Abstract
BACKGROUND:
Up to 40% of combat casualties with a truncal injury die of massive hemorrhage before reaching a surgeon. This hemorrhage can be prevented with damage control resuscitation (DCR) methods, which are focused on replacing shed whole blood by empirically transfusing blood components in a 1:1:1:1 ratio of platelets:fresh frozen plasma:erythrocytes:cryoprecipitate (PLT:FFP:RBC: CRYO). Measurement of hemostatic function with rotational thromboelastometry (ROTEM) may allow optimization of the type and quantity of blood products transfused. Our hypothesis was that incorporating ROTEM measurements into DCR methods at the US Role 3 hospital at Bagram Airfield, Afghanistan would change the standard transfusion ratios of 1:1:1:1 to a product mix tailored specifically for the combat causality.
METHODS:
This retrospective study collected data from the Department of Defense Trauma Registry to compare transfusion practices and outcomes before and after ROTEM deployment to Bagram Airfield. Over the course of six months, 134 trauma patients received a transfusion (pre-ROTEM) and 85 received a transfusion and underwent ROTEM testing (post-ROTEM). Trauma teams received instruction on ROTEM use and interpretation, with no provision of a specific transfusion protocol, to supplement their clinical judgment and practice.
RESULTS:
The pre and post groups were not significantly different in terms of mortality, massive transfusion protocol activation, mean injury severity score, or coagulation measurements. Despite the difference in size, each group received an equal total number of transfusions. However, the post-ROTEM group received a significant increase in PLT and CRYO transfusions ratios, 4× and 2×, respectively.
CONCLUSION:
The introduction of ROTEM significantly improved adherence to DCR practices. The transfusion differences suggest that aggressive DCR without thromboelastometry data may result in reduced hemostatic support and underestimate the need for PLT and CRYO. Thus, future controlled trials should include ROTEM-guided coagulation management in trauma resuscitation.
Keywords: Combat, transfusion, thromboelastometry, damage control resuscitation
Trauma-induced hemorrhage and coagulopathy are the leading causes of combat death. Research from modern conflicts has documented that 24% of these deaths may be potentially survivable.1 Military trauma research has thus focused on decreasing bleeding in the early postinjury period with tourniquets and hemostatic dressings.2,3 Despite these advances, only one third of hemorrhagic wounds are amenable to a tourniquet, as the majority of patients experience junctional (axilla/groin) or truncal hemorrhage.4 Moreover, at least 38% of transfused combat causalities have acute traumatic coagulopathy (ATC, defined as international normalized ratio [INR], > 1.2), which is also a significant risk factor for mortality.5 Therefore, optimization of coagulopathy management represents a critical capability gap in the treatment of traumatic combat injuries.
In severely injured casualties, the lethal triad of hypothermia, acidosis, and coagulopathy precede imminent death.6 Conventional resuscitation practices focus on rapid reversal of acidosis, prevention of hypothermia, and surgical techniques to control hemorrhage. This practice has often neglected the direct treatment of traumatic coagulopathy that can be aggravated by resuscitation, hemodilution, and hypothermia. Laboratories often cannot provide coagulation test results in a timely manner and blood banks may be unable to deliver specific blood products such as fresh frozen plasma (FFP), platelets (PLT), and cryoprecipitate (CRYO) when needed.7 However, recent conflicts have directly addressed the entire lethal triad with damage control resuscitation (DCR) policies adopted in combat hospitals since 2006.1 DCR consists of two parts initiated immediately upon arrival to a combat hospital. First, a limitation of resuscitation goals to a systolic blood pressure of 80 mm Hg to 90 mm Hg, preventing rebleeding from damaged vessels and reducing fluid and vasoactive agent support requirements.8 Second, intravascular volume restoration is accomplished by using rapidly available thawed FFP as a primary resuscitation fluid, targeting a 1:1 ratio with PRBCs.9 These DCR policies have led to a significant decrease in the amount of fluid resuscitation and mortality among potentially salvageable combat causalities and civilian trauma patients.4
The American College of Pathology and the British National Blood Transfusion service define the presence of coagulopathy as a prothrombin time (PT) longer than 18 seconds (INR, > 1.2) or an activated partial thromboplastin time (APTT) longer than 60 seconds.10,11 These standard coagulation laboratory tests typically entail turnaround times of 45 minutes, which results in a significant delay in diagnosis, monitoring therapy, or delivering a therapeutic intervention. Rotational thromboelastometry (ROTEM) provides a rapid and point-of-care method to measure clot formation in whole blood. Additionally, studies document successful use in a military setting.10 Measurement of patient hemostatic function with ROTEM may allow tailoring of therapy to optimize component use.
Several studies have evaluated the use of thromboelastography and thromboelastometry (TEG/ROTEM) to guide transfusion decisions in trauma.12–15 Even fewer have documented the use of TEG/ROTEM with DCR policies or were conducted in an active combat setting.16 However, there is an ongoing multicenter RCT (NCT02593877) and a single institution RCT which documented that utilization of a goal-directed TEG-guided massive transfusion protocol compared with conventional coagulation assays significantly improved survival and reduced the transfusion of FFP and PLT.17 Therefore, the purpose of this study was to evaluate in an active combat setting the effects of the use of ROTEM on the resuscitation procedure, blood product transfusions, and ratios. The hypothesis of this study is that ROTEM use in a military setting would facilitate personalization of transfusion strategies for severely injured combat casualties and produce blood product ratios that differ from empiric 1:1:1:1 prescription of DCR. Our secondary hypothesis was that use of ROTEM would reduce the amount of blood products transfused and mortality from combat-related injury.
MATERIALS AND METHODS
Study Design
The US Army Medical Research and Materiel Command Institutional Review Board approved the study protocol. Our primary goal was to determine the effect of ROTEM on DCR practices. Using the Department of Defense Trauma Registry (DoDTR), we collected data on transfusions before (pre-ROTEM) and after (post-ROTEM) implementation. The Department of Defense established the DoDTR database to capture data prospectively from multiple nonintegrated clinical and administrative systems. This database provides comprehensive data collection from the point of injury through discharge from military treatment facilities for non-US military patients and from point of injury through rehabilitation for US patients. The ROTEM device arrived at the Craig Theater Hospital at Bagram Airfield (BAF) in November 2011. This retrospective study protocol compared all subjects, transfused with at least one blood product, for three months proceeding to define ROTEM effects upon DCR clinical practice pre-ROTEM (August 2011 to October 2011) versus post-ROTEM (November 2011 to January 2012). We specifically chose this interval because of minimal changes in operations, wounding patterns, or clinical team rotation that could have affected the results. Therefore, the only change to transfusion therapy during this interval was the use of ROTEM analysis along with standard care to direct DCR therapy.
