Significance Statement
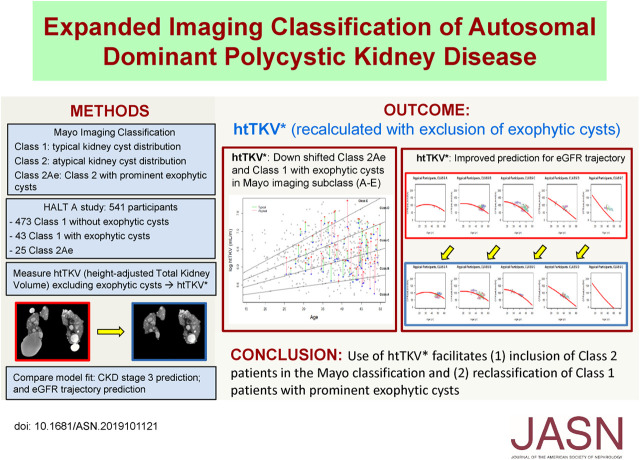
Accurate prediction of risk for disease progression is crucial for clinical management of autosomal dominant polycystic kidney disease (ADPKD). The Mayo imaging classification of ADPKD uses height-adjusted total kidney volume (htTKV) and age to identify patients at highest risk. Because the current Mayo classification applies only to patients with typical diffuse cystic disease (class 1) and poorly predicts eGFR decline for the remaining 5%–10% of patients with atypical morphology (class 2), imaging-based risk modeling remains unresolved. The authors report an expanded imaging classification model in which use of a recalculated htTKV value that excludes prominent exophytic cysts improved prediction for eGFR trajectory. Using a recalculated htTKV may allow inclusion of class 2 patients in the Mayo classification of ADPKD and reclassification of class 1 patients with prominent exophytic cysts.
Keywords: ADPKD, clinical trial, kidney volume, risk factors
Visual Abstract
Abstract
Background
The Mayo Clinic imaging classification of autosomal dominant polycystic kidney disease (ADPKD) uses height-adjusted total kidney volume (htTKV) and age to identify patients at highest risk for disease progression. However, this classification applies only to patients with typical diffuse cystic disease (class 1). Because htTKV poorly predicts eGFR decline for the 5%–10% of patients with atypical morphology (class 2), imaging-based risk modeling remains unresolved.
Methods
Of 558 adults with ADPKD in the HALT-A study, we identified 25 patients of class 2A with prominent exophytic cysts (class 2Ae) and 43 patients of class 1 with prominent exophytic cysts; we recalculated their htTKVs to exclude exophytic cysts. Using original and recalculated htTKVs in association with imaging classification in logistic and mixed linear models, we compared predictions for developing CKD stage 3 and for eGFR trajectory.
Results
Using recalculated htTKVs increased specificity for developing CKD stage 3 in all participants from 82.6% to 84.2% after adjustment for baseline age, eGFR, BMI, sex, and race. The predicted proportion of class 2Ae patients developing CKD stage 3 using a cutoff of 0.5 for predicting case status was better calibrated to the observed value of 13.0% with recalculated htTKVs (45.5%) versus original htTKVs (63.6%). Using recalculated htTKVs reduced the mean paired difference between predicted and observed eGFR from 17.6 (using original htTKVs) to 4.0 ml/min per 1.73 m2 for class 2Ae, and from −1.7 (using original htTKVs) to 0.1 ml/min per 1.73 m2 for class 1.
Conclusions
Use of a recalculated htTKV measure that excludes prominent exophytic cysts facilitates inclusion of class 2 patients and reclassification of class 1 patients in the Mayo classification model.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic kidney disease and the fourth leading cause of ESKD.1 Clinical management of patients with ADPKD is challenging in that the presentations of ADPKD vary significantly between and within affected families, and the progression of disease continues for several decades before reaching ESKD. Therefore, accurate risk prediction at early stages of disease is crucial in providing care for patients to receive appropriate treatment to result in the most effective outcome before severe disease progression. Major factors predicting disease progression in ADPKD include genotype, kidney function, sex, age, and total kidney volume (TKV). Among these factors, TKV is recognized as the most valuable predictor of ADPKD progression and is widely used as an end point in many clinical trials.2,3 A recent study reported that height-adjusted TKV (htTKV) considered in conjunction with age is a significant independent predictor of the risk for CKD progression in patients with ADPKD.4
One model for predicting patients with ADPKD at high risk of disease progression is the Mayo imaging classification5 using patient age and htTKV. In this model, a patient with ADPKD is first assessed qualitatively according to cystic and parenchymal appearance in kidney imaging. Class 1 (90%–95% of patients) is assigned when kidney appearance is considered “typical” with bilateral diffuse cystic involvement throughout kidney parenchyma and no kidney atrophy. Class 2 (5%–10% of patients) is considered when kidney appearance is “atypical” including those with unilateral, asymmetric, segmental, or lopsided cystic distribution (class 2A), and rarely those with substantial parenchymal atrophy (class 2B).
In the Mayo imaging classification model, a class 1 patient is further stratified into 5 subclasses (1A–1E) on the basis of the annual growth rate in htTKV (<1.5%, 1.5%–3%, 3%–4.5%, 4.5%–6%, or >6% per year) estimated from patient age and a theoretical starting htTKV (150 ml/m). This classification model has been found to be a strong predictor of subsequent eGFR decrease.5 However, class 2 patients are excluded from the Mayo imaging prediction model because htTKVs in these patients do not predict eGFR.5,6 Thus, predicting the progression risk for class 2 patients remains unresolved.
The majority of class 2 patients present with prominent exophytic cysts protruding from kidney parenchyma in contrast to class 1 patients with bilateral diffuse cysts infiltrating throughout kidney parenchyma. These prominent exophytic cysts affect not only the qualitative morphology of the kidney, making it appear atypical, but also the overestimation of TKV, which includes the volumes of exophytic cysts. We postulate that, in class 2Ae patients, measuring kidney volume without including exophytic cysts (i.e., recalculated TKV) has a greater power for predicting progression than the original htTKV. Thus, we used recalculated TKVs in both class 2Ae and class 1 patients with prominent exophytic cysts, and investigated the feasibility of a single model that best predicts prognosis for both typical and atypical patients with ADPKD.
CONCISE METHODS
Study Participants
The study protocol of the Halt Polycystic Kidney Disease (HALT-PKD) study and the baseline characteristics of this population have been reported in detail.7,8 Briefly, the HALT-PKD trials were prospective, randomized, double-blind, placebo-controlled, multicenter, intervention trials testing whether multilevel blockade of the renin-angiotensin-aldosterone system using angiotensin-converting enzyme inhibitors plus angiotensin receptor blocker (lisinopril plus telmisartan) combination therapy would delay progression of renal disease compared with an angiotensin-converting enzyme inhibitor (lisinopril plus placebo) monotherapy in studies A and B, and whether intensive BP control (95−110/60−75 mm Hg) would delay progression as compared with standard control (120−130/70−80 mm Hg) in study A. The protocol for the HALT study was approved by the institutional review board at each study site. This article reports on study A participants only, because magnetic resonance imaging (MRI) was not performed in study B.
