Significance Statement
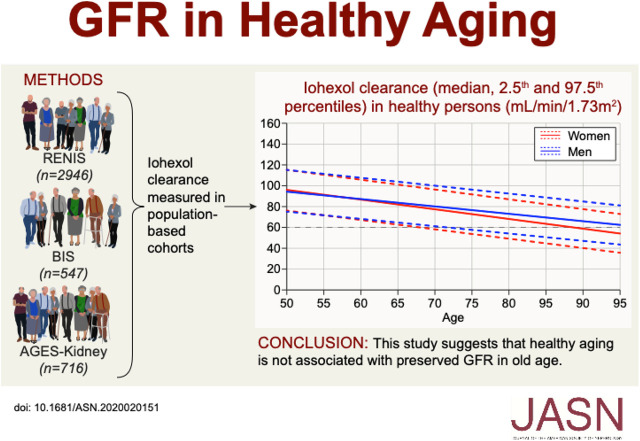
In populations, mean GFR is lower in older age, but whether healthy aging is associated with preserved rather than lower GFR in some individuals is unknown. In a meta-analysis of three large European-based cohorts, the authors investigated the cross-sectional association of being healthy (defined as having no major chronic disease or risk factors for CKD), age, and iohexol clearance measurements. The mean and the 97.5th percentile of the GFR distribution were higher in older persons who were healthy than in those who were unhealthy, but lower than in middle-aged people who were healthy. The GFR-age association was more negative in women than in men. These results suggest that, although being healthy is associated with higher GFR in old age, healthy aging is probably not associated with preserved GFR in old age.
Keywords: epidemiology and outcomes, geriatric nephrology, glomerular filtration rate, glomerular hyperfiltration, renal dysfunction, renal function decline
Visual Abstract
Abstract
Background
Population mean GFR is lower in older age, but it is unknown whether healthy aging is associated with preserved rather than lower GFR in some individuals.
Methods
We investigated the cross-sectional association between measured GFR, age, and health in persons aged 50–97 years in the general population through a meta-analysis of iohexol clearance measurements in three large European population-based cohorts. We defined a healthy person as having no major chronic disease or risk factors for CKD and all others as unhealthy. We used a generalized additive model to study GFR distribution by age according to health status.
Results
There were 935 (22%) GFR measurements in persons who were healthy and 3274 (78%) in persons who were unhealthy. The mean GFR was lower in older age by −0.72 ml/min per 1.73 m2 per year (95% confidence interval [95% CI], −0.96 to −0.48) for men who were healthy versus −1.03 ml/min per 1.73 m2 per year (95% CI, −1.25 to −0.80) for men who were unhealthy, and by −0.92 ml/min per 1.73 m2 per year (95% CI, −1.14 to −0.70) for women who were healthy versus −1.22 ml/min per 1.73 m2 per year (95% CI, −1.43 to −1.02) for women who were unhealthy. For healthy and unhealthy people of both sexes, both the 97.5th and 2.5th GFR percentiles exhibited a negative linear association with age.
Conclusions
Healthy aging is associated with a higher mean GFR compared with unhealthy aging. However, both the mean and 97.5 percentiles of the GFR distribution are lower in older persons who are healthy than in middle-aged persons who are healthy. This suggests that healthy aging is not associated with preserved GFR in old age.
The Global Burden of Disease study found that aging was responsible for 43% of the increased loss of disability-adjusted life-years caused by CKD between 1990 and 2016.1 Improved survival for the oldest age groups combined with an almost exponential increase in CKD prevalence with age indicate that this trend will probably continue.2
Because of the age-related reduction in population mean GFR, there is an ongoing debate about whether the CKD definition should be changed to incorporate age-varying GFR thresholds.3,4 However, it is not known whether lower mean GFR in older people is caused by natural senescence or by diseases associated with lower GFR in the elderly. Some longitudinal studies have found a preserved or improved rather than lowered GFR in a significant proportion of aging persons.5 Although this suggests that good health may prevent age-related GFR decline, studies of kidney biopsy specimens from living kidney donors demonstrate that a reduction in nephron number occurs from a young age even in the absence of disease.6
Our current knowledge about aging and GFR in the general population mainly comes from cross-sectional studies performed decades ago, which have been summarized in a detailed review by Delanaye et al.7 Few, if any, of these studies were population based, and the number of participants older than 65 years was very small.7 Because the prevalence of chronic disease is higher at older age, we have little knowledge about the effects of natural aging versus disease on GFR in the last decades of life. Although there are some large population-based studies based on GFR estimated from serum creatinine,8–15 this approach is problematic in older people because of confounding by sarcopenia and other non-GFR–related factors.16–20
We performed a meta-analysis of individual participant data from four population-based studies in three European cohorts where GFR had been measured using plasma iohexol clearance in persons aged between 50 and 97 years. Our aims were to study the association of GFR with age in healthy people and predict reference intervals for GFR in healthy aging across the studied age range. Because it would not be possible to perform a population-based study with a high number of truly healthy individuals in the oldest age groups, we designed the study to use a generalized additive regression model to adjust for the association of comorbidity and risk factors with GFR and to predict the distribution of GFR in hypothetically healthy persons.
Methods
Study Population
This cross-sectional investigation was a collaboration between population-based studies in Europe that measured GFR using exogenous filtration markers. Information about eligible studies was obtained from the European Kidney Function Consortium and from a search of literature databases. Three eligible cohorts were identified: the Renal Iohexol Clearance Survey (RENIS) in Tromsø 6 (RENIS-T6; n=1632);21 the Berlin Initiative Study (BIS; n=610);22 and the Age, Gene/Environment Susceptibility–Kidney Study (AGES-Kidney; n=819) (Figure 1).23 The RENIS cohort included a repeated GFR measurement in the RENIS follow-up study (RENIS-FU) after a mean follow-up of 5.6 years (n=1329).24 The examinations in RENIS-T6 and RENIS-FU were both included in this investigation.
Figure 1.
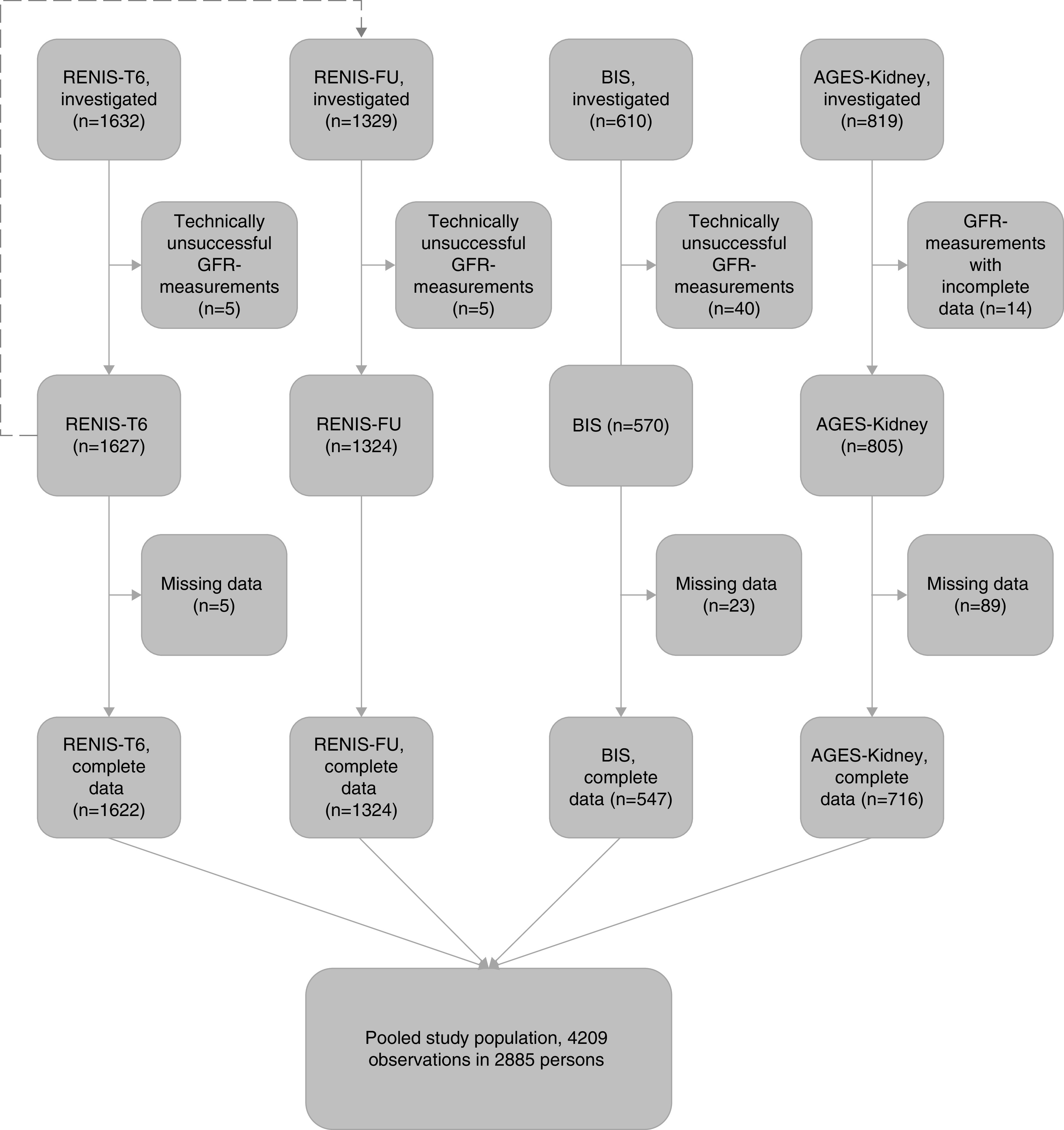
Inclusion of participants from RENIS-T6, RENIS-FU, BIS, and AGES-Kidney in the meta-analysis. The dashed arrow from RENIS-T6 to RENIS-FU indicates the repeated measurements of GFR in the RENIS cohort after a mean follow-up of 5.6 years.
