Abstract
Purpose of review.
Diabetes is a common and prevalent medical condition as it affects many lives around the globe. Specifically, type-2 Diabetes (T2D) is characterized by chronic systemic inflammation alongside hyperglycemia and insulin resistance in the body, which can result in atherosclerotic legion formation in the arteries and thus progression of related conditions called diabetic vasculopathies. T2D patients are especially at risk for vascular injury; adjunct in many of these patients heir cholesterol and triglyceride levels reach dangerously high levels and accumulate in the lumen of their vascular system.
Recent findings.
Microvascular and macrovascular vasculopathies as complications of diabetes can accentuate the onset of organ illnesses, thus it is imperative that research efforts help identify more effective methods for prevention and diagnosis of early vascular injuries. Current research into vasculopathy identification/treatment will aid in the amelioration of diabetes-related symptoms and thus reduce the large number of deaths that this disease accounts annually.
Summary.
This review aims to showcase the evolution and effects of diabetic vasculopathy from development to clinical disease as macrovascular and microvascular complications with a concerted reference to sex-specific disease progression as well.
Keywords: diabetes, atherosclerosis, vascular injury, macrovascular, microvascular
2. Introduction
According to recent data from the CDC, in the US, over 646,000 deaths per year are directly related to cardiovascular disease (CVD) [1]. CVD deaths account for the highest percentage of deaths in the US, as 48% of all US adults are currently living with some form of heart disease [1]. Cardiovascular disease risk is often accentuated by adjacent conditions such as Diabetes, atherosclerosis, and hypertension [1]. From the aforementioned set of diseases, Diabetes, uniquely as a worldwide epidemic has taken millions of lives around the world [2]. The prevalence of this disease is expected to increase 1.6-fold by 2030 [3]. Diabetes is a chronic inflammatory multisystem disease generally characterized by hyperglycemia and insulin resistance and can be a consequence of genetic predisposition as well as environmental factors [4]. Another common cause are drug-associated conditions, as occurs in chronic systemic steroid treatment [4]. There are multiple types of diabetes, type-2 diabetes (T2D) being the most common in the world (90% in the US) [5, 6]. T2D is a chronic inflammatory disease with high incidence in adults with chronic hyperglycemia and insulin resistance. T2D shares many pathologic conditions with other chronic degenerative diseases. Recent discoveries show there is a strong correlation between atherosclerotic disease in T2D patients. The systemic inflammatory state in T2D cause hemodynamic disruption via alteration of vascular permeability and increase vascular thrombus formation which therefore increases risk for atherosclerosis development [7]. Up to 25% of diabetic patients have at least one associated comorbidity [8]. Patients with different endocrinopathies often converge in having been diagnosed with Diabetes and therefore share commonly associated clinical symptoms. Insulin resistance is observed in around 80% of patients with different types of endocrinopathies as well as 40% present with glucose intolerance [9]. Diabetic patients have more aggressive forms of vascular disease [10]. Diabetes is a significant and modifiable risk factor for atherosclerosis [11]. Atherosclerosis is the most commonly observed diabetic vasculopathy, also referred to as diabetic atherosclerosis, but other diseases like hypertension and heart failure are also included and account for many diabetic complications such as nephropathy, retinopathy, neuropathy, etc. [12]. Atherosclerosis is a chronic CVD, adversely influenced by increased serum lipid levels of small lipoprotein particles, which accumulate inside the wall of a vessel specifically in the sub intimal space [13]. Dysregulation from macrophage IGF1R causes atherosclerotic lesion and plaque vulnerability. This results in build-up of plaques that cause serious cardiovascular complications [14]. Atherosclerosis is prevalent in all developed nations and the leading cause of mortality and disability in the US [15]. Additionally, both diabetes and atherosclerosis have a greater likelihood of end-organ ischemia, as well as increased morbidity and mortality following vascular interventions [16]. High levels of low-density lipoprotein cholesterol in the bloodstream can cause significant cardiac macrovascular complications such as myocardial infarction resulting from inadequate blood flow [17]. Other aortic vascular diseases can also be present, such as aortic calcification, commonly associated to aging and lifestyle risk factors [18]. Aneurisms and diabetes also have a high association, also related to atherosclerosis [19]. Aneurisms mostly occur in the abdominal aorta, were high matrix metalloproteinase (MMP) activity and elastin loss modifies aortic wall integrity and function[12]. Vascular injury is a common pathologic characteristic found in almost all patients with diabetic vasculopathy. The definition of vascular injury is described as trauma affecting the vessels that carry blood to a target organ or tissue. Vascular injury does not only pertain to mechanical forces in the vasculature, but to the dynamic and pathologic mechanisms that inflict such damage. Systemic inflammation is a major component of all chronic diseases which present with characteristic vascular injury as a pathologic feature [20]. Vascular injury commonly occurs sub clinically and often undiagnosed until clinical signs are identified. Therefore, an early sign of disease progression is identified as endothelial dysfunction, which we will talk more extensively in the following section [21]. Depending on the severity and size of the affected vessels, diabetic vasculopathy complications are classified as macro or microvascular in nature [22]. Diabetic vasculopathy complications are studied then differently as microvascular and macrovascular damage [23]. Research has discovered a strong relationship between insulin resistance, inflammation, diabetes and CVD [9].Treatment options for severe atherosclerosis include percutaneous balloon angioplasty and stenting, endarterectomy, or bypass grafting [24]. Complication-free effective therapeutic modalities are non-existent in the clinical setting to prevent restenosis in atherosclerotic disease. Such complications are an alarming problem that causes significant morbidity and mortality [25]. Therefore, there is a pressing need to develop better ways to treat atherosclerosis and/or prevent restenosis after surgical interventions [16, 26]. Diabetic patients have more aggressive forms of vascular diseases, a greater need to undergo revascularization procedures [27, 28]. Yet, diabetic patients are more prone to therapeutic failure following intervention due to heightened restenosis rates and adverse outcomes [11]. Results from the PRESTO trial and NHLBI showed that diabetic patients have higher rates of in-stent restenosis [29]. Diabetes predicted a greater need for target vessel revascularization and was independently associated with death, undergoing percutaneous coronary angioplasty and stenting [30, 31]. This paper will review both macro and microvascular injury that occur in diabetic atherosclerosis with a particular reference to sex-specific differences in disease progression (Figure 1).
Figure 1.
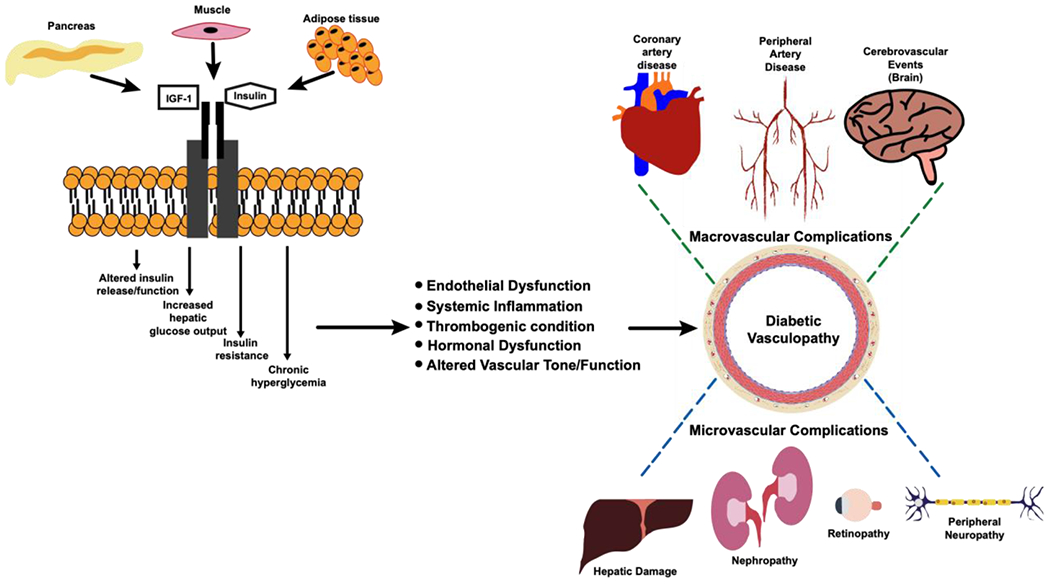
Diagram of the progression of macro/microvascular diabetic vasculopathy complications
3. Diabetes, atherosclerosis and vascular injury
Most of the pathologic changes that occur in diabetic vasculopathies have an intricate relationship between each other [20, 32]. As both diabetes and atherosclerosis are chronic diseases, vascular injury occurs in both of these conditions, which initiates the pathologic process of injury repair [3, 9]. Most patients present with multiple associated comorbidities but regardless, vascular injury is almost always present in diabetic vasculopathy patients [21, 33]. From the perspective of our review, we will describe endothelial dysfunction, systemic inflammation, thrombogenic conditions as well as altered vascular tone/function as the common pathologic features of diabetic vasculopathies We will individually review them as key components to micro and macrovascular injury and their complications.. We will also discuss hormonal dysfunction as an important topic that guides recent attempts to understand sex-disparities in diabetic vasculopathies. We will try to illustrate how common pathologic findings often occur in both micro and macrovascular vascular injury. We start by illustrating the pathobiology of diabetic vasculopathy after development of the diabetic phenotype and how it progresses into macro/micro vascular damage in Figure 1.
Endothelial Dysfunction.