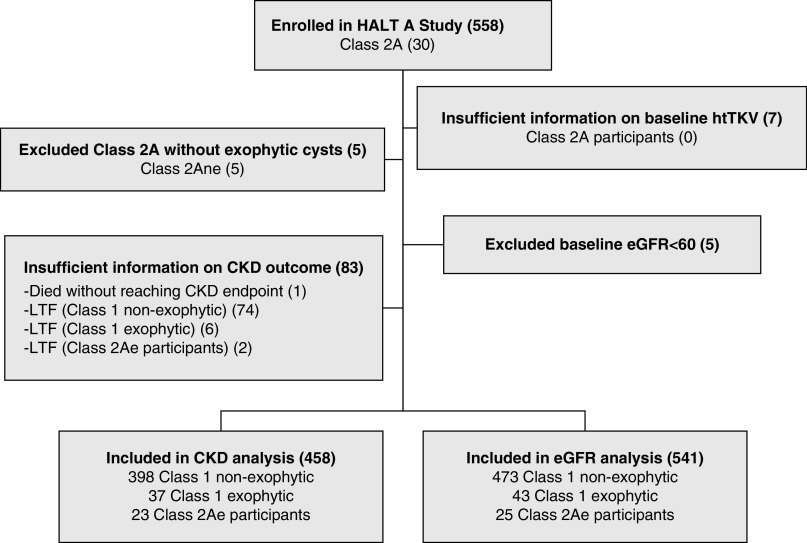
There were 558 participants enrolled in the HALT-A study. Seven participants missing baseline htTKV records were excluded from analysis due to lack of baseline images (n=5) or the patient’s height (n=2). Five participants with eGFR <60 at baseline were excluded. Eighty-three participants who died (n=1) or were lost to follow-up (n=82) before reaching CKD end points were excluded from the CKD stage analysis, but were included in the eGFR analysis. After these exclusion criteria, 463 participants for the CKD stage analysis and 516 participants for the eGFR analysis were left for imaging evaluation.
Evaluation of Imaging Classification and Baseline TKV Measurement
The baseline MRIs of 516 participants were reviewed and compared with the imaging classification for the HALT-A study reported in a previous study.6 Image classifications were performed blinded to clinical data. Patients were classified as typical (class 1) and atypical (class 2) on the basis of prespecified imaging findings. Class 1 patients with ADPKD were further stratified into five subclasses on the basis of htTKV and age as previously described.5 Class 2 patients were classified depending on the absence or presence of kidney atrophy into class 2A (no atrophic kidney) or class 2B (atrophic kidney). Class 2A patients were further divided into two groups according to their kidney cyst distribution pattern: class 2Ae (with prominent exophytic cysts) and class 2Ane (with no prominent exophytic cysts). An exophytic cyst is defined as a cyst in which the center of the cyst is situated outside of the expected contour of overall kidney parenchyma. The imaging findings of class 2Ane are characterized by scattered cortical cysts or asymmetric diffuse cysts without dominant exophytic cysts.
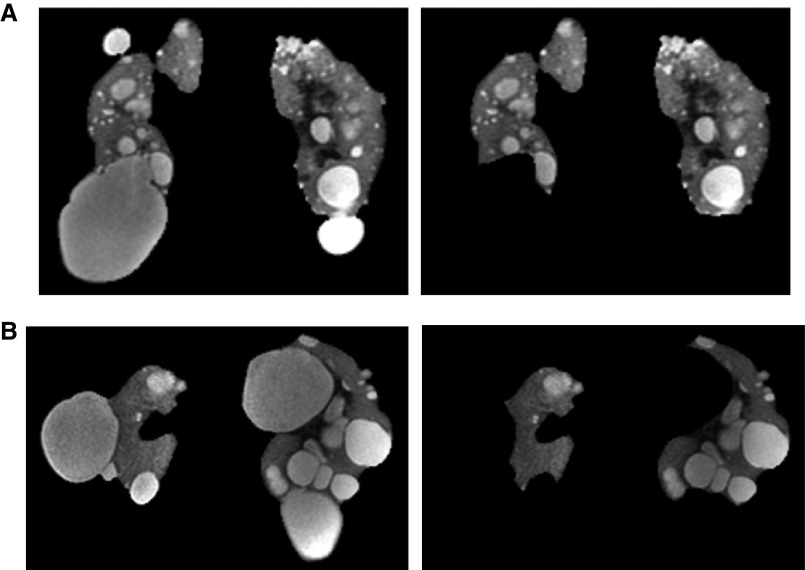
Baseline TKVs were previously measured for the HALT study.7,8 For class 2Ae patients, TKVs were recalculated by subtracting the cyst volumes of exophytic cysts from the original baseline TKVs (Figure 1). The cyst volume of exophytic cysts in each kidney was measured using a region-based thresholding method on T2-weighted MRI images.9,10 On each MRI image slice, a binary signal intensity map was generated by determining a threshold signal intensity that differentiates bright cysts and gray kidney parenchymal regions. The total exophytic cyst volume was calculated from each set of contiguous images by summing the products of the bright exophytic cyst areas measured and the slice thickness. The images of class 1 patients were also reviewed to determine the presence of prominent exophytic cysts. For class 1 with prominent exophytic cysts, TKV was recalculated using the same measurement method.
Figure 1.
Coronal T2-weighted MRIs from two class 2Ae patients. Images show the regions included in the TKV determination before (left) and after (right) excluding exophytic cysts. (A) 35-year-old PKD1 woman whose baseline TKV reduced from 956 ml to 631 ml after exclusion of exophytic cysts, and (B) 42-year-old PKD1 man whose baseline TKV reduced from 2080 ml to 845 ml after exclusion of exophytic cysts.
Statistical Analyses
Descriptive Statistics
For all patients in class 1, class 1 with prominent exophytic cysts, and class 2Ae before and after exclusion of exophytic cysts, baseline htTKV was described using the mean, median, SD, and interquartile range. In addition, the Mayo imaging classification of each patient was graphically depicted according to patient age and htTKV. For class 2Ae and class 1 with prominent exophytic cysts, changes in the imaging classification before and after the exclusion of exophytic cysts were determined.
