Abstract
To be highly reliable, synaptic transmission needs postsynaptic receptors (Rs) in precise apposition to the presynaptic release sites. At inhibitory synapses, the postsynaptic protein gephyrin self-assembles to form a scaffold that anchors glycine and GABAARs to the cytoskeleton, thus ensuring the accurate accumulation of postsynaptic receptors at the right place. This protein undergoes several post-translational modifications which control protein–protein interaction and downstream signaling pathways. In addition, through the constant exchange of scaffolding elements and receptors in and out of synapses, gephyrin dynamically regulates synaptic strength and plasticity.
The aim of the present review is to highlight recent findings on the functional role of gephyrin at GABAergic inhibitory synapses. We will discuss different approaches used to interfere with gephyrin in order to unveil its function. In addition, we will focus on the impact of gephyrin structure and distribution at the nanoscale level on the functional properties of inhibitory synapses as well as the implications of this scaffold protein in synaptic plasticity processes. Finally, we will emphasize how gephyrin genetic mutations or alterations in protein expression levels are implicated in several neuropathological disorders, including autism spectrum disorders, schizophrenia, temporal lobe epilepsy and Alzheimer's disease, all associated with severe deficits of GABAergic signaling.
This article is part of a Special Issue entitled: Honoring Ricardo Miledi - outstanding neuroscientist of XX-XXI centuries.
Abbreviations: AD, Alzheimer Disease; ASDs, Autism Spectrum Disorders; Cys, cysteine; GABA, γ-aminobutyric acid; GBD, gephyrin-binding domain; GlyR, glycine receptor; GPHN, gene encoding for gephyrin; GRAIL, Gene Relationships Across Implicated Loci; iLTP, inhibitory long-term synaptic potentiation; IATC, Intracellular Antibodies Capture Technology; IPSCs, inhibitory postsynaptic currents; m, miniature; Moco, Molybdenum cofactor; NMDA, N-methyl-D- aspartate; PIN1, Peptidyl-prolyl Isomerase 1; PSD, postsynaptic density; R, receptor; scFv, single-chain antibody fragment; shRNAs, small hairpin RNAs; TM, transmembrane domain; Tyr, tyrosine; vGAT, vesicular GABA transporter
Key words: gephyrin, glycine and GABAARs, structural organization, synaptic plasticity, neuropsychiatric disorders
Graphical abstract
Highlights
-
•
Post-translational modifications of gephyrin clusters shape GABAergic transmission.
-
•
The subsynaptic nanoscale distribution of gephyrin affects GABAergic inhibitory synaptic plasticity.
-
•
Hampering gephyrin function with selective intrabodies reduces the probability of GABA release.
-
•
Dynamic changes of gephyrin govern lateral diffusion of GABAA receptors and synaptic plasticity processes.
-
•
Impaired gephyrin function is often associated with neurodevelopmental and neurodegenerative disorders.
Introduction
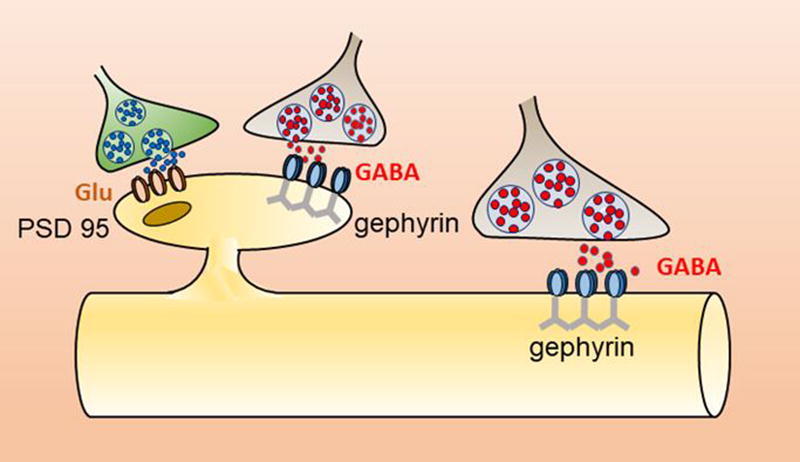
Synapses are highly specialized structures responsible for information's flow between neurons. Excitatory or inhibitory neurotransmitters, released from presynaptic nerve endings, bind to selective postsynaptic receptors and transduce biochemical signals into electrical ones. Speed and reliability of synaptic transmission are ensured by scaffold proteins, essential components of postsynaptic densities that, by interacting with the cytoskeleton, guarantee the accurate accumulation of postsynaptic receptors at the right place, anchoring them in precise apposition to the presynaptic release sites. This interaction does provide the physical constraints necessary not only for maintaining receptors at synapses but also for regulating the constant exchange of receptors and scaffolding elements in and out of post-synaptic sites (Hanus et al., 2006, Okabe, 2007, Sheng and Hoogenraad, 2007, Newpher and Ehlers, 2008, Choquet and Triller, 2013, Petrini and Barberis, 2014). Postsynaptic specializations exhibit substantial differences at excitatory and inhibitory synapses. While at excitatory synapses postsynaptic densities (PSDs) are localized on dendritic spines and comprise a dense and rich network of interacting proteins, at inhibitory synapses PSDs are localized mainly on dendritic shafts and on the cell body and are less elaborated (Sheng and Kim, 2011). However, inhibitory synapses directly impinging onto dendritic spines have been also described (Chen et al., 2012, van Versendaal et al., 2012, Chiu et al., 2013, Villa et al., 2016).
At inhibitory synapses, gephryin is the main scaffold molecule that anchors both glycine (Gly) and GABAA receptors to the subsynaptic cytoskeleton, in precise apposition to the presynaptic release sites. Originally purified in association with Gly receptors by Betz group (Pfeiffer et al., 1982), gephyrin has been extensively characterized from a structural, biochemical and functional point of view, thus leading to a deep, though still incomplete, understanding of its clustering properties and of its impact on inhibitory synaptic transmission and plasticity.
Here, we review how the regulation of gephyrin properties and clustering shapes GABAergic synaptic transmission and tonic GABAA-mediated inhibition. We summarize recent technological advancements (including intrabodies) used to silence this scaffold protein with an eye towards the physiological impact of such manipulations. We also overview super-resolution microscopy data concerning the nanoscale distribution of gephyrin and its role in synaptic transmission. We discuss gephyrin dynamics in the framework of inhibitory synaptic plasticity in vitro and in vivo. Finally, we examine gephyrin dysfunctions in neurodevelopmental and neurodegenerative disorders.
Gephyrin at inhibitory synapses: features, regulations and roles
Gephyrin is a 93-kDa protein initially identified as a tubulin-binding molecule that co-purifies with GlyRs (Pfeiffer et al., 1982, Prior et al., 1992, Kirsch et al., 1993). In vertebrates gephyrin is structurally organized into an N-terminal G-domain and a C-terminal E-domain connected by a central linker region or C-domain (Prior et al., 1992). The GPHN gene encoding for gephyrin is highly conserved and exhibits a complex intron-exon structure. The alternative splicing of GPHN leads to several gephyrin splice variants expressed both in neuronal and in non-neuronal tissues (Ramming et al., 2000). The structure of the N- and C-terminal domains of gephyrin is similar to the bacterial proteins MogA and MoeA, involved in the biosynthesis of the molybdenum cofactor (Moco), which is essential for the activity of various metabolically important enzymes such as aldehyde oxidase, xanthine oxidoreductase, and sulfite oxidase, crucial for survival (Nawrotzki et al., 2012). Patients suffering from hereditary Moco deficiency present severe neurological abnormalities, microcephaly, and mental retardation (Kirsch et al., 1993, Schwarz, 2016). Moreover, they display symptoms that resemble those seen upon loss of inhibitory neurotransmission, including myoclonus and epileptic seizures (Yu and Pearl, 2013). Crystal structure studies of gephyrin have revealed that while the G-domain has an intrinsic tendency to trimerize the E-domain to dimerize (Schwarz et al., 2001, Sola et al., 2001, Sola et al., 2004). Based on these observations it was initially proposed that the full-length protein, via the concomitant utilization of both oligomerization domains, forms a planar hexagonal lattice underneath the synaptic membrane (Sola et al., 2001, Sola et al., 2004), which exposes a high number of binding sites for the recruitment and clustering of GlyRs and a subset of GABAARs (Maric et al., 2011, Mukherjee et al., 2011, Tretter et al., 2012, Kowalczyk et al., 2013, Brady and Jacob, 2015). Deciphering the mechanism by which gephyrin can accommodate both types of inhibitory receptors has been quite challenging and it is far from being fully understood. These receptors belong to the Cys-loop pentameric ligand-gated ion channel superfamily, and therefore they share a similar structural organization. Each subunit of the receptor complex is composed by a large N-terminal ligand binding domain, followed by four transmembrane (TM) domains, a large intracellular loop connecting TM3 and TM4 and a small C-terminal region (Moss, 2001, Thompson et al., 2010). The highly unstructured intracellular loop of the GlyR β subunit and of the GABAARs α1, α2, α3, α5, β2 and β3 subunits (Maric et al., 2011, Mukherjee et al., 2011, Tretter et al., 2012, Kowalczyk et al., 2013, Brady and Jacob, 2015) allows inhibitory receptors binding to a hydrophobic pocket of the gephyrin E domain (Sola et al., 2004, Kim et al., 2006). Structural studies, validated by mutational and biophysical analyses, further identified specific signature sequences shared by the two types of receptors (Maric et al., 2011) as well as differences in their binding motifs that underline the diverse affinity and modality of interaction (Maric et al., 2014, Mukherjee et al., 2011, Tretter et al., 2012; Grünewald et al., 2018). Moreover it has been recently demonstrated that also specific sequences of the C domain contribute to the formation of the GlyR-gephyrin complex (Grünewald et al., 2018). Moreover, atomic force microscopy (AFM) and small-angle X-ray scattering (SAXS), have revealed differences in the folding of the intrinsically unstructured C-domain which may account for the observed conformational variability of gephyrin (Sander et al., 2013). This feature in turn can influence gephyrin folding plasticity and its ability to affect GABAA and Gly receptor dynamics and stabilization at inhibitory synapses.
