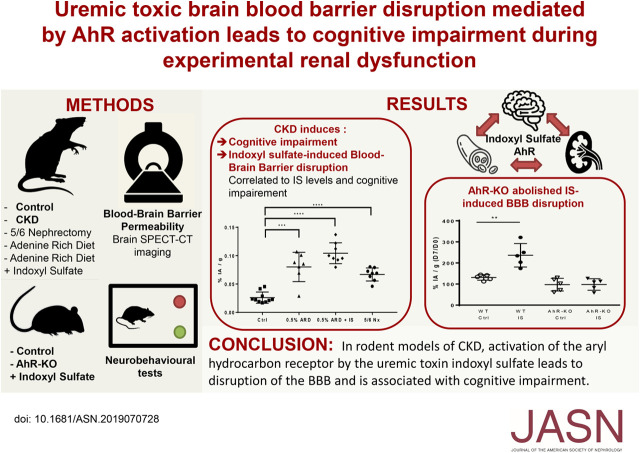
Significance Statement
Uremic toxicity may play a role in the elevated risk of developing cognitive impairment observed in patients with CKD. Some uremic toxins, such as indoxyl sulfate, are agonists of the transcription factor aryl hydrocarbon receptor (AhR). The authors found that cognitive impairment in three models of CKD in rats is correlated with serum levels of indoxyl sulfate as well as blood-brain barrier disruption as detected by SPECT/CT imaging. Using AhR−/− knockout mice, the authors described for the first time that indoxyl sulfate–induced activation of AhR is responsible for blood-brain barrier disruption. These findings demonstrate that blood-brain barrier disruption seems to be an important mechanism involved in cognitive impairment in the context of CKD and that AhR may be a promising therapeutic target to prevent cognitive impairment in CKD.
Keywords: chronic kidney disease, uremia, dementia
Visual Abstract
Abstract
Background
Uremic toxicity may play a role in the elevated risk of developing cognitive impairment found among patients with CKD. Some uremic toxins, like indoxyl sulfate, are agonists of the transcription factor aryl hydrocarbon receptor (AhR), which is widely expressed in the central nervous system and which we previously identified as the receptor of indoxyl sulfate in endothelial cells.
Methods
To characterize involvement of uremic toxins in cerebral and neurobehavioral abnormalities in three rat models of CKD, we induced CKD in rats by an adenine-rich diet or by 5/6 nephrectomy; we also used AhR−/− knockout mice overloaded with indoxyl sulfate in drinking water. We assessed neurologic deficits by neurobehavioral tests and blood-brain barrier disruption by SPECT/CT imaging after injection of 99mTc-DTPA, an imaging marker of blood-brain barrier permeability.
Results
In CKD rats, we found cognitive impairment in the novel object recognition test, the object location task, and social memory tests and an increase of blood-brain barrier permeability associated with renal dysfunction. We found a significant correlation between 99mTc-DTPA content in brain and both the discrimination index in the novel object recognition test and indoxyl sulfate concentrations in serum. When we added indoxyl sulfate to the drinking water of rats fed an adenine-rich diet, we found an increase in indoxyl sulfate concentrations in serum associated with a stronger impairment in cognition and a higher permeability of the blood-brain barrier. In addition, non-CKD AhR−/− knockout mice were protected against indoxyl sulfate–induced blood-brain barrier disruption and cognitive impairment.
Conclusions
AhR activation by indoxyl sulfate, a uremic toxin, leads to blood-brain barrier disruption associated with cognitive impairment in animal models of CKD.
Major adverse cardiovascular events and mortality are greatly increased in patients with CKD.1 Recent observations highlight that patients with CKD have a higher risk of developing cognitive impairment.2−7 Cognitive impairment is frequent, appears early with CKD, and worsens parallel with the decline of the GFR. These disorders are compared with a form of early-onset vascular dementia3,8−10 but with limited cerebral structural damage because patients with CKD have minimal alteration of cerebral volumes in magnetic resonance imaging, not correlated to cognitive impairment.11 There is currently no treatment to improve cognitive disorders during CKD, and these disorders are only partly reversible after kidney transplantation,12 especially in frail patients.13 This risk of developing cognitive impairment is generally explained by the high prevalence of both symptomatic and subclinical ischemic cerebrovascular lesions,14−18 white matter lesions, and microbleeds,15,19 and it could be worsened by significant alteration in cerebral oxygenation and blood flow in hemodialysis.20 However, other potential mechanisms such as direct toxicity of uremic toxins or cerebral endothelial dysfunction could be involved.
In fact, plasmatic uremic toxin contents can rise from 10 to 100 times higher in patients with CKD than those seen in the general population. Among the uremic toxins, indole solutes belong to the family of uremic toxins bound to proteins and are difficult to purify by dialysis.14 The accumulation of indoles is associated with a higher risk of cardiovascular events21 and may play an important role in abnormalities of cognitive functions.22,23 Recently, Assem et al.22 implied that indoxyl sulfate (IS) could be a major player in the neurologic effect of kidney failure. Moreover, Lin et al.24 highlighted a correlation between serum IS concentrations and cognitive disorders in patients on hemodialysis. Our team identified the receptor of IS in endothelial cells, aryl hydrocarbon receptor (AhR). AhR modulates many signaling pathways. Activation of AhR by IS causes endothelial dysfunction leading to a procoagulant state by inducing endothelial inflammation and an increase in oxidative stress.21,25−27 In addition, AhR is widely expressed in the central nervous system, and its activation has been shown to play a detrimental role in both cognitive function28 and stroke-induced brain injury in mice with normal renal function.29
Recent work suggests that blood-brain barrier (BBB) disruption is an important mechanism involved in acute and chronic neurodegenerative processes.30 Particularly, BBB disruption may be an early biomarker of human cognitive dysfunction in patients without CKD.31 Surprisingly, the integrity of BBB in patients with CKD has been poorly explored, and few reports suggest BBB permeability in animal models.19 However, to date, no link between BBB disruption, IS accumulation, and cognitive impairment has been made in the context of CKD.
Thus, we challenged the hypothesis that neurocognitive disorders may be associated with IS-induced BBB disruption in rodent models of CKD.
Methods
All procedures using animals were approved by the institution’s Animal Care and Use Committee (Project #15636–2018122710383826 VI, CE14; Aix-Marseille Université), and they were conducted according to European Union Directive 2010/63 and to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rat experiments were conducted in 5-week-old male Sprague-Dawley rats (Envigo). The animals were housed two per cage with enrichment and free access to food and water. The temperature and the humidity of the air were controlled and varied between 40% and 60%, respectively; a light cycle of 7–19 hours was carried out. Several daily passages of competent personnel were carried out in the animal facility to control the welfare of the animals and for the identification of the end points. The study protocol is summarized in Figure 1.
Figure 1.
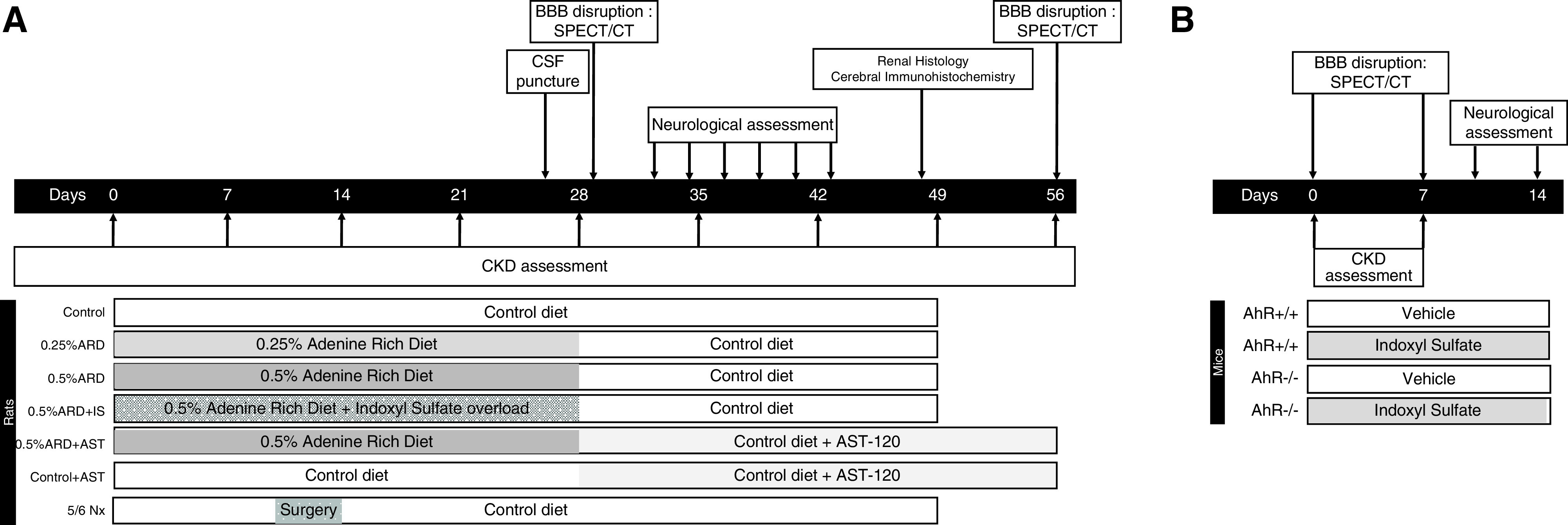
Experimental protocol of the study. Description and timing of the experiments conducted in (A) rats and (B) mice. 5/6 Nx, 5/6 nephrectomy; AhR+/+, wild type; AhR-/-, Aryl Hydrocarbon Receptor knock-out; BBB, blood-brain barrier; CSF, cerebro spinal fluid.
