Abstract
In this work, the degradation of chloroquine (CLQ), an antiviral and antimalarial drug, using electro-Fenton oxidation was investigated. Due to the importance of hydrogen peroxide (H2O2) generation during electro-Fenton oxidation, effects of pH, current density, molecular oxygen (O2) flow rate, and anode material on H2O2 generation were evaluated. H2O2 generation was enhanced by increasing the current density up to 60 mA/cm2 and the O2 flow rate up to 80 mL/min at pH 3.0 and using carbon felt cathode and boron-doped diamond (BDD) anode. Electro-Fenton-BDD oxidation achieved the total CLQ depletion and 92% total organic carbon (TOC) removal. Electro-Fenton-BDD oxidation was more effective than electro-Fenton-Pt and anodic oxidation using Pt and BDD anodes. The efficiency of CLQ depletion by electro-Fenton-BDD oxidation raises by increasing the current density and Fe2+ dose; however it drops with the increase of pH and CLQ concentration. CLQ depletion follows a pseudo-first order kinetics in all the experiments. The identification of CLQ degradation intermediates by chromatography methods confirms the formation of 7-chloro-4-quinolinamine, oxamic, and oxalic acids. Quantitative amounts of chlorides, nitrates, and ammonium ions are released during electro-Fenton oxidation of CLQ. The high efficiency of electro-Fenton oxidation derives from the generation of hydroxyl radicals from the catalytic decomposition of H2O2 by Fe2+ in solution, and the electrogeneration of hydroxyl and sulfates radicals and other strong oxidants (persulfates) from the oxidation of the electrolyte at the surface BDD anode. Electro-Fenton oxidation has the potential to be an alternative method for treating wastewaters contaminated with CLQ and its derivatives.
Keywords: Chloroquine, Electro-fenton, H2O2 generation, Boron-doped diamond, Hydroxyl radicals
Highlights
-
•
Chloroquine, an antiviral drug, has the potential to be persistent pollutant in water.
-
•
Effective H2O2 generation was obtained by pairing carbon felt cathode and BDD anode.
-
•
Electro-Fenton-BDD depleted chloroquine from water independently of operating conditions.
-
•
Chloroquine degradation leads to the formation of aromatic intermediates and carboxylic acids.
-
•
Electro-Fenton achieved the release of Cl− ions and conversion of organic nitrogen to NO3 − and NH4 +.
1. Introduction
Chloroquine (CLQ), a generic pharmaceutical drug, is recommended as the primary antimalarial prevention drug (Frosch et al., 2011; Lee et al., 2011; Price et al., 2014) and to treat diseases such as amoebic dysentery (Singh et al., 2011, 2013), and rheumatism (lupus erythematosus) (Furst, 1996; Howard, 2007; Schrezenmeier and Dörner, 2020). Recently, national and international health organizations permitted the treatment of Coronavirus (COVID-19) in certain hospitalized patients by chloroquine (Cortegiani et al., 2020; Devaux et al., 2020; J. Gao et al., 2020). The emergency authorization use of antimalarial drugs including CLQ requires manufacturing this drug in larger scale to fight COVID-19 that infected millions of people in the planet within few months. Accordingly, large quantities of wastewaters contaminated with CLQ will be discharged into the environment. CLQ has high potential to being persistent, bioaccumulate, and transfer to living organisms in intensified toxic forms owing to its antiviral and antibacterial characteristics. The high risks of natural water contamination due to the large production and utilization of CLQ, necessitates more attention to limit its hazardous effects on human health and environment (ozone depleting substance, bioaccumulation, and persistence).
Few are the studies reported in literature about the degradation and fate of CLQ in water (Ahmad et al., 2016; Coelho et al., 2017; Doddaga; Peddakonda, 2013; Karim et al., 1994; Nord et al., 1991). Mostly, the studies cited in literature have been focused on the photochemical stability of HCQ in water and none of them investigated its removal from water. Coelho et al. (2017) investigated the forced degradation of CLQ in water by alkaline hydrolysis and chemical oxidation with diluted H2O2. The degradation of CLQ into simpler molecules was confirmed by high performance liquid chromatography (HPLC) (Coelho et al., 2017); however, the degradation products themselves can pause substantial environmental concerns due to their high toxicity and bioresistance (Ahmad et al., 2016; Doddaga; Peddakonda, 2013). The growing interests on CLQ to prevent a diversity of diseases including the new quickly spreading COVID-19, requires an urgent search for an efficient water treatment method having the ability to destroy this micropollutant and remove it from wastewaters or at least convert it into less harmful and easy biodegrade substances before its discharge into the environment.
Advanced oxidation processes (AOPs) have attracted an increasing interest to substitute and complement with the traditional wastewater treatment methods due their higher efficacy in destroying a myriad of organic pollutants in water (Asghar et al., 2015a; Boczkaj and Fernandes, 2017; Cheng et al., 2016; Deng and Zhao, 2015; Pignatello et al., 2006). The reasons are related to their competency to produce large quantities of powerful oxidizing radicals among them hydroxyl radicals (HO•) (Kanakaraju et al., 2018; Miklos et al., 2018; Wang and Xu, 2012). Being unstable with short residence time (Gligorovski et al., 2015; Peralta et al., 2014; Xiang et al., 2011), these radical species react immediately in a non-selective mode with organic pollutants and convert them into harmless compounds and occasionally to valuable products (Badmus et al., 2018; Tayo et al., 2018; Vallejo et al., 2015). AOPs are based on redox reactions between oxidants and reductants in solution and/or combination of chemical reactants with physical activating methods (Cheng et al., 2016; Gągol et al., 2018). Particularly, AOPs based on chemical and photochemical decomposition of hydrogen peroxide (H2O2) to produce HO• radicals have drawn more attention due to their high efficacy, cost-effectiveness, and possibility to scale up (Ahmed et al., 2011, 2009; Asghar et al., 2015b; Bensalah et al., 2018; Bokare and Choi, 2014; Pham et al., 2012). Fenton and Fenton-like (Babuponnusami and Muthukumar, 2014a; Jiang et al., 2010), photo-Fenton (Clarizia et al., 2017; Dbira et al., 2014), and electro-Fenton (Brillas and Martınez-Huitle, 2009; Hou et al., 2018) processes are among H2O2-based AOPs most investigated for water treatment in small- and large-scale applications.
In Fenton and photo-Fenton processes, the addition of desired amounts of H2O2 as reactant is required among other things (pH control, addition of Fe2+ catalyst) (Asghar et al., 2015a; Babuponnusami and Muthukumar, 2014b), while in electro-Fenton process, H2O2 is electrogenerated in situ from the cathodic reduction of molecular oxygen (O2) (Bensalah et al., 2013). The in situ electrogeneration of H2O2 results in virtuous control of the oxidation, lessens the safety risks associated with the transport and storage of this hazardous and unstable chemical, and then reduces the overall costs of the treatment. The electrodes’ materials play a crucial role in the improvement of the effectiveness of electro-Fenton oxidation (El-Ghenymy et al., 2012; Guinea et al., 2010; Moreira et al., 2013; Pinheiro et al., 2019; Yang et al., 2017; Zhang et al., 2018). Especially, the kinetics and current efficiency of the production of H2O2 from the electrochemical reduction of O2 at the surface of the cathode is extensively influenced by the cathode’s material. Brillas et al. (2009) stated that Hg, C-graphite, carbon-PTFE O2 diffusion, carbon felt and others materials can reduce O2 into H2O2 in water with high current efficiency. Carbon felt a 3D material, is a cost-effective cathode material for electro-Fenton oxidation (Gong et al., 2016; Huong Le et al., 2017; Yu et al., 2015, 2014). Furthermore, recent studies showed that pairing carbon felt cathode with boron doped diamond anode (BDD) could boost the efficacy of electro-Fenton oxidation (Borràs et al., 2013; El-Ghenymy et al., 2015; Ruiz et al., 2011). BDD anode is known by its capability to produce HO• radicals and other strong oxidants from the oxidation of the electrolyte (Bensalah et al., 2015; Groenen Serrano, 2018; Marselli et al., 2003; Michaud et al., 2003). The supplementary production of strong oxidants at BDD anode and the continuous generation of H2O2 and regeneration of the catalyst (Fe2+) by electrochemical reduction of O2 and Fe3+ ions at the cathode offer to this type of AOP an outstanding effectiveness compared to other AOPs in the destruction of organic pollutants in water (Bensalah et al., 2015; Groenen Serrano, 2018; Marselli et al., 2003).