The RENIS cohort included participants between 50 and 63 years of age at baseline from the sixth wave of a series of population surveys in the municipality of Tromsø in northern Norway.21 The response rate in the sixth Tromsø Study was 74% for persons eligible for RENIS.25 BIS recruited persons from the German healthcare fund Allgemeine Ortskrankenkasse Nordost in Berlin. The response rate for the total BIS cohort was 8.1%. AGES-Kidney is a substudy of the AGES-II-Reykjavik Study, which was a follow-up of the population-based Reykjavik Study in Iceland.23 The response rate in the AGES-II-Reykjavik Study was 71%,26 and for those eligible for AGES-Kidney the response rate was 65%.23
The inclusion criteria for the three cohorts were similar, except AGES-Kidney excluded individuals receiving active cancer treatment, and BIS excluded persons that required nursing care during daytime and nighttime. RENIS excluded persons with self-reported diabetes, cardiovascular disease, or kidney disease at baseline in RENIS-T6, but diabetes diagnosed by hemoglobin A1c ≥6.5% (≥48 mmol/mol) at baseline and incident cases during follow-up were included. 21,22,23 People receiving RRT were excluded from all three cohorts.
The people invited to AGES-Kidney and RENIS comprised random samples of the general population, and those invited to BIS comprised a random sample from the Allgemeine Ortskrankenkasse Nordost, which provides insurance coverage to almost 50% of people older than 70 years in Berlin.22 Although participation was voluntary, the three cohorts of examined persons were all representative of their source populations. The RENIS cohort has been found similar to all eligible patients in the sixth Tromsø Study with respect to key variables.27 The mean eGFR, calculated using the Modification of Diet in Renal Disease equation, was 89.3 versus 90.6 ml/min per 1.73 m2 for women, and 93.1 versus 93.2 ml/min per 1.73 m2 for men in RENIS and the Tromsø Study, respectively.
The prevalence of the most important chronic diseases in BIS was similar to that of all persons older than 70 years in the Allgemeine Ortskrankenkasse Nordost.28 Also, the prevalence of diabetes,29 myocardial infarction,30 angina pectoris,30 stroke,31 and cancer32 were of a similar order of magnitude as in other German studies of chronic diseases in older adults.
The participants in AGES-Kidney were younger, had lower systolic BP, and were less likely to be current smokers or have cardiovascular disease or diabetes than participants in AGES-II-Reykjavik who were not included.23 The mean eGFR was 65.7 versus 64.1 ml/min per 1.73 m2 among those who participated and those who did not.23 More detailed information about the cohorts can be found in previous publications.21–24
Technically unsuccessful measurements were excluded from the investigations (RENIS-T6, n=5; RENIS-FU, n=5; AGES-Kidney, n=14; BIS, n=40; Figure 1).21,23,24,33
This study was approved by the ethical review boards of the three respective investigations, and the study was approved by the Icelandic National Bioethics Committee (VSN 00-063). The study adhered to the Declaration of Helsinki. All of the subjects provided informed written consent.
Data
Data on morbidity, smoking habits and medication use were obtained through questionnaires in all three cohorts. The use of individual classes of antihypertensive medications was registered in the following dichotomous variables: β-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium-channel blockers, loop diuretics, thiazide diuretics, mineralocorticoid receptor antagonists, other diuretics, and other antihypertensive drugs including α-blockers. The use of lipid-lowering medications, antidiabetic drugs, and cardiac glycosides (digoxin/digitoxin) was also registered. Smokers were categorized as current, previous, or never.
Definitions
Diabetes was defined as either self-reported diabetes, use of antidiabetic medication, or hemoglobin A1c ≥6.5% (≥48 mmol/mol).
Details about urinary creatinine and albumin measurements in all three cohorts have been given previously.23,28,34 The urinary albumin-creatinine ratio (ACR) was classified in the categories <10, ≥10 and <30, and ≥30 mg/g, corresponding to the categories optimal, high normal, and high/very high/nephrotic.35
In RENIS and AGES-Kidney, BP was measured as described previously.23,36 BP in BIS was measured according to the European Society of Cardiology/European Society of Hypertension (ESC/ESH) recommendations.37 Subjects with an office systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or those who were on antihypertensive medications were categorized as having hypertension according to the ESC/ESH guidelines.37
GFR Measurements
GFR was measured as plasma iohexol clearance in all three cohorts. Multiple-sample protocols were used in AGES-Kidney and BIS, and a single-sample protocol was used in RENIS. Details of the GFR measurement methods, including an investigation of agreement between the multiple- and single-sample protocols, have been previously reported.38 Substantial agreement between the methods was found. Iohexol concentration was measured with high-performance liquid chromatography in all three cohorts.38 The iohexol assays of the three studies were calibrated by reanalyzing thawed samples in the laboratory of the Department of Medical Biochemistry, University Hospital of North Norway (UNN; Tromsø, Norway). The results of the calibration have been reported previously.38 No calibration was found to be necessary for the BIS and RENIS samples, but the following equation was used to calibrate the AGES-Kidney results to the UNN laboratory: log(iohexolUNN)=−0.091+1.025×log(iohexolAGES).38 This calibration resulted in a mean difference in GFR of only 0.87 ml/min from the original results.
GFR was indexed to 1.73 m2 body surface area. Body surface area was estimated using the equation developed by Dubois and Dubois.39
Serum creatinine was analyzed using the same isotope dilution mass spectrometry-traceable enzymatic assay (CREA Plus; Roche Diagnostics, Mannheim, Germany) in all three cohorts.33,36,40 eGFR was calculated from serum creatinine (eGFRcrea) using the CKD Epidemiology Collaboration equation.41
Statistical Analyses
Characteristics of the study participants were provided at the time of the GFR measurements, i.e., characteristics for the RENIS cohort was reported for both the baseline (RENIS-T6) and the follow-up (RENIS-FU) examination. Quantile regression was used for testing the unadjusted difference in median GFR across health status categories.
“Health status” was defined as a dichotomous variable where a healthy person was defined as a person with no history of myocardial infarction, angina pectoris, coronary revascularization procedures, stroke, cancer, diabetes, hypertension, current smoking, or use of lipid-lowering medication or cardiac glycosides; body mass index <30 kg/m2; and ACR <30 mg/g. Because information about heart failure was not available in BIS and RENIS, it was not included in the definition. However, most persons with heart failure caused by coronary heart disease would have been classified as unhealthy and medications commonly used for heart failure such as angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, β-blockers, loop diuretics, and mineralocorticoid receptor blockers were classified as antihypertensive drugs that would also have caused a participant to be categorized as unhealthy. Additionally, we included cardiac glycosides as another indicator of heart failure.
This investigation was designed as a meta-analysis with individual participant data using a one-stage statistical analysis.42 The associations between GFR indexed for body surface area and age, sex, health status, and cohort membership were explored in generalized additive regression models for location, scale, and shape (GAMLSS) using the gamV procedure from the mgcViz-package in R.43,44 GAMLSS is a new regression method suitable for modeling the age-dependent distribution of variables. A detailed explanation of the method can be found in the Supplemental Methods (Supplemental Figure 1, Supplemental References). In brief, the mean and SD of the GFR distribution are modeled as separate functions of the independent variables in the same regression model. These functions may be linear or nonlinear. In addition to investigating the association of the independent variables with the mean of the GFR distribution as in ordinary regression methods, GAMLSS makes it possible to investigate their association with the SD of the distribution. This means that the association of any percentile of the GFR distribution across age can be studied instead of focusing only on the mean, similar to quantile regression.
We analyzed the associations between GFR indexed for body surface area as the dependent variable and age, sex, and health status as independent variables. We adjusted for the correlation between the first (RENIS-T6) and second (RENIS-FU) GFR measurement for the same individual in the RENIS cohort by including a random intercept for each participant in all models. Adjustment for cohort effects were made by including random coefficients for cohort membership and for the interaction between cohort membership and other independent variables. We assumed the same cohort effects for both rounds (RENIS-T6 and RENIS-FU) of RENIS. The variance-covariance structure of the random effects was assumed to be independent in all models. The GFR-age association was defined as the difference in mean GFR per 1-year older age and is represented by the coefficient for the age variable in the regression model. A negative GFR-age association signifies a lower population mean GFR by older age. The regression coefficient for the interaction between age and health status represents the association between health status and the GFR-age association.
