Significance Statement
Although generally effective at raising hemoglobin levels to treat dialysis-dependent CKD anemia, erythropoiesis-stimulating agents such as darbepoetin alfa have safety issues and are less effective in patients with inflammation. In this randomized controlled trial in stable Japanese patients on hemodialysis previously treated with erythropoiesis-stimulating agents, the authors compared roxadustat—an oral hypoxia-inducible factor prolyl hydroxylase inhibitor previously shown to be effective in treating CKD anemia—with darbepoetin alfa. The study found that roxadustat was effective in maintaining hemoglobin within target levels and that its efficacy was noninferior to darbepoetin alfa. Consistent with previous findings, roxadustat showed an acceptable safety profile. These data confirm that oral roxadustat is a valid alternative to injectable erythropoiesis-stimulating agents for dialysis-dependent CKD anemia.
Keywords: chronic kidney disease, roxadustat, anemia, darbepoetin alfa, hemodialysis, clinical trial
Abstract
Background
Roxadustat is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor approved in China for dialysis-dependent CKD anemia.
Methods
This phase 3, 24-week, double-blind, double-dummy study evaluated roxadustat’s noninferiority to darbepoetin alfa for hemodialysis-dependent CKD anemia. We randomly assigned Japanese patients to oral roxadustat three times weekly or to darbepoetin alfa injections once weekly, titrating doses to maintain hemoglobin between 10–12 g/dl. The primary end point was change of average hemoglobin from baseline to weeks 18–24 (∆Hb18–24). Secondary end points were average hemoglobin and proportion of patients with hemoglobin between 10–12 g/dl (maintenance rate) at weeks 18–24, and iron parameters. Safety assessments included treatment-emergent adverse events and adjudicated ophthalmologic findings.
Results
We randomly assigned 303 patients to roxadustat (n=151) or darbepoetin alfa (n=152). The difference between roxadustat and darbepoetin alfa in ∆Hb18–24 was −0.02 g/dl (95% confidence interval, –0.18 to 0.15), confirming roxadustat’s noninferiority to darbepoetin alfa. Average hemoglobin at weeks 18–24 with roxadustat was 10.99 g/dl (95% confidence interval: 10.88 to 11.10), confirming its efficacy. Among patients with one or more hemoglobin value during weeks 18–24, the maintenance rate was 95.2% with roxadustat and 91.3% with darbepoetin alfa. Serum iron, ferritin, and transferrin saturation remained clinically stable with roxadustat; transferrin and total iron binding capacity increased through week 4 before stabilizing. Common treatment-emergent adverse events were nasopharyngitis, shunt stenosis, diarrhea, contusion, and vomiting. The proportion of patients with new or worsening retinal hemorrhage was 32.4% with roxadustat and 36.6% with darbepoetin alfa. We observed no clinically meaningful changes in retinal thickness groups.
Conclusions
Roxadustat maintained hemoglobin within 10–12 g/dl in patients on hemodialysis and was noninferior to darbepoetin alfa. Treatment-emergent adverse events were consistent with previous reports.
Clinical Trial registry name and registration number
A Study of Intermittent Oral Dosing of ASP1517 in Hemodialysis Chronic Kidney Disease Patients with Anemia, NCT02952092 (ClinicalTrials.gov)
Anemia is a complication of CKD resulting from a decreased synthesis of erythropoietin by the impaired kidneys and an altered iron metabolism.1 The prevalence of anemia is twice as high in patients with CKD (15.4%) than in the general population (7.6%) and increases with the severity of CKD.2 Erythropoiesis-stimulating agents (ESAs), including darbepoetin alfa (DA), are currently available for the treatment of CKD anemia; however, safety concerns and adverse effects associated with higher doses and hemoglobin (Hb) goals in CKD patients with cancer, diabetes, and cardiovascular disease have resulted in a decrease in the doses of ESAs used globally.3,4 Moreover, ESAs are administered parenterally and their efficacy is reduced in patients with inflammation.5 Consequently, alternative therapeutic strategies for anemia in CKD are currently being investigated, and the availability of an orally administered treatment option may represent an advantage for some patients.
Hypoxia-inducible factor (HIF) is an oxygen-sensitive transcription factor that regulates erythropoiesis. In the presence of normal oxygen tension, HIF-α subunits are marked for degradation by the activity of HIF prolyl hydroxylase enzymes; whereas in the presence of reduced oxygen tension, the activity of these enzymes is suppressed, which allows HIF-α to dimerize with HIF-β and accumulate, leading to increased erythropoiesis, transferrin receptor expression, and iron absorption.6 HIF prolyl hydroxylase inhibitors (HIF-PHIs) represent a new strategy to increase Hb levels by activating the body’s natural response to hypoxia, independent of cellular oxygen levels.7,8 Roxadustat (ASP1517, FG-4592, AZD9941) is an orally active HIF-PHI that has shown safety and efficacy in phase 2 trials in patients with CKD on dialysis9–11 and those not on dialysis.10,12–14 Roxadustat was approved in December 2018 in China for treatment of dialysis-dependent CKD anemia and is currently being investigated in Japan, the United States, and Europe.
This study was conducted to evaluate the noninferiority of roxadustat to DA when both drugs are titrated to maintain Hb levels of 10–12 g/dl in Japanese CKD patients with renal anemia on hemodialysis (HD). The safety of roxadustat was assessed by monitoring treatment-emergent adverse events (TEAEs) and through detailed ophthalmologic investigations, including adjudicated examination of retinal vascular findings before and after treatment.
Materials and Methods
Study Design
This was a phase 3, multicenter, randomized, double-blind study with DA as an active comparator (ClinicalTrials.gov: NCT02952092) conducted from November 2016 to March 2018 at 58 Japanese sites. Patients were randomized (1:1) to oral roxadustat three times weekly or DA injections once weekly for up to 24 weeks. In this study, there was no formal washout period; the treatment period began on the day of dialysis after the longest dialysis interval in the week when ESA had been administered (i.e., generally within 1–2 weeks). This study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice, the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use guidelines, and applicable laws and regulations. The protocol was approved by the relevant institutional review board at each site and all subjects provided written informed consent.
Study Population
Patients were aged ≥20 years with CKD anemia, receiving stable HD three times weekly for >12 weeks. Before randomization, patients were receiving intravenous (IV) short-acting recombinant human erythropoietin (rHuEPO) or DA for >8 weeks, had Hb levels within 10–12 g/dl, and transferrin saturation (TSAT) ≥20% or serum ferritin ≥100 ng/ml. Exclusion criteria included any untreated retinal neovascular lesion; untreated macular edema; uncontrolled hypertension; anemia that was not CKD related; concurrent autoimmune disease with inflammation that could have affected erythropoiesis; and elevated aspartate aminotransferase, alanine aminotransferase, or total bilirubin. A full list of eligibility criteria is reported in the Supplemental Methods.
Study Drug Administration
Assignment was implemented by a web-based randomization system (EPS Corporation, Tokyo, Japan), conducted with a dynamic allocation method using a biased-coin minimization approach. Study site, Hb levels, ESA dose, previous/concurrent retinal vascular disorder, and diabetes mellitus were assignment factors. To maintain blinding, a double-dummy design was used; only the drug assignment manager and designated staff had access to the randomization code. Patients were assigned to the initial dose of roxadustat (70 mg or 100 mg) or DA (10–60 µg) based on the average weekly dose of short-acting rHuEPO or DA before randomization (Supplemental Table 1). Roxadustat was self-administered at 2- or 3-day intervals (e.g., Monday, Wednesday, and Friday or Tuesday, Thursday, and Saturday).