Unstructured protein domains are privileged target sites for post-translational modifications such as phosphorylation and, indeed, all known gephyrin phosphorylation sites map to the linker region of gephyrin. Given the documented impact of phosphorylation events on the multimerization of gephyrin it is not surprising that they also affect GABAAR dynamics and synaptic stabilization. Extracellular signal-regulated kinase 1/2 (ERK1/2)-mediated phosphorylation of gephyrin at serine 268 (S268) was shown to reduce scaffold size and miniature inhibitory postsynaptic currents (mIPSC) amplitude (Tyagarajan et al., 2013) while blocking glycogen synthase kinase 3β (GSK3β)-dependent phosphorylation of gephyrin at serine 270 significantly increases mIPSC amplitude and frequency (Tyagarajan et al., 2011). Unexpectedly, both phosphorylation events, although promoting opposite effects on synaptic morphology, similarly altered the GABAAR diffusion properties (Battaglia et al., 2018), further emphasizing the complexity of gephyrin scaffolding regulation.
Gephyrin undergoes additional post-translational modifications including palmitoylation (Dejanovic et al., 2014a), S-nitrosylation (Dejanovic and Schwarz, 2014), acetylation (Herweg and Schwarz, 2012, Tyagarajan et al., 2013) and SUMOylation (Ghosh et al., 2016). Palmitoylation is a process that adds a small lipid tail to surface-exposed cysteine residues, allowing the protein to be anchored at the plasma membrane. Gephyrin palmitoylation is mediated by palmitoyl acyltransferase DHHC-12 and involves Cys212 and Cys284, both residues located in the C-domain (Dejanovic et al., 2014a). Over-expression of this enzyme increases the size of gephyrin clusters and consequently the strength of GABAergic transmission. In contrast, preventing gephyrin palmitoylation leads to non-synaptic mislocalized gephyrin clusters (Dejanovic et al., 2014a). Interestingly, the reduced GABAergic transmission due to the expression of a palmitoylation-deficient gephyrin mutant in the basolateral amygdala leads to severe anxiety disorders in rats (Shen et al., 2019).
Gephyrin directly interacts with neuronal nitric oxide synthase (nNOS) and it gets physiologically S-nitrosylated, a modification that negatively affects the cluster size and the overall surface expression of synaptic GABAARs (Dejanovic and Schwarz, 2014).
More recently, Ghosh et al. (2016) have discovered the existence of a phosphorylation-dependent SUMOylation of gephyrin that competes with its acetylation (Ghosh et al., 2016) for regulating its scaffolding properties. These observations place gephyrin at the convergence of several signaling pathways, which may operate with diverse kinetics in different subcellular localizations, neuronal microcircuits and/or brain regions.
By interacting with different proteins, gephyrin further contributes to regulate synapse formation and plasticity. Gephyrin interactors, including tubulin (Langosch et al., 1992), neuroligin2 (NLG2, Poulopoulos et al., 2009), collybistin (Papadopoulos et al., 2007), the peptidyl–prolyl cis–trans isomerase NIMA-interacting protein1 PIN1 (Antonelli et al., 2014), the translation regulator rapamycin and RAFT1 (Sabatini et al., 1999), the actin-associated proteins such as Profilin1 and 2, the mammalian enabled (Mena)/vasodilator stimulated phosphoprotein (VASP) (Giesemann et al., 2003) and IQSEC3 (Um et al., 2016) have been extensively reviewed (Tyagarajan and Fritschy, 2014, Zacchi et al., 2014, Choii and Ko, 2015, Chiu et al., 2019). Interestingly, for some of these interactors, phosphorylation controls their ability to be recruited by gephyrin. One example is given by neuroligin1 (NLG1), the cell adhesion molecule enriched at glutamatergic synapses (Varley et al., 2011, Giannone et al., 2013) which shares, with all NLG family members, the presence of a gephyrin-binding module (GBD) in its intracellular domain and therefore is potentially able to enroll gephyrin at excitatory synapses. Phosphorylation of Tyrosine (Tyr) 782 embedded in the GBD of NLG1, promoted by neurexin-adhesion signaling, was shown to preclude NLG1/gephyrin interaction while favoring PSD-95 recruitment. Such a ligand-induced phospho-tyrosine “switch” could represent a very sensitive mechanism in synaptogenesis, when early neuronal contacts, relying on neurexin/neuroligin adhesion, may be primed to assemble functional inhibitory vs excitatory synapses.
It is worth mentioning that in in vitro hippocampal neurons subjected to transient oxygen–glucose deprivation or ischemic damage, activation of a calpain-dependent gephyrin cleavage by oxytotoxic stimulation leads to the production of truncated forms of gephyrin with consequent reduction of GABAA receptors clustering and downregulation of GABAergic synaptic transmission (Costa et al., 2016).
Some synapse do not require gephyrin as an essential player for GABAARs accumulation, as suggested by GABAARs subunit specific clustering observed in the absence of gephyrin (gephyrin KO mice) (Fischer et al., 2000, Kneussel et al., 2001). Those pieces of evidence suggest the involvement of other GABAAR clustering proteins. For instance, the dystrophin–glycoprotein complex ensures α1-containing GABAA receptor clustering in a subset of GABAergic synapses in cortical neurons and cerebellar Purkinje cells (Panzanelli et al., 2011). In cortical and hippocampal neurons in culture, the scaffold protein GIT1 interacts with βPIX to anchor synaptic GABAA receptors and tunes synaptic strength in a gephyrin-independent manner (Smith et al., 2014). Recently, the tetraspanin LH4 protein, a member of the GARLHs auxiliary subunits, also known as LHFPL4, has been reported to mediate a cell-type specific anchoring of GABAA receptors by forming a stable complex with GABAAR γ2 subunit and NLG2 (Davenport et al., 2017, Martenson et al., 2017, Yamasaki et al., 2017).
Synaptic distribution of gephyrin at the nanoscale level
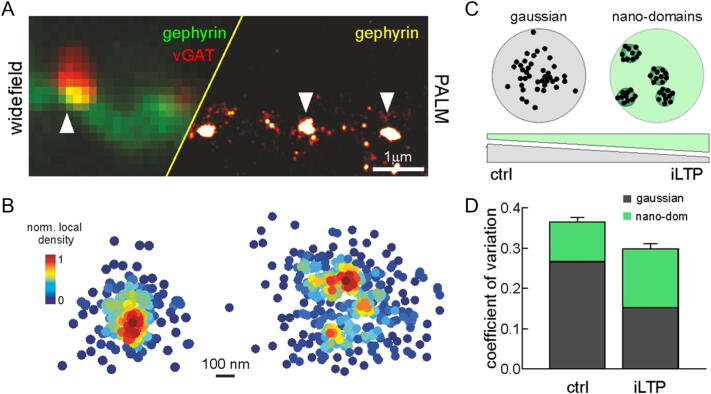
When visualized with epifluorescence microscopy, gephyrin postsynaptic clusters appear as round-shaped and rather uniform structures. However, the use of super-resolution microscopy allowing sub-diffraction limit spatial resolution (25–30 nm) revealed that the distribution of gephyrin in the postsynaptic area is considerably inhomogeneous, indicating that the gephyrin lattice is a dynamic puzzle of gephyrin nano-domains of different size and density (Specht et al., 2013, Pennacchietti et al., 2017, Specht, 2019). Indeed, whereas some synapses show continuous and uniformly distributed gephyrin puncta (single-spot synapses), other synapses exhibit unevenly distributed gephyrin localizations segmented into nano-domains (multi-spot synapses) (Specht et al., 2013, Pennacchietti et al., 2017; Fig. 1A, B).
Fig. 1.
Gephyrin at the nanoscale level. (A) Wide-field (left) and photoactivated localization microscopy (PALM, right) of the same dendritic portion of a hippocampal neuron expressing gephyrin-mEos3.2 and immunoprobed for vGAT to localize inhibitory synapse (arrowheads). vGAT immunoreactivity is not shown on the right. Scale bar 1 μm. (B) Nanoscale organization of synaptic gephyrin in single-spot (Gaussian, left) and multi-spot (nano-domains, right) clusters. Scale bar, 100 nm. (C) Top, Schematized examples of Gaussian and nano-domains organization of the inhibitory postsynaptic disk. Bottom, During iLTP, the fraction of synapses exhibiting gephyrin nano-domains is increased. (D) Impact of synapse nanofragmentation during iLTP on simulated IPSC variability. [Modified from Pennacchietti et al., 2017].