The most commonly found early sign of vascular injury in diabetic vasculopathy is endothelial damage that produces marked dysfunction [34]. The impairment of the endothelium to maintain and regulate vascular tone is known as endothelial dysfunction. Such condition characterized primarily by a prothrombotic and proinflammatory state with diminished dilation of the blood vessels [35]. Patients that have been followed longitudinally for endothelial dysfunction appear to have increased risk of carotid artery disease (CAD) and specifically atherosclerosis-related events [36–38]. Chronic hyperglycemia from T2D contributes to vascular injury; vascular endothelial cells are very vulnerable to glucose toxicity [39, 40]. Glycolysis end products increase specific glucotoxicity of endothelial cells; endothelial dysfunction chronically promotes pro-atherogenic/inflammatory conditions within the vascular system [41]. However, it appears that there is much more to just hyperglycemic damage because diabetic patients even under glycemic control still fare worse in regards to CVD [42]. Together with glucose toxicity, endothelial-derived NO alteration disrupts vasodilation and homeostasis [43]. Importantly, oxidative stress has been consistently indicative as an endothelial dysfunction trademark [44, 45]. The pathologic condition promoted by diabetes and increase accumulation of dense/small LDL oxidated particles as occurs in atherosclerosis promotes overexpression of chemotactic proteins [15]. Endothelial dysfunction then alters adhesion molecule expression and endothelial cell junction altered morphology [46]. Recent findings suggest that endothelial dysfunction can be evidenced early in disease progression using promising serum biomarkers like proBNP, high sensitive Troponin, and lyso-GB3 as well as exosome release analysis [47, 48]. Therefore, the endothelial dysfunction makes a pro-inflammatory scenario that contributes to the following subsection in our review.
Systemic inflammation.
The pro-inflammatory conditions from T2D are promoted via altered regulation of forkhead transcription factor activation capable of eliciting substantial circulating inflammatory factors and cellularity [49]. Blood stasis and vascular permeability increase in a pro-inflammatory state due to vascular injury resulting from endothelial dysfunction [50]. As immune cells are activated in the periphery, they migrate-guided by these pro-inflammatory conditions to the site of inflammation in the vascular injury of the affected vessel [51]. Resident mononuclear cells and specifically macrophages in the intimal and medial layers of blood vessels form reactive oxygen species and foam cell formation (lipid-rich macrophages) respectively [52]. Cells that are recruited to the site of vascular injury take advantage of suitable vascular conditions due to enhanced cell infiltration and transmigration due to systemic inflammation [53, 54]. Systemic interplay between inflammation and endothelial dysfunction promotes an injury repair imbalance that facilitates progression of microvascular and macrovascular complications [55]. There have been multiple theories as to how systemic inflammation in T2D patients accelerate vascular disease. Recent findings indicate that there is also robust infectious mechanism that proposes an alternative approach to characteristic diabetic vasculopathy development [7]. Increased circulating neutrophils and monocyte/macrophages means higher reactive oxygen species as well as matrix degradation [40, 56]. Oxidative stress, matrix degradation via MMP released from macrophages and necrotic core degradation create the perfect storm: thrombus formation [57].
Thrombogenic conditions.
Atherosclerotic plaques are vulnerable sites for rupture mostly because of their lipi-rich cores as well as thin fibrous cap formation around the plaque [58]. Diabetic patients have systemic inflammation and more severe endothelial dysfunction meaning more platelets and increased vascular stasis resulting in a thrombogenic milieu [59]. Buildup of plaque progresses rapidly in T2D patients within the vascular system, possessing a high thromobogenic risk [15, 60]. Pronounced pro-thrombotic effects due to alteration of vascular homeostasis creates a serious risk for disease complications but is mostly seen in chronic and advanced stages of vascular injury [20]. Migration of inflammatory cells to the intima as well as vascular smooth muscle cell (VSMC) proliferation are important for thrombogenic conditions [12]. When foam cells die via apoptosis, they promote a necrotic core in vascular atherosclerotic lesions [61]. When a diabetic atherosclerotic lesion develops into a plaque, the thromboembolic risk is greatly increased [18]. Such vulnerable plaques for rupture have a characteristic thin fibrous cap that ruptures often as thrombus is formed [40].
Hormonal dysfunction.
Hormonal dysfunction has recently been identified as a factor influencing sex disparities in diabetic vasculopathies. Although some concepts had already been studied, the recently proposed role in hormonal dysfunction goes far beyond the estrogen-associated variation that contribute to known differences in diabetic vasculopathy progression as we have discussed in the above subsections. Hypothalamus Pituitary Axis (HPA) dysfunction is associated with many chronic and degenerative diseases [62, 63]. Disparities due to HPA dysfunction appear to have a clear relationship to estrogen and androgen regulation via glucocorticoid disruption [64–66]. Not only estrogen dependent effects control this alteration but appear to have a clear role in HPA dysfunction. We will illustrate more on the sex-specific differences in diabetic vasculopathies in a following section of this review. Hormonal dysfunction apparently does not unequivocally promote vascular injury; more importantly vascular injury has an important role for HPA disruption and consequent alteration of diabetic vasculopathy progression [67, 68]. As well, alteration of the HPA alters other pathways in the bradykinin and renin-angiotensin systems that alter vascular function as well as vascular wall stiffness and complexity (regulators of vascular tone) [69, 70].
Altered vascular tone and vascular function.
Vascular injury promotes an imbalance in vascular tone regulation via dysregulation of the Angiotensin II system, which stimulates reactive oxygen species to cause increased vasoconstriction [71–73]. T2D patients have altered liver and kidney function, which sequentially disrupts the renin-angiotensin system even further [74]. Endothelial dysfunction, thrombogenic conditions and systemic inflammation make the functional alteration of the vasculature more pronounced in diabetic vasculopathy but it is often observed as a late symptom in diabetic vascular injury [9]. An early promoter of alteration in vascular tone is the NO-dependent vasoactive dysregulation that alters oncotic and osmotic pressure [16]. Vascular stiffness is normally regulated by VSMC function in homeostatic/physiologic conditions [71]. Diabetic patients have increased VSMC proliferation and switch to a more synthetic phenotype consistent with vascular dysfunction, which can produce medial layer thickening and narrowed lumen of the artery which results in ischemia [68]. Altered vascular resistance contributes to blood stasis and also disruption in the venous system, promoting vascular tone dysfunction. The relaxation and constriction defects due to vascular damage in diabetic patients set such patients in high risk for hypertensive disease [75]. Altogether, vascular tone alteration directly affects cardiac function and vascular response to stress that contributes to high risk of CAD and heart failure together with diabetes and atherosclerosis [57].
4. The macrovascular injury in diabetic vasculopathy
Diabetic patients very frequently present with localized and systemic vascular complications known as diabetic vasculopathies that are accelerated by many comorbidities for example hypertension [12]. Studies have shown that the risk of developing CAD, peripheral artery disease (PAD), and cerebrovascular disease is increased 2- to 4-fold in patients with diabetes [76]. The morbidity and mortality related to vascular disease are also worse in diabetics, accounting for the majority of deaths in these patients [31]. The increase in advanced glycation products and systemic inflammation make comorbidities commonly associated with diabetes more harmful [22]. The hyperglycemic and insulin resistance environment in diabetic patients makes it suitable for pro-atherogenic conditions systemically by allowing for plaque buildup and subsequent diabetic vasculopathies by alteration of vascular tone and permeability making it easier for inflammatory cells to transmigrate and lipids to accumulate inside vessel wall via disrupted endothelium (Figure 1). These diabetic vasculopathies can be characterized as both micro vascular and macrovascular based on their location and effect on the body as in different levels of severity [7]. As well, the likelihood of the end organ to produce clinical signs of disease classifies such complications. Most macrovascular events take longer to have evident clinical signs but when they are present it is life threatening. This highlights the importance of early detection of vascular injury as non-invasively as possible but accurately [36, 51]. Tissues like the heart, with elevated contractility get high risk of development of macrovascular complications because arteries like the coronaries become occluded even from physiologic contractility, this is worst together with pathologic vascular occlusion [77]. Table 1 shows the common diagnostic and therapeutic tools available in macrovascular complications [78, 79]. The next subsections will describe the common macrovascular complications associated with diabetes and associated clinical symptoms that serve as a link to macrovascular injury [78].
Table 1.
Macro/microvascular diabetic vasculopathy complications with clinical manifestations and major risk factors.
| Complication | Type of Complication | Clinical Manifestations | Major Risk Factor | Diagnostic Tools Used |
|---|---|---|---|---|
| Cardiac Complications (ASCVD) | Macrovascular | Angina, shortness of breath, tachycardia, STEMI, NSTEMI | Type 2 DM/Atherosclerotic buildup, Hypertension (W. Fan et al., 2019) | Traditional: lipid screening Nontraditional: C-reactive proteins, lipoprotein particles, apolipoproteins. (Curry et al., 2018) |
| Peripheral Artery Disease | Macrovascular | Cramping, numbness, discoloration of distal limbs, weak pulse in affected limb, ischemic resting pain | Atherosclerotic buildup, Tobacco usage (Powell et al., 1997) | Ankel-Brachial Index or Digital Subtraction Angiography (C. Marques et al., 2018) |
| Cerebrovascular events, Carotid Artery Disease | Macrovascular | Dizziness, dysarthria, numbness, headaches, stroke | Atherosclerotic buildup | Bruit testing, ultrasound, CT angiogram, MRI, Carotid Doppler or cerebral angiogram. (Ringer, 2018) |
| Nephropathy | Microvascular | Macroalbuminuria, Microalbuminuria, Oliguria, Anuria | Type 2 DM/Atherosclerosis, hypertension, drug-induced (Fowler, 2008) | Albumin evaluation, glomerular filtration rate, random spot albumin/creatinine ratio, renal biopsy, four-hour urine collection. (Roett et al., 2012) |
| Liver Disease (Non-alcoholic Fatty Liver Disease) | Microvascular | Jaundice, ascitis, gynecomastia | Type 2 DM/Atherosclerosis/Hypertension/Obesity (MH et al., 2018) | Liver enzyme screening and liver imaging. (Wilkins et al., 2013) |
| Retinopathy | Microvascular | Red/Gray/White dots on retina. | Type 2 DM/Atherosclerosis (Preferred Practice Patterns Committee 2014) | Fluorescein Angiography or Optical Coherence Tomography to examine macula (Boyd, 2018) |
| Peripheral Neuropathy | Microvascular | Symetric Polyneuropathy: Buring, tingling, numbness, pain, sensory loss to touch, vibration, and temperature. Mononeuropathy: severe muscle pain and atrophy. Autonomic Neuropathy: digestive/urination issues and tachycardia. Most prominent at night. | Type 2 DM/Atherosclerosis, genetic predisposition (Fowler, 2008) | Skin exam, circulation analysis, Loss of Protective Sensation (LOPS) test, pinprick test, ankle reflex, pressure/vibration perception, Nerve Conduction Velocity and Electromyography tests. (Harrar, 2017) (Jardon-Reyez et al., 2018) |
Coronary Artery disease
More than 60% of all diabetes related deaths are due to cardiovascular disease [60]. One of the most common macrovascular diabetic vasculopathy is CAD, characterized by reduced blood flow in the coronary arteries [9, 60]. Hyperglycemia, increase in circulating inflammatory markers, and elevated cholesterol levels in diabetic patients create a progressive environment for CAD [80]. Hyperglycemia in diabetic patients also causes increased vascular permeability and blood stasis as we had mentioned due to vascular injury. Coronary artery vessel lumen is easily compromised due to atherosclerotic plaque development in diabetic patients [76]. Diabetic patients have high incidence of developing a myocardial infarction within the first 10 years of disease presentation [81, 82]. Atherosclerotic anatomical plaque buildup in patients normally affects the coronary arteries as the second most common place of disease presentation after abdominal aorta in the systemic vasculature [81]. Lifestyle modification as treatment for both diabetes and atherosclerotic have proven ineffective. T2D There are no stand alone pharmacologic interventions able to completely alleviate the associated comorbidities of diabetic vasculopathies. CAD is commonly treated with Carotid Endarterectomy (CEA), specifically an eversion CEA (ECEA), which involves surgical removal of the material blocking the blood flow in the artery [83]. Other alternatives to CAD treatment are balloon angioplasty, STENT placement and MICS CABG (Minimally Invasive Cardiac Surgery Coronary Artery Bypass Graft) which is a bypass surgery to restore blood flow to an obstructed coronary artery [82].
