Abstract
Although the catalytic carboxylation of unactivated alkyl electrophiles has reached remarkable levels of sophistication, the intermediacy of (phenanthroline)Ni(I)–alkyl species—complexes proposed in numerous Ni-catalyzed reductive cross-coupling reactions—has been subject to speculation. Herein we report the synthesis of such elusive (phenanthroline)Ni(I) species and their reactivity with CO2, allowing us to address a long-standing question related to Ni-catalyzed carboxylation reactions.
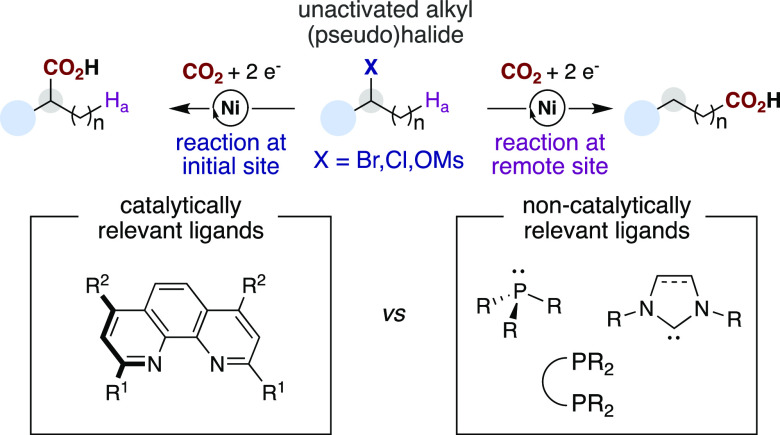
Over the past decade, Ni-catalyzed reductive carboxylation reactions involving organic (pseudo)halides and carbon dioxide have received considerable attention as methodologies for the preparation of many synthetically useful carboxylic acids.1 Among the wide variety of Ni-catalyzed reductive carboxylation reactions developed to date, the carboxylation of unactivated alkyl (pseudo)halides possessing β-hydrogens was found to be particularly challenging.2 This is likely due to the propensity of the alkylnickel intermediates that are formed via C(sp3)–X scission (X = Br, Cl, OSO2R) to undergo unproductive reduction, β-hydride elimination, and homocoupling reactions.3 Although nickel catalysts supported by (di)phosphine or N-heterocyclic carbene ligands are routinely employed in a myriad of Ni-catalyzed C–C and C–heteroatom bond-forming reactions,4 only finely tuned 1,10-phenanthroline derivatives—phen ligands—have enabled the carboxylation of unactivated alkyl electrophiles either at the initial C(sp3)–X site or at remote C(sp3)–H bonds via chain-walking of the Ni catalyst along the alkyl side chain (Scheme 1).2,5 Furthermore, a careful analysis of the literature indicates that phen ligands are also crucial for a wide number of Ni-catalyzed cross-couplings of unactivated alkyl halides, indicating that the importance of these ligands extends beyond carboxylation reactions.4,6
Scheme 1. Carboxylation of Unactivated Alkyl Electrophiles.
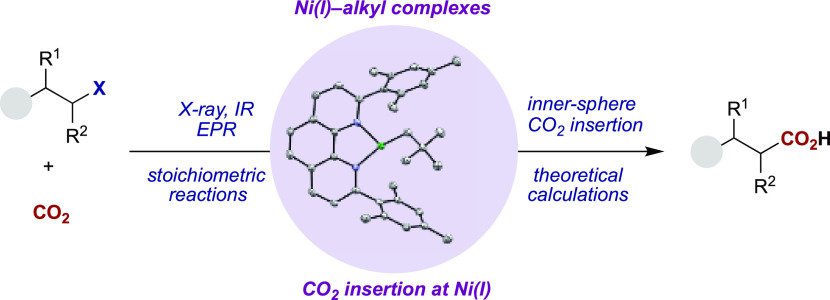
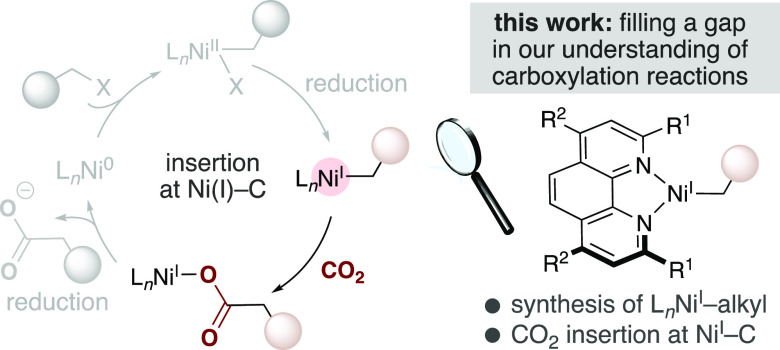
Despite significant advances in methodology design, the mechanism of the Ni-catalyzed reductive carboxylation of unactivated alkyl (pseudo)halides with CO2 is poorly understood. At present, our knowledge is primarily based on studies using aryl (pseudo)halide substrates. These suggest that CO2 insertion at a (phen)Ni(I)–alkyl complex is a crucial elementary step (Scheme 2, left).7,8 However, it is worth noting that no (phen)Ni(I)–alkyl complexes have been structurally characterized or even observed spectroscopically, probably because of the fleeting nature and high reactivity of these paramagnetic species.9 Elegant efforts toward this goal were recently described by Diao, and cultimated in the synthesis of (diphosphine)Ni(I)–alkyl complexes and investigations into their reactivity with CO2.10,11 Unfortunately, diphosphine ligands have not been shown to facilitate the Ni-catalyzed carboxylation of unactivated alkyl (pseudo)halides (Scheme 1).2,12 Therefore, a study aimed at preparing well-defined Ni(I)–alkyl complexes bearing catalytically relevant phen ligands would represent (a) an opportunity to study the reactivity of elusive Ni(I)–alkyl complexes supported by nitrogen-donor ligands, (b) a foundation for investigating the mechanistic intricacies of catalytic reductive carboxylation reactions, and (c) a starting point for understanding the speciation of Ni catalysts supported by phen ligands in related cross-coupling and chain-walking reactions.4 Herein we report the realization of these goals through the synthesis and isolation of Ni(I)–alkyl complexes bearing phen ligands, which has enabled us to obtain experimental evidence for rapid CO2 insertion at Ni(I)–carbon bonds (Scheme 2, right). These results not only shed light on a long-speculated mechanistic step but also support efforts to exploit and expand the reactivity of (phen)Ni(I)–alkyl intermediates through photoredox or electrochemical methodologies.2a,2b,4,13
Scheme 2. Proposed Reductive Carboxylation Mechanism via CO2 Insertion at Phen-Ligated Ni(I)–Alkyl Species.