Analysis of CKD Prognosis Associated with Changes in Imaging Classification
This analysis uses a logistic regression model. Development of CKD stage 3 (CKD3) by the end of the HALT study (at 5 years of follow-up) was first modeled (unadjusted) using class 1 subjects only as a function of htTKV. The odds ratios (ORs) and 95% confidence intervals (CIs) were presented in terms of a 1-SD change to evaluate whether volume recalculation changed the estimated association between htTKV and CKD3. The logistic model was then run adjusting for baseline eGFR, baseline age, sex, body mass index, and race. The logistic models (unadjusted and adjusted) were also used to assess differences in the predicted probability of CKD3 versus non-CKD3. The area under the Receiver Operating Characteristic (ROC) curve was also calculated and compared between original and recalculated htTKVs between typical (class 1) and atypical (class 2Ae) participants, using unadjusted and adjusted models. Finally, model calibration and fit were assessed by (1) summarizing the mean predicted probability by patient status separately for original and recalculated htTKVs (using an unadjusted or adjusted model), (2) summarizing the predicted proportion of patients (using a cutoff of 0.5 for predicted cases) for original and recalculated htTKVs (using an unadjusted or adjusted model), and (3) conducting the Hosmer–Lemeshow test for model fit.
Analysis of eGFR Trajectories Across Different Imaging Cohorts
This analysis uses a linear mixed model (with a random intercept and random slope to account for within-subject correlation and individual differences) to assess the eGFR trajectories. Predicted eGFR curves (specific to imaging classification) were fitted for the class 1 participants. To summarize differences between predicted and observed (using independent observations from each subject), 1000 bootstrap samples were generated using (1) a randomly selected subject and (2) a randomly selected time point (both with replacement). Bootstrapping is a standard method to assess bias and variability without assuming an underlying distribution.11 For each bootstrap sample, the paired difference (to assess bias) and squared paired difference (to assess variability) were calculated between the observed eGFR and predicted eGFR. Then, the mean and 2.5 and 97.5 percentiles of the bootstrap distribution were computed to get 95% CIs. The analysis was run for (1) class 2Ae participants only, (2) both class 2Ae and class 1 participants, and (3) class 1 participants only. The participants who were excluded from the CKD stage analysis due to insufficient information on CKD outcome were included in the eGFR trajectory analysis. For class 2Ae and class 1 patients with prominent exophytic cysts, the analysis was performed first using the original htTKV and imaging classification, and then repeated using the remeasured htTKV and imaging classification.
All tests were done with a two-sided significance level of 0.05 and STATA version 1412 was used for all statistical analyses.
RESULTS
Imaging Classification and Baseline htTKV Changes
The imaging evaluation of the 458 participants yielded 435 typical (class 1) and 30 atypical patients (class 2: all were class 2A patients with no class 2B) in agreement with a previous study.6 The main clinical, laboratory, and genetic characteristics of the HALT-A cohort at baseline have been reported in detail previously.6,8 Of 30 class 2A patients, 25 contained prominent exophytic cysts (class 2Ae) and 5 were without prominent cysts (class 2Ane). Two class 2Ae patients were removed from the CKD stage analysis due to insufficient information on CKD end point. Thus, the final CKD stage analysis included a total of 458 patients: 435 typical (class 1, with 43 having prominent exophytic cysts) and 23 atypical (class 2Ae) (Figure 2). There were 230 men and 205 women in class 1, whereas 7 men and 16 women were in class 2Ae. The genotypes for class 2Ae were PKD1 truncating (n=2), PKD1 nontruncating (n=9), and PKD2 or no mutation detected (n=9 or n=2, respectively). Five of the PKD1 nontruncating participants were scored as mutation strength group 3.13 One class 2Ae participant had no DNA sample. Three of the class 2Ae participants, two women and one man, reached CKD3, with their lowest recorded eGFRs ranging from 54.3 to 57.6 ml/min per 1.73 m2 at ages of 41–47 years. Their genotypes were PKD1 truncating (n=1), PKD1 nontruncating (n=1), and PKD2 or no mutation detected (n=1).
Figure 2.
Flowchart of the study design and study participants. There were a total of 558 participants enrolled in the HALT-A study including 521 class 1 and 30 class 2A. Seven participants missing baseline htTKV records were excluded. Five class 2A patients without exophytic cysts were excluded. For the CKD stage analysis, 83 participants who died or were lost to follow-up (LTF) before reaching CKD end points were excluded, resulting in a total of 458 participants, including 23 class 2Ae participants, in the analysis. For the eGFR analysis, five participants whose eGFR was <60 at baseline were excluded, resulting in a total of 541 participants, including 25 class 2Ae participants, in the analysis.
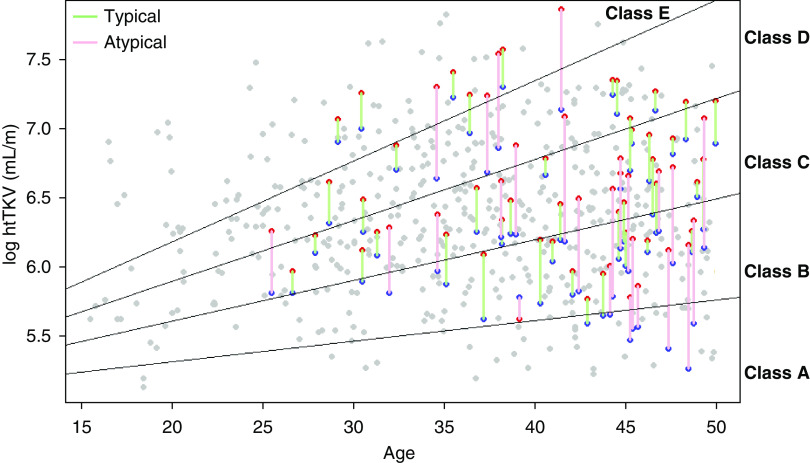
The baseline htTKVs of the class 1 and class 2Ae participants before and after exclusion of exophytic cysts are shown in Table 1. The baseline htTKVs of class 2Ae and class 1 participants with prominent exophytic cysts decreased with the exclusion of exophytic cysts (mean htTKV changed from 874.7 to 459.2 ml/m in class 2Ae and from 847.0 to 672.0 in class 1 with prominent exophytic cysts). Figure 3 shows the five imaging subclasses of all participants, including 25 class 2Ae participants before and after exclusion of exophytic cysts. With recalculated htTKVs smaller than original htTKVs, 23 of 25 class 2Ae participants were downgraded to lower subclasses: 4 (from 1E to 1D), 2 (from 1D to 1C), 1 (from 1D to 1B), 9 (from 1C to 1B), and 7 (from 1B to 1A). Two class 2Ae participants were unchanged in class (1B and 1C). Compared with class 2Ae, class 1 participants with prominent exophytic cysts shifted more moderately to lower subclasses due to recalculated htTKVs. Fourteen of 43 class 1 participants with prominent exophytic cysts were downgraded by one subclass: 1 (from 1E to 1D), 3 (from 1D to 1C), 8 (from 1C to 1B), and 2 (from 1B to 1A). Twenty-nine participants were unchanged in class.
Table 1.
