Abstract
Differences in device design may have an effect on platelet damage and associated clinical complications. We aimed to compare device specific platelet functionality in 26 heart failure patients supported with three continuous-flow left ventricular assist devices (CF-LVADs): HeartMate II (n=8), Jarvik 2000 (n=9) and HeartWare (n=9). Intraplatelet reactive oxygen species (ROS) generation, mitochondrial damage and platelet apoptosis were compared between device types before and after the implantation at every week up to 1 month. Overall, the baseline characteristics, demographics, routine laboratory values were comparable between the three device groups. Intraplatelet ROS, mitochondrial damage and platelet apoptosis significantly elevated in the HeartWare group in comparison to the other two device groups after implantation. The major bleeding, infections, systemic inflammatory response syndrome and right ventricular failure were found to be more common among the HeartWare group than others. Intraplatelet ROS and platelet damage levels were returned to baseline in both the HeartMate II and Jarvik groups whereas in HeartWare group they remained elevated. The patients with the Jarvik and the HeartMate II experienced less clinical complications and the platelet functionality is not compromised by these devices. Data from this study suggests that the CF-LVAD design may exert different effects on platelet function.
Keywords: Heart failure, left ventricular assist devices, intraplatelet reactive oxygen species, mitochondrial damage, platelet apoptosis
Introduction
CF-LVAD therapy has evolved into a standard therapy for patients with advanced heart failure (HF), either as a destination therapy or a bridge to cardiac transplantation or a bridge to myocardial recovery.1 Despite demonstrating significant improvements in survival with contemporary CF-LVADs compared to the older, pulsatile devices. Non-surgical bleeding, major infections, transient ischemic attack or stroke, systemic inflammatory response syndrome (SIRS), renal dysfunction, respiratory failure, right ventricular failure continues to be the most frequently reported complications.2–5
In United States, the most common implantable 2nd generation pumps are the Heart-Mate II and the Jarvik 2000.6–9 Up to date, the HeartMate II is the most successful second-generation pump worldwide and approved as bridge to transplant and as destination therapy.10–13 While device complications were comparable between 2nd and 3rd generation CF-LVADs, it was reported that patients with 3rd generation CF-LVAD (HeartWare HVAD) experienced a significantly higher incidence of thrombosis, stroke and gastrointestinal bleeding.14
As platelets have well recognized roles in hemostasis and thrombosis, altered platelet functionality or platelet damage may lead to unrestrained bleeding or other associated complications. Exposure of blood to elevated shear stresses, which have been demonstrated to exist in these CF-LVADs based on rotary blood pump technology, can not only result in platelet activation, but also trigger apoptosis events, including mitochondrial transmembrane potential depolarization and phosphatidylserine exposure.15 It is reasonable to assume that long-term exposure to high shear stress flow environment in CF-LVADs may have an additive role in platelet damage. Despite increasing experience with newer generation devices, there has been little research comparing the design effects of CF-LVADs on platelet functionality of patients. In this single center study, we measured the intraplatelet reactive oxygen species (ROS), mitochondrial damage and platelet apoptosis in serially collected blood samples from patients supported with either HeartMate II or Jarvik or HeartWare CF-LVAD to examine device specific changes in platelet functionality.
Materials and Methods
Subjects
We recruited 26 HF patients with NYHA class IV undergoing CF-LVAD implantation as bridge to transplant or destination therapy and 11 healthy volunteers as the control group. The CF-LVADs implanted included the HeartMate II (Thoratec Corp, Pleasanton, CA) in 8 patients, the Jarvik 2000 (Jarvik Heart, New York, NY) in 9 patients, and the HeartWare HVAD (HeartWare Inc, Framingham, MA) in 9 patients. Seven patients received the Levitronix CentriMag for right ventricular support along with either the HeartMate II (n=2) or the Jarvik 2000 (n=1) or HeartWare HVAD (n=4). The duration of RVAD supports for these patients was in between 1 to 25 days. Jarvik is the preferred device for patients with prior sternotomy and preserved RV function due to its ability to be implanted via left thoracotomy (relatively preserved RV function is preferred because this approach doesn’t allow access for direct RVAD insertion). Since February 2013 we have also preferred it for destination therapy patients due to the availability of a post-auricular pedestal connector, which should reduce the risk of exit site infection. The HeartWare is our preferred device for BTT. Hence it is more frequently used in younger, more acutely ill, non-ischemic patients, who have a tendency toward increased risk of RV failure. The Heartmate II is used primarily for destination therapy patient who don’t qualify for the Jarvik or HeartWare trials, or who receive the device via the control arm of one of those trials. All procedures involving collection of human blood were approved by the Institutional Review Board (IRB). All patients and volunteers gave their written informed consent and were informed about the aim of the study.