Peripheral Artery Disease
PAD is a macrovascular diabetic vasculopathy characterized by buildup of plaque in the peripheral vascular system affecting over 12% of Americans over 60 years old [84]. PAD compared to CAD mostly differs due to the anatomic location were events occur (PAD> extremities, CAD> coronaries) [10, 11]. Vessels affected that are not supplying the brain or the hearts are classified under PAD. Early signs of PAD are seen with claudication, limb ischemia, weak peripheral pulses and vascular dermatologic lesions [85–87]. Classifications such as the Fontaine make stratification of limb ischemia reliable, it classifies patients merely by their clinical symptoms [88]. One of the most common and financially heavy clinical presentations of PAD is diabetic foot [89]. It is estimated that billions of dollars are designated to treating this high incidence complication in T2D patients [90]. The pathognomonic finding in diabetic foot is diabetic ulcers that occur more frequently in the lower extremities [91]. Such ulcers are sometimes non-disclosed by patients due to the nature of associated peripheral neuropathy associated in T2D patients [92]. Almost 1 in 4 patients with T2D will have diabetic vasculopathy presenting as diabetic foot [93]. Risk of amputation in diabetic patients increased almost 4 times as age of disease progresses and in many cases amputation can be evaded by timely and accurate intervention [94].
Most patients with PAD require invasive interventions at the time of presentation if lifestyle intervention is not effective, diabetic patients have worst outcome following vascular interventions [7].Smoking, hypertension and other risks also play an important role, but appear not to accelerate disease progression as fast as diabetes does, especially T2D [15]. For those with severe PAD, the patency depends on the nature and location of the disease (PAD is significantly worse below the kneecap) [14, 84]. PAD affects mostly the abdominal aorta due to atherosclerotic disease along with the iliac and femoral arteries, most of the vascular bypass interventions are therefore ileo-femoral and femoral-popliteal. T2D patients with severe cases of PAD that undergo surgical revascularization of arteries to re-establish adequate lumen blood flow typically present with worsened outcomes which can lead to the possibility of amputation [84]. Besides the already mentioned treatment options for CAD in the subsection above, bypass grafting, cryoplasty, laser atherectomy and angiogenesis-based MultiGeneAngio are alternatives [10, 95].
Cerebrovascular events
Blood flow to the central nervous system is affected mostly due to atherosclerotic vascular disease and more specifically thromboembolic events associated with diabetic atherosclerotic plaque buildup [96, 97]. Carotid disease due to carotid atherosclerotic plaque rupture is an important complication of diabetic vasculopathies leading to the risk of stroke due to thromboembolic events [98]. Upstream blood flow to the brain from the carotid arteries and in the Willis circle compromises important areas in the central nervous system that cause severe debilitating complications [99]. T2D patients have increased risk of cerebrovascular events and worst outcomes following such events [100]. The combination of diabetes, atherosclerosis along with hypertension also lead to many non-thromboembolic cerebrovascular events as in aneurism rupture and hemorrhagic events [101]. The increasing likelihood of developing obesity alongside diabetes induced an environment of hyperglycemia, hypertension and dyslipidemia, which cause pathological changes to blood vessels and can lead to a stroke [102].
The scope of this review relies on vascular injury, so we will not surpass into anatomic-specific clinical symptoms of cerebrovascular events. In this particular case, the anatomical affection of vascular disease vary greatly on the affected location. Hemiplegia, hemiparesia, headache are some examples of clinical signs of cerebrovascular events [103, 104]. The aim in cerebrovascular events is to prevent and timely diagnose them because reversals of neurologic complications are many times non-viable [105, 106]. Although most of this subsection reviews the macrovascular injury that occurs in diabetic vasculopathy, there are also some findings illustrating microvascular injury in the brain [107]. For example, Cilostazol protects against microvascular brain injury in a rat model of type-2 diabetes [108].
5. The microvascular injury in diabetic vasculopathy
Microvascular diabetic injury complications include a field of conditions that cause significant damage at what could be considered a slow and steady pace [22, 32]. The pathobiological characteristic of microvascular damage is basement membrane thickening in different systems mostly but not limited to the eyeball, kidney disease and mostly peripheral neuropathy [78, 109]. Basement membrane thickening creates a hypoxic condition due to altered vascular diffusion, homeostatic alteration and maintained glucotoxicity in small vessels and capillaries [110, 111]. From these systems, clinical symptoms such as renal failure, blindness, amputations, and predict future cardiovascular issues [77, 111]. Understanding early clinical symptoms has the potential to significantly reduce the number of deaths by cardiovascular events by promoting early treatment [32]. Furthermore, given the severity of atherosclerosis in diabetes, these patients have a greater likelihood of end-organ ischemia [112]. Table 1 shows the common diagnostic and therapeutic tools available in microvascular complications. The next subsections will describe the common microvascular complications associated with diabetes with associated clinical symptoms as well as the link to microvascular injury.
Hepatic damage, altered homeostasis
Pathognomonic features of diabetic liver vasculopathy damage belong to a plethora of pathologies known as non-alcoholic fatty liver disease [113, 114]. Different metabolic pathways are included, but steatosis and necroinflammation are common findings in diabetic microvascular liver injury [32, 115]. Insulin resistance that occurs in diabetes affects the liver greatly, as well as preexisting hypertension and altered liver function [116]. Additionally, bilirubin has recently been associated as a predictor of diabetic vasculopathy [117]. The NASH study correlates diabetic predisposition as well as being a male to liver fibrosis. In diabetic rats with liver disease compared to control and diabetic rats not treated with curcumin, it appears to reverse liver damage giving high importance to liver redox imbalance in pre-clinical models of diabetes [118, 119]. Importantly, a relationship has been established between diabetic rats and liver mitochondrial dysfunction [120]. In regards to liver damage, ethanolic extract of M. cocanensis nimmo leaves (EEMCNL) ameliorates symptoms of hepatic damage in diabetic patients [121]. Animals with induced diabetes were compared to control animals and results showed that inadequate levels of protein, urea, creatinine, and uric acid all returned to their normal levels in diabetic animals treated with EEMCNL [121]. Inhibition of Notch signaling using Sunitinib controls hepatocyte morphology as well as vascular liver damage in a model of type 1 diabetes in streptozotocin-induced diabetic mice [122]. Nrf2 activation via HDAC3 inhibition potentiates the role of redox balance in liver damage of diabetic rats [119]. The relationship between diabetic vasculopathy in the liver and redox imbalance is very promising, as it elevates the interest in studying models of liver damage to aim towards diabetic atherosclerotic disease, which is limited.
Nephropathy: Micro albuminuria and the road to macro albuminuria
Chronic hyperglycemia and lipid accumulation in the basement membrane, as well as renal artery constriction are the primary issues in diabetic vasculopathy patients that contribute to diabetic nephropathy [123]. Chronic kidney failure is the most common diabetic vasculopathy in the US [124, 125]. In regards to nephropathy, only macro albuminuria is considered as true nephropathy because micro albuminuria is commonly associated as an incidental finding [126]. Patients with diabetes commonly present with microvascular kidney damage, which can result in many long-term complications [127]. Comorbidities associated with diabetes as we have mentioned extensively in this review, atherosclerosis and hypertension worsen disease progression [128, 129]. Diabetic nephropathy is very commonly associated with primary hypertension [130]. Capillaries around the glomeruli are damaged and such injury causes progressives and marked albuminuria [126]. Histologically, the pathognomonic feature in the kidney is associated with Kimmelstiel Wilson nodular glomerulosclerosis due to obvious sclerosis (elevated TNF beta and protein kinase C) [131] and basement membrane thickening (increase in vascular permeability). Such histologic findings are also associated with hyperglycemia which causes glycosylation of proteins in the mesangium [132]. In the clinic, the classification for micro albuminuria in diabetic vasculopathy progresses goes from minimally detected to gross albumin in the urine (>300mg/day) as stratified in current guidelines [126, 133]. First line of treatment goes into dialysis via the peritoneum or in its case hemodialysis [74, 134]. Severe cases with associated low glomerular filtration rate and severe high creatinine are treated with renal transplantation, which has historical bad prognosis mostly when associated with other comorbidities, but is still a common occurrence [135].
