Abstract
In the context of the COVID-19 pandemic, several drugs have been repurposed as potential candidates for the treatment of COVID-19 infection. While preliminary choices were essentially based on in vitro potency, clinical translation into effective therapies may be challenging due to unfavorable in vivo pharmacokinetic properties at the doses chosen for this new indication of COVID-19 infection. However, available pharmacokinetic and pharmacokinetic-pharmacodynamic studies suffer from severe limitations leading to unreliable conclusions, especially in term of dosing optimization.
In this paper we propose to highlight these limitations and to identify some of the major requirements that need to be addressed in designing PK and PK-PD studies in this era of COVID. A special attention should be paid to pre-analytical and analytical requirements and to the proper collection of covariates affecting dose-exposure relationships (co-medications, use of specific organ support techniques and other clinical and para-clinical data). We also promote the development of population PK and PK-PD models specifically dedicated to COVID-19 patients since those previously developed for other diseases (SEL, malaria, HIV) and clinical situations (steady-state, non-ICU patients) are not representative of severe patients.
Therefore, implementation of well-designed PK and PD studies targeted to COVID-19 patients is urgently needed. For that purpose we call for multi-institutional collaborative work and involvement of clinical pharmacologists in multidisciplinary research consortia.
Keywords: COVID-19, Pharmacokinetics, Pharmacodynamics, PK-PD
1. Introduction
In the context of the COVID-19 pandemic, several existing approved drugs and experimental antiviral agents have been repurposed as potential antiviral candidates for the treatment of COVID-19 infection. While preliminary choices were essentially based on in vitro potency, clinical translation into effective therapies may be challenging due to unfavorable in vivo pharmacokinetic (PK) properties (i.e. plasma protein binding, tissue distribution, drug interactions) at the doses chosen for this new indication of COVID-19 infection. The particular conditions of COVID-19 infection (cytokine storm, multi-visceral failure and life-threatening prognosis), patient co-morbidities (i.e. obesity, diabetes, cardiovascular complications) and the requirement for a short and rapidly effective treatment further complicate the choice of the ideal candidate.
Remdesivir, chloroquine derivatives (essentially hydroxychloroquine (HCQ) due to a better safety profile than chloroquine) and the anti-HIV agent lopinavir (LPV) were among the first to be tested due to an in vitro antiviral activity demonstrated against SARS-CoV-2 or other similar respiratory viruses (i.e. SARS-CoV, MERS-CoV). Although remdesivir is not yet commercially available, other agents are already easily accessible to clinical investigators as they are part of the therapeutic armamentarium of other diseases (i.e. systemic lupus erythematosus (SLE) for HCQ and human immunodeficiency virus (HIV) treatment for LPV) probably explaining the large number of ongoing clinical trials worldwide. Favipiravir, ribavirin, tocilizumab, ivermectin, nafamostat and other agents have also been proposed for treatment of COVID-19 infection either as antivirals or immunomodulatory agents (Sanders et al., 2020).
In the area of infectious diseases and antiviral drugs, pharmacological properties are of particular importance for treatment choices, evaluation and optimization. Indeed, suboptimal antiviral response may be a consequence of inadequate exposure and/or poor PK-PD properties of the studied drug. From the HIV pandemic, we have learned that maintaining sufficient plasma drug exposure is critical to stop virus replication and avoid emergence of resistances (González de Requena et al., 2005). This has led to the implementation of strategies to optimize dosing regimen such as the PK “boosting”, used in the lopinavir/ritonavir (LPV/r) association. In this area, increasing knowledge on the PK and pharmacokinetic-pharmacodynamic (PK-PD) relationships of antiretrovirals has also demonstrated its usefulness in treatment optimization through the use of therapeutic drug monitoring (Boffito et al., 2005). In the context of COVID-19 infection, optimizing drug exposure at the site of infection, i.e. in the respiratory tract, is probably the key to successful treatment.
Accurate collection of PK and PD data is therefore of primary importance, especially for these repurposed drugs. We believe that extrapolation of PK data from other clinical situations may require specific caution due to different physiopathological conditions. In the context of the current global emergency, the number of clinical trials is rapidly increasing in order to quickly generate the data required for efficient patient healthcare. However, we have found that some of the pharmacology data published so far are somewhat disappointing, due to a lack of information permitting adequate comprehension of the dose-exposure and dose-effect relationships (Gautret et al., 2020; Perinel et al., 2020) and the poor representativeness of data used for simulations of effective dosing regimens (Garcia-Cremades et al., 2020; Perinel et al., 2020; Yao et al., 2020).
For these reasons and on behalf of the Clinical Pharmacology Committee of the French agency for AIDS and viral hepatitis research (ANRS) and the Therapeutic Drug Monitoring and Treatment Personalization working group of the French Society of Pharmacology and Therapeutics (SFPT), we believe that there is an urgent need for clarifications and improvements in order to generate high-quality PK and PK-PD data for the drugs to be used for COVID-19 treatment.
2. Limitations of available PK and PK-PD data
Few studies have already described PK and PK-PD in potential treatments for COVID-19 infection and only a small fraction of ongoing clinical trials have planned to do so. We were only able to find 18 out of the 1546 registered in clinicaltrials.gov (Table 1 ) (www.clinicaltrials.gov, accessed on: May 18, 2020). The list presented here is representative of the current situation though not necessarily exhaustive. The situation is particularly striking for the commonly used repurposed drugs HCQ and LPV for which only 2 trials, one in children and another in adults, were designed to measure concentration data (Table 1). PK analyses are preferentially planned for new drugs (i.e. monoclonal antibodies) not yet approved in another indication, such as to take advantage of COVID-19 infection to acquire PK data in humans. Perinel et al. (2020) and Gautret et al. (2020) have reported HCQ blood or serum concentrations from COVID-19 patients. However, in these two papers, very little methodological, demographic, clinical, paraclinical and even dosing information that could help to better understand the dose-exposure relationship in COVID-19 patients is available. Perinel et al. have succinctly described a small population of intensive care unit (ICU) patients receiving 200 mg of HCQ three times daily without loading dose in which HCQ whole blood concentrations were measured (Perinel et al., 2020). A similar dosing regimen was used by Gautret et al. (2020) but serum concentrations were determined instead. Apparently, for their study, they have reported the sum of HCQ and its metabolite concentrations. Whether the approximation used to quantify HCQ metabolite is valid (Gautret et al., 2020) and whether this metabolite is active on SARS-CoV-2 require further evaluation. Anyway, simply summing-up the concentrations of the active moiety and its metabolite cannot effectively contribute to comprehension of PK and PK-PD relationships since they may present different PK and/or PD properties and consequently misrepresent the adequate drug exposure (Tett, 1993). These preliminary observations also raise the question of the selection of the adequate biological matrix (blood vs plasma or serum) for PK assessment of HCQ.
Table 1.
