Abstract
A major goal of translational toxicology is to identify adverse chemical effects and determine whether they are conserved or divergent across experimental systems. Translational toxicology encompasses assessment of chemical toxicity across multiple life stages, determination of toxic mode-of-action, computational prediction modeling, and identification of interventions that protect or restore health following toxic chemical exposures. The zebrafish is increasingly used in translational toxicology because it combines the genetic and physiological advantages of mammalian models with the higher-throughput capabilities and genetic manipulability of invertebrate models. Here, we review recent literature demonstrating the power of the zebrafish as a model for addressing all four activities of translational toxicology. Important data gaps and challenges associated with using zebrafish for translational toxicology are also discussed.
Keywords: Adults, developmental toxicology, disease modeling, gene editing, gut microbiome, hazard identification, interventions, juveniles, life stages, mode-of-action, molecular toxicology, predictive toxicity, toxicity testing
1.0. Introduction
A main goal of toxicology is to determine the potential for and the mechanisms by which xenobiotic agents cause harm to biological systems. While the human is a predominant target species of interest, for most xenobiotics there is limited human data. To address this data gap, it is often necessary to extrapolate data integrated from diverse species across varying levels of organization, including computational, biochemical, in vitro, and in vivo systems. In vivo models offer a distinct advantage by enabling assessment of integrative effects across organ systems, and across different life stages. However, all experimental models fail to recapitulate some aspects of human biology so it is important to understand and account for the limitations of any given model (Figure 1A).
Figure 1: Comparisons of model organisms to humans.
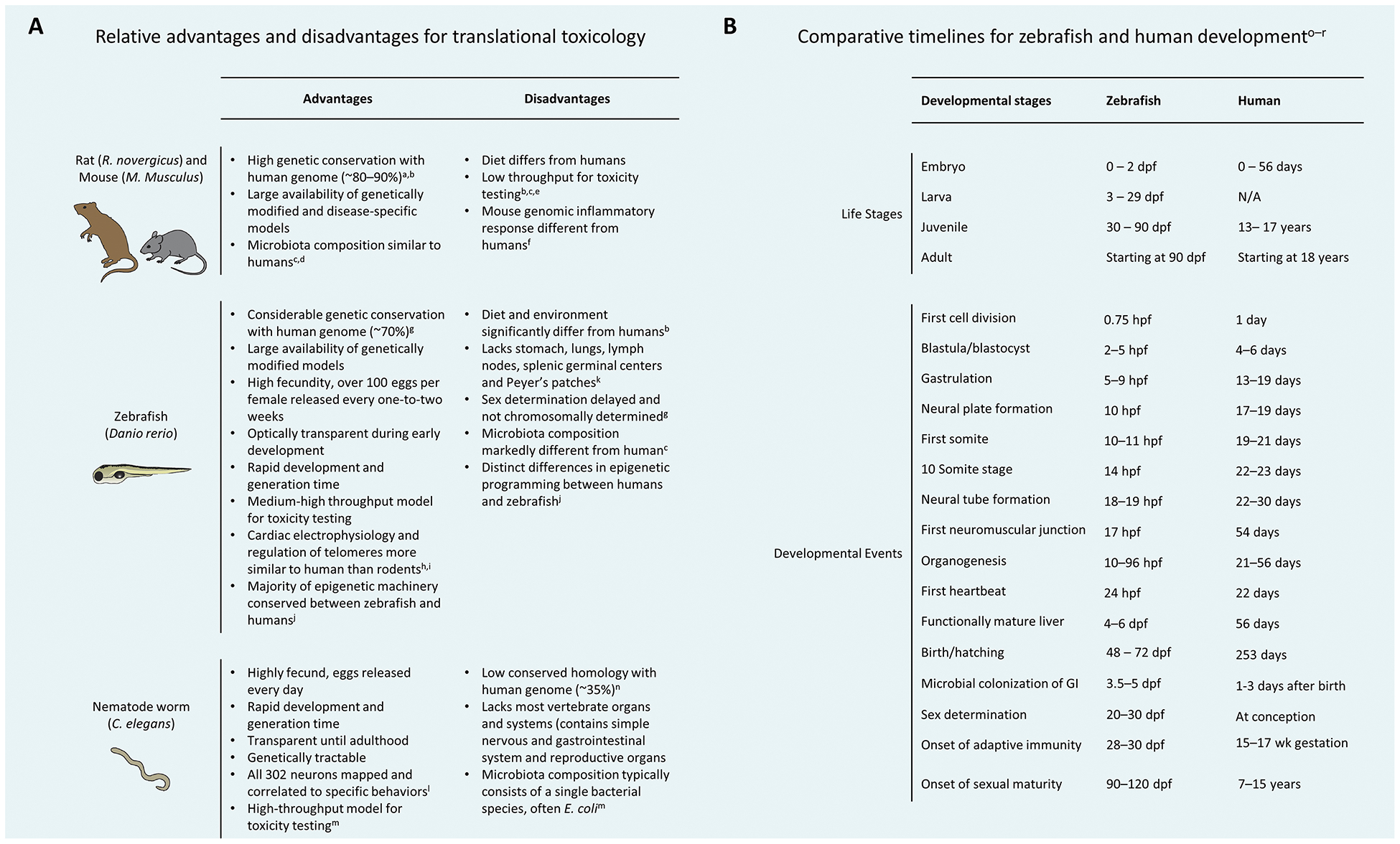
(A) Strengths and weaknesses of widely used translational toxicology animal models. (B) Comparative timelines for zebrafish and human development (https://zfin.org/zf_info/zfbook/stages/). The timing of all developmental events in zebrafish is influenced by temperature. Abbreviations: dpf = days post-fertilization; hpf = hours post-fertilization; wk = weeks. aKeane et al. 2011 Nature 477:289–294; bFritz et al. 2013 Microbiome 1:14; cKostic et al. 2013 Genes Dev 27:701–718; dNagpal et al. 2018 Front Microbiol 9:2897; eBedell et al. 1997 Genes Dev 11:11–43; fSeok et al. 2013 Proc Natl Acad Sci U S A 110:3507–3512; gHowe et al. 2013 Nature 496:498–503; hMacRae and Peterson 2015 Nat Rev Drug Discov 14:721–731; iCayuela et al. 2018 Front Cell Dev Biol 6(178); jAluru et al. 2018 Environ Epigenet 4:dvy005; kGoldsmith and Jobin (2012) J Biomed Biotechnol 2012: 817341; lBargmann 2006 Worm Book 1–29; mClark and Walker 2018 Cell Mol Life Sci 75:93–101; nKim et al. 2018 Genetics 210:445–461; oKimmel et al. 1995 Dev Dyn 203:253–310; pO’Rahilly et al. 1979 Anat Embryol (Berl) 157:167–176; qPhelps et al. 2017 Sci Rep 7:11244; rRawls et al. 2007 Proc Natl Acad Sci U S A 104:7622–7627.
Due to genetic and physiologic conservation between zebrafish and humans (Box 1) and the relevance of this small aquatic vertebrate to translational toxicology (Box 2), the zebrafish has become a widely used model for toxicological research [1,2] that is increasingly being used to address long-standing challenges in toxicology. For example, zebrafish are a powerful model for studying the toxicity of chemical mixtures, as exemplified by a recent study in which gene expression changes and lethality were quantified in embryonic zebrafish exposed to multiple concentrations of three different pesticides, either individually or as binary or tertiary mixtures [3]. The authors concluded that the quantitative and qualitative effects of the mixtures would not have been predicted based on changes elicited by exposure to individual chemicals. Another long-standing challenge – sex differences in toxic outcomes – was recently studied in zebrafish exposed to perfluorooctane sulfonate (PFOS). Transcriptomic analysis of multiple organs revealed that PFOS altered expression of genes associated with fatty acid metabolism and neural function in a manner that varied not only according to the target organ, and concentration and duration of PFOS exposure, but also sex [4]. In a separate study, wildtype female zebrafish were found to be significantly more sensitive to the behavioral effects of chronic ethanol exposure than long fin striped females or males [5], suggesting that sex and genetic background interact to determine toxic outcome. These studies suggest the potential for using zebrafish to identify specific gene × environment interactions that influence individual susceptibility for adverse outcomes [6].
