Significance Statement
Immunosuppression and a high prevalence of comorbidities among patients with ESKD on dialysis raise concerns that such patients may have an elevated risk of severe coronavirus disease 2019 (COVID-19). However, the outcomes for COVID-19 in this patient population are not clear. In their study of 59 patients with ESKD and COVID-19 receiving dialysis at a New York City medical center, the authors found that although the presentation of patients on dialysis with COVID-19 was similar to that of the general population, these patients have poor outcomes, including 31% overall mortality and 75% mortality among those requiring mechanical ventilation. In addition, higher levels of inflammatory markers associated with severe disease. This information will help inform care of patients on dialysis who develop COVID-19 and reinforces the importance of infection control measures when treating this vulnerable population.
Keywords: coronavirus, dialysis, Epidemiology and outcomes, ESRD, COVID-19
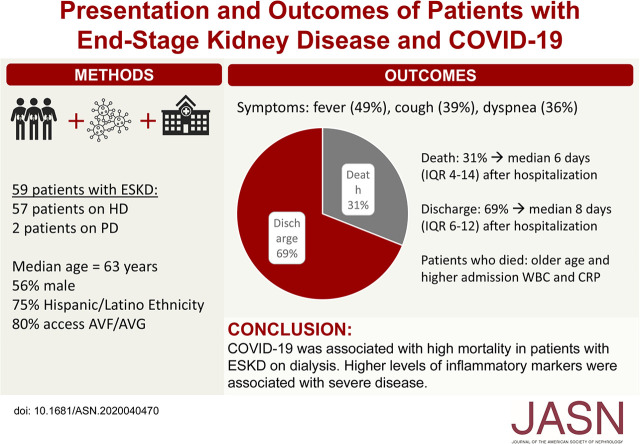
Visual Abstract
Abstract
Background
The relative immunosuppression and high prevalence of comorbidities in patients with ESKD on dialysis raise concerns that they may have an elevated risk of severe coronavirus disease 2019 (COVID-19), but outcomes for COVID-19 in such patients are unclear.
Methods
To examine presentation and outcomes of COVID-19 in patients with ESKD on dialysis, we retrospectively collected clinical data on 59 patients on dialysis who were hospitalized with COVID-19. We used Wilcoxon rank sum and Fischer exact tests to compare patients who died versus those still living.
Results
Two of the study’s 59 patients were on peritoneal dialysis, and 57 were on hemodialysis. Median age was 63 years, with high prevalence of hypertension (98%) and diabetes (69%). Patients who died were significantly older than those still living (median age, 75 versus 62 years) and had a higher median Charlson comorbidity index (8 versus 7). The most common presenting symptoms were fever (49%) and cough (39%); initial radiographs most commonly showed multifocal or bilateral opacities (59%). By end of follow-up, 18 patients (31%) died a median 6 days after hospitalization, including 75% of patients who required mechanical ventilation. Eleven of those who died had advanced directives against intubation. The remaining 41 patients (69%) were discharged home a median 8 days after admission. The median initial white blood cell count was significantly higher in patients who died compared with those still living (7.5 versus 5.7×103/μl), as was C-reactive protein (163 versus 80 mg/L).
Conclusions
The association of COVID-19 with high mortality in patients with ESKD on dialysis reinforces the need to take appropriate infection control measures to prevent COVID-19 spread in this vulnerable population.
