Significance Statement
Information about the type of health care costs associated with CKD across all stages in a general population with a substantial comorbidity burden is lacking. Using electronic medical records of an integrated delivery system, the authors evaluated health care costs in patients with CKD, with or without diabetes mellitus, cardiovascular disease, and heart failure, and by eGFR level. Despite high use of currently available therapies, inpatient costs were an increasing proportion of the total health care costs with each higher eGFR category, regardless of the presence of comorbidities. This trend began in earlier stages of CKD and escalated as kidney function declined. Additional therapies to reduce CKD incidence, slow CKD progression, and lower the risk of hospitalizations are needed to benefit patients and reduce the economic burden of CKD.
Keywords: chronic kidney disease, Economic Impact, Epidemiology and outcomes
Abstract
Background
CKD is associated with higher health care costs that increase with disease progression. However, research is lacking on the type of health care costs associated with CKD across all stages in a general population with a substantial comorbidity burden.
Methods
Using electronic medical records of an integrated delivery system, we evaluated health care costs by expenditure type in general and in patients with CKD by eGFR and presence of comorbidities. We categorized 146,132 patients with eGFR data in 2016 or 2017 and examined nonmutually exclusive groups according to presence of diabetes mellitus, cardiovascular disease, or heart failure. We used 1 year of follow-up data to calculate outpatient, inpatient, emergency, pharmaceutical, dialysis, and total health care costs by eGFR (Kidney Disease Improving Global Outcomes–defined eGFR categories), adjusted for age, sex, and nonwhite race.
Results
Mean total health care costs among patients with CKD without comorbidities were 31% higher than among patients without CKD ($7374 versus $5631, respectively). Hospitalizations accounted for 35% of total costs among those with CKD and no comorbidities but up to 55% among patients with CKD and heart failure. The proportion of costs attributable to hospitalizations accelerated with declining kidney function, reaching as high as 66%.
Conclusions
Poorer kidney function and the presence of diabetes mellitus, cardiovascular disease, or heart failure drive substantial health care costs and increase the proportion of costs attributable to inpatient care. The large contribution of inpatient costs begins in earlier stages of CKD and escalates as kidney function declines. Additional therapies to reduce CKD incidence, slow CKD progression, and lower hospitalization risk are needed to benefit patients and reduce CKD’s economic burden.
An estimated 15% of the United States adult population has CKD,1 a prevalence that can be expected to grow along with the obesity-driven diabetes epidemic and an aging population.2 Patients with CKD often have other comorbid diseases, including cardiovascular disease (CVD),3 diabetes mellitus (DM),4 and heart failure (HF).5 Although the high health care costs of CKD are often attributed to RRTs and ESKD, prior studies suggest excess costs begin to accrue at earlier stages of CKD. For example, according to the 2018 Annual Data Report of the US Renal Data System (USRDS), Medicare expenditures for beneficiaries with ESKD amounted to $35 billion in 2016, whereas spending for patients with CKD exceeded $79 billion with progressively higher costs at more severe CKD stages.6 One recent study also used Medicare data to report progressively higher costs across stages,7 and another study found higher costs of care across more severe CKD stages among commercially insured versus Medicare patients receiving a renin-angiotensin-aldosterone system inhibitor prescription,8 suggesting that studies only on the basis of Medicare data may underestimate actual costs for patients with CKD.
Research on the type of health care costs associated with CKD across all stages from a general population with substantial comorbidity burden, especially regarding DM, CVD, and HF, is lacking. A better understanding of health care costs associated with CKD and how they are allocated is needed to optimize patient care.
Methods
Study subjects were members of Kaiser Permanente Northwest (KPNW), an integrated delivery system serving approximately 550,000 individuals in a 100-mile radius around Portland, Oregon. In its provision of medical care, KPNW uses comprehensive electronic medical records (EMRs) that include encounter diagnoses, laboratory tests and results, and pharmaceutical dispenses. As a result of enrolling people whose insurance is self-paid, provided through employment, or provided by Medicare or Medicaid, KPNW membership is representative of the service area.9 The KPNW Institutional Review Board approved the study with a waiver of informed consent.