These findings are in line with electron microscopy studies demonstrating that, at symmetric inhibitory synapses, the postsynaptic density (PSD) can be fragmented (Lushnikova et al., 2011). Super resolution investigations have also allowed the quantitative characterization of gephyrin clusters at inhibitory synapses. It has been estimated that in cultured spinal cord and hippocampal neurons the size of synaptic gephyrin clusters is ~ 0.04–0.05 mm2 (Specht et al., 2013, Pennacchietti et al., 2017, Battaglia et al., 2017), containing ~ 200–300 gephyrin molecules (Specht et al., 2013, Patrizio et al., 2017, Battaglia et al., 2017). However, the number of gephyrin molecules quantified in vivo at spinal cord inhibitory synapses was ~ 600 (Specht et al., 2013). This discrepancy might be ascribed to a slower maturation of spinal cord neurons in culture as compared to in vivo conditions (Specht et al., 2013). In contrast, in vivo cortical inhibitory synapses displayed ~ 130 molecules of gephyrin, hence emphasizing a considerable variability in the abundance of gephyrin molecules among synapses in different neuronal subtypes. Interestingly, at the nanoscale level, clusters of GABAA receptor are spatially correlated with those of gephyrin (Specht et al., 2013, Crosby et al., 2019), thus confirming a close interaction and an architectural interdependence between these synaptic components. The number of GABAA receptors at inhibitory central synapses has been estimated in the range from tens to few hundreds (Nusser et al., 1997). Although it seems that GABAA receptors bind gephyrin trimeric complexes, the exact stoichiometry of GABAA receptors-gephyrin interactions is not fully clarified yet. This lack of knowledge precludes the estimation of the nominal occupancy of gephyrin binding sites by GABAA receptors, as it has been already done for GlyR (~ 50%) (Patrizio et al., 2017, Specht, 2019). Additionally, at spinal cord synapses, the scenario would be further complicated by the competition between GABAA receptors and Gly receptors for gephyrin binding sites (Specht et al., 2013).
By using both electron microscopy and super-resolution approaches, it has been shown that the expression of inhibitory long-term synaptic potentiation (iLTP), induced by either oxygen–glucose deprivation or brief NMDA applications, was accompanied by the relative increase of multi-spot synapses with respect to the single-spot ones, indicating that the increase of synaptic gephyrin during iLTP is associated with gephyrin fragmentation (Lushnikova et al., 2011, Pennacchietti et al., 2017; Fig. 1C). Recently, a three-color super-resolution approach allowed to resolve the nanoscale organization of the inhibitory synapse, whereby gephyrin, GABAARs and the active-zone protein RIM (Rab-3 interacting molecule) are arranged in nanodomains (Crosby et al., 2019). In line with the enhanced nano-fragmentation of gephyrin previously observed during inhibitory synaptic potentiation (Pennacchietti et al., 2017), Crosby et al. (2019) show an activity-dependent recruitment of additional gephyrin-GABAARs-RIM nanodomains at inhibitory synapses. However, the molecular mechanisms leading to gephyrin nanoscale rearrangements and the functional role of nano-domains remain elusive. A first attempt to address the latter point exploited a computer model simulation approach to study simulated inhibitory unitary synaptic currents in relation to the GABAARs compaction within the postsynaptic disk (Pennacchietti et al., 2017; Fig. 1D). In the case of GABAAR compartmentalized in nano-domains, simulated IPSCs showed a lower trial-to-trial variability with respect to a condition whereby synaptic GABAARs were uniformly diffused in the postsynaptic disk. In the light of the increased fragmentation of the inhibitory PSD during inhibitory synaptic potentiation, those data suggest that iLTP expression may involve a reduction of the inhibitory unitary current fluctuations besides the already known increase of synaptic current amplitude.
Recently, the impact of plasticity-related manipulations of gephyrin phosphorylation (known to modulate gephyrin clusters size) has been studied at the nanoscale level (Battaglia et al., 2018). Clusters of phosphomimetic gephyrin mutants were smaller and exhibited higher molecule density as compared to wild types, indicating that gephyrin compaction, controlled by gephyrin phosphorylation, may be an additional determinant for the inhibitory PSD architecture. Overall, at nanoscale level, the postsynaptic structure of GABAergic synapses appears more difficult to predict than that of glycinergic synapses (which shows high affinity gephyrin-GlyR interactions) (Maric et al., 2011; Grünewald et al., 2018) or that of glutamatergic synapses (which shows modular PDZ domain architecture) (MacGillavry et al., 2011). Nevertheless, GABAergic synapses share with glutamatergic synapses the nanoscale coordination between presynaptic and postsynaptic structural elements (MacGillavry et al., 2013, Crosby et al., 2019) and a nanoscale rearrangement during the expression of synaptic plasticity (Nair et al., 2013,; Haas et al., 2018, Hruska et al., 2018, Tang et al., 2016, Barberis, 2019). Although the functional role of synaptic proteins nano-compartmentalization is only beginning to be deciphered (Pennacchietti et al., 2017), current data suggest that the coordination of synaptic elements at the nanoscale level represents a fundamental determinant for synaptic plasticity. Future work aimed at analyzing the synaptic dynamics at single molecule level is expected to provide novel and more integrated insights of the principles that regulate the size, the organization and the function of synapses.
Functional role of gephyrin on GABAA-mediated postsynaptic currents
Assessing the functional role of gephyrin on synaptic transmission has been made difficult by the fact that gephyrin knock-out mice die at birth due to breathing failure (Feng et al., 1998). Therefore, a number of molecular tools have been used to hamper gephyrin function, thus allowing to investigate its functional role in inhibitory synaptic transmission. These include small hairpin RNAs or microRNAs targeting gephyrin mRNA, or intracellular antibody domains (intrabodies) targeting gephyrin at the protein level.
Small hairpin RNAs (shRNAs) have been used to knock-down gephyrin at both GABAergic and glycinergic synapses. Hippocampal neurons in culture, treated with shRNA against gephyrin exhibited a decreased number of synaptic GABAAR clusters while leaving their total number unchanged (Jacob et al., 2005). In line with these findings, a decrease in amplitude and frequency of spontaneous GABAergic synaptic currents was observed upon disruption of gephyrin clusters (Yu et al., 2007). However, no changes in amplitude of currents induced by direct application of GABA were detected, suggesting a selective effect on postsynaptic GABAA receptor clusters. In spinal cord neurons in culture, reducing gephyrin levels with a phosphorothioated gephyrin antisense oligonucleotide, caused a reduction in both amplitude and frequency of miniature glycinergic currents while in the same neurons, only the frequency but not the amplitude of miniature GABAergic currents was affected (van Zundert et al., 2005). Blockade of gephyrin expression resulted in synaptic GABAA receptors becoming highly sensitive to zinc and insensitive to benzodiazepines, suggesting that gephyrin is required for the insertion and stabilization of GABAAR clusters containing the γ2 subunit.
A strong reduction in gephyrin levels (more than 90%) was obtained also by expressing, in organotypic hippocampal slices, a micro-RNA directed against the mRNA coding for this protein. This produced a reduction in amplitude of inhibitory postsynaptic currents (IPSCs), evoked in CA1 principal cells by stimulation of parvalbumin- and somatostatin-positive GABAergic interneurons (Nguyen et al., 2016, Horn and Nicoll, 2018). It must be noted that microRNAs might have pleiotropic effects, because each microRNA intrinsically targets a large number of diverse mRNAs.
Hampering protein function at post-translational level may represent an attractive alternative for interfering with gephyrin function in a more spatially localized manner.
One strategy to achieve post-translational protein silencing is provided by the intracellular antibody (intrabody) approach (Biocca and Cattaneo, 1995), which is recently emerging as a unique strategy to tackle the complexities of protein interaction networks such as that involving gephyrin (Cattaneo and Chirichiella, 2019). In this approach, small recombinant antibody domains selected to bind the target protein can be equipped with various signals to achieve a protein retargeting, with fluorescent moieties for live imaging purposes or with effector functions to achieve the targeted degradation of the protein (Gross et al., 2016; Melchionna and Cattaneo, 2007).
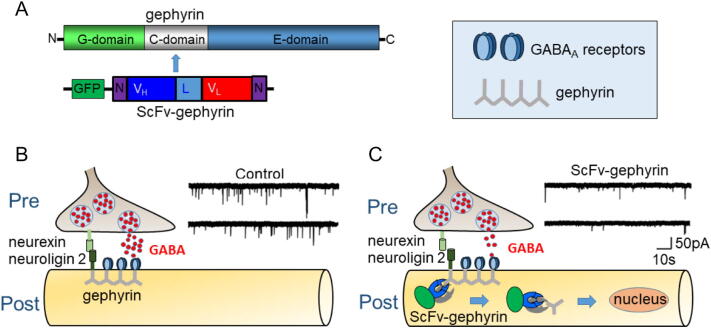
The Intracellular Antibodies Capture Technology (IATC, Visintin et al., 1999) has been used to efficiently and selectively remove gephyrin from Gly and GABAAR clusters by providing an anti-gephyrin single-chain antibody fragment (scFv) of a nuclear localization signal (Zacchi et al., 2008; Fig. 2).
Fig. 2.
Gephyrin declusterization impairs GABAergic synaptic transmission. (A) The intrabody against gephyrin (ScFv-gephyrin) binds the C-domain of gephyrin (aa 153–348). GFP: green fluorescent protein; N: nuclear localization signal; VH: intrabody heavy chain; VL: intrabody light chain: (B) At inhibitory synapses located mainly on dendritic shafts, the scaffold protein gephyrin is responsible for maintaining GABAARs in front of the presynaptic release sites; gephyrin interacts also with the postsynaptic adhesion molecule neuroligin 2 which binds to its presynaptic partner neurexin to ensure the cross talk between the post and presynaptic specializations. (C) ScFv-Gephyrin sequesters gephyrin and retargets it towards the nucleus. Note the reduction in frequency and amplitude of mIPSCs after scFv-gephyrin expression. This may be due to alterations in gephyrin-neuroligin 2 interaction [Modified from: Marchionni et al., 2009].