Distribution of baseline htTKV before and after exclusion of exophytic cysts
| Original versus Recalculated htTKV | Group | n | Mean (ml/m) | Median (ml/m) | SD (ml/m) | IQR (ml/m) |
|---|---|---|---|---|---|---|
| Original htTKV | All | 458 | 709.5 | 594.5 | 410.1 | 480 |
| Class 1 | 435 | 700.7 | 589 | 401.1 | 491 | |
| Class 1 with exophytic cysts | 43 | 847.0 | 732.7 | 409.7 | 657.9 | |
| Class 2Ae | 23 | 874.7 | 706 | 538.0 | 654 | |
| Recalculated htTKV | All | 458 | 673.8 | 566 | 388.6 | 434 |
| Class 1 | 435 | 685.2 | 574 | 391.2 | 455 | |
| Class 1 with exophytic cysts | 43 | 672.0 | 513.6 | 337.3 | 550.4 | |
| Class 2Ae | 23 | 459.2 | 388.4 | 257.4 | 241.4 |
IQR, interquartile range.
Figure 3.
Mayo imaging classification of all participants, including class 2Ae and class1 participants with prominent exophytic cysts, before and after exclusion of exophytic cysts. The 541 class 1 participants were stratified into five subclasses (1A–1E) on the basis of their baseline htTKV and age. The htTKVs of each of the 25 class 2Ae participants are depicted by red points (before exclusion of exophytic cysts) and blue points (after exclusion of exophytic cysts) connected by red lines. With the recalculated htTKVs, 23 of 25 class 2Ae participants were downgraded to milder imaging classes (from red to blue data points): 4 (from 1E to 1D), 2 (from 1D to 1C), 1 (from 1D to 1B), 9 (from 1C to 1B), and 7 (from 1B to 1A). Two class 2Ae participants were unchanged in class (1B and 1C). The htTKVs of each of the 43 class 1 participants with prominent exophytic cysts are depicted by red and blue points connected by green lines. With the recalculated htTKVs, 14 of 43 class 1 participants with prominent exophytic cysts were downgraded by one subclass: 1 (from 1E to 1D), 3 (from 1D to 1C), 8 (from 1C to 1B), and 2 (from 1B to 1A). Twenty-nine participants were unchanged in class.
Analysis of CKD Prognosis Associated with Changes in Imaging Classification
With the use of original htTKVs, the inclusion of the class 2Ae group lowered the magnitude of association between htTKV and odds of CKD3 associated with a 1-SD change in htTKV from 2.90 (2.35) (unadjusted and adjusted, respectively) for class 1 participants to 2.61 (1.99) for all participants (Table 2). In contrast, with the use of recalculated htTKVs excluding exophytic cysts, the inclusion of the class 2Ae group increased the magnitude of association between htTKV and odds of CKD3 slightly to 2.99 (2.56) for all participants. However, all 95% CIs overlapped and all P values were <0.001, implying that the differences did not affect the significance of the model.
Table 2.
Logistic models and ORs for developing CKD stage 3 associated with 1-SD change in htTKV
| Model | Group | htTKV | n | OR | P>|z| | 95% CI |
|---|---|---|---|---|---|---|
| Unadjusted | Class 1 | Original | 435 | 2.90 | <0.001 | 2.21 to 3.81 |
| All | Original | 458 | 2.61 | <0.001 | 2.02 to 3.36 | |
| All | Recalculated | 458 | 2.99 | <0.001 | 2.28 to 3.91 | |
| Adjusteda | Class 1 | Original | 425b | 2.35 | <0.001 | 1.7 to 3.26 |
| All | Original | 447b | 1.99 | <0.001 | 1.49 to 2.68 | |
| All | Recalculated | 447b | 2.56 | <0.001 | 1.85 to 3.54 |
Covariates in the adjusted model: baseline age, eGFR, body mass index, sex, and race.
Observations lost due to missing covariates.
The predicted probability of reaching CKD3 for the class 2Ae group using the original versus recalculated htTKVs is shown in Figure 4A. The predictions with the original htTKVs were always higher than those with the recalculated htTKVs in both unadjusted and adjusted models, because htTKVs had a positive coefficient and because the original htTKVs were always greater than the corresponding recalculated htTKVs. The predicted probability of reaching CKD3 for class 1 with prominent exophytic cysts using the original versus recalculated htTKVs is shown in Figure 4B. Sensitivity (using a cutoff of 0.5 for predicting case status) changed from 46.0% (95% CI, 38.9 to 53.2) and 74.2% (95% CI, 67.5 to 80.2) (unadjusted and adjusted, respectively) with original htTKVs to 49.0% (95% CI, 41.9 to 56.1) and 75.3% (95% CI, 68.6 to 81.2) with recalculated htTKVs, whereas specificity changed from 82.6% (95% CI, 77.4 to 87) and 82.6% (95% CI, 77.4 to 87.1) with original htTKVs to 83.7% (95% CI, 78.6 to 88) and 84.2% (95% CI, 79.1 to 88.5) with recalculated htTKVs. The areas under the ROC curve in unadjusted and adjusted models computed with the recalculated htTKVs were not significantly different from those with the original htTKVs. However, the areas under the ROC curve were significantly different (with nonoverlapping 95% CIs) between unadjusted and adjusted models for both original and recalculated htTKVs (Supplemental Table 1).
Figure 4.