Mouse experiments were conducted with AhR knockout (AhR−/−) mice (B6.129-Ahrtm1Bra/J)32 purchased from Jackson Laboratories and maintained as a breeding colony in the animal care facility at the Faculty of Medicine of Marseille. C57BL/6J mice were used as experimental controls. Genotypes were confirmed by PCR analysis of DNA from tail clippings.
Induction and Assessment of CKD
CKD was induced by subjecting the rats to an adenine-rich diet (ARD) at 0.25% and 0.5% (Safe Nutrition) for 28 days. We challenged a separate animal group of 0.5% ARD (0.5% ARD + IS) rats with IS by adding IS in the drinking water during day 14 (D14)–D28 (1 g of IS per liter of water).33 IS was purchased as a potassium salt (Sigma-Aldrich); an equivalent concentration of KCl in drinking water was used as control.
In another separate animal group, after 4 weeks of 0.5% ARD diet, oral sorbent 8% AST-120 (Kureha) was added mixed with standard food following the protocol previously described34 for another 4 weeks (D28–D46).
A second model of renal failure was obtained by 5/6 nephrectomy as described in the literature.35 Briefly, ablation of the upper and lower poles of the left kidney was performed after subcostal laparotomy and clamping of the renal pedicle, and total right nephrectomy was performed 4 days later. The surgery was performed under anesthesia with 100 μl 3% sevoflurane. Intraoperative analgesia was provided by 2 mg/ml ropivacaine subcutaneously, and postoperative analgesia was by injection of 0.05 mg/kg buprenorphine subcutaneously.36 Postoperative pain was assessed using a grid of facial expressions validated in the rat.37 In case of pain, another injection of 0.05 mg/kg buprenorphine was given on D1 and D2 postsurgery.
BP of the animals was measured at D28 with a rat-adapted monitor after a weekly habituation.
In mice, IS overload was given in drinking water (1 g/L) for the duration of the experiments (14 days). Food and water were provided ad libitum.
The severity of CKD was assessed using classic biochemical markers serum urea and creatinine. The serum levels of IS, indole acetic acid, and paracresyl sulfate were assessed by high performance liquid chromatography as previously described38 (Shimadzu LC2 device). Blood samples were collected by catheterization of the caudal vein under anesthesia by 3% sevoflurane. The serum was extracted after centrifugation (2500×g for 15 minutes at 21°C). Blood samples were stored at −80°C.
Aryl hydrocarbon receptor activating potential (AhR-AP) in serum was quantified by reporter-luciferase gene bioluminescence gene and expressed as percentage activation of FICZ (positive control)39 on human HEPG2 cell culture.
After euthanasia, the kidneys were fixed in 4% formaldehyde for 48 hours and then, kept in 70% ethanol to be included in paraffin. The interstitial lesions were assessed with optic microscopy (station Ellipse Ni-E; Nikon) after hematoxylin-eosin staining according to the score described by Wan et al.40 for each kidney by two trained physicians blinded to the interventions. Renal fibrosis was assessed by histochemical Sirius red staining. Quantification of Sirius red was performed using ImageJ 1.51 analysis software.
Central Nervous System Evaluation
Indoles and paracresyl sulfate levels in cerebrospinal fluid (CSF) of 0.5% ARD rats were measured by high performance liquid chromatography.38 CSF samples were taken by stereotaxic puncture of magna cisterna at D28 using a specific stereotaxic device for rats under 3% sevoflurane anesthesia. CSF samples were stored at −80°C.
Neurobehavioral Tests
The behavioral tests used are all validated tests and widely used in the literature for the evaluation of neurologic deficits in rats.41,42 In order to avoid any influence of anesthesia, we had to allow animals to recover from SPECT-CT imaging for at least 3 days before performing neurobehavioral assessment.
The novel object recognition (NOR) test was used to evaluate exploratory behaviors, learning, and animal memory.43,44 Object recognition was measured by the difference between the exploration times of familiar objects and new objects in a given enclosure. In this work, we set up a protocol to characterize the learning performance in the short term and thus, the potential learning deficit.45 The rats were placed in a 1×1×0.25-m arena. Different objects were used of different sizes, shapes, and colors. The test was conducted in three phases. (1) In the habituation phase, the day before the test the animals were placed in the southeast corner of the empty arena, and they were able to explore for 30 minutes. The arena was cleaned after each rat pass. (2) In the learning phase, on the day of the test two identical objects were placed in specific places in the arena, and the rats explored the space for 3 minutes. This sequence was repeated three times 30 minutes apart. (3) In the test phase, one of the familiar objects was replaced by a new object (same location). The animal explored the arena and the objects. The recognition time of each object was measured. Exploration is defined as the animal having its head <0.05 m around the object while looking at it, sniffing it, or touching it. Data were collected by video tracking software (Ethovision XT 8.5; Noldus). The total time of exploration of each object has been recorded. A discrimination index has been measured, which corresponds to the time spent exploring the new object in relation to the total exploration time [(n−a)/(n+a)].
The object location (OL) task was performed to assess a rat’s ability to recognize that an object that it had experienced before had changed location in order to explore a spatial memory ability.46 In the learning phase, the rat was exposed to objects, which were placed in the far corners of the arena. The animal was allowed to explore both objects during a sample phase of 3 minutes, and the amount of time of exploration of each object was recorded by the experimenter. This sequence was repeated three times 30 minutes apart. In the test phase, one object was placed in the same position as it had occupied in the sample phase; the second object was placed in the corner adjacent to the original position so that the two objects were in diagonal corners. Thus, both objects in the test phase were equally familiar, but one was in a new location. The position of the moved object was counterbalanced between rats. Intact OL memory is evidenced by subjects spending more time investigating the object in the new location.
The social memory test assessed cognition, namely the ability to recognize novel versus familiar animals, in rodent models of CNS disorders.47 Over the course of multiple exposures, rodents will become habituated to intruders and no longer find them as interesting as a completely novel intruder. During testing, the subject was given four 1-minute exposures in its home cage to the same intruder 10 minutes apart. In the fifth trial, the subject encountered an entirely novel intruder. All test trials were videotaped and subsequently analyzed for total body investigation, anogenital investigation, perioral investigation, and body investigation.
The NOR and OL tests were performed in mice as previously described.48 Briefly, all animals were handled for a week and then habituated to the empty arena for 25 min the day prior to the test. Then, the acquisition and test phases were separated by a 1-hour delay of retention time in both tests.
NOR Task
In the acquisition phase, duplicate objects were placed near two corners in the arena. Each subject was allowed a total of either 40 seconds of object investigation or 5 minutes in the arena. During the test (duration of 5 minutes), the animal was replaced in the arena and presented with objects in the same positions as at acquisition: one object was the third copy of the object used at acquisition, and the other was a novel object. Object positions and the objects used as novel or familiar were counterbalanced.
OL Task
In the acquisition phase, the mouse was allowed to investigate duplicate objects for 5 minutes. During the test (duration of 5 minutes), one object was placed in the same position that it had occupied at acquisition, whereas an identical object was placed in the corner diagonally opposite. The position of the moved object was counterbalanced between mice. Intact OL memory is evidenced by subjects spending more time investigating the object in the new location.
Isotopic Imaging
BBB permeability was assessed by SPECT-CT after injection of 99mTc-coupled diethylene-triamine-penta-acetic acid (99mTc-DTPA) as previously described.49 At D30, rats received, under anesthesia by 3% sevoflurane, an intravenous injection of 100 µl of radioactive tracer (20 MBq) in isotonic and pyrogen-free solution; 30 minutes after injection, the SPECT-CT acquisition was done for 25 minutes under anesthesia with 3% sevoflurane using a Mediso NanoSpect camera and the Mediso Nucline 1.02 software. Activity was measured in the anatomic regions by the Invicro VivoQuant 3.5 and Invicro InvivoScope 2.00 reconstruction software. The cerebral acquisitions were placed on a three-dimensional cerebral atlas (CERMEP Living Imaging).50 A quantitative index of BBB permeability was defined as the cerebral content of 99mTc-DTPA, expressed as a percentage of the total amount of injected activity and corrected per gram of brain tissue. In mice, brain activity was quantified as percentage of injected activity by gram of brain using the same process as for rats. We studied the evolution of BBB permeability between D0 and D7 and expressed the results as a percentage of activities D7/D0.
Statistical Analyses
Results are expressed in mean ± SD. Mean comparisons were done by Mann–Whitney test and Kruskal–Wallis test (for repeated values over time), and calculation of correlation was by Spearman test and linear regression. All tests were nonparametric and bilateral. Statistical analysis was done using Microsoft Excel and GraphPad Prism 7.0 software; a P value of 0.05 was considered significant.
Ethics
This study was conducted following European Directive 2010/63/EU and the Declaration of Helsinki, and it was approved by local and national ethics committees and registered under the number APAFiS #15636.