For this study, we identified all adult members aged 18 years and older who had a serum creatinine value recorded in 2016 or 2017 (n=174,303). Because hepatitis C, HIV/AIDS, and cancers (other than of the kidney, bladder, or urinary tract) generate very large costs that are unrelated to CKD, CVD, HF, or DM, we excluded 28,171 patients with these conditions to avoid cost distortions. For the remaining 146,132 patients, we used the first available serum creatinine value in the study period to calculate eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation and defined the date of this value as the index date.10 Values <60 ml/min per 1.73 m2 were confirmed with a second value 3–12 months later. We categorized these values using the Kidney Disease Improving Global Outcomes categories (G1, normal or high: ≥90 ml/min per 1.73 m2; G2, mildly decreased: 60–89 ml/min per 1.73 m2; G3a, mildly to moderately decreased: 45–59 ml/min per 1.73 m2; G3b, moderately to severely decreased: 30–44 ml/min per 1.73 m2; G4, severely decreased: 15–29 ml/min per 1.73 m2; and G5, kidney failure: <15 ml/min per 1.73 m2 or diagnosis of ESKD with dialysis or transplant).11 Because albuminuria data were rarely available for patients without DM, we do not include any patients from stage G2 in our definition of CKD.
From EMR diagnoses entered any time prior to the index date, we identified patients with DM (International Classification of Diseases, 10th Revision [ICD-10] codes E11–E13), CVD (I21, I22, I25, G45, G46, I63, and I65–I67), or HF (I50) and analyzed nine groups: (1) all patients; (2) no CKD; (3) all CKD (with or without DM, CVD, or HF); (4) CKD only (without DM, CVD, or HF); (5) CKD and DM; (6) CKD and CVD; (7) CKD, DM, and CVD; (8) CKD and HF; and (9) CKD, DM, and HF. Groups were not mutually exclusive, and therefore, patients could fall into more than one group.
The main outcomes were annualized inpatient, outpatient, emergency, pharmacy, dialysis, and total health care costs for each group overall and by eGFR stage (G1–G5) during the 1-year period following the index date adjusted for age, sex, and race/ethnicity. We calculated annual utilization and costs on a per member per month basis. To annualize these data when <12 full months of data were available due to death or other disenrollment, we divided a patient’s summed utilization and costs by number of months of eligibility and then multiplied by 12. The cost estimates are then weighted by number of months of eligibility to mitigate the effect of high end-of-life costs. Our costing method was developed and validated for research and risk-adjustment purposes by the KPNW Center for Health Research,12 and it has been recently described in detail elsewhere.13 Briefly, this costing algorithm assigns an average cost per unit of service on the basis of general ledger information. For outpatient costs, this method creates standard costs for office visits by specialty/department and type of provider (MD versus Physician Assistant/Nurse Practitioner). The number of visits per department per clinician type is multiplied by the appropriate unit cost. Pharmaceutical costs were on the basis of retail prices within the respective service areas. Hospitalization costs were calculated by multiplying the average daily cost per assigned diagnosis-related groups by the length of stay. Costs for medical services incurred at facilities not owned by KPNW were on the basis of the amount paid to the nonplan provider. All costs are reported in 2019 United States dollars.
It is well known that comorbidities increase costs, and therefore, our focus was on how costs are distributed. Because our analysis groups were not mutually exclusive, each group was analyzed independently; therefore, between-group statistical tests were not performed, but 95% confidence intervals (95% CIs) are presented. We defined directly attributable CKD-related costs as inpatient, outpatient, or emergency encounters with a primary diagnosis of CKD (ICD-10 N17–N19). CKD-related pharmaceutical costs included dispenses for renin-angiotensin-aldosterone system inhibitors, sodium glucose cotransporter-2 inhibitors, phosphate binders, calcimimetics, erythropoiesis-stimulating agents, vitamin D analogs, and iron supplements. We further evaluated inpatient costs by examining the primary discharge diagnoses to determine the most frequent reasons for admissions.
Health care costs are rarely normally distributed. Although log transformation can be used to normalize the data, sample means perform equally well and are unlikely to lead to inappropriate conclusions.14 Furthermore, modeling log costs results in more statistical significance than modeling mean costs, and therefore, results from ordinary least squares models are conservative. Therefore, for ease of interpretation, we used generalized linear models to adjust mean costs by age, sex, and nonwhite race/ethnicity with the LSMEANS option in PROC GLM (SAS version 9.4; SAS Institute, Cary, NC). Because the groups are not mutually exclusive, an independent model is constructed for each comorbidity group. Thus, the results of the models do not fully account for the differing distribution of the adjustors.
Results
Of the total 146,132 patients, 14.5% (n=21,252) had CKD (all CKD), and of those, approximately half (48.8%; n=10,380) had CKD without CVD, DM, or HF (CKD only) (Table 1). Mean age of all patients was 59.4±14.3 years, and 55% were women; mean age of patients with CKD was 73.3±11.2 years, and 60.5% were women. Mean follow-up time was 11.3±2.2 months.
Table 1.