Box 1. Relevance of zebrafish to human biology and disease.
The zebrafish expresses gene orthologs for >70% of human genes. 82% of human disease-causing proteins, anti 85% of known human drug targets [71]. Zebrafish proteins resemble their human counterparts, particularly within functional domains. For example, while the zebrafish glucocorticoid receptor is only 54% identical to the human glucocorticoid receptor, the ligand binding domain is 74% identical and its pharmacologic properties closely resemble those of the human [72], There is also considerable anatomic and physiologic conservation between zebrafish and humans. The zebrafish possesses counterparts of most human organ systems, and zebrafish organs largely perform the same functions as their human analogs, Physiologic mechanisms are well conserved at the molecular and cellular levels, and some cases (e.g., cardiac electrophysiology), the zebrafish is a better model of the human than rodents (reviewed in [72]). The zebrafish has many of the same sensory modalities as humans, including vision, olfaction, taste, touch, balance and hearing, and it exhibits an extensive behavioral repertoire, ranging from simple stimulus-response behaviors to complex behaviors such as sleep, pain, affective and depressive-like behavior, locomotion, social interactions and cognitive behaviors [16]
Zebrafish are widely used to model diverse human diseases. Because targeted gene mutations can be generated and phenotyped more efficiently in zebrafish than in rodents, (here is significant interest in using zebrafish to investigate rare genetic disorders. For example, using a scnllab mutant zebrafish that recapitulates critical clinical features of Dravet syndrome, a chemical library screen identified the 5-HT2B receptor as a novel therapeutic target for this rare genetic seizure disorder [73]. In a second example, zebrafish expressing human type I collagen gene mutations were engineered to investigate human genetic skeletal dysplasias because unlike mouse models, zebrafish bone mutants survive into adulthood [74], Using micro-computed tomography (μCT) for detailed and rapid skeletal phenotyping of zebrafish mutants and systematic collagen analysis by SDS/PAGE and mass-spectrometry, the authors demonstrated that zebrafish and human type i collagen are compositionaliy and functionally related, and that expression in the zebrafish of select human mutations in type I collagen gives rise to phenotypic variability that mirrors the clinical variability associated with the human disease [74].
Box 2. Zebrafish is a powerful model for toxicology research.
The zebrafish model is particularly well suited for molecular and developmental toxicology studies. Key advantages include: (i) the zebrafish genome has been completely sequenced and is highly homologous to the human genome [71]; (ii) powerful gene-editing techniques continue to be developed and optimized for use in zebrafish [75]; and (iii) zebrafish embryo and larvae are optically transparent, which enables visualization of the dynamic changes in individual cells and organs in vivo across a broad range of developmental stages [76].
Another significant advantage of zebrafish is that toxic outcomes can be measured at the molecular (e.g., mRNA or protein expression), structural (e.g., cells and organs) and systems level, including Structural [76] and electrophysiological [77] parameters of neural circuitry and behavior, This enables molecular effects to be anchored to phenotypic outcomes.
In contrast to cell-based assays that provide limited toxicokinetic information, zebrafish can reveal cnticat insights about the absorption, distribution, metabolism, and excretion (ADME) of xenobiotics. Zebrafish have a functional liver, kidneys, and blood-brain barrier, expression conserved tissue-specific transporters, and exhibit both phase I and II metabolism (reviewed in [72]).
Zebrafish are easily exposed by direct addition of chemicals to the house media (referred to as water-borne exposures). This is a significant advantage for screening studies, especially with the availability of robotics that automate delivery of compound into multi-well plates. However, nominal media concentrations are not necessarily representative of internal dosimetry [78,79], and this remains an important challenge in the use of zebrafish for toxicity testing. Compounds that do not readily dissolve in aqueous solutions represent another challenge for using waterborne exposures; however, this can be overcome by injecting chemicals directly into zebrafish [80].
The zebrafish is also proving to be a strong model for addressing emerging questions in toxicology, such as the influence of xenobiotics in the developmental origins of health and disease (DOHaD), epigenetic mechanisms of toxicity [7], and the role of the microbiome in modifying toxic effects of xenobiotics [8,9]. For example, the effects of bisphenol A (BPA) or the replacement chemicals BPAF, BPB, BPF, or BPS on developmental toxicity and microbiome community structure were recently studied in zebrafish [10]. Chemical potency was conserved in a zebrafish developmental toxicity assay when compared to previously reported estrogen receptor activity in both human in vitro and zebrafish reporter systems [10]. However, an inverse relationship between zebrafish developmental toxicity and chemical-dependent microbiome disruption was observed indicating that traditional toxicology tests fail to capture microbiome-dependent effects. Through the use of colonized, microbe-free axenic, and conventionalized zebrafish, recent work has shown that host-associated microbes biotransform xenobiotic agents into metabolites with unknown toxicity profiles [11,12].
2.0. Evaluating zebrafish for translational toxicology research
Translational toxicology broadly refers to the determination of toxicological effects as conserved or divergent across different experimental systems. Four activities are proposed to comprise translational toxicology [13]. First, assessing chemical toxicity across multiple life stages. Second, identifying chemical mode of action and relevance of key events across models. Third, using data from one model to predict chemical toxicity in other systems. Fourth, deploying models to develop and evaluate interventions to protect or restore a healthy status following chemical exposure. Here, we discuss recent evidence collected in zebrafish that encompasses these four activities (Figure 2).
Figure 2: Translational toxicology in zebrafish.
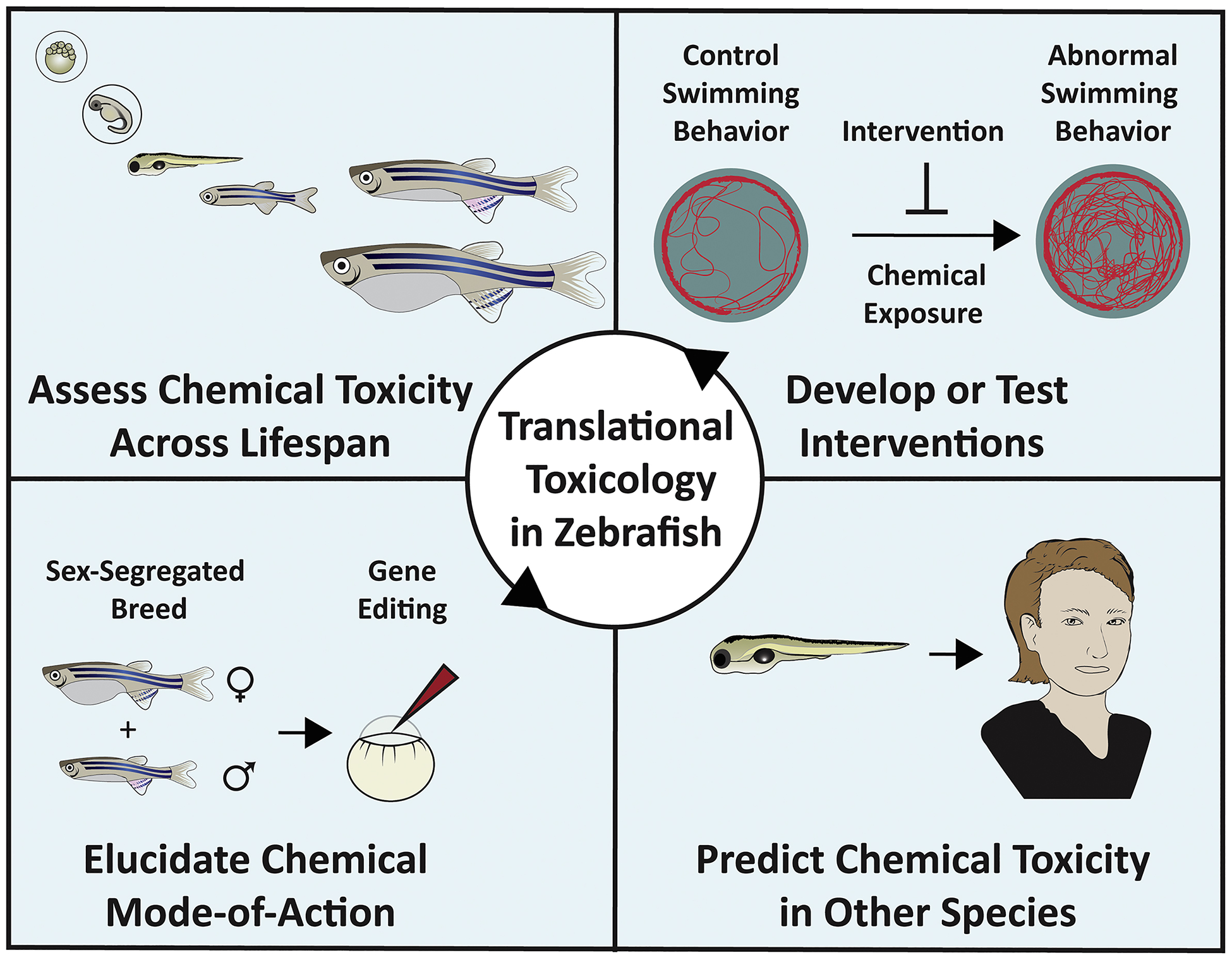
Zebrafish can be used to assess the four components of translational toxicological research, including assessment of chemical toxicity across life stages, delineation of chemical mode of action, development or testing of interventions that block chemical-dependent toxicity outcomes and restore health, or prediction of chemical toxicity in other systems, including humans.
