Abstract
Background:
Firefighting foam–contaminated ground water, which contains high levels of perfluoroalkyl substances (PFAS), is frequently found around airports. In 2018 it was detected that employees at a municipal airport in northern Sweden had been exposed to high levels of short-chain PFAS along with legacy PFAS (i.e., PFOA, PFHxS, and PFOS) through drinking water.
Objectives:
In this study, we aimed to describe the PFAS profile in drinking water and biological samples (paired serum and urine) and to estimate serum half-lives of the short-chain PFAS together with legacy PFAS.
Methods:
Within 2 weeks after provision of clean water, blood sampling was performed in all 26 airport employees. Seventeen of them were then followed up monthly for 5 months. PFHxA, PFHpA, PFBS, PFPeS, and PFHpS together with legacy PFAS in water and biological samples were quantified using LC/MS/MS. Half-lives were estimated by assuming one compartment, first-order elimination kinetics.
Results:
The proportions of PFHxA, PFHpA, and PFBS were higher in drinking water than in serum. The opposite was found for PFHxS and PFOS. The legacy PFAS accounted for about 50% of total PFAS in drinking water and 90% in serum. Urinary PFAS levels were very low compared with serum. PFBS showed the shortest half-life {average 44 d [95% confidence interval (CI): 37, 55 d]}, followed by PFHpA [62 d (95% CI: 51, 80 d)]. PFPeS and PFHpS showed average half-lives as 0.63 and 1.46 y, respectively. Branched PFOS isomers had average half-lives ranging from 1.05 to 1.26 y for different isomers. PFOA, PFHxS, and linear PFOS isomers showed average half-lives of 1.77, 2.87, and 2.93 y, respectively.
Discussion:
A general pattern of increasing half-lives with increasing chain length was observed. Branched PFOS isomers had shorter half-lives than linear PFOS isomers. https://doi.org/10.1289/EHP6785
Introduction
Perfluoroalkyl substances (PFAS) are persistent man-made chemicals. Drinking-water sources have been contaminated around production facilities, airports, and air force bases worldwide, affecting employees drinking the polluted water as well as the general population in adjacent communities (Domingo et al. 2012; Fromme et al. 2009). Efforts have been made to reduce the emission of PFAS into the environment through the phase-out of most long-chain PFAS, especially perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) and related compounds, by voluntary and regulatory actions over the last two decades. Among the PFAS, long-chain homologs of perfluorinated carboxylic acids (PFCAs) have eight or more carbons in the carbon chain, and among perfluorinated sulfonic acids (PFSAs), a carbon chain length of six carbons or more (ITRC 2020). Due to their persistence and bioaccumulation capability, however, PFAS exposure and their associated potential toxic effects will exist long term. To address potential adverse health effects, exposure, epidemiology, and experimental studies have mainly focused on the long-chain legacy PFOA and PFOS (EFSA 2018). Less is known about short-chain PFAS and more complex structures that are now suggested as replacements by industry due to their lower bioaccumulation potential (Buck et al. 2011). There are very little data on human exposure levels and health effects of short-chain PFAS.
Serum elimination half-lives of PFOA, PFOS, and perfluorohexane sulfonic acid (PFHxS) have been estimated in a general Swedish population with high PFAS exposure through drinking water when biomonitoring started 6 months after clean water was provided (Li et al. 2018). The average half-lives were estimated to be 2.7 y for PFOA, 3.4 y for PFOS, and 5.3 y for PFHxS, with marked interindividual variation (Li et al. 2018). The estimates were in the same range as reported by others (Brede et al. 2010; Olsen et al. 2007; Worley et al. 2017). To date, studies on the serum half-lives of short-chain PFAS are very scarce. Olsen et al. (2009) reported 25.8 d as a half-life of perfluorobutane sulfonic acid (PFBS, C4) based on data from 7 occupationally exposed workers followed up to 180 d. For perfluorohexanoic acid (PFHxA, C6) and perfluoroheptanoic acid (PFHpA, C7), the apparent elimination half-lives were reported to be 14–49 d (Russell et al. 2013) and 70 d (Russell et al. 2015a) in 11 occupationally exposed subjects. A more rapid elimination of shorter-chain PFAS has been attributed to weaker affinities for renal transport proteins responsible for reabsorption and higher water solubility (Han et al. 2012).
Arvidsjaur, a small municipality in the northern part of Sweden, has one municipal waterworks for residential drinking-water supply. The regional airport, however, has its own water supply (Figure 1). In mid-August 2018, PFAS for the first time was included in the regular water quality surveillance. A first report obtained on 31 August 2018 indicated that the drinking water was contaminated with PFAS at both the municipality waterworks and at the airport. Warnings to not drink or cook with tap water were immediately issued and clean water from tanks was immediately supplied. However, confirmatory samples from 3 September 2018 showed that only the airport drinking water was contaminated and that the municipal drinking water had very low levels of PFAS (Table 1).
Figure 1.
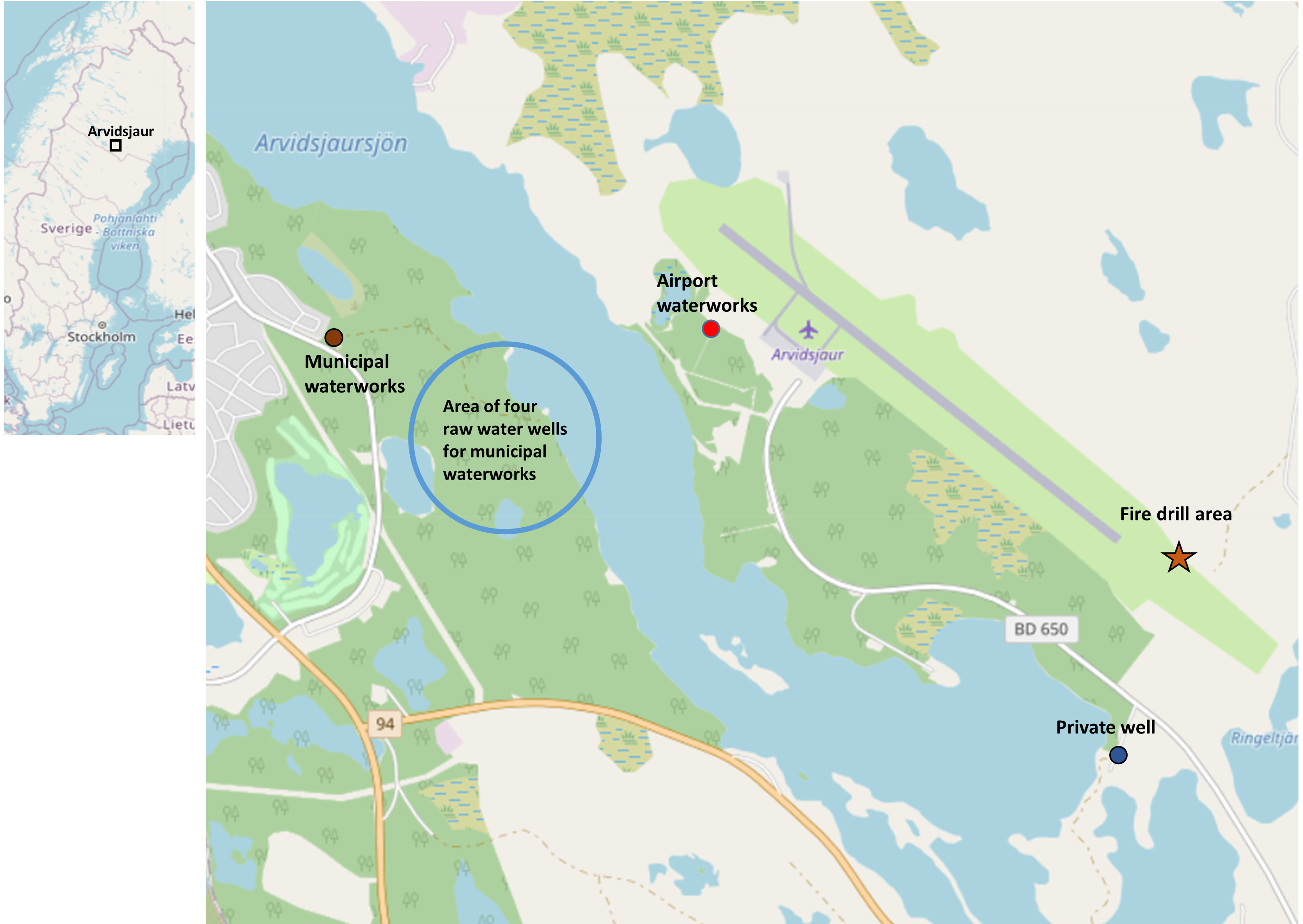
Map of Arvidsjaur municipality, Sweden, and the regional airport, with information of fire drill area, two waterworks, the private well, and area of four raw-water wells for municipal waterworks. Background map data: © OpenStreetMap contributors, CC BY-SA.
Table 1.