Patient Data Collection
The patient population included civilians and both US and foreign military personnel. All combat casualties who received at least one transfusion within 24 hours from admission were included in the study. Moreover, the study did not exclude any casualty who received ROTEM analysis. The DoDTR database provided clinical outcomes such as the Injury Severity Score (ISS), Abbreviated Injury Scale (AIS) scores, primary cause of death, time of death, and mortality at hospital discharge, and vital signs on admission to BAF. The database also provides quantitative laboratory and patient data including hematocrit, PLT count, base deficit, INR, systolic blood pressure, temperature, heart rate, and vital signs upon admission to BAF. Additional data included the total amount of crystalloid and blood products (RBC, FFP, CRYO, recombinant FVIIa, apheresis PLT [aPLT], and fresh whole blood [FWB] units) administered, within 24 hours from admission, at the combat support hospital. As in previous studies,9 we defined a massive transfusion as 10 or more RBC units (including both stored RBC and FWB units) given to one patient within 24 hours. One apheresis PLT unit is equal in content to approximately six units of whole blood-derived PLTs.18 In accordance to previous studies, the PLT contribution from FWB was included in the calculation of apheresis PLT units transfused.19 Because one unit of FWB has approximately one unit of RBCs, one sixth unit of PLT and one unit of FFP, the total amount of RBC, PLTand FFP units transfused was set as the number of both stored component and FWB units transfused.
Reported outcomes for all patients in this study were the proportion of patients receiving a blood transfusion, total units transfused, and the ratios of products FFP:RBC, PLT:RBC, and CRYO:RBC. For US military patients, the study collected mortality and hospital discharge data, throughout all levels of care including discharge from acute care hospitals in the United States. For non-US military patients, the study collected mortality and hospital discharge data only from the combat support hospital at BAF. Discharge or transfer criteria were the same for civilian, non-US, and US military patients, which included stable surgical repair, stable hemodynamics, and no requirement for use of vasoactive agents or mechanical ventilation. Additional clinical outcomes documented for both groups included the length of stay, ventilator days, age, sex, nature of the trauma (blunt or penetrating), number of shocked patients (defined as base deficit greater than 5 upon arrival to the hospital), coagulopathy of trauma (INR, > 1.2), and the number of massive transfusions.20
Clinical Coagulation Monitoring
Upon arrival to the hospital, a sample of blood for ROTEM analysis was collected into a 2.7-mL citrate vacutainer (0.109 M buffered sodium citrate, 3.2%; Becton Dickinson, Plymouth, UK) and processed in the hospital laboratory. Additional laboratory coagulation tests (PT, PTT, fibrinogen, and complete blood count) were also performed. An arterial blood gas analysis for base deficit was performed at the same time as the ROTEM sample collection.
ROTEM Analysis
Within minutes of collection, blood samples were processed on a ROTEM delta instrument (Pentapharm GmbH, Munich, Germany). Treating physicians had access to the results but no standard protocol to guide transfusions. The detailed methodology and parameters of ROTEM analysis have been previously described in other studies.10,21 Several ROTEM tests are available, but the four tests used clinically were EXTEM, INTEM, FIBTEM, and APTEM to define the function of the extrinsic and intrinsic coagulation systems, fibrinogen function, and fibrinolysis respectively. Briefly, the assays combine 20 μL calcium chloride and 300 μL of whole blood with either 20 μL of tissue factor activator or 20 μL of ellagic acid activator, for the EXTEM and INTEM, respectively. FIBTEM, measures fibrinogen levels and fibrin polymerization, and is similar to EXTEM except for the addition of cytochalasin D to block PLT function and isolate the effects of fibrinogen on clot strength. APTEM contains the plasmin inhibitor aprotinin that prevents fibrinolysis; therefore, in combination with EXTEM, it can confirm hyperfibrinolysis. All pipetting steps and the mixing of reagents with samples are performed with the automated electronic pipette program. In the post-ROTEM periods, the following parameters were collected for each test performed: clotting time (CT), alpha angle (alpha), clot formation time (CFT), maximum clot firmness (MCF), and clot lysis at 30 minutes (LI30). Interpretation of results was based on comparison of patient results with manufacturer-provided normal ranges.
Statistical Analysis
Data are presented as counts and percentages, mean (standard deviation) or median [interquartile range] for pre- and post-ROTEM periods. For continuous, normally distributed data, a Student’s t test was performed for comparisons of means. For non-normally distributed data, a non-parametric Wilcoxon test was performed. For categorical data, the χ2 and Fischer exact tests were used. For proportions, we used the Clopper and Pearson method to calculate an “exact” confidence interval and error bars. Significance was set at p less than 0.05. Comparison of overall mortality among the pre- and post-ROTEM groups was calculated using survival analysis based on the Cox model. A hazard ratio was estimated, adjusted on potential confounders associated with death in univariate analysis with p less than 0.25 using step-wise backward method (presence of shock and ISS). We tested the proportionality of the variables using the test of proportional hazards. Statistical analysis was done with JMP version 10 (SAS Institute Inc., Cary, NC) and GraphPad Prism Version 7.0b.
RESULTS
Between August 2011 and January 2012, 970 trauma patients were admitted to the Craig Theater Hospital, BAF in Afghanistan (Table 1). Of these, 134 (21%) patients received a blood transfusion in the pre-ROTEM period (August 2011 to October 2011) and 85 (26%) patients in the post-ROTEM period (November 2011 to January 2012). There was no significant difference between the pre- and post-ROTEM groups in age, gender (majority were male), mean ISS, distributions of blunt and penetrating trauma, mean base deficit in shocked patients, mean INR, and the number of patients with coagulopathy of trauma (INR > 1.2). All outcomes are contained in Table 1. Up to 27% of transfused patients met the criteria for shocked in the pre-ROTEM compared with 17% in the post-ROTEM group (p = 0.14). Moreover, there was no significant change in coagulopathy of trauma with an incidence of 50% in pre-ROTEM compared to 61% in the post-ROTEM group (p = 0.28). To compare to other studies of trauma, we compiled a data set to calculate a Trauma-Related Injury Severity Score (TRISS) mortality prediction. Unfortunately, we only had complete values to calculate a TRISS for 81 patients in the pre-ROTEM and 52 patients in the post-ROTEM groups. Based on this sample, there was no difference between the TRISS predictions within the groups or between predicted mortality and actual mortality for blunt and penetrating trauma, respectively. Please see Table 1 for data on TRISS scores for each group. Lastly, the number of massive transfusions was similar between pre- and post-ROTEM at 5% and 6%, respectively (p = 0.55).