Our study began by establishing a route to Ni(I)–halide complexes bearing phen ligands L1 or L2. The choice of these ligands was not arbitrary, as substituents adjacent to the nitrogen donor atoms are critical in Ni-catalyzed reductive carboxylation reactions of unactivated alkyl (pseudo)halides.2 Steric shielding by the bulky mesityl substituents of L1 may help to stabilize our targeted Ni(I)–alkyl complexes, which are likely highly reactive.9,14 Additionally, L2 is employed in the Ni-catalyzed chain-walking carboxylation of alkyl bromides.2c We envisioned that (L)Ni(I)–alkyl species could be accessed by alkylation of inner-sphere Ni(I)–halide complexes with an appropriate organometallic reagent. However, at the outset of our investigations, it was unclear whether an inner-sphere (L)Ni(I)–halide precursor could be obtained, as the most closely related reported species bearing a phen ligand was the outer-sphere halide complex [Ni(L)2]Cl, formed via oxidation of Ni(0)L2 (L = 2,9-dimethylphen) with AgCl.8,14,16 In order to avoid the synthesis of Ni(0)L2 complexes and the purification steps required to remove oxidation byproducts, we hypothesized that inner sphere (L)Ni(I)X (X = Br, Cl) might be obtained via comproportionation of (L)NiX2 with [Ni(COD)2] in the presence of 1 equiv of bulky L.15,16 This was indeed the case, and deep-blue (L)Ni(I)X species were obtained in high yields (Figure 1, left). The presence of the inner-sphere halide ligand was confirmed by X-ray crystallographic analysis of 1-Cl and 2-Cl. In addition, the axial electron paramagnetic resonance (EPR) spectra of the four (L)Ni(I)X complexes at 77 K support the presence of a Ni-centered radical. These results are noteworthy, as they represent examples of Ni(I) complexes bearing phen ligands with the halide directly coordinated to the Ni center.16,17 With a reliable route to (L1,L2)Ni(I)X in hand, we turned our attention to accessing the targeted Ni(I)–alkyl complexes via alkylation. An initial survey of the stability of the resulting Ni(I)–alkyl products was carried out by monitoring these reactions using EPR spectroscopy. As expected, the choice of alkyl group, reaction temperature, and ligand employed all influenced the reaction outcome. For example, reactions with EtMgBr and MeMgCl resulted in negligible amounts of new metal-centered radicals, if any. Analysis of these reactions by 1H NMR spectroscopy indicated the presence of Ni(0)Ln complexes, suggesting decomposition pathways arising from β-hydride elimination, reduction, and/or homolytic cleavage.18
Figure 1.
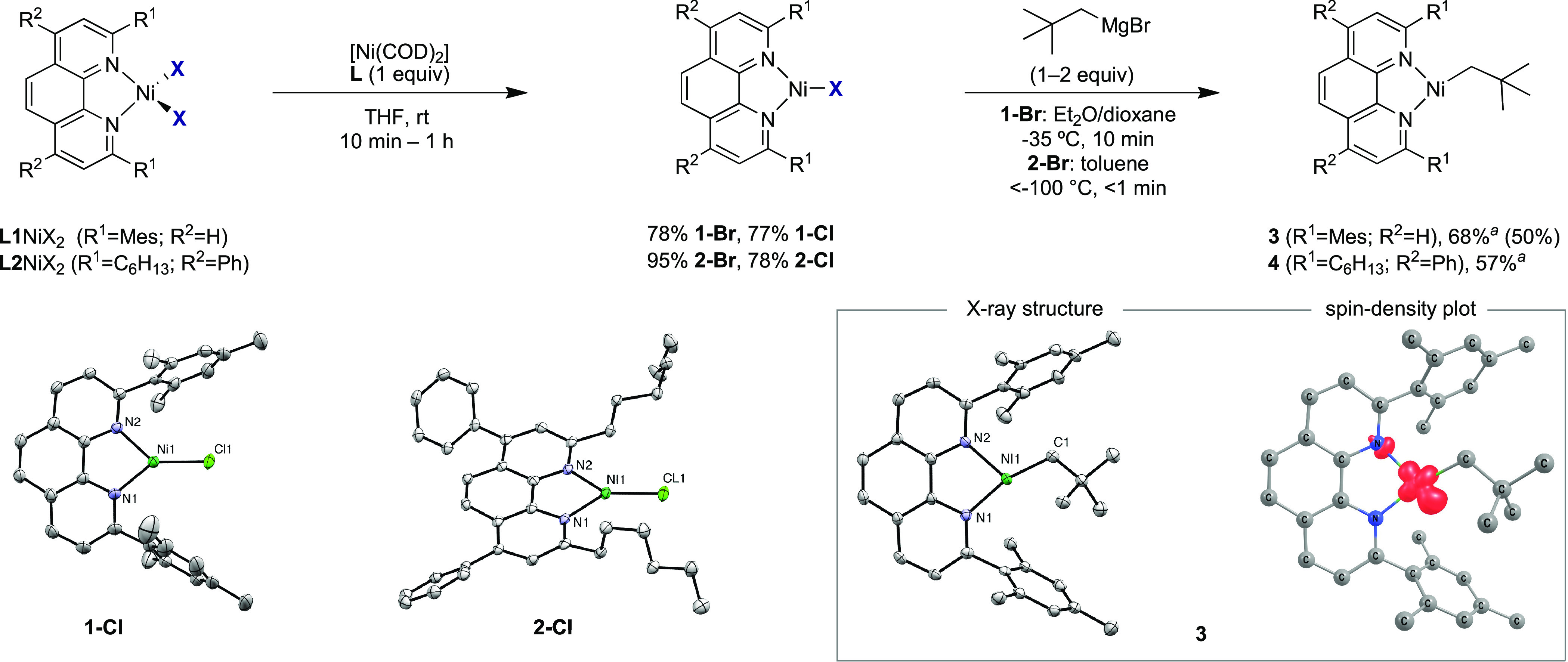
(top) Synthesis of Ni(I)–X and Ni(I)–alkyl complexes. aYield determined by EPR spectroscopy against Cu(II) standards. All other yields are isolated yields (0.010 mmol scale for 3). (bottom left and center) X-ray structures with thermal ellipsoids drawn at the 50% probability level (see the Supporting Information for details). Selected distances (Å) and angles (deg): 1-Cl: Ni1–Cl1 2.1064(6), N1–Ni1–Cl1 140.24(6), N2–Ni1–Cl1 136.32(6). 2-Cl: Ni–Cl1 2.1417(9), N1–Ni–Cl1 133.80(8), N2–Ni–Cl1 142.61(9). 3: Ni–C1 1.961(3), N1–Ni–C1 156.74(14), C1–Ni–N2 114.25(13). (bottom right) Calculated spin-density plot of 3 with a spin population of 0.94 on Ni (PBE-D3BJ/def2-TZVP, isovalue = 0.01; Figure S33).