Levels of measured PFAS in the airport drinking water, municipal drinking water, and from the private well.
| PFAS (chain length) | Airport drinking water (ng/L)a | Municipal drinking water (ng/L)a | Drinking water from private wella |
|---|---|---|---|
| Perfluorinated carboxylic acids (PFCAs) | |||
| PFBA (C4) | 60 | 2.2 | |
| PFPeA (C5) | 180 | 0.63 | |
| PFHxA (C6) | 330 | 0.84 | |
| PFHpA (C7) | 97 | 0.33 | |
| Linear PFOA (C8) | 210 | 0.53 | |
| Branched PFOA (C8) | 88 | ||
| Total PFOA (C8) | 300 | 0.53 | |
| PFNA (C9)b | |||
| PFDA (C10)b | |||
| PFUnDA (C11)b | |||
| PFDoDA (C12)b | |||
| Perfluorinated sulfonic acids (PFSAs) | |||
| PFBS (C4) | 200 | 0.5 | |
| PFPeS (C5) | 180 | ||
| PFHxS (C6) | 710 | 0.32 | 4.6 |
| PFHpS (C7) | 16 | ||
| Linear PFOS (C8) | 62 | 1.7 | |
| Branched PFOS (C8) | 64 | 0.24 | |
| Total PFOS (C8) | 130 | 1.9 | |
| Fluorotelomer sulfonic acid | |||
| [6:2 FTS (C6)] | 6.6 | ||
| Sum of 11 PFASc | 2,000 | 12 | |
Note: PFAS, perfluoroalkyl substances; PFBA, perfluorobutanoic acid; PFBS, perfluorobutane sulfonic acid; PFDA, perfluorodecanoic acid; PFDoDA, perfluorododecanoic acid; PFHpA, perfluoroheptanoic acid; PFHpS, perfluoroheptane sulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFPeA, perfluoropentanoic acid; PFOS, perfluorooctane sulfonic acid; PFPeS, perfluoropentane sulfonic acid; PFUnDA, perfluoroundecanoic acid; 6:2 FTS, fluorotelomer sulfonic acid.
The data were generated from the reports of Arvidsjaur municipality, updated, and shared with authors in December 2019. Multiple water samples were taken at different locations from 31 August to 3 September 2018. We reported results from water samples obtained on 3 September 2018.
Levels of PFNA, PFDA, PFUnDA and PFDoDA were below detection levels and not further included in the statistical analysis in this paper.
Sum of 11 PFAS: PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFBS, PFHxS, PFOS, 6:2 FTS.
The source of contamination of the airport drinking water was assumed to be from firefighting foam. Notably high concentrations of short-chain PFAS, such as perfluorobutanoic acid (PFBA), perfluoropentanoic acid (PFPeA), PFHxA, PFBS, and perfluoropentane sulfonic acid (PFPeS), were found in addition to the legacy PFOA, PFHxS, and PFOS.
Our research group was contacted by the Arvidsjaur municipality. Biomonitoring of all employees at the airport was initiated 11 d after PFAS-free drinking water was supplied to the airport. This population was considered highly relevant for a study on the elimination of short-chain PFAS because the subjects were recruited almost immediately after the abrupt end of the exposure.
In the present study, we aimed to a) describe the PFAS profile in drinking water as well as in paired serum and urine samples, and b) estimate serum half-lives of short-chain PFAS including PFHxA, PFHpA, PFBS, and PFPeS and the long-chain perfluoroheptane sulfonic acid (PFHpS), along with legacy PFOA, PFHxS, and PFOS, but distinguishing between linear and branched PFOS.
Material and Methods
Study Setting
The regional airport in Arvidsjaur was established in 1990. During a few years in the early 2000s, pilots were trained at the airfield and the number of yearly landings rose to 20,000. Thereafter, the traffic was reduced markedly and has stabilized to around 1,200 landings per year. The fire drill area at the airfield is used by the airport fire-fighting staff and by the municipal fire brigade. No accidents with fires have occurred at the airport.
Immediately after the drinking-water contamination was revealed on 31 August 2018, clean bottled water was supplied to the employees and passengers at the airport until granular activated carbon filters were installed for tap water filtration after about 2 months. Drinking-water quality was regularly checked afterward, and no elevated levels of PFAS were further noticed.
Study Population
All 26 employees at the airport were invited for a blood sampling between 11 and 14 September 2018 (i.e., within 11 to 14 d after the termination of contaminated drinking-water exposure). Information on age, home address, employment history, working tasks, and sick leave and vacation days in August and September were collected by questionnaire. In addition, data on the number of glasses of water consumed per day, local fish consumption, and history of blood donation and medication were collected. For female employees, questions about menstruation, pregnancy, and duration of breast-feeding were asked.
Because municipal drinking water did not show elevated PFAS levels (Table 1), we are confident that there was no longer ongoing drinking-water exposure at home as long as people had no other source of drinking water. Based on the linkage of self-reported home address and municipal waterworks information, we were able to identify private wells. One airport employee living in the vicinity of the airport had a private well (Figure 1) with low PFAS levels in the water (Table 1).
The PFAS analysis in serum from the first sampling was immediately performed and personal results were reported to the employees. Most of the employees showed elevated serum PFAS levels, especially for short-chain PFAS. Twenty-one employees with PFBS level above the limit of detection (LOD) were invited for a 4-month follow-up (from 16 October 2018 to 22 January 2019) and 17 volunteered. These 17 employees submitted four rounds of blood and first morning urine samples in parallel, at 1-month intervals. In total, there were five rounds of serum sampling (started from September) and four rounds of urine sampling (from October). For modeling of elimination, all samples were reanalyzed in a single batch. We obtained 83 serum samples with a median count of 5 (range: 4–5) samples per employee, and 59 urine samples with median of 4 (range: 1–4) samples per employee. Only one employee provided a single urine sample. The study was approved by the Regional Ethical Review Board in Lund, Sweden. All employees provided written informed consent.
For comparison with Swedish general background PFAS serum levels, we referred to the PFAS levels observed in a reference population from Karlshamn, a municipality in southern Sweden without PFAS contamination in the municipal drinking water. In 2016, 226 individuals were sampled. Fifty-nine individuals were randomly selected for analyzing other short-chain PFAS than the legacy ones, using the same modified method as for the airport employees. Fifty-eight individuals within the same age range as the airport employees (i.e., ) were included in the comparison. For a detailed description of this population see Li et al. (2018).
Chemical Analysis
PFAS analysis in drinking water.
Drinking-water sampling and analyses were performed by a commercial water analysis company, SYNLAB Analytics & Services (SYNLAB). The first water samplings of outgoing drinking water from the municipal waterworks and from the airport waterworks were performed on 15 August 2018. High levels of PFAS in water samples from both locations were reported on 31 August 2018. Resampling was immediately performed at different locations from 31 August to 3 September 2018, revealing that only the airport drinking water was contaminated while the municipal outgoing drinking water had a sum of 11 PFAS [i.e., PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFBS, PFHxS, PFOS, fluorotelomer sulfonic acid (6:2FTS)] that was (Table 1). The reasons for the mistake on 15 August 2018 were never clarified. For the locations of water sampling, see Figure 1. In all, there were five separate water samples from the municipal raw and outgoing drinking water and six samples from the airport during this 2.5-week period. We here report results from the water samples obtained on 3 September 2018.
PFAS analysis in drinking water was performed according to German standard methods for determination of selected polyfluorinated compounds in water using liquid chromatography–tandem mass spectrometry (LC/MS/MS) after solid–liquid extraction (F 42) (DIN 38407-42) (German Institute for Standardization 2011). SYNLAB is accredited by SWEDAC (the national accreditation body of Sweden) according to SS-EN ISO/IEC 17025. At our request, SNYLAB expanded their report from the original 11 PFAS to 15 PFAS in December 2019. The 15 PFAS were PFBA, PFPeA, PFHxA, PFHpA, PFOA, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA); perfluoroundecanoic acid (PFUnDA), perfluorododecanoic acid (PFDoDA), PFBS, PFPeS, PFHxS, PFHpS, PFOS, and 6:2 FTS. The LODs for PFAS in water sample are listed in Table S3.
PFAS analysis in serum and urine samples.
Venous whole blood samples were obtained and were centrifuged for 10 min at at room temperature to isolate the serum on site. The serum samples were collected in BD Vacutainer® Plus plastic serum tubes without gel (BD). Urine samples were collected in polypropylene screw cap tubes (Sarstedt). Serum and urine samples were frozen at after sampling and transported to Lund using cold chain logistics. Serum and urine samples were stored at and , respectively, before analysis.
Chemicals.
An overview of the PFAS analytes and internal standards with abbreviations is given in Table S1. All native and isotopically labeled standards (PFAC-MXC, MPFAC-C-ES, P1MHpS, P5MHpS, and P6MHpS) were purchased from Wellington Laboratories as diluted reference standards. PFAC-MXC was used for the native PFC stock solution. Stable isotope–labeled PFC Standards Solution MPFAC-C-ES was used for internal standards. To quantify the branched PFOS, methanol solutions of sodium perfluoro-1-methylheptane sulfonate (P1MHpS; 1m-PFOS), sodium perfluoro-5-methylheptane sulfonate (P5MHpS; 5m-PFOS), and sodium perfluoro-6-methylheptane sulfonate (P6MHpS; 6m-PFOS) were used.
Acetonitrile (high-performance liquid chromatography grade), ammonium acetate and methanol (LC-MS grade) were purchased from Merck. Water was purchased from a Milli-Q Integral 5 system (Millipore).
Instrumentation.
Quantitative analysis was conducted using triple quadrupole linear ion trap mass spectrometer equipped with TurboIonSpray sources (QTRAP ; AB Sciex) coupled to an LC/MS/MS system (UFLCXR; Shimadzu Corporation). Nitrogen was used as the nebulizer, auxiliary, curtain, and collision gas. The MS analyses were carried out using selected reaction monitoring (SRM) in negative ion mode. The SRM parameters for the data acquisition are shown in Table S2. All data acquisition was performed using Analyst® software (version 1.6.3; AB Sciex), and data processing was performed using MultiQuant™ software (version 2.1; AB Sciex).
Sample preparation: Calibration standards, quality control samples and chemical blanks.
Standard solutions were prepared by further dilution of diluted reference standards in methanol. For the calibration standards, the blank matrix, fetal bovine serum, (FBS; Gibco) was used for the serum samples. Human normal urine obtained from the colleagues at our lab was used for the urine analysis. These were prepared in the same way as the samples, except for the addition of of the diluted standard solutions.