The Akaike information criterion (AIC) was used to compare the fit of different regression models.45 This criterion scores the models for fit to the data, but penalizes the score for the number of independent variables and complexity of the model. A lower AIC indicates a better fit.
We first analyzed both the mean and the SD of the GFR distribution as functions of the main effects of age, sex, and the health status variable. We also included the interaction between age and health status and a random sex-specific effect for cohort membership to adjust for possible differences in GFR distribution between the cohorts.
In this model, statistically significant main effects of all of the independent variables and the age-health status interaction were found for the mean of GFR (P<0.05). The cohort random effect was statistically significant for men, but not for women. This means that there is variation in the GFR level between the cohorts for men, but not for women. For the SD of GFR, there was no main effect of sex and no sex-specific cohort random effect (P≥0.05). Accordingly, in the SD part of the model, the sex-specific cohort random effect was removed and a common cohort random effect for both sexes (P<0.05) was retained. The AIC for this model was 33380.43.
To investigate the possibility of different associations between age and GFR distributions across the cohorts, we included random effects for the interaction between age and cohort in the functions for both the mean and the SD of GFR. The effect was statistically significant for the mean, but not for the SD. This means that the GFR-age associations may differ between the cohorts, and adding this effect to the function for the mean of the model improved the fit (AIC=33306.61).
Next, we tested interactions between age and sex, and health status and sex, for both the mean and SD in this model. Only the interaction between age and sex for the mean was statistically significant (P=0.005). This interaction also improved the fit (AIC=33302.43) and was included in the model.
Possible nonlinear effects of age were tested by adding smooth terms for the interaction between age and health status, and age and sex in separate models. This was done for both the mean and SD of GFR, but model fit was no better for these models than for the model without nonlinear terms (AIC=33302.98 for the interaction between age and health status; AIC=33304.35 for age and sex). In a separate model, we also tested the effect of the three-way interaction between age, sex, and health status on the GFR mean, which was not statistically significant (P=0.34).
The final model was used to predict and plot the 2.5th percentile, median, and 97.5th percentiles for the GFR distribution for individuals aged 50–95 years.46 We predicted the percentiles separately for men and women by health status. The random cohort effects in the model were set to zero in these predictions, which means that the predictions represent an average between the three cohorts.
We used R version 3.5.1 (https://www.r-project.org/). Statistical significance was set at P<0.05.
Results
The combined RENIS, BIS, and AGES-Kidney cohorts comprise 4326 GFR measurements in 3002 people. Of these, 4209 (97%) observations in 2885 persons had complete data sets without missing data (Supplemental Table 1) (Figure 1). Observations with missing data were omitted from the study population because multiple imputation methods for generalized additive regression models are not available.
Baseline characteristics of the study population are shown in Table 1. RENIS-T6 and RENIS-FU combined covered the age range 50–70 years, and the age ranges for BIS and AGES-Kidney were 70–97 and 74–93 years, respectively. The differences between the cohorts shown in Table 1 reflect the variation in age and in inclusion criteria. AGES-Kidney and BIS are similar, except for higher prevalence of diabetes and ACR ≥30 mg/g in BIS than in AGES-Kidney. The number of persons with body mass index <20 kg/m2 was 26 (1.6%) in RENIS-T6, 31 (2.3%) in RENIS-FU, 4 (0.7%) in BIS, and 15 (2.1%) in AGES-Kidney.
Table 1.
Characteristics of the population-based cohorts
| Characteristic | RENIS-T6a | RENIS-FUa | BIS | AGES-Kidney |
|---|---|---|---|---|
| Number of participants, n (%) | 1622 (100.0) | 1324 (100.0) | 547 (100.0) | 716 (100.0) |
| Age, yr (SD) | 58.1 (3.8) | 63.6 (4.0) | 78.4 (6.2) | 80.3 (4.1) |
| Male sex, n (%) | 797 (49.1) | 657 (49.6) | 311 (56.9) | 317 (44.3) |
| Body weight, kg (SD) | 79.7 (14.4) | 79.4 (14.3) | 77.3 (14.0) | 77.1 (14.1) |
| Height, cm (SD) | 170.6 (8.7) | 170.6 (8.7) | 166.2 (8.5) | 167.7 (9.4) |
| Body mass index, kg/m2 (SD) | 27.3 (4.0) | 27.2 (4.1) | 27.9 (4.3) | 27.4 (4.3) |
| Body surface area, m2 (SD) | 1.9 (0.2) | 1.9 (0.2) | 1.9 (0.2) | 1.9 (0.2) |
| Cardiovascular disease, n (%) | ||||
| Myocardial infarction | 1 (0.1) | 18 (1.4) | 83 (15.2) | 89 (12.4) |
| Myocardial revascularization | 5 (0.3) | 26 (2.0) | 93 (17.0) | 113 (15.8) |
| Angina pectoris | 2 (0.1) | 12 (0.9) | 56 (10.2) | 60 (8.4) |
| Stroke | 3 (0.2) | 24 (1.8) | 42 (7.7) | 53 (7.4) |
| Diabetes, n (%) | 19 (1.2) | 42 (3.2) | 136 (24.9) | 81 (11.3) |
| Cancer, n (%) | 76 (4.7) | 120 (9.1) | 123 (22.5) | 134 (18.7) |
| Hypertension, n (%)b | 692 (42.7) | 693 (52.3) | 503 (92.0) | 623 (87.0) |
| Systolic BP, mm Hg (SD) | 129.7 (17.6) | 130.7 (17.0) | 144.9 (21.5) | 142.3 (20.3) |
| Diastolic BP, mm Hg (SD) | 83.4 (9.8) | 81.9 (9.3) | 82.3 (13.0) | 69.6 (10.7) |
| Antihypertensive medication, n (%) | 298 (18.4) | 420 (31.7) | 425 (77.7) | 524 (73.2) |
| Digoxin or digitoxin, n (%) | 1 (0.1) | 6 (0.5) | 18 (3.3) | 24 (3.4) |
| Lipid-lowering medication, n (%) | 106 (6.5) | 232 (17.5) | 202 (36.9) | 287 (40.1) |
| Antidiabetic medication, n (%) | 0 (0.0) | 11 (0.8) | 99 (18.1) | 44 (6.1) |
| Smoking, n (%) | ||||
| Never | 503 (31.0) | 432 (32.6) | 263 (48.1) | 295 (41.2) |
| Current | 344 (21.2) | 177 (13.4) | 32 (5.9) | 42 (5.9) |
| Previous | 775 (47.8) | 715 (54.0) | 252 (46.1) | 379 (52.9) |
| Absolute GFR, ml/min (SD) | 104.0 (20.1) | 98.5 (19.8) | 64.8 (19.2) | 66.7 (19.4) |
| Body surface area–indexed GFR, ml/min per 1.73 m2 (SD) | 94.0 (14.4) | 89.1 (14.5) | 60.5 (16.3) | 61.9 (16.6) |
| CKD-EPI estimate of GFR based on creatinine, ml/min per 1.73 m3 (SD) | 94.9 (9.5) | 88.2 (10.5) | 68.8 (17.1) | 65.5 (17.1) |
| Urinary ACR ≥30.0 mg/g, n (%) | 24 (1.5) | 26 (2.0) | 126 (23.0) | 110 (15.4) |
| Urinary ACR ≥300.0 mg/g, n (%) | 1 (0.1) | 2 (0.2) | 19 (3.5) | 15 (2.1) |
Data are shown as mean (SD) or n (%). CKD-EPI, CKD Epidemiology Collaboration.
RENIS-T6 and RENIS-FU are the baseline and follow-up examinations of the RENIS cohort.
Office systolic BP ≥140 mm Hg, office diastolic BP ≥90 mm Hg, or the use of antihypertensive medications.
A total of 513 (32%), 360 (27%), 18 (3%), and 44 (6%) participants were categorized as healthy in RENIS-T6, RENIS-FU, BIS, and AGES-Kidney, respectively (Table 2). Baseline characteristics according to health status are shown in Supplemental Table 2. A scatterplot of all of the GFR measurements versus age according to health status is presented in Figure 2.
Table 2.
GFR (ml/min per 1.73 m2) according to health status of participants in the population-based cohorts
| Study | Number of Participants | Median | 2.5th Percentile | 97.5th Percentile | ||||
|---|---|---|---|---|---|---|---|---|
| Unhealthy | Healthya | Unhealthy | Healthya | Unhealthy | Healthya | Unhealthy | Healthya | |
| RENIS-T6b | 1109 | 513 | 94.2 | 93.1 | 65.6 | 63.0 | 123.1 | 118.9 |
| RENIS-FUb | 964 | 360 | 89.2 | 90.1 | 57.5 | 66.5 | 117.5 | 115.3 |
| BIS | 529 | 18 | 60.4 | 69.8c | 29.7 | 44.2 | 88.7 | 96.2 |
| AGES-Kidney | 672 | 44 | 62.5 | 72.4c | 26.2 | 42.4 | 90.5 | 98.4 |
| Total | 3274 | 935 | 82.6 | 90.8 | 34.0 | 59.7 | 117.8 | 118.0 |
Healthy was defined as no cardiovascular disease, cancer, diabetes, hypertension, smoking, lipid-lowering medication, or digoxin, as well as body mass index <30 kg/m2 and urinary ACR <30 mg/g.