Results
The 0.5% ARD Induced Uremic Toxins Accumulation and AhR Activation
We confirmed the induction of CKD after 28 days of 0.5% ARD in rats by increases in both serum creatinine and urea (Supplemental Figure 1, Supplemental Material). At D0, the mean serum IS level was 20.0±6.2 µmol/L. There was a significant increase in IS over time compared with the control group occurring since D21 in the 0.25% ARD group (n=8; P=0.007) and since D7 in the 0.5% ARD group (n=8; P=0.001). At D35, IS level was higher in the 0.5% ARD group (68.4±22.1 µmol/L; n=7) compared with the control and 0.25% ARD group: 15.4±11.1 µmol/L (n=8; P=0.00005) and 44.6±12.8 µmol/L (P=0.04), respectively; the IS level was higher in the 0.25% ARD group than in the control group (n=8; P=0.002) (Figure 2A). The serum concentrations of indole acetic acid and paracresyl sulfate were not different in the three groups regardless of the time (data not shown).
Figure 2.
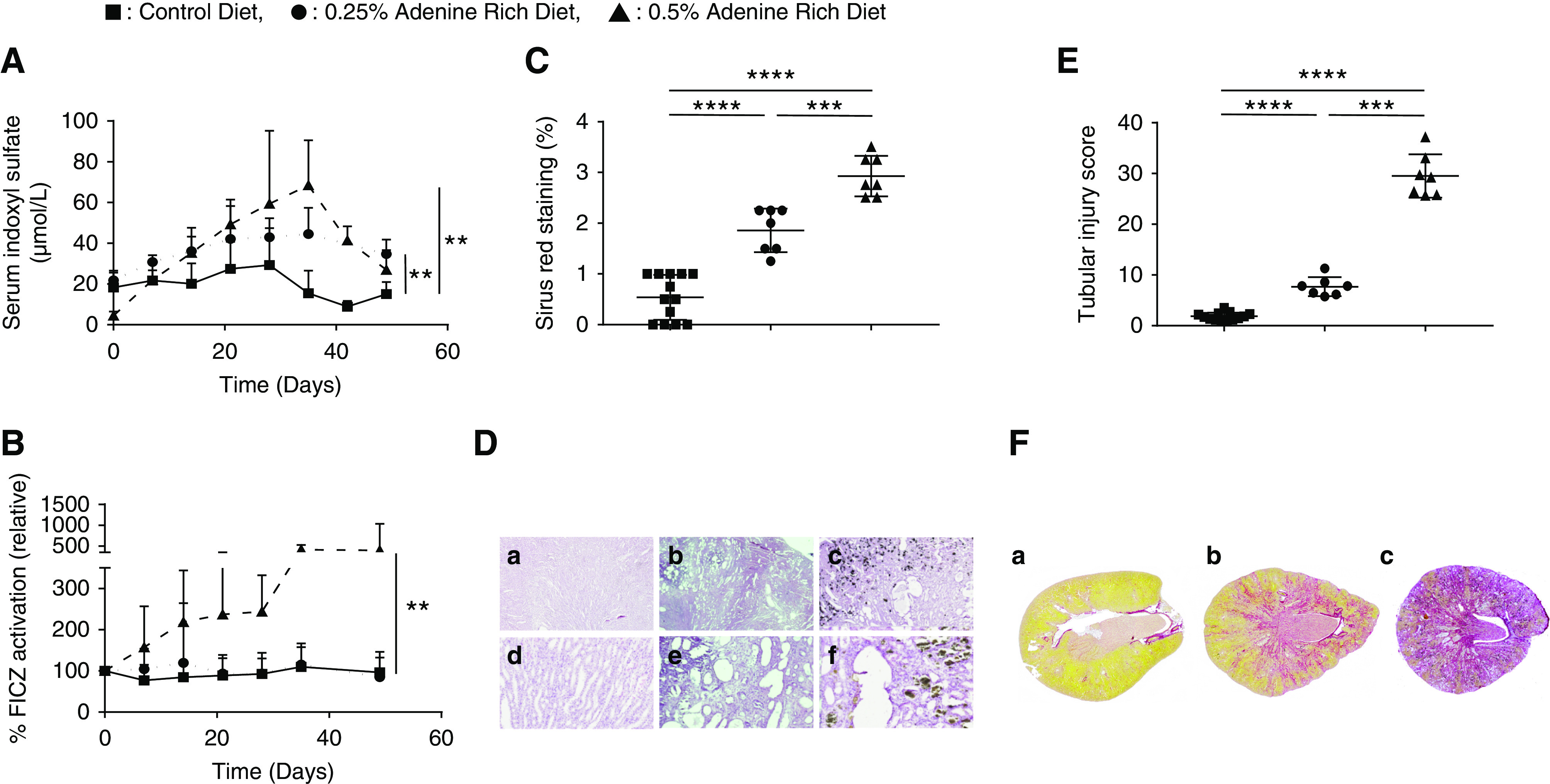
ARD induced dose-dependent irreversible tubular lesion and fibrosis; the 0.5% ARD induced uremic toxins accumulation and AhR activation. Biologic and histologic evaluation of control or 0.25% and 0.5% ARD rats. (A) Serum IS over time. (B) Relative AhR activation by the rats’ serum (by percentage of FICZ). (C) Tubular injury score after hematoxylin-eosin staining. (D) Renal histology in optical microscopy after hematoxylin-eosin staining in (D, a and d) control rats, (D, b and e) 0.25% ARD rats, and (D, c and f) 0.5% ARD rats. Original magnification, x20 in D, a–c; x100 in D, d–f. (E) Sirius red staining. (F) Renal histology in optical microscopy after Sirius red staining on full histologic section in (F, a) control rats, (F, b) 0.25% ARD rats, and (F, c) 0.5% ARD rats. **P<0.01; ***P<0.001; ****P<0.0001.
At D0, the mean AhR activation by the rats’ serum was 13.7%±8.3% FICZ (n=32). Relative serum AhR activation relative to the J0 value as a function of time was significantly greater in the 0.5% ARD group (n=8) compared with the control group (n=8; P=0.002) and with the 0.25% ARD group (n=8; P=0.02) (Figure 2B). At D28, the relative AhR activation was significantly higher in 0.5% ARD group (244.0±88.4; n=8) compared with control (92.5%±37.7%; n=8; P=0.005) and with 0.25% group (93.6%±50.5%; n=8; P=0.002). There was no significant difference between the control and 0.25% ARD groups (P=0.33) (Figure 2B).
ARD Induced Dose-Dependent Irreversible Tubular Lesion and Fibrosis
After hematoxylin-eosin staining, average tubular lesion score was 0.54±0.44 in the control group (n=13) versus 1.86±0.43 in the 0.25% ARD group (n=7; P=0.00003) and 2.93±0.40 in the 0.5% ARD group (n=7; P=0.00003). In addition, intratubular precipitation of adenine crystals was observed in the 0.5% ARD group (Figure 2, C and D). After Sirius red staining, average staining percentage per slide was 1.84%±0.75% in the control group (n=13) versus 7.69%±1.89% in the 0.25% ARD group (n=7; P=0.00003) and 29.52%±4.27% in the 0.5% ARD group (n=7; P=0.00003) (Figure 2, E and F).
Renal Failure Induced Neurobehavioral Alteration
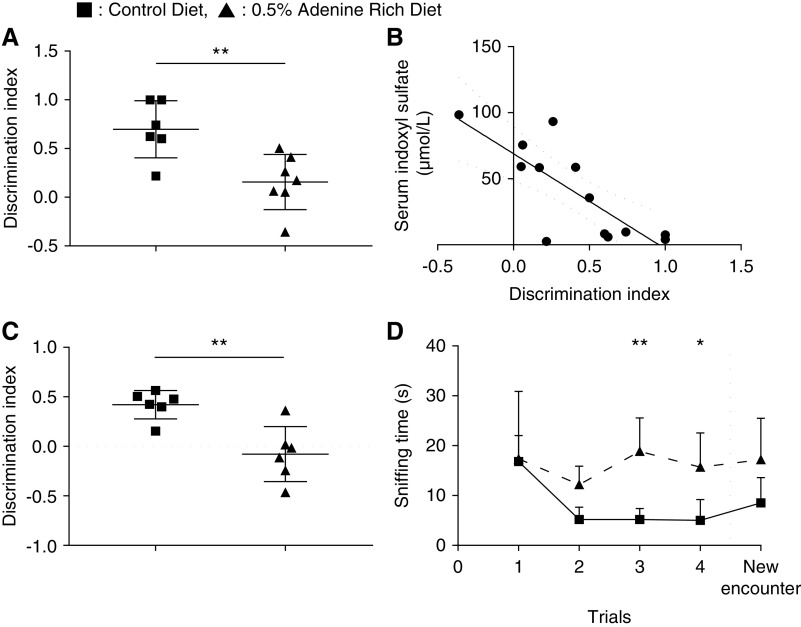
The 0.5% ARD rats did not develop any somatosentive or sensorimotor impairment (Supplemental Figure 2). The discrimination index of the NOR test was lower in the 0.5% ARD group than in the control group: 0.16±0.28 versus 0.70±0.29 (n=7; P=0.008) (Figure 3A). It was correlated with serum IS (r=−0.70; P=0.01; R2=0.62) (Figure 3B). The discrimination index of the OL test was lower in the 0.5% ARD group than in the control group: −0.08±0.11 versus 0.42±0.06 (n=6; P=0.003) (Figure 3C). The sniffing time in the social memory test was higher in 0.5% ARD rats compared with control rats in trial 3: 18.8±6.7 and 5.2±2.2 seconds (n=6; P=0.004), respectively. It was also higher in trial 4: 15.7±6.9 and 5.0±4.2 seconds, respectively (n=6; P=0.04); however, it was not different between the two groups in trial 5 with the new encounter: 17.2±8.3 and 8.5±5.1 seconds (n=6; P=0.14) (Figure 3D).