Anticoagulation/Antiplatelet Treatment
After CF-LVAD implantation, anticoagulation was initiated with a titrated heparin dose with the goal for partial thromboplastin time of 40–45s once chest drainage was less than 30 mL/h for at least 4 hours. Thereafter the goal was aimed to have an anti-Xa activity level of 0.1–0.15 U/mL. The anticoagulation medication was subsequently converted to warfarin with a targeted international normalized ratio (INR) from 1.8 to 2.3 for the HeartMate II, 2 to 3 for the Jarvik and the HeartWare. Antiplatelet agents were added to the anticoagulation regimen and the dosage was titrated based on measurements of platelet function using a platelet function analyzer (PFA-100® (Dade Behring, Inc, Deerfield, IL) and thrombelastogram (TEG) (TEG® 5000 Thrombelastograph® Hemostasis Analyzer System, Haemonetics Corporation, Braintree, MA). All the patients received pentoxifylline to improve RBC deformability in the hope of mitigating shear-induced hemolysis.
Collection and Preparation of Blood Sample
EDTA/Citrate-anticoagulated blood samples from the HF patients were collected before CF-LVAD implant surgery (baseline/pre-operative: Pre-OP) and at day 7, 14, 21 and 30 (Post-operative duration 1, 2, 3 weeks and 1 month: POD-1W, 2W, 3W and 1M) after the implant surgery. Based on the expected post-transfusion recovery and life span of transfused platelets, we had collected blood samples after 2–5 days of platelet transfusion (if any). Blood samples from the healthy donors were collected once. All the blood samples from the HF patients and the healthy volunteers were aliquoted and processed immediately according to the standardized study protocol.
Platelet Function Measurements
Measurements of platelet function with the PFA-100® were performed according to the manufacturer’s instructions using the collagen/adenosine-5′-diphosphate (CAPD) cartridge and the collagen/epinephrine (CEPI) cartridge. We also used the TEG parameters to assess the platelet function. The TEG parameters: TEG-maximum amplitude (TEG-MA), kinetic time (TEG-KT) and angle (TEG-Angle) were analyzed.
Measurement of Intraplatelet ROS
Platelet rich plasma (PRP) was prepared by centrifugation and was loaded with 2′,7′-dichlorofluorescein (H2DCF-DA; Sigma), a cell-permeable non-fluorescent dye that is cleaved by intracellular esterases to H2DCF, rendering it membrane-impermeable, and then emits fluorescent energy in the presence of ROS. Generation of ROS in the platelets resulted in green fluorescence that was quantified in fluorescence channel-1(FL1) and was expressed as mean fluorescence intensity (MFI) in arbitrary unit. Intraplatelet ROS was also visualized by immunofluorescence microscopy using the commercially available ROS detection kit (cat no: ENZ-51011, Mercodia Inc, Winston Salem, NC, USA).
Measurement of Platelet Mitochondrial Damage
Platelet mitochondrial damage was measured by flow cytometry using mitochondrial membrane potential (ΔΨm)-sensitive dye, tetramethylrhodamine ethyl ester (TMRE), a commercially available MitoPT® TMRE Assay Kit (cat no: 9103, ImmunoChemistry Technologies, LLC, Bloomington, MN). TMRE is a cell permeant, positively-charged, red-orange dye that readily accumulates in active mitochondria due to their relative negative charge. Depolarized or inactive mitochondria have decreased membrane potential and fail to sequester TMRE. Thus TMRE measured the mitochondrial integrity of platelets by monitoring the loss of fluorescence intensity and displayed as % of depolarized ΔΨm platelets.
Detection of Platelet Apoptosis
Platelet-surface exposure of phosphatidylserine (PS) was determined using flow cytometry with the Annexin V-FITC Apoptosis Detection Kit (cat no: K101–100, BioVision, Inc., Milpitas, California) according to the manufacturer’s instruction. Platelet apoptosis was determined by flow cytometry where both annexin V-FITC (FL1) and PE-conjugated mouse anti-CD41 IgG (FL2) stains were positive and was expressed as mean fluorescence intensity (MFI) in arbitrary unit.
Statistical Analyses
The data are presented as mean±SD or SE (standard deviation or standard Error) or median with interquartile range (IQR) and statistically analyzed using SPSS statistical software (Statistical Package for Social Sciences for windows, release 18.0; SPSS Inc., Chicago, IL, USA). Statistical differences were determined by using Chi-square test, Student’s t-test and Mann-Whitney U test, as applicable. Univariate analysis was carried out using Spearman’s rank correlation test to find out the relation between two measurable parameters as continuous variables, and the result was expressed as ρ (rho) value. Statistical significance was assigned at P < 0.05.
Results
Demography and Clinical Characteristics
Comparative analyses of demographic and clinical characteristics of the HF patients in the HeartMate II, Jarvik 2000 and HeartWare groups before implantation were summarized in Table 1. All the three groups were comparable with respect to demography, vital signs, past medical history, etiology of heart disease and echocardiographic parameters.
Table 1.