When transfected in cultured hippocampal neurons this construct induced a marked decrease in the number of gephyrin clusters associated with a significant reduction in the number of synaptic γ2-subunit containing GABAARs (Marchionni et al., 2009). These effects were associated with a slowdown of the onset kinetics, a reduction in amplitude and in frequency of miniature inhibitory postsynaptic currents.
Interestingly, changes in the onset kinetics, similar to those observed in mice lacking collybistin, a guanine nucleotide exchange factor required for gephyrin-dependent GABAA receptor clustering (Papadopoulos et al., 2007), may be due to modifications in the gating properties of GABAARs, particularly to a reduced entry into their desensitized state. However, this should be associated with an increase and not a decrease in current amplitude (Jones and Westbrook, 1995). The reduced current amplitude observed by Marchionni et al. (2009) may result from the concomitant reduction of GABA release from presynaptic GABAergic terminals, in line with the reduced immunoreactivity for vGAT, a vesicular GABA transporter. Moreover, paired recordings from interconnected GABAergic interneurons and principal cells have demonstrated a reduced probability of GABA release, possibly mediated by a trans-synaptic mechanism involving neuroligin 2 (Varley et al., 2011), an adhesion molecule belonging to the neuroligin family, known to trans-synaptically interact with presynaptic neurexins to ensure the crosstalk between the post- and presynaptic specializations (Südhof, 2008). Although the precise mechanism by which gephyrin regulates the gating properties of GABAARs is still unknown, the possibility that confining receptors into clusters may enhance their allosteric interactions with intracellular proteins (via specific domains in the intracellular loop between TM3 and TM4) as suggested for GlyRs (Legendre et al., 2002) cannot be excluded. This would lead to conformational changes and enhanced desensitization, particularly in case of high receptor density. Hampering gephyrin function with scFv induced also a significant reduction of a GABAA-mediated tonic conductance, suggesting the involvement not only of synaptic but also extrasynaptic gephyrin clusters (Marchionni et al., 2009)
More recently, another intrabody-based approach for the post-translational silencing of gephyrin (GFE3) has been developed by fusing an E3 ligase, which mediates the ubiquitination and rapid degradation of proteins, with a recombinant antibody-like protein (FingR) that binds to gephyrin (Gross et al., 2016). When expressed in dissociated hippocampal neurons GFE3 caused a reduction in frequency and amplitude of mIPSCs (Gross et al., 2016). Furthermore, in gigantocellular reticular neurons, a dose-dependent reduction in glycinergic synaptic strength was obtained by loading the patch pipette with increasing concentrations of a” super-binding” peptide targeting the intracellular receptor-gephyrin interaction, thus decreasing gephyrin-mediated recruitment of GlyRs at postsynaptic sites (Maric et al., 2017).
Gephyrin dynamics during synaptic plasticity
GABAergic synapses have been shown to undergo several types of synaptic plasticity expressed at both pre and postsynaptic sites (Chiu et al., 2019). At postsynaptic level, receptor lateral diffusion plays a key role in this process, allowing surface receptors to rapidly exchange between synaptic and extrasynaptic domains (Choquet and Triller, 2013). Since transient receptor-scaffold interactions govern the “diffusion trapping” of the receptors at postsynaptic sites, changes in the properties, distribution and dynamics of synaptic scaffold proteins are expected to impact into synaptic strength (Choquet and Triller, 2013, Petrini and Barberis, 2014). Indeed, at GABAergic synapses, several studies have shown that, during inhibitory synaptic potentiation, gephyrin accumulation at synaptic sites is enhanced (Petrini et al., 2014, Bannai et al., 2015, Flores et al., 2015, Battaglia et al., 2017), whereas the synaptic dispersal of gephyrin is associated with inhibitory synaptic depression (Bannai et al., 2009, Muir et al., 2010, Niwa et al., 2012, Bannai et al., 2015). In particular, the dynamics of gephyrin have been studied during the expression of inhibitory long-term potentiation (iLTP) induced by brief NMDA applications (Petrini et al., 2014). Live-cell imaging experiments revealed that during the early phase of iLTP expression (30 min) gephyrin was recruited from extrasynaptic to synaptic sites, indicating that fast gephyrin redistribution between specific sub-neuronal compartments contributes to inhibitory synaptic plasticity (Petrini et al., 2014). Interestingly, simultaneous imaging of GABAAR and gephyrin during the expression of this chemically-induced synaptic plasticity showed that gephyrin increase at synapses did not precede the accumulation of GABAARs, ruling against the hypothesis that GABAARs require pre-existing synaptic gephyrin in order to be anchored and clustered at synapses. Along the same line, the time course of both GABAAR and gephyrin synaptic decrease was studied during the depression of inhibitory synapses induced by the application of 4-aminopyridine (Niwa et al., 2012). This study revealed that the dispersal of GABAARs preceded that of synaptic gephyrin, indicating that receptors may diffuse away from the synapses independently from gephyrin de-clustering. These studies suggest that while gephyrin is fundamental for the regulation of GABAAR mobility and clustering, other scaffold/anchoring proteins are likely to contribute to the rearrangement of the inhibitory PSD both in basal conditions and during the expression of inhibitory synaptic plasticity.
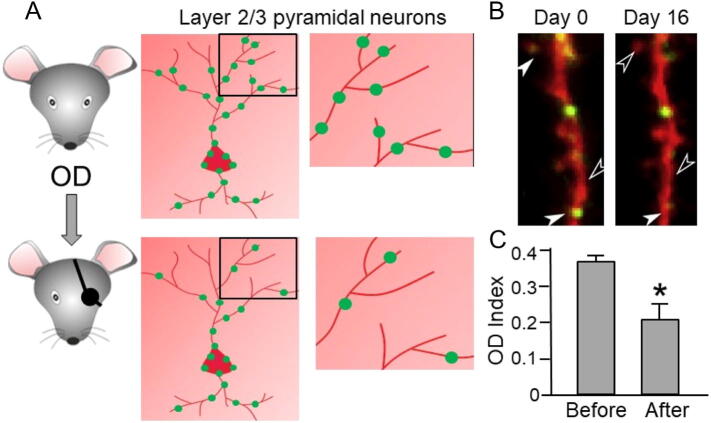
Remarkably, studies tackling the in vivo dynamics of gephyrin over days by two-photon imaging of inhibitory synapses, identified by fluorescent markers, revealed that dendritic gephyrin clusters are continuously assembled and disassembled. In layer 2/3 of the mouse visual cortex, dendritic gephyrin puncta imaged at time interval of 4 days exhibited substantial dynamism, with synapses directly impinging onto dendritic spines being significantly more dynamic with respect to those formed onto the dendritic shaft (Chen et al., 2012, van Versendaal et al., 2012). In the same studies, such basal inhibitory synapse turnover was altered upon the sensory manipulation of the animal (Fig. 3).
Fig. 3.
Ocular dominance plasticity induces inhibitory synapses pruning. (A) Ocular dominance (OD) plasticity in adult mouse induces a loss of inhibitory synapses (green spots) from distal dendrites (preferentially innervated by thalamic inputs) in layer 2/3 pyramidal neurons in the visual cortex. (B) A representative image of a dendritic portion of a layer 2/3 pyramidal neuron electroporated with cytosolic red fluorescent protein and GFP-gephyrin (green puncta) and imaged in vivo at different time points after OD plasticity protocol. Empty arrows indicate the loss of GFP-gephyrin puncta while filled arrows indicate GFP-gephyrin puncta still present. (C) Ocular Dominance Index (OD Index) decreases significantly following OD plasticity protocol. [Modified from van Versendaal et al., 2012].
Ocular dominance plasticity induced an increase of inhibitory synapses dynamism, leading to an overall loss of inhibitory inputs that, similar to naïve animals, was more pronounced at inhibitory synapses located at spines (Chen et al., 2012, van Versendaal et al., 2012). It is worth noting that in layer 2–3 pyramidal cells, spines possessing an inhibitory synapse are preferentially innervated by thalamic inputs (van Versendaal et al., 2012) and are more likely located in the distal region of apical dendrites (Chen et al., 2012), thus indicating that these inputs are strategically located in particular sub-neuronal domains in order to absolve to specific dendritic integration and computation functions. In addition, in sub-regions of pyramidal cells dendrites (about 10 μm), synaptic gephyrin puncta dynamics correlated with that of spines, suggesting that dendritic glutamatergic and GABAergic synapses can be locally coordinated in small dendritic portions (Chen et al., 2012). More recently, the remodeling of excitatory and inhibitory synapses has been studied with daily intervals (Villa et al., 2016), thus considerably increasing the temporal resolution as compared to previous attempts (Chen et al., 2012, van Versendaal et al., 2012). By using an analogous experimental approach as described above, the degree of gephyrin synaptic turnover was found to be markedly higher with respect to that measured at 4 days sampling time, revealing that gephyrin puncta, especially those at glutamatergic spines, are among the most dynamic synaptic elements in cortical dendrites. In addition, gephyrin clusters at spines could disappear and reappear in the same location several times, indicating the presence of a reversible GABAergic structural plasticity mechanism that is expected to modulate “on demand” the strength of specific glutamatergic spines. Intriguingly, at doubly-innervated spines where glutamatergic synapses are substantially stable, inhibitory synapses are the most dynamic, thus highlighting a fundamental role of inhibition in the coordinated flexibility of such persistent glutamatergic inputs.