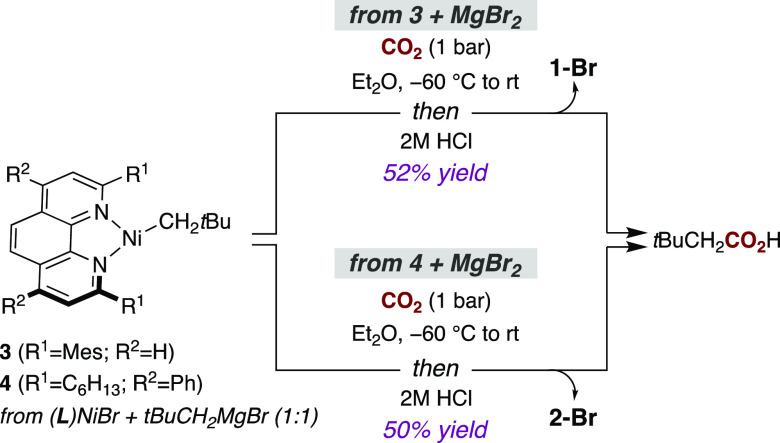
Gratifyingly, the reactions of 1-Br and 2-Br with neopentylMgBr resulted in new rhombic EPR spectra, suggesting that the desired alkylation may have taken place.19 Low-temperature crystallization (−35 °C, Et2O/pentane) furnished deep-green crystals suitable for X-ray diffraction, allowing us to identify three-coordinate [(L1)Ni(I)CH2tBu] (3) (Figure 1, right). Density functional theory (DFT) calculations support the Ni(I) description, with one unpaired electron centered on Ni (Figures 1 and S33). The synthesis of 3 is particularly noteworthy: to the best of our knowledge, it is the first Ni(I)–alkyl complex to be obtained with a catalytically relevant phen ligand. The Ni–C bond distance of 1.961(3) Å is similar to that of Ni(I) complexes bearing phosphine or NHC ligands.10,20 The Ni coordination plane is offset by ca. 23° from the mean plane through L1, presumably because of the steric bulk of the neopentyl ligand. Interestingly, the N–Ni–C angles in 3 are 114.25(13)° and 156.74(14)°. The distortion of 3 to this T-shaped geometry is similar to that observed in a related diphosphine species [(dtbpe)Ni(CH2tBu)] (dtbpe = 1,2-bis(di-tert-butylphosphino)ethane) (110.97(8)° and 157.82(8)°).20a This geometry is electronically favored for a range of three-coordinate Ni(I) complexes and differs from the Y-shaped geometry of 1-Cl and 2-Cl.21,22 We propose that the geometry of the latter complexes is due to the π-donating nature of the chloride ligand, which has been shown to favor Y-shaped complexes.22 Alkylation of 2-Br at low temperature gave [(L2)Ni(I)CH2tBu] (4) in 57% yield as estimated by EPR spectroscopy against a Cu(II) standard. Unfortunately, the thermal instability of 4 prevented its isolation or characterization by X-ray diffraction.