This work aims to investigate the degradation of CLQ in water by electro-Fenton oxidation using carbon felt as cathode material, and Pt and BDD as anode materials. The results will offer significant information needed in the future to depollute large quantities of wastewaters contaminated with CLQ drug and its metabolites especially this drug is adopted as the first treatment of COVID-19 by many health organizations. The electrogeneration of H2O2 during electrolysis was investigated at different operating conditions. The degradation of CLQ by electro-Fenton oxidation under various conditions (pH, current density, CLQ concentration, Fe2+ concentration) was monitored by HPLC analysis and total organic carbon (TOC) measurement. The analysis of organic and inorganic intermediates and final products was conducted using HPLC and ion chromatography (IC).
2. Materials and methods
2.1. Chemicals
CLQ N4-(7-Chloro-4-quinolyl)-N1,N1-diethyl-1,4-pentanediamine Diphosphate (see Table 1 ) was purchased from VWR (with purity ≥ 98%). 7-chloro-4-quinolinamine (4-Amino-7-chloroquinoline) (CQLA) was obtained from Sigma-Aldrich (see Table 1). Oxalic acid (OAA) (anhydrous, ≥ 98.0) and oxamic acid (OAMA) (anhydrous, ≥ 97.0) were received from VWR (see Table 1). 30% (by mass) H2O2 solutions were purchased from VWR. Analytical grade FeSO4·7H2O, Na2SO3, and Ti(SO4)2 were used as received from Sigma-Aldrich. The other chemicals used for pH adjustment and in chromatography analysis are HPLC analytical grade from Sigma Aldrich or Fluka. All aqueous solutions were prepared in deionized water obtained from Mill-Q™ system having 18 mΩ cm−1 resistivity.
Table 1.
Chemical formulas and structures of CLQ and its intermediates.
| Substance | Chemical formula | Chemical structure |
|---|---|---|
| Chloroquine | C18H26ClN3 |  |
| 7-chloro-4-quinolinamine | C9H7ClN2 |  |
| Oxamic acid | C2H3NO3 |  |
| Oxalic acid | C2H2O4 |  |
2.2. Analytical methods
All the samples withdrawn at desired times, underwent a filtration through 0.2 μm membrane filters before analysis. The pH was monitored using a pH-meter (Seven Compact S210, Mettler Toledo®). TOC and total nitrogen (TN) analysis was conducted using Skalar FormacsHT TOC/TN analyzer. UV–Visible spectrophotometer (PerkinElmer Lambda 5) was used for rapid measurements of CLQ concentration at 278 nm using a 1 cm-quartz cells. H2O2 concentration was evaluated by colorimetric method (at 420 nm) using titanium (IV) sulfate (Ti(SO4)2) for lower concentrations than 50 mg/L, while volumetric titration method with KMnO4 was used for H2O2 concentration > 50 mg/L (Eisenberg, 1943; Klassen et al., 1994). Active chlorine was measured by DPD colorimetric method using N,N-diethyl-p-phenylenediamine (Rice et al., 2017). Chlorides and nitrates were monitored using Dionex ICS 2000 ion chromatograph equipped with EGC eluent generator, Ion Pac AS 19 (4 mm 250 mm) analytical separation column, ASRS 300 mm–4mm suppressor, and DS6 conductometric cell. Ammonium ions were analyzed by ion-selective electrode for ammonium ion (ELIT 8051 PVC membrane). CLQ and CQLA concentrations were measured by HPLC using Shimadzu 20A Gradient LC System with UV-VIS Detector equipped with Shim-pack GWS C18 (150x4.6, 5 μm) separation column. The separation was performed using a mobile phase composed of a mixture of eluent A (0.1% H3PO4 in water) and eluent B (acetonitrile, CH3CN) in gradient elution mode at a fixed flow rate of 1 mL/min and constant column temperature at 40 °C. By injecting 10 μL of each sample, the gradient elution begun with 90% of eluent A during 5 min, then eluent A decreased to 40% within 15 min, and after that the elution gradient remains constant (40% A+60% B) until the end of analysis. The UV detector was set at a wavelength of 340 nm. Oxalic and oxamic acids were measured by HPLC using a Supelcogel H column (mobile phase, 0.15% phosphoric acid solution; flow rate, 0.15 mL/min) with UV detection at 210 nm. Linear calibration curves based on external standardization were obtained in chromatography analysis for all analytes with regression coefficients higher than 98%.
2.3. Experimental setup
A single-compartment glass electrochemical cell with a double jacket was used in all the electrochemical experiments. The temperature was maintained to 25 °C by water circulation. The cathode materials were made from carbon felt (Carbone Loraine, 15 × 4 × 0.5 cm3) and stainless steel in electro-Fenton oxidation and electrochemical oxidation, respectively. The anode materials used were BDD and platinum (Pt). Pt electrodes were obtained from Advent Research Materials (Oxford, England, UK). BDD anodes were purchased from Adamant Technologies (Neuchatel, Switzerland). They were fabricated by hot filament chemical vapor deposition (HF CVD) technique of boron-doped diamond thin film deposited on single-crystal p-type Si (100) substrates (0.1 Ω cm Siltronix) as described elsewhere (Nasr et al., 2005). The cathode was attached to the wall of the electrochemical cell and the anode was placed in vertical position in the center 2 cm distant from the cathode. A fixed geometric area of 30 cm2 for each electrode was immersed in the solution. The homogenization of the solutions was assured by continuous stirring using a magnetic stirrer (Thermo Scientific™ S88854105) at 300 rpm in all the experiments. The pH was adjusted to a desired value by adding aliquots of 0.1 M H2SO4 or 0.1 M NaOH solutions. After pH adjustment and addition of an amount of the catalyst (FeSO4·7H2O) when needed, pure oxygen was continuously bubbled into 400 mL solution (0.05 M Na2SO4) nearby the cathode at a fixed flow rate in the range 60–240 mL/min. Electro-Fenton and anodic oxidation experiments were performed under galvanostatic mode (constant current density). The electrodes were connected to a digital dc power supply (Monacor PS-430) providing current and voltage in the ranges 0–30 A and 0–20 V. The current intensity applied during each experiment was maintained to a constant value using the power supply. A potentiometer (Micronal B474) was used to measure the cell voltage during the experiments. At certain time-periods, samples were withdrawn from the solution and then filtered by 0.45-mm membrane for analysis. During electrochemical oxidation experiments, BDD (or Pt) and stainless steel plates (effective area of 30 cm2) were inserted into the solution in parallel position and an inter-electrode gap of 2 cm.
3. Results and discussions
-
1.
Generation of H2O2 in electro-Fenton process
The generation of H2O2 by electrochemical reduction of O2 at the carbon felt cathode is main important stage in electro-Fenton process. The efficacy of this process depends largely on the rate of H2O2 generation and the amount of H2O2 available in solution to react with Fe2+ ions and produce HO• radicals (Eqs (1), (2))) (Brillas et al., 2009; Yu et al., 2015, 2014).
| (1) |
| (2) |
Fig. 1 presents the effects of pH, current density, O2 flow rate, and anode material on the changes of H2O2 concentration with time during the electrolysis of 0.05 M Na2SO4 aqueous solutions. It is remarkable that the graphs of H2O2 concentration via time have the same trend: A rapid linear increase in H2O2 concentration from the beginning of the electrolysis to reach a maximum after 90–120 min, and then it is maintained to almost a constant value for a large plateau until the end (300 min). This result corresponds to steady state conditions at which the rate of generation of H2O2 is equal to the rate of its destruction. This is probably due to the limited solubility of O2, mass/charge transfer kinetic limitations, and competition between the main reaction of generation of H2O2 (Eq. (1)) and secondary reactions (Eqs. (3), (4), (5), (6)))
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
Fig. 1.
Effects of (a) initial pH, (b) current density, (c) O2 flow rate, and (d) anode material on the changes of H2O2 concentration with time during electrolysis of 0.05 M Na2SO4. Experimental conditions: (a) j = 60 mA/cm2, O2 flow rate = 80 mL/min, Anode: Pt (30 cm2), cathode: Carbon felt (30 cm2), T = 25 °C, stirring: 300 rpm; (b) pH = 3.0, O2 flow rate = 80 mL/min, Anode: Pt (30 cm2), cathode: Carbon felt (30 cm2), T = 25 °C, stirring: 300 rpm; (c) j = 60 mA/cm2, pH = 3.0, Anode: Pt (30 cm2), cathode: Carbon felt (30 cm2), T = 25 °C, stirring: 300 rpm (d) j = 60 mA/cm2, pH = 3.0, O2 flow rate = 80 mL/min, Anode: BDD/Pt/Graphite (30 cm2), cathode: Carbon felt (30 cm2), T = 25 °C, stirring: 300 rpm.
