family functioning and IPV/SRB‐related attitudes and norms, were entered in multivariate regression models.
Results: Rate of IPV perpetration was 51%; rates of SRB ranged from 33% to 49%. IPV perpetration was correlated with SRBs (rs = 0.30 to 0.36). Bivariate analyses revealed common correlates associated with a lower likelihood of IPV perpetration and SRB: greater equitable gender beliefs and ability to negotiate sexual consent; positive norms related to condom use; and safer attitudes towards sex and condoms. Other correlates were unique to IPV perpetration (e.g. food insecurity) or SRB (e.g. violence exposure). Multivariate models revealed that higher food security, better family communication and safer attitudes towards sex, and lower violence exposure, more equitable gender beliefs, and higher sexual consent negotiation ability were associated with lower odds IPV perpetration and SRB respectively.
Conclusions: Gender and sexual risk‐related norm perceptions/misperceptions, beliefs and attitudes possibly explain the association between IPV and SRB among adolescent boys in South Africa and should be focused by prevention efforts. Addressing IPV in an adolescent‐tailored manner needs also to include targeting family factors. More research is needed to further uncover unique risk factors relating to IPV perpetration and SRB during adolescence, including access to resources and violence/trauma exposure.
OAD0204
An alarming prevalence of gender‐based violence experiences among HIV high risk population in Cambodia
P. Chhoun1, C. Wieten1, S. Tuot1, C. Brody2, S. Kros3, S. Yi1,4,5
1KHANA Center for Population Health Research, Chamkarmorn, Cambodia, 2Touro University California, Public Health Program, College of Education and Health Sciences, Vallejo, United States, 3Ministry of Health, Provincial Health Department, Siem Reap, Cambodia, 4National University of Singapore, Saw Swee Hock School of Public Health, Singapore, Singapore, 5Touro University California, Center for Global Health Research, Vallejo, United States
Background: A growing body of international research identifies women working in the sex and entertainment industry as a high‐risk group for exposure to gender‐based violence (GBV). Female entertainment workers (FEWs) are one of the HIV high‐risk population in Cambodia. Despite the recognized vulnerability of these women, their experiences with GBV remain understudied. This study aims to examine the prevalence of GBV among one HIV high risk population in Cambodia and identify factors associated with their victimization.
Methods: A cross‐sectional study was conducted as part of the mid‐term survey for the Mobile Link project in November 2019. A structured questionnaire was programmed in Kobo Humanitarian Response Platform and offline‐used for face‐to‐face interviews with 600 FEWs from three provinces and a capital city in Cambodia. The study participants were recruited from different entertainment venues using a stratified random sampling method. The questionnaire collected data on socio‐demographic characteristics, gender inequity norm and GBV. Bivariate and multivariable logistic regression analyses were performed to identify risk factors for GBV victimization.
Results: Of the total, 60.5% women had experienced a form of GBV during their lifetime, of whom 37.5% experienced it in the past six months. The prevalence of emotional abuse, forced substance use, physical abuse and forced sex was 51.5%, 25.0%, 20.6% and 2.9% respectively. Forced substance use and forced sex were mainly perpetrated by clients, physical abuse by intimate partners and emotional abuse by others. FEWs victimized by clients (RRR = 0.19, 95% CI = 0.07 to 0.53) and others (RRR = 0.11, 95% CI = 0.03 to 0.44) were less likely to be married compared to victims of intimate partner violence. Factors associated with sexual harassment were working in beer gardens (AOR = 2.39, 95% CI = 1.20 to 4.73) and restaurants/cafés (AOR = 1.65, 95% CI = 1.01 to 2.69), and having high adherence to gender inequity norms (AOR = 3.21, 95% CI = 1.42 to 7.25).
Conclusions: FEWs in Cambodia experience high levels and unique forms of GBV as they are confronted with different types of perpetrators. Interventions need to be tailored to fit the specific needs and experiences of FEWs working in different entertainment venues. Interventions aimed at reducing client‐perpetrated violence should specifically focus on forced substance use and forced sex, while physical abuse by intimate partners should also be addressed.
OAD0205
Gender‐based violence perpetration by male sexual partners of adolescent girls and young women in Haiti: Demographic and HIV correlates
O. Desinor1, J. McOwen1, K. Francoise2, E. Felker‐Kantor3, J. Michel4, K. Andrinopoulos3
1United States Agency for International Development, Health, Port au Prince, Haiti, 2Ministry of Public Health and Population, Port au Prince, Haiti, 3Tulane School of Public Health and Tropical Medicine, Global Community Health and Behavioral Sciences, New Orleans, United States, 4Pentagone Consulting Group, Port au Prince, Haiti
Background: Globally, women who experience gender‐based violence (GBV) have been shown to be at higher risk for HIV. Male perpetrators of GBV report higher risk HIV behaviours (multiple partners, sex worker patronage and inconsistent condom use). GBV is potentially an important determinant of HIV vulnerability for AGYW in Haiti, but limited information exists about the characteristics of men who perpetrate GBV.
Methods: A cross‐sectional survey was administered to adult men in Port‐au‐Prince (PaP) (n = 500) and St. Marc (n = 300) reporting an AGYW sexual partner in the last 12 months. Men were recruited using respondent‐driven‐sampling, and asked to report on their HIV‐related behaviours, and perpetration of emotional and sexual/physical violence with their most recent AGYW partner. Statistical analysis included bivariate and multivariate logistic regression with appropriate RDS sampling weights. Results are presented separately for each city.
Results: The most common form of emotional violence reported by men was trying to control what their partner does (72.5% in PaP, 64.9% in St. Marc). Emotional violence perpetration was more common among men with higher levels of education (adjusted odds ratio (AOR) 11.09, p = 0.000 in PaP and 5.55, p = 0.092 in St. Marc), and higher income in PaP (AOR 2.20, p = 0.004). Men who reported emotional violence perpetration in PaP were more likely to use condoms with their AGYW partner (AOR 1.79, p = 0.071). In terms of physical violence, 7.5% of participants in PaP and 7.0% of participants in St. Marc reported ever having hit, pushed, slapped, punched, or kicked their AGYW sexual partner. A higher proportion reported ever having forced their AGYW partner to have sex (17.2% of participants in PaP and 19.8% in St. Marc). In multivariate analysis, men in St. Marc who report physical/sexual violence perpetration were more likely to report high‐risk sexual behaviour (multiple concurrent partnerships and being six or more years older than their partner) (AOR 3.06, p = 0.011) and less likely to report condom use (AOR 0.39, p = 0.009).
Conclusions: In Haiti physical/sexual violence perpetration is linked to higher risk sexual behaviour for men and may increase their partner's HIV vulnerability. It is important to include GBV interventions in HIV programming.
OAD0206
Rational reasoning and (non) disclosure of sexual assault by female students in a university in Eswatini: Implications for HIV prevention
F. Shabalala1, N. Masilela2, P. Ndlangamandla2, S. Masuku2, R. Fielding‐Miller3
1University of Eswatini, Community Health Nursing, Mbabane, Eswatini, 2University of Eswatini, Community Health Nursing Science, Mbabane, Eswatini, 3University of California, Global Health and Medicine, San Diego, United States
Background: Sexual violence is strongly linked to an increased risk of HIV acquisition, and one in three women around the world will experience some form of sexual violence in their lifetime. While post‐exposure prophylaxis medications, counselling and other forms of support that may decrease some of the attendant risk of HIV are available to survivors of assault in many global settings, they can only be accessed if survivors choose to disclose that an assault has happened. Increasing safe and supported sexual assault disclosure is an important step in addressing the links between HIV and gender‐based violence. We analysed a sample of female university students in Eswatini who had experienced sexual assault in their lifetime to identify factors associated with the choice to disclose.
Methods: Participants were a random sample of female students enrolled fulltime at the University of Eswatini, drawn from a list of all students at time of study. Analyses were conducted on a subsample of women (n = 188) who reported experiencing sexual assault in their lifetime. We assessed the prevalence and correlates of disclosure, testing the hypothesis that financial reliance on a perpetrator would be a strong predictor of nondisclosure.
Results: We sampled 372 female students. Of these, 51% (n = 188) reported lifetime penetrative sexual assault. Only 43% of survivors (n = 80) reported ever disclosing their assault to anyone. In our analyses, economic variables were not associated with disclosure. Believing one's friends would support her if she was assaulted by a boyfriend was associated with disclosure (OR 2.16, 95% CI: 1.18 to 3.92) and believing she would be supported if assaulted by a stranger was marginally associated (OR 1.79, 95% CI: 0.99 to 3.25). Among participants who never disclosed, 10% cited financial reliance on their perpetrator. Being responsible for a child was marginally associated with nondisclosure because of financial reliance (OR 3.3, 95% CI: 0.93 to 11.81).
Conclusions: The majority of sexual assault survivors never disclosed their assault to anyone. Programmes to reduce HIV risk for survivors of sexual assault must consider both women's social and financial landscapes to create holistic policies.
OAD0302
Characterizing strategies used by HIV‐infected smallholder farmers to mitigate the effects of climate change in the Nyanza region of Kenya
T. Nicastro1, C.R. Cohen2, N. Beyeler3, S. Jawouro4, G. Odhiambo4, E.A. Bukusi4, S.D. Weiser5
1University of California, National Center of Excellence in Women's Health, San Francisco, United States, 2University of California, Obstetrics and Gynecology, San Francisco, United States, 3University of California, Institute for Global Health Science, San Francisco, United States, 4Kenya Medical Research Institute, Kisumu, Kenya, 5University of California, HIV and Infectious Diseases, San Francisco, United States
Background: Severe weather events pose risks to HIV health among infected individuals relying on farming for livelihoods and food. Little information exists on strategies rural people living with HIV (RPLHIV) adopt to cope with severe weather events. We used qualitative methods to characterize strategies used by HIV‐infected farmers to mitigate climate change impacts.
Methods: We interviewed 40 HIV‐infected‐individuals in 2018 enrolled within the Shamba Maisha cluster‐randomized control trial, a multisectoral agricultural and financial intervention to improve HIV health outcomes among RPLHIV in Kisumu, Homa Bay and Migori counties in Kenya (NCT02815579). We used purposive sampling to select participants from diverse geographies. In‐depth interviews were conducted in participants’ native language, transcribed, translated into English and double‐coded. Thematic content analysis followed an integrated inductive‐deductive approach.
Results: Participants reported severe weather (droughts and flooding) became more severe over time, leading to significant losses in livestock, crop yields, infrastructure and income, posing threats to their HIV health through increased food insecurity, lack of money for transportation and displacement from flooded homes. Mitigation strategies included short‐term coping such as reduced number of meals, eating lower‐quality food, walking long distances to clinic, displacement to safer regions, and skipping medications if no food was available. People also described longer‐term adaptation strategies such as farming plot expansion, farming infrastructure investments, individual requests for larger distributions of medications, allocating more money towards savings, diversifying crops, and securing non‐farming employment. According to participants, these efforts were driven by their complete dependence on farming to support their basic needs, while also noting the importance of farming yields and availability of food in order to adhere to their ART medications. Gender may play a role in mitigation strategies utilized because male farmers may have more access to some resources than female farmers, such as land, farming machinery, and available time.
Conclusions: These data provide useful information on how RPLHIV adapt to impacts of severe weather events, which can help guide development of climate responsive support systems, including those that prevent wide‐spread interruptions in ART adherence. More work is needed on gender‐specific adaptation strategies so that interventions and programmes are responsive to the needs of all RPLHIV.
OAD0303
Randomized controlled trial of nurse‐led lay village women on behavioural and nutritional intervention for women living with HIV/AIDS in India: 18 months follow‐up
A. Nyamathi1
1University of California, Sue & Bill Gross School of Nursing, Irvine, United States
Background: Long‐Term Impact of a nurse‐led behavioural and nutrition intervention, supported by Asha (lay village women), and focused on improving the health of WLH/A in India. Health parameters include Depressive Symptoms, CD4 levels, Body Mass Index [BMI]) and Haemoglobin. Women Living with HIV/AIDS (WLH/A) in rural India face extreme health disparities, challenging adherence to Antiretroviral (ART) Treatment. Nutritional deficits including anaemia exacerbate disease progression.
Methods: After extensive formative research, we conducted a four‐arm quasi‐experimental trial with 600 women recruited from primary‐health centres. The four programmes each included group‐education sessions and Asha support and differed on the nutritional component: 1) Asha‐supported standard education (SE) alone; 2) SE + nutrition education (+NE); 3) SE + nutrition supplements (+NS); or 4) SE + nutrition education and supplements (+NENS). The intervention was delivered over 6 months. Assessments occurred at baseline, and month 6 (post‐intervention), 12, and 18, with 100% retention. Multilevel modelling examined effects of programme over time.
Results: At baseline, mean age was 34 years and CD4 level was 447.4. 100% of the women were anaemic. At 18‐month follow‐up, Programme 4 experienced greatest improvements in CD4 counts compared to the Programme 1. For BMI, Programmes 3 and 4 exhibited greater gains compared to Programme 1. All programmes improved depressive symptom scores and ART adherence from baseline to 18‐month follow‐up; no severe anaemia at 18‐months.
Conclusions: A low‐cost Nurse‐led and Asha‐supported behavioural and nutritional intervention improved health parameters sustained at 18‐month follow‐up. Future research should explore this model in other communities and infectious diseases.
OAD0304
How does HIV risk differ by co‐occurring structural factors? A latent class analysis of structural vulnerability indicators among cisgender female sex workers in Baltimore, Maryland, USA
C. Tomko1, K. Schneider2, D.F. Nestadt1, R.H. White1, R. Musci2, M. Kaufman1, S.G. Sherman1
1Johns Hopkins Bloomberg School of Public Health, Health, Behavior, and Society, Baltimore, United States, 2Johns Hopkins Bloomberg School of Public Health, Mental Health, Baltimore, United States
Background: Structural vulnerability (SV) posits that a group's social position can constrain behaviours owing to conflict with existing power structures, elevating risk for health disparities. Little research has examined how HIV risk differs by the co‐occurrence of SV indicators (e.g. violence, economic strains) among female sex workers (FSW), a key population in the HIV epidemic grossly understudied in the US.
Methods: We recruited 385 cisgender FSW 18 years+ in Baltimore, Maryland via mobile van. Participants completed survey, HIV rapid test and self‐administered chlamydia and gonorrhoea tests. Using latent class analysis, we sought to identify typologies of SV based on clustering of SV indicators experienced in the past 6 months: unstable housing, financially dependent on someone else, client‐perpetrated physical or sexual violence and hungry “because there was not enough food” at least weekly. Number of latent classes was determined by relevant fit statistics (AIC, BIC, LRT). Mplus commands dcat and dcon performed bivariate tests of significance between latent classes and categorical and continuous variables, respectively, while accounting for class.
Results: Participants were a median 37 years old, 36% Black and 58% injected drugs in the past 6 months. Baseline HIV prevalence was 7% with 16% and 18% testing positive for gonorrhoea and chlamydia respectively. A 3‐class model emerged: economic factors (housing, financial dependence) only (E); economic and hunger (EH); highest SV (HSV) (Figure 1). Significant differences between classes include: condomless sex with clients (p = 0.002), injecting drugs (p < 0.001), chlamydia infection (p = 0.04), internalized sex work stigma (p = 0.03) and depression (p < 0.001) and PTSD (p < 0.001) symptoms.
Abstract OAD0304‐Figure 1.
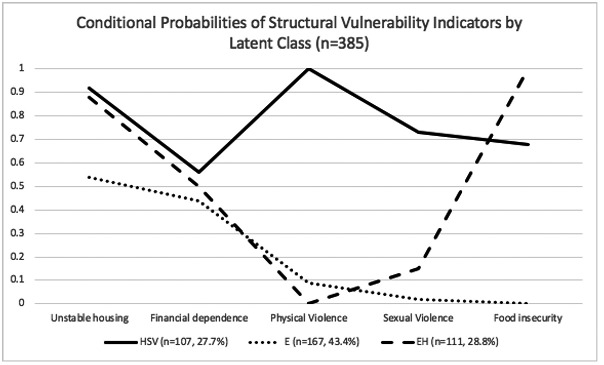
Conclusions: Clear patterns of SV and HIV risk exist among FSW in the US. Results demonstrate the importance of employing a social determinants of health perspective in reducing HIV burden in this population, with the study providing nuances as to how to target subgroups to potentiate interventions’ impacts among this key population.
OAD0305
Gaining traction: Promising shifts in gender norms and intimate partner violence during an HIV prevention trial in South Africa
A. Gottert1, J. Pulerwitz1, N. Haberland1, R. Mathebula2, D. Rebombo2,3, K. Spielman1, R. West4, A. Julien5, R. Twine6, D. Peacock2,7,8, M.‐S. Kang Dufour4, F.X. Gómez‐Olivé6, A. Pettifor5,6, S.A. Lippman4,6, K. Kahn6
1Population Council, HIV and AIDS Program, Washington, United States, 2Sonke Gender Justice, Agincourt, South Africa, 3Independent Consultant, Johannesburg, South Africa, 4University of California, Division of Prevention Science, Department of Medicine, San Francisco, United States, 5University of North Carolina at Chapel Hill, Department of Epidemiology, Chapel Hill, United States, 6University of the Witwatersrand, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, Agincourt, South Africa, 7Promundo, Washington, United States, 8School of Public Health and Family Medicine, University of Cape Town, Division of Social and Behavioural Sciences, Cape Town, South Africa
Background: HIV and violence prevention programmes increasingly seek to transform gender norms among participants, yet how to do so at the community level, and subsequent pathways to behaviour change, remain poorly understood. We assessed shifts in endorsement of equitable gender norms, and intimate partner violence (IPV), during a three‐year community‐based trial of an HIV “treatment as prevention” intervention in rural South Africa.
Methods: Cross‐sectional household surveys were conducted with men and women ages 18 to 49 years, in eight intervention and seven control communities, at 2014‐baseline (n = 1149) and 2018‐endline (n = 1189). Gender norms were measured by the GEM Scale. Intent‐to‐treat analyses assessed intervention effects and change over time. Qualitative research with 59 community members and 38 staff examined the change process.
Results: Two‐thirds of men and half of women in intervention communities had heard of the intervention/seen the logo; half of these had attended two‐day workshop(s). Regression analyses showed a 15% improvement in GEM score over time, irrespective of the intervention, among men (p < 0.001) and women (p < 0.001). Younger men (ages 18 to 29) also had decreased odds of reporting past‐year IPV perpetration over time (aOR 0.40; p < 0.05), while younger women had lower odds of reporting IPV over time in intervention versus control communities (aOR 0.53; p < 0.05). Qualitative data suggest that gender norms shifts may be linked to rapidly increasing media access (via satellite TV/smartphones) and consequent exposure to serial dramas modelling equitable relationships. Workshop activities that fostered couple‐communication skill‐building and critical reflection around gender norms further supported IPV reductions.
Abstract OAD0305‐Figure 1.
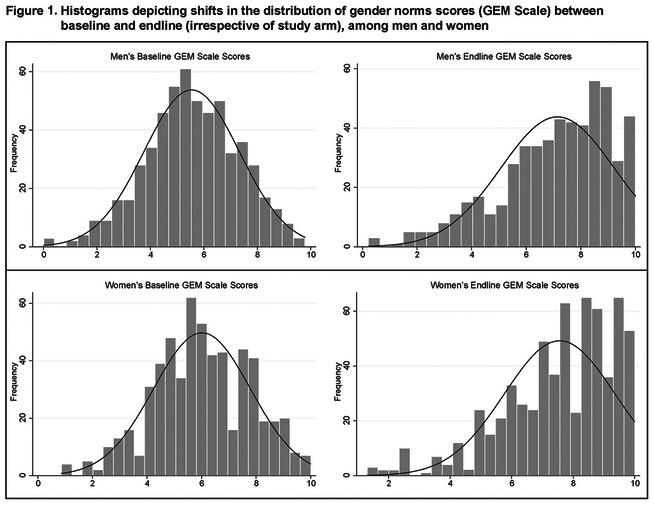
Conclusions: There was a population‐level shift towards greater endorsement of equitable gender norms between 2014 to 2018, potentially linked with escalation in media access. There was also an intervention effect on reported IPV among women, although not among men. Societal‐level gender norm shifts can create enabling environments for interventions to find new traction for violence and HIV‐related behaviour change.
OAD0306
Modifying social action theory to conceptualize social and structural factors and their impacts on HIV treatment engagement
S.M. Topp1, C. Mwamba2, L.K. Beres3, I. Sikazwe2, C.B. Holmes4, M.E. Herce5,2, A. Sharma2
1James Cook University, Townsville, Australia, 2Centre for Infectious Disease Research in Zambia, Lusaka, Zambia, 3Johns Hopkins University, Baltimore, United States, 4Georgetown University, Washington DC, United States, 5University of North Carolina, Chapel Hill, United States
Background: Long term engagement and retention in HIV treatment is an ongoing challenge to national treatment programmes, yet behavioural models on which many retention‐promoting programmes are built, do not adequately account for the role of social and structural determinants. Based on secondary analysis, we interrogated Ewart's Social Action Theory as a promising approach to conceptualizing not just which, but how, social and structural factors and their interaction influence HIV treatment engagement.
Methods: Thematic summaries from three empirical qualitative data sets documenting patient and provider experiences of HIV treatment engagement and disengagement in Zambia (2012/13, 2015/16) and Malawi (2018) were analysed for congruence with Social Action Theory (SAT). We conducted iterative comparison of thematic summaries to theoretical constructs relating to context, self‐change processes and action‐states respectively.
Results: Qualitative validation demonstrated a high degree of congruence with SAT across data sets. Patients’ experience of illness (physiological state) community HIV‐related knowledge and attitudes, gender and cultural norms (relationship systems), work place and health facility contexts (organisational systems) and experiences of poverty and food security (socioeconomic settings) combined to create a critical “context” in which individuals’ operated. Individual's knowledge and understanding which informed both imagined and real possibilities of HIV and treatment (generative capabilities), personal acceptance or denial of status and expectations of seeking treatment (motivations), capacity to disclose as well as relationships with health providers (social interactions) combined to form dynamic responses influencing engagement decision behaviours. The relative influence of spouses or close family members (social interdependence), combined with immediate physiological outcomes of treatment (side effects, recovery and/or illness), completed a dynamic loop feeding back into the “contextual” experience of illness and personal affect.
Conclusions: The challenge of how to strengthen engagement in HIV treatment in both general and targeted epidemics remains ubiquitous, yet also paradoxically context‐specific. Social Action Theory extends current models by more consciously linking social and structural factors (particularly structural poverty, power‐dynamics and health system drivers) to well‐recognized behavioural drivers of engagement. It provides a promising tool for conceptualizing “whole‐of‐system” planning for improving HIV retention accounting for the dynamic relationship between factors while remaining sufficiently flexible to use across political, cultural and geographic settings.
OAD0402
A new generation of drug users in St. Petersburg, Russia? A preliminary testing of theory of drug generations based on a mixed‐methods pilot study of young hard drug users
P. Meylakhs1, S. Friedman2, D. Ompad3
1National Research University Higher School of Economics, International Centre for Health Economics, Management, and Policy, St. Petersburg, Russian Federation, 2NYU Langone, New York, United States, 3NYU College of Global Public Health, New York, United States
Background: Russia has a widespread injection drug use epidemic with high prevalence of HIV and HCV among people who inject drugs (PWID). Thus, HIV prevalence among PWID in St. Petersburg is around 60%, HCV – 95%. Most research to date was concentrated on older cohorts, mostly opioid users, of PWID while young drug users in Russia have not received proper attention. The goal of the pilot study was to gain some preliminary understanding of possible drug generation's change. Our theoretical approach was “drug generation theory,” according to which drug generations succeed each other when a previously fashionable drug falls into disrepute.
Methods: Mixed methods study of young (age 18 to 26) hard (opiates, stimulants, NPS) drug users in St. Petersburg using HIV and HCV oral tests (OraQuick) in addition to behavioural data (10 semi‐structured interviews and 40 structured interviews).
Results: Almost half (49%) of the sample used amphetamines, 21% used amphetamines and mephedrone (NPS) – also a stimulant (thus, 70% of the sample used only stimulants). Only 18% ever used opioid (and only episodically). Mean IDU experience was 4.2 years. 0 HIV cases and 2 HCV cases were detected among 30 PWID subsample. None of the participants shared a syringe in the last 12 months. Opioid use, syringe sharing and HIV and HCV statuses were heavily stigmatized. The informants avoided older (30 + ) PWID. Qualitative data show some of the participants used opioids episodically but were disappointed by their effects.
Conclusions: These data indicate that a new generation of drug users in St. Petersburg may have emerged. Though the sample was small, the discrepancies—0% versus 65% on HIV and 7% versus 95% on HCV can hardly be attributed to chance. Thus, this cohort seems to be much safer in its injection practices than older PWID cohorts. It is also opiate aversive in comparison to older cohorts. Thus, this generation of drug users can be called “amphetamine generation” or given the rapid spread and popularity of stimulant type NPS “stimulants generation.” The pilot data give some confirmation to the theory of drug generation's change. However, given the small sample size these conclusions are very preliminary.
OAD0403
HIV and hepatitis C virus co‐infection among people who inject drugs in Cambodia: Findings from a national survey using respondent driven sampling method
C.H. Saing1, P. Chhoun2, N. Chann3, S. Tuot2, P. Mun3, S. Eng2, S.C. Choub2, S. Yi2,1,4
1National University of Singapore, Saw Swee Hock School of Public Health, Singapore, Singapore, 2KHANA Center for Population Health Research, Phnom Penh, Cambodia, 3National Center for HIV/AIDS, Dermatiology and STD, Surveillance Unit, Phnom Penh, Cambodia, 4Touro University California, Center for Global Health Research, Vallejo, United States
Background: Despite the evidence of the relationship between human immunodeficiency virus (HIV) and hepatitis C virus (HCV) in people who inject drugs globally, studies on the co‐infection among this key population remain scarce in resource‐poor countries. This study was therefore conducted to explore the prevalence of and factors associated with HIV/HCV co‐infection among people who inject drugs in Cambodia.
Methods: This study was conducted in 2017 as part of the National Integrated Biological and Behavioral Survey. The Respondent Driven Sampling method was used to recruit participants in 12 provinces for face‐to‐face interviews and HIV and HCV testing. Weighted multivariable logistic regression analysis was conducted to identify risk factors associated with HIV/HCV co‐infection. This study was approved by the National Ethics Committee for Health Research.
Results: This study included 286 people who inject drugs with a mean age of 31.6 (SD = 7.5) years. The prevalence of HIV and HCV was 15.2% and 30.4% respectively. Almost one in ten (9.4%) of the total study population were co‐infected with HIV and HCV. After adjustment, the odds of HIV/HCV co‐infection was significantly higher among participants who were female (AOR = 2.17, 95% CI = 1.03 to 6.08), were in the older age group of 35 and older (AOR = 3.67, 95% CI = 1.04 to 9.80), were widowed/divorced/separated (AOR = 3.25, 95% CI = 1.76 to 13.94), were living on the streets (AOR = 4.83, 95% CI = 1.23 to 9.02), and had received methadone maintenance therapy in the past year (AOR = 4.02, 95% CI = 1.13 to 18.96) compared to their respective reference group. The odds was significantly lower among participants who reported having attained ≥10 years of formal education compared to those who had attained only primary education or lower (AOR = 0.68, 95% CI = 0.15 to 0.96).
Conclusions: The prevalence of HIV/HCV con‐infection among people who inject drugs in Cambodia is considerably high, particularly in older and more vulnerable subgroups. Tailor‐made interventions are required to increase access to culturally sensitive harm reduction interventions to prevent both HIV and HCV infection. In addition, there is an opportunity to expand HCV screening, diagnosis and treatment in this key population given its small population size and the availability of new directly acting antiviral agents in the country.
OAD0404
Effects of cigarette smoking and substance use on HIV viral suppression over time in a cohort of young men who have sex with men
M. Kalmin1, H. Aralis2, M. Li1, M. Javanbakht3, P. Gorbach3, S. Shoptaw1
1UCLA, Family Medicine, Los Angeles, United States, 2UCLA, Biostatistics, Los Angeles, United States, 3UCLA, Epidemiology, Los Angeles, United States
Background: Cigarette smoking and substance use behaviours often co‐occur in HIV‐positive populations. Although smoking and substance use have been linked to HIV viral suppression (VS), limited data are available on their independent and joint effects on VS longitudinally. Determining whether there is an interaction between these behaviours on VS over time could inform syndemic approaches in managing HIV.
Methods: The Men who have sex with Men and Substance Use Cohort at UCLA Linking Infections, Noting Effects (mSTUDY) is a cohort study primarily among men of color in Los Angeles, CA. This analysis included mSTUDY participants enrolled from 2014 to 2018 who were HIV‐positive, reported being prescribed antiretroviral therapy (ART), and had available data on smoking and substance use. Independent and joint effects of time‐varying smoking (at least one cigarette/day) and substance use (opiates, fentanyl, cocaine, amphetamine‐type stimulants or nitrites) on VS (viral load < 200 copies/mL) at each six‐month follow‐up visit were estimated using a mixed‐effects logistic regression model, accounting for repeated measures and adjusting for time, age, race/ethnicity, employment and history of psychiatric illness.
Results: Among 227 HIV‐positive participants with a median follow‐up of two years, 126 (56%) reported smoking, 181 (80%) reported using substances other than cannabis and 205 (90%) experienced viral suppression at least once over follow‐up. At each visit, participants who reported smoking had significantly decreased adjusted odds of experiencing VS compared to nonsmokers (aOR = 0.59, 95% CI: 0.36 to 0.97). Similarly, participants who reported using substances other than cannabis had less than half the adjusted odds of experiencing VS compared to those reporting either cannabis only or no drug use (aOR = 0.46, 95% CI: 0.28 to 0.74). There was not a significant interaction between smoking and substance use on VS at each six‐month visit.
Conclusions: Reported cigarette smoking and substance use other than cannabis independently reduced the odds of experiencing VS at each six‐month visit. However, individuals who reported both smoking and using substances other than cannabis did not experience an enhanced decreased odds of VS compared to either smoking or substance use alone. Further research will focus on ways to understand the roles of smoking and substance use over time in this study population.
OAD0405
PrEP awareness and perceived HIV stigma among people who inject drugs, San Francisco, 2018
D. Veloso1, H. Xie1, J. Lin1, D. Miller1, W. McFarland1,2
1San Francisco Department of Public Health, Center for Public Health Research, San Francisco, United States, 2University of California, San Francisco, United States
Background: “Getting to Zero” efforts require achieving zero HIV stigma. Unfortunately, interventions to reduce stigma lag behind HIV treatment to reduce mortality and PrEP to prevent infection. Research has shown HIV stigma as a barrier to prevention options such as PrEP; however, the direction of this association may go both ways. Education on PrEP may reduce HIV stigma as people at risk become aware of effective ways to prevent infection. Meanwhile, awareness of PrEPs ability to prevent HIV transmission is low among people who inject drugs (PWID) compared to other populations at risk. We therefore analysed data from a community‐based survey of PWID in San Francisco to illuminate the effects of PrEP awareness on perceptions of HIV stigma.
Methods: PWID were recruited through respondent‐driven sampling from July to December 2018. Eligibility criteria included San Francisco residence, 18 years of age or older and injection of drugs within the past twelve months. Participants completed a structured survey including questions on PrEP awareness and ranking of how strongly they agreed with the statement, “Most people in San Francisco would discriminate against someone with HIV.”
Results: Among 464 participants, 38.1% were ≥50 years old, 55.0% were non‐white, 66.5% were male‐identified, 78.0% were unstably housed and 9.1% had previously tested HIV positive. Among HIV‐negative PWID, 37.2% were aware that PrEP could prevent HIV transmission from sharing injection equipment, and 38.8% agreed that most people would discriminate against someone with HIV. Black/African Americans (OR 3.61, 95% CI 2.14 to 6.08; p < 0.01) and Hispanics (OR 1.93, 95% CI: 1.05 to 3.57; p = 0.035) had greater perceptions of HIV stigma compared to white PWID. Those who perceived less HIV stigma were more likely to be aware of PrEP (OR 1.55, 95% CI 1.04 to 2.30, p = 0.030) and know that PrEP can prevent HIV transmission through sharing injection equipment (OR 1.66, 95% CI 1.09 to 2.51 p = 0.018).
Conclusions: Increased promotion of PrEP for prevention of transmission through sharing injection equipment is needed among PWID together hand‐in‐hand with HIV stigma reduction programmes. Getting to zero HIV infections may increasingly depend upon HIV stigma reduction as the remaining infections increasingly occur among groups experiencing intersecting stigma and discrimination.
OAD0406
Associations of recreational drug use with HIV‐related sexual risk behaviours among men who have sex with men in Japan: Results from the cross‐sectional LASH study
T. Miwa1, M. Yamaguchi2, T. Ohtsuki1, C. Wakabayashi3, S. Nosaka1, Y. Ikushima1, M. Tarui1
1PLACE, Tokyo, Japan, 2Bunan Hospital, Saitama, Japan, 3Saitama Prefectural University, Department of Health Sciences, Saitama, Japan
Background: While there are indications that recreational drug use promotes high‐risk sexual behaviours among men who have sex with men (MSM), there is a scarcity of recent research focusing in Japan which has made a series of legislative changes surrounding drug control over the past decade. This research aims to assess recreational drug use patterns among MSM in Japan and evaluate their potential associations with HIV‐related sexual risk behaviours.
Methods: Between September 2016 and October 2016, study participants were recruited in a cross‐sectional behavioural survey through a geosocial networking application for MSM. Participants were asked to complete an anonymous, self‐administered online questionnaire which included information on sexual behaviours and drug use.
Results: The mean age of the 6921 respondents who were included in the analysis was 33.8 (95% CI: 33.6 to 34.0). 25.4% (1756/6921) of them reported that they had used recreational drugs some time in their life, and 11.3% (780/6921) in the past six months. The most commonly used drugs in the past six months were erectile dysfunction drugs (7.6%), alkyl nitrites (4.1%) and codeine‐containing cough medicines (1.8%). Drug users were more likely than non‐drug users to be older, have lower education level, self‐identify as homosexual (gay), drink alcohol almost every day, know their HIV status, and have a better knowledge of HIV/STI. Recreational drug use in the past six months were independently associated with each of the following high‐risk sexual behaviours in the same period: (i) > 5 sexual partners (aOR = 2.70, 95% CI: 2.30 to 3.17); (ii) unprotected anal intercourse (aOR = 2.88, 95% CI: 2.43 to 3.42); (iii) group sex (aOR = 2.60, 95% CI: 2.22 to 3.05); and (iv) sex work (aOR = 2.30, 95% CI: 1.67 to 3.16).
Conclusions: This study suggests that recreational drug use is common among MSM in Japan amid tighter controls in the country. Furthermore, drug users were more likely to report high‐risk sexual behaviours despite having a better knowledge of HIV/STI. Instead of merely prohibiting the use of drugs, it is important for the public and the private sector to work in concert to develop community outreach programmes to minimize the harm caused by drug use.
OAD0502
The role of popular opinion leaders in distributing HIV/syphilis self‐tests among men who have sex with men in China
N. Yang1, Z. Yi2, W. Huang3, D. Wu4,5,6,7, W. Tang6,7,5
1University of Minnesota Medical School, Minneapolis, United States, 2Centers for Disease Control, Department of HIV Control, Zhuhai, China, 3Emory University, Atlanta, United States, 4London School of Hygiene and Tropical Medicine, Clinical Research Department, London, United Kingdom, 5Social Entrepreneurship to Spur Health, Guangzhou, China, 6University of North Carolina at Chapel Hill‐Project China, Guangzhou, China, 7Dermatology Hospital of Southern Medical University, Guangzhou, China
Background: Novel strategies are needed to increase HIV testing, especially among key populations like men who have sex with men (MSM). Targeting MSM popular opinion leaders (POL) for distributing self‐tests may increase access to HIV testing.
Methods: This was a secondary analysis of a cohort study in Zhuhai, China. Men 16 years or older, born biologically male, ever had sex with a man and applying for HIV/syphilis dual self‐test kits were enrolled as indexes. Indexes who scored in the top 15% of a sexual influencer scale were deemed POL (Cronbach alpha 0.87). All indexes received up to five self‐tests per application and were encouraged to distribute self‐tests throughout their social networks. Recipients (alters) were instructed to upload their self‐test results and complete a survey. The primary outcome was the average alters recruited per index. Poisson regression was used to calculate the rate ratio (RR) of recruitment by POL versus non‐POL.
Results: From June 17, 2018 to November 12, 2019, 371 indexes successfully applied for self‐tests, 64 of whom were POL and 307 were non‐POL. Compared to non‐POL, more POL had disclosed their MSM status (86% vs. 67%, p < 0.01) and were MSM community volunteers (20% vs. 3%, p < 0.01). Eighty percent of all indexes had prior HIV testing, with no significant difference between POL and non‐POL. Two hundred seventy‐eight alters returned a verified test result. The average recruitment was 1.7 alters per POL index, versus 0.5 alters per non‐POL index (RR 3.19, 95% CI 2.51 to 4.05). POL were also more efficient than non‐POL at recruiting first‐time testers (RR 2.57, 95% CI 1.73 to 3.83), HIV‐positive alters (RR 5.48, 95% CI 1.99 to 15.12) and syphilis‐positive alters (RR 4.80, 95% CI 1.20 to 19.18). Alters of POL were more likely than alters of non‐POL to live rurally (47% vs. 25%, p < 0.01), have a below‐college education (55% vs. 41%, p = 0.02) and have multiple male sexual partners in the past 6 months (43% vs. 30%, p = 0.03).
Conclusions: POL were more efficient than non‐POL at distributing self‐tests among MSM, and reached alters with higher risk for HIV/syphilis but less access to testing. Future randomized control trials are warranted to explore POL targeting for self‐test distribution.
OAD0503
Household couples‐based HIV self‐testing is effective in promoting male testing, identifying discordant couples and linking positive partners to care: Results from the Wenza Huru study in Kisarawe, Tanzania
J. Mbwambo1, C. Bailey2, V. Fonner2, T. Rutayuga1, K. O'Reilly2, J. Ntogwisangu1, I. Hamidu1, B. Singh2, M. Sweat2
1Muhimbili University of Health and Allied Sciences, Psychiatry, Dar es Salaam, Tanzania, United Republic of, 2Medical University of South Carolina, Psychiatry and Behavioral Sciences, Charleston, United States
Background: Many HIV infections in sub‐Saharan Africa occur within stable couples, yet engaging couples in testing together is uncommon. Men are less likely to test for HIV than women. Serodiscordant couples are typically overlooked in HIV prevention despite being high‐risk. HIV self‐testing has been proven highly acceptable, accurate and preferred over clinic‐based testing. We assessed whether engaging couples as a dyad in HIV self‐testing would promote testing uptake, disclosure of test results, identification of serodiscordant couples and engagement in care for positive partners.
Methods: In Kisarawe, Tanzania we recruited 446 cohabitating couples (aged 18 years or older, with at least one member aged 55 years or less) via door‐to‐door sampling. Couples received pre‐test education and two OraQuick® Rapid HIV‐1/2 test kits, then answered a brief survey. Two‐weeks later we returned, offered rapid blood‐based HIV testing and conducted an additional survey. Post‐test referral and counselling were offered if HIV + . At six months, a random subset of 30 participants completed a brief follow‐up survey to assess care engagement for HIV+ participants.
Results: 65% of households had a cohabiting couple, 76% of couples were home when contacted and 89% of those couples accepted self‐test kits. We enrolled 446 couples (N = 892). The 2‐week follow‐up rate was 89% (n = 796). Of those, 97% (n = 775) had used the self‐test kit, 72% (n = 558) tested together with their partner and 97% (n = 748) disclosed their self‐test result to the partner. Over 90% of participants found the kits easy to use and trustworthy, were satisfied with the experience, and would recommend self‐testing to a friend. HIV prevalence was 3.9% (n = 31), two‐thirds (n = 20) of whom were positive partners in serodiscordant relationships. The overall serodiscordancy rate was 5.2% (n = 20/388 couples with complete data). Only 25% of negative partners reported knowing their partner's positive status prior to self‐testing. Among 22 HIV+ persons completing the six‐month follow‐up survey, 82% (n = 18) enrolled in HIV care.
Conclusions: Household‐based couples HIV self‐testing was highly acceptable, increased male testing, almost universally facilitated couples jointly testing, identified a large number of discordant couples, yielded high disclosure of test results within couples, and resulted in high rates of linkage to care for HIV‐infected participants.
OAD0504
High acceptability of HIV self‐testing in an online randomized controlled trial for men who have sex with men in England and Wales
T.C. Witzel1, L. McCabe2, M.M. Gabriel2, P. Weatherburn1, D. Ward2, A.J. Rodger3, Y. Collaco Moraes2, C. Bonell1, R. Pebody4, J. Harbottle5, M. Gafos6, A. Speakman3, F. Lampe3, A.N. Phillips3, D.T. Dunn2, S. McCormack2, F.M. Burns3
1London School of Hygiene and Tropical Medicine, Department of Public Health, Environments and Society, London, United Kingdom, 2Medical Research Council Clinical Trials Unit, University College London, London, United Kingdom, 3University College London, Institute for Global Health, United Kingdom, 4NAM Aidsmap, London, United Kingdom, 5SH24, London, United Kingdom, 6London School of Hygiene and Tropical Medicine, Department of Global Health and Development, London, United Kingdom
Background: SELPHI is an online randomized controlled trial evaluating whether free HIV self‐testing (HIVST) increases the rate of HIV diagnoses compared to standard of care. SELPHI recruited 10,111 cis and trans men who have sex with men (MSM), =>16 years old, without diagnosed HIV, reporting lifetime anal sex and resident in England or Wales. We describe baseline characteristics and intervention acceptability.
Methods: Individuals, recruited through geo‐location hook‐up apps and social media, registered with SELPHI then completed an online enrolment survey before randomisation. Initial randomisation was to Baseline Test (BT) arm to receive one free HIVST kit (BioSURETM) or no HIVST kit (nBT). Post‐randomisation follow‐up surveys were sent at two‐weeks asking about kit use and at three‐months asking about kit use and intervention acceptability.
Results: 10,111 men were randomized (6049 BT; 4062 nBT); median age 33 years (IQR 26 to 44); 89% white; 20% born outside UK; <1% trans men; 47% degree educated; 15% never tested for HIV; 8% ever used PrEP; 4% currently using PrEP.
Of 6049 randomized to BT, 65% (n = 3895) completed a two‐week survey, by which time 96% (n = 3728) had received the kit and 84% (n = 3128) had used it. Kit use rose to 93% (n = 4262) after 3‐months. Men over the age of 46 were least likely to have used their kit, while those with lower educational qualifications and black ethnicity were most likely (Table 1).
Acceptability was high: 97% (3584/3682) found the instructions easy to understand, 97% (3538/3630) the test simple to use and 98% (3625/3687) reported an overall good experience.
Abstract OAD0504‐Table 1.
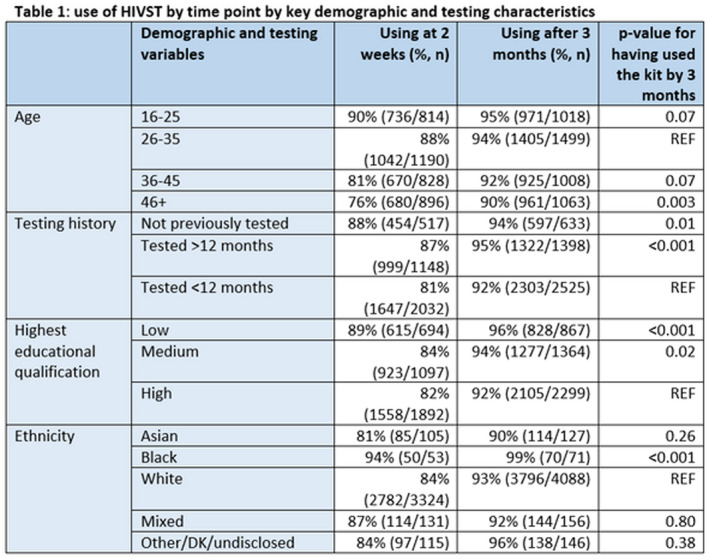
Conclusions: HIVST was acceptable and uptake substantial; the vast majority of participants reported using their kit by 3‐months. Encouragingly, men with least educational qualifications and those of black ethnicity were most likely to use their kits, ameliorating known health inequalities related to access to testing.
OAD0505
Approach to scale and optimize case finding to minimize the gap in UNAIDS first 90 target in Nepal
P.K. Thakur1, M. Cassell2, U. Shrestha1, Y.R. Sapkota1, D.P. Bhandari1, K. Bam1, A. Shrestha1, R.P. Khanal1, B. Shrestha1
1FHI 360, Kathmandu, Nepal, 2FHI 360, Vietnam, Vietnam
Background: The HIV epidemic in Nepal is concentrated among members of key populations who face both elevated infection risks and obstacles to accessing lifesaving HIV services. While 56% of all people living with HIV (PLHIV) in Nepal have an HIV diagnosis and are receiving treatment, an estimated 8000 to 9000 do not know their status. The USAID‐ and PEPFAR‐supported LINKAGES project in Nepal introduced index testing to both improve PLHIV support and focus testing services among individuals more likely to have had exposure to HIV.
Description: Over the course of a year, the project expanded capacities to implement index testing in 17 districts in Nepal. In the process of providing treatment support in community and clinical settings, index testing was offered to both newly and previously identified PLHIV. A provider‐facing tracking tool was developed to confidentially support and monitor successful referrals of the biological children and sexual and injecting partners of PLHIV to testing, treatment and prevention services. We analysed programme data through this tool to identify opportunities to improve beneficiary support and programme performance.
Lessons learned: From October 2018 to September 2019, index testing accounted for only 5% of the overall project‐supported testing volume (2835 of 54,518 individuals tested) but accounted for 44% of the number of individuals newly diagnosed with HIV (423 of 972). Of the individuals newly diagnosed through index testing, 87% (366 of 423) were successfully linked to treatment which accounted for 40% of total individuals linked to treatment (366 of 905). Index testing has distinguished itself as an efficient strategy to focus and enhance testing services to close gaps in ensuring 90% of all PLHIV know their status in Nepal.
Conclusions/Next steps: The relatively low volume of index testing compared to other testing approaches suggests important opportunities for further expansion. The project will continue this expansion, seeking guidance from both index clients and providers for ongoing improvement. In addition, the high case‐detection rate among the contacts of index clients suggests a need to prioritize prevention and HIV pre‐exposure prophylaxis (PrEP) support among these networks.
OAD0506
Improving HIV testing using a community‐based HIV+parenting programme in rural Lesotho: A cluster‐randomized controlled trial
M. Tomlinson1, M. Marlow1, S. Skeen1, S. Mofokeng1, M. Makhetha1, S. Gordon1, L. Sherr2, L. Cluver3
1Stellenbosch University, Institute for Life Course Health Research, Department of Global Health, Cape Town, South Africa, 2University College London, London, United Kingdom, 3University of Oxford, Oxford, United Kingdom
Background: Since 1990, the lives of 48 million children under the age of 5 have been saved because of increased investments in reducing child mortality. However, despite these unprecedented gains, more than 200 million children in low and middle income countries (LMIC) cannot meet their developmental potential. Lesotho has high levels of poverty, HIV and malnutrition, all of which affect child development outcomes.
Methods: In this cluster‐randomized trial, we assessed the effectiveness of a manualized, HIV+parenting intervention – a early‐years parenting plus intervention that included psychosocial stimulation, HIV testing and nutrition components. We randomly assigned 34 clusters (villages) to either the intervention or wait‐list control arm (17 clusters per arm). Participants within villages were caregiver‐child dyads, where the child was 12 to 60 months of age at the baseline assessment. The intervention consisted of eight weekly group sessions delivered at local village preschools, followed by a ninth top‐up session one month later. Thereafter, mobile health events were hosted in both intervention and control clusters, offering HIV testing and other health services to all community members. The primary outcome was child HIV testing rates, as reported by their caregivers.
Results: 1040 children and their caregivers (531 intervention; 509 control) were enrolled into the study. The intervention group showed higher child HIV‐testing at three months and one‐year post‐intervention. The intervention group showed improved child receptive language at three months and one‐year post‐intervention. The intervention group showed improved child language development at one‐year post‐intervention. Child attention did not differ significantly between groups.
Conclusions: Community‐based, integrated child health and development interventions, delivered to caregivers by trained community health workers, can improve targeted health behaviour, such as child HIV testing, and child language development one year after the end of intervention.
OAD0602
Prevalence, trends and correlates of HIV pre‐exposure prophylaxis (PrEP) use during sexual events by sexual minority men in Canada's three largest metropolitan areas
N. Lachowsky1,2,3, Z. Cui3, N. Bacani3, J. Sang3,4, A. Lal3, M. Messier‐Peet5, H. Apelian5, A. Parlette6, S.W. Noor6, S. Skakoon‐Sparling6, J. Jollimore2, D. Grace7, D.M. Moore3,4, G. Lambert5, J. Cox8, T.A. Hart, the Engage Study Team
1University of Victoria, Public Health & Social Policy, Victoria, Canada, 2Community Based Research Centre Society, Vancouver, Canada, 3British Columbia Centre for Excellence in HIV/AIDS, Vancouver, Canada, 4University of British Columbia, Vancouver, Canada, 5Direction Régionale de Santé Publique de Montréal, Montreal, Canada, 6Ryerson University, Toronto, Canada, 7University of Toronto, Toronto, Canada, 8McGill University, Montreal, Canada
Background: While most behavioural research uses individuals as the unit of analysis, sexual event‐level analyses provide more granularity. We examined prevalence, trends and correlates of sexual event‐level PrEP‐use among urban Canadian gay, bisexual and other men who have sex with men (gbMSM).
Methods: Beginning 02/2017, sexually active gbMSM (cisgender and transgender) ≥16 years of age were recruited into a prospective cohort study using respondent‐driven sampling (RDS) in Vancouver, Montreal and Toronto. Follow‐up data were collected every six to twelve months (depending on site) to 08/2019. At each visit, participants completed computer‐assisted self‐interview, including questions on up to their five most recent sexual encounters (different partners; past 6 months). Participants reported event‐level PrEP‐use for themselves and their partners. We used general estimating equations accounting for clustering by repeated visits and multiple events/participant to evaluate temporal trends (6‐monthly prevalence) and correlates of PrEP‐use. Multivariable models were built using backward selection to minimize QIC. Analyses applied RDS‐II weights.
Results: 2449 participants completed 4672 study visits and reported on 15,071 sexual events, of which 31.6% included event‐level PrEP‐use. There was a significant temporal increase in PrEP‐use (11.9% during 08/2016 to 02/2017 to 43.6% during 03/2019 to 08/2019, OR = 1.34, 95% CI:1.24 to 1.44). Overall, PrEP‐use was higher in Toronto (34.9%, AOR = 1.72, 95% CI:1.25 to 2.38) and Vancouver (42.3%, AOR = 1.86, 95% CI:1.34 to 2.57) compared with Montreal (22.9%). PrEP‐use varied by participant‐partner HIV status: 44.8% (n = 684/1526) if serodifferent, 42.6% if serosame (n = 3074/7208) and 18.7% (n = 1003/5356) if status unknown partner. PrEP‐use was positively associated with younger age (<30 vs. 45 + : AOR = 2.05, 95% CI:1.36 to 3.08), higher income (>$60,000CAD vs. <$30,000CAD: AOR = 1.52, 95% CI:1.10 to 2.08), postsecondary education (AOR = 1.75, 95% CI:1.08 to 2.81), one‐time versus romantic partner (AOR = 2.85, 95% CI:2.19 to 3.70), poppers‐use (AOR = 1.62, 95% CI:1.32 to 1.98) and expecting sex with that partner again (AOR = 1.50, 95% CI:1.23 to 1.82). PrEP‐use was negatively associated with Indigenous race/ethnicity (AOR = 0.12, 95% CI:0.03 to 0.41), bisexual identity (AOR = 0.34, 95% CI:0.20 to 0.59), living with HIV (AOR = 0.22, 95% CI:0.15 to 0.34) and condom‐use (e.g. condom‐protected receptive anal sex: AOR = 0.43, 95% CI:0.33 to 0.58).
Conclusions: Event‐level PrEP‐use increased threefold over the 2.5‐year study period, approaching half of sexual events in the final time period; publicly funded PrEP access varied over time, by jurisdiction (e.g. publicly funded in Vancouver in 01/2018). Given differences by geography and social determinants (i.e. income, education, race/ethnicity, sexual orientation), comparisons across jurisdictions should inform PrEP policy, service delivery and health promotion.
OAD0603
Trans‐forming PrEP in Vietnam: Rethinking service delivery to enhance access among transgender women
K. Green1, T. Tan Nguyen2, M.C. Hoang Vu3, T. Thi Tran1, T. Minh Thi Nguyen4, A.T. Duong5, T. Minh Ngo6, B. Ngoc Vu1, H. Ngo1, H.A. Doan1
1PATH, HIV & TB, Hanoi, Vietnam, 2My Home, Ho Chi Minh City, Vietnam, 3Vietnam Network of Transgender People, Hanoi, Vietnam, 4Vietnam Administration of HIV/AIDS Control, HIV & TB, Hanoi, Vietnam, 5Venus, Hanoi, Vietnam, 6USAID, Hanoi, Vietnam
Background: Although HIV prevalence among transgender women (TGW) who have sex with men in Vietnam is high (18%), awareness and uptake of PrEP is very limited. PrEP services were first made available in March 2017, and while there was a significant increase in PrEP enrolment among men who have sex with men and HIV sero‐discordant couples, enrolment among TGW remained consistently low with an average of 3.4 new enrolments monthly over the first year. A rapid assessment identified that TGW were worried that PrEP would reduce the efficacy of feminizing hormones, and/or lead to severe side‐effects.
Description: TGW leaders and the USAID/PATH Healthy Markets team co‐formulated and advanced three key actions: 1) directly addressing hormone‐PrEP drug interaction concerns through online content (primarily through a dedicated Facebook page), engagement with TGW peer experts (online and in‐person) and small events that enabled Q&A with TGW peer experts and health workers; 2) training PrEP clinic staff and community providers in transgender competent care (provided by Tangerine Clinic in Bangkok) 3) offering routine hormone level testing and counselling at PrEP clinics.
Lessons learned: Through these combined efforts, average monthly TGW PrEP enrolment increased to 25.7 new clients per month – a 7.6 fold increase pre‐intervention, as of the last quarter of measurement from September to December, 2019. TGW reported feeling more knowledgeable about PrEP and having greater confidence in taking it. However, while new enrolment increased substantially, early PrEP continuation at month three was 74% during the same time period, lower than the average. Continuation rate across all populations (84.7%).
Abstract OAD0603‐Figure 1.
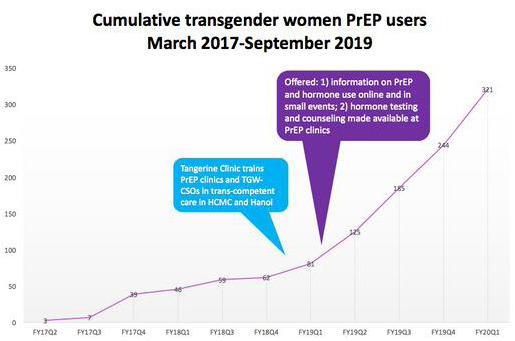
Conclusions/Next steps: To ensure PrEP is acceptable and accessible to TGW in Vietnam, services that aim to reach them need to offer trans‐competent care and make efforts to address underlying concerns about PrEP use. In addition, TGW who wish to remain on PrEP may need additional support than is currently offered.
OAD0604
HIV risk perception and salience are paradoxically associated with Pre‐Exposure Prophylaxis (PrEP) discontinuation among adolescent girls and young women in Lesotho
T. Chakare1, A. Rozario1, N. Nonyana1, J. Berg1, M. Strachan2, R. Heffron3
1Jhpiego, TSEPO, Maseru, Lesotho, 2Jhpiego, MER, Baltimore, United States, 3University of Washington, Epidermiology, Maseru, Lesotho
Background: Adolescent girls and young women (AGYW) are disproportionally infected with HIV in Lesotho with an annual incidence of 1.5% compared to 0.13% for their male counterparts. Despite the excellent protection provided by oral pre‐exposure prophylaxis (PrEP), 78% of new AGYW PrEP users in Lesotho discontinue PrEP in the first month of use.
Methods: We conducted a cross‐sectional survey of current PrEP clients and recent drop‐outs in three districts of Lesotho (Maseru, Berea, Leribe). An interviewer‐administered questionnaire assessed demographics, sexual behaviour, experiences with PrEP, depression symptoms using the PHQ‐9, lifestyle choices and a composite measure of salience and perceptions of HIV risk. Using univariate logistic regression, we identified factors associated with continued PrEP use and we adjusted statistically significant associations by age (18 to 21 or 22 to 24) which was determined a priori to be the most influential confounding factor.
Results: One hundred and ninety‐three (193) AGYW participated, of which 40 were new PrEP clients, 65 were continuing use without interruption, 72 had discontinued PrEP and not restarted and 16 were restarting. Of the discontinuers, only 12.5% felt they were no longer at risk of HIV infection, but none were “doing other things to prevent HIV infection”. Among the 72 who had discontinued, reasons reported for stopping varied: negative experience with provider (30.6%); side effects (19.4%); partner disapproval (5.6%); and, concern about being mistaken as HIV‐positive (5.5%). Discontinuers showed a higher prevalence of depressive symptoms (37.5% vs. 30.8, p = 0.4) and recreational drug use (5.6% v 1.5%, p = 0.2) than continuers, although not statistically significant. Having a higher HIV perception and salience score was associated with greater likelihood of being a discontinuer (median 9 vs. 7, p = 0.02) and remained significant after adjusting for age (p = 0.04).
Conclusions: The majority of AGYW (88%) stopped PrEP despite feeling they were still at infection risk due to a mix of service‐, product‐ and community‐level factors. HIV risk perception and salience were significantly higher among discontinuers than current users suggesting an unmet need among the former. Motivators other than risk need to be identified to compel AGYW to stay on PrEP, particularly those who are aware of their risk and attach importance to it.
OAD0605
Who is being diagnosed with syphilis while on PrEP in Brazil?
I. Ornelas Pereira1, C. Habsckot Dutra de Barros1, G.F. Mendes Pereira1, M. Araújo de Freitas1, A.R. Pati Pascom1
1Ministry of Health of Brazil, Department of Diseases of Chronic Condition and STI, Brasili, Brazil
Background: Preexposure prophylaxis (PrEP) is safe and highly protective against HIV when adherence is ideal. Considering that individuals who can benefit from PrEP are also likely to be at increased STIs risk, we aimed to describe the profile of users diagnosed with syphilis while on PrEP and predictors of diagnosis after the second follow‐up visit in the Brazilian daily dosing PrEP programme (BPP).
Methods: We used programmatic data from the Ministry of Health of Brazil, including individuals with two or more visits in BPP, between January 2018 and November 2019. We considered a syphilis diagnosis when it occurred on the second or any other subsequent visit. Multivariable logistic regression model was used to assess the likelihood of syphilis diagnosis while on PrEP considering demographic and behavioural predictors.
Results: Among 8566 enrolled PrEP users, 786 (9%) had a syphilis diagnosis at second visit or later. Median age of diagnosed users was 33 yo (IQR 28 to 39). Users from 40 to 49 years old were 48% more likely to be diagnosed for syphilis (aOR1.477,95% CI:1.100 to 1.983) as well as users who reported, in the first visit, having had STI symptoms in the past 6 months (aOR:1.486,95% CI:1.248 to 1.769). Compared to heterosexual cis men, transwomen were more than six times more likely to be diagnosed for syphilis (aOR:6046; 95% CI: 5.599 to 14.063), and more than 5 times for MSM (aOR:5.627;95% CI: 2.625 to 12.062).Users who reported, in the first visit, having more than 10 sexual partners were 109% more likely to be diagnosed (aOR 2.087,95% CI:1.673 to 2.603) than those who reported only one partner. No association was found with condom use frequency.
Abstract OAD0605‐Figure 1.
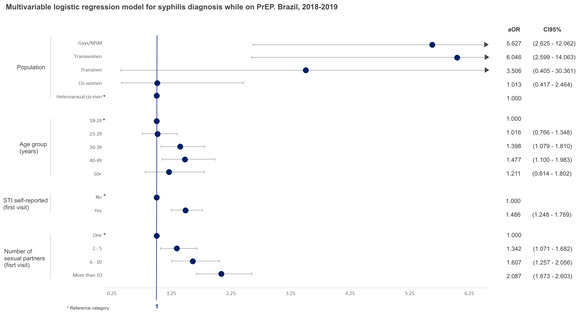
Conclusions: It is crucial to take advantage of PrEP services to improve diagnosis and treatment of other STIs. Therefore, understanding characteristics of users most‐likely to be diagnosed with syphilis while on PrEP may help health services to target the most affected populations with a more comprehensive “combination prevention” approach.
OAD0606
Just4Us: A theory‐based PrEP uptake intervention study for PrEP‐eligible women in two highly affected U.S. cities shows favourable PrEP‐use intentions but many barriers along the PrEP cascade
A.M. Teitelman1, H.V. Tieu2, A. Davis3, R. Lipsky1, C. Darlington1, E. Iwu4, B. Brawner1, P. Shaw3, K. Bond5, V. Frye6, B. Koblin3
1University of Pennsylvania, School of Nursing, Family and Community Health, Philadelphia, United States, 2New York Blood Center, New York City, United States, 3University of Pennsylvania, Philadelphia, United States, 4Rutgers University, New Brunswick, United States, 5New York Medical College, Health Behavior and Community Health, Hawthorn, United States, 6City University of New York School of Medicine, New York, United States
Background: Merely 2% of women eligible for PrEP take it in the U.S. We conducted an R34 feasibility pilot to assess preliminary efficacy of a theory‐based PrEP uptake intervention for women. Formative research guided intervention development.
Methods: Women ages 18 to 55 years were recruited from community sites (e.g. drug treatment, shelters), online and through participant referral. Eligibility criteria were consistent with U.S. guidelines for PrEP initiation. Based on the Integrated Behavioral Model and the Theory of Vulnerable Populations, the Just4Us (J4U) intervention included an in‐person, individually tailored, technology‐enhanced, 1 to 1.5 hour‐long information, motivation, skill‐building, problem‐solving and referral session with follow‐up phone calls to support linkage to community‐based PrEP care. The control arm (C) received a packet of handouts on PrEP facts, cost and PrEP providers. From 11/2018 to 10/2019, 83 women were enrolled and randomized 3:1 (61: J4U; 22: C). Participants completed baseline, immediate post‐intervention and three‐month follow‐up (3MFU) computer‐assisted surveys, which included some open‐ended questions. Descriptive analyses were conducted.
Results: At baseline: mean age was 37 years (SD:12); 79% were Black, 26% Latina, 83% had recent economic insecurity; 50% recent drug use. At 3MFU (90% retention, n = 75) there was a limited difference in having made an appointment to see a provider for PrEP (J4U: 25/54 [46%]; C: 9/21 [43%]); or PrEP initiation (J4U; 6/54 [11%]; C:2/21 [10%]) between study arms. Among those who had not yet initiated PrEP at 3MFU (n = 67), slightly more Just4Us participants (21/48; 44%) planned to start PrEP in next 3 months than control participants (7/19; 37%). Uptake barriers identified included: concern about PrEP side effects; perceived adherence inability; low perceived HIV risk; structural barriers, i.e. competing material priorities (e.g. housing, money, time, immediate health issues), provider discomfort with PrEP and/or insurance issues.
Conclusions: The biggest step‐off along the PrEP care cascade was between making an appointment for starting PrEP with their preferred provider and starting PrEP among both groups. Key personal and structural barriers were identified, notably limited provider PrEP knowledge. Just4Us shows promise as a woman‐focused PrEP‐uptake intervention. Next steps are intervention refinement based on these results and a study with a larger sample and longer follow‐up to assess the efficacy of Just4Us.
OAD0702
Effects of financial incentives for clinic attendance on HIV viral suppression among adults initiating antiretroviral therapy in Tanzania: A three‐arm randomized controlled trial
C. Fahey1, P. Njau2, E. Katabaro3, R. Mfaume2, N. Ulenga4, N. Mwenda5, P. Bradshaw1, W. Dow1, N. Jewell1, N. Padian1, S. McCoy1
1University of California, Berkeley, United States, 2Tanzania Ministry of Health, Community Development, Gender, Elderly and Children, Dodoma, Tanzania, United Republic of, 3Health for a Prosperous Nation, Shinyanga, Tanzania, United Republic of, 4Management and Development for Health, Dar es Salaam, Tanzania, United Republic of, 5Rasello, Dar es Salaam, Tanzania, United Republic of
Background: Several trials demonstrate that financial incentives promote retention in HIV care and may improve antiretroviral therapy (ART) adherence. However, few evaluations have assessed impacts on a related biological outcome, nor compared the effectiveness of different incentive sizes. Moreover, complex delivery mechanisms used in previous studies may prove difficult to implement in practice. We sought to determine the effects of small, automated financial incentives for clinic attendance on viral suppression (VS) among patients starting ART in Tanzania.
Methods: We conducted a three‐arm parallel‐group randomized controlled trial. At four clinics in Shinyanga region, we recruited HIV‐positive adults (≥18 years) who initiated ART ≤30 days prior. Participants were individually allocated (1:1:1) to usual care (control group) or to additionally receive a monthly cash incentive, conditional on clinic attendance, in one of two amounts: 10,000 TZS (approximately US $4.50) or 22,500 TZS (≈US $10.00). Cash transfers were delivered for up to six months via mobile health technology (mHealth), which monitored attendance and automatically disbursed mobile payments. We evaluated the relationship between incentive size and VS (<1000 copies/mL) at six months using logistic regression.
Results: From April 24 to December 14, 2018, we randomized 530 patients (184 control; 172 smaller incentive; 174 larger incentive). At six months, approximately 73.0% of participants in the control group remained on ART and achieved VS, compared to 82.9% in the smaller incentive group [risk difference (RD) = 9.9, 95% CI: 1.2 to 18.5] and 86.1% in the larger incentive group (RD = 13.1, 95% CI: 4.5 to 21.5); the incentive groups did not significantly differ (RD = 3.2, 95% CI: −4.6 to 11.0). Testing for trend showed a positive relationship between increasing incentive size and VS (OR = 1.10 per 2500 TZS, 95% CI: 1.03 to 1.17, p‐trend = 0.003), although the pairwise comparisons suggest a threshold effect. Improvements were additionally found for all pre‐specified secondary outcomes, including retention on ART, VS among those retained on ART and appointment attendance.
Conclusions: Small, automated financial incentives improved retention in care and viral suppression among adults starting ART in Tanzania. These findings strengthen the evidence for implementing incentives within standard HIV care.
OAD0703
An RCT in Zimbabwe found an intervention increased parental disclosure of HIV and improved parent, child and family outcomes
M. Lightfoot1, G. Kumalo‐Sakutukwa1, L. Pollack1, A. Chingono2
1University of California, San Francisco, United States, 2University of Zimbabwe, Harare, Zimbabwe
Background: Globally, HIV status disclosure remains a key challenge facing parents living with HIV (PLH), who especially face challenges in disclosing their status to their non‐adult children. Disclosure of one's status to family members is an important strategy for improving treatment and care outcomes. Robust studies are needed to test the impact of disclosure‐support on family outcomes in sub‐Saharan Africa where most families living with HIV reside. Our study examines the efficacy of an intervention to increase PLHs disclosure of their HIV status to their children and examines the impact of disclosure on family outcomes.
Methods: We conducted a randomized controlled trial with 326 families (one parent and one randomly chosen adolescent child aged 10 – 18 years) recruited from 19 health centres in Mutoko District (Zimbabwe). PLH were assigned to either an: 1) experimental condition, 3‐session disclosure intervention (n = 168) or 2) attention control condition, a 3‐session nutrition intervention (n = 158). The intervention's impact was assessed over 18 months (recruitment, 3, 6, 12 and 18 months). The culturally tailored experimental and control interventions were conducted only with the parents and were delivered by study nurses.
Results: PLH were predominantly female (79%) and had a mean age of 43.65. Children were 54% male and 48% were 10 to 13 years of age. Almost all PLH completed all 3 sessions of the intervention (89% disclosure, 93% nutrition) and 97% of parents and 94% of children completed the 18‐month follow‐up. Significantly more parents in the intervention condition (71.4%) reported disclosing to their child at the 3 month assessment compared to those in the control condition (26.8%; χ2 = 62.51, df = 1, p < 0.001). Overall, at 18 months, parents and children in the intervention arm had better outcomes over the nutrition only arm, including better psychological functioning, and parental health behaviour and coping, and fewer adolescent delinquent behaviours.
Conclusions: Our intervention provided families with important options for planning, care and support. Our study also provides guidance to organizations, such as ministries of health, on how to improve disclosure. Our findings lay the groundwork for future culturally tailored disclosure interventions.
OAD0704
Is reluctance to restart ART a risk of ATI trials?
G.E. Henderson1, T. Jupimai2, N. Ormsby1, S. Rennie1, R.J. Cadigan1, K. Kuczynski1, S.C. Isaacson1, N. Phanuphak3, E. Kroon3, J. Ananworanich4, D. Colby3, C. Sacdalan3, P. Prueksakaew3, T. Luekasemsuk3, H.L. Peay5
1University of North Carolina, Department of Social Medicine, Chapel Hill, United States, 2Chulalongkorn University, Center of Excellence for Pediatric Infectious Diseases and Vaccines, Bangkok, Thailand, 3Thai Red Cross AIDS Research Centre, Bangkok, Thailand, 4University of Amsterdam, Amsterdam, Netherlands, 5RTI International, Early Education, Disability, and Health Program, Durham, United States
Background: Controversies surround analytic treatment interruption (ATI) in HIV remission trials. ATI is presented as a risk during informed consent, but it has also been perceived by volunteers as a benefit. Further, some acutely diagnosed individuals perceive themselves to be particularly suited for ATI and the potential for long‐term remission. Given these findings, is it possible that trials with ATI unintentionally foster reluctance to resume ART?
Methods: From 2016 to 2019, we conducted longitudinal interviews with 54 participants in 4 HIV remission trials with ATI, assessing ATI experiences and attitudes about restarting ART. These trials recruited from the Thai SEARCH010 cohort, who are mainly male/MSM and diagnosed with acute HIV infection. For 34 participants in the two most recent trials, employing additional coding and thematic analyses, we explored factors that might predict reluctance to restart ART, including the primary reason for testing (after experiencing acute retroviral syndrome [ARS] or triggered by worry about a risky event), and ongoing side effects and/or psychosocial challenges with ART.
Results: Participants in all 4 trials described ATI as a way to challenge their bodies. At trials’ end, all but one participant experienced viral rebound. Response to their own rebound varied, from expecting this outcome, to regret about their “failed body.” Most were disappointed but reported adjusting quickly. We found no evidence of more negative attitudes about ART after ATI, nor did participants report having or anticipating additional problems with adherence. In contrast, many participants indicated that rebound confirmed the importance of ART for their health. Among the smaller group in the two most recent trials, we found no support for associations among perceived ARS symptoms at diagnosis, pre‐trial difficulties with ART and attitudes about restarting ART.
Conclusions: It would be a major concern for trials with ATI if participants did not restart their ART. Despite expectations that pre‐trial challenges with ARS and/or ART and experiences with ATI might impact ART resumption, we found no supporting evidence. In contrast, our data suggest that viral rebound during ATI may reinforce the need for ART adherence in acutely diagnosed individuals. Additional research in other remission trial populations is needed.
OAD0705
Psychosocial care bundles to improve the mental health of people living with HIV in Taiwan
S.‐P. Lin1, C.‐Y. Hsieh2, L.‐F. Chen2, S.‐P. Huang2, Y.‐H. Liu2, Y.‐F. Chen2, Z.‐Y. Shi1, Q. Ma3
1Taichung Veterans General Hospital, Internal Medicine, Infectious Disease, Taichung, Taiwan, Province of China, 2Taichung Veterans General Hospital, Department of Nursing, Taichung, Taiwan, Province of China, 3University at Buffalo, NYS Center of Excellence in Bioinformatics and Life Sciences Department of Pharmacy Practice, Buffalo, United States
Background: Depression and anxiety are among the most common comorbidities among people living with HIV (PLWH). These mental health complications are associated with suboptimal outcomes including high mortality. Evidence has suggested that psychological interventions could improve mental health, quality of life and HIV care outcomes. This study aimed to evaluate the effectiveness of bundle‐designed psychosocial interventions in the reduction of anxiety and depression in PLWH enrolled in the Taiwan VA system.
Methods: This prospective cohort study tested the effectiveness of a bundle‐designed psychosocial intervention package (HIV‐STAR) mainly including adherence, psychology, social support and individual case management. Anxiety and depression evaluation was performed using the Hospital Anxiety and Depression Scale (HADS) at the hospital admission and discharge. McNemar test was used to assess changes of HADS before and after HIV‐STAR and between HIV‐STAR and control. Logistic regression was performed to identify risk factors.
Results: Among the 97 PLWH enrolled after screening, 36% were positive for anxiety and 30% for depression. The overall incidence of anxiety and/or depression was significantly decreased from 46% at admission to 23% at discharge after HIV‐STAR with an average intervention duration of 14 days, (p < 0.001). The mean score of HADS was 11.2 (SD, 7.4) at the admission and 8.1 (SD, 6.2) at the discharge (p < 0.001). The historical control group without HIV‐STAR did not show marked improvement. In multivariate analysis, female sex (OR = 23.64; 95% CI, 1.43 to 392.26), current recreational drug use (OR = 3.07; 95% CI, 1.16 to 8.13) and risk group for HIV infection other than MSM (OR = 3.32; 95% CI, 1.02 to 10.86) were statistically significant associated factors with high HADS (≥8.0) at the discharge.
Conclusions: The high prevalence of mental health complications among PLWH in Taiwan underscores the importance of integrated psychosocial care. While the newly developed HIV‐STAR has provided an effective intervention to reduce anxiety and depression in general, more advanced psychosocial care bundles will be warranted to address specific risk factors for intervention‐resistant anxiety and depression.
OAD0706
Factors increasing use of sexual and reproductive health services among people living with HIV (PLH) in Peru. New needs and concerns from PLH and sexual and reproductive health providers
C. Sandoval1, J.‐P. Jirón1, J. Enciso1, C. Cáceres1
1Universidad Peruana Cayetano Heredia, Centro de Investigación Interdisciplinaria de Sexualidad, Sida y Sociedad, Lima, Peru
Background: The rise of biomedical prevention has minimized efforts in social/behavioural prevention in HIV/STIs and sexual and reproductive health (SRH), sometimes failing to provide a broad perspective for client‐provider interaction. To develop a Brief Sexuality Communication based on the Motivational Interviewing Model (MI) and Information, Motivation and Behavioral skills (IMB) framework, we conducted a formative study to assess the feasibility of implementing MI in public health facilities. We report findings for PLH.
Methods: 18 In‐depth‐interviews with providers, 23 Focus‐Groups with Key‐populations, including 6 FG with PLH. Goal was to identify and understand PLHs SRH needs and perceptions, including provider‐client interaction. Interviews were recorded, transcribed and analysed using the Dedoose qualitative software.
Results: PLH explained that a trust‐based interaction is crucial to attending SRH services. Otherwise, a discussion on sexuality would be impossible. They claimed providers must be sensitized on PLH issues, as some still blame them for their diagnosis based on prejudices about their sexual orientation, gender identity or sexual behaviour. Moreover, in other services than SRH they use old markers (“white code”) in their clinical records, leading to a stigmatizing treat. PLH expressed new SRH needs, they are concerned about to have children because sometimes providers tell them to avoid have children due to their diagnosis and their “life‐style”, women want to know about sexual consent and sexual/physical violence.
Providers lack training and sensitization on sexual‐diversity, gender‐identity and gender‐based violence. Their practice is still influenced by prejudices on sexuality stigmatizing PLH, so they avoid in‐depth discussions about sexuality. However providers seemed willing to implement intervention thinking it would help fulfil PLH clients’ expectations. Some realized that if people are treated with respect and care, they will demand SRH services.
Conclusions: Trust/friendly based interaction is crucial in a client‐provider interaction where PLH can establish effective sexual/SRH communication. Sometimes their experiences suggest a degree of discrimination based on a stigmatized PLH condition. New PLH needs on SRH appear related to sexual and gender issues that must be address in public health system. Providers’ efforts and dedication are valued, but it depends on each professional's ability to establish empathy and trust.
OAD0802
Predictors of attempted suicide among youth living with perinatal HIV infection and perinatal HIV exposed uninfected peers
P. Kreniske1, C. Mellins1, C. Dolezal1, S. Espinel1, C.‐S. Leu2, P. Fisher3, J. Raymond1, R. Robbins1, N. Nguyen1, A. Wiznia4, E. Abrams5
1HIV Center for Clinical and Behavioral Studies at Columbia University and New York State Psychiatric Institute, New York, United States, 2Mailman School of Public Health, Columbia University, New York, United States, 3New York State Psychiatric Institute, New York, United States, 4Albert Einstein College of Medicine, Jacobi Medical Center, Bronx, United States, 5ICAP at Columbia University, Mailman School of Public Health, New York, United States
Background: Suicide is a global crisis and attempted suicide is a leading risk factor for completed suicide. Our recently published analysis from a longitudinal study (CASAH) of youth living with perinatally acquired HIV infection (YLPHIV) and perinatally HIV‐exposed but uninfected peers (YPHEU), showed significantly more YLPHIV attempted suicide versus YPHEU (24% vs. 13%). To inform preventive interventions for these populations, we examined psychosocial and sociodemographic predictors of attempted suicide.
Methods: YLPHIV and YPHEU participants in CASAH were recruited from four medical centres in New York City (n = 340; mean age 12.5 years at baseline) and interviewed every 12 to 18 months with 7 follow‐ups (FU) to date (mean age 24.5 years at FU 7). We compared youth who did and did not report a suicide attempt on a structured psychiatric interview at any FU on the following baseline variables; gender, sexuality, race, ethnicity, age, HIV status, city stress inventory score (CSI), negative stressful‐life events, spirituality, social problem‐solving inventory score, Tennessee self‐concept score (TSCS), Child Depression Inventory (CDI) and among only YLPHIV, HIV‐stigma – measured by the Social Impact Scale. We used two backward stepwise logistic regression models, one for the overall sample, and one for only YLPHIV, and each model predicted lifetime suicide attempt with baseline demographic and psychosocial variables.
Results: At baseline, 51% of participants were female, 65% Black and 42% Latinx. In the overall sample those who attempted suicide at any FU were more likely to: be YLPHIV (Adjusted Odds Ratio (AOR) 1.96 95% CI 1.06 to 3.62), Black (AOR = 3.00, 95% CI 1.35 to 6.69), Latinx (AOR = 2.88, 95% CI 1.29 to 6.40); have lower family self‐concept (AOR = 0.37, 95% CI 0.21 to 0.65); better social self‐concept (AOR = 1.85, 95% CI 1.17 to 2.93); and higher depression scores (AOR = 1.06, 95% CI 1.00 to 1.13). In the second model, among only YLPHIV, attempted suicide was associated with lower personal self‐concept (AOR = 0.33, 95% CI 0.15 to 0.71), less spirituality (AOR = 0.42, 95% CI 0.20 to 0.90) and greater HIV stigma (AOR = 3.18, 95% CI 1.06 to 9.52).
Conclusions: Our analyses indicate mental health services should address YPHEU and YLPHIV self‐concept and depression and stigma for YLPHIV. In addition, we see an urgent need for routine integration of suicide risk assessment into treatment for YLPHIV.
OAD0803
Younger initiation of selling sex and depressive symptoms among female sex workers in eSwatini
A. Grosso1,2, R. Fielding‐Miller3, S. Matse4, Z. Mnisi4, B. Sithole5, S. Baral6
1Rutgers Institute for Health, Health Care Policy and Aging Research, New Brunswick, United States, 2Rutgers School of Public Health, Urban‐Global Public Health, New Brunswick, United States, 3University of California, Department of Medicine, San Diego, United States, 4Ministry of Health, Mbabane, Eswatini, 5Health Communication Capacity Collaborative, Mbabane, Eswatini, 6Johns Hopkins Bloomberg School of Public Health, Center for Public Health and Human Rights, Baltimore, United States
Background: Youth who sell sex likely have complex mental health needs that may persist into adulthood and potentiate HIV transmission and acquisition risks. Preliminary evidence from female sex workers (FSW) in Malawi suggests negative mental health outcomes are more common among those initiating sex work as minors (MacLean, et al. 2018), but additional research is needed on this understudied topic in Africa.
Methods: FSW aged 18 + recruited through venue‐based sampling from October‐December 2014 in eSwatini completed a survey including a question about the age at which they started selling sex and the Patient Health Questionnaire (PHQ‐9) to measure depressive symptoms. Younger initiators were defined as those who started selling sex prior to age 18. Bivariate and multivariable logistic regression analyses were conducted to assess associations.
Results: 16.62%(128/770) of FSW with complete data on the PHQ‐9 and age of initiation started selling sex as minors. Younger initiators had higher mean and median PHQ‐9 scores and greater depression severity (Table 1). The prevalence of probable depression (PHQ‐9 score ≥10) was 55.47%(71/128) among younger initiators, compared to 40.65%(381/642) among older initiators (p = 0.002). Younger initiators were more likely to have probable depression (aOR 1.56;95% CI 1.03 to 2.37; p = 0.037) after controlling for number of years selling sex, days per month selling sex, frequency of past‐month condom failure and anticipated healthcare stigma. Being orphaned before age 18 and carrying condoms less often were significantly associated with younger initiation but not probable depression. FSW who started selling sex to feed themselves or their families and those who did not know their HIV status were more likely to have probable depression, but these factors were not correlated with younger initiation.
Abstract OAD0803‐Table 1. Depression severity by younger or older age of initiation of selling sex among female sex workers in eSwatini, 2014
| PHQ‐9 score | Severity % (n) | ||||||
|---|---|---|---|---|---|---|---|
| Median | Mean | Minimal [0 to 4] | Mild [5 to 9] | Moderate [15 to 19] | Moderately severe [15 to 19] | Severe [20 to 27] | |
| Started selling sex < 18 (n = 128) | 11 | 10.45 | 20.31 (26) | 24.22 (31) | 30.47 (39) | 14.84 (19) | 10.16 (13) |
| Started selling sex 18 + (n = 642) | 8 | 8.93 | 28.82 (185) | 30.52 (196) | 20.72 (133) | 10.59 (68) | 9.35 (60) |
| Total (n = 770) | 9 | 9.18 | 27.40 (211) | 29.48 (227) | 22.34 (172) | 11.30 (87) | 9.49 (73) |
Abstract OAD0803‐Table 2 Associations between probable depression and younger initiation of selling sex among female sex workers in eSwatini, 2014
| Dependent variables ↓ | Independent variables→ | Selling sex < 18 | Probable depression | Current age | Orphaned < 18 | Number of years selling sex | Days per month selling sex | Number of times condom slipped off or broke in the last month | Frequency of carrying condoms when selling sex | Ever afraid of or avoided seeking healthcare due to fear of someone learning they sell sex | Does not know her HIV status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Selling sex < 18 | Odds ratio (95% Confidence Interval) | – | 1.82 (1.24, 2.67)* | 0.87 (0.83, 0.91)* | 3.38 (1.89, 6.01)* | 1.09 (1.05, 1.13)* | 1.03 (1.00, 1.05)* | 1.13 (1.01, 1.25)* | 0.76 (0.62, 0.93)* | 1.66 (1.11, 2.50)* | 1.34 (0.81, 2.19) |
| Adjusted odds ratio (95% Confidence Interval) | – | 1.54 (1.02, 2.34)* | ‐ | ‐ | 1.09 (1.05, 1.13)* | 1.02 (0.99, 1.05) | 1.05 (0.94, 1.18) | ‐ | 1.41 (0.90, 2.20) | ‐ | |
| Probable depression | Odds ratio (95% Confidence Interval) | 1.82 (1.24, 2.67)* | – | 1.00 (0.98, 1.03) | 0.89 (0.51, 1.55) | 1.03 (1.00, 1.06)* | 1.02 (1.00, 1.04)* | 1.28 (1.15, 1.42)* | 0.89 (0.75, 1.07) | 3.26 (2.32, 4.58)* | 2.51 (1.68, 3.77)* |
*p < 0.05.
Conclusions
Depression among FSW in eSwatini was highly prevalent and linked to experiences of selling sex before age 18. Scaling up mental health interventions in the African context is needed for this key population.
OAD0804
Designing for hope: Addressing adherence by looking beyond the pill. A co‐creation approach to addressing multidimensional factors that impact ART adherence and retention among adolescents living with HIV in Mozambique
S. Carey1, B. Kolada1, B. Olmedo1, C. Shadwick1, H. Singhal1, J. Falcao2, E.J. Abrams2,3, L. Weinstein1
1IDEO.org, New York, United States, 2ICAP at Columbia University, Mailman School of Public Health, New York, United States, 3Vagelos College of Physicians & Surgeons, Columbia University, New York, United States
Background: Retention rates, adherence to antiretroviral treatment (ART) and viral suppression are alarmingly low among adolescents living with HIV (ALHIV), who are often poorly equipped to manage their disease during a time of rapid physical and psychological development. The CombinADO study aims to develop and test an adolescent‐focused intervention to improve 90‐90‐90 targets among ALHIV in Mozambique. Formative work led by IDEO.org employed human‐centred design (HCD) — a novel methodology that involves co‐creating solutions with adolescents and testing them through rapid prototyping.
Methods: In late 2019, IDEO.org led a 4‐month co‐creation and rapid prototyping process in and around 2 health facilities (HF) in Nampula, Mozambique. The HCD approach began with design research and co‐creation activities — including individual interviews and focus groups using interactive methods like card sorting and storytelling. Findings were synthesized to inform the development of potential interventions. During the prototyping phase, the team sought to learn about effective strategies for engaging ALHIV and HF staff, collect further feedback from ALHIV to iterate the interventions and test variations and combinations of the interventions.
Results: The team interviewed 52 participants during the initial phase, which yielded 13 key insights used to inform potential interventions. Ninety‐six participants tested 12 potential interventions during the rapid prototyping phase. The most critical finding was that social support alone is not sufficient to drive adherence among ALHIV. The journey of adherence relies on a more intrinsic foundation: hope for a future worth living for. In order to shift pill‐taking behaviour among adolescents, results suggested that ALHIV require support with three key inputs: contextually appropriate and culturally connected medical literacy; an increased sense of belonging through peer connection; and ongoing demystification and destigmatization messaging within the wider community.
Conclusions: HCD research allows for deep insight into the motivations, experiences and needs of young people that impact adherence and retention among ALHIV. Co‐creation builds trust among ALHIV and engages them in shaping the solutions that will be available to them—building ownership and confidence. Prototyping allows for multiple iterations before implementation—maximizing learning and improvements prior to a pilot investment.
OAD0805
Factors associated with poor adherence among non‐virological suppressing school going adolescents: Lessons from The Aids Support Organization (TASO) in Masaka, Uganda
G. Jjuuko1
1The AIDS Support Organization (TASO), Community Systems, Kampala, Uganda
Background: According to the 90‐90‐90 UNAIDS ambitious target by 2020, viral load suppression is key among patients on antiretroviral therapy (ART). Whereas there is a growing number of people on ART, limited information is known about virological non‐suppression and its major determinants among HIV‐positive school going adolescents enrolled in many resource‐limited settings. We investigated the factors leading to poor adherence among adolescents with non‐suppressed viral load attending the Adolescent HIV/AIDS care clinic at The Aids Support Organization (TASO) in Masaka.
Description: Between January and December 2017, we identified adolescents with non‐suppressed viral load attending the HIV clinic specifically those in upper primary and secondary school. Blood samples were taken to the central government laboratory for analysis and non – virological suppression was considered as having ≥1000 copies/mL of blood. A six‐month viral load testing interval followed by three months repeat for the non ‐suppressors was the selection criteria. Through one on one and group counselling by trained counsellors, we identified adolescent with poor adherence (below 95%) to explore the causes.
Adolescents were grouped in age ranges of 10 to 13, 14 to 17 and 18 to 19 years, respectively, and to each group a trained counsellor, clinician and adolescent peer educator was attached to facilitate intensive adherence counselling. Information on social demographic characteristics and causes of poor adherence was collected using an interview guided questionnaire, data were analysed using Stata 14.
Lessons learned: Out of 355 adolescents on ART, 325 (91.8%) had their viral loads taken; 127 (39%) had non‐ suppressed viral load, of which 47 (37%) were boys and 80 (63%) were girls. 17 (13.4%) of the non‐suppressors had adherence above 95%, 110 (86.6%) had adherence below 95%. Reasons for non‐adherence were; 54 (42.5%) joined a candidate class for National promotional exams, 20 (15.7%) changed care takers, 17 (13.4%) joined a new school, 15 (11.8%) joined boarding school, 13 (10.2%) took a self‐drug holiday, 8 (6.3%) missed morning doses and 119 (94%) of all had not disclosed to any one at school.
Conclusions/Next steps: Non‐disclosure among School going adolescents is the leading cause of poor adherence hence there is need for interventions that promote disclosure.
OAD0806
Adolescent HIV research participation in low‐ and middle‐income countries: Ethical challenges and solutions from seven countries and a scoping review
S. Day1, B.G. Kapogiannis2, T.D. Ruel3, S.K. Shah4,5, E.C. Wilson6,7, D.F. Conserve8, J.D. Tucker1,9,10
1University of North Carolina at Chapel Hill, Institute for Global Health and Infectious Diseases, Chapel Hill, United States, 2Eunice Kennedy Shriver National Institute of Child Health and Human Development, Maternal and Pediatric Infectious Diseases Branch, Bethesda, United States, 3University of California, Division of Pediatric Infectious Diseases and Global Health, Department of Pediatrics, San Francisco, United States, 4Northwestern University Feinberg School of Medicine, Department of Pediatrics, Chicago, United States, 5Ann & Robert H. Lurie Children's Hospital of Chicago, Mary Ann & J. Milburn Smith Child Health Research, Outreach, and Advocacy Center, Stanley Manne Children's Research Institute, Chicago, United States, 6University of California, Department of Epidemiology and Statistics, San Francisco, United States, 7San Francisco Department of Public Health, Trans Research Unit for Equity (TRUE), San Francisco, United States, 8University of South Carolina, Department of Health Promotion, Education, and Behavior, Columbia, United States, 9University of North Carolina at Chapel Hill, School of Medicine, Chapel Hill, United States, 10London School of Hygiene and Tropical Medicine, Faculty of Infectious Diseases, London, United Kingdom
Background: Countries have varying ethical and legal guidance for adolescent participation in HIV research. Parental permission requirements effectively exclude some groups of adolescents from research. Excluding youth with or at risk of acquiring HIV can have the unintended consequences of limiting access to innovative prevention and care, and impairing progress in addressing the epidemic in low‐ and middle‐income countries (LMICs). We examined ethical and practical challenges in adolescent research participation across seven LMICs and potential solutions for including adolescents in HIV‐related research in LMICs.