Next, we turned our attention to an investigation of CO2 insertion into the Ni(I)–C bond en route to Ni(I) carboxylate complexes, proposed to be the key elementary step in the catalytic carboxylation of alkyl (pseudo)halides (Figure 2).7,8 Prior to these insertion experiments, however, an anion metathesis reaction between 1-Cl and tBuCH2CO2K was performed to obtain reference EPR and IR spectra of the proposed CO2 insertion product (Figure 2, left). Gratifyingly, spectroscopic analysis of the reaction mixture showed the formation of a complex distinct from both 3 and 1-Cl and supported the formation of Ni(I)–carboxylate complex 5. For example, the band in the IR spectrum at 1543 cm–1 is suggestive of a νasym carboxylate stretch.23 Furthermore, although repeated attempts to crystallize 5 did not provide crystals suitable for X-ray diffraction, the observed stretching frequency combined with the absence of signals between 1200 and 1400 cm–1 suggests κ2 coordination of the carboxylate to the Ni(I) center.23a This was supported by DFT calculations that suggested a pseudotetrahedral geometry for 5 (Figure 3, right) with a computed stretching frequency of 1484 cm–1 (Figure S36). With these results in hand, we next investigated the reaction between 3 and CO2 (1 bar) at −60 °C (Figure 2, right). Analysis by EPR spectroscopy (77 K) showed the disappearance of the rhombic signal of 3 and the appearance of a new pseudoaxial signal with gx, gy > gz that very closely resembles the spectrum of 5 (Figure 2, center). Importantly, comparison of the IR spectrum of the product of direct CO2 insertion with that of the anion metathesis product showed an identical νasym carboxylate stretch at 1543 cm–1. Particularly illustrative was the disappearance of this signal and the appearance of new signals at lower wavenumber when the reaction was performed with 13CO2, providing evidence that 5 was formed via CO2 insertion into the Ni(I)–C bond. Calculations predicted a 34 cm–1 shift to lower wavenumbers upon incorporation of 13C, consistent with the observed shift of 38 cm–1 to a band at 1505 cm–1 (Figures S18 and S37). Insertion was also corroborated indirectly by quenching in situ-generated 5 with dilute HCl and observing a 52% yield of tert-butylacetic acid (Scheme 3, top). These observations are consistent with DFT calculations indicating facile CO2 insertion into the Ni(I)–C bond of 3 with a free energy barrier of 7.7 kcal mol–1 relative to 3 and free CO2 (Figure 3, middle). The calculations argue against the formation of a stable Ni–CO2 adduct before insertion (Figure S38), with interactions between Ni and CO2 first becoming significant at the carboxylation transition state (TS), where CO2 is significantly bent (137°) and interacts with Ni in a η2(C,O) fashion (Figure 3, center). Notably, an alternative outer-sphere insertion where CO2 does not interact with Ni in the transition state is predicted to have a barrier of 22.7 kcal mol–1, a 15.0 kcal mol–1 penalty compared to the inner-sphere pathway (Figure 3, center).24 Although an inner-sphere pathway has been calculated for the Ni/PCp3-catalyzed reductive carboxylation of benzyl halides,25 our data contrast with the outer-sphere pathway suggested for (Xantphos)Ni(I)–methyl and (PCP pincer)Ni(II)–methyl complexes.9,11 While one might argue that the bulky neopentyl group in 3 disfavors an outer-sphere pathway, we note that the inner-sphere pathway at the less sterically encumbered [(L1)Ni(I)Me] was still found to be favored computationally by 6.6 kcal mol–1 (Figure S39). This finding is important, as it supports the notion that the ancillary ligand can influence the mechanism of CO2 insertion.24,26
Figure 2.
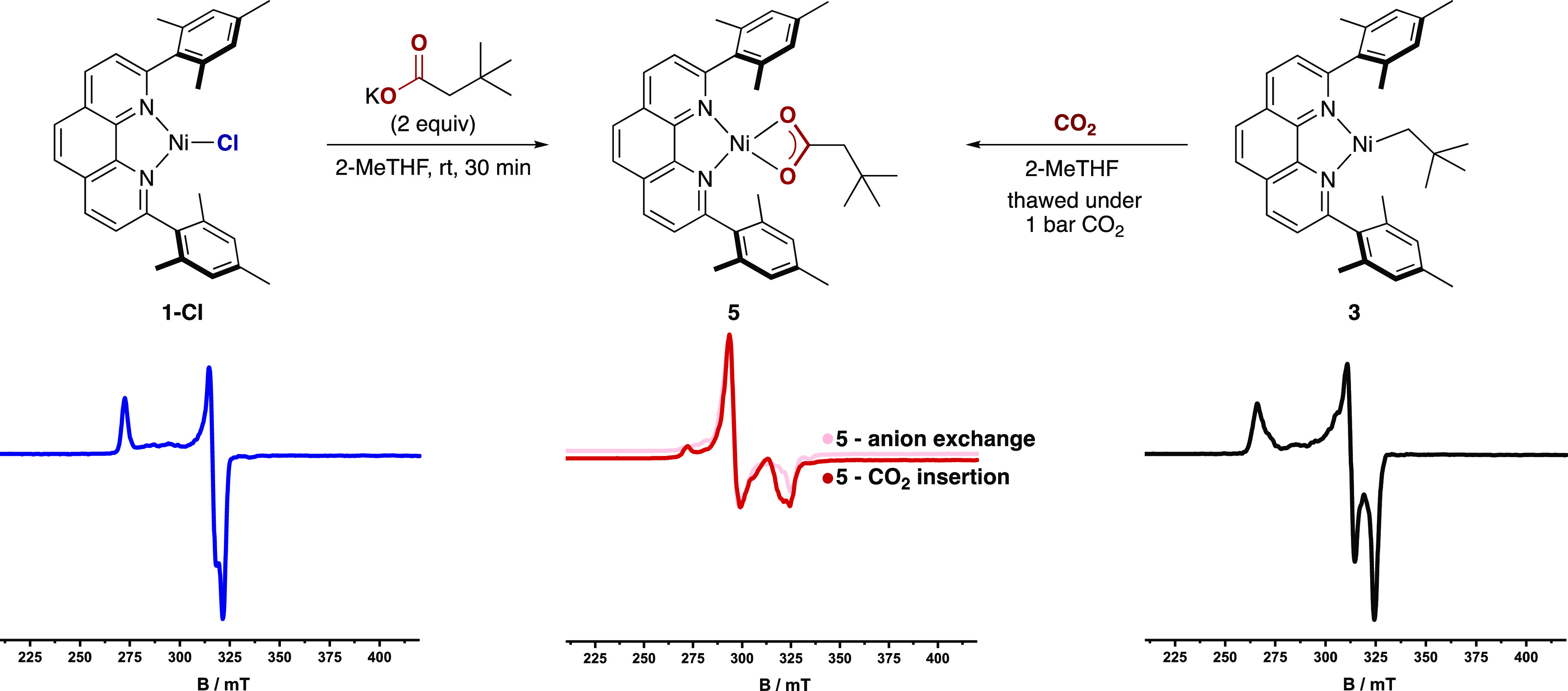
CO2 insertion at Ni(I). (top) Anion metathesis reaction (left) and CO2 insertion into 3 (right). (bottom) Changes in the 77 K X-band EPR spectra of 1-Cl (left, gx = 2.084, gy = 2.119, gz = 2.461) after anion metathesis and after CO2 insertion at 3 (right, gx = 2.065, gy = 2.145, gz = 2.519) to form 5 (center, gx = 2.299, gy = 2.272, gz = 2.064).
Figure 3.