Figure 3.
Renal failure induced neurobehavioral alteration. Neurobehavioral evaluation of control or 0.5% ARD rats. (A) Discrimination index of the NOR test. (B) Correlation between discrimination index of the NOR test and serum IS levels. (C) Discrimination index of the OL test. (D) Sniffing time in social recognition test. *P<0.05; **P<0.01.
BBB Permeability Was Increased in 0.5% ARD Rats
The cerebral content of 99mTc-DTPA, the imaging biomarker of BBB permeability, was significantly higher in the 0.5% ARD group compared with the control group: 0.086%±0.030% and 0.026%±0.010% IA/g, respectively (n=7; P<0.001) (Figure 4A).
Figure 4.
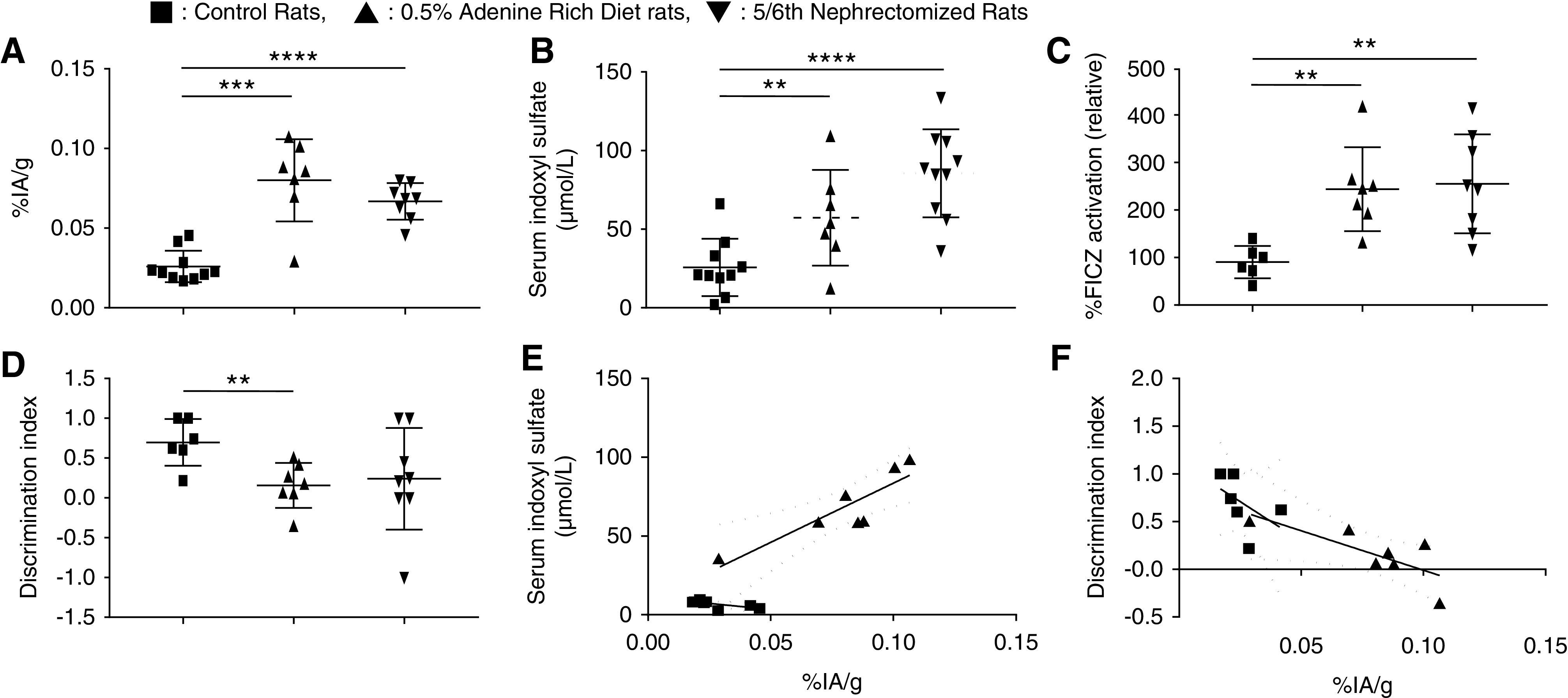
The 5/6 nephrectomy induced comparable outcomes with 0.5% ARD on kidney function, uremic toxins accumulation, AhR activation, neurobehavioral impairment, and BBB permeability in rats. Evaluation of the effects of CKD induced by 0.5% ARD or 5/6 nephrectomy in rats on (A) brain content of 99mTc-DTPA cerebral scintigraphy in percentage of injected activity per gram of brain (% IA/g). (B) Serum indoxyl levels at D28. (C) Relative AhR activation by the rats’ serum (by percentage of FICZ) at D28. (D) Discrimination index of the NOR test. (E) Correlation between discrimination index of the NOR test and brain content in 99mTc-DTPA cerebral scintigraphy expressed as percentage of injected activity per gram of brain (% IA/g). (F) Correlation between serum IS levels and brain content in 99mTc-DTPA cerebral scintigraphy expressed as percentage of injected activity per gram of brain (% IA/g). **P<0.01; ***P<0.001; ****P<0.0001.
The 5/6 Nephrectomy Induced Comparable Outcomes with 0.5% ARD on Kidney Function, Uremic Toxins Accumulation, AhR Activation, Neurobehavioral Impairment, and BBB Permeability in Rats
Mean serum creatinine levels were not different in 0.5% ARD and 5/6 nephrectomy groups at D28: 106.9±18.2 versus 93.5±17.7 µmol/L (n=8; P=0.19); however, they were higher in these groups compared with the control group: 42.3±9.7 µmol/L (n=8; P=0.0001 and P=0.00005, respectively). Serum IS was significantly increased over time in the 5/6 nephrectomy group compared with the control group (n=8; P<0.001) but not different from the 0.5% ARD group (n=8; P=0.17). At D28, serum IS in the 5/6 nephrectomy group was higher than in the control group: 85.7±31.8 and 25.7±18.2 µmol/L, respectively (n=10; P<0.001); however, it was not different from the 0.5% ARD group: 64.8 μmol ±28.1 µmol/L (n=8; P=0.35) (Figure 4B). The serum concentrations of indole acetic acid and paracresyl sulfate were not different in the three groups regardless of the time. The relative AhR activation in the 5/6 nephrectomy group was higher than in the control group: 255.1%±104.2% and 90.4%±34.1%, respectively (n=8; P=0.001); however, it was not different from in the 0.5% ARD group: 244.0%±88.4% (n=8; P>0.99) (Figure 4C). The discrimination index of the NOR test tended to be decreased in the 5/6 nephrectomy group compared with the control group: 0.24±0.64 and 0.70±0.29, respectively (n=8; P=0.06); also, it was not different from the 0.5% ARD group (P=0.34) (Figure 4D). Brain content of 99mTc-DTPA was significantly higher in the 5/6 nephrectomy group than in the control group: 0.066%±0.012% and 0.026%±0.009% IA/g, respectively (n=8; P=0.00005) (Figure 4A).
BBB Permeability Was Correlated with Cognitive Impairment and Serum IS Levels
The cerebral fixation of 99mTc-DTPA was inversely correlated with the discrimination index of NOR (r=−0.90; P<0.001; R2=0.70). This association remains significant in the ARD group (r=−0.75; P=0.05; R2=0.56) (Figure 4E). 99mTc-DTPA content was correlated with serum IS levels (r=0.68; P=0.006; R2=0.87); this association remains significant in the ARD group (r=0.82; P=0.01; R2=0.76) (Figure 4F).
IS Overload Increased Cognitive Impairment and BBB Disruption in 0.5% ARD Rats
The average weekly water intake per rat was not different between the two adenine groups (n=8; P>0.99). At D28, serum creatinine levels were not different between ARD and ARD + IS groups: 124.5±18.9 and 124.3±11.1 µmol/L, respectively (n=8; P=0.69). Serum IS over time increased in the 0.5% ARD + IS group compared with the control group (n=8; P=0.00003) and with the 0.5% ARD group (n=8; P<0.001). The IS level was higher in the 0.5% ARD + IS group compared with the control group (424.6±95.4 and 25.7±18.2 μmol/L, respectively; n=8; P=0.0005) and with the 0.5% ARD group (64.8±28.1 μmol/L; n=8; P=0.00005) (Figure 5A). The serum concentrations of indole acetic acid and serum paracresyl sulfate were not different in the three groups regardless of the time. The relative AhR activation was higher in the 0.5% ARD + IS group than in the control group (1049.0%±355.5% and 90.4%±34.1%, respectively; n=8; P<0.001) and higher than in the 0.5% ARD group (244.0%±88.4%; n=8; P<0.001) (Figure 5B). The discrimination index of the NOR test was lower in the 0.5% ARD + IS group than in the control group (−0.65±0.24 and 0.70±0.29, respectively; n=8; P=0.002) and lower than in the 0.5% ARD group (P=0.001) (Figure 5C). Brain content of 99mTc-DTPA was higher in the 0.5% ARD + IS group than in the control group (0.104%±0.018% and 0.026%±0.009% IA/g, respectively; n=8; P=0.00005) and higher than in the 0.5% ARD group (n=8; P=0.05) (Figure 5D).