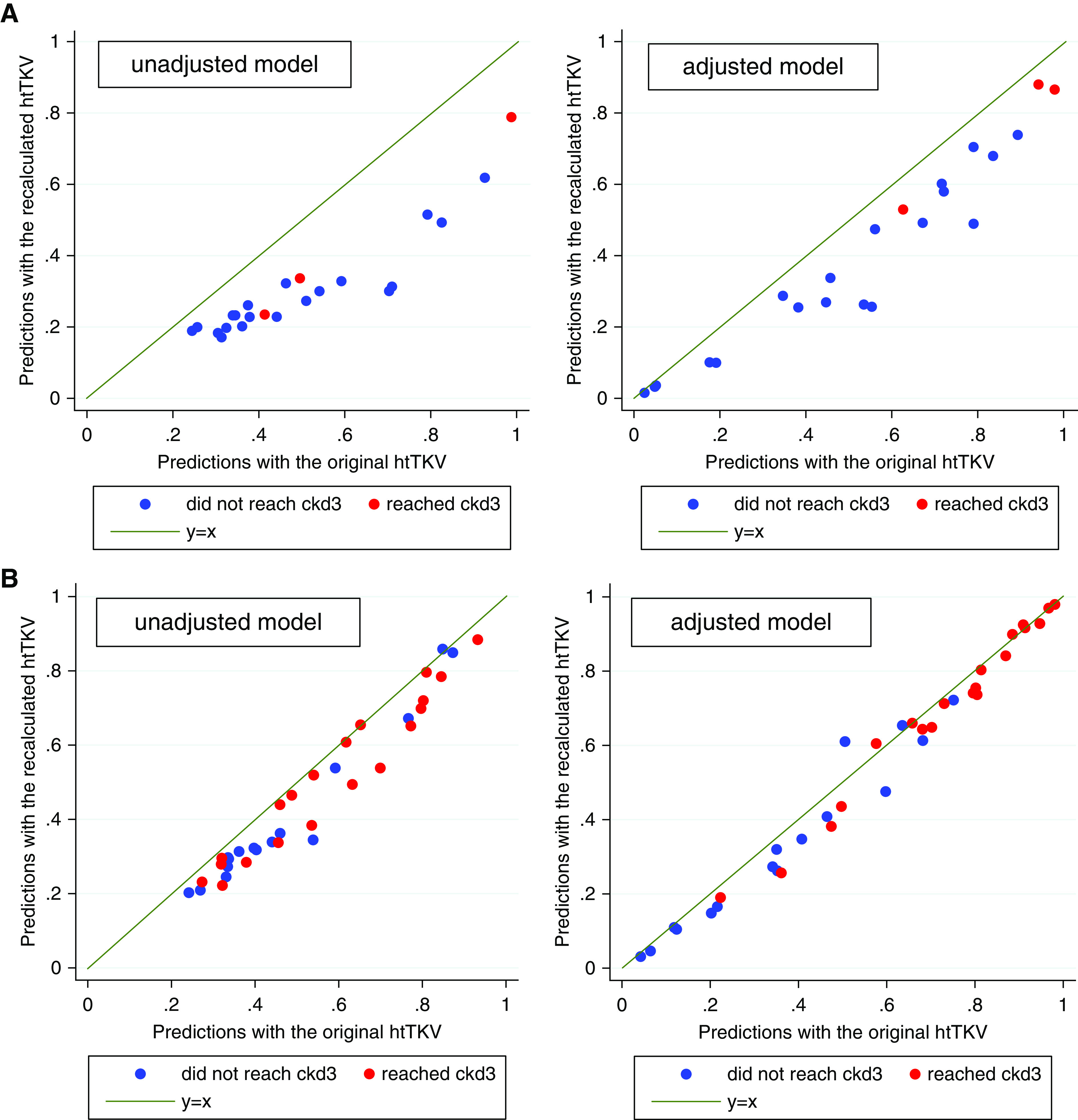
Predicted probability of reaching CKD3 using original versus recalculated baseline htTKVs in unadjusted and adjusted models for (A) class 2Ae and (B) class 1 participants with prominent exophytic cysts. All data points are below the diagonal because htTKV has a positive coefficient and recalculated htTKVs are always less than the original htTKVs.
The mean predicted probabilities by case status, separately for original and recalculated htTKVs in the unadjusted and adjusted models, are summarized in Table 3. The actual development of CKD3 was 13.0% for class 2Ae and 43.7% for all participants. Although predicted probabilities were, on average (by 15%−40%), higher for patients, the difference between predictions in patients (i.e., participants who reached CKD) and controls (who did not reach CKD) was not affected by the recalculated htTKVs. The predicted %CKD3s using a cutoff of 0.5 for predicted cases for the original and recalculated htTKVs in the unadjusted and adjusted models are summarized in Supplemental Table 2. Although there was no significant change in overall %predicted cases, the recalculated htTKVs lowered (nonsignificantly, with overlapping 95% CIs) the predicted %CKD3 for class 2Ae participants from 39.1% (95% CI, 19.71 to 61.46) and 63.6% (95% CI, 40.66 to 82.8) (unadjusted and adjusted, respectively) with the original htTKVs to 17.4% (95% CI, 4.95 to 38.78) and 45.45% (95% CI, 24.39 to 67.79) with the recalculated htTKVs. Finally, the goodness of model fit using the Hosmer–Lemeshow test (where a significant result implies lack of fit) is summarized in Supplemental Table 3. The goodness of fit for class 2Ae participants was significantly better with the recalculated htTKVs than with the original htTKVs, with P values going from 0.014 and 0.003 (unadjusted and adjusted, respectively) with the original htTKVs to 0.83 and 0.31 with the recalculated htTKVs.
Table 3.
Mean (SD) predicted probability for developing CKD3 with original versus recalculated htTKV
| Model | All Controlsa | All Patientsb | Class 2Ae Controls | Class 2Ae Patients |
|---|---|---|---|---|
| Unadjusted, original htTKV | 0.37 (0.16) | 0.52 (0.20) | 0.49 (0.20) | 0.63 (0.31) |
| Unadjusted, recalculated htTKV | 0.36 (0.17) | 0.54 (0.22) | 0.29 (0.13) | 0.46 (0.30) |
| Adjusted, original htTKVc | 0.26 (0.23) | 0.66 (0.26) | 0.48 (0.28) | 0.84 (0.19) |
| Adjusted, recalculated htTKVc | 0.25 (0.23) | 0.68 (0.26) | 0.36 (0.24) | 0.77 (0.20) |
Controls are participants who did not reach CKD.
Patients are participants who reached CKD.
Covariates in the adjusted model: baseline age, eGFR, body mass index, sex, and race.
Analysis of eGFR Trajectories Across Different Imaging Cohorts
When the Mayo imaging subclass model (class 1A–1E) was applied to both the original and recalculated htTKVs, the predicted eGFRs using the recalculated imaging subclass in class 2Ae were much closer to the observed eGFRs than the predicted eGFRs using the original imaging subclass (Table 4). The mean paired difference between the predicted and observed eGFR was reduced from 17.6 ml/min per 1.73 m2 (95% CI, 10.3 to 24.9) with the original imaging subclass to 4.0 ml/min per 1.73 m2 (95% CI, −1.5 to 9.2) with the recalculated imaging subclass. For class 1 participants, the mean paired difference also improved from −1.7 (95% CI, −3.5 to 0.04) to 0.1 (95% CI, −1.6 to 1.9), where the change was small in part because the difference with the original imaging class was already small and in part because only 43 of 516 class 1 participants had prominent exophytic cysts. The variance of the estimate decreased significantly for class 2Ae (from 27.20 to 7.94) but there was little change for class 1.
Table 4.
Mean paired difference and variance between predicted and observed eGFR
| Original versus Reclassified Imaging Class | Group | n | Mean Paired Difference (ml/min per 1.73 m2) | 95% CI (Paired Difference) | Mean Variance | 95% CI (Variance) |
|---|---|---|---|---|---|---|
| Original imaging class | All | 541 | −0.9 | −2.7 to 1 | 0.77 | 0.66 to 0.89 |
| Class 1 | 516 | −1.7 | −3.5 to 0.04 | 0.78 | 0.66 to 0.91 | |
| Class 2Ae | 25 | 17.6 | 10.3 to 24.9 | 27.20 | 13.6 to 43.8 | |
| Reclassified imaging class | All | 541 | 0.1 | −1.7 to 1.9 | 0.73 | 0.63 to 0.85 |
| Class 1 | 516 | 0.1 | −1.6 to 1.9 | 0.77 | 0.65 to 0.90 | |
| Class 2Ae | 25 | 4.0 | −1.5 to 9.2 | 7.94 | 4.54 to 11.64 |
Overall, when the Mayo imaging subclass model (class 1A–1E) was applied to all 541 participants, the mean paired difference between the predicted and observed eGFR was reduced from −0.9 ml/min per 1.73 m2 (95% CI, −2.7 to 1.0) with the original imaging subclass to 0.1 ml/min per 1.73 m2 (95% CI, −1.7 to 1.9) with the recalculated imaging subclass. The variance of the estimate also decreased slightly (from 0.77 to 0.73). Whereas these improvements in model fit were not statistically significant, the overall fit with the recalculated imaging subclass became similar across class 1 and class 2Ae (i.e., overlapping 95% CIs between class 2Ae and class 1) as compared with the fit with the original imaging subclass, in that the fit of class 2Ae was significantly worse than that of class 1 (i.e., with nonoverlapping 95% CIs).