The high dynamism of gephyrin clustering is also emphasized in the mechanisms of GABAergic synapses formation. Indeed, in layer 2–3 pyramidal neurons of developing somatosensory organotypic slices, it has been demonstrated that the focal dendritic photorelease of caged GABA was able to readily induce the formation of gephyrin puncta that subsequently matured in GABAergic synapses (Oh et al., 2016). Such induction of inhibitory synapse formation was mediated by the depolarizing action of GABA (typically observed in early postnatal age, Cherubini et al., 1991) that determined the engagement of L- and T-type voltage-gated calcium channels. This mechanism also occurred in vivo, since the optogenetic activation of somatostatin interneurons (SOM-INs) generated the formation of new gephyrin clusters in close vicinity (< 2 μm) of presynaptic boutons of somatostatin-positive interneurons (Oh et al., 2016).
Finally, in vivo experiments allowed to determine the role of gephyrin in controlling neuronal connectivity involved in motor control. It is known that one of the major roles of inhibitory interneurons in the zebrafish spinal cord is to prevent the co-contraction of opposing muscles by inhibiting contralateral motoneurons (Hirata et al., 2011). In keeping with this, the elimination of endogenous gephyrin from the spinal cord of zebrafish with the GFE3 molecular tool described above, led to motor incoordination, possibly caused by the co-contraction of opposing muscles (Gross et al., 2016).
Overall these data show that gephyrin clustering is an important substrate of inhibitory synaptic plasticity and that the dynamism of gephyrin clusters is a powerful mechanism in neuronal networks for the selective and versatile tuning of inhibition both in space and time in relation to specific neuronal activity states.
Gephyrin alterations associated with neuropsychiatric and neurodegenerative disorders
Mutations in GPHN or alterations in protein expression levels have been found in several neuropathological states including neurodevelopmental and neurodegenerative disorders. Using high resolution microarray data from a cohort of 5384 patients with neurodevelopmental disorders, hemizygous microdeletions at chromosome 14q23 (de novo or inherited), overlapping GPHN exons 3–5, which encode for the G-domain of gephyrin, were identified in six unrelated subjects affected by Autism Spectrum Disorders (ASDs), schizophrenia or temporal lobe epilepsy (Lionel et al., 2013). Microdeletions in the GPHN exons 2–3 and 5–9, encoding gephyrin G-domain, have been found also in two patients affected by idiopathic generalized epilepsy (Dejanovic et al., 2014b). Deletions in exons 5–9 impair oligomerization and perturb in a dominant-negative manner the formation of gephyrin clusters at GABAergic synapses while those in exons 2–3 cause a frameshift resulting in a premature stop-codon after exon 1. The GPHN hemizygous missense mutation (G375D), found in a patient with a form of epileptic encephalopathy resembling the Dravet's syndrome, but lacking the typical mutation in the SCN1A gene, further strengthens the association of GPHN with epilepsy (Dejanovic et al., 2015). Although properly folded, when expressed in cultured hippocampal neurons, gephyrin G375D led to alterations in gephyrin clusterization with severe impairment of GABAergic synaptic function. The mutated gephyrin did not affect oligomerization, but reduced the binding affinity to GABAA and GlyRs. In addition gephyrin G375D was unable to synthetize the molybdenum cofactor, essential for the activation of MoCo-dependent enzymes.
A reduced expression of gephyrin was found in hippocampal and adjacent cortical tissues collected during surgery from patients affected by drug-resistant forms of temporal lobe epilepsy (TLE, Fang et al., 2011). A similar downregulation of gephyrin was observed in animal models of TLE, particularly during the acute period (Fang et al., 2011). Immunohistochemical and western blot analysis of the hippocampus of patients with intractable forms of TLE unveiled four gephyrin splice variants lacking several exons in their G domains with dominant negative effects leading to oligomerization deficits and alterations of GABAA receptors clustering (Förstera et al., 2010). Interestingly, the extensive cell loss occurring in the hilus and in the CA1 region of the hippocampus following seizures induced by ipsilateral injection of kainate, an animal model of TLE, was associated with hypertrophy of granule cells and a marked increase of postsynaptic GABAA receptor clusters (Knuesel et al., 2001). GABAergic synapses reorganization in the dentate gyrus was paralleled by overexpression of gephyrin and dystrophin suggesting that these proteins are both involved in postsynaptic clustering of GABAA receptors.
GPHN was among the nine highly penetrant genetic risk loci for schizophrenia (Lencz et al., 2007). Using a statistical approach “Gene Relationships Across Implicated Loci” (GRAIL), GPHN was shown to play a causal role in schizophrenia, on the basis of its functional connections with other risk genes (Raychaudhuri et al., 2009).
Regarding neurodegenerative disorders, the analysis of post mortem brain tissues from Alzheimer Disease (AD) patients has allowed unveiling a tight association between gephyrin dysfunction and neurodegenerative diseases (Agarwal et al., 2008). An abnormal accumulation of gephyrin, co-localized with β-amyloid plaques, was detected in brain samples from AD patients (Hales et al., 2013). Biochemical and proteomic analysis revealed a shift in gephyrin's solubility with accumulation of a lower molecular weight isoform (a product of gephyrin cleavage), and a reduced amount of the full length gephyrin fragment. These modifications may severely alter GABAergic transmission. In keeping with this, an age-dependent decrease of GABAergic currents, associated with a reduction of mRNA and GABAAR proteins was found in Xenopus oocytes transplanted with cell membranes isolated from temporal cortices of AD patients (Limon et al., 2012). Biphasic alterations of gephyrin levels have been described in the APPPS1 mouse, an animal model of AD, at different ages (Kiss et al., 2016). While 3-month-old animals exhibit, before plaques formation, an enhanced expression of gephyrin at inhibitory synapses in the CA1 region of the hippocampus and in the dentate gyrus, adult transgenic animals display decreased levels of the scaffold protein in hippocampal areas devoid of amyloid plaques. This is in accordance with the increased activation of genes encoding for synaptic proteins, including those involved in inhibitory synapses organization, observed in AD patients before the onset of cognitive deficits (Bossers et al., 2010). Decreased gephyrin levels were also found in other AD mouse models, namely AD TG2576 and TG-SwDI as compared to matched controls by using western blot analysis (Liang et al., 2014).
The impairment of gephyrin function common to several neuropsychiatric disorders points to this inhibitory synapse scaffold protein as a possible target for therapeutic interventions.
Open questions and future directions
Gephyrin is the main organizer of GABAARs which, upon activation by GABA released from interneurons, exert a powerful control on network excitability and oscillatory behavior crucial for information processing (Buzsáki et al., 2003; Klausberger and Somogyi, 2008). GABAARs can be localized on the soma and on the dendrites of principal cells and interneurons, at synaptic and extrasynaptic sites to regulate phasic and tonic inhibition, respectively, or on presynaptic axon terminals to regulate transmitter release. While, as highlighted in this review, new data on gephyrin structure and distribution at nanoscale level have contributed to better understand its function, many aspects related to its temporal and spatial dynamics during plasticity processes are still largely unknown. Does gephyrin bind to extrasynaptic GABAARs? Does this scaffold regulate dynamic changes between synaptic and extrasynaptic GABAA receptors particularly during activity-dependent synaptic plasticity processes? How does gephyrin interact with GABAARs to form synaptic and extrasynaptic clusters early in development, when GABA exerts a depolarizing and excitatory action on its targets?
How, during critical periods of brain development, does gephyrin contribute to experience-dependent re-shaping of neuronal circuits to adapt GABAergic transmission to changing contingencies?
A closer examination of gephyrin function will be essential to answer these open questions. Furthermore, the knowledge of the multifaceted gephyrin roles at inhibitory synapses will help designing new drugs for the treatment of brain disorders caused by dysfunctions of inhibitory synaptic transmission.
Acknowledgments
Acknowledgments
This work was supported by grants from Telethon (GGP 16083) to EC and AB and from the European Union's Horizon 2020 Framework Programme for Research and Innovation under the Specific Grant Agreement No. 785907 (Human Brain Project SGA2) to EC and AC. We wish to thank our colleagues who contributed to the original work reported in this review. We want to dedicate this paper to Ricardo Miledi for the constructive discussions and suggestions on this interesting topic during his visits to Trieste and Rome.
Conflict of interest
None.