2.1. Assessing chemical toxicity across the zebrafish lifespan
Zebrafish pass through four major life stages: embryonic, larval, juvenile, and adult. Zebrafish are considered embryos from fertilization until hatching, which can occur between 48–96 hours post-fertilization (hpf), at which point they are considered larvae. Zebrafish transition to the juvenile stage at ~30 days post-fertilization (dpf) (https://zfin.org/zf_info/zfbook/stages/), which corresponds to the age when many laboratory-bred strains have determined their sex. Sexual maturity and the ability to produce offspring signals the adult stage, which occurs by ~90–120 dpf. Compared to humans, the key molecular and cellular transitions that occur during the development and maturation of most major organ systems are similar in terms of sequence, but occur more rapidly in zebrafish (Figure 1B).
Zebrafish embryos and larvae are widely used for developmental toxicology studies for theoretical reasons – the molecular and cellular mechanisms of early development are among the most conserved between zebrafish and humans [14] – and practical considerations – zebrafish develop rapidly and external to the mother and for the first 7 dpf, obtain most of their nutrients from the yolk sac. Zebrafish are therefore readily adapted to higher throughput formats that deploy 96- or 384-well plates and automated tools for image acquisition, processing, and associated analyses. Because of its relatively short life cycle, the zebrafish offers significant advantages for assessing transgenerational (e.g., epigenetic) effects [7] and differential vulnerability to toxic effects across the lifespan. With regard to the latter, a recent evaluation of embryonic (3 hpf), larval (3 dpf), juvenile (30 dpf) and adult (3 month old) zebrafish exposed to varying concentrations of four different strobilurin fungicides revealed that the larval stage was the most susceptible [15]. Whether this reflects toxicokinetic or toxicodynamic mechanisms has yet to be determined.
In contrast, there are significantly fewer examples of juvenile and adult zebrafish being used for toxicology research. This may be because unlike embryonic and larval zebrafish, juvenile and adult zebrafish cannot be maintained in multi-well format plates for prolonged periods of time and they are not optically transparent. Despite these limitations, juvenile and larval zebrafish are advantageous for toxicological studies of phenotypes not exhibited at earlier life stages, such as sex, reproductive function, and adaptive immunity (Figure 1B), and behaviors that cannot be readily assessed in younger fish, including learning, memory, social, and anxiety-like behaviors [16]. For example, adult zebrafish were recently used to evaluate the therapeutic and toxic effects of the antidepressant amitriptyline [17]. Adult zebrafish are also gaining traction as models for studying chemical effects on phenotypes and diseases associated with aging, including various cancers [18]. An adult zebrafish model was used to screen novel small molecule therapeutics for liver cancer to identify compounds with a better therapeutic index than the standard of care, sorafenib [19]. Adult zebrafish were also recently validated as a model for evaluating drug-induced kidney injury [20]. Larval zebrafish have only one pair of nephrons whereas the adult zebrafish kidney has several hundred nephrons with similar histological structure and physiological function as the mammalian kidney (reviewed in [20]). The renal pathology observed in adult zebrafish exposed to nephrotoxic levels of gentamicin or doxorubicin was similar to that seen in mammals, and a screen of 28 chemicals with known nephrotoxicity and 14 with no known nephrotoxicity in humans demonstrated that 16 of the nephrotoxic chemicals and none of the negative controls caused drug-induced kidney injury in adult zebrafish.
2.2. Using zebrafish to define chemical mode-of-action
A significant strength of the zebrafish is that molecular insights into chemical mode-of-action can be obtained using diverse approaches ranging from chemical screens to elucidate structure-activity relationships (SARs) to genetic manipulation that identifies molecular targets of xenobiotics. Chemical screens in zebrafish have revealed novel mechanistic information about compounds with unknown modes-of-action via phenotypic mapping to compounds with known modes-of-action. For example, in a screen of 14,000 compounds, automated behavior testing of zebrafish coupled with a barcoding-based computational approach was used to identify novel neuroactive compounds that shared behavioral profiles with compounds with known modes-of-action [21]. This strategy has since been applied to identify chemicals that regulate zebrafish sleep/wake cycles [22], passive and active threat response [23], addiction [24], and psychosis [25]. Phenotypic SARs have been identified for the developmental toxicity of oxygenated, hydroxylated, or heterocyclic polycyclic aromatic hydrocarbon (PAH) derivatives [26]. In the same study, a relationship between behavior phenotypes and specific PAH substitutions was not apparent [26]. More recently, a smaller-scale comparison of alkyl sulphonic acid, alkyl carboxylic acid, or branched or ether containing per- and polyfluoroalkyl substances (PFAS) showed that exposure to alkyl sulfonic acid PFAS with more than four fluorinated carbons caused hyperactivity and the potency for this structural subclass of PFAS correlated with fluorinated carbon chain length [27]. Quantitative SARs (QSARs) have also recently been used to predict zebrafish acute toxicity for neutral [28] or ionizable [29] organic chemicals.
Medium-to-high-throughput zebrafish screens coupled with automated morphological and behavioral phenotyping represent a powerful strategy to identify SARs that illuminate phenotypic readouts particularly sensitive to chemical disruption. Subsequent unbiased pathway-level assessment and gene editing can then be used to solve mode-of-action in vivo. Unbiased [30–37] or targeted RNA sequencing [38] are routinely used to identify chemical-dependent perturbations in zebrafish at the level of genes and pathways. As an example of the translational potential of this approach, changes in gene expression following exposure to three hepatotoxic compounds were compared across whole zebrafish, mouse and rat livers, in vitro mouse and rat hepatocytes, and primary human hepatocytes [39]. While specific changes in gene expression were not generally conserved across models, shared pathway-level perturbations were identified demonstrating that the zebrafish has the capacity to identify pathway-level transcriptomic-based disruptions that indicate liver toxicity in a suite of mammalian models [39]. The observed lack of concordance on the gene level likely stems from comparing profiles obtained from whole zebrafish homogenates versus liver-specific human cells or tissues. Future studies should consider isolating specific cell types via cell sorting or microdissecting specific tissues from zebrafish to enable cross-species transcriptomic comparisons based on similar cell or tissue types.