Demographic and baseline clinical characteristics of HF patients prior to HeartMate II, Jarvik and HeartWare implantation
| Characteristics | Pre-implant HF patients (n=26) |
||
|---|---|---|---|
| HeartMate II (n=8) | Jarvik (n=9) | HeartWare (n=9) | |
| Demography | |||
| Age in years, median (IQR) | 62 (57–76) | 62 (23–70) | 62 (25–71) |
| Sex, n(% male) | 6 (75.0%) | 9 (100%) | 6 (66.7%) |
| Race | |||
| Caucasian white, n(%) | 3 (37.5%) | 4 (44.4%) | 3 (33.3%) |
| Black, n(%) | 5 (62.5%) | 4 (44.4%) | 5 (55.6%) |
| Hispanic or Latino, n(%) | - | 1 (11.1%) | 1 (11.1%) |
| Height in meter, median (IQR) | 1.7 (1.6–1.9) | 1.8 (1.7–1.9) | 1.8 (1.7–1.9) |
| Weight in kilograms, median (IQR) | 84.0 (48.1–127.9) | 89.6 (76.6–119.4) | 78.9 (53.0–109.1) |
| Body mass index (kg/m2), median (IQR) | 27.7 (17.0–38.2) | 29.2 (24.2–39.0) | 24.4 (19.5–32.2) |
| Body surface area (m2), median (IQR) | 1.9 (1.5–2.5) | 2.2 (1.9–2.4) | 2.0 (1.6–2.3) |
| History of smoking, n(%) | 2 (25%) | 2 (22.2%) | 2 (22.2%) |
| History of substance abuse | |||
| Ethyl alcohol abuse, n(%) | 2 (25%) | 2 (22.2%) | 2 (22.2%) |
| Drug abuse, n(%) | 2 (25%) | 2 (22.2%) | 2 (22.2%) |
| Vital signs | |||
| Systolic blood pressure (mmHg), mean±SD | 95.7±20.0 | 100.4±12.1 | 98.5±22.7 |
| Diastolic blood pressure (mmHg), mean±SD | 56.3±8.8 | 58.6±7.2 | 69.0±25.3 |
| Past medical history | |||
| Heart failure, n(%) | 8 (100%) | 9 (100%) | 9 (100%) |
| Diabetes mellitus, n(%) | 2 (25%) | 3 (33.3%) | 3 (33.3%) |
| Hypertension, n(%) | 4 (50%) | 5 (55.6%) | 4 (44.4%) |
| Etiology of heart disease | |||
| Ischemic cardiomyopathy, n(%) | 3 (37.5%) | 5 (55.6%) | 1 (11.1%) |
| Non-ischemic cardiomyopathy, n(%) | 5 (62.5%) | 3 (33.3%) | 5 (55.6%) |
| Idiopathic Cardiomyopathy, n(%) | - | 1 (11.1%) | 3 (33.3%) |
| Echocardiographic parameters | |||
| Left ventricular end diastolic diameter (mm), mean±SD | 71.4±11.0 | 61.4±9.2 | 77.7±10.5 |
| Left ventricular ejection fraction, (%), mean±SD | 12.5±4.2 | 15.0±3.2 | 12.5±5.0 |
We prefer the Jarvik for patients with prior sternotomy. These are typically ischemics. The preponderance of nonischemics in the other two device groups explains the slightly higher LVEDD vs the Jarvik. All patients had severe LV dysfunction (EF <20%); the difference between Jarvik (15%) and the other two groups (12.5%) is clinically and statistically insignificant. Also as stated above, the preferred technique for Jarvik insertion is via left lateral thoracotomy, which does not allow access to the RA/RV/PA for direct RVAD insertion. Thus, we preferentially select patients with better RV function for that device. Similarly, most Heartmate II patients are destination therapy patients, in which one also preferentially selects those with better RV function due to the lack of a permanent RV support device. Unfortunately our echo lab does not report RVEDD, RVEF, or TAPSE.
Adverse Events after Implantation
Table 2 lists adverse events and clinical complications in these patients after CF-LVAD implantation. Non-surgical bleeding was found to be more frequent in the HeartWare group compared to the other two groups. Besides bleeding; major infections, SIRS, RV failure were also predominated in HeartWare group.
Table 2.
Adverse events of HF patients after HeartMate II, Jarvik and HeartWare HVAD implantation
| Adverse events | Pre-implant HF patients (n=26) |