Methods: We report lessons from the field for seven countries with adolescent HIV studies in the PATC3H consortium. Supported by the U.S. NIH Eunice Kennedy Shriver National Institute of Child Health and Development, the consortium comprises HIV prevention and treatment studies among adolescents in Brazil, Kenya, Mozambique, Nigeria, South Africa, Uganda and Zambia. We describe the ethical‐legal frameworks for adolescent research participation in these countries and their associated ethical and practical challenges. PATC3H researchers reviewed seven scenarios to clarify these ethical‐legal considerations. Finally, we conducted a scoping review to supplement PATC3H experiences on strategies to enhance adolescent participation in LMIC HIV studies.
Results: Consortium researchers identified many ongoing challenges, including limited guidance for determining whether adolescents can consent to research without parental permission, regulations that fail to account for the complexity of adolescent‐lived experiences (e.g. key population identities, related stigmas), and exclusion of many adolescents under 18 years old. We identified several strategies to enhance adolescent participation in LMICs’ HIV studies, including adolescent independent consent, selective waiving of parental consent and surrogate decision‐making. Independent consent and waiving of parental consent under select study conditions can enhance participation among at‐risk adolescents, including sexual and/or gender minorities. Additionally, surrogate decision‐makers (i.e. individuals providing consent in place of a parent or guardian) can be beneficial when parental/guardian involvement may be inappropriate or unavailable. Each of these solutions has been implemented in resource‐constrained settings and helped to broaden adolescent participation.
Conclusions: Despite multiple barriers and uncertainties, we identified several practical strategies to enhance ethical participation of adolescents in LMIC HIV studies. This analysis supports the feasibility of expanding adolescent HIV research in LMICs.
OAD0902
Challenges faced by a population ageing with HIV: Baseline data from the CORE healthy ageing initiative (CHAI)
O. Adeyemi1, A. Cameron1, S. Porter1, J. Barnes1, J. Catrambone1, CHAI 2.0 study group
1Cook County Health, Chicago, United States
Background: As people with HIV (PWH) age, there are increasing medical and psychosocial comorbidities that impact quality of life (QOL). The CORE healthy ageing initiative 2.0 (CHAI 2.0) was designed to better understand and address the medical and psychosocial needs including social isolation among PWH > 60 years receiving care at the CORE centre, Chicago.
Methods: Between 2/1/19 and 12/17/19, a needs assessment survey was distributed to PWH > 60 years identified during clinic visits by CHAI peer navigators (ages 62 and 65). We present the cross‐sectional analysis on 331 PWH who completed CHAI 2.0 baseline survey.
Results: 415 PWH > 60 years with a median age of 64 years (60—82) were surveyed. Seventy percent (n = 287) were male, 30% female. Eighty‐three percent (n = 339) were African American, 9% white and 6% Hispanic/Latino. Seventy‐nine percent (n = 318) had been diagnosed with HIV > 10 years; 52%>20 yrs. Eighty‐two percent reported undetectable viral loads. 24% reporting taking > 6 medications daily. current smoking was 37%, hypertension 50%, depression 30%, hyperlipidaemia 25%, diabetes 20% and kidney disease 14%. Thirty percent had > 1 fall in the last 12 months. Fifty‐five percent lived alone, 41% reported feeling lonely sometimes in the last month. Self‐rated good/excellent in 57% and 37% had concerns about getting older with HIV. The top five concerns were money concerns (41%), living with HIV (37%), other medical concerns (30%), housing (29%) and who will care for me in old age (24%). Other concerns: memory issues (23%), stigma (21%), retirement planning (18%), loneliness (14%), finding a partner (14%), sexual health (14%) and mental health concerns (13%). 22% reported had not disclosed their HIV status to anyone outside of clinic staff.
Abstract OAD0902‐Table 1.
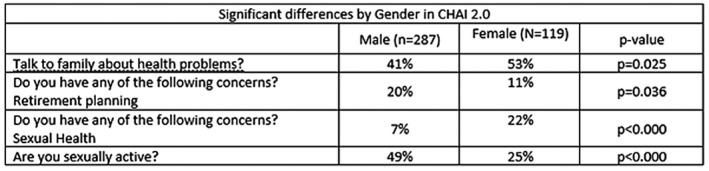
Conclusions: Among older, predominantly African American PWH, 55% lived alone. The interplay of polypharmacy, social isolation and comorbidities increase the risk of falls and other adverse outcomes. Programmes addressing these issues remain important to optimizing QOL in PWH.
OAD0903
Clinical and sociodemographic characteristics associated with poor self‐rated health across multiple domains among older adults living with HIV
D. Short1, F. Spinelli2, C. Okoli3, P. De Los Rios4
1ViiV Healthcare, Innovation and Implementation Science, London, United Kingdom, 2ViiV Healthcare, Medical Affairs North America, Research Triangle Park, United States, 3ViiV Healthcare, Global Medical Team, London, United Kingdom, 4ViiV Healthcare, Global Medical Sciences, Research Triangle Park, United States
Background: Efforts to improve the wellbeing of older adults have sometimes focused on a single aspect of health; holistic approaches must however consider all aspects. We characterized older persons living with HIV (PLHIV) who consistently reported sub‐optimal health on multiple domains.
Methods: The 24‐country 2019 Positive Perspectives survey included 648 PLHIV aged ≥50 years. Self‐rated health was assessed across four health domains (physical/mental/sexual/overall), each of which was dichotomized as optimal (“Good”/“Very good”) or sub‐optimal (“Neither good nor poor”/“Poor”/“Very poor”). We tallied the number of domains suboptimal health was reported (overall domain also included to account for unmeasured subdomains e.g. intellectual/emotional). Multinomial logistic regression among all older adults (n = 648) measured for associations between health domains and various sociodemographic/clinical characteristics including past ART drug‐drug interactions (DDIs), resistance, side effects, adherence and polypharmacy (≥5 pills/day or taking medicines for ≥5 conditions), adjusting for gender and disease duration (p < 0.05).
Results: Median disease duration was 19 years. Overall, 82.7% reported ≥1 comorbidity, 54.6% polypharmacy, 10.8% past DDI, 16.7% past resistance and 7.7% were very treatment‐experienced (changed ART ≥4 times, including ≥once in past year because of resistance/poor tolerability). Common co‐morbidities were hypertension (32.4%), hypercholesterolaemia (30.4%), mental illness (26.5%) and insomnia (24.4%). Overall, 45.4% (294/648) reported suboptimal physical health, 39.4% (255/648) suboptimal mental health, 61.7% (400/648) suboptimal sexual health and 47.2% (306/648) suboptimal overall health; Within mutually exclusive groups, 24.1% (156/648) reported suboptimal health on all domains. 24.4% (158/648) reported optimal health on all domains, 22.7% (147/648) on three domains only, 11.9% (77/648) on two domains only and 17.0% (110/648) on one domain only. The strongest predictors of reporting sub‐optimal health on all domains included having ≥2 comorbidities (AOR = 10.24, 95% CI = 4.85 to 21.63), being dissatisfied with treatment (AOR = 9.83, 95% CI = 5.12 to 18.86), missing ART for ≥5 days/past month (AOR = 7.52, 95% CI = 3.52 to 16.07) and experiencing gastrointestinal ART side effects (AOR = 6.72, 95% CI = 3.48 to 12.99).
Conclusions: One‐quarter of older adults reported suboptimal health on all domains; groups at greatest risk included those reporting poor adherence, polypharmacy, gastrointestinal side effects and treatment dissatisfaction. Treatment optimization as part of holistic care may improve overall wellbeing.
OAD0904
Killing two birds with one stone – Responding to health challenges of the elderly living with HIV at AIDS Information Centre
I. Nume1, H. Kizito2
1AIDS Information Centre, Prevention, Kampala, Uganda, 2AIDS Information Centre, Programing, Kampala, Uganda
Background: Global HIV/AIDS statistics (UNAIDS 2018) estimated 37.9 million people were living with HIV with an estimated 3.6 million aged 50 years or older (UNAIDS 2013). The majority of these (2.9 million) are in low‐and middle‐income countries where the percentage of adults 50 years or older living with HIV is above 10%. In high‐income countries almost one‐third of adults living with HIV are 50 years or older.
Elderly patients attending ART clinics have faced a number of challenges related to their HIV status although there have been limited interventions to address them. In sub‐Saharan Africa this group has particularly been neglected despite the distinctive healthcare and socio‐economic needs.
Description: The elderly clinic was started (July 2019) in response to challenges identified during a support group meeting for the elderly living with HIV specifically to address HIV‐related issues and promote screening and management of non‐communicable diseases (NCDs). This clinic was composed of clients aged 50 years and above. Only elderly clients had visits scheduled on Fridays in order to reduce waiting time and allow adequate time for psychosocial support and comprehensive clinical reviews. Staff were sensitized to periodically update the list of elderly clients and the screening and psychosocial support these clients required.
Lessons learned:
● From July to December 2019 the number of elderly clients in the clinic increased from 118 (69 males, 49 females) to 130 (75 males, 55 females).
● Through basic screening we were able to newly identify clients with diabetes (7), hypertension (10), prostate disorders (3), mental illnesses namely depression (20), anxiety disorders (1) and mild dementia (15). Other ailments previously undocumented included arthritis and erectile dysfunction.
● Most clients could not afford some of the screening tests and where medically advised were referred to public facilities. However, the majority did not go because of socioeconomic reasons.
● Health workers lack knowledge about geriatric care and NCDs therefore not all clients are comprehensively screened.
Conclusions/Next steps: The elderly living with HIV require:
● Health workers trained to adequately respond to their health challenges.
● Provision of subsidized/no cost comprehensive screening and standard healthcare packages
OAD0905
Assessing the factor structure and psychometric properties of the HIV/AIDS resilience assessment tool in a sample of NYC‐based HIV‐positive gay men aged 50 to 69: The Gold studies
K. Krause1,2, P. Halkitis3,2
1Rutgers University School of Public Health, Social and Behavioral Sciences, Newark, United States, 2Center for Health, Identity, Behavior and Prevention Studies, Newark, United States, 3Rutgers University School of Public Health, Departments of Biostatistics and Urban‐Global Health, Piscataway, United States
Background: By 2025, people over 50 will constitute the majority of those living with HIV/AIDS (PLWHA) in the United States and similar projections are expected globally within the next decade. This ageing population of PLWHA face different physical, mental and psychosocial health challenges related to living with HIV/AIDS, the general ageing process and the long‐term impact of being on antiretroviral treatment. Emergent literature suggests that resilience may act as a buffer to the negative impact of these myriad challenges. However, measuring resilience among PLWHA has been inconsistent. Given the variance of understanding and conceptualizing resilience in PLWHA, theoretically designed and validated instruments are needed specifically within the lens this population. To address this gap in the literature, we developed and examined the initial factor structure and psychometric properties of the 10‐item HIV Resilience Assessment Tool (H‐RAT).
Methods: Data for the present cross‐sectional study are drawn from n = 250 gay HIV‐positive men aged 50 to 69 living in New York City. Participants were sociodemographically diverse with regard to race/ethnicity, SES, age and education. Exploratory (EFA) and Confirmatory Factor Analyses (CFA) along with tests of reliability and validity were conducted in this sample.
Results: Results from the EFA indicated that a three‐factor model was the most parsimonious solution based on eigenvalues and model fit. The items were examined for their underlying relationships and the three factors were labelled: adaptive coping, optimism and effective coping. Taken together, the 10 items produced a Cronbach's alpha of 0.84 with the three subscales producing a Cronbach's alpha of at least 0.72. Convergent and discriminant validity were established using other psychosocial (e.g. grit, loneliness, etc.) and physical (e.g. BMI and blood pressure) outcomes.
Conclusions: The H‐RAT is a psychometrically sound instrument to assess resilience among PLWHA. With three subscales compromising the H‐RAT (adaptive coping, optimism and effective coping) the multidimensional tool can be used in future research and clinical settings. Looking forward, we recommend continued testing in different populations of PLWHA to ascertain its stability within different groups, geographic locations and over time. The H‐RAT will help clinicians, researchers and practitioners move towards a more holistic strengths‐based approach to working with PLWHA.
OAD0906
“Is it HIV or just old age?” Uncertainties of “successful” ageing with HIV
C. Howard1, L. Fitzgerald2, A. Mutch2
1Queensland Positive People, Brisbane, Australia, 2University of Queensland, Brisbane, Australia
Background: Globally, the population of people living with HIV is ageing. In Australia, around half of all people living with HIV (PLHIV) are now over 50. Understanding “successful ageing” for PLHIV is a critical question for researchers, HIV communities and policy makers.
Methods: Living Positive in Queensland (LPQ), is a participatory qualitative longitudinal study examining ageing in people living long term with HIV. LPQ, one of the largest research projects of its kind to be undertaken internationally, interviewed 73 participants annually over three years. Inductive thematic analysis was used to draw themes from over 200 interviews. This presentation discusses participants’ perceptions and experiences of ageing.
Results: Participants described uncertainty about ageing, expressing ambivalence in the face of debates surrounding adverse HIV ageing discourses and unknown futures. Alongside uncertainties about health and increasing comorbidities, participants described uncertainty about social determinants of “successful ageing.” Older participants, particularly those from the Pre‐HAART era, experienced cumulative disadvantage related to disrupted employment trajectories, limited resources, long‐term welfare access and limited social support arising from service cuts and the corresponding fracturing of communities. These issues generated worries about living and ageing in disadvantage.
Care for older people was often considered synonymous with residential aged‐care. Having experienced stigma and discrimination in healthcare settings, many were concerned about discrimination in aged‐care settings and worried the aged‐care sector would not respond to the needs of PLHIV. Some participants described “back up plans” of treatment non‐adherence when confronted with accessing aged‐care.
Conclusions: Ageing with HIV is biosocial, lived within diverse intersections of embodied experiences of HIV, generational, social and locational contexts. “Successful ageing” as it is currently portrayed in the broader ageing literature must move beyond individual actions and acknowledge the role of social determinants of health. HIV and ageing literacy; quality and culturally competent aged‐care services; and coordination and partnership between the aged‐care sector and HIV communities are urgently needed. The presentation will consider how policy and programme responses must integrate these elements in the development of services to move beyond the biomedical to address the social aspects of health and support “Healthy ageing” for PLHIV.
OAE0102
The effectiveness of implementing universal HIV treatment: A regression discontinuity analysis from Zambia
A. Mody1, I. Sikazwe2, I. Eshun‐Wilson1, A. Mwila3, L. Mulenga4, M. Herce2,5, K. Mweebo3, P. Somwe2, M. Wa Mwanza2, T. Savory2, K. Sikombe2, L. Beres6, J. Pry2,1, C. Holmes7, C. Bolton‐Moore2,8, E. Geng1
1Washington University in St. Louis, Division of Infectious Diseases, St. Louis, United States, 2Centre for Infectious Diseases Research in Zambia, Lusaka, Zambia, 3U.S. Centers for Disease Control and Prevention, Lusaka, Zambia, 4Zambia Ministry of Health, Lusaka, Zambia, 5University of North Carolina, Division of Infectious Diseases, Chapel Hill, United States, 6Johns Hopkins University School of Public Health, Baltimore, United States, 7Georgetown University, Center for Global Health and Quality, Washington, D.C., United States, 8University of Alabama, Division of Infectious Diseases, Birmingham, United States
Background: Universal treatment for all persons living with HIV (PLWH) has only been assessed under experimental conditions in cluster‐randomized trials, but the public health effectiveness of actually implementing treat‐all policies on the HIV care cascade under real‐world conditions is not known.
Methods: We used a regression discontinuity design (RDD) to assess the real‐world effectiveness of Zambia's January 1, 2017 adoption of universal HIV treatment. Using data from Zambia's routine electronic medical record, we analysed ART‐naïve adults newly enrolling in HIV care between January 1, 2016 to December 31, 2018 at 58 clinics supported by CDC/PEPFAR and the Centre for Infectious Disease Research in Zambia. We excluded patients enrolling 30 days prior to, and 90 days after, implementation to minimize bias from cross‐over and clinic‐to‐clinic variations in guideline uptake. Under the assumption that those presenting immediately before and after this period are balanced on both measured and unmeasured characteristics, we estimated the effects of implementing treat‐all on both ART initiation and retention in care on ART at 12 months (defined as any clinic attendance 9 to 15 months after enrolment and 6 months on ART). We also performed an instrumental variable (IV) analysis to obtain unbiased estimates of the effect of same‐day ART initiation on 12‐month retention.
Results: Among 77,361 newly enrolling HIV patients (62.1% female, median age 32 years [IQR 26 to 39], median CD4 286 cells/µL [IQR 147 to 465]), implementing universal treatment increased same‐day ART initiation from 42.2% to 78.9% (risk difference [RD] +36.7%, 95% CI 35.2 to 38.3%), ART initiation by 1 month from 69.6% to 89.1% (RD + 19.6%, 95% CI 18.3 to 20.8%), and 12‐month retention in care on ART from 53.4% to 62.7% (RD + 9.1%, 95% CI 7.4 to 10.8%). An IV analysis demonstrated that same‐day ART initiation due to universal treatment led to a 13.4% (95% CI 11.7 to 15.1%) increase in 12‐month retention on ART.
Conclusions: Implementing universal HIV treatment in Zambia substantially increased same‐day and overall ART initiation among newly enrolling patients. Retention in care also improved, but overall levels remained suboptimal and were lower than in randomized trials. Strategies that leverage the short‐term impacts of universal treatment to cultivate long‐term treatment success are needed.
OAE0103
Understanding preferences for HIV care among patients experiencing homelessness or unstable housing: Results of a discrete choice experiment
M. Conte1,2,3, I. Eshun‐Wilson4, E. Geng4, E. Imbert1, M. Hickey1, D. Havlier1, M. Gandhi1, A. Clemenzi‐Allen1,5
1Division of HIV, ID and Global Medicine, University of California, San Francisco, United States, 2Institute for Global Health Sciences, University of California, San Francisco, United States, 3Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, United States, 4Division of Infectious Diseases, Washington University, St Louis, United States, 5San Francisco Department of Public Health, San Francisco, United States
Background: Homelessness and unstable housing (HUH) negatively impact primary care visit attendance, viral suppression and overall survival rates among people living with HIV (PLWH). To incorporate patient preferences into solutions for more effective care for HUH‐PLWH, we quantified patient preferences and financial trade‐offs across multiple possible HIV‐service domains for this programme using a discrete choice experiment (DCE).
Methods: The San Francisco General Hospital's “Ward 86” HIV clinic has a 37% prevalence of HUH. We sequentially sampled Ward 86 patients reporting HUH who had missed primary care visit in the last year and recent viraemia to conduct a DCE. Subjects chose between two hypothetical clinics which varied by five service attributes: patient‐centred care team (“get to know me as a person” vs. not), gift cards ($10, $15 or $20/visit), drop‐in versus scheduled visits, distance to clinic (2 vs. 20 blocks) and direct phone communication to care team versus front‐desk staff. We estimated relative utility (i.e. preference) for attribute levels using mixed‐effects logistic regression and calculated the monetary trade‐off of preferred options.
Results: Of 65 individuals enrolled, 61% were > 40 years‐old; 45% white; 77% male; 46% heterosexual; 56% lived outdoors or in emergency housing and 44% in temporary housing, Strongest preferences were for having patient‐centred providers (β = 3.80; 95% CI 2.57 to 5.02) and drop‐in clinic appointments (β = 1.33; 95% CI 0.85 to 1.80), with a willingness to trade $32.79 (95% CI 14.75 to 50.81) and $11.45 (95% CI 2.95 to 19.95) in gift cards/visit, respectively, for each component (Figure 1).
Abstract OAE0103‐Figure 1.
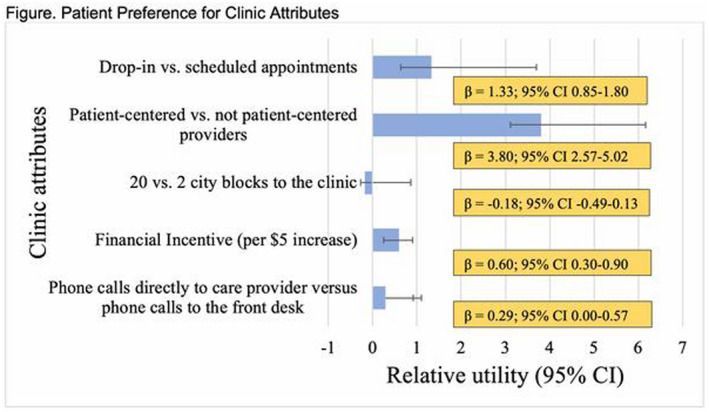
Conclusions: HUH‐PLWH, who live on the economic margins and who often lack basic subsistence, were nevertheless willing to trade significant financial gain in a DCE to have a personal relationship with and immediate access to the primary care team. These findings can inform “Ending the HIV Epidemic” by guiding innovative programming to improve retention in HIV care.
OAE0104
Drug shops are an effective strategy to reach adolescent girls and young women with HIV self‐testing and contraception: A randomized trial in Tanzania
L.A. Hunter1, J.X. Liu2, A. Rao3, S. Napierala4, A. Kalinjila5, A. Mnyippembe5, K. Hassan5, R. Mfaume6, P. Njau5,7, S.I. McCoy1
1University of California, School of Public Health, Berkeley, United States, 2University of California, Institute for Health and Aging, Bixby Center for Global Reproductive Health, San Francisco, United States, 3CVS Health, Design and Innovation Lab, Boston, United States, 4RTI International, Berkeley, United States, 5Health for a Prosperous Nation, Dar es Salaam, Tanzania, United Republic of, 6Shinyanga Regional Medical Office, Shinyanga, Tanzania, United Republic of, 7Ministry of Health, Community Development, Gender, Elderly, and Children, National AIDS Control Programme, Dar es Salaam, Tanzania, United Republic of
Background: Adolescent girls and young women (AGYW, ages 15 to 24) comprise 25% of new adult HIV infections in sub‐Saharan Africa and disproportionately bear 44% of all reported unintended births. Located in nearly every community, drug shops are extensions of the health system which offer unparalleled reach of health services to underserved populations. Thus, we designed and evaluated a girl‐friendly intervention to deliver HIV self‐testing (HIVST) and contraception to AGYW at privately owned drug shops in Tanzania.
Methods: We conducted a 4‐month randomized trial at 20 drug shops in Shinyanga, Tanzania, to determine if the Malkia Klabu (“Queen Club”) intervention increased AGYW patronage, provision of HIVST and contraception and health facility referrals to AGYW. Drug shops were randomized 1:1 to the intervention or comparison arm. Both intervention and comparison shops were provided with OraQuick HIVST kits to give AGYW customers for free. Intervention shops implemented Malkia Klabu, a loyalty programme designed for AGYW using behavioural economics and human‐centred design. We measured AGYW patronage through time‐location surveys at randomly selected 3‐hour blocks at baseline (n = 109) and endline (n = 246). In intent‐to‐treat analyses, we used Poisson regression to estimate rate ratios via a difference‐in‐differences approach. We measured HIVST and contraception distribution and referrals with monitoring data. The trial was pre‐registered (clinicaltrials.gov: NCT04045912).
Results: Drug shops implementing Malkia Klabu had higher AGYW patronage at endline than comparison shops (mean AGYW per survey 2.86 vs. 0.91; rate ratio: 3.16; 95% confidence interval: 1.94, 5.16). Over the study period, intervention shops distributed 140% more HIVST kits to AGYW (1275 vs. 532), provided more contraception (5237 vs. 148 products) and made more referrals for HIV services (71 vs. 2) and family planning (379 vs. 43) to AGYW than comparison arm shops. No adverse events were reported.
Conclusions: The Malkia Klabu intervention dramatically increased AGYW patronage and HIVST and contraception distribution, despite HIVST being freely available at all participating shops. A future effectiveness and sustainability study is warranted to evaluate Malkia Klabu's impact on HIV diagnoses and unintended pregnancy among AGYW, assess its potential for scale up, and confirm underlying theories for behaviour change.
OAE0105
The impact of immediate ART initiation on patients’ healthcare expenditures: A stepped‐wedge cluster‐randomized trial in Eswatini
J.I. Steinert1, P. Geldsetzer2,2, S. Khan3, E. Mafara3, C. Wong3, K. Mlambo3, A. Hettema3, F. Walsh3, C. Lejeune3, S. Mazibuko4, V. Okello4, O. Ogbouji5, J.‐W. De Neve6, S. Vollmer7, T. Bärnighausen6
1University of Oxford, Social Policy and Intervention, Oxford, United Kingdom, 2Harvard T.H. Chan School of Public Health, Department of Global Health and Population, Cambridge, United States, 3Clinton Health Access Initiative, Boston, United States, 4Ministry of Health of the Kingdom of Eswatini, Mbabane, Eswatini, 5Duke Global Health Institute, Durham, United States, 6Heidelberg Institute of Global Health, Heidelberg, Germany, 7University of Göttingen, Göttingen, Germany
Background: Healthcare expenditures for HIV care pose a major economic burden on households in sub‐Saharan Africa. Immediate initiation of antiretroviral therapy (ART) for all HIV‐positive patients is thought to have important health benefits but it is unknown how this profound change in HIV care provision will affect patients’ healthcare expenditures. This study, therefore, aims to determine the causal impact of immediate ART initiation on patients’ healthcare expenditures in Eswatini.
Methods: This stepped‐wedge cluster‐randomized controlled trial took place from September 1 2014 to August 31 2017. Fourteen public‐sector healthcare facilities in rural and semi‐urban Eswatini were paired and then randomly assigned to transition at one of seven time points from the standard of care (ART eligibility at CD4 counts of < 350 cells/mm3 until September 2016 and < 500 cells/mm3 thereafter) to the immediate ART for all intervention (EAAA). During each of the study's eight steps, we administered a questionnaire to a random sample of HIV patients at each healthcare facility. The primary outcome was total patient‐borne healthcare expenditures during the preceding 12 months. We used mixed‐effects negative binomial regressions adjusted for secular trends and clustering at the facility level. This study is registered with ClinicalTrials.gov, number NCT03789448.
Results: 2261 participants were interviewed over the study period. Participants in the EAAA phase reported a 45% decrease (RR: 0.55, 95% CI: 0.39, 0.77, p < 0.001) – or a mean reduction of 8.73 USD (95% CI: −14.39, −3.09), in absolute terms – in their total past‐year healthcare expenditures compared to the standard‐of‐care phase. Patients’ healthcare expenditures for private and traditional healthcare providers were 93% (RR 0.07, 95% CI: 0.01, 0.77, p < 0.001) lower in the EAAA than the standard of care phase. Self‐reported health status was similar between study phases.
Conclusions: Despite a higher frequency of HIV care visits for newly initiated ART patients, immediate ART initiation lowered patients’ healthcare expenditures, at least in part because they sought less care from private and traditional healthcare providers. This study adds an important economic argument to the World Health Organisation's recommendation for countries to abolish CD4‐count‐based eligibility thresholds for ART.
OAE0106
Root cause analysis as quality improvement tool for identification of barriers to improve retention in HIV care: The case of Uganda
W. Bikokye Kafeero1, B. Elur1, D. Bogere2, S. Sendagala1, J. Ssendiwala3, P. Namukanja2, A. Namale2, J. Calnan4, A.C. Awor1, J. Ward1
1Division of Global HIV and TB Centers for Disease Control and Prevention, Strategic Information Branch, Kampala, Uganda, 2Division of Global HIV and TB Centers for Disease Control and Prevention, Health Services Branch, Kampala, Uganda, 3Makerere University, School of Public Health, Kampala, Uganda, 4USAID, HIV/AIDS Services, Kampala, Uganda
Background: In Uganda, 81% of PLHIV know their HIV status, 89% on ART and 78% virally suppressed (UNAIDS 2018). With more HIV+ clients starting treatment, there was a need to address the gaps in retention and viral suppression to enable achievement of the 95‐95‐95 targets by 2030. Continuous quality improvement (CQI) efforts to close the quality gaps in the HIV/AIDS care cascade have been previously implemented at small scale with pockets of success for, however, these efforts have not yield significantly visible results at national level.
Description: As of Dec 2018, Uganda reported 1004,162 PLHIV receiving ART services. Between Oct‐Dec 2018, 36,702 patients were reported as lost to follow‐up (LTFU) Through implementation of Root Cause Analysis (RCA) initiative starting Jan 2019, patients initially categorized as LTFU were traced and interviewed by community peers or reached by phone by facility staff using a customized tool. The RCA as a tool for CQI was implemented to identify barriers to retention in care. The barriers identified and their frequencies ranked using Pareto analysis.
Lessons learned: Of the 36,702 patients initially categorized as LTFU, 5008 were traced by community peers or reached on phone by facility staff; 95% (4758) were > 15 years, 60% (3005) females. From the RCA, of those LTFU were a result of lost clients reported lack of transport or long distance, 19% (950), forgot appointment; 15% (758) while travel away from home, sickness, or work accounted for 12%, 10% and 8.3% respectively.
Abstract OAE0106‐Figure 1
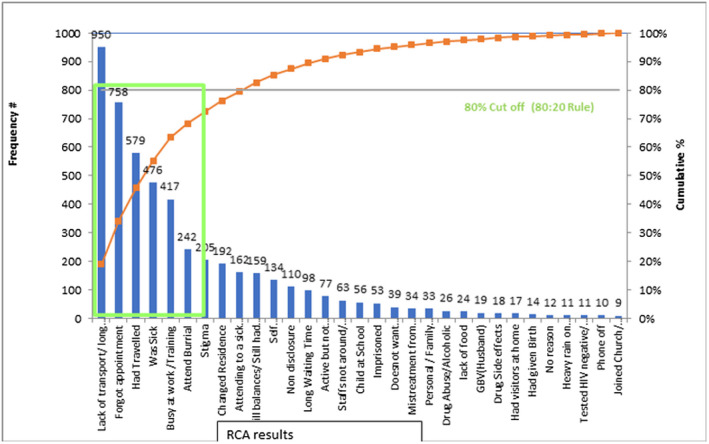
Conclusions/Next steps: Large‐scale implementation RCA is feasible and useful in identifying gaps in service quality that impact programming. For retention, while roll out of differentiated service delivery may address transport challenges, further analysis is required to determine best solutions Disclaimer.
OAE0202
Sustaining progress in prevention of mother‐to‐child HIV transmission services – how low‐volume sites have an impact in Tanzania
R. van de Ven1, C. Nnko1, R. Lyimo1, B. Kilama1, D. Kajoka2, J. Kalimunda3, S. Kimambo1
1Elizabeth Glaser Pediatric AIDS Foundation, Dar es Salaam, Tanzania, United Republic of, 2Ministry of Health, Community Development, Gender, Elderly and Children, PMTCT Unit, Dodoma, Tanzania, United Republic of, 3USAID Tanzania, Dar es Salaam, Tanzania, United Republic of
Background: Prevention of mother‐to‐child transmission of HIV (PMTCT) services have been successfully rolled out across all health facilities offering maternal and child health services in Tanzania. However, in recent years, progress in PMTCT services uptake appears to have slowed. As part of the PEPFAR “pivot”, which shifts attention and PEPFAR resources to high‐volume sites, the Elizabeth Glaser Pediatric AIDS Foundation also shifted its direct site‐level support from both high‐ and low‐volume sites to high‐volume sites only.
Methods: A retrospective analysis was conducted for a two‐year period (October 2017 to September 2019) to assess the uptake of PMTCT services nationally. We used the national DHIS2 database to extract aggregated, routine PMTCT data at national level, compiled the standard PMTCT indicators and analysed the yearly performance trend. For the six EGPAF supported regions, we also compared PEPFAR supported sites to non‐supported sites.
Results: Comparing the periods October 2017‐September 2018 and October 2018‐September 2019, nationally the uptake of HIV testing among pregnant women remained at 97% (2181,015/2254,107). However, antiretroviral therapy (ART) initiation among newly identified women dropped from 90% (35,350/39,467) to 80% (28,240/35,500) and the uptake of early infant HIV diagnosis (EID) dropped from 57% (47,403/82,772) to 54% (46,069/85,020). During the October 2018 to September 2019 period, the uptake of HIV testing among pregnant women between EGPAF‐supported high‐volume sites (n = 417) versus non‐supported low‐volume sites (n = 1285) remained the same (97.1% vs. 97.7%), but there is a significant difference in ART uptake (99% vs. 49%, p < 0.0001) and EID testing uptake (72% vs. 26%, p < 0.0001). These non‐supported low‐volume sites covered 45% (3015/6647) of the newly identified HIV‐positive pregnant women within the year.
Conclusions: While the PEPFAR pivot has shifted efforts and resources for high‐quality ART service delivery models to high‐volume sites, services are not sustained at the low‐volume non‐supported sites. As these sites still cover nearly half of the newly identified pregnant women living with HIV, it has a negative impact on reaching elimination. Therefore, PMTCT programmes need consistent support across all service delivery platforms and a reboot to address the challenges at low‐volume sites to sustain the progress towards reaching elimination.
OAE0203
Life after PEPFARS direct service support: Programme sustainability among South African HIV/AIDS organizations funded by PEPFAR
J. Chiliza1,2, F. Feeley III1, R. Laing1, A. Brennan1, D. Jackson3, J. Arendse4, K. Riffenberg1, L. Tabbaa1
1Boston University, Department of Global Health, Boston, United States, 2Boston University, Boston, United States, 3University of the Western Cape, Bellville, South Africa, 4Western Cape Government Health, Cape Town, South Africa
Background: Public health practitioners have little guidance of how toplan for the sustainability of donor sponsored programmes. The literature is broad and provides no consensus on a definition of sustainability. This study used a robust mixed‐methods methodology to develop a list of programme sustainability factors to inform donor‐funded programmes.
Methods: This study examined 61 health facilities in the Western Cape, South Africa, supported by four PEPFAR non‐governmental organizations (NGOs) from 2007 to 2012. Retention in Care (RIC) was used to determine health facility performance. Sustainability was measured by comparing RIC during PEPFAR direct service, to RIC in the post PEPFAR period (2012 to 2015). Crude and adjusted risk differences were calculated to estimate the association between the type of government ownership, PEPFAR NGO support, ART treatment policy change, size of ART patient cohort, human resource transition and our outcome of RIC at 12 and 24 months on ART.
Forty‐three semi‐structured in‐depth interviews were conducted with key informants. The qualitative data were used to examine how predictor variables were operationalized at a health facility and NGO level.
Results: Though the linear regression models showed no difference in RIC pre and post 2012, our graphed descriptive results showed a dip in RIC among the majority of the study facilities in 2012/2013. The RIC decrease was likely due to PEPFARs move from direct service to technical assistance: the decrease in the numbers of community health workers (CHWs) and a change in HIV treatment eligibility guidelines.
Our qualitative results suggest the following lessons for the sustainability of future programmes:
• Sufficient and stable resources (i.e. financial, human resources, technical expertise, equipment, physical space)
• Investment in organizations that understood the local context and have strong relationships with local government
• Strong leadership at a health facility level.
• Some disease specific staff (i.e. clinical, administrative, community)
• Joint planning and formalized skill transfer:
• Local positive perceived value of the programme
• Stable financial and political support for the programme
Conclusions: Sustainability is complex, context dependent and reliant on various processes and outcomes. This study suggests additional health facility and community level staff should be employed in the health system to ensure RIC sustainability.
OAE0204
Key populations doing it for themselves: The rise of social enterprise approaches to increase financial sustainability of the community‐based HIV response
Y. Vu1, K. Green1, B. Vu1, T. Ngo2, T. Tran1, K.O. Pham3, T. Le4, K. Do5, S. Pham6, P.A. Nguyen3
1PATH, Healthy Markets Project, Hanoi, Vietnam, 2USAID, HIV Prevention, Hanoi, Vietnam, 3CSIP, Hanoi, Vietnam, 4Glink, Ho Chi Minh, Vietnam, 5Galant, Ho Chi Minh, Vietnam, 6G3VN, Ho Chi Minh, Vietnam
Background: Despite a complex HIV epidemic, with a rise in HIV infections among men who have sex with men and transgender women, external financing for HIV prevention has declined significantly over the past ten years in Vietnam. Declining resources directly impacted the viability of fledgling key population (KP)‐led civil‐society organizations (CSOs) that were the backbone of community HIV prevention efforts.
Description: Starting in 2014, the USAID/PATH Healthy Markets (HM) project partnered with 25 KP‐led organizations to co‐grow areas of organizational health and wealth. This included: 1) measuring progress towards sustainability through a locally developed social enterprise organizational capacity assessment tool; 2) developing and implementing an organizational growth plan; 3) CSO mentoring from a local social enterprise incubator; and 4) supporting to generate market insights and accessing capital. Three distinct KP‐led business (KPLB) models emerged: CSOs with integrated sales activities (mainly condoms); legally registered social enterprises selling health goods and services; and private clinics offering HIV and related health services.
Lessons learned: A 2019 assessment of organizational capacity and financial viability of a sample of nine KPLB found that all but one broke even by month eight of operations, with private clinics taking the longest time, and 100% reporting annual increases in sales and revenue. Overall profit for the nine KPLB increased from US$73,791 to US$129,685 between 2016 and 2018. All private clinics, 75% of social enterprises, and 67% of CSOs achieved their financial sustainability goals. The KPLB reported that the joint capacity assessment, business training and tailored mentoring were most valuable in enabling their transition from CSO to a KPLB.
Conclusions/Next steps: As donor funds decline, and where public financing for KP CSOs is not assured, enabling financial independence is essential to sustain the presence of community‐led HIV and related health service providers.
OAE0205
Sustainability, HIV financing and transition preparedness: Building financing literacy to strengthen the HIV community response
P.C. Loh1, M.F. Teh1, G. Gray1
1Australian Federation of AIDS Organisations (AFAO), NSW, Australia
Background: HIV‐financing and transition planning are relatively new and technical areas for key‐population (KP) communities. Challenges remain for communities to understand the scope of HIV‐financing, funding sustainability and the urgency of transition planning. These knowledge areas are sometimes perceived as too technocratic, with low community literacy resulting in limited empowerment to drive advocacy and community mobilization.
Description: The Sustainable HIV Financing in Transition (SHIFT) Programme was a two‐year (2017 to 2018) Global Fund advocacy programme implemented by the Australian Federation of AIDS Organisations (AFAO) aimed at empowering civil society organizations and KP networks to influence domestic HIV funding processes. SHIFTs objectives were to ensure a sustainable, cost‐effective and strategically allocated funding for HIV in four transition countries (Malaysia, Philippines, Indonesia and Thailand).
Lessons learned: A crucial component of ensuring transition preparedness and sustainability of HIV responses is the meaningful inclusion of, and buy in from, KP groups. Two main challenges were noted:
Limited meaningful KP inclusion in country transition decision‐making processes: While there was KP representation on CCM and transition planning working groups, there is a lack of inclusion of their voices or is often tokenistic. As KP are perceived as not technically qualified in these knowledge areas, their inputs were often put aside, with discussions dominated by policymakers and government technocrats.
Limited community‐literacy and awareness around HIV‐financing and transition preparednes: HIV‐financing information and other key strategic data to advocate for KP investment and allocatively efficient funding are often dense and difficult to understand, given that community representatives have limited interest and capacity for technical jargon. HIV‐financing information and other data need to be made more readily available and accessible at the community‐level. Capacity development activities are needed to help KP communities better understand and utilize data for programmatic and financial advocacy.
Conclusions/Next steps: An empowered and informed civil‐society, crucial to the success of a sustainable response in HIV financing, requires increased community understanding, awareness and engagement on HIV‐financing and transition. Technical HIV‐financing and transition planning information need to be distilled into community‐friendly knowledge products. Communities must be trained on how to use and transform this information into advocacy for effective increased KP‐investments and allocatively efficient funding policies.
OAE0206
HIV integration for a more sustainable and resilient HIV response in low and middle‐income countries
C. Mann1, M. Hijazi1, S. Baker1, H. Marqusee1, C.A. Nguyen2, R. Stanley3, C. Piña4
1USAID, Washington, D.C., United States, 2USAID, Hanoi, Vietnam, 3USAID, Phnom Penh, Cambodia, 4USAID, Santo Domingo, Dominican Republic
Background: Much of the HIV/AIDS response has been financed by external donors, stabilizing the epidemic for many countries. This led to programmatic and external financing shifts as many countries approach sustained epidemic control. Now countries must increase domestic financing but minimize or eliminate out‐of‐pocket spending at point of care. Leveraging well‐functioning social health insurance (SHI) or social protection schemes to include HIV/AIDS is one approach with recent success to increase domestic financing. This provides more sustainable and resilient HIV/AIDS service delivery systems.
Description: The PEPFAR‐funded Sustainable Financing Initiative for HIV/AIDS (SFI) supports several countries to integrate HIV/AIDS services into SHI or social protection benefits package. In Vietnam, the project focused on integrating donor‐supported HIV treatment centres into the public health system; enrolling people living with HIV/AIDS (PLHIV) into SHI; and domestically financed antiretroviral (ARVs) procurement. In Cambodia, despite a challenging political environment, SFI supported passing a HIV/AIDS policy circular through evidence generation, continued engagement with government and identifying champions for policy achievement. In the Domican Republic (DR), the project is working with government to integrate ARV financing into their SHI.
Lessons learned: In Vietnam, this work resulted in $5.9 million in ARVs procured domestically, enrolling 90% of HIV patients in SHI (36% in 2016), and integrating 87% of outpatient facilities into SHI‐supported facilities. This provided savings of $5.9 million to PEPFAR with increased government contributions; a 3:1 return on investment. In Cambodia, the HIV/AIDS circular provides a 6‐pronged approach for a more sustainable and equitable HIV/AIDS response. This includes all PLHIV being eligible for the Health Equity Fund (HEF) – providing free access to all health services and social protection schemes. SFI is supporting circular implementation, including a cost analysis for including PLHIV into HEF. In the DR, financing ARVs using SHI ensures commodity sustainability with shifting $3.7 million annually from government financing to regular insurance contributions, and potential savings by transferring procurement responsibilities.
Conclusions/Next steps: HIV integration into health insurance and social protection schemes provide a sustainable way to increase domestic financing while increasing coverage for care and treatment.
OAE0302
The effect of health insurance to HIV‐positive caregivers caring for orphans and vulnerable children in Tanzania
K. Tani1, A. Exavery1, T. Mbwambo1, J. Charles1, E. Jerre2
1Pact Tanzania, Monitoring Evaluation Research and Learning, Dar es Salaam, Tanzania, United Republic of, 2Pact Tanzania, Chief of Part, Dar es Salaam, Tanzania, United Republic of
Background: Sustainable Development Goal 3 promotes universal health coverage to achieve well‐being for all. In Tanzania, only 32% of the 55 million population are covered by health insurance, of which 72% is community health fund, 23% is national health insurance and 3% is private insurance. This study explores the relation of having health insurance coverage and enrolling into HIV Care and Treatment Clinics (CTC) while holding other factors constant.
Methods: A PEPFAR‐funded orphans and vulnerable children (OVC) project collected individual and household data between April 2017 and September 2019 using a project‐specific Family and Child Assets Assessment tool. The data were collected by lay community social welfare volunteers at household level during screening of the household at enrolment and repeated after two years of service delivery. Data were analysed for caregivers with known HIV status. The respondents who did not know their HIV status and/or refused to disclose were excluded from the analysis. The multivariate logistic regression was examined using Stata.
Results: Of the 129,406 caregivers who declared their HIV status, 32.4% self‐reported as HIV+ and, of these, 91.4% were enrolled in a CTC. Health insurance coverage was 15.8% at enrolment, and 24% at two‐year reassessment. At enrolment (before OVC services) the presence of health insurance had no influence on HIV+ enrolment to CTC (OR = 1.05 CI = 0.89 to 1.24). At reassessment, the result depicted that HIV+ covered by health insurance were more likely to be enrolled to CTC (OR = 0.1.61 CI = 1.47 to 1.77). These effects were adjusted for respondent ability to cover the emergency medical needs (self‐reported), age, sex and residence.
Conclusions: Although caregivers received a variety of needs‐based services once enrolled into the project (including case management, counselling, economic strengthening and escorted referrals),the inclusion of health insurance in the package of services opened up more demand and utilization of healthcare. The data suggest that insurance contributes to uptake of HIV services, even though the HIV services are free, because general health services were made more accessible With insurance the HIV+ can acquire the needed health services where available, without the barrier of limited resources, and this includes access to health services that complement their HIV care.
OAE0303
Leveraging private providers to improve and extend HIV treatment access in South Africa: Cost implications for universal healthcare in South Africa
L. Long1,2,3, S. Girdwood2,3, K. Govender2,3, N. Lekodeba3,2, S. Kgowedi3,2, G. Meyer‐Rath1,2,3, J. Miot2,3
1Boston University, Global Health, Boston, United States, 2University of Witwatersrand, Department of Internal Medicine, Johannesburg, South Africa, 3Wits Health Consortium, Health Economics and Epidemiology Research Office, Johannesburg, South Africa
Background: Despite significant gains towards HIV epidemic control in South Africa, expanding treatment access remains a priority. The proposed South African National Health Insurance aims to re‐engineer primary healthcare by leveraging private‐sector providers to achieve universal healthcare. We conducted a cost‐outcome analysis to explore the possible implications of expanding HIV access using private providers.
Methods: Four sites in South Africa's Gauteng province were included: two government, primary health clinics (PHC) (PUB1&PUB2), one NGO‐run PHC which accesses public‐sector drugs and laboratory tests (PRV1) and one contracted doctor model which utilizes private clinicians to manage public sector patients (PRV2). We sampled adult HIV‐positive patients initiating, or newly presenting for, HIV treatment at sites in 2017 and 2018 and followed them for 12 months. Retention in care with viral suppression (IC suppressed) at 12 months was the primary outcome. Bottom‐up costing from the provider perspective was based on patient‐level resource usage. PRV1 charged patients a means tested fee of <USD5 per visit; PRV2 charged a donor‐covered annual capitation fee per patient paid quarterly based on attendance. Costs are reported in 2019 USD.
Results: Sites reported similar mean age, days in care and number of visits. The public sites performed both the best (64%) and worst (33%) in terms of IC suppressed, with the private sites falling between. In the private models, uptake is higher in men and those most at need (lowest CD4); cost is variable but similar once non‐clinical staff is excluded. Costs for non‐clinical staff performing services that were largely not HIV‐related drove the higher average cost for PRV1.
Abstract OAE0303‐Table 1. Cohort demographics, outcomes and costs
| Public Site 1 (PUB1), n = 76 | Public Site 2 (PUB2), n = 75 | Private Site 1 (PRV1), n = 75 | Private Site 2 (PRV2), n = 75 | |
|---|---|---|---|---|
| Male, % | 22% | 28% | 39% | 47% |
| Baseline CD4, mean | 425 | 333 | 282 | 440 |
| In care (IC) | 61 (80%) | 55 (73%) | 47 (63%) | 58 (77%) |
| Suppressed, n (%) | 49 (64%) | 25 (33%) | 31 (41%) | 42 (56%) |
| Suppression unknown, n (%) | 6 (8%) | 26 (35%) | 11 (15%) | 9 (12%) |
| Unsuppressed, n (%) | 6 (8%) | 4 (5%) | 5 (7%) | 7 (9%) |
| Not in care (NIC), n (%) | 15 (20%) | 20 (27%) | 28 (37%) | 17 (23%) |
| Lost after 1 visit, n (%) | 4 (5%) | 3 (4%) | 10 (13%) | 3 (4%) |
| Lost after > 1 visit, n (%) | 11 (14%) | 17 (23%) | 18 (24%) | 14 (19%) |
| Avg total cost/px – all (12 mon) | $290 | $188 | $357 | $239 |
| Drugs – HIV | $79 | $76 | $71 | $69 |
| Drugs – Other | $15 | $4 | $13 | $13 |
| Laboratory tests | $44 | $24 | $47 | $41 |
| Staff costs – Clinical | $100 | $52 | $87 | – |
| Staff costs – Non clinical | $15 | $20 | $80 | – |
| Fixed costs | $36 | $13 | $59 | $116* |
| Avg total cost/px – IC suppressed | $332 | $230 | $475 | $282 |
| Avg total cost/px – IC suppression unknown | $324 | $226 | $511 | $259 |
| Avg total cost/px – IC unsuppressed | $279 | $234 | $481 | $278 |
| Avg total cost/px – NIC | $144 | $77 | $145 | $106 |
*Capitated annual fee.
Conclusions
If we are to reach the goal of universal HIV treatment access we need to utilize existing resources across sectors. Using private providers to move towards universal healthcare may expand HIV treatment access to under‐reached populations without significantly increasing costs nor reducing outcomes.
OAE0304
Funeral and life insurance in South Africa: How type 2 diabetes mellitus survival can inform access to and affordability of life and funeral insurance in adults with HIV‐1
L. Sarkin1, J. Ball2, G. Maartens1, M. Cotton3, J. Nachega4,5,6,7, R. Leisegang3,1
1University of Cape Town, Division of Clinical Pharmacology, Cape Town, South Africa, 2Medscheme (Pty) Ltd, Aid for AIDS, Cape Town, South Africa, 3Stellenbosch University, FAM‐CRU, Department of Paediatrics & Child Health, Tygerberg, South Africa, 4Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology, Baltimore, United States, 5Johns Hopkins Bloomberg School of Public Health, Department of International Health, Baltimore, United States, 6University of Pittsburgh Graduate School of Public Health, Departments of Epidemiology, Infectious Diseases and Microbiology, Pittsburgh, United States, 7Stellenbosch University, Department of Medicine and Centre for Infectious Disease, Tygerberg, South Africa
Background: Funeral insurance, and to some extent, life insurance are common in South Africa, and access to affordable policies for people living with HIV (PLWH) is important. Insurability is measured by relative rather than absolute mortality or life expectancy, but these data are not commonly reported.
Methods: Using private medical scheme data from South Africa, we identified 3 patient groups: PLWH on antiretroviral therapy (ART), HIV‐negative with type 2 Diabetes Mellites and on treatment (DM2) and a control group with neither. Relative all‐cause mortality risk (relative risk) was estimated using a generalized linear model (GLM) assuming a Poisson error distribution and with expected numbers of deaths based on the control cohort mortality according to age, gender and population group specified as on offset; for PLWH, current CD4 count, viral load, baseline CD4 count and time on ART are also included.
Results: In the ART group, 8920 deaths were observed recorded in 77,325 patients starting ART between 2000 and 2013 contributing 315,341 person‐years of observation (PYO) (median follow‐up of 3.23 years [IQR 2.04;5.30]). In the DM2 group, 7970 deaths were recorded in 67,705 patients starting antihyperglycaemic therapy over the same period contributed 365,547 PYO (median follow‐up of 6.20 years [IQR 3.85;9.53]). Our relative risk ratios compared with Kaulich‐Bartz et al. (2013) from a high‐income setting. Using our methodology, 90% in the ART group had a relative risk from 6 months within the insurance industry threshold (i.e. <5 when compared to the control) and a lower or comparable relative risk to the DM2 group from 12 months – see Figure 1.
Abstract OAE0304‐Figure 1.
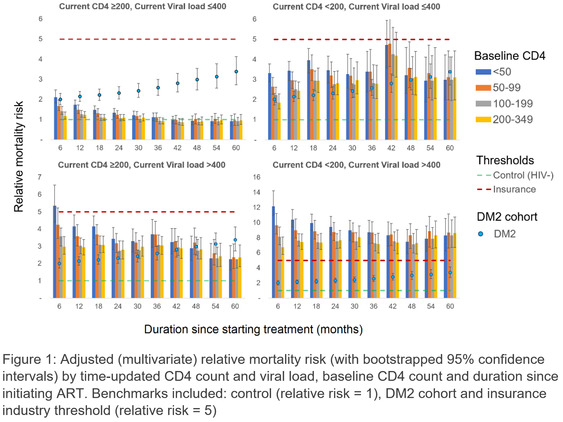
Conclusions: Most PLWH have both insurable and comparable relative risk to DM2. Both current VL and CD4 were clearly prognostic over the whole period and therefore are likely to remain a requirement for life insurance, but not for HIV programmes or funeral policies.
OAE0305
The influence of healthcare financing on cardiovascular disease prevention in people living with HIV
A. Webel1, J. Schexnayder1, C.R. Rentrope1, H. Bosworth2, C. Hileman3, N.L. Okeke2, C. Longenecker1
1Case Western Reserve University, Cleveland, United States, 2Duke University, School of Medicine, Durham, United States, 3The MetroHealth System, Cleveland, United States
Background: People living with HIV (PWH) are diagnosed with age‐related comorbidities including cardiovascular disease (CVD) at higher than expected rates. Medical management of comorbidities frequently occurs in HIV specialty clinics. In recent years, changes in the healthcare financing for PWH in the U.S. has been dynamic. There is little evidence examining how healthcare financing characteristics shape primary and secondary CVD prevention among PWH. Our purpose was to examine the perspectives of PWH and their healthcare providers on how healthcare financing influences CVD prevention.
Methods: As part of the NHLBI‐funded PreCLUDE initiative, we conducted in‐depth, semi‐structured interviews with 34 multidisciplinary healthcare providers and 51 PWH at 3 U.S. HIV clinics from October, 2018 to March, 2019. Using Braun and Clark's (2006) thematic analysis framework, we examined barriers and enablers of CVD prevention for PWH related to healthcare financing.
Results: Three themes emerged across sites and disciplines: (1) Health systems organized around relative value units (RVUs) experience pressures that may disincentivize CVD prevention efforts by HIV specialty care providers. Increasingly, HIV clinics are internally co‐locating services such as smoking cessation and cardiovascular health clinics to prevent CVD. Yet, this expansion of services strains clinic personnel and processes in a way that threatens their effectiveness. (2) Grant‐based services enable locally tailored CVD prevention strategies but are limited by the funder's priorities. (3) While commercial insurances support innovative CVD prevention tools, PWH with these payers experience increased barriers compared to public insurances. Examples include potential discomfort in being referred to new primary care providers, co‐pays for specialty visits with one's longstanding HIV provider, and challenges in medication authorization due to HIV and CVD drug interactions.
Conclusions: As healthcare financing for PWH evolves, an understanding of the effects of various payers on patient and provider behaviour and responses of the healthcare systems in which this care is provided, is important. HIV specialty clinics can consider implementing comprehensive CVD prevention strategies into everyday HIV care that align with a dynamic reimbursement landscape. HIV clinics should also be at the forefront of advocating for healthcare delivery and reimbursement models responsive to the evolving medical needs of PWH.
OAE0306
Towards universal health coverage among people living with HIV in Nigeria: Are they willing to enrol in a health insurance scheme?
B. Olakunde1, T. Oladele1, D. Ndukwe1, S. Wakdok1, D. Adeyinka2,3
1National Agency for the Control of AIDS, Department of Community Prevention & Care Services, Abuja, Nigeria, 2Federal Ministry of Health, Department of Public Health, Abuja, Nigeria, 3University of Saskatchewan, Department of Community Health and Epidemiology, Saskatoon, Canada
Background: High out‐of‐pocket expenditures for HIV‐related services can limit access to care, and also result in financial catastrophe, particularly among the poor. While these consequences can be avoided by providing financial protection through a health insurance scheme, it is largely unknown whether people living with HIV (PLHIV) in Nigeria will be willing to participate in it. In this study, we assessed willingness of PLHIV in Nigeria to enrol in and pay for a health insurance scheme.
Methods: The study was a cross‐sectional survey of 229 PLHIV 18 years and older receiving antiretroviral therapy in three secondary health facilities in the Federal Capital Territory, Nigeria. Data on sociodemographic characteristics, financial burden of HIV care, knowledge of health insurance scheme, willingness to enrol for health insurance scheme, and the premium respondents were willing to pay was collected using a pre‐tested semi‐structured self‐administered questionnaire. We performed descriptive statistics, and linear regression analyses to examine factors associated with the premium the respondents were willing to pay.
Results: Approximately 67% (129/193) indicated willingness to enrol in an insurance scheme. The maximum monthly premium the respondents (n = 126) were willing to pay ranged from N100 ($0.3) to N1000 ($3.2), with a median of N500 ($1.6). In the bivariate analyses, gender, education, marital status and religion were significantly associated with the monthly premium the respondents were willing to pay. In the multivariate linear regression model which contained the significant factors at bivariate level, gender, education and marital status remained significant. Females were willing to pay N108 ($0.4) more than males (p = 0.014). Those who were single were willing to pay N141 ($0.5) more than the married respondents (p=0.004). Compared to those with tertiary education, those with secondary education were willing to pay N124 ($0.4) less (p=0.011).
Conclusions: About two‐thirds of PLHIV in our study indicated interest in risk pooling to cover HIV services. However, they were only willing to pay a little premium. More information about health insurance and its benefits may improve willingness of PLHIV in Nigeria to enrol. Poor PLHIV may also require subsidies for enrolment into health insurance schemes.
OAE0402
Treatment outcomes in a community pharmacy anti‐retroviral therapy programme
Y. Kambai Avong1, G. Ayodele Kayode2, E. Bosede Avong3, B. Jatau1, C. Mensah4, P. Dakum4
1Institute of Human Virology Nigeria, Clinical, Abuja, Nigeria, 2Institute of Human Virology Nigeria, Research, Abuja, Nigeria, 3APIN, Clinical, Makurdi, Nigeria, 4Institute of Human Virology Nigeria, Administration, Abuja, Nigeria
Background: The use of community‐based models for scaling up HIV treatment has been recommended by the World Health Organization. In a community base project providing antiretroviral therapy, we investigated retention in care, adherence to medications and virologic suppression among the participants who willingly chose to refill prescriptions in registered Community Pharmacies (Co‐Pharm). Viral suppression (<20 copies/mL) was the measure of outcome.
Methods: This is a non‐randomized intervention study. Adults living with HIV that were virologically suppressed (i.e. having a viral load of less than 20 copies/mL) were recruited and enrolled in a community‐based programme where patients refilled prescription in registered Co‐Pharm in northern Nigeria [Nasarawa state, Katsina state, Kano state and the Federal Capital Territory (FCT), Abuja, Nigeria], from January 2017 to June 2019. Sociodemographic and treatment data (medication regimen, prescription refill, retention in care and viral load) were collected. Baseline virologic suppression before patients devolved to the Co‐Pharm was compared with the patients’ virologic suppression data after 12 months of devolvement. Descriptive statistics and multivariable linear regression analysis were applied.
Results: Twenty nine public hospitals and 64 registered community pharmacies were recruited. Of the 2938 patients included in the analysis; 56.7% (1665) were men and mean age was 53 years [SD = 3.2]. Majority of the patients were retained in care [98.1% (2882)] and 85% (2497) had optimal adherence (≥95%). Baseline log viral load was 3.7 (SD = 1.2); no significant difference in median viral load (VL) before and after participants devolved to the Co‐Pharm [before median VL = 2.9 log copies/mL [IQR=2.9 to 7.1] Vs after median VL = 2.9 log copies/mL [IQR = 2.9 to 10].
Conclusions: Patients remained virologically stable with optimal adherence and retention in care. This suggests that patients who are already virologically suppressed may remain stable even if they are devolved from the hospitals to the Co‐Pharm. Registered Co‐Pharm linked to public hospitals may therefore provide a viable option for treating patients who are virologically suppressed. In developing countries, over‐crowded hospitals could be decongested by allowing patients who are virologically suppressed to devolve to the Co‐Pharm.
OAE0403
Building capacity for management of patients on advanced ART regimens through guided practice using the ECHO tele‐monitoring model in Kenya
J. Humphrey1, S. Ali2, M. Alera3, S. Goodrich1, D. Litzelman1,4, A. Gardner1,2
1Indiana University School of Medicine, Indianapolis, United States, 2Moi University College of Health Sciences, Eldoret, Kenya, 3Academic Model Providing Access to Healthcare, Eldoret, Kenya, 4Regenstrief Institute, Indiana University Center for Global Health, Indianapolis, United States
Background: Increasing numbers of people with HIV are transitioning to advanced (i.e. second‐ and third‐line) antiretroviral therapy (ART) regimens in resource‐limited settings. Interventions are needed to scale‐up the clinical capacity for management of these complex cases when virologic failure occurs. We evaluated the feasibility of implementing Project ECHOs teleECHO clinic training model at the Academic Model Providing Access to Healthcare in Kenya.
Methods: From March‐July 2019, HIV clinical staff at 19 public facilities throughout western Kenya participated in a teleECHO curriculum developed and led by HIV management experts in Eldoret, Kenya. Eight weekly sessions utilizing a case‐based curriculum presented patients failing advanced ART regimens, followed by expert‐led didactics based on Kenya National HIV treatment guidelines. Clinical officers (COs) at each site were purposefully sampled to complete pre‐ and post‐intervention semi‐structured surveys to investigate their knowledge and self‐efficacy regarding the management of patients on advanced ART and the barriers/facilitators to implementing the intervention. The data were analysed using descriptive and thematic analyses and paired t‐tests. Viral suppression (<40 copies/mL) among the patients discussed was assessed at six months post‐intervention.
Results: A total of 245 clinical staff (68% female; median age 38 years; 68% with > 5 years of experience providing HIV services) participated in the intervention (average 58 participants/session), including: nurses (22%), COs (25%), counsellors (32%), nutritionists (7%), social workers (4%) and other staff (10%). Among 32 COs surveyed, pre/post surveys demonstrated improved ability and self‐efficacy to monitor patients on second‐ and third‐line ART, construct a multi‐disciplinary team plan, and switch ART for patients failing second‐line ART (p < 0.05 for all). Facilitators to implementation included the interactive nature of the sessions, provision of pre‐paid internet bundles and regular access to expert consultants. Barriers included unstable internet connectivity at rural sites, technology issues and schedule interruptions. Of 16 patients ages 4 to 64 years discussed during the sessions, the median number of months with continuous pre‐intervention viraemia was 47 (interquartile range 15 to 53), and 5 of 10 patients with an available viral load achieved suppression.
Conclusions: The teleECHO model is a feasible and scalable tool to improve the management of patients failing advanced ART regimens in resource‐limited settings.
OAE0404
Improving antiretroviral therapy initiation in hospital and after discharge in Johannesburg, South Africa
M. Bisnauth1, K. Rees2, H. Struthers3, J. McIntyre4, N. Davies5
1University of Witwatersrand/Anova Health Institute, Public Health, Johannesburg, South Africa, 2University of Witwatersrand, Community Health, Johannesburg, South Africa, 3University of Cape Town, Division of Infectious Diseases and HIV Medicine, Johannesburg, South Africa, 4University of Cape Town, School of Public Health and Family Medicine, Johannesburg, South Africa, 5Anova Health Institute, Clinical Research, Johannesburg, South Africa
Background: In South Africa, despite increasing access to antiretroviral therapy (ART), HIV‐related mortality has changed very little over the last 5 years. Many people living with HIV are identified for the first time during an admission to hospital because of advanced HIV and related illnesses. Although same‐day ART initiation has been implemented since October 2016, up to 40% of people diagnosed in hospital are either clinically ineligible or not psychologically ready to start antiretroviral therapy (ART) during their admission. After discharge, these clients often struggle to link to care and treatment at their local primary healthcare facility (PHC), leading to delays in ART initiation, and further morbidity and mortality.
Description: We implemented a linkage to care model (Figure 1) at the two largest hospitals, in Johannesburg, South Africa. The model supported people who were identified as needing ART to either initiate ART during their hospital admission or link to ART initiation at their local PHC as soon as possible after discharge. We used routine data to measure linkage rates before and after implementation.
Abstract OAE0404‐Figure 1.
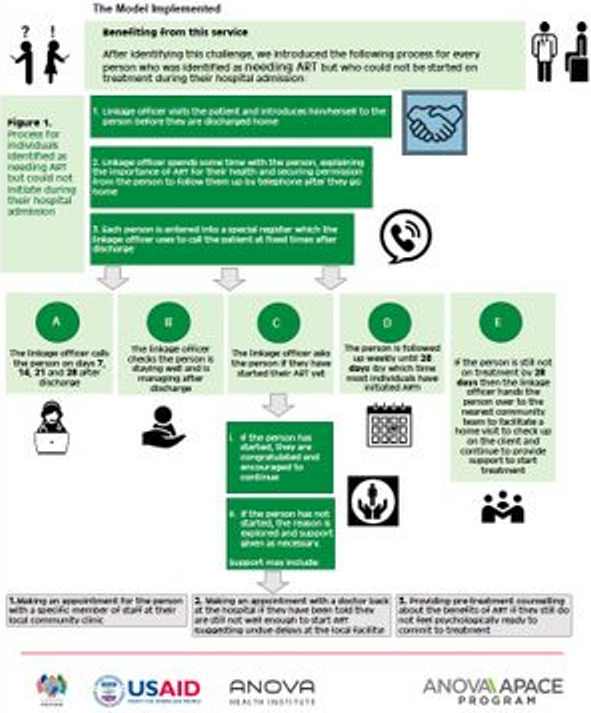
Lessons learned: Before implementing the model, an average of 55% of clients needing ART were confirmed to have initiated treatment following hospital admission. After implementation, over 90% of clients had initiated ART within 28‐days post‐discharge (549 clients over 2 months). Poorly established referral pathways and communication between hospitals and PHCs can undermine linkage to ART care but a structured post‐discharge client support model can help overcome these barriers. Delayed ART initiation is common due to acute clinical complications or lack of client psychological readiness to commit to lifelong treatment.
Conclusions/Next steps: Our model successfully improved linkage to ART and strengthened referral pathways between hospitals and PHCs following discharge. This model will be scaled‐up to all hospitals in the district to minimize loss to follow‐up and positively impact HIV‐related morbidity and mortality.
OAE0405
Healthcare workers perspectives on client volumes and workload with differentiated service delivery models in the Kingdom of Eswatini
A. Hughey1, J. Tailor2, A. Hettema1, M. Rabkin2, P. Preko3, R. Kuwengwa4, W. Reidy3, S. Shongwe5, N. Lukhele6, H. Kambale4
1Clinton Health Access Initiative, Mbabane, Eswatini, 2ICAP, Columbia University Mailman School of Public Health, New York, United States, 3ICAP, CQUIN, New York, United States, 4Ministry of Health, Eswatini National AIDS Program, Mbabane, Eswatini, 5ICAP, CQUIN, Mbabane, Eswatini, 6World Health Organization, Mbabane, Eswatini
Background: In response to overcrowding at health facilities (HF) and the importance of client‐centred care, Eswatini is scaling up less‐intensive differentiated service delivery models (DSDM) for adults and adolescents doing well on antiretroviral therapy (ART). DSDM are anticipated to improve the satisfaction of both clients and healthcare workers (HCW) but little data are available on the HCW experience of DSDM implementation, including HCW perceptions on how clinic workload has been impacted by DSDM.
Methods: We conducted a mixed‐methods study to explore HCW perspectives on the impact of DSDM on client volumes and HCW workload. Between August and October 2019, we administered 172 quantitative surveys and conducted 20 semi‐structured in‐depth‐interviews (IDI) with HCW representing multiple cadres, including expert clients, at 39 purposively selected HF in Eswatini. Quantitative data were analysed using Stata 12 and interview transcripts were coded and analysed using Dedoose.
Results: Respondents included 78 nurses (45%), 53 expert clients (31%), 16 nursing assistants (9%) and smaller numbers of medical officers, community health workers, counsellors, pharmacy staff and others. When asked about the impact of DSDM on the daily volume of ART clients, among the 67% of respondents stating there was an impact, 78% perceived a decrease while 22% perceived an increase in client volume. In IDIs, many HCWs described shorter client queues and waiting times. Reflecting on their individual workloads, 64% felt their workload had decreased with the advent of DSDM, 16% noted no change and 18% reported an increased workload. In IDIs, HCW noted that some DSDM were more labor intensive than others, noting the need for increased documentation for Community Antiretroviral Groups, pre‐packing of medication and preparation of files for Fast Track, and working on Saturdays for Teen Club.
Conclusions: The majority of HCWs reported that DSD scale‐up has decreased their workloads by reducing the volume of ART clients at HFs, but there is substantial heterogeneity in their responses. Understanding the impact of different DSDM and the impact on different HCW cadres will be important as DSDM are scaled‐up nationwide.
OAE0406
POP‐UP clinic: A multicomponent model of care for people living with HIV (PLHIV) who experience homelessness or unstable housing (HUH)
E. Imbert1, M.D. Hickey1, A. Clemenzi‐Allen2, M. Conte3, E.R. Riley1, D.V. Havlir1, M. Gandhi1
1University of California, Medicine, Division of HIV, ID and Global Medicine, San Francisco, United States, 2San Francisco Department of Public Health, San Francisco, United States, 3Zucker School of Medicine at Hofstra/Northwell, Hempstead, United States
Background: In San Francisco, homelessness is the single greatest risk factor for HIV viraemia. At San Francisco General Hospital's “Ward 86” HIV clinic, over one‐third of patients experience HUH, with increasing housing instability associated with higher likelihood of viraemia, higher frequency of drop‐in and emergency room visits and lower adherence to primary care. UNAIDS “Getting to Zero” and U.S. “End the Epidemic” goals will not be achieved without innovative models of care and greater housing access for PLHIV‐HUH. We launched “POP‐UP” in January 2019, a no‐appointment (“low‐threshold”), incentivized primary care clinic, to address structural and individual‐level barriers to care for PLHIV‐HUH.
Methods: POP‐UP eligibility includes 1) HIV RNA ≥200 copies/mL or off ART, 2) HUH and 3) ≥1 missed primary care appointment and ≥2 drop‐in visits in the prior 12 months. Patients are identified through the electronic health record and clinic‐based referrals. POP‐UP provides drop‐in primary care, which includes mental health and substance use treatment, housing assistance and case management, financial incentives and patient navigation with frequent contact. We describe programme uptake, ART initiation, return to care by 90 days post‐enrolment, and cumulative incidence of first instance of viral suppression (HIV RNA < 200) at 6 months post‐enrolment, estimated via Kaplan‐Meier.
Results: Sixty‐four patients were enrolled into POP‐UP from January‐December 2019: 83% cis‐men, 11% cis‐women, 6% transgender/non‐binary; 47% white, 36% black, 8% Latinx; 55% street homeless; 100% with a substance use disorder; 76% with a mental health disorder; and 39% with CD4 < 200. Among the 64 patients enrolled, 59 (92%) restarted ART, most at enrolment (median 0, IQR 0 to 12 days); 59 (92%) returned for follow‐up within 90 days. Cumulative incidence of viral suppression at 6 months post‐enrolment was 60% (95% CI 47 to 74%). Nine patients were unenrolled from the programme (3 died, 1 moved, 2 transferred back to PCP, 3 for threatening behaviour).
Conclusions: The POP‐UP programme at Ward 86 demonstrates early success in engaging viraemic PLHIV‐HUH in care and improving viral suppression. Low‐threshold, high‐contact primary care programmes offering comprehensive services and incentives similar to POP‐UP may improve patient outcomes for this vulnerable population in other urban settings.
OAE0502
Transition to dolutegravir‐based regimens improves overall viral‐load suppression in the national ART cohort in Malawi and closes the gender gap
T. Hamisi1, T. Chimpandule1,2, A. Jahn1,2,3, B. Chiwandira1, T. Kalua1, R. Nyirenda1
1Ministry of Health, Department of HIV and AIDS, Lilongwe, Malawi, 2International Training and Education Center for Health, Department of HIV and AIDS, Lilongwe, Malawi, 3University of Washington, Department for Global Health, Seattle, United States
Background: Malawi introduced dolutegravir (DTG)‐based first‐line regimens in January 2019, targeting all new and existing patients > 20 kg, but excluding women of reproductive potential due to concerns of risk of neural tube defects. Most adults transitioned from tenofovir/lamivudine/efavirenz (TLE) to tenofovir/lamivudine/dolutegravir; children 20 to 30 kg to abacavir/lamivudine+dolutegravir. Confirmation of viral load suppression (VLS) was not required before transition due to limited monitoring capacity.
VL monitoring is scheduled 6 months from ART initiation and every 12 months thereafter. VLS rates were already high and it was unclear if DTG would yield additional population benefits.
Description: Before DTG‐transition, 97% of all 805,254 patients on ART were on efavirenz‐ or nevirapine‐based regimens and 92% of these were on TLE. Around 42% of patients received routine VL testing in the 12 months before transition; 89% of 334,233 results were < 1000 copies/mL. VLS among women was 92 to 95% in age groups ≥25 years and it was consistently lower in men. VLS was much lower among children and adolescents, and boys had 5 to 12% lower VLS rates than girls.
By September 2019, 61% of all patients were on DTG‐based regimens. VLS among the 311,956 results collected since the start of transition had increased to 93%. The VL gender gap in adults had disappeared. Boys and male adolescents showed the greatest increase in VLS.
Lessons learned: National lab information system (LIMS) data suggest an early increase in VLS following transition to DTG. The greatest increase was among patients selected for an early unconditional transition, where pre‐transition VLS had been unsatisfactory.
Abstract OAE0502‐Figure 1.
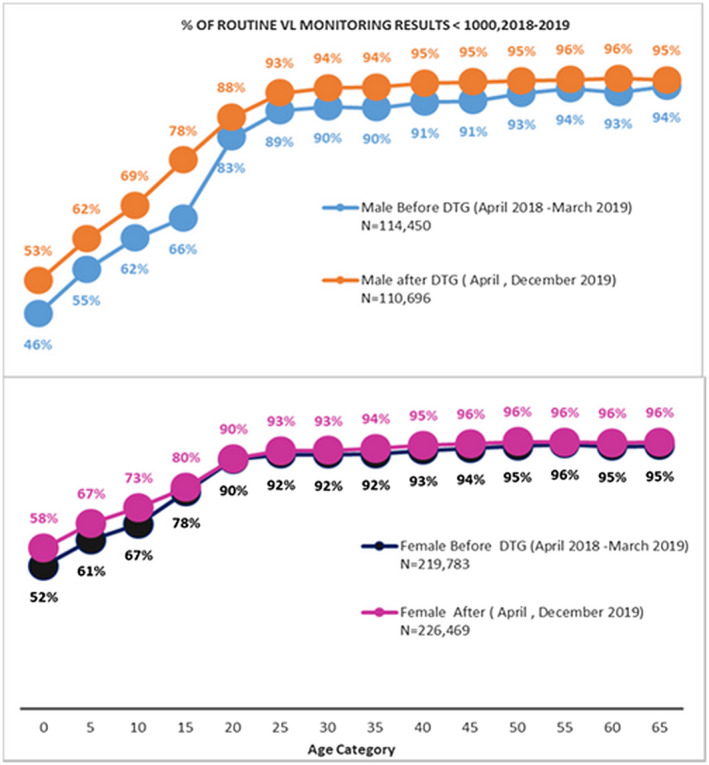
Conclusions/Next steps: Completion of the DTG transition for all patients over the coming months may further increase VLS, approaching 95%. Ongoing transition of children < 20 kg from nevirapine to lopinavir may improve VLS until convenient DTG‐based formulations become available.
Inclusion of current regimen and further patient characteristics in LIMS will add value for cross‐sectional and longitudinal programme monitoring.
OAE0503
“Missing men” or missed opportunity? Men's frequent use of health services in Malawi
K. Dovel1,2, K. Balakasi2, S. Gupta1,2, M. Mphande2, I. Robson2, P. Kalande2, E. Lungu2,2, A. Amberbir2,1, J. van Oosterhout2,1, S. Khan3, N. Doi3, B. Nichols4,5
1University of California, David Geffen School of Medicine, Division of Infectious Diseases, Department of Medicine, Westwood, United States, 2Partners in Hope, Science Department, Lilongwe, Malawi, 3Clinton Health Access Initiative, Boston, United States, 4Boston University, Department of Global Health, School of Public Health, Boston, United States, 5University of the Witwatersrand, Health Economics and Epidemiology Research Office, Department of Internal Medicine, School of Clinical Medicine, Faculty of Health Sciences, Johannesburg, South Africa
Background: Men are underrepresented in HIV testing across sub‐Saharan Africa. Community‐based strategies are prioritized for reaching men, however, little is known about the frequency of men's facility attendance for non‐HIV services, and if men attending facilities are offered HIV testing.
Methods: We conducted a cross‐sectional, community representative survey with men (15 to 64 years) from 36 villages in rural Malawi. We used staged sampling to randomly select individuals using census data, and stratified by village and age. Primary outcomes were facility attendance (as client or guardian who supports services for others) and HIV testing within 24 months. Descriptive statistics were conducted to examine facility visits among men in need of HIV testing.
Results: 1187/1254 of men completed a survey, of whom 884 (74%) were adults (25 + years). 67 (6%) were known positive and excluded from analyses. 87% of young (≤24 years) and 91% of adult (25 + years) men attended a facility visit within 24 months. 81% of facility visits were to outpatient departments. 58% of young and 38% of adult men were in need of HIV testing (i.e. tested > 24 months ago or never tested). Among those in need of testing, ~81% of young and ~77% of adult men visited a facility within 24 months for a non‐HIV visit (Figure 1). Guardian visits comprised the majority of visits made. Only ~15% of men in need of testing were offered HIV testing services during recent facility visits. Reasons for not testing during recent facility visits were: not offered testing (32%); not at risk of HIV (19%); and (3) not ready to test (14%).
Abstract OAE0503‐Figure 1.
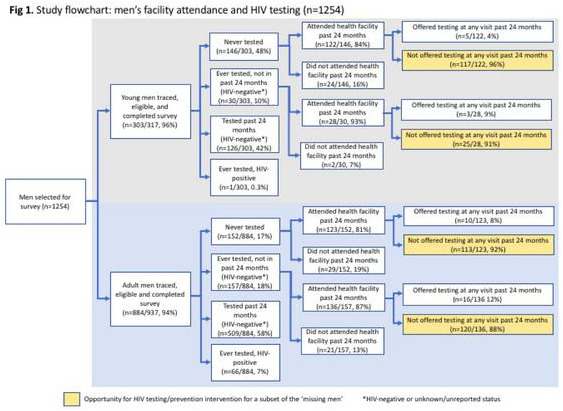
Conclusions: Most men regularly attended health facilities, especially outpatient departments. Men in need of testing were especially likely to attend facilities as a guardian, but few were offered HIV testing. HIV case finding interventions should capitalize on men's routine facility visits to reach the general male population.
OAE0504
Factors encouraging men to test for HIV for the first time in HPTN 071 (PopART) communities in Zambia
M.M. Phiri1, R. Kumar12, T. Gachie12, S. Floyd2, A. Schaap12, K. Shanaube1, S. Fidler3, S. Griffith4, R. Hayes2, H. Ayles12, HPTN071 Study Team
1Zambart, Lusaka, Zambia, 2London School of Hygiene and Tropical Medicine, London, United Kingdom, 3Imperial College, London, United Kingdom, 4FHI 360, Durham, United States
Background: HPTN071 (PopART) was a 3‐arm, community‐randomized trial in 21 Zambian and South African communities that demonstrated a reduction in HIV incidence following implementation of a combination prevention package including universal testing and treatment. The intervention was delivered in three annual rounds (ARs) of home‐based, door‐to‐door visits including HIV testing services by a pair of Community‐HIV‐care‐Providers (CHiPs) working in zones. We aim to determine whether household/CHiP‐related factors influenced men to test for HIV for the first time in 8 Zambian intervention communities during AR3.
Methods: The outcome was acceptance of HIV testing among men >18 years who had “never‐tested” during AR3 (September 2016 to December 2017). Individuals were considered “never‐tested” if they: never self‐reported previous HIV testing, did not verbally confirm their HIV‐positive status in current or previous rounds, and did not test with CHiPs during previous rounds. A multi‐level logistic model (zone/household‐nesting) was fitted adjusting for: community, whether other adults in the household accepted testing, whether participant was resident in previous ARs, whether CHiPs were intervention community residents, CHiP pair gender composition, and age of CHiP pair in relation to participant.
Results: During AR3, 6605/9172 previously “never‐tested” men (72.0%) accepted HIV testing. Factors associated with accepting testing are shown in Table 1. Uptake of testing was highest in mixed‐gender households where another man or woman tested for HIV (aOR = 21.68; 95% CI:16.06 to 29.28), and households of adult men where another man tested for HIV (aOR = 14.85;95% CI: 12.12 to 18.2). The age of the CHiP pair affected uptake of first‐time testing. Men had higher odds of testing when approached by a CHiP pair in which both were >5 years older than the participant (aOR = 2.28;95% CI:1.82 to 2.87), compared to a pair of younger CHiPs.
Abstract OAE0504‐Table 1.
| Description | n/N (%) | Adjusted odds ratio (95% CI) | p‐value |
|---|---|---|---|
| Any adult accepts testing in the participant's household | |||
| No other adult accepted testing in participant's household/bachelors | 433/1642 (26.4) | Reference | |
| Another adult man or woman accepted testing in participant's household of adult men and women | 5259/6505 (80.8) | 14.85 (12.12, 18.2) | <0.001 |
| Another adult man accepted testing in participant's household of only adult men | 913/1025 (89.1) | 21.68 (16.06, 29.28) | <0.001 |
| CHiP pair and male participant age difference | |||
| Both CHiPs >5 years younger to participant | 399/708 (56.4) | Reference | |
| Both CHiPs >5 years older to participant | 3401/4440 (76.6) | 2.28 (1.82, 2.87) | <0.001 |
| At least 1 CHiP is a peer of participant (within ±5 years) | 2133/3028 (70.4) | 1.84 (1.46, 2.30) | <0.001 |
| Mixed age pair: older (>5 years) and younger (>5 years) CHiPs | 443/658 (67.3) | 1.76 (1.29, 2.40) | <0.001 |
Conclusions: Identifying positive role‐models, such as older (and therefore respected) health providers, or other adults in the household who accept testing, increases first‐time testing among men who have never‐tested before.
OAE0505
Implementation of HIV self‐testing (HIVST) to reach men in rural Umkhanyakude, KwaZulu‐Natal, South Africa
N. Sithole1, A.E. Shapiro2, M. Krows2, O. Koole1, T.T. Schaafsma2, D. Pillay1, C.L. Celum2, R.V. Barnabas2
1Africa Health Research Institute, Mtubatuba, South Africa, 2University of Washington, Seattle, United States
Background: African women have higher rates of HIV testing, HIV prevalence and ART coverage, and consequently men have lower survival rates. KwaZulu–Natal, South Africa has one of the highest HIV prevalence rates globally, where persons <35 years and men account for most of the people who have not been tested for HIV. HIV self‐testing (HIVST) may overcome some of the barriers of facility‐based HIV testing in order to identify HIV positive young persons and men and link them to care.
Methods: Teams made up of a nurse, clinic research assistant, and 4 recruiters distributed HIVST kits in rural Umkhanyakude, KwaZulu‐Natal from August to November 2018 with a focus on testing men. Places where men could be found such as workplaces (farms), social venues, taxi ranks and homesteads were used as HIVST distribution points. Community sensitization was done through community advisory boards (CABs). In areas without CABs, permission to distribute kits was granted by the local chiefs. The Department of Health assisted with confirmatory testing and linkage at their facilities, and a 24‐hour cell phone number was provided in case of an emergency.
Results: Over 11 weeks , we distributed 2634 HIVST kits with 2052 (78%) kits distributed to aged <35 years and 582 (22%) kits distributed to aged ≥35 years. 2591 (98%) kits were distributed to males and 43 (2%) were distributed to females. Of those, 2107/2634 (80%) used the HIVST kits and provided results to the study team, among whom 157/2107 (7%) tested positive. Of those who tested positive, 153/157 (97%) were males .102/157 (65%) did a confirmatory test and were initiated on ART. No emergencies were reported.
Conclusions: Large scale distribution of HIVST kits targeting men in rural Umkhanyakude is feasible, acceptable in the community, and effective at reaching men who have not tested for HIV. While two‐thirds of persons who tested HIV positive initiated ART, additional linkage strategies are needed for those who do not link after HIVST. This testing strategy should be used as a tool to reach men in order to achieve 95 coverage in the UNAIDS testing and care cascade in KwaZulu‐Natal.
OAE0506
Understanding men who have sex with men (MSM) using human‐centred design approach in Zimbabwe
K. Chatora1, C. Chang2, H. Sithole1, Z. Adell2, N. Kunaka1, K. Riger2, C. Johnson1, H. Ndondo1, N. Taruberekera1
1Population Services International, Harare, Zimbabwe, 2IDEO.org, San Francisco, United States
Background: Like many African countries, Zimbabwe has a paucity of research on men who have sex with men (MSM). Against this backdrop, PSI Zimbabwe employed human‐centred design (HCD) approaches to understand the lives of MSM and co‐create solutions to improve their uptake of HIV prevention and treatment services. We explored the MSM journey to accessing services and created distinct archetypes based on barriers and motivators to service uptake.
Description: We used HCD techniques involving immersions, co‐designing and prototyping solutions with the MSM community. We conducted 18 focus group discussions, 20 one on one interviews, 3 hotspot immersions and 3 observations across two urban areas over a two‐week period. A total of 65 men from the MSM community and 5 service providers participated in this exploration. Thematic analysis was used to analyse the data.
Lessons learned: We identified 6 archetypes and a journey map detailing how each archetype accesses services. These archetypes included: The Glass Box ‐ Identifies as gay only within the MSM community for fear of stigma; The Subtle Champion – Is an advocate providing social and health‐related support to others; The Flag Bearer ‐ Openly gay and unconcerned with societal stigma; The Dual Life ‐ Conforms to heterosexual societal expectations and embraces his sexuality only in safe spaces; and The Conflicted Heart ‐ Fighting the fact that he has just realized his attraction to men. Although the journey was fairly the same across all the archetypes there were some differences in experiences by different archetypes which resulted in archetypes with more comfort self‐ identifying as MSM, like the Flag Bearer, being most likely to engage with HIV services. A gay man relates to HIV when a man he had sex with dies from HIV. He contemplates getting tested but fears social fall out. After testing, the need to disclose his MSM activity and a lack of provider empathy prevent him from returning.
Conclusions/Next steps: MSM archetypes and their journey to the uptake of HIV prevention and treatment services differ based on their mindsets and behaviour. Understanding these archetypes presents an opportunity to tailor‐make the provision of HIV services and mobilization activities to their unique needs.
OAE0602
Domestic public spending in low‐and‐middle‐income countries 2006 to 2018: Levels and trends
D. Mattur1, J.A. Izazola Licea1, J. Javadekar2
1UNAIDS, Strategic Information, Geneva, Switzerland, 2University of Geneva, Institute of Economics and Econometrics, Geneva, Switzerland
Background: Domestic public investments in HIV/AIDS have consistently increased annually in LMICs. Understanding the correlates and predictors of domestic public spending on HIV can inform sustainability plans.
Methods: Country reported domestic public AIDS spending data from Global AIDS Monitoring were considered. A panel of data using data from 2005 to 2018 from 114 low‐and‐middle‐income countries were used for regression analysis resulting in 1224 country year data points. All the estimations are based on panel data random effects models.
A panel data regression using reports to UNAIDS for 2005 to 2018 from 114 low‐and‐middle‐income countries were used for the analysis resulting in 1224 country year data points. All the regression estimates are based on panel data random effects models.
Results: There are significant positive associations between the log of GDP per capita (1.058, <0.001) of a country and its level of domestic public spending on HIV. The ART coverage (15.75, <0.001) and the HIV prevalence (0.05, <0.01) were also significant predictors. No significant effect was found for ODA for HIV or other independent variables.
The domestic public resources per person living with HIV in 2018 was US$184 in East and Southern Africa, US$50.6 in West and Central Africa, US$363 in Asia and the Pacific, US$209 in the Caribbean, US$659 in Easter Europe and central Asia, US$ 1406 in Latin America, and US$479 in Middle East and North Africa.
Domestic public spending has increased 70% between 2010 and 2018. In 2018, domestic resources (public and private) constituted 56% of the global AIDS resources; the majority of these being public funds. With the observed flat lining of international resources, sustained and efficient domestic public spending will be key in achieving fast‐track targets to end AIDS by 2030.
There are still large gaps in donor dependency across geographies, for example while 95% of AIDS resources in Latin America come from domestic resources on the other extreme of dependency the share of domestic resources is 38% and 27% in West Central Africa and the Caribbean respectively.
Conclusions: The main determinants of domestic public spending for HIV are, as expected: ability to pay (GDP per capita), burden of disease (HIV prevalence) and ART coverage.
OAE0603
Scaling up antiretroviral therapy in a resource‐limited setting: Mexico's experience
M. Valenzuela‐Lara1, E. León‐Juárez2, P. Uribe‐Zúñiga3, C. Magis‐Rodríguez4
1Emory University, Epidemiology, Atlanta, United States, 2National Center for the Prevention and Control of HIV and AIDS (Censida), Mexico City, Mexico, 3National Institute for Women (Inmujeres), Mexico City, Mexico, 4Universidad Nacional Autónoma de México, Departamento de Salud Pública, Mexico City, Mexico
Background: In 2013, the National Institute of Public Health of Mexico concluded that if the observed trends of antiretroviral therapy (ART) costs were maintained, and the number of people living with HIV (PLWH) on ART grew at the same observed rate, the current financial mechanisms in the Ministry of Health (MoH) would result insufficient by 2018. At the same time, evidence regarding the initiation of ART “as soon as possible” rapidly accumulated.
Description: As part of the national efforts to expand ART coverage and improve the optimal allocation of resources, the MoH implemented four key strategies: (1) a medical drug prescription monitoring group, which included the development and implementation of a prescription electronic algorithm according to the clinical guidelines, (2) encouraged competition among generic antiretroviral drug makers, (3) improved forecast accuracy and (4) data transparency.
Lessons learned: The prescription‐monitoring group improve the quality of the prescription and allowed a more rational use of ART. The improvement of forecast accuracy and data transparency showed a more stable and attractive market which incentive competition. Finally, the number of available generic ARVs increase by 71% and achieved important savings. For example, the acquisition of efavirenz through a state‐owned enterprise achieved a price reduction of 62% between 2014 and 2018; and the acquisition of the generics EFV/TDX/3TC and TDX/3TC, coformulations that were used in 74,188 of the 95,732 people on ART by the end of 2018, reached price reductions of 66 and 44%, resulting in savings of up to $68 million USD in 2019.
Conclusions/Next steps: These strategies allowed a 68% increase in the number of people on ART between 2013 and 2018, while the expenditure only increased by 29%, generating savings of up to $125.4 million USD and contributing greatly to the sustainability of the universal access to ART programme.
Abstract OAE0603‐Figurte 1. Annual costs of ART and PLWH on ART in Mexico's Ministry of Health between 2013 and 2018.