Retinopathy
Diabetic retinopathy (DR) is the most frequent microvascular complication of diabetes with a rate percentage of up to 30% in all-diabetic patients [136]. It is the number one cause of blindness in the US. Micro aneurisms due to neoangiogenesis in the retina are the most commonly observed pathologic finding that causes blindness and intermittent blurry vision [136]. Diabetic retinopathy is very closely associated with the endothelin system in vascular injury and chronic damage [109]. Macular damage, macular edema, cataracts and glaucoma are some of the common diseases associated with microvascular retinopathy in diabetic vasculopathy [28]. Diagnostic tools for DR often used beside accurate prevention rely on angiograms with fluorescence advances but in many instances the damage is extensive by the time they are accurately assessed [137].
Peripheral Neuropathy.
Diabetic peripheral neuropathy is the second common microvascular vasculopathy that causes neuropathic complications on the entire peripheral neuron and can present in many forms [138]. Most of the mechanisms that guide neuropathic damage due to diabetic vasculopathy like glucotoxicity and basement membrane lipid accumulation are key factors for peripheral neuropathy because they cause demyelination but it is the chronic microscopic damage that affects motor and sensitivity neurons in the periphery the most. The exact mechanisms that guide peripheral damage are not well understood. The microvascular complications affect the neurons peripherally in what is described as a stocking-glove anatomical distribution pattern and attribute characteristic neurologic symptomatology [139]. Clinical signs include loss of function, sensitivity loss (vibration, pain, temperature) with deep tendon reflexes often seen as an initial sign [138]. Gastroparesias and dyskinesias are some of the most representative clinical symptoms, although many times present late in the disease [140]. Most of these are hard to treat and are a very common complains between diabetic patients. Neurological examination is a key part of the peripheral neuropathy assessment as it considers a wide range of tests with selective sensitivity and interpretation [28, 52, 141]. Clinical signs are very important to accurately and timely assess neuropathy, but most of the clinical signs are subclinical and not as abruptly presented as compared to cerebrovascular events [131]. A few of these neurological examinations include electromyography, which is a common study done to perform nerve conduction function and assess damage of diabetic neuropathy [138]. Dermatologic examination is another examination associated with neuropathy analysis because loss of sensor nerve function denies patients of correct self-assessment of wounds and lesions that often end up in limb ischemia as well as extremity amputations [27].
6. Sex-specific differences in diabetic vasculopathy
Diabetes and atherosclerosis affect specific populations differently [67]. Increased risk to diabetic vasculopathies is non-modifiable associated to genetic predisposition, aging and importantly sex [142]. Although all of the aforementioned are important, it appears that the less variable and straightforward to asses biological risk factor is sex [143]. Sex-specific metabolic characteristics have major implications in physiological versus pathological cardiovascular changes. Men are more prone to earlier diabetic phenotype development than women; more men become pre diabetic earlier than women [144]. Premenopausal women have less risk of CVD compared to males during adulthood [67]. The estrogen-dependent protective role is reversed in postmenopausal women when the risk for CAD is increased and matches risk in males [67]. Diabetic females lose pre-menopausal cardio-protection for high risk of fatal CVD as well as occurs in the physiologic post menopausal phase [145]. Moreover, diabetes also disproportionately affects racial/ethnic minorities with major sex-specific differences as occur in the hispanic community [146]. Recently identified sex-specific genes, such as ASXL1 and ASXL2 appear to have a big role in normal cardiovascular development and maybe in CVD risk [147]. In sex-specific disease progression, males have worst age-associated diabetic disease complications as in nephropathy [144]. Related to diabetic associated diseases, EPHA4 polymorphism increases VSMC contractility in females increasing the risk to hypertension [68]. It is also implicated that genes with evidenced sex-specific differences serve as early predictors of the pathologic and physiologic disparities in male versus female CVD risk stratification [148]. In relationship to research, in a recent sex-specific study, pups from high fat diet fed females transferred to foster mothers induced higher risk of sex-specific obesity, diabetes and adiposity [149, 150]. Even in the intrauterine period, insulin related proteins: Insulin-Like Growth Factor-1 (IGF1) and the IGF1 receptor (IGF1R) appear to have a sex-specific regulation [151, 152]. Both of these proteins appear to have an important role in regulating macrophage atherogenic activity [13, 153] and cardiac contractility [154]. Dysregulation from macrophage IGF1R causes atherosclerotic lesion and plaque vulnerability worsening in apoE KO mice [155]. In medium caliber vessels, IGF binding proteins in humans appear to regulate VSMC function [156]. In clinical settings, there are marked sex-related differences for vulnerable atherosclerotic disease and CAD [157]. The PROMISE study assessed how many interacting factors from high-risk atherosclerotic plaque and specific population predictors can have promising implications. Their approach helps clinicians assess atherosclerotic conditions with follow up within such high-risk populations in mind [158]. Exogenous hormone use has been recently identified as a biomarker for diabetic vasculopathy injury [61]. Such biomarkers were surprisingly associated mostly to cardiovascular risk but not myocardial infarction risk [61]. For cardiac diseases associated with diabetic microvascular injury such as atrial fibrillation, females have increased associated miRNAs levels as well as associated pathologic aortic changes [18]. In animal models, female diabetic Wistar rats after a induced stroke injury have increased neurodegeneration compared to males [141]. Estrogen is a sex-dependent factor with the strongest evidence effect on CVD [66, 159]. Estrogen vascular receptor is deterministic in disease progression, but particularly in premenopausal females under hormonal therapy [65]. Polymorphisms in the estrogen receptor alpha in young girls with type-1 diabetes appear to not be useful in prognosis of vascular complications [160]. Estrogen receptors outside of the nucleus appear to have an important role in T2D via regulation of beta islet cell regulation [161]. Female abdominal circumference and visceral adiposity predispose females for nephropathy due to increased vascular stiffness compared to males [143]. The physiologic and pathologic involvement of estrogen precursors from the liver has a role systemically in vascular function regulation [162]. In contrast to estrogen effects, growth hormone-deficient children have increased risk of atherosclerosis [163].
7. Recent findings in diabetic vasculopathies diagnosis and treatment
Continual research on complications due to diabetic vascular injury lends itself to new discoveries regarding treatments and diagnostic techniques [77]. Effective ways to identify disease characteristics before they progress into more serious or fatal conditions is fundamental. Macrovascular vasculopathy diagnosis techniques have undergone several improvements in the past few years [78, 79]. Table 1 shows current diagnostic and therapeutic tools available in macro/micro diabetic vasculopathy complications. Aims towards diabetic vasculopathy can detect arterial wall thickening and decreased blood flow [57]. Current diagnosis/treatment for PAD includes: resting ankle-brachial index (ABI) test, lifestyle modifications (exercise and smoking cessation), antiplatelet therapy, ACE inhibitors, and statins [84]. Cilostazol is the only FDA approved drug for PAD as it improves PAD symptoms [164] but does not improve overall CVD mortality [165]. In regards to lipid lowering therapies, recent research has shown that pro-protein convertase subtilisin/kexin type 9 (PCSK9) inhibitors function as excellent therapeutic agents for lowering LDL-C with concomitant statin usage which could reduce lipid buildup and thus CAD [17]. Alirocumab and Evolocumab are two drugs functioning as PCSK9 inhibitors that have been approved by the FDA and reduce LDL levels by up to 60% and reduce ASCVD events with statin usage [17]. Recent research also concludes that glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a cAMP/PKA/Rho-dependent mechanism [75]. Research in surgical approaches has shown a modified ECEA treatment for carotid disease is known as a Q-modified ECEA, modifies the surgical approach of the technique [83]. Results concluded much lower levels of swelling, scar length, and numbness post-surgery in QCEA patients compared to ECEA patients [83]. When talking about retinopathy findings, recent findings suggest a new form of fluorescein angiography with OCT (OCTA) provide efficient forms of diagnosis/identification for DR [136] Treatment for DR revolves around hypertensive and glycemic control, Fenofibrate for dyslipidemic diabetic patients as well as anti-VEGF agents such as Aflibercept does also present a notable decline in DR progression [136]. Recent studies on diabetic neuropathy and S100B/S100P (calcium-modulated) confirm them as significant indicators of peripheral neuropathy in diabetic patients [166]. Some recent studies also suggest that dietary non-hemoglobin iron supplements can help to restrict progression or development of peripheral diabetic neuropathy [52]. A recent study on 4-O-methylhonokiol (MH) revealed that this ingredient, when induced with a gavage on mice with insulin resistance, significantly prevented renal lipid accumulation and increased levels of proteinuria along with final fibrosis [127]. This may potentially be related to the NRF2/SOD2 anti-oxidative stress cycle and could act as a future treatment for patients with nephropathy [127]. Exosome treatment from urine-derived stem cells (current treatment for nephropathy) and adipose-derived stem cells (experimental treatment for nephropathy) has recently been proven useful for chronic renal disease [167]. Insulin-producing pancreatic cell therapies using stem cells as well as their multi/pluripotent properties are a novel and very interesting approach to diabetic vasculopathy treatment. For the most current diabetic treatments, different approaches to stem cell discoveries give promise to target therapeutics [168]. Adipose-derived stem cells significantly decreased symptoms of diabetic nephropathy: serum creatinine levels, cell apoptosis, and podocyte injury [167]. Inhibition of the Smad1/mTOR signaling pathway in the podocyte has potential for use as a nephropathy treatment in the future [167].