References
- Agarwal S, Tannenberg RK, Dodd PR. Reduced expression of the inhibitory synapse scaffolding protein gephyrin in Alzheimer's disease. J Alzheimers Dis. 2008;14:313–321. doi: 10.3233/jad-2008-14305. [DOI] [PubMed] [Google Scholar]
- Antonelli R, Pizzarelli R, Pedroni A, Fritschy JM, Del Sal G, Cherubini E, Zacchi P. Pin1-dependent signalling negatively affects GABAergic transmission by modulating neuroligin2/gephyrin interaction. Nat Commun doi. 2014 doi: 10.1038/ncomms6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H, Lévi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Bannai H, Niwa F, Sherwood MW, Shrivastava AN, Arizono M, Miyamoto A, Sugiura K, Lévi S. Bidirectional control of synaptic GABAAR clustering by glutamate and calcium. Cell Rep. 2015;13:2768–2780. doi: 10.1016/j.celrep.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis A. (2019) Postsynaptic plasticity of GABAergic synapses. Neuropharmacology pii: S0028-3908(19)30169–8. doi: 10.1016/j.neuropharm. [DOI] [PubMed]
- Battaglia S, Renner M, Russeau M, Côme E, Tyagarajan SK, Lévi S. (2018) Activity-dependent inhibitory synapse scaling is determined by gephyrin phosphorylation and subsequent regulation of GABAA receptor diffusion. eNeuro 5.Pii:0203-17.2017. doi: 10.1523/ENEURO. [DOI] [PMC free article] [PubMed]
- Biocca S, Cattaneo A. Intracellular immunization: antibody targeting to subcellular compartments. Trends Cell Biol. 1995;5:248–252. doi: 10.1016/s0962-8924(00)89019-4. [DOI] [PubMed] [Google Scholar]
- Bossers K, Wirz KT, Meerhoff GF, Essing AH, van Dongen JW, Houba P, Kruse CG, Verhaagen J. Concerted changes in transcripts in the prefrontal cortex precede neuropathology in Alzheimer's disease. Brain. 2010;133:3699–3723. doi: 10.1093/brain/awq258. [DOI] [PubMed] [Google Scholar]
- Brady ML, Jacob TC. Synaptic localization of α5 GABA (a) receptors via gephyrin interaction regulates dendritic outgrowth and spine maturation. Dev Neurobiol. 2015;75:1241–1251. doi: 10.1002/dneu.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Buhl DL, Harris KD, Csicsvari J, Czéh B, Morozov A. Hippocampal network patterns of activity in the mouse. Neuroscience. 2003;116:201–211. doi: 10.1016/s0306-4522(02)00669-3. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Chirichiella M. (2019) Targeting the post-translational proteome with intrabodies. Trends Biotechnol pii: S0167-7799(18)30319–6. doi: 10.1016/j.tibtech.2018.11.009. [DOI] [PubMed]
- Chen JL, Villa KL, Cha JW, So PT, Kubota Y, Nedivi E. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 2012;74:361–373. doi: 10.1016/j.neuron.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GC, Higley MJ. Compartmentalization of GABAergic inhibition by dendritic spines. Science. 2013;340:759–762. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CQ, Barberis A, Higley MJ. Preserving the balance: diverse forms of long-term GABAergic synaptic plasticity. Nat Rev Neurosci doi. 2019 doi: 10.1038/s41583-019-0141-5. [DOI] [PubMed] [Google Scholar]
- Choii G, Ko J. Gephyrin: a central GABAergic synapse organizer. Exp Mol Med. 2015;47 doi: 10.1038/emm.2015.5. [DOI] [PubMed] [Google Scholar]
- Choquet D, Triller A. The dynamic synapse. Neuron. 2013;80:691–703. doi: 10.1016/j.neuron.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Costa JT, Mele M, Baptista MS, Gomes JR, Ruscher K, Nobre RJ, de Almeida LP, Wieloch T. Gephyrin cleavage in in vitro brain ischemia decreases GABAA receptor clustering and contributes to neuronal death. Mol Neurobiol. 2016;53:3513–3527. doi: 10.1007/s12035-015-9283-2. [DOI] [PubMed] [Google Scholar]
- Crosby KC, Gookin SE, Garcia JD, Hahm KM, Dell'Acqua ML, Smith KR. Nanoscale subsynaptic domains underlie the organization of the inhibitory synapse. Cell Rep. 2019;26:3284–3297. doi: 10.1016/j.celrep.2019.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport EC, Pendolino V, Kontou G, McGee TP, Sheehan DF, López-Doménech G, Farrant M, Kittler JT. An essential role for the tetraspanin LHFPL4 in the cell-type-specific targeting and clustering of synaptic GABAA receptors. Cell Rep. 2017;21:70–83. doi: 10.1016/j.celrep.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejanovic B, Schwarz G. Neuronal nitrioxide synthase-dependent S-nitrosylation of gephyrin regulates gephyrin clustering at GABAergic synapses. J Neurosci. 2014;34:7763–7768. doi: 10.1523/JNEUROSCI.0531-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejanovic B, Semtner M, Ebert S, Lamkemeyer T, Neuser F, Lüscher B, Meier JC, Schwarz G. (2014a) Palmitoylation of gephyrin controls receptor clustering and plasticity of GABAergic synapses. PLoS biol Jul 15;12(7):e1001908. doi: 10.1371/journal.pbio.1001908. [DOI] [PMC free article] [PubMed]
- Dejanovic B, Lal D, Catarino CB, Arjune S, Belaidi AA, Trucks H, Vollmar C, Surges R. Exonic microdeletions of the gephyrin gene impair GABAergic synaptic inhibition in patients with idiopathic generalized epilepsy. Neurobiol Dis. 2014;67:88–96. doi: 10.1016/j.nbd.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Dejanovic B, Djémié T, Grünewald N, Suls A, Kress V, Hetsch F, Craiu D, Zemel M. Simultaneous impairment of neuronal and metabolic function of mutated gephyrin in a patient with epileptic encephalopathy. EMBO Mol Med. 2015;7:1580–1594. doi: 10.15252/emmm.201505323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Shen L, Yin H, Pan YM, Wang L, Chen D, Xi ZQ, Xiao Z. Downregulation of gephyrin in temporal lobe epilepsy neurons in humans and a rat model. Synapse. 2011;65:1006–1014. doi: 10.1002/syn.20928. [DOI] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- Fischer F, Kneussel M, Tintrup H, Haverkamp S, Rauen T, Betz H, Wässle H. Reduced synaptic clustering of GABA and glycine receptors in the retina of the gephyrin null mutant mouse. J Comp Neurol. 2000;427:634–648. doi: 10.1002/1096-9861(20001127)427:4<634::aid-cne10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Flores CE, Nikonenko I, Mendez P, Fritschy JM, Tyagarajan SK, Muller D. Activity-dependent inhibitory synapse remodeling through gephyrin phosphorylation. Proc Natl Acad Sci U S A. 2015;112:E65–E72. doi: 10.1073/pnas.1411170112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstera B, Belaidi AA, Jüttner R, Bernert C, Tsokos M, Lehmann TN, Horn P, Dehnicke C. Irregular RNA splicing curtails postsynaptic gephyrin in the cornu ammonis of patients with epilepsy. Brain. 2010;133:3778–3794. doi: 10.1093/brain/awq298. [DOI] [PubMed] [Google Scholar]
- Ghosh H, Auguadri L, Battaglia S, Simone Thirouin Z, Zemoura K, Messner S, Acuña MA. Several posttranslational modifications act in concert to regulate gephyrin scaffolding and GABAergic transmission. Nat Commun. 2016;7:13365. doi: 10.1038/ncomms13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone G, Mondin M, Grillo-Bosch D, Tessier B, Saint-Michel E, Czöndör K, Sainlos M, Choquet D. Neurexin-1β binding to neuroligin-1 triggers the preferential recruitment of PSD-95 versus gephyrin through tyrosine phosphorylation of neuroligin-1. Cell Rep. 2013;3:1996–2007. doi: 10.1016/j.celrep.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Giesemann T, Schwarz G, Nawrotzki R, Berhörster K, Rothkegel M, Schlüter K, Schrader N, Schindelin H. Complex formation between the postsynaptic scaffolding protein gephyrin, profilin, and Mena: a possible link to the microfilament system. J Neurosci. 2003;23:8330–8339. doi: 10.1523/JNEUROSCI.23-23-08330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GG, Straub C, Perez-Sanchez J, Dempsey WP, Junge JA, Roberts RW, Trinh le A, Fraser SE. An E3-ligase-based method for ablating inhibitory synapses, Nat Methods. 2016;13:673–678. doi: 10.1038/nmeth.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald N., Jan A., Salvatico C., Kress V., Renner M., Triller A., Specht C.G., Schwarz G. Sequences flanking the gephyrin-binding site of glyRβ tune receptor stabilization at synapses. eNeuro. 2018 Feb 19;5(1) doi: 10.1523/ENEURO.0042-17.2018. pii: ENEURO.0042-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KT, Compans B, Letellier M, Bartol TM, Grillo-Bosch D, Sejnowski TJ, Sainlos M, Choquet D et al. (2018) Pre–post synaptic alignment through neuroligin-1 tunes synaptic transmission efficiency. Elife 7, e31755. 10.7554/eLife.31755. pii. [DOI] [PMC free article] [PubMed]