RENIS-T6 and RENIS-FU are the baseline and follow-up examinations of the same cohort.
P<0.05 for difference between unhealthy and healthy category in the BIS and AGES-Kidney cohorts.
Figure 2.
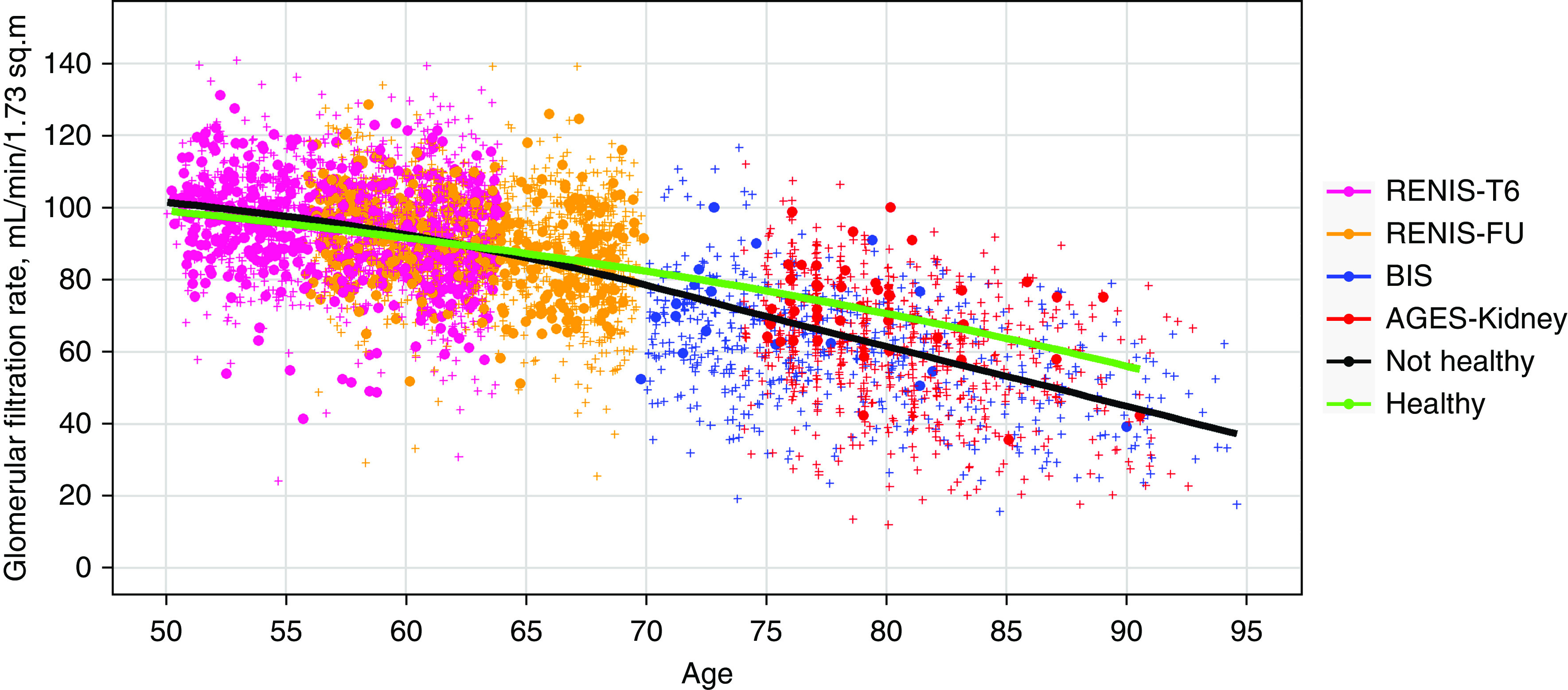
Unadjusted GFR according to cohort and health status. Body surface area–indexed GFR measured as plasma iohexol clearance and plotted against age in the RENIS, BIS, and AGES-Kidney cohorts (n=4209). The marker colors indicate cohort membership. Filled circles indicate measurements in persons who were healthy and crosses in persons who were unhealthy. Measurements for both the baseline (RENIS-T6) and the follow-up examinations (RENIS-FU) of the same persons in the RENIS cohort are shown. The red and green curves represent unadjusted locally estimated scatterplot smoothing fits to measurements in people who were unhealthy and healthy, respectively.
Table 2 shows the observed median and the 2.5th and 97.5th percentiles for GFR according to the health status variable for each of the cohorts. In BIS and AGES-Kidney, there was a statistically significant higher GFR for healthy versus unhealthy persons (P<0.05), but not in RENIS-T6 or RENIS-FU.
Using GAMLSS regression, we analyzed the mean and the SD of the GFR distribution as functions of age, sex, cohort, and the health status variable (Table 3, Supplemental Table 3). The intercept of the model corresponds to the GFR estimate for a 50-year-old woman who is unhealthy (Table 3). Being healthy was associated with a slightly lower mean GFR of 3.25 ml/min per 1.73 m2 at age 50 years, but with a markedly higher GFR-age association of 0.30 ml/min per 1.73 m2 per year, as represented by the coefficient for the interaction between age and health status in Table 3 (P<0.001). Consequently, GFR was higher for healthy than for unhealthy people at all ages >61 years (Figure 3). The GFR-age association was less negative for men than for women, being respectively −0.72 (95% CI, −0.96 to −0.48) versus −0.92 (95% CI, −1.14 to −0.70) ml/min per 1.73 m2 per year for people who were healthy, and −1.03 (95% CI, −1.25 to −0.80) versus −1.22 (95% CI, −1.43 to −1.02) ml/min per 1.73 m2 per year for those who were unhealthy (P=0.005 for the interaction between sex and the GFR-age association). The difference in GFR-age association between men and women was also observed when we stratified by age younger or older than 70 years (Supplemental Figure 2) and by cohort, although this was not statistically significant (0.12, 0.12, and 0.16 ml/min per 1.73 m2 per year in RENIS, AGES-Kidney, and BIS, respectively).
Table 3.
General additive mixed model analysis of GFR mean and SD in three population-based cohorts
| Variable | β (95% CI) | P Value |
|---|---|---|
| Effect of independent variables on mean GFR | ||
| 50-year-old female who was unhealthy (intercept) | 98.91 (97.43 to 100.39) | <0.001 |
| Age, per yr | −1.22 (−1.43 to −1.02) | <0.001 |
| Healthy (yes/no)a | −3.25 (−4.86 to −1.63) | <0.001 |
| Male sex | −0.82 (−8.54 to 6.90) | 0.84 |
| Interaction between age and being healthya | 0.30 (0.18 to 0.43) | <0.001 |
| Interaction between age and male sex | 0.20 (0.06 to 0.34) | 0.005 |
| Effect of independent variables on the SD of GFR | ||
| 50-year-old female who was unhealthy (intercept) | 12.40 (10.53 to 14.61) | <0.001 |
| Percentage change associated with each independent variable | ||
| Age, per yr | −0.1% (−0.6% to 0.3%) | 0.52 |
| Healthy (yes/no)a | −18.6% (−23.9% to −13.0%) | <0.001 |
| Male sex | 1.4% (−3.5% to 6.6%) | 0.59 |
GFR measured in ml/min per 1.73 m2. Model adjusted for random cohort effects and for random intercepts for each participant, see Supplemental Table 3 for random effect results. Fixed effects of health status, age, and sex.
Healthy was defined as no cardiovascular disease, cancer, diabetes, hypertension, smoking, lipid-lowering medication, or digoxin, as well as body mass index <30 kg m−2 and urinary ACR <30 mg/g.
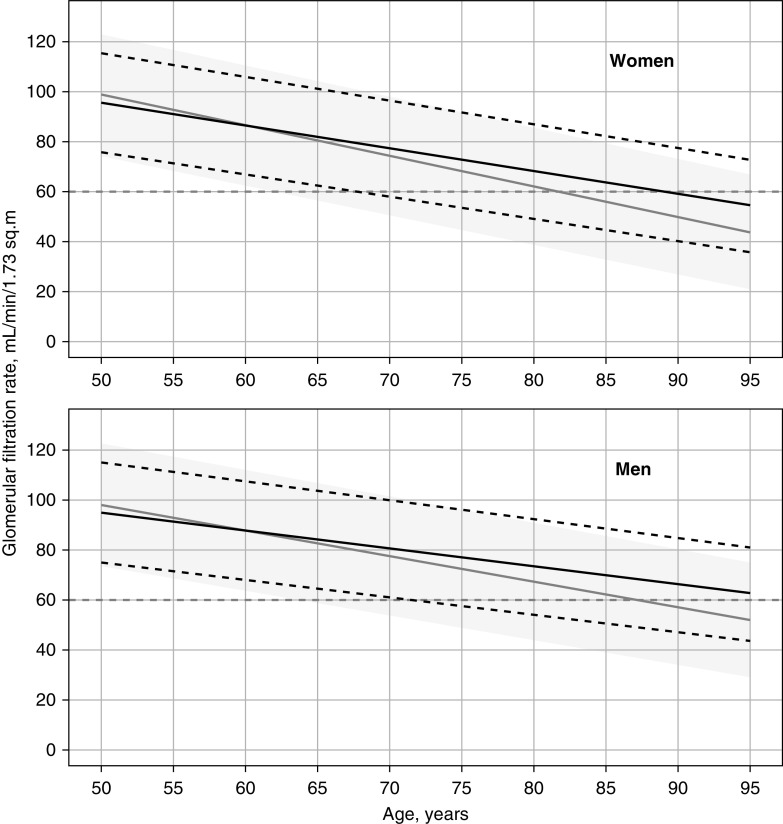
Figure 3.