Pharmacological issues relative to COVID-19 infection in clinical trials: 18 studies found for: Pharmacokinetic | COVID-19.
| Clinical trial Identifier | Official Title | Primary outcome | Experimental drug | Pharmacological |
|---|---|---|---|---|
| NCT04345614 | A Randomized Controlled Open-Label Study of CM4620 Injectable Emulsion in Patients with Severe COVID-19 Pneumonia | Safety, efficacy, and the PK profile of CM4620-IE in patients with severe COVID-19 pneumonia. | CM4620-Injectable Emulsion | M4620-IE serum concentration |
| NCT04357613 | A RANDOMIZED NON-COMPARATIVE PHASE 2 PILOT STUDY TESTING THE VALUE OF IMATINIB MESYLATE AS AN EARLY TREATMENT OF COVID-19 DISEASE IN AGED HOSPITALIZED PATIENTS. | To evaluate the benefit of early imatinib therapy to prevent severe COVID-19 disease in hospitalized aged patients. [Time Frame: 30 days] | Imatinib 800 mg/d during 14days | To evaluate plasma levels of imatinib [Time Frame: 14 days] Imatinib trough level |
| NCT04346628 | A Phase 2 Randomized, Open Label Study of Oral Favipiravir Compared to Standard Supportive Care in Subjects With Mild COVID-19 | Time until cessation of oral shedding of SARS-CoV-2 virus [ Time Frame: Up to 28 days ] | Favipiravir administered orally, 1800 mg on the first dose (day 1) followed by 800 mg twice daily for the next 9 days (days 2–10). | Cmax of favipiravir [Time Frame: Days 1 and 10 (samples taken 30 min prior to and 1 h following favipiravir administration)] |
| NCT04358549 | Open Label, Randomized, Controlled Phase 2 Proof-of-Concept Study of the Use of Favipiravir v. Standard of Care in Hospitalized Subjects With COVID-19 | Time to viral clearance [Time Frame: Day 29]; To determine the effect of favipiravir + SOC v. SOC on viral clearance of COVID-19 as measured by nasopharyngeal and oropharyngeal sampling | Day 1: favipiravir 1800 mg BID plus Standard of Care (SOC) Days 2–14: 1000 mg BID plus SOC. For subjects with Child-Pugh A liver impairment: Days 2–14: 800 mg BID plus SOC | Plasma PK of favipiravir |
| NCT03891420 | A Phase 1b Double-blind, Placebo-controlled, Dose-ranging Study to Evaluate the Safety, Pharmacokinetics, and Anti-viral Effects of Galidesivir Administered Via Intravenous Infusion to Subjects With Yellow Fever or COVID-19 | Number of subjects with 1- treatment emergent adverse events and serious adverse events 2- change in laboratory parameters |
Galidesivir IV infusion | Exposure of galidesivir as measured by plasma concentrations |
| NCT03648372 | An Open Label, Dose-Escalation, Phase I Study to Evaluate the Safety, Tolerability and Pharmacokinetics of TAK-981 in Adult Patients With Advanced or Metastatic Solid Tumors or Relapsed/Refractory Hematologic Malignancies and in a Subset With Coronavirus Disease 2019 | COVID-19 proof of concept: Once the Safety Lead-in is complete and a TAK-981 dose and regimen is selected by the Safety Monitoring Committee (SMC), the randomized COVID-19 proof of concept will begin with participants randomized to Arm A: COVID-19 standard of care (SOC), or Arm B COVID-19 SOC + TAK-981. | TAK-981 Intravenous infusion | TAK-981 COVID-19 safety lead-in: TAK-981, intravenously, administered as 60 min-infusion, once on Days 1 and 4. The starting dose of TAK-981 will be 60 mg (mg). |
| NCT04371640 | A Randomized, Double-Blinded, Placebo-Controlled Trial Evaluating the Virological Efficacy, Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Sirolimus Adjuvant Therapy in Patients With COVID-19 | Change in SARS-COV-2 viral burden from baseline to day 7 of treatment [Time Frame: Baseline, and days 1, 2, 3, 4, 5, 6, & 7 post-dose for all patients] SARS-COV-2 viral burden will be quantified for both arms using a qRT-PCR |
Sirolimus + standard medical care Day 1: 10 mg Days 2–7: 5 mg Intervention: Drug: Sirolimus 1 MG/ML |
PK of sirolimus |
| NCT04363736 | A Phase-II, Open-Label, Randomized, Multicenter Study to Investigate the Pharmacodynamics, Pharmacokinetics, Safety, and Efficacy of 8 mg/kg or 4 mg/kg Intravenous Tocilizumab in Patients With Moderate to Severe COVID-19 Pneumonia | Concentration of C-Reactive Protein (CRP) [Time Frame: Day 7] | Participants will receive intravenous (IV) tocilizumab (TCZ) at a dose of 8 mg/kg vs 4 mg/kg in addition to standard-of-care treatment. | Concentration of C-Reactive Protein (CRP) as surrogate marker of TCZ PK |
| NCT04320615 | A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia | Clinical Status Assessed Using a 7-Category Ordinal Scale [ Time Frame: Day 28 ] | Participants will receive 1 intravenous (IV) infusion of TCZ, dosed at 8 mg/kg, up to a maximum dose 800 mg. Up to 1 additional dose may be given if clinical symptoms worsen or show no improvement. | Serum Concentration of TCZ [Time Frame: Up to 60 days] |
| NCT04158648 | A Multicenter, Open-Label Study to Evaluate the Safety, Efficacy, Pharmacokinetics, and Pharmacodynamics of Emicizumab in Patients With Mild or Moderate Hemophilia A Without FVIII Inhibitors | 17 primary outcomes | 4 loading doses of emicizumab 3 mg/kg will be administered subcutaneously once a week (QW) for 4 weeks followed by participant's preference of one of the following maintenance regimens: 1.5 mg/kg QW, 3 mg/kg once every 2 weeks (Q2W), or 6 mg/kg once every 4 weeks (Q4W). | Plasma Trough Concentration (Ctrough) of Emicizumab Over Time [Time Frame: Pre-dose at Weeks 1, 2, 3, 4, 5, 9, 13, 17, 21, 25, 29, 33, 37, 41, 45, and 49, and every 12 weeks thereafter until study completion/discontinuation (up to approximately 30 months)] |
| NCT04278404 | Pharmacokinetics, Pharmacodynamics, and Safety Profile of Understudied Drugs | Usual PK parameters | Aminocaproic acid, Amiodarone, Bosentan, Budesonide, Cefdinir, Cefepime, Ceftazidime, Clindamycin, Clobazam, Dexamethasone, Dexmedetomidine, Dextroamphetamine/Amphetamine- Immediate Release, Fosfomycin Furosemide, Gabapentin Guanfacine, Hydrocortisone Labetalol, Meropenem, Metformin Milrinone, Nalbuphine, Nicardipine, Nifedipine, Oseltamivir, Oxycodone, Risperidone, Sertraline, Sevelamer Carbonate/Sevelamer, Hydrochloride, Spironolactone, Terbutaline, Tranexamic acid Voriconazole, Zolpidem Dextroamphetamine/Amphetamine - Extended Release, Azithromycin Chloroquine, Hydroxychloroquine, Lopinavir/Ritonavir, Ribavirin, Tocilizumab |
The POP02 study is collecting bodily fluid samples (i.e., whole blood, effluent samples) of children prescribed the following drugs of interest per standard of care: The prescribing of drugs to children is not part of this protocol. Participants will receive DOIs as prescribed by their treating provider. |
| NCT04392219 | A Randomized, Double-Blind, Placebo-Controlled, First-in-Human Study Designed to Evaluate the Safety, Tolerability, and Pharmacokinetics of EIDD-2801 Following Oral Administration to Healthy Volunteers | Safety and Tolerability of Single Ascending Dose (SAD) of EIDD-2801 (Part 1) and Multiple Ascending Dose (MAD) of EIDD-2801 (Part 3): Adverse Events [ Time Frame: From screening through study completion, up to 15 days ] Number and severity of treatment emergent adverse events |