After first being identified in Wuhan, China in December 2019, the novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 quickly became a global pandemic. Currently, the United States has more cases of COVID-19 than any other nation, and New York City has become the epicenter of the pandemic with over 100,000 cases and almost 7000 confirmed deaths.1 Although the spectrum of disease has ranged from asymptomatic to fatal multiorgan failure, the mechanisms underlying these interperson differences in disease course are not well understood. Reports from multiple international investigators have identified patient characteristics associated with a higher risk of severe disease and death, including older age, men, underlying cardiac or pulmonary disease, diabetes, and hypertension.2−5 Among these, age has also been consistently identified as a risk factor for death from COVID-19 in the United States, where early reports found that 80% of deaths in over 4000 cases up to March 2020 occurred in individuals older than 65 years.6 In addition, underlying CKD has been identified as a risk factor for mortality, with a Chinese cohort demonstrating that elevated baseline serum creatinine was associated with a hazard ratio for death of 3.97.7
The demographics and comorbidities of patients with ESKD on dialysis in our network, as well as their relative immunocompromised status, raised the possibility that these patients might be at an increased risk of severe COVID-19 disease. In our network, mean age is about 64 years, with 51% of patients 65 years or older, and there is a high prevalence of diabetes (67%), coronary artery disease (55%), heart failure (54%), and chronic obstructive pulmonary disease (32%), with an average of 5.3 comorbidities per patient. Further, 12 of every 13 patients on dialysis receive in-center hemodialysis, which precludes the ability to practice adequate social distancing while also requiring the need for frequent travel despite shelter in place orders in a city that relies heavily on public transportation.8
Given these concerns for increased exposure to COVID-19 and higher risk of severe disease among patients receiving dialysis, we sought to examine the presentation, clinical course, outcomes, and risk factors for more severe disease in this patient population. Here, we present our early experience with COVID-19 in patients on dialysis admitted to our institution.
Methods
We identified 59 consecutive patients with ESKD on dialysis admitted to Columbia University Irving Medical Center for COVID-19 from March 9, 2020 to April 8, 2020. Demographics, clinical data, details of their hospital course, and follow up through April 29, 2020 were obtained from the medical record. Diagnosis of COVID-19 was made exclusively on the basis of positive severe acute respiratory syndrome coronavirus 2 PCR testing on nasopharyngeal swabs. Baseline characteristics of patients who died versus those who are still living were compared using Wilcoxon rank sum tests and Fischer exact tests. Given that 11 of the patients who died had advanced directives against intubation and mechanical ventilation, we also used descriptive statistics to present characteristics of those who received mechanical ventilation, those who did not receive mechanical ventilation, and those who died with a “Do Not Intubate” advanced directive in place. This study was approved by the Institutional Review Board of Columbia University Irving Medical Center.
During the initial phase of the pandemic in New York, all patients with COVID-19 had bedside hemodialysis performed by one dedicated dialysis nurse. After the proportion of patients on dialysis with COVID-19 exceeded 50% on March 30, 2020 (due to an increase in both patients with ESKD and patients with AKI), medically stable patients with COVID-19 received dialysis in our inpatient hemodialysis unit, with an initial staffing ratio of one dialysis nurse for every two patients that was eventually expanded to one dialysis nurse for every three patients in order to increase the availability of nurses for bedside dialysis. Disinfection was performed before starting each non–COVID-19 shift as per Centers for Disease Control and Prevention guidelines. During this time, patients with hemodynamic instability and/or respiratory distress continued to receive bedside hemodialysis or continuous RRT. Although we initially considered routinely reducing dialysis frequency to twice weekly for all patients with COVID-19, this plan was not broadly implemented for all patients due to concern for hyperkalemia and volume overload.
Results
We identified 59 patients with ESKD on dialysis, including 2 patients who were on peritoneal dialysis and 57 patients on hemodialysis (Table 1). The median age of our cohort was 63 years (interquartile range [IQR], 56–78), with a slightly higher prevalence of men (56%), and 75% had Hispanic ethnicity. All but one patient had hypertension, 69% had diabetes, 46% had coronary artery disease, 54% were overweight or obese, 17% had pulmonary disease, and 32% were current or former smokers. Additionally, 73% of patients were using a statin, and 22% were taking an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker at the time of admission. Eight percent were previous kidney transplant recipients with failed allografts, none of whom were on chronic immunosuppression at the time of admission. Most patients (80%) dialyzed via arteriovenous fistula or graft. Compared with patients who are still living, those who died were older (median, 75 years; IQR, 61–81 versus median, 62 years; IQR, 54–73; P=0.04) and had higher median Charlson comorbidity index (8; IQR, 6–10 versus 7; IQR, 5–8; P=0.03). Otherwise, there were no differences in demographic characteristics between groups.
Table 1.