Doses were adjusted to maintain the Hb levels within 10–12 g/dl. Roxadustat dose was evaluated every even week starting from week 4 in accordance with the dose-adjusting rules (Supplemental Tables 2, 3, and 4). Roxadustat dose was not to exceed 3 mg/kg or 300 mg, whichever was lower, and, as a general rule, doses were to be maintained for ≥4 weeks. DA dose was evaluated every even week (Supplemental Tables 2, 3, and 4). Dose evaluation between treatment arms differed but was in line with dosing requirements. The use of statins was allowed with the recommendation that doses not exceed the proposed maximum doses. Phosphate binders were to be dosed ≥1 hour before or after roxadustat. Based on Japanese treatment guidelines, concomitant IV iron was allowed in both arms at the discretion of the investigator only to maintain TSAT ≥20% and/or serum ferritin ≥100 ng/ml when TSAT was <20% or serum ferritin was <100 ng/ml. Oral iron was allowed based on individual patient needs, without any restrictions.
Study Outcomes and Assessments
The primary efficacy end point was the change of average Hb levels from baseline to weeks 18–24 (ΔHb18–24). Secondary efficacy end points included the average of all Hb levels during weeks 18–24; maintenance rate of the target Hb level (proportion of patients achieving an average Hb level of 10–12 g/dl during weeks 18–24); and iron parameters including serum iron, serum ferritin, serum transferrin, total iron binding capacity (TIBC), TSAT, soluble transferrin receptor, and Hb in reticulocytes. The exploratory end point hepcidin was summarized using mean, SD, minimum, maximum, and median by treatment arm, and by visit. Additionally, a within-patient change was calculated as the postbaseline measurement minus the baseline measurement and was summarized in the same way. The safety of the roxadustat treatment arm and DA treatment arm were comparatively evaluated in a double-blind manner (double-dummy method). Safety assessments included TEAEs; findings from laboratory tests, vital signs, and 12-lead electrocardiograms; and changes in ophthalmologic findings (color fundus photography and optical coherence tomography [OCT]). Fundus photography included four wide-angle color fundus photographs and OCT was conducted to capture images of the macula and its surrounding area. The images of the ophthalmologic examination were centrally assessed by two blinded independent graders. If the primary graders’ results were in disagreement, a final adjudication of the images was conducted by another independent grader. All TEAEs occurring during or after the patient discontinued the study were followed up until they were resolved, judged to be no longer clinically significant, or until they became chronic to the extent that they could be fully characterized. The schedule of assessments is reported in Supplemental Figure 1.
Statistical Methods
Sample Size
A sample size of 103 for each group would provide 90% power to demonstrate noninferiority of roxadustat to DA, assuming an SD of 1.1 g/dl and a difference of −0.25 g/dl in ΔHb18–24 between groups. Noninferiority of roxadustat to DA was demonstrated if the lower limit of the 95% confidence interval (CI) of the difference in the least squares (LS) mean of ΔHb18–24 between roxadustat and DA was above the noninferiority margin of −0.75 g/dl. To allow for an estimated drop-out rate of 30%, 150 patients per group was planned. This sample size would provide 99% power to confirm the efficacy of roxadustat, which would be achieved if the 95% CI of the average Hb levels during weeks 18–24 was within 10–12 g/dl.
Statistical Analyses
The ΔHb18–24 was analyzed by a mixed model of repeated measurements with an unstructured covariance matrix within patients that considered randomization groups, visit, baseline Hb, ESA dose before registration, previous or concurrent retinal vascular disorder, diabetes mellitus, and visit by randomization group interaction as explanatory variables, where the visit variable was dealt with as a categoric variable without grouping in the mixed model.
The primary analyses of the primary efficacy end point, confirmation of roxadustat’s efficacy and noninferiority to DA, were conducted using the per protocol set (PPS), which included patients who received treatment for ≥18 weeks, received ≥70% of treatment, and had Hb measurements at baseline and at four or more time points between weeks 18–24. A secondary analysis of the primary end point was conducted using the full analysis set (FAS), which included all patients who received one or more dose of study drug and who had one or more efficacy measurement, by the same form of statistical model as that for the primary analysis; that is, a likelihood modeling was used for incorporating all observed values from week 0 through week 24 of all subjects with and without missing data. Analysis of covariance and analyses taking into account multiple missing data mechanisms were conducted on the PPS as sensitivity analysis of the primary end point. The primary analysis of the primary efficacy end point in the PPS, the secondary analysis of the primary end point in the FAS, and sensitivity analyses of the primary end point in the PPS, were prespecified. All secondary efficacy end points were analyzed using the FAS. The secondary end points were not hypothesized, but were summarized in a descriptive manner without prespecified noninferiority margins. The average Hb levels during weeks 18–24 were calculated using data from patients with at least one Hb value during weeks 18–24. For the by-visit summary of Hb level and iron parameters, summary statistics were calculated at each visit using data from patients who had observations at the visit. The safety analysis set (SAF) included all patients who received at least one dose of study drug. Descriptive statistics were used to summarize demographics, baseline characteristics, and all iron parameters.
To evaluate the effect of roxadustat in patients with inflammation, a subgroup analysis of average Hb levels and the allocated doses of the study drug per administration by visit was conducted using high-sensitivity C-reactive protein (hs-CRP; i.e., <3.000 mg/L and ≥3.000 mg/L) as a factor, where the threshold of 3.000 mg/L represents the upper limit of the normal hs-CRP range. A subgroup analysis of new or worsening retinal hemorrhages was also conducted using the absence or presence of one or more retinal hemorrhage at baseline as a factor.
Results
Patient Disposition and Demographics
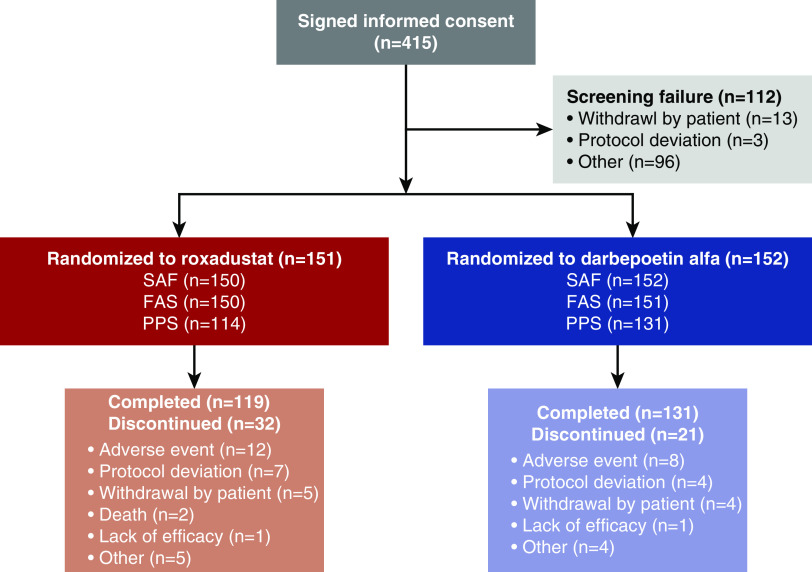
Of 415 patients screened, 112 were screen failures (protocol deviation, n=3 [0.7%]; withdrawal by patient, n=13 [3.1%]; other, n=96 [23.1%]), and 303 were randomized to receive either roxadustat (n=151) or DA (n=152). A total of 250 (82.5%) patients (roxadustat, n=119 [78.8%]; DA, n=131 [86.2%]) completed the study and 53 (17.5%) patients (roxadustat, n=32 [21.2%]; DA, n=21 [13.8%]) discontinued. Across all randomized patients, the leading reasons for discontinuation were adverse events (roxadustat, n=12 [7.9%]; DA, n=8 [5.3%]), protocol deviations (roxadustat, n=7 [4.6%]; DA, n=4 [2.6%]), and withdrawal by the patient (roxadustat, n=5 [3.3%]; DA, n=4 [2.6%]) (Figure 1). Patient demographics and baseline characteristics were similar in the roxadustat and DA groups (Table 1).