Figure 5.
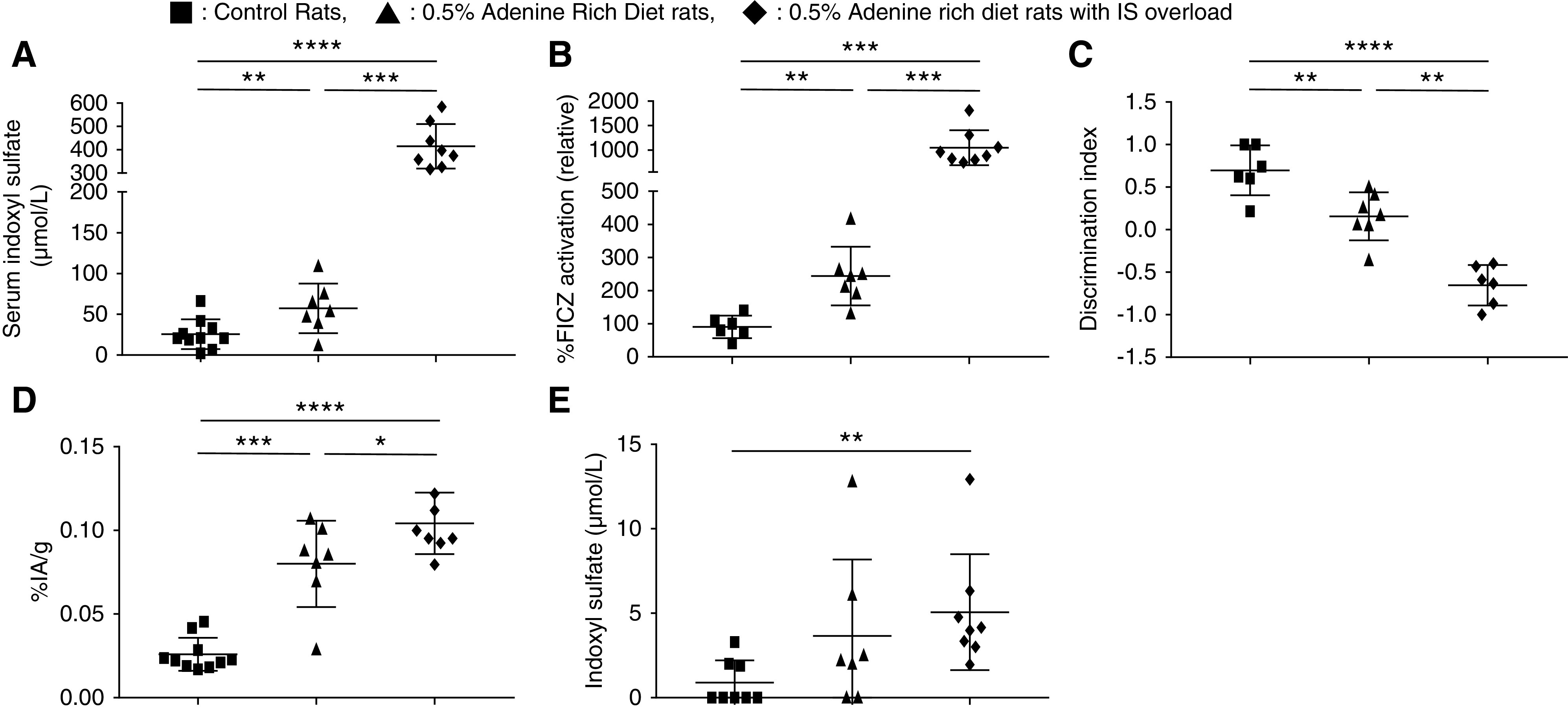
IS overload increased cognitive impairment and BBB disruption in 0.5% ARD rats. Evaluation of ARD rats with IS overload compared with control or 0.5% ARD rats. (A) Serum indoxyl levels at D28. (B) Relative AhR activation by the rats’ serum (by percentage of FICZ) at D28. (C) Discrimination index of the NOR test. (D) Brain content of 99mTc-DTPA cerebral scintigraphy in percentage of injected activity by gram of brain (% IA/g). (E) IS levels in CSF. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
IS Increased in CSF of 0.5% ARD Rats Challenged with IS
Compared with the control group, IS levels were not different in rats’ CSF in the 0.5% ARD group (0.9±1.3 and 3.7±4.5 µmol/L, respectively; n=7; P=0.10), but they increased in the 0.5% ARD + IS group (5.1±3.4; n=8; P=0.001) (Figure 5E). Paracresyl sulfate and indole acetic acid levels were not detectable in the CSF of the three rat populations (data not shown).
Oral Sorbent AST-120 Treatment after ARD Decreased Serum Indoxyl Sulfate but Did Not Reverse BBB Dysfunction
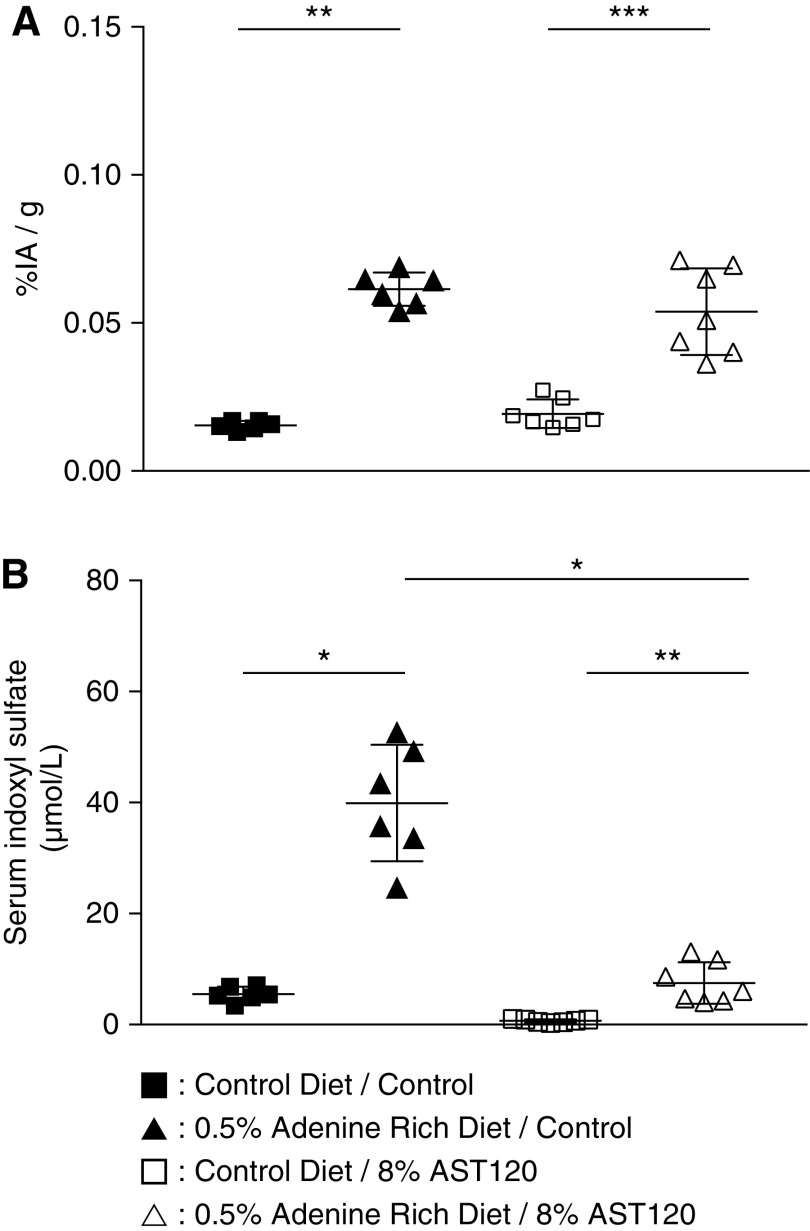
AST-120 treatment after 0.5% ARD induced a significant decrease in serum IS level compared with control 0.5% ARD rats: 7.5±3.7 versus 39.9±10.5 μmol/L (n=7; P=0.04), respectively (Figure 6A). Serum IS level remained higher in 0.5% ARD rats than in control rats: 5.5±1.4 μmol/L (n=7; P=0.002). There was no difference in brain content of 99mTc-DTPA between 0.5% ARD rats whether they were treated with AST-120 or not: 0.054%±0.001% and 0.061%±0.005% IA/g (n=7; P=0.77), respectively. Interestingly, brain content in 99mTc-DTPA remained higher in the 0.5% ARD rats than in the control rats: 0.015%±0.001% IA/g (n=7–6; P=0.0006) (Figure 6B).
Figure 6.
Oral sorbent AST-120 treatment after ARD decreased serum indoxyl sulfate but did not reverse BBB dysfunction in rats. Evaluation of a 4-week treatment with oral sorbent AST-120 after ARD in rats. (A) BBB dysfunction measured by brain content of 99mTc-DTPA cerebral scintigraphy expressed as percentage of injected activity per gram of brain (% IA/g). (B) Serum IS levels. *P<0.05; **P<0.01; ***P<0.001.