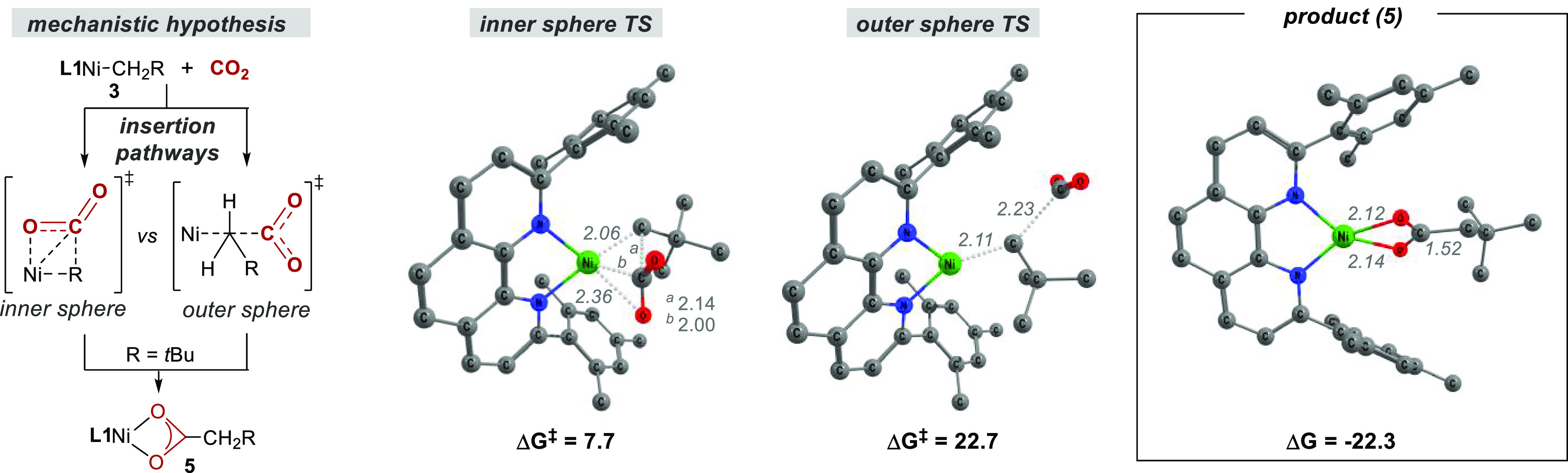
Optimized TS geometries for inner-sphere vs outer-sphere CO2 insertion and the optimized geometry of 5 (PBE-D3BJ/def2-TZVP/IEFPCM, H atoms omitted, distances in Å, energies in kcal mol–1 relative to 3 + free CO2, 298.15 K).
Scheme 3. CO2 Insertion En Route to tBuCH2CO2H.
Given the relevance of L2 in Ni-catalyzed carboxylation reactions, CO2 insertion at 4 was also studied. Although the sensitivity of 4 prevented workup to remove MgBr2 (the byproduct obtained by reacting (L2)Ni(I)Br with 1 equiv of neopentylMgBr), a 50% yield of tert-butylacetic acid was obtained upon exposure of a cold solution of 4 to CO2 (1 bar) and then to dilute HCl (Scheme 3, bottom). Interestingly, this reaction mixture rapidly turned blue upon CO2 addition, and only 2-Br was observed by EPR spectroscopy. This suggested that the L2 carboxylate complex [(L2)Ni(I)O2CCH2tBu] (6) resulting from CO2 insertion at Ni(I) underwent halide exchange with MgBr2 to form blue 2-Br. This was confirmed by the addition of MgBr2 to salt-free 5. Given the wide number of Ni-catalyzed reductive coupling reactions that employ MgX2 (X = Br, Cl) additives,27 the formation of 2-Br from in situ-generated 6 provides support for the formation of Ni(I) halide complexes prior to reduction to the propagating Ni(0)Ln species.28
In conclusion, we have investigated the synthesis and CO2 insertion reactivity of Ni(I)–alkyl complexes bearing catalytically relevant phen ligands. We have obtained experimental evidence for the rapid insertion of CO2 into Ni(I)–C bonds, a long-presumed elementary step in the reductive carboxylation of alkyl (pseudo)halides. Given the widespread use of phen ligands in Ni-catalyzed reactions, these results are expected to guide new investigations into the catalytic relevance of Ni(I)–alkyl complexes. Further investigations along these lines are currently underway in our laboratories.
Acknowledgments
R.M., R.J.S., and C.O. thank ICIQ, FEDER/MICIU-AEI/PGC2018-096839-B-100, and funding from “la Caixa” Foundation (ID 100010434) under Agreement LCF/BQ/SO15/52260010 for financial support. R.J.S. sincerely thanks “la Caixa” for a predoctoral fellowship. N.H. acknowledges support from the NIH NIGMS under Award R01GM120162. K.H.H. and M.F.O. acknowledge the Research Council of Norway (Grant 262695), the Tromsø Research Foundation (Grant TFS2016KHH), NordForsk (Grant 85378), and Notur (Grants nn9330k and nn4654k). We also gratefully acknowledge the X-ray Diffraction Research Support Area at ICIQ, Dr. Brandon Q. Mercado at Yale University for solving the structure of 1-Cl, and Dr. Georgiana Stoica from the Spectroscopy and Material Characterization Unit at ICIQ for her help with EPR studies. We also thank Dr. Megan Mohadjer Beromi and Dave Charboneau for insightful discussions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c04695.
