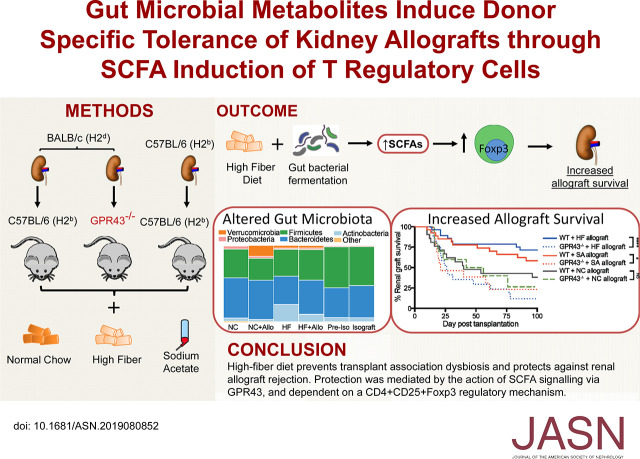
Significance Statement
The gut microbiome is known to affect immune responses in autoimmunity and cancer, but little is known about its role in transplant immunity. In a mouse model, the authors observed dysbiosis after kidney transplantation in the absence of antibiotics or other drugs. A high-fiber diet prevented dysbiosis and afforded protection against allograft rejection, as did supplementation with the short-chain fatty acids sodium acetate or sodium butyrate, microbial metabolites produced by gut fermentation of dietary fiber. This protection was dependent on the G protein–coupled receptor GPR43 and T regulatory cells. These findings show how the microbiome can be modified to retard alloimmunity in a mouse model of kidney transplantation, and provide a rationale to explore this strategy in humans as a means to facilitate transplant acceptance.
Keywords: transplantation, acute allograft rejection, tolerance, chronic allograft rejection, immunology
Visual Abstract
Abstract
Background
Short-chain fatty acids derived from gut microbial fermentation of dietary fiber have been shown to suppress autoimmunity through mechanisms that include enhanced regulation by T regulatory cells (Tregs).
Methods
Using a murine kidney transplantation model, we examined the effects on alloimmunity of a high-fiber diet or supplementation with the short-chain fatty acid acetate. Kidney transplants were performed from BALB/c(H2d) to B6(H2b) mice as allografts in wild-type and recipient mice lacking the G protein–coupled receptor GPR43 (the metabolite-sensing receptor of acetate). Allograft mice received normal chow, a high-fiber diet, or normal chow supplemented with sodium acetate. We assessed rejection at days 14 (acute) and 100 (chronic), and used 16S rRNA sequencing to determine gut microbiota composition pretransplantation and post-transplantation.
Results
Wild-type mice fed normal chow exhibited dysbiosis after receiving a kidney allograft but not an isograft, despite the avoidance of antibiotics and immunosuppression for the latter. A high-fiber diet prevented dysbiosis in allograft recipients, who demonstrated prolonged survival and reduced evidence of rejection compared with mice fed normal chow. Allograft mice receiving supplemental sodium acetate exhibited similar protection from rejection, and subsequently demonstrated donor-specific tolerance. Depletion of CD25+ Tregs or absence of the short-chain fatty acid receptor GPR43 abolished this survival advantage.
Conclusions
Manipulation of the microbiome by a high-fiber diet or supplementation with sodium acetate modified alloimmunity in a kidney transplant model, generating tolerance dependent on Tregs and GPR43. Diet-based therapy to induce changes in the gut microbiome can alter systemic alloimmunity in mice, in part through the production of short-chain fatty acids leading to Treg cell development, and merits study as a potential clinical strategy to facilitate transplant acceptance.
Kidney transplantation remains the best treatment for end-stage kidney failure, but life-long immunosuppression is required to prevent rejection.1 Current immunosuppressive strategies remain only partially effective, increase recipient susceptibility to infection and cancer, and paradoxically contribute to premature graft failure.2 Establishing donor-specific allograft tolerance while maintaining adequate immunity to protect against infection and cancer would limit the burden of current treatment, but is only rarely achieved in humans. With growing knowledge of the gut microbiomes capacity to influence systemic host immune responses,3 modulation of the gut microbiota, such as by diet, offers a novel pathway to favorably influence the host response to alloantigens.
The clinical relevance of the gut microbiota in human immunity has been demonstrated most profoundly in cancer studies, where the antitumor response of immune checkpoint inhibition was revealed to be dependent on specific gut microbiota, with nonresponsive mice converted to a responder phenotype by gut microbiota transfer.4–6 In experimental autoimmunity, spontaneous development of diabetes in a nonobese diabetic mouse model of type 1 diabetes required both a proinflammatory gut microbiota and an intact toll-like receptor signaling cascade to facilitate development of insulitis,7 with susceptibility transferrable from affected to unaffected mice by microbiota transfer.8 Kidney allograft rejection shares common pathways to those causing insulitis in nonobese diabetic mice, including the toll-like receptor 4–MyD88 pathway that we have previously reported to be required for the development of ischemia reperfusion injury9 and full development of acute allograft rejection in experimental kidney transplantation.10,11
Several experimental models of allograft rejection have demonstrated altered rejection kinetics on the basis of microbiota composition; however, results have been variable. Survival of skin allografts in C57BL/6 mice differs between vendors and is both microbiota dependent and transferrable.12 Delayed rejection kinetics of cardiac allografts are seen in both gnotobiotic and antibiotic pretreated mice.13 However, similar antibiotic depletion of gut microbiota accelerated rejection of murine aortic allografts,14 suggesting greater complexity to the microbiota–immunity axis.
These findings suggest that the gut microbiota is likely to play a significant role in the immune responses that determine the fate of a kidney allograft: tolerance or rejection. Several potential mechanisms exist: (1) interaction between gut organisms, their metabolites, and immune cells in the gut wall, mediated by pathogen-associated molecular pattern/innate receptor interactions15; (2) disruption of gut homeostasis by microbiota leading to an inflammatory milieu that alters immune cell maturation16; and (3) production of metabolites, including short-chain fatty acids (SCFAs) that modulate cell function through specific G protein–coupled receptors (GPRs)17 or through inhibition of histone deacetylase (HDAC) activity, affecting gene transcription. Diet remains the largest exogenous determinant of gut microbiota composition, and can predictably and sustainably alter each of the above mechanisms. In particular, high intake of dietary fibers, which are in turn fermented by colonic bacteria to form SCFAs, has shown promising outcomes in experimental autoimmunity.18,19
Here, we examined the effects of a high-fiber (HF) diet on the gut microbiome and the alloimmune response to kidney transplantation, using a fully MHC-mismatched, murine kidney transplantation model, and explored the role of the SCFA acetate and its metabolite-sensing receptor GPR43 in mediating this response.
Methods
Study Design
Animals
Male BALB/c (H2-Kd) kidney donor, B10Br (H2-Kk) skin donor, and C57BL/6 (H2-Kb) recipient mice were obtained from the Animal Resource Centre (Perth, Australia). GPR43−/− mice on C57BL/6 backgrounds have been described,18 and were obtained from our animal facility. Life-sustaining kidney transplants were performed on wild-type (WT) C57BL/6 and GPR43−/− mice who received a kidney from a BALB/c donor as allografts or from a C57BL/6 donor as isografts. Male mice aged 10–16 weeks were used in all experiments and were housed in a specific pathogen-free facility within the University of Sydney. Animal care and experiments were conducted following established guidelines for animal care and were approved by the Animal Ethics Committee of the University of Sydney.
Survival Experiment
WT+HF allografts (n=28), WT+ sodium acetate (SA) allografts (n=27), and WT+ sodium butyrate (SB) allografts (n=21) were compared with WT allografts (n=21) and isografts (n=13) to establish a survival curve up to day 100 post-transplant.
For allograft recipients surviving to day 200, a proportion of WT+SA allografts (n=4) and WT allografts (n=4) received full-thickness skin grafts from three sources: BALB/c (H2-Kd kidney donor–matched skin allograft), C57BL/6 (H2-Kb syngeneic), and B10Br (H2-Kk third-party allografts). Skin grafts were observed regularly for signs of rejection up to day 100 after skin transplantation.
Time-Course Study
To examine for intragraft events and mechanisms, groups of mice were euthanized early (day 14) and late (day 100) to investigate for evidence of acute and chronic rejection, respectively.
Diets and Acetate Treatments
HF (SF11–029) enriched in guar gum and cellulose, zero fiber (SF09–28), and normal mouse chow (irradiated mouse chow) were purchased from Specialty Feeds (Glen Forrest, WA, Australia) (Supplemental Table 1). Mice received normal chow (NC) upon arrival at our facility, followed by specific diets ad libitum commencing 2 weeks before and continuing throughout experiments.
The dose of SA and butyrate was adapted from Andrade-Oliveira et al.20 Briefly, 150 mM of either SA or SB solution (Sigma-Aldrich) was administered to mice as an intraperitoneal injection at a dose of 200 mg/kg, 30 minutes before and immediately after transplantation, and then daily for 2 weeks. SA (150 mM) or SB (100 mM) were then administered in their drinking water ad libitum until 100 days post-transplantation.
CD25+ Cell Depletion Study
To deplete CD4+CD25+ cells in vivo, mice received rat anti-mouse CD25 mAb (clone PC-61, rat IgG1; BioXcell, West Lebanon, NH) or isotype rat IgG, 0.5 mg per mouse, via intraperitoneal injection on days −2 and 0 post-transplantation (n=17). CD25+ cell depletion was confirmed between days 14 and 21 after kidney transplantation, by staining white blood cells with anti-CD25 (clone 7D4 or 3C7; BD Biosciences Pharmingen, CA) as previously described.11,21 Survival was assessed to day 100 post-transplantation.
Kidney Transplantation
Heterotopic kidney transplants and skin transplant were performed as previously described,11 and the detailed method is described in the Supplemental Material. Briefly, the left kidney of the donor animal was removed together with the ureter and vessels en masse and placed in the left iliac fossa of the recipient animal after an ipsilateral nephrectomy. No immunosuppressive therapy was administered. The recipient’s right native kidney was removed at day 3–7, rendering the graft to be life-sustaining. Animals with technical graft failure or wound infection became overtly ill (and were euthanized) or died within 4 days of the contralateral nephrectomy and were removed from the study.
Skin Transplantation
Full-thickness tail skin grafts were placed on graft beds prepared on recipients at day 200 after kidney allograft transplantation. Grafts were covered with protective bandages for 7 days. Rejection was defined as graft necrosis of ≥90% of the transplant skin area. Recipients that accepted their allograft demonstrated preserved graft size and hair growth.
Sample Harvest
Blood, kidney tissue, and urine were harvested at day 14 and day 100 after kidney transplant, as previously described.11,21 Mouse fecal samples were collected under sterile conditions immediately after extrusion, frozen on dry ice after retrieval, and stored at −80°C.