||
|---|---|---|---|
| HeartMate II (n =8) | Jarvik (n =9) | HeartWare (n =9) | |
| Non-surgical bleeding | 1 | 1 | 5 |
| Major infections | 0 | 1 | 3 |
| Stroke | 1 | 3 | 2 |
| SIRS | 2 | 1 | 4 |
| Renal dysfunction | 2 | 3 | 2 |
| Respiratory failure | 2 | 1 | 2 |
| RV failure | 2 | 1 | 4 |
| RVAD support required | 2 | 1 | 4 |
| Replacement of VADs | 0 | 0 | 0 |
| Device malfunction | 1 | 0 | 0 |
Laboratory Hematology, Blood Chemistry and Platelet Function Tests
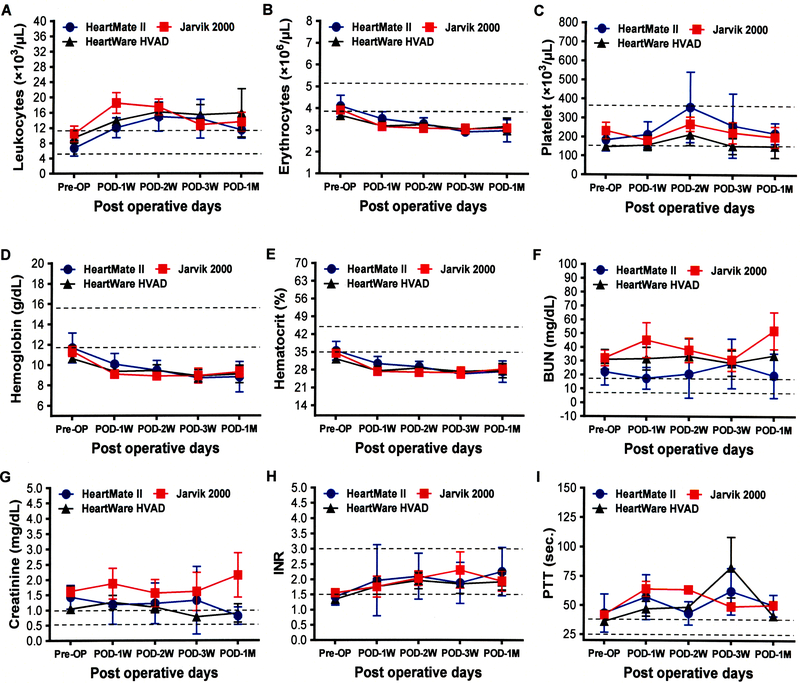
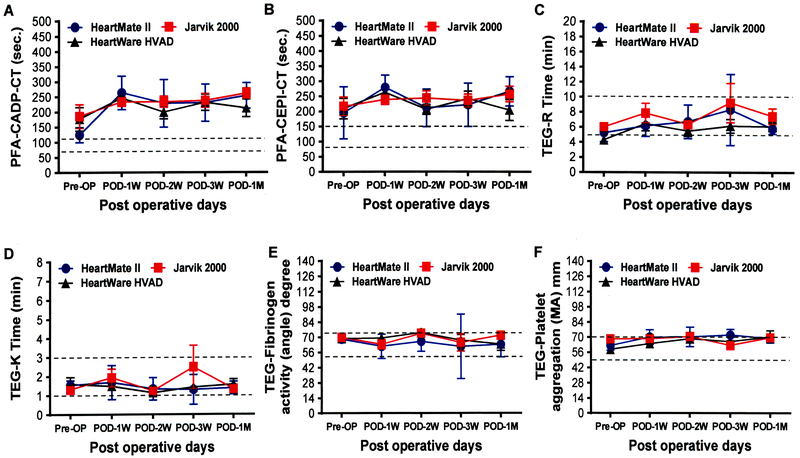
The routine laboratory hematologic and blood chemistry tests of patients in each device group before and after implantation are summarized in Figure 1. There were no significant differences in the hematology and blood chemistry parameters between the device groups before and after implantation. The change in PFA 100 and TEG data during the three types of device support over the time period is depicted in the Figure 2. There was no significant difference in the severity of the primary hemostatic defect indicated by these tests between the device groups.
Figure 1.
Comparison of routine laboratory hematology and blood chemistry before and after implantation with three different kinds of CF-LVADs. Data are expressed as mean±SD. The dotted lines in each line diagram indicating the normal high and normal low values of each parameter measured.
Figure 2.
Comparison of platelet function and whole blood hemostasis tests parameters before and after implantation with three different kinds of CF-LVADs. Data are expressed as mean±SD. The dotted lines in each line diagram indicating the normal high and normal low values of each parameter measured.
Change in intraplatelet ROS
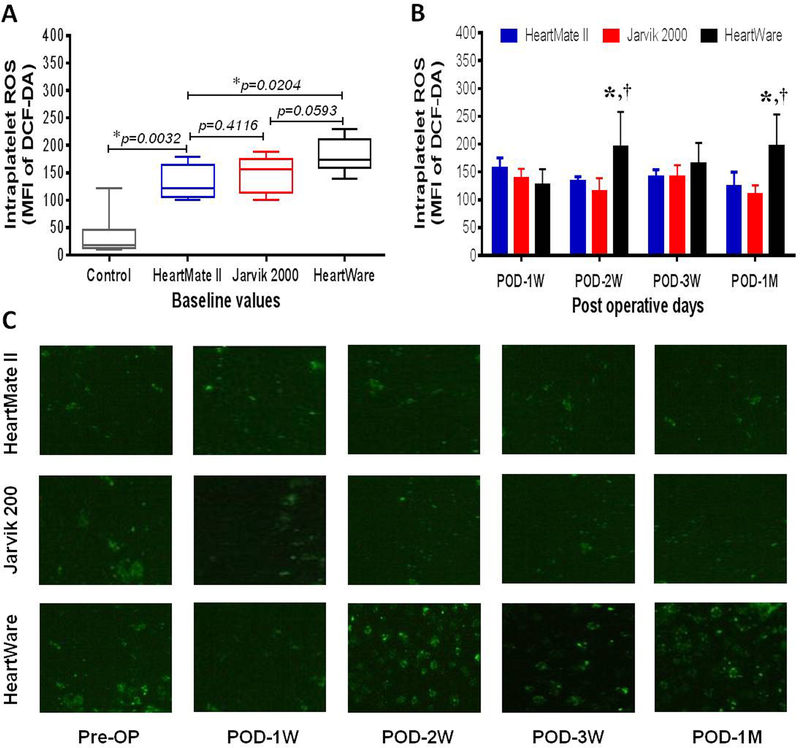
Flow cytometric analysis shows that the intraplatelet ROS among all the HF patients prior to CF-LVAD implantation is 4-times higher than that in the healthy volunteers (144.1 ± 8.5 vs 36.1 ± 11.2, p <0.0001 in Student’s t-test). However, when we divided the patients according to CF-LVAD types, the level of the intraplatelet ROS was higher in HeartWare group in comparison to those in the HeartMate II and Jarvik groups (Figure 3A). The post implant measurement shows that the levels of the intraplatelet ROS slightly increased in the HeartMate II and HeartWare groups and slightly decreased in the Jarvik group throughout the study period. While examining the temporal trend of each device group, we noticed significantly higher intraplatelet ROS in the HeartWare group at POD 2 weeks and POD 1 month in comparison to the other two device groups (Figure 3B). At one month after CF-LVAD implantation, we noticed that the level of the intraplatelet ROS in the HeartWare group was 1.6-fold and 1.8-fold higher compared with those in the HeartMate II and Jarvik groups, respectively (Figure 3B). The immunofluorescence microscopic examination of the intraplatelet ROS of the patients from the three device groups was in general agreement with the flow cytometric evaluation. The difference in the fluorescence intensity of ROS positive platelets before and after CF-LVAD implantation in all three groups is shown in the figure 3C. More ROS positive platelets (green spots) were observed in the HeartWare group compared to those in the HeartMate II and Jarvik groups.