Author Contributions
# C.O. and M.F.O. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- For selected reviews, see:; a Burkart M. D.; Hazari N.; Tway C. L.; Zeitler E. L. Opportunities and Challenges for Catalysis in Carbon Dioxide Utilization. ACS Catal. 2019, 9, 7937. 10.1021/acscatal.9b02113. [DOI] [Google Scholar]; b Yang Y.; Lee J.-W. Toward Ideal Carbon Dioxide Functionalization. Chem. Sci. 2019, 10, 3905. 10.1039/C8SC05539D. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Tortajada A.; Juliá-Hernández F.; Börjesson M.; Moragas T.; Martin R. Transition-Metal-Catalyzed Carboxylation Reactions with Carbon Dioxide. Angew. Chem., Int. Ed. 2018, 57, 15948. 10.1002/anie.201803186. [DOI] [PubMed] [Google Scholar]; d Yeung C. Photoredox Catalysis as a Strategy for CO2 Incorporation: Direct Access to Carboxylic Acids from a Renewable Feedstock. Angew. Chem., Int. Ed. 2019, 58, 5492. 10.1002/anie.201806285. [DOI] [PubMed] [Google Scholar]; e Artz J.; Müller T. E.; Thenert K.; Kleinekorte J.; Meys R.; Sternberg A.; Bardow A.; Leitner W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434. 10.1021/acs.chemrev.7b00435. [DOI] [PubMed] [Google Scholar]; f Cokoja M.; Bruckmeier C.; Rieger B.; Herrmann W. A.; Kühn F. E. Transformation of Carbon Dioxide with Homogeneous Transition-Metal Catalysts: A Molecular Solution to a Global Challenge?. Angew. Chem., Int. Ed. 2011, 50, 8510. 10.1002/anie.201102010. [DOI] [PubMed] [Google Scholar]; g Huang K.; Sun C. L.; Shi Z.-J. Transition-Metal-Catalyzed C–C Bond Formation through the Fixation of Carbon Dioxide. Chem. Soc. Rev. 2011, 40, 2435. 10.1039/c0cs00129e. [DOI] [PubMed] [Google Scholar]; h Carbon Dioxide as Chemical Feedstock; Aresta M., Ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]; i Sakakura T.; Choi J. C.; Yasuda H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365. 10.1021/cr068357u. [DOI] [PubMed] [Google Scholar]
- For selected references, see:; a Sahoo B.; Bellotti P.; Juliá-Hernández F.; Meng Q. – Y.; Crespi S.; König B.; Martin R. Site-Selective Remote sp3 C–H Carboxylation Enabled by the Merger of Photoredox and Nickel Catalysis. Chem. - Eur. J. 2019, 25, 9001. 10.1002/chem.201902095. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Meng Q. – Y.; Wang S.; König B. Carboxylation of Aromatic and Aliphatic Bromides and Triflates with CO2 by Dual Visible-Light Nickel Catalysis. Angew. Chem., Int. Ed. 2017, 56, 13426. 10.1002/anie.201706724. [DOI] [PubMed] [Google Scholar]; c Juliá-Hernández F.; Moragas T.; Cornella J.; Martin R. Remote Carboxylation of Halogenated Aliphatic Hydrocarbons with Carbon Dioxide. Nature 2017, 545, 84. 10.1038/nature22316. [DOI] [PubMed] [Google Scholar]; d Börjesson M.; Moragas T.; Martin R. Ni-Catalyzed Carboxylation of Unactivated Alkyl Chlorides with CO2. J. Am. Chem. Soc. 2016, 138, 7504. 10.1021/jacs.6b04088. [DOI] [PubMed] [Google Scholar]; e Wang X.; Liu Y.; Martin R. Ni-Catalyzed Divergent Cyclization/Carboxylation of Unactivated Primary and Secondary Alkyl Halides with CO2. J. Am. Chem. Soc. 2015, 137, 6476. 10.1021/jacs.5b03340. [DOI] [PubMed] [Google Scholar]; f Liu Y.; Cornella J.; Martin R. Ni-Catalyzed Carboxylation of Unactivated Primary Alkyl Bromides and Sulfonates with CO2. J. Am. Chem. Soc. 2014, 136, 11212. 10.1021/ja5064586. [DOI] [PubMed] [Google Scholar]
- For selected reviews on the use of unactivated alkyl electrophiles in cross-coupling reactions, see:; a Kambe N.; Iwasaki T.; Terao J. Pd-Catalyzed Cross-Coupling Reactions of Alkyl Halides. Chem. Soc. Rev. 2011, 40, 4937. 10.1039/c1cs15129k. [DOI] [PubMed] [Google Scholar]; b Hu X. Nickel-Catalyzed Cross-Coupling of Non-Activated Alkyl Halides: A Mechanistic Perspective. Chem. Sci. 2011, 2, 1867. 10.1039/c1sc00368b. [DOI] [Google Scholar]; c Jana R.; Pathak T. P.; Sigman M. S. Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-Organometallics as Reaction Partners. Chem. Rev. 2011, 111, 1417. 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For excellent authoritative reviews on Ni catalysis, see:; a Diccianni J. B.; Diao T. Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions. Trends Chem. 2019, 1, 830. 10.1016/j.trechm.2019.08.004. [DOI] [Google Scholar]; b Richmond E.; Moran J. Recent Advances in Nickel Catalysis Enabled by Stoichiometric Metallic Reducing Agents. Synthesis 2018, 50, 499. 10.1055/s-0036-1591853. [DOI] [Google Scholar]; c Ananikov V. P. Nickel: The “Spirited Horse” of Transition Metal Catalysis. ACS Catal. 2015, 5, 1964. 10.1021/acscatal.5b00072. [DOI] [Google Scholar]; d Tasker S.; Standley E.; Jamison T. Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299. 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a recent review on Ni-catalyzed chain-walking reactions, see:Janssen-Müller D.; Sahoo B.; Sun S.-Z.; Martin R. Tackling Remote sp3 C–H Functionalization via Ni-Catalyzed “Chain-walking” Reactions. Isr. J. Chem. 2020, 60, 195. 10.1002/ijch.201900072. [DOI] [Google Scholar]
- For selected reviews on Ni-catalyzed cross-coupling reactions, including those that make use of phen-type ligands, see:; a Goldfogel M. J.; Huang L.; Weix D. J.. Cross-Electrophile Coupling. In Nickel Catalysis in Organic Synthesis; Ogoshi S., Ed.; Wiley, 2019; pp 183–222. [Google Scholar]; b Gu J.; Wang X.; Xue W.; Gong H. Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Other Electrophiles: Concept and Mechanistic Considerations. Org. Chem. Front. 2015, 2, 1411. 10.1039/C5QO00224A. [DOI] [Google Scholar]; c Hu X. Nickel-Catalyzed Cross Coupling of Non-Activated Alkyl Halides: A Mechanistic Perspective. Chem. Sci. 2011, 2, 1867. 10.1039/c1sc00368b. [DOI] [Google Scholar]