TABLE 1.
Demographics and Outcomes
| Demographics | Pre-ROTEM | Post-ROTEM | p |
|---|---|---|---|
| Transfused patients % (n) | 21% (134) | 26% (85) | 0.10 |
| Mean age, y | 24 [21–29] (131) | 25 [21–29] (84) | 0.29 |
| Sex: males % (n) | 93% (124) | 95% (81) | 0.57 |
| Penetrating trauma | 81% (109/134) | 91% (77/85°) | 0.08 |
| Blunt trauma | 16% (21/134) | 8% (7/85) | 0.14 |
| ISS | 21 [14–29] (134) | 24 [17–35] (85) | 0.11 |
| ISS≥15 %(n) | 75% (100) | 79% (67) | 0.52 |
| Shocked patients BD≥5 % (n) | 27% (40/117) | 17% (16/70) | 0.14 |
| Mean BD in shocked patient | 7.0 [6–8.8] | 8.0 [5.3–11.8] | 0.45 |
| Mean INR (n) | 1.2 [1–1.3] (107) | 1.2 [1–1.4] (46) | 0.40 |
| CoT patients INR≥1.2% (n) | 50% (54/107) | 61% (28/46) | 0.74 |
| Mean INR in CoT patients | 1.3 [1.2–1.6] | 1.4 [1.2–1.5] | 0.74 |
| Admit hematocrit, % (n) | 36.4 [31–41.3] (110) | 37.5 [34–43.2] (49) | 0.09 |
| Admit PLT count, × 103 (n) | 203 [145–272] (107) | 160 [132–229] (46) | 0.10 |
| Massive transfusions, % (n) | 5% (7/134) | 6% (5/85) | 0.55 |
| Outcomes | Pre-ROTEM | Post-ROTEM | p |
| Hospital days (n) | 2 [1–7] (134) | 2 [1–3] (85) | 0.07 |
| ICU days (n) | 1 [1–3] (134) | 1 [1–3] (85) | 0.70 |
| Ventilation-free days (n) | 1 [0–4] (134) | 0 [0–2] (85) | 0.16 |
| Overall mortality % (n) | 5.2% (7/134) | 4.7% (4/85) | 1.00 |
| Blunt injury mortality, % (n) | 4.8% (1/21) | 14% (1/7) | 0.44 |
| TRISS predicted blunt mortality % (n) | 1.7 (81) | 4.5 (52) | 0.56 |
| Penetrating injury mortality % (n) | 4.6% (5/109) | 3.9% (3/77) | 1.00 |
| TRISS predicted penetrating % (n) | 2.1 (81) | 4.6 (52) | 0.64 |
All values documented are MEDIAN [IQR] (n of observations) unless otherwise specified.
BD, base deficit; CoT, coagulopathy of trauma as defined as admission INR > 1.2, ED, emergency department or arrival information, ICU, intensive care unit.
Before the introduction of ROTEM to DCR at BAF, the overall blood product transfusion volume was 9.9 units/day compared with 12.7 units/day in the post-ROTEM period. The increased volume of blood products consisted of PLTand CRYO transfusions (Table 2). FWB use was low in each group (10 units transfused in the pre-ROTEM period and 11 units in the post-ROTEM period). We document the proportion of patients who received each blood product in the pre- and post-ROTEM periods during the first 24 hours of admission in Figure 1. The proportion of patients who received a PLT (p = 0.003) or CRYO (p < 0.0001) transfusion rose significantly in the post-ROTEM group. In terms of resource allocation, it is also clear in Figure 2 that the number of PLT and CRYO units distributed over the post-ROTEM period significantly increased when compared to the pre-ROTEM period while number of transfused patients decreased. Overall, the increased number of blood product transfusions did not increase length of hospital stay, length of intensive care unit stay, ventilation-free days, or mortality. For all listed in Table 1, we adjusted on ISS, presence of shock (BD > =5), there was no significant change in overall mortality between groups (hazard ratio, 0.6; confidence interval, 0.1–3.1; p = 0.54), test of proportional hazards verified (p = 0.39); see Table, Supplemental Digital Content 1, http://links.lww.com/TA/A979). Figure 3 documents the effects of ROTEM implementation on DCR transfusion ratios. Figure 3A documents the ratio of blood products to RBCs for patients who received a full set of transfusions. After the introduction of ROTEM analysis, physicians transfused 4× the PLTs and 2× CRYO, which was much closer to achieving the 1:1:1:1 prescription of DCR. There was no significant change in FFP:pRBC ratio between groups (p = 0.20). Lastly, the post-ROTEM group received significantly more fluid resuscitation compared with the pre-ROTEM group (p = 0.0001).
TABLE 2.
Transfusions Within 24 Hours From Admission
| Product | Pre-ROTEM | Post-ROTEM | p |
|---|---|---|---|
| Transfusions (all patients) | |||
| Per patient transfusion | |||
| Packed red blood cells | 2 [1–3] | 2 [1–4] | 0.32 |
| FFP | 1 [0–2] | 2 [0–3.5] | 0.13 |
| Apheresis PLTs | 0 [0–0] | 0 [0–1.6] | <0.01 |
| CRYO | 0 [0–0] | 0 [0–0.5] | <0.01 |
| Crystalloid (L) | 2 [1.3–4.0] | 3.5 [1.2–5.3] | <0.01 |
| Colloids (L) | 0 [0–0.3] | 0 [0–0.5] | <0.01 |
| Transfusions (patients who received the specific blood product) | |||
| Per patient transfusion | |||
| Packed red blood cells | 2 [1–4] | 3 [2–4.5] | 0.06 |
| FFP | 2 [1–4] | 2 [1–5] | 0.23 |
| Apheresis PLTs | 2 [1–6] | 2 [1–6.5] | 0.51 |
| CRYO | 7.5 [1.7–10] | 10 [1.5–20] | 0.15 |
All values documented as median [IQR].
Figure 1.