For serum, three quality control (QC) samples (QC1, QC2, and QC3) were prepared in-house. QC1 was prepared by pooling equal volumes of three serum samples containing different levels of PFAS. Similarly, QC2 was prepared using another three samples and pooled with equal volumes. QC3 was prepared with additional spiking with PFAS standard solutions. For urine, one QC sample was used, and the sample was prepared by pooling equal volumes of three urine samples from individuals with high levels of PFAS in serum.
Chemical blanks were prepared from Milli-Q water and were prepared in the same way as the samples. Two sets of each QC samples and three sets of chemical blanks were added to each batch, where one batch corresponded to a 96-well plate.
Sample preparation: Preparation of serum samples.
The serum samples, QC samples, and chemical blanks were prepared in 96-well plates with flat-bottom glass vials (Biotech solutions). Serum samples were thawed for 1–2 h and mixed on a vortex shaker for approximately 10 s two times. Once after all samples were thawed and a second time before pipetting the serum to the plate. Before pipetting, the tip was pre-wetted several times, drawing a volume into the tip and dispensing it back into the tube. Sample preparation procedures for serum samples consisted of serum mixed with water, methanol, isotopically labeled internal standards in 50% acetonitrile (see Table S1), and acetonitrile. Thereafter, samples were mixed vigorous by shaking for 30 min and centrifuged at for 10 min at room temperature. The supernatant () was transferred to 96-well plates with conical glass vials (MicroLiter Analytical Supplies) and again centrifuged at for 10 min before analysis. The QC samples and chemical blanks were prepared following the same procedure as the serum samples. The calibration standards were prepared in FBS the same way as the samples, except for the addition of diluted reference standards of all analyzed compounds in of the methanol solution.
Aliquots of of the sample were analyzed using LC/MS/MS. For the analysis, a C18 column (, , Genesis Lightning) was used prior to the injector to filter the mobile phases. The analytical column was an Aquity BEH (, Waters). The mobile phases (A and B) were A: ammonium acetate in water:methanol (50:50); and B: methanol. The mobile phase was kept at 0% B for 2.5 min after injection. A gradient was then applied over 11 min to 90% B, where it was then kept for 1.5 min. The column was then conditioned at 0% B for 2 min. A diverter valve was used, and the column effluent was diverted to the MS between 1.1 and 14.0 min. The flow rate was , and the column was maintained at 60°C. The analysis was performed in negative ion mode. The ion source temperature was at 350°C.
Sample prepration: Preparation of urine samples.
The urine samples, QC sample, and chemical blanks were prepared in 96-well plates with conical glass vials (MicroLiter Analytical Supplies). The samples were thawed 1–2 h, then mixed by vortex shaker and manual turning. Before pipetting, the tip was pre-wetted several times, drawing a volume into the tip and dispensing it back into the tube. For the urine samples, urine was mixed with of methanol and of a 50% acetonitrile solution containing isotopically labeled internal standards (see Table S1). Then samples were shaken vigorously for 30 min and centrifuged at for 10 min at room temperature. The QC sample and chemical blanks were prepared following the same procedure as the urine samples. Calibration standards were prepared in blank urine, the same way as the samples, except for the addition of diluted reference standards of all analyzed compounds in the of methanol solution.
Aliquots of of the sample were analyzed using LC/MS/MS. For the analysis, a C18 column (, , Genesis Lightning) was used prior to the injector to filter the mobile phases. The analytical column was an Aquity BEH (, Waters). The mobile phases were A: ammonium acetate in water:methanol (50:50); and B: methanol. The mobile phase was kept at 0% B for 2.5 min after injection. A gradient was then applied over 11 min to 90% B, where it was then kept for 1.5 min. The column was then conditioned at 0% B for 2 min. A diverter valve was used, and the column effluent was diverted to the MS between 1.1 and 14.0 min. The flow rate was , and the column was maintained at 60°C. The analysis was performed in negative ion mode. The ion source temperature was at 350°C.
Quantification.
The concentrations of PFAS were determined by peak area ratios between analyte and internal standard. The quantifier transitions are shown in Table S2. The total, nonisomer-specific compounds for all PFAS except for PFOS are reported. For the branched PFOS, 1m-PFOS could be separated from the other isomers and evaluated using a calibration curve of 1m-PFOS. Perfluoro-2/6-methylheptanesulfonate (2/6m-PFOS) could not be separated, thus the sum of 2/6m-PFOS was evaluated using a calibration curve of 6m-PFOS. The sum of perfluoro-3/4/5-methylheptanesulfonate (3/4/5m-PFOS) was evaluated using a calibration curve of 5m-PFOS.
The limits of detection (LODs) were determined as the concentrations corresponding to the average plus three times the standard deviation of the concentrations in chemical blank samples. The LODs for each PFAS in serum and urine samples, respectively, are shown in Table S3. The results of three QC samples used in serum analysis are listed in Table S4.
The analyses of PFOS and PFOA are part of a QC program between analytical laboratories coordinated by H. Drexler, Institute and Outpatient Clinic for Occupational, Social and Environmental Medicine, University of Erlangen-Nuremberg, Germany. The laboratory also participated in the European Human Biomonitoring Initiative (HBM4EU) QA/QC program, and its successful performance has resulted in its qualification as an HBM4EU laboratory for the analysis of PFPeA, PFHpA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFBS, PFHxS, PFHpS, and PFOS.
In this paper, we excluded PFPeA, PFNA, PFDA, PFUnDA, and PFDoDA from further analysis. The level of PFPeA was below LOD in all serum samples. The levels for the other PFAS were below LOD in the water sample, indicating that exposure through the drinking water was negligible, and therefore not relevant for half-life estimation in the present study.
Calculated Variables
The ratio of serum and water concentration for each PFAS (serum/water ratio) was calculated based on median serum level in the first serum sample obtained from all 26 airport employees divided by the drinking-water concentration. This ratio is an indicator of PFAS accumulation in body.
Urinary PFAS concentrations were specific-gravity adjusted. The ratio of urine and serum concentration for each PFAS (urine/serum ratio) at each sampling was calculated based on PFAS concentrations in paired serum and urine samples from 17 employees. Then the personal average urine/serum ratio was calculated using the mean value of repeated samplings for each employee.
Modeling of PFAS Elimination and Estimating of Half-Life
Serum PFAS concentrations were log-transformed. Time was calculated as the elapsed days between supply of clean bottled water and first blood sampling. We assumed one compartment, first-order elimination kinetics. In this kinetic model, the body is considered as one homogeneous volume in which mixing is instantaneous and from which PFAS could be absorbed, transferred, and eliminated according to the rate of elimination that is proportional to the concentration of PFAS in the body. Two methods were used to estimate serum elimination rate constant (k) and half-life for each PFAS:
Method 1: For estimation of the population average k, a linear mixed model was used as reported previously (Li et al. 2018) but without the random slope in the model. Briefly, in the linear mixed model
is the serum concentration of PFAS for individual i at sampling round j; is the subject-specific intercept (random intercept); is the time; is the average slope; is a vector of fixed covariates for individual i; stands for the fixed effect coefficients; and is the random error term. We modified the model by excluding the subject-specific slope (random slope) due to a failure to converge after inclusion, which may be due to the limited number of study subjects. The negative value of the average slope () is the population average k, and average half-life is calculated as .
In the linear mixed model, we included only age and sex as covariates. We based covariate selection on factors that could conceivably confound the PFAS half-life estimation. Working task, duration of employment, number of glasses of water per day, blood donation, and pregnancy before mitigation of PFAS exposure from drinking water can influence the initial serum levels but would not be expected to influence the PFAS half-life. Medication use was checked, and no one reported usage of cholestyramine [a medication that influences PFAS elimination (Genuis et al. 2010, 2013)], therefore, medication use was not included in the model.
A sensitivity analysis was performed using the same linear mixed model but with as the serum levels after subtracting general background levels (using the median levels obtained from the nonexposed reference population). If the serum PFAS levels after subtraction were lower than the half of the background levels, they were replaced with half of the background levels.
Method 2: For estimation of individual k, linear regression was used to derive the slope () of the logarithm of serum concentrations vs. time for each employee. The negative value of the slope () for each individual was equivalent to the individual k, and individual serum elimination half-life was calculated as for each PFAS. If was positive (i.e., PFAS concentrations tended to increase over the five samplings), the half-life was not calculated.
For comparison of PFAS initial levels between sexes, the Mann-Whitney U-test was used on original PFAS scales. All statistical analyses were performed in IBM SPSS (version 25.0; IBM).
Results
Basic Characteristics for the Study Population
Table 2 shows the basic characteristics and initial serum concentrations of PFAS for the employees who attended only the first sampling and those who attended repeated samplings, together with the corresponding background PFAS levels observed in the reference population (Li et al. 2018). PFHxS showed the highest serum concentration in the airport employees, with a median level of about 102–225 times higher than the level observed in the reference population. In addition, the median PFPeS concentration, although lower than PFHxS, was about 175–380 times higher than the maximum level in the reference population. As expected, the nine employees not included (five of them were not invited) in the further sampling showed much lower serum levels of short-chain PFAS (i.e., PFHpA, PFBS, PFPeS, and PFHpS), as well as of legacy PFOA, PFHxS, and PFOS.
Table 2.