Figure 1.
Characteristics of patients. For screen failures, “other” generally indicates that a patient was excluded based on inclusion/exclusion criteria. For patients who discontinued after randomization, other refers to cancer (n=1), met withdrawal criteria (n=1), withdrew due to lack of study drug (n=1), and rheumatoid arthritis (n=1) in patients on DA; and met withdrawal criteria (n=2), eye surgery (n=2), and safety (n=1) in patients on roxadustat.
Table 1.
Patient demographics and baseline characteristics (FAS)
| Parameter | Roxadustat (n=150) | DA (n=151) |
|---|---|---|
| Sex, n (%) | ||
| Male | 101 (67.3) | 107 (70.9) |
| Female | 49 (32.7) | 44 (29.1) |
| Age, yr | ||
| Mean (SD) | 64.6 (11.7) | 64.9 (10.1) |
| Median | 65.5 | 66.0 |
| Min, max | 24, 89 | 37, 85 |
| Weight (after HD), kg | ||
| Mean (SD) | 57.82 (11.97) | 58.78 (12.90) |
| Median | 57.10 | 58.10 |
| Min, max | 36.8, 99.7 | 36.3, 107.0 |
| HD vintage, mo | ||
| Mean (SD) | 92.77 (89.78) | 99.66 (101.63) |
| Median | 59.45 | 54.34 |
| Min, max | 3.4, 488.7 | 4.3, 422.4 |
| Previous ESA medication, n (%) | ||
| rHuEPO | 43 (28.7) | 46 (30.5) |
| DA | 107 (71.3) | 105 (69.5) |
| Previous rHuEPO dose, IU/wk | ||
| Mean (SD) | 4691.86 (2436.29) | 4777.17 (2246.09) |
| Median | 4500.00 | 4500.00 |
| Min, max | 1500.0, 9000.0 | 1500.0, 9000.0 |
| Previous DA dose, µg/wk | ||
| Mean (SD) | 18.00 (14.62) | 19.00 (15.76) |
| Median | 15.00 | 15.00 |
| Min, max | 2.5, 120.0 | 0.8, 120.0 |
| Hb, g/dl | ||
| Mean (SD) | 11.02 (0.56) | 11.01 (0.60) |
| Median | 11.00 | 11.00 |
| Min, max | 9.7, 12.1 | 10.0, 12.2 |
| hs-CRP, mg/L | ||
| Mean (SD) | 1.3246 (2.4124) | 1.4622 (2.2948) |
| Median | 0.5650 | 0.5640 |
| Min, max | 0.050, 19.000 | 0.050, 13.400 |
| hs-CRP group, mg/L, n (%) | ||
| <3.000 | 136 (90.7) | 129 (85.4) |
| ≥3.000 | 14 (9.3) | 22 (14.6) |
| Iron, µmol/L | ||
| Mean (SD) | 12.1 (5.1) | 12.6 (4.5) |
| Median | 11.0 | 11.0 |
| Min, max | 5, 46 | 4, 27 |
| Ferritin, ng/ml | ||
| Mean (SD) | 102.31 (83.45) | 96.28 (75.14) |
| Median | 83.10 | 84.30 |
| Min, max | 6.9, 521.0 | 9.0, 477.0 |
| TSAT, % | ||
| Mean (SD) | 28.28 (11.70) | 29.04 (10.18) |
| Median | 25.20 | 26.90 |
| Min, max | 12.7, 93.4 | 14.1, 66.5 |
| Iron repletion, n (%) | ||
| Ferritin <100 ng/ml and TSAT <20% | 28 (18.7) | 12 (7.9) |
| Ferritin <100 ng/ml and TSAT ≥20% | 68 (45.3) | 81 (53.6) |
| Ferritin ≥100 ng/ml and TSAT <20% | 10 (6.7) | 10 (6.6) |
| Ferritin ≥100 ng/ml and TSAT ≥20% | 44 (29.3) | 48 (31.8) |
| Transferrin, g/L | ||
| Mean (SD) | 1.802 (0.327) | 1.810 (0.297) |
| Median | 1.770 | 1.810 |
| Min, max | 1.17, 2.96 | 1.14, 2.52 |
| Reticulocyte Hb, pg | ||
| Mean (SD) | 34.71 (2.00) | 35.10 (2.34) |
| Median | 34.75 | 35.30 |
| Min, max | 29.1, 41.4 | 23.4, 40.0 |
| Previous or concurrent retinal vascular disorder, n (%) | ||
| Absent | 88 (58.7) | 94 (62.3) |
| Present | 62 (41.3) | 57 (37.7) |
| Diabetes mellitus, n (%) | ||
| Absent | 96 (64.0) | 97 (64.2) |
| Present | 54 (36.0) | 54 (35.8) |
Min, minimum; max, maximum.
Treatment Compliance and Exposure
In the SAF, the mean (median, SD) treatment compliance during participation in the study was 99.23% (100%, 1.99) in the roxadustat group and 100% (100%, 0.0) in the DA group. The mean (median, SD) duration of exposure was 146.7 (168.0, 45.8) days and 154.7 (168.0, 37.4) days in the roxadustat and DA groups, respectively, and the mean (median, SD) dose per administration at week 23 was 67.1 (50.0, 41.9) mg and 31.4 (15.0, 35.8) µg in the roxadustat and DA groups, respectively. The mean (SD) number of changes in study drug dosing was 2.8 (1.4) with roxadustat and 2.3 (2.3) with DA.
Efficacy Outcomes
Primary End Point
In the PPS, the LS mean of ΔHb18–24 was −0.04 g/dl (95% CI, −0.16 to 0.08 g/dl) and −0.03 g/dl (95% CI, −0.14 to 0.09 g/dl) for roxadustat and DA, respectively, with the estimated difference of –0.02 g/dl (95% CI, –0.18 to 0.15 g/dl) between the two groups; the lower limit of the 95% CI was above the predefined noninferiority margin of –0.75 g/dl, confirming the noninferiority of roxadustat to DA. In the roxadustat group, the mean of average Hb levels during weeks 18–24 was 10.99 g/dl and its 95% CI (10.88 to 11.10 g/dl) was within the prespecified reference range of 10–12 g/dl, thus confirming the efficacy of roxadustat.
Similar results were observed with a secondary analysis in the FAS. The mean of average Hb levels during weeks 18–24 was 11.00 g/dl (95% CI, 10.89 to 11.10 g/dl) in the roxadustat group. The LS mean of ΔHb18–24 was –0.07 g/dl (95% CI, –0.19 to 0.05 g/dl) and –0.06 g/dl (95% CI, –0.18 to 0.05 g/dl) for the roxadustat and DA groups, respectively, and the estimated difference between the LS means of the two groups was –0.01 g/dl (95% CI, –0.18 to 0.16 g/dl). Sensitivity analyses in the PPS also provided similar estimated differences between the LS means of the two groups (Supplemental Table 5), suggesting the primary analysis result was robust against the missing data mechanism.
Secondary End Points
In the FAS, the mean of average Hb levels during weeks 18–24 was 11.00 g/dl (SD, 0.60; 95% CI, 10.89 to 11.10 g/dl) and 10.95 g/dl (SD, 0.63; 95% CI, 10.84 to 11.05 g/dl) for the roxadustat and DA groups, respectively, and the mean difference between the two groups was 0.05 g/dl (95% CI, –0.10 to 0.20 g/dl). The mean Hb levels during the study are shown in Figure 2.