AhR Activation Was Involved in BBB Disruption and Cognitive Impairment
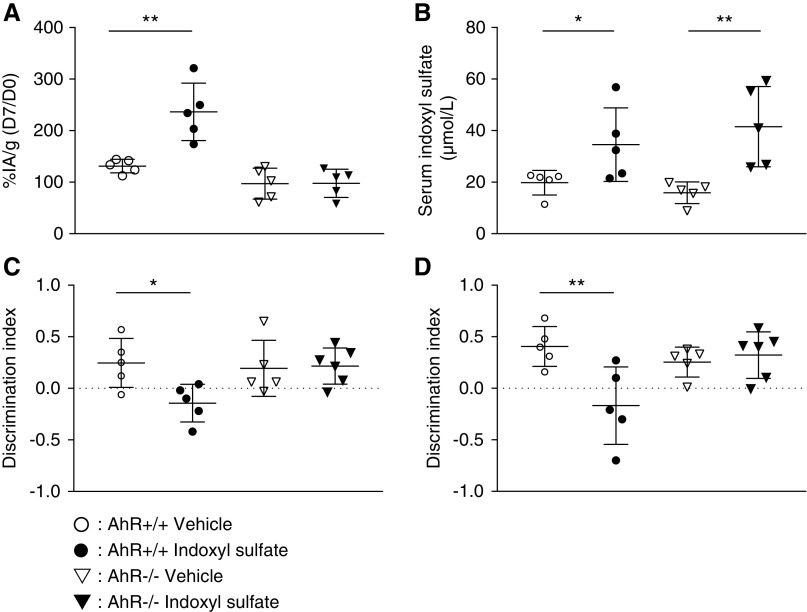
Compared with control AhR+/+ mice, the cerebral content of the 99mTc-DTPA ratio between D7 and D0 (D7/D0) was significantly higher after IS administration (236%±56% and 131%±13%, respectively; n=5; P=0.008) (Figure 7A). There was no difference in 99mTc-DTPA ratio D7/D0 between AhR−/− mice whether they were supplemented with IS or not (97%±30% versus 98%±27%; n=5; P>0.99). In wild-type mice, serum IS at D7 was higher after IS administration than in controls (34.6±14.2 versus 19.8±4.7 µmol/L; n=5; P=0.04). In AhR knockout mice, serum IS at D7 was higher after IS administration than in controls (41.5.0±15.6 versus 15.9±4.2; n=5; P=0.002) (Figure 7B). The discrimination index in the NOR test was lower in the wild-type mice submitted to IS challenge than in the control group: −0.14±0.18 and 0.25±0.24, respectively (n=5; P=0.03). The discrimination index in the NOR test was not impaired in AhR−/− mice challenged with IS compared with AhR−/− control mice: 0.22±0.17 and 0.19±0.27, respectively (n=5; P>0.99) (Figure 7C). The discrimination index in the OL test was lower in the AhR+/+ mice submitted to IS challenge than in AhR+/+ control mice: −0.17±0.38 and 0.41±0.19, respectively (n=5; P=0.01). The discrimination index in the OL test was not impaired in AhR−/− mice challenged with IS compared with in AhR−/− control mice: 0.32±0.22 and 0.25±0.14, respectively (n=5; P=0.84). (Figure 7D).
Figure 7.
AhR activation was involved in indoxyl sulfate-induced BBB disruption and cognitive impairment in mice. (A) Relative brain content of 99mTc-DTPA cerebral scintigraphy in percentage of injected activity by gram of brain (% IA/g) in wild-type (AhR+/+) and AhR knockout (AhR-/-) mice at D7 compared with D0. (B) Serum IS in AhR+/+ and AhR-/- mice at D7. (C) Relative AhR activation in vitro by the mice’s serum expressed in percentage of the reference agonist FICZ. (D) Cognitive assessment by discrimination index in the NOR test and the OL test. *P<0.05; **P<0.01.
Discussion
We report cognitive impairment associated with BBB permeability in three models of CKD in rats. We first highlighted neurobehavioral alterations in both 0.5% ARD and 5/6 nephrectomized rats. These animals did not develop sensory-motor impairment, but we showed impaired short-term learning performance in the NOR test, impaired spatial memory in the OL test, and impaired social memory, revealing an impaired cognitive function. This is consistent with clinical reports about cognitive function in patients with CKD,3,8,9 without the somatosensorial or sensorimotor impairment, but to our knowledge, there are very few publications about cognitive dysfunction in animal models of CKD.51,52 For example, Mazumder et al.53,54 highlighted a decrease in cognitive function using a similar NOR paradigm in mice fed with 0.3% ARD. Interestingly, we showed a strong correlation with a significant linear regression between 99mTc-DTPA content in brain and discrimination index in NOR test. This statistical link suggests that the BBB leakage could contribute to cognitive dysfunction in CKD rats. Interestingly, there is an increasing literature highlighting a link between BBB disruption and cognitive dysfunction in various conditions, such as aging55 and diabetes. Recently, Nation et al.31 suggested that BBB disruption may be a predictive marker of cognitive impairment in Alzheimer disease.
A few animal models of acute and chronic renal failure have shown BBB disruption in the setting of uremia,19,53,56 but underlying mechanisms remain unclear. In addition to ARD models, we performed 5/6 nephrectomy in rats in order to confirm that the BBB dysfunction and cognitive impairment described in ARD rats were the results of CKD and not of ARD by itself. Here, we report a strong correlation with a significant linear regression between IS in serum and 99mTc-DTPA in brain. This statistical link suggested that IS could contribute to BBB disruption. Thus, we challenged this hypothesis by an overload of IS in drinking water of 0.5% ARD rats. This induced a massive accumulation of IS in the serum in rats followed by an increase in BBB disruption compared with 0.5% ARD rats, suggesting a strong involvement of IS in BBB disruption. We challenged mice without CKD with IS in drinking water and confirmed that IS alone is sufficient to induce BBB disruption. The lack of increased BBB permeability in AhR−/− mice challenged with IS validated the role of AhR in response to IS in BBB. Moreover, these experiments confirmed that IS-treated mice developed cognitive dysfunction in both the NOR test and the OL task, and these impairments were prevented in AhR−/− mice. These results validate for the first time the involvement of the IS-AhR pathway in cognitive impairment and BBB dysfunction in the context of CKD.
Then, we confirmed that a 4-week uremic toxin sorbent AST-120 treatment decreased ARD-induced IS elevation in serum but did not resolve BBB permeability. These results suggest that the effect of CKD on BBB permeability impairment is persistent because CKD is established. These findings may be consistent with clinical outcomes because renal transplantation does not completely improve cognitive disorders in patients with CKD.12
Interestingly, we studied the activation of the AhR pathway by 0.5% ARD rats’ serum ex vivo using AhR-AP, which evaluates the overall load of AhR agonists in serum. We showed that 0.5% ARD rats’ serum displayed a significant elevation of AhR-AP associated with IS elevation in serum, which is consistent with previous reports in human and mice.26 Furthermore, the addition of IS in drinking water of 0.5% ARD rats increased their serum AhR-AP. These results are in favor of the involvement of IS-AhR in the vascular hypothesis of the development of cognitive disorders in CKD.3 Published data suggest a direct toxicity of IS in brain parenchyma,57 especially oxidative stress, neuroinflammation, and apoptosis in neuron and astrocytes in vitro and in vivo in non-CKD mice. Moreover, 0.2% ARD in mice led to an increase in IS levels in brain,58 and the authors suggested that this increase may be responsible for CKD-related cognitive decline. Unfortunately, cognitive function was not assessed in this model. Consistently, we demonstrated an increase in CSF levels of IS in 0.5% ARD rats challenged with IS in drinking water. This increase was associated with a higher cognitive impairment compared with 0.5% ARD rats. These results suggest a direct toxicity mediated by IS in CSF. However, we did not observe any increase in IS in CSF of ARD rats, which is not in favor of a potential direct toxicity of IS on brain tissue in this models. It is important to note that we showed a BBB dysfunction that was relatively moderate compared with the BBB breakdown that we previously described in other conditions, such as stroke49 or subarachnoidal hemorrhage.59 This suggests that IS could leak through BBB moderately. These results are in accordance with our hypothesis because BBB permeability is more impaired in ARD rats challenged with IS compared with ARD rats. Notwithstanding, we cannot rule out effectiveness of efflux transporters such as OAT. For example, IS is an OAT3 substrate, and OAT3 is expressed in the brain.60
Thus, we suggest that IS-mediated BBB dysfunction and subsequent cognitive dysfunction resulted from endothelial toxicity through AhR activation. Uremic toxins have been reported to directly alter the integrity of both large- and small-vessel endothelial cells.61 To date, no data are available about IS toxicity in BBB models in vitro. However, IS decreased cerebral endothelial cell viability in vitro associated with a decrease in NO production and an increase in ROS production,62 highlighting an IS-induced oxidant stress on endothelial cells.63 This is consistent with data reporting an AhR-mediated oxidative stress pathway in human vascular endothelial cells.64 IS also induced increased TNF-α and IL-6 in mice serum in vivo57 and in human central nervous system cells,65 and it induced increased IL-6 expression in human vascular endothelial cells in vitro.66 These results suggest a potential role of inflammatory cytokines as a mediator of IS effect. In addition, recent experiments highlight that uremia alters cell-to-cell junctions, leading to increased endothelial damage. Surprisingly, we did not show any decrease in tight junction proteins’ expression in CKD rats’ brains by western blot (Supplemental Figure 3). This may be explained by an alteration of phosphorylation and subcellular distribution of occludine, which increases the permeability of tight junctions without decreasing protein expression.67 Other junction proteins on BBB may also be involved. For example, Maciel et al.68 demonstrated a significant decrease in VE-cadherin gene and protein expressions in endothelial cells treated with IS in vitro. Specific studies focusing on the characterization of the reorganization of junction proteins of BBB during CKD are needed.