Description of the 26 airport employees and PFAS levels measured in the first serum samples, together with PFAS levels measured in the reference group.
| Categories | Employees participated in first blood sampling only | Employees participated in first blood sampling and repeated blood and urine sampling | Reference groupa |
|---|---|---|---|
| Basic characteristics | |||
| Counts (N) | 9 | 17 | 58 |
| Female [N (%)] | 1 (11) | 6 (35) | 37 (64) |
| Age [median (range)] | 33 (22–61) | 50 (24–62) | 34 (22–49) |
| Years of current employment [median (range)] | 2 (1–27) | 10 (1–28) | NAb |
| Total working days from 1 August to 11 September 2018 [median (range)] | 29 (1–40) | 39 (20–42) | NA |
| Pregnancy previously [yes (%)] | 1 (100) | 2 (33) | NA |
| Year of last pregnancy | 2018 | 2010 and 1999 | NA |
| Month of breast-feeding (median) | 6 | Both 6 | NA |
| PFCAs in first serum sample { [median (range)]}c | |||
| PFHxA (C6) | 0.38 (0.23–1.1) | 0.37 (0.16–0.92) | NA |
| PFHpA (C7) | 0.17 (0.07–1.3) | 0.53 (0.20–2.2) | NA |
| PFOA (C8) | 6.5 (2.9–11) | 13 (5.0–31) | 1.5 (0.26–4.6) |
| PFSAs in first serum sample { [median (range)]} | |||
| PFBS (C4) | 0.10 (–1.0)d | 0.46 (0.22–1.3) | NA |
| PFPeS (C5) | 3.5 (1.4–11) | 7.6 (3.6–17) | (–0.02) |
| PFHxS (C6) | 60 (17–85) | 133 (21–402) | 0.59 (–1.2) |
| PFHpS (C7) | 0.97 (0.28–1.9) | 1.6 (0.49–6.2) | (–0.16) |
| L-PFOS (C8) | 6.5 (5.4–13) | 11 (5.5–28) | 3.0 (0.30–9.8) |
| 1m-PFOS (C8) | 0.52 (0.25–2.7) | 1.0 (0.32–4.1) | 0.18 (–0.59) |
| 3/4/5m-PFOS (C8) | 2.9 (1.2–6.7) | 4.9 (1.7–17) | 0.79 (0.10–2.2) |
| 2/6m-PFOS (C8) | 1.8 (0.84–3.2) | 2.6 (1.0–7.5) | 0.46 (–1.4) |
Note: L-PFOS, linear perfluorooctane sulfonic acid; LOD, limit of detection; PFAS, perfluoroalkyl substances; PFBS, perfluorobutane sulfonic acid; PFCA, perfluorinated carboxylic acid; PFHpA, perfluoroheptanoic acid; PFHpS, perfluoroheptane sulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFOA, perfluorooctanoic acid; PFPeS, perfluoropentane sulfonic acid; PFSA, perfluorinated sulfonic acid; 1m-PFOS, branched, perfluoro-1-methylheptanesulfonate; 2/6m-PFOS, branched, sum of perfluoro-2/6-methylheptanesulfonate; 3/4/5m-PFOS, branched, sum of perfluoro-3/4/5-methylheptanesulfonate.
The reference group was from Karlshamn, Sweden, a municipality without PFAS contamination in drinking water (Li et al. 2018). This group was sampled in 2016. A subset of 59 individuals were randomly selected for analyzing other short-chain PFAS than the legacy ones using the same method as the airport employees, and 58 within the same age range as the airport employees (i.e., 22–62 years of age) were included in the comparison.
No data available or PFAS was not measured.
PFBA and PFPeA were elevated in the drinking water (Table 1) but below the LOD in all the serum samples, so they were not presented in the present paper.
LODs are listed in Table S3.
The initial PFAS levels for males and females are listed in Table S5. There was no difference between sexes for most of the measured PFAS. Only PFHxA showed higher levels in males than females.
Comparison between PFAS Levels in Drinking Water and in First Serum Samples
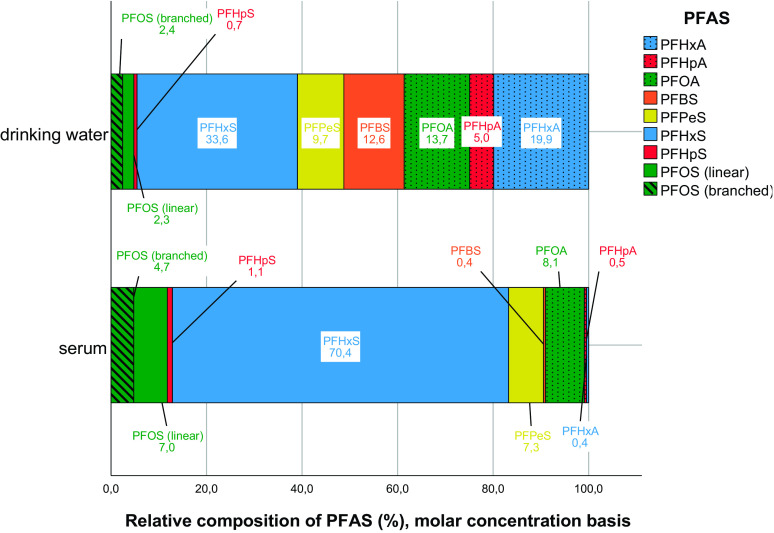
The different patterns of PFAS levels in the drinking water and in serum are illustrated in Figure 2, expressed on a molar-concentration basis, and in Figure S1 on a weight-concentration basis. The corresponding summary data are listed in Table S6. The proportions of short-chain PFHxA and PFBS were higher in the water (20% and 13%, respectively) than in serum (0.4% for both). In contrast, PFHxS and PFOS (linear and branched) showed higher proportions in serum (70% and 5–7%, respectively) than in drinking water (34% and around 2.4%, respectively). In total, the legacy PFOA, PFHxS, and PFOS accounted for about 50% of total PFAS in water and 90% in serum.
Figure 2.
Composition (molar-concentration basis) of perfluorinated carboxylic acids (PFCAs, dotted bars) and perfluorinated sulfonic acids (PFSAs, open bars; branched PFOS in hatched bar) in the airport drinking water and in the first serum samples obtained from the 26 airport employees (detailed data are presented in Table S6). PFCAs and PFSAs with same number of carbons in the carbon chain (i.e., PFHxA vs. PFHxS, PFHpA vs. PFHpS, and PFOA vs. PFOS) are illustrated with the same color. The relative composition (percentage) for each PFAS is embedded in the box. Although PFBA and PFPeA were elevated in the drinking water, they were below the LOD in all the serum samples, so they were not presented. Note: PFAS, perfluoroalkyl substances; PFBS, perfluorobutane sulfonic acid; PFHpA, perfluoroheptanoic acid; PFHpS, perfluoroheptane sulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFPeS, perfluoropentane sulfonic acid.
Levels of PFAS measured in the drinking water at the airport and in the first serum samples taken from all 26 employees at the airport, together with the calculated serum/water ratio, are given in Table 3. For both PFCAs and PFSAs, an increasing serum/water ratio with increasing carbon chain length is suggested, indicating more bioaccumulation in the human body for the longer-chain PFAS.
Table 3.
PFAS Levels measured in the airport drinking water and in the first serum samples from all 26 airport employees.
| PFAS | Airport drinking water (ng/mL) | Serum level in the first samples {ng/mL [median (range)]} | Serum/water ratioa |
|---|---|---|---|
| PFCAsb | |||
| PFHxA | 0.33 | 0.38 (0.16–1.1) | 1.15 |
| PFHpA | 0.097 | 0.46 (0.07–2.2) | 4.74 |
| PFOA | 0.30 | 9.1 (2.9–31) | 30.3 |
| PFSAs | |||
| PFBS | 0.20 | 0.33 (–1.3) | 1.65 |
| PFPeS | 0.18 | 6.9 (1.4–17) | 38.3 |
| PFHxS | 0.71 | 76 (17–402) | 107 |
| PFHpS | 0.016 | 1.3 (0.28–6.2) | 81.3 |
| L-PFOS | 0.062 | 9.5 (5.4–28) | 153 |
| B-PFOS | 0.064 | 6.4 (2.2–28) | 100 |
Note: B-PFOS, branched PFOS; L-PFOS, linear PFOS; LOD, limit of detection; PFAS, perfluoroalkyl substances; PFBS, perfluorobutane sulfonic acid; PFCA, perfluorinated carboxylic acid; PFHpA, perfluoroheptanoic acid; PFHpS, perfluoroheptane sulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFOA, perfluorooctanoic acid; PFPeS, perfluoropentane sulfonic acid; PFSA, perfluorinated sulfonic acid.
Serum/water ratio was calculated as median serum level divided by drinking-water level.
PFBA and PFPeA were elevated in the drinking water but below the LOD in all the serum samples, so no ratio is presented.
Urine Levels of PFAS and Urine/Serum Ratios
Table 4 presents the descriptive analysis of PFAS concentrations in serum and urine samples for all samplings. In general, the urine concentrations of PFAS were very low compared with serum levels. The urinary PFAS levels for each sampling are presented in Table S7 and illustrated in Figure S2.
Table 4.
PFAS Levels measured in the paired serum and urine samples obtained from the second to the fifth samplings from 17 airport employees and calculated urine/serum ratio.
| PFAS | Serum level {ng/mL [median (range)]}a | Specific gravity adjusted urine level {ng/mL [median (range)]}a | Personal average urine/serum ratio [median (range)]b |
|---|---|---|---|
| PFCAs | |||
| PFHpA | NAc | 0.025 (–0.080) |
0.086 (0.018–0.32) |
| PFOA | 10 (4.1–28) | 0.031 (0.010–0.13) |
0.0032 (0.00066–0.0067) |
| PFSAs | |||
| PFPeS | 6.5 (1.8–14) | 0.072 (0.021–0.12) |
0.0089 (0.0057–0.026) |
| PFHxS | 118 (17–399) | 0.092 (0.025–0.73) |
0.00093 (0.00060–0.0018) |
| L–PFOS | 10 (4.1–24) |
(–0.084) |
0.00092 (0.000035–0.0056) |
| 2/6m-PFOS | 2.1 (0.57–8.1) |
(–1.6) |
0.0051 (0.00059–0.032) |
Note: L-PFOS, linear PFOS; LOD, limit of detection; NA, not applicable; PFAS, perfluoroalkyl substances; PFCA, perfluorinated carboxylic acid; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFOA, perfluorooctanoic acid; PFPeS, perfluoropentane sulfonic acid; PFSA, perfluorinated sulfonic acid; 2/6m-PFOS, branched, sum of perfluoro-2/6-methylheptanesulfonate.