Bacteria 16S rRNA Sequencing and Bioinformatics
DNA was extracted from feces collected under sterile conditions with the QIAamp DNA Stool Mini Kit (QIAGEN) according to the manufacturer’s instructions. DNA was sequenced using tagged amplicons spanning the V4 region of bacterial 16S rRNA gene (515f/806r) on the Illumina MiSeq platform (2×250 bp) at the Ramaciotti Centre for Genomic (University of New South Wales, Sydney, NSW, Australia). Data were deposited in the European Nucleotide Archive under accession number PRJEB34109. Demultiplexed reads were processed using the QIIME 1.9.1 software.22 Briefly, paired-end reads were joined using the fast-q algorithm with no mismatches allowed. Operational taxonomic units (OTUs) were picked using 97% similarity with taxonomy assigned using the Greengenes v13_8 database, and de novo OTU picking was performed on sequences that did not match the reference database. Chimeric sequences were identified using ChimeraSlayer, and OTUs with total abundance of <0.01% were filtered from the table. Rarefaction analysis was used to compare the adequacy of sequencing depth to ensure that the microbiota populations were sufficiently sampled such that additional sampling would produce few additional OTUs (Supplemental Figure 1). For α-diversity analysis, samples were rarefied to a read depth of 46,397 using the Calypso workflow, which was also used to visualize the taxonomic make-up of microbial communities.23 The weighted UniFrac distance matrix was used to compare differences in microbial community composition between sample groups and visualized using a principal coordinate analysis plot,24 with the significance in the divergence of community structure assessed by Adonis (9999 permutations). The differential abundance of microbiota species in response to dietary change and transplantation was determined using the Kruskal–Wallis nonparametric test on libraries normalized by cumulative sum scaling25 through Calypso, and also on complete libraries using the negative binomial DESeq226 model (R package, phyloseq v1.29.0),27 with an false discovery rate–adjusted P value <0.01 for significance.
Assessment of Kidney Function
Serum creatinine was measured using the modified Jaffe rate reaction by the Biochemistry Department of The Royal Prince Alfred Hospital (Sydney, NSW, Australia). Total urinary protein was assessed by the Bradford method using a commercially available kit (Bio-Rad Laboratories, Gladesville, Australia) according to the manufacturer’s instructions.
Histology
Periodic acid–Schiff or Picro-Sirius red staining was performed to assess tubulitis, glomerulosclerosis and interstitial fibrosis, and interstitial collagen deposition. Previously described scoring systems11,28 were applied for each histologic parameter and have been included in the Supplemental Material.
Immunohistochemistry and Immunofluorescence
The detailed method for immunohistochemistry and immunofluorescence for C4d, including antibody clones and quantification, is included in the Supplemental Material. Briefly, acetone-fixed frozen sections were prepared, blocked, and exposed to a primary antibody for 60 minutes with concentration-matched IgG used as an isotype-negative control, followed by incubation with the appropriate biotinylated secondary antibody or anti-rat IgG conjugated with Alexa Fluor 488. Vector stain ABC kit (Vector Laboratories, Burlingame, CA) was applied to the tissue followed by 3,3′diaminobenzidine substrate-chromogen solution (Dako North America) and counterstained. Quantification was performed by scoring the number of positive cells in 20 consecutive high-power fields (HPFs), or by percentage of positive staining per HPF using a digital image analysis program.
RNA Extraction and Complementary DNA Synthesis
Total RNA was extracted from kidney tissue and cells using TRIzol (Invitrogen, Mulgrave, VIC, Australia). Complementary DNA was synthesized using oligo d(T)16 (Applied Biosystems) primers and the SuperScript III Reverse transcription kit (Invitrogen) as per the manufacturer’s instructions.
HDAC Activity
To determine the activity of HDAC in kidney allograft tissue, equal quantities of nuclear fraction proteins (20.0 μg) were analyzed using a Fluorometric HDAC Activity Assay Kit (k330–100; BioVision) as per the manufacturer’s instructions.
Real-Time PCR
Specific TaqMan primers and probes for IFN-γ, TNF, TGF-β, IL-4, IL-6, IL-10, IL-12, IL-23, granzyme A, granzyme B, perforin, CCL2, CCL5, CXCL9, CXCL10, indoleamine 2,3-dioxygenase, MMP-2, MMP-9, TIMP-1, TIMP-2, fibronectin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) have all been described previously.9,11,29 Complementary DNA was amplified in 1× Universal Master Mix (Applied Biosystems) with gene-specific primers and probes using either a Rotor-Gene 6000 system (Corbett Life Science) or Roche LightCycler 480 (Roche Applied Science, Penzberg, Germany). Acquired data sets were analyzed using the accompanying Rotor-Gene 6000 Analysis Software v1.7 or LightCycler 480 SW v1.5.1, respectively. All results are expressed as a fold expression normalized to GAPDH expression.
Statistical Analyses
Survival curves were constructed via the Kaplan–Meier method, and comparisons between graft survival times were analyzed with the log-rank test. Statistical differences between two groups were analyzed by unpaired, two-tailed t tests and multiple groups were compared using one- or two-way ANOVA with post hoc Tukey correction, using GraphPad Prism 7.0 software. A P value of <0.05 was considered to be statistically significant. Data are presented as mean±SEM.
Results
Transplantation-Associated Dysbiosis Was Prevented by an HF Diet
We first examined the effect of kidney transplantation, in the absence of antibiotics or immunosuppression, on the composition of the gut microbiota and determined the effect of dietary fiber content on this effect. WT mice were fed either NC or an HF diet enriched with guar gum and cellulose. After 2 weeks on their respective diet, groups of mice then received a kidney allograft (NC+Allo n=10, HF+Allo n=16) or isograft (n=9), and were single-housed and observed on their allocated diets for a further 2 weeks. Fecal samples were obtained immediately before transplantation and at 2 weeks post-transplantation to enable microbial analysis by sequencing of the V4 region of the 16S rRNA gene. After quality trimming, filtering, and removal of nonbacterial sequences, a total of 6,398,328 reads were generated from 68 fecal collections, with an average of 94,093±28,665 reads per sample.
Gut microbiota α-diversity was evaluated using the nonparametric Shannon diversity index, reflecting both the richness of species and their relative abundance. After transplant, NC fed mice experienced a significant loss in microbial diversity (Shannon diversity index: NC, 4.04±0.04; NC+Allo, 3.60±0.08; P<0.01), with isograft controls exhibiting a similar although less pronounced change (Shannon diversity index: Pre-Isograft, 3.93±0.12; isograft, 3.45±0.12; P<0.01). In contrast, diversity was modestly expanded post-transplant in HF-fed mice (Shannon diversity index: HF, 3.54±0.11; HF+Allo, 3.88±0.05; P<0.01) (Figure 1A). The richness of gut microbial communities was similarly reduced in NC-fed allograft and isograft recipients, yet remained unchanged in HF-fed allograft recipients (Supplemental Figure 2).
Figure 1.
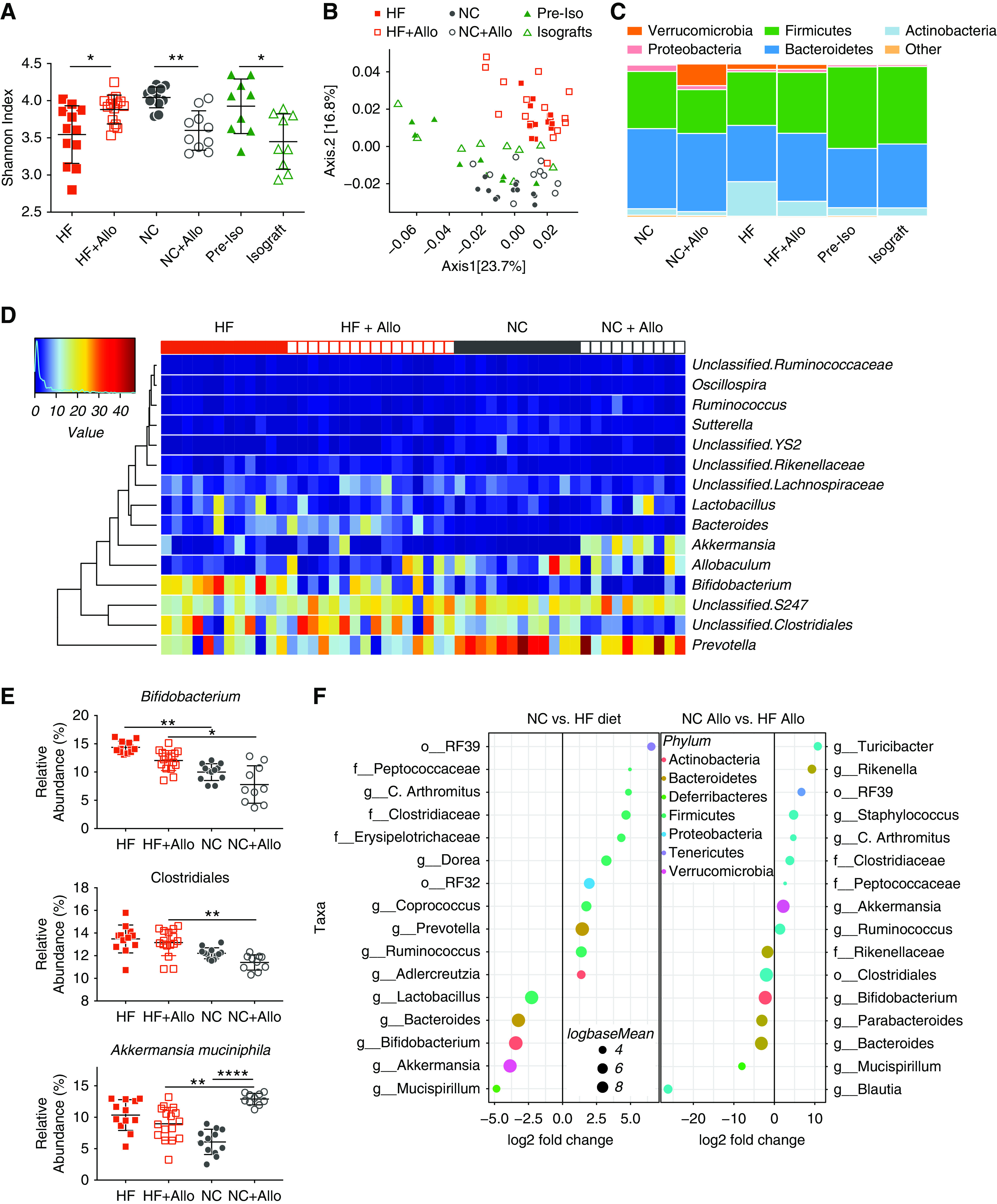
HF diet altered the gut microbial community structure and prevented transplant-associated dysbiosis. Fecal DNA analysis was performed on mice fed an NC (n=12) or HF (n=12) diet for 2 weeks. Mice then received a kidney allograft and continued on a NC diet (NC+Allo, n=10) or HF diet (HF+Allo, n=16) with fecal microbiota analyzed 14 days post-transplant. (A) Shannon diversity index demonstrated a change in bacterial diversity from pre- to post-transplant, with a reduction in diversity in NC-fed mice after transplant (P<0.01) compared with an increase in HF-fed mice (P<0.01). Isograft controls fed NC pre- and post-transplant (n=9 for both groups) also exhibited reduced diversity after transplantation by Shannon diversity index. (B) Principal coordinate analysis of the weighted UniFrac distance demonstrated significant modulation of the microbiota community postallograft (Adonis: NC versus NC+Allo, R2=0.28; P<0.001; HF versus HF+Allo R2=0.16; P=0.002), with significant dissimilarities between treatment groups (Adonis: NC+Allo versus HF+Allo, R2=0.31; P<0.001), but no significant change in isografts pre- versus post-transplant. (Adonis: preisograft versus isograft, R2=0.1; P=0.14). (C) Relative abundance of the dominant phyla in treatment groups and isograft controls, demonstrating shifts in microbial composition postallograft, and stability after isograft surgery. (D) Heatmap of the dominant microbiota genera of allograft animals. (E) Relative abundance after cumulative sum squaring (CSS) normalization of significant SCFA-producing OTUs and the mucin-degrading genus Akkermansia. (F) DESeq2 analysis demonstrating differential abundance of OTUs (false discovery rate–adjusted P<0.01) between dietary groups pre- and post-transplant. OTUs were assigned to their lowest described classification (y axis) and color-coded by phylum. Bubble size represents a log fold-change in the log base mean of the recorded OTU, with the x axis values demonstrating log2 fold-change in relative abundance. Fecal microbiota composition was assessed by 16S rRNA sequencing of the V4 region. Data are shown as the mean±SEM. Statistical analysis by ANOVA with Tukey post hoc analysis (A), and Kruskal–Wallis nonparametric testing (E). *P<0.05; **P<0.01; ****P<0.0001.