Figure 2.
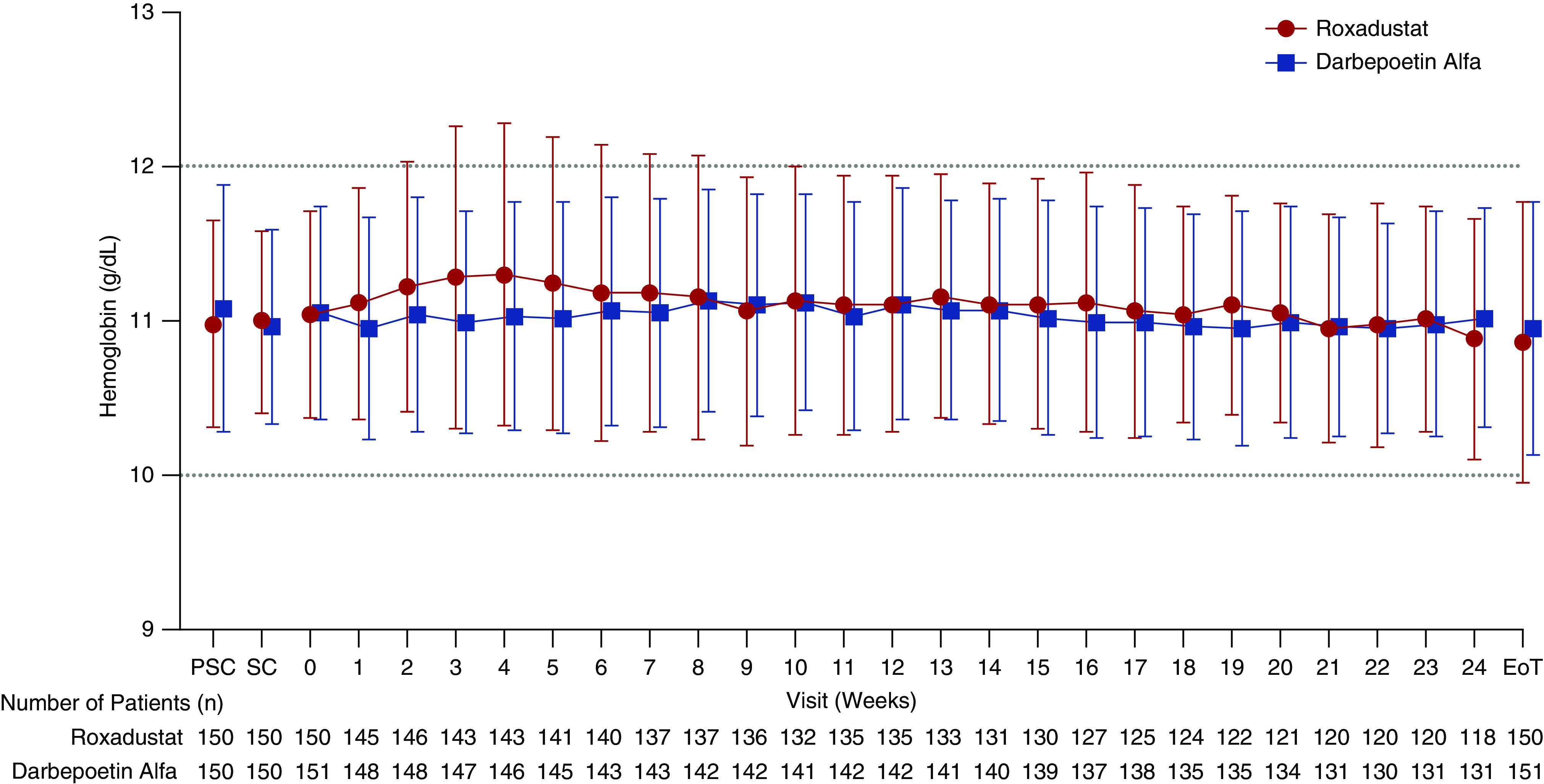
Mean (SD) Hb levels were comparable between groups throughout the study (FAS). EoT, end of treatment; PSC, prescreening; SC, screening.
The maintenance rates of the target Hb level (average Hb level of 10–12 g/dl during weeks 18–24) in the FAS were 79.3% (95% CI, 72.0% to 85.5%) and 83.4% (95% CI, 76.5% to 89.0%) for the roxadustat and DA groups, respectively, and the difference between the two groups was –4.1% (95% CI, –13.6% to 5.3%) (Supplemental Table 6). Among patients with at least one Hb value during weeks 18–24, the maintenance rates of the target Hb level were 95.2% (95% CI, 89.8% to 98.2%) and 91.3% (95% CI, 85.3% to 95.4%) for the roxadustat and DA groups, respectively, and the difference between the two groups was 3.9% (95% CI, –2.9% to 10.7%). In the roxadustat group, mean hematocrit values increased from week 0 through week 4, decreased through week 8, and then became stable through week 24, whereas, in the DA group, hematocrit levels remained stable at each visit (Supplemental Figure 2).
Throughout the study, the mean values of serum iron, soluble transferrin receptor, TSAT, reticulocyte Hb content, and ferritin remained clinically stable in both treatment groups. In the roxadustat group, the mean levels of TIBC and transferrin increased from week 0 through week 4 and then remained stable through the end of treatment, whereas, in the DA group, levels remained stable throughout the treatment period (Table 2). The use of oral and IV iron before the study was generally similar to iron usage during the study. The percentage of patients in the SAF who used oral iron was 8.9% (roxadustat, 10.0%; DA, 7.9%) before the study and 9.3% (roxadustat, 9.3%; DA, 9.2%) during the study, whereas the percentage of those who used IV iron was 25.8% (roxadustat, 26.7%; DA, 25.0%) before and 21.5% (roxadustat, 22.7%; DA, 20.4%) during the study.
Table 2.
Mean levels of iron parameters and changes from week 0 to end of treatment (FAS)
| Parameter | Roxadustat (n=150) | DA (n=151) |
|---|---|---|
| Serum iron, µmol/L | ||
| Wk 0 | 12.1 (5.1) | 12.6 (4.5) |
| EoT | 13.3 (5.3) | 11.7 (5.3) |
| Change from wk 0 to EoT | 1.2 (6.4) | −0.9 (5.5) |
| Transferrin, g/L | ||
| Wk 0 | 1.802 (0.327) | 1.810 (0.297) |
| EoT | 2.220 (0.522) | 1.915 (0.373) |
| Change from wk 0 to EoT | 0.418 (0.393) | 0.105 (0.288) |
| TIBC, µmol/L | ||
| Wk 0 | 43.4 (7.0) | 43.5 (6.2) |
| EoT | 51.1 (10.5) | 45.1 (7.6) |
| Change from wk 0 to EoT | 7.8 (8.1) | 1.6 (5.7) |
| Soluble transferrin receptor, nmol/L | ||
| Wk 0 | 22.14 (7.07) | 22.91 (7.29) |
| EoT | 23.84 (12.44) | 27.18 (14.06) |
| Change from wk 0 to EoT | 1.70 (10.55) | 4.28 (11.31) |
| TSAT, % | ||
| Wk 0 | 28.28 (11.70) | 29.04 (10.18) |
| EoT | 27.19 (12.30) | 26.60 (12.64) |
| Change from wk 0 to EoT | −1.09 (13.84) | −2.44 (13.83) |
| Hb in reticulocytes, pg | ||
| Wk 0 | 34.71 (2.00) | 35.10 (2.34) |
| EoT | 34.25 (3.02) | 33.69 (3.26) |
| Change from Wk 0 to EoT | −0.46 (2.87) | −1.41 (2.70) |
| Ferritin, ng/ml | ||
| Wk 0 | 102.31 (83.45) | 96.28 (75.14) |
| EoT | 98.33 (98.13) | 77.54 (82.82) |
| Change from wk 0 to EoT | −3.98 (78.41) | −18.75 (64.64) |
Data are presented as mean (SD). EoT, end of treatment.