- a García-López D.; Pavlovic L.; Hopmann K. H. To Bind or Not to Bind: Mechanistic Insights into C–CO2 Bond Formation with Late Transition Metals. Organometallics 2020, 39, 1339. 10.1021/acs.organomet.0c00090. [DOI] [Google Scholar]; b Obst M.; Pavlovic L.; Hopmann K. H. Carbon-Carbon Bonds with CO2: Insights from Computational Studies. J. Organomet. Chem. 2018, 864, 115. 10.1016/j.jorganchem.2018.02.020. [DOI] [Google Scholar]
- Somerville R. J.; Martin R.. Relevance of Ni(I) in Catalytic Carboxylation Reactions. In Nickel Catalysis in Organic Synthesis; Ogoshi S., Ed.; Wiley, 2019; pp 285–330. [Google Scholar]
- For a recent example of Ni(I)–aryl complexes bearing N-donor ligands, see:Mohadjer Beromi M.; Brudvig G. W.; Hazari N.; Lant H. M. C.; Mercado B. Q. Synthesis and Reactivity of Paramagnetic Polypyridyl Ni Complexes Relevant to C(Sp2)–C(Sp3) Coupling Reactions. Angew. Chem., Int. Ed. 2019, 58, 6094. 10.1002/anie.201901866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diccianni J. B.; Hu C. T.; Diao T. Insertion of CO2 Mediated by a (Xantphos)NiI–Alkyl Species. Angew. Chem., Int. Ed. 2019, 58, 13865. 10.1002/anie.201906005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For examples of CO2 insertion at PCP and PCN (pincer)Ni(II)–Me complexes, see:; a Mousa A. H.; Polukeev A. V.; Hansson J.; Wendt O. F. Carboxylation of the Ni–Me Bond in an Electron-Rich Unsymmetrical PCN Pincer Nickel Complex. Organometallics 2020, 39 (9), 1553. 10.1021/acs.organomet.9b00817. [DOI] [Google Scholar]; b Mousa A. H.; Bendix J.; Wendt O. F. Synthesis, Characterization, and Reactivity of PCN Pincer Nickel Complexes. Organometallics 2018, 37 (15), 2581–2593. 10.1021/acs.organomet.8b00333. [DOI] [Google Scholar]; c Jonasson K. J.; Wendt O. F. Synthesis and Characterization of a Family of POCOP Pincer Complexes with Nickel: Reactivity towards CO2 and Phenylacetylene. Chem. - Eur. J. 2014, 20, 11894. 10.1002/chem.201403246. [DOI] [PubMed] [Google Scholar]; d Schmeier T. J.; Hazari N.; Incarvito C. D.; Raskatov J. A. Exploring the Reactions of CO2 with PCP Supported Nickel Complexes. Chem. Commun. 2011, 47, 1824. 10.1039/C0CC03898A. [DOI] [PubMed] [Google Scholar]
- For control experiments using tBuXantphos in the catalytic carboxylation of unactivated alkyl halides, see the Supporting Information.
- For reviews on Ni-catalyzed reductive cross-coupling reactions, see:; a Diccianni J.; Lin Q.; Diao T. Mechanisms of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization. Acc. Chem. Res. 2020, 53, 906. 10.1021/acs.accounts.0c00032. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gu J.; Wang X.; Xue W.; Gong H. Nickel-Catalyzed Reductive Coupling of Alkyl Halides with other Electrophiles: Concept and Mechanistic Considerations. Org. Chem. Front. 2015, 2, 1411. 10.1039/C5QO00224A. [DOI] [Google Scholar]; c Weix J. D. Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res. 2015, 48, 1767. 10.1021/acs.accounts.5b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Moragas T.; Correa A.; Martin R. Metal-Catalyzed Reductive Coupling Reactions of Organic Halides with Carbonyl-Type Compounds. Chem. - Eur. J. 2014, 20, 8242. 10.1002/chem.201402509. [DOI] [PubMed] [Google Scholar]; e Knappke C. E. I.; Grupe S.; Gärtner D.; Corpet M.; Gosmini C.; Jacobi von Wangelin A. Reductive Cross-Coupling Reactions between Two Electrophiles. Chem. - Eur. J. 2014, 20, 6828. 10.1002/chem.201402302. [DOI] [PubMed] [Google Scholar]
- For the first reports of L1 complexes, see:; a Schmittel M.; Lüning U.; Meder M.; Ganz A.; Michel C.; Herderich M. Synthesis of Sterically Encumbered 2,9-Diaryl Substituted Phenanthrolines. Key Building Blocks for the Preparation of Mixed (Bis-Heteroleptic) Phenanthroline Copper(I) Complexes. Heterocycl. Commun. 1997, 3, 493. 10.1515/HC.1997.3.6.493. [DOI] [Google Scholar]; b Schmittel M.; Ganz A. Stable Mixed Phenanthroline Copper(I) Complexes. Key Building Blocks for Supramolecular Coordination Chemistry. Chem. Commun. 1997, 999. 10.1039/a701509g. [DOI] [Google Scholar]
- During the course of our studies, a comproportionation route to inner-sphere Ni(I)–halide complexes was reported. See:Zarate C.; Yang H.; Bezdek M. J.; Hesk D.; Chirik P. J. Ni(I)–X Complexes Bearing a Bulky α-Diimine Ligand: Synthesis, Structure, and Superior Catalytic Performance in the Hydrogen Isotope Exchange in Pharmaceuticals. J. Am. Chem. Soc. 2019, 141, 5034. 10.1021/jacs.9b00939. [DOI] [PubMed] [Google Scholar]
- During the course of our studies, an inner-sphere bromide complex bearing a bulky bipyridine ligand that was obtained via reduction of a Ni(II) complex was reported. See:Lin Q.; Diao T. Mechanism of Ni-Catalyzed Reductive 1,2-Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc. 2019, 141, 17937. 10.1021/jacs.9b10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2-Br and L1 were competent as the precatalyst and ligand, respectively, for the chain-walking carboxylation of 2-bromoheptane. See the Supporting Information and ref (2c) for details.
