Abstract
Parent-offspring conflict—conflict over resource distribution within families due to differences in genetic relatedness—is the biological foundation for many psychological phenomena. In genomic imprinting disorders, parent-specific genetic expression is altered causing imbalances in behaviors influenced by parental investment. We use this natural experiment to test the theory that parent-offspring conflict contributed to the evolution of vocal music by moderating infant demands for parental attention. Individuals with Prader-Willi syndrome, a genomic imprinting disorder resulting from increased relative maternal genetic contribution, show enhanced relaxation responses to song, consistent with reduced demand for parental investment (Mehr et al., 2017, Psychological Science). We report the necessary complementary pattern here: individuals with Angelman syndrome, a genomic imprinting disorder resulting from increased relative paternal genetic contribution, demonstrate a relatively reduced relaxation response to song, suggesting increased demand for parental attention. These results support the extension of genetic conflict theories to psychological resources like parental attention.
Keywords: Parent-offspring conflict, Angelman syndrome, genomic imprinting, music, evolution
1. Introduction
The psychology of parents and infants is shaped by the dynamics of parent-offspring conflict (Trivers, 1974). For all species that reproduce sexually and produce multiple offspring that compete for care, parents and offspring will have conflicting interests over the optimal distribution of parental investment. This is the result of asymmetric fitness interests within the family: an offspring is more related to themselves than to a sibling, while a parent is equally related to each of their offspring. Moreover, a mother is always certain about her relatedness to each of her offspring (and any future offspring), while a father needn’t be genetically tied to more than one of that mother’s children. Therefore, from the perspective of a gene in a mother, all of her children are equally likely to have inherited identical copies of that gene. Assuming equal offspring quality, the fitness of a maternally derived gene is maximized through the equitable division of investment across her lifetime reproductive output. However, because there is a smaller chance that any given maternal sibling will share the same paternally derived DNA, genes derived from the father benefit from maternal investment directed relatively more towards the offspring in which they are found, even if this comes at a cost to that mother’s overall reproductive success (Wilkins & Haig, 2003).
This asymmetry in genetic interests between parents and offspring drives natural selection toward mechanisms to manage parent-offspring conflict. The resulting effects have been documented across the animal kingdom, including disputes over weaning in apes (Mandalaywala, Higham, Heistermann, Parker, & Maestripieri, 2014), sibling rivalry in birds (Lougheed & Anderson, 1999), and maternal care in insects (Kölliker et al., 2015). In humans, parent-offspring conflict affects many psychological phenomena including mate choice (Apostolou, 2008; Buunk, Park, Justin, & Dubbs, 2008; Khosrotehrani, Johnson, Guégan, Stroh, & Bianchi, 2005), time spent engaging with children (Bugental, Beaulieu, & Silbert-Geiger, 2010), and child abuse and homicide (Daly & Wilson, 1999).
One molecular mechanism that has likely resulted from the selective pressures of parent-offspring conflict is genomic imprinting (Haig, 2000): an epigenetic phenomenon in which a gene differs in its expression depending on the sex of the parent from whom it was inherited (Reik & Walter, 2001). Because most adaptive problems are not antagonistic (from the perspective of parental fitness interests), autosomal alleles are generally under selection for their overall effect on fitness regardless of the sex of parental origin. However, natural selection favors differing optimal levels of expression for alleles that affect the distribution of resources within the family, because one’s paternally-derived alleles and maternally-derived alleles are not equally likely to be found across all relatives. That is, genes that affect the distribution of rivalrous parental investment are likely to be imprinted, such that differing maternal and paternal interests can be expressed. Paternally-derived alleles are less likely to be present in mother (or maternal relatives) than are maternally derived alleles, and have been selected to demand a higher level of maternal investment for the offspring in which they are found (Moore & Haig, 1991). By contrast, a maternally derived allele in an offspring would benefit from demanding fewer resources from mother, thereby optimizing its propagation by increasing overall maternal reproductive output. The kinship theory of genomic imprinting posits that genomic imprinting is a mechanism regulating gene expression that allows genes to instantiate these differing optimal strategies (Haig, 2000). If the optimal level of expression for a given allele is higher when inherited from fathers rather than mothers, selection will have acted to produce a paternally-expressed gene; if the reverse, a maternally-expressed gene (Wilkins & Haig, 2003).
The balance between the antagonistic effects of maternally expressed and paternally expressed genes produces the successful development we see in typical individuals, whereas maladaptive phenotypes are expected in conditions in which expression of imprinted genes is perturbed (Peters, 2014). Both experimentally-derived and naturally occurring cases of imprinting imbalance are generally associated with perturbations of resource distribution phenotypes, such as resource exchange in utero (Haig 1993); embryonic demands of endosperm tissue in plants (Haig & Westoby, 1989, 1991); and early feeding behaviors in humans and other mammals (Brown & Consedine, 2004; Crespi, 2010; Haig, 2014; Haig & Wharton, 2003; Kotler, Balko, Berall, & Haig, 2016).