Characteristics and presentation of patients with dialysis-dependent ESKD and COVID-19 admitted to a single center in New York City by vital status
| Characteristic | n (Column %) or Median (IQR) | P Value | ||
|---|---|---|---|---|
| All | Still Living | Deceased | ||
| n | 59 | 41 (69%) | 18 (31%) | |
| Demographics on admission | ||||
| Age, yr | 63 (56–78) | 62 (54–73) | 75 (61–81) | 0.04 |
| Men | 33 (56) | 23 (56) | 10 (56) | 0.99 |
| Race | ||||
| White | 11 (19) | 7 (17) | 4 (22) | 0.86 |
| Black | 15 (25) | 11 (27) | 4 (22) | |
| Other/no answer | 33 (56) | 23 (56) | 10 (56) | |
| Hispanic ethnicity | 44 (75) | 31 (76) | 13 (72) | 0.76 |
| Charlson comorbidity index | 7 (5–8) | 7 (5–8) | 8 (6–10) | 0.03 |
| Body mass index, kg/m2, n=57 | 25.2 (22.5–29.5) | 25 (22.5–30.3) | 27.7 (21.8–29.2) | 0.74 |
| Body mass index ≥25 kg/m2, n=57 | 31 (54) | 21/41 (51) | 10/16 (63) | 0.56 |
| Hypertension | 58 (98) | 41 (100) | 17 (94) | 0.31 |
| Diabetes mellitus | 41 (69) | 29 (71) | 12 (67) | 0.77 |
| Coronary artery disease | 27 (46) | 17 (41) | 10 (56) | 0.40 |
| Pulmonary disease | 10 (17) | 8 (20) | 2 (11) | 0.71 |
| Smoker | ||||
| Current | 2 (3) | 2 (5) | 0 (0) | 0.20 |
| Former | 17 (29) | 9 (22) | 8 (44) | |
| Prior kidney transplant | 5 (8) | 4 (10) | 1 (6) | 0.99 |
| ACEi or ARB use | 13 (22) | 10 (24) | 3 (17) | 0.74 |
| Statin use | 43 (73) | 29 (71) | 14 (78) | 0.75 |
| Dialysis access | ||||
| AVF/AVG | 47 (80) | 32 (78) | 15 (83) | 0.63 |
| Dialysis catheter | 10 (17) | 7 (17) | 3 (17) | |
| Peritoneal dialysis | 2 (3) | 2 (5) | 0 (0) | |
| COVID-19 presentation | ||||
| Symptom onset to admission, d, n=48a | 3 (2–5) | 3 (2–4) | 3 (2–6) | 0.38 |
| Admitted after extended dialysis break, n=57b | 9/57 (16) | 7 (18) | 2 (11) | 0.70 |
| Presenting symptoms | ||||
| Fever | 29 (49) | 20 (49) | 9 (50) | 0.58 |
| Cough | 23 (39) | 17 (41) | 6 (33) | 0.39 |
| Dyspnea (exertional or rest) | 21 (36) | 14 (34) | 7 (39) | 0.77 |
| Fatigue/malaise | 13 (22) | 9 (22) | 4 (22) | 0.99 |
| Gastrointestinal | 9 (15) | 6 (15) | 3 (17) | 0.99 |
| Chills | 6 (10) | 5 (12) | 1 (6) | 0.40 |
| Myalgia | 4 (7) | 3 (7) | 1 (6) | 0.64 |
| Altered mentation | 5 (8) | 5 (12) | 0 (0) | 0.31 |
| None of the above symptoms | 3 (5) | 1 (2) | 2 (11) | 0.22 |
| Predominant initial CXR findings | ||||
| Multifocal/bilateral opacities | 35 (59) | 26 (63) | 9 (50) | 0.18 |
| No acute findings | 11 (19) | 8 (20) | 3 (17) | |
| Unilateral opacity | 6 (10) | 3 (7) | 3 (17) | |
| Pleural effusion | 4 (7) | 1 (2) | 3 (17) | |
| Pulmonary edema | 3 (5) | 3 (7) | 0 (0) | |
Pulmonary disease includes asthma (n=4), chronic obstructive pulmonary disease (n=2), idiopathic pulmonary fibrosis (n=1), lung cancer (n=2), and unspecified restrictive lung disease (n=1). Patients were considered to have been admitted after an extended (i.e., 2-day) dialysis break if they received outpatient dialysis Monday/Wednesday/Friday and were admitted on a Sunday or if they received outpatient dialysis Tuesday/Thursday/Saturday and were admitted on a Monday. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AVF, arteriovenous fistula; AVG, arteriovenous graft; CXR, chest X-ray.