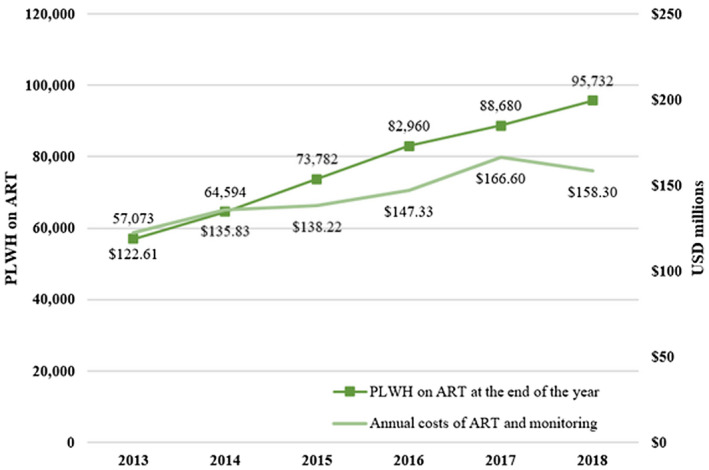
OAE0604
Making ARV medicines affordable: Community advocacy good practice on reducing ARV price in Indonesia
A. Wardhana1
1Indonesia AIDS Coalition, Jakarta, Indonesia
Background: Indonesia has an increasing HIV rate. Fueled by stigma and discrimination against the AIDS‐affected population, the number of people living with human immunodeficiency virus (PLHIV) in the country is among the highest in the region. According to a government survey, roughly 640,000 people are living with HIV in Indonesia, with only 124,813 (19.49 percent) of them undergoing crucial antiretroviral (ARV) treatment. The country has the lowest antiretroviral therapy (ART) coverage in the region. Since 2004, ARV treatment has been given for free since the government provided full subsidies for ARV medicine procurement. However, due to the inefficiency of the procurement system, the price of ARV medicines procured by the government is among the highest in the world.
Description: A rational price structure analysis was developed in 2016, supported by many partners both nationally and internationally, to assess the cause of this exorbitant price as well as to recalculate the rational price. These findings, which was compiled into a briefing paper, was circulated widely to stakeholders. Several press conferences also were conducted to raise awareness on this issue to a broader society.
Lessons learned: During the MoH meeting in November 2019, it was decided that GoI will procure the ARV TLE by price of IDR 210.000 (UDS 15) per bottle. This price is 48% lower than the usual price. With total of 48.981 PLHIV consuming these packed regimens, the government will have an estimated IDR 114 billion of saving, which is roughly USD 8 million per year. This savings will be able to add 45.482 PLHIV on treatment, using the same regimen.
Conclusions/Next steps: The price reduction that resulted from the patient group's advocacy efforts by using price structure analysis is a certifiable success. This bold action should trigger more government measures to lower other ARV regimens’ prices. This could be replicated with other medicines as well. IAC plan to replicate this success to analyse other medicines to ensure that medicines price for HIV and Tb cheaper thus can expand access to more people who need it.
OAE0605
Introducing Result‐Based Financing (RBF) model to scale up Opioid Substitution Therapy (OST) in Ukraine
Z. Islam1, T. Prokhorova1, V. Kolomiets1, O. Masiuk1, K. Shavchenko1, N. Ivanova1
1Alliance for Public Health, Treatment, Procurement and Supply Management, Kyiv, Ukraine
Background: Ukraine is implementing OST using methadone and buprenorphine since 2008, however, scale up and achieving national target for OST has been a challenge due to legal barriers and lack of motivation from the HCFs. Currently, only 4.2% of estimated PWID receives OST in Ukraine. Financial mechanism introduced under the health care reform in 2016 was an appropriate opportunity to introduce RBF model in order to scale up OST target in the country.
Description: RBF model within OST services is implemented under the modalities of direct contracting with 3 health care facilities and it aimed to improve access to OST for PWIDs by ensuring high‐quality services for clients; improve retention and introduce enhance monitoring mechanism for all OST services within new RBF model. Using incentivized monthly payments, simple billing system and direct contracting with the HCF for achieving target, has increased motivation of health care providers to scale up and improved retention in the programme.
Lessons learned: 1038 OST patients were enrolled under the RBF service model from 2016 till 2019 in 3 different sites. The project selected 3 HCF sites similar to 3 RBF sites in terms of number of patients and the package of services provided. All results showed RBF‐model indicators increased compared with HCF sites: patient's growth – by 16.4%, retention rate – by 24.1 %, number of patients on ART by 9.8%, retention rate of OST patients who received drugs within 7 to 10 days – by 17.5%.
Abstract OAE0605‐Table 1.
| Indicator | RBF model sites | Other HCFs | Difference between RBF model sites and Other HCFs |
|---|---|---|---|
| Number of patients as of 30.09.2016 | 751 | 660 | |
| Number of patients as of 30.09.2019 | 1038 | 804 | |
| Patient's enrolment growth | 38.20% | 21.80% | 16.4% |
| Number of patients with HIV (as of 30.09.2019) | 319 | 317 | |
| Number of patients on ART (as of 30.09.2019) | 316 (99.1%) | 283 (89.3%) | 9.8% |
| Percentage of OST patients who received drugs within 7 to 10 days (as of 30.09.2019) | 711 (68.5%) | 410 (51%) | 17.5% |
| Retention rate of OST patients receiving treatment continuously for at least 6 months | 74.10% | 50.00% | 24.1% |
Conclusions/Next steps: While the payment for OST service provider is still under discussion and development of health care reform led by newly formed national health services, it is evident that a system of RBF method can be implemented within the government healthcare system of Ukraine. RBF is proven to improve attitudes of Head Doctors and representatives of oblast authorities towards acceptance and contributing to OST scale‐up. RBF model should be implemented in all OST sites in Ukraine.
OAE0606
Results from a performance‐based financing (PBF) pilot to incentivize HIV case‐finding and viral load sample collection in Haut Katanga in the Democratic Republic of the Congo (DRC)
D. Canagasabey1, I. Thior1, V. Cikobe2, N. Meyer1, J. Diarra2, P. Kibwa2, J.‐C. Kiluba2, L. Mueller Scott1
1PATH, Washington DC, United States, 2PATH, Lubumbashi, Congo, Democratic Republic of the
Background: In a time of stagnating international HIV/AIDS financing and increasingly ambitious targets to enable ending AIDS as a public health threat by 2030, identifying cost‐effective and efficient financing methods is critical to ensure value for money and achieve more with available resources. Through the USAID‐funded Integrated HIV/AIDS Project (IHAP), PATH piloted a PBF mechanism to incentivize efficient HIV case‐finding and increase viral load sample collection.
Description: In October 2018, IHAP introduced a PBF mechanism across 106 project‐supported health facilities in Haut Katanga Province. Instead of receiving 100% of monthly stipends regardless of site‐level achievement against monthly targets, under the PBF mechanism, 40% of providers’ monthly stipends were paid based on site‐level achievement against select indicators, including:
Number of newly diagnosed people living with HIV (PLHIV).
Number of viral load samples collected and transported to laboratories for analysis.
Programmatic data were used to compare facility achievement in HIV testing, new PLHIV identified, testing yield, and viral load sample collection between the pre‐pilot period (October 2017 through September 2018) and the pilot period (October 2018 through September 2019).
Lessons learned: Programmatic data showed an improvement in targeted indicators with the introduction of PBF. Incentivizing identification of HIV‐positive individuals resulted in more efficient HIV testing, with less individuals tested but more PLHIV identified during the pilot period, leading to an increase in testing yield from 4.1% during the pre‐pilot period to 5.6% during the pilot period. There was also a 52% increase in the number of viral load samples collected by facilities during the pilot period.
Abstract OAE0606‐Table 1.
| Indicators | Pre‐pilot period (October 2017 to September 2018) | Pilot period (October 2018 to September 2019) | ||
|---|---|---|---|---|
| Mean | Median (Interquartile range) | Mean | Median (Interquartile range) | |
| Number of people tested | 40,587 | 40,897 (37,602 – 43,571) | 37,705 | 36,300 (35,104 to 40,306) |
| Number of new PLHIV identified | 1674 | 1648 (1382 to 1967) | 2127 | 2006 (1646 – 2608) |
| Testing yield | 4% | 4% (3% to 5%) | 5% | 5% (4% to 6%) |
| Number of viral load samples collected | 3521 | 3505 (2647 to 4395) | 5363 | 5441 (4588 to 6138) |
Conclusions/Next steps: Our analysis shows an improvement in HIV case‐finding and viral load sample collection between the pre‐pilot and pilot periods, suggesting that PBF positively affected facility achievement in targeted programmatic areas. Based on these results, IHAP is continuing implementation of PBF with project‐supported facilities to incentivize behaviours to support the DRC achieve epidemic control.
OAE0702
Low proportion and retention rates among Thai men who have sex with men using event driven PrEP
T. Chinbunchorn1, G. Pungpapong1, O. Nampaisarn1, S. Teerasakulpisal1, S. Janyam2, T. Sangprasert3, P. Chanlearn4, M. Avery5, S. Mills5, R. Vannakit6, P. Phanuphak1, N. Phanuphak1, R. Ramautarsing1
1Thai Red Cross AIDS Research Centre, Prevention, Bangkok, Thailand, 2Service Workers in Group, Bangkok, Thailand, 3Rainbow Sky Association of Thailand, Bangkok, Thailand, 4Mplus, Chiang Mai, Thailand, 5FHI360 and LINKAGES, Bangkok, Thailand, 6Office of Publich Health, USAID Regional Development Mission Asia, Bangkok, Thailand
Background: Men who have sex with men (MSM) can take oral pre‐exposure prophylaxis (PrEP) either daily or event‐driven (ED) to prevent HIV acquisition. However, ED‐PrEP, defined as PrEP initiated 2 to 24 hours in advance of sex, uptake in Thailand has been low. We compared characteristics and retention of daily and ED‐PrEP clients to profile the clients and their respective retention to PrEP.
Methods: Data from June 2018 to June 2019 were analysed from Thailand's Princess PrEP programme, which is implemented in 8 community‐based clinics. All MSM clients were offered the choice between ED and daily PrEP. Retention was determined at 1, 3 and 6 months after initiation. Effective use was defined as taking ED‐PrEP as instructed, or taking more than 4 pills per week for daily PrEP.
Results: 2655 MSM clients initiated PrEP, 2516 (94.8%) daily, 139 (5.2%) ED‐PrEP. Median age was 30 years (IQR 25 to 35), 93.6% reported inconsistent condom use, 61.8% reported using any drugs including alcohol during sex. Reasons for choosing daily PrEP included: frequent sexual intercourse (36.6%), and not being able to predict or delay sexual intercourse (43.5%). Reasons for choosing ED‐PrEP included: infrequent sexual intercourse (49.4%), being able to predict or delay sexual intercourse (32.8%), unwilling to take pills everyday (9.8%), and worried about side effects of daily PrEP (4.6%). Retention at Month 1, 3 and 6 was 31.6%, 35.2%, and 38.4%, respectively, for daily PrEP and 18.7%, 22.3%, and 23.7%, respectively, for ED‐PrEP (p < 0.001). During 3830 daily PrEP visits, 1.5% reported taking <4 pills/week, among them 0.6% reported inconsistent condom use. During 190 ED‐PrEP visits, 5.3% reported taking ED‐PrEP incorrectly during their sexual encounters, and 5.3% also reported condomless sex during those periods.
Conclusions: Low proportions of clients choose ED‐PrEP and significantly lower retention rates compared to daily PrEP were observed. Clients who engage in condomless sex while incorrectly taking ED‐PrEP should have counselled to understand potential risks. ED‐PrEP knowledge distribution, emphasising retention and adherence, should occur concurrently with daily PrEP promotion as an alternative. Strategies such as technology‐assisted pill reminder notifications should be explored to further facilitate ED‐PrEP roll‐out.
OAE0703
PrEPTECH ‐ integration of an on‐ and off‐line holistic PrEP solution for youth
R. Braun1, B. Sheoran1, L. Idelson1, J. Lykens1, J. Klausner2
1YTH Initiative, ETR, Oakland, United States, 2UCLA, David Geffen School of Medicine and Fielding School of Public Health, Los Angeles, United States
Background: In the United States, young men who have sex with men (YMSM) and transgender youth (TY) of colour represent a high number of new HIV diagnoses annually. HIV pre‐exposure prophylaxis (PrEP) is effective and acceptable to YMSM and TY of colour; yet, PrEP uptake is low in those communities because of barriers including stigma, cost, adherence concerns, and medical distrust. PrEPTECH, a telehealth‐based approach to PrEP initiation, may be a solution to those barriers.
Description: To pilot PrEPTECH, we enrolled 25 HIV‐uninfected YMSM, aged 18 to 25 years, from San Francisco into a 180‐day longitudinal study between November 2016 and May 2017. Participants received cost‐free PrEP services through telehealth [i.e. telemedicine visits, home delivery of Truvada, and STI testing kits], except for 2 laboratory visits. Participants completed online survey assessments at 90 and 190 days querying PrEPTECH features and experiences, as well as PrEP adherence and stigma. The pilot demonstrated that PrEPTECH was confidential, fast, convenient, and easy to use, supporting participants to effectively access and maintain a PrEP regimen.
Lessons learned: Based on feedback from participants, we made several changes to the PrEPTECH platform to improve the user experience, and better reach key populations affected by HIV. We added an online pharmacy for PrEP distribution, in‐home STI/HIV testing, and a telehealth platform to conveniently connect users to a PrEP provider. We also expanded the eligible participant definition to include TY [including but not limited to transgender men, transgender women, and gender non‐conforming individuals], as well as increase the eligible age range to 15 through 27.
Conclusions/Next steps: Telehealth programmes such as PrEPTECH can increase PrEP access for YMSM and TY of colour by eliminating barriers inherent in traditional clinic‐based models, support quick and convenient PrEP initiation, and transition users to a sustainable PrEP provider. Given promising pilot study findings, we are launching a multi‐site randomized controlled trial to test the efficacy of PrEPTECH in enhancing PrEP uptake by comparing it to a knowledge‐focused online website. We are also exploring opportunities to launch PrEPTECH in other countries most affected by HIV, including South Africa and India.
OAE0704
Monitoring characteristics of episodic HIV pre‐exposure prophylaxis (PrEP) use among over 40,000 clients in sub‐Saharan African countries prescribed daily oral PrEP: Indefinite, continuous use neither the reality nor the goal
J. Reed1, P. Shrestha2, J. Mutegi3, D. Were3, A. Musau3, B. Wakhutu3, T. Chakare4, A. Rozario4, N.M. Nonyana4, R. Manda5, L. Maile6, A. Christensen7, J. Daud7, B. Burure7, R. Eakle8,9, K. Curran1, D. Mohan2
1Jhpiego an affiliate of Johns Hopkins University, Baltimore, United States, 2Johns Hopkins Bloomberg School of Public Health, Baltimore, United States, 3Jhpiego, Nairobi, Kenya, 4Jhpiego, Maseru, Lesotho, 5USAID Lesotho, Maseru, Lesotho, 6Lesotho Ministry of Health, Maseru, Lesotho, 7Jhpiego, Dar es Salaam, Tanzania, United Republic of, 8United States Agency for International Development, Washington DC, United States, 9London School of Hygiene and Tropical Medicine, London, United Kingdom
Background: Substantial drop‐off rates of oral pre‐exposure prophylaxis (PrEP) use within the first year are reported by many programmes, which may be misconstrued as programme failure. However, PrEP use may be non‐continuous and still effective, since HIV risk fluctuates. Real‐world PrEP use phenomena, like restarting and cyclical use, and the temporal characteristics of these use patterns are not well described.
Methods: We analysed demographic and clinical data routinely collected during client visits. A > 14‐day delay in returning for refill was defined as PrEP discontinuation. Clients resuming PrEP after discontinuation were deemed a restart. The initial start and subsequent restart(s) of PrEP defined the beginning of independent use cycle(s), with each continuing for as long as refills were obtained without delay. Using prescriptions as a proxy for actual use, we characterized duration on/off PrEP, and modelled the likelihood of spending time (in months) off PrEP using ordinal regression.
Results: Through May 2019, Jhpiego‐supported PrEP programmes in Kenya, Lesotho, and Tanzania initiated 41,459 clients on a daily dosing (not event‐driven) PrEP regimen. Among these, 10,809 (26.1%) discontinued and subsequently restarted PrEP at least once, with 20.7%, 27.5%, and 51.8% remaining off of PrEP for <30, 30 to 60, and 61 + days, respectively. The median days’ duration of use for the first versus subsequent use cycle(s) was 222 days. With each increase in cycle number, clients were 12.5% (11.1 to 14.8), 45.8% (26.4 to 60.1) and 24.1% (7.6 to 37.6) less likely to stay off PrEP for an extra month, in Kenya, Tanzania, and Lesotho programmes, respectively. Females 15 to 24 years were 40.1% and 57.2% less likely to stay off PrEP for an extra month than the general population in Kenya and Lesotho, respectively.
Conclusions: PrEP users frequently cycle on and off PrEP, which may effectively protect them against HIV risk that is periodic. While duration of use did not increase with cycle number, the duration spent off PrEP did decrease in all countries with subsequent use cycles, suggesting normalization of use with experience, particularly by young women. With the likely introduction of event‐driven PrEP on the horizon, more nuanced measures of use are needed if successful use of PrEP is to be meaningfully distinguished from HIV treatment.
OAE0705
Oral pre‐exposure prophylaxis (PrEP) and family planning (FP) integration to improve PrEP continuation among adolescent girls and young women (AGYW) in Kenya
D. Were1, A. Musau1, P. Ongwen1, M. Strachan2
1Jhpiego, Nairobi, Kenya, 2Jhpiego, Baltimore, United States
Background: Despite steady progress in uptake among AGYW, PrEP continuation remains a significant challenge in Kenya, with majority of the new PrEP initiates discontinuing their PrEP use within the first month. This study analyses PrEP continuation outcomes among AGYW within the context of integrated PrEP and FP delivery.
Description: Jilinde, a four‐year PrEP scale‐up project in Kenya, delivers PrEP for AGYW in Migori County through twelve sites that include public and private health facilities, drop in centres and community safe spaces. Demand creation for PrEP is conducted by peer educators at the community and AGYW referred to the diverse service delivery points for uptake and follow‐up monitoring. PrEP is provided as part of integrated services that includes pregnancy and HIV prevention interventions. PrEP and FP counselling and services are offered concomitantly by the same provider during the same session, while follow‐up services are synchronized.
Lessons learned: Overall, 2662 AGYW initiated PrEP between May 2017 and November 2019, of these 991 (37%) revisited at month‐one. Further, there was an upward increase of FP uptake from 53 PrEP clients in 2017 to 618 in 2019, contributed by increasing PrEP uptake. Although continuation remained a consistent challenge, Jilinde observed slightly better month‐one continuation rates among AGYW who concurrently initiated both PrEP and FP (39.4%), compared to AGYW who only initiated PrEP (36.2%). Routine service delivery data from Jilinde elucidates that AGYW using FP, compared to those who do not, have lower odds of discontinuation at month 1 (OR 0.71 (0.53 to 0.93), (p = 0.01) and similarly lower odds at month 3 (OR 0.55 (0.30 to 0.98), (p = 0.04). This suggests that delivering PrEP combined with FP to AGYW might be working synergistically to improve persistence for both interventions.
Conclusions/Next steps: AGYW demonstrate an appetite for PrEP, but struggle with persistence. When PrEP is paired with FP, there is promise for better persistence compared to PrEP offered alone. These findings suggest that pairing biomedical HIV prevention interventions such as PrEP with sexual and reproductive health services has the potential to optimize continuation outcomes, but warrants further investigation.
OAE0706
The use of community‐based approach and telemedicine as an alternative option in providing HIV pre‐exposure prophylaxis in the Philippines
J.D. Rosadiño1, J. Gonzales1, J.O. Corciega1, R. Pagtakhan1, C. Lagman1
1LoveYourself, Inc., Manila, Philippines
Background: The World Health Organization recommended the use of PrEP in addition to other prevention methods may be an option to halt and reverse the HIV epidemic in the Philippines. However, roll‐out efforts and scaling up PrEP use remains low because of various barriers. This results in very few facilities offering PrEP. Learning from the experiences from its demonstration project, LoveYourself, in this project, aims to offer PrEP in various locations in the country through an alternative method of service delivery: community‐based approach and telemedicine.
Description: LoveYourself has partnered with Hi‐Precision Diagnostics, a medical diagnostics facility with over 50 branches all over the Philippines, in order to provide other access points of all the diagnostic tests needed in order to access PrEP. A client goes to a Hi‐Precision facility, and access tests for HIV, Hepatitis B, Syphilis, and serum creatinine. Once done, the results will be sent directly to LoveYourself. LoveYourself then schedules a telemedicine session with the client in order to provide: a consultation with a physician, and PrEP coaching session with a trained LoveYourself volunteer. The coaching sessions include information about PrEP, such as uptake, adherence, and side‐effects management. It also includes counselling for HIV, STI, and sexual health management. After the session, the PrEP is delivered to the client via courier, with options for both door‐to‐door delivery and pick‐up at delivery points.
Lessons learned: The partnership of LoveYourself and Hi‐Precision effectively adds over 50 access points of PrEP in the Philippines. About 14.1% of people who expressed interest to obtain PrEP via LoveYourself channels have expressed to get it via this process. The clients who have accessed this process have also provided their feedback on how to further improve this process. This process has also provided ways for people who want to access PrEP conveniently, especially to some clients who prefer to access PrEP discreetly.
Conclusions/Next steps: While the project aims to effectively offer multiple access points for PrEP in the Philippines, this process has also provided a new avenue for the community to access PrEP in a convenient way. Further promotions are needed to make more people aware of this process.
OAE0802
Leading from the community: How key population organizations in Vietnam transformed from peer support groups to clinical service providers
B. Vu1, T. Le2, S. Le3, P. Nguyen4, H. Vu5, D. Nguyen6, K. Green1, H. Phan7, H. Ngo1, A. Doan1, T. Tran1, G. Ha1, A. Tran1, T. Nguyen8, L. Hang8, T. Ngo9, P. Tran8, H. Nguyen1, T. Phan1, B. Nguyen1, H. Van10, L. La11, G. Nguyen12
1PATH, HIV/TB, Hanoi, Vietnam, 2Glink Social Enterprise, Ho Chi Minh City, Vietnam, 3G3VN Social Enterprise, Ho Chi Minh City, Vietnam, 4Blue Sky Social Enterprise, Ho Chi Minh City, Vietnam, 5Vietnam MSM‐TG Community Social Enterprise, Hanoi, Vietnam, 6Green Trust Community‐Based Organization, Hanoi, Vietnam, 7Vietnam Administration for HIV/AIDS Control, Hanoi, Vietnam, 8LIFE Center, Ho Chi Minh City, Vietnam, 9USAID, Hanoi, Vietnam, 10Ho Chi Minh City Center for Disease Control, Ho Chi Minh City, Vietnam, 11Hanoi Center for Disease Control, Hanoi, Vietnam, 12Dong Nai Center for Disease Control, Dong Nai, Vietnam
Background: Reaching the last mile to achieve 95‐95‐95 and the goal of ending AIDS by 2030 requires breakthrough approaches. Key population (KP) leaders, government authorities and USAID/PATH Healthy Markets (HM) partnered to pilot and scale up KP–led HIV services as an critical way to increase reach and service uptake in Vietnam.
Description: Four key steps were employed to introduce and scale‐up KP‐led HIV services in Vietnam from late 2015 onward: 1) KP community‐based organizations (CBOs) were enabled for the first time to pilot and then scale HIV lay and self‐testing; 2) KP delivery of assisted partner notification services; 3) registration of first‐ever KP owned and led clinics offering HIV and other health services; and 4) launching PrEP services. The scope of KP‐led HIV services expanded along with the transformation of KP groups from self‐help groups to CBOs and social enterprises. In parallel, HM advocated for policy change by engaging the Ministry of Health (MOH) to endorse pilots, develop national guidelines, and amending regulations to facilitate nationwide scale‐up.
Lessons learned: KP‐led lay HIV testing reached first‐time and infrequent testers and yielded high HIV‐positive results. From Dec 2015 to Sept 2019, HM tested 124,285 clients through lay testing, 11,450 clients through self‐testing, and 15,961 sexual and injecting partners through index testing, with HIV‐positivity rates of 4.6%, 6%, and 9.2%, and antiretroviral therapy initiation of 94.8%, 94.1%, and 99.4%, respectively. KPs preferred PrEP services delivered by KP‐led organizations. Among 3631 PrEP users from Mar 2017 to Sept 2019, KP‐led private clinics enrolled the majority of the users (81.4%). Promising pilot results served as a powerful tool for policy advocacy. As a result, Vietnam MOH approved national guidelines and nationwide scale‐up plans for community HIV testing in 33 provinces and PrEP in 26 provinces out of 63 province of the country.
Conclusions/Next steps: KP‐led HIV services are feasible, acceptable, and preferable by KPs and are therefore a critical addition to accelerating attainment of 95‐95‐95 and ending AIDS goals in Vietnam. KP‐led HIV services has now been integrated as an essential part of the national action plan and national ending AIDS by 2030 strategy.
OAE0803
Scaling up a lay counsellor‐delivered transdiagnostic mental health intervention (CETA) to improve the HIV care cascade in Sofala, Mozambique
W. Hammett1,2, A. Muanido3, V. Cumbe4,5, C. Mukunta3, N. Manaca3, L. Hicks2, S. Dorsey6, B. Wagenaar1,2,7
1University of Washington, Department of Global Health, Seattle, United States, 2Health Alliance International, Seattle, United States, 3Health Alliance International, Beira, Mozambique, 4Sofala Provincial Health Directorate, Ministry of Health, Department of Mental Health, Beira, Mozambique, 5Eduardo Mondlane University, Faculty of Medicine, Maputo, Mozambique, 6University of Washington, Department of Psychology and Behavioral Sciences, Seattle, United States, 7University of Washington, Department of Epidemiology, Seattle, United States
Background: Evidence shows that common mental illnesses are associated with poor HIV treatment outcomes and that lay‐counsellor‐delivered mental health therapies can improve symptoms of common mental illness among HIV+ patients in low‐ and middle‐income countries (LMICs). Yet, few LMICs have scaled‐up lay‐counsellor‐delivered therapies for HIV+ patients, and few studies have shown that such therapies can improve the HIV care cascade. The present study examined the scale‐up of a lay‐counsellor‐delivered psychological therapy (CETA) delivered in routine HIV care and its effect on mental health symptoms and HIV care cascade outcomes in Mozambique.
Description: CETA was integrated into routine public HIV care in five high‐flow urban facilities in Beira City, Sofala, Mozambique. Beginning in May 2019, all newly diagnosed adult (18 + ) HIV+ patients were screened for common mental illness and those with clinically significant symptoms were offered weekly individual psychological therapy sessions. Mental symptoms were tracked at each visit and HIV care cascade outcomes were collected for CETA patients and compared with facility averages.
Lessons learned: 59% (148/250) of newly diagnosed HIV+ patients showed clinically significant mental symptoms and 16% (20/127) had suicidal ideation. Mental health symptoms of CETA‐enrolled patients decreased 56% after 4 sessions and 90% after 6 sessions; suicidal ideation decreased to 0% after 4 sessions. The combined rate of ART initiation among CETA participants was 98%. Among CETA patients attending 2 or more sessions, the ART initiation rate was 100%. One‐month retention among CETA participants was 69%, compared to the combined one‐month retention rate of 60% for all HIV+ patients (those offered CETA + those not offered CETA). Three‐month retention among CETA patients was 83%, compared to 64% among all HIV+ patients.
Conclusions/Next steps: Over 50% of newly diagnosed HIV+ patients in Mozambique suffer from clinically significant mental health symptoms and over 15% experience suicidal ideation. After 4 CETA sessions, mental health symptoms decreased over 50% and suicidal ideation decreased 100%. Compared to all HIV+ patients, CETA‐enrolled patients had 9% higher one‐month retention in HIV care (69%) and 19% higher three‐month HIV retention (83%). CETA is a promising approach to reduce symptoms of common mental illness among HIV+ patients and improve HIV care cascade outcomes in areas with high HIV prevalence.
OAE0804
Nurse practitioner‐led MDR‐TB/HIV treatment may offer a safe approach to improve access to care: Results from a longitudinal cohort in Kwa Zulu‐Natal, South Africa
J.E. Farley1, O. Heidari1, P.D. Stamper1, K. Lowensen1, C. Budhathoki1, N. Ndjeka2
1Johns Hopkins University School of Nursing, The REACH Initiative, Baltimore, United States, 2National Department of Health South Africa, Drug Resistant TB Program, Pretoria, South Africa
Background: Tuberculosis (TB) remains the leading cause of death among people living with HIV, with multidrug resistant (MDR) TB worsening outcomes. Delays in linkage to treatment and availability of a competent clinician are contributing factors. Nurse‐led models are proven effective in both TB and HIV programmes, yet evidence of safety and treatment outcomes are limited in MDR‐TB/HIV populations.
Methods: We evaluated a longitudinal cohort treated by clinical nurse practitioners (CNPs) between 2016 and 2018 from two MDR‐TB outpatient clinics in Kwa Zulu‐Natal, South Africa. The cohort was nested within a cluster randomized trial evaluating case management for MDR‐TB patients. The CNPs were experienced in HIV management before hire and received a nationally accredited, one‐week, MDR‐TB training plus a one‐month supervised treatment period. After training, medical officers were available for consultation and referral, but did not complete routine audits. A standardized, five‐drug, weight‐based, MDR‐TB regimen was utilized per South African guidelines. Descriptive and univariate statistics were used to compare treatment success to a composite negative outcome (loss to follow‐up, death or treatment failure).
Results: CNPs treated 120 (22%) of the 546 participants enrolled at the two sites. These participants were male (55.8%) with a median age of 35.3 years (IQR: 28.9 to 42.1) and a median BMI of 20.1 (IQR 17.9 to 23.0). The majority were living with HIV (75.8%) with a median CD4 count of 233 (IQR = 111.5 to 409), with 55% on ART at baseline. MDR‐TB treatment success occurred in 70% of CNP (84/120) patients. A negative outcome was associated with being: male (41.8% vs. 15.4%, p = 0.002); older (39.6 years vs. 33.2 years, p = 0.003); and having a lower BMI (18.8 vs. 20.8, p = 0.004). HIV status did not significantly impact outcome. A drug‐by‐drug prescription review demonstrated excellent guideline adherence. A single drug was documented to be under the recommended dose in 49/1200 (4.1%) prescriptions and only one patient had a single drug (<1%) prescribed over the recommended dosing.
Conclusions: Treatment success in this cohort was better than both South African and WHO estimates for the same period. CNP adherence to weight‐based dosing was high. CNP‐led treatment programmes may offer a safe approach to improve access to care without compromising outcomes.
OAE0805
Nurses and outreach workers are important but undervalued care providers in HIV care and harm reduction programmes in Kazakhstan
S. Primbetova1, T. Hunt2, T. McCrimmon2, M. Darisheva1, A. Terlikbayeva1, P. Gulyaev1, A. Kuskulov1, Y. Rozental1, D. Bekishev1, L. Gilbert2, E. Wu2, G. Mergenova1, D.A. Goddard‐Eckrich2, N. El‐Bassel2
1Global Health Research Center of Central Asia, Almaty, Kazakhstan, 2Columbia University, School of Social Work, New York, United States
Background: In Kazakhstan nurses and outreach workers in primary health care are one of the largest providers of HIV services and harm reduction programmes to key populations. For this NIDA‐funded Implementation research study (Bridge), we examined syringe exchange programme (SEP) workers’ roles in HIV care, job climate, level of job demands and satisfaction, training needs, and ability to perform their job on HIV care in SEPs.
Methods: Structured surveys were conducted with 24 nurses and 44 outreach workers (OW) employed in 24 trust points ‐ SEPs in 4 cities of Kazakhstan: Almaty (n = 25), Shymkent (n = 21), Karaganda (n = 12), Temirtau (n = 10). Surveys included sociodemographics, organizational readiness to change assessment (ORCA) RAPHIS and TCU Survey of Organizational Functioning. Descriptive analyses were conducted and data was collected using a tablet assigned to respondents.
Results: Results indicate that the major motivation to work was to help people who use drugs (80.88%); however, 32.84% felt like they were not making any differences and 19.41% felt organizational structure and procedures at work were a barrier. Respondents complained on limited resources: lack of computers (58.21%), human resources (28.36%), inadequate office and supplies (35.82%), staff turnover (31.35%). Participants expressed a tremendous need for training in assessing clients’ needs (67.16%), increasing clients’ participation in treatment (77.61%), and improving rapport with clients (62.68%). Respondents perceived they were not efficient enough due to security (22.39%), too slow in making changes (34.33%), stress and strain (23.89%), pressure (29.85%) and heavy workload (32.83%) that reduces programme effectiveness.
Conclusions: In order to improve HIV testing and treatment cascade among PWID, nurses and outreach workers in SEPs need to be engaged in HIV linkage to care and treatment adherence services. There are two main issues they face at the trust points: organizational and individual. Staff reported that they are undervalued for their demanding and important job, underpaid, experience lack of training on how to work with PWID living with HIV, rapid HIV testing, linkage to care and HIV treatment. Additional training on HIV care, institutional changes and better funding will improve nurses and outreach workers level of job satisfaction and decrease job demands and improve treatment outcomes among PWID.
OAE0806
Informal providers can increase uptake of HIV testing among adults of unknown serostatus: Results from a cluster randomized pilot study in southwestern Uganda
R. Sundararajan1, D. Nabukalu2, R. King3, J. Mwanga‐Amumpaire2
1Weill Cornell Medicine, New York, United States, 2Mbarara University of Science and Technology, Mbarara, Uganda, 3University of California, San Francisco, United States
Background: Human immunodeficiency virus (HIV) infection and transmission continue unabated, despite biomedical advances in prevention, diagnosis, and treatment. In low resource settings, such as rural Uganda, a major barrier to epidemic control is poor engagement with HIV testing. Lack of HIV testing uptake has been attributed to frequent use of informal healthcare providers, such as traditional healers, who do not routinely discuss or offer HIV testing.
Methods: We conducted a cluster randomized trial in southwestern Uganda in August 2019‐January 2020. Traditional healers were randomized to offer point‐of‐care HIV testing (Oraquick©) with pre‐ and post‐test counselling (n = 9 clusters) versus protocolized usual care (n = 8 clusters). Usual care entailed offering HIV education with referral to existing clinic‐based testing services. Adults receiving care from participating healers were eligible for participation if sexually active and reported not receiving an HIV test within the prior 12 months. The primary outcome was receipt of an HIV test within 90 days of study enrolment. We conducted qualitative interviews with key informants at 90 days follow‐up to gather contextual information regarding outcomes.
Results: 433 participants were enrolled (intervention = 250, control = 183). Participant age, income and gender were similar among study arms. HIV testing was received significantly more often among participants treated by traditional healers randomized to the intervention group (100% vs. 17%, adjusted risk ratio 5.90, 95% CI 4.3 to 8.1, p < 0.01). Ten (4%) participants in the intervention arm were newly diagnosed as HIV‐infected, compared to no participants in the control arm (p = 0.02). Four of these 10 HIV‐positive participants linked to HIV care within 90 days of enrolment. Intervention participants described the testing programme as highly acceptable. Participants in the control arm reported lack of funds and time to travel to the biomedical clinic as primary barriers to HIV testing.
Conclusions: Informal providers, such as traditional healers, can effectively increase uptake of HIV testing in endemic regions. Our novel approach holds promise to identify HIV‐infected adults in communities where conventional biomedical outreach has limited impact. Further work is needed to understand low rates of linkage to care among newly diagnosed HIV‐infected participants.
OAF0102
Maintaining epidemic control in Namibia: Designing the optimal package. Lessons from ACS support to the Namibian Government
E. Owino1, C. Jones2
1University of Nairobi, Nairobi, Kenya, 2University of Nambia, Windhoek, Namibia
Background: Namibia has achieved 94% of people living with HIV knowing their status, 96% of those HIV‐positive persons were on ART and 95% of those have achieved viral suppression (by 2019).The Namibian Government wanted to develop a priority package of services for epidemic control, the process was driven by the reducing donor funding.
Description: From August to December 2019, the African Collaborative for Health Financing Solutions’ (ACS) supported the Ministry of Health to work through the 10‐step process suggested by Glassman et al (2016) to devise a package of HIV/AIDS services for epidemic control. Semi‐structured interviews were conducted with 40 stakeholders critical to the HIV/AIDS response in Namibia to map existing HIV/AIDS‐related interventions and identify the services needed to maintain epidemic control. Through a very collaborative process, ACS facilitated the agreement of the goal of the package of services, the definition of selection criteria, the shaping of package options based on the country epidemiological profile, and the determination of priority services.
Lessons learned: By the end of the process, we identified that:
1) A political economy analysis is critical to understand the role of all stakeholders involved in HIV/AIDS interventions to ensure a balanced consensus on the priority services.
2) Openness and regular communication among civil society, academia, government agencies and development partners were the critical catalysers of the process.
3) Clarification and country‐specific adaptation of the terminologies (such as epidemic control, fast tracking, critical vs. noncritical) is essential at the outset of the process given the sensitivity regarding the Namibian context.
4) The engagement of local political networks and technical stakeholders from the beginning was instrumental to mobilize necessary resources to sustain the prioritization process.
Conclusions/Next steps: An inclusive stakeholders’ engagement not only provides sound knowledge, critical thinking and hand‐on‐experience, but it serves as a vital ingredient to secure country ownership and therefore improving sustainability. As Namibia is one of the pioneers in developing an HIV epidemic‐maintenance package, the lessons learned on its experience, especially those related to the drivers of an effective process, should be of inspiration for countries that have similar context or want to go through similar approach.
OAF0103
Programmes shaping policies: How partnerships and programme data were used to transform the policy environment for key populations
R. Wilcher1, R. Dayton1, H. Mahler1
1FHI 360, HIV, Durham, United States
Background: Since 2014, the USAID/PEPFAR‐supported LINKAGES project led by FHI 360 has delivered HIV services to key populations (KPs)—female sex workers, men who have sex with men, people who inject drugs, and transgender people—in more than 30 countries. Throughout the project, LINKAGES forged partnerships and used programme data to advocate for policy changes that increase KPs’ access to HIV services.
Description: LINKAGES was characterized by strategic partnerships with host‐country governments and funders, a focus on KP community‐led services, and the use of data to understand and improve programme performance. This approach created meaningful opportunities for KP members to have their voices heard, and for project staff and local partners to advocate with government stakeholders and country representatives from Global Fund and PEPFAR for supportive policies to engage KPs in services across the cascade.
Lessons learned: LINKAGES contributed to enabling policy environments in 22 countries. In each, LINKAGES facilitated updates to national HIV and/or STI policies and guidelines to better address the needs of, and incorporate evidence‐based recommendations for, KPs. In 20 countries, LINKAGES programme data showing the effectiveness of interventions, including the enhanced peer outreach approach, HIV self‐testing, index testing, and peer navigation, led to endorsement of these approaches in government strategies, PEPFAR country operational plans, or Global Fund grants. In 13 countries, LINKAGES removed policy barriers to KP service uptake. For example, in Botswana, Kenya, Malawi, and Lesotho, LINKAGES increased treatment initiation by gaining government approval for antiretroviral therapy provision at KP‐led drop‐in‐centres. LINKAGES also influenced policy‐level processes by forming national KP technical working groups (nine countries), strengthening national data systems to include KP‐specific data (19 countries), and mobilizing domestic resources for KP services (six countries). In Angola, Botswana, and Malawi, LINKAGES contributed to successful KP decriminalization efforts.
Conclusions/Next steps: By leveraging the project's routine data, working closely with local decision‐makers, and amplifying KP voices, LINKAGES contributed to policy environments that enabled successful HIV programming, including in criminalized settings. These policy changes not only resulted in immediate improvements in service uptake but are also likely to have a sustained impact on epidemic control efforts and KP individuals’ quality of life.
OAF0104
Greater involvement of people living with HIV in research: The experience of the regional study on violence and women living with HIV in Latin America
M. Negrete1, D. Luciano2, A. Cano3, M. Iacono4, M.J. Vazquez5, F. Hale6, J. Salas7, M. Alvarez8, M. Arends9, N. Sanchez4, M. Cabezas10, B. Chete11, G. Flores12, F. Garcia13, M.L. Herreira14, L. Lopez15, B. Ramirez16
1Development Connections, Research, Asuncion, Paraguay, 2Development Connections, Washington, United States, 3ICW Latina, General Secretariat, Managua, Nicaragua, 4ICW Latina, Regional Coordination, Managua, Nicaragua, 5Salamander Trust, Research, Barcelona, Spain, 6Salamander Trust, London, United Kingdom, 7HIVOS, Monitoring and Evaluation, Mexico DF, Mexico, 8HIVOS, Advocacy, San Jose, Costa Rica, 9HIVOS, Program Development, San Jose, Costa Rica, 10ICW Latina, Country Representative, Cochabamba, Bolivia, 11ICW Latina, Country Representative, Guatemala, Guatemala, 12ICW Latina, Country Representative, Lima, Peru, 13ICW Latina, Country Representative, Santo Domingo, Dominican Republic, 14ICW Latina, Country Representative, Asunción, Paraguay, 15ICW Latina, Country Representative, Cali, Colombia, 16ICW Latina, Country Representative, Tegucigalpa, Honduras
Background: The Regional Study on Violence and Women Living with HIV (WLHIV) in Latin America carried out by ICW Latina, Hivos, Development Connections and Salamander Trust in 2018, followed the GIPA principle. We documented WLHIV participation using the following criteria: knowledge and skills used, skills development, autonomy of decision‐making, and ownership.
Description: 1) Design and preparation of the Study. The Study methodology and data collection tools were discussed with ICW Latina representatives from 15 countries in a regional meeting. Using their insights, concepts and operational definitions were revised including types of intimate partner relationships, partners’ controlling behaviours and types of violence: emotional, economic, related to activism, perpetrated by State agents and organized crime.
2) Implementation. The representatives of ICW Latina in the 7 selected countries (Bolivia, Colombia, Dominican Republic, Guatemala, Honduras, Paraguay and Peru) participated in a five‐week online course on research protocol and piloting the questionnaire. They coordinated the country study including: selecting and training the research team, budget management, interinstitutional planning for participants’ recruitment, submitting the protocol to Ethical Committees (Dominican Republic, Guatemala), planning and supervising field work, and overseeing the adherence to ethical guidelines. The regional research team provided technical support throughout this phase.
Lessons learned: a) The involvement of 37 WLHIV ensured the quality of the study's, developed their skills for conducting research and strengthened interinstitutional alliances. The findings were used to design Regional Guidelines for Addressing Violence Against WLHIV; b) Fostering ownership led to a greater use of the findings for policy/programme development, advocacy, and capacity building at country level. The study and guidelines are being disseminated through webinars, conferences, social media, websites, @bulletins. Advocacy materials and local adaptation of the regional guidelines were developed in Guatemala, and a proposal for capacity building in Paraguay; c) It is critical to include emotional support and referrals to services for the field researchers.
Conclusions/Next steps: Advocacy, dissemination and interinstitutional collaboration will continue aiming to translate the study into action. Activities will be integrated into the national plans of the 3‐year (2019 to 2022) regional project ALEP coordinated by Hivos.
OAF0105
Is pre‐exposure prophylaxis suitable for Filipino cis‐women? A review of literature, policies, and clinical guidelines to introduce PrEP as a reproductive health service for cis‐women in the Philippines
R.S. Regner1, P. Eustaquio1, J.D. Rosadiño2, R. Llauderes2, L. Yarcia3, A. Bandola4
1Love Yourself Inc., Mandaluyong, Philippines, 2Love Yourself, Mandaluyong, Philippines, 3HIV‐AIDS Support House, Quezon, Philippines, 4University of the Philippines, General Hospital, Obstetrics and Gynecology Infectious Diseases, Manila, Philippines
Background: There has been a focus on HIV prevention for men who have sex with men (MSM) and transgender women (TGW) in the Philippines notwithstanding the three‐fold increase in new infection among cis‐women in the past 5 years. Among all the cis‐women cases, 90% are of reproductive‐age upon diagnosis. Cis‐women are less likely considered for HIV prevention measures considering that present epidemiologic data reflect TGW and MSM as priority populations for HIV and AIDS interventions. Thus, prevention opportunities for cis‐women are limited. The objective of this study was to appraise policies and guidelines that would provide more options for prevention for cis‐women.
Methods: The legal and medical framework for reproductive health services cis‐women was appraised through law and policy review and medical literature search to determine availability and accessibility to PrEP for Filipino cis‐women.
Results: Only the Reproductive Health Act of 2012 and HIV/AIDS Policy of 2018 ensure HIV‐prevention services. Among currently available RH services, only screening and male‐condoms function as HIV prevention. Surveys done among sexually active, reproductive‐aged, unmarried cis‐women consistently show majority (52 to 62%) knew that male‐condoms decrease HIV‐transmission yet there is low‐uptake (3 to 9%). 62 to 72% of female sex workers (FSW) do not use male‐condom due to partner objection. Aside from FSW to whom HIV screening is encouraged, studies show low uptake of HIV screening among cis‐women (3%). Neither PrEP nor PEP is included in the local STI guidelines for pregnant women. These are consistent with one systematic review of literature on HIV prevention strategies in Filipino cis‐women which revealed fixation of literature to no other biomedical strategy but male‐condom usage.
Conclusions: A concentrated epidemic may lead to lapses in service delivery, neglecting those who are not considered high risk. Given acceptable knowledge yet low uptake of male‐condom and HIV screening demands further studies to analyse this disparity and efforts to increase the uptake. In addition, the inclusion of PrEP as another option is both promising and empowering. Although WHO recommendation suggests PrEP provision among high‐risk groups, it also emphasized provision to those who desire it. Its integration into RH‐service may ensure government‐stewardship, financial‐sustainability, and access through the community‐based service delivery model.
OAF0106
Harmonization of the legal environment on adolescent sexual and reproductive health and rights in East and Southern Africa
R. Tallarico1
1UNFPA, East and Southern Africa Regional Office, Johannesburg, South Africa
Background: The aim of the study commissioned by UNFPA and conducted by the University of Pretoria was to assess the progress in laws, policies and other related sources that directly or indirectly either impede or enable ASRHR. The study measures the legal provisions of the East and Southern Africa countries against the international, continental and regional treaties and commitments.
Methods: The legal review was conducted through a detailed perusal of various laws and policies in 23 ESA countries, as well as through the study of other sources that set out the legal environment for ASRHR in the relevant countries. The legislative and policy provisions that impact on ASRHR are varied and the desktop review identified 10 themes of relevant laws and policies to guide the assessment.
Results: The study captured a number of relevant findings negatively impacting on ASRHR. To mention a few:
All ESA countries do not have clear provisions that set the minimum age of consent to sexual activity in legislation.
All 23 countries have set ages of consent to marriage. However, there is disharmony between the legislative provisions and the international standards; and between statutory and customary law provisions.
The ages of consent to access HIV services are in the majority of the countries not provided for in laws; in some countries, however, the ages are provided for in policies.
Sexual diversity is not recognized in the majority of the ESA countries and this is evident from legislative provisions that still criminalize sexual activity between men (variously referred to as acts against the order of nature, or unnatural acts).
Although the majority of the ESA countries have provisions in their diverse policies indicating that CSE is key and should be implemented, only a handful appear to have CSE curricula in schools aligned to the international standards.
Conclusions: A particular challenge in the region is the coexistence of customary law, religious law and civil law. The harmonization of provisions directly influencing ASRHR must ensure that contradictions between laws and policies are removed, and that the amended law is infused with the rights‐based approach evident in the many of the policies.
OAF0202
Progress towards 90‐90‐90 targets in Canadian correctional facilities, 2019
J. Smith1, K. Meghnath1, O. Varsaneux1, E. Kom1
1Correctional Services Canada, Ottawa, Canada
Background: In support of global targets established by the Joint United Nations Programme on HIV/AIDS (UNAIDS), Correctional Service of Canada (CSC) has compiled 90‐90‐90 estimates for the federal inmate population for 2019.
Methods: Federal inmates are offered voluntary HIV testing on admission and throughout incarceration. Data collected from CSCs enhanced surveillance system were analysed to estimate the proportion of inmates in federal custody as of December 2019 that were aware of their HIV status, on treatment, and virally suppressed (<250 copies/mL). Results were stratified by Indigenous status and gender.
Results: In 2019, the HIV prevalence amongst federal inmates was estimated to be 0.95%, and 88% of inmates were aware of their status. Overall, ninety‐eight percent were on treatment and of those, 93% had achieved viral suppression. The majority of inmates (84%) living with HIV were diagnosed in the community prior to incarceration. Over one‐quarter of inmates living with HIV (28%) were diagnosed within the past 5 years.
Indigenous persons and women account for a disproportionately high number of HIV cases. Eleven percent of inmates living with HIV were women, and half (50%) self‐identified as Indigenous.
These subgroups account for 5% and 30% of the overall inmate population respectively.
Ninety‐two percent of Indigenous inmates and 94% of women offenders were aware of their status. All Indigenous inmates known to be living with HIV were on treatment (100%) and 92% had achieved viral suppression. Of the female inmates known to be living with HIV, 92% were on treatment and 87% had achieved viral suppression.
Conclusions: This analysis shows that a high proportion of inmates with HIV are on treatment and have achieved viral suppression. Almost 90% of inmates were aware of their HIV status. Women offenders and inmates of Indigenous ancestry were disproportionately affected by HIV. CSC is committed to continuing to monitor these indicators and working towards increasing testing uptake in order to achieve the UNAIDS 90‐90‐90 targets for the federal inmate population.
OAF0203
Supporting the judicial response to HIV and TB in Africa: The Africa Regional Judges’ Forum
L. Ferguson1, M. Lambert‐Peck1, K. Zacharias1, D. Owolabi2, D. Patel2, A. Saha2, S. Gruskin1
1University of Southern California, Institute on Inequalities in Global Health, Los Angeles, United States, 2UNDP RSCA, Istanbul, Turkey
Background: Building on the work of the Global Commission on HIV and the Law, UNDP Regional Service Centre for Africa (UNDP RSCA) initiated regional work to address HIV‐ and TB‐related legal barriers. The Africa Regional Judges’ Forum, now in its sixth year, is a response to judges’ requests for capacity building on issues surrounding HIV, TB and the law across the region. Judges have full ownership of fora proceedings, while UNDP RSCA acts as its Secretariat, providing technical and financial support.
Description: The forum convenes judges and magistrates representing 16 sub‐Saharan countries to share successes, challenges, and advancements in human rights‐based responses to HIV and TB. The goal is to support a new generation of judicial leaders able to preside over legal cases relating to HIV, TB, and human rights. Medical experts, scientists, civil society, representatives of key populations, TB survivors, and other community groups are invited to participate in the fora to maximize understanding of the issues being discussed. An evaluation was recently conducted comprising a review of relevant documents and key informant interviews with stakeholders including participating judges, UNDP staff and consultants.
Lessons learned: Judges are uniquely positioned to ensure that law is used appropriately in HIV responses. The forum acts as a safe space for peer discussion among judges, for judges to learn directly from key populations about the impact of the law on their lives, and for discussing the latest advancements in science and medicine. Cases addressing exclusion and inequalities, human rights and HIV/TB are shared to promote cross‐country learning. Recently, Kenyan judges who participated ruled that the overbroad criminalization of HIV transmission was unconstitutional and that the imprisonment of patients with TB was unlawful and beyond the parameters of public health legislation, and Botswanan judges ruled on the need to provide HIV treatment to foreign prisoners with HIV.
Conclusions/Next steps: The sustained demand from the judges for the continuation of this forum demonstrates its relevance and acceptability for bringing HIV science and lived experience into the legal sphere. This is a replicable model for cultivating regional, cross‐country knowledge transfers through engendering peer‐to‐peer collaboration, thereby supporting positive change in HIV‐related legal environments.
OAF0204
Involvement of law enforcement agencies in responding to GBV against LGBTI people and in reducing their vulnerability for better access to healthcare for key populations in the city of Douala
J. Mandeng1, J. Ngando2
1Alternatives‐Cameroun, Management, Douala, Cameroon, 2Alternatives‐Cameroun, Genre te Droits Humains, Douala, Cameroon
Background: In 2018, a national report indicated 1134 cases of violence and violations of rights against LGBTI people in Cameroon, an increasing number. The impunity that surrounds this violence has the effect of legitimizing it, and of creating a psychosis among the victims, as well as consequences for their physical and mental health. Alternatives Cameroon decided to work with law enforcement forces to better respond to cases of violence against LGBTI people, and to reduce their vulnerability.
Description: We started by listing some cases of documented violence which did not receive any response. We targeted two police stations in which we had already sensitized the officers during the workshops on human rights and HIV. Then we went to the commissioners of these establishments to submit these cases to them and organize a response. They provided us with inspectors who coached our clients on how to file a complaint, avoiding the use of words which could incriminate them, and then they recorded the complaints, thus allowing the opening of legal proceedings.
Lessons learned: The collaboration with the police has allowed some of our beneficiaries to have the courage to file a complaint, and better still, to succeed, which strengthens their courage to resort to the police. in the event of violence against them. On the other hand, we were able to team up with fifteen police and gendarmes for the management of future cases of violence. The latter recently referred us to an MSM who was brought into their services. We tested positive for HIV and put him in care.
Conclusions/Next steps: In a win‐win logic, we have successfully offered to the police forces with whom we collaborate, to often visit their posts to carry out HIV testing campaigns in their favour and in favour of those who may be detained there. In return, we can always count on these police forces to accompany us in certain night activities, in order to ensure our safety and that of the beneficiaries. We also intend to involve them in a collective complaint procedure in favour of victims of violence without any procedure having been initiated.
OAF0205
The battle of defeating HIV stigma and discrimination continues: Lessons from the South African Human Rights Commission's use of litigation strategy in the protection of human rights
L. Lotz1
1South African Human Rights Commission, Durban, South Africa
Background: According to Statistics South Africa (Stats SA) 2019, approximately 7/97 million people are HIV positive. Evidence is overwhelming on the importance, impact on society, link to systemic discrimination on the grounds of positive HIV status, clearly stringent measures need to be taken to ensure that HIV positive people are able to be productively employed and that their basic human rights are at all times respected. The South African Human Rights Commission (SAHRC) used the litigation strategy in challenges discriminatory practices and policies of the South African National Defence Force (SANDF) In respect of the right to equal treatment of HIV‐positive individuals.
Description: In promoting the protection of human rights the SAHRC used the litigation strategy against the SANDF in the Western Cape High Court by seeking redress under the Promotion of Equality and Prevention of Unfair Discrimination Act 52 of 2002 in that a reservist was unfairly and unlawfully discriminated against on the basis of his HIV‐positive status. The SANDF had failed to deploy the reservist on naval vessels on account of his status, contrary to its own policy in respect of members who are HIV positive. He complained to the SAHRC that the discrimination has been ongoing.
Lessons learned: Lessons were learned on how to use different strategies for using rights to impact on HIV stigma and discrimination, and to achieve social change. In order to achieve maximum success in advancing social change, litigation and other strategies can be used. The SAHRC successfully used the litigation strategy in combination with other strategies (i.e. public information; advice and assistance; and social mobilization and advocacy), as empowered by its constitutional mandate, to secure redress and to enforce the protection of human rights.
Conclusions/Next steps: By litigating the SAHRC impacted on social change by holding government institutions accountable and in protecting the human dignity and right to equality of all who are or may be affected by HIV. The outcome of the litigation strategy found that the SANDFs implementation of its policies relating to HIV‐positive members is inadequate. Accordingly, the court found that SANDF had unfairly, unlawfully and unjustifiably discriminated against the reservist.
OAF0206
Scaling up public health approach to law enforcement to remove human rights‐related abuses against key populations in Ghana
J. Blantari1, M. Salifu1, E. Awotwi2
1Ghana Police Hospital, Ghana Police AIDS Control Programme, Accra, Ghana, 2United Nations Population Fund, Accra, Ghana
Background: Police are a critical sector in determining the risk environment for HIV in most key affected populations (PAPs), especially Sex Workers, Men who Have Sex with Men; People who inject Drugs, and other marginalized communities. In the global response to HIV, the key importance of the police's role has been recognized. In most developing countries however, police continue to serve as barriers to effective HIV responses and their role in human rights violations against KAPs Targeted programmes with the Ghana Police seeks to change most of these situations.
Description:
• A three prongs approach was adopted for this intervention:
• Buy‐in from the Police Hierarchy
• In service training and sensitization for serving personnel
• Pre‐service training for recruits/students
Lessons learned: Three meetings with the top hierarchy of the Ghana Police Service were held to solicit their buy‐in. These were: one on one with the Inspector‐General of police and two separate meetings with the Police Management Board (POMAB).
Intense in‐service sensitization meetings were held across 22 Global Fund Implementation Districts spread across 9 out of the 16 Political Regions. Topics treated in these sessions include Human Rights abuses, arrest procedures, SGBV, and the review of a video which highlights HR abuses. 100 master trainers from the Police Team including personnel who serve as UN trainers have also been sensitized to undertake step down trainings in the affected Regions across the country.
Curriculum drawing from the key sectors has been produced to serve as a textbook for all 7 police training institutions in Ghana to equip all personnel who would pass through them.
Conclusions/Next steps: It is expected that by the end of the 3‐year programme, 70% of those trained would become champions of the Public Health Approach to law enforcement, particularly in relation to protecting the rights of key populations. It is also expected that there is sustained change in Police policies, culture and practice with peer education (combined with law and policy reform) and increased partnerships with partners such as the Global Fund/WAPCAS and other key stakeholders to promote the Public Health Approach to Law Enforcement.
OAF0302
Effect of homophobic attack to attainment of 90‐90‐90 goals in Uganda: Experiences from TASO Jinja clinic
L. Oucul1, D. Kagimu2, H. Amongin3, B.M. Etukoit4, K. Mugisha1
1The AIDS Support Organization (TASO), Program and Capacity Development, Kampala, Uganda, 2The AIDS Support Organization (TASO), Medical, Kampala, Uganda, 3The AIDS Support Organization, Psycho‐Social, Jinja, Uganda, 4The AIDS Support Organization TASO, Advocacy, Kampala, Uganda
Background: In Uganda, the prevalence of HIV is 6.2% (UPHIA, 2017) and key populations are disproportionately affected. Men who have sex with Men HIV prevalence is double at 12.7% (Crane Survey 2017). MSM continue to face stigma and discrimination due to social, legal and policy environment. Cases of homophobic attack seem to be on resurgence after the repeal of anti homosexual act. We share experience of an attack on one MSM that is likely to affect the attainment of 90‐90‐90 targets at one of our centres of excellence offering HIV/AIDS services to key populations through the Deeper Engagement Grant (DEG).
Description: TASO began implementing the DEG project in June 2018. The goal of the project is universal access to health for all: Health and empowered LGBT communities. To reach the LGBT, the centre uses the peer model, moonlight clinics to offer HTS and combination of other services, monthly adherence club meetings for HIV positive LGBT.
On 5th October 2019, the LBGT peer attached to TASO Jinja clinic was attacked and murdered in his residential room by un ‐known assailant. This murder left the entire LGBT community and health workers in fear. To mitigate the impact of loss, TASO organized a grief and bereavement counselling session for close peers to the deceased. Staff and peers were trained on security and safety.
Lessons learned: By November 2019, TASO Jinja clinic had registered 388 LGBT, 231 were reached with HIV Testing Services, 17 diagnosed positive, and 14 were initiated on ART. After the attack, 2 HIV positive LGBT were relocated, 02 who were on intensive adherence counselling due to high viral load went into hiding, and 05 missed their appointments. Only 05 of 14 HIV positive LGBT on ART are still active. No LGBT has since turned up for their routine monthly adherence meetings following the death of their fellow peer.
Conclusions/Next steps: Resurgence of homophobic attacks in Uganda is likely to dent the attainment of 90‐90‐90. Programmes providing services to marginalized communities like LGBT in repressive environment should offer training on security and safety for LGBT groups and health workers to mitigate the effects of such attacks.
OAF0303
Re‐envisioning the social enablers of the global response: An evidence‐based framework to inform investments and targets for HIV‐related stigma and discrimination, legal environment and social justice, and gender equality
J.A. Izazola Licea1, A. Stangl2,3, T. Pliakas4
1UNAIDS, Strategic Information, Geneva, Switzerland, 2Hera Solutions, Baltimore, United States, 3Johns Hopkins Bloomberg School of Public Health, International Health Department, Baltimore, United States, 4LSHTM, London, United Kingdom
Background: There is consensus that social enablers, like reducing HIV‐related stigma and discrimination, modify the effectiveness of HIV prevention, care and treatment services and are important for achieving global HIV goals. While several social enablers were included in the 2011 HIV Investment Framework, it was not possible to set evidence‐based funding and programmatic targets for countries to pursue due to the paucity of evidence on what interventions constituted social enablers, and for those interventions that were defined, their effects on HIV outcomes. Over the past decade, significant progress has been made to develop and test interventions to address structural impediments to HIV services, including seven widely promoted human rights programmes. Based on this evidence, UNAIDS has been leading a process since June 2019 to re‐envision the social enablers and more accurately include them in HIV modelling.
Description: The process began with a broad, multi‐stakeholder technical consultation which identified evidence and elements to be highlighted as social enablers of the HIV response. Following the consultation, an in‐depth review of the literature was conducted to gather the latest evidence on each social enabler to inform the updated framework and allow estimation of impact on HIV outcomes and resource needs. The resulting framework differentiates enablers based on the 3 S's of the HIV response: services, systems and society. The social context can greatly influence how well countries are able to implement HIV systems and services. We propose that national governments invest in four social enablers to strengthen their HIV responses: (1) HIV‐related stigma and discrimination, (2) the legal environment and access to social justice, (3) gender equality, and (4) links with other SDGs.
Lessons learned: While there is increased evidence documenting direct and indirect effects of social enablers on HIV outcomes, there are still gaps that limit quantitative analyses. The wide consultations allowed for the inclusion of key social enablers regardless of data availability. Better modelling is now expected to predict the impact of scaling up social enabling interventions on HIV services.
Conclusions/Next steps: The new framework for social enablers may galvanize advocacy to increase programme effectiveness, and improve quantitative statistical or modelling efforts documenting or estimating impact.
OAF0304
Medical mistrust, discrimination, and sexual violence among a sample of women at risk for HIV
J.K. Stockman1, K.M. Anderson1, A. Fernandez DeSoto1, M. Young Karris1
1University of California, Department of Medicine, Division of Infectious Diseases and Global Public Health, La Jolla, United States
Background: Women of colour are disproportionately affected by HIV, representing the majority of new diagnoses among women. Medical mistrust and discrimination are barriers to accessing HIV prevention methods. These barriers may be further heightened for survivors of sexual violence. We sought to understand medical mistrust and discrimination in the context of sexual violence and HIV risk among racial/ethnic groups in San Diego, California, USA.
Methods: The THRIVE Study is a case‐control study with follow‐up, of racially and ethnically diverse girls and women aged 14 to 45. Eligible participants are consensually sexually active or have experienced recent sexual violence. At study visits, participants complete an interviewer‐administered survey with measures on demographics, medical mistrust and suspicion, discrimination, and lack of support subscales, everyday discrimination, and history of sexual violence (ever/never forced/threatened sex). Using baseline data for 48 participants, descriptive statistics examine differences in medical mistrust and experience of discrimination by racial/ethnic group and history of sexual violence.
Results: Women identifying as Black or African American (AA) had higher levels of medical mistrust compared to their non‐Black/AA counterparts (34.07±9.96 vs. 24.03±7.47; p < 0.001). Black/AA women also had higher levels of suspicion (12.71±2.9 vs. 8.1±3.5; p < 0.01) and lack of support (9.1±2.6 vs. 6.0±2.2; p < 0.001) compared to non‐Black/AA women. Compared to women in all other racial/ethnic groups, non‐significantly lower medical mistrust was observed among Hispanic/Latina, Asian, and White women. Survivors of forced or threatened sex had higher medical mistrust than non‐survivors (30.9±9.9 vs. 24.8±8.4; p < 0.05), and marginally significantly higher levels of suspicion (11.1±4.4 vs. 8.6±4.2; p = 0.063) and lack of support (7.8±3.1 vs. 6.4±2.3; p = 0.10). Among Black/AA women, survivorship did not significantly impact medical mistrust or sub‐scales. Compared to non‐survivors, Hispanic/Latina survivors had higher medical mistrust (34.0±11.1 vs. 21.9±5.2; p < 0.01), suspicion (12.2±4.7 vs. 7.2±2.0; p < 0.01), and discrimination (10.0±2.7 vs. 6.9±2.1; p < 0.05) while Asian survivors and White survivors had non‐significantly higher medical mistrust.
Conclusions: Our study findings highlight the need to account for racial/ethnic differences and sexual violence history as relates to medical mistrust and aspects of discrimination. This is imperative in the development of structural interventions for HIV prevention as we address the HIV epidemic in the United States.
OAF0305
Undetectable = Untransmittable (U=U) to drive stigma reduction and epidemic control in Vietnam: A global model for political and programme innovation
A. Nguyen1, J. Blandford1, C.D. Hoang2, L.H. Nguyen2, H.T.T. Phan2, N.T. Do2, T.H. Do2, T. Pollack3, D.D. Do4, P.A. Nguyen5, T.D. Thanh6, G.M. Le7, L.H. Tong8, N. Thi Tuyet Vo3, H.B. Tran3, P.T. Nguyen3
1U.S. Centers for Disease Control and Prevention, Division of Global HIV and TB, Hanoi, Vietnam, 2Ministry of Health, Vietnam Authority on AIDS Control, Hanoi, Vietnam, 3The Partnership for Health Advancement in Vietnam, Beth Israel Deaconess Medical Center, Hanoi, Vietnam, 4Vietnam Network of People Living With HIV, Hanoi, Vietnam, 5Vietnam Network of People Living with HIV, Ho Chi Minh City, Vietnam, 6Lighthouse Social Enterprise, Hanoi, Vietnam, 7Hanoi Medical University, Hanoi, Vietnam, 8The Lab Saigon, Ho Chi Minh City, Vietnam
Background: Effective ART with sustained viral load (VL) suppression provides complete protection against sexual transmission of HIV. In Vietnam, the Ministry of Health (MOH), Vietnam Network of People Living with HIV (PLHIV), and community leaders have rapidly and comprehensively leveraged Undetectable = Untransmittable (U=U), K=K in Vietnamese, as a programme catalyst and driver for stigma and discrimination reduction and meeting epidemic control goals. K=K is a versatile concept beyond reducing stigma and drives our Vietnam programme priorities for case finding and ART initiation.
Description: Since its 2017 inception, the K=K movement ushered in MOH policies to document VL suppression <200 mL/copies as treatment success and mandate integration of K=K messaging into health practice to support 95‐95‐95 goals. Following two successful municipal campaigns in Hanoi and Ho Chi Minh City, the current national campaign celebrates K=K as transformative for individuals, couples, and communities – directly confronting established public perceptions around HIV. Grants to community‐based organizations ensured widespread dissemination of the K=K message to key population and PLHIV networks, especially young urban men who have sex with men.
Lessons learned: Coordinated MOH and community commitment is critical to mainstream K=K into HIV programme strategy. Despite global endorsements, healthcare providers were reluctant to inform patients of the benefits of K=K. Simple, visually impactful materials clarified K=K messaging, addressing concerns vis‐a‐vis PMTCT and blood transmission and STI prevention. Initial campaigns were conducted first in cities where success would influence broader commitment and leveraged Vietnam's impressive viral suppression rates. In response to these developments, MOH officially endorsed K=K and issued national implementation guidelines. Community fora confirmed regionally nuanced messaging and preferred platforms for effective dissemination, as well as the national campaign design.
Conclusions/Next steps: K=K revolutionized the HIV response in health and community settings. As of September 2019, Vietnam is the first PEPFAR country to disseminate official K=K guidance and to document 95% VL suppression <200 mL/copies among ART patients. In the next phase, Vietnam will unite messages of effective ART for those living with HIV and pre‐exposure prophylaxis for those at substantial risk so that the preventive use of ARVs offers a clear path to HIV epidemic control in Vietnam.
OAF0306
Cumulative effect of fear of stigma from health professionals and family/neighbours and health care avoidance among PLHIV in Morocco: Results from the Stigma Index Survey Morocco (2016)
R.M. Delabre1, A. Ben Moussa1,2, V. Villes1, M. Elkhammas1,2, L. Ouarsas1,2, D. Rojas Castro1,3, M. Karkouri1,2
1Coalition PLUS, Community‐Based Research Laboratory, Pantin, France, 2Association de Lutte Contre le Sida (ALCS), Casablanca, Morocco, 3Aix Marseille Univ, INSERM, IRD, SESSTIM, Sciences Economiques & Sociales de la Santé & Traitement de l'Information Médicale, Marseille, France
Background: Since 2015, entry into the HIV care cascade in Morocco has been facilitated by community screening and the “test and treat” strategy. However, experience and/or fear of stigma among people living with HIV (PLHIV) can hinder entry into the care system. Through the Stigma Index Morocco survey, we identified factors associated with having avoided health services for fear of stigma.
Methods: ALCS, Coalition PLUS member, in collaboration with the Ministry of Health, UNAIDS and the Global Fund, conducted the Stigma Index survey in Morocco (March‐June 2016) among PLHIV using temporal cluster sampling. The questionnaire addressed several themes, including experiences of stigma and discrimination and health‐seeking behaviour. Factors associated with avoiding HIV testing and treatment services for fear of stigma were assessed using multinomial logistic regression models. We compared people who did not avoid health services for fear of stigma (reference) to people who avoided health services for fear of stigma from (A) health personnel or family/neighbours and (B) health personnel and family/neighbours (cumulative effect).
Results: Among 583 participants, 280 (48.0%) were women and median age was 36 [IQR 29 to 43]. Half avoided health services for fear of stigma by health personnel and/or family/neighbours: (A) n = 228, 39.1% and (B) n = 68, 11.7%. After adjustments, having been excluded from social activities ((A) aOR[95% CI] = 1.70 [1.10; 2.61]; (B) 2.63 [1.39; 5.00]), having been discriminated against by PLHIV ((A) 1.87 [1.12; 3.13]; (B) 3.35 [1.63; 6.88]) and not having had access to antiretroviral treatment ((A) 1.76 [1.16; 2.68]; (B) 2.18 [1.11; 4.27]) were associated with having avoided health services for fear of stigma by health personnel and/or family/neighbours. Being female (2.85 [1.48; 5.47]) and having discussed sexual and reproductive health with a health professional (4.56 [2.38; 8.71]) were associated with having avoided health services for fear of the two sources of stigma.
Conclusions: Results demonstrate a cumulative effect of fear of stigma and discrimination at the community and health service levels among PLHIV in Morocco. PLHIV who have experienced discrimination seek to avoid reproducing the experience at the expense of their health. These findings have informed ongoing implemented actions within the community and the health sector to improve entry and retention in care among PLHIV in Morocco.
OAF0402
Breaking down human‐rights related barriers to HIV and TB services in 20 countries. Soon everywhere?
R. Jurgens1, A. Iovita1, H. Lim1, G. Arustamyan1, J. Csete2, S. Timberlake3
1The Global Fund to Fight AIDS, TB and Malaria, Community, Rights and Gender Department, Le Grand Saconnex, Switzerland, 2Columbia University, School of Public Health, New York, United States, 3Consultant, Geneva, Switzerland
Background: The six‐year Breaking Down Barriers (BDB) initiative of the Global Fund to Fight AIDS, TB and Malaria seeks to vastly scale up programmes to reduce stigma, discrimination, gender inequality and other human rights‐related barriers to HIV, TB and malaria services. Many programmes to address these barriers have been small‐scale and unsustainable. Twenty countries from all Global Fund regions were chosen for intensive BDB support to create the conditions for comprehensive responses to these barriers.
Description: In 2018 to 2019, independent research teams conducted desk reviews followed by in‐country rapid assessments of existing human rights‐related barriers in the 20 BDB countries and of existing programmes aiming to reduce them. Barriers to HIV services were assessed in all countries, those related to TB in 13 countries and to malaria in two countries. These largely qualitative baseline assessments included focus group discussions and in‐depth interviews with key populations and their organizations, other NGOs, policy‐makers and other stakeholders. Local experts were part of the research teams. The researchers also assessed the cost of existing programmes and outlined a costed comprehensive response to the barriers identified. Baseline assessments were used to design country‐owned multi‐year plans for achieving a comprehensive response, funded in part by catalytic funding from the Global Fund.
Lessons learned: In all countries these barriers were found to be numerous and severe. Key populations face marginalization by undue criminalization and have inadequate access to justice, the stigma of HIV and TB continues to hamper access to care, and gender inequality remains profound. Many programmes to address these barriers were poorly funded, not brought to scale, not strategically coordinated, and generally not understood to be central to successful disease programmes. Key population organizations were often found to need technical and management support. But the $78 million in catalytic funding and matched funds from governments represents a quantum jump in support for scaled‐up programmes to reduce human rights‐related barriers.
Conclusions/Next steps: For the first time in the history of the HIV epidemic, 20 countries, with support from the Global Fund, are seeking to comprehensively address human rights‐related barriers. Discussions are starting on how to break down barriers, everywhere.
OAF0403
Utilizing individual level data to assess the relationship between prevalent HIV infection and punitive same sex policies and legal barriers across 10 countries in Sub‐Saharan Africa
C. Lyons1, D. Diouf2, J.O. Twahirwa Rwema1, S. Kouanda3, A. Simplice4, A. Kouame5, Z. Mnisi6, U. Tamoufe7, B. Cham8, I. Ba2, M.A. Djaló9, T. Mothopeng10, I.M. Njindam11, J. Loum8, S. Matse12, J.P. Enama13, E.‐P. Obodou14, E. Karita15, R. Nowak16, T. Crowell17, O. Ky‐Zerbo18, C. Beyrer1, S. Baral1
1Johns Hopkins School of Public Health, Epidemiology, Baltimore, United States, 2Enda Sante, Dakar, Senegal, 3Institut de Recherche en Sciences de la Santé, Ouagadougou, Burkina Faso, 4ONG Arc‐en‐Ciel, Lome, Togo, 5Ministère de la Sante et de l'Hygiène Publique, Abidjan, Cote D'Ivoire, 6Ministry of Health, Health Research Department, Strategic Information Division, Mbabane, Eswatini, 7Metabiota, Yaoundé, Cameroon, 8ActionAid, Banjul, Gambia, 9Enda Santé, Bissau, Guinea‐Bissau, 10People's Matrix Association, Maseru, Lesotho, 11Johns Hopkins School of Public Health, Yaounde, Cameroon, 12Health Research Department, Mbabane, Eswatini, 13The Pact, Yaounde, Cameroon, 14Enda Sante, Abidjan, Cote D'Ivoire, 15Project San Fancisco, Kigali, Rwanda, 16University of Maryland, Institute of Human Virology, Baltimore, United States, 17Walter Reed Army Institute of Research, U.S. Military HIV Research Program, Silver Spring, United States, 18Programme d'Appui au Monde Associatif et Communautaire, Ouagadougou, Burkina Faso
Background: Gay men other men who have sex with men (MSM) have consistently been shown to be disproportionately affected by HIV across epidemic settings in Sub‐Saharan Africa. Evidence from systematic reviews suggests the association of legislation and engagement in HIV services, however empiric, individual‐level data, to assess this relationship remains limited. In response, the aims of this study are to use individual data from MSM from ten different countries across Sub‐Saharan Africa to examine the relationship between HIV and legal environments.
Methods: Respondent driven sampling was used to recruit 8113 MSM over the period of 2011 to 2018 across 10 countries: Burkina Faso, Cameroon, Côte d'Ivoire, Gambia, Guinea‐Bissau, Nigeria, Senegal, eSwatini, Rwanda, and Togo. Interviewer‐administered socio‐behavioural questionnaires and biological testing for HIV were conducted. Same‐sex policy categorization was based on ILGA defined legal approach: Not criminalized and not protected; criminalized (<8 years imprisonment); and severe criminalization (>10 years imprisonment). Legal barriers to civil‐society‐organizations (CSO) is defined as legal barriers to the registration or operation of sexual orientation‐related CSOs. Individual‐level data were pooled across countries and multivariable logistic regression models used to measure the association between legal status and HIV.
Results: HIV prevalence among MSM in contexts without criminalization was 8.4% (567/3170); 19.7% (341/1729) in criminalized settings; and 51.8% (422/815) in severely criminalized setting (Table 1). Same sex policies was associated with HIV (p < 0.001). When compared to non‐criminalized settings, criminalized (aOR: 2.31; 95% CI: 1.06, 5.03), and severely criminalized settings (aOR: 8.10;95% CI: 5.31, 12.34) were associated with increased odds of HIV. Legal barriers to CSO engagement was associated with increased odds of HIV (aOR:5.59; 95% CI: 3.41, 9.17).
Abstract OAF0403‐Table 1.
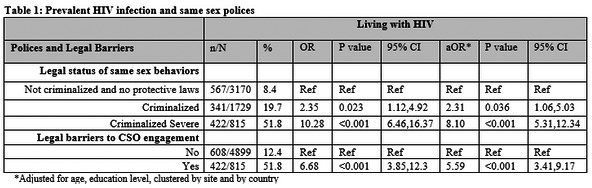
Conclusions: Consistently, same‐sex policies was associated with prevalent individual HIV infection MSM with the magnitude of this relationship the strongest in the most punitive settings. These results provide empiric data of how laws potentiate suboptimal individual HIV outcomes among MSM across Sub‐Saharan Africa and the potential for decriminalization to optimize HIV prevention efforts.
OAF0404
Early warning signs: Assessing the impact of the expanded Mexico City policy on communities most affected by HIV
L. Orza1, L. Stackpool‐Moore2, S. Tuot3
1Frontline AIDS, Brighton, United Kingdom, 2Watipa, Geneva, Switzerland, 3KHANA, Phnom Penh, Cambodia
Background: On 23rd January 2017 President Trump signed an Executive Order to re‐instate the Mexico City Policy – or Global Gag Rule (GGR) ‐‐ with unprecedented application to all US global health assistance, including PEPFAR funds; an approximately 16‐fold expansion on previous iterations of the policy. The HIV community mobilized to express concern for what the expanded Global Gag Rule (GGR) could mean for the health and rights of people most affected by HIV, to ensure that negative impacts of the policy are documented, to support advocacy for its permanent rescindment. Frontline AIDS and Watipa, with support from Sida, conducted a study in Malawi and Cambodia to explore the impact of the GGR on HIV and key population services, and on HIV‐SRHR integration.
Description: The study combined desk research, service data analysis and community engagement for qualitative data collection. In Malawi 16 organizations were engaged through 10 one‐to‐one interviews and one focus group discussion. Quantitative data were analysed from two service sites, provided by organizations that work with sex workers and men who have sex with men. In Cambodia, three focus group discussions took place with a mixed group of health care facility workers and civil society organizations; representatives from civil society organizations only; and, entertainment workers. Eight one‐to‐one qualitative interviews were also undertaken.
Lessons learned: Findings from both countries suggest that:
1.The policy has created some disruption to HIV programmes, outreach services, and referrals to safe, tailored, integrated services for marginalised people, including sex workers, transgender people and men who have sex with men.
2.The policy has created an environment of mistrust, confusion, and isolation among civil society actors, and tightened the space for advocacy on comprehensive SRHR.
3.These changes have compromised access to HIV prevention, testing and treatment services for marginalized people.
Conclusions/Next steps: While quantifying the effect of the GGR on the HIV response remains challenging, findings from this study by Frontline AIDS and partners is consistent with others’ research documenting the impact of the policy on HIV programmes and communities most affected by HIV. These findings contribute to the building of a collective advocacy agenda to mitigate and ultimately permanently rescind the policy.
OAF0405
Assessing the status of drug rehabilitation practices in selected districts of Nepal, from a health and human rights perspective by MoHA, GoN in partnership with YV FDDR and Mainline Foundation
R. Thapa1, M. Ghimire1
1Youth Vision, Health and Rights for the Key Population, Kathmandu, Nepal
Background: In Nepal, PWUD are often marginalized groups who lack access to rights‐based and evidence‐based treatment. As a result of stigmatization of health care providers, social exclusion and criminalizing laws, many human rights violations have reported in treatment centres. This is the first study assessing health and rights‐based treatment in an unique collaboration with Ministry of Home Affairs, the umbrella organization for Rehabilitation centres (FDDR), a local organization Youth Vision and an international NGO (Mainline).
Methods: 85 Drug treatment and Rehabilitation centres were assessed on 9 areas ‐ Adequate water, Enough Toilets, Living Cleanliness Hygiene; Cafeteria/Kitchen Cleanliness Hygiene; Nutrition and full to eat Meal System; Free and Open space Main Area; Playground/Physical Fitness Provision; 1 person 1 bed full accommodation and Proper Assembly Hall; through a standardized checklist, based on a likert scale of 1 to 5 with a score of 1 being ‘unacceptable’ quality and a score of 5 being an appropriate feature of excellent quality.
85 FGDs, 38 KII, 85 interviews with treatment providers and 8 stakeholder interviews, quality and rights‐based treatment services was assessed.
Results: 3.5% had serious water supply problems; 16.5% had inadequate Toilet Facilities versus number of clients housed. 21.2% had unacceptable living conditions, residential, living cleanliness and general hygiene; 17.6% had unacceptable kitchen cleanliness and hygiene. 17.6% had poor nutrition and meal systems, 13% were congested and badly laid out for a rehab. 20.0% had no playground or provision for physical exercise. 36.5% did not always provide 1 person‐1 bed full accommodation and instead, a roll‐out mattress. 25.9% did not have appropriate space to gather as a group with little drug treatment programme materials. 44.7% were in bad physical condition, some were make‐shift huts with tin walls and roofs, others ad‐hoc structures.
Conclusions: Despite improvements in access to rights‐based treatment‐ there are still human rights violations. Only around 10% of the overall rehabs are actually able to comply with SOPs, guidelines and succeeding amendments. The Government of Nepal is currently interested to review current national guidelines and should be regularly monitored and followed up with close scrutiny by involved civil society organizations.
OAF0406
Assessing a human rights‐based approach to HIV in Kenya
N. Sircar1, A. Maleche2, T. Saoyo2
1University of California, Center for Tobacco Control Research and Education, San Francisco, United States, 2Kenya Legal and Ethical Issues Network, Nairobi, Kenya
Background: Kenya's health authorities expanded HIV testing and notification services through increased capacity and training to reach vulnerable populations that are under‐testing and experiencing stigma and/or discrimination: young women, men who have sex with men (MSM), female sex workers (FSW), and persons who inject drugs (PWID). While Kenya promotes a human rights‐based approach (HRBA) to HIV services to help build trust and encourage testing, little was known about programme, policy, and practice implementation with respect to privacy, confidentiality, and dignity. This exploratory, qualitative study evaluated Kenya's implementation of a HRBA to HIV through assessing perspectives on human rights and health care interactions.
Methods: This study included 4 focus group discussions and 16 in‐depth interviews with individuals from Key and Affected Populations (KAP), and HIV care providers or policy experts (HPs). Data were collected from four sites (Nairobi, Mombasa, Homa Bay, and Kisumu counties) from May to July 2019. We analysed data using grounded theory, and applied a rights analysis to the data codes and themes to evaluate Kenya's approach in their HRBA.
Results: A majority of 52 total participants identified female (58%); 36% as male, and 6% were not identified by sex. Most participants self‐identified as from KAPs: MSM (21%), FSW (20%), young women (21%), and PWID (23%), with 15% being non‐KAP HPs. The KAP participants conveyed mixed perspectives about interacting with providers regarding privacy and confidentiality, with mistrust and fears of disrespect being expressed for government‐related facilities. Community‐based organizations with health services were highly regarded and KAPs acknowledged improvement in some provider interactions. HPs acknowledge their need to better engage with KAPs and undergo improved, consistent training on the HRBA to overcome known trust and confidence barriers.
Conclusions: Kenya is increasing the rate of HIV testing and notification among KAPs with community partnerships. Challenges remain in building KAP trust and confidence for HPs and the health care system generally. By identifying opportunities for KAPs to collaborate with HPs, expanding community‐based organizations’ reach, and raising legal literacy to better recognize human rights and effectuate the HRBA to HIV programmes, policies, and practices, more KAPs may utilize HIV services and help identify other at‐risk individuals.
Poster Discussion
PDA0102
PD‐1 expression is linked to NK cell dysfunction and advanced liver fibrosis in HIV/HCV‐coinfected individuals
M.L. Polo1, A. Urioste1, V. Gonzalez Polo1, G. Poblete2, A. Martinez2, A. Sisto2, M.J. Rolon2, G. Turk1, N. Laufer12
1INBIRS‐UBA‐CONICET, Ciudad de Buenos Aires, Argentina, 2Hospital Fernandez, Buenos Aires, Argentina
Background: Liver disease is one of the leading causes of morbi‐mortality in people living with HIV/HCV. Response to HCV therapy and fibrosis regression after HCV clearance is diminished in cirrhotic patients, together with increased risk of hepatocellular carcinoma. Natural killer cells (NK) are associated with tumoral and viral control, and also amelioration of liver fibrosis; however, frequency and degranulation of NK cells are reduced in cirrhotic HIV/HCV‐coinfected individuals. Here, we aim to further study NK cell activation and exhaustion in progressive stages of hepatic fibrosis secondary to HIV/HCV coinfection.
Methods: Blood samples from ART‐treated HCV/HIV‐coinfected participants with different liver fibrosis levels (mild: METAVIR F0/F1 n = 16; advanced: F4 n = 18, assessed by transient elastography) were collected and PBMCs purified. Immunophenotyping (CD25, CD69, NKp46, NKG2D, and PD‐1 expression) and degranulation capacity (CD107a assay) of NK cells were studied by flow cytometry. Clinical and experimental data were analysed using non‐parametric statistics.
Results: All participants had undetectable HIV viral load (VL) with a median LT‐CD4 count of 646 cells/μl (IQR 394 to 849). Overall, 47% were female, median age: 49 years (IQR 46.75 to 53), time of known HIV infection: 20 years (IQR 17.5 to 23), HCV infection: 14 years (IQR 10.5 to 20), time on ART: 11.5 years (IQR 7.75 to 18.35). HCV genotype was predominantly 1a, HCV VL: 6.96 log10 copies (IQR 5.91 to 16). None of the above parameters differed between groups. Frequency of NK/PD‐1 + cells (p = 0.006) as well as PD‐1 expression per NK cell (p = 0.002) were upregulated in F4 participants. In both groups, PD‐1 was confined to CD56dim subset (F0/F1: p = 0.002; F4: p = 0.015) and was associated with higher CD69 and CD25 expression. PD‐1 expression on NK cells inversely correlated with NK cell frequency (r = −0.50; p = 0.01) and degranulation capacity (r = −0.63; p = 0.002). Additionally, PD‐1 expression positively correlated with APRI score (r = 0.53; p = 0.02), liver stiffness (r = 0.51; p = 0.01), and AST levels (r = 0.52; p = 0.02), and negatively with albumin (r = −0.6; p = 0.01) and prothrombin time (r = −0.57; p = 0.01).
Conclusions: Cirrhosis is associated to NK cell exhaustion in HIV/HCV‐coinfected individuals. Potential interventions to improve NK cell function may have relevant implications to boost HCV treatment success in cirrhotic individuals, as well as potential NK cell‐based immunotherapies targeted to modulate liver fibrosis or counteract malignant transformation.
PDA0103
The CARD8 rs2043211 genetic variant and IL‐33 plasma levels are associated with TB‐HIV/IRIS onset in Brazilian individuals
N.B.R. De Sá1, N.C.S. De Sousa1, M. Ribeiro‐Alves2, T.P. Da Silva1, J.H. Pilotto1, V.C. Rolla3, C.B.W. Giacoia‐Gripp1, L.M. De‐Oliveira‐Pinto4, D. Scott‐Algara5, M.G. Morgado1, S.L.M. Teixeira1
1FIOCRUZ, Oswaldo Cruz Institute ‐ Laboratory of AIDS and Molecular Immunology, Rio de Janeiro, Brazil, 2FIOCRUZ, National Institute of Infectious Diseases Evandro Chagas ‐ Laboratory of Clinical Research on STD/AIDS, Rio de Janeiro, Brazil, 3FIOCRUZ, National Institute of Infectious Diseases Evandro Chagas ‐ Clinical Research Laboratory on Mycobacteria, Rio de Janeiro, Brazil, 4FIOCRUZ, Oswaldo Cruz Institute ‐ Laboratory of Viral Immunology, Rio de Janeiro, Brazil, 5Institut Pasteur, Unité de Biologie Cellulaire des Lymphocytes, Paris, France
Background: Inflammasomes are multi‐protein complexes of receptors and sensors that mediate innate immune responses and induce inflammation. Tuberculosis (TB) and aids are the leading causes of infectious disease death worldwide in which inflammation plays a major role in disease progression. In some TB‐HIV‐infected individuals, treated simultaneously for both diseases, a pathological inflammatory reaction, named immune reconstitution inflammatory syndrome (IRIS), may occur. The search of risk factors for IRIS is of relevance for clinical management. We investigated the role of single‐base polymorphisms (SNPs) of the NLRP3, CARD8, and IL‐1 β inflammasomes genes, as well as the profile of their related proinflammatory cytokines (IL‐1β, IL‐18, IL‐33, and IL‐6) in the susceptibility/resistance to TB‐HIV coinfection outcomes.
Methods: Patients were divided into four groups: TB‐HIV (n = 88; 11 of them with IRIS), HIV (n = 20), TB (n = 24) and healthy controls (n = 24). These patients were followed up at INI/FIOCRUZ and HGNI, Rio de Janeiro, Brazil, from 2006 to 2016. SNPs genotyping of the cellular inflammasomes were determined by Real‐Time PCR, and plasma concentrations of cytokines were measured by ELISA kits. Protection/risk estimations were performed by unconditional logistic regression models.
Results: Significant differences in the plasma cytokine levels and their relationships with the SNPs were observed among the groups. Regarding the TB‐HIV individuals, the A/T genotype (p = 0.034), allele T (p = 0.030) and carrier‐T (p = 0.030) in the CARD8 rs2043211 polymorphism were associated with non‐IRIS, while IL‐33 plasma levels tended to be slightly higher among the TB‐HIV/IRIS individuals (p = 0.055).
Conclusions: These results provide new insights into the role of innate immunity in the physiopathology of TB‐HIV/IRIS, and as of our knowledge, this is the first study demonstrating an association between the CARD8 rs2043211 polymorphism and plasma levels of IL‐33 with IRIS in TB‐HIV coinfected individuals. The functional role of such molecules in IRIS pathogenesis is still to be demonstrated. These results, associated with previous data of HLA and KIR polymorphisms in this study group, contribute to the discussion of the impact of host genes in TB‐HIV individuals and the IRIS outcome.
PDA0104
Association of age and cervical cancer screening results in 11 urban health centres in Lusaka, Zambia
J. Pry1,2, M. Duran‐Frigola3,4, J. Matambo3, K. Taghavi5, J. Mubita3, O. Chituwo6, I. Sikazwe3, C. Bolton‐Moore3,7, A. Manasyan3,7
1Washington University, School of Medicine, St. Louis, United States, 2Centre for Infectious Disease Research Zambia, Implementation Science Unit, Lusaka, Zambia, 3Centre for Infectious Disease Research Zambia, Lusaka, Zambia, 4Institute for Research in Biomedicine, Structural Bioinformatics & Network Biology Lab, Barcelona, Spain, 5University of Bern, Bern, Switzerland, 6Centers for Disease Control and Prevention, Center for Global Health, Division of Global HIV and TB, Lusaka, Zambia, 7University of Alabama, School of Medicine, Birmingham, United States
Background: Cervical cancer is the leading cancer‐related cause of death among women in Zambia and poses an even greater threat to HIV‐positive women. The World Health Organization global strategy towards the elimination of cervical cancer requires 70% of women aged 35 to 45 years to be screened of which 90% should receive treatment by 2030. To identify screening gaps to achieving this goal we evaluated the effect of age on visual inspection with acetic acid (VIA) screening results in Zambia.