Once key phenotypes and pathway-level perturbations have been identified, zebrafish can easily be used for mechanistic research, with the goal of identifying causative events that link chemical exposure to phenotypic outcomes. Alternatively, molecular toxicology approaches can be used to disprove dogma related to assumed or predicted modes-of-action. One recent example of the latter was the demonstration of the non-essentiality of PPARγ for ciglitazone-dependent dorsoventral patterning defects in early zebrafish development [40]. Injection of an anti-sense oligonucleotide morpholino into single cell stage zebrafish to transiently suppress the generation of PPARy protein revealed that defects in patterning elicited by ciglitazone exposure occurred via a PPARy-independent mechanism [40]. While some researchers have argued that morpholino knockdown phenotypes can be more severe than mutant phenotypes because of off-target effects [41], lack of concordance between morpholino knockdowns and stable gene knockouts may be more complex and involve genetic compensatory mechanisms specific to gene knockouts, but not knockdowns, at least at certain loci [42]. Nevertheless, gene editing approaches (e.g., CRISPR/Cas9) are now widely used to discover mutations that cause phenotypes and define toxicological modes-of-action. This endeavor is aided by a wide array of mutant zebrafish available via the Zebrafish Mutation Project [43] and the generation of cell type-specific mutant zebrafish lines [44]. While concerns regarding off-target effects of CRISPR/Cas9-based gene editing have been raised [45], recent whole exome sequencing evidence obtained across two generations of zebrafish derived from the same founding mutant pair failed to show evidence of off-target, de novo mutations [46], supporting the use of CRISPR/Cas9-based gene editing to uncover mechanisms by which toxicants elicit adverse outcomes.
There are several recent examples of using gene editing to solve toxicological mode-of-action in zebrafish. In an elegant study that integrated human hepatocellular carcinoma samples, human hepatocyte culture, and zebrafish, CRISPR/Cas9-dependent knockout of G protein-coupled receptor-1 (gper-1) was sufficient to block liver growth in 17-beta-estradiol exposed zebrafish [47]. This work identified gper-1 as a fundamental hepatic estrogen sensor. Given data from human studies demonstrating a causal link between gper signaling and atherosclerosis, heart failure, reproduction, metabolic disorders, cancer, and menopause (reviewed in [48]), this research broadly illustrates that zebrafish can be used to elucidate human-relevant toxicity mechanisms [47]. CRISPR/Cas9-dependent gene knockout was also effectively deployed to show that the efflux transporter multi-resistance-associated protein-1 (mrp1) functions to efflux both cadmium and benzo[a]pyrene [49]. Increased compound accumulation, mortality and, at lower concentrations, increased incidence of pericardial edema and failure to hatch, was observed in mrp1 mutant zebrafish exposed to either compound [49]. Perturbation of arylhydrocarbon receptor (ahr)-dependent signaling represents a well-studied molecular mechanism by which xenobiotic exposure triggers adverse outcomes. Mutant zebrafish lines have revealed the essentiality of ahr2 [50] and sox9b [51] in mediating TCDD-dependent effects on zebrafish heart development. Morpholino-mediated knockdown of the long non-coding RNA slincR was used to demonstrate that slincR repressed sox9b expression as part of the mechanism by which TCDD induced vascular hemorrhage in zebrafish [52]. Mutant ahr2 zebrafish have also been leveraged to reveal the essentiality of the receptor for mono-substituted isopropylated triaryl phosphate, a component of Firemaster 550, to cause a heart looping defect [53].
2.3. Retrofitting zebrafish toxicity data to build or evaluate predictive toxicity models
Historically, zebrafish toxicity data has been used for hazard identification and chemical prioritization [54–59]. To fully leverage available zebrafish toxicity data, its ability to predict toxicity in humans must be defined. An early key paper calculated overall concordances between developmental toxicants in zebrafish and rat (52%) or rabbit (47%) guideline studies [60]. Interestingly, the percentage of concordant chemicals identified between rat and rabbit studies was similar (58%), indicating that at least for the evaluated set of chemicals, zebrafish toxicity data was generally as predictive as the calculated concordance between two widely used mammalian models [60]. A subsequent meta-analysis compared zebrafish toxicity data on 443 chemicals, 19 aggregated toxicity phenotypes (e.g. cardiovascular), and 57 individual toxicity phenotypes (e.g. pericardial edema) to guideline toxicity data collected in rat, mouse, and rabbit [61]. Zebrafish LC50 values were highly correlated with acute mammalian inhalation toxicity with zebrafish LC50 values roughly 180% more sensitive than their mammalian counterparts [61]. From a developmental perspective, zebrafish hatching rate, pericardial edema, and decreased heart rate positively correlated with rabbit lowest effect levels (LELs) for prenatal loss [61]. In an interesting twist, the authors incorporated human exposure values to rank chemicals based on the integration of zebrafish toxicity data and human exposure estimates [61]. The resulting hazard index identified 14 chemicals where exposure levels in humans occur at concentrations that cause toxicity in zebrafish and therefore deserve further scrutiny [61].
A refinement of toxicity concordances that considers SARs is critical to understanding chemical blind-spots in the zebrafish test system. For example, several compounds identified as reference compounds for developmental neurotoxicity because of documented human developmental toxicity were negative hits in a screen of 91 compounds for teratological and behavioral effects in larval zebrafish [54]. These included lead acetate trihydrate, valproic acid sodium salt, and toluene. Whether these compounds are true negatives or the lack of toxic effect is due to toxicokinetic (e.g. reduced bioavailability due to minimal uptake of the compound, lack of metabolic activation, or photoinactivation of the compound) or toxicodynamic (e.g. deficient target expression) differences in developing zebrafish versus mammalian models remains to be determined.
These observations are relevant to a second challenge in establishing concordance between zebrafish and mammalian toxicity data, which is that most of the zebrafish data used in these analyses compare nominal media concentrations to phenotypic outcomes. Tissue dose in zebrafish as a result of waterborne exposure is affected by diverse physicochemical properties [28,29,56]. If a compound fails to provoke phenotypic effects in zebrafish, paired analytical chemistry data is necessary to demonstrate chemical uptake and confirm the assumption that a chemical is negative for the measured toxicity outcome. For example, a recent study showed that GenX, an emerging PFAS compound of public health concern, was unstable in dimethylsulfoxide (DMSO), a solvent widely used in zebrafish chemical screening studies. Without tissue dose measurements, this compound would have been assumed to be negative for a number of developmental toxicity and developmental neurotoxicity endpoints [27].
The zebrafish also contributes to translational toxicology as a tool for evaluating computational models developed using in vitro and biochemical data generated with human cells or receptors. A computational model predicting xenobiotic disruption of blood vessel development [62] was subsequently validated using a transgenic zebrafish assay for evaluating chemical-dependent effects on vessel development [63]. Comparison of human amino acid sequence similarities for members of the predictive signature in the computational model to the zebrafish analog demonstrated biological domain-specific differences in protein sequence conservation [64], and the zebrafish assay proved zebrafish are particularly adept at detecting vascular disruptors associated with chemokine and/or extracellular matrix disruption in human in vitro assays [64].
2.4. Testing interventions in zebrafish
Zebrafish are increasingly used in phenotypic screens to identify compounds that reverse or suppress adverse effects of genetic mutations. For example, a zebrafish expressing a germline mutation in the vhl gene was used to identify pharmacologic approaches for reversing the loss of vision associated with von-Hippel Lindau (VHL) syndrome [65], a rare disease characterized by vision loss associated with retinal capillary hemangioblastomas (tumors of retinal blood vessels). Zebrafish nullizygous for vhl, which were developed because Vhl knockout is embryolethal in mice, exhibit ectopic ocular blood vessels and aberrant eye development associated with an absent optokinetic response and significantly reduced visual motor response [65]. Sunitinib malate, an anti-angiogenic compound approved for cancer treatment, was found to reverse the ocular behavioral and morphological phenotypes in the vhl knockout zebrafish [65]. The methods used in this study were not high throughput; however, an automated system for histological analyses in zebrafish was recently described in which a commercially available platform that automates the transfer of zebrafish larvae from multi-well plates was combined with a customized spinning disk confocal microscope interfaced to software for high resolution image acquisition and analysis [66]. Using this system to screen 175 chemicals in Tg(mbp:eGFP) larvae, a transgenic zebrafish line that expressed enhanced green fluorescent protein (eGFP) in myelinating oligodendrocytes, three novel compounds that significantly altered myelination were identified [66].