Part 1: Subjects will be randomized to receive a single oral dose of EIDD-2801 or Placebo. Part 3: Subjects will be randomized to receive twice daily dosing either EIDD-2801 or Placebo. |
Pharmacokinetics (PK) of EIDD-2801 when given as Single Doses (Part 2): Maximum observed concentration Cmax [ Time Frame: Day 1 through Day 18 ] Multiple pharmacokinetic variables of EIDD-2801 will be assessed and may include, but are not limited to: Maximum observed concentration Cmax |
| NCT04346199 | A Phase 2, Open Label, Randomized Study of the Efficacy and Safety of Acalabrutinib With Best Supportive Care Versus Best Supportive Care in Subjects Hospitalized With COVID-19 | Subject alive and free of respiratory failure [ Time Frame: Day 14 ] | Acalabrutinib- administered orally | Plasma PK parameters of acalabrutinib and its active metabolite ACP- 5862 [ Time Frame: 28 days after last dose ] |
| NCT04380688 | A Phase 2, Open Label, Randomized Study of the Efficacy and Safety of Acalabrutinib With Best Supportive Care Versus Best Supportive Care in Subjects Hospitalized With COVID-19 | Occurrence of Adverse Events and Serious Adverse Events [Time Frame: 28 days after last dose] Subject alive and free of respiratory failure [Time Frame: Day 14] |
Acalabrutinib- administered orally | Plasma PK parameters of acalabrutinib and its active metabolite ACP- 5862 [Time Frame: 28 days after last dose] |
| NCT04350736 | A Phase 1, Double-blind, Randomized, Placebo-controlled, Sponsor-open, SAD and MAD Study in Healthy Subjects to Evaluate the Safety, Tolerability, and PK of Inhaled TD-0903, a Potential Treatment for ALI Associated With COVID-19 | Safety and Tolerability of MAD of TD-0903: Adverse Events [ Time Frame: Day 1 to Day 14 ] Number and severity of treatment emergent adverse events |
TD-0903 Study drug to be administered by inhalation |
Plasma PK parameters of SAD and MAD TD-0903 |
| NCT04369469 | A Phase 3 Open-label, Randomized, Controlled Study to Evaluate the Efficacy and Safety of Intravenously Administered Ravulizumab Compared With Best Supportive Care in Patients With COVID-19 Severe Pneumonia, Acute Lung Injury, or Acute Respiratory Distress Syndrome | Survival (based on all-cause mortality) at Day 29 [ Time Frame: Baseline, Day 29 ] | Weight-based doses of ravulizumab will be administered intravenously on Days 1, 5, 10, and 15. Other Names: Ultomiris ALXN1210 |
PK parameters of ravulizumab |
| NCT04379271 | A Prospective, Multi-Center, Randomized, Placebo-Controlled, Double-Blinded Study to Evaluate the Efficacy, Safety and Tolerability of IMU-838 as Addition to Investigator's Choice of Standard of Care Therapy, in Patients With Coronavirus Disease 19 | Proportion of patients without any need for INV until end-of-study (EoS) [ Time Frame: Throughout the Study (Day 0 to Day 28) ] Clinical |
twice-daily (BID) oral 22.5 mg IMU-838 (45 mg/day + SoC) | Morning trough plasma levels of IMU-838 on Days 0, 1, 2, 3, 6, 14, and 28 [ Time Frame: on Days 0, 1, 2, 3, 6, 14, and 28 ] |
| NCT04315948 | Multi-center, Adaptive, Randomized Trial of the Safety and Efficacy of Treatments of COVID-19 in Hospitalized Adults | Percentage of subjects reporting each severity rating on a 7-point ordinal scale [ Time Frame: Day 15 ]
|
Remdesivir will be administered as a 200 mg intravenous loading dose on Day 1, followed by a 100 mg once-daily intravenous maintenance dose for the duration of the hospitalization up to a 10 days total course. Lopinavir/ritonavir (400 lopinavir mg/100 mg ritonavir) will be administered every 12 h for 14 days in tablet form. For patients who are unable to take medications by mouth, the lopinavir/ritonavir (400 lopinavir mg/100 mg ritonavir) will be administered as a 5-mL suspension every 12 h for 14 days via a pre-existing or newly placed nasogastric tube. Interferon ß1a will be administered subcutaneously at the dose of 44 μg for a total of 3 doses in 6 days (day 1, day 3, day 6). Hydroxychloroquine will be administered orally as a loading dose of 400 mg twice daily for one day followed by 400 mg once daily for 9 days. The loading dose of hydroxychloroquine through a nasogastric tube will be increased to 600 mg twice a day for one day, followed by a maintenance dose of 400 mg once a day for 9 days |
Plasma concentration of lopinavir [ Time Frame: Days 1, 3, 5, 8 and 11 ] On Day 1, plasma concentration 4 h after the first administration (peak), and before the second administration (trough at H12) On Days 3, 5, 8 and 11, trough plasma concentration (before dose administration) while hospitalized Plasma concentration of hydroxychloroquine [ Time Frame: Days 1, 3, 5, 8 and 11 ] On Day 1, plasma concentration 4 h after the first administration (peak), and before the second administration (trough at H12) On Days 3, 5, 8 and 11, trough plasma concentration (before dose administration) while hospitalized |
Some of these papers (Garcia-Cremades et al., 2020; Perinel et al., 2020; Yao et al., 2020) have built dosing recommendations for HCQ based on PK models previously developed in other diseases (SEL, malaria) and clinical situations (steady-state, non-ICU patients), which raises questions about the relevance of such recommendations. Perinel et al. (2020) have graphically compared measured HCQ blood concentrations in COVID-19 patients to PK simulations obtained with a HCQ population PK model initially developed for rheumatoid arthritis (RA) patients (Carmichael et al., 2003). Results clearly demonstrate the inadequacy of a population PK model developed for stable chronic RA patients to describe actual PK data obtained in ICU patients. This point has also been confirmed by Martin-Blondel et al. (Martin-Blondel et al., n.d.) in their attempt to describe HCQ plasma concentrations obtained in COVID-19 patients using a PK model initially developed for SLE patients. As a consequence, we believe that due to probably large PK differences affecting either clearance or volume of distribution as demonstrated with other anti-infective drugs, this type of PK model should not be used to simulate any dosing regimens for ICU patients (Roberts et al., 2014). Whether these models could be applied to less severe COVID patients, at the early stage of the disease, requires confirmation. It is likely that models developed in healthy volunteers may be appropriate in the prophylaxis setting. The population PK-PD model developed by Garcia-Cremades et al. (2020) to describe viral decline and QTc prolongation after HCQ administration in COVID-19 patients, may theoretically enable achievement of more robust simulation results. For the PK part of the model, they used a population PK model initially developed using plasma concentrations obtained from healthy Korean volunteers and