The UniFrac distance matrix was used to compare the effects of both diet and transplantation on the community structure of the gut microbiota, and weighted UniFrac distances were plotted using principal coordinate analysis, as shown in Figure 1B. The fecal microbiota of mice clustered separately according to diet, with subsequent shifts in microbial community structure occurring in both groups after transplantation. Adonis (9999 permutations) confirmed that both transplant and diet accounted for the significant variation in bacterial communities encountered between groups (R2=0.49; P<0.001). In comparison, NC-fed isograft controls exhibited no change in β-diversity after surgery (P=0.14; Figure 1B), suggesting that shifts in the microbial landscape seen in allografts are predominantly driven by the host–donor immune response, rather than by ischemia-reperfusion injury.
The relative abundance of the dominant bacteria at the phylum level is shown in Figure 1C. Post-transplant, alterations in the fecal microbiota were relatively consistent within the treatment groups, despite the single-housing of transplanted mice (Figure 1, C and D). Bacteroidetes and Firmicutes were the dominant phyla both pre- and post-transplant, regardless of diet. The next most-dominant phylum differed significantly according to diet. Verrucomicrobia (represented by a single genus, Akkermansia muciniphila), was significantly expanded in NC-fed mice post-transplant. This pattern, and in particular the expansion of Verrucomicrobia seen post-transplant (mean relative abundance: NC, 0.3%±0.11%; NC+Allo, 13.72%±1.77%), closely resembled that seen in nontransplant mice on a zero-fiber diet, previously reported as dysbiotic18,30 (Supplemental Figure 3). In contrast, HF-fed mice yielded only a small population of Verrucomicrobia (relative abundance 2.78%±1.09%), but displayed marked expansion of Actinobacteria both pre- and post-transplantation (Figure 1, C and D).
Differential abundance testing revealed significant differences in OTU population sizes between dietary groups, which was further modified after transplantation (Figure 1E). The microbiota of mice fed an HF diet exhibited significant increases in SCFA producing bacteria, including Bifidobacterium spp. (phylum Actinobacteria) and Bacteroides spp. Similarly, an unclassified commensal Clostridiales sp. was more abundant in the HF group compared with NC-fed mice, before and particularly after transplantation. The increase in Verrucomicrobia seen in transplanted NC-fed mice was exclusively because of the expansion of the mucus-degrading bacteria A. muciniphila. In contrast, isograft controls demonstrated relatively stable bacterial communities, with no significant shifts at the phylum level (Figure 1C) and differential abundance testing at the genus level revealing only a single Parabacteroides genus to have an altered relative abundance in response to isograft surgery (Supplemental Table 2).
The univariate differential abundance of recorded OTUs was confirmed using a negative binomial distribution model as implemented by DESeq2. Taxa were considered to be have differed significantly in their abundance if their false discovery rate–adjusted P value was <0.01 (Figure 1F, Supplemental Tables 2, 3, and 4).
HF Diet Prolonged Kidney Allograft Survival
To determine the effect of an HF diet on allograft survival, we transplanted BALB/c (H2-Kd) kidneys into C57BL/6 (H2-Kb) nephrectomized recipients as WT allografts, whose survival was dependent upon sustained function of the allograft. Recipients were fed HF or NC, commencing 2 weeks before transplantation and continuing throughout the experiments. Survival to 100 days was observed in all C57BL/6 isografts. WT mice on NC diets rejected their allografts with a mean graft survival of 40 days, whereas WT+HF allografts displayed prolonged survival (P<0.05; Figure 2A).
Figure 2.
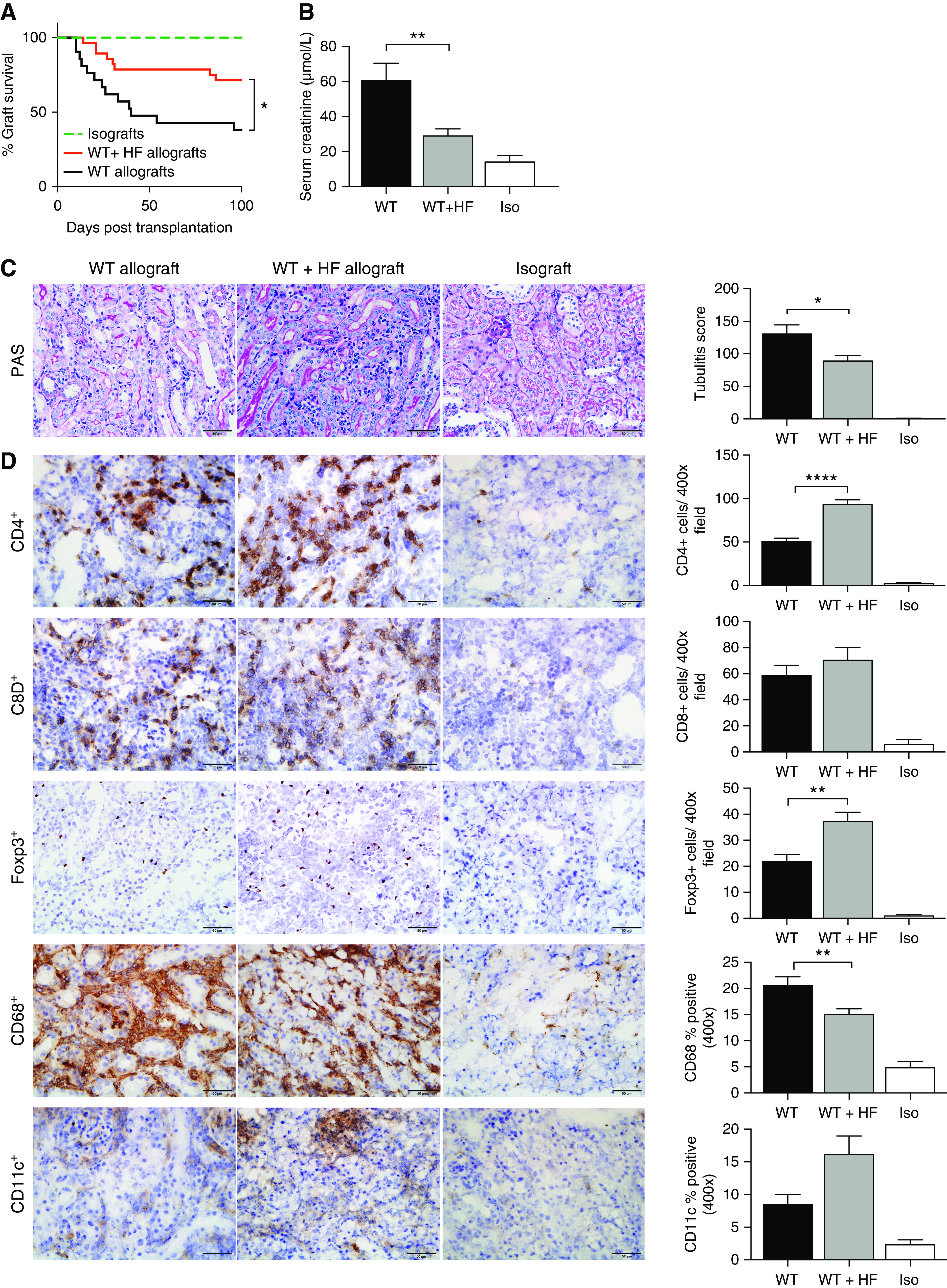
HF diet prolonged renal allograft survival and attenuated acute allograft rejection at day 14 after kidney transplant. (A) Survival to day 100 was observed in all isografts (n=13), but in only eight WT allografts (n=21), with a mean survival of 40 days. HF-fed mice showed enhanced survival (HF n=20 of 28). (B) At day 14, impaired renal function was evident in WT allografts (n=7) with an increase in serum creatinine as compared with isografts (n=5). Mice fed an HF diet (n=9) were partially protected from allograft dysfunction with lower serum creatinine (P<0.01). (C) Tubulitis was present in both WT and HF-fed allograft mice, although it was significantly attenuated in the HF group (tubulitis score P<0.05). (D) Representative photomicrographs of WT and WT+HF allograft sections at day 14 post-transplant demonstrate significant inflammatory cell infiltrates as compared with isografts. Increased numbers of CD4+ and Foxp3+ cells and lesser numbers of CD68+ macrophages were evident for WT+HF allografts compared with WT allografts. Scale bar, 50.0 μm. Data are shown as mean±SEM. Statistical analysis by log-rank test and ANOVA with Tukey post hoc analysis. *P<0.05; **P<0.01; ****P<0.0001.
HF Diet Protected against Acute Allograft Rejection
As allograft rejection in this model characteristically occurs in the acute phase (day 14), and in surviving mice, the chronic phase (day 100), further transplant experiments were undertaken to examine the effect of diet at these timepoints.
At day 14, WT allografts exhibited kidney dysfunction, with a four-fold increase in serum creatinine as compared with isografts (WT, 61.1±9.3 μmol/L; isografts, 14.4±3.4 μmol/L), as well as severe tubulitis on histologic examination of the transplanted kidneys (mean tubulitis score: WT, 131.6±12.9; isografts, 0.8±0.4; Figure 2, B and C). WT+HF allografts were protected against acute rejection, with lower serum creatinine (WT+HF, 29.3±3.6 μmol/L; Figure 2B) and a 30% reduction in tubulitis score compared with WT allografts (WT+HF, 89.9%±7.1%; Figure 2C).
Immunohistochemical assessment of allografts at day 14 revealed significant accumulation of CD4+ and CD8+ T cells, CD68+ macrophages, Foxp3+ T regulatory cells (Tregs) and CD11c+ dendritic cells in WT allografts as compared with isografts (Figure 2D). Compared with WT allografts, WT+HF allografts showed an increase in CD4+ T cells, including an increase in Foxp3+ Tregs (P<0.01), and a reduction in CD68+ macrophages (P<0.01).