Moreover, we did not find any change in serum indole acetic acid or paracresyl sulfate in our models. Velenosi et al.34 reported an increase in serum pCS levels along with IS levels in rats fed with 0.7% ARD during 5 weeks. It is likely that our model induced milder CKD, but higher concentrations or duration of ARD would have resulted in severe weight loss and increased mortality.69,70 These results may not be incompatible with the behavioral cognitive assessment considered in our study with rats. To our knowledge, no other studies report indole acetic acid concentrations in rat CKD models. Finally, we did not assess other uremic toxins accumulation, like guanidino compounds, which may also have a direct neurotoxicity.71
In conclusion, we report for the first time in the literature the existence of a BBB permeability in three models of CKD in rats. The increased permeability of the BBB in animals with CKD is inversely correlated with cognitive performance and correlated with circulating IS levels. IS-induced activation of AhR is a key phenomenon that may explain BBB disruption in the context of CKD.
Disclosures
S. Burtey reports personal fees from Otsuka, personal fees from Bayer, and personal fees from BMS outside the submitted work. All remaining authors have nothing to disclose.
Funding
This research was funded by Société Française de Néphrologie, Dialyse et Transplantation (French Society of Nephrology) grant end-stage renal disease and dialysis in 2018.
Supplementary Material
Acknowledgments
We thank Ayano Konagai (Kureha Corporation, Tokyo, Japan) for providing AST-120. We also thank the Plateforme de Biochimie, Unité Mixte de Recherche 1149 Inserm, Université Paris Diderot, Equipe de Recherche Labellisée Centre Nationale pour la Recherche Scientifique 8252, Faculté de Médecine Site Bichat, Paris for performing biochemical tests.
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
M. Bobot, P. Brige, S. Burtey, F. Dignat-George, B. Guillet, and G. Hache designed the study; L. Balasse, M. Bobot, C. Cérini, S. Chopinet, S. Fernandez, P. Garrigue, G. Hache, N. McKay, A. Moyon, S. Poitevin, and L. Thomas carried out experiments; M. Bobot, B. Guillet, G. Hache, and L. Thomas analyzed the data; M. Bobot drafted the manuscript and the figures; S. Burtey, B. Guillet, and G. Hache revised it critically; and L. Balasse, M. Bobot, P. Brige, P. Brunet, S. Burtey, C. Cérini, S. Chopinet, F. Dignat-George, S. Fernandez, P. Garrigue, B. Guillet, G. Hache, N. McKay, A. Moyon, S. Poitevin, and L. Thomas read and approved the final manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019070728/-/DCSupplemental.
Supplemental Figure 1. Clinical and biologic evaluation of control or 0.25% and 0.5% adenine-rich diet rats.
Supplemental Figure 2. Neurologic evaluation of rats fed with control or 0.5% adenine-rich diet.
Supplemental Figure 3. Western blot analysis of occludin, claudin-5, and zonula occludens-1 expressions in brains of control or 0.5% adenine-rich diet rats.
References
- 1.Gansevoort R, Correa-Rotter R, Hemmelgarn B, Jafar T, Heerspink H, Mann J, et al.: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Gaxatte C, Daroux M, Bloch J, Puisieux F, Deramecourt V, Boulanger E: [Cognitive impairment and chronic kidney disease: Which links?]. Nephrol Ther 7: 10–17, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Bugnicourt J-M, Godefroy O, Chillon J-M, Choukroun G, Massy Z: Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J Am Soc Nephrol 24: 353–363, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Yaffe K, Ackerson L, Kurella Tamura M, Le Blanc P, Kusek J, Sehgal A, et al. Chronic Renal Insufficiency Cohort Investigators : Chronic kidney disease and cognitive function in older adults: Findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 58: 338–345, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A, Montgomery R, Bedros V, Lesko J, Mahnken J, Chakraborty S, et al.: Subclinical cognitive impairment and listing for kidney transplantation. Clin J Am Soc Nephrol 14: 567–575, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns C, Knopman D, Tupper D, Davey C, Slinin Y, Lakshminarayan K, et al.: Prevalence and risk of severe cognitive impairment in advanced chronic kidney disease. J Gerontol A Biol Sci Med Sci 73: 393–399, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gesualdo G, Duarte J, Zazzetta M, Kusumota L, Say K, Pavarini S, et al.: Cognitive impairment of patients with chronic renal disease on hemodialysis and its relationship with sociodemographic and clinical characteristics. Dement Neuropsychol 11: 221–226, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan A, Kiernan M: Neurological complications of chronic kidney disease. Nat Rev Neurol 5: 542–551, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, et al.: US renal data system 2010 annual data report. Am J Kidney Dis 57[1 Suppl 1]: A8, e1–e526, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Seliger S, Siscovick D, Stehman-Breen C, Gillen D, Fitzpatrick A, Bleyer A, et al.: Moderate renal impairment and risk of dementia among older adults: The Cardiovascular Health Cognition study. J Am Soc Nephrol 15: 1904–1911, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Hartung E, Erus G, Jawad A, Laney N, Doshi J, Hooper S, et al.: Brain magnetic resonance imaging findings in children and young adults with CKD. Am J Kidney Dis 72: 349–359, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Wen J, Qi R, Zhong J, Schoepf U, Varga-Szemes A, et al.: Re-establishing brain networks in patients with ESRD after successful kidney transplantation. Clin J Am Soc Nephrol 13: 109–117, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu N, Gross A, Shaffer A, Haugen C, Norman S, Xue Q-L, et al.: Frailty and changes in cognitive function after kidney transplantation. J Am Soc Nephrol 30: 336–345, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravera M, Bussalino E, Paoletti E, Bellasi A, Di Lullo L, Fusaro M: Haemorragic and thromboembolic risk in CKD patients with non valvular atrial fibrillation: Do we need a novel risk score calculator? Int J Cardiol 274: 179–185, 2019. [DOI] [PubMed] [Google Scholar]
- 15.Toyoda K, Ninomiya T: Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol 13: 823–833, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Micozkadioglu H, Ozelsancak R, Giray S, Arlier Z: CKD is associated with recurrent ischemia but not with hemorrhagic transformation in acute ischemic stroke patients. Ren Fail 36: 217–221, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Shima H, Ishimura E, Naganuma T, Ichii M, Yamasaki T, Mori K, et al.: Decreased kidney function is a significant factor associated with silent cerebral infarction and periventricular hyperintensities. Kidney Blood Press Res 34: 430–438, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Yao H, Takashima Y, Hashimoto M, Uchino A, Yuzuriha T: Subclinical cerebral abnormalities in chronic kidney disease. Contrib Nephrol 179: 24–34, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Lau W, Nunes A, Vasilevko V, Floriolli D, Lertpanit L, Savoj J, et al.: Chronic kidney disease increases cerebral microbleeds in mouse and man. Transl Stroke Res 11: 122–134, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polinder-Bos H, Elting J, Aries M, García D, Willemsen A, van Laar P, et al.: Changes in cerebral oxygenation and cerebral blood flow during hemodialysis - a simultaneous near-infrared spectroscopy and positron emission tomography study. J Cereb Blood Flow Metab 40: 328–340, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dou L, Sallée M, Cerini C, Poitevin S, Gondouin B, Jourde-Chiche N, et al.: The cardiovascular effect of the uremic solute indole-3 acetic acid. J Am Soc Nephrol 26: 876–887, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assem M, Lando M, Grissi M, Kamel S, Massy Z, Chillon J-M, et al.: The impact of uremic toxins on cerebrovascular and cognitive disorders. Toxins (Basel) 10: E303, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stinghen A, Pecoits-Filho R: Vascular damage in kidney disease: Beyond hypertension. Int J Hypertens 2011: 232683, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y-T, Wu P-H, Liang S-S, Mubanga M, Yang Y-H, Hsu Y-L, et al.: Protein-bound uremic toxins are associated with cognitive function among patients undergoing maintenance hemodialysis. Sci Rep 9: 20388, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gondouin B, Cerini C, Dou L, Sallée M, Duval-Sabatier A, Pletinck A, et al.: Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int 84: 733–744, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Dou L, Poitevin S, Sallée M, Addi T, Gondouin B, McKay N, et al.: Aryl hydrocarbon receptor is activated in patients and mice with chronic kidney disease. Kidney Int 93: 986–999, 2018. [DOI] [PubMed] [Google Scholar]