Nine patient records were missing these data or patient was unsure.
Two patients on peritoneal dialysis were not included.
The most common presenting symptom overall was fever (49%) followed by cough (39%), dyspnea (36%), and fatigue/malaise (22%) (Table 1). Patients less commonly reported gastrointestinal symptoms (15%), chills (10%), myalgia (7%), or altered mentation (8%). Initial chest radiographs showed multifocal or bilateral opacities in the majority of patients (59%), although six patients (10%) had unilateral opacities and notably, 11 patients (19%) had no acute radiographic findings (Table 1). Only 16% of patients were admitted after a long dialysis break, a proportion that was similar between groups.
Eight patients (14%) received mechanical ventilation, with median time from admission to intubation of 1.5 days (range, 0–9) (Table 2). No other patients were admitted to the intensive care unit. Of these patients, three (38%) were treated with continuous RRT while in the intensive care unit (Table 2). Three patients (38%) were extubated after a median of 6 days (range, 1–10) of mechanical ventilation. Given the proportion of patients who died but had advanced directives declining intubation and mechanical ventilation, comparisons between those who did and did not receive mechanical ventilation were not performed.
Table 2.
Single-center outcomes of patients with ESKD receiving dialysis hospitalized with COVID-19
| Outcome | n (%) or Median (IQR) | |||
|---|---|---|---|---|
| All | No Mechanical Ventilation | Mechanical Ventilation | Died with “Do Not Intubate” Advanced Directive | |
| n | n=59 | n=40 (68%) | n=8 (14%) | n=11 (19%) |
| Total no. of inpatient hemodialysis sessions per patient | 2 (2–4) | 2 (2–4) | 2 (0–5) | 2 (0–3) |
| Received continuous RRT | 3 (5) | 0 (0) | 3 (38) | 0 (0) |
| Days between admission and intubation | — | — | 1.5 (range, 0–9) | — |
| Extubated | — | — | 3 (38) | — |
| Days between intubation and extubation, n=3 | — | — | 6 (range, 1–10) | — |
| Anti–COVID-19 therapies | ||||
| Hydroxychloroquine | 34 (58) | 21 (53) | 5 (63) | 8 (73) |
| Tocilizumab | 3 (5) | 2 (5) | 1 (13) | 0 (0) |
| Died in the hospital | 18 (31) | 1 (3) | 6 (75) | 11 (100) |
| Days between admission and death | 6 (4–14) | 15 | 13 (4–21) | 6 (4–8) |
| Discharged from hospital | 41 (69) | 39 (98) | 2 (25) | — |
| Days between admission and discharge | 8 (6–12) | 8 (5–11) | 9 and 19 | — |
By the end of follow-up, 18 patients (31%) had died a median of 6 days (IQR, 4–14) after hospital admission, including 75% of patients who required mechanical ventilation (Table 2). Of the 12 patients who died without receiving mechanical ventilation, all but 1 had documented advanced directives against intubation (“Do Not Intubate” orders) (Supplemental Table 1). Overall, patients who died had higher initial values of white blood cell count (median, 7.5 versus 5.7×1000/μl; P=0.04), lactate dehydrogenase (median, 507 versus 312 U/L; P=0.04), and C-reactive protein (median, 163 versus 80.3 mg/L; P=0.01) than those who are still living (Supplemental Table 2). Peak values of white blood cell count and several inflammatory markers were also higher among patients who died, although these test results were not available for all patients.