GFR according to sex and health status. Predicted median (bold black line) and 2.5th and 97.5th percentiles (dashed black lines) as a function of age for healthy women (upper panel) and men (lower panel). The predicted median (gray line) and 95% interpercentile intervals (dark gray band) are shown for persons classified as unhealthy for comparison. The gray dashed line indicates the 60 ml/min per 1.73 m2 level.
There was no statistically significant association between the SD of the GFR distribution and older age (Table 3). This means that although mean GFR was lower at higher age, the variation in GFR at any given age for a given health status was more or less the same. However, persons who were healthy had a 19% lower SD of their GFR distribution than those who were unhealthy (P<0.001), indicating that GFR varies less in people who are healthy than in those who are unhealthy (Table 3).
The random effects in the model demonstrate there were differences between the cohorts with regard to the association between GFR and male sex (P=0.003), in the GFR-age association (P=0.03), and in the SD of the GFR distribution (P<0.001) (Supplemental Table 3). Because we had only three different cohorts, which may be considered few for the estimation of random effects, we replaced the random cohort effects with fixed effects and reanalyzed the model. The point estimates of the fixed effects model were very similar to the random effects model (Supplemental Table 4).
We repeated the analyses of the model shown in Table 3 with nonindexed GFR, measured in ml/min, as the dependent variable and height and weight added as independent variables. The effect of being healthy on the GFR-age association was very similar to that in the primary analysis (0.32 [95% CI, 0.20 to 0.45] versus 0.30 [95% CI, 0.18 to 0.43] ml/min per 1.73 m2 per year; Table 3).
Results of stratified analyses according to sex and age using the final model can be found in Supplemental Figure 2. The 95% CI for the GFR-age association for persons who were healthy was consistently lower than zero in all of the subgroups. The confidence interval for healthy persons >70 years of age was wide due to the small sample size in this category.
The predicted median and 95% reference intervals for GFR in a healthy person based on the final GAMLSS model (Table 3) have been plotted against age for men and women in Figure 3. The group defined as unhealthy is shown in gray for comparison. In people who were healthy, the 60 ml/min per 1.73 m2 threshold intersects the 2.5th percentile at age 67.1 years for women and 70.8 years for men (Figure 3). Table 4 presents predicted median GFR values and 95% reference ranges for 5-year intervals in healthy men and women. Predicted tenth, 25th, 75th, and 90th percentiles of the GFR distribution can be found in Supplemental Table 5.
Table 4.
Predicted percentiles of GFR (ml/min per 1.73 m2) for healthy women and men according to age group
| Age Group (yr) | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of GFR Measurements | Median | 2.5th Percentile | 97.5th Percentile | Number of GFR Measurements | Median | 2.5th Percentile | 97.5th Percentile | |
| 50–54 | 226 | 93.4 | 73.7 | 113.1 | 217 | 93.0 | 73.1 | 113.0 |
| 55–59 | 405 | 88.8 | 69.2 | 108.3 | 423 | 89.4 | 69.6 | 109.3 |
| 60–64 | 566 | 84.2 | 64.7 | 103.6 | 521 | 85.8 | 66.1 | 105.5 |
| 65–69 | 296 | 79.6 | 60.3 | 98.9 | 293 | 82.2 | 62.7 | 101.8 |
| 70–74 | 129 | 75.0 | 55.8 | 94.1 | 102 | 78.6 | 59.2 | 98.0 |
| 75–79 | 253 | 70.4 | 51.4 | 89.4 | 225 | 75.0 | 55.7 | 94.3 |
| 80–84 | 164 | 65.8 | 46.9 | 84.7 | 188 | 71.4 | 52.2 | 90.6 |
| 85–89 | 68 | 61.2 | 42.4 | 79.9 | 79 | 67.8 | 48.8 | 86.8 |
| ≥90 | 20 | 56.6 | 38.0 | 75.2 | 34 | 64.2 | 45.3 | 83.1 |
Estimates corresponding to Figure 3.
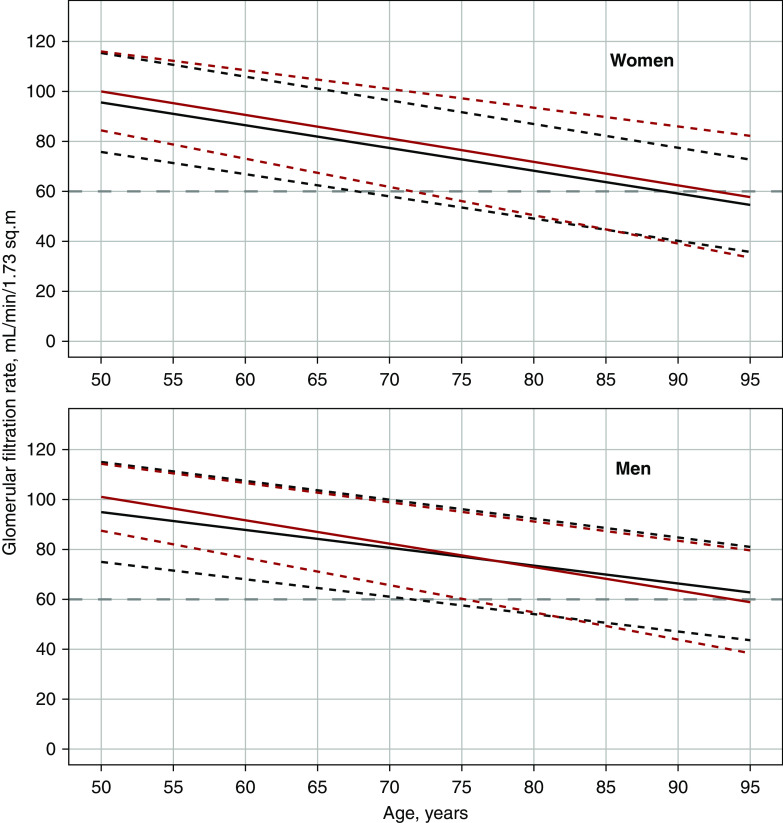
The final GAMLSS model in Table 3 was reanalyzed using eGFRcrea as the dependent variable instead of iohexol clearance (Supplemental Table 6). The predicted median and 95% reference interval for eGFRcrea in a person who was healthy are compared with iohexol clearance in Figure 4. Contrary to iohexol clearance, there was no sex difference in the eGFRcrea-age association. Also, the SD of the eGFRcrea distribution demonstrated an increase with both age and female sex, which was not seen with iohexol clearance. However, the eGFRcrea-age association was still negative for the 97.5th percentiles in both sexes (Figure 4).
Figure 4.
Measured and estimated GFR in healthy persons according to sex. Predicted medians (bold lines) and 2.5th and 97.5th percentiles (dashed lines) of iohexol clearance (black) and eGFR based on creatinine (red) as functions of age for healthy women (upper panel) and men (lower panel). The gray dashed line indicates the 60 ml/min per 1.73 m2 level.
Discussion
We found a negative linear association between population mean GFR and older age, even in persons defined as healthy by a set of very stringent criteria. The point estimate for the GFR-age association in healthy persons was −0.72 and −0.92 ml/min per 1.73 m2 per year in men and women, respectively. Previous cross-sectional estimates of the GFR-age association have varied widely,47–55 probably because of differences between the study populations. In potential living kidney donors, point estimates between −0.5 and −0.9 ml/min per 1.73 m2 per year have been found.51–53,56–58
If adjustment for health status had eliminated the GFR-age association, this would have supported the hypothesis that poor health fully accounts for the finding of lower mean GFR in old age.5 It is noteworthy that the SD of GFR did not increase with age in persons who were healthy, as would be expected if a subset of this group had unrecognized kidney disease, whereas others aged with preserved GFR. If even a minority of exceptionally healthy individuals aged with preserved rather than lower GFR, one would expect no or only a weak age association for the 97.5th percentile of the GFR distribution. Although the absence of these findings in this study does not refute the hypothesis, it suggests that age or other factors may contribute to the association. A similar finding regarding the 95th percentile was observed in a study of potential living kidney donors by Chakkera et al.59 The results are also consistent with histologic findings in biopsies from living kidney donors, which indicate that nephron number is lower at older age even in people who are apparently healthy, although the study by Denic et al.6 only included persons younger than 75 years and may not be representative of older healthy people in the general population.