Figure 3.
Comparison of change in intraplatelet ROS levels in controls and before and after implantation with three different kinds of CF-LVADs. (A) Box-whisker plots showing control and baseline values of intraplatelet ROS in patients with different CF-LVAD settings. The lines across each box plot represent the median value. The lines that extend from the top and the bottom of each box represent the lowest and highest observations still inside the lower and upper limit of confidence. *p<0.05 is considered significant in Mann-Whitney U test. (B) Change in post-operative levels of intraplatelet ROS between different devices specific patients during the study period. Data are expressed as mean±SD. *,p<0.05 compared with HeartMate II and †,p<0.05 compared with Jarvik in Student’s t-test. (C) Immunofluorescence microscopy showing DCF-DA positive platelets (green) as an indicator of intraplatelet ROS generation. Note higher fluorescence intensity among HF patient supported by HeartWare in comparison to HeartMate II and Jarvik. Magnification×1000.
Change in platelet mitochondrial damage
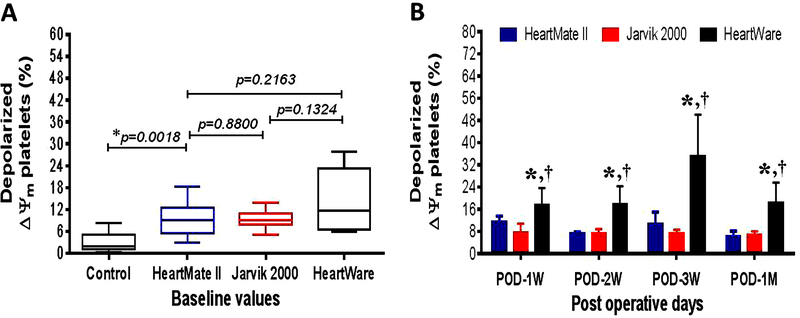
Compared to the healthy volunteers, HF patients showed 4-fold higher % of depolarized ΔΨm platelets prior to CF-LVAD implantation (11.0 ± 1.2 vs 2.8 ± 0.7, p<0.001 in Student’s t-test), indicating a pre-existing condition of platelet mitochondrial damage in these patients. Differences between the device groups were not statistically significant at baseline (Figure 4A). The post-implant depolarized ΔΨm platelets in the HeartMate II and Jarvik groups did not show any significant change in comparison to the baseline although a decreasing tendency was evident with increasing post implant time. In contrast, we noticed a consistent increase in the depolarized ΔΨm platelets in the HeartWare group, and highest at the 3rd weeks after implantation, indicating platelet mitochondrial damage was more prominent in the HF patients supported with the HeartWare CF-LVAD (Figure 4B). Throughout the follow-up period after CF-LVAD implantation, the depolarized ΔΨm platelets always remained significantly higher in HeartWare group compared to the other two groups (Figure 4B).
Figure 4.
Comparison of change in % depolarized ΔΨm platelets in controls and before and after implantation with three different kinds of CF-LVADs. (A) Box-whisker plots showing control and baseline values of % depolarized ΔΨm platelets in patients with different CF-LVAD settings. The lines across each box plot represent the median value. The lines that extend from the top and the bottom of each box represent the lowest and highest observations still inside the lower and upper limit of confidence. *p<0.05 is considered significant in Mann-Whitney U test. (B) Change in post-operative levels of % depolarized ΔΨm platelets between different devices specific patients during the study period. Data are expressed as mean±SD. *,p<0.05 compared with HeartMate II and †,p<0.05 compared with Jarvik in Student’s t-test.