Fig. 1 a presents the effect of initial pH on the changes of H2O2 concentration with time during electrolysis of 0.05 M Na2SO4 using Carbon felt cathode and Pt anode at j = 60 mA/cm2, O2 flow rate = 80 mL/min, T = 25 °C, and stirring at 300 rpm. As it can be seen from Fig. 1 a, the initial pH affected both the rate of H2O2 generation and its maximum concentration. The average rate of H2O2 generation decreased from 1.67 mg/min at pH 3.0 to 1.43, 1.13, 0.38, 0.27 mg/min at pH 5.2, 7.1, 9.4, and 12.0, respectively (the average rate was calculated from the slope of the linear ascending part of the graph). H2O2 concentration decreased from 150 mg/L at pH 3.0 to 136, 108, 60.2, and 45.6 mg/L at pH 5.2, 7.1, 9.2, and 12.0, respectively (see Figure S1.a). The drop of the average rate of H2O2 generation and its generated concentration at neutral and alkaline pH can be explained by the acceleration reactions of cathodic reduction and disproportionation of H2O2 at neutral and alkaline conditions (Eqs. (3), (7))) (Shemer and Linden, 2006; Teymori et al., 2020). A value of pH around 3.0 is optimal for H2O2 generation by electrochemical reduction of O2 at carbon-felt cathode.
Fig. 1 b presents the effect of current density on the changes of H2O2 concentration with time during electrolysis of 0.05 M Na2SO4 using Carbon felt cathode and Pt anode at pH = 3.0, O2 flow rate = 80 mL/min, T = 25 C, stirring at 300 rpm. Fig. 1 b demonstrates that the increase of current density from 20 to 100 mA/cm2 enhanced the generation of H2O2; however, at 200 mA/cm2 a decrease in H2O2 generation was observed. The accumulated H2O2 concentration passed from 25.8 mg/L at 20 mA/cm2 to 70.1, 150, 165, and 136 mg/L at 40, 60, 100, and 200 mA/cm2, respectively. The average rates of H2O2 generation were 0.25, 0.74, 1.67, 1.83, and 1.48 at current densities of 20, 40, 60, 100, and 200 mA/cm2, respectively (see Figure S1.b). It seems that at higher current density than 60 mA/cm2, the electrochemical reduction of O2 to H2O (Eq. (5)) starts to compete with the main reaction of H2O2 generation (Eq. (1)). In addition, at high current density, the electro-generated H2O2 can be oxidized at the anode (Eq. (8)).
| (8) |
Fangke et al. (Yu et al., 2014) reported similar results related to the effect of current density on H2O2 generation during electrolysis using graphite felt cathode. Several studies (Y. Gao et al., 2020; Özcan et al., 2008; Wang et al., 2019; Zhou et al., 2013) correlated the decrease of H2O2 generation at high current density with the decrease in the cathode potential with the increase of current density, which results in greater competition between the reduction of oxygen to H2O2 and to H2O (Eqs. (1), (5))). A current density of 60 mA/cm2 is cost-effective to generate H2O2 from the reduction of O2 at carbon-felt cathode.
Fig. 1 c presents the effect of O2 flow rate on the changes of H2O2 concentration with time during electrolysis of 0.05 M Na2SO4 using Carbon felt cathode and Pt anode at j = 60 mA/cm2, pH = 3.0, T = 25 °C, and stirring at 300 rpm. It is noticeably observed that H2O2 can be generated even without bubbling O2 in the solution (O2 flow rate = 0 mL/min) with a low average rate of 0.24 mg/L and its accumulated concentration reached 43.8 mg/L. This generation of H2O2 in absence of O2 bubbling resulted from the electrochemical reduction of the dissolved O2 from air and the additional O2 electrogenerated at the anode from water discharge (Y. Gao et al., 2020; Özcan et al., 2008; Wang et al., 2019; Zhou et al., 2013). The increase of O2 flow rate from 40 mL/min to 80 mL/min increased the average rate of H2O2 generation and the accumulated H2O2 concentration from 0.84 mg/min and 94.9 mg/L to 1.67 mg/min and 150 mg/L, respectively (see Figure S1.c). Further increase in O2 flow rate does not have a significant effect on H2O2 generation. Bubbling O2 in a solution increases the concentration of O2 in water. In addition, the increase in the O2 flow rate improves the mass transfer kinetics. When the solution is saturated in O2 (saturated solution contains 8.3 mg O2/L at 1.0 atm and 25 °C), the increase of O2 flow rate (>80 mL/min) will not affect the accumulated H2O2 concentration. A flow rate of 80 mL/min of pure oxygen is adequate to produce high-accumulated H2O2 concentration.
Fig. 1 d presents the changes of H2O2 concentration with time during electrolysis of 0.05 M Na2SO4 using Carbon felt cathode and different anode materials (Pt, BDD, graphite) at j = 60 mA/cm2, pH = 3.0, O2 flow rate = 80 mL/min, T = 25 °C, and stirring at 300 rpm. The average rates of H2O2 generation were 2.75, 1.67 and 1.49 mg/min for BDD, Pt, and graphite anodes, respectively. The accumulated H2O2 concentrations measured at the end of electrolysis were 218, 150, and 133 mg/L for BDD, Pt, and graphite anodes, respectively (see Figure S1.d). BDD anode showed better H2O2 generation compared with Pt and graphite anode materials. This result can be explained by the large voltage window and the high over potential of O2 evolution of BDD anode (Marselli et al., 2003; Michaud et al., 2003). BDD anode, in fact, can produce large amounts of hydroxyl radicals from water oxidation that combine together to form H2O2 (Eqs. (9), (10))) (Marselli et al., 2003; Michaud et al., 2003). Similar results were reported in the literature confirming higher performance of BDD anode in electro-Fenton oxidation (Borràs et al., 2013; El-Ghenymy et al., 2015; Pereira et al., 2016; Ruiz et al., 2011).
| (9) |
| (10) |
Pairing carbon felt cathode with BDD anode generates additional amount of H2O2 compared to the other configurations. Accordingly, the combination of anodic oxidation using BDD and the electrogeneration of H2O2 by reduction of O2 at carbon felt electrode would result in high efficacy to electro-Fenton oxidation of organic pollutants.
-
2.
Efficiency of electro-Fenton oxidation in the degradation of CLQ
Fig. 2 presents the changes of CLQ concentration with time during the electrochemical treatment of 125 mg/L CLQ aqueous solutions by anodic oxidation using Pt (Electrolysis-Pt) and BDD (Electrolysis-BDD) anodes and stainless steel cathode and by electro-Fenton oxidation (O2 flow rate = 80 mL/min; Fe2+: 10 mg/L) using carbon felt cathode and Pt (Electro-Fenton-Pt) and BDD (Electro-Fenton-BDD) anodes holding the other experimental conditions unvaried (0.05 M Na2SO4, j = 60 mA/cm2, pH = 3.0, T = 25 °C, stirring at 300 rpm). CLQ concentration decreased with time in all the experiments, but at different extent. The efficiency of CLQ degradation (in terms of kinetics and % of CLQ depletion) is increasing in the order: Electrolysis-Pt < Electrolysis-BDD < Electro-Fenton-Pt < Electro-Fenton-BDD. Assuming pseudo-first order kinetics for CLQ degradation (exponential decay of HCQ concentration), the rate constants, kobs calculated for a pseudo-first order degradation, were 0.001, 0.006, 0.011, and 0.029 min−1 for electrolysis-Pt, electrolysis-BDD, electro-Fenton-Pt, and electro-Fenton-BDD, respectively. Electro-Fenton-BDD achieved the complete depletion CLQ after 180 min; however, 84%, 68%, and 17% of CLQ were removed during the same period of time (180 min), electro-Fenton-Pt, electrolysis-BDD, and electrolysis-Pt, respectively. The anode material has a significant influence on CLQ degradation in both anodic oxidation and electro-Fenton.
Fig. 2.
Changes of CLQ concentration with time during electrochemical treatment of 125 mg/L CLQ aqueous solutions by anodic oxidation and electro-Fenton oxidation. Experimental conditions: Electrolyte: 0.05 M Na2SO4, j = 60 mA/cm2, pH = 3.0, T = 25 °C, stirring = 300 rpm; Electrolysis-Pt: Anode: Pt, Cathode: Stainless steel; Electrolysis-BDD: Anode: BDD, Cathode: Stainless steel; Electro-Fenton-Pt: Anode Pt, Cathode: Carbon felt, O2 flow rate: 80 mL/min, Fe2+: 10 mg/L; Electro-Fenton-BDD: Anode: BDD, Cathode: Carbon felt, O2 flow rate: 80 mL/min, Fe2+: 10 mg/L.