- Hales CM, Rees H, Seyfried NT, Dammer EB, Duong DM, Gearing M, Montine TJ, Troncoso JC. Abnormal gephyrin immunoreactivity associated with Alzheimer disease pathologic changes. J Neuropathol Exp Neurol. 2013;72:1009–1015. doi: 10.1097/01.jnen.0000435847.59828.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus C, Ehrensperger MV, Triller A. Activity-dependent movements of postsynaptic scaffolds at inhibitory synapses. J Neurosci. 2006;26:4586–4595. doi: 10.1523/JNEUROSCI.5123-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herweg J, Schwarz G. Splice-specific glycine receptor binding, folding, and phosphorylation of the scaffolding protein gephyrin. J Biol Chem. 2012;287:12645–12656. doi: 10.1074/jbc.M112.341826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Takahashi M, Yamada K, Ogino K. The biological role of the glycinergic synapse in early zebrafish motility. Neurosci Res. 2011;71:1–11. doi: 10.1016/j.neures.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Horn ME, Nicoll RA. Somatostatin and parvalbumin inhibitory synapses onto hippocampal pyramidal neurons are regulated by distinct mechanisms. Proc Natl Acad Sci U S A. 2018;115:589–594. doi: 10.1073/pnas.1719523115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska M, Henderson N, Le Marchand SJ, Jafri H, Dalva MB. Synaptic nanomodules underlie the organization and plasticity of spine synapses. Nat Neurosci. 2018;21:671–682. doi: 10.1038/s41593-018-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Kim EY, Schrader N, Smolinsky B, Bedet C, Vannier C, Schwarz G, Schindelin H. Deciphering the structural framework of glycine receptor anchoring by gephyrin. EMBO J. 2006;25:1385–1395. doi: 10.1038/sj.emboj.7601029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch J, Malosio ML, Wolters I, Betz H. Distribution of gephyrin transcripts in the adult and developing rat brain. Eur J Neurosci. 1993;5:1109–1117. doi: 10.1111/j.1460-9568.1993.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Kiss E, Gorgas K, Schlicksupp A, Groß D, Kins S, Kirsch J, Kuhse J. Biphasic alteration of the inhibitory synapse scaffold protein gephyrin in early and late stages of an Alzheimer disease model. Am J Pathol. 2016;86:2279–2291. doi: 10.1016/j.ajpath.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Brandstätter JH, Gasnier B, Feng G, Sanes JR, Betz H. Gephyrin-independent clustering of postsynaptic GABA(A) receptor subtypes. Mol Cell Neurosci. 2001;17:973–982. doi: 10.1006/mcne.2001.0983. [DOI] [PubMed] [Google Scholar]
- Knuesel I, Zuellig RA, Schaub MC, Fritschy JM. Alterations in dystrophin and utrophin expression parallel the reorganization of GABAergic synapses in a mouse model of temporal lobe epilepsy. Eur J Neurosci. 2001;6:1113–11124. doi: 10.1046/j.0953-816x.2001.01476.x. [DOI] [PubMed] [Google Scholar]
- Kowalczyk S, Winkelmann A, Smolinsky B, Förstera B, Neundorf I, Schwarz G, Meier JC. Direct binding of GABAA receptor β2 and β3 subunits to gephyrin. Eur J Neurosci. 2013;37:544–554. doi: 10.1111/ejn.12078. [DOI] [PubMed] [Google Scholar]
- Langosch D, Hoch W, Betz H. The 93 kDa protein gephyrin and tubulin associated with the inhibitory glycine receptor are phosphorylated by an endogenous protein kinase. FEBS Lett. 1992;298:113–117. doi: 10.1016/0014-5793(92)80034-e. [DOI] [PubMed] [Google Scholar]
- Legendre P, Muller E, Badiu CI, Meier J, Vannier C, Triller A. Desensitization of homomeric alpha1 glycine receptor increases with receptor density. Mol Pharmacol. 2002;62:817–827. doi: 10.1124/mol.62.4.817. [DOI] [PubMed] [Google Scholar]
- Lencz T, Lambert C, DeRosse P, Burdick KE, Morgan TV, Kane JM, Kucherlapati R, Malhotra AK. Runs of homozygosity reveal highly penetrant recessive loci in schizophrenia. Proc Natl Acad Sci U S A. 2007;104:19942–19947. doi: 10.1073/pnas.0710021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, López-Valdés HE, Martínez-Coria H, Lindemeyer AK, Shen Y, Shao XM, Olsen RW. Dihydromyricetin ameliorates behavioral deficits and reverses neuropathology of transgenic mouse models of Alzheimer's disease. Neurochem Res. 2014;39:1171–1181. doi: 10.1007/s11064-014-1304-4. [DOI] [PubMed] [Google Scholar]
- Limon A, Reyes-Ruiz JM, Miledi R. Loss of functional GABA(A) receptors in the Alzheimer diseased brain. Proc Natl Acad Sci U S A. 2012;109:10071–10076. doi: 10.1073/pnas.1204606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionel AC, Vaags AK, Sato D, Gazzellone MJ, Mitchell EB, Chen HY, Costain G, Walker S. Rare exonic deletions implicate the synaptic organizer Gephyrin (GPHN) in risk for autism, schizophrenia and seizures. Hum Mol Genet. 2013;22:2055–2066. doi: 10.1093/hmg/ddt056. [DOI] [PubMed] [Google Scholar]
- Lushnikova I, Skibo G, Muller D, Nikonenko I. Excitatory synaptic activity is associated with a rapid structural plasticity of inhibitory synapses on hippocampal CA1 pyramidal cells. Neuropharmacology. 2011;60:757–764. doi: 10.1016/j.neuropharm.2010.12.014. [DOI] [PubMed] [Google Scholar]
- MacGillavry HD, Kerr JM, Blanpied TA. Lateral organization of the postsynaptic density. Mol Cell Neurosci. 2011;48:321–331. doi: 10.1016/j.mcn.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillavry HD, Song Y, Raghavachari S, Blanpied TA. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron. 2013;78:615–622. doi: 10.1016/j.neuron.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni I, Kasap Z, Mozrzymas JW, Sieghart W, Cherubini E, Zacchi P. New insights on the role of gephyrin in regulating both phasic and tonic GABAergic inhibition in rat hippocampal neurons in culture. Neuroscience. 2009;164:552–562. doi: 10.1016/j.neuroscience.2009.07.063. [DOI] [PubMed] [Google Scholar]
- Maric HM, Mukherjee J, Tretter V, Moss SJ, Schindelin H. Gephyrin-mediated γ-aminobutyric acid type A and glycine receptor clustering relies on a common binding site. J Biol Chem. 2011;286:42105–42114. doi: 10.1074/jbc.M111.303412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric HM, Kasaragod VB, Hausrat TJ, Kneussel M, Tretter V, Strømgaard K, Schindelin H. Molecular basis of the alternative recruitment of GABA(A) versus glycine receptors through gephyrin. Nat Commun. 2014;5:5767. doi: 10.1038/ncomms6767. [DOI] [PubMed] [Google Scholar]
- Maric HM, Hausrat TJ, Neubert F, Dalby NO, Doose S, Sauer M, Kneussel M, Strømgaard K. Gephyrin-binding peptides visualize postsynaptic sites and modulate neurotransmission. Nat Chem Biol. 2017;13:153–160. doi: 10.1038/nchembio.2246. [DOI] [PubMed] [Google Scholar]
- Martenson JS, Yamasaki T, Chaudhury NH, Albrecht D, Tomita S. (2017) Assembly rules for GABAA receptor complexes in the brain. Elife 6. Pii: e30826. doi: 10.7554/eLife.27443. [DOI] [PMC free article] [PubMed]
- Melchionna T, Cattaneo A. A protein silencing switch by ligand-induced proteasome-targeting intrabodies. J Mol Biol. 2007;374:641–654. doi: 10.1016/j.jmb.2007.09.053. [DOI] [PubMed] [Google Scholar]
- Moss SJ. Smart TG. Constructing inhibitory synapses Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- Muir J, Arancibia-Carcamo IL, Macaskill AF, Smith KR, Griffin LD, Kittler JT. NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the gamma2 subunit. Proc Natl Acad Sc. U S A. 2010;107:16679–16684. doi: 10.1073/pnas.1000589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, Davies PA. The residence time of GABA(A)Rs at inhibitory synapses is determined by direct binding of the receptor α1 subunit to gephyrin. J Neurosci. 2011;31:14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair D, Hosy E, Petersen JD, Constals A, Giannone G, Choquet D, Sibarita JB. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J Neurosci. 2013;33:13204–13224. doi: 10.1523/JNEUROSCI.2381-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrotzki R, Islinger M, Vogel I, Völkl A, Kirsch J. Expression and subcellular distribution of gephyrin in non-neuronal tissues and cells. Histochem Cell Biol. 2012;137:471–482. doi: 10.1007/s00418-012-0914-7. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QA, Horn ME, Nicoll RA (2016) Distinct roles for extracellular and intracellular domains in neuroligin function at inhibitory synapses. Elife Nov 2;5. Pii: e19236. doi: 10.7554/eLife.19236. [DOI] [PMC free article] [PubMed]
- Niwa F, Bannai H, Arizono M, Fukatsu K, Triller A, Mikoshiba K. Gephyrin-independent GABA(A)R mobility and clustering during plasticity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z., Cull-Candy S., Farrant M. Differences in synaptic GABA(A) receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- Oh WC, Lutzu S, Castillo PE, Kwon HB. De novo synaptogenesis induced by GABA in the developing mouse cortex. Science. 2016;353:1037–1040. doi: 10.1126/science.aaf5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S. Molecular anatomy of the postsynaptic density. Mol Cell Neurosci. 2007;34:503–518. doi: 10.1016/j.mcn.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Panzanelli P, Gunn BG, Schlatter MC, Benke D, Tyagarajan SK, Scheiffele P, Belelli D, Lambert JJ et al. Distinct mechanisms regulate GABAA receptor and gephyrin clustering at perisomatic and axo-axonic synapses on CA1 pyramidal cells. J Physiol 589:4959–4980. [DOI] [PMC free article] [PubMed]