Characteristics of all patients and by presence of CKD with and without DM, CVD, and HF
| Characteristic | Analysis Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All Patients | No CKD | All CKD | CKD Only | CKD and DM | CKD and CVD | CKD, DM, and CVD | CKD and HF | CKD, DM, and HF | |
| Sample, n | 146,132 | 124,880 | 21,252 | 10,380 | 7625 | 4708 | 2280 | 3338 | 2386 |
| Sample, % | 100 | 85.5 | 14.5 | 7.1 | 5.2 | 3.2 | 1.6 | 2.3 | 1.6 |
| CKD, % | — | 0 | 100.0 | 48.8 | 35.9 | 22.2 | 10.7 | 15.7 | 11.2 |
| Age, yr | 59.4 (14.3) | 57.0 (13.4) | 73.3 (11.2) | 71.8 (11.6) | 72.8 (10.3) | 77.0 (10) | 75.2 (9.7) | 77.9 (11.0) | 75.4 (10.5) |
| Women, % | 55.0 | 54.1 | 60.5 | 65.7 | 56.0 | 48.0 | 46.5 | 57.2 | 53.8 |
| Hispanic, % | 6.0 | 6.6 | 2.9 | 2.4 | 4.0 | 2.8 | 3.7 | 3.0 | 3.7 |
| Non-Hispanic black, % | 1.1 | 1.1 | 1.4 | 1.1 | 2.0 | 1.4 | 1.6 | 1.5 | 1.6 |
| Current smoker, % | 9.2 | 9.9 | 4.9 | 5.3 | 4.3 | 4.6 | 3.7 | 3.9 | 3.7 |
| Systolic BP, mm Hg | 129 (14) | 129 (14) | 133 (14) | 133 (14) | 134 (14) | 132 (15) | 132 (15) | 129 (15) | 130 (15) |
| Diastolic BP, mm Hg | 74 (10) | 74 (10) | 71 (9) | 73 (9) | 69 (9) | 68 (9) | 67 (8) | 67 (9) | 66 (9) |
| Body mass index, kg/m2 | 30.9 (7.4) | 30.9 (7.4) | 31.0 (7.4) | 29.9 (6.7) | 33.3 (7.9) | 30.2 (7.0) | 32.1 (7.4) | 31.5 (8.5) | 33.9 (8.8) |
| LDL cholesterol, mg/dl | 114 (37) | 116 (37) | 102 (38) | 115 (37) | 88 (34) | 88 (35) | 84 (32) | 87 (34) | 81 (31) |
| HDL cholesterol, mg/dl | 53 (18) | 53 (18) | 51 (17) | 55 (17) | 45 (14) | 46 (15) | 42 (13) | 46 (16) | 41 (13) |
| Triglycerides, mg/dl | 165 (129) | 164 (130) | 174 (120) | 158 (95) | 203 (145) | 179 (148) | 205 (175) | 170 (124) | 192 (146) |
| eGFR, ml/min per 1.73 m2 | 82 (20) | 88 (15) | 49 (12) | 52 (10) | 47 (13) | 46 (13) | 44 (14) | 44 (13) | 42 (14) |
| Hemoglobin, g/dl | 13.7 (1.5) | 13.8 (1.4) | 12.9 (1.7) | 13.3 (1.5) | 12.5 (1.7) | 12.6 (1.7) | 12.2 (1.6) | 12.3 (1.7) | 12.0 (1.7) |
| ACEi/ARB, % | 39.3 | 28.9 | 60.9 | 48.9 | 76.5 | 69.4 | 74.6 | 64.4 | 67.9 |
| β-Blockers, % | 23.6 | 15.2 | 49.2 | 33.9 | 59.1 | 77.7 | 81.7 | 83.4 | 85.0 |
| Statins, % | 37.8 | 27.2 | 62.5 | 44.8 | 82.0 | 84.9 | 88.7 | 76.5 | 86.9 |
| Diabetes, % | 21.3 | 18.8 | 35.9 | 0.0 | 100.0 | 48.4 | 100.0 | 51.5 | 100.0 |
| CVD, % | 9.1 | 6.9 | 22.2 | 0.0 | 29.9 | 100.0 | 100.0 | 55.5 | 61.4 |
| HF, % | 4.3 | 2.3 | 15.7 | 0.0 | 22.5 | 39.3 | 46.1 | 100.0 | 100.0 |
| ESKD, % | 0.7 | 0.0 | 4.7 | 2.9 | 7.5 | 6.8 | 10.3 | 7.5 | 11.2 |
| Months of follow-up | 11.3 (2.2) | 11.2 (2.1) | 11.2 (2.5) | 11.5 (1.9) | 11.0 (2.7) | 10.5 (3.3) | 10.4 (3.5) | 9.8 (4.0) | 9.8 (4.0) |
| 12 mo of follow-up, % | 90.0 | 90.2 | 88.8 | 92.9 | 87.0 | 80.5 | 79.2 | 72.1 | 73.0 |
Groups are not mutually exclusive. ACEi, angiotensin-converting-enzyme inhibitors; ARB, angiotension receptor blockers.