The graphical representations of eGFR trajectories show a consistent trend in improvement in model fit (Figure 5). First, the trajectories for class E atypical participants with original htTKVs (as shown in the last graph on the right in the second row of graphs in Figure 5B) are all above the predicted trajectory from typical participants, showing a clear bias. However, with recalculated htTKVs, class E atypical participants downgrade to class D, as shown in the fourth graph in the third row of graphs in Figure 5C. These downgraded observed trajectories closely overlap with the predicted trajectory. The same trend in improvement in model fit with recalculated htTKVs is observed for (1) atypical participants in class D with original htTKVs downgraded to class C and (2) atypical participants in class C with original htTKVs downgraded to class B.
Figure 5.
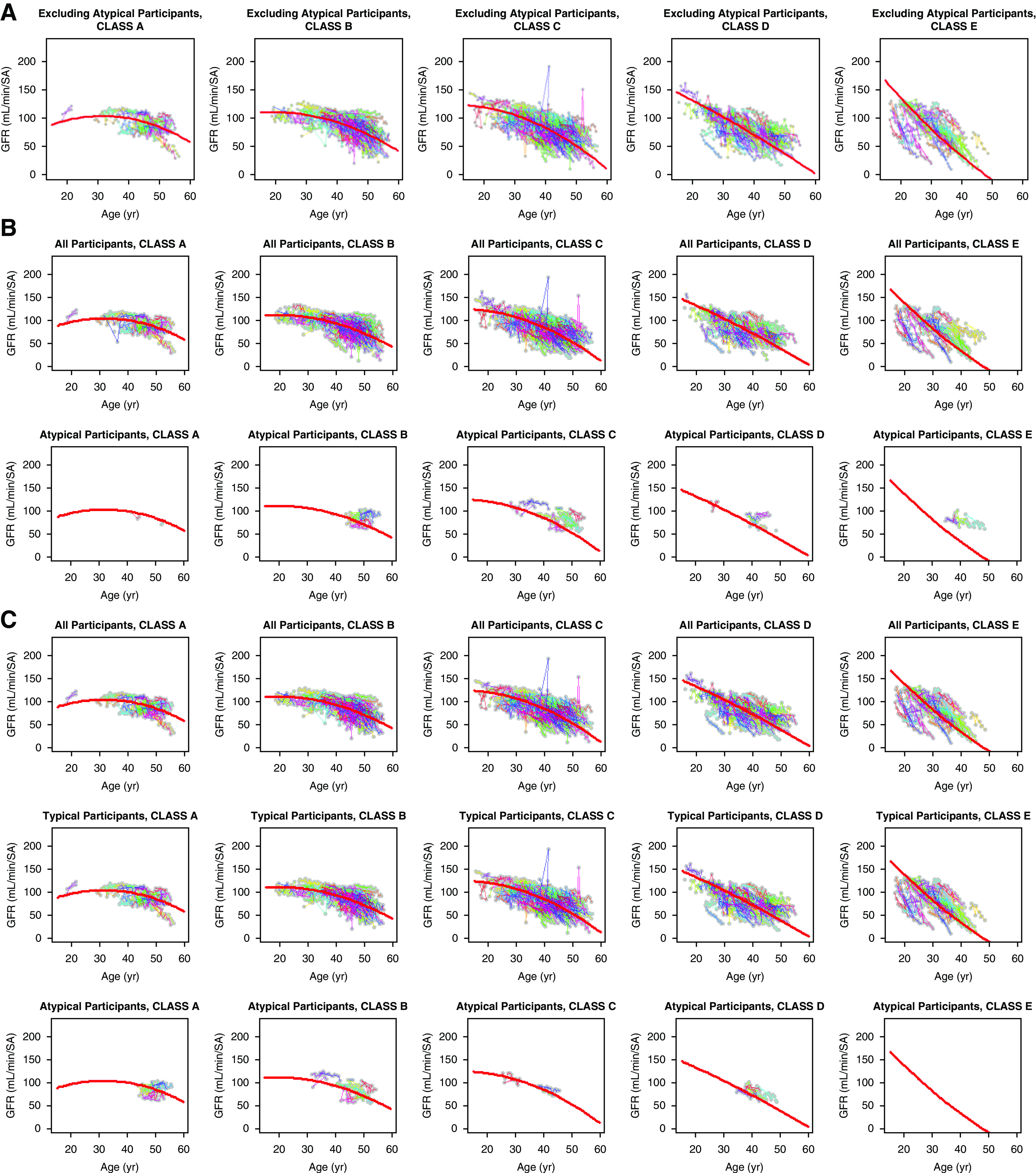
eGFR trajectories. eGFR trajectories are shown for (A) class 1 participants only, (B) all and class 2Ae participants with original imaging classes, and (C) all, class 1, and class 2Ae participants with recalculated imaging classes. The data from class 1 only were used to fit the predicted curves, the trend of which was then assessed on the other groups of participants.
DISCUSSION
The Mayo imaging classification of ADPKD is a widely accepted tool that uses htTKV and age to identify patients at highest risk for disease progression. However, the current Mayo imaging classification is applicable only to patients who have typical disease with diffuse cystic involvement (class 1). Class 2 patients with ADPKD (5%–10% of patients) were excluded in this risk prediction model because htTKVs do not predict eGFR decline. The imaging-based risk prediction for class 2 has yet to be determined. The majority of class 2 patients have focal cystic disease (class 2A), whereas a few are older individuals with atrophic kidneys with cysts (class 2B). For instance, in the HALT-A study, all class 2 patients were class 2A.
The original description of class 2A includes four subtypes: unilateral, segmental, asymmetric, and lopsided.5 An exophytic cyst pattern was not in the original description, because exophytic cysts are present in both class 1 (although uncommon) and class 2, and because the presence of exophytic cysts itself may or may not dominate the overall pattern of cyst distribution and classification. Within class 2A, prominent exophytic cysts are most common in the lopsided subtype. Nevertheless, the original description of class 2A lopsided, “bilateral distribution of renal cysts with mild replacement of kidney tissue with atypical cysts where ≤5 cysts account for ≥50% TKV (the largest cyst diameter is used to estimate individual cyst volume),” does not specify what “atypical cysts” are. In addition, the description, “where ≤5 cysts account for ≥50% TKV” is too restricted because the numbers and sizes of exophytic cysts are variable. Thus, our study focused on the analysis of exophytic cysts that are objectively discernible and quantifiable, although not specifically described under the original class 2A subtype.