8. Conclusion and future perspectives
There is still a big gap in clinical practice to accurately diagnose and triage patients that will most likely develop diabetic vasculopathies. Our efforts in modern medicine take us in a path of direct estimation of disease progression but we still fall short to timely and accurate diagnose them. This review tries to evidence the importance and close relationship from vascular injury in diabetic vasculopathy. The main pathobiological component in diabetic vasculopathies is vascular injury. Many efforts are currently directed to point of care testing of early vascular injury in order to eradicate the high incidence of such as atherosclerosis, hypertension and T2D and any associated comorbidities. And although many chronic diseases present with characteristics of vascular injury such as endothelial dysfunction, altered vascular tone and inflammation there is a strong correlation of lower complications with early diagnosis from current clinical testing. It is important to conclude that macrovascular and microvascular injuries are pathologic forces that synergize in the development of diabetic vasculopathy as well as other important entities, as occurs in metabolic syndrome. In this review, we also illustrated the sex-specific differences in the role of diabetic vasculopathies in vascular injury. Such differences can guide meaningful efforts to find alternative therapeutic targets evidenced from sex-protective pathways.
Acknowledgements.
Supported by the Collaborative Research Travel Grant (Burroughs Wellcome Fund) to RIM, and the NHLBI (K01HL145354)to EMB. EMB was a KL2 scholar partially supported by the UNC Clinical and Translational Science Award-K12 Scholars Program (KL2TR002490) and by the UNC-NORC Pilot & Feasibility Grant (P30DK056350).
Conflicts of Interest
Dr. Mota reports grants from Burroughs Wellcome Fund, during the conduct of the study.
Mr. Samuel Morgan has nothing to disclose.
Dr. Bahnson reports grants from NIH, during the conduct of the study
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Human and Animal rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
References
- 1.Benjamin EJ, et al. , Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation, 2019. 139(10): p. e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt AM, Highlighting Diabetes Mellitus: The Epidemic Continues. Arterioscler Thromb Vasc Biol, 2018. 38(1): p. e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowley WR, et al. , Diabetes 2030: Insights from Yesterday, Today, and Future Trends. Popul Health Manag, 2017. 20(1): p. 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mihai B, et al. , Rare types of diabetes mellitus. Rev Med Chir Soc Med Nat Iasi, 2012. 116(3): p. 700–7. [PubMed] [Google Scholar]
- 5.Lee AK, et al. , Number and Characteristics of US Adults Meeting Prediabetes Criteria for Diabetes Prevention Programs: NHANES 2007-2016. J Gen Intern Med, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greene J, Dividing Diabetes by Cluster Instead of Types. Manag Care, 2018. 27(6): p. 29–30. [PubMed] [Google Scholar]
- 7.Rahman S, et al. , Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes Metab, 2007. 9(6): p. 767–80. [DOI] [PubMed] [Google Scholar]
- 8.● Lin PJ, Pope E, and Zhou FL, Comorbidity Type and Health Care Costs in Type 2 Diabetes: A Retrospective Claims Database Analysis. Diabetes Ther, 2018. 9(5): p. 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu W, Tian M, and Zhou Y, The relationship between insulin resistance, adiponectin and C-reactive protein and vascular endothelial injury in diabetic patients with coronary heart disease. Exp Ther Med, 2018. 16(3): p. 2022–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiruvoipati T, Kielhorn CE, and Armstrong EJ, Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World J Diabetes, 2015. 6(7): p. 961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maranghi M, et al. , Atherosclerosis renal artery stenosis and in-stent restenosis in a diabetic patient: Targeting on diabetic dyslipidemia is a key intervention. J Endocrinol Invest, 2010. 33(4): p. 284–5. [DOI] [PubMed] [Google Scholar]
- 12.Cooper ME, et al. , Mechanisms of diabetic vasculopathy: an overview. Am J Hypertens, 2001. 14(5 Pt 1): p. 475–86. [DOI] [PubMed] [Google Scholar]
- 13.von der Thusen JH, et al. , IGF-1 has plaque-stabilizing effects in atherosclerosis by altering vascular smooth muscle cell phenotype. Am J Pathol, 2011. 178(2): p. 924–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun J, et al. , Subclinical carotid atherosclerosis: short-term natural history of lipid-rich necrotic core--a multicenter study with MR imaging. Radiology, 2013. 268(1): p. 61–8. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira-Santos M, et al. , Atherosclerotic plaque metabolism in high cardiovascular risk subjects - A subclinical atherosclerosis imaging study with (18)F-NaFPET-CT. Atherosclerosis, 2017. 260: p. 41–46. [DOI] [PubMed] [Google Scholar]
- 16.Bahnson ES, et al. , Targeted Nitric Oxide Delivery by Supramolecular Nanofibers for the Prevention of Restenosis After Arterial Injury. Antioxid Redox Signal, 2016. 24(8): p. 401–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon DL, et al. , Recent Updates on the Use of PCSK9 Inhibitors in Patients with Atherosclerotic Cardiovascular Disease. Curr Atheroscler Rep, 2019. 21(5): p. 16. [DOI] [PubMed] [Google Scholar]
- 18.Dudink E, et al. , Vascular Calcification and not Arrhythmia in Idiopathic Atrial Fibrillation Associates with Sex Differences in Diabetic Microvascular Injury miRNA Profiles. Microrna, 2019. 8(2): p. 127–134. [DOI] [PubMed] [Google Scholar]
- 19.Wolstenhulme S, et al. , Agreement between objective and subjective assessment of image quality in ultrasound abdominal aortic aneurism screening. Br J Radiol, 2015. 88(1046): p. 20140482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asfour V, Smythe E, and Attia R, Vascular injury at laparoscopy: a guide to management. J Obstet Gynaecol, 2018. 38(5): p. 598–606. [DOI] [PubMed] [Google Scholar]
- 21.Bohlen HG, Mechanisms for early microvascular injury in obesity and type II diabetes. Curr Hypertens Rep, 2004. 6(1): p. 60–5. [DOI] [PubMed] [Google Scholar]
- 22.Murayama H, et al. , Relationship of patient background with macro- and microvascular complications: a 2-year post-marketing surveillance of vildagliptin in nearly 20,000 Japanese diabetic patients. Expert Opin Pharmacother, 2019: p. 1–11. [DOI] [PubMed] [Google Scholar]
- 23.Mitranun W, et al. , Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients. Scand J Med Sci Sports, 2014. 24(2): p. e69–76. [DOI] [PubMed] [Google Scholar]
- 24.Kolos I, et al. , Modern medical treatment with or without carotid endarterectomy for severe asymptomatic carotid atherosclerosis. J Vasc Surg, 2015. 62(4): p. 914–22. [DOI] [PubMed] [Google Scholar]
- 25.Dorenkamp M, et al. , Cost-effectiveness of paclitaxel-coated balloon angioplasty in patients with drug-eluting stent restenosis. Clin Cardiol, 2013. 36(7): p. 407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X, et al. , Bilayered Nanoparticles with Sequential Release of VEGF Gene and Paclitaxel for Restenosis Inhibition in Atherosclerosis. ACS Appl Mater Interfaces, 2017. 9(33): p. 27522–27532. [DOI] [PubMed] [Google Scholar]
- 27.Chou C, et al. , Combination of Vascular Intervention Surgery and Free Tissue Transfer for Critical Diabetic Limb Salvage. Ann Plast Surg, 2016. 77 Suppl 1: p. S16–21. [DOI] [PubMed] [Google Scholar]
- 28.McVicar CM, et al. , Intervention with an erythropoietin-derived peptide protects against neuroglial and vascular degeneration during diabetic retinopathy. Diabetes, 2011. 60(11): p. 2995–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh M, et al. , Geographical differences in the rates of angiographic restenosis and ischemia-driven target vessel revascularization after percutaneous coronary interventions: results from the Prevention of Restenosis With Tranilast and its Outcomes (PRESTO) Trial. J Am Coll Cardiol, 2006. 47(1): p. 34–9. [DOI] [PubMed] [Google Scholar]
- 30.Jadue TA, Gonzalez LR, and Irarrazabal LLM, [Meta-analysis of coronary artery bypass surgery compared to percutaneous transluminal angioplasty with stent in diabetic patients]. Rev Med Chil, 2012. 140(5): p. 640–8. [DOI] [PubMed] [Google Scholar]
- 31.Naito R, et al. , Clinical Outcomes in Diabetic Patients Who Underwent Percutaneous Coronary Intervention during the Plain Old Balloon Angioplasty (POBA)-, Bare Metal Stents (BMS)- and Drug-eluting Stents (DES)-eras from 1984 to 2010. Intern Med, 2017. 56(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.●● Avogaro A and Fadini GP, Microvascular complications in diabetes: A growing concern for cardiologists. Int J Cardiol, 2019. [DOI] [PubMed] [Google Scholar]; Gives a great perspective on the importance on subclinical microvascular complications in diabetic patients
- 33.Phillips JA 3rd, Dominant-negative diabetes insipidus and other endocrinopathies. J Clin Invest, 2003. 112(11): p. 1641–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiggenhauser LM and Kroll J, Vascular damage in obesity and diabetes: Highlighting links between endothelial dysfunction and metabolic disease in zebrafish and man. Curr Vasc Pharmacol, 2018. [DOI] [PubMed] [Google Scholar]
- 35.Endemann DH and Schiffrin EL, Endothelial dysfunction. J Am Soc Nephrol, 2004. 15(8): p. 1983–92. [DOI] [PubMed] [Google Scholar]