While the kinship theory has traditionally been applied to conflicts over material resources, parents and children are also likely to have conflicting interests over psychological resources, including parental attention (Mehr, Kotler, Howard, Haig, & Krasnow, 2017; Mehr & Krasnow, 2017; Oliver et al., 2007). Human infants are born relatively altricial, relying on parents to provide food and safety. To access these resources, a child must attract and maintain their parent’s attention. Attention is a limited resource, as attention spent on one child cannot be equally spent on another or on other tasks; thus, like the case of limited material resources, parent-offspring conflict is expected to occur over the amount of attentional investment a parent should deliver to any particular offspring. Consequently, infants are expected to “demand” more attention, on average, than a parent would “prefer” to provide.
Unlike material resources, attention is a covert property of the mind that can only be signaled through observable behavior. When parties have conflicting interests over a signaled property a co-evolutionary arms race can occur (Krebs & Dawkins, 1984). Just as peacocks evolved increasingly elaborate tails to display genetic quality, satisfying peahens’ co-evolving choosiness (Darwin, 1871), traits that more honestly signal parental attention could co-evolve with infants’ attentional demands. Mehr and Krasnow (2017) proposed that infant-directed song evolved in this way: infant-directed song is proposed to be an honest signal of attention, and thus a likely target of this arms race, because it can signal attention in the absence of vision, honestly signals the singer’s proximity and orientation, and increases the costs of engaging in other activities that detract attention away from the offspring.
This proposal is testable by studying the psychology of people with atypical expression of typically imprinted genes, referred to as genomic imprinting disorders. These disorders present a naturally-occurring example of the maladaptive developmental effects of imbalanced maternal/paternal patterns of genomic imprinting. Many phenotypes resulting from these disorders are consistent with atypical resource demands, making them a model for examining traits related to parent-offspring conflict in humans (Kotler et al., 2016; Kotler & Haig, 2018; Mehr et al., 2017; Úbeda, 2008). Consistent with Mehr and Krasnow’s (2017) proposal, musical responses of individuals with Prader-Willi syndrome are altered, relative to typical development (Mehr et al., 2017). Prader-Willi syndrome (PWS) is a rare genomic imprinting disorder wherein individuals only express the maternally inherited component of the short arm of chromosome 15. Characteristic symptoms including reduced nursing, increased sleep, and altered endocronology are indicative of reduced demands for maternal investment (Haig, 2014; Haig & Wharton, 2003; Kotler et al., 2016; Úbeda, 2008). Relative to the typically developing population, people with PWS show an enhanced relaxation response (decrease in heart rate) specifically in response to song (Mehr et al., 2017); this suggests a reduction in demand for attention, as the enhanced relaxation response implies easier satiation of their attentional demand.
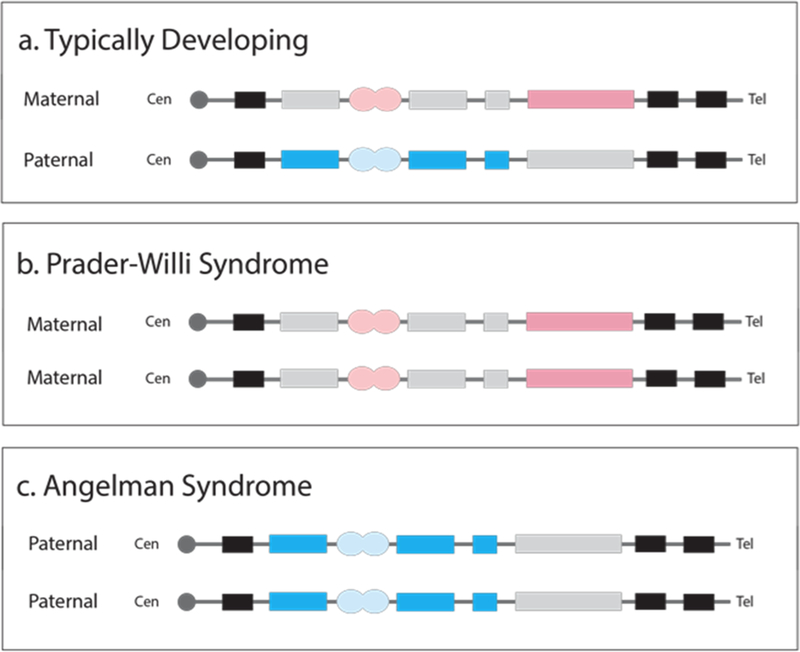
However, these results could be spuriously related to the numerous developmental perturbations resulting from PWS. If they are attributable to parent-offspring conflict over attentional resources, we should find the complementary effect in a population with the complementary genomic disorder. In the present experiment, we contrast our prior results with data from people lacking maternally-expressed genes at the same chromosomal location as PWS, in its “sister disorder”, Angelman syndrome (AS, Chamberlain & Lalande, 2010; Knoll et al., 1989; see Figure 1). Though their presenting phenotypes are highly disparate, many symptoms present in these disorders follow predictions made by the kinship theory of genomic imprinting (Haig & Wharton, 2003; Oliver et al., 2007; Úbeda, 2008), making behavior in AS a prime candidate to compare against behavior in PWS.
Fig. 1.