Median and range obtained from the second to the fifth serum or urine samplings.
The urine/serum ratio was first calculated for each study subject at each sampling (i.e., each subject had four urine/serum ratios for each PFAS). Personal average urine/serum ratio was then calculated as the mean value of four ratios for each subject. Median and range were obtained from the personal average urine/serum ratio.
PFAS was not measured.
For each PFAS compound, there was a substantial variation of the urine/serum ratio within and between individuals over the follow-up time. The variation for PFPeS (representing short-chain PFAS) and L-PFOS (representing long-chain PFAS) is illustrated in Figure S3. Overall, there was no consistent pattern of variation over time. At the group level, the urine/serum ratio was highest for PFHpA, followed by PFPeS and 2/6m-PFOS, indicating that short-chained PFAS and 2/6m-PFOS are excreted more rapidly through the urine, whereas PFOA, PFHxS, and L-PFOS are excreted more slowly through the urine (Table 4). In general, for both PFCAs and PFSAs, there was a decreased urine/serum ratio together with an increased serum/water ratio with the increment of chain length.
Estimation of Serum Elimination Half-Lives
The population average elimination rate and half-life are shown in Table 5, and individual estimates for each employee are listed in Table 6 (for short-chain PFAS) and Table 7 (for long-chain PFAS). In general, short-chain PFAS such as PFHpA, PFBS, and PFPeS showed shorter average half-lives of about 44–230 d, whereas long-chain legacy PFAS showed much longer average half-lives of 1.5–2 y (Table 5). PFHxA (C6), however, did not significantly decrease within 5 months. The estimated average constant elimination rate of PFHxA (i.e., the slope) from the mixed model was not significantly different from zero (Table 5). Correspondingly, 53% (9 of 17) of the individuals did not show a decrement in serum concentration of PFHxA during the 5-month follow-up (Table 6). PFHxS and L-PFOS showed the longest estimated half-lives in the airport workers (all about 2.9 y). All the branched PFOS showed half-lives that, on average, were shorter than L-PFOS; 2/6m-PFOS showed the shortest half-life. For the PFAS with same number of carbon atoms in the molecule, PFSAs showed longer half-lives than PFCAs, for example, PFHpS had on average about a 1.3-y longer half-life than the corresponding PFHpA, and PFOS had on average about a 1.2-y longer half-life than PFOA. The pattern of half-lives of different PFAS were somewhat consistent with the serum/water ratio and urine/serum ratio, indicating that PFAS with shorter estimated half-lives were corresponding to lower serum/water ratios and larger urine/serum ratios. The median and distribution of PFAS levels in serum for each sampling are presented in Table S8. There were clear trends of decrements for short-chain PFAS over time, whereas the decrements of long-chain PFAS were less evident. The sample size is too small to detect whether there were departures from linearity.
Table 5.
Population average constant elimination rate (yearly) and half-life (year) for each PFAS.
| PFAS | Model with original serum levelsa | Model with serum levels after subtraction of background exposureb | ||||
|---|---|---|---|---|---|---|
| Constant elimination rate (95% CI) | Half-life (95% CI) | -Value | Constant elimination rate (95% CI) | Half-life (95% CI) | -Value | |
| PFHxA | 0.43 (, 1.1) | 1.63 (NA) | 0.2 | NAc | NA | |
| PFHpA | 4.11 (3.13, 5.09) | 0.17 (0.14, 0.22)d | NA | NA | ||
| PFOA | 0.39 (0.3, 0.49) | 1.77 (1.43, 2.31) | 0.002 | 0.47 (0.35, 0.58) | 1.48 (1.19, 1.96) | |
| PFBS | 5.96 (4.65, 7.27) | 0.12 (0.1, 0.15)d | 0.003 | NA | NA | |
| PFPeS | 1.1 (0.68, 1.51) | 0.63 (0.46, 1.01) | 0.004 | 1.1 (0.68, 1.52) | 0.63 (0.46, 1.01) | |
| PFHxS | 0.24 (0.16, 0.33) | 2.86 (2.1, 4.47) | 0.005 | 0.24 (0.16, 0.33) | 2.84 (2.08, 4.43) | 0.002 |
| PFHpS | 0.48 (0.11, 0.84) | 1.46 (0.83, 6.25) | 0.014 | 0.51 (0.12, 0.9) | 1.35 (0.77, 5.71) | 0.014 |
| L-PFOS | 0.24 (0.07, 0.4) | 2.91 (1.71, 9.63) | 0.007 | 0.41 (0.11, 0.71) | 1.69 (0.98, 6.04) | 0.010 |
| 1m-PFOS | 0.55 (0.09, 1.01) | 1.27 (0.69, 7.64) | 0.022 | 0.75 (0.15, 1.36) | 0.92 (0.51, 4.77) | 0.018 |
| 3/4/5m-PFOS | 0.64 (0.18, 1.1) | 1.09 (0.63, 3.96) | 0.011 | 0.83 (0.24, 1.42) | 0.83 (0.49, 2.84) | 0.011 |
| 2/6m-PFOS | 0.66 (0.29, 1.04) | 1.04 (0.67, 2.42) | 0.001 | 0.95 (0.39, 1.5) | 0.73 (0.46, 1.76) | 0.002 |
Note: CI, confidence interval; L-PFOS, linear perfluorooctane sulfonic acid; NA, not applicable; PFAS, perfluoroalkyl substances; PFBS, perfluorobutane sulfonic acid; PFHpA, perfluoroheptanoic acid; PFHpS, perfluoroheptane sulfonic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexane sulfonic acid; PFOA, perfluorooctanoic acid; PFPeS, perfluoropentane sulfonic acid; 1m-PFOS, branched, perfluoro-1-methylheptanesulfonate; 2/6m-PFOS, branched, sum of perfluoro-2/6-methylheptanesulfonate; 3/4/5m-PFOS, branched, sum of perfluoro-3/4/5-methylheptanesulfonate.
Linear mixed model with age and sex as covariates. Serum PFAS levels were log-transformed. The constant elimination rate denotes the negative value of the slope () from the model, and half-life is calculated as .
Sensitivity analysis using same linear mixed model. Serum PFAS levels were first subtracted with the median levels from the reference population (background exposure levels, presented in Table 2). If the levels after subtraction were lower than half of the corresponding background levels, they were replaced with half of the background levels.
No background serum PFAS levels available.
Half-life and 95% CI in days: PFHpA: 62 d (95% CI: 51–80 d); PFBS: 44 d (95% CI: 37–55 d).
Table 6.
Individual constant elimination rate (yearly) and half-life (year) in the 17 airport employees for short-chain PFAS.
| Subject | Sample counts | PFHxA | PFHpA | PFBS | PFPeS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elimination ratea | HLa | -Valuea | Elimination rate | HL | -Value | Elimination rate | HL | -Value | Elimination rate | HL | -Value | ||
| 1 | 5 | 0.2 | 4.1 | 0.78 | 2.1 | 0.3 | 0.02 | 3.2 | 0.2 | 0.03 | 0.7 | 1.0 | 0.02 |
| 2 | 5 | NAb | 0.87 | 3.7 | 0.2 | 0.01 | 4.0 | 0.2 | 0.02 | 0.6 | 1.2 | 0.04 | |
| 3 | 5 | 1.6 | 0.4 | 0.04 | 1.7 | 0.4 | 0.03 | 5.1 | 0.1 | 0.00 | 0.7 | 0.9 | 0.03 |
| 4 | 5 | NA | 0.57 | 3.2 | 0.2 | 0.14 | 3.1 | 0.2 | 0.44 | 0.8 | 0.8 | 0.05 | |
| 5 | 5 | NA | 0.24 | 1.3 | 0.5 | 0.02 | 3.0 | 0.2 | 0.00 | 0.4 | 1.7 | 0.11 | |
| 6 | 4 | NA | 0.04 | 4.1 | 0.2 | 0.03 | 2.9 | 0.2 | 0.43 | 0.9 | 0.7 | 0.08 | |
| 7 | 5 | 1.5 | 0.5 | 0.27 | 4.6 | 0.1 | 0.00 | 6.2 | 0.1 | 0.02 | 0.7 | 0.9 | 0.01 |
| 8 | 5 | NA | 0.44 | 5.4 | 0.1 | 0.02 | 4.4 | 0.2 | 0.14 | 0.5 | 1.4 | 0.07 | |
| 9 | 5 | NA | 0.88 | 7.8 | 0.1 | 0.01 | 11.7 | 0.1 | 0.00 | 0.7 | 1.0 | 0.00 | |
| 10 | 5 | 0.4 | 1.9 | 0.75 | 2.2 | 0.3 | 0.04 | 3.8 | 0.2 | 0.03 | 0.6 | 1.2 | 0.08 |
| 11 | 5 | 2.8 | 0.2 | 0.03 | 2.6 | 0.3 | 0.01 | 7.1 | 0.1 | 0.02 | 2.0 | 0.3 | 0.13 |
| 12 | 5 | NA | 0.64 | 6.7 | 0.1 | 0.00 | 5.4 | 0.1 | 0.01 | 1.1 | 0.6 | 0.00 | |
| 13 | 5 | NA | 0.25 | 5.4 | 0.1 | 0.00 | 6.2 | 0.1 | 0.02 | 0.8 | 0.9 | 0.06 | |
| 14 | 4 | NA | 0.87 | 6.6 | 0.1 | 0.02 | 10.7 | 0.1 | 0.01 | 1.2 | 0.6 | 0.08 | |
| 15 | 5 | 2.7 | 0.3 | 0.09 | 3.9 | 0.2 | 0.02 | 5.7 | 0.1 | 0.01 | 2.5 | 0.3 | 0.07 |
| 16 | 5 | 1.1 | 0.6 | 0.08 | 0.9 | 0.7 | 0.17 | 3.6 | 0.2 | 0.01 | 0.4 | 1.6 | 0.07 |
| 17 | 5 | 2.5 | 0.3 | 0.06 | 5.2 | 0.1 | 0.04 | 10.4 | 0.1 | 0.02 | 2.4 | 0.3 | 0.06 |
Note: HL, half-life; PFBS, perfluorobutane sulfonic acid; PFHpA, perfluoroheptanoic acid; PFHxA, perfluorohexanoic acid; PFPeS, perfluoropentane sulfonic acid.