Zebrafish are also being leveraged to screen for compounds that mitigate the adverse effects of xenobiotics. A screen of 2,271 small molecules identified 120 compounds that prevented cardiotoxicity in doxorubicin-exposed zebrafish, and subsequent SAR and target enrichment analyses of the seven most effective compounds identified CYP1A1 as a putative target [67]. This was corroborated by showing that cyp1a knockout zebrafish larvae were resistant to doxorubicin-induced cardiotoxicity [67]. In a separate study, the age-dependent sensitivity of zebrafish to cyanide was leveraged to identify novel therapeutic targets for cyanide poisoning [68]. Initial studies revealed that zebrafish embryos are highly resistant to cyanide during the first 3 dpf but become progressively more sensitive as the larvae mature. Unbiased transcriptomic and metabolomic analyses revealed age-dependent differences in energy metabolism during cyanide exposure [68]. This observation led to the identification of compounds that modulate the pyruvate dehydrogenase complex and the small molecule sodium glyoxylate as potential prophylactic treatments for modulating sensitivity to cyanide poisoning [68].
3.0. Major data gaps and summary
Zebrafish are widely used for hazard identification and chemical prioritization [27,32,33,54,56,58,59,61,64]. To improve the use of zebrafish toxicity data in human risk assessment, several data gaps need to be addressed. First, harmonization of common toxicity assays and assessments are necessary to overcome variability in zebrafish data that are due to differences in testing protocols [69]. Additionally, recent advances in developmental toxicity SARs [26,56] and chemical uptake [28,29] need to be expanded, and large-scale SAR analysis of more sensitive behavior endpoints, such as hyperactivity [27], are needed.
A major hindrance to the identification of relevant target pathways in zebrafish is the widespread use of whole animal transcriptomic data, due in part to the technical difficulties associated with obtaining organ- or cell type-specific expression data. Future work should capitalize on the ability to sort specific populations of cells from transgenic zebrafish to increase the ability to detect xenobiotic-dependent transcriptional effects on sensitive, but low abundance cell types. In addition, while there are a growing number of examples in zebrafish [47,49,51,70], more studies should consider using gene editing to characterize toxicity mechanisms.
Perhaps the area ripest for gains is the development of computational models to predict human toxicity from zebrafish toxicity data. Here, toxicokinetic data must be more routinely gathered in phenotypic zebrafish studies, both to identify true negative compounds [27] and to serve as the basis for dose extrapolation to human-relevant exposure scenarios.
In summary, the zebrafish is an exceptional model for the illumination of chemical-dependent toxic effects that are conserved or divergent across different experimental systems. Because of the inherent power of the system for medium-to-high throughput chemical-genetic screens, the zebrafish represents a powerful experimental system for assessing chemical toxicity across lifespan, identification of chemical mode-of-action, generation of datasets for the prediction of chemical toxicity in humans, and rapid assessment of interventions to prevent chemical toxicity in exposed organisms. Researchers who focus on translational research using zebrafish may ultimately have a deep impact on the protection of both human health and the environment.
Funding
This work was supported by the Helmholtz Association First-time Appointments of Excellent Female Scientists [Grant to TT] and by the National Institutes of Health [grant numbers P30 ES023513, R21 NS110647, and R01 ES014901 to PJL].
Abbreviations:
- AhR
arylhydrocarbon receptor
- BPA
bisphenol A
- BPAF
bisphenol AF
- BPB
bisphenol B
- BPF
bisphenol F
- BPS
bisphenol S
- CYP1A1
cytochrome P450, family 1, subfamily A, polypeptide1
- DOHaD
developmental origins of health and disease
- dpf
days post-fertilization
- DMSO
dimethylsulfoxide
- GenX
ammonium salt of hexafluoropropylene oxide dimer acid fluoride
- eGFP
enhanced green fluorescent protein
- gper-1
G protein-coupled receptor 1
- hpf
hours post fertilization
- LELs
lowest effect levels
- mrp1
multi-resistance-associated protein-1
- PFAS
per- and polyfluoroalkyl substances
- PFOS
perfluorooctane sulfonate
- SARs
structure activity relationships
- slincR
sox9b long intergenic noncoding RNA
- sox9b
SRY-box transcription factor 9b
- QSARs
Quantitative SARs
- TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- VHL
von-Hippel Lindau syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Annotated References
- 1.Cassar S, Adatto I, Freeman JL, Gamse JT, Iturria I, Lawrence C, Muriana A, Peterson RT, Van Cruchten S, Zon LI: Use of zebrafish in drug discovery toxicology. Chem Res Toxicol (2020) 33(1):95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horzmann KA, Freeman JL: Making waves: New developments in toxicology with the zebrafish. Toxicol Sci (2018) 163(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen W, Lou B, Xu C, Yang G, Yu R, Wang X, Li X, Wang Q, Wang Y: Lethal toxicity and gene expression changes in embryonic zebrafish upon exposure to individual and mixture of malathion, chlorpyrifos and lambda-cyhalothrin. Chemosphere (2020) 239(124802. [DOI] [PubMed] [Google Scholar]
- 4.Khazaee M, Guardian MGE, Aga DS, Ng CA: Impacts of sex and exposure duration on gene expression in zebrafish following perfluorooctane sulfonate exposure. Environ Toxicol Chem (2020) 39(2):437–449. [DOI] [PubMed] [Google Scholar]
- 5.Dlugos CA, Brown SJ, Rabin RA: Gender differences in ethanol-induced behavioral sensitivity in zebrafish. Alcohol (2011) 45(1):11–18. [DOI] [PubMed] [Google Scholar]
- 6.Volgin AD, Yakovlev OA, Demin KA, de Abreu MS, Alekseeva PA, Friend AJ, Lakstygal AM, Amstislavskaya TG, Bao W, Song C, Kalueff AV: Zebrafish models for personalized psychiatry: Insights from individual, strain and sex differences, and modeling gene × environment interactions. J Neurosci Res (2019) 97(4):402–413. [DOI] [PubMed] [Google Scholar]
- 7.Aluru N: Epigenetic effects of environmental chemicals: Insights from zebrafish. Curr Opin Toxicol (2017) 6(26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertotto LB, Catron TR, Tal T: Exploring interactions between xenobiotics, microbiota, and neurotoxicity in zebrafish. Neurotoxicology (2020) 76(235–244. [DOI] [PubMed] [Google Scholar]
- 9.Catron TR, Gaballah S, Tal T: Using zebrafish to investigate interactions between xenobiotics and microbiota. Current Pharmacology Reports (2019) 5(468–480. [Google Scholar]; ● This study is the first of its kind to document an inverse relationship between host developmental toxicity and microbiome disruption. The rank order of bisphenol compounds was shared for zebrafish developmental toxicity, zebrafish estrogen receptor activity, and estrogen receptor activity in multiple human in vitro and biochemical ToxCast assays. This study shows that traditional toxicity testing fails to capture whether chemical exposures cause microbiota disruption.