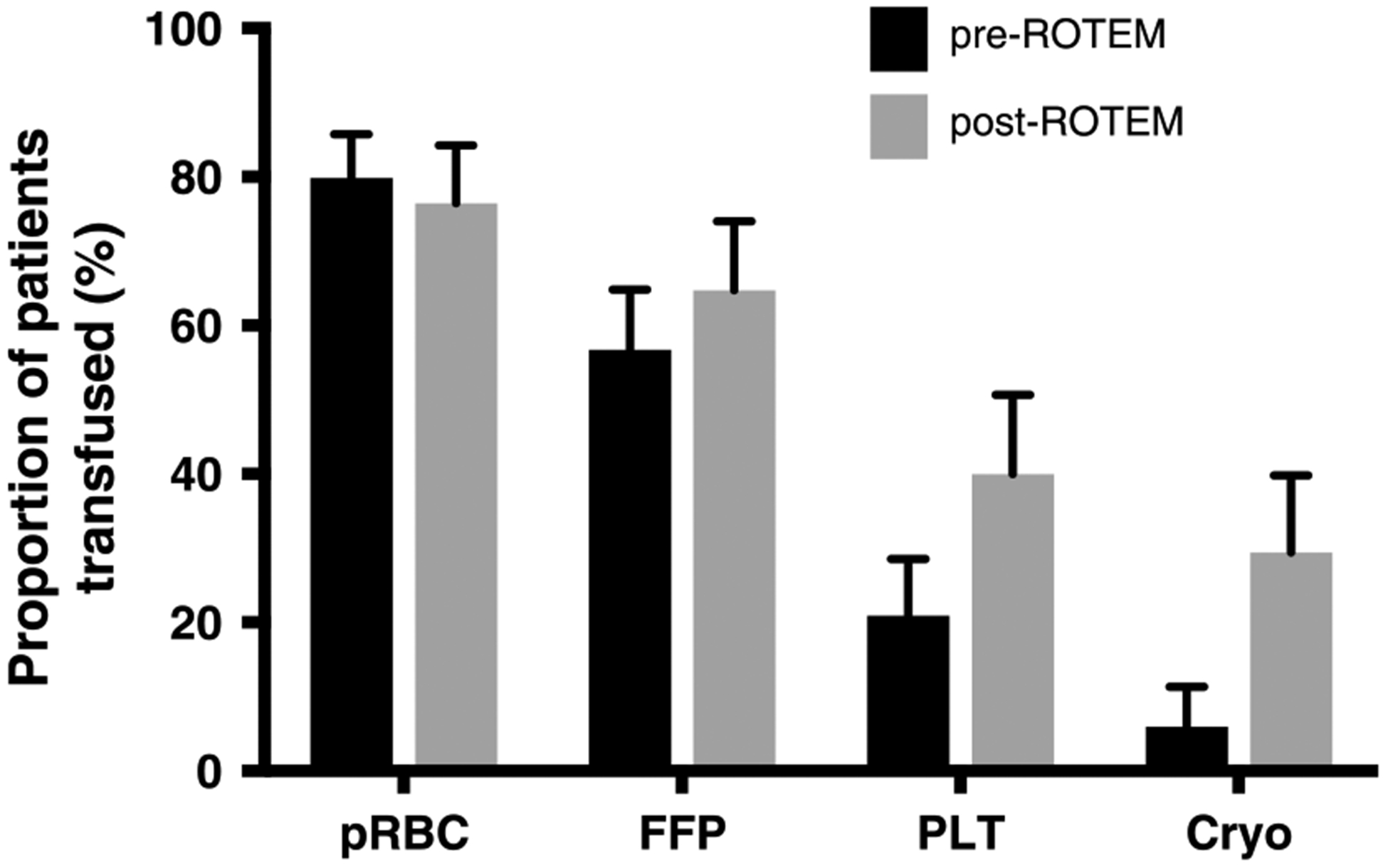
Percentage of transfused patients receiving 1+ unit of each product pre- and post-ROTEM. Data represented in percentage of total patients who received a transfusion over the pre- and post-3 month study period, n = 134 and n = 85, respectively.
Figure 2.
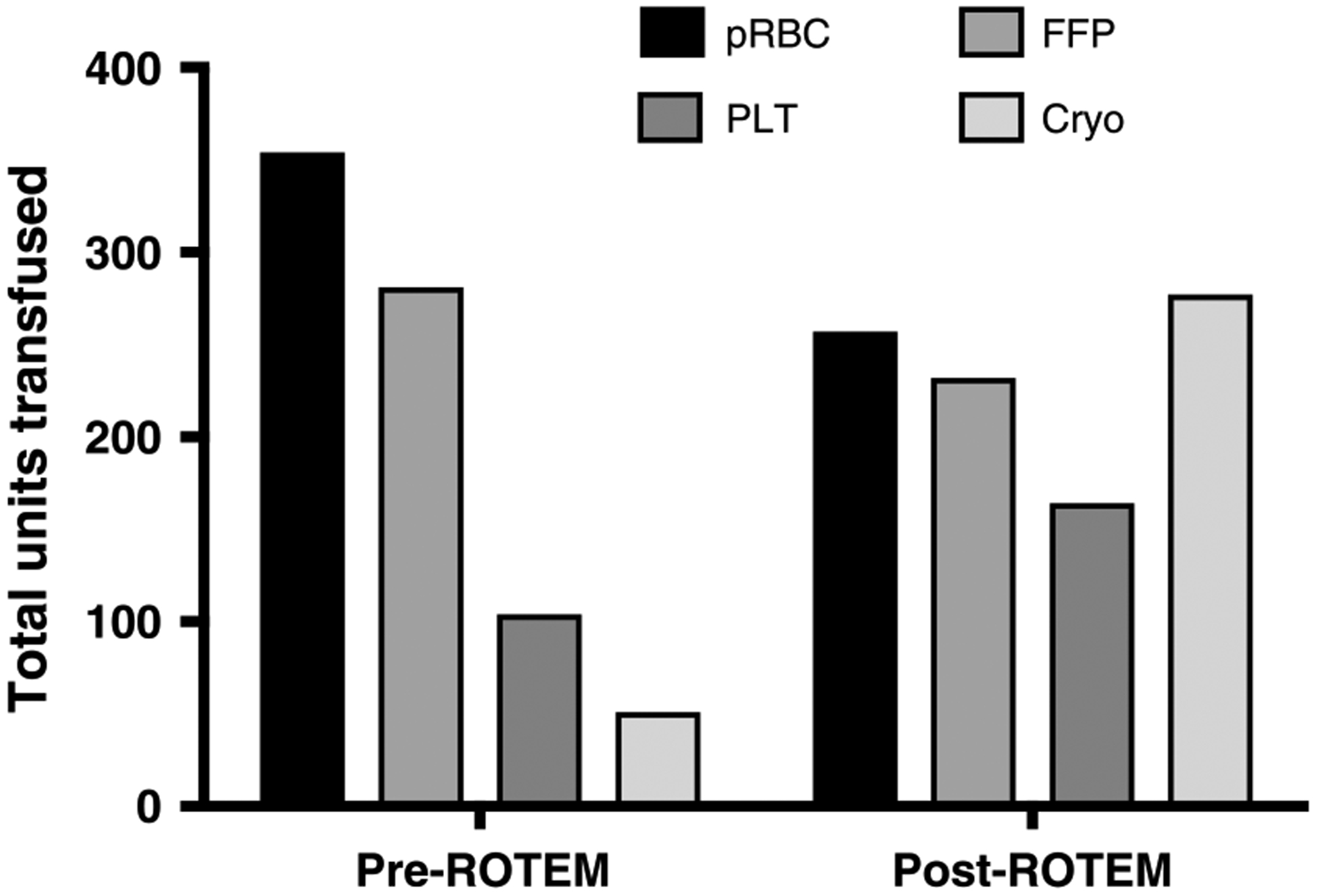
Total blood products (units) transfused in the pre- and post-ROTEM periods. Data represented as mean ± SD.