Variations in genomic imprinting on chromosome 15q11–13. Typically developing individuals express a balanced maternally- and paternally-expressed contribution. Prader-Willi syndrome (PWS) is characterized by an overexpression of maternally-expressed alleles. Angelman syndrome (AS) is characterized by an overexpression of paternally-expressed alleles in the same region. There are multiple molecular mechanisms by which these errors occur; schematic represents imbalanced imprinted contribution. Black = unimprinted genes; blue = paternally-expressed genes; pink = maternally-expressed genes; grey = unexpressed alleles; circles = imprinting centers.
2. Material and methods
2.1. Angelman Syndrome
AS is associated with heightened social engagement, motor difficulties including ataxia and muscular hypotonia (Williams et al., 2004), hyperactivity (Margolis, Sell, Zbinden, & Bird, 2015), vastly reduced sleep in infancy (Buiting, 2010), and microcephaly (resulting in severe cognitive delays and speech limitations; Williams et al., 2004). While people with PWS demonstrate a complex symptom profile including hypersomnolence, hypotonia, and an overall lack of social engagement (Buiting, 2010; Koenig, Klin, & Schultz, 2004; Whittington & Holland, 2004), their cognitive delays are much less significant than we see in PWS. Most pertinent to this study, linguistic and motor development is significantly more affected in individuals with AS relative to those with PWS. More information about AS is available in Chamberlain and Lalande (2010), Clayton-Smith and Laan (2003) and Cassidy, Dykens, and Williams (2000).
2.2. Participants
This research was approved by the Committee on the Use of Human Subjects in Research at Harvard University. Participants with AS were recruited through the Angelman Syndrome Foundation, a national organization focused on advancing the awareness and treatment of AS, as well as a specialized AS clinic at Cincinnati Children’s Hospital. Clinical characterization of participants with AS was confirmed via copies of genetic testing conducted as part of participants’ normal treatment regimen. Participants were screened for hearing impairments via parental report. Data were collected on-site at our facility on Harvard University campus or with a mobile laboratory. All participants were provided with small gifts in exchange for their participation.
2.3. Samples
Data reported here were gathered from 29 people with AS (11 female, age in years: M = 13.1, SD = 9.09, range: 2.10–34.0). We recruited 36 participants but excluded 1 participant who did not tolerate wearing the heart rate monitor, 2 participants due to a technical malfunction resulting in loss of the physiological data, and 4 participants whose motion during the experiment produced uninterpretable heart rate data.
The rarity of AS and the difficulty of psychophysiological testing in this population precluded us from determining a fixed sample size before conducting the experiments. We did not use any stopping rule for data collection; rather, we tested the maximum number of participants with AS available. We tested children and adults with AS rather than infants, because, although the effects we predict are targeted toward infancy, they likely persist through childhood and adulthood (Mehr et al., 2017). Unlike other infant-specific adaptations which become maladaptive later in life, a response to infant-directed song is unlikely to be under negative selection pressure later in development (see discussion in Mehr & Krasnow, 2017). Further, the rarity of AS, which occurs in approximately 1/10,000–1/15,000 births (Cassidy, Dykens, & Williams, 2000) and which is often undiagnosed until late infancy, makes the testing of infants prohibitively difficult. Therefore, while we focused our recruitment efforts towards younger individuals, we tested individuals of all ages who could provide genetic testing confirming the molecular origins of their AS diagnosis.
2.4. Listening session
Methods were similar to our previous work (Mehr et al., 2017) except as noted below. Participants listened to a series of high-fidelity recordings of a female vocalist singing or speaking the lyrics of 12 songs (all recordings available at http://osf.io/unhwb). Participants listened to a counterbalanced set of songs, with each set containing 6 sung samples and 6 spoken samples; in this way, each participant only heard one instance of each set of lyrics (either sung or spoken), so that no lyrics were repeated in a given listening session. The twelve songs vary on a number of features: some are infant directed (lullabies and play songs), others are adult-directed; some were selected because they were predicted to be familiar to participants, others unfamiliar. After each listening session we asked parents to self-report whether or not their children were familiar with each song from which the lyrics were taken. The songs were presented in random order with 10 seconds of silence before and after each song. At the end of the 12 trials, the experimenter asked the participant’s caregiver to report whether each song was familiar (“yes”, “no”, and “unsure”), and whether not the participant seemed to enjoyed it (“disliked it a lot”, “disliked it a little”, “it was just OK”, “liked it a little” and “liked it a lot”). Participants were seated either alone or with a caregiver, at the participant’s discretion.
Participants sat such that the position of the participant’s head was approximately centered between two powered speakers (M-Audio AV40, placed on IsoAcoustics isolation stands, and driven by a Focusrite Forte audio interface). While participants were free to move around the testing space, caregivers were instructed to encourage the participants to stay centered in front of the speakers as much as possible. Any caregiver who accompanied the participant into the room listened to classical music through passive noise-cancelling headphones (KAT Percussion KTUI26) to ensure that they were unable to hear and respond to the experimental tracks being played to the participant.
The listening session continued over 12 trials. Participants could take breaks whenever necessary: if the participant was excessively active, in any distress, or if the caregiver requested it, a break was taken until the participant was ready to proceed. This occurred on 31 of 372 trials (where each trial consisted of listening to one song), all of which were excluded from analysis, for the following reasons: 3 trials for outside noise interference (noise from siblings in the adjoining room), and 28 trials for excessive participant movement or distress. Trial exclusion decisions were made blind to the condition (song vs. speech) of each trial and to any data collected from the participant on each trial. As a result of the significant physical and developmental delays present in the AS population, two measures used in prior work with the PWS population (pitch discrimination and periodic movement assessments) could not be used with this group.