Assessment of gene expression relevant to rejection by real-time PCR of kidney tissue obtained 14 days after transplantation revealed significant upregulation of proinflammatory cytokines (TNF-α, IL-6), Th1 (IFN-γ), and Th2 (IL-4) cytokines, chemokines (CCL2, CCL5, and CXCL10), and cytotoxic molecules (perforin and granzyme), in addition to regulatory cytokines (TGF-β and IL-10), in WT allografts versus isografts (Figure 3). Compared with WT allografts, WT+HF allografts exhibited significantly greater upregulation of IL-10 (WT, 1072±119 IL-10/GAPDH×1000; WT+HF, 1595±67 IL-10/GAPDH×1000; P<0.001) and diminished production of IL-23 (WT, 2000±250 IL-23/GAPDH×1000; WT+HF, 760±76 IL-23/GAPDH×1000; P<0.001).
Figure 3.
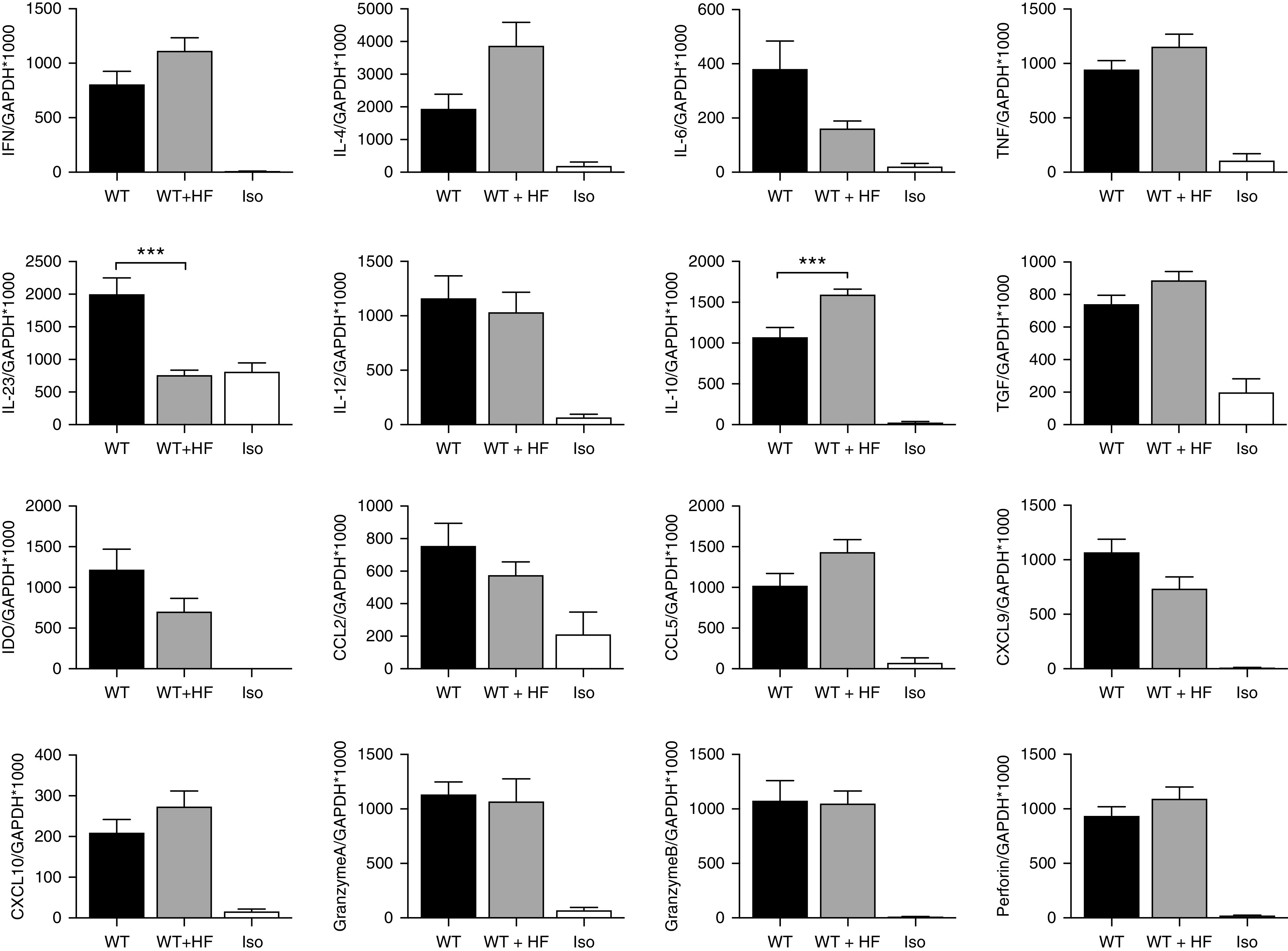
HF diet resulted in altered mRNA gene expression in transplanted kidneys at day 14 post-transplant. Inflammatory cytokine mRNA was upregulated in both WT (n=7) and WT+HF (n=9) allografts compared with isografts (n=5). Expression of the regulatory cytokine IL-10 was significantly increased and expression of proinflammatory cytokine IL-23 was decreased in WT+HF compared with WT allografts. Data are shown as the mean±SEM. Statistical analysis by ANOVA with Tukey post hoc analysis. ***P<0.001.
HF Diet Protected against Chronic Allograft Rejection
Groups of transplanted mice who survived to day 100 were euthanized to assess for evidence of chronic allograft rejection. A minority of WT allografts survived to day 100 and these exhibited severe chronic rejection, manifest by marked increases in serum creatinine (WT, 48.8±9.5 μmol/L; isograft, 13.0±1.8 μmol/L; Figure 4A), and proteinuria compared with isografts (WT, 1.81±0.4 mg/16 h; isograft, 0.15±0.05 mg/16 h; Figure 4B). Severe histologic changes of chronic rejection were evident in WT allografts, including glomerulosclerosis, interstitial fibrosis and tubular atrophy, and extensive chronic inflammatory cell infiltration and deposition of collagen shown by Picro-Sirius red staining (Figure 4C). The majority of WT+HF allograft mice survived to day 100 and exhibited significantly less kidney dysfunction, with serum creatinine (28.4±2.1 μmol/L) and urine protein (0.62±0.12 mg/16 h) not statistically different to isografts (Figure 4, A and B). WT+HF allograft histology revealed modest degrees of inflammation, glomerulosclerosis, tubulointerstitial fibrosis, and collagen deposition, all of which were reduced in severity compared with WT allografts (Figure 4C). At day 100 post-transplant, there was no difference in the accumulation of CD4+ T cells, CD8+ T cells, CD68+ macrophages, CD11c+ dendritic cells, or Foxp3+ Tregs (WT, 30.54±3.3 cells/HPF; WT+HF, 27.50±2.3 cells/HPF; P=0.67) in the allografts of WT and WT+HF fed mice, as determined by immunohistochemistry (Figure 4D). Prominent C4d staining was present diffusely in almost all cross-sections of peritubular capillaries (PTCs) with a bright, broad, linear pattern in WT allografts, and this remained unaltered in WT+HF mice (Figure 4E).
Figure 4.
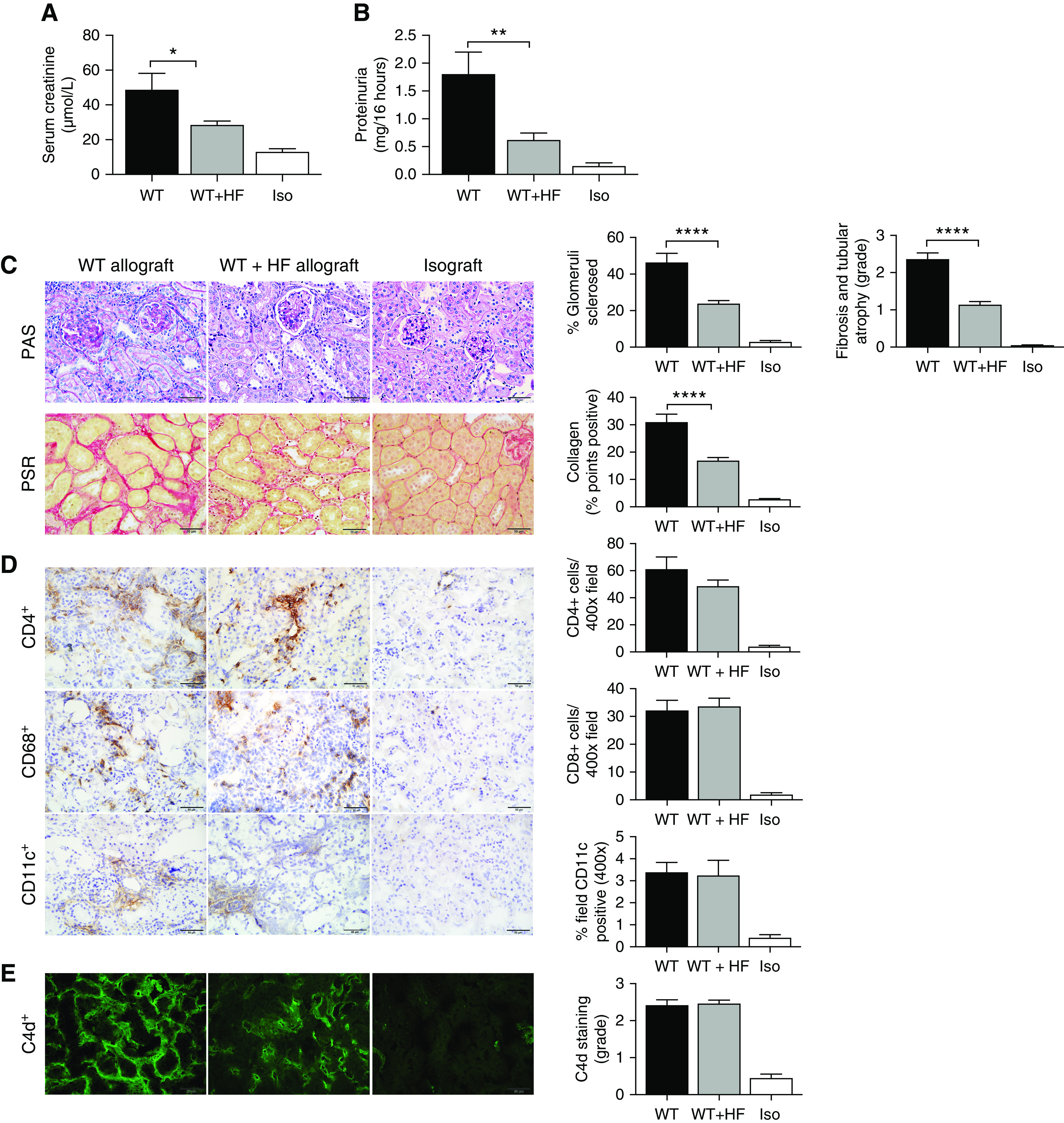
HF diet attenuated chronic allograft damage at day 100 after kidney transplant. (A) Serum creatinine and (B) urinary protein excretion were significantly reduced in WT+HF (n=12) allografts compared with WT allografts (n=8) at day 100 post-transplant. (C) Representative photomicrographs of the histologic changes on periodic acid–Schiff (PAS); Picro-Sirius Red (PSR); immunochemistry staining for CD4, CD8, CD68, and CD11c; and fluorescence staining for C4d in WT allografts, WT+HF allografts, and isografts (n=5). PAS and PSR sections showed significant glomerulosclerosis, interstitial fibrosis and tubular atrophy, and interstitial collagen deposition in WT allografts compared with isografts, all of which were significantly reduced in WT+HF allografts compared to WT allografts. (D) CD4, CD8, and CD11c infiltrates, and (E) C4d deposition, were significantly and equally increased in WT and WT+HF allografts compared with isografts. Photomicrographs at ×400; scale bar, 50.0 μm. Data are shown as the mean±SEM. Statistical analysis by ANOVA with Tukey post hoc analysis. *P<0.05; **P<0.01; ****P<0.0001.