Methods: We abstracted VIA screening data from the Cervical Cancer Prevention Program in Zambia registers (January 1, 2010–June 30, 2019) at 11 clinics in Lusaka Province. We conducted a mixed‐effects logistic regression analysis to assess VIA results allowing random effects at the clinic and individual level. Post‐estimation modelling was used to calculate adjusted predictive probabilities of VIA‐positive results by HIV status and age.
Results: We included 204,225 VIA screening results from 183,194 women. Of 204,225 VIA screenings, 21,326 (10.4%) were positive, and median patient age was 34 years (interquartile range, 28 to 42 years). The predictive probability of screening positive was highest among HIV‐positive patients aged 20 to 29 years (18.6%; 95% confidence interval [CI]: 14.2% to 22.9%) followed by those younger than 20 years (18.4%; 95% CI: 9.6% to 27.3%; Figure 1).
Abstract PDA0104‐Figure 1.
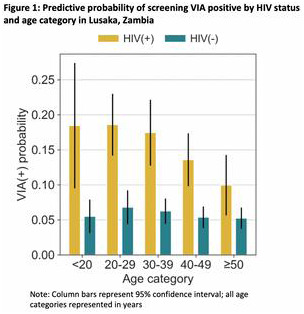
Conclusions: Almost one in five HIV‐positive women aged ≤29 years screened VIA positive in Lusaka. To optimize progress toward cervical cancer elimination, in Zambia, a differentiated model that focuses on HIV‐positive women, especially younger women (≤29 years), as a high‐risk group could be considered.
PDA0105
Modelling endothelial function in vitro and via blood sampling to assess cardiovascular risk in people living with HIV
A.A. Khawaja1, R.T. Maughan1, K.E. Paschalaki1, K.A. Taylor1, A.O. Lovell2, C. Pericleous1, J.C. Mason1, A.M. Randi1, M. Boffito2,3, M. Emerson1
1Imperial College London, National Heart and Lung Institute, London, United Kingdom, 2Imperial College London, Department of Infectious Disease, London, United Kingdom, 3Chelsea and Westminster NHS Trust, London, United Kingdom
Background: Cardiovascular (CV) disease, which is driven by endothelial and platelet dysfunction, is more prevalent among people living with HIV (PLWH) and has been associated with some antiretroviral (ARV) use. Determining effects of ARVs upon platelet function via blood sampling is commonplace, however examining the impact on endothelial cells, without using invasive sampling methods, is more complex. We therefore evaluated in vitro models of endothelial dysfunction and developed methods to isolate and phenotype endothelial‐derived microparticles (EMP) and endothelial ‘progenitor’ cells (endothelial colony‐forming cells, ECFCs), both of which may be isolated from blood and allow for detailed analysis of endothelial function in patients or clinical trial participants.
Methods: Human coronary artery endothelial cells (HCAECs) were treated with plasma Cmax concentrations of ABC or tenofovir disoproxil fumarate (TDF), stimulated with TNF‐alpha to mimic inflammation, and inflammatory and pro‐thrombotic properties assessed by flow cytometry. EMP were isolated from cell culture supernatants and plasma from human subjects, and characterized using flow cytometry. ECFCs were isolated in the presence of ARVs using whole blood of healthy subjects taking PrEP in order to establish protocols in a relevant population exposed to daily ARVs. Statistical significance was determined by one‐way ANOVA with Tukey's multiple comparison test.
Results: ABC treatment enhanced levels of TNF‐alpha‐induced inflammatory ICAM‐1 and pro‐thrombotic TF expression compared to TDF (+1.9‐ and +1.2‐fold, p < 0.05) in HCAECs. ABC treatment led to greater numbers of ICAM‐1+ and TF+ EMP compared to TAF (+2.1‐ and +3.3‐fold, p < 0.05) in HCAECs and EMP from the blood of human subjects taking ARVs at therapeutic doses were successfully isolated and their inflammatory and thrombotic properties determined. We were able to isolate viable ECFCs from PrEP users in similar numbers to those obtained from ARV‐naïve donors in earlier studies.
Conclusions: ABC enhanced the inflammatory and thrombotic properties of cultured HCAEC suggesting that this model may be used predictively to evaluate the cardiovascular risk profile or ARVs. In the context of clinical studies, EMPs and ECFCs are suggested as useful tools for determining the effects of ARVs and HIV infection per se upon vascular endothelial thrombo‐inflammatory properties and therefore cardiovascular health.
PDA0106
Innate lymphoid cells are reduced in pregnant HIV positive women and are associated with preterm birth
C. Akoto1, C. Chan1, C. Tshivuila‐Matala1, K. Ravi1, W. Zhang1, M. Vatish1, S. Norris2, J. Hemelaar1
1University of Oxford, Nuffield Department of Women's & Reproductive Health, Oxford, United Kingdom, 2University of the Witwatersrand, South African Medical Research Council Developmental Pathways for Health Research Unit, Department of Paediatrics, School of Clinical Medicine, Johannesburg, South Africa
Background: Preterm birth is the leading cause of neonatal and child mortality worldwide. Globally, 1.4 million pregnant women are estimated to be living with HIV/AIDS, the majority of whom live in sub‐Saharan Africa. Maternal HIV infection and antiretroviral treatment (ART) have been associated with increased rates of preterm birth, but the underlying mechanisms remain unknown. Acute HIV infection is associated with a rapid depletion of all three subsets of innate lymphoid cells (ILCs), ILC1s, ILC2s and ILC3s, which is not reversed by ART. ILCs have been found at the maternal‐foetal interface and we therefore investigated the potential association between maternal HIV infection, peripheral ILC frequencies and preterm birth.
Methods: We conducted flow‐cytometric analysis of peripheral blood samples from 46 HIV‐positive (HIV+) and 45 HIV‐negative (HIV‐) pregnant women enrolled in a prospective pregnancy cohort study in Soweto, South Africa. Frequencies of ILC1s, ILC2s and ILC3s were compared between women with and without HIV infection, and between women with and without PTB or spontaneous preterm labour (Sp‐PTL).
Results: We show that maternal HIV infection is associated with reduced levels of all three ILC subsets. Preterm birth was also associated with lower levels of all three ILC subsets in early pregnancy. ILC frequencies were lowest in HIV positive women who experienced preterm birth. Moreover, ILC levels were reduced in pregnancies resulting in spontaneous onset of preterm labour and in extreme preterm birth (<28 weeks gestation).
Conclusions: Our findings suggest that reduced ILC frequencies may be a link between maternal HIV infection and preterm birth. In addition, ILC frequencies in early pregnancy may serve as predictive biomarkers for women who are at risk of delivering preterm.
PDA0107
Youth perinatal HIV‐associated cognitive impairment: Associations with childhood trauma
N. Phillips1, D. Stein1, L. Myer2, H. Zar3, J. Hoare1
1University of Cape Town, Psychiatry and Mental Health, Cape Town, South Africa, 2University of Cape Town, Public Health and Family Medicine, Cape Town, South Africa, 3University of Cape Town, Child Health, Cape Town, South Africa
Background: Exposure to childhood trauma is associated with cognitive impairment in non‐clinical populations. The association between childhood trauma and cognitive impairment in the context of perinatal HIV‐infection has not been published to‐date. This study was nested within the Cape Town Adolescent Antiretroviral Cohort (CTAAC) neuro sub‐study, a longitudinal cohort of perinatally ARV‐treated HIV‐infected youth from public healthcare facilities across Cape Town, South Africa. The purpose was to examine the association between childhood trauma and HIV‐associated cognitive impairment among perinatally HIV‐infected youth.
Methods: HIV‐infected youth and HIV‐uninfected controls completed a comprehensive neuropsychological battery and the Childhood Trauma Questionnaire (CTQ). We then assessed associations between cognitive impairment in various domains and CTQ scores by means of a simple bivariate correlation.
Results: Results represent data from 36‐month CTAAC follow‐ups, which includes 122 HIV‐infected and 35 HIV‐uninfected controls between 12 and 15 years old. Independent samples t‐test show no statistically significant differences in self‐reported childhood trauma between HIV‐infected youth and controls (i.e.: both groups showed low – moderate levels of trauma). Within the HIV‐infected group CTQ total scores were significantly correlated with impaired working memory (r = .228, p = .023) and processing speed (r = .238, p = .016). The CTQ subscale of emotional abuse was significantly correlated with the domains of attention, working memory and processing speed, yet the CTQ subscale of emotional neglect was only correlated with impaired processing speed (r = .204, p = .041). The CTQ subscales of physical abuse and neglect and sexual abuse were not significantly correlated with any of the cognitive domains. In the control group childhood trauma on the physical neglect subscale correlated with impaired general intellectual function and emotional abuse correlated with impaired motor coordination.
Conclusions: The majority of HIV‐infected youth in South Africa live in very low socioeconomic environments and are exposed to numerous risk factors, the most significant of which is childhood trauma. Given the association between childhood trauma and cognitive impairment, limiting childhood trauma should be a major public health concern. These findings suggest that low – moderate trauma within the HIV‐infected group is associated with more cognitive problems compared to controls. This study provides preliminary data to further investigate the relationship between childhood trauma and HIV‐associated cognitive impairment.
PDA0202
High Y‐chromosome DNA concentrations are associated with increased cervical cytokine concentrations and activated cervical HIV target cell frequencies
J. Jewanraj1,2, S. Ngcapu1,2, V. Ramsuran1,2,3, A. Mtshali1,2, L. Mansoor1, S. Abdool Karim1,4, Q. Abdool Karim1,4, J.‐A. Passmore1,5,6, L. Liebenberg1,2
1Centre for the AIDS Programme of Research in South Africa (CAPRISA), Durban, South Africa, 2School of Laboratory Medicine and Medical Science, College of Health Sciences, University of KwaZulu‐Natal, Durban, South Africa, 3KwaZulu‐Natal Research and Innovation Sequencing Platform (KRISP), Durban, South Africa, 4Department of Epidemiology, Columbia University, New York City, United States, 5Institute of Infectious Disease and Molecular Medicine IDM, University of Cape Town, Cape Town, South Africa, 6National Health Laboratory Service, Cape Town, South Africa
Background: Semen is the carrier for spermatozoa and the primary vector for heterosexual transmission of HIV to women during intercourse. Semen induces cytokine production and immune cell recruitment at the female genital tract (FGT) in order to facilitate conception. Since genital inflammation increases HIV susceptibility in women, semen‐induced alterations at the FGT may also have implications for HIV risk. Here we investigated the contribution of semen exposure to biomarkers of inflammation associated with HIV acquisition.
Methods: Genital specimens were collected every 6 months (average 5 ± 1 visits) from 149 HIV‐negative women participating in the CAPRISA 008 tenofovir gel open‐label extension trial (n = 693 specimens). Y‐chromosome DNA (YcDNA) was extracted using a Human Y‐chromosome DNA detection kit and quantified using the Quantifiler Trio DNA quantification kit in cervicovaginal lavage (CVL) pellet specimens. In matched CVL supernatant specimens, YcDNA concentrations were compared with concentrations of 48 cytokines and 9 matrix metalloproteinases (MMPs; epithelial barrier function proteins) determined by multiplexed enzyme‐linked immunosorbent assay (ELISA), and with the frequencies of cervix‐derived NK cells and HIV T cell targets determined by flow cytometry.
Results: A total of 175/233 (75%) genital specimens with detectable YcDNA had a yield sufficient for quantitation. In multivariable linear mixed model analyses, higher YcDNA concentrations were associated with elevated concentrations of growth factors (IL‐7, IL‐9, PDGF‐ββ, VEGF, G‐CSF), pro‐inflammatory/chemotactic (IL‐12p70, IL‐6, IP‐10), anti‐inflammatory (IL‐10), and adaptive response cytokines (IFN‐γ, IL‐13, IL‐4). Increased concentrations of MMPs (MMP‐1, MMP‐2, MMP‐3, MMP‐7, MMP‐10, and MMP‐13) involved in the degradation and repair of the extracellular matrix were associated with higher YcDNA concentration. Additionally, higher YcDNA concentrations were also associated with significantly increased frequencies of activated HIV target cells (CD4 + CCR5 + HLA‐DR+) at the FGT.
Conclusions: Higher YcDNA concentrations were associated with raised levels of cytokines and MMPs, and with greater frequencies of HIV target cells at the female genital mucosa. Considering the association between YcDNA and these established biomarkers of genital inflammation and HIV risk, semen‐induced alterations at the FGT may, therefore, have implications for HIV susceptibility in women.
PDA0203
CyTOF analysis reveals that HIV‐1 upregulates expression of multiple RNA and DNA sensors in subsets of primary CD4 + T cells
A. George1,2, J. Frouard1,2, T. Ma1,2, W. Greene1,3, N. Roan1,2
1Gladstone Institute of Virology and Immunology, San Francisco, United States, 2University of California, Department of Urology, San Francisco, United States, 3University of California, Departments of Medicine, Microbiology, and Immunology, San Francisco, United States
Background: While CD4 + T cells are the primary targets of human immunodeficiency virus (HIV), not all are equally susceptible to infection. Cell permissiveness to infection is determined, in part, by innate immune sensing and host restriction of HIV. We set out to determine if cells that fuse to HIV differentially express viral sensors and restriction factors, and whether HIV infection modulates expression of viral sensors and restriction factors in primary CD4 + T cells.
Methods: We developed and validated a 41‐parameter CyTOF panel that includes 23 intracellular viral sensing proteins and restriction factors. Activated human PBMCs from seronegative individuals were exposed to a CCR5‐tropic transmitted/founder HIV‐1 reporter virus, and then HIV‐fused cells, identified using a Blam‐vpr‐based viral fusion assay, were sorted by flow cytometry and analysed by CyTOF. Concurrently, cells from the same donor PBMCs were exposed to HIV and productively infected cells were identified three days later by CyTOF.
Results: Relative to mock‐treated CD4 + T cells, HIV‐fused CD4 + T cells expressed lower levels of a variety of HIV sensors, including IFI16 and cGAS, as well as restriction factors, including SAMHD1 and IFITM1. Comparison of HIV‐fused cells to productively infected cells revealed that HIV directly upregulated expression of the restriction factors SAMHD1 and IFITM1, the RNA sensors RIGI and TLR7, and the DNA sensors IFI16 and cGAS within infected cells.
Conclusions: These data suggest that HIV preferentially enters CD4 + T cells expressing low levels of viral sensor and restriction factors, which may facilitate the completion of the viral life cycle. Following entry, HIV upregulates expression levels of many of these factors but this upregulation is insufficient to prevent productive infection. These results suggest that enhancing innate immune recognition of HIV may be necessary to fully restrict replication of the virus.
PDA0205
TILRR modulates production of proinflammatory cytokines and promote leukocytes migration and may be a novel target to prevent HIV‐1 vaginal infection
M. Kashem1,2, X. Ren3, H. Li4, F. Lin3, F. Plummer1, M. Luo1,2,4
1University of Manitoba, Medical Microbiology and Infectious Diseases, Winnipeg, Canada, 2JC Wilt Infectious Diseases Research Center, Winnipeg, Canada, 3University of Manitoba, Biosystems Engineering, Winnipeg, Canada, 4National Microbiology Laboratory, Winnipeg, Canada
Background: Toll‐like Interleukin‐1 receptor regulator (TILRR), a splice variant of FREM1, is an IL‐1R1 co‐receptor and an important modulator of inflammatory responses. Our previous studies showed the minor allele of FREM1 SNP rs1552896 is significantly associated with resistance to HIV‐1 infection in the Pumwani sex worker cohort. Women with the minor allele of rs1552896 expressed no or very low TILRR RNA, whereas the major allele of rs1552896 expressed a significant amount of TILRR RNA. Since TILRR modulates many inflammation responsive genes, it could play an important role in modulating immune cell migration in response to infection through its influence on proinflammatory cytokines secretion by epithelial cells. In this study, we investigated the effect of TILRR‐ overexpressed cervical epithelial cells supernatants on the migration of HIV‐1 target cells using two different migration approaches.
Methods: We conducted the migration experiments using a novel microfluidic real‐time migration device and a transwell migration method. THP‐1 (monocytes), MOLT‐4 (lymphocytes), and primary human T‐cells were used as target cells to investigate the effect of TILRR on their migration behaviour. Cervico‐epithelial cell (HeLa) transfected with either TILRR or vector‐only control, and parental cell culture supernatants were used as chemoattractants.
Results: The results showed that TILRR‐ overexpressed HeLa cell supernatant significantly attracted more monocytes (THP‐1) than vector‐only control (>20% higher of % relative migration, PRM = 59.83 ± 5.00 vs. 32.70 ± 4.37, p = 0.0021) in transwell assay. Similar to THP‐1 cells, significantly higher amount of MOLT‐4 (47.72 ± 6.13 vs. 14.95 ± 6.68, p = 0.0033), and Primary T‐cells (72.98 ± 3.08 vs. 40.16 ± 2.00, p = 0.0001) were also migrated to the TILRR‐ overexpressed HeLa culture supernatant. Moreover, the microfluidic real‐time assay showed that the migration distance of THP‐1 and primary T‐cells was significantly longer towards TILRR‐transfected HeLa culture supernatants than to the vector‐only control supernatants.
Conclusions: Our study, for the first time, demonstrated that TILRR overexpressed cell culture supernatant significantly influences the migration of leukocytes. Thus, TILRR could play an important role in recruiting HIV‐1 target cells at mucosal surfaces through its modulation on the production of multiple proinflammatory cytokines that may lead to increased susceptibility to HIV‐1 vaginal infection. TILRR may be a novel target to reduce/prevent HIV‐1 vaginal infection.
PDA0206
Th17 cells are early targets of SIV during acute infection in rhesus macaque vaginal challenge model
M.S. Arif1, S. Xiao1, D. Thakkar1, G.C. Cianci1, R. Lorenzo‐Redondo2, M. Halkett1, K.K. Halavaty1, M. Becker1, A.M. Carias1, D. Maric1, R. Veazey3, T. Hope1
1Feinberg School of Medicine, Northwestern University, Department of Cell and Developmental Biology, Chicago, United States, 2Feinberg School of Medicine, Northwestern University, Department of Medicine, Chicago, United States, 3Tulane National Primate Research Center, Tulane University School of Medicine, Covington, United States
Background: Identification of infected cells immediately after mucosal HIV/SIV transmission is critical to design effective prevention strategies. Previous studies have identified CD4 + T‐cells, especially, Th17s, as early targets of HIV/SIV in the female reproductive tract (FRT). Here we used rhesus macaque (RM) vaginal challenge model to identify early targets of infection and to follow the infected cell phenotype changes over time.
Methods: 12 female RMs were challenged intravaginally with a non‐replicative luciferase reporter, LiCH, and SIVmac239 mixture. Animals were sacrificed 48‐, 72‐, or 96‐hours post‐challenge. Macroscopic luciferase signal detected by in vivo imaging system (IVIS) allowed us to identify FRT regions likely containing infected cells. IVIS positive and negative tissues were serially cryosectioned for immunofluorescence staining and RNA isolation. Infected cells were phenotyped microscopically to identify Th17s (CD3 + CCR6 + ), other T‐cells (CD3 + CCR6‐), immature dendritic cells (iDCs)(CD3‐CCR6 + ), and other cells (CD3‐CCR6‐). RNA was extracted from infected and non‐infected adjacent tissue sections for RNA‐Seq.
Results: Phenotyping of > 5000 SIV‐infected cells in FRT of eight RMs sacrificed at 72 hr and 96 hr post‐challenge identified infection throughout FRT in 3/4 and 4/4 of 72 hr and 96 hr animals, respectively. Comparing the two time points, proportion of infected Th17s remains constant (85 vs. 70%), however, we can detect an increase in infection rate of iDCs (from 10 to 30%) and other T‐cells (from 1 to 3%) as infection progressed. The use of serial sectioning allowed us to identify spread of infection across multiple cryosections of the same tissue. Also, plotting the coordinates of infected cells from multiple sections allowed us to visualize the infected cells in three‐dimensional space and to follow the infected cells dissemination over the time.
Conclusions: These findings support our previous data demonstrating the entire FRT is susceptible to infection and that Th17s are the predominant early targets. In our future work, we hope to compare the distribution of infected cells together with the transcriptome profiles between infected and non‐infected tissues at different time points to help understand dynamics and kinetics of virus distribution and dissemination during acute infection.
PDA0207
Interferon‐α modulates the host glycosylation machinery during treated HIV infection
L. Giron1, F. Colomb1, E. Papasavvas1, L. Azzoni1, X. Yin1, A. Anzurez1, M. Fair1, K. Mounzer2, J. Kostman2, P. Tebas3, U. O'Doherty3, Q. Liu1, M. Betts3, L.J. Montaner1, M. Abdel‐Mohsen1
1The Wistar Institute, Philadelphia, United States, 2Philadelphia FIGHT, Philadelphia, United States, 3University of Pennsylvania, Philadelphia, United States
Background: A comprehensive understanding of host factors modulated by the key antiviral cytokine interferon‐α (IFNα) is imperative for harnessing its beneficial effects while avoiding its detrimental side‐effects, during chronic diseases such as HIV infection. Cytokines modulate host glycosylation, and the host glycome (circulating glycans and cell‐surface glycans) plays a critical role in mediating several cellular processes and immunological functions. However, the impact of IFNα on host glycosylation machinery has never been characterized.
Methods: We assessed the impact of pegylated IFNα2a therapy on circulating IgG glycomes and isolated CD8 + T and NK cell‐surface glycomes of 18 HIV‐mono‐infected individuals on suppressive antiretroviral therapy, using capillary electrophoresis and lectin microarrays. Plasma levels of sCD14 and sCD163 were measured by ELISA. CD8 + T cell and K562‐stimulated NK cell phenotypes were profiled using flow cytometry. Integrated HIV DNA in CD4 + T cells was measured by qPCR. Wilcoxon test and Spearman's correlations were used for statistical analysis. False discovery rates (FDR) were calculated to account for multiple comparisons.
Results: Interactome analysis highlighted significant interactions that support a model in which a) IFNα increases the proportion of pro‐inflammatory, bisected GlcNAc glycans (known to enhance FcγR binding) within the IgG glycome (FDR < 0.02), which in turn b) increases inflammation (as measured by sCD14 and sCD163; p < 0.03), which c) leads to lower levels of CD8 + T cell functionality (perforin, Eomes, and TNFα expression) but higher degranulation (CD107) (p < 0.02, Figure). IFNα‐mediated induction of bisected GlcNac associated with a poor reduction of HIV integrated DNA (p = 0.02, rho = −0.78). Examining cell‐surface glycomes, IFNα increases the levels of T antigen (Gal‐GalNAc) on CD8 + T cells (FDR = 0.01). This induction is associated with lower CD8 + T degranulation (p < 0.02, rho < −0.8). Last, IFNα increases the levels of fucose on NK cells (p < 0.05). This induction is associated with higher expression of Eomes, T‐bet, and IFNγ upon K562 stimulation (p = 0.048, rho > 0.8).
Conclusions: IFNα causes host glycomic alterations that are known to mediate inflammatory responses. These alterations are associated with mainly detrimental, but also beneficial, consequences of IFNα on innate and adaptive immune functions. Manipulating glycan‐lectin interactions may represent a strategy to enhance the impact of IFNα on immunity while avoiding its detrimental side‐effects.
PDB0102
Time to viral rebound after interruption of modern antiretroviral therapies
J. Li1, E. Aga2, R. Bosch2, M. Pilkinton3, L. MacLaren4, M. Keefer5, E. Acosta6, L. Fox7, L. Bar8, J. Mellors9, R. Coombs10, A. Landay11, B. Macatangay9, S. Deeks12, R. Gandhi13, D. Smith14, AIDS Clinical Trials Group (ACTG) A5345 Study Team
1Brigham and Women's Hospital, Harvard Medical School, Cambridge, United States, 2Harvard T.H. Chan School of Public Health, Boston, United States, 3Vanderbilt University Medical Center, Nashville, United States, 4Whitman Walker Health, Washington, DC, United States, 5University of Rochester, Rochester, United States, 6University of Alabama, Birmingham, United States, 7National Institute of Allergy and Infectious Diseases, Rockville, United States, 8ACTG Community Scientific Subcommittee, Los Angeles, United States, 9University of Pittsburgh, Pittsburgh, United States, 10University of Washington, Seattle, United States, 11Rush University, Chicago, United States, 12University of California, San Francisco, San Francisco, United States, 13Massachusetts General Hospital, Harvard Medical School, Boston, United States, 14University of California, San Diego, San Diego, United States
Background: Strategies for ART‐free HIV remission require validation through treatment interruption (TI) studies to confirm a delay in HIV rebound, but there is uncertainty whether modern ART regimens may impact this outcome. We compared HIV rebound timing in A5345 to historic TI studies.
Methods: A5345 is a prospective study enrolling individuals who initiated ART during chronic or early HIV infection, on suppressive ART for ≥2 years with CD4 count ≥500 cells/mm3 and nadir CD4 count ≥200 cells/mm3. Participants on a non‐nucleoside reverse transcriptase inhibitor (NNRTI)‐based regimen were switched to a protease inhibitor (PI) or integrase strand transfer inhibitor (INSTI)‐based regimen before the TI. During TI, viral loads were monitored twice weekly and participants restarted ART upon two successive viral loads ≥1000 copies/mL. We compared the chronic‐treated participants of A5345 with chronic‐treated participants on PI regimens from placebo arms of 4 historic ACTG TI studies.
Results: Thirty‐three chronic‐treated A5345 participants interrupted ART and were compared to 61 participants from historic studies. There were no significant differences between the groups in age (median 46 vs. 43 years), sex (88% vs. 87% male), nadir or baseline CD4 count (median 783 vs. 852 cells/mm3), or pre‐ART viral loads (median 4.5 vs. 4.4 log10 copies/mL). All participants of the historic studies were on older PI‐based regimens while 94% of A5345 participants were on INSTI‐based ART. The median time to viral rebound ≥1000 copies/mL in A5345 was 22 days. Acute retroviral syndrome was diagnosed in three (9%) participants. There were no differences between A5345 vs. historic studies in the percentage of participants with viral rebound by either TI week 4 (73% vs. 79%, p = 0.61) or week 8 (97% vs. 95%, p = 1.0). There was no significant association of ART duration or CD4 count with timing of HIV rebound in A5345; higher pre‐ART HIV RNA was associated with shorter time to rebound (Spearman r = −0.37, p = 0.09). All participants re‐suppressed after ART re‐initiation.
Conclusions: For chronic‐treated individuals, virologic suppression by modern ART regimens did not result in a significant delay in the time to HIV rebound after ART interruption. Novel strategies will be needed to achieve ART‐free HIV remission.
PDB0103
Is DTG + 3TC effective and safe in clinical practice? Evidence from real world data
Y. Punekar1, D. Parks2, J. van Wyk1, A. de Ruiter1
1ViiV Healthcare, Brentford, United Kingdom, 2GlaxoSmithKline, Philadelphia, United States
Background: RCT evidence has shown dolutegravir (DTG) + lamivudine (3TC) is an efficacious and durable regimen with a good safety profile in treatment‐naïve and treatment‐experienced HIV‐infected individuals. Several observational studies have concluded that it is effective in clinical practice. The objective of this meta‐analysis was to estimate effectiveness and safety of DTG + 3TC in treatment‐experienced, virologically suppressed people living with HIV (PLHIV) by combining real‐world evidence (RWE) from clinical practice.
Methods: A systematic literature review of PubMed and Embase along with 24 regional and international conferences was conducted between 2013 and Dec 2019 to identify non‐RCT studies of DTG + 3TC in PLHIV. Eligible published articles presenting outcomes of interest were identified and extracted. Identified studies were included if they had acceptable level of publications bias and heterogeneity determined using funnel plots and I2 statistics, respectively. One‐arm meta‐analyses using the Dersimonian and Laird method were conducted to estimate effect sizes for viral failure, viral suppression, and discontinuations for DTG + 3TC.
Results: A total of 7 DTG + 3TC studies (n = 1800 patients) reported data on treatment experienced virologically suppressed PLHIV on outcomes of interest at different timepoints. Results showed that among patients switching to DTG + 3TC treatment ≥90% maintained virological suppression (ITT) with ≤1% viral failures.
Abstract PDB0103‐Table 1. Proportion of patients with viral failure, virological suppression and discontinuations at week 48
| DTG + 3TC | ||||
|---|---|---|---|---|
| Viral Failure (n = 1800) | Virological suppression ITT (n = 1800) | Virological suppression PP (n = 1552) | Discontinuations (n = 1800) | |
| Week 48 (Mean [95% CI]) | 0.008 [0.004 to 0.014] | 0.906 [0.849 to 0.951] | 0.990 [0.983 to 0.995] | 0.089 [0.048 to 0.139] |
CI, confidence interval; ITT, intention to treat; PP, per protocol.
Abstract PDB0103‐Table 2. Proportion of patients with viral failure, virological suppression and discontinuations at week 96
| DTG + 3TC | ||||
|---|---|---|---|---|
| Viral Failure (n = 904) | Virological suppression ITT (n = 904) | Virological suppression PP (n = 767) | Discontinuations (n = 904) | |
| Week 96 (Mean [95% CI]) | 0.005 [0.001 to 0.013] | 0.930 [0.831 to 0.990] | 0.995 [0.976 to 1.000] | 0.057 [0.004 to 0.151] |
CI, confidence interval; ITT, intention to treat; PP, per protocol.
Conclusions
DTG + 3TC is an effective and durable antiretroviral regimen with low rates of discontinuation in treatment experienced patients in clinical practice.
PDB0104
Comparative efficacy and safety of a combination therapy of dolutegravir and lamivudine versus 3‐drug antiretroviral regimens in treatment‐naïve HIV‐1 infected patients at 96 weeks: A systematic review and network meta‐analysis
K. Nickel1, N. Halfpenny2, S. Snedecor3, Y. Punekar4
1Pharmerit International, Berlin, Germany, 2Pharmerit International, Rotterdam, Netherlands, 3Pharmerit International, Bathesda, United States, 4ViiV Healthcare, Brentford, United Kingdom
Background: Dolutegravir+lamivudine (DTG + 3TC) has been shown to be comparable to guideline recommended 3‐drug antiretroviral treatments (ARTs) in HIV up to week 48. The objective of this analyses was to compare the efficacy and safety of DTG + 3TC to 3‐drug ARTs up to 96 weeks (WK96).
Methods: Randomized controlled trials (RCTs) of treatment‐naïve HIV‐1 infected patients reporting outcomes at WK96 were identified by systematic review. The proportion of patients achieving virologic suppression (VS) to <50 RNA copies/mL at WK96 for all patients and those with baseline viral load > 100,000 RNA copies/mL was compared between DTG + 3TC and guideline recommended ARTs using a fixed‐effect Bayesian network meta‐analysis framework. Other outcomes examined were CD4 + cell count change from baseline, treatment discontinuations and safety (adverse events [AEs], serious AEs [SAEs], and drug‐related AEs [DRAEs]).
Results: The network included 11 ARTs from 11 RCTs with 7991 patients. ARTs containing tenofovir disoproxil/emtricitabine (TDF/FTC) and tenofovir alafenamide (TAF)/FTC were combined by their core agent to maintain network connectivity. The treatment difference for viral suppression at WK96 for DTG + 3TC compared to the other 10 ARTs ranged from −3% (−7%, 0%) versus DTG+TDF (or) TAF/FTC to 13% (4%, 23%) versus ritonavir‐boosted darunavir (DRV/r) + TDF/FTC. On other outcomes DTG + 3TC was broadly similar to all 3‐drug ARTs with some statistically significant benefits on SAEs and DRAEs.
Abstract pdb0104‐fig-0001-a
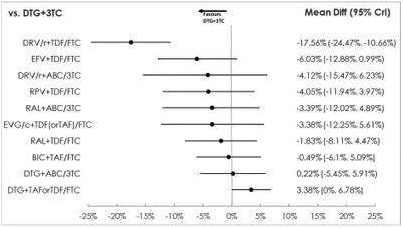
Abstract PDB0104‐Figure 1. Difference in proportions in viral suppression (95% credible intervals) at WK96, 3‐drug ARTs versus DTG + 3TC.
Conclusions: DTG + 3TC offers comparable and durable efficacy and safety to guideline recommended 3‐drug regimen with reduced exposure to ARTs for naïve patients starting treatment.
PDB0105
Switching to dolutegravir plus lamivudine (DTG + 3TC) is non‐inferior to and as safe as continuing standard triple antiretroviral therapy (TAR)
J. Rojas1, J.L. Blanco1, E. Negredo2, P. Domingo3, J. Tiraboschi4, E. Ribera5, N. Abdulghani6, J. Puig2, G. Mateo3, D. Podzamczer4, M. Gutierrez3, E. de Lazzari1, R. Paredes2, E. Martinez1, DOLAM Study Team
1Hospital Clínic, Barcelona, Spain, 2Hospital Germans Trías i Pujol, Badalona, Spain, 3Hospital de Sant Pau, Barcelona, Spain, 4Hospital de Bellvitge, L'Hospitalet, Spain, 5Hospital Vall d'Hebron, Barcelona, Spain, 6Hospital Arnau de Vilanova, Lleida, Spain
Background: It is uncertain whether virally suppressed patients on standard triple ART can be safely switched to DTG + 3TC.
Methods: HIV‐1‐infected adults with <50 copies/mL for ≥12 months, no viral failure or resistance mutations to study drugs, CD4 nadir >200 cells/mm3, and HBsAg negative were randomized 1:1 (stratified by baseline third agent ) to continue triple antiretroviral therapy (control) or to switch to DTG + 3TC. Primary end‐point was the proportion of patients with HIV‐1 RNA ≥ 50 copies/mL at week 48 (FDA Snapshot, 8% non‐inferiority margin). Blips, adverse effects, weight and body fat (DXA scan), and sleep quality (PSQI) were secondary end‐points.
Results: 265 patients randomized (DTG + 3TC: 131; control: 134). Baseline: age 46 years; women 14%; CD4 712 cells/mm3; weight 75 kg; trunk fat 10,134 gr; limbs fat 7615 gr; poor sleep quality (defined by PSQI > 5) 40%. At week 48, subjects with HIV‐1 RNA ≥ 50 copies mL 2.4% (3/125) DTG + 3TC versus 0.8% (1/126) control [difference 1.6%; 95% CI −2.3 to 6.1] per‐protocol, and 2.3% (3/131) DTG + 3TC versus 0.7% (1/134) control [difference 1.5%; 95% CI −2.1 to 5.8] intention‐to‐treat demonstrating non‐inferiority. There were no differences between DTG + 3TC versus control in incidence of blips or number of patients with ≥1 blip, and overall or serious (none drug‐related) adverse events. Weight (kg) change (mean, SD) at week 48 was 1.55 (3.98) DTG + 3TC versus 0.08 (3.95) control (p = 0.005) with no differences among strata; there were no differences in regional fat (gr) (mean, SD): trunk, −115 (4413) versus 499 (2056) (p = 0.68); limbs, 620 (1308) versus 1166 (4220) (p = 0.98) or PSQI changes over time (p = 0.46).
Abstract PDB0105‐Table 1.
| Snapshot outcomes week 48 | DTG/3TC (n = 131) | Triple ART (n = 134) |
|---|---|---|
| VL < 50 copies/mL, n (%) | 122 (93.1) | 125 (93.3) |
| VL ≥ 50 copies/mL, n (%) | 3 (2.3)* | 1 (0.7) |
| No virologic data, n (%) | 6 (4.6) | 8 (6.0) |
| Discontinued therapy | 3 (2.3) | 4 (3.0) |
| Lost of follow‐up | 2 (1.5) | 2 (1.5) |
| Patient's decision | 1 (0.8) | 2 (1.5) |
*No resistance mutations detected in all 3 patients. Two maintained DTG + 3TC and had HIV‐1 RNA < 50 copies mL at week 48.
Conclusions
Switching to DTG + 3TC in virologically suppressed patients was non‐inferior to and as safe as continuing triple ART at 48 weeks. Although weight increased with DTG + 3TC relative to triple ART, regional fat changes did not differ between arms.
PDB0106
Optimizing the management of patients with virologic non‐suppression
G. Alemnji1, Y. Obeng‐Aduasare2, H. Chun3, G. McTigue4, A. Mishra2, S. Bunga5, D. Ellenberger6, J. Katoro5, A. Russell3, L. Deng7, C. Godfrey8, S. Cavanaugh8, H. Watts9
1Office of the Global AIDS Coordinator and Health Diplomacy (OGAC), Laboratory, Washington, United States, 2Office of the U.S. Global AIDS Coordinator and Health Diplomacy (OGAC), ICPI, Washington, United States, 3Centers for Diseases Control and Prevention, Care and Treatment, Atlanta, United States, 4Brown University, Rhodes ISland, United States, 5Centers for Diseases Control and Prevention, Care and Treatment, Juba, South Sudan, 6Centers for Diseases Control and Prevention, Laboratory, Atlanta, United States, 7Centers for Diseases Control and Prevention, National Public Health Laboratory, Juba, South Sudan, 8Office of the U.S. Global AIDS Coordinator and Health Diplomacy (OGAC), Care and Treatment, Washington, United States, 9Office of the U.S. Global AIDS Coordinator and Health Diplomacy (OGAC), HIV Prevention, Washington, United States
Background: Some programmes continue to face challenges meeting HIV viral supression targets, with incomplete rates of repeat viral load (VL) testing in patients with virologic non‐suppression (PVNS), and persistent VL non‐suppression. Recent data show that interventions such as enhanced adherence counselling (EAC) and the use of Point‐of‐Care (POC) testing may improve outcomes in PVNS.
Methods: Significant efforts are underway in PEPFAR‐supported countries to improve VL coverage and suppression. Generally, PLHIV on ART for at least 6 months with VL results ≥1000 copies/mL receive EAC, with follow‐up VL testing. Data from 15 countries were assessed using Wilcoxon‐Signed Rank Test for the proportion of individuals with VL suppression over the period 2017 to 2019, and the average number of PVNS per facility. Site‐level high VL cascade were reviewed.
Results:
Abstract PDB0106‐Figure 1.
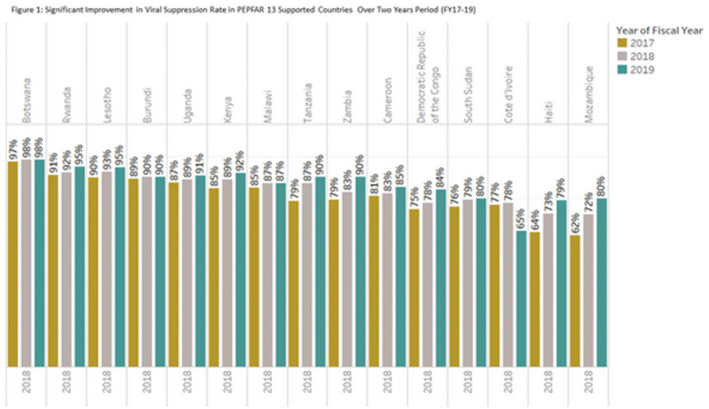
VL suppression rates improved significantly in 13 of the 15 countries over the three‐year period observed (Figure 1, p < 0.05). Data from 2, 021 treatment facilities in some selected countries averaged 200 PVNS per facility. Site level high VL cascades highlighted low rates of PVNS EAC receipt, repeat VL testing, and ART switch, with prolonged times between interventions (Figure 2 South Sudan).
Abstract PDB0106‐Figure 2.
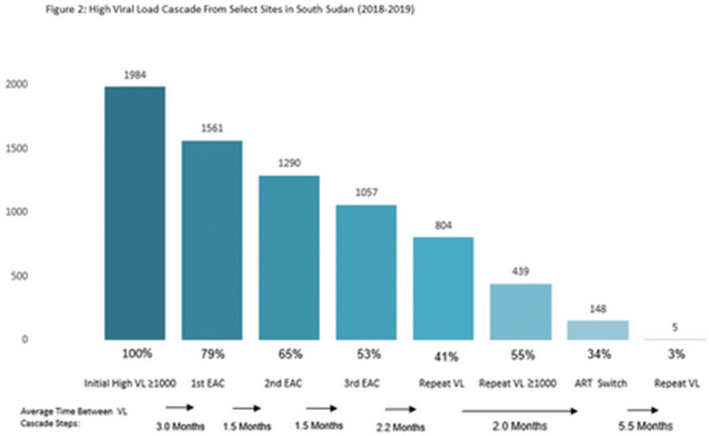
Conclusions: The management of PVNS continues to be a challenge and is critical to achieving epidemic control. There is a continued need for differentiated client‐centred models that include interventions for PVNS care, such as the delivery of quality EAC, ART switch, optimized use of both laboratory‐based and POC instruments for VL re‐testing, and timely results availability and utilization for improved patient and programme outcomes.
PDB0202
Darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) in treatment‐naïve (AMBER) and virologically suppressed (EMERALD) patients with neurologic and/or psychiatric comorbidities: Week 96 subgroup analysis
J. Bushen1, D. Luo2, R.B. Simonson1, R. Burress1, K. Brown1, D. Anderson1, K. Dunn1
1Janssen Scientific Affairs, LLC, Titusville, United States, 2Janssen Research & Development, LLC, Titusville, United States
Background: Patients with HIV‐1 and neurologic and/or psychiatric comorbidities (NPCs) face unique challenges including suboptimal adherence and possible exacerbation of NPCs, requiring individualized treatment approaches for optimal health outcomes.
Methods: This analysis evaluated 96‐week efficacy/safety in patients with versus without NPCs at baseline from two trials: AMBER (ClinicalTrials.gov:NCT02431247; treatment‐naïve patients randomized to initiate D/C/F/TAF 800/150/200/10 mg or control regimen) and EMERALD (ClinicalTrials.gov:NCT02269917; virologically suppressed patients randomized to switch to D/C/F/TAF or continue their boosted protease inhibitor‐based regimen). NPCs were based on verbatim medical history terms, coded and defined as those within the MedDRAv22 system organ class Nervous System Disorders or Psychiatric Disorders. In this analysis, efficacy was assessed by virologic response (HIV‐1 RNA < 50copies/mL) at Week 96 by intent‐to‐treat FDA snapshot analysis in patients randomized to receive D/C/F/TAF.
Results: Overall, 88/362 (AMBER) and 294/763 (EMERALD) patients receiving D/C/F/TAF had baseline NPCs, with psychiatric comorbidities more common than neurologic (Table 1). High virologic response rates (80 to 91%) were observed at Week 96, regardless of NPCs (Table 1). Small response rate differences in AMBER patients with versus without NPCs were driven by discontinuation for reasons other than virologic failure with last HIV‐1 RNA ≥ 50 copies/mL; notably, no treatment‐emergent resistance was detected among AMBER patients with NPCs. Across both studies, most AEs were grade 1 and discontinuation rates due to D/C/F/TAF‐related AEs were low and similar for patients with and without NPCs (Table 1). In both studies, patients with NPCs had higher overall rates of neurologic/psychiatric AEs; however, incidences of D/C/F/TAF‐related neurologic or psychiatric AEs were similar regardless of NPCs. There were no neurologic or psychiatric D/C/F/TAF‐related serious AEs.
Abstract PDB0202‐Table 1.
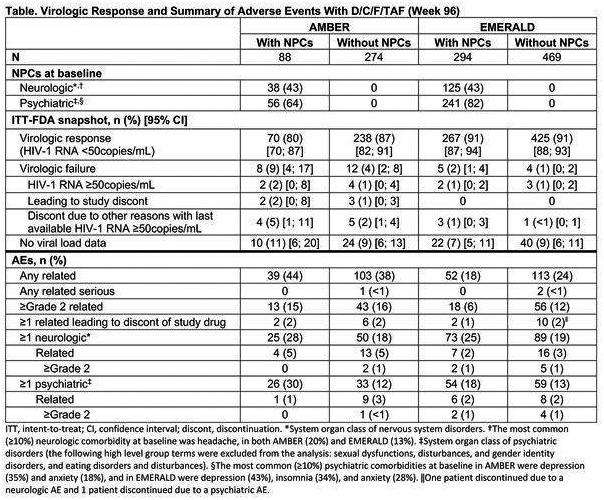
Conclusions: Patients with and without NPCs receiving D/C/F/TAF over 96 weeks had high virologic response rates and low D/C/F/TAF‐related discontinuation rates. Patients with NPCs tolerated D/C/F/TAF well without additional central nervous system‐related burden, indicating that D/C/F/TAF may be a suitable option for these patients.
PDB0203
Task‐shared, nurse‐delivered, cognitive behavioural therapy for adherence and depression (CBT‐AD) in HIV clinics in Kayelitsha, South Africa: A randomized controlled trial
S. Safren1,2, C. O'Cleirigh3,4,2, L. Andersen5, J. Magidson6, J. Lee1, S. Bainter1, N. Musinguzi7, S.A. Kagee8, J. Joska5
1University of Miami, Psychology, Miami, United States, 2Fenway Health, The Fenway Institute, Boston, United States, 3Massachusetts General Hosptial, Psychiatry/Behavioral Medicine, Boston, United States, 4Harvard Medical School, Psychiatry/Psychology, Boston, United States, 5University of Cape Town, Cape Town, South Africa, 6University of Maryland, College Park, United States, 7Mbarara University of Science and Technology, Mbarara, Uganda, 8Stellenbosch University, Stellenbosch, South Africa
Background: Depression is a highly prevalent condition among people living with HIV (PLWH) both globally and in Sub‐Saharan Africa (SSA), home to the highest number of PLWH. Depression is a consistent and robust predictor of non‐adherence to antiretroviral therapy (ART). Yet, there is a significant mental health treatment gap, with insufficient numbers of mental health providers. Task sharing, originally developed in SSA to expand ART availability, may also be a useful solution to increase access to depression treatment (e.g. by nurses who are more available).
Methods: Methods: This study is a two‐arm randomized controlled trial for virally unsuppressed PLWH and clinical depression (N = 161) in primary HIV care clinics in Khayelitsha, South Africa. It is comparing a task‐shared cognitive behavioural therapy for adherence and depression (CBT‐AD) administered by nurses (supervised by a clinical psychologist) with standard of care (SOC) clinic‐based adherence counselling and support. Primary outcomes (baseline to acute post‐treatment; 4‐months) were the Hamilton Depression Rating Scale (HAMD) administered by a blinded independent evaluator, and weekly adherence via real‐time monitoring (Wisepill). For the depression analyses, we used linear mixed models using maximum likelihood for missing data. For weekly adherence, we used a generalized estimating equation model (uses all available data) with robust standard errors, censoring Wisepill non‐usage.
Results: CBT‐AD showed superiority on both outcomes. While both groups improved in depression, there was a significant interaction such that the CBT‐AD condition improved by an estimated 4.88 points (CI: −7.86, −1.87, p = .0016) more than SOC. Wisepill usage was variable (non‐usage); however, there was also a significant time by condition interaction (est = 1.38, CI: .38, 3.60, p = .000) such that the SOC started off lower than CBT‐AD, and had a significant decrease in adherence over this time period (est = 1.26, CI: −1.79, −0.73, p = .000), and the CBT‐AD condition maintained their higher adherence. The uncensored adherence (wisepill) analysis yielded a similar pattern of results.
Conclusions: Nurse‐delivered CBT‐AD using a task sharing model was effective in improving depression and ART adherence for virally unsuppressed PLWH. Longer term follow‐up, with behavioural and biological outcomes are needed, as are future implementation science trials and analysis of cost‐effectiveness, to translate findings into clinical practice.
PDB0204
High burden of depression among clients initiating same‐day antiretroviral therapy at the Anonymous Clinic, Bangkok Thailand
P. Jomja1, T. Chinbunchorn1, S. Thitipatarakorn1, P. Seekaew2, S. Amatavete1, M. Avery3, S. Mills3, R. Vannakit4, P. Phanuphak1, N. Phanuphak1, R. Ramautarsing1
1Thai Red Cross AIDS Research Centre, Bangkok, Thailand, 2Columbia University, New York, United States, 3FHI 360 and LINKAGES, Bangkok, Thailand, 4USAID Regional Development Mission Asia, Office of Public Health, Bangkok, Thailand
Background: Depressive disorders are common, but often underdiagnosed among people living with HIV. We implemented a depression‐screening tool into our Same‐Day antiretroviral therapy (SDART) service at the Thai Red Cross AIDS Research Centre (TRCARC) to determine the prevalence of depression and its association with clients’ decision to accept or reject SDART.
Methods: Clients at the TRCARC underwent processes for eligibility determination for ART initiation per national guidelines on the same day as HIV diagnosis. As part of the ART initiation process the Thai version of the Patient Health Questionnaire (PHQ‐9), a depression screening tool, was implemented, which was completed electronically by the client. A score of 0 to 4 represents no depression, 5 to 8 mild depression, 9 to 14 moderate depression, 15 to 19 moderately severe depression, and ≥20 represents severe depression.
Results: Between June and November 2019, 879 clients were enrolled in the SDART programme, 657 (74.7%) were men who have sex with men, 95 (10.8%) cis‐gender women, 82 (9.3%) heterosexual men, and 45 (5.1%) transgender women. Median (interquartile range) age was 29 (24 to 36). A total of 859 (97.7%) accepted SDART. Of 833 clients who completed the Thai PHQ‐9, 471 clients (56.5%) had no depression, 217 (26.1%) had mild depression, 119 (14.3%) had moderate depression, 22 (2.6%) had moderately severe depression and 4 (0.5%) had severe depression. Among clients with scores indicative of moderate, moderately severe or severe depression, only 2 (1.4%) rejected SDART. There was no relationship between the presence of moderate to severe depression and the decision to accept SDART (p = 0.688). The most common reasons for rejecting SDART were: 1) difficulty travelling to healthcare units (33.3%), 2) fear of ART side effects (27.3) and 3) had a job which prohibits taking ART at regular times (21.4%).
Conclusions: Acceptability of SDART was high, and although the severity of depression did not impact this, we found a high burden of depression among our clients, with almost half of all clients having at least mild depression. HIV diagnosis could be utilized as an opportunity for diagnosis of depression, and routine self‐screening for depression during ART initiation using the PHQ‐9 can be implemented effectively.
PDB0205
Longitudinal study of neurocognitive disorders and associated structural brain changes in adolescent HIV
J. Hoare1, J.‐P. Fouche2, N. Phillips1, L. Myer2, S. Heany1, H. Zar2, D. Stein1
1University of Cape Town, Psychiatry and Mental Health, Cape Town, South Africa, 2University of Cape Town, Cape Town, South Africa
Background: Neurocognitive disorders (NCD) despite ART are well known in perinatally infected HIV+ adolescents (PHIV) but there are few data on longitudinal changes in NCD and brain structure in PHIV over time.
Methods: Within this sub‐study of the Cape Town Adolescent Antiretroviral Cohort, PHIV on ART > 6 m completed baseline and 3‐year follow‐up assessments including a comprehensive neurocognitive battery assessing function in 10 domains. We applied the youth HIV‐associated NCD diagnostic criteria to classify each as having either a major NCD, a minor NCD, or no impairment. Diffusion tensor imaging and structural brain magnetic resonance imaging was done to determine fractional anisotropy (FA), mean diffusivity (MD), grey and white matter volumes, cortical thickness and cortical surface area. In analysis we examined changes over the 3‐year period in NCD and neurostructural measures in PHIV compared to age‐ and sex‐matched HIV‐ controls.
Results: Overall 122 PHIV ages 9 to 12 years (mean CD4 cell count 953 cells/µL and 85.3% VL < 50 copies/mL) and 37 age‐matched HIV‐ controls completed baseline and 3‐year follow‐up assessments. 48% PHIV had a NCD at baseline and 60% at follow‐up: NCD diagnosis was stable over time in 60 (49%) of participants, 22 (18%) improved NCD status and 40 (33%) deteriorated. At baseline, PHIV with major NCD showed the highest whole brain MD (p = .007); at follow‐up whole brain grey (p = .004) and white matter volumes (p = .032) were lowest in PHIV, with whole brain MD remaining highest in PHIV with a major NCD (p = .02). Higher MD is suggestive of inflammation and myelin loss. In addition significant regional brain changes were observed at follow‐up compared to baseline in PHIV versus controls. Structural changes over time were observed mainly in cortical surface area of the bilateral orbitofrontal, anterior cingulate, medial orbitofrontal, middle frontal, superior temporal, transverse temporal gyri and insula (all p < .05). White matter microstructural changes over time were observed in the internal capsule, cerebral peduncle and the cingulum (all p < .05).
Conclusions: NCD and brain structural alterations in PHIV increased over the 3 years of follow‐up compared to HIV‐ controls. Studying the participants who improved versus deteriorated over time may provide insight into future interventions for NCD in PHIV.
PDB0206
Assessing neurocognitive functioning among adolescents and young adults with perinatally acquired HIV in Thailand: Support for the NeuroScreen app
R.N. Robbins1, T. Kulvadee2, T. Puthanakit3, N. Wipaporn3, A.F. Santoro1, C.A. Mellins1, K.M. Malee4, R. Paul5, L. Aurpibul6
1HIV Center for Clinical and Behavioral Studies at Columbia University and New York State Psychiatric Institute, New York, United States, 2Faculty of Humanities, Chiang Mai University, Department of Psychology, Chiang Mai, Thailand, 3Faculty of Medicine, Chulalongkorn University, Department of Pediatrics, Bangkok, Thailand, 4Northwestern University Feinberg School of Medicine, Department of Psychiatry and Behavioral Science, Chicago, United States, 5Missouri Institute of Mental Health/University of Missouri‐St. Louis, Missouri, St. Louis, United States, 6Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand
Background: Adolescents and young adults (AYA) living with perinatally acquired HIV (PHIV) often perform worse on selective neurocognitive tests compared to their uninfected peers, including tests of working memory, processing speed and executive functioning. Assessing their neurocognition is challenged in low‐ and middle‐income countries (LMICs), such as Thailand, due to resource constraints (e.g. lack of easy‐to‐use, validated tools). This study examined if a novel, highly automated (e.g. tests automatically timed and scored), brief (25 minutes), easy‐to‐use by any staff, tablet‐based neurocognitive assessment (NeuroScreen) adapted for Thailand could detect test performance differences between AYA with PHIV and uninfected‐AYAs.
Methods: NeuroScreen underwent translation by bilingual (English and Thai) psychologists, and was reviewed by Thai AYA with and without PHIV, and Thai clinical staff. Thai AYA (50 PHIV, 49 uninfected‐AYA) recruited from similar communities were administered the Thai‐language version of the NeuroScreen app, consisting of 12 tests of processing speed, working memory, executive functioning, learning, delayed recall, and motor speed. Independent samples T‐tests were computed examining group differences.
Results: Median age was 18 years (IQR 16 to 20), 60% were female. Groups did not differ by sex or age. Table 1 presents means and standard deviations and T‐test results for each NeuroScreen test by HIV‐status group. AYA with PHIV performed significantly worse on: five tests of processing speed, one of two tests of working memory, and the one test of executive functioning. AYA with PHIV mean performance was lower on all remaining tests, though not statistically significantly different than uninfected‐AYAs.
Abstract PDB0206‐Table 1
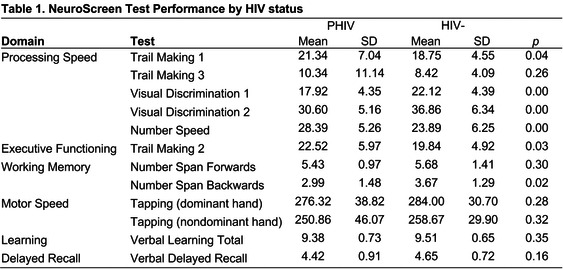
Conclusions: Results indicate that the NeuroScreen app detects performance differences in neurocognitive domains known to be vulnerable to PHIV, including processing speed, executive function, and working memory. NeuroScreen demonstrates potential as an assessment tool for Thai AYA with PHIV and LMICs.
PDB0302
Incidence and risk factors for STIs among MSM on PrEP ‐ A post‐hoc analysis of the ANRS IPERGAY trial
J. Zeggagh1,2, R. Bauer3,4, C. Delaugerre5, D. Carette3, L. Fressard6, I. Charreau3, C. Chidiac7, G. Pialloux8, C. Tremblay9, E. Cua10, O. Robineau11, F. Raffi12, L. Meyer13, J.M. Molina114, ANRS IPERGAY Study Group
1Hospital Saint‐Louis, APHP, Infectious Diseases Department, Paris, France, 2University of Paris Diderot, Paris, France, 3INSERM, SC 10‐US 19, Villejuif, France, 4Paris 11 University, Kremlin Bicêtre, France, 5Hospital Saint‐Louis, Laboratory of virology, INSERM UMR 941, paris, France, 6Aix Marseille University, IRD, UMR‐S912, Marseille, France, 7University Claude Bernard Lyon 1 and Hospices Civils de Lyon, Infectious Diseases Department, Lyon, France, 8Hospital Tenon, APHP, Infectious Diseases Department, Paris, France, 9Centre de Recherche du Centre Hospitalier de l'Université de Montréal, Montréal, Canada, 10Hospital de l'Archet, Infectious Diseases Department, Nice, France, 11Hospital Gustave Dron, Infectious Diseases Department, Tourcoing, France, 12CHU Hôtel Dieu, Infectious Diseases Department, Nantes, France, 13Paris Saclay University, Paris, France, 14University of Paris Diderot, INSERM UMR 941, Paris, France
Background: High rates of STIs have been reported among MSM on PrEP. Our objective was to assess, within the setting of the ANRS IPERGAY trial, the incidence of bacterial STIs over time and baseline risk factors associated with STIs.
Methods: Data from all participants enrolled in the ANRS IPERGAY trial were used. Participants were enrolled in February 2012 and switched to open‐label PrEP ( with TDF/FTC) in November 2014. Study visits were scheduled every 8 weeks.
Participants were screened for STI (syphilis, chlamydia, and gonorrhoea) at baseline and at least every 6 months at the physician's discretion. All bacterial STIs reported in the database were analysed with their location (pharyngeal, rectal, urine). STIs incidence was calculated yearly. Cox proportional hazards model regression was used to explore associations between participant's characteristics at baseline and first STI occurrence.
Results: Between February 2012 and June 2016, 429 participants were enrolled with a median follow‐up of 23 months (range: 0 to 51). At baseline, median age: 35 years, 99% MSM, 91% white, 72% had post‐secondary education, 46% used recreational drug, 27% use GHB, 35% use erectile drugs, 27% had STI, median number of sexual partners in prior two months: 8, and 35% had condomless receptive anal sex at last intercourse.
Overall STIs incidence was 74, 33, 13, 32 and 30 per 100 PY for all STIs, rectal STIs, syphilis, gonorrhoea and chlamydia, respectively. STI incidence significantly increased from February 2012 (55 per 100 PY) to June 2016 (90 per 100 PY) (p < 0.001).
One hundred and sixty‐seven participants (39%) accounted for 557 (86%) of all STIs reported while 170 participants (40%) did not experience any STI during follow‐up.
Baseline risk factors associated with STIs occurrence are shown in the Table 1.
Abstract PDB0302‐Table 1.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| N/Ntot | HRCox [IC] | p Cox | HRCox[IC] | p Cox | |
| STI at baseline | 115/429 | 1.46 [1.12;1.9] | 0.005 | 1.45 [1.11;1.89] | 0.007 |
| Number of Partners, ≥8 | 240/429 | 1.45 [1.13;1.87] | 0.004 | 1.39 [1.07;1.82] | 0.015 |
| Sex Party | 181/419 | 1.42 [1.11;1.82] | 0.005 | 1.09 [0.83;1.43] | 0.529 |
| Use of GHB | 117/413 | 1.76 [1.35;2.29] | <0.001 | 1.53 [1.15;2.04] | 0.004 |
| Use of erectile drugs | 151/420 | 1.62 [1.26;2.07] | <0.001 | 1.32 [1.01;1.74] | 0.045 |
Conclusions: STI incidence was high and increased during the IPERGAY trial, but most STIs were concentrated in a high‐risk group that should be targeted for future interventions.
PDB0303
Persistently high rates of sexually transmitted infections in the DISCOVER HIV PrEP trial
L. Gorgos1, J. Coll2, P. Mallon3, S. Morris4, Y. Shao5, P. Wong5, R. Ebrahimi5, M. Das5, S. McCallister5, D.M. Brainard5, C.C. Carter5, I. Brar6, M. Prins7
1Southwest Care Center, Santa Fe, United States, 2BCN Checkpoint, Barcelona, Spain, 3Mater Misericordiae, University Hospital, Dublin, Ireland, 4University of California, San Diego AVRC, San Diego, United States, 5Gilead Sciences Inc., Foster City, United States, 6Henry Ford Hospital, Detroit, United States, 7Public Health Service of Amsterdam (GGD), Amsterdam, Netherlands
Background: In DISCOVER, F/TAF was noninferior to F/TDF for HIV PrEP in MSM and transgender women. Here, we report STI outcomes during the trial through 96 weeks of follow‐up.
Methods: DISCOVER randomized 5387 participants 1:1 to receive blinded daily F/TAF or F/TDF for PrEP. Participation required documented HIV risk, determined by history of rectal STI, syphilis, or sexual behaviours. STI testing (gonorrhoea, chlamydia and syphilis) and sexual behaviour computer‐assisted self‐interview (covering the preceding 3 months) occurred at screening and every 12 weeks. Statistical analyses for categorical variables used the CMH test, and comparisons of HIV incidence used a Poisson model.
Results: At baseline, 430 (16.1%) of participants in the F/TAF arm and 421 (15.8%) in the T/TDF arm tested positive for gonorrhoea or chlamydia at any anatomic site; 299 (11.3%) and 279 (10.5%) tested positive at the rectum. The proportion of participants positive for gonorrhoea and chlamydia by visit is found in the Figure. The overall incidence rate of gonorrhoea or chlamydia at any anatomic site was 85.7 and 83.1 per 100 person‐years (PY) for F/TAF and F/TDF respectively, and for rectal gonorrhoea and chlamydia was 47.5 and 46.9 per 100 PY. The 96‐week prevalence of syphilis was 14.8% and 15.2% in the F/TAF and F/TDF arms, and the incidence rate was 9.9 and 9.3 per 100PY, respectively. The rate of HIV acquisition was higher in participants with a history of rectal gonorrhoea, rectal chlamydia or syphilis at screening (0.61 vs. 0.12 per 100 PY, p < 0.001). The mean (SD) number of condomless receptive anal sex partners was 3.6 (5.9) versus 3.4 (6.2) at screening and 3.8 (7.3) versus 3.9 (7.8) at week 96 for F/TAF and F/TDF respectively.
Abstract PDB0303‐Figure 1.
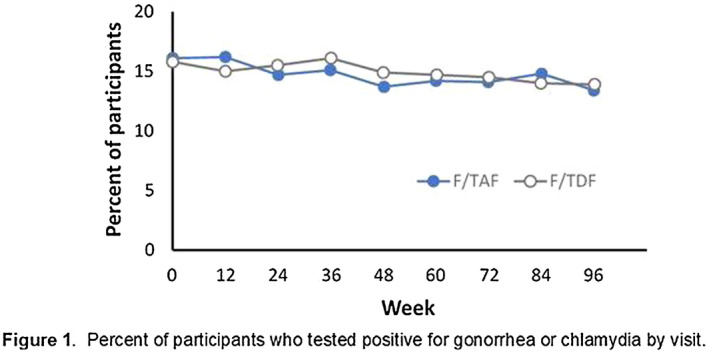
Conclusions: STI acquisition rates, especially rectal gonorrhoea and chlamydia, remained stably high through 96 weeks of follow‐up. In combination with sexual behaviour data, this argues against risk compensation in DISCOVER.
PDB0304
Pooled pharyngeal, rectal and urine samples for the Point‐of‐Care detection of Chlamydia trachomatis and Neisseria gonorrhoeae by lay‐providers in Key Population‐Led Health Services in Thailand
N. Thammajaruk1, S. Promthong1, P. Posita1, S. Chumseng1, O. Nampaisan1, P. Srivachiraroj1, J. Peelay1, O. Fungfoosri2, P. Thapwong3, T. Pankam4, S. Pattanachaiwit4, C. Arsaman4, M. Avery5, S. Mills5, R. Vannakit6, P. Phanuphak1, N. Phanuphak1, R. Ramautarsing1
1PREVENTION, The Thai Red Cross AIDS Research Centre, Bangkok, Thailand, 2Rainbow Sky Association of Thailand, Bangkok, Thailand, 3The Service Workers In Group Foundation, Bangkok, Thailand, 4Anonymous Clinic, The Thai Red Cross AIDS Research Centre, Bangkok, Thailand, 5FHI360 and LINKAGES, Bangkok, Thailand, 6USAID Regional Development Mission Asia, Office of Public Health, Bangkok, Thailand
Background: In Thailand, Chlamydia Trachomatis (CT) and Neisseria gonorrhoeae (NG) screening for at‐risk populations is not routinely provided. Screening for CT/NG among men who have sex with men (MSM) and transgender women (TGW) must include pharyngeal, rectal and urine samples, leading to high costs (25 dollars per compartment). We integrated Cepheid GeneXpert for CT/NG testing in the Key Population‐Led Health Services model. Trained key‐population lay‐providers performed CT/NG sampling and testing in community‐based organizations (CBOs). We present the performance of pooling samples from three compartments for CT/NG detection.
Methods: Between August and October 2019, 199 MSM or TGW were recruited from 2 CBOs in Bangkok, Thailand. First‐catch urine samples were self‐collected by participants, KP‐lay providers collected pharyngeal and rectal swabs. GeneXpert was used for separate testing of three compartments of 199 participants, and for testing of single participant pooled urine, pharyngeal and rectal samples from a subset of 50 participants. Performance of separate and pooled samples by GeneXpert were compared with the laboratory‐based standard of care (SoC – Abbott RealTime).
Results: Compared with SoC, sensitivity and specificity of GeneXpert were 100% and 100% for pharyngeal CT, 100% and 99.4% for rectal CT, 100% and 99% for urethral CT, 93.3% and 98.9% for pharyngeal NG, 100% and 99.5% for rectal NG, and 100% and 100% for urethral NG, respectively. Sensitivity and specificity of pooled samples were 100% and 100% for CT and 88.9% and 100% for NG, respectively, compared with SoC. One pharyngeal NG infection was missed using pooled sampling. Cohen's Kappa agreement for pooled samples was 100% for CT and 98% for NG when compared with SoC.
Conclusions: Pooled sampling from three compartments among MSM and TGW showed excellent performance for detection of CT. Sensitivity for detection of NG was lower for pooled specimens compared to single‐site testing due to a missed infection in the pharynx, where bacterial loads can be lower compared to other compartments. Nonetheless, agreement between pooled sampling, single‐site and SoC was good, and pooled sampling significantly reduces costs. Pooled sampling in CBOs by KP‐lay providers should be implemented to facilitate access to CT/NG testing services for at‐risk populations in Thailand.
PDB0305
Cytomegalovirus retinitis in advanced HIV patients: Screening and care in Mozambique
A.G. Gutierrez1, C.M. Siufi1, N. Tamayo Antabak1, G. Muvale2, H.V. Andela1, I.C.B. Magaia2, P. Saranchuk3, L. Molfino1, A. Guadarram1, I. Ciglenecki4, D. Heiden3, A. Couto2, A. Loarec1
1Médecins Sans Frontières, Maputo, Mozambique, 2Ministry of Health, Maputo, Mozambique, 3SEVA Foundation, Berkeley, United States, 4Médecins Sans Frontières, Geneva, Switzerland
Background: AIDS‐related cytomegalovirus retinitis (CMVR) is a late‐stage opportunistic infection. In Africa, CMVR burden is largely unknown, due to lack of screening and limited access to treatment. In Maputo, Médecins Sans Frontières with the Ministry of Health supports patients with advanced HIV disease (AHD) at the referral centre of Alto Mae (CRAM) and in Jose Macamo hospital (JM). Here we describe CMVR activities as part of the AHD package of care.
Methods: CMVR screening was introduced for adult HIV‐positive patients with CD4 count < 100 cells/µl and patients with visual complaints in February 2019.
Retinal examination is performed by trained non‐ophthalmologist physicians on fully dilated pupils using indirect ophthalmoscopy. Patients with active CMVR are treated with oral valganciclovir. We analysed routine data, collected between February and November 2019.
Results: Among eligible patients with CD4 < 100 cells/µl, 120/160 (75%) were screened in CRAM and 245/721 (34%) in JM.
In CRAM, 8/120 patients were diagnosed with CMVR (6.6%; 6 active and 2 inactive CMVR) and 13/245 in JM (5.3%; 11 active and 2 inactive CMVR), with total CMVR prevalence of 5.7% (21/365). Median age was 40 years (IQR 33 to 49), 76% were women (n:16). At diagnosis time, median CD4 was 21 cells/µl (IQR: 15 to 45), with 76% of CMVR patients reporting ART history (n:16).
Mortality was high among hospitalized CMVR patients: 7/13 (54%) patients died prior to treatment initiation, and 2 were lost‐to‐follow‐up. Eight patients with active CMVR were treated with oral valganciclovir. At the end of the follow‐up period, 6 patients were alive in care (75%) and 2 (25%) died. Overall, 9 (43%) CMVR patients died, some while being treated for co‐existent opportunistic infections.
Conclusions: This first report of AIDS‐related CMV retinitis in Mozambique revealed a 5.7% CMVR prevalence among screened patients with AHD. Mortality among patients with CMVR was high. Although CMVR diagnosis with indirect ophthalmoscopy was feasible, we faced limitations to screen all at risk patients. Also, ophthalmic screening doesn't allow for diagnosis of other CMV end‐organ disease or viraemia. There is urgent need for early and easy diagnosis of CMV infection, such as detection of CMV viraemia, and better access to treatment with valganciclovir.
PDB0306
Incidence of HCV reinfection among HIV‐positive MSM and its association with sexual risk behaviour: A longitudinal analysis
A.M. Newsum1, A. Matser1, J. Schinkel2, M. van der Valk2, K. Brinkman3, A. van Eeden4, F.N. Lauw5, B.J. Rijnders6, T.J. van de Laar7, M. van de Kerkhof1, C. Smit8, A. Boyd1, J.E. Arends9, M. Prins1, MSM Observational Study of Acute Infection with hepatitis C (MOSAIC) study group
1GGD Amsterdam, Amsterdam, Netherlands, 2Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands, 3OLVG, Department of Internal Medicine and Infectious Diseases, Amsterdam, Netherlands, 4DC Kliniek Lairesse, Amsterdam, Netherlands, 5Medical Center Jan van Goyen, Department of Internal Medicine, Amsterdam, Netherlands, 6Erasmus MC, University Medical Center, Department of Internal Medicine and Infectious Diseases, Rotterdam, Netherlands, 7Laboratory of Medical Microbiology, OLVG, Amsterdam, Netherlands, 8Stichting HIV Monitoring, Amsterdam, Netherlands, 9University Medical Center Utrecht, Department of Internal Medicine, Utrecht, Netherlands
Background: HIV‐positive men who have sex with men (MSM) are at high risk of hepatitis C virus (HCV) reinfection following clearance of HCV. Risk factors for reinfection, which include sexual risk behaviour, have yet to be comprehensively assessed.
Methods: Using data from a prospective observational cohort study among HIV‐positive MSM with an acute HCV infection (MOSAIC) in the Netherlands, the incidence of HCV reinfection following spontaneous clearance or successful treatment was assessed. A univariable Bayesian exponential survival model was used to identify risk factors associated with HCV reinfection. Prior distributions of hazards ratios (HR) were based on the anticipated strength of association for a given risk factor and together with the data, were used to estimate the posterior distribution of HR and 95% credible intervals (CrI) for each risk factor with Markov Chain Monte Carlo methods.
Results: Overall, 122 HIV‐positive MSM who had a spontaneously cleared or successfully treated HCV infection between 2003 and 2017 were included. During a median follow‐up of 1.4 years (interquartile range 0.5 to 3.8), 34 HCV reinfections were observed in 28 patients. The incidence of HCV reinfection was 11.5/100 person‐years and among those with reinfection, median time to reinfection was 1.3 years (interquartile range 0.6 to 2.7). HCV reinfection was associated with receptive condomless anal intercourse (posterior‐HR = 4.27, 95% CrI 1.86 to 9.78), sharing of sex toys (posterior‐HR = 4.91, 95% CrI 2.27 to 10.31), group sex (posterior‐HR = 2.80, 95% CrI 1.33 to 5.98), anal rinsing before sex (posterior‐HR = 2.47, 95% CrI 1.14 to 5.43), ≥10 casual sex partners in the last 6 months (posterior‐HR = 2.81, 95% CrI 1.26 to 6.50), nadir CD4 cell count < 200 cells/mm3 (posterior‐HR = 2.22, 95% CrI 1.05 to 4.81), and recent CD4 cell count < 500 cells/mm3 (posterior‐HR = 3.60, 95% CrI 1.73 to 7.64).
Conclusions: Incidence of HCV reinfection was high and strongly associated with sexual risk behaviour. These results highlight the need for interventions to reduce risk behaviour and prevent HCV reinfections among HIV‐positive MSM.
PDB0402
HIV‐1 DNA testing in viraemic patients demonstrates a greater ability to detect drug resistance compared to plasma virus testing
D. Curanovic1, S. Martens1, M. Rodriguez1, H. Hammill2, C. Petropoulos1, C. Walworth1
1Monogram Biosciences, San Francisco, United States, 2The Woman's Hospital of Texas, Houston, United States
Background: Treatment guidelines recommend drug resistance testing on plasma virus to guide the selection of antiretroviral therapy in patients with HIV‐1 viraemia > 500 copies/mL. However, drug resistance mutations (DRMs) in plasma virus represent only the selective pressure imposed by the failing regimen and may miss DRMs reflective of prior regimens. HIV‐1 DNA testing derives drug resistance information from whole blood and offers a resistance testing option when plasma virus is undetectable. HIV‐1 DNA testing may also have the ability to capture DRMs in actively replicating virus in viraemic patients.
Methods: Resistance test results derived from paired whole blood (DNA) and plasma (RNA) samples obtained from the same patient on the same day were compared for 89 patients with HIV. DRM and antiretroviral susceptibility concordance was assessed between 103 paired tests. All samples had viral loads (VLs) > 500 copies/mL and were stratified to assess the impact of viral load on concordance. Resistance to individual antiretroviral drugs and drug classes was also assessed.
Results: The mean patient age was 38; 88% were female, of whom 19% were pregnant. The mean VL was 132,487 copies/mL. HIV‐1 DNA testing captured 505/548 (92%) of all mutations reported by plasma virus testing and identified 128 additional DRMs. HIV‐1 DNA testing demonstrated an average 94% concordance in 103 paired comparisons with plasma HIV‐1 DRMs. HIV‐1 DNA testing also captured 210/240 (88%) of the resistance calls reported by plasma virus testing, and identified 80 additional resistance calls. Plasma virus DRMs identified at VLs > 10,000 copies/mL were more likely to be detected in the DNA compartment compared with plasma virus DRMs identified at VLs < 10,000 copies/mL. Viral load level did not affect the percentage of DRMs on HIV‐1 DNA reports derived from previously archived virus. HIV‐1 DNA testing identified more resistance than plasma virus testing across all drug classes, including individual mutations such as M184V and K103N.
Conclusions: These findings demonstrate that HIV‐1 DNA testing largely recaptures plasma virus DRMs, and identifies additional mutations in previously archived virus. HIV‐1 DNA testing has clinical utility in viraemic patients, especially those who have no or limited prior resistance reports.
PDB0403
HIV‐infected treatment‐experienced children and adolescents from Sub‐Saharan Africa: Clinical outcomes on third‐line antiretroviral treatment in the New Horizons drug donation programme
A. Tiam1,2, R. Machecano1, K. Walner3, E. Magongo4, L. Chirwa5, R. Masaba6, P. Khumalo7, C. Lwaka8, R. Tumwebaze9, L. Mulenga5, L. Mofenson1, N. Rakhmanina3,10, New Horizons Study Team
1Elizabeth Glaser Pediatric AIDS Foundation, Research, Washington, United States, 2University of Bergen, Center for International Health, Bergen, Norway, 3Elizabeth Glaser Pediatric AIDS Foundation, TLPO, Washington, United States, 4Ministry of Health, AIDS Control Program, Kampala, Uganda, 5University Teaching Hospital, Infectious Disease, Lusaka, Zambia, 6Elizabeth Glaser Pediatric AIDS Foundation, Research, Nairobi, Kenya, 7Elizabeth Glaser Pediatric AIDS Foundation, Research, Mbabane, Eswatini, 8Elizabeth Glaser Pediatric AIDS Foundation, Program, Nairobi, Kenya, 9Elizabeth Glaser Pediatric AIDS Foundation, Research, Kampala, Uganda, 10Children's Hospital/George Washington University, Washington, United States
Background: Viral load (VL) scale‐up has highlighted significant rates of treatment failure among HIV‐infected children and adolescents on ART in sub‐Saharan Africa. The aim of this study was to describe virologic and immunologic characteristics of children and adolescents with treatment failure on second‐line antiretroviral treatment (ART) and to describe their clinical outcomes on third‐line ART.
Methods: This is an observational cohort study collecting prospective data from patients aged 0 to 24 years on third‐line ART in Eswatini, Kenya, Uganda, and Zambia. We collected data from clinical record of patients initiated on darunavir (DRV) and/or etravirine (ETR) as part of third‐line ART through the New Horizons drug donation programme sponsored by Johnson & Johnson. Baseline demographic, clinical and laboratory data (CD4 cell count, HIV RNA VL, genotypic resistance) were collected at the starting point of initiating third‐line ART and summarized using descriptive statistics and median (IQR).
Results: From December 2018 to November 2019, 152 participants were enrolled; 57.2% (87/152) were male; median (min‐max) age at initiation of third line was 12.8 (1.3 – 21.8) years. Prior second‐line ART was PI‐based, including lopinavir/ritonavir in 67.1%(n = 102) and atazanavir/ritonavir in 17.8% (n = 27). The NRTI backbone included lamivudine plus zidovudine in 20.4%, abacavir in 53.3%, or TDF in 23.0%. Most participants with available VL assessment (85.5%; n = 130/152) had elevated VL within six months prior to switching: median (min‐max) VL was 4.8 log (1.3 – 6.5). Of the 123 patients with baseline resistance results, 89 (68.5%) had thymidine analogue mutations (TAMs), 62.9% (n = 56/89) TAM1 and 73.0% (n = 65/89) TAM2 pathways. PI resistance mutations were observed in 71 (79.8%), with 70.4% (n = 50/71) having accumulated > 3 PI mutations. At six months on DRV/ETR‐based third‐line ART, of the 58 participants with VL results, 72.4% (n = 42/58) had viral suppression. At twelve months on third‐line ART, of 36 participants with VL results, 80.6% (n = 29/36) had viral suppression.
Conclusions: Treatment‐experienced paediatric and adolescent patients failing a second‐line PI‐based ART had high level HIV viraemia and high levels of NRTI thymidine analogue and PI mutations. Among those patients with VL results available at 6 and 12 months on third‐line ART containing DRV and/or ETR, the majority achieved virologic suppression.
PDB0404
Deep sequencing with unique molecular identifiers for evaluation of HIV‐1 drug resistance in the DISCOVER pre‐exposure prophylaxis trial
S. Cox1, U. Parikh2, A. Heaps2, B. Goetz2, J. Mellors2, M. Das1, C. Callebaut1
1Gilead Sciences, Foster City, United States, 2University of Pittsburgh, Pittsburgh, United States
Background: The DISCOVER study is an ongoing randomized, double‐blind study of pre‐exposure prophylaxis (PrEP) using daily FTC/TAF (F/TAF; Descovy; DVY) or FTC/TDF (F/TDF; Truvada; TVD) in men or transgender women who have sex with men. Of the 5335 randomized participants evaluated for HIV‐1 infection, 24 (0.4%) became infected through a median of 120 Weeks on study. Here we present standard and ultrasensitive resistance testing from the DISCOVER participants who acquired HIV infection.
Methods: Plasma samples from participants who became HIV‐1 infected and had a viral load of > 400 copies/mL were tested with the Monogram GenoSure™ MG assay, using Sanger sequencing to analyse the protease (PR) and reverse transcriptase (RT) genes for any known resistance mutations (at ≥ 15 to 20% of the viral population). Identification of minor variants was evaluated using ultrasensitive resistance testing (at ≥ 1% of the viral population) that employed unique molecular identifiers for amplification of viral variants followed by next generation sequencing (UMI‐NGS) to analyse RT codons 63 to 131 and 152 to 211 (University of Pittsburgh).
Results: By standard sequencing, 4/20 HIV‐positive participants tested had M184V, all in the F/TDF group and all with suspected baseline infection; 2 of these 4 also had M184I present. Six participants had additional mutations conferring resistance to non‐study drugs including NRTI, NNRTI, and PI, which were presumed to be transmitted.
By UMI‐NGS, 23/24 HIV participants with HIV had samples available and 21/23 were successfully analysed. The four participants with M184V each had M184I also detected; K65R was detected in 1 participant at very low levels. One participant on F/TAF had the M184V mutation present at 2%. Two out of 3 participants with samples that had viral loads < 400 copies/mL were successfully tested and neither had resistance to study drugs.
Conclusions: Using standard sequencing, M184V was detected in 4 participants, all in the F/TDF arm. Using ultrasensitive UMI‐NGS testing, similar results were observed, with the addition of one participant with M184V in the F/TAF arm. Overall, drug resistance in the DISCOVER study was most commonly seen in participants with suspected baseline infections and in only 1 individual who became infected while on study.
PDB0405
Pretreatment low‐frequency HIV drug resistance mutations in antiretroviral naive individuals in Botswana
D. Maruapula1, C. Rowley2, K. Molebatsi3, S. Moyo4, S. Gaseitsiwe4
1Botswana Harvard AIDS Institute Partnership, Research, Gaborone, Botswana, 2Harvard T.H Chan School of Public Health, Immunology and Infectious Diseases, Boston, United States, 3University of Botswana, Statistics, Gaborone, Botswana, 4Botswana Harvard AIDS Institute, Gaborone, Botswana
Background: The commonly used Sanger population‐based sequencing (SPBS) assay misses low‐frequency HIV drug resistance mutations present at less than 20% of the viral population. Detecting and monitoring low‐frequency HIV drug resistance mutations at treatment initiation is important for patient management. In this study, we aimed to determine the baseline prevalence of HIV low‐frequency drug resistance mutations among antiretroviral naïve individuals in Botswana.
Methods: Baseline plasma samples from 93 viraemic treatment naïve participants were extracted and Pol gene was amplified. The purified PCR product was used for targeted Next Generation Sequencing (NGS) using illumina MiSeq platform. Raw sequences were analysed using Genome Detective platform and Geneious Software where amino acid frequency cut‐offs > 1% were selected to report low‐level HIV‐1 drug resistance mutations. Results of NGS were compared with SPBS data for concordance. Mutations were identified and evaluated according to the Stanford HIV‐1 Drug Resistance database.
Results: Next‐Generation sequencing identified all 9 drug resistance mutations detected by SPBS. However, NGS detected 39 additional low‐frequency HIV drug resistance mutations at < 20% of the viral quasispecies. Of the 39, 33 low‐frequency HIV drug resistance mutations were detected at a frequency of ≥ 1%; 4 were detected at a frequency of ≥ 5%; and 2 were detected at a frequency of ≥ 10%. Among the low‐frequency HIV drug resistance mutations, 4 (10%) were to NNRTIs: 17 (44%) to NRTIs and 18 (46%) to PR inhibitors. Table 1 shows the distribution of the HIV drug resistance mutations detected in baseline samples.
Abstract PDB0405‐Table 1.
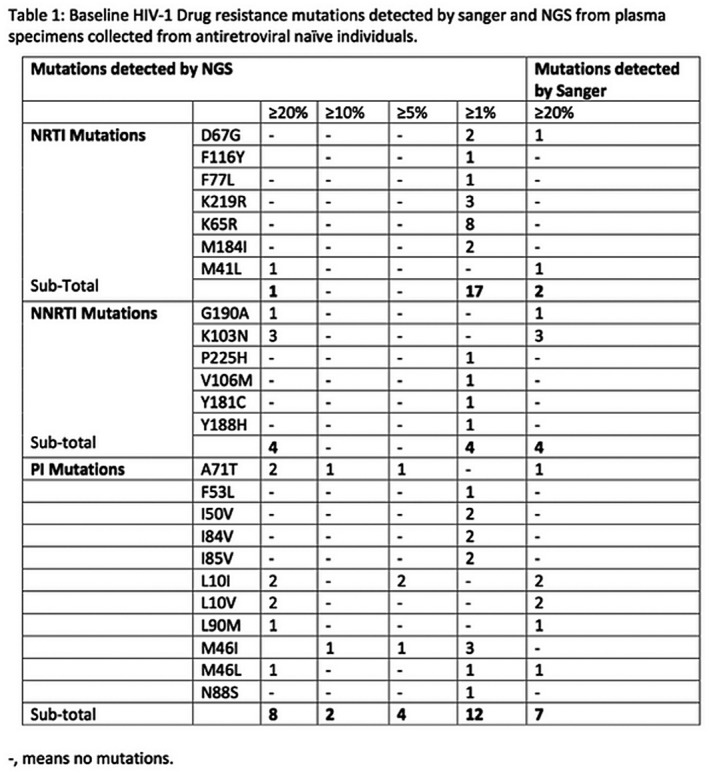
Conclusions: This is the first time in Botswana NGS has been used in a large population to detect HIV pre‐treatment low‐level drug resistance mutations. Our results revealed the presence of HIV low‐frequency drug resistance mutations in baseline samples. The impact of low frequency major DRMs warrants further investigation.
PDB0406
Doravirine resistance profile in clinical isolates and impact of baseline NNRTI resistance‐associated mutations observed in treatment‐naïve participants from phase 3 clinical trials
E. Asante‐Appiah1, J. Lai2, Q. Li1, D. Yang2, E.A. Martin1, P. Sklar1, D. Hazuda1, C.J. Petropoulos2, C. Walworth2, J.A. Grobler1
1Merck & Co., Inc, Kenilworth, United States, 2Monogram Biosciences, South San Francisco, United States
Background: Doravirine (DOR), a novel NNRTI with activity against viruses bearing common NNRTI resistance‐associated mutations (RAMs), is approved for the treatment of HIV‐1 infection. This study evaluated the activity of DOR in a large panel of clinical samples submitted for routine drug resistance testing and retrospectively analysed the impact of baseline RAMs in treatment‐naïve (TN) participants from DOR clinical trials to extend our understanding of its resistance profile.
Methods: Genotype and phenotype data from 4070 TN and treatment‐experienced samples evaluated for DOR susceptibility between August 2018 and August 2019 at Monogram Biosciences were analysed. RAM prevalence and susceptibility to all FDA‐approved NNRTIs was compared. To evaluate clinical significance, the prevalence of baseline RAMs and the proportion of participants achieving HIV‐1 RNA < 50 copies/mL at weeks 48 and 96 was retrospectively assessed in participants from the Phase 3 clinical trials, DRIVE‐FORWARD and DRIVE‐AHEAD.
Results: Using established biological and clinical cut‐offs for approved NNRTIs and a DOR biological cut‐off of 3‐fold, the percentage of samples susceptible to DOR, NVP, EFV, RPV and ETR was 92.5%, 77.5%, 81.5%, 89.5% and 91.5%, respectively. A 5‐fold DOR cut‐off increased susceptible samples to 94.5%. Individual DOR RAMs ranged from 0.02 to 2.29%. Among 228 samples (5%) resistant to NVP, EFV, RPV and ETR, 28.5% remained susceptible to DOR. The prevalence and DOR median fold‐change (FC) in samples bearing common NNRTI RAMs (n > 50 isolates) were as follows: K103N (14.3%, 1.25), V106I (5.4%, 1.28), Y181C (5.3%, 2.23), V108I (3.1%, 2.15), K101E (3.0%, 1.46), G190A (2.4%, 1.82), E138K (1.4%, 1.64), K103N/Y181C (1.3%, 3.06).
Common NRTI RAMs increased DOR susceptibility by ~2‐fold in the absence of NNRTI RAMs; DOR hyper‐susceptibility (FC ≤ 0.4) was often observed.
In DRIVE‐FORWARD and DRIVE‐AHEAD, the most prevalent NNRTI RAM at baseline was V106I, which was detected in 5 TN participants (0.7%); all 5 achieved HIV‐1 RNA < 50 copies/mL at weeks 48 and 96. Other RAMs were detected in 1 or 2 participants only.
Conclusions: Clinical samples with common NNRTI RAMs show high susceptibility to DOR. In phase 3 clinical trials, DOR demonstrated high efficacy in a small group of TN participants with common NNRTI RAMs.
PDB0407
Surveillance of transmitted HIV drug resistance among treatment‐naïve children under 18 months in Brazil (2009 to 2018)
A. Alberto Cunha Mendes Ferreira1, R. Elisa Gonçalves Gonçalves Pinho1, R. Castro de Albuquerque1, T. Cherem Morelli1, R. Vianna Brizolara1, A. Francisca Kolling1, M. Camelo Madeira de Moura1, A. Sposito Tresse1, N. Mendonça Collaço Véras1, L. Martins de Aquino1, A.R. Pati Pascom1, T. Dahrug Barros1, L. Neves da Silveira1, F. Fernades Fonseca1, G. Mosimann Júnior1, G. Fernando Mendes Pereira1, M. Araújo de Freitas1, V. Iida Avelino‐Silva2
1Ministry of Health of Brazil, Department of Chronic Disease and Sexually Transmitted Infectious, Brasilia, Brazil, 2Faculdade de Medicina USP, São Paulo, Brazil
Background: Children living with HIV (CLWHIV) are exposed to antiretroviral drugs antepartum, intrapartum or postpartum, which can lead to HIV drug resistance (HIV‐DR). Brazilian guidelines recommend the use of genotyping tests for all CLWHIV before ART initiation, which are fully provided by the national public health system, in order to support the choice of the most adequate ART regimen. The aim of this study is to evaluate the prevalence of HIV‐DR in CLWHIV treatment‐naïve in Brazil.
Methods: The national genotyping database from the Ministry of Health of Brazil was used for this analysis. HIV pol sequences from treatment‐naïve infants under 18 months from 2009 to 2018 were selected. The Stanford HIVdb Programme was used to assess the presence of HIV‐DR.
Results: In period of analysis, 838 HIV pol sequences were identified (median age: 5 months; IQR:3 to 9 months. The HIV‐DR prevalence for Nevirapine (NVP): 18.74% (CI95%: 14.27 to 22.83), Efavirenz (EFZ): 18.74% (CI95%: 14.2 to 22.68); Lamivudine (3TC): 5.37% (CI95%: 3.15 to 7.53), Zidovudine (AZT): 6.92% (CI95%: 4.92 to 8.92), Abacavir (ABC): 7.16% (CI95%: 4.9 to 9.44), Lopinavir/ritonavir (LPV/r): 3.34% (CI95%: 2.16 to 4.24). Only three CLWHIV showed resistance to Darunavir/r. The highest prevalence identified were to NVP and EFZ – Non nucleoside reverse transcriptase inhibitors (NNRTI) (Figure 1). In addition, 3.7% (n = 31) presented HIV‐DR to AZT+ABC, 5.4% (n = 45) to ABC + 3TC, 2.02% (n = 17) to 3TC+AZT, 2.02% (n = 17) were resistant to all NRTI (AZT, ABV, TDF), 1.79% (n = 15) to AZT + 3TC+NVP, 3.7% (n = 31) to AZT+ABC+NVP and 4.3% (n = 36) to 3TC+ABC+NVP.