2.5. Physiological monitoring
We measured heart rate using the Empatica E4, a physiological monitoring wristband that yields clinical-grade heart rate via photoplethysmography measured at 64Hz (Garbarino, Lai, Bender, Picard, & Tognetti, 2015). Inter-beat intervals were computed by Empatica’s proprietary algorithm, which automatically imputes missing data from the photoplethysmograph signal and corrects for motion artifacts. Participants wore one device on each ankle, wrapped in physiotherapy tape to discourage them from tampering with the devices. Before processing any data, we visualized the heart rate signal from both devices and selected one of the two devices’ signals to analyze, on the basis of signal quality alone; this decision was made blind to the order, content, and timing of stimulus presentation, so it could not bias the results of any analyses. Because the measure of interest was the change in heart rate from before music listening to after music listening, we normalized heart rate values in the 10-second post-stimulus period to the 10-second pre-stimulus period from the same trial, as in our previous work, and scores were interpreted as standard-deviation changes in heart rate as a result of listening to the stimulus (see Mehr et al., 2017).
Heart rate data were missing for portions of each participant’s listening session, of variable lengths, for a number of reasons (e.g., excessive motion or participants attempting to remove the device), thereby necessitating data cleaning. We used two data cleaning methods identical to those in Mehr et al (2017). First, on the assumption that standard deviations computed on sparse data have low precision, data from post-stimulus periods where fewer than 5 heart rate observations were present in the corresponding pre-stimulus period were dropped. This eliminated 20.4% of heart rate observations and 1 participant from the sample. Second, to reduce the impact of extreme values, we trimmed the data of all observations with |z| > 5. This resulted in removal of a further 0.85% of heart rate observations.
We also note that all heart rate measures that we undertook are within-subjects; that is, comparing average heart rate during a given interval to an immediately prior baseline interval for the same subject. This approach helps to rule out cohort-level bias toward or against our hypothesis.
2.6. Analysis strategy
We used t-tests and linear regression to examine the relation between cohort and heart rate. All regressions were bootstrapped with 40,000 replications and stratified by cohort so as to adjust for the cohorts’ different sizes. To ensure that no findings were attributable to the presence of influential observations, we validated all findings with planned sensitivity analyses (Cohen, Cohen, West, & Aiken, 2003). We also confirmed that all results held when excluding participants who had very few valid heart rate observations after data cleaning, to ensure that lack of precision in these participants did not bias the results toward or away from our hypotheses. Consistent with our analysis for the PWS cohort (Mehr et al., 2017), the data from infant-directed, adult, and play songs were collapsed; given the rarity of AS diagnoses there was insufficient power to conduct sub-analyses. In Results, we analyze data from the AS cohort in conjunction with prior data from both PWS and typically developing participants; those data are taken from our previous work with these cohorts (Mehr et al., 2017) and are publicly available at http://osf.io/unhwb. A combined dataset including those data and the AS data collected and reported here is available at https://osf.io/zbrn5/?view_only=a23f65160a3649ec94d021b91703e237 [view-only link for peer review].
3. Results
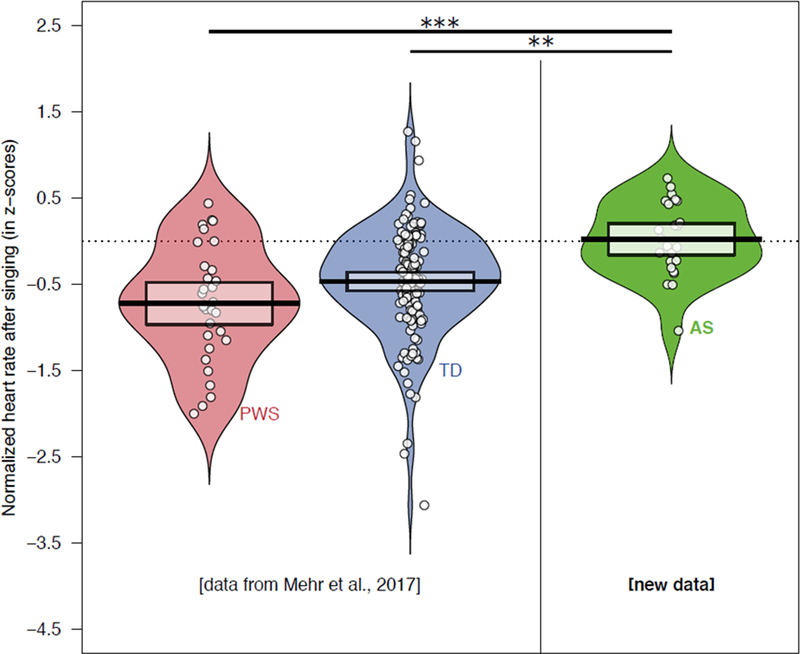
In our previous work, we found much larger changes in heart rate after listening to songs in both typically developing people (M = −0.46, SD = 0.65, 95% CI = [−0.56, −0.35]) and people with PWS (M = −0.72, SD = 0.67, 95% CI = [−0.97, −0.48]) (Mehr et al., 2017). In sharp contrast to both groups, participants with AS showed no decrease in heart rate after listening to songs (Figure 2; M = −0.11, SD = 0.59, 95% CI = [−0.34, 0.12]; comparison to 0: t(26) = 0.98, p = 0.34; these and the above values are reported in z-scores, normalized relative to the 10 second prestimulus period, see Methods). Heart rate in AS after music listening was significantly higher than both other cohorts (comparison between AS and typically developing cohorts: Satterthwaite’s t(39.0) = 2.74, p = .009; between AS and PWS cohorts: Satterthwaite’s t(56.0) = 3.70, p = .0005). These results confirm the predictions laid out above.