This can be explained by the larger electrochemical activity of BDD than Pt anode in 0.05 M Na2SO4 enabling the direct oxidation of organic molecules on the surface of BDD anode (Martínez-Huitle and Panizza, 2018; Panizza et al., 2008). BDD anode can produce large amounts of hydroxyl radicals (HO•) from the electrochemical oxidation of water (Eq. (9)), which are weakly adsorbed on BDD surface resulting in immediate and non-selective reactions with the organic pollutant molecules (Marselli et al., 2003; Michaud et al., 2003). Furthermore, the electrochemical oxidation of sulfate ions at BDD anode yields the formation of strong oxidants (sulfate radicals, SO4 −• and persulfate ions, S2O8 2−) as shown by the following reactions (Eqs. (11), (12))) (Michaud et al., 2000; Serrano et al., 2002):
| (11) |
| (12) |
These oxidants participate in the degradation of organic pollutants in solution. Furthermore, Michaud et al. (2003) stated that O3 and H2O2 could also be produced by anodic oxidation of water at high current density.
In addition, the results demonstrate higher efficiency of electro-Fenton oxidation compared to anodic oxidation (for Pt and BDD anodes) in degrading HCQ. This is probably due to larger production of hydroxyl radicals from catalytic decomposition of the electrogenerated H2O2 by Fe2+ in solution. In the case of BDD, anodic oxidation of water at the anode produces supplementary hydroxyl radicals, which clarifies the better performance of electro-Fenton with BDD anode than with Pt anode.
The highest efficiency of electro-Fenton oxidation using BDD anode compared to the other electrochemical methods can be explained by the contribution of different pathways in CLQ degradation including (i) the mediated oxidation by hydroxyl radicals produced by Fenton reaction between H2O2 electrogenerated at carbon felt cathode and Fe2+ ions in solution, (ii) the indirect oxidation via hydroxyl and sulfate radicals electrogenerated at the BBD anode from water oxidation locally close to BDD surface; (iii) the indirect oxidation via sulfate radicals electrogenerated from direct oxidation of sulfate ions at BDD surface and/or by the reaction of sulfate ions with HO• radicals produced at BDD surface from water discharge at BDD surface (de Freitas Araújo et al., 2020; Escalona-Durán et al., 2020); (iv) mediated chemical oxidation in solution by the inorganic strong oxidants (persulfate ions, O3, H2O2) produced by anodic and cathodic reactions involving the electrolyte, (v) direct electrochemical oxidation of CLQ at the surface of BDD anode.
-
3.
Effects of operating conditions on electro-Fenton oxidation of CLQ
Fig. 3 presents the changes of the normalized concentration ([CLQ]t/[CLQ]0) with time (/[CLQ]0 is the initial CLQ concentration at t = 0 s, [CLQ]t is CLQ concentration at an instant t) during electro-Fenton oxidation of 0.05 M Na2SO4 aqueous solutions containing different CLQ concentrations using carbon felt cathode and BDD anode keeping the other operating conditions fixed (j = 60 mA/cm2, pH = 3.0, O2 flow rate = 80 mL/min, [Fe2+] = 10 mg/L, T = 25 °C, stirring = 300 rpm). As it can been seen, the normalized CLQ concentration decreased exponentially with time for all the initial concentrations studied indicating a pseudo-first order kinetics for CLQ degradation by electro-Fenton-BDD oxidation. It is important to report that CLQ was completely depleted by electro-Fenton-BDD oxidation in regardless of CLQ concentration (for [CLQ] ≤ 250 mg/L. The increase of initial concentration increased the time required to deplete CLQ from water: 90 min for [CLQ] = 34 mg/L, 120 min for [CLQ] = 75 mg/L, 180 min for [CLQ] = 125 mg/L, 240 min for [CLQ] = 200, and 300 min for [CLQ] = 250 mg/L. Moreover, the inlet graph of Fig. 3 shows that the pseudo-first order rate constant kobs decreased with the increase of CLQ concentration for [CLQ] < 200 mg/L, and then it remained almost constant [CLQ] ≥ 200 mg/L. This results indicates that, for [CLQ] < 200 mg/L, the reaction is not truly first order reaction (although good first order fitting was observed for each concentration). For [CLQ] < 200 mg/L, the generated amount of hydroxyl radicals is high enough and it stays unchanged during the electro-Fenton-BDD oxidation, which does not affect the kinetics of CLQ degradation; while for [CLQ] ≥ 200 mg/L, the amount of hydroxyl radicals becomes the critical parameter that determines CLQ degradation kinetics.
Fig. 3.
Changes of normalized concentration ([CLQ]t/[CLQ]0) with time (/[CLQ]0 during electro-Fenton oxidation of 0.05 M Na2SO4 aqueous solutions containing different CLQ concentrations using carbon felt cathode and BDD anode. Inlet graph: the changes of pseudo-first order rate constant kobsversus CLQ concentration. Experimental conditions: j = 60 mA/cm2, pH = 3.0, O2 flow rate = 80 mL/min, [Fe2+] = 10 mg/L, T = 25 °C, stirring = 300 rpm.
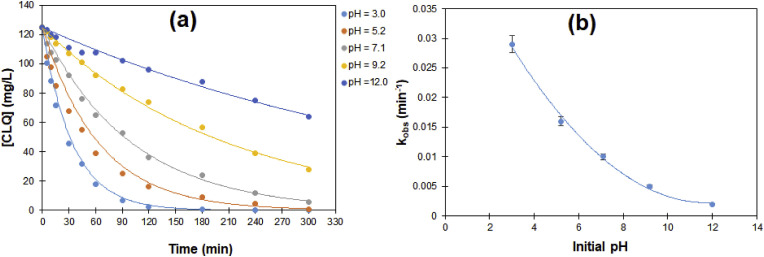
Fig. 4 a presents the effect of pH on the changes of CLQ concentration with time during electro-Fenton oxidation of 125 mg/L CLQ aqueous solutions using carbon felt cathode and BDD anode holding the other operating parameters fixed (Electrolyte: 0.05 M Na2SO4, j = 60 mA/cm2, O2 flow rate = 80 mL/min, [Fe2+] = 10 mg/L, T = 25 °C, stirring = 300 rpm). The profile of CLQ concentration with time exhibited an exponential decrease with time for all the pH values in the range 3.0–12.0. The complete depletion was achieved for pH = 3.0 after 180, while the percentages of CLQ depletion after the same period of time were 93, 81, 54, 29% at pH values 5.2, 7.1, 9.4, and 12.0, respectively. The rate of CLQ depletion decreased with the increase of pH value from 3.0 to 12.0 as shown in Fig. 4 b. These results are in good correlation with the results presented in Fig. 1 a, where it was shown that the highest H2O2 generation occurred at pH = 3.0. The higher efficiency of electro-Fenton oxidation in depleting CLQ at pH = 3.0 is due to larger production of hydroxyl radicals from catalytic decomposition of H2O2 by Fe2+.
Fig. 4.
Changes of: (a) CLQ concentration with time at different pH values during electro-Fenton oxidation of 125 mg/L CLQ aqueous solutions using carbon felt cathode and BDD anode, and (b) pseudo-first order rate constant kobsversus initial pH. Experimental conditions: Electrolyte: 0.05 M Na2SO4, j = 60 mA/cm2, O2 flow rate = 80 mL/min, [Fe2+] = 10 mg/L, T = 25 °C, stirring = 300 rpm.
Fig. 5 presents the effect of current density on the changes of CLQ concentration with time during electro-Fenton oxidation of 125 mg/L CLQ aqueous solutions using carbon felt cathode and BDD anode holding the other operating parameters fixed (Electrolyte: 0.05 M Na2SO4, pH = 3.0, O2 flow rate = 80 mL/min, [Fe2+] = 10 mg/L, T = 25 °C, stirring = 300 rpm). The complete depletion of CLQ was accomplished after 120 min at 200 mA/cm2. After 120 min of the starting of electro-Fenton experiments, the % of CLQ depletion was 49.6, 72.8, 98.1, and 98.6 at 20, 40, 60, and 100 mA/cm2, respectively. Fitting the data to pseudo-first order kinetics showed that the rate constant kobs increased linearly with current density between 20 and 100 mA/cm2, then it became almost unvaried with the increase of the current density (see inlet graph in Fig. 5).