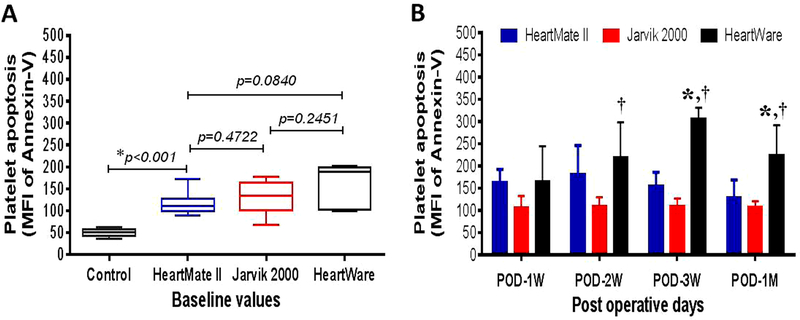
Change in platelet apoptosis
Similar to the intraplatelet ROS and mitochondrial damage, platelet apoptotic was also found to be a pre-existing condition in all the HF patients compared to the healthy volunteers (134.6 ± 8.5 vs.50.6 ± 2.7, p<0.001 in Student’s t-test). Differences between the device groups were not statistically significant at baseline (Figure 5A). Compared to the baseline value, the post-implant apoptotic platelets in the HeartMate II group increased slightly up to POD 2 weeks and at the end of the study almost returned to the baseline level. In the Jarvik group, we did not notice any change in platelet apoptosis throughout the study period compared to the baseline level. In contrast, we noticed a consistent increase in platelet apoptosis in the HeartWare group, maximum at POD 3 weeks, indicating platelet apoptosis was more prominent in the HF patients with the HeartWare CF-LVAD (Figure 4B). Comparing the overall trends in platelet apoptosis after CF-LVAD implantation, it was found that the HeartWare group had significantly higher levels of platelet apoptosis at POD 2 weeks, 3 weeks and one month compared to HeartMate II and Jarvik groups (Figure 5B).
Figure 5.
Comparison of change in platelet apoptosis in controls and before and after implantation with three different kinds of CF-LVADs. (A) Box-whisker plots showing control and baseline values of platelet apoptosis in patients with different CF-LVAD settings. The lines across each box plot represent the median value. The lines that extend from the top and the bottom of each box represent the lowest and highest observations still inside the lower and upper limit of confidence. *p<0.05 is considered significant in Mann-Whitney U test. (B) Change in post-operative levels of platelet apoptosis between different devices specific patients during the study period. Data are expressed as mean±SD. *,p<0.05 compared with HeartMate II and †,p<0.05 compared with Jarvik in Student’s t-test.
Relationship between intraplatelet ROS and platelet damage
To examine the relationships between the intraplatelet ROS and platelet damage, we conducted Spearman’s nonparametric correlation test between the indication parameters for intraplatelet ROS and platelet damage in all the HF patients supported by CF-LVAD. When the intraplatelet ROS values are plotted against the mitochondrial damage values, we noticed a statistically significant positive association between the intraplatelet ROS and platelet apoptosis (ρ=0.4024, 95%C.I=0.13–0.62, p=0.0042 for intraplatelet ROS with mitochondrial damage and ρ=0.5314, 95 %C.I.=0.30–0.71, p<0.0001 for intraplatelet ROS with platelet apoptosis).
Device, clinical complications and platelet dysfunction
Considering the small sample size in each group, we did not notice any significant change in platelet dysfunctionality between different LVAD versus RVAD. However, we compared the data for the RVAD patients to the rest of the cohort, independent of LVAD types. RVAD patients were obviously sicker and the presence of a 2nd pump would logically cause increased platelet damage. To figure out any role of RVAD support after different CF-LVAD implantation, we need to increase our sample size in future study.
Considering the small sample size in each group, at present it is not possible to compare device specific clinical complications and their effects on platelet dysfunctionality. Every patient had different combinations of clinical outcomes that might change the platelet function. Considering the already stated correlation between sepsis and RVF and platelet activation, we found that only two patients had sepsis and RV failure at a time in the HeartWare group. These patients obviously had higher intraplatelet ROS, mitochondrial damage and platelet apoptosis when compared to rest of the patients without sepsis and RV failure after CF-LVAD implantation.
Discussion
Oxidative stress, defined as an excess production of ROS relative to antioxidant defense, has been shown to play an important role in the pathophysiology of cardiac remodeling and heart failure.16–18 The current study focused on the intraplatelet oxidative stress that is associated with platelet functionality in HF patients supported with CF-LVADs. We noticed excess production of intraplatelet ROS in the HF patients prior to CF-LVAD implantation when compared to healthy volunteers. Oxidative stress has been reported to increase with aging, life-style factors (substance abuse/smoking), diabetes and hypertension.19–22 This might explain why these HF patients had preexisting higher intraplatelet ROS. However, the knowledge of how implanted CF-LVADs affect the production of ROS and its association with platelet mitochondrial integrity and survival is limited.