Forty-one patients (69%) were discharged from the hospital a median of 8 days (IQR, 6–12) after admission, including two patients who previously received mechanical ventilation and were discharged after 9 and 19 days of hospitalization. No discharged patients have been readmitted to date.
Discussion
Patients with advanced age and with comorbid conditions such as diabetes, pulmonary disease, and cardiovascular disease are at the highest risk of severe manifestations of COVID-19.2−4,9 Given the immunocompromised nature of ESKD and the high comorbidity burden seen in patients with kidney failure, patients with ESKD are among the most vulnerable populations—especially given the impractical nature of social distancing rules for patients who need in-center hemodialysis.10 Herein, we describe our early experience with patients with ESKD and COVID-19.
Although we found high overall mortality of 31%, several factors may have affected this finding. A high number of patients who died had expressed wishes against intubation and mechanical ventilation, which likely reflects the fact that dialysis centers in the United States are required to inform patients about their right to an advanced directive and document whether an advanced directive has been completed.11 It is not possible for us to ascertain the likely outcome had these patients also received mechanical ventilation. Additionally, our data are therefore unable to shed light on the risk of death among patients who do not require ventilation. A lower threshold for hospital admission for patients on dialysis with COVID-19 may also be present, leading to higher likelihood of admission in mild disease due to concerns related to their comorbidities and reluctance of outpatient dialysis centers to continue to treat these patients while risking exposure to other patients or staff at their facilities. These patients may also present to the hospital earlier in their disease course due to inability to receive dialysis at their usual outpatient centers. Unfortunately, outcomes for those patients who received mechanical ventilation were poor, with 75% mortality in this group.
An early report from California described an atypical presentation of COVID-19 in a patient on dialysis, whose initial symptoms were primarily gastrointestinal distress.12 In contrast, similar to the general population, the most common presenting symptoms in our cohort were fever, reported by about half of patients, and cough, although a small number of patients had no typical symptoms of COVID-19 (two of whom were admitted after falls).3,9 None of the patients who eventually required mechanical ventilation and few of those who died had initial chest radiographs demonstrating pulmonary edema or pleural effusions. Rather, over half of patients in our cohort had multifocal infiltrates, and the remainder had unilateral infiltrates or no acute radiographic findings, similar to the general population.13 Further, only 16% of patients were admitted following their weekly two-day hiatus in the dialysis schedule. Together, these findings argue against the suggestion that patients on dialysis have an increased risk of severely symptomatic COVID-19 primarily due to chronic underlying volume overload.
Patients who died were older and had a higher Charlson comorbidity index compared with those who are still living, consistent with general population findings that patients with a higher burden of chronic illness are at an increased risk of severe disease. Similar to prior studies in the general population, we also found a relationship between higher white blood cell count on presentation and severe COVID-19.14−16 However, we found no significant differences in absolute lymphocyte count between groups despite prior identification of lymphopenia as a predictor of severe disease.14,15 Notably, other cohorts of patients at higher risk of severe disease, such as transplant recipients, have no found associations between either of these laboratory values and COVID-19 outcomes.17,18 It is possible that the utility of these tests differs in high-risk subgroups compared with the general population. Patients who died had significantly higher admission values of C-reactive protein and higher peak values of several inflammatory markers compared with those who did not, consistent with our understanding that clinical deterioration is mediated by an inflammatory response.17,19,20 However, our ability to draw inferences from these data is limited by missing values.