Fig. 5.
Changes of CLQ concentration with time at different current densities (20–200 mA/cm2) during electro-Fenton oxidation of 125 mg/L CLQ aqueous solutions using carbon felt cathode and BDD anode. Inlet: pseudo-first order rate constant kobsversus current density. Experimental conditions: Electrolyte: 0.05 M Na2SO4, pH = 3.0, O2 flow rate = 80 mL/min, [Fe2+] = 10 mg/L, T = 25 °C, stirring = 300 rpm.
The specific electric charge consumption calculated from the following formula:
where j is the current density, A is the electrode area, t is the time required for complete depletion, V the volume of the reactor, and [CLQ] is CLQ concentration. The estimated values of Q are illustrated in Table 2 . The results showed that similar specific electrical charge consumption are required during the electro-Fenton oxidation at current density ≤60 mA/cm2. Increasing the current density to values higher than 60 mA/cm2, increases significantly the specific electrical charge consumption.
Table 2.
Estimated specific electric charge consumption during electro-Fenton oxidation of 125 mg/L CLQ aqueous solutions using carbon felt cathode and BDD anode at different current densities (same experimental conditions than Fig. 2).
| Current density (mA/cm2) | Time required for complete CLQ depletion (min) | Q (Ah/g HCQ) |
|---|---|---|
| 20 | 320 | 129.0 |
| 40 | 240 | 131.9 |
| 60 | 210 | 128.5 |
| 100 | 150 | 152.2 |
| 200 | 120 | 241.7 |
It can be concluded that the increase of current from 20 to 60 mA/cm2 enhanced the kinetics and efficiency of electro-Fenton oxidation of CLQ; however, higher current density than 60 mA/cm2 showed minor improvement and higher specific electric charge consumption. Increasing the current density up to 60 mA/cm2 (i) enhances the generation of H2O2 from electrochemical reduction of O2 at the cathode (Fig. 1b) producing larger amounts of HO• radicals by catalytic decomposition with Fe2+ in solution, (ii) generates substantial HO• radicals from the discharge of water at BDD anode, (iii) produces strong oxidants from the anodic oxidation of sulfate at BDD anode (sulfate radicals and persulfate ions) that participate in the degradation of CLQ and its intermediates, (iv) accelerates the direct anodic oxidation of CLQ and its intermediates on the surface of BDD electrode. However, higher current densities than 60 mA/cm2 accelerates the secondary reactions of H2O2 disproportionation in solution, H2 evolution at the cathode and O2 evolution at the anode, and makes them highly competitive with the primary reactions of generation of H2O2 at the cathode and HO• radicals at the anode. This results in generating similar amount of HO• radicals to attack the same organic pollution than at 60 mA/cm2, which rises the electrical energy requirements, and then augments the overall costs of the electro-Fenton oxidation.
Fig. 6 a presents the effect of Fe2+ dose on the changes of CLQ concentration during with time during the first 60 min of electro-Fenton oxidation of 125 mg/L CLQ aqueous solutions using carbon felt cathode and BDD and Pt anodes using the same operating parameters (Electrolyte: 0.05 M Na2SO4, j = 60 mA/cm2, pH = 3.0, O2 flow rate = 80 mL/min, T = 25 °C, stirring = 300 rpm). The degradation of CLQ occurred even in absence of Fe2+ and 8% and 51% of CLQ were removed by electro-Fenton-Pt and electro-Fenton-BDD after 60 min. This can be explained by the contribution of HO• radicals produced at the anode surface in the degradation of CLQ. The addition of Fe2+ enhanced the efficiency of CLQ degradation for both electro-Fenton-Pt and electro-Fenton-BDD; however, the highest % CLQ depletion by electro-Fenton-Pt did not exceed that obtained by electro-Fenton-BDD in absence of Fe2+. This result confirms the important contribution of anodic oxidation using BDD on the overall efficiency of electro-Fenton oxidation (Borràs et al., 2013; El-Ghenymy et al., 2015; Pereira et al., 2016; Ruiz et al., 2011).
Fig. 6.
Changes of: (a) CLQ concentration with time at different Fe2+ doses during electro-Fenton oxidation of 125 mg/L CLQ aqueous solutions using carbon felt cathode and BDD anode, and (b) pseudo-first order rate constant kobsversus Fe2+ dose. Experimental conditions: Electrolyte: 0.05 M Na2SO4, j = 60 mA/cm2, pH = 3.0, O2 flow rate = 80 mL/min, T = 25 °C, stirring = 300 rpm.
The increase of Fe2+ dose increased the % CLQ depletion for both electro-Fenton-Pt and electro-Fenton-BDD. After 60 min electro-Fenton-Pt oxidation, the % CLQ depletion increased from 8% in absence of Fe2+ to 26.4, 33.6, and 37.6 in presence of 5, 10, and 20 mg Fe/L, respectively. For the same period of time electro-Fenton-BDD achieved 51% in absence of Fe2+ and 75.2, 85.6, and 92.8 in presence of 5, 10, and 20 mg Fe/L, respectively. The enhancement of % CLQ depletion by increasing Fe2+ dose is mainly due to the acceleration of Fenton reaction (H2O2 decomposition into HO• radicals by Fe2+) and the rapid regeneration of Fe2+ catalyst by the electrochemical reduction of Fe3+ at carbon felt cathode. The rate constant kobs obtained from fitted data to pseudo-first order kinetics increases with the increase of Fe2+ dose. This increase is more important for electro-Fenton-BDD as shown in Fig. 6 b. However, higher Fe2+ doses than 10 mg Fe/L had less impact on the kinetics and efficiency of electro-Fenton, which can be due to partial precipitation of Fe3+ as Fe(OH)3 and formation Fe-oxalate complexes decelerating the regeneration of Fe2+ catalyst.
-
4.
Intermediates of CLQ degradation by electro-Fenton oxidation
Fig. 7 a presents the changes of the concentrations (in mg C/L) of CLQ, TOC, and intermediates (calculated from mass balance) during the electro-Fenton oxidation of 125 mg/L CLQ aqueous solutions using carbon felt cathode and BDD and Pt anodes using under the optimized operating conditions (Electrolyte: 0.05 M Na2SO4, j = 60 mA/cm2, pH = 3.0, O2 flow rate = 80 mL/min, Fe2+ = 10 mg/L, T = 25 °C, stirring = 300 rpm). CLQ and TOC concentrations decreased continuously with time with different rates, while the intermediates concentration increased from the beginning of the treatment to reach a maximum after 60 min, and then decreased with the same profile than TOC. The continuous decrease of TOC indicates that the organic carbon is transformed into CO2 from the beginning electro-Fenton-BDD. Assuming pseudo-first order kinetics for CLQ and TOC, the rate constant determined by fitting was 0.029 and 0.008 min−1 for CLQ and TOC, respectively. This results confirms the formation of organic intermediates at the first stages of CLQ the degradation by electro-Fenton oxidation. The overlapping of the profiles of TOC and the intermediates demonstrates the persistence of certain organic intermediates until the end of the treatment (300 min), where 92% TOC was eliminated.
Fig. 7.
Changes with time of: (a) TOC and intermediates, (b) Chlorides and active chlorine, (c) Nitrogen intermediates, (d) organic intermediates during electro-Fenton oxidation of 125 mg/L CLQ aqueous solutions using carbon felt cathode and BDD anode. Experimental conditions: Electrolyte: 0.05 M Na2SO4, j = 60 mA/cm2, pH = 3.0, O2 flow rate = 80 mL/min, Fe2+ = 10 mg/L T = 25 °C, stirring = 300 rpm.