- 36.Adams A, Bojara W, and Schunk K, Early Diagnosis and Treatment of Coronary Heart Disease in Asymptomatic Subjects With Advanced Vascular Atherosclerosis of the Carotid Artery (Type III and IV b Findings Using Ultrasound) and Risk Factors. Cardiol Res, 2018. 9(1): p. 22–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell CC, et al. , Carotid artery ultrasound texture, cardiovascular risk factors, and subclinical arterial disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Br J Radiol, 2018. 91(1084): p. 20170637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.● Polak JF, Szklo M, and O’Leary DH, Carotid Intima-Media Thickness Score, Positive Coronary Artery Calcium Score, and Incident Coronary Heart Disease: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc, 2017. 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]; Emphasizes the importance of intima-media thickness assessment in carotid arteries from ethnic populations
- 39.Choi SY, et al. , Tonicity-Responsive Enhancer-Binding Protein Mediates Hyperglycemia-Induced Inflammation and Vascular and Renal Injury. J Am Soc Nephrol, 2018. 29(2): p. 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Q, et al. , Alleviation of hyperglycemia induced vascular endothelial injury by exenatide might be related to the reduction of nitrooxidative stress. Biomed Res Int, 2013. 2013: p. 843657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quincozes-Santos A, et al. , Fluctuations in glucose levels induce glial toxicity with glutamatergic, oxidative and inflammatory implications. Biochim Biophys Acta Mol Basis Dis, 2017. 1863(1): p. 1–14. [DOI] [PubMed] [Google Scholar]
- 42.DeRubertis BG, et al. , Reduced primary patency rate in diabetic patients after percutaneous intervention results from more frequent presentation with limb-threatening ischemia. J Vasc Surg, 200847(1): p. 101–8. [DOI] [PubMed] [Google Scholar]
- 43.Jiang M, et al. , ALA/LA ameliorates glucose toxicity on HK-2 cells by attenuating oxidative stress and apoptosis through the ROS/p38/TGF-betal pathway. Lipids Health Dis, 2017. 16(1): p. 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bajaj HS, et al. , Biomarkers of vascular injury and endothelial dysfunction after recent glucose intolerance in pregnancy. Diab Vasc Dis Res, 2018. 15(5): p. 449–457. [DOI] [PubMed] [Google Scholar]
- 45.Jia M, et al. , Beneficial effects of apple peel polyphenols on vascular endothelial dysfunction and liver injury in high choline-fed mice. Food Funct, 2017. 8(3): p. 1282–1292. [DOI] [PubMed] [Google Scholar]
- 46.Hansson GK, Robertson AK, and Soderberg-Naucler C, Inflammation and atherosclerosis. Annu Rev Pathol, 2006. 1: p. 297–329. [DOI] [PubMed] [Google Scholar]
- 47.Yang B, et al. , High-Resolution Magnetic Resonance Imaging Confirmed Atherosclerosis of an Intracranial Penetrating Artery: A Case Report. J Stroke Cerebrovasc Dis, 2018. 27(7): p. e121–e124. [DOI] [PubMed] [Google Scholar]
- 48.Loso J, et al. , Serum Biomarkers of Endothelial Dysfunction in Fabry Associated Cardiomyopathy. Front Cardiovasc Med, 2018. 5: p. 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei L, et al. , Development of an inflammation imaging tracer, (111)In-DOTA-DAPTA, targeting chemokine receptor CCR5 and preliminary evaluation in an ApoE(−/−) atherosclerosis mouse model. J Nucl Cardiol, 2018. [DOI] [PubMed] [Google Scholar]
- 50.Chistiakov DA, Orekhov AN, and Bobryshev YV, Endothelial Barrier and Its Abnormalities in Cardiovascular Disease. Front Physiol, 2015. 6: p. 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bykov AT, et al. , [Early diagnostics, prophylaxis, and non-pharmacological treatment of the preclinical stages of atherosclerosis and arterial hypertension]. Vopr Kurortol Fizioter Lech Fiz Kult, 2015. 92(5): p. 18–21. [DOI] [PubMed] [Google Scholar]
- 52.Paeschke S, et al. , The Role of Iron and Nerve Inflammation in Diabetes Mellitus Type 2-Induced Peripheral Neuropathy. Neuroscience, 2019. [DOI] [PubMed] [Google Scholar]
- 53.Collins RG, et al. , P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med, 2000. 191(1): p. 189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.● Meester EJ, et al. , Imaging of atherosclerosis, targeting LFA-1 on inflammatory cells with (111)In-DANBIRT. J Nucl Cardiol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van den Oever IA, et al. , Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm, 2010. 2010: p. 792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paffett ML, et al. , Ozone Inhalation Impairs Coronary Artery Dilation via Intracellular Oxidative Stress: Evidence for Serum-Borne Factors as Drivers of Systemic Toxicity. Toxicol Sci, 2015. 146(2): p. 244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clerkin KJ, Ali ZA, and Mancini DM, New developments for the detection and treatment of cardiac vasculopathy. Curr Opin Cardiol, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menezes LJ, et al. , Investigating vulnerable atheroma using combined (18)F-FDG PET/ET angiography of carotid plaque with immunohistochemical validation. J Nucl Med, 2011. 52(11): p. 1698–703. [DOI] [PubMed] [Google Scholar]
- 59.Pechlivani N and Ajjan RA, Thrombosis and Vascular Inflammation in Diabetes: Mechanisms and Potential Therapeutic Targets. Front Cardiovasc Med, 2018. 5: p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Low Wang CC, et al. , Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation, 2016. 133(24): p. 2459–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.●● Pletsch-Borba L, et al. , Biomarkers of vascular injury in relation to myocardial infarction risk: A population-based study. Sci Rep, 2019. 9(1): p. 3004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Highlights the use of biomarkers assesment for risk between vascular injury and importantly CVD
- 62.Krysiak R, Szkrobka W, and Okopien B, Effect of Metformin on Hypothalamic-Pituitary-Thyroid Axis Activity in Elderly Antipsychotic-Treated Women With Type 2 Diabetes and Subclinical Hypothyroidism: A Preliminary Study. J Clin Pharmacol, 2018. 58(5): p. 586–592. [DOI] [PubMed] [Google Scholar]
- 63.Tirabassi G, et al. , Influence of the hypothalamic-pituitary-adrenal axis dysregulation on the metabolic profile of patients affected by diabetes mellitus-associated late onset hypogonadism. Nutr Metab Cardiovasc Dis, 2016. 26(1): p. 53–9. [DOI] [PubMed] [Google Scholar]
- 64.Bayramci NS, et al. , Investigation of glucocorticoid receptor and calpain-10 gene polymorphisms in Turkish patients with type 2 diabetes mellitus. Turk J Med Sci, 2017. 47(5): p. 1568–1575. [DOI] [PubMed] [Google Scholar]
- 65.Khalil RA, Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem Pharmacol, 2013. 86(12): p. 1627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmed MA and Hassanein KM, Effects of estrogen on hyperglycemia and liver dysfunction in diabetic male rats. Int J Physiol Pathophysiol Pharmacol, 2012. 4(3): p. 156–66. [PMC free article] [PubMed] [Google Scholar]
- 67.Prospective Studies, C. and C. Asia Pacific Cohort Studies, Sex-specific relevance of diabetes to occlusive vascular and other mortality: a collaborative meta-analysis of individual data from 980 793 adults from 68 prospective studies. Lancet Diabetes Endocrinol, 2018. 6(7): p. 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Z, et al. , EPHA4 regulates vascular smooth muscle cell contractility and is a sex-specific hypertension risk gene in individuals with type 2 diabetes. J Hypertens, 2019. 37(4): p. 775–789. [DOI] [PubMed] [Google Scholar]
- 69.Pimenta DC, et al. , Mass spectrometric analysis of the individual variability of Bothrops jararaca venom peptide fraction. Evidence for sex-based variation among the bradykinin-potentiating peptides. Rapid Commun Mass Spectrom, 2007. 21(6): p. 1034–42. [DOI] [PubMed] [Google Scholar]
- 70.Weinberg J, Diniz CR, and Mares-Guia M, Influence of sex and sexual hormones in the bradykinin-receptor interaction in the guinea pig ileum. Biochem Pharmacol, 1976. 25(4): p. 433–7. [DOI] [PubMed] [Google Scholar]
- 71.Dewitte A, et al. , Doppler resistive index to reflect regulation of renal vascular tone during sepsis and acute kidney injury. Crit Care, 2012. 16(5): p. R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klychnikova EV, et al. , [Correlation between biochemical parameters of oxidative stress, endogenous intoxication and regulation of vascular tone in patients with burn injury]. Anesteziol Reanimatol, 2015. 60(1): p. 45–9. [PubMed] [Google Scholar]
- 73.Montezano AC, et al. , Angiotensin II and vascular injury. Curr Hypertens Rep, 2014. 16(6): p. 431. [DOI] [PubMed] [Google Scholar]
- 74.Shen J, et al. , Protection against death and renal failure by renin-angiotensin system blockers in patients with diabetes and kidney disease. J Renin Angiotensin Aldosterone Syst, 2016. 17(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang D, et al. , Glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a cAMP/PKA/Rho-dependent mechanism. Diabetes, 2013. 62(5): p. 1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hetterich H, et al. , AHA classification of coronary and carotid atherosclerotic plaques by grating-based phase-contrast computed tomography. Eur Radiol, 2016. 26(9): p. 3223–33. [DOI] [PubMed] [Google Scholar]
- 77.Zoungas S, et al. , Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia, 2014. 57(12): p. 2465–74. [DOI] [PubMed] [Google Scholar]
- 78.Gedebjerg A, et al. , Prevalence of micro- and macrovascular diabetes complications at time of type 2 diabetes diagnosis and associated clinical characteristics: A cross-sectional baseline study of 6958 patients in the Danish DD2 cohort. J Diabetes Complications, 2018. 32(1): p. 34–40. [DOI] [PubMed] [Google Scholar]
- 79.Triches C, et al. , [Macrovascular diabetic complications: clinical characteristics, diagnosis and management]. Arq Bras Endocrinol Metabol, 2009. 53(6): p. 698–708. [DOI] [PubMed] [Google Scholar]
- 80.Bosevski M, Carotid artery disease in diabetic patients. Pril (Makedon Akad Nauk Umet Odd Med Nauki), 2014. 35(3): p. 149–61. [PubMed] [Google Scholar]