The observation of a high proportion of persons with nondeclining GFR in some longitudinal studies may be explained by a failure to use statistical methods that take measurement error into account.8,10,12,60–62 This will overestimate the variability of the rate at which GFR declines and the proportion of persons with nondeclining GFR. Also, none of these studies used precise methods to measure GFR,8–15,60–66 and, except for one small Swedish study,67 they were not population based.60,61,63–66 The Baltimore Longitudinal Study of Aging is often referred to as having found that a third of a group of persons who were healthy experienced no decline in GFR. However, the study may have been biased by the use of creatinine clearance for assessing GFR and the inclusion of patients with diabetes in the healthy group.60
Being unhealthy was associated with a slightly higher GFR in the regression model (Table 3), which indicates that mean GFR for persons who were unhealthy was higher than for those who were healthy among the youngest participants (Figure 3). One explanation for this paradoxic finding may be the glomerular hyperfiltration associated with obesity, smoking, elevated BP, and other risk factors.68–73 Previous longitudinal results from the RENIS cohort and Pima Indians in the United States have indicated that hyperfiltration is independently associated with a more rapid decline in GFR.74
The GFR-age association was less negative in men than in women (Table 3). This finding was consistent across all three cohorts and in models adjusting for differences in health status between the sexes. Previous population-based studies,9,12 small studies using measured GFR,51,52,54,75–77 and studies of persons with established CKD62,78–81 have yielded mixed results about sex differences in GFR-age associations, but most of them did not fully adjust for comorbidity and risk factors. Our findings may partly explain the higher prevalence of CKD in women than in men, but do not explain why more men than women initiate RRT.82 However, the findings should be interpreted with caution because of the lack of longitudinal data.
Statistically significant random effects demonstrated differences in mean GFR for men and in GFR-age associations across the three cohorts (Supplemental Table 3). Selection bias relative to the source populations in the RENIS and AGES-Kidney cohorts seems unlikely because these cohorts had high participation rates and eGFR similar to those who did not participate.23,25,26 The participation rate in BIS was lower, but comparisons with the source population indicate the cohort was representative.28 The most probable explanation for the heterogeneity may be unmeasured confounders. Unmeasured morbidity, risk factors, and medications are the most likely possibilities; but differences in genetic, epigenetic, or environmental factors (e.g., the dietary intake of protein) cannot be excluded. There are also other indications of geographic variations in kidney disease within Europe. One study of CKD prevalence found that Norway had the lowest and Northeastern Germany the highest prevalence,83 and that these differences could not be explained by variations in hypertension, diabetes, or obesity.
One method for defining reference intervals for physiologic or biochemical parameters is to take the 95th interpercentile range of their distribution in a population of persons who are healthy. For age-dependent parameters, it is almost impossible to find cohorts of sufficient size for the oldest age groups, which include very few individuals who are truly healthy.7 Accordingly, most previous studies of reference intervals for GFR have included few individuals older than 70 years and have made no distinction between healthy subjects and others.53,84–86 By contrast, we used a statistical model to estimate the effects of disease and risk factors and to predict percentiles of GFR in persons who were healthy and between age 50 and 95 years. Because we adjusted for the heterogeneity between the cohorts, the predictions represent an average of the observations in three different geographic regions.
A comparison between classification of low GFR based on the 2.5th percentile for persons who are healthy in this study and the currently accepted criterion for CKD stage 3–5 (GFR <60 ml/min per 1.73 m2) shows that the criterion underestimates the prevalence of low GFR in women younger than 67.1 years and in men younger than 70.8 years, and overestimates the prevalence at higher ages (Figure 3). However, this is the result of applying a low percentile and a rather strict definition of “healthy” to assess the effect of age on GFR under optimal physiologic conditions. In addition to reference intervals for GFR in people who are healthy, an optimal classification system for CKD should be based on the risk of adverse outcomes at different levels of GFR. Further research of GFR as a risk factor in old age is needed.87
In clinical practice, eGFRcrea is the most commonly used method for assessing GFR. Similar to iohexol clearance, both the median and 97.5th percentile of eGFRcrea had negative associations with age (Figure 4). However, there were several differences between eGFRcrea and iohexol clearance when modeling their relationship with age (Supplemental Table 6). A study of potential kidney donors also found differences between the 95th percentiles of eGFRcrea and measured GFR.59 This indicates that measured GFR should be used for establishing reference intervals for GFR.
The principal strength of this study is that we used measured GFR instead of eGFR and that the cohorts are population based. To our knowledge, the three cohorts included in the study are the only ones in Europe with these characteristics. The number of included persons far exceeds that of previous studies using measured GFR, especially in the oldest age groups. Although the low number of persons who were healthy in the highest age groups may have limited the power of statistical tests for the interaction between age, health status, and other variables, it seems unlikely that we have failed to include a significant number of very old persons who were healthy with preserved GFR that would have changed our conclusion.
A consensus about an operational definition of healthy does not exist. Although it could have been made more stringent, we believe that the definition used in this investigation is very conservative and excludes most common conditions that affect GFR. One exception is that the definition does not explicitly include heart failure because this information was not available in BIS and RENIS. However, we believe that the inclusion of other cardiovascular disease and medications as indicators of heart failure ensured that very few of these patients were misclassified as healthy (see Methods). Another possible limitation of the definition is that it does not explicitly exclude persons with specific types of kidney disease, or persons with CKD based on kidney damage other than albuminuria as ascertained by kidney imaging or urine sediment examination. However, the criteria excluding persons with albuminuria and hypertension will probably also exclude most of these people. Therefore, we assume that this limitation had only a small effect on our estimates.
The heterogeneity between the three cohorts is a reason for caution in applying the results to other populations. We cannot exclude a survivor bias in the estimates of the GFR distribution, especially in the older age groups. However, this would support rather than weaken our conclusion of a lower GFR in older people who are healthy. Due to the cross-sectional design of our study, the GFR-age associations apply at the population level, whereas the rates of change at the individual level could not be estimated. In principle, GFR-age associations observed over time in follow-up of individuals may differ from those observed across cohorts of individuals at different ages.88 However, even allowing for great variation and possible nonlinear individual GFR trajectories, the finding that the 2.5th percentile in 50-year-old people who are healthy is similar to the 97.5th percentile in 95-year-old people who are healthy (Figure 3) suggests that aging with preserved GFR across this age span must be very uncommon. Although difficult to perform, longitudinal studies of GFR decline would be of value to confirm these findings at the individual level. Another limitation was the difference between the cohorts regarding inclusion of subjects and available data on morbidity and risk factors. Statistical methods have been used to adjust for these differences.
We conclude that there is a negative linear GFR-age association in people who are healthy and aged between 50 and 95 years, and an even more negative association for people with chronic diseases and CKD risk factors. Although it can only conclusively be demonstrated in longitudinal studies with repeated GFR measurements in the same individuals, this finding suggests that healthy aging is not associated with preserved GFR in old age.
Disclosures
Dr. Eriksen reports grants from Boehringer-Ingelheim during the conduct of the study. Dr. Inker reports grants and other from Omeros Corporation, grants from Otsuka, grants from Reata Pharmaceuticals, grants from Retrophin, and other from Tricida, outside the submitted work. Dr. Levey reports grants from National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study, grants from the National Kidney Foundation, and grants from Siemens, outside the submitted work. Dr. Levey also reports being a Kidney Disease Improving Global Outcomes CKD Guideline Workgroup Member. Dr. Schaeffner reports grants from DDnÄ Institut für Disease Management, grants from Kuratorium für Dialyse und Nierentransplantation foundation of preventive medicine, during the conduct of the study, and reports receiving honoraria for lectures from Fresenius Kabi, Fresenius Medical Care, and Siemens Healthineers, outside the submitted work. Dr. van der Giet received funding from the Deutsche Forschungsgemeinschaft, Else-Kröner Fresenius Stiftung, Federal Ministry of Education and Research (BMBF), and Jackstädt-Stiftung. Dr. van der Giet received lecture fees from Bayer, Berlin Chemie, CVRX, IEM, Otsuka, Servier, and Vifor. Dr. van der Giet has a consulting agreement with IEM and Charité Research Organization. All remaining authors have nothing to disclose.
Funding
AGES-Kidney was funded by National Institutes of Health, National Institute on Aging (NIA) contract N01-AG-1-2100; the NIA Intramural Research Program; Hjartavernd (the Icelandic Heart Association); and Althingi (the Icelandic Parliament). BIS was funded by the Kuratorium für Dialyse und Nierentransplantation (KfH) foundation of preventive medicine and the DDnÄ Institut für Disease Management e.V. RENIS-T6 and RENIS-FU were funded by Helse Nord RHF (Northern Norway Regional Health Authority), Universitetet i Tromsø The Arctic University of Norway, and by a grant from Boehringer-Ingelheim.
Supplementary Material
Acknowledgments
B.O. Eriksen, R. Palsson, N. Ebert, T. Melsom, O.S. Indridason, and E. Schaeffner are grateful to their comembers of the European Kidney Function Consortium for advice and support. We thank all of the participants in the AGES-Kidney, BIS, and RENIS-FU cohorts for their contributions to this investigation.
N. Ebert, B.O. Eriksen, O.S. Indridason, T. Melsom, R. Palsson, and E. Schaeffner designed the study; N. Ebert, B.O. Eriksen, V. Gudnason, O.S. Indridason, L.A. Inker, A.S. Levey, T. Melsom, R. Palsson, E. Schaeffner, and M. van der Giet organized the GFR measurements and collected the data; B.O. Eriksen analyzed the data, made the figures, and drafted the paper; N. Ebert, B.O. Eriksen, V. Gudnason, O.S. Indridason, L.A. Inker, T.G. Jenssen, A.S. Levey, T. Melsom, R. Palsson, H. Tighiouart, E. Schaeffner, and M. van der Giet interpreted the data and revised the paper. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020020151/-/DCSupplemental.