Over two thirds of the patients in our cohort were discharged home by the end of follow-up, a median of 8 days after admission. Further, patients required a median of two dialysis sessions during their admissions. Although this number may be small overall, it represents a substantial contribution to the need for RRT in the hospital at a time when resources needed to provide these treatments are limited due to the high proportion of patients with severe COVID-19 cases who develop AKI.7,21 Given the reluctance of many outpatient dialysis centers to treat patients with COVID-19 due to risk of exposing patients and staff and the significant measures needed to prevent spread within units, dialysis organizations have created COVID-19 outpatient dialysis units in New York City to treat these patients until infection resolution.22
In our inpatient dialysis unit, all patients wore face masks throughout their dialysis sessions, regardless of COVID-19 test result. All nurses and physicians wore personal protective equipment (PPE) during dialysis procedures, including gowns, gloves, and N95 masks covered by surgical masks. By reusing N95 masks and covering them with standard surgical masks, we avoided a shortage of PPE. However, in order to preserve PPE and reduce provider exposure, only the attending physician (and not the fellow or nurse practitioner) examined patients with COVID-19. Early in the pandemic, if a patient was diagnosed with COVID-19 after previously having an inpatient dialysis session without droplet and contact isolation, dialysis nurses who took care of that patient were quarantined due to presumed exposure. Dialysis nurses with comorbidities associated with a higher risk of severe COVID-19, in particular older age, were assigned to patients without COVID-19, and physicians with such comorbidities did not round on the dialysis service. One provider developed symptoms of COVID-19 after rounding on the inpatient dialysis service but tested negative for COVID-19 by RT-PCR and returned to work after isolation. Five of a total 34 nurses (27 full time and 7 per diem) developed COVID-19 symptoms and tested positive. All returned to work after isolation and symptom resolution.
Limitations of this study include a relatively small sample size, the observational nature of our data, and missing laboratory tests in some patients. Additionally, we are unable to determine the true total number of patients with ESKD affected with COVID-19, including those who were asymptomatic or only mildly symptomatic and never tested due to the limited availability of testing for patients with mild cases. Although median age and prevalence of comorbidities such as diabetes or coronary artery disease in our cohort are similar to those of our broader ESKD network, additional data are needed to determine which demographic factors or comorbidities are associated with the need for hospitalization among patients on dialysis who develop COVID-19. Similarly, although a high proportion of patients in our cohort was of Hispanic ethnicity compared with our typical hospital census (35%–40%), this finding is not unexpected on the basis of our hospital’s location in Washington Heights, New York City, where 71% of residents have Hispanic ethnicity.23 More data are therefore needed to determine whether there is an association between ethnicity and need for hospitalization or COVID-19 severity. Further, we lack valuable information on the site of exposure (in-center versus community-acquired exposure) of COVID-19. A reliance on the medical record to retrospectively identify patient comorbidities may lead to inaccurate prevalence estimates and assessment of comorbidity severity. Approximately the same proportion of individuals in each group received hydroxychloroquine at the recommendation of the infectious disease team, whereas three patients received the IL-6 antagonist tocilizumab. Given the small number of patients receiving these treatments and the selection bias in their use, further conclusions about their efficacy cannot be drawn from our data.
In conclusion, hospitalized patients with ESKD and COVID-19 displayed high mortality, although many who died had advanced directives against intubation. This study reinforces the need to consider the ESKD population as a high-risk, highly vulnerable population and the need to take appropriate infection control measures to prevent the spread of COVID-19 in this group.
Disclosures
All authors have nothing to disclose.
Funding
Dr. Husain was supported by National Center for Advancing Translational Sciences grant KL2 TR001874.
Supplementary Material
Acknowledgments
Dr. Husain, Dr. Mohan, Dr. Stevens, and Dr. Valeri designed the study; Dr. Robbins-Juarez and Dr. Valeri collected the data; Dr. Husain, Dr. Mohan, and Dr. Valeri analyzed the data; Dr. Ahn, Dr. Gharavi, Dr. Husain, Dr. Mohan, Dr. Radhakrishnan, Dr. Rao, Dr. Robbins-Juarez, Dr. Stevens, and Dr. Valeri interpreted the data; Dr. Ahn, Dr. Gharavi, Dr. Husain, Dr. Mohan, Dr. Radhakrishnan, Dr. Rao, Dr. Robbins-Juarez, Dr. Stevens, and Dr. Valeri drafted and revised the paper; and Dr. Ahn, Dr. Gharavi, Dr. Husain, Dr. Mohan, Dr. Radhakrishnan, Dr. Rao, Dr. Robbins-Juarez, Dr. Stevens, and Dr. Valeri approved the final version of the manuscript. Dr. Mohan is supported by National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK114893, R01-MD014161, and U01-DK116066. Dr. Gharavi reports grants from the Renal Research Institute.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020040470/-/DCSupplemental.