The results of HPLC analysis for CQLA (7-chloro-4-quinolinamine), OMA (oxamic acid), and OAA (oxalic acid) expected as intermediates of degradation are given in Fig. 7 b. The profiles of CQLA and OMA concentrations are similar with a rapid increase to reach maxima of 5.3 and 6.1 mg C/L at 30 and 60 min, and then they rapidly decreased to disappear after 120 and 240 min, respectively. OAA exhibited a slow accumulation to reach a maximum concentration of 12.3 mg C/L after 120 min, and then underwent a sluggish decrease to persist at 7.4 mg C/L at the end of the treatment. These results endorse the formation of aromatic intermediates including CQLA at the first stages of the electro-Fenton oxidation of CLQ. These intermediates undergo a rapid oxidative of the aromatic rings into aliphatic intermediates including carboxylic acids (OMA and OAA). The latter are slowly degraded and take longtime to be mineralized due to the formation of stable complexes with Fe2+/Fe3+ that resist to HO• radicals attack as mentioned in literature by several authors (El-Ghenymy et al., 2015; Garcia-Segura and Brillas, 2011; Gong et al., 2016). The mineralization of the target compound was confirmed by the release of inorganic nitrogen ions and chlorides as shown Fig. 7, Fig. 8 d. The organic nitrogen was mainly released in the form of nitrates, NO3 − and ammonium ions, NH4 + as shown in Fig. 7 c. Nitrates and ammonium ions started to form after 30 min of the starting of the electrolysis indicating that the release of nitrogen does not happen at the first stages of CLQ degradation by electro-Fenton oxidation. After that, NO3 − and NH4 + concentrations raised to reach plateaus after 120 min at 9.7 and 4.0 mg N/L, respectively. The total nitrogen (TN) declined a little bit from 16.8 to 15.0 mg N/L at the end of electrolysis indicating that a small part of organic nitrogen was volatilized in the form of NH3, NOx, and chloramines (Dbira et al., 2015; Zöllig et al., 2015). In contrast, chlorides were released from the beginning of the treatment as shown in the changes of the concentration of chlorides presented in Fig. 7 d. Chlorides concentration increased rapidly to reach a maximum value of 12.0 mg Cl/L (84% of total chlorine) after 120 min, then it remained stable until the end of the treatment. A small amount of active chlorine (HClO and ClO−) was measured during the treatment with a maximum of 2.3 mg Cl/L (16% of total chlorine). Active chlorine is formed by reaction of HO• radicals with chlorides in solution or at immediate vicinity of BDD surface (Martínez-Huitle and Panizza, 2018). Based on these results a simple mechanism for CLQ degradation by electro-Fenton oxidation is proposed in Fig. 8. CLQ degradation starts by dealkylation of the aromatic ring and formation of CQLA, followed by the release of chloride ions. The aromatic intermediates undergo an oxidative ring opening to form aliphatic carboxylic acids among them oxamic and oxalic acids and release of organic nitrogen as nitrates and ammonium ions. The latter are slowly mineralized into CO2.
Fig. 8.
Simple mechanism for CLQ degradation by electro-Fenton oxidation.
4. Conclusion
This work demonstrates that electro-Fenton oxidation using carbon felt cathode and BDD anode accomplished the complete removal of chloroquine drug, CLQ, and 92% TOC removal under optimized operational conditions (0.05 M Na2SO4, pH = 3.0, j = 60 mA/cm2, O2 flow rate = 80 mL/min, T = 25 °C, stirring = 300 rpm). The efficiency of electro-Fenton oxidation is in good correlation with the generation of H2O2 by electrochemical reduction of O2 at carbon felt cathode. Higher H2O2 generation was achieved with electron-Fenton-BDD compared to electron-Fenton-Pt and anodic oxidation using Pt and BDD anodes. The most cost-effective H2O2 generation was obtained at pH = 3, j = 60 mA/cm2, O2 flow rate = 80 mL/min using carbon felt cathode and BDD anode. The kinetics of CLQ depletion follows a pseudo-first order reaction for all the operational conditions. The rate constant decreases with the increase of CLQ concentration and pH; however it increases with the increase of current density, and Fe2+ dose. The increase of current density up 60 mA/cm2 enhances CLQ degradation, but higher current densities than 60 mA/cm2 increases the specific electrical charge consumption. The addition of 10 mg/L Fe2+ was optimal to deplete CLQ in a reasonable time and without formation of Fe(OH)3 precipitate. HPLC analysis identified some of CLQ degradation intermediates including CQLA as an aromatic intermediate and OMA and OAA as carboxylic acids. The mineralization of CLQ drug was confirmed by the release of chloride and inorganic nitrogen ions (nitrates and ammonium). CLQ degradation by electro-Fenton oxidation involves several oxidation pathways including the mediated oxidation by HO• radicals produced in solution by catalytic decomposition of H2O2 with Fe2+, the mediated oxidation by HO• and sulfate radicals electrogenerated at the surface of BDD anode, mediated oxidation by strong oxidants generated by anodic oxidation of electrolyte (persulfates), and direct electrochemical oxidation of CLQ and its intermediates at the surface of BDD anode.
CRediT authorship contribution statement
Sondos Midassi: Investigation, Data curation, Writing - original draft. Ahmed Bedoui: Data curation, Supervision, Resources, Project administration. Nasr Bensalah: Conceptualization, Supervision, Validation, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Shane Snyder
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2020.127558.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ahmad I., Ahmed S., Anwar Z., Sheraz M.A., Sikorski M. Photostability and photostabilization of drugs and drug products. Int. J. Photoenergy. 2016 doi: 10.1155/2016/8135608. [DOI] [Google Scholar]
- Ahmed B., Limem E., Abdel-Wahab A., Nasr B. Photo-Fenton treatment of actual agro-industrial wastewaters. Ind. Eng. Chem. Res. 2011 doi: 10.1021/ie200266d. [DOI] [Google Scholar]
- Ahmed B., Mohamed H., Limem E., Nasr B. Degradation and mineralization of organic pollutants contained in actual pulp and paper mill wastewaters by a UV/H 2O 2 process. Ind. Eng. Chem. Res. 2009 doi: 10.1021/ie801755u. [DOI] [Google Scholar]
- Asghar A., Raman A.A.A., Daud W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: a review. J. Clean. Prod. 2015 doi: 10.1016/j.jclepro.2014.09.010. [DOI] [Google Scholar]
- Asghar A., Raman A.A.A., Daud W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: a review. J. Clean. Prod. 2015 doi: 10.1016/j.jclepro.2014.09.010. [DOI] [Google Scholar]
- Babuponnusami A., Muthukumar K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014 doi: 10.1016/j.jece.2013.10.011. [DOI] [Google Scholar]
- Babuponnusami A., Muthukumar K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014 doi: 10.1016/j.jece.2013.10.011. [DOI] [Google Scholar]
- Badmus K.O., Tijani J.O., Massima E., Petrik L. Treatment of persistent organic pollutants in wastewater using hydrodynamic cavitation in synergy with advanced oxidation process. Environ. Sci. Pollut. Res. 2018 doi: 10.1007/s11356-017-1171-z. [DOI] [PubMed] [Google Scholar]
- Bensalah N., Bedoui A., Chellam S., Abdel-Wahab A. Electro-Fenton treatment of photographic processing wastewater. Clean. 2013;41 doi: 10.1002/clen.201200521. [DOI] [Google Scholar]
- Bensalah N., Chair K., Bedoui A. Efficient degradation of tannic acid in water by UV/H2O2 process. Sustain. Environ. Res. 2018 doi: 10.1016/j.serj.2017.04.004. [DOI] [Google Scholar]
- Bensalah N., Dbira S., Bedoui A. The contribution of mediated oxidation mechanisms in the electrolytic degradation of cyanuric acid using diamond anodes. J. Environ. Sci. (China) 2015;45 doi: 10.1016/j.jes.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Boczkaj G., Fernandes A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: a review. Chem. Eng. J. 2017 doi: 10.1016/j.cej.2017.03.084. [DOI] [Google Scholar]