Fig. 2.
Normalized heart rates after listening to singing, from the Prader-Willi syndrome (PWS) and typically developing (TD) cohorts (from previous work), and from the Angelman syndrome (AS) cohort. The circles show averaged values for individual subjects and are jittered to avoid overlap; the violin plots show kernel density estimations of the heart rate values; the horizontal lines indicate the group means; and the shaded boxes show the 95% confidence intervals of the means. The dotted line indicates 0, i.e., no change in heart rate from before songs to after songs. While typically developing people and people with PWS showed significant decreases in heart rate after music listening, relative to the baseline period before each song, participants with AS did not. Moreover, average heart rates after music listening were substantially higher in the AS cohort than in both the PWS and TD cohorts. ***p < .001; **p < .01
A comparable pattern of results was found in heart rate responses after listening to speech. Participants with AS showed no significant decrease in heart rate after speech listening (M = 0.03, SD = 0.57, 95% CI = [−0.21, 0.26]), and compared to our previous cohorts, heart rates were significantly higher than both typically developing people (M = −0.44, SD = 0.75, 95% CI = [−0.70, −0.18]; Satterthwaite’s t(33.8) = 4.19, p = .0002) and people with PWS (M = −0.50, SD = 0.60, 95% CI = [−0.60, −0.40]; Satterthwaite’s t(56.9) = 2.72, p = .009).
Because AS is associated with short attention span, and because heart rate deceleration is known to be related to orienting response (Graham & Clifton, 1966), we tested for the presence of trial-wise order effects in the AS data as a control analysis. We found no relation between heart rate and trial order overall (r = .033, p = .63), for singing trials alone (r < .01, p = .98), or for speaking trials alone (r = .06, p = .53). Visual inspection of scatterplots did not suggest any nonlinear relation between heart rate and trial order.
To examine the degree to which the heart rate results in AS reflected general patterns of responses to auditory stimuli, as opposed to an altered response to music relative to the other cohorts, we proceeded with a multiple regression analysis to estimate cohort-level differences in the within-subjects change in heart rate after song listening vs. speech listening. Combining datasets across the present and previous results, these measures, unsurprisingly, were significantly correlated (r = .33, p < .0001, N = 195). Thus, we proceeded with the regression analysis and used it to compare estimates of cohort differences in heart rate response after music listening, adjusting for heart rate response after speech listening. For simplicity, we used a no-constant model predicting normalized heart rate after song listening from indicator variables for the three cohorts, as well as the normalized heart rate after speech listening. The coefficients for each indicator variable are thus interpretable as the estimated mean cohort heart rate response to music listening, adjusting for the response to speech listening.
The results showed clear differences between the three cohorts in adjusted heart rate responses to music listening. The model was significant (Wald χ2(4) = 131.8, R2 = .436, p = 1.60 × 10−27) with cohort-level effects following the same pattern as the raw comparisons reported above: adjusting for heart rate response to speech listening, the AS cohort had no estimated change in heart rate following song listening (β = 0.02, z = 0.20, p = .840), the typically developing cohort had a moderate, negative estimated change in heart rate (β = −0.32, z = 4.69, p = 1.37 × 10−06), and the PWS cohort had a stronger, negative estimated change in heart rate (β = −0.58, z = 4.79, p = 8.34 × 10−07). Linear combinations confirmed that the estimated heart rate after song listening in AS was significantly higher than both the typically developing cohort (βdiff = 0.34, 95% CI = [0.13, 0.56], z = 3.11, p = .0018) and the PWS cohort (βdiff = 0.60, 95% CI = [0.31, 0.89], z = 4.03, p = .00005), after adjusting for heart rate response to speech listening.
In sum, not only is AS associated with a pattern of heart rate responses to song that is different than both typically developing people and people with PWS, but the pattern of responses is not attributable to some general difference in heart rate response to auditory stimuli. That is, after adjusting for heart rate responses to speech, heart rate responses to music listening in AS are substantially higher than those in PWS and typical development — showing a complete absence of the relaxation effect we previously demonstrated in PWS.
4. Discussion
The kinship theory of genomic imprinting has been applied to a variety of human phenomena, from nursing to the pace of human maturation (Haig, 2014; Kotler & Haig, 2018; Úbeda, 2008), but few empirical tests of its psychological predictions have been attempted (see Oliver et al., 2007). The results presented here, in conjunction with our prior work (Mehr et al., 2017), demonstrate that the effect of listening to vocal music on physiological relaxation depends in part on the relative contribution of genes expressed in the q11–13 region of chromosome 15. Specifically, the absence of maternally-expressed genes in this region attenuates relaxation responses to song, while the absence of corresponding paternally-expressed genes potentiates relaxation responses to the same stimuli. This work provides evidence for the predictive power of the kinship theory in the psychological domain and supports the role of parent-offspring conflict in the evolution of infant-directed song (Mehr & Krasnow, 2017).