Figure 3.
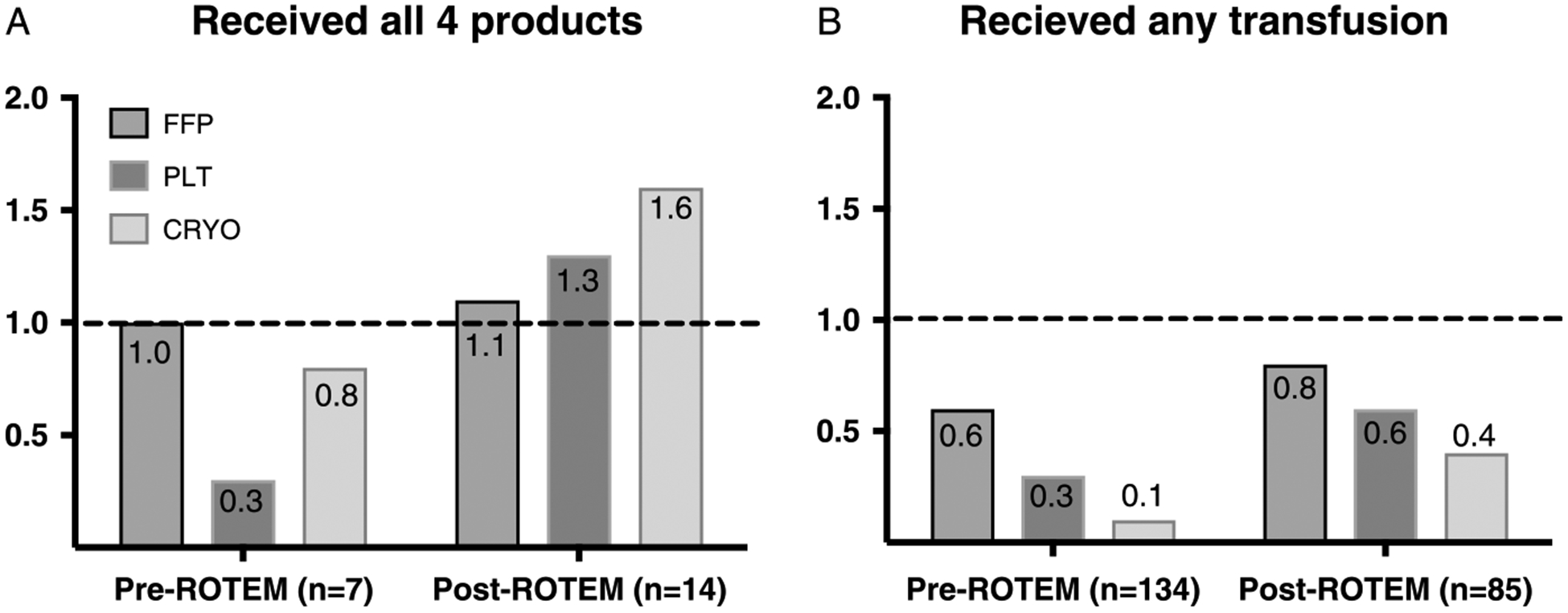
Transfusion ratios in the pre- and post-ROTEM periods. A, A graphical representation of FFP:pRBC, PLT:pRBC, and FFP:pRBC ratios for patients who received all four blood products (n = 7, pre-ROTEM and n = 14, post-ROTEM). B, A graphical representation of FFP:pRBC, PLT:pRBC, and FFP:pRBC ratios for all trauma patients.
ROTEM analyses were performed on individual patients with the following frequencies: INTEM, 233; EXTEM, 26; and FIBTEM, 30, and the results are compared with published normal ranges. Of 331 trauma admissions in the post-ROTEM period, ROTEM findings supported treatment with CRYO, FFP, PLT, or tranexamic acid in 85 (26%) patients. An additional 16 ROTEM analyses confirmed hyperfibrinolysis, by combining APTEM and EXTEM. These results further identified eight patients who had evidence of hypofibrinogenemia (low EXTEM and FIBTEM MCF; 1 of these had hyperfibrinolysis by LI30). Four patients had ROTEM findings consistent with PLT dys-function (low EXTEM and normal FIBTEM MCF). Sixty-four patients had low INTEM MCF values and nine patients had hyperfibrinolysis by LI30 (LI30 < 94 or significant increase in MCF on APTEM compared to EXTEM; one diagnosed by APTEM). Please see Figure 4, for study ROTEM results and the normal reference ranges for INTEM and EXTEM CT, MCF, and LI30. The INTEM results are in Figure 4A documenting that MCF had greater than 10% of values outside the normal range (16% of samples). Moreover, the EXTEM results in Figure 4B document that patients had greater than 10% of MCF values outside normal range (17% of samples). Additionally in Figure 4C, ROTEM documented extended CFT with over 36% of patients demonstrating values over the normal range in the INTEM analysis and 23% of patients in the EXTEM analysis. Lastly in Figure 4D, 30% of patients demonstrated below normal alpha angle on INTEM analysis and 43% on EXTEM analysis.
Figure 4.