- 10.Catron TR, Keely SP, Brinkman NE, Zurlinden TJ, Wood CE, Wright JR, Phelps D, Wheaton E, Kvasnicka A, Gaballah S, Lamendella R et al. : Host developmental toxicity of bpa and bpa alternatives is inversely related to microbiota disruption in zebrafish. Toxicol Sci (2019) 167(2):468–483. [DOI] [PubMed] [Google Scholar]
- 11.Catron TR, Swank A, Wehmas LC, Phelps D, Keely SP, Brinkman NE, McCord J, Singh R, Sobus J, Wood CE, Strynar M et al. : Microbiota alter metabolism and mediate neurodevelopmental toxicity of 17beta-estradiol. Sci Rep (2019) 9(1):7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weitekamp CA, Phelps D, Swank A, McCord J, Sobus JR, Catron T, Keely S, Brinkman N, Zurlinden T, Wheaton E, Strynar M et al. : Triclosan-selected host-associated microbiota perform xenobiotic biotransformations in larval zebrafish. Toxicol Sci (2019) [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes C, Waters M, Allen D, Obasanjo I: Translational toxicology: A developmental focus for integrated research strategies. BMC Pharmacol Toxicol (2013) 14:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comte A, Roux J, Robinson-Rechavi M: Molecular signaling in zebrafish development and the vertebrate phylotypic period. Evol Dev (2010) 12(2):144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang J, Wu S, Lv L, Liu X, Chen L, Zhao X, Wang Q: Mitochondrial dysfunction, apoptosis and transcriptomic alterations induced by four strobilurins in zebrafish (danio rerio) early life stages. Environ Pollut (2019) 253:722–730. [DOI] [PubMed] [Google Scholar]
- 16.Orger MB, de Polavieja GG: Zebrafish behavior: Opportunities and challenges. Annu Rev Neurosci (2017) 40:125–147. [DOI] [PubMed] [Google Scholar]
- 17.Demin KA, Kolesnikova TO, Khatsko SL, Meshalkina DA, Efimova EV, Morzherin YY, Kalueff AV: Acute effects of amitriptyline on adult zebrafish: Potential relevance to antidepressant drug screening and modeling human toxidromes. Neurotoxicol Teratol (2017) 62:27–33. [DOI] [PubMed] [Google Scholar]
- 18.Cayuela ML, Claes KBM, Ferreira MG, Henriques CM, van Eeden F, Varga M, Vierstraete J, Mione MC: The zebrafish as an emerging model to study DNA damage in aging, cancer and other diseases. Front Cell Dev Biol (2018) 6:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin HS, Huang YL, Wang YS, Hsiao E, Hsu TA, Shiao HY, Jiaang WT, Sampurna BP, Lin KH, Wu MS, Lai GM et al. : Identification of novel anti-liver cancer small molecules with better therapeutic index than sorafenib via zebrafish drug screening platform. Cancers (Basel) (2019) 11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato Y, Tonomura Y, Hanafusa H, Nishimura K, Fukushima T, Ueno M: Adult zebrafish model for screening drug-induced kidney injury. Toxicol Sci (2020) 174(2):241–253. [DOI] [PubMed] [Google Scholar]; ● Drug-induced kidney disease is a common toxicity observed in drug development, and current in vitro systems for screening compounds for adverse effects on renal function have serious limitations. This work validates the adult zebrafish as a model for screening compounds for potential to induce kidney injury early in the drug development process.
- 21.Kokel D, Bryan J, Laggner C, White R, Cheung CY, Mateus R, Healey D, Kim S, Werdich AA, Haggarty SJ, Macrae CA et al. : Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol (2010) 6(3):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S, Haggarty SJ, Kokel D, Rubin LL, Peterson RT, Schier AF: Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science (2010) 327(5963):348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rennekamp AJ, Huang XP, Wang Y, Patel S, Lorello PJ, Cade L, Gonzales AP, Yeh JR, Caldarone BJ, Roth BL, Kokel D et al. : Sigma1 receptor ligands control a switch between passive and active threat responses. Nat Chem Biol (2016) 12(7):552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosse GD, Peterson RT: Development of an opioid self-administration assay to study drug seeking in zebrafish. Behav Brain Res (2017) 335:158–166. [DOI] [PubMed] [Google Scholar]
- 25.Bruni G, Rennekamp AJ, Velenich A, McCarroll M, Gendelev L, Fertsch E, Taylor J, Lakhani P, Lensen D, Evron T, Lorello PJ et al. : Zebrafish behavioral profiling identifies multitarget antipsychotic-like compounds. Nat Chem Biol (2016) 12(7):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geier MC, Chlebowski AC, Truong L, Massey Simonich SL, Anderson KA, Tanguay RL: Comparative developmental toxicity of a comprehensive suite of polycyclic aromatic hydrocarbons. Arch Toxicol (2018) 92(2):571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaballah S, Swank A, Sobus JR, Howey XM, Schmid J, Catron T, McCord J, Hines E, Strynar M, Tal T: Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to GenX and other PFAS. Environ Health Perspect (2020) 128(4):47005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kluver N, Vogs C, Altenburger R, Escher BI, Scholz S: Development of a general baseline toxicity qsar model for the fish embryo acute toxicity test. Chemosphere (2016) 164:164–173. [DOI] [PubMed] [Google Scholar]
- 29.Kluver N, Bittermann K, Escher BI: Qsar for baseline toxicity and classification of specific modes of action of ionizable organic chemicals in the zebrafish embryo toxicity test. Aquat Toxicol (2019) 207:110–119. [DOI] [PubMed] [Google Scholar]
- 30.Aluru N, Karchner SI, Glazer L: Early life exposure to low levels of AhR agonist PCB126 (3,3’,4,4’,5-pentachlorobiphenyl) reprograms gene expression in adult brain. Toxicol Sci (2017) 160(2):386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aluru N, Krick KS, McDonald AM, Karchner SI: Developmental exposure to PCB153 (2,2’,4,4’,5,5’-hexachlorobiphenyl) alters circadian rhythms and the expression of clock and metabolic genes. Toxicol Sci (2020) 173(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dasgupta S, Reddam A, Liu Z, Liu J, Volz DC: High-content screening in zebrafish identifies perfluorooctanesulfonamide as a potent developmental toxicant. Environ Pollut (2020) 256:113550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haggard DE, Noyes PD, Waters KM, Tanguay RL: Transcriptomic and phenotypic profiling in developing zebrafish exposed to thyroid hormone receptor agonists. Reprod Toxicol (2018) 77:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddam A, Mitchell CA, Dasgupta S, Kirkwood JS, Vollaro A, Hur M, Volz DC: MRNA-sequencing identifies liver as a potential target organ for triphenyl phosphate in embryonic zebrafish. Toxicol Sci (2019) [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sant KE, Venezia OL, Sinno PP, Timme-Laragy AR: Perfluorobutanesulfonic acid disrupts pancreatic organogenesis and regulation of lipid metabolism in the zebrafish, Danio rerio. Toxicol Sci (2019) 167(1):258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shankar P, Geier MC, Truong L, McClure RS, Pande P, Waters KM, Tanguay RL: Coupling genome-wide transcriptomics and developmental toxicity profiles in zebrafish to characterize polycyclic aromatic hydrocarbon (PAH) hazard. Int J Mol Sci (2019) 20(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vliet SM, Dasgupta S, Volz DC: Niclosamide induces epiboly delay during early zebrafish embryogenesis. Toxicol Sci (2018) 166(2):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balik-Meisner MR, Mav D, Phadke DP, Everett LJ, Shah RR, Tal T, Shepard PJ, Merrick BA, Paules RS: Development of a zebrafish s1500+ sentinel gene set for high-throughput transcriptomics. Zebrafish (2019) 16(4):331–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Driessen M, Vitins AP, Pennings JLA, Kienhuis AS, van de Water B, van der Ven LTM: A transcriptomics-based hepatotoxicity comparison between the zebrafish embryo and established human and rodent in vitro and in vivo models using cyclosporine a, amiodarone and acetaminophen. Toxicology Letters (2015) 232(2):403–412. [DOI] [PubMed] [Google Scholar]
- 40.Cheng V, Dasgupta S, Reddam A, Volz DC: Ciglitazone-a human PPARgamma agonist-disrupts dorsoventral patterning in zebrafish. PeerJ (2019) 7:e8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, DeSantis DF et al. : Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell (2015) 32(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi A, Kontarakis Z, Gerri C, Nolte H, Holper S, Kruger M, Stainier DY: Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature (2015) 524(7564):230–233. [DOI] [PubMed] [Google Scholar]
- 43.Kettleborough RNW, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, Sealy I, White RJ, Herd C, Nijman IJ, Fenyes F et al. : A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature (2013) 496(7446):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gawdzik JC, Yue MS, Martin NR, Elemans LMH, Lanham KA, Heideman W, Rezendes R, Baker TR, Taylor MR, Plavicki JS: Sox9b is required in cardiomyocytes for cardiac morphogenesis and function. Sci Rep (2018) 8(1):13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ et al. : DNA targeting specificity of RNA-guided CAS9 nucleases. Nat Biotechnol (2013) 31(9):827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mooney MR, Davis EE, Katsanis N: Analysis of single nucleotide variants in crisprcas9 edited zebrafish exomes shows no evidence of off-target inflation. Front Genet (2019) 10:949. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● Using whole exome sequencing on two generations of offspring from the same founding pair and their CRISPR-Cas9-edited offspring, the authors measured transmission of variants to the next generation and found no evidence of an increase in off-target effects. This study shows that CRISPR-Cas9 genome editing does not cause an increase in unwanted point mutations, which has previously been raised as a potential barrier to the use of this gene-editing technology.