Day 100 WT allografts demonstrated significant upregulation of cytokines (IFN-γ, IL-4, IL-6, IL-10, IL-12, IL-23, TNF, TGF-β), chemokines (CCL5, CXCL9, CXCL10), and genes involved in tissue re-modeling (fibronectin, TIMP-1, TIMP-2, MMP-2, MMP-9) as compared with isografts. Despite a reduction in chemokine CXCL9 expression in HF allografts (WT, 171±49; WT+HF, 140±20 CXCL9/GAPDH*1000; P<0.05) no other significant difference in mRNA expression was evident between the WT+HF and WT groups (Supplemental Figure 4).
Acetate Supplementation Prolonged Allograft Survival by Promoting Donor-Specific Tolerance
As dietary supplementation with HF has been shown to increase colonic and serum SCFA concentrations,18 we hypothesized that SCFAs were key mediators of the protective effects of the HF diet in our kidney allograft model. We sought to test this by examining the effect of direct SA supplementation. We transplanted BALB/c kidneys into C57BL/6 recipients who received 200 mg/kg SA via intraperitoneal injection on the day of transplantation and for 14 days post-transplantation, followed by oral 150 mM SA solution via drinking water indefinitely (SA allografts) and compared outcomes with WT allografts. As SA is not the only SCFA reported to mediate proregulatory immune responses, the experiment was also performed with SB as described above (SB allografts).
Both SA allografts and SB allografts experienced superior survival to WT allografts, similar to the effects of an HF diet but without reaching significance (Figure 5A). SA allografts euthanized at post-transplant day 14 showed preservation of kidney function (creatinine: WT, 61.1±9.3 μmol/L; WT+SA, 32.0±1.3 μmol/L; P<0.01; Figure 5B) and significantly less tubulitis than WT allografts (mean tubultis score: WT, 118.4±9.9; WT+SA, 76.3±4.8; P<0.001; Figure 5C). Immunohistochemical staining revealed greater accumulation of CD4+ T cells in WT+SA allografts as compared with WT allografts (P<0.0001), although not CD8+ cells, along with an increase in CD4+Foxp3+ Tregs and CD11c+ cells (P<0.01 and P<0.05, respectively), with no significant difference in the abundance of CD68+-stained cells (Figure 5D).
Figure 5.
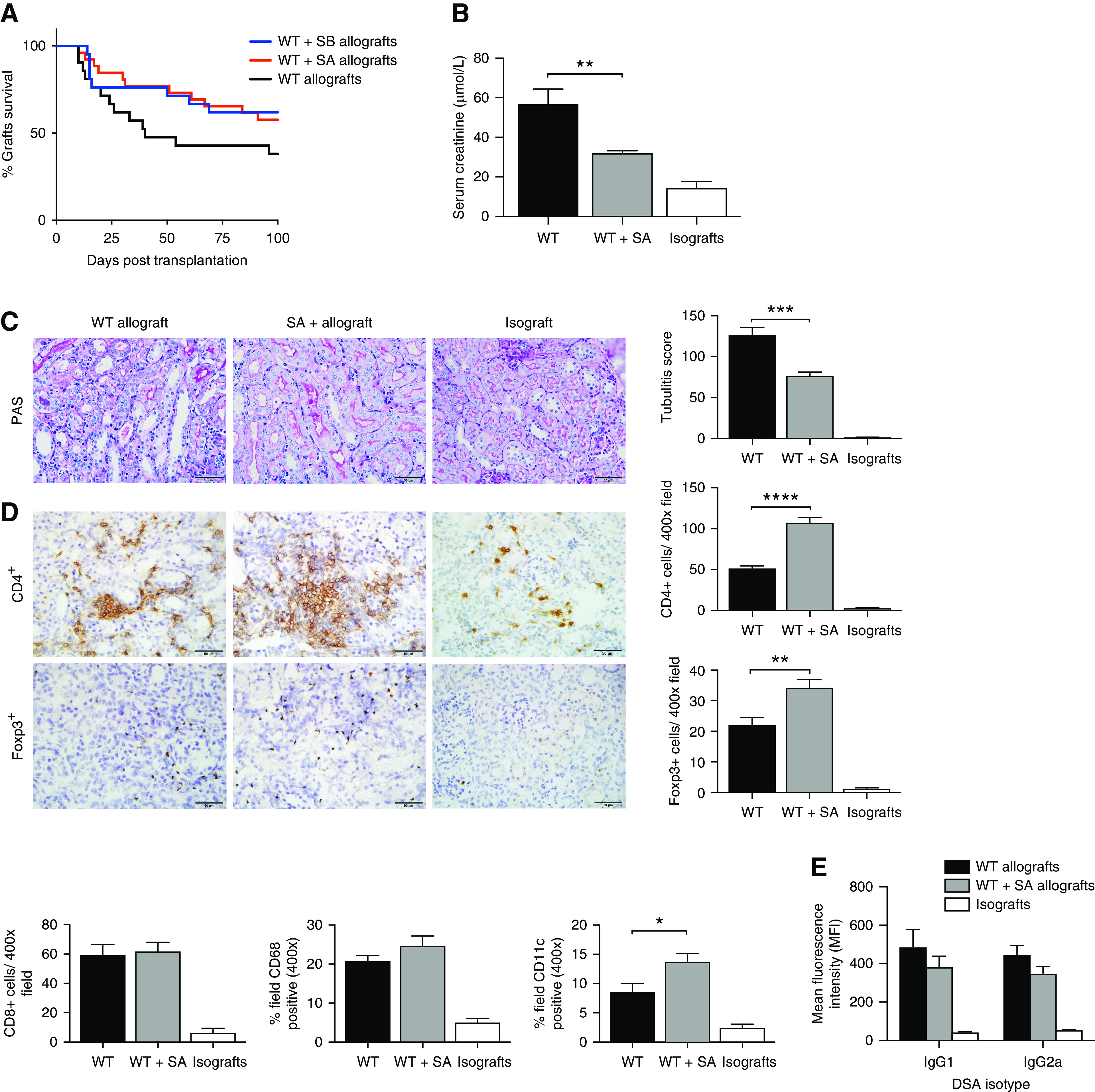
SCFA supplementation attenuated acute allograft rejection. (A) WT+SA allografts (n=27) and WT+SB allografts (n=21) exhibited a trend toward improved survival compared with WT allografts (n=21), without reaching significance. At day 14 post-transplant, WT+SA allografts (n=9) demonstrated improved graft function with lower serum creatinine (P<0.05) (B), and less tubulitis (C) compared with WT allografts (n=7) (P<0.01). (D) Representative photomicrographs and analysis of cellular infiltrate by IHC showed a marked increase in CD4+ and Foxp3+ T cells, as well as CD11c+ cells in WT+SA allografts compared with WT allografts (isografts n=5). (E) Serum donor-specific antibody titers were evaluated at 14 days post-transplantation. WT (n=13) and SA-supplemented (n=9) allograft mice demonstrated elevated donor-specific IgG1 and IgG2a compared with isografts (n=5). Scale bar, 50.0 μm. Data are shown as the mean±SEM. Statistical analysis by one-way ANOVA with Tukey post hoc analysis. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Measured at day 14 post-transplant, donor-specific antibody levels in both WT and WT+SA allograft mice were significantly elevated compared with isograft counterparts, although they did not differ significantly from each other (Figure 5E).
Examination of allograft tissue by RT-PCR revealed a similar pattern of gene expression to that observed in HF allografts, including upregulation of IL-10 (WT, 683±52 IL-10/GAPDH×1000; WT+SA, 993±98 IL-10/GAPDH×1000; P<0.05), but a marked reduction in IL-6 expression (WT, 7253±1323 IL-6/GAPDH×1000; WT+SA, 306±51 IL-6/GAPDH×1000; P<0.0001) (Figure 6).
Figure 6.
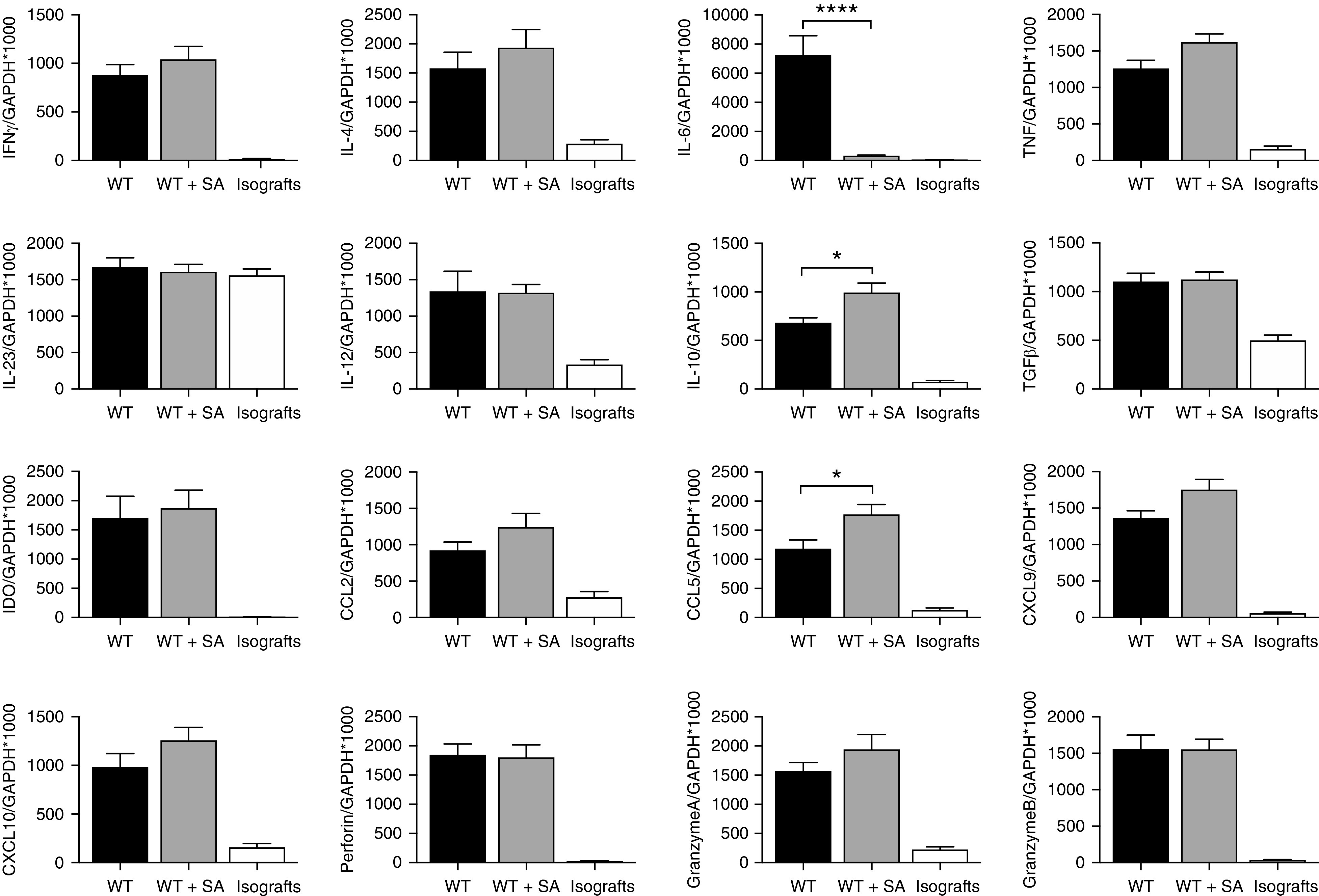
SCFA supplementation altered allograft mRNA expression at D14. WT+SA allografts (n=9) showed upregulation of most Th1 (IFN-g) and Th2 (IL-4) cytokines in a pattern consistent with WT allografts (n=7). Inflammatory cytokine IL-6 was significantly reduced whereas expression of the regulatory cytokine IL-10 was significantly increased in WT+SA allografts compared with WT allografts (isografts n=5). Data are shown as the mean±SEM. Statistical analysis by one-way ANOVA. *P<0.05; ****P<0.0001.