- 81.Benjamin EJ, et al. , Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation, 2017. 135(10): p. e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bashore TM, et al. , 2012 American College of Cardiology Foundation/Society for Cardiovascular Angiography and Interventions expert consensus document on cardiac catheterization laboratory standards update: A report of the American College of Cardiology Foundation Task Force on Expert Consensus documents developed in collaboration with the Society of Thoracic Surgeons and Society for Vascular Medicine. J Am Coll Cardiol, 2012. 59(24): p. 2221–305. [DOI] [PubMed] [Google Scholar]
- 83.Jiang Q, et al. , Reduced facial swelling and incision numbness after Q-modified eversion carotid endarterectomy in patients with severe carotid stenosis. World Neurosurg, 2019. [DOI] [PubMed] [Google Scholar]
- 84.● Firnhaber JM and Powell CS, Lower Extremity Peripheral Artery Disease: Diagnosis and Treatment. Am Fam Physician, 2019. 99(6): p. 362–369. [PubMed] [Google Scholar]
- 85.Paraskevas KF, et al. , Screening for peripheral artery disease in dialysis patients: an opportunity for early disease detection and timely initiation of appropriate therapeutic measures. Int Urol Nephrol, 2011. 43(1): p. 143–5. [DOI] [PubMed] [Google Scholar]
- 86.Tagawa S, et al. , Determination of Early and Late Endothelial Progenitor Cells in Peripheral Circulation and Their Clinical Association with Coronary Artery Disease. Int J Vasc Med, 2015. 2015: p. 674213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim BG, et al. , Impact of peripheral artery disease on early and late outcomes of transcatheter aortic valve implantation in patients with severe aortic valve stenosis. Int J Cardiol, 2018. 255: p. 206–211. [DOI] [PubMed] [Google Scholar]
- 88.Skorkowska-Telichowska K, et al. , Insufficient modification of atherosclerosis risk factors in PAD patients. Adv Clin Exp Med, 2018. 27(6): p. 819–826. [DOI] [PubMed] [Google Scholar]
- 89.Parker CN, et al. , Differences between national and international guidelines for the management of diabetic foot disease. Diabetes Metab Res Rev, 2019. 35(2): p. e3101. [DOI] [PubMed] [Google Scholar]
- 90.Cherviakov IV, et al. , [Differentiated approach to treatment of decompensated lower limb ischaemia with the use of the WIFI classification system]. Angiol Sosud Khir, 2019. 25(1): p. 9–16. [DOI] [PubMed] [Google Scholar]
- 91.Vaquero Morillo F, The impact of peripheral arterial disease: A proposal for a new classification. Cir Esp, 2016. 94(5): p. 266–73. [DOI] [PubMed] [Google Scholar]
- 92.Hanrahan CJ, et al. , Diagnostic Accuracy of Noncontrast MR Angiography Protocols at 3T for the Detection and Characterization of Lower Extremity Peripheral Arterial Disease. J Vasc Interv Radiol, 2018. 29(11): p. 1585–1594 e2. [DOI] [PubMed] [Google Scholar]
- 93.Behrendt CA, et al. , Radiation Dosage for Percutaneous PAD Treatment is Different in Cardiovascular Disciplines: Results From an Eleven Year Population Based Registry in the Metropolitan Area of Hamburg. Eur J Vasc Endovasc Surg, 2017. 53(2): p. 215–222. [DOI] [PubMed] [Google Scholar]
- 94.Sevestre MA, et al. , Pilot safety study of perivascular injection of tissue-engineered allogeneic aortic endothelial cells in patients undergoing minimally invasive peripheral revascularization. J Vasc Surg, 2014. 59(6): p. 1597–606. [DOI] [PubMed] [Google Scholar]
- 95.Flugelman MY, et al. , Phase Ib Safety, Two-Dose Study of MultiGeneAngio in Patients with Chronic Critical Limb Ischemia. Mol Ther, 2017. 25(3): p. 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hanhart J, Comaneshter DS, and Vinker S, Mortality after a cerebrovascular event in age-related macular degeneration patients treated with bevacizumab ocular injections. Acta Ophthalmol, 2018. 96(6): p. e732–e739. [DOI] [PubMed] [Google Scholar]
- 97.Barra S, et al. , [Prediction of cerebrovascular event risk following myocardial infarction]. Rev Port Cardiol, 2011. 30(7–8): p. 655–63. [DOI] [PubMed] [Google Scholar]
- 98.Di Minno MND, et al. , Impact of cardiovascular and immunologic variables on subclinical carotid atherosclerosis in subjects with anti-phospholipid antibodies. Data Brief, 2018. 19: p. 1799–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao FF, et al. , A Correlational Study on Cerebral Microbleeds and Carotid Atherosclerosis in Patients with Ischemic Stroke. J Stroke Cerebrovasc Dis, 2018. 27(8): p. 2228–2234. [DOI] [PubMed] [Google Scholar]
- 100.Tanaka A, et al. , Differential effect of concomitant antidiabetic agents on carotid atherosclerosis: a subgroup analysis of the PROLOGUE study. Heart Vessels, 2019. 34(2): p. 375–384. [DOI] [PubMed] [Google Scholar]
- 101.Yao L, et al. , Association of carotid atherosclerosis and stiffness with abdominal aortic aneurysm: The atherosclerosis risk in communities (ARIC) study. Atherosclerosis, 2018. 270: p. 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen R, Ovbiagele B, and Feng W, Diabetes and Stroke: Epidemiology, Pathophysiology, Pharmaceuticals and Outcomes. Am J Med Sci, 2016. 351(4): p. 380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Venketasubramanian N, et al. , Two-year vascular event rates in patients with symptomatic cerebrovascular disease: the REACH registry. Cerebrovasc Dis, 2011. 32(3): p. 254–60. [DOI] [PubMed] [Google Scholar]
- 104.Saadatnia M, et al. , Prevalence and Prognosis of Cerebrovascular Accidents and its Subtypes Among Patients with Systemic Lupus Erythematosus in Isfahan, Iran: A Hospital Clinic-based Study. Int J Prev Med, 2014. 5(1): p. 123–6. [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar A, et al. , Multicenter cross-sectional study of asymptomatic peripheral arterial disease among patients with a single previous coronary or cerebrovascular event in the Arabian Gulf. Curr Med Res Opin, 2014. 30(9): p. 1725–32. [DOI] [PubMed] [Google Scholar]
- 106.Kurosaki Y, et al. , Asymptomatic Carotid T1-High-Intense Plaque as a Risk Factor for a Subsequent Cerebrovascular Ischemic Event. Cerebrovasc Dis, 2017. 43(5–6): p. 250–256. [DOI] [PubMed] [Google Scholar]
- 107.Ly H, et al. , Brain microvascular injury and white matter disease provoked by diabetes-associated hyperamylinemia. Ann Neurol, 2017. 82(2): p. 208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsukamoto Y, et al. , Cilostazol protects against microvascular brain injury in a rat model of type 2 diabetes. Neurosci Res, 2017. 117: p. 48–53. [DOI] [PubMed] [Google Scholar]
- 109.Sorrentino FS, et al. , Diabetic retinopathy and endothelin system: microangiopathy versus endothelial dysfunction. Eye (Lond), 2018. 32(7): p. 1157–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Borroni RG, et al. , Involvement of dermal microvascular basement membrane in senile purpura: quantitative immunohistochemical study. J Eur Acad Dermatol Venereol, 2016. 30(10): p. e63–e65. [DOI] [PubMed] [Google Scholar]
- 111.Sava P, et al. , Human microvascular pericyte basement membrane remodeling regulates neutrophil recruitment. Microcirculation, 2015. 22(1): p. 54–67. [DOI] [PubMed] [Google Scholar]
- 112.Coughlan MT, Cooper ME, and Forbes JM, Renal microvascular injury in diabetes: RAGE and redox signaling. Antioxid Redox Signal, 2007. 9(3): p. 331–42. [DOI] [PubMed] [Google Scholar]
- 113.Mohamed J, et al. , Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ Med J, 2016. 16(2): p. e132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masarone M, et al. , Liver biopsy in type 2 diabetes mellitus: Steatohepatitis represents the sole feature of liver damage. PLoS One, 2017. 12(6): p. e0178473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saxena A, Sachin K, and Bhatia AK, System Level Meta-analysis of Microarray Datasets for Elucidation of Diabetes Mellitus Pathobiology. Curr Genomics, 2017. 18(3): p. 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adeyemi DO, et al. , Anti-hepatotoxic activities of Hibiscus sabdariffa L. in animal model of streptozotocin diabetes-induced liver damage. BMC Complement Altern Med, 2014. 14: p. 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moon JS, Role of Bilirubin in Diabetic Vascular Complications: Can Bilirubin Predict More than Just Liver Disease? Diabetes Metab J, 2015. 39(5): p. 384–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khimmaktong W, et al. , Study of curcumin on microvasculature characteristic in diabetic rat’s liver as revealed by vascular corrosion cast/scanning electron microscope (SEM) technique. J Med Assoc Thai, 2012. 95 Suppl 5: p. S133–41. [PubMed] [Google Scholar]
- 119.Zhang J, et al. , HDAC3 inhibition in diabetic mice may activate Nrf2 preventing diabetes-induced liver damage and FGF21 synthesis and secretion leading to aortic protection. Am J Physiol Endocrinol Metab, 2018. 315(2): p. E150–E162. [DOI] [PubMed] [Google Scholar]
- 120.Dobias L, et al. , Effect of sulodexide on vascular responses and liver mitochondrial function in diabetic rats. Physiol Res, 2015. 64 Suppl 4: p. S497–505. [DOI] [PubMed] [Google Scholar]
- 121.Balakrishnan BB, et al. , Moringa concanensis Nimmo extracts ameliorates hyperglycemia-mediated oxidative stress and upregulates PPARgamma and GLUT4 gene expression in liver and pancreas of streptozotocin-nicotinamide induced diabetic rats. Biomed Pharmacother, 2019. 112: p. 108688. [DOI] [PubMed] [Google Scholar]
- 122.Acikgoz E, et al. , Repression of the Notch pathway prevents liver damage in streptozotocin-induced diabetic mice. Folia Histochem Cytobiol, 2017. 55(3): p. 140–148. [DOI] [PubMed] [Google Scholar]