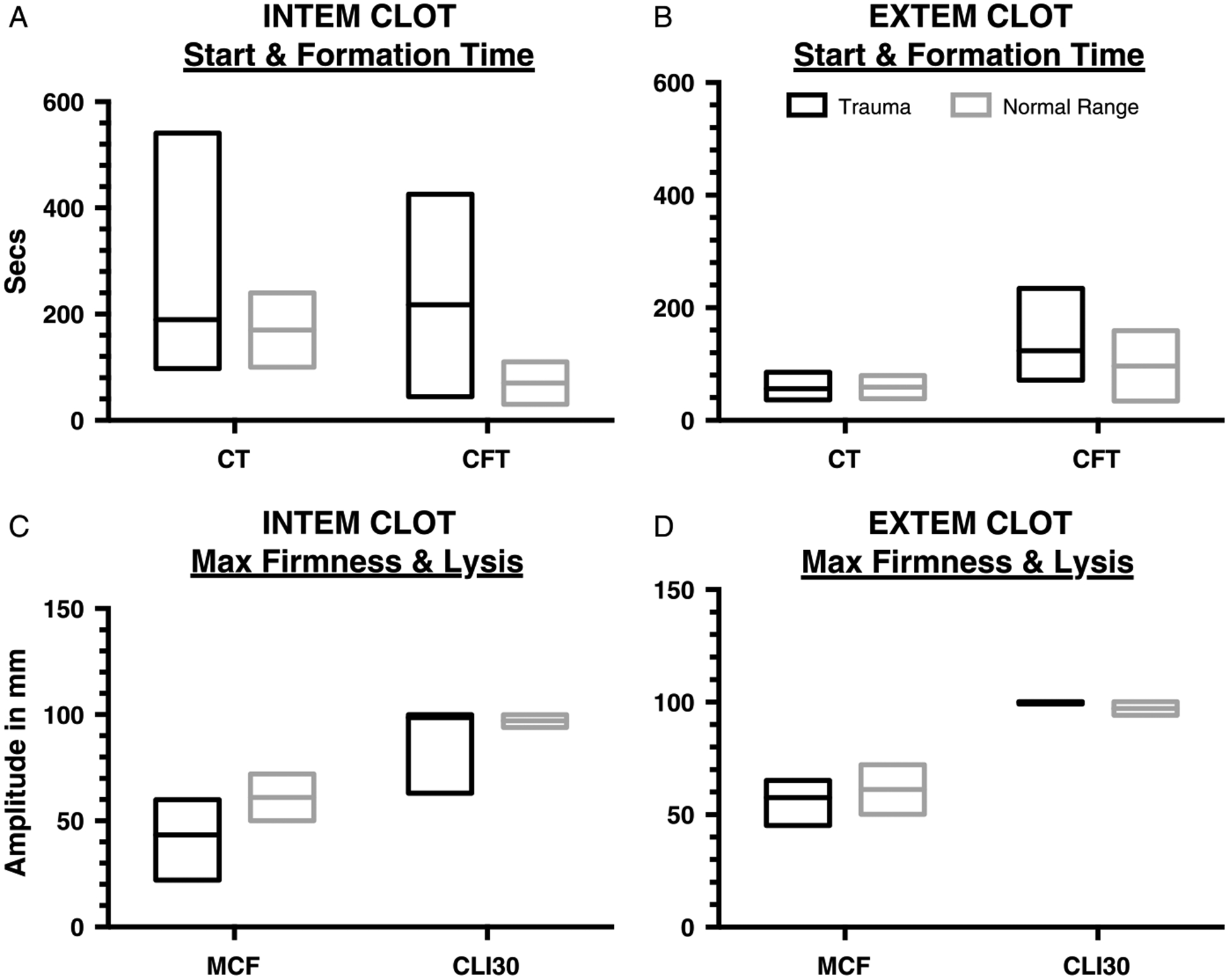
ROTEM test results for all admitted trauma patients. A, An interleaved plot of INTEM CT and CFT plotted next to the standard reference range. B, An interleaved plot of EXTEM CT and CFT plotted next to the standard references range. These results are consistent with other studies that coagulopathy of trauma influences INTEM greater than EXTEM. C, An interleaved plot of INTEM Maximum Clot Firmness (MCF) and Clot Lysis Inhibition at 30 minutes (CLI30) plotted next to the standard reference range. INTEM MCF had 16% of values lower than normal. D, An interleaved plot of EXTEM MCF and CLI30 plotted next to the standard reference ranges. EXTEM MCF had 17% of values lower than normal.
DISCUSSION
Trauma is the leading cause of morbidity and mortality for patients younger than 44 years.22 Current DCR guidelines recommend early and aggressive correction of coagulation abnormalities, without the usual coagulation testing, to decrease mortality and morbidity4 (see Appendix, Supplemental Digital Content 2, http://links.lww.com/TA/A980). Combat hospitals that use DCR practices with a 1:1:1 blood product ratio (FFP: RBC:PLTS) have improved survival to discharge, presumably by decreasing severe hemorrhage.9 However, this practice does not diagnose early trauma coagulopathies or direct patient-specific treatment. Early coagulopathy of trauma (COT) is also an indicator of mortality, present in up to 24% in some studies, and part of the lethal triad in trauma that includes acidosis and hypothermia.5 Moreover, the diagnosis of COT is difficult because laboratory-based clotting tests are logistically challenging to do on the battlefield or even as point-of-care tests in the emergency room because of sample preparation time and bench space requirements. Therefore, there is a great interest in identifying early COT by confirming and testing the reliability and accuracy of rapid point-of-care diagnostic coagulation devices used in combat support hospitals and in civilian trauma bays.