Among patients with CKD, the distribution of eGFR categories was markedly different depending on comorbidity burden (Figure 1). When none were present, 76.0% of patients were stage G3a versus about 55% in those with DM or CVD and just 37.8% among patients with CKD with DM and HF. Correspondingly, 16.6% of the patients in this latter group were stage G4, and 11.2% were stage G5, whereas the percentages of patients with CKD without comorbidities in stages G4 and G5 were 2.5% and 2.9%, respectively.
Figure 1.

Distribution of Kidney Disease Improving Global Outcomes eGFR categories were markedly different for patients with and without CKD and patients with CKD and DM, CVD, or HF. The comorbidity groups are not mutually exclusive.
Mean total annualized health care costs were about 31% higher among patients with CKD only ($7334; 95% CI, $6746 to $8003) than among patients without CKD ($5631; 95% CI, $5497 to $5768) (Figure 2, Supplemental Table 1). In the presence of DM, CVD, or HF, health care costs of all types were substantially greater. Inpatient costs accounted for roughly one third of total costs, representing 30% of total costs in the absence of CKD and 35% among those with CKD and no comorbidities. However, when DM, CVD, or HF was present, inpatient costs ranged from 44% (CKD and DM) to 55% (CKD and HF) of total costs. Interestingly, costs were somewhat lower for patients age 65 years and older in the presence of CKD with or without other comorbidities. Costs of all types that could be directly attributed to care for nondialysis CKD represented a relatively small proportion of the total. Most of the directly attributable costs were for dialysis.
Figure 2.
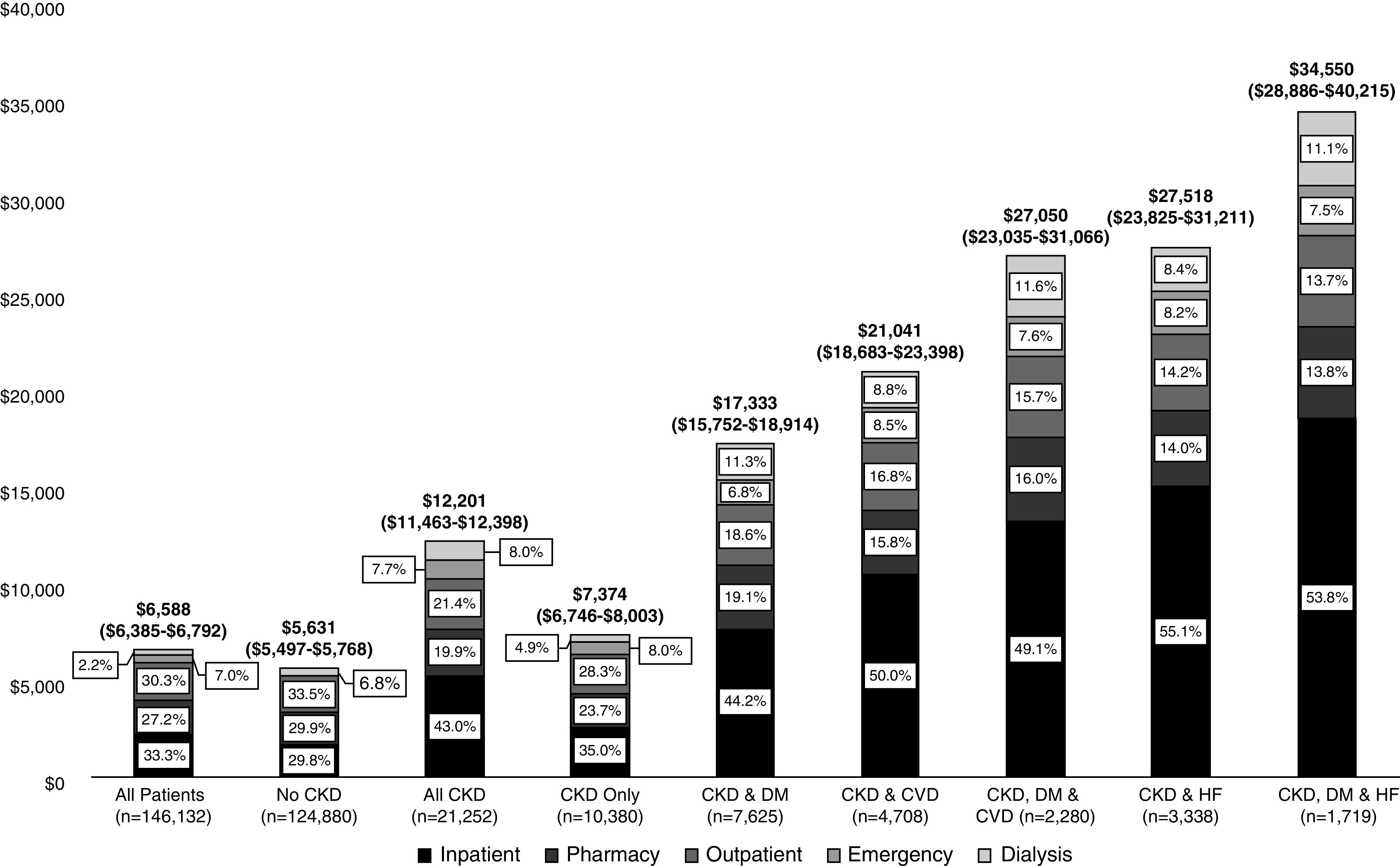
Annualized mean medical costs in total (95% CIs) and by resource adjusted for age, sex, and race/ethnicity were higher for patients with versus without CKD and much greater for patients with CKD and DM, CVD, or HF. The comorbidity groups are not mutually exclusive.
Health care costs by resource and by eGFR category are displayed in Figure 3 for patients with CKD only (Figure 3A), patients with CKD and DM (Figure 3B), patients with CKD and CVD (Figure 3C), and patients with CKD and HF (Figure 3D). The ordering reflects the relative costs, with patients in Figure 3A incurring the lowest costs and those in Figure 3D incurring the highest costs, a pattern that was consistent across eGFR categories. Costs for