At day 100 post-transplant, SB allograft mice displayed prolonged survival similar to SA supplemented mice, but exhibited higher markers of allograft injury (serum creatinine); therefore, mechanistic studies were explored on SA-supplemented mice alone.
Acetate Protected against Chronic Allograft Rejection
The majority of SA allograft mice survived to day 100, at which time their serum creatinine and urinary protein excretion were not significantly different to isografts and were markedly lower than WT allografts (creatinine: WT, 48.8±9.5 μmol/L; WT+SA, 28.5±1.8 μmol/L; P<0.05; and proteinuria: WT, 1.21±0.14 mg/16 h, WT+SA, 0.39±0.09 mg/16 h; P<0.0001; Figure 7A). Histologic studies showed a significant reduction in glomerulosclerosis, interstitial fibrosis and tubular atrophy, and collagen deposition in SA allografts compared with WT allografts (Figure 7B). Immunohistochemistry staining revealed a reduction in inflammatory infiltrate in SA allografts, with modestly reduced CD4+ T cell and CD11c+ dendritic cell accumulation when compared with WT allografts (P<0.01 and P<0.0001, respectively, Figure 7C). Gene expression of key cytokines in SA allografts revealed a three-fold increase in IL-10 expression compared with WT allografts (WT, 347±70 IL-10/GAPDH×1000; WT+SA, 1090±180 IL-10/GAPDH×1000; P<0.01; Figure 7D). C4d staining of both SA and WT allografts revealed prominent staining of peritubular capillaries with no difference between groups (Figure 7E).
Figure 7.
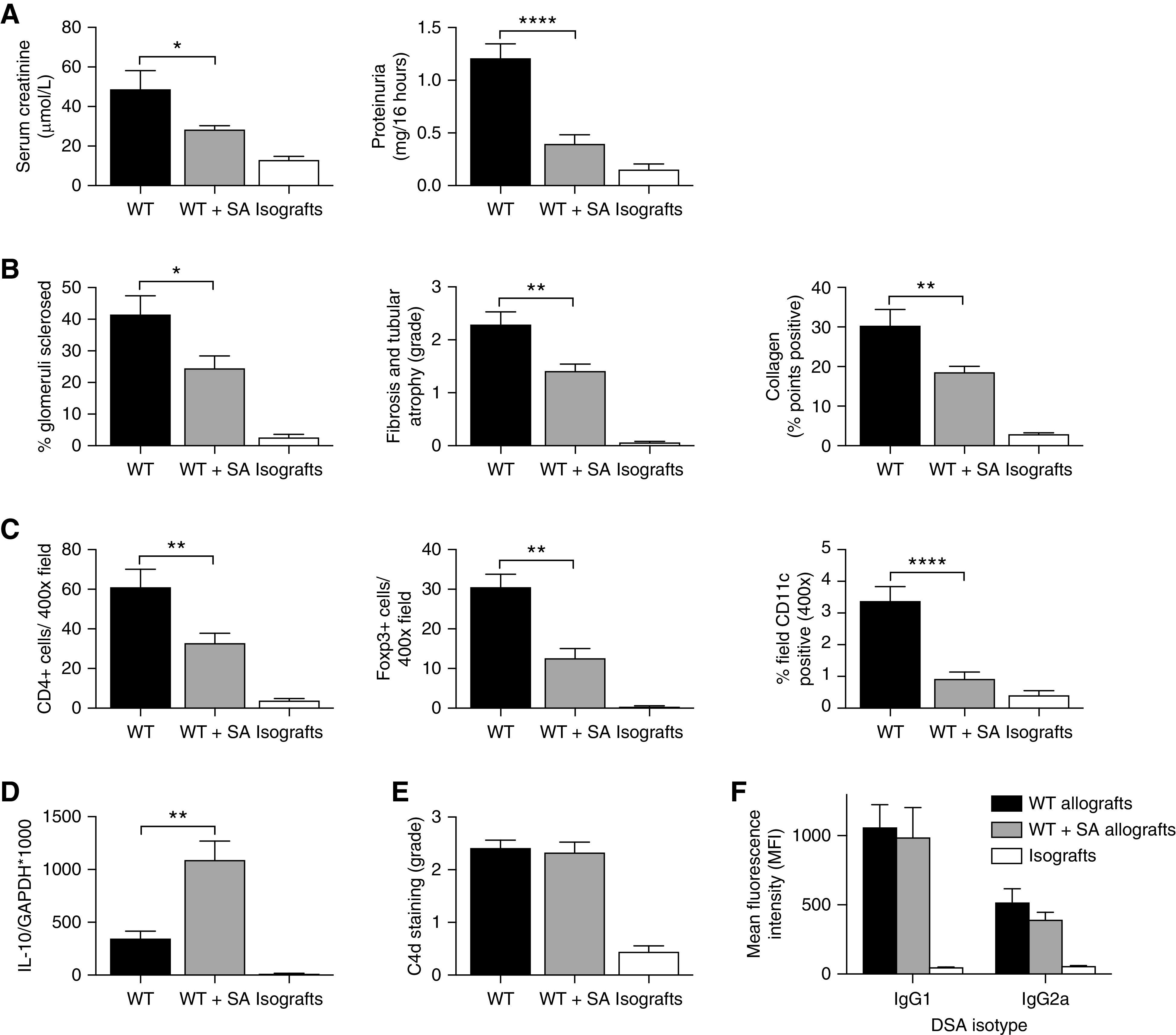
SA supplementation protected against chronic allograft injury at day 100 post-transplant. (A) SA supplementation resulted in a significant decrease in serum creatinine and urinary protein excretion in WT+SA allograft mice (n=11) at day 100 post-transplant, compared with WT allograft mice (n=8). (B) Glomerulosclerosis, interstitial fibrosis and tubular atrophy, and interstitial collagen deposition were markedly decreased in WT+SA allografts compared with WT allografts. (C) Immunohistochemistry staining showed a decrease in CD4+, Foxp3+, and CD11c+ infiltrates in WT+SA allografts compared with WT allografts. (D) Expression of the regulatory cytokine IL-10 was significantly increased in WT+SA allografts compared with WT allografts. (E) Prominent C4d staining was present diffusely in peritubular capillaries of both WT and WT+SA allografts compared with isografts (n=5). (F) At day 100 post-transplant, serum donor-specific antibody titers were evaluated in WT (n=12) and SA-supplemented (n=11) allograft mice, with all groups demonstrating elevated donor-specific IgG1 and IgG2a compared with isografts (n=5). Data are shown as the mean±SEM. Statistical analysis by one-way ANOVA. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Donor-specific antibody levels were assessed in the serum of transplanted animals harvested at day 100 (Figure 7F). Both WT and WT+SA allograft mice demonstrated similar elevations of donor-specific IgG1 by mean fluorescene intensity (MFI) (MFI: WT, 1061±0.5; WT+SA, 988.1±215.8; P=0.95) and IgG2a (MFI: WT, 519.3±96.4; WT+SA, 394.5±51.6; P=0.46), greater than those seen in isograft mice but not significantly divergent from each other.
SA Supplementation Promoted Donor Antigen–Specific Tolerance
To test for dominant immune tolerance, a subset of both WT allograft and SA allograft survivors received skin grafts from BALB/c (donor-matched allograft), C57BL/6 (isograft), and B10Br (third-party allograft) mice at least 200 days post-kidney transplant (Figure 8A). Skin isografts remained intact on both sets of animals. WT mice rejected donor-matched and third-party skin allografts at 12–14 days and died within 34–107 days after skin allografts were performed. SA allografts accepted donor-matched skin allografts (H-2d) for over 100 days, rejected B10Br (H-2k) third-party allografts by day 15, and survived for over 100 days after placement of skin grafts, at which time kidney function remained normal (serum creatinine 21±3 µmol/L).
Figure 8.