In our study, we noticed that there were differences in the pattern of intraplatelet ROS generation with respect to the types of CF-LVADs. The patients supported with the HeartWare CF-LAVD had a significantly higher level of the intraplatelet ROS compared to the HeartMate II and Jarvik CF-LVADs. The elevation of the intraplatelet ROS generation in this group is not clearly understood. The non-physiological high shear stress is often induced at the blade tip of the rotary blood pumps. The tip gap of the Jarvik and HeartMate II is in the range of 100 to 150 micrometers. Although the gap between the impeller and housing of the HVAD is large (radial direction), its hydrodynamic bearing might have more profound effect on platelet damage because the area between the impeller and top housing for the hydrodynamic bearing is much large compared with that of the axial flow pump. The gap size of the hydrodynamic bearing is unknown. Thus the HVAD might induce a higher level of platelet injury. However, it is interesting to note that the post implant clinical complications (major infections, SIRS and RV failure) in the patients supported with the HeartWare were more frequent. It may suggest that there was an association of these post implant complications with ROS generation. It has been reported that the production of ROS was found to be elevated during inflammation, infection and right heart failure.23–25
Platelets are the primary mediators of hemostasis and can be a target of ROS during activation. These conditions can affect platelet physiology, leading, as an ultimate event, to the cell number modification.26 Our previous study indicated that there is a difference in platelet count between non-bleeding and bleeding patients after the CF-LVAD implantation.27 In the present study, pre versus post implant platelet counts were found to be somewhat altered in numbers in all the three groups but remained within normal range throughout the study. Under the normal circumstance, platelets contain fully functional mitochondria which regulate the pro-thrombotic function of platelets through not only energy generation, but also redox signalling and the initiation of apoptosis.28 We noticed higher mitochondrial damage at baseline in all the three groups of HF patients in comparison to the healthy volunteers. After CF-LVAD implantation, the trends of mitochondrial damage were minimized in the HeartMate II and Jarvik groups. Interestingly, an opposite trend was noticed in the HeartWare group indicating that mitochondrial damage was more pronounced. A similar trend was also noticed in platelet apoptosis because both the mitochondrial damage and platelet apoptosis are related to each other. The higher mitochondrial damage and platelet apoptosis in the HeartWare group might attribute to the bleeding complications as more than half of the patients in the HeartWare group encountered non-surgical bleeding during the study period. Significantly higher incidences of GI bleeding in the patients supported with the HeartWare CF-LVAD was also reported by others compared to the HeartMate II and Jarvik CF-LVADs.14 In our study, we noticed that more HVAD patients required RVAD support. It might also be suggested that the disease state of the HVAD patients might be more severe compared to the Jarvik and HeartMate II patients although there were no difference in the demographic parameters.
The principal function of platelets is to prevent bleeding, thus dysfunctional platelets can be a cause of major bleeding events in CF-LVAD patients. In agreement with this we have recently reported the rheologic disturbance in the codomain of the glycoprotein Ibα that may point to platelet defects in the presence of CF-LVAD in the circulation system,27 Although platelets are anuclear, they do undergo apoptosis, a process of programmed cell death. The events of platelet apoptosis via the intrinsic pathway include only the cytoplasmic events initiated by the increased rate of endogenous reactive oxygen species (ROS).29 We noticed significantly higher intraplatelet ROS generation in all the three groups of CF-LVAD patients as a preexisting condition. The Spearman’s rank correlation test established a significant positive association between intraplatelet ROS and percentage of depolarized ΔΨm and apoptotic platelets in all the HF patients irrespective of device types. In this case, the primary target of oxidative stress may be mitochondrial DNA and its damage leads to the down regulation of electron transport chain, eventually leading to an intensification of ROS generation and formation of mitochondrial permeability transition pore resulting in the inner transmembrane potential depolarization.30 Ultimately there is externalization of phosphatidylserine, which is a signal from the apoptotic cells for phagocytosis.30,31
Study Limitation
We acknowledge that our study has some limitations. This was a single-center study of a small number of patients in each group who were screened for quantification of intraplatelet ROS, mitochondrial damage and platelet damage. Not all CF-LVAD-supported patients were enrolled for this study. The effects of antiplatelet drugs or other medication on intraplatelet ROS and platelet damage may need to be explored in future study involving higher patients in each CF-LAVD group. A larger cohort followed for a longer period of time is also needed.
Conclusion
This study provides first evidence that the response of oxidative stress and platelet damage varied from devise to device after implantation. The patients in the HeartWere group experienced higher mitochondrial damage and concomitant platelet apoptosis.
Acknowledgments
Conflicts of Interests and Source of Funding: The authors have no conflicts of interest to report. The described research was partially sponsored by the National Institutes of Health (Grant R01 HL 088100).