The current study highlights the utility that clinical and basic research communities offer one another. For basic science researchers, the clinical literature and its practitioners hold deep expertise in phenomena and populations that present unique opportunities to test theory. For clinicians, this study shows how basic science theories can help reveal otherwise counterintuitive features of well-documented disorders, which have the potential to better design treatment interventions. The data reported here are consistent with archetypal AS phenotype in other arenas, including feeding, energetics and growth (Haig, 2010; Kotler et al., 2016; Wilkins & Haig, 2003). For example, while individuals with PWS show hypersomnelence, people with an AS diagnosis are known to be hyperactive, often experiencing childhood and infant sleep disturbance (Clayton-Smith & Laan, 2003). Similarly, infants with AS demonstrate food-focused behavior, while young infants with PWS must be tube-fed for lack of interest in feeding (Berry, Leitner, Clarke, & Einfeld, 2005; Haig, 2010; McAllister, Whittington, & Holland, 2011). Overall the pattern is one of decreased demands for parental investment in PWS and increased demands for parental investment in AS. Consistent with this pattern, while listening to song in PWS was more relaxing than in typical development (indicating a higher level of satiation to parental investment via attention), in AS we found the opposite pattern: no decrease in heart rate whatsoever, and, relative to PWS and typically-developing people, higher heart rate after music listening. Moreover, while the current study examines only the auditory component of song, these effects are likely to interact with other behaviors like touch, rocking, or bouncing, which often occur in conjunction with singing to a child: we likely underestimate the differences in responses to song between AS, PWS, and typical development. We are not suggesting that the evolution of infant-directed song evolved independently of, or prior to, these behaviors; rather, they are likely to have evolved in conjunction with one another, all with the goal of signaling parental attention.
We note three weaknesses of this work that can be addressed in future studies. First, while the sample size is consistent with other studies conducted with AS populations (Mount, Oliver, Berg, & Horsler, 2011; Oliver et al., 2007) given the rarity of AS diagnoses, it was small relative to both PWS and typically developing groups. Second, while the differences in findings across AS and PWS confirm predicted parent-of-origin effects, extending the comparison to include individuals with non-imprinted genetic disorders (e.g., Down syndrome) would provide additional certainty that our findings are not being caused by genetic abnormalities broadly, but specifically by differences in maternal versus paternal imprinting patterns. Last, because we were unable to measure pitch discrimination and periodic movement in response to song due to the cognitive and physical limitations of the AS population (Williams et al., 2006), the full extent of an altered psychology of music in AS remains unknown. Individuals with AS suffer functionally severe cognitive delays, making them unable to cognitively process pitch discrimination questions; moreover, their non-verbal nature and disabled muscle coordination makes first-person responses nonviable. The lack of muscle coordination, hypermotic movements and motor ataxia consistently present in individuals with AS, along with the fact that most participants sat close to a parent (who could move independently of the participant) made periodic motion difficult or impossible to assess in our listening session. The development of new assessments of pitch perception and motion response appropriate for impaired individuals could address this issue.
The kinship theory of genomic imprinting and the parent-offspring conflict theory from which it derives have been used to make predictions about a variety of traits related to parent-offspring dynamics, with implications for psychology. These results show the value of applying work from theoretical biology to the psychological domain: our results confirm the prediction that genomic imprinting is implicated in the conflict over psychological resources like parental attention.
The results may also have applications in music therapy, which is frequently recommended for individuals with imprinting disorders (Grolla et al., 2011; Phelan, 2008). Anecdotally, clinicians and parents report that individuals with both PWS and AS enjoy and engage with music, consistent with our findings. Our results suggest that understanding group differences in musical responses may produce more effective interventions: for instance, music may be more suited to increase engagement for individuals with AS, rather than to moderate behavioral outbursts, as it is in other groups including PWS. The application of evolutionary theory to clinical questions such as these allows for more accurate predictions and moves us towards more effective clinical outcomes.
Funding
This work was supported by a Harvard University Department of Psychology seed grant to M.M.K.; the Harvard Data Science Initiative (S.A.M.); the National Institutes of Health Director’s Early Independence Award DP5OD024566 (S.A.M.); the Harvard Graduate School of Education/Harvard University Presidential Scholarship (S.A.M.); and the Natural Sciences and Engineering Research Council of Canada Post-Graduate Scholarship (J.K.).
Footnotes
Data Availability
New data collected and reported here are available at https://osf.io/zbrn5/?view_only=eb20db2aa95b44a5b9af32744323b17b [view-only link for peer review]. This paper also includes data from our previous work, which are publicly available at http://osf.io/unhwb.