- Bokare A.D., Choi W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard Mater. 2014 doi: 10.1016/j.jhazmat.2014.04.054. [DOI] [PubMed] [Google Scholar]
- Borràs N., Arias C., Oliver R., Brillas E. Anodic oxidation, electro-Fenton and photoelectro-Fenton degradation of cyanazine using a boron-doped diamond anode and an oxygen-diffusion cathode. J. Electroanal. Chem. 2013 doi: 10.1016/j.jelechem.2012.11.012. [DOI] [Google Scholar]
- Brillas E., Martınez-Huitle C.A. Applied Catalysis B : environmental Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods : a general review. Appl. Catal. B Environ. 2009 doi: 10.1016/j.apcatb.2008.09.017. [DOI] [Google Scholar]
- Brillas E., Sirés I., Oturan M.A. Electro-fenton process and related electrochemical technologies based on fenton’s reaction chemistry. Chem. Rev. 2009 doi: 10.1021/cr900136g. [DOI] [PubMed] [Google Scholar]
- Cheng M., Zeng G., Huang D., Lai C., Xu P., Zhang C., Liu Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: a review. Chem. Eng. J. 2016 doi: 10.1016/j.cej.2015.09.001. [DOI] [Google Scholar]
- Clarizia L., Russo D., Di Somma I., Marotta R., Andreozzi R. Homogeneous photo-Fenton processes at near neutral pH: a review. Appl. Catal. B Environ. 2017 doi: 10.1016/j.apcatb.2017.03.011. [DOI] [Google Scholar]
- Coelho A.S., Chagas C.E.P., de Pádua R.M., Pianetti G.A., Fernandes C. A comprehensive stability-indicating HPLC method for determination of chloroquine in active pharmaceutical ingredient and tablets: identification of oxidation impurities. J. Pharmaceut. Biomed. Anal. 2017 doi: 10.1016/j.jpba.2017.06.023. [DOI] [PubMed] [Google Scholar]
- Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020 doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbira S., Bedoui A., Bensalah N. Investigations on the degradation of triazine herbicides in water by photo-fenton process. Am. J. Anal. Chem. 2014 doi: 10.4236/ajac.2014.58059. [DOI] [Google Scholar]
- Dbira S., Bensalah N., Bedoui A., Cañizares P., Rodrigo M.A. Treatment of synthetic urine by electrochemical oxidation using conductive-diamond anodes. Environ. Sci. Pollut. Res. 2015 doi: 10.1007/s11356-014-3831-6. [DOI] [PubMed] [Google Scholar]
- de Freitas Araújo K.C., da Silva D.R., dos Santos E.V., Varela H., Martínez-Huitle C.A. Investigation of persulfate production on BDD anode by understanding the impact of water concentration. J. Electroanal. Chem. 2020 doi: 10.1016/j.jelechem.2020.113927. [DOI] [Google Scholar]
- Deng Y., Zhao R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Reports. 2015 doi: 10.1007/s40726-015-0015-z. [DOI] [Google Scholar]
- Devaux C.A., Rolain J.-M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddaga S., Peddakonda R. Chloroquine-N-oxide, a major oxidative degradation product of chloroquine: identification, synthesis and characterization. J. Pharmaceut. Biomed. Anal. 2013 doi: 10.1016/j.jpba.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Eisenberg G.M. Colorimetric determination of hydrogen peroxide. Ind. Eng. Chem. - Anal. Ed. 1943 doi: 10.1021/i560117a011. [DOI] [Google Scholar]
- El-Ghenymy A., Centellas F., Rodríguez R.M., Cabot P.L., Garrido J.A., Sirés I., Brillas E. Comparative use of anodic oxidation, electro-Fenton and photoelectro-Fenton with Pt or boron-doped diamond anode to decolorize and mineralize Malachite Green oxalate dye. Electrochim. Acta. 2015 doi: 10.1016/j.electacta.2015.09.078. [DOI] [Google Scholar]
- El-Ghenymy A., Garrido J.A., Centellas F., Arias C., Cabot P.L., Rodríguez R.M., Brillas E. Electro-fenton and photoelectro-fenton degradation of sulfanilic acid using a boron-doped diamond anode and an air diffusion cathode. J. Phys. Chem. 2012 doi: 10.1021/jp300442y. [DOI] [PubMed] [Google Scholar]
- Escalona-Durán F., Ribeiro da Silva D., Martínez-Huitle C.A., Villegas-Guzman P. The synergic persulfate-sodium dodecyl sulfate effect during the electro-oxidation of caffeine using active and non-active anodes. Chemosphere. 2020 doi: 10.1016/j.chemosphere.2020.126599. [DOI] [PubMed] [Google Scholar]
- Frosch A.E., Venkatesan M., Laufer M.K. Patterns of chloroquine use and resistance in sub-Saharan Africa: a systematic review of household survey and molecular data. Malar. J. 2011 doi: 10.1186/1475-2875-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst D.E. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus. 1996 doi: 10.1177/0961203396005001041. [DOI] [PubMed] [Google Scholar]
- Gągol M., Przyjazny A., Boczkaj G. Wastewater treatment by means of advanced oxidation processes based on cavitation – a review. Chem. Eng. J. 2018 doi: 10.1016/j.cej.2018.01.049. [DOI] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020 doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gao Y., Zhu W., Wang C., Zhao X., Shu M., Zhang J., Bai H. Enhancement of oxygen reduction on a newly fabricated cathode and its application in the electro-Fenton process. Electrochim. Acta. 2020 doi: 10.1016/j.electacta.2019.135206. [DOI] [Google Scholar]
- Garcia-Segura S., Brillas E. Mineralization of the recalcitrant oxalic and oxamic acids by electrochemical advanced oxidation processes using a boron-doped diamond anode. Water Res. 2011 doi: 10.1016/j.watres.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Gligorovski S., Strekowski R., Barbati S., Vione D. Environmental implications of hydroxyl radicals (•OH) Chem. Rev. 2015 doi: 10.1021/cr500310b. [DOI] [PubMed] [Google Scholar]
- Gong Y., Li J., Zhang Y., Zhang M., Tian X., Wang A. Partial degradation of levofloxacin for biodegradability improvement by electro-Fenton process using an activated carbon fiber felt cathode. J. Hazard Mater. 2016 doi: 10.1016/j.jhazmat.2015.10.064. [DOI] [PubMed] [Google Scholar]
- Groenen Serrano K. Electrochemical Water and Wastewater Treatment. 2018. Indirect electrochemical oxidation using hydroxyl radical, active chlorine, and peroxodisulfate. [DOI] [Google Scholar]
- Guinea E., Garrido J.A., Rodríguez R.M., Cabot P.L., Arias C., Centellas F., Brillas E. Degradation of the fluoroquinolone enrofloxacin by electrochemical advanced oxidation processes based on hydrogen peroxide electrogeneration. Electrochim. Acta. 2010 doi: 10.1016/j.electacta.2009.11.040. [DOI] [Google Scholar]
- Hou C., Shen J., Jiang X., Zhang D., Sun X., Li J., Han W., Liu X., Wang L. Enhanced anoxic biodegradation of pyridine coupled to nitrification in an inner loop anoxic/oxic-dynamic membrane bioreactor (A/O-DMBR) Bioresour. Technol. 2018 doi: 10.1016/j.biortech.2018.07.105. [DOI] [PubMed] [Google Scholar]
- Howard B. XPharm: the Comprehensive Pharmacology Reference. 2007. Hydroxychloroquine. [DOI] [Google Scholar]
- Huong Le T.X., Bechelany M., Cretin M. Carbon felt based-electrodes for energy and environmental applications: a review. Carbon N. Y. 2017 doi: 10.1016/j.carbon.2017.06.078. [DOI] [Google Scholar]
- Jiang C., Pang S., Ouyang F., Ma J., Jiang J. A new insight into Fenton and Fenton-like processes for water treatment. J. Hazard Mater. 2010 doi: 10.1016/j.jhazmat.2009.09.125. [DOI] [PubMed] [Google Scholar]
- Kanakaraju D., Glass B.D., Oelgemöller M. Advanced oxidation process-mediated removal of pharmaceuticals from water: a review. J. Environ. Manag. 2018 doi: 10.1016/j.jenvman.2018.04.103. [DOI] [PubMed] [Google Scholar]
- Karim E.I.A., Ibrahim K.E.E., Abdelrahman A.N., Fell A.F. Photodegradation studies on chloroquine phosphate by high-performance liquid chromatography. J. Pharmaceut. Biomed. Anal. 1994 doi: 10.1016/0731-7085(93)E0026-J. [DOI] [PubMed] [Google Scholar]
- Klassen N.V., Marchington D., McGowan H.C.E. H2O2 determination by the I3− method and by KMnO4 titration. Anal. Chem. 1994 doi: 10.1021/ac00090a020. [DOI] [Google Scholar]
- Lee S.J., Silverman E., Bargman J.M. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat. Rev. Nephrol. 2011 doi: 10.1038/nrneph.2011.150. [DOI] [PubMed] [Google Scholar]