patients in category G3a were lowest in Figure 3A and highest in Figure 3D, as were costs in categories G3b, G4, and G5. Inpatient admissions were the largest contributor to total costs even in the early stages of CKD, ranging from 35% to 56% in eGFR category G3a. The proportion of costs attributable to inpatient admissions increased exponentially with declining kidney function, reaching from 40% to 65% in G4 until category G5 when the high costs of dialysis manifested, declining to 23%–37%. However, absolute costs of inpatient care were equal to or greater than in G5 than in G4 (Supplemental Table 2).
Figure 3.

Annualized mean medical costs in total (95% CIs) and by resource adjusted for age, sex, and race/ethnicity for patients with CKD and (A) no comorbidities, (B) DM, (C) CVD, and (D) HF increased dramatically with declining kidney function. The comorbidity groups are not mutually exclusive.
The two most common reasons for hospitalization in all groups were conditions related to CVD (primary discharge diagnosis of ischemic heart disease or cerebrovascular disease) and bacterial, viral, or respiratory infections (Table 2). Among patients with comorbidities, 13%–22% of all admissions had a CVD-related primary discharge diagnosis, and 11%–21% had a primary discharge diagnosis of bacterial, viral, or respiratory infection.
Table 2.
Proportion of inpatient admissions that were due to CVD-related conditions or infections among patients with CKD by eGFR category with or without DM, CVD, or HF
| Analysis Group, Primary Reason for Hospitalization | eGFR Categorya | |||
|---|---|---|---|---|
| G3a, % | G3b, % | G4, % | G5, % | |
| All CKD | ||||
| CVD related | 18.2 | 15.4 | 15.0 | 14.9 |
| Infections | 14.2 | 18.4 | 12.2 | 17.6 |
| CKD Only | ||||
| CVD related | 16.7 | 14.1 | 22.2 | 10.3 |
| Infections | 12.0 | 18.5 | 11.1 | 15.4 |
| CKD and DM | ||||
| CVD related | 17.7 | 15.8 | 13.4 | 15.5 |
| Infections | 15.2 | 17.5 | 12.1 | 18.6 |
| CKD and CVD | ||||
| CVD related | 21.9 | 19.8 | 15.4 | 16.6 |
| Infections | 15.1 | 16.7 | 11.2 | 15.3 |
| CKD and DM and CVD | ||||
| CVD related | 22.1 | 21.2 | 13.8 | 16.1 |
| Infections | 14.9 | 14.6 | 10.9 | 16.5 |
| CKD and HF | ||||
| CVD related | 16.4 | 13.8 | 14.3 | 14.2 |
| Infections | 15.7 | 19.0 | 10.9 | 20.0 |
| CKD and DM and HF | ||||
| CVD related | 15.5 | 13.8 | 13.0 | 13.3 |
| Infections | 14.2 | 18.5 | 10.5 | 21.1 |
Groups are not mutually exclusive.