- It is worth noting that Ni(L2)2 was detected when L2 complexes were employed (see ref (2c)). For reactions with L1-bearing complexes, unusual [Ni(L1)]3 trimers and [Ni(L4)]4 tetramers crystallized from the reaction mixtures (see the Supporting Information for details).
- Reactions between 1-Cl or 2-Cl and neopentylMgBr also form 3.
- a Kitiachvili K. D.; Mindiola D. J.; Hillhouse G. L. Preparation of Stable Alkyl Complexes of Ni(I) and Their One-Electron Oxidation to Ni(II) Complex Cations. J. Am. Chem. Soc. 2004, 126, 10554. 10.1021/ja047052z. [DOI] [PubMed] [Google Scholar]; b Laskowski C. A.; Bungum D. J.; Baldwin S. M.; Del Ciello S. A.; Iluc V. M.; Hillhouse G. L. Synthesis and Reactivity of Two-Coordinate Ni(I) Alkyl and Aryl Complexes. J. Am. Chem. Soc. 2013, 135, 18272. 10.1021/ja4095236. [DOI] [PubMed] [Google Scholar]
- For a selection of T-shaped Ni(I) complexes, see:; a Kogut E.; Wiencko H. L.; Zhang L.; Cordeau D. E.; Warren T. H. A Terminal Ni(III)-Imide with Diverse Reactivity Pathways. J. Am. Chem. Soc. 2005, 127, 11248. 10.1021/ja0533186. [DOI] [PubMed] [Google Scholar]; b Eckert N. A.; Dinescu A.; Cundari T. R.; Holland P. L. A T-Shaped Three-Coordinate Nickel(I) Carbonyl Complex and the Geometric Preferences of Three-Coordinate d9 Complexes. Inorg. Chem. 2005, 44, 7702. 10.1021/ic0510213. [DOI] [PubMed] [Google Scholar]; c Iluc V. M.; Hillhouse G. L. Three-Coordinate Nickel Carbene Complexes and Their One-Electron Oxidation Products. J. Am. Chem. Soc. 2014, 136, 6479. 10.1021/ja501900j. [DOI] [PubMed] [Google Scholar]
- For information about the geometries of three-coordinate d9 complexes, see:; a Alvarez S. Bonding and Stereochemistry of Three-Coordinated Transition Metal Compounds. Coord. Chem. Rev. 1999, 193–195, 13. 10.1016/S0010-8545(99)00085-5. [DOI] [Google Scholar]; b Jean Y.; Marsden C. T.. Molecular Orbitals of Transition Metal Complexes; Oxford University Press, 2005. [Google Scholar]
- a Deacon G. B.; Phillips R. J. Relationships between the Carbon-Oxygen Stretching Frequencies of Carboxylato Complexes and the Type of Carboxylate Coordination. Coord. Chem. Rev. 1980, 33, 227. 10.1016/S0010-8545(00)80455-5. [DOI] [Google Scholar]; b Nara M.; Torii H.; Tasumi M. Correlation between the Vibrational Frequencies of the Carboxylate Group and the Types of Its Coordination to a Metal Ion: An Ab Initio Molecular Orbital Study. J. Phys. Chem. 1996, 100, 19812. 10.1021/jp9615924. [DOI] [Google Scholar]
- Hazari N.; Heimann J. E. Carbon Dioxide Insertion into Group 9 and 10 Metal-Element σ Bonds. Inorg. Chem. 2017, 56, 13655. 10.1021/acs.inorgchem.7b02315. [DOI] [PubMed] [Google Scholar]
- Sayyed F. B.; Sakaki S. The crucial roles of MgCl2 as a non-innocent additive in the Ni-catalyzed carboxylation of benzyl halide with CO2. Chem. Commun. 2014, 50, 13026. 10.1039/C4CC04962D. [DOI] [PubMed] [Google Scholar]
- For an example where the nature of the ligand affects the CO2 insertion pathway, see ref (7).
- For selected examples in which MgX2 additives were employed in Ni-catalyzed reductive cross-coupling reactions, see:; a Ye Y.; Chen H.; Yao K.; Gong H. Iron-Catalyzed Reductive Vinylation of Tertiary alkyl Oxalates with Activated Vinyl Halides. Org. Lett. 2020, 22, 2070. 10.1021/acs.orglett.0c00561. [DOI] [PubMed] [Google Scholar]; b Gao M.; Sun D.; Gong H. Ni-Catalyzed Reductive C–O Bond Arylation of Oxalates Derived from α-Hydroxy Esters with Aryl Halides. Org. Lett. 2019, 21, 1645. 10.1021/acs.orglett.9b00174. [DOI] [PubMed] [Google Scholar]; c Wang X.; Ma G.; Peng Y.; Pitsch C. E.; Moll B. J.; Ly T. D.; Wang X.; Gong H. Ni-Catalyzed Reductive Coupling of Electron-Rich Aryl Iodides with Tertiary Alkyl Halides. J. Am. Chem. Soc. 2018, 140, 14490. 10.1021/jacs.8b09473. [DOI] [PubMed] [Google Scholar]; d Fujihara T.; Horimoto Y.; Mizoe T.; Sayyed F. B.; Tani Y.; Terao J.; Sakaki S.; Tsuji Y. Nickel-Catalyzed Double Carboxylation of Alkynes Employing Carbon Dioxide. Org. Lett. 2014, 16, 4960. 10.1021/ol502538r. [DOI] [PubMed] [Google Scholar]; e Leon T.; Correa A.; Martin R. Nickel-Catalyzed Direct Carboxylation of Benzyl Halides. J. Am. Chem. Soc. 2013, 135, 1221. 10.1021/ja311045f. [DOI] [PubMed] [Google Scholar]
- Charboneau D. J.; Brudvig G. W.; Hazari N.; Lant H. M. C.; Saydjari A. K. Development of an Improved System for the Carboxylation of Aryl Halides through Mechanistic Studies. ACS Catal. 2019, 9, 3228. 10.1021/acscatal.9b00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.