Abstract PDB0407‐Figure 1.
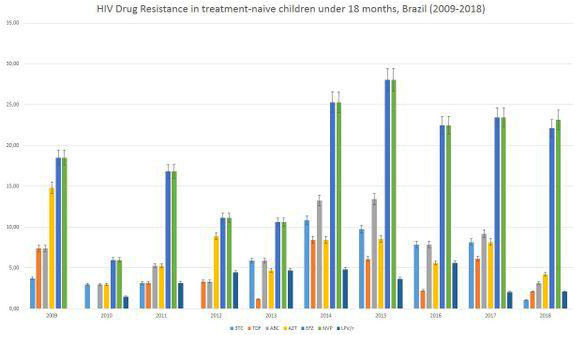
Conclusions: The high prevalence of transmitted HIV‐DR to NNTRI in CLWHIV is a matter of concern because there is a limited number of treatment options for this population. The incorporation of new drugs, such as integrase inhibitors, as prophylaxis and treatment, is essential to tackle resistance to NNTRI and improve treatment outcomes in this age group.
PDC0102
High PrEP adherence in men who have sex with men and transgender women cohort in Manila, Philippines: Evidence of HIV protective levels from tenofovir blood concentrations in project PrEPPY
G.‐M. Santos1,2, R. Ditangco3, C. Lagman4, D. Rosadiño4, K. Jonas5, L. Vi‐Le6, Y.‐R.J. Lo7, F. van Griensven1,8
1University of California, San Francisco, United States, 2San Francisco Department of Public Health, San Francisco, United States, 3AIDS Research Group, Research Institute for Tropical Medicine, Department of Health, Manila, Philippines, 4LoveYourself Foundation, Manila, Philippines, 5Maastricht University, Maastricht, United States, 6World Health Organization, Regional Office for the Western Pacific, Manila, Philippines, 7World Health Organization, Representative Office for Malaysia, Singapore, and Brunei Darussalam, Kuala Lumpur, Malaysia, 8Thai Red Cross AIDS Research Center, Bangkok, Thailand
Background: Pre‐exposure prophylaxis (PrEP) significantly reduces HIV infections. Efforts to increase PrEP access are underway in the Philippines, where an accelerating HIV epidemic disproportionately affects men who have sex with men (MSM) and transgender women (TGW). PrEPs efficacy is contingent on adherence; however, studies on PrEP adherence using objective measures remain limited in the Philippines. We tested banked human plasma to examine tenofovir concentrations in a cohort of PrEP‐using MSM/TGW in Metro Manila, the region with the highest number of new HIV diagnoses in the Philippines.
Methods: We enrolled 250 participants in a prospective cohort of PrEP‐naïve, HIV‐uninfected MSM/TGW in “Project PrEPPY.” Participants received daily oral PrEP for 12 months. Self‐reported PrEP adherence was monitored using daily diaries. Using a computer algorithm, we randomly selected 50 banked plasma samples collected at 6‐ and 12‐month visits for tenofovir concentrations testing using liquid chromatography‐electrospray tandem mass spectrometry. We classified samples as having HIV protective concentrations using previously established cut‐offs (tenofovir concentrations > 40 ng/mL). This cut‐off has been estimated to have 88% protective effect against HIV and was demonstrated to be consistent with steady‐state daily dosing.
Results: Participants that had their plasma samples randomly selected for testing had similar baseline sociodemographic (age, gender, income, education : p > 0.05) characteristics as non‐randomly selected participants. The prevalence of HIV protective concentrations from plasma samples was similar (p ≥ 0.99) overtime: 92.9% (95% CI = 73.7 to 98.4%) at 6‐month, and 90.9% (95% CI = 67.3% to 98.0%) at 12‐month visits. The proportion of participants who reported taking ≥ 85% of their PrEP daily doses was 88% during follow‐up, whereas 99.6% reported taking 4 pills per week (protective drug‐level adherence). There were no HIV seroconversions during the follow‐up (187.6 person years).
Conclusions: This is the first study to demonstrate high prevalence of HIV protective tenofovir blood concentrations levels in a PrEP‐using cohort in the Philippines. Data from our objective marker of PrEP adherence demonstrate drug concentrations consistent with high protective effects against HIV during follow‐up. Results also corroborate the high levels of self‐reported adherence. Taken together, these findings support high acceptability of PrEP among MSM/TGW in Manila and underscore the important role PrEP can play in slowing down the HIV epidemic in the Philippines.
PDC0103
Adherence to daily and event‐driven pre‐exposure prophylaxis among men who have sex with men in Taiwan
H.‐J. Wu1, S.W.‐W. Ku2, C.‐W. Li3, Y.‐T. Chu2, Y.‐W. Tang4, A.‐C. Chung5,6, N.‐Y. Ko4,5,6, C. Strong1
1College of Medicine, National Cheng Kung University, Department of Public Health, Tainan, Taiwan, Province of China, 2Taipei Veterans General Hospital, Division of Infectious Diseases, Department of Medicine, Taipei, Taiwan, Province of China, 3National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Division of Infectious Diseases, Department of Medicine, Tainan, Taiwan, Province of China, 4National Cheng‐Kung University Hospital, College of Medicine, National Cheng‐Kung University, Department of Nursing, Tainan, Taiwan, Province of China, 5Taiwan Love and Hope Association, Kaohsiung, Taiwan, Province of China, 6Healing, Empowerment, Recovery of Chemsex Health Center, Kaohsiung, Taiwan, Province of China
Background: WHO has recommended both daily and event‐driven (ED) pre‐exposure prophylaxis (PrEP) to men who have sex with men (MSM). While switching between two dosing regimens has not been uncommon, real‐world data reporting PrEP users’ adherence and its correctness related to each regimen were limited.
Methods: A multi‐centre, prospective cohort study was conducted at hospital‐based clinics in three urban cities in Taiwan between 1st January 2018 and 15th December 2019. At each visit, participants reported their choice of PrEP dosing regimen for the past month and the number of pills taken within five days of the last anal intercourse. We defined correct PrEP use as followed: 1) taking two pills on day X (i.e. the day having sex) or the day X‐1, and at least one pill on the day X, X + 1 and X + 2 for ED regimen 2) at least one pill every day for five days for daily regimen. Missed doses with ED regimen were counted as pre‐coital and post‐coital respectively.
Results: There were 374 MSM participants with a total of 1054 visits. ED PrEP was reported in nearly half of the visits (48.7%). There were 53 MSM who reported 81 regimen switches: 46 from daily to ED and 35 from ED to daily. Overall, PrEP were taken correctly in 83.5% visits. The proportion of correct PrEP use was higher with daily use than ED use (92.2% vs 74.3%, p < 0.001). Among 132 visits reported incorrect ED use, 65.9% missed pre‐coital doses, 20.4% missed post‐coital doses, and 13.6% missed both pre‐coital and post‐coital doses.
Conclusions: Lower adherence was more likely to be observed in ED than daily PrEP use. Missing pills before sex suggests potential difficulty for MSM to predict sexual acts. Given the high acceptability of ED dosing regimen in Taiwan, understanding barriers to PrEP adherence and developing an effective intervention for both ED and daily PrEP users are urgently needed.
PDC0104
Project My PrEP: Results from a PrEP demonstration project among high‐risk men who have sex with men (MSM) in Kuala Lumpur, Malaysia
M. Akbar1, I. Azwa2, M. Chong1, O. Hangchen2, S. Basri2, A. Yap3, R. Tai4, H. Lim1, J. Ying Ru‐Lo5, F. van Griensven6,7, A. Kamarulzaman1,2
1University of Malaya, Center of Excellence for Research in AIDS, Kuala Lumpur, Malaysia, 2University of Malaya, Infectious Disease Unit, Department of Medicine, Faculty of Medicine, Kuala Lumpur, Malaysia, 3The Red Clinic, Kuala Lumpur, Philippines, 4Pink Triangle Foundation, Community Health Care Clinic, Kuala Lumpur, Malaysia, 5World Health Organization, Representative Office for Malaysia, Singapore, and Brunei Darussalam, Kuala Lumpur, Malaysia, 6University of California at San Francisco, Department of Epidemiology and Biostatistics, San Francisco, United States, 7Thai Red Cross AIDS Research Center, Bangkok, Thailand
Background: In recent years, HIV prevalence among MSM in Kuala Lumpur has been increasing. Additional HIV prevention measures are urgently necessary. The MyPrEP project aims to evaluate feasibility of daily PrEP among MSM in Kuala Lumpur.
Methods: Prospective participants were recruited through the internet and social media. Men were eligible if ≥ 18 years old, Malaysian, HIV uninfected (by 4th Gen HIV Ag/Ab combo and POC HIV VL), had normal renal function, and reported a history of high‐risk behaviour. Men were seen at three different sites around Kuala Lumpur for a duration of 12 months. Daily generic oral TDF/FTC was provided free of charge. Behavioural and adherence data and STI laboratory test results were collected at baseline and every 3 months thereafter.
Results: From March to October 2018, 381 men were screened of whom 186 (49%) did not meet demographic or behavioural criteria. Of the remaining 195 men, 14 (7%) were HIV infected, 8 (2%) had medical pre‐conditions, 23 (12%) declined and 150 (77%) were enrolled. Most participants were > 25 years (79%), of Chinese ethnicity (57%) and had at least a university degree (90%). One‐year retention was 96% (139/150), daily pill adherence (self‐reports and pill counts), 88% (7 doses/wk) and protective drug level adherence (≥4 doses/wk), 99%. Multiple (>1) male sexual partners were reported by 71% at baseline and by 85% at 12 months, inconsistent condom‐use by 81% and 86%, and “chemsex” by 51% and 44%. The mean number of anal sex partners increased from 2.5 to 3.6 during the same time period. Prevalence of rectal chlamydia declined from 19% at baseline to 10% at 12 months, of rectal gonorrhoea from 10% to 9% and of syphilis from 11% to 9%. None of these differences were statistically significant. No new HIV infections were detected during the course of the project.
Conclusions: The MyPrEP demonstration project showed feasibility of daily PrEP among MSM in Kuala Lumpur. Adherence and retention were high and no new HIV infections occurred during follow‐up. No dramatic changes in HIV risk behaviours were reported. The project provided an excellent opportunity to diagnose and treat asymptomatic STI in a high‐risk population.
PDC0105
High persistence of daily oral PrEP among 18 to 26 year old Thai men who sell sex: Preliminary results of the COPE4YMSM study
A. Hickey1,2, B. Weir3, C. Dun3, A. Wirtz3, S.H.H. Mon3, T. Chemnasiri1,2, A. Warapornmongkholkul1,2, C. Ungsedhapand1,2, W. Sukwicha1,2, S. Pattanasin1,2, A. Varangrat1,2, E.F. Dunne1,2, M. Thigpen1,2, T. Holtz1,2, S. Janyam4, D. Linjongrat5, A. Adeyeye6, C. Beyrer3
1U.S. Centers for Disease Control and Prevention, Division of HIV/AIDS Prevention, Atlanta, United States, 2Thailand Ministry of Public Health and U.S. Centers for Disease Control and Prevention Collaboration (TUC), Nonthaburi, Thailand, 3Johns Hopkins Bloomberg School of Public Health, Baltimore, United States, 4Service Workers In Group Foundation (SWING), Bangkok and Pattaya, Thailand, 5Rainbow Sky Association of Thailand (RSAT), Bangkok, Thailand, 6National Institute of Allergy and Infectious Diseases (NIAID), Prevention Science Program, Division of AIDS (DAIDS), Bethesda, United States
Background: Sustained use of HIV pre‐exposure prophylaxis (PrEP) over time (persistence) has been a significant challenge among many populations, including young men who have sex with men (YMSM). PrEP persistence among YMSM who sell sex is unknown.
Methods: COPE4YMSM, a Thai‐US collaborative study, (NIH/NIAID, R01 AI118505) is assessing the effectiveness of an open‐label offer of combination HIV prevention with or without daily oral Tenofovir‐Emtricitabine (Truvada) for PrEP and mobile phone‐based text message adherence support among 18 to 26 year old MSM who sell or exchange sex. We partner with key population‐led service providers, SWING and RSAT, to enable persistence through community mobilization and social media. Participants may choose to take PrEP or not, in combination with regular HIV testing, risk reduction counselling, and condom and lubricant provision. We analysed PrEP initiation, study retention, and PrEP adherence based on text message self‐report of doses of PrEP in the last week. PrEP persistence was defined as self‐reported continued use of PrEP 12 months after initiation. A random sample of DBS specimens from those reporting good PrEP adherence in the last 7 days (>4 doses of PrEP in the last week) were analysed for intracellular tenofovir diphosphate (TFV‐DP).
Results: We enrolled 856 HIV‐uninfected, at‐risk young male sex workers, of whom 590 (68.9%) initiated PrEP within 30 days of enrolment. Among men initiating PrEP at baseline, retention in the study was good, at 75.9% at 12 months. PrEP adherence was also high, with over 98% of participants reporting 4 or more doses of PrEP/week. Self‐reported use in the last 7 days had a high positive predictive value relative to intracellular TFV‐DP levels; among samples from participants with good self‐reported adherence (n = 62), 79.0% had protective levels of TFV‐DP in their DBS samples (kappa agreement = 78.3%, chi‐square = 79.2, p < 0.001).
Conclusions: Using an open‐label combination prevention approach in partnership with key population‐led providers can successfully engage and sustain PrEP use among young MSW at risk in Thailand. Over two‐thirds of YMSW aged 18 to 26 years persisted on PrEP at 12 months after initiation, significantly higher than other reports.
PDC0106
Youth‐focused strategies to promote adherence to pre‐exposure prophylaxis among adolescent men who have sex with men and transgender women at‐risk for HIV in Thailand
W.N. Songtaweesin1, S. Kawichai1, N. Phanuphak2, T.R. Cressey3,4,5, P. Wongharn1, C. Saisaengjan1, T. Chinbunchorn2, S. Janyam6, D. Linjongrat7, T. Puthanakit1,8, CE‐PID ‐ TRC Adolescent Study Team
1Chulalongkorn University, Center of Excellence for Pediatric Diseases and Vaccines, Faculty of Medicine, Bangkok, Thailand, 2Thai Red Cross AIDS Research Center, Prevention, Bangkok, Thailand, 3Chiang Mai University, PHPT/IRD UMI 174, Faculty of Associated Medical Sciences, Chiang Mai, Thailand, 4Harvard T.H. Chan School of Public Health, Department of Immunology and Infectious Diseases, Boston, United States, 5University of Liverpool, Department of Molecular and Clinical Pharmacology, Liverpool, United Kingdom, 6The Service Workers IN Group Foundation, Bangkok, Thailand, 7Rainbow Sky Association Thailand, Bangkok, Thailand, 8Chulalongkorn University, Department of Pediatrics, Faculty of Medicine, Bangkok, Thailand
Background: Strategies are urgently needed to curb the increasing incidence of HIV in young men who have sex with men (YMSM) and transgender women (YTGW) worldwide. We assessed the impact of youth‐friendly services and a mobile phone application (app) on adherence to pre‐exposure prophylaxis (PrEP) in YMSM and YTGW in Thailand.
Methods: A randomized control trial was conducted in YMSM and YTGW aged 15 to 19 years. Participants were provided daily oral TDF/FTC and condoms and randomization to receive either youth‐friendly services (standard of care, SOC) or SOC plus a PrEP app (SOC+APP), whose features included self‐assessment of HIV acquisition risk activities, point rewards, and reminders for PrEP and clinic appointments. Clinic visits occurred at 0, 1, 3, 6 months and telephone contact at 2, 4, and 5 months. Sexually transmitted infection (STI) screening was performed at baseline and month 6, and HIV testing at all visits. PrEP adherence was evaluated with intracellular tenofovir diphosphate (TFV‐DP) concentrations in dried blood spots (DBS) samples at months 3 and 6. The primary endpoint was ‘PrEP adherence’ defined as a TFV‐DP DBS concentrations ≥ 700fmol/punch [equivalent to ≥ 4 doses of TDF/week at either month 3 and/or 6.
Results: Between March 2018 and June 2019, 489 adolescents were screened, 27 (6%) tested HIV positive and 200 (41%) were enrolled and initiated PrEP. Of these, 147 were YMSM (74%) and 53 YTGW (26%). At baseline, median age was 18 years (IQR 17 to 19), 84% reported inconsistent condom use in the past month, and prevalence of STIs was 23%. Retention at 6‐months was 73%. In the SOC+APP arm, median app time use was 3 months (IQR 1 to 5). PrEP adherence was 49.1% overall (45.8% in SOC and 52.4% in SOC+APP arm, p = 0.40). YMSM were 3.6 times (adjusted OR 95% CI 1.41 to 9.05) more likely to adhere to PrEP than YTGW. No HIV seroconversions occurred during 75 person years of follow‐up.
Conclusions: PrEP implementation in adolescents is feasible through youth friendly services with high retention rates at 6‐months. YTGW may require more support for PrEP adherence than YMSM. App use in this trial did not affect PrEP adherence.
PDC0107
Assessing the performance of international preexposure prophylaxis (PrEP) eligibility guidelines in a cohort of Chinese MSM, Beijing, China 2009 to 2016
E.W. Hall1, L. Wang2, X. Huang3, P.S. Sullivan1, A.J. Siegler4
1Emory University, Department of Epidemiology, Atlanta, United States, 2Emory University/Blued, Beijing, China, 3Beijing You'An Hospital, Center for Infectious Diseases, Beijing, China, 4Emory University, Behavioral Sciences and Health Education, Atlanta, United States
Background: Evidence is needed for China to design a PrEP eligibility assessment tool to facilitate the development of national guidelines. We assessed performance of international PrEP eligibility criteria to predict future HIV seroconversion among MSM in Beijing, China.
Methods: Participants were MSM aged ≥18 years who enrolled in a cohort study between July 2009 and March 2016. Participants completed HIV testing, syphilis testing, and a questionnaire on recent sexual health behaviours at each follow‐up visit and were followed until HIV seroconversion or dropout. We assessed PrEP eligibility at the most recent follow‐up visit prior to the final study visit. Participants were classified as either indicated or not indicated for PrEP based on criteria from each of the following guidelines: European AIDS Clinical Society (EACS), Korean Society for AIDS (KSA), Southern African HIV Clinicians Society (SA), Taiwan Centers for Disease Control, British HIV Association (UK), United States Public Health Service clinical (USPHSC) and risk score (USPHSR), and the World Health Organization (WHO). To compare guideline performance, we calculated sensitivity, specificity, Youden's Index (YI), and Matthew's Correlation Coefficient (MCC). For each guideline, performance measures were compared to random allocation of PrEP by randomly selecting a proportion of participants equal to the proportion indicated.
Results: There were 287 (17.3%) incident HIV seroconversions among 1663 MSM. The number of men indicated for PrEP ranged from 556 (33.4%, USPHSC) to 1569 (94.2%, KSA). Compared to random allocation, sensitivity ranged from slightly worse (−4.7%, USPHSR) to 30.2% better than random (USPHSC). Across all guidelines, specificity was not meaningfully better than random allocation. EACS guidelines had the highest binary classification performance measures (YI = 0.129, MCC = 0.100).
Conclusions: The performance of most international guidelines were slightly better than random PrEP allocation, but none performed well. For settings in which international guidelines perform poorly, alternative indication approaches should be considered.
Abstract PDC0107‐Table 1.
| Guidelines | n | Sensitivity | Specificity | Matthew's correlation coefficient | Youden's index | ||||
|---|---|---|---|---|---|---|---|---|---|
| Guidelines (95% CI) | Random (95% BI) | Guidelines (95% CI) | Random (95% BI) | Guidelines | Random (95% BI) | Guidelines | Random (95% BI) | ||
| EACS | 657 | 0.502 (0.442, 0.561) | 0.394 (0.345, 0.446) | 0.627 (0.601, 0.653) | 0.605 (0.594, 0.616) | 0.100 | −0.001 (−0.047, 0.048) | 0.129 | −0.002 (−0.061, 0.062) |
| KSA | 1569 | 0.972 (0.946, 0.988) | 0.944 (0.920, 0.969) | 0.063 (0.050, 0.077) | 0.057 (0.052, 0.062) | 0.057 | 0.002 (−0.047, 0.050) | 0.035 | 0.001 (−0.029, 0.030) |
| SA | 1296 | 0.840 (0.792, 0.880) | 0.780 (0.735, 0.822) | 0.233 (0.211, 0.257) | 0.221 (0.211, 0.230) | 0.067 | 0.001 (−0.049, 0.047) | 0.073 | 0.001 (−0.053, 0.052) |
| Taiwan | 918 | 0.624 (0.565, 0.680) | 0.551 (0.498, 0.603) | 0.463 (0.436, 0.490) | 0.448 (0.437, 0.459) | 0.066 | −0.001 (−0.049, 0.047) | 0.087 | −0.002 (−0.065, 0.061) |
| UK | 849 | 0.533 (0.474, 0.592) | 0.512 (0.456, 0.564) | 0.494 (0.467, 0.521) | 0.490 (0.478, 0.501) | 0.021 | 0.002 (−0.049, 0.049) | 0.027 | 0.002 (−0.065, 0.065) |
| USPHSC | 556 | 0.436 (0.377, 0.495) | 0.334 (0.286, 0.383) | 0.687 (0.662, 0.711) | 0.666 (0.656, 0.676) | 0.098 | 0.000 (−0.047, 0.047) | 0.122 | 0.000 (−0.059, 0.059) |
| USPHSR | 1244 | 0.714 (0.658, 0.766) | 0.749 (0.700, 0.794) | 0.245 (0.222, 0.269) | 0.252 (0.242, 0.262) | −0.036 | 0.001 (−0.050, 0.049) | −0.041 | 0.001 (−0.058, 0.056) |
| WHO | 734 | 0.544 (0.484, 0.602) | 0.439 (0.390, 0.495) | 0.580 (0.553, 0.606) | 0.558 (0.548, 0.570) | 0.094 | −0.002 (−0.047, 0.049) | 0.124 | −0.003 (−0.062, 0.065) |
PDC0202
Progress and challenges towards reaching 90‐90‐90 targets among key populations in Botswana
W. Dikobe1, L. Okui1, C. Akolo2, R. Kereng1, M. Gilbert‐Lephodisa1, O. Komotere1, D. Kanyenvu3, K. Kusi3, B. Nkomo3, J. Ngidi4, S. Chishala4, J. Bolebantswe3, C. Petlo3, M. Mine5, M. Merrigan2
1FHI360, Gaborone, Botswana, 2FHI360, Washington, United States, 3Ministry of Health and Wellness, Dept of HIV/AIDS and Prevention, Gaborone, Botswana, 4Botswana Harvard, HIV Reference Laboratory, Gaborone, Botswana, 5Ministry of Health and Wellness, National Health Laboratory, Gaborone, Botswana
Background: Botswana has made great strides towards the 90‐90‐90 goals, however, achieving these targets for key populations (KPs) remains a challenge. KPs, such as men who have sex with men (MSM) and female sex workers (FSWs), have limited access to HIV prevention, care, and treatment services due to stigma and discrimination. Using data from the first and second Biological and Behavioral Surveillance Surveys (BBSS1, BBSS2), we analysed progress towards reaching the targets among KPs in Botswana.
Methods: To examine progress between 2012 (BBSS1) and 2017 (BBSS2), comparisons were made for the three districts represented in both surveys: Gaborone, Francistown, and Chobe. KP members aged 16–64 years responded to a questionnaire and were tested for sexually transmitted infections, including HIV. HIV testing was done with all participants regardless of documented status.
Results: HIV prevalence among FSWs remained high from 2012 (61.9%) to 2017 (51.5%), with no significant change (p = 0.19). Among MSM, HIV prevalence increased significantly from 13.1% to 19.1% (p = 0.016). This may be due to the slightly older sample in 2017 compared to 2012 (28 vs. 23 years). Individuals who self‐reported as HIV positive increased for FSW (68.4% vs. 45.1%, p = 0.008) and for MSM (41% vs. 16.9%, p = 0.016). Additionally, those living with HIV and on ART significantly increased from 10.6% to 60.3% (p = 0.000) for FSW and from 5.1% to 37.2% (p = 0.0000) for MSM. Only 59.5% of all FSWs and 35.9% of MSM, living with HIV who know their status reported taking ART daily in 2017.
Abstract PDC0202‐Table 1. Progress towards 90‐90‐90 targets in Botswana+++
| First 90 (%) | Second 90 (%) | Third 90 (%) | |||
|---|---|---|---|---|---|
| % PLHIV who know their HIV status | % PLHIV who know their status who are on treatment * | % PLHIV who are on treatment | % PLHIV who know their status, reporting taking treatment daily * | % PLHIV reporting taking treatment daily* | |
| General Population | 85 | >85 | >81 | >89 (with VLS) | >73 (VLS) |
| FSW (2012) | 45.1 (35.5 to 55.1) | 24.9 (13.8 to 36) | 10.6 (6.3 to 17.2)) | Not asked | Not asked |
| FSW (2017) | 68.4 (53.4 to 80.4) | 87.8 (81.2 to 92.2) | 60.3 (49.5 to 70.1) | 99.2 (97.7 to 99.7) | 59.5 (49.1 to 69.1) |
| MSM (2012) | 16.9(9.3 to 28.9) | ||||
| 13(1.8 to 27.9) | 5.1(1.6 to to 14.9) | Not asked | Not asked | ||
| MSM (2017) | 41.0(30.6 to 52.4) | ||||
| 82.1(66.0 to 91.4) | 37.2(27.1 to 48.5) | 97.2(81.3 to 99.6) | 35.9(25.9 to 47.3) |
Conclusions: Despite the significant improvements between BBSS1 and BBSS2, KPs continues to be highly affected by HIV and are far from reaching the 90‐90‐90 goals compared to the general population. FSW are at 68‐60‐60, while MSM are at 41‐37‐36 towards targets. For countries to achieve epidemic control, ongoing investments in programmes tailored to the needs of KPs are needed.
PDC0203
Food insecurity highly prevalent and associated with early PrEP non‐adherence among trans and non‐binary people in the San Francisco Bay Area: The STAY Study
A. Liu1,2, E. Vittinghoff2, J. Gagliano1, C. Turner1, H. Xie1, J. Rapues1, S. Pardo1, P. Anderson3, C. Rodriguez1, Z.‐J. Eskman4, E. Palafox5, R. Lin61, B. Makoni5, E. Rodriguez7, P. von Felten1, S. Buchbinder12, H. Scott1,2, S. Arayasirikul1,2, E.C. Wilson1,2, STAY Study Team
1San Francisco Department of Public Health, San Francisco, United States, 2University of California, San Francisco, United States, 3University of Colorado, Aurora, United States, 4San Francisco Community Health Center, San Francisco, United States, 5Tri City Health Center, Fremont, United States, 6Tom Waddell Urban Health Center, San Francisco, United States, 7Castro Mission Health Center, San Francisco, United States
Background: Few studies have evaluated real‐world PrEP delivery in trans and non‐binary populations. We describe baseline characteristics and early adherence in participants enrolled in one of the first PrEP demonstration projects for transgender communities – the STAY Study.
Methods: The STAY Study enrolled HIV‐uninfected trans women, trans men, and non‐binary individuals across 5 trans‐affirmative clinics in the San Francisco Bay Area and offered participants 48 weeks of PrEP, along with peer navigation, bi‐directional SMS support, and panel management. Tenofovir‐diphosphate (TFV‐DP) levels in dried blood spots (DBS) collected at 4 and 12 weeks were analysed to assess early adherence. Correlates of early adherence (TFV‐DP ≥ 700) were evaluated using multivariable logistic regression.
Results: From August 2017 to May 2019, 193 individuals were screened and 159 enrolled. Median age was 35 (IQR 27 to 46); 26% were Latino/a, 25% White, 14% Black, 8% Asian, and 27% multirace/other. Overall, 86% were transwomen or women, 6% were transmen or men, and 8% were non‐binary. Half completed high‐school; 92% had a primary care provider/health insurance, and 82% were taking gender‐affirming hormones. At baseline, 80% reported food insecurity; 8% were homeless, 15% lived in a motel/hotel/boarding house, and 50% rented a house/apartment/room. In the past year, 68% reported condomless anal/vaginal sex and 22% reported an STI. Retention was 87% at week 4 and 83% at week 12. Among 60 participants with DBS testing at week 12, 55% had levels consistent with 4 to 7 doses/week, 15% 2 to 3 doses/week, 25% <2 doses/week, and 5% were undetectable. In a multivariable model, food insecurity (AOR 0.26, 95% CI 0.07 to 0.94) and those who were multiracial/other (AOR 0.11, 95% CI 0.03 to 0.45) were less likely to have protective levels, while those living in a motel, hotel, or boarding house (compared with those who were homeless/in a shelter) were more likely to have protective levels (AOR 8.47, 1.56 to 45.85). Use of gender‐affirming hormones was not associated with TFV‐DP.
Conclusions: Over half of STAY participants with DBS tested during early follow‐up had protective PrEP levels. Food insecurity was highly prevalent and associated with lower PrEP adherence, while relative housing stability was associated with higher protection, highlighting the impact of structural factors on PrEP adherence in this population.
PDC0204
Rates and trends of HIV diagnoses among Indigenous peoples in Canada, Australia, New Zealand, and the United States from 2009 to 2017
K. Koehn1,2, C. Cassidy‐Matthews3,2, M. Pearce4,5, C. Aspin6, H. Pruden4, J. Ward7, R. Hogg2,1, V. Nicholson2
1Simon Fraser University, Faculty of Health Sciences, Burnaby, Canada, 2BC Centre for Excellence in HIV/AIDS, Vancouver, Canada, 3University of British Columbia, School of Population and Public Health, Vancouver, Canada, 4BC Centre for Disease Control, Vancouver, Canada, 5First Nations Health Authority, Vancouver, Canada, 6Health Quality and Safety Commission, Wellington, New Zealand, 7University of Queensland, School of Public Health, Brisbane, Australia
Background: While Indigenous peoples of the Anglo‐settler states of Canada, Australia, the USA, and New Zealand have experienced similar histories of colonization and resistance to the health impacts of ongoing oppression, few cross‐national comparisons of HIV diagnoses have been conducted. The objective of this study is to compare rates and trends of HIV diagnoses among Indigenous peoples in Canada (First Nations, Métis, Inuit, and Other Non‐Specified), Australia (Torres Strait Islanders and Aboriginal), the USA (American Indian, Alaska Native, Native Hawaiian, and Other Pacific Islanders), and New Zealand (Māori).
Methods: We employed publicly available surveillance data from 2009 to 2017 to estimate the rate per 100,000 of HIV diagnoses. Estimated annual percent change (EAPC) in diagnosis rates was calculated using Poisson regression. The four countries have passive population‐based HIV surveillance programmes. Population estimates from respective census programmes were used as rate denominators. Estimated annual HIV diagnosis rate per 100,000 and EAPC were calculated for total Indigenous peoples, women, and men.
Results: As of 2017, rates of HIV were highest in Canada (16.22, 95% CI: 14.30, 18.33) and lowest in New Zealand (1.36, 95% CI: 0.65, 2.50). Australia had a rate of 3.81 (95% CI: 2.59, 5.40) and the USA had a rate of 3.22 (95% CI: 2.85, 3.63). HIV diagnosis rates among the total Indigenous population decreased in Canada (−7.92 EAPC, 95% CI: −9.34, −6.49) and in the USA (−4.25 EAPC, 95% CI: −5.75, −2.73), but increased in Australia (5.10 EAPC, 95% CI: 0.39, 10.08). No significant trends over time were observed in New Zealand (2.23 EAPC, 95% CI: −4.48, 9.47).
Conclusions: We found elevated but decreasing rates of HIV diagnoses in Canada compared to Australia, the USA, and New Zealand. While there are limitations to conducting cross‐national comparisons, there are substantial differences in HIV diagnosis rates in these four countries that may be reflective of divergent country‐level policies and systems that affect the health status of Indigenous peoples.
PDC0205
Effect of targeted counselling on retention to HIV pre‐ exposure prophylaxis among men who have sex with men within Nairobi City County, Kenya
M. Akolo1, J. Kimani2, R. Gichuki3, J. Osero4, A. Kibera4, L. Gelmon2
1PHDA, Clinical, Nairobi, Kenya, 2PHDA/University of Nairobi/Manitoba, Nairobi, Kenya, 3AMREF, Nairobi, Kenya, 4Kenyatta, Nairobi, Kenya
Background: In Sub Saharan African countries, MSMs have 19.3 folds higher odds of being HIV infected compared with the general population. Kenya MSM have a HIV prevalence of 18.2. PrEP protects up to 90% of HIV infection in those who adhere well. However, retention of high‐risk MSM on PrEP in Kenya has proved to be a challenge.
Methods: Experimental design was used. The two facilities within Nairobi serving Men having sex with Men were purposely selected. Eligible Men who have Sex with Men and had just enrolled into PrEP within one week were selected through simple random sampling and randomized to either arm using computer generated randomization table During the study participants in the intervention arm received targeted counselling as an intervention which included assessment and counselling on depression, PrEP adherence, alcohol consumption and Short message reminder to come to the facility. The control arm followed the government prescription of issuing PrEP in reliance to oral self report on adherence with no other intervention. The two groups were followed for six months; month one after PrEP initiation, month three and month six and retention calculated and compared among the two groups.
Results: 84 study participants were enrolled on each arm. At month one intervention arm had retained 82 (97.6%) of its initial study participants compared to 68 (81.0%) control group with a significance difference (p < 0.001). At month three retention gap widened; intervention arm had retained 77 (91.7%) compared to 26 (31.0%) control group with a clear significant difference (p < 0.001) At month six the intervention arm retention reduced to 58 (69.0%) and the control arm dropped farther to 16 (19.0%) with still a significant difference (p < 0.001). Each intervention indicator was considered in relation to its effect on MSM retention to PrEP as follows; Depression assessment counselling showed significant association with MSM retention to PrEP at p = 0.004. Alcohol consumption counselling also showed significant association with PrEP retention (p = 0.002), while pill adherence counselling showed a significant association with MSM retention on PrEP (p < 0.001).
Conclusions: Targeted counselling sessions should be incorporated into ministry of Health PrEP dissemination package among MSM to improve on retention.
PDC0206
Closing the HIV identification gap for men: The impact of assisted partner notification services in a real‐world setting in Kenya
F. Odhiambo1, C. Dande1, R. Onyango1, G. Nyanaro1, E. Mulwa1, M. Aluda1, M. Agallo1, F. Miruka2, A. Aoko2, E.A. Bukusi1, C.R. Cohen3
1Kenya Medical Research Institute, Center for Microbiology Research‐Research Care and Training Program, Nairobi, Kenya, 2Centre for Disease Control and Prevention, Division of Global HIV and TB, Kisumu, Kenya, 3University of California, Department of Obstetrics, Gynecology and Reproductive Sciences, San Francisco, United States
Background: Globally in 2018, 23.3 million persons living with HIV were receiving antiretroviral treatment. Despite this, progress in identification and linkage of HIV‐infected men has lagged behind. We compared the impact of assisted Partner Notification Services (aPNS) in improving identification and linkage of men with undiagnosed HIV infection with facility testing strategies.
Methods: We conducted a retrospective analysis of routine programme data collected from 61 health facilities in Kisumu County, Kenya between October 2018 and September 2019. Records of male clients > 15 years of age who received HIV testing services through either aPNS or facility testing approaches, were included in the analysis. We compared the proportions of yield in the aPNS vs facility testing using risk ratios (RR) with confidence intervals (CIs) computed by Traditional (log‐transformation) method and linkage to treatment using Chi‐square method.
Results: A total of 12,220 men underwent HIV testing through aPNS and 90,127 through facility testing giving HIV positive yields of 12.3% (n = 1503) and 0.6% (n = 540), respectively. Most of the HIV‐positive males were aged > 25 years in both aPNS (92%) and facility testing (77%) groups. The overall HIV‐positive yield was 20.6‐fold (95% CI, 18.74 to 22.73) higher in aPNS compared to facility testing and highest among men aged 20 to 24 years (Table 1). Similarly, overall linkage to HIV treatment was 84% vs 78%, (p = 0.001) in aPNS compared to facility testing respectively and highest among men aged 20 to 24 years.
Abstract PDC0206‐Table 1. Comparison of HIV‐positive yield and linkage to treatment between aPNS and facility testing among men by age category—Kisumu, Kenya.
| Age categories | HIV testing modality | Total Tested | Total HIV positive | % yield (95% CI) | RR (95% CI) | % Linkage to treatment (95% CI) | p‐value |
|---|---|---|---|---|---|---|---|
| Overall | aPNS | 12,220 | 1503 | 12.30 (11.72 to 12.89) | 20.53 (18.64 to 22.61) | 83.70 (81.77 to 85.50) | P = 0.001 |
| Facility testing | 90,127 | 540 | 0.60 (0.55 to 0.65) | 77.8 (74.1 to 81.1) | |||
| 15 to 19 years | aPNS | 594 | 12 | 2.02 (1.10 to to 3.41) | 20.37 (9.94 to 41.78) | 100.00 (77.91 to 100.00) | P = 0.034 |
| Facility testing | 19,165 | 19 | 0.10 (0.06 to 0.15) | 73.68 (50.94 to 89.66) | |||
| 20 to 24 years | aPNS | 1032 | 92 | 8.91 (7.29 to 10.77) | 42.64 (28.92 to 62.87) | 93.48 (86.93 to 97.31) | P < 0.001 |
| Facility testing | 16,261 | 34 | 0.21 (0.15 to 0.29) | 5.88 (1.00 to 18.10) | |||
| 25 + years | aPNS | 10,594 | 1399 | 13.21 (12.57 to 13.86) | 14.83 (13.41 to 16.41) | 82.92 (80.88 to 84.85) | P = 0.492 |
| Facility testing | 54,701 | 487 | 0.89 (0.81 to 0.97) | 82.96 (79.32 to 86.19) |
Conclusions: Our data suggest that aPNS implemented in a real‐world setting identified more HIV‐infected men and led to greater linkage to treatment. Thus, scaling up aPNS may be an acceptable and efficient model to achieve near universal uptake of HIV testing amongst men at high risk of HIV infection and linking them to HIV treatment.
PDC0207
Uneven progress in Europe and Central Asia towards the 90‐90‐90 goals
T. Noori1, A. Brown2, R. Hayes3, C. Gowar3, D. Gold3, V. Delpech2, A. Pharris1, Dublin Declaration monitoring group
1European Centre for Disease Prevention and Control, Solna, Sweden, 2Public Health England, London, United Kingdom, 3National AIDS Trust, London, United Kingdom
Background: The objective of this study was to assess how countries in Europe and Central Asia (ECA) are progressing towards the UNAIDS 90‐90‐90 targets by 2020: 90% of all people living with HIV (PLHIV) know their status; 90% of those diagnosed are receiving antiretroviral treatment (ART); 90% of those on ART are virally suppressed.
Methods: National data for the most recent year available were submitted in 2019 to the European Centre for Disease Prevention and Control by national focal points from 52 countries in ECA. Estimates followed standard definitions on these measures. Longitudinal data for the period 2015 to 2018 were extracted from UNAIDS Global AIDS Monitoring database.
Results: Eighty percent of PLHIV were diagnosed in the 43 countries reporting data (country range: 46% to 98%), totalling 438,000 people living with undiagnosed HIV in ECA. Sixty‐five percent of those diagnosed in the region are on ART (country range: 40% to 100%), while 86% of those on ART are virally suppressed (country range: 42% to 99%). In the 36 countries in the region able to report data on all measures, 44% of the estimated PLHIV were virally suppressed (country range: 22% to 87%), totalling 1.2 million people living with unsuppressed viral load in ECA (Figure 1). From 2015 to 2018, the number of undiagnosed PLHIV in the region declined from 580,000 to 480,000, the number not on treatment declined from 1230,600 to 1096,000 and the number not virally suppressed declined from 1387,000 to 1303,000.
Conclusions: There is considerable diversity in progress towards reaching the 90‐90‐90 targets across ECA, with a few countries exceeding the targets while others lag far behind. Overall, one in five PLHIV in the region are unaware of their HIV infection and nearly two in five diagnosed are not on treatment. These individuals are at risk of ill health and passing on the virus. Currently ECA are not on track to reach the 90‐90‐90 targets. There has been progress on reducing the proportion of undiagnosed in the region, but significant challenges regarding treatment and viral suppression remain.
PDC0302
HIV remains the leading cause of death among adults in Zambia
S. Kamocha1, P. Minchella1, A. Wolkon1
1Centers for Disease Control and Prevention, Lusaka, Zambia
Background: HIV is thought to be a leading cause of death in Zambia despite improved access to treatment and palliative care. Nationally representative data on mortality are not available due to an inadequate vital statistics system. Using population‐based mortality data, we describe HIV/AIDS cause‐specific mortality.
Methods: Stratified cluster sampling methodology was used to select 350 rural and urban clusters in Zambia and baseline censuses were implemented in each cluster in 2011 and 2016 to gather information on deaths. VA interviews were conducted at households where deaths occurred during the prior 12 months and where they were identified prospectively for 12 months following the censuses. Probable cause of death was determined by two physicians who independently reviewed each VA questionnaire.
Results:
Abstract PDC0302‐Table 1.
| Deaths due to HIV | Deaths due to other causes | |||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||
| n | % | n | % | n | % | n | % | |
| 0 to 4 | 386 | 51.3 | 366 | 48.7 | 20,196 | 55.9 | 15,959 | 44.1 |
| 5 to 14 | 78 | 26.6 | 214 | 73.4 | 6866 | 54.8 | 5672 | 45.2 |
| 15+ | 17,020 | 60.0 | 11,340 | 40.0 | 68,681 | 59.5 | 46,757 | 40.5 |
| Total | 17,483 | 59.5 | 11,920 | 40.5 | 95,743 | 58.3 | 68,389 | 41.7 |
The census covered 136,834 households and identified 193,534 deaths. HIV/AIDS was the leading cause of death among adults; 29,403 (19.7%) of all deaths were due to HIV/AIDS. Nearly 60% of all deaths (17,483, 59.5%) were among men. Deaths due to HIV/AIDS have declined from 20.3% to 15.2% among all ages. Among adults, deaths due to HIV/AIDS have declined from 28.4% in 2011 to 19.7% in 2016. In 2016, there were more deaths due to HIV/AIDS among adult males (17,020, 60.0%) compared to females (11,340, 40.0%).
Conclusions: Despite a decline of deaths due to HIV/AIDS from 28.4% in 2011 to 19.7% in 2016, the leading cause of death among Zambian adults is HIV/AIDS. Adult males appear to be more affected by deaths due to HIV/AIDS compared to their female counterparts (60.0% vs 40.0%). Overall, the decline in deaths due to HIV/AIDS shows that progress has been made. However, there is need to accelerate efforts to improve access to ARVs and to improve retention. Interventions to improve linkage to care for adult males need to continue to receive support.
PDC0303
Use of verbal autopsy to determine HIV and TB co‐morbidity: findings from the South African National Cause‐of‐Death Validation Project
P. Groenewald1, D. Bradshaw1, M. Maqungo1, N. Nannan1, R. Laubscher1, D. Morof2, M. Cheyip3, E. Nichols4, C. Rao5, J. Price6, J. Joubert1, NCODV Team
1South African Medical Research Council, Burden of Disease Research Unit, Cape Town, South Africa, 2Centers for Disease Control and Prevention, Division of Global HIV/AID and TB, Pretoria, South Africa, 3Centers for Disease Control and Prevention, Division of Global HIV/AIDS and TB, Pretoria, South Africa, 4Centers for Disease Control and Prevention, National Center for Health Statistics, Hyattsville, United States, 5Australian National University, Department of Global Health, Research School of Population Health, Canberra, Australia, 6Oxford University, Nuffield Department of Primary Care Health Sciences, Oxford, United Kingdom
Background: In South Africa, despite death registration of 90%, HIV deaths are under‐reported, or misclassified to immediate causes of death such as TB. Vital statistics for 2016 reported that 4.9% of deaths were due to HIV, and 6.7% were due to TB. HIV and TB co‐morbidity rates are unknown because reporting is limited to 3‐character International Classification of Disease (ICD) codes. We assessed the use of verbal autopsies to more accurately ascertain the proportion of deaths caused by HIV and TB.
Methods: We analysed data from the 2017/2018 National Cause‐Of‐Death validation project. Interviewers conducted verbal autopsies with next‐of‐kin using 2016 World Health Organization (WHO) standardized instruments. Physicians completed the WHO standard medical certificate of cause of death. These were coded to ICD‐10, and the underlying cause of death was selected using Iris automated software.
Results: HIV was the underlying cause of death for 22.7% of deaths and TB for 7.0%. Proportions of HIV deaths were similar for men and women; however, there were more TB deaths among men than women. HIV disease resulting in TB (B20.0) accounted for 49.3% of all HIV‐related deaths (604/1224) and 61.5% of all TB‐related deaths (604/982; Table 1). Verbal autopsies indicated that of the 1224 HIV deaths, 125 (10.2%) individuals had received antiretroviral therapy but had discontinued treatment at some point.
Abstract PDC0303‐Table 1. Characteristics of deaths and proportions with HIV and TB as underlying cause, South Africa National Cause‐of‐Death Validation Project (2017–2018)
| Sex | All deaths | Median age, years | HIV UCOD | TB UCOD | ||||
|---|---|---|---|---|---|---|---|---|
| HIV no TB (B20.1‐B24)* | HIV with TB (B20.0)* | (A15‐A19, B90)* | ||||||
| n (%) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | ||
| Male | 2808 (52.1) | 51.6 | 303 | 10.8 (9.7–12.0) | 350 | 12.5 (11.3–13.7) | 247 | 8.8 (7.8–9.9) |
| Female | 2580 (47.9) | 56.4 | 317 | 12.3 (11.0–13.6) | 254 | 9.8 (8.7–11.1) | 131 | 5.1 (4.3–6.0) |
| Total | 5388 (100) | 53.7 | 620 | 11.5 (10.7–12.4) | 604 | 11.2 (10.4–12.1) | 378 | 7.0 (6.3–7.7) |
*ICD Code.
CI, confidence interval; UCOD, underlying cause of death; HIV, human immunodeficiency virus; TB, tuberculosis, ICD, International Classification of Disease.
Conclusions
In South Africa, implementing verbal autopsy may help identify misclassified HIV‐related deaths (22.7% vs 4.9%) and HIV and TB co‐morbidity deaths that are not otherwise reported in official statistics. If integrated into routine vital registration systems, verbal autopsies have the potential to improve cause‐of‐death statistics.
PDC0304
The burden of advanced HIV disease in a concentrated HIV epidemic: A historical analysis of the Medecins Sans Frontieres HIV cohort in Myanmar
T. Homan1, P. Thit1, H. They Mar1, M.P. Thandar1, M. Sangma1, T.T. Thwe1, A. Spina2, R. Kremer3, A. Lenglet3, A. Mesic3
1Médecins Sans Frontières, Medical, Yangon, Myanmar, 2Medecins Sans Frontieres UK, Medical, London, United Kingdom, 3Medecins Sans Frontieres Amsterdam, Medical, Amsterdam, Netherlands
Background: Globally, one‐third of those enrolled on HIV treatment, present with advanced HIV disease (AHD). AHD is associated with increased morbidity, mortality, risk of onward transmission and public health costs. Médécins Sans Frontières (MSF) has delivered 15 years of HIV care in Myanmar and has extensive experience in the treatment of AHD patients. We describe burden of AHD in MSF Myanmar cohort and the main risk factors for mortality.
Methods: We conducted a retrospective cohort analysis, using routinely collected patient‐level data. The study population included patients presenting with AHD (CD4 < =200 cells/mL and/or WHO‐stage 3/4) in MSF Myanmar cohort from 2003 to 2018). We included antiretroviral treatment (ART)‐naïve patients that initiated ART at MSF and patients returning to care after being lost‐to‐follow‐up (LTFU) from MSF HIV cohort. For both groups we compared patients with AHD to those without. We performed mortality and a risk factor analyses using Cox regression.
Results: 34,242 ART naïve patients were enrolled during the study period and 25,435 (74.3%) presented with AHD. In this group 33.9% presented with tuberculosis, 1.6% with cryptococcal meningitis, 1% with ocular cytomegalovirus infection and 0.1 % had penicilliosis at enrolment. ART naïve patients presenting with AHD at enrolment were at 3.4 higher risk of death than ART naïve patients who did not present with AHD at enrolment. The main risk factors for death were documented users of injecting drugs and sex work, while age 40 to 65 was a protective factor. There were 7811 patients who returned to care after being LTFU; 4707 (60.3%) patients presented with AHD. Those with AHD were at a 2.16 higher risk of death than those without AHD when returning to care. Sex work, male gender and tuberculosis were risk factors for death.
Conclusions: In Myanmar, there was a high proportion of advanced HIV disease among ART naïve and people returning to care after being LTFU. Patients with AHD had a higher risk of death during treatment and mortality risk factors were associated with vulnerable key populations. Our findings advocate for the introduction of AHD packages of care in models of care designed for concentrated HIV epidemics that target key populations.
PDC0305
Impact of HIV test‐and‐treat policy on the incidence of TB among HIV populations in East‐Central Uganda
R. Nyinoburyo1, A. Muhwezi2, N. Tumwesigye3
1University Research Co., LLC, TB/HIV, Jinja, Uganda, 2University Research Co., LLC, Health Systems Strengthening, Jinja, Uganda, 3University Research Co., LLC, Jinja, Uganda
Background: Early initiation of antiretroviral therapy (ART) is known to reduce the risk of Tuberculosis by up to 67%. Uganda adopted the test‐and ‐treat policy for HIV in 2016 as a strategy for early ART initiation. However, the impact of this policy on TB incidence in programme settings has not been studied in the Ugandan setting. This study aimed to determine the incidence of TB in the test‐and‐treat era compared to the pre‐test‐and‐treat era.
Methods: A cross‐sectional analysis of retrospective data of adult (>15 years) HIV/AIDS patients receiving ART at a large hospital in East‐Central Uganda between 01.01.2005 and 31.12.2018. Data on TB status and the year of starting ART were collected. Patients who had TB before starting ART were excluded. Year of starting ART was categorized into; era1‐before 2009 (ART eligibility; CD4 ≤ 350 cells/µL), era2‐2009 to 2015 (ART eligibility; CD4 ≤ 500 cells/µL) and the test‐and‐treat era (ART start irrespective of CD4 count). Incident TB was calculated for each of the 3 eras. Odds ratios were determined for association of ART start era and TB incidence.
Results: 3941 patients were enrolled in this study; 70% were female, the median age was 38 years (IQR 29 to 46). 648 patients started ART in era1, 2247 started in era2 while 1046 started in the test‐and‐treat era. A total of 383 participants developed TB while receiving ART; 242 (63%) male and 141 (37%) female. Sixteen 16%(104/648) of era1 ART patients developed TB compared to 9.5%(213/2247) in era2 and 6.3% (66/1046) in the test‐and‐treat era. Chi‐square = 44, p < 0.0001 showing a significant association between TB incidence and era of starting ART. The odds of TB during era1 were 2.5 times those of the test‐and‐treat era (95% CI; 1.6 to 3.9), while the odds of TB in era2 were 1.6 times those of the test‐and‐treat era (95% CI; 1.02 to 2.24).
Conclusions: Tuberculosis is still incident in the test‐and‐treat era for HIV. However, the incidence has decreased with subsequent eras of early ART initiation and was lowest in the era of test‐and‐treat HIV policy. The test and treat policy has had a positive impact on reducing the incidence of TB among PLHIV in programme settings in Uganda.
PDC0306
Reducing TB/HIV co‐infections: Trends in TPT and HIV testing in PEPFAR‐supported countries in Africa
T. Al‐Samarrai1, E. Wong1, M. Peterson2, C. Nichols3, P. Vinayak1, S. Cavanaugh1
1US Department of State, Office of the Global AIDS Coordinator, Washington, United States, 2Centers for Disease Control and Prevention, Division of Global HIV and Tuberculosis, Atlanta, United States, 3US Agency for International Development, Washington, United States
Background: Tuberculosis (TB) and HIV co‐infection continues to be a major global health concern. TB is the leading cause of death among people living with HIV (PLHIV), while HIV also greatly increases the risk of latent TB advancing to active disease. The President's Emergency Program for AIDS Relief (PEPFAR) is committed to reducing TB/HIV co‐infections, setting ambitious targets to treat all PLHIV with TB preventive therapy (TPT) by 2021. In PEPFAR supported countries, HIV testing among presumptive or confirmed TB patients is also critical for HIV case finding and linkage to treatment.
Methods: We analysed programmatic data from PEPFAR‐supported countries in Africa. We conducted a descriptive analysis of TPT completion data from October 2016 to September 2019 of 18 African countries; one country reported less than 150 completions overall and was excluded from further analysis. To assess HIV testing among confirmed and presumptive TB patients (TB patients), we analysed data reported from 21 African countries between October 2017 and September 2019.
Results: Between fiscal years FY17‐FY19, 4459,397 PLHIV on ART were initiated and 2947,724 (66%) completed TPT in 17 PEPFAR‐supported African countries. TPT completion rates ranged from 0 to 101% (average country completion 50%) in FY17, 14 to 89% in FY18 (average 62%), and 29 to 91% in FY19 (average 70%). In FY17, 4/17 countries achieved completion rates of ≥ 70% while in FY19, 10/17 countries achieved completion rates ≥ 70%. In Kenya and Tanzania > 90% of PLHIV on ART have completed a course of TPT. In FY2019, 93% of TB patients had documented HIV tests across 21 African PEPFAR countries (range: 70 to 99%). Five of 21 (24%) countries analysed had HIV testing coverage < 90% in 9/21 (42%) countries. Of those tested, an average 35% of TB patients were HIV positive across countries (range: 10 to 81%), with 9% (range: 2.6 to 18.6%) of those tested newly identified as HIV positive.
Conclusions: These results indicate high frequencies of TB/HIV co‐infection persist in these countries. While HIV testing among suspected and confirmed TB patients should be routine in PEPFAR countries, there are persistent gaps which need to be addressed, particularly for children < 15 years of age. TPT for PLHIV should be scaled up across all PEPFAR‐supported countries yet there continues to be significant variation in coverage. Further analyses should be performed to characterize context specific reasons for poor TPT completion and HIV testing among TB patients. Number of TPT Completions Over Time and Overall Percent Completion in African PEPFAR‐Supported Countries, FY17‐FY2019.
Abstract PDC0306‐Figure 1.
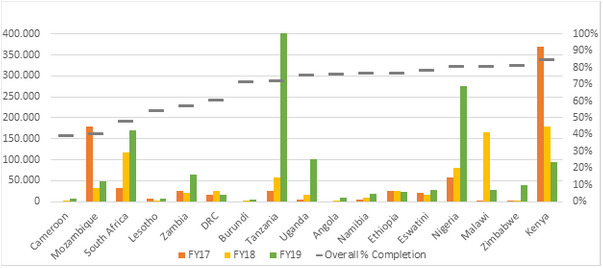
Abstract PDC0306‐Figure 2. Testing of TB Patients for HIV by Age in African PEPFAR‐Supported Countries, FY2019.
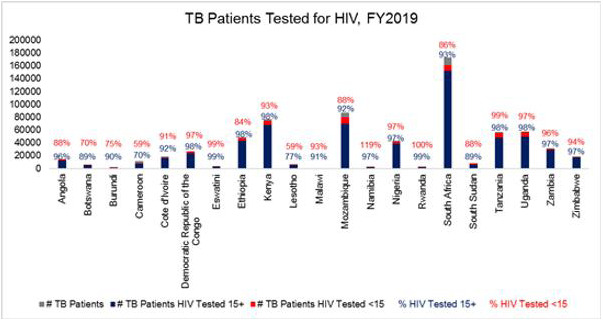
PDC0402
Preventing HIV and achieving pregnancy among HIV‐discordant couples using safer conception strategies in Zimbabwe
J. Brown1,2,3, S. Gitome4, B. Mateveke5, T. Chirenda5, G. Chareka5, C. Chasakara5, C. Murombedzi5, A. Matubu5, N. Mgodi5, P. Musara5, T. Makurumure6, C. Smith‐Hughes3, Z.M. Chirenje2,5, F. Mhlanga5, SAFER Study Group
1University of California, Epidemiology and Biostatistics, San Francisco, United States, 2University of California, Obstetrics, Gynecology, and Reproductive Sciences, San Francisco, United States, 3University of California, Global Health Sciences, San Francisco, United States, 4Kenya Medical Research Institute, Centre for Microbiology Research, Nairobi, Kenya, 5University of Zimbabwe, Obstetrics and Gynecology, Clinical Trials Research Centre, Harare, Zimbabwe, 6Mercy‐Care Fertility Centre, Harare, Zimbabwe
Background: Safer conception strategies are needed to minimize HIV transmission risk among HIV‐discordant couples desiring pregnancy. Few studies have evaluated the use and effectiveness of safer conception strategies among HIV‐discordant couples. We measured the uptake and clinical outcomes of four safer conception strategies among discordant couples in Zimbabwe planning to get pregnant.
Methods: We enrolled HIV‐discordant couples desiring conception into a prospective, non‐randomized pilot study. Couples were given a choice of one or more safer conception strategies: antiretroviral therapy with viral load monitoring (ART/VL), oral pre‐exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate/emtricitabine, home‐based vaginal insemination (VI) for couples with an HIV‐positive female, and semen washing (SW) for couples with an HIV‐positive male. Couples were taught to identify the fertile period and counselled to always use condoms, except for those using ART/VL or PrEP, who had condomless sex during the fertile period. Participants were followed monthly for up to 12 months of pregnancy attempts, quarterly during pregnancy, and 12 weeks post‐delivery. At each visit self‐reported data on strategy use, urine for pregnancy testing, and blood for HIV antibody testing, or viral load if HIV positive were obtained. Newborns from HIV‐positive females were tested for HIV using DNA PCR at 6 and 12 weeks.
Results: Twenty‐three discordant couples were followed from April 2017 to June 2019 with no loss‐to‐follow‐up. Twelve couples had an HIV‐positive female partner. Median age was 31 years for females, 34 years for males. At enrolment, all couples chose ART/VL, and all couples chose at least one additional strategy: (PrEP [n = 17/23;74%; 8/17 were female]), VI (n = 3/12;25%), SW (n = 4/11;36%). During follow‐up, three couples switched from ART/VL+SW to ART/VL+PrEP, and one from ART/VL+PrEP+VI to ART/VL+PrEP. One female discontinued PrEP due to an adverse reaction. Half (n = 12/23;52%) of the couples achieved pregnancy, with 10 pregnancies reaching term. All participants were virally suppressed prior to pregnancy attempts, and two participants (9%) had detectable viral load during follow‐up. There were no cases of horizontal or vertical transmission.
Conclusions: All four safer conception strategies appear safe and effective. When offered a choice, discordant couples desiring pregnancy seek a combination of HIV prevention strategies, with ART/VL plus PrEP being the most frequently selected.
PDC0403
HIV and syphilis prevalence and sexual risk‐taking behaviours among in‐ and out‐of‐school adolescent girls and young women in Uganda: Results from a national survey
J.K. Matovu1,2, J. Bukenya3, L. Mugenyi3, A. Nyabigambo3, D. Lubogo3, R. Kisa3, S. Kisaka3, D. Kasozi3, D. Serwadda3, I. Murungi4, R.K. Wanyenze3
1Makerere University School of Public Health, Disease Control and Environmental Health, Kampala, Uganda, 2Busitema University Faculty of Health Sciences, Community and Public Health, Mbale, Uganda, 3Makerere University School of Public Health, Kampala, Uganda, 4The AIDS Support Organization, Kampala, Uganda
Background: Studies suggest that in‐school girls may be at a less risk of HIV infection than their out‐of‐school counterparts. However, few studies have examined HIV and syphilis prevalence or sexual risk‐taking behaviours of in‐ and out‐of‐school AGYW as part of the same study. To address this gap, we assessed sexual risk‐behaviours and HIV and syphilis prevalence among in‐ and out‐of‐school adolescent girls and young women (AGYW) aged 10 to 24 years to inform the design of age‐appropriate HIV prevention interventions.
Methods: This was a cross‐sectional study conducted in 233 villages and 80 schools in 20 districts between July and August 2018. Districts were selected from 10 geographically demarcated regions based on background HIV prevalence and presence/absence of HIV interventions. We collected data on socio‐demographic, sexual, health and behavioural characteristics and diagnosed HIV and syphilis using rapid diagnostic test kits. Data were entered into EpiData (version 3.1) and analysed using STATA (version 14.1).
Results: Of 8236 (97.2%) AGYW enrolled into the study, 50.3% (n = 4139) were in‐school. In‐school AGYW were significantly less likely to have ever had sex (35.2% vs. 73.1%, Risk Ratio [RR]=0.48; 95% Confidence Interval [95% CI]: 0.46, 0.50); or to have initiated sex before age 15 (17.5% vs. 30.3%, RR = 0.58; 95% CI: 0.51, 0.65). In‐school AGYW were significantly more likely to report that they used a condom or other contraceptive methods to prevent pregnancy at first sex (65.1% vs. 41.1%, RR = 1.58; 95% CI: 1.50, 1.68) and to report that they used a condom at last sex (55.3% vs. 20.7%, RR = 2.67; 95% CI: 2.46, 2.91) than their out‐of‐school counterparts. Overall, 1.0% (n = 106) had HIV while 1.2% (n = 105) had syphilis. HIV and syphilis prevalence increased with age, and were higher among out‐of‐school than in‐school AGYW (HIV prevalence: 1.6% vs. 0.6%; syphilis prevalence: 1.9% vs. 0.6%).
Conclusions: We found low overall HIV and syphilis prevalence among AGYW. However, both HIV and syphilis prevalence were higher among out‐of‐school than in‐school AGYW, possibly due to the very high‐risk behaviours reported by out‐of‐school AGYW compared to their in‐school counterparts. Targeted risk reduction programmes including interventions aimed at keeping girls in school may help to tame the HIV tide among AGYW in Uganda.
PDC0404
Are HIV and syphilis syndemic in pregnant women in Brazil? Hot‐spot analysis of the two epidemics
M.C. Cambou1, E. Saad2, K. McBride3, K. Nielsen‐Saines2
1UCLA, Infectious Diseases, Los Angeles, United States, 2UCLA David Geffen School of Medicine, Pediatric Infectious Diseases, Los Angeles, United States, 3UCLA Fielding School of Public Health, Health Policy and Management, Los Angeles, United States
Background: Despite enhanced public health efforts to eradicate HIV and syphilis mother‐to‐child transmission (MTCT), rates of congenital syphilis have quadrupled in Brazil in the past decade. Geographic information system (GIS) and hot‐spot analysis, often underutilized research techniques, may provide better understandings of epidemic patterns.
Methods: Aggregate data provided by the Brazilian Ministry of Health/SINAN for all pregnant women diagnosed with HIV or syphilis between January 1, 2010 and December 31, 2018 was analysed. ArcGIS software was used to map the annual incidence of HIV and syphilis diagnosed in pregnancy by state. Hot‐spot analysis was performed to identify state‐specific clusters.
Results: From 2010 to 2018, 66,632 pregnant women were diagnosed with HIV, 271,209 were diagnosed with syphilis, and 150,414 infants were diagnosed with congenital syphilis. While the annual, national incidence of HIV diagnosis in pregnancy remained stable, syphilis incidence increased six‐fold, from 3.5 per 1000 live births in 2010, to 21.4 per 1000 live births in 2018 (r = 0.97). Rio Grande do Sul had the highest incidence of HIV in 2018 (9.2 per 1000 live births), and hot‐spots of HIV incidence were identified in three Southern states (p < 0.01). The incidence of syphilis was significantly higher than HIV, and there was little overlap between HIV and syphilis incidence by state (r = 0.25). While syphilis incidence exceeded 30 per 1000 live births in 2018 in Acre, Mato Grosso do Sul, Rio de Janeiro, and Espirito Santo, only the last two states in Southeastern Brazil were spatial clusters in hot‐spot analysis.
Abstract PDC0404‐Figure 1.
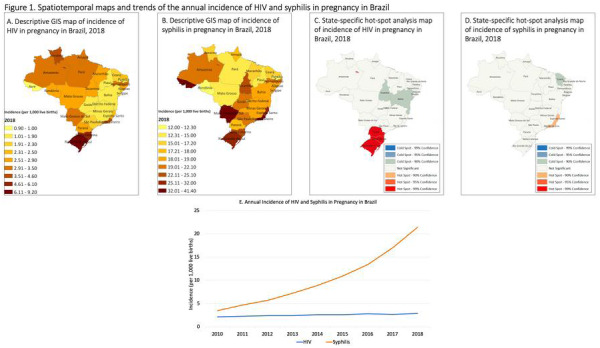
Conclusions: HIV and syphilis epidemics in Brazil are not syndemic in pregnant women. There is a spatial cluster of HIV in the South, while syphilis is increasing throughout the country, particularly in the Southeastern coast. Combating HIV hot‐spots alone is not sufficient to curtail syphilis MTCT. Monitoring geographic variation allows for improved targeted efforts.
PDC0405
HIV, STIs and pregnancy among women of reproductive age in a Lake Victoria fishing community: A population‐based study
A.D. Peer1, J. Kagaayi2, R. Ssekubugu2, J. Jackson1, G. Kigozi2, S. Kalibala2, R.H. Gray3, M.J. Wawer3, J. Mpagazi2, S. Kiboneka2, S.J. Reynolds4, A.A. Tobian1, C.A. Gaydos1, T.C. Quinn4, M.K. Grabowski1
1Johns Hopkins University School of Medicine, Baltimore, United States, 2Rakai Health Sciences Program, Kalisizo, Uganda, 3Johns Hopkins University Bloomberg School of Public Health, Baltimore, United States, 4National Institute for Allergy and Infectious Diseases, Laboratory of Immunoregulation, Division of Intramural Research, Bethesda, United States
Background: Despite sequelae including miscarriage and neonatal death, population‐based data on sexually transmitted infections (STI) during pregnancy are limited in sub‐Saharan Africa. The prevalence of HIV and four curable STIs (Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), Trichomonas vaginalis (TV), and Treponema pallidum (syphilis)) were measured among women in a Lake Victoria fishing community in southern Uganda.
Methods: We compared population‐level prevalence of NG, CT, TV, and syphilis among pregnant and non‐pregnant sexually active women of childbearing age (15 to 49) who participated in the Rakai Community Cohort Study May‐July 2019. CT and NG testing were conducted by nucleic acid amplification testing (Abbott RealTime CT/NG m2000). Point‐of‐care testing was performed for TV (OSOM Trichomonas) and syphilis (Anti‐TP SDBioline syphilis 3.0), with confirmatory rapid plasma reagin (RPR) titres (Cypress Diagnostics). RPR titres ≥ 1:8 were classified as active syphilis. All participants received treatment when indicated. Associations between STIs, pregnancy, and HIV status were assessed with multivariable modified Poisson regression and reported as age adjusted prevalence risk ratios (adjPRR) with 95% confidence intervals (CI).
Results: 432 women met inclusion criteria, with 11% (n = 47) pregnant. Among pregnant women, HIV prevalence was 32% (n = 15), NG 13% (n = 6), CT 13% (n = 6), and TV 26% (n = 12). Syphilis reactivity was 17% (n = 8), and 6.4% (n = 3) had titres indicative of active infection. Prevalence of ≥ 1 active STI infection (NG, CT, TV, or active syphilis) was 34% (n = 16). Among non‐pregnant women (n = 385), HIV prevalence was 49% (n = 189), NG 9.6% (n = 37), CT 10% (n = 40), and TV 18% (n = 68). Syphilis reactivity was 27% (n = 108), and 9.6% (n = 37) had active infection. The age‐adjusted relative risks of active STI in pregnant versus non‐pregnant and HIV‐positive versus HIV‐negative women were 1.31 (95% CI: 0.74 to 2.12) and 1.64 (95% CI: 1.12 to 2.40), respectively. Pregnant women with HIV were 62% more likely to have ≥ 1 STIs compared to pregnant women without HIV (adjPRR = 1.62; 95% CI: 1.11 to 2.39).
Conclusions: These data highlight the very high burden of STIs among women in Lake Victoria fishing communities, particularly among pregnant women. There is an urgent need for effective integrated STI screening and treatment in antenatal care and HIV treatment programmes in this population.
PDC0406
Sexual partner types of adolescent girls and young women identified from latent class analysis (LCA) and incident HIV‐infection in Rakai, Uganda
N. Nguyen1, R. Tsong2, T. Lutalo3, A. Zhang2, Y. Wei2, I. Chen2, J. Li2, F. Nalugoda3, C. Kennedy4, M.K. Grabowski5,6, J. Santelli7, S. Hoffman1
1HIV Center for Clinical and Behavioral Studies at Columbia University and New York State Psychiatric Institute, New York, United States, 2Columbia University Mailman School of Public Health, Department of Biostatistics, New York, United States, 3Rakai Health Sciences Program, Kalisizo, Uganda, 4Johns Hopkins Bloomberg School of Public Health, Department of International Health, Baltimore, United States, 5Johns Hopkins School of Medicine, Baltimore, United States, 6Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology, Baltimore, United States, 7Heilbrunn Department of Population and Family Health, Columbia University Mailman School of Public Health, New York, United States
Background: Sexual partners play a critical role in HIV acquisition among adolescent girls and young women (AGYW). We identified sexual partner types and associations with incident HIV infection among AGYW ages 15 to 24 during years 2005 to 2013 of the Rakai Community Cohort Study.
Methods: At each survey round, AGYW reported the following partner (age, concurrency, likelihood of HIV‐infection, high‐risk occupation) and partnership characteristics (cohabitation, condom use, and alcohol before sex with AGYW) and offered HIV testing. Characteristics were used to identify sexual partner types using Latent Class Analysis (LCA). Among HIV‐negative AGYW, we estimated incident rate ratios (IRR) and 95% confidence intervals (CI) for each LCA‐identified partner type and incident HIV infection using a Poisson GEE model, controlling for other partner types, sexual behaviour and demographic risk factors, and repeated observations.
Results: In total 7742 AGYW reported 12,649 sexual partners. Of those AGYW, 2691 were eligible for the HIV‐incidence analysis, contributing 7262 total person‐years of follow‐up. Overall, 90 AGYW became newly HIV infected, for an incidence rate of 12.38 per 1000 person‐years (95% CI: 6.56, 23.37). We identified four sexual partner types (Figure). Compared to the reference group (AGYW with ‘somewhat older cohabiting’ partners), AGYW with ‘somewhat older non‐cohabiting’ partners had 4.21 times the rate of HIV infection (95% CI: 2.34, 7.59), while AGYW with ‘similar‐age non‐cohabiting’ partners had 3.83 times the rate (95% CI: 1.90, 7.73), and AGYW with ‘much older cohabiting’ partners had 1.62 times the rate (95% CI: 0.94, 2.82).
Abstract PDC0406‐Figure 1.
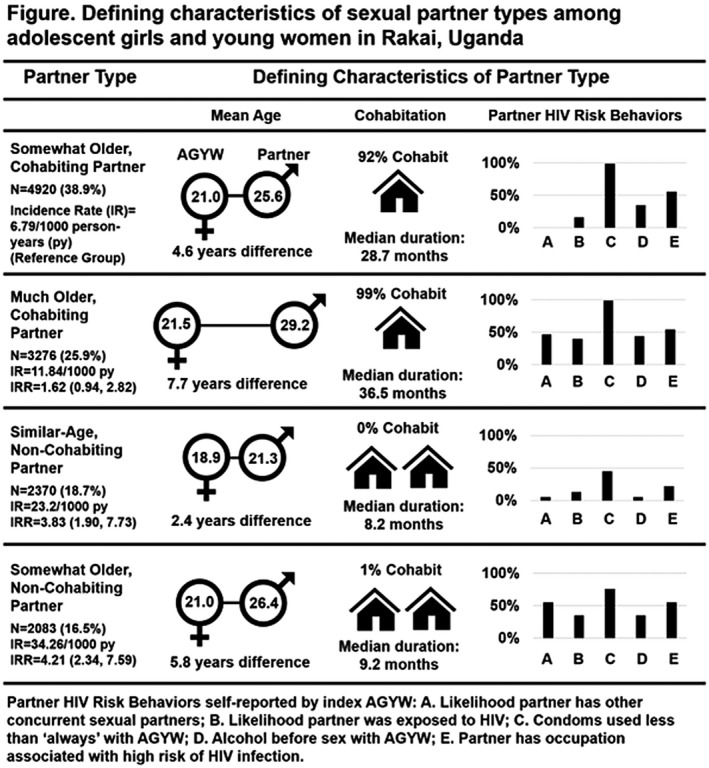
Conclusions: Partner types derived from LCA were strongly associated with incident HIV‐infection among AGYW, though some partner types were associated with lower than expected HIV‐infection rates given reported risk behaviours, while others were higher. These findings highlight the importance of examining partner characteristics together in the context of sexual partnerships to understand HIV risk in this vulnerable population.
PDC0407
Characteristics of older male partners of adolescent girls and young women (AGYW) in four Eastern and Southern African countries, PHIA 2015 to 2017
S. Hoffman1,2, J. Mantell2, C. Wang3, I. Mushamiri1, A. Low1,3
1Mailman School of Public Health, Columbia University, Epidemiology, New York, United States, 2HIV Center for Clinical and Behavioral Studies at Columbia University and New York State Psychiatric Institute, Psychiatry, New York, United States, 3Mailman School of Public Health, Columbia University, ICAP, New York, United States
Background: Accumulating evidence indicates that sexual partnerships between older men and adolescent girls and young women (AGYW aged 15 to 24) contribute to ongoing high HIV incidence among AGYW in Eastern and Southern Africa. However, little is known about characteristics of older men who partner with AGYW.
Methods: Participants were from Population‐based HIV Impact Assessment (PHIA) household surveys in Eswatini, Tanzania, Zambia, Malawi. Past‐year sexually active men aged 25 to 59 with known age of their three most recent female partners were included. HIV status was obtained via rapid test; HIV‐1 RNA with real‐time PCR. We constructed separate logistic regression models by male partner age‐group (25 to 34 years, 35 to 44 years, 45 to 59 years) adjusted by continuous age to compare characteristics of men partnering with AGYW versus those partnering only with same age‐group or older women. Analyses were adjusted for survey design; Taylor Series method was used for variance estimation. We tested for interactions by country in a pooled model.
Results: Of 17,110 sexually active men, 32.3% reported ≥1 past‐year AGYW partner: this was highest among men aged 25 to 34 (58.5%). HIV prevalence was highest in those over age 45 (12.0%). Viral load suppression among those HIV positive increased with age. Men who were not married/living together, had > 1 partner, and bought/sold sex in the past year had higher odds of partnering with an AGYW. HIV‐positive men did not have higher odds of partnering with an AGYW (OR = 0.81; 95% CI: 0.67 to 0.99), but among HIV‐positive men, virally unsuppressed men had higher odds of doing so than those virally suppressed (OR = 1.89; 95% CI: 1.42 to 2.52) (Table 1). Patterns were consistent across age‐group, but heterogeneity by country was observed (not shown).
Abstract PDC0407‐Table 1.
| Characteristic associated with odds of partnering with AGYW | All men aged 25 to 59 OR (95% CI) | Men aged 25 to 34 OR (95% CI) | Men aged 35 to 44 OR (95% CI) | Men aged 45 to 59 OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Marital status (reference = married/living together) | ||||||||
| Never married | 2.15 | (1.72 to 2.7) | 1.67 | (1.31 to 2.13) | 3.49 | (2.1 to 5.79) | 0.72 | (0.21 to 2.43) |
| Widowed | 1.94 | (1.47 to 2.57) | 1.62 | (1.18 to 2.21) | 2.55 | (1.68 to 3.88) | 2.53 | (1.27 to 5.05) |
| Divorced or separated | 3.41 | (1.62 to 7.2) | 2.58 | (0.46 to 14.54) | 2.73 | (1.03 to 7.24) | 2.33 | (0.7 to 7.78) |
| Number of past‐year partners (> 2 vs. 1) | 4.19 | (2.19 to 3.42) | 2.22 | (1.62 to 3.05) | 4.06 | (2.91 to 5.66) | 3.38 | (1.78 to 6.41) |
| Bought/sold sex, past 12 months (yes vs. no) | 2.74 | (2.19 to 3.42) | 2.22 | (1.62 to 3.05) | 4.06 | (2.91 to 5.66) | 3.38 | (1.78 to 6.41) |
| HIV Status (positive vs. negative) | 0.81 | (0.67 to 0.99) | 1.01 | (0.74 to 1.37) | 0.76 | (0.54 to 1.09) | 0.58 | (0.32 to 1.05) |
| Virally unsuppressed, among HIV+ (reference = virally suppressed) | 1.89 | (1.42 to 2.52) | 2.3 | (1.58 to 3.35) | 1.31 | (0.59 to 2.93) | 1.31 | (0.59 to 2.93) |
Conclusions: Older partners may present an HIV‐risk to AGYW not only because they have higher HIV prevalence, but also because those who partner with AGYW (versus those who do not) engage in more risk behaviours and are more likely to be virally unsuppressed.
PDC0408
Higher Colon Tissue Infectivity in HIV Seronegative Cisgender Women compared to Cisgender Men on Candidate Oral Antiretroviral (ARV) Pre‐Exposure Prophylaxis (PrEP) Regimens in HPTN 069
R. Sekabira1, K. Yuhas2, I. McGowan3, R. Metter Brand3, M. Marzinke4, K. Mayer5, R. J. Landovitz6, T. Wilkin7, K.R. Amico8, Y. Manabe4, I. Frank9, A. R. Kekitiinwa10, R. M. Gulick7, C. W. Hendrix4
1Baylor, Research Pharmacy, Kampala, Uganda, 2Fred Hutchinson Cancer Research Center, Seattle, United States, 3University of Pittsburgh, Medical School, Pittsburgh, United States, 4Johns Hopkins University School of Medicine, Baltimore, United States, 5Fenway Health/Harvard Medical School, Boston, United States, 6University of California, Center for Clinical AIDS Research and Education, Los Angeles, United States, 7Weill Cornell Medicine, New York, United States, 8University of Michigan, School of Public Health, Ann Arbor, United States, 9University of Pennsylvania, Infectious Disease Division, Philadelphia, United States, 10Baylor, Kampala, Uganda
Background: HPTN 069 randomized HIV‐negative men and women to one of four candidate daily oral PrEP regimens for 48 weeks: tenofovir disoproxil fumarate (TDF) + emtricitabine (FTC), maraviroc (MVC) only, MVC+FTC, and MVC+TDF. In this tissue substudy, we compare susceptibility of colon tissue to HIV infection ex vivo between men and women.
Methods: Plasma, peripheral blood mononuclear cells (PBMC), and colon tissue were collected for drug concentrations. Colon biopsy “explants” were challenged with HIV ex vivo followed by tissue culture supernatant collection over two weeks for p24 antigen measurement; results were summarized as cumulative biopsy‐weight adjusted p24 (Cum p24 pg/mL/mg). Assessments were made at baseline (no drug), week 24 and 48 (on study drugs), and week 49 (one week after the last dose). Comparisons used Wilcoxon with exact significance.
Results: This substudy included 12 women and 59 men. Compared to men, women's median week 24 and 48 colon tissue MVC concentrations were 42% lower (p = 0.08) and 57% lower for FTC (p = 0.004), but higher for TFV diphosphate (p = 0.002). Blood ARV concentrations and recent daily adherence (90% overall based on PBMC drug concentration benchmarks) did not differ by sex. Women had higher explant p24 expression at all visits (Figure) compared to men, which ranged from 2‐fold (p = 0.046) to 16‐fold (p = 0.016). Two‐fold male‐female differences existed before any drugs were taken and were largest (10‐ to 16‐fold) when participants were taking the drug daily (week 24 and 48). The male‐female p24 differences were not statistically significant in the MVC only arm (minimal, variable p24 suppression) or the TDF+FTC arm (near maximal p24 suppression).
Abstract PDC0408‐Figrue 1.
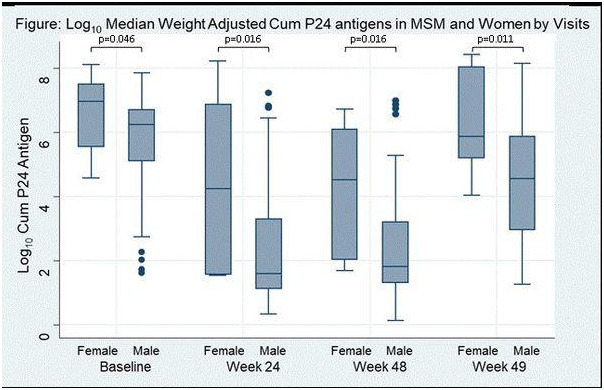
Conclusions: Colon explants from women have higher HIV replication after ex vivo HIV challenge compared to men ‐ with and without the PrEP study drugs. This is not due to adherence differences. Male‐female differences in tissue concentrations may partly provide an explanation.
PDD0102
Savings and loans groups as a potential community tool for viral load suppression in resource‐limited settings: An intervention study from Mozambique
M. Lisboa1, S. Culuze2, A. Cambule2, S. Greenberg1
1United States Agency for International Development (USAID), Integrated Health Office, Maputo, Mozambique, 2World Vision Mozambique, Health and Wellbeing ‐ SCIP Ogumaniha, USAID Funded Project, Quelimane, Mozambique
Background: Village savings and loans groups (VSLGs) are a sustainable community‐based intervention, comprised of HIV‐positive and ‐negative participants, that improve resilience at the household and community levels. Evidence supports that VSLG members report heightened awareness levels of health problems and healthy behaviours as well as enhanced social cohesion, community support systems, and solidarity. We aimed to assess the impact of VSLG membership on continuous retention to HIV treatment and viral load suppression (VLS) among HIV‐positive members over a 12‐month period.
Methods: A randomized, two‐arm, unblinded, non‐inferiority design with 12‐months follow‐up (from January to December 2018) of newly enrolled HIV treatment and previously lost to follow‐up clients was conducted in Zambézia, Mozambique. The control arm received a standard facility‐based package of HIV care while the intervention arm received the same package coupled with VSLG participation. In addition to financial activities, VSLG meetings included facilitated sessions on various health‐related topics, including HIV‐related stigma and discrimination, social support and solidarity, and HIV treatment retention and VLS. Patient‐level assessments of continuous retention over a 12‐month period and VLS were performed. Associations between VSLG status and retention and VLS were calculated using logistic regression.
Results: Among 677 patients included in the study, 47% (321) were in the intervention arm, from which 57% were female and 49% were aged 25 to 34 years. Within the analysed 12‐month period, 93% of VSLG members had not experienced any treatment interruptions as compared to 47% in the control arm. VSLG members were far more likely than the control group to have a documented viral load test (78% vs. 40%) and to experience VLS (94% vs. 55%). Patients in the intervention arm were nearly six times more likely to achieve VLS (aOR = 5.6; 95% CI: 2.6 to 11.8) when compared to the control arm, adjusting for socio‐demographic and clinical factors.
Conclusions: These results suggest that promoting VSLGs among people living with HIV may be an effective way to reaching HIV epidemic control. Scaling‐up VSLGs should be considered not only as a sustainable community tool for economic and social support strengthening, but also as a platform for better communication for improved HIV outcomes and stigma and discrimination reduction.
PDD0103
#MenOfPrEP: Engagement of online community influencers and the use of sex‐positive messaging in creating awareness of HIV pre‐exposure prophylaxis among men who have sex with men in the Philippines
J.D. Rosadiño1, P.V. Junio1, J.O. Corciega1, R. Pagtakhan1, C. Lagman1
1LoveYourself, Inc., Manila, Philippines
Background: The WHO recommended the use of antiretroviral pre‐exposure prophylaxis (PrEP) in addition to other prevention methods may be an option to halt and reverse this epidemic in the Philippines. However, PrEP awareness is low among Filipino MSMs. The project aims to bring PrEP awareness to MSMs in the Philippines by engaging online community influencers and using sex‐positive messaging.
Description: A communications plan was designed to determine the tone and feel of the campaign, together with its accompanying key messages. The messages focused on PrEP information, access, and its impact on the lifestyle of prospective clients. The visual theme and the key messages developed are sex‐positive, with a motivational tone of espousing self‐empowerment. The developed plan was then cascaded to people who have the following qualifications: a known member of the MSM community and has an established online presence. The following were then shared with the influencer as part of the campaign development: A PrEP 101 briefer, the photoshoot, and an “influencer package” containing FAQs should they receive inquiries regarding PrEP on their personal accounts. After the campaign development, the materials (together with its appropriate captions/key messages) were then posted on LoveYourself's social media channels (Facebook, Twitter, and Instagram), and shared by the influencers. All posts contain the registration link for PrEP access in LoveYourself community centres and its affiliates.
Lessons learned: A total of 20 MSM community influencers agreed to participate in the campaign, even without monetary compensation. Each post has received unique engagements ranging from 8000 to 30,000. These numbers are organic, and no advertising/boosting funds were spent. The sign‐up link for PrEP registration was accessed 25,000 times. Inquiries regarding PrEP in LoveYourself's social media channels increased by 2000%. The campaign has helped increase the number of PrEP enrolees under LoveYourself's care from 50 to 750 in 4 months.
Conclusions/Next steps: It was seen that a sex‐positive campaign powered by the community is effective in bringing awareness of PrEP. Population‐specific variants of the campaign (#WomenOfPrEP for cisgender and transgender women, for instance) are recommended in order to create PrEP campaigns that are targeted and diverse.
PDD0104
Employing syndemic theory in pre‐exposure prophylaxis (PrEP) access among transgender women of colour in South Florida
S. Kiplagat1, Y. Mariano1, J. Dévieux1, M. Jean‐Gilles1, S. Johnson1, M.J. Trepka1, M. Padilla1, D. Stephens1, A. Lint2, Y. Ocon3, F. Duberli3, C. Lewis4, E. Cyrus1
1Florida International University, Miami, United States, 2Arianna's Center, Miami, United States, 3Survivor's Pathway, Miami, United States, 4Empower U Community Center, Miami, United States
Background: Although transgender women of colour (TWOC) are disproportionately at high risk of acquiring HIV, they remain underrepresented in HIV screening and engagement in HIV prevention including pre‐exposure prophylaxis (PrEP). Syndemics of poverty, substance use, violence and mental health further restrict engagement in HIV prevention. Therefore, the objective of this study was to employ a syndemic theoretical framework to identify barriers and facilitators of PrEP among TWOC in South Florida.
Methods: In September‐January 2020, eight in‐depth interviews and two focus groups were conducted among transgender adult women living in Miami‐Dade and Broward counties in South Florida. Participants were recruited through convenience sampling, active recruitment, print advertisements and emails to transgender organizations listservs. Content analysis approaches were developed for coding categories and themes. The codes were developed independently using NVivo before comparison, discussion, and differences were reconciled to identify and analyse themes.
Results: The mean age of participants was 42.2 years old; 82.4% were transgender Latinas and 17.6% were African American. Discrimination and stigma by providers and the wider society, and limited economic opportunities were identified as primary barriers to accessing HIV screening and PrEP healthcare services. Lack of employment opportunities, due to prejudice, stigma and discrimination was a recurring theme. As a result, many participants were vulnerable to economic insecurity. The high cost of PrEP medication as well as stigma and discrimination by healthcare providers limiting engagement in HIV prevention also emerged as a theme. The participants also noted that under‐representation of transgender women in clinical trials has led to inadequate data on side effects including dermatological side effects forcing them to discontinue PrEP. Emerging themes also included physical violence, psychological violence and sexual abuse from peers, partners, and systems from early childhood. Effects of violence were compounded by the syndemics of poverty, substance misuse, mental health issues, and engagement in transactional sex work. Consequently, HIV screening and engagement in HIV prevention is often a low health priority among TWOC.
Conclusions: Economic constraints, stigma, discrimination, and violence are associated with systematic marginalization of TWOC in their local communities, inhibiting HIV screening use of PrEP. The development of tailored interventions should consider these syndemic factors.
PDD0105
Community influencers are key to HIV prevention for Latino homeless populations: A strategy for getting to zero among immigrants
J. Zepeda1, R. Melendez2, M.G. Alaniz1
1San Francisco AIDS Foundation, San Francisco, United States, 2San Francisco State University, Sociology and Sexuality Studies, San Francisco, United States
Background: In San Francisco, 20% of new HIV diagnoses in 2018 were among people without housing, with the proportion growing in recent years. Nearly a quarter (24%) of HIV cases from 2009 to 2018 were Latinx. To reduce HIV transmission in San Francisco and “Get to Zero”, we must invest in developing effective ways to reach these populations, since they may not be aware of or may be distrustful of available services. Instead of relying on individuals to access services, community‐based recruitment strategies may successfully bring services to individuals.
Description: In 2018, San Francisco AIDS Foundation's Latino Programs piloted the Todos Somos Familiaproject with the support of a Kaiser Permanente Community Benefit Grant. The project trained 16 formerly and currently homeless Latino immigrants as community HIV influencers, or promotores de salud, to share information about navigating drug use programmes and mental health services, overdose prevention, applying for housing, and accessing legal assistance and HIV services. During outreach, influencers also shared HIV prevention information including how to access HIV testing, advantages of routine sexual health screenings, and the benefits of PrEP, PEP, and condoms. Influencers were each paid $494; clients receiving case management were provided $120 for completing 8 contacts with influencers. Over 6 months, 16 influencers made 376 health education contacts with Latino homeless individuals; 175 of those individuals made a second contact with project staff and HIV screening referrals. Of those 175 who made a second contact with SFAF: 25 accessed case management, 19 accessed legal services, 7 entered methamphetamine treatment, 10 entered psychiatric treatment, 2 re‐connected with HIV care, 2 clients were diagnosed with cancer and sought treatment, 8 took a PrEP orientation class, and 1 received an overdose reversal.
Lessons learned: Paying formerly and currently homeless immigrant individuals to reach their communities with health information and services is an effective way to share HIV and health services. Formerly and currently homeless Latino individuals successfully engage with other Latino homeless individuals organically and are social influencers‐‐securing survival for themselves and communicating skills to others.
Conclusions/Next steps: SFAF, in collaboration with other Latino organizations, seeks to replicate and expand the project.
PDD0106
The Cedar Project: Intergenerational child apprehension and HIV health and wellness among young Indigenous people who have used drugs in two Canadian cities ‐ a mixed methods study
K. Jongbloed1, S. Pooyak2, M.E. Pearce1, A. Mazzuca1, R. Sharma1, W.M. Christian3, M. Teegee4, L. Demerais5, R.T. Lester1, M.T. Schechter1, P.M. Spittal1, For the Cedar Project Partnership
1University of British Columbia, Vancouver, Canada, 2Aboriginal HIV/AIDS Community‐Based Research Collaborative Centre (AHA Centre), Victoria, Canada, 3Splatsin te Secwepemc, Enderby, Canada, 4Carrier Sekani Family Services, Prince George, Canada, 5The Cedar Project, Vancouver, Canada
Background: Wellbeing is eroded when Indigenous children are forcefully removed from families and communities, as they have been through Canada's residential school and child apprehension systems. Despite prevalence of intergenerational child apprehensions among Indigenous people involved in substance use, there is a paucity of research on child apprehension as a determinant of HIV‐related health. We explored how child apprehension experiences shaped HIV health and wellness among young Indigenous people who have used drugs in two Canadian cities.