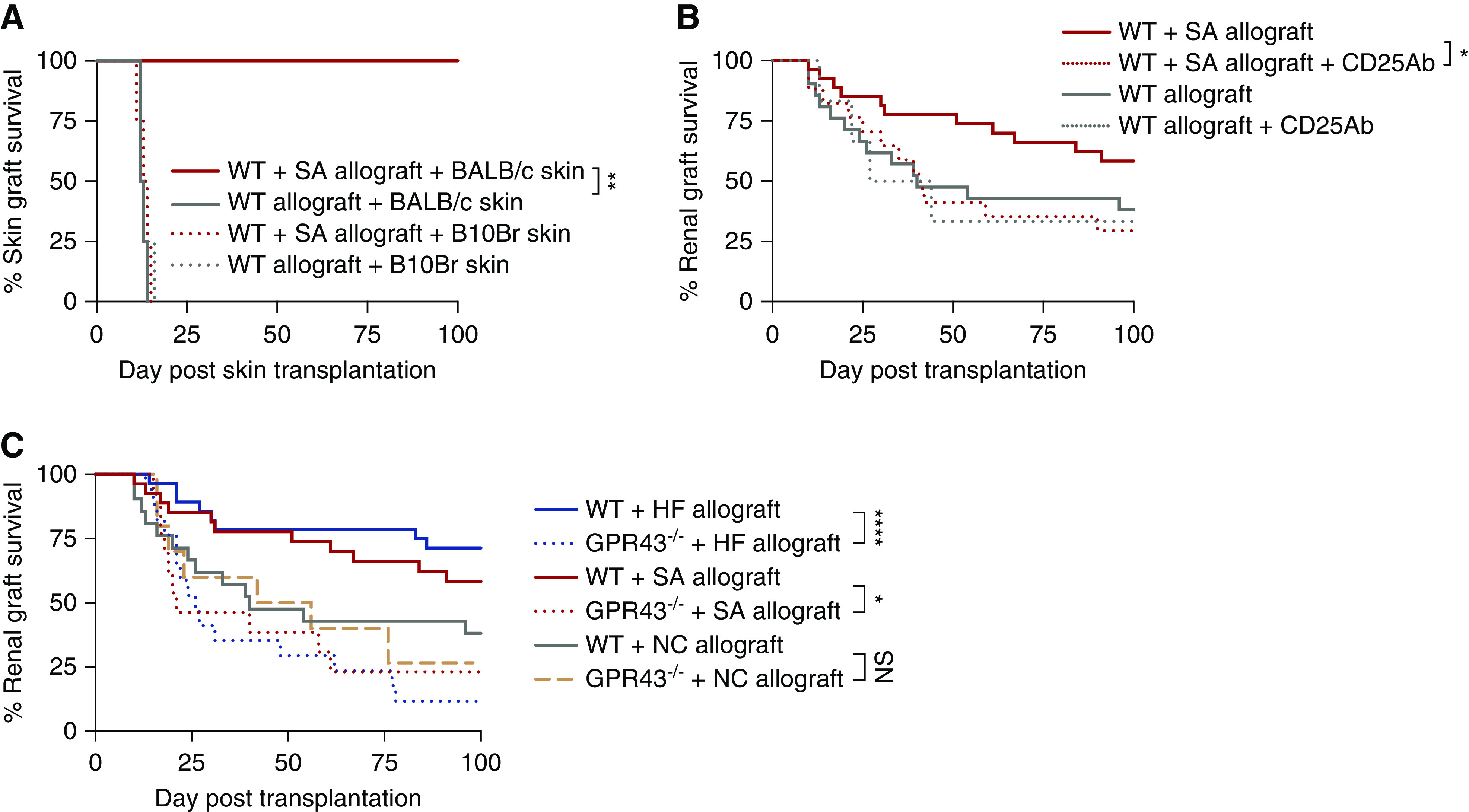
SA promoted donor antigen–specific tolerance of kidney allografts, dependent on a CD4+CD25+Foxp3+ regulatory mechanism and signaling via GPR43. (A) Skin graft survival curves for skin challenges in WT and WT+SA kidney allograft acceptors. A subset of WT (n=4) and SA-supplemented (n=4) allograft mice who survived to over day 200 after kidney transplant received skin grafts from donor-matched (BALB/c), isografts (C57BL/6), and third-party (B10Br) mice. Skin isografts remained intact on all animals, whereas third-party skin allografts were promptly rejected by day 15 by WT and WT+SA kidney allograft acceptors. WT kidney allograft acceptors rejected donor-matched skin grafts at 12–14 days, whereas WT+SA kidney allograft acceptors accepted donor-matched skin grafts for >100 days. (B) A depleting mAb to CD25 was given to WT (n=6) and SA-supplemented (n=17) allograft mice on days −2 and 0 post-transplant. The survival benefit seen in SA-supplemented allograft recipients (n=27) was annulled after depletion of CD25+ cells (P<0.05), whereas administration of anti-CD25 Ab did not alter allograft rejection kinetics in WT allograft recipients. (C) SA supplementation and HF diet was ineffective in GPR43−/− recipients of WT allografts (n=13 for SA, n=17 for HF), with no survival advantage compared with WT recipients of kidney allograft (n=21), whereas GPR43−/− allograft recipients fed NC (n=10) had graft survival comparable to WT mice fed NC. Statistical analysis by log-rank test. *P<0.05; **P<0.01; ****P<0.0001.
SA-Mediated Donor-Specific Tolerance of Kidney Allografts Was Dependent on a CD4+CD25+Foxp3+ Regulatory Mechanism
As a greater number of Treg cells, both proportionally and absolute, were observed in HF allografts and SA allografts at day 14 post-transplant, we hypothesized that long-term allograft tolerance may be dependent on immune regulation mediated by CD4+CD25+ Tregs. To examine this, we treated SA allografts with an anti-CD25 mAb (clone: PC61). CD4+CD25+Foxp3+ cells were depleted by >90% compared with control antibody–treated mice, and the allograft survival benefit conferred by SA was abrogated (Figure 8B). Administration of anti-CD25 antibody to WT allograft recipients did not alter allograft rejection kinetics.
GPR43 Was Critical for HF and Acetate-Mediated Protection against Allograft Rejection
Acetate preferentially binds the metabolite-sensing GPRs, such as GPR43, to elicit cellular responses. To determine whether GPR43 was required to mediate the benefit seen in HF and SA supplemented allograft mice, we next transplanted BALB/c WT kidneys into GPR43−/− mice on a C57BL/6 background, who received an HF diet or SA supplementation as previously described (as GPR43−/−+HF and GPR43−/−+SA allografts, respectively). Both HF and SA supplementation was ineffective in GPR43−/− allograft recipients, with abrogation of the survival advantage seen in HF and SA allograft mice (Figure 8C), demonstrating that signaling of acetate via GPR43 was critical to mitigate graft rejection. Graft survival was unchanged in NC-fed GPR43−/− allograft recipients, as compared with NC-fed WT allograft recipients, suggesting maintenance of the alloimmune response in GPR43−/− mice.
As SCFAs are known to epigenetically alter gene expression by inhibiting HDACs, we next determined whether HDAC activity was upregulated in SA supplemented allografts. Compared with WT allografts, SA supplemented allografts did not demonstrate significant changes in HDAC activity measured at day 14 post-transplant (P=0.27; Supplemental Figure 5), indicating that the beneficial effects of acetate in our transplant model were not attributable to alternations in histone acetylation alone.
Discussion
Using an established and reproducible kidney transplant model, we found considerable alteration in gut microbiota composition after allograft transplantation, which occurred in the absence of immunosuppression or antibiotic intervention and did not occur in isograft recipients. This transplant-induced dysbiosis was ameliorated by maintenance on an HF diet. Mice fed HF were partially protected from allograft rejection, exhibiting prolonged survival and reduced evidence of both acute and chronic allograft rejection. Diet-related production of SCFAs by the gut microbiome has been shown to modify pathogenic immune responses in several experimental settings.18,30,31 Our findings that dietary supplementation with SA afforded similar protection against allograft rejection to HF diet, and that deficiency of GPR43, a receptor for acetate, rendered mice resistant to the protective effects of both HF diet and acetate supplementation, strongly suggests that gut-derived acetate is important in retarding alloimmunity in the context of kidney transplantation. Enhanced immune regulation is another proposed mechanism of immune-modulation by the gut microbiome, and this also appears to be important in transplantation as the protective effects of SCFA were negated by depletion of CD4+CD25+ T-cells.
The gut microbiome has a modulatory role in directing host systemic immune responses and has coevolved with the mammalian immune system to form a necessary symbiotic relationship.15,32 The process of transplantation is highly immunogenic, provoking an innate response to surgical tissue injury and ischemia-reperfusion injury to the implanted organ, followed by T cell recognition of nonself HLA, causing a powerful adaptive response.9 Given this profound stimulus to host immunity, it is predictable that transplantation would have reciprocal effects on the gut microbiota.33 Although clinical studies have demonstrated shifts in microbial composition after kidney transplantation with possible links to allograft outcomes,34,35 it is unclear what contribution exposure to allogenic antigen plays in addition to the role of antibiotics, immunosuppression, surgery, and resolution of the uremic environment in causing this shift.
The shift in bacterial community structure in our model after transplantation confirmed the development of transplant-induced dysbiosis. In the absence of antibiotics and other drugs, potential causes of dysbiosis in our model were reduced to transplant surgery and the alloimmune response. That isograft controls demonstrated no significant change in the gut bacterial landscape and only a transient reduction in α-diversity postsurgery identifies the allograft immune response as the prime driver of transplant dysbiosis.
Diet is the dominant exogenous factor known to affect composition of the gut microbiome and is the key determinant of its diversity and richness.36 Murine host microbial communities are diet-, colony-, and facility-specific; however, the composition of the microbiota in our NC-fed mice were consistent with previous observations of C57BL/6 gut microbiota.4,12 After transplantation, shifts in microbiota structure were seen in both NC- and HF-fed mice. Two key differences between NC- and HF-fed mice were notable and potentially underpin the protective capacity of the HF diet: first, the relative abundance of bacteria known to produce SCFAs; and second, the balance between mucous-producing and mucous-degrading bacteria.
The relative abundance of bacteria known to produce SCFAs, including Bifidobacterium, Clostridium, and Bacteroidetes spp., were significantly increased in transplanted mice on an HF diet compared with NC. Bifidobacteria spp. have been shown to offer protection from kidney ischemia-reperfusion injury in a murine model.20 Furthermore, our results are consistent with the recent findings of Bromberg et al.,37 who demonstrated that lone transfer of a Bifidobacteria sp. was sufficient to improve outcomes in murine cardiac allografts.
We demonstrated that the ability of acetate to limit allograft rejection was dependent on its action on the metabolite-sensing receptor GPR43, with transplanted GPR43−/− mice not protected by diet. Our findings are consistent with a growing body of data highlighting the importance of GPR43 in mediating immunomodulatory effects of SCFA.17,38 We have previously demonstrated that acetate can ameliorate murine dextran sulfate sodium (DSS) induced colitis via GPR43 activation on nonhematopoietic cells,18 and more recently, signaling via GPR43 was shown to be a critical determinant of the severity of experimental graft versus host disease.39 The benefit of SCFA in both models was dependent on GPR43-mediated ERK phosphorylation and activation of the NLRP3 inflammasome in intestinal epithelial cells, rather than direct activation of GPR43 on hemopoietic cells. GPR43 is known to be expressed by multiple cell types, including antigen-presenting cells, polymorphonuclear cells, B cells, and T cells, in addition to epithelial cell types, including renal tubular cells, and thus may modulate both local and systemic immunity through numerous pathways.17,40,41 Although the effects of microbiota-derived acetate on local resident cells is of significance in experimental colitis and graft versus host disease, the importance of circulating Treg cells in mediating alloimmunity in transplantation highlights the influence of acetate on modulating adaptive immunity to affect distant organ sites.
In health, the mucous-degrading actions of A. muciniphila are counterbalanced by bacterial species, including Lactobacillus and Bifidobacterium, which protect the colonic mucous barrier from diet-induced, microbiota-related degradation.42,43 In NC-fed mice, we found this balance to be disturbed because of an excess of A. muciniphila and reduction in the relative abundance of Lactobacillus and Bifidobacterium, as opposed to HF-fed mice, where balance was preserved. The colonic mucosal layer is critical for gut–immune interactions, providing a protective barrier against harmful gut bacteria as well as an environment that enables the trafficking of T cells required for maturation. Disruption of the mucous barrier leads to translocation of microbiota, pathobionts, and their products to the epithelial surface, causing inflammation and directing immune cell maturation toward a proinflammatory phenotype.44 Thus, the promotion of mucous-protective over mucous-degrading bacterial communities offers a second mechanism by which the HF diet may have retarded rejection.