References
- 1.Birks EJ, Tansley PD, Hardy J, et al. : Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med 355:1873–1884,2006. [DOI] [PubMed] [Google Scholar]
- 2.Lietz K: Destination therapy: patient selection and current outcomes. J Card Surg 25:462–471,2010. [DOI] [PubMed] [Google Scholar]
- 3.Lahpor J, Khaghani A, Hetzer R, et al. : European results with a continuous-flow ventricular assist device for advanced heart failure patients. Eur J Cardiothorac Surg 37:357–361,2010. [DOI] [PubMed] [Google Scholar]
- 4.Crow S, John R, Boyle A, et al. : Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg 137:208–215,2009. [DOI] [PubMed] [Google Scholar]
- 5.Stern DR, Kazam J, Edwards P, et al. : Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg 25:352–356,2010. [DOI] [PubMed] [Google Scholar]
- 6.Siegenthaler MP, Martin J, van de Loo A, et al. : Implantation of the permanent Jarvik-2000 left ventricular assist device: a single-center experience. J Am Coll Cardiol 39:1764–1772,2002. [DOI] [PubMed] [Google Scholar]
- 7.Frazier OH, Myers TJ, Westaby S, et al. : Use of the Jarvik 2000 left ventricular assist system as a bridge to heart transplantation or as destination therapy for patients with chronic heart failure. Ann Surg 237:631–636,2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haj-Yahia S, Birks EJ, Rogers P, et al. : Midterm experience with the Jarvik 2000 axial flow left ventricular assist device. J Thorac Cardiovasc Surg 134:199–203,2007. [DOI] [PubMed] [Google Scholar]
- 9.Nawata K, Nishimura T, Kyo S, et al. : Outcomes of midterm circulatory support by left ventricular assist device implantation with descending aortic anastomosis. J Artif Organs 13:197–201,2010. [DOI] [PubMed] [Google Scholar]
- 10.Slaughter MS, Rogers JG, Milano CA, et al. : HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 361:2241–2251,2009. [DOI] [PubMed] [Google Scholar]
- 11.Rogers JG, Aaronson KD, Boyle AJ, et al. : Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol 55:1826–1834,2010. [DOI] [PubMed] [Google Scholar]
- 12.Kirklin JK, Naftel DC, Kormos RL, et al. : Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant 29:1–10,2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagani FD, Miller LW, Russell SD, et al. : Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol 54:312–321,2009. [DOI] [PubMed] [Google Scholar]
- 14.Lalonde SD, Alba AC, Rigobon A, et al. : Clinical differences between continuous flow ventricular assist devices: a comparison between HeartMate II and HeartWare HVAD. J Card Surg 28:604–610,2013. [DOI] [PubMed] [Google Scholar]
- 15.Leytin V, Allen DJ, Mykhaylov S, et al. : Pathologic high shear stress induces apoptosis events in human platelets. Biochem Biophys Res Commun 320:303–310,2004. [DOI] [PubMed] [Google Scholar]
- 16.Belch JJ, Bridges AB, Scott N, et al. : Oxygen free radicals and congestive heart failure. Br Heart J 65:245–248,1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsutsui H, Kinugawa S, Matsushima S: Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301:H2181–190,2011. [DOI] [PubMed] [Google Scholar]
- 18.Mondal NK, Sorensen E, Hiivala N, et al. : Oxidative stress, DNA damage and repair in heart failure patients after implantation of continuous flow left ventricular assist devices. Int J Med Sci 10:883–893,2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andriollo-Sanchez M, Hininger-Favier I, Meunier N, et al. : Age-related oxidative stress and antioxidant parameters in middle-aged and older European subjects: the ZENITH study. Eur J Clin Nutr Suppl2:S58–62,2005. [DOI] [PubMed] [Google Scholar]
- 20.Donohue FJ: Ageing, smoking and oxidative stress. Thorax 61:461–462,2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giacco F, Brownlee M: Oxidative stress and diabetic complications. Circ Res 107:1058–1070,2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison DG, Gongora MC: Oxidative stress and hypertension. Med Clin North Am 93:621–635,2009. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee A, Mondal NK, Das D, et al. : Neutrophilic inflammatory response and oxidative stress in premenopausal women chronically exposed to indoor air pollution from biomass burning. Inflammation 35:671–683,2012. [DOI] [PubMed] [Google Scholar]
- 24.Hakim J: Reactive oxygen species and inflammation. C R Seances Soc Biol Fil 187:286–295,1993. [PubMed] [Google Scholar]
- 25.Redout EM, Wagner MJ, Zuidwijk MJ, et al. : Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res 75:770–781,2007. [DOI] [PubMed] [Google Scholar]
- 26.Pietraforte D, Vona R, Marchesi A, et al. : Redox control of platelet functions in physiology and pathophysiology. Antioxid Redox Signal 21:177–193,2014. [DOI] [PubMed] [Google Scholar]
- 27.Hu J, Mondal NK, Sorensen EN, et al. : Platelet glycoprotein Ibα ectodomain shedding and non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist devices. J Heart Lung Transplant 33:71–79,2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zharikov S, Shiva S: Platelet mitochondrial function: from regulation of thrombosis to biomarker of disease. Biochem Soc Trans 41:118–123,2013. [DOI] [PubMed] [Google Scholar]
- 29.Lopez JJ, Salido GM, Gómez-Arteta E, et al. : Thrombin induces apoptotic events through the generation of reactive oxygen species in human platelets. J Thromb Haemost 5:1283–1291,2007. [DOI] [PubMed] [Google Scholar]
- 30.Leytin V Apoptosis in the anucleate platelet. Blood Rev 26:51–63,2012. [DOI] [PubMed] [Google Scholar]
- 31.Gyulkhandanyan AV, Mutlu A, Freedman J, et al. : Markers of platelet apoptosis: methodology and applications. J Thromb Thrombolysis 33:397–411,2012. [DOI] [PubMed] [Google Scholar]