Given the lack of an imaging-based prediction model for class 2 relative to class 1, it seems reasonable to explore the effect of prominent exophytic cysts (class 2Ae) that affect not only the imaging class differentiation but also the calculation of TKV. Whereas exophytic cysts in class 1 are uncommon and do not add substantially to TKV, prominent exophytic cysts in class 2Ae greatly contribute to escalation of the TKV value. When we consider an inverse correlation between TKV and GFR,14 an overestimated TKV value in class 2Ae would project a GFR lower than the actual GFR. The premise of this study is that exclusion of exophytic cysts from computation of TKV may restore TKV’s predictability of eGFR for class 2Ae similar to that of class 1. Furthermore, we reviewed all class 1 participants to identify class 1 with prominent exophytic cysts and recalculated their TKVs.
The effect of the recalculated htTKVs after exclusion of exophytic cysts in class 2Ae (and, secondarily, class 1) participants was assessed in terms of two analyses: (1) prediction of CKD3 outcome and (2) prediction of eGFR trajectory. The logistic models and ORs for developing CKD3 at the end of the HALT study revealed that the use of recalculated htTKVs increased ORs slightly (and nonsignificantly, with overlapping 95% CIs) for CKD3 prognosis. The specificity for CKD3 prognosis and the area under the ROC curve computed with recalculated htTKVs were also greater than, but very similar to, those with original htTKVs. The sensitivity and area under the ROC curve were significantly different (with nonoverlapping 95% CIs) in unadjusted and adjusted models, but were not significantly different in models with original and recalculated htTKVs. The recalculated htTKVs also yielded a better calibrated model for class 2Ae and class 1 participants with prominent exophytic cysts in the predicted %CKD3 (using a cutoff of 0.5 for predicted cases).
The goodness of fit for class 2Ae participants was significantly better with recalculated htTKVs than that with original htTKVs. For the prediction of eGFR trajectory, the predicted eGFRs using the recalculated imaging subclasses were significantly closer to the observed eGFRs than the predicted eGFRs using the original imaging subclasses. All other results with recalculated htTKVs showed the expected trends in improvement over original htTKVs, but were not statistically significant. These findings show some common trends in improving model utility with recalculated htTKVs for both typical and atypical participants with prominent exophytic cysts.
Compared with class 1 participants, class 2Ae participants had the genotype distribution associated with milder disease. For example, nearly one half of class 2Ae participants were PKD2 (compared with approximately 15% in the whole population). Ten of the 12 participants who were PKD1 were nontruncating (approximately 35% in the whole population), and five of those that were nontruncating were scored as mutation score group 3 (i.e., the weaker group of nontruncating mutations that make up about one third of nontruncating mutations). It was proposed that merging classes 1A and 2 (lowest severity), 1B and 1C (intermediate severity), and 1D and 1E (highest severity) detected stronger beneficial effects on TKV increase, and eGFR decline, in class 1D and E with a smaller number of patients.6 However, we may have to reconsider this approach of grouping class 2A patients into class 1A as a low-severity category. When 25 class 2Ae participants in our study were stratified according to the Mayo imaging classification for class 1 (1A–1E), 22 of 25 class 2Ae participants were downgraded to the immediate lower subclass with recalculated htTKVs after exclusion of exophytic cysts. One participant dropped by two subclasses (from 1D to 1B), whereas two participants were unchanged in class (1B and 1C). Nevertheless, it is noteworthy that seven participants remained in classes 1C (n=3) and 1D (n=4) even with recalculated htTKVs. Furthermore, three class 2Ae participants developed CKD stage 3. Therefore, moving some class 2A patients to a low-severity group may not be justified.
Clinicians currently either decline to prognosticate for class 2 patients or simply include them in class 1A. Including them in the standard Mayo imaging classification, but using recalculated htTKVs, would therefore improve on this approach. Until larger data sets become available to develop more accurate models, we recommend using recalculated htTKVs for class 2Ae patients to guide clinical care. Furthermore, use of recalculated htTKVs would improve the imaging classification for class 1 patients with prominent exophytic cysts.
A practical implemental question is how to measure TKVs devoid of exophytic cysts in class 2Ae. Although TKVs were measured slice-by-slice on MRI in our study, TKVs may be approximated using the ellipsoid method on computed tomography or MRI.5 The ellipsoid method typically uses three linear measurements: length on the longitudinal plane, and width and depth on the transverse plane of kidneys, on computed tomography or MRI. Each linear measurement typically spans from one edge to the opposite edge of the kidney for class 1 TKV measurement to encompass the entire kidney volume. For class 2Ae TKV measurement, just as in class 1 TKV measurement, we propose that the three longest linear measurements for the ellipsoid method be delineated on each kidney over the largest cross-sectional longitudinal and transverse imaging planes. In this approach, the end points of the linear measurements for class 2Ae should not be extended to the outer edges of exophytic cysts, but at the expected outer contours of kidney parenchyma avoiding exophytic cysts (Supplemental Figure 1, Supplemental Table 4). Nevertheless, when the shape of the kidney greatly deviates from ellipsoid because of large exophytic cysts, the slice-by-slice method for TKV is preferred over the ellipsoid method to precisely measure TKV excluding exophytic cysts.
The study has limitations. First, the determination of imaging class and exophytic cysts was on the basis of qualitative subjective assessment of a small number of patients. However, the assessment was performed by imaging experts with a high degree of agreement.6 We also arbitrarily defined exophytic cysts as cysts whose centers were outside the boundaries of kidney parenchyma. Although this definition seems reasonable, it lacks an objective biologic basis. Second, we excluded from the study class 2A without prominent exophytic cysts (class 2Ane). This lack of test cases with other atypical class 2 patterns (i.e., unilateral, segmental, and asymmetric) limits this study. However, in practice, it is difficult to objectively define and quantify these nonexophytic atypical cyst patterns. Third, it may be ideal to develop a model that accurately predicts prognosis for class 2A patients alone without using the class 1 imaging model framework, because it is possible that the model for class 2A may be completely different from the model for class 1. However, given the small sample size (30 class 2A: 5.4% in the HALT database), an independent imaging classification model for class 2A could not be meaningfully achieved. Validation of our study results would require a large ADPKD MRI database from another independent cohort.
In conclusion, patients with ADPKD, with typical or atypical imaging classification including prominent exophytic cysts, showed improvements in the predictions for developing CKD stage 3 and eGFR trajectories when we used htTKVs recalculated the after exclusion of prominent exophytic cysts, in comparison with the predictions using the original htTKVs. This finding implies that the use of recalculated htTKVs facilitates not only the inclusion of class 2 patients with prominent exophytic cysts in the Mayo classification, but also the reclassification of class 1 patients with prominent exophytic cysts.