The elimination rate and -value were obtained from linear regression. Serum PFAS levels were log-transformed. The elimination rate denotes the negative value of the slope () from the model, and half-life is calculated as .
Not applicable because the elimination rate was negative, indicating PFAS levels did not decrease during the 5-month follow-up.
Table 7.
Individual constant elimination rate (yearly) and half-life (year) in the 17 airport employees for long-chain PFAS.
| Subject | Sample counts | PFOA | PFHxS | PFHpS | L-PFOS | 1m-PFOS | 3/4/5m-PFOS | 2/6m-PFOS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elimination ratea | HLa | -Valuea | Elimination rate | HL | -Value | Elimination rate | HL | -Value | Elimination rate | HL | -Value | Elimination rate | HL | -Value | Elimination rate | HL | -Value | Elimination rate | HL | -Value | ||
| 1 | 5 | 0.3 | 2.4 | 0.17 | 0.2 | 3.5 | 0.19 | 0.5 | 1.3 | 0.31 | NA | 0.30 | 0.3 | 2.0 | 0.10 | 0.4 | 1.9 | 0.13 | NA | 0.76 | ||
| 2 | 5 | 0.2 | 2.9 | 0.27 | 0.3 | 2.0 | 0.09 | 0.2 | 3.4 | 0.64 | 0.4 | 1.7 | 0.07 | 0.1 | 5.4 | 0.83 | 0.3 | 2.1 | 0.11 | 0.5 | 1.3 | 0.20 |
| 3 | 5 | 0.6 | 1.2 | 0.04 | 0.5 | 1.5 | 0.09 | 0.2 | 3.0 | 0.34 | 0.5 | 1.3 | 0.21 | 0.8 | 0.9 | 0.09 | 0.4 | 1.7 | 0.15 | 0.7 | 1.0 | 0.12 |
| 4 | 5 | 0.3 | 2.3 | 0.15 | 0.1 | 9.6 | 0.75 | 0.001 | 526 | 1.00 | NA | 0.24 | NA | 0.78 | 0.1 | 11 | 0.87 | 0.0 | 51 | 0.97 | ||
| 5 | 5 | 0.3 | 2.2 | 0.15 | 0.1 | 4.7 | 0.20 | NA | 0.19 | 0.6 | 1.2 | 0.13 | 0.1 | 5.9 | 0.75 | 0.2 | 3.1 | 0.16 | 0.4 | 1.6 | 0.56 | |
| 6 | 4 | 0.2 | 2.9 | 0.39 | NAb | 0.42 | 0.1 | 6.1 | 0.43 | 0.04 | 17 | 0.87 | NA | 0.51 | 0.04 | 19 | 0.49 | 0.4 | 1.7 | 0.04 | ||
| 7 | 5 | 0.4 | 1.6 | 0.03 | 0.3 | 2.3 | 0.02 | NA | 0.88 | 0.006 | 108 | 0.92 | NA | 0.03 | 0.1 | 4.8 | 0.02 | 1.1 | 0.6 | 0.29 | ||
| 8 | 5 | 0.007 | 100 | 0.98 | NA | 0.94 | NA | 0.42 | 0.23 | NA | 0.13 | NA | 0.24 | NA | 0.44 | |||||||
| 9 | 5 | 0.4 | 1.9 | 0.05 | NA | 0.81 | 0.05 | 15 | 0.69 | 0.4 | 1.8 | 0.21 | NA | 0.10 | 0.1 | 10 | 0.70 | 0.2 | 4.2 | 0.50 | ||
| 10 | 5 | 0.3 | 2.1 | 0.25 | 0.4 | 1.9 | 0.11 | 0.4 | 1.8 | 0.46 | NA | 0.32 | 0.6 | 1.2 | 0.10 | 0.2 | 4.0 | 0.52 | NA | 0.97 | ||
| 11 | 5 | 0.5 | 1.5 | 0.12 | 0.2 | 3.0 | 0.39 | 1.7 | 0.4 | 0.20 | 0.1 | 4.7 | 0.56 | 1.9 | 0.4 | 0.15 | 2.0 | 0.4 | 0.15 | 1.7 | 0.4 | 0.10 |
| 12 | 5 | 0.7 | 1.0 | 0.00 | 0.4 | 1.6 | 0.04 | 0.6 | 1.1 | 0.08 | 0.8 | 0.9 | 0.01 | 0.03 | 26 | 0.95 | 0.5 | 1.3 | 0.09 | 1.7 | 0.4 | 0.03 |
| 13 | 5 | 0.4 | 1.6 | 0.02 | 0.5 | 1.4 | 0.01 | NA | 0.70 | 0.2 | 3.7 | 0.34 | 1.1 | 0.6 | 0.08 | 0.5 | 1.5 | 0.04 | 1.0 | 0.7 | 0.20 | |
| 14 | 4 | 0.4 | 1.6 | 0.40 | 0.6 | 1.2 | 0.31 | 1.0 | 0.7 | 0.21 | 0.3 | 2.0 | 0.67 | 1.7 | 0.4 | 0.08 | 0.3 | 2.0 | 0.55 | 1.5 | 0.5 | 0.44 |
| 15 | 5 | 0.6 | 1.1 | 0.04 | 0.3 | 2.2 | 0.06 | 1.6 | 0.4 | 0.09 | 0.5 | 1.3 | 0.01 | 1.3 | 0.6 | 0.18 | 2.2 | 0.3 | 0.08 | 1.2 | 0.6 | 0.18 |
| 16 | 5 | 0.3 | 2.7 | 0.19 | 0.04 | 19 | 0.89 | 0.1 | 12 | 0.61 | 0.6 | 1.2 | 0.05 | NA | 0.02 | 0.3 | 2.0 | 0.12 | NA | 0.81 | ||
| 17 | 5 | 0.6 | 1.2 | 0.09 | 0.4 | 1.6 | 0.07 | 1.7 | 0.4 | 0.13 | 0.3 | 2.1 | 0.19 | 2.2 | 0.3 | 0.12 | 1.9 | 0.4 | 0.13 | 1.9 | 0.4 | 0.10 |
Note: HL, half-life; L-PFOS, linear perfluorooctane sulfonic acid; PFAS, perfluoroalkyl substances; PFHpS, perfluoroheptane sulfonic acid; PFHxS, perfluorohexane sulfonic acid; PFOA, perfluorooctanoic acid; 1m-PFOS, branched, perfluoro-1-methylheptanesulfonate; 2/6m-PFOS, branched, sum of perfluoro-2/6-methylheptanesulfonate; 3/4/5m-PFOS, branched, sum of perfluoro-3/4/5-methylheptanesulfonate.
The elimination rate and -value were obtained from linear regression. Serum PFAS levels were log-transformed. The elimination rate denotes the negative value of the slope () from the model, and half-life is calculated as .
Not applicable because the elimination rate was negative, indicating PFAS levels did not decrease during the 5-month follow-up.
The sensitivity analysis using serum PFAS levels after subtracting general background levels are listed in the right part of Table 5. For PFPeS and PFHxS, which were much higher in the airport workers compared with the reference population, the estimates after subtraction of background levels were quite close to estimates without subtraction of background exposure. For PFOA and PFOS, with less contrast (approximately 5-fold), the estimation of half-lives were shortened after subtraction of background levels. The reductions of half-life estimation were 8% for PFHpS, 16% for PFOA, 42% for L-PFOS, and around 28% for branched PFAS. For most of the PFAS, the individual elimination rates were not correlated with their initial serum levels ( from Spearman’s correlation test). Only PFHpA showed a significant positive correlation (Spearman’s , ).
Discussion
In the 17 airport employees sampled between 2 weeks to 5 months after the end of exposure to PFAS-contaminated drinking water, we determined the average half-life for PFPeS as 0.63 y (i.e., 230 d) and PFHpS as 1.46 y. The shortest estimated half-life was for PFBS (0.12 y; i.e., 44 d) and the longest half-lives were observed for PFHxS and L-PFOS (2.86 and 2.91 y, respectively). The pattern of estimated half-lives was consistent with the observed PFAS serum/water ratios and urine/serum ratios of various PFAS, indicating that the lower the rate of renal clearance, the higher the serum/water ratio and the longer the half-life.
Short-chain PFCAs with chains of less than eight carbons and PFSAs with less than six carbons have been shown to be excreted faster compared with the long-chain legacy PFOS and PFOA (Conder et al. 2008). The rapid excretion and lower bioaccumulation of short-chain PFAS has made it difficult to find an exposed population that can be used to study human half-lives. The major strength of the present study is that first serum samples were taken within only 2 weeks after the exposure to the highly contaminated water was terminated. This provided us with an opportunity to address half-lives of some short-chain PFAS in human blood. We were confident that all individuals in the study no longer had ongoing drinking-water exposure at home after thoroughly checking water supply information at each individual’s home address. Moreover, we used two methods to estimate PFAS elimination rate at the population level and individual level, respectively. The mixed model is preferable for a population average estimation and provides more robust estimates when random error of sampling exists. The linear regression for each individual estimates served as an alternative method to show interindividual variations. Because up to only five measurements were used in each regression, the statistical power was limited and, therefore, not considered as main results.