References
- Berry RJ, Leitner RP, Clarke AR, & Einfeld SL (2005). Behavioral aspects of Angelman syndrome: A case control study. American Journal of Medical Genetics Part A, 132A(1), 8–12. 10.1002/ajmg.a.30154 [DOI] [PubMed] [Google Scholar]
- Brown W, & Consedine N (2004). Just how happy is the happy puppet? An emotion signaling and kinship theory perspective on the behavioral phenotype of children with Angelman syndrome. Medical Hypotheses, 63(3), 377–85. 10.1016/j.mehy.2004.05.010 [DOI] [PubMed] [Google Scholar]
- Bugental DB, Beaulieu DA, & Silbert-Geiger A (2010). Increases in parental investment and child health as a result of an early intervention. Journal of Experimental Child Psychology, 106(1), 30–40. 10.1016/j.jecp.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Buiting K (2010). Prader-Willi syndrome and Angelman syndrome. American Journal of Medical Genetics, Part C: Seminars in Medical Genetics, 154(3), 365–376. 10.1002/ajmg.c.30273 [DOI] [PubMed] [Google Scholar]
- Cassidy SB, Dykens E, & Williams CA (2000). Prader-Willi and Angelman syndromes: sister imprinted disorders. American Journal of Medical Genetics, 97(2), 136–46. [DOI] [PubMed] [Google Scholar]
- Cassidy SB, Schwartz S, Miller JL, & Driscoll DJ (2012). Prader-Willi syndrome. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 14(1), 10–26. 10.1038/gim.0b013e31822bead0 [DOI] [PubMed] [Google Scholar]
- Chamberlain SJ, & Lalande M (2010). Angelman syndrome, a genomic imprinting disorder of the brain. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 30(30), 9958–63. 10.1523/JNEUROSCI.1728-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton-Smith J, & Laan L (2003). Angelman syndrome: a review of the clinical and genetic aspects. Journal of Medical Genetics, 40(2), 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FK, & Clifton RK (1966). Heart-rate change as a component of the orienting response. Psychological Bulletin, 65(5), 305–20. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West LS, & Aiken SG (2003). Applied multiple regression/correlation analysis for the behavioral sciences. L. Erlbaum Associates. [Google Scholar]
- Corbeil M, Trehub SE, & Peretz I (2016). Singing Delays the Onset of Infant Distress. Infancy, 21(3), 373–391. 10.1111/infa.12114 [DOI] [Google Scholar]
- Crespi B (2010). The strategies of the genes: genomic conflicts, attachment theory, and development of the social brain In Petronis A & Mill J (Eds.), Brain, behavior and epigenetics. Springer Publishing. [Google Scholar]
- Garbarino M, Lai M, Bender D, Picard RW, & Tognetti S (2015). Empatica E3 - A wearable wireless multi-sensor device for real-time computerized biofeedback and data acquisition. In Proceedings of the 2014 4th International Conference on Wireless Mobile Communication and Healthcare - “Transforming Healthcare Through Innovations in Mobile and Wireless Technologies”, MOBIHEALTH 2014 (pp. 39–42). 10.1109/MOBIHEALTH.2014.7015904 [DOI] [Google Scholar]
- Grolla E, Andrighetto G, Parmigiani P, Hladnik U, Ferrari G, Bernardelle R, … Dolcetta D (2011). Specific treatment of Prader-Willi syndrome through cyclical rehabilitation programmes. Disability and Rehabilitation, 33(19–20), 1837–47. 10.3109/09638288.2010.549288 [DOI] [PubMed] [Google Scholar]
- Haig D (2000). The Kinship Theory of Genomic Imprinting. Annual Review of Ecology and Systematics, 31, 9–32. [Google Scholar]
- Haig D (2010). Transfers and transitions: parent-offspring conflict, genomic imprinting, and the evolution of human life history. Proceedings of the National Academy of Sciences, 107, 1731–5. 10.1073/pnas.0904111106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D (2014). Troubled sleep: Night waking, breastfeeding and parent-offspring conflict. Evolution, Medicine, and Public Health, 2014(1), 32–39. 10.1093/emph/eou005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D, & Westoby M (1989). Parent-specific gene expression and the triploid endosperm. The American Naturalist, 134(1), 147–155. [Google Scholar]
- Haig D, & Westoby M (1991). Genomic imprinting in endosperm: its effect on seed development in crosses between species, and between different ploidies of the same species, and its implications. Philosophical Transactions: Biological Sciences, 333(1266), 1–13. [Google Scholar]
- Haig D, & Wharton R (2003). Prader-Willi syndrome and the evolution of human childhood. American Journal of Human Biology : The Official Journal of the Human Biology Council, 15(3), 320–9. 10.1002/ajhb.10150 [DOI] [PubMed] [Google Scholar]
- Knoll JH, Nicholls RD, Magenis RE, Graham JM, Lalande M, Latt SA, … Latt SA (1989). Angelman and Prader-Willi syndromes share a common chromosome 15 deletion but differ in parental origin of the deletion. American Journal of Medical Genetics, 32(2), 285–290. 10.1002/ajmg.1320320235 [DOI] [PubMed] [Google Scholar]