Exploratory End Point
In the FAS, no remarkable changes in the mean hepcidin values were observed in either of the two treatment groups throughout the study. The mean (SD) change in hepcidin level from week 0 to end of treatment was 2.308 (27.279) ng/ml for roxadustat and –0.600 (27.061) ng/ml for DA (Supplemental Table 7).
Subgroup Analysis
Among patients with hs-CRP <3.000 mg/L, the mean (SD) ΔHb18–24 was −0.03 (0.79) g/dl and −0.03 (0.88) g/dl in the roxadustat and DA groups, respectively, whereas, among patients with hs-CRP ≥3.000 mg/L, the mean (SD) ΔHb18–24 was −0.13 (0.81) g/dl and −0.18 (0.94) g/dl in the roxadustat and DA groups, respectively (Figure 3).
Figure 3.
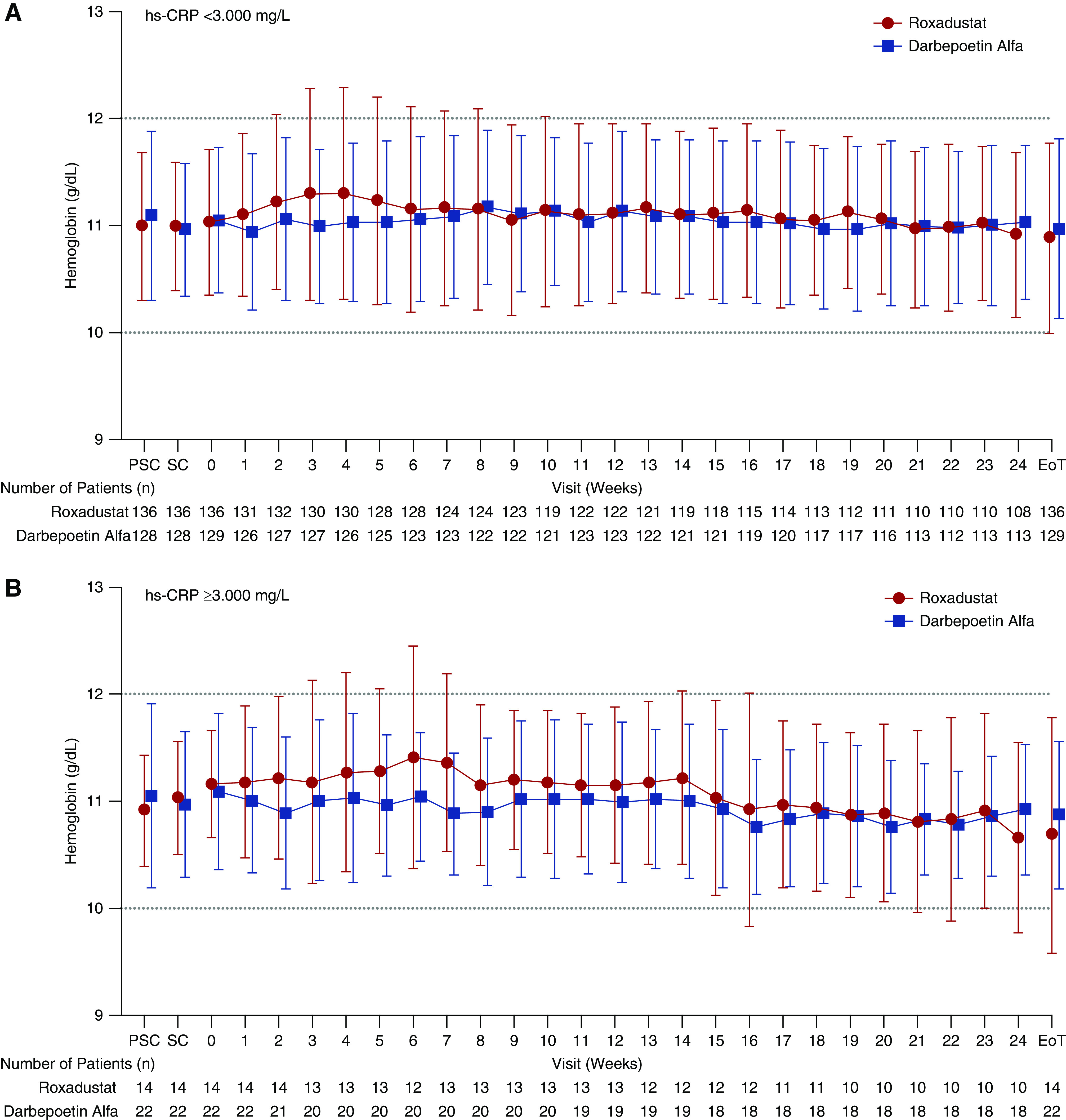
Mean (SD) Hb levels were similar between groups throughout the study when stratified by hs-CRP (FAS). EoT, end of treatment; PSC, prescreening; SC, screening.
In the DA group, in patients with hs-CRP ≥3.000 mg/L, there was a trend for higher median doses of DA used to maintain target Hb levels compared with patients with hs-CRP <3.000 mg/L; in the roxadustat group, this trend was not visible (Figure 4).
Figure 4.
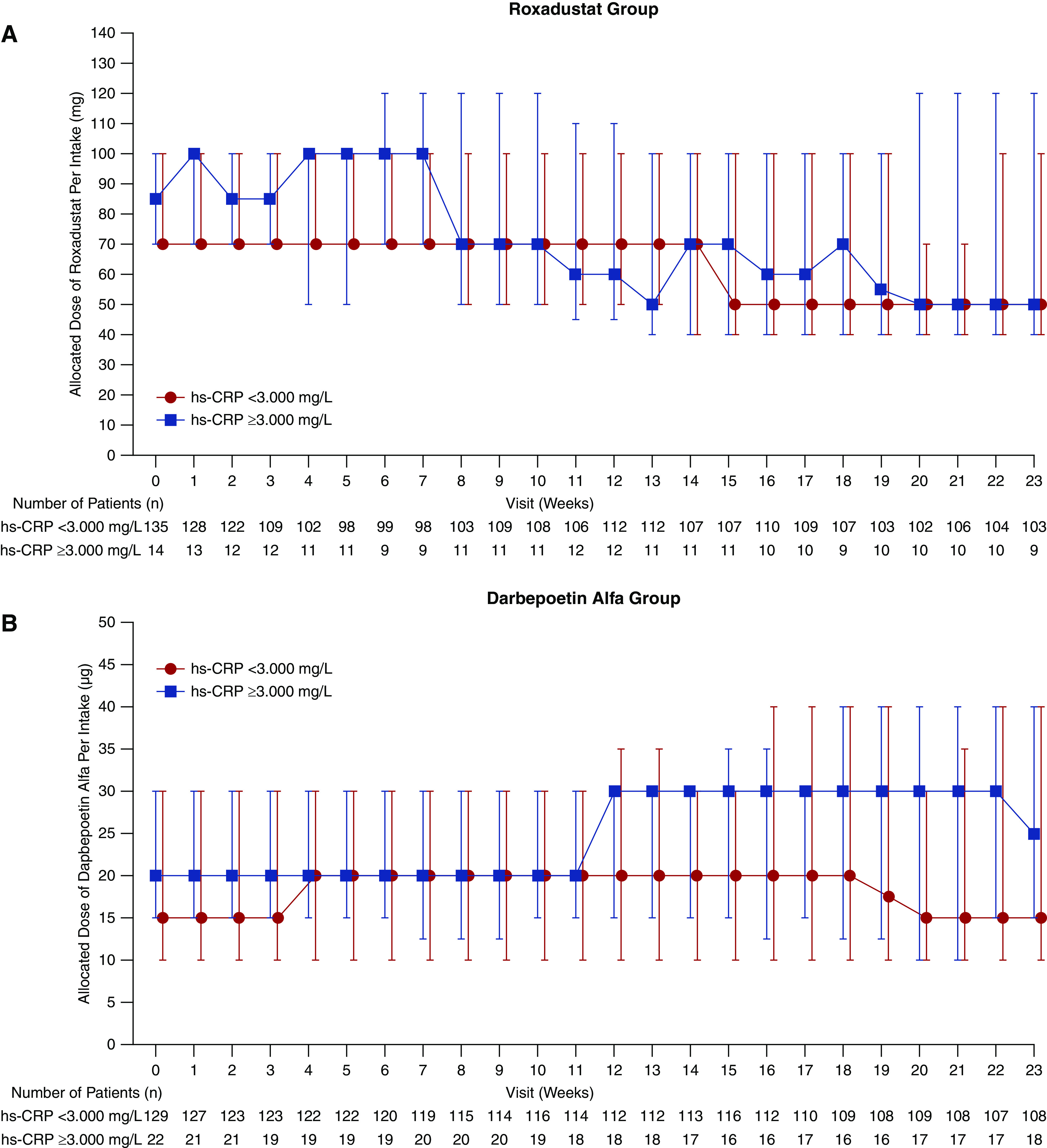
Median (interquartile range) allocated dose of study drug per administration required to maintain target Hb levels was similar in the roxadustat group and slightly different in the DA group when stratified by hs-CRP (FAS). DA was IV administered once per week to patients at the completion of dialysis, on the dialysis day after the longest dialysis interval, for a maximum of 24 weeks. The last dose was administered at completion of dialysis on the day of the week 23 visit.
Safety
The proportion of patients who reported TEAEs was similar in the roxadustat (129/150; 86.0%) and DA (126/152; 82.9%) groups (Table 3, Supplemental Table 8). The incidence of serious TEAEs was 20.7% (31/150) and 14.5% (22/152) in the roxadustat and DA groups, respectively (Supplemental Table 8). Serious TEAEs considered potentially drug related were reported in 3.3% (5/150) and 3.9% (6/152) of patients in the roxadustat and DA groups, respectively. The incidence of TEAEs leading to withdrawal of treatment and, in turn, withdrawal from the study, was 8.7% (13/150) and 5.3% (8/152) in the roxadustat and DA groups, respectively. Two deaths were reported during the study, both of which occurred in the roxadustat group (Table 3): one 64-year-old patient with a 41-year history of dialysis and concomitant chronic heart failure and a history of acute pericarditis died from acute myocardial infarction, and one 75-year-old patient with a history of hypertension, angina pectoris, hyperkalemia, and dyslipidemia died from cardiopulmonary arrest due to congestive heart failure. Common (incidence ≥5%) TEAEs included nasopharyngitis, shunt stenosis, diarrhea, contusion, and vomiting. The incidences of nasopharyngitis and vomiting were higher in the roxadustat group than in the DA group (Table 3). No clinically significant changes were observed in the clinical laboratory evaluations, vital signs, or 12-lead electrocardiograms.
Table 3.
Overview of TEAEs (SAF)
| Parameter | Roxadustat (n=150) | DA (n=152) |
|---|---|---|
| TEAEs | 129 (86.0) | 126 (82.9) |
| Serious TEAEs | 31 (20.7) | 22 (14.5) |
| Drug-related serious TEAEsa | 5 (3.3) | 6 (3.9) |
| TEAEs leading to withdrawal of treatment | 13 (8.7) | 8 (5.3) |
| Drug-related TEAEs leading to withdrawal of treatment | 8 (5.3) | 5 (3.3) |
| Deaths | 2 (1.3) | 0 |
| TEAEs occurring in ≥5% patients in either group, n (%)b | ||
| Gastrointestinal disorders | 42 (28.0) | 28 (18.4) |
| Diarrhea | 11 (7.3) | 12 (7.9) |
| Vomiting | 10 (6.7) | 3 (2.0) |
| Infections/infestations | 67 (44.7) | 58 (38.2) |
| Nasopharyngitis | 52 (34.7) | 40 (26.3) |
| Injury, poisoning, and procedural complications | 41 (27.3) | 45 (29.6) |
| Shunt stenosisc | 11 (7.3) | 13 (8.6) |
| Contusion | 10 (6.7) | 10 (6.6) |
Data are presented as n (%).
Drug-related TEAEs were defined as those TEAEs whose relationship to the study drugs could not be ruled out based on the investigator assessment.
MedDRA Version 19.0 system organ class and preferred term.
Includes arteriovenous fistula stenosis and arteriovenous graft stenosis.
Ophthalmologic Evaluations
Retinal hemorrhage was reported as a TEAE in 3.3% (5/150) and 3.9% (6/152) of patients in the roxadustat and DA groups, respectively. Color fundus photography images, assessed by an independent blinded central reviewer, revealed new or worsening retinal hemorrhages during treatment in 32.4% (46/142) and 36.6% (53/145) of patients in the roxadustat and DA groups, respectively (Table 4). No clinically meaningful changes in retinal thickness were observed by OCT, as assessed by independent blinded central reviewers, from week 0 through the end of treatment in either of the treatment groups.
Table 4.
Overview of ophthalmologic evaluations by an independent blinded central reviewer (SAF)
| Parameter | Roxadustat (n=150) | DA (n=152) |
|---|---|---|
| New or worsening retinal hemorrhage, n (%) | ||
| Treatment perioda | 46/142 (32.4) | 53/145 (36.6) |
| At wk 12 | 31/132 (23.5) | 35/136 (25.7) |
| At wk 24 | 30/113 (26.5) | 34/126 (27.0) |
| At EoT | 34/142 (23.9) | 42/145 (29.0) |
| New or worsening retinal hemorrhage by subgroup, n (%) | ||
| No retinal hemorrhages at baseline | ||
| Treatment perioda | 18/94 (19.1) | 24/96 (25.0) |
| At wk 12 | 11/85 (12.9) | 15/90 (16.7) |
| At wk 24 | 10/71 (14.1) | 16/84 (19.0) |
| At EoT | 13/94 (13.8) | 18/96 (18.8) |
| One or more retinal hemorrhage at baseline | ||
| Treatment perioda | 28/48 (58.3) | 29/49 (59.2) |
| At wk 12 | 20/47 (42.6) | 20/46 (43.5) |
| At wk 24 | 20/42 (47.6) | 18/42 (42.9) |
| At EoT | 21/48 (43.8) | 24/49 (49.0) |
EoT, end of treatment.
Detected throughout the entire treatment period.
Discussion
This double-blind, double-dummy study compared the efficacy of the HIF-PHI, roxadustat, with DA in CKD patients with renal anemia on HD who were converted from short-acting rHuEPO or DA to roxadustat. In the roxadustat group, the 95% CI of average Hb levels during weeks 18–24 was within the target range of 10–12 g/dl, therefore confirming the efficacy of roxadustat. The estimated difference in the LS mean of ΔHb18–24 between the roxadustat and DA groups confirmed the noninferiority of roxadustat to DA. The means of Hb levels were similar in the two treatment groups and remained within the target range in both treatment groups throughout the study. Moreover, the maintenance rate of the target Hb levels during weeks 18–24 was comparable between treatment groups. The mean treatment compliance was high in both treatment groups (roxadustat, 99.23%; DA, 100%).
A subgroup analysis suggested that, in the DA group, the median allocated dose of DA required to maintain target Hb levels was higher among patients with higher hs-CRP (≥3.000 mg/L) compared with those with lower hs-CRP (<3.000 mg/L). Conversely, the median allocated dose of roxadustat required to maintain the target Hb levels in the roxadustat group did not appear to be associated with hs-CRP levels. The allocated dose of roxadustat was higher in the higher hs-CRP versus lower hs-CRP group during the first 7 weeks of treatment and then became similar in the two groups, whereas the allocated dose of DA remained higher in the higher hs-CRP group from week 12 through the end of the study. Although the number of patients with higher hs-CRP (≥3.000 mg/L) was limited in this study, this observation provides preliminary evidence that the dose requirement of roxadustat may not be affected by inflammation to the same extent that the dose requirement of ESAs is; however, further investigation is required before any firm conclusions can be made regarding the relationship between roxadustat dosing and inflammation. Markers of iron bioavailability remained clinically stable in both treatment groups throughout the study, and the proportion of patients who used oral or IV iron was similar before and during the study in both treatment groups; there was no conscious effort to use less iron in either group. Among those treated with roxadustat, the levels of TIBC and transferrin increased from week 0 to week 4 and then remained stable throughout the end of treatment. Together, these findings suggest an improved iron metabolism with roxadustat.
In line with previous studies, roxadustat was generally well tolerated in patients with CKD who are HD dependent and have anemia.9–11 The proportions of overall TEAEs reported throughout the study were similar between the DA and roxadustat groups; however, a higher proportion of patients in the roxadustat group reported drug-related TEAEs (22.0% versus 13.2%) and serious TEAEs (20.7% versus 14.5%). A possible reason for this difference could be that 69.5% of patients in the DA group had received treatment with DA for ≥8 weeks before the study, which may have introduced selection bias favoring patients who tolerated DA.
Angiogenesis may be mediated via proangiogenic factors such as vascular endothelial growth factor, which can be induced by HIF-1α. Indeed, some reports have suggested angiogenesis may be associated with the development or progression of retinopathy.15,16 Considering the potential relationship between HIF-PHIs (which activate the HIF pathway) and retinal disease, ophthalmologic findings were examined. In this study, the rate of new or worsening retinal hemorrhages was comparable in the DA and roxadustat groups, and no clinically meaningful changes in retinal thickness were observed. The ophthalmologic findings suggest that treatment with roxadustat does not negatively affect retinal neovascularization in this population of patients on HD, 36.0% of whom have diabetes.
The findings of this study confirm those from previous studies showing that roxadustat is effective in the treatment of anemia in both patients with CKD who are nondialysis dependent10,12–14 and those who are HD dependent,9–11 and are consistent with results from a recent phase 3 study in Chinese patients on HD or peritoneal dialysis.17 Moreover, the results of this study are consistent with previous findings showing that inflammation, as indicated by elevated hs-CRP and frequently observed in patients with CKD, is associated with ESA hyporesponsiveness.5 The effect of roxadustat in patients with inflammation has been shown in two previous phase 2 studies. Chen et al.10 reported that treatment with roxadustat significantly decreased the levels of hepcidin in both patients who are dialysis dependent and those who are nondialysis dependent. Another study comparing epoetin alfa with roxadustat showed that hs-CRP levels were correlated with higher pre-enrollment doses of epoetin alfa, whereas, in the same patients after a 19-week treatment, no association between the roxadustat dose required to maintain target Hb levels and hs-CRP levels was observed in the last 7 weeks of treatment.11 In this study, investigators aimed to keep Hb levels within a target range, making it difficult to observe differences in Hb between groups. That said, the ability of roxadustat to maintain target Hb levels in the presence of inflammation, with few dose modifications, may represent an advantage of roxadustat compared with ESA treatment. Furthermore, the convenience of oral administration for roxadustat, supported by the high treatment compliance observed in this study, may provide an advantage for patients with CKD who are on peritoneal dialysis or not on dialysis, reducing the need of frequent hospital visits.17
This study does have limitations to be considered. Although the study was designed to assess the noninferiority of roxadustat’s efficacy to DA, a placebo group was not included for comparison, which may limit the accuracy of roxadustat’s efficacy and safety. Moreover, this study is not powered to provide a definitive conclusion regarding overall safety or cardiovascular outcomes. In addition, although the Hb response to roxadustat was relatively stable throughout the study period, studies of longer duration are needed to determine if these effects will remain consistent over time. Whereas previous studies have investigated the efficacy and safety of roxadustat in the United States9,11,12,14 and China,9,10 this study was conducted in Japanese patients, and therefore may not be fully generalizable to other ethnicities. Furthermore, because enrollment included only those patients who had previously been treated with ESAs for >8 weeks before prescreening and who had Hb levels between 10–12 g/dl, a selection bias may have favored patients who tolerated and responded well to ESAs, precluding a true head-to-head comparison. Specifically, the fact that patients in this study were previously treated with ESAs may have reduced the occurrence of side effects reported in the DA arm. Lastly, the short treatment period used in this study does not permit any definitive conclusions regarding safety.
Despite its efficacy for the treatment of CKD anemia, ESA therapy has been associated with safety concerns and the disadvantage of administration by injection, which prompted numerous clinical studies investigating safer and more convenient therapies.
Overall, this study demonstrated the efficacy of orally administered roxadustat and its noninferiority to DA in maintaining the levels of Hb within the target range in Japanese patients with anemia on HD. Roxadustat displayed a manageable safety profile with no increased risk of ophthalmologic abnormalities; however, further investigation will be required to firmly establish the safety profile of roxadustat in this population. Additional large, long-term phase 3 studies of roxadustat are currently underway to confirm and extend these efficacy- and safety-related findings to other CKD populations.
Data Sharing Statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Disclosures
Dr. Akizawa reports personal fees from Astellas, Bayer Yakuhin LD., GlaxoSmithKline, JT Pharmaceuticals, Kissei Pharmaceutical Co. Ltd., Kyowa Hakko Kirin, and Chugai Pharmaceutical Co. Ltd during the conduct of the study and reports personal fees from Ono Pharmaceutical Co. Ltd., Fuso Pharmaceutical Industries, Ltd., Nipro Corporation, and Torii Pharmaceutical Co. Ltd. outside of the submitted work. Dr. Yamaguchi and Dr. Majikawa are employees of Astellas Pharma Inc. Dr. Reusch is an employee of Astellas Pharma Europe B.V. and reports other from Astellas Pharma Europe B.V., outside the submitted work. All remaining authors have nothing to disclose.
Funding
This study was funded by Astellas Pharma, Inc.
Supplementary Material
Acknowledgments
The authors would like to thank all principal investigators and staff from each participating clinical site: Shigeru Miyazaki (Shinrakuen Hospital), Teruki Kondo (Nagano Chuo Hospital), Yasuhiro Onodera (Sapporo Tokushukai Hospital), Shigeaki Nishimura (Ehime Prefectural Central Hospital), Sumi Hidaka (Shonan Kamakura General Hospital), Satoru Matsuoka (Rakuwakai Otowa Kinen Hospital), Takeshi Nishida (Matsubara Tokushukai Hospital), Yoshinari Tsuruta (Meiyo Clinic Hemodialysis Center), Hideo Araki (Fukui Prefectural Hospital), Atsushi Kyan (Shirakawa Kosei General Hospital), Kazue Ueki (Sanshikai Toho Hospital), Shinji Ako (Matsumoto City Hospital), Takeaki Shinzato (Medical Juridical Person Kenshokai Shinzato Clinic Urakami), Hidetoshi Yoshinaga (Social Medical Corporation the Chiyukai Foundation Fukuoka Wajiro Hospital), Muneo Tomizawa (Hanyu General Hospital), Tadashi Iitsuka (Ibaraki Seinan Medical Center Hospital), Hajime Inoue (Kaikoukai Healthcare Corporation Ama Kyoritsu Clinic), Hirotake Kasuga (Kaikoukai Central Clinic), Satoshi Ota (Toyama City Hospital), Kiyoshi Izumino (Fujikoshi Hospital), Isoji Sasagawa (Yamagata Tokushukai Hospital), Zenzo Fujii (St. Hill Hospital), Haruyuki Ogura (Medical Corporation Bishinkai Kurosawa Hospital), Shunichi Umeda (Iida Hospital), Soichi Uekihara (Japanese Red Cross Kumamoto Hospital), Taku Miyoshi (Kumamoto General Hospital), Toshiro Shibata (Takayama Red Cross Hospital), Ryota Ikee (Medical Corporation H.N. Medic Kitahiroshima), Masayoshi Yamaha (Daiyukai Health System Daiyukai Dai-ichi Hospital), Yoshiro Fujita (Chubu Rosai Hospital), Yoichi Iwafuchi (Sanjo General Hospital), Jun Ino (Todachuo General Hospital), Akihiko Nakamura (Osafune Clinic), Keiichiro Mishima (Gunmaken Saiseikai Maebashi Hospital), Hideaki Yoshida (JR Sapporo Hospital), Toshiya Okumura (Tonami General Hospital), Akikazu Yamamoto (Hakuyoukai Medical Corporation, Hakuyoukai Hospital), Takayuki Toda (Tsuchiura Kyodo General Hospital), Hiroshi Kikuchi (Kikuchi Medical Clinic), Hideya Niimura (Ueyama Hospital), Norio Nagase (Kawashima Dialysis Clinic; present affiliation, Aizumi Kawashima Clinic), Sumiko Homma (Japanese Red Cross Koga Hospital), Jun Madarame (Moriya Keiyu Hospital), Yoshitaka Maeda (JA Toride Medical Center), Shinji Fujita (Sapporo Century Hospital), Akira Suga (Tenjin Clinic, Medical Corporation Shinwakai), Hiroaki Shimosaka (Hakuyoukai Medical Corporation Tajimi Clinic), Takahiro Shimodaira (Kizankai Memorial Hospital, Medical Corporation Kizankai), Toshiaki Suzuki (Asagaya Suzuki Clinic), Kenji Takada (Tsukuba-Gakuen Hospital), Masaki Oomoto (Saiseikai Imabari Hospital), Nobuyuki Miyake (Shirakawa Hospital), Masahiko Ogihara (Ogihara Clinic), Atsushi Ueda (Hitachi General Hospital), and Ryoichi Miyazaki (Fujita Memorial Hospital).
Medical writing/editorial support was provided by Patrick Tucker and Elizabeth Hermans from OPEN Health Medical Communications, Chicago, IL, and was funded by the study sponsor.
Roxadustat is being developed by FibroGen, AstraZeneca, and Astellas.
Dr. Akizawa, Dr. Majikawa, and Dr. Reusch were responsible for conceptualizing and designing of the study; Dr. Majikawa was responsible for data acquisition; all authors were responsible for data analysis/interpretation, drafting the work or revising it for important intellectual content, providing final approval of the work to be published, and agree to be accountable for all aspects of the work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019060623/-/DCSupplemental.
Supplemental Table 1. Dose conversion between mean weekly doses of short-acting rHuEPO or darbepoetin alfa before study registration and initial dose of roxadustat or darbepoetin alfa.
Supplemental Table 2. Dose-adjusting criteria.
Supplemental Table 3. Dose-adjustment steps for roxadustat.
Supplemental Table 4. Dose-adjustment steps for darbepoetin alfa.
Supplemental Table 5. Sensitivity analysis: change of average Hb levels of weeks 18 to 24 from baseline (per protocol set).
Supplemental Table 6. Hemoglobin levels by category (full analysis set).
Supplemental Table 7. Hepcidin (ng/mL) levels by visit (full analysis set).
Supplemental Table 8. Serious TEAEs (safety analysis set).
Supplemental Figure 1. Schedule of assessments.
Supplemental Figure 2. Mean and SD plot of hematocrit (fraction) (full analysis set).
References
- 1.Babitt JL, Lin HY: Mechanisms of anemia in CKD. J Am Soc Nephrol 23: 1631–1634, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stauffer ME, Fan T: Prevalence of anemia in chronic kidney disease in the United States. PLoS One 9: e84943, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Vecchio L, Locatelli F: An overview on safety issues related to erythropoiesis-stimulating agents for the treatment of anaemia in patients with chronic kidney disease. Expert Opin Drug Saf 15: 1021–1030, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Akizawa T, Okumura H, Alexandre AF, Fukushima A, Kiyabu G, Dorey J: Burden of anemia in chronic kidney disease patients in japan: A literature review. Ther Apher Dial 22: 444–456, 2018. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DW, Pollock CA, Macdougall IC: Erythropoiesis-stimulating agent hyporesponsiveness. Nephrology (Carlton) 12: 321–330, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Gupta N, Wish JB: Hypoxia-inducible factor prolyl hydroxylase inhibitors: A potential new treatment for anemia in patients with ckd. Am J Kidney Dis 69: 815–826, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan JM, Sharma N, Dikdan S: Hypoxia-inducible factor and its role in the management of anemia in chronic kidney disease. Int J Mol Sci 19: E389, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locatelli F, Fishbane S, Block GA, Macdougall IC: Targeting hypoxia-inducible factors for the treatment of anemia in chronic kidney disease patients. Am J Nephrol 45: 187–199, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Besarab A, Chernyavskaya E, Motylev I, Shutov E, Kumbar LM, Gurevich K, et al.: Roxadustat (fg-4592): Correction of anemia in incident dialysis patients. J Am Soc Nephrol 27: 1225–1233, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N, Qian J, Chen J, Yu X, Mei C, Hao C, et al.: Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant 32: 1373–1386, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provenzano R, Besarab A, Wright S, Dua S, Zeig S, Nguyen P, et al.: Roxadustat (fg-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: A phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis 67: 912–924, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Besarab A, Provenzano R, Hertel J, Zabaneh R, Klaus SJ, Lee T, et al.: Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant 30: 1665–1673, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akizawa T, Iwasaki M, Otsuka T, Reusch M, Misumi T: Roxadustat treatment of chronic kidney disease-associated anemia in japanese patients not on dialysis: A phase 2, randomized, double-blind, placebo-controlled trial. Adv Ther 36: 1438–1454, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Provenzano R, Besarab A, Sun CH, Diamond SA, Durham JH, Cangiano JL, et al.: Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (fg-4592) for the treatment of anemia in patients with ckd. Clin J Am Soc Nephrol 11: 982–991, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D, Lv FL, Wang GH: Effects of HIF-1α on diabetic retinopathy angiogenesis and VEGF expression. Eur Rev Med Pharmacol Sci 22: 5071–5076, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Takagi H, Watanabe D, Suzuma K, Kurimoto M, Suzuma I, Ohashi H, et al.: Novel role of erythropoietin in proliferative diabetic retinopathy. Diabetes Res Clin Pract 77[Suppl 1]: S62–S64, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Akizawa T, Otsuka T, Reusch M, Ueno M: Intermittent oral dosing of roxadustat in peritoneal dialysis chronic kidney disease patients with anemia: A randomized, phase 3, multicenter, open-label study. Ther Apher Dial 24: 115–125, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.