Our study also has some limitations. Principally, that the number of study subjects was relatively small, although we succeeded in obtaining five blood samples and four urine samples from most subjects during 5 months. For long-chain PFAS with long half-lives, only 5 months is relatively short for estimating the half-life. However, the study was sufficient to estimate the half-life for several PFAS with reasonable precision. The statistical power for individual estimates would still benefit from further follow-up with more sampling points.
For short-chain PFAS, limited human studies about half-lives are available. In the present study, the individual serum half-life of PFBS ranged from 21.9 to 87.6 d, with an average estimation of 43.8 d. The estimation of PFBS by Olsen et al. (2009) was 25.8 d (geometric mean) with a range of 13.1–45.7 d. Although the average estimation was about half compared with our study, the ranges overlapped; four to six subjects in the study by Olsen et al. (2009) had a half-life estimation within the range observed in the present study, with one more subject showing 21.2 d as the PFBS half-life. Their follow-up of 180 d was a little longer. Further, PFBS serum levels reported by Olsen et al. (2009) (average level: ) were much higher than in the present study (median of initial level: ). Given the large variation in exposure levels between the two studies, a dose-dependency of PFBS serum elimination half-life cannot be ruled out.
For PFHpA, the present study showed a similar half-life (0.17 y, corresponding to 62 d) to that reported by Russell et al. (2015a), who calculated the first-order apparent elimination half-life of 70 d (geometric mean) in whole blood based on repeated samples from five ski wax technicians after occupational exposure during the skiing season. Another study performed in a Chinese population reported estimated half-lives of 0.82 and 1.0 y (geometric mean for young females and for older females plus males, respectively) for PFHpA (Zhang et al. 2013). The latter study was based only on single time-paired serum (or whole blood) and urine samples and assumed renal clearance as total clearance in their calculation, so it is not strictly comparable. The underestimation of total clearance would lead to an overestimation of half-life.
For PFHxA, about half of the airport employees did not show a clear decrease of serum concentration (i.e., 53% had no individual estimated half-life). A reason could be that we measured PFHxA in serum, which only presents a very small fraction of PFHxA in blood (although all serum levels were ). Unlike the other PFAS we present, PFHxA is more bound to blood cells and whole blood would therefore be a more suitable blood matrix to determine PFHxA (Poothong et al. 2017). Several other studies also failed to detect PFHxA in serum/plasma (Christensen et al. 2016; Eriksson et al. 2017; Salihovic et al. 2015). Russell et al. (2013) reported an apparent elimination half-life of PFHxA in whole blood from seven ski wax technicians as 32 d with a range of 14–49 d. Due to the fact that PFHxA was measured in serum in the present study, which did not adequately reflect the real body burden level, our estimation of the half-life is, therefore, unreliable.
For the short-chain PFPeS with a five-carbon chain length, our half-life estimation (0.63 y) was slightly shorter than the observation from the highly exposed general population from Ronneby, Sweden, which showed an average half-life of 1.0 y [95% confidence interval (CI): 0.9, 1.0 y] (Li et al. 2019). However, for the long-chain PFHpS with a seven-carbon chain length, the estimated half-life from this study (1.46 y) was much shorter than the Ronneby population [4.7 y (95% CI: 4.3, 5.3 y)] (Li et al. 2019). No other study was available for further comparison.
A shorter half-life estimation in the present study than the corresponding half-lives derived over 2 y in the Ronneby population was also observed for other PFAS compounds. The half-lives of PFOA (1.77 y), PFHxS (2.86 y), and L-PFOS (2.91 y) were each shorter compared with findings of Li et al. (2018) (2.7, 5.3, and 3.4 y for total PFOS, respectively). The difference in study population (e.g., age range, sex composition) may be one explanation. In addition, the follow-up period was different between two studies. The first serum samples in the Ronneby population were obtained 6 months after the exposure stopped. Therefore, the elimination rate in the first 6 months could not be directly compared between these populations. There is some evidence of a possible nonlinear clearance process of PFAS from the literature, mainly about PFOA, showing that the estimated half-life appeared to decrease with increased follow-up time. For instance, based on a 5-y follow-up, Olsen et al. (2007) estimated an average half-life of PFOA of 3.8 y in retired fluorochemical production workers, whereas Brede et al. (2010) reported a half-life of PFOA of 3.3 y based on a 2-y follow-up and Bartell et al. (2010) reported of 2.3 y with a 1-y follow-up. Our PFOA half-life of 1.77 y is consistent with this pattern that elimination is faster in the early time window after exposure stopped and becomes slower after 1 or 2 y.
Half-life estimation can also be influenced by ongoing exposure, which could contribute to explaining the different half-lives reported in different studies. Our sensitivity analysis of estimations with and without subtraction of general background level also clearly showed that for PFPeS and PFHxS, much higher initial serum levels compared with the background levels, the estimates did not differ much. For PFOA and PFOS, however, the estimated half-lives were shortened after subtracting background levels. The result is in line with the findings of Russell et al. (2015b) that if the background exposure compared to the contaminated level is not small, then ignoring the background exposure will lead to an overestimated of half-life. The half-life comparison between studies is summarized in Table 8.
Table 8.
Summary of PFAS half-lives in the present study and in other studies referred to in the discussion.
| PFAS | Half-life in the present study | Half-life in other study | Study population | Year of study | Follow-up time | Reference |
|---|---|---|---|---|---|---|
| PFHpA | 0.17 y (62 d) | 70 d | 5 ski wax technicians | 2007–2011 | 7 months | Russell et al. 2015a |
| PFOA | 1.77 y | 2.7 y | 106 Swedes | 2014–2016 | 2 y | Li et al. 2018 |
| 3.8 y | 26 retired fluorochemical production workers | 1999–2004 | 5 y | Olsen et al. 2007 | ||
| 3.3 y | 138 Germans | 2006–2008 | 2 y | Brede et al. 2010 | ||
| 2.3 y | 200 Americans | 2007–2008 | 1 y | Bartell et al. 2010 | ||
| PFBS | 0.12 y (44 d) | 25.8 d | 6 3M employees | 2004 | 3 months | Olsen et al. 2009 |
| PFPeS | 0.63 y | 1.0 y | 108 Swedes | 2014–2019 | 5 y | Li et al. 2019 |
| PFHxS | 2.86 y | 5.3 y | 106 Swedes | 2014–2016 | 2 y | Li et al. 2018 |
| L-PFOS | 2.91 y | 3.4 y | 106 Swedes | 2014–2016 | 2 y | Li et al. 2018 |
Note: L-PFOS, linear perfluorooctane sulfonic acid; PFAS, perfluoroalkyl substances; PFBS, perfluorobutane sulfonic acid; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFOA, perfluorooctanoic acid; PFPeS, perfluoropentane sulfonic acid.
The average serum/water ratio of PFOA observed in our study (30.3) was lower than in other studies, which ranged from 100 to 231 (Post et al. 2009; Hoffman et al. 2011; Zhang et al. 2019). In those studies, PFOA was measured in the tap water supplied by public water systems (Post et al. 2009; Zhang et al. 2019) or in private wells (Hoffman et al. 2011). It is reasonable to assume that their population was exposed to PFOA-contaminated drinking water at home. In our study, however, the individuals were only exposed at work and had a PFAS-free water supply at home. Therefore, the contribution from drinking water to serum level may be less compared with those who are constantly exposed at home.
Although PFAS are eliminated through urine to different degrees, urinary levels of PFAS are generally low in adults. In our study, despite the fact that 11 PFAS were successfully measured in serum, only 6 PFAS (PFHpA, PFOA, PFPeS, PFHxS, L-PFOS, and 2/6m-PFOS) could be detected and quantified in urine samples. Moreover, the urine/serum ratios were observed to be low (all ) among the airport employees. Our observed urine/serum ratios of PFOS (0.00092) and PFOA (0.0032) were similar to the findings of a Chinese study (Zhang et al. 2015), which reported urine/serum ratios of PFOS of 0.0004 for pregnant women and 0.013 for nonpregnant women and urine/serum ratios of PFOA of 0.0011 and 0.0028 for pregnant and nonpregnant women, respectively. Our results showed that the urine/serum ratio for PFOA was larger than linear PFOS, suggesting that PFOA is excreted faster through urine than PFOS. Our results also showed a lower urine/serum ratio of L-PFOS than 2/6m-PFOS, indicating that 2/6m-PFOS is preferentially excreted through urine compared with L-PFOS, which is consistent with other studies that reported a smaller renal clearance of L-PFOS than branched PFOS except 1m-PFOS (Zhang et al. 2013; Zhou et al. 2014).
In general, for both PFCAs and PFSAs, there was a decreased serum/water ratio and increased urine/serum ratio with decreasing chain length, indicating a faster excretion of PFAS with shorter chain length and a corresponding shorter half-life. For instance, short-chain PFPeS had a 10 times higher urine/serum ratio than long-chain L-PFOS. Correspondingly, PFPeS had a half-life 4 times shorter than L-PFOS. The shorter carbon chain length, the less accumulation, and shorter half-lives may be attributed to the difference in potential binding affinities to different organic anion transporter proteins (Han et al. 2012; Weaver et al. 2010). In animal models, it has been found that one organic anion transport protein (OATP 1a1) expressed in the kidney had a stronger interaction with long-chain PFAS and led to higher reabsorption and lower elimination levels (Yang et al. 2009). To some extent, higher water solubility of short-chain PFAS compared with longer ones may also be associated with faster excretion (Bhhatarai and Gramatica 2011). One exception to the above conclusion that PFAS elimination and half-lives is dependent on chain length was C6 PFHxS, which had a longer half-life than C7 PFHpS and a half-life similar to that of C8 PFOS. The reason is unclear, but such findings have been observed in other studies as well (Li et al. 2018; Olsen et al. 2007).