- Koenig K, Klin A, & Schultz R (2004). Deficits in social attribution ability in Prader-Willi syndrome. Journal of Autism and Developmental Disorders, 34(5), 573–82. [DOI] [PubMed] [Google Scholar]
- Kotler J, Balko K, Berall G, & Haig D (2016). Nutritional Phases in Prader-Willi Syndrome: Evolutionary and Clinical Interpretations. Journal of Evolutionary Medicine, 4, 1–7. 10.4303/jem/235968 [DOI] [Google Scholar]
- Kotler J, & Haig D (2018). The tempo of human childhood: a maternal foot on the accelerator and a paternal foot on the brake. Evolutionary Anthropology, 1–12. 10.1002/evan.21579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs J, & Dawkins R (1984). Animal Signals: Mind-Reading and Manipulation In Krebs J & Davies NB (Eds.), Behavioural Ecology: An Evolutionary Approach (2nd ed., pp. 380–402). Oxford: Blackwell Scientific Publications. [Google Scholar]
- Margolis SS, Sell GL, Zbinden MA, & Bird LM (2015). Angelman Syndrome. Neurotherapeutics : The Journal of the American Society for Experimental NeuroTherapeutics. 10.1007/s13311-015-0361-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister CJ, Whittington JE, & Holland AJ (2011). Development of the eating behaviour in Prader-Willi Syndrome: advances in our understanding. International Journal of Obesity (2005), 35(2), 188–97. 10.1038/ijo.2010.139 [DOI] [PubMed] [Google Scholar]
- Mehr SA, Kotler J, Howard R, Haig D, & Krasnow MM (2017). Genomic imprinting is implicated in the psychology of music. Psychological Science, 28(10), 1455–1467. 10.1177/0956797617711456 [DOI] [PubMed] [Google Scholar]
- Mehr SA, & Krasnow MM (2017). Parent-offspring conflict and the evolution of infant-directed song. Evolution and Human Behavior, 38(5), 674–684. [Google Scholar]
- Mehr SA, Singh M, York H, Glowacki L, & Krasnow MM (2018). Form and Function in Human Song. Current Biology, 28(3), 356–368.e5. 10.1016/j.cub.2017.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehr SA, Song LA, & Spelke ES (2016). For 5-Month-Old Infants, Melodies Are Social. Psychological Science, 27(4), 486–501. 10.1177/0956797615626691 [DOI] [PubMed] [Google Scholar]
- Mehr SA, & Spelke ES (2018). Shared musical knowledge in 11-month-old infants. Developmental Science, 21(2). 10.1111/desc.12542 [DOI] [PubMed] [Google Scholar]
- Moore T, & Haig D (1991). Genomic imprinting in mammalian development: a parental tug-of-war. Trends in Genetics : TIG, 7(2), 45–9. 10.1016/0168-9525(91)90230-N [DOI] [PubMed] [Google Scholar]
- Mount R, Oliver C, Berg K, & Horsler K (2011). Effects of adult familiarity on social behaviours in Angelman syndrome. Journal of Intellectual Disability Research, 55(3), 339–350. 10.1111/j.1365-2788.2010.01364.x [DOI] [PubMed] [Google Scholar]
- Oliver C, Horsler K, Berg K, Bellamy G, Dick K, & Griffiths E (2007). Genomic imprinting and the expression of affect in Angelman syndrome: what’s in the smile? Journal of Child Psychology and Psychiatry, and Allied Disciplines, 48(6), 571–9. 10.1111/j.1469-7610.2007.01736.x [DOI] [PubMed] [Google Scholar]
- Phelan MC (2008). Deletion 22q13.3 syndrome. Orphanet Journal of Rare Diseases, 3, 14 10.1186/1750-1172-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, & Walter J (2001). Genomic imprinting: parental influence on the genome. Nature Reviews Genetics, 2(1), 21–32. 10.1038/35047554 [DOI] [PubMed] [Google Scholar]
- Trainor LJ, Wu L, & Tsang CD (2004). Long-term memory for music: Infants remember tempo and timbre. Developmental Science, 7(3), 289–296. 10.1111/j.1467-7687.2004.00348.x [DOI] [PubMed] [Google Scholar]
- Trehub SE, Unyk AM, & Trainor LJ (1993). Adults identify infant-directed music across cultures. Infant Behavior and Development, 16(2), 193–211. 10.1016/0163-6383(93)80017-3 [DOI] [Google Scholar]
- Trivers R (1974). Parent-Offspring Conflict. American Zoologist, 14(1), 249–264. [Google Scholar]
- Úbeda F (2008). Evolution of genomic imprinting with biparental care: implications for Prader-Willi and Angelman syndromes. PLoS Biology, 6(8), 1678–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington J, & Holland T (2004). Prader-Willi syndrome: Development and manifestations. Cambridge: Cambridge University Press. [Google Scholar]
- Wilkins JF, & Haig D (2003). Inbreeding, Maternal Care and Genomic Imprinting. Journal of Theoretical Biology, 221, 559–564. 10.1006/jtbi.2003.3206. [DOI] [PubMed] [Google Scholar]
- Williams CA, Beaudet AL, Clayton-Smith J, Knoll JH, Kyllerman M, Laan LA, … Wagstaff J (2006). Angelman Syndrome 2005: Updated Consensus for Diagnostic Criteria. American Journal of Medical Genetics Part A, 140A(5), 413–418. 10.1002/ajmg.a [DOI] [PubMed] [Google Scholar]