- 47.Chaturantabut S, Shwartz A, Evason KJ, Cox AG, Labella K, Schepers AG, Yang S, Acuna M, Houvras Y, Mancio-Silva L, Romano S et al. : Estrogen activation of g-protein-coupled estrogen receptor 1 regulates phosphoinositide 3-kinase and mtor signaling to promote liver growth in zebrafish and proliferation of human hepatocytes. Gastroenterology (2019) 156(6):1788–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● Using gene editing, the authors identified G-protein coupled estrogen receptor 1 (GPER1)-dependent mechanisms in estrogen effects on development, regeneration and tumorigenesis in zebrafish liver and demonstrates a similar mechanism mediates estrogen effects on human primary hepatocytes. The findings of this study identify GPER1 as a potential target for liver cancer treatment.
- 48.Zimmerman MA, Budish RA, Kashyap S, Lindsey SH: Gper-novel membrane oestrogen receptor. Clin Sci (Lond) (2016) 130(12):1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian J, Hu J, Chen M, Yin H, Miao P, Bai P, Yin J: The use of mrp1-deficient (Danio rerio) zebrafish embryos to investigate the role of mrp1 in the toxicity of cadmium chloride and benzo[a]pyrene. Aquat Toxicol (2017) 186(123–133. [DOI] [PubMed] [Google Scholar]; ● Using CRISPR-Cas9-dependent gene editing in zebrafish, the authors show that the mrp1 transporter gene is critical for mitigating the toxic effects of cadmium and benzo[a]pyrene via cell efflux. This study leveraged cutting-edge gene editing techniques to reveal new knowledge about mechanisms of detoxification, and provides a tool to evaluate a key mechanism by which zebrafish, and potentially other organisms, combat xenobiotic toxicity.
- 50.Souder JP, Gorelick DA: ahr2, but not ahr1a or ahr1b, is required for craniofacial and fin development and TCDD-dependent cardiotoxicity in zebrafish. Toxicol Sci (2019) 170(1):25–44. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● Using genetic and pharmacologic approaches, the authors demonstrated that ahr2, but not ahr1a or ahr1b, is required for normal development of adult zebrafish fins and craniofacial structures. From a toxicological perspective, the authors confirmed that ahr2 mediates TCDD-dependent toxicity and demonstrated that loss of ahr1a or ahrb was not sufficient to block TCDD toxicity. This study used gene editing to elucidate the role of ahr genes in the context of TCDD-dependent toxicity and examine their crosstalk with estrogen receptor genes.
- 51.Hofsteen P, Plavicki J, Johnson SD, Peterson RE, Heideman W: Sox9b is required for epicardium formation and plays a role in TCDD-induced heart malformation in zebrafish. Mol Pharmacol (2013) 84(3):353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia GR, Shankar P, Dunham CL, Garcia A, La Du JK, Truong L, Tilton SC, Tanguay RL: Signaling events downstream of AhR activation that contribute to toxic responses: The functional role of an AhR-dependent long noncoding RNA (slincr) using the zebrafish model. Environ Health Perspect (2018) 126(11):117002. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● The authors demonstrate recruitment of a long noncoding RNA (slincR) represses sox9b transcription in response to TCDD exposure, identify several PAHs that upregulate slincR expression, and identify potential mouse and human orthologs of slincR. This zebrafish study provides greater insight into ligand-specific mechanisms of AhR-mediated toxicity that may be relevant in humans.
- 53.Haggard DE, Das SR, Tanguay RL: Comparative toxicogenomic responses to the flame retardant MITP in developing zebrafish. Chem Res Toxicol (2017) 30(2):508–515. [DOI] [PubMed] [Google Scholar]
- 54.Dach K, Yaghoobi B, Schmuck MR, Carty DR, Morales KM, Lein PJ: Teratological and behavioral screening of the national toxicology program 91-compound library in zebrafish (Danio rerio). Toxicol Sci (2019) 167(1):77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaballah SG, Swank A, Sobus JR, Howey XM, Schmid J, Catron T, McCord J, Hines E, Strynar M, Tal T: Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to GenX and other PFAS. Environmental Health Perspectives (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Padilla S, Corum D, Padnos B, Hunter DL, Beam A, Houck KA, Sipes N, Kleinstreuer N, Knudsen T, Dix DJ, Reif DM: Zebrafish developmental screening of the ToxCast Phase I chemical library. Reprod Toxicol (2012) 33(2):174–187. [DOI] [PubMed] [Google Scholar]
- 57.Teixido E, Kiessling TR, Krupp E, Quevedo C, Muriana A, Scholz S: Automated morphological feature assessment for zebrafish embryo developmental toxicity screens. Toxicol Sci (2019) 167(2):438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● The authors developed new image analysis software that allows for unbiased and automated quantification of morphological endpoints during zebrafish development, which has been an obstacle in this field in the past.
- 58.Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL: Multidimensional in vivo hazard assessment using zebrafish. Toxicol Sci (2014) 137(1):212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Volz DC, Hipszer RA, Leet JK, Raftery TD: Leveraging embryonic zebrafish to prioritize ToxCast testing. Environ Sci Tech Let (2015) 2(7):171–176. [Google Scholar]
- 60.Sipes NS, Martin MT, Reif DM, Kleinstreuer NC, Judson RS, Singh AV, Chandler KJ, Dix DJ, Kavlock RJ, Knudsen TB: Predictive models of prenatal developmental toxicity from ToxCast high-throughput screening data. Toxicol Sci (2011) 124(1):109–127. [DOI] [PubMed] [Google Scholar]
- 61.Ducharme NA, Reif DM, Gustafsson JA, Bondesson M: Comparison of toxicity values across zebrafish early life stages and mammalian studies: Implications for chemical testing. Reprod Toxicol (2015) 55:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● A meta-analysis strategy revealed that certain zebrafish developmental toxicity endpoints accurately predict relative acute toxicity of specific in vivo rodent model guideline study outcomes. Importantly, the authors leveraged zebrafish toxicity data and human exposure data to identify high priority compounds. This study provides a roadmap for prioritizing toxicity testing of environmental contaminants relevant to human health based, in part, on zebrafish developmental toxicity endpoints.
- 62.Kleinstreuer N, Dix D, Rountree M, Baker N, Sipes N, Reif D, Spencer R, Knudsen T: A computational model predicting disruption of blood vessel development. PLoS Comput Biol (2013) 9(4):e1002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tal TL, McCollum CW, Harris PS, Olin J, Kleinstreuer N, Wood CE, Hans C, Shah S, Merchant FA, Bondesson M, Knudsen TB et al. : Immediate and long-term consequences of vascular toxicity during zebrafish development. Reprod Toxicol (2014) 48:51–61. [DOI] [PubMed] [Google Scholar]
- 64.Tal T, Kilty C, Smith A, LaLone C, Kennedy B, Tennant A, McCollum CW, Bondesson M, Knudsen T, Padilla S, Kleinstreuer N: Screening for angiogenic inhibitors in zebrafish to evaluate a predictive model for developmental vascular toxicity. Reprod Toxicol (2017) 70:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward R, Ali Z, Slater K, Reynolds AL, Jensen LD, Kennedy BN: Pharmacological restoration of visual function in a zebrafish model of von-hippel Lindau disease. Dev Biol (2020) 457(2):226–234. [DOI] [PubMed] [Google Scholar]; ●● von-Hippel Lindau (VHL) syndrome is a rare, systemic disease in which loss-of-function mutations in the tumor suppressor gene VHL leads to vision loss associated with retinal capillary hemangioblastomas (tumors of retinal blood vessels). Genetic knockout of Vhl in mice is embryolethal, therefore, in this study, the authors engineered vhl knockout zebrafish as an alternative vertebrate model of VHL syndrome. The authors not only demonstrate that vhl null zebrafish exhibit pathological lesions in the eye and deficits in vision similar to human VHL patients, but also identify sunitinib malate as a novel therapeutic agent for treating VHL-associated vision loss.