- Papadopoulos T, Korte M, Eulenburg V, Kubota H, Retiounskaia M, Harvey RJ, Harvey K, O'Sullivan GA. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. EMBO J. 2007;26:3888–3899. doi: 10.1038/sj.emboj.7601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizio A, Renner M, Pizzarelli R, Triller A, Specht CG. Alpha subunit-dependent glycine receptor clustering and regulation of synaptic receptor numbers. Sci Rep. 2017;7(1):10899. doi: 10.1038/s41598-017-11264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchietti F, Vascon S, Nieus T, Rosillo C, Das S, Tyagarajan SK, Diaspro A, Del Bue A. Nanoscale molecular reorganization of the inhibitory postsynaptic density is a determinant of GABAergic synaptic potentiation. J Neurosci. 2017;37:1747–1756. doi: 10.1523/JNEUROSCI.0514-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Barberis A. (2014) Diffusion dynamics of synaptic molecules during inhibitory postsynaptic plasticity. Front cell Neurosci Sep 23;8:300. doi: 10.3389/fncel. [DOI] [PMC free article] [PubMed]
- Petrini EM, Ravasenga T, Hausrat TJ, Iurilli G, Olcese U, Racine V, Sibarita JB, Jacob TC. Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat Comm. 2014;5:3921. doi: 10.1038/ncomms4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer F, Graham D, Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982;257:9389–9393. [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Prior P, Schmitt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maulet Y, Werner P. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron. 1992;8:1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- Ramming M, Kins S, Werner N, Hermann A, Betz H, Kirsch J. Diversity and phylogeny of gephyrin: tissue-specific splice variants, gene structure, and sequence similarities to molybdenum cofactor-synthesizing and cytoskeleton-associated proteins. Proc Natl Acad Sci U S A. 2000;97:10266–10271. doi: 10.1073/pnas.97.18.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri S, Plenge RM, Rossin EJ, Ng AC; International Schizophrenia Consortium, Purcell SM, Sklar P, Scolnick EM et al Identifying relationships among genomic disease regions: predicting genes at pathogenic SNP associations and rare deletions. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM, Barrow RK, Blackshaw S, Burnett PE, Lai MM, Field ME, Bahr BA, Kirsch J. Interaction of RAFT1 with gephyrinrequired for rapamycin-sensitive signaling. Science. 1999;284:1161–1164. doi: 10.1126/science.284.5417.1161. [DOI] [PubMed] [Google Scholar]
- Sander B, Tria G, Shkumatov AV, Kim EY, Grossmann JG, Tessmer I, Svergun DI, Schindelin H. Structural characterization of gephyrin by AFM and SAXS reveals a mixture of compact and extended states. Acta Crystallogr D Biol Crystallogr. 2013;69:2050–2060. doi: 10.1107/S0907444913018714. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Molybdenum cofactor and human disease. Curr Opin Chem Biol. 2016;31:179–187. doi: 10.1016/j.cbpa.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Schwarz G, Schrader N, Mendel R, Hechet HJ, Schindelin H. Cristal structure of human gephyrin and plant Cnx1 G domains: comparative analysis and functional implications. J Mol Biol. 2001;312:405–418. doi: 10.1006/jmbi.2001.4952. [DOI] [PubMed] [Google Scholar]
- Shen ZC, Wu PF, Wang F, Xia ZX, Deng Q, Nie TL, Zhang SQ, Zheng HL. Gephyrin palmitoylation in basolateral amygdala mediates the anxiolytic action of benzodiazepine. Biol Psychiatry. 2019;85:202–213. doi: 10.1016/j.biopsych.2018.09.024. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim E. The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol doi. 2011 doi: 10.1101/cshperspect.a005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Davenport EC, Wei J, Li X, Pathania M, Vaccaro V, Yan Z, Kittler JT. GIT1 and βPIX are essential for GABA(A) receptor synaptic stability and inhibitory neurotransmission. Cell Rep. 2014;9:298–310. doi: 10.1016/j.celrep.2014.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola M, Kneussel M, Heck IS, Betz H, Weissenhorn W. X-ray crystal structures of the trimeric N-terminal domain of gephyrin. J Biol Chem. 2001;276:25294–25301. doi: 10.1074/jbc.M101923200. [DOI] [PubMed] [Google Scholar]
- Sola M, Bavro VN, Timmins J, Franz T, Ricard-Blum S, Schoehn G, Ruigrok RW, Paarmann I. Structural basis of dynamic glycine receptor clustering by gephyrin. EMBO J. 2004;23:2510–2519. doi: 10.1038/sj.emboj.7600256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht CG. (2019) Fractional occupancy of synaptic binding sites and the molecular. Plasticity of inhibitory synapses. Neuropharmacology pii: S0028-3908(19)30007–3. doi: 10.1016/j.neuropharm.2019.01.008. [DOI] [PubMed]
- Specht CG, Izeddin I, Rodriguez PC, El Beheiry M, Rostaing P, Darzacq X, Dahan M, Triller A. Quantitative nanoscopy of inhibitory synapses: counting gephyrin molecules and receptor binding sites. Neuron. 2013;79:308–321. doi: 10.1016/j.neuron.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Chen H, Li TP, Metzbower SR, MacGillavry HD, Blanpied TA. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature. 2016;536:210–214. doi: 10.1038/nature19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Lester HA, Lummis SC. The structural basis of function in Cys-loop receptors. Q Rev Biophys. 2010;43:449–499. doi: 10.1017/S0033583510000168. [DOI] [PubMed] [Google Scholar]
- Tretter V, Mukherjee J, Maric HM, Schindelin H, Sieghart W, Moss SJ. (2012) Gephyrin the enigmatic organizer at GABAergic synapses. Front Cell Neurosci May 15;6:23. doi: 10.3389/fncel.2012.00023. [DOI] [PMC free article] [PubMed]
- Tyagarajan SK, Fritschy JM. Gephyrin: a master regulator of neuronal function? Nat Rev Neurosci. 2014;15:141–156. doi: 10.1038/nrn3670. [DOI] [PubMed] [Google Scholar]
- Tyagarajan SK, Ghosh H, Yévenes GE, Nikonenko I, Ebeling C, Schwerdel C, Sidler C, Zeilhofer HU. Regulation of GABAergic synapse formation and plasticity by GSK3beta-dependent phosphorylation of gephyrin. Proc Natl Acad Sci U S A. 2011;108:379–384. doi: 10.1073/pnas.1011824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagarajan SK, Ghosh H, Yévenes GE, Imanishi SY, Zeilhofer HU, Gerrits B, Fritschy JM. Extracellular signal-regulated kinase and glycogen synthase kinase 3β regulate gephyrin postsynaptic aggregation and GABAergic synaptic function in a calpain-dependent mechanism. J Biol Chem. 2013;288:9634–9647. doi: 10.1074/jbc.M112.442616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW, Choii G, Park D, Kim D, Jeon S, Kang H, Mori T, Papadopoulos T. IQ motif and SEC7 domain-containing protein 3 (IQSEC3) interacts with gephyrin to promote inhibitory synapse formation. J Biol Chem. 2016;291:10119–10130. doi: 10.1074/jbc.M115.712893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Versendaal D, Rajendran R, Saiepour MH, Klooster J, Smit-Rigter L, Sommeijer JP, De Zeeuw CI, Hofer SB. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron. 2012;74:374–383. doi: 10.1016/j.neuron.2012.03.015. [DOI] [PubMed] [Google Scholar]
- van Zundert B, Castro P, Aguayo LG. Glycinergic and GABAergic synaptic transmission are differentially affected by gephyrin in spinal neurons. Brain Res. 2005;1050:40–47. doi: 10.1016/j.brainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Varley ZK, Pizzarelli R, Antonelli R, Stancheva SH, Kneussel M, Cherubini E, Zacchi P. Gephyrin regulates GABAergic and glutamatergic synaptic transmission in hippocampal cell cultures. J Biol Chem. 2011;286:20942–20951. doi: 10.1074/jbc.M111.234641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa KL, Berry KP, Subramanian J, Cha JW, Oh WC, Kwon HB, Kubota Y, So PT, Nedivi E. Inhibitory synapses are repeatedly assembled and removed at persistent sites in vivo. Neuron. 2016;89:756–769. doi: 10.1016/j.neuron.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin M, Tse E, Axelson H, Rabbitts TH, Cattaneo A. Selection of antibodies for intracellular function using a two-hybrid in vivo system. Proc Natl Acad Sci U S A. 1999;96:11723–11728. doi: 10.1073/pnas.96.21.11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T, Hoyos-Ramirez E, Martenson JS, Morimoto-Tomita M, Tomita S. GARLH family proteins stabilize GABAA receptors at synapses. Neuron. 2017;93:1138–1152. doi: 10.1016/j.neuron.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, Pearl PL. (2013) Metabolic causes of epileptic encephalopathy. Epilepsy Res Treat :124934. doi: 10.1155/2013/124934. [DOI] [PMC free article] [PubMed]
- Yu W, Jiang M, Miralles CP, Li RW, Chen G, de Blas AL. Gephyrin clustering is required for the stability of GABAergic synapses. Mol Cell Neurosci. 2007;36:484–500. doi: 10.1016/j.mcn.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchi P, Dreosti E, Visintin M, Moretto-Zita M, Marchionni I, Cannistraci I, Kasap Z, Betz H. Gephyrin selective intrabodies as a new strategy for studying inhibitory receptor clustering. J Mol Neurosci. 2008;34:141–148. doi: 10.1007/s12031-007-9018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchi P, Antonelli R, Cherubini E. (2014) Gephyrin phosphorylation in the functional organization and plasticity of GABAergic synapses. Front cell Neurosci Apr 9;8:103. doi: 10.3389/fncel.2014.00103. [DOI] [PMC free article] [PubMed]