- 123.Lim A, Diabetic nephropathy - complications and treatment. Int J Nephrol Renovasc Dis, 2014. 7: p. 361–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tan WS, et al. , Modeling heart failure risk in diabetes and kidney disease: limitations and potential applications of transverse aortic constriction in high-fat-fed mice. Am J Physiol Regul Integr Comp Physiol, 2018. 314(6): p. R858–R869. [DOI] [PubMed] [Google Scholar]
- 125.Woo V, et al. , The role of sodium glucose cotransporter-2 (SGLT-2) inhibitors in heart failure and chronic kidney disease in type 2 diabetes. Curr Med Res Opin, 2019: p. 1–13. [DOI] [PubMed] [Google Scholar]
- 126.Wang Q, et al. , Association of microalbuminuria with diabetes is stronger in people with prehypertension compared to those with ideal blood pressure. Nephrology (Carlton), 2018. 23(7): p. 690–696. [DOI] [PubMed] [Google Scholar]
- 127.Ma T, et al. , 4-O-methylhonokiol ameliorates type 2 diabetes-induced nephropathy in mice likely by activation of AMPK-mediated fatty acid oxidation and Nrf2-mediated anti-oxidative stress. Toxicol Appl Pharmacol, 2019. [DOI] [PubMed] [Google Scholar]
- 128.Aguilar D, Heart Failure, Diabetes Mellitus, and Chronic Kidney Disease: A Clinical Conundrum. Circ Heart Fail, 2016. 9(7). [DOI] [PubMed] [Google Scholar]
- 129.Bello NA, et al. , Increased risk of stroke with darbepoetin alfa in anaemic heart failure patients with diabetes and chronic kidney disease. Eur J Heart Fail, 2015. 17(11): p. 1201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abbas S, et al. , Association of GSTP1 gene polymorphism with diabetic nephropathy and comorbidities (hypertension and dyslipidemia) in Type 2 diabetes mellitus. Br J Biomed Sci, 2019. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y, et al. , Role of Protease-Activated Receptor 2 in Regulating Focal Segmental Glomerulosclerosis. Cell Physiol Biochem, 2017. 41(3): p. 1147–1155. [DOI] [PubMed] [Google Scholar]
- 132.Muller-Hocker J, et al. , A case of idiopathic nodular glomerulosclerosis mimicking diabetic glomerulosclerosis (Kimmelstiel-Wilson type). Pathol Res Pract, 2002. 198(5): p. 375–9. [DOI] [PubMed] [Google Scholar]
- 133.Ucgul Atilgan C, et al. , Effect of microalbuminuria on macular thickness in patients with type-2 diabetes mellitus. Eur J Ophthalmol, 2018: p. 1120672118811256. [DOI] [PubMed] [Google Scholar]
- 134.Hippisley-Cox J and Coupland C, Diabetes treatments and risk of amputation, blindness, severe kidney failure, hyperglycaemia, and hypoglycaemia: open cohort study in primary care. BMJ, 2016. 352: p. i1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ito T, et al. , Effectiveness of Preceding Solo Kidney Transplantation for Type 1 Diabetes With End-Stage Renal Failure. Transplant Proc, 2018. 50(10): p. 3249–3254. [DOI] [PubMed] [Google Scholar]
- 136.Corcostegui B, et al. , Update on Diagnosis and Treatment of Diabetic Retinopathy: A Consensus Guideline of the Working Group of Ocular Health (Spanish Society of Diabetes and Spanish Vitreous and Retina Society). J Ophthalmol, 2017. 2017: p. 8234186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rasta SH, Nikfarjam S, and Javadzadeh A, Detection of retinal capillary nonperfusion in fundus fluorescein angiogram of diabetic retinopathy. Bioimpacts, 2015. 5(4): p. 183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Juster-Switlyk K and Smith AG, Updates in diabetic peripheral neuropathy. F1000Res, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kirschke J, et al. , Glove-and-stocking-like keratoderma with hyperhidrosis and perioral erythema. Clin Exp Dermatol, 2007. 32(4): p. 477–8. [DOI] [PubMed] [Google Scholar]
- 140.Rivera LR, et al. , Damage to enteric neurons occurs in mice that develop fatty liver disease but not diabetes in response to a high-fat diet. Neurogastroenterol Motil, 2014. 26(8): p. 1188–99. [DOI] [PubMed] [Google Scholar]
- 141.Ward R, et al. , Poststroke cognitive impairment and hippocampal neurovascular remodeling: the impact of diabetes and sex. Am J Physiol Heart Circ Physiol, 2018. 315(5): p. H1402–H1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mieritz MG, et al. , A Longitudinal Study of Growth, Sex Steroids, and IGF-1 in Boys With Physiological Gynecomastia. J Clin Endocrinol Metab, 2015. 100(10): p. 3752–9. [DOI] [PubMed] [Google Scholar]
- 143.Earle KA, et al. , Sex differences in vascular stiffness and relationship to the risk of renal functional decline in patients with type 2 diabetes. Diab Vasc Dis Res, 2017. 14(4): p. 304–309. [DOI] [PubMed] [Google Scholar]
- 144.Werner KB, et al. , Male sex and vascular risk factors affect cystatin C-derived renal function in older people without diabetes or overt vascular disease. Age Ageing, 2014. 43(3): p. 411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maric-Bilkan C, Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin Sci (Lond), 2017. 131(9): p. 833–846. [DOI] [PubMed] [Google Scholar]
- 146.Herrera-Lopez EE, et al. , The rs1256031 of estrogen receptor beta gene is associated with type 2 diabetes. Diabetes Metab Syndr, 2018. 12(5): p. 631–633. [DOI] [PubMed] [Google Scholar]
- 147.McGinley AL, et al. , Additional sex combs-like family genes are required for normal cardiovascular development. Genesis, 2014. 52(7): p. 671–86. [DOI] [PubMed] [Google Scholar]
- 148.Eriksson AL, et al. , Genetic variations in sex steroid-related genes as predictors of serum estrogen levels in men. J Clin Endocrinol Metab, 2009. 94(3): p. 1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kojima S, Catavero C, and Rinaman L, Maternal high-fat diet increases independent feeding in pre-weanling rat pups. Physiol Behav, 2016. 157: p. 237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ullah R, et al. , Postnatal feeding with high-fat diet induces obesity and precocious puberty in C57BL/6J mouse pups: a novel model of obesity and puberty. Front Med, 2017. 11(2): p. 266–276. [DOI] [PubMed] [Google Scholar]
- 151.Dehmel S, et al. , Intrauterine smoke exposure deregulates lung function, pulmonary transcriptomes, and in particular insulin-like growth factor (IGF)-1 in a sex-specific manner. Sci Rep, 2018. 8(1): p. 7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Toriola AT, et al. , Association of serum 25-hydroxyvitamin D (25-OHD) concentrations with maternal sex steroids and IGF-1 hormones during pregnancy. Cancer Causes Control, 2011. 22(6): p. 925–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sukhanov S, et al. , IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol, 2007. 27(12): p. 2684–90. [DOI] [PubMed] [Google Scholar]
- 154.Ceylan-Isik AF, Li Q, and Ren J, Insulin-like growth factor I (IGF-1) deficiency ameliorates sex difference in cardiac contractile function and intracellular Ca(2+) homeostasis. Toxicol Lett, 2011. 206(2): p. 130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Higashi Y, et al. , Insulin-Like Growth Factor-1 Receptor Deficiency in Macrophages Accelerates Atherosclerosis and Induces an Unstable Plaque Phenotype in Apolipoprotein E-Deficient Mice. Circulation, 2016. 133(23): p. 2263–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang J, et al. , The expression of IGFs and IGF binding proteins in human carotid atherosclerosis, and the possible role of IGF binding protein-1 in the regulation of smooth muscle cell proliferation. Atherosclerosis, 2012. 220(1): p. 102–9. [DOI] [PubMed] [Google Scholar]
- 157.Pena JM and Min JK, Coronary artery disease: Sex-related differences in CAD and plaque characteristics. Nat Rev Cardiol, 2016. 13(6): p. 318–9. [DOI] [PubMed] [Google Scholar]
- 158.Desai MY and Schoenhagen P, Noninvasive testing strategies in symptomatic, intermediate-risk CAD patients: a perspective on the “PROMISE” trial and its potential implementation in clinical practice. Cardiovasc Diagn Ther, 2015. 5(2): p. 166–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Klein BE, Klein R, and Moss SE, Exogenous estrogen exposures and changes in diabetic retinopathy. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care, 1999. 22(12): p. 1984–7. [DOI] [PubMed] [Google Scholar]
- 160.Slominski B, et al. , Estrogen receptor alpha gene polymorphism and vascular complications in girls with type 1 diabetes mellitus. Mol Cell Biochem, 2018. 437(1–2): p. 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tiano JP, et al. , Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. J Clin Invest, 2011. 121(8): p. 3331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miller VM and Duckles SP, Vascular actions of estrogens: functional implications. Pharmacol Rev, 2008. 60(2): p. 210–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Binay C, et al. , Growth hormone and the risk of atherosclerosis in growth hormone-deficient children. Growth Horm IGF Res, 2015. 25(6): p. 294–7. [DOI] [PubMed] [Google Scholar]
- 164.Pande RL, et al. , A pooled analysis of the durability and predictors of treatment response of cilostazol in patients with intermittent claudication. Vasc Med, 2010. 15(3): p. 181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hiatt WR, Money SR, and Brass EP, Long-term safety of cilostazol in patients with peripheral artery disease: the CASTLE study (Cilostazol: A Study in Long-term Effects). J Vasc Surg, 2008. 47(2): p. 330–336. [DOI] [PubMed] [Google Scholar]
- 166.Afarideh M, et al. , Associations of Serum S100B and S100P with the Presence and Classification of Diabetic Peripheral Neuropathy in Adults With Type 2 Diabetes: A Case-Cohort Study. Can J Diabetes, 2019. [DOI] [PubMed] [Google Scholar]
- 167.Jin J, et al. , Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther, 2019. 10(1): p. 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Peng BY, et al. , Addressing Stem Cell Therapeutic Approaches in Pathobiology of Diabetes and Its Complications . J Diabetes Res, 2018. 2018: p. 7806435. [DOI] [PMC free article] [PubMed] [Google Scholar]