Methods: This exploratory sequential mixed‐methods study took place within the Cedar Project cohort involving young Indigenous people who have used drugs in British Columbia, Canada. In‐depth interviews addressing HIV cascade of care experiences involved 12 participants living with HIV in 2016. Interpretive description identified themes. Based on qualitative findings, longitudinal generalized linear mixed effects models involving 52 participants tested for relationships between intergenerational child apprehension and HIV viral suppression using data collected between 2011 and 2014.
Results: Child apprehension experiences were a central concern for participants; 78.8% had been apprehended as children and, among parents, 60.5% had experienced their own child(ren) being apprehended. Themes highlighting intersections with HIV included: (1) impact of removal from families on long‐term health and wellbeing; (2) re/connecting with family; (3) intersections of substance use, apprehension, and HIV; (4) stress and demands of maintaining/regaining custody; and (5) traditional wellness practices being valued but complicated. Being apprehended (aOR: 0.23; 95% CI: 0.06 to 0.82) and having a child apprehended (aOR: 0.24; 95% CI: 0.07 to 0.77) were significantly associated with reduced odds of HIV treatment success (viral suppression).
Conclusions: Young Indigenous people who have used drugs were over 75% less likely to be virally suppressed if they were apprehended from their parents as children, or their own children had been apprehended. To our knowledge, this is the first study to demonstrate statistical links between intergenerational child apprehensions and negative HIV outcomes among young Indigenous people with HIV. Respecting Indigenous rights to self‐determination over child welfare processes is urgent. HIV care for young Indigenous people who have used drugs must acknowledge and address ongoing impacts of intergenerational child apprehension experiences. Supporting parenting and family connections are essential to culturally safe, healing‐centred HIV care.
PDD0107
Patient‐centred, patient‐provider engagement as mediator of effects of healthcare discrimination, depression and pain on later pain‐related outcomes and quality‐of‐life among African Americans with HIV who use drugs
T.‐Y. Tseng1, M. Mitchell2, M.C. Beach3, G. Chander3, A. Knowlton1
1Johns Hopkins Bloomberg School of Public Health, Health, Behavior and Society, Baltimore, United States, 2Friends Research Institute, Baltimore, United States, 3Johns Hopkins School of Medicine, Baltimore, United States
Background: Chronic pain is a prevalent, under‐addressed comorbidity among people living with HIV (PLHIV) that adversely impacts their quality‐of‐life (QOL). Because pain may compound discrimination in healthcare and problematic (self‐medicating) coping , racial/ethnic minority PLHIV who use drugs experience particular disparities in pain and treatment access. Patient‐centred patient‐provider engagement (PCE), characterized by provider respect and shared treatment decision making, is associated with patients’ improved well‐being, and is a metric of healthcare quality. We examined whether PCE mediates the effects of discrimination, depression, and pain on later mental health‐related QOL (MHRQOL) and other pain‐related outcomes among African American PLHIV who use drugs.
Methods: 331 PLHIV (95.8% African American, 42.6% female) with current or former drug use recruited from HIV clinics and community venues in Baltimore, Maryland, USA completed 3 semi‐annual surveys. In structural equation modelling, PLHIV's 12‐month pain‐related outcomes (MHRQOL, fear of doctor's disbelief that they are in pain, and substance use for unrelieved pain) were regressed on PCE (a latent mediator assessed at 6‐months) and baseline depression, pain intensity and healthcare discrimination experiences. Analysis adjusted for baseline assessment of outcomes and changes in HIV care provider between baseline and 6‐month.
Results: Baseline depression, discrimination, substance use for pain, and fear of doctor's disbelief of their pain were all associated with reduced PCE at 6‐months. There were significant indirect paths from baseline discrimination to higher chances of substance use for pain and lower MHRQOL at 12‐month, and from baseline depression to lower MHRQOL and higher chances of reporting fear of doctor's disbelief in their pain at 12‐month, mediated through reduced PCE.
Abstract PDD0107‐Figure 1.
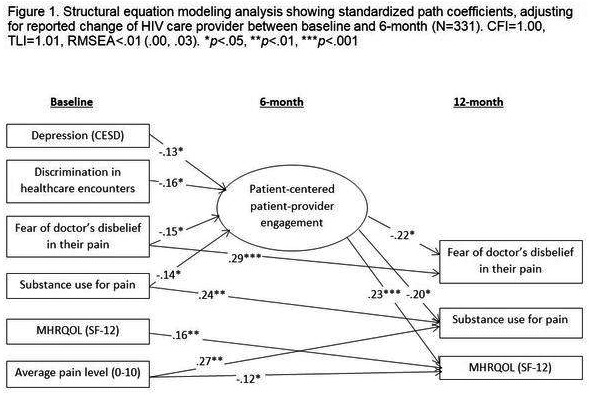
Conclusions: Findings highlight the important role of patient‐centred engagement in chronic pain management to enhance mental health and quality‐of‐life for PLHIV. Integrative interventions are needed to address co‐occurring pain and behavioural health problems, and to improve quality of patient‐clinician relationships.
PDD0202
Online dating patterns, sexual behaviours, and relationship characteristics among single young men who have sex with men: Latent profile analysis
S.K. Choi1, J.A. Bauermeister1
1University of Pennsylvania, Family and Community Health, Philadelphia, United States
Background: Geosocial networking applications (GNA) are a popular way to find dates and hookups among young men who have sex with men (YMSM; ages 18 to 24). Despite the increased use of GNA and its purported association with HIV risk behaviours in the literature, few studies have examined whether YMSM can be classified into different GNA use patterns. This person‐centred approach may unmask heterogeneity of GNA using patterns among YMSM, and examine whether these GNA patterns are differentially associated with HIV risk correlates.
Methods: 180 YMSM (mean age 21.67) who completed the baseline survey of an online HIV intervention trial delivering dating and partner‐seeking behaviour contents. Latent Profile Analysis (LPA) was used to identify the number of online dating usage profiles based on the distribution of four variables: frequency of GNA use for dating and hookups, respectively, and perceptions of the usefulness of GNA for dating and hookups. Using LPA, multinomial logistic regression (MLR) was used to assess associations with sexual behaviours and relationship characteristics. Mplus was used for LPA and SAS was used for MLR.
Results: Based on fit indices, a 3‐latent‐profile solution was selected. Profile1 (Low Utility Users; 50.8%) spent the least amount of time in GNA and did not consider GNA as useful to meeting partners. Profile2 (Hookup Seeker; 11.8%) used GNA almost every day for hookups and found GNAs useful to meet partners. Profile 3 (Dates; 37.4%) spent more time in GNAs seeking dates over hookups, yet acknowledged that GNA were useful to hookup. In MLR, Hookup Seekers (profile 2) were more likely than Low Utility Users (profile 1) to report higher scores in sexual sensation seeking and lower relationship commitment. They also reported a greater number of recent sexual partners, receptive/insertive anal intercourse, and a greater likelihood of having sex with a partner met online (ps < 0.05).
Conclusions: YMSM reported different GNA use profiles and, in turn, reported differences in HIV risk correlates. Interventions using technology to reduce HIV risk among YMSM who meet partners online may explore tailoring based on GNA profiles and offer risk reduction strategies that align with users’ frequency and perceived usefulness of GNA when seeking partners online.
PDD0204
Using mixed methods in discrete choice experiments to determine patients’ preferences for HIV and TB services
Y. Hirsch‐Moverman1, J.E. Mantell2, J.M. Zech1, M. Strauss3, G. George3, T. Masvawure4, M. Rabkin1,2
1ICAP at Columbia University, New York, United States, 2Columbia University Irving Medical Center and the New York State Psychiatric Institute, Division of Gender, Sexuality and Health, Psychiatry, New York, United States, 3Heatlh Economics and HIV and AIDS Research Division (HEARD), University of KwaZulu‐Natal, Durban, South Africa, 4College of the Holy Cross, Health Studies Program, Center for Interdisciplinary Studies, Worcester, United States
Background: The discrete choice experiment (DCE) is a quantitative method increasingly used to understand patient preferences in healthcare. Participants make choices in a series of hypothetical scenarios that force trade‐offs to reveal how patients prioritize different attributes (e.g. cost, location, clinic hours) and attribute levels (e.g. $1 vs. $3 vs. $5 for cost). Aggregate ranked preferences are useful for formulating patient‐centred policies and designing programmes that maximize uptake. However, there is limited guidance on developing attributes/levels used in DCE scenarios.
Description: We used qualitative and quantitative methods to refine DCE designs exploring HIV services in Zimbabwe and TB services in Eswatini. For each, we developed an initial list of literature‐derived attributes/levels. For the HIV‐related DCE, we then conducted eight focus group discussions (FGD) with adults on antiretroviral therapy (ART) to identify key attributes/levels of ART service delivery. Participants also placed stickers on a list alongside attributes they felt most important; attributes with more stickers were selected for inclusion. Choice card content and design were validated in two follow‐up FGDs. The TB‐related DCE examined preferences for TB preventive treatment in children in Eswatini. The initial list of literature‐derived attributes/levels was explored via 80 in‐depth interviews with children, caregivers and healthcare providers. Using participatory ranking methods (PRM), we asked participants to select the three most important attributes. Scores were tallied and the highest ranked attributes included in the DCE.
Lessons learned: Using mixed methods ensured inclusion of the most important context‐specific attributes/levels in each DCE. Qualitative data enabled assessment of content validity and validation of DCE results; PRM allowed systematic reduction, refinement and validation of attributes/levels. Qualitative analysis also allowed us to combine some attributes, such as number of pills and pill size in Eswatini. In both DCEs, participant preferences were consistent in qualitative interviews, DCEs and surveys: HIV – provider interactions, individual‐ versus group‐based models, clinic visit cost, visit frequency, and wait time; TB – clinic visit cost, wait time, pill formulation/size, pill taste, dosing frequency, treatment duration and visit frequency, and clinic hours.
Conclusions/Next steps: A rigorous mixed‐methods process for developing attributes can improve the validity of DCEs, reducing the chances of excluding important variables.
PDD0205
Expanding the use of the ECHO model to improve access to high‐quality care and treatment for people living with HIV in Malawi
M. Phoso1, T. Maphosa1, K. Ashburn2, R. Kanyenda1, H. Nkhoma1, M. Chilombe1, K. Kwashie1, S. Hrapcak3, N. Buono4, S. Phiri5, R. Nyirenda6, A. Auld4, E. Kim4, A. Maida4
1Elizabeth Glaser Pediatric AIDS Foundation, Lilongwe, Malawi, 2Elizabeth Glaser Pediatric AIDS Foundation, Washington DC, United States, 3US Centers for Disease Control and Prevention, Division of Global HIV and TB, Center for Global Health, Atlanta, United States, 4Centers for Disease Control and Prevention, Lilongwe, Malawi, 5Lighthouse Trust, Lilongwe, Malawi, 6Ministry of Health, Department of HIV, Lilongwe, Malawi
Background: Despite task‐shifting for antiretroviral treatment (ART) initiation and follow‐up to improve access to treatment at the primary care level, targeted capacity building activities to optimize clinical management have limited reach in Malawi. We implemented the project ‘Extension for Community Healthcare Outcomes’ (ECHO) to assess acceptability and improvement in clinical knowledge and capability among providers caring for people living with HIV (PLHIV) in Malawi.
Description: Project ECHO used proven adult learning techniques and interactive video technology connecting ART service providers in five high‐volume facilities in Malawi (one ‘hub’ and four ‘spokes’). From November 2018―September 2019, weekly, hour‐long sessions covering 25 didactic topics and collaborative case presentations were conducted. The sessions utilized case‐based learning and mentorship to increase the expertise of local health‐care workers to manage PLHIV. The evaluation consisted of a pre‐ and post‐intervention assessment; 29 clinical providers completed paper questionnaires and surveys before and after the project period, assessing HIV knowledge, perceived behavioural capability, and acceptability of ECHO. Change in knowledge was defined as (post‐evaluation/pre‐evaluation) knowledge scores * 100 across subject areas.
Lessons learned: Improvements in overall knowledge were noted, with a mean post‐test score of 67% compared to the pre‐test score of 59%, though this was not statistically significant. Knowledge gains were highest in the topics 1st and 2nd line ART failure (+41.1%), contraception and family planning (+31.6%) and pulmonary tuberculosis (+31.2%). Negative knowledge efficacy was observed in topics covering HIV and Hepatitis B (−50.1%), HIV and neurology (−19.0%), and HIV and the heart (−16.7%). Topic areas with negative knowledge efficacy had lower participation (attendance rates ≤ 50%). Participants reported improved perceived behavioural capability in all topics, with an increase in the number considering themselves experts. Most (86%) felt ECHO has improved the quality of care in their clinics. Participants were motivated to use innovative technology to expand communication for health providers.
Conclusions/Next steps: Project ECHO proved acceptable and showed overall positive gains in knowledge efficacy and capacity of clinical HIV providers. The model can be adopted nationally to increase real‐time, cost‐effective, high‐quality training in HIV services in Malawi. Strategies to increase attendance are key in implementing successful ECHO.
PDD0206
eMpower” a technology solution to social problem: A tool in hands of grassroots workers to increase efficiency
V. Arumugam1, H. Rosenara1, S. Kandaswamy1, R. Prasad1
1India HIV/AIDS Alliance, New Delhi, India
Background: In India, 2.1 million PLHIVs are estimated of which 1.4 million are taking regular treatment from the national ART programme. With the support of Global fund, India HIV/AIDS Alliance implements Vihaan Care and Support programme since 2018 through 310 care and support centres (CSCs) across India through differentiated care service model. Vihaan programme registered 85% of 1.3 million PLHIV and provide services through 1822 outreach workers and peer counsellors. With the huge clients load (Average ORW client load is 992), outreach workers were facing challenge to identify the needy clients and provide required services using hard copy data sheets. User friendly eMpower application (android‐based mobile/tablet application) has been developed to resolve this issue.
Description: eMpower tablet‐based IT application has been designed and provided to 1822 community ORWs/peer counsellors across India with client's prioritization, GIS, evidence collection, pop‐up reminders, in‐built validation and offline entry options. Priority clients were allotted to ORWs as per their geographical area coverage through regular synchronization between tablets and CMIS.ORWs provided needed services to their clients and updated the data in tablet which further synchronizes once in fortnight with CMIS. Tablets were managed centrally using MDM (mobile device management) system.
Lessons learned: eMpower tablet‐based application, has increased the efficiency of outreach workers in providing need‐based services and capture quality data in a timely manner as the follow up rate has increased from 42%(n = 10,80,031) before tablet implementation to 52%(n = 12,29,665) after tablet implementation as per September 2019. There is an evident increase in achievements between December 2017 and September 2019 related to TB screening (13,318/month to 149,035 clients/month), and HIV testing of partner/family members (453/month to 4311/month) and lost to follow clients brought back to the treatment (10,550/month to 16,779/month).
Conclusions/Next steps: eMpower tablet application utilization provided clear evidence that user friendly technology solution is important to prioritise and provide needful services to the PLHIV community in the high load setting. It increases the efficiency of the work of the field workers in the community. This application can be scaled up in different regions in HIV and associated service provision and data collection.
PDD0207
Leveraging low‐cost mobile technologies to increase community participation and sustainability of the HIV response: The case of OVC care in eastern and northern Uganda.
E. Kazibwe1, N. Kamwesigye1, J. Barry2, D. Kaufa1, A. Mugume1
1World Education, Inc., The Bantwana Initiative, Kampala, Uganda, 2World Education, Inc., The Bantwana Initiative, Boston, United States
Background: Government spending on the HIV response in Uganda remains below the recommendations of the WHO ($84 USD per capita) and Abuja Declaration (15% of GDP). The bulk of the deficit in healthcare financing is shouldered by donor agencies that emphasize country ownership and sustainable interventions. This paradigm requires innovative approaches to reach priority populations with quality healthcare using fewer resources while inculcating elements of sustainability.
Description: Since 2016, under USAID/Uganda Better Outcomes for Children and Youth in Eastern and Northern Uganda, the Bantwana Initiative of World Education, Inc. has leveraged its commercial partnership with MTN Uganda to create a Closed User Group (CUG), an in‐service network connecting health, social welfare, and child protection actors as part of an integrated referral network to ensure rapid response to child protection issues and comprehensive access to core services.
Since 2016, under USAID/Uganda Better Outcomes for Children and Youth in Eastern and Northern Uganda, the Bantwana Initiative of World Education, Inc. has leveraged its commercial partnership with MTN Uganda to create a Closed User Group (CUG), an in‐service network connecting health, social welfare, and child protection actors as part of an integrated referral network to ensure rapid response to child protection issues and comprehensive access to core services.
The monthly CUG subscription cost of $1.74 USD per user (paid by the project) allows users to communicate at no cost using voice and text messages within the group. The initiative has been scaled up to 22 districts, connecting more than 3600 actors.
Lessons learned: Programme evidence suggests that the majority of CUG‐registered users were utilizing the service to consult on child abuse cases (92%), coordinate critical child protection activities (88%), report child abuse cases (87%), mobilize community actors for case conferences (84%), follow‐up on reported cases (76%), and refer of cases to tertiary care providers (73%).
A 2019 study indicated that by improving communication and coordination among the multiple actors, the CUG has enabled quick identification of cases of child neglect and HIV‐exposed children and youth, mobilization, and referral to support services like HIV screening, ART referral, and adherence and viral load tracking ‐‐ providing evidence that the CUG improves coordination between communities and health service providers.
Referrals for cases of HIV and child abuse and neglect have improved, with a completion rate of 95% across the continuum of response.
Conclusions/Next steps: Leveraging low‐cost mobile technologies that link multilateral stakeholder efforts in national HIV response mechanisms can be part of a strategic support package to sustain and expand gains towards Uganda's epidemic control and social development goals.
PDD0302
Leveraging social networks and technology for HIV prevention and treatment with transgender women
I.W. Holloway1, S. Jordan1, S. Dunlap1, A. Ritterbusch1, C.J. Reback2
1University of California, Social Welfare, Los Angeles, United States, 2Friends Research Institute, Los Angeles, United States
Background: Transgender women (“trans women”) are disproportionately impacted by HIV; yet there are few interventions tailored to trans women. There has been increasing interest in how social networks may influence health behaviours. This study employed qualitative methods to better understand how trans women's social networks and technology‐based networking platforms may be leveraged in developing health promotion strategies for this high‐priority population.
Methods: Five 60‐minute, audio‐recorded focus groups were conducted with 39 trans women. Participants were eligible if they identified as a trans woman; were assigned male at birth; were at least 18 years of age; and used either alcohol or illicit substance or engaged in condomless anal sex in the past 12 months. Four of the focus groups were comprised of community members and consumers of social services (N = 31) and one of the focus groups were comprised of trans women service providers (N = 8). . During the focus groups, open‐ended questions focused on social network composition and use of technology for socialization, partner seeking, and health information. Audio recordings were transcribed, coded using an iterative, open coding process and analysed.
Results: Participants were racially and ethnically diverse with majority (74.4%) identifying as Black/African American and Latinx, ranging from ages 20 to 72, with a mean age of 37 (SD = 11.90). [CJR1] Most participants were connected to other trans women online where they exchanged health information. Qualitative data support the crucial role of these social networks for social support and health seeking information. Participants used technology to break isolation and to exchange health‐related information and advice. Participants’ described their social networks as stratified across class, racial, and generational differences.
Conclusions: Technology served as a crucial resource for collectively organizing resources and establishing relationships with other trans women. The strength of existing networks supports the development of network‐driven HIV prevention intervention strategies for trans women. Policymakers and practitioners should invest in the knowledge and expertise of trans women in using technology to organize health resources in the development of technology‐based HIV prevention and care interventions.
PDD0303
Longitudinal associations between place of sex work, depression and HIV vulnerabilities among sex workers in Baltimore, Maryland: A social geography of sex work approach to guide HIV prevention cascade optimization
C. Logie1, R. Hamilton White2, N. Galai2, C. Tomko2, S.G. Sherman2
1University of Toronto, Social Work, Toronto, Canada, 2Johns Hopkins University, Baltimore, United States
Background: Sex workers’ work environments influence sex worker‐client dynamics and HIV vulnerabilities. Cross‐sectional studies have documented violence, HIV risks, and depression associated with sex work in public compared to indoor spaces. In exploring where women reported sex with clients, we examined longitudinal associations between public place of sex work (PPSW) and health outcomes (HIV vulnerabilities, depression) among sex workers.
Methods: This cohort study involved five data collection points over one year among cisgender women sex workers (N = 246) in Baltimore, Maryland. We conducted bivariate analyses to examine associations between currently conducting any sex work in a public place (PPSW, e.g. car, abandoned house, street, park/forest, public bathroom) vs. exclusively indoor sex work (ISW, e.g. house, motel, dance club) with sociodemographic, substance use (e.g. injection drug use [IDU], crack use), past 3‐month condom coercion (e.g. client condom refusal/removal), and baseline clinically significant depression (CES‐D‐10; cut off of 10). We used logistic regressions with generalized estimating equations and exchangeable correlation structure to examine longitudinal associations between PPSW and subsequent condom coercion or depression adjusting for sociodemographics and substance use.
Results: Among participants (race/ethnicity: White: 67.5%; Black/African American: 22.8%; Latina/other ethnicity: n = 9.8%), most reported daily IDU (58.5%), daily crack use (62.2%), and homelessness (62.2%). Over three‐quarters (88.6%) reported any PPSW at baseline. PPSW was associated with increased odds of past 3‐month condom coercion (AOR: 1.85, 95% CI: 1.16 to 2.94, p = 0.01) and depression (AOR: 1.41, 95% CI: 1.02 to 1.97, p = 0.04) compared to ISW. PPSW was dynamic, reported by n = 218 at baseline, 3‐month (n = 133/155), 6‐month (n = 103/130), 9‐month (n = 93/119), 12‐month (n = 77/99), and was associated with not completing all study visits (see lasagne plot).
Abstract PDD0303‐Figure 1.
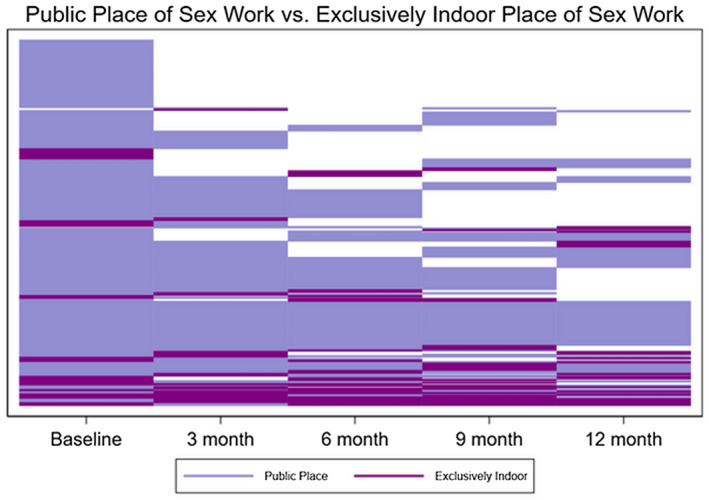
Conclusions: Public environments of sex work were associated with sexual (condom coercion) and mental (depression) health disparities. Public sex work confers additional health risks in contexts of illegality. Interventions to optimize HIV prevention cascade engagement can address these social geographies of sex work.
PDD0304
Creating communities of emergency responders to reduce violence against sex workers and increase access to justice and HIV services: Lessons learned from the Hands Off programme
S. Bouwmeester1, P. Chakuvinga2, M. Mogale3, A. Mashumba4, I. van Beekum1
1Aidsfonds, International Sex Work Programme, Amsterdam, Netherlands, 2Sisonke, Cape Town, South Africa, 3North Star Alliance, Durban, South Africa, 4BONELA, Gaborone, Botswana
Background: Gender‐based violence is a key risk factor increasing sex workers’ vulnerability to HIV. Violence impedes sex workers’ access to services. Despite evidence showing that a reduction of 25% of HIV infections among sex workers may be achieved when violence is reduced, there remains a lack of investment in programmes directly addressing violence as a structural barrier to HIV prevention. Under the Aidsfonds’ Hands Off programme (2014 to 2019) sex worker‐led organizations and service providers implemented various strategies to reduce violence against sex workers and increase access to services in Southern Africa.
Description: As a distinctive component of the programme, 65 different community‐led crisis response systems were set up, among which drop‐in centres and help lines. Trained sex worker paralegals escorted their peers to report cases, and provided court support, information and counselling. Crisis response teams consisting of sex workers, healthcare workers, police and influential community members ensured efficient referral and follow‐up to HIV, psychosocial and legal services. Litigation lawyers supported in bringing case before court.
Lessons learned: An independent evaluation of the programme showed that innovative crisis response systems such as help lines directly operated by police and multidisciplinary local response teams have increased access to services for sex workers. Over 81.000 sex workers have accessed healthcare, psychosocial and legal services. Partners learned that using decentralized systems leads to more effective referrals and follow‐up. In places where paralegals were responsible for supporting sex workers, sex workers received rapid assistance far more than they had in the past. Paralegals documented 1.500 cases of violence, contributing to an evidence‐informed advocacy strategy for sex workers’ rights. Over 235 cases were brought before court, illegal arrests have decreased and perpetrators have faced consequences for violence. However, to significantly reduce individual and structural violence, community‐led crisis response needs to be implemented parallel to interventions strengthening a rights‐based sex worker movement and supporting police engagement.
Conclusions/Next steps: Coupled with movement building and police engagement, community‐led response systems are a powerful strategy to reduce sex workers’ vulnerability to HIV. Substantially larger investment in programmes that focus on addressing violence as a structural barrier to HIV prevention is needed.
PDD0305
Roll out of innovative “Social Network Model” integrated with Index Testing contributed to reaching “Hard‐to‐reach”, HIV case detection and Linkage to ART among PWID in Northeast India
P. Goswami1, N. Singh2, B. George2, G. Shreenivas2, P. Choudhury2
1FHI 360, Research and M&E, New Delhi, India, 2FHI 360, New Delhi, India
Background: Reaching, testing and treating “hard‐to‐reach” individuals at risk of HIV infection is vital for achieving UNAIDS’ 90‐90‐90 goals. Northeast India, which has poor roads, uneven topography and inadequate facilities, has a large population of people who inject drugs (PWID), but traditional government‐run interventions in this difficult environment have achieved low case finding (0.4%). FHI 360 implemented an innovative “Social Network Model” under the CDC‐ and PEPFAR‐funded Project Sunrise to reach, test and treat hard‐to‐reach PWID.
Description: The Social Network Model used respondent‐driven chain referral to reach, test and treat PWID not already covered by government‐supported Targeted Interventions (TI). PWID who self‐reported large injecting networks were selected as seeds to recruit clients from their networks and refer them for HIV screening. Seeds, and any subsequently recruited clients, were given four coupons to distribute among injecting partners not covered by TI. For each new client tested, the recruiter received US $1, and clients were compensated for travel to HIV testing. Clients confirmed positive were linked to ART. HIV‐positive clients were also given index testing coupons to recruit their sexual partners, spouses and children, who were offered motivational counselling and HIV testing.
Lessons learned: 42 initial seeds from two districts in northeast India generated contact with 1476 previously unreached PWID, all of whom were tested. 13.8% were confirmed HIV positive, a case‐finding rate 35 times higher than under the TI model. 72% positive clients were linked to ART, while 24% positive and negative clients were linked to prevention services including OST. Index testing carried out with all PLHIV contributed to 16.7% positivity. Focusing on the high‐risk population led to higher reach, case detection and treatment.
Abstract PDD0305‐Table 1.
| Parameters | Churachandpur | Aizawl | Total |
|---|---|---|---|
| Initial seeds | 14 | 28 | 42 |
| Recruited and tested for HIV | 810 | 666 | 1476 |
| PLHIV newly diagnosed | 82 (10.1%) | 121 (18.2%) | 203 (13.8%) |
| PLHIV initiated on ART | 72 (88%) | 74 (61%) | 146 (72%) |
| Contacts of PLHIV tested for HIV | 132 (1:1.6) | 119 (1:1) | 251 (1:1.2) |
| Contacts of PLHIV diagnosed HIV positive | 22 (16.7%) | 20 (16.8%) | 42 (16.7%) |
Conclusions/Next steps: SNM integrated with index testing identified HIV‐positive PWID at a much higher rates than traditional programmes, though challenges remain linking all positive clients to ART. This model has been adopted by National AIDS Control Program and is now being scaled up across India.
PDD0306
Prevalence of depression and key associated factors among female sex workers living with HIV in Durban, South Africa
A. Bhardwaj1, C. Comins2, V. Guddera3, M. Mcingana3, K. Young3, R. Phetlhu3, N. Mulumba3, S. Mishra4, H. Hausler3, S. Schwartz2, S. Baral2
1Johns Hopkins Bloomberg School of Public Health, Population, Family and Reproductive Health, Baltimore, United States, 2Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology, Baltimore, United States, 3 TB HIV Care, Durban, South Africa, 4University of Toronto, Toronto, Canada
Background: More than half of all female sex workers (FSW) in South Africa are estimated to be living with HIV, however, emerging data have demonstrated that up to 80% of FSW in KwaZulu Natal also are clinically depressed. The syndemic framing of HIV and depression among FSW has been described, but there are limited data characterizing the upstream structural determinants of depression among FSW who are living with HIV.
Methods: Non‐pregnant, cisgender women (>18 years), enrolled in the Siyaphambili study in Durban, South Africa reporting sex work as their primary source of income and diagnosed with HIV for ≥ 6 months, were eligible for enrolment. Analyses was guided using an adaptation of stress‐diathesis model. Robust Poisson regression models were used to asses univariate associations with depression (PHQ 10). Variables aligned with the conceptual framework and significantly associated with depression in bivariate analyses (p < 0.1) were included in the multivariate robust Poisson regression model.
Results: Of the 1207 FSW enrolled, nearly all were South African, 27% were homeless (n = 326) the median age was 31 years [IQR 27 to 37]. A total of 387 (32%) participants reported symptoms of depression defined as a PHQ9 score greater than or equal to 10. Age, education, housing, viral suppression, experiences of sexual and physical violence, alcohol use, anticipated and internalized stigmas were associated with depression and included in multivariate analyses. In the multivariate regression, significant increases in the prevalence of depression were observed for participants who were homeless (PR = 1.25,95% Cl 1.03–1.52), had ever experienced sexual violence (PR = 1.54, 95% Cl 1.28 to 1.85) and reported internalized stigma (PR = 1.08, 95% Cl 1.00–1.16). Of the 1207 FSW enrolled, nearly all were South African, 27% were homeless (n = 326) the median age was 31 years [IQR27 to 37]. A total of 387 (32%) participants reported symptoms of depression defined as a PHQ9 score greater than or equal to 10. Age, education, housing, viral suppression, experiences of sexual and physical violence, alcohol use, anticipated and internalized stigmas were associated with depression and included in multivariate analyses. In the multivariate regression, significant increases in the prevalence of depression were observed for participants who were homeless (PR = 1.25,95% Cl 1.03–1.52), had ever experienced sexual violence (PR = 1.54, 95% Cl 1.28 to 1.85) and reported internalized stigma (PR = 1.08, 95% Cl 1.00–1.16).
Conclusions: Taken together, these results reinforce the structural foundation of syndemics of HIV and depression among FSW in South Africa and depression with independent predictors including unstable housing, violence, and stigmas. Given the relationship between depression and adherence among PLWH, these results suggest the need to integrate mental health and HIV programmes to optimize outcomes for both.
PDD0307
Seeing regulars associated with lower odds of sexual violence and client condom refusal amongst sex workers in Metro Vancouver, Canada (2010 to 2019): Implications for HIV prevention and removal of ‘end‐demand’ criminalization
B. McBride1, S.M. Goldenberg1, K. Shannon1, M. Braschel1, S. Moreheart1, AESHA Project
1Centre for Gender and Sexual Health Equity, Vancouver, Canada
Background: Globally, individuals who purchase sex services (i.e. clients) are commonly misrepresented as violent and exploitative, and are criminalized under ‘end‐demand’ legislation in 50 + countries. While qualitative research has documented heterogeneous relationships between clients and sex workers, there remain significant gaps in quantitative evidence examining the impacts of seeing different types of clients (i.e. regulars vs. one‐time) on sex workers’ safety and HIV/STI risks.
Methods: Analyses drew on longitudinal data (2010 to 2019) from a community‐based open cohort of 900 + indoor, street‐based, and online sex workers in Vancouver (AESHA; An Evaluation of Sex Workers’ Health Access) which has included experiential staff (e.g. interviewers, nurses, coordinators) since the project's inception in 2010. Our objectives were to (1) describe characteristics of women seeing mostly regulars (defined as 75% to 100% of clients are repeat clients) in the past 6 months, and (2) identify independent effects of seeing mostly regulars on odds of experiencing sexual violence and client condom refusal over a 9‐year period.
Results: Among 942 sex workers, 58.5% (n = 551) saw mostly regulars at some point over the 9‐year study. HIV prevalence was 14.1% at baseline. Women who reported inconsistent condom use for vaginal/anal sex with clients had lower odds of seeing mostly regulars (OR 0.80, 95% CI 0.67 to 0.96). In multivariable analysis, recent and longterm immigrants and those in in‐call venues (e.g. massage parlours) had lower odds of seeing regulars, while those soliciting independently and working in informal indoor settings (i.e. apartments) had higher odds of seeing regulars. In separate multivariable confounder models, seeing mostly regulars was independently associated with reduced odds of experiencing sexual violence (AOR 0.71, 95% CI 0.52 to 0.97) and client condom refusal (AOR 0.73, 95% CI 0.60 to 0.88), after adjusting for key confounders.
Conclusions: Our results disrupt common stereotypes of clients as inherently violent or ‘risky’ and suggest that within criminalized environments, sex workers may see mostly regulars as a strategy for enhancing safety and reducing HIV risk. Full decriminalization of sex work, including removal of ‘end‐demand’ client criminalization, is needed to enable sex workers to choose clients and organize their work according to their needs, towards HIV prevention and the full achievement of sex workers’ labour and human rights.
PDD0402
Building resilience for children affected by parental HIV in China: Efficacy of the multi‐level ChildCARE intervention at 18‐, 24‐, 30‐, and 36‐months
S. Harrison1, X. Li2, C. Cai3, J. Zhao4, G. Zhao5
1University of South Carolina, Department of Psychology, Columbia, United States, 2University of South Carolina, Department of Health Promotion, Education, and Behavior, Columbia, United States, 3University of South Carolina, College of Pharmacy, Department of Clinical Pharmacy and Outcomes Sciences, Columbia, United States, 4Henan University, College of Educational Sciences, Kaifeng, China, 5Henan Normal University, Department of Psychology, Xinxiang, China
Background: Global literature indicates that children affected by parental HIV are at risk for a range of negative developmental outcomes, including psychosocial, behavioural, educational, and health‐related challenges. Yet despite these hardships, many youth with parents who are living with HIV or who have died from AIDS‐related causes go on to experience positive later life outcomes. Building on four decades of theory and empirical research on resilience, the Child‐Caregiver‐Advocacy Resilience (ChildCARE) was developed to enhance the resilience of children affected by HIV in rural central China. Notably, the ChildCARE intervention provides psychosocial intervention at three levels, including the child‐level (i.e. peer‐group sessions), caregiver‐level (i.e. positive parenting training, caregiver support), and community‐level (i.e. local advocacy, stigma reduction efforts).
Methods: Efficacy of the ChildCARE intervention was evaluated through a four‐arm cluster‐based randomized controlled trial in Henan, China. In total, 790 child and caregiver dyads were assigned to one of four intervention conditions: control, child‐only intervention, child+caregiver intervention, and child+caregiver+community intervention. This study utilized difference score modelling to compare resilience‐related outcomes between children assigned to the control condition and those who received the full intervention package (i.e. child+caregiver+community intervention). Specifically, we evaluated whether children who received the multi‐level intervention displayed increases in psychosocial wellbeing and resilience‐related outcomes at 18‐, 24‐, 30‐, and 36‐months.
Results: Children assigned to the intervention group displayed higher resilience and enhanced psychosocial functioning when compared to children assigned to the control group at multiple time points after the ChildCARE intervention concluded. Notably, change scores from baseline to the final study assessment point (i.e. 36‐months) indicated that youth assigned to the child+caregiver+community intervention displayed increased resilience (p = 0.010), higher levels of post‐traumatic growth (p = 0.033), and better emotional regulation skills (p = 0.049) than those in the control group. Positive trends were noted for other constructs, including self‐esteem and use of positive coping skills.
Conclusions: There is increasing support for psychosocial HIV interventions that target not only vulnerable individuals, but also their social support systems and broader community networks. Current findings provide support for the efficacy of one such intervention‐‐ChildCARE‐‐in enhancing the resilience of children made vulnerable by parental HIV in China.
PDD0403
The national prevalence of viral load suppression and associated factors among children receiving ART in Malawi
A. Ahimbisibwe1, T. Maphosa1, G. Woelk2, H. Nkhoma1, S. Zimba1, J. Sunguti1, R. Kanyenda1, V. Sampathkumar1, E. Kim3, A. Maida3, N. Wandonda3, T. Mekonnen3, T. Kalua4, B. Bighnoli5, B. Mvula5, M. Kagoli6, G. Bello6,7
1Elizabeth Glaser Pediatric AIDS Foundation, Lilongwe, Malawi, 2Elizabeth Glaser Pediatric AIDS Foundation HQ, Washington, United States, 3Centers for Disease Control and Prevention, Center for Global Health, Division of Global HIV & TB, Lilongwe, Malawi, 4Ministry of Health, Department of HIV and AIDS, Lilongwe, Malawi, 5Ministry of Health, National Reference Laboratory, Lilongwe, Malawi, 6Ministry of Health, Epidemiology Unit, Lilongwe, Malawi, 7I‐Tech, Lilongwe, Malawi
Background: Impressive gains have been made in expanding access to antiretroviral therapy (ART) in Malawi. However, challenges remain in viral load suppression (VLS) among children. We assessed national VLS among HIV‐positive children aged < 18 years on ART for at least 6 months.
Methods: Between June 2018 and January 2019, we conducted a cross‐sectional survey that employed a two‐stage, cluster‐sampling design where 36 study health facilities providing ART were selected nationally using probability proportion to size. Blood samples were collected through heel or finger prick or venipuncture for viral load (VL) analysis at the National Reference Laboratory on the Abbott m2000 platform. VL > 1000 copies/ml was considered high VL. Demographic data were abstracted from routine facility‐based medical records. Counts and proportions were used for the descriptive analysis while bivariate analysis was conducted using the Chi square test.
Results: Of 806 children with VL results, (median age 10 years, interquartile range 7 to 13), 516 (64%) had suppressed VL. The majority (73%) of children with high VL were aged between 6 and 14 years old, with the age group of 10 to 14 years contributing 60% of high VL cases. Similar to children with suppressed VL, the majority (70%) of children with high VL had been on ART >=3 years. Of the children with high VL, 94% were on non‐nucleoside reverse transcriptase inhibitor (NNRTI)‐based regimens (98% nevirapine, 2% efavirenz as a tail), while 80% were on zidovudine (AZT) as nucleoside reverse transcriptase inhibitor (NRTI) backbone. The majority of children with no ART exposure for prevention of mother‐to‐child transmission (PMTCT) (66%) had high VL compared to those with PMTCT exposure (52%; p = 0.03). A higher proportion of children on abacavir (ABC)‐based regimens were virally suppressed compared to children on any other NRTI‐based regimen (53% vs. 30%, respectively; p = 0.02). The proportions of children on NNRTI and protease inhibitor (PI)‐based regimens with suppressed VL were 37% and 38%, respectively. Of 806 children with VL results, (median age 10 years, interquartile range 7 to 13), 516 (64%) had suppressed VL. The majority (73%) of children with high VL were aged between 6 and 14 years old, with the age group of 10 to 14 years contributing 60% of high VL cases. Similar to children with suppressed VL, the majority (70%) of children with high VL had been on ART >=3 years. Of the children with high VL, 94% were on non‐nucleoside reverse transcriptase inhibitor (NNRTI)‐based regimens (98% nevirapine, 2% efavirenz as a tail), while 80% were on zidovudine (AZT) as nucleoside reverse transcriptase inhibitor (NRTI) backbone. The majority of children with no ART exposure for prevention of mother‐to‐child transmission (PMTCT) (66%) had high VL compared to those with PMTCT exposure (52%; p = 0.03). A higher proportion of children on abacavir (ABC)‐based regimens were virally suppressed compared to children on any other NRTI‐based regimen (53% vs. 30%, respectively; p = 0.02). The proportions of children on NNRTI and protease inhibitor (PI)‐based regimens with suppressed VL were 37% and 38%, respectively.
Conclusions: VLS among children was suboptimal. The study showed no difference in suppression rates between children on NNRTI‐ and PI‐based regimens, while a higher proportion of children on ABC‐based regimens were virally suppressed compared to children on any other NRTI‐based regimens.
PDD0404
Pathways to HIV disclosure and optimal PMTCT outcomes among pregnant and postpartum women living with HIV in the Democratic Republic of Congo
A. Adedimeji1, C. Mpody2,3, N. Ravelomanana4, M. Tabala4, F. Malongo4, B. Kawende4, P. Babakazo4, M. Yotebieng1, CQI‐PMTCT Study Group
1Albert Einstein College of Medicine, Epidemiology and Population Health, Bronx, United States, 2Ohio State University, Epidemiology, Columbus, United States, 3Ohio State University, Columbus, United States, 4The University of Kinshasa, School of Public Health, Kinshasa, Congo, Democratic Republic of the
Background: Disclosure of HIV status in the context of PMTCT may increase social support and adherence to ART and thus, contribute to improved outcomes for women living with HIV (WLWH) and their infants. We examined factors associated with and the pathways mediating HIV disclosure among pregnant and post‐partum WLWH receiving routine PMTCT care.
Methods: We performed a cross‐sectional analysis, utilizing baseline data from a cluster‐randomized trial to evaluate the effect of continuous quality interventions on long‐term ART outcomes among pregnant and postpartum WLWH. We enrolled all pregnant and postpartum (≤1‐year post‐delivery) WLWH receiving ART in the 3 clinics with the highest caseload within each of the 35 provincial health zones in Kinshasa, from November 2016 to July 2019. For our outcome, we derived 3 binary indicators of HIV status disclosure to (i) anyone, (ii) a sexual partner, and (iii) family or friends that are important sources of social support. We performed logistic regression to estimate the association of disclosure of HIV status with demographic and clinical characteristics, using a general estimating equation to account for clustering at the health facility and health zone level.
Results: Of the 2775 WLWH enrolled, 2754 who provided information on HIV disclosure were retained in the analysis. Participants’ ages range from <=24 (16%), 25 to 34 (53%) and > 35 (31%). The majority were married (69%), had secondary education (87%), no income (90%) and multiparous (95%). About 58% were diagnosed with HIV prior to the most recent pregnancy and 55% initiated ART >=12 months. About one‐third (35%) experienced intimate partner violence (IPV). HIV status disclosure ranges from 52% to anyone, 34% to a sexual partner and 35% to family/friend. Factors associated with disclosure included older age, marriage, multiparity, tertiary education, >= 13 months on ART and history of IPV. Participants who exhibited symptoms of depression were less likely to disclose to anyone (OR: 0.98; 95% CI: 0.97, 0.99) or sexual partner (OR:0.98, 95% CI: 0.96, 1.00).
Conclusions: Pregnant and postpartum WLWH face difficult decisions regarding disclosure of HIV status. Depression and IPV complicate the disclosure processes, but the pathways through which these limit optimal PMTCT outcomes are less understood and warrants further investigation.
PDD0405
Caregiver characteristics and predictors of viral suppression among children living with HIV on ART in Kenya
B. Odeny1, R. Patel1, L. Abuogi2, I. Mukui3, E. Kinywa4, P. Oyaro5
1University of Washington, Seattle, United States, 2University of Colorado, Denver, Denver, United States, 3Ministry of Health, Nairobi, Kenya, 4Department of Health, Kisumu, Kenya, 5Health Innovations Kenya, Kisumu, Kenya
Background: Caregivers play a vital role in ensuring antiretroviral therapy (ART) adherence, retention in care, and viral suppression among children living with HIV (CLHIV). Caregiver characteristics may strongly predict viral suppression among CLHIV.
Methods: We analysed pre‐enrolment data for caregivers and children (<15 years old) enrolled from March to December 2019 in an on‐going randomized controlled trial, Opt4Kids, which assesses the impact of point‐of‐care viral load (VL) testing on VL suppression among CLHIV on ART and conducted in five facilities located in Kisumu County, Kenya. Clinical and sociodemographic characteristics were obtained through self‐report, standardized questionnaires, and clinical records. We used multivariable logistic regression models adjusting for various characteristics to assess associations between caregiver characteristics and the children's viral suppression (defined as VL < 1000copies/ml).
Results: Overall, 704 children were enrolled with a median age of children of 9 years (interquartile range [IQR] 6 to 11) and caregivers of 36 years (IQR 31 to 43). A majority of caregivers (69%) had attained at least primary education. The biological mother was the most common primary caregiver (68%) while 23% had someone other than their biological parent as their caregiver. A total of 568 caregivers (81%) were living with HIV and among these, 45% reported being virologically suppressed. Most children (78%) were virologically suppressed. Children with virologically suppressed caregivers were more likely to achieve viral suppression than children who had caregivers without viral suppression (adjusted odds ratio [AOR] = 7.53, 95% confidence interval [CI] 1.32 to 43.03, p < 0.017). Compared to children who had their biological mother as the primary caregiver, children with other caregivers were less likely to achieve viral suppression (AOR = 0.26, 95% CI 0.08 to 0.82, p = 0.016).
Conclusions: Our results indicate viral suppression of caregivers living with HIV and type of caregiver are associated with viral suppression in CLHIV. Targeted support of specific groups of caregivers may help improve viral suppression in CLHIV.
PDD0406
Understanding emotional distress among perinatally acquired HIV infected adolescents in Pune, India: Examining mediating and moderating effects
A. Verma1, K.K. Kota2, S. Bangar3, G. Rahane1, N. Yenbhar1, S. Sahay1
1ICMR‐National AIDS Research Institute, Division of Social and Behavioural Research Sciences, Pune, India, 2Georgia State University, Department of Health Policy & Behavioural Sciences, Atlanta, United States, 3ICMR‐National AIDS Research Institute, Division of Epidemiology, Pune, India
Background: Development of emotional distress (ED) among adolescents living with HIV (ALHIV) affects their adherence behaviour, social and psychological functioning. Data on stressors among ALHIV demonstrate the gap pertaining to the predictors of ED experienced by ‘adolescents living with perinatal HIV (ALPH)’ in the socio‐cultural milieu of India. This study aimed to examine the underlying mechanisms leading towards ED among Indian ALPH through mediation and moderation analysis.
Methods: This mixed‐method study was conducted (2014 to 17) to explore the psycho‐social issues and predictors of ED among Indian ALPH. Forty‐three qualitative interviews with ALPH, parents/guardians and health care providers were followed by a cross‐sectional survey among 100 ALPH (10 to 19 years). Distress sub‐scale of Weinberger's Adjustment Inventory was used to measure ED. Qualitative data were analysed using grounded theory in QSR NUD*IST Version 6.0 and survey data in SPSS 25.0. Mediation and moderation models were tested using Hayes PROCESS macros v3.0. The study was approved by the institutional ethics committee.
Results: Strong parental control, compulsive asexuality, internalized stigma by ALPH, and anger on parents were the major themes emerged from qualitative component. These themes led to survey tool constructs viz., HIV awareness, parental control (PC), hypervigilance, adolescent‐parent relationship (APR), adolescent‐parent communication (APC), body image and perceived negatively different from peers (PNDP). ED was found to be high among ALPH and significantly associated with PNDP (B = 3.91; 95% CI: 0.43 to 7.40; p = 0.03), anger (B = 0.22; 95% CI: 0.04 to 0.41; p = 0.02), body image (B = 0.37; 95% CI: 0.06 to 0.68; p = 0.02), and hypervigilance (B = 0.57; 95% CI: 0.17 to 0.96; p = 0.006). Anger was partially mediating the pathway between PNDP and ED while body image and hypervigilance had moderating effects on the relationship between PNDP and ED. The findings emphasize the need for mental health interventions for Indian ALPH.
Conclusions: The study provides empirical evidence that negative self‐perception, anger, body image issues and hypervigilance by primary caregivers are the predictors of ED among ALPH. It is critical to intervene early before an ALPH develops ED. Focused counselling on body image issues and self‐perception is critical for living a ‘normal’ life by ALPH. Primary caregivers need to build skills to draw a line between protection and over protection.
PDD0407
Individual and facility‐level factors associated with interruption in HIV care and treatment among pregnant and postpartum women in the Kabeho Study in Kigali, Rwanda
E. Nawar1, K. Andrinopoulos2, T. Carton3, R. Machekano1, E. Bobrow4, A. Asiimwe5, P. Mugwaneza6
1Elizabeth Glaser Pediatric AIDS Foundation, Washington DC, United States, 2Tulane University School of Public Health and Tropical Medicine, New Orleans, United States, 3Louisiana Public Health Institute, New Orleans, United States, 4Mathematica, Washington DC, United States, 5National Early Childhood Development Program, Kigali, Rwanda, 6Rwanda Ministry of Health, Kigali, Rwanda
Background: Pregnancy and postpartum are challenging periods where interruptions in HIV care and treatment (starting, stopping, and returning to care over time) are common. The absence of ART is strongly associated with high viral load and vertical transmission. Understanding determinants for interruptions in care could inform interventions aimed at continuous retention to prevent negative health outcomes related to high viral load.
Methods: The Kigali Antiretroviral and Breastfeeding Assessment for the Elimination of HIV (Kabeho) study was an observational, prospective cohort of 608 HIV‐positive women enrolled in their third trimester of pregnancy or within two weeks post‐delivery between April 2013 and May 2014. Multivariate logistic models adjusted for clustering by facility were used to examine the odds of an interruption (one or more missed visits followed by returning to care). Models included the following individual characteristics ascertained from interviews at enrolment: age, marital status, travel time to facility, education, CD4 results, disclosure of HIV status to partner, and household size. Facility characteristics captured during site assessments such as frequency and types of services offered were also included.
Results: Women who attended facilities that offered select services had much lower odds of having an interruption as compared to women who attended facilities that did not offer those services (Table 1). None of the individual characteristics examined were associated with interruptions.
Abstract PDD0407‐Table 1. Adjusted odds ratios and 95% confidence intervals from logistic models examining individual and facility‐level characteristics among women enrolled in the Kabeho Study; Kigali, Rwanda; 2015 to 2017.
| Facility level characteristics [individual characteristics not shown] | OR (95% CI) |
|---|---|
| ANC, PMTCT, and ART services offered all 5 days per week | 0.54 (0.32, 0.92) |
| Services not offered all 5 days | Reference |
| Retention support (telephone reminders, transportation reimbursement/support, or defaulter tracing system) | 0.30 (0.12, 0.76) |
| No retention support | Reference |
| Peer counselling | 0.31 (0.23, 0.42) |
| No peer counselling | Reference |
| Infant feeding counselling | 0.20 (0.15, 0.26) |
| No infant feeding counselling | Reference |
Conclusions: Our study suggests that health facilities may be more effective targets for interventions to improve retention than individuals. The lack of services offered was strongly associated with interruptions. Studies aimed at assessing health care utilization and motivation may be an effective means to identify the services that most encourage continuous engagement of pregnant and postpartum women to reduce the likelihood of vertical transmission.
PDD0502
Improving post‐operative follow‐up of voluntary medical male circumcision clients using a toll‐free line: Lessons from Rwenzori Region‐Uganda
F.F. Tendo1, P. Elyanu2, N. Mukiza1, J. Balungi1, A. Kekitinwa3
1Baylor College of Medicine Children's Foundation, Medical and Psychosocial, Kampala, Uganda, 2Baylor College of Medicine Children's Foundation, Research and Knowledge Management, Kampala, Uganda, 3Baylor College of Medicine Children's Foundation, Executive Director, Kampala, Uganda
Background: Voluntary Medical Male Circumcision (VMMC) is one of the effective biomedical HIV prevention interventions implemented in Uganda. Mobile outreaches have been used to increase uptake of VMMC. However, there are challenges managing post‐operative complications for clients circumcised during the outreaches since the VMMC teams are mobile, and client's homes may be far from health facilities which may lead to inadequate post‐operative follow‐up and management of the adverse events. Baylor‐Uganda implemented a VMMC call centre to aid in post‐operative follow‐up and link clients to care in rwenzori Region. We describe the implementation and impact of the toll‐free on follow‐up of client's post‐operation from July 2018 to June 2019.
Description: A VMMC toll‐free line was set‐up in July 2018, marketing of the line was done through radio talk shows, posters and engraved wristbands given to males after circumcision. The line is manned by a trained doctor, communication experts and counsel, who on receiving a call, give advice and depending on the query, link the client to the responsible follow‐up officer in specific VMMC camps. The project provided a standby vehicle to pick clients who required urgent medical attention then a follow‐up call is made 24‐hours after the initial call to monitor progress. Data from calls were recorded, descriptive statistics generated and shared monthly. We used proportions to analyse adverse events reported by the callers.
Lessons learned: Between July 2018 and June 2019, we received 2001 VMMC calls, 87% of the callers were males and the median age 16 years (IQR:12,22). Of the 2001, 1181 (59%) were given advise online especially on wound care and did not need linking to a VMMC officer while 820 (41%) had adverse events and were linked. Of the clients linked, 96% successfully received care within 24 hours.
Mild adverse events (AEs) reported were pain (10%) and swelling of the penis (15%) whereas moderate AEs included; wound disruption (40%),Abscess formation (11%),wound infection (10%),inability to urinate (9%), excessive bleeding (3%) and sexual dysfunction (2%).
Conclusions/Next steps: The toll‐free line service is effective in follow‐up of VMMC clients post‐operation. Other VMMC partners and the Ministry of Health should consider adopting this approach nationally.
PDD0503
Increased yield in voluntary medical male circumcision (VMMC) using HIV testing service screening tool in Zambia
J. Masiye1, G. Sishekanu Liche1, L. Aladesanmi1, J. Banda1, K. Asiedu1, O. Chituwo2, R. Kamboyi3, J. Siachobe1
1Jhpiego, Affiliate of Johns Hopkins University, Jhpiego, Lusaka, Zambia, 2Centers for Disease Prevention and Control, CDC/DDPHSIS/CGH/DGHT, Lusaka, Zambia, 3Ministry of Health, Health Services, Lusaka, Zambia
Background: VMMC prevalence in Zambia is 21% and HIV prevalence among males is 9.3%. The VMMC package includes HIV Testing Services (HTS) and has been touted as a unique opportunity to reach men thus contributing towards the UNAIDS 95‐95‐95 target. Universal testing for VMMC clients remains the gold standard, yield among VMMC clients is consistently low, suggesting opportunities for better targeting and resource optimization. Ministry of Health developed an HIV risk screening tool to identify and test clients most at risk. The screening tools has been primarily used in other HTS service points but newly introduced in VMMC.
Description: Since April 2015, Jhpiego implemented a 5‐year PEPFAR/CDC funded project that aims to increase VMMC coverage to 80%. The national HTS screening tool was rolled out in 58 VMMC facilities in 5 provinces in May 2019. Facility staff were trained before implementation. We assessed changes in HIV testing rates and yield among VMMC clients during 5 months of implementation (May – September 2019).
Lessons learned: Testing rate declined and yield increased; a 64% reduction in testing rates (100% to 36%) in the 10 to 14 age‐group and minimal increase in yield (0.2%), the 40 to 49 age‐group equally recorded a 60% drop in testing with more increase in yield (2.3%). The yield improvements are prominent in age‐groups older than 15 to 29, suggesting the tool helps target HTS for higher risk men, and decreases testing rates for men less likely to be HIV‐positive. These low yield rates below 3% are the highest recorded in the last 2 years.
Abstract PDD0503‐Figure 1.
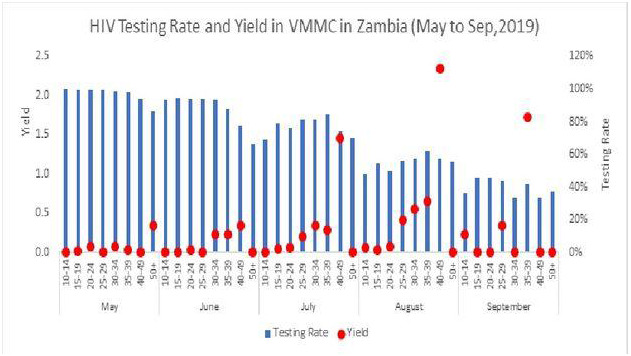
Conclusions/Next steps: The tool has resulted in reduced testing and increased yield among VMMC clients, it appears to be an effective approach for targeting HTS to the highest risk clients, particularly older age groups. The use of the tool should be expanded and monitored closely to ensure we are not turning away high‐risk clients.
PDD0504
Behaviour design methodology for uptake of voluntary medical male circumcision among fisherfolks in the lake zone of Tanzania
C. Mejia1, M. Swai2, E. Zimmerman3, E. McElwee3, L. Mphuru2, Z. Lweno2
1IntraHealth International, Chapel Hill, United States, 2IntraHealth International, Dar es Salaam, Tanzania, United Republic of, 3Ideas42, New York, United States
Background: Voluntary medical male circumcision (VMMC) is highly effective and among PEPFAR's highest HIV prevention priorities. HIV prevalence among fisherfolks in Tanzania's Lake Zone Region is estimated at 14%, three times higher than national HIV prevalence; 26% ‐ 54% of adult men from the general population in the Lake Zone Regions are uncircumcised. VMMC outreach approaches that have succeeded among the general population have not been as effective among fisherfolks in Lake Victoria islands; thus, nonconventional multidisciplinary solutions are needed to reach this segment of the population. Behavioural design (BD) leverages insights from social psychology, economics, and neuroscience to understand individuals’ decisions and actions. IntraHealth International, in collaboration with ideas42, used BD to create tools to increase VMMC uptake among fisherfolks.
Description: BD occurred in four‐phases: problem definition, diagnosis, design, and testing. We generated over 100 hypotheses about behavioural drivers of VMMC uptake, which were investigated through interviews with providers, clients, and community members. Confirmed common behavioural drivers included:•Time inconsistent preferences: immediate costs of VMMC related to lost wages and wait times outweigh longer‐term benefits.•Zero risk bias: men's motivation to avoid any risk of jeopardizing sexual performance, even if they perceive that risk to be remote.•Descriptive social norms: men perceive circumcision to be uncommon among peers and conform to this norm.•Availability heuristic: vivid stories of fears or complications related to VMMC come easily to mind, leading men to overestimate risks.
We designed protypes (job aids) for community health workers to support them in addressing these and other behavioural drivers through outreach and sensitization activities.
Lessons learned: BD can be employed to address cognitive biases and other behavioural tendencies contributing to low uptake of VMMC among priority populations. Solutions can reframe choices to allay fears about sexual performance, provide identity cues that show accessing VMMC as consistent with strength and masculinity, reduce immediate costs, and connect VMMC to salient, immediate benefits.
Conclusions/Next steps: Implementers and researchers must understand the context of service provision and learn directly from priority populations what drives their behaviour so that behaviourally informed interventions can be harnessed to achieve HIV prevention goals.
PDD0505
Condom use among men who have sex with men within Metro Manila, Philippines: Associations with attitude towards its use and sexual health outcomes
J. Cadelina1,2
1LoveYourself, Inc., Administration, Mandaluyong City, Philippines, 2De La Salle University, Behavioural Science Department, Manila, Philippines
Background: The purpose of this study was to determine and explain the level of condom use among men who have sex with men (MSM) within Metro Manila. It also intended to determine the associations between the level of attitude on condoms towards their level of condom use and the association of the direction of condom use attitude, level of sexual health knowledge, level of condom access and the associations of their level of condom use and sexual health outcomes.
Methods: The research used a quantitative methodological approach utilizing an online self‐administered survey questionnaire as a data collection tool using non‐probability sampling of 415 MSM. The data gathered were analysed using descriptive statistics to find means scores, frequencies and percentages. Meanwhile, a non parametric inferential statistics were used to test the association of variables.
Results: Findings revealed that men who have sex with men have a high level of knowledge, high level of condom access, high level of condom use and good level of sexual health outcomes but with a negative attitude towards condom use. However, only one fourth of the respondents have knowledge on the new concept of undetectable equals unstransmittable, where in a person living with HIV who have undetectable viral load by taking antiretroviral drugs cannot transmit HIV sexually. Findings also revealed that sexual health knowledge and condom access have significant but negative association with condom use attitude while no significant association was found between the level of condom use attitude and condom use implying that attitudes has nothing to do with MSM's condom use practices. However, a significant positive correlation was found between condom use and the level of sexual health outcomes.
Conclusions: Despite the high level of knowledge, condom access, condom use, and sexual health outcomes among men who have sex with men within Metro Manila, their condom use attitudes were negative and the researcher recommends further investigation to gather insights on the factors affecting their negative attitudes towards condom use. Furthermore, continuation and scaling up of condom distribution programmes are recommended while behavioural change communication strategies should focus on changing the negative attitudes of MSM on condom use.
PDD0506
End‐user research for the development of an implant to prevent unintended pregnancy and HIV prevention: Qualitative insights from South Africa and Zimbabwe
M.K. Shapley‐Quinn1, W. Makoni2, E. Luecke1, E. Mbatsane3, E. Odoom1, E. Krogstad4, L. Johnson5, A. van der Straten1,6
1RTI International, Women's Global Health Imperative, San Francisco, United States, 2Pangaea Zimbabwe AIDS Trust, Harare, Zimbabwe, 3Setshaba Research Centre, Soshanguve, South Africa, 4University of Cape Town, Desmond Tutu HIV Centre, Cape Town, South Africa, 5RTI International, Research Triangle Park, United States, 6University of California, Center for AIDS Prevention Studies, San Francisco, United States
Background: The SCHIELD (Subcutaneous Contraceptive and HIV Implant Engineered for Long‐Acting Delivery) programme aims to develop a Multipurpose Prevention Technology (MPT) implant for HIV and pregnancy prevention. An assessment was undertaken to provide rapid implant attribute and acceptability feedback to the product development team from potential end‐users.
Methods: Twelve Focus Group Discussions (FGDs) were conducted with women aged 18 to 34 in Soshanguve, South Africa and Harare, Zimbabwe along with quantitative demographic and preference data collection with each participant. FGDs were stratified by contraceptive implant experience as well as parity and transactional sex. Frequencies were run on quantitative data and debriefing reports of FGDs were analysed to summarize emerging themes.
Results: 110 women (median age 24) were enrolled, and they overwhelmingly supported the idea of an MPT implant. Preferred duration varied by participant type and country. Women who engaged in transactional sex in Soshanguve and parous women at both sites favoured a longer lasting implant (3 years), whereas women who engaged in transactional sex in Harare and nulliparous women generally preferred shorter durations (≤2 years). Participants had mixed reactions to possible menstrual changes related to use of a hormonal contraceptive, citing impact on daily activities and economic considerations (e.g. ability to engage in transactional sex, cost of menstrual products). These were considered possible barriers to future uptake and persistence with an MPT implant. Participants anticipated situations where their desire to conceive could rapidly change, necessitating a quickly reversible contraceptive component, whereas the need for quick reversibility for the HIV prevention indication was not anticipated. This drove preference – especially in Harare – for separate rods for each indication, with easy removability of the contraceptive portion while the HIV prevention portion could be left in place (preference for separate rods: Harare = 70%, Soshanguve = 48%).
Conclusions: An MPT implant system for prevention of HIV and unintended pregnancy was highly desirable to potential end‐users, and optimal duration may vary by personal and country context. Furthermore, product developers should consider the importance of return to fertility and tolerance for menstrual changes in early stages of product development when it is more feasible to modify attributes of an MPT product containing a hormonal contraceptive.
PDD0507
A call to improve understanding of Undetectable equals Untransmissible (U=U) slogan in Brazil
T.S. Torres1, L.M.S. Marins1, D.R.B. Bezerra1, V.G. Veloso1, B. Grinsztejn1, P.M. Luz1
1Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil
Background: The correct understanding of U=U (undetectable equals untransmissible) is key in the fight against the HIV epidemic as it empowers people living with HIV, improving adherence and decreasing self‐stigma. We assessed the correct understanding of U=U in Brazil in 2019 by population groups.
Methods: Adult (age ≥ 18y) Brazilian residents were recruited to complete a web‐based survey through advertisements on a geosocial network dating app (Grindr), Facebook and WhatsApp Groups during October/2019. Participants were grouped as: people living with HIV (PLWH), HIV‐negative/unknown gay or bisexual cisgender men (GBM) and other populations (POP). Understanding of U=U was accessed with the question: “With regards to HIV‐infected individuals transmitting HIV through sexual contact, how accurate do you believe the slogan U=U (undetectable equals untransmissible) is?” Responses were dichotomized into “correct” (completely correct) and “incorrect” (partially correct, partially incorrect, completely incorrect and I don't know what undetectable means). Logistic regression models were used to assess the factors associated with the correct understanding of U=U by group.
Results: A total of 2311 individuals accessed the questionnaire, 234 (10%) did not meet inclusion criteria and 1690 (73%) completed the survey. Of these, 347 (20%) were PLWH, 785 (46%) GBM and 558 (33%) POP. More PLWH had a correct understanding of U=U (79%, 274/347), compared to 44%(347/785) GBM and 17%(96/558) POP (Chi‐square test p < 0.01). Among PLWH and GBM, black race and lower income were associated with decreased odds of correct understanding, respectively (Table 1).
Abstract PDD0507‐Table 1.
| PLWH aOR (95% CI) | GBM aOR (95% CI) | POP aOR (95% CI) | ||
|---|---|---|---|---|
| Gender/Orientation | Category A (Ref: Category B)a | 0.74 (0.36 to 1.49) | 2.16 (1.48 to 3.18) | 3.12 (1.18 to 8.02) |
| Age | ≤ 35 years (Ref: >35 years) | 2.64 (1.40 to 5.20) | 2.00 (1.45 to 3.18) | 1.15 (0.68 to 1.92) |
| Race | Black (Ref. Otherb) | 0.34 (0.16 to 0.71) | 1.22 (0.76 to 1.94) | 0.79 (0.32 to 1.74) |
| Family Income | Middle/High (Ref. Lowc) | 1.59 (0.85 to 2.99) | 1.55 (1.09 to 2.22) | 0.77 (0.45 to 1.35) |
| Schooling | ≤ Secondary school (Ref. > Secondary school) | 0.94 (0.50 to 1.78) | 0.96 (0.66 to 1.38) | 1.06 (0.62 to 1.78) |
| Living in Capital Cities | Yes (Ref. No) | 1.35 (0.72 to 2.49) | 1.44 (1.02 to 2.04) | 1.26 (0.77 to 2.11) |
| Steady Partner | Yes (Ref. No) | 2.58 (1.39 to 5.01) | 1.11 (0.80 to 1.56) | 1.38 (0.84 to 2.32) |
| Ever testing HIV | Yes (Ref. No) | NA | 1.54 (1.00 to 2.40) | 2.26 (1.28 to 4.20) |
a For PLWH, Category A= GBM and Category B= other; For GBM, Category A= Gay and Category B= Bisexual; For POP, Category A= Transgender/Non‐Binary and Category B= Cisgender;
b Other = White, Asian, Native or Pardo (Mixed‐black);
c Low income is equivalent to R$1996.00 or USD468.00 per month; NA: not applicable.
Conclusions
There was significant difference in U=U knowledge across population groups, with average and very poor understanding among GBM and the general population, respectively. Correct understanding of the slogan needs to be promoted in more vulnerable populations such as PLWH of black race and GBM of lower income, and more broadly among older individuals and the general population, in an effort to decrease stigma against PLWH.
PDE0102
Capacity building for community health workers in Vietnam: Results of an intervention trial
L. Li1, L.‐J. Liang2, C. Lin1, L.Q. Pham1, T.A. Nguyen3
1University of California, Center for Community Health ‐ Semel Institute, Los Angeles, United States, 2University of California, Statistics Core, Department of Medicine, Los Angeles, United States, 3National Institute of Hygiene and Epidemiology, Hanoi, Vietnam
Background: Community health workers (CHW) can play an essential role in addressing the needs of people living with HIV (PLH) and people with substance use disorders (PSUD); this is particularly critical in low‐ and middle‐income countries. Coordination and networking among CHW and with providers of other service agencies need to be strengthened so that that CHW can be equipped with relevant knowledge and supported from their professional peers. In this study, we report preliminary outcomes of a capacity‐building intervention targeting CHW in Vietnam. The aim was to enhance their networking and collaboration to support HIV and addiction treatment initiation, retention, and adherence.
Methods: From 2017 to 2019, we conducted a cluster randomized controlled trial in four provinces of Vietnam. Intervention CHW participated in the intervention programme for integrated service delivery, including two in‐person group sessions followed by online group communications to network and support each other. All the CHW participants completed assessments with an audio computer‐assisted self‐interview method. The outcome measures include two multi‐item scales. Interaction with providers in other service sectors was measured by an eight‐item scale developed for this study. Confidence in HIV/drug‐related service provision was measured by CHW ratings in confidence in providing services to PLH and PSUD in several service areas. Mixed‐effects regressions models were performed to evaluate intervention outcomes based on the data collected at baseline, 3‐, 6‐, 9‐, and 12‐month follow‐up.
Results: We observed no significant differences in baseline CHW demographic characteristics, work background, and training between the intervention and control conditions. Intervention CHW showed greater improvement in interaction with providers in other service sectors (p = 0.004) and confidence in HIV and drug‐related service provision (p = 0.025) than control CHW at the 6‐month follow‐up. The intervention effects on both outcome measures remained at the end of the study (12‐month, p < 0.05).
Conclusions: Our study indicated that the intervention focusing on capacity building and networking has the potential to improve cross‐agency communication and collaboration among CHW. With the support of a professional network, CHW will be more equipped to provide HIV and drug‐related services in community settings.
PDE0103
A community‐based experience of a viral load (VL) sample collection campaign to enhance Dolutegravir (DTG) containing antiretroviral therapy (ART) regimen switch among female sex workers (FSWs) in Malawi
C. Kwitonda1, E. Nicco1, D. Van Laeken1, E. Chang1, K. Chawinga1, D. Muambeta1, I. Kwamwanza2, G. Momba2, R. Chimenya2, T. Ellman3, L. O'Connell3
1MSF OCB, Limbe, Malawi, 2MOH, Mwanza, Malawi, 3SAMU, Cape Town, South Africa
Background: The MSF FSWs Project provides HIV TB and sexual and reproductive health (SRH) services for Malawian FSWs through a community‐based peer‐led model. National VL monitoring policy is VL testing for all those on ART at 6 months and yearly. VL coverage among FSWs in the MSF cohort was 45% prior to the campaign. The additional importance of VL in ensuring correct switch at the time of DTG rollout adds urgency to improve VL coverage. We conducted a campaign to reach enrolled FSWs eligible for VL based on peer‐led community outreach in Dedza, Mwamza and Zalewa Districts. This descriptive data analysis presents the outcome of the intervention.
Description: From Aug. to Nov. 2019, FSWs eligible for VL sample collection in the 3 districts were mobilized by peers to have dried blood spot (DBS) VL tests according to Malawian guidelines. Tracing was achieved using micro‐planning tools and an eligible list was extracted from programme database. Data were collected on paper register, encoded into an excel sheet and an analysis was performed using STATA 13.
Lessons learned: 252 DBS samples were collected during the campaign, reaching 71% of the 355 eligible FSW. . Among those reached, 39% had never previously had a VL and 80% were VL suppressed (<1000 copies/mL). VL suppression rate on DBS was 45,6% (21/46), 86% (123/143) and 83,5% (56/63) in Zalewa, Mwanza, and Dedza. Of 252 FSWs reached by the campaign 220 (87%) returned for results and DTG‐based regimen switch, increasing the speed of DTG switch among FSWs.
Conclusions/Next steps: A campaign led through peer‐based community mobilization is an effective method to rapidly increase coverage of VL testing among FSWs on ART in Malawi, increasing VL coverage from 44,7% to 64,4% among FSWs enrolled in the MSF cohort in the 3 sites. All reached during the campaign received a VL. The observed percentage of VL suppression (80%) indicates remaining gaps in achieving the 90‐90‐90 target for FSWs. While Malawi protocol at the time of the campaign pragmatically recognized that DTG switch should not be delayed by awaiting VL results, our approach demonstrates that VL guided switch can be rapidly achieved in this population.
PDE0104
Positive effects of intensified preventive calls/home visits on early retention among adults newly initiated on antiretroviral therapy in Zambézia Province, Mozambique
C. De Schacht1, G. Amorim2, S. Van Rompaey1, J. Matsimbe3, A. Naftal Fernando4, J. A. Tique1, I. Torres1, L. Dique5, E. Ntasis1, C.W. Wester6,7
1Friends in Global Health, Maputo, Mozambique, 2Vanderbilt University Medical Center (VUMC), Department of Statistics, Nashville, United States, 3Friends in Global Health, Quelimane, Quelimane, Mozambique, 4Provincial Health Directorate, Quelimane, Mozambique, 5Centers for Disease Control and Prevention (CDC), Maputo, Mozambique, 6Vanderbilt Institute of Global Health, Nashville, United States, 7Vanderbilt University Medical Center (VUMC), Department of Medicine, Nashville, United States
Background: In Mozambique, early retention rates among antiretroviral therapy (ART)‐treated adults remained low in 2018, ranging from 65% to 69% at one and three months, respectively. To bolster rates, patients newly initiated on ART received services in the first three months including phone calls and/or monthly supportive home visits performed (for non‐pregnant/lactating adults) by Counsellors and Peer Educators, and (for pregnant/lactating women [PLW]) by Mentor Mothers. In February 2019, activities were intensified in 20 health facilities in Zambézia Province, focusing on technical support to counsellors and volunteers, data triangulation and weekly process measures monitoring. The effect on early retention was evaluated.
Methods: Routinely collected aggregated programme data extracted from electronic patient database of HIV‐positive adults initiating ART between September 2018 and August 2019 were evaluated. Retention was defined as returning for ≥ 1 ART pick‐up within 33 days (1‐m retention) and 61 to 120 days (3‐m retention) post‐initiation. Trend analysis was done using generalized linear mixed effects models to account for site‐level clustering, adjusting for covariates (urban/rural, patient volume, district).
Results: Analysis included 19,750 patients. Overall, one‐ and three‐month retention rates increased with the intervention from 61% to 93%, and 76% to 91%, respectively (Figure 1). In observation period, odds of being retained at one‐month increased by 1.92 (95% CI: 1.67 to 2.20) and we predicted a continuous retention rate increase of 1.23 (95% CI: 1.18 to 1.29) post‐observation period. The change was less substantial (OR 1.01, 95% CI: 0.87 to 1.16) for 3‐m retention, but the increased rate was still significantly higher (OR 1.25, 95% CI: 1.19 to 1.31).
Abstract PDE0104‐Figure 1.
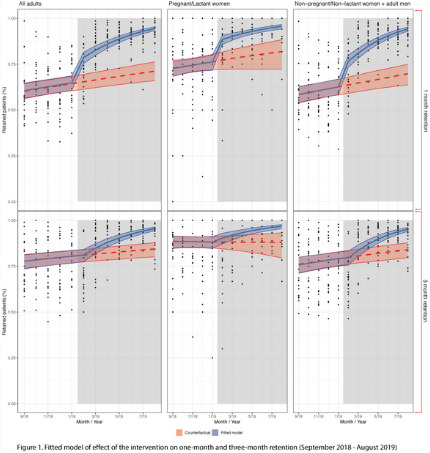
Conclusions: Improved psychosocial support implementation appeared to have a significant effect on early retention. Counsellors and volunteers ensured procedural fidelity through clear identification of roles/responsibilities and creating a feedback loop regarding performance. High quality community support should start as early as possible to prevent lost to follow‐up in this critical post‐ART initiation window.
PDE0105
Community‐based antiretroviral therapy delivery associated with viral suppression and retention in care in South Africa
L. Violette1, J. Dorward2, J. Quame‐Amaglo3, N. Garrett2,4, P. Drain1,3,5
1University of Washington, Department of Medicine, Seattle, United States, 2University of KwaZulu‐Natal, Centre for the AIDS Programme of Research in South Africa (CAPRISA), Durban, South Africa, 3University of Washington, Department of Global Health, Seattle, United States, 4University of KwaZulu‐Natal, School of Nursing and Public Health, Durban, South Africa, 5University of Washington, Department of Epidemiology, Seattle, United States
Background: We assessed South Africa's differentiated community‐based antiretroviral therapy (ART) delivery programme (CCMDD) for people living with HIV (PLHIV) for participation rates and to determine the association between CCMDD enrolment and HIV care outcomes.
Methods: The STREAM trial enrolled PLHIV in Durban who were clinically stable on ART for six months and randomized participants to receive either point‐of‐care viral load (VL) testing (Xpert® HIV‐1 VL, Cepheid) and task‐shifting to an enrolled nurse or standard laboratory VL testing. Six months after STREAM enrolment, non‐pregnant participants with two consecutive undetectable (<40 copies/mL) VLs were eligible for referral into the CCMDD programme. At 12 months after STREAM enrolment, participants were reassessed for eligibility. We used Poisson models with robust standard errors to evaluate the association between CCMDD enrolment and viral suppression (<200 copies/mL) and/or retention in care.
Results:
Abstract PDE0105‐Table 1. Centralized Chronic Medication Dispensing and Distribution (CCMDD) Programme enrolment and HIV care outcomes at study exit in the STREAM Study, n = 390
| HIV care outcomes | Not enrolled in CCMDDa N = 264 (68%) | Enrolled in CCMDDa N = 126 (32%) | RR (95% CI) | p‐value | aRRb (95% CI) | p‐value |
|---|---|---|---|---|---|---|
| HIV viral load < 200 copies/mL and retained in care at the clinic | 204 (77.3) | 119 (94.4) | 1.22 (1.13 to 1.32) | <0.001 | 1.19 (1.09 to 1.29) | <0.001 |
| HIV viral load < 200 copies/mL | 218 (82.6) | 126 (100.0) | 1.21 (1.15 to 1.28) | <0.001 | 1.20 (1.12 to 1.29) | <0.001 |
| Retained in care at the clinic | 226 (85.6) | 119 (94.4) | 1.10 (1.03 to 1.18) | 0.003 | 1.08 (1.01 to 1.16) | 0.017 |
a Enrolment into CCMDD is defined as first enrolment prior to the study exit visit.
b Adjusted for study randomization arm, continuous age, and gender.
Among 390 participants, 176 (45%) were eligible, 168 (43%) were referred, and 144 (37%) enrolled into CCMDD. Participants obtained their ART within the expected window at 93% (265/286) of scheduled CCMDD visits. Reasons ART was not obtained included missed appointments (9/21, 43%) and ART package not being available at the pick‐up point (6/21, 29%). Of 135 participants reassessed for CCMDD consideration six months after initial eligibility, 14 (10%) became CCMDD‐ineligible due to pregnancy (n = 5), detectable viral loads (n = 3), and declined re‐referral (n = 6). After adjusting for study randomization arm, age, and gender, CCMDD enrolment prior to study exit was significantly associated with viral suppression (<200 copies/mL) and retention in care at the clinic (RR = 1.19 p < 0.001), viral suppression alone (RR = 1.20 p < 0.001) and retention in care alone (RR = 1.08 p = 0.017).
Conclusions: Among clinically stable participants, those enrolled in the CCMDD programme had higher rates of viral suppression and retention in care, indicating that the community‐based ART delivery model did not negatively impact HIV care outcomes. The CCMDD programme should be promoted for clinically stable PLHIV in South Africa.
PDE0106
High satisfaction with Key Population‐Led Xpress service delivery for follow‐up of pre‐exposure prophylaxis clients in community‐based organizations in Thailand
N. Thammajaruk1, P. Posita1, S. Promthong1, O. Nampaisan1, T. Sangpasert2, P. Meekrue3, T. Pasansai4, P. Palee5, P. Brutrat6, M. Avery7, S. Mills7, R. Vannakit8, P. Phanuphak1, N. Phanuphak1, R. Ramautarsing1
1PREVENTION, The Thai Red Cross AIDS Research Centre, Bangkok, Thailand, 2Rainbow Sky Association of Thailand, Bangkok, Thailand, 3The Service Workers In Group Foundation, Bangkok, Thailand, 4Mplus Foundation, Chiang Mai, Thailand, 5Caremat, Chiang Mai, Thailand, 6The Service Workers In Group Foundation, Pattaya, Chonburi, Thailand, 7FHI360 and LINKAGES, Bangkok, Thailand, 8USAID Regional Development Mission Asia, Office of Public Health, Bangkok, Thailand
Background: Thailand has achieved significant scale‐up of HIV pre‐exposure prophylaxis (PrEP) under a community‐based service delivery model with lay providers; however, PrEP retention has been suboptimal. Xpress service flow was implemented to reduce the one‐hour wait time for PrEP follow‐up visits and potentially improve service retention. Here we report clients’ experiences with Xpress services.
Methods: Xpress service flow was designed by staff of community‐based organizations (CBOs) working across 6 Thai provinces, and was launched at 6 CBOs in June 2019. Eligibility assessment was performed by counsellors, and clients were considered eligible if they had good adherence, no acute HIV symptom and willing to use Xpress service. If eligible, clients skipped post‐test counselling during that visit, and received their HIV test result through a channel of their choosing (LINE, SMS or email), resulting in an average visit duration of 30 minutes. A satisfaction survey using a 5‐point Likert scale was conducted among clients using the Xpress service on the day of service utilization.
Results: Between August and December 2019, 898 clients came for PrEP follow‐up, all were eligible and used the Xpress service, of whom 341 completed the satisfaction survey. A majority indicated to be satisfied or very satisfied with service accessibility (98.5%), duration (95.9%) and convenience (97.7%). Nearly all clients agreed or strongly agreed that enough attention was paid to them during Xpress services (98.2%), that Xpress services fit their lifestyle better than regular services (98.8%), that they preferred Xpress over regular services (97.1%), and that receiving test results through LINE/SMS/email is private and safe enough (94.4%). Of the 898 clients who used the Xpress flow, one seroconverted. This client was immediately contacted by CBO staff and linked to treatment within one day.
Conclusions: The Xpress option to optimize the flow and duration of services for PrEP follow‐up clients results in very high client satisfaction and should be offered as a service option for eligible clients, and longitudinal clinical outcomes like retention in care and adherence should be assessed in the future to inform service optimization. The Xpress option should also be expanded to other services to improve service utilization.
PDE0202
Variations in community‐based HIV testing outcomes by testing approach: An updated systematic review of the evidence for community‐based HIV testing
P. Stankard1, A. Groves2, S. Bowler2, M. Jamil3, S. Shaw4, L. Gebrekristos2, C. Quinn3, R. Baggaley3, C. Johnson3
1Stankard Consulting LLC, Washington DC, United States, 2Drexel University, Dornsife School of Public Health, Philadelphia, United States, 3World Health Organization, Global HIV, Hepatitis and STIs Programme, Geneva, Switzerland, 4Independent Consultant, Washington DC, United States
Background: Approximately 8.1 million people with HIV (21%) are undiagnosed. Efforts to scale‐up HIV testing among those unreached, using efficient and effective approaches, is essential for achieving the 95‐95‐95 global targets. WHO recommended community‐based HIV testing services (CB‐HTS) in 2013 and, in 2015, recommended lay provider testing to further support implementation. Here we examine differentiated CB‐HTS to guide future implementation.
Methods: We conducted a global systematic review following PRISMA, searching eight electronic databases, for studies on CB‐HTS published/presented between January 2015 and July 2018. This analysis presents studies reporting on CB‐HTS outcomes among the general population. We calculated pooled proportions using random effects models.
Results: 228/13,218 unique studies were included in this review. HIV testing uptake across CB‐HTS approaches was 83% (95% CI: 78 to 87%). Over a third were first time testers (37%;CI: 28 to 46%) and over half were male (61%;CI: 57 to 65%). Pooled HIV positivity was 6% (CI: 5 to 7%) and over three‐quarters of those diagnosed HIV+ were new diagnoses (78%;CI: 67 to 87%).
Results varied by CB‐HTS approach (Figure 1). Compared with home‐based testing, outreach had greater uptake (O: 90%, CI: 77 to 98% vs H: 83%, CI: 77 to 88%), reached more first time (O: 40%, CI: 34 to 46% vs H: 32%, CI: 16 to 51%) and male testers (O: 61%, CI: 55 to 66% vs H: 55%, CI: 49 to 61%), and resulted in a greater percentage of new HIV+ diagnoses (O: 77%, CI: 38 to 100% vs H: 71%, CI: 58 to 83%).
Site‐specific approaches varied greatly. School‐based testing found lower uptake (65%, CI: 63 to 67%) and reach among first‐time (34%, CI: 32 to 37%) and male testers (24%, CI: 22 to 26%). Pooled HIV positivity was also low (0%, CI: 0 to 1%). Workplace models demonstrated similarly low uptake (55%, CI: 6 to 91%), but reached high proportions of men (97%, CI: 84 to 100%) and first‐time testers (52%, CI: 30 to 74%). Pooled positivity was 7% (CI: 4 to 11%) and 94%(CI: 63 to 100%) of HIV+ diagnoses were new.
Abstract PDE0202‐Figure 1.
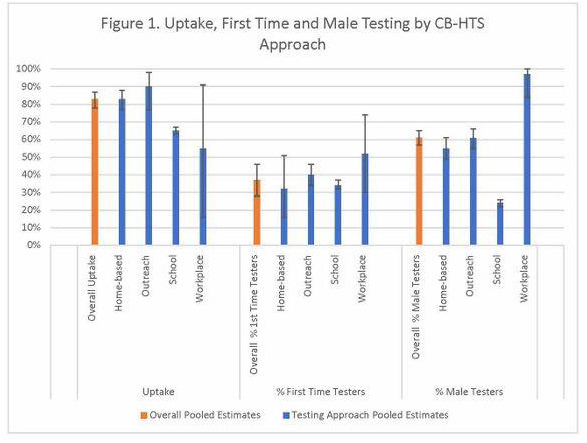
Conclusions: CB‐HTS remains an effective way to reach first‐time testers and men, and to identify new HIV infections. There is considerable variability by testing approach. Programmes must consider their context and select a strategic mix of CB‐HTS approaches to reach those in need of HIV testing, prevention and treatment services.
PDE0203
An innovative “1 + 1” HIV self‐service testing in cooperation with community‐based organizations or voluntary testing and counselling sites in five provinces, China
Y. Lyu1, M. Chen2, J. He3, S. Liang4, X. Hu5, Y. Yao6, J. Yao1, Y. Jiang1
1National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China, 2Yunnan Center for Disease Control and Prevention, Kunming, China, 3Hunan Provincial Center for Disease Control and Prevention, Changsha, China, 4Guangxi Province Centers for Disease Control and Prevention, Nanning, China, 5Xinjiang Uygur Autonomous Region Center for Disease Control and Prevention, Urumchi, China, 6Guizhou Center for Disease Control and Prevention, Guiyang, China
Background: In order to meet the needs of both fast and accurate testing results in the anonymous state, as well as professional medical service, we developed the “1 + 1” HIV self‐service testing (including finger‐prick rapid testing and dried blood spot self‐collection testing), and evaluated its acceptability and feasibility in five Province, China.
Methods: From March to December 2019, in cooperation with local community‐based organization or voluntary testing and counselling sites, 9868 “1 + 1” HIV self‐service testing packages were distributed to the participants for free. When got the package, according to the instructions, participants performed finger‐prick rapid testing, collected their dried blood spot (DBS) in private, completed the questionnaire and mailed the specimen and questionnaire back to the designated laboratory. The DBS specimens were tested with HIV‐1 antibody ELISA and the results were uploaded to the web‐based platform within five working days.
Results: A total of 4196 (42.5%) urine specimens and questionnaires were mailed back. Of these 4196 participants, 2016 had performed finger‐prick rapid testing and the positive rate was 8.4% (169/2016). 416 out of 4196 (9.9%) dried blood spot specimens were determined HIV‐1 antibody positive. CDC staff reached 401 of 416 (96.4%) HIV‐1 antibody‐positive participants, and provided a western blot diagnostic assay kit to test their venous blood specimens. Among them, 100% reported being confirmed HIV antibody positive. 352 out of 401 (87.8%) participants were confirmed as newly diagnosed. 93.3% (388/416) HIV‐1 antibody‐positive participants and 63.2% (2390/3780) HIV‐1 antibody‐negative participants searched for their test results by logging onto the website with the unique identification code.
Conclusions: This study showed that “1 + 1” HIV self‐service testing was an efficient approach supplemental to the existing HIV testing and counselling system through eliminating the key barriers of conventional PITC and VCT testing, could help medical professionals to establish a linkage with individuals with high‐risk sexual behaviour, effectively identify undiagnosed HIV infection, and timely refer and confirm those who screened HIV positive in private.
PDE0204
Combining enhanced peer outreach approach with Index Testing: A better strategy for reaching key populations at high risk in HIV concentrated settings in India
K. Krishnan1, M.R. Parthasarathy1, A.K. Paulraj1, G.S. Shreenivas1, J. Bhaisya2, B. George1
1FHI 360, LINKAGES, New Delhi, India, 2USAID, Health, New Delhi, India
Background: The USAID/PEPFAR funded LINKAGES project led by FHI 360 and implemented in six high‐burden districts for HIV in India, explored combining the enhanced peer outreach approach (EPOA) among hidden networks of men who have sex with men (MSM) with index testing to reach spouses and stable sexual partners and achieve India's 90‐90‐90 goals. HIV prevalence among MSM is reported as 2.69%.
Description: EPOA adapts respondent driven sampling to reach networks of key populations (KPs) beyond the catchment geographies of the National HIV Control Program, while index testing expands HIV testing from HIV diagnosed KP members to undiagnosed high‐risk individuals particularly spouses and stable sexual partners. Stakeholders were consulted on locally appropriate and community‐friendly strategies for peer‐based outreach, HIV screening, accompanied referral for confirmatory testing, and antiretroviral therapy (ART) initiation. HIV testing was performed through community‐based approaches either at drop‐in centres or community events. Confirmatory tests were conducted at the government testing centres. The WHO Partner Notification Framework was adapted for index testing. A differential package of services for MSM based on risk was developed. Risk assessment targeting high‐risk MSM and the documentation system were strengthened. Onsite mentorship and supportive supervision were provided.
Lessons learned: During 2018 to 2019, 12,896 new MSM clients were reached through EPOA, with a case detection rate of 6.09% (n = 786). From the index MSM clients found HIV positive, 334 spouses were notified and tested for HIV with a case detection rate of 51.79% (n = 173). In addition, 1229 regular partners were notified and tested with a case detection rate of 17.49% (n = 215). ART was initiated among 148 spouses (85.54%) and 190 regular partners (88.37%).