Korean patients receiving HCQ for treatment of vivax malaria (Lim et al., 2009). To demonstrate the adequacy of this model for COVID-19 patients, they used published serum concentrations obtained in non-severe and non-ICU COVID-19 patients drawn from the study by Gautret et al. (2020) described above. Although some differences between serum and plasma concentrations cannot be ruled out (Bergqvist and Domeij-Nyberg, 1983), they showed that HCQ concentrations from COVID-19 patients fell within the lower range of expected population profiles, thereby suggesting that this model could be appropriate to describe the PK of HCQ, at least in non-ICU COVID-19 patients. However, one should note that the concentration data set used for this model validation expressed HCQ concentration as the sum of the active moiety and its metabolite (Gautret et al., 2020), consequently leading to systematic overestimation of actual exposure to HCQ. Other limitations of this paper apply to the PD parts of the model since viral kinetic data are from SARS-CoV-1 and the concentration/QTc prolongation data were obtained from a study with chloroquine. Limitations also apply to the study by Yao et al. (2020), who developed a PB-PK model to simulate different dosing regimens for HCQ in COVID-19 patients. Indeed, HCQ concentrations in lung were simulated by incorporating in their model blood-to-lung concentration data obtained by analyzing tissue homogenates after dosing in rats (McChesney, 1983). This approach may provide unreliable diffusion data in lungs (Mouton et al., 2008; Nix et al., 1991) and consequently unreliable simulations at the site of infection. Similar limitations of this lung model have been discussed by Fan et al. (2020) and by Yeo et al. (2020).
Similarly, a steady-state population PK model initially developed from HIV patients receiving standard dose LPV/r has been used to simulate LPV total and free concentrations for comparison with various virological endpoints, i.e. actual IC50 values for HIV-1, MERS-CoV and SARS-CoV (Smith et al., 2020). From a PK perspective, this model is probably unable to describe actual LPV concentrations currently measured in COVID-19 patients, which are as recently reported (Gregoire et al., 2020) much higher than those observed in HIV patients. Furthermore, this recent paper showed that despite very high plasma LPV concentrations observed in patients, the free fraction, representing the active part of the drug, was not affected (Gregoire et al., 2020).
Concerning favipiravir, a recent comment proposes to evaluate higher doses for SARS-CoV-2 than in Ebola disease, based on both in vitro EC50 and PK simulations data associated with a close monitoring of adverse events and plasma concentrations (Eloy et al., 2020). However, favipiravir is a prodrug, and determination of tissue and intracellular exposure of the activate metabolite favipiravir-ribofuranosyl-5-triphosphate would be required in order to better characterize PK-PD relationship (Du and Chen, 2020).
No such attempts have been made with remdesivir, yet. Furthermore, data on the diffusion of these drugs into the pulmonary tract are lacking.
3. Recommendations for improvements
We propose here to identify some of the major requirements that need to be addressed in designing PK and PK-PD studies in this era of COVID.
4. Pre-analytical and analytical requirements
Pre-analytical and analytical steps should be strictly controlled to guarantee the necessary accuracy of measured concentrations. For LPV, well-standardized sampling and assay procedures have been defined for the monitoring of HIV patients and we believe that these procedures may adequately apply to COVID-19 patients. By contrast, this is more confusing with HCQ since it could be analyzed either in whole blood (Carmichael et al., 2003) or in plasma (Morita et al., 2016; Tett et al., 1989). In the area of anti-infective drugs, plasma may appear as the preferred matrix since free plasma concentration is in equilibrium with tissue concentrations and the putative site of action. Moreover, some PK-PD relationships have been commonly developed for antivirals using plasma concentration as a surrogate for the concentrations at the site of action (Rizk et al., 2012). However, as regards plasma, it appears that the pre-analytical step is of particular importance for chloroquine derivatives since early observations have shown that chloroquine could be released from blood cells, leading to overestimation of plasma concentrations (Bergqvist and Domeij-Nyberg, 1983). Preliminary pre-analytical data suggest a similar pattern with HCQ when blood is not rapidly centrifuged and plasma separated. Moreover, HCQ blood-to-plasma ratio presents wide between-subject variability according to disease context and equilibrium distribution (Morita et al., 2016; Tett et al., 1988) suggesting that extrapolation from one to another is not reliable. Pre-analytical treatment of plasma for remdesivir assay requires an acidic stabilization step (Gilead, personal communication). Regarding the analytical step, robust specific and sensitive assays targeting individual analytes (active moiety and metabolites) have already been developed and validated for clinical pharmacokinetics. In the event of matrix modification (blood vs. plasma), a partial validation could be considered (European Medicines Agency. Guideline on bioanalytical method validation, 2011). In addition, inter-laboratory comparisons should be organized, especially in case of multicenter PK studies.
5. Collection of covariates affecting dose-exposure relationships
In order to comprehensively understand and describe dose-exposure relationships, collection of accurate dosing information, demographic, clinical and para-clinical data is mandatory.
Regarding dosing information, galenic forms and routes of administration are important data. It is common practice in the setting of an ICU, where the patient is unable to swallow solid oral dosage forms, to administer drugs through enteral feeding tubes. In this situation, the administration of crushed tablets in the feeding tube may have an impact on bioavailability, which may alter the dosing-exposure relationship of the studied drug. This has been demonstrated in the HIV setting where crushing tablets of LPV have led to a 45% decrease in AUC (Best et al., 2011). Surprisingly, crushing tablet does not appear to have a significant impact on the oral bioavailability of HCQ (Sanofi, personal communication).
The dosing regimen may include a loading dose that is particularly important in view of more rapidly achieving effective concentrations. This point is critical for HCQ due to its very long half-life (Lê et al., 2020; Tett et al., 1989). A loading dose has also been suggested for LPV (Smith et al., 2020) and is required for remdesivir (European Medicines Agency. Summary on remdesivir compassionate use. April 2020).