It should be noticed that even though short-chain PFAS showed a lower serum/water ratio than the legacy long-chain PFAS, they were clearly detected in the sera of the airport employees 2 weeks and longer after the cessation of exposure from the contaminated drinking water. For example, PFPeS showed much higher serum levels among the exposed employees even 5 months after exposure ceased than the levels observed in general population without PFAS exposure through drinking water (median vs. ; i.e., ). Consequently, populations with high daily exposure to short-chain PFAS from highly contaminated drinking water will have clearly elevated serum levels of these PFAS above background as long as exposure continues. Therefore, high short-chain PFAS contamination of drinking water may be a serious environmental health problem that should be taken into account in future epidemiological studies.
Conclusion
In the present study of 17 airport employees from Arvidsjaur, Sweden, who had been exposed to PFAS through drinking water at work, the estimated average half-lives after abrupt cessation of drinking-water exposure, ranged from 44 d for short-chain PFBS to 2.9 y for PFHxS and PFOS. A general pattern of increasing half-lives with chain length was observed. Branched PFOS had shorter half-lives than linear PFOS. Both PFOA and PFOS half-lives in the present study were shorter than published estimates, suggesting a possible time-dependent PFAS elimination process, with more rapid elimination in the first few months after the end of exposure.
Supplementary Material
Acknowledgments
We acknowledge all the personnel at Arvidsjaur Airport who willingly participated in the study. Blood sampling was performed at Arjeploghälsan, in collaboration with personnel at the local health center laboratory. We also acknowledge K. Forsell, Occupational and Environmental Medicine, Umeå University Hospital, for his inputs to this study. The study was funded by the Swedish Environmental Protection Agency and Swedish Research Council FORMAS grant 2017-00875.
References
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. 2010. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect 118(2):222–228, PMID: 20123620, 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhhatarai B, Gramatica P. 2011. Prediction of aqueous solubility, vapor pressure and critical micelle concentration for aquatic partitioning of perfluorinated chemicals. Environ Sci Technol 45(19):8120–8128, PMID: 20958003, 10.1021/es101181g. [DOI] [PubMed] [Google Scholar]
- Brede E, Wilhelm M, Göen T, Müller J, Rauchfuss K, Kraft M, et al. 2010. Two-year follow-up biomonitoring pilot study of residents’ and controls’ PFC plasma levels after PFOA reduction in public water system in Arnsberg, Germany. Int J Hyg Environ Health 213(3):217–223, PMID: 20488749, 10.1016/j.ijheh.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541, PMID: 21793199, 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KY, Raymond M, Thompson BA, Anderson HA. 2016. Perfluoroalkyl substances in older male anglers in Wisconsin. Environ Int 91:312–318, PMID: 27003842, 10.1016/j.envint.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Conder JM, Hoke RA, De Wolf W, Russell MH, Buck RC. 2008. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ Sci Technol 42(4):995–1003, PMID: 18351063, 10.1021/es070895g. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Jogsten IE, Eriksson U, Martorell I, Perelló G, Nadal M, et al. 2012. Human dietary exposure to perfluoroalkyl substances in Catalonia, Spain. Temporal trend. Food Chem 135(3):1575–1582, PMID: 22953896, 10.1016/j.foodchem.2012.06.054. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority). 2018. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J 16(1):e05194, 10.2903/j.efsa.2018.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson U, Mueller JF, Toms LL, Hobson P, Kärrman A. 2017. Temporal trends of PFSAs, PFCAs and selected precursors in Australian serum from 2002 to 2013. Environ Pollut 220(pt A):168–177, PMID: 27726977, 10.1016/j.envpol.2016.09.036. [DOI] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D. 2009. Perfluorinated compounds—exposure assessment for the general population in western countries. Int J Hyg Environ Health 212(3):239–270, PMID: 18565792, 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Genuis SJ, Birkholz D, Ralitsch M, Thibault N. 2010. Human detoxification of perfluorinated compounds. Public Health 124(7):367–375, PMID: 20621793, 10.1016/j.puhe.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Genuis SJ, Curtis L, Birkholz D. 2013. Gastrointestinal elimination of perfluorinated compounds using cholestyramine and Chlorella pyrenoidosa. ISRN Toxicol 2013:657849, PMID: 24106616, 10.1155/2013/657849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German Institute for Standardization. 2011. German standard methods for the examination of water, waste water and sludge--Jointly determinable substances (group F)–Part 42: Determination of selected polyfluorinated compounds (PFC) in water–Method using high performance liquid chromatography and mass spectrometric detection (HPLC/MS-MS) after solid-liquid extraction (F 42). https://global.ihs.com/doc_detail.cfm?document_name=DIN%2038407%2D42&item_s_key=00566149 [accessed 7 July 2020]. [Google Scholar]
- Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW. 2012. Renal elimination of perfluorocarboxylates (PFCAs). Chem Res Toxicol 25(1):35–46, PMID: 21985250, 10.1021/tx200363w. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Webster TF, Bartell SM, Weisskopf MG, Fletcher T, Vieira VM. 2011. Private drinking water wells as a source of exposure to perfluorooctanoic acid (PFOA) in communities surrounding a fluoropolymer production facility. Environ Health Perspect 119(1):92–97, PMID: 20920951, 10.1289/ehp.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITRC (Interstate Technology Regulatory Council). 2020. Naming conventions and physical and chemical properties of per- and polyfluoroalkyl substances (PFAS). https://pfas-1.itrcweb.org/fact_sheets_page/PFAS_Fact_Sheet_Naming_Conventions_April2020.pdf [accessed 7 July 2020].
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75(1):46–51, PMID: 29133598, 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu Y, Scott K, Lindh C, Jakobsson K, Fletcher T. 2019. Half-lives of PFOA, PFPeS, PFHxS, PFHpS and PFOS after end of exposure to contaminated drinking water. Environ Epidemiol 3:237, 10.1097/01.EE9.0000608476.06577.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Chang SC, Noker PE, Gorman GS, Ehresman DJ, Lieder PH, et al. 2009. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology 256(1–2):65–74, PMID: 19059455, 10.1016/j.tox.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Poothong S, Thomsen C, Padilla-Sanchez JA, Papadopoulou E, Haug LS. 2017. Distribution of novel and well-known poly- and perfluoroalkyl substances (PFASs) in human serum, plasma, and whole blood. Environ Sci Technol 51(22):13388–13396, PMID: 29056041, 10.1021/acs.est.7b03299. [DOI] [PubMed] [Google Scholar]
- Post GB, Louis JB, Cooper KR, Boros-Russo BJ, Lippincott RL. 2009. Occurrence and potential significance of perfluorooctanoic acid (PFOA) detected in New Jersey public drinking water systems. Environ Sci Technol 43(12):4547–4554. 15, PMID: 19603675, 10.1021/es900301s. [DOI] [PubMed] [Google Scholar]
- Russell MH, Himmelstein MW, Buck RC. 2015a. Inhalation and oral toxicokinetics of 6:2 FTOH and its metabolites in mammals. Chemosphere 120:328–335, PMID: 25180935, 10.1016/j.chemosphere.2014.07.092. [DOI] [PubMed] [Google Scholar]
- Russell MH, Nilsson H, Buck RC. 2013. Elimination kinetics of perfluorohexanoic acid in humans and comparison with mouse, rat and monkey. Chemosphere 93(10):2419–2425, PMID: 24050716, 10.1016/j.chemosphere.2013.08.060. [DOI] [PubMed] [Google Scholar]
- Russell MH, Waterland RL, Wong F. 2015b. Calculation of chemical elimination half-life from blood with an ongoing exposure source: the example of perfluorooctanoic acid (PFOA). Chemosphere 129:210–216, PMID: 25149361, 10.1016/j.chemosphere.2014.07.061. [DOI] [PubMed] [Google Scholar]
- Salihovic S, Kärrman A, Lind L, Lind PM, Lindström G, van Bavel B. 2015. Perfluoroalkyl substances (PFAS) including structural PFOS isomers in plasma from elderly men and women from Sweden: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Environ Int 82:21–27, PMID: 26001496, 10.1016/j.envint.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Weaver YM, Ehresman DJ, Butenhoff JL, Hagenbuch B. 2010. Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicol Sci 113(2):305–314, PMID: 19915082, 10.1093/toxsci/kfp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley RR, Moore SM, Tierney BC, Ye X, Calafat AM, Campbell S, et al. 2017. Per-and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ Int 106:135–143, PMID: 28645013, 10.1016/j.envint.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-H, Glover KP, Han X. 2009. Organic anion transporting polypeptide (Oatp) 1a1-mediated perfluorooctanoate transport and evidence for a renal reabsorption mechanism of Oatp1a1 in renal elimination of perfluorocarboxylates in rats. Toxicol Lett 190(2):163–171, PMID: 19616083, 10.1016/j.toxlet.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Zhang S, Kang Q, Peng H, Ding M, Zhao F, Zhou Y, et al. 2019. Relationship between perfluorooctanoate and perfluorooctane sulfonate blood concentrations in the general population and routine drinking water exposure. Environ Int 126:54–60, PMID: 30776750, 10.1016/j.envint.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Zhang T, Sun H, Qin X, Gan Z, Kannan K. 2015. PFOS and PFOA in paired urine and blood from general adults and pregnant women: assessment of urinary elimination. Environ Sci Pollut Res Int 22(7):5572–5579, PMID: 25367642, 10.1007/s11356-014-3725-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin JW. 2013. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol 47(18):10619–10627, PMID: 23980546, 10.1021/es401905e. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Shi Y, Vestergren R, Wang T, Liang Y, Cai Y. 2014. Highly elevated serum concentrations of perfluoroalkyl substances in fishery employees from Tangxun Lake, China. Environ Sci Technol 48(7):3864–3874, PMID: 24588690, 10.1021/es4057467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.