Supplemental Table 1. Missing data in RENIS-T6, RENIS-FU, BIS and AGES-Kidney.
Supplemental Table 2. Characteristics of the study population according to health status.
Supplemental Table 3. General additive mixed model analysis of GFR mean and standard deviation in three population-based cohorts. Fixed and random effects.
Supplemental Table 4. General additive mixed model analysis of GFR mean and SD in three population-based cohorts. Fixed cohort effects.
Supplemental Table 5. Predicted percentiles of GFR (ml/min per 1.73 m2) for healthy women and men according to age group.
Supplemental Table 6. General additive mixed model analysis of creatinine-based eGFR mean and SD in three population-based cohorts. Fixed and random effects.
Supplemental Figure 1. Histogram of the residuals for the final GAMLSS model.
Supplemental Figure 2. GFR-age association for the GAMLSS models, stratified according to age and sex.
References
- 1.Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, et al.: Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 94: 567–581, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R, Bunaye Z, Bekele DM, Light RP: Competing risk factor analysis of end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 28: 569–575, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Inker LA, Coresh J: Chronic kidney disease in older people. JAMA 314: 557–558, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Delanaye P, Jager KJ, Bökenkamp A, Christensson A, Dubourg L, Eriksen, et al.: CKD: A Call for an Age-Adapted Definition. J Am Soc Nephrol 30: 1785–1805, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muntner P: Longitudinal measurements of renal function. Semin Nephrol 29: 650–657, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, et al.: Single-nephron glomerular filtration rate in healthy adults. N Engl J Med 376: 2349–2357, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delanaye P, Schaeffner E, Ebert N, Cavalier E, Mariat C, Krzesinski JM, et al.: Normal reference values for glomerular filtration rate: what do we really know? Nephrol Dial Transplant 27: 2664–2672, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Lauretani F, Semba RD, Bandinelli S, Miller ER 3rd, Ruggiero C, Cherubini A, et al.: Plasma polyunsaturated fatty acids and the decline of renal function. Clin Chem 54: 475–481, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmelgarn BR, Zhang J, Manns BJ, Tonelli M, Larsen E, Ghali WA, et al.: Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int 69: 2155–2161, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, et al.: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halbesma N, Jansen DF, Stolk RP, De Jong PE, Gansevoort RT; PREVEND Study group : Changes in renal risk factors versus renal function outcome during follow-up in a population-based cohort study. Nephrol Dial Transplant 25: 1846–1853, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Kronborg J, Solbu M, Njølstad I, Toft I, Eriksen BO, Jenssen T: Predictors of change in estimated GFR: A population-based 7-year follow-up from the tromso study. Nephrol Dial Transplant 23: 2818–2826, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Robinson-Cohen C, Katz R, Mozaffarian D, Dalrymple LS, de Boer I, Sarnak M, et al.: Physical activity and rapid decline in kidney function among older adults. Arch Intern Med 169: 2116–2123, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peralta CA, Jacobs DR Jr., Katz R, Ix JH, Madero M, Duprez DA, et al.: Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60 mL/min/1.73 m(2): The multi-ethnic study of atherosclerosis (MESA). Am J Kidney Dis 59: 41–49, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malmgren L, McGuigan FE, Berglundh S, Westman K, Christensson A, Åkesson K: Declining estimated glomerular filtration rate and its association with mortality and comorbidity over 10 years in elderly women. Nephron 130: 245–255, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Mathisen UD, Melsom T, Ingebretsen OC, Jenssen T, Njølstad I, Solbu MD, et al.: Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol 22: 927–937, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, et al.: Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65: 1416–1421, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, et al.: Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 75: 652–660, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schei J, Stefansson VTN, Mathisen UD, Eriksen BO, Solbu MD, Jenssen TG, et al.: Residual associations of inflammatory markers with eGFR after accounting for measured GFR in a community-based cohort without CKD. Clin J Am Soc Nephrol 11: 280–286, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melsom T, Fuskevåg OM, Mathisen UD, Strand H, Schei J, Jenssen T, et al.: Estimated GFR is biased by non-traditional cardiovascular risk factors. Am J Nephrol 41: 7–15, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Eriksen BO, Mathisen UD, Melsom T, Ingebretsen OC, Jenssen TG, Njølstad I, et al.: Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int 78: 1305–1311, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Schaeffner ES, van der Giet M, Gaedeke J, Tölle M, Ebert N, Kuhlmann MK, et al.: The Berlin initiative study: The methodology of exploring kidney function in the elderly by combining a longitudinal and cross-sectional approach. Eur J Epidemiol 25: 203–210, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Inker LA, Okparavero A, Tighiouart H, Aspelund T, Andresdottir MB, Eiriksdottir G, et al.: Midlife blood pressure and late-life GFR and albuminuria: An elderly general population cohort. Am J Kidney Dis 66: 240–248, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melsom T, Schei J, Stefansson VT, Solbu MD, Jenssen TG, Mathisen UD, et al.: Prediabetes and risk of glomerular hyperfiltration and albuminuria in the general nondiabetic population: A prospective cohort study. Am J Kidney Dis 67: 841–850, 2016. [DOI] [PubMed] [Google Scholar]
- 25.Grønn KW: Tromsøundersøkelsen - Tromsø 6, Tromsø, Norway, UiT The Arctic University of Norway, 2014 [Google Scholar]
- 26.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, et al.: Age, gene/environment susceptibility-reykjavik study: Multidisciplinary applied phenomics. Am J Epidemiol 165: 1076–1087, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksen BO, Melsom T, Mathisen UD, Jenssen TG, Solbu MD, Toft I: GFR normalized to total body water allows comparisons across genders and body sizes. J Am Soc Nephrol 22: 1517–1525, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebert N, Jakob O, Gaedeke J, van der Giet M, Kuhlmann MK, Martus P, et al.: Prevalence of reduced kidney function and albuminuria in older adults: The Berlin initiative study. Nephrol Dial Transplant 32: 997–1005, 2017. [DOI] [PubMed] [Google Scholar]
- 29.Tamayo T, Brinks R, Hoyer A, Kuß OS, Rathmann W: The prevalence and incidence of diabetes in Germany. Dtsch Arztebl Int 113: 177–182, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gößwald A, Schienkiewitz A, Nowossadeck E, Busch MA: [Prevalence of myocardial infarction and coronary heart disease in adults aged 40-79 years in Germany: Results of the German health interview and examination survey for adults (DEGS1)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 56: 650–655, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Busch MA, Schienkiewitz A, Nowossadeck E, Gößwald A: [Prevalence of stroke in adults aged 40 to 79 years in Germany: Results of the German health interview and examination survey for adults (DEGS1)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 56: 656–660, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Jacob L, Breuer J, Kostev K: Prevalence of chronic diseases among older patients in German general practices. Ger Med Sci 14: Doc03, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaeffner ES, Ebert N, Delanaye P, Frei U, Gaedeke J, Jakob O, et al.: Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 157: 471–481, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Melsom T, Stefansson V, Schei J, Solbu M, Jenssen T, Wilsgaard T, et al.: Association of increasing GFR with change in albuminuria in the general population. Clin J Am Soc Nephrol 11: 2186–2194, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, Coresh J: Chronic kidney disease. Lancet 379: 165–180, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Eriksen BO, Stefansson VTN, Jenssen TG, Mathisen UD, Schei J, Solbu MD, et al.: Elevated blood pressure is not associated with accelerated glomerular filtration rate decline in the general non-diabetic middle-aged population. Kidney Int 90: 404–410, 2016. [DOI] [PubMed] [Google Scholar]
- 37.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al.; ESC Scientific Document Group: 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 39: 3021–3104, 2018. [DOI] [PubMed] [Google Scholar]
- 38.Eriksen BO, Schaeffner E, Melsom T, Ebert N, van der Giet M, Gudnason V, et al.: Comparability of plasma iohexol clearance across population-based cohorts [published online ahead of print December 23, 2019]. Am J Kidney Dis 10.1053/j.ajkd.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 39.DuBois D, Dubois EF: The measurement of the surface area of man. Arch Intern Med (Chic) 15: 868–881, 1915 [Google Scholar]
- 40.Björk J, Grubb A, Gudnason V, Indridason OS, Levey AS, Palsson R, et al.: Comparison of glomerular filtration rate estimating equations derived from creatinine and cystatin C: Validation in the age, gene/environment susceptibility-reykjavik elderly cohort. Nephrol Dial Transplant 33: 1380–1388, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burke DL, Ensor J, Riley RD: Meta-analysis using individual participant data: One-stage and two-stage approaches, and why they may differ. Stat Med 36: 855–875, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood SN: Generalized Additive Models. An Introduction with R, Boca Raton, FL, CRC Press, 2017 [Google Scholar]