- Marselli B., Garcia-Gomez J., Michaud P., Rodrigo M.A., Comninellis C. Electrogeneration of hydroxyl radicals on boron-doped diamond electrodes. J. Electrochem. Soc. 2003 doi: 10.1149/1.1553790. [DOI] [Google Scholar]
- Martínez-Huitle C.A., Panizza M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018 doi: 10.1016/j.coelec.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Michaud P.A., Mahé E., Haenni W., Perret A., Comnineiiis C. Preparation of peroxodisulfuric acid using boron-doped diamond thin film electrodes. Electrochem. Solid State Lett. 2000 doi: 10.1149/1.1390963. [DOI] [Google Scholar]
- Michaud P.A., Panizza M., Ouattara L., Diaco T., Foti G., Comninellis C. Electrochemical oxidation of water on synthetic boron-doped diamond thin film anodes. J. Appl. Electrochem. 2003 doi: 10.1023/A:1024084924058. [DOI] [Google Scholar]
- Miklos D.B., Remy C., Jekel M., Linden K.G., Drewes J.E., Hübner U. Evaluation of advanced oxidation processes for water and wastewater treatment – a critical review. Water Res. 2018 doi: 10.1016/j.watres.2018.03.042. [DOI] [PubMed] [Google Scholar]
- Moreira F.C., Garcia-Segura S., Vilar V.J.P., Boaventura R.A.R., Brillas E. Decolorization and mineralization of Sunset Yellow FCF azo dye by anodic oxidation, electro-Fenton, UVA photoelectro-Fenton and solar photoelectro-Fenton processes. Appl. Catal. B Environ. 2013 doi: 10.1016/j.apcatb.2013.03.023. [DOI] [Google Scholar]
- Nasr B., Abdellatif G., Cañizares P., Sáez C., Lobato J., Rodrigo M.A. Electrochemical oxidation of hydroquinone, resorcinol, and catechol on boron-doped diamond anodes. Environ. Sci. Technol. 2005 doi: 10.1021/es0500660. [DOI] [PubMed] [Google Scholar]
- Nord K., Karlsen J., Tønnesen H.H. Photochemical stability of biologically active compounds. IV. Photochemical degradation of chloroquine. Int. J. Pharm. 1991 doi: 10.1016/0378-5173(91)90375-X. [DOI] [Google Scholar]
- Özcan A., Şahin Y., Savaş Koparal A., Oturan M.A. Carbon sponge as a new cathode material for the electro-Fenton process: comparison with carbon felt cathode and application to degradation of synthetic dye basic blue 3 in aqueous medium. J. Electroanal. Chem. 2008 doi: 10.1016/j.jelechem.2008.01.002. [DOI] [Google Scholar]
- Panizza M., Brillas E., Comninellis C. Application of boron-doped diamond electrodes for wastewater treatment. J. Environ. Eng. Manag. 2008 [Google Scholar]
- Peralta E., Roa G., Hernandez-Servin J.A., Romero R., Balderas P., Natividad R. Hydroxyl Radicals quantification by UV spectrophotometry. Electrochim. Acta. 2014 doi: 10.1016/j.electacta.2014.02.047. [DOI] [Google Scholar]
- Pereira G.F., El-Ghenymy A., Thiam A., Carlesi C., Eguiluz K.I.B., Salazar-Banda G.R., Brillas E. Effective removal of Orange-G azo dye from water by electro-Fenton and photoelectro-Fenton processes using a boron-doped diamond anode. Separ. Purif. Technol. 2016 doi: 10.1016/j.seppur.2016.01.029. [DOI] [Google Scholar]
- Pham A.L.T., Doyle F.M., Sedlak D.L. Kinetics and efficiency of H2O2 activation by iron-containing minerals and aquifer materials. Water Res. 2012 doi: 10.1016/j.watres.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatello J.J., Oliveros E., MacKay A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006 doi: 10.1080/10643380500326564. [DOI] [Google Scholar]
- Pinheiro V.S., Paz E.C., Aveiro L.R., Parreira L.S., Souza F.M., Camargo P.H.C., Santos M.C. Mineralization of paracetamol using a gas diffusion electrode modified with ceria high aspect ratio nanostructures. Electrochim. Acta. 2019 doi: 10.1016/j.electacta.2018.10.097. [DOI] [Google Scholar]
- Price R.N., von Seidlein L., Valecha N., Nosten F., Baird J.K., White N.J. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect. Dis. 2014 doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice E.W., Baird R.B., Eaton A.D. 4500-Cl chlorine (residual). Stand. Methods exam. Water wastewater. 2017. [DOI]
- Ruiz E.J., Hernández-Ramírez A., Peralta-Hernández J.M., Arias C., Brillas E. Application of solar photoelectro-Fenton technology to azo dyes mineralization: effect of current density, Fe2+ and dye concentrations. Chem. Eng. J. 2011 doi: 10.1016/j.cej.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020 doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- Serrano K., Michaud P.A., Comninellis C., Savall A. Electrochemical preparation of peroxodisulfuric acid using boron doped diamond thin film electrodes. Electrochim. Acta. 2002 doi: 10.1016/S0013-4686(02)00688-6. [DOI] [Google Scholar]
- Shemer H., Linden K.G. Degradation and by-product formation of diazinon in water during UV and UV/H2O2 treatment. J. Hazard Mater. 2006 doi: 10.1016/j.jhazmat.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Singh R., Adhikari D.R., Patil B.P., Talathi N.R., Hanamshetti S.R., Joshi R.M. Amoebic liver abscess: an appraisal. Int. Surg. 2011 doi: 10.9738/CC9.1. [DOI] [PubMed] [Google Scholar]
- Singh S., Chaudhary P., Saxena N., Khandelwal S., Poddar D.D., Biswal U.C. Treatment of liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Ann. Gastroenterol. 2013 [PMC free article] [PubMed] [Google Scholar]
- Tayo L.L., Caparanga A.R., Doma B.T., Liao C.H. A review on the removal of pharmaceutical and personal care products (PPCPs) using advanced oxidation processes. J. Adv. Oxid. Technol. 2018 doi: 10.26802/jaots.2017.0079. [DOI] [Google Scholar]
- Teymori M., Khorsandi H., Aghapour A.A., Jafari S.J., Maleki R. Electro-Fenton method for the removal of Malachite Green: effect of operational parameters. Appl. Water Sci. 2020 doi: 10.1007/s13201-019-1123-5. [DOI] [Google Scholar]
- Vallejo M., Fresnedo San Román M., Ortiz I., Irabien A. Overview of the PCDD/Fs degradation potential and formation risk in the application of advanced oxidation processes (AOPs) to wastewater treatment. Chemosphere. 2015 doi: 10.1016/j.chemosphere.2014.05.077. [DOI] [PubMed] [Google Scholar]
- Wang J.L., Xu L.J. Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012 doi: 10.1080/10643389.2010.507698. [DOI] [Google Scholar]
- Wang Y., Zhou W., Gao J., Ding Y., Kou K. Oxidative modification of graphite felts for efficient H2O2 electrogeneration: enhancement mechanism and long-term stability. J. Electroanal. Chem. 2019 doi: 10.1016/j.jelechem.2018.11.051. [DOI] [Google Scholar]
- Xiang Q., Yu J., Wong P.K. Quantitative characterization of hydroxyl radicals produced by various photocatalysts. J. Colloid Interface Sci. 2011 doi: 10.1016/j.jcis.2011.01.093. [DOI] [PubMed] [Google Scholar]
- Yang W., Zhou M., Cai J., Liang L., Ren G., Jiang L. Ultrahigh yield of hydrogen peroxide on graphite felt cathode modified with electrochemically exfoliated graphene. J. Mater. Chem. 2017 doi: 10.1039/c7ta01534h. [DOI] [Google Scholar]
- Yu F., Zhou M., Yu X. Cost-effective electro-Fenton using modified graphite felt that dramatically enhanced on H2O2 electro-generation without external aeration. Electrochim. Acta. 2015 doi: 10.1016/j.electacta.2015.02.166. [DOI] [Google Scholar]
- Yu F., Zhou M., Zhou L., Peng R. A novel electro-fenton process with H2O2 generation in a rotating disk reactor for organic pollutant degradation. Environ. Sci. Technol. Lett. 2014 doi: 10.1021/ez500178p. [DOI] [Google Scholar]
- Zhang Z., Meng H., Wang Y., Shi L., Wang X., Chai S. Fabrication of graphene@graphite-based gas diffusion electrode for improving H2O2 generation in Electro-Fenton process. Electrochim. Acta. 2018 doi: 10.1016/j.electacta.2017.11.048. [DOI] [Google Scholar]
- Zhou L., Hu Z., Zhang C., Bi Z., Jin T., Zhou M. Electrogeneration of hydrogen peroxide for electro-Fenton system by oxygen reduction using chemically modified graphite felt cathode. Separ. Purif. Technol. 2013 doi: 10.1016/j.seppur.2013.03.038. [DOI] [Google Scholar]
- Zöllig H., Fritzsche C., Morgenroth E., Udert K.M. Direct electrochemical oxidation of ammonia on graphite as a treatment option for stored source-separated urine. Water Res. 2015 doi: 10.1016/j.watres.2014.11.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.