The purpose of this paper was to evaluate the before and after effects of implementing ROTEM, a rapid-diagnostic point-of-care coagulation device, on the DCR practices in the combat support hospital at Bagram Air Base, Afghanistan. Our hypothesis was that that ROTEM use in a military setting would recognize early coagulopathy of trauma in critically wounded soldiers, thus leading to customized therapy that would diverge from empiric 1:1:1:1-based transfusion. ROTEM, an FDA-approved point-of-care diagnostic device, has been studied in civilian trauma. Da Luz et al published a descriptive, systematic review on the effect of TEG and ROTEM on diagnosis, transfusion guidance, and mortality in trauma.23 They found over 50 studies that met inclusion criteria for the review, but only three studies had a low risk of bias in patient selection, index test, reference standard for flow, and timing. Most of these studies were also single center and 20% had concerns for applicability. However, the review documented that TEG/ROTEM measurements were associated with early coagulopathies (e.g., hypercoagulability, hyperfibrinolysis, and PLT dysfunction) not diagnosed by routine screening coagulation tests. Moreover, one study reviewed presented a ROTEM-based transfusion algorithm that reduced blood-product transfusion,24 but very few, if any studies found an association between TEG/ROTEM-based resuscitation and decreases in mortality. These results are consistent with previous combat studies documenting no mortality difference in the pre-ROTEM vs. post-ROTEM groups (Table 1). Although this was a single center and homogenous study (Table 1), it has high applicability to the type of injuries seen at an active combat support hospital. The groups are comparable because there was no difference in the coagulopathy of trauma (INR > 1.2), massive transfusion protocols, or shocked as measured by base deficit. There was also no significant difference in the mean injury severity score despite the lower numbers of patients in the post-ROTEM group. There was no difference in blunt or penetrating traumatic injury mortality in each of our groups. There was no difference in the groups for their calculated TRISS-predicted mortality, which accurately predicts survival for blunt and penetrating trauma injuries. This is in contrast to previous combat studies that documented ROTEM analysis can predict a change in TRISS score.24
In 2014, a randomized controlled trial documented, using intent-to-treat analysis, that thromboelastography (TEG) vs. conventional coagulation assays improved the management of massive transfusion protocols (MTP) for civilian trauma patients.25 This study documented that a TEG-guided MTP can improve overall survival compared to conventional coagulation assays (p < 0.049) and decrease the total amount of blood transfusions during the early phase of resuscitation. Moreover, a 2014 consensus panel recommended early viscoelastic testing during the early phases of trauma.26 In 2015, the Pragmatic, Randomized Optimal Platelet and Plasma Ratio study27 was published reporting on the effectiveness and safety of using the clinical practice guideline of DCR 1:1:1 blood product ratio (n = 338) compared with a 1:1:2 ratio (n = 342). Although, the DCR ratio of 1:1:1 did not decrease 24-hour or 30-day mortality, it did significantly decrease the primary cause of death, exsanguination, within the first 24 hours (p < 0.03). Our retrospective study did not detect changes in mortality, documented that early ROTEM analysis significantly increased the amount and type of blood products transfused at a combat support hospital. The significance of this finding is that ROTEM analysis may decrease mortality by facilitating a strong adherence to DCR clinical practice guidelines, which overall will decrease mortality from the coagulopathy of trauma.4,9,28 We do not know the direct reason why the pre-hospital crystalloid and colloid resuscitation was significantly higher in the post-ROTEM group. However, previous publications have documented that up to 40% of fluid-resuscitated traumatic hemorrhagic shock patients do not have evidence of coagulopathy on admission.29 Moreover, this study compared coagulopathy markers to healthy controls and found only differences in thrombin-antithrombin complexes and markers of fibrinolysis, not PT or PLT count. This may explain part of the increase in CRYO transfusion but not the increase in PLT transfusion in the post-ROTEM group. We suggest that the DCR as practiced in combat support hospitals may still result in suboptimal hemostatic support, reduced PLTs and CRYO, and need for additional fluid resuscitation without ROTEM guidance.
In 2015, the Cochrane Collaboration published a diagnostic test accuracy review on TEG or ROTEM to determine whether they could accurately identify coagulopathy of trauma in adult patients who were bleeding. Their search in early 2013 found only three studies (UK, France, and Afghanistan) that met criteria for analysis. The Cochrane review concluded that no evidence was available on the accuracy of TEG and very little on the accuracy of ROTEM.30 Later in 2013, Chapman and Moore et al31 published an article documenting that post injury hyperfibrinolysis, defined as LY30 of 3% or greater on rapid thrombelastography, was associated with high mortality and large use of blood products. In 2015, they also published a World Trauma Association Plenary Paper defining a rapid thrombelastography, with a “diamond-shaped” tracing, that when applied to 572 unmatched patients had a 100% positive predictive value for mortality.32 For ROTEM analysis, a 2011 cohort study of blunt trauma patients (n = 334) documented that standard coagulation tests (e.g., INR, > 1.2) correlated with ROTEM thresholds (FIBTEM MCF <7, EXTEM MCF <45, EXTEM CFT >200, and EXTEM CT > 100).33 In February of 2015, experts published guidelines on the use of viscoelastic testing to guide transfusion therapy for early trauma resuscitation.26 The panel concluded that testing should be early and rapid, focusing on fibrinolysis due to its linkage to mortality. Despite these recommendations, the panel requested further studies because there was insufficient evidence to recommend exact thresholds for FFP, CRYO, PLT, or to withhold blood product transfusion.
Our study identified three patients out of 35 EXTEMS who had prolonged CFT (172, 230, and 234) and low MCF (45, 45, and 47). Additionally, a large number of INTEM MCF (n = 77 of 502) was below the normal value of 72. Further-more, a recent retrospective analysis of a civilian trauma registry (n = 358) documented a 75% sensitivity for EXTEM CT > 79 s with an INR, > 1.5 and EXTEM MCF <30 with PLT count less than 100.34 In our post-ROTEM group, 35 EXTEM were performed with 6% having prolonged CT which is a close approximation of the 9% of patients who had INR greater than 1.5. Although none of the 35 EXTEM reported MCF values were less than 30, over 17% had MCF less than 50, which does not correlate with the 11% of patients who had a PLT count less than 100. However, 92% of consensus panel participants agreed that providers consider PLT transfusion in patients with EXTEM MCF < 50.26 Overall, our ROTEM analysis is similar to previous studies that have documented ROTEM usefulness in rapidly identifying coagulopathy of trauma. Further adaption of these tests may decrease time to diagnosis, improve DCR therapies, and facilitate the evaluation of new surgical and pharmaceutical interventions.
Our results have several limitations inherent to retrospective “before and after” studies, which include incomplete data collection, lack of standard timing for measuring variables, and lack of a standardized transfusion protocol based on ROTEM measurements. Other influencing variables were the differences in the hematocrit level measured in the emergency department (p = 0.04) and the amount of prehospital fluid resuscitation. This leads us to conclude that the ROTEM results informed the trauma team to consider transfusing CRYO and PLTs to correct traumatic or iatrogenic coagulopathies. Lastly, the study was observational and not controlled as compared with other studies that compared TEG vs. common coagulation assays.25 Clearly, the next step is a randomized controlled trial comparing ROTEM-guided versus common coagulation assay management to optimize DCR in a combat support hospital.
In a 6-month, before and after study of a combat support hospital, ROTEM measurements prompted the trauma team to significantly increase the amounts of CRYO and PLT transfused. We conclude that this is the first study to document that the deployment of a ROTEM unit in a combat support hospital can improve the coagulation management of critical combat causalities to provide a more hemostatic resuscitation. Future trials will be able to confirm whether ROTEM can improve clinical outcomes by informing providers on how to provide optimal and patient-specific DCR.
Supplementary Material
Acknowledgments
Conflicts of Interest and Source of Funding: There were no conflicts of interest in the creation of this document. The source of the funding is the Department of Defense.