Conclusions/Next steps: Results show that combining the hidden peer led network testing approach with index testing enhances the coverage of high‐risk KP members and improves HIV case detection. The blended approach extends into high‐risk individuals, specifically spouses and stable sexual partners of MSM, who are otherwise beyond the reach of the National HIV Program, contributing towards the 90‐90‐90 goals. Adapting locally appropriate and community centric outreach, disclosure and partner notification for HIV concentrated settings are pre‐requisites for such combination approaches.
PDE0205
Reaching the ‘first’ 95 ‐ Uptake and yield of community‐based HIV testing service modalities in South Africa
N. Nyoni1, C. Magezi1
1FHI 360, Capacity Development and Support, Pretoria, South Africa
Background: Despite high HIV burden in South Africa, testing coverage remains low. Community‐based HIV testing services play improves diagnosis and linkage to HIV care for marginalized gaps. The Accelerated‐Targeted Community‐based HIV Testing Services (ATC‐HTS) project, funded by USAID, aimed to increase case‐finding of HIV‐positive individuals to link and retain them in care and treatment. The project focused on priority populations and undiagnosed HIV‐positive individuals in targeted communities in Mpumalanga and Free State provinces, Africa.
Description: To achieve high testing coverage, mobile, home‐based and index testing modalities were implemented. The results compared HTS uptake and yield according to the modalities implemented. Using routine HTS data, from October 2017 to September 2018, the impact of the different modalities in identifying undiagnosed HIV infections. Index testing targets sexual partners and biological children (off‐shoots) of positive index clients identified through TIER.Net registers, mobile testing and home‐based testing through door‐to‐door campaigns.
Lessons learned: Approximately 100,000 people were tested: 56,026 – home‐based HTS (56.1%), 35,692 – mobile (35.7%) and 8328 – index (8.2%) testing. Home‐based testing modality achieved higher uptake, compared to the other two modalities of testing: a higher proportion of < 18 were tested through home‐based than index testing (91% vs. 9%) and a higher proportion of adult men than index‐testing (88% vs. 12%).
Of 8328 index off‐shoots tested for HIV, 1630 (20%) tested positive while 1533 tested positive through home‐based modality and 1376 through mobile testing. In comparison, Index testing was the most efficient for case identification with 20% positivity rate, compared to 2% for both mobile and home‐based testing.
Of the 4539 HIV‐positive individuals, 92% of clients tested positive through community‐based testing and were linked to care, confirmed through TIER.Net.
Conclusions/Next steps: Index testing is the most efficient modality for identifying HIV‐positive individuals. It allows testing for sex partners and biological children of index clients resulting in increased yield. This model reached PLHIV who would not ordinarily access HTS through conventional health facility HTS modalities.
PDE0206
HIV services closer to the communities: community‐level interventions to optimize HIV case findings and treatment initiation in Nepal
A. Shrestha1, H. Subhani1, K. Bam1, R. Khanal1, B. Shrestha1, R. Didiya2, A. Bhattachan3
1FHI 360/LINKAGES Nepal Project, Kathmandu, Nepal, 2National Association of People Living with HIV in Nepal, Kathmandu, Nepal, 3National Center for AIDS and STD Control, Kathmandu, Nepal
Background: In 2018, 20% of the estimated total number of people living with HIV (PLHIV) in Nepal did not know their status, and 30 percent who knew their status were not enrolled in antiretroviral therapy (ART). We describe the efforts of community lay providers for HIV screening through test‐for‐triage and peer navigators facilitating ART initiation to increase case finding and treatment in the USAID/PEPFAR‐funded LINKAGES Nepal Project.
Methods: Based on LINKAGES Nepal programme data for HIV prevention, care, support and treatment in 17 high‐burden districts, we calculated the case finding through test‐for‐triage by mobilizing community lay providers (including key populations), and the use of HIV‐positive peer navigators to facilitate treatment (October 2018–September 2019). Peer navigators accompanied those identified as positive for ART initiation including adherence support. Test‐for‐triage is a process where the lay‐providers do HIV screening in the community as a part of a cost‐effective and targeted approach. Test‐for‐triage were used for differentiated outreach and index testing. Index testing was offered to trace sexual, injecting partners and risk network referrals, and U=U messages were communicated to discordant couples.
Results: Compared with no test‐for‐triage approach in FY18, case finding increased from two cases to 549 cases in FY19; also in FY19, case finding through index testing using test‐for‐triage increased to 18% compared to those from static clinics (2.7%), where beneficiaries need to find time to come for testing. Compared to no peer navigation support in FY18, ART initiation increased by 14% point in FY19 (75% to 89%). This demonstrates human‐centred approach for HIV epidemic control where rather than beneficiaries going to services, services go to the beneficiaries.
Conclusions: Mobilization of community lay providers and peer navigators helps risk groups to know of their HIV status rather than visiting the clinic and helps to initiate ART. Their role is crucial to fill the gap in Nepal's HIV cascade.
PDE0302
Faith‐engaged community posts associated with over 1200% increase in new HIV case ascertainment, with high linkage and retention, Zambia
G. Makangila1, A. Mwango2, M. Shah3, N. Kancheya4, K. Nkwemu4, I. Zulu3, I. Essiet‐Gibson3, L. Erickson Mamane3, S. Agolory4, S. Hillis5
1Circle of Hope, Lusaka, Zambia, 2Catholic Relief Services, Lusaka, Zambia, 3Centers for Disease Control and Prevention, Atlanta, United States, 4Centers for Disease Control and Prevention, Lusaka, Zambia, 5Office of the Global AIDS Coordinator, Washington DC, United States
Background: Recent data indicate that gaps in HIV testing uptake and antiretroviral therapy (ART) coverage in Lusaka, particularly for men and children, are related to their being less likely to access health facilities, preferring to receive HIV services from trusted individuals. Catholic Relief Services and Circle of Hope (an Assembly of God affiliate) addressed this by engaging and deploying local faith leaders to de‐centralized community posts (CPs). We considered whether this model was associated with increases in case‐finding, linkage and retention, particularly for men and children.
Description: Each of 21 CPs was located in a high‐activity setting with minimal branding and served by a multi‐disciplinary team of community health workers and a clinician. CRS and COH collaborated in a programme to increase case‐finding, linkage and retention. The intervention leveraged trusted relationships of faith leaders to identify individuals at higher risk for HIV infection.
Lessons learned: During the 19 months following introduction of CPs (March 2018–September 2019), as compared to the 17 months before, the median number of new HIV cases identified per month increased 1087% overall (from 46 to 500), 1494% in men (from 16 to 239), and substantively in children (from 0 to 10). During the programme period, testing yield for men was 27.0% and for children, 5.5%. Of the 11,457 clients identified as new HIV cases at CPs, >96% were linked and >92% were retained on ART as of September 2019.
Key programme components include:
• Hiring – harnessed social infrastructure, with > 90% of staff serving as trusted faith leaders in local congregations;
• Senior Management support ‐ through daily meetings and WhatsApp
• Celebration – quarterly events distribute non‐monetary awards
• Leveraging faith leaders’ close relationships with those at risk in the community (such as those with marital/partner conflict, familial illness, bereavement or attendance at healing services)
• Shared core values – training with continuous reinforcement – ‘RECIPE’ – Responsibility, Empathy, Compassion, Integrity, Passion, Ethics
Conclusions/Next steps: We report that implementation of this faith‐engaged CP model can result in substantial improvements in case‐finding, linkage and retention. Expanding this model to other contexts may help advance epidemic control in Zambia and beyond.
PDE0303
Pilot evidence‐based interventions to reduce methamphetamine use in Vietnam: Promising outcomes and lessons learned
H.D. Hoe1, N.H. Anh1, N.T. Trang1, S. Larkins2, S. Shoptaw3, L.M. Giang1
1Hanoi Medical University, Center for Research and Training on Substance Abuse and HIV, Hanoi, Vietnam, 2University of California, Integrated Substance Abuse Programs, Los Angeles, United States, 3University of California, Departments of Family Medicine and Psychiatry and Biobehavioral Sciences, Los Angeles, United States
Background: Methamphetamine has gradually replaced opioids to be the first drug of abuse in Asia‐Pacific. Methamphetamine use is associated with HIV risk behaviours and lower uptake of methadone maintenance treatment. Little evidence exists to guide the effective implementation of evidence‐based interventions (EBIs) to reduce methamphetamine use in resource‐poor settings like Vietnam.
Description: Between 2018 and 2019, VHATTC at Hanoi Medical University has piloted four combinations of EBIs on methadone patients and people who use drugs (PWUD) not in treatment. The project aims to assess the feasibility of interventions and preliminary treatment outcomes. These EBIs included motivational interviewing, contingency management, Matrix group therapy, SMS messaging and on‐site psychiatric treatment. Participants with methamphetamine‐positive urinalysis and/or at moderate/high risk with methamphetamine were recruited into intervention programmes that lasted between 8 and 16 weeks. Participants were tested for methamphetamine twice every week throughout the interventions.
Lessons learned: The retention rate of 288 patients receiving interventions remained at 90% in all EBIs combinations, except for MSM who used methamphetamine. The reduction of both methamphetamine and heroin use across EBIs combinations was consistent. Among high‐risk methadone patients (n = 56), methamphetamine use reduced from 39.3% to 6%, opioid use from 28.6% to 4% after 16 weeks. Among PWUD not in treatment, methamphetamine use reduced from 49.2% to 31% after 8 weeks. Among HIV‐positive methadone patients (n = 51), methamphetamine use reduced from 54.9% to 12.5% and viral load decreased after 12 weeks. In all combinations, participants’ mental health improved after interventions. Findings indicate that EBIs implementation is feasible in Vietnam. Methadone providers were able to adopt intervention techniques with online and on‐site assistance. When confidentiality was ensured, all patients agreed to be tested for methamphetamine. SMS messaging might work to sustain participants’ achievements in resource‐poor settings. Moderate‐risk users responded better to all EBIs combinations.
Conclusions/Next steps: EBIs have been proved to be effective in Vietnam. We will assist Vietnam's Ministry of Health to develop implementation guidelines for methamphetamine interventions in methadone clinics. Strategies to retain MSM who use methamphetamine in care and other EBIs like family interventions among adolescents using drugs need to be piloted.
PDE0304
Violence, confidentiality breaches, and hard to reach clinics impede adolescent treatment adherence
L. Cluver1,2, Y. Shenderovich2, E. Toska3,2,4, W. Rudgard2, S. Zhou3, M. Orkin2,5, R. Haghighat2, L. Sherr6, L. Gulaid7, A. Armstrong7
1University of Cape Town, Psychiatry and Mental Health, Cape Town, South Africa, 2University of Oxford, Department of Social Policy and Intervention, Oxford, United Kingdom, 3University of Cape Town, AIDS and Society Research Unit, Cape Town, South Africa, 4University of Cape Town, Department of Sociology, Cape Town, South Africa, 5University of Witwatersrand, MRC‐NRF Developmental Pathways to Health Research Unit, School of Clinical Medicine, Johannesburg, South Africa, 6University College London, Research Department of Global Health, London, United Kingdom, 7UNICEF Eastern and Southern Africa Regional Office, Nairobi, Kenya
Background: We urgently need effective strategies to increase treatment adherence amongst Africa's adolescents living with HIV, but lack evidence of social, structural and clinical factors impacting adherence.
Methods: A prospective 3‐year study of n = 1060 adolescents living with HIV (55% female, mean age 13.6) in one health district of South Africa's Eastern Cape. We traced all adolescents ever initiated on treatment (90% uptake) in all 73 government health facilities, with standardized questionnaires and clinical records. Study retention was 94% (18 months) and 91% (36 months), with 3% mortality. Ethical approvals were received from University of Cape Town, University of Oxford, Provincial government. Analyses used logistic random‐effects and fixed‐effects models (estimating semi‐elasticities) to investigate predictors of adherence.
Outcome: past‐week self‐reported ART adherence. Potential predictors included: Healthcare: medication stockouts, confidentiality of records, travel time to clinic and clinic waiting time; Structural family factors: orphanhood, changes of primary caregiver, household size and (non)biological relationship to primary caregiver; and Family care factors: physical or emotional violence victimization, domestic violence, good caregiver supervision and caregiver‐adolescent communication. Controls were: age, study round, gender, urban/rural, (in)formal home, poverty, vertical/horizontal infection and recent ART initiation.
Results: Adherence was associated with undetectable viral load (baseline OR 1.45, 95% CI 1.02;2.07; 18‐month OR 1.47, CI 1.03;2.11). Rates of consistent adherence from baseline were 45% at 18‐month and 37% at 36‐month follow‐up. Three factors independently predicted changes in individual's adherence from baseline to 18‐month follow‐up (p < 0.05): emotional or physical violence (OR 0.46, CI 0.33;0.65), travel time to clinic > 1 hour (OR 0.50, CI 0.29;0.86), and perceived non‐confidentiality of clinical information (OR 0.64; CI 0.46;0.88). Average probability of adherence was reduced by becoming exposed to violence (by 27%), >1 hour travel to clinic (24%), and non‐confidentiality (15%).
Conclusions: Findings highlight three modifiable areas for intervention. Violence victimization had the greatest impact, suggesting urgent need for effective violence prevention caregiving programmes. ART distribution through adherence clubs or closer to home may be valuable. Modifying patient flow and mentoring health workers may improve confidentiality. Adolescents need access, trust and care to survive, and their voices will need to inform most relevant solutions.
PDE0305
Implementation of the “First Friendly Practice for Trans‐Female People” at Arzobispo Loayza National Hospital. A public‐private partnership, Lima, Peru 2019
M. Muñoz1, E. Matos2, N. Bravo1, M. Caballero1, J.C. Minano1, R. Carrasco1, S. Ramirez1, M. Guevara1, L. Mestanza1, J.L. Sebastian1
1AIDS Healthcare Foundation, Lima, Peru, 2Arzobispo Loayza Hospital, Lima, Peru, 3Continental University, Lima, Peru
Background: Trans‐gender population is considered at high risk for HIV infection due to multiple factors.
AHF Peru works with trans‐female population since 2013, and since 2017, has implemented a “friendly” external consultant for Trans‐female patients at Arzobispo Loayza National Hospital (HNAL), with a high impact in the attention of the HIV Trans‐Female population.
Abstract PDE0305‐Figure 1.

Methods: A cross‐sectional, retrospective and descriptive study, evaluates the impact of this intervention in the quality of the attention of the Trans female population in Peru.
The work was executed through II phases of implementation:
Phase I: Approach of the female trans population to the friendly service considering a trans female hostess , support with diagnosis and timely linkage to HIV positive through a trans linker, provision of hormones, ensuring a comprehensive health system to 100%.
Phase II: The trans‐female population of Phase I, replicated the intervention and expanded the population by adding the approach to the system, HNAL assumed 100% of the cost of the hormonal treatment.
Results: Before the start of this project, only 06 Trans female person were attended in the HNAL in the period of one year.
Since the start of the implementation of consultant, there was an increase of around 3000% (182) in the number of patients. 87 (48%) were detected as reactive to HIV.
After 10 months of starting care at the “First Friendly Practice for Trans‐Female People”, the MoH made the purchase and delivery of hormones to the HNAL. Currently, the hormone treatment is guaranteed for free for all female transwomen.
Conclusions: Adapting the offer of a differentiated and friendly practice for the trans‐female population, identifying their needs and values their health priorities, generates confidence and approach of female Trans population to the health system.
PDE0306
‘MTV Shuga’: Can mass media communication HIV prevention and sexual health in adolescent girls and young women in rural South Africa?
M. Shahmanesh1, N. Mthiyane2, N. Chimbindi2, T. Zuma2, J. Dreyer2, I. Birdthistle3, S. Floyd3, N. Kyegombe3, S. Danaviah2, T. Smit2, G. Harling1, J. Seeley3, K. Baisley3
1University College London, London, United Kingdom, 2Africa Health Research Institute, Durban, South Africa, 3London School of Hygiene and Tropical Medicine, London, United Kingdom
Background: Adolescent girls and young women (AGYW) in South Africa are at high risk of HIV and early pregnancy. MTV‐Shuga, a mass‐media edu‐drama, improved some sexual health outcomes in a randomized trial amongst young people in Nigeria. We used a national free‐to‐air TV screening of MTV‐Shuga (the “Down South” series), concurrent with the roll‐out of a large scale‐up of combination HIV prevention for AGYW (“DREAMS”), to test the hypothesis that mass‐media edu‐drama can improve the sexual health of AGYW in a rural and resource‐constrained area of KwaZulu‐Natal was evaluated.
Methods: We followed a representative population‐based prospective cohort of females aged 13 to 23 (between May 2017 and September 2019). We measured the relationship between exposure to MTV‐Shuga (i.e. reported seeing ≥ 1 of 24 episodes; able to recall any storyline) and incident HSV‐2; incident pregnancy; condom use at last sex; uptake of HIV‐testing and contraception; and awareness of HIV Pre‐Exposure Prophylaxis (PrEP).
Results: Of 2184 (85.5%) eligible participants that were surveyed at baseline, 2016 (92.3%) had at least one follow‐up visit. MTV‐Shuga exposure at baseline was low – 308 (14.1%) reported seeing ≥ 1 episode and 121 (5.5%) recalled any storyline. Teenage pregnancy and incident HSV‐2 were high: 9.1 (95% CI: 9.2 to 11.4) and 15.3 (95% CI: 13.5 to 17.3) per 100 person‐years respectively. MTV‐Shuga exposed AGYW were from wealthier households, urban areas, and more likely to have been received DREAMS interventions (all p < 0.001). After adjusting for these confounders, watching MTV‐Shuga was associated with significantly greater awareness of PrEP (aOR = 2.06, 95% CI: 1.57 to 2.70), contraception uptake (aOR = 2.08, 95% CI: 1.45 to 2.98), consistent condom use (aOR = 1.84, 95% CI: 1.24 to 2.93), and lower probability of early pregnancy (aOR = 0.49, 0.26 to 0.81). Watching MTV‐Shuga was not associated with HIV testing (aOR = 1.02, 95% CI: 0.77 to 1.21) or acquiring HSV to 2 (aOR = 1.01, 95% CI: 0.68 to 1.51).
Conclusions: In a setting where AGYW remain at high risk for STI, HIV and early pregnancy, the minority who watched the MTV‐Shuga edu‐drama had better HIV prevention and sexual health outcomes. Further work is needed to explore the pathways through which MTV‐Shuga synergizes with social norms and interventions on the ground to improve demand and uptake of HIV prevention and sexual health technologies.
PDE0307
A Quality Improvement Collaborative (QIC) for HIV‐positive adolescents to improve immediate ART initiation at 25 Health Facilities (HF) in Lusaka, Zambia
G. Dougherty1, R. Boccanera2, M.A. Boyd3, T. Gantt2, S.C. Kasonka4, P. Kasonde4, N. Kaetano4, C. Madevu‐Matson5, P. Milimo4, M. Mwamba4, B. Senyana5, F. Tsiouris5, L. Walker5, N. Zyongwe6, A. Zulu4, M. Rabkin5
1ICAP at Columbia University, Mailman School of Public Health, New York, United States, 2U.S. Health Resources and Services Administration (HRSA), Bethesda, United States, 3U.S. Centers for Disease Control and Prevention (CDC), Lusaka, Zambia, 4ICAP Zambia, Lusaka, Zambia, 5ICAP at Columbia University, New York, United States, 6Ministry of Health, Quality Assurance, Lusaka, Zambia
Background: HIV testing and rapid antiretroviral therapy (ART) initiation are life‐saving interventions for adolescents living with HIV (ALWH). In Zambia, the time between HIV diagnosis and ART for ALWH often exceeds the national standard of two weeks, despite rollout of national guidelines, training and adequate ART supply.
Methods: In collaboration with the Zambian MOH, HRSA and CDC Zambia, ICAP at Columbia University designed and implemented a QIC to increase the proportion of ALWH (age 10 to 19) starting ART within two weeks of diagnosis at 25 HF in Lusaka between August 2018 and July 2019. Key indicators were collected at baseline and throughout QIC implementation, which included training on QI methods for 107 HF staff and leaders, monthly QI coaching visits, and quarterly workshops. Each HF QI team identified contextually appropriate interventions; used QI methods and tools to conduct rapid tests of change; and analysed progress using run charts. QI teams presented their performance and shared best practices at joint quarterly learning sessions.
Results: During the 12‐month implementation period, QI teams tested interventions focused on: health worker training, data quality, patient education, workflow processes and community engagement. 205,232 adolescents were tested for HIV during this time: 3355 (2%) were positive. ART initiation within 2 weeks of diagnosis improved from a median of 24% at baseline to a median of 95% during the final six months of the QIC. Same‐day ART initiation improved from a median of 27% at baseline to a median of 94% during the final six months of the QIC.
Abstract PDE0307‐Figure 1.
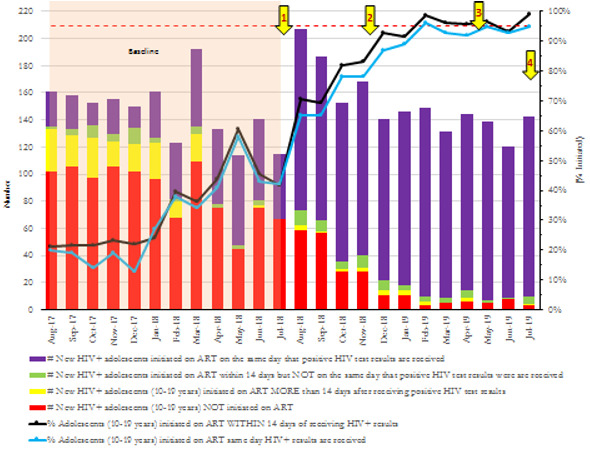
Conclusions: The QIC approach improved immediate ART initiation for ALWH by helping QI teams generate local innovations to identify and link ALWH to ART. In addition to building QI capacity and improving targeted outcomes, the QIC resulted in a “change package” of successful initiatives that will be disseminated within Zambia.
PDE0402
Improving access to quality HIV services in 11 West African countries: Impact of a regional community treatment observatory
G. Oberth1, S. Baptiste2, W. Jallow3, A. Manouan4, P. Garcia4, A. Traore4, J. Murara4, R. Boka5, V. Keïpo4
1University of Cape Town, Center for Social Science Research, Cape Town, South Africa, 2International Treatment Preparedness Coalition (ITPC), Johannesburg, South Africa, 3International Treatment Preparedness Coalition (ITPC), Gaborone, Botswana, 4International Treatment Preparedness Coalition (ITPC), Abidjan, Cote D'Ivoire, 5International Treatment Preparedness Coalition ‐ West Africa (ITPC‐WA), Abidjan, Cote D'Ivoire
Background: In West and Central Africa, 64% of people living with HIV (PLHIV) are aware of their status, 51% are accessing antiretroviral therapy (ART), and 39% are virally suppressed. Progress is stymied by low demand for services, frequent stock‐outs, weak health systems and poor quality of care. In 2017, the International Treatment Preparedness Coalition (ITPC) established a Regional Community Treatment Observatory in West Africa to increase accountability for the 90‐90‐90 targets.
Methods: ITPC trained and supported national networks of PLHIV to collect and analyse facility‐level HIV treatment data from 125 health centres in 11 West African countries. From January 2018 to June 2019, the treatment observatory completed 1781 monthly monitoring reports, 1501 interviews and 143 focus group discussions. Feedback was provided to patients, health centre staff and government decision‐makers through real‐time alerts, quarterly reports and multi‐stakeholder dialogues.
Results: At the monitored health centres, the frequency of ART stock‐outs decreased from 23.6% (95% CI 19.9 to 27.2) in the first six‐month period, to 16.4% (95% CI 13.6 to 19.3) in the second, to 15.2% (95% CI 12.3 to 18.1) in the third. In one country, the average duration of stock‐outs fell from 52.9 days (95% CI 33.4 to 86.3), to 32.9 days (95% CI 24.1 to 41.8), to 22.5 days (95% CI 9.4 to 35.6). The number of viral load tests performed more than doubled, from 16,532 in the first period, to 31,472 in the second, to 33,376 in the third. The rate of viral suppression increased dramatically, from 48.3%, to 67.9%, to 77.4%, respectively. In the third period, 30% of viral load results were returned within two weeks, up from 26% in the first period and 27% in the second. While quality of care steadily improved – from 3.8 (out of 5), to 4.0, to 4.2 – young women were twice as likely as the general population to say that unfriendly health workers were a barrier to services.
Conclusions: When communities of PLHIV are activated to monitor HIV services, access and quality improves. The treatment observatory changed the way that networks of PLHIV were perceived, creating a culture of collective problem‐solving among patients, healthcare workers and policy‐makers. The approach should be expanded to achieve global targets.
PDE0403
Implementing a “Low Dose High Frequency” capacity building approach for HIV service delivery in Uganda's military health facilities
D. Bwayo1
1URC, Program, Kampala, Uganda
Background: Evolving evidence indicates that Continuous capacity building (CB) is critical for improving health workers’ knowledge and is used to inform HIV care guidelines. The lack of standard appropriately tailored CB content and delivery mechanisms contributes to non‐adherence to evidence‐based practices with less than optimal HIV care service delivery. The Low Dose High Frequency (LDHF) CB approach has been shown to be comparatively effective and impactful. We describe the URC‐Department of Defense HIV/AIDS Prevention Program LDHF approach and its effect on HIV services within the Uganda military health services.
Description: We implemented a LDHF approach that entailed; initial start‐up training of health workers followed by bi‐monthly on‐site mentorship, coaching and feedback session at 28 military health facilities over a 6‐month period. Using mixed methods, we assessed health workers’ response to the approach and adherence to HIV guidelines. Data were abstracted from 541 client records with 12 health workers interviewed.
Lessons learned: Overall, health workers were positive to the LDHF approach which resulted into improvement in quality of care. Prescriptions for recommended first line ART regimen improved from 82% to 95%; timely due viral load test ordering increased from 45% to 80%; timely initiation of adherence counselling for non‐ suppressed clients increased from 32% to 55%; and appropriate switching of patients on failing regimes improved from 23% to 51%. Key barriers to adherence to guidelines raised by the health workers were; burdensome reporting requirements, work overload, complex guidelines, lack of capacity in paediatric guidelines, inability to timely follow‐up of some patients and frequent changes in existing guidelines.
Conclusions/Next steps: The LDHF CB model was acceptable to health workers and results in adherence to HIV guidelines. However, comprehensive adherence to the guidelines requires addressing other health system and patient‐related factors that cannot be resolved by the LDHF approach alone.
PDE0404
Are ART patients who miss appointments really lost? Determining true patient outcomes through tracing in 7 regions of Namibia
L. Bikinesi1, I.C. Pietersen2, S. Hong2, T. Negussie1, B. Seolwane3, N. Hamunime1, L. Ashipala1, E. Dziuban2, G. Mutandi2, A. Knutson4, D. Cockburn3, A. Besa3
1Ministry of Health and Social Services, Windhoek, Namibia, 2Centers for Disease Control and Prevention, Windhoek, Namibia, 3Development AID from People to People, Windhoek, Namibia, 4Centers for Disease Control and Prevention, Atlanta, United States
Background: Namibia's standard of care for antiretroviral therapy (ART) includes phone and physical tracing of treatment defaulters. Development Aid from People to People Namibia (DAPP) is a community‐based PEPFAR implementing partner providing support to the Ministry of Health and Social Services with community tracing. DAPP obtains from public health facilities a list of patients who missed their appointments from seven days to a month. Tracing is conducted telephonically and physically. Transfers are verified with the health facilities.
Methods: Programme data were analysed for DAPP from seven high‐burden regions for the period of October 2018‐September 2019. Key analytic outcomes were traced and untraced; with traced further classified into alive, died and unable to locate; and with those alive classified into confirmed missing, confirmed active, silent transfer, facility transfer out and unable to locate; and with those confirmed missing further classified into re‐engaged and confirmed active, promised to return to care and refused.
Results: Results: The graph below shows tracing outcomes.
Abstract PDE0404‐Figure 1.
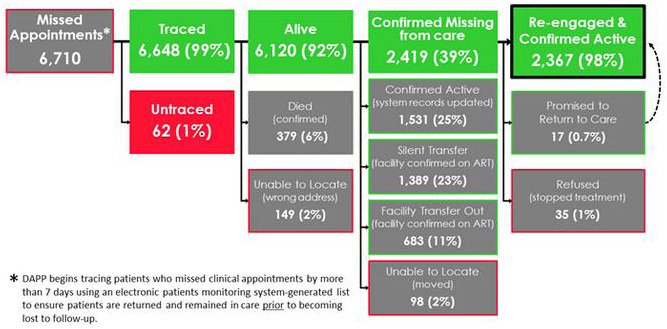
Conclusions: Most patients thought to be missing appointments were still active in care in the same ART clinic or at another ART clinic. Of those patients truly missing, most were able to be re‐engaged into care through tracing. This model of tracing is being scaled throughout all ART clinics in Namibia.
PDE0405
Integration of key population classification into national routine HIV testing services in Mozambique, 2019
J. De Seleme1, A. Couto1, N. Chicuecue1, G. Amane1, R. Hoek1, M. Cunha1, H. Magaia1, I. Sathane1
1Ministry of Health of Mozambique, STI and HIV/AIDS Control Program, Maputo Cidade, Mozambique
Background: Though over one‐quarter of new HIV infections are among key populations (KP), there is a dearth of national‐level cascade programme data for KP in higher prevalence countries. Expansion of HIV Testing Services (HTS) in Mozambique with provision of KP‐specific care has created an opportunity to collect timely national programme data on KP, specifically female sex workers (FSW), men who have sex with men (MSM), people who inject drugs (PWID) and prisoners. This information can provide essential, timely information for optimal resource allocation, programmatic decisions, and contribute to the body of knowledge on KP in the region.
Description: In March 2019, the Ministry of Health updated HTS data tools to enable collection of key population status and test result data at all public HTS facilities in Mozambique (n = 1634). HTS providers were trained using a KP package that included sensitization materials and a risk behaviour classification algorithm to identify and record KP status on HTS paper‐based forms, which are aggregated at the clinic level and digitized into a national database.
Lessons learned: We analysed HTS routine programme data from April to December 2019. Of 2709,331 persons receiving HIV counselling and testing during this time, 37,223 (1.4%) were identified as KP; 503 (0.02%) were PWID and 6022 (0.22%) were prisoners. Among females tested, 26,471 (1.8%) were FSW. Among males tested, 4227 (0.4%) were MSM. The proportion testing positive was highest among PWID (18%) and lowest among prisoners (11%). Among MSM, 15% were positive compared to 6% of males not classified as MSM. Among FSW, 13% tested positive for HIV compared to 6% among non‐FSW classified women.
Conclusions/Next steps: Mozambique is among the first high HIV prevalence countries to collect national programme data on KP status at all public HTS sites. Data confirm feasibility of capturing KP status country‐wide. The granular level and timely information on KP at facility, district and national level can complement other surveillance data such as KP size estimations and behavioural surveillance surveys to better assess the roll of KP in national and sub‐national HIV epidemics, and helps with planning and allocating prevention and treatment resources appropriately.
PDE0406
Modelling differentiated HIV services for transgender people: Experiences from Maharashtra, India
J. Kirubakaran1, S. Acharya2, L. Gabhane3, S. Katkar2, M. Setia4, S. Panyam5, S. Talwar6, S. Mathew7, P. Goswami8, G. Shreenivas8, J. Baishya9, B. George8, P.S. Keskar2
1Consultant Public Health Expert, New Delhi, India, 2Mumbai District AIDS Control Society (MDACS), Mumbai, India, 3Maharashtra State AIDS Control Society (MSACS), Mumbai, India, 4Consultant, Epidemiologist, Mumbai, India, 5Consultant Monitoring and Evaluation Expert, Hyderabad, India, 6Consultant Program Management Expert, Mumbai, India, 7Consultant Strategic Information Expert, Bengaluru, India, 8FHI360, New Delhi, India, 9USAID/India, New Delhi, India
Background: In India, HIV services for key populations are delivered through targeted interventions (TIs) implemented by nongovernmental organizations and funded by the National AIDS Control Organization (NACO). To maximize impact, optimized service delivery approaches are needed to meet the differentiated preferences of key population members with elevated risks.
Methods: In collaboration with Maharashtra/Mumbai AIDS Control Societies and NACO, we developed and validated a model to prioritize service delivery for transgender people under the PEPFAR/USAID‐supported and FHI 360‐led LINKAGES project. We analysed routinely collected programme data from two transgender TIs in Maharashtra, covering demographics, risk behaviour, vulnerabilities and biological outcomes from April 2016–March 2018. Individuals’ behavioural data prior to HIV testing were linked to their test results, generating 3938 data points. We used penalized regular logistic regression analyses to estimate the odds ratio, 95% confidence intervals of HIV positivity, and prospective explanatory variables; the best model was used for dominance analyses to estimate the weights. The final model was applied prospectively in two transgender TIs to study their efficiency in segmenting transgender people for differentiated prevention services.
Results: In the data set for generating the model, the HIV positivity proportion was 0.94%. The factors associated with HIV positivity were being transgender for less than three years (p < 0.001) and ever having missed a condom in the last 10 sex acts (p = 0.01), which were assigned the highest weights. We used an optimal cutoff for identifying high‐priority and moderate‐priority groups; the sensitivity for HIV positivity was 83.8%, and negative predictive value was 99.8%. At this cutoff, the proportion of HIV positivity in the high‐priority group was significantly higher compared with the moderate group (p = 0.007). Among the 1785 transgender people prospectively categorized into priority groups, 1276 (71.5%) were considered high priority. All 27 HIV cases were from the high‐priority group (p < 0.001).
Conclusions: The model demonstrated effective, precise categorization of transgender people at increased HIV risk, supporting the need for differentiated efforts for priority subgroups. Based on this successful experience, a national‐level model has been developed as part of NACOs revised/revamped TI strategies.
PDE0407
A national electronic system to support antiretroviral (ART) initiation of people living with HIV in Brazil at site level
R. Vianna Brizolara1, T. Cherem Morelli2, P. Emília Adamy1, A. Alberto Cunha Mendes Ferreira1, R. Castro de Albuquerque1, A. Francisca Kolling1, M. Camelo Madeira de Moura1, A. Sposito Tresse1, G. Fernando Mendes Pereira1, M. Araújo de Freitas1
1Ministry of Health of Brazil, Department of Chronic Diseases and Sexually Transmitted Infections, Brasília, Brazil, 2Ministry of Health of Brazil, Department of Chronic Disease and Sexually Transmitted Infections, Brasília, Brazil
Background: After national implementation of Test and Start strategy, Brazilian Ministry of Health (MOH) developed an electronic system to support immediate ART initiation of people living with HIV (PLHIV) in Brazil linked to care at site level. The Clinical Monitoring System of People Living with HIV (SIMC) was launched in 2013 and was made available countrywide.
Description: HIV services of the national health system are granted access electronically through SIMC to on‐line lists of HIV‐positive individuals older than 12 y.o with at least one VL exam who have not started ART. Lists are automatically generated on a monthly basis by the MOH, with the linkage of the national ART with the laboratory VL database. Lists of patients are specific for each HIV service, so that health care workers have access to information of their own patients only. The situation of each patient in the list is analysed by HIV services and outreach activities are conducted so that patients have their treatment started. The use of SIMC counts entirely with the existing structure of the National Health System and does not require additional funds. By December 2019, 2948 health workers from 1692 health services had access to the system.
Lessons learned: From December 2013 to December 2019, SIMC identified 209,746 PLHIV who had not started ART: 82,3% (172,526) were analysed ‐ 73,5% (154,215) started treatment over the period, 2,8% (5949) were dead, 0,4% (743) were transferred, 2,9% (6065) were not located, 0,6% (1274) refusal treatment, 1,2% (2488) were duplicates and 0,9% (1792) others. 17,7% (37,220) have not yet been analysed. In the same period, time from linkage to care and ART initiation in Brazil decreased from 182 to 33 days.
Conclusions/Next steps: SIMC has proven to be useful to support ART initiation at site level and stands as an important national strategy to improve access to care and treatment services and reduce time from diagnosis to ART initiation. Moving forward, in 2020 MOH will include in SIMC individuals below 12 y.o and link other national surveillance systems, in order to include all PLWHIV who were tested positive for HIV but did not have a VL exam.
PDE0502
Cost‐based estimated prices for key HIV, HCV, and MDR‐TB medicines
M. Barber1, D. Gotham2, A. Hill3
1Harvard University, Global Health and Population, Boston, United States, 2Independent, London, United Kingdom, 3University of Liverpool, Department of Translational Medicine, Liverpool, United Kingdom
Background: Prices for treatments for HIV and coinfections vary substantially between countries, with high prices limiting treatment access in many settings. Scaling up of generic HIV manufacture has enabled massive expansion of global treatment programmes. The cost of active pharmaceutical ingredient (API) is a significant determinant of the cost of production. The aim of this analysis was to estimate cost‐based prices that could be achieved with robust generic competition for WHO‐recommended treatments for key HIV, hepatitis C virus (HCV) and multidrug‐resistant tuberculosis (MDR‐TB) medicines, and to compare them with current prices.
Methods: Active pharmaceutical ingredient (API) price and volume data were collected from an online database (Panjiva) of export data from India. Cost‐of‐production was then calculated using an established algorithm, accounting for API costs, excipients (API × 2 × $2.63), formulation ($0.01/pill), tax obligations on profit assuming manufacture in India (27%), and a 10% profit margin. List prices for key HIV, TB and HCV drugs were extracted from national drug price databases in seven countries for comparison.
Results: Table 1 shows current prices of antiretrovirals for HIV (per year), direct‐acting antivirals for HCV (per 12 week course), and solid oral formulations for tuberculosis (per month). API costs/kg were $1500 for dolutegravir (DTG), $5000 for tenofovir alafenamide (TAF), $150 for tenofovir disoproxil fumarate (TDF), $900 for darunavir (DRV), $700 for sofosbuvir (SOF), $600 for daclatasvir (DCV), $6000 for velpatasvir (VEL), $250 for moxifloxacin (MFX) and $100 for linezolid (LZD). There were inadequate data to determine glecaprevir+pibrentasvir (G+P) API prices.
Abstract PDE0502‐Table 1.
| Estimated cost‐based price | Argentina | Brazil | India | Russian Federation | Thailand | Ukraine | USA (Veterans Affairs) | |
|---|---|---|---|---|---|---|---|---|
| DTG | $35 | $7408 | $3687 | $574 | $1672 | $3695 | $4467 | $12,520 |
| TAF | $25 | $3717 | – | $252 | – | – | – | $9026 |
| TDF | $23 | $2807 | $1913 | $174 | $40 | $131 | $1832 | $264 |
| DRV | $455 | $6162 | $1955 | $841 | – | $1410 | – | $6441 |
| SOF+VEL | $85 | $37,499 | $13,632 | – | – | – | – | $17,965 |
| SOF+DCV | $31 | $30,012 | $25,732 | $41 | $8976 | $7021 | $78 | $111,659 |
| G+P | – | $24,085 | $12,724 | – | – | – | – | $19,014 |
| MFX | $4 | $93 | $62 | $6 | $43 | $91 | $33 | $70 |
| LZD | $5 | $9 | $45 | $2 | $3 | $11 | $12 | $5260 |
HIV (yearly), HCV (per course), TB (monthly). USD.
Conclusions: Current prices for medicines are up to 1000 times more than cost‐based estimated generic prices. Originator prices are often incongruous with country income level, with implications for access and scale‐up of treatment programmes.
PDE0503
Healthcare resource use and related cost of non‐HIV comorbidities management in people living with HIV in a Spanish cohort from 2007 to 2017
Y. Milanes Guisado1, F. Jodar2, D. Sánchez Pardo3, J. Moreno3, P. Viciana1, C. Roca1
1Institute of Biomedicine of Seville/Virgen del Rocio Hospital, Infectious Diseases, Seville, Spain, 2Institute of Biomedicine of Seville, Medical Informatics and Health Economics, Seville, Spain, 3Institute of Biomedicine of Seville/Virgen del Rocio Hospital, Medical Informatics and Health Economics, Seville, Spain
Background: Few studies have assessed the economic impact of non‐HIV comorbidities in people living with HIV (PLHIV) during a long follow‐up period. Our objective was to estimate the cost and healthcare resources use associated to the prevalence of comorbidities in PLHIV in a Spanish cohort along 11 years.
Methods: From 2007 to 2017, patients with at least one follow‐up visit were included. PLHIV were categorized into two groups according to time of diagnosis: before 2007 (Pre_2007 group) and after 2007 (Post_2007). The cost categories evaluated were Hospitalizations, Ambulatory costs, Emergency visits, Laboratory testing, HIV Antiretroviral therapy (ART), and Other Non‐HIV tests. Generalized linear models and cross‐validation were used to evaluate predictors of total care cost for overall population and by stratified groups.
Results: The study included 2.803 PLHIV (21.385 p‐y), 82.9% were men, median age at entry was 39 years (Interquartile Range (IQR): 32 to 44). 1.660 (59.2%) were included in Pre_2007 Group and 1.143 in Post_2007, 8.9 ± 3.40 and 5.8 ± 2.8 years of follow‐up, respectively (p < 0.001). Overall, 1.623 (57.9%) presented at least one non‐HIV comorbidity, 826 (29.3%) had 3 or more comorbidities during follow‐up. The most prevalent non‐HIV comorbidities were hypertension (28.5%), Diabetes (13.7%), Bacterial infections (13.4%) and Cardiovascular diseases (12.8%). The presence of comorbidities increases total healthcare cost by 47%. The highest cost increase of 65% was observed in PLHIV with three or more comorbidities compared to PLHIV with no comorbidities (over the 11‐year period, 115.435 ± 57.870€ vs. 69.795 ± 47.931€, p < 0.001). The mean annual cost per patient was higher in Pre_2007 group with comorbidities compared to the Post_2007 cohort (14.218 ± 11.117€ vs. 12.159 ± 8.207€, p < 0.001 for interaction); due to a higher mean age and longer follow‐up. Patterns are similar if ART costs are excluded. There was a positive correlation between the presence of comorbidities and healthcare costs in PLHIV, both for the overall population and for stratified groups for adjusted models.
Conclusions: The presence of comorbidities increases the total healthcare cost of PLHIV, being more relevant in older patients. Both clinical and economic decision‐makers should consider and evaluate cost of comorbidities when evaluating HIV treatment guidelines or recommendations.
PDE0504
The clinical and economic impact of genotypic resistance testing after virologic failure on first‐line tenofovir‐lamivudine‐dolutegravir in South Africa
E.P. Hyle1,2, C.M. Dugdale1,2, A.C. Bangs1, L.‐G. Bekker3, M.J. Siedner1,2, S.M. McCluskey1,2, P.E. Sax2,4, A.L. Ciaranello1,2, J.A. Scott1, R.T. Gandhi1,2, M.C. Weinstein5, K.A. Freedberg1,2, R. Wood3, R.P. Walensky1,2
1Massachusetts General Hospital, Department of Medicine, Boston, United States, 2Harvard Medical School, Boston, United States, 3The Desmond Tutu HIV Center, University of Cape Town, Cape Town, South Africa, 4Brigham and Women's Hospital, Medicine, Boston, United States, 5Harvard T. H. Chan School of Public Health, Department of Health Policy and Management, Boston, United States
Background: Treatment‐emergent resistance is rare when tenofovir‐lamivudine‐dolutegravir (TLD) is used as first‐line ART. We examined the clinical and economic impact of genotypic resistance testing (GRT) for adults in South Africa with virologic failure (VF) on first‐line TLD despite 3 m of enhanced adherence counselling (EAC).
Methods: We used the CEPAC‐International model to compare four strategies among adults with VF on TLD after EAC: 1) “GRT” to distinguish people with susceptible virus (VFS) who continue TLD from people with dolutegravir‐resistant virus (VFR), who switch to second‐line ART (LPV/r+AZT/3TC); 2) “Immediate switch” to second‐line; 3) “EAC+,” additional EAC while continuing TLD with switch to second‐line for anyone with persistent VF at 6 m; 4) “TLD,” remaining on TLD indefinitely. We assumed 1% of VF were VFR and mean ART adherence was better among VFR (85%) than VFS (78%). We estimated 48‐wk virologic suppression based on trial data and adherence (Table 1). Costs included TLD ($70/yr), LPV/r+AZT/3TC ($270/yr) and genotypes (RT and IN, $290/total). Outcomes were life expectancy (LE), HIV‐related costs, and incremental cost‐effectiveness ratios (ICERs, Δ$/ΔLE). Sensitivity analyses included %VFR, ART effectiveness (Table), second‐line cost ($60 to 270/year) and genotype cost ($50 to 290/total).
Abstract PDE0504‐Table 1. Selected model input parameters and modelled outcomes regarding the clinical and economic impact of genotypic resistance testing after virologic failure on first‐line tenofovir‐lamivudine‐dolutegravir in South Africa
| Model input parameters | VFR, base case (range) | VFS, base case (range) |
|---|---|---|
| Prevalence among those with VF | 1% (0 to 20%) | 99% (80 to 100%) |
| 48‐week suppression | TLD: 35% (25 to 59%) LPV/r+AZT/3TC: 73% (62 to 85%) | TLD: 68% (50 to 71%) LPV/r+AZT/3TC: 60% (50 to 70%) |
| Modelled outcomes | Undisc. LY | Undisc. Costs ($) | Disc. LY | Disc. Costs ($) | ICER ($/YLS)a |
|---|---|---|---|---|---|
| TLD | 26.13 | 14,400 | 15.97 | 9000 | – |
| GRT | 26.35 | 14,800 | 16.08 | 9400 | 3100 |
| EAC+ | 26.02 | 17,500 | 15.90 | 10,700 | Dominated |
| Immediate switch | 25.52 | 19,200 | 15.61 | 12,000 | Dominated |
Disc, discounted; GRT, genotypic resistance testing; ICER, incremental cost‐effectiveness ratio; LPV/r+AZT/3TC, lopinavir‐ritonavir+zidovudine‐lamivudine; LY, life years; TLD, tenofovir‐lamivudine‐dolutegravir; Undisc, undiscounted; VFR, virologic failure with resistant virus; VFS, virologic failure with susceptible virus; YLS, year‐of‐life‐saved.
a We calculated ICERs using LYs and costs discounted at 3%/year. We considered strategies to be cost‐effective if ICER<$1175/YLS (Woods et al. 2015) or ‘dominated’ if clinical outcomes were worse at higher cost.
Results
GRT resulted in longer LE at lower costs than EAC+ and Immediate switch (Table). GRT became cost‐effective versus TLD (ICER<$1175/YLS) at genotype cost ≤$60 or VFR prevalence ≥5%. In sensitivity analyses, GRT remained clinically preferred unless virologic suppression for VFS was higher on second‐line than TLD; even then, GRT remained the most economically efficient strategy, unless second‐line also cost less than TLD.
Conclusions: A strategy offering genotypic resistance testing after virologic failure on TLD resulted in the best clinical outcomes at lower cost than EAC+ or Immediate switch and was cost‐effective versus TLD at lower genotype cost (≤$60) or ≥ 5% dolutegravir resistance. Scaling up capacity for genotype in resource‐limited settings is a rational addition to TLD rollout.
PDE0505
Preparing for scale‐up: The changing cost and cost‐effectiveness of HIV self‐testing integration into community‐based mobile outreach and index HIV testing models in Lesotho
M. d'Elbée1, M.C. Makhetha2, M. Jubilee3, K.M. Maphatsoe‐Pule2, M. Taole2, S. Nako2, C. Nkomo2, A. Machinda2, M. Tlhomola4, L. Sande5,1, L. Corbett5,6, C.C. Johnson7, K. Hatzold8, G. Meyer‐Rath9,10, F. Terris‐Prestholt1
1London School of Hygiene and Tropical Medicine, Global Health and Development, London, United Kingdom, 2Population Services International, Maseru, Lesotho, 3John Snow, Inc, Lusaka, Zambia, 4Ministry of Health, Maseru, Lesotho, 5Malawi Liverpool Wellcome Trust Research Programme, Blantyre, Malawi, 6London School of Hygiene and Tropical Medicine, Department of Clinical Research, London, United Kingdom, 7World Health Organisation, Department of HIV, Hepatitis and STIs, Geneva, Switzerland, 8Population Services International, Johannesburg, South Africa, 9Health Economics and Epidemiology Research Office ‐ University of the Witwatersrand, Department of Internal Medicine, Johannesburg, South Africa, 10Boston University, School of Public Health, Boston, United States
Background: In Lesotho, 25.6% of adults are living with HIV (PLHIV) with only 81% aware of their status. HIV self‐testing (HIVST) was added to existing mobile outreach and index testing (HTS) in five priority districts in 2017. We investigated costs and yield as the programme evolved (Figure 1).
Methods: We evaluated programme costs before/after HIVST addition (Period 1 and 2: Figure 1), and after encouraging clients to use onsite HIVST booths (Period 3) allowing multiple clients to self‐test concurrently and immediately link to confirmatory testing. We estimated full economic costs for mobile HTS, including central costs, and only incremental cost of adding HIVST onto HTS as well as overall cost‐effectiveness of all testing.
Results: The introduction of onsite HIVST increased HIV yield in all districts (Figure 1). For both HTS and HIVST programmes, the drivers of costs are personnel and testing supplies (Table 1). Costs per new HIV‐positive case identified increased between period 1 and period 2 but was the lowest in period 3 when onsite HIVST was introduced.
Abstract PDE0505‐Figure 1. Outcomes of the ongoing HIV testing programme between May 2017 and April 2019.
Yield corresponds to new HIV‐positive cases among clients tested with HTS, including confirmatory testing following a reactive self‐test.
Abstract PDE0505‐Figure 1.
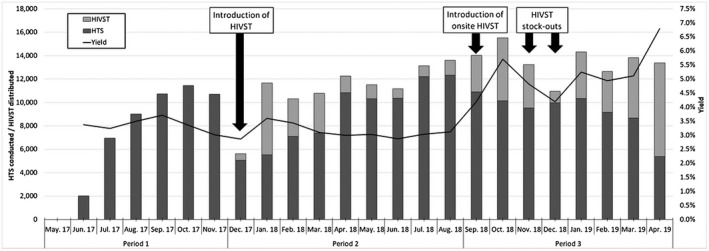
Abstract PDE0505‐Table 1. Three‐month averages of costs and outcomes of the HTS/HIVST programme by period
| US$ 2019 | Period 1 | Period 2 | Period 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost inputs | HTS | % | HTS | % | HIVST | % | HTS | % | HIVST | % |
| Personnel & Per diems | $546,031 | 67% | $614,262 | 65% | $72,445 | 75% | $781,795 | 74% | $23,121 | 33% |
| Supplies (rapid tests, HIVST kits, consumables) | $115,657 | 14% | $86,126 | 9% | $17,396 | 18% | $75,490 | 7% | $34,510 | 49% |
| Others (start‐up and central costs, capital (vehicle), and other recurrent costs: fuel, waste, etc.) | $157,953 | 19% | $245,824 | 26% | $7395 | 7% | $203,146 | 19% | $12,941 | 18% |
| Total costs – HTS and HIVST programme | $819,641 | 100% | $946,212 | 100% | $97,236 | 100% | $1060,431 | 100% | $70,572 | 100% |
| Total costs ‐ HIV testing programme | $819,641 | $1043,448 | $1131,003 | |||||||
| Total number of new HIV‐positive cases identified | 858 | 836 | 1392 | |||||||
| Cost per new HIV‐positive case identified | $955 | $1248 | $813 | |||||||
Conclusions: Continuous programme learning is critical for sustainable scale‐up. The introduction of HIVST improved overall programme efficiency and cost‐effectiveness once onsite self‐testing became available. Additionally, our HIVST incremental costs assume that the existing HTS programme is adequately funded, which should considered when planning for scale‐up.
PDE0506
Single versus multiple tablet regimens for first‐line antiretroviral treatment of HIV: A real‐world cost‐effectiveness analysis using a patient cohort in Brazil
J. de Oliveira Costa1,2, W. Botha1, M.d.G. Braga Ceccato3, S. Pearson1, S. Goodall4, F. de Assis Acurcio3,2, ECOART Project Group
1UNSW Sydney, Centre for Big Data Research in Health, Sydney, Australia, 2Universidade Federal de Minas Gerais, Post‐Graduation Program in Public Health, Belo Horizonte, Brazil, 3Universidade Federal de Minas Gerais, Department of Social Pharmacy, Belo Horizonte, Brazil, 4University of Technology Sydney, Centre for Health Economics Research and Evaluation, Sydney, Australia
Background: Single‐tablet regimens (STR) are as effective in improving antiretroviral therapy outcomes and cost‐effective when compared with multiple‐table regimens (MTR) based on clinical trial data. However, there is currently no evidence on the real‐world cost‐effectiveness of STR in the Brazilian context.
Methods: Data from 440 people initiating antiretroviral therapy in 2014 and 2015 in Belo Horizonte, Brazil were analysed. We compared the STR containing tenofovir, lamivudine, efavirenz to multiple‐tablet regimens with the same components (MTR‐SC) or different components (MTR‐Other). We assigned effectiveness and costs to the initiating therapy and defined effectiveness as the probability of achieving viral suppression (viral load < 50 copies/ml) after 12 months of therapy. The cost analysis was adjusted for censoring and a public payer perspective was adopted, which included direct medical costs.
Results: A total of 185 (42.0%) patients initiated STR, 189 (43.0%) MTR‐SC and 66 MTR‐Other. Overall, 64.3% of patients achieved viral suppression and the average annual cost per patient was US$ 1544 (SD 3803). STR was as effective but a lower cost option when compared to MTR, hence it dominates MTR (Table 1). The lower cost of STR was driven by a lower utilization of specialists’ visits, laboratory exams, and by the lower cost of antiretroviral therapy, despite no differences in the number of dispensations among groups. STR is the optimal choice for payers with a willingness to pay threshold below US$ 19,500 to 21,500 (Figure 1).
Abstract PDE0506‐Table 1.
| Outcomes after 12 months of follow‐up | STR (n = 185) | MTR‐SC (n = 189) | MTR‐Other (n = 66) | p‐value* |
|---|---|---|---|---|
| Suppressed viral load, % (95% CI) | 62.7 (55.7; 69.7) | 65.1 (58.2; 71.9) | 66.6 (55.0; 78.3) | 0.812 |
| Total mean cost per patient, US$ (SD) | 1102 (2776) | 1572 (3453) | 2706 (6283) | 0.013 |
| Cost per responder ratio (95% IC) | 1757 (1178; 2461) | 2415 (1788; 3289) | 4059 (2202; 7084) | – |
| Incremental cost‐effectiveness ratio, US$/responder | Reference | 19,583 | 41,128 | – |
1 US$, 1.996 R$; CI, Confidence interval; efavirenz; lamivudine; MTR‐Other, multi tablet regimen with different components of STR; MTR‐SC, multi tablet regimen same components of STR; SD, Standard deviation; STR, Single tablet regimen containing tenofovir disoproxil fumarate.
*χ2 test or Student‐t tests, where appropriate.
Abstract PDE0506‐Figure 1.
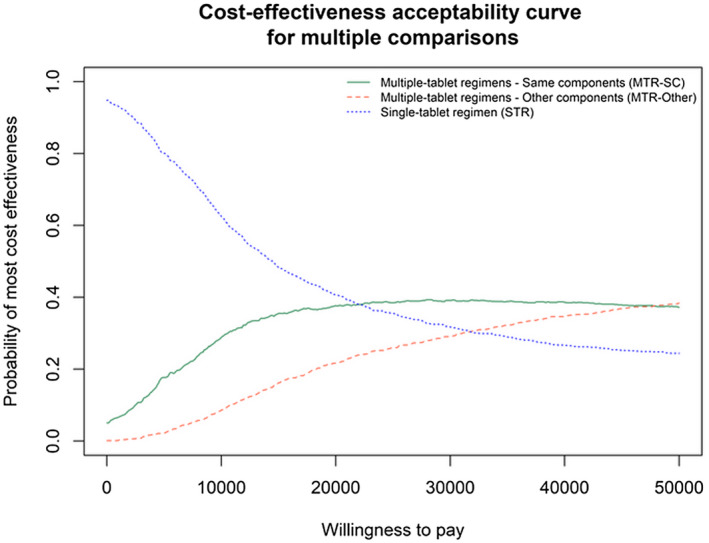
Conclusions: We identified that generic STR is the most cost‐effective regimen when compared to all other multiple‐tablet regimens available in the period analysed. This regimen should be the standard comparator for future evaluations and the preferred first‐line therapy in the Brazilian health system.
PDE0507
The impact and cost‐effectiveness of expanding cryptococcal antigen screening to include individuals with CD4 100 to 200 cells/µL in Botswana
C. Muthoga1,2, M.W. Tenforde2,3,4, P. Ponatshego1,2, J. Ngidi1,5, M. Mine5, B. Larson6, J.N. Jarvis1,7
1Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana, 2Botswana‐UPenn Partnership, Gaborone, Botswana, 3University of Washington School of Medicine, Seattle, United States, 4University of Washington School of Public Health, Seattle, United States, 5National Health Laboratory, Gaborone, Botswana, 6Boston University School of Public Health, Boston, United States, 7London School of Hygiene & Tropical Medicine, London, United Kingdom
Background: Cryptococcal antigen (CrAg) screening and pre‐emptive fluconazole for CrAg‐positives reduces incident cryptococcal meningitis (CM) and all‐cause mortality in persons with advanced HIV starting antiretroviral therapy (ART). The WHO conditionally recommended increasing CrAg screening thresholds from CD4 < 100 to < 200 cells/µL in those initiating or re‐initiating ART, but the benefit of this increase in CD4 threshold is unknown. We evaluated the marginal impact and cost‐effectiveness of reflex CrAg screening among patients with CD4 100 to 200 cells/µL in Botswana, a country with a mature ART programme still performing CD4 count monitoring post‐ART initiation.
Methods: We developed a decision analytic model to evaluate laboratory‐based CrAg screening at CD4 counts of 100 to 200 cells/µL using local CD4 distribution, CrAg prevalence, titre and ART status data from 2019. We estimated CM cases and deaths averted, and cost per disability‐adjusted life year (DALY) averted with nationwide implementation of CrAg screening in this group compared to the current policy of no screening.
Results: An estimated 34,775/650,000 (5.35%) CD4 tests nationwide in 2019 were 100 to 200 cells/µL; of these, 2.5% were CrAg positive and eligible for pre‐emptive therapy with 20% having a high CrAg titre (>1: 160) indicating higher risk of CM progression. Only 15% were ART‐naïve; 25% of ART‐experienced were classified as defaulters/treatment failures. Without screening, 129 CM cases (36 in ART‐naïve) and 77 CM‐related deaths (21 in ART‐naïve) occur. With screening and pre‐emptive fluconazole for ART‐naïve patients only, an estimated 6 deaths and 123 DALYs are averted at a cost of US$1385/DALY averted. With treatment extended to ART‐naïve and ART‐experienced, 43 deaths and 919 DALYs are averted at a cost of US$244/DALY averted.
Conclusions: In a mature ART programme with routine CD4 monitoring, a low proportion of CrAg‐positive patients with a CD4 100 to 200 cells/µL were ART‐naïve. Pre‐emptive treatment in ART‐naïve has only a marginal impact and modest cost per death or DALY averted. Assuming a benefit in treating ART‐experienced individuals (a proportion of whom are reinitiating ART), screening and pre‐emptive treatment has greater impact and is more cost‐effective. CrAg screening in the CD4 100 to 200 cells/µL group should include ART‐experienced individuals, who now make up a majority of CM cases.
PDF0102
Strong in diversity: Building intersectionality in creating an enabling environment for HIV work in East Java, Indonesia
D. Oetomo1
1GAYa NUSANTARA Foundation, Surabaya, Indonesia
Background: In the past three years Indonesia has experienced a politicized moral panic involving homophobia, transphobia, demonization of PLHIV and people who use drugs, and more generally other religious and social minorities. Human Rights Watch summarized the problem for HIV work among sexual minorities in its report: https://www.hrw.org/report/2018/07/01/scared‐public‐and‐now‐no‐privacy/human‐rights‐and‐public‐health‐impacts‐indonesias. Civil society has fought back intersectionally to create and maintain equity and equality, including advocating for enabling environment for work on HIV and STIs as well as sexual and reproductive health and rights more generally.
Description: GAYa NUSANTARA Foundation, Surabaya, East Java, Indonesia, has in 2018 to 2019 collaborated with (inter)faith organizations, media, human rights organizations, AIDS service organizations, academics and politicians to mainstream diversity in SOGIESC to partners in eight key municipalities or districts in East Java Province (total number of municipalities and districts: 38).
The project identifies existing sexual minority communities, often through AIDS service organizations; primes them to intersectionally and strategically work with key stakeholders; and creates local networks to work on issues of diversity in SOGIESC in a proactive and a reactive way (i.e. responding to crises such as negative government policies or disturbances by religious militias). This is done through preliminary work, and follow‐up workshops.
Particularly on faith issues, a workshop with progressive faith leaders and theologians as well as members of diverse SOGIESC communities in 2018 resulted in a master document on progressive interpretation of Christian and Islamic texts, currently in print. This will be used for campaigns, both offline and online, to create a conducive social environment.
Lessons learned: 1. Trans women communities are most ready to engage, while in some locations gay men are also ready. People with non‐normative SOGIESC find one another and build networks, mostly through internet platforms.
2. Sexual minority communities are not in touch with key stakeholders and vice versa. The project brings them together to build advocacy and crisis response networks.
3. Allies exist in all localities. Knowledge of SOGIESC diversity and commitment to pluralism and human rights, vary.
Conclusions/Next steps: The project can be replicated in other localities. A similar project is on the drawing board in Eastern Indonesia and other localities in East Java.
PDF0103
Trans Task Force Team against violence and harassment towards marginalized transgender community across Pakistan, 2019
M.K.A. Choudhry1, K.A. Khan1
1Sub Rang Society, Karachi, Pakistan
Background: Established in 2016 and legally registered in 2018; Sub Rang Society (SRS) is working towards empowering transgender community in Pakistan to get their basic and equal human rights granted in Constitution of Pakistan as well as prescribed in Universal Declaration of Human Rights (UDHR).
Description: In 2019 a total of 11 transgender lost their lives in Pakistan. To fight against the harassment and violence related issues with transgender of Karachi, SRS has formulated TRANS TASK FORCE TEAM (TTFT) which is actively working across 19 towns of Karachi; against the issues faced by TGs and other sexually marginalized community members.
It was decided that two representative from each town will be selected for a point of contact, to build capacity and spread awareness of Trans Rights and legal Procedures within the trans community of their respective districts.
Lessons learned: TTFT tackles root causes of violence against transgender by working with civil society, local institutions and governments and helps change community attitudes towards transgender, supporting them to realize their potential and advocate for their rights.
TTFT will strive to minimize the cases of violence and harassment within Karachi city with the help of TTFT irrespective of Hijra Culture and their sects; work with unity and also build the capacity of TTFT to raise their voice and report the cases by TGs themselves.
Conclusions/Next steps: Support safety, justice and autonomy of all victims and survivors of violence.
Work to meet the needs of underserved and marginalized trans community.
Create a forum to enhance the response of sexual violence prevention initiatives among TTFT response team.: Create social media to empower TTFT with the members of the group for follow‐up, furthermore to create awareness regarding laws and policies of state.
TTFT will engage trans community and their allies to educate masses to reduces violence, stigma, discrimination and bring positive change in society towards marginalized trans community.
Efforts are also be made by focusing on policies and efforts to engage trans community by examining and challenging destructive notions of gender and power in post‐conflict settings, while being guided by the voices and inputs of community.
PDF0104
Sexual violence against women and girls fuelling the spread of HIV in urban communities than rural communities in Ondo State, Nigeria
F. Bamigboye1, D. Faponle2
1Kids & Teens Resource Centre, Programmes, Akure, Nigeria, 2Kids & Teens Resource Centre, M&E, Akure, Nigeria
Background: Majority of HIV Prevention Programmes in Nigeria promote safe sex through condom use while ignoring the realities of sexual violence and gender inequalities which increases the susceptibility/vulnerability of women and girls to HIV. Victims of gender‐based violence more often suffer sexual and reproductive health consequences including unwanted pregnancies, unsafe abortion, traumatic fistula, sexually transmitted infections that could lead to death or prevent survivors from achieving economic prosperity due to social stigma or physical and psychological trauma caused by the violence. This paper analysed and reveals the relationship between sexual violence and the spread of HIV in urban settings.
Description: An Adolescent Girls and Young Women Program was carried out in Akure (urban) and Bamikemo (rural) communities of Ondo State between February and August 2019 where SRHR issues were discussed among 52 adolescents (10 to 14) and Young Women (15 to 24).10 In‐depth Interviews with parents and community leaders, Focused Group Discussions with 12 adolescents and another one with 15 young women were conducted in both the rural and urban communities. Also, the youth leaders (2) and women leaders (2) and Community heads were interviewed.
Lessons learned: Focus Group Discussions and interviews revealed gender‐based violence (structural), male superiority (cultural) and economic disadvantage (financial) as factors increasing the susceptibility of Adolescent Girls and Young Women to HIV. 73% of the women and girls in the Urban areas reported sexual violence as the definite cause of the spread of HIV/STIs while 58% in the rural communities named Poverty, ignorance (63%) and abuse of trust (42%).Interviews with the youth leaders in the Urban revealed male superiority and economic power as major drivers of HIV while the rural area revealed cultural bias. Women leaders were of the opinion that gender inequalities was at the centre of problems they experience in life.
Conclusions/Next steps: Sexual Violence undermines the health, dignity and autonomy of its victims, yet it remain shrouded in a culture of silence. The power of protection and choice should be placed in the hands of women through women empowerment and female condoms availability/accessibility. The culture of acceptance of rape as something excusable or blame able should be shattered through policy advocacy &value clarification.
PDF0105
International gender affirming medical intervention to avoid persecution and violence in El Salvador
A. Montano1
1ALDES, San Francisco, United States
Background: Life expectancy for Central American transwomen is 35 years. They suffer sexual violence, extortion by criminal gangs and police. School bullying forces them to abandon their education. Families force them out of their homes at an early age. They are coerced into sex work or selling drugs. They are hate crime victims which are rarely investigated and criminals go ‘scot‐free.’
Verónica, Trans woman at ASTRANS (Salvadoran human rights NGO), states: “We know we can be killed. We do not know if we will come back home, or come to work the next day . . .”
Gender‐affirming services are vital; ‘passing’ as a woman or man protects against violence. Yet, sex change surgeries are not available. Trans Salvadorans must rely on hormones, but access is limited. Many transwomen inject oil into their breasts, leading to severe medical complications. Others purchase hormones, but without medical supervision, it's dangerous.
Description: ASTRANS has the only Non‐Surgical Gender Affirmation clinic managed by ‘queer’ staff. They offer free‐hormone therapy and social‐psycho support to one hundred (100) trans patients, approx. % are HIV + . ASTRANS teaches interpreting HIV lab results, avoiding self‐medication, reproductive options, legal/advocacy issues, the nature of human trafficking to reduce risk, and PrEP.
Lessons learned:
• Having a gender‐affirming clinic avoids dangerous self‐inflicted treatments like injecting oil in breasts as a desperate measure to enhance feminization.
• Free hormone treatment helps avoid discrimination and violence.
• HIV treatment and gender‐affirming hormones in one location, and staff who reflect the clientele, fosters trust.
• Clients that have a ‘safe space’ clinic engage and adhere to HIV care.
Conclusions/Next steps:
1. HIV care and gender affirming clinics accelerate ending the HIV/AIDS epidemic.
2. The ASTRANS clinic model saves trans lives.
3. The Salvadoran ASTRANS clinic model needs replication.
4. US researchers should collaborate with El Salvador and expand mental health supports, gender enhancing treatment, HIV prevention, evaluation and PrEP.
PDF0106
Reasons why younger queer face vulnerability and risks while seeking asylum in Kenya: Studying three safe spaces, August 2019‐January 2020
T. Mumba1, T. Muyunga‐Mukasa2, Refugees
1Refugee Independence Support Organisation, Communication Office, Nairobi, Kenya, 2Advocacy Network Africa, Advocacy, Mobilisation and Education, Nairobi, Kenya
Background: Kenya is considered to be a more progressive country than Uganda when it comes to asylum seeking by Queer refugees from other African countries. Younger Queer refugee's face a triple stigma based on age, status conditions of asylum seeking. Kenya bases its refugee reception conditions on her Immigration Act (Cap. 172) and the international instruments. However, younger Queer refugees meet bias and prejudices as they seek asylum. This study aimed at finding out what these biases and prejudices were as they navigated access to social services while awaiting resettlement in Kenya.
Methods: Through Respondent driven sampling, Questionnaire administering and Focus Group discussion with 74 younger MSM living in Nyeri, Nairobi and Nakuru as refugees from Uganda, Rwanda, Burundi and Tanzania.
Results: All respondents stated that what was on the ground was different from what they expected. 24 (18 to 25 years) reported they were suspected of being impostors or telling lies; recruited into the vice; and asked questions that were off the script of eligibility Such questions were probing whether they were recruited into homosexuality or trafficked. This led to 22 (18 to 22 years) being denied registration in time. This caused delays in acquiring National IDs. This raised vulnerability to them.
Conclusions: Age and status of person impact presentation and case processing for Queer refugees during asylum seeking. When they are younger they are said to be recruited into homosexuality. This study report was based on self reporting, further research using a bigger sample and service providers can throw more light on reasons for delays in refugee status processing, integration in host communities, productivity and awareness levels or cultural sensitivity of providers as far as Sexuality, Orientation, Gender Identity, Gender Expression and Sexual Characteristics (SOGIESC) is concerned.
PDF0202
Ending discrimination in HIV/TB programmes: Lessons learned from the Global Fund's Breaking Down Barriers initiative
J. Amon1, N. Sun1, R. Jurgens2, A. Iovita2, G. Arustamyan2, O.b.o. BDBI MTA Evaluation Team1
1Drexel University, Office of Global Health, Philadelphia, United States, 2Global Fund, CRG, Geneva, Switzerland
Background: The Global Fund's 2017 to 2022 Strategy recognizes that addressing HIV and TB requires scaling‐up programmes to remove human rights‐related barriers to health services and end discrimination. Supporting this goal, the five‐year Breaking Down Barriers (BDB) initiative has funded HIV and TB programmes in 20 countries focused upon: stigma and discrimination reduction; training for healthcare providers; sensitization of law‐makers and law enforcement agents; reducing HIV‐related gender discrimination, harmful gender norms and violence against women and girls in all their diversity; legal literacy; legal services and monitoring and reforming laws, regulations and policies. Additional programmes for TB include: mobilizing and empowering patient and community groups; addressing overly broad policies regarding involuntary isolation or detention for failure to adhere to TB treatment; and making efforts to remove service barriers in prisons.
Description: In 2019 to 2020, a mid‐term evaluation was conducted that assessed efforts under the initiative's 20 countries to identify progress towards a comprehensive response to reduce human rights‐related barriers to service access. In addition to a descriptive assessment of barriers and facilitating factors for the implementation of human rights programmes, the assessment examined programme integration into national plans and existing health services, and identified emerging evidence of increased coverage, access and/or retention of key and vulnerable populations as a result of the BDB Initiative. Key informant interviews with government officials, donors, key populations and their organizations, other NGOs, policy‐makers and other stakeholders formed the basis of the assessment.
Lessons learned: Eliminating discrimination is an often proclaimed goal and a frequently underfunded objective. The Global Fund's $78 million investment in the BDB initiative represents a significant step towards scaling up evidence‐based programmes to address discrimination and other human rights‐related barriers to access HIV and TB services. Innovative programmes targeting discrimination, legal services and law reform will be highlighted.
Conclusions/Next steps: The BDB initiative has resulted in a sharp increase in funding for human rights‐related programmes addressing discrimination. Emerging evidence suggests that key populations are increasingly able to access HIV and TB programmes and be retained in care.
PDF0203
The obligation of the state to ensure freedom from torture and abuse for women who use drugs during pregnancy and childbirth
L. Vorontsova1
1Central Asian Association of People Living with HIV, Almaty, Kazakhstan
Background: The estimated number of women who use drugs in Kazakhstan is 21,726. 87% of women who use drugs and psychotropic substances are at the fertile age. Drug‐addicted pregnant women in Kazakhstan experience pain and suffering that amounted to torture. Because in Kazakhstan there are no separate normative legal acts on the provision of this medical care doctors do not have clear algorithms for managing such patients. Support methods available in the form of methadone therapy are not available to women in maternity hospitals. Methadone therapy is not available in some regions of the country. Because of the stigma, pregnant women who use drugs do not want to seek medical help. Women do not always have access to medical services, including drug treatment, prenatal and postnatal care.
Objective: to obtain information on the realization of the right to freedom from torture and ill‐treatment for drug‐addicted women during pregnancy and childbirth in Kazakhstan.
Hypothesis: women who use drugs receive necessary and timely medical care during pregnancy and childbirth without discrimination and abuse.
Methods: To conduct the study, we used a mixed‐method approach that combines the use of primarily qualitative data.
Information collection methods:
• Analysis of the laws of the country and international standards
• Focus groups and a survey of women who use drugs
• In‐depth interviews with experts (practicing psychotherapists‐narcologists, representatives of NGOs)
• Documentation cases of human rights violations
Results:
Abstract PDF0203‐Figure 1.
Conclusions: Due to the stigma and lack of appropriate medical protocols, pregnant women who use drags in Kazakhstan are not always able to use the necessary medical services, including drug treatment, prenatal and postnatal care. This leads to the fact that women experience pain and suffering. Kazakhstan needs a comprehensive approach that includes working with the medical staff of women's clinics and maternity hospitals on issues of stigma and discrimination against women who use drugs.
PDF0204
Breaking down barriers: Engaging and training lawyers to improve health rights and access to care for people living with HIV
M. Pedrola1, A. S. Benzaken2, F. Rick2, G. Alaniz Gatius1
1AIDS Healthcare Foundation Argentina, Buenos Aires, Argentina, 2AIDS Healthcare Foundation, Manaus, Brazil
Background: Even though HIV care in Argentina is provided free of charge, the fragmented health system makes it difficult for people living with HIV (PLHIV) to identify which sector is responsible for their healthcare. Therefore, PLHIV need support from lawyers to access HIV care. Beyond stigma and discrimination, the scarcity of lawyers specialized in health rights contributes to increased barriers to healthcare. AIDS Healthcare Foundation (AHF) Argentina, together with Foundation FUNDALEIS (a leading NGO in health rights) began training lawyers countrywide in health rights to help PLHIV access HIV care.
Methods: In 2018, AHF started this initiative in 16 of 24 Argentine provinces, where AHF partners identified lawyers to attend training sessions. Following the training, a network of specialized lawyers was created, and support was offered free of charge.
Results: After a total of 32 lawyers from 14 provinces completed three courses, the National Network of Lawyers for the Right to Health (NNLRH) (specializing in HIV) was established. It provided 156 free consultations related to HIV care, 149/156 were resolved after the first consultation or by extrajudicial actions. Only seven went to court, which resulted in favourable decisions for all clients.
PLHIV still face constrains or unawareness about their rights due to myths, fears and resistance, which prevents them from searching for lawyers and exercising their rights to submit claims for care.
Conclusions: This initiative showed an effective method for breaking down barriers and increasing retention by improving access to the health system. It also reinforced the role of lawyers on expanding access to HIV care by promoting health rights. Marketing campaigns on rights and NNLRH support are also required to encourage PLHIV to seek access to improve their wellbeing.
Further training sessions for lawyers on stigma and discrimination, and for PLHIV on health rights, can empower communities to support this initiative, which will further improve and expand access to HIV care.
PDF0205
Protecting health and biometric data is a human rights necessity
M. Johnson1, J. Mukherjee2,3,4, G. Jerome5, W. Lambert6
1Partners In Health, Strategic Information & Systems, Boston, United States, 2Partners In Health, Chief Medical Officer, Boston, United States, 3Harvard Medical School, Department of Global Health and Social Medicine, Boston, United States, 4Brigham and Women's Hopsital, Division of Global Health Equity, Boston, United States, 5Partners In Health, Boston, United States, 6Zanmi Lasante, Director of Research, Education, and Strategic Information, Port au Prince, Haiti
Background: Partners In Health is a network of 10 international NGOs unified by our work in healthcare delivery, health system strengthening and global policy advocacy. PIH provides care and advocacy to people living with HIV/AIDS and TB through a partnership with PIH‐affiliated INGOs and MOH partners in Haiti, Peru, Rwanda, Lesotho, Malawi, Liberia, Sierra Leone, Kazakhstan, Mexico and Navajo Nation. PIH has been a pioneer in the use of electronic health records to support clinical care and quality monitoring for PLHIV since 1990, co‐founding Open Medical Record System (OpenMRS) in 2004, now in use in 65 countries.
Description: While data security has always been a priority for the health sector, recent expansion in data‐sharing requirements and biometric identification have raised questions about the important responsibility INGOs hold in ensuring data protection of personally identifiable health data, especially biometric data which can never be de‐identified or anonymized. We have been involved in projects capturing fingerprint biometrics and GPS location of community‐based activities in multiple countries. The pathways for opting out of this data collection are not clearly communicated to PLHIV, clinicians, or INGOs, and government regulations lack specific guidance.
Lessons learned: In our partnerships with governments, we have observed sensitive data collection may in some cases undermine the principles of privacy and human rights for all PLHIV, especially those who are most vulnerable. While we recognize the significant potential for longitudinal health records and biometric data to improve access to high‐quality HIV treatment, we are concerned that when data are shared without stringent governance mechanisms and without patient consent PLHIV may be exposed to current and future harms including identity theft, criminal prosecution, harassment or death. Our experience has been that most discussions and policies regarding data security, sharing and privacy involve IT, legal and administrative staff, without involving clinicians and PLHIV to ensure that their rights are protected.
Conclusions/Next steps: Upholding the responsibility of healthcare delivery organizations requires protecting the human rights of vulnerable patients. Protection must include development and implementation of practices that safeguard private health data, especially biometrics and facial recognition, empowering PLHIV to make informed decisions regarding the use of their data.
PDF0206
Global perspectives towards a rights‐based approach to self‐care interventions for sexual and reproductive health and HIV: Implications for advancing universal health coverage
C. Logie1, M. Narasimhan2, A. Gauntley1, A. Pauchari3, N. Siegfried4
1University of Toronto, Social Work, Toronto, Canada, 2World Health Organization, Geneva, Switzerland, 3Centre for Human Progress, Delhi, India, 4South African Medical Research Council, Cape Town, South Africa
Background: Across the globe there are 400 million persons without access to essential sexual and reproductive healthcare services, signalling the urgent need for innovative solutions to realize universal health coverage (UHC). Self‐care strategies can harness the ability of individuals to manage their health by improving their autonomy and agency. The 2019 World Health Organization's (WHO) consolidated guideline on self‐care interventions for sexual and reproductive health and rights (SRHR) provides recommendations regarding self‐care interventions alongside good practice statements to guide service delivery. These self care strategies include HPV self‐sampling and HIV self testing. Perspectives from healthcare providers and users are key as we move from conceptual development to programmatic implementation.
Description: Three WHO expert consultations to inform the guideline included a survey to explore both healthcare provider and client perspectives on awareness of, access to, preferences and concerns around self‐care interventions for SRHR. These data were collected via an online survey hosted on the website of the WHO Department of Sexual and Reproductive Health and Research and shared through a range of global listservs.
Lessons learned: There were 326 participants who provided qualitative responses to open‐ended questions, these included healthcare providers (n = 242) and lay persons (n = 70) from 77 countries. Participants were mostly women (66.9%) and were from the African Region (34.5%), Region of the Americas (32.5%), South‐East Asia Region (5.6%), European Region (19.8%), Eastern Mediterranean Region (4.8%) and the Western Pacific Region (2.8%). Participants perceived multiple benefits of self‐care SRHR interventions, including: reduced exposure to stigma, increased confidentiality, reduced access barriers, empowerment, self‐confidence and informed decision‐making. Concerns include insufficient knowledge, stigma, affordability and side‐effects. Implementation considerations included innovative approaches to linkages with health services as needed.
Conclusions/Next steps: Self‐care interventions are especially promising for the area of HIV testing (HIV self‐testing), and care management (e.g. mobile apps for ART adherence) as well as elimination of cervical cancer through better screening tools (HPV self‐sampling). As many of these strategies do not necessitate direct contact with healthcare professionals, they may be ideal for key populations who face discrimination when accessing sexual and reproductive health services or for communities in which sexual and reproductive health remain highly stigmatized topics.
Late Breaking
OAALB0101
Induction of cross‐neutralizing antibodies and protection from heterologous tier‐2 SHIV challenge by an mRNA‐based vaccine in macaques
P. Zhang1, M. Prabhakaran2, S. Ding3, Y. Tsybovsky4, E. Narayanan5, A. Carfi5, S. Himansu5, Y. Lin1, D. Rogers1, Q. Liu1, H. Miao1, X. Chen2, E.K. Sarfo2, D.R. Ambrozak2, R. Gautam6, M.A. Martin6, D. Weiss7, J. Misamore7, J.R. Mascola2, A. Finzi3, A. McDermott2, P. Lusso1
1NIH, LIR, NIAID, Bethesda, United States, 2NIH, VRC, Bethesda, United States, 3University of Montreal, Montreal, Canada, 4NIH, ATRF, NCI, Frederick, United States, 5Moderna Inc, Cambridge, United States, 6NIH, LMM, NIAID, Bethesda, United States, 7Bioqual Inc., Rockville, United States
Background: Despite intensive research over the past four decades, an HIV‐1 vaccine capable of inducing broadly neutralizing antibodies (bNAbs) and protection from heterologous tier‐2 strains is still wanting.
Methods: We tested the immunogenicity and efficacy of an mRNA‐based vaccine that expresses native, membrane‐anchored HIV‐1 envelope (Env) glycoproteins in combination with SIVmac239 Gag in order to promote the in vivo formation of virus like particles (VLP). Rhesus macaques were immunized with 8 sequential mRNA immunizations or mRNA followed by protein boosts with purified homologous SOSIP trimers over a period of one year. The Envs included WITO (a clade‐B transmitter‐founder strain) BG505 (clade A) and DU422 (clade C).
Results: Env trimer‐binding antibodies (Abs) were readily induced after the second immunization, showing increasing titers and durability after each booster injection. Following the third heterologous boost, cross‐neutralizing Abs against tier‐2 viruses of different clades started to appear in all animals, reaching higher and more stable titers after the last immunization. After challenge with heterologous tier‐2 SHIV (AD8) by 12 repeated low‐dose rectal inoculations, significant protection was observed in a group of animals, with no difference between those immunized with mRNA vs. mRNA+protein. Extensive immunologic analyses identified three significant correlates of protection: i) Abs to a glycanated CD4‐binding site (CD4‐BS) gp120 core protein; ii) Abs to the AD8 Env trimer expressed on the surface of infected cells; iii) Abs mediating ADCC against the closed AD8 Env trimer. Abs to both the CD4‐BS and the trimer apex were visualized in the serum of protected animals by electron microscopy polyclonal mapping. By single B‐cell cloning and antibody amplification from a protected macaque, we derived a panel of mAbs that share genetic similarities with previously identified macaque and human bNAbs against the CD4‐BS. Detailed functional characterization of these mAbs will be presented at the Conference.
Conclusions: These results provide evidence that extensive immunization with mRNA encoding multiple heterologous membrane‐anchored HIV‐1 Envs can induce cross‐clade neutralization and partial protection from heterologous tier‐2 virus challenge.
Funding: Supported in part by the intramural research programs of the NIAID DIR and VRC, and by the NIH Office of AIDS Research (OAR).
OAALB0102
HIV envelope BG505 SOSIP immunization induces autologous virus and CD4 binding site‐specific B cell lineage antibody precursor responses in infant rhesus macaques
A.N. Nelson1,2, M. Dennis1, C.C. LaBranche3, J. Eudailey1, D.C. Montefiori3, R.W. Sanders4,5, J.P. Moore4, G.G. Fouda1,2, K.K.A. Van Rompay6, K. De Paris7, S.R. Permar1,2
1Duke University Medical Center, Human Vaccine Institute, Durham, United States, 2Duke University Medical Center, Pediatrics, Durham, United States, 3Duke University Medical Center, Surgery, Durham, United States, 4Weill Medical College of Cornell University, Microbiology and Immunology, New York, United States, 5University of Amsterdam, Medical Microbiology, Amesterdam, Netherlands, 6University of California, Davis, California National Primate Research Center, Davis, United States, 7University of North Carolina, Microbiology and Immunology, Chapel Hill, United States
Background: More than 30% of new HIV infections globally occur among youth ages 15 to 24 years. Thus, a vaccine that induces protective antibodies prior to sexual debut is critical for prevention of HIV in this population. A successful HIV vaccine will likely require induction of broadly neutralizing antibodies (bnAbs), yet this remains a challenge. Native conformation envelope (Env) trimers, such as BG505 SOSIP.664, are ideal immunogens given their enhanced ability to elicit antibody responses against vulnerable sites of the HIV Env that are the targets of bnAbs. As HIV‐infected children have been shown to develop bnAbs earlier and at a higher frequency than adults do, the infant immune landscape may be more amenable to the induction of bnAb B cell lineages. Furthermore, immunization in childhood provides the opportunity for long term, multi‐dose immunization prior to adolescence. The goal of our study was to assess the ability of a B cell lineage‐designed HIV Env trimer to induce bnAb lineages in early life.
Methods: Infant rhesus macaques (RMs) received 50mg of either the BG505 wild type (WT) SOSIP trimer or the BG505 germline‐targeting (GT1.1) SOSIP trimer (n=5/group) with the 3M‐052‐SE adjuvant at 0, 6, and 12 weeks of age. All 10 infant RMs were then boosted with the BG505 WT SOSIP trimer at weeks 26 and 52, mimicking a pediatric immunization schedule of multiple vaccine boosts within the first two years of life.
Results: Both immunization strategies induced durable, high magnitude binding antibody responses with the peak IgG responses at week 14. Plasma neutralization responses against BG505 tier 1 and 2 virus variants were enhanced after the 4th immunization at week 28 compared to week 14. Two GT1.1‐immunized infants exhibited a plasma HIV neutralization signature reflective of CD4 binding site‐specific bnAb precursor development. Moreover, this bnAb precursor neutralization signature continued to rise following immunization while other neutralization responses declined, potentially indicating continued maturation of this lineage.
Conclusions: A multi‐dose immunization regimen in infants with a B cell lineage designed SOSIP trimer is a promising strategy for inducing HIV bnAbs throughout childhood to elicit protective HIV immunity prior to adolescence, when sexual HIV exposure risk begins.
OAALB0103
CCR5 antibody blockade protects rhesus macaques from rectal SHIV acquisition
X. Chang1, G. Webb1, J. Reed1, C. Pessoa1, H. Wu1, N. Burnett2, M. Fischer2, C. Moats2, J. Smedley2, D. Burger3, N. Pourhassen3, J. Greene1, J. Sacha1
1Oregon Health and Science University, Vaccine and Gene Therapy Institute, Beaverton, United States, 2Oregon Health and Science University, Oregon National Primate Research Center, Beaverton, United States, 3CytoDyn, Inc., Vancouver, United States
Background: Adherence remains a challenge to the success of pre‐exposure prophylaxis (PrEP) in preventing HIV acquisition. Thus, new approaches are urgently needed. The primary use of the CCR5 coreceptor by mucosally transmitted virus, together with the resistance to infection observed in CCR5‐delta 32 homozygous people, suggests that CCR5‐blocking reagents might be effective PrEP agents. Leronlimab is a CCR5‐specific monoclonal antibody with excellent safety and adherence profile used in over 800 HIV+ people. We hypothesize that leronlimab can protect from the sexual transmission of HIV.
Methods: To determine if subcutaneous leronlimab at the lowest and highest doses tested in clinical trials (10 mg/kg or 50 mg/kg in rhesus macaques via allometric scaling) could prevent sexual transmission of SHIV, we conducted a study in macaques using low‐dose intra‐rectal SHIVSF162P3 challenges. Three groups of six macaques received either no treatment or leronlimab at 10 mg/kg weekly or 50 mg/kg bi‐monthly. We monitored longitudinally for plasma viremia, CCR5 occupancy, cell‐associated virus, and anti‐SHIV immune responses.
Results: Following eight weekly challenges, all animals treated with 50 mg/kg were protected from acquisition, while all control animals became infected (p=0.0005). Four animals treated with 10 mg/kg were fully protected, while two animals were infected following the last two challenges (p=0.001). Of these two infected animals, one developed anti‐leronlimab antibodies resulting in rapid leronlimab clearance, while the other maintained CCR5 occupancy with the lowest longitudinally viral loads. Colon and duodenum biopsies taken from the protected animals after completion of all challenges showed complete CCR5 occupancy and absence of cell‐associated viral DNA and RNA. Following leronlimab uncoating from CCR5, protected animals remained aviremic and lacked anti‐SHIV immune responses for at least six weeks before sacrifice, indicating sterilizing protection from rectal SHIV acquisition.
Conclusions: These results support the efficacy of leronlimab as PrEP, with the potential development of long‐acting leronlimab to improve adherence.
Abstract OAALB0103‐Figure 1.
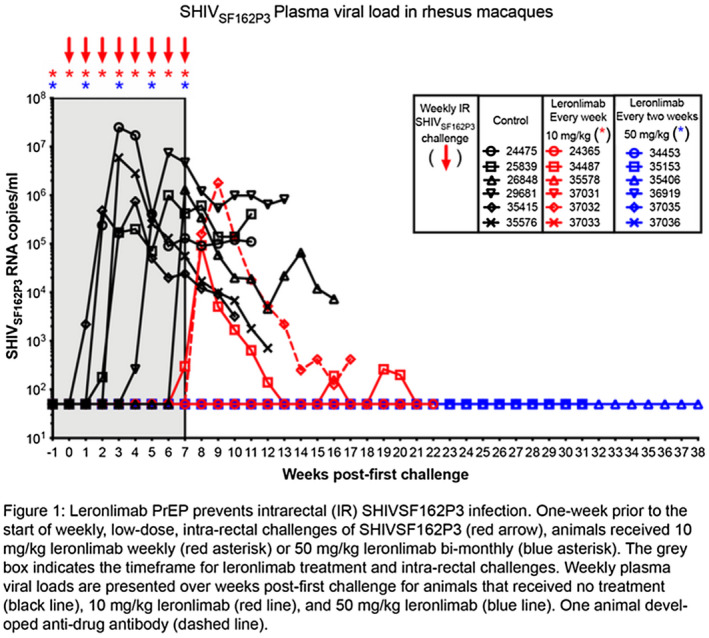
OAALB0104
Vaccination against HIV‐1 with interbilayer cross‐linked multilamellar vesicles carrying SOSIP trimer
C. Park1,2, J. Bazzill2,3, J. Moon1,2,3
1University of Michigan, Department of Biomedical Engineering, Ann Arbor, United States, 2University of Michigan, Biointerfaces Institute, Ann Arbor, United States, 3University of Michigan, Department of Pharmaceutical Sciences, Ann Arbor, United States
Background: Successful vaccination against human immunodeficiency virus‐1 (HIV‐1) has been an elusive goal. Prior research has shown that nanoparticle systems can facilitate antigen delivery to lymph nodes and enhance immune responses. However, during encapsulation of protein antigens in nanoparticles, the conformation of epitopes can be altered, thus negatively impacting the potency of the vaccines. We have previously developed interbilayer cross‐linked multilamellar vesicles (ICMV) as a vaccine delivery platform [1]. Here, we optimized our ICMV technology to deliver HIV envelope glycoprotein (BG505 SOSIP.664 Env Trimer).