Overall, our findings with regard to the effect of diet and the microbiota on kidney allograft rejection do not suggest that any one specific bacterial species may be sufficient to determine transplant survival or rejection, but raise the possibility that the balance between beneficial and nonbeneficial species may be the most important determinant of a favorable clinical outcome.5 Furthermore, our findings support the notion that greater overall diversity may not be as important for maintaining health as the balance between specific bacterial species.45
The known capacity of the gut microbiome to enhance immune regulation was also evident in our studies. The protection afforded by both HF and direct supplementation of acetate was associated with an influx of Tregs and elevated IL-10 gene expression within allografts. SCFAs have the potential to regulate tissue inflammation through their effects on multiple cell types such as Tregs, DCs, and macrophages.46 Supplementation with acetate caused pronounced suppression of IL-6 expression in allograft kidneys, which, coupled with upregulation of IL-10, likely represents the establishment of a local immunoregulatory environment. Similarly, allograft gene profiles obtained from HF-fed, WT mice indicated an environment conducive to Treg generation or expansion. The expansion of Tregs found with HF diet and SCFA supplementation in our studies is in keeping with earlier observations that SCFA can induce colonic and peripheral Treg expansion acting through GPR43 expressed on colonic T cells.38,47 Clostridium species, which were augmented by HF and diminished after transplantation on NC, have been identified as important promoters of immune regulation with some commensal clusters shown to bias naïve T cell development toward a regulatory phenotype.48 In our model, early depletion of CD25+ T cells was sufficient to abrogate the survival advantage conferred by acetate, suggesting that natural Tregs are required for the protective effect. Additional actions of dietary metabolites on Treg induction and functionality in our model are likely, and further experiments examining Tregs at numerous post-transplant timepoints and the adoptive transfer of CD4+CD25+Foxp3+ T cell populations to untreated allograft recipients may provide important insight into the mechanistic role of SCFA-enhanced Tregs in kidney transplantation.
Our data suggest dietary modification of the microbiome may be a simple and safe means to enhance Treg generation in vivo as a strategy to enhance organ tolerance. Whether such modifications can be made in humans and what their effect is remains to be seen. Research in the future may determine whether modifying the microbiome with diet-based prebiotic therapies, probiotics, a combination of the two (synbiotics), or directed therapy with fecal microbiota transplant or specific SCFA supplementation could be a useful adjunct to current treatment regimens. Recent reports suggest that even small changes in gut microbial communities can enhance or impair the response to immunomodulatory treatments.4,5,49 Given the toxic off-target effects of current immunosuppressive treatments, achieving an enhanced therapeutic response with reduced drug exposure would therefore be advantageous in the clinic.
In summary, our results demonstrate that dietary supplementation with HF promotes renal allograft survival and limits transplant-induced dysbiosis. The protective effect is mediated through increased microbial SCFA production and is dependent on Tregs through GPR43 signaling.
Disclosures
None.
Funding
This work was supported by the Australian National Health and Medical Research Council (project grant APP1162764). The project grant was awarded to Prof. Chadban, A/Prof Wu and A/Prof Macia.
Supplementary Material
Acknowledgments
We gratefully acknowledge the expertise of Dr. Brian Howden who performed the kidney transplant surgeries, and Dr. Jin Ma who performed the histone deacetylase assay.
Dr. Singer and A/Prof. Wu contributed equally to this paper. A/Prof. Wu, A/Prof. Macia, and Prof. Chadban conceived and designed the study. Dr. Singer, Dr. Kwan, Dr. Loh, Dr. Lai, and Dr. Li performed the experiments. Dr. Wang performed transplant surgeries. Dr. Singer, A/Prof. Wu, and Prof. Chadban analyzed the data. Dr. Singer constructed the figures. Dr. Singer and Dr. Tan performed microbiota analysis. Dr. Singer, A/Prof. Wu and Prof. Chadban drafted and revised the paper. Prof. Alexander revised the paper. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Can Diet Induce Transplantation Tolerance?” on pages 1417–1418.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019080852/-/DCSupplemental.
Supplemental Figure 1. Multiple sample rarefaction curves.
Supplemental Figure 2. Richness of gut microbial communities in allograft and isograft mice.
Supplemental Figure 3. Dominant phylum of WT C57Bl/6 mice fed a zero-fiber diet.
Supplemental Figure 4. Cytokine and chemokine mRNA expression in allografts at D100.
Supplemental Figure 5. HDAC activity in renal allografts.
Supplemental Table 1. Nutritional parameters of HF and normal mouse chow used in experiments.
Supplemental Table 2. DESeq2 analysis of differential microbial abundance in NC-fed isograft pre- and post-transplant.
Supplemental Table 3. DESeq2 analysis of differential microbial abundance in NC- and HF-fed mice.
Supplemental Table 4. DESeq2 analysis of differential microbial abundance in NC+Allo and HF+Allo mice.
References
- 1.Nankivell BJ, Alexander SI: Rejection of the kidney allograft. N Engl J Med 363: 1451–1462, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Halloran PF: Immunosuppressive drugs for kidney transplantation. N Engl J Med 351: 2715–2729, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid Y, Hand TW: Role of the microbiota in immunity and inflammation. Cell 157: 121–141, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al.: Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350: 1084–1089, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, et al.: The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359: 104–108, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al.: Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359: 91–97, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Burrows MP, Volchkov P, Kobayashi KS, Chervonsky AV: Microbiota regulates type 1 diabetes through Toll-like receptors. Proc Natl Acad Sci U S A 112: 9973–9977, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al.: Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455: 1109–1113, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, et al.: TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwan TK, Chadban SJ, Wu H: Toll-like receptor 4 deficiency improves short-term renal function but not long-term graft survival in a fully MHC-mismatched murine model of renal allograft transplantation. Transplantation 100: 1219–1227, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Noordmans GA, O’Brien MR, Ma J, Zhao CY, Zhang GY, et al.: Absence of MyD88 signaling induces donor-specific kidney allograft tolerance. J Am Soc Nephrol 23: 1701–1716, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntosh CM, Chen L, Shaiber A, Eren AM, Alegre M-L: Gut microbes contribute to variation in solid organ transplant outcomes in mice. Microbiome 6: 96, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lei YM, Chen L, Wang Y, Stefka AT, Molinero LL, Theriault B, et al.: The composition of the microbiota modulates allograft rejection. J Clin Invest 126: 2736–2744, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rey K, Manku S, Enns W, Van Rossum T, Bushell K, Morin RD, et al.: Disruption of the gut microbiota with antibiotics exacerbates acute vascular rejection. Transplantation 102: 1085–1095, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R: Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Lennon G, Balfe A, Earley H, Devane LA, Lavelle A, Winter DC, et al.: Influences of the colonic microbiome on the mucous gel layer in ulcerative colitis. Gut Microbes 5: 277–285, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al.: Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al.: Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 6: 6734, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al.: Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, et al.: Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol 26: 1877–1888, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwan T, Chadban SJ, Ma J, Bao S, Alexander SI, Wu H: IL-17 deficiency attenuates allograft injury and prolongs survival in a murine model of fully MHC-mismatched renal allograft transplantation. Am J Transplant 15: 1555–1567, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al.: QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zakrzewski M, Proietti C, Ellis JJ, Hasan S, Brion M-J, Berger B, et al.: Calypso: A user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics 33: 782–783, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozupone C, Knight R: UniFrac: A new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71: 8228–8235, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulson JN, Stine OC, Bravo HC, Pop M: Differential abundance analysis for microbial marker-gene surveys. Nat Methods 10: 1200–1202, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Love MI, Huber W, Anders S: Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMurdie PJ, Holmes S: phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8: e61217, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McWhinnie DL, Thompson JF, Taylor HM, Chapman JR, Bolton EM, Carter NP, et al.: Morphometric analysis of cellular infiltration assessed by monoclonal antibody labeling in sequential human renal allograft biopsies. Transplantation 42: 352–358, 1986. [DOI] [PubMed] [Google Scholar]
- 29.Wu H, Craft ML, Wang P, Wyburn KR, Chen G, Ma J, et al.: IL-18 contributes to renal damage after ischemia-reperfusion. J Am Soc Nephrol 19: 2331–2341, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, et al.: Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep 15: 2809–2824, 2016. [DOI] [PubMed] [Google Scholar]
- 31.Berer K, Martínez I, Walker A, Kunkel B, Schmitt-Kopplin P, Walter J, et al.: Dietary non-fermentable fiber prevents autoimmune neurological disease by changing gut metabolic and immune status. Sci Rep 8: 10431, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Round JL, Mazmanian SK: The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9: 313–323, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maynard CL, Elson CO, Hatton RD, Weaver CT: Reciprocal interactions of the intestinal microbiota and immune system. Nature 489: 231–241, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fricke WF, Maddox C, Song Y, Bromberg JS: Human microbiota characterization in the course of renal transplantation. Am J Transplant 14: 416–427, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Lee JR, Muthukumar T, Dadhania D, Toussaint NC, Ling L, Pamer E, et al.: Gut microbial community structure and complications after kidney transplantation: A pilot study. Transplantation 98: 697–705, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Z, Knight R: Dietary effects on human gut microbiome diversity. Br J Nutr 113[Suppl]: S1–S5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bromberg JS, Hittle L, Xiong Y, Saxena V, Smyth EM, Li L, et al.: Gut microbiota-dependent modulation of innate immunity and lymph node remodeling affects cardiac allograft outcomes. JCI Insight 3: e121045, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, et al.: The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujiwara H, Docampo MD, Riwes M, Peltier D, Toubai T, Henig I, et al.: Microbial metabolite sensor GPR43 controls severity of experimental GVHD. Nat Commun 9: 3674, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi M, Mikami D, Kimura H, Kamiyama K, Morikawa Y, Yokoi S, et al.: Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun 486: 499–505, 2017. [DOI] [PubMed] [Google Scholar]
- 41.Covington DK, Briscoe CA, Brown AJ, Jayawickreme CK: The G-protein-coupled receptor 40 family (GPR40-GPR43) and its role in nutrient sensing. Biochem Soc Trans 34: 770–773, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Schroeder BO, Birchenough GM, Ståhlman M, Arike L, Johansson ME, Hansson GC, et al.: Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 23: 27–40.e27, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM: The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325–2340, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, et al.: Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342: 447–453, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, et al.: Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359: 1151–1156, 2018. [DOI] [PubMed] [Google Scholar]
- 46.Crespo-Salgado J, Vehaskari VM, Stewart T, Ferris M, Zhang Q, Wang G, et al.: Intestinal microbiota in pediatric patients with end stage renal disease: A Midwest Pediatric Nephrology Consortium study. Microbiome 4: 50, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al.: Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504: 451–455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al.: Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331: 337–341, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z, Liu L, Tang H, Jiao W, Zeng S, Xu Y, et al.: Immunosuppressive effect of the gut microbiome altered by high-dose tacrolimus in mice. Am J Transplant 18: 1646–1656, 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.