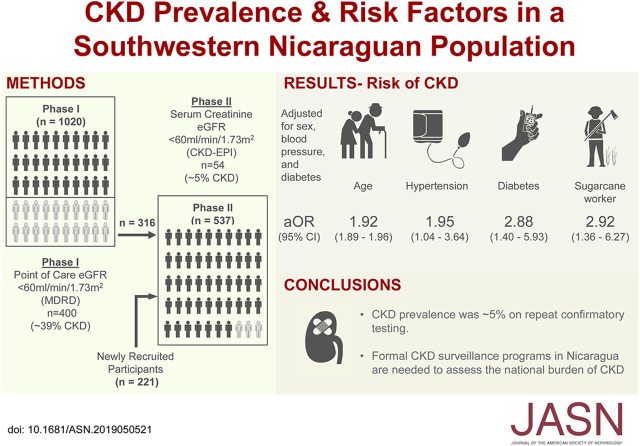
Significance Statement
Most studies of Mesoamerican nephropathy have focused on regions in El Salvador and northwest Nicaragua and on agricultural workers, but information regarding prevalence and risk factors for CKD in Nicaragua’s general population is sparse. In a study of community-dwelling individuals in southwestern Nicaragua, the authors screened 1242 participants for CKD (defined as <60 ml/min per 1.73 m2). Risk factors for prevalent CKD included age, diabetes, and hypertension. Current or former workers in the sugarcane industry (but not other types of agriculture) had a twofold-increased odds of CKD. CKD prevalence in southwestern Nicaragua is about 5% among the general population but is not consistent across Nicaragua. Formal CKD surveillance programs in Nicaragua are needed to assess the overall burden of CKD nationally, with a focus on agricultural workers.
Keywords: Mesoamerican nephropathy, Nicaragua, chronic kidney disease, renal insufficiency, public health, epidemiology
Visual Abstract
Abstract
Background
Studies have described Mesoamerican nephropathy among agricultural workers of El Salvador and northwestern Nicaragua. Data on prevalence and risk factors for CKD beyond agricultural workers and in other regions in Nicaragua are sparse.
Methods
We recruited participants from 32 randomly selected communities in the Department of Rivas’s ten municipalities in two phases. In phase 1, we screened participants using a field-based capillary creatinine measuring system and collected self-reported information on lifestyle and occupational, exposure, and health histories. Two years later, in phase 2, we enrolled 222 new participants, performing serum creatinine testing in these participants and confirmatory serum creatinine testing in phase 1 participants.
Results
We enrolled 1242 of 1397 adults (89%) living in 533 households (median age 41 years; 43% male). We confirmed CKD (eGFR<60 ml/min per 1.73 m2) in 53 of 1227 (4.3%) evaluable participants. In multivariable testing, risk factors for prevalent CKD included age (odds ratio [OR], 1.92; 95% confidence interval [95% CI], 1.89 to 1.96) and self-reported history of hypertension (OR, 1.95; 95% CI, 1.04 to 3.64), diabetes (OR, 2.88; 95% CI, 1.40 to 5.93), or current or past work in the sugarcane industry (OR 2.92; 95% CI, 1.36 to 6.27).
Conclusions
Adjusted CKD prevalence was about 5% with repeat confirmatory testing in southwest Nicaragua, lower than in the northwest region. Risk factors included diabetes, hypertension, and current or prior work in the sugarcane industry but not in other forms of agricultural work. Formal CKD surveillance programs in Nicaragua are needed to assess the overall burden of CKD nationally, with a focus on agricultural workers.
There is an increased awareness of a recently recognized form of CKD of unknown origin in Central America over the past decade.1,2 The disease, also termed Mesoamerican nephropathy, disproportionately affects young male agricultural workers living in the Pacific lowlands of Central America with hotspots identified in El Salvador and northwest Nicaragua.3,4 Mesoamerican nephropathy is typically not associated with the traditional risk factors for CKD such as diabetes and hypertension. Putative causes of the disease include heat stress and dehydration,5 occupational or environmental exposures,6,7 fructose toxicity, infectious disease,8,9 and genetic susceptibility.10 Kidney biopsy specimens from persons affected with Mesoamerican nephropathy in El Salvador and Nicaragua show chronic tubulointerstitial damage with11 or without8 evidence of glomerular ischemia and glomerulosclerosis.
A lack of health care information in other regions in Nicaragua makes it difficult to estimate national CKD prevalence. Most studies in these hotspots are cross-sectional and include predominantly young adults. The proportion of decreased eGFR has widely divergent prevalence, from 9%–42% in men to 1%–10% in women. Published cross-sectional studies were executed with important design differences, even in geographically proximate communities. Study differences include classification of participants with CKD on the basis of determination of eGFR at a single time point, selected study populations,12−13 and varying measurements of creatinine and definitions of CKD.14,15 Almost all studies of CKD prevalence in Nicaraguan communities12,13,15–17were conducted in the Pacific northwest region, where sugarcane is the primary agricultural crop and which is a presumed hotspot for Mesoamerican nephropathy.4 One study conducted in the central interior of Nicaragua found lower rates of CKD and differed from other studies in both altitude of the region (>1000 m above sea level) and having coffee farming as the main agricultural crop.14,18–20 An important question facing health care providers and administrators is whether CKD is limited to identified geographic hotspots or is more ubiquitous throughout Nicaragua. Despite a lack of more robust and geographically distributed studies, Mesoamerican nephropathy was widely publicized and characterized as an epidemic,10,21 with a call made for additional research22 and intervention.23,24
It is important to assess the burden of CKD across Nicaragua with important health service implications as well as to address risk factors leading to CKD specific to Nicaragua. The Department of Rivas borders the Pacific Ocean in the southwest of Nicaragua and shares many of the features of the northwestern regions that are associated with Mesoamerican nephropathy, including low altitude and similar occupations, agricultural crops, climate, and population demographics. In 2012, health care providers and administrators in Rivas were concerned about CKD in their region and supported studying of the Rivas region.25 The aim of this project was to create a representative geographic-based cohort to estimate prevalence of CKD in the Department of Rivas.
Methods
Study Design and Data Collection
Detailed descriptions of study design and population are available in a published methods paper25 and important design features are summarized here.
The study was conducted in two phases. Subject recruitment was designed to minimize the potential for recruitment bias regarding CKD status and involved randomization at the community level. Within each randomly selected community, participants were recruited from sequential households and an attempt was made to enroll all eligible members of each household. Both phase 1 (2012) and phase 2 (2014) were conducted in July and August, before the sugarcane harvest period. The Institutional Review Board of the Dirección General de Docencia e Investigaciónes of the Ministry of Health of Nicaragua approved the protocol and granted permission to enroll participants from these municipalities (Altagracia, Belén, Buenos Aires, Cárdenas, Moyogalpa, Potosí, Rivas, San Jorge, San Juan del Sur, and Tola) for phase 1 of the study. Phase 2 of the study was approved by the Institutional Review Board of the Centro Nacional de Diagnostico y Referencia of the Ministry of Health of Nicaragua. All participants gave informed consent, and the study was performed in adherence with the Declaration of Helsinki.
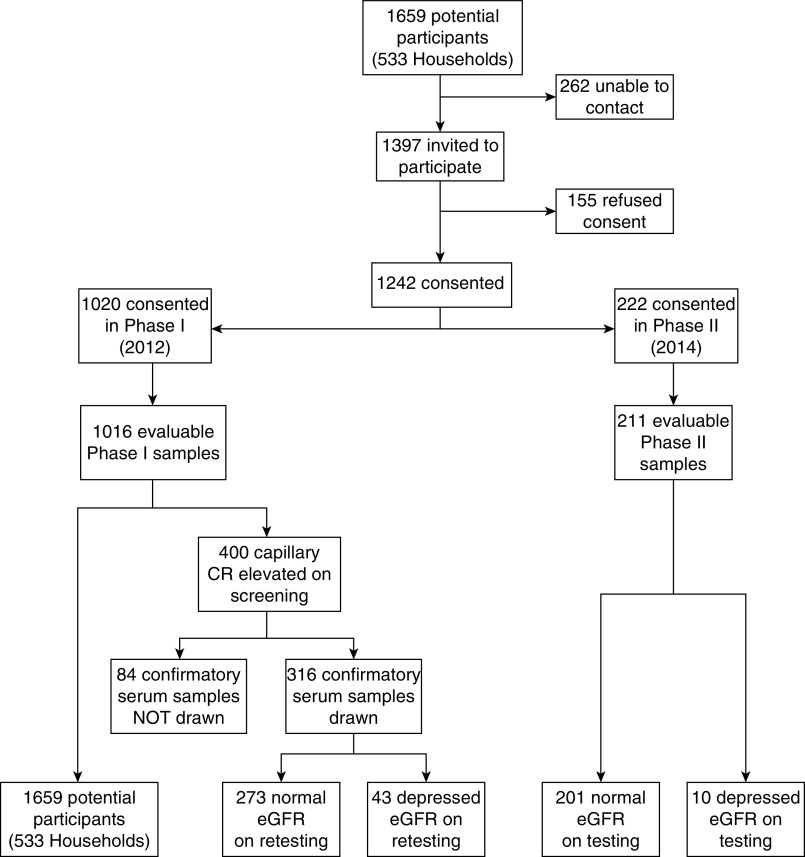
To facilitate recruitment and screening in phase 1, participants were initially screened using a point-of-care capillary creatinine–measuring system, the StatSensor Express (Nova Biomedical, Waltham, MA),26 and then follow-up of those with elevated creatinine by venous blood sampling for creatinine was carried out, using standard laboratory procedures to confirm the presence of CKD. In phase 1, 1020 participants consented and were screened for CKD (Figure 1, Strengthening the Reporting of Observational studies in Epidemiology diagram). Of the 1016 evaluable participants, 400 had an eGFR of <60 ml/min per 1.73 m2 calculated with the Modification of Diet in Renal Disease (MDRD) equation. Phase 2 allowed for repeat testing of participants to confirm CKD and also enrollment of new participants from the same communities as well as from four additional communities not visited during phase 1 that make-up the Department of Rivas. All enrolled participants in both phases had a urine dipstick test performed and completed a questionnaire to ascertain exposures relevant to CKD and Mesoamerican nephropathy.
Figure 1.
Study population of the Rivas Cohort Study, Nicaragua.
The initial phase conducted point-of-care testing with participants due to cost and transport issues, and serum samples were not routinely collected. Participants visited in phase 2 had height and weight measured for body mass index determination using a portable mechanical scale and measuring tape. Blood samples were obtained by trained phlebotomists. Blood samples were placed on ice immediately after collection; centrifuged at 3000 × g for 15 minutes within 3 hours; refrigerated at 4°C until weekly transport to the International Organization for Standardization-certified Centro Nacional de Diagnostico y Referencia in Managua, a division of the Ministry of Health (Ministerio de Salud); and then stored at −80°C. Serum creatinine was measured using a kinetic-rate Jaffe method and calculations were made to calibrate to an isotopic dilution mass spectrometry standard. Urine dipstick testing was done immediately on voided specimens using Rapid Response 10 Parameter (10SG) Urinalysis Reagent Test Strips (BTNX Inc., Markham, ON).
Although the MDRD method was used for screening participants, all statistical analyses reported use the eGFR calculated with the Chronic Kidney Disease Epidemiology Collaboration equation.27 CKD stage is defined only on the basis of eGFR determinations using the Kidney Disease: Improving Global Outcomes definition, when the eGFR<60 ml/min per 1.73 m2 separated by at least 3 months was used to define CKD.28
Statistical Analyses
Comparisons between groups were performed with use of the t test for continuous measures and the chi-squared test (or Fisher’s exact test) for categoric measures. Stratum-specific prevalence was calculated for those with and without CKD (eGFR<60 versus eGFR≥60 ml/min per 1.73 m2) and compared by chi-squared test. Crude and adjusted (for sex, age, self-reported high BP, and self-reported diabetes determined a priori) prevalence odds ratios (ORs) were calculated using simple and multivariable logistic regression and presented with their corresponding 95% confidence intervals (95% CIs). Participants with only serum creatinine in phase 2 and a single time point of assessment were included in a sensitivity analysis of CKD prevalence estimates.
Results
The age distribution (mean 40.4 years, range 17.4–101.8) of the study population is similar to that of the Department of Rivas as determined in the 2005 census29 (Table 1). Of the 400 participants with eGFR<60 ml/min per 1.73 m2 (MDRD) in phase 1, 316 had confirmatory serum creatinine determined via venipuncture during phase 2. A total of 84 participants were not retested; ten had died since the initial visit in phase 1 and the remainder refused to participate, had moved, or were not at home—but were known to be alive and well with no known kidney disease by family members and neighbors. Among those participants who could not be recontacted in phase 2, there was no significant difference in age, prevalence of self-reported hypertension, or proportion male. The only difference was that rates of self-reported diabetes were lower in those that were not available for recontact.
Table 1.
Comparison of demographics among Nicaragua 2005 census,29 Rivas 2005 census,29 and the Rivas Cohort Study participants
| Indicator | Nicaragua 2005 Census | Rivas 2005 Census | Study Participants | |||
|---|---|---|---|---|---|---|
| N | % | N | % | Na | % | |
| Total | 2,627,737 | 51.1 | 84,944 | 54.4 | 1080 | |
| Age | ||||||
| 20–29 | 951,701 | 36.2 | 28,088 | 33.1 | 336 | 31.1 |
| 30–39 | 632,253 | 24.1 | 19,178 | 22.6 | 275 | 25.5 |
| 40–49 | 451,622 | 17.2 | 14,935 | 17.6 | 181 | 16.8 |
| 50–59 | 278,984 | 10.6 | 9844 | 11.6 | 135 | 12.5 |
| 60–69 | 165,848 | 6.3 | 6110 | 7.2 | 81 | 7.5 |
| 70–79 | 96,194 | 3.7 | 4243 | 5.0 | 50 | 4.6 |
| 80+ | 51,135 | 1.9 | 2546 | 3.0 | 22 | 2.0 |
| Sex | ||||||
| Male | 1,255,294 | 47.8 | 41,581 | 49.0 | 472 | 43.8 |
| Female | 1,372,443 | 52.2 | 43,363 | 51.0 | 608 | 56.3 |
Excludes 84 participants enrolled in phase 1 and not retested in phase 2; 63 enrolled individuals whose age is <20 years as comparable census data are not available.
CKD was confirmed in 43 of the 316 participants that were retested 2 years later. Observed prevalence of CKD in the cohort was 4.2% (43 cases of 1016 screened participants), but this estimate excludes consideration of the 84 participants who screened positive by capillary creatinine in phase 1 but were not available for retesting (Table 2). If the same rate of CKD was observed, then it is estimated that there would be an additional 11 cases of CKD (13.6% × 84 participants) that went unobserved. When combined with the observed cases, the “adjusted CKD prevalence” was 5.3% (54 cases of 1016 screened participants). Proteinuria (>30 mg/dl) was found by dipstick in 6.2% of tested participants, but was more common in CKD cases (26.0%; 13 of 50 participants tested) than in non-CKD cases (5.2%; 53 of 1023 participants tested; P<0.001).
Table 2.
Prevalence of CKD in Rivas, Nicaragua from 2012 to 2014
| Variable | Phase 1, 2012a | Phase 2, 2014b | Combined |
|---|---|---|---|
| Total evaluable (n) | 1016 | 211 | 1227 |
| Screened with CKD by MDRD (n) | 400 (39%) | ||
| Not retested (n) | 84 | – | – |
| Retested (n) | 316 | – | – |
| Confirmed CKD by serum creatinine (n) | 43 | 10 | 53 |
| Stage 3A (eGFR 45–59) (n) | 21 | 5 | 26 |
| Stage 3B (eGFR 30–44) (n) | 12 | 4 | 16 |
| Stage 4 (eGFR 15–29) (n) | 8 | 1 | 9 |
| Stage 5 (eGFR<15) (n) | 2 | 0 | 2 |
| Confirmed not CKD (n) | 273 | ||
| CKD observed prevalence | 4.2% (43 of 1016) | 4.7% (10 of 211) | 4.3% (53 of 1227) |
| CKD adjusted prevalencec | 5.3% (54 of 1016) | 4.7% (10 of 211) | 5.2% ([54+10] of 1227) |
–, not applicable.
CKD defined as eGFR<60 ml/min per 1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration equation.
New participants enrolled.
Adjusted for participants who were screened positive for CKD in phase 1 but were not available for retesting in phase 2.
Prevalence estimates differed significantly by diabetes and hypertension status (Table 3). There were no significant differences by sex, alcohol intake, water intake, or agricultural work. In univariable and multivariable testing (Table 4), risk factors for CKD included age (OR, 1.92; 95% CI, 1.89 to 1.96), self-reported history of hypertension (OR, 1.95; 95% CI, 1.04 to 3.64), and diabetes (OR, 2.88; 95% CI, 1.40 to 5.93). There was a strong association (OR, 2.9; 95% CI, 1.36 to 6.27, adjusted for sex, age, diabetes, and hypertension) of current or past work in the sugarcane industry and CKD in the 174 participants who reported work in this sector.
Table 3.
Stratum-specific prevalence of CKD by demographic characteristics and potential risk factors in Rivas, Nicaragua
| Characteristic | N (%) | Stratum-Specific Prevalence (95% CI) | P Value | |
|---|---|---|---|---|
| eGFR<60 ml/min per 1.73 m2 | eGFR≥60 ml/min per 1.73 m2 | |||
| Study population | 1143 | n=53 | n=1090 | |
| Mean eGFR±SD | 42.0±13.2 | 95.6±19.6 | ||
| Demographic characteristics | ||||
| Sex | 0.4 | |||
| Female | 642 (56.2) | 50.9 (36.8 to 64.9) | 56.4 (53.4 to 59.4) | |
| Male | 501 (43.8) | 49.1 (35.1 to 63.2) | 43.6 (40.6 to 46.6) | |
| Age (continuous) | 1143 (100) | 62.2±13.3 | 38.7±16.0 | <0.001 |
| Age (categoric) | <0.001 | |||
| <20 | 63 (5.5) | 0 | 5.8 (4.5 to 7.3) | |
| 20–29 | 336 (29.4) | 0 | 30.8 (28.1 to 33.7) | |
| 30–39 | 275 (24.1) | 7.6 (2.1 to 18.2) | 24.9 (22.3 to 27.5) | |
| 40–49 | 181 (15.8) | 11.3 (4.3 to 23.0) | 16.1 (13.9 to 18.4) | |
| 50–59 | 135 (11.8) | 28.3 (13.2 to 35.6) | 11.0 (9.2 to 13.0) | |
| 60–69 | 81 (7.1) | 24.5 (13.8 to 38.3) | 6.2 (4.9 to 7.8) | |
| 70–79 | 50 (4.4) | 18.9 (9.4 to 32.0) | 3.7 (2.7 to 5.0) | |
| 80+ | 22 (1.9) | 9.4 (3.1 to 20.7) | 1.6 (0.9 to 2.5) | |
| Municipalities | 0.1 | |||
| Altagracia | 41 (3.6) | 1.9 (0.05 to 10.1) | 3.7 (2.6 to 5.0) | |
| Belen | 136 (11.9) | 11.3 (4.3 to 23.0) | 11.9 (10.1 to 14.0) | |
| Buenos Aires | 97 (8.5) | 3.8 (0.5 to 13.0) | 8.7 (7.1 to 10.6) | |
| Cardenas | 104 (9.1) | 11.3 (4.3 to 23.0) | 9.0 (7.4 to 10.9) | |
| Moyogalpa | 40 (3.5) | 1.9 (0.05 to 10.1) | 3.6 (2.6 to 4.9) | |
| Potosi | 143 (12.5) | 15.1 (6.8 to 27.6) | 12.4 (10.5 to 14.5) | |
| Rivas | 167 (14.6) | 17.0 (8.1 to 29.8) | 14.5 (12.5 to 16.7) | |
| San Jorge | 145 (12.7) | 11.3 (4.3 to 23.0) | 12.8 (10.8 to 14.9) | |
| San Juan | 124 (10.9) | 1.9 (0.05 to 10.1) | 11.3 (9.5 to 13.3) | |
| Tola | 146 (12.8) | 24.5 (13.8 to 38.3) | 12.2 (10.3 to 14.3) | |
| Water sourcea | ||||
| Well | 543 (47.5) | 43.4 (29.8 to 57.7) | 47.8 (44.8 to 50.9) | 0.5 |
| Piped | 523 (45.8) | 54.7 (40.5 to 68.4) | 45.5 (42.5 to 48.5) | 0.2 |
| Potential risk factors | ||||
| Alcohol consumption | ||||
| No | 998 (87.3) | 92.3 (81.5 to 97.9) | 89.1 (87.1 to 90.9) | 0.5 |
| Yes | 120 (10.5) | 7.7 (2.1 to 18.5) | 10.9 (9.1 to 12.9) | |
| Alcohol consumption volume | 0.8 | |||
| 0 drinks/d | 998 (87.3) | 92.3 (81.5 to 97.9) | 89.1 (87.1 to 90.9) | |
| 1–2 drinks/d | 44 (3.9) | 1.9 (0.05 to 10.3) | 4.0 (2.9 to 5.4) | |
| 3–4 drinks/d | 18 (1.6) | 1.9 (0.05 to 10.3) | 1.6 (0.9 to 2.5) | |
| 5+ drinks/d | 58 (5.1) | 3.9 (0.5 to 13.2) | 5.3 (4.0 to 6.8) | |
| Ever smoked | 0.1 | |||
| No | 751 (65.7) | 64.4 (48.8 to 78.1) | 75.4 (72.6 to 78.1) | |
| Yes | 251 (22.0) | 35.6 (21.9 to 51.2) | 24.6 (21.9 to 27.4) | |
| Self-reported diabetes | <0.001 | |||
| No | 1063 (93.0) | 75.5 (61.7 to 86.2) | 93.9 (92.4 to 95.3) | |
| Yes | 79 (7.0) | 24.5 (13.8to 38.3) | 6.1 (4.7 to 7.7) | |
| Self-reported high BP | <0.001 | |||
| No | 840 (73.5) | 41.5 (28.1 to 55.9) | 75.1 (72.4 to 77.7) | |
| Yes | 302 (26.4) | 58.5 (44.1 to 71.9) | 24.9 (22.3 to 27.6) | |
| Number of years worked in agricultureb | 0.1 | |||
| Never | 590 (51.6) | 52.4 (36.4 to 68.0) | 59.2 (56.0 to 62.3) | |
| 0–4 | 135 (11.8) | 11.9 (4.0 to 25.6) | 13.5 (11.4 to 15.9) | |
| 5–9 | 60 (5.3) | 2.4 (0.1 to 12.6) | 6.2 (4.7 to 7.9) | |
| 10–14 | 69 (6.0) | 4.8 (0.6 to 16.2) | 7.0 (5.5 to 8.8) | |
| ≥15 | 148 (13.0) | 28.6 (15.7 to 44.6) | 14.2 (12.0 to 16.5) | |
| Daily water consumption | 0.8 | |||
| ≤1 L water/d | 451 (39.5) | 36.5 (23.6 to 51.0) | 40.2 (37.3 to 43.3) | |
| 2–3 L water/d | 533 (46.6) | 48.1 (36.8 to 63.2) | 47.3 (44.3 to 50.3) | |
| 4–5 L water/d | 126 (11.0) | 15.4 (6.9 to 28.1) | 11.0 (9.2 to 13.0) | |
| 6–7 L water/d | 12 (1.1) | 0 | 1.1 (0.6 to 1.9) | |
| 8–9 L water/d | 4 (0.4) | 0 | 0.4 (0.1 to 1.0) | |
Sample sizes (N) for some risk factor subgroups vary due to missing data.
Categories are not mutually exclusive.
Missing duration of agricultural work in 141 individuals.
Table 4.
Multivariable model of associations between CKD and potential risk factors in Rivas, Nicaragua
| Covariates | Unadjusted POR (95% CI) | P Value | Adjusted POR (95% CI)a | P Value |
|---|---|---|---|---|
| Sex | ||||
| Female | Referent | Referent | ||
| Male | 1.25 (0.72 to 2.16) | 0.4 | 1.40 (0.76 to 2.58) | 0.2 |
| Age (continuous, 10-yr increase) | 2.04 (2.00 to 2.07) | <0.001 | 1.92 (1.89 to 1.96) | <0.001 |
| Self-reported diabetes | ||||
| No | Referent | Referent | ||
| Yes | 5.04 (2.57 to 9.88) | <0.001 | 2.88 (1.40 to 5.93) | 0.04 |
| Self-reported high BP | ||||
| No | Referent | Referent | ||
| Yes | 4.25 (2.42 to 7.47) | <0.001 | 1.95 (1.04 to 3.64) | 0.004 |
| Worked in sugarcane | ||||
| No | Referent | Referent | ||
| Yes | 2.95 (1.61 to 5.43) | 0.0005 | 2.92 (1.36 to 6.27) | 0.006 |
| Worked in agricultureb | ||||
| No | Referent | Referent | ||
| Yes | 1.53 (0.88 to 2.68) | 0.1 | 1.53 (0.76 to 3.09) | 0.2 |
| Number of years worked in agriculture | ||||
| Never | Referent | Referent | ||
| 0–4 | 0.99 (0.37 to 2.67) | 0.9 | 1.47 (0.49 to 4.47) | 0.5 |
| 5–9 | 0.44 (0.06 to 3.31) | 0.4 | 0.65 (0.08 to 5.37) | 0.7 |
| 10–14 | 0.77 (0.18 to 3.35) | 0.7 | 0.97 (0.20 to 4.76) | 0.9 |
| ≥15 | 2.28 (1.10 to 4.72) | 0.03 | 1.49 (0.59 to 3.74) | 0.4 |
| Ever smoked | ||||
| No | Referent | Referent | ||
| Yes | 1.70 (0.91 to 3.18) | 0.09 | 1.46 (0.63 to 3.39) | 0.4 |
POR, prevalence odds ratio.
Logistic regression model adjusted for sex, continuous age, self-reported high BP, and diabetes.
Defined as any of the following: current work in agriculture, ever worked in agriculture, or ever worked in sugarcane.
Discussion
In a population sampling of southwestern coastal Nicaragua, in the Department of Rivas, we report that the prevalence of CKD is about 5% and is typically associated with older age, diabetes, and hypertension. There was also a higher CKD prevalence among those who had current or past work in the sugarcane industry; however, other agricultural work was not associated with increased odds of CKD. This study employed geographic sampling techniques with recruitment of random households in specific sampling tracts and the logistics of subject recruitment in the community, to maximize enrollment and retention in a rural region of Nicaragua and to ensure that the study population was reflective of the general population. Additionally, there was a very small loss to follow-up of 6% over 2 years; yet, key information on mortality and report of kidney disease were available from other household members.
In a random general population of southwestern Nicaragua, the observed prevalence of CKD was 4.3% and in contrast with most previous studies that show a higher prevalence of CKD in population samples from Nicaragua and El Salvador. Planned recruitment strategies reduced the likelihood of over-sampling or enrichment of the study population with agricultural (specifically sugarcane) workers, for example, and so it is more reflective of the general population. Recent studies in artisanal brick layers from Las Paz Centro,30 agricultural workers, young adults under age 30, and other regional populations in the northwest report higher prevalence of CKD,31,32 reflective of the higher-risk region and occupations at risk and not reflective of the general population. Alternatively, the prevalence of CKD found in Rivas may reflect a true geographic difference from other studies conducted in northwestern coastal Nicaragua and El Salvador. Indeed, a low prevalence of CKD is reported in a high-altitude coffee-growing village of central Nicaragua.14 The Department of Rivas shares many of the features of the Northwestern regions that are associated with Mesoamerican nephropathy including low altitude inhabitant occupations, agricultural crops, climate and population demographics. These shared features argue against the CKD prevalance differences reported in each region.
An important feature of this study, and one that makes it unique in relation to other studies in this region, is that kidney function was assessed twice and 2 years apart. This is critical because we identified 400 participants initially with possible CKD and, upon retesting after 2 years, almost 70% no longer had advanced CKD. The high number of participants no longer having CKD could be due to false-positive findings, likely from the lack of precision of the point-of-care testing26 or resolution of AKI. Nonetheless, frequent surveillance of high-risk agricultural workers is important to screen for the true burden of CKD in Nicaragua. Additionally, participants were enrolled in July and August, before the sugarcane harvest season, in order to minimize this potential bias. Another critical feature of the study methods is that creatinine values used for subject classification were determined at a central laboratory and were not reliant on field-based measurements using capillary testing devices. The main limitations of this study include the inability to classify participants with milder forms of CKD with eGFR from 60 to 90 ml/min per 1.73 m2 and that proteinuria was not available in all participants. Proteinuria by dipstick was more common among those with CKD and should be added to any screening program. A further limitation was that less than half of the study cohort worked within an agricultural industry, with the majority having only a mild-to-moderate yearly exposure. Lastly, we were unable to retest 84 of the 400 participants who screened positive in phase 1, ten of whom had died, and they may have suffered from kidney disease; however, reports from household members denied any kidney disease. Due to this lack of retesting, an adjusted model presuming similar CKD prevalence was incorporated as the clinical characteristics were similar. This may have increased the overall prevalence reported.
CKD prevalence is 5% in southwestern Nicaragua among the general population and is associated with diabetes and hypertension. Prior or current work in the sugarcane industry was also independently and significantly associated with CKD; however, the overall prevalence remained low. Rates of CKD are not consistent across Nicaragua or among high-risk agricultural workers. The Rivas cohort established in southwest Nicaragua33 reflects a growing awareness for CKD surveillance programs in countries with emerging economies,34 where a lack of reliable health care data is a major obstacle to understanding CKD burden.
Disclosures
All authors have nothing to disclose.
Funding
This project was funded primarily by small private contributions managed by the “Newton-San Juan Del Sur Sister City Project” (http://sanjuandelsursistercityproject.wordpress.com/). Additional support was provided by the Medical Alumni Association at the University of Toronto Faculty of Medicine and the American Society of Nephrology (Dr. Minnings and Dr. Parekh). Dr. Parekh is funded by the Canada Research Chair in CKD Epidemiology. The funders had no role in the design, conduct, analysis, or writing up of the study. The corresponding author had full access to the data and took the decision to submit for publication.
Supplementary Material
Acknowledgments
Dr. Ferguson, Dr. Amador, Dr. Brooks, Dr. Kaufman, and Dr. L. Fiore conceived the study. Field work was managed by Dr. Madeline Fiore, Dr. Minnings, and Ms. Mosco. Validation of capillary creatinine devices was performed by Ms. Melissa Fiore, Dr. L. Fiore, and Dr. Kerns. Dr. Amador communicated with Nicaraguan authorities and institutional review boards. Dr. Leatherman did the statistical analysis. All authors contributed to editing of the manuscript. Dr. L. Fiore and Dr. Parekh wrote the first and final drafts of the manuscript.
Data collected for the study, including deidentified individual participant data and a data dictionary defining each field in the set, will be made available to researchers with an institutional review board–approved protocol. A signed data access agreement that specifies how the data may be used is required. Generally, data are available without cost and publications that result from data analyses must acknowledge the original work. Data will be available indefinitely from the time of publication and can be obtained by contacting the corresponding author. Additional, related documents, including study procedures and data collection forms, are published and available elsewhere25 or will be supplied by the corresponding author.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019050521/-/DCSupplemental.
Supplemental Table 1. Published epidemiologic studies of Mesoamerican nephropathy.
Supplemental Figure 1. Map of Nicaragua showing Department of Rivas; location of participant recruitment.
References
- 1.Cohen J: Mesoamerica’s mystery killer. Science 344: 143–147, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RJ, Sánchez-Lozada LG: Chronic kidney disease: Mesoamerican nephropathy--new clues to the cause. Nat Rev Nephrol 9: 560–561, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Brooks DR, Ramirez-Rubio O, Amador JJ: CKD in Central America: A hot issue. Am J Kidney Dis 59: 481–484, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Martín-Cleary C, Ortiz A: CKD hotspots around the world: Where, why and what the lessons are. A CKJ review series. Clin Kidney J 7: 519–523, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Trabanino R, Jarquín E, Wesseling C, Johnson RJ, González-Quiroz M, Weiss I, et al.: Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador--A cross-shift study of workers at risk of Mesoamerican nephropathy. Environ Res 142: 746–755, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Laws RL, Brooks DR, Amador JJ, Weiner DE, Kaufman JS, Ramírez-Rubio O, et al.: Changes in kidney function among Nicaraguan sugarcane workers. Int J Occup Environ Health 21: 241–250, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wesseling C, Aragón A, González M, Weiss I, Glaser J, Rivard CJ, et al.: Heat stress, hydration and uric acid: A cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open 6: e011034, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer RSB, Vangala C, Truong L, Mandayam S, Chavarria D, Granera Llanes OM, et al.: Early detection of acute tubulointerstitial nephritis in the genesis of Mesoamerican nephropathy. Kidney Int 93: 681–690, 2018. [DOI] [PubMed] [Google Scholar]
- 9.Murray KO, Fischer RS, Chavarria D, Duttmann C, Garcia MN, Gorchakov R, et al.: Mesoamerican nephropathy: A neglected tropical disease with an infectious etiology? Microbes Infect 17: 671–675, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Correa-Rotter R, Wesseling C, Johnson RJ: CKD of unknown origin in Central America: The case for a Mesoamerican nephropathy. Am J Kidney Dis 63: 506–520, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wijkström J, Leiva R, Elinder CG, Leiva S, Trujillo Z, Trujillo L, et al.: Clinical and pathological characterization of Mesoamerican nephropathy: A new kidney disease in Central America. Am J Kidney Dis 62: 908–918, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Lebov JF, Valladares E, Peña R, Peña EM, Sanoff SL, Cisneros EC, et al.: A population-based study of prevalence and risk factors of chronic kidney disease in León, Nicaragua. Can J Kidney Health Dis 2: 6, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanoff SL, Callejas L, Alonso CD, Hu Y, Colindres RE, Chin H, et al.: Positive association of renal insufficiency with agriculture employment and unregulated alcohol consumption in Nicaragua. Ren Fail 32: 766–777, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laux TS, Bert PJ, Barreto Ruiz GM, González M, Unruh M, Aragon A, et al.: Nicaragua revisited: Evidence of lower prevalence of chronic kidney disease in a high-altitude, coffee-growing village. J Nephrol 25: 533–540, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donnell JK, Tobey M, Weiner DE, Stevens LA, Johnson S, Stringham P, et al.: Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant 26: 2798–2805, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raines N, González M, Wyatt C, Kurzrok M, Pool C, Lemma T, et al.: Risk factors for reduced glomerular filtration rate in a Nicaraguan community affected by Mesoamerican nephropathy. MEDICC Rev 16: 16–22, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Torres C, Aragón A, González M, López I, Jakobsson K, Elinder CG, et al.: Decreased kidney function of unknown cause in Nicaragua: A community-based survey. Am J Kidney Dis 55: 485–496, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Orantes CM, Herrera R, Almaguer M, Brizuela EG, Hernández CE, Bayarre H, et al.: Chronic kidney disease and associated risk factors in the Bajo Lempa region of El Salvador: Nefrolempa study, 2009. MEDICC Rev 13: 14–22, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Peraza S, Wesseling C, Aragon A, Leiva R, García-Trabanino RA, Torres C, et al.: Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis 59: 531–540, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Kupferman J, Ramírez-Rubio O, Amador JJ, López-Pilarte D, Wilker EH, Laws RL, et al.: Acute kidney injury in sugarcane workers at risk for mesoamerican nephropathy. Am J Kidney Dis 72: 475–482, 2018. [DOI] [PubMed] [Google Scholar]
- 21.Weiner DE, McClean MD, Kaufman JS, Brooks DR: The Central American epidemic of CKD. Clin J Am Soc Nephrol 8: 504–511, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Wesseling C, Crowe J, Hogstedt C, Jakobsson K, Lucas R, Wegman DH: The epidemic of chronic kidney disease of unknown etiology in Mesoamerica: A call for interdisciplinary research and action. Am J Public Health 103: 1927–1930, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correa-Rotter R, García-Trabanino R: Mesoamerican nephropathy. Semin Nephrol 39: 263–271, 2019. [DOI] [PubMed] [Google Scholar]
- 24.Orduñez P, Silva LC: Pesticides and the epidemic of CKD in Central America. Am J Kidney Dis 64: 477, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Minnings K, Fiore M, Mosco M, Ferguson R, Leatherman S, Kerns E, et al.: The Rivas Cohort Study: Design and baseline characteristics of a Nicaraguan cohort. BMC Nephrol 17: 93, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minnings K, Kerns E, Fiore M, Fiore M, Parekh RS, DuBois J, et al.: Chronic kidney disease prevalence in Rivas, Nicaragua: Use of a field device for creatinine measurement. Clin Biochem 48: 456–458, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Stevens LA, Claybon MA, Schmid CH, Chen J, Horio M, Imai E, et al.: Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int 79: 555–562, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members : Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158: 825–830, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Desarrollo EINdId: Caracterización sociodemográfica del Departamento de Rivas. VIII Censo de población y IV de vivienda, 2005. Available at: http://www.inide.gob.ni/censos2005/MONOGRAFIASD/RIVAS.pdf. Accessed April 19, 2018
- 30.Gallo-Ruiz L, Sennett CM, Sánchez-Delgado M, García-Urbina A, Gámez-Altamirano T, Basra K, et al.: Prevalence and risk factors for CKD among brickmaking workers in La Paz Centro, Nicaragua. Am J Kidney Dis 74: 239–247, 2019. [DOI] [PubMed] [Google Scholar]
- 31.Fischer RSB, Vangala C, Mandayam S, Chavarria D, García-Trabanino R, Garcia F, et al.: Clinical markers to predict progression from acute to chronic kidney disease in Mesoamerican nephropathy. Kidney Int 94: 1205–1216, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Quiroz M, Smpokou ET, Pearce N, Caplin B, Nitsch D: Identification of young adults at risk of an accelerated loss of kidney function in an area affected by Mesoamerican nephropathy. BMC Nephrol 20: 21, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.González-Quiroz M, Camacho A, Faber D, Aragón A, Wesseling C, Glaser J, et al.: Rationale, description and baseline findings of a community-based prospective cohort study of kidney function amongst the young rural population of Northwest Nicaragua. BMC Nephrol 18: 16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes MD, Dalal S, Volmink J, Adebamowo CA, Njelekela M, Fawzi WW, et al.: Non-communicable diseases in sub-Saharan Africa: The case for cohort studies. PLoS Med 7: e1000244, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.