Methods: Rabbits were prime immunized on day 0 with 30 µg of SOSIP and 50 µg MPLA and boosted on day 28 and 84, each with 12.4 µg of SOSIP and 20.6 µg of MPLA by subcutaneous injections at four sites on both caudal thighs. Blood samples were collected from marginal ear vein on day 105 and 169 for neutralization study.
Results: New ICMV formulation with a mean diameter of ~300 nm allows for ~30% loading efficiency of SOSIP. ICMV preserves the conformational epitopes in SOSIP protein complex as confirmed by PAGE and direct immunofluorescence staining with HIV‐1 neutralizing antibodies. Rabbits were immunized three times with SOSIP‐containing ICMVs, leading to elicitation of robust humoral immune responses. SOSIP‐ICMV immune sera neutralized homologous tier 1A (MW965.26) and 2 (BG505/T332N) viral entry to TZM‐bl cells, which was not observed from soluble control (Figure).
Conclusions: ICMV may be a promising vaccine platform for vaccination against HIV‐1 and other infectious pathogens.
Acknowledgement : This work was supported by NIH R01AI127070. We thank Dr. John Moore and Rogier Sanders for providing BG505 SOSIP.664 Env Trimer.
Reference: [1] J. J. Moon et al., Nature Materials 2011 10:243‐251
Abstract OAALB0104‐Figure 1.
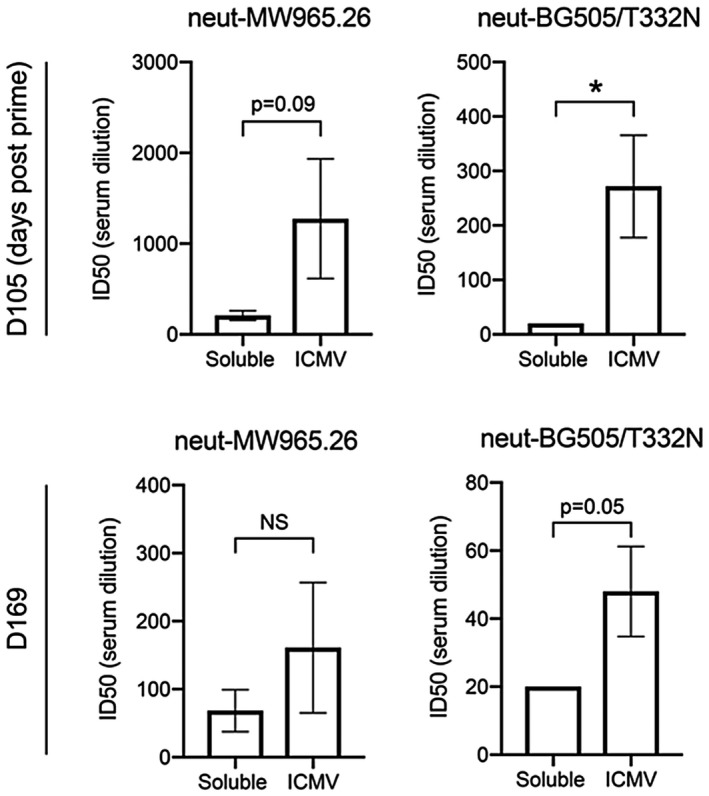
OABLB0101
Safety, pharmacokinetics and efficacy of low‐dose E/C/F/TAF in virologically suppressed children ≥2 years old living with HIV
E. Natukunda1, A. Liberty2, R. Strehlau3, E. Hellstrom4, J.G. Hakim5, H. Kaur6, H. Maxwell6, P. German6, Y. Shao6, D.M. Brainard6, C. Pikora6
1Joint Clinical Research Centre, Kampala, Uganda, 2Chris Hani Baragwanath Academic Hospital, Soweto, South Africa, 3University of the Witwatersrand, Empilweni Services and Research Unit, Rahima Moosa Mother and Child Hospital, Department of Paediatrics and Child Health, Faculty of Health Sciences, Johannesburg, South Africa, 4Be Part Yoluntu Centre, Western Cape, South Africa, 5University of Zimbabwe, Avondale, Zimbabwe, 6Gilead Sciences Inc., Foster City, United States
Background: Elvitegravir(EVG)/cobicistat/emtricitabine/tenofovir alafenamide(TAF) (E/C/F/TAF) is a single‐tablet regimen (STR) approved for HIV treatment in children ≥6y and ≥25 kg. Previous data reported no bone or renal toxicity of E/C/F/TAF in 50 children 6‐<12y. We report safety, pharmacokinetics (PK), and efficacy from interim analyses of the first clinical trial of low‐dose E/C/F/TAF tablet in young children with HIV.
Methods: Virologically suppressed children ≥2y, 14‐<25 kg, HIV‐1 RNA <50 c/mL for ≥6 months, CD4 ≥200 cells/μL received low‐dose E/C/F/TAF 90/90/120/6mg once daily in a, single‐arm, open‐label trial. Adverse events AE, laboratory data, and proportion of participants with virologic suppression were assessed through W24. Steady‐state PK of E/C/F/TAF was evaluated; EVG AUCtau and TAF AUCtau in children were compared to those in E/C/F/TAF‐treated adults 150/150/200/10 mg.
Results: 27 children were enrolled; median age 6y (range 3‐9y), median weight 19 kg (15‐24 kg), 63% female, 89% Black, median CD4 count 1061 cells/μL (383‐2401 cells/μL),. Most common AEs were upper respiratory tract infection (6 participants [22%]), cough (5 [19%), decreased appetite (4 [15%]). All AEs were grade 1 or 2; no child discontinued STR for AE. 27 participants (100%) maintained HIV‐1 RNA <50 c/mL by M=E at W16, with 10/11 (91%) at W24 (one participant had HIV‐1 RNA between 200 to <400c/mL). Mean change (% change) in CD4 count from baseline was ‐95 cells/mL (‐0.3%) at W24. EVG and TAF geometric mean AUCtau estimates were modestly (<2‐fold) higher in children vs adults (table). Exposures of all analytes remained within range of historical data. Most children found swallowability, acceptability, and palatability favorable at all timepoints assessed.
Conclusions: E/C/F/TAF low‐dose STR was acceptable with high virologic suppression. E/C/F/TAF exposures in young children were within range of adult historical data. Safety and efficacy of low‐dose STR in young children are consistent with full‐strength STR efficacy in older populations.
Abstract oablb0101‐fig-0001-a.png
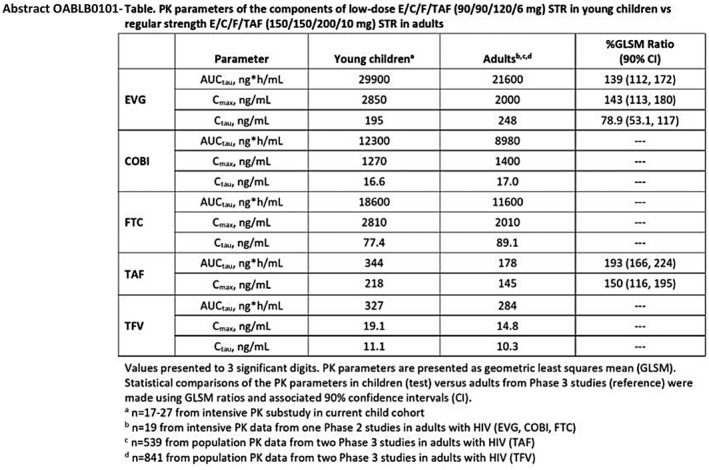
OABLB0102
Clinical outcomes by HIV serostatus, CD4 count, and viral suppression among people hospitalized with COVID‐19 in the Bronx, New York
V.V. Patel1, U.R. Felsen2, M. Fisher3, M.J. Fazzari4, M.S. Ginsberg4, R. Beil1, M.J. Akiyama1, K. Anastos1, D.B. Hanna4
1Albert Einstein College of Medicine, Montefiore Health System, Division of General Internal Medicine, Department of Medicine, Bronx, United States, 2Albert Einstein College of Medicine, Montefiore Health System, Division of Infectious Diseases, Department of Medicine, Bronx, United States, 3Albert Einstein College of Medicine, Montefiore Health System, Division of Nephrology, Department of Medicine, Bronx, United States, 4Albert Einstein College of Medicine, Montefiore Health System, Department of Epidemiology and Population Health, Bronx, United States
Background: It is unknown whether people living with HIV (PLWH) are at greater risk for adverse outcomes due to COVID‐19 than people without HIV infection or if, among PLWH, outcomes are associated with CD4 count and viral suppression (VS). Bronx County in New York City is an epicenter of both the HIV epidemic and COVID‐19 in the United States.
Methods: We conducted a retrospective cohort study of SARS‐CoV‐2 PCR‐positive patients admitted to a large tertiary academic health system in the Bronx, New York between March 10 and May 11, 2020. HIV‐related data came from the Einstein‐Rockefeller‐CUNY Center for AIDS Research Clinical Cohort Database[DH1] . Outcomes assessed were in‐hospital intubation, acute kidney injury (AKI), mortality, and length of stay (LOS). To compare outcomes between PLWH and those without HIV infection we used multivariable regression models, adjusting for age, gender, and race/ethnicity. Outcomes were explored among PLWH according to CD4 count and VS.
Results: Among 4,662 patients, median age (IQR) was 65 (54‐76); 47% were female. Most were either non‐Hispanic Black (36%) or Hispanic (37%), and 80% had public health insurance. Overall, 77 (1.7%) were PLWH, among whom the most recent HIV viral load was undetectable (<40 copies/mL) in 83%; most recent CD4 was <200 cells/uL in 16%, 200‐499 cells/uL in 44%), and ≥500 cells/uL in 40%. Overall, 10/77 (13%) PLWH and 634/4585 (14%) without HIV were intubated, and 29/77 (38%) PLWH and 1881/4585 (41%) without HIV developed AKI. In‐hospital mortality was 14/77 (18%) among PLWH and 1037/4585 (23%) among those without HIV. Hospital LOS was 5 days (3‐9) for both PLWH and those without HIV who were discharged. HIV status was not significantly associated with mortality, intubation, AKI or LOS. In exploratory analyses among PLWH with CD4 count available (N=73), higher CD4 count was associated with intubation (adjusted odds ratio 1.36 per 100 cells/uL, 95% CI 1.02‐1.82). None of the 10 viremic PLWH were intubated, versus 10/57 (18%) among suppressed PLWH.
Conclusions: In hospitalized patients with COVID‐19, there were no significant differences in intubation, AKI, mortality, and LOS between PLWH and without HIV. Our preliminary findings regarding intubation among PLWH warrant further examination.
OABLB0103
The predicted risk of adverse pregnancy outcomes from treatment‐induced obesity in the ADVANCE trial
S.F. Asif1, A. Qavi1, E. Baxevanidi1, K. McCann1, C. Serenata2, W.D.F. Venter2, N. Chandiwana2, S. Sokhela2, A. Hill3
1Imperial College London, Faculty of Medicine, London, United Kingdom, 2Wits Reproductive Health and HIV Institute, Ezintsha, University of the Witwatersrand, Johannesburg, South Africa, 3Liverpool University, Department of Translational Medicine, Liverpool, United Kingdom
Background: Dolutegravir (DTG) is associated with obesity, especially when combined with tenofovir alafenamide (TAF) in black females. Obesity increases the risk of adverse pregnancy outcomes (APOs). We aimed to predict the 10‐year risk of APOs caused by DTG‐induced obesity, using the ADVANCE trial as a model.
Methods: A systematic review was performed, evaluating the association between pre‐pregnancy obesity and APOs. The relative risk (RR) for each APO in women with obese (≥30kg/m2) versus normal BMIs (18.5‐24.9kg/m2) was calculated. To model the risk prediction, 1000 pregnant women with normal baseline BMIs were allocated to each treatment‐arm of ADVANCE (TAF/FTC/DTG, TDF/FTC/DTG, TDF/FTC/EFV). The ADVANCE treatment‐induced obesity rates were applied to this model to calculate the number of obese and normal BMI women at 96‐weeks. This was combined with APO RRs to predict the number of women experiencing APOs with each treatment at 96‐weeks.
Results: At 96‐weeks, the percentage of women becoming obese was 14% for TAF/FTC/DTG, 8% for TDF/FTC/DTG and 2% for TDF/FTC/EFV. The RR of APOs in women with obese versus normal BMIs was significantly higher for most APOs. Women were predicted to have a higher risk of APOs with DTG‐regimens compared to TDF/FTC/EFV, noticeably with TAF/FTC/DTG. From baseline to 96‐weeks, the predicted increase in APOs for women receiving TAF/FTC/DTG, TDF/FTC/DTG, and TDF/FTC/EFV was 9.9%, 5.2% and 0.9%, respectively.
Abstract OABLB0103‐Table 1.
| APO | Relative Risk | TAF/FTC/DTG | TDF/FTC/DTG | TDF/FTC/EFV | ||||
|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | Baseline | 96‐weeks | Baseline | 96‐weeks | Baseline | 96‐weeks | |
| Preterm delivery | 1.33 | (1.19,1.48) | 70 | 73 | 70 | 71 | 70 | 70 |
| Gestational Hypertension | 3.68 | (2.97,4.55) | 28 | 39 | 28 | 34 | 28 | 29 |
| Gestational diabetes mellitus | 4.31 | (3.18,5.85) | 16 | 23 | 16 | 19 | 16 | 16 |
| Pre‐eclampsia | 4.06 | (3.09,5.33) | 25 | 35 | 25 | 30 | 25 | 26 |
| Postpartum haemorrhage | 1.23 | (1.01,1.50) | 112 | 115 | 112 | 114 | 112 | 112 |
| Caesarean section | 1.64 | (1.55,1.73) | 213 | 232 | 213 | 224 | 213 | 215 |
| Small‐for‐gestational‐age infants | 0.84 | (0.76,0.93) | 89 | 87 | 89 | 88 | 89 | 89 |
| Large‐for‐gestational‐age infants | 2.04 | (1.65,2.52) | 134 | 154 | 134 | 145 | 134 | 137 |
| Low birthweight infants | 1.01 | (0.56,1.80) | 64 | 65 | 64 | 64 | 64 | 64 |
| Macrosomia | 2.48 | (2.10,2.93) | 31 | 37 | 31 | 34 | 31 | 31 |
| Stillbirth | 1.39 | (1.01,1.92) | 4 | 4 | 4 | 4 | 4 | 4 |
| Neonatal death | 1.57 | (1.00,2.48) | 2 | 2 | 2 | 2 | 2 | 2 |
| Neural tube defect | 2.53 | (1.15,5.55) | 0 | 0 | 0 | 0 | 0 | 0 |
Conclusions: Treatment‐emergent obesity with TAF/FTC/DTG and to a lesser extent, TDF/FTC/DTG, could increase the risk of APOs in black females. The increase in APOs with TAF/FTC/DTG was almost double that of TDF/FTC/DTG. These predictions are likely underestimates of the APO risk with DTG‐induced obesity as weight gain continues past Week 96. Longer‐term follow‐up of women and their infants is required.
OABLB0104
Immunologic characteristics of acute COVID‐19 in people with HIV
H. Ho1, M. Peluso2, C. Margus1, J.P. Matias Lopes1, C. He1, M. Gaisa1, G. Osorio1, J. Aberg1, M. Mullen1
1Icahn School of Medicine at Mount Sinai, New York, United States, 2University of California, San Francisco, San Francisco, United States
Background: Data on the immunologic impact of SARS‐CoV‐2 co‐infection in people living with HIV (PLWH) are limited.
Methods: We conducted a retrospective study of clinical and immunologic outcomes of COVID‐19 in 93 PLWH presenting to 5 New York City emergency departments who tested positive for SARS‐CoV‐2 by nucleic acid amplification.
Results: Median previous CD4+ T lymphocyte count was 554 cells/uL, and 57/68 individuals (83.8%) had recent plasma HIV‐1 RNA measurements below 50 copies/mL. Sixty‐two of 89 (69.6%) were on antiretroviral therapy (ART) that included tenofovir. At presentation, PLWH with COVID‐19 demonstrated significant lymphopenia and decreased CD4+ T cell counts. Levels of inflammatory markers, including C‐reactive protein (CRP), fibrinogen, and D‐dimer were commonly elevated. Serum cytokine profiles during acute COVID‐19 were characterized by elevated interleukin (IL)‐6, IL‐8, and TNF‐alpha, but not IL‐1b. Of 72 PLWH hospitalized with COVID‐19, 16 (22.2%) died, 48 (66.7%) recovered, and 8 (11.1%) remained hospitalized at the time of analysis. Those who died had significantly lower nadir absolute lymphocyte counts during COVID‐19 compared with those who recovered. Peak inflammatory markers including CRP, fibrinogen, and IL‐6 were significantly higher in those who died; there were non‐significant trends toward IL‐8 and TNF‐alpha elevations. No difference was observed in age, sex, BMI, duration of HIV infection, nadir, preceding, or presenting CD4+ T cell count, or viral suppression preceding or during the COVID‐19 presentation. A greater proportion in the recovered group was on a regimen containing tenofovir, but the difference was not statistically significant.
Conclusions: PLWH who died of COVID‐19 had significantly higher levels of soluble markers of immune activation and inflammation and more severe lymphopenia than those who survived. Our findings indicate that a subset of PLWH are capable of mounting profound inflammatory responses that have been noted to correlate with poor outcomes in people without HIV. Taken together, these findings raise important concerns that PLWH remain at risk for severe manifestations of COVID‐19 despite ART, and that prominent immune dysregulation in a subset of PLWH during infection is associated with worse outcomes. Further studies are warranted to determine whether inflammatory pathways are exacerbated or potentiated in some PLWH compared with the general population.
OABLB0105
Faster virological suppression with dolutegravir versus efavirenz in pregnancy does not lower the risk of HIV mother‐to‐child‐transmission: A meta‐analysis of 5 clinical trials in 1074 pregnant women
S.F. Asif1, E. Baxevanidi1, W.D.F. Venter2, C. Serenata2, A. Hill3
1Imperial College London, Faculty of Medicine, London, United Kingdom, 2Wits Reproductive Health and HIV Institute, Ezintsha, University of the Witwatersrand, Johannesburg, South Africa, 3Liverpool University, Department of Translational Medicine, Liverpool, United Kingdom
Background: Dolutegravir (DTG) is part of the updated first‐line antiretroviral treatment. However, literature surrounding the safety and efficacy of DTG in pregnant women remains insufficient as most early drug trials excluded this population from enrolment. A meta‐analysis was conducted to collate the data emerging from newer RCTs that measure pregnancy outcomes with DTG use.
Methods: Clinicaltrials.gov was searched for RCTs that compared DTG+2 NRTIs against the control treatment, EFV+2 NRTIs, in pregnant women. The primary endpoints included viral suppression, the number of stillbirths, neonatal deaths and HIV mother‐to‐child‐transmissions (MTCT). Secondary endpoints included the number of mothers and infants experiencing ≥1 adverse event, preterm births and small‐for‐gestational‐age infants. Using the Mantel‐Haenszel test with random‐effects modelling, risk difference and odds ratio were calculated.
Results: The following five trials were selected: DOLPHIN‐1, DOLPHIN‐2, IMPAACT 2010, ADVANCE and NAMSAL to provide a sample of 1074 pregnant women for analysis. Preterm births and viral suppression rates were the only endpoints with a significant difference between treatments. The odds of viral suppression were almost 3 times higher in women using DTG (OR: 2.90, 95% CI:1.54, 5.46). The risk of preterm births was 4% higher in women using EFV (RD: ‐0.04, 95% CI:‐0.07, ‐0.00). Interestingly, no significant difference was found between the treatments regarding the number of MTCTs. Two cases were reported in IMPAACT 2010 and three cases in DOLPHIN‐2, all occurring in the DTG‐arms. As viral suppression rates were significantly lower with EFV, a significantly higher number of MTCTs had been expected with EFV use.
Conclusions: Greater and faster rates of viral suppression were seen in pregnant women who used DTG over EFV but, all five cases of HIV MTCT occurred in the DTG‐arms, versus none in the EFV‐arms. There was minimal difference between the safety of the treatments. These results should be confirmed in larger studies with longer‐term follow‐up.
Abstract OABLB0105‐Figure 1.
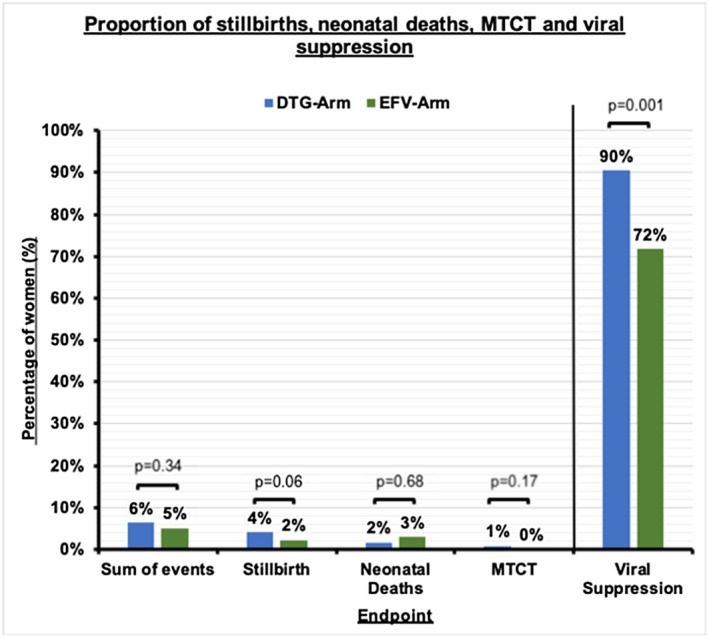
OACLB0101
HPTN 083 interim results: Efficacy of pre‐exposure prophylaxis (PrEP) containing long‐acting injectable cabotegravir (CAB‐LA) is maintained across regions and key populations
B. Grinsztejn1, D. Donnell2, M. Clement3, B. Hanscom2, L. Cottle2, L. Coelho4, R. Cabello5, S. Chariyalestak6, E. Dunne7,8, I. Frank9, A. Gaur10, P. Gonzalez11, T.V. Ha12, J. Hinojosa13, E. Kallas14, C. Kelley15, M. Losso16, J. Valdez Madruga17, K. Middelkoop18, N. Phanuphak19, J. Gallardo20, B. Santos21, O. Sued22, J. Valencia Huanami23, C. Hucks‐Ortiz24, N. Cardozo22, L. Monteiro25, K. Promjantuek19, X. Mvula26, D. Jones27, S. Eshleman28, C. Blanchette29, J. Lucas29, H. Scott30, S. Fields31, K. Gomez‐Feliciano29, A. Jennings29, J. Rooney32, W. Spreen33, D. Margolis33, A. Rinehart33, A. Adeyeye34, M. Cohen35, M. McCauley29, R. Landovitz36, C. Vela13, for the HPTN 083 Study Team
1Instituto Nacional de Infectologia Evandro Chagas‐Fiocruz, Rio de Janeiro, Brazil, 2Fred Hutchinson Cancer Institute, Seattle, United States, 3Louisiana State University, Baton Rouge, United States, 4Instituto de Pesquisa Clinica Evandro Changas‐Fiocruz, Rio de Janeiro, Brazil, 5Via Libre Clinical Research Center, Lima, Peru, 6Chiang Mai University, Research Institute for Health Sciences, Chiang Mai, Thailand, 7Center for Disease Control and Prevention, Atlanta, United States, 8Silom Clinic, Bangkok, Thailand, 9University of Pennsylvania, Philadelphia, United States, 10St. Jude Children's Research Hospital, Memphis, United Kingdom, 11Asociacion Civil Impacta Salud y Educacion, Lima, Peru, 12Health Center of Pho Yen, Pho Yen, Vietnam, 13Asociación Civil Selva Amazónica, Iquitos, Peru, 14University of Sao Paulo, Sao Paulo, Brazil, 15Emory University School of Medicine, Atlanta, United States, 16Hospital General de Agudos JM Ramos Mejia, Buenos Aires, Argentina, 17Sao Paulo DST/AIDS CRS, Sao Paulo, Brazil, 18Groote Schuur Hospital, Cape Town, South Africa, 19Thai Red Cross AIDS Research Centre, Bangkok, Thailand, 20CITBM CRS, Lima, Peru, 21Hospital Nossa Senhora da Conceição, Porto Alegre, Brazil, 22Fundación Huésped, Buenos Aires, Argentina, 23Barranco CRS, Lima, Peru, 24JWCH Institute, Los Angeles, United States, 25Instituto de Pesquisa Clinica Evandro Chagas‐Fiocruz, Rio de Janeiro, Brazil, 26Desmond Tutu HIV Centre, University of Cape Town, Cape Town, South Africa, 27St. Jude Children's Research Hospital, Memphis, United States, 28Johns Hopkins University School of Medicine, Baltimore, United States, 29FHI 360, Durham, United States, 30San Francisco Department of Public Health, San Francisco, United States, 31New York Institute of Technology, Old Westbury, United States, 32Gilead Sciences, Foster City, United States, 33ViiV Healthcare, Durham, United States, 34Division of AIDS, NIAID, NIH, Clinical Prevention Research Branch, Rockville, United States, 35University of North Carolina School of Medicine, Department of Medicine, Chapel Hill, United States, 36University of California Los Angeles, UCLA Center for Clinical AIDS Research & Education, Los Angeles, United States
Background: HPTN 083 is a phase 2b/3 randomized multicenter double‐blind, double‐dummy clinical trial of long‐acting cabotegravir (CAB) compared to daily oral TDF/FTC for HIV PrEP; primary results have been presented. In this analysis we compare HIV incidence and efficacy of CAB‐LA versus TDF/FTC in targeted subpopulations.
Methods: HIV‐negative cisgender men who have sex with men (MSM) and transgender women (TGW) who have sex with men at increased HIV risk were randomized 1:1 to either active CAB + placebo TDF/FTC or active TDF/FTC + placebo CAB. Participants were offered open‐label daily oral TDF/FTC after their last injection. Enrollment was pre‐specified to include at least: 50% participants under age 30; 10% TGW; and 50% Black in the United States (US). Hazard ratios (HR) were estimated using Cox proportional hazard models.
Results: Of the 4566 participants enrolled, 67% were < 30 years, 12% were TGW, 50% of the US population was Black. Fifty‐two incident HIV infections were observed, 44 among participants < 30 years old (11 in the CAB‐LA arm versus 33 in the TDF/FTC arm; HR: 0.32, 95%CI: 0.16, 0.63). Nine infections were observed among TGW, 2 in the CAB‐LA arm and 7 in the TDF/FTC arm (HR 0.29, 95% CI: 0.06,1.41). Among Black US participants, 4 infections were observed in the CAB‐LA arm versus 15 in the TDF/FTC arm (HR 0.28, 95%CI:0.10,0.83). Across regions, the HR (CAB‐LA vs TDF/FTC) varied from 0.19 (95% CI:0.07,0.56) in the US to 0.54 (95% CI: 0.20,1.46) in Latin America (Figure).
Conclusions: HPTN 083 is the first study demonstrating efficacy for a long‐acting PrEP agent. Pre‐specified key subpopulations historically underrepresented in PrEP registrational trials were successfully enrolled and retained, fostering inclusion and equity in scientific development. CAB provided estimates of high levels of protection regardless of gender, region, or age.
Abstract OACLB0101‐Figure 1.
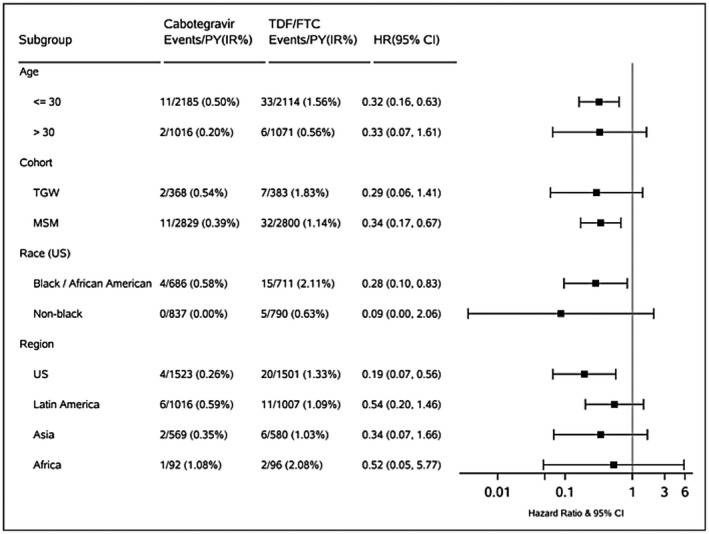
OACLB0102
HIV infection and unsafe injection practice among children in Sindh, Pakistan: A case‐control study of an outbreak
F. Mir1, V. Simms2, S.F. Mahmood3, S.H. Abidi4, A.A. Nathwani5, S.A. Shaikh6, B. Achakzai7, Q. Saeed7, P. Khan2, H. Weiss2, R.A. Ferrand8
1The Aga Khan University, Section Pediatric Infectious Disease, Pediatrics and Child Health, The Aga Khan University, Karachi, Pakistan, 2London School of Hygiene & Tropical Medicine, Infectious Disease Epidemiology, London, United Kingdom, 3The Aga Khan University, Section Infectious Disease, Medicine, Karachi, Pakistan, 4The Aga Khan University, Department of Basic and Biological Sciences, Karachi, Pakistan, 5The Aga Khan University, Pediatrics and Child Health, Karachi, Pakistan, 6Ministry of Health, Sindh, Sindh AIDS Control Programme, Karachi, Pakistan, 7National AIDS Control Program, Islamabad, Pakistan, 8London School of Hygiene & Tropical Medicine, Clinical Research, London, United Kingdom
Background: An HIV outbreak unfolded in Larkana District, Sindh, Pakistan in April 2019. By December 2019, 1167 children had tested positive for HIV. Our study evaluates risk factors for HIV in this population.
Methods: We conducted a household‐based, individually‐matched case‐control study. Cases (children aged <16 years registered for pediatric HIV care in Larkana City) and controls (HIV‐uninfected children matched 1:1 by age, sex and neighbourhood) were sampled concurrently. Serum was collected from all participants for hepatitis B and C serology. Mothers of all participants were tested for HIV. Adjusted odds ratios (aOR) were estimated using conditional logistic regression to assess factors associated with HIV infection.
Results: From 3 July to 26 December 2019, 403 HIV cases and 403 individually‐matched controls were recruited. Prevalence of HBV surface antigen and HCV antibodies were 18.4% (95% CI 14.7‐22.5) and 6.5% (95% CI 4.3‐9.3) respectively among cases, and 5.2% (3.3‐7.9) and 1.0% (0.3‐2.5) respectively among controls. Only 7.0% of cases had HIV positive mothers. In the 6 months prior to HIV testing, 228 (56.6%) cases and 32 (7.9%) controls reported >10 injections, and 294 (72.7%) cases and 78 (19.3%) controls had received an infusion. At least one blood transfusion was reported in 56 (13.9%) cases and 3 (0.7%) controls. HIV infection was independently associated with mother's occupation, history of blood transfusion (aOR 115.6, 95%CI 6.4‐2091), and history of more injections/infusions (aOR 1.5, 95%CI 1.2‐1.9), more than 1 visit to a government hospital (aOR19.9, 95%CI 2.6‐155.2), and more visits to private clinics (aOR 3.1, 95%CI 1.9‐5.2) in the 6 months prior to HIV testing.
Conclusions: HIV infection was associated with blood transfusion, multiple recent injections or infusions, and more visits to healthcare facilities in this population. This establishes that the most likely mode of transmission in this outbreak was parenteral (unsafe injection).
Funding Department of Pediatrics and Child Health, the Aga Khan University, Karachi
OACLB0103
Impact of physical distancing due to COVID‐19 on HIV pre‐exposure prophylaxis (PrEP) use and sexual behaviour among gay and bisexual men in Australia: Implications for trends in HIV and other sexually transmissible infections
M. Hammoud1, A. Grulich1, L. Maher1, M. Holt2, L. Degenhardt3, F. Jin1, D. Murphy1, B. Bavinton1, T. Lea2, B. Haire1, A. Bourne4, P. Saxton5, S. Vaccher1, J. Ellard6, B. Mackie7, C. Batrouney8, N. Bath9, G. Prestage1
1University of New South Wales UNSW, Kirby Institute, Sydney, Australia, 2University of New South Wales UNSW, Centre for Social Research in Health, Sydney, Australia, 3University of New South Wales UNSW, National Drug and Alcohol Research Centre NDARC, Sydney, Australia, 4La Trobe University, Australian Research Centre in Sex, Health and Society, Melbourne, Australia, 5University of Auckland., Department of Social and Community Health, School of Population Health, Auckland, New Zealand, 6Australian Federation of AIDS Organisations AFAO, Australian Federation of AIDS Organisations AFAO, Sydney, Australia, 7ACON Health, ACON Health, Sydney, Australia, 8Thorne Harbour Health, Thorne Harbour Health, Melbourne, Australia, 9National LGBTI Health Alliance, National LGBTI Health Alliance, Sydney, Australia
Background: In late‐March 2020, Australian state and federal governments introduced physical distancing measures to combat COVID‐19. We investigated the impact of physical distancing measures on HIV pre‐exposure prophylaxis (PrEP) use and sexual behaviour among gay and bisexual men (GBM) in Australia.
Methods: In April 2020, 940 participants in an ongoing cohort study responded to questions about COVID‐19 and changes in PrEP use and sexual behaviours before and following enactment of physical distancing measures.
Results: Mean age was 43.9 years (SD: 13.4). Among the 664 men who reported sex with either fuckbuddies or casual partners, 82.8% had ceased sex with those partners entirely following COVID‐19 and the average number of partners per day decreased from 0.089 (SD: 0.144) to 0.031 (SD: 0.097; p<0.001), representing a reduction of 65.2%.
Among non‐HIV positive GBM, 48.2% reported PrEP use prior to COVID‐19 physical distancing measures. Among those, 58.4% continued to use PrEP and 41.6% ceased use since physical distancing restrictions were imposed. Men who ceased PrEP use were more likely to cease having sex with casual partners (90.8% vs 74.8%; p<0.001) and with fuckbuddies (88.2% vs 64.4%; p<0.001).
Most (86.0%) indicated that the reason for their cessation of PrEP use was ‘I'm not having sex’ but 17.0% also indicated that they had found it more difficult to access PrEP during social distancing restrictions.
Conclusions: The dramatic decreases in PrEP use and sexual activity observed in these data will likely result in short‐term reductions in new HIV and sexually transmissible infections diagnoses in the short term, but they may be transient as COVID‐19 physical distancing restrictions are eased, and reinstated, over time. These dramatic reductions in sexual activity may be difficult to sustain throughout physical distancing restrictions.
On‐demand PrEP messaging could be usefully deployed to GBM who have ceased their PrEP use during physical distancing measures. The possibility of continued non‐use of PrEP, or lack of preparedness for recommencement of PrEP when physical distancing measures eventually ease may lead to short term increases in HIV infections. Policy responses and harm reduction interventions will need to be appropriately targeted as GBM engage with a ‘new normal’.
OACLB0104
Impact of COVID‐19 on HIV preexposure prophylaxis care at a Boston community health center
D. Krakower1, P. Solleveld1, K. Levine2, K. Mayer2
1Beth Israel Deaconess Medical Center, Boston, United States, 2The Fenway Institute, Boston, United States
Background: COVID‐19 has impeded healthcare in the US since March 2020. We described the impact of COVID‐19 on HIV preexposure prophylaxis (PrEP) care at a Boston community health center specializing in sexual healthcare.
Methods: We extracted electronic healthcare data for patients with at least one active PrEP prescription during January‐April 2020. We described trends in PrEP initiations and refill lapses (i.e., lack of refill before end of prior prescription), testing for gonorrhea/chlamydia (GC/CT) and HIV, and telehealth. We assessed covariates associated with refill lapses in April 2020 using chi‐squared tests.
Results: Of 3520 PrEP patients, the mean (SD) age was 36.9 (11.2), 72.7% were white, 13.6% Latinx, 92.1% cisgender men and 12.9% publically insured. From January to April, PrEP initiations decreased by 72.1% (122/month to 34/month), refill lapses increased by 278% (140/month to 407/month), and the number of PrEP patients decreased by 17.9% (Figure 1). GC/CT and HIV tests each decreased by 85.1% (1058/month to 158/month for GC/CT and 1014/month to 151/month for HIV), while GC/CT test positivity rates increased slightly (12.3% to 15.8%; Figure 2); the only HIV diagnosis among PrEP patients was in January. Clinical encounters decreased by 26.3% (1419 to 1046) and transitioned from 0% to 97.7% telehealth. Refill lapses were associated with being ≤26y (p=0.001), non‐white (p=0.001), Latinx (p=0.049), and publically insured (p=0.002).
Conclusions: COVID‐19 was associated with disruptions in PrEP care, especially among vulnerable subpopulations, despite high use of telehealth. Studies to understand whether changes in PrEP care reflect decreased sexual risk or barriers to optimal healthcare are needed.
Abstract OACLB0104‐Figure 1.
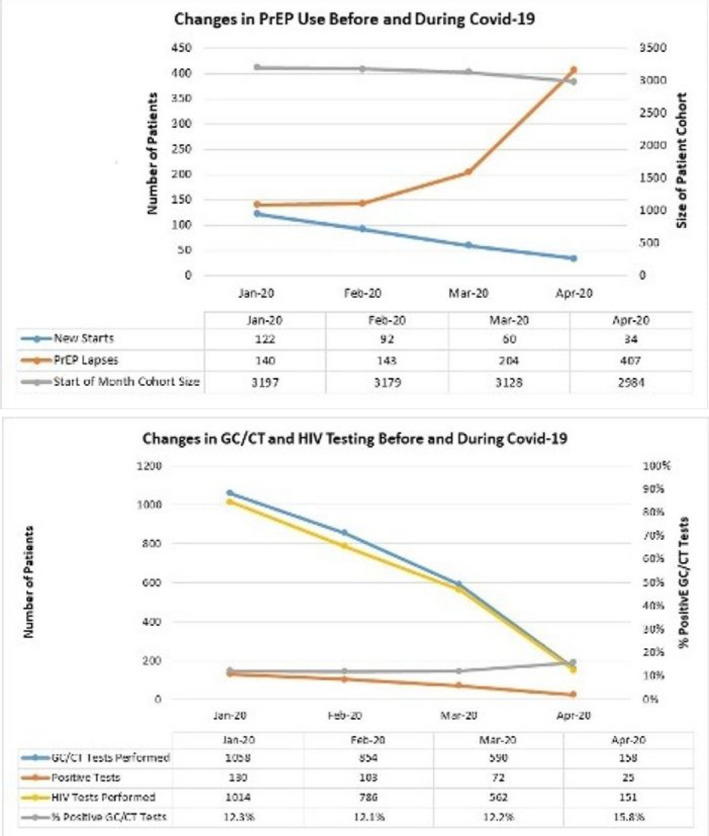
OACLB0105
Cluster randomized trial of an HIV self‐testing intervention to promote partner testing and safer sexual behavior among women at high risk of HIV infection in Kenya
H. Thirumurthy1, E. Bair1, P. Ochwal2, N. Marcus1, S. Maman3, S. Napierala4, K. Agot2
1Perelman School of Medicine, University of Pennsylvania, Department of Medical Ethics and Health Policy, Philadelphia, United States, 2Impact Research and Development Organization, Kisumu, Kenya, 3University of North Carolina at Chapel Hill, Department of Health Behavior, Chapel Hill, United States, 4RTI International, Women's Global Health Imperative, Berkeley, United States
Background: HIV self‐testing (HIVST) can overcome barriers to HIV testing, but its potential as an HIV prevention strategy for women in sub‐Saharan Africa has not been assessed. We examined whether provision of multiple self‐tests to high‐risk women promotes partner testing, facilitates safer sexual behavior, and reduces HIV risk (NCT03135067).
Methods: Between 2017‐2018, we recruited 2,090 HIV‐negative women ≥18 years with ≥2 partners from 66 clusters in Siaya County, Kenya including beach communities and sex worker hotspots. In clusters randomized to the intervention, participants received self‐tests at regular intervals during the study. In control clusters, participants received referral cards for facility‐based testing. Follow‐up visits occurred at 6‐month intervals, for up to 24 months, and concluded in March 2020. HIV incidence, partner testing, couples testing, HIV‐positive partners identified, and sexual behavior were compared between study groups.
Results: Participants’ mean age was 27.1 years, 64.5% were married, and 66.6% reported sex work as an income source. Mean follow‐up duration was 19.1 months. Intervention participants received an average of 16.8 self‐tests. HIV incidence did not differ between the intervention and control groups (1.2 vs. 1.0 per person‐year, HR 1.17; 95% CI 0.54, 2.51; p=0.69). At each follow‐up, the intervention significantly increased primary partner and couples testing in the past 6 months (Figure). The intervention also identified 1.79 times more HIV‐positive sexual partners per participant (p<0.001). At 6 months, intervention participants were more likely to report that condom use resulted from ≥1 partners testing HIV‐positive or declining testing (11.6% vs. 6.2%; p<0.001); however, this difference did not persist at 12‐24 months.
Conclusions: Provision of multiple self‐tests to high‐risk women in Kenya led to increased awareness of sexual partners’ HIV status and modestly safer sexual behavior, but did not affect HIV incidence. This approach can support achievement of UNAIDS 95‐95‐95 targets and should be accompanied with additional HIV prevention interventions.
Abstract OACLB0105‐Figure 1.
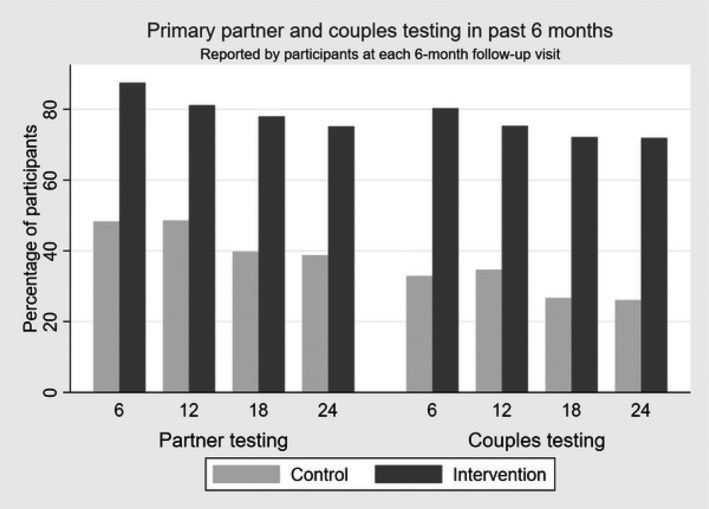
OADLB0101
Impact of COVID‐19 related shelter‐in‐place orders on PrEP access, usage and HIV risk behaviors in the United States
S. Brawley1, J. Dinger2, C. Nguyen3, J. Anderson3
1American Academy of HIV Medicine, Policy and Programs, Washington, United States, 2University of California San Francisco, School of Pharmacy, San Francisco, United States, 3Gilead Sciences, Medical Affairs, Foster City, United States
Background: In 2020, USA implemented shelter‐in‐place orders (SIPOs) during the COVID‐19 outbreak. These orders led to reduced staff and hours or completely closed healthcare facilities. We sought to understand how SIPOs impacted PrEP access, use, and HIV risk behaviors among PrEP users or modified PrEP providers’ practice to understand how SIPOs may impact the future of PrEP delivery.
Methods: Electronic convenience sample surveys of PrEP users and prescribers were simultaneously deployed across a 25‐day period at the height of SIPO implementation. PrEP user survey link was sent via social media and PrEP advocates. The provider survey link was emailed to more than 2500 providers from the Academy's database. Summary analysis of cohort groups for insights and trends
Results: 409 PrEP users under SIPOs responded, approximately 33% reported discontinuing PrEP. Many discontinued users (83%) stopped voluntarily and of those, 85% due to low perceived HIV risk, with only 11 participants citing inability to access PrEP medications. Post‐SIPO HIV risk varied among respondents (Figure 1.) Of 189 prescribers, 95% reported being able to prescribe PrEP during SIPOs despite >90% reporting practice‐site restrictions. While some PrEP users discontinued PrEP, among providers: 90% recommended no PrEP regimen changes to patients; 68% implemented telemedicine practices; and 59% indicated refilling PrEP medications while postponing routine HIV/STI and laboratory tests to be completed as soon as possible; 15% opted to completely forgo testing and lab monitoring. One in five providers encountered PrEP users with suspected STIs for which they could not obtain a test and half (47%) elected to treat empirically.
Conclusions: During USA SIPOs, PrEP users were able to access PrEP via telemedicine while some discontinued due to perceived low HIV risk and self‐reported reduced risk behaviors. Despite in‐person visits being limited, providers were able to prescribe PrEP, however, PrEP users had limited access to HIV/STI testing.
Abstract OADLB0101‐Figure 1.
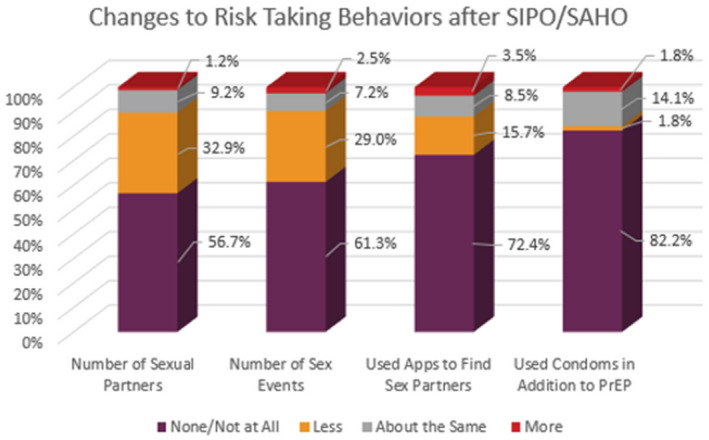
OADLB0102
Experiences of participants in a decentralized HIV medication distribution program in South Africa during the COVID‐19 pandemic
J. Jarolimova1,2, B. Bunda2, S. Govere3, N. Wara2, L. Bogart4, H. Thulare3, R. Parker5,6,7, I. Bassett1,2,6,7
1Massachusetts General Hospital, Division of Infectious Diseases, Boston, United States, 2Massachusetts General Hospital, Medical Practice Evaluation Center, Boston, United States, 3AIDS Healthcare Foundation, Durban, South Africa, 4RAND Corporation, Santa Monica, United States, 5Massachusetts General Hospital, Biostatistics Center, Boston, United States, 6Harvard University, Center for AIDS Research CFAR, Boston, United States, 7Harvard Medical School, Boston, United States
Background: Restrictions on social interaction and mobility during the COVID‐19 pandemic could adversely impact long‐term HIV care, particularly in areas of high HIV prevalence such as South Africa. De‐centralized antiretroviral therapy (ART) programs, which allow stable patients to collect ART at community‐based pick‐up points, have the potential to improve care by decongesting clinics and decreasing barriers to ART access. We aimed to evaluate the experiences of people receiving HIV care through a decentralized program in South Africa during the COVID‐19 pandemic.
Methods: We telephoned a random subsample of 604 participants enrolled in a larger prospective cohort study of the Central Chronic Medicine Dispensing and Distribution Program (CCMDD) in urban Kwazulu‐Natal. A semi‐structured telephone interview included questions about general concerns regarding the COVID‐19 pandemic and national lockdown, and changes in access to HIV care and ART.
Results: We completed interviews with 280 participants (46%) of whom 66% were female, median age was 37y, and median time of enrollment in the CCMDD program was 6.7 months. The most common concern among participants regarding the COVID‐19 pandemic was food running out, cited by 113 (40%) of participants, followed by becoming infected (n = 99; 35%), being unable to work (n = 85, 30%), and money running out (n= 80; 29%). Primary concerns regarding ART pick‐up in the future were COVID‐19 infection risk (91, 33%), transportation availability (63, 22%), and safety (58, 21%). Twenty (7%) of 278 participants had recently delayed picking up their ART due to COVID‐19. Among those with available data, 40 (30% of 143) reported an increase in time to travel to their medication pick‐up point, and 71 (49% of 144) reported an increase in wait‐times.
Conclusions: Participants in a decentralized ART program in South Africa identified socioeconomic concerns and fear of COVID‐19 infection during the national lockdown for COVID‐19 mitigation. While a small number had delayed picking up ART due to COVID‐19, a significant proportion had concerns about accessibility and safety of medication pick‐up going forward, and many reported new barriers to picking up their medication. Strategies for maintaining access to longitudinal HIV care during the COVID‐19 pandemic in South Africa are needed.
OADLB0103
Safety and preliminary effectiveness of the Tu'Washindi intervention to increase PrEP use among Kenyan adolescent girls and young women at risk of intimate partner violence: A pilot cluster‐randomized controlled trial
S.T. Roberts1, E.N. Browne1, A.M. Minnis1, M. Hartmann1, S. Otticha2, E.T. Montgomery1, S. Ohaga2, K. Agot2
1RTI International, Women's Global Health Imperative, Berkeley, United States, 2Impact Research and Development Organization, Kisumu, Kenya
Background: Oral pre‐exposure prophylaxis (PrEP) has potential to reduce HIV acquisition among adolescent girls and young women (AGYW) in sub‐Saharan Africa, a priority population for epidemic control. However, intimate partner violence (IPV) and low relationship power may create significant challenges to PrEP use. The Tu'Washindi intervention aimed to increase PrEP uptake and adherence among AGYW enrolled in the DREAMS Initiative in Siaya County, Kenya, where IPV and gender inequity are highly prevalent.
Methods: Our multi‐level, community‐based intervention was piloted in a cluster‐randomized controlled trial conducted at 6 pair‐matched DREAMS Safe Spaces. Three intervention components were delivered over 6 months: an 8‐session empowerment‐based support club, community sensitization targeted towards male partners, and a couples’ PrEP education event. Participants were ages 17‐24, HIV‐uninfected, and either eligible for, or already taking, PrEP. PrEP uptake and IPV were assessed over 6 months of follow‐up through interviewer‐administered questionnaires, and adherence was assessed with Wisepill electronic monitoring devices. PrEP uptake, adherence and IPV were compared using Poisson and negative binomial regression models adjusting for matched sites.
Results: We enrolled 103 AGYW; median age was 22 years (IQR 20‐23), 95% had a primary partner, 32% were currently taking PrEP, and 44% reported IPV in the past 3 months. Retention was 97% at Month 6. Compared to control arm participants, those in the intervention arm were more likely to initiate PrEP, if not already using it at enrollment (68% vs. 32%, aRR 2.28, 95% CI 1.19‐4.38, p=0.01), and those taking PrEP had higher adherence (25% vs.13%, aRR 1.86, 95% CI 1.10‐3.13, p=0.02). Twenty percent of participants reported IPV during study follow‐up. There were trends towards fewer IPV events per participant (aRR 0.62, 95% CI 0.24‐1.57, p=0.31) and fewer events resulting in injury (aRR 0.22, 95% CI 0.05‐1.06, p=0.06) in the intervention versus control arm.
Conclusions: Tu'Washindi shows promise in promoting PrEP uptake and adherence among AGYW without concomitant increases in IPV, however adherence was still suboptimal. Further research is needed to determine whether these gains translate to increases in the proportion of AGYW with protective levels of PrEP adherence and to evaluate the potential for the intervention to reduce IPV risk.
OADLB0104
Effectiveness of the Sista2Sista program on improving HIV and other sexual and reproductive health outcomes among vulnerable adolescent girls and young women in Zimbabwe
G. Oberth1, T. Chinhengo2, T. Katsande3, R. Mhonde3, P. Kasere3, B. Chihumela3, D. Hanisch3, B. Madzima4
1University of Cape Town, Center for Social Science Research, Cape Town, South Africa, 2United Nations Population Fund UNFPA, East and Southern Africa Regional Office, Johannesburg, South Africa, 3United Nations Population Fund (UNFPA), Harare, Zimbabwe, 4Ministry of Health and Child Care, Harare, Zimbabwe
Background: In Zimbabwe, adolescent girls and young women (AGYW) experience high rates of HIV infection, gender‐based violence, and other sexual and reproductive health challenges. They also face barriers to accessing health information, services and support. In 2013, the Ministry of Health and Child Care partnered with the United Nations Population Fund to design and implement the Sista2Sista program. Sista2Sista is a structured peer group behavioral intervention aimed at improving health outcomes among vulnerable in‐ and out‐of‐school AGYW.
Methods: Program data was analyzed for 91,612 AGYW aged 10‐24 years who were enrolled in the Sista2Sista program in Zimbabwe between 2013 and 2019. Logistic regression was used to determine odds ratios (OR) with 95% confidence intervals (CI) and evaluate Sists2Sista program exposure as a factor in the following variables, defined a priori: HIV testing, marriage, school attendance, family planning, pregnancy and reported sexual abuse.
Results: A total of 58,471 AGYW (63.82%) graduated from the Sista2Sista program by completing at least 30 out of 40 exercises. Compared to those with fewer than 30 exercises, graduates were more likely to take an HIV test (2.78 OR 95% CI 2.52‐3.10), less likely to get married (0.63 OR 95% CI 0.55‐0.73) and less likely to drop out of school (0.60 OR 95% CI 0.53‐0.69). At higher thresholds of program completion, additional positive outcomes were observed. Participants who completed all 40 exercises were more likely to go back to school (1.41 OR 95% CI 1.18‐1.69), report the use a family planning method (1.38 OR 95% CI 1.21‐1.56), and come forward to report sexual abuse (1.76 OR 95% CI 1.17‐2.66). They were also less likely to become pregnant as adolescents (0.41 OR 95% CI 0.24‐0.72). Participants who benefited from individual counselling sessions were more likely to graduate from the program.
Conclusions: The Sista2Sista program had a positive effect on a range of HIV and other sexual and reproductive health outcomes among vulnerable AGYW in Zimbabwe. Strategies to retain participants in the program for longer should be explored. These might include layering structural elements into the program (e.g. school support) as well as intensifying individual counselling.
OADLB0105
The #Stayhome #Selftest campaign. Rapid pivot of HIV testing services to enable continuity of care in Hanoi, Vietnam during the COVID‐19 lock‐down
K. Green1, T. Thi Tran1, M.T. Le2, H. Ha3, T. Tan Nguyen4, B. Vu Ngoc1, D. Hong Anh1, S. Vo Hai5, A. Khanh Tran1, H. Thai Nguyen1, T. Phan Minh1, T. Ngo Minh6
1PATH, Hanoi, Vietnam, 2Glink, Hanoi, Vietnam, 3National MSM & TG Network, Hanoi, Vietnam, 4My Home, Ho Chi Minh City, Vietnam, 5Vietnam Administration of HIV/AIDS Control, Hanoi, Vietnam, 6USAID, Hanoi, Vietnam
Background: With SARS‐CoV‐2 transmission on the rise in Hanoi, Vietnam in late March, facility‐and community‐based HIV testing was largely halted through most of April. Even before restrictions were put in place, the number of individuals seeking an HIV test declined through March. Leading up to COVID‐19, differentiated HIV testing options were available to key populations (KP), such as self‐testing (HIVST), lay‐testing, and facility‐based testing. Both blood‐based and oral fluid HIVST are available in Vietnam.
Description: Starting in April, KP‐led organizations, private clinic partners, and the USAID/PATH Healthy Markets project crowd‐sourced ideas from KP for re‐framing HIV testing during COVID‐19. The resulting “#Stayhome #Selftest” campaign offered KP the option to place an order for an HIV self‐test kit by phone, text, or an online ordering platform. An online risk screening tool further guided individuals in self‐determining their need for an HIVST. HIVST kits were delivered using local courier options (e.g. Grab), post, or home drop‐off depending on client preference and location, while applying a universal mask use policy.
Lessons learned: The coordinated effort to offer HIVST through online orders and home‐delivery resulted in 877 HIVST kits being couriered, posted, or otherwise delivered to those seeking an HIV test in Hanoi in April. This reflected a 97% increase in the monthly average number of HIVST kits that were distributed in the three previous months. The majority of those ordering a test were young men who have sex with men between the ages of 19‐24, of whom 28% had never tested for HIV before, and 85% of whom said it was online content that prompted them to seek an HIV test.
Conclusions/Next steps: HIVST is an empowering self‐care tool, allowing individuals to seek an HIV test based on their own assessment of risk and need. It also provides testing options for those who may be reluctant to go to a clinic for an HIV test due to COVID‐19. Increasing access to HIV self‐testing during and after the COVID‐19 lock‐down is an essential measure to ensure that those at risk of HIV, including those who have never tested for HIV before, have continued access to COVID‐19 safe HIV testing.
OAELB0101
Continuity of ART provision during COVID‐19 lockdown, a TASO Masaka community ART delivery experience
I. Magala1, E. Muwanga2
1The AIDS Support Organization, Community systems and Linkages, Kampala, Uganda, 2The AIDS Support Organisation, Pyscho‐social, Kampala, Uganda
Background: TASO Masaka has 8020 ART clients in care of which 65% of these receive their drugs through the community arm of drug distribution. The outbreak of COVID ‐19 in Uganda with now 55 cases recorded has come with preventive measures to reduce transmissions. These include social distancing and lockdown where movement from one place to another is restricted to a few professionals hence affecting ART clients who seek ART care at TASO Masaka. The procedures of obtaining travel permits are cumbersome given that one must seek approval from the Resident District Commissioners office which is far away for most clients given the geographical scope of the district. It is against such a background that TASO Masaka developed community strategies of reaching out its clients amidst the lock down.
Description: Monitoring and evaluation department classified clients based on ART delivery models, for clients receiving drugs in Community drug distribution points the group leaders were called and ensured that drugs were to be delivered while maintaining social distancing measures. Those receiving drugs at facility and leave far away from the centre, the Monitoring and evaluation team had to cluster clients based on their sub counties, parishes and villages. The list is shared with the phyco‐social department for follow up, files are retrieved and phone calls are made to schedule appointments for ART delivery. This can be at home or near by agreed central location where clients can receive drugs.
For clients outside the catchment areas appropriate referrals are made to near by health centers and updating of client's records is done at the parent facility.
Lessons learned: During the lockdown clients have had uninterrupted drugs refills in their communities.
Referrals to near by health facilities have enabled continued adherence to ART among clients.
New community structures are emerging for those clients who were receiving drugs at facility.
Conclusions/Next steps: A community ART delivery approach has enabled service providers to continually provide care to clients amidst COVID Lock down.
The created community structures can be maintained to handle ART delivery during complicated situations.
OAELB0102
Twenty‐four month retention and viral load outcomes from a non‐inferiority cluster randomized trial of extending ART dispensing intervals to 6‐monthly in adherence clubs
T. Cassidy1,2, C. Keene1, K. Lebelo1, A. Grimsrud3, B. Sibanda4, S. Ndlovu4, H. Hayes5, C. Orrell6,7, E. Roberts5, N. Zokufa1, T. Mutseyekwa1, J. Voget5, R. Gerstenhaber4, L. Wilkinson8
1Medecins Sans Frontieres, Khayelitsha, Cape Town, South Africa, 2University of Cape Town, Division of Public Health Medicine, Cape Town, South Africa, 3Internation AIDS Society, Cape Town, South Africa, 4Medecins Sans Frontieres, Cape Town, Cape Town, South Africa, 5Western Cape Provincial Department of Health, Cape Town, South Africa, 6University of Cape Town, Department of Medicine, Cape Town, South Africa, 7University of Cape Town, The Desmond Tutu HIV Centre, Cape Town, South Africa, 8University of Cape Town, Center of Infectious Diseases and Epidemiological Research, Cape Town, South Africa
Background: Patients and health systems could benefit from reduced visit frequency by increasing ART refills. Antiretroviral therapy (ART) adherence clubs (AC) support clinically stable patients’ retention through lay healthcare worker‐led group ART refills and psychosocial support. We conducted a non‐inferiority cluster randomized control trial comparing standard of care (SoC) ACs and 6‐month refill intervention ACs in Khayelitsha, South Africa.
Methods: Existing ACs were randomized to either SoC or intervention ACs. SoC ACs met five times annually receiving 2‐month refills with a 4‐month refill over year‐end; one AC visit included an annual blood draw followed by clinical assessment at the next visit. Intervention ACs met twice annually receiving 6‐month refills, with an individual blood collection anytime 3‐30 days before the annual clinical assessment AC visit. Study enrolment took place in 2017 with the first study visits in October/November 2017 and patients followed for 24‐months. Retention was defined as a visit on or within 3‐months after the 24‐month scheduled appointment. Viral load (VL) completion (12‐24 months after enrolment) and suppression(<400copies/mL) at analysis closure are presented by group. We conducted a Cox proportional hazards regression analysis to compare attrition (death or loss to follow‐up) using robust standard errors to account for clustering.
Results: A total of 2,150 patients in 88 ACs were included; 977 in 40 intervention ACs (22% male) and 1,173 in 48 SoC ACs (24% male). Twenty‐four month retention was high in both arms; 93.1% (95% CI: 91.2‐94.7%) in intervention ACs and 94.0% (95% CI:92.4‐95.2%) in SoC ACs, with no significant difference between groups (Hazard Ratio 1.09, 95% CI: 0.54‐2.19). Among those retained at 24 months, viral load completion was slightly higher in the intervention arm (848/897;94.5% [CI:92.9‐95.8%] vs. 972/1089;89.3% [CI:85.6‐92.1%]) and suppression was similar between arms (817/848; 96.3% [95%CI: 94.6‐97.5%] vs. 948/972; 97.5%[95%CI: 96.4‐98.3%]).
Conclusions: After 24‐months, non‐inferior retention and viral load outcomes were observed between the intervention ACs with 6‐month ART refills and the SoC ACs. These findings are consistent with the 12‐month outcomes and provider further reassurance that clinically stable patients can achieve good outcomes with fewer ART visits and longer ART refills.
OAELB0103
SWING‐led effort to sustain essential needs and HIV services of vulnerable sex workers in Thailand through COVID‐19 pandemic
S. Janyam1, C. Phaengnongyang1, N. Pungpapong2, R. Vannakit3, N. Rittiwong1, S. Sukthongsa1, S. Sumalu1, D. Phuengsamran4, C. Songsamphan5
1SWING Foundation, Bangkok, Thailand, 2Winchester College, Winchester, United Kingdom, 3Independent Researcher, Bangkok, Thailand, 4Mahidol University, Nakhonpathom, Thailand, 5Thammasat University, Bangkok, Thailand
Background: Around 200,000 sex workers in Thailand immediately became jobless due to COVID‐19 public health measures. Sex workers could not access the government's financial aid for informal workers as their job is deemed non‐existent/illegal. We described how an agile community‐led effort has enabled sex workers in Thailand to access essential needs and HIV services during the pandemic.
Description: SWING, a sex worker‐led organisation, empowers the lives of sex workers through health, educational, and legal support. SWING staff are current/ex‐sex workers trained to be lay providers delivering HIV services to their peers. COVID‐19 enthused these lay providers to cook and deliver meals to sex workers and their families. The “COVID‐19 Fund for Sex Workers” was rapidly set up asking for money and essential supply donations to provide immediate support to sex workers in Bangkok and Pattaya.
Lessons learned: From April 11 to 22 May 2020, SWING received almost 2 million THB (≈62,500USD) from individuals and various industries. Over this period, SWING has provided support to 560 Thai and non‐Thai sex workers (385 cis‐women, 96 trans‐women, 79 cis‐men), aged 18‐65 years, in Bangkok and Pattaya. Ready‐to‐eat meals and food supplies were delivered to these service workers three times a week. Over 80% of them had to rely on sex work income to support their children and families.
Temporary jobs were created for sex workers to aid other homeless people during these times. Some entertainment venues, inspired by SWING, also acted as temporary kitchens to support sex workers. A small entrepreneur group has also helped SWING to conduct crowdsourcing through the vending of SWING merchandise.
Realizing that many service workers have been struggling to continue their job during COVID‐19, SWING has also organized mobile clinics to provide HIV/COVID‐19 laboratory screening and referral services to these sex workers. A life‐saving guide and toolkit for sex workers during COVID‐19 is currently under development.
Conclusions/Next steps: Sex workers in Thailand have survived through COVID‐19 pandemic due to true leadership, agility, and solidarity within their own community. Sex work has been de‐stigmatized through public donation campaign which proved to fill in support gaps not covered by the Thai government.
OAELB0104
Global interruptions in HIV prevention and treatment services as a result of the response to COVID‐19: Results from a social media‐based sample of men who have sex with men
A. Rao1, K. Rucinski1, B. Ackerman2, S. Wallach1, A. Garner3, G.‐M. Santos4, S. Howell3, C. Beyrer1, S. Baral1
1Johns Hopkins Bloomberg School of Public Health, Epidemiology, Baltimore, United States, 2Johns Hopkins Bloomberg School of Public Health, Biostatistics, Baltimore, United States, 3Hornet, San Francisco, United States, 4University of California, San Francisco, San Francisco, United States
Background: Globally, the coronavirus pandemic has necessitated a range of population‐based measures in order to stem the spread of infection and reduce COVID‐19 morbidity and mortality. These measures may be associated with disruptions to other health services including for gay men and other men who have sex with men(MSM) at risk for or living with HIV. Here, we assess the relationship between intensity and breadth of COVID‐19 mitigation strategies and interruptions to HIV prevention and treatment services for MSM.
Methods: Data for this study were collected as part of a COVID‐19 disparities survey implemented by the gay social networking app, Hornet (16/Apr/2020‐4/May/2020). Data were assessed for countries where ≥100 participants completed the survey (Brazil/France/Indonesia/Mexico/Taiwan/Turkey/UK/USA), to evaluate the association between stringency of COVID‐19 mitigation strategies and HIV‐service engagement (n=1929) using mixed‐effects models with clustering by country. Stringency was measured using the Oxford Government Response Tracker Stringency Index; each country received a score (0‐100) based on the number and strictness of nine indicators related to school and workplace closures and travel bans.
Results: The median age of participants was 36(IQR:28‐47); 13%(246/1929) are living with HIV. The median stringency score was 70.5(Range:[29.36,Taiwan]‐[89.41,France]). For each indicator of prevention, increasing stringency of response was associated with decreased access to services. For every one‐point increase in stringency, there was a 4% reduction in the odds of access to in‐person testing, and a 3% reduction in the odds of access to self‐testing, to PrEP, and to condoms(Table 1). Among those living with HIV, having health insurance (government(aOR: 4.86,[95%CI:1.58,14.9]); private(aOR: 4.47,[95%CI 1.45,13.8])) was independently associated with access to antiretroviral treatment.
Conclusions: More stringent government responses were associated with decreased access to HIV services. Innovative strategies, like mobile‐service delivery or telehealth, may be needed to minimize the service interruptions from these types of government responses on MSM communities and ensure continuity of care.
Abstract oaelb0104‐fig-0001-a
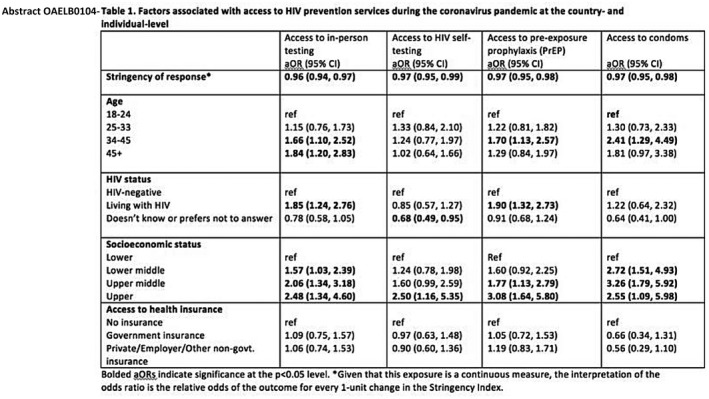
OAELB0105
Reaching UNAIDS 95‐95‐95 targets worldwide: Predicted benefits and treatment costs with generic manufacture
K. Heath1, J. Levi2, M.J. Barber3, D. Gotham4, A. Hill5
1Macfarlane Burnet Institute, Melbourne, Australia, 2Homerton Hospital, London, United Kingdom, 3Harvard T. H. Chan School of Public Health, Harvard University, Boston, United States, 4Independent Researcher, London, United Kingdom, 5Department of Translational Medicine, University of Liverpool, Liverpool, United Kingdom
Background: UNAIDS aims for HIV testing, treatment and viral suppression rates to be 90%‐90%‐90%, respectively, by 2020 and 95%‐95%‐95% by 2025. Patented drug prices remain a barrier to HIV treatment. Generic alternatives are being produced and exported from countries without patent barriers at a fraction of the cost. Global access to generic alternatives could reduce expenditure and improve clinical outcomes.
Methods: Epidemiological and demographic HIV data were compiled for 160 countries from UNAIDS, publications and country reports. The cost of generic TDF/3TC/DTG was estimated using the Panjiva database; shipment values of active pharmaceutical ingredients from India were used to estimate finished product costs, including excipient costs, tax, and 10% profit. We estimated the cost of generic drugs with (a) current, (b) 90‐90‐90 and (c) 95‐95‐95 service coverage. Weighted log‐linear regression of the relationship between antiretroviral coverage and (i) HIV infections, (ii) deaths, and (iii) mother‐to‐child transmission (MTCT) rate was used to estimate the effects of increased service coverage. Annual antiretroviral sales were compiled from pharmaceutical quarterly financial sales reports, adjusted for inflation and GDP.
Results: The estimated cost of generic TDF/3TC/DTG was $59 per person per year. The TDF component was $20, 3TC was $27 and DTG was $12. 95‐95‐95 generic coverage at this price would cost $2 billion annually. Estimated 2019 global HIV drug expenditure was $36.25 billion ($700 million per week).
| Service coverage | |||
|---|---|---|---|
| Current | 90‐90‐90 | 95‐95‐95 | |
| Cost of treatment with generics in 160 countries | $1.34 billion | $1.81 billion | $2.00 billion |
| Number receiving ART | 22.63 million | 30.42 million | 33.67 million |
| Estimated number of new infections in adults | 1.16 million | 0.74 million | 0.68 million |
| Estimated number of HIV‐related deaths in adults | 530,000 | 240,000 | 200,000 |
| Estimated number of mother‐to‐child‐transmissions | 179,000 | 71,000 | 58,000 |
Conclusions: Reaching the UNAIDS 95‐95‐95 targets could prevent 480,000 new adult HIV infections, 121,000 infant HIV infections and 330,000 adult deaths per year, compared to current treatment coverage levels. The annual cost of 95‐95‐95 treatment coverage for the 160 countries in this study is equivalent to only three weeks of global sales at current prices; significant savings could be made by switching to quality‐assured generics. Generic drug access is paramount to reduce HIV infections and deaths.
OAXLB0101
HPTN083 interim results: Pre‐exposure prophylaxis (PrEP) containing long‐acting injectable cabotegravir (CAB‐LA) is safe and highly effective for cisgender men and transgender women who have sex with men (MSM,TGW)
R.J. Landovitz1, D. Donnell2, M. Clement3, B. Hanscom2, L. Cottle2, L. Coelho4, R. Cabello5, S. Chariyalestak6, E. Dunne7, I. Frank8, J. Gallardo9, A. Gaur10, P. Gonzalez11, V. Ha12, J. Hinojosa13, E. Kallas14, C. Kelley15, M. Losso16, J. Valdez Madruga17, K. Middelkoop18, N. Phanuphak19, B. Santos20, O. Sued21, J. Valencia Huanami22, E.T. Overton23, S. Swaminathan24, C. del Rio25, R. Gulick26, P. Richardson27, P. Sullivan28, E. Piwowar‐Manning28, M. Marzinke28, J. Marrazzo29, E. Daar30, A. Asmelash31, P. Anderson32, S. Eshleman33, C. Blanchette31, J. Lucas31, C. Psaros34, S. Safren35, J. Sugarman36, H. Scott37, J. Eron38, S. Fields39, K. Gomez‐Feliciano31, A. Jennings31, K. Shin40, J. Rooney41, W. Spreen42, D. Margolis42, A. Rinehart42, A. Adeyeye43, M. Cohen44, M. McCauley31, B. Grinsztejn45, for the HPTN 083 Study Team
1University of California Los Angeles, UCLA Center for Clinical AIDS Research & Education, Los Angeles, United States, 2Fred Hutchinson Cancer Institute, Seattle, United States, 3Louisiana State University, Baton Rouge, United States, 4Instituto de Pesquisa Clinica Evandro Changas‐Fiocruz, Rio de Janeiro, Brazil, 5Via Libre Clinical Research Center, Lima, Peru, 6Chiang Mai University, Research Institute for Health Sciences, Chiang Mai, Thailand, 7Center for Disease Control and Prevention, Silom Clinic Bangkok Thailand, Atlanta, United States, 8University of Pennsylvania, Philadelphia, United States, 9CITBM CRS, Lima, Peru, 10St. Jude Children's Research Hospital, Memphis, United States, 11Asociacion Civil Impacta Salud y Educacion, Lima, Peru, 12Yen Hoa Clinic, Hanoi, Vietnam, 13Asociación Civil Selva Amazónica, Iquitos, Peru, 14University of Sao Paulo, Sao Paulo, Brazil, 15Emory University School of Medicine, Atlanta, United States, 16Hospital General de Agudos JM Ramos Mejia, Buenos Aires, Argentina, 17Sao Paulo DST/AIDS CRS, Sao Paulo, Brazil, 18Groote Schuur Hospital, Cape Town, South Africa, 19Thai Red Cross AIDS Research Centre, Bangkok, Thailand, 20Hospital Nossa Senhora da Conceição, Porto Alegre, Brazil, 21Fundación Huésped, Buenos Aires, Argentina, 22Barranco CRS, Lima, Peru, 23University of Alabama at Birmingham, Birmingham, United States, 24Rutgers New Jersey Medical School, Newark, United States, 25Emory University Rollins School of Public Health, Atlanta, United States, 26Cornell University Weill Medical College, Division of Infectious Diseases, New York, United States, 27Johns Hopkins University, Department of Pathology, Baltimore, United States, 28Johns Hopkins University, Baltimore, United States, 29University of Alabama at Birmingham School of Medicine, Birmingham, United States, 30Lundquist Institute, Harbor‐UCLA Medical Center, Los Angeles, United States, 31FHI 360, Washington, United States, 32University of Colorado Anschutz Campus, Aurora, United States, 33Johns Hopkins University School of Medicine, Baltimore, United States, 34Massachusetts General Hospital, Boston, United States, 35University of Miami, Miami, United States, 36Johns Hopkins Berman Institute of Bioethics, Baltimore, United States, 37San Francisco Department of Public Health, San Francisco, United States, 38University of North Carolina at Chapel Hill, Division of Infectious Diseases, Chapel Hill, United States, 39New York Institute of Technology, Old Westbury, United States, 40Division of AIDS, Pharmaceutical Affairs Branch, Rockville, United States, 41Gilead Sciences, Foster City, United States, 42ViiV Healthcare, Durham, United States, 43Division of AIDS, NIAID, NIH, Clinical Prevention Research Branch, Rockville, United States, 44University of North Carolina School of Medicine, Department of Medicine, Chapel Hill, United States, 45Instituto de Pesquisa Clinica Evandro Chagas‐Fiocruz, Rio de Janeiro, Brazil
Background: HPTN 083 is a Phase 2b/3 randomized multicenter double‐blind, double‐dummy, clinical trial evaluating safety and efficacy of long‐acting injectable cabotegravir (CAB) compared to daily oral TDF/FTC for HIV PrEP. The blinded trial was stopped at a pre‐planned interim DSMB review in May 2020.
Methods: HIV‐uninfected MSM and TGW at increased HIV risk were randomized 1:1 to either active CAB +TDF/FTC placebo (oral cabotegravir(CAB) for 5 weeks, then IM injections every 8 weeks for 148 weeks) or active TDF/FTC+CAB placebo (oral placebo for 5 weeks, then placebo injections on the same schedule). All participants were offered daily oral TDF/FTC for 48 weeks after last injection. The primary endpoints were incident HIV infection and grade 2 or higher clinical and laboratory events.
Results: Participants were enrolled at 43 sites in Africa, Asia, Latin America, and the US (N=4566); median age: 26y (IQR 22‐32); 12%TGW (n=567); 50% of US participants were Black (n=844). Participant retention at 6, 12, and 24 months was 91%, 87%, and 74%, respectively. Fifty‐two incident HIV infections were observed over 6385 person‐years, with overall HIV incidence 0.81% (95%CI 0.61‐1.07); 39 infections were in the TDF/FTC arm (incidence 1.22%, 95%CI 0.87‐1.67); 13 infections were in the CAB arm (incidence 0.41%, 95%CI 0.22‐0.69%); HR: 0.34 (95%CI 0.18‐0.62). Blinded study product injections covered 92% of person‐years. Adherence to oral TDF/FTC was high; in a random subset of 372 TDF/FTC participants 87% had plasma samples with detectable concentrations, and 75% had concentrations consistent with daily dosing. CAB and TDF/FTC were both well tolerated; most adverse events were mild/moderate and balanced between arms. Injection site reactions, pyrexia, and hypertension were significantly more common in CAB participants, nausea was significantly more common in TDF/FTC participants. Injection intolerance led to discontinuation in 46 (2.2%) active CAB‐LA recipients and was associated with the severity of the intolerance/reaction.
Conclusions: CAB and TDF/FTC were both safe and highly effective for PrEP in HPTN083, with estimated HIV incidence in the CAB arm 66% lower than in the TDF/FTC arm. CAB is the first injectable agent proven effective for HIV PrEP; a companion trial in cisgender women is ongoing.
OAXLB0102
Update on neural tube defects with antiretroviral exposure in the Tsepamo study, Botswana
R. Zash1, L. Holmes2, M. Diseko3, D. Jacobson4, G. Mayondi3, A. Isaacson3, S. Davey3, J. Mabuta3, M. Mmalane3, T. Gaolathe3, S. Lockman5, J. Makhema3, R. Shapiro4
1Beth Israel Deaconess Medical Center, Boston, United States, 2Mass General Hospital for Children, Boston, United States, 3Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana, 4Harvard TH Chan School of Public Health, Boston, United States, 5Brigham and Women's Hospital, Boston, United States
Background: The Tsepamo study last reported neural tube defect (NTD) data collected through March 2019 (Zash et al, NEJM 2019), with 0.3% prevalence following exposure to dolutegravir at conception compared with 0.1% following exposure to non‐DTG antiretrovirals at conception. The study is ongoing and data collected through April 2020 are now reported.
Methods: The Tsepamo Study conducts birth outcomes surveillance study at government hospitals throughout Botswana, currently covering ~70% of all births. Midwives perform surface examinations of all live births and stillbirths and describe abnormalities. Research assistants photograph major abnormalities after maternal consent, which are reviewed by a birth defects expert blinded to exposures. Prevalence of NTDs was determined by maternal HIV and antiretroviral exposure status (95%CI by Wilson method) and the primary analysis evaluated prevalence differences by exposure status (95%CI by Newcombe method).
Results: Since March 2019, 39,200 additional births were recorded, including 1908 additional DTG conception exposures. Since August 2014, there have been a total of 158,244 deliveries; 153,899 (97.2%) had an evaluable infant surface exam, with 126 (0.08%, 95%CI 0.07%, 0.09%) NTDs identified (83 with photo, 43 by description only). Among women on dolutegravir at conception, 7/3591 NTDs occurred (0.19%; 95%CI 0.09%, 0.40%): 3 myelomeningoceles, 1 anencephaly, 2 encephaloceles, and 1 iniencephaly. In comparison, NTDs occurred in 21/19,361 (0.11%; 95%CI 0.07%, 0.17%) women delivering on any non‐dolutegravir antiretrovirals from conception, 8/10,958 (0.07%; 95%CI 0.03%, 0.17%) on efavirenz from conception, 2/4,581 (0.04%; 95%CI 0.1%, 0.16%) on dolutegravir started in pregnancy, and 87/119,630 (0.07%; 95%CI 0.06, 0.09%) HIV‐uninfected women. NTD prevalence differed non‐significantly between dolutegravir and any non‐dolutegravir antiretrovirals from conception (0.09% difference; 95%CI ‐0.03%, 0.30%).
Conclusions: After a period of decline since the original safety signal, prevalence of NTDs among infants born to women on dolutegravir at conception may be stabilizing at approximately 2 per 1000.
Abstract OAXLB0102‐Figure 1.
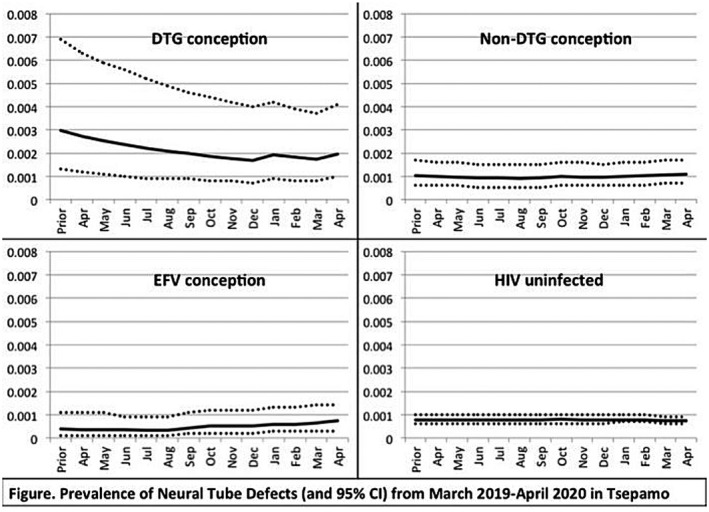
OAXLB0103
Community HIV testing continuity in the context of COVID‐19 lockdown and social distancing
R. Dekova1, N. Mdluli1
1Population Services International, Health Services, Mbabane, Eswatini
Background: Eswatini entered into a national lockdown at the end of March 2020 in response to the spread of SARS‐CoV‐2. Non‐essential industries stopped operating, and the Ministry of Health announced a halt to community HIV testing services (those taking place outside of healthcare facilities). Focus shifted to providing HIV self‐testing kits near to essential businesses, including pharmacies and food stores. The latest data indicate that Eswatini is one of six countries who have achieved the 90‐90‐90 cascade goals. It is thus especially important in the country's context of a generalised epidemic to not undo this achievement. The objective of this paper is to analyse this new approach in offering HIV testing.
Description: PSI healthcare workers distributed oral HIV self‐tests in front of food stores, pharmacies, and in their own residential communities. A risk assessment was conducted for each client, as well as screening for contacts to receive an HIV self‐test. Contact details were collected from consenting clients for follow‐up, where they were provided with further support and linked to HIV prevention or treatment services.
Lessons learned: Over 24 days, 7,997 HIVST kits were distributed near to pharmacies, shops and residential communities of distributors. The kits distributed were evenly split between male and female recipients. With regards to testing history, 38% of all recipients had not tested for HIV in the year prior to receiving the self‐test, and 17% of recipients had never tested for HIV before. Further breakdown reveals that 56% of the recipients who had never tested for HIV prior were men, and 15% were above age 40.
Conclusions/Next steps: The advent of Covid‐19 brought temporary HTS policy changes to Eswatini. The new HTS strategy had positive results, with more than half of the HIV self‐tests reaching clients who had never tested before, or who had not tested in the past year, which is the guideline for testing frequency in Eswatini. Furthermore, the larger proportion of those with poor testing behaviour were men, who are a target group for HIV self‐testing. Though new, this is a strategy that should continue, with more attention given to locations all over the country, for more equitable access.
OAXLB0104
The ADVANCE trial: Phase 3, randomised comparison of TAF/FTC+DTG, TDF/FTC+DTG or TDF/FTC/EFV for first‐line treatment of HIV‐1 infection
F. Venter1, S. Sokhela1, L. Fairlie2, C. Serenata1, N. Mashabane1, M. Masenya2, A. Qavi3, K. McCann3, B. Simmons3, P. Clayden4, A. Hill5
1University of the Witwatersrand, Ezintsha, Wits RHI, Johannesburg, South Africa, 2University of the Witwatersrand, Wits RHI, Johannesburg, South Africa, 3Imperial College London, Faculty of Medicine, London, United Kingdom, 4HIV i‐Base, London, United Kingdom, 5Liverpool University, Department of Pharmacology, Liverpool, United Kingdom
Background: In low‐ and middle‐income countries, most treatment‐naïve people living with HIV (PLWH) take tenofovir disoproxil fumarate (TDF) with emtricitabine FTC (or lamivudine (3TC)) and efavirenz (EFV). Dolutegravir (DTG) and tenofovir alafenamide (TAF) are recommended in international guidelines, but clinical experience with these ARVs in sub‐Saharan Africa is limited. In South Africa, over 10% of patients have transmitted NNRTI drug resistance.
Methods: We conducted a 96‐week, open‐label randomised trial in South Africa, comparing TAF/FTC+DTG, TDF/FTC+DTG and TDF/FTC/EFV. Inclusion criteria included age ≥12 years, no prior ART >30 days, creatinine clearance >60 mL/min (>80 mL/min if <19 years), and HIV‐1 RNA >500 copies/mL. Pregnancy and tuberculosis (TB) were exclusion criteria. There was no screening for baseline drug resistance, consistent with South African treatment guidelines. The primary treatment failure endpoint was 96‐week HIV‐1 RNA >50 copies/mL, discontinuation or missing data (Intent‐to‐treat population, non‐inferiority margin ‐10%, significance level p=0.017, adjusted for multiple comparisons). We report 96‐week efficacy and safety data.
Results: We randomised 1053 PLWH between February 2017 and May 2018: 99% black, 59% female, mean age 32 years, with mean CD4 336 cells/uL. At week 96, the percentage of participants with HIV RNA <50 copies/mL was 78.6% for TAF/FTC+DTG, 78.3% for TDF/FTC+DTG and 73.5% for TDF/FTC/EFV. In the on‐treatment analysis, 96% of participants on TAF/FTC+DTG, 95.7% on TDF/FTC+DTG and 95.5% on TDF/FTC/EFV had HIV RNA <50 copies/mL at Week 96. Both DTG arms demonstrated non‐inferior efficacy versus the EFV arm. Overall, 206/244 (84%) of treatment failures were from discontinuation. Clinical adverse events and laboratory abnormalities were similar between treatment arms.
Table 1. ADVANCE trial results at Week 96
| Treatment arm | TAF/FTC+DTG | TDF/FTC+DTG | TDF/FTC/EFV |
|---|---|---|---|
| n | n=351 | n=351 | n=351 |
| Week 96 Efficacy | |||
| HIV RNA <50 copies/mL | 276 (78.6%) | 275(78.3%) | 258 (73.5%) |
| HIV RNA ≥50 copies/mL | 11 (3.1%) | 14 (4.0%) | 15 (4.3%) |
| Discontinuation for adverse events | 2 (0.6%) | 1 (0.3%) | 10 (2.8%) |
| Discontinuation for other reasons | 64 (18.2%) | 62 (17.7%) | 80 (22.8%) |
| Treatment‐emergent drug resistance | 0 (0.0%) | 2 (0.6%) | 14 (4.0%) |
| Female mean weight change | +8.1kg | +4.8kg | +3.2kg |
| Male mean weight change | +5.4kg | +3.6kg | +1.1kg |
| Treatment emergent obesity | 47 (18%) | 28 (11%) | 18 (8%) |
| Treatment emergent metabolic syndrome | 23 (8%) | 16 (6%) | 10 (4%) |
Conclusions: In the ADVANCE study, TAF/FTC+DTG and TDF/FTC+DTG demonstrated non‐inferior efficacy versus TDF/FTC/EFV, with low rates of virologic failure in all three arms despite country‐level background NRTI/NNRTI resistance.
OAXLB0105
The first long‐term remission of chronic HIV‐1 infection without myeloablation?
R. Diaz1, L. Giron2, J. Galinskas3, J. Hunter1, S. Samer4, D. Dias1, L.M. Janini3, I.L. Shytaj5, M.C. Sucupira1, M. Tarek6, A. Savarino7
1UNIFESP, Infectious Diseases, Sao Paulo, Brazil, 2Wistar Institute, Philadelphia, United States, 3UNIFESP, Sao Paulo, Brazil, 4Emory University/Yerkes National Primate Research Center, Atlanta, United States, 5Heidelberg University Hospital, Infectious Diseases, Heidelberg, Germany, 6Armed Forces College of Medicine, Bioinformatics Department, Cairo, Egypt, 7Italian Institute of Health, Infectious Diseases, Rome, Italy
Background: A 34 yo Brazilian male received HIV diagnosis on October 11th, 2012. Baseline CD4+ T cell count was 372 cells/microliter and viral load (VL) was 20,221 cp/mL, suggesting chronic HIV infection. On December 12st, 2012 he started antiretroviral treatment with TDF/3TC/EFV maintaining VL below detection limits (BDL) since them. In 2016, he was enrolled in clinical trial NCT02961829 as one of five individuals under highly intensified ART (baseline ART+dolutegravir+maraviroc) and nicotinamide (500 mg twice daily) for 48 weeks. Nicotinamide (NAM) was chosen because of inhibition of immune exhaustion‐related lymphocyte apoptosis related to its inhibitory effects on PARPs, and potential multiple mechanisms of antilatency such as Class III HDACs inhibition (NAM) and SUV39 Deacetylation (NAD). Maraviroc was also chosen due to potential HIV antilatency property.
Methods: Viral DNA was measured as an estimate of the viral reservoir by published qPCR techniques. Antibody quantitation was performed using the Abbott ARCHITECT HIV Ag/Ab Combo assay (Abbott, IL, USA). Mathematical modelling performed according Conway et al., 2015.
Results: Among 30 participating patients from NCT02961829, this study subject was the only experiencing viral load blips during experimental treatment, at weeks 16 (VL BDL with target detected) and 24 (56 cp/mL). Viral DNA showed low‐level positivity in PBMCs and rectal biopsy at baseline and week 48. Antibody quantitation over time (RLU [S/CO] in duplicates) was 91.8 (baseline), 75.6 (week 12), 60.8 (w24), 56.8 (w36) and 58.0 (w48). In March 28th 2019, he underwent analytical treatment interruption (ATI). HIV Plasma VL performed every 3 weeks after ATI was BDL up to 57 weeks, and total HIV DNA on PBMCs was undetectable pre‐ATI and 57 weeks post‐ATI. EIA rapid test kit (TR DPP HIV 1/2 Bio‐Manguinhos) on February 3rd 2020 was negative. Mathematical modelling (Conway et al., 2015) showed that the antiapoptotic and antiproliferative effects might improve clearance of productively infected cells, but only the additional contribution of the antilatency effect might induce long‐term remission.
Conclusions: Although still an isolated case, this might represent the first long‐term HIV remission without myeloablation/stem cell transplantation. Further analyses such as viral cultivation and sequential HIV antibody profile/detection are ongoing.