- 44.Fasiolo M, Nedellec R, Goude Y, Wood SN: Scalable visualisation methods for modern Generalized Additive Models. arXiv 2018 [Google Scholar]
- 45.Burnham KP: Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, New York, Springer, 2002 [Google Scholar]
- 46.Fasiolo M, Wood SN, Zaffran M, Nedellec R, Goude Y: Fast calibrated additive quantile regression [published online ahead of print March 11, 2020]. J Am Stat Assoc 10.1080/01621459.2020.1725521 [DOI] [Google Scholar]
- 47.Davies DF, Shock NW: Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 29: 496–507, 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Granerus G, Aurell M: Reference values for 51Cr-EDTA clearance as a measure of glomerular filtration rate. Scand J Clin Lab Invest 41: 611–616, 1981. [DOI] [PubMed] [Google Scholar]
- 49.Bäck SE, Ljungberg B, Nilsson-Ehle I, Borgå O, Nilsson-Ehle P: Age dependence of renal function: Clearance of iohexol and p-amino hippurate in healthy males. Scand J Clin Lab Invest 49: 641–646, 1989. [DOI] [PubMed] [Google Scholar]
- 50.Fehrman-Ekholm I, Skeppholm L: Renal function in the elderly (>70 years old) measured by means of iohexol clearance, serum creatinine, serum urea and estimated clearance. Scand J Urol Nephrol 38: 73–77, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, et al.: Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis 43: 112–119, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Grewal GS, Blake GM: Reference data for 51Cr-EDTA measurements of the glomerular filtration rate derived from live kidney donors. Nucl Med Commun 26: 61–65, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Poggio ED, Rule AD, Tanchanco R, Arrigain S, Butler RS, Srinivas T, et al.: Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int 75: 1079–1087, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma YC, Zuo L, Chen L, Su ZM, Meng S, Li JJ, et al.: Distribution of measured GFR in apparently healthy Chinese adults. Am J Kidney Dis 56: 420–421, 2010. [DOI] [PubMed] [Google Scholar]
- 55.Jafar TH, Islam M, Jessani S, Bux R, Inker LA, Mariat C, et al.: Level and determinants of kidney function in a South Asian population in Pakistan. Am J Kidney Dis 58: 764–772, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaillard F, Courbebaisse M, Kamar N, Rostaing L, Del Bello A, Girerd S, et al.: The age-calibrated measured glomerular filtration rate improves living kidney donation selection process. Kidney Int 94: 616–624, 2018. [DOI] [PubMed] [Google Scholar]
- 57.Peters AM, Perry L, Hooker CA, Howard B, Neilly MDJ, Seshadri N, et al.: Extracellular fluid volume and glomerular filtration rate in 1878 healthy potential renal transplant donors: Effects of age, gender, obesity and scaling. Nephrol Dial Transplant 27: 1429–1437, 2012. [DOI] [PubMed] [Google Scholar]
- 58.Pottel H, Hoste L, Yayo E, Delanaye P: Glomerular filtration rate in healthy living potential kidney donors: A meta-analysis supporting the construction of the full age spectrum equation. Nephron 135: 105–119, 2017. [DOI] [PubMed] [Google Scholar]
- 59.Chakkera HA, Denic A, Kremers WK, Stegall MD, Larson JJ, Ravipati H, et al.: Comparison of high glomerular filtration rate thresholds for identifying hyperfiltration [published online ahead of print November 6, 2018]. Nephrol Dial Transplant 10.1093/ndt/gfy332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985. [DOI] [PubMed] [Google Scholar]
- 61.Jiang S, Sun X, Gu H, Chen Y, Xi C, Qiao X, et al.: Age-related change in kidney function, its influencing factors, and association with asymptomatic carotid atherosclerosis in healthy individuals--a 5-year follow-up study. Maturitas 73: 230–238, 2012. [DOI] [PubMed] [Google Scholar]
- 62.Sesso R, Prado F, Vicioso B, Ramos LR: Prospective study of progression of kidney dysfunction in community-dwelling older adults. Nephrology (Carlton) 13: 99–103, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Baba M, Shimbo T, Horio M, Ando M, Yasuda Y, Komatsu Y, et al.: Longitudinal study of the decline in renal function in healthy subjects. PLoS One 10: e0129036, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen E, Nardi Y, Krause I, Goldberg E, Milo G, Garty M, et al.: A longitudinal assessment of the natural rate of decline in renal function with age. J Nephrol 27: 635–641, 2014. [DOI] [PubMed] [Google Scholar]
- 65.Imai E, Horio M, Yamagata K, Iseki K, Hara S, Ura N, et al.: Slower decline of glomerular filtration rate in the Japanese general population: A longitudinal 10-year follow-up study. Hypertens Res 31: 433–441, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Mänttäri M, Tiula E, Alikoski T, Manninen V: Effects of hypertension and dyslipidemia on the decline in renal function. Hypertension 26: 670–675, 1995. [DOI] [PubMed] [Google Scholar]
- 67.Larsson M, Jagenburg R, Landahl S: Renal function in an elderly population. A study of S-creatinine, 51Cr-EDTA clearance, endogenous creatinine clearance and maximal tubular water reabsorption. Scand J Clin Lab Invest 46: 593–598, 1986. [DOI] [PubMed] [Google Scholar]
- 68.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S: Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant 27: 1821–1825, 2012. [DOI] [PubMed] [Google Scholar]
- 69.Maeda I, Hayashi T, Sato KK, Koh H, Harita N, Nakamura Y, et al.: Cigarette smoking and the association with glomerular hyperfiltration and proteinuria in healthy middle-aged men. Clin J Am Soc Nephrol 6: 2462–2469, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinto-Sietsma SJ, Mulder J, Janssen WM, Hillege HL, de Zeeuw D, de Jong PE: Smoking is related to albuminuria and abnormal renal function in nondiabetic persons. Ann Intern Med 133: 585–591, 2000. [DOI] [PubMed] [Google Scholar]
- 71.Tomaszewski M, Charchar FJ, Maric C, McClure J, Crawford L, Grzeszczak W, et al.: Glomerular hyperfiltration: A new marker of metabolic risk. Kidney Int 71: 816–821, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Lee J, Kim HJ, Cho B, Park JH, Choi HC, Lee CM, et al.: Abdominal adipose tissue was associated with glomerular hyperfiltration among non-diabetic and normotensive adults with a normal body mass index. PLoS One 10: e0141364, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taal MW, Brenner BM: Predicting initiation and progression of chronic kidney disease: Developing renal risk scores. Kidney Int 70: 1694–1705, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Melsom T, Nair V, Schei J, Mariani L, Stefansson VTN, Harder JL, et al.: Correlation between baseline GFR and subsequent change in GFR in Norwegian adults without diabetes and in Pima Indians. Am J Kidney Dis 73: 777–785, 2019. [DOI] [PubMed] [Google Scholar]
- 75.Wesson L: Physiology of the human kidney, New York, Grune & Stratton, 1969 [Google Scholar]
- 76.Slack TK, Wilson DM: Normal renal function: CIN and CPAH in healthy donors before and after nephrectomy. Mayo Clin Proc 51: 296–300, 1976. [PubMed] [Google Scholar]
- 77.Hamilton D, Riley P, Miola U, Mousa D, Popovich W, al Khader A: Total plasma clearance of 51Cr-EDTA: Variation with age and sex in normal adults. Nucl Med Commun 21: 187–192, 2000. [DOI] [PubMed] [Google Scholar]
- 78.Neugarten J, Acharya A, Silbiger SR: Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol 11: 319–329, 2000. [DOI] [PubMed] [Google Scholar]
- 79.Jafar TH, Schmid CH, Stark PC, Toto R, Remuzzi G, Ruggenenti P, et al.: The rate of progression of renal disease may not be slower in women compared with men: A patient-level meta-analysis. Nephrol Dial Transplant 18: 2047–2053, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Ricardo AC, Yang W, Sha D, Appel LJ, Chen J, Krousel-Wood M, et al.; CRIC Investigators: Sex-related disparities in CKD progression. J Am Soc Nephrol 30: 137–146, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eriksen BO, Ingebretsen OC: The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int 69: 375–382, 2006. [DOI] [PubMed] [Google Scholar]
- 82.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ: Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 14: 151–164, 2018. [DOI] [PubMed] [Google Scholar]
- 83.Brück K, Stel VS, Gambaro G, Hallan S, Völzke H, Ärnlöv J, et al.; European CKD Burden Consortium: CKD prevalence varies across the European general population. J Am Soc Nephrol 27: 2135–2147, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pottel H, Delanaye P, Weekers L, Selistre L, Goffin K, Gheysens O, et al.: Age-dependent reference intervals for estimated and measured glomerular filtration rate. Clin Kidney J 10: 545–551, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yayo E, Ayé M, Yao C, Gnionsahé A, Attoungbré M-L, Cavalier E, et al.: Measured (and estimated) glomerular filtration rate: Reference values in west africa. Nephrol Dial Transplant 33: 1176–1180, 2018. [DOI] [PubMed] [Google Scholar]
- 86.Price CP, Finney H: Developments in the assessment of glomerular filtration rate. Clin Chim Acta 297: 55–66, 2000. [DOI] [PubMed] [Google Scholar]
- 87.Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, et al.; Chronic Kidney Disease Prognosis Consortium: Age and association of kidney measures with mortality and end-stage renal disease. JAMA 308: 2349–2360, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hofer SM, Sliwinski MJ, Flaherty BP: Understanding ageing: Further commentary on the limitations of cross-sectional designs for ageing research. Gerontology 48: 22–29, 2002 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.