CVD-related conditions include ischemic heart disease and cerebrovascular disease. Infections include bacterial, viral, and respiratory infections.
Discussion
In this analysis of the annual health care costs of 146,880 adults in an integrated delivery system, of which 21,252 had prevalent CKD, we found that inpatient costs accounted for the predominant portion of total health care costs regardless of comorbidity burden or predialysis eGFR category. In absolute terms, inpatient costs were an increasing proportion of the total health care cost with each higher eGFR category, including G5, regardless of comorbidity group. As expected, consistent patterns of increasing total health care costs by progressing stages of CKD and by increasing comorbidity burden were observed. Not surprisingly, prevalence of DM, CVD, and HF was associated with substantially increased health care costs, but the relative increase in health care costs across stages of CKD regardless of the presence of one or more of these comorbidities was remarkably similar with a consistent pattern of increase in total costs of care at higher (more severe) stages of CKD.
According to the Centers for Disease Control and Prevention, hospital costs account for approximately one third of total costs in the United States.15 This is consistent with our data, where inpatient costs accounted for 33.2% of total costs for all patients, suggesting that the structure of costs by resource we observed in our data is representative. However, we found that hospital costs contributed substantially >33% at progressively higher eGFR categories and when comorbidities were present, ranging from 43.5% among patients with DM in category G3a to 66% of the total for category G4 patients with DM and HF. A previous EMR-based study found similar proportions by CKD stage.8 It is thus apparent that reducing costs of care for patients with CKD, even among those with early stages of CKD, would be best achieved by avoiding hospitalizations and/or further progression of kidney disease. Moreover, because CVD-related hospitalizations were the single most common reason for admission, accounting for 11%–22% among patients with CKD and comorbidities, emphasizing strategies that minimize cardiovascular events is warranted.
The USRDS publishes an annual report that includes health care expenditures for Medicare patients by CKD stage.6 Overall, the costs we report are lower, but there are important differences in methodology. As noted, the USRDS uses only Medicare data, whereas our study includes adults of all ages. More importantly, the USRDS defines CKD and eGFR categories using International Classification of Diseases, 9th Revision, Clinical Modification and International Classification of Diseases, 10th Revision, Clinical Modification diagnoses. Studies have consistently shown diagnosis codes to be insensitive markers of CKD when compared with eGFR values16,17 and especially poor for identifying or comparing early CKD stages.18,19 We speculate that the use of diagnosis codes by the USRDS identifies sicker patients with more advanced CKD, which could explain the higher costs in their analyses as well as the lower relative differences between stages. Although our overall costs were lower than a recent EMR-based study, the relative increase in costs across stages was remarkably similar, suggesting that our results accurately portray the relationship between medical costs and eGFR categories.8 For example, we found that costs were 1.9 times greater in eGFR category G3b and 3.7 times greater in category G4 compared without CKD (G1 and G2). The comparable ratios in Golestaneh et al.8 were 1.9 and 3.2, respectively, among patients ≥65 years old. Consistent with that study, we found that costs for elderly patients were lower than for those under 65 years old. This was primarily due to lower average (across group) pharmaceutical costs and dialysis costs, probably because elderly patients were less likely to pursue aggressive treatments for health conditions, including declining kidney function. Slowing the progression of CKD especially among younger patients could, therefore, lead to substantial cost savings.
Patients with CKD often have DM, CVD, or HF, all of which are known to be responsible for enormous medical costs.20–22 CKD with or without these conditions is also known to be associated with higher health care costs,6,23 but to our knowledge, there are currently no studies of the type of costs associated with DM, CVD, and HF in conjunction with CKD by eGFR category evaluated simultaneously in the same setting. Our analyses contribute important data about the relative costs of CKD with and without these conditions and allow for comparisons by comorbid disease states. HF (with or without DM and CVD) was particularly expensive, more than quadrupling health care costs relative to patients with CKD without any of these conditions, but both DM and CVD more than doubled costs (2.35 and 2.85 times greater, respectively). We cannot determine the temporal order in which the comorbidities we examined occurred from this analysis, and it is unclear whether effective treatment of CKD would affect the costs associated with the comorbidities. Furthermore, because analysis groups were assigned at baseline and costs were assessed over the ensuing 12 months, it is possible that some of those costs could be attributed to the development of new comorbidities or to progression of kidney disease. In any case, treatment of CKD clearly requires a multidisciplinary approach. We note that costs among patients without comorbidities and mild CKD (G3A) are only modestly elevated compared with patients without CKD. Prevention of CKD progression and development of comorbidities could supply substantial long-term cost savings, whereas costs at later CKD stages or after comorbidities develop might best be minimized with effective therapies that minimize hospitalizations.