Supplemental Table 1. Characteristics and presentation of patients with ESKD and coronavirus disease 2019 admitted to a single center in New York City by mechanical ventilation status.
Supplemental Table 2. Laboratory results of patients with ESKD and coronavirus disease 2019 admitted to a single center in New York City by vital status.
References
- 1.NYC: COVID-19: Data, 2020. Available at: https://www1.nyc.gov/site/doh/covid/covid-19-data.page. Accessed April 14, 2020
- 2.CDC: Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1–30, 2020, 2020. Available at: https://www.cdc.gov/mmwr/volumes/69/wr/mm6915e3.htm. Accessed April 14, 2020 [DOI] [PMC free article] [PubMed]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19 : Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al.: Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al.: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395: 1054–1062, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC COVID-19 Response Team : Severe outcomes among patients with Coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep 69: 343–346, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al.: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.End-Stage Renal Disease Network of New York: 2018 Annual Report, 2020. Available at: https://network2.esrd.ipro.org/wp-content/uploads/sites/3/2019/06/NW2-2018-Annual-Report_Final-approved-for-web.pdf. Accessed April 15, 2020
- 9.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. : Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [published online ahead of print March 13, 2020] JAMA Intern Med doi:10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurts C, Panzer U, Anders HJ, Rees AJ: The immune system and kidney disease: Basic concepts and clinical implications. Nat Rev Immunol 13: 738–753, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Medicare & Medicaid Services (CMS), HHS : Medicare and medicaid programs; conditions for coverage for end-stage renal disease facilities. Final rule. Fed Regist 73: 20369–20484, 2008 [PubMed] [Google Scholar]
- 12.Ferrey AJ, Choi G, Hanna RM, Chang Y, Tantisattamo E, Ivaturi K, et al.: A case of novel coronavirus disease 19 in a chronic hemodialysis patient presenting with gastroenteritis and developing severe pulmonary disease [published online ahead of print March 28, 2020]. Am J Nephrol doi:10.1159/000507417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, et al.: Frequency and distribution of chest radiographic findings in COVID-19 positive patients [published online ahead of print March 27, 2019]. Radiology doi:10.1148/radiol.202020116032216717 [Google Scholar]
- 14.Ruan Q, Yang K, Wang W, Jiang L, Song J: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46: 846–848, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al.: Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 8: 475–481, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al.: Clinical characteristics of Covid-19 in New York City [published online ahead of print April 17, 2020]. N Engl J Med doi:10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Columbia University Kidney Transplant Program: Early description of coronavirus 2019 disease in kidney transplant recipients in New York [published online ahead of print April 28, 2020]. J Am Soc Nephrol doi:10.1681/ASN.2020030375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, et al.: COVID-19 in solid organ transplant recipients: Initial report from the US epicenter [published online ahead of print April 24, 2020]. Am J Transplant doi:10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Gao Y, Qiao L, Wang W, Chen D: Inflammatory response cells during acute respiratory distress syndrome in patients with coronavirus disease 2019 (COVID-19) [published online ahead of print April 13, 2020]. Ann Intern Med doi:10.7326/L20-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye Q, Wang B, Mao J: Cytokine storm in COVID-19 and treatment [published online ahead of print April 10, 2020]. J Infect doi:10.1016/j.jinf.2020.03.03732283152 [Google Scholar]
- 21.Burgner A, Ikizler TA, Dwyer JP: COVID-19 and the inpatient dialysis unit: Managing resources during contingency planning pre-crisis. Clin J Am Soc Nephrol 15: 720–722, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kliger AS, Silberzweig J: Mitigating risk of COVID-19 in dialysis facilities. Clin J Am Soc Nephrol 15: 707–709, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Population Division–New York City Department of City Planning: Table PL-P3A NTA: Total population by mutually exclusive race and Hispanic origin, 2011. Available at: https://www1.nyc.gov/assets/planning/download/pdf/data-maps/nyc-population/census2010/t_pl_p3a_nta.pdf. Accessed April 30, 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.