Knowledge of co-administered drugs is likewise of primary importance, particularly in the context of ICU, where many medications are involved or when complications such as secondary infections are present, requiring co-administration of antibacterials and antifungals (Sanders et al., 2020). Co-medications are also frequent in patients particularly at risk for severe COVID-19, i.e. chronic disease patients such as obese and/or diabetic patients. Indeed, drug-drug interactions may occur at each step of the ADME process, leading to altered pharmacokinetics and, possibly, in increased variability of drug exposure. Divalent cations, such as calcium or magnesium, may interfere with the absorption of chloroquine (McElnay et al., 1982), thereby reducing its bioavailability. Similarly, LPV and HCQ, which undergo CYP mediated metabolism (Liverpool HIV group. Liverpool COVID-19 Interactions. https://www.covid19-druginteractions.org/, 2020), could be victims of drug-drug interactions affecting both bioavailability and clearance. Although remdesivir is also a substrate for CYP isoenzymes in vitro, its metabolism is likely to be predominantly mediated by hydrolase activity (Liverpool HIV group. Liverpool COVID-19 Interactions. https://www.covid19-druginteractions.org/, 2020), but this does not stave off inter- nor intra-patient variability.
Anthropometric characteristics, which are currently involved in PK variability, should be collected as well. Body weight has been described as a significant covariate of HCQ clearance in Japanese patients suffering from systemic lupus erythematosus (Morita et al., 2016). Whether this relationship is valid in COVID-19 patients requires confirmation.
The use of specific organ support techniques (Zhang et al., 2020) such as renal replacement therapy (hemodialysis, hemofiltration, hemodiafiltration) or ventilation support (high-flow oxygen therapy, noninvasive ventilation, invasive mechanical ventilation and extracorporeal membrane oxygenation, ECMO) should be reported in order to assess their impact on drug exposure. A significant impact of hemodialysis on the pharmacokinetics of HCQ (Tett et al., 1989) and LPV (Gupta et al., 2008) is unlikely but requires confirmation since steady-state conditions are not achieved in COVID-19 patients. An impact of ECMO on chloroquine pharmacokinetics has been described (Bagate et al., 2017) but no convincing data exist yet for LPV (Ghazi Suliman et al., 2017). However, a significant impact of ECMO should be considered since several studies have shown altered PK profiles in this situation (Ha and Sieg, 2017). These data need to be confirmed in COVID-19 patients, especially considering differences in steady-state attainment.
Importantly, severe COVID-19 is associated with a systemic hyper-inflammation state, the so-called cytokine storm, which is associated with highly elevated C-reactive protein (CRP) levels, hyperferritinaemia and increased cytokine levels (IL-1β, IL-2, IL-6, IL-17, IL-8, TNF and CCL2) (McGonagle et al., 2020). The impact of inflammation on the pharmacokinetics of drugs has already been highlighted. For example, several studies have reported a positive association between CRP levels and voriconazole or tacrolimus concentrations (Bonneville et al., 2020; Gautier-Veyret et al., 2019). The extent of increase in voriconazole concentration could be explained by downregulation of CYP isoenzymes by inflammatory stimuli leading to reduced metabolism (Morgan, 2009). Impact may also occur through altered expression of membrane transporters (Seifert et al., 2017). Preliminary data suggest a major decrease of LPV clearance in COVID-19 patients, highlighting the putative role of inflammation in these PK alterations.
6. Development of population PK and PK-PD models
Population approaches, which rely on PK-PD modeling, appear particularly appealing in this situation because these models can handle sparse data originating from different sources (ICU, non-ICU, dialysis patients) and different dosing regimens. Further, covariates as described above can be included in these population models to identify PK and pharmacodynamic (PD) variability factors. Finally, population-PK and PK-PD models have proven useful in the area of infectious diseases, providing a valuable tool to explore and predict efficient dosing regimens (Jumah et al., 2018). However, we believe that the development of population PK models specifically dedicated to acute COVID-19 patients is urgently needed due to major physiopathological differences with chronically ill HIV, RA and SEL patients. For that purpose, a collaborative multicenter study within clinical centers involved in clinical trials with these drugs may enable collection of the amount of data necessary to develop such a PK model. Such a collaborative project is ongoing within our groups.
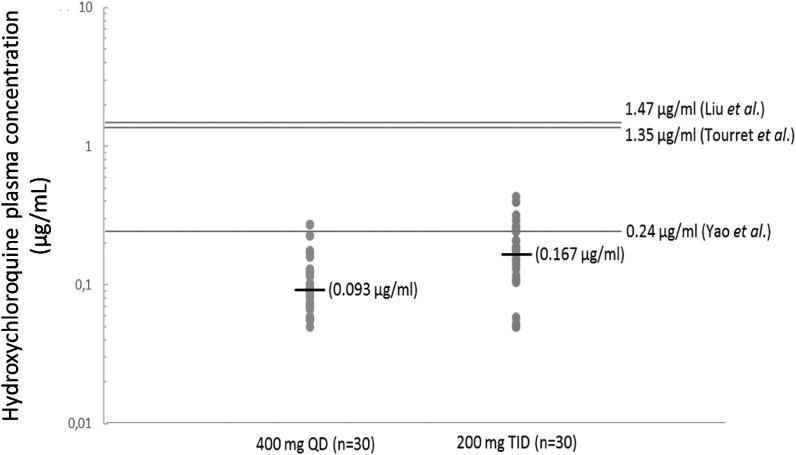
Current dosing regimens proposed for these repurposed drugs are empirical since they have not been specifically developed for COVID-19 but rather for HIV, malaria and chronic inflammatory diseases. In order to develop more efficient dosing regimens, we also need reliable PD data to relate the concentration at the site of infection to viral sensitivity through the development of PK-PD models. Viral sensitivity could be determined through the measurement of EC50 or EC90 either in presence or in absence of human serum. Addition of human serum enables representation of the impact of drug protein binding on drug potency. EC50 is available for HCQ (Liu et al., 2020; Yao et al., 2020), LPV (Choy et al., 2020), favipiravir (Eloy et al., 2020), remdesivir (Choy et al., 2020) and other drugs such as ivermectin (Table 2 ). But, as observed in Table 2, translation into effective therapies may be challenging due to unfavorable in vivo PK properties. For example, ivermectin, total (bound and unbound) plasma concentrations are >45 times lower than the in vitro EC50. Since ivermectin is highly bound to plasma protein and its accumulation into human lung unknown (Schmith et al., 2020), the likelihood for ivermectin to reach IC50 at the site of action after the approved dose is low. The large variability reported in EC50 values between studies, mainly explained by differences in experimental conditions or mechanism of action, may also contribute to the difficulty of determining the accurate target concentration in vivo. For example, with HCQ, we observed high variability between EC50 values (i.e. for HCQ: from 0.72 μM ≈ 0.24 μg/mL to more than 15 μM ≈ 5 μg/mL) depending on multiplicity of infections (MOIs), incubation duration, presence/absence of human serum, etc …, which may be of PK relevance for a drug presenting such a narrow therapeutic index. As reported in Fig. 1 , HCQ median plasma concentrations could be 1.5 to 15 times lower than the in vitro EC50 depending on the scheme of administration and EC50 values. Moreover, the choice of experimental cells (Vero E6 cells or pulmonary epithelial cells) may be crucial for such respiratory viruses, although no consensus has yet arisen, and extrapolations from other viruses appear speculative (Smith et al., 2020). Finally, regarding the lack of specificity/sensitivity of the current virological marker (i.e viral load in the upper respiratory tract) to assess antiviral activity, the combination of multiple PD parameters could be of interest. The relationships between viral load and clinically relevant endpoints is mostly unknown. Indeed, many clinical endpoints could be considered: mortality, recovery, hospital discharge, respiratory failure, need for oxygenation or mechanical ventilation, time-to-intubation, hospitalization, duration of hospital-stay, ICU admission, time-to-improvement, severity scores (SOFA), etc. Selecting the best clinically relevant endpoint may depend upon several factors such as its clinical relevance, its measurement's reliability, disease severity and statistical considerations.