- 66.Early JJ, Cole KL, Williamson JM, Swire M, Kamadurai H, Muskavitch M, Lyons DA: An automated high-resolution in vivo screen in zebrafish to identify chemical regulators of myelination. Elife (2018) 7:35136. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● This paper describes an exciting advance in automating histologic analysis of zebrafish, which has been a significant bottleneck in using histologic endpoints in higher throughput screening assays. The authors demonstrated the feasibility of the system by screening a small library of 175 chemicals for effects on myelination in larvae of transgenic zebrafish that expressed eGFP in myelinating oligodendrocytes Tg(mbp:eGFP). Three novel compounds that strongly enhanced the number of myelinating oligodendrocytes were identified. Importantly, this imaging platform and analysis pipeline appears flexible enough to adapt to high-resolution imaging-based screens of diverse phenotypes.
- 67.Lam PY, Kutchukian P, Anand R, Imbriglio J, Andrews C, Padilla H, Vohra A, Lane S, Parker DL Jr., Cornella Taracido I, Johns DG et al. : Cyp1 inhibition prevents doxorubicin-induced cardiomyopathy in a zebrafish heart-failure model. Chembiochem (2020) [Online pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sips PY, Shi X, Musso G, Nath AK, Zhao Y, Nielson J, Morningstar J, Kelly AE, Mikell B, Buys E, Bebarta V et al. : Identification of specific metabolic pathways as druggable targets regulating the sensitivity to cyanide poisoning. PLoS One (2018) 13(6):e0193889. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● Cyanide causes rapid toxicity via inhibition of cytochrome c oxidase-dependent cellular respiration, and while there are approved antidotes for cyanide poisoning, these are effective only when administered during the acute phase of cyanide poisoning. In this study, the authors leveraged their observation that zebrafish embryos are highly resistant to cyanide during the first 3 dpf but become progressively more sensitive to identify significant age-dependent differences in energy metabolism during cyanide exposure that led to the identification and validation of small molecules that modulate the tricarboxylic acid cycle and related metabolic processes as potential antidotes for cyanide poisoning.
- 69.Hamm JT, Ceger P, Allen D, Stout M, Maull EA, Baker G, Zmarowski A, Padilla S, Perkins E, Planchart A, Stedman D et al. : Characterizing sources of variability in zebrafish embryo screening protocols. ALTEX (2019) 36(1):103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia GR, Bugel SM, Truong L, Spagnoli S, Tanguay RL: Ahr2 required for normal behavioral responses and proper development of the skeletal and reproductive systems in zebrafish. PLoS One (2018) 13(3):e0193484. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● The authors generated a zebrafish ahr2 null line using the CRISPR-Cas9 system to demonstrate that ahr2 is required for zebrafish reproduction and fertility, development of adult fins and skeletal structures, as well as larval and adult behavioral responses. The ahr2 null line was also resistant to TCDD-dependent toxicity, confirming that disruption of AhR2-dependent signaling is a key event in this chemical’s mode of action.
- 71.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S et al. : The zebrafish reference genome sequence and its relationship to the human genome. Nature (2013) 496(7446):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacRae CA, Peterson RT: Zebrafish as tools for drug discovery. Nat Rev Drug Discov (2015) 14(10):721–731. [DOI] [PubMed] [Google Scholar]
- 73.Griffin AL, Jaishankar P, Grandjean JM, Olson SH, Renslo AR, Baraban SC: Zebrafish studies identify serotonin receptors mediating antiepileptic activity in Dravet syndrome. Brain Commun (2019) 1(1):fcz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gistelinck C, Kwon RY, Malfait F, Symoens S, Harris MP, Henke K, Hawkins MB, Fisher S, Sips P, Guillemyn B, Bek JW et al. : Zebrafish type i collagen mutants faithfully recapitulate human type i collagenopathies. Proc Natl Acad Sci U S A (2018) 115(34):E8037–E8046. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● Type I collagenopathies are a heterogenous group of connective tissue disorders, caused by genetic defects in type I collagen. Inherent to these disorders is a large clinical variability, for which the underlying molecular basis remains undefined. By systematically analyzing skeletal phenotypes in a large set of type I collagen zebrafish mutants, the authors show that zebrafish phenocopy different forms of human type I collagenopathies, suggesting a similar pathogenetic basis. This study illustrates the potential of zebrafish as a tool to further dissect the molecular basis of phenotypic variability in human type I collagenopathies, to improve diagnostic strategies, and enhance the discovery of novel therapeutic targets for treating these disorders.
- 75.Wu RS, Lam II, Clay H, Duong DN, Deo RC, Coughlin SR: A rapid method for directed gene knockout for screening in G0 zebrafish. Dev Cell (2018) 46(1):112–125. [DOI] [PubMed] [Google Scholar]
- 76.Miller GW, Chandrasekaran V, Yaghoobi B, Lein PJ: Opportunities and challenges for using the zebrafish to study neuronal connectivity as an endpoint of developmental neurotoxicity. Neurotoxicology (2018) 67(102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu J, Baraban SC: Network properties revealed during multi-scale calcium imaging of seizure activity in zebrafish. eNeuro (2019) 6(1):e0041. [DOI] [PMC free article] [PubMed] [Google Scholar]; ●● Dynamic tracking of neural activity across integrated neuronal networks at the level of the brain is critical to understanding how seizures initiate and propagate. The authors addressed this data gap by using a well-established larval zebrafish model of seizure activity and fast confocal imaging of larvae expressing genetically encoded calcium indicator (GCaMP). They found that seizure activity rapidly propagates from anterior-to-posterior brain regions in the zebrafish brain, and that neuronal subpopulations are active during interictal-like periods in a manner similar to that seen in human EEG recordings. Collectively, this work suggests the potential for non-invasive optical imaging approaches to advance understanding of the network basis underlying seizures and facilitate the development of methods to suppress these events.
- 78.Quevedo C, Behl M, Ryan K, Paules RS, Alday A, Muriana A, Alzualde A: Detection and prioritization of developmentally neurotoxic and/or neurotoxic compounds using zebrafish. Toxicol Sci (2019) 168(1):225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bandara SB, Carty DR, Singh V, Harvey DJ, Vasylieva N, Pressly B, Wulff H, Lein PJ: Susceptibility of larval zebrafish to the seizurogenic activity of GABA type A receptor antagonists. Neurotoxicology (2020) 76:220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]; ● While zebrafish are increasingly used to study seizure disorders, most studies rely on locomotor assays to assess seizurogenic activity, which has raised significant questions about the zebrafish as a valid model of human seizure disorders. In this study, the authors demonstrated that GABA type A receptor antagonists known to cause status epilepticus in mammalian models, also caused sustained locomotor responses and seizure-like electrical activity in the brain of larval zebrafish. The authors also showed that these chemical-induced responses were attenuated by positive allosteric modulators of the GABA-A receptor. These data validate the zebrafish as a tool for screening chemical libraries for both pro- and anti-seizure activity.
- 80.Schubert S, Keddig N, Hanel R, Kammann U: Microinjection into zebrafish embryos (Danio rerio) - a useful tool in aquatic toxicity testing. Environ Sci Eur (2014) 26:32. [Google Scholar]