Footnotes
Publisher's Disclaimer: DOD Disclaimer: The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
This study was presented at the 71st annual meeting of the American Association for the Surgery of Trauma, September 12–15, 2012, in Kauai, HI and at the 20th annual meeting of the Military Health System Research Symposium, August 15–18, 2013, Fort Lauderdale, FL.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
REFERENCES
- 1.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–S437. [DOI] [PubMed] [Google Scholar]
- 2.Beekley AC, Sebesta JA, Blackbourne LH, Herbert GS, Kauvar DS, Baer DG, Walters TJ, Mullenix PS, Holcomb JB. 31st Combat Support Hospital Research Group. Prehospital tourniquet use in Operation Iraqi Freedom: effect on hemorrhage control and outcomes. J Trauma. 2008;64(Suppl 2): S28–S37; discussion S. [DOI] [PubMed] [Google Scholar]
- 3.King DR. Thirty consecutive uses of a hemostatic bandage at a US Army combat support hospital and forward surgical team in Operation Iraqi Freedom. J Trauma. 2011;71(6):1775–1778. [DOI] [PubMed] [Google Scholar]
- 4.Langan NR, Eckert M, Martin MJ. Changing patterns of in-hospital deaths following implementation of damage control resuscitation practices in US forward military treatment facilities. JAMA Surg. 2014;149(9):904–912. [DOI] [PubMed] [Google Scholar]
- 5.Niles SE, McLaughlin DF, Perkins JG, Wade CE, Li Y, Spinella PC, Holcomb JB. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64(6):1459–1463; discussion 63–5. [DOI] [PubMed] [Google Scholar]
- 6.Moore EE. Thomas G. Orr Memorial Lecture. Staged laparotomy for the hypothermia, acidosis, and coagulopathy syndrome. Am J Surg. 1996;172(5): 405–410. [DOI] [PubMed] [Google Scholar]
- 7.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007; 62(2):307–310. [DOI] [PubMed] [Google Scholar]
- 8.Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Filipescu D, Hunt BJ, Komadina R, Nardi G, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17(2):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. [DOI] [PubMed] [Google Scholar]
- 10.Doran CM, Woolley T, Midwinter MJ. Feasibility of using rotational thromboelastometry to assess coagulation status of combat casualties in a deployed setting. J Trauma. 2010;69(Suppl 1):S40–S48. [DOI] [PubMed] [Google Scholar]
- 11.British Committee for Standards in Haematology; Stainsby D, MacLennan S, Thomas D, Isaac J, Hamilton PJ. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135(5):634–641. [DOI] [PubMed] [Google Scholar]
- 12.Kashuk JL, Moore EE, Wohlauer M, Johnson JL, Pezold M, Lawrence J, Biffl WL, Burlew CC, Barnett C, Sawyer M, et al. Initial experiences with point-of-care rapid thrombelastography for management of life-threatening postinjury coagulopathy. Transfusion. 2012;52(1):23–33. [DOI] [PubMed] [Google Scholar]
- 13.Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ Jr, Mattox KL, Suliburk J. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378–385; discussion 85–6. [DOI] [PubMed] [Google Scholar]
- 14.Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, Stanworth S, Brohi K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10(7):1342–1351. [DOI] [PubMed] [Google Scholar]
- 15.Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, Allaouchiche B, Negrier C. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007; 5(2):289–295. [DOI] [PubMed] [Google Scholar]
- 16.Woolley T, Midwinter M, Spencer P, Watts S, Doran C, Kirkman E. Utility of interim ROTEM(®) values of clot strength, A5 and A10, in predicting final assessment of coagulation status in severely injured battle patients. Injury. 2013;44(5):593–599. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drews RE. Critical issues in hematology: anemia, thrombocytopenia, coagulopathy, and blood product transfusions in critically ill patients. Clin Chest Med. 2003;24(4):607–622. [DOI] [PubMed] [Google Scholar]
- 19.Mohr R, Martinowitz U, Lavee J, Amroch D, Ramot B, Goor DA. The hemostatic effect of transfusing fresh whole blood versus platelet concentrates after cardiac operations. J Thorac Cardiovasc Surg. 1988;96(4):530–534. [PubMed] [Google Scholar]
- 20.Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, Schreiber MA, Bulger EM, Muskat P, Alarcon LH, et al. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S40–S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benson G Rotational thromboelastometry and its use in directing the management of coagulopathy in the battle injured trauma patient. J Perioper Pract. 2014;24(1–2):25–28. [DOI] [PubMed] [Google Scholar]
- 22.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31(7):1507–1511. [DOI] [PubMed] [Google Scholar]
- 23.Da Luz LT, Nascimento B, Shankarakutty AK, Rizoli S, Adhikari NK. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Crit Care. 2014;18(5):518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schochl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, Kozek-Langenecker S, Solomon C. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14(2):R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2015;263:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaba K, Rizoli S, Veigas PV, Callum J, Davenport R, Hess J, Maegele M. Viscoelastic Testing in Trauma Consensus Panel. 2014 Consensus conference on viscoelastic test-based transfusion guidelines for early trauma resuscitation: report of the panel. J Trauma Acute Care Surg. 2015;78(6): 1220–1229. [DOI] [PubMed] [Google Scholar]
- 27.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holcomb JB, Zarzabal LA, Michalek JE, Kozar RA, Spinella PC, Perkins JG, Matijevic N, Dong JF, Pati S, Wade CE, et al. Increased platelet: RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 Suppl 3):S318–S328. [DOI] [PubMed] [Google Scholar]
- 29.Delano MJ, Rizoli SB, Rhind SG, Cuschieri J, Junger W, Baker AJ, Dubick MA, Hoyt DB, Bulger EM. Prehospital resuscitation of traumatic hemorrhagic shock with hypertonic solutions worsens hypocoagulation and hyperfibrinolysis. Shock. 2015;44(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, Zhelev Z, Hyde C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev. 2015;2:CD010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013;75(6):961–967; discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman MP, Moore EE, Moore HB, Gonzalez E, Morton AP, Chandler J, Fleming CD, Ghasabyan A, Silliman CC, Banerjee A, et al. The “Death Diamond”: rapid thrombelastography identifies lethal hyperfibrinolysis. J Trauma Acute Care Surg. 2015;79(6):925–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tauber H, Innerhofer P, Breitkopf R, Westermann I, Beer R, El Attal R, Strasak A, Mittermayr M. Prevalence and impact of abnormal ROTEM (R) assays in severe blunt trauma: results of the ‘Diagnosis and Treatment of Trauma-Induced Coagulopathy (DIA-TRE-TIC) study’. Br J Anaesth. 2011;107(3):378–387. [DOI] [PubMed] [Google Scholar]
- 34.David JS, Durand M, Levrat A, Lefevre M, Rugeri L, Geay-Baillat MO, Inaba K, Bouzat P. Correlation between laboratory coagulation testing and thromboelastometry is modified during management of trauma patients. J Trauma Acute Care Surg. 2016;81:319–327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