The current data precede the President’s Executive Order—Advancing American Kidney Health (AAKH).24 The high-level goals of the AAKH include reducing the number of Americans developing ESKD, an increase in the number on home dialysis, and doubling the number of organs available for transplantation. Although reducing ESKD will certainly lower costs, other programs to achieve the AAKH objectives, such as increasing dialysis and transplants, could lead to higher costs. Thus, our results may provide important baseline information for assessing the effect of the AAKH initiative.
Strengths of our study include the ability to capture complete medical utilization, and therefore overall health care costs and the structure of these costs, with sufficient sample size to examine costs by categories of eGFR among patients with and without comorbidities. We assigned categories of eGFR using laboratory values collected during the course of actual clinical practice. Although we confirmed low eGFR values with a second recorded measure, these often occurred at irregular intervals, which could have led to some misclassification. In addition, to ensure we began accumulating costs at the first recognized manifestation of CKD, we used the first eGFR<60 ml/min per 1.73 m2 to assign stages. We acknowledge that this may have resulted in misclassification but not in a systematic way. That is, patients with a first value in stage G3a and a second value in G4 would be “underclassified” (as G3a), whereas patients with a first value in G4 and a second in G3a would be “overclassified” (as G4). We relied on EMR diagnoses to assign comorbidity status, which may be subject to misclassification. However, our data represent the working medical record used by practicing clinicians who are authorized to both add and remove diagnoses to ensure accuracy.
We used data from a single integrated health system that uses population and patient management systems that aggressively manage BP with outreach programs to increase renin-angiotensin-aldosterone system blockade use, especially among high-risk patients. These along with other cost controls and resource allocations may differ from other settings and may, therefore, limit generalizability. In addition, because our costing methods assign average unit costs to an encounter, the cost estimates do not fully capture the difference in intensity of encounters. Comparing absolute costs should be done with caution. Nevertheless, because the relative cost differences between eGFR categories and comorbidity states reflect cost allocations within the system, they are likely to generalize to other United States systems and settings. Furthermore, because each comorbidity group was analyzed (and adjusted) independently, the results do not completely account for the differing distributions of the adjustors between the categories. However, there is substantial overlap of patients in the groups, thus minimizing the effect of our approach.
In conclusion, we found that total costs of care increased substantially with worsening categories of eGFR. Inpatient care and more specifically, CVD-related admissions were responsible for the dominant proportion of costs at all CKD stages. Although comorbidities were associated with still higher costs, the patterns across eGFR categories were consistent regardless of the presence of DM, CVD, or HF. Given the significant economic burden evident in all CKD stages, development and implementation of effective measures to avoid further progression of CKD and to reduce the need for inpatient care are critical to cost-reduction strategies.
Disclosures
K. Brodovicz is an employee of Boehringer Ingelheim. All remaining authors have nothing to disclose.
Funding
A. Déruaz-Luyet and A. Ustyugova report other from Boehringer Ingelheim International GmbH during the conduct of the study. G. Nichols reports grants from Boehringer Ingelheim during the conduct of the study.
Supplementary Material
Acknowledgments
G. Nichols had full access to the data, designed the study, guided the analyses, and drafted the manuscript; M. O’Keeffe-Rosetti conducted the economic analyses; K. Brodovicz, A. Déruaz-Luyet, and A. Ustyugova participated in the design of the study and provided comments and edits to the draft; and K. Brodovicz, A. Déruaz-Luyet, G. Nichols, M. O’Keeffe-Rosetti, and A. Ustyugova approved the final submission. A. Déruaz-Luyet reports other from Boehringer Ingelheim International GmbH outside the submitted work. G. Nichols reports grants from Bristol-Myers Squibb, grants from Merck and Co., grants from Janssen Pharmaceutical Affairs, and grants from Amarin Corp outside the submitted work. A. Ustyugova reports other from Boehringer Ingelheim GmbH outside the submitted work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019121308/-/DCSupplemental.
Supplemental Table 1. Total annualized health care costs (95% confidence interval) and CKD-related costs by resource for all analysis groups in total and among patients age 65 years and older adjusted for age, sex, and race/ethnicity.
Supplemental Table 2. Annualized health care costs in total (95% confidence intervals) and by eGFR category and resource adjusted for age, sex, and race/ethnicity among patients with CKD only; patients with CKD and diabetes mellitus; patients with CKD and cardiovascular disease; patients with CKD, diabetes mellitus, and cardiovascular disease; patients with CKD and heart failure; and patients with CKD, diabetes mellitus, and heart failure.
References
- 1.Centers of Disease Control and Prevention: Chronic kidney disease in the United States, 2019, 2019. Available at: www.cdc.gov/kidneydisease/pdf/2019_National-Chronic-Kidney-Disease-Fact-Sheet.pdf. Accessed April 9, 2019
- 2.Anderson S, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kaysen GA, et al.; workshop participants: Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol 20: 1199–1209, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al.: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Alicic RZ, Rooney MT, Tuttle KR: Diabetic kidney disease: Challenges, progress, and possibilities. Clin J Am Soc Nephrol 12: 2032–2045, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuegel C, Bansal N: Heart failure in patients with kidney disease. Heart 103: 1848–1853, 2017. [DOI] [PubMed] [Google Scholar]
- 6.US Renal Data System : US Renal Data System 2018 Annual Data Report Epidemiology of Kidney Disease in the United States: Chapter 7: Healthcare expenditures for persons with CKD. Am J Kidney Dis 73[Suppl 1]: S133–S158, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D: Medical costs of CKD in the Medicare population. J Am Soc Nephrol 24: 1478–1483, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golestaneh L, Alvarez PJ, Reaven NL, Funk SE, McGaughey KJ, Romero A, et al.: All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care 23[Suppl]: S163–S172, 2017. [PubMed] [Google Scholar]
- 9.Sukumaran L, McCarthy NL, Li R, Weintraub ES, Jacobsen SJ, Hambidge SJ, et al.: Demographic characteristics of members of the Vaccine Safety Datalink (VSD): A comparison with the United States population. Vaccine 33: 4446–4450, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Kidney Foundation : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Chapter 1: Definition and classification of CKD. Kidney Int Suppl 3: 19–62, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornbrook MC, Goodman MJ, Fishman PA, Meenan RT, O’Keeffe-Rosetti M, Bachman DJ: Building health plan databases to risk adjust outcomes and payments. Int J Qual Health Care 10: 531–538, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Nichols GA, Philip S, Reynolds K, Granowitz CB, O’Keeffe-Rosetti M, Fazio S: Comparison of medical care utilization and costs among patients with statin-controlled low-density lipoprotein cholesterol with versus without hypertriglyceridemia. Am J Cardiol 122: 1128–1132, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Briggs A, Nixon R, Dixon S, Thompson S: Parametric modelling of cost data: Some simulation evidence. Health Econ 14: 421–428, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Centers of Disease Control and Prevention : National Center for Health Statistics, FastStats, Health Expenditures, 2019. Available at: https://www.cdc.gov/nchs/fastats/health-expenditures.htm. Accessed November 12, 2019
- 16.Kern EF, Maney M, Miller DR, Tseng CL, Tiwari A, Rajan M, et al.: Failure of ICD-9-CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res 41: 564–580, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronksley PE, Tonelli M, Quan H, Manns BJ, James MT, Clement FM, et al.; Alberta Kidney Disease Network: Validating a case definition for chronic kidney disease using administrative data. Nephrol Dial Transplant 27: 1826–1831, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Winkelmayer WC, Schneeweiss S, Mogun H, Patrick AR, Avorn J, Solomon DH: Identification of individuals with CKD from Medicare claims data: A validation study. Am J Kidney Dis 46: 225–232, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Cipparone CW, Withiam-Leitch M, Kimminau KS, Fox CH, Singh R, Kahn L: Inaccuracy of ICD-9 codes for chronic kidney disease: A study from two practice-based research networks (PBRNs). J Am Board Fam Med 28: 678–682, 2015. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association : Economic costs of diabetes in the U.S. in 2017. Diabetes Care 41: 917–928, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee: Heart disease and stroke statistics-2019 update: A report from the American Heart Association [published correction appears in Circulation 141: e33, 2020]. Circulation 139: e56–e528, 2019. [DOI] [PubMed] [Google Scholar]
- 22.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al.; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council: Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ Heart Fail 6: 606–619, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith DH, Gullion CM, Nichols G, Keith DS, Brown JB: Cost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO population. J Am Soc Nephrol 15: 1300–1306, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Knight R: A patient’s perspective on advancing American kidney health initiative. Clin J Am Soc Nephrol 14: 1795–1797, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