- 27.Kolachalama V, Shashar M, Alousi F, Shivanna S, Rijal K, Belghasem M, et al.: Uremic solute-aryl hydrocarbon receptor-tissue factor axis associates with thrombosis after vascular injury in humans. J Am Soc Nephrol 29: 1063–1072, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latchney S, Hein A, O’Banion M, DiCicco-Bloom E, Opanashuk L: Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory. J Neurochem 125: 430–445, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuartero M, Ballesteros I, de la Parra J, Harkin A, Abautret-Daly A, Sherwin E, et al.: L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation 130: 2040–2051, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Sweeney M, Zhao Z, Montagne A, Nelson A, Zlokovic B: Blood-brain barrier: From physiology to disease and back. Physiol Rev 99: 21–78, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nation D, Sweeney M, Montagne A, Sagare A, D’Orazio L, Pachicano M, et al.: Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25: 270–276, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt J, Su G, Reddy J, Simon M, Bradfield C: Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A 93: 6731–6736, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akiyama Y, Kikuchi K, Saigusa D, Suzuki T, Takeuchi Y, Mishima E, et al.: Indoxyl sulfate down-regulates SLCO4C1 transporter through up-regulation of GATA3. PLoS One 8: e66518, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velenosi T, Hennop A, Feere D, Tieu A, Kucey A, Kyriacou P, et al.: Untargeted plasma and tissue metabolomics in rats with chronic kidney disease given AST-120. Sci Rep 6: 22526, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffin K, Picken M, Bidani A: Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol 4: 2023–2031, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Flecknell P: Rodent analgesia: Assessment and therapeutics. Vet J 232: 70–77, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Sotocinal S, Sorge R, Zaloum A, Tuttle A, Martin L, Wieskopf J, et al.: The rat grimace scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain 7: 55, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calaf R, Cerini C, Génovésio C, Verhaeghe P, Jourde-Chiche N, Bergé-Lefranc D, et al.: Determination of uremic solutes in biological fluids of chronic kidney disease patients by HPLC assay. J Chromatogr B Analyt Technol Biomed Life Sci 879: 2281–2286, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, et al.: The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem 284: 2690–2696, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Wan X, Hou L-J, Zhang L-Y, Huang W-J, Liu L, Zhang Q, et al.: IKKα is involved in kidney recovery and regeneration of acute ischemia/reperfusion injury in mice through IL10-producing regulatory T cells. Dis Model Mech 8: 733–742, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S, Zhen G, Meloni B, Campbell K, Winn H: Rodent stroke model guidelines for preclinical stroke trials (1st edition). J Exp Stroke Transl Med 2: 2–27, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schallert T, Leasure J, Kolb B: Experience-associated structural events, subependymal cellular proliferative activity, and functional recovery after injury to the central nervous system. J Cereb Blood Flow Metab 20: 1513–1528, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Pellegrini L, Bennis Y, Velly L, Grandvuillemin I, Pisano P, Bruder N, et al.: Erythropoietin protects newborn rat against sevoflurane-induced neurotoxicity. Paediatr Anaesth 24: 749–759, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Bartolini L, Casamenti F, Pepeu G: Aniracetam restores object recognition impaired by age, scopolamine, and nucleus basalis lesions. Pharmacol Biochem Behav 53: 277–283, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Ennaceur A, Delacour J: A new one-trial test for neurobiological studies of memory in rats. 1. Behavioral data. Behav Brain Res 31: 47–59, 1988. [DOI] [PubMed] [Google Scholar]
- 46.Hattiangady B, Mishra V, Kodali M, Shuai B, Rao X, Shetty A: Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Front Behav Neurosci 8: 78, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coutellier L, Beraki S, Ardestani P, Saw N, Shamloo M: Npas4: A neuronal transcription factor with a key role in social and cognitive functions relevant to developmental disorders. PLoS One 7: e46604, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barker G, Warburton E: Object-in-place associative recognition memory depends on glutamate receptor neurotransmission within two defined hippocampal-cortical circuits: A critical role for AMPA and NMDA receptors in the hippocampus, perirhinal, and prefrontal cortices. Cereb Cortex 25: 472–481, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garrigue P, Giacomino L, Bucci C, Muzio V, Filannino M, Sabatier F, et al.: Single photon emission computed tomography imaging of cerebral blood flow, blood-brain barrier disruption, and apoptosis time course after focal cerebral ischemia in rats. Int J Stroke 11: 117–126, 2016. [DOI] [PubMed] [Google Scholar]
- 50.Lancelot S, Roche R, Slimen A, Bouillot C, Levigoureux E, Langlois J-B, et al.: A multi-atlas based method for automated anatomical rat brain MRI segmentation and extraction of PET activity. PLoS One 9: e109113, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujisaki K, Tsuruya K, Yamato M, Toyonaga J, Noguchi H, Nakano T, et al.: Cerebral oxidative stress induces spatial working memory dysfunction in uremic mice: Neuroprotective effect of tempol. Nephrol Dial Transplant 29: 529–538, 2014. [DOI] [PubMed] [Google Scholar]
- 52.Haruyama N, Fujisaki K, Yamato M, Eriguchi M, Noguchi H, Torisu K, et al.: Improvement in spatial memory dysfunction by telmisartan through reduction of brain angiotensin II and oxidative stress in experimental uremic mice. Life Sci 113: 55–59, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Mazumder M, Giri A, Kumar S, Borah A: A highly reproducible mice model of chronic kidney disease: Evidences of behavioural abnormalities and blood-brain barrier disruption. Life Sci 161: 27–36, 2016. [DOI] [PubMed] [Google Scholar]
- 54.Mazumder M, Paul R, Bhattacharya P, Borah A: Neurological sequel of chronic kidney disease: From diminished Acetylcholinesterase activity to mitochondrial dysfunctions, oxidative stress and inflammation in mice brain. Sci Rep 9: 3097, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montagne A, Barnes S, Sweeney M, Halliday M, Sagare A, Zhao Z, et al.: Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85: 296–302, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu M, Liang Y, Chigurupati S, Lathia J, Pletnikov M, Sun Z, et al.: Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol 19: 1360–1370, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adesso S, Magnus T, Cuzzocrea S, Campolo M, Rissiek B, Paciello O, et al.: Indoxyl sulfate affects glial function increasing oxidative stress and neuroinflammation in chronic kidney disease: Interaction between astrocytes and microglia. Front Pharmacol 8: 370, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato E, Saigusa D, Mishima E, Uchida T, Miura D, Morikawa-Ichinose T, et al.: Impact of the oral adsorbent AST-120 on organ-specific accumulation of uremic toxins: LC-MS/MS and MS imaging techniques. Toxins (Basel) 10: 19, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lagier D, Tonon D, Garrigue P, Guillet B, Giacomino L, Martin J-C, et al.: Thromboxane-prostaglandin receptor antagonist, terutroban, prevents neurovascular events after subarachnoid haemorrhage: A nanoSPECT study in rats. Crit Care 23: 42, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H: Expression cloning and characterization of a novel multispecific organic anion transporter. J Biol Chem 272: 18526–18529, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P: Vascular incompetence in dialysis patients—protein-bound uremic toxins and endothelial dysfunction. Semin Dial 24: 327–337, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Stinghen A, Chillon J-M, Massy Z, Boullier A: Differential effects of indoxyl sulfate and inorganic phosphate in a murine cerebral endothelial cell line (bEnd.3). Toxins (Basel) 6: 1742–1760, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, et al.: The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost 5: 1302–1308, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Watanabe I, Tatebe J, Namba S, Koizumi M, Yamazaki J, Morita T: Activation of aryl hydrocarbon receptor mediates indoxyl sulfate-induced monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells. Circ J 77: 224–230, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Adesso S, Paterniti I, Cuzzocrea S, Fujioka M, Autore G, Magnus T, et al.: AST-120 reduces neuroinflammation induced by indoxyl sulfate in glial cells. J Clin Med 7: 365, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adelibieke Y, Yisireyili M, Ng H-Y, Saito S, Nishijima F, Niwa T: Indoxyl sulfate induces IL-6 expression in vascular endothelial and smooth muscle cells through OAT3-mediated uptake and activation of AhR/NF-κB pathway. Nephron, Exp Nephrol 128: 1–8, 2014. [DOI] [PubMed] [Google Scholar]
- 67.Kawedia J, Jiang M, Kulkarni A, Waechter H, Matlin K, Pauletti G, et al.: The protein kinase A pathway contributes to Hg2+-induced alterations in phosphorylation and subcellular distribution of occludin associated with increased tight junction permeability of salivary epithelial cell monolayers. J Pharmacol Exp Ther 326: 829–837, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maciel R, Cunha R, Busato V, Franco C, Gregório P, Dolenga C, et al.: Uremia impacts VE-cadherin and ZO-1 expression in human endothelial cell-to-cell junctions. Toxins (Basel) 10: 404, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diwan V, Brown L, Gobe G: Adenine-induced chronic kidney disease in rats. Nephrology (Carlton) 23: 5–11, 2018. [DOI] [PubMed] [Google Scholar]
- 70.Diwan V, Mistry A, Gobe G, Brown L: Adenine-induced chronic kidney and cardiovascular damage in rats. J Pharmacol Toxicol Methods 68: 197–207, 2013. [DOI] [PubMed] [Google Scholar]
- 71.D’Hooge R, Pei Y, Marescau B, De Deyn P: Convulsive action and toxicity of uremic guanidino compounds: Behavioral assessment and relation to brain concentration in adult mice. J Neurol Sci 112: 96–105, 1992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.