Table 2.
Available EC50 against SARS-CoV-2 and corresponding total plasma concentrations obtained in human.
| Repurposed drug | In vitro antiviral activity against SARS-CoV-2 | Plasma total concentrations in human (SARS-CoV-2 infected patients) | Plasma total concentrations in human (others populations) |
|---|---|---|---|
| Lopinavir | EC50 = 26.63 μM (16.75 μg/mL) (Choy et al., 2020) EC50 = 15.27 μM (9.60 μg/mL) (Jeon et al., 2020) |
400/100 mg BID: Ctrough [11.4–30.8 μg/mL] 400/100 mg QD: Ctrough [8.7–18.3 μg/mL](Gregoire et al., 2020) |
|
| Remdesivir | EC50 = 0.77 μM (0.46 μg/mL) (Wang et al., 2020) EC50 = 23.15 μM (13.95 μg/mL) (Choy et al., 2020) EC50 = 8.24 μM (4.97 μg/mL) (Jeon et al., 2020) EC50 = 165 ± 0.79 μM (99.43 μg/mL) (Touret et al., 2020) |
Healthy subjects 100 mg QD for 5–10 days: Mean (SD) GS-443902 C24 in PBMCs = 10.2 (5.5) μM (Day 5) (European Medicines Agency. Summary on remdesivir compassionate use. April 2020) |
|
| Hydroxychloroquine | EC50 = 0.72 μM (0.24 μg/mL) (Yao et al., 2020) EC50 = 4.51 μM (1.47 μg/mL) (Liu et al., 2020) EC50 = 4.17 μM (1.35 μg/mL) (Touret et al., 2020) |
200 mg TID MD without LD: Ctrough = 0.09 ± 0.01 (H8 on day 2) to 0.19 ± 0.06 (H8 on day 6) μg/mL 400mgx2 LD on day 1 followed by 400 × 1 MD: Ctrough = 0.09 ± 0.12 (H12 on day 2) to 0.13 ± 0.14 (H12 on day 6) μg/mL (Martin-Blondel et al., n.d.) |
|
| Favipiravir | EC50 = 9.4 μg/mL EC50 = 40–80 μg/mL (Eloy et al., 2020) EC50 > 100 μM (>15.71 μg/mL) (Choy et al., 2020) EC50 > 500 μM (>78.55 μg/mL) (Jeon et al., 2020) |
Ebola patients 6000 mg on day 0 followed by 1200 mg BID for 9 days: Median (range) Ctrough = 46.1 (2.3–106.9) μg/mL on day 2 and 25.9 (0–173.2) μg/mL on day 4 (Nguyen et al., 2017) |
|
| Ivermectin | EC50 = 2 μM (1750 ng/mL) (Caly et al., 2020) |
Onchocerciacis patients Single dose of 150 μg/kg PO: Cmax = 38.2 ± 5.8 ng/mL (Okonkwo et al., 1993) |
*Remdesivir is a prodrug rapidly converted to a circulating monophosphate nucleoside analog (GS-441524) which inside cells undergoes rapid conversion to the pharmacologically active analog of adenosine triphosphate (GS-443902) that inhibits viral RNA polymerases. LD = loading dose, MD = maintenance dose.
Fig. 1.
Observed hydroxychloroquine plasma concentrations (median) after 3–5 days of treatment in patients treated for SARS-CoV-2 infection depending on the scheme of administration: 400 mg QD after a loading dose of 400mgx2 on day 1 and 200 mg TID without any loading dose (C. Solas, internal data from the Pharmacokinetics and Toxicology Laboratory). Solid line represent in vitro EC50 described against SARS-CoV-2 at T48h post incubation.
In order to assess drug penetration to the site of action, determination of drug concentration in bronchoalveolar lavage could be proposed as a surrogate for lung concentrations. Urea or albumin should be used as a marker of dilution to determine the volume of epithelial lining fluid recovered and samples should be preferably collected at multiple time points throughout the dosing interval (Rodvold et al., 2011). To our knowledge, corresponding data for COVID-19 treatment candidates are scarce. A case study in a single HIV patient (Atzori et al., 2003) reported significant LPV concentration in BAL at steady-state. ELF and total plasma concentrations were 14.4 and 8.1 μg/mL, respectively. The corresponding ratio of ELF over total plasma concentrations was 1.8, suggesting an accumulation of LPV in ELF requiring confirmation in COVID-19 patients. Data of the same order of magnitude but with less accumulation were also reported by Boffito et al. (2002).
7. Conclusion
Available PK and PK-PD studies suffer from severe limitations leading to unreliable conclusions, especially in term of dosing optimization. At this time, there is still no high-quality evidence to support the use of these repurposed drugs for the treatment of COVID-19. Therefore, implementation of well-designed PK and PD studies specifically targeted to COVID-19 patients is urgently needed in order to increase our comprehension of the dose-exposure-effect relationships of the repurposed drugs. Without these data, efficient evaluation and development of effective dosing regimens will remain difficult. Other scientific societies (Baker et al., 2020) and organizations (Hartman et al., 2020; Rayner et al., 2020) have also recently issued call to action for the appropriate application of clinical pharmacology principles in the search for COVID-19 treatments. Further, to accelerate the production of up-to-date PK and PD data and the development of meaningful PK and PK-PD models, we also call for multi-institutional collaborative work and involvement of clinical pharmacologists in multidisciplinary research consortia.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
Gilles Peytavin has received travel grants, consultancy fees, honoraria, or study grants from various pharmaceutical companies, including Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Others: None.
Acknowledgments
We wish to thank Jeffrey Arsham, an American medical translator, for editing the English of our original manuscript.
ANRS-AC43 Clinical Pharmacology and SFPT Therapeutic Drug Monitoring and Treatment Personalization Committees: Zoubir Djerada, Matthieu Grégoire, Florian Lemaitre, Damien Montange, Patrice Muret, Jean-Marc Treluyer.
References
- Atzori C., Villani P., Regazzi M., Maruzzi M., Cargnel A. Detection of intrapulmonary concentration of lopinavir in an HIV-infected patient. AIDS. 2003;17:1710–1711. doi: 10.1097/00002030-200307250-00022. [DOI] [PubMed] [Google Scholar]
- Bagate F., Radu C., Mekontso Dessap A., de Prost N. Early extracorporeal membrane oxygenation for cardiovascular failure in a patient with massive chloroquine poisoning. Am. J. Emerg. Med. 2017;35 doi: 10.1016/j.ajem.2016.08.058. 380.e3-380.e4. [DOI] [PubMed] [Google Scholar]
- Baker E.H., Gnjidic D., Kirkpatrick C.M.J., Pirmohamed M., Wright D.F.B., Zecharia A.Y. A call for the appropriate application of clinical pharmacological principles in the search for safe and efficacious COVID-19 (SARS-COV-2) treatments. Br. J. Clin. Pharmacol. 2020 doi: 10.1111/bcp.14416. [DOI] [PubMed] [Google Scholar]
- Bergqvist Y., Domeij-Nyberg B. Distribution of chloroquine and its metabolite desethyl-chloroquine in human blood cells and its implication for the quantitative determination of these compounds in serum and plasma. J. Chromatogr. 1983;272:137–148. doi: 10.1016/s0378-4347(00)86110-1. [DOI] [PubMed] [Google Scholar]
- Best B.M., Capparelli E.V., Diep H., Rossi S.S., Farrell M.J., Williams E., Lee G., van den Anker J.N., Rakhmanina N. Pharmacokinetics of lopinavir/ritonavir crushed versus whole tablets in children. J. Acquir. Immune Defic. Syndr. 2011;58:385–391. doi: 10.1097/QAI.0b013e318232b057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffito M., Acosta E., Burger D., Fletcher C.V., Flexner C., Garaffo R., Gatti G., Kurowski M., Perno C.F., Peytavin G., Regazzi M., Back D. Current status and future prospects of therapeutic drug monitoring and applied clinical pharmacology in antiretroviral therapy. Antivir. Ther. (Lond.) 2005;10:375–392. [PubMed] [Google Scholar]
- Boffito M., Hoggard P.G., Back D.J., Bonora S., Maiello A., Lucchini A., Di Perri G. Lopinavir measurement in pleural effusion in a human immunodeficiency virus type 1-infected patient with kaposi's sarcoma. Antimicrob. Agents Chemother. 2002;46:3684–3685. doi: 10.1128/aac.46.11.3684-3685.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville E., Gautier-Veyret E., Ihl C., Hilleret M.-N., Baudrant M., Fonrose X., Stanke-Labesque F. Unexpected overdose blood concentration of tacrolimus: keep in mind the role of inflammation. Br. J. Clin. Pharmacol. 2020 doi: 10.1111/bcp.14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J., Catton M., Jans D., Wagstaff K. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael S.J., Charles B., Tett S.E. Population pharmacokinetics of hydroxychloroquine in patients with rheumatoid arthritis. Ther. Drug Monit. 2003;25:671–681. doi: 10.1097/00007691-200312000-00005. [DOI] [PubMed] [Google Scholar]
- Choy K., Wong A., Kaewpreedee P., Sia S., Chen D., Hui K., Chu D., Chan M., Cheung P., Huang X., Peiris M., Yen H. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y.-X., Chen X.-P. Response to “Dose rationale for favipiravir use in patients infected with SARS-CoV-2. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloy P., Solas C., Touret F., Mentré F., Malvy D., de Lamballerie X., Guedj J. Dose rationale for favipiravir use in patients infected with SARS-CoV-2. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1877. [DOI] [PubMed] [Google Scholar]
- Fan J., Zhang X., Liu J., Yang Y., Zheng N., Liu Q., Bergman K., Reynolds K., Huang S.-M., Zhu H., Wang Y. Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cremades M., Solans B.P., Hughes E., Ernest J.P., Wallender E., Aweeka F., Luetkemeyer A., Savic R.M. Optimizing hydroxychloroquine dosing for patients with COVID-19: an integrative modeling approach for effective drug repurposing. Clin. Pharmacol. Ther. 2020 doi: 10.1002/cpt.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier-Veyret E., Truffot A., Bailly S., Fonrose X., Thiebaut-Bertrand A., Tonini J., Cahn J.-Y., Stanke-Labesque F. Inflammation is a potential risk factor of voriconazole overdose in hematological patients. Fundam. Clin. Pharmacol. 2019;33:232–238. doi: 10.1111/fcp.12422. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi Suliman M.A., Ogungbenro K., Kosmidis C., Ashworth A., Barker J., Szabo-Barnes A., Davies A., Feddy L., Fedor I., Hayes T., Stirling S., Malagon I. The effect of veno-venous ECMO on the pharmacokinetics of ritonavir, darunavir, tenofovir and lamivudine. J. Crit. Care. 2017;40:113–118. doi: 10.1016/j.jcrc.2017.03.010. [DOI] [PubMed] [Google Scholar]
- González de Requena D., Bonora S., Garazzino S., Sciandra M., D'Avolio A., Raiteri R., Marrone R., Boffito M., De Rosa F.G., Sinicco A., Di Perri G. Nevirapine plasma exposure affects both durability of viral suppression and selection of nevirapine primary resistance mutations in a clinical setting. Antimicrob. Agents Chemother. 2005;49:3966–3969. doi: 10.1128/AAC.49.9.3966-3969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire M., Le Turnier P., Gaborit B., Veyrac G., Lecomte R., Boutoille D., Canet E., Imbert B., Bellouard R., Raffi F. Lopinavir pharmacokinetics in COVID-19 patients. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkaa195. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.K., Rosenkranz S.L., Cramer Y.S., Koletar S.L., Szczech L.A., Amorosa V., Hall S.D. The pharmacokinetics and pharmacogenomics of efavirenz and lopinavir/ritonavir in HIV-infected persons requiring hemodialysis. AIDS. 2008;22:1919–1927. doi: 10.1097/QAD.0b013e32830e011f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M.A., Sieg A.C. Evaluation of altered drug pharmacokinetics in critically ill adults receiving extracorporeal membrane oxygenation. Pharmacotherapy. 2017;37:221–235. doi: 10.1002/phar.1882. [DOI] [PubMed] [Google Scholar]
- Hartman D., Kern S., Brown F., Minton S.K., Rayner C.R. Time to step up: a call to action for the clinical and quantitative pharmacology community to accelerate therapeutics for COVID-19. Clin. Transl. Sci. 2020 doi: 10.1111/cts.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S., Shum D., Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother. 2020 doi: 10.1128/AAC.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumah M.T.B., Vasoo S., Menon S.R., De P.P., Neely M., Teng C.B. Pharmacokinetic/pharmacodynamic determinants of vancomycin efficacy in enterococcal bacteremia. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.01602-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê M.P., Peiffer-Smadja N., Guedj J., Neant N., Mentre F., Ader F., Yazdanpanah Y., Peytavin G., on behalf of C-20-5 15 DisCoVeRy French Steering Committee, F Rationale of a loading dose initiation for hydroxychloroquine treatment in COVID-19 1 infection in DisCoVeRy trial. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkaa191. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.-S., Im J.-S., Cho J.-Y., Bae K.-S., Klein T.A., Yeom J.-S., Kim T.-S., Choi J.-S., Jang I.-J., Park J.-W. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob. Agents Chemother. 2009;53:1468–1475. doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blondel, G., Ruiz, S., Murris, M., Faguer, S., Duhalde, V., Eyvrard, F., Izopet, J., Mansuy, J.M., Rolland, Y., Delavigne, K., Guimbaud, R., Pugnet, G., Conil, J.M., Georges, B., Delobel, P., Minville, V., Silva Sifontes, S., Concordet, D., Gandia, P., n.d. Hydroxychloroquine in COVID-19 patients: what still needs to be known about the kinetics. Clin. Infect. Dis.. 10.1093/cid/ciaa558. [DOI] [PMC free article] [PubMed]
- McChesney E. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am. J. Med. 1983 doi: 10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- McElnay J.C., Mukhtar H.A., D'Arcy P.F., Temple D.J., Collier P.S. The effect of magnesium trisilicate and kaolin on the in vivo absorption of chloroquine. J. Trop. Med. Hyg. 1982;85:159–163. [PubMed] [Google Scholar]
- McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E.T. Impact of infectious and inflammatory disease on cytochrome P450-mediated drug metabolism and pharmacokinetics. Clin. Pharmacol. Ther. 2009;85:434–438. doi: 10.1038/clpt.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S., Takahashi T., Yoshida Y., Yokota N. Population pharmacokinetics of hydroxychloroquine in Japanese patients with cutaneous or systemic lupus erythematosus. Ther. Drug Monit. 2016;38:259–267. doi: 10.1097/FTD.0000000000000261. [DOI] [PubMed] [Google Scholar]
- Mouton M., Theuretzbacher U., Craig W., Tulkens P., Derendorf H., Cars O. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 2008 doi: 10.1093/jac/dkm476. [DOI] [PubMed] [Google Scholar]
- Nguyen T.H.T., Guedj J., Anglaret X., Laouénan C., Madelain V., Taburet A.-M., Baize S., Sissoko D., Pastorino B., Rodallec A., Piorkowski G., Carazo S., Conde M.N., Gala J.-L., Bore J.A., Carbonnelle C., Jacquot F., Raoul H., Malvy D., de Lamballerie X., Mentré F., JIKI study group Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix D.E., Goodwin S.D., Peloquin C.A., Rotella D.L., Schentag J.J. Antibiotic tissue penetration and its relevance: models of tissue penetration and their meaning. Antimicrob. Agents Chemother. 1991;35:1947–1952. doi: 10.1128/AAC.35.10.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo P.O., Ogbuokiri J.E., Ofoegbu E., Klotz U. Protein binding and ivermectin estimations in patients with onchocerciasis. Clin. Pharmacol. Therapeut. 1993;53:426–430. doi: 10.1038/clpt.1993.46. [DOI] [PubMed] [Google Scholar]
- Perinel S., Launay M., Botelho-Nevers É., Diconne É., Louf-Durier A., Lachand R., Murgier M., Page D., Vermesch R., Thierry G., Delavenne X. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner C.R., Smith P.F., Hershberger K., Wesche D. Optimizing COVID-19 candidate therapeutics: thinking without borders. Clin. Transl. Sci. 2020 doi: 10.1111/cts.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk M.L., Hang Y., Luo W.-L., Su J., Zhao J., Campbell H., Nguyen B.-Y.T., Sklar P., Eron J.J., Wenning L. Pharmacokinetics and pharmacodynamics of once-daily versus twice-daily raltegravir in treatment-naive HIV-infected patients. Antimicrob. Agents Chemother. 2012;56:3101–3106. doi: 10.1128/AAC.06417-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J.A., Abdul-Aziz M.H., Lipman J., Mouton J.W., Vinks A.A., Felton T.W., Hope W.W., Farkas A., Neely M.N., Schentag J.J., Drusano G., Frey O.R., Theuretzbacher U., Kuti J.L., International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect. Dis. 2014;14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodvold K.A., George J.M., Yoo L. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin. Pharmacokinet. 2011;50:637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Schmith V., Zhou J., Lohmer L. The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin. Pharmacol. Therapeut. 2020 doi: 10.1002/cpt.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert S.M., Castillo-Mancilla J.R., Erlandson K.M., Anderson P.L. Inflammation and pharmacokinetics: potential implications for HIV-infection. Expet Opin. Drug Metabol. Toxicol. 2017;13:641–650. doi: 10.1080/17425255.2017.1311323. [DOI] [PubMed] [Google Scholar]
- Smith P.F., Dodds M., Bentley D., Yeo K., Rayner C. Dosing will be a key success factor in repurposing antivirals for COVID-19. Br. J. Clin. Pharmacol. 2020 doi: 10.1111/bcp.14314. [DOI] [PubMed] [Google Scholar]
- Tett S.E. Clinical pharmacokinetics of slow-acting antirheumatic drugs. Clin. Pharmacokinet. 1993;25:392–407. doi: 10.2165/00003088-199325050-00005. [DOI] [PubMed] [Google Scholar]
- Tett S.E., Cutler D.J., Day R.O., Brown K.F. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br. J. Clin. Pharmacol. 1989;27:771–779. doi: 10.1111/j.1365-2125.1989.tb03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tett S.E., Cutler D.J., Day R.O., Brown K.F. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br. J. Clin. Pharmacol. 1988;26:303–313. doi: 10.1111/j.1365-2125.1988.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touret F., Gilles M., Barral K., Nougairède A., Decroly E., Lamballerie X. de, Coutard B. 2020. In Vitro Screening of a FDA Approved Chemical Library Reveals Potential Inhibitors of SARS-CoV-2 Replication. bioRxiv. 2020.04.03.023846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.-F., Bo L., Lin Y., Li F.-X., Sun S., Lin H.-B., Xu S.-Y., Bian J., Yao S., Chen X., Meng L., Deng X. Response of Chinese anesthesiologists to the COVID-19 outbreak. Anesthesiology. 2020 doi: 10.1097/ALN.0000000000003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo K.R., Zhang M., Pan X., Ke A.B., Jones H.M., Wesche D., Almond L.M. Impact of disease on plasma and lung exposure of chloroquine, hydroxy-chloroquine and azithromycin: application of PBPK modelling. Clin. Pharmacol. Therapeut. 2020 doi: 10.1002/cpt.1955. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]