Disclosures
Dr. Bae reports personal fees from Kadmon and Otsuka Pharmaceuticals, outside the submitted work. Dr. Harris reports grants from Otsuka Pharmaceuticals, and other support from Amgen, Inc., Bayer AG, EMD Millipore Corporation (aka EMDMerck KGaA), Genzyme Corporation, GlaxoSmithKline LLC, Mitobridge, Inc., Otsuka Pharmaceuticals, Regulus, and Vertex Pharmaceuticals, outside the submitted work. Dr. Perrone reports grants and personal fees from Otsuka Pharmaceuticals, Reata, and Sanofi-Genzyme; personal fees from Goldfinch, Palladio Biosciences, and Vertex Pharmaceuticals; grants from Kadmon; and other support from UpToDate, outside the submitted work. Dr. Torres reports grants from Acceleron Pharma, Inc., Blueprint Medicines, Otsuka Pharmaceuticals, Palladio Biosciences, and Regulus Therapeutics, and other support from Mironid, Otsuka Pharmaceuticals, Palladio Biosciences, Sanofi-Genzyme, and Vertex Pharmaceuticals, outside the submitted work. Dr. Yu reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study, and personal fees from Otsuka Pharmaceuticals and Regulus Therapeutics, outside the submitted work. All remaining authors have nothing to disclose.
Funding
This work has been supported by National Institute of Diabetes and Digestive and Kidney Diseases grants DK62402 (to Dr. Torres), DK082230, DK62411 (to Dr. Perrone), DK62410, DK62408 (to Dr. Chapman), DK62401 (to Washington University in St. Louis), and DK090728 (to the Mayo Translational PKD Center); National Center for Research Resources General Clinical Research Centers grants RR000585 (to the Mayo Clinic), RR000039 (to Emory University), RR000054 (to Tufts Medical Center), RR000051 (to the University of Colorado), RR023940 (to the University of Kansas Medical Center), and RR001032 (to Beth Israel Deaconess Medical Center); National Center for Advancing Translational Sciences Clinical and Translational Science awards RR024150 and TR00135 (to the Mayo Clinic), RR025008 and TR000454 (to Emory University), RR025752 and TR001064 (to Tufts University), RR025780 and TR001082 (to the University of Colorado), RR025758 and TR001102 (to Beth Israel Deaconess Medical Center), RR033179 and TR000001 (to the University of Kansas Medical Center), and RR024989 and TR000439 (to Cleveland Clinic); the Zell Family Foundation (to the University of Colorado); and the PKD Foundation.
Supplementary Material
Acknowledgments
We thank the patients involved in the study for their participation and contribution.
The results presented in this paper have not been published previously in whole or in part, except in abstract form. These data were presented at American Society of Nephrology Kidney Week in Washington DC in November 2019.
Dr. Bae, Dr. Landsittel, Dr. Yu, and Dr. Shi designed the study. Dr. Chapman, Dr. Yu, Dr. Brosnahan, Dr. Perrone, Dr. Steinman, Dr. Torres, and Dr. Braun acquired clinical and imaging data. Dr. Srivastava, Dr. Tao, Dr. Landsittel, Dr. Bae, Dr. Abebe, Dr. Irazabal, Dr. Harris, and Dr. Shi analyzed the data. Dr. Srivastava, Dr. Tao, Dr. Landsittel, Dr. Bae, and Dr. Shi made the figures. Dr. Bae and Dr. Landsittel drafted the manuscript. All authors reviewed, revised, and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019101121/-/DCSupplemental.
Supplemental Table 1. Area under ROC curve with original versus recalculated htTKV.
Supplemental Table 2. Predicted %CKD with original versus recalculated htTKV.
Supplemental Table 3. Goodness of fit P value according to Hosmer–Lemeshow test.
Supplemental Table 4. TKV (ml) measured by slice-by-slice versus ellipsoid methods in class 2Ae before and after exclusion of exophytic cysts.
Supplemental Figure 1. TKV measurement using ellipsoid method in two patients with class 2Ae. These MRI images are from the same two patients illustrated in Figure 1. Three longest linear measurements are delineated on each kidney over the largest cross-sectional longitudinal and transverse imaging planes before (left) and after (right) excluding exophytic cysts. (A) TKV was measured 723 ml before and 551 ml after exclusion of exophytic cysts (as a comparison, TKV in Figure 1 was 956 ml before and 631 ml after exclusion of exophytic cysts). (B) TKV was measured 2119 ml before and 857 ml after exclusion of exophytic cysts (as a comparison, TKV in Figure 1 was 2080 ml before and 845 ml after exclusion of exophytic cysts).
References
- 1.Ong AC, Devuyst O, Knebelmann B, Walz G; ERA-EDTA Working Group for Inherited Kidney Diseases : Autosomal dominant polycystic kidney disease: The changing face of clinical management [published correction appears in Lancet 385: 2576, 2015]. Lancet 385: 1993–2002, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Xue C, Zhou C, Mei C: Total kidney volume: The most valuable predictor of autosomal dominant polycystic kidney disease progression. Kidney Int 93: 540–542, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Alam A, Dahl NK, Lipschutz JH, Rossetti S, Smith P, Sapir D, et al.: Total kidney volume in autosomal dominant polycystic kidney disease: A biomarker of disease progression and therapeutic efficacy. Am J Kidney Dis 66: 564–576, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Yu ASL, Shen C, Landsittel DP, Harris PC, Torres VE, Mrug M, et al. Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) : Baseline total kidney volume and the rate of kidney growth are associated with chronic kidney disease progression in Autosomal Dominant Polycystic Kidney Disease. Kidney Int 93: 691–699, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, et al. CRISP Investigators : Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irazabal MV, Abebe KZ, Bae KT, Perrone RD, Chapman AB, Schrier RW, et al. HALT Investigators : Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: The HALT-PKD clinical trial. Nephrol Dial Transplant 32: 1857–1865, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman AB, Torres VE, Perrone RD, Steinman TI, Bae KT, Miller JP, et al.: The HALT polycystic kidney disease trials: Design and implementation. Clin J Am Soc Nephrol 5: 102–109, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, et al. HALT-PKD Trial Investigators : Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 371: 2255–2266, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae KT, Grantham JJ: Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 6: 96–106, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Bae KT, Zhu F, Chapman AB, Torres VE, Grantham JJ, Guay-Woodford LM, et al. Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) : Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol 1: 64–69, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Efron B, Tibshirani RJ: An Introduction to the Bootstrap, Boca Raton, FL, CRC Press, 1994 [Google Scholar]
- 12.StataCorp : Stata Statistical Software: Release 14, College Station, TX, StataCorp LP, 2015 [Google Scholar]
- 13.Heyer CM, Sundsbak JL, Abebe KZ, Chapman AB, Torres VE, Grantham JJ, et al. HALT PKD and CRISP Investigators : Predicted mutation strength of nontruncating PKD1 mutations aids genotype-phenotype correlations in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 2